User login
SUDEP Risk Decreases During Long-Term VNS Treatment
For patients with refractory epilepsy who receive vagus nerve stimulation (VNS) therapy, the risk of sudden unexpected death in epilepsy (SUDEP) decreases significantly during long-term follow-up, according to research published online ahead of print January 16 in Epilepsia. This finding “has not been previously reported in the literature and would be of value for patients at risk of SUDEP,” said the authors.
Long-Term Data Are Lacking
Most epidemiologic studies of SUDEP have had cross-sectional designs. Consequently, neurologists have lacked information about potential changes in the rate of SUDEP over time. Cyberonics, a Houston-based company, records information about implantation of its VNS device when the implanting facilities provide this information to it. To understand the evolution of SUDEP risk over time, Philippe Ryvlin, MD, Head of the Department of Clinical Neurosciences at Vaud University Hospital in Lausanne, Switzerland, and colleagues analyzed information from the company’s database.
For their study, the authors examined patients who underwent device implantation for epilepsy between November 16, 1988, and December 31, 2012. Eligible participants were US citizens or residents at the time of implantation and had a Social Security Number and a known date of birth. To ascertain patients’ vital status and cause of death, Dr. Ryvlin and colleagues submitted data to the Centers for Disease Control and Prevention’s National Death Index (NDI). The NDI included an underlying cause and as many as 20 contributory causes for each death.
The investigators defined SUDEP according to the criteria published by Annegers in 1997. To adjudicate SUDEP, they examined NDI data and death reports that Cyberonics recorded in a subset of patients. For patients for whom a death report was unavailable, the investigators performed adjudication by extrapolation.
Rate of SUDEP Decreased by One-Fourth
According to the database, 57,551 patients underwent implantation with the VNS device during the study period. A total of 40,443 (70%) participants met the researchers’ inclusion criteria. Patients’ average age at implantation was 30.8, and 15% of patients were under age 12. Half of participants were male.
The median duration of follow-up was 7.6 years. In all, 2,864 (7%) participants underwent explantation or had their devices turned off before the cutoff date, and 3,689 (9%) patients died during the study period.
A total of 953 (25.8%) of the deaths were associated with underlying and contributory causes considered compatible with SUDEP. Adjudication per protocol resulted in 632 (66.3%) cases of definite, probable, and possible SUDEP. The consensus conclusion among investigators resulted in 638 (66.9%) SUDEP cases. Adjudication by extrapolation resulted in 667 (70.0%) SUDEP cases and 286 (30.0%) non-SUDEP cases.
The crude and age-adjusted rates of SUDEP during years 3 to 10 of follow-up (2.10/1,000 patient years and 1.68/1,000 patient years, respectively) were significantly lower than those observed during the first two years of follow-up (2.74/1,000 patient years and 2.47/1,000 patient years, respectively). The crude rate ratio of SUDEP was 0.77, and the age-adjusted rate ratio of SUDEP was 0.68.
Biomarkers of SUDEP Would Aid Research
Because the study did not include a control group, and the database did not have preimplantation baseline information or data about individual responses to VNS therapy, the analysis does not clarify the role of VNS in the rate of SUDEP. Factors such as attrition, natural evolution, aging, or changes in medications or medical practice over time could explain the study findings, said Dr. Ryvlin.
The reasons for which SUDEP risk decreases over time should be investigated further, said the authors. A three-year randomized controlled trial would require at least 28,000 patients in each arm to compare adjunctive VNS treatment and standard treatment, but this level of enrollment is not feasible. “Novel biomarkers highly predictive of SUDEP will be needed to make prospective studies of SUDEP prevention feasible in an enriched population,” said Dr. Ryvlin. “Until then, only large retrospective cohorts, such as the study presented here, can help us make progress in SUDEP prevention, an issue that one should acknowledge when weighing the limitations and value of currently available data.”
—Erik Greb
Suggested Reading
Ryvlin P, So EL, Gordon CM, et al. Long-term surveillance of SUDEP in drug-resistant epilepsy patients treated with VNS therapy. Epilepsia. 2018 Jan 16 [Epub ahead of print].
For patients with refractory epilepsy who receive vagus nerve stimulation (VNS) therapy, the risk of sudden unexpected death in epilepsy (SUDEP) decreases significantly during long-term follow-up, according to research published online ahead of print January 16 in Epilepsia. This finding “has not been previously reported in the literature and would be of value for patients at risk of SUDEP,” said the authors.
Long-Term Data Are Lacking
Most epidemiologic studies of SUDEP have had cross-sectional designs. Consequently, neurologists have lacked information about potential changes in the rate of SUDEP over time. Cyberonics, a Houston-based company, records information about implantation of its VNS device when the implanting facilities provide this information to it. To understand the evolution of SUDEP risk over time, Philippe Ryvlin, MD, Head of the Department of Clinical Neurosciences at Vaud University Hospital in Lausanne, Switzerland, and colleagues analyzed information from the company’s database.
For their study, the authors examined patients who underwent device implantation for epilepsy between November 16, 1988, and December 31, 2012. Eligible participants were US citizens or residents at the time of implantation and had a Social Security Number and a known date of birth. To ascertain patients’ vital status and cause of death, Dr. Ryvlin and colleagues submitted data to the Centers for Disease Control and Prevention’s National Death Index (NDI). The NDI included an underlying cause and as many as 20 contributory causes for each death.
The investigators defined SUDEP according to the criteria published by Annegers in 1997. To adjudicate SUDEP, they examined NDI data and death reports that Cyberonics recorded in a subset of patients. For patients for whom a death report was unavailable, the investigators performed adjudication by extrapolation.
Rate of SUDEP Decreased by One-Fourth
According to the database, 57,551 patients underwent implantation with the VNS device during the study period. A total of 40,443 (70%) participants met the researchers’ inclusion criteria. Patients’ average age at implantation was 30.8, and 15% of patients were under age 12. Half of participants were male.
The median duration of follow-up was 7.6 years. In all, 2,864 (7%) participants underwent explantation or had their devices turned off before the cutoff date, and 3,689 (9%) patients died during the study period.
A total of 953 (25.8%) of the deaths were associated with underlying and contributory causes considered compatible with SUDEP. Adjudication per protocol resulted in 632 (66.3%) cases of definite, probable, and possible SUDEP. The consensus conclusion among investigators resulted in 638 (66.9%) SUDEP cases. Adjudication by extrapolation resulted in 667 (70.0%) SUDEP cases and 286 (30.0%) non-SUDEP cases.
The crude and age-adjusted rates of SUDEP during years 3 to 10 of follow-up (2.10/1,000 patient years and 1.68/1,000 patient years, respectively) were significantly lower than those observed during the first two years of follow-up (2.74/1,000 patient years and 2.47/1,000 patient years, respectively). The crude rate ratio of SUDEP was 0.77, and the age-adjusted rate ratio of SUDEP was 0.68.
Biomarkers of SUDEP Would Aid Research
Because the study did not include a control group, and the database did not have preimplantation baseline information or data about individual responses to VNS therapy, the analysis does not clarify the role of VNS in the rate of SUDEP. Factors such as attrition, natural evolution, aging, or changes in medications or medical practice over time could explain the study findings, said Dr. Ryvlin.
The reasons for which SUDEP risk decreases over time should be investigated further, said the authors. A three-year randomized controlled trial would require at least 28,000 patients in each arm to compare adjunctive VNS treatment and standard treatment, but this level of enrollment is not feasible. “Novel biomarkers highly predictive of SUDEP will be needed to make prospective studies of SUDEP prevention feasible in an enriched population,” said Dr. Ryvlin. “Until then, only large retrospective cohorts, such as the study presented here, can help us make progress in SUDEP prevention, an issue that one should acknowledge when weighing the limitations and value of currently available data.”
—Erik Greb
Suggested Reading
Ryvlin P, So EL, Gordon CM, et al. Long-term surveillance of SUDEP in drug-resistant epilepsy patients treated with VNS therapy. Epilepsia. 2018 Jan 16 [Epub ahead of print].
For patients with refractory epilepsy who receive vagus nerve stimulation (VNS) therapy, the risk of sudden unexpected death in epilepsy (SUDEP) decreases significantly during long-term follow-up, according to research published online ahead of print January 16 in Epilepsia. This finding “has not been previously reported in the literature and would be of value for patients at risk of SUDEP,” said the authors.
Long-Term Data Are Lacking
Most epidemiologic studies of SUDEP have had cross-sectional designs. Consequently, neurologists have lacked information about potential changes in the rate of SUDEP over time. Cyberonics, a Houston-based company, records information about implantation of its VNS device when the implanting facilities provide this information to it. To understand the evolution of SUDEP risk over time, Philippe Ryvlin, MD, Head of the Department of Clinical Neurosciences at Vaud University Hospital in Lausanne, Switzerland, and colleagues analyzed information from the company’s database.
For their study, the authors examined patients who underwent device implantation for epilepsy between November 16, 1988, and December 31, 2012. Eligible participants were US citizens or residents at the time of implantation and had a Social Security Number and a known date of birth. To ascertain patients’ vital status and cause of death, Dr. Ryvlin and colleagues submitted data to the Centers for Disease Control and Prevention’s National Death Index (NDI). The NDI included an underlying cause and as many as 20 contributory causes for each death.
The investigators defined SUDEP according to the criteria published by Annegers in 1997. To adjudicate SUDEP, they examined NDI data and death reports that Cyberonics recorded in a subset of patients. For patients for whom a death report was unavailable, the investigators performed adjudication by extrapolation.
Rate of SUDEP Decreased by One-Fourth
According to the database, 57,551 patients underwent implantation with the VNS device during the study period. A total of 40,443 (70%) participants met the researchers’ inclusion criteria. Patients’ average age at implantation was 30.8, and 15% of patients were under age 12. Half of participants were male.
The median duration of follow-up was 7.6 years. In all, 2,864 (7%) participants underwent explantation or had their devices turned off before the cutoff date, and 3,689 (9%) patients died during the study period.
A total of 953 (25.8%) of the deaths were associated with underlying and contributory causes considered compatible with SUDEP. Adjudication per protocol resulted in 632 (66.3%) cases of definite, probable, and possible SUDEP. The consensus conclusion among investigators resulted in 638 (66.9%) SUDEP cases. Adjudication by extrapolation resulted in 667 (70.0%) SUDEP cases and 286 (30.0%) non-SUDEP cases.
