User login
At 52 weeks, complete hair regrowth rates still climbing on deuruxolitinib
BERLIN – at the annual congress of the European Academy of Dermatology and Venereology.
With response curves still climbing at follow-up to date, the results are “truly, truly remarkable,” said Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
Deuruxolitinib is a JAK inhibitor that has specificity for the 1 and 2 subtypes. At 24 weeks in the phase 3 THRIVE-AA1 and THRIVE-AA2 trials, presented at the American Academy of Dermatology annual meeting earlier this year, about 40% of those on the 12-mg twice-daily dose and 32% of those on the 8-mg twice-daily dose achieved a Severity of Alopecia Tool (SALT) score of ≤ 20%, signifying 80% or greater hair regrowth at 24 weeks. The placebo response was 0%.
By 52 weeks, the proportion had climbed to 62% among those on continuous deuruxolitinib whether maintained on the 8-mg or 12-mg twice daily doses. Among patients on placebo, 58.4% reached this endpoint after being switched at 24 weeks to the 12-mg twice daily dose. Of the patients on placebo switched to 8 mg twice daily, the 52-week response was 45.2%, according to Dr. King.
There were 741 patients available at 52 weeks for this on-going analysis. The mean SALT scores at entry exceeded 80%, meaning complete or near complete hair loss. The substantial proportion of patients who met the primary endpoint of SALT ≤ 20 at the end of the blinded period was encouraging, but Dr. King said that the 52-week results are important, not only showing the response was sustained, but that greater regrowth occurs over time.
“Alopecia takes time to treat,” said Dr. King, summarizing the lesson from these data. Moreover, he added that the long-term data are likely to under represent the absolute benefit even if no further growth is achieved with even longer follow-up. One reason is that missing long-term data were accounted for with a last-observation-carried-forward approach.
In other words, “this is the floor when considering response at 52 weeks,” Dr. King said. “In the real world, where adjunctive measures such as intralesional Kenalog [triamcinolone acetonide] or topical treatments are added, we are likely to do even better,” he added.
Adverse events remained low
Treatment-emergent adverse events remained low with “nothing particularly surprising,” Dr. King said. The rate of serious adverse events over 52 weeks was less than 2% on either dose of deuruxolitinib. The proportion of patients who discontinued treatment because of an adverse event was 0.7% in the 8-mg twice-daily arm and 1.1% in the 12-mg twice-daily arm.
Most approved oral JAK inhibitors carry a boxed warning based on a trial conducted with the relatively nonspecific tofacitinib. The trial enrolled older patients with rheumatoid arthritis at risk for thrombotic events, raising questions about its relevance to selective JAK inhibitors employed for other indications. There was only one thrombosis observed in the 52-week alopecia areata follow-up in a patient on deuruxolitinib. Dr. King noted that this patient, who was obese and was on the higher of the two doses, had multiple comorbidities, including systemic lupus erythematosus.
There were no major adverse cardiac events reported in long-term follow-up or cases of tuberculosis. The rate of opportunistic infections was 0.1% in the 8-mg twice-daily arm and 0.2% in the 12-mg twice-daily arm. Serious infections were observed in 0.6% and 0.4% of these two arms, respectively. There were four malignancies (0.5%) in each of the two study arms.
Of the side effects likely to be related to deuruxolitinib, acne was observed in about 10% of patients on either dose. The mechanism is unclear, but Dr. King reported this has been commonly observed with other JAK inhibitors.
Asked his opinion about the optimal starting dose of deuruxolitinib, Dr. King said, “in my mind, the efficacy of 8 mg is so impressive that I would not struggle at all in starting there,” noting that the higher dose could be considered with a slow or inadequate response.
Two JAK inhibitors are already approved
If approved for alopecia areata, deuruxolitinib will be the third JAK inhibitor available for this indication, following the recent approvals of baricitinib and ritlecitinib.
Calling JAK inhibitors “a major advance in the treatment of alopecia areata, particularly for those patients with severe, refractory disease,” Lynne Goldberg, MD, professor of dermatology at Boston University, and director of the hair clinic, Boston Medical Center, said that the proportion of patients with SALT scores ≤ 20 at 52-weeks is “huge.”
She is generally comfortable with the safety of the JAK inhibitors for alopecia areata.
“I believe that, in general, these medications are well tolerated in the alopecia areata population, particularly in otherwise healthy, young patients,” she said, indicating the benefit-to-risk ratio is particularly acceptable when disease is severe.
“This disease has tremendous emotional and functional implications, and many patients with severe or recurrent disease are willing to chance the side effects to live with a full head of hair,” she said. She added that well-informed patients can “make their own, individual assessment.”
Dr. King has financial relationships with approximately 20 pharmaceutical companies, including Concert Pharmaceuticals, which makes deuruxolitinib and provided funding for this study. Dr. Goldberg reports no financial conflicts relevant to this topic.
BERLIN – at the annual congress of the European Academy of Dermatology and Venereology.
With response curves still climbing at follow-up to date, the results are “truly, truly remarkable,” said Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
Deuruxolitinib is a JAK inhibitor that has specificity for the 1 and 2 subtypes. At 24 weeks in the phase 3 THRIVE-AA1 and THRIVE-AA2 trials, presented at the American Academy of Dermatology annual meeting earlier this year, about 40% of those on the 12-mg twice-daily dose and 32% of those on the 8-mg twice-daily dose achieved a Severity of Alopecia Tool (SALT) score of ≤ 20%, signifying 80% or greater hair regrowth at 24 weeks. The placebo response was 0%.
By 52 weeks, the proportion had climbed to 62% among those on continuous deuruxolitinib whether maintained on the 8-mg or 12-mg twice daily doses. Among patients on placebo, 58.4% reached this endpoint after being switched at 24 weeks to the 12-mg twice daily dose. Of the patients on placebo switched to 8 mg twice daily, the 52-week response was 45.2%, according to Dr. King.
There were 741 patients available at 52 weeks for this on-going analysis. The mean SALT scores at entry exceeded 80%, meaning complete or near complete hair loss. The substantial proportion of patients who met the primary endpoint of SALT ≤ 20 at the end of the blinded period was encouraging, but Dr. King said that the 52-week results are important, not only showing the response was sustained, but that greater regrowth occurs over time.
“Alopecia takes time to treat,” said Dr. King, summarizing the lesson from these data. Moreover, he added that the long-term data are likely to under represent the absolute benefit even if no further growth is achieved with even longer follow-up. One reason is that missing long-term data were accounted for with a last-observation-carried-forward approach.
In other words, “this is the floor when considering response at 52 weeks,” Dr. King said. “In the real world, where adjunctive measures such as intralesional Kenalog [triamcinolone acetonide] or topical treatments are added, we are likely to do even better,” he added.
Adverse events remained low
Treatment-emergent adverse events remained low with “nothing particularly surprising,” Dr. King said. The rate of serious adverse events over 52 weeks was less than 2% on either dose of deuruxolitinib. The proportion of patients who discontinued treatment because of an adverse event was 0.7% in the 8-mg twice-daily arm and 1.1% in the 12-mg twice-daily arm.
Most approved oral JAK inhibitors carry a boxed warning based on a trial conducted with the relatively nonspecific tofacitinib. The trial enrolled older patients with rheumatoid arthritis at risk for thrombotic events, raising questions about its relevance to selective JAK inhibitors employed for other indications. There was only one thrombosis observed in the 52-week alopecia areata follow-up in a patient on deuruxolitinib. Dr. King noted that this patient, who was obese and was on the higher of the two doses, had multiple comorbidities, including systemic lupus erythematosus.
There were no major adverse cardiac events reported in long-term follow-up or cases of tuberculosis. The rate of opportunistic infections was 0.1% in the 8-mg twice-daily arm and 0.2% in the 12-mg twice-daily arm. Serious infections were observed in 0.6% and 0.4% of these two arms, respectively. There were four malignancies (0.5%) in each of the two study arms.
Of the side effects likely to be related to deuruxolitinib, acne was observed in about 10% of patients on either dose. The mechanism is unclear, but Dr. King reported this has been commonly observed with other JAK inhibitors.
Asked his opinion about the optimal starting dose of deuruxolitinib, Dr. King said, “in my mind, the efficacy of 8 mg is so impressive that I would not struggle at all in starting there,” noting that the higher dose could be considered with a slow or inadequate response.
Two JAK inhibitors are already approved
If approved for alopecia areata, deuruxolitinib will be the third JAK inhibitor available for this indication, following the recent approvals of baricitinib and ritlecitinib.
Calling JAK inhibitors “a major advance in the treatment of alopecia areata, particularly for those patients with severe, refractory disease,” Lynne Goldberg, MD, professor of dermatology at Boston University, and director of the hair clinic, Boston Medical Center, said that the proportion of patients with SALT scores ≤ 20 at 52-weeks is “huge.”
She is generally comfortable with the safety of the JAK inhibitors for alopecia areata.
“I believe that, in general, these medications are well tolerated in the alopecia areata population, particularly in otherwise healthy, young patients,” she said, indicating the benefit-to-risk ratio is particularly acceptable when disease is severe.
“This disease has tremendous emotional and functional implications, and many patients with severe or recurrent disease are willing to chance the side effects to live with a full head of hair,” she said. She added that well-informed patients can “make their own, individual assessment.”
Dr. King has financial relationships with approximately 20 pharmaceutical companies, including Concert Pharmaceuticals, which makes deuruxolitinib and provided funding for this study. Dr. Goldberg reports no financial conflicts relevant to this topic.
BERLIN – at the annual congress of the European Academy of Dermatology and Venereology.
With response curves still climbing at follow-up to date, the results are “truly, truly remarkable,” said Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn.
Deuruxolitinib is a JAK inhibitor that has specificity for the 1 and 2 subtypes. At 24 weeks in the phase 3 THRIVE-AA1 and THRIVE-AA2 trials, presented at the American Academy of Dermatology annual meeting earlier this year, about 40% of those on the 12-mg twice-daily dose and 32% of those on the 8-mg twice-daily dose achieved a Severity of Alopecia Tool (SALT) score of ≤ 20%, signifying 80% or greater hair regrowth at 24 weeks. The placebo response was 0%.
By 52 weeks, the proportion had climbed to 62% among those on continuous deuruxolitinib whether maintained on the 8-mg or 12-mg twice daily doses. Among patients on placebo, 58.4% reached this endpoint after being switched at 24 weeks to the 12-mg twice daily dose. Of the patients on placebo switched to 8 mg twice daily, the 52-week response was 45.2%, according to Dr. King.
There were 741 patients available at 52 weeks for this on-going analysis. The mean SALT scores at entry exceeded 80%, meaning complete or near complete hair loss. The substantial proportion of patients who met the primary endpoint of SALT ≤ 20 at the end of the blinded period was encouraging, but Dr. King said that the 52-week results are important, not only showing the response was sustained, but that greater regrowth occurs over time.
“Alopecia takes time to treat,” said Dr. King, summarizing the lesson from these data. Moreover, he added that the long-term data are likely to under represent the absolute benefit even if no further growth is achieved with even longer follow-up. One reason is that missing long-term data were accounted for with a last-observation-carried-forward approach.
In other words, “this is the floor when considering response at 52 weeks,” Dr. King said. “In the real world, where adjunctive measures such as intralesional Kenalog [triamcinolone acetonide] or topical treatments are added, we are likely to do even better,” he added.
Adverse events remained low
Treatment-emergent adverse events remained low with “nothing particularly surprising,” Dr. King said. The rate of serious adverse events over 52 weeks was less than 2% on either dose of deuruxolitinib. The proportion of patients who discontinued treatment because of an adverse event was 0.7% in the 8-mg twice-daily arm and 1.1% in the 12-mg twice-daily arm.
