User login
Case Study - Foot and Hand Tapping
Nikesh Ardeshna, MD, MS, FAES
Case
A 73-year-old right-handed male presents with a history of mild depression (since his retirement about 2 years prior to the office visit) and benign prostatic hypertrophy. For about 8 months, the patient had episodes of right hand tapping, or right foot tapping accompanied by staring, and sometimes by what was described as a pronounced swallow/gulp. The total duration of these symptoms was less than one minute. The patient denied having any falls, major illness, or head trauma prior to the onset of symptoms. On initial evaluation the patient was diagnosed with anxiety. He elected not to start any medication.
The symptoms continued, and the patient was not aware that they were occurring. For example, one episode occurred at the dinner table with guests. The patient tapped on the adjacent dinner plate, and the guests thought he was playing a joke. The patient’s wife took him for a re-evaluation, and an episode occurred in the physician’s office.
The physician ordered a routine electroencephalogram (EEG). The EEG showed frequent left frontal temporal sharp and slow waves. No seizures were recorded. The patient was referred to an epileptologist. He was started on lacosamide, with a slowly escalating dose. Since being on a therapeutic dose the patient has not experienced any events, as reported by others.
Question 1: What is the patient’s diagnosis?
- Anxiety
- Depression
- Tics
- Partial epilepsy
- Unknown
Answer: d) Partial Epilepsy
Partial epilepsy (recurrent partial seizures) can manifest with a combination of tapping, chewing, staring, and blinking, but it is not limited to these symptoms. These abnormal, unintended movements are automatisms.
Question 2: Which adverse events may take greater precedence in the elderly and must be considered when choosing which anti-seizure medication to prescribe?
- Drowsiness
- Cognitive slowing/slow processing
- Unsteady gait
- Double vision
- All of the above
Answer: e) All of the above
Different anti-seizure drugs (ASDs) can have different adverse events. But, many potential adverse events are common to all ASDs although to differing extents, including but not limited to sleepiness/drowsiness and cognitive slowing/memory loss. Elderly patients are more prone to falls, and may have preexisting memory or cognitive issues. Adverse events should be minimized to the greatest extent possible in the elderly. Physicians should remember that each individual/case is unique.
Question 3: Which of the following demonstrates the correct match of a cause (etiology) of epilepsy and when seizures may begin due to that cause:
- Stroke – 6 months
- Brain tumor – 3 months
- Dementia – 1 year
- Idiopathic (unknown etiology) – childhood years
- None of the above
Answer: e) None of the above
The above is only a partial list of causes of epilepsy. For symptomatic epilepsy – epilepsy due to a stroke, brain tumor, or dementia, there is not a specific timeframe in which/by which the seizures have to begin. Also, a significant number of cases of epilepsy are of unknown cause (idiopathic), and in these situations seizures can begin at any age, as is the case above.
Nikesh Ardeshna, MD, MS, FAES
Case
A 73-year-old right-handed male presents with a history of mild depression (since his retirement about 2 years prior to the office visit) and benign prostatic hypertrophy. For about 8 months, the patient had episodes of right hand tapping, or right foot tapping accompanied by staring, and sometimes by what was described as a pronounced swallow/gulp. The total duration of these symptoms was less than one minute. The patient denied having any falls, major illness, or head trauma prior to the onset of symptoms. On initial evaluation the patient was diagnosed with anxiety. He elected not to start any medication.
The symptoms continued, and the patient was not aware that they were occurring. For example, one episode occurred at the dinner table with guests. The patient tapped on the adjacent dinner plate, and the guests thought he was playing a joke. The patient’s wife took him for a re-evaluation, and an episode occurred in the physician’s office.
The physician ordered a routine electroencephalogram (EEG). The EEG showed frequent left frontal temporal sharp and slow waves. No seizures were recorded. The patient was referred to an epileptologist. He was started on lacosamide, with a slowly escalating dose. Since being on a therapeutic dose the patient has not experienced any events, as reported by others.
Question 1: What is the patient’s diagnosis?
- Anxiety
- Depression
- Tics
- Partial epilepsy
- Unknown
Answer: d) Partial Epilepsy
Partial epilepsy (recurrent partial seizures) can manifest with a combination of tapping, chewing, staring, and blinking, but it is not limited to these symptoms. These abnormal, unintended movements are automatisms.
Question 2: Which adverse events may take greater precedence in the elderly and must be considered when choosing which anti-seizure medication to prescribe?
- Drowsiness
- Cognitive slowing/slow processing
- Unsteady gait
- Double vision
- All of the above
Answer: e) All of the above
Different anti-seizure drugs (ASDs) can have different adverse events. But, many potential adverse events are common to all ASDs although to differing extents, including but not limited to sleepiness/drowsiness and cognitive slowing/memory loss. Elderly patients are more prone to falls, and may have preexisting memory or cognitive issues. Adverse events should be minimized to the greatest extent possible in the elderly. Physicians should remember that each individual/case is unique.
Question 3: Which of the following demonstrates the correct match of a cause (etiology) of epilepsy and when seizures may begin due to that cause:
- Stroke – 6 months
- Brain tumor – 3 months
- Dementia – 1 year
- Idiopathic (unknown etiology) – childhood years
- None of the above
Answer: e) None of the above
The above is only a partial list of causes of epilepsy. For symptomatic epilepsy – epilepsy due to a stroke, brain tumor, or dementia, there is not a specific timeframe in which/by which the seizures have to begin. Also, a significant number of cases of epilepsy are of unknown cause (idiopathic), and in these situations seizures can begin at any age, as is the case above.
Nikesh Ardeshna, MD, MS, FAES
Case
A 73-year-old right-handed male presents with a history of mild depression (since his retirement about 2 years prior to the office visit) and benign prostatic hypertrophy. For about 8 months, the patient had episodes of right hand tapping, or right foot tapping accompanied by staring, and sometimes by what was described as a pronounced swallow/gulp. The total duration of these symptoms was less than one minute. The patient denied having any falls, major illness, or head trauma prior to the onset of symptoms. On initial evaluation the patient was diagnosed with anxiety. He elected not to start any medication.
The symptoms continued, and the patient was not aware that they were occurring. For example, one episode occurred at the dinner table with guests. The patient tapped on the adjacent dinner plate, and the guests thought he was playing a joke. The patient’s wife took him for a re-evaluation, and an episode occurred in the physician’s office.
The physician ordered a routine electroencephalogram (EEG). The EEG showed frequent left frontal temporal sharp and slow waves. No seizures were recorded. The patient was referred to an epileptologist. He was started on lacosamide, with a slowly escalating dose. Since being on a therapeutic dose the patient has not experienced any events, as reported by others.
Question 1: What is the patient’s diagnosis?
- Anxiety
- Depression
- Tics
- Partial epilepsy
- Unknown
Answer: d) Partial Epilepsy
Partial epilepsy (recurrent partial seizures) can manifest with a combination of tapping, chewing, staring, and blinking, but it is not limited to these symptoms. These abnormal, unintended movements are automatisms.
Question 2: Which adverse events may take greater precedence in the elderly and must be considered when choosing which anti-seizure medication to prescribe?
- Drowsiness
- Cognitive slowing/slow processing
- Unsteady gait
- Double vision
- All of the above
Answer: e) All of the above
Different anti-seizure drugs (ASDs) can have different adverse events. But, many potential adverse events are common to all ASDs although to differing extents, including but not limited to sleepiness/drowsiness and cognitive slowing/memory loss. Elderly patients are more prone to falls, and may have preexisting memory or cognitive issues. Adverse events should be minimized to the greatest extent possible in the elderly. Physicians should remember that each individual/case is unique.
Question 3: Which of the following demonstrates the correct match of a cause (etiology) of epilepsy and when seizures may begin due to that cause:
- Stroke – 6 months
- Brain tumor – 3 months
- Dementia – 1 year
- Idiopathic (unknown etiology) – childhood years
- None of the above
Answer: e) None of the above
The above is only a partial list of causes of epilepsy. For symptomatic epilepsy – epilepsy due to a stroke, brain tumor, or dementia, there is not a specific timeframe in which/by which the seizures have to begin. Also, a significant number of cases of epilepsy are of unknown cause (idiopathic), and in these situations seizures can begin at any age, as is the case above.
MDedge Daily News: Where the latest HCV drug combos fit in
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Psychiatrists urged to take lead in recognizing physician burnout
NEW YORK – The proportion of physicians who commit suicide each year is greater than the proportion of Americans who die of an opioid overdose, according to a series of sobering statistics on physician burnout presented at the American Psychiatric Association annual meeting.
“There are about 400 physician suicides very year, which is proportional rate that is about twice the suicide rate in the general population,” reported Darrell G. Kirch, MD, president and chief executive officer of the Association of American Medical Colleges, Washington.
Burnout is variably defined, but characterizations typically include emotional exhaustion, a high sense of depersonalization, and a low sense of personal accomplishment. In the 2015 study, the overall rate of burnout when assessed via the Maslach Burnout Inventory was 54.4%.
At 40%, the rate of burnout found among psychiatrists is lower than the mean and places them toward the bottom of the list in the rank order among specialists. Yet, 40% is still a large proportion. Moreover, Dr. Kirch believes that psychiatrists have an important role to play in recognizing and addressing this condition in others.
“ in the organization you work in,” Dr. Kirch said. The campaign to which he referred was a call to action launched last year by the National Academy of Medicine (NAM), the Association of American Medical Colleges, and the Accreditation Council for Graduate Medical Education. Led by NAM, it is called the Action Collaborative on Clinician Well-Being and Resilience.
In the 18 months since it was launched, “more than 150 organizations, including the APA, have made commitment statements and are supporting the work of the collaborative around improving clinician well-being,” Dr. Kirch reported.
Many tools already generated by this collaboration can be found in the NAM website at nam.edu/clinicianwellbeing. This section of the website not only includes data about burnout as well as presentation slides for download on the topic, but it is expected to be “a growing repository for solutions that work,” Dr. Kirch said.
This was not news to those who attended the APA symposium. When Dr. Kirch asked the audience who had treated a colleague for burnout, almost every hand was raised. This would not be surprising, except that the leaders in this field so far have typically not been psychiatrists, Dr. Kirch said.
For example, an increasing number of medical centers are following the lead of Stanford (Calif.) University, which appointed Tate D. Shanafelt, MD, as its first chief wellness officer. However, to the knowledge of Dr. Kirch, none of the appointments have gone to a psychiatrist.
“It strikes me that if you need a chief wellness officer who not only can understand the dynamics of burnout but can understand what a short path it is from being burned out to being depressed, suicidal, or addicted, who would be better suited than a psychiatrist? I really think that in many ways, this may be a career path for psychiatrists,” Dr. Kirch said.
Even with the appointment of chief wellness officers, the problem will not soon go away. Although Dr. Kirch believes interventions are needed from the beginning of medical training, he acknowledged that eliminating burnout “is a heavy lift, because there is no single solution.” Listing regulatory burdens, administrative burdens, lack of support staff, and a sense of isolation among documented causes of burnout, he believes identifying all of the solutions to relieve stress and improve clinical satisfaction “will be a journey.”
Dr. Kirch reported no potential conflicts of interest.
NEW YORK – The proportion of physicians who commit suicide each year is greater than the proportion of Americans who die of an opioid overdose, according to a series of sobering statistics on physician burnout presented at the American Psychiatric Association annual meeting.
“There are about 400 physician suicides very year, which is proportional rate that is about twice the suicide rate in the general population,” reported Darrell G. Kirch, MD, president and chief executive officer of the Association of American Medical Colleges, Washington.
Burnout is variably defined, but characterizations typically include emotional exhaustion, a high sense of depersonalization, and a low sense of personal accomplishment. In the 2015 study, the overall rate of burnout when assessed via the Maslach Burnout Inventory was 54.4%.
At 40%, the rate of burnout found among psychiatrists is lower than the mean and places them toward the bottom of the list in the rank order among specialists. Yet, 40% is still a large proportion. Moreover, Dr. Kirch believes that psychiatrists have an important role to play in recognizing and addressing this condition in others.
“ in the organization you work in,” Dr. Kirch said. The campaign to which he referred was a call to action launched last year by the National Academy of Medicine (NAM), the Association of American Medical Colleges, and the Accreditation Council for Graduate Medical Education. Led by NAM, it is called the Action Collaborative on Clinician Well-Being and Resilience.
In the 18 months since it was launched, “more than 150 organizations, including the APA, have made commitment statements and are supporting the work of the collaborative around improving clinician well-being,” Dr. Kirch reported.
Many tools already generated by this collaboration can be found in the NAM website at nam.edu/clinicianwellbeing. This section of the website not only includes data about burnout as well as presentation slides for download on the topic, but it is expected to be “a growing repository for solutions that work,” Dr. Kirch said.
This was not news to those who attended the APA symposium. When Dr. Kirch asked the audience who had treated a colleague for burnout, almost every hand was raised. This would not be surprising, except that the leaders in this field so far have typically not been psychiatrists, Dr. Kirch said.
For example, an increasing number of medical centers are following the lead of Stanford (Calif.) University, which appointed Tate D. Shanafelt, MD, as its first chief wellness officer. However, to the knowledge of Dr. Kirch, none of the appointments have gone to a psychiatrist.
“It strikes me that if you need a chief wellness officer who not only can understand the dynamics of burnout but can understand what a short path it is from being burned out to being depressed, suicidal, or addicted, who would be better suited than a psychiatrist? I really think that in many ways, this may be a career path for psychiatrists,” Dr. Kirch said.
Even with the appointment of chief wellness officers, the problem will not soon go away. Although Dr. Kirch believes interventions are needed from the beginning of medical training, he acknowledged that eliminating burnout “is a heavy lift, because there is no single solution.” Listing regulatory burdens, administrative burdens, lack of support staff, and a sense of isolation among documented causes of burnout, he believes identifying all of the solutions to relieve stress and improve clinical satisfaction “will be a journey.”
Dr. Kirch reported no potential conflicts of interest.
NEW YORK – The proportion of physicians who commit suicide each year is greater than the proportion of Americans who die of an opioid overdose, according to a series of sobering statistics on physician burnout presented at the American Psychiatric Association annual meeting.
“There are about 400 physician suicides very year, which is proportional rate that is about twice the suicide rate in the general population,” reported Darrell G. Kirch, MD, president and chief executive officer of the Association of American Medical Colleges, Washington.
Burnout is variably defined, but characterizations typically include emotional exhaustion, a high sense of depersonalization, and a low sense of personal accomplishment. In the 2015 study, the overall rate of burnout when assessed via the Maslach Burnout Inventory was 54.4%.
At 40%, the rate of burnout found among psychiatrists is lower than the mean and places them toward the bottom of the list in the rank order among specialists. Yet, 40% is still a large proportion. Moreover, Dr. Kirch believes that psychiatrists have an important role to play in recognizing and addressing this condition in others.
“ in the organization you work in,” Dr. Kirch said. The campaign to which he referred was a call to action launched last year by the National Academy of Medicine (NAM), the Association of American Medical Colleges, and the Accreditation Council for Graduate Medical Education. Led by NAM, it is called the Action Collaborative on Clinician Well-Being and Resilience.
In the 18 months since it was launched, “more than 150 organizations, including the APA, have made commitment statements and are supporting the work of the collaborative around improving clinician well-being,” Dr. Kirch reported.
Many tools already generated by this collaboration can be found in the NAM website at nam.edu/clinicianwellbeing. This section of the website not only includes data about burnout as well as presentation slides for download on the topic, but it is expected to be “a growing repository for solutions that work,” Dr. Kirch said.
This was not news to those who attended the APA symposium. When Dr. Kirch asked the audience who had treated a colleague for burnout, almost every hand was raised. This would not be surprising, except that the leaders in this field so far have typically not been psychiatrists, Dr. Kirch said.
For example, an increasing number of medical centers are following the lead of Stanford (Calif.) University, which appointed Tate D. Shanafelt, MD, as its first chief wellness officer. However, to the knowledge of Dr. Kirch, none of the appointments have gone to a psychiatrist.
“It strikes me that if you need a chief wellness officer who not only can understand the dynamics of burnout but can understand what a short path it is from being burned out to being depressed, suicidal, or addicted, who would be better suited than a psychiatrist? I really think that in many ways, this may be a career path for psychiatrists,” Dr. Kirch said.
Even with the appointment of chief wellness officers, the problem will not soon go away. Although Dr. Kirch believes interventions are needed from the beginning of medical training, he acknowledged that eliminating burnout “is a heavy lift, because there is no single solution.” Listing regulatory burdens, administrative burdens, lack of support staff, and a sense of isolation among documented causes of burnout, he believes identifying all of the solutions to relieve stress and improve clinical satisfaction “will be a journey.”
Dr. Kirch reported no potential conflicts of interest.
REPORTING FROM APA
Small-Cell Lung Cancer
From the Karmanos Cancer Institute, Detroit, MI (Dr. Mamdani) and the Indiana University School of Medicine, Indianapolis, IN (Dr. Jalal).
Abstract
- Objective: To review the clinical aspects and current practices of management of small cell lung cancer (SCLC).
- Methods: Review of the literature.
- Results: SCLC is an aggressive cancer of neuroendocrine origin with a very strong association with smoking. Approximately 25% of patients present with limited-stage disease while the remaining majority of patients have extensive-stage disease, defined as disease extending beyond one hemithorax at the time of diagnosis. SCLC is often associated with endocrine or neurologic paraneoplastic syndromes. The treatment of limited-stage disease consists of platinum-based chemotherapy administered concurrently with radiation. Patients with partial or complete response should be offered prophylactic cranial radiation (PCI). Extensive-stage disease is largely treated with platinum-based chemotherapy and the role of PCI is more controversial. The efficacy of second-line chemotherapy after disease progression on platinum based chemotherapy is limited.
- Conclusion: Despite a number of advances in the treatment of various malignancies over the period of past several years, the prognosis of patients with SCLC remains poor. There have been a number of clinical trials utilizing novel therapeutic agents to improve outcomes of these patients; however, few of them have shown marginal success in a very select subgroup of patients.
Key words: lung cancer; small-cell lung cancer.
Small-cell lung cancer (SCLC) is an aggressive cancer of neuroendocrine origin, accounting for approximately 15% of all lung cancer cases, with approximately 33,000 patients diagnosed annually [1]. The incidence of SCLC in the United States has steadily declined over the past 30 years presumably because of decrease in the percentage of smokers and change to low-tar filter cigarettes [2]. Although the incidence of SCLC has been decreasing, the incidence in women is increasing and the male-to-female incidence ratio is now 1:1 [3]. Nearly all cases of SCLC are associated with heavy tobacco exposure, making it a heterogeneous disease with complex genomic landscape consisting of thousands of mutations [4,5]. Despite a number of advances in the treatment of non-small cell lung cancer over the past decade, the therapeutic landscape of SCLC remains narrow with median overall survival (OS) of 9 months in patients with advanced disease.
Case Study
Initial Presentation
A 61-year-old man presents to the emergency department with progressive shortness of breath and cough over the period of past 6 weeks. He also reports having had 20-lb weight loss over the same period of time. He is a current smoker and has been smoking one pack of cigarettes per day since the age of 18 years. A chest x-ray performed in the emergency department shows a right hilar mass. Computed tomography (CT) scan confirms the presence of a 4.5 cm right hilar mass with presence of enlarged mediastinal lymph nodes bilaterally.
What are the next steps in diagnosis?
SCLC is characterized by rapid growth and early hematogenous metastases. Consequently, only 25% of patients have limited-stage disease at the time of diagnosis. According to the VA staging system, limited-stage disease is defined as tumor that is confined to one hemithorax and can be encompassed within one radiation field. This typically includes mediastinal lymph nodes and ipsilateral supraclavicular lymph nodes. Extensive-stage disease is the presentation in 75% of the patients where the disease extends beyond one hemithorax. Extensive-stage disease includes presence of malignant pleural effusion and/or distant metastasis [6]. The Veterans Administration Lung Study Group (VALG) classification and staging system is more commonly used compared to the AJCC TNM staging system since it is less complex, directs treatment decisions, and correlates closely with prognosis. Given its propensity to metastasize quickly, none of the currently available screening methods have proven to be successful in early detection of SCLC. Eighty-six percent of the 125 patients that were diagnosed with SCLC while undergoing annual low-dose chest CT scans on National Lung Cancer Screening Trial had advanced disease at diagnosis [7,8]. These results highlight the fact that he majority of the SCLC develop in the interval between annual screening imaging.
SCLC frequently presents with a large hilar mass that is symptomatic. In addition, SCLC usually presents with centrally located tumors and bulky mediastinal adenopathy. Common symptoms include shortness of breath and cough. SCLC is commonly located submucosally in the bronchus and therefore hemoptysis is not a very common symptom at the time of presentation. Patients may present with superior vena cava (SVC) syndrome from local compression by the tumor. Not infrequently, SCLC is associated with paraneoplastic syndromes (PNS) owing to the ectopic secretion of hormones or antibodies by the tumor cells. The PNS can be broadly categorized into endocrine and neurologic; and are summarized in Table 1.
The common sites of metastases include brain, liver, and bone. Therefore, the staging workup should include fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT scan. Contrast-enhanced CT scan of chest and abdomen and bone scan can be obtained for staging in lieu of PET scan. Due to the physiologic FDG uptake, cerebral metastases cannot be assessed with sufficient certainty using the PET-CT. Therefore, brain imaging with contrast enhanced CT or MRI is also necessary. Although the incidence of metastasis to bone marrow is less than 10%, bone marrow aspiration and biopsy is warranted in case of unexplained cytopenias, especially when associated with teardrop red cells or nucleated red cells on peripheral blood smear indicative of marrow infiltrative process. The tissue diagnosis is established by obtaining a biopsy of the primary tumor or one of the metastatic sites. In case of localized disease, bronchoscopy (if necessary, with endobronchial ultrasound) with biopsy of centrally located tumor and/or lymph node is required. Histologically, SCLC consists of monomorphic cells, a high nucleus:cytoplasmic ratio, and confluent necrosis. The tumor cells are positive for chromogranin, synaptophysin, and CD56 by immunohistochemistry. Very frequently the cells are also positive for TTF1. Although serum tumor markers, including neuron-specific enolase (NSE) and progastrin-releasing peptide (prGRP), are frequently elevated in patients with SCLC, they are of limited value in clinical practice owing to their lack of sensitivity and specificity.
Case Continued
The patient underwent FDG-PET scan that showed the presence of hypermetabolic right hilar mass in addition to enlarged and hypermetabolic bilateral mediastinal lymph nodes. There were no other areas of FDG avidity. His brain MRI did not show any evidence of brain metastasis. Thus, he was confirmed to have limited-stage SCLC.
What is the standard of care for limited-stage SCLC?
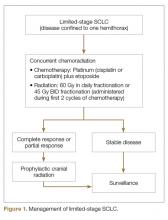
SCLC is exquisitely sensitive to both chemotherapy and radiation, especially at the time of initial presentation. The standard of care for the treatment of limited stage SCLC is 4 cycles of platinum-based chemotherapy in combination with thoracic radiation started within the first 2 cycles of chemotherapy (Figure 1).
Choice of Chemotherapy
Etoposide and cisplatin is the most commonly used initial combination chemotherapy regimen [9]. This combination has largely replaced anthracycline-based regimens given its favorable efficacy and toxicity profile [10–12]. Several small randomized trials have shown comparable efficacy of carboplatin and etoposide in extensive stage SCLC [13–15]. A meta-analysis of 4 randomized trials, including 663 patients with SCLC, comparing cisplatin-based versus carboplatin-based regimens where 32% of patients had limited stage disease and 68% had extensive stage disease showed no statistically significant difference in the response rate, progression free survival (PFS), or OS between the two regimens [16]. Therefore, in clinical practice carboplatin is frequently used instead of cisplatin in patients with extensive-stage disease. In patients with limited-stage disease, cisplatin is still the drug of choice. However, the toxicity profile of the two regimens is different. Cisplatin based regimens are more commonly associated with neuropathy, nephrotoxicity, and chemotherapy induced nausea/vomiting [13], while carboplatin-based regimens are more myelosuppressive [17]. In addition, the combination of thoracic radiation with either of these regiments is associated with higher risk of esophagitis, pneumonitis, and myelosuppression [18]. The use of myeloid growth factors is not recommended in patients undergoing concurrent chemoradiation [19]. Of note, intravenous (IV) etoposide is always preferred over oral etoposide, especially in curative setting given unreliable absorption and bioavailability of oral formulations.
Thoracic Radiation
The addition of thoracic radiation to platinum-etoposide chemotherapy improves local control and OS. Two meta-analyses of 13 trials including more than 2000 patients have shown 25% to 30% decrease in local failure and 5% to 7% increase in 2-year OS with chemoradiation compared to chemotherapy alone in limited stage SCLC [20,21]. Early (with the first 2 cycles) concurrent thoracic radiation is superior to delayed and/or sequential radiation in terms of local control and OS [18,22,23]. The dose and fractionation of thoracic radiation in limited-stage SCLC has remained a controversial issue. The ECOG/RTOG randomized trial compared 45 Gy radiation delivered twice daily over a period of 3 weeks with once a day over 5 weeks, concurrently with chemotherapy. The twice a day regimen led to 10% improvement in 5-year OS (26% vs 16%), but higher incidence of grade 3 and 4 adverse events [24]. Despite the survival advantage demonstrated by hyperfractionated radiotherapy, the results need to be interpreted with caution because the radiation doses are not biologically equivalent. In addition the difficult logistics of patients receiving radiation twice a day has limited the routine implementation of this strategy. Subsequently, another randomized phase III trial (CONVERT) compared 45 Gy twice daily with 66 Gy once daily radiation in this setting. This trial did not show any difference in OS. The patients in twice daily arm had higher incidence of grade 4 neutropenia [25]. Considering the results of these trials, both strategies—45 Gy fractionated twice daily or 60 Gy fractionated once daily, delivered concurrently with chemotherapy—are acceptable in the setting of limited-stage SCLC. However, quite often hyperfractionated regimen is not feasible for the patients and many radiation oncology centers. Hopefully the CALBG 30610 study, which is ongoing, will clarify the optimal radiation schedule for limited-stage disease.
Prophylactic Cranial Irradiation
Approximately 75% of patients with limited-stage disease experience disease recurrence and brain is the site of recurrence in approximately half of these patients. Prophylactic cranial irradiation (PCI) consisting of 25 Gy radiation delivered in 10 fractions has been shown to be effective in decreasing the incidence of cerebral metastases [26–28]. Although individual small studies have not shown survival benefit of PCI because of small sample size and limited power, a meta-analysis of these studies has shown 25% decrease in the 3-year incidence of brain metastasis and 5.4% increase in 3-year OS [27]. The majority of patients included in these studies had limited-stage disease. Therefore, PCI is the standard of care for patients with limited-stage disease who attain a partial or complete response to chemoradiation.
Role of Surgery
Surgical resection may be an acceptable choice in a very limited subset of patients with peripherally located small (< 5 cm) tumors where mediastinal lymph nodes have been confirmed to be uninvolved with complete mediastinal staging [29,30]. Most of the data in this setting are derived from retrospective studies [31,32]. A 5-year OS of 40% to 60% has been has been reported with this strategy in patients with clinical stage I disease. In general, when surgery is considered, lobectomy with mediastinal lymph node dissection followed by chemotherapy (if no nodal involvement) or chemoradiation (if nodal involvement) is recommended [33,34]. Wedge or segmental resections are not considered to be optimum surgical options.
Case Continued
The patient received 4 cycles of cisplatin and etoposide along with 70 Gy radiation concurrently with the first 2 cycles of chemotherapy. His post-treatment CT scans showed partial response (PR). The patient underwent PCI 6 weeks after completion of treatment. Eighteen months later, the patient comes to the clinic for routine follow-up. He is doing generally well except for mildly decreased appetite and unintentional loss of 5 lb weight. His CT scans demonstrate multiple hypodense liver lesions ranging from 7 mm to 2 cm in size and a 2 cm left adrenal gland lesion highly concerning for metastasis. FDG PET scan confirmed the adrenal and liver lesions to be hypermetabolic. In addition, the PET showed multiple FDG avid bone lesions throughout the spine. Brain MRI was negative for any brain metastasis.
What is the standard of care for extensive-stage SCLC?
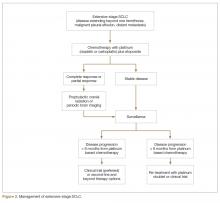
For extensive-stage SCLC, chemotherapy is the mainstay of treatment, with the goals of treatment being prolongation of survival, prevention or alleviation of cancer-related symptoms, and improvement in quality of life. The combination of etoposide with a platinum agent (carboplatin or cisplatin) is the preferred first-line treatment option (Figure 2).
Multiple attempts at improving first-line chemotherapy in extensive-stage disease have failed to show any meaningful difference in OS. For example, addition of ifosfamide, palifosfamide, cyclophosphamide, taxane, or anthracycline to platinum doublet failed to show improvement in OS and led to more toxicity [39–42]. Additionally, the use of alternating or cyclic chemotherapies in an attempt to curb drug resistance has also failed to show survival benefit [43–45]. The addition of antiangiogenic agent bevacizumab to standard platinum-based doublet has not yielded prolongation of OS in SCLC and led to unacceptably higher rate of tracheoesophageal fistula when used in conjunction with chemoradiation in limited-stage disease [46–51]. Finally, the immune checkpoint inhibitor ipilimumab in combination with platinum plus etoposide failed to improve PFS or OS compared to platinum plus etoposide alone in a recent phase III trial and maintenance pembrolizumab after completion of platinum-based chemotherapy did not improve PFS [52,53].
Patients with extensive-stage disease who have brain metastasis at the time of diagnosis can be treated with systemic chemotherapy first if brain metastases are asymptomatic and there is significant extracranial disease burden. In that case, whole brain radiotherapy should be given after completion of systemic therapy.
Second-Line Therapy
Despite being exquisitely chemo-sensitive, SCLC is associated with very poor prognosis largely because of invariable disease progression following first-line therapy and lack of effective second-line treatment options that can lead to appreciable disease control. The choice of second-line treatment is predominantly determined by the time of disease relapse since first-line platinum based therapy. If this interval is 6 months or longer, re-treatment utilizing the same platinum doublet is appropriate. However, if the interval is 6 months or less, second-line systemic therapy options should be explored. Unfortunately, the response rate tends to be less than 10% with most of the second-line therapies in platinum-resistant disease (defined as disease progression within 3 months of receiving platinum-based therapy). If the disease progression occurs between 3 to 6 months since platinum-based therapy, the response rate with second-line chemotherapy is in the range of 25% [54,55]. A number of second-line chemotherapy options have been explored in small studies, including topotecan, irinotecan, paclitaxel, docetaxel, temozolomide, vinorelbine, oral etoposide, gemcitabine, bendamustine, and CAV (cyclophosphamide, adriamycin, vincristine) (Table 2).
Immunotherapy
The role of immune checkpoint inhibitors in the treatment of SCLC is evolving and currently there are no FDA-approved immunotherapy agents in SCLC. A recently conducted phase I/II trial (CheckMate 032) of anti-PD-1 antibody nivolumab with or without anti-CTLA-1 antibody ipilimumab in patients with relapsed SCLC reported a response rate of 10% with nivolumab 3 mg/kg and 21% with nivolumab 1 mg/kg + ipilimumab 3 mg/kg. The 2-year OS was 26% with the combination and 14% with single agent nivolumab [56,57]. Only 18% of patients had PD-L1 expression of ≥ 1% and the response rate did not correlate with PD-L1 status. The rate of grade 3 or 4 adverse events was approximately 20% and only 10% of patients discontinued treatment because of toxicity. Based on these data, nivolumab plus ipilimumab is now included in the NCCN guidelines as one of the options for patients with SCLC who experience disease relapse within 6 months of receiving platinum-based therapy; however, it is questionable whether routine use of this combination is justified based on currently available data. However the evidence for the combination of nivolumab and ipilimumab remains limited. This efficacy and toxicity data of both randomized and nonrandomized cohorts were presented together making it hard to interpret the results.
Another phase Ib study (KEYNOTE-028) utilizing anti-PD-1 antibody pembrolizumab 10 mg/kg IV every 2 weeks in patients with relapsed SCLC after receiving one or more prior lines of therapy and PD-L1 expression of ≥ 1% showed a response rate of 33% with median duration of response of 19 months and 1-year OS of 38% [58]. Although only 28% of screened patients had PD-L1 expression of ≥ 1% , these results indicated that at least a subset of SCLC patients are able to achieve durable responses with immune checkpoint inhibition. A number of clinical trials utilizing immune checkpoint inhibitors in various combinations and settings are currently underway.
Role of Prophylactic Cranial Irradiation
The role of PCI in extensive-stage SCLC is not clearly defined. A randomized phase III trial conducted by EORTC comparing PCI with no PCI in patients with extensive-stage SCLC who had attained partial or complete response to initial platinum-based chemotherapy showed decrease in the incidence of symptomatic brain metastasis and improvement in 1-year OS with PCI. However, this trial did not require mandatory brain imaging prior to PCI and therefore it is unclear if some patients in the PCI group had asymptomatic brain metastasis prior to enrollment and therefore received therapeutic benefit from brain radiation. Additionally, the dose and fractionation of PCI was not standardized across patient groups. A more recent phase III study conducted in Japan that compared PCI (25Gy in 10 fractions) with no PCI reported no difference in survival between the two groups. As opposed to EORTC study, the Japanese study did require baseline brain imaging to confirm absence of brain metastasis prior to enrollment. In addition, the patients in the control arm underwent periodic brain MRI to allow early detection of brain metastasis [59]. Given the emergence of the new data, the impact of PCI on survival in patients with extensive-stage SCLC is unproven and PCI likely has a role in a highly select small group of patients with extensive-stage SCLC. PCI is not recommended for patients with poor performance status (ECOG PS 3–4) or underlying neurocognitive disorders [33,60]. NMDA receptor antagonist memantine can be used in patients undergoing PCI to delay the occurrence of cognitive dysfunction [61]. Memantine 20 mg daily delayed time to cognitive decline and reduced the rate of decline in memory, executive function, and processing speed compared to placebo in patients receiving whole brain radiation [61].
Role of Radiation
A subset of patients with extensive-stage SCLC may benefit from consolidative thoracic radiation after completion of platinum-based chemotherapy. A randomized trial including patients who achieved complete or near complete response after 3 cycles of cisplatin plus etoposide compared thoracic radiation in combination with continued chemotherapy versus chemotherapy alone [62]. The median OS was longer with the addition of thoracic radiation compared to chemotherapy alone. Another phase III trial did not show improvement in 1-year OS with consolidative thoracic radiation, but 2-year OS and 6-month PFS were longer [63]. In general, consolidative thoracic radiation benefits patients who have residual thoracic disease and low-bulk extrathoracic disease that has responded to systemic therapy [64]. In addition, patients who initially presented with bulky symptomatic thoracic disease should also be considered for consolidative radiation.
Similar to other solid tumors, radiation should be utilized for palliative purposes in patients with painful bone metastasis, spine cord compression, or brain metastasis. Surgery is generally not recommended for spinal cord compression given the short life expectancy with extensive stage disease. Whole brain radiotherapy is preferred over SRS because of frequent presence of micrometastasis even in the setting of one or two radiographically evident brain metastasis.
Novel Therapies
A very complex genetic landscape of SCLC accounts for its resistance to conventional therapy and a high recurrence rate; however, at the same time this complexity can form the basis for effective targeted therapy for the disease. One of the major limitations to the development of targeted therapies in SCLC is limited availability of tissue owing to small tissue samples and frequent presence of significant necrosis in the samples. In recent years, several different therapeutic strategies and targeted agents have been under investigation for their potential role in SCLC. Several of them, including EGFR TKIs, BCR-ABL TKIs, mTOR inhibitors, and VEGF inhibitors, have been unsuccessful in showing a survival advantage in this disease. Several others including PARP inhibitors, cellular developmental pathway inhibitors and antibody drug conjugates are being tested. A phase I study of veliparib combined with cisplatin and etoposide in patients with previously untreated extensive-stage SCLC demonstrated complete response in 14.3%, partial response in 57.1%, and stable disease in 28.6% of patients with acceptable safety profile [65]. So far, none of these agents are approved for use in SCLC and the majority are in early phase clinical trials [66].
One of the emerging targets in the treatment of SCLC is DLL3. DLL3 is expressed on > 80% SCLCL tumor cells and cancer stem cells. Rovalpituzumab tesirine (ROVA-T) is an antibody drug conjugate consisting of humanized anti-DLL3 monoclonal antibody linked to SC-DR002, a DNA-crosslinking agent. A phase I trial of ROVA-T in patients with relapsed SCLC after 1 or 2 prior lines of therapies reported a response rate of 31% in patients with DLL3 expression of ≥ 50%. The median duration of response and mPFS were 4.6 months [67]. ROVA-T is currently in later phases of clinical trials and has a potential to serve as one of the options for patients with extensive-stage disease after disease progression on platinum-based therapy.
Response Assessment/Surveillance
For patients undergoing treatment for limited-stage SCLC, response assessment with contrast-enhanced CT of the chest/abdomen should be performed after completion of 4 cycles of chemotherapy and thoracic radiation. The surveillance guidelines consist of history, physical exam, and imaging every 3 months during 1st 2 years, every 6 months during the 3rdyear, and annually thereafter. If PCI is not performed, brain MRI or contrast enhanced CT scan should be performed every 3 to 4 months during the first 2 years of follow-up. For extensive-stage disease, response assessment should be performed after every 2 cycles of therapy. After completion of therapy, history, physical exam, and imaging should be done every 2 months during the 1st year, every 3 to 4 months during year 2 and 3, every 6 months during years 4 and 5, and annually thereafter. Routine use of PET scan for surveillance is not recommended. Any new pulmonary nodule should prompt evaluation for a second primary lung malignancy. Finally, smoking cessation counseling is an integral part of management of any patient with SCLC and should be included with every clinic visit.
Corresponding author: Hirva Mamdani, MD, Karmanos Cancer Institute, 4100 John R, Detroit, MI 48201, [email protected].
Financial disclosures: None.
1. American Cancer Society. Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Published 2017. Accessed April 11, 2018.
2. Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539–44.
3. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute website. https://seer.cancer.gov/csr/1975_2014/. Updated April 2, 2018. Accessed April 11, 2018.
4. Varghese AM, Zakowski MF, Yu HA, et al. Small-cell lung cancers in patients who never smoked cigarettes. J Thorac Oncol 2014;9:892–6.
5. Pleasance ED, Stephens PJ, O’Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010;463:184–90.
6. Green RA, Humphrey E, Close H, Patno ME. Alkylating agents in bronchogenic carcinoma. Am J Med 1969;46:516–25.
7. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409.
8. Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920–31.
9. Evans WK, Shepherd FA, Feld R, et al. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol 1985;3:1471–7.
10. Pujol JL, Carestia L, Daurés JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer 2000;83:8–15.
11. Mascaux C, Paesmans M, Berghmans T, et al; European Lung Cancer Working Party (ELCWP). A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 2000;30:23–36.
12. Sundstrøm S, Bremnes RM, Kaasa S, et al; Norwegian Lung Cancer Study Group. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol 2002;20:4665–72.
13. Hatfield LA, Huskamp HA, Lamont EB. Survival and toxicity after cisplatin plus etoposide versus carboplatin plus etoposide for extensive-stage small-cell lung cancer in elderly patients. J Oncol Pract 2016;12(7):666–73.
14. Okamoto H, Watanabe K, Kunikane H, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer 2007;97:162–9.
15. Skarlos DV, Samantas E, Kosmidis P, et al. Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small-cell lung cancer. A Hellenic Co-operative Oncology Group study. Ann Oncol 1994;5:601–7.
16. Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data J Clin Oncol 2012;30:1692–8.
17. Bishop JF, Raghavan D, Stuart-Harris R, et al. Carboplatin (CBDCA, JM-8) and VP-16-213 in previously untreated patients with small-cell lung cancer. J Clin Oncol 1987;5:1574–8.
18. Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002;20:3054–60.
19. Bunn PA Jr, Crowley J, Kelly K, et al. Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: a prospective phase III randomized study of the Southwest Oncology Group. J Clin Oncol 1995;13:1632–41.
20. Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618–24.
21. Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10(6):890–5.
22. Murray N, Coy P, Pater JL, Hodson I, Arnold A, Zee BC, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1993;11:336–44.
23. De Ruysscher D, Lueza B, Le Péchoux C, et al. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol 2016;27(10):1818–28.
24. Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340(4):265–71.
25. Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18(8):1116–25.
26. Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 1995;87(3):183–90.
27. Aupérin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476–84.
28. Le Péchoux C, Dunant A, Senan S, et al; Prophylactic Cranial Irradiation (PCI) Collaborative Group. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009;10(5):467–74.
29. Schneider BJ, Saxena A, Downey RJ. Surgery for early-stage small cell lung cancer. J Natl Compr Canc Netw 2011;9(10):1132–9.
30. Inoue M, Nakagawa K, Fujiwara K, et al. Results of preoperative mediastinoscopy for small cell lung cancer. Ann Thorac Surg 2000;70:1620–3.
31. Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol 2008;3:1267–71.
32. Inoue M, Miyoshi S, Yasumitsu T, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg 2000;70:1615–9.
33. Yang CF, Chan DY, Speicher PJ, Gulack BC, Wang X, Hartwig MG, et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol 2016;34:1057–64.
34. Shepherd FA, Evans WK, Feld R, et al. Adjuvant chemotherapy following surgical resection for small-cell carcinoma of the lung. J Clin Oncol 1988;6:832–8.
35. Noda K, Nishiwaki Y, Kawahara M, et al; Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85–91.
36. Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530–5.
37. Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. J Clin Oncol 1999;17:1794–801.
38. Zhou H, Zeng C, Wei Y, Zhou J, Yao W. Duration of chemotherapy for small cell lung cancer: a meta-analysis. PloS One 2013;8:e73805.
39. Loehrer PJ Sr, Ansari R, Gonin R, et al. Cisplatin plus etoposide with and without ifosfamide in extensive small-cell lung cancer: a Hoosier Oncology Group study. J Clin Oncol 1995 Oct;13:2594–9.
40. Pujol JL, Daurés JP, Riviére A, et al. Etoposide plus cisplatin with or without the combination of 4’-epidoxorubicin plus cyclophosphamide in treatment of extensive small-cell lung cancer: a French Federation of Cancer Institutes multicenter phase III randomized study. J Natl Cancer Inst 2001;93:300–8.
41. Berghmans T, Scherpereel A, Meert AP, et al; European Lung Cancer Working Party (ELCWP). A phase III randomized study comparing a chemotherapy with cisplatin and etoposide to a etoposide regimen without cisplatin for patients with extensive small-cell lung cancer. Front Oncol 2017;7:217.
42. Jalal SI, Lavin P, Lo G, et al. Carboplatin and etoposide with or without palifosfamide in untreated extensive-stage small-cell lung cancer: a Multicenter, Adaptive, Randomized Phase III Study (MATISSE). J Clin Oncol 2017;35:2619–23.
43. Fukuoka M, Furuse K, Saijo N, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst 1991;83:855–61.
44. Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 1992;10(2):282–91.
45. Miles DW, Earl HM, Souhami RL, Harper PG, Rudd R, Ash CM, et al. Intensive weekly chemotherapy for good-prognosis patients with small-cell lung cancer. J Clin Oncol 1991;9:280–5.
46. Petrioli R, Roviello G, Laera L, et al. Cisplatin, etoposide, and bevacizumab regimen followed by oral etoposide and bevacizumab maintenance treatment in patients with extensive-stage small cell lung cancer: a single-institution experience. Clin Lung Cancer 2015;16:e229–34.
47. Spigel DR, Greco FA, Zubkus JD, et al. Phase II trial of irinotecan, carboplatin, and bevacizumab in the treatment of patients with extensive-stage small-cell lung cancer. J Thorac Oncol 2009;4(12):1555–60.
48. Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011;29(16):2215–22.
49. Horn L, Dahlberg SE, Sandler AB, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol 2009;27(35):6006–11.
50. Tiseo M, Boni L, Ambrosio F, et al. Italian, multicenter, phase III, randomized study of cisplatin plus etoposide with or without bevacizumab as first-line treatment in extensive-disease small-cell lung cancer: the GOIRC-AIFA FARM6PMFJM trial. J Clin Oncol 2017;35:1281–7.
51. Pujol JL, Lavole A, Quoix E, et al. Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trialdagger. Ann Oncol 2015;26:908–14.
52. Gadgeel SM, Ventimiglia J, Kalemkerian GP, et al. Phase II study of maintenance pembrolizumab (pembro) in extensive stage small cell lung cancer (ES-SCLC) patients (pts). J Clin Oncol 2017;35(15_suppl):8504-. [Can’t find the rest of the reference]
53. Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol 2016;34:3740–8.
54. Owonikoko TK, Behera M, Chen Z, et al. A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol 2012;7:866–72.
55. Postmus PE, Berendsen HH, van Zandwijk N, et al. Retreatment with the induction regimen in small cell lung cancer relapsing after an initial response to short term chemotherapy. Eur J Cancer Clin Oncol 1987;23:1409–11.
56. Hellmann MD, Ott PA, Zugazagoitia J, Ready NE, Hann CL, Braud FGD, et al. Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): First report of a randomized expansion cohort from CheckMate 032. J Clin Oncol 2017;35(15_suppl):8503-. [Can’t find the rest of the reference]
57. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95.
58. Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the Phase Ib KEYNOTE-028 study. J Clin Oncol 2017;35:3823–9.
59. Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:663–71.
60. Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol 2009;27(1):78–84.
61. Brown PD, Pugh S, Laack NN, et al; Radiation Therapy Oncology Group (RTOG). Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429–37.
62. Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol 1999;17(7):2092–9.
63. Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36–42.
64. Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, et al. Radiotherapy for extensive stage small-cell lung cancer - authors’ reply. Lancet 2015;385:1292–3.
65. Owonikoko TK, Dahlberg SE, Khan SA, et al. A phase 1 safety study of veliparib combined with cisplatin and etoposide in extensive stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E2511). Lung Cancer. 2015 ;89:66–70.
66. Mamdani H, Induru R, Jalal SI. Novel therapies in small cell lung cancer. Translational lung cancer research. 2015;4:533–44.
67. Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017;18:42–51.
From the Karmanos Cancer Institute, Detroit, MI (Dr. Mamdani) and the Indiana University School of Medicine, Indianapolis, IN (Dr. Jalal).
Abstract
- Objective: To review the clinical aspects and current practices of management of small cell lung cancer (SCLC).
- Methods: Review of the literature.
- Results: SCLC is an aggressive cancer of neuroendocrine origin with a very strong association with smoking. Approximately 25% of patients present with limited-stage disease while the remaining majority of patients have extensive-stage disease, defined as disease extending beyond one hemithorax at the time of diagnosis. SCLC is often associated with endocrine or neurologic paraneoplastic syndromes. The treatment of limited-stage disease consists of platinum-based chemotherapy administered concurrently with radiation. Patients with partial or complete response should be offered prophylactic cranial radiation (PCI). Extensive-stage disease is largely treated with platinum-based chemotherapy and the role of PCI is more controversial. The efficacy of second-line chemotherapy after disease progression on platinum based chemotherapy is limited.
- Conclusion: Despite a number of advances in the treatment of various malignancies over the period of past several years, the prognosis of patients with SCLC remains poor. There have been a number of clinical trials utilizing novel therapeutic agents to improve outcomes of these patients; however, few of them have shown marginal success in a very select subgroup of patients.
Key words: lung cancer; small-cell lung cancer.
Small-cell lung cancer (SCLC) is an aggressive cancer of neuroendocrine origin, accounting for approximately 15% of all lung cancer cases, with approximately 33,000 patients diagnosed annually [1]. The incidence of SCLC in the United States has steadily declined over the past 30 years presumably because of decrease in the percentage of smokers and change to low-tar filter cigarettes [2]. Although the incidence of SCLC has been decreasing, the incidence in women is increasing and the male-to-female incidence ratio is now 1:1 [3]. Nearly all cases of SCLC are associated with heavy tobacco exposure, making it a heterogeneous disease with complex genomic landscape consisting of thousands of mutations [4,5]. Despite a number of advances in the treatment of non-small cell lung cancer over the past decade, the therapeutic landscape of SCLC remains narrow with median overall survival (OS) of 9 months in patients with advanced disease.
Case Study
Initial Presentation
A 61-year-old man presents to the emergency department with progressive shortness of breath and cough over the period of past 6 weeks. He also reports having had 20-lb weight loss over the same period of time. He is a current smoker and has been smoking one pack of cigarettes per day since the age of 18 years. A chest x-ray performed in the emergency department shows a right hilar mass. Computed tomography (CT) scan confirms the presence of a 4.5 cm right hilar mass with presence of enlarged mediastinal lymph nodes bilaterally.
What are the next steps in diagnosis?
SCLC is characterized by rapid growth and early hematogenous metastases. Consequently, only 25% of patients have limited-stage disease at the time of diagnosis. According to the VA staging system, limited-stage disease is defined as tumor that is confined to one hemithorax and can be encompassed within one radiation field. This typically includes mediastinal lymph nodes and ipsilateral supraclavicular lymph nodes. Extensive-stage disease is the presentation in 75% of the patients where the disease extends beyond one hemithorax. Extensive-stage disease includes presence of malignant pleural effusion and/or distant metastasis [6]. The Veterans Administration Lung Study Group (VALG) classification and staging system is more commonly used compared to the AJCC TNM staging system since it is less complex, directs treatment decisions, and correlates closely with prognosis. Given its propensity to metastasize quickly, none of the currently available screening methods have proven to be successful in early detection of SCLC. Eighty-six percent of the 125 patients that were diagnosed with SCLC while undergoing annual low-dose chest CT scans on National Lung Cancer Screening Trial had advanced disease at diagnosis [7,8]. These results highlight the fact that he majority of the SCLC develop in the interval between annual screening imaging.
SCLC frequently presents with a large hilar mass that is symptomatic. In addition, SCLC usually presents with centrally located tumors and bulky mediastinal adenopathy. Common symptoms include shortness of breath and cough. SCLC is commonly located submucosally in the bronchus and therefore hemoptysis is not a very common symptom at the time of presentation. Patients may present with superior vena cava (SVC) syndrome from local compression by the tumor. Not infrequently, SCLC is associated with paraneoplastic syndromes (PNS) owing to the ectopic secretion of hormones or antibodies by the tumor cells. The PNS can be broadly categorized into endocrine and neurologic; and are summarized in Table 1.
The common sites of metastases include brain, liver, and bone. Therefore, the staging workup should include fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT scan. Contrast-enhanced CT scan of chest and abdomen and bone scan can be obtained for staging in lieu of PET scan. Due to the physiologic FDG uptake, cerebral metastases cannot be assessed with sufficient certainty using the PET-CT. Therefore, brain imaging with contrast enhanced CT or MRI is also necessary. Although the incidence of metastasis to bone marrow is less than 10%, bone marrow aspiration and biopsy is warranted in case of unexplained cytopenias, especially when associated with teardrop red cells or nucleated red cells on peripheral blood smear indicative of marrow infiltrative process. The tissue diagnosis is established by obtaining a biopsy of the primary tumor or one of the metastatic sites. In case of localized disease, bronchoscopy (if necessary, with endobronchial ultrasound) with biopsy of centrally located tumor and/or lymph node is required. Histologically, SCLC consists of monomorphic cells, a high nucleus:cytoplasmic ratio, and confluent necrosis. The tumor cells are positive for chromogranin, synaptophysin, and CD56 by immunohistochemistry. Very frequently the cells are also positive for TTF1. Although serum tumor markers, including neuron-specific enolase (NSE) and progastrin-releasing peptide (prGRP), are frequently elevated in patients with SCLC, they are of limited value in clinical practice owing to their lack of sensitivity and specificity.
Case Continued
The patient underwent FDG-PET scan that showed the presence of hypermetabolic right hilar mass in addition to enlarged and hypermetabolic bilateral mediastinal lymph nodes. There were no other areas of FDG avidity. His brain MRI did not show any evidence of brain metastasis. Thus, he was confirmed to have limited-stage SCLC.
What is the standard of care for limited-stage SCLC?
SCLC is exquisitely sensitive to both chemotherapy and radiation, especially at the time of initial presentation. The standard of care for the treatment of limited stage SCLC is 4 cycles of platinum-based chemotherapy in combination with thoracic radiation started within the first 2 cycles of chemotherapy (Figure 1).
Choice of Chemotherapy
Etoposide and cisplatin is the most commonly used initial combination chemotherapy regimen [9]. This combination has largely replaced anthracycline-based regimens given its favorable efficacy and toxicity profile [10–12]. Several small randomized trials have shown comparable efficacy of carboplatin and etoposide in extensive stage SCLC [13–15]. A meta-analysis of 4 randomized trials, including 663 patients with SCLC, comparing cisplatin-based versus carboplatin-based regimens where 32% of patients had limited stage disease and 68% had extensive stage disease showed no statistically significant difference in the response rate, progression free survival (PFS), or OS between the two regimens [16]. Therefore, in clinical practice carboplatin is frequently used instead of cisplatin in patients with extensive-stage disease. In patients with limited-stage disease, cisplatin is still the drug of choice. However, the toxicity profile of the two regimens is different. Cisplatin based regimens are more commonly associated with neuropathy, nephrotoxicity, and chemotherapy induced nausea/vomiting [13], while carboplatin-based regimens are more myelosuppressive [17]. In addition, the combination of thoracic radiation with either of these regiments is associated with higher risk of esophagitis, pneumonitis, and myelosuppression [18]. The use of myeloid growth factors is not recommended in patients undergoing concurrent chemoradiation [19]. Of note, intravenous (IV) etoposide is always preferred over oral etoposide, especially in curative setting given unreliable absorption and bioavailability of oral formulations.
Thoracic Radiation
The addition of thoracic radiation to platinum-etoposide chemotherapy improves local control and OS. Two meta-analyses of 13 trials including more than 2000 patients have shown 25% to 30% decrease in local failure and 5% to 7% increase in 2-year OS with chemoradiation compared to chemotherapy alone in limited stage SCLC [20,21]. Early (with the first 2 cycles) concurrent thoracic radiation is superior to delayed and/or sequential radiation in terms of local control and OS [18,22,23]. The dose and fractionation of thoracic radiation in limited-stage SCLC has remained a controversial issue. The ECOG/RTOG randomized trial compared 45 Gy radiation delivered twice daily over a period of 3 weeks with once a day over 5 weeks, concurrently with chemotherapy. The twice a day regimen led to 10% improvement in 5-year OS (26% vs 16%), but higher incidence of grade 3 and 4 adverse events [24]. Despite the survival advantage demonstrated by hyperfractionated radiotherapy, the results need to be interpreted with caution because the radiation doses are not biologically equivalent. In addition the difficult logistics of patients receiving radiation twice a day has limited the routine implementation of this strategy. Subsequently, another randomized phase III trial (CONVERT) compared 45 Gy twice daily with 66 Gy once daily radiation in this setting. This trial did not show any difference in OS. The patients in twice daily arm had higher incidence of grade 4 neutropenia [25]. Considering the results of these trials, both strategies—45 Gy fractionated twice daily or 60 Gy fractionated once daily, delivered concurrently with chemotherapy—are acceptable in the setting of limited-stage SCLC. However, quite often hyperfractionated regimen is not feasible for the patients and many radiation oncology centers. Hopefully the CALBG 30610 study, which is ongoing, will clarify the optimal radiation schedule for limited-stage disease.
Prophylactic Cranial Irradiation
Approximately 75% of patients with limited-stage disease experience disease recurrence and brain is the site of recurrence in approximately half of these patients. Prophylactic cranial irradiation (PCI) consisting of 25 Gy radiation delivered in 10 fractions has been shown to be effective in decreasing the incidence of cerebral metastases [26–28]. Although individual small studies have not shown survival benefit of PCI because of small sample size and limited power, a meta-analysis of these studies has shown 25% decrease in the 3-year incidence of brain metastasis and 5.4% increase in 3-year OS [27]. The majority of patients included in these studies had limited-stage disease. Therefore, PCI is the standard of care for patients with limited-stage disease who attain a partial or complete response to chemoradiation.
Role of Surgery
Surgical resection may be an acceptable choice in a very limited subset of patients with peripherally located small (< 5 cm) tumors where mediastinal lymph nodes have been confirmed to be uninvolved with complete mediastinal staging [29,30]. Most of the data in this setting are derived from retrospective studies [31,32]. A 5-year OS of 40% to 60% has been has been reported with this strategy in patients with clinical stage I disease. In general, when surgery is considered, lobectomy with mediastinal lymph node dissection followed by chemotherapy (if no nodal involvement) or chemoradiation (if nodal involvement) is recommended [33,34]. Wedge or segmental resections are not considered to be optimum surgical options.
Case Continued
The patient received 4 cycles of cisplatin and etoposide along with 70 Gy radiation concurrently with the first 2 cycles of chemotherapy. His post-treatment CT scans showed partial response (PR). The patient underwent PCI 6 weeks after completion of treatment. Eighteen months later, the patient comes to the clinic for routine follow-up. He is doing generally well except for mildly decreased appetite and unintentional loss of 5 lb weight. His CT scans demonstrate multiple hypodense liver lesions ranging from 7 mm to 2 cm in size and a 2 cm left adrenal gland lesion highly concerning for metastasis. FDG PET scan confirmed the adrenal and liver lesions to be hypermetabolic. In addition, the PET showed multiple FDG avid bone lesions throughout the spine. Brain MRI was negative for any brain metastasis.
What is the standard of care for extensive-stage SCLC?
For extensive-stage SCLC, chemotherapy is the mainstay of treatment, with the goals of treatment being prolongation of survival, prevention or alleviation of cancer-related symptoms, and improvement in quality of life. The combination of etoposide with a platinum agent (carboplatin or cisplatin) is the preferred first-line treatment option (Figure 2).
Multiple attempts at improving first-line chemotherapy in extensive-stage disease have failed to show any meaningful difference in OS. For example, addition of ifosfamide, palifosfamide, cyclophosphamide, taxane, or anthracycline to platinum doublet failed to show improvement in OS and led to more toxicity [39–42]. Additionally, the use of alternating or cyclic chemotherapies in an attempt to curb drug resistance has also failed to show survival benefit [43–45]. The addition of antiangiogenic agent bevacizumab to standard platinum-based doublet has not yielded prolongation of OS in SCLC and led to unacceptably higher rate of tracheoesophageal fistula when used in conjunction with chemoradiation in limited-stage disease [46–51]. Finally, the immune checkpoint inhibitor ipilimumab in combination with platinum plus etoposide failed to improve PFS or OS compared to platinum plus etoposide alone in a recent phase III trial and maintenance pembrolizumab after completion of platinum-based chemotherapy did not improve PFS [52,53].
Patients with extensive-stage disease who have brain metastasis at the time of diagnosis can be treated with systemic chemotherapy first if brain metastases are asymptomatic and there is significant extracranial disease burden. In that case, whole brain radiotherapy should be given after completion of systemic therapy.
Second-Line Therapy
Despite being exquisitely chemo-sensitive, SCLC is associated with very poor prognosis largely because of invariable disease progression following first-line therapy and lack of effective second-line treatment options that can lead to appreciable disease control. The choice of second-line treatment is predominantly determined by the time of disease relapse since first-line platinum based therapy. If this interval is 6 months or longer, re-treatment utilizing the same platinum doublet is appropriate. However, if the interval is 6 months or less, second-line systemic therapy options should be explored. Unfortunately, the response rate tends to be less than 10% with most of the second-line therapies in platinum-resistant disease (defined as disease progression within 3 months of receiving platinum-based therapy). If the disease progression occurs between 3 to 6 months since platinum-based therapy, the response rate with second-line chemotherapy is in the range of 25% [54,55]. A number of second-line chemotherapy options have been explored in small studies, including topotecan, irinotecan, paclitaxel, docetaxel, temozolomide, vinorelbine, oral etoposide, gemcitabine, bendamustine, and CAV (cyclophosphamide, adriamycin, vincristine) (Table 2).
Immunotherapy
The role of immune checkpoint inhibitors in the treatment of SCLC is evolving and currently there are no FDA-approved immunotherapy agents in SCLC. A recently conducted phase I/II trial (CheckMate 032) of anti-PD-1 antibody nivolumab with or without anti-CTLA-1 antibody ipilimumab in patients with relapsed SCLC reported a response rate of 10% with nivolumab 3 mg/kg and 21% with nivolumab 1 mg/kg + ipilimumab 3 mg/kg. The 2-year OS was 26% with the combination and 14% with single agent nivolumab [56,57]. Only 18% of patients had PD-L1 expression of ≥ 1% and the response rate did not correlate with PD-L1 status. The rate of grade 3 or 4 adverse events was approximately 20% and only 10% of patients discontinued treatment because of toxicity. Based on these data, nivolumab plus ipilimumab is now included in the NCCN guidelines as one of the options for patients with SCLC who experience disease relapse within 6 months of receiving platinum-based therapy; however, it is questionable whether routine use of this combination is justified based on currently available data. However the evidence for the combination of nivolumab and ipilimumab remains limited. This efficacy and toxicity data of both randomized and nonrandomized cohorts were presented together making it hard to interpret the results.
Another phase Ib study (KEYNOTE-028) utilizing anti-PD-1 antibody pembrolizumab 10 mg/kg IV every 2 weeks in patients with relapsed SCLC after receiving one or more prior lines of therapy and PD-L1 expression of ≥ 1% showed a response rate of 33% with median duration of response of 19 months and 1-year OS of 38% [58]. Although only 28% of screened patients had PD-L1 expression of ≥ 1% , these results indicated that at least a subset of SCLC patients are able to achieve durable responses with immune checkpoint inhibition. A number of clinical trials utilizing immune checkpoint inhibitors in various combinations and settings are currently underway.
Role of Prophylactic Cranial Irradiation
The role of PCI in extensive-stage SCLC is not clearly defined. A randomized phase III trial conducted by EORTC comparing PCI with no PCI in patients with extensive-stage SCLC who had attained partial or complete response to initial platinum-based chemotherapy showed decrease in the incidence of symptomatic brain metastasis and improvement in 1-year OS with PCI. However, this trial did not require mandatory brain imaging prior to PCI and therefore it is unclear if some patients in the PCI group had asymptomatic brain metastasis prior to enrollment and therefore received therapeutic benefit from brain radiation. Additionally, the dose and fractionation of PCI was not standardized across patient groups. A more recent phase III study conducted in Japan that compared PCI (25Gy in 10 fractions) with no PCI reported no difference in survival between the two groups. As opposed to EORTC study, the Japanese study did require baseline brain imaging to confirm absence of brain metastasis prior to enrollment. In addition, the patients in the control arm underwent periodic brain MRI to allow early detection of brain metastasis [59]. Given the emergence of the new data, the impact of PCI on survival in patients with extensive-stage SCLC is unproven and PCI likely has a role in a highly select small group of patients with extensive-stage SCLC. PCI is not recommended for patients with poor performance status (ECOG PS 3–4) or underlying neurocognitive disorders [33,60]. NMDA receptor antagonist memantine can be used in patients undergoing PCI to delay the occurrence of cognitive dysfunction [61]. Memantine 20 mg daily delayed time to cognitive decline and reduced the rate of decline in memory, executive function, and processing speed compared to placebo in patients receiving whole brain radiation [61].
Role of Radiation
A subset of patients with extensive-stage SCLC may benefit from consolidative thoracic radiation after completion of platinum-based chemotherapy. A randomized trial including patients who achieved complete or near complete response after 3 cycles of cisplatin plus etoposide compared thoracic radiation in combination with continued chemotherapy versus chemotherapy alone [62]. The median OS was longer with the addition of thoracic radiation compared to chemotherapy alone. Another phase III trial did not show improvement in 1-year OS with consolidative thoracic radiation, but 2-year OS and 6-month PFS were longer [63]. In general, consolidative thoracic radiation benefits patients who have residual thoracic disease and low-bulk extrathoracic disease that has responded to systemic therapy [64]. In addition, patients who initially presented with bulky symptomatic thoracic disease should also be considered for consolidative radiation.
Similar to other solid tumors, radiation should be utilized for palliative purposes in patients with painful bone metastasis, spine cord compression, or brain metastasis. Surgery is generally not recommended for spinal cord compression given the short life expectancy with extensive stage disease. Whole brain radiotherapy is preferred over SRS because of frequent presence of micrometastasis even in the setting of one or two radiographically evident brain metastasis.
Novel Therapies
A very complex genetic landscape of SCLC accounts for its resistance to conventional therapy and a high recurrence rate; however, at the same time this complexity can form the basis for effective targeted therapy for the disease. One of the major limitations to the development of targeted therapies in SCLC is limited availability of tissue owing to small tissue samples and frequent presence of significant necrosis in the samples. In recent years, several different therapeutic strategies and targeted agents have been under investigation for their potential role in SCLC. Several of them, including EGFR TKIs, BCR-ABL TKIs, mTOR inhibitors, and VEGF inhibitors, have been unsuccessful in showing a survival advantage in this disease. Several others including PARP inhibitors, cellular developmental pathway inhibitors and antibody drug conjugates are being tested. A phase I study of veliparib combined with cisplatin and etoposide in patients with previously untreated extensive-stage SCLC demonstrated complete response in 14.3%, partial response in 57.1%, and stable disease in 28.6% of patients with acceptable safety profile [65]. So far, none of these agents are approved for use in SCLC and the majority are in early phase clinical trials [66].
One of the emerging targets in the treatment of SCLC is DLL3. DLL3 is expressed on > 80% SCLCL tumor cells and cancer stem cells. Rovalpituzumab tesirine (ROVA-T) is an antibody drug conjugate consisting of humanized anti-DLL3 monoclonal antibody linked to SC-DR002, a DNA-crosslinking agent. A phase I trial of ROVA-T in patients with relapsed SCLC after 1 or 2 prior lines of therapies reported a response rate of 31% in patients with DLL3 expression of ≥ 50%. The median duration of response and mPFS were 4.6 months [67]. ROVA-T is currently in later phases of clinical trials and has a potential to serve as one of the options for patients with extensive-stage disease after disease progression on platinum-based therapy.
Response Assessment/Surveillance
For patients undergoing treatment for limited-stage SCLC, response assessment with contrast-enhanced CT of the chest/abdomen should be performed after completion of 4 cycles of chemotherapy and thoracic radiation. The surveillance guidelines consist of history, physical exam, and imaging every 3 months during 1st 2 years, every 6 months during the 3rdyear, and annually thereafter. If PCI is not performed, brain MRI or contrast enhanced CT scan should be performed every 3 to 4 months during the first 2 years of follow-up. For extensive-stage disease, response assessment should be performed after every 2 cycles of therapy. After completion of therapy, history, physical exam, and imaging should be done every 2 months during the 1st year, every 3 to 4 months during year 2 and 3, every 6 months during years 4 and 5, and annually thereafter. Routine use of PET scan for surveillance is not recommended. Any new pulmonary nodule should prompt evaluation for a second primary lung malignancy. Finally, smoking cessation counseling is an integral part of management of any patient with SCLC and should be included with every clinic visit.
Corresponding author: Hirva Mamdani, MD, Karmanos Cancer Institute, 4100 John R, Detroit, MI 48201, [email protected].
Financial disclosures: None.
From the Karmanos Cancer Institute, Detroit, MI (Dr. Mamdani) and the Indiana University School of Medicine, Indianapolis, IN (Dr. Jalal).
Abstract
- Objective: To review the clinical aspects and current practices of management of small cell lung cancer (SCLC).
- Methods: Review of the literature.
- Results: SCLC is an aggressive cancer of neuroendocrine origin with a very strong association with smoking. Approximately 25% of patients present with limited-stage disease while the remaining majority of patients have extensive-stage disease, defined as disease extending beyond one hemithorax at the time of diagnosis. SCLC is often associated with endocrine or neurologic paraneoplastic syndromes. The treatment of limited-stage disease consists of platinum-based chemotherapy administered concurrently with radiation. Patients with partial or complete response should be offered prophylactic cranial radiation (PCI). Extensive-stage disease is largely treated with platinum-based chemotherapy and the role of PCI is more controversial. The efficacy of second-line chemotherapy after disease progression on platinum based chemotherapy is limited.
- Conclusion: Despite a number of advances in the treatment of various malignancies over the period of past several years, the prognosis of patients with SCLC remains poor. There have been a number of clinical trials utilizing novel therapeutic agents to improve outcomes of these patients; however, few of them have shown marginal success in a very select subgroup of patients.
Key words: lung cancer; small-cell lung cancer.
Small-cell lung cancer (SCLC) is an aggressive cancer of neuroendocrine origin, accounting for approximately 15% of all lung cancer cases, with approximately 33,000 patients diagnosed annually [1]. The incidence of SCLC in the United States has steadily declined over the past 30 years presumably because of decrease in the percentage of smokers and change to low-tar filter cigarettes [2]. Although the incidence of SCLC has been decreasing, the incidence in women is increasing and the male-to-female incidence ratio is now 1:1 [3]. Nearly all cases of SCLC are associated with heavy tobacco exposure, making it a heterogeneous disease with complex genomic landscape consisting of thousands of mutations [4,5]. Despite a number of advances in the treatment of non-small cell lung cancer over the past decade, the therapeutic landscape of SCLC remains narrow with median overall survival (OS) of 9 months in patients with advanced disease.
Case Study
Initial Presentation
A 61-year-old man presents to the emergency department with progressive shortness of breath and cough over the period of past 6 weeks. He also reports having had 20-lb weight loss over the same period of time. He is a current smoker and has been smoking one pack of cigarettes per day since the age of 18 years. A chest x-ray performed in the emergency department shows a right hilar mass. Computed tomography (CT) scan confirms the presence of a 4.5 cm right hilar mass with presence of enlarged mediastinal lymph nodes bilaterally.
What are the next steps in diagnosis?
SCLC is characterized by rapid growth and early hematogenous metastases. Consequently, only 25% of patients have limited-stage disease at the time of diagnosis. According to the VA staging system, limited-stage disease is defined as tumor that is confined to one hemithorax and can be encompassed within one radiation field. This typically includes mediastinal lymph nodes and ipsilateral supraclavicular lymph nodes. Extensive-stage disease is the presentation in 75% of the patients where the disease extends beyond one hemithorax. Extensive-stage disease includes presence of malignant pleural effusion and/or distant metastasis [6]. The Veterans Administration Lung Study Group (VALG) classification and staging system is more commonly used compared to the AJCC TNM staging system since it is less complex, directs treatment decisions, and correlates closely with prognosis. Given its propensity to metastasize quickly, none of the currently available screening methods have proven to be successful in early detection of SCLC. Eighty-six percent of the 125 patients that were diagnosed with SCLC while undergoing annual low-dose chest CT scans on National Lung Cancer Screening Trial had advanced disease at diagnosis [7,8]. These results highlight the fact that he majority of the SCLC develop in the interval between annual screening imaging.
SCLC frequently presents with a large hilar mass that is symptomatic. In addition, SCLC usually presents with centrally located tumors and bulky mediastinal adenopathy. Common symptoms include shortness of breath and cough. SCLC is commonly located submucosally in the bronchus and therefore hemoptysis is not a very common symptom at the time of presentation. Patients may present with superior vena cava (SVC) syndrome from local compression by the tumor. Not infrequently, SCLC is associated with paraneoplastic syndromes (PNS) owing to the ectopic secretion of hormones or antibodies by the tumor cells. The PNS can be broadly categorized into endocrine and neurologic; and are summarized in Table 1.
The common sites of metastases include brain, liver, and bone. Therefore, the staging workup should include fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT scan. Contrast-enhanced CT scan of chest and abdomen and bone scan can be obtained for staging in lieu of PET scan. Due to the physiologic FDG uptake, cerebral metastases cannot be assessed with sufficient certainty using the PET-CT. Therefore, brain imaging with contrast enhanced CT or MRI is also necessary. Although the incidence of metastasis to bone marrow is less than 10%, bone marrow aspiration and biopsy is warranted in case of unexplained cytopenias, especially when associated with teardrop red cells or nucleated red cells on peripheral blood smear indicative of marrow infiltrative process. The tissue diagnosis is established by obtaining a biopsy of the primary tumor or one of the metastatic sites. In case of localized disease, bronchoscopy (if necessary, with endobronchial ultrasound) with biopsy of centrally located tumor and/or lymph node is required. Histologically, SCLC consists of monomorphic cells, a high nucleus:cytoplasmic ratio, and confluent necrosis. The tumor cells are positive for chromogranin, synaptophysin, and CD56 by immunohistochemistry. Very frequently the cells are also positive for TTF1. Although serum tumor markers, including neuron-specific enolase (NSE) and progastrin-releasing peptide (prGRP), are frequently elevated in patients with SCLC, they are of limited value in clinical practice owing to their lack of sensitivity and specificity.
Case Continued
The patient underwent FDG-PET scan that showed the presence of hypermetabolic right hilar mass in addition to enlarged and hypermetabolic bilateral mediastinal lymph nodes. There were no other areas of FDG avidity. His brain MRI did not show any evidence of brain metastasis. Thus, he was confirmed to have limited-stage SCLC.
What is the standard of care for limited-stage SCLC?
SCLC is exquisitely sensitive to both chemotherapy and radiation, especially at the time of initial presentation. The standard of care for the treatment of limited stage SCLC is 4 cycles of platinum-based chemotherapy in combination with thoracic radiation started within the first 2 cycles of chemotherapy (Figure 1).
Choice of Chemotherapy
Etoposide and cisplatin is the most commonly used initial combination chemotherapy regimen [9]. This combination has largely replaced anthracycline-based regimens given its favorable efficacy and toxicity profile [10–12]. Several small randomized trials have shown comparable efficacy of carboplatin and etoposide in extensive stage SCLC [13–15]. A meta-analysis of 4 randomized trials, including 663 patients with SCLC, comparing cisplatin-based versus carboplatin-based regimens where 32% of patients had limited stage disease and 68% had extensive stage disease showed no statistically significant difference in the response rate, progression free survival (PFS), or OS between the two regimens [16]. Therefore, in clinical practice carboplatin is frequently used instead of cisplatin in patients with extensive-stage disease. In patients with limited-stage disease, cisplatin is still the drug of choice. However, the toxicity profile of the two regimens is different. Cisplatin based regimens are more commonly associated with neuropathy, nephrotoxicity, and chemotherapy induced nausea/vomiting [13], while carboplatin-based regimens are more myelosuppressive [17]. In addition, the combination of thoracic radiation with either of these regiments is associated with higher risk of esophagitis, pneumonitis, and myelosuppression [18]. The use of myeloid growth factors is not recommended in patients undergoing concurrent chemoradiation [19]. Of note, intravenous (IV) etoposide is always preferred over oral etoposide, especially in curative setting given unreliable absorption and bioavailability of oral formulations.
Thoracic Radiation
The addition of thoracic radiation to platinum-etoposide chemotherapy improves local control and OS. Two meta-analyses of 13 trials including more than 2000 patients have shown 25% to 30% decrease in local failure and 5% to 7% increase in 2-year OS with chemoradiation compared to chemotherapy alone in limited stage SCLC [20,21]. Early (with the first 2 cycles) concurrent thoracic radiation is superior to delayed and/or sequential radiation in terms of local control and OS [18,22,23]. The dose and fractionation of thoracic radiation in limited-stage SCLC has remained a controversial issue. The ECOG/RTOG randomized trial compared 45 Gy radiation delivered twice daily over a period of 3 weeks with once a day over 5 weeks, concurrently with chemotherapy. The twice a day regimen led to 10% improvement in 5-year OS (26% vs 16%), but higher incidence of grade 3 and 4 adverse events [24]. Despite the survival advantage demonstrated by hyperfractionated radiotherapy, the results need to be interpreted with caution because the radiation doses are not biologically equivalent. In addition the difficult logistics of patients receiving radiation twice a day has limited the routine implementation of this strategy. Subsequently, another randomized phase III trial (CONVERT) compared 45 Gy twice daily with 66 Gy once daily radiation in this setting. This trial did not show any difference in OS. The patients in twice daily arm had higher incidence of grade 4 neutropenia [25]. Considering the results of these trials, both strategies—45 Gy fractionated twice daily or 60 Gy fractionated once daily, delivered concurrently with chemotherapy—are acceptable in the setting of limited-stage SCLC. However, quite often hyperfractionated regimen is not feasible for the patients and many radiation oncology centers. Hopefully the CALBG 30610 study, which is ongoing, will clarify the optimal radiation schedule for limited-stage disease.
Prophylactic Cranial Irradiation
Approximately 75% of patients with limited-stage disease experience disease recurrence and brain is the site of recurrence in approximately half of these patients. Prophylactic cranial irradiation (PCI) consisting of 25 Gy radiation delivered in 10 fractions has been shown to be effective in decreasing the incidence of cerebral metastases [26–28]. Although individual small studies have not shown survival benefit of PCI because of small sample size and limited power, a meta-analysis of these studies has shown 25% decrease in the 3-year incidence of brain metastasis and 5.4% increase in 3-year OS [27]. The majority of patients included in these studies had limited-stage disease. Therefore, PCI is the standard of care for patients with limited-stage disease who attain a partial or complete response to chemoradiation.
Role of Surgery
Surgical resection may be an acceptable choice in a very limited subset of patients with peripherally located small (< 5 cm) tumors where mediastinal lymph nodes have been confirmed to be uninvolved with complete mediastinal staging [29,30]. Most of the data in this setting are derived from retrospective studies [31,32]. A 5-year OS of 40% to 60% has been has been reported with this strategy in patients with clinical stage I disease. In general, when surgery is considered, lobectomy with mediastinal lymph node dissection followed by chemotherapy (if no nodal involvement) or chemoradiation (if nodal involvement) is recommended [33,34]. Wedge or segmental resections are not considered to be optimum surgical options.
Case Continued
The patient received 4 cycles of cisplatin and etoposide along with 70 Gy radiation concurrently with the first 2 cycles of chemotherapy. His post-treatment CT scans showed partial response (PR). The patient underwent PCI 6 weeks after completion of treatment. Eighteen months later, the patient comes to the clinic for routine follow-up. He is doing generally well except for mildly decreased appetite and unintentional loss of 5 lb weight. His CT scans demonstrate multiple hypodense liver lesions ranging from 7 mm to 2 cm in size and a 2 cm left adrenal gland lesion highly concerning for metastasis. FDG PET scan confirmed the adrenal and liver lesions to be hypermetabolic. In addition, the PET showed multiple FDG avid bone lesions throughout the spine. Brain MRI was negative for any brain metastasis.
What is the standard of care for extensive-stage SCLC?
For extensive-stage SCLC, chemotherapy is the mainstay of treatment, with the goals of treatment being prolongation of survival, prevention or alleviation of cancer-related symptoms, and improvement in quality of life. The combination of etoposide with a platinum agent (carboplatin or cisplatin) is the preferred first-line treatment option (Figure 2).
Multiple attempts at improving first-line chemotherapy in extensive-stage disease have failed to show any meaningful difference in OS. For example, addition of ifosfamide, palifosfamide, cyclophosphamide, taxane, or anthracycline to platinum doublet failed to show improvement in OS and led to more toxicity [39–42]. Additionally, the use of alternating or cyclic chemotherapies in an attempt to curb drug resistance has also failed to show survival benefit [43–45]. The addition of antiangiogenic agent bevacizumab to standard platinum-based doublet has not yielded prolongation of OS in SCLC and led to unacceptably higher rate of tracheoesophageal fistula when used in conjunction with chemoradiation in limited-stage disease [46–51]. Finally, the immune checkpoint inhibitor ipilimumab in combination with platinum plus etoposide failed to improve PFS or OS compared to platinum plus etoposide alone in a recent phase III trial and maintenance pembrolizumab after completion of platinum-based chemotherapy did not improve PFS [52,53].
Patients with extensive-stage disease who have brain metastasis at the time of diagnosis can be treated with systemic chemotherapy first if brain metastases are asymptomatic and there is significant extracranial disease burden. In that case, whole brain radiotherapy should be given after completion of systemic therapy.
Second-Line Therapy
Despite being exquisitely chemo-sensitive, SCLC is associated with very poor prognosis largely because of invariable disease progression following first-line therapy and lack of effective second-line treatment options that can lead to appreciable disease control. The choice of second-line treatment is predominantly determined by the time of disease relapse since first-line platinum based therapy. If this interval is 6 months or longer, re-treatment utilizing the same platinum doublet is appropriate. However, if the interval is 6 months or less, second-line systemic therapy options should be explored. Unfortunately, the response rate tends to be less than 10% with most of the second-line therapies in platinum-resistant disease (defined as disease progression within 3 months of receiving platinum-based therapy). If the disease progression occurs between 3 to 6 months since platinum-based therapy, the response rate with second-line chemotherapy is in the range of 25% [54,55]. A number of second-line chemotherapy options have been explored in small studies, including topotecan, irinotecan, paclitaxel, docetaxel, temozolomide, vinorelbine, oral etoposide, gemcitabine, bendamustine, and CAV (cyclophosphamide, adriamycin, vincristine) (Table 2).
Immunotherapy
The role of immune checkpoint inhibitors in the treatment of SCLC is evolving and currently there are no FDA-approved immunotherapy agents in SCLC. A recently conducted phase I/II trial (CheckMate 032) of anti-PD-1 antibody nivolumab with or without anti-CTLA-1 antibody ipilimumab in patients with relapsed SCLC reported a response rate of 10% with nivolumab 3 mg/kg and 21% with nivolumab 1 mg/kg + ipilimumab 3 mg/kg. The 2-year OS was 26% with the combination and 14% with single agent nivolumab [56,57]. Only 18% of patients had PD-L1 expression of ≥ 1% and the response rate did not correlate with PD-L1 status. The rate of grade 3 or 4 adverse events was approximately 20% and only 10% of patients discontinued treatment because of toxicity. Based on these data, nivolumab plus ipilimumab is now included in the NCCN guidelines as one of the options for patients with SCLC who experience disease relapse within 6 months of receiving platinum-based therapy; however, it is questionable whether routine use of this combination is justified based on currently available data. However the evidence for the combination of nivolumab and ipilimumab remains limited. This efficacy and toxicity data of both randomized and nonrandomized cohorts were presented together making it hard to interpret the results.
Another phase Ib study (KEYNOTE-028) utilizing anti-PD-1 antibody pembrolizumab 10 mg/kg IV every 2 weeks in patients with relapsed SCLC after receiving one or more prior lines of therapy and PD-L1 expression of ≥ 1% showed a response rate of 33% with median duration of response of 19 months and 1-year OS of 38% [58]. Although only 28% of screened patients had PD-L1 expression of ≥ 1% , these results indicated that at least a subset of SCLC patients are able to achieve durable responses with immune checkpoint inhibition. A number of clinical trials utilizing immune checkpoint inhibitors in various combinations and settings are currently underway.
Role of Prophylactic Cranial Irradiation
The role of PCI in extensive-stage SCLC is not clearly defined. A randomized phase III trial conducted by EORTC comparing PCI with no PCI in patients with extensive-stage SCLC who had attained partial or complete response to initial platinum-based chemotherapy showed decrease in the incidence of symptomatic brain metastasis and improvement in 1-year OS with PCI. However, this trial did not require mandatory brain imaging prior to PCI and therefore it is unclear if some patients in the PCI group had asymptomatic brain metastasis prior to enrollment and therefore received therapeutic benefit from brain radiation. Additionally, the dose and fractionation of PCI was not standardized across patient groups. A more recent phase III study conducted in Japan that compared PCI (25Gy in 10 fractions) with no PCI reported no difference in survival between the two groups. As opposed to EORTC study, the Japanese study did require baseline brain imaging to confirm absence of brain metastasis prior to enrollment. In addition, the patients in the control arm underwent periodic brain MRI to allow early detection of brain metastasis [59]. Given the emergence of the new data, the impact of PCI on survival in patients with extensive-stage SCLC is unproven and PCI likely has a role in a highly select small group of patients with extensive-stage SCLC. PCI is not recommended for patients with poor performance status (ECOG PS 3–4) or underlying neurocognitive disorders [33,60]. NMDA receptor antagonist memantine can be used in patients undergoing PCI to delay the occurrence of cognitive dysfunction [61]. Memantine 20 mg daily delayed time to cognitive decline and reduced the rate of decline in memory, executive function, and processing speed compared to placebo in patients receiving whole brain radiation [61].
Role of Radiation
A subset of patients with extensive-stage SCLC may benefit from consolidative thoracic radiation after completion of platinum-based chemotherapy. A randomized trial including patients who achieved complete or near complete response after 3 cycles of cisplatin plus etoposide compared thoracic radiation in combination with continued chemotherapy versus chemotherapy alone [62]. The median OS was longer with the addition of thoracic radiation compared to chemotherapy alone. Another phase III trial did not show improvement in 1-year OS with consolidative thoracic radiation, but 2-year OS and 6-month PFS were longer [63]. In general, consolidative thoracic radiation benefits patients who have residual thoracic disease and low-bulk extrathoracic disease that has responded to systemic therapy [64]. In addition, patients who initially presented with bulky symptomatic thoracic disease should also be considered for consolidative radiation.
Similar to other solid tumors, radiation should be utilized for palliative purposes in patients with painful bone metastasis, spine cord compression, or brain metastasis. Surgery is generally not recommended for spinal cord compression given the short life expectancy with extensive stage disease. Whole brain radiotherapy is preferred over SRS because of frequent presence of micrometastasis even in the setting of one or two radiographically evident brain metastasis.
Novel Therapies
A very complex genetic landscape of SCLC accounts for its resistance to conventional therapy and a high recurrence rate; however, at the same time this complexity can form the basis for effective targeted therapy for the disease. One of the major limitations to the development of targeted therapies in SCLC is limited availability of tissue owing to small tissue samples and frequent presence of significant necrosis in the samples. In recent years, several different therapeutic strategies and targeted agents have been under investigation for their potential role in SCLC. Several of them, including EGFR TKIs, BCR-ABL TKIs, mTOR inhibitors, and VEGF inhibitors, have been unsuccessful in showing a survival advantage in this disease. Several others including PARP inhibitors, cellular developmental pathway inhibitors and antibody drug conjugates are being tested. A phase I study of veliparib combined with cisplatin and etoposide in patients with previously untreated extensive-stage SCLC demonstrated complete response in 14.3%, partial response in 57.1%, and stable disease in 28.6% of patients with acceptable safety profile [65]. So far, none of these agents are approved for use in SCLC and the majority are in early phase clinical trials [66].
One of the emerging targets in the treatment of SCLC is DLL3. DLL3 is expressed on > 80% SCLCL tumor cells and cancer stem cells. Rovalpituzumab tesirine (ROVA-T) is an antibody drug conjugate consisting of humanized anti-DLL3 monoclonal antibody linked to SC-DR002, a DNA-crosslinking agent. A phase I trial of ROVA-T in patients with relapsed SCLC after 1 or 2 prior lines of therapies reported a response rate of 31% in patients with DLL3 expression of ≥ 50%. The median duration of response and mPFS were 4.6 months [67]. ROVA-T is currently in later phases of clinical trials and has a potential to serve as one of the options for patients with extensive-stage disease after disease progression on platinum-based therapy.
Response Assessment/Surveillance
For patients undergoing treatment for limited-stage SCLC, response assessment with contrast-enhanced CT of the chest/abdomen should be performed after completion of 4 cycles of chemotherapy and thoracic radiation. The surveillance guidelines consist of history, physical exam, and imaging every 3 months during 1st 2 years, every 6 months during the 3rdyear, and annually thereafter. If PCI is not performed, brain MRI or contrast enhanced CT scan should be performed every 3 to 4 months during the first 2 years of follow-up. For extensive-stage disease, response assessment should be performed after every 2 cycles of therapy. After completion of therapy, history, physical exam, and imaging should be done every 2 months during the 1st year, every 3 to 4 months during year 2 and 3, every 6 months during years 4 and 5, and annually thereafter. Routine use of PET scan for surveillance is not recommended. Any new pulmonary nodule should prompt evaluation for a second primary lung malignancy. Finally, smoking cessation counseling is an integral part of management of any patient with SCLC and should be included with every clinic visit.
Corresponding author: Hirva Mamdani, MD, Karmanos Cancer Institute, 4100 John R, Detroit, MI 48201, [email protected].
Financial disclosures: None.
1. American Cancer Society. Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Published 2017. Accessed April 11, 2018.
2. Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539–44.
3. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute website. https://seer.cancer.gov/csr/1975_2014/. Updated April 2, 2018. Accessed April 11, 2018.
4. Varghese AM, Zakowski MF, Yu HA, et al. Small-cell lung cancers in patients who never smoked cigarettes. J Thorac Oncol 2014;9:892–6.
5. Pleasance ED, Stephens PJ, O’Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010;463:184–90.
6. Green RA, Humphrey E, Close H, Patno ME. Alkylating agents in bronchogenic carcinoma. Am J Med 1969;46:516–25.
7. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409.
8. Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920–31.
9. Evans WK, Shepherd FA, Feld R, et al. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol 1985;3:1471–7.
10. Pujol JL, Carestia L, Daurés JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer 2000;83:8–15.
11. Mascaux C, Paesmans M, Berghmans T, et al; European Lung Cancer Working Party (ELCWP). A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 2000;30:23–36.
12. Sundstrøm S, Bremnes RM, Kaasa S, et al; Norwegian Lung Cancer Study Group. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol 2002;20:4665–72.
13. Hatfield LA, Huskamp HA, Lamont EB. Survival and toxicity after cisplatin plus etoposide versus carboplatin plus etoposide for extensive-stage small-cell lung cancer in elderly patients. J Oncol Pract 2016;12(7):666–73.
14. Okamoto H, Watanabe K, Kunikane H, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer 2007;97:162–9.
15. Skarlos DV, Samantas E, Kosmidis P, et al. Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small-cell lung cancer. A Hellenic Co-operative Oncology Group study. Ann Oncol 1994;5:601–7.
16. Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data J Clin Oncol 2012;30:1692–8.
17. Bishop JF, Raghavan D, Stuart-Harris R, et al. Carboplatin (CBDCA, JM-8) and VP-16-213 in previously untreated patients with small-cell lung cancer. J Clin Oncol 1987;5:1574–8.
18. Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002;20:3054–60.
19. Bunn PA Jr, Crowley J, Kelly K, et al. Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: a prospective phase III randomized study of the Southwest Oncology Group. J Clin Oncol 1995;13:1632–41.
20. Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618–24.
21. Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10(6):890–5.
22. Murray N, Coy P, Pater JL, Hodson I, Arnold A, Zee BC, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1993;11:336–44.
23. De Ruysscher D, Lueza B, Le Péchoux C, et al. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol 2016;27(10):1818–28.
24. Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340(4):265–71.
25. Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18(8):1116–25.
26. Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 1995;87(3):183–90.
27. Aupérin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476–84.
28. Le Péchoux C, Dunant A, Senan S, et al; Prophylactic Cranial Irradiation (PCI) Collaborative Group. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009;10(5):467–74.
29. Schneider BJ, Saxena A, Downey RJ. Surgery for early-stage small cell lung cancer. J Natl Compr Canc Netw 2011;9(10):1132–9.
30. Inoue M, Nakagawa K, Fujiwara K, et al. Results of preoperative mediastinoscopy for small cell lung cancer. Ann Thorac Surg 2000;70:1620–3.
31. Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol 2008;3:1267–71.
32. Inoue M, Miyoshi S, Yasumitsu T, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg 2000;70:1615–9.
33. Yang CF, Chan DY, Speicher PJ, Gulack BC, Wang X, Hartwig MG, et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol 2016;34:1057–64.
34. Shepherd FA, Evans WK, Feld R, et al. Adjuvant chemotherapy following surgical resection for small-cell carcinoma of the lung. J Clin Oncol 1988;6:832–8.
35. Noda K, Nishiwaki Y, Kawahara M, et al; Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85–91.
36. Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530–5.
37. Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. J Clin Oncol 1999;17:1794–801.
38. Zhou H, Zeng C, Wei Y, Zhou J, Yao W. Duration of chemotherapy for small cell lung cancer: a meta-analysis. PloS One 2013;8:e73805.
39. Loehrer PJ Sr, Ansari R, Gonin R, et al. Cisplatin plus etoposide with and without ifosfamide in extensive small-cell lung cancer: a Hoosier Oncology Group study. J Clin Oncol 1995 Oct;13:2594–9.
40. Pujol JL, Daurés JP, Riviére A, et al. Etoposide plus cisplatin with or without the combination of 4’-epidoxorubicin plus cyclophosphamide in treatment of extensive small-cell lung cancer: a French Federation of Cancer Institutes multicenter phase III randomized study. J Natl Cancer Inst 2001;93:300–8.
41. Berghmans T, Scherpereel A, Meert AP, et al; European Lung Cancer Working Party (ELCWP). A phase III randomized study comparing a chemotherapy with cisplatin and etoposide to a etoposide regimen without cisplatin for patients with extensive small-cell lung cancer. Front Oncol 2017;7:217.
42. Jalal SI, Lavin P, Lo G, et al. Carboplatin and etoposide with or without palifosfamide in untreated extensive-stage small-cell lung cancer: a Multicenter, Adaptive, Randomized Phase III Study (MATISSE). J Clin Oncol 2017;35:2619–23.
43. Fukuoka M, Furuse K, Saijo N, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst 1991;83:855–61.
44. Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 1992;10(2):282–91.
45. Miles DW, Earl HM, Souhami RL, Harper PG, Rudd R, Ash CM, et al. Intensive weekly chemotherapy for good-prognosis patients with small-cell lung cancer. J Clin Oncol 1991;9:280–5.
46. Petrioli R, Roviello G, Laera L, et al. Cisplatin, etoposide, and bevacizumab regimen followed by oral etoposide and bevacizumab maintenance treatment in patients with extensive-stage small cell lung cancer: a single-institution experience. Clin Lung Cancer 2015;16:e229–34.
47. Spigel DR, Greco FA, Zubkus JD, et al. Phase II trial of irinotecan, carboplatin, and bevacizumab in the treatment of patients with extensive-stage small-cell lung cancer. J Thorac Oncol 2009;4(12):1555–60.
48. Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011;29(16):2215–22.
49. Horn L, Dahlberg SE, Sandler AB, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol 2009;27(35):6006–11.
50. Tiseo M, Boni L, Ambrosio F, et al. Italian, multicenter, phase III, randomized study of cisplatin plus etoposide with or without bevacizumab as first-line treatment in extensive-disease small-cell lung cancer: the GOIRC-AIFA FARM6PMFJM trial. J Clin Oncol 2017;35:1281–7.
51. Pujol JL, Lavole A, Quoix E, et al. Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trialdagger. Ann Oncol 2015;26:908–14.
52. Gadgeel SM, Ventimiglia J, Kalemkerian GP, et al. Phase II study of maintenance pembrolizumab (pembro) in extensive stage small cell lung cancer (ES-SCLC) patients (pts). J Clin Oncol 2017;35(15_suppl):8504-. [Can’t find the rest of the reference]
53. Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol 2016;34:3740–8.
54. Owonikoko TK, Behera M, Chen Z, et al. A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol 2012;7:866–72.
55. Postmus PE, Berendsen HH, van Zandwijk N, et al. Retreatment with the induction regimen in small cell lung cancer relapsing after an initial response to short term chemotherapy. Eur J Cancer Clin Oncol 1987;23:1409–11.
56. Hellmann MD, Ott PA, Zugazagoitia J, Ready NE, Hann CL, Braud FGD, et al. Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): First report of a randomized expansion cohort from CheckMate 032. J Clin Oncol 2017;35(15_suppl):8503-. [Can’t find the rest of the reference]
57. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95.
58. Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the Phase Ib KEYNOTE-028 study. J Clin Oncol 2017;35:3823–9.
59. Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:663–71.
60. Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol 2009;27(1):78–84.
61. Brown PD, Pugh S, Laack NN, et al; Radiation Therapy Oncology Group (RTOG). Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429–37.
62. Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol 1999;17(7):2092–9.
63. Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36–42.
64. Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, et al. Radiotherapy for extensive stage small-cell lung cancer - authors’ reply. Lancet 2015;385:1292–3.
65. Owonikoko TK, Dahlberg SE, Khan SA, et al. A phase 1 safety study of veliparib combined with cisplatin and etoposide in extensive stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E2511). Lung Cancer. 2015 ;89:66–70.
66. Mamdani H, Induru R, Jalal SI. Novel therapies in small cell lung cancer. Translational lung cancer research. 2015;4:533–44.
67. Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017;18:42–51.
1. American Cancer Society. Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Published 2017. Accessed April 11, 2018.
2. Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539–44.
3. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute website. https://seer.cancer.gov/csr/1975_2014/. Updated April 2, 2018. Accessed April 11, 2018.
4. Varghese AM, Zakowski MF, Yu HA, et al. Small-cell lung cancers in patients who never smoked cigarettes. J Thorac Oncol 2014;9:892–6.
5. Pleasance ED, Stephens PJ, O’Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010;463:184–90.
6. Green RA, Humphrey E, Close H, Patno ME. Alkylating agents in bronchogenic carcinoma. Am J Med 1969;46:516–25.
7. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409.
8. Aberle DR, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013;369:920–31.
9. Evans WK, Shepherd FA, Feld R, et al. VP-16 and cisplatin as first-line therapy for small-cell lung cancer. J Clin Oncol 1985;3:1471–7.
10. Pujol JL, Carestia L, Daurés JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer 2000;83:8–15.
11. Mascaux C, Paesmans M, Berghmans T, et al; European Lung Cancer Working Party (ELCWP). A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 2000;30:23–36.
12. Sundstrøm S, Bremnes RM, Kaasa S, et al; Norwegian Lung Cancer Study Group. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol 2002;20:4665–72.
13. Hatfield LA, Huskamp HA, Lamont EB. Survival and toxicity after cisplatin plus etoposide versus carboplatin plus etoposide for extensive-stage small-cell lung cancer in elderly patients. J Oncol Pract 2016;12(7):666–73.
14. Okamoto H, Watanabe K, Kunikane H, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer 2007;97:162–9.
15. Skarlos DV, Samantas E, Kosmidis P, et al. Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small-cell lung cancer. A Hellenic Co-operative Oncology Group study. Ann Oncol 1994;5:601–7.
16. Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data J Clin Oncol 2012;30:1692–8.
17. Bishop JF, Raghavan D, Stuart-Harris R, et al. Carboplatin (CBDCA, JM-8) and VP-16-213 in previously untreated patients with small-cell lung cancer. J Clin Oncol 1987;5:1574–8.
18. Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002;20:3054–60.
19. Bunn PA Jr, Crowley J, Kelly K, et al. Chemoradiotherapy with or without granulocyte-macrophage colony-stimulating factor in the treatment of limited-stage small-cell lung cancer: a prospective phase III randomized study of the Southwest Oncology Group. J Clin Oncol 1995;13:1632–41.
20. Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992;327:1618–24.
21. Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10(6):890–5.
22. Murray N, Coy P, Pater JL, Hodson I, Arnold A, Zee BC, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1993;11:336–44.
23. De Ruysscher D, Lueza B, Le Péchoux C, et al. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol 2016;27(10):1818–28.
24. Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340(4):265–71.
25. Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18(8):1116–25.
26. Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 1995;87(3):183–90.
27. Aupérin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476–84.
28. Le Péchoux C, Dunant A, Senan S, et al; Prophylactic Cranial Irradiation (PCI) Collaborative Group. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009;10(5):467–74.
29. Schneider BJ, Saxena A, Downey RJ. Surgery for early-stage small cell lung cancer. J Natl Compr Canc Netw 2011;9(10):1132–9.
30. Inoue M, Nakagawa K, Fujiwara K, et al. Results of preoperative mediastinoscopy for small cell lung cancer. Ann Thorac Surg 2000;70:1620–3.
31. Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. J Thorac Oncol 2008;3:1267–71.
32. Inoue M, Miyoshi S, Yasumitsu T, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg 2000;70:1615–9.
33. Yang CF, Chan DY, Speicher PJ, Gulack BC, Wang X, Hartwig MG, et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol 2016;34:1057–64.
34. Shepherd FA, Evans WK, Feld R, et al. Adjuvant chemotherapy following surgical resection for small-cell carcinoma of the lung. J Clin Oncol 1988;6:832–8.
35. Noda K, Nishiwaki Y, Kawahara M, et al; Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85–91.
36. Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530–5.
37. Chute JP, Chen T, Feigal E, et al. Twenty years of phase III trials for patients with extensive-stage small-cell lung cancer: perceptible progress. J Clin Oncol 1999;17:1794–801.
38. Zhou H, Zeng C, Wei Y, Zhou J, Yao W. Duration of chemotherapy for small cell lung cancer: a meta-analysis. PloS One 2013;8:e73805.
39. Loehrer PJ Sr, Ansari R, Gonin R, et al. Cisplatin plus etoposide with and without ifosfamide in extensive small-cell lung cancer: a Hoosier Oncology Group study. J Clin Oncol 1995 Oct;13:2594–9.
40. Pujol JL, Daurés JP, Riviére A, et al. Etoposide plus cisplatin with or without the combination of 4’-epidoxorubicin plus cyclophosphamide in treatment of extensive small-cell lung cancer: a French Federation of Cancer Institutes multicenter phase III randomized study. J Natl Cancer Inst 2001;93:300–8.
41. Berghmans T, Scherpereel A, Meert AP, et al; European Lung Cancer Working Party (ELCWP). A phase III randomized study comparing a chemotherapy with cisplatin and etoposide to a etoposide regimen without cisplatin for patients with extensive small-cell lung cancer. Front Oncol 2017;7:217.
42. Jalal SI, Lavin P, Lo G, et al. Carboplatin and etoposide with or without palifosfamide in untreated extensive-stage small-cell lung cancer: a Multicenter, Adaptive, Randomized Phase III Study (MATISSE). J Clin Oncol 2017;35:2619–23.
43. Fukuoka M, Furuse K, Saijo N, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst 1991;83:855–61.
44. Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 1992;10(2):282–91.
45. Miles DW, Earl HM, Souhami RL, Harper PG, Rudd R, Ash CM, et al. Intensive weekly chemotherapy for good-prognosis patients with small-cell lung cancer. J Clin Oncol 1991;9:280–5.
46. Petrioli R, Roviello G, Laera L, et al. Cisplatin, etoposide, and bevacizumab regimen followed by oral etoposide and bevacizumab maintenance treatment in patients with extensive-stage small cell lung cancer: a single-institution experience. Clin Lung Cancer 2015;16:e229–34.
47. Spigel DR, Greco FA, Zubkus JD, et al. Phase II trial of irinotecan, carboplatin, and bevacizumab in the treatment of patients with extensive-stage small-cell lung cancer. J Thorac Oncol 2009;4(12):1555–60.
48. Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011;29(16):2215–22.
49. Horn L, Dahlberg SE, Sandler AB, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol 2009;27(35):6006–11.
50. Tiseo M, Boni L, Ambrosio F, et al. Italian, multicenter, phase III, randomized study of cisplatin plus etoposide with or without bevacizumab as first-line treatment in extensive-disease small-cell lung cancer: the GOIRC-AIFA FARM6PMFJM trial. J Clin Oncol 2017;35:1281–7.
51. Pujol JL, Lavole A, Quoix E, et al. Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trialdagger. Ann Oncol 2015;26:908–14.
52. Gadgeel SM, Ventimiglia J, Kalemkerian GP, et al. Phase II study of maintenance pembrolizumab (pembro) in extensive stage small cell lung cancer (ES-SCLC) patients (pts). J Clin Oncol 2017;35(15_suppl):8504-. [Can’t find the rest of the reference]
53. Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol 2016;34:3740–8.
54. Owonikoko TK, Behera M, Chen Z, et al. A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol 2012;7:866–72.
55. Postmus PE, Berendsen HH, van Zandwijk N, et al. Retreatment with the induction regimen in small cell lung cancer relapsing after an initial response to short term chemotherapy. Eur J Cancer Clin Oncol 1987;23:1409–11.
56. Hellmann MD, Ott PA, Zugazagoitia J, Ready NE, Hann CL, Braud FGD, et al. Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): First report of a randomized expansion cohort from CheckMate 032. J Clin Oncol 2017;35(15_suppl):8503-. [Can’t find the rest of the reference]
57. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95.
58. Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the Phase Ib KEYNOTE-028 study. J Clin Oncol 2017;35:3823–9.
59. Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:663–71.
60. Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol 2009;27(1):78–84.
61. Brown PD, Pugh S, Laack NN, et al; Radiation Therapy Oncology Group (RTOG). Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429–37.
62. Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol 1999;17(7):2092–9.
63. Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36–42.
64. Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, et al. Radiotherapy for extensive stage small-cell lung cancer - authors’ reply. Lancet 2015;385:1292–3.
65. Owonikoko TK, Dahlberg SE, Khan SA, et al. A phase 1 safety study of veliparib combined with cisplatin and etoposide in extensive stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E2511). Lung Cancer. 2015 ;89:66–70.
66. Mamdani H, Induru R, Jalal SI. Novel therapies in small cell lung cancer. Translational lung cancer research. 2015;4:533–44.
67. Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017;18:42–51.
A Practical Approach to Management of the Patient with Inflammatory Bowel Disease Following Tumor Necrosis Factor Antagonist Failure
From the Division of Gastroenterology University of Washington, Seattle, WA (Dr. Tiderington), and the Division of Gastroenterology, Hepatology and Nutrition, The Ohio State University Wexner Medical Center and The Ohio State University Inflammatory Bowel Disease Center, Columbus, OH (Dr. Afzali).
Abstract
- Objective: To provide a practical approach to the management of patients with inflammatory bowel disease (IBD) following tumor necrosis factor (TNF) alpha antagonist failure.
- Methods: Review of the literature.
- Results: TNF alpha antagonists play a central role in the treatment of IBD. Unfortunately, some patients will not respond to therapy with TNF antagonists, and others will lose response during treatment. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. In this paper we review the mechanisms of drug failure, the use of reactive therapeutic drug monitoring to guide clinical decision making, and propose an evidence-based method for managing this common clinical scenario.
- Conclusion: Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Key words: TNF antagonists; therapeutic drug monitoring; biologic failure; Crohn’s disease treatment; ulcerative colitis treatment.
Ulcerative colitis and Crohn’s disease are the two types of inflammatory bowel disease (IBD), and they are characterized by chronic, immunologically mediated inflammation involving the gastrointestinal tract [1]. Guided by an understanding of the role of tumor necrosis factor (TNF) alpha in the pathogenesis of IBD, TNF antagonists have played a central role in modern treatment algorithms [2]. Unfortunately, roughly one third of patients will not have a clinical response when given induction dosing of the currently available anti-TNF agents, and of those who do respond to treatment, up to one half will lose response to treatment within the first year [3]. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. Once the clinician has confirmed that active IBD is present based on endoscopic, cross-sectional imaging and/or biochemical markers of inflammation, the next step is to identify the cause of the treatment failure, as this guides management. Here we review the body of literature that guides our understanding of treatment failure as well as therapeutic drug monitoring and propose an evidence-based algorithm for managing this common clinical scenario.
Defining Treatment Failure
Patients who receive anti-TNF therapy but demonstrate active IBD should be classified as having either primary nonresponse or secondary loss of response. Primary nonresponse is defined as having either no response, or only partial response, to induction with anti-TNF therapy [4]. Data from pivotal trials and meta-analyses suggest that about one third of patients will not show any clinical response to induction with anti-TNF therapies, with response typically being defined using composite endpoints favoring clinical symptoms and only sometimes incorporating endoscopic findings [5]. An additional one third of patients will have only a partial response, without remission. Patients with ulcerative colitis are at a slightly increased risk of primary nonresponse compared to patients with Crohn’s disease. Though the time frame for defining primary nonresponse is different for each agent because each agent has a slightly different induction schedule, in general the maximal response to therapy is typically seen by week 12, and it is reasonable to use this as a time cutoff [6].
Secondary loss of response is likewise defined as recrudescence of clinically active disease after an initial response. In general, the presence of secondary loss of response should not be invoked until week 12 of therapy. In most pivotal trials, secondary loss of response was seen in roughly half of patients at 1 year. In clinical practice, however, particularly as therapeutic drug monitoring has become more common, the observed rates of secondary loss of response have been lower [6].
Applying these definitions appropriately is important because it dictates the next steps in management. When a patient presents with symptoms suggesting active IBD while on anti-TNF therapy, either during induction when primary nonresponse is possible, or in maintenance when secondary loss of response would be invoked, the first step is to determine if active IBD is the etiology for the presenting symptoms. The initial evaluation should rule out common infectious causes of symptoms mimicking IBD. In particular, Clostridium difficile infection should be ruled out with stool testing. In certain circumstances, ruling out cytomegalovirus (CMV) colitis is important, so an endoscopic evaluation with colonic biopsies should be considered. In the absence of infectious colitis, the presence of active inflammation can often be identified endoscopically, or can be inferred from noninvasive markers with a fair degree of certainty. Fecal calprotectin is an ideal choice for this purpose. In ulcerative colitis it is estimated to have a sensitivity of 0.88 and a specificity of 0.79 for the prediction of endoscopically active disease. The estimated sensitivity for detecting endoscopically active Crohn’s disease is essentially the same (0.87), and the specificity is only slightly lower (0.67). C-reactive protein demonstrates a better specificity (0.92), but has a marginal sensitivity (0.49) [7]. Other etiologies for the patient’s symptoms should also be considered, including medication side effects including use of nonsteroidal anti-inflammatory medications, bile acid malabsorption, small intestinal bacterial overgrowth (SIBO), irritable bowel syndrome (IBS), diverticular disease, ischemic colitis, fibrostenotic strictures, and cancer, depending on comorbidities and the history of present illness.
Once it has been determined that active IBD is the etiology for the patient’s symptoms, the patient should be classified as having either primary nonresponse or secondary loss of response as described above. For the clinician, the next question is how to alter or optimize therapy.
The decision of how to optimize therapy will largely depend on which anti-TNF therapy the patient is currently receiving, and whether they are receiving it as monotherapy or as combination therapy with an immunomodulator. Optimizing therapy will involve either increasing the dose or frequency of administration of the anti-TNF therapy, increasing the dose of azathioprine if indicated, adding an immunomodulator if the patient is on anti-TNF monotherapy, changing to a different anti-TNF agent, or changing to a different class of medication with a different mechanism of action. The recently released American Gastroenterological Association (AGA) guidelines on therapeutic drug monitoring in IBD provide a framework for making these decisions [8]. In general, the clinical choice will be dictated by the etiology of the drug failure.
Types of TNF Antagonist Drug Failure
Our understanding of the causes of biologic treatment failure are evolving but are typically classified as due to mechanistic failure, non-immune-mediated pharmacokinetic failure, or immune-mediated pharmacokinetic failure [9]. Differentiating between these classes of treatment failure requires therapeutic drug monitoring (TDM), which will be discussed in more detail below.
Mechanistic failure is encountered when the underlying biology does not favor a response to a particular therapy. Studies indicate a strong association between particular genetic phenotypes and the probability of a response to induction with anti-TNF agents [10]. This suggests that some individuals have IBD driven by a biochemical inflammatory cascade in which TNF features prominently, while others have alternative mechanistic drivers of inflammation without significantly elevated TNF levels. Mechanistic failure will typically present as primary nonresponse, but can also be seen in patients with secondary loss of response. Mechanistic failure can be elucidated clinically by the use of TDM. In the case of mechanistic failure, active disease is seen in the presence of adequate drug level, without the presence of anti-drug antibodies. The AGA recommends considering switching to a biologic with a different mechanism of action when mechanistic failure is identified [8].
Non-immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to a drug at adequate drug levels experiences suboptimal drug levels because of pharmacokinetic factors. In the case of anti-TNF therapy, this can be conceptualized as either an increased clearance of anti-TNF from the body (eg, in patients with significant hypoalbuminemia or severe colitis), a reduction in the average serum anti-TNF level because of the redistribution of drug in patients with a large body mass index, or inadequate saturation of the total body burden of TNF-alpha in subjects with a high baseline level of inflammation [11]. Non-immune-mediated pharmacokinetic failure can also be identified clinically through TDM. In this case, active disease is seen in the presence of a suboptimal drug level, without the presence of anti-drug antibodies. The AGA recommends considering dose-escalation of the current TNF antagonist when non-immune-mediated pharmacokinetic failure is identified [8], as this can improve clinical response in an estimated 82% of patients [9].
Finally, immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to the current biologic therapy when at adequate drug concentration levels experiences suboptimal drug levels because of increased drug clearance mediated by anti-drug antibodies [9]. Because anti-TNF agents are monoclonal antibodies, they are inherently immunogenic, and it is well established that episodic dosing and lower serum drug concentrations are strong risk factors for the development of anti-drug antibodies [12]. When anti-drug antibodies are present, and are associated with both a decreased serum drug concentration and active inflammatory bowel disease, immune-mediate pharmacokinetic failure can be invoked. When anti-drug antibodies are present, but at a low level, the AGA recommends dose escalation of current TNF antagonist. When anti-drug antibodies are present at a high level, the AGA recommends considering either the addition of an immunomodulator (if not already being used), or changing to a different class of biologic therapy [8]. This recommendation is based in part on data showing that the proportion of patients with sustained anti-drug antibodies during the first year of therapy with an TNF antagonist is likely between 14% and 20% for those on monotherapy, but between 1% and 5% for those on concomitant immunomodulatory therapy [13,14].
Therapeutic Drug Monitoring of Anti-TNF Agents
As described above, TDM, which is the process of testing the patient’s serum for both the concentration of the TNF antagonist and for the presence and concentration of anti-drug antibodies, can help differentiate between mechanistic failure, non-immune-mediated pharmacokinetic failure, and immune-mediated pharmacokinetic failure (Table 1).
Therapeutic drug monitoring can be classified as either proactive or reactive. Proactive TDM is performed during induction or maintenance therapy when the patient does not have signs or symptoms of active disease to suggest a loss of response. Theoretically, this would allow dose modification and optimization, including dose de-escalation in certain circumstances, and could thus provide cost savings with minimal impact on clinical outcomes. The TAXIT trial provides the most robust evaluation of proactive TDM in TNF antagonist therapy. In this study, patients with Crohn’s disease or ulcerative colitis who had a stable clinical response while on maintenance infliximab were first dose optimized proactively to a target trough concentration of 3–7 μg/mL, then randomized to having dose modifications made based on clinical factors alone, defined as reactive monitoring, or dose modifications based on proactive monitoring, performed by checking the drug concentration and antibody levels before each infusion. At 1 year there was no statistically significant difference in the proportion of patients in remission. In addition, some patients in the proactive TDM group were able to have a dose reduction without a subsequent flare of disease, thus providing cost savings [15]. This study suggests that proactive TDM may have a role in drug optimization, particularly with respect to cost-effectiveness, but provides only indirect evidence of a clinical benefit, since all subjects enrolled in the study were proactively dose optimized prior to randomization. This study had a limited follow-up time of 1 year so was not able to assess for longer-term benefits and risks associated with proactive TDM.
More recently, a large, multicenter, retrospective cohort study provided additional evidence that proactive TDM may provide a clinical benefit in addition to cost savings. This study retrospectively evaluated consecutive patients receiving maintenance infliximab for Crohn’s disease between 2006 and 2015, with a median follow-up time of 2.4 years. They were classified as having had either proactive TDM or reactive TDM. Proactive TDM was associated with statistically significant reductions in the risk of treatment failure (hazard ratio [HR] 0.16, 95% confidence interval [CI] 0.09–0.27), the need for surgery (HR 0.30, 95% CI 0.11–0.80), hospitalization (HR 0.16, 95% CI 0.07–0.33), and anti-drug antibody formation (HR 0.25, 95% CI 0.07–0.84) [16].
To date, however, no randomized controlled trials have been published comparing proactive TDM to reactive TDM in treatment-naive patients. Because of the paucity of prospective studies, the AGA currently makes no recommendation regarding the use of proactive TDM in clinical practice. However, the current AGA guidelines do recommend reactive TDM in the setting of secondary loss of response based on the results of one randomized controlled trial (RCT) and several observational studies. The RCT was small (n = 69), and enrolled patients with Crohn’s disease on maintenance therapy with infliximab. Similar to the TAXIT trial, the study did not show a statistically significant difference in rates of clinical remission when subjects were randomized to either empiric dose escalation (to 5 mg/kg every 4 weeks) based on symptoms, or to dose escalations based on the results of reactive TDM. Also similar to the TAXIT trial, it showed an estimated cost savings of about 34% based on local prices in Denmark for reactive TDM over empiric dose escalation [17].
Meanwhile, the observational studies for reactive TDM provided additional support to the clinical benefit of reactive TDM, but also to the underlying hypotheses that drive reactive TDM, namely that subjects with mechanistic failure benefit from a change in drug class, those with non-immune-mediated pharmacokinetic failure benefit from dose escalation, and that those with immune-mediated pharmacokinetic failure may benefit from either dose escalation or a change in mechanism of action, depending on antibody titers. Specifically, on pooled analysis of 2 of these studies, 82% of subjects who were found to have non-immune-mediated pharmacokinetic failure responded to empiric dose escalation, whereas only 8% of subjects who were found to have immune-mediated pharmacokinetic failure with high anti-drug antibody titers responded to dose escalation [9]. Likewise, in a retrospective study involving subjects who were being treated with infliximab and then had reactive TDM performed, when non-immune-mediated pharmacokinetic failure was identified, a clinical response was seen in 86% of subjects who underwent dose escalation, and only 33% among those who were switched to a different anti-TNF (P < 0.016). Conversely, dose escalation resulted in a clinical response only 17% of the time when anti-drug antibodies were detectable, compared to a 92% response rate when the subject was switched to a different anti-TNF (P < 0.004) [18].
Interpreting the Results of Reactive Therapeutic Drug Monitoring
The implementation of reactive TDM involves obtaining a serum drug and antibody level and then interpreting what those results suggest about the mechanism of drug failure, in order to decide on a course of action. The serum drug level should be a trough concentration, so it should be obtained just prior to a timed dose, while on a stable treatment regimen. Exactly what serum drug concentration we should be targeting in reactive therapeutic drug monitoring remains an area of investigation. No RCTs have been published. There is ample observational, cross-sectional data from cohorts of patients on maintenance therapy, though heterogeneity in study design and study populations, as well as use of different assays, limit interpretation of the data. In a secondary analysis of data from 6 observational studies of patients on infliximab maintenance therapy, there was a highly statistically significant concentration-dependent trend in rates of clinical remission depending on the measured infliximab trough concentration, with 96% of those with infliximab > 7 μg/mL in remission, 92% of those with infliximab > 5 μg/mL in remission, and 75% of those with infliximab > 1 μg/mL in remission. Likewise, data from 4 studies of patients receiving adalimumab showed a statistically significant concentration-dependent trend in clinical remission, with 90% of those with adalimumab trough concentrations > 7.5 μg/mL being in clinical remission, compared with only 83% of those with concentrations > 5 μg/mL. Similarly, data from 9 studies suggested that a certolizumab trough concentration > 20 μg/mL was associated with a 75% probability of being in clinical remission, compared to a 60% probability when the trough concentration was > 10 μg/mL [9]. Based on these analyses, the AGA suggests target trough concentrations for reactive therapeutic drug monitoring of anti-TNF agents of ≥ 5 μg/mL for infliximab, ≥ 7.5 μg/mL for adalimumamb, and ≥ 20 μg/mL for certolizumab. They did not suggest a target trough concentration for golimumab because of insufficient evidence [8].
When interpreting TDM test results, it is important to know if the test you have used is drug-sensitive or drug-tolerant (Table 2). Drug-sensitive tests will be less likely to reveal the presence of anti-drug antibodies when the drug level is above a certain threshold. A post-hoc analysis of the TAXIT trial recently suggested that subjects who have antibodies detected on a drug-tolerant test which were not detected on a drug-sensitive test are more likely to respond to higher doses of infliximab [19]. It follows that there should be a threshold anti-drug antibody titer below which someone who has immune-mediated pharmacokinetic failure will still respond to TNF antagonist dose escalation, but above which they will fail to respond to dose escalation. To be sure, our understanding of the clinical implications of a drug-tolerant test demonstrating an adequate drug level while also detectable anti-drug antibodies is evolving. Complicating the issue further is the fact that anti-drug antibody concentrations cannot be compared between assays because of assay-specific characteristics. As such, though the presence of low antibody titers and high antibody titers seems to be clinically important, recommendations cannot yet be made on how to interpret specific thresholds. Furthermore, development of transient versus sustained antibodies requires further clinical investigation to determine impact and treatment algorithms.
Optimizing Therapy
Once you have determined the most likely cause of drug failure, the next step is to make a change in medical therapy.
When switching within class (to another anti-TNF agent), the choice of which agent to use next will largely depend on patient preference (route of administration, infusion versus injection), insurance, and costs of treatment. When making the decision to switch within class, it should be kept in mind that the probability of achieving remission is modestly reduced compared to the probability seen in anti-TNF-naive patients [20], and even more so when the patient is switching to their third anti-TNF agent [21]. Thus, for the patient who has already previously switched from one TNF antagonist to a second TNF antagonist, it may be better to switch to a different class of biologic rather than attempting to capture a clinical remission with a third TNF antagonist.
When adding an immunomodulator (azathioprine or methotrexate), the expectation is that the therapy will increase the serum concentration of the anti-TNF agent [14] and/or reduce the ongoing risk of anti-drug antibody formation [22]. There could also be a direct treatment effect on the bowel disease by the immunomodulator.
When switching to an alternate mechanism of action, the currently FDA-approved options include the biologic agents vedolizumab (for both moderate-to-severe ulcerative colitis and moderate-to-severe Crohn’s disease) and ustekinumab (for moderate-to-severe Crohn’s disease), as well as the recently FDA-approved oral, small-molecule JAK1 and JAK3 inhibitor tofacitinib (for moderate-to-severe ulcerative colitis). Prospective comparative effectiveness studies for these agents are lacking and are unlikely to be performed in part due to the cost and time required to accomplish these studies. A recent post-hoc analysis of clinical trials data suggests that there are no significant differences in the rates of clinical response, clinical remission, or in adverse outcomes to vedolizumab or ustekinumab when they are used in patients who have failed anti-TNF therapy [23]. Thus, one cannot be recommended over the other, and the decision of which to use is usually guided by patient preference and insurance coverage.
Meanwhile, the role of tofacitinib in the treatment algorithm of patients who have failed anti-TNF therapy remains unclear. The phase III clinical trials OCTAVE 1, OCTAVE 2, and OCTAVE Sustain showed efficacy for both the induction and maintenance of remission in patients with moderate-to-severe ulcerative colitis who had previously failed anti-TNF agents. However, there remain concerns about the safety profile of tofacitinib compared to vedolizumab and ustekinumab, particularly regarding herpes zoster infection, dyslipidemia, and adverse cardiovascular events. Notable findings from the tofacitinib induction trials include robust rates of clinical remission (18.5% vs 8.2% for placebo in Octave 1, and 16.6% vs 3.6% in Octave 2, P < 0.001 for both comparisons) and mucosal healing (31.3% vs 15.6% for placebo in Octave 1, and 28.4% and 11.6% in Octave 2, P < 0.001 for both comparisons) after 8 weeks of induction therapy [24]. These results suggest that tofacitinib, or other JAK inhibitors that become approved in the future, may be excellent oral agents for the induction of remission in moderate-to-severe ulcerative colitis, and may demonstrate a better side effect profile than steroids. Whether cost factors (compared to steroid therapy) will limit the role of JAK-inhibitor therapy in induction, and whether safety concerns will limit their use in maintenance therapy, remains to be seen.
Off-Label Rescue Therapy and Surgery
Though the armamentarium of IBD therapies has expanded greatly over the past 2 decades, and will continue to do so for the foreseeable future, there are still a limited selection of therapies available to patients. Patients who have failed anti-TNF therapy, and subsequently fail vedolizumab and/or ustekinumab, have limited options. These options include clinical trials, off-label small molecule rescue therapy, and surgery. Patients who are felt to require any of these options should be referred to a tertiary center for evaluation by a gastroenterologist specializing in the treatment of IBD and/or a colorectal surgeon specializing in the surgical management of IBD.
Tacrolimus
Tacrolimus, a macrolide calcineurin inhibitor, has been studied as a small molecule therapy for IBD, though not in randomized controlled trials. There is biological plausibility for its use as a disease modifying agent. Mucosal T cells in subjects with active Crohn’s disease have been found to express increased levels of mRNA encoding IL-2, and tacrolimus acts primarily by reducing IL-2 production [25]. The largest observational cohort evaluating the use of tacrolimus, published by Thin et al, included patients with both ulcerative colitis (n = 24) and Crohn’s disease (n = 11) who had moderate to severely active IBD. All patients had failed dose-optimized thiopurine therapy, 89% had primary nonresponse or secondary loss of response to at least one anti-TNF agent, and 74% were either steroid-refractory or steroid-dependent at the time tacrolimus was started. With close monitoring, they targeted a tacrolimus trough of 8–12 ng/mL. At 30 days, 66% had a clinical response, and 40% were in clinical remission. At 90 days, 60% had a clinical response, and 37% were in clinical remission. At 1 year, 31% had a clinical response, and 23% were in clinical remission. Of those in clinical remission at 1 year, 88% were either off of steroids or on less than 5 mg of prednisone per day. Renal impairment was seen in 25% of patients, including severe renal impairment in 11%, requiring drug cessation. Infectious complications were seen in 9% of patients. Headaches, tremor, and pancreatitis were also observed, though less commonly. The majority of patients ultimately had a surgical intervention, particularly if they were steroid-refractory at baseline, but the time to surgery was delayed in those who achieved a response or remission in the first 90 days of tacrolimus therapy. The authors suggested that while tacrolimus may lack clear long-term benefit in patients with medically refractory IBD, a therapeutic trial should be considered in select patients with the goal of medical and nutritional optimization before surgical intervention [26].
Cyclosporine
Cyclosporine, which also exerts its effect by inhibiting IL-2 production, has an established role in the management of severe ulcerative colitis. Data from randomized, placebo-controlled trials, along with numerous open label observational studies, have shown that intravenous cyclosporine can induce remission and potentially obviate the need for urgent/emergent colectomy in steroid-refractory patients who are hospitalized with severe ulcerative colitis [27,28]. Its use in maintenance therapy remains controversial, however. Older observational data suggest that even among those who have an initial clinical response to cyclosporine induction, 33% will undergo colectomy by 1 year, and 88% will undergo colectomy by 7 years [27). Though the concomitant administration of a thiopurine may delay the need for colectomy [29,30], cyclosporine seems to be, at best, a temporizing therapy for patients with severe ulcerative colitis. Studies on the use of cyclosporine for the induction of remission in Crohn’s disease have been less robust, as have studies on the use of cyclosporine for the maintenance of remission in Crohn’s disease [31]. Dose-dependent toxicity also remains a concern, particularly when being considered as maintenance therapy. Though some observational data suggest that the absolute risks of serious side effects from maintenance cyclosporine are small, cyclosporine is still generally avoided as a maintenance therapy [30].
Mycophenolate Mofetil
Mycophenolate mofetil (MMF), which inhibits both B and T cell proliferation by inhibiting de novo purine synthesis, has been studied in both Crohn’s disease and ulcerative colitis. Studies have been small, observational, and heterogeneous. On the whole, they suggest that MMF does have some efficacy, but it is not necessarily more effective than azathioprine and may have a slightly increased risk of side effects [32]. Given that the side effects of MMF include diarrhea, and an IBD-like enterocolitis (MMF-induced colitis) when given to subjects without an established diagnosis of IBD, it is likely best to avoid using the drug in patients with IBD [33].
Surgery
Finally, when medical management has failed, or when fibrostenotic and/or penetrating complications of inflammatory bowel disease are present, surgery should be considered. Surgery can provide a cure in patients with ulcerative colitis, and can induce remission in patients with Crohn’s disease. Managing IBD medications around the time of surgery is always challenging. Multiple large, retrospective cohort studies have suggested that the risk for postoperative infectious complications, anastomotic leaks, and thrombotic complications do not differ between those who receive anti-TNF therapy within several months of surgery and those who do not. Nevertheless, some surgeons may prefer to time elective surgery halfway between doses of anti-TNF therapy. Additionally, there is some data to suggest that patients who are on both thiopurines and anti-TNF agents have an increased risk of postoperative complications compared to those who are on anti-TNF agents alone [34].
After a surgical evaluation, a plan of action should be formulated in a multidisciplinary fashion to determine how medical management will proceed. For those with an established diagnosis of ulcerative colitis, medical therapy can often be stopped postoperatively and the patient can be monitored prospectively for pouch complications including possible new-onset Crohn’s disease. For those who undergo surgery for the management of Crohn’s disease, though a resection completed with negative margins does induce remission, nearly 90% can be expected to have histologic, endoscopic, or clinical recurrence by 1 year. A randomized controlled trial showed that postoperative anti-TNF therapy can reduce this risk to 9% [35]. Unfortunately, a subsequently conducted large, multicenter, randomized controlled trial comparing postoperative infliximab to placebo was terminated early because of a lack of a statistically significant difference in clinical recurrence between the 2 groups at week 74. However, this lack of demonstrated efficacy may have been obscured by the relatively mild phenotype of the enrolled participants, who had a median CDAI score of 105.5 at baseline [36]. Based on available data, the AGA does conditionally recommend postoperative anti-TNF and/or thiopurine therapy for those patients with Crohn’s disease who are in a surgically induced remission [37]. The patients who are most likely to benefit from postoperative medical therapy are those who have the highest risk of recurrence, namely those who were young at the time of diagnosis, had a short disease duration prior to surgery, have multiple sites of disease, and who use tobacco products [34].
Emerging and Future Options
Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Based on our improved, and evolving understanding of the pathogenesis of IBD, several new biochemical targets have emerged, offering novel ways to modulate the cytokine cascade which drives IBD [38]. Well over a dozen phase II and phase III trials for IBD therapeutic agents are ongoing, including biologic agents targeting interleukin-23, β7-Integrin, and MAdCAM-1, as well as small molecule agents targeting the JAK/STAT pathway and the sphingosine-1-phosphate receptor modulators [39]. As new agents are approved, it may be possible to develop a more patient-centered approach to care by targeting therapies to the particular pathogenesis of each patient’s disease. Nevertheless, integrating these therapies into practice algorithms will remain a challenge in the absence of meaningful comparative effectiveness trials [40].
Conclusion
When evaluating a patient who seems to have failed anti-TNF therapy for IBD, the first step is to confirm that active inflammatory disease is present. This process includes ruling out other potential causes of the patient’s symptoms, including infectious colitis, and ideally includes obtaining objective evidence of inflammation, whether through non-invasive biomarkers, an endoscopic evaluation and/or cross-sectional imaging. Once active IBD is confirmed, reactive therapeutic drug monitoring can help elucidate the likely mechanism of drug failure, which in turn can guide medical decision making.
Corresponding author: Anita Afzali MD, MPH, The Ohio State University Wexner Medical Center, 395 West 12th Ave, Room 280, Columbus, OH 43210, [email protected].
Financial disclosures: Dr. Afzali has served as a speaker/consultant for Abbvie, UCB, Takeda, Pfizer, Janssen; on the advisory board of Abbvie, UCB; received grant support from UCB; and is a board member of IBD Horizons.
1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78.
2. Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 2012;61:918–32.
3. Mitrev N, Leong RW. Therapeutic drug monitoring of anti-tumour necrosis factor-α agents in inflammatory bowel disease. Expert Opin Drug Saf 2017;16:303–17.
4. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015;21:182–97.
5. Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015;148:37–51.
6. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis 2010;4:355–66.
7. Mosli MH, Zou G, Garg SK, et al. C-Reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:802–19.
8. Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–34.
9. Vande Casteele N, Herfarth H, Katz J, et al. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology 2017;153:835–57.
10. López-Hernández R, Valdés M, Campillo JA, et al. Genetic polymorphisms of tumour necrosis factor alpha (TNF-α) promoter gene and response to TNF-α inhibitors in Spanish patients with inflammatory bowel disease. Int J Immunogenet 2014;41:63–8.
11. Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012;91:635–46.
12. Hindryckx P, Novak G, Vande Casteele N, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: a practical overview. Drugs 2017;77:363–77.
13. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95.
14. Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther 2009;30:210–26.
15. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9.
16. Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–8.
17. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27.
18. Afif W, Loftus EV Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9.
19. Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut 2017.
20. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther 2015;41:613–23.
21. Gisbert JP, Chaparro M. Use of a third anti-TNF after failure of two previous anti-TNFs in patients with inflammatory bowel disease: is it worth it? Scand J Gastroenterol 2015;50:379–86.
22. Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012;10:1079–87.
23. Kawalec P, Moćko P. An indirect comparison of ustekinumab and vedolizumab in the therapy of TNF-failure Crohn’s disease patients. J Comp Eff Res 2017;7:101–11.
24. Sandborn WJ, Su C, Panes J, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:1723–36.
25. Yarkoni S, Sagiv Y, Kaminitz A, Askenasy N. Interleukin 2 targeted therapy in inflammatory bowel disease. Gut 2009;58:1705–6.
26. Thin LW, Murray K, Lawrance IC. Oral tacrolimus for the treatment of refractory inflammatory bowel disease in the biologic era. Inflamm Bowel Dis 2013;19:1490–8.
27. Moskovitz DN, Van Assche G, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:760–5.
28. Arts J, D’Haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis 2004;10:73–8.
29. Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol 1999;94:1587–92.
30. Cheifetz AS, Stern J, Garud S, et al. Cyclosporine is safe and effective in patients with severe ulcerative colitis. J Clin Gastroenterol 2011;45:107–12.
31. Lazarev M, Present DH, Lichtiger S, et al. The effect of intravenous cyclosporine on rates of colonic surgery in hospitalized patients with severe Crohn’s colitis. J Clin Gastroenterol 2012;46:764–7.
32. Renna S, Cottone M, Orlando A. Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease. World J Gastroenterol 2014;20:9675–90.
33. Izower MA, Rahman M, Molmenti EP, et al. Correlation of abnormal histology with endoscopic findings among mycophenolate mofetil treated patients. World J Gastrointest Endosc 2017;9:405–10.
34. Ferrari L, Krane MK, Fichera A. Inflammatory bowel disease surgery in the biologic era. World J Gastrointest Surg 2016;8:363–70.
35. Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441–50.
36. Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology 2016;150:1568–78.
37. Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute technical review on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:277–95.
38. Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology 2017;152:374–88.
39. Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci 2017;38:127–42.
40. Khanna R, Feagan BG. Emerging therapies for inflammatory bowel diseases. Dig Dis 2016;34 Suppl 1:67–
From the Division of Gastroenterology University of Washington, Seattle, WA (Dr. Tiderington), and the Division of Gastroenterology, Hepatology and Nutrition, The Ohio State University Wexner Medical Center and The Ohio State University Inflammatory Bowel Disease Center, Columbus, OH (Dr. Afzali).
Abstract
- Objective: To provide a practical approach to the management of patients with inflammatory bowel disease (IBD) following tumor necrosis factor (TNF) alpha antagonist failure.
- Methods: Review of the literature.
- Results: TNF alpha antagonists play a central role in the treatment of IBD. Unfortunately, some patients will not respond to therapy with TNF antagonists, and others will lose response during treatment. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. In this paper we review the mechanisms of drug failure, the use of reactive therapeutic drug monitoring to guide clinical decision making, and propose an evidence-based method for managing this common clinical scenario.
- Conclusion: Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Key words: TNF antagonists; therapeutic drug monitoring; biologic failure; Crohn’s disease treatment; ulcerative colitis treatment.
Ulcerative colitis and Crohn’s disease are the two types of inflammatory bowel disease (IBD), and they are characterized by chronic, immunologically mediated inflammation involving the gastrointestinal tract [1]. Guided by an understanding of the role of tumor necrosis factor (TNF) alpha in the pathogenesis of IBD, TNF antagonists have played a central role in modern treatment algorithms [2]. Unfortunately, roughly one third of patients will not have a clinical response when given induction dosing of the currently available anti-TNF agents, and of those who do respond to treatment, up to one half will lose response to treatment within the first year [3]. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. Once the clinician has confirmed that active IBD is present based on endoscopic, cross-sectional imaging and/or biochemical markers of inflammation, the next step is to identify the cause of the treatment failure, as this guides management. Here we review the body of literature that guides our understanding of treatment failure as well as therapeutic drug monitoring and propose an evidence-based algorithm for managing this common clinical scenario.
Defining Treatment Failure
Patients who receive anti-TNF therapy but demonstrate active IBD should be classified as having either primary nonresponse or secondary loss of response. Primary nonresponse is defined as having either no response, or only partial response, to induction with anti-TNF therapy [4]. Data from pivotal trials and meta-analyses suggest that about one third of patients will not show any clinical response to induction with anti-TNF therapies, with response typically being defined using composite endpoints favoring clinical symptoms and only sometimes incorporating endoscopic findings [5]. An additional one third of patients will have only a partial response, without remission. Patients with ulcerative colitis are at a slightly increased risk of primary nonresponse compared to patients with Crohn’s disease. Though the time frame for defining primary nonresponse is different for each agent because each agent has a slightly different induction schedule, in general the maximal response to therapy is typically seen by week 12, and it is reasonable to use this as a time cutoff [6].
Secondary loss of response is likewise defined as recrudescence of clinically active disease after an initial response. In general, the presence of secondary loss of response should not be invoked until week 12 of therapy. In most pivotal trials, secondary loss of response was seen in roughly half of patients at 1 year. In clinical practice, however, particularly as therapeutic drug monitoring has become more common, the observed rates of secondary loss of response have been lower [6].
Applying these definitions appropriately is important because it dictates the next steps in management. When a patient presents with symptoms suggesting active IBD while on anti-TNF therapy, either during induction when primary nonresponse is possible, or in maintenance when secondary loss of response would be invoked, the first step is to determine if active IBD is the etiology for the presenting symptoms. The initial evaluation should rule out common infectious causes of symptoms mimicking IBD. In particular, Clostridium difficile infection should be ruled out with stool testing. In certain circumstances, ruling out cytomegalovirus (CMV) colitis is important, so an endoscopic evaluation with colonic biopsies should be considered. In the absence of infectious colitis, the presence of active inflammation can often be identified endoscopically, or can be inferred from noninvasive markers with a fair degree of certainty. Fecal calprotectin is an ideal choice for this purpose. In ulcerative colitis it is estimated to have a sensitivity of 0.88 and a specificity of 0.79 for the prediction of endoscopically active disease. The estimated sensitivity for detecting endoscopically active Crohn’s disease is essentially the same (0.87), and the specificity is only slightly lower (0.67). C-reactive protein demonstrates a better specificity (0.92), but has a marginal sensitivity (0.49) [7]. Other etiologies for the patient’s symptoms should also be considered, including medication side effects including use of nonsteroidal anti-inflammatory medications, bile acid malabsorption, small intestinal bacterial overgrowth (SIBO), irritable bowel syndrome (IBS), diverticular disease, ischemic colitis, fibrostenotic strictures, and cancer, depending on comorbidities and the history of present illness.
Once it has been determined that active IBD is the etiology for the patient’s symptoms, the patient should be classified as having either primary nonresponse or secondary loss of response as described above. For the clinician, the next question is how to alter or optimize therapy.
The decision of how to optimize therapy will largely depend on which anti-TNF therapy the patient is currently receiving, and whether they are receiving it as monotherapy or as combination therapy with an immunomodulator. Optimizing therapy will involve either increasing the dose or frequency of administration of the anti-TNF therapy, increasing the dose of azathioprine if indicated, adding an immunomodulator if the patient is on anti-TNF monotherapy, changing to a different anti-TNF agent, or changing to a different class of medication with a different mechanism of action. The recently released American Gastroenterological Association (AGA) guidelines on therapeutic drug monitoring in IBD provide a framework for making these decisions [8]. In general, the clinical choice will be dictated by the etiology of the drug failure.
Types of TNF Antagonist Drug Failure
Our understanding of the causes of biologic treatment failure are evolving but are typically classified as due to mechanistic failure, non-immune-mediated pharmacokinetic failure, or immune-mediated pharmacokinetic failure [9]. Differentiating between these classes of treatment failure requires therapeutic drug monitoring (TDM), which will be discussed in more detail below.
Mechanistic failure is encountered when the underlying biology does not favor a response to a particular therapy. Studies indicate a strong association between particular genetic phenotypes and the probability of a response to induction with anti-TNF agents [10]. This suggests that some individuals have IBD driven by a biochemical inflammatory cascade in which TNF features prominently, while others have alternative mechanistic drivers of inflammation without significantly elevated TNF levels. Mechanistic failure will typically present as primary nonresponse, but can also be seen in patients with secondary loss of response. Mechanistic failure can be elucidated clinically by the use of TDM. In the case of mechanistic failure, active disease is seen in the presence of adequate drug level, without the presence of anti-drug antibodies. The AGA recommends considering switching to a biologic with a different mechanism of action when mechanistic failure is identified [8].
Non-immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to a drug at adequate drug levels experiences suboptimal drug levels because of pharmacokinetic factors. In the case of anti-TNF therapy, this can be conceptualized as either an increased clearance of anti-TNF from the body (eg, in patients with significant hypoalbuminemia or severe colitis), a reduction in the average serum anti-TNF level because of the redistribution of drug in patients with a large body mass index, or inadequate saturation of the total body burden of TNF-alpha in subjects with a high baseline level of inflammation [11]. Non-immune-mediated pharmacokinetic failure can also be identified clinically through TDM. In this case, active disease is seen in the presence of a suboptimal drug level, without the presence of anti-drug antibodies. The AGA recommends considering dose-escalation of the current TNF antagonist when non-immune-mediated pharmacokinetic failure is identified [8], as this can improve clinical response in an estimated 82% of patients [9].
Finally, immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to the current biologic therapy when at adequate drug concentration levels experiences suboptimal drug levels because of increased drug clearance mediated by anti-drug antibodies [9]. Because anti-TNF agents are monoclonal antibodies, they are inherently immunogenic, and it is well established that episodic dosing and lower serum drug concentrations are strong risk factors for the development of anti-drug antibodies [12]. When anti-drug antibodies are present, and are associated with both a decreased serum drug concentration and active inflammatory bowel disease, immune-mediate pharmacokinetic failure can be invoked. When anti-drug antibodies are present, but at a low level, the AGA recommends dose escalation of current TNF antagonist. When anti-drug antibodies are present at a high level, the AGA recommends considering either the addition of an immunomodulator (if not already being used), or changing to a different class of biologic therapy [8]. This recommendation is based in part on data showing that the proportion of patients with sustained anti-drug antibodies during the first year of therapy with an TNF antagonist is likely between 14% and 20% for those on monotherapy, but between 1% and 5% for those on concomitant immunomodulatory therapy [13,14].
Therapeutic Drug Monitoring of Anti-TNF Agents
As described above, TDM, which is the process of testing the patient’s serum for both the concentration of the TNF antagonist and for the presence and concentration of anti-drug antibodies, can help differentiate between mechanistic failure, non-immune-mediated pharmacokinetic failure, and immune-mediated pharmacokinetic failure (Table 1).
Therapeutic drug monitoring can be classified as either proactive or reactive. Proactive TDM is performed during induction or maintenance therapy when the patient does not have signs or symptoms of active disease to suggest a loss of response. Theoretically, this would allow dose modification and optimization, including dose de-escalation in certain circumstances, and could thus provide cost savings with minimal impact on clinical outcomes. The TAXIT trial provides the most robust evaluation of proactive TDM in TNF antagonist therapy. In this study, patients with Crohn’s disease or ulcerative colitis who had a stable clinical response while on maintenance infliximab were first dose optimized proactively to a target trough concentration of 3–7 μg/mL, then randomized to having dose modifications made based on clinical factors alone, defined as reactive monitoring, or dose modifications based on proactive monitoring, performed by checking the drug concentration and antibody levels before each infusion. At 1 year there was no statistically significant difference in the proportion of patients in remission. In addition, some patients in the proactive TDM group were able to have a dose reduction without a subsequent flare of disease, thus providing cost savings [15]. This study suggests that proactive TDM may have a role in drug optimization, particularly with respect to cost-effectiveness, but provides only indirect evidence of a clinical benefit, since all subjects enrolled in the study were proactively dose optimized prior to randomization. This study had a limited follow-up time of 1 year so was not able to assess for longer-term benefits and risks associated with proactive TDM.
More recently, a large, multicenter, retrospective cohort study provided additional evidence that proactive TDM may provide a clinical benefit in addition to cost savings. This study retrospectively evaluated consecutive patients receiving maintenance infliximab for Crohn’s disease between 2006 and 2015, with a median follow-up time of 2.4 years. They were classified as having had either proactive TDM or reactive TDM. Proactive TDM was associated with statistically significant reductions in the risk of treatment failure (hazard ratio [HR] 0.16, 95% confidence interval [CI] 0.09–0.27), the need for surgery (HR 0.30, 95% CI 0.11–0.80), hospitalization (HR 0.16, 95% CI 0.07–0.33), and anti-drug antibody formation (HR 0.25, 95% CI 0.07–0.84) [16].
To date, however, no randomized controlled trials have been published comparing proactive TDM to reactive TDM in treatment-naive patients. Because of the paucity of prospective studies, the AGA currently makes no recommendation regarding the use of proactive TDM in clinical practice. However, the current AGA guidelines do recommend reactive TDM in the setting of secondary loss of response based on the results of one randomized controlled trial (RCT) and several observational studies. The RCT was small (n = 69), and enrolled patients with Crohn’s disease on maintenance therapy with infliximab. Similar to the TAXIT trial, the study did not show a statistically significant difference in rates of clinical remission when subjects were randomized to either empiric dose escalation (to 5 mg/kg every 4 weeks) based on symptoms, or to dose escalations based on the results of reactive TDM. Also similar to the TAXIT trial, it showed an estimated cost savings of about 34% based on local prices in Denmark for reactive TDM over empiric dose escalation [17].
Meanwhile, the observational studies for reactive TDM provided additional support to the clinical benefit of reactive TDM, but also to the underlying hypotheses that drive reactive TDM, namely that subjects with mechanistic failure benefit from a change in drug class, those with non-immune-mediated pharmacokinetic failure benefit from dose escalation, and that those with immune-mediated pharmacokinetic failure may benefit from either dose escalation or a change in mechanism of action, depending on antibody titers. Specifically, on pooled analysis of 2 of these studies, 82% of subjects who were found to have non-immune-mediated pharmacokinetic failure responded to empiric dose escalation, whereas only 8% of subjects who were found to have immune-mediated pharmacokinetic failure with high anti-drug antibody titers responded to dose escalation [9]. Likewise, in a retrospective study involving subjects who were being treated with infliximab and then had reactive TDM performed, when non-immune-mediated pharmacokinetic failure was identified, a clinical response was seen in 86% of subjects who underwent dose escalation, and only 33% among those who were switched to a different anti-TNF (P < 0.016). Conversely, dose escalation resulted in a clinical response only 17% of the time when anti-drug antibodies were detectable, compared to a 92% response rate when the subject was switched to a different anti-TNF (P < 0.004) [18].
Interpreting the Results of Reactive Therapeutic Drug Monitoring
The implementation of reactive TDM involves obtaining a serum drug and antibody level and then interpreting what those results suggest about the mechanism of drug failure, in order to decide on a course of action. The serum drug level should be a trough concentration, so it should be obtained just prior to a timed dose, while on a stable treatment regimen. Exactly what serum drug concentration we should be targeting in reactive therapeutic drug monitoring remains an area of investigation. No RCTs have been published. There is ample observational, cross-sectional data from cohorts of patients on maintenance therapy, though heterogeneity in study design and study populations, as well as use of different assays, limit interpretation of the data. In a secondary analysis of data from 6 observational studies of patients on infliximab maintenance therapy, there was a highly statistically significant concentration-dependent trend in rates of clinical remission depending on the measured infliximab trough concentration, with 96% of those with infliximab > 7 μg/mL in remission, 92% of those with infliximab > 5 μg/mL in remission, and 75% of those with infliximab > 1 μg/mL in remission. Likewise, data from 4 studies of patients receiving adalimumab showed a statistically significant concentration-dependent trend in clinical remission, with 90% of those with adalimumab trough concentrations > 7.5 μg/mL being in clinical remission, compared with only 83% of those with concentrations > 5 μg/mL. Similarly, data from 9 studies suggested that a certolizumab trough concentration > 20 μg/mL was associated with a 75% probability of being in clinical remission, compared to a 60% probability when the trough concentration was > 10 μg/mL [9]. Based on these analyses, the AGA suggests target trough concentrations for reactive therapeutic drug monitoring of anti-TNF agents of ≥ 5 μg/mL for infliximab, ≥ 7.5 μg/mL for adalimumamb, and ≥ 20 μg/mL for certolizumab. They did not suggest a target trough concentration for golimumab because of insufficient evidence [8].
When interpreting TDM test results, it is important to know if the test you have used is drug-sensitive or drug-tolerant (Table 2). Drug-sensitive tests will be less likely to reveal the presence of anti-drug antibodies when the drug level is above a certain threshold. A post-hoc analysis of the TAXIT trial recently suggested that subjects who have antibodies detected on a drug-tolerant test which were not detected on a drug-sensitive test are more likely to respond to higher doses of infliximab [19]. It follows that there should be a threshold anti-drug antibody titer below which someone who has immune-mediated pharmacokinetic failure will still respond to TNF antagonist dose escalation, but above which they will fail to respond to dose escalation. To be sure, our understanding of the clinical implications of a drug-tolerant test demonstrating an adequate drug level while also detectable anti-drug antibodies is evolving. Complicating the issue further is the fact that anti-drug antibody concentrations cannot be compared between assays because of assay-specific characteristics. As such, though the presence of low antibody titers and high antibody titers seems to be clinically important, recommendations cannot yet be made on how to interpret specific thresholds. Furthermore, development of transient versus sustained antibodies requires further clinical investigation to determine impact and treatment algorithms.
Optimizing Therapy
Once you have determined the most likely cause of drug failure, the next step is to make a change in medical therapy.
When switching within class (to another anti-TNF agent), the choice of which agent to use next will largely depend on patient preference (route of administration, infusion versus injection), insurance, and costs of treatment. When making the decision to switch within class, it should be kept in mind that the probability of achieving remission is modestly reduced compared to the probability seen in anti-TNF-naive patients [20], and even more so when the patient is switching to their third anti-TNF agent [21]. Thus, for the patient who has already previously switched from one TNF antagonist to a second TNF antagonist, it may be better to switch to a different class of biologic rather than attempting to capture a clinical remission with a third TNF antagonist.
When adding an immunomodulator (azathioprine or methotrexate), the expectation is that the therapy will increase the serum concentration of the anti-TNF agent [14] and/or reduce the ongoing risk of anti-drug antibody formation [22]. There could also be a direct treatment effect on the bowel disease by the immunomodulator.
When switching to an alternate mechanism of action, the currently FDA-approved options include the biologic agents vedolizumab (for both moderate-to-severe ulcerative colitis and moderate-to-severe Crohn’s disease) and ustekinumab (for moderate-to-severe Crohn’s disease), as well as the recently FDA-approved oral, small-molecule JAK1 and JAK3 inhibitor tofacitinib (for moderate-to-severe ulcerative colitis). Prospective comparative effectiveness studies for these agents are lacking and are unlikely to be performed in part due to the cost and time required to accomplish these studies. A recent post-hoc analysis of clinical trials data suggests that there are no significant differences in the rates of clinical response, clinical remission, or in adverse outcomes to vedolizumab or ustekinumab when they are used in patients who have failed anti-TNF therapy [23]. Thus, one cannot be recommended over the other, and the decision of which to use is usually guided by patient preference and insurance coverage.
Meanwhile, the role of tofacitinib in the treatment algorithm of patients who have failed anti-TNF therapy remains unclear. The phase III clinical trials OCTAVE 1, OCTAVE 2, and OCTAVE Sustain showed efficacy for both the induction and maintenance of remission in patients with moderate-to-severe ulcerative colitis who had previously failed anti-TNF agents. However, there remain concerns about the safety profile of tofacitinib compared to vedolizumab and ustekinumab, particularly regarding herpes zoster infection, dyslipidemia, and adverse cardiovascular events. Notable findings from the tofacitinib induction trials include robust rates of clinical remission (18.5% vs 8.2% for placebo in Octave 1, and 16.6% vs 3.6% in Octave 2, P < 0.001 for both comparisons) and mucosal healing (31.3% vs 15.6% for placebo in Octave 1, and 28.4% and 11.6% in Octave 2, P < 0.001 for both comparisons) after 8 weeks of induction therapy [24]. These results suggest that tofacitinib, or other JAK inhibitors that become approved in the future, may be excellent oral agents for the induction of remission in moderate-to-severe ulcerative colitis, and may demonstrate a better side effect profile than steroids. Whether cost factors (compared to steroid therapy) will limit the role of JAK-inhibitor therapy in induction, and whether safety concerns will limit their use in maintenance therapy, remains to be seen.
Off-Label Rescue Therapy and Surgery
Though the armamentarium of IBD therapies has expanded greatly over the past 2 decades, and will continue to do so for the foreseeable future, there are still a limited selection of therapies available to patients. Patients who have failed anti-TNF therapy, and subsequently fail vedolizumab and/or ustekinumab, have limited options. These options include clinical trials, off-label small molecule rescue therapy, and surgery. Patients who are felt to require any of these options should be referred to a tertiary center for evaluation by a gastroenterologist specializing in the treatment of IBD and/or a colorectal surgeon specializing in the surgical management of IBD.
Tacrolimus
Tacrolimus, a macrolide calcineurin inhibitor, has been studied as a small molecule therapy for IBD, though not in randomized controlled trials. There is biological plausibility for its use as a disease modifying agent. Mucosal T cells in subjects with active Crohn’s disease have been found to express increased levels of mRNA encoding IL-2, and tacrolimus acts primarily by reducing IL-2 production [25]. The largest observational cohort evaluating the use of tacrolimus, published by Thin et al, included patients with both ulcerative colitis (n = 24) and Crohn’s disease (n = 11) who had moderate to severely active IBD. All patients had failed dose-optimized thiopurine therapy, 89% had primary nonresponse or secondary loss of response to at least one anti-TNF agent, and 74% were either steroid-refractory or steroid-dependent at the time tacrolimus was started. With close monitoring, they targeted a tacrolimus trough of 8–12 ng/mL. At 30 days, 66% had a clinical response, and 40% were in clinical remission. At 90 days, 60% had a clinical response, and 37% were in clinical remission. At 1 year, 31% had a clinical response, and 23% were in clinical remission. Of those in clinical remission at 1 year, 88% were either off of steroids or on less than 5 mg of prednisone per day. Renal impairment was seen in 25% of patients, including severe renal impairment in 11%, requiring drug cessation. Infectious complications were seen in 9% of patients. Headaches, tremor, and pancreatitis were also observed, though less commonly. The majority of patients ultimately had a surgical intervention, particularly if they were steroid-refractory at baseline, but the time to surgery was delayed in those who achieved a response or remission in the first 90 days of tacrolimus therapy. The authors suggested that while tacrolimus may lack clear long-term benefit in patients with medically refractory IBD, a therapeutic trial should be considered in select patients with the goal of medical and nutritional optimization before surgical intervention [26].
Cyclosporine
Cyclosporine, which also exerts its effect by inhibiting IL-2 production, has an established role in the management of severe ulcerative colitis. Data from randomized, placebo-controlled trials, along with numerous open label observational studies, have shown that intravenous cyclosporine can induce remission and potentially obviate the need for urgent/emergent colectomy in steroid-refractory patients who are hospitalized with severe ulcerative colitis [27,28]. Its use in maintenance therapy remains controversial, however. Older observational data suggest that even among those who have an initial clinical response to cyclosporine induction, 33% will undergo colectomy by 1 year, and 88% will undergo colectomy by 7 years [27). Though the concomitant administration of a thiopurine may delay the need for colectomy [29,30], cyclosporine seems to be, at best, a temporizing therapy for patients with severe ulcerative colitis. Studies on the use of cyclosporine for the induction of remission in Crohn’s disease have been less robust, as have studies on the use of cyclosporine for the maintenance of remission in Crohn’s disease [31]. Dose-dependent toxicity also remains a concern, particularly when being considered as maintenance therapy. Though some observational data suggest that the absolute risks of serious side effects from maintenance cyclosporine are small, cyclosporine is still generally avoided as a maintenance therapy [30].
Mycophenolate Mofetil
Mycophenolate mofetil (MMF), which inhibits both B and T cell proliferation by inhibiting de novo purine synthesis, has been studied in both Crohn’s disease and ulcerative colitis. Studies have been small, observational, and heterogeneous. On the whole, they suggest that MMF does have some efficacy, but it is not necessarily more effective than azathioprine and may have a slightly increased risk of side effects [32]. Given that the side effects of MMF include diarrhea, and an IBD-like enterocolitis (MMF-induced colitis) when given to subjects without an established diagnosis of IBD, it is likely best to avoid using the drug in patients with IBD [33].
Surgery
Finally, when medical management has failed, or when fibrostenotic and/or penetrating complications of inflammatory bowel disease are present, surgery should be considered. Surgery can provide a cure in patients with ulcerative colitis, and can induce remission in patients with Crohn’s disease. Managing IBD medications around the time of surgery is always challenging. Multiple large, retrospective cohort studies have suggested that the risk for postoperative infectious complications, anastomotic leaks, and thrombotic complications do not differ between those who receive anti-TNF therapy within several months of surgery and those who do not. Nevertheless, some surgeons may prefer to time elective surgery halfway between doses of anti-TNF therapy. Additionally, there is some data to suggest that patients who are on both thiopurines and anti-TNF agents have an increased risk of postoperative complications compared to those who are on anti-TNF agents alone [34].
After a surgical evaluation, a plan of action should be formulated in a multidisciplinary fashion to determine how medical management will proceed. For those with an established diagnosis of ulcerative colitis, medical therapy can often be stopped postoperatively and the patient can be monitored prospectively for pouch complications including possible new-onset Crohn’s disease. For those who undergo surgery for the management of Crohn’s disease, though a resection completed with negative margins does induce remission, nearly 90% can be expected to have histologic, endoscopic, or clinical recurrence by 1 year. A randomized controlled trial showed that postoperative anti-TNF therapy can reduce this risk to 9% [35]. Unfortunately, a subsequently conducted large, multicenter, randomized controlled trial comparing postoperative infliximab to placebo was terminated early because of a lack of a statistically significant difference in clinical recurrence between the 2 groups at week 74. However, this lack of demonstrated efficacy may have been obscured by the relatively mild phenotype of the enrolled participants, who had a median CDAI score of 105.5 at baseline [36]. Based on available data, the AGA does conditionally recommend postoperative anti-TNF and/or thiopurine therapy for those patients with Crohn’s disease who are in a surgically induced remission [37]. The patients who are most likely to benefit from postoperative medical therapy are those who have the highest risk of recurrence, namely those who were young at the time of diagnosis, had a short disease duration prior to surgery, have multiple sites of disease, and who use tobacco products [34].
Emerging and Future Options
Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Based on our improved, and evolving understanding of the pathogenesis of IBD, several new biochemical targets have emerged, offering novel ways to modulate the cytokine cascade which drives IBD [38]. Well over a dozen phase II and phase III trials for IBD therapeutic agents are ongoing, including biologic agents targeting interleukin-23, β7-Integrin, and MAdCAM-1, as well as small molecule agents targeting the JAK/STAT pathway and the sphingosine-1-phosphate receptor modulators [39]. As new agents are approved, it may be possible to develop a more patient-centered approach to care by targeting therapies to the particular pathogenesis of each patient’s disease. Nevertheless, integrating these therapies into practice algorithms will remain a challenge in the absence of meaningful comparative effectiveness trials [40].
Conclusion
When evaluating a patient who seems to have failed anti-TNF therapy for IBD, the first step is to confirm that active inflammatory disease is present. This process includes ruling out other potential causes of the patient’s symptoms, including infectious colitis, and ideally includes obtaining objective evidence of inflammation, whether through non-invasive biomarkers, an endoscopic evaluation and/or cross-sectional imaging. Once active IBD is confirmed, reactive therapeutic drug monitoring can help elucidate the likely mechanism of drug failure, which in turn can guide medical decision making.
Corresponding author: Anita Afzali MD, MPH, The Ohio State University Wexner Medical Center, 395 West 12th Ave, Room 280, Columbus, OH 43210, [email protected].
Financial disclosures: Dr. Afzali has served as a speaker/consultant for Abbvie, UCB, Takeda, Pfizer, Janssen; on the advisory board of Abbvie, UCB; received grant support from UCB; and is a board member of IBD Horizons.
From the Division of Gastroenterology University of Washington, Seattle, WA (Dr. Tiderington), and the Division of Gastroenterology, Hepatology and Nutrition, The Ohio State University Wexner Medical Center and The Ohio State University Inflammatory Bowel Disease Center, Columbus, OH (Dr. Afzali).
Abstract
- Objective: To provide a practical approach to the management of patients with inflammatory bowel disease (IBD) following tumor necrosis factor (TNF) alpha antagonist failure.
- Methods: Review of the literature.
- Results: TNF alpha antagonists play a central role in the treatment of IBD. Unfortunately, some patients will not respond to therapy with TNF antagonists, and others will lose response during treatment. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. In this paper we review the mechanisms of drug failure, the use of reactive therapeutic drug monitoring to guide clinical decision making, and propose an evidence-based method for managing this common clinical scenario.
- Conclusion: Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Key words: TNF antagonists; therapeutic drug monitoring; biologic failure; Crohn’s disease treatment; ulcerative colitis treatment.
Ulcerative colitis and Crohn’s disease are the two types of inflammatory bowel disease (IBD), and they are characterized by chronic, immunologically mediated inflammation involving the gastrointestinal tract [1]. Guided by an understanding of the role of tumor necrosis factor (TNF) alpha in the pathogenesis of IBD, TNF antagonists have played a central role in modern treatment algorithms [2]. Unfortunately, roughly one third of patients will not have a clinical response when given induction dosing of the currently available anti-TNF agents, and of those who do respond to treatment, up to one half will lose response to treatment within the first year [3]. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. Once the clinician has confirmed that active IBD is present based on endoscopic, cross-sectional imaging and/or biochemical markers of inflammation, the next step is to identify the cause of the treatment failure, as this guides management. Here we review the body of literature that guides our understanding of treatment failure as well as therapeutic drug monitoring and propose an evidence-based algorithm for managing this common clinical scenario.
Defining Treatment Failure
Patients who receive anti-TNF therapy but demonstrate active IBD should be classified as having either primary nonresponse or secondary loss of response. Primary nonresponse is defined as having either no response, or only partial response, to induction with anti-TNF therapy [4]. Data from pivotal trials and meta-analyses suggest that about one third of patients will not show any clinical response to induction with anti-TNF therapies, with response typically being defined using composite endpoints favoring clinical symptoms and only sometimes incorporating endoscopic findings [5]. An additional one third of patients will have only a partial response, without remission. Patients with ulcerative colitis are at a slightly increased risk of primary nonresponse compared to patients with Crohn’s disease. Though the time frame for defining primary nonresponse is different for each agent because each agent has a slightly different induction schedule, in general the maximal response to therapy is typically seen by week 12, and it is reasonable to use this as a time cutoff [6].
Secondary loss of response is likewise defined as recrudescence of clinically active disease after an initial response. In general, the presence of secondary loss of response should not be invoked until week 12 of therapy. In most pivotal trials, secondary loss of response was seen in roughly half of patients at 1 year. In clinical practice, however, particularly as therapeutic drug monitoring has become more common, the observed rates of secondary loss of response have been lower [6].
Applying these definitions appropriately is important because it dictates the next steps in management. When a patient presents with symptoms suggesting active IBD while on anti-TNF therapy, either during induction when primary nonresponse is possible, or in maintenance when secondary loss of response would be invoked, the first step is to determine if active IBD is the etiology for the presenting symptoms. The initial evaluation should rule out common infectious causes of symptoms mimicking IBD. In particular, Clostridium difficile infection should be ruled out with stool testing. In certain circumstances, ruling out cytomegalovirus (CMV) colitis is important, so an endoscopic evaluation with colonic biopsies should be considered. In the absence of infectious colitis, the presence of active inflammation can often be identified endoscopically, or can be inferred from noninvasive markers with a fair degree of certainty. Fecal calprotectin is an ideal choice for this purpose. In ulcerative colitis it is estimated to have a sensitivity of 0.88 and a specificity of 0.79 for the prediction of endoscopically active disease. The estimated sensitivity for detecting endoscopically active Crohn’s disease is essentially the same (0.87), and the specificity is only slightly lower (0.67). C-reactive protein demonstrates a better specificity (0.92), but has a marginal sensitivity (0.49) [7]. Other etiologies for the patient’s symptoms should also be considered, including medication side effects including use of nonsteroidal anti-inflammatory medications, bile acid malabsorption, small intestinal bacterial overgrowth (SIBO), irritable bowel syndrome (IBS), diverticular disease, ischemic colitis, fibrostenotic strictures, and cancer, depending on comorbidities and the history of present illness.
Once it has been determined that active IBD is the etiology for the patient’s symptoms, the patient should be classified as having either primary nonresponse or secondary loss of response as described above. For the clinician, the next question is how to alter or optimize therapy.
The decision of how to optimize therapy will largely depend on which anti-TNF therapy the patient is currently receiving, and whether they are receiving it as monotherapy or as combination therapy with an immunomodulator. Optimizing therapy will involve either increasing the dose or frequency of administration of the anti-TNF therapy, increasing the dose of azathioprine if indicated, adding an immunomodulator if the patient is on anti-TNF monotherapy, changing to a different anti-TNF agent, or changing to a different class of medication with a different mechanism of action. The recently released American Gastroenterological Association (AGA) guidelines on therapeutic drug monitoring in IBD provide a framework for making these decisions [8]. In general, the clinical choice will be dictated by the etiology of the drug failure.
Types of TNF Antagonist Drug Failure
Our understanding of the causes of biologic treatment failure are evolving but are typically classified as due to mechanistic failure, non-immune-mediated pharmacokinetic failure, or immune-mediated pharmacokinetic failure [9]. Differentiating between these classes of treatment failure requires therapeutic drug monitoring (TDM), which will be discussed in more detail below.
Mechanistic failure is encountered when the underlying biology does not favor a response to a particular therapy. Studies indicate a strong association between particular genetic phenotypes and the probability of a response to induction with anti-TNF agents [10]. This suggests that some individuals have IBD driven by a biochemical inflammatory cascade in which TNF features prominently, while others have alternative mechanistic drivers of inflammation without significantly elevated TNF levels. Mechanistic failure will typically present as primary nonresponse, but can also be seen in patients with secondary loss of response. Mechanistic failure can be elucidated clinically by the use of TDM. In the case of mechanistic failure, active disease is seen in the presence of adequate drug level, without the presence of anti-drug antibodies. The AGA recommends considering switching to a biologic with a different mechanism of action when mechanistic failure is identified [8].
Non-immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to a drug at adequate drug levels experiences suboptimal drug levels because of pharmacokinetic factors. In the case of anti-TNF therapy, this can be conceptualized as either an increased clearance of anti-TNF from the body (eg, in patients with significant hypoalbuminemia or severe colitis), a reduction in the average serum anti-TNF level because of the redistribution of drug in patients with a large body mass index, or inadequate saturation of the total body burden of TNF-alpha in subjects with a high baseline level of inflammation [11]. Non-immune-mediated pharmacokinetic failure can also be identified clinically through TDM. In this case, active disease is seen in the presence of a suboptimal drug level, without the presence of anti-drug antibodies. The AGA recommends considering dose-escalation of the current TNF antagonist when non-immune-mediated pharmacokinetic failure is identified [8], as this can improve clinical response in an estimated 82% of patients [9].
Finally, immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to the current biologic therapy when at adequate drug concentration levels experiences suboptimal drug levels because of increased drug clearance mediated by anti-drug antibodies [9]. Because anti-TNF agents are monoclonal antibodies, they are inherently immunogenic, and it is well established that episodic dosing and lower serum drug concentrations are strong risk factors for the development of anti-drug antibodies [12]. When anti-drug antibodies are present, and are associated with both a decreased serum drug concentration and active inflammatory bowel disease, immune-mediate pharmacokinetic failure can be invoked. When anti-drug antibodies are present, but at a low level, the AGA recommends dose escalation of current TNF antagonist. When anti-drug antibodies are present at a high level, the AGA recommends considering either the addition of an immunomodulator (if not already being used), or changing to a different class of biologic therapy [8]. This recommendation is based in part on data showing that the proportion of patients with sustained anti-drug antibodies during the first year of therapy with an TNF antagonist is likely between 14% and 20% for those on monotherapy, but between 1% and 5% for those on concomitant immunomodulatory therapy [13,14].
Therapeutic Drug Monitoring of Anti-TNF Agents
As described above, TDM, which is the process of testing the patient’s serum for both the concentration of the TNF antagonist and for the presence and concentration of anti-drug antibodies, can help differentiate between mechanistic failure, non-immune-mediated pharmacokinetic failure, and immune-mediated pharmacokinetic failure (Table 1).
Therapeutic drug monitoring can be classified as either proactive or reactive. Proactive TDM is performed during induction or maintenance therapy when the patient does not have signs or symptoms of active disease to suggest a loss of response. Theoretically, this would allow dose modification and optimization, including dose de-escalation in certain circumstances, and could thus provide cost savings with minimal impact on clinical outcomes. The TAXIT trial provides the most robust evaluation of proactive TDM in TNF antagonist therapy. In this study, patients with Crohn’s disease or ulcerative colitis who had a stable clinical response while on maintenance infliximab were first dose optimized proactively to a target trough concentration of 3–7 μg/mL, then randomized to having dose modifications made based on clinical factors alone, defined as reactive monitoring, or dose modifications based on proactive monitoring, performed by checking the drug concentration and antibody levels before each infusion. At 1 year there was no statistically significant difference in the proportion of patients in remission. In addition, some patients in the proactive TDM group were able to have a dose reduction without a subsequent flare of disease, thus providing cost savings [15]. This study suggests that proactive TDM may have a role in drug optimization, particularly with respect to cost-effectiveness, but provides only indirect evidence of a clinical benefit, since all subjects enrolled in the study were proactively dose optimized prior to randomization. This study had a limited follow-up time of 1 year so was not able to assess for longer-term benefits and risks associated with proactive TDM.
More recently, a large, multicenter, retrospective cohort study provided additional evidence that proactive TDM may provide a clinical benefit in addition to cost savings. This study retrospectively evaluated consecutive patients receiving maintenance infliximab for Crohn’s disease between 2006 and 2015, with a median follow-up time of 2.4 years. They were classified as having had either proactive TDM or reactive TDM. Proactive TDM was associated with statistically significant reductions in the risk of treatment failure (hazard ratio [HR] 0.16, 95% confidence interval [CI] 0.09–0.27), the need for surgery (HR 0.30, 95% CI 0.11–0.80), hospitalization (HR 0.16, 95% CI 0.07–0.33), and anti-drug antibody formation (HR 0.25, 95% CI 0.07–0.84) [16].
To date, however, no randomized controlled trials have been published comparing proactive TDM to reactive TDM in treatment-naive patients. Because of the paucity of prospective studies, the AGA currently makes no recommendation regarding the use of proactive TDM in clinical practice. However, the current AGA guidelines do recommend reactive TDM in the setting of secondary loss of response based on the results of one randomized controlled trial (RCT) and several observational studies. The RCT was small (n = 69), and enrolled patients with Crohn’s disease on maintenance therapy with infliximab. Similar to the TAXIT trial, the study did not show a statistically significant difference in rates of clinical remission when subjects were randomized to either empiric dose escalation (to 5 mg/kg every 4 weeks) based on symptoms, or to dose escalations based on the results of reactive TDM. Also similar to the TAXIT trial, it showed an estimated cost savings of about 34% based on local prices in Denmark for reactive TDM over empiric dose escalation [17].
Meanwhile, the observational studies for reactive TDM provided additional support to the clinical benefit of reactive TDM, but also to the underlying hypotheses that drive reactive TDM, namely that subjects with mechanistic failure benefit from a change in drug class, those with non-immune-mediated pharmacokinetic failure benefit from dose escalation, and that those with immune-mediated pharmacokinetic failure may benefit from either dose escalation or a change in mechanism of action, depending on antibody titers. Specifically, on pooled analysis of 2 of these studies, 82% of subjects who were found to have non-immune-mediated pharmacokinetic failure responded to empiric dose escalation, whereas only 8% of subjects who were found to have immune-mediated pharmacokinetic failure with high anti-drug antibody titers responded to dose escalation [9]. Likewise, in a retrospective study involving subjects who were being treated with infliximab and then had reactive TDM performed, when non-immune-mediated pharmacokinetic failure was identified, a clinical response was seen in 86% of subjects who underwent dose escalation, and only 33% among those who were switched to a different anti-TNF (P < 0.016). Conversely, dose escalation resulted in a clinical response only 17% of the time when anti-drug antibodies were detectable, compared to a 92% response rate when the subject was switched to a different anti-TNF (P < 0.004) [18].
Interpreting the Results of Reactive Therapeutic Drug Monitoring
The implementation of reactive TDM involves obtaining a serum drug and antibody level and then interpreting what those results suggest about the mechanism of drug failure, in order to decide on a course of action. The serum drug level should be a trough concentration, so it should be obtained just prior to a timed dose, while on a stable treatment regimen. Exactly what serum drug concentration we should be targeting in reactive therapeutic drug monitoring remains an area of investigation. No RCTs have been published. There is ample observational, cross-sectional data from cohorts of patients on maintenance therapy, though heterogeneity in study design and study populations, as well as use of different assays, limit interpretation of the data. In a secondary analysis of data from 6 observational studies of patients on infliximab maintenance therapy, there was a highly statistically significant concentration-dependent trend in rates of clinical remission depending on the measured infliximab trough concentration, with 96% of those with infliximab > 7 μg/mL in remission, 92% of those with infliximab > 5 μg/mL in remission, and 75% of those with infliximab > 1 μg/mL in remission. Likewise, data from 4 studies of patients receiving adalimumab showed a statistically significant concentration-dependent trend in clinical remission, with 90% of those with adalimumab trough concentrations > 7.5 μg/mL being in clinical remission, compared with only 83% of those with concentrations > 5 μg/mL. Similarly, data from 9 studies suggested that a certolizumab trough concentration > 20 μg/mL was associated with a 75% probability of being in clinical remission, compared to a 60% probability when the trough concentration was > 10 μg/mL [9]. Based on these analyses, the AGA suggests target trough concentrations for reactive therapeutic drug monitoring of anti-TNF agents of ≥ 5 μg/mL for infliximab, ≥ 7.5 μg/mL for adalimumamb, and ≥ 20 μg/mL for certolizumab. They did not suggest a target trough concentration for golimumab because of insufficient evidence [8].
When interpreting TDM test results, it is important to know if the test you have used is drug-sensitive or drug-tolerant (Table 2). Drug-sensitive tests will be less likely to reveal the presence of anti-drug antibodies when the drug level is above a certain threshold. A post-hoc analysis of the TAXIT trial recently suggested that subjects who have antibodies detected on a drug-tolerant test which were not detected on a drug-sensitive test are more likely to respond to higher doses of infliximab [19]. It follows that there should be a threshold anti-drug antibody titer below which someone who has immune-mediated pharmacokinetic failure will still respond to TNF antagonist dose escalation, but above which they will fail to respond to dose escalation. To be sure, our understanding of the clinical implications of a drug-tolerant test demonstrating an adequate drug level while also detectable anti-drug antibodies is evolving. Complicating the issue further is the fact that anti-drug antibody concentrations cannot be compared between assays because of assay-specific characteristics. As such, though the presence of low antibody titers and high antibody titers seems to be clinically important, recommendations cannot yet be made on how to interpret specific thresholds. Furthermore, development of transient versus sustained antibodies requires further clinical investigation to determine impact and treatment algorithms.
Optimizing Therapy
Once you have determined the most likely cause of drug failure, the next step is to make a change in medical therapy.
When switching within class (to another anti-TNF agent), the choice of which agent to use next will largely depend on patient preference (route of administration, infusion versus injection), insurance, and costs of treatment. When making the decision to switch within class, it should be kept in mind that the probability of achieving remission is modestly reduced compared to the probability seen in anti-TNF-naive patients [20], and even more so when the patient is switching to their third anti-TNF agent [21]. Thus, for the patient who has already previously switched from one TNF antagonist to a second TNF antagonist, it may be better to switch to a different class of biologic rather than attempting to capture a clinical remission with a third TNF antagonist.
When adding an immunomodulator (azathioprine or methotrexate), the expectation is that the therapy will increase the serum concentration of the anti-TNF agent [14] and/or reduce the ongoing risk of anti-drug antibody formation [22]. There could also be a direct treatment effect on the bowel disease by the immunomodulator.
When switching to an alternate mechanism of action, the currently FDA-approved options include the biologic agents vedolizumab (for both moderate-to-severe ulcerative colitis and moderate-to-severe Crohn’s disease) and ustekinumab (for moderate-to-severe Crohn’s disease), as well as the recently FDA-approved oral, small-molecule JAK1 and JAK3 inhibitor tofacitinib (for moderate-to-severe ulcerative colitis). Prospective comparative effectiveness studies for these agents are lacking and are unlikely to be performed in part due to the cost and time required to accomplish these studies. A recent post-hoc analysis of clinical trials data suggests that there are no significant differences in the rates of clinical response, clinical remission, or in adverse outcomes to vedolizumab or ustekinumab when they are used in patients who have failed anti-TNF therapy [23]. Thus, one cannot be recommended over the other, and the decision of which to use is usually guided by patient preference and insurance coverage.
Meanwhile, the role of tofacitinib in the treatment algorithm of patients who have failed anti-TNF therapy remains unclear. The phase III clinical trials OCTAVE 1, OCTAVE 2, and OCTAVE Sustain showed efficacy for both the induction and maintenance of remission in patients with moderate-to-severe ulcerative colitis who had previously failed anti-TNF agents. However, there remain concerns about the safety profile of tofacitinib compared to vedolizumab and ustekinumab, particularly regarding herpes zoster infection, dyslipidemia, and adverse cardiovascular events. Notable findings from the tofacitinib induction trials include robust rates of clinical remission (18.5% vs 8.2% for placebo in Octave 1, and 16.6% vs 3.6% in Octave 2, P < 0.001 for both comparisons) and mucosal healing (31.3% vs 15.6% for placebo in Octave 1, and 28.4% and 11.6% in Octave 2, P < 0.001 for both comparisons) after 8 weeks of induction therapy [24]. These results suggest that tofacitinib, or other JAK inhibitors that become approved in the future, may be excellent oral agents for the induction of remission in moderate-to-severe ulcerative colitis, and may demonstrate a better side effect profile than steroids. Whether cost factors (compared to steroid therapy) will limit the role of JAK-inhibitor therapy in induction, and whether safety concerns will limit their use in maintenance therapy, remains to be seen.
Off-Label Rescue Therapy and Surgery
Though the armamentarium of IBD therapies has expanded greatly over the past 2 decades, and will continue to do so for the foreseeable future, there are still a limited selection of therapies available to patients. Patients who have failed anti-TNF therapy, and subsequently fail vedolizumab and/or ustekinumab, have limited options. These options include clinical trials, off-label small molecule rescue therapy, and surgery. Patients who are felt to require any of these options should be referred to a tertiary center for evaluation by a gastroenterologist specializing in the treatment of IBD and/or a colorectal surgeon specializing in the surgical management of IBD.
Tacrolimus
Tacrolimus, a macrolide calcineurin inhibitor, has been studied as a small molecule therapy for IBD, though not in randomized controlled trials. There is biological plausibility for its use as a disease modifying agent. Mucosal T cells in subjects with active Crohn’s disease have been found to express increased levels of mRNA encoding IL-2, and tacrolimus acts primarily by reducing IL-2 production [25]. The largest observational cohort evaluating the use of tacrolimus, published by Thin et al, included patients with both ulcerative colitis (n = 24) and Crohn’s disease (n = 11) who had moderate to severely active IBD. All patients had failed dose-optimized thiopurine therapy, 89% had primary nonresponse or secondary loss of response to at least one anti-TNF agent, and 74% were either steroid-refractory or steroid-dependent at the time tacrolimus was started. With close monitoring, they targeted a tacrolimus trough of 8–12 ng/mL. At 30 days, 66% had a clinical response, and 40% were in clinical remission. At 90 days, 60% had a clinical response, and 37% were in clinical remission. At 1 year, 31% had a clinical response, and 23% were in clinical remission. Of those in clinical remission at 1 year, 88% were either off of steroids or on less than 5 mg of prednisone per day. Renal impairment was seen in 25% of patients, including severe renal impairment in 11%, requiring drug cessation. Infectious complications were seen in 9% of patients. Headaches, tremor, and pancreatitis were also observed, though less commonly. The majority of patients ultimately had a surgical intervention, particularly if they were steroid-refractory at baseline, but the time to surgery was delayed in those who achieved a response or remission in the first 90 days of tacrolimus therapy. The authors suggested that while tacrolimus may lack clear long-term benefit in patients with medically refractory IBD, a therapeutic trial should be considered in select patients with the goal of medical and nutritional optimization before surgical intervention [26].
Cyclosporine
Cyclosporine, which also exerts its effect by inhibiting IL-2 production, has an established role in the management of severe ulcerative colitis. Data from randomized, placebo-controlled trials, along with numerous open label observational studies, have shown that intravenous cyclosporine can induce remission and potentially obviate the need for urgent/emergent colectomy in steroid-refractory patients who are hospitalized with severe ulcerative colitis [27,28]. Its use in maintenance therapy remains controversial, however. Older observational data suggest that even among those who have an initial clinical response to cyclosporine induction, 33% will undergo colectomy by 1 year, and 88% will undergo colectomy by 7 years [27). Though the concomitant administration of a thiopurine may delay the need for colectomy [29,30], cyclosporine seems to be, at best, a temporizing therapy for patients with severe ulcerative colitis. Studies on the use of cyclosporine for the induction of remission in Crohn’s disease have been less robust, as have studies on the use of cyclosporine for the maintenance of remission in Crohn’s disease [31]. Dose-dependent toxicity also remains a concern, particularly when being considered as maintenance therapy. Though some observational data suggest that the absolute risks of serious side effects from maintenance cyclosporine are small, cyclosporine is still generally avoided as a maintenance therapy [30].
Mycophenolate Mofetil
Mycophenolate mofetil (MMF), which inhibits both B and T cell proliferation by inhibiting de novo purine synthesis, has been studied in both Crohn’s disease and ulcerative colitis. Studies have been small, observational, and heterogeneous. On the whole, they suggest that MMF does have some efficacy, but it is not necessarily more effective than azathioprine and may have a slightly increased risk of side effects [32]. Given that the side effects of MMF include diarrhea, and an IBD-like enterocolitis (MMF-induced colitis) when given to subjects without an established diagnosis of IBD, it is likely best to avoid using the drug in patients with IBD [33].
Surgery
Finally, when medical management has failed, or when fibrostenotic and/or penetrating complications of inflammatory bowel disease are present, surgery should be considered. Surgery can provide a cure in patients with ulcerative colitis, and can induce remission in patients with Crohn’s disease. Managing IBD medications around the time of surgery is always challenging. Multiple large, retrospective cohort studies have suggested that the risk for postoperative infectious complications, anastomotic leaks, and thrombotic complications do not differ between those who receive anti-TNF therapy within several months of surgery and those who do not. Nevertheless, some surgeons may prefer to time elective surgery halfway between doses of anti-TNF therapy. Additionally, there is some data to suggest that patients who are on both thiopurines and anti-TNF agents have an increased risk of postoperative complications compared to those who are on anti-TNF agents alone [34].
After a surgical evaluation, a plan of action should be formulated in a multidisciplinary fashion to determine how medical management will proceed. For those with an established diagnosis of ulcerative colitis, medical therapy can often be stopped postoperatively and the patient can be monitored prospectively for pouch complications including possible new-onset Crohn’s disease. For those who undergo surgery for the management of Crohn’s disease, though a resection completed with negative margins does induce remission, nearly 90% can be expected to have histologic, endoscopic, or clinical recurrence by 1 year. A randomized controlled trial showed that postoperative anti-TNF therapy can reduce this risk to 9% [35]. Unfortunately, a subsequently conducted large, multicenter, randomized controlled trial comparing postoperative infliximab to placebo was terminated early because of a lack of a statistically significant difference in clinical recurrence between the 2 groups at week 74. However, this lack of demonstrated efficacy may have been obscured by the relatively mild phenotype of the enrolled participants, who had a median CDAI score of 105.5 at baseline [36]. Based on available data, the AGA does conditionally recommend postoperative anti-TNF and/or thiopurine therapy for those patients with Crohn’s disease who are in a surgically induced remission [37]. The patients who are most likely to benefit from postoperative medical therapy are those who have the highest risk of recurrence, namely those who were young at the time of diagnosis, had a short disease duration prior to surgery, have multiple sites of disease, and who use tobacco products [34].
Emerging and Future Options
Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Based on our improved, and evolving understanding of the pathogenesis of IBD, several new biochemical targets have emerged, offering novel ways to modulate the cytokine cascade which drives IBD [38]. Well over a dozen phase II and phase III trials for IBD therapeutic agents are ongoing, including biologic agents targeting interleukin-23, β7-Integrin, and MAdCAM-1, as well as small molecule agents targeting the JAK/STAT pathway and the sphingosine-1-phosphate receptor modulators [39]. As new agents are approved, it may be possible to develop a more patient-centered approach to care by targeting therapies to the particular pathogenesis of each patient’s disease. Nevertheless, integrating these therapies into practice algorithms will remain a challenge in the absence of meaningful comparative effectiveness trials [40].
Conclusion
When evaluating a patient who seems to have failed anti-TNF therapy for IBD, the first step is to confirm that active inflammatory disease is present. This process includes ruling out other potential causes of the patient’s symptoms, including infectious colitis, and ideally includes obtaining objective evidence of inflammation, whether through non-invasive biomarkers, an endoscopic evaluation and/or cross-sectional imaging. Once active IBD is confirmed, reactive therapeutic drug monitoring can help elucidate the likely mechanism of drug failure, which in turn can guide medical decision making.
Corresponding author: Anita Afzali MD, MPH, The Ohio State University Wexner Medical Center, 395 West 12th Ave, Room 280, Columbus, OH 43210, [email protected].
Financial disclosures: Dr. Afzali has served as a speaker/consultant for Abbvie, UCB, Takeda, Pfizer, Janssen; on the advisory board of Abbvie, UCB; received grant support from UCB; and is a board member of IBD Horizons.
1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78.
2. Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 2012;61:918–32.
3. Mitrev N, Leong RW. Therapeutic drug monitoring of anti-tumour necrosis factor-α agents in inflammatory bowel disease. Expert Opin Drug Saf 2017;16:303–17.
4. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015;21:182–97.
5. Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015;148:37–51.
6. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis 2010;4:355–66.
7. Mosli MH, Zou G, Garg SK, et al. C-Reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:802–19.
8. Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–34.
9. Vande Casteele N, Herfarth H, Katz J, et al. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology 2017;153:835–57.
10. López-Hernández R, Valdés M, Campillo JA, et al. Genetic polymorphisms of tumour necrosis factor alpha (TNF-α) promoter gene and response to TNF-α inhibitors in Spanish patients with inflammatory bowel disease. Int J Immunogenet 2014;41:63–8.
11. Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012;91:635–46.
12. Hindryckx P, Novak G, Vande Casteele N, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: a practical overview. Drugs 2017;77:363–77.
13. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95.
14. Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther 2009;30:210–26.
15. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9.
16. Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–8.
17. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27.
18. Afif W, Loftus EV Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9.
19. Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut 2017.
20. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther 2015;41:613–23.
21. Gisbert JP, Chaparro M. Use of a third anti-TNF after failure of two previous anti-TNFs in patients with inflammatory bowel disease: is it worth it? Scand J Gastroenterol 2015;50:379–86.
22. Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012;10:1079–87.
23. Kawalec P, Moćko P. An indirect comparison of ustekinumab and vedolizumab in the therapy of TNF-failure Crohn’s disease patients. J Comp Eff Res 2017;7:101–11.
24. Sandborn WJ, Su C, Panes J, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:1723–36.
25. Yarkoni S, Sagiv Y, Kaminitz A, Askenasy N. Interleukin 2 targeted therapy in inflammatory bowel disease. Gut 2009;58:1705–6.
26. Thin LW, Murray K, Lawrance IC. Oral tacrolimus for the treatment of refractory inflammatory bowel disease in the biologic era. Inflamm Bowel Dis 2013;19:1490–8.
27. Moskovitz DN, Van Assche G, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:760–5.
28. Arts J, D’Haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis 2004;10:73–8.
29. Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol 1999;94:1587–92.
30. Cheifetz AS, Stern J, Garud S, et al. Cyclosporine is safe and effective in patients with severe ulcerative colitis. J Clin Gastroenterol 2011;45:107–12.
31. Lazarev M, Present DH, Lichtiger S, et al. The effect of intravenous cyclosporine on rates of colonic surgery in hospitalized patients with severe Crohn’s colitis. J Clin Gastroenterol 2012;46:764–7.
32. Renna S, Cottone M, Orlando A. Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease. World J Gastroenterol 2014;20:9675–90.
33. Izower MA, Rahman M, Molmenti EP, et al. Correlation of abnormal histology with endoscopic findings among mycophenolate mofetil treated patients. World J Gastrointest Endosc 2017;9:405–10.
34. Ferrari L, Krane MK, Fichera A. Inflammatory bowel disease surgery in the biologic era. World J Gastrointest Surg 2016;8:363–70.
35. Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441–50.
36. Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology 2016;150:1568–78.
37. Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute technical review on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:277–95.
38. Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology 2017;152:374–88.
39. Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci 2017;38:127–42.
40. Khanna R, Feagan BG. Emerging therapies for inflammatory bowel diseases. Dig Dis 2016;34 Suppl 1:67–
1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78.
2. Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 2012;61:918–32.
3. Mitrev N, Leong RW. Therapeutic drug monitoring of anti-tumour necrosis factor-α agents in inflammatory bowel disease. Expert Opin Drug Saf 2017;16:303–17.
4. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015;21:182–97.
5. Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015;148:37–51.
6. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis 2010;4:355–66.
7. Mosli MH, Zou G, Garg SK, et al. C-Reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:802–19.
8. Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–34.
9. Vande Casteele N, Herfarth H, Katz J, et al. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology 2017;153:835–57.
10. López-Hernández R, Valdés M, Campillo JA, et al. Genetic polymorphisms of tumour necrosis factor alpha (TNF-α) promoter gene and response to TNF-α inhibitors in Spanish patients with inflammatory bowel disease. Int J Immunogenet 2014;41:63–8.
11. Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012;91:635–46.
12. Hindryckx P, Novak G, Vande Casteele N, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: a practical overview. Drugs 2017;77:363–77.
13. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95.
14. Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther 2009;30:210–26.
15. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9.
16. Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–8.
17. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27.
18. Afif W, Loftus EV Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9.
19. Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut 2017.
20. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther 2015;41:613–23.
21. Gisbert JP, Chaparro M. Use of a third anti-TNF after failure of two previous anti-TNFs in patients with inflammatory bowel disease: is it worth it? Scand J Gastroenterol 2015;50:379–86.
22. Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012;10:1079–87.
23. Kawalec P, Moćko P. An indirect comparison of ustekinumab and vedolizumab in the therapy of TNF-failure Crohn’s disease patients. J Comp Eff Res 2017;7:101–11.
24. Sandborn WJ, Su C, Panes J, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:1723–36.
25. Yarkoni S, Sagiv Y, Kaminitz A, Askenasy N. Interleukin 2 targeted therapy in inflammatory bowel disease. Gut 2009;58:1705–6.
26. Thin LW, Murray K, Lawrance IC. Oral tacrolimus for the treatment of refractory inflammatory bowel disease in the biologic era. Inflamm Bowel Dis 2013;19:1490–8.
27. Moskovitz DN, Van Assche G, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:760–5.
28. Arts J, D’Haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis 2004;10:73–8.
29. Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol 1999;94:1587–92.
30. Cheifetz AS, Stern J, Garud S, et al. Cyclosporine is safe and effective in patients with severe ulcerative colitis. J Clin Gastroenterol 2011;45:107–12.
31. Lazarev M, Present DH, Lichtiger S, et al. The effect of intravenous cyclosporine on rates of colonic surgery in hospitalized patients with severe Crohn’s colitis. J Clin Gastroenterol 2012;46:764–7.
32. Renna S, Cottone M, Orlando A. Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease. World J Gastroenterol 2014;20:9675–90.
33. Izower MA, Rahman M, Molmenti EP, et al. Correlation of abnormal histology with endoscopic findings among mycophenolate mofetil treated patients. World J Gastrointest Endosc 2017;9:405–10.
34. Ferrari L, Krane MK, Fichera A. Inflammatory bowel disease surgery in the biologic era. World J Gastrointest Surg 2016;8:363–70.
35. Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441–50.
36. Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology 2016;150:1568–78.
37. Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute technical review on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:277–95.
38. Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology 2017;152:374–88.
39. Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci 2017;38:127–42.
40. Khanna R, Feagan BG. Emerging therapies for inflammatory bowel diseases. Dig Dis 2016;34 Suppl 1:67–
The Management of Hypertension in Elderly Patients with Chronic Kidney Disease
From the Division of Nephrology, University of New Mexico Health Sciences Center, Albuquerque, NM.
Abstract
- Objective: To review the available literature regarding hypertension and chronic kidney disease (CKD) in the elderly and provide a framework for clinical management of hypertension in this subset of the elderly population.
- Methods: Review of the available literature.
- Results: Though several large, well-designed randomized trials exist examining the treatment of isolated hypertension in the elderly, these trials have uniformly excluded patients with CKD, thus reducing the generalizability of these results to this subgroup. CKD in the elderly is poorly studied overall, and whether CKD in the elderly is an expected product of senescence or a pathology from modifiable risk factors is debatable. Concern exists regarding the increased potential of acute kidney injury events and a more rapid progression of CKD with more aggressive hypertension lowering in elderly patients.
- Conclusion: Though data is limited regarding hypertension treatment in the subset of elderly patients with CKD, given the consistent benefits in cardiovascular reduction with hypertension treatment in the general elderly population, it is likewise recommended that elderly patients with hypertension and CKD receive antihypertensive therapy, though with more careful monitoring for adverse renal effects. We provide a practical approach to management for this clinical scenario.
Chronic kidney disease (CKD) is an increasingly recognized finding in elderly patients, with approximately half of all patients over the age of 70 meeting the most common currently accepted definition of CKD stage III, an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2 [1]. Whether this finding is a result of normal physiologic aging or whether it represents a true disease process in elderly patients has been a matter of considerable debate [2–4]. Nonetheless, the decline in eGFR in elderly patients has important implications regarding drug dosing and the potential risk of acute kidney injury (AKI) in this population [5–9]. Additionally, elderly patients with reduced GFR may have an increased risk of cardiovascular events and progression to end-stage kidney disease (ESKD), though extensive studies are lacking in this population [10–13].
In contrast, isolated hypertension and its treatment in the elderly population has now been extensively evaluated in several well-designed, prospective randomized studies, with generally favorable results arguing for the treatment of hypertension in elderly individuals [14–17]. Unfortunately, however, these studies have uniformly excluded patients with CKD in their study designs. Thus, the impact of aggressive hypertension management in elderly patients with CKD is unknown. As a considerable proportion of CKD in this population has been felt secondary to vascular disease and poor overall vascular health, many have questioned whether aggressive blood pressure reduction, particularly in patients with wide pulse pressure as an indicator of vascular disease, may result in decreased overall renal perfusion and greater risk for AKI, and thus potentially accelerate renal decline in this population [18–22].
In this paper, we review the epidemiology and physiology of renal disease in the elderly, provide an analysis of the available data regarding management of hypertension in the elderly, and suggest an approach to management of hypertension in this specific patient population. Though a multitude of age cutoffs defining elderly have been proposed, for the purposes of this paper we define elderly as age greater than 65 years unless otherwise specified.
Definition of CKD
The currently accepted definition of CKD represents any composite of pathology resulting in impaired kidney function, defined as a drop of GFR < 60 mL/min/1.73 m2 for 3 months or longer, or a higher GFR but with evidence of structural or functional abnormalities, such as proteinuria [23]. However, there are key aspects and important limitations to the above diagnostic criteria to be considered in the elderly patient population. Importantly, the most commonly used GFR estimation equations use serum creatinine as the marker for impaired renal function. As serum creatinine levels are also determined by overall muscle mass, significant error in estimating GFR can occur using these equations in elderly patients, who may have widely varying degrees of musculature and thus creatinine production. Additionally, these equations were often derived using all CKD or mostly CKD patients, which may result in healthy individuals having a higher GFR at the same serum creatinine levels than CKD patients, thus incorrectly classifying many patients with normal kidney function as having CKD [24].
Serum cystatin C has been proposed as an alternative surrogate marker for impaired kidney function, particularly in the elderly, as it is not affected by muscle mass. However, cystatin C levels are affected by obesity, inflammation, and atherosclerosis, and thus equations using this marker to determine GFR also face some limitations in the elderly population [25]. Evidence comparing various GFR estimating equations in the elderly suggest that formulas that use a combination of serum creatinine and cystatin C do best at predicting GFR when compared to gold standard techniques, such as iohexol clearance, though it is important to note that yet the ideal GFR estimating equation for elderly patients has not been determined [26–28].
It has been suggested that given these limitations and potential to underestimate GFR in the elderly population, a lower GFR reference range of 45 mL/min/1.73 m2 be used in the absence of other signs of kidney damage given the multiple unique characteristics of the aging kidneys, as we will explore in this review [29]. In general, we are in agreement with this suggestion that all elderly patients with a creatinine based estimated GFR of < 45 mL/min/1.73 m2 can safely be assumed to have CKD, and it is our opinion that elderly patients with a GFR > 45 mL/min/1.73 m2 but less < 60 mL/min/1.73 m2, without other signs of structural of functional renal disease such as proteinuria, have additional evaluation for the presence of impaired renal function, including but not limited to the addition of cystatin C to estimate GFR.
Epidemiology of CKD in the Elderly
According to the Centers for Disease Control and Prevention (CDC), the number of elderly patients in the United States is expected to double in the next 25 years to 72 million patients, representing approximately 20% of the adult population by 2030 [30]. Analysis of the National Health and Nutrition Examination Surveys (NHANES) from 1999–2004 revealed an overall prevalence of CKD in the US population of 13.1%. However, when sub-grouped into patients greater than or equal to 70 years of age, the prevalence of CKD in this population increased to a staggering 47.5% [31]. Likewise, analysis of other elderly populations from Canada, China, Italy, and Spain indicated a roughly 3- to 7-fold increase in CKD prevalence in those elderly populations compared to younger patients [3]. Additionally, according to the United States Renal Data System there is evidence of a progressive rise in the number of end-stage renal disease (ESRD) patients enrolled in Medicare-funded programs over the past decades [32]. In extrapolating these estimates, it is conceivable to predict that approximately 30 million elderly patients may have CKD in the United States by year 2030, with enormous implications to treatment recommendations and healthcare associated costs.
The Aging Kidney and Expected Rate of Nephron Loss
A progressive, age-related decline in GFR has been demonstrated in many studies. In an earlier analysis of the Baltimore Longitudinal Study of Aging by Lindeman et al, a decline in measured creatinine clearance of 0.75 mL/min/year was demonstrated. It is important to note that in this analysis, patients with suspected pre-existing renal or urologic disease and those on diuretics or other antihypertensives were excluded from analysis, and a normal Gaussian distribution of creatinine clearance slopes versus time was demonstrated, suggesting the GFR loss was a process of normal aging [4].
In support of the theory of a physiologic age-related decline in renal function, a study by Rule et al analyzed potential kidney transplant donors for age-related decline in renal function and determined an approximately 6.3 mL/min/1.73 m2 decline in GFR for each decade. In this investigation, core needle biopsies were obtained at the time of donation and transplantation. The investigators found a progressive increase in the histologic prevalence of nephrosclerosis with each age group analyzed, increasing from 2.7% at ages 18 to 29 to 16% for ages 30 to 39, 28% for ages 40 to 49, 44% for ages 50 to 59, 58% for ages 60 to 69, and finally 73% in donors older than age 70. It is important to note that this study only examined live kidney donors, a group heavily screened and selected on the basis of optimal health, thus strongly arguing for progressive renal decline as a consequence of “normal” aging. Furthermore, though controlled hypertensive patients (treated with 2 or less medications) were allowed to be donors in this study, exclusion of this group had only a minimal impact on the findings of the study [33].
However, whether this age-related decline is purely a result of normal senescence or is a consequence of modifiable risk factors that could alter this outcome remains debatable. Additionally, vascular disease is clearly implicated in more accelerated renal decline. This concept was well demonstrated in an analysis of the longitudinal Age, Gene/Environment Susceptibility – Reykjavik Study, which showed that although age was associated with both reduced GFR and albuminuria, reduced GFR and albuminuria in elderly patients (mean age 80.8 yr) was strongly associated with midlife systolic and diastolic blood pressure, thus suggesting that potentially modifiable vascular pathology may play a much stronger role in CKD in the elderly than aging alone [34].
Finally, it has been hypothesized that reduced nephron mass at birth may contribute to CKD in the elderly [29]. Reduced nephron mass appears to be associated with low birth weight and prematurity, and this has been associated with an increased risk for ESRD later in life [35,36].
Hypertension in the Elderly
Pathophysiology
Age-associated hypertension is felt to arise from several mechanisms and hemodynamic changes. Systolic blood pressure has been noted to progressively rise with age, whereas diastolic blood pressure rises to the 5th or 6th decade, after which it appears to slowly decline. This pattern is felt likely secondary to increasing large vessel stiffness from collagen deposition and calcification with aging, and fracturing and degradation of elastin fibers. As large vessels become less distensible, pulse pressure and pulse wave velocity increases with this drop in diastolic BP, with less forward flow seen in diastole, leading to decreased organ perfusion. Additionally and alternatively, concentric left ventricular hypertrophy develops with aging, leading to reduced cardiac output from decreased stroke volume, which may also contribute to reduced organ perfusion [37,38]. These findings have led many to speculate that hypertension in the elderly may actually serve as a protective mechanism to maintain organ perfusion, and have led to great concern regarding excessive lowering of diastolic blood pressure and increasing of pulse pressure in this population with antihypertensive therapy. This theory was initially corroborated with a sub-analysis of the Systolic Hypertension in the Elderly Program (SHEP) where an increase in pulse pressure by 10 mm Hg was accompanied by increased risk of stroke and congestive heart failure in the treatment arm [39]. Nonetheless, the bulk of evidence continues to support a lower overall risk of cardiovascular events with treatment of hypertension in elderly patients, and general expert consensus recommends treatment with gradual reduction to normal levels of systolic blood pressure accompanied by careful monitoring for adverse effects [40,41].
In addition to these above changes, reduced GFR in elderly likewise results in impaired natriuresis, thereby fostering hypertension via volume expansion. Age-related arteriolosclerosis may result in renal artery stenosis, resulting in decreased renal perfusion and upregulation of the renin-angiotensin-aldosterone cascade. Further challenging treatment decisions is the frequent development of autonomic dysregulation in the elderly, a major risk factor for falls and cardiovascular events [40].
The result of these abnormalities is that roughly 65% of patients greater than the age of 60 have at least isolated systolic hypertension [42]. Similarly corresponding to the underlying physiology highlighted above, rising pulse pressure, rather than systolic or diastolic blood pressure, appears to be the greatest risk factor for cardiovascular events in the elderly population [43,44]. In an interesting analysis of the Framingham Heart Study by Franklin et al, the authors noted that in patients < 50 years of age, diastolic blood pressure was the strongest risk factor for events. However, at age 50 to 59, a change occurred where all 3 blood pressure indexes were comparable risk predictors, and then from age 60 years and on pulse pressure became the superior predictor, with diastolic blood pressure being negatively correlated to cardiovascular risk, highlighting the potential importance for organ perfusion during diastole in this group [45].
Likewise, in the elderly population pulse pressure also appears to be inversely related to GFR, suggesting that vascular stiffness and the reduced forward flow in diastole may contribute to microvascular damage and CKD [46]. In elderly patients with untreated isolated systolic hypertension, increasing systolic blood pressure (a reflection of rising pulse pressure) was associated with the greatest risk of renal decline when compared to diastolic blood pressure, pulse, and mean arterial pressure [47]. In the normal state, high renal blood flow and low renal arterial resistance can contribute to regular large intrarenal pressure variations. Because of vascular stiffness, these pressure variations increase with time, increasing up to 4-fold in the elderly compared with young peers, and likely contribute to renal damage seen in older patients [48].
Treatment
In comparison to the paucity of randomized trials examining CKD progression in the elderly, 4 very large, well designed randomized trials (SHEP, MRC trial, Syst-Eur trial, and HYVET) specifically examining the treatment of hypertension in the elderly have now been conducted [14–17] and confirmed earlier and smaller trials demonstrating the benefits of treatment of hypertension in the elderly [49,50]. In addition to this, several of the other large landmark hypertension trials such as ALLHAT, ACCOMPLISH, and the SPRINT trial included a considerable number of elderly patients [51–53]. Though the primary aim of those trials was not to determine the effects of hypertension treatment in the elderly per se, sub-analysis of this population in these trials has further added to our knowledge of this condition.
In the largest initial trial of hypertension in the elderly (SHEP), the researchers randomized 4376 patients over the age of 60 with an average blood pressure of 170/77 mm Hg into a treatment versus placebo arm. Such a study would be inconceivable today due to the consistent benefit derived from antihypertensive therapy now demonstrated in multiple trials. An achieved systolic blood pressure of 143 mm Hg in the treatment arm versus 155 mm Hg in the placebo arm was obtained. Stroke and nonfatal cardiac events were significantly reduced with treatment. The development of renal dysfunction occurred in 7 patients in the treatment arm and 11 patients in the placebo arm, a nonsignificant difference. As we have noted previously, however, patients with pre-existing kidney disease were excluded from the study [14]. A subsequent analysis of the SHEP trial results by Vaccarino et al, however, showed that in patients on treatment who developed an increase in pulse pressure of 10 mm Hg or more carried a 23% higher risk for developing heart failure and a 24% higher risk for stroke. This effect was not seen in the placebo arm [39].
Shortly following the publication of the SHEP results, the Medical Research Council trial of treatment of hypertension in older adults (MRC) further confirmed the initial findings by demonstrating a 25% reduction in stroke and a 17% reduction in all cardiac events in 4396 patients aged 65 to 74 with a systolic blood pressure greater than 160 mm Hg randomized to treatment of hypertension with either atenolol or a diuretic combination of amiloride and hydrochlorothiazide versus placebo. Like SHEP, however, patients with pre-existing renal disease were excluded, and no report of renal outcomes was published in the initial results [15]. Similarly, the Systolic Hypertension in Europe Trial (Syst-Eur) revealed a 42% reduction in stroke and a 26% reduction in all cardiac endpoints in 4695 patients with a systolic blood pressure of greater than 160 mm Hg randomized to receive nitrendipine with addition of enalapril and hydrochlorothiazide as required. However, CKD patients were likewise excluded in this trial [16].
Finally, the Hypertension in the Very Elderly Trial (HYVET) was unique in that it sought to enroll only patients greater than 80 years of age, a significant departure from the earlier hypertension in elderly trials. This trial randomized 3845 patients, again with a systolic blood pressure of 160 mm Hg or greater, to a placebo arm versus a treatment arm of the thiazide type diuretic indapamide, with addition of the ACE inhibitor perindopril if blood pressure was still greater than 150 mm Hg on monotherapy. Despite the older age of the participants in this trial, patients still benefited from blood pressure reduction with a 30% reduction in rate of stroke, a 21% reduction in the rate of death from any cause, and an impressive 64% reduction in the rate of heart failure [17]. These findings from HYVET, combined with the earlier SHEP, MRC and Syst-Eur trials, confirmed that treatment of hypertension in the elderly of any age should be attempted.
Recomendations for Managing Hypertension in the Elderly with CKD
Though a lack of data exists regarding the treatment of hypertension in elderly patients with the comorbidity of CKD, given the consistent and robust data that exists demonstrating a reduction in cardiovascular risk and mortality in the general elderly population without renal impairment, it is our opinion that elderly patients with CKD and hypertension should receive antihypertensive treatment. This opinion is supported by the fact that in the recently published SPRINT trial, 28.1% of patients in the standard treatment arm (targeting a blood pressure of less than 140 mm Hg), and 28.4% of patients in the intensive treatment arm (blood pressure target less than 120 mm Hg) had CKD, and similarly 28.2% of the trial participants in each group were greater than the age of 75. The percentage of patients with both CKD and age greater than 75 years was not reported in the initial trial results, though it is assumed a significant portion of these patients had both CKD and age greater than 75 years. It is nonetheless reassuring that patients with CKD in the SPRINT trial, as well as those with age > 75 years, both seemed to derive the same benefit in cardiovascular and mortality benefit in the intensive treatment arm compared to the standard treatment arm [53].
It should be noted, however, that though cardiovascular events and mortality were lower in the more intensive treatment arm of the SPRINT trial, CKD progression did not differ between the two treatment groups. Additionally, the risk of acute kidney injury was significantly greater in the intensive treatment arm when compared to the standard treatment arm, with 3.8% of patients in the intensive treatment arm suffering AKI compared to 2.3% in the standard arm [22]. Thus, it should be understood by both the clinician and the elderly patient with hypertension and CKD that the goal of more aggressively lowering blood pressure is to prevent cardiovascular events and not slow renal disease progression.
The recently published 2017 hypertension guidelines by the American College of Cardiology/American Heart Association is the most comprehensive set of hypertension treatment recommendations published to date and includes a section regarding patients with CKD as well as a section on the elderly [54]. Regarding CKD, the guidelines recommend a goal blood pressure of less than 130/80 mm Hg in patients with CKD, and that patients with macroalbuminuria (defined as a daily urine protein excretion of greater than 300 mg/dL or a urine albumin to creatinine ratio of 300 mg/g) be treated with and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB). We feel these are reasonable recommendations for CKD targets and agree with the guideline, with the understanding that the target of 130/80 mm Hg is based largely on the SPRINT data. It is important to recognize that in the Action to Control Cardiovascular Risk in Diabetes trial (ACCORD), a more intensive blood pressure target of 120 mm Hg did not result in further improvement in cardiovascular events compared to a traditional target of 140/90 mm Hg [55]. However, given the larger and more robust sample size from SPRINT, we feel the target of 130/80 mm Hg is warranted and therefore should be the first target for elderly patients with CKD. With this goal in mind, it has been our clinical experience that some elderly patients with CKD have difficulty tolerating this goal, either from the development of worsening of GFR, acute kidney injury events, or due to orthostatic hypotension. Additionally, it should be noted that patients with orthostatic hypotension were excluded from SPRINT, though an increase in falls was not seen in the primary study. Therefore, for patients who are unable to tolerate the SPRINT goal of 130/80 mm Hg, an individualized goal of at least less than 160 mm Hg systolic and ideally less than 140 mm Hg, reflecting achieved blood pressure endpoints from earlier trials, may be a reasonable alternative [55]. The recent hypertension guidelines also recommend that for elderly adults with a high burden of comorbidities or limited life expectancy, “clinical judgement, patient preference, and at team-based approach to risk/benefit is reasonable for decisions regarding intensity of BP lowering and choice of antihypertensive drugs.” We agree that all treatment decisions must be individualized based upon each patient’s clinical scenario, and that a guideline is only a general aid for treatment decisions, not a mandate for care.
Therefore, with the acknowledgement that there is a lack of literature specifically examining blood pressure goals in elderly patients with CKD, it is our opinion based on available evidence that the following suggestions constitute a reasonable approach to this scenario: (1) a blood pressure target of less than 130/80 mm Hg should be sought as the primary blood pressure target; (2) if the patient cannot tolerate this due to rapidly declining GFR, acute kidney injury, orthostatic hypotension and or falls; or in other situations where this is not a practical a goal, individualized goal of at ideally less than 140 mm Hg, though at least less than 160 mm Hg systolic, could be considered; (3) the clinician should attempt careful and gradual reduction of blood pressure, with no more than one agent added or one escalation of medication dose attempted per visit; (4) the patient should have close follow up-after medication changes with an adjustment period of at least 4 weeks before additional medication or dose escalations are made; (5) if CKD is accompanied by albuminuria (daily urine protein excretion of greater than 300 mg/dL or a urine albumin to creatinine ratio of 300 mg/g) an ACEI or ARB should be used in management; (6) a rise in serum creatinine of up to 30% of baseline after addition of an ACEI may be acceptable; however, a rise greater than this amount should prompt discontinuation of the drug and evaluation for renal artery stenosis; (7) frequent monitoring of creatinine is required, with repeat chemistry performed after medication adjustments; (8) patients with a high pulse pressure should be monitored especially closely for symptoms or changes in renal function; and finally (9) individualized treatment and clinical judgement, with the patient being an informed participant, should take priority over all other recommendations and guidelines. We feel that further research in this growing subgroup of elderly patients is needed and will be sought, and we expect recommendations will continue to evolve as future literature becomes available.
Corresponding author: Jonathan G. Owen, MD, MSC04 2785, 1 University of New Mexico, Albuquerque, NM 87131, [email protected].
Financial disclosures: None.
Author contributions: conception and design, JGO; analysis and interpretation of data, KA, FXR, JGO; drafting of article, KA, FXR, JGO; critical revision of the article, KA, FXR, JGO; collection and assembly of data, KA, FXR, JGO.
1. Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 2012;157:471–81.
2. Wetzels JF, Kiemeney LA, Swinkels DW, et al. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int 2007;72:632–7.
3. Minutolo R, Borrelli S, De Nicola L. CKD in the elderly: kidney senescence or blood pressure-related nephropathy? Am J Kidney Dis 2015;66:184–6.
4. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985; 33:278–85.
5. Gill J, Malyk R, Djurdiev O, Levin A. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group – a cautionary tale. Nephrol Dial Transplant 2007;22:2894–9.
6. Spruill WJ, Wade WE, Cobb HH 3rd. Comparison of estimated glomerular filtration rate with estimated creatinine clearance in the dosing of drugs requiring adjustments in elderly patients with declining renal function. Am J Geriatr Pharmacother 2008;6:153–60.
7. Dowling TC, Wang ES, Ferruci L, Sorkin JD. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: impact on renal drug dosing. Pharmacotherapy 2013;33:912–21.
8. Ballew SH, Chen Y, Daya NR, Godino JG, Windham BG, McAdams-DeMarco M, Coresh J, Selvin E, Grams ME. Frailty, kidney function, and polypharmacy: the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis 2017;69:228–36.
9. Grams ME, Sang Y, Ballew SH, et al; CKD Prognosis Consortium. A meta-analysis of the association of estimated GFR, albuminuria, race, and sex with acute kidney injury. Am J Kidney Dis 2015;66:591–601.
10. Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011;80:93–104.
11. Masson P, Webster AC, Hong M, et al. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant 2015;30:1162–9.
12. Tellez-Plaza M, Orozco-Beltran D, Gil-Guillen V, et al; ESCARVAL STUDY GROUP. Renal function and attributable risk of death and cardiovascular hospitalization in patients with cardiovascular risk factors from a registry-based cohort: the Estudio Cardiovascular Valencia-risk study. J Hypertens 2016;34:2266–73.
13. Smink PA, Lambers-Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012;60:804–11.
14. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the systolic hypertension in the elderly program (SHEP). JAMA 1991;265:3255–64.
15. Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ 1992;304:405–12.
16. Staessen JA, Faggard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997;350:757–64.
17. Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–98.
18. Fesler P, Safar ME, du Cailar G, et al. Pulse pressure is an independent determinant of renal function decline during treatment of essential hypertension. J Hypertens 2007;25:1915–20.
19. Weir MR, Dworkin LD. Antihypertensive drugs, dietary salt, and renal protection: how low should you go and with which therapy? Am J Kidney Dis 1998;32:1–22.
20. Obi Y, Kalantar-Zadeh K, Shintani A, et al. Estimated glomerular filtration rate and the risk-benefit profile of intensive blood pressure control amongst nondiabetic patients: a post hoc analysis of a randomized clinical trial. J Intern Med 2017;283:314–27.
21. Peralta CA, McClure LA, Scherzer R, et al. Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: a post hoc analysis of the secondary prevention of small subcortical strokes (SPS3) randomized trial. Circulation 2016;133:584–91.
22. Rocco MV, Sink KM, Lovato LC, et al; SPRINT Research Group. Effects of intensive blood pressure treatment on acute kidney injury events in the systolic blood pressure intervention trial (SPRINT). Am J Kidney Dis 2018;71:352–61.
23. Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013;3:19–62.
24. Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 2004;141:929–37.
25. Stevens LA, Schmid CH, Green T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 2009;75:652–60.
26. Biork J, Grubb A, Gudnason V, et al. Comparison of glomerular filtration rate estimating equations derived from creatinine and cystatin C: validation in the age, gene/environment susceptibility-Reykjavik elderly cohort. Nephrol Dial Transplant 2017.
27. Bevc S, Hojs N, Hois R, et al. Estimation of glomerular filtration rate in elderly chronic kidney disease patients: comparison of three novel sophisticated equations and simple cystatin C equation. Therapeutic Apheresis and Dialysis 2017;21:126–32.
28. Pottel H, Delanaye P, Schaeffner E, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant 2017;32:497–507.
29. Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis 2016;23:19–28.
30. US Department of Health and Human Services, Centers for Disease Control and Prevention. The state of aging and health in America 2013. Centers for Disease Control and Prevention website. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf. Published 2013. Accessed April 5, 2018.
31. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47.
32. United Stated Renal Data System. USRD 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. United States Renal Data System website. https://www.usrds.org/atlas13.aspx . Published 2013. Accessed April 5, 2018.
33. Rule AD, Amer H, Cornell LD, et al. The association between age and nephroclerosis on renal biopsy among healthy adults. Ann Intern Med 2010;152:561–7.
34. Inker LA, Okparavero A, Tighiouart H, et al. Midlife blood pressure and late-life GFR and albuminuria: an elderly general population cohort. Am J Kidney Dis 2015;66:240–8.
35. Vikse BE, Irgens LM, Leivestad T, et al. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 2008;19:151–7.
36. Luyckx VA, Bertram JF, Brenner BM, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013;382:273–83.
37. Franklin SS, Gustin W 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96:308–15.
38. Messerli FH, Sundgaard-Riise K, Ventura HO, et al. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983;2:983–6.
39. Vaccarino V, Berger AK, Abramson J, et al. Pulse pressure and risk of cardiovascular events in the systolic hypertension in the elderly program. Am J Cardiol 2001;88:980–6.
40. Aronow WS, Harrington RA, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Forciea MA, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents. Circulation 2011;123:2434–506.
41. Chaudhry SI, Krumholz HM, Foody JM. Systolic hypertension in older persons. JAMA 2004;292:1074–80.
42. Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension 1995;25: 305–13.
43. Blacher J, Staessen JA, Girerd X, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med 2000;160:1085–90.
44. Franklin SS, Lopez VA, Wong ND, et al. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation 2009;119:243–50.
45. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001;103:1245–9.
46. Verhave JC, Fesler P, du Cailar G, et al. Elevated pulse pressure is associated with low renal function in elderly patients with isolated systolic hypertension. Hypertension 2005;45:586–91.
47. Young JH, Klaq MJ, Muntner P, et al. Blood pressure and decline in kidney function: findings from the systolic hypertension in elderly program (SHEP). J Am Soc Nephrol 2002;13:2776–82.
48. O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–4.
49. Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986;293:1145–8.
50. Dahlof B, Lindholm LH, Hansson L, et al. Morbidity and mortality in the Swedish trial in old patients with hypertension (STOP-Hypertension). Lancet 1991;338:1281–5.
51. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2002;288:2981–97.
52. Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators. Benazapril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008;359:2417–28.
53. SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373: 2103–16.
54. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 2017.
55. ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85.
From the Division of Nephrology, University of New Mexico Health Sciences Center, Albuquerque, NM.
Abstract
- Objective: To review the available literature regarding hypertension and chronic kidney disease (CKD) in the elderly and provide a framework for clinical management of hypertension in this subset of the elderly population.
- Methods: Review of the available literature.
- Results: Though several large, well-designed randomized trials exist examining the treatment of isolated hypertension in the elderly, these trials have uniformly excluded patients with CKD, thus reducing the generalizability of these results to this subgroup. CKD in the elderly is poorly studied overall, and whether CKD in the elderly is an expected product of senescence or a pathology from modifiable risk factors is debatable. Concern exists regarding the increased potential of acute kidney injury events and a more rapid progression of CKD with more aggressive hypertension lowering in elderly patients.
- Conclusion: Though data is limited regarding hypertension treatment in the subset of elderly patients with CKD, given the consistent benefits in cardiovascular reduction with hypertension treatment in the general elderly population, it is likewise recommended that elderly patients with hypertension and CKD receive antihypertensive therapy, though with more careful monitoring for adverse renal effects. We provide a practical approach to management for this clinical scenario.
Chronic kidney disease (CKD) is an increasingly recognized finding in elderly patients, with approximately half of all patients over the age of 70 meeting the most common currently accepted definition of CKD stage III, an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2 [1]. Whether this finding is a result of normal physiologic aging or whether it represents a true disease process in elderly patients has been a matter of considerable debate [2–4]. Nonetheless, the decline in eGFR in elderly patients has important implications regarding drug dosing and the potential risk of acute kidney injury (AKI) in this population [5–9]. Additionally, elderly patients with reduced GFR may have an increased risk of cardiovascular events and progression to end-stage kidney disease (ESKD), though extensive studies are lacking in this population [10–13].
In contrast, isolated hypertension and its treatment in the elderly population has now been extensively evaluated in several well-designed, prospective randomized studies, with generally favorable results arguing for the treatment of hypertension in elderly individuals [14–17]. Unfortunately, however, these studies have uniformly excluded patients with CKD in their study designs. Thus, the impact of aggressive hypertension management in elderly patients with CKD is unknown. As a considerable proportion of CKD in this population has been felt secondary to vascular disease and poor overall vascular health, many have questioned whether aggressive blood pressure reduction, particularly in patients with wide pulse pressure as an indicator of vascular disease, may result in decreased overall renal perfusion and greater risk for AKI, and thus potentially accelerate renal decline in this population [18–22].
In this paper, we review the epidemiology and physiology of renal disease in the elderly, provide an analysis of the available data regarding management of hypertension in the elderly, and suggest an approach to management of hypertension in this specific patient population. Though a multitude of age cutoffs defining elderly have been proposed, for the purposes of this paper we define elderly as age greater than 65 years unless otherwise specified.
Definition of CKD
The currently accepted definition of CKD represents any composite of pathology resulting in impaired kidney function, defined as a drop of GFR < 60 mL/min/1.73 m2 for 3 months or longer, or a higher GFR but with evidence of structural or functional abnormalities, such as proteinuria [23]. However, there are key aspects and important limitations to the above diagnostic criteria to be considered in the elderly patient population. Importantly, the most commonly used GFR estimation equations use serum creatinine as the marker for impaired renal function. As serum creatinine levels are also determined by overall muscle mass, significant error in estimating GFR can occur using these equations in elderly patients, who may have widely varying degrees of musculature and thus creatinine production. Additionally, these equations were often derived using all CKD or mostly CKD patients, which may result in healthy individuals having a higher GFR at the same serum creatinine levels than CKD patients, thus incorrectly classifying many patients with normal kidney function as having CKD [24].
Serum cystatin C has been proposed as an alternative surrogate marker for impaired kidney function, particularly in the elderly, as it is not affected by muscle mass. However, cystatin C levels are affected by obesity, inflammation, and atherosclerosis, and thus equations using this marker to determine GFR also face some limitations in the elderly population [25]. Evidence comparing various GFR estimating equations in the elderly suggest that formulas that use a combination of serum creatinine and cystatin C do best at predicting GFR when compared to gold standard techniques, such as iohexol clearance, though it is important to note that yet the ideal GFR estimating equation for elderly patients has not been determined [26–28].
It has been suggested that given these limitations and potential to underestimate GFR in the elderly population, a lower GFR reference range of 45 mL/min/1.73 m2 be used in the absence of other signs of kidney damage given the multiple unique characteristics of the aging kidneys, as we will explore in this review [29]. In general, we are in agreement with this suggestion that all elderly patients with a creatinine based estimated GFR of < 45 mL/min/1.73 m2 can safely be assumed to have CKD, and it is our opinion that elderly patients with a GFR > 45 mL/min/1.73 m2 but less < 60 mL/min/1.73 m2, without other signs of structural of functional renal disease such as proteinuria, have additional evaluation for the presence of impaired renal function, including but not limited to the addition of cystatin C to estimate GFR.
Epidemiology of CKD in the Elderly
According to the Centers for Disease Control and Prevention (CDC), the number of elderly patients in the United States is expected to double in the next 25 years to 72 million patients, representing approximately 20% of the adult population by 2030 [30]. Analysis of the National Health and Nutrition Examination Surveys (NHANES) from 1999–2004 revealed an overall prevalence of CKD in the US population of 13.1%. However, when sub-grouped into patients greater than or equal to 70 years of age, the prevalence of CKD in this population increased to a staggering 47.5% [31]. Likewise, analysis of other elderly populations from Canada, China, Italy, and Spain indicated a roughly 3- to 7-fold increase in CKD prevalence in those elderly populations compared to younger patients [3]. Additionally, according to the United States Renal Data System there is evidence of a progressive rise in the number of end-stage renal disease (ESRD) patients enrolled in Medicare-funded programs over the past decades [32]. In extrapolating these estimates, it is conceivable to predict that approximately 30 million elderly patients may have CKD in the United States by year 2030, with enormous implications to treatment recommendations and healthcare associated costs.
The Aging Kidney and Expected Rate of Nephron Loss
A progressive, age-related decline in GFR has been demonstrated in many studies. In an earlier analysis of the Baltimore Longitudinal Study of Aging by Lindeman et al, a decline in measured creatinine clearance of 0.75 mL/min/year was demonstrated. It is important to note that in this analysis, patients with suspected pre-existing renal or urologic disease and those on diuretics or other antihypertensives were excluded from analysis, and a normal Gaussian distribution of creatinine clearance slopes versus time was demonstrated, suggesting the GFR loss was a process of normal aging [4].
In support of the theory of a physiologic age-related decline in renal function, a study by Rule et al analyzed potential kidney transplant donors for age-related decline in renal function and determined an approximately 6.3 mL/min/1.73 m2 decline in GFR for each decade. In this investigation, core needle biopsies were obtained at the time of donation and transplantation. The investigators found a progressive increase in the histologic prevalence of nephrosclerosis with each age group analyzed, increasing from 2.7% at ages 18 to 29 to 16% for ages 30 to 39, 28% for ages 40 to 49, 44% for ages 50 to 59, 58% for ages 60 to 69, and finally 73% in donors older than age 70. It is important to note that this study only examined live kidney donors, a group heavily screened and selected on the basis of optimal health, thus strongly arguing for progressive renal decline as a consequence of “normal” aging. Furthermore, though controlled hypertensive patients (treated with 2 or less medications) were allowed to be donors in this study, exclusion of this group had only a minimal impact on the findings of the study [33].
However, whether this age-related decline is purely a result of normal senescence or is a consequence of modifiable risk factors that could alter this outcome remains debatable. Additionally, vascular disease is clearly implicated in more accelerated renal decline. This concept was well demonstrated in an analysis of the longitudinal Age, Gene/Environment Susceptibility – Reykjavik Study, which showed that although age was associated with both reduced GFR and albuminuria, reduced GFR and albuminuria in elderly patients (mean age 80.8 yr) was strongly associated with midlife systolic and diastolic blood pressure, thus suggesting that potentially modifiable vascular pathology may play a much stronger role in CKD in the elderly than aging alone [34].
Finally, it has been hypothesized that reduced nephron mass at birth may contribute to CKD in the elderly [29]. Reduced nephron mass appears to be associated with low birth weight and prematurity, and this has been associated with an increased risk for ESRD later in life [35,36].
Hypertension in the Elderly
Pathophysiology
Age-associated hypertension is felt to arise from several mechanisms and hemodynamic changes. Systolic blood pressure has been noted to progressively rise with age, whereas diastolic blood pressure rises to the 5th or 6th decade, after which it appears to slowly decline. This pattern is felt likely secondary to increasing large vessel stiffness from collagen deposition and calcification with aging, and fracturing and degradation of elastin fibers. As large vessels become less distensible, pulse pressure and pulse wave velocity increases with this drop in diastolic BP, with less forward flow seen in diastole, leading to decreased organ perfusion. Additionally and alternatively, concentric left ventricular hypertrophy develops with aging, leading to reduced cardiac output from decreased stroke volume, which may also contribute to reduced organ perfusion [37,38]. These findings have led many to speculate that hypertension in the elderly may actually serve as a protective mechanism to maintain organ perfusion, and have led to great concern regarding excessive lowering of diastolic blood pressure and increasing of pulse pressure in this population with antihypertensive therapy. This theory was initially corroborated with a sub-analysis of the Systolic Hypertension in the Elderly Program (SHEP) where an increase in pulse pressure by 10 mm Hg was accompanied by increased risk of stroke and congestive heart failure in the treatment arm [39]. Nonetheless, the bulk of evidence continues to support a lower overall risk of cardiovascular events with treatment of hypertension in elderly patients, and general expert consensus recommends treatment with gradual reduction to normal levels of systolic blood pressure accompanied by careful monitoring for adverse effects [40,41].
In addition to these above changes, reduced GFR in elderly likewise results in impaired natriuresis, thereby fostering hypertension via volume expansion. Age-related arteriolosclerosis may result in renal artery stenosis, resulting in decreased renal perfusion and upregulation of the renin-angiotensin-aldosterone cascade. Further challenging treatment decisions is the frequent development of autonomic dysregulation in the elderly, a major risk factor for falls and cardiovascular events [40].
The result of these abnormalities is that roughly 65% of patients greater than the age of 60 have at least isolated systolic hypertension [42]. Similarly corresponding to the underlying physiology highlighted above, rising pulse pressure, rather than systolic or diastolic blood pressure, appears to be the greatest risk factor for cardiovascular events in the elderly population [43,44]. In an interesting analysis of the Framingham Heart Study by Franklin et al, the authors noted that in patients < 50 years of age, diastolic blood pressure was the strongest risk factor for events. However, at age 50 to 59, a change occurred where all 3 blood pressure indexes were comparable risk predictors, and then from age 60 years and on pulse pressure became the superior predictor, with diastolic blood pressure being negatively correlated to cardiovascular risk, highlighting the potential importance for organ perfusion during diastole in this group [45].
Likewise, in the elderly population pulse pressure also appears to be inversely related to GFR, suggesting that vascular stiffness and the reduced forward flow in diastole may contribute to microvascular damage and CKD [46]. In elderly patients with untreated isolated systolic hypertension, increasing systolic blood pressure (a reflection of rising pulse pressure) was associated with the greatest risk of renal decline when compared to diastolic blood pressure, pulse, and mean arterial pressure [47]. In the normal state, high renal blood flow and low renal arterial resistance can contribute to regular large intrarenal pressure variations. Because of vascular stiffness, these pressure variations increase with time, increasing up to 4-fold in the elderly compared with young peers, and likely contribute to renal damage seen in older patients [48].
Treatment
In comparison to the paucity of randomized trials examining CKD progression in the elderly, 4 very large, well designed randomized trials (SHEP, MRC trial, Syst-Eur trial, and HYVET) specifically examining the treatment of hypertension in the elderly have now been conducted [14–17] and confirmed earlier and smaller trials demonstrating the benefits of treatment of hypertension in the elderly [49,50]. In addition to this, several of the other large landmark hypertension trials such as ALLHAT, ACCOMPLISH, and the SPRINT trial included a considerable number of elderly patients [51–53]. Though the primary aim of those trials was not to determine the effects of hypertension treatment in the elderly per se, sub-analysis of this population in these trials has further added to our knowledge of this condition.
In the largest initial trial of hypertension in the elderly (SHEP), the researchers randomized 4376 patients over the age of 60 with an average blood pressure of 170/77 mm Hg into a treatment versus placebo arm. Such a study would be inconceivable today due to the consistent benefit derived from antihypertensive therapy now demonstrated in multiple trials. An achieved systolic blood pressure of 143 mm Hg in the treatment arm versus 155 mm Hg in the placebo arm was obtained. Stroke and nonfatal cardiac events were significantly reduced with treatment. The development of renal dysfunction occurred in 7 patients in the treatment arm and 11 patients in the placebo arm, a nonsignificant difference. As we have noted previously, however, patients with pre-existing kidney disease were excluded from the study [14]. A subsequent analysis of the SHEP trial results by Vaccarino et al, however, showed that in patients on treatment who developed an increase in pulse pressure of 10 mm Hg or more carried a 23% higher risk for developing heart failure and a 24% higher risk for stroke. This effect was not seen in the placebo arm [39].
Shortly following the publication of the SHEP results, the Medical Research Council trial of treatment of hypertension in older adults (MRC) further confirmed the initial findings by demonstrating a 25% reduction in stroke and a 17% reduction in all cardiac events in 4396 patients aged 65 to 74 with a systolic blood pressure greater than 160 mm Hg randomized to treatment of hypertension with either atenolol or a diuretic combination of amiloride and hydrochlorothiazide versus placebo. Like SHEP, however, patients with pre-existing renal disease were excluded, and no report of renal outcomes was published in the initial results [15]. Similarly, the Systolic Hypertension in Europe Trial (Syst-Eur) revealed a 42% reduction in stroke and a 26% reduction in all cardiac endpoints in 4695 patients with a systolic blood pressure of greater than 160 mm Hg randomized to receive nitrendipine with addition of enalapril and hydrochlorothiazide as required. However, CKD patients were likewise excluded in this trial [16].
Finally, the Hypertension in the Very Elderly Trial (HYVET) was unique in that it sought to enroll only patients greater than 80 years of age, a significant departure from the earlier hypertension in elderly trials. This trial randomized 3845 patients, again with a systolic blood pressure of 160 mm Hg or greater, to a placebo arm versus a treatment arm of the thiazide type diuretic indapamide, with addition of the ACE inhibitor perindopril if blood pressure was still greater than 150 mm Hg on monotherapy. Despite the older age of the participants in this trial, patients still benefited from blood pressure reduction with a 30% reduction in rate of stroke, a 21% reduction in the rate of death from any cause, and an impressive 64% reduction in the rate of heart failure [17]. These findings from HYVET, combined with the earlier SHEP, MRC and Syst-Eur trials, confirmed that treatment of hypertension in the elderly of any age should be attempted.
Recomendations for Managing Hypertension in the Elderly with CKD
Though a lack of data exists regarding the treatment of hypertension in elderly patients with the comorbidity of CKD, given the consistent and robust data that exists demonstrating a reduction in cardiovascular risk and mortality in the general elderly population without renal impairment, it is our opinion that elderly patients with CKD and hypertension should receive antihypertensive treatment. This opinion is supported by the fact that in the recently published SPRINT trial, 28.1% of patients in the standard treatment arm (targeting a blood pressure of less than 140 mm Hg), and 28.4% of patients in the intensive treatment arm (blood pressure target less than 120 mm Hg) had CKD, and similarly 28.2% of the trial participants in each group were greater than the age of 75. The percentage of patients with both CKD and age greater than 75 years was not reported in the initial trial results, though it is assumed a significant portion of these patients had both CKD and age greater than 75 years. It is nonetheless reassuring that patients with CKD in the SPRINT trial, as well as those with age > 75 years, both seemed to derive the same benefit in cardiovascular and mortality benefit in the intensive treatment arm compared to the standard treatment arm [53].
It should be noted, however, that though cardiovascular events and mortality were lower in the more intensive treatment arm of the SPRINT trial, CKD progression did not differ between the two treatment groups. Additionally, the risk of acute kidney injury was significantly greater in the intensive treatment arm when compared to the standard treatment arm, with 3.8% of patients in the intensive treatment arm suffering AKI compared to 2.3% in the standard arm [22]. Thus, it should be understood by both the clinician and the elderly patient with hypertension and CKD that the goal of more aggressively lowering blood pressure is to prevent cardiovascular events and not slow renal disease progression.
The recently published 2017 hypertension guidelines by the American College of Cardiology/American Heart Association is the most comprehensive set of hypertension treatment recommendations published to date and includes a section regarding patients with CKD as well as a section on the elderly [54]. Regarding CKD, the guidelines recommend a goal blood pressure of less than 130/80 mm Hg in patients with CKD, and that patients with macroalbuminuria (defined as a daily urine protein excretion of greater than 300 mg/dL or a urine albumin to creatinine ratio of 300 mg/g) be treated with and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB). We feel these are reasonable recommendations for CKD targets and agree with the guideline, with the understanding that the target of 130/80 mm Hg is based largely on the SPRINT data. It is important to recognize that in the Action to Control Cardiovascular Risk in Diabetes trial (ACCORD), a more intensive blood pressure target of 120 mm Hg did not result in further improvement in cardiovascular events compared to a traditional target of 140/90 mm Hg [55]. However, given the larger and more robust sample size from SPRINT, we feel the target of 130/80 mm Hg is warranted and therefore should be the first target for elderly patients with CKD. With this goal in mind, it has been our clinical experience that some elderly patients with CKD have difficulty tolerating this goal, either from the development of worsening of GFR, acute kidney injury events, or due to orthostatic hypotension. Additionally, it should be noted that patients with orthostatic hypotension were excluded from SPRINT, though an increase in falls was not seen in the primary study. Therefore, for patients who are unable to tolerate the SPRINT goal of 130/80 mm Hg, an individualized goal of at least less than 160 mm Hg systolic and ideally less than 140 mm Hg, reflecting achieved blood pressure endpoints from earlier trials, may be a reasonable alternative [55]. The recent hypertension guidelines also recommend that for elderly adults with a high burden of comorbidities or limited life expectancy, “clinical judgement, patient preference, and at team-based approach to risk/benefit is reasonable for decisions regarding intensity of BP lowering and choice of antihypertensive drugs.” We agree that all treatment decisions must be individualized based upon each patient’s clinical scenario, and that a guideline is only a general aid for treatment decisions, not a mandate for care.
Therefore, with the acknowledgement that there is a lack of literature specifically examining blood pressure goals in elderly patients with CKD, it is our opinion based on available evidence that the following suggestions constitute a reasonable approach to this scenario: (1) a blood pressure target of less than 130/80 mm Hg should be sought as the primary blood pressure target; (2) if the patient cannot tolerate this due to rapidly declining GFR, acute kidney injury, orthostatic hypotension and or falls; or in other situations where this is not a practical a goal, individualized goal of at ideally less than 140 mm Hg, though at least less than 160 mm Hg systolic, could be considered; (3) the clinician should attempt careful and gradual reduction of blood pressure, with no more than one agent added or one escalation of medication dose attempted per visit; (4) the patient should have close follow up-after medication changes with an adjustment period of at least 4 weeks before additional medication or dose escalations are made; (5) if CKD is accompanied by albuminuria (daily urine protein excretion of greater than 300 mg/dL or a urine albumin to creatinine ratio of 300 mg/g) an ACEI or ARB should be used in management; (6) a rise in serum creatinine of up to 30% of baseline after addition of an ACEI may be acceptable; however, a rise greater than this amount should prompt discontinuation of the drug and evaluation for renal artery stenosis; (7) frequent monitoring of creatinine is required, with repeat chemistry performed after medication adjustments; (8) patients with a high pulse pressure should be monitored especially closely for symptoms or changes in renal function; and finally (9) individualized treatment and clinical judgement, with the patient being an informed participant, should take priority over all other recommendations and guidelines. We feel that further research in this growing subgroup of elderly patients is needed and will be sought, and we expect recommendations will continue to evolve as future literature becomes available.
Corresponding author: Jonathan G. Owen, MD, MSC04 2785, 1 University of New Mexico, Albuquerque, NM 87131, [email protected].
Financial disclosures: None.
Author contributions: conception and design, JGO; analysis and interpretation of data, KA, FXR, JGO; drafting of article, KA, FXR, JGO; critical revision of the article, KA, FXR, JGO; collection and assembly of data, KA, FXR, JGO.
From the Division of Nephrology, University of New Mexico Health Sciences Center, Albuquerque, NM.
Abstract
- Objective: To review the available literature regarding hypertension and chronic kidney disease (CKD) in the elderly and provide a framework for clinical management of hypertension in this subset of the elderly population.
- Methods: Review of the available literature.
- Results: Though several large, well-designed randomized trials exist examining the treatment of isolated hypertension in the elderly, these trials have uniformly excluded patients with CKD, thus reducing the generalizability of these results to this subgroup. CKD in the elderly is poorly studied overall, and whether CKD in the elderly is an expected product of senescence or a pathology from modifiable risk factors is debatable. Concern exists regarding the increased potential of acute kidney injury events and a more rapid progression of CKD with more aggressive hypertension lowering in elderly patients.
- Conclusion: Though data is limited regarding hypertension treatment in the subset of elderly patients with CKD, given the consistent benefits in cardiovascular reduction with hypertension treatment in the general elderly population, it is likewise recommended that elderly patients with hypertension and CKD receive antihypertensive therapy, though with more careful monitoring for adverse renal effects. We provide a practical approach to management for this clinical scenario.
Chronic kidney disease (CKD) is an increasingly recognized finding in elderly patients, with approximately half of all patients over the age of 70 meeting the most common currently accepted definition of CKD stage III, an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2 [1]. Whether this finding is a result of normal physiologic aging or whether it represents a true disease process in elderly patients has been a matter of considerable debate [2–4]. Nonetheless, the decline in eGFR in elderly patients has important implications regarding drug dosing and the potential risk of acute kidney injury (AKI) in this population [5–9]. Additionally, elderly patients with reduced GFR may have an increased risk of cardiovascular events and progression to end-stage kidney disease (ESKD), though extensive studies are lacking in this population [10–13].
In contrast, isolated hypertension and its treatment in the elderly population has now been extensively evaluated in several well-designed, prospective randomized studies, with generally favorable results arguing for the treatment of hypertension in elderly individuals [14–17]. Unfortunately, however, these studies have uniformly excluded patients with CKD in their study designs. Thus, the impact of aggressive hypertension management in elderly patients with CKD is unknown. As a considerable proportion of CKD in this population has been felt secondary to vascular disease and poor overall vascular health, many have questioned whether aggressive blood pressure reduction, particularly in patients with wide pulse pressure as an indicator of vascular disease, may result in decreased overall renal perfusion and greater risk for AKI, and thus potentially accelerate renal decline in this population [18–22].
In this paper, we review the epidemiology and physiology of renal disease in the elderly, provide an analysis of the available data regarding management of hypertension in the elderly, and suggest an approach to management of hypertension in this specific patient population. Though a multitude of age cutoffs defining elderly have been proposed, for the purposes of this paper we define elderly as age greater than 65 years unless otherwise specified.
Definition of CKD
The currently accepted definition of CKD represents any composite of pathology resulting in impaired kidney function, defined as a drop of GFR < 60 mL/min/1.73 m2 for 3 months or longer, or a higher GFR but with evidence of structural or functional abnormalities, such as proteinuria [23]. However, there are key aspects and important limitations to the above diagnostic criteria to be considered in the elderly patient population. Importantly, the most commonly used GFR estimation equations use serum creatinine as the marker for impaired renal function. As serum creatinine levels are also determined by overall muscle mass, significant error in estimating GFR can occur using these equations in elderly patients, who may have widely varying degrees of musculature and thus creatinine production. Additionally, these equations were often derived using all CKD or mostly CKD patients, which may result in healthy individuals having a higher GFR at the same serum creatinine levels than CKD patients, thus incorrectly classifying many patients with normal kidney function as having CKD [24].
Serum cystatin C has been proposed as an alternative surrogate marker for impaired kidney function, particularly in the elderly, as it is not affected by muscle mass. However, cystatin C levels are affected by obesity, inflammation, and atherosclerosis, and thus equations using this marker to determine GFR also face some limitations in the elderly population [25]. Evidence comparing various GFR estimating equations in the elderly suggest that formulas that use a combination of serum creatinine and cystatin C do best at predicting GFR when compared to gold standard techniques, such as iohexol clearance, though it is important to note that yet the ideal GFR estimating equation for elderly patients has not been determined [26–28].
It has been suggested that given these limitations and potential to underestimate GFR in the elderly population, a lower GFR reference range of 45 mL/min/1.73 m2 be used in the absence of other signs of kidney damage given the multiple unique characteristics of the aging kidneys, as we will explore in this review [29]. In general, we are in agreement with this suggestion that all elderly patients with a creatinine based estimated GFR of < 45 mL/min/1.73 m2 can safely be assumed to have CKD, and it is our opinion that elderly patients with a GFR > 45 mL/min/1.73 m2 but less < 60 mL/min/1.73 m2, without other signs of structural of functional renal disease such as proteinuria, have additional evaluation for the presence of impaired renal function, including but not limited to the addition of cystatin C to estimate GFR.
Epidemiology of CKD in the Elderly
According to the Centers for Disease Control and Prevention (CDC), the number of elderly patients in the United States is expected to double in the next 25 years to 72 million patients, representing approximately 20% of the adult population by 2030 [30]. Analysis of the National Health and Nutrition Examination Surveys (NHANES) from 1999–2004 revealed an overall prevalence of CKD in the US population of 13.1%. However, when sub-grouped into patients greater than or equal to 70 years of age, the prevalence of CKD in this population increased to a staggering 47.5% [31]. Likewise, analysis of other elderly populations from Canada, China, Italy, and Spain indicated a roughly 3- to 7-fold increase in CKD prevalence in those elderly populations compared to younger patients [3]. Additionally, according to the United States Renal Data System there is evidence of a progressive rise in the number of end-stage renal disease (ESRD) patients enrolled in Medicare-funded programs over the past decades [32]. In extrapolating these estimates, it is conceivable to predict that approximately 30 million elderly patients may have CKD in the United States by year 2030, with enormous implications to treatment recommendations and healthcare associated costs.
The Aging Kidney and Expected Rate of Nephron Loss
A progressive, age-related decline in GFR has been demonstrated in many studies. In an earlier analysis of the Baltimore Longitudinal Study of Aging by Lindeman et al, a decline in measured creatinine clearance of 0.75 mL/min/year was demonstrated. It is important to note that in this analysis, patients with suspected pre-existing renal or urologic disease and those on diuretics or other antihypertensives were excluded from analysis, and a normal Gaussian distribution of creatinine clearance slopes versus time was demonstrated, suggesting the GFR loss was a process of normal aging [4].
In support of the theory of a physiologic age-related decline in renal function, a study by Rule et al analyzed potential kidney transplant donors for age-related decline in renal function and determined an approximately 6.3 mL/min/1.73 m2 decline in GFR for each decade. In this investigation, core needle biopsies were obtained at the time of donation and transplantation. The investigators found a progressive increase in the histologic prevalence of nephrosclerosis with each age group analyzed, increasing from 2.7% at ages 18 to 29 to 16% for ages 30 to 39, 28% for ages 40 to 49, 44% for ages 50 to 59, 58% for ages 60 to 69, and finally 73% in donors older than age 70. It is important to note that this study only examined live kidney donors, a group heavily screened and selected on the basis of optimal health, thus strongly arguing for progressive renal decline as a consequence of “normal” aging. Furthermore, though controlled hypertensive patients (treated with 2 or less medications) were allowed to be donors in this study, exclusion of this group had only a minimal impact on the findings of the study [33].
However, whether this age-related decline is purely a result of normal senescence or is a consequence of modifiable risk factors that could alter this outcome remains debatable. Additionally, vascular disease is clearly implicated in more accelerated renal decline. This concept was well demonstrated in an analysis of the longitudinal Age, Gene/Environment Susceptibility – Reykjavik Study, which showed that although age was associated with both reduced GFR and albuminuria, reduced GFR and albuminuria in elderly patients (mean age 80.8 yr) was strongly associated with midlife systolic and diastolic blood pressure, thus suggesting that potentially modifiable vascular pathology may play a much stronger role in CKD in the elderly than aging alone [34].
Finally, it has been hypothesized that reduced nephron mass at birth may contribute to CKD in the elderly [29]. Reduced nephron mass appears to be associated with low birth weight and prematurity, and this has been associated with an increased risk for ESRD later in life [35,36].
Hypertension in the Elderly
Pathophysiology
Age-associated hypertension is felt to arise from several mechanisms and hemodynamic changes. Systolic blood pressure has been noted to progressively rise with age, whereas diastolic blood pressure rises to the 5th or 6th decade, after which it appears to slowly decline. This pattern is felt likely secondary to increasing large vessel stiffness from collagen deposition and calcