User login
Clinical Challenges - March 2018 What's your diagnosis?
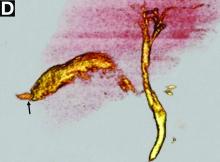
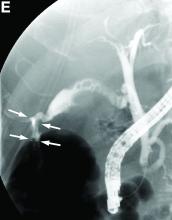
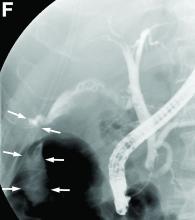
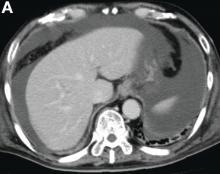
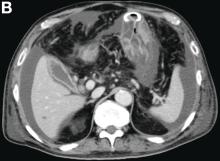
The diagnosis: Spontaneous gallbladder perforation
References
1. Ausania, F., Guzman Suarez, S., Alvarez Garcia, H. et al. Gallbladder perforation: morbidity, mortality and preoperative risk prediction. Surg Endosc. 2015;29:955-60.
2. Niemeier, O.W. Acute free perforation of the gall-bladder. Ann Surg. 1934;99:922-4.
3. Hyodo, T., Kumano, S., Kushihata, F. et al. CT and MR cholangiography: advantages and pitfalls in perioperative evaluation of biliary tree. Br J Radiol. 2012;85:887-96.
The diagnosis: Spontaneous gallbladder perforation
References
1. Ausania, F., Guzman Suarez, S., Alvarez Garcia, H. et al. Gallbladder perforation: morbidity, mortality and preoperative risk prediction. Surg Endosc. 2015;29:955-60.
2. Niemeier, O.W. Acute free perforation of the gall-bladder. Ann Surg. 1934;99:922-4.
3. Hyodo, T., Kumano, S., Kushihata, F. et al. CT and MR cholangiography: advantages and pitfalls in perioperative evaluation of biliary tree. Br J Radiol. 2012;85:887-96.
The diagnosis: Spontaneous gallbladder perforation
References
1. Ausania, F., Guzman Suarez, S., Alvarez Garcia, H. et al. Gallbladder perforation: morbidity, mortality and preoperative risk prediction. Surg Endosc. 2015;29:955-60.
2. Niemeier, O.W. Acute free perforation of the gall-bladder. Ann Surg. 1934;99:922-4.
3. Hyodo, T., Kumano, S., Kushihata, F. et al. CT and MR cholangiography: advantages and pitfalls in perioperative evaluation of biliary tree. Br J Radiol. 2012;85:887-96.
Published previously in Gastroenterology (2016;151[1]:40-2).
What is your diagnosis and treatment?
Can Online Patient Journals Improve MS Care?
SAN DIEGO—Continuous collection of patient-reported outcomes via a web-based platform may help neurologists identify patients with multiple sclerosis (MS) with active disease who would benefit from additional care coordination, according to a study described at the ACTRIMS 2018 Forum.
MS has a “variable and unpredictable disease course,” said Alexis Ahmad, Director of Clinical Trials for the Georgetown University Department of Neurology in Washington, DC, and colleagues. “Given the chronic and complex nature of MS, patients living with this disease require continuous and specialized care.”
The MS and Neuroimmunology Center at Georgetown University aims to become the first MS center recognized as a Patient-Centered Specialty Practice by the National Committee for Quality Assurance.
To assess whether a web-based platform to capture patient-reported outcomes can help MS practices meet Patient-Centered Specialty Practice standards and achieve MS care goals, the researchers analyzed patient responses in their center’s MS-Advance Study. The MS-Advance Study includes patients from the practice who use a web-based platform to relay patient-reported outcomes in between and at clinic visits.
Patients completed scales and questionnaires at baseline (eg, Modified Fatigue Impact Scale, Patient Health Questionnaire-9, Work Productivity and Activity Improvement Questionnaire, and Treatment Satisfaction Questionnaire for Medication) and journal entries about relapses, mood, energy, nutrition, sleep, and health service or resource utilization. The investigators analyzed responses from patients who completed at least one patient-reported outcome or journal entry between January and August 2017.
Five hundred twenty patients agreed to report outcomes using the online platform; 310 patients completed one or more patient-reported outcome or journal entry. In all, 259 patients reported daily MS sympto
“It is challenging for providers and clinicians to incorporate routine review of patient-reported outcomes into their clinic schedules,” the researchers noted. Strategies for reviewing patient-reported outcomes in clinic and eliciting more complete and timely patient reports are warranted, they said.
“Use of an online platform provides an opportunity to learn about patients’ daily experiences. Understanding these experiences can inform care strategies to address total functionality and other clinical outcomes.”
—Jake Remaly
SAN DIEGO—Continuous collection of patient-reported outcomes via a web-based platform may help neurologists identify patients with multiple sclerosis (MS) with active disease who would benefit from additional care coordination, according to a study described at the ACTRIMS 2018 Forum.
MS has a “variable and unpredictable disease course,” said Alexis Ahmad, Director of Clinical Trials for the Georgetown University Department of Neurology in Washington, DC, and colleagues. “Given the chronic and complex nature of MS, patients living with this disease require continuous and specialized care.”
The MS and Neuroimmunology Center at Georgetown University aims to become the first MS center recognized as a Patient-Centered Specialty Practice by the National Committee for Quality Assurance.
To assess whether a web-based platform to capture patient-reported outcomes can help MS practices meet Patient-Centered Specialty Practice standards and achieve MS care goals, the researchers analyzed patient responses in their center’s MS-Advance Study. The MS-Advance Study includes patients from the practice who use a web-based platform to relay patient-reported outcomes in between and at clinic visits.
Patients completed scales and questionnaires at baseline (eg, Modified Fatigue Impact Scale, Patient Health Questionnaire-9, Work Productivity and Activity Improvement Questionnaire, and Treatment Satisfaction Questionnaire for Medication) and journal entries about relapses, mood, energy, nutrition, sleep, and health service or resource utilization. The investigators analyzed responses from patients who completed at least one patient-reported outcome or journal entry between January and August 2017.
Five hundred twenty patients agreed to report outcomes using the online platform; 310 patients completed one or more patient-reported outcome or journal entry. In all, 259 patients reported daily MS sympto
“It is challenging for providers and clinicians to incorporate routine review of patient-reported outcomes into their clinic schedules,” the researchers noted. Strategies for reviewing patient-reported outcomes in clinic and eliciting more complete and timely patient reports are warranted, they said.
“Use of an online platform provides an opportunity to learn about patients’ daily experiences. Understanding these experiences can inform care strategies to address total functionality and other clinical outcomes.”
—Jake Remaly
SAN DIEGO—Continuous collection of patient-reported outcomes via a web-based platform may help neurologists identify patients with multiple sclerosis (MS) with active disease who would benefit from additional care coordination, according to a study described at the ACTRIMS 2018 Forum.
MS has a “variable and unpredictable disease course,” said Alexis Ahmad, Director of Clinical Trials for the Georgetown University Department of Neurology in Washington, DC, and colleagues. “Given the chronic and complex nature of MS, patients living with this disease require continuous and specialized care.”
The MS and Neuroimmunology Center at Georgetown University aims to become the first MS center recognized as a Patient-Centered Specialty Practice by the National Committee for Quality Assurance.
To assess whether a web-based platform to capture patient-reported outcomes can help MS practices meet Patient-Centered Specialty Practice standards and achieve MS care goals, the researchers analyzed patient responses in their center’s MS-Advance Study. The MS-Advance Study includes patients from the practice who use a web-based platform to relay patient-reported outcomes in between and at clinic visits.
Patients completed scales and questionnaires at baseline (eg, Modified Fatigue Impact Scale, Patient Health Questionnaire-9, Work Productivity and Activity Improvement Questionnaire, and Treatment Satisfaction Questionnaire for Medication) and journal entries about relapses, mood, energy, nutrition, sleep, and health service or resource utilization. The investigators analyzed responses from patients who completed at least one patient-reported outcome or journal entry between January and August 2017.
Five hundred twenty patients agreed to report outcomes using the online platform; 310 patients completed one or more patient-reported outcome or journal entry. In all, 259 patients reported daily MS sympto
“It is challenging for providers and clinicians to incorporate routine review of patient-reported outcomes into their clinic schedules,” the researchers noted. Strategies for reviewing patient-reported outcomes in clinic and eliciting more complete and timely patient reports are warranted, they said.
“Use of an online platform provides an opportunity to learn about patients’ daily experiences. Understanding these experiences can inform care strategies to address total functionality and other clinical outcomes.”
—Jake Remaly
Tenecteplase May Be Superior to Alteplase Before Thrombectomy
LOS ANGELES—Tenecteplase may be superior to alteplase for thrombolysis in patients with acute ischemic stroke who are scheduled to undergo thrombectomy, according to research presented at the International Stroke Conference 2018.
Comparing Two Thrombolytic Agents
The results came from the Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK) trial, a multicenter study of 202 patients in Australia and New Zealand. It was conducted from 2015 to 2017 at 18 hospitals. All enrolled patients received thrombolytic treatment within 4.5 hours of stroke onset and underwent endovascular thrombectomy within six hours of onset.
Investigators examined patients’ blood flow after they had received thrombolysis and undergone an initial angiogram, but before they underwent thrombectomy. The proportion of patients with robust blood flow, which was defined as a Thrombolysis in Cerebral Infarction (TICI) score of 2b or 3 or no retrievable thrombus, was 22% in the patients treated with
After the researchers adjusted the data, they found that tenecteplase was associated with 2.6 times greater odds of robust reperfusion, compared with alteplase. The result was statistically significant for the noninferiority and superiority of tenecteplase, said Bruce C. Campbell, MBBS, PhD, Professor of Neurology at the University of Melbourne and head of hyperacute stroke at Royal Melbourne Hospital.
These results, however, failed to show a significant between-group difference for improvement in NIH Stroke Scale score at three days after enrollment. This outcome occurred in 72% of the patients who received tenecteplase and 69% of patients who received alteplase. The 90-day modified Rankin Scale score was 0 to 2 or unchanged from baseline in 65% of the tenecteplase group and 52% of the alteplase group, a difference that did not reach statistical significance. All patients who enrolled were selected to undergo thrombectomy if their angiogram showed continued occlusion. Dr. Campbell did not report the number of patients who underwent this intervention, however.
The safety outcomes of death, symptomatic intracranial hemorrhage, and parenchymal hematoma occurred at statistically similar rates in both treatment arms.
Ongoing Research
Tenecteplase is a genetically modified tissue plasminogen activator with enhanced fibrin specificity that increases the drug’s half-life and allows for bolus administration, unlike alteplase, which needs continuous infusion. In the United States, the wholesale price per vial of tenecteplase is about $3,000 cheaper than that of alteplase, said Dr. Campbell.
Further data are needed before tenecteplase is ready for routine use, said Dr. Campbell. Two studies in progress are comparing tenecteplase with alteplase in patients with acute ischemic stroke who will not undergo endovascular thrombectomy. Dr. Campbell is leading a study that compares 0.25-mg/kg and 0.40-mg/kg doses of tenecteplase.
“I don’t think the data [that Dr. Campbell reported] are sufficient to say that tenecteplase is equivalent to alteplase. This [question] was studied in a select group of patients who had large-vessel occlusions and were transported to receive mechanical thrombectomy,” said William J. Powers, MD, Professor and Chair of Neurology at the University of North Carolina in Chapel Hill, who was not involved in the study. Most of the data on the efficacy of thrombolysis in patients with ischemic stroke have involved alteplase, he noted. Tenecteplase has FDA marketing approval only for treating patients with an acute myocardial infarction. Alteplase has FDA approval for treating acute ischemic stroke. Both drugs are marketed by Genentech.
Reports in recent years have suggested that treatment with tenecteplase appears to be at least as good as treatment with alteplase in patients with ischemic stroke. For example, a randomized trial that included 75 patients with ischemic stroke selected by imaging at three Australian centers found that treatment with tenecteplase produced a significant 24% improvement in the rate of arterial reperfusion and an average five-point improvement in NIH Stroke Scale score.
In addition, results from the NOR-TEST study showed that among 1,100 patients randomized at 13 Norwegian centers, the primary outcome of a 90-day modified Rankin Scale score of 0 to 1 was achieved by 64% of patients who received tenecteplase and 63% of patients who received alteplase.
In the EXTEND-IA TNK trial, tenecteplase appeared to act better than alteplase and had the extra advantage of being administered as a bolus injection, said Jeffrey L. Saver, MD, Professor of Neurology and Director of the Stroke Unit at the University of California, Los Angeles, in an interview at the meeting. “Alteplase is delivered as a drip, and it is often hard to get patients with an IV infusion out of the hospital quickly when you have to transport the patient. You need a nurse in the ambulance monitoring the drip. With tenecteplase, you administer the bolus and can then send the patient without an IV line, said Dr. Saver, who was not involved in the EXTEND-IA TNK study.
“Two other trials of tenecteplase, compared with alteplase, are now underway, so we will soon have a much larger evidence base for tenecteplase. This is the first large, multicenter, randomized trial to show an advantage for tenecteplase, but it failed to show a significant advantage for change in NIH Stroke Scale score. The results show a strong signal of benefit, but we need additional data from other trials.”
—Mitchel L. Zoler
Suggested Reading
Campbell BC, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before endovascular thrombectomy (EXTEND-IA TNK): A multicenter, randomized, controlled study. Int J Stroke. 2017 Sep 27 [Epub ahead of print].
Logallo N, Kvistad CE, Thomassen L. Therapeutic potential of tenecteplase in the management of acute ischemic stroke. CNS Drugs. 2015;29(10):811-818.