Most approved oral JAK inhibitors carry a boxed warning based on a trial conducted with the relatively nonspecific tofacitinib. The trial enrolled older patients with rheumatoid arthritis at risk for thrombotic events, raising questions about its relevance to selective JAK inhibitors employed for other indications. There was only one thrombosis observed in the 52-week alopecia areata follow-up in a patient on deuruxolitinib. Dr. King noted that this patient, who was obese and was on the higher of the two doses, had multiple comorbidities, including systemic lupus erythematosus.
There were no major adverse cardiac events reported in long-term follow-up or cases of tuberculosis. The rate of opportunistic infections was 0.1% in the 8-mg twice-daily arm and 0.2% in the 12-mg twice-daily arm. Serious infections were observed in 0.6% and 0.4% of these two arms, respectively. There were four malignancies (0.5%) in each of the two study arms.
Of the side effects likely to be related to deuruxolitinib, acne was observed in about 10% of patients on either dose. The mechanism is unclear, but Dr. King reported this has been commonly observed with other JAK inhibitors.
Asked his opinion about the optimal starting dose of deuruxolitinib, Dr. King said, “in my mind, the efficacy of 8 mg is so impressive that I would not struggle at all in starting there,” noting that the higher dose could be considered with a slow or inadequate response.
Two JAK inhibitors are already approved
If approved for alopecia areata, deuruxolitinib will be the third JAK inhibitor available for this indication, following the recent approvals of baricitinib and ritlecitinib.
Calling JAK inhibitors “a major advance in the treatment of alopecia areata, particularly for those patients with severe, refractory disease,” Lynne Goldberg, MD, professor of dermatology at Boston University, and director of the hair clinic, Boston Medical Center, said that the proportion of patients with SALT scores ≤ 20 at 52-weeks is “huge.”
She is generally comfortable with the safety of the JAK inhibitors for alopecia areata.
“I believe that, in general, these medications are well tolerated in the alopecia areata population, particularly in otherwise healthy, young patients,” she said, indicating the benefit-to-risk ratio is particularly acceptable when disease is severe.
“This disease has tremendous emotional and functional implications, and many patients with severe or recurrent disease are willing to chance the side effects to live with a full head of hair,” she said. She added that well-informed patients can “make their own, individual assessment.”
Dr. King has financial relationships with approximately 20 pharmaceutical companies, including Concert Pharmaceuticals, which makes deuruxolitinib and provided funding for this study. Dr. Goldberg reports no financial conflicts relevant to this topic.
At THE EADV CONGRESS
Tapinarof effective for AD in patients as young as 2 years
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
BERLIN – of age, according to results of two pivotal trials presented at the at the annual congress of the European Academy of Dermatology and Venereology.
If approved for AD, one advantage of tapinarof cream relative to topical corticosteroids is potential use “without restrictions on duration, extent, or site of application,” reported Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research, George Washington University, Washington.
Tapinarof cream, 1%, an aryl hydrocarbon receptor agonist, was approved in 2022 for treating plaque psoriasis in adults.
In the two phase 3 trials, ADORING 1 and ADORING 2, which were presented together at the meeting, the primary endpoint was Validated Investigator Global Assessment (vIGA) for AD of 0 (clear) or 1 (almost clear) at 8 weeks. For this endpoint and all secondary endpoints, the relative advantage of the active cream over the vehicle alone was about the same in both studies.
For example, the vIGA clear or almost clear response was met by 45.4% and 46.4% of those in the experimental arm of ADORING 1 and 2, respectively, but only 13.9% and 18.0% in the control arms (P < .0001 for both).
For the secondary endpoint of Eczema Area and Severity Index (EASI75), signifying 75% clearance of skin lesions, the response rates were 55.8% and 59.1% in the two trials, but only 22.9% and 24.1% in the respective control arms (P < .0001 for both).
The two identically designed trials randomized patients with moderate to severe AD in a 2:1 ratio to tapinarof cream or vehicle alone. There were 407 patients ages 2-81 years in ADORING I and 406 in ADORING 2. Patients were instructed to apply the active cream or vehicle once per day.
The safety data for tapinarof in these studies was generally consistent with the experience with this agent in plaque psoriasis. According to Dr. Silverberg, there was a modest increase in reports of headache early in this study, but these were transient. Follicular events were also more common on tapinarof than on its vehicle, but Dr. Silverberg said that the rate of discontinuations for adverse events, although low in both arms, was numerically lower in the active treatment arm in both trials.
“There were reports of contact dermatitis in the psoriasis studies, but we have not seen this in the AD trials,” Dr. Silverberg said.
Itch control evaluated
In a separate presentation of ADORING 1 and 2 results, Eric Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, provided detailed information about itch control, which was evaluated with the Peak Pruritus–Numerical Rating Scale (PP-NRS).
“The PP-NRS considers a person’s worst itch over the past 24 hours based on an 11-point scale,” explained Dr. Simpson, who said that patients scored itch daily with comparisons made at weeks 1, 2, 4, and 8.
Over time, pruritus scores fell in both groups, but reductions were far steeper among those in the active treatment arms.
“In ADORING 1, there were greater reductions in itch as early as day 1,” Dr. Simpson reported. Although the differences in itch were not detected until day 2 in ADORING 2, the differences were already significant and clinically meaningful in both studies by the end of the first week.
By week 8, the mean reductions in PP-NRS scores were 2.6 and 2.4 in the vehicle arms of ADORING 1 and 2, respectively. In the treatment arm, the reduction was 4.1 points in both arms (P < .0001 for both studies).
Forty-eight–week follow-up planned
More than 90% of patients in both studies have rolled over into the open-label extension ADORING 3 trial, with a planned follow-up of 48 weeks, according to Dr. Silverberg, who said that those in the placebo arm have been crossed over to tapinarof.
The response and the safety appear to be similar in adults and children, although Dr. Silverberg said that further analyses of outcomes by age are planned. He noted that there is also an ongoing study of tapinarof in children with plaque psoriasis.
In AD in particular, Dr. Silverberg said there is “an unmet need” for a topical nonsteroidal anti-inflammatory. While topical corticosteroids are a mainstay of AD therapy in children as well as adults, he noted the limitations of these drugs, including that they can only be applied for limited periods.
Tapinarof binds to the aryl hydrocarbon receptor (AhR), which regulates immune function in the skin and is expressed in many skin cell types. By inhibiting AhR, tapinarof blocks cytokine activation and has an antioxidant effect.
Adelaide A. Hebert, MD, professor and director of pediatric dermatology, McGovern Medical School at UTHealth, Houston, has participated in clinical studies of tapinarof for AD, and said she has been impressed with its efficacy and tolerability in children as well as adults. In the case of children, parents, as well as patients, “valued the rapid onset of disease control, the once-daily application regimen, and the itch control,” she said in an interview after the meeting.
If approved, Dr. Hebert said, “this novel steroid-free medication has the potential to change the management arena for pediatric and adult patients with moderate to severe atopic dermatitis.”
The recent introduction of new systemic therapies for AD, such as JAK inhibitors, has increased options for AD control, but “we still need effective and safe topical therapies, especially in children and young adults,” said Sonja Ständer, MD, head of the Interdisciplinary Center for Chronic Pruritus, University of Münster (Germany). Author of a comprehensive review article on AD in the New England Journal of Medicine 2 years ago, Dr. Ständer said results from the phase 3 topical tapinarof trials, as well as the phase 3 topical ruxolitinib trials, which were also presented as late breakers at the 2023 EADV meeting, provide “hope that an alternative to topical steroids will soon be available.”
Based on their safety and rapid control of itch in children with AD, “these will complement our current portfolio of topical therapies very well and have the potential to replace topical steroids early in therapy or to replace them altogether,” she told this news organization.
Dermavant Sciences, manufacturer of tapinarof, anticipates filing for Food and Drug Administration approval for AD in the first quarter of 2024, according to a company statement.
Dr. Silverberg and Dr. Simpson reported financial relationships with multiple pharmaceutical companies, including Dermavant, which provided funding for the ADORING trials. Dr. Hebert has financial relationship with more than 15 pharmaceutical companies, including Dermavent and other companies that have or are developing therapies for AD. Dr. Ständer reported financial relationships with Beiersdorf, Eli Lilly, Galderma, Kiniksa, Pfizer, and Sanofi.
AT THE EADV CONGRESS
Hidradenitis suppurativa: Two anti-IL17A/F therapies yield positive results
BERLIN – In separate trials conducted in patients with hidradenitis suppurativa (HS), two biologics that inhibit the activity of interleukin-17A (IL-17A) and IL-17F were associated with highly encouraging rates of control.
One of the trials evaluated a nanobody inhibitor, sonelokimab, a molecule with a substantially smaller size than traditional monoclonal antibodies (40 kilodaltons vs. 150 kilodaltons). After 24 weeks of treatment, the most effective of the two study doses almost doubled the proportion of patients with complete resolution of draining tunnels (41.1% vs. 23.8%; P < .05) relative to placebo.
“I think the size of sonelokimab is important,” Brian Kirby, MD, a consultant dermatologist at St. Vincent’s Hospital, Dublin, said at the annual congress of the European Academy of Dermatology and Venereology. “We think the smaller size results in better penetration of inflamed tissue,” he added, noting that penetration of abscesses, fistulae, and tunnels has been recognized in the past as a potential weakness of the larger monoclonal antibodies.
The other set of anti-17-A/F set of data were generated by a pooled 48-week maintenance from the BE HEARD I and II trials with bimekizumab. The 16-week data from these two trials were presented at the annual meeting of the American Academy of Dermatology earlier this year.
IL-17A/F trials
Both the
In the sonelokimab trial, called MIRA, 234 adults with HS were randomized in a 2:2:2:1 ratio to one of the two experimental arms, placebo, or a reference arm with the tumor necrosis factor (TNF) inhibitor adalimumab. Nearly 64% had Hurley stage II HS.
The primary endpoint was a 75% or greater reduction in total abscesses and nodules with no increase in draining tunnel count (HiSCR75) from baseline. Dr. Kirby said that this is more rigorous than the HiSCR50 endpoint more commonly used in HS clinical trials. Treatments were administered every 2 weeks for the first 8 weeks of a planned follow-up of 24 weeks and then every 4 weeks thereafter.
At 16 weeks, according to the data Dr. Kirby presented, both doses of sonelokimab were more active than placebo, but Dr. Kirby reported that the lower dose performed better for most objective endpoints.
For example, the HiSCR75 was reached by 43.3% of those randomized to the 120-mg dose (P < .001 vs. placebo), 34.8% of those randomized to the 240-mg dose (P <.01), and 14.7% of those randomized to placebo.
For HiSCR50, response rates were 65.7%, 53.0%, and 27.9%, for the 120-mg, 240-mg, and placebo arms, respectively. Again, both the lower dose (P < .001) and the higher dose (P < .01) were significantly superior to placebo.
On the International Hidradenitis Suppurativa Severity Score System (IHS4), which counts nodules and abscesses, score reductions were 19.3, 14.5, and 7.9 for the lower dose, higher dose, and placebo, respectively, with a greater statistical advantage for the lower relative to the higher dose over placebo (P <.001 vs. P <.01).
However, patient-focused outcomes were not necessarily greater for the lower dose. For the patient-completed measure, the Numerical Rating Scale 50% reduction in skin pain (NRS50), the proportion of patients responding at 12 weeks was numerically greater for the 240-mg dose (41.3%) than with the 120-mg dose (32.0%), although both reached the same statistical advantage (P < .001) over the 4.3% who reached this level of response on placebo.