The crude and age-adjusted rates of SUDEP during years 3 to 10 of follow-up (2.10/1,000 patient years and 1.68/1,000 patient years, respectively) were significantly lower than those observed during the first two years of follow-up (2.74/1,000 patient years and 2.47/1,000 patient years, respectively). The crude rate ratio of SUDEP was 0.77, and the age-adjusted rate ratio of SUDEP was 0.68.
Biomarkers of SUDEP Would Aid Research
Because the study did not include a control group, and the database did not have preimplantation baseline information or data about individual responses to VNS therapy, the analysis does not clarify the role of VNS in the rate of SUDEP. Factors such as attrition, natural evolution, aging, or changes in medications or medical practice over time could explain the study findings, said Dr. Ryvlin.
The reasons for which SUDEP risk decreases over time should be investigated further, said the authors. A three-year randomized controlled trial would require at least 28,000 patients in each arm to compare adjunctive VNS treatment and standard treatment, but this level of enrollment is not feasible. “Novel biomarkers highly predictive of SUDEP will be needed to make prospective studies of SUDEP prevention feasible in an enriched population,” said Dr. Ryvlin. “Until then, only large retrospective cohorts, such as the study presented here, can help us make progress in SUDEP prevention, an issue that one should acknowledge when weighing the limitations and value of currently available data.”
—Erik Greb
Suggested Reading
Ryvlin P, So EL, Gordon CM, et al. Long-term surveillance of SUDEP in drug-resistant epilepsy patients treated with VNS therapy. Epilepsia. 2018 Jan 16 [Epub ahead of print].
ACOG app and applets: Tools to augment your practice
The American College of Obstetricians and Gynecologists (ACOG) is a nonprofit organization of women’s health care physicians advocating the highest standards of practice, continuing member education, and public awareness of women’s health care issues.1 The organization has long recognized the impact that social media and mobile technology would have for itself as well as its membership. ACOG published a Social Media Guide in 2012, featuring a section on how to use apps in ObGyn practice and provided a list of apps for ObGyns and their patients.2
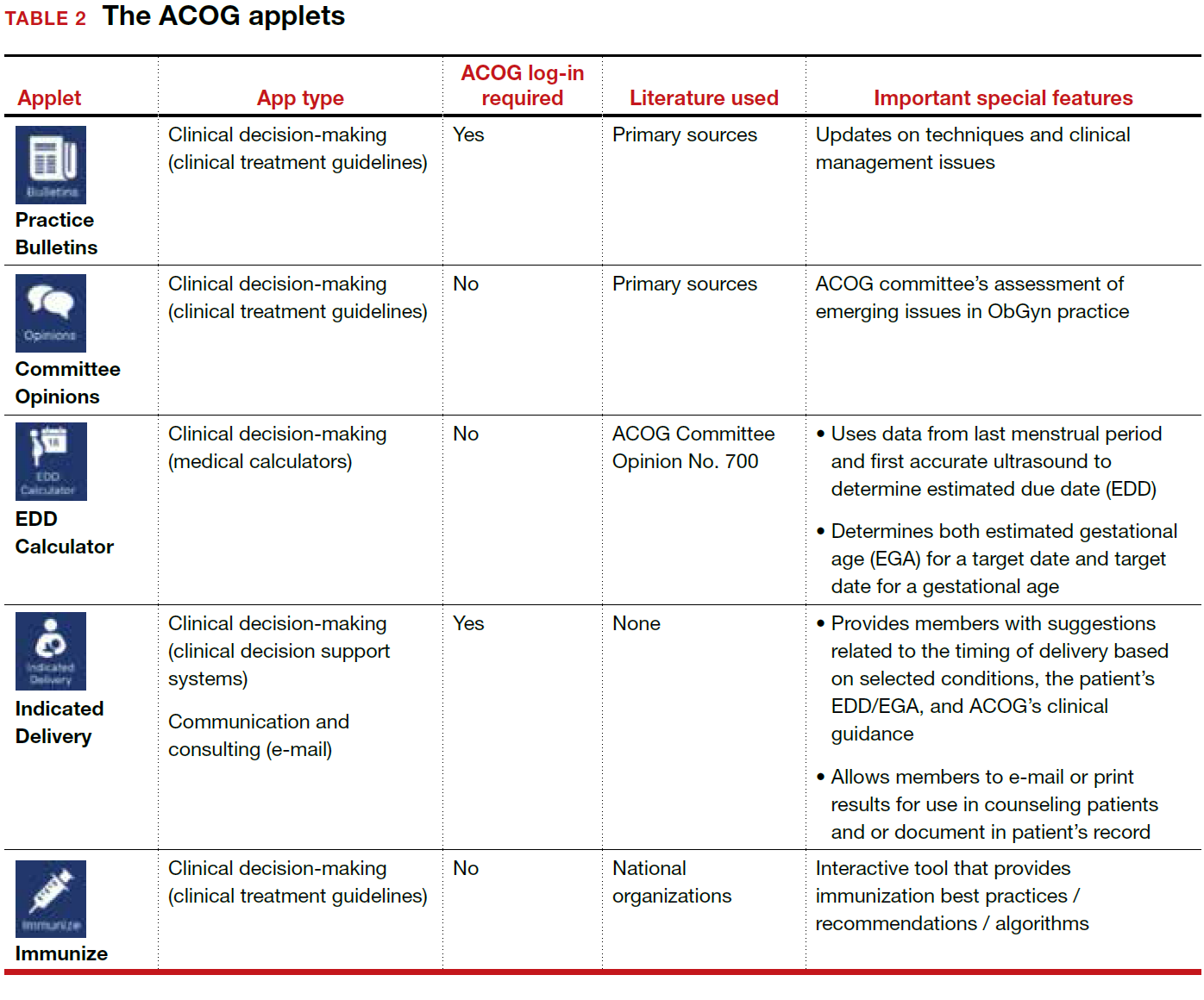
ACOG introduced its own app 4 years ago and has since updated the app several times, most recently on December 6, 2017. The ACOG app has a useful search function, a home button, and a place for users to email feedback (TABLE 1). The app most importantly contains several applets (small applications designed to perform a specific function within the main application). These applets encompass 3 types of apps for health care providers: clinical decision-making apps (Practice Bulletins, Committee Opinions, an Estimated Due Date Calculator that was featured in a prior review,3 Indicated Delivery, and Immunize) (TABLE 2), reference and information gathering apps (Today’s Headlines), and member support apps (ACOG Contacts, Careers, Annual Meeting, Districts, Council on Resident Education in Obstetrics and Gynecology [CREOG], and Website).4
This review will focus on the main ACOG app, which is evaluated by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).5 In addition, the clinical decision-making applets will be highlighted in a second table. I commend ACOG for developing these useful tools to augment their members’ practices. Of note, for the Practice Bulletins and Indicated Delivery applets, users will need to input their ACOG log-in access information.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- The American College of Obstetricians and Gynecologists web site. https://www.acog.org/About-ACOG. Updated 2017. Accessed February 12, 2018.
- ACOG today. The American College of Obstetricians and Gynecologists https://www.acog.org/-/media/ACOG-Today /acogToday201211.pdf. Published November 2012. Accessed February 12, 2018.
- Chen KT. Three good apps for calculating the date of delivery. OBG Manag. 2017;29(1):45–46.
- Ventola CL. Mobile devices and apps for health care professionals: Uses and benefits. P T. 2014;39(5):356–364.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478–1483.
The American College of Obstetricians and Gynecologists (ACOG) is a nonprofit organization of women’s health care physicians advocating the highest standards of practice, continuing member education, and public awareness of women’s health care issues.1 The organization has long recognized the impact that social media and mobile technology would have for itself as well as its membership. ACOG published a Social Media Guide in 2012, featuring a section on how to use apps in ObGyn practice and provided a list of apps for ObGyns and their patients.2
ACOG introduced its own app 4 years ago and has since updated the app several times, most recently on December 6, 2017. The ACOG app has a useful search function, a home button, and a place for users to email feedback (TABLE 1). The app most importantly contains several applets (small applications designed to perform a specific function within the main application). These applets encompass 3 types of apps for health care providers: clinical decision-making apps (Practice Bulletins, Committee Opinions, an Estimated Due Date Calculator that was featured in a prior review,3 Indicated Delivery, and Immunize) (TABLE 2), reference and information gathering apps (Today’s Headlines), and member support apps (ACOG Contacts, Careers, Annual Meeting, Districts, Council on Resident Education in Obstetrics and Gynecology [CREOG], and Website).4
This review will focus on the main ACOG app, which is evaluated by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).5 In addition, the clinical decision-making applets will be highlighted in a second table. I commend ACOG for developing these useful tools to augment their members’ practices. Of note, for the Practice Bulletins and Indicated Delivery applets, users will need to input their ACOG log-in access information.


Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The American College of Obstetricians and Gynecologists (ACOG) is a nonprofit organization of women’s health care physicians advocating the highest standards of practice, continuing member education, and public awareness of women’s health care issues.1 The organization has long recognized the impact that social media and mobile technology would have for itself as well as its membership. ACOG published a Social Media Guide in 2012, featuring a section on how to use apps in ObGyn practice and provided a list of apps for ObGyns and their patients.2
ACOG introduced its own app 4 years ago and has since updated the app several times, most recently on December 6, 2017. The ACOG app has a useful search function, a home button, and a place for users to email feedback (TABLE 1). The app most importantly contains several applets (small applications designed to perform a specific function within the main application). These applets encompass 3 types of apps for health care providers: clinical decision-making apps (Practice Bulletins, Committee Opinions, an Estimated Due Date Calculator that was featured in a prior review,3 Indicated Delivery, and Immunize) (TABLE 2), reference and information gathering apps (Today’s Headlines), and member support apps (ACOG Contacts, Careers, Annual Meeting, Districts, Council on Resident Education in Obstetrics and Gynecology [CREOG], and Website).4
This review will focus on the main ACOG app, which is evaluated by a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).5 In addition, the clinical decision-making applets will be highlighted in a second table. I commend ACOG for developing these useful tools to augment their members’ practices. Of note, for the Practice Bulletins and Indicated Delivery applets, users will need to input their ACOG log-in access information.


Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- The American College of Obstetricians and Gynecologists web site. https://www.acog.org/About-ACOG. Updated 2017. Accessed February 12, 2018.
- ACOG today. The American College of Obstetricians and Gynecologists https://www.acog.org/-/media/ACOG-Today /acogToday201211.pdf. Published November 2012. Accessed February 12, 2018.