Logallo N, Novotny V, Assmus J, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017;16(10):781-788.
Parsons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Eng J Med. 2012;366(12):1099-1107.
LOS ANGELES—Tenecteplase may be superior to alteplase for thrombolysis in patients with acute ischemic stroke who are scheduled to undergo thrombectomy, according to research presented at the International Stroke Conference 2018.
Comparing Two Thrombolytic Agents
The results came from the Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK) trial, a multicenter study of 202 patients in Australia and New Zealand. It was conducted from 2015 to 2017 at 18 hospitals. All enrolled patients received thrombolytic treatment within 4.5 hours of stroke onset and underwent endovascular thrombectomy within six hours of onset.
Investigators examined patients’ blood flow after they had received thrombolysis and undergone an initial angiogram, but before they underwent thrombectomy. The proportion of patients with robust blood flow, which was defined as a Thrombolysis in Cerebral Infarction (TICI) score of 2b or 3 or no retrievable thrombus, was 22% in the patients treated with
After the researchers adjusted the data, they found that tenecteplase was associated with 2.6 times greater odds of robust reperfusion, compared with alteplase. The result was statistically significant for the noninferiority and superiority of tenecteplase, said Bruce C. Campbell, MBBS, PhD, Professor of Neurology at the University of Melbourne and head of hyperacute stroke at Royal Melbourne Hospital.
These results, however, failed to show a significant between-group difference for improvement in NIH Stroke Scale score at three days after enrollment. This outcome occurred in 72% of the patients who received tenecteplase and 69% of patients who received alteplase. The 90-day modified Rankin Scale score was 0 to 2 or unchanged from baseline in 65% of the tenecteplase group and 52% of the alteplase group, a difference that did not reach statistical significance. All patients who enrolled were selected to undergo thrombectomy if their angiogram showed continued occlusion. Dr. Campbell did not report the number of patients who underwent this intervention, however.
The safety outcomes of death, symptomatic intracranial hemorrhage, and parenchymal hematoma occurred at statistically similar rates in both treatment arms.
Ongoing Research
Tenecteplase is a genetically modified tissue plasminogen activator with enhanced fibrin specificity that increases the drug’s half-life and allows for bolus administration, unlike alteplase, which needs continuous infusion. In the United States, the wholesale price per vial of tenecteplase is about $3,000 cheaper than that of alteplase, said Dr. Campbell.
Further data are needed before tenecteplase is ready for routine use, said Dr. Campbell. Two studies in progress are comparing tenecteplase with alteplase in patients with acute ischemic stroke who will not undergo endovascular thrombectomy. Dr. Campbell is leading a study that compares 0.25-mg/kg and 0.40-mg/kg doses of tenecteplase.
“I don’t think the data [that Dr. Campbell reported] are sufficient to say that tenecteplase is equivalent to alteplase. This [question] was studied in a select group of patients who had large-vessel occlusions and were transported to receive mechanical thrombectomy,” said William J. Powers, MD, Professor and Chair of Neurology at the University of North Carolina in Chapel Hill, who was not involved in the study. Most of the data on the efficacy of thrombolysis in patients with ischemic stroke have involved alteplase, he noted. Tenecteplase has FDA marketing approval only for treating patients with an acute myocardial infarction. Alteplase has FDA approval for treating acute ischemic stroke. Both drugs are marketed by Genentech.
Reports in recent years have suggested that treatment with tenecteplase appears to be at least as good as treatment with alteplase in patients with ischemic stroke. For example, a randomized trial that included 75 patients with ischemic stroke selected by imaging at three Australian centers found that treatment with tenecteplase produced a significant 24% improvement in the rate of arterial reperfusion and an average five-point improvement in NIH Stroke Scale score.
In addition, results from the NOR-TEST study showed that among 1,100 patients randomized at 13 Norwegian centers, the primary outcome of a 90-day modified Rankin Scale score of 0 to 1 was achieved by 64% of patients who received tenecteplase and 63% of patients who received alteplase.
In the EXTEND-IA TNK trial, tenecteplase appeared to act better than alteplase and had the extra advantage of being administered as a bolus injection, said Jeffrey L. Saver, MD, Professor of Neurology and Director of the Stroke Unit at the University of California, Los Angeles, in an interview at the meeting. “Alteplase is delivered as a drip, and it is often hard to get patients with an IV infusion out of the hospital quickly when you have to transport the patient. You need a nurse in the ambulance monitoring the drip. With tenecteplase, you administer the bolus and can then send the patient without an IV line, said Dr. Saver, who was not involved in the EXTEND-IA TNK study.
“Two other trials of tenecteplase, compared with alteplase, are now underway, so we will soon have a much larger evidence base for tenecteplase. This is the first large, multicenter, randomized trial to show an advantage for tenecteplase, but it failed to show a significant advantage for change in NIH Stroke Scale score. The results show a strong signal of benefit, but we need additional data from other trials.”
—Mitchel L. Zoler
Suggested Reading
Campbell BC, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before endovascular thrombectomy (EXTEND-IA TNK): A multicenter, randomized, controlled study. Int J Stroke. 2017 Sep 27 [Epub ahead of print].
Logallo N, Kvistad CE, Thomassen L. Therapeutic potential of tenecteplase in the management of acute ischemic stroke. CNS Drugs. 2015;29(10):811-818.
Logallo N, Novotny V, Assmus J, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017;16(10):781-788.
Parsons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Eng J Med. 2012;366(12):1099-1107.
LOS ANGELES—Tenecteplase may be superior to alteplase for thrombolysis in patients with acute ischemic stroke who are scheduled to undergo thrombectomy, according to research presented at the International Stroke Conference 2018.
Comparing Two Thrombolytic Agents
The results came from the Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK) trial, a multicenter study of 202 patients in Australia and New Zealand. It was conducted from 2015 to 2017 at 18 hospitals. All enrolled patients received thrombolytic treatment within 4.5 hours of stroke onset and underwent endovascular thrombectomy within six hours of onset.
Investigators examined patients’ blood flow after they had received thrombolysis and undergone an initial angiogram, but before they underwent thrombectomy. The proportion of patients with robust blood flow, which was defined as a Thrombolysis in Cerebral Infarction (TICI) score of 2b or 3 or no retrievable thrombus, was 22% in the patients treated with
After the researchers adjusted the data, they found that tenecteplase was associated with 2.6 times greater odds of robust reperfusion, compared with alteplase. The result was statistically significant for the noninferiority and superiority of tenecteplase, said Bruce C. Campbell, MBBS, PhD, Professor of Neurology at the University of Melbourne and head of hyperacute stroke at Royal Melbourne Hospital.
These results, however, failed to show a significant between-group difference for improvement in NIH Stroke Scale score at three days after enrollment. This outcome occurred in 72% of the patients who received tenecteplase and 69% of patients who received alteplase. The 90-day modified Rankin Scale score was 0 to 2 or unchanged from baseline in 65% of the tenecteplase group and 52% of the alteplase group, a difference that did not reach statistical significance. All patients who enrolled were selected to undergo thrombectomy if their angiogram showed continued occlusion. Dr. Campbell did not report the number of patients who underwent this intervention, however.
The safety outcomes of death, symptomatic intracranial hemorrhage, and parenchymal hematoma occurred at statistically similar rates in both treatment arms.
Ongoing Research
Tenecteplase is a genetically modified tissue plasminogen activator with enhanced fibrin specificity that increases the drug’s half-life and allows for bolus administration, unlike alteplase, which needs continuous infusion. In the United States, the wholesale price per vial of tenecteplase is about $3,000 cheaper than that of alteplase, said Dr. Campbell.
Further data are needed before tenecteplase is ready for routine use, said Dr. Campbell. Two studies in progress are comparing tenecteplase with alteplase in patients with acute ischemic stroke who will not undergo endovascular thrombectomy. Dr. Campbell is leading a study that compares 0.25-mg/kg and 0.40-mg/kg doses of tenecteplase.
“I don’t think the data [that Dr. Campbell reported] are sufficient to say that tenecteplase is equivalent to alteplase. This [question] was studied in a select group of patients who had large-vessel occlusions and were transported to receive mechanical thrombectomy,” said William J. Powers, MD, Professor and Chair of Neurology at the University of North Carolina in Chapel Hill, who was not involved in the study. Most of the data on the efficacy of thrombolysis in patients with ischemic stroke have involved alteplase, he noted. Tenecteplase has FDA marketing approval only for treating patients with an acute myocardial infarction. Alteplase has FDA approval for treating acute ischemic stroke. Both drugs are marketed by Genentech.
Reports in recent years have suggested that treatment with tenecteplase appears to be at least as good as treatment with alteplase in patients with ischemic stroke. For example, a randomized trial that included 75 patients with ischemic stroke selected by imaging at three Australian centers found that treatment with tenecteplase produced a significant 24% improvement in the rate of arterial reperfusion and an average five-point improvement in NIH Stroke Scale score.
In addition, results from the NOR-TEST study showed that among 1,100 patients randomized at 13 Norwegian centers, the primary outcome of a 90-day modified Rankin Scale score of 0 to 1 was achieved by 64% of patients who received tenecteplase and 63% of patients who received alteplase.
In the EXTEND-IA TNK trial, tenecteplase appeared to act better than alteplase and had the extra advantage of being administered as a bolus injection, said Jeffrey L. Saver, MD, Professor of Neurology and Director of the Stroke Unit at the University of California, Los Angeles, in an interview at the meeting. “Alteplase is delivered as a drip, and it is often hard to get patients with an IV infusion out of the hospital quickly when you have to transport the patient. You need a nurse in the ambulance monitoring the drip. With tenecteplase, you administer the bolus and can then send the patient without an IV line, said Dr. Saver, who was not involved in the EXTEND-IA TNK study.
“Two other trials of tenecteplase, compared with alteplase, are now underway, so we will soon have a much larger evidence base for tenecteplase. This is the first large, multicenter, randomized trial to show an advantage for tenecteplase, but it failed to show a significant advantage for change in NIH Stroke Scale score. The results show a strong signal of benefit, but we need additional data from other trials.”
—Mitchel L. Zoler
Suggested Reading
Campbell BC, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before endovascular thrombectomy (EXTEND-IA TNK): A multicenter, randomized, controlled study. Int J Stroke. 2017 Sep 27 [Epub ahead of print].
Logallo N, Kvistad CE, Thomassen L. Therapeutic potential of tenecteplase in the management of acute ischemic stroke. CNS Drugs. 2015;29(10):811-818.
Logallo N, Novotny V, Assmus J, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017;16(10):781-788.
Parsons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Eng J Med. 2012;366(12):1099-1107.
Arm-placement indication for CGM approved by FDA
The Food and Drug Administration has approved a new indication for the Guardian Sensor 3 continuous glucose monitor (CGM), allowing patients to position it on their arms, according to an announcement by Medtronic, the sensor’s manufacturer.
Although previously only the abdomen had FDA approval as a CGM site, findings from at least one study have shown that most patients prefer to position their sensors elsewhere. This new indication gives these patients more flexibility in where they can place their CGMs. The site of CGM placement did not affect the failure rate or noncompliance.
The sensor works in tandem with an automated insulin delivery system known as the MiniMed 670G system, also manufactured by Medtronic. This system is approved for people with type 1 diabetes aged 14 years and older and adjusts basal insulin levels every 5 minutes – “based on real-time data delivered by the Guardian Sensor 3,” according to Medtronic.
Read more in Medtronic’s press release.
Correction, 3/21/18: A previous version of this article contained a photo of a device that was not the Guardian 3 Sensor.
The Food and Drug Administration has approved a new indication for the Guardian Sensor 3 continuous glucose monitor (CGM), allowing patients to position it on their arms, according to an announcement by Medtronic, the sensor’s manufacturer.
Although previously only the abdomen had FDA approval as a CGM site, findings from at least one study have shown that most patients prefer to position their sensors elsewhere. This new indication gives these patients more flexibility in where they can place their CGMs. The site of CGM placement did not affect the failure rate or noncompliance.
The sensor works in tandem with an automated insulin delivery system known as the MiniMed 670G system, also manufactured by Medtronic. This system is approved for people with type 1 diabetes aged 14 years and older and adjusts basal insulin levels every 5 minutes – “based on real-time data delivered by the Guardian Sensor 3,” according to Medtronic.
Read more in Medtronic’s press release.
Correction, 3/21/18: A previous version of this article contained a photo of a device that was not the Guardian 3 Sensor.
The Food and Drug Administration has approved a new indication for the Guardian Sensor 3 continuous glucose monitor (CGM), allowing patients to position it on their arms, according to an announcement by Medtronic, the sensor’s manufacturer.
Although previously only the abdomen had FDA approval as a CGM site, findings from at least one study have shown that most patients prefer to position their sensors elsewhere. This new indication gives these patients more flexibility in where they can place their CGMs. The site of CGM placement did not affect the failure rate or noncompliance.
The sensor works in tandem with an automated insulin delivery system known as the MiniMed 670G system, also manufactured by Medtronic. This system is approved for people with type 1 diabetes aged 14 years and older and adjusts basal insulin levels every 5 minutes – “based on real-time data delivered by the Guardian Sensor 3,” according to Medtronic.
Read more in Medtronic’s press release.
Correction, 3/21/18: A previous version of this article contained a photo of a device that was not the Guardian 3 Sensor.