For the Dermatology Life Quality Index (DLQI) and the Patient Global Impression of Severity (PGI-S), improvements from baseline were similar for the lower and higher dose, although there was a modest numerical and statistical advantage for the higher dose over placebo (P < .001 vs. P <.01).
The HiSCR50 (57.6%) and HiSCR75 (36.4%) responses were both lower for those randomized to the TNF inhibitor adalimumab relative to sonelokimab, but the smaller number of patients in this arm prohibited a statistical comparison.
Although oral candidiasis was more common among patients receiving either dose of sonelokimab than placebo, these were of mild to moderate severity. Dr. Kirby said that there were no unexpected safety issues, and sonelokimab was generally well tolerated.
The results are encouraging, but Dr. Kirby acknowledged that data are now needed to confirm that resolution of tunnels and fistulae is greater with a nanobody inhibitor of IL-17A/F than other targeted therapies. Even if this is validated, he said studies are needed to prove that the small relative molecule size is the reason behind the benefits.
Forty-eight–week bimekizumab data
From the pooled BE HEARD I and BE HEARD II maintenance data, the major message is that the robust responses observed at 16 weeks versus placebo were maintained at 48 weeks. More than 75% of patients retained a HiSCR50 response and more than 55% achieved a HiSCR75 response at the 48-week follow-up. The durable response was also reflected in other measures, according to Christos C. Zouboulis, MD, PhD, director of the department of dermatology, Brandenburg Medical School, Neuruppin, Germany.
“Improvements in disease severity were seen over time,” Dr. Zouboulis reported. “The majority of patients with severe HS at baseline shifted to mild to moderate disease according to the IHS4 classification.”
To the degree that both sonelokimab and bimekizumab target IL-17A/F, these data are mutually reinforcing. Dr. Kirby said that there is a sizable body of data implicating IL-17A/F in driving HS, and the activity of inhibitors in support the clinical value of IL-17A/F suppression.
On Oct. 18, shortly after the EADV meeting concluded, the Food and Drug Administration approved bimekizumab for treating moderate to severe plaque psoriasis, the first approved indication in the United States. In the European Union, it was approved for psoriasis in 2021, and for psoriatic arthritis and ankylosing spondylitis in June 2023.
Dr. Kirby has financial relationships with more than 10 pharmaceutical companies, including MoonLake, which is developing sonelokimab and sponsored the MIRA trial. Dr. Christos, president of the European HS Foundation, has financial relationships with multiple pharmaceutical companies, including UCB, which makes bimekizumab and provided funding for the BE HEARD I and II trials.
BERLIN – In separate trials conducted in patients with hidradenitis suppurativa (HS), two biologics that inhibit the activity of interleukin-17A (IL-17A) and IL-17F were associated with highly encouraging rates of control.
One of the trials evaluated a nanobody inhibitor, sonelokimab, a molecule with a substantially smaller size than traditional monoclonal antibodies (40 kilodaltons vs. 150 kilodaltons). After 24 weeks of treatment, the most effective of the two study doses almost doubled the proportion of patients with complete resolution of draining tunnels (41.1% vs. 23.8%; P < .05) relative to placebo.
“I think the size of sonelokimab is important,” Brian Kirby, MD, a consultant dermatologist at St. Vincent’s Hospital, Dublin, said at the annual congress of the European Academy of Dermatology and Venereology. “We think the smaller size results in better penetration of inflamed tissue,” he added, noting that penetration of abscesses, fistulae, and tunnels has been recognized in the past as a potential weakness of the larger monoclonal antibodies.
The other set of anti-17-A/F set of data were generated by a pooled 48-week maintenance from the BE HEARD I and II trials with bimekizumab. The 16-week data from these two trials were presented at the annual meeting of the American Academy of Dermatology earlier this year.
IL-17A/F trials
Both the
In the sonelokimab trial, called MIRA, 234 adults with HS were randomized in a 2:2:2:1 ratio to one of the two experimental arms, placebo, or a reference arm with the tumor necrosis factor (TNF) inhibitor adalimumab. Nearly 64% had Hurley stage II HS.
The primary endpoint was a 75% or greater reduction in total abscesses and nodules with no increase in draining tunnel count (HiSCR75) from baseline. Dr. Kirby said that this is more rigorous than the HiSCR50 endpoint more commonly used in HS clinical trials. Treatments were administered every 2 weeks for the first 8 weeks of a planned follow-up of 24 weeks and then every 4 weeks thereafter.
At 16 weeks, according to the data Dr. Kirby presented, both doses of sonelokimab were more active than placebo, but Dr. Kirby reported that the lower dose performed better for most objective endpoints.
For example, the HiSCR75 was reached by 43.3% of those randomized to the 120-mg dose (P < .001 vs. placebo), 34.8% of those randomized to the 240-mg dose (P <.01), and 14.7% of those randomized to placebo.
For HiSCR50, response rates were 65.7%, 53.0%, and 27.9%, for the 120-mg, 240-mg, and placebo arms, respectively. Again, both the lower dose (P < .001) and the higher dose (P < .01) were significantly superior to placebo.
On the International Hidradenitis Suppurativa Severity Score System (IHS4), which counts nodules and abscesses, score reductions were 19.3, 14.5, and 7.9 for the lower dose, higher dose, and placebo, respectively, with a greater statistical advantage for the lower relative to the higher dose over placebo (P <.001 vs. P <.01).
However, patient-focused outcomes were not necessarily greater for the lower dose. For the patient-completed measure, the Numerical Rating Scale 50% reduction in skin pain (NRS50), the proportion of patients responding at 12 weeks was numerically greater for the 240-mg dose (41.3%) than with the 120-mg dose (32.0%), although both reached the same statistical advantage (P < .001) over the 4.3% who reached this level of response on placebo.
For the Dermatology Life Quality Index (DLQI) and the Patient Global Impression of Severity (PGI-S), improvements from baseline were similar for the lower and higher dose, although there was a modest numerical and statistical advantage for the higher dose over placebo (P < .001 vs. P <.01).
The HiSCR50 (57.6%) and HiSCR75 (36.4%) responses were both lower for those randomized to the TNF inhibitor adalimumab relative to sonelokimab, but the smaller number of patients in this arm prohibited a statistical comparison.
Although oral candidiasis was more common among patients receiving either dose of sonelokimab than placebo, these were of mild to moderate severity. Dr. Kirby said that there were no unexpected safety issues, and sonelokimab was generally well tolerated.
The results are encouraging, but Dr. Kirby acknowledged that data are now needed to confirm that resolution of tunnels and fistulae is greater with a nanobody inhibitor of IL-17A/F than other targeted therapies. Even if this is validated, he said studies are needed to prove that the small relative molecule size is the reason behind the benefits.
Forty-eight–week bimekizumab data
From the pooled BE HEARD I and BE HEARD II maintenance data, the major message is that the robust responses observed at 16 weeks versus placebo were maintained at 48 weeks. More than 75% of patients retained a HiSCR50 response and more than 55% achieved a HiSCR75 response at the 48-week follow-up. The durable response was also reflected in other measures, according to Christos C. Zouboulis, MD, PhD, director of the department of dermatology, Brandenburg Medical School, Neuruppin, Germany.
“Improvements in disease severity were seen over time,” Dr. Zouboulis reported. “The majority of patients with severe HS at baseline shifted to mild to moderate disease according to the IHS4 classification.”
To the degree that both sonelokimab and bimekizumab target IL-17A/F, these data are mutually reinforcing. Dr. Kirby said that there is a sizable body of data implicating IL-17A/F in driving HS, and the activity of inhibitors in support the clinical value of IL-17A/F suppression.
On Oct. 18, shortly after the EADV meeting concluded, the Food and Drug Administration approved bimekizumab for treating moderate to severe plaque psoriasis, the first approved indication in the United States. In the European Union, it was approved for psoriasis in 2021, and for psoriatic arthritis and ankylosing spondylitis in June 2023.
Dr. Kirby has financial relationships with more than 10 pharmaceutical companies, including MoonLake, which is developing sonelokimab and sponsored the MIRA trial. Dr. Christos, president of the European HS Foundation, has financial relationships with multiple pharmaceutical companies, including UCB, which makes bimekizumab and provided funding for the BE HEARD I and II trials.
BERLIN – In separate trials conducted in patients with hidradenitis suppurativa (HS), two biologics that inhibit the activity of interleukin-17A (IL-17A) and IL-17F were associated with highly encouraging rates of control.
One of the trials evaluated a nanobody inhibitor, sonelokimab, a molecule with a substantially smaller size than traditional monoclonal antibodies (40 kilodaltons vs. 150 kilodaltons). After 24 weeks of treatment, the most effective of the two study doses almost doubled the proportion of patients with complete resolution of draining tunnels (41.1% vs. 23.8%; P < .05) relative to placebo.
“I think the size of sonelokimab is important,” Brian Kirby, MD, a consultant dermatologist at St. Vincent’s Hospital, Dublin, said at the annual congress of the European Academy of Dermatology and Venereology. “We think the smaller size results in better penetration of inflamed tissue,” he added, noting that penetration of abscesses, fistulae, and tunnels has been recognized in the past as a potential weakness of the larger monoclonal antibodies.
The other set of anti-17-A/F set of data were generated by a pooled 48-week maintenance from the BE HEARD I and II trials with bimekizumab. The 16-week data from these two trials were presented at the annual meeting of the American Academy of Dermatology earlier this year.
IL-17A/F trials
Both the
In the sonelokimab trial, called MIRA, 234 adults with HS were randomized in a 2:2:2:1 ratio to one of the two experimental arms, placebo, or a reference arm with the tumor necrosis factor (TNF) inhibitor adalimumab. Nearly 64% had Hurley stage II HS.
The primary endpoint was a 75% or greater reduction in total abscesses and nodules with no increase in draining tunnel count (HiSCR75) from baseline. Dr. Kirby said that this is more rigorous than the HiSCR50 endpoint more commonly used in HS clinical trials. Treatments were administered every 2 weeks for the first 8 weeks of a planned follow-up of 24 weeks and then every 4 weeks thereafter.
At 16 weeks, according to the data Dr. Kirby presented, both doses of sonelokimab were more active than placebo, but Dr. Kirby reported that the lower dose performed better for most objective endpoints.
For example, the HiSCR75 was reached by 43.3% of those randomized to the 120-mg dose (P < .001 vs. placebo), 34.8% of those randomized to the 240-mg dose (P <.01), and 14.7% of those randomized to placebo.
For HiSCR50, response rates were 65.7%, 53.0%, and 27.9%, for the 120-mg, 240-mg, and placebo arms, respectively. Again, both the lower dose (P < .001) and the higher dose (P < .01) were significantly superior to placebo.
On the International Hidradenitis Suppurativa Severity Score System (IHS4), which counts nodules and abscesses, score reductions were 19.3, 14.5, and 7.9 for the lower dose, higher dose, and placebo, respectively, with a greater statistical advantage for the lower relative to the higher dose over placebo (P <.001 vs. P <.01).
However, patient-focused outcomes were not necessarily greater for the lower dose. For the patient-completed measure, the Numerical Rating Scale 50% reduction in skin pain (NRS50), the proportion of patients responding at 12 weeks was numerically greater for the 240-mg dose (41.3%) than with the 120-mg dose (32.0%), although both reached the same statistical advantage (P < .001) over the 4.3% who reached this level of response on placebo.
For the Dermatology Life Quality Index (DLQI) and the Patient Global Impression of Severity (PGI-S), improvements from baseline were similar for the lower and higher dose, although there was a modest numerical and statistical advantage for the higher dose over placebo (P < .001 vs. P <.01).
The HiSCR50 (57.6%) and HiSCR75 (36.4%) responses were both lower for those randomized to the TNF inhibitor adalimumab relative to sonelokimab, but the smaller number of patients in this arm prohibited a statistical comparison.