- Chen KT. Three good apps for calculating the date of delivery. OBG Manag. 2017;29(1):45–46.
- Ventola CL. Mobile devices and apps for health care professionals: Uses and benefits. P T. 2014;39(5):356–364.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478–1483.
- The American College of Obstetricians and Gynecologists web site. https://www.acog.org/About-ACOG. Updated 2017. Accessed February 12, 2018.
- ACOG today. The American College of Obstetricians and Gynecologists https://www.acog.org/-/media/ACOG-Today /acogToday201211.pdf. Published November 2012. Accessed February 12, 2018.
- Chen KT. Three good apps for calculating the date of delivery. OBG Manag. 2017;29(1):45–46.
- Ventola CL. Mobile devices and apps for health care professionals: Uses and benefits. P T. 2014;39(5):356–364.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478–1483.
Preoperative penicillin allergy tests could decrease SSI
Patients with reported penicillin allergies are significantly more likely to develop surgical site infections, according to a study conducted at Massachusetts General Hospital in Boston.
With new evidence reporting 90%-99% of patients with a reported allergy are not actually allergic, conducting a preoperative allergy test could improve treatment choice and decrease the risk of SSI, as well as the notable financial burden associated with it. Thus, “systematic, preoperative penicillin allergy evaluations in surgical patients may not only improve antibiotic choice but also decrease SSI risk,” according to Kimberly Blumenthal, MD, the quality director for the department of allergy and immunology at Massachusetts General Hospital, and her fellow investigators.
Surgeries performed were hip arthroplasty, knee arthroplasty, hysterectomy, colon surgery, or coronary artery bypass grafting.
Of the patients studied, 922 (11%) reported a penicillin allergy; most had minor reactions, such as rashes (37.5%) or urticaria (18%). “Only 5 reactions to penicillin represented contraindications to receiving a beta-lactam; the vast majority of patients would have tolerated first-line recommended cephalosporin prophylaxis had allergy evaluation been pursued,“ according to Dr. Blumenthal and her colleagues.
Overall, a total of 241 (2.7%) patients contracted an SSI. In a multivariate analysis, patients who had reported a penicillin allergy were 50% more likely to develop an SSI than those who had no reported allergy (adjusted odds ratio, 1.5; P = .04).
Risk may even be higher than 50% in the general health care population because this health center has a relatively low rate of SSIs, compared with many other hospitals, Dr. Blumenthal and her fellow investigators stated.
The increased risk primarily concerns the treatment used because those with a reported allergy were more likely than those without the allergy to be given clindamycin (48.8% vs. 3.1%, respectively), vancomycin (34.7% vs. 3.3%), gentamicin (24% vs. 2.8%), or fluoroquinolones (6.8% vs. 1.3%) instead of the most commonly used antibiotic, cefazolin (12.2% vs. 92.4%).
Patients given antibiotics other than cefazolin were usually given treatment outside of the perioperative window, which could severely increase the likelihood for developing an SSI, according to investigators. Of patients given vancomycin, 97.5% did not receive their treatment in the recommended time frame, compared with 1.7% of those given cefazolin.
“Increased odds of SSI among patients reporting a penicillin allergy in this cohort was entirely due to the use of beta-lactam–alternative perioperative antibiotics,” wrote to Dr. Blumenthal and her colleagues. “Patients with reported penicillin allergy in this study were not only less likely to receive the most effective perioperative antibiotic, they were also less likely to receive prophylaxis in the recommended time frame for optimal tissue concentration.”
While allergy assessments before surgery are currently recommended, there are no specifically outlined methods for these evaluations, which leads many providers to take what has been deemed the safer route of giving patients beta-lactam–alternative antibiotics instead, Dr. Blumenthal and her colleagues suggested.
The research was supported by the National Institutes of Health, and the investigators reported having no relevant conflicts.
SOURCE: Blumenthal K et al. Clin Infect Dis. 2018 Jan 18;66(3):329-36.
Patients with reported penicillin allergies are significantly more likely to develop surgical site infections, according to a study conducted at Massachusetts General Hospital in Boston.
With new evidence reporting 90%-99% of patients with a reported allergy are not actually allergic, conducting a preoperative allergy test could improve treatment choice and decrease the risk of SSI, as well as the notable financial burden associated with it. Thus, “systematic, preoperative penicillin allergy evaluations in surgical patients may not only improve antibiotic choice but also decrease SSI risk,” according to Kimberly Blumenthal, MD, the quality director for the department of allergy and immunology at Massachusetts General Hospital, and her fellow investigators.
Surgeries performed were hip arthroplasty, knee arthroplasty, hysterectomy, colon surgery, or coronary artery bypass grafting.
Of the patients studied, 922 (11%) reported a penicillin allergy; most had minor reactions, such as rashes (37.5%) or urticaria (18%). “Only 5 reactions to penicillin represented contraindications to receiving a beta-lactam; the vast majority of patients would have tolerated first-line recommended cephalosporin prophylaxis had allergy evaluation been pursued,“ according to Dr. Blumenthal and her colleagues.
Overall, a total of 241 (2.7%) patients contracted an SSI. In a multivariate analysis, patients who had reported a penicillin allergy were 50% more likely to develop an SSI than those who had no reported allergy (adjusted odds ratio, 1.5; P = .04).
Risk may even be higher than 50% in the general health care population because this health center has a relatively low rate of SSIs, compared with many other hospitals, Dr. Blumenthal and her fellow investigators stated.
The increased risk primarily concerns the treatment used because those with a reported allergy were more likely than those without the allergy to be given clindamycin (48.8% vs. 3.1%, respectively), vancomycin (34.7% vs. 3.3%), gentamicin (24% vs. 2.8%), or fluoroquinolones (6.8% vs. 1.3%) instead of the most commonly used antibiotic, cefazolin (12.2% vs. 92.4%).
Patients given antibiotics other than cefazolin were usually given treatment outside of the perioperative window, which could severely increase the likelihood for developing an SSI, according to investigators. Of patients given vancomycin, 97.5% did not receive their treatment in the recommended time frame, compared with 1.7% of those given cefazolin.
“Increased odds of SSI among patients reporting a penicillin allergy in this cohort was entirely due to the use of beta-lactam–alternative perioperative antibiotics,” wrote to Dr. Blumenthal and her colleagues. “Patients with reported penicillin allergy in this study were not only less likely to receive the most effective perioperative antibiotic, they were also less likely to receive prophylaxis in the recommended time frame for optimal tissue concentration.”
While allergy assessments before surgery are currently recommended, there are no specifically outlined methods for these evaluations, which leads many providers to take what has been deemed the safer route of giving patients beta-lactam–alternative antibiotics instead, Dr. Blumenthal and her colleagues suggested.
The research was supported by the National Institutes of Health, and the investigators reported having no relevant conflicts.
SOURCE: Blumenthal K et al. Clin Infect Dis. 2018 Jan 18;66(3):329-36.
Patients with reported penicillin allergies are significantly more likely to develop surgical site infections, according to a study conducted at Massachusetts General Hospital in Boston.
With new evidence reporting 90%-99% of patients with a reported allergy are not actually allergic, conducting a preoperative allergy test could improve treatment choice and decrease the risk of SSI, as well as the notable financial burden associated with it. Thus, “systematic, preoperative penicillin allergy evaluations in surgical patients may not only improve antibiotic choice but also decrease SSI risk,” according to Kimberly Blumenthal, MD, the quality director for the department of allergy and immunology at Massachusetts General Hospital, and her fellow investigators.
Surgeries performed were hip arthroplasty, knee arthroplasty, hysterectomy, colon surgery, or coronary artery bypass grafting.
Of the patients studied, 922 (11%) reported a penicillin allergy; most had minor reactions, such as rashes (37.5%) or urticaria (18%). “Only 5 reactions to penicillin represented contraindications to receiving a beta-lactam; the vast majority of patients would have tolerated first-line recommended cephalosporin prophylaxis had allergy evaluation been pursued,“ according to Dr. Blumenthal and her colleagues.
Overall, a total of 241 (2.7%) patients contracted an SSI. In a multivariate analysis, patients who had reported a penicillin allergy were 50% more likely to develop an SSI than those who had no reported allergy (adjusted odds ratio, 1.5; P = .04).
Risk may even be higher than 50% in the general health care population because this health center has a relatively low rate of SSIs, compared with many other hospitals, Dr. Blumenthal and her fellow investigators stated.
The increased risk primarily concerns the treatment used because those with a reported allergy were more likely than those without the allergy to be given clindamycin (48.8% vs. 3.1%, respectively), vancomycin (34.7% vs. 3.3%), gentamicin (24% vs. 2.8%), or fluoroquinolones (6.8% vs. 1.3%) instead of the most commonly used antibiotic, cefazolin (12.2% vs. 92.4%).
Patients given antibiotics other than cefazolin were usually given treatment outside of the perioperative window, which could severely increase the likelihood for developing an SSI, according to investigators. Of patients given vancomycin, 97.5% did not receive their treatment in the recommended time frame, compared with 1.7% of those given cefazolin.
“Increased odds of SSI among patients reporting a penicillin allergy in this cohort was entirely due to the use of beta-lactam–alternative perioperative antibiotics,” wrote to Dr. Blumenthal and her colleagues. “Patients with reported penicillin allergy in this study were not only less likely to receive the most effective perioperative antibiotic, they were also less likely to receive prophylaxis in the recommended time frame for optimal tissue concentration.”
While allergy assessments before surgery are currently recommended, there are no specifically outlined methods for these evaluations, which leads many providers to take what has been deemed the safer route of giving patients beta-lactam–alternative antibiotics instead, Dr. Blumenthal and her colleagues suggested.
The research was supported by the National Institutes of Health, and the investigators reported having no relevant conflicts.
SOURCE: Blumenthal K et al. Clin Infect Dis. 2018 Jan 18;66(3):329-36.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Patients with reported penicillin allergies are at higher risk of developing a surgical site infection.
Major finding: Having a penicillin allergy was associated with a 50% increased risk of developing a surgical site infection, compared with those without the allergy (adjusted odds ratio, 1.5; P = .04).
Study details: Retrospective cohort study of 8,385 patients operated on at Massachusetts General Hospital, Boston, during 2010-2014.
Disclosures: The research was supported by the National Institutes of Health, and the investigators reported having no relevant conflicts.
Source: Blumenthal K et al. Clin Infect Dis. 2018 Jan 18;66(3):329-36.