Product Update: Vistara; Ultravision trocar; CompuFlo Epidural; and Philips ultrasound
PRENATAL SCREENING FOR SINGLE-GENE DISORDERS
Vistara®, a non-invasive prenatal test (NIPT) from Natera, Inc, screens for single-gene disorders after 9 weeks’ gestation. Complementing the Panorama® NIPT, Vistara tests for major anatomic abnormalities and chromosome imbalances that have a combined incidence rate of 1 in 600 (higher than Down syndrome). These mutations can cause severe conditions affecting skeletal, cardiac, and neurologic systems, such as Noonan syndrome, osteogenesis imperfecta, craniosynostosis syndromes, achondroplasia, and Rett syndrome. Standard NIPT commonly cannot detect these de novo (not inherited) mutations. Ultrasound exams may either completely miss the disorders or identify nonspecific findings later in pregnancy.
Natera says that Vistara has a combined analytical sensitivity of >99% and a combined analytical specificity of >99% in validation studies.
FOR MORE INFORMATION, VISIT: https://www.natera.com/vistara
ELECTROSTATIC SURGICAL SMOKE REMOVAL
The UltravisionTM Trocar device from Alesi Surgical Technologies uses a low-energy electrostatic charge to eliminate the surgical smoke generated by cutting instruments during laparoscopic surgery. Electrostatic precipitation accelerates the natural process of sedimentation; Ultravision creates negatively charged gas ions that draw water vapor and particulate matter away from the surgical site toward “positive” patient tissue.
Alesi says that bench studies comparing Ultravision with a vacuum-system when using monopolar, bipolar, and ultrasonic instruments show that its device is faster and more efficient than smoke evacuation. When switched on before cutting, Ultravision precipitates 99% of particles within 30 seconds. After 1 minute of continuous use, Ultravision precipitates 99.9% of particles, independent of particle size, from 7 nm to 10 µm. Smoke evacuation removes 30.2% of particles after 1 minute, according to Alesi.
FOR MORE INFORMATION, VISIT: http://www.alesi-surgical.com
PRESSURE-SENSING TECHNOLOGY FOR EPIDURALS
The CompuFlo®Epidural from Milestone Scientific uses pressure-sensing technology to identify the epidural space, and provides a computer-controlled drug delivery system.
Knowing the precise needle location during an epidural injection procedure provides a measure of safety not available to physicians who use conventional syringes. Milestone says that its CompuFlo Epidural allows anesthesiologists to use both hands to advance and direct the needle, and to confirm the epidural space with 99% accuracy on the first attempt.
CompuFlo Epidural differentiates tissue types for the medical professional via visual and audio feedback, leading to precise location guidance as the needle advances toward the intended area. It also allows for controlled needle exit pressure, precise flow rate and drug volumes, and patient treatment documentation.
FOR MORE INFORMATION, VISIT: https://www.milestonescientific.com/products/compuflo-epidural
OBGYN ULTRASOUND INNOVATIONS
Philips recently announced enhancements to its EPIQ 7 and 5 and Affiniti 70 ultrasound systems. According to Philips, the eL18-4 transducer provides high-detail resolution and image uniformity with penetration for enhanced diagnostic quality in 1st- and 2nd-trimester obstetric exams. aBiometry AssistAI, with anatomical intelligence of fetal anatomy, streamlines fetal measurement by preplacing measurement cursors on selected structures. The new TouchVue control-panel interface on TrueVue allows practitioners to interact with finger gestures and to direct 3D-volume rotation and internal light-source position. The 2D Tilt feature offered on the 3D9-v3 transducer provides lateral scanning of anatomic structures that are off-axis without having to manually angle the transducer.
These new features complement the existing suite of Philips ObGyn ultrasound visualization tools: TrueVue, GlassVue, aRevealAI, and MaxVue.
FOR MORE INFORMATION, VISIT: https://www.usa.philips.com/healthcare/resources/feature-detail/ultrasound-truevue-imaging
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
PRENATAL SCREENING FOR SINGLE-GENE DISORDERS
Vistara®, a non-invasive prenatal test (NIPT) from Natera, Inc, screens for single-gene disorders after 9 weeks’ gestation. Complementing the Panorama® NIPT, Vistara tests for major anatomic abnormalities and chromosome imbalances that have a combined incidence rate of 1 in 600 (higher than Down syndrome). These mutations can cause severe conditions affecting skeletal, cardiac, and neurologic systems, such as Noonan syndrome, osteogenesis imperfecta, craniosynostosis syndromes, achondroplasia, and Rett syndrome. Standard NIPT commonly cannot detect these de novo (not inherited) mutations. Ultrasound exams may either completely miss the disorders or identify nonspecific findings later in pregnancy.
Natera says that Vistara has a combined analytical sensitivity of >99% and a combined analytical specificity of >99% in validation studies.
FOR MORE INFORMATION, VISIT: https://www.natera.com/vistara
ELECTROSTATIC SURGICAL SMOKE REMOVAL
The UltravisionTM Trocar device from Alesi Surgical Technologies uses a low-energy electrostatic charge to eliminate the surgical smoke generated by cutting instruments during laparoscopic surgery. Electrostatic precipitation accelerates the natural process of sedimentation; Ultravision creates negatively charged gas ions that draw water vapor and particulate matter away from the surgical site toward “positive” patient tissue.
Alesi says that bench studies comparing Ultravision with a vacuum-system when using monopolar, bipolar, and ultrasonic instruments show that its device is faster and more efficient than smoke evacuation. When switched on before cutting, Ultravision precipitates 99% of particles within 30 seconds. After 1 minute of continuous use, Ultravision precipitates 99.9% of particles, independent of particle size, from 7 nm to 10 µm. Smoke evacuation removes 30.2% of particles after 1 minute, according to Alesi.
FOR MORE INFORMATION, VISIT: http://www.alesi-surgical.com
PRESSURE-SENSING TECHNOLOGY FOR EPIDURALS
The CompuFlo®Epidural from Milestone Scientific uses pressure-sensing technology to identify the epidural space, and provides a computer-controlled drug delivery system.
Knowing the precise needle location during an epidural injection procedure provides a measure of safety not available to physicians who use conventional syringes. Milestone says that its CompuFlo Epidural allows anesthesiologists to use both hands to advance and direct the needle, and to confirm the epidural space with 99% accuracy on the first attempt.
CompuFlo Epidural differentiates tissue types for the medical professional via visual and audio feedback, leading to precise location guidance as the needle advances toward the intended area. It also allows for controlled needle exit pressure, precise flow rate and drug volumes, and patient treatment documentation.
FOR MORE INFORMATION, VISIT: https://www.milestonescientific.com/products/compuflo-epidural
OBGYN ULTRASOUND INNOVATIONS
Philips recently announced enhancements to its EPIQ 7 and 5 and Affiniti 70 ultrasound systems. According to Philips, the eL18-4 transducer provides high-detail resolution and image uniformity with penetration for enhanced diagnostic quality in 1st- and 2nd-trimester obstetric exams. aBiometry AssistAI, with anatomical intelligence of fetal anatomy, streamlines fetal measurement by preplacing measurement cursors on selected structures. The new TouchVue control-panel interface on TrueVue allows practitioners to interact with finger gestures and to direct 3D-volume rotation and internal light-source position. The 2D Tilt feature offered on the 3D9-v3 transducer provides lateral scanning of anatomic structures that are off-axis without having to manually angle the transducer.
These new features complement the existing suite of Philips ObGyn ultrasound visualization tools: TrueVue, GlassVue, aRevealAI, and MaxVue.
FOR MORE INFORMATION, VISIT: https://www.usa.philips.com/healthcare/resources/feature-detail/ultrasound-truevue-imaging
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
PRENATAL SCREENING FOR SINGLE-GENE DISORDERS
Vistara®, a non-invasive prenatal test (NIPT) from Natera, Inc, screens for single-gene disorders after 9 weeks’ gestation. Complementing the Panorama® NIPT, Vistara tests for major anatomic abnormalities and chromosome imbalances that have a combined incidence rate of 1 in 600 (higher than Down syndrome). These mutations can cause severe conditions affecting skeletal, cardiac, and neurologic systems, such as Noonan syndrome, osteogenesis imperfecta, craniosynostosis syndromes, achondroplasia, and Rett syndrome. Standard NIPT commonly cannot detect these de novo (not inherited) mutations. Ultrasound exams may either completely miss the disorders or identify nonspecific findings later in pregnancy.
Natera says that Vistara has a combined analytical sensitivity of >99% and a combined analytical specificity of >99% in validation studies.
FOR MORE INFORMATION, VISIT: https://www.natera.com/vistara
ELECTROSTATIC SURGICAL SMOKE REMOVAL
The UltravisionTM Trocar device from Alesi Surgical Technologies uses a low-energy electrostatic charge to eliminate the surgical smoke generated by cutting instruments during laparoscopic surgery. Electrostatic precipitation accelerates the natural process of sedimentation; Ultravision creates negatively charged gas ions that draw water vapor and particulate matter away from the surgical site toward “positive” patient tissue.
Alesi says that bench studies comparing Ultravision with a vacuum-system when using monopolar, bipolar, and ultrasonic instruments show that its device is faster and more efficient than smoke evacuation. When switched on before cutting, Ultravision precipitates 99% of particles within 30 seconds. After 1 minute of continuous use, Ultravision precipitates 99.9% of particles, independent of particle size, from 7 nm to 10 µm. Smoke evacuation removes 30.2% of particles after 1 minute, according to Alesi.
FOR MORE INFORMATION, VISIT: http://www.alesi-surgical.com
PRESSURE-SENSING TECHNOLOGY FOR EPIDURALS
The CompuFlo®Epidural from Milestone Scientific uses pressure-sensing technology to identify the epidural space, and provides a computer-controlled drug delivery system.
Knowing the precise needle location during an epidural injection procedure provides a measure of safety not available to physicians who use conventional syringes. Milestone says that its CompuFlo Epidural allows anesthesiologists to use both hands to advance and direct the needle, and to confirm the epidural space with 99% accuracy on the first attempt.
CompuFlo Epidural differentiates tissue types for the medical professional via visual and audio feedback, leading to precise location guidance as the needle advances toward the intended area. It also allows for controlled needle exit pressure, precise flow rate and drug volumes, and patient treatment documentation.
FOR MORE INFORMATION, VISIT: https://www.milestonescientific.com/products/compuflo-epidural
OBGYN ULTRASOUND INNOVATIONS
Philips recently announced enhancements to its EPIQ 7 and 5 and Affiniti 70 ultrasound systems. According to Philips, the eL18-4 transducer provides high-detail resolution and image uniformity with penetration for enhanced diagnostic quality in 1st- and 2nd-trimester obstetric exams. aBiometry AssistAI, with anatomical intelligence of fetal anatomy, streamlines fetal measurement by preplacing measurement cursors on selected structures. The new TouchVue control-panel interface on TrueVue allows practitioners to interact with finger gestures and to direct 3D-volume rotation and internal light-source position. The 2D Tilt feature offered on the 3D9-v3 transducer provides lateral scanning of anatomic structures that are off-axis without having to manually angle the transducer.
These new features complement the existing suite of Philips ObGyn ultrasound visualization tools: TrueVue, GlassVue, aRevealAI, and MaxVue.
FOR MORE INFORMATION, VISIT: https://www.usa.philips.com/healthcare/resources/feature-detail/ultrasound-truevue-imaging
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
VIDEO: Cannabinoids in dermatology
SAN DIEGO – To date, most of the research on cannabinoids has been outside of dermatology, but these agents may eventually play an important role in the treatment of dermatologic diseases, according to Adam Friedman, MD, director of translational research, department of dermatology, at George Washington University, Washington.
for diseases like dermatomyositis, scleroderma, and lupus, Dr. Friedman said in a video interview at the annual meeting of the American Academy of Dermatology.
In this area, most progress has been made with a synthetic cannabinoid, ajulemic acid (also known as anabasum), which is designed to go after CB2 cannabinoid receptors, which have the anti-inflammatory effects, and not the CB1 receptors, which have the psychoactive effects, he explained. Results of phase 2 studies of ajulemic acid in dermatomyositis and systemic sclerosis have been “very promising,” he noted.
In collaboration with Albert Einstein College of Medicine, New York, he and his associates have studied the topical application of an endocannabinoid, anandamide (AEA), in nanoparticles in an animal model of cutaneous lupus. “We found that we can actually reverse the very classic, almost chronic cutaneous-like symptoms that we see in these animals if they go untreated,” he said.
In the interview, Dr. Friedman, who spoke about the potential of cannabinoids for the treatment of inflammatory and neoplastic diseases of the skin at the meeting, said that it is actually surprising that most research with cannabinoids to date has been outside of dermatology, “because our skin is chock full of cannabinoids; chock full of expression of cannabinoid receptors.”
Dr. Friedman disclosed that he has invented the nanotechnology licensed to Zylo Therapeutics. He is a member of the Dermatology News advisory board.

SAN DIEGO – To date, most of the research on cannabinoids has been outside of dermatology, but these agents may eventually play an important role in the treatment of dermatologic diseases, according to Adam Friedman, MD, director of translational research, department of dermatology, at George Washington University, Washington.
for diseases like dermatomyositis, scleroderma, and lupus, Dr. Friedman said in a video interview at the annual meeting of the American Academy of Dermatology.
In this area, most progress has been made with a synthetic cannabinoid, ajulemic acid (also known as anabasum), which is designed to go after CB2 cannabinoid receptors, which have the anti-inflammatory effects, and not the CB1 receptors, which have the psychoactive effects, he explained. Results of phase 2 studies of ajulemic acid in dermatomyositis and systemic sclerosis have been “very promising,” he noted.
In collaboration with Albert Einstein College of Medicine, New York, he and his associates have studied the topical application of an endocannabinoid, anandamide (AEA), in nanoparticles in an animal model of cutaneous lupus. “We found that we can actually reverse the very classic, almost chronic cutaneous-like symptoms that we see in these animals if they go untreated,” he said.