Although oral candidiasis was more common among patients receiving either dose of sonelokimab than placebo, these were of mild to moderate severity. Dr. Kirby said that there were no unexpected safety issues, and sonelokimab was generally well tolerated.
The results are encouraging, but Dr. Kirby acknowledged that data are now needed to confirm that resolution of tunnels and fistulae is greater with a nanobody inhibitor of IL-17A/F than other targeted therapies. Even if this is validated, he said studies are needed to prove that the small relative molecule size is the reason behind the benefits.
Forty-eight–week bimekizumab data
From the pooled BE HEARD I and BE HEARD II maintenance data, the major message is that the robust responses observed at 16 weeks versus placebo were maintained at 48 weeks. More than 75% of patients retained a HiSCR50 response and more than 55% achieved a HiSCR75 response at the 48-week follow-up. The durable response was also reflected in other measures, according to Christos C. Zouboulis, MD, PhD, director of the department of dermatology, Brandenburg Medical School, Neuruppin, Germany.
“Improvements in disease severity were seen over time,” Dr. Zouboulis reported. “The majority of patients with severe HS at baseline shifted to mild to moderate disease according to the IHS4 classification.”
To the degree that both sonelokimab and bimekizumab target IL-17A/F, these data are mutually reinforcing. Dr. Kirby said that there is a sizable body of data implicating IL-17A/F in driving HS, and the activity of inhibitors in support the clinical value of IL-17A/F suppression.
On Oct. 18, shortly after the EADV meeting concluded, the Food and Drug Administration approved bimekizumab for treating moderate to severe plaque psoriasis, the first approved indication in the United States. In the European Union, it was approved for psoriasis in 2021, and for psoriatic arthritis and ankylosing spondylitis in June 2023.
Dr. Kirby has financial relationships with more than 10 pharmaceutical companies, including MoonLake, which is developing sonelokimab and sponsored the MIRA trial. Dr. Christos, president of the European HS Foundation, has financial relationships with multiple pharmaceutical companies, including UCB, which makes bimekizumab and provided funding for the BE HEARD I and II trials.
AT THE EADV CONGRESS
Quitting tobacco can improve lung health in COPD
Reducing exposure to tobacco smoke may reduce the burden of chronic obstructive pulmonary disease, and public health measures are needed, according to a new Tobacco Knowledge Summary from the World Health Organization.
“Smoking is a major risk factor for COPD and leads to airway inflammation and remodeling associated with lung destruction,” and contributes to approximately 70% of COPD cases worldwide, according to the statement.
Types of tobacco exposure include not only traditional smoked tobacco products (cigarettes, cigars, pipes, water pipes, kreteks, and bidis), but also smokeless tobacco, heated tobacco products, and electronic nicotine delivery systems; the addition of chemicals and flavors can increase the appeal of tobacco products and promote addiction, the authors wrote. Hookahs and water pipes “are at least as detrimental to lung health as smoking cigarettes and should not be considered as a safe alternative,” they added.
The risk of COPD extends to new e-cigarette products, the authors noted. A study in the American Journal of Preventive Medicine showed that current users of e-cigarettes had a 75% increased risk of developing COPD compared with individuals who have never used e-cigarettes.
Individuals with COPD also face an increased risk of cardiovascular disease and type 2 diabetes, and smokers with COPD who quit not only improve their COPD but also reduce their risk of developing these conditions, the authors said.
Mechanism of action explored
The authors noted how tobacco smoking may cause COPD when inhaled particles are deposited through the airway.
Growing evidence suggests that extracellular vesicles may play a role in the development of lung disorders such as COPD, and cigarette smoke can have an impact through this channel. A study published in the American Journal of Respiratory and Critical Care Medicine offered evidence of a potential link between exposure to cigarette smoke and the generation of a unique extracellular vesicle population that could promote the development of lung damage. In the study, Matthew C. Madison, MD, of the University of Alabama, Birmingham, and colleagues examined activity in extracellular vesicles from the bronchoalveolar lavage (BAL) fluid of smoke-exposed mice and human smokers who were otherwise healthy.
The researchers found that airway extracellular vesicles in mice or humans exposed to cigarette smoke had the ability to cause rapid lung damage when transferred into naive recipient mice. The results provide a new model that can inform preclinical COPD research, they wrote.
Public health action needed
“In recognition of COPD and Lung Cancer Awareness Month, the World Health Organization (WHO) emphasizes the impact of various forms of tobacco use on COPD,” Dharani K. Narendra, MD, of Baylor College of Medicine, Houston, said in an interview.
“This article focuses on the different types of tobacco exposure, the health care burden associated with COPD, and the risk of developing lung cancer. It also addresses the high-risk groups, especially youth, underscoring the importance of public education and the implementation of restrictions on tobacco use to combat these growing concerns,” she said.
“Education, awareness, and targeted interventions are essential for smoking cessation and COPD management,” said Dr. Narendra. “These elements are key to informing the public about smoking risks, encouraging behavioral change, and ultimately reducing the incidence of smoking-related diseases,” she emphasized.
The WHO statement called for population-level interventions including brief advice to tobacco users, toll-free quit lines, pharmacological interventions, use of messaging and chatbots to provide quit support, and the WHO quit tobacco mobile app.
“It is imperative that all tobacco users, particularly those living in low- to middle-income countries, have access to comprehensive cessation support aligned with WHO recommendations,” the authors wrote.
Finally, the authors emphasized the need to protect children and teens from the dangers of tobacco use through product regulation and to expose the tobacco industry’s marketing tactics.
“The article offers a comprehensive look at different types of tobacco exposure and their contribution to the development of COPD,” Dr. Narendra told this news organization. “Notably, it presents groundbreaking evidence of a strong association between the use of electronic nicotine delivery systems (ENDS) and heated tobacco products to development of COPD; additionally, it provides valuable guidance on smoking cessation resources for physicians to help patients quit smoking,” she said.
Looking ahead, more research is needed on “developing and sustaining state-specific or population-specific interventions for effective smoking cessation programs, and reducing the burden of COPD,” Dr. Narendra said.
The study by Madison and colleagues was supported by the National Heart, Lung, and Blood Institute, the National Institute of General Medical Science, the U.S. Veterans Affairs Administration, the Cystic Fibrosis Foundation Research Development Program, and the Veterans Affairs Merit grant.
Additional financial support came from Imperial College London, a Wellcome Trust Senior Research Fellowship, and Rosetrees Trust/The Stoneygate Trust.
Dr. Narendra had no financial conflicts to disclose but serves as a member of the editorial board of CHEST Physician.
Reducing exposure to tobacco smoke may reduce the burden of chronic obstructive pulmonary disease, and public health measures are needed, according to a new Tobacco Knowledge Summary from the World Health Organization.
“Smoking is a major risk factor for COPD and leads to airway inflammation and remodeling associated with lung destruction,” and contributes to approximately 70% of COPD cases worldwide, according to the statement.
Types of tobacco exposure include not only traditional smoked tobacco products (cigarettes, cigars, pipes, water pipes, kreteks, and bidis), but also smokeless tobacco, heated tobacco products, and electronic nicotine delivery systems; the addition of chemicals and flavors can increase the appeal of tobacco products and promote addiction, the authors wrote. Hookahs and water pipes “are at least as detrimental to lung health as smoking cigarettes and should not be considered as a safe alternative,” they added.
The risk of COPD extends to new e-cigarette products, the authors noted. A study in the American Journal of Preventive Medicine showed that current users of e-cigarettes had a 75% increased risk of developing COPD compared with individuals who have never used e-cigarettes.
Individuals with COPD also face an increased risk of cardiovascular disease and type 2 diabetes, and smokers with COPD who quit not only improve their COPD but also reduce their risk of developing these conditions, the authors said.
Mechanism of action explored
The authors noted how tobacco smoking may cause COPD when inhaled particles are deposited through the airway.
Growing evidence suggests that extracellular vesicles may play a role in the development of lung disorders such as COPD, and cigarette smoke can have an impact through this channel. A study published in the American Journal of Respiratory and Critical Care Medicine offered evidence of a potential link between exposure to cigarette smoke and the generation of a unique extracellular vesicle population that could promote the development of lung damage. In the study, Matthew C. Madison, MD, of the University of Alabama, Birmingham, and colleagues examined activity in extracellular vesicles from the bronchoalveolar lavage (BAL) fluid of smoke-exposed mice and human smokers who were otherwise healthy.
The researchers found that airway extracellular vesicles in mice or humans exposed to cigarette smoke had the ability to cause rapid lung damage when transferred into naive recipient mice. The results provide a new model that can inform preclinical COPD research, they wrote.
Public health action needed
“In recognition of COPD and Lung Cancer Awareness Month, the World Health Organization (WHO) emphasizes the impact of various forms of tobacco use on COPD,” Dharani K. Narendra, MD, of Baylor College of Medicine, Houston, said in an interview.
“This article focuses on the different types of tobacco exposure, the health care burden associated with COPD, and the risk of developing lung cancer. It also addresses the high-risk groups, especially youth, underscoring the importance of public education and the implementation of restrictions on tobacco use to combat these growing concerns,” she said.
“Education, awareness, and targeted interventions are essential for smoking cessation and COPD management,” said Dr. Narendra. “These elements are key to informing the public about smoking risks, encouraging behavioral change, and ultimately reducing the incidence of smoking-related diseases,” she emphasized.
The WHO statement called for population-level interventions including brief advice to tobacco users, toll-free quit lines, pharmacological interventions, use of messaging and chatbots to provide quit support, and the WHO quit tobacco mobile app.
“It is imperative that all tobacco users, particularly those living in low- to middle-income countries, have access to comprehensive cessation support aligned with WHO recommendations,” the authors wrote.
Finally, the authors emphasized the need to protect children and teens from the dangers of tobacco use through product regulation and to expose the tobacco industry’s marketing tactics.
“The article offers a comprehensive look at different types of tobacco exposure and their contribution to the development of COPD,” Dr. Narendra told this news organization. “Notably, it presents groundbreaking evidence of a strong association between the use of electronic nicotine delivery systems (ENDS) and heated tobacco products to development of COPD; additionally, it provides valuable guidance on smoking cessation resources for physicians to help patients quit smoking,” she said.
Looking ahead, more research is needed on “developing and sustaining state-specific or population-specific interventions for effective smoking cessation programs, and reducing the burden of COPD,” Dr. Narendra said.
The study by Madison and colleagues was supported by the National Heart, Lung, and Blood Institute, the National Institute of General Medical Science, the U.S. Veterans Affairs Administration, the Cystic Fibrosis Foundation Research Development Program, and the Veterans Affairs Merit grant.
Additional financial support came from Imperial College London, a Wellcome Trust Senior Research Fellowship, and Rosetrees Trust/The Stoneygate Trust.
Dr. Narendra had no financial conflicts to disclose but serves as a member of the editorial board of CHEST Physician.
Reducing exposure to tobacco smoke may reduce the burden of chronic obstructive pulmonary disease, and public health measures are needed, according to a new Tobacco Knowledge Summary from the World Health Organization.
“Smoking is a major risk factor for COPD and leads to airway inflammation and remodeling associated with lung destruction,” and contributes to approximately 70% of COPD cases worldwide, according to the statement.
Types of tobacco exposure include not only traditional smoked tobacco products (cigarettes, cigars, pipes, water pipes, kreteks, and bidis), but also smokeless tobacco, heated tobacco products, and electronic nicotine delivery systems; the addition of chemicals and flavors can increase the appeal of tobacco products and promote addiction, the authors wrote. Hookahs and water pipes “are at least as detrimental to lung health as smoking cigarettes and should not be considered as a safe alternative,” they added.
