User login
Taming or teaching the tiger? Myths and management of childhood aggression
How to deal with aggression delivered by a child’s peers is a common concern and social dilemma for both parents and children. How does a child ward off aggressive peers without getting hurt or in trouble while also not looking weak or whiny? What can parents do to stop their child from being hurt or frightened but also not humiliate them or interfere with their learning important life skills by being over protective?
Children do not want to fight, but they do want to be treated fairly. Frustration, with its associated feelings of anger, is the most common reason for aggression. Being a child is certainly full of its frustrations because, while autonomy and desires are increasing, opportunities expand at a slower rate, particularly for children with developmental weaknesses or economic disadvantage. Fear and a lack of coping skills are other major reasons for resorting to aggressive responses.
Physical bullying affects 21% of students in grades 3-12 and is a risk factor for aggression at all ages. A full one-third of 9th-12th graders report having been in a physical fight in the last year. In grade school age and adolescence, factors known to be associated with peer aggression include the humiliation of school failure, substance use, and anger from experiencing parental or sibling aggression.
One would think a universal goal of parents would be to raise their children to get along with others without fighting. Unfortunately, some parents actually espouse childrearing methods that directly or indirectly make fighting more likely.
Essentially all toddlers and preschoolers can be aggressive at times to get things they want (instrumental) or when angry in the beginning of their second year of life; this peaks in the third year and typically declines after age 3 years. But for some 10% of children, aggression remains high. What parent and child factors set children up for such persistent aggression?
Parents have many reasons for how they raise their children, but some myths about parenting that persist promote aggression.
“My child will love me more if I am more permissive.”
Infants and toddlers develop self-regulation skills better when it is gradually expected of them with encouragement and support from their parents. Parents may feel that they are showing love to their toddler by having a “relaxed” home with few limits and no specific bedtime or rules. These parents also may “rescue” their child from frustrating situations by giving in to their demands or removing them from even mildly stressful situations.
These strategies can interfere with the progressive development of frustration tolerance, a key life skill. A lack of routines, inadequate sleep or food, overstimulation by noise, frightening experiences (including fighting in the home or neighborhood), or violent media exposure sets toddlers up to be out of control and thereby increases dysregulation. In addition, the dysregulated child may then act up, which can invoke punishment from that same parent.
Frustrating toddlers with inconsistent expectations and arbitrary punishment, a common result of low structure, makes the child feel insecure and leads to aggression. Instead, children need small doses of frustration appropriate to their age and encouragement from a supportive adult to problem solve. You can praise (or model), cheering on a child with words such as “Are you stuck? You can do it! Try again,” instead of instantly solving problems for them.
“Spare the rod and spoil the child.”
Parents may feel that they are promoting obedience when they use corporal punishment, thinking this will keep the child out of trouble in society. Instead, corporal punishment is associated with increased aggression toward peers, as well as defiance toward parents. These effects are especially strong when mothers are distant emotionally. As pediatricians, we can educate people on the importance of warm parenting, redirection instead of punishment for younger children, and using small, logical consequences or time out when needed for aggression.
“Just ignore bullies.”
It is a rare child who can follow the command to “ignore” a bully without turning red or getting tears in his or her eyes – making them appealing targets. We can coach parents and kids how to disarm bullies by standing tall, putting hands on hips, making eye contact, and asking the peer a question such as “I do not understand what you’re trying to accomplish.” Learning martial arts also teaches children that they are powerful (but not to fight outside the class) so they can present themselves in this way. Programs that encourage children to get together to confront bullies supported by a school administration that uses comprehensive assessment and habilitation strategies for aggressive students are most effective in reducing aggression in schools. Anonymous reporting (for example, by using a cell phone app, such as STOPit) empowers students to report bullying or fights to school staff without risking later retribution from the peer.
“Tough teachers help kids fall in line.”
While peer fights generally increase from 2nd to 4th grade before declining, student fighting progressively increases when teachers use reprimands, rather than praise, to manage their classes. Children look to teachers to learn more than what is in books – how to be respectful and in control without putting others down. The most effective classroom management includes clear, fair rules; any correction should be done privately to avoid shaming students. Students dealt with this way are less likely to be angry and take it out on others. Of course, appropriate services helping every child experience success in learning is the foundation of positive behavior in school.
“Children with ADHD won’t learn self-regulation if they are treated with medicine.”
Children who show “low effortful control” or higher “dysregulation” are both more aggressive and also less likely to decline in aggression in early childhood. ADHD is a neurological condition characterized by such dysregulation and low effortful control. Children with ADHD often have higher and more persistent aggression. These tendencies also result in impulsive behaviors that can irritate peers and adults and can result in correction and criticism, further increasing aggression. Children with ADHD who are better controlled, often with the help of medication, have more positive interactions at school and at home, receive more praise and less correction, and develop more reasoned interaction patterns.
“I am the parent, and my child should do what I say.”
When adults step in to stop a fight, they are rarely in a position to know what actually happened between the kids. Children may quickly learn how to entrap a sibling or peer to look like the perpetrator in order to get them in trouble and/or avoid consequences for themselves, especially if large or harsh punishments are being used.
While it can seem tricky to treat children who are very different in age or development equally, having parents elicit or at least verbalize each child’s point of view is part of how children learn respect and mediation skills. Parents who refrain from taking sides or dictating how disputes should be resolved leave the chance for the children to acquire these component skills of negotiation. This does not mean there are no consequences, just that a brief discussion comes first.
When fighting is a pediatric complaint, you have a great opportunity to educate families in evidence-based ways that can both prevent and reduce their child’s use of aggression.
In one effective 90-minute training program, parents were taught basic mediation principles: to give ground rules and ask their children to agree to them, to ask each child to describe what happened and identify their disagreements and common ground, to encourage the children to discuss their goals in the fight and feelings about the issues, and to encourage the children to come up with suggestions to resolve their disputes and help them assess the practical aspects of their ideas. Praise should be used each time a child uses even some of these skills. Parents in this program also were given communication strategies, such as active listening, reflecting, and reframing, to help children learn to take the others’ perspective. In a follow up survey a month later, children of parents in the intervention group were seen to use these skills in real situations that might otherwise have been fights.
When aggression persists, mindfulness training, cognitive-behavioral techniques, social-emotional approaches, or peer mentoring programs delivered through individual counseling or school programs are all ways of teaching kids important interaction skills to reduce peer aggression. Remember, 40% of severe adult aggression begins before age 8 years, so preventive education or early referral to mental health services is key.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at [email protected].
How to deal with aggression delivered by a child’s peers is a common concern and social dilemma for both parents and children. How does a child ward off aggressive peers without getting hurt or in trouble while also not looking weak or whiny? What can parents do to stop their child from being hurt or frightened but also not humiliate them or interfere with their learning important life skills by being over protective?
Children do not want to fight, but they do want to be treated fairly. Frustration, with its associated feelings of anger, is the most common reason for aggression. Being a child is certainly full of its frustrations because, while autonomy and desires are increasing, opportunities expand at a slower rate, particularly for children with developmental weaknesses or economic disadvantage. Fear and a lack of coping skills are other major reasons for resorting to aggressive responses.
Physical bullying affects 21% of students in grades 3-12 and is a risk factor for aggression at all ages. A full one-third of 9th-12th graders report having been in a physical fight in the last year. In grade school age and adolescence, factors known to be associated with peer aggression include the humiliation of school failure, substance use, and anger from experiencing parental or sibling aggression.
One would think a universal goal of parents would be to raise their children to get along with others without fighting. Unfortunately, some parents actually espouse childrearing methods that directly or indirectly make fighting more likely.
Essentially all toddlers and preschoolers can be aggressive at times to get things they want (instrumental) or when angry in the beginning of their second year of life; this peaks in the third year and typically declines after age 3 years. But for some 10% of children, aggression remains high. What parent and child factors set children up for such persistent aggression?
Parents have many reasons for how they raise their children, but some myths about parenting that persist promote aggression.
“My child will love me more if I am more permissive.”
Infants and toddlers develop self-regulation skills better when it is gradually expected of them with encouragement and support from their parents. Parents may feel that they are showing love to their toddler by having a “relaxed” home with few limits and no specific bedtime or rules. These parents also may “rescue” their child from frustrating situations by giving in to their demands or removing them from even mildly stressful situations.
These strategies can interfere with the progressive development of frustration tolerance, a key life skill. A lack of routines, inadequate sleep or food, overstimulation by noise, frightening experiences (including fighting in the home or neighborhood), or violent media exposure sets toddlers up to be out of control and thereby increases dysregulation. In addition, the dysregulated child may then act up, which can invoke punishment from that same parent.
Frustrating toddlers with inconsistent expectations and arbitrary punishment, a common result of low structure, makes the child feel insecure and leads to aggression. Instead, children need small doses of frustration appropriate to their age and encouragement from a supportive adult to problem solve. You can praise (or model), cheering on a child with words such as “Are you stuck? You can do it! Try again,” instead of instantly solving problems for them.
“Spare the rod and spoil the child.”
Parents may feel that they are promoting obedience when they use corporal punishment, thinking this will keep the child out of trouble in society. Instead, corporal punishment is associated with increased aggression toward peers, as well as defiance toward parents. These effects are especially strong when mothers are distant emotionally. As pediatricians, we can educate people on the importance of warm parenting, redirection instead of punishment for younger children, and using small, logical consequences or time out when needed for aggression.
“Just ignore bullies.”
It is a rare child who can follow the command to “ignore” a bully without turning red or getting tears in his or her eyes – making them appealing targets. We can coach parents and kids how to disarm bullies by standing tall, putting hands on hips, making eye contact, and asking the peer a question such as “I do not understand what you’re trying to accomplish.” Learning martial arts also teaches children that they are powerful (but not to fight outside the class) so they can present themselves in this way. Programs that encourage children to get together to confront bullies supported by a school administration that uses comprehensive assessment and habilitation strategies for aggressive students are most effective in reducing aggression in schools. Anonymous reporting (for example, by using a cell phone app, such as STOPit) empowers students to report bullying or fights to school staff without risking later retribution from the peer.
“Tough teachers help kids fall in line.”
While peer fights generally increase from 2nd to 4th grade before declining, student fighting progressively increases when teachers use reprimands, rather than praise, to manage their classes. Children look to teachers to learn more than what is in books – how to be respectful and in control without putting others down. The most effective classroom management includes clear, fair rules; any correction should be done privately to avoid shaming students. Students dealt with this way are less likely to be angry and take it out on others. Of course, appropriate services helping every child experience success in learning is the foundation of positive behavior in school.
“Children with ADHD won’t learn self-regulation if they are treated with medicine.”
Children who show “low effortful control” or higher “dysregulation” are both more aggressive and also less likely to decline in aggression in early childhood. ADHD is a neurological condition characterized by such dysregulation and low effortful control. Children with ADHD often have higher and more persistent aggression. These tendencies also result in impulsive behaviors that can irritate peers and adults and can result in correction and criticism, further increasing aggression. Children with ADHD who are better controlled, often with the help of medication, have more positive interactions at school and at home, receive more praise and less correction, and develop more reasoned interaction patterns.
“I am the parent, and my child should do what I say.”
When adults step in to stop a fight, they are rarely in a position to know what actually happened between the kids. Children may quickly learn how to entrap a sibling or peer to look like the perpetrator in order to get them in trouble and/or avoid consequences for themselves, especially if large or harsh punishments are being used.
While it can seem tricky to treat children who are very different in age or development equally, having parents elicit or at least verbalize each child’s point of view is part of how children learn respect and mediation skills. Parents who refrain from taking sides or dictating how disputes should be resolved leave the chance for the children to acquire these component skills of negotiation. This does not mean there are no consequences, just that a brief discussion comes first.
When fighting is a pediatric complaint, you have a great opportunity to educate families in evidence-based ways that can both prevent and reduce their child’s use of aggression.
In one effective 90-minute training program, parents were taught basic mediation principles: to give ground rules and ask their children to agree to them, to ask each child to describe what happened and identify their disagreements and common ground, to encourage the children to discuss their goals in the fight and feelings about the issues, and to encourage the children to come up with suggestions to resolve their disputes and help them assess the practical aspects of their ideas. Praise should be used each time a child uses even some of these skills. Parents in this program also were given communication strategies, such as active listening, reflecting, and reframing, to help children learn to take the others’ perspective. In a follow up survey a month later, children of parents in the intervention group were seen to use these skills in real situations that might otherwise have been fights.
When aggression persists, mindfulness training, cognitive-behavioral techniques, social-emotional approaches, or peer mentoring programs delivered through individual counseling or school programs are all ways of teaching kids important interaction skills to reduce peer aggression. Remember, 40% of severe adult aggression begins before age 8 years, so preventive education or early referral to mental health services is key.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at [email protected].
How to deal with aggression delivered by a child’s peers is a common concern and social dilemma for both parents and children. How does a child ward off aggressive peers without getting hurt or in trouble while also not looking weak or whiny? What can parents do to stop their child from being hurt or frightened but also not humiliate them or interfere with their learning important life skills by being over protective?
Children do not want to fight, but they do want to be treated fairly. Frustration, with its associated feelings of anger, is the most common reason for aggression. Being a child is certainly full of its frustrations because, while autonomy and desires are increasing, opportunities expand at a slower rate, particularly for children with developmental weaknesses or economic disadvantage. Fear and a lack of coping skills are other major reasons for resorting to aggressive responses.
Physical bullying affects 21% of students in grades 3-12 and is a risk factor for aggression at all ages. A full one-third of 9th-12th graders report having been in a physical fight in the last year. In grade school age and adolescence, factors known to be associated with peer aggression include the humiliation of school failure, substance use, and anger from experiencing parental or sibling aggression.
One would think a universal goal of parents would be to raise their children to get along with others without fighting. Unfortunately, some parents actually espouse childrearing methods that directly or indirectly make fighting more likely.
Essentially all toddlers and preschoolers can be aggressive at times to get things they want (instrumental) or when angry in the beginning of their second year of life; this peaks in the third year and typically declines after age 3 years. But for some 10% of children, aggression remains high. What parent and child factors set children up for such persistent aggression?
Parents have many reasons for how they raise their children, but some myths about parenting that persist promote aggression.
“My child will love me more if I am more permissive.”
Infants and toddlers develop self-regulation skills better when it is gradually expected of them with encouragement and support from their parents. Parents may feel that they are showing love to their toddler by having a “relaxed” home with few limits and no specific bedtime or rules. These parents also may “rescue” their child from frustrating situations by giving in to their demands or removing them from even mildly stressful situations.
These strategies can interfere with the progressive development of frustration tolerance, a key life skill. A lack of routines, inadequate sleep or food, overstimulation by noise, frightening experiences (including fighting in the home or neighborhood), or violent media exposure sets toddlers up to be out of control and thereby increases dysregulation. In addition, the dysregulated child may then act up, which can invoke punishment from that same parent.
Frustrating toddlers with inconsistent expectations and arbitrary punishment, a common result of low structure, makes the child feel insecure and leads to aggression. Instead, children need small doses of frustration appropriate to their age and encouragement from a supportive adult to problem solve. You can praise (or model), cheering on a child with words such as “Are you stuck? You can do it! Try again,” instead of instantly solving problems for them.
“Spare the rod and spoil the child.”
Parents may feel that they are promoting obedience when they use corporal punishment, thinking this will keep the child out of trouble in society. Instead, corporal punishment is associated with increased aggression toward peers, as well as defiance toward parents. These effects are especially strong when mothers are distant emotionally. As pediatricians, we can educate people on the importance of warm parenting, redirection instead of punishment for younger children, and using small, logical consequences or time out when needed for aggression.
“Just ignore bullies.”
It is a rare child who can follow the command to “ignore” a bully without turning red or getting tears in his or her eyes – making them appealing targets. We can coach parents and kids how to disarm bullies by standing tall, putting hands on hips, making eye contact, and asking the peer a question such as “I do not understand what you’re trying to accomplish.” Learning martial arts also teaches children that they are powerful (but not to fight outside the class) so they can present themselves in this way. Programs that encourage children to get together to confront bullies supported by a school administration that uses comprehensive assessment and habilitation strategies for aggressive students are most effective in reducing aggression in schools. Anonymous reporting (for example, by using a cell phone app, such as STOPit) empowers students to report bullying or fights to school staff without risking later retribution from the peer.
“Tough teachers help kids fall in line.”
While peer fights generally increase from 2nd to 4th grade before declining, student fighting progressively increases when teachers use reprimands, rather than praise, to manage their classes. Children look to teachers to learn more than what is in books – how to be respectful and in control without putting others down. The most effective classroom management includes clear, fair rules; any correction should be done privately to avoid shaming students. Students dealt with this way are less likely to be angry and take it out on others. Of course, appropriate services helping every child experience success in learning is the foundation of positive behavior in school.
“Children with ADHD won’t learn self-regulation if they are treated with medicine.”
Children who show “low effortful control” or higher “dysregulation” are both more aggressive and also less likely to decline in aggression in early childhood. ADHD is a neurological condition characterized by such dysregulation and low effortful control. Children with ADHD often have higher and more persistent aggression. These tendencies also result in impulsive behaviors that can irritate peers and adults and can result in correction and criticism, further increasing aggression. Children with ADHD who are better controlled, often with the help of medication, have more positive interactions at school and at home, receive more praise and less correction, and develop more reasoned interaction patterns.
“I am the parent, and my child should do what I say.”
When adults step in to stop a fight, they are rarely in a position to know what actually happened between the kids. Children may quickly learn how to entrap a sibling or peer to look like the perpetrator in order to get them in trouble and/or avoid consequences for themselves, especially if large or harsh punishments are being used.
While it can seem tricky to treat children who are very different in age or development equally, having parents elicit or at least verbalize each child’s point of view is part of how children learn respect and mediation skills. Parents who refrain from taking sides or dictating how disputes should be resolved leave the chance for the children to acquire these component skills of negotiation. This does not mean there are no consequences, just that a brief discussion comes first.
When fighting is a pediatric complaint, you have a great opportunity to educate families in evidence-based ways that can both prevent and reduce their child’s use of aggression.
