User login
Phase 2 study tests low-dose maintenance therapy for ALL
Researchers at MD Anderson Cancer Center in Houston are studying the safety and clinical effectiveness of low-dose inotuzumab ozogamicin in controlling acute lymphocytic leukemia (ALL).
The phase 2 study (NCT03441061), which was launched on Feb. 15, will include up to 40 adult patients with B-cell ALL who are in complete remission with molecular failure or molecular relapse at any point after 3 months of frontline therapy. The study is looking first at relapse-free survival, and secondarily at overall survival and minimal residual disease negativity rate overall and after the first cycle. In addition, the researchers will consider the safety of the drug in this setting.
Inotuzumab ozogamicin (Besponsa) was approved by the Food and Drug Administration in August 2017 for the treatment of adults with relapsed or refractory B-cell precursor ALL.
During the open-label, single-arm study, patients can receive up to six cycles of the drug and each cycle is 28 (plus or minus 7) days. Eligible patients will receive inotuzumab ozogamicin on days 1,8, and 15 of cycle 1 and days 1 and 8 of cycles 2-6.
If a patient chooses to undergo a stem cell transplant from a donor, that patient will receive only two to three cycles of the drug. Study participants may also be taken off treatment after cycle 2 if the disease has had no response.
The study is being conducted at MD Anderson Cancer Center and is expected to be completed in February 2023. The study is sponsored by Pfizer.
Researchers at MD Anderson Cancer Center in Houston are studying the safety and clinical effectiveness of low-dose inotuzumab ozogamicin in controlling acute lymphocytic leukemia (ALL).
The phase 2 study (NCT03441061), which was launched on Feb. 15, will include up to 40 adult patients with B-cell ALL who are in complete remission with molecular failure or molecular relapse at any point after 3 months of frontline therapy. The study is looking first at relapse-free survival, and secondarily at overall survival and minimal residual disease negativity rate overall and after the first cycle. In addition, the researchers will consider the safety of the drug in this setting.
Inotuzumab ozogamicin (Besponsa) was approved by the Food and Drug Administration in August 2017 for the treatment of adults with relapsed or refractory B-cell precursor ALL.
During the open-label, single-arm study, patients can receive up to six cycles of the drug and each cycle is 28 (plus or minus 7) days. Eligible patients will receive inotuzumab ozogamicin on days 1,8, and 15 of cycle 1 and days 1 and 8 of cycles 2-6.
If a patient chooses to undergo a stem cell transplant from a donor, that patient will receive only two to three cycles of the drug. Study participants may also be taken off treatment after cycle 2 if the disease has had no response.
The study is being conducted at MD Anderson Cancer Center and is expected to be completed in February 2023. The study is sponsored by Pfizer.
Researchers at MD Anderson Cancer Center in Houston are studying the safety and clinical effectiveness of low-dose inotuzumab ozogamicin in controlling acute lymphocytic leukemia (ALL).
The phase 2 study (NCT03441061), which was launched on Feb. 15, will include up to 40 adult patients with B-cell ALL who are in complete remission with molecular failure or molecular relapse at any point after 3 months of frontline therapy. The study is looking first at relapse-free survival, and secondarily at overall survival and minimal residual disease negativity rate overall and after the first cycle. In addition, the researchers will consider the safety of the drug in this setting.
Inotuzumab ozogamicin (Besponsa) was approved by the Food and Drug Administration in August 2017 for the treatment of adults with relapsed or refractory B-cell precursor ALL.
During the open-label, single-arm study, patients can receive up to six cycles of the drug and each cycle is 28 (plus or minus 7) days. Eligible patients will receive inotuzumab ozogamicin on days 1,8, and 15 of cycle 1 and days 1 and 8 of cycles 2-6.
If a patient chooses to undergo a stem cell transplant from a donor, that patient will receive only two to three cycles of the drug. Study participants may also be taken off treatment after cycle 2 if the disease has had no response.
The study is being conducted at MD Anderson Cancer Center and is expected to be completed in February 2023. The study is sponsored by Pfizer.
FROM CLINICALTRIALS.GOV
NASH rapidly overtaking hepatitis C as cause of liver cancer
Researchers reported on their analysis of past prevalence of HCV, NASH, and alcoholic cirrhosis and prediction of future trends and their effect on hepatocellular carcinoma in the Feb. 24 online edition of the Journal of Clinical and Experimental Hepatology.
The analysis, based on data from the National Health and Nutrition Examination Survey and the Organ Procurement and Transplantation Network, shows that the prevalence of HCV has been in steady decline since 2005 and that decline is forecast to continue. From a prevalence of 3.22 million cases in 2005, researchers have forecasted a decline to 1.06 million cases by 2025.
At the same time, even a conservative linear model for the changing prevalence of NASH forecast a rapid increase from 1.37 million cases in 2005 to 17.95 million in 2025. The exponential model suggested an increase from 2.41 million in 2005 to 42.34 million in 2025.
In terms of the effect on the prevalence of hepatocellular carcinoma (HCC), the modeling suggested cases of HCV-related liver cancer were predicted to peak at around 29,000 cases in 2015 then decline to fewer than 18,000 cases by 2025. In contrast, the prevalence of HCC from NASH is forecast to increase from between 5000 and 6000 cases in 2005 to 45,000 in 2025 by the conservative linear model or even as high as 106,000 cases according to the exponential model. It overtook HCV infection as a cause of liver cancer by around 2015.
“Despite the lack of existing data off of which to work, the general trends of our prediction models are consistent with the documented trends of liver transplant etiology, as well as 2010 insurance data indicating nonalcoholic fatty liver disease/NASH as the leading etiology associated with HCC,” wrote Osmanuddin Ahmed, MD, from the Rush University Medical Center in Chicago and his coauthors.
The study used liver transplant data as a proxy for the prevalence of hepatocellular carcinoma and also took into account the natural history of the disease. Between 5% and 20% of untreated HCV infections will go on to develop into cirrhosis, and of patients with HCV-related cirrhosis, around 15% will develop HCC within 10 years. In the case of NASH, the authors cited research suggesting that around 35% of patients go on to develop progressive fibrosis, that progression to cirrhosis takes around 29 years, and that the risk of progression to HCC ranged from 2.4% over 7 years to 12.8% over 3 years.
“A higher proportion of patients with NASH develop cirrhosis, but of those who develop cirrhosis, the probability of developing HCC is higher in patients with HCV,” the authors wrote. “In contrast, HCV progression to HCC rarely occurs in noncirrhotic patients.”
The authors wrote that it was important to explore projected trends in the etiology of hepatocellular carcinoma to inform the development of screening, diagnostic, and treatment approaches, particularly given potential differences in the pathology, natural history, and treatment options for NASH-related and HCV-related liver cancer.
“Histologically, NASH shares characteristics with alcoholic liver disease, primarily proinflammatory fat accumulation in parenchymal cells, [and] key players in NASH progression to HCC are suggested to include genetic modifications, proinflammatory high-fat and/or high-fructose diets, and oxidative and endoplasmic cellular stresses,” they wrote. “In HCV progression to HCC, the presence of the HCV core protein may induce HCC without the prerequisite load of genetic errors normally required for cancer development, skipping or accelerating some of the classic steps of cancer induction.”
The authors did note that their model represented a base scenario that assumed the environmental and genetic factors driving NASH would continue along the path of current trends.
“Therefore, the possibility exists that our models underestimate the response of the medical community in addressing the rising nonalcoholic fatty liver disease/NASH epidemic.”
No funding sources or conflicts of interest were declared.
SOURCE: Ahmed O et al. J Clin Exp Hepatology. 2018 Feb 24. doi: 10.1016/j.jceh.2018.02.006.
Researchers reported on their analysis of past prevalence of HCV, NASH, and alcoholic cirrhosis and prediction of future trends and their effect on hepatocellular carcinoma in the Feb. 24 online edition of the Journal of Clinical and Experimental Hepatology.
The analysis, based on data from the National Health and Nutrition Examination Survey and the Organ Procurement and Transplantation Network, shows that the prevalence of HCV has been in steady decline since 2005 and that decline is forecast to continue. From a prevalence of 3.22 million cases in 2005, researchers have forecasted a decline to 1.06 million cases by 2025.
At the same time, even a conservative linear model for the changing prevalence of NASH forecast a rapid increase from 1.37 million cases in 2005 to 17.95 million in 2025. The exponential model suggested an increase from 2.41 million in 2005 to 42.34 million in 2025.
In terms of the effect on the prevalence of hepatocellular carcinoma (HCC), the modeling suggested cases of HCV-related liver cancer were predicted to peak at around 29,000 cases in 2015 then decline to fewer than 18,000 cases by 2025. In contrast, the prevalence of HCC from NASH is forecast to increase from between 5000 and 6000 cases in 2005 to 45,000 in 2025 by the conservative linear model or even as high as 106,000 cases according to the exponential model. It overtook HCV infection as a cause of liver cancer by around 2015.
“Despite the lack of existing data off of which to work, the general trends of our prediction models are consistent with the documented trends of liver transplant etiology, as well as 2010 insurance data indicating nonalcoholic fatty liver disease/NASH as the leading etiology associated with HCC,” wrote Osmanuddin Ahmed, MD, from the Rush University Medical Center in Chicago and his coauthors.
The study used liver transplant data as a proxy for the prevalence of hepatocellular carcinoma and also took into account the natural history of the disease. Between 5% and 20% of untreated HCV infections will go on to develop into cirrhosis, and of patients with HCV-related cirrhosis, around 15% will develop HCC within 10 years. In the case of NASH, the authors cited research suggesting that around 35% of patients go on to develop progressive fibrosis, that progression to cirrhosis takes around 29 years, and that the risk of progression to HCC ranged from 2.4% over 7 years to 12.8% over 3 years.
“A higher proportion of patients with NASH develop cirrhosis, but of those who develop cirrhosis, the probability of developing HCC is higher in patients with HCV,” the authors wrote. “In contrast, HCV progression to HCC rarely occurs in noncirrhotic patients.”
The authors wrote that it was important to explore projected trends in the etiology of hepatocellular carcinoma to inform the development of screening, diagnostic, and treatment approaches, particularly given potential differences in the pathology, natural history, and treatment options for NASH-related and HCV-related liver cancer.
“Histologically, NASH shares characteristics with alcoholic liver disease, primarily proinflammatory fat accumulation in parenchymal cells, [and] key players in NASH progression to HCC are suggested to include genetic modifications, proinflammatory high-fat and/or high-fructose diets, and oxidative and endoplasmic cellular stresses,” they wrote. “In HCV progression to HCC, the presence of the HCV core protein may induce HCC without the prerequisite load of genetic errors normally required for cancer development, skipping or accelerating some of the classic steps of cancer induction.”
The authors did note that their model represented a base scenario that assumed the environmental and genetic factors driving NASH would continue along the path of current trends.
“Therefore, the possibility exists that our models underestimate the response of the medical community in addressing the rising nonalcoholic fatty liver disease/NASH epidemic.”
No funding sources or conflicts of interest were declared.
SOURCE: Ahmed O et al. J Clin Exp Hepatology. 2018 Feb 24. doi: 10.1016/j.jceh.2018.02.006.
Researchers reported on their analysis of past prevalence of HCV, NASH, and alcoholic cirrhosis and prediction of future trends and their effect on hepatocellular carcinoma in the Feb. 24 online edition of the Journal of Clinical and Experimental Hepatology.
The analysis, based on data from the National Health and Nutrition Examination Survey and the Organ Procurement and Transplantation Network, shows that the prevalence of HCV has been in steady decline since 2005 and that decline is forecast to continue. From a prevalence of 3.22 million cases in 2005, researchers have forecasted a decline to 1.06 million cases by 2025.
At the same time, even a conservative linear model for the changing prevalence of NASH forecast a rapid increase from 1.37 million cases in 2005 to 17.95 million in 2025. The exponential model suggested an increase from 2.41 million in 2005 to 42.34 million in 2025.
In terms of the effect on the prevalence of hepatocellular carcinoma (HCC), the modeling suggested cases of HCV-related liver cancer were predicted to peak at around 29,000 cases in 2015 then decline to fewer than 18,000 cases by 2025. In contrast, the prevalence of HCC from NASH is forecast to increase from between 5000 and 6000 cases in 2005 to 45,000 in 2025 by the conservative linear model or even as high as 106,000 cases according to the exponential model. It overtook HCV infection as a cause of liver cancer by around 2015.
“Despite the lack of existing data off of which to work, the general trends of our prediction models are consistent with the documented trends of liver transplant etiology, as well as 2010 insurance data indicating nonalcoholic fatty liver disease/NASH as the leading etiology associated with HCC,” wrote Osmanuddin Ahmed, MD, from the Rush University Medical Center in Chicago and his coauthors.
The study used liver transplant data as a proxy for the prevalence of hepatocellular carcinoma and also took into account the natural history of the disease. Between 5% and 20% of untreated HCV infections will go on to develop into cirrhosis, and of patients with HCV-related cirrhosis, around 15% will develop HCC within 10 years. In the case of NASH, the authors cited research suggesting that around 35% of patients go on to develop progressive fibrosis, that progression to cirrhosis takes around 29 years, and that the risk of progression to HCC ranged from 2.4% over 7 years to 12.8% over 3 years.
“A higher proportion of patients with NASH develop cirrhosis, but of those who develop cirrhosis, the probability of developing HCC is higher in patients with HCV,” the authors wrote. “In contrast, HCV progression to HCC rarely occurs in noncirrhotic patients.”
The authors wrote that it was important to explore projected trends in the etiology of hepatocellular carcinoma to inform the development of screening, diagnostic, and treatment approaches, particularly given potential differences in the pathology, natural history, and treatment options for NASH-related and HCV-related liver cancer.
“Histologically, NASH shares characteristics with alcoholic liver disease, primarily proinflammatory fat accumulation in parenchymal cells, [and] key players in NASH progression to HCC are suggested to include genetic modifications, proinflammatory high-fat and/or high-fructose diets, and oxidative and endoplasmic cellular stresses,” they wrote. “In HCV progression to HCC, the presence of the HCV core protein may induce HCC without the prerequisite load of genetic errors normally required for cancer development, skipping or accelerating some of the classic steps of cancer induction.”
The authors did note that their model represented a base scenario that assumed the environmental and genetic factors driving NASH would continue along the path of current trends.
“Therefore, the possibility exists that our models underestimate the response of the medical community in addressing the rising nonalcoholic fatty liver disease/NASH epidemic.”
No funding sources or conflicts of interest were declared.
SOURCE: Ahmed O et al. J Clin Exp Hepatology. 2018 Feb 24. doi: 10.1016/j.jceh.2018.02.006.
FROM THE JOURNAL OF CLINICAL AND EXPERIMENTAL HEPATOLOGY
Key clinical point: NASH is rapidly eclipsing HCV infection as the leading contributor to liver cancer in the United States.
Major finding: The prevalence of HCV infection is forecast to decline to 1.06 million cases by 2025 while the prevalence of NASH is projected to increase to as many as 42.34 million cases by 2025.
Data source: Analysis based on data from the National Health and Nutrition Examination Survey and the Organ Procurement and Transplantation Network.
Disclosures: No funding sources or conflicts of interest were declared.
Source: Ahmed O et al. J Clin Exp Hepatology. 2018 Feb 24. doi: 10.1016/j.jceh.2018.02.006.
Ira Turner, MD
Firearm home storage practices pose a risk for suicidal teens
according to the findings of a cross-sectional analysis.
“For homes with children and guns, the odds are roughly 2 to 1 that firearms are not stored in accordance with recommendations promulgated by the [American Academy of Pediatrics], regardless of whether children in the home have a history of self-harm risk factors” wrote John Scott of Northeastern University, Boston, and his colleagues. “Given the prevalence of household firearms in the United States, our findings suggest that millions of U.S. children are placed at substantially higher risk of fatal firearm injury, especially suicide, than would be the case were parents to follow guidelines first put forward by the AAP.”
The information from the study was collected from a cross-sectional analysis of a nationally representative, online survey of U.S. adults from March to April of 2015. Of the 7,318 adults invited to participate, 3,949 completed the survey (a 55% response rate). The survey was conducted by the firm Growth for Knowledge to learn about firearm ownership, storage practices, and firearm use in adults. The adults selected for participation were drawn from Growth for Knowledge’s Knowledge Panel.
Survey participants were asked a variety of questions pertaining to gun ownership, such as: “Do you or does anyone else you live with currently own any type of gun?”and “Do you personally own a gun?” After determining gun ownership and style of gun (long rifle vs. handgun), survey respondents were questioned about how they stored their guns: Were they kept locked, unloaded, and away from ammo? Or were they stored unlocked and loaded?
After ascertaining information about firearm possession and storage practices, the researchers asked respondents if they were caregivers for children under the age of 18 and whether or not these children had any of the following medical conditions determined to be self-harm risk factors: depression, mental health conditions other than depression, or ADHD.
The researchers found that firearms were present in 42% of households, regardless of whether a child had a history of self harm risk factors (44%). Only one-third of household firearms were securely locked and unloaded. The proportion of parents who store at least one gun loaded and unlocked is only slightly lower among parents with children who have self-harm risk factors, compared with parents with children who have no history of self-harm risk (12% vs. 20%, respectively). Parents whose children have a history of depression, other mental health issues, and ADHD are also not significantly more likely to store all household firearms locked, compared with those whose children do not have such a history (62% vs. 52%, respectively).
Online surveys such as the one in this study rely on data that are reported by non-health experts, so the potential for a parent to misclassify their child’s mental or behavioral health issues is a distinct possibility. Like most self-reported data, social desirability bias may play a role in determining how parents responded to the survey regarding their children’s health.
While storage practices do not seem to change based on child self harm risk factors, the American Academy of Pediatrics has issued recommendations that may help convince some parents.
“Guidelines intended to reduce firearm injury to children, first issued by the American Academy of Pediatrics (AAP) in 1992, assert that whereas the safest home for a child is one without firearms, risk can be reduced substantially, although not eliminated, by storing all household firearms locked, unloaded, and separate from ammunition” Mr. Scott and his associates wrote. The study was published in Pediatrics.
The authors indicated they had no relevant financial disclosures. The study was supported in part by grants from Fund for a Safer Future, the Joyce Foundation, and the U.S. Department of Veteran Affairs.
SOURCE: Scott J et al. Pediatrics. 2018 February 26. doi: 10.1542/peds.2017-2600.
Adolescent suicide is an issue that weighs heavily on families and society as a whole and is only worsening. From 1999 to 2014, the suicide rate among 10- to 14-year-olds tripled, and doubled among 15- to 25-year-olds. Among these age groups, this is the second leading cause of death.
How guns and mental health interact is a current concern for many people in the United States. The primary focus is how to reduce injuries and deaths through background checks and restricted gun sales to high-risk individuals. While these interventions may reduce some issues associated with gun-related injuries, it may not do as much to protect youth at risk for self-inflicted harm. This responsibility falls to caretakers and parents who must understand the role they play in controlling access to firearms for at-risk youth.
A hypothetical framework proposes a three-part strategy to restrict gun access to at-risk teenagers. First, parents must be aware of the risk that unlocked guns in the home pose, and how drugs and alcohol can exacerbate the risk for self harm. Second, be ready to discuss access to guns in the house and, if needed, reduce access by utilizing safer storage practices in the home, or by using an outside storage facility. Third, families should seek to provide treatment and care for teenagers suffering from mental health issues with the aim of working toward remission and reducing self-harm risk.
Pediatricians and other health care professionals also play a critical role in preventing youth suicide. Routine screening of all adolescents for depression is recommended by Bright Futures and the U.S. Preventive Services Task Force. This screening process also presents the opportunity to ask about and discuss the presence of firearms and their storage in the home with patients and parents.
The increase in youth firearm suicide over the past 2 decades is indicative of a failure by both health care and public health systems to adequately address prevention of these unfortunate injuries and deaths.
David C. Grossman, MD, MPH, is a senior investigator with Kaiser Permanente Washington Health Research Institute and a Washington pediatrician. He wrote an editorial to accompany the article by Scott et al. (Pediatrics. 2018 Feb 26. doi: 10.1542/peds.2017-3884).
Adolescent suicide is an issue that weighs heavily on families and society as a whole and is only worsening. From 1999 to 2014, the suicide rate among 10- to 14-year-olds tripled, and doubled among 15- to 25-year-olds. Among these age groups, this is the second leading cause of death.
How guns and mental health interact is a current concern for many people in the United States. The primary focus is how to reduce injuries and deaths through background checks and restricted gun sales to high-risk individuals. While these interventions may reduce some issues associated with gun-related injuries, it may not do as much to protect youth at risk for self-inflicted harm. This responsibility falls to caretakers and parents who must understand the role they play in controlling access to firearms for at-risk youth.