In the interview, Dr. Friedman, who spoke about the potential of cannabinoids for the treatment of inflammatory and neoplastic diseases of the skin at the meeting, said that it is actually surprising that most research with cannabinoids to date has been outside of dermatology, “because our skin is chock full of cannabinoids; chock full of expression of cannabinoid receptors.”
Dr. Friedman disclosed that he has invented the nanotechnology licensed to Zylo Therapeutics. He is a member of the Dermatology News advisory board.

SAN DIEGO – To date, most of the research on cannabinoids has been outside of dermatology, but these agents may eventually play an important role in the treatment of dermatologic diseases, according to Adam Friedman, MD, director of translational research, department of dermatology, at George Washington University, Washington.
for diseases like dermatomyositis, scleroderma, and lupus, Dr. Friedman said in a video interview at the annual meeting of the American Academy of Dermatology.
In this area, most progress has been made with a synthetic cannabinoid, ajulemic acid (also known as anabasum), which is designed to go after CB2 cannabinoid receptors, which have the anti-inflammatory effects, and not the CB1 receptors, which have the psychoactive effects, he explained. Results of phase 2 studies of ajulemic acid in dermatomyositis and systemic sclerosis have been “very promising,” he noted.
In collaboration with Albert Einstein College of Medicine, New York, he and his associates have studied the topical application of an endocannabinoid, anandamide (AEA), in nanoparticles in an animal model of cutaneous lupus. “We found that we can actually reverse the very classic, almost chronic cutaneous-like symptoms that we see in these animals if they go untreated,” he said.
In the interview, Dr. Friedman, who spoke about the potential of cannabinoids for the treatment of inflammatory and neoplastic diseases of the skin at the meeting, said that it is actually surprising that most research with cannabinoids to date has been outside of dermatology, “because our skin is chock full of cannabinoids; chock full of expression of cannabinoid receptors.”
Dr. Friedman disclosed that he has invented the nanotechnology licensed to Zylo Therapeutics. He is a member of the Dermatology News advisory board.

REPORTING FROM AAD 18
Health Care Use May Elucidate MS Prodrome
SAN DIEGO—In the five years before multiple sclerosis (MS) symptom onset, patients are more likely to see a physician or be admitted to the hospital for problems related to the nervous system, sensory organs, musculoskeletal system, and genitourinary system, compared with controls, according to research presented at the ACTRIMS 2018 Forum.
In addition, patients with MS are more likely to see psychiatrists and urologists and to fill prescriptions related to the musculoskeletal system or the genitourinary system in the five years before MS onset.
“It is possible to measure and phenotype the MS prodrome five years before the clinical recognition of MS,” said Elaine Kingwell, PhD, a research associate in the Division of Neurology at the University of British Columbia in Vancouver, and colleagues. “Our findings inform the etiologically relevant time window and suggest that an earlier recognition and diagnosis of MS is feasible.”
A prior study by the researchers had identified increased health care use before a first demyelinating event among patients
Phenotypic Characteristics
To investigate the phenotypic characteristics of the MS prodrome, Dr. Kingwell and colleagues examined the medical diagnoses and therapeutic drug classes related to inpatient and outpatient health care encounters five years before the clinical recognition of MS. The researchers performed a population-based matched cohort study using linked health administrative and clinical data in four Canadian provinces between 1989 and 2014.
The investigators compared the reasons for physician and hospital encounters, using ICD-10 chapters or physician specialty, and prescriptions filled, using Anatomical Therapeutic Chemical System, level 1 drug classes. The researchers matched people with MS with as many as five people without any demyelinating disease by sex, year of birth, and postal code.
The study included two cohorts of people with MS. One cohort encompassed patients with a first demyelinating disease–related claim (ie, the administrative cohort), and the other cohort comprised patients with a neurologist-confirmed diagnosis of MS (ie, the clinical cohort). The researchers compared inpatient and outpatient encounters between cases and controls in the five years before the cases’ first demyelinating claim or clinically reported symptom onset.
Fewer Pregnancy-Related Encounters
The administrative cohort included 13,951 cases and 66,940 controls (about 73% women; average age, 43). The clinical cohort included 3,202 cases and 16,006 controls (about 74% women; average age, 36.5). Compared with controls, in the five years before the first demyelinating claim or symptom onset, cases had more physician and hospital encounters for the nervous system (rate ratio [RR] = 1.70–4.75), sensory organs (RR = 1.40–2.28), musculoskeletal system (RR = 1.19–1.70), and genitourinary system (RR = 1.17–1.59). Cases also had more encounters with psychiatrists (RR = 1.48–1.66) and urologists (RR = 1.49–1.80), and a higher proportion of filled prescriptions for hormonal preparations and for drugs related to the musculoskeletal or genitourinary systems (1.1–1.5 times higher). In contrast, cases had fewer pregnancy-related encounters, compared with controls (RR = 0.78–0.88).
“Research into factors that might cause or prevent MS should take the MS prodrome into account,” the researchers said.
—Jake Remaly
Suggested Reading
Wijnands JMA, Kingwell E, Zhu F, et al. Health-care use before a first demyelinating event suggestive of a multiple sclerosis prodrome: a matched cohort study. Lancet Neurol. 2017;16(6):445-451.
SAN DIEGO—In the five years before multiple sclerosis (MS) symptom onset, patients are more likely to see a physician or be admitted to the hospital for problems related to the nervous system, sensory organs, musculoskeletal system, and genitourinary system, compared with controls, according to research presented at the ACTRIMS 2018 Forum.
In addition, patients with MS are more likely to see psychiatrists and urologists and to fill prescriptions related to the musculoskeletal system or the genitourinary system in the five years before MS onset.
“It is possible to measure and phenotype the MS prodrome five years before the clinical recognition of MS,” said Elaine Kingwell, PhD, a research associate in the Division of Neurology at the University of British Columbia in Vancouver, and colleagues. “Our findings inform the etiologically relevant time window and suggest that an earlier recognition and diagnosis of MS is feasible.”
A prior study by the researchers had identified increased health care use before a first demyelinating event among patients
Phenotypic Characteristics
To investigate the phenotypic characteristics of the MS prodrome, Dr. Kingwell and colleagues examined the medical diagnoses and therapeutic drug classes related to inpatient and outpatient health care encounters five years before the clinical recognition of MS. The researchers performed a population-based matched cohort study using linked health administrative and clinical data in four Canadian provinces between 1989 and 2014.
The investigators compared the reasons for physician and hospital encounters, using ICD-10 chapters or physician specialty, and prescriptions filled, using Anatomical Therapeutic Chemical System, level 1 drug classes. The researchers matched people with MS with as many as five people without any demyelinating disease by sex, year of birth, and postal code.
The study included two cohorts of people with MS. One cohort encompassed patients with a first demyelinating disease–related claim (ie, the administrative cohort), and the other cohort comprised patients with a neurologist-confirmed diagnosis of MS (ie, the clinical cohort). The researchers compared inpatient and outpatient encounters between cases and controls in the five years before the cases’ first demyelinating claim or clinically reported symptom onset.
Fewer Pregnancy-Related Encounters
The administrative cohort included 13,951 cases and 66,940 controls (about 73% women; average age, 43). The clinical cohort included 3,202 cases and 16,006 controls (about 74% women; average age, 36.5). Compared with controls, in the five years before the first demyelinating claim or symptom onset, cases had more physician and hospital encounters for the nervous system (rate ratio [RR] = 1.70–4.75), sensory organs (RR = 1.40–2.28), musculoskeletal system (RR = 1.19–1.70), and genitourinary system (RR = 1.17–1.59). Cases also had more encounters with psychiatrists (RR = 1.48–1.66) and urologists (RR = 1.49–1.80), and a higher proportion of filled prescriptions for hormonal preparations and for drugs related to the musculoskeletal or genitourinary systems (1.1–1.5 times higher). In contrast, cases had fewer pregnancy-related encounters, compared with controls (RR = 0.78–0.88).
“Research into factors that might cause or prevent MS should take the MS prodrome into account,” the researchers said.
—Jake Remaly
Suggested Reading
Wijnands JMA, Kingwell E, Zhu F, et al. Health-care use before a first demyelinating event suggestive of a multiple sclerosis prodrome: a matched cohort study. Lancet Neurol. 2017;16(6):445-451.
SAN DIEGO—In the five years before multiple sclerosis (MS) symptom onset, patients are more likely to see a physician or be admitted to the hospital for problems related to the nervous system, sensory organs, musculoskeletal system, and genitourinary system, compared with controls, according to research presented at the ACTRIMS 2018 Forum.
In addition, patients with MS are more likely to see psychiatrists and urologists and to fill prescriptions related to the musculoskeletal system or the genitourinary system in the five years before MS onset.
“It is possible to measure and phenotype the MS prodrome five years before the clinical recognition of MS,” said Elaine Kingwell, PhD, a research associate in the Division of Neurology at the University of British Columbia in Vancouver, and colleagues. “Our findings inform the etiologically relevant time window and suggest that an earlier recognition and diagnosis of MS is feasible.”
A prior study by the researchers had identified increased health care use before a first demyelinating event among patients
Phenotypic Characteristics
To investigate the phenotypic characteristics of the MS prodrome, Dr. Kingwell and colleagues examined the medical diagnoses and therapeutic drug classes related to inpatient and outpatient health care encounters five years before the clinical recognition of MS. The researchers performed a population-based matched cohort study using linked health administrative and clinical data in four Canadian provinces between 1989 and 2014.
The investigators compared the reasons for physician and hospital encounters, using ICD-10 chapters or physician specialty, and prescriptions filled, using Anatomical Therapeutic Chemical System, level 1 drug classes. The researchers matched people with MS with as many as five people without any demyelinating disease by sex, year of birth, and postal code.
The study included two cohorts of people with MS. One cohort encompassed patients with a first demyelinating disease–related claim (ie, the administrative cohort), and the other cohort comprised patients with a neurologist-confirmed diagnosis of MS (ie, the clinical cohort). The researchers compared inpatient and outpatient encounters between cases and controls in the five years before the cases’ first demyelinating claim or clinically reported symptom onset.
Fewer Pregnancy-Related Encounters
The administrative cohort included 13,951 cases and 66,940 controls (about 73% women; average age, 43). The clinical cohort included 3,202 cases and 16,006 controls (about 74% women; average age, 36.5). Compared with controls, in the five years before the first demyelinating claim or symptom onset, cases had more physician and hospital encounters for the nervous system (rate ratio [RR] = 1.70–4.75), sensory organs (RR = 1.40–2.28), musculoskeletal system (RR = 1.19–1.70), and genitourinary system (RR = 1.17–1.59). Cases also had more encounters with psychiatrists (RR = 1.48–1.66) and urologists (RR = 1.49–1.80), and a higher proportion of filled prescriptions for hormonal preparations and for drugs related to the musculoskeletal or genitourinary systems (1.1–1.5 times higher). In contrast, cases had fewer pregnancy-related encounters, compared with controls (RR = 0.78–0.88).
“Research into factors that might cause or prevent MS should take the MS prodrome into account,” the researchers said.
—Jake Remaly
Suggested Reading
Wijnands JMA, Kingwell E, Zhu F, et al. Health-care use before a first demyelinating event suggestive of a multiple sclerosis prodrome: a matched cohort study. Lancet Neurol. 2017;16(6):445-451.
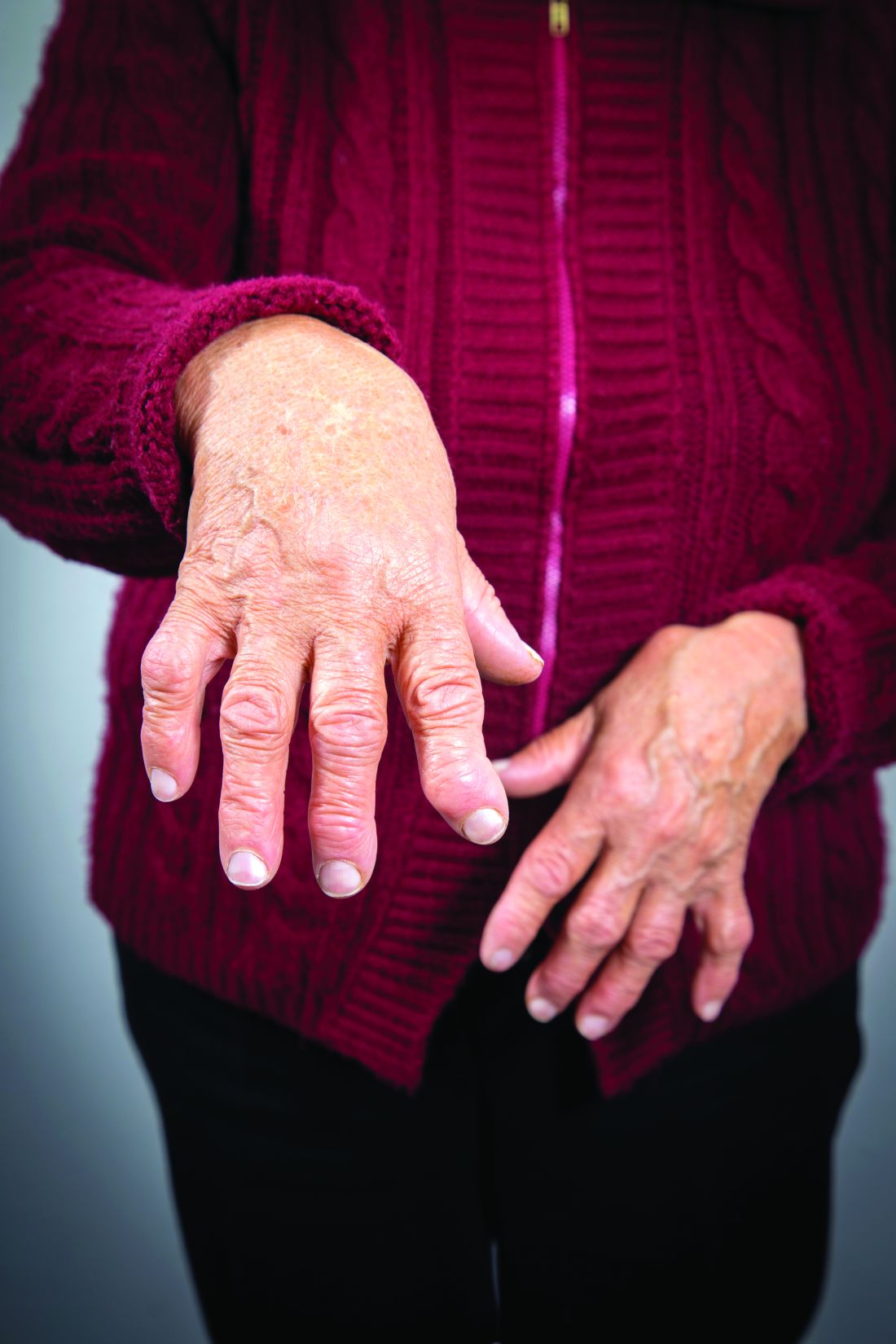
Researchers identify three distinct clinical-histologic-genetic subtypes in RA
Researchers have identified three different synovial subtypes of rheumatoid arthritis that exhibit different mechanisms of pain and correlate with specific clinical phenotypes.