VIDEO: The return of Kaposi’s sarcoma
SAN DIEGO – Dermatologists, who served as crucial sentinels during the early years of the AIDS epidemic, should be alert for dermatologic signs and symptoms of HIV infection, according to Toby Maurer, MD, professor of clinical dermatology at the University of California, San Francisco.
“We’re now seeing a lot of HIV-infected patients presenting once again with skin symptoms,” including new-onset psoriasis, poorly controlled seborrheic dermatitis, and even Kaposi’s sarcoma, she said in a video interview at the annual meeting of the American Academy of Dermatology.
The upswing in cases of Kaposi’s sarcoma “comes as a shock to many dermatologists; they thought Kaposi’s sarcoma was a thing of the past,” added Dr. Maurer, who presented on HIV-associated skin conditions at the meeting.
“My whole plea is to remember that HIV has not gone away, that it keeps showing up, and that the skin symptoms absolutely show up,” she said. “It’s not on the radar as much as it should be.”
In her presentation, Dr. Maurer, who is also chief of dermatology at San Francisco General Hospital, said HIV and HIV medications have a variety of impacts on skin. For example, psoriasis gets worse when patients are off medication and better when they’re on it, she said, while molluscum contagiosum and herpes simplex can actually worsen when patients start HIV drugs. And, she said, a late start of AIDS drugs can worsen eczema.
In the interview, she discussed the impact of starting antiretrovirals late into the infection, when CD4 counts are low, on skin conditions, as well as possible reasons behind the increase in Kaposi’s sarcoma, and interactions between systemic dermatologic medications and some antiretrovirals.
Dr. Maurer reports no relevant disclosures.
SAN DIEGO – Dermatologists, who served as crucial sentinels during the early years of the AIDS epidemic, should be alert for dermatologic signs and symptoms of HIV infection, according to Toby Maurer, MD, professor of clinical dermatology at the University of California, San Francisco.
“We’re now seeing a lot of HIV-infected patients presenting once again with skin symptoms,” including new-onset psoriasis, poorly controlled seborrheic dermatitis, and even Kaposi’s sarcoma, she said in a video interview at the annual meeting of the American Academy of Dermatology.
The upswing in cases of Kaposi’s sarcoma “comes as a shock to many dermatologists; they thought Kaposi’s sarcoma was a thing of the past,” added Dr. Maurer, who presented on HIV-associated skin conditions at the meeting.
“My whole plea is to remember that HIV has not gone away, that it keeps showing up, and that the skin symptoms absolutely show up,” she said. “It’s not on the radar as much as it should be.”
In her presentation, Dr. Maurer, who is also chief of dermatology at San Francisco General Hospital, said HIV and HIV medications have a variety of impacts on skin. For example, psoriasis gets worse when patients are off medication and better when they’re on it, she said, while molluscum contagiosum and herpes simplex can actually worsen when patients start HIV drugs. And, she said, a late start of AIDS drugs can worsen eczema.
In the interview, she discussed the impact of starting antiretrovirals late into the infection, when CD4 counts are low, on skin conditions, as well as possible reasons behind the increase in Kaposi’s sarcoma, and interactions between systemic dermatologic medications and some antiretrovirals.
Dr. Maurer reports no relevant disclosures.
SAN DIEGO – Dermatologists, who served as crucial sentinels during the early years of the AIDS epidemic, should be alert for dermatologic signs and symptoms of HIV infection, according to Toby Maurer, MD, professor of clinical dermatology at the University of California, San Francisco.
“We’re now seeing a lot of HIV-infected patients presenting once again with skin symptoms,” including new-onset psoriasis, poorly controlled seborrheic dermatitis, and even Kaposi’s sarcoma, she said in a video interview at the annual meeting of the American Academy of Dermatology.
The upswing in cases of Kaposi’s sarcoma “comes as a shock to many dermatologists; they thought Kaposi’s sarcoma was a thing of the past,” added Dr. Maurer, who presented on HIV-associated skin conditions at the meeting.
“My whole plea is to remember that HIV has not gone away, that it keeps showing up, and that the skin symptoms absolutely show up,” she said. “It’s not on the radar as much as it should be.”
In her presentation, Dr. Maurer, who is also chief of dermatology at San Francisco General Hospital, said HIV and HIV medications have a variety of impacts on skin. For example, psoriasis gets worse when patients are off medication and better when they’re on it, she said, while molluscum contagiosum and herpes simplex can actually worsen when patients start HIV drugs. And, she said, a late start of AIDS drugs can worsen eczema.
In the interview, she discussed the impact of starting antiretrovirals late into the infection, when CD4 counts are low, on skin conditions, as well as possible reasons behind the increase in Kaposi’s sarcoma, and interactions between systemic dermatologic medications and some antiretrovirals.
Dr. Maurer reports no relevant disclosures.
REPORTING FROM AAD 18
An either/or choice is not a good strategy for pain
An either/or choice is not a good strategy for pain
I found Dr. Barbieri’s editorial on postpartum opioid use and breastfeeding interesting, but one key issue was not addressed: Following this guidance means that new mothers have to choose between breastfeeding and pain control. You may explain to a patient with 2-day cesarean delivery pain, “If you take pain medicine while breastfeeding, it can adversely affect the baby. So we will give you acetaminophen.” While some moms will deal with it, others will stop breastfeeding. With the increasing pressure to advocate for breastfeeding, this strategy is likely not realistic.
R. Lee Toler, DO
Bolivia, North Carolina
My pain management protocol
While presently in an office-based setting, back in my inpatient practice days I would order oxycodone plus acetaminophen for 1 to 2 days postoperative cesarean delivery, and only 1 day after normal spontaneous delivery if the patient had a large perineal repair or multiparous involution pain. Otherwise, it was ibuprofen 800 mg, then 400 to 600 mg on discharge home.
Gabrielle Long, CNM
Mohegan Lake, New York
Respect women’s postsurgical pain management needs
There is a real disrespect for pain control for women, such as after a cesarean delivery. I would like to see any male have major surgery through a large muscle like the uterus and not need significant pain control options!
Anne V. Hale, MD
El Paso, Texas
Dr. Barbieri responds
I agree with Ms. Long that most postpartum patients, including many who have had a cesarean delivery, can achieve adequate pain control with the use of parenteral and oral nonsteroidal anti-inflammatory drugs (NSAIDs) and oral acetaminophen. Drs. Toler and Hale are concerned that postpartum pain control might be suboptimal if opioids are underprescribed. However, in many developed countries obstetricians do not use opioid pain medicine for postpartum pain management, relying on NSAIDs and acetaminophen. Given the success of this approach, I think we can significantly reduce the use of opioids by postpartum women in the United States by optimizing our use of nonopioid medications.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
An either/or choice is not a good strategy for pain
I found Dr. Barbieri’s editorial on postpartum opioid use and breastfeeding interesting, but one key issue was not addressed: Following this guidance means that new mothers have to choose between breastfeeding and pain control. You may explain to a patient with 2-day cesarean delivery pain, “If you take pain medicine while breastfeeding, it can adversely affect the baby. So we will give you acetaminophen.” While some moms will deal with it, others will stop breastfeeding. With the increasing pressure to advocate for breastfeeding, this strategy is likely not realistic.
R. Lee Toler, DO
Bolivia, North Carolina
My pain management protocol
While presently in an office-based setting, back in my inpatient practice days I would order oxycodone plus acetaminophen for 1 to 2 days postoperative cesarean delivery, and only 1 day after normal spontaneous delivery if the patient had a large perineal repair or multiparous involution pain. Otherwise, it was ibuprofen 800 mg, then 400 to 600 mg on discharge home.
Gabrielle Long, CNM
Mohegan Lake, New York
Respect women’s postsurgical pain management needs
There is a real disrespect for pain control for women, such as after a cesarean delivery. I would like to see any male have major surgery through a large muscle like the uterus and not need significant pain control options!
Anne V. Hale, MD
El Paso, Texas
Dr. Barbieri responds
I agree with Ms. Long that most postpartum patients, including many who have had a cesarean delivery, can achieve adequate pain control with the use of parenteral and oral nonsteroidal anti-inflammatory drugs (NSAIDs) and oral acetaminophen. Drs. Toler and Hale are concerned that postpartum pain control might be suboptimal if opioids are underprescribed. However, in many developed countries obstetricians do not use opioid pain medicine for postpartum pain management, relying on NSAIDs and acetaminophen. Given the success of this approach, I think we can significantly reduce the use of opioids by postpartum women in the United States by optimizing our use of nonopioid medications.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
An either/or choice is not a good strategy for pain
I found Dr. Barbieri’s editorial on postpartum opioid use and breastfeeding interesting, but one key issue was not addressed: Following this guidance means that new mothers have to choose between breastfeeding and pain control. You may explain to a patient with 2-day cesarean delivery pain, “If you take pain medicine while breastfeeding, it can adversely affect the baby. So we will give you acetaminophen.” While some moms will deal with it, others will stop breastfeeding. With the increasing pressure to advocate for breastfeeding, this strategy is likely not realistic.
R. Lee Toler, DO
Bolivia, North Carolina
My pain management protocol
While presently in an office-based setting, back in my inpatient practice days I would order oxycodone plus acetaminophen for 1 to 2 days postoperative cesarean delivery, and only 1 day after normal spontaneous delivery if the patient had a large perineal repair or multiparous involution pain. Otherwise, it was ibuprofen 800 mg, then 400 to 600 mg on discharge home.
Gabrielle Long, CNM
Mohegan Lake, New York
Respect women’s postsurgical pain management needs
There is a real disrespect for pain control for women, such as after a cesarean delivery. I would like to see any male have major surgery through a large muscle like the uterus and not need significant pain control options!
Anne V. Hale, MD
El Paso, Texas
Dr. Barbieri responds
I agree with Ms. Long that most postpartum patients, including many who have had a cesarean delivery, can achieve adequate pain control with the use of parenteral and oral nonsteroidal anti-inflammatory drugs (NSAIDs) and oral acetaminophen. Drs. Toler and Hale are concerned that postpartum pain control might be suboptimal if opioids are underprescribed. However, in many developed countries obstetricians do not use opioid pain medicine for postpartum pain management, relying on NSAIDs and acetaminophen. Given the success of this approach, I think we can significantly reduce the use of opioids by postpartum women in the United States by optimizing our use of nonopioid medications.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Effects of LDs and Migraine Treatment History
Typical practices of obtaining new baselines every 2 years in the high school population can be applied to athletes with a history of special education or learning disorders (LD) and headache/migraine treatment, a recent study found. This study examined the test-retest reliability of the 4- and 2-factor structures (ie, memory and speed) of ImPACT (Immediate Post-Concussion Assessment and Cognitive Test) over a 2-year interval across multiple groups with premorbid conditions, including those with a history of special education or LDs (n=114), treatment history for headache/migraine (n=81), and a control group (n= 792).