The risk of COPD extends to new e-cigarette products, the authors noted. A study in the American Journal of Preventive Medicine showed that current users of e-cigarettes had a 75% increased risk of developing COPD compared with individuals who have never used e-cigarettes.
Individuals with COPD also face an increased risk of cardiovascular disease and type 2 diabetes, and smokers with COPD who quit not only improve their COPD but also reduce their risk of developing these conditions, the authors said.
Mechanism of action explored
The authors noted how tobacco smoking may cause COPD when inhaled particles are deposited through the airway.
Growing evidence suggests that extracellular vesicles may play a role in the development of lung disorders such as COPD, and cigarette smoke can have an impact through this channel. A study published in the American Journal of Respiratory and Critical Care Medicine offered evidence of a potential link between exposure to cigarette smoke and the generation of a unique extracellular vesicle population that could promote the development of lung damage. In the study, Matthew C. Madison, MD, of the University of Alabama, Birmingham, and colleagues examined activity in extracellular vesicles from the bronchoalveolar lavage (BAL) fluid of smoke-exposed mice and human smokers who were otherwise healthy.
The researchers found that airway extracellular vesicles in mice or humans exposed to cigarette smoke had the ability to cause rapid lung damage when transferred into naive recipient mice. The results provide a new model that can inform preclinical COPD research, they wrote.
Public health action needed
“In recognition of COPD and Lung Cancer Awareness Month, the World Health Organization (WHO) emphasizes the impact of various forms of tobacco use on COPD,” Dharani K. Narendra, MD, of Baylor College of Medicine, Houston, said in an interview.
“This article focuses on the different types of tobacco exposure, the health care burden associated with COPD, and the risk of developing lung cancer. It also addresses the high-risk groups, especially youth, underscoring the importance of public education and the implementation of restrictions on tobacco use to combat these growing concerns,” she said.
“Education, awareness, and targeted interventions are essential for smoking cessation and COPD management,” said Dr. Narendra. “These elements are key to informing the public about smoking risks, encouraging behavioral change, and ultimately reducing the incidence of smoking-related diseases,” she emphasized.
The WHO statement called for population-level interventions including brief advice to tobacco users, toll-free quit lines, pharmacological interventions, use of messaging and chatbots to provide quit support, and the WHO quit tobacco mobile app.
“It is imperative that all tobacco users, particularly those living in low- to middle-income countries, have access to comprehensive cessation support aligned with WHO recommendations,” the authors wrote.
Finally, the authors emphasized the need to protect children and teens from the dangers of tobacco use through product regulation and to expose the tobacco industry’s marketing tactics.
“The article offers a comprehensive look at different types of tobacco exposure and their contribution to the development of COPD,” Dr. Narendra told this news organization. “Notably, it presents groundbreaking evidence of a strong association between the use of electronic nicotine delivery systems (ENDS) and heated tobacco products to development of COPD; additionally, it provides valuable guidance on smoking cessation resources for physicians to help patients quit smoking,” she said.
Looking ahead, more research is needed on “developing and sustaining state-specific or population-specific interventions for effective smoking cessation programs, and reducing the burden of COPD,” Dr. Narendra said.
The study by Madison and colleagues was supported by the National Heart, Lung, and Blood Institute, the National Institute of General Medical Science, the U.S. Veterans Affairs Administration, the Cystic Fibrosis Foundation Research Development Program, and the Veterans Affairs Merit grant.
Additional financial support came from Imperial College London, a Wellcome Trust Senior Research Fellowship, and Rosetrees Trust/The Stoneygate Trust.
Dr. Narendra had no financial conflicts to disclose but serves as a member of the editorial board of CHEST Physician.
Conditional recommendations rule in new SARD-associated interstitial lung disease guidelines
SAN DIEGO – In the spring of 2024, the American College of Rheumatology is expected to release guidelines to help inform the screening, monitoring, and treatment of interstitial lung disease (ILD) in people with systemic autoimmune rheumatic diseases (SARDs).
The guidelines, which were previewed during a session at the ACR’s annual meeting, will include 50 recommendations, 3 of which met criteria for a strong rating:
- For people with SARDs at increased risk of developing ILD, the authors strongly recommend against screening with surgical lung biopsy.
- For people with systemic sclerosis (SSc)-related ILD, the authors strongly recommend against glucocorticoids as a first-line ILD treatment.
- For people with SSc-related ILD progression despite an initial ILD treatment, the authors strongly recommend against using long-term glucocorticoids.
Elana J. Bernstein, MD, MSc, a rheumatologist who directs the Columbia/New York-Presbyterian Scleroderma Center, and Sindhu R. Johnson, MD, a rheumatologist who directs the Toronto Scleroderma Program at the University of Toronto, provided a sneak peek of the recommendations to attendees before anticipated publication in Arthritis & Rheumatology and Arthritis Care & Research. For now, guideline summaries for screening and monitoring and treatment are currently available, and three manuscripts are under peer review: one about screening and monitoring, one about treatment, and one about the patient panel that participated in the effort.
“ILD is a significant cause of morbidity and mortality in people with SARDs,” said Dr. Bernstein, who is co-first author of the guidelines. “People with systemic sclerosis, rheumatoid arthritis, idiopathic inflammatory myopathies, mixed connective tissue disease, and Sjögren’s disease are at greatest risk of developing ILD.”
Pediatric patients with SARDs excluded
The guidelines’ population of interest was people 17 years of age and older who were diagnosed with SARDs with a high risk of ILD. Pediatric patients with SARDs were excluded from the endeavor, as were those with systemic lupus erythematosus, antineutrophil cytoplasmic antibody–associated vasculitis, sarcoidosis, ankylosing spondylitis, undifferentiated connective tissue disease, interstitial pneumonia with autoimmune features, and those with unclassifiable ILD.
In the realm of screening, the guideline authors conditionally recommend two screening tests for patients considered at increased risk of ILD: pulmonary function tests and high-resolution chest CT (HRCT). Pulmonary function tests should include spirometry, lung volumes, and diffusion capacity. “Office spirometry alone is insufficient,” said Dr. Johnson, who served as lead author of the guidelines. And while a HRCT scan is recommended, “some patients may present to the emergency room with acute onset shortness of breath, and they may receive a CT angiogram to screen for pulmonary embolism,” she said. “It’s important to note that CT angiograms are performed in incomplete inspiration to maximize pulmonary artery enhancement. This may produce atelectasis that may obscure or mimic ILD. As a result, CTA studies are often inadequate to screen for ILD.”
Once a patient is diagnosed with ILD, three tests are recommended for monitoring: pulmonary function testing (every 3-6 months the first year in patients with IIM and SSc, then less frequently once stable, and every 3-12 months in the first year in patients with RA, SjD, and MCTD, then less frequently once stable); ambulatory desaturation testing every 3-12 months; and HRCT as needed. Dr. Johnson noted that while that the screening of ILD lies within the realm of rheumatologists, “once a patient is diagnosed, we are encouraged to comanage these patients with pulmonologists,” she said. “Ambulatory desaturation testing is not an infrequent test in the hands of pulmonologists. This is where co-management can be helpful.” She characterized a 6-minute walk test with continuous oximetry as “insufficient and is not synonymous with ambulatory desaturation testing. Ambulatory desaturation testing includes up titration of oxygen if a patient desaturates.”
The guidelines conditionally recommend against using chest radiography, 6-minute walk test distance, ambulatory desaturation testing, and bronchoscopy for ILD screening, and there is a strong recommendation against surgical lung biopsy. “However, there are unique circumstances where these tests may be considered,” Dr. Johnson said. “For example, ambulatory desaturation testing may be helpful if a patient is unable to perform a pulmonary function test. Bronchoscopy may be used to rule out infection, sarcoidosis, lymphoma, or alveolar hemorrhage, and surgical lung biopsy may be considered if you’re trying to rule out a malignancy.”
Similarly, several tests are conditionally recommended against for the monitoring of ILD, including chest radiography, the 6-minute walk test distance, and bronchoscopy. “But there are unique circumstances where they may be considered,” she said. “The 6-minute walk test may be used if a patient is unable to perform a pulmonary function test or if they’re being assessed for lung transplantation. Bronchoscopy may be used to rule out infection or alveolar hemorrhage.”
Preferred treatment options described
First-line treatment recommendations for ILD were based on the best available published evidence, voting panel expertise, and patient preferences. For SSc, the preferred treatment options include mycophenolate (CellCept), tocilizumab (Actemra), or rituximab (Rituxan and biosimilars), while additional options include cyclophosphamide, nintedanib (Ofev), and azathioprine. For myositis, the preferred treatment options include mycophenolate, azathioprine, rituximab, or calcineurin inhibitors, while additional options include a Janus kinase (JAK) inhibitor or cyclophosphamide. For MCTD, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include tocilizumab or cyclophosphamide. For RA and Sjögren’s, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include cyclophosphamide. Dr. Johnson emphasized that there was low certainty evidence to recommend one treatment over another. “Many situations might lead a provider to choose a different option for ILD treatment, such as the presence of comorbidities or extra-pulmonary disease,” she said. “So, while our guidelines were focused on effectiveness for ILD, providers may choose therapies that will help ILD and other disease manifestations.”
The guidelines conditionally recommend a short course of glucocorticoids as a bridging therapy or for treatment of a flare of ILD in patients with myositis, MCTD, RA, and Sjögren’s. The panel strongly recommends against the use of glucocorticoids in patients with SSc due to the concern for inducing a scleroderma renal crisis. “While this may be common knowledge for rheumatologists, it may not be common knowledge for pulmonologists,” she said. “So here is an opportunity to educate our pulmonology colleagues in our consultation notes.”
The guidelines also include recommendations for progression of ILD, which was defined using the INBUILD trial criteria. Mycophenolate is conditionally recommended to be the first ILD treatment for all SARDs when progression occurs, if it wasn’t the first ILD treatment used. “If it was, then other medications that rheumatologists are used to can be considered as the next ILD treatment in the face of progression: rituximab, nintedanib, tocilizumab, and cyclophosphamide,” she said. The guidelines include a conditional recommendation against long-term glucocorticoid use in myositis, MCTD, RA, and Sjögren’s, plus a strong recommendation against long-term glucocorticoid use in SSc. Finally, there is a conditional recommendation of referral for lung transplant evaluation at the appropriate time at experienced centers.
Another group of recommendations has to do with cases of rapidly progressive ILD, which is characterized by rapid progression from no oxygen or a patient’s baseline oxygen requirement to a high oxygen requirement or intubation usually within days to weeks without a documented cause, such as infection or heart failure. “In cases of rapidly progressive ILD, which typically occurs in the setting of anti-MDA5 antibodies, there is a conditional recommendation for IV glucocorticoids plus two additional therapies: traditionally rituximab and mycophenolate,” Dr. Johnson said. “However, what may be new to some clinicians is combination IVIG [intravenous immunoglobulin] and a calcineurin inhibitor, notably tacrolimus,” she said. “This is the situation where experience at expert centers is influencing our guidelines in advance of data.”
A patient panel provided input
For the undertaking, a core team that included six rheumatologists; one pulmonologist; one thoracic radiologist; one expert on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology; and two literature review experts developed clinically relevant population, intervention, comparator, and outcomes (PICO) questions. The literature review team included 13 rheumatologists, 8 pulmonologists, and 3 methodologists. Finally, a 21-member patient panel was convened to share their values and preferences regarding screening, monitoring, and treatment of SARD-related ILD. Of these, Dr. Bernstein said that 4 were at risk for ILD and 17 had been diagnosed with ILD. Next, the literature review team conducted a systematic review and used the GRADE methodology to rate the available evidence as high, moderate, low, or very low. Then, a voting panel comprising 13 rheumatologists, 10 pulmonologists, 1 radiologist, and 3 patients from the patient panel cast votes for each PICO question and made final recommendations.