The findings could be clinically meaningful and may help guide optimal treatment strategies for patients, as well as provide a better understanding of the cause of pain in patients with high tender and swollen joint counts but little tissue inflammation, according to the research team led by Dana E. Orange, MD, of the Hospital for Special Surgery and Rockefeller University in New York.
The report was published in Arthritis & Rheumatology.
The assessment of the synovium in rheumatoid arthritis (RA) has the potential to provide guidance on optimal treatment strategies, they noted, but its classification has not yet factored into current diagnosis or treatment guidelines of RA.
In total, the research team analyzed 20 histologic features on 129 synovial tissue samples.
The researchers used machine learning integration to identify three distinct molecular subtypes of RA from a consensus clustering of the 500 most variable genes expressed in a subset of 45 synovial samples, including 39 from RA patients. The subtypes were high inflammatory, low inflammatory, and a mixed phenotype.
The researchers then took the histologic features that best corresponded to each subtype to develop a histology scoring algorithm that predicted the three gene expression subtypes (using only histology features), each of which were each associated with levels of erythrocyte sedimentation rate, C-reactive protein, and autoantibodies.
The histologic features that most strongly defined the high inflammatory subtype included three plasma cell features: binucleate plasma cells, plasma cell percentage, and Russell bodies. Patients with a high inflammatory synovial subtype also exhibited higher levels of markers of systemic inflammation and autoantibodies. For example, C-reactive protein was significantly correlated with pain in the high inflammatory group.
“This suggests that pain is associated with inflammation in patients with high inflammatory subtype and that pain may be driven by distinct mechanisms in the other patients,” the study authors wrote.
The low inflammatory subgroup was characterized by high neuronal and glycoprotein gene expression. But in this group, pain scores were not associated with elevated inflammatory markers.
“It is interesting that this subtype is characterized by a paucity of inflammatory infiltrates, yet maintains high pain scores and multiple tender/swollen joints – this too is consistent with other findings of patients with established RA,” the research team noted.
The mixed subtype shared features with both the high and low subtypes, the researchers said.
“Our work suggests that RA patients with longstanding disease and poor response to anti-inflammatory treatment may warrant synovial biopsy to determine their inflammatory subtype,” the researchers concluded.
Several research institutions and the Accelerating Medicines Partnership in Rheumatoid Arthritis and Lupus Network, a public-private partnership involving several pharmaceutical companies, patient advocacy groups, and the National Institutes of Health, funded the study.
SOURCE: Orange D et al. Arthritis Rheumatol. 2018 Feb 22. doi: 10.1002/art.40428.
Researchers have identified three different synovial subtypes of rheumatoid arthritis that exhibit different mechanisms of pain and correlate with specific clinical phenotypes.
The findings could be clinically meaningful and may help guide optimal treatment strategies for patients, as well as provide a better understanding of the cause of pain in patients with high tender and swollen joint counts but little tissue inflammation, according to the research team led by Dana E. Orange, MD, of the Hospital for Special Surgery and Rockefeller University in New York.
The report was published in Arthritis & Rheumatology.
The assessment of the synovium in rheumatoid arthritis (RA) has the potential to provide guidance on optimal treatment strategies, they noted, but its classification has not yet factored into current diagnosis or treatment guidelines of RA.
In total, the research team analyzed 20 histologic features on 129 synovial tissue samples.
The researchers used machine learning integration to identify three distinct molecular subtypes of RA from a consensus clustering of the 500 most variable genes expressed in a subset of 45 synovial samples, including 39 from RA patients. The subtypes were high inflammatory, low inflammatory, and a mixed phenotype.
The researchers then took the histologic features that best corresponded to each subtype to develop a histology scoring algorithm that predicted the three gene expression subtypes (using only histology features), each of which were each associated with levels of erythrocyte sedimentation rate, C-reactive protein, and autoantibodies.
The histologic features that most strongly defined the high inflammatory subtype included three plasma cell features: binucleate plasma cells, plasma cell percentage, and Russell bodies. Patients with a high inflammatory synovial subtype also exhibited higher levels of markers of systemic inflammation and autoantibodies. For example, C-reactive protein was significantly correlated with pain in the high inflammatory group.
“This suggests that pain is associated with inflammation in patients with high inflammatory subtype and that pain may be driven by distinct mechanisms in the other patients,” the study authors wrote.
The low inflammatory subgroup was characterized by high neuronal and glycoprotein gene expression. But in this group, pain scores were not associated with elevated inflammatory markers.
“It is interesting that this subtype is characterized by a paucity of inflammatory infiltrates, yet maintains high pain scores and multiple tender/swollen joints – this too is consistent with other findings of patients with established RA,” the research team noted.
The mixed subtype shared features with both the high and low subtypes, the researchers said.
“Our work suggests that RA patients with longstanding disease and poor response to anti-inflammatory treatment may warrant synovial biopsy to determine their inflammatory subtype,” the researchers concluded.
Several research institutions and the Accelerating Medicines Partnership in Rheumatoid Arthritis and Lupus Network, a public-private partnership involving several pharmaceutical companies, patient advocacy groups, and the National Institutes of Health, funded the study.
SOURCE: Orange D et al. Arthritis Rheumatol. 2018 Feb 22. doi: 10.1002/art.40428.
Researchers have identified three different synovial subtypes of rheumatoid arthritis that exhibit different mechanisms of pain and correlate with specific clinical phenotypes.
The findings could be clinically meaningful and may help guide optimal treatment strategies for patients, as well as provide a better understanding of the cause of pain in patients with high tender and swollen joint counts but little tissue inflammation, according to the research team led by Dana E. Orange, MD, of the Hospital for Special Surgery and Rockefeller University in New York.
The report was published in Arthritis & Rheumatology.
The assessment of the synovium in rheumatoid arthritis (RA) has the potential to provide guidance on optimal treatment strategies, they noted, but its classification has not yet factored into current diagnosis or treatment guidelines of RA.
In total, the research team analyzed 20 histologic features on 129 synovial tissue samples.
The researchers used machine learning integration to identify three distinct molecular subtypes of RA from a consensus clustering of the 500 most variable genes expressed in a subset of 45 synovial samples, including 39 from RA patients. The subtypes were high inflammatory, low inflammatory, and a mixed phenotype.
The researchers then took the histologic features that best corresponded to each subtype to develop a histology scoring algorithm that predicted the three gene expression subtypes (using only histology features), each of which were each associated with levels of erythrocyte sedimentation rate, C-reactive protein, and autoantibodies.
The histologic features that most strongly defined the high inflammatory subtype included three plasma cell features: binucleate plasma cells, plasma cell percentage, and Russell bodies. Patients with a high inflammatory synovial subtype also exhibited higher levels of markers of systemic inflammation and autoantibodies. For example, C-reactive protein was significantly correlated with pain in the high inflammatory group.
“This suggests that pain is associated with inflammation in patients with high inflammatory subtype and that pain may be driven by distinct mechanisms in the other patients,” the study authors wrote.
The low inflammatory subgroup was characterized by high neuronal and glycoprotein gene expression. But in this group, pain scores were not associated with elevated inflammatory markers.
“It is interesting that this subtype is characterized by a paucity of inflammatory infiltrates, yet maintains high pain scores and multiple tender/swollen joints – this too is consistent with other findings of patients with established RA,” the research team noted.
The mixed subtype shared features with both the high and low subtypes, the researchers said.
“Our work suggests that RA patients with longstanding disease and poor response to anti-inflammatory treatment may warrant synovial biopsy to determine their inflammatory subtype,” the researchers concluded.
Several research institutions and the Accelerating Medicines Partnership in Rheumatoid Arthritis and Lupus Network, a public-private partnership involving several pharmaceutical companies, patient advocacy groups, and the National Institutes of Health, funded the study.
SOURCE: Orange D et al. Arthritis Rheumatol. 2018 Feb 22. doi: 10.1002/art.40428.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Mechanisms of pain may differ in RA patients with different synovial subtypes.
Major findings:
Study details: Twenty histologic features were assessed on 129 synovial tissue samples from 123 RA patients and 6 OA patients.
Disclosures: Several research institutions and the Accelerating Medicines Partnership in Rheumatoid Arthritis and Lupus Network, a public-private partnership involving several pharmaceutical companies, patient advocacy groups, and the National Institutes of Health, funded the study.
Source: Orange D et al. Arthritis Rheumatol. 2018 Feb 22. doi: 10.1002/art.40428.
Factors critical to reducing US maternal mortality and morbidity
More women die from pregnancy complications in the United States than in any other developed country. The United States is the only industrialized nation with a rising maternal mortality rate.
Those 2 sentences should stop us all in our tracks.
In fact, the United States ranks 47th globally with the worst maternal mortality rate. More than half these deaths are likely preventable, with suicide and drug overdose the leading causes of maternal death in many states. All this occurs despite our advanced medical system, premier medical colleges and universities, embrace of high-tech medical advances, and high percentage of gross domestic product spent on health care.
Need more numbers? According to a 2016 report in Obstetrics and Gynecology, the United States saw a 26% increase in the maternalmortality rate (unadjusted) in only 15 years: from 18.8 deaths per 100,000 live births in 2000 to 23.8 in 2014 (FIGURE 1).1
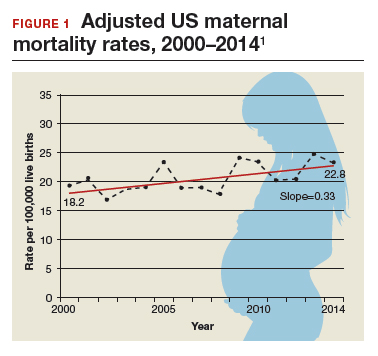
This problem received federal attention when, in 2000, the US Department of Health and Human Services launched Healthy People 2010. That health promotion and disease prevention agenda set a goal of reducing maternal mortality to 3.3 deaths per 100,000 live births by 2010, a goal clearly not met.
Considerable variations by race and by state
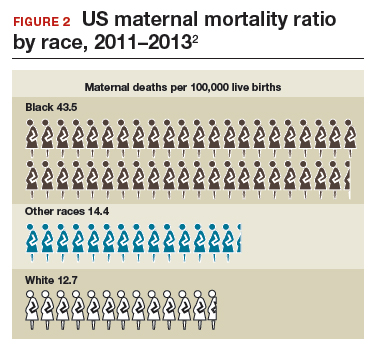
The racial disparities in maternal mortality are staggering and have not improved in more than 20 years: African American women are 3.4 times more likely to die than non-Hispanic white women of pregnancy-related complications. In 2011–2013, the maternal mortality ratio for non-Hispanic white women was 12.7 deaths per 100,000 live births compared with 43.5 deaths for non-Hispanic black women (FIGURE 2).2 American Indian or Alaska Native women, Asian women, and some Latina women also experience higher rates than non-Hispanic white women. The rate for American Indian or Alaska Native women is 16.9 deaths per 100,000 live births.3
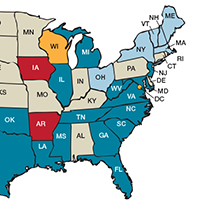
Some states are doing better than others, showing that there is nothing inevitable about the maternal mortality crisis. Texas, for example, has seen the highest rate of maternal mortality increase. Its rate doubled from 2010 to 2012, while California reduced its maternal death rate by 30%, from 21.5 to 15.1, during roughly the same period.1
This is a challenge of epic proportions, and one that the American College of Obstetricians and Gynecologists (ACOG), under the leadership of President Haywood Brown, MD, and Incoming President Lisa Hollier, MD, is determined to meet, ensuring that a high maternal death rate does not become our nation’s new normal.
Dr. Brown put it this way, “ACOG collaborative initiatives such as Levels of Maternal Care (LOMC) and implementation of OB safety bundles for hemorrhage, hypertension, and thromboembolism through the AIM [Alliance for Innovation on Maternal Health] Program target maternal morbidity and mortality at the community level. Bundles have also been developed to address the disparity in maternal mortality and for the opiate crisis.”
ACOG is making strides in putting in place nationwide meaningful, evidence-driven systems and care approaches that are proven to reduce maternal mortality and morbidity, saving mothers’ lives and keeping families whole.