A hypothetical framework proposes a three-part strategy to restrict gun access to at-risk teenagers. First, parents must be aware of the risk that unlocked guns in the home pose, and how drugs and alcohol can exacerbate the risk for self harm. Second, be ready to discuss access to guns in the house and, if needed, reduce access by utilizing safer storage practices in the home, or by using an outside storage facility. Third, families should seek to provide treatment and care for teenagers suffering from mental health issues with the aim of working toward remission and reducing self-harm risk.
Pediatricians and other health care professionals also play a critical role in preventing youth suicide. Routine screening of all adolescents for depression is recommended by Bright Futures and the U.S. Preventive Services Task Force. This screening process also presents the opportunity to ask about and discuss the presence of firearms and their storage in the home with patients and parents.
The increase in youth firearm suicide over the past 2 decades is indicative of a failure by both health care and public health systems to adequately address prevention of these unfortunate injuries and deaths.
David C. Grossman, MD, MPH, is a senior investigator with Kaiser Permanente Washington Health Research Institute and a Washington pediatrician. He wrote an editorial to accompany the article by Scott et al. (Pediatrics. 2018 Feb 26. doi: 10.1542/peds.2017-3884).
Adolescent suicide is an issue that weighs heavily on families and society as a whole and is only worsening. From 1999 to 2014, the suicide rate among 10- to 14-year-olds tripled, and doubled among 15- to 25-year-olds. Among these age groups, this is the second leading cause of death.
How guns and mental health interact is a current concern for many people in the United States. The primary focus is how to reduce injuries and deaths through background checks and restricted gun sales to high-risk individuals. While these interventions may reduce some issues associated with gun-related injuries, it may not do as much to protect youth at risk for self-inflicted harm. This responsibility falls to caretakers and parents who must understand the role they play in controlling access to firearms for at-risk youth.
A hypothetical framework proposes a three-part strategy to restrict gun access to at-risk teenagers. First, parents must be aware of the risk that unlocked guns in the home pose, and how drugs and alcohol can exacerbate the risk for self harm. Second, be ready to discuss access to guns in the house and, if needed, reduce access by utilizing safer storage practices in the home, or by using an outside storage facility. Third, families should seek to provide treatment and care for teenagers suffering from mental health issues with the aim of working toward remission and reducing self-harm risk.
Pediatricians and other health care professionals also play a critical role in preventing youth suicide. Routine screening of all adolescents for depression is recommended by Bright Futures and the U.S. Preventive Services Task Force. This screening process also presents the opportunity to ask about and discuss the presence of firearms and their storage in the home with patients and parents.
The increase in youth firearm suicide over the past 2 decades is indicative of a failure by both health care and public health systems to adequately address prevention of these unfortunate injuries and deaths.
David C. Grossman, MD, MPH, is a senior investigator with Kaiser Permanente Washington Health Research Institute and a Washington pediatrician. He wrote an editorial to accompany the article by Scott et al. (Pediatrics. 2018 Feb 26. doi: 10.1542/peds.2017-3884).
according to the findings of a cross-sectional analysis.
“For homes with children and guns, the odds are roughly 2 to 1 that firearms are not stored in accordance with recommendations promulgated by the [American Academy of Pediatrics], regardless of whether children in the home have a history of self-harm risk factors” wrote John Scott of Northeastern University, Boston, and his colleagues. “Given the prevalence of household firearms in the United States, our findings suggest that millions of U.S. children are placed at substantially higher risk of fatal firearm injury, especially suicide, than would be the case were parents to follow guidelines first put forward by the AAP.”
The information from the study was collected from a cross-sectional analysis of a nationally representative, online survey of U.S. adults from March to April of 2015. Of the 7,318 adults invited to participate, 3,949 completed the survey (a 55% response rate). The survey was conducted by the firm Growth for Knowledge to learn about firearm ownership, storage practices, and firearm use in adults. The adults selected for participation were drawn from Growth for Knowledge’s Knowledge Panel.
Survey participants were asked a variety of questions pertaining to gun ownership, such as: “Do you or does anyone else you live with currently own any type of gun?”and “Do you personally own a gun?” After determining gun ownership and style of gun (long rifle vs. handgun), survey respondents were questioned about how they stored their guns: Were they kept locked, unloaded, and away from ammo? Or were they stored unlocked and loaded?
After ascertaining information about firearm possession and storage practices, the researchers asked respondents if they were caregivers for children under the age of 18 and whether or not these children had any of the following medical conditions determined to be self-harm risk factors: depression, mental health conditions other than depression, or ADHD.
The researchers found that firearms were present in 42% of households, regardless of whether a child had a history of self harm risk factors (44%). Only one-third of household firearms were securely locked and unloaded. The proportion of parents who store at least one gun loaded and unlocked is only slightly lower among parents with children who have self-harm risk factors, compared with parents with children who have no history of self-harm risk (12% vs. 20%, respectively). Parents whose children have a history of depression, other mental health issues, and ADHD are also not significantly more likely to store all household firearms locked, compared with those whose children do not have such a history (62% vs. 52%, respectively).
Online surveys such as the one in this study rely on data that are reported by non-health experts, so the potential for a parent to misclassify their child’s mental or behavioral health issues is a distinct possibility. Like most self-reported data, social desirability bias may play a role in determining how parents responded to the survey regarding their children’s health.
While storage practices do not seem to change based on child self harm risk factors, the American Academy of Pediatrics has issued recommendations that may help convince some parents.
“Guidelines intended to reduce firearm injury to children, first issued by the American Academy of Pediatrics (AAP) in 1992, assert that whereas the safest home for a child is one without firearms, risk can be reduced substantially, although not eliminated, by storing all household firearms locked, unloaded, and separate from ammunition” Mr. Scott and his associates wrote. The study was published in Pediatrics.
The authors indicated they had no relevant financial disclosures. The study was supported in part by grants from Fund for a Safer Future, the Joyce Foundation, and the U.S. Department of Veteran Affairs.
SOURCE: Scott J et al. Pediatrics. 2018 February 26. doi: 10.1542/peds.2017-2600.
according to the findings of a cross-sectional analysis.
“For homes with children and guns, the odds are roughly 2 to 1 that firearms are not stored in accordance with recommendations promulgated by the [American Academy of Pediatrics], regardless of whether children in the home have a history of self-harm risk factors” wrote John Scott of Northeastern University, Boston, and his colleagues. “Given the prevalence of household firearms in the United States, our findings suggest that millions of U.S. children are placed at substantially higher risk of fatal firearm injury, especially suicide, than would be the case were parents to follow guidelines first put forward by the AAP.”
The information from the study was collected from a cross-sectional analysis of a nationally representative, online survey of U.S. adults from March to April of 2015. Of the 7,318 adults invited to participate, 3,949 completed the survey (a 55% response rate). The survey was conducted by the firm Growth for Knowledge to learn about firearm ownership, storage practices, and firearm use in adults. The adults selected for participation were drawn from Growth for Knowledge’s Knowledge Panel.
Survey participants were asked a variety of questions pertaining to gun ownership, such as: “Do you or does anyone else you live with currently own any type of gun?”and “Do you personally own a gun?” After determining gun ownership and style of gun (long rifle vs. handgun), survey respondents were questioned about how they stored their guns: Were they kept locked, unloaded, and away from ammo? Or were they stored unlocked and loaded?
After ascertaining information about firearm possession and storage practices, the researchers asked respondents if they were caregivers for children under the age of 18 and whether or not these children had any of the following medical conditions determined to be self-harm risk factors: depression, mental health conditions other than depression, or ADHD.
The researchers found that firearms were present in 42% of households, regardless of whether a child had a history of self harm risk factors (44%). Only one-third of household firearms were securely locked and unloaded. The proportion of parents who store at least one gun loaded and unlocked is only slightly lower among parents with children who have self-harm risk factors, compared with parents with children who have no history of self-harm risk (12% vs. 20%, respectively). Parents whose children have a history of depression, other mental health issues, and ADHD are also not significantly more likely to store all household firearms locked, compared with those whose children do not have such a history (62% vs. 52%, respectively).
Online surveys such as the one in this study rely on data that are reported by non-health experts, so the potential for a parent to misclassify their child’s mental or behavioral health issues is a distinct possibility. Like most self-reported data, social desirability bias may play a role in determining how parents responded to the survey regarding their children’s health.
While storage practices do not seem to change based on child self harm risk factors, the American Academy of Pediatrics has issued recommendations that may help convince some parents.
“Guidelines intended to reduce firearm injury to children, first issued by the American Academy of Pediatrics (AAP) in 1992, assert that whereas the safest home for a child is one without firearms, risk can be reduced substantially, although not eliminated, by storing all household firearms locked, unloaded, and separate from ammunition” Mr. Scott and his associates wrote. The study was published in Pediatrics.
The authors indicated they had no relevant financial disclosures. The study was supported in part by grants from Fund for a Safer Future, the Joyce Foundation, and the U.S. Department of Veteran Affairs.
SOURCE: Scott J et al. Pediatrics. 2018 February 26. doi: 10.1542/peds.2017-2600.
FROM PEDIATRICS
Key clinical point: Millions of children at risk for self harm live in a household with unsecured firearms.
Major finding: One in three parents stored all household firearms locked and unloaded.
Study details: A cross-sectional analysis of a nationally representative, online survey of 3,949 U.S. adults concerning gun ownership and gun storage practices from March to April of 2015.
Disclosures: The authors indicated they have no relevant financial disclosures. The study was supported in part by grants from Fund for a Safer Future, the Joyce Foundation, and the U.S. Department of Veteran Affairs.
Source: Scott J et al. Pediatrics. 2018 February 26. doi: 10.1542/peds.2017-2600.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review
Bacterial bloodstream infections (BSIs) are a major cause of morbidity and mortality in the United States. Approximately 600,000 BSI cases occur annually, resulting in 85,000 deaths,1 at a cost exceeding $1 billion.2 Traditionally, BSIs have been managed with intravenous antimicrobials, which rapidly achieve therapeutic blood concentrations, and are viewed as more potent than oral alternatives. Indeed, for acutely ill patients with bacteremia and sepsis, timely intravenous antimicrobials are lifesaving.3
However, whether intravenous antimicrobials are essential for the entire treatment course in BSIs, particularly for uncomplicated episodes, is controversial. Patients that are clinically stable or have been stabilized after an initial septic presentation may be appropriate candidates for treatment with oral antimicrobials. There are costs and risks associated with extended courses of intravenous agents, such as the necessity for long-term intravenous catheters, which entail risks for procedural complications, secondary infections, and thrombosis. A prospective study of 192 peripherally inserted central catheter (PICC) episodes reported an overall complication rate of 30.2%, including central line-associated BSIs (CLABSI) or venous thrombosis.4 Other studies also identified high rates of thrombosis5 and PICC-related CLABSI, particularly in patients with malignancy, where sepsis-related complications approach 25%.6 Additionally, appropriate care of indwelling catheters requires significant financial and healthcare resources.
Oral antimicrobial therapy for bacterial BSIs offers several potential benefits. Direct economic and healthcare workforce savings are expected to be significant, and procedural and catheter-related complications would be eliminated.7 Moreover, oral therapy provides antimicrobial stewardship by reducing the use of broad-spectrum intravenous agents.8 Recent infectious disease “Choosing Wisely” initiatives recommend clinicians “prefer oral formulations of highly bioavailable antimicrobials whenever possible”,9 and this approach is supported by the Centers for Disease Control and Prevention antibiotic stewardship program.10 However, the expected savings and benefits of oral therapy would be lost should they be less effective and result in treatment failure or relapse of the primary BSI. Pathogen susceptibility, gastrointestinal absorption, oral bioavailability, patient tolerability, and adherence with therapy need to be carefully considered before choosing oral antimicrobials. Thus, oral antimicrobial therapy for BSI should be utilized in carefully selected circumstances.
In this narrative review, we highlight areas where oral therapy is safe and effective in treating bloodstream infections, as well as offer guidance to clinicians managing patients experiencing BSI. Given the lack of robust clinical trials on this subject, the evidence for performing a systematic review was insufficient. Thus, the articles and recommendations cited in this review were selected based on the authors’ experiences to represent the best available evidence.
Infection Source Control
Pathogen and Antimicrobial Factors
Patient Factors
Evidence Regarding Bloodstream Infections due to Gram-Negative Rods
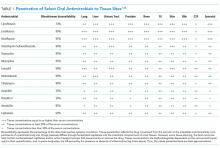
BSIs due to gram-negative rods (GNRs) are common and cause significant morbidity and mortality. GNRs represent a broad and diverse array of pathogens. We focus on the Enterobacteriaceae family and Pseudomonas aeruginosa, because they are frequently encountered in clinical practice.1
Gram-Negative Rods, Enterobacteriaceae Family
The Enterobacteriaceae family includes Escherichia coli, Klebsiella, Salmonella, Proteus, Enterobacter, Serratia, and Citrobacter species. The range of illnesses caused by Enterobacteriaceae is as diverse as the family, encompassing most body sites. Although most Enterobacteriaceae are intrinsically susceptible to antibiotics, there is potential for significant multi-drug resistance. Of particular recent concern has been the emergence of Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBL) and even carbapenem-resistant strains.14
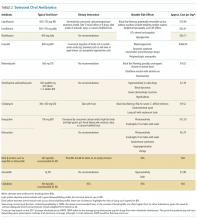
However, Enterobacteriaceae species susceptible to oral antimicrobials are often suitable candidates for oral BSI therapy. Among 106 patients with GNR BSI treated with a highly bioavailable oral antibiotic (eg, levofloxacin), the treatment failure rate was only 2% (versus 14% when an antimicrobial with only moderate or low bioavailability was selected).15 Oral treatment of Enterobacteriaceae BSIs secondary to urinary tract infection has been best studied. A prospective randomized, controlled trial evaluated oral versus intravenous ciprofloxacin amongst 141 patients with severe pyelonephritis or complicated urinary tract infections, in which the rate of bacteremia was 38%.16 Notably, patients with obstruction or renal abscess were excluded from the trial. No significant differences in microbiological failure or unsatisfactory clinical responses were found between the IV and oral treatment groups. Additionally, a Cochrane review reported that oral antibiotic therapy is no less effective than intravenous therapy for severe UTI, although data on BSI frequency were not provided.17
Resistance to fluoroquinolones such as ciprofloxacin has been identified as a risk factor for GNR BSI oral treatment failure, highlighting the importance of confirming susceptibilities before committing to an oral treatment plan.18,19 Even ESBL Enterobacteriaceae can be considered for treatment with fluoroquinolones if susceptibilities allow.20
The ideal duration of therapy for GNR BSI is an area of active research. A recent retrospective trial showed no difference in all-cause mortality or recurrent BSI in GNR BSI treated for 8 versus 15 days.21 A recent meta-analysis suggested that 7 days of therapy was noninferior to a longer duration therapy (10–14 days) for pyelonephritis, in which a subset was bacteremic.22 However, another trial reported that short course therapy for GNR BSI (<7 days) is associated with higher risk of treatment failure.22 Further data are needed.
Gram-Negative Rods, Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common pathogen, intrinsically resistant to many antimicrobials, and readily develops antimicrobial resistance during therapy. Fluoroquinolones (such as ciprofloxacin, levofloxacin, and delafloxacin) are the only currently available oral agents with antipseudomonal activity. However, fluoroquinolones may not achieve blood concentrations appropriate for P. aeruginosa treatment at standard doses, while higher dose regimens may be associated with increased risk for undesirable side effects.24,25 Currently, given the minimal trial data comparing oral versus intravenous therapy for P. aeruginosa BSIs, and multiple studies indicating increased mortality when P. aeruginosa is treated inappropriately,26,27 we prefer a conservative approach and consider oral therapy a less-preferred option.
Evidence Regarding Bloodstream Infections due to Gram-Positive Cocci
The majority of bloodstream infections in the United States, and the resultant morbidity and mortality, are from gram-positive cocci (GPCs) such as Staphylococcus, Streptococcus, and Enterococcus species.1
Gram-Positive Cocci, Streptococcus pneumoniae
Of the approximately 900,000 annual cases of S. pneumoniae infection in the United States, approximately 40,000 are complicated by BSI, with 70% of those cases being secondary to pneumococcal pneumonia.28 In studies on patients with pneumococcal pneumonia, bacteremic cases generally fare worse than those without bacteremia.29,30 However, several trials demonstrated comparable outcomes in the setting of bacteremic pneumococcal pneumonia when switched early (within 3 days) from intravenous to oral antibiotics to complete a 7-day course.31,32 Before pneumococcal penicillin resistance became widespread, oral penicillin was shown to be effective, and remains an option for susceptible strains.33 It is worth noting, however, that other trials have shown a mortality benefit from treating bacteremic pneumococcal pneumonia initially with dual-therapy including a β-lactam and macrolide such as azithromycin. This observation highlights the importance of knowing the final susceptibility data prior to consolidating to monotherapy with an oral agent, and that macrolides may have beneficial anti-inflammatory effects, though further research is needed.34,35
Although the evidence for treating bacteremic pneumococcal pneumonia using a highly active and absorbable oral agent is fairly robust, S. pneumoniae BSI secondary to other sites of infection sites is less well studied and may require a more conservative approach.
Gram-Positive Cocci, β-hemolytic Streptococcus species
β-Hemolytic Streptococci include groups A to H, of which groups A (S. pyogenes) and B (S. agalactiae) are the most commonly implicated in BSIs.36 Group A Streptococcus (GAS) is classically associated with streptococcal pharyngitis and Group B Streptococcus (GBS) is associated with postpartum endometritis and neonatal meningitis, though both are virulent organisms with a potential to cause invasive infection throughout the body and in all age-groups. Up to 14% of GAS and 41% GBS BSIs have no clear source;37,38 given these are skin pathogens, such scenarios likely represent invasion via microabrasion. As β-hemolytic streptococcal BSI is often observed in the context of necrotizing skin and soft tissue infections, surgical source control is particularly important.39 GAS remains exquisitely susceptible to penicillin, and intravenous penicillin remains the mainstay for invasive disease; GBS has higher penicillin resistance rates than GAS.40 Clindamycin should be added when there is concern for severe disease or toxic shock.41 Unfortunately, oral penicillin is poorly bioavailable (approximately 50%), and there has been recent concern regarding inducible clindamycin resistance in GAS.42 Thus, oral penicillin V and/or clindamycin is a potentially risky strategy, with no clinical trials supporting this approach; however, they may be reasonable options in selected patients with source control and stable hemodynamics. Amoxicillin has high bioavailability (85%) and may be effective; however, there is lack of supporting data. Highly bioavailable agents such as levofloxacin and linezolid have GAS and GBS activity43 and might be expected to produce satisfactory outcomes. Because no clinical trials have compared these agents with intravenous therapy for BSI, caution is advised. Although bacteriostatic against Staphylococcus, linezolid is bactericidal against Streptococcus.44 Fluoroquinolone resistance amongst β-hemolytic Streptococcus is rare (approximately 0.5%) but does occur.45
Gram-Positive Cocci, Staphylococcus Species
Staphylococcus species include S. aureus (including methicillin susceptible and resistant strains: MSSA and MRSA, respectively) and coagulase-negative species, which include organisms such as S. epidermidis. S. aureus is the most common cause of BSI mortality in the United States,1 with mortality rates estimated at 20%–40% per episode.46 Infectious disease consultation has been associated with decreased mortality and is recommended.47 The guidelines of the Infectious Diseases Society of America for the treatment of MRSA recommend the use of parenteral agents for BSI.48 It is important to consider if a patient with S. aureus BSI has infective endocarditis.