Researchers found:
- Significant improvement on all 4 composites were observed for the control group over a 2-year interval, whereas significant differences were observed only on visual motor speed for the LD and headache/migraine treatment history groups.
- The 2-factor structure has potential to increase test-retest reliability.
Two-year test-retest reliability in high school athletes using the four- and two-factor ImPACT composite structures: The effects of learning disorders and headache/migraine treatment history. Clin Neuropsychol. 2018;33(2):256-226. doi:10.1093/arclin/acx059.
Typical practices of obtaining new baselines every 2 years in the high school population can be applied to athletes with a history of special education or learning disorders (LD) and headache/migraine treatment, a recent study found. This study examined the test-retest reliability of the 4- and 2-factor structures (ie, memory and speed) of ImPACT (Immediate Post-Concussion Assessment and Cognitive Test) over a 2-year interval across multiple groups with premorbid conditions, including those with a history of special education or LDs (n=114), treatment history for headache/migraine (n=81), and a control group (n= 792).
Researchers found:
- Significant improvement on all 4 composites were observed for the control group over a 2-year interval, whereas significant differences were observed only on visual motor speed for the LD and headache/migraine treatment history groups.
- The 2-factor structure has potential to increase test-retest reliability.
Two-year test-retest reliability in high school athletes using the four- and two-factor ImPACT composite structures: The effects of learning disorders and headache/migraine treatment history. Clin Neuropsychol. 2018;33(2):256-226. doi:10.1093/arclin/acx059.
Typical practices of obtaining new baselines every 2 years in the high school population can be applied to athletes with a history of special education or learning disorders (LD) and headache/migraine treatment, a recent study found. This study examined the test-retest reliability of the 4- and 2-factor structures (ie, memory and speed) of ImPACT (Immediate Post-Concussion Assessment and Cognitive Test) over a 2-year interval across multiple groups with premorbid conditions, including those with a history of special education or LDs (n=114), treatment history for headache/migraine (n=81), and a control group (n= 792).
Researchers found:
- Significant improvement on all 4 composites were observed for the control group over a 2-year interval, whereas significant differences were observed only on visual motor speed for the LD and headache/migraine treatment history groups.
- The 2-factor structure has potential to increase test-retest reliability.
Two-year test-retest reliability in high school athletes using the four- and two-factor ImPACT composite structures: The effects of learning disorders and headache/migraine treatment history. Clin Neuropsychol. 2018;33(2):256-226. doi:10.1093/arclin/acx059.
ECTRIMS and EAN Publish Recommendations for Treating MS
The European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Academy of Neurology (EAN) have published a guideline to offer up-to-date, evidence-based recommendations for the treatment of adult patients with MS. The guideline is intended to fill a perceived need for a comprehensive document that includes information about recently approved MS therapies and helps clinicians and patients resolve difficulties in everyday clinical practice. It was published in the February issue of Multiple Sclerosis.
Authors Addressed 10 Questions
Xavier Montalban, MD, PhD, Chair and Director of the Department of Neurology and Neuroimmunology at Vall d’Hebron University Hospital in Barcelona, and colleagues agreed to investigate 10 questions related to treatment efficacy, response criteria, strategies to address suboptimal response and safety concerns, and treatment of MS in pregnancy. They developed the guideline following the recommendations of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group, along with EAN recommendations for writing a neurologic management guideline. Literature searches relied upon databases such as the Cochrane Central Register of Controlled Trials, Excerpta Medica, and Medical Literature Analysis and Retrieval System Online.
The authors evaluated data for all disease-modifying treatments (DMTs) approved by the European Medicines Agency at the time of publication. They did not consider combination therapies, complementary or alternative medicine, or symptomatic treatment. Although focused on Europe, the guideline does not account for regulatory or organizational issues specific to any European country.
Strong Recommendations
Dr. Montalban and colleagues agreed upon 21 recommendations and consensus statements. The recommendations were categorized as strong or weak, according to the quality of evidence and the risk–benefit balance. The authors formulated consensus statements on questions for which evidence was insufficient to support a formal recommendation.
The first of the guideline’s three strong recommendations is that neurologists offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS. The second is to offer early treatment with DMTs to patients with active relapsing-remitting MS, as defined by clinical relapses or MRI activity. The authors defined active lesions as contrast-enhancing lesions or new or unequivocally enlarging T2 lesions assessed at least annually. This recommendation also applies to patients with CIS who fulfill current diagnostic criteria for MS. The third strong recommendation is to offer a more efficacious drug to patients treated with interferon or glatiramer acetate who show evidence of disease activity.
Weak Recommendations
Nine of the guideline’s recommendations are categorized as weak. For example, neurologists should consider treating patients with active secondary progressive MS with interferon beta-1a or -1b, taking into account the treatments’ “dubious efficacy,” as well as their safety and tolerability, according to the authors. Neurologists should consider mitoxantrone, ocrelizumab, and cladribine for this population. The authors recommend considering ocrelizumab as a treatment for patients with primary progressive MS.
As a way of monitoring treatment response, the authors recommend that neurologists consider combining MRI with clinical measures when evaluating disease evolution in treated patients. When making treatment decisions in the event of safety concerns, neurologists should consider the possibility that disease activity may resume or rebound if treatment is stopped, particularly with natalizumab, said Dr. Montalban and colleagues. Continuation of DMT treatment should be considered for patients who are clinically stable, who have stable MRI, and who have no problems with safety or tolerability, according to the guideline.
“For women planning a pregnancy, if there is a high risk of disease reactivation, consider using interferon or glatiramer acetate until pregnancy is confirmed,” said the authors. “In some very specific (active) cases, continuing this treatment during pregnancy could also be considered.” Delaying pregnancy is advisable for women with persistent high disease activity. If such a woman decides to become pregnant or has an unplanned pregnancy, neurologists may consider treatment with natalizumab throughout pregnancy after a full discussion with the patient of the potential implications. “Treatment with alemtuzumab could be an alternative therapeutic option for planned pregnancy in very active cases, provided that a four-month interval is strictly observed from the latest infusion until conception,” said Dr. Montalban and colleagues.
Consensus Statements
Several of the guideline’s consensus statements relate to the monitoring of treatment response. For patients treated with DMTs, the authors recommend performing a standardized reference brain MRI within six months of treatment onset, and comparing it with a subsequent brain MRI performed 12 months after starting treatment. The measurement of new or unequivocally enlarging T2 lesions is the preferred MRI method of gauging treatment response, supplemented by gadolinium-enhancing lesions. To monitor treatment safety, the authors recommend performing a standardized reference brain MRI every year in patients at low risk for progressive multifocal leukoencephalopathy (PML), and every three to six months in patients at high risk for PML.
If the neurologist and patient have decided to switch therapies, they should consider the patient’s characteristics and comorbidities, drug safety profiles, and disease severity when choosing a new DMT. If treatment of a highly efficacious drug is stopped because of safety or efficacy problems, the neurologist should consider starting another highly efficacious drug, according to the guideline. Disease activity, the drug’s half-life and biologic activity, and the potential for resumed disease activity or rebound should be considered when choosing a new therapy, said Dr. Montalban and colleagues.
—Erik Greb
Suggested Reading
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96-120.
The European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Academy of Neurology (EAN) have published a guideline to offer up-to-date, evidence-based recommendations for the treatment of adult patients with MS. The guideline is intended to fill a perceived need for a comprehensive document that includes information about recently approved MS therapies and helps clinicians and patients resolve difficulties in everyday clinical practice. It was published in the February issue of Multiple Sclerosis.
Authors Addressed 10 Questions
Xavier Montalban, MD, PhD, Chair and Director of the Department of Neurology and Neuroimmunology at Vall d’Hebron University Hospital in Barcelona, and colleagues agreed to investigate 10 questions related to treatment efficacy, response criteria, strategies to address suboptimal response and safety concerns, and treatment of MS in pregnancy. They developed the guideline following the recommendations of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group, along with EAN recommendations for writing a neurologic management guideline. Literature searches relied upon databases such as the Cochrane Central Register of Controlled Trials, Excerpta Medica, and Medical Literature Analysis and Retrieval System Online.
The authors evaluated data for all disease-modifying treatments (DMTs) approved by the European Medicines Agency at the time of publication. They did not consider combination therapies, complementary or alternative medicine, or symptomatic treatment. Although focused on Europe, the guideline does not account for regulatory or organizational issues specific to any European country.
Strong Recommendations
Dr. Montalban and colleagues agreed upon 21 recommendations and consensus statements. The recommendations were categorized as strong or weak, according to the quality of evidence and the risk–benefit balance. The authors formulated consensus statements on questions for which evidence was insufficient to support a formal recommendation.
The first of the guideline’s three strong recommendations is that neurologists offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS. The second is to offer early treatment with DMTs to patients with active relapsing-remitting MS, as defined by clinical relapses or MRI activity. The authors defined active lesions as contrast-enhancing lesions or new or unequivocally enlarging T2 lesions assessed at least annually. This recommendation also applies to patients with CIS who fulfill current diagnostic criteria for MS. The third strong recommendation is to offer a more efficacious drug to patients treated with interferon or glatiramer acetate who show evidence of disease activity.
Weak Recommendations
Nine of the guideline’s recommendations are categorized as weak. For example, neurologists should consider treating patients with active secondary progressive MS with interferon beta-1a or -1b, taking into account the treatments’ “dubious efficacy,” as well as their safety and tolerability, according to the authors. Neurologists should consider mitoxantrone, ocrelizumab, and cladribine for this population. The authors recommend considering ocrelizumab as a treatment for patients with primary progressive MS.
As a way of monitoring treatment response, the authors recommend that neurologists consider combining MRI with clinical measures when evaluating disease evolution in treated patients. When making treatment decisions in the event of safety concerns, neurologists should consider the possibility that disease activity may resume or rebound if treatment is stopped, particularly with natalizumab, said Dr. Montalban and colleagues. Continuation of DMT treatment should be considered for patients who are clinically stable, who have stable MRI, and who have no problems with safety or tolerability, according to the guideline.