Read about the AIM Program’s initiatives
ACOG’s AIM Program established to make an impact
The AIM Program (www.safehealthcare foreverywoman.org) is bringing together clinicians, public health officials, hospital administrators, patient safety organizations, and advocates to eliminate preventable maternal mortality throughout the United States. With funding and support from the US Health Resources and Services Administration, AIM is striving to:
- reduce maternal mortality by 1,000 deaths by 2018
- reduce severe maternal morbidity
- assist states and hospitals to improve outcomes
- create and encourage use of maternal safety bundles (evidence-based tool kits to guide the best care).
AIM offers participating physicians and hospitals online learning modules, checklists, work plans, and links to tool kits and published resources. Implementation data is shared with hospitals and states to further improve care. Physicians participating in AIM can receive Part IV maintenance of certification; continuing education units will soon be offered for nurses. In the future, AIM-participating hospitals may be able to receive reduced liability protection costs, too.
To date, 17 states are participating in the AIM initiative (FIGURE 3), with more states ready to enroll.4 States must demonstrate a commitment to lasting change to participate. Each AIM state must have an active maternal mortality review committee (MMRC); committed leadership from public health, hospital associations, and provider associations; and a commitment to report AIM data.
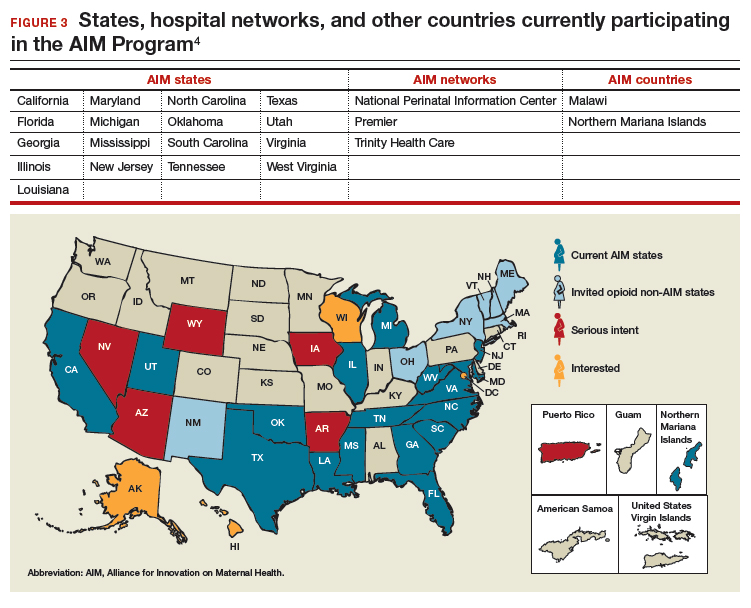
AIM thus far has released 9 obstetric patient safety bundles, including:
- reducing disparities in maternity care
- severe hypertension in pregnancy
- safe reduction of primary cesarean birth
- prevention of venous thromboembolism
- obstetric hemorrhage
- maternal mental health
- patient, family, and staff support following a severe maternal event
- postpartum care basics
- obstetric care of women with opioid use disorder (in use by Illinois, Massachusetts, Maryland, New Jersey, Maine, New Hampshire, Vermont, New York, Ohio, Oklahoma, Tennessee, Texas, and Virginia).
Read about how active MMRCS are critical to success
Review committees are critical to success
In use in many states, MMRCs are groups of local ObGyns, nurses, social workers, and other health care professionals who review specific cases of maternal deaths from their local area and recommend local solutions to prevent future deaths. MMRCs can be a critically important source of data to help us understand the underlying causes of maternal mortality.
Remember California’s success in reducing its maternal mortality rate, previously mentioned? That state was an early adopter of an active MMRC and has worked to bring best practices to maternity care throughout the state.
While every state should have an active MMRC, not every state does. ACOG is working with states, local leaders, and state and federal legislatures to help develop MMRCs in every state.
Dr. Brown pointed out that, “For several decades, Indiana had a legislatively authorized multidisciplinary maternal mortality review committee that I actively participated in and led in the late 1990s. The authorization for the program lapsed in the early 2000s, and the Indiana MMRC had to shut down. Bolstering the federal government’s capacity to help states like Indiana rebuild MMRCs, or start them from scratch, will help state public health officials, hospitals, and physicians take better care of moms and babies.”
Dr. Hollier explained, “In Texas, I chair our Maternal Mortality and Morbidity Task Force, which was legislatively authorized in 2013 in response to the rising rate of maternal death. The detailed state-based maternal mortality reviews provide critical information: verification of vital statistics data, assessment of the causes and contributing factors, and determination of pregnancy relatedness. These reviews identify opportunities for prevention and implementation of the most appropriate interventions to reduce maternal mortality on a local level. Support of essential review functions at the federal level would also enable data to be combined across jurisdictions for national learning that was previously not possible.”
Pending legislation will strengthen efforts
ACOG is working to enact into law the Preventing Maternal Deaths Act, HR 1318 and S1112. This is bipartisan legislation under which the Centers for Disease Control and Prevention would help states create or expand MMRCs and will require the Department of Health and Human Services to research ways to reduce disparities in maternal health outcomes.
Acknowledgement
The author thanks Jean Mahoney, ACOG’s Senior Director, AIM, for her generous assistance.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- MacDorman MF, Declerq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128(3):447–455.
- Centers for Disease Control and Prevention. Pregnancy mortality surveillance system. www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Updated November 9, 2017. Accessed February 16, 2018.
- Singh GK. Maternal mortality in the United States, 1935−2007: Substantial racial/ethnic, socioeconomic, and geographic disparities persist. A 75th Anniversary Publication. Health Resources and Services Administration, Maternal and Child Health Bureau. Rockville, Maryland: US Department of Health and Human Services; 2010. https://www.hrsa.gov/sites/default/files/ourstories/mchb75th/mchb75maternalmortality.pdf. Accessed February 16, 2018.
- Council on Patient Safety in Women’s Health Care. Alliance for Innovation on Maternal Health Program: AIM states and systems. http://safehealthcareforeverywoman.org/aim-states-systems-2/#link_tab-1513011413196-9. Accessed February 20, 2018.
More women die from pregnancy complications in the United States than in any other developed country. The United States is the only industrialized nation with a rising maternal mortality rate.
Those 2 sentences should stop us all in our tracks.
In fact, the United States ranks 47th globally with the worst maternal mortality rate. More than half these deaths are likely preventable, with suicide and drug overdose the leading causes of maternal death in many states. All this occurs despite our advanced medical system, premier medical colleges and universities, embrace of high-tech medical advances, and high percentage of gross domestic product spent on health care.
Need more numbers? According to a 2016 report in Obstetrics and Gynecology, the United States saw a 26% increase in the maternalmortality rate (unadjusted) in only 15 years: from 18.8 deaths per 100,000 live births in 2000 to 23.8 in 2014 (FIGURE 1).1

This problem received federal attention when, in 2000, the US Department of Health and Human Services launched Healthy People 2010. That health promotion and disease prevention agenda set a goal of reducing maternal mortality to 3.3 deaths per 100,000 live births by 2010, a goal clearly not met.
Considerable variations by race and by state
The racial disparities in maternal mortality are staggering and have not improved in more than 20 years: African American women are 3.4 times more likely to die than non-Hispanic white women of pregnancy-related complications. In 2011–2013, the maternal mortality ratio for non-Hispanic white women was 12.7 deaths per 100,000 live births compared with 43.5 deaths for non-Hispanic black women (FIGURE 2).2 American Indian or Alaska Native women, Asian women, and some Latina women also experience higher rates than non-Hispanic white women. The rate for American Indian or Alaska Native women is 16.9 deaths per 100,000 live births.3

Some states are doing better than others, showing that there is nothing inevitable about the maternal mortality crisis. Texas, for example, has seen the highest rate of maternal mortality increase. Its rate doubled from 2010 to 2012, while California reduced its maternal death rate by 30%, from 21.5 to 15.1, during roughly the same period.1
This is a challenge of epic proportions, and one that the American College of Obstetricians and Gynecologists (ACOG), under the leadership of President Haywood Brown, MD, and Incoming President Lisa Hollier, MD, is determined to meet, ensuring that a high maternal death rate does not become our nation’s new normal.
Dr. Brown put it this way, “ACOG collaborative initiatives such as Levels of Maternal Care (LOMC) and implementation of OB safety bundles for hemorrhage, hypertension, and thromboembolism through the AIM [Alliance for Innovation on Maternal Health] Program target maternal morbidity and mortality at the community level. Bundles have also been developed to address the disparity in maternal mortality and for the opiate crisis.”
ACOG is making strides in putting in place nationwide meaningful, evidence-driven systems and care approaches that are proven to reduce maternal mortality and morbidity, saving mothers’ lives and keeping families whole.
Read about the AIM Program’s initiatives
ACOG’s AIM Program established to make an impact
The AIM Program (www.safehealthcare foreverywoman.org) is bringing together clinicians, public health officials, hospital administrators, patient safety organizations, and advocates to eliminate preventable maternal mortality throughout the United States. With funding and support from the US Health Resources and Services Administration, AIM is striving to:
- reduce maternal mortality by 1,000 deaths by 2018
- reduce severe maternal morbidity
- assist states and hospitals to improve outcomes
- create and encourage use of maternal safety bundles (evidence-based tool kits to guide the best care).
AIM offers participating physicians and hospitals online learning modules, checklists, work plans, and links to tool kits and published resources. Implementation data is shared with hospitals and states to further improve care. Physicians participating in AIM can receive Part IV maintenance of certification; continuing education units will soon be offered for nurses. In the future, AIM-participating hospitals may be able to receive reduced liability protection costs, too.
To date, 17 states are participating in the AIM initiative (FIGURE 3), with more states ready to enroll.4 States must demonstrate a commitment to lasting change to participate. Each AIM state must have an active maternal mortality review committee (MMRC); committed leadership from public health, hospital associations, and provider associations; and a commitment to report AIM data.

AIM thus far has released 9 obstetric patient safety bundles, including:
- reducing disparities in maternity care
- severe hypertension in pregnancy
- safe reduction of primary cesarean birth
- prevention of venous thromboembolism
- obstetric hemorrhage
- maternal mental health
- patient, family, and staff support following a severe maternal event
- postpartum care basics
- obstetric care of women with opioid use disorder (in use by Illinois, Massachusetts, Maryland, New Jersey, Maine, New Hampshire, Vermont, New York, Ohio, Oklahoma, Tennessee, Texas, and Virginia).
Read about how active MMRCS are critical to success
Review committees are critical to success
In use in many states, MMRCs are groups of local ObGyns, nurses, social workers, and other health care professionals who review specific cases of maternal deaths from their local area and recommend local solutions to prevent future deaths. MMRCs can be a critically important source of data to help us understand the underlying causes of maternal mortality.
Remember California’s success in reducing its maternal mortality rate, previously mentioned? That state was an early adopter of an active MMRC and has worked to bring best practices to maternity care throughout the state.
While every state should have an active MMRC, not every state does. ACOG is working with states, local leaders, and state and federal legislatures to help develop MMRCs in every state.
Dr. Brown pointed out that, “For several decades, Indiana had a legislatively authorized multidisciplinary maternal mortality review committee that I actively participated in and led in the late 1990s. The authorization for the program lapsed in the early 2000s, and the Indiana MMRC had to shut down. Bolstering the federal government’s capacity to help states like Indiana rebuild MMRCs, or start them from scratch, will help state public health officials, hospitals, and physicians take better care of moms and babies.”
Dr. Hollier explained, “In Texas, I chair our Maternal Mortality and Morbidity Task Force, which was legislatively authorized in 2013 in response to the rising rate of maternal death. The detailed state-based maternal mortality reviews provide critical information: verification of vital statistics data, assessment of the causes and contributing factors, and determination of pregnancy relatedness. These reviews identify opportunities for prevention and implementation of the most appropriate interventions to reduce maternal mortality on a local level. Support of essential review functions at the federal level would also enable data to be combined across jurisdictions for national learning that was previously not possible.”
Pending legislation will strengthen efforts
ACOG is working to enact into law the Preventing Maternal Deaths Act, HR 1318 and S1112. This is bipartisan legislation under which the Centers for Disease Control and Prevention would help states create or expand MMRCs and will require the Department of Health and Human Services to research ways to reduce disparities in maternal health outcomes.
Acknowledgement
The author thanks Jean Mahoney, ACOG’s Senior Director, AIM, for her generous assistance.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
More women die from pregnancy complications in the United States than in any other developed country. The United States is the only industrialized nation with a rising maternal mortality rate.
Those 2 sentences should stop us all in our tracks.
In fact, the United States ranks 47th globally with the worst maternal mortality rate. More than half these deaths are likely preventable, with suicide and drug overdose the leading causes of maternal death in many states. All this occurs despite our advanced medical system, premier medical colleges and universities, embrace of high-tech medical advances, and high percentage of gross domestic product spent on health care.
Need more numbers? According to a 2016 report in Obstetrics and Gynecology, the United States saw a 26% increase in the maternalmortality rate (unadjusted) in only 15 years: from 18.8 deaths per 100,000 live births in 2000 to 23.8 in 2014 (FIGURE 1).1

This problem received federal attention when, in 2000, the US Department of Health and Human Services launched Healthy People 2010. That health promotion and disease prevention agenda set a goal of reducing maternal mortality to 3.3 deaths per 100,000 live births by 2010, a goal clearly not met.