Oral antibiotic therapy for S. aureus BSI is not currently standard practice. Although trimethoprim-sulfamethoxazole (TMP-SMX) has favorable pharmacokinetics and case series of using it successfully for BSI exist,49 TMP-SMX showed inferior outcomes in a randomized trial comparing oral TMP-SMX with intravenous vancomycin in a series of 101 S. aureus infections.50 This observation has been replicated.51 Data on doxycycline or clindamycin for S. aureus BSI are limited, and IDSA guidelines advise against their use in this setting because they are predominantly bacteriostatic.48 Linezolid has favorable pharmacokinetics, with approximately 100% bioavailability, and S. aureus resistance to linezolid is rare.52 Several randomized trials have compared oral linezolid with intravenous vancomycin for S. aureus BSI; for instance, Stevens et al. randomized 460 patients with S. aureus infection (of whom 18% had BSI) to linezolid versus vancomycin and observed similar clinical cure rates.53 A pooled analysis showed oral linezolid was noninferior to vancomycin specifically for S. aureus BSI.54 However, long-term use is often limited by hematologic toxicity, peripheral or optic neuropathy (which can be permanent), and induced serotonin syndrome. Additionally, linezolid is bacteriostatic, not bactericidal against S. aureus. Using oral linezolid as a first-line option for S. aureus BSI would not be recommended; however, it may be used as a second-line treatment option in selected cases. Tedizolid has similar pharmacokinetics and spectrum of activity with fewer side effects; however, clinical data on its use for S. aureus BSI are lacking.55 Fluoroquinolones such as levofloxacin and the newer agent delafloxacin have activity against S. aureus, including MRSA, but on-treatment emergence of fluoroquinolone resistance is a concern, and data on delafloxacin for BSI are lacking.56,57 Older literature suggested the combination of ciprofloxacin and rifampin was effective against right-sided S. aureus endocarditis,58 and other oral fluoroquinolone-rifamycin combinations have also been found to be effective59 However, this approach is currently not a standard therapy, nor is it widely used. Decisions on the duration of therapy for S. aureus BSI should be made in conjunction with an infectious diseases specialist; 14 days is currently regarded as a minimum.47,48
Published data regarding oral treatment of coagulase-negative Staphylococcus (CoNS) BSI are limited. Most CoNS bacteremia and up to 80% Staphylococcus epidermidis bacteremia represent blood culture contamination, though true infection from CoNS is not uncommon, particularly in patients with indwelling catheters.60 An exception is the CoNS species Staphylococcus lugdunensis, which is more virulent, and bacteremia with this organism usually warrants antibiotics. Oral antimicrobial therapy is currently not a standard treatment practice for CoNS BSI that is felt to represent true infection; however, linezolid has been successfully used in case series.61
Gram-Positive Cocci, Enterococcus
E. faecium and E. faecalis are commonly implicated in BSI.1 Similar to S. aureus, infective endocarditis must be ruled out when treating enterococcus BSI; a scoring system has been proposed to assist in deciding if such patients require echocardiography.62 Intravenous ampicillin is a preferred, highly effective agent for enterococci treatment when the organism is susceptible.44 However, oral ampicillin has poor bioavailability (50%), and data for its use in BSI are lacking. For susceptible strains, amoxicillin has comparable efficacy for enterococci and enhanced bioavailability (85%); high dose oral amoxicillin could be considered, but there is minimal clinical trial data to support this approach. Fluoroquinolones exhibit only modest activity against enterococci and would be an inferior choice for BSI.63 Although often sensitive to oral tetracyclines, data on their use in enterococcal BSI are insufficient. Nitrofurantoin can be used for susceptible enterococcal urinary tract infection; however, it does not achieve high blood concentrations and should not be used for BSI.
There is significant data comparing oral linezolid with intravenous daptomycin for vancomycin-resistant enterococci (VRE) BSI. In a systematic review including 10 trials using 30-day all-cause mortality as the primary outcome, patients treated with daptomycin demonstrated higher odds of death (OR 1.61, 95% CI 1.08–2.40) compared with those treated with linezolid.64 However, more recent data suggested that higher daptomycin doses than those used in these earlier trials resulted in improved VRE BSI outcomes.65 A subsequent study reported that VRE BSI treatment with linezolid is associated with significantly higher treatment failure and mortality compared with daptomycin therapy.66 Further research is needed, but should the side-effect profile of linezolid be tolerable, it remains an effective option for oral treatment of enterococcal BSIs.
Evidence Regarding Anaerobic Bacterial Blood Stream Infection
Anaerobic bacteria include Bacteroides, Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Veillonella, and Clostridium. Anaerobes account for approximately 4% of bacterial BSIs, and are often seen in the context of polymicrobial infection.67 Given that anaerobes are difficult to recover, and that antimicrobial resistance testing is more labor intensive, antibiotic therapy choices are often made empirically.67 Unfortunately, antibiotic resistance amongst anaerobes is increasing.68 However, metronidazole remains highly active against a majority of anaerobes, with only a handful of treatment failures reported,69 and has a highly favorable pharmacokinetic profile for oral treatment. Oral metronidazole remains an effective choice for many anaerobic BSIs. Considering the polymicrobial nature of many anaerobic infections, source control is important, and concomitant GNR infection must be ruled out before using metronidazole monotherapy.
Clindamycin has significant anaerobic activity, but Bacteroides resistance has increased significantly in recent years, as high as 26%-44%.70 Amoxicillin-clavulanate has good anaerobic coverage, but bioavailability of clavulanate is limited (50%), making it inferior for BSI. Bioavailability is also limited for cephalosporins with anaerobic activity, such as cefuroxime. Moxifloxacin is a fluoroquinolone with some anaerobic coverage and a good oral pharmacokinetic profile, but Bacteroides resistance can be as high as 50%, making it a risky empiric choice.68
Conclusions
Bacterial BSIs are common and result in significant morbidity and mortality, with high associated healthcare costs. Although BSIs are traditionally treated with intravenous antimicrobials, many BSIs can be safely and effectively cured using oral antibiotics. When appropriately selected, oral antibiotics offer lower costs, fewer side effects, promote antimicrobial stewardship, and are easier for patients. Ultimately, the decision to use oral versus intravenous antibiotics must consider the characteristics of the pathogen, patient, and drug.
Disclosures
None of the authors report any conflicts of interest.
1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501-509. PubMed
2. Kilgore M, Brossette S. Cost of bloodstream infections. Am J Infect Control. 2008;36(10):S172.e1-3. PubMed
3. Youkee D, Hulme W, Roberts T, Daniels R, Nutbeam T, Keep J. Time Matters: Antibiotic Timing in Sepsis and Septic Shock. Crit Care Med. 2016;44(10):e1016-1017. PubMed
4. Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;28;6:18. PubMed
5. Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, Waybill PN. Venous Thrombosis Associated with the Placement of Peripherally Inserted Central Catheters. J Vasc Interv Radiol. 2000;11(10):1309-1314. PubMed
6. Cheong K, Perry D, Karapetis C, Koczwara B. High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004;34(5):234-238. PubMed
7. Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006;90(6):1197-1222. PubMed
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014;5(2):83-87. PubMed
9. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
10. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
11. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
12. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393-407. PubMed
13. Cua YM, Kripalani S. Medication Use in the Transition from Hospital to Home. Ann Acad Med Singapore. 2008;37(2):136. PubMed
14. Paterson DL. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am J Med. 2006; 119(6):S20-28.
15. Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. PubMed
16. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M Oral vs Intravenous Ciprofloxacin in the Initial Empirical Management of Severe Pyelonephritis or Complicated Urinary Tract Infections: A Prospective Randomized Clinical Trial. Arch Intern Med. 1999;159(1):53-58. PubMed
17. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003237. PubMed
18. Brigmon MM, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Impact of fluoroquinolone resistance in Gram-negative bloodstream infections on healthcare utilization. Clin Microbiol Infect. 2015;21(9):843-849. PubMed
19. Ortega M, Marco F, Soriano A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63(3):568-574. PubMed
20. Lo CL, Lee CC, Li CW, et al. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2017;50(3):355-361. PubMed
21. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the Outcomes of Adults With Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score–Matched Cohort. Clin Infect Dis. 2017; cix767. doi.org/10.1093/cid/cix767 PubMed
22. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection-- 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183-2191. PubMed
23. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 2017;45(5):613-620. PubMed
24. Zelenitsky S, Ariano R, Harding G, Forrest A. Evaluating Ciprofloxacin Dosing for Pseudomonas aeruginosa Infection by Using Clinical Outcome-Based Monte Carlo Simulations. Antimicrob Agents Chemother. 2005;49(10):4009-4014. PubMed
25. Cazaubon Y, Bourguignon L, Goutelle S, Martin O, Maire P, Ducher M. Are ciprofloxacin dosage regimens adequate for antimicrobial efficacy and prevention of resistance? Pseudomonas aeruginosa bloodstream infection in elderly patients as a simulation case study. Fundam Clin Pharmacol. 2015;29(6):615-624. PubMed
26. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob Agents Chemother. 2005;49(4):1306-1311. PubMed
27. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of Combination Antimicrobial Therapy for Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756-2764. PubMed
28. The Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Emerging Infections Program Network Streptococcus pneumoniae, 2013. https://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Published November, 2014. Accessed September 26, 2017.
29. Brandenburg JA, Marrie TJ, Coley CM, et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000;15(9):638-646. PubMed
30. Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000;79(4):210-221. PubMed
31. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001; 161(6):848-850. PubMed
32. Oosterheert JJ, Bonten MJM, Schneider MME, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333(7580):1193. PubMed
33. Austrian R, Winston AL. The efficacy of penicillin V (phenoxymethyl-penicillin) in the treatment of mild and of moderately severe pneumococcal pneumonia. Am J Med Sci. 1956;232(6):624-628. PubMed
34. Waterer GW, Somes GW, Wunderink RG. Monotherapy May Be Suboptimal for Severe Bacteremic Pneumococcal Pneumonia. Arch Intern Med. 2001; 161(15):1837-1842. PubMed
35. Baddour LM, Yu VL, Klugman KP, et al. International Pneumococcal Study Group. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440-444. PubMed
36. Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic streptococcus group g: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112(8):622-626. PubMed
37. Davies HD, McGeer A, Schwartz B, Green, et al; Ontario Group A Streptococcal Study Group. Invasive Group A Streptococcal Infections in Ontario, Canada. N Engl J Med. 1996;335(8):547-554. PubMed
38. Farley MM, Harvey C, Stull T, et al. A Population-Based Assessment of Invasive Disease Due to Group B Streptococcus in Nonpregnant Adults. N Engl J Med. 1993;328(25):1807-1811. PubMed
39. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478-486. PubMed
40. Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo JJ. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994;38(9):2183-2186. PubMed
41. Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096-1100. PubMed
42. Chen I, Kaufisi P, Erdem G. Emergence of erythromycin- and clindamycin-resistant Streptococcus pyogenes emm 90 strains in Hawaii. J Clin Microbiol. 2011;49(1):439-441. PubMed
43. Biedenbach DJ, Jones RN. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25(1):47–51. PubMed
44. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT. Sanford Guide To Antimicrobial Therapy 2017. Dallas, TX. Antimicrobial Theapy, Inc, 2017.
45. Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn Microbiol Infect Dis. 2006;55(2):119-127. PubMed
46. Shurland S, Zhan M, Bradham DD, Roghmann M-C. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):2739. PubMed
47. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56(4):527-535. PubMed
48. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55. PubMed
49. Adra M, Lawrence KR. Trimethoprim/Sulfamethoxazole for Treatment of Severe Staphylococcus aureus Infections. Ann Pharmacother. 2004;38(2):338-341. PubMed
50. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117(5):390-398. PubMed
51. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ. 2015;350:h2219. PubMed
52. Sánchez García M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260-2264. PubMed
53. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481-1490. PubMed
54. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923-929. PubMed
55. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health-Syst Pharm. 2014;71(8):621-633. PubMed
56. Gade ND, Qazi MS. Fluoroquinolone Therapy in Staphylococcus aureus Infections: Where Do We Stand? J Lab Physicians. 2013;5(2):109-112. PubMed
57. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71(3):821-829. PubMed
58. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071-1073. PubMed
59. Schrenzel J, Harbarth S, Schockmel G, et al. A Randomized Clinical Trial to Compare Fleroxacin-Rifampicin with Flucloxacillin or Vancomycin for the Treatment of Staphylococcal Infection. Clin Infect Dis. 2004;39(9):1285-1292. PubMed
60. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. PubMed
61. Antony SJ, Diaz-Vasquez E, Stratton C. Clinical experience with linezolid in the treatment of resistant gram-positive infections. J Natl Med Assoc. 2001;93(10):386-391. PubMed
62. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis. 2015;60(4):528-535. PubMed
63. Martínez-Martínez L, Joyanes P, Pascual A, Terrero E, Perea EJ. Activity of eight fluoroquinolones against enterococci. Clin Microbiol Infect. 1997;3(4):497-499. PubMed
64. Balli EP, Venetis CA, Miyakis S. Systematic Review and Meta-Analysis of Linezolid versus Daptomycin for Treatment of Vancomycin-Resistant Enterococcal Bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-739. PubMed
70. Snydman DR, Jacobus NV, McDermott LA, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007;51(5):1649-1655. PubMed
69. Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis. 2010;50 Suppl 1:S26-33. PubMed
68. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2012;56(3):1247-1252. PubMed
67. Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26(6):1413-1417. PubMed
66. Britt NS, Potter EM, Patel N, Steed ME. Comparison of the Effectiveness and Safety of Linezolid and Daptomycin in Vancomycin-Resistant Enterococcal Bloodstream Infection: A National Cohort Study of Veterans Affairs Patients. Clin Infect Dis. 2015;61(6):871-878. PubMed
65. Chuang YC, Lin HY, Chen PY, et al. Effect of Daptomycin Dose on the Outcome of Vancomycin-Resistant, Daptomycin-Susceptible Enterococcus faecium Bacteremia. Clin Infect Dis. 2017;64(8):1026-1034. PubMed
Bacterial bloodstream infections (BSIs) are a major cause of morbidity and mortality in the United States. Approximately 600,000 BSI cases occur annually, resulting in 85,000 deaths,1 at a cost exceeding $1 billion.2 Traditionally, BSIs have been managed with intravenous antimicrobials, which rapidly achieve therapeutic blood concentrations, and are viewed as more potent than oral alternatives. Indeed, for acutely ill patients with bacteremia and sepsis, timely intravenous antimicrobials are lifesaving.3
However, whether intravenous antimicrobials are essential for the entire treatment course in BSIs, particularly for uncomplicated episodes, is controversial. Patients that are clinically stable or have been stabilized after an initial septic presentation may be appropriate candidates for treatment with oral antimicrobials. There are costs and risks associated with extended courses of intravenous agents, such as the necessity for long-term intravenous catheters, which entail risks for procedural complications, secondary infections, and thrombosis. A prospective study of 192 peripherally inserted central catheter (PICC) episodes reported an overall complication rate of 30.2%, including central line-associated BSIs (CLABSI) or venous thrombosis.4 Other studies also identified high rates of thrombosis5 and PICC-related CLABSI, particularly in patients with malignancy, where sepsis-related complications approach 25%.6 Additionally, appropriate care of indwelling catheters requires significant financial and healthcare resources.
Oral antimicrobial therapy for bacterial BSIs offers several potential benefits. Direct economic and healthcare workforce savings are expected to be significant, and procedural and catheter-related complications would be eliminated.7 Moreover, oral therapy provides antimicrobial stewardship by reducing the use of broad-spectrum intravenous agents.8 Recent infectious disease “Choosing Wisely” initiatives recommend clinicians “prefer oral formulations of highly bioavailable antimicrobials whenever possible”,9 and this approach is supported by the Centers for Disease Control and Prevention antibiotic stewardship program.10 However, the expected savings and benefits of oral therapy would be lost should they be less effective and result in treatment failure or relapse of the primary BSI. Pathogen susceptibility, gastrointestinal absorption, oral bioavailability, patient tolerability, and adherence with therapy need to be carefully considered before choosing oral antimicrobials. Thus, oral antimicrobial therapy for BSI should be utilized in carefully selected circumstances.
In this narrative review, we highlight areas where oral therapy is safe and effective in treating bloodstream infections, as well as offer guidance to clinicians managing patients experiencing BSI. Given the lack of robust clinical trials on this subject, the evidence for performing a systematic review was insufficient. Thus, the articles and recommendations cited in this review were selected based on the authors’ experiences to represent the best available evidence.
Infection Source Control
Pathogen and Antimicrobial Factors
Patient Factors
Evidence Regarding Bloodstream Infections due to Gram-Negative Rods
BSIs due to gram-negative rods (GNRs) are common and cause significant morbidity and mortality. GNRs represent a broad and diverse array of pathogens. We focus on the Enterobacteriaceae family and Pseudomonas aeruginosa, because they are frequently encountered in clinical practice.1
Gram-Negative Rods, Enterobacteriaceae Family
The Enterobacteriaceae family includes Escherichia coli, Klebsiella, Salmonella, Proteus, Enterobacter, Serratia, and Citrobacter species. The range of illnesses caused by Enterobacteriaceae is as diverse as the family, encompassing most body sites. Although most Enterobacteriaceae are intrinsically susceptible to antibiotics, there is potential for significant multi-drug resistance. Of particular recent concern has been the emergence of Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBL) and even carbapenem-resistant strains.14
However, Enterobacteriaceae species susceptible to oral antimicrobials are often suitable candidates for oral BSI therapy. Among 106 patients with GNR BSI treated with a highly bioavailable oral antibiotic (eg, levofloxacin), the treatment failure rate was only 2% (versus 14% when an antimicrobial with only moderate or low bioavailability was selected).15 Oral treatment of Enterobacteriaceae BSIs secondary to urinary tract infection has been best studied. A prospective randomized, controlled trial evaluated oral versus intravenous ciprofloxacin amongst 141 patients with severe pyelonephritis or complicated urinary tract infections, in which the rate of bacteremia was 38%.16 Notably, patients with obstruction or renal abscess were excluded from the trial. No significant differences in microbiological failure or unsatisfactory clinical responses were found between the IV and oral treatment groups. Additionally, a Cochrane review reported that oral antibiotic therapy is no less effective than intravenous therapy for severe UTI, although data on BSI frequency were not provided.17
Resistance to fluoroquinolones such as ciprofloxacin has been identified as a risk factor for GNR BSI oral treatment failure, highlighting the importance of confirming susceptibilities before committing to an oral treatment plan.18,19 Even ESBL Enterobacteriaceae can be considered for treatment with fluoroquinolones if susceptibilities allow.20
The ideal duration of therapy for GNR BSI is an area of active research. A recent retrospective trial showed no difference in all-cause mortality or recurrent BSI in GNR BSI treated for 8 versus 15 days.21 A recent meta-analysis suggested that 7 days of therapy was noninferior to a longer duration therapy (10–14 days) for pyelonephritis, in which a subset was bacteremic.22 However, another trial reported that short course therapy for GNR BSI (<7 days) is associated with higher risk of treatment failure.22 Further data are needed.
Gram-Negative Rods, Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common pathogen, intrinsically resistant to many antimicrobials, and readily develops antimicrobial resistance during therapy. Fluoroquinolones (such as ciprofloxacin, levofloxacin, and delafloxacin) are the only currently available oral agents with antipseudomonal activity. However, fluoroquinolones may not achieve blood concentrations appropriate for P. aeruginosa treatment at standard doses, while higher dose regimens may be associated with increased risk for undesirable side effects.24,25 Currently, given the minimal trial data comparing oral versus intravenous therapy for P. aeruginosa BSIs, and multiple studies indicating increased mortality when P. aeruginosa is treated inappropriately,26,27 we prefer a conservative approach and consider oral therapy a less-preferred option.
Evidence Regarding Bloodstream Infections due to Gram-Positive Cocci
The majority of bloodstream infections in the United States, and the resultant morbidity and mortality, are from gram-positive cocci (GPCs) such as Staphylococcus, Streptococcus, and Enterococcus species.1
Gram-Positive Cocci, Streptococcus pneumoniae
Of the approximately 900,000 annual cases of S. pneumoniae infection in the United States, approximately 40,000 are complicated by BSI, with 70% of those cases being secondary to pneumococcal pneumonia.28 In studies on patients with pneumococcal pneumonia, bacteremic cases generally fare worse than those without bacteremia.29,30 However, several trials demonstrated comparable outcomes in the setting of bacteremic pneumococcal pneumonia when switched early (within 3 days) from intravenous to oral antibiotics to complete a 7-day course.31,32 Before pneumococcal penicillin resistance became widespread, oral penicillin was shown to be effective, and remains an option for susceptible strains.33 It is worth noting, however, that other trials have shown a mortality benefit from treating bacteremic pneumococcal pneumonia initially with dual-therapy including a β-lactam and macrolide such as azithromycin. This observation highlights the importance of knowing the final susceptibility data prior to consolidating to monotherapy with an oral agent, and that macrolides may have beneficial anti-inflammatory effects, though further research is needed.34,35
Although the evidence for treating bacteremic pneumococcal pneumonia using a highly active and absorbable oral agent is fairly robust, S. pneumoniae BSI secondary to other sites of infection sites is less well studied and may require a more conservative approach.