“For women planning a pregnancy, if there is a high risk of disease reactivation, consider using interferon or glatiramer acetate until pregnancy is confirmed,” said the authors. “In some very specific (active) cases, continuing this treatment during pregnancy could also be considered.” Delaying pregnancy is advisable for women with persistent high disease activity. If such a woman decides to become pregnant or has an unplanned pregnancy, neurologists may consider treatment with natalizumab throughout pregnancy after a full discussion with the patient of the potential implications. “Treatment with alemtuzumab could be an alternative therapeutic option for planned pregnancy in very active cases, provided that a four-month interval is strictly observed from the latest infusion until conception,” said Dr. Montalban and colleagues.
Consensus Statements
Several of the guideline’s consensus statements relate to the monitoring of treatment response. For patients treated with DMTs, the authors recommend performing a standardized reference brain MRI within six months of treatment onset, and comparing it with a subsequent brain MRI performed 12 months after starting treatment. The measurement of new or unequivocally enlarging T2 lesions is the preferred MRI method of gauging treatment response, supplemented by gadolinium-enhancing lesions. To monitor treatment safety, the authors recommend performing a standardized reference brain MRI every year in patients at low risk for progressive multifocal leukoencephalopathy (PML), and every three to six months in patients at high risk for PML.
If the neurologist and patient have decided to switch therapies, they should consider the patient’s characteristics and comorbidities, drug safety profiles, and disease severity when choosing a new DMT. If treatment of a highly efficacious drug is stopped because of safety or efficacy problems, the neurologist should consider starting another highly efficacious drug, according to the guideline. Disease activity, the drug’s half-life and biologic activity, and the potential for resumed disease activity or rebound should be considered when choosing a new therapy, said Dr. Montalban and colleagues.
—Erik Greb
Suggested Reading
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96-120.
The European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Academy of Neurology (EAN) have published a guideline to offer up-to-date, evidence-based recommendations for the treatment of adult patients with MS. The guideline is intended to fill a perceived need for a comprehensive document that includes information about recently approved MS therapies and helps clinicians and patients resolve difficulties in everyday clinical practice. It was published in the February issue of Multiple Sclerosis.
Authors Addressed 10 Questions
Xavier Montalban, MD, PhD, Chair and Director of the Department of Neurology and Neuroimmunology at Vall d’Hebron University Hospital in Barcelona, and colleagues agreed to investigate 10 questions related to treatment efficacy, response criteria, strategies to address suboptimal response and safety concerns, and treatment of MS in pregnancy. They developed the guideline following the recommendations of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group, along with EAN recommendations for writing a neurologic management guideline. Literature searches relied upon databases such as the Cochrane Central Register of Controlled Trials, Excerpta Medica, and Medical Literature Analysis and Retrieval System Online.
The authors evaluated data for all disease-modifying treatments (DMTs) approved by the European Medicines Agency at the time of publication. They did not consider combination therapies, complementary or alternative medicine, or symptomatic treatment. Although focused on Europe, the guideline does not account for regulatory or organizational issues specific to any European country.
Strong Recommendations
Dr. Montalban and colleagues agreed upon 21 recommendations and consensus statements. The recommendations were categorized as strong or weak, according to the quality of evidence and the risk–benefit balance. The authors formulated consensus statements on questions for which evidence was insufficient to support a formal recommendation.
The first of the guideline’s three strong recommendations is that neurologists offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS. The second is to offer early treatment with DMTs to patients with active relapsing-remitting MS, as defined by clinical relapses or MRI activity. The authors defined active lesions as contrast-enhancing lesions or new or unequivocally enlarging T2 lesions assessed at least annually. This recommendation also applies to patients with CIS who fulfill current diagnostic criteria for MS. The third strong recommendation is to offer a more efficacious drug to patients treated with interferon or glatiramer acetate who show evidence of disease activity.
Weak Recommendations
Nine of the guideline’s recommendations are categorized as weak. For example, neurologists should consider treating patients with active secondary progressive MS with interferon beta-1a or -1b, taking into account the treatments’ “dubious efficacy,” as well as their safety and tolerability, according to the authors. Neurologists should consider mitoxantrone, ocrelizumab, and cladribine for this population. The authors recommend considering ocrelizumab as a treatment for patients with primary progressive MS.
As a way of monitoring treatment response, the authors recommend that neurologists consider combining MRI with clinical measures when evaluating disease evolution in treated patients. When making treatment decisions in the event of safety concerns, neurologists should consider the possibility that disease activity may resume or rebound if treatment is stopped, particularly with natalizumab, said Dr. Montalban and colleagues. Continuation of DMT treatment should be considered for patients who are clinically stable, who have stable MRI, and who have no problems with safety or tolerability, according to the guideline.
“For women planning a pregnancy, if there is a high risk of disease reactivation, consider using interferon or glatiramer acetate until pregnancy is confirmed,” said the authors. “In some very specific (active) cases, continuing this treatment during pregnancy could also be considered.” Delaying pregnancy is advisable for women with persistent high disease activity. If such a woman decides to become pregnant or has an unplanned pregnancy, neurologists may consider treatment with natalizumab throughout pregnancy after a full discussion with the patient of the potential implications. “Treatment with alemtuzumab could be an alternative therapeutic option for planned pregnancy in very active cases, provided that a four-month interval is strictly observed from the latest infusion until conception,” said Dr. Montalban and colleagues.
Consensus Statements
Several of the guideline’s consensus statements relate to the monitoring of treatment response. For patients treated with DMTs, the authors recommend performing a standardized reference brain MRI within six months of treatment onset, and comparing it with a subsequent brain MRI performed 12 months after starting treatment. The measurement of new or unequivocally enlarging T2 lesions is the preferred MRI method of gauging treatment response, supplemented by gadolinium-enhancing lesions. To monitor treatment safety, the authors recommend performing a standardized reference brain MRI every year in patients at low risk for progressive multifocal leukoencephalopathy (PML), and every three to six months in patients at high risk for PML.
If the neurologist and patient have decided to switch therapies, they should consider the patient’s characteristics and comorbidities, drug safety profiles, and disease severity when choosing a new DMT. If treatment of a highly efficacious drug is stopped because of safety or efficacy problems, the neurologist should consider starting another highly efficacious drug, according to the guideline. Disease activity, the drug’s half-life and biologic activity, and the potential for resumed disease activity or rebound should be considered when choosing a new therapy, said Dr. Montalban and colleagues.
—Erik Greb
Suggested Reading
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96-120.
Consider thalassemia traits in patients with iron deficiency
Consider thalassemia traits in patients with iron deficiency
The editorial is an excellent review of iron deficiency as an associated finding with adverse health and pregnancy outcomes. However, one genetic issue appears to have escaped comment. In Florida, our African American patients hav
Your recommendation to routinely screen for ferritin deficit is laudable as a general health care practice. If the screening result is normal, however, consider thalassemia carrier states as a secondary explanation as well as a genetic issue requiring partner testing. Aggressive iron loading of a nondeficient anemic patient can risk excess absorption, storage, and ultimate organ compromise in later life if continued indefinitely.
Richard P. Perkins, MD
Fort Myers, Florida
Patient education is key to managing iron deficiency
Forty years ago, my professors expounded on how some people could not absorb iron and that the answer was intravenous iron infusion. After writing a few prescriptions, however, I found that I no longer had patients with absorptive problems once I learned to carefully, and with visual aids, explain the iron story and meticulously monitor compliance. I have been through the “slow Fe” and the “prenatal vitamins have iron” nonsense. Ferrous sulfate is about as good as anything. I have explained the theory of vitamin C−assist and found that telling people to avoid taking iron with meals is folly.
I suggest that the iron story is complete. Rather than wasting money on further research, we should spend funds on teaching young physicians to educate patients and monitor compliance. In recent years, I have found that a daily text message to the patient frequently is very helpful.
Robert W. Jackson, MD
Washougal, Washington
Dr. Barbieri responds
I thank Drs. Perkins and Jackson for their helpful recommendations for the management of iron deficiency anemia. I agree with Dr. Perkins that screening for thalassemia is an important part of preconception and prenatal care. In the editorial’s table on page 10 discussing the differential diagnosis of anemia, we mentioned the importance of hemoglobin electrophoresis and measurement of vitamin B12 and folate levels to identify cases of anemia caused by thalassemia or vitamin deficiency. I agree with Dr. Jackson that oral iron supplementation along with patient education can resolve most cases of iron deficiency in early and mid-pregnancy. However, in the last few weeks of pregnancy there may not be sufficient time for oral iron supplementation to be effective in resolving iron deficiency anemia. In this situation and in patients at high risk for malabsorption, including women with prior gastric bypass, intravenous iron might be the best approach to resolving the anemia.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Consider thalassemia traits in patients with iron deficiency
The editorial is an excellent review of iron deficiency as an associated finding with adverse health and pregnancy outcomes. However, one genetic issue appears to have escaped comment. In Florida, our African American patients hav
Your recommendation to routinely screen for ferritin deficit is laudable as a general health care practice. If the screening result is normal, however, consider thalassemia carrier states as a secondary explanation as well as a genetic issue requiring partner testing. Aggressive iron loading of a nondeficient anemic patient can risk excess absorption, storage, and ultimate organ compromise in later life if continued indefinitely.
Richard P. Perkins, MD
Fort Myers, Florida
Patient education is key to managing iron deficiency
Forty years ago, my professors expounded on how some people could not absorb iron and that the answer was intravenous iron infusion. After writing a few prescriptions, however, I found that I no longer had patients with absorptive problems once I learned to carefully, and with visual aids, explain the iron story and meticulously monitor compliance. I have been through the “slow Fe” and the “prenatal vitamins have iron” nonsense. Ferrous sulfate is about as good as anything. I have explained the theory of vitamin C−assist and found that telling people to avoid taking iron with meals is folly.
I suggest that the iron story is complete. Rather than wasting money on further research, we should spend funds on teaching young physicians to educate patients and monitor compliance. In recent years, I have found that a daily text message to the patient frequently is very helpful.