Considerable variations by race and by state
The racial disparities in maternal mortality are staggering and have not improved in more than 20 years: African American women are 3.4 times more likely to die than non-Hispanic white women of pregnancy-related complications. In 2011–2013, the maternal mortality ratio for non-Hispanic white women was 12.7 deaths per 100,000 live births compared with 43.5 deaths for non-Hispanic black women (FIGURE 2).2 American Indian or Alaska Native women, Asian women, and some Latina women also experience higher rates than non-Hispanic white women. The rate for American Indian or Alaska Native women is 16.9 deaths per 100,000 live births.3

Some states are doing better than others, showing that there is nothing inevitable about the maternal mortality crisis. Texas, for example, has seen the highest rate of maternal mortality increase. Its rate doubled from 2010 to 2012, while California reduced its maternal death rate by 30%, from 21.5 to 15.1, during roughly the same period.1
This is a challenge of epic proportions, and one that the American College of Obstetricians and Gynecologists (ACOG), under the leadership of President Haywood Brown, MD, and Incoming President Lisa Hollier, MD, is determined to meet, ensuring that a high maternal death rate does not become our nation’s new normal.
Dr. Brown put it this way, “ACOG collaborative initiatives such as Levels of Maternal Care (LOMC) and implementation of OB safety bundles for hemorrhage, hypertension, and thromboembolism through the AIM [Alliance for Innovation on Maternal Health] Program target maternal morbidity and mortality at the community level. Bundles have also been developed to address the disparity in maternal mortality and for the opiate crisis.”
ACOG is making strides in putting in place nationwide meaningful, evidence-driven systems and care approaches that are proven to reduce maternal mortality and morbidity, saving mothers’ lives and keeping families whole.
Read about the AIM Program’s initiatives
ACOG’s AIM Program established to make an impact
The AIM Program (www.safehealthcare foreverywoman.org) is bringing together clinicians, public health officials, hospital administrators, patient safety organizations, and advocates to eliminate preventable maternal mortality throughout the United States. With funding and support from the US Health Resources and Services Administration, AIM is striving to:
- reduce maternal mortality by 1,000 deaths by 2018
- reduce severe maternal morbidity
- assist states and hospitals to improve outcomes
- create and encourage use of maternal safety bundles (evidence-based tool kits to guide the best care).
AIM offers participating physicians and hospitals online learning modules, checklists, work plans, and links to tool kits and published resources. Implementation data is shared with hospitals and states to further improve care. Physicians participating in AIM can receive Part IV maintenance of certification; continuing education units will soon be offered for nurses. In the future, AIM-participating hospitals may be able to receive reduced liability protection costs, too.
To date, 17 states are participating in the AIM initiative (FIGURE 3), with more states ready to enroll.4 States must demonstrate a commitment to lasting change to participate. Each AIM state must have an active maternal mortality review committee (MMRC); committed leadership from public health, hospital associations, and provider associations; and a commitment to report AIM data.

AIM thus far has released 9 obstetric patient safety bundles, including:
- reducing disparities in maternity care
- severe hypertension in pregnancy
- safe reduction of primary cesarean birth
- prevention of venous thromboembolism
- obstetric hemorrhage
- maternal mental health
- patient, family, and staff support following a severe maternal event
- postpartum care basics
- obstetric care of women with opioid use disorder (in use by Illinois, Massachusetts, Maryland, New Jersey, Maine, New Hampshire, Vermont, New York, Ohio, Oklahoma, Tennessee, Texas, and Virginia).
Read about how active MMRCS are critical to success
Review committees are critical to success
In use in many states, MMRCs are groups of local ObGyns, nurses, social workers, and other health care professionals who review specific cases of maternal deaths from their local area and recommend local solutions to prevent future deaths. MMRCs can be a critically important source of data to help us understand the underlying causes of maternal mortality.
Remember California’s success in reducing its maternal mortality rate, previously mentioned? That state was an early adopter of an active MMRC and has worked to bring best practices to maternity care throughout the state.
While every state should have an active MMRC, not every state does. ACOG is working with states, local leaders, and state and federal legislatures to help develop MMRCs in every state.
Dr. Brown pointed out that, “For several decades, Indiana had a legislatively authorized multidisciplinary maternal mortality review committee that I actively participated in and led in the late 1990s. The authorization for the program lapsed in the early 2000s, and the Indiana MMRC had to shut down. Bolstering the federal government’s capacity to help states like Indiana rebuild MMRCs, or start them from scratch, will help state public health officials, hospitals, and physicians take better care of moms and babies.”
Dr. Hollier explained, “In Texas, I chair our Maternal Mortality and Morbidity Task Force, which was legislatively authorized in 2013 in response to the rising rate of maternal death. The detailed state-based maternal mortality reviews provide critical information: verification of vital statistics data, assessment of the causes and contributing factors, and determination of pregnancy relatedness. These reviews identify opportunities for prevention and implementation of the most appropriate interventions to reduce maternal mortality on a local level. Support of essential review functions at the federal level would also enable data to be combined across jurisdictions for national learning that was previously not possible.”
Pending legislation will strengthen efforts
ACOG is working to enact into law the Preventing Maternal Deaths Act, HR 1318 and S1112. This is bipartisan legislation under which the Centers for Disease Control and Prevention would help states create or expand MMRCs and will require the Department of Health and Human Services to research ways to reduce disparities in maternal health outcomes.
Acknowledgement
The author thanks Jean Mahoney, ACOG’s Senior Director, AIM, for her generous assistance.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- MacDorman MF, Declerq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128(3):447–455.
- Centers for Disease Control and Prevention. Pregnancy mortality surveillance system. www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Updated November 9, 2017. Accessed February 16, 2018.
- Singh GK. Maternal mortality in the United States, 1935−2007: Substantial racial/ethnic, socioeconomic, and geographic disparities persist. A 75th Anniversary Publication. Health Resources and Services Administration, Maternal and Child Health Bureau. Rockville, Maryland: US Department of Health and Human Services; 2010. https://www.hrsa.gov/sites/default/files/ourstories/mchb75th/mchb75maternalmortality.pdf. Accessed February 16, 2018.
- Council on Patient Safety in Women’s Health Care. Alliance for Innovation on Maternal Health Program: AIM states and systems. http://safehealthcareforeverywoman.org/aim-states-systems-2/#link_tab-1513011413196-9. Accessed February 20, 2018.
- MacDorman MF, Declerq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128(3):447–455.
- Centers for Disease Control and Prevention. Pregnancy mortality surveillance system. www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Updated November 9, 2017. Accessed February 16, 2018.
- Singh GK. Maternal mortality in the United States, 1935−2007: Substantial racial/ethnic, socioeconomic, and geographic disparities persist. A 75th Anniversary Publication. Health Resources and Services Administration, Maternal and Child Health Bureau. Rockville, Maryland: US Department of Health and Human Services; 2010. https://www.hrsa.gov/sites/default/files/ourstories/mchb75th/mchb75maternalmortality.pdf. Accessed February 16, 2018.
- Council on Patient Safety in Women’s Health Care. Alliance for Innovation on Maternal Health Program: AIM states and systems. http://safehealthcareforeverywoman.org/aim-states-systems-2/#link_tab-1513011413196-9. Accessed February 20, 2018.
Research Changes Understanding of Posttraumatic Headache
NAPLES, FL—A growing literature on posttraumatic headache suggests that many of the accepted principles of onset, course, and treatment should be re-examined. Data suggest that pathogenesis is multidimensional, and neurologists still lack evidence-based treatments, according to a lecture delivered at the 45th Annual Meeting of the Southern Clinical Neurological Society.
When Does Onset Occur?
To begin with, research is calling the current definition of posttraumatic headache into question. Data culled from head injuries in the military, professional sports, and everyday trauma show that headache onset more than seven days after the trauma is not the exception it once was believed to be.
“Regarding soldiers in particular, only 37% report posttraumatic headache onset within seven days of their injury,” reported Bert B. Vargas, MD, Director of the Sports Neurology and Concussion Program at the University of Texas Southwestern Medical Center in Dallas. “The rest of them presented up to several weeks later.”
Of these others, 20% developed headache from one week to one month after the head trauma, and the remaining patients, more than 40% of the total, noted onset of headache more than a month later. “This is compelling evidence that seven days is perhaps not really the right timeframe” when considering a diagnosis of posttraumatic headache and suggests a need to revisit existing diagnostic criteria, said Dr. Vargas.
A New Understanding of Phenotype
The accepted phenotypes of posttraumatic headache have also shifted over the past several years, according to Dr. Vargas. Although tension-like headache was considered characteristic of most posttraumatic headache in years past, researchers familiar with International Classification of Headache Disorders (ICHD) criteria for migraine and other headache disorders found that the tension-type phenotype represented only about 20% of posttraumatic headache. Migraine (∼40%) and probable migraine (∼25%) are the most representative phenotypes, Dr. Vargas said. About 10% of patients have a cervicogenic phenotype, while other phenotypes, like cluster headache, are less common.
Prognosis has also been revisited. More rigorous follow-up shows that a one-to-two-week recovery period is not as typical as once was believed, said Dr. Vargas. An examination of military and athletic injuries suggests that half or more patients continue to have recurring headaches at three months, and as much as one-third have them at the end of one year. In one study, 24% of patients still had recurring headaches at four years. A more recent study reported that at five years after injury, as much as 36% of patients may experience headache several times per week or daily.
Research Clarifies Pathophysiology
The close association between posttraumatic headache and migraine is consistent with the underlying pathophysiology derived from experimental models. In these models, the depolarization caused by concussive force produces a shift in ions that can disrupt neuronal metabolic function, Dr. Vargas said. Similar metabolic changes are associated with migraine aura. These changes include potassium efflux and sodium influx. The increased energy demand produced by activation of ion pumps can be complicated by diminished cerebral blood flow, thus impairing the cell’s drive to maintain homeostasis.
In this cascade of events, which includes cortical spreading depression, headache pain for posttraumatic headache and migraine is believed to be generated by activation of glial cells and release of factors such as calcitonin gene-related peptide (CGRP) that are implicated in pain signaling. “If the accepted pathophysiologies are correct, then what is happening on an intracellular level after concussion is similar to what is seen in migraine aura,” said Dr. Vargas.
Potential Changes in Treatment
Regarding medications commonly used for prophylaxis of posttraumatic headache, “we see a great deal of overlap with medications that are commonly used to treat migraine,” Dr. Vargas said. The evidence supporting the benefit of these agents is generally derived from small, retrospective, open-label studies, however. In one retrospective study in soldiers, topiramate outperformed tricyclic antidepressants, propranolol, and valproate with regard to decrease in headache frequency and Migraine Disability Assessment score in patients with posttraumatic headache. In this study, triptans outperformed nontriptans for acute treatment at two hours. The response rate was better, however, for posttraumatic headache associated with blunt trauma, relative to blast trauma (86% vs 66%).
This difference is potentially important, because experimental studies of blunt and blast concussions suggest that they may be different. “In rodent models, blunt force injury has been shown to result in mast cell degranulation and decrease in the actual density of the mast cells on the ipsilateral and contralateral side of the injury within 72 hours,” said Dr. Vargas. Blast injuries in rodents, in contrast, produce “a delayed and bilateral mast cell degranulation at day seven.” Although further degranulation occurs after this point, the persistence in mast cell density suggests that “the cascades of events that ensue after blunt trauma and blast injuries may be different and may have a meaningful influence on treatment and our expectations for recovery timelines,” said Dr. Vargas.
Even if the best treatments for blast and blunt posttraumatic headache differ, however, there is a lack of well-conducted clinical trials for either condition. Based on available evidence and his own experience, Dr. Vargas concluded that all or most of the therapies used for acute treatment and prophylaxis of migraine are effective in at least some patients with posttraumatic headache. As a precaution, “despite excellent evidence that it is an effective migraine prophylactic medication, I find myself avoiding topiramate as a first-line treatment,” due to concern that this agent may exacerbate the cognitive dysfunction frequently associated with concussion, said Dr. Vargas. Despite some headache specialists’ belief that extended-release topiramate has less effect on cognitive function, Dr. Vargas is not aware of any head-to-head study confirming that the drug reduces this risk.
Despite the evidence that migraine medications offer relief in posttraumatic headache, they are not the first choice for many clinicians, said Dr. Vargas. In one study, between 2% and 5% of patients with posttraumatic headache received triptans. In an ongoing concussion registry in Texas that has now enrolled more than 2,000 patients, “we have observed frequent use of either nonsteroidal anti-inflammatory drugs or acetaminophen,” but initial treatment with migraine-specific medications, such as triptans, is not common, said Dr. Vargas. The data from this registry suggest that many patients, particularly those with a migraine phenotype, appear to report suboptimal pain control.
“Our registry data support other studies showing that migraine-specific medications may be underutilized in posttraumatic headache—including those with migrainous features,” said Dr. Vargas, who helped develop the concussion registry. “An important question that we must address is whether more aggressive treatment directed toward headache phenotype leads to better short- and long-term outcomes.
“Additionally, although current expert opinion suggests that treatment of posttraumatic headache should be based on treatment algorithms based on headache phenotype, well-designed prospective studies are needed to address this question.”
More rigorously defined treatment algorithms have become an urgent need in the context of growing evidence that posttraumatic headache can result in significant morbidity. Dr. Vargas cited one study in which 18.7% of soldiers with persistent posttraumatic headache returned to combat. Also, concern about the long-term consequences of posttraumatic headache from sports-related concussion is growing. In the context of the frequency of posttraumatic headache, Dr. Vargas believes there is an urgent need for objective studies to improve care.
Dr. Vargas reported financial relationships with Amgen, Alder, Avanir, Lilly, Pernix, and Upsher-Smith.
—Ted Bosworth
Erickson JC. Treatment outcomes of chronic post-traumatic headaches after mild head trauma in US soldiers: an observational study. Headache. 2011;51(6):932-944.
Packard RC. Treatment of chronic daily posttraumatic headache with divalproex sodium. Headache. 2000;40(9):736-739.
NAPLES, FL—A growing literature on posttraumatic headache suggests that many of the accepted principles of onset, course, and treatment should be re-examined. Data suggest that pathogenesis is multidimensional, and neurologists still lack evidence-based treatments, according to a lecture delivered at the 45th Annual Meeting of the Southern Clinical Neurological Society.
When Does Onset Occur?
To begin with, research is calling the current definition of posttraumatic headache into question. Data culled from head injuries in the military, professional sports, and everyday trauma show that headache onset more than seven days after the trauma is not the exception it once was believed to be.
“Regarding soldiers in particular, only 37% report posttraumatic headache onset within seven days of their injury,” reported Bert B. Vargas, MD, Director of the Sports Neurology and Concussion Program at the University of Texas Southwestern Medical Center in Dallas. “The rest of them presented up to several weeks later.”
Of these others, 20% developed headache from one week to one month after the head trauma, and the remaining patients, more than 40% of the total, noted onset of headache more than a month later. “This is compelling evidence that seven days is perhaps not really the right timeframe” when considering a diagnosis of posttraumatic headache and suggests a need to revisit existing diagnostic criteria, said Dr. Vargas.
A New Understanding of Phenotype
The accepted phenotypes of posttraumatic headache have also shifted over the past several years, according to Dr. Vargas. Although tension-like headache was considered characteristic of most posttraumatic headache in years past, researchers familiar with International Classification of Headache Disorders (ICHD) criteria for migraine and other headache disorders found that the tension-type phenotype represented only about 20% of posttraumatic headache. Migraine (∼40%) and probable migraine (∼25%) are the most representative phenotypes, Dr. Vargas said. About 10% of patients have a cervicogenic phenotype, while other phenotypes, like cluster headache, are less common.
Prognosis has also been revisited. More rigorous follow-up shows that a one-to-two-week recovery period is not as typical as once was believed, said Dr. Vargas. An examination of military and athletic injuries suggests that half or more patients continue to have recurring headaches at three months, and as much as one-third have them at the end of one year. In one study, 24% of patients still had recurring headaches at four years. A more recent study reported that at five years after injury, as much as 36% of patients may experience headache several times per week or daily.
Research Clarifies Pathophysiology
The close association between posttraumatic headache and migraine is consistent with the underlying pathophysiology derived from experimental models. In these models, the depolarization caused by concussive force produces a shift in ions that can disrupt neuronal metabolic function, Dr. Vargas said. Similar metabolic changes are associated with migraine aura. These changes include potassium efflux and sodium influx. The increased energy demand produced by activation of ion pumps can be complicated by diminished cerebral blood flow, thus impairing the cell’s drive to maintain homeostasis.
In this cascade of events, which includes cortical spreading depression, headache pain for posttraumatic headache and migraine is believed to be generated by activation of glial cells and release of factors such as calcitonin gene-related peptide (CGRP) that are implicated in pain signaling. “If the accepted pathophysiologies are correct, then what is happening on an intracellular level after concussion is similar to what is seen in migraine aura,” said Dr. Vargas.
Potential Changes in Treatment
Regarding medications commonly used for prophylaxis of posttraumatic headache, “we see a great deal of overlap with medications that are commonly used to treat migraine,” Dr. Vargas said. The evidence supporting the benefit of these agents is generally derived from small, retrospective, open-label studies, however. In one retrospective study in soldiers, topiramate outperformed tricyclic antidepressants, propranolol, and valproate with regard to decrease in headache frequency and Migraine Disability Assessment score in patients with posttraumatic headache. In this study, triptans outperformed nontriptans for acute treatment at two hours. The response rate was better, however, for posttraumatic headache associated with blunt trauma, relative to blast trauma (86% vs 66%).
This difference is potentially important, because experimental studies of blunt and blast concussions suggest that they may be different. “In rodent models, blunt force injury has been shown to result in mast cell degranulation and decrease in the actual density of the mast cells on the ipsilateral and contralateral side of the injury within 72 hours,” said Dr. Vargas. Blast injuries in rodents, in contrast, produce “a delayed and bilateral mast cell degranulation at day seven.” Although further degranulation occurs after this point, the persistence in mast cell density suggests that “the cascades of events that ensue after blunt trauma and blast injuries may be different and may have a meaningful influence on treatment and our expectations for recovery timelines,” said Dr. Vargas.
Even if the best treatments for blast and blunt posttraumatic headache differ, however, there is a lack of well-conducted clinical trials for either condition. Based on available evidence and his own experience, Dr. Vargas concluded that all or most of the therapies used for acute treatment and prophylaxis of migraine are effective in at least some patients with posttraumatic headache. As a precaution, “despite excellent evidence that it is an effective migraine prophylactic medication, I find myself avoiding topiramate as a first-line treatment,” due to concern that this agent may exacerbate the cognitive dysfunction frequently associated with concussion, said Dr. Vargas. Despite some headache specialists’ belief that extended-release topiramate has less effect on cognitive function, Dr. Vargas is not aware of any head-to-head study confirming that the drug reduces this risk.
Despite the evidence that migraine medications offer relief in posttraumatic headache, they are not the first choice for many clinicians, said Dr. Vargas. In one study, between 2% and 5% of patients with posttraumatic headache received triptans. In an ongoing concussion registry in Texas that has now enrolled more than 2,000 patients, “we have observed frequent use of either nonsteroidal anti-inflammatory drugs or acetaminophen,” but initial treatment with migraine-specific medications, such as triptans, is not common, said Dr. Vargas. The data from this registry suggest that many patients, particularly those with a migraine phenotype, appear to report suboptimal pain control.
“Our registry data support other studies showing that migraine-specific medications may be underutilized in posttraumatic headache—including those with migrainous features,” said Dr. Vargas, who helped develop the concussion registry. “An important question that we must address is whether more aggressive treatment directed toward headache phenotype leads to better short- and long-term outcomes.
“Additionally, although current expert opinion suggests that treatment of posttraumatic headache should be based on treatment algorithms based on headache phenotype, well-designed prospective studies are needed to address this question.”
More rigorously defined treatment algorithms have become an urgent need in the context of growing evidence that posttraumatic headache can result in significant morbidity. Dr. Vargas cited one study in which 18.7% of soldiers with persistent posttraumatic headache returned to combat. Also, concern about the long-term consequences of posttraumatic headache from sports-related concussion is growing. In the context of the frequency of posttraumatic headache, Dr. Vargas believes there is an urgent need for objective studies to improve care.
Dr. Vargas reported financial relationships with Amgen, Alder, Avanir, Lilly, Pernix, and Upsher-Smith.
—Ted Bosworth
Erickson JC. Treatment outcomes of chronic post-traumatic headaches after mild head trauma in US soldiers: an observational study. Headache. 2011;51(6):932-944.
Packard RC. Treatment of chronic daily posttraumatic headache with divalproex sodium. Headache. 2000;40(9):736-739.
NAPLES, FL—A growing literature on posttraumatic headache suggests that many of the accepted principles of onset, course, and treatment should be re-examined. Data suggest that pathogenesis is multidimensional, and neurologists still lack evidence-based treatments, according to a lecture delivered at the 45th Annual Meeting of the Southern Clinical Neurological Society.
When Does Onset Occur?
To begin with, research is calling the current definition of posttraumatic headache into question. Data culled from head injuries in the military, professional sports, and everyday trauma show that headache onset more than seven days after the trauma is not the exception it once was believed to be.
“Regarding soldiers in particular, only 37% report posttraumatic headache onset within seven days of their injury,” reported Bert B. Vargas, MD, Director of the Sports Neurology and Concussion Program at the University of Texas Southwestern Medical Center in Dallas. “The rest of them presented up to several weeks later.”
Of these others, 20% developed headache from one week to one month after the head trauma, and the remaining patients, more than 40% of the total, noted onset of headache more than a month later. “This is compelling evidence that seven days is perhaps not really the right timeframe” when considering a diagnosis of posttraumatic headache and suggests a need to revisit existing diagnostic criteria, said Dr. Vargas.
A New Understanding of Phenotype
The accepted phenotypes of posttraumatic headache have also shifted over the past several years, according to Dr. Vargas. Although tension-like headache was considered characteristic of most posttraumatic headache in years past, researchers familiar with International Classification of Headache Disorders (ICHD) criteria for migraine and other headache disorders found that the tension-type phenotype represented only about 20% of posttraumatic headache. Migraine (∼40%) and probable migraine (∼25%) are the most representative phenotypes, Dr. Vargas said. About 10% of patients have a cervicogenic phenotype, while other phenotypes, like cluster headache, are less common.
Prognosis has also been revisited. More rigorous follow-up shows that a one-to-two-week recovery period is not as typical as once was believed, said Dr. Vargas. An examination of military and athletic injuries suggests that half or more patients continue to have recurring headaches at three months, and as much as one-third have them at the end of one year. In one study, 24% of patients still had recurring headaches at four years. A more recent study reported that at five years after injury, as much as 36% of patients may experience headache several times per week or daily.
Research Clarifies Pathophysiology
The close association between posttraumatic headache and migraine is consistent with the underlying pathophysiology derived from experimental models. In these models, the depolarization caused by concussive force produces a shift in ions that can disrupt neuronal metabolic function, Dr. Vargas said. Similar metabolic changes are associated with migraine aura. These changes include potassium efflux and sodium influx. The increased energy demand produced by activation of ion pumps can be complicated by diminished cerebral blood flow, thus impairing the cell’s drive to maintain homeostasis.
In this cascade of events, which includes cortical spreading depression, headache pain for posttraumatic headache and migraine is believed to be generated by activation of glial cells and release of factors such as calcitonin gene-related peptide (CGRP) that are implicated in pain signaling. “If the accepted pathophysiologies are correct, then what is happening on an intracellular level after concussion is similar to what is seen in migraine aura,” said Dr. Vargas.
Potential Changes in Treatment
Regarding medications commonly used for prophylaxis of posttraumatic headache, “we see a great deal of overlap with medications that are commonly used to treat migraine,” Dr. Vargas said. The evidence supporting the benefit of these agents is generally derived from small, retrospective, open-label studies, however. In one retrospective study in soldiers, topiramate outperformed tricyclic antidepressants, propranolol, and valproate with regard to decrease in headache frequency and Migraine Disability Assessment score in patients with posttraumatic headache. In this study, triptans outperformed nontriptans for acute treatment at two hours. The response rate was better, however, for posttraumatic headache associated with blunt trauma, relative to blast trauma (86% vs 66%).
This difference is potentially important, because experimental studies of blunt and blast concussions suggest that they may be different. “In rodent models, blunt force injury has been shown to result in mast cell degranulation and decrease in the actual density of the mast cells on the ipsilateral and contralateral side of the injury within 72 hours,” said Dr. Vargas. Blast injuries in rodents, in contrast, produce “a delayed and bilateral mast cell degranulation at day seven.” Although further degranulation occurs after this point, the persistence in mast cell density suggests that “the cascades of events that ensue after blunt trauma and blast injuries may be different and may have a meaningful influence on treatment and our expectations for recovery timelines,” said Dr. Vargas.
Even if the best treatments for blast and blunt posttraumatic headache differ, however, there is a lack of well-conducted clinical trials for either condition. Based on available evidence and his own experience, Dr. Vargas concluded that all or most of the therapies used for acute treatment and prophylaxis of migraine are effective in at least some patients with posttraumatic headache. As a precaution, “despite excellent evidence that it is an effective migraine prophylactic medication, I find myself avoiding topiramate as a first-line treatment,” due to concern that this agent may exacerbate the cognitive dysfunction frequently associated with concussion, said Dr. Vargas. Despite some headache specialists’ belief that extended-release topiramate has less effect on cognitive function, Dr. Vargas is not aware of any head-to-head study confirming that the drug reduces this risk.
Despite the evidence that migraine medications offer relief in posttraumatic headache, they are not the first choice for many clinicians, said Dr. Vargas. In one study, between 2% and 5% of patients with posttraumatic headache received triptans. In an ongoing concussion registry in Texas that has now enrolled more than 2,000 patients, “we have observed frequent use of either nonsteroidal anti-inflammatory drugs or acetaminophen,” but initial treatment with migraine-specific medications, such as triptans, is not common, said Dr. Vargas. The data from this registry suggest that many patients, particularly those with a migraine phenotype, appear to report suboptimal pain control.
“Our registry data support other studies showing that migraine-specific medications may be underutilized in posttraumatic headache—including those with migrainous features,” said Dr. Vargas, who helped develop the concussion registry. “An important question that we must address is whether more aggressive treatment directed toward headache phenotype leads to better short- and long-term outcomes.
“Additionally, although current expert opinion suggests that treatment of posttraumatic headache should be based on treatment algorithms based on headache phenotype, well-designed prospective studies are needed to address this question.”
More rigorously defined treatment algorithms have become an urgent need in the context of growing evidence that posttraumatic headache can result in significant morbidity. Dr. Vargas cited one study in which 18.7% of soldiers with persistent posttraumatic headache returned to combat. Also, concern about the long-term consequences of posttraumatic headache from sports-related concussion is growing. In the context of the frequency of posttraumatic headache, Dr. Vargas believes there is an urgent need for objective studies to improve care.
Dr. Vargas reported financial relationships with Amgen, Alder, Avanir, Lilly, Pernix, and Upsher-Smith.
—Ted Bosworth
Erickson JC. Treatment outcomes of chronic post-traumatic headaches after mild head trauma in US soldiers: an observational study. Headache. 2011;51(6):932-944.
Packard RC. Treatment of chronic daily posttraumatic headache with divalproex sodium. Headache. 2000;40(9):736-739.