The review of evidence left the guidelines authors with 241 PICO questions, “which is a lot,” Dr. Bernstein said. “To put this in perspective, some guidelines address only 10 or 15 PICO questions. Fortunately, we had a dedicated group of experts who were up to the challenge.” Dr. Johnson emphasized that the forthcoming guidelines should not be used by insurers to mandate a specific order of prescribing. “Clinicians must retain the latitude to prescribe medications based on individual patient factors and preferences,” she said.
Dr. Bernstein disclosed that she is an adviser to, a consultant for, and has received grant or research support from Boehringer Ingelheim and has also received grant or research support from Kadmon and Pfizer. Dr. Johnson disclosed that she has received research support from the American College of Rheumatology to develop these guidelines. She has also been an investigator for trials sponsored by Bristol-Myers Squibb, Roche, and Boehringer Ingelheim and has mitigated these relevant conflicts of interest 1 year prior to the development of these guidelines, and will continue to do so for the foreseeable future.
SAN DIEGO – In the spring of 2024, the American College of Rheumatology is expected to release guidelines to help inform the screening, monitoring, and treatment of interstitial lung disease (ILD) in people with systemic autoimmune rheumatic diseases (SARDs).
The guidelines, which were previewed during a session at the ACR’s annual meeting, will include 50 recommendations, 3 of which met criteria for a strong rating:
- For people with SARDs at increased risk of developing ILD, the authors strongly recommend against screening with surgical lung biopsy.
- For people with systemic sclerosis (SSc)-related ILD, the authors strongly recommend against glucocorticoids as a first-line ILD treatment.
- For people with SSc-related ILD progression despite an initial ILD treatment, the authors strongly recommend against using long-term glucocorticoids.
Elana J. Bernstein, MD, MSc, a rheumatologist who directs the Columbia/New York-Presbyterian Scleroderma Center, and Sindhu R. Johnson, MD, a rheumatologist who directs the Toronto Scleroderma Program at the University of Toronto, provided a sneak peek of the recommendations to attendees before anticipated publication in Arthritis & Rheumatology and Arthritis Care & Research. For now, guideline summaries for screening and monitoring and treatment are currently available, and three manuscripts are under peer review: one about screening and monitoring, one about treatment, and one about the patient panel that participated in the effort.
“ILD is a significant cause of morbidity and mortality in people with SARDs,” said Dr. Bernstein, who is co-first author of the guidelines. “People with systemic sclerosis, rheumatoid arthritis, idiopathic inflammatory myopathies, mixed connective tissue disease, and Sjögren’s disease are at greatest risk of developing ILD.”
Pediatric patients with SARDs excluded
The guidelines’ population of interest was people 17 years of age and older who were diagnosed with SARDs with a high risk of ILD. Pediatric patients with SARDs were excluded from the endeavor, as were those with systemic lupus erythematosus, antineutrophil cytoplasmic antibody–associated vasculitis, sarcoidosis, ankylosing spondylitis, undifferentiated connective tissue disease, interstitial pneumonia with autoimmune features, and those with unclassifiable ILD.
In the realm of screening, the guideline authors conditionally recommend two screening tests for patients considered at increased risk of ILD: pulmonary function tests and high-resolution chest CT (HRCT). Pulmonary function tests should include spirometry, lung volumes, and diffusion capacity. “Office spirometry alone is insufficient,” said Dr. Johnson, who served as lead author of the guidelines. And while a HRCT scan is recommended, “some patients may present to the emergency room with acute onset shortness of breath, and they may receive a CT angiogram to screen for pulmonary embolism,” she said. “It’s important to note that CT angiograms are performed in incomplete inspiration to maximize pulmonary artery enhancement. This may produce atelectasis that may obscure or mimic ILD. As a result, CTA studies are often inadequate to screen for ILD.”
Once a patient is diagnosed with ILD, three tests are recommended for monitoring: pulmonary function testing (every 3-6 months the first year in patients with IIM and SSc, then less frequently once stable, and every 3-12 months in the first year in patients with RA, SjD, and MCTD, then less frequently once stable); ambulatory desaturation testing every 3-12 months; and HRCT as needed. Dr. Johnson noted that while that the screening of ILD lies within the realm of rheumatologists, “once a patient is diagnosed, we are encouraged to comanage these patients with pulmonologists,” she said. “Ambulatory desaturation testing is not an infrequent test in the hands of pulmonologists. This is where co-management can be helpful.” She characterized a 6-minute walk test with continuous oximetry as “insufficient and is not synonymous with ambulatory desaturation testing. Ambulatory desaturation testing includes up titration of oxygen if a patient desaturates.”
The guidelines conditionally recommend against using chest radiography, 6-minute walk test distance, ambulatory desaturation testing, and bronchoscopy for ILD screening, and there is a strong recommendation against surgical lung biopsy. “However, there are unique circumstances where these tests may be considered,” Dr. Johnson said. “For example, ambulatory desaturation testing may be helpful if a patient is unable to perform a pulmonary function test. Bronchoscopy may be used to rule out infection, sarcoidosis, lymphoma, or alveolar hemorrhage, and surgical lung biopsy may be considered if you’re trying to rule out a malignancy.”
Similarly, several tests are conditionally recommended against for the monitoring of ILD, including chest radiography, the 6-minute walk test distance, and bronchoscopy. “But there are unique circumstances where they may be considered,” she said. “The 6-minute walk test may be used if a patient is unable to perform a pulmonary function test or if they’re being assessed for lung transplantation. Bronchoscopy may be used to rule out infection or alveolar hemorrhage.”
Preferred treatment options described
First-line treatment recommendations for ILD were based on the best available published evidence, voting panel expertise, and patient preferences. For SSc, the preferred treatment options include mycophenolate (CellCept), tocilizumab (Actemra), or rituximab (Rituxan and biosimilars), while additional options include cyclophosphamide, nintedanib (Ofev), and azathioprine. For myositis, the preferred treatment options include mycophenolate, azathioprine, rituximab, or calcineurin inhibitors, while additional options include a Janus kinase (JAK) inhibitor or cyclophosphamide. For MCTD, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include tocilizumab or cyclophosphamide. For RA and Sjögren’s, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include cyclophosphamide. Dr. Johnson emphasized that there was low certainty evidence to recommend one treatment over another. “Many situations might lead a provider to choose a different option for ILD treatment, such as the presence of comorbidities or extra-pulmonary disease,” she said. “So, while our guidelines were focused on effectiveness for ILD, providers may choose therapies that will help ILD and other disease manifestations.”
The guidelines conditionally recommend a short course of glucocorticoids as a bridging therapy or for treatment of a flare of ILD in patients with myositis, MCTD, RA, and Sjögren’s. The panel strongly recommends against the use of glucocorticoids in patients with SSc due to the concern for inducing a scleroderma renal crisis. “While this may be common knowledge for rheumatologists, it may not be common knowledge for pulmonologists,” she said. “So here is an opportunity to educate our pulmonology colleagues in our consultation notes.”
The guidelines also include recommendations for progression of ILD, which was defined using the INBUILD trial criteria. Mycophenolate is conditionally recommended to be the first ILD treatment for all SARDs when progression occurs, if it wasn’t the first ILD treatment used. “If it was, then other medications that rheumatologists are used to can be considered as the next ILD treatment in the face of progression: rituximab, nintedanib, tocilizumab, and cyclophosphamide,” she said. The guidelines include a conditional recommendation against long-term glucocorticoid use in myositis, MCTD, RA, and Sjögren’s, plus a strong recommendation against long-term glucocorticoid use in SSc. Finally, there is a conditional recommendation of referral for lung transplant evaluation at the appropriate time at experienced centers.
Another group of recommendations has to do with cases of rapidly progressive ILD, which is characterized by rapid progression from no oxygen or a patient’s baseline oxygen requirement to a high oxygen requirement or intubation usually within days to weeks without a documented cause, such as infection or heart failure. “In cases of rapidly progressive ILD, which typically occurs in the setting of anti-MDA5 antibodies, there is a conditional recommendation for IV glucocorticoids plus two additional therapies: traditionally rituximab and mycophenolate,” Dr. Johnson said. “However, what may be new to some clinicians is combination IVIG [intravenous immunoglobulin] and a calcineurin inhibitor, notably tacrolimus,” she said. “This is the situation where experience at expert centers is influencing our guidelines in advance of data.”
A patient panel provided input
For the undertaking, a core team that included six rheumatologists; one pulmonologist; one thoracic radiologist; one expert on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology; and two literature review experts developed clinically relevant population, intervention, comparator, and outcomes (PICO) questions. The literature review team included 13 rheumatologists, 8 pulmonologists, and 3 methodologists. Finally, a 21-member patient panel was convened to share their values and preferences regarding screening, monitoring, and treatment of SARD-related ILD. Of these, Dr. Bernstein said that 4 were at risk for ILD and 17 had been diagnosed with ILD. Next, the literature review team conducted a systematic review and used the GRADE methodology to rate the available evidence as high, moderate, low, or very low. Then, a voting panel comprising 13 rheumatologists, 10 pulmonologists, 1 radiologist, and 3 patients from the patient panel cast votes for each PICO question and made final recommendations.
The review of evidence left the guidelines authors with 241 PICO questions, “which is a lot,” Dr. Bernstein said. “To put this in perspective, some guidelines address only 10 or 15 PICO questions. Fortunately, we had a dedicated group of experts who were up to the challenge.” Dr. Johnson emphasized that the forthcoming guidelines should not be used by insurers to mandate a specific order of prescribing. “Clinicians must retain the latitude to prescribe medications based on individual patient factors and preferences,” she said.
Dr. Bernstein disclosed that she is an adviser to, a consultant for, and has received grant or research support from Boehringer Ingelheim and has also received grant or research support from Kadmon and Pfizer. Dr. Johnson disclosed that she has received research support from the American College of Rheumatology to develop these guidelines. She has also been an investigator for trials sponsored by Bristol-Myers Squibb, Roche, and Boehringer Ingelheim and has mitigated these relevant conflicts of interest 1 year prior to the development of these guidelines, and will continue to do so for the foreseeable future.
SAN DIEGO – In the spring of 2024, the American College of Rheumatology is expected to release guidelines to help inform the screening, monitoring, and treatment of interstitial lung disease (ILD) in people with systemic autoimmune rheumatic diseases (SARDs).
The guidelines, which were previewed during a session at the ACR’s annual meeting, will include 50 recommendations, 3 of which met criteria for a strong rating:
- For people with SARDs at increased risk of developing ILD, the authors strongly recommend against screening with surgical lung biopsy.
- For people with systemic sclerosis (SSc)-related ILD, the authors strongly recommend against glucocorticoids as a first-line ILD treatment.
- For people with SSc-related ILD progression despite an initial ILD treatment, the authors strongly recommend against using long-term glucocorticoids.
Elana J. Bernstein, MD, MSc, a rheumatologist who directs the Columbia/New York-Presbyterian Scleroderma Center, and Sindhu R. Johnson, MD, a rheumatologist who directs the Toronto Scleroderma Program at the University of Toronto, provided a sneak peek of the recommendations to attendees before anticipated publication in Arthritis & Rheumatology and Arthritis Care & Research. For now, guideline summaries for screening and monitoring and treatment are currently available, and three manuscripts are under peer review: one about screening and monitoring, one about treatment, and one about the patient panel that participated in the effort.