Gram-Positive Cocci, β-hemolytic Streptococcus species
β-Hemolytic Streptococci include groups A to H, of which groups A (S. pyogenes) and B (S. agalactiae) are the most commonly implicated in BSIs.36 Group A Streptococcus (GAS) is classically associated with streptococcal pharyngitis and Group B Streptococcus (GBS) is associated with postpartum endometritis and neonatal meningitis, though both are virulent organisms with a potential to cause invasive infection throughout the body and in all age-groups. Up to 14% of GAS and 41% GBS BSIs have no clear source;37,38 given these are skin pathogens, such scenarios likely represent invasion via microabrasion. As β-hemolytic streptococcal BSI is often observed in the context of necrotizing skin and soft tissue infections, surgical source control is particularly important.39 GAS remains exquisitely susceptible to penicillin, and intravenous penicillin remains the mainstay for invasive disease; GBS has higher penicillin resistance rates than GAS.40 Clindamycin should be added when there is concern for severe disease or toxic shock.41 Unfortunately, oral penicillin is poorly bioavailable (approximately 50%), and there has been recent concern regarding inducible clindamycin resistance in GAS.42 Thus, oral penicillin V and/or clindamycin is a potentially risky strategy, with no clinical trials supporting this approach; however, they may be reasonable options in selected patients with source control and stable hemodynamics. Amoxicillin has high bioavailability (85%) and may be effective; however, there is lack of supporting data. Highly bioavailable agents such as levofloxacin and linezolid have GAS and GBS activity43 and might be expected to produce satisfactory outcomes. Because no clinical trials have compared these agents with intravenous therapy for BSI, caution is advised. Although bacteriostatic against Staphylococcus, linezolid is bactericidal against Streptococcus.44 Fluoroquinolone resistance amongst β-hemolytic Streptococcus is rare (approximately 0.5%) but does occur.45
Gram-Positive Cocci, Staphylococcus Species
Staphylococcus species include S. aureus (including methicillin susceptible and resistant strains: MSSA and MRSA, respectively) and coagulase-negative species, which include organisms such as S. epidermidis. S. aureus is the most common cause of BSI mortality in the United States,1 with mortality rates estimated at 20%–40% per episode.46 Infectious disease consultation has been associated with decreased mortality and is recommended.47 The guidelines of the Infectious Diseases Society of America for the treatment of MRSA recommend the use of parenteral agents for BSI.48 It is important to consider if a patient with S. aureus BSI has infective endocarditis.
Oral antibiotic therapy for S. aureus BSI is not currently standard practice. Although trimethoprim-sulfamethoxazole (TMP-SMX) has favorable pharmacokinetics and case series of using it successfully for BSI exist,49 TMP-SMX showed inferior outcomes in a randomized trial comparing oral TMP-SMX with intravenous vancomycin in a series of 101 S. aureus infections.50 This observation has been replicated.51 Data on doxycycline or clindamycin for S. aureus BSI are limited, and IDSA guidelines advise against their use in this setting because they are predominantly bacteriostatic.48 Linezolid has favorable pharmacokinetics, with approximately 100% bioavailability, and S. aureus resistance to linezolid is rare.52 Several randomized trials have compared oral linezolid with intravenous vancomycin for S. aureus BSI; for instance, Stevens et al. randomized 460 patients with S. aureus infection (of whom 18% had BSI) to linezolid versus vancomycin and observed similar clinical cure rates.53 A pooled analysis showed oral linezolid was noninferior to vancomycin specifically for S. aureus BSI.54 However, long-term use is often limited by hematologic toxicity, peripheral or optic neuropathy (which can be permanent), and induced serotonin syndrome. Additionally, linezolid is bacteriostatic, not bactericidal against S. aureus. Using oral linezolid as a first-line option for S. aureus BSI would not be recommended; however, it may be used as a second-line treatment option in selected cases. Tedizolid has similar pharmacokinetics and spectrum of activity with fewer side effects; however, clinical data on its use for S. aureus BSI are lacking.55 Fluoroquinolones such as levofloxacin and the newer agent delafloxacin have activity against S. aureus, including MRSA, but on-treatment emergence of fluoroquinolone resistance is a concern, and data on delafloxacin for BSI are lacking.56,57 Older literature suggested the combination of ciprofloxacin and rifampin was effective against right-sided S. aureus endocarditis,58 and other oral fluoroquinolone-rifamycin combinations have also been found to be effective59 However, this approach is currently not a standard therapy, nor is it widely used. Decisions on the duration of therapy for S. aureus BSI should be made in conjunction with an infectious diseases specialist; 14 days is currently regarded as a minimum.47,48
Published data regarding oral treatment of coagulase-negative Staphylococcus (CoNS) BSI are limited. Most CoNS bacteremia and up to 80% Staphylococcus epidermidis bacteremia represent blood culture contamination, though true infection from CoNS is not uncommon, particularly in patients with indwelling catheters.60 An exception is the CoNS species Staphylococcus lugdunensis, which is more virulent, and bacteremia with this organism usually warrants antibiotics. Oral antimicrobial therapy is currently not a standard treatment practice for CoNS BSI that is felt to represent true infection; however, linezolid has been successfully used in case series.61
Gram-Positive Cocci, Enterococcus
E. faecium and E. faecalis are commonly implicated in BSI.1 Similar to S. aureus, infective endocarditis must be ruled out when treating enterococcus BSI; a scoring system has been proposed to assist in deciding if such patients require echocardiography.62 Intravenous ampicillin is a preferred, highly effective agent for enterococci treatment when the organism is susceptible.44 However, oral ampicillin has poor bioavailability (50%), and data for its use in BSI are lacking. For susceptible strains, amoxicillin has comparable efficacy for enterococci and enhanced bioavailability (85%); high dose oral amoxicillin could be considered, but there is minimal clinical trial data to support this approach. Fluoroquinolones exhibit only modest activity against enterococci and would be an inferior choice for BSI.63 Although often sensitive to oral tetracyclines, data on their use in enterococcal BSI are insufficient. Nitrofurantoin can be used for susceptible enterococcal urinary tract infection; however, it does not achieve high blood concentrations and should not be used for BSI.
There is significant data comparing oral linezolid with intravenous daptomycin for vancomycin-resistant enterococci (VRE) BSI. In a systematic review including 10 trials using 30-day all-cause mortality as the primary outcome, patients treated with daptomycin demonstrated higher odds of death (OR 1.61, 95% CI 1.08–2.40) compared with those treated with linezolid.64 However, more recent data suggested that higher daptomycin doses than those used in these earlier trials resulted in improved VRE BSI outcomes.65 A subsequent study reported that VRE BSI treatment with linezolid is associated with significantly higher treatment failure and mortality compared with daptomycin therapy.66 Further research is needed, but should the side-effect profile of linezolid be tolerable, it remains an effective option for oral treatment of enterococcal BSIs.
Evidence Regarding Anaerobic Bacterial Blood Stream Infection
Anaerobic bacteria include Bacteroides, Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Veillonella, and Clostridium. Anaerobes account for approximately 4% of bacterial BSIs, and are often seen in the context of polymicrobial infection.67 Given that anaerobes are difficult to recover, and that antimicrobial resistance testing is more labor intensive, antibiotic therapy choices are often made empirically.67 Unfortunately, antibiotic resistance amongst anaerobes is increasing.68 However, metronidazole remains highly active against a majority of anaerobes, with only a handful of treatment failures reported,69 and has a highly favorable pharmacokinetic profile for oral treatment. Oral metronidazole remains an effective choice for many anaerobic BSIs. Considering the polymicrobial nature of many anaerobic infections, source control is important, and concomitant GNR infection must be ruled out before using metronidazole monotherapy.
Clindamycin has significant anaerobic activity, but Bacteroides resistance has increased significantly in recent years, as high as 26%-44%.70 Amoxicillin-clavulanate has good anaerobic coverage, but bioavailability of clavulanate is limited (50%), making it inferior for BSI. Bioavailability is also limited for cephalosporins with anaerobic activity, such as cefuroxime. Moxifloxacin is a fluoroquinolone with some anaerobic coverage and a good oral pharmacokinetic profile, but Bacteroides resistance can be as high as 50%, making it a risky empiric choice.68
Conclusions
Bacterial BSIs are common and result in significant morbidity and mortality, with high associated healthcare costs. Although BSIs are traditionally treated with intravenous antimicrobials, many BSIs can be safely and effectively cured using oral antibiotics. When appropriately selected, oral antibiotics offer lower costs, fewer side effects, promote antimicrobial stewardship, and are easier for patients. Ultimately, the decision to use oral versus intravenous antibiotics must consider the characteristics of the pathogen, patient, and drug.
Disclosures
None of the authors report any conflicts of interest.
Bacterial bloodstream infections (BSIs) are a major cause of morbidity and mortality in the United States. Approximately 600,000 BSI cases occur annually, resulting in 85,000 deaths,1 at a cost exceeding $1 billion.2 Traditionally, BSIs have been managed with intravenous antimicrobials, which rapidly achieve therapeutic blood concentrations, and are viewed as more potent than oral alternatives. Indeed, for acutely ill patients with bacteremia and sepsis, timely intravenous antimicrobials are lifesaving.3
However, whether intravenous antimicrobials are essential for the entire treatment course in BSIs, particularly for uncomplicated episodes, is controversial. Patients that are clinically stable or have been stabilized after an initial septic presentation may be appropriate candidates for treatment with oral antimicrobials. There are costs and risks associated with extended courses of intravenous agents, such as the necessity for long-term intravenous catheters, which entail risks for procedural complications, secondary infections, and thrombosis. A prospective study of 192 peripherally inserted central catheter (PICC) episodes reported an overall complication rate of 30.2%, including central line-associated BSIs (CLABSI) or venous thrombosis.4 Other studies also identified high rates of thrombosis5 and PICC-related CLABSI, particularly in patients with malignancy, where sepsis-related complications approach 25%.6 Additionally, appropriate care of indwelling catheters requires significant financial and healthcare resources.
Oral antimicrobial therapy for bacterial BSIs offers several potential benefits. Direct economic and healthcare workforce savings are expected to be significant, and procedural and catheter-related complications would be eliminated.7 Moreover, oral therapy provides antimicrobial stewardship by reducing the use of broad-spectrum intravenous agents.8 Recent infectious disease “Choosing Wisely” initiatives recommend clinicians “prefer oral formulations of highly bioavailable antimicrobials whenever possible”,9 and this approach is supported by the Centers for Disease Control and Prevention antibiotic stewardship program.10 However, the expected savings and benefits of oral therapy would be lost should they be less effective and result in treatment failure or relapse of the primary BSI. Pathogen susceptibility, gastrointestinal absorption, oral bioavailability, patient tolerability, and adherence with therapy need to be carefully considered before choosing oral antimicrobials. Thus, oral antimicrobial therapy for BSI should be utilized in carefully selected circumstances.
In this narrative review, we highlight areas where oral therapy is safe and effective in treating bloodstream infections, as well as offer guidance to clinicians managing patients experiencing BSI. Given the lack of robust clinical trials on this subject, the evidence for performing a systematic review was insufficient. Thus, the articles and recommendations cited in this review were selected based on the authors’ experiences to represent the best available evidence.
Infection Source Control
Pathogen and Antimicrobial Factors
Patient Factors
Evidence Regarding Bloodstream Infections due to Gram-Negative Rods
BSIs due to gram-negative rods (GNRs) are common and cause significant morbidity and mortality. GNRs represent a broad and diverse array of pathogens. We focus on the Enterobacteriaceae family and Pseudomonas aeruginosa, because they are frequently encountered in clinical practice.1
Gram-Negative Rods, Enterobacteriaceae Family
The Enterobacteriaceae family includes Escherichia coli, Klebsiella, Salmonella, Proteus, Enterobacter, Serratia, and Citrobacter species. The range of illnesses caused by Enterobacteriaceae is as diverse as the family, encompassing most body sites. Although most Enterobacteriaceae are intrinsically susceptible to antibiotics, there is potential for significant multi-drug resistance. Of particular recent concern has been the emergence of Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBL) and even carbapenem-resistant strains.14
However, Enterobacteriaceae species susceptible to oral antimicrobials are often suitable candidates for oral BSI therapy. Among 106 patients with GNR BSI treated with a highly bioavailable oral antibiotic (eg, levofloxacin), the treatment failure rate was only 2% (versus 14% when an antimicrobial with only moderate or low bioavailability was selected).15 Oral treatment of Enterobacteriaceae BSIs secondary to urinary tract infection has been best studied. A prospective randomized, controlled trial evaluated oral versus intravenous ciprofloxacin amongst 141 patients with severe pyelonephritis or complicated urinary tract infections, in which the rate of bacteremia was 38%.16 Notably, patients with obstruction or renal abscess were excluded from the trial. No significant differences in microbiological failure or unsatisfactory clinical responses were found between the IV and oral treatment groups. Additionally, a Cochrane review reported that oral antibiotic therapy is no less effective than intravenous therapy for severe UTI, although data on BSI frequency were not provided.17
Resistance to fluoroquinolones such as ciprofloxacin has been identified as a risk factor for GNR BSI oral treatment failure, highlighting the importance of confirming susceptibilities before committing to an oral treatment plan.18,19 Even ESBL Enterobacteriaceae can be considered for treatment with fluoroquinolones if susceptibilities allow.20
The ideal duration of therapy for GNR BSI is an area of active research. A recent retrospective trial showed no difference in all-cause mortality or recurrent BSI in GNR BSI treated for 8 versus 15 days.21 A recent meta-analysis suggested that 7 days of therapy was noninferior to a longer duration therapy (10–14 days) for pyelonephritis, in which a subset was bacteremic.22 However, another trial reported that short course therapy for GNR BSI (<7 days) is associated with higher risk of treatment failure.22 Further data are needed.
Gram-Negative Rods, Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common pathogen, intrinsically resistant to many antimicrobials, and readily develops antimicrobial resistance during therapy. Fluoroquinolones (such as ciprofloxacin, levofloxacin, and delafloxacin) are the only currently available oral agents with antipseudomonal activity. However, fluoroquinolones may not achieve blood concentrations appropriate for P. aeruginosa treatment at standard doses, while higher dose regimens may be associated with increased risk for undesirable side effects.24,25 Currently, given the minimal trial data comparing oral versus intravenous therapy for P. aeruginosa BSIs, and multiple studies indicating increased mortality when P. aeruginosa is treated inappropriately,26,27 we prefer a conservative approach and consider oral therapy a less-preferred option.
Evidence Regarding Bloodstream Infections due to Gram-Positive Cocci
The majority of bloodstream infections in the United States, and the resultant morbidity and mortality, are from gram-positive cocci (GPCs) such as Staphylococcus, Streptococcus, and Enterococcus species.1
Gram-Positive Cocci, Streptococcus pneumoniae
Of the approximately 900,000 annual cases of S. pneumoniae infection in the United States, approximately 40,000 are complicated by BSI, with 70% of those cases being secondary to pneumococcal pneumonia.28 In studies on patients with pneumococcal pneumonia, bacteremic cases generally fare worse than those without bacteremia.29,30 However, several trials demonstrated comparable outcomes in the setting of bacteremic pneumococcal pneumonia when switched early (within 3 days) from intravenous to oral antibiotics to complete a 7-day course.31,32 Before pneumococcal penicillin resistance became widespread, oral penicillin was shown to be effective, and remains an option for susceptible strains.33 It is worth noting, however, that other trials have shown a mortality benefit from treating bacteremic pneumococcal pneumonia initially with dual-therapy including a β-lactam and macrolide such as azithromycin. This observation highlights the importance of knowing the final susceptibility data prior to consolidating to monotherapy with an oral agent, and that macrolides may have beneficial anti-inflammatory effects, though further research is needed.34,35
Although the evidence for treating bacteremic pneumococcal pneumonia using a highly active and absorbable oral agent is fairly robust, S. pneumoniae BSI secondary to other sites of infection sites is less well studied and may require a more conservative approach.
Gram-Positive Cocci, β-hemolytic Streptococcus species
β-Hemolytic Streptococci include groups A to H, of which groups A (S. pyogenes) and B (S. agalactiae) are the most commonly implicated in BSIs.36 Group A Streptococcus (GAS) is classically associated with streptococcal pharyngitis and Group B Streptococcus (GBS) is associated with postpartum endometritis and neonatal meningitis, though both are virulent organisms with a potential to cause invasive infection throughout the body and in all age-groups. Up to 14% of GAS and 41% GBS BSIs have no clear source;37,38 given these are skin pathogens, such scenarios likely represent invasion via microabrasion. As β-hemolytic streptococcal BSI is often observed in the context of necrotizing skin and soft tissue infections, surgical source control is particularly important.39 GAS remains exquisitely susceptible to penicillin, and intravenous penicillin remains the mainstay for invasive disease; GBS has higher penicillin resistance rates than GAS.40 Clindamycin should be added when there is concern for severe disease or toxic shock.41 Unfortunately, oral penicillin is poorly bioavailable (approximately 50%), and there has been recent concern regarding inducible clindamycin resistance in GAS.42 Thus, oral penicillin V and/or clindamycin is a potentially risky strategy, with no clinical trials supporting this approach; however, they may be reasonable options in selected patients with source control and stable hemodynamics. Amoxicillin has high bioavailability (85%) and may be effective; however, there is lack of supporting data. Highly bioavailable agents such as levofloxacin and linezolid have GAS and GBS activity43 and might be expected to produce satisfactory outcomes. Because no clinical trials have compared these agents with intravenous therapy for BSI, caution is advised. Although bacteriostatic against Staphylococcus, linezolid is bactericidal against Streptococcus.44 Fluoroquinolone resistance amongst β-hemolytic Streptococcus is rare (approximately 0.5%) but does occur.45
Gram-Positive Cocci, Staphylococcus Species
Staphylococcus species include S. aureus (including methicillin susceptible and resistant strains: MSSA and MRSA, respectively) and coagulase-negative species, which include organisms such as S. epidermidis. S. aureus is the most common cause of BSI mortality in the United States,1 with mortality rates estimated at 20%–40% per episode.46 Infectious disease consultation has been associated with decreased mortality and is recommended.47 The guidelines of the Infectious Diseases Society of America for the treatment of MRSA recommend the use of parenteral agents for BSI.48 It is important to consider if a patient with S. aureus BSI has infective endocarditis.
Oral antibiotic therapy for S. aureus BSI is not currently standard practice. Although trimethoprim-sulfamethoxazole (TMP-SMX) has favorable pharmacokinetics and case series of using it successfully for BSI exist,49 TMP-SMX showed inferior outcomes in a randomized trial comparing oral TMP-SMX with intravenous vancomycin in a series of 101 S. aureus infections.50 This observation has been replicated.51 Data on doxycycline or clindamycin for S. aureus BSI are limited, and IDSA guidelines advise against their use in this setting because they are predominantly bacteriostatic.48 Linezolid has favorable pharmacokinetics, with approximately 100% bioavailability, and S. aureus resistance to linezolid is rare.52 Several randomized trials have compared oral linezolid with intravenous vancomycin for S. aureus BSI; for instance, Stevens et al. randomized 460 patients with S. aureus infection (of whom 18% had BSI) to linezolid versus vancomycin and observed similar clinical cure rates.53 A pooled analysis showed oral linezolid was noninferior to vancomycin specifically for S. aureus BSI.54 However, long-term use is often limited by hematologic toxicity, peripheral or optic neuropathy (which can be permanent), and induced serotonin syndrome. Additionally, linezolid is bacteriostatic, not bactericidal against S. aureus. Using oral linezolid as a first-line option for S. aureus BSI would not be recommended; however, it may be used as a second-line treatment option in selected cases. Tedizolid has similar pharmacokinetics and spectrum of activity with fewer side effects; however, clinical data on its use for S. aureus BSI are lacking.55 Fluoroquinolones such as levofloxacin and the newer agent delafloxacin have activity against S. aureus, including MRSA, but on-treatment emergence of fluoroquinolone resistance is a concern, and data on delafloxacin for BSI are lacking.56,57 Older literature suggested the combination of ciprofloxacin and rifampin was effective against right-sided S. aureus endocarditis,58 and other oral fluoroquinolone-rifamycin combinations have also been found to be effective59 However, this approach is currently not a standard therapy, nor is it widely used. Decisions on the duration of therapy for S. aureus BSI should be made in conjunction with an infectious diseases specialist; 14 days is currently regarded as a minimum.47,48
Published data regarding oral treatment of coagulase-negative Staphylococcus (CoNS) BSI are limited. Most CoNS bacteremia and up to 80% Staphylococcus epidermidis bacteremia represent blood culture contamination, though true infection from CoNS is not uncommon, particularly in patients with indwelling catheters.60 An exception is the CoNS species Staphylococcus lugdunensis, which is more virulent, and bacteremia with this organism usually warrants antibiotics. Oral antimicrobial therapy is currently not a standard treatment practice for CoNS BSI that is felt to represent true infection; however, linezolid has been successfully used in case series.61
Gram-Positive Cocci, Enterococcus
E. faecium and E. faecalis are commonly implicated in BSI.1 Similar to S. aureus, infective endocarditis must be ruled out when treating enterococcus BSI; a scoring system has been proposed to assist in deciding if such patients require echocardiography.62 Intravenous ampicillin is a preferred, highly effective agent for enterococci treatment when the organism is susceptible.44 However, oral ampicillin has poor bioavailability (50%), and data for its use in BSI are lacking. For susceptible strains, amoxicillin has comparable efficacy for enterococci and enhanced bioavailability (85%); high dose oral amoxicillin could be considered, but there is minimal clinical trial data to support this approach. Fluoroquinolones exhibit only modest activity against enterococci and would be an inferior choice for BSI.63 Although often sensitive to oral tetracyclines, data on their use in enterococcal BSI are insufficient. Nitrofurantoin can be used for susceptible enterococcal urinary tract infection; however, it does not achieve high blood concentrations and should not be used for BSI.