Robert W. Jackson, MD
Washougal, Washington
Dr. Barbieri responds
I thank Drs. Perkins and Jackson for their helpful recommendations for the management of iron deficiency anemia. I agree with Dr. Perkins that screening for thalassemia is an important part of preconception and prenatal care. In the editorial’s table on page 10 discussing the differential diagnosis of anemia, we mentioned the importance of hemoglobin electrophoresis and measurement of vitamin B12 and folate levels to identify cases of anemia caused by thalassemia or vitamin deficiency. I agree with Dr. Jackson that oral iron supplementation along with patient education can resolve most cases of iron deficiency in early and mid-pregnancy. However, in the last few weeks of pregnancy there may not be sufficient time for oral iron supplementation to be effective in resolving iron deficiency anemia. In this situation and in patients at high risk for malabsorption, including women with prior gastric bypass, intravenous iron might be the best approach to resolving the anemia.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Consider thalassemia traits in patients with iron deficiency
The editorial is an excellent review of iron deficiency as an associated finding with adverse health and pregnancy outcomes. However, one genetic issue appears to have escaped comment. In Florida, our African American patients hav
Your recommendation to routinely screen for ferritin deficit is laudable as a general health care practice. If the screening result is normal, however, consider thalassemia carrier states as a secondary explanation as well as a genetic issue requiring partner testing. Aggressive iron loading of a nondeficient anemic patient can risk excess absorption, storage, and ultimate organ compromise in later life if continued indefinitely.
Richard P. Perkins, MD
Fort Myers, Florida
Patient education is key to managing iron deficiency
Forty years ago, my professors expounded on how some people could not absorb iron and that the answer was intravenous iron infusion. After writing a few prescriptions, however, I found that I no longer had patients with absorptive problems once I learned to carefully, and with visual aids, explain the iron story and meticulously monitor compliance. I have been through the “slow Fe” and the “prenatal vitamins have iron” nonsense. Ferrous sulfate is about as good as anything. I have explained the theory of vitamin C−assist and found that telling people to avoid taking iron with meals is folly.
I suggest that the iron story is complete. Rather than wasting money on further research, we should spend funds on teaching young physicians to educate patients and monitor compliance. In recent years, I have found that a daily text message to the patient frequently is very helpful.
Robert W. Jackson, MD
Washougal, Washington
Dr. Barbieri responds
I thank Drs. Perkins and Jackson for their helpful recommendations for the management of iron deficiency anemia. I agree with Dr. Perkins that screening for thalassemia is an important part of preconception and prenatal care. In the editorial’s table on page 10 discussing the differential diagnosis of anemia, we mentioned the importance of hemoglobin electrophoresis and measurement of vitamin B12 and folate levels to identify cases of anemia caused by thalassemia or vitamin deficiency. I agree with Dr. Jackson that oral iron supplementation along with patient education can resolve most cases of iron deficiency in early and mid-pregnancy. However, in the last few weeks of pregnancy there may not be sufficient time for oral iron supplementation to be effective in resolving iron deficiency anemia. In this situation and in patients at high risk for malabsorption, including women with prior gastric bypass, intravenous iron might be the best approach to resolving the anemia.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Online psoriasis consultations shown equivalent to office visits
SAN DIEGO – Online consultations between dermatologists, patients with psoriasis, and the patients’ primary care physicians were as effective as in-person consultations in successfully treating the disease in a multicenter, randomized study of 296 patients.
“Innovative telehealth delivery models that emphasize collaboration, quality, and efficiency can be transformative to improving patient-centered outcomes in chronic disease,” April W. Armstrong, MD, said at the annual meeting of the American Academy of Dermatology. The online model she tested fostered “increased patient engagement” and provided “comprehensive specialist support,” said Dr. Armstrong, director of the psoriasis program at the University of Southern California, Los Angeles.
To objectively assess whether online consultations are as effective as in-person examinations, Dr. Armstrong and her associates at three U.S. centers randomized adult psoriasis patients from across the disease spectrum to receive 1 year of dermatology care either in person or online. Patients enrolled in the online arm received training in taking digital images of their skin lesions and uploading the data for remote access by their dermatologist and primary care physician. The frequency of in-person and online consultations was left to the discretion of each patient and his or her physician.
Among the 148 patients randomized to each arm, 17 in the online group and 13 in the in-person group withdrew from the study or were lost to follow-up. The researchers analyzed the results on an intention-to-treat basis.
They assessed three parameters of treatment efficacy that they measured at baseline and then every 3 months out to 1 year: Psoriasis Area and Severity Index, body surface area score, and patient global self-assessment. A comparison of changes between the two treatment arms after 1 year for the first two measures met the study’s prespecified definition of equivalence, Dr. Armstrong reported. The third measure, a patient’s global self-assessment, showed lower patient-assessed disease severity after 1 year among the patients managed online, compared with those managed in person.
The incidence of adverse events and serious adverse events was similar in the two treatment arms.
Dr. Armstrong had no relevant financial disclosures.
Source: Armstrong A et al. AAD 2018, abstract 6730..
SAN DIEGO – Online consultations between dermatologists, patients with psoriasis, and the patients’ primary care physicians were as effective as in-person consultations in successfully treating the disease in a multicenter, randomized study of 296 patients.
“Innovative telehealth delivery models that emphasize collaboration, quality, and efficiency can be transformative to improving patient-centered outcomes in chronic disease,” April W. Armstrong, MD, said at the annual meeting of the American Academy of Dermatology. The online model she tested fostered “increased patient engagement” and provided “comprehensive specialist support,” said Dr. Armstrong, director of the psoriasis program at the University of Southern California, Los Angeles.
To objectively assess whether online consultations are as effective as in-person examinations, Dr. Armstrong and her associates at three U.S. centers randomized adult psoriasis patients from across the disease spectrum to receive 1 year of dermatology care either in person or online. Patients enrolled in the online arm received training in taking digital images of their skin lesions and uploading the data for remote access by their dermatologist and primary care physician. The frequency of in-person and online consultations was left to the discretion of each patient and his or her physician.
Among the 148 patients randomized to each arm, 17 in the online group and 13 in the in-person group withdrew from the study or were lost to follow-up. The researchers analyzed the results on an intention-to-treat basis.
They assessed three parameters of treatment efficacy that they measured at baseline and then every 3 months out to 1 year: Psoriasis Area and Severity Index, body surface area score, and patient global self-assessment. A comparison of changes between the two treatment arms after 1 year for the first two measures met the study’s prespecified definition of equivalence, Dr. Armstrong reported. The third measure, a patient’s global self-assessment, showed lower patient-assessed disease severity after 1 year among the patients managed online, compared with those managed in person.
The incidence of adverse events and serious adverse events was similar in the two treatment arms.
Dr. Armstrong had no relevant financial disclosures.
Source: Armstrong A et al. AAD 2018, abstract 6730..
SAN DIEGO – Online consultations between dermatologists, patients with psoriasis, and the patients’ primary care physicians were as effective as in-person consultations in successfully treating the disease in a multicenter, randomized study of 296 patients.
“Innovative telehealth delivery models that emphasize collaboration, quality, and efficiency can be transformative to improving patient-centered outcomes in chronic disease,” April W. Armstrong, MD, said at the annual meeting of the American Academy of Dermatology. The online model she tested fostered “increased patient engagement” and provided “comprehensive specialist support,” said Dr. Armstrong, director of the psoriasis program at the University of Southern California, Los Angeles.
To objectively assess whether online consultations are as effective as in-person examinations, Dr. Armstrong and her associates at three U.S. centers randomized adult psoriasis patients from across the disease spectrum to receive 1 year of dermatology care either in person or online. Patients enrolled in the online arm received training in taking digital images of their skin lesions and uploading the data for remote access by their dermatologist and primary care physician. The frequency of in-person and online consultations was left to the discretion of each patient and his or her physician.
Among the 148 patients randomized to each arm, 17 in the online group and 13 in the in-person group withdrew from the study or were lost to follow-up. The researchers analyzed the results on an intention-to-treat basis.
They assessed three parameters of treatment efficacy that they measured at baseline and then every 3 months out to 1 year: Psoriasis Area and Severity Index, body surface area score, and patient global self-assessment. A comparison of changes between the two treatment arms after 1 year for the first two measures met the study’s prespecified definition of equivalence, Dr. Armstrong reported. The third measure, a patient’s global self-assessment, showed lower patient-assessed disease severity after 1 year among the patients managed online, compared with those managed in person.
The incidence of adverse events and serious adverse events was similar in the two treatment arms.
Dr. Armstrong had no relevant financial disclosures.
Source: Armstrong A et al. AAD 2018, abstract 6730..
REPORTING FROM AAD 18
Key clinical point: Online physician telemonitoring of psoriasis patients was equivalent to in-person management.
Major finding: After 1 year, changes in the Psoriasis Area and Severity Index in the two arms met the prespecified definition of equivalence.
Study details: A multicenter, randomized trial with 296 psoriasis patients in which outcomes were compared for online monitoring and in-person examinations.
Disclosures: Dr. Armstrong had no relevant financial disclosures.
Source: Armstrong A et al. AAD 18, abstract 6730.
Deeply entrenched gender bias in academic medicine is treatable
TAMPA, FLA. – Gender bias that disadvantages women from rising in academic medicine might require specific habit-changing strategies rather than efforts that draw on goodwill alone, according to new follow-up data from a randomized trial discussed and reevaluated at the annual meeting of the American College of Psychiatrists.
One premise of this trial, supported by other research, is that entrenched gender stereotypes drive both male and female behavior and must be addressed directly for change, said Molly Carnes, MD, professor of psychiatry at the University of Wisconsin, Madison.
The initial results of the trial, which randomized academic departments at the University of Wisconsin to participate in habit-changing workshops or to serve as controls, were published almost 3 years ago (Acad Med. 2015 Feb;90[2]:221-30). It is the most recent follow-up (Devine et al. J Exp Soc Psychol. 2017 Nov;73:211-5) that corroborates that long-term changes are possible with intervention.
The published findings showed that when 1,137 faculty members from 46 departments in the experimental arm were compared with 1,153 faculty members from 46 departments in the control arm, there were significant improvements in the experimental arm in surveyed attitudes reflecting personal bias awareness (P = .001) and willingness to support gender equity (P = .013).
These changes in attitude translated into concrete changes in new female faculty hires in the most recent analysis. From 32% in a 2-year period before the workshops, the new female hires climbed to 46% in the 2-year period after the workshops – a relative increase of 44% in the departments participating in the experimental arm. In the control departments, female new faculty hires remained at 32% in both time periods.
“Basically, there are 20 new women faculty members at the University of Wisconsin because of this study,” Dr. Carnes said.
. The result was a fundamental change in culture within departments randomized to the experimental arm, according to data generated by a variety of study analyses.