“ILD is a significant cause of morbidity and mortality in people with SARDs,” said Dr. Bernstein, who is co-first author of the guidelines. “People with systemic sclerosis, rheumatoid arthritis, idiopathic inflammatory myopathies, mixed connective tissue disease, and Sjögren’s disease are at greatest risk of developing ILD.”
Pediatric patients with SARDs excluded
The guidelines’ population of interest was people 17 years of age and older who were diagnosed with SARDs with a high risk of ILD. Pediatric patients with SARDs were excluded from the endeavor, as were those with systemic lupus erythematosus, antineutrophil cytoplasmic antibody–associated vasculitis, sarcoidosis, ankylosing spondylitis, undifferentiated connective tissue disease, interstitial pneumonia with autoimmune features, and those with unclassifiable ILD.
In the realm of screening, the guideline authors conditionally recommend two screening tests for patients considered at increased risk of ILD: pulmonary function tests and high-resolution chest CT (HRCT). Pulmonary function tests should include spirometry, lung volumes, and diffusion capacity. “Office spirometry alone is insufficient,” said Dr. Johnson, who served as lead author of the guidelines. And while a HRCT scan is recommended, “some patients may present to the emergency room with acute onset shortness of breath, and they may receive a CT angiogram to screen for pulmonary embolism,” she said. “It’s important to note that CT angiograms are performed in incomplete inspiration to maximize pulmonary artery enhancement. This may produce atelectasis that may obscure or mimic ILD. As a result, CTA studies are often inadequate to screen for ILD.”
Once a patient is diagnosed with ILD, three tests are recommended for monitoring: pulmonary function testing (every 3-6 months the first year in patients with IIM and SSc, then less frequently once stable, and every 3-12 months in the first year in patients with RA, SjD, and MCTD, then less frequently once stable); ambulatory desaturation testing every 3-12 months; and HRCT as needed. Dr. Johnson noted that while that the screening of ILD lies within the realm of rheumatologists, “once a patient is diagnosed, we are encouraged to comanage these patients with pulmonologists,” she said. “Ambulatory desaturation testing is not an infrequent test in the hands of pulmonologists. This is where co-management can be helpful.” She characterized a 6-minute walk test with continuous oximetry as “insufficient and is not synonymous with ambulatory desaturation testing. Ambulatory desaturation testing includes up titration of oxygen if a patient desaturates.”
The guidelines conditionally recommend against using chest radiography, 6-minute walk test distance, ambulatory desaturation testing, and bronchoscopy for ILD screening, and there is a strong recommendation against surgical lung biopsy. “However, there are unique circumstances where these tests may be considered,” Dr. Johnson said. “For example, ambulatory desaturation testing may be helpful if a patient is unable to perform a pulmonary function test. Bronchoscopy may be used to rule out infection, sarcoidosis, lymphoma, or alveolar hemorrhage, and surgical lung biopsy may be considered if you’re trying to rule out a malignancy.”
Similarly, several tests are conditionally recommended against for the monitoring of ILD, including chest radiography, the 6-minute walk test distance, and bronchoscopy. “But there are unique circumstances where they may be considered,” she said. “The 6-minute walk test may be used if a patient is unable to perform a pulmonary function test or if they’re being assessed for lung transplantation. Bronchoscopy may be used to rule out infection or alveolar hemorrhage.”
Preferred treatment options described
First-line treatment recommendations for ILD were based on the best available published evidence, voting panel expertise, and patient preferences. For SSc, the preferred treatment options include mycophenolate (CellCept), tocilizumab (Actemra), or rituximab (Rituxan and biosimilars), while additional options include cyclophosphamide, nintedanib (Ofev), and azathioprine. For myositis, the preferred treatment options include mycophenolate, azathioprine, rituximab, or calcineurin inhibitors, while additional options include a Janus kinase (JAK) inhibitor or cyclophosphamide. For MCTD, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include tocilizumab or cyclophosphamide. For RA and Sjögren’s, the preferred treatment options include mycophenolate, azathioprine, or rituximab, while additional options include cyclophosphamide. Dr. Johnson emphasized that there was low certainty evidence to recommend one treatment over another. “Many situations might lead a provider to choose a different option for ILD treatment, such as the presence of comorbidities or extra-pulmonary disease,” she said. “So, while our guidelines were focused on effectiveness for ILD, providers may choose therapies that will help ILD and other disease manifestations.”
The guidelines conditionally recommend a short course of glucocorticoids as a bridging therapy or for treatment of a flare of ILD in patients with myositis, MCTD, RA, and Sjögren’s. The panel strongly recommends against the use of glucocorticoids in patients with SSc due to the concern for inducing a scleroderma renal crisis. “While this may be common knowledge for rheumatologists, it may not be common knowledge for pulmonologists,” she said. “So here is an opportunity to educate our pulmonology colleagues in our consultation notes.”
The guidelines also include recommendations for progression of ILD, which was defined using the INBUILD trial criteria. Mycophenolate is conditionally recommended to be the first ILD treatment for all SARDs when progression occurs, if it wasn’t the first ILD treatment used. “If it was, then other medications that rheumatologists are used to can be considered as the next ILD treatment in the face of progression: rituximab, nintedanib, tocilizumab, and cyclophosphamide,” she said. The guidelines include a conditional recommendation against long-term glucocorticoid use in myositis, MCTD, RA, and Sjögren’s, plus a strong recommendation against long-term glucocorticoid use in SSc. Finally, there is a conditional recommendation of referral for lung transplant evaluation at the appropriate time at experienced centers.
Another group of recommendations has to do with cases of rapidly progressive ILD, which is characterized by rapid progression from no oxygen or a patient’s baseline oxygen requirement to a high oxygen requirement or intubation usually within days to weeks without a documented cause, such as infection or heart failure. “In cases of rapidly progressive ILD, which typically occurs in the setting of anti-MDA5 antibodies, there is a conditional recommendation for IV glucocorticoids plus two additional therapies: traditionally rituximab and mycophenolate,” Dr. Johnson said. “However, what may be new to some clinicians is combination IVIG [intravenous immunoglobulin] and a calcineurin inhibitor, notably tacrolimus,” she said. “This is the situation where experience at expert centers is influencing our guidelines in advance of data.”
A patient panel provided input
For the undertaking, a core team that included six rheumatologists; one pulmonologist; one thoracic radiologist; one expert on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology; and two literature review experts developed clinically relevant population, intervention, comparator, and outcomes (PICO) questions. The literature review team included 13 rheumatologists, 8 pulmonologists, and 3 methodologists. Finally, a 21-member patient panel was convened to share their values and preferences regarding screening, monitoring, and treatment of SARD-related ILD. Of these, Dr. Bernstein said that 4 were at risk for ILD and 17 had been diagnosed with ILD. Next, the literature review team conducted a systematic review and used the GRADE methodology to rate the available evidence as high, moderate, low, or very low. Then, a voting panel comprising 13 rheumatologists, 10 pulmonologists, 1 radiologist, and 3 patients from the patient panel cast votes for each PICO question and made final recommendations.
The review of evidence left the guidelines authors with 241 PICO questions, “which is a lot,” Dr. Bernstein said. “To put this in perspective, some guidelines address only 10 or 15 PICO questions. Fortunately, we had a dedicated group of experts who were up to the challenge.” Dr. Johnson emphasized that the forthcoming guidelines should not be used by insurers to mandate a specific order of prescribing. “Clinicians must retain the latitude to prescribe medications based on individual patient factors and preferences,” she said.
Dr. Bernstein disclosed that she is an adviser to, a consultant for, and has received grant or research support from Boehringer Ingelheim and has also received grant or research support from Kadmon and Pfizer. Dr. Johnson disclosed that she has received research support from the American College of Rheumatology to develop these guidelines. She has also been an investigator for trials sponsored by Bristol-Myers Squibb, Roche, and Boehringer Ingelheim and has mitigated these relevant conflicts of interest 1 year prior to the development of these guidelines, and will continue to do so for the foreseeable future.
AT ACR 2023
2023 USPSTF mammography age to start screening in average-risk patients: What’s new is old again
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.
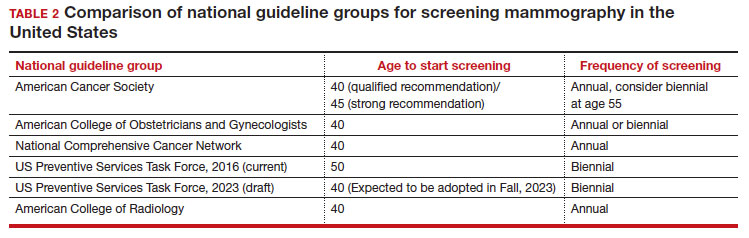
In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14
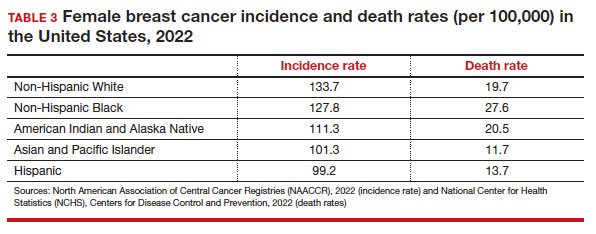
While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.
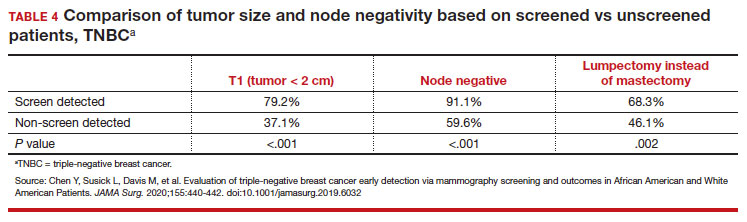
Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.

In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14

While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.

Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.

In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14

While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.

Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
Real-world study confirms benefits of erenumab for migraine prevention
Key clinical point: This real-world study confirms the efficacy and safety of erenumab in patients with migraine associated with extreme unmet needs.
Major finding: Overall, 52.9%, 58.5%, 57.0%, and 58.8% of patients receiving erenumab achieved ≥50% reduction in monthly migraine days at 3, 6, 9, and 12 months, respectively, with significant reductions in the proportion of patients with chronic migraine at all time points compared with baseline (P < .001). At month 3, 57.3% of patients changed from chronic migraine to episodic migraine. No treatment-related serious adverse events were reported.
Study details: Findings are from a 1-year prospective, real-word study including 140 patients with migraine and previous migraine preventive treatment failures who received ≥1 dose of erenumab.
Disclosures: This study did not receive funding from any source. Several authors declared receiving personal fees for consultancy activities or research support from various sources, and some authors declared no conflicts of interest.
Source: Lanteri-Minet M et al. One-year prospective real-world assessment of effectiveness and safety of erenumab in migraine prevention: Results of the French FHU INOVPAIN registry study. J Headache Pain. 2023;24:152 (Nov 8). doi: 10.1186/s10194-023-01680-4
Key clinical point: This real-world study confirms the efficacy and safety of erenumab in patients with migraine associated with extreme unmet needs.
Major finding: Overall, 52.9%, 58.5%, 57.0%, and 58.8% of patients receiving erenumab achieved ≥50% reduction in monthly migraine days at 3, 6, 9, and 12 months, respectively, with significant reductions in the proportion of patients with chronic migraine at all time points compared with baseline (P < .001). At month 3, 57.3% of patients changed from chronic migraine to episodic migraine. No treatment-related serious adverse events were reported.
Study details: Findings are from a 1-year prospective, real-word study including 140 patients with migraine and previous migraine preventive treatment failures who received ≥1 dose of erenumab.
Disclosures: This study did not receive funding from any source. Several authors declared receiving personal fees for consultancy activities or research support from various sources, and some authors declared no conflicts of interest.
Source: Lanteri-Minet M et al. One-year prospective real-world assessment of effectiveness and safety of erenumab in migraine prevention: Results of the French FHU INOVPAIN registry study. J Headache Pain. 2023;24:152 (Nov 8). doi: 10.1186/s10194-023-01680-4
Key clinical point: This real-world study confirms the efficacy and safety of erenumab in patients with migraine associated with extreme unmet needs.
Major finding: Overall, 52.9%, 58.5%, 57.0%, and 58.8% of patients receiving erenumab achieved ≥50% reduction in monthly migraine days at 3, 6, 9, and 12 months, respectively, with significant reductions in the proportion of patients with chronic migraine at all time points compared with baseline (P < .001). At month 3, 57.3% of patients changed from chronic migraine to episodic migraine. No treatment-related serious adverse events were reported.
Study details: Findings are from a 1-year prospective, real-word study including 140 patients with migraine and previous migraine preventive treatment failures who received ≥1 dose of erenumab.
Disclosures: This study did not receive funding from any source. Several authors declared receiving personal fees for consultancy activities or research support from various sources, and some authors declared no conflicts of interest.
Source: Lanteri-Minet M et al. One-year prospective real-world assessment of effectiveness and safety of erenumab in migraine prevention: Results of the French FHU INOVPAIN registry study. J Headache Pain. 2023;24:152 (Nov 8). doi: 10.1186/s10194-023-01680-4
Heavy secondhand smoke exposure tied to higher risk for severe headaches or migraine
Key clinical point: Heavy secondhand smoke (SHS) exposure was positively associated with a higher risk for severe headaches or migraine in adults who never smoked.
Major finding: Heavy SHS exposure (serum cotinine level 1-10 ng/mL) was positively associated with severe headaches or migraine (adjusted odds ratio 2.02; P = .011). No significant association was observed between low SHS exposure (serum cotinine level 0.05-0.99 ng/mL) and headaches or migraine (P = .226).
Study details: This nationwide cross-sectional study included 4560 adults who had never smoked, of which 20% had severe headaches or migraine.
Disclosures: This study did not receive any funding from external sources. The authors declared no conflicts of interest.
Source: Wu J, Yang P, et al. Association between secondhand smoke exposure and severe headaches or migraine in never-smoking adults. Headache. 2023 (Nov 8). doi: 10.1111/head.14640
Key clinical point: Heavy secondhand smoke (SHS) exposure was positively associated with a higher risk for severe headaches or migraine in adults who never smoked.
Major finding: Heavy SHS exposure (serum cotinine level 1-10 ng/mL) was positively associated with severe headaches or migraine (adjusted odds ratio 2.02; P = .011). No significant association was observed between low SHS exposure (serum cotinine level 0.05-0.99 ng/mL) and headaches or migraine (P = .226).
Study details: This nationwide cross-sectional study included 4560 adults who had never smoked, of which 20% had severe headaches or migraine.
Disclosures: This study did not receive any funding from external sources. The authors declared no conflicts of interest.
Source: Wu J, Yang P, et al. Association between secondhand smoke exposure and severe headaches or migraine in never-smoking adults. Headache. 2023 (Nov 8). doi: 10.1111/head.14640
Key clinical point: Heavy secondhand smoke (SHS) exposure was positively associated with a higher risk for severe headaches or migraine in adults who never smoked.
Major finding: Heavy SHS exposure (serum cotinine level 1-10 ng/mL) was positively associated with severe headaches or migraine (adjusted odds ratio 2.02; P = .011). No significant association was observed between low SHS exposure (serum cotinine level 0.05-0.99 ng/mL) and headaches or migraine (P = .226).
Study details: This nationwide cross-sectional study included 4560 adults who had never smoked, of which 20% had severe headaches or migraine.
Disclosures: This study did not receive any funding from external sources. The authors declared no conflicts of interest.
Source: Wu J, Yang P, et al. Association between secondhand smoke exposure and severe headaches or migraine in never-smoking adults. Headache. 2023 (Nov 8). doi: 10.1111/head.14640
Heart rate variability may help predict treatment response in chronic migraine
Key clinical point: Patients with chronic migraine have autonomic dysfunction, the extent of which is evaluated using heart rate variability (HRV) analysis, and a preserved function is associated with a superior response to flunarizine preventive treatment.
Major finding: Most heart-rate variability (HRV) parameters, except the ratios of low-frequency (LF) band and LF/high-frequency band, were significantly lower in patients with migraine vs control individuals (P < .001). The response to flunarizine treatment was superior in patients with normal HRV, as exemplified by higher reductions in monthly headache days after 3 months in those with normal vs lower HRV (─9.7 days vs ─6.2 days; P = .026).
Study details: This cross-sectional, prospective study included 81 prophylaxis-naive patients with chronic migraine and 58 age- and gender-matched control individuals. Patients with migraine initiated flunarizine as a preventive treatment for 12 weeks.
Disclosures: This study was supported by grants from the National Science and Technology Council, Taiwan, and others. SJ Wang declared being a principal investigator and receiving personal fees as an advisor or speaker from various sources.
Source: Chuang CH et al. Abnormal heart rate variability and its application in predicting treatment efficacy in patients with chronic migraine: An exploratory study. Cephalalgia. 2023 (Oct 18). doi: 10.1177/03331024231206781
Key clinical point: Patients with chronic migraine have autonomic dysfunction, the extent of which is evaluated using heart rate variability (HRV) analysis, and a preserved function is associated with a superior response to flunarizine preventive treatment.
Major finding: Most heart-rate variability (HRV) parameters, except the ratios of low-frequency (LF) band and LF/high-frequency band, were significantly lower in patients with migraine vs control individuals (P < .001). The response to flunarizine treatment was superior in patients with normal HRV, as exemplified by higher reductions in monthly headache days after 3 months in those with normal vs lower HRV (─9.7 days vs ─6.2 days; P = .026).
Study details: This cross-sectional, prospective study included 81 prophylaxis-naive patients with chronic migraine and 58 age- and gender-matched control individuals. Patients with migraine initiated flunarizine as a preventive treatment for 12 weeks.
Disclosures: This study was supported by grants from the National Science and Technology Council, Taiwan, and others. SJ Wang declared being a principal investigator and receiving personal fees as an advisor or speaker from various sources.
Source: Chuang CH et al. Abnormal heart rate variability and its application in predicting treatment efficacy in patients with chronic migraine: An exploratory study. Cephalalgia. 2023 (Oct 18). doi: 10.1177/03331024231206781
Key clinical point: Patients with chronic migraine have autonomic dysfunction, the extent of which is evaluated using heart rate variability (HRV) analysis, and a preserved function is associated with a superior response to flunarizine preventive treatment.
Major finding: Most heart-rate variability (HRV) parameters, except the ratios of low-frequency (LF) band and LF/high-frequency band, were significantly lower in patients with migraine vs control individuals (P < .001). The response to flunarizine treatment was superior in patients with normal HRV, as exemplified by higher reductions in monthly headache days after 3 months in those with normal vs lower HRV (─9.7 days vs ─6.2 days; P = .026).
Study details: This cross-sectional, prospective study included 81 prophylaxis-naive patients with chronic migraine and 58 age- and gender-matched control individuals. Patients with migraine initiated flunarizine as a preventive treatment for 12 weeks.
Disclosures: This study was supported by grants from the National Science and Technology Council, Taiwan, and others. SJ Wang declared being a principal investigator and receiving personal fees as an advisor or speaker from various sources.
Source: Chuang CH et al. Abnormal heart rate variability and its application in predicting treatment efficacy in patients with chronic migraine: An exploratory study. Cephalalgia. 2023 (Oct 18). doi: 10.1177/03331024231206781
Remote electrical neuromodulation: A pill-free, needle-free option for long-term migraine management
Key clinical point: This real-world study confirms the safety, efficacy, and tolerability of remote electrical neuromodulation (REN) for long-term management of acute migraine, thus establishing REN as a valuable comprehensive treatment for this chronic disease.
Major finding: Overall, 74.1% and 26.0% of patients achieved consistent pain relief and pain freedom with REN, respectively, and 70.2% and 33.7% achieved functional disability relief and functional disability freedom, respectively. The incidence of device-related adverse events (dAE) was low, ie, 1.96%, which included 0.49% negligible, 1.22% moderate, and 0.24% mild AE. No severe AE were reported, and all patients continued treatment despite dAE.
Study details: This real-world evidence study included 409 patients with migraine treated for 12 consecutive months with REN, a self-administered device used at the onset of migraine headache or aura for acute treatment.
Disclosures: This study was funded by Theranica Bio-Electronics Ltd. M Weinstein and A Synowiec declared serving as consultants for Theranica. A Stark-Inbar and A Ironi declared being employees of and hold stock options in Theranica. A Mauskop had no conflicts of interest to disclose.
Source: Synowiec A et al. One-year consistent safety, utilization, and efficacy assessment of remote electrical neuromodulation (REN) for migraine treatment. Adv Ther. 2023 (Oct 19). doi: 10.1007/s12325-023-02697-6
Key clinical point: This real-world study confirms the safety, efficacy, and tolerability of remote electrical neuromodulation (REN) for long-term management of acute migraine, thus establishing REN as a valuable comprehensive treatment for this chronic disease.
Major finding: Overall, 74.1% and 26.0% of patients achieved consistent pain relief and pain freedom with REN, respectively, and 70.2% and 33.7% achieved functional disability relief and functional disability freedom, respectively. The incidence of device-related adverse events (dAE) was low, ie, 1.96%, which included 0.49% negligible, 1.22% moderate, and 0.24% mild AE. No severe AE were reported, and all patients continued treatment despite dAE.
Study details: This real-world evidence study included 409 patients with migraine treated for 12 consecutive months with REN, a self-administered device used at the onset of migraine headache or aura for acute treatment.
Disclosures: This study was funded by Theranica Bio-Electronics Ltd. M Weinstein and A Synowiec declared serving as consultants for Theranica. A Stark-Inbar and A Ironi declared being employees of and hold stock options in Theranica. A Mauskop had no conflicts of interest to disclose.
Source: Synowiec A et al. One-year consistent safety, utilization, and efficacy assessment of remote electrical neuromodulation (REN) for migraine treatment. Adv Ther. 2023 (Oct 19). doi: 10.1007/s12325-023-02697-6
Key clinical point: This real-world study confirms the safety, efficacy, and tolerability of remote electrical neuromodulation (REN) for long-term management of acute migraine, thus establishing REN as a valuable comprehensive treatment for this chronic disease.
Major finding: Overall, 74.1% and 26.0% of patients achieved consistent pain relief and pain freedom with REN, respectively, and 70.2% and 33.7% achieved functional disability relief and functional disability freedom, respectively. The incidence of device-related adverse events (dAE) was low, ie, 1.96%, which included 0.49% negligible, 1.22% moderate, and 0.24% mild AE. No severe AE were reported, and all patients continued treatment despite dAE.
Study details: This real-world evidence study included 409 patients with migraine treated for 12 consecutive months with REN, a self-administered device used at the onset of migraine headache or aura for acute treatment.
Disclosures: This study was funded by Theranica Bio-Electronics Ltd. M Weinstein and A Synowiec declared serving as consultants for Theranica. A Stark-Inbar and A Ironi declared being employees of and hold stock options in Theranica. A Mauskop had no conflicts of interest to disclose.
Source: Synowiec A et al. One-year consistent safety, utilization, and efficacy assessment of remote electrical neuromodulation (REN) for migraine treatment. Adv Ther. 2023 (Oct 19). doi: 10.1007/s12325-023-02697-6