There is significant data comparing oral linezolid with intravenous daptomycin for vancomycin-resistant enterococci (VRE) BSI. In a systematic review including 10 trials using 30-day all-cause mortality as the primary outcome, patients treated with daptomycin demonstrated higher odds of death (OR 1.61, 95% CI 1.08–2.40) compared with those treated with linezolid.64 However, more recent data suggested that higher daptomycin doses than those used in these earlier trials resulted in improved VRE BSI outcomes.65 A subsequent study reported that VRE BSI treatment with linezolid is associated with significantly higher treatment failure and mortality compared with daptomycin therapy.66 Further research is needed, but should the side-effect profile of linezolid be tolerable, it remains an effective option for oral treatment of enterococcal BSIs.
Evidence Regarding Anaerobic Bacterial Blood Stream Infection
Anaerobic bacteria include Bacteroides, Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Veillonella, and Clostridium. Anaerobes account for approximately 4% of bacterial BSIs, and are often seen in the context of polymicrobial infection.67 Given that anaerobes are difficult to recover, and that antimicrobial resistance testing is more labor intensive, antibiotic therapy choices are often made empirically.67 Unfortunately, antibiotic resistance amongst anaerobes is increasing.68 However, metronidazole remains highly active against a majority of anaerobes, with only a handful of treatment failures reported,69 and has a highly favorable pharmacokinetic profile for oral treatment. Oral metronidazole remains an effective choice for many anaerobic BSIs. Considering the polymicrobial nature of many anaerobic infections, source control is important, and concomitant GNR infection must be ruled out before using metronidazole monotherapy.
Clindamycin has significant anaerobic activity, but Bacteroides resistance has increased significantly in recent years, as high as 26%-44%.70 Amoxicillin-clavulanate has good anaerobic coverage, but bioavailability of clavulanate is limited (50%), making it inferior for BSI. Bioavailability is also limited for cephalosporins with anaerobic activity, such as cefuroxime. Moxifloxacin is a fluoroquinolone with some anaerobic coverage and a good oral pharmacokinetic profile, but Bacteroides resistance can be as high as 50%, making it a risky empiric choice.68
Conclusions
Bacterial BSIs are common and result in significant morbidity and mortality, with high associated healthcare costs. Although BSIs are traditionally treated with intravenous antimicrobials, many BSIs can be safely and effectively cured using oral antibiotics. When appropriately selected, oral antibiotics offer lower costs, fewer side effects, promote antimicrobial stewardship, and are easier for patients. Ultimately, the decision to use oral versus intravenous antibiotics must consider the characteristics of the pathogen, patient, and drug.
Disclosures
None of the authors report any conflicts of interest.
1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501-509. PubMed
2. Kilgore M, Brossette S. Cost of bloodstream infections. Am J Infect Control. 2008;36(10):S172.e1-3. PubMed
3. Youkee D, Hulme W, Roberts T, Daniels R, Nutbeam T, Keep J. Time Matters: Antibiotic Timing in Sepsis and Septic Shock. Crit Care Med. 2016;44(10):e1016-1017. PubMed
4. Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;28;6:18. PubMed
5. Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, Waybill PN. Venous Thrombosis Associated with the Placement of Peripherally Inserted Central Catheters. J Vasc Interv Radiol. 2000;11(10):1309-1314. PubMed
6. Cheong K, Perry D, Karapetis C, Koczwara B. High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004;34(5):234-238. PubMed
7. Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006;90(6):1197-1222. PubMed
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014;5(2):83-87. PubMed
9. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
10. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
11. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
12. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393-407. PubMed
13. Cua YM, Kripalani S. Medication Use in the Transition from Hospital to Home. Ann Acad Med Singapore. 2008;37(2):136. PubMed
14. Paterson DL. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am J Med. 2006; 119(6):S20-28.
15. Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. PubMed
16. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M Oral vs Intravenous Ciprofloxacin in the Initial Empirical Management of Severe Pyelonephritis or Complicated Urinary Tract Infections: A Prospective Randomized Clinical Trial. Arch Intern Med. 1999;159(1):53-58. PubMed
17. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003237. PubMed
18. Brigmon MM, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Impact of fluoroquinolone resistance in Gram-negative bloodstream infections on healthcare utilization. Clin Microbiol Infect. 2015;21(9):843-849. PubMed
19. Ortega M, Marco F, Soriano A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63(3):568-574. PubMed
20. Lo CL, Lee CC, Li CW, et al. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2017;50(3):355-361. PubMed
21. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the Outcomes of Adults With Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score–Matched Cohort. Clin Infect Dis. 2017; cix767. doi.org/10.1093/cid/cix767 PubMed
22. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection-- 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183-2191. PubMed
23. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 2017;45(5):613-620. PubMed
24. Zelenitsky S, Ariano R, Harding G, Forrest A. Evaluating Ciprofloxacin Dosing for Pseudomonas aeruginosa Infection by Using Clinical Outcome-Based Monte Carlo Simulations. Antimicrob Agents Chemother. 2005;49(10):4009-4014. PubMed
25. Cazaubon Y, Bourguignon L, Goutelle S, Martin O, Maire P, Ducher M. Are ciprofloxacin dosage regimens adequate for antimicrobial efficacy and prevention of resistance? Pseudomonas aeruginosa bloodstream infection in elderly patients as a simulation case study. Fundam Clin Pharmacol. 2015;29(6):615-624. PubMed
26. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob Agents Chemother. 2005;49(4):1306-1311. PubMed
27. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of Combination Antimicrobial Therapy for Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756-2764. PubMed
28. The Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Emerging Infections Program Network Streptococcus pneumoniae, 2013. https://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Published November, 2014. Accessed September 26, 2017.
29. Brandenburg JA, Marrie TJ, Coley CM, et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000;15(9):638-646. PubMed
30. Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000;79(4):210-221. PubMed
31. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001; 161(6):848-850. PubMed
32. Oosterheert JJ, Bonten MJM, Schneider MME, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333(7580):1193. PubMed
33. Austrian R, Winston AL. The efficacy of penicillin V (phenoxymethyl-penicillin) in the treatment of mild and of moderately severe pneumococcal pneumonia. Am J Med Sci. 1956;232(6):624-628. PubMed
34. Waterer GW, Somes GW, Wunderink RG. Monotherapy May Be Suboptimal for Severe Bacteremic Pneumococcal Pneumonia. Arch Intern Med. 2001; 161(15):1837-1842. PubMed
35. Baddour LM, Yu VL, Klugman KP, et al. International Pneumococcal Study Group. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440-444. PubMed
36. Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic streptococcus group g: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112(8):622-626. PubMed
37. Davies HD, McGeer A, Schwartz B, Green, et al; Ontario Group A Streptococcal Study Group. Invasive Group A Streptococcal Infections in Ontario, Canada. N Engl J Med. 1996;335(8):547-554. PubMed
38. Farley MM, Harvey C, Stull T, et al. A Population-Based Assessment of Invasive Disease Due to Group B Streptococcus in Nonpregnant Adults. N Engl J Med. 1993;328(25):1807-1811. PubMed
39. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478-486. PubMed
40. Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo JJ. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994;38(9):2183-2186. PubMed
41. Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096-1100. PubMed
42. Chen I, Kaufisi P, Erdem G. Emergence of erythromycin- and clindamycin-resistant Streptococcus pyogenes emm 90 strains in Hawaii. J Clin Microbiol. 2011;49(1):439-441. PubMed
43. Biedenbach DJ, Jones RN. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25(1):47–51. PubMed
44. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT. Sanford Guide To Antimicrobial Therapy 2017. Dallas, TX. Antimicrobial Theapy, Inc, 2017.
45. Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn Microbiol Infect Dis. 2006;55(2):119-127. PubMed
46. Shurland S, Zhan M, Bradham DD, Roghmann M-C. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):2739. PubMed
47. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56(4):527-535. PubMed
48. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55. PubMed
49. Adra M, Lawrence KR. Trimethoprim/Sulfamethoxazole for Treatment of Severe Staphylococcus aureus Infections. Ann Pharmacother. 2004;38(2):338-341. PubMed
50. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117(5):390-398. PubMed
51. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ. 2015;350:h2219. PubMed
52. Sánchez García M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260-2264. PubMed
53. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481-1490. PubMed
54. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923-929. PubMed
55. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health-Syst Pharm. 2014;71(8):621-633. PubMed
56. Gade ND, Qazi MS. Fluoroquinolone Therapy in Staphylococcus aureus Infections: Where Do We Stand? J Lab Physicians. 2013;5(2):109-112. PubMed
57. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71(3):821-829. PubMed
58. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071-1073. PubMed
59. Schrenzel J, Harbarth S, Schockmel G, et al. A Randomized Clinical Trial to Compare Fleroxacin-Rifampicin with Flucloxacillin or Vancomycin for the Treatment of Staphylococcal Infection. Clin Infect Dis. 2004;39(9):1285-1292. PubMed
60. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. PubMed
61. Antony SJ, Diaz-Vasquez E, Stratton C. Clinical experience with linezolid in the treatment of resistant gram-positive infections. J Natl Med Assoc. 2001;93(10):386-391. PubMed
62. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis. 2015;60(4):528-535. PubMed
63. Martínez-Martínez L, Joyanes P, Pascual A, Terrero E, Perea EJ. Activity of eight fluoroquinolones against enterococci. Clin Microbiol Infect. 1997;3(4):497-499. PubMed
64. Balli EP, Venetis CA, Miyakis S. Systematic Review and Meta-Analysis of Linezolid versus Daptomycin for Treatment of Vancomycin-Resistant Enterococcal Bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-739. PubMed
70. Snydman DR, Jacobus NV, McDermott LA, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007;51(5):1649-1655. PubMed
69. Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis. 2010;50 Suppl 1:S26-33. PubMed
68. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2012;56(3):1247-1252. PubMed
67. Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26(6):1413-1417. PubMed
66. Britt NS, Potter EM, Patel N, Steed ME. Comparison of the Effectiveness and Safety of Linezolid and Daptomycin in Vancomycin-Resistant Enterococcal Bloodstream Infection: A National Cohort Study of Veterans Affairs Patients. Clin Infect Dis. 2015;61(6):871-878. PubMed
65. Chuang YC, Lin HY, Chen PY, et al. Effect of Daptomycin Dose on the Outcome of Vancomycin-Resistant, Daptomycin-Susceptible Enterococcus faecium Bacteremia. Clin Infect Dis. 2017;64(8):1026-1034. PubMed
1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501-509. PubMed
2. Kilgore M, Brossette S. Cost of bloodstream infections. Am J Infect Control. 2008;36(10):S172.e1-3. PubMed
3. Youkee D, Hulme W, Roberts T, Daniels R, Nutbeam T, Keep J. Time Matters: Antibiotic Timing in Sepsis and Septic Shock. Crit Care Med. 2016;44(10):e1016-1017. PubMed
4. Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;28;6:18. PubMed
5. Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, Waybill PN. Venous Thrombosis Associated with the Placement of Peripherally Inserted Central Catheters. J Vasc Interv Radiol. 2000;11(10):1309-1314. PubMed
6. Cheong K, Perry D, Karapetis C, Koczwara B. High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004;34(5):234-238. PubMed
7. Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006;90(6):1197-1222. PubMed
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014;5(2):83-87. PubMed
9. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
10. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
11. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
12. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393-407. PubMed
13. Cua YM, Kripalani S. Medication Use in the Transition from Hospital to Home. Ann Acad Med Singapore. 2008;37(2):136. PubMed
14. Paterson DL. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am J Med. 2006; 119(6):S20-28.
15. Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. PubMed
16. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M Oral vs Intravenous Ciprofloxacin in the Initial Empirical Management of Severe Pyelonephritis or Complicated Urinary Tract Infections: A Prospective Randomized Clinical Trial. Arch Intern Med. 1999;159(1):53-58. PubMed
17. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003237. PubMed
18. Brigmon MM, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Impact of fluoroquinolone resistance in Gram-negative bloodstream infections on healthcare utilization. Clin Microbiol Infect. 2015;21(9):843-849. PubMed
19. Ortega M, Marco F, Soriano A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63(3):568-574. PubMed
20. Lo CL, Lee CC, Li CW, et al. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2017;50(3):355-361. PubMed
21. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the Outcomes of Adults With Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score–Matched Cohort. Clin Infect Dis. 2017; cix767. doi.org/10.1093/cid/cix767 PubMed
22. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection-- 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183-2191. PubMed
23. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 2017;45(5):613-620. PubMed
24. Zelenitsky S, Ariano R, Harding G, Forrest A. Evaluating Ciprofloxacin Dosing for Pseudomonas aeruginosa Infection by Using Clinical Outcome-Based Monte Carlo Simulations. Antimicrob Agents Chemother. 2005;49(10):4009-4014. PubMed
25. Cazaubon Y, Bourguignon L, Goutelle S, Martin O, Maire P, Ducher M. Are ciprofloxacin dosage regimens adequate for antimicrobial efficacy and prevention of resistance? Pseudomonas aeruginosa bloodstream infection in elderly patients as a simulation case study. Fundam Clin Pharmacol. 2015;29(6):615-624. PubMed
26. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob Agents Chemother. 2005;49(4):1306-1311. PubMed
27. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of Combination Antimicrobial Therapy for Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756-2764. PubMed
28. The Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Emerging Infections Program Network Streptococcus pneumoniae, 2013. https://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Published November, 2014. Accessed September 26, 2017.
29. Brandenburg JA, Marrie TJ, Coley CM, et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000;15(9):638-646. PubMed
30. Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000;79(4):210-221. PubMed
31. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001; 161(6):848-850. PubMed
32. Oosterheert JJ, Bonten MJM, Schneider MME, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333(7580):1193. PubMed
33. Austrian R, Winston AL. The efficacy of penicillin V (phenoxymethyl-penicillin) in the treatment of mild and of moderately severe pneumococcal pneumonia. Am J Med Sci. 1956;232(6):624-628. PubMed
34. Waterer GW, Somes GW, Wunderink RG. Monotherapy May Be Suboptimal for Severe Bacteremic Pneumococcal Pneumonia. Arch Intern Med. 2001; 161(15):1837-1842. PubMed
35. Baddour LM, Yu VL, Klugman KP, et al. International Pneumococcal Study Group. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440-444. PubMed
36. Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic streptococcus group g: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112(8):622-626. PubMed
37. Davies HD, McGeer A, Schwartz B, Green, et al; Ontario Group A Streptococcal Study Group. Invasive Group A Streptococcal Infections in Ontario, Canada. N Engl J Med. 1996;335(8):547-554. PubMed
38. Farley MM, Harvey C, Stull T, et al. A Population-Based Assessment of Invasive Disease Due to Group B Streptococcus in Nonpregnant Adults. N Engl J Med. 1993;328(25):1807-1811. PubMed
39. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478-486. PubMed
40. Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo JJ. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994;38(9):2183-2186. PubMed
41. Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096-1100. PubMed
42. Chen I, Kaufisi P, Erdem G. Emergence of erythromycin- and clindamycin-resistant Streptococcus pyogenes emm 90 strains in Hawaii. J Clin Microbiol. 2011;49(1):439-441. PubMed
43. Biedenbach DJ, Jones RN. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25(1):47–51. PubMed
44. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT. Sanford Guide To Antimicrobial Therapy 2017. Dallas, TX. Antimicrobial Theapy, Inc, 2017.
45. Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn Microbiol Infect Dis. 2006;55(2):119-127. PubMed
46. Shurland S, Zhan M, Bradham DD, Roghmann M-C. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):2739. PubMed
47. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56(4):527-535. PubMed
48. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55. PubMed
49. Adra M, Lawrence KR. Trimethoprim/Sulfamethoxazole for Treatment of Severe Staphylococcus aureus Infections. Ann Pharmacother. 2004;38(2):338-341. PubMed
50. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117(5):390-398. PubMed
51. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ. 2015;350:h2219. PubMed
52. Sánchez García M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260-2264. PubMed
53. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481-1490. PubMed
54. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923-929. PubMed
55. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health-Syst Pharm. 2014;71(8):621-633. PubMed
56. Gade ND, Qazi MS. Fluoroquinolone Therapy in Staphylococcus aureus Infections: Where Do We Stand? J Lab Physicians. 2013;5(2):109-112. PubMed
57. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71(3):821-829. PubMed
58. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071-1073. PubMed
59. Schrenzel J, Harbarth S, Schockmel G, et al. A Randomized Clinical Trial to Compare Fleroxacin-Rifampicin with Flucloxacillin or Vancomycin for the Treatment of Staphylococcal Infection. Clin Infect Dis. 2004;39(9):1285-1292. PubMed
60. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. PubMed
61. Antony SJ, Diaz-Vasquez E, Stratton C. Clinical experience with linezolid in the treatment of resistant gram-positive infections. J Natl Med Assoc. 2001;93(10):386-391. PubMed
62. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis. 2015;60(4):528-535. PubMed
63. Martínez-Martínez L, Joyanes P, Pascual A, Terrero E, Perea EJ. Activity of eight fluoroquinolones against enterococci. Clin Microbiol Infect. 1997;3(4):497-499. PubMed
64. Balli EP, Venetis CA, Miyakis S. Systematic Review and Meta-Analysis of Linezolid versus Daptomycin for Treatment of Vancomycin-Resistant Enterococcal Bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-739. PubMed
70. Snydman DR, Jacobus NV, McDermott LA, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007;51(5):1649-1655. PubMed
69. Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis. 2010;50 Suppl 1:S26-33. PubMed
68. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2012;56(3):1247-1252. PubMed
67. Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26(6):1413-1417. PubMed
66. Britt NS, Potter EM, Patel N, Steed ME. Comparison of the Effectiveness and Safety of Linezolid and Daptomycin in Vancomycin-Resistant Enterococcal Bloodstream Infection: A National Cohort Study of Veterans Affairs Patients. Clin Infect Dis. 2015;61(6):871-878. PubMed
65. Chuang YC, Lin HY, Chen PY, et al. Effect of Daptomycin Dose on the Outcome of Vancomycin-Resistant, Daptomycin-Susceptible Enterococcus faecium Bacteremia. Clin Infect Dis. 2017;64(8):1026-1034. PubMed
© 2018 Society of Hospital Medicine
The Harm We Do: The Environmental Impact of Medicine
Healthcare is a “dirty” business with widespread effects on the environment. In the US, healthcare is estimated to generate 9.8% of our greenhouse gases and 9% of our particulate matter emissions.1 Hazardous wastes must be incinerated, emitting carbon dioxide, nitrogen oxides, and volatile substances into the atmosphere.2 Similarly, hospitals are responsible for 7% of commercial water use in the US.3 Conventional water treatment systems are not designed to remove heavy metals, pharmaceuticals, and disinfectants in hospital wastewaters; these compounds have been detected in rivers and streams throughout the US.4,5 Furthermore, pharmaceutical compounds such as antibiotics, anti-epileptics, and narcotics have even been isolated in our drinking water.5
As hospitalists, we are the directors of inpatient care, yet we only witness brief moments in the lives of our patients and the products we use for their care. For example, we are unaware of particulate matter emissions needed to power an extra imaging study or the contribution of unused materials to a growing landfill. However, pollution, including that from our clinical practice, is detrimental to human health in many ways. Exposure to particulate matter and toxic wastes has been linked to increased rates of reproductive and developmental disorders, cancer, and respiratory disease. 6 Particles <2.5 µm in diameter can diffuse through alveoli into the bloodstream, contributing to heart disease, stroke, and lung disease.7 Climate change has been linked to a wide range of adverse cardiovascular, respiratory, infectious, and mental health outcomes.8,9 These examples of the health impacts of pollution are illustrative but not exhaustive.