“When we looked at questions about department climate, we found that both male and female faculty members in the experimental groups were significantly more likely to say they fit in their department, they felt respected for their research and scholarship by their colleagues, and they felt comfortable raising personal and family issues even if they conflicted with departmental activities,” Dr. Carnes said.
This general attitude change is important, because Dr. Carnes emphasized that women share the cultural biases that can result in reduced female career opportunities in clinical and academic medicine. In addition, women generally are aware that stereotypical positive “agentic” adjectives for men, such as decisive, competitive, and ambitious, often are viewed negatively and generate backlash when applied to women. They therefore act on this awareness.
“Stereotype-based bias is a habit that can be broken, but it requires more than good intentions,” said Dr. Carnes, who emphasized that “gender-based assumptions and stereotypes are deeply embedded in the patterns of thinking of both men and women.”
As one example, Dr. Carnes cited her work evaluating female resident behavior when leading in-hospital code resuscitations. There are data to show that there is no difference in the effectiveness of male and female resident code leaders, but women typically feel that the assertive, aggressive behavior required for code leadership is “counternormative.” After the code, some women feel compelled to apologize to team members for being demanding or assertive, a step that Dr. Carnes attributed at least in part to fear of backlash from stepping out of gender-expected behavior.
The fix is not necessarily suppression of gender-related attributes. Dr. Carnes cited evidence that the stereotypical positive communal adjectives for women, such as nurturing, supportive, and sympathetic, might explain why studies suggest that women are more likely than men to be transformational leaders who inspire team members to contribute beyond their own self-interest in achieving goals.
Ultimately, the fix is replacement of stereotypes that impair men as well as women from defusing biases that “lead to subtle unintentional advantages in academic career advancement for Jack not afforded to Jill,” Dr. Carnes said. Based on the low numbers of female leaders in academic medicine decades after medical schools began enrolling women in substantial numbers, she concluded that meaningful change in gender bias is not likely to occur without implementation of specific proactive strategies aimed at challenging current perceptions. Her published study confirms that such strategies can help.
Dr. Carnes reported no conflicts of interest.
Patricia Devine et al. in a recent study published in the Journal of Experimental Social Psychology tested the effect of one 2.5-hour workshop that sought to positively influence the mental habit of gender bias, which exists in our academic world (and elsewhere) in both men and women.
Bevra H. Hahn, MD, is Distinguished Professor of Medicine (emeritus) at the University of California, Los Angeles.
Patricia Devine et al. in a recent study published in the Journal of Experimental Social Psychology tested the effect of one 2.5-hour workshop that sought to positively influence the mental habit of gender bias, which exists in our academic world (and elsewhere) in both men and women.
Bevra H. Hahn, MD, is Distinguished Professor of Medicine (emeritus) at the University of California, Los Angeles.
Patricia Devine et al. in a recent study published in the Journal of Experimental Social Psychology tested the effect of one 2.5-hour workshop that sought to positively influence the mental habit of gender bias, which exists in our academic world (and elsewhere) in both men and women.
Bevra H. Hahn, MD, is Distinguished Professor of Medicine (emeritus) at the University of California, Los Angeles.
TAMPA, FLA. – Gender bias that disadvantages women from rising in academic medicine might require specific habit-changing strategies rather than efforts that draw on goodwill alone, according to new follow-up data from a randomized trial discussed and reevaluated at the annual meeting of the American College of Psychiatrists.
One premise of this trial, supported by other research, is that entrenched gender stereotypes drive both male and female behavior and must be addressed directly for change, said Molly Carnes, MD, professor of psychiatry at the University of Wisconsin, Madison.
The initial results of the trial, which randomized academic departments at the University of Wisconsin to participate in habit-changing workshops or to serve as controls, were published almost 3 years ago (Acad Med. 2015 Feb;90[2]:221-30). It is the most recent follow-up (Devine et al. J Exp Soc Psychol. 2017 Nov;73:211-5) that corroborates that long-term changes are possible with intervention.
The published findings showed that when 1,137 faculty members from 46 departments in the experimental arm were compared with 1,153 faculty members from 46 departments in the control arm, there were significant improvements in the experimental arm in surveyed attitudes reflecting personal bias awareness (P = .001) and willingness to support gender equity (P = .013).
These changes in attitude translated into concrete changes in new female faculty hires in the most recent analysis. From 32% in a 2-year period before the workshops, the new female hires climbed to 46% in the 2-year period after the workshops – a relative increase of 44% in the departments participating in the experimental arm. In the control departments, female new faculty hires remained at 32% in both time periods.
“Basically, there are 20 new women faculty members at the University of Wisconsin because of this study,” Dr. Carnes said.
. The result was a fundamental change in culture within departments randomized to the experimental arm, according to data generated by a variety of study analyses.
“When we looked at questions about department climate, we found that both male and female faculty members in the experimental groups were significantly more likely to say they fit in their department, they felt respected for their research and scholarship by their colleagues, and they felt comfortable raising personal and family issues even if they conflicted with departmental activities,” Dr. Carnes said.
This general attitude change is important, because Dr. Carnes emphasized that women share the cultural biases that can result in reduced female career opportunities in clinical and academic medicine. In addition, women generally are aware that stereotypical positive “agentic” adjectives for men, such as decisive, competitive, and ambitious, often are viewed negatively and generate backlash when applied to women. They therefore act on this awareness.
“Stereotype-based bias is a habit that can be broken, but it requires more than good intentions,” said Dr. Carnes, who emphasized that “gender-based assumptions and stereotypes are deeply embedded in the patterns of thinking of both men and women.”
As one example, Dr. Carnes cited her work evaluating female resident behavior when leading in-hospital code resuscitations. There are data to show that there is no difference in the effectiveness of male and female resident code leaders, but women typically feel that the assertive, aggressive behavior required for code leadership is “counternormative.” After the code, some women feel compelled to apologize to team members for being demanding or assertive, a step that Dr. Carnes attributed at least in part to fear of backlash from stepping out of gender-expected behavior.
The fix is not necessarily suppression of gender-related attributes. Dr. Carnes cited evidence that the stereotypical positive communal adjectives for women, such as nurturing, supportive, and sympathetic, might explain why studies suggest that women are more likely than men to be transformational leaders who inspire team members to contribute beyond their own self-interest in achieving goals.
Ultimately, the fix is replacement of stereotypes that impair men as well as women from defusing biases that “lead to subtle unintentional advantages in academic career advancement for Jack not afforded to Jill,” Dr. Carnes said. Based on the low numbers of female leaders in academic medicine decades after medical schools began enrolling women in substantial numbers, she concluded that meaningful change in gender bias is not likely to occur without implementation of specific proactive strategies aimed at challenging current perceptions. Her published study confirms that such strategies can help.
Dr. Carnes reported no conflicts of interest.
TAMPA, FLA. – Gender bias that disadvantages women from rising in academic medicine might require specific habit-changing strategies rather than efforts that draw on goodwill alone, according to new follow-up data from a randomized trial discussed and reevaluated at the annual meeting of the American College of Psychiatrists.
One premise of this trial, supported by other research, is that entrenched gender stereotypes drive both male and female behavior and must be addressed directly for change, said Molly Carnes, MD, professor of psychiatry at the University of Wisconsin, Madison.
The initial results of the trial, which randomized academic departments at the University of Wisconsin to participate in habit-changing workshops or to serve as controls, were published almost 3 years ago (Acad Med. 2015 Feb;90[2]:221-30). It is the most recent follow-up (Devine et al. J Exp Soc Psychol. 2017 Nov;73:211-5) that corroborates that long-term changes are possible with intervention.
The published findings showed that when 1,137 faculty members from 46 departments in the experimental arm were compared with 1,153 faculty members from 46 departments in the control arm, there were significant improvements in the experimental arm in surveyed attitudes reflecting personal bias awareness (P = .001) and willingness to support gender equity (P = .013).
These changes in attitude translated into concrete changes in new female faculty hires in the most recent analysis. From 32% in a 2-year period before the workshops, the new female hires climbed to 46% in the 2-year period after the workshops – a relative increase of 44% in the departments participating in the experimental arm. In the control departments, female new faculty hires remained at 32% in both time periods.
“Basically, there are 20 new women faculty members at the University of Wisconsin because of this study,” Dr. Carnes said.
. The result was a fundamental change in culture within departments randomized to the experimental arm, according to data generated by a variety of study analyses.
“When we looked at questions about department climate, we found that both male and female faculty members in the experimental groups were significantly more likely to say they fit in their department, they felt respected for their research and scholarship by their colleagues, and they felt comfortable raising personal and family issues even if they conflicted with departmental activities,” Dr. Carnes said.
This general attitude change is important, because Dr. Carnes emphasized that women share the cultural biases that can result in reduced female career opportunities in clinical and academic medicine. In addition, women generally are aware that stereotypical positive “agentic” adjectives for men, such as decisive, competitive, and ambitious, often are viewed negatively and generate backlash when applied to women. They therefore act on this awareness.
“Stereotype-based bias is a habit that can be broken, but it requires more than good intentions,” said Dr. Carnes, who emphasized that “gender-based assumptions and stereotypes are deeply embedded in the patterns of thinking of both men and women.”
As one example, Dr. Carnes cited her work evaluating female resident behavior when leading in-hospital code resuscitations. There are data to show that there is no difference in the effectiveness of male and female resident code leaders, but women typically feel that the assertive, aggressive behavior required for code leadership is “counternormative.” After the code, some women feel compelled to apologize to team members for being demanding or assertive, a step that Dr. Carnes attributed at least in part to fear of backlash from stepping out of gender-expected behavior.
The fix is not necessarily suppression of gender-related attributes. Dr. Carnes cited evidence that the stereotypical positive communal adjectives for women, such as nurturing, supportive, and sympathetic, might explain why studies suggest that women are more likely than men to be transformational leaders who inspire team members to contribute beyond their own self-interest in achieving goals.
Ultimately, the fix is replacement of stereotypes that impair men as well as women from defusing biases that “lead to subtle unintentional advantages in academic career advancement for Jack not afforded to Jill,” Dr. Carnes said. Based on the low numbers of female leaders in academic medicine decades after medical schools began enrolling women in substantial numbers, she concluded that meaningful change in gender bias is not likely to occur without implementation of specific proactive strategies aimed at challenging current perceptions. Her published study confirms that such strategies can help.
Dr. Carnes reported no conflicts of interest.
REPORTING FROM THE COLLEGE 2018