In one effective 90-minute training program, parents were taught basic mediation principles: to give ground rules and ask their children to agree to them, to ask each child to describe what happened and identify their disagreements and common ground, to encourage the children to discuss their goals in the fight and feelings about the issues, and to encourage the children to come up with suggestions to resolve their disputes and help them assess the practical aspects of their ideas. Praise should be used each time a child uses even some of these skills. Parents in this program also were given communication strategies, such as active listening, reflecting, and reframing, to help children learn to take the others’ perspective. In a follow up survey a month later, children of parents in the intervention group were seen to use these skills in real situations that might otherwise have been fights.
When aggression persists, mindfulness training, cognitive-behavioral techniques, social-emotional approaches, or peer mentoring programs delivered through individual counseling or school programs are all ways of teaching kids important interaction skills to reduce peer aggression. Remember, 40% of severe adult aggression begins before age 8 years, so preventive education or early referral to mental health services is key.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at [email protected].
Vaccines: Effectiveness vs. efficacy
During the influenza portion of the Feb. 21, 2018, Centers for Diseases Control and Prevention’s Advisory Committee on Immunization Practices meeting, two pleas from the audience asked the CDC/ACIP to make messages very clear about how protective influenza vaccine really is.
We hear apparently conflicting percentages from Australia, Canada, Europe, and the United States from the many stories/press releases in the news media and from official public health outlets. And the gloomiest ones get the most exposure.1 It can be confusing even for medical care providers who are supposed to advise families on such matters.
A key misunderstanding in many medical and lay news stories is about what vaccine effectiveness and vaccine efficacy really mean. What? Aren’t those the same thing? Nope. They are quite different. And are we sure of what those 95% confidence intervals (CI) mean? Let’s review the “math” so we can explain this to families.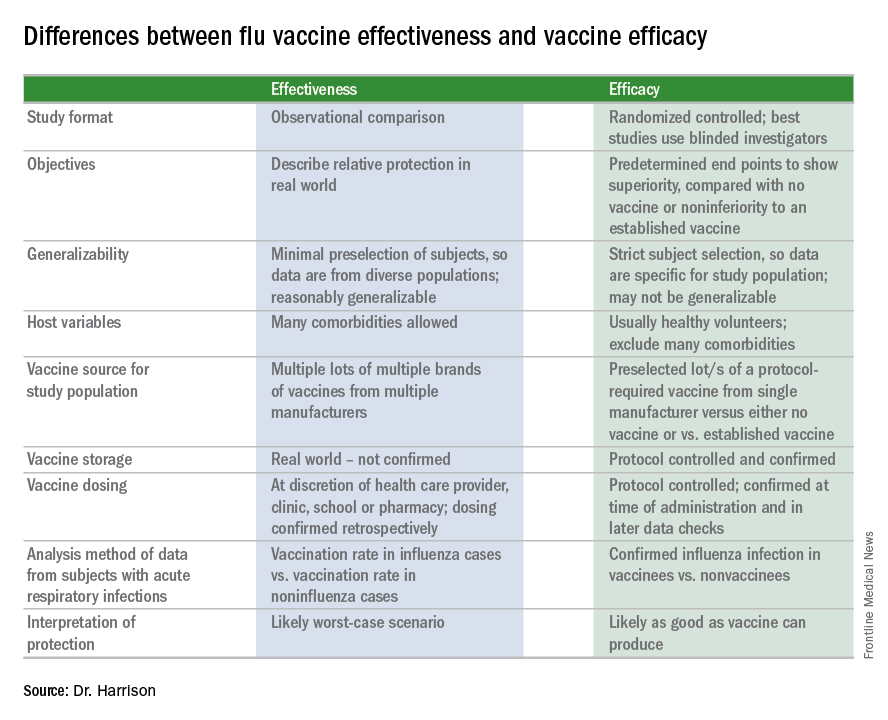
Vaccine effectiveness (VE)2,3
The first thing to know is that the CDC and similar public health agencies in other countries do not report vaccine efficacy. Instead, the percentage reported is VE during (interim estimated VE) and just after (final adjusted VE) each influenza season. This means that VE is generally a retrospective analysis of data, most of which were collected prospectively. Further, VE is likely the worst case scenario. VE is a measure of real-world benefit to patients for whom vaccine is recommended, by analyzing specific geographically diverse populations (population-based) without excluding most underlying illness or comorbidities (note that immunosuppressed persons are excluded). Subjects in VE studies receive their vaccine in the real world and, therefore, vaccinees may receive their vaccines from any number of the usual outlets (e.g., primary care provider, urgent care or emergency department, public health department, pharmacy, school, or nursing home). There are multiple lots of multiple brands from multiple vaccine manufacturers. Children who need two doses of influenza vaccine do not necessarily receive those doses according to the package insert’s schedule. VE studies do not have the capability to confirm that vaccine was stored, handled, and administered in a precisely correct manner according to manufacturer’s and CDC’s recommendations.
VE is calculated using a “test-negative” (case-control) analysis of patients presenting with acute respiratory infections (ARIs). People who are not in vaccine research can find this methodology confusing. Briefly, the VE compares the odds of vaccination in ARIs due to confirmed influenza to the odds of vaccination in ARIs not due to influenza. Additional statistical tools can adjust VE for specific factors. VE is also calculated by factors of interest, such as age, gender, pregnancy, influenza type, region of the country, presence of asthma or other comorbidity, etc. Whether the VE value is the “truth in the universe” is related to having enough subjects in each analyzed group and the degree to which the studied populations actually represent the whole country. So, VE is more accurate when there are large subject numbers.
Remember also that VE is usually calculated from outpatients, so it does not really measure all the benefits of vaccination. Prevention rates for severe influenza (such as influenza hospitalizations) are higher but usually unavailable until after the entire season.
VE studies generally measure real-world and likely worst-case-scenario benefit for the overall population being protected against outpatient influenza medical visits.
Vaccine efficacy2,3
Vaccine efficacy measures how the vaccine performs under ideal circumstances in a regimented protocol in relatively normal hosts – likely the best-case-scenario benefit. Vaccine efficacy is the percent difference in confirmed influenza episodes in vaccinees getting the “experimental” vaccine vs. episodes in nonvaccinees (or vaccinees getting an established vaccine). Vaccine efficacy, therefore, is usually calculated based on prospective well-controlled studies under ideal circumstances in subjects who received their vaccines on time per the recommended schedule. Most such studies are performed on otherwise healthy children or adults, with most comorbidities excluded. The “experimental” vaccine is generally from a single manufacturer from a single lot, and chain-of-custody is well controlled. The vaccine is administered at selected research sites according to a strict protocol; vaccine storage is ensured to be as recommended.
Confidence intervals
To assess whether the “protection” is “significant,” the calculations derive 95% confidence intervals (CI). If the 95% CI range is wide, such as many tens of percents, then there is less confidence that the calculation is correct. And if the lower CI is less than 0, then the result is not significant. For example, a VE of 20% is not highly protective, but can be significant if the 95% CI ranges from 10 to 28 (the lower value of 10 is above zero). It would not be significant if the 95% CI lower limit was –10. Values for seasons 2004-2005 and 2005-2006 were similar to this. Consider however that a VE of 55% seems great, but may not be significant if the 95% CI range is –20 to 89 (the lower value is less than zero). In the ideal world, the VE would be greater than 50% and the 95% CI range would be tight with the lower CI value far above zero; for example, VE of 70% with 95% CI ranging from 60 to 80. The 2010-2011 season was close to this.
Type and age-specific VE
Aside from overall VE, there are subset analyses that can be revealing. This year there are the concerning mid-season VE estimates of approximately 25% for the United States and 17% in Canada, for one specific type, H3N2, which unfortunately has been the dominant circulating U.S. type. That number is what everybody seems to have focused on. But remember influenza B becomes dominant late in most seasons (increasing at the time of writing this article). Interim 2017-2018 VE for influenza B was in the mid 60% range, making the box plot near 40% overall.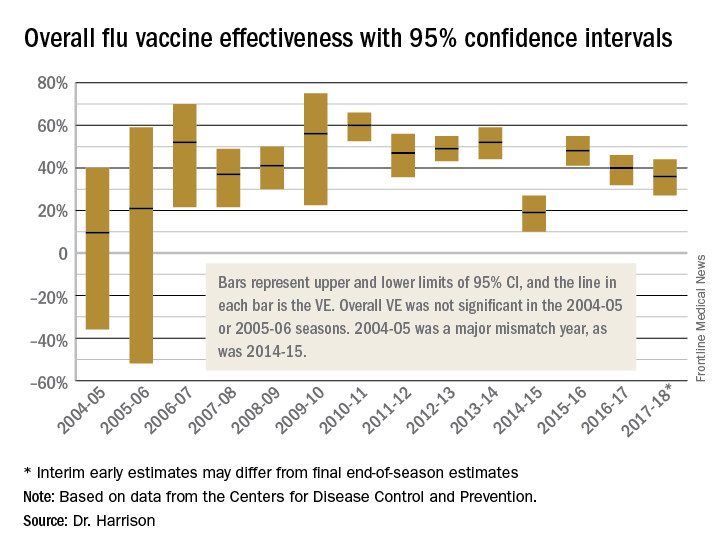
Age-related VE analysis can show difference; for example, the best benefit for H3N2 this season has been in young children and the worst in elderly and 9- to 17-year-olds.
Take-home message
The simplest way to think of overall VE is that it is the real-world, worst-case-scenario value for influenza protection by vaccine against the several circulating types of influenza.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Children’s Mercy Hospital receives grant funding for Dr. Harrison’s work as an investigator from GSK for MMR and rotavirus vaccine studies, from Merck for in vitro and clinical antibiotic studies, from Allergan for clinical antibiotic studies, from Pfizer for pneumococcal seroepidemiology studies, and from Regeneron for RSV studies. Dr. Harrison received support for travel and to present seroepidemiology data at one meeting. Email him at [email protected].
References
1. MMWR Weekly. 2017 Feb 17;66(6):167-71.
2. Dev Biol Stand. 1998;95:195-201.
3. Lancet Infect Dis. 2012 Jan;12(1):36-44.
During the influenza portion of the Feb. 21, 2018, Centers for Diseases Control and Prevention’s Advisory Committee on Immunization Practices meeting, two pleas from the audience asked the CDC/ACIP to make messages very clear about how protective influenza vaccine really is.
We hear apparently conflicting percentages from Australia, Canada, Europe, and the United States from the many stories/press releases in the news media and from official public health outlets. And the gloomiest ones get the most exposure.1 It can be confusing even for medical care providers who are supposed to advise families on such matters.
A key misunderstanding in many medical and lay news stories is about what vaccine effectiveness and vaccine efficacy really mean. What? Aren’t those the same thing? Nope. They are quite different. And are we sure of what those 95% confidence intervals (CI) mean? Let’s review the “math” so we can explain this to families.
Vaccine effectiveness (VE)2,3
The first thing to know is that the CDC and similar public health agencies in other countries do not report vaccine efficacy. Instead, the percentage reported is VE during (interim estimated VE) and just after (final adjusted VE) each influenza season. This means that VE is generally a retrospective analysis of data, most of which were collected prospectively. Further, VE is likely the worst case scenario. VE is a measure of real-world benefit to patients for whom vaccine is recommended, by analyzing specific geographically diverse populations (population-based) without excluding most underlying illness or comorbidities (note that immunosuppressed persons are excluded). Subjects in VE studies receive their vaccine in the real world and, therefore, vaccinees may receive their vaccines from any number of the usual outlets (e.g., primary care provider, urgent care or emergency department, public health department, pharmacy, school, or nursing home). There are multiple lots of multiple brands from multiple vaccine manufacturers. Children who need two doses of influenza vaccine do not necessarily receive those doses according to the package insert’s schedule. VE studies do not have the capability to confirm that vaccine was stored, handled, and administered in a precisely correct manner according to manufacturer’s and CDC’s recommendations.
VE is calculated using a “test-negative” (case-control) analysis of patients presenting with acute respiratory infections (ARIs). People who are not in vaccine research can find this methodology confusing. Briefly, the VE compares the odds of vaccination in ARIs due to confirmed influenza to the odds of vaccination in ARIs not due to influenza. Additional statistical tools can adjust VE for specific factors. VE is also calculated by factors of interest, such as age, gender, pregnancy, influenza type, region of the country, presence of asthma or other comorbidity, etc. Whether the VE value is the “truth in the universe” is related to having enough subjects in each analyzed group and the degree to which the studied populations actually represent the whole country. So, VE is more accurate when there are large subject numbers.
Remember also that VE is usually calculated from outpatients, so it does not really measure all the benefits of vaccination. Prevention rates for severe influenza (such as influenza hospitalizations) are higher but usually unavailable until after the entire season.
VE studies generally measure real-world and likely worst-case-scenario benefit for the overall population being protected against outpatient influenza medical visits.
Vaccine efficacy2,3
Vaccine efficacy measures how the vaccine performs under ideal circumstances in a regimented protocol in relatively normal hosts – likely the best-case-scenario benefit. Vaccine efficacy is the percent difference in confirmed influenza episodes in vaccinees getting the “experimental” vaccine vs. episodes in nonvaccinees (or vaccinees getting an established vaccine). Vaccine efficacy, therefore, is usually calculated based on prospective well-controlled studies under ideal circumstances in subjects who received their vaccines on time per the recommended schedule. Most such studies are performed on otherwise healthy children or adults, with most comorbidities excluded. The “experimental” vaccine is generally from a single manufacturer from a single lot, and chain-of-custody is well controlled. The vaccine is administered at selected research sites according to a strict protocol; vaccine storage is ensured to be as recommended.
Confidence intervals
To assess whether the “protection” is “significant,” the calculations derive 95% confidence intervals (CI). If the 95% CI range is wide, such as many tens of percents, then there is less confidence that the calculation is correct. And if the lower CI is less than 0, then the result is not significant. For example, a VE of 20% is not highly protective, but can be significant if the 95% CI ranges from 10 to 28 (the lower value of 10 is above zero). It would not be significant if the 95% CI lower limit was –10. Values for seasons 2004-2005 and 2005-2006 were similar to this. Consider however that a VE of 55% seems great, but may not be significant if the 95% CI range is –20 to 89 (the lower value is less than zero). In the ideal world, the VE would be greater than 50% and the 95% CI range would be tight with the lower CI value far above zero; for example, VE of 70% with 95% CI ranging from 60 to 80. The 2010-2011 season was close to this.
Type and age-specific VE
Aside from overall VE, there are subset analyses that can be revealing. This year there are the concerning mid-season VE estimates of approximately 25% for the United States and 17% in Canada, for one specific type, H3N2, which unfortunately has been the dominant circulating U.S. type. That number is what everybody seems to have focused on. But remember influenza B becomes dominant late in most seasons (increasing at the time of writing this article). Interim 2017-2018 VE for influenza B was in the mid 60% range, making the box plot near 40% overall.
Age-related VE analysis can show difference; for example, the best benefit for H3N2 this season has been in young children and the worst in elderly and 9- to 17-year-olds.
Take-home message
The simplest way to think of overall VE is that it is the real-world, worst-case-scenario value for influenza protection by vaccine against the several circulating types of influenza.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Children’s Mercy Hospital receives grant funding for Dr. Harrison’s work as an investigator from GSK for MMR and rotavirus vaccine studies, from Merck for in vitro and clinical antibiotic studies, from Allergan for clinical antibiotic studies, from Pfizer for pneumococcal seroepidemiology studies, and from Regeneron for RSV studies. Dr. Harrison received support for travel and to present seroepidemiology data at one meeting. Email him at [email protected].
References
1. MMWR Weekly. 2017 Feb 17;66(6):167-71.
2. Dev Biol Stand. 1998;95:195-201.
3. Lancet Infect Dis. 2012 Jan;12(1):36-44.
During the influenza portion of the Feb. 21, 2018, Centers for Diseases Control and Prevention’s Advisory Committee on Immunization Practices meeting, two pleas from the audience asked the CDC/ACIP to make messages very clear about how protective influenza vaccine really is.
We hear apparently conflicting percentages from Australia, Canada, Europe, and the United States from the many stories/press releases in the news media and from official public health outlets. And the gloomiest ones get the most exposure.1 It can be confusing even for medical care providers who are supposed to advise families on such matters.
A key misunderstanding in many medical and lay news stories is about what vaccine effectiveness and vaccine efficacy really mean. What? Aren’t those the same thing? Nope. They are quite different. And are we sure of what those 95% confidence intervals (CI) mean? Let’s review the “math” so we can explain this to families.
Vaccine effectiveness (VE)2,3
The first thing to know is that the CDC and similar public health agencies in other countries do not report vaccine efficacy. Instead, the percentage reported is VE during (interim estimated VE) and just after (final adjusted VE) each influenza season. This means that VE is generally a retrospective analysis of data, most of which were collected prospectively. Further, VE is likely the worst case scenario. VE is a measure of real-world benefit to patients for whom vaccine is recommended, by analyzing specific geographically diverse populations (population-based) without excluding most underlying illness or comorbidities (note that immunosuppressed persons are excluded). Subjects in VE studies receive their vaccine in the real world and, therefore, vaccinees may receive their vaccines from any number of the usual outlets (e.g., primary care provider, urgent care or emergency department, public health department, pharmacy, school, or nursing home). There are multiple lots of multiple brands from multiple vaccine manufacturers. Children who need two doses of influenza vaccine do not necessarily receive those doses according to the package insert’s schedule. VE studies do not have the capability to confirm that vaccine was stored, handled, and administered in a precisely correct manner according to manufacturer’s and CDC’s recommendations.
VE is calculated using a “test-negative” (case-control) analysis of patients presenting with acute respiratory infections (ARIs). People who are not in vaccine research can find this methodology confusing. Briefly, the VE compares the odds of vaccination in ARIs due to confirmed influenza to the odds of vaccination in ARIs not due to influenza. Additional statistical tools can adjust VE for specific factors. VE is also calculated by factors of interest, such as age, gender, pregnancy, influenza type, region of the country, presence of asthma or other comorbidity, etc. Whether the VE value is the “truth in the universe” is related to having enough subjects in each analyzed group and the degree to which the studied populations actually represent the whole country. So, VE is more accurate when there are large subject numbers.
Remember also that VE is usually calculated from outpatients, so it does not really measure all the benefits of vaccination. Prevention rates for severe influenza (such as influenza hospitalizations) are higher but usually unavailable until after the entire season.
VE studies generally measure real-world and likely worst-case-scenario benefit for the overall population being protected against outpatient influenza medical visits.
Vaccine efficacy2,3
Vaccine efficacy measures how the vaccine performs under ideal circumstances in a regimented protocol in relatively normal hosts – likely the best-case-scenario benefit. Vaccine efficacy is the percent difference in confirmed influenza episodes in vaccinees getting the “experimental” vaccine vs. episodes in nonvaccinees (or vaccinees getting an established vaccine). Vaccine efficacy, therefore, is usually calculated based on prospective well-controlled studies under ideal circumstances in subjects who received their vaccines on time per the recommended schedule. Most such studies are performed on otherwise healthy children or adults, with most comorbidities excluded. The “experimental” vaccine is generally from a single manufacturer from a single lot, and chain-of-custody is well controlled. The vaccine is administered at selected research sites according to a strict protocol; vaccine storage is ensured to be as recommended.
Confidence intervals
To assess whether the “protection” is “significant,” the calculations derive 95% confidence intervals (CI). If the 95% CI range is wide, such as many tens of percents, then there is less confidence that the calculation is correct. And if the lower CI is less than 0, then the result is not significant. For example, a VE of 20% is not highly protective, but can be significant if the 95% CI ranges from 10 to 28 (the lower value of 10 is above zero). It would not be significant if the 95% CI lower limit was –10. Values for seasons 2004-2005 and 2005-2006 were similar to this. Consider however that a VE of 55% seems great, but may not be significant if the 95% CI range is –20 to 89 (the lower value is less than zero). In the ideal world, the VE would be greater than 50% and the 95% CI range would be tight with the lower CI value far above zero; for example, VE of 70% with 95% CI ranging from 60 to 80. The 2010-2011 season was close to this.
Type and age-specific VE
Aside from overall VE, there are subset analyses that can be revealing. This year there are the concerning mid-season VE estimates of approximately 25% for the United States and 17% in Canada, for one specific type, H3N2, which unfortunately has been the dominant circulating U.S. type. That number is what everybody seems to have focused on. But remember influenza B becomes dominant late in most seasons (increasing at the time of writing this article). Interim 2017-2018 VE for influenza B was in the mid 60% range, making the box plot near 40% overall.
Age-related VE analysis can show difference; for example, the best benefit for H3N2 this season has been in young children and the worst in elderly and 9- to 17-year-olds.
Take-home message
The simplest way to think of overall VE is that it is the real-world, worst-case-scenario value for influenza protection by vaccine against the several circulating types of influenza.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Children’s Mercy Hospital receives grant funding for Dr. Harrison’s work as an investigator from GSK for MMR and rotavirus vaccine studies, from Merck for in vitro and clinical antibiotic studies, from Allergan for clinical antibiotic studies, from Pfizer for pneumococcal seroepidemiology studies, and from Regeneron for RSV studies. Dr. Harrison received support for travel and to present seroepidemiology data at one meeting. Email him at [email protected].
References
1. MMWR Weekly. 2017 Feb 17;66(6):167-71.
2. Dev Biol Stand. 1998;95:195-201.
3. Lancet Infect Dis. 2012 Jan;12(1):36-44.
Supreme Court declines to hear DACA case
The U.S. Supreme Court has declined to decide for now whether the Deferred Action for Childhood Arrivals (DACA) program should continue, turning down a request by the Trump administration to hear the case early. The Feb. 26 decision allows the DACA program to remain in effect under a district court ruling and sends the case back to the appeals court.
The Trump administration has called DACA, an Obama administration policy that protected from deportation immigrants who were brought to the United States illegally as children and authorized them to work in the United States, an “unconstitutional overreach of executive branch power” by the former administration that deliberately sought to undermine the legislative branch.
President Trump announced in September 2016 that he would phase out the DACA program and gave Congress 6 months to pass legislation that would replace DACA or preserve some of its provisions or he would terminate it. They have made little progress toward agreeing on replacement legislation for DACA since then.
A number of plaintiffs sued the Trump administration over the program’s rescission, including the University of California, several states, and a group of individuals who remain in the United States because of DACA. The plaintiffs alleged the rescission violated the Administrative Procedure Act because it was an abuse of discretion and deprived those affected by DACA of “constitutionally protected property and liberty interests without due process of law.”
In a Jan. 9 opinion, the United States District Court for the Northern District of California sided with the plaintiffs, ruling that they were likely to succeed on their claims that the rescission “was arbitrary, capricious, an abuse of discretion, or not otherwise in accordance with law.” The court ordered the government to continue accepting DACA renewal applications while the challenges continued through the court system. Rather than pursuing an appeal, the U.S. Department of Justice requested that the Supreme Court take up the case.
In its Feb. 26 order, the high court declined to hear the case, writing, “it is assumed the court of appeals will act expeditiously to decide this case.”
Jirayut New Latthivongskorn, a co-plaintiff in the case and a medical student with DACA status at the University of California, San Francisco, said the Supreme Court made the right decision. Mr. Latthivongskorn, who came to the United States from Thailand when he was 9 years old, is cofounder of Pre-Health Dreamers, a network of undocumented students who plan to pursue medical careers. He is in his third year at UCSF and is slated to graduate in 2019 after completing the university’s Program in Medical Education for the Urban Underserved.
The Department of Justice expressed disappointment at the Supreme Court’s decision not to take up the case and said it will continue to strongly defend the case.
“While we were hopeful for a different outcome, the Supreme Court very rarely grants certiorari before judgment, though in our view it was warranted for the extraordinary injunction requiring the Department of Homeland Security to maintain DACA,” DOJ spokesman Devin O’Malley said in a statement. “We will continue to defend DHS’ lawful authority to wind down DACA in an orderly manner.”
The U.S. Supreme Court has declined to decide for now whether the Deferred Action for Childhood Arrivals (DACA) program should continue, turning down a request by the Trump administration to hear the case early. The Feb. 26 decision allows the DACA program to remain in effect under a district court ruling and sends the case back to the appeals court.
The Trump administration has called DACA, an Obama administration policy that protected from deportation immigrants who were brought to the United States illegally as children and authorized them to work in the United States, an “unconstitutional overreach of executive branch power” by the former administration that deliberately sought to undermine the legislative branch.
President Trump announced in September 2016 that he would phase out the DACA program and gave Congress 6 months to pass legislation that would replace DACA or preserve some of its provisions or he would terminate it. They have made little progress toward agreeing on replacement legislation for DACA since then.
A number of plaintiffs sued the Trump administration over the program’s rescission, including the University of California, several states, and a group of individuals who remain in the United States because of DACA. The plaintiffs alleged the rescission violated the Administrative Procedure Act because it was an abuse of discretion and deprived those affected by DACA of “constitutionally protected property and liberty interests without due process of law.”
In a Jan. 9 opinion, the United States District Court for the Northern District of California sided with the plaintiffs, ruling that they were likely to succeed on their claims that the rescission “was arbitrary, capricious, an abuse of discretion, or not otherwise in accordance with law.” The court ordered the government to continue accepting DACA renewal applications while the challenges continued through the court system. Rather than pursuing an appeal, the U.S. Department of Justice requested that the Supreme Court take up the case.
In its Feb. 26 order, the high court declined to hear the case, writing, “it is assumed the court of appeals will act expeditiously to decide this case.”
Jirayut New Latthivongskorn, a co-plaintiff in the case and a medical student with DACA status at the University of California, San Francisco, said the Supreme Court made the right decision. Mr. Latthivongskorn, who came to the United States from Thailand when he was 9 years old, is cofounder of Pre-Health Dreamers, a network of undocumented students who plan to pursue medical careers. He is in his third year at UCSF and is slated to graduate in 2019 after completing the university’s Program in Medical Education for the Urban Underserved.
The Department of Justice expressed disappointment at the Supreme Court’s decision not to take up the case and said it will continue to strongly defend the case.
“While we were hopeful for a different outcome, the Supreme Court very rarely grants certiorari before judgment, though in our view it was warranted for the extraordinary injunction requiring the Department of Homeland Security to maintain DACA,” DOJ spokesman Devin O’Malley said in a statement. “We will continue to defend DHS’ lawful authority to wind down DACA in an orderly manner.”
The U.S. Supreme Court has declined to decide for now whether the Deferred Action for Childhood Arrivals (DACA) program should continue, turning down a request by the Trump administration to hear the case early. The Feb. 26 decision allows the DACA program to remain in effect under a district court ruling and sends the case back to the appeals court.
The Trump administration has called DACA, an Obama administration policy that protected from deportation immigrants who were brought to the United States illegally as children and authorized them to work in the United States, an “unconstitutional overreach of executive branch power” by the former administration that deliberately sought to undermine the legislative branch.
President Trump announced in September 2016 that he would phase out the DACA program and gave Congress 6 months to pass legislation that would replace DACA or preserve some of its provisions or he would terminate it. They have made little progress toward agreeing on replacement legislation for DACA since then.
A number of plaintiffs sued the Trump administration over the program’s rescission, including the University of California, several states, and a group of individuals who remain in the United States because of DACA. The plaintiffs alleged the rescission violated the Administrative Procedure Act because it was an abuse of discretion and deprived those affected by DACA of “constitutionally protected property and liberty interests without due process of law.”
In a Jan. 9 opinion, the United States District Court for the Northern District of California sided with the plaintiffs, ruling that they were likely to succeed on their claims that the rescission “was arbitrary, capricious, an abuse of discretion, or not otherwise in accordance with law.” The court ordered the government to continue accepting DACA renewal applications while the challenges continued through the court system. Rather than pursuing an appeal, the U.S. Department of Justice requested that the Supreme Court take up the case.
In its Feb. 26 order, the high court declined to hear the case, writing, “it is assumed the court of appeals will act expeditiously to decide this case.”
Jirayut New Latthivongskorn, a co-plaintiff in the case and a medical student with DACA status at the University of California, San Francisco, said the Supreme Court made the right decision. Mr. Latthivongskorn, who came to the United States from Thailand when he was 9 years old, is cofounder of Pre-Health Dreamers, a network of undocumented students who plan to pursue medical careers. He is in his third year at UCSF and is slated to graduate in 2019 after completing the university’s Program in Medical Education for the Urban Underserved.
The Department of Justice expressed disappointment at the Supreme Court’s decision not to take up the case and said it will continue to strongly defend the case.
“While we were hopeful for a different outcome, the Supreme Court very rarely grants certiorari before judgment, though in our view it was warranted for the extraordinary injunction requiring the Department of Homeland Security to maintain DACA,” DOJ spokesman Devin O’Malley said in a statement. “We will continue to defend DHS’ lawful authority to wind down DACA in an orderly manner.”
Atypical Presentation of Acquired Angioedema
To the Editor:
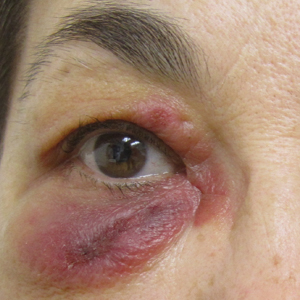
A 65-year-old woman with B-cell marginal zone lymphoma presented with asymptomatic swelling and redness of the upper and lower eyelids of 1 week’s duration that was unresponsive to topical corticosteroids for presumptive allergic contact dermatitis. She denied any lip or tongue swelling, abdominal pain, or difficulty breathing or swallowing. Diagnosis of acquired angioedema (AAE) was confirmed on laboratory analysis, which showed C1q levels less than 3.6 mg/dL (reference range, 5.0–8.6 mg/dL), complement component 4 levels less than 8 mg/dL (reference range, 14–44 mg/dL), and C1 esterase inhibitor (C1-INH) levels of 3 mg/dL (reference range, 12–30 mg/dL).
A review of the patient’s medical record showed chronic thrombocytopenia secondary to previous chemotherapy. It was determined that the patient’s ecchymosis and purpura of the eyelids was secondary to a low platelet count resulting in bleeding into the area of angioedema (Figure). Serum protein electrophoresis did not demonstrate a monoclonal spike, and flow cytometry showed persistent B-cell leukemia without evidence of an aberrant T-cell antigenic profile. The edema and purpura of the eyelids spontaneously resolved over days, and the patient has had no recurrences to date. She was prescribed icatibant for treatment of future acute AAE attacks.

The common pathway of AAE involves the inability of C1-INH to stop activation of the complement, fibrinolytic, and contact systems. Failure to control the contact system leads to increased bradykinin production resulting in vasodilation and edema. Diagnosis of hereditary angioedema (HAE) types 1 and 2 can be confirmed in the setting of low complement component 4 and C1-INH functional levels and normal C1q levels; in AAE, C1q levels also are low.1,2
The malignancies most frequently associated with AAE are non-Hodgkin lymphomas (eg, nodal marginal zone lymphoma, splenic marginal zone lymphoma), such as in our patient, as well as monoclonal gammopathies.2 Triggers of AAE include trauma (eg, surgery, strenuous exercise), infection, and use of certain medications such as angiotensin-converting enzyme inhibitors and estrogen, but most episodes are spontaneous. Swelling of any cutaneous surface can occur in the setting of AAE. Mucosal involvement appears to be limited to the upper airway and gastrointestinal tract. Edema of the upper airway mucosa can lead to asphyxiation. In these cases, asphyxia can occur rapidly, and therefore all patients with upper airway involvement should present to the emergency room or call 911. Pain from swelling in the gastrointestinal tract can mimic an acute abdomen.3
Newly developed targeted therapies for HAE also appear to be effective in treating AAE. A summary of available treatments for angioedema is provided in the Table. Human plasma C1-INH can be used intravenously to treat acute attacks or can be given prophylactically to prevent attacks, but large doses may be necessary due to consumption of the protein.1,3 The risk of bloodborne disease as a result of treatment exists, but screening and processing during production of the plasma makes this unlikely. Ecallantide is a reversible inhibitor of plasma kallikrein.1,3 Rapid onset and subcutaneous dosing make it useful for treatment of acute AAE attacks. Because anaphylaxis has been reported in up to 3% of patients, ecallantide includes a boxed warning indicating that it must be administered by a health care professional with appropriate medical support to manage anaphylaxis and HAE.4 Icatibant is a selective competitive antagonist of bradykinin receptor B2. It can be administered subcutaneously by the patient, making it ideal for rapid treatment of angioedema.1,3 Adverse events include pain and irritation at the injection site.
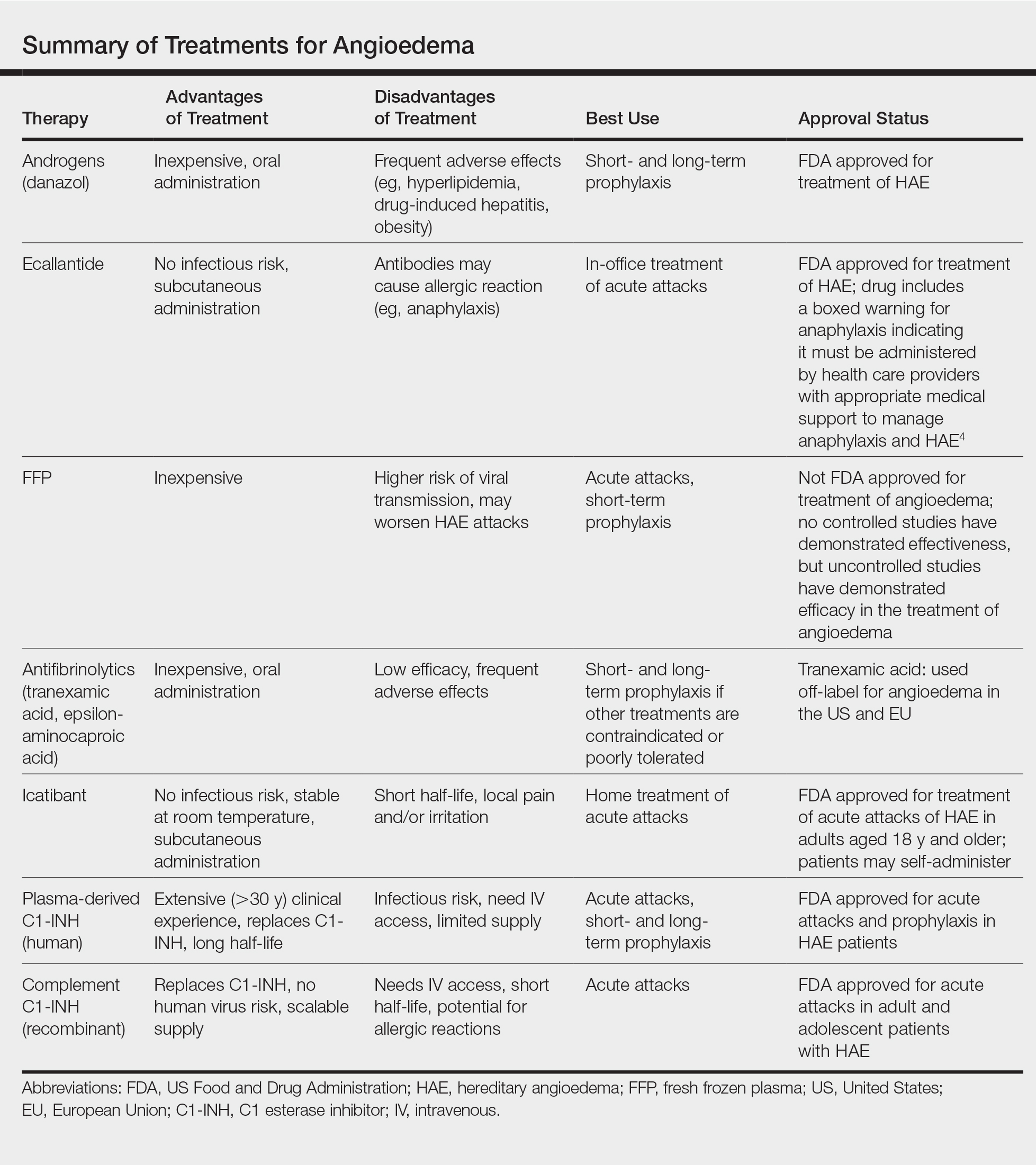
The most appropriate therapy for AAE is treatment of the underlying malignancy. Recognition and proper treatment of AAE is essential, as bradykinin-induced angioedema (AAE, HAE and angiotensin-converting enzyme inhibitor induced angioedema) does not respond to antihistamines and corticosteroids and instead requires therapy as discussed above.
- Craig T, Riedl M, Dykewicz MS, et al. When is prophylaxis for hereditary angioedema necessary? Ann Allergy Asthma Immunol. 2009;102:366-372.
- Cugno M, Castelli R, Cicardi M. Angioedema due to acquired C1-inhibitor deficiency: a bridging connection between autoimmunity and lymphoproliferation. Autoimmun Rev. 2008;8:156-159.
- Buyantseva LV, Sardana N, Craig TJ. Update on treatment of hereditary angioedema. Asian Pac J Allergy Immunol. 2012;30:89-98.
- Kalbitor [package insert]. Burlington, MA: Dyax Corp; 2015.
To the Editor:
A 65-year-old woman with B-cell marginal zone lymphoma presented with asymptomatic swelling and redness of the upper and lower eyelids of 1 week’s duration that was unresponsive to topical corticosteroids for presumptive allergic contact dermatitis. She denied any lip or tongue swelling, abdominal pain, or difficulty breathing or swallowing. Diagnosis of acquired angioedema (AAE) was confirmed on laboratory analysis, which showed C1q levels less than 3.6 mg/dL (reference range, 5.0–8.6 mg/dL), complement component 4 levels less than 8 mg/dL (reference range, 14–44 mg/dL), and C1 esterase inhibitor (C1-INH) levels of 3 mg/dL (reference range, 12–30 mg/dL).
A review of the patient’s medical record showed chronic thrombocytopenia secondary to previous chemotherapy. It was determined that the patient’s ecchymosis and purpura of the eyelids was secondary to a low platelet count resulting in bleeding into the area of angioedema (Figure). Serum protein electrophoresis did not demonstrate a monoclonal spike, and flow cytometry showed persistent B-cell leukemia without evidence of an aberrant T-cell antigenic profile. The edema and purpura of the eyelids spontaneously resolved over days, and the patient has had no recurrences to date. She was prescribed icatibant for treatment of future acute AAE attacks.

The common pathway of AAE involves the inability of C1-INH to stop activation of the complement, fibrinolytic, and contact systems. Failure to control the contact system leads to increased bradykinin production resulting in vasodilation and edema. Diagnosis of hereditary angioedema (HAE) types 1 and 2 can be confirmed in the setting of low complement component 4 and C1-INH functional levels and normal C1q levels; in AAE, C1q levels also are low.1,2
The malignancies most frequently associated with AAE are non-Hodgkin lymphomas (eg, nodal marginal zone lymphoma, splenic marginal zone lymphoma), such as in our patient, as well as monoclonal gammopathies.2 Triggers of AAE include trauma (eg, surgery, strenuous exercise), infection, and use of certain medications such as angiotensin-converting enzyme inhibitors and estrogen, but most episodes are spontaneous. Swelling of any cutaneous surface can occur in the setting of AAE. Mucosal involvement appears to be limited to the upper airway and gastrointestinal tract. Edema of the upper airway mucosa can lead to asphyxiation. In these cases, asphyxia can occur rapidly, and therefore all patients with upper airway involvement should present to the emergency room or call 911. Pain from swelling in the gastrointestinal tract can mimic an acute abdomen.3
Newly developed targeted therapies for HAE also appear to be effective in treating AAE. A summary of available treatments for angioedema is provided in the Table. Human plasma C1-INH can be used intravenously to treat acute attacks or can be given prophylactically to prevent attacks, but large doses may be necessary due to consumption of the protein.1,3 The risk of bloodborne disease as a result of treatment exists, but screening and processing during production of the plasma makes this unlikely. Ecallantide is a reversible inhibitor of plasma kallikrein.1,3 Rapid onset and subcutaneous dosing make it useful for treatment of acute AAE attacks. Because anaphylaxis has been reported in up to 3% of patients, ecallantide includes a boxed warning indicating that it must be administered by a health care professional with appropriate medical support to manage anaphylaxis and HAE.4 Icatibant is a selective competitive antagonist of bradykinin receptor B2. It can be administered subcutaneously by the patient, making it ideal for rapid treatment of angioedema.1,3 Adverse events include pain and irritation at the injection site.

The most appropriate therapy for AAE is treatment of the underlying malignancy. Recognition and proper treatment of AAE is essential, as bradykinin-induced angioedema (AAE, HAE and angiotensin-converting enzyme inhibitor induced angioedema) does not respond to antihistamines and corticosteroids and instead requires therapy as discussed above.
To the Editor:
A 65-year-old woman with B-cell marginal zone lymphoma presented with asymptomatic swelling and redness of the upper and lower eyelids of 1 week’s duration that was unresponsive to topical corticosteroids for presumptive allergic contact dermatitis. She denied any lip or tongue swelling, abdominal pain, or difficulty breathing or swallowing. Diagnosis of acquired angioedema (AAE) was confirmed on laboratory analysis, which showed C1q levels less than 3.6 mg/dL (reference range, 5.0–8.6 mg/dL), complement component 4 levels less than 8 mg/dL (reference range, 14–44 mg/dL), and C1 esterase inhibitor (C1-INH) levels of 3 mg/dL (reference range, 12–30 mg/dL).
A review of the patient’s medical record showed chronic thrombocytopenia secondary to previous chemotherapy. It was determined that the patient’s ecchymosis and purpura of the eyelids was secondary to a low platelet count resulting in bleeding into the area of angioedema (Figure). Serum protein electrophoresis did not demonstrate a monoclonal spike, and flow cytometry showed persistent B-cell leukemia without evidence of an aberrant T-cell antigenic profile. The edema and purpura of the eyelids spontaneously resolved over days, and the patient has had no recurrences to date. She was prescribed icatibant for treatment of future acute AAE attacks.

The common pathway of AAE involves the inability of C1-INH to stop activation of the complement, fibrinolytic, and contact systems. Failure to control the contact system leads to increased bradykinin production resulting in vasodilation and edema. Diagnosis of hereditary angioedema (HAE) types 1 and 2 can be confirmed in the setting of low complement component 4 and C1-INH functional levels and normal C1q levels; in AAE, C1q levels also are low.1,2
The malignancies most frequently associated with AAE are non-Hodgkin lymphomas (eg, nodal marginal zone lymphoma, splenic marginal zone lymphoma), such as in our patient, as well as monoclonal gammopathies.2 Triggers of AAE include trauma (eg, surgery, strenuous exercise), infection, and use of certain medications such as angiotensin-converting enzyme inhibitors and estrogen, but most episodes are spontaneous. Swelling of any cutaneous surface can occur in the setting of AAE. Mucosal involvement appears to be limited to the upper airway and gastrointestinal tract. Edema of the upper airway mucosa can lead to asphyxiation. In these cases, asphyxia can occur rapidly, and therefore all patients with upper airway involvement should present to the emergency room or call 911. Pain from swelling in the gastrointestinal tract can mimic an acute abdomen.3
Newly developed targeted therapies for HAE also appear to be effective in treating AAE. A summary of available treatments for angioedema is provided in the Table. Human plasma C1-INH can be used intravenously to treat acute attacks or can be given prophylactically to prevent attacks, but large doses may be necessary due to consumption of the protein.1,3 The risk of bloodborne disease as a result of treatment exists, but screening and processing during production of the plasma makes this unlikely. Ecallantide is a reversible inhibitor of plasma kallikrein.1,3 Rapid onset and subcutaneous dosing make it useful for treatment of acute AAE attacks. Because anaphylaxis has been reported in up to 3% of patients, ecallantide includes a boxed warning indicating that it must be administered by a health care professional with appropriate medical support to manage anaphylaxis and HAE.4 Icatibant is a selective competitive antagonist of bradykinin receptor B2. It can be administered subcutaneously by the patient, making it ideal for rapid treatment of angioedema.1,3 Adverse events include pain and irritation at the injection site.

The most appropriate therapy for AAE is treatment of the underlying malignancy. Recognition and proper treatment of AAE is essential, as bradykinin-induced angioedema (AAE, HAE and angiotensin-converting enzyme inhibitor induced angioedema) does not respond to antihistamines and corticosteroids and instead requires therapy as discussed above.
- Craig T, Riedl M, Dykewicz MS, et al. When is prophylaxis for hereditary angioedema necessary? Ann Allergy Asthma Immunol. 2009;102:366-372.
- Cugno M, Castelli R, Cicardi M. Angioedema due to acquired C1-inhibitor deficiency: a bridging connection between autoimmunity and lymphoproliferation. Autoimmun Rev. 2008;8:156-159.
- Buyantseva LV, Sardana N, Craig TJ. Update on treatment of hereditary angioedema. Asian Pac J Allergy Immunol. 2012;30:89-98.
- Kalbitor [package insert]. Burlington, MA: Dyax Corp; 2015.
- Craig T, Riedl M, Dykewicz MS, et al. When is prophylaxis for hereditary angioedema necessary? Ann Allergy Asthma Immunol. 2009;102:366-372.
- Cugno M, Castelli R, Cicardi M. Angioedema due to acquired C1-inhibitor deficiency: a bridging connection between autoimmunity and lymphoproliferation. Autoimmun Rev. 2008;8:156-159.
- Buyantseva LV, Sardana N, Craig TJ. Update on treatment of hereditary angioedema. Asian Pac J Allergy Immunol. 2012;30:89-98.
- Kalbitor [package insert]. Burlington, MA: Dyax Corp; 2015.
Practice Points
- Late-onset angioedema without urticaria can be secondary to acquired angioedema with C1 esterase inhibitor deficiency (C1-INH).
- Most patients with angioedema with C1-INH inhibitor deficiency will have either a monoclonal gammopathy or a lymphoma.
Pediatric Migraine/Headache and Sleep Disturbances
Assessment and treatment of sleep problems in pediatric patients with chronic headache is important, with several contextual and headache diagnostic factors influencing the severity of sleep disturbance, according to a recent retrospective chart review. Researchers evaluated 527 patients, aged 7-17 years, with a primary headache diagnosis of migraine (n=278), tension-type headache (TTH; n=157), and new daily persistent-headache (NDPH; n=92). Patients completed measures of disability, anxiety, and depression and their parents completed measures of sleep disturbance. They found:
- Sleep disturbance was greater in patients with TTH (10.34 ± 5.94) and NDPH (11.52 ± 6.40) than migraine (8.31 ± 5.89).
- Across patient groups, greater sleep disturbance was significantly associated with higher levels of functional disability, anxiety, and depression.
- Additionally, higher pain levels were significantly associated with greater sleep disturbance among TTH patients, with this association non-significant among the other headache groups.
- When simultaneously examining demographic, pain-related, and emotional distress factors, older age, higher levels of disability and depression, and NDPH diagnosis were all significant predictors of greater sleep disturbance.
Pediatric headache and sleep disturbance: A comparison of diagnostic groups. Headache. 2018;58(2):217-228. doi:10.1111/head.13207.
Assessment and treatment of sleep problems in pediatric patients with chronic headache is important, with several contextual and headache diagnostic factors influencing the severity of sleep disturbance, according to a recent retrospective chart review. Researchers evaluated 527 patients, aged 7-17 years, with a primary headache diagnosis of migraine (n=278), tension-type headache (TTH; n=157), and new daily persistent-headache (NDPH; n=92). Patients completed measures of disability, anxiety, and depression and their parents completed measures of sleep disturbance. They found:
- Sleep disturbance was greater in patients with TTH (10.34 ± 5.94) and NDPH (11.52 ± 6.40) than migraine (8.31 ± 5.89).
- Across patient groups, greater sleep disturbance was significantly associated with higher levels of functional disability, anxiety, and depression.
- Additionally, higher pain levels were significantly associated with greater sleep disturbance among TTH patients, with this association non-significant among the other headache groups.
- When simultaneously examining demographic, pain-related, and emotional distress factors, older age, higher levels of disability and depression, and NDPH diagnosis were all significant predictors of greater sleep disturbance.
Pediatric headache and sleep disturbance: A comparison of diagnostic groups. Headache. 2018;58(2):217-228. doi:10.1111/head.13207.
Assessment and treatment of sleep problems in pediatric patients with chronic headache is important, with several contextual and headache diagnostic factors influencing the severity of sleep disturbance, according to a recent retrospective chart review. Researchers evaluated 527 patients, aged 7-17 years, with a primary headache diagnosis of migraine (n=278), tension-type headache (TTH; n=157), and new daily persistent-headache (NDPH; n=92). Patients completed measures of disability, anxiety, and depression and their parents completed measures of sleep disturbance. They found:
- Sleep disturbance was greater in patients with TTH (10.34 ± 5.94) and NDPH (11.52 ± 6.40) than migraine (8.31 ± 5.89).
- Across patient groups, greater sleep disturbance was significantly associated with higher levels of functional disability, anxiety, and depression.
- Additionally, higher pain levels were significantly associated with greater sleep disturbance among TTH patients, with this association non-significant among the other headache groups.
- When simultaneously examining demographic, pain-related, and emotional distress factors, older age, higher levels of disability and depression, and NDPH diagnosis were all significant predictors of greater sleep disturbance.
Pediatric headache and sleep disturbance: A comparison of diagnostic groups. Headache. 2018;58(2):217-228. doi:10.1111/head.13207.
Hidradenitis suppurativa: An underdiagnosed skin problem of women
In recent decades the practice of medicine has drifted away from the performance of a physical examination during most patient encounters and evolved toward the more intensive use of history, imaging, and laboratory studies to guide management decisions. For example, it is common for a woman to present to an emergency department with abdominal or pelvic pain and undergo a computerized tomography scan before an abdominal and pelvic examination is performed. Some authorities believe that the trend to reduce the importance of the physical examination has gone way too far and resulted in a reduction in the quality of health care.1,2
Many skin diseases only can be diagnosed by having the patient disrobe and examining the skin. Gynecologists are uniquely positioned to diagnose important skin diseases because, while performing a reproductive health examination, they may be the first clinicians to directly examine the anogenital area and inner thighs. Skin diseases that are prevalent and can be diagnosed while performing an examination of the anogenital region include lichen sclerosus (LS) and hidradenitis suppurativa (HS). The prevalence of each of these conditions is in the range of 1% to 4% of women.3–5
Failure to examine the anogenital area and insufficient attention to the early signs of LS and HS may result in a long delay in the diagnosis.6 In 1 survey, of 517 patients with HS, there was a 7-year interval between the onset of the disease and the diagnosis by a clinician.7 Delay in diagnosis results in increased scarring, which makes it more difficult to effectively treat the disease. In this editorial, I will focus on the pathogenesis, diagnosis, and treatment of HS.
Diagnosis, presentation, and staging
Hidradenitis suppurativa (from the Greek, hidros means sweat and aden means glands) is a painful, chronic, relapsing, inflammatory skin disorder affecting the follicular unit. It is manifested by nodules, pustules, sinus tracts, and scars, usually in intertriginous areas. The diagnosis is made by history and physical examination. The 3 cardinal features of HS are 1) deep-seated nodules, comedones, and fibrosis; 2) typical anatomic location of the lesions in the axillae, inguinocrural, and anogenital regions, and 3) chronic relapsing course.8
Disease severity is often assessed using the Hurley staging system:
- stage I: abscess formation without sinus tracts or scarring (FIGURE)
- stage II: recurrent abscesses with tract formation and scarring, widely separated lesions
- stage III: diffuse or near-diffuse involvement or multiple interconnected tracts and abscesses.
In one report, stage I, II, and III disease was diagnosed in 65%, 31%, and 4% of cases, respectively, indicating that most HS is diagnosed instage I and suitable for treatment by a gynecologist.9
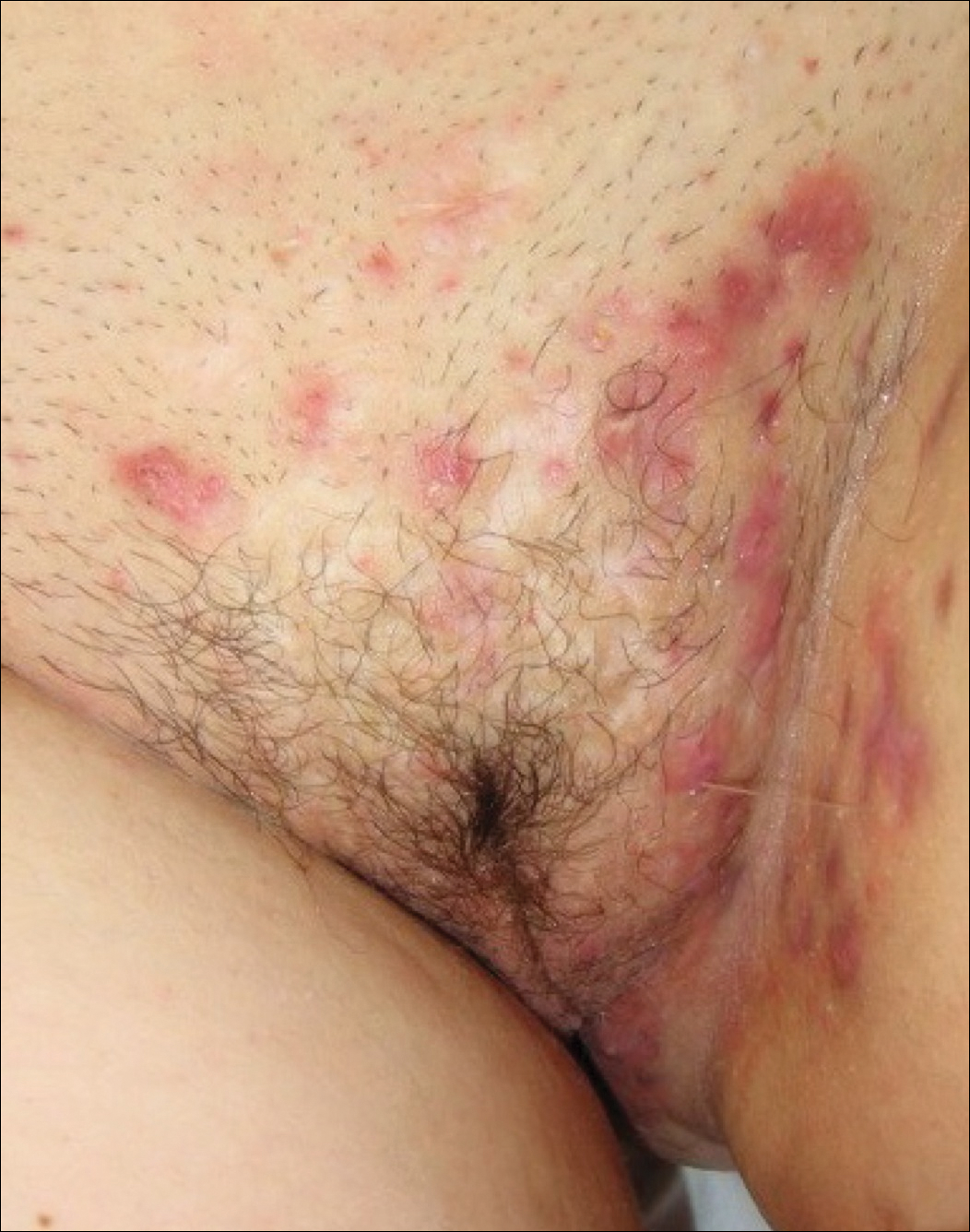
HS typically presents after puberty and women are more commonly affected than men. In one case series including 232 women with HS the regions most commonly affected were: axillae, inguinofemoral, urogenital, and buttocks in 79%, 77%, 51%, and 40% of cases, respectively.10 Risk factors for HS include obesity, cigarette smoking, tight fitting clothing, and chronic friction across the affected skin area.5
Pathogenesis
The pathophysiology of HS is thought to begin with occlusion of the follicle, resulting in follicle rupture deep in the dermis, thereby triggering inflammation, bacterial infection, and scarring. Dermal areas affected by HS have high concentrations of cytokines, including tumor necrosis factor (TNF)–alpha, interleukin (IL)-1-beta, IL-23, and IL-32.11,12 Once HS becomes an established process, it is difficult to treat because the dermal inflammatory process and scarring provides a microenvironment that facilitates disease progression. Hence early detection and treatment may result in optimal long-term outcomes.
Read about management of HS by stage
Treatment
Many recommended treatments for HS have not been formally tested in large randomized trials. A recent Cochrane review identified only 12 high-quality trials and the median number of participants was 27 per trial.13 Consequently, most treatment recommendations are based on expert opinion. Recommended treatments include smoking cessation, weight loss, topical and systemic antibiotics, antiandrogens, anti-inflammatory biologics (adalimumab and infliximab), and surgery. Smoking cessation and weight loss are strongly recommended in the initial treatment of HS. Bariatric surgery and significant postprocedure weight loss has been reported to cause a reduction in disease activity.14
Stage I management. For the initial treatment of stage I HS, clindamycin gel 1% applied twice daily to affected areas is recommended.15 Recommended oral antibiotic treatments include tetracycline 500 mg twice daily for 12 weeks16 or doxycycline 100 mg or 200 mg given daily for 10 weeks or clindamycin 300 mg twice daily plus rifampicin 600 mg once daily for 10 weeks.17,18 These antibiotics have both antimicrobial and anti-inflammatory effects.
Hormonal interventions that suppress androgen production or action may help reduce HS disease activity. For women with HS who also need contraception, an estrogen-progestin contraceptive may help reduce HS disease activity in up to 50% of individuals.19 The 5-alpha reductase inhibitor finasteride, at high doses (5 to 15 mg daily), has been reported to reduce HS disease activity.20,21 Finasteride is a teratogen, and the FDA strongly recommends against its use by women. Spironolactone, an anti-mineralocorticoid and antiandrogen, at a dose of 100 mg daily has been reported to reduce disease activity in about 50% of treated individuals and is FDA approved for use in women.22 Among reproductive-age women, spironolactone, which is a teratogen, only should be prescribed to women using an effective form of contraceptive. HS is often associated with obesity and insulin resistance. Metformin 500 mg three times daily has been reported to decrease disease activity.23,24
Stage II or III management. For Hurley stage II or III HS, referral to a dermatologist is warranted. There is evidence that too few people with HS are referred to a dermatologist.25 For severe HS resistant to oral medications, anti-TNF monoclonal antibody treatment with adalimumab (Humira) or infliximab (Remicade) is effective. Adalimumab is administered by subcutaneous injection and is US Food and Drug Administration (FDA)–approved to treat HS. Following a loading dose, adalimumab is administered weekly at a dose of 40 mg.26 Infliximab, which is not FDA approved to treat HS, is administered by intravenous infusion at a dose of 5 mg/kg at weeks 0, 2, and 6, and then every 8 weeks.27
Surgical management. HS is sometimes treated surgically with laser destruction of lesions, punch debridement, or wide excision of diseased tissue.28,29 There are no high quality clinical trials of surgical treatment of HS. Punch debridement can be performed using a 5- to 7-mm circular skin punch to deeply excise the inflamed follicle. Wide excision can be followed by wound closure with advancement flaps or split-thickness skin grafting. Wound closure by secondary intention is possible but requires many weeks or months of burdensome dressing changes to complete the healing process. Recurrence is common following surgical therapy and ranges from 30% with deroofing or laser treatment to 6% following wide excision and skin graft closure of the wound.30
Physical examination vital to early diagnosis
Delay in diagnosis of an active disease process has many causes, including nonperformance of a physical examination. In a web-based survey of physicians’ experiences with oversights related to the physical examination, 3 problems frequently reported were: nonperformance of any portion of the physical examination, failure to undress the patient to examine the skin, and failure to examine the abdomen and anogenital region in a patient with abdominal or pelvic pain.31 Oversights in the physical examination frequently caused a delay in diagnosis and treatment. With both LS and HS, patients may not recognize that they have a skin disease, or they may be embarrassed to show a clinician a skin change they have noticed. Early diagnosis and treatment are essential to achieving a good outcome and make a tremendous difference in the quality of life for the patient. Physical examination is a skill we have learned through diligent study and experience in practice. We can use these skills to greatly improve the lives of our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In recent decades the practice of medicine has drifted away from the performance of a physical examination during most patient encounters and evolved toward the more intensive use of history, imaging, and laboratory studies to guide management decisions. For example, it is common for a woman to present to an emergency department with abdominal or pelvic pain and undergo a computerized tomography scan before an abdominal and pelvic examination is performed. Some authorities believe that the trend to reduce the importance of the physical examination has gone way too far and resulted in a reduction in the quality of health care.1,2
Many skin diseases only can be diagnosed by having the patient disrobe and examining the skin. Gynecologists are uniquely positioned to diagnose important skin diseases because, while performing a reproductive health examination, they may be the first clinicians to directly examine the anogenital area and inner thighs. Skin diseases that are prevalent and can be diagnosed while performing an examination of the anogenital region include lichen sclerosus (LS) and hidradenitis suppurativa (HS). The prevalence of each of these conditions is in the range of 1% to 4% of women.3–5
Failure to examine the anogenital area and insufficient attention to the early signs of LS and HS may result in a long delay in the diagnosis.6 In 1 survey, of 517 patients with HS, there was a 7-year interval between the onset of the disease and the diagnosis by a clinician.7 Delay in diagnosis results in increased scarring, which makes it more difficult to effectively treat the disease. In this editorial, I will focus on the pathogenesis, diagnosis, and treatment of HS.
Diagnosis, presentation, and staging
Hidradenitis suppurativa (from the Greek, hidros means sweat and aden means glands) is a painful, chronic, relapsing, inflammatory skin disorder affecting the follicular unit. It is manifested by nodules, pustules, sinus tracts, and scars, usually in intertriginous areas. The diagnosis is made by history and physical examination. The 3 cardinal features of HS are 1) deep-seated nodules, comedones, and fibrosis; 2) typical anatomic location of the lesions in the axillae, inguinocrural, and anogenital regions, and 3) chronic relapsing course.8
Disease severity is often assessed using the Hurley staging system:
- stage I: abscess formation without sinus tracts or scarring (FIGURE)
- stage II: recurrent abscesses with tract formation and scarring, widely separated lesions
- stage III: diffuse or near-diffuse involvement or multiple interconnected tracts and abscesses.
In one report, stage I, II, and III disease was diagnosed in 65%, 31%, and 4% of cases, respectively, indicating that most HS is diagnosed instage I and suitable for treatment by a gynecologist.9

HS typically presents after puberty and women are more commonly affected than men. In one case series including 232 women with HS the regions most commonly affected were: axillae, inguinofemoral, urogenital, and buttocks in 79%, 77%, 51%, and 40% of cases, respectively.10 Risk factors for HS include obesity, cigarette smoking, tight fitting clothing, and chronic friction across the affected skin area.5
Pathogenesis
The pathophysiology of HS is thought to begin with occlusion of the follicle, resulting in follicle rupture deep in the dermis, thereby triggering inflammation, bacterial infection, and scarring. Dermal areas affected by HS have high concentrations of cytokines, including tumor necrosis factor (TNF)–alpha, interleukin (IL)-1-beta, IL-23, and IL-32.11,12 Once HS becomes an established process, it is difficult to treat because the dermal inflammatory process and scarring provides a microenvironment that facilitates disease progression. Hence early detection and treatment may result in optimal long-term outcomes.
Read about management of HS by stage
Treatment
Many recommended treatments for HS have not been formally tested in large randomized trials. A recent Cochrane review identified only 12 high-quality trials and the median number of participants was 27 per trial.13 Consequently, most treatment recommendations are based on expert opinion. Recommended treatments include smoking cessation, weight loss, topical and systemic antibiotics, antiandrogens, anti-inflammatory biologics (adalimumab and infliximab), and surgery. Smoking cessation and weight loss are strongly recommended in the initial treatment of HS. Bariatric surgery and significant postprocedure weight loss has been reported to cause a reduction in disease activity.14
Stage I management. For the initial treatment of stage I HS, clindamycin gel 1% applied twice daily to affected areas is recommended.15 Recommended oral antibiotic treatments include tetracycline 500 mg twice daily for 12 weeks16 or doxycycline 100 mg or 200 mg given daily for 10 weeks or clindamycin 300 mg twice daily plus rifampicin 600 mg once daily for 10 weeks.17,18 These antibiotics have both antimicrobial and anti-inflammatory effects.
Hormonal interventions that suppress androgen production or action may help reduce HS disease activity. For women with HS who also need contraception, an estrogen-progestin contraceptive may help reduce HS disease activity in up to 50% of individuals.19 The 5-alpha reductase inhibitor finasteride, at high doses (5 to 15 mg daily), has been reported to reduce HS disease activity.20,21 Finasteride is a teratogen, and the FDA strongly recommends against its use by women. Spironolactone, an anti-mineralocorticoid and antiandrogen, at a dose of 100 mg daily has been reported to reduce disease activity in about 50% of treated individuals and is FDA approved for use in women.22 Among reproductive-age women, spironolactone, which is a teratogen, only should be prescribed to women using an effective form of contraceptive. HS is often associated with obesity and insulin resistance. Metformin 500 mg three times daily has been reported to decrease disease activity.23,24
Stage II or III management. For Hurley stage II or III HS, referral to a dermatologist is warranted. There is evidence that too few people with HS are referred to a dermatologist.25 For severe HS resistant to oral medications, anti-TNF monoclonal antibody treatment with adalimumab (Humira) or infliximab (Remicade) is effective. Adalimumab is administered by subcutaneous injection and is US Food and Drug Administration (FDA)–approved to treat HS. Following a loading dose, adalimumab is administered weekly at a dose of 40 mg.26 Infliximab, which is not FDA approved to treat HS, is administered by intravenous infusion at a dose of 5 mg/kg at weeks 0, 2, and 6, and then every 8 weeks.27
Surgical management. HS is sometimes treated surgically with laser destruction of lesions, punch debridement, or wide excision of diseased tissue.28,29 There are no high quality clinical trials of surgical treatment of HS. Punch debridement can be performed using a 5- to 7-mm circular skin punch to deeply excise the inflamed follicle. Wide excision can be followed by wound closure with advancement flaps or split-thickness skin grafting. Wound closure by secondary intention is possible but requires many weeks or months of burdensome dressing changes to complete the healing process. Recurrence is common following surgical therapy and ranges from 30% with deroofing or laser treatment to 6% following wide excision and skin graft closure of the wound.30
Physical examination vital to early diagnosis
Delay in diagnosis of an active disease process has many causes, including nonperformance of a physical examination. In a web-based survey of physicians’ experiences with oversights related to the physical examination, 3 problems frequently reported were: nonperformance of any portion of the physical examination, failure to undress the patient to examine the skin, and failure to examine the abdomen and anogenital region in a patient with abdominal or pelvic pain.31 Oversights in the physical examination frequently caused a delay in diagnosis and treatment. With both LS and HS, patients may not recognize that they have a skin disease, or they may be embarrassed to show a clinician a skin change they have noticed. Early diagnosis and treatment are essential to achieving a good outcome and make a tremendous difference in the quality of life for the patient. Physical examination is a skill we have learned through diligent study and experience in practice. We can use these skills to greatly improve the lives of our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In recent decades the practice of medicine has drifted away from the performance of a physical examination during most patient encounters and evolved toward the more intensive use of history, imaging, and laboratory studies to guide management decisions. For example, it is common for a woman to present to an emergency department with abdominal or pelvic pain and undergo a computerized tomography scan before an abdominal and pelvic examination is performed. Some authorities believe that the trend to reduce the importance of the physical examination has gone way too far and resulted in a reduction in the quality of health care.1,2
Many skin diseases only can be diagnosed by having the patient disrobe and examining the skin. Gynecologists are uniquely positioned to diagnose important skin diseases because, while performing a reproductive health examination, they may be the first clinicians to directly examine the anogenital area and inner thighs. Skin diseases that are prevalent and can be diagnosed while performing an examination of the anogenital region include lichen sclerosus (LS) and hidradenitis suppurativa (HS). The prevalence of each of these conditions is in the range of 1% to 4% of women.3–5
Failure to examine the anogenital area and insufficient attention to the early signs of LS and HS may result in a long delay in the diagnosis.6 In 1 survey, of 517 patients with HS, there was a 7-year interval between the onset of the disease and the diagnosis by a clinician.7 Delay in diagnosis results in increased scarring, which makes it more difficult to effectively treat the disease. In this editorial, I will focus on the pathogenesis, diagnosis, and treatment of HS.
Diagnosis, presentation, and staging
Hidradenitis suppurativa (from the Greek, hidros means sweat and aden means glands) is a painful, chronic, relapsing, inflammatory skin disorder affecting the follicular unit. It is manifested by nodules, pustules, sinus tracts, and scars, usually in intertriginous areas. The diagnosis is made by history and physical examination. The 3 cardinal features of HS are 1) deep-seated nodules, comedones, and fibrosis; 2) typical anatomic location of the lesions in the axillae, inguinocrural, and anogenital regions, and 3) chronic relapsing course.8
Disease severity is often assessed using the Hurley staging system:
- stage I: abscess formation without sinus tracts or scarring (FIGURE)
- stage II: recurrent abscesses with tract formation and scarring, widely separated lesions
- stage III: diffuse or near-diffuse involvement or multiple interconnected tracts and abscesses.
In one report, stage I, II, and III disease was diagnosed in 65%, 31%, and 4% of cases, respectively, indicating that most HS is diagnosed instage I and suitable for treatment by a gynecologist.9

HS typically presents after puberty and women are more commonly affected than men. In one case series including 232 women with HS the regions most commonly affected were: axillae, inguinofemoral, urogenital, and buttocks in 79%, 77%, 51%, and 40% of cases, respectively.10 Risk factors for HS include obesity, cigarette smoking, tight fitting clothing, and chronic friction across the affected skin area.5
Pathogenesis
The pathophysiology of HS is thought to begin with occlusion of the follicle, resulting in follicle rupture deep in the dermis, thereby triggering inflammation, bacterial infection, and scarring. Dermal areas affected by HS have high concentrations of cytokines, including tumor necrosis factor (TNF)–alpha, interleukin (IL)-1-beta, IL-23, and IL-32.11,12 Once HS becomes an established process, it is difficult to treat because the dermal inflammatory process and scarring provides a microenvironment that facilitates disease progression. Hence early detection and treatment may result in optimal long-term outcomes.
Read about management of HS by stage
Treatment
Many recommended treatments for HS have not been formally tested in large randomized trials. A recent Cochrane review identified only 12 high-quality trials and the median number of participants was 27 per trial.13 Consequently, most treatment recommendations are based on expert opinion. Recommended treatments include smoking cessation, weight loss, topical and systemic antibiotics, antiandrogens, anti-inflammatory biologics (adalimumab and infliximab), and surgery. Smoking cessation and weight loss are strongly recommended in the initial treatment of HS. Bariatric surgery and significant postprocedure weight loss has been reported to cause a reduction in disease activity.14
Stage I management. For the initial treatment of stage I HS, clindamycin gel 1% applied twice daily to affected areas is recommended.15 Recommended oral antibiotic treatments include tetracycline 500 mg twice daily for 12 weeks16 or doxycycline 100 mg or 200 mg given daily for 10 weeks or clindamycin 300 mg twice daily plus rifampicin 600 mg once daily for 10 weeks.17,18 These antibiotics have both antimicrobial and anti-inflammatory effects.
Hormonal interventions that suppress androgen production or action may help reduce HS disease activity. For women with HS who also need contraception, an estrogen-progestin contraceptive may help reduce HS disease activity in up to 50% of individuals.19 The 5-alpha reductase inhibitor finasteride, at high doses (5 to 15 mg daily), has been reported to reduce HS disease activity.20,21 Finasteride is a teratogen, and the FDA strongly recommends against its use by women. Spironolactone, an anti-mineralocorticoid and antiandrogen, at a dose of 100 mg daily has been reported to reduce disease activity in about 50% of treated individuals and is FDA approved for use in women.22 Among reproductive-age women, spironolactone, which is a teratogen, only should be prescribed to women using an effective form of contraceptive. HS is often associated with obesity and insulin resistance. Metformin 500 mg three times daily has been reported to decrease disease activity.23,24
Stage II or III management. For Hurley stage II or III HS, referral to a dermatologist is warranted. There is evidence that too few people with HS are referred to a dermatologist.25 For severe HS resistant to oral medications, anti-TNF monoclonal antibody treatment with adalimumab (Humira) or infliximab (Remicade) is effective. Adalimumab is administered by subcutaneous injection and is US Food and Drug Administration (FDA)–approved to treat HS. Following a loading dose, adalimumab is administered weekly at a dose of 40 mg.26 Infliximab, which is not FDA approved to treat HS, is administered by intravenous infusion at a dose of 5 mg/kg at weeks 0, 2, and 6, and then every 8 weeks.27
Surgical management. HS is sometimes treated surgically with laser destruction of lesions, punch debridement, or wide excision of diseased tissue.28,29 There are no high quality clinical trials of surgical treatment of HS. Punch debridement can be performed using a 5- to 7-mm circular skin punch to deeply excise the inflamed follicle. Wide excision can be followed by wound closure with advancement flaps or split-thickness skin grafting. Wound closure by secondary intention is possible but requires many weeks or months of burdensome dressing changes to complete the healing process. Recurrence is common following surgical therapy and ranges from 30% with deroofing or laser treatment to 6% following wide excision and skin graft closure of the wound.30
Physical examination vital to early diagnosis
Delay in diagnosis of an active disease process has many causes, including nonperformance of a physical examination. In a web-based survey of physicians’ experiences with oversights related to the physical examination, 3 problems frequently reported were: nonperformance of any portion of the physical examination, failure to undress the patient to examine the skin, and failure to examine the abdomen and anogenital region in a patient with abdominal or pelvic pain.31 Oversights in the physical examination frequently caused a delay in diagnosis and treatment. With both LS and HS, patients may not recognize that they have a skin disease, or they may be embarrassed to show a clinician a skin change they have noticed. Early diagnosis and treatment are essential to achieving a good outcome and make a tremendous difference in the quality of life for the patient. Physical examination is a skill we have learned through diligent study and experience in practice. We can use these skills to greatly improve the lives of our patients.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Migraine Severity, Obesity Link Examined in Women
Associations of migraine severity and presence of associated features with inhibitory control varied by body mass index (BMI) in overweight/obese women with migraine, according to a recent study. These findings, therefore, warrant consideration of weight status in clarifying the role of migraine in executive functioning. Women (n=124) aged 18–50 years with overweight/obesity BMI=35.1 ± 6.4 kg/m2 and migraine completed a 28-day smartphone-based headache diary assessing migraine headache severity (attack frequency, pain intensity) and frequency of associated features (aura, photophobia, phonophobia, nausea). They then completed computerized measures of inhibitory control during an interictal (headache-free) period. Researchers found:
- Participants with higher migraine attack frequency performed worse on the Flanker test (accuracy and reaction time).
- Migraine attack frequency and pain intensity interacted with BMI to predict slower Stroop and/or Flanker Reaction Time (RT).
- More frequent photophobia, phonophobia, and aura were independently related to slower RT on the Stroop and/or Flanker tests, and BMI moderated the relationship between the occurrence of aura and Stroop RT.
The role of migraine headache severity, associated features and interactions with overweight/obesity in inhibitory control. Int J Neurosci. 2018;128(1):63-70. doi:10.1080/00207454.2017.1366474.
Associations of migraine severity and presence of associated features with inhibitory control varied by body mass index (BMI) in overweight/obese women with migraine, according to a recent study. These findings, therefore, warrant consideration of weight status in clarifying the role of migraine in executive functioning. Women (n=124) aged 18–50 years with overweight/obesity BMI=35.1 ± 6.4 kg/m2 and migraine completed a 28-day smartphone-based headache diary assessing migraine headache severity (attack frequency, pain intensity) and frequency of associated features (aura, photophobia, phonophobia, nausea). They then completed computerized measures of inhibitory control during an interictal (headache-free) period. Researchers found:
- Participants with higher migraine attack frequency performed worse on the Flanker test (accuracy and reaction time).
- Migraine attack frequency and pain intensity interacted with BMI to predict slower Stroop and/or Flanker Reaction Time (RT).
- More frequent photophobia, phonophobia, and aura were independently related to slower RT on the Stroop and/or Flanker tests, and BMI moderated the relationship between the occurrence of aura and Stroop RT.
The role of migraine headache severity, associated features and interactions with overweight/obesity in inhibitory control. Int J Neurosci. 2018;128(1):63-70. doi:10.1080/00207454.2017.1366474.
Associations of migraine severity and presence of associated features with inhibitory control varied by body mass index (BMI) in overweight/obese women with migraine, according to a recent study. These findings, therefore, warrant consideration of weight status in clarifying the role of migraine in executive functioning. Women (n=124) aged 18–50 years with overweight/obesity BMI=35.1 ± 6.4 kg/m2 and migraine completed a 28-day smartphone-based headache diary assessing migraine headache severity (attack frequency, pain intensity) and frequency of associated features (aura, photophobia, phonophobia, nausea). They then completed computerized measures of inhibitory control during an interictal (headache-free) period. Researchers found:
- Participants with higher migraine attack frequency performed worse on the Flanker test (accuracy and reaction time).
- Migraine attack frequency and pain intensity interacted with BMI to predict slower Stroop and/or Flanker Reaction Time (RT).
- More frequent photophobia, phonophobia, and aura were independently related to slower RT on the Stroop and/or Flanker tests, and BMI moderated the relationship between the occurrence of aura and Stroop RT.
The role of migraine headache severity, associated features and interactions with overweight/obesity in inhibitory control. Int J Neurosci. 2018;128(1):63-70. doi:10.1080/00207454.2017.1366474.
Carcinoma Erysipeloides of Papillary Serous Ovarian Cancer Mimicking Cellulitis of the Abdominal Wall
To the Editor:
A 40-year-old woman with a history of stage IIIC ovarian cancer presented with progressing abdominal erythema and pain of 1 month’s duration. She had been diagnosed 4 years prior with grade 3, poorly differentiated papillary serous carcinoma involving the bilateral ovaries, uterine tubes, uterus, and omentum with lymphovascular invasion. She underwent tumor resection and debulking followed by paclitaxel plus platinum-based chemotherapy. The cancer recurred 2 years later with carcinomatous ascites. She declined chemotherapy but underwent therapeutic paracentesis.
One month prior to presentation, the patient developed a small, tender, erythematous patch on the abdomen. Her primary physician started her on cephalexin for presumed cellulitis without improvement. The erythema continued to spread on the abdomen with worsening pain, which prompted her presentation to the emergency department. She was admitted and started on intravenous vancomycin.
On admission to the hospital, the patient was cachexic and afebrile with a white blood cell count of 10,400/µL (reference range, 4500–11,000/µL). Physical examination revealed a well-demarcated, 15×20-cm, erythematous, blanchable, indurated plaque in the periumbilical region (Figure 1). The plaque was tender to palpation with guarding but no increased warmth. Punch biopsies of the abdominal skin revealed carcinoma within the lymphatic channels in the deep dermis and dilated lymphatics throughout the overlying dermis (Figure 2). These findings were diagnostic for carcinoma erysipeloides. Tissue and blood cultures were negative for bacterial, fungal, or mycobacterial growth. Vancomycin was discontinued, and she was discharged with pain medication. She declined chemotherapy due to the potential side effects and elected to continue symptomatic management with palliative paracentesis. After she was discharged, she underwent a tunneled pleural catheterization for recurrent malignant pleural effusions.
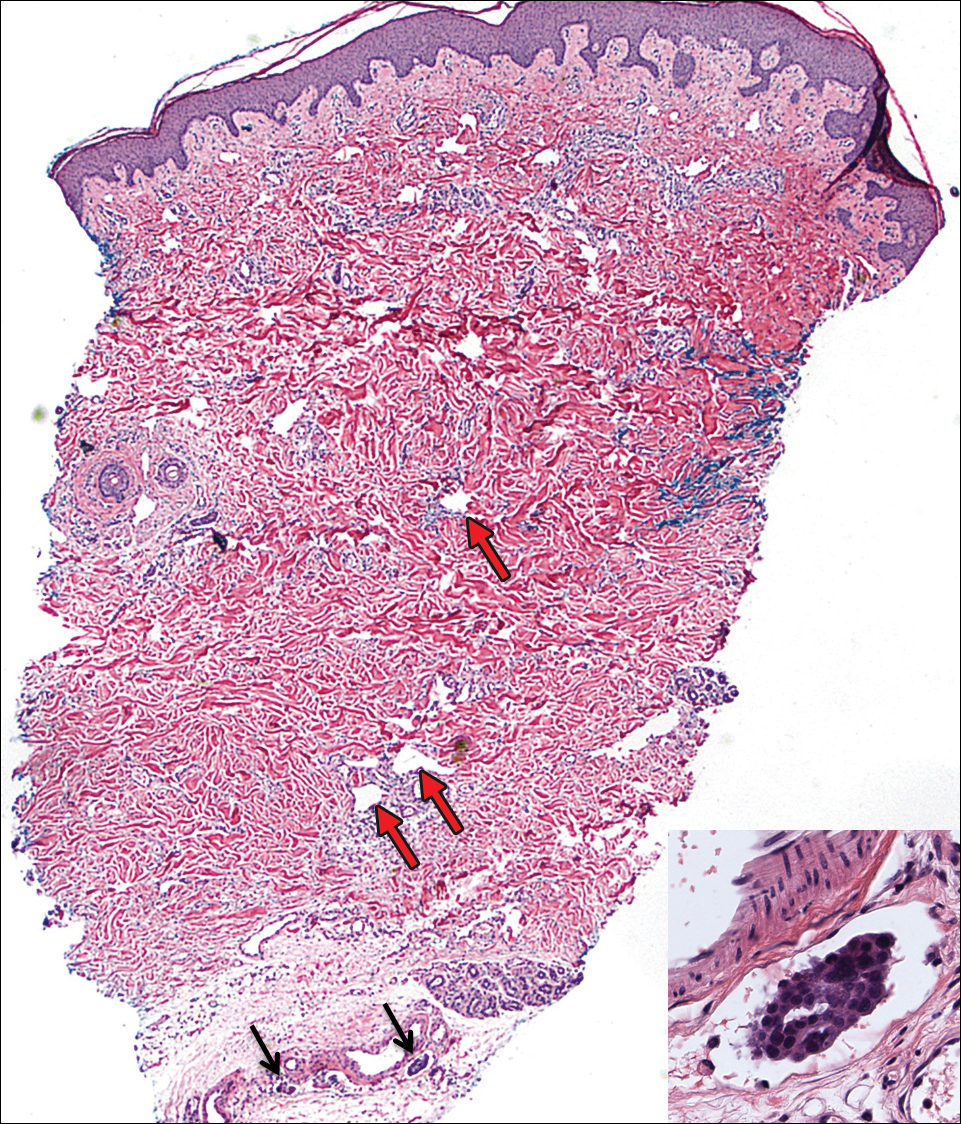
Carcinoma erysipeloides is a rare cutaneous metastasis secondary to internal malignancy that presents as well-demarcated areas of erythema and is sometimes misdiagnosed as cellulitis or erysipelas. Histology is notable for lymphovascular congestion without inflammation. Carcinoma erysipeloides most commonly is associated with breast cancer, but it also has been described in cancers of the prostate, larynx, stomach, lungs, thyroid, parotid gland, fallopian tubes, cervix, pancreas, and metastatic melanoma.1-5 While the pathogenesis of carcinoma erysipeloides is poorly understood, it is thought to occur by direct spread of tumor cells from the lymph nodes to the cutaneous lymphatics, causing obstruction and edema.
Ovarian cancer has the highest mortality of all gynecologic cancers and often is associated with delayed diagnosis. Cutaneous metastasis is a late manifestation often presenting as subcutaneous nodules.6,7 Carcinoma erysipeloides is an even rarer presentation of ovarian cancer, with a poor prognosis and a median survival of 18 months.8 A PubMed search of articles indexed for MEDLINE using the term carcinoma erysipeloides revealed 9 cases of carcinoma erysipeloides from ovarian cancer: 1 describing erythematous papules, plaques, and zosteriform vesicles on the upper thighs to the lower abdomen,9 and 8 describing erythematous plaques on the breasts.8,10 We report a case of carcinoma erysipeloides associated with stage IIIc ovarian cancer localized to the abdominal wall mimicking cellulitis. Our report reminds clinicians of this important diagnosis in ovarian cancer and of the importance of a skin biopsy to expedite a definitive diagnosis. Immunohistochemistry using ovarian tumor markers (eg, paired-box gene 8, cancer antigen 125) is an additional tool to accurately identify malignant cells in skin biopsy.8,10 Once diagnosed, primary treatment for carcinoma erysipeloides is treatment of the underlying malignancy.
- Cormio G, Capotorto M, Di Vagno G, et al. Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol Oncol. 2003;90:682-685.
- Kim MK, Kim SH, Lee YY, et al. Metastatic skin lesions on lower extremities in a patient with recurrent serous papillary ovarian carcinoma: a case report and literature review. Cancer Res Treat. 2012;44:142-145.
- Karmali S, Rudmik L, Temple W, et al. Melanoma erysipeloides. Can J Surg. 2005;48:159-160.
- Godinez-Puig V, Frangos J, Hollmann TJ, et al. Carcinoma erysipeloides of the breast in a patient with advanced ovarian carcinoma. Clin Infect Dis. 2012;54:575-576.
- Hazelrigg DE, Rudolph AH. Inflammatory metastic carcinoma. carcinoma erysipelatoides. Arch Dermatol. 1977;113:69-70.
- Cowan LJ, Roller JI, Connelly PJ, et al. Extraovarian stage IV peritoneal serous papillary carcinoma presenting as an asymptomatic skin lesion—a case report and literature review. Gynecol Oncol. 1995;57:433-435.
- Schonmann R, Altaras M, Biron T, et al. Inflammatory skin metastases from ovarian carcinoma—a case report and review of the literature. Gynecol Oncol. 2003;90:670-672.
- Klein RL, Brown AR, Gomez-Castro CM, et al. Ovarian cancer metastatic to the breast presenting as inflammatory breast cancer: a case report and literature review. J Cancer. 2010;1:27-31.
- Lee HC, Chu CY, Hsiao CH. Carcinoma erysipeloides from ovarian clear-cell carcinoma. J Clin Oncol. 2007;25:5828-5830.
- Godinez-Puig V, Frangos J, Hollmann TJ, et al. Photo quiz. rash in a patient with ovarian cancer. Clin Infect Dis. 2012;54:538, 575-576.
To the Editor:
A 40-year-old woman with a history of stage IIIC ovarian cancer presented with progressing abdominal erythema and pain of 1 month’s duration. She had been diagnosed 4 years prior with grade 3, poorly differentiated papillary serous carcinoma involving the bilateral ovaries, uterine tubes, uterus, and omentum with lymphovascular invasion. She underwent tumor resection and debulking followed by paclitaxel plus platinum-based chemotherapy. The cancer recurred 2 years later with carcinomatous ascites. She declined chemotherapy but underwent therapeutic paracentesis.
One month prior to presentation, the patient developed a small, tender, erythematous patch on the abdomen. Her primary physician started her on cephalexin for presumed cellulitis without improvement. The erythema continued to spread on the abdomen with worsening pain, which prompted her presentation to the emergency department. She was admitted and started on intravenous vancomycin.
On admission to the hospital, the patient was cachexic and afebrile with a white blood cell count of 10,400/µL (reference range, 4500–11,000/µL). Physical examination revealed a well-demarcated, 15×20-cm, erythematous, blanchable, indurated plaque in the periumbilical region (Figure 1). The plaque was tender to palpation with guarding but no increased warmth. Punch biopsies of the abdominal skin revealed carcinoma within the lymphatic channels in the deep dermis and dilated lymphatics throughout the overlying dermis (Figure 2). These findings were diagnostic for carcinoma erysipeloides. Tissue and blood cultures were negative for bacterial, fungal, or mycobacterial growth. Vancomycin was discontinued, and she was discharged with pain medication. She declined chemotherapy due to the potential side effects and elected to continue symptomatic management with palliative paracentesis. After she was discharged, she underwent a tunneled pleural catheterization for recurrent malignant pleural effusions.


Carcinoma erysipeloides is a rare cutaneous metastasis secondary to internal malignancy that presents as well-demarcated areas of erythema and is sometimes misdiagnosed as cellulitis or erysipelas. Histology is notable for lymphovascular congestion without inflammation. Carcinoma erysipeloides most commonly is associated with breast cancer, but it also has been described in cancers of the prostate, larynx, stomach, lungs, thyroid, parotid gland, fallopian tubes, cervix, pancreas, and metastatic melanoma.1-5 While the pathogenesis of carcinoma erysipeloides is poorly understood, it is thought to occur by direct spread of tumor cells from the lymph nodes to the cutaneous lymphatics, causing obstruction and edema.
Ovarian cancer has the highest mortality of all gynecologic cancers and often is associated with delayed diagnosis. Cutaneous metastasis is a late manifestation often presenting as subcutaneous nodules.6,7 Carcinoma erysipeloides is an even rarer presentation of ovarian cancer, with a poor prognosis and a median survival of 18 months.8 A PubMed search of articles indexed for MEDLINE using the term carcinoma erysipeloides revealed 9 cases of carcinoma erysipeloides from ovarian cancer: 1 describing erythematous papules, plaques, and zosteriform vesicles on the upper thighs to the lower abdomen,9 and 8 describing erythematous plaques on the breasts.8,10 We report a case of carcinoma erysipeloides associated with stage IIIc ovarian cancer localized to the abdominal wall mimicking cellulitis. Our report reminds clinicians of this important diagnosis in ovarian cancer and of the importance of a skin biopsy to expedite a definitive diagnosis. Immunohistochemistry using ovarian tumor markers (eg, paired-box gene 8, cancer antigen 125) is an additional tool to accurately identify malignant cells in skin biopsy.8,10 Once diagnosed, primary treatment for carcinoma erysipeloides is treatment of the underlying malignancy.
To the Editor:
A 40-year-old woman with a history of stage IIIC ovarian cancer presented with progressing abdominal erythema and pain of 1 month’s duration. She had been diagnosed 4 years prior with grade 3, poorly differentiated papillary serous carcinoma involving the bilateral ovaries, uterine tubes, uterus, and omentum with lymphovascular invasion. She underwent tumor resection and debulking followed by paclitaxel plus platinum-based chemotherapy. The cancer recurred 2 years later with carcinomatous ascites. She declined chemotherapy but underwent therapeutic paracentesis.
One month prior to presentation, the patient developed a small, tender, erythematous patch on the abdomen. Her primary physician started her on cephalexin for presumed cellulitis without improvement. The erythema continued to spread on the abdomen with worsening pain, which prompted her presentation to the emergency department. She was admitted and started on intravenous vancomycin.
On admission to the hospital, the patient was cachexic and afebrile with a white blood cell count of 10,400/µL (reference range, 4500–11,000/µL). Physical examination revealed a well-demarcated, 15×20-cm, erythematous, blanchable, indurated plaque in the periumbilical region (Figure 1). The plaque was tender to palpation with guarding but no increased warmth. Punch biopsies of the abdominal skin revealed carcinoma within the lymphatic channels in the deep dermis and dilated lymphatics throughout the overlying dermis (Figure 2). These findings were diagnostic for carcinoma erysipeloides. Tissue and blood cultures were negative for bacterial, fungal, or mycobacterial growth. Vancomycin was discontinued, and she was discharged with pain medication. She declined chemotherapy due to the potential side effects and elected to continue symptomatic management with palliative paracentesis. After she was discharged, she underwent a tunneled pleural catheterization for recurrent malignant pleural effusions.


Carcinoma erysipeloides is a rare cutaneous metastasis secondary to internal malignancy that presents as well-demarcated areas of erythema and is sometimes misdiagnosed as cellulitis or erysipelas. Histology is notable for lymphovascular congestion without inflammation. Carcinoma erysipeloides most commonly is associated with breast cancer, but it also has been described in cancers of the prostate, larynx, stomach, lungs, thyroid, parotid gland, fallopian tubes, cervix, pancreas, and metastatic melanoma.1-5 While the pathogenesis of carcinoma erysipeloides is poorly understood, it is thought to occur by direct spread of tumor cells from the lymph nodes to the cutaneous lymphatics, causing obstruction and edema.
Ovarian cancer has the highest mortality of all gynecologic cancers and often is associated with delayed diagnosis. Cutaneous metastasis is a late manifestation often presenting as subcutaneous nodules.6,7 Carcinoma erysipeloides is an even rarer presentation of ovarian cancer, with a poor prognosis and a median survival of 18 months.8 A PubMed search of articles indexed for MEDLINE using the term carcinoma erysipeloides revealed 9 cases of carcinoma erysipeloides from ovarian cancer: 1 describing erythematous papules, plaques, and zosteriform vesicles on the upper thighs to the lower abdomen,9 and 8 describing erythematous plaques on the breasts.8,10 We report a case of carcinoma erysipeloides associated with stage IIIc ovarian cancer localized to the abdominal wall mimicking cellulitis. Our report reminds clinicians of this important diagnosis in ovarian cancer and of the importance of a skin biopsy to expedite a definitive diagnosis. Immunohistochemistry using ovarian tumor markers (eg, paired-box gene 8, cancer antigen 125) is an additional tool to accurately identify malignant cells in skin biopsy.8,10 Once diagnosed, primary treatment for carcinoma erysipeloides is treatment of the underlying malignancy.
- Cormio G, Capotorto M, Di Vagno G, et al. Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol Oncol. 2003;90:682-685.
- Kim MK, Kim SH, Lee YY, et al. Metastatic skin lesions on lower extremities in a patient with recurrent serous papillary ovarian carcinoma: a case report and literature review. Cancer Res Treat. 2012;44:142-145.
- Karmali S, Rudmik L, Temple W, et al. Melanoma erysipeloides. Can J Surg. 2005;48:159-160.
- Godinez-Puig V, Frangos J, Hollmann TJ, et al. Carcinoma erysipeloides of the breast in a patient with advanced ovarian carcinoma. Clin Infect Dis. 2012;54:575-576.
- Hazelrigg DE, Rudolph AH. Inflammatory metastic carcinoma. carcinoma erysipelatoides. Arch Dermatol. 1977;113:69-70.
- Cowan LJ, Roller JI, Connelly PJ, et al. Extraovarian stage IV peritoneal serous papillary carcinoma presenting as an asymptomatic skin lesion—a case report and literature review. Gynecol Oncol. 1995;57:433-435.
- Schonmann R, Altaras M, Biron T, et al. Inflammatory skin metastases from ovarian carcinoma—a case report and review of the literature. Gynecol Oncol. 2003;90:670-672.
- Klein RL, Brown AR, Gomez-Castro CM, et al. Ovarian cancer metastatic to the breast presenting as inflammatory breast cancer: a case report and literature review. J Cancer. 2010;1:27-31.
- Lee HC, Chu CY, Hsiao CH. Carcinoma erysipeloides from ovarian clear-cell carcinoma. J Clin Oncol. 2007;25:5828-5830.
- Godinez-Puig V, Frangos J, Hollmann TJ, et al. Photo quiz. rash in a patient with ovarian cancer. Clin Infect Dis. 2012;54:538, 575-576.
- Cormio G, Capotorto M, Di Vagno G, et al. Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol Oncol. 2003;90:682-685.
- Kim MK, Kim SH, Lee YY, et al. Metastatic skin lesions on lower extremities in a patient with recurrent serous papillary ovarian carcinoma: a case report and literature review. Cancer Res Treat. 2012;44:142-145.
- Karmali S, Rudmik L, Temple W, et al. Melanoma erysipeloides. Can J Surg. 2005;48:159-160.
- Godinez-Puig V, Frangos J, Hollmann TJ, et al. Carcinoma erysipeloides of the breast in a patient with advanced ovarian carcinoma. Clin Infect Dis. 2012;54:575-576.
- Hazelrigg DE, Rudolph AH. Inflammatory metastic carcinoma. carcinoma erysipelatoides. Arch Dermatol. 1977;113:69-70.
- Cowan LJ, Roller JI, Connelly PJ, et al. Extraovarian stage IV peritoneal serous papillary carcinoma presenting as an asymptomatic skin lesion—a case report and literature review. Gynecol Oncol. 1995;57:433-435.
- Schonmann R, Altaras M, Biron T, et al. Inflammatory skin metastases from ovarian carcinoma—a case report and review of the literature. Gynecol Oncol. 2003;90:670-672.
- Klein RL, Brown AR, Gomez-Castro CM, et al. Ovarian cancer metastatic to the breast presenting as inflammatory breast cancer: a case report and literature review. J Cancer. 2010;1:27-31.
- Lee HC, Chu CY, Hsiao CH. Carcinoma erysipeloides from ovarian clear-cell carcinoma. J Clin Oncol. 2007;25:5828-5830.
- Godinez-Puig V, Frangos J, Hollmann TJ, et al. Photo quiz. rash in a patient with ovarian cancer. Clin Infect Dis. 2012;54:538, 575-576.
Practice Points
- Carcinoma erysipeloides is a rare cutaneous marker of metastatic ovarian cancer.
- Clinicians should be aware of carcinoma erysipeloides in ovarian cancer and maintain a low threshold for biopsy for accurate diagnosis and management planning.
Metastatic Melanoma and Prostatic Adenocarcinoma in the Same Sentinel Lymph Node
To the Editor:
Sentinel lymph node (SLN) biopsies routinely are performed to detect regional metastases in a variety of malignancies, including breast cancer, squamous cell carcinoma, Merkel cell carcinoma, and melanoma. Histologic examination of an SLN occasionally enables detection of other unsuspected underlying diseases that typically are inflammatory in nature. Although concomitant hematolymphoid malignancy, particularly chronic lymphocytic leukemia, has been reported in SLNs, collision of 2 different solid tumors in the same SLN is rare.1,2 We report a unique case documenting collision of both metastatic melanoma and prostatic adenocarcinoma detected in an SLN to raise awareness of the diagnostic challenges occurring in patients with coexisting malignancies.
A 71-year-old man with a history of metastatic prostatic adenocarcinoma to the bone presented for treatment of a melanoma that was newly diagnosed by an outside dermatologist. The patient’s medical history was notable for radical prostatectomy performed 15 years prior for treatment of a prostatic adenocarcinoma (Gleason score unknown) followed by bilateral orchiectomy performed 7 years later after his serum prostate-specific antigen (PSA) level began to rise, with no response to goserelin (a gonadotropin-releasing hormone agonist) therapy. Two years prior to the diagnosis of metastatic disease, his PSA level started to rise again and the patient received bicalutamide with little improvement, followed by 8 cycles of docetaxel. His PSA level improved and he most recently was being treated with abiraterone acetate. The patient’s latest computed tomography scan showed that the bony metastases secondary to prostatic adenocarcinoma had progressed. His serum PSA level was 105 ng/mL (reference range, <4.0 ng/mL) at the current presentation, elevated from 64 ng/mL one year prior.
Recently, the patient had noted a changing pigmented skin lesion on the left side of the flank. The patient described the lesion as a “black mole” first appearing 2 years prior, which had begun to ooze, change shape, and become darker and more nodular. A shave biopsy revealed a primary cutaneous malignant melanoma at least 3.4 mm in depth with ulceration and a mitotic rate of 15/mm2. No molecular studies were performed on the melanoma. Standard treatment via wide local excision and sentinel lymphadenectomy was planned.
Lymphoscintigraphy revealed 3 left draining axillary lymph nodes. The patient was treated with wide local excision and left axillary SLN biopsy. Five SLNs and 3 non-SLNs were excised. Per protocol, all SLNs were examined pathologically with serial sections: 2 hematoxylin and eosin–stained levels, S-100, and melan-A immunohistochemical stains. No residual melanoma was identified in the wide-excision specimen. Examination of the left axillary SLNs revealed metastatic melanoma in 3 of 5 SLNs. Two SLNs demonstrated total replacement by metastatic melanoma. A third SLN revealed a metastatic malignant neoplasm occupying 75% of the nodal area (Figure, A). S-100 and melan-A immunohistochemical staining were negative in this nodule but revealed small aggregates and isolated tumor cells distinct from this nodule that were diagnostic of micrometastatic melanoma (Figures, B and C). The tumor cells in the large nodule were histologically distinct from the melanoma and were instead composed of nests of epithelioid cells with clear cytoplasm (Figure, D). Upon further immunohistochemical staining, this tumor was strongly positive for AE1/AE3 keratin and PIN4 cocktail (cytokeratin 5, cytokeratin 15, p63, and p504s/alpha-methylacyl-CoA-racemase)(Figure, E) with focal positivity for PSA and prostatic acid phosphatase, diagnostic of metastatic adenocarcinoma of prostate origin.

A positron emission tomography scan performed a few days after the discovery of metastatic prostatic adenocarcinoma in the SLNs showed expected postoperative changes (eg, increased activity from procedure-related inflammation) in the left side of the flank and axilla as well as moderately hypermetabolic left supraclavicular lymph nodes suspicious for viable metastatic disease. Subsequent fine-needle aspiration of the aforementioned lymph nodes revealed metastatic prostatic adenocarcinoma. The preoperative lymphoscintigraphy at the time of SLN biopsy did not show drainage to the left supraclavicular nodal basin.
Based on a discussion of the patient’s case during a multidisciplinary tumor board consultation, the benefit of performing completion lymph node dissection for melanoma management did not outweigh the risks. Accordingly, the patient received adjuvant radiation therapy to the axillary nodal basin. He was started on ketoconazole and zoledronic acid therapy for metastatic prostate adenocarcinoma and was alive with disease at 6-month follow-up. The finding of both metastatic melanoma and prostate adenocarcinoma detected in an SLN after wide excision and SLN biopsy for cutaneous melanoma is a unique report of collision of these 2 tumors. Rare cases of collision between 2 solid tumors occurring in the same lymph node have involved prostate adenocarcinoma as one of the solid tumor components.1,3 Detection of tumor collision on lymph node biopsy between prostatic adenocarcinoma and urothelial carcinoma has been documented in 2 separate cases.1 Three additional cases of concurrent prostatic adenocarcinoma and colorectal adenocarcinoma identified on lymph node biopsy have been reported.1,3 Although never proven statistically, it is likely that these concurrent diagnoses are due to the high incidences of prostate and colorectal adenocarcinomas in the general US population; they are ranked first and third, respectively, for cancer incidence in US males.4
As demonstrated in the current case and the available literature, immunohistochemical stains play a vital role in the detection of tumor collision phenomena as well as identification of histologic source of the metastases. Furthermore, thorough histopathologic examination of biopsy specimens in the context of a patient’s clinical history remains paramount in obtaining an accurate diagnosis. Earlier identification of second malignancies in SLNs can alert the clinician to the presence of relapse of a known concurrent malignancy before it is clinically apparent, enhancing the possibility of more effective treatment of earlier disease. As has been demonstrated for lymphoma and melanoma, in rare cases awareness of the possibility of a second malignancy in the SLN can result in earlier initial diagnosis of undiscovered malignancy.2
- Sughayer MA, Zakarneh L, Abu-Shakra R. Collision metastasis of breast and ovarian adenocarcinoma in axillary lymph nodes: a case report and review of the literature. Pathol Oncol Res. 2009;15:423-427.
- Farma JM, Zager JS, Barnica-Elvir V, et al. A collision of diseases: chronic lymphocytic leukemia discovered during lymph node biopsy for melanoma. Ann Surg Oncol. 2013;20:1360-1364.
- Wade ZK, Shippey JE, Hamon GA, et al. Collision metastasis of prostatic and colonic adenocarcinoma: report of 2 cases. Arch Pathol Lab Med. 2004;128:318-320.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30.
To the Editor:
Sentinel lymph node (SLN) biopsies routinely are performed to detect regional metastases in a variety of malignancies, including breast cancer, squamous cell carcinoma, Merkel cell carcinoma, and melanoma. Histologic examination of an SLN occasionally enables detection of other unsuspected underlying diseases that typically are inflammatory in nature. Although concomitant hematolymphoid malignancy, particularly chronic lymphocytic leukemia, has been reported in SLNs, collision of 2 different solid tumors in the same SLN is rare.1,2 We report a unique case documenting collision of both metastatic melanoma and prostatic adenocarcinoma detected in an SLN to raise awareness of the diagnostic challenges occurring in patients with coexisting malignancies.
A 71-year-old man with a history of metastatic prostatic adenocarcinoma to the bone presented for treatment of a melanoma that was newly diagnosed by an outside dermatologist. The patient’s medical history was notable for radical prostatectomy performed 15 years prior for treatment of a prostatic adenocarcinoma (Gleason score unknown) followed by bilateral orchiectomy performed 7 years later after his serum prostate-specific antigen (PSA) level began to rise, with no response to goserelin (a gonadotropin-releasing hormone agonist) therapy. Two years prior to the diagnosis of metastatic disease, his PSA level started to rise again and the patient received bicalutamide with little improvement, followed by 8 cycles of docetaxel. His PSA level improved and he most recently was being treated with abiraterone acetate. The patient’s latest computed tomography scan showed that the bony metastases secondary to prostatic adenocarcinoma had progressed. His serum PSA level was 105 ng/mL (reference range, <4.0 ng/mL) at the current presentation, elevated from 64 ng/mL one year prior.
Recently, the patient had noted a changing pigmented skin lesion on the left side of the flank. The patient described the lesion as a “black mole” first appearing 2 years prior, which had begun to ooze, change shape, and become darker and more nodular. A shave biopsy revealed a primary cutaneous malignant melanoma at least 3.4 mm in depth with ulceration and a mitotic rate of 15/mm2. No molecular studies were performed on the melanoma. Standard treatment via wide local excision and sentinel lymphadenectomy was planned.
Lymphoscintigraphy revealed 3 left draining axillary lymph nodes. The patient was treated with wide local excision and left axillary SLN biopsy. Five SLNs and 3 non-SLNs were excised. Per protocol, all SLNs were examined pathologically with serial sections: 2 hematoxylin and eosin–stained levels, S-100, and melan-A immunohistochemical stains. No residual melanoma was identified in the wide-excision specimen. Examination of the left axillary SLNs revealed metastatic melanoma in 3 of 5 SLNs. Two SLNs demonstrated total replacement by metastatic melanoma. A third SLN revealed a metastatic malignant neoplasm occupying 75% of the nodal area (Figure, A). S-100 and melan-A immunohistochemical staining were negative in this nodule but revealed small aggregates and isolated tumor cells distinct from this nodule that were diagnostic of micrometastatic melanoma (Figures, B and C). The tumor cells in the large nodule were histologically distinct from the melanoma and were instead composed of nests of epithelioid cells with clear cytoplasm (Figure, D). Upon further immunohistochemical staining, this tumor was strongly positive for AE1/AE3 keratin and PIN4 cocktail (cytokeratin 5, cytokeratin 15, p63, and p504s/alpha-methylacyl-CoA-racemase)(Figure, E) with focal positivity for PSA and prostatic acid phosphatase, diagnostic of metastatic adenocarcinoma of prostate origin.

A positron emission tomography scan performed a few days after the discovery of metastatic prostatic adenocarcinoma in the SLNs showed expected postoperative changes (eg, increased activity from procedure-related inflammation) in the left side of the flank and axilla as well as moderately hypermetabolic left supraclavicular lymph nodes suspicious for viable metastatic disease. Subsequent fine-needle aspiration of the aforementioned lymph nodes revealed metastatic prostatic adenocarcinoma. The preoperative lymphoscintigraphy at the time of SLN biopsy did not show drainage to the left supraclavicular nodal basin.
Based on a discussion of the patient’s case during a multidisciplinary tumor board consultation, the benefit of performing completion lymph node dissection for melanoma management did not outweigh the risks. Accordingly, the patient received adjuvant radiation therapy to the axillary nodal basin. He was started on ketoconazole and zoledronic acid therapy for metastatic prostate adenocarcinoma and was alive with disease at 6-month follow-up. The finding of both metastatic melanoma and prostate adenocarcinoma detected in an SLN after wide excision and SLN biopsy for cutaneous melanoma is a unique report of collision of these 2 tumors. Rare cases of collision between 2 solid tumors occurring in the same lymph node have involved prostate adenocarcinoma as one of the solid tumor components.1,3 Detection of tumor collision on lymph node biopsy between prostatic adenocarcinoma and urothelial carcinoma has been documented in 2 separate cases.1 Three additional cases of concurrent prostatic adenocarcinoma and colorectal adenocarcinoma identified on lymph node biopsy have been reported.1,3 Although never proven statistically, it is likely that these concurrent diagnoses are due to the high incidences of prostate and colorectal adenocarcinomas in the general US population; they are ranked first and third, respectively, for cancer incidence in US males.4
As demonstrated in the current case and the available literature, immunohistochemical stains play a vital role in the detection of tumor collision phenomena as well as identification of histologic source of the metastases. Furthermore, thorough histopathologic examination of biopsy specimens in the context of a patient’s clinical history remains paramount in obtaining an accurate diagnosis. Earlier identification of second malignancies in SLNs can alert the clinician to the presence of relapse of a known concurrent malignancy before it is clinically apparent, enhancing the possibility of more effective treatment of earlier disease. As has been demonstrated for lymphoma and melanoma, in rare cases awareness of the possibility of a second malignancy in the SLN can result in earlier initial diagnosis of undiscovered malignancy.2
To the Editor:
Sentinel lymph node (SLN) biopsies routinely are performed to detect regional metastases in a variety of malignancies, including breast cancer, squamous cell carcinoma, Merkel cell carcinoma, and melanoma. Histologic examination of an SLN occasionally enables detection of other unsuspected underlying diseases that typically are inflammatory in nature. Although concomitant hematolymphoid malignancy, particularly chronic lymphocytic leukemia, has been reported in SLNs, collision of 2 different solid tumors in the same SLN is rare.1,2 We report a unique case documenting collision of both metastatic melanoma and prostatic adenocarcinoma detected in an SLN to raise awareness of the diagnostic challenges occurring in patients with coexisting malignancies.
A 71-year-old man with a history of metastatic prostatic adenocarcinoma to the bone presented for treatment of a melanoma that was newly diagnosed by an outside dermatologist. The patient’s medical history was notable for radical prostatectomy performed 15 years prior for treatment of a prostatic adenocarcinoma (Gleason score unknown) followed by bilateral orchiectomy performed 7 years later after his serum prostate-specific antigen (PSA) level began to rise, with no response to goserelin (a gonadotropin-releasing hormone agonist) therapy. Two years prior to the diagnosis of metastatic disease, his PSA level started to rise again and the patient received bicalutamide with little improvement, followed by 8 cycles of docetaxel. His PSA level improved and he most recently was being treated with abiraterone acetate. The patient’s latest computed tomography scan showed that the bony metastases secondary to prostatic adenocarcinoma had progressed. His serum PSA level was 105 ng/mL (reference range, <4.0 ng/mL) at the current presentation, elevated from 64 ng/mL one year prior.
Recently, the patient had noted a changing pigmented skin lesion on the left side of the flank. The patient described the lesion as a “black mole” first appearing 2 years prior, which had begun to ooze, change shape, and become darker and more nodular. A shave biopsy revealed a primary cutaneous malignant melanoma at least 3.4 mm in depth with ulceration and a mitotic rate of 15/mm2. No molecular studies were performed on the melanoma. Standard treatment via wide local excision and sentinel lymphadenectomy was planned.
Lymphoscintigraphy revealed 3 left draining axillary lymph nodes. The patient was treated with wide local excision and left axillary SLN biopsy. Five SLNs and 3 non-SLNs were excised. Per protocol, all SLNs were examined pathologically with serial sections: 2 hematoxylin and eosin–stained levels, S-100, and melan-A immunohistochemical stains. No residual melanoma was identified in the wide-excision specimen. Examination of the left axillary SLNs revealed metastatic melanoma in 3 of 5 SLNs. Two SLNs demonstrated total replacement by metastatic melanoma. A third SLN revealed a metastatic malignant neoplasm occupying 75% of the nodal area (Figure, A). S-100 and melan-A immunohistochemical staining were negative in this nodule but revealed small aggregates and isolated tumor cells distinct from this nodule that were diagnostic of micrometastatic melanoma (Figures, B and C). The tumor cells in the large nodule were histologically distinct from the melanoma and were instead composed of nests of epithelioid cells with clear cytoplasm (Figure, D). Upon further immunohistochemical staining, this tumor was strongly positive for AE1/AE3 keratin and PIN4 cocktail (cytokeratin 5, cytokeratin 15, p63, and p504s/alpha-methylacyl-CoA-racemase)(Figure, E) with focal positivity for PSA and prostatic acid phosphatase, diagnostic of metastatic adenocarcinoma of prostate origin.

A positron emission tomography scan performed a few days after the discovery of metastatic prostatic adenocarcinoma in the SLNs showed expected postoperative changes (eg, increased activity from procedure-related inflammation) in the left side of the flank and axilla as well as moderately hypermetabolic left supraclavicular lymph nodes suspicious for viable metastatic disease. Subsequent fine-needle aspiration of the aforementioned lymph nodes revealed metastatic prostatic adenocarcinoma. The preoperative lymphoscintigraphy at the time of SLN biopsy did not show drainage to the left supraclavicular nodal basin.
Based on a discussion of the patient’s case during a multidisciplinary tumor board consultation, the benefit of performing completion lymph node dissection for melanoma management did not outweigh the risks. Accordingly, the patient received adjuvant radiation therapy to the axillary nodal basin. He was started on ketoconazole and zoledronic acid therapy for metastatic prostate adenocarcinoma and was alive with disease at 6-month follow-up. The finding of both metastatic melanoma and prostate adenocarcinoma detected in an SLN after wide excision and SLN biopsy for cutaneous melanoma is a unique report of collision of these 2 tumors. Rare cases of collision between 2 solid tumors occurring in the same lymph node have involved prostate adenocarcinoma as one of the solid tumor components.1,3 Detection of tumor collision on lymph node biopsy between prostatic adenocarcinoma and urothelial carcinoma has been documented in 2 separate cases.1 Three additional cases of concurrent prostatic adenocarcinoma and colorectal adenocarcinoma identified on lymph node biopsy have been reported.1,3 Although never proven statistically, it is likely that these concurrent diagnoses are due to the high incidences of prostate and colorectal adenocarcinomas in the general US population; they are ranked first and third, respectively, for cancer incidence in US males.4
As demonstrated in the current case and the available literature, immunohistochemical stains play a vital role in the detection of tumor collision phenomena as well as identification of histologic source of the metastases. Furthermore, thorough histopathologic examination of biopsy specimens in the context of a patient’s clinical history remains paramount in obtaining an accurate diagnosis. Earlier identification of second malignancies in SLNs can alert the clinician to the presence of relapse of a known concurrent malignancy before it is clinically apparent, enhancing the possibility of more effective treatment of earlier disease. As has been demonstrated for lymphoma and melanoma, in rare cases awareness of the possibility of a second malignancy in the SLN can result in earlier initial diagnosis of undiscovered malignancy.2
- Sughayer MA, Zakarneh L, Abu-Shakra R. Collision metastasis of breast and ovarian adenocarcinoma in axillary lymph nodes: a case report and review of the literature. Pathol Oncol Res. 2009;15:423-427.
- Farma JM, Zager JS, Barnica-Elvir V, et al. A collision of diseases: chronic lymphocytic leukemia discovered during lymph node biopsy for melanoma. Ann Surg Oncol. 2013;20:1360-1364.
- Wade ZK, Shippey JE, Hamon GA, et al. Collision metastasis of prostatic and colonic adenocarcinoma: report of 2 cases. Arch Pathol Lab Med. 2004;128:318-320.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30.
- Sughayer MA, Zakarneh L, Abu-Shakra R. Collision metastasis of breast and ovarian adenocarcinoma in axillary lymph nodes: a case report and review of the literature. Pathol Oncol Res. 2009;15:423-427.
- Farma JM, Zager JS, Barnica-Elvir V, et al. A collision of diseases: chronic lymphocytic leukemia discovered during lymph node biopsy for melanoma. Ann Surg Oncol. 2013;20:1360-1364.
- Wade ZK, Shippey JE, Hamon GA, et al. Collision metastasis of prostatic and colonic adenocarcinoma: report of 2 cases. Arch Pathol Lab Med. 2004;128:318-320.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30.
Practice Points
- Immunohistochemical stains play a vital role in the detection of tumor collision phenomena as well as identification of histologic sources of metastases.
- Thorough histopathologic examination of biopsy specimens in the context of a patient’s clinical history remains paramount in obtaining an accurate diagnosis, enhancing the possibility of more effective treatment of earlier disease.
Updates on health and care utilization by TGNC youth
As we providers begin to gain a better understanding of the complexities of gender identity and expression, studies examining the health of transgender and gender-nonconforming (TGNC) youth are emerging. Multiple studies have demonstrated the mental health disparities that TGNC youth face, but more studies examining other health risks and disparities are needed.
Prevalence of TGNC students higher than expected
Previous studies looking at prevalence rates of TGNC youth often dichotomized gender identities into binary (masculine or feminine) groups and were not inclusive of nonbinary and questioning identities. This may have led to underestimation of the size of this population.2,3 This study assessed for TGNC identities by asking, “Do you consider yourself transgender, genderqueer, gender fluid, or unsure about your gender identity?” Given the prevalence of TGNC identities in this sample, it is likely that TGNC youth will be encountered in general pediatric practice. As such, it is important that we as providers continue to build our competency in working with this population.
Statistically significant differences in health status were identified
Almost two-thirds (62%) of TGNC youth identified their health as poor, fair, or good as opposed to very good or excellent, compared with one-third (33.1%) of cisgender youth. Over half (52%) of TGNC youth reported staying home from school because of illness at least once in the past month, compared with 43% of cisgender youth. About 60% of TGNC youth reported a preventive medical check-up in the past year, compared with 65% of cisgender youth. In terms of long-term health problems, TGNC youth reported higher rates of long-term physical (25% vs. 15%) and mental health (59% vs. 17%) problems than did their cisgender peers.
Role of perceived gender expression
A unique aspect of this study was that it sought to examine the effect of perceived gender expression (the way others interpret a person’s gender presentation; their appearance, style, dress, or the way they walk or talk) on health status and care utilization. Categories of perceived gender expression included very or mostly feminine, somewhat feminine, equally feminine and masculine, somewhat masculine, or very or mostly masculine. The prevalence of TGNC adolescents with an equally feminine and masculine gender expression was highest for both those assigned male (29%) and assigned female (41%) at birth, compared with other perceived gender presentations.
TGNC youth who were perceived to have a gender expression that was incongruent with the sex assigned at birth were at higher risk of reporting poor health status. For example, in TGNC participants who were assigned male at birth, those perceived as equally feminine and masculine (49%) or somewhat masculine (58%) were significantly more likely to report having poorer general health than those with a very masculine perceived gender expression (32%).
Suggestions for providers
The authors of the study and the accompanying commentary by Daniel Shumer, MD, MPH, suggest that there are things we as health care providers can do to address these barriers.
- Recognize that health disparities exist in this population. Individuals perceived as gender nonconforming may be vulnerable to discrimination and have difficulty accessing and receiving heath care, compared with their cisgender peers.
- Screen for health risks and identify barriers to care for TGNC youth while promoting and bolstering wellness within this community.
- Continue to promote access to gender affirming care. Data suggest that children who receive gender affirming care achieve mental health status similar to that of their cisgender peers.3,4,5
- Continue to develop an understanding of how youth understand and express gender.
- Nonbinary youth face unique barriers when accessing health affirming services because of fears that their gender identity may be misunderstood. These barriers lead to delays in seeking health care services, which may lead to poorer outcomes. As providers, educating ourselves about these diverse identities and being respectful of all patients’ identities can help reduce these barriers.
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at Ohio State University, both in Columbus. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Pediatrics. 2018 Feb 5. doi: 10.1542/peds.2017-1683.
2. J Adolesc Health. 2017 Oct;61(4):521-6.
3. Pediatrics. 2018. doi: 10.1542/peds.2017-4079.
4. Pediatrics. 2014 Oct;134(4):696-704.
5. Pediatrics. 2016 Mar;137(3):e20153223.
As we providers begin to gain a better understanding of the complexities of gender identity and expression, studies examining the health of transgender and gender-nonconforming (TGNC) youth are emerging. Multiple studies have demonstrated the mental health disparities that TGNC youth face, but more studies examining other health risks and disparities are needed.
Prevalence of TGNC students higher than expected
Previous studies looking at prevalence rates of TGNC youth often dichotomized gender identities into binary (masculine or feminine) groups and were not inclusive of nonbinary and questioning identities. This may have led to underestimation of the size of this population.2,3 This study assessed for TGNC identities by asking, “Do you consider yourself transgender, genderqueer, gender fluid, or unsure about your gender identity?” Given the prevalence of TGNC identities in this sample, it is likely that TGNC youth will be encountered in general pediatric practice. As such, it is important that we as providers continue to build our competency in working with this population.
Statistically significant differences in health status were identified
Almost two-thirds (62%) of TGNC youth identified their health as poor, fair, or good as opposed to very good or excellent, compared with one-third (33.1%) of cisgender youth. Over half (52%) of TGNC youth reported staying home from school because of illness at least once in the past month, compared with 43% of cisgender youth. About 60% of TGNC youth reported a preventive medical check-up in the past year, compared with 65% of cisgender youth. In terms of long-term health problems, TGNC youth reported higher rates of long-term physical (25% vs. 15%) and mental health (59% vs. 17%) problems than did their cisgender peers.
Role of perceived gender expression
A unique aspect of this study was that it sought to examine the effect of perceived gender expression (the way others interpret a person’s gender presentation; their appearance, style, dress, or the way they walk or talk) on health status and care utilization. Categories of perceived gender expression included very or mostly feminine, somewhat feminine, equally feminine and masculine, somewhat masculine, or very or mostly masculine. The prevalence of TGNC adolescents with an equally feminine and masculine gender expression was highest for both those assigned male (29%) and assigned female (41%) at birth, compared with other perceived gender presentations.
TGNC youth who were perceived to have a gender expression that was incongruent with the sex assigned at birth were at higher risk of reporting poor health status. For example, in TGNC participants who were assigned male at birth, those perceived as equally feminine and masculine (49%) or somewhat masculine (58%) were significantly more likely to report having poorer general health than those with a very masculine perceived gender expression (32%).
Suggestions for providers
The authors of the study and the accompanying commentary by Daniel Shumer, MD, MPH, suggest that there are things we as health care providers can do to address these barriers.
- Recognize that health disparities exist in this population. Individuals perceived as gender nonconforming may be vulnerable to discrimination and have difficulty accessing and receiving heath care, compared with their cisgender peers.
- Screen for health risks and identify barriers to care for TGNC youth while promoting and bolstering wellness within this community.
- Continue to promote access to gender affirming care. Data suggest that children who receive gender affirming care achieve mental health status similar to that of their cisgender peers.3,4,5
- Continue to develop an understanding of how youth understand and express gender.
- Nonbinary youth face unique barriers when accessing health affirming services because of fears that their gender identity may be misunderstood. These barriers lead to delays in seeking health care services, which may lead to poorer outcomes. As providers, educating ourselves about these diverse identities and being respectful of all patients’ identities can help reduce these barriers.
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at Ohio State University, both in Columbus. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Pediatrics. 2018 Feb 5. doi: 10.1542/peds.2017-1683.
2. J Adolesc Health. 2017 Oct;61(4):521-6.
3. Pediatrics. 2018. doi: 10.1542/peds.2017-4079.
4. Pediatrics. 2014 Oct;134(4):696-704.
5. Pediatrics. 2016 Mar;137(3):e20153223.
As we providers begin to gain a better understanding of the complexities of gender identity and expression, studies examining the health of transgender and gender-nonconforming (TGNC) youth are emerging. Multiple studies have demonstrated the mental health disparities that TGNC youth face, but more studies examining other health risks and disparities are needed.
Prevalence of TGNC students higher than expected
Previous studies looking at prevalence rates of TGNC youth often dichotomized gender identities into binary (masculine or feminine) groups and were not inclusive of nonbinary and questioning identities. This may have led to underestimation of the size of this population.2,3 This study assessed for TGNC identities by asking, “Do you consider yourself transgender, genderqueer, gender fluid, or unsure about your gender identity?” Given the prevalence of TGNC identities in this sample, it is likely that TGNC youth will be encountered in general pediatric practice. As such, it is important that we as providers continue to build our competency in working with this population.
Statistically significant differences in health status were identified
Almost two-thirds (62%) of TGNC youth identified their health as poor, fair, or good as opposed to very good or excellent, compared with one-third (33.1%) of cisgender youth. Over half (52%) of TGNC youth reported staying home from school because of illness at least once in the past month, compared with 43% of cisgender youth. About 60% of TGNC youth reported a preventive medical check-up in the past year, compared with 65% of cisgender youth. In terms of long-term health problems, TGNC youth reported higher rates of long-term physical (25% vs. 15%) and mental health (59% vs. 17%) problems than did their cisgender peers.
Role of perceived gender expression
A unique aspect of this study was that it sought to examine the effect of perceived gender expression (the way others interpret a person’s gender presentation; their appearance, style, dress, or the way they walk or talk) on health status and care utilization. Categories of perceived gender expression included very or mostly feminine, somewhat feminine, equally feminine and masculine, somewhat masculine, or very or mostly masculine. The prevalence of TGNC adolescents with an equally feminine and masculine gender expression was highest for both those assigned male (29%) and assigned female (41%) at birth, compared with other perceived gender presentations.
TGNC youth who were perceived to have a gender expression that was incongruent with the sex assigned at birth were at higher risk of reporting poor health status. For example, in TGNC participants who were assigned male at birth, those perceived as equally feminine and masculine (49%) or somewhat masculine (58%) were significantly more likely to report having poorer general health than those with a very masculine perceived gender expression (32%).
Suggestions for providers
The authors of the study and the accompanying commentary by Daniel Shumer, MD, MPH, suggest that there are things we as health care providers can do to address these barriers.
- Recognize that health disparities exist in this population. Individuals perceived as gender nonconforming may be vulnerable to discrimination and have difficulty accessing and receiving heath care, compared with their cisgender peers.
- Screen for health risks and identify barriers to care for TGNC youth while promoting and bolstering wellness within this community.
- Continue to promote access to gender affirming care. Data suggest that children who receive gender affirming care achieve mental health status similar to that of their cisgender peers.3,4,5
- Continue to develop an understanding of how youth understand and express gender.
- Nonbinary youth face unique barriers when accessing health affirming services because of fears that their gender identity may be misunderstood. These barriers lead to delays in seeking health care services, which may lead to poorer outcomes. As providers, educating ourselves about these diverse identities and being respectful of all patients’ identities can help reduce these barriers.
Dr. Chelvakumar is an attending physician in the division of adolescent medicine at Nationwide Children’s Hospital and an assistant professor of clinical pediatrics at Ohio State University, both in Columbus. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Pediatrics. 2018 Feb 5. doi: 10.1542/peds.2017-1683.
2. J Adolesc Health. 2017 Oct;61(4):521-6.
3. Pediatrics. 2018. doi: 10.1542/peds.2017-4079.
4. Pediatrics. 2014 Oct;134(4):696-704.
5. Pediatrics. 2016 Mar;137(3):e20153223.