The environmental impact of US healthcare accounts for an estimated 470,000 disability-adjusted life years lost; this figure is on par with the burden of preventable medical errors.1 Clearly, change is necessary at all levels in the healthcare system to address our impact on human health. Fortunately, healthcare systems and hospital administrators have begun to address this issue. This perspective describes sustainability efforts in hospitals and healthcare systems and seeks to motivate hospitalists to build upon these efforts.
EFFORTS BY HOSPITALS AND HEALTHCARE SYSTEMS
With the ability to affect change from the top down, health systems are playing an important role in healthcare’s environmental sustainability. Ambitiously, Kaiser Permanente outlined eight environmental stewardship goals, which include becoming net carbon positive and recycling, reusing, or composting 100% of their non-hazardous waste by 2025.10 The Cleveland Clinic has pledged to become carbon neutral within the next 10 years.11 Other healthcare systems may follow suite. Many “green” interventions aimed at reducing waste and pollution also protect population health and reduce hospital operating costs.
From 2011 to 2015, a group of Boston Hospitals decreased energy use by 9.4% compared with a historical growth of 1.5% per year and saved over 15 million dollars.12 Similarly, Virginia Mason reduced landfill waste by reprocessing single-use medical devices, thereby decreasing purchasing costs by $3 million.13 As part of a regional campaign to protect the St. Croix River, Hudson Hospital and Clinic in Wisconsin saved over $20,000 with new recycling and waste reduction programs.13 Notably, these programs not only benefit hospitals but also patients and payers by reducing costs of care.
ROLE OF THE HOSPITALIST
These examples illustrate that a greener healthcare industry is achievable. Despite the potential benefits, sustainability efforts in US hospitals are the exception, not the rule, and the diffusion of such innovations must be encouraged from within.
In addition to the moral case for environmentally sustainable healthcare,14,15 such efforts can also improve our quality of care. The conversation around healthcare waste has focused on costs. Yet, examining our waste from a new perspective may reveal new ways to increase the value of patient care while protecting population health. Our communities and families are not immune to the health impacts of pollution, including that generated by our industry. However, predicted effects of climate change including altered patterns of vector-borne disease and frequent hurricanes and forest fires are upon us, affecting our communities, hospitals, and health delivery enterprise today. These challenges represent educational, academic, and economic opportunities that hospitalists should embrace.
RECOMMENDATIONS FOR ACTION
Education and Awareness
The first step to engagement is to promote awareness of the effects of healthcare waste. Physicians remain one of the most trusted sources of information about the health impacts of climate change.16 By educating ourselves, we can spread accurate knowledge to our patients and communities. Furthermore, we have the ability to advocate for our hospitals to follow institutions such as Kaiser Permanente and the Cleveland Clinic.
Given that hospitalists play a key role in educating students and residents, they are ideal vehicles for such dissemination. Education should begin in medical and nursing schools, where curricula detailing the importance and impact of healthcare pollution may be introduced. As hospitalists, we should champion such efforts.
Measurement and Amelioration
Second, resource use, waste production, and areas for improvement must be systematically quantified. At a national level, the Sustainable Development Unit of the National Health System (NHS) measures and reports water use, waste production, and energy consumption of the UK’s healthcare sector. Consequently, the NHS has surpassed their 2015 goal of reducing their carbon footprint by 10%.17 By establishing a baseline understanding of our carbon emissions, waste production, and water consumption, areas where physicians and hospitals can target improvement can similarly be identified.
Hospitalists appreciate the practical tradeoffs between clinical work and change efforts; thus, they are critical in establishing pragmatic policies. Physicians, often in collaboration with environmental engineers, have used evidence-based methods such as life-cycle analysis (LCA) to evaluate the environmental impacts of the pharmaceuticals and procedures that they use.18-20 An LCA is a cost-benefit analysis that examines multiple parameters of a product, namely, emissions, water use, costs, and waste production, from production to disposal. For example, an LCA of disposable custom packs for hysterectomies, vaginal deliveries, and laryngeal masks found costs savings and environmental benefits from choosing reusable over single-use items and removing unnecessary materials such as extra towels in this setting. 18-20 By considering the full life cycle of a procedure, LCAs reveal important information about the value and safety of care. LCAs, along with other sustainable design strategies, are tools that can provide hospitalists with new insights for quality improvement.
Public Reporting
Numerous physicians are known for educating their communities about the impacts of pollution on health. Recently, a pediatrician brought the presence of lead in Flint’s water supply to the public’s attention, instigating government action and policy change.21 A group called Utah Physicians for a Healthy Environment publishes online summaries of peer-reviewed information on air pollution and health. The Huma Lung Foundation led by a pulmonologist in Chennai, India, is working with a local radio station to report daily air quality measurements along with health advisories for the city.
We must now extend this paradigm to encompass transparency about healthcare’s practices and their impact on health. Indeed, the public is comfortable with this idea: a survey of 1011 respondents in the UK found that 92% indicated that the healthcare system should be environmentally sustainable.22 One idea may be a public-facing scorecard for hospitals, akin to publicly reported quality metrics. We can look to the example of the SDU and corporations such as Apple, which publicly report their carbon emissions, waste production, water use, and other metrics of their environmental impact. By galvanizing efforts to quantify and report our impact, hospitalists have the opportunity to be a role model for the industry and increase trust within their communities.
Individual Actions
What can a hospitalist do today? First, simple measures, like turning off idle electronics, recycling appropriately, or avoiding the use of unnecessary supplies or tests, are behavioral steps in the right direction. Second, just as education, goal setting, and feedback have met success in improving hand hygiene,23 we must begin the hard work of developing programs to monitor our environmental impact. Individual hospitalist carbon scores may help intensify efforts and spur improvement. Finally, we should learn and celebrate each other’s success. Renewed focus on this topic with increased reporting of interventions and outcomes is needed.
CONCLUSIONS
As hospitalists, we must look within ourselves to protect our planet and advocate for solutions that assure a sustainable future. By recognizing that a healthy environment is crucial to human health, we can set an example for other industries and create a safer world for our patients. Eliminating the harm we do is the first step in this process.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Chopra is supported by a Career Development Award from the Agency for Healthcare Quality and Research (1-K08-HS-022835-01).
1. Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. Ahmad S, ed. PLoS One. 2016;11(6):e0157014. doi:10.1371/journal.pone.0157014. PubMed
2. Windfeld ES, Brooks MS-L. Medical waste management–A review. J Environ Manage. 2015;163:98-108. doi:10.1016/j.jenvman.2015.08.013. PubMed
3. Environmental Protection Agency. Saving Water in Hospitals. Available at: https://www.epa.gov/sites/production/files/2017-01/documents/ws-commercial-factsheet-hospitals.pdf. Accessed December 9, 2017.
4. Kolpin DW, Furlong ET, Meyer MT, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999−2000: A national reconnaissance. Environ Sci & Technol 2002;36(6):1202-1211. PubMed
5. Deo, RP, Halden, RU. Pharmaceuticals in the built and natural water environment of the United States. Water. 2013;5(3):1346-1365. doi:10.3390/w5031346.
6. Lim SS, Vos T, Flaxman AD, Danaei G, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013; 380: 2224-60. PubMed
7. Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American heart association. Circulation. 2010;121(21):2331-2378. doi:10.1161/CIR.0b013e3181dbece1. PubMed
8. Watts N, Adger WN, Ayeb-Karlsson S, et al. The Lancet countdown: tracking progress on health and climate change. Lancet. 2017;389(10074):1151-1164. doi:10.1016/S0140-6736(16)32124-9. PubMed
9. Whitmee S, Haines A, Beyrer C, et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet. 2015;386(10007):1973–2028. PubMed
10. Kaiser Permanente. Environmental Stewardship. Available at: https://share.kaiserpermanente.org/article/environmental-stewardship-overview/. Accessed December 2, 2017.
11. Health Facilities Management Magazine. Cleveland Clinic makes carbon-neutrality its newest sustainability goal. Available at: https://www.hfmmagazine.com/articles/3210-cleveland-clinic-makes-carbon-neutrality-its-newest-sustainability-goal?lipi=urn%3Ali%3Apage%3Ad_flagship3_feed%3BHXuZOUrpQUu0OQ3RcUQqEg%3D%3D. Accessed December 2, 2017.
12. Healthcare without Harm. Metropolitan Boston Health Care Energy & Greenhouse Gas Profile: 2011 through 2015, and 2020 Projection. Available at: https://noharm-uscanada.org/sites/default/files/documents-files/4723/Report-Boston%20Health%20Care%20Energy%20Profile-May%202017.pdf Accessed December 9, 2017.
13. Practice Greenhealth. Advancing sustainability in healthcare: a collection of special case studies. Available at: https://practicegreenhealth.org/sites/default/files/upload-files/hhi.case_.studies.pdf. Accessed July 22, 2017.
14. Macpherson C, Hill J. Are physicians obliged to lead environmental sustainability efforts in health care organizations? AMA J Ethics. 2017;19(12):1164-1173. doi:10.1001/journalofethics.2017.19.12.ecas2-1712. PubMed
15. American Nurses Association. ANA’s principles of environmental health for nursing practice with implementation strategies. Available at: http://www.nursingworld.org/MainMenuCategories/WorkplaceSafety/Healthy-Nurse/ANAsPrinciplesofEnvironmentalHealthforNursingPractice.pd. Accessed December 9, 2017.
16. Maibach EW, Kreslake JM, Roser-Renouf C, et al. Do Americans understand that global warming is harmful to human health? Evidence from a national survey. Ann Glob Health. 2015;81(3):396-409. doi:10.1016/j.aogh.2015.08.010. PubMed
17. Healthcare without Harm. Reducing Healthcare’s Climate Footprint: opportunities for European Hospitals & Health Systems. Available at: https://noharm-europe.org/sites/default/files/documents-files/4746/HCWHEurope_Climate_Report_Dec2016.pdf. Accessed May 22, 2017.
18. Campion, N, Thiel, CL, Woods, et al. Sustainable healthcare and environmental life-cycle impacts of disposable supplies: a focus on disposable custom packs. J Clean Prod. 2015;94:46-55. doi:10.1016/j.jclepro.2015.01.076.
19. Eckelman M, Mosher M, Gonzalez A, et al. Comparative life cycle assessment of disposable and reusable laryngeal mask airways: Anesth Analg. 2012;114(5):1067-1072. doi:10.1213/ANE.0b013e31824f6959. PubMed
20. Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci & Technol. 2015;49(3):1779-1786. doi:10.1021/es504719g. PubMed
21. Hanna-Attisha M, LaChance J, Sadler RC, et al. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. PubMed
22. Sustainable Development Unit. Sustainability and the NHS, Public Health and Social Care system–Ipsos Mori survey. Available at: https://www.sduhealth.org.uk/policy-strategy/reporting/ipsos-mori.aspx. Accessed December 9, 2017.
23. Luangasanatip N, Hongsuwan M, Limmathurotsakul D, et al. Comparative efficacy of interventions to promote hand hygiene in hospital: systematic review and network meta-analysis. BMJ. 2015;351:h3728. doi:10.1136/bmj.h3728. PubMed
Healthcare is a “dirty” business with widespread effects on the environment. In the US, healthcare is estimated to generate 9.8% of our greenhouse gases and 9% of our particulate matter emissions.1 Hazardous wastes must be incinerated, emitting carbon dioxide, nitrogen oxides, and volatile substances into the atmosphere.2 Similarly, hospitals are responsible for 7% of commercial water use in the US.3 Conventional water treatment systems are not designed to remove heavy metals, pharmaceuticals, and disinfectants in hospital wastewaters; these compounds have been detected in rivers and streams throughout the US.4,5 Furthermore, pharmaceutical compounds such as antibiotics, anti-epileptics, and narcotics have even been isolated in our drinking water.5
As hospitalists, we are the directors of inpatient care, yet we only witness brief moments in the lives of our patients and the products we use for their care. For example, we are unaware of particulate matter emissions needed to power an extra imaging study or the contribution of unused materials to a growing landfill. However, pollution, including that from our clinical practice, is detrimental to human health in many ways. Exposure to particulate matter and toxic wastes has been linked to increased rates of reproductive and developmental disorders, cancer, and respiratory disease. 6 Particles <2.5 µm in diameter can diffuse through alveoli into the bloodstream, contributing to heart disease, stroke, and lung disease.7 Climate change has been linked to a wide range of adverse cardiovascular, respiratory, infectious, and mental health outcomes.8,9 These examples of the health impacts of pollution are illustrative but not exhaustive.
The environmental impact of US healthcare accounts for an estimated 470,000 disability-adjusted life years lost; this figure is on par with the burden of preventable medical errors.1 Clearly, change is necessary at all levels in the healthcare system to address our impact on human health. Fortunately, healthcare systems and hospital administrators have begun to address this issue. This perspective describes sustainability efforts in hospitals and healthcare systems and seeks to motivate hospitalists to build upon these efforts.
EFFORTS BY HOSPITALS AND HEALTHCARE SYSTEMS
With the ability to affect change from the top down, health systems are playing an important role in healthcare’s environmental sustainability. Ambitiously, Kaiser Permanente outlined eight environmental stewardship goals, which include becoming net carbon positive and recycling, reusing, or composting 100% of their non-hazardous waste by 2025.10 The Cleveland Clinic has pledged to become carbon neutral within the next 10 years.11 Other healthcare systems may follow suite. Many “green” interventions aimed at reducing waste and pollution also protect population health and reduce hospital operating costs.
From 2011 to 2015, a group of Boston Hospitals decreased energy use by 9.4% compared with a historical growth of 1.5% per year and saved over 15 million dollars.12 Similarly, Virginia Mason reduced landfill waste by reprocessing single-use medical devices, thereby decreasing purchasing costs by $3 million.13 As part of a regional campaign to protect the St. Croix River, Hudson Hospital and Clinic in Wisconsin saved over $20,000 with new recycling and waste reduction programs.13 Notably, these programs not only benefit hospitals but also patients and payers by reducing costs of care.
ROLE OF THE HOSPITALIST
These examples illustrate that a greener healthcare industry is achievable. Despite the potential benefits, sustainability efforts in US hospitals are the exception, not the rule, and the diffusion of such innovations must be encouraged from within.
In addition to the moral case for environmentally sustainable healthcare,14,15 such efforts can also improve our quality of care. The conversation around healthcare waste has focused on costs. Yet, examining our waste from a new perspective may reveal new ways to increase the value of patient care while protecting population health. Our communities and families are not immune to the health impacts of pollution, including that generated by our industry. However, predicted effects of climate change including altered patterns of vector-borne disease and frequent hurricanes and forest fires are upon us, affecting our communities, hospitals, and health delivery enterprise today. These challenges represent educational, academic, and economic opportunities that hospitalists should embrace.
RECOMMENDATIONS FOR ACTION
Education and Awareness
The first step to engagement is to promote awareness of the effects of healthcare waste. Physicians remain one of the most trusted sources of information about the health impacts of climate change.16 By educating ourselves, we can spread accurate knowledge to our patients and communities. Furthermore, we have the ability to advocate for our hospitals to follow institutions such as Kaiser Permanente and the Cleveland Clinic.
Given that hospitalists play a key role in educating students and residents, they are ideal vehicles for such dissemination. Education should begin in medical and nursing schools, where curricula detailing the importance and impact of healthcare pollution may be introduced. As hospitalists, we should champion such efforts.
Measurement and Amelioration
Second, resource use, waste production, and areas for improvement must be systematically quantified. At a national level, the Sustainable Development Unit of the National Health System (NHS) measures and reports water use, waste production, and energy consumption of the UK’s healthcare sector. Consequently, the NHS has surpassed their 2015 goal of reducing their carbon footprint by 10%.17 By establishing a baseline understanding of our carbon emissions, waste production, and water consumption, areas where physicians and hospitals can target improvement can similarly be identified.
Hospitalists appreciate the practical tradeoffs between clinical work and change efforts; thus, they are critical in establishing pragmatic policies. Physicians, often in collaboration with environmental engineers, have used evidence-based methods such as life-cycle analysis (LCA) to evaluate the environmental impacts of the pharmaceuticals and procedures that they use.18-20 An LCA is a cost-benefit analysis that examines multiple parameters of a product, namely, emissions, water use, costs, and waste production, from production to disposal. For example, an LCA of disposable custom packs for hysterectomies, vaginal deliveries, and laryngeal masks found costs savings and environmental benefits from choosing reusable over single-use items and removing unnecessary materials such as extra towels in this setting. 18-20 By considering the full life cycle of a procedure, LCAs reveal important information about the value and safety of care. LCAs, along with other sustainable design strategies, are tools that can provide hospitalists with new insights for quality improvement.
Public Reporting
Numerous physicians are known for educating their communities about the impacts of pollution on health. Recently, a pediatrician brought the presence of lead in Flint’s water supply to the public’s attention, instigating government action and policy change.21 A group called Utah Physicians for a Healthy Environment publishes online summaries of peer-reviewed information on air pollution and health. The Huma Lung Foundation led by a pulmonologist in Chennai, India, is working with a local radio station to report daily air quality measurements along with health advisories for the city.
We must now extend this paradigm to encompass transparency about healthcare’s practices and their impact on health. Indeed, the public is comfortable with this idea: a survey of 1011 respondents in the UK found that 92% indicated that the healthcare system should be environmentally sustainable.22 One idea may be a public-facing scorecard for hospitals, akin to publicly reported quality metrics. We can look to the example of the SDU and corporations such as Apple, which publicly report their carbon emissions, waste production, water use, and other metrics of their environmental impact. By galvanizing efforts to quantify and report our impact, hospitalists have the opportunity to be a role model for the industry and increase trust within their communities.
Individual Actions
What can a hospitalist do today? First, simple measures, like turning off idle electronics, recycling appropriately, or avoiding the use of unnecessary supplies or tests, are behavioral steps in the right direction. Second, just as education, goal setting, and feedback have met success in improving hand hygiene,23 we must begin the hard work of developing programs to monitor our environmental impact. Individual hospitalist carbon scores may help intensify efforts and spur improvement. Finally, we should learn and celebrate each other’s success. Renewed focus on this topic with increased reporting of interventions and outcomes is needed.
CONCLUSIONS
As hospitalists, we must look within ourselves to protect our planet and advocate for solutions that assure a sustainable future. By recognizing that a healthy environment is crucial to human health, we can set an example for other industries and create a safer world for our patients. Eliminating the harm we do is the first step in this process.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Chopra is supported by a Career Development Award from the Agency for Healthcare Quality and Research (1-K08-HS-022835-01).
Healthcare is a “dirty” business with widespread effects on the environment. In the US, healthcare is estimated to generate 9.8% of our greenhouse gases and 9% of our particulate matter emissions.1 Hazardous wastes must be incinerated, emitting carbon dioxide, nitrogen oxides, and volatile substances into the atmosphere.2 Similarly, hospitals are responsible for 7% of commercial water use in the US.3 Conventional water treatment systems are not designed to remove heavy metals, pharmaceuticals, and disinfectants in hospital wastewaters; these compounds have been detected in rivers and streams throughout the US.4,5 Furthermore, pharmaceutical compounds such as antibiotics, anti-epileptics, and narcotics have even been isolated in our drinking water.5
As hospitalists, we are the directors of inpatient care, yet we only witness brief moments in the lives of our patients and the products we use for their care. For example, we are unaware of particulate matter emissions needed to power an extra imaging study or the contribution of unused materials to a growing landfill. However, pollution, including that from our clinical practice, is detrimental to human health in many ways. Exposure to particulate matter and toxic wastes has been linked to increased rates of reproductive and developmental disorders, cancer, and respiratory disease. 6 Particles <2.5 µm in diameter can diffuse through alveoli into the bloodstream, contributing to heart disease, stroke, and lung disease.7 Climate change has been linked to a wide range of adverse cardiovascular, respiratory, infectious, and mental health outcomes.8,9 These examples of the health impacts of pollution are illustrative but not exhaustive.
The environmental impact of US healthcare accounts for an estimated 470,000 disability-adjusted life years lost; this figure is on par with the burden of preventable medical errors.1 Clearly, change is necessary at all levels in the healthcare system to address our impact on human health. Fortunately, healthcare systems and hospital administrators have begun to address this issue. This perspective describes sustainability efforts in hospitals and healthcare systems and seeks to motivate hospitalists to build upon these efforts.
EFFORTS BY HOSPITALS AND HEALTHCARE SYSTEMS
With the ability to affect change from the top down, health systems are playing an important role in healthcare’s environmental sustainability. Ambitiously, Kaiser Permanente outlined eight environmental stewardship goals, which include becoming net carbon positive and recycling, reusing, or composting 100% of their non-hazardous waste by 2025.10 The Cleveland Clinic has pledged to become carbon neutral within the next 10 years.11 Other healthcare systems may follow suite. Many “green” interventions aimed at reducing waste and pollution also protect population health and reduce hospital operating costs.
From 2011 to 2015, a group of Boston Hospitals decreased energy use by 9.4% compared with a historical growth of 1.5% per year and saved over 15 million dollars.12 Similarly, Virginia Mason reduced landfill waste by reprocessing single-use medical devices, thereby decreasing purchasing costs by $3 million.13 As part of a regional campaign to protect the St. Croix River, Hudson Hospital and Clinic in Wisconsin saved over $20,000 with new recycling and waste reduction programs.13 Notably, these programs not only benefit hospitals but also patients and payers by reducing costs of care.
ROLE OF THE HOSPITALIST
These examples illustrate that a greener healthcare industry is achievable. Despite the potential benefits, sustainability efforts in US hospitals are the exception, not the rule, and the diffusion of such innovations must be encouraged from within.
In addition to the moral case for environmentally sustainable healthcare,14,15 such efforts can also improve our quality of care. The conversation around healthcare waste has focused on costs. Yet, examining our waste from a new perspective may reveal new ways to increase the value of patient care while protecting population health. Our communities and families are not immune to the health impacts of pollution, including that generated by our industry. However, predicted effects of climate change including altered patterns of vector-borne disease and frequent hurricanes and forest fires are upon us, affecting our communities, hospitals, and health delivery enterprise today. These challenges represent educational, academic, and economic opportunities that hospitalists should embrace.
RECOMMENDATIONS FOR ACTION
Education and Awareness
The first step to engagement is to promote awareness of the effects of healthcare waste. Physicians remain one of the most trusted sources of information about the health impacts of climate change.16 By educating ourselves, we can spread accurate knowledge to our patients and communities. Furthermore, we have the ability to advocate for our hospitals to follow institutions such as Kaiser Permanente and the Cleveland Clinic.
Given that hospitalists play a key role in educating students and residents, they are ideal vehicles for such dissemination. Education should begin in medical and nursing schools, where curricula detailing the importance and impact of healthcare pollution may be introduced. As hospitalists, we should champion such efforts.
Measurement and Amelioration
Second, resource use, waste production, and areas for improvement must be systematically quantified. At a national level, the Sustainable Development Unit of the National Health System (NHS) measures and reports water use, waste production, and energy consumption of the UK’s healthcare sector. Consequently, the NHS has surpassed their 2015 goal of reducing their carbon footprint by 10%.17 By establishing a baseline understanding of our carbon emissions, waste production, and water consumption, areas where physicians and hospitals can target improvement can similarly be identified.
Hospitalists appreciate the practical tradeoffs between clinical work and change efforts; thus, they are critical in establishing pragmatic policies. Physicians, often in collaboration with environmental engineers, have used evidence-based methods such as life-cycle analysis (LCA) to evaluate the environmental impacts of the pharmaceuticals and procedures that they use.18-20 An LCA is a cost-benefit analysis that examines multiple parameters of a product, namely, emissions, water use, costs, and waste production, from production to disposal. For example, an LCA of disposable custom packs for hysterectomies, vaginal deliveries, and laryngeal masks found costs savings and environmental benefits from choosing reusable over single-use items and removing unnecessary materials such as extra towels in this setting. 18-20 By considering the full life cycle of a procedure, LCAs reveal important information about the value and safety of care. LCAs, along with other sustainable design strategies, are tools that can provide hospitalists with new insights for quality improvement.
Public Reporting
Numerous physicians are known for educating their communities about the impacts of pollution on health. Recently, a pediatrician brought the presence of lead in Flint’s water supply to the public’s attention, instigating government action and policy change.21 A group called Utah Physicians for a Healthy Environment publishes online summaries of peer-reviewed information on air pollution and health. The Huma Lung Foundation led by a pulmonologist in Chennai, India, is working with a local radio station to report daily air quality measurements along with health advisories for the city.
We must now extend this paradigm to encompass transparency about healthcare’s practices and their impact on health. Indeed, the public is comfortable with this idea: a survey of 1011 respondents in the UK found that 92% indicated that the healthcare system should be environmentally sustainable.22 One idea may be a public-facing scorecard for hospitals, akin to publicly reported quality metrics. We can look to the example of the SDU and corporations such as Apple, which publicly report their carbon emissions, waste production, water use, and other metrics of their environmental impact. By galvanizing efforts to quantify and report our impact, hospitalists have the opportunity to be a role model for the industry and increase trust within their communities.
Individual Actions
What can a hospitalist do today? First, simple measures, like turning off idle electronics, recycling appropriately, or avoiding the use of unnecessary supplies or tests, are behavioral steps in the right direction. Second, just as education, goal setting, and feedback have met success in improving hand hygiene,23 we must begin the hard work of developing programs to monitor our environmental impact. Individual hospitalist carbon scores may help intensify efforts and spur improvement. Finally, we should learn and celebrate each other’s success. Renewed focus on this topic with increased reporting of interventions and outcomes is needed.
CONCLUSIONS
As hospitalists, we must look within ourselves to protect our planet and advocate for solutions that assure a sustainable future. By recognizing that a healthy environment is crucial to human health, we can set an example for other industries and create a safer world for our patients. Eliminating the harm we do is the first step in this process.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Chopra is supported by a Career Development Award from the Agency for Healthcare Quality and Research (1-K08-HS-022835-01).
1. Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. Ahmad S, ed. PLoS One. 2016;11(6):e0157014. doi:10.1371/journal.pone.0157014. PubMed
2. Windfeld ES, Brooks MS-L. Medical waste management–A review. J Environ Manage. 2015;163:98-108. doi:10.1016/j.jenvman.2015.08.013. PubMed
3. Environmental Protection Agency. Saving Water in Hospitals. Available at: https://www.epa.gov/sites/production/files/2017-01/documents/ws-commercial-factsheet-hospitals.pdf. Accessed December 9, 2017.
4. Kolpin DW, Furlong ET, Meyer MT, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999−2000: A national reconnaissance. Environ Sci & Technol 2002;36(6):1202-1211. PubMed
5. Deo, RP, Halden, RU. Pharmaceuticals in the built and natural water environment of the United States. Water. 2013;5(3):1346-1365. doi:10.3390/w5031346.
6. Lim SS, Vos T, Flaxman AD, Danaei G, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013; 380: 2224-60. PubMed
7. Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American heart association. Circulation. 2010;121(21):2331-2378. doi:10.1161/CIR.0b013e3181dbece1. PubMed
8. Watts N, Adger WN, Ayeb-Karlsson S, et al. The Lancet countdown: tracking progress on health and climate change. Lancet. 2017;389(10074):1151-1164. doi:10.1016/S0140-6736(16)32124-9. PubMed
9. Whitmee S, Haines A, Beyrer C, et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet. 2015;386(10007):1973–2028. PubMed
10. Kaiser Permanente. Environmental Stewardship. Available at: https://share.kaiserpermanente.org/article/environmental-stewardship-overview/. Accessed December 2, 2017.
11. Health Facilities Management Magazine. Cleveland Clinic makes carbon-neutrality its newest sustainability goal. Available at: https://www.hfmmagazine.com/articles/3210-cleveland-clinic-makes-carbon-neutrality-its-newest-sustainability-goal?lipi=urn%3Ali%3Apage%3Ad_flagship3_feed%3BHXuZOUrpQUu0OQ3RcUQqEg%3D%3D. Accessed December 2, 2017.
12. Healthcare without Harm. Metropolitan Boston Health Care Energy & Greenhouse Gas Profile: 2011 through 2015, and 2020 Projection. Available at: https://noharm-uscanada.org/sites/default/files/documents-files/4723/Report-Boston%20Health%20Care%20Energy%20Profile-May%202017.pdf Accessed December 9, 2017.
13. Practice Greenhealth. Advancing sustainability in healthcare: a collection of special case studies. Available at: https://practicegreenhealth.org/sites/default/files/upload-files/hhi.case_.studies.pdf. Accessed July 22, 2017.
14. Macpherson C, Hill J. Are physicians obliged to lead environmental sustainability efforts in health care organizations? AMA J Ethics. 2017;19(12):1164-1173. doi:10.1001/journalofethics.2017.19.12.ecas2-1712. PubMed
15. American Nurses Association. ANA’s principles of environmental health for nursing practice with implementation strategies. Available at: http://www.nursingworld.org/MainMenuCategories/WorkplaceSafety/Healthy-Nurse/ANAsPrinciplesofEnvironmentalHealthforNursingPractice.pd. Accessed December 9, 2017.
16. Maibach EW, Kreslake JM, Roser-Renouf C, et al. Do Americans understand that global warming is harmful to human health? Evidence from a national survey. Ann Glob Health. 2015;81(3):396-409. doi:10.1016/j.aogh.2015.08.010. PubMed
17. Healthcare without Harm. Reducing Healthcare’s Climate Footprint: opportunities for European Hospitals & Health Systems. Available at: https://noharm-europe.org/sites/default/files/documents-files/4746/HCWHEurope_Climate_Report_Dec2016.pdf. Accessed May 22, 2017.
18. Campion, N, Thiel, CL, Woods, et al. Sustainable healthcare and environmental life-cycle impacts of disposable supplies: a focus on disposable custom packs. J Clean Prod. 2015;94:46-55. doi:10.1016/j.jclepro.2015.01.076.
19. Eckelman M, Mosher M, Gonzalez A, et al. Comparative life cycle assessment of disposable and reusable laryngeal mask airways: Anesth Analg. 2012;114(5):1067-1072. doi:10.1213/ANE.0b013e31824f6959. PubMed
20. Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci & Technol. 2015;49(3):1779-1786. doi:10.1021/es504719g. PubMed
21. Hanna-Attisha M, LaChance J, Sadler RC, et al. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. PubMed
22. Sustainable Development Unit. Sustainability and the NHS, Public Health and Social Care system–Ipsos Mori survey. Available at: https://www.sduhealth.org.uk/policy-strategy/reporting/ipsos-mori.aspx. Accessed December 9, 2017.
23. Luangasanatip N, Hongsuwan M, Limmathurotsakul D, et al. Comparative efficacy of interventions to promote hand hygiene in hospital: systematic review and network meta-analysis. BMJ. 2015;351:h3728. doi:10.1136/bmj.h3728. PubMed
1. Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. Ahmad S, ed. PLoS One. 2016;11(6):e0157014. doi:10.1371/journal.pone.0157014. PubMed
2. Windfeld ES, Brooks MS-L. Medical waste management–A review. J Environ Manage. 2015;163:98-108. doi:10.1016/j.jenvman.2015.08.013. PubMed
3. Environmental Protection Agency. Saving Water in Hospitals. Available at: https://www.epa.gov/sites/production/files/2017-01/documents/ws-commercial-factsheet-hospitals.pdf. Accessed December 9, 2017.
4. Kolpin DW, Furlong ET, Meyer MT, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999−2000: A national reconnaissance. Environ Sci & Technol 2002;36(6):1202-1211. PubMed
5. Deo, RP, Halden, RU. Pharmaceuticals in the built and natural water environment of the United States. Water. 2013;5(3):1346-1365. doi:10.3390/w5031346.
6. Lim SS, Vos T, Flaxman AD, Danaei G, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013; 380: 2224-60. PubMed
7. Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American heart association. Circulation. 2010;121(21):2331-2378. doi:10.1161/CIR.0b013e3181dbece1. PubMed
8. Watts N, Adger WN, Ayeb-Karlsson S, et al. The Lancet countdown: tracking progress on health and climate change. Lancet. 2017;389(10074):1151-1164. doi:10.1016/S0140-6736(16)32124-9. PubMed
9. Whitmee S, Haines A, Beyrer C, et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet. 2015;386(10007):1973–2028. PubMed
10. Kaiser Permanente. Environmental Stewardship. Available at: https://share.kaiserpermanente.org/article/environmental-stewardship-overview/. Accessed December 2, 2017.
11. Health Facilities Management Magazine. Cleveland Clinic makes carbon-neutrality its newest sustainability goal. Available at: https://www.hfmmagazine.com/articles/3210-cleveland-clinic-makes-carbon-neutrality-its-newest-sustainability-goal?lipi=urn%3Ali%3Apage%3Ad_flagship3_feed%3BHXuZOUrpQUu0OQ3RcUQqEg%3D%3D. Accessed December 2, 2017.
12. Healthcare without Harm. Metropolitan Boston Health Care Energy & Greenhouse Gas Profile: 2011 through 2015, and 2020 Projection. Available at: https://noharm-uscanada.org/sites/default/files/documents-files/4723/Report-Boston%20Health%20Care%20Energy%20Profile-May%202017.pdf Accessed December 9, 2017.
13. Practice Greenhealth. Advancing sustainability in healthcare: a collection of special case studies. Available at: https://practicegreenhealth.org/sites/default/files/upload-files/hhi.case_.studies.pdf. Accessed July 22, 2017.
14. Macpherson C, Hill J. Are physicians obliged to lead environmental sustainability efforts in health care organizations? AMA J Ethics. 2017;19(12):1164-1173. doi:10.1001/journalofethics.2017.19.12.ecas2-1712. PubMed
15. American Nurses Association. ANA’s principles of environmental health for nursing practice with implementation strategies. Available at: http://www.nursingworld.org/MainMenuCategories/WorkplaceSafety/Healthy-Nurse/ANAsPrinciplesofEnvironmentalHealthforNursingPractice.pd. Accessed December 9, 2017.
16. Maibach EW, Kreslake JM, Roser-Renouf C, et al. Do Americans understand that global warming is harmful to human health? Evidence from a national survey. Ann Glob Health. 2015;81(3):396-409. doi:10.1016/j.aogh.2015.08.010. PubMed
17. Healthcare without Harm. Reducing Healthcare’s Climate Footprint: opportunities for European Hospitals & Health Systems. Available at: https://noharm-europe.org/sites/default/files/documents-files/4746/HCWHEurope_Climate_Report_Dec2016.pdf. Accessed May 22, 2017.
18. Campion, N, Thiel, CL, Woods, et al. Sustainable healthcare and environmental life-cycle impacts of disposable supplies: a focus on disposable custom packs. J Clean Prod. 2015;94:46-55. doi:10.1016/j.jclepro.2015.01.076.
19. Eckelman M, Mosher M, Gonzalez A, et al. Comparative life cycle assessment of disposable and reusable laryngeal mask airways: Anesth Analg. 2012;114(5):1067-1072. doi:10.1213/ANE.0b013e31824f6959. PubMed
20. Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci & Technol. 2015;49(3):1779-1786. doi:10.1021/es504719g. PubMed
21. Hanna-Attisha M, LaChance J, Sadler RC, et al. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283-290. PubMed
22. Sustainable Development Unit. Sustainability and the NHS, Public Health and Social Care system–Ipsos Mori survey. Available at: https://www.sduhealth.org.uk/policy-strategy/reporting/ipsos-mori.aspx. Accessed December 9, 2017.
23. Luangasanatip N, Hongsuwan M, Limmathurotsakul D, et al. Comparative efficacy of interventions to promote hand hygiene in hospital: systematic review and network meta-analysis. BMJ. 2015;351:h3728. doi:10.1136/bmj.h3728. PubMed
© 2018 Society of Hospital Medicine
MDedge Daily News: Do tryptophan deficits fuel eating disorders?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Does a lack of tryptophan fuel eating disorders? How long to wait for results in ankylosing spondylitis, clarithromycin and heart disease may be a deadly duo, and why substance use disorder needs gender-specific treatment.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Does a lack of tryptophan fuel eating disorders? How long to wait for results in ankylosing spondylitis, clarithromycin and heart disease may be a deadly duo, and why substance use disorder needs gender-specific treatment.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Does a lack of tryptophan fuel eating disorders? How long to wait for results in ankylosing spondylitis, clarithromycin and heart disease may be a deadly duo, and why substance use disorder needs gender-specific treatment.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Expanded UCB product can stand alone
SALT LAKE CITY—The expanded umbilical cord blood (UCB) product NiCord can be used as a stand-alone graft, according to research presented at the 2018 BMT Tandem Meetings.
Researchers found that a single NiCord unit provided “robust” engraftment in a phase 1/2 study of patients with high-risk hematologic malignancies.
NiCord recipients had quicker neutrophil and platelet engraftment than matched control subjects who received standard myeloablative UCB transplant (single or double).
Mitchell Horwitz, MD, of the Duke University Medical Center in Durham, North Carolina, presented these results at the meeting as abstract 49.* The research was sponsored by Gamida Cell, the company developing NiCord.
“[NiCord] is an ex vivo expanded cell product that’s derived from an entire unit of umbilical cord blood,” Dr Horwitz explained. “It’s manufactured starting with a CD133-positive selection, which is the progenitor cell population that’s cultured, and a T-cell containing CD133-negative fraction that is provided also at the time of transplant.”
“The culture system contains nicotinamide—that’s the active ingredient in the culture. And that’s supplemented with cytokines—thrombopoietin, IL-6, FLT-3 ligand, and stem cell factor. The culture is 21 days.”
Previous research showed that double UCB transplant including a NiCord unit could provide benefits over standard double UCB transplant. This led Dr Horwitz and his colleagues to wonder if NiCord could be used as a stand-alone graft.
So the team evaluated the safety and efficacy of NiCord alone in 36 adolescents/adults with high-risk hematologic malignancies.
Patients had acute myelogenous leukemia (n=17), acute lymphoblastic leukemia (n=9), myelodysplastic syndrome (n=7), chronic myelogenous leukemia (n=2), and Hodgkin lymphoma (n=1).
Most patients had intermediate (n=15) or high-risk (n=13) disease. They had a median age of 44 (range, 13-63) and a median weight of 75 kg (range, 41-125).
Treatment
For conditioning, 19 patients received thiotepa, busulfan, and fludarabine. Fifteen patients received total body irradiation and fludarabine with or without cyclophosphamide or thiotepa. And 2 patients received clofarabine, fludarabine, and busulfan.
Most patients had a 4/6 human leukocyte antigen (HLA) match (n=26), 8 had a 5/6 HLA match, and 2 had a 6/6 HLA match.
The median total nucleated cell dose was 2.4 x 107/kg prior to expansion of the UCB unit and 3.7 x 107/kg after expansion. The median CD34+ cell dose was 0.2 x 106/kg and 6.3 x 106/kg, respectively.
“CD34 cells were expanded 33-fold in the 3-week culture system,” Dr Horwitz noted. “That translated to a median CD34 dose of 6.3 x 106/kg, a dose comparable to what would be obtained from an adult donor graft.”
Engraftment
There was 1 case of primary graft failure and 2 cases of secondary graft failure. One case of secondary graft failure was associated with an HHV-6 infection, and the other was due to a lethal adenovirus infection.
Of those patients who engrafted, 97% achieved full donor chimerism, and 3% had mixed chimerism.
Dr Horwitz and his colleagues compared engraftment results in the NiCord recipients to results in a cohort of patients from the CIBMTR registry who underwent UCB transplants from 2010 to 2013. They had similar characteristics as the NiCord patients—age, conditioning regimen, disease status, etc.
In total, there were 148 CIBMTR registry patients, 20% of whom received a single UCB unit.
The median time to neutrophil engraftment was 11.5 days (range, 6-26) with NiCord and 21 days in the CIBMTR matched control cohort (P<0.001). The cumulative incidence of neutrophil engraftment was 94.4% and 89.7%, respectively.
The median time to platelet engraftment was 34 days (range, 25-96) with NiCord and 46 days in the CIBMTR controls (P<0.001). The cumulative incidence of platelet engraftment was 80.6% and 67.1%, respectively.
“There’s a median 10-day reduction in neutrophil recovery [and] 12-day reduction in time to platelet recovery [with NiCord],” Dr Horwitz noted. “There is evidence of robust and durable engraftment with a NiCord unit, with one patient now over 7 years from his first transplant on the pilot trial.”
Relapse, survival, and GVHD
Dr Horwitz reported other outcomes in the NiCord recipients without making comparisons to the CIBMTR matched controls.
The estimated 2-year rate of non-relapse mortality in NiCord recipients was 23.8%, and the estimated 2-year incidence of relapse was 33.2%.
The estimated disease-free survival was 49.1% at 1 year and 43.0% at 2 years. The estimated overall survival was 51.2% at 1 year and 2 years.
At 100 days, the rate of grade 2-4 acute GVHD was 44.0%, and the rate of grade 3-4 acute GVHD was 11.1%.
The estimated 1-year rate of mild to severe chronic GVHD was 40.5%, and the estimated 2-year rate of moderate to severe chronic GVHD was 9.8%.
Dr Horwitz said these “promising results” have led to the launch of a phase 3 registration trial in which researchers are comparing NiCord to standard single or double UCB transplant. The trial is open for accrual.
*Information in the abstract differs from the presentation.
SALT LAKE CITY—The expanded umbilical cord blood (UCB) product NiCord can be used as a stand-alone graft, according to research presented at the 2018 BMT Tandem Meetings.
Researchers found that a single NiCord unit provided “robust” engraftment in a phase 1/2 study of patients with high-risk hematologic malignancies.
NiCord recipients had quicker neutrophil and platelet engraftment than matched control subjects who received standard myeloablative UCB transplant (single or double).
Mitchell Horwitz, MD, of the Duke University Medical Center in Durham, North Carolina, presented these results at the meeting as abstract 49.* The research was sponsored by Gamida Cell, the company developing NiCord.
“[NiCord] is an ex vivo expanded cell product that’s derived from an entire unit of umbilical cord blood,” Dr Horwitz explained. “It’s manufactured starting with a CD133-positive selection, which is the progenitor cell population that’s cultured, and a T-cell containing CD133-negative fraction that is provided also at the time of transplant.”
“The culture system contains nicotinamide—that’s the active ingredient in the culture. And that’s supplemented with cytokines—thrombopoietin, IL-6, FLT-3 ligand, and stem cell factor. The culture is 21 days.”
Previous research showed that double UCB transplant including a NiCord unit could provide benefits over standard double UCB transplant. This led Dr Horwitz and his colleagues to wonder if NiCord could be used as a stand-alone graft.
So the team evaluated the safety and efficacy of NiCord alone in 36 adolescents/adults with high-risk hematologic malignancies.
Patients had acute myelogenous leukemia (n=17), acute lymphoblastic leukemia (n=9), myelodysplastic syndrome (n=7), chronic myelogenous leukemia (n=2), and Hodgkin lymphoma (n=1).
Most patients had intermediate (n=15) or high-risk (n=13) disease. They had a median age of 44 (range, 13-63) and a median weight of 75 kg (range, 41-125).
Treatment
For conditioning, 19 patients received thiotepa, busulfan, and fludarabine. Fifteen patients received total body irradiation and fludarabine with or without cyclophosphamide or thiotepa. And 2 patients received clofarabine, fludarabine, and busulfan.
Most patients had a 4/6 human leukocyte antigen (HLA) match (n=26), 8 had a 5/6 HLA match, and 2 had a 6/6 HLA match.
The median total nucleated cell dose was 2.4 x 107/kg prior to expansion of the UCB unit and 3.7 x 107/kg after expansion. The median CD34+ cell dose was 0.2 x 106/kg and 6.3 x 106/kg, respectively.
“CD34 cells were expanded 33-fold in the 3-week culture system,” Dr Horwitz noted. “That translated to a median CD34 dose of 6.3 x 106/kg, a dose comparable to what would be obtained from an adult donor graft.”
Engraftment
There was 1 case of primary graft failure and 2 cases of secondary graft failure. One case of secondary graft failure was associated with an HHV-6 infection, and the other was due to a lethal adenovirus infection.
Of those patients who engrafted, 97% achieved full donor chimerism, and 3% had mixed chimerism.
Dr Horwitz and his colleagues compared engraftment results in the NiCord recipients to results in a cohort of patients from the CIBMTR registry who underwent UCB transplants from 2010 to 2013. They had similar characteristics as the NiCord patients—age, conditioning regimen, disease status, etc.
In total, there were 148 CIBMTR registry patients, 20% of whom received a single UCB unit.
The median time to neutrophil engraftment was 11.5 days (range, 6-26) with NiCord and 21 days in the CIBMTR matched control cohort (P<0.001). The cumulative incidence of neutrophil engraftment was 94.4% and 89.7%, respectively.
The median time to platelet engraftment was 34 days (range, 25-96) with NiCord and 46 days in the CIBMTR controls (P<0.001). The cumulative incidence of platelet engraftment was 80.6% and 67.1%, respectively.
“There’s a median 10-day reduction in neutrophil recovery [and] 12-day reduction in time to platelet recovery [with NiCord],” Dr Horwitz noted. “There is evidence of robust and durable engraftment with a NiCord unit, with one patient now over 7 years from his first transplant on the pilot trial.”
Relapse, survival, and GVHD
Dr Horwitz reported other outcomes in the NiCord recipients without making comparisons to the CIBMTR matched controls.
The estimated 2-year rate of non-relapse mortality in NiCord recipients was 23.8%, and the estimated 2-year incidence of relapse was 33.2%.
The estimated disease-free survival was 49.1% at 1 year and 43.0% at 2 years. The estimated overall survival was 51.2% at 1 year and 2 years.
At 100 days, the rate of grade 2-4 acute GVHD was 44.0%, and the rate of grade 3-4 acute GVHD was 11.1%.
The estimated 1-year rate of mild to severe chronic GVHD was 40.5%, and the estimated 2-year rate of moderate to severe chronic GVHD was 9.8%.
Dr Horwitz said these “promising results” have led to the launch of a phase 3 registration trial in which researchers are comparing NiCord to standard single or double UCB transplant. The trial is open for accrual.
*Information in the abstract differs from the presentation.
SALT LAKE CITY—The expanded umbilical cord blood (UCB) product NiCord can be used as a stand-alone graft, according to research presented at the 2018 BMT Tandem Meetings.
Researchers found that a single NiCord unit provided “robust” engraftment in a phase 1/2 study of patients with high-risk hematologic malignancies.
NiCord recipients had quicker neutrophil and platelet engraftment than matched control subjects who received standard myeloablative UCB transplant (single or double).
Mitchell Horwitz, MD, of the Duke University Medical Center in Durham, North Carolina, presented these results at the meeting as abstract 49.* The research was sponsored by Gamida Cell, the company developing NiCord.
“[NiCord] is an ex vivo expanded cell product that’s derived from an entire unit of umbilical cord blood,” Dr Horwitz explained. “It’s manufactured starting with a CD133-positive selection, which is the progenitor cell population that’s cultured, and a T-cell containing CD133-negative fraction that is provided also at the time of transplant.”
“The culture system contains nicotinamide—that’s the active ingredient in the culture. And that’s supplemented with cytokines—thrombopoietin, IL-6, FLT-3 ligand, and stem cell factor. The culture is 21 days.”
Previous research showed that double UCB transplant including a NiCord unit could provide benefits over standard double UCB transplant. This led Dr Horwitz and his colleagues to wonder if NiCord could be used as a stand-alone graft.
So the team evaluated the safety and efficacy of NiCord alone in 36 adolescents/adults with high-risk hematologic malignancies.
Patients had acute myelogenous leukemia (n=17), acute lymphoblastic leukemia (n=9), myelodysplastic syndrome (n=7), chronic myelogenous leukemia (n=2), and Hodgkin lymphoma (n=1).
Most patients had intermediate (n=15) or high-risk (n=13) disease. They had a median age of 44 (range, 13-63) and a median weight of 75 kg (range, 41-125).
Treatment
For conditioning, 19 patients received thiotepa, busulfan, and fludarabine. Fifteen patients received total body irradiation and fludarabine with or without cyclophosphamide or thiotepa. And 2 patients received clofarabine, fludarabine, and busulfan.
Most patients had a 4/6 human leukocyte antigen (HLA) match (n=26), 8 had a 5/6 HLA match, and 2 had a 6/6 HLA match.
The median total nucleated cell dose was 2.4 x 107/kg prior to expansion of the UCB unit and 3.7 x 107/kg after expansion. The median CD34+ cell dose was 0.2 x 106/kg and 6.3 x 106/kg, respectively.
“CD34 cells were expanded 33-fold in the 3-week culture system,” Dr Horwitz noted. “That translated to a median CD34 dose of 6.3 x 106/kg, a dose comparable to what would be obtained from an adult donor graft.”
Engraftment
There was 1 case of primary graft failure and 2 cases of secondary graft failure. One case of secondary graft failure was associated with an HHV-6 infection, and the other was due to a lethal adenovirus infection.
Of those patients who engrafted, 97% achieved full donor chimerism, and 3% had mixed chimerism.
Dr Horwitz and his colleagues compared engraftment results in the NiCord recipients to results in a cohort of patients from the CIBMTR registry who underwent UCB transplants from 2010 to 2013. They had similar characteristics as the NiCord patients—age, conditioning regimen, disease status, etc.
In total, there were 148 CIBMTR registry patients, 20% of whom received a single UCB unit.
The median time to neutrophil engraftment was 11.5 days (range, 6-26) with NiCord and 21 days in the CIBMTR matched control cohort (P<0.001). The cumulative incidence of neutrophil engraftment was 94.4% and 89.7%, respectively.
The median time to platelet engraftment was 34 days (range, 25-96) with NiCord and 46 days in the CIBMTR controls (P<0.001). The cumulative incidence of platelet engraftment was 80.6% and 67.1%, respectively.
“There’s a median 10-day reduction in neutrophil recovery [and] 12-day reduction in time to platelet recovery [with NiCord],” Dr Horwitz noted. “There is evidence of robust and durable engraftment with a NiCord unit, with one patient now over 7 years from his first transplant on the pilot trial.”
Relapse, survival, and GVHD
Dr Horwitz reported other outcomes in the NiCord recipients without making comparisons to the CIBMTR matched controls.
The estimated 2-year rate of non-relapse mortality in NiCord recipients was 23.8%, and the estimated 2-year incidence of relapse was 33.2%.
The estimated disease-free survival was 49.1% at 1 year and 43.0% at 2 years. The estimated overall survival was 51.2% at 1 year and 2 years.
At 100 days, the rate of grade 2-4 acute GVHD was 44.0%, and the rate of grade 3-4 acute GVHD was 11.1%.
The estimated 1-year rate of mild to severe chronic GVHD was 40.5%, and the estimated 2-year rate of moderate to severe chronic GVHD was 9.8%.
Dr Horwitz said these “promising results” have led to the launch of a phase 3 registration trial in which researchers are comparing NiCord to standard single or double UCB transplant. The trial is open for accrual.
*Information in the abstract differs from the presentation.
FDA approves abemaciclib plus aromatase inhibitor as initial therapy
Abemaciclib (Verzenio) in combination with an aromatase inhibitor has been approved as initial endocrine-based therapy for postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer, the US Food and Drug Administration announced in a press release.
Approval was based on the results of the MONARCH 3 study, a randomized, double-blind, placebo-controlled, multicenter clinical trial in postmenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer. A total of 493 patients were randomized to receive either abemaciclib 150 mg or placebo orally twice daily, plus the treating physician’s choice of letrozole or anastrozole. The estimated median progression-free survival (PFS) (RECIST 1.1) was 28.2 months (95% CI: 23.5, not reached) for patients receiving abemaciclib and 14.8 months (95% CI: 11.2, 19.2) for those receiving placebo (HR 0.540; 95% CI: 0.418, 0.698; p<0.0001).
The most common adverse reactions that were seen in at least 20% of patients receiving abemaciclib in MONARCH 3 and were reported at a rate more than 2% higher than the rates seen in the placebo arm were diarrhea, neutropenia, fatigue, infections, nausea, abdominal pain, anemia, vomiting, alopecia, decreased appetite, and leukopenia.
The recommended starting dose of abemaciclib in combination with an aromatase inhibitor is 150 mg twice daily orally with or without food.
Abemaciclib (Verzenio) is manufactured by Eli Lilly.
Full prescribing information is available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208855s000lbl.pdf.
Abemaciclib (Verzenio) in combination with an aromatase inhibitor has been approved as initial endocrine-based therapy for postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer, the US Food and Drug Administration announced in a press release.
Approval was based on the results of the MONARCH 3 study, a randomized, double-blind, placebo-controlled, multicenter clinical trial in postmenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer. A total of 493 patients were randomized to receive either abemaciclib 150 mg or placebo orally twice daily, plus the treating physician’s choice of letrozole or anastrozole. The estimated median progression-free survival (PFS) (RECIST 1.1) was 28.2 months (95% CI: 23.5, not reached) for patients receiving abemaciclib and 14.8 months (95% CI: 11.2, 19.2) for those receiving placebo (HR 0.540; 95% CI: 0.418, 0.698; p<0.0001).
The most common adverse reactions that were seen in at least 20% of patients receiving abemaciclib in MONARCH 3 and were reported at a rate more than 2% higher than the rates seen in the placebo arm were diarrhea, neutropenia, fatigue, infections, nausea, abdominal pain, anemia, vomiting, alopecia, decreased appetite, and leukopenia.
The recommended starting dose of abemaciclib in combination with an aromatase inhibitor is 150 mg twice daily orally with or without food.
Abemaciclib (Verzenio) is manufactured by Eli Lilly.
Full prescribing information is available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208855s000lbl.pdf.
Abemaciclib (Verzenio) in combination with an aromatase inhibitor has been approved as initial endocrine-based therapy for postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer, the US Food and Drug Administration announced in a press release.
Approval was based on the results of the MONARCH 3 study, a randomized, double-blind, placebo-controlled, multicenter clinical trial in postmenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer. A total of 493 patients were randomized to receive either abemaciclib 150 mg or placebo orally twice daily, plus the treating physician’s choice of letrozole or anastrozole. The estimated median progression-free survival (PFS) (RECIST 1.1) was 28.2 months (95% CI: 23.5, not reached) for patients receiving abemaciclib and 14.8 months (95% CI: 11.2, 19.2) for those receiving placebo (HR 0.540; 95% CI: 0.418, 0.698; p<0.0001).
The most common adverse reactions that were seen in at least 20% of patients receiving abemaciclib in MONARCH 3 and were reported at a rate more than 2% higher than the rates seen in the placebo arm were diarrhea, neutropenia, fatigue, infections, nausea, abdominal pain, anemia, vomiting, alopecia, decreased appetite, and leukopenia.
The recommended starting dose of abemaciclib in combination with an aromatase inhibitor is 150 mg twice daily orally with or without food.
Abemaciclib (Verzenio) is manufactured by Eli Lilly.
Full prescribing information is available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208855s000lbl.pdf.
HCV infection tied to premature ovarian senescence and a high miscarriage rate
Premenopausal women with hepatitis C virus (HCV) showed increased ovarian senescence, which was associated with a lower chance of live birth. Such women also had a greater risk of infertility, as reported in the Journal of Hepatology.
Researchers examined three cohort studies, which comprised an age-matched prospectively enrolled cohort study of 100 women who were HCV positive and had chronic liver disease, 50 women who were HBV positive and had CLD, and 100 healthy women; 1,998 HCV-infected women enrolled in the Platform for the Study of Viral Hepatitis Therapies (PITER) trial from Italy; and 6,085 women infected with HCV plus 20,415 uninfected women from a United States database, according to Aimilia Karampatou, MD, of the University of Bologna, Modena, Italy, and colleagues.
In the second group examined, the women from the PITER trial, miscarriages occurred in 42% of the HCV-infected women with 44.6% of these women experiencing multiple miscarriages. The total fertility rate, defined as the average number of children that would be born in a lifetime, was 0.7 for the HCV-infected women, compared with 1.37 in the general Italian population.
Infertility data from the large U.S. study was assessed from a total of 27,525 women (20,415 HCV negative and HIV negative; 6,805 HCV positive; and 305 HCV positive/HIV positive). Women with HCV showed a significantly higher probability of infertility compared with uninfected controls (odds ratio, 2.44), and those women dually infected with HCV and HIV were affected even more (OR, 3.64).
Primarily based on the observations of AMH, which in many of the HCV-positive women fell into the menopausal range, the researchers suggested that “the reduced reproductive capacity of women who are HCV positive is related to failing ovarian function and subsequent follicular depletion in the context of a more generalized dysfunction of other fertility-related factors.”
With regard to the effect of antiviral therapy, AMH levels remained stable in women who attained a sustained virologic response but continued to fall in those for whom the therapy was a failure.
“HCV infection significantly and negatively affects many aspects of fertility. It remains to be assessed whether antiviral therapy at a very early age can positively influence the occurrence of miscarriages and can prevent ovarian senescence because the latter has broader health implications than simply preserving fertility,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Karampatou A et al. J Hepatology. 2018;68:33-41.
Premenopausal women with hepatitis C virus (HCV) showed increased ovarian senescence, which was associated with a lower chance of live birth. Such women also had a greater risk of infertility, as reported in the Journal of Hepatology.
Researchers examined three cohort studies, which comprised an age-matched prospectively enrolled cohort study of 100 women who were HCV positive and had chronic liver disease, 50 women who were HBV positive and had CLD, and 100 healthy women; 1,998 HCV-infected women enrolled in the Platform for the Study of Viral Hepatitis Therapies (PITER) trial from Italy; and 6,085 women infected with HCV plus 20,415 uninfected women from a United States database, according to Aimilia Karampatou, MD, of the University of Bologna, Modena, Italy, and colleagues.
In the second group examined, the women from the PITER trial, miscarriages occurred in 42% of the HCV-infected women with 44.6% of these women experiencing multiple miscarriages. The total fertility rate, defined as the average number of children that would be born in a lifetime, was 0.7 for the HCV-infected women, compared with 1.37 in the general Italian population.
Infertility data from the large U.S. study was assessed from a total of 27,525 women (20,415 HCV negative and HIV negative; 6,805 HCV positive; and 305 HCV positive/HIV positive). Women with HCV showed a significantly higher probability of infertility compared with uninfected controls (odds ratio, 2.44), and those women dually infected with HCV and HIV were affected even more (OR, 3.64).
Primarily based on the observations of AMH, which in many of the HCV-positive women fell into the menopausal range, the researchers suggested that “the reduced reproductive capacity of women who are HCV positive is related to failing ovarian function and subsequent follicular depletion in the context of a more generalized dysfunction of other fertility-related factors.”
With regard to the effect of antiviral therapy, AMH levels remained stable in women who attained a sustained virologic response but continued to fall in those for whom the therapy was a failure.
“HCV infection significantly and negatively affects many aspects of fertility. It remains to be assessed whether antiviral therapy at a very early age can positively influence the occurrence of miscarriages and can prevent ovarian senescence because the latter has broader health implications than simply preserving fertility,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Karampatou A et al. J Hepatology. 2018;68:33-41.
Premenopausal women with hepatitis C virus (HCV) showed increased ovarian senescence, which was associated with a lower chance of live birth. Such women also had a greater risk of infertility, as reported in the Journal of Hepatology.
Researchers examined three cohort studies, which comprised an age-matched prospectively enrolled cohort study of 100 women who were HCV positive and had chronic liver disease, 50 women who were HBV positive and had CLD, and 100 healthy women; 1,998 HCV-infected women enrolled in the Platform for the Study of Viral Hepatitis Therapies (PITER) trial from Italy; and 6,085 women infected with HCV plus 20,415 uninfected women from a United States database, according to Aimilia Karampatou, MD, of the University of Bologna, Modena, Italy, and colleagues.
In the second group examined, the women from the PITER trial, miscarriages occurred in 42% of the HCV-infected women with 44.6% of these women experiencing multiple miscarriages. The total fertility rate, defined as the average number of children that would be born in a lifetime, was 0.7 for the HCV-infected women, compared with 1.37 in the general Italian population.
Infertility data from the large U.S. study was assessed from a total of 27,525 women (20,415 HCV negative and HIV negative; 6,805 HCV positive; and 305 HCV positive/HIV positive). Women with HCV showed a significantly higher probability of infertility compared with uninfected controls (odds ratio, 2.44), and those women dually infected with HCV and HIV were affected even more (OR, 3.64).
Primarily based on the observations of AMH, which in many of the HCV-positive women fell into the menopausal range, the researchers suggested that “the reduced reproductive capacity of women who are HCV positive is related to failing ovarian function and subsequent follicular depletion in the context of a more generalized dysfunction of other fertility-related factors.”
With regard to the effect of antiviral therapy, AMH levels remained stable in women who attained a sustained virologic response but continued to fall in those for whom the therapy was a failure.
“HCV infection significantly and negatively affects many aspects of fertility. It remains to be assessed whether antiviral therapy at a very early age can positively influence the occurrence of miscarriages and can prevent ovarian senescence because the latter has broader health implications than simply preserving fertility,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Karampatou A et al. J Hepatology. 2018;68:33-41.
FROM THE JOURNAL OF HEPATOLOGY
Key clinical point: HCV-positive women appear to undergo increased ovarian senescence.
Major finding: The fertility rate of HCV-positive women was 0.7 vs. 1.37 in the general population.
Study details: Three separate studies together comprising more than 30,000 HCV-infected and -uninfected women.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Karampatou A et al. J Hepatology. 2018;68:33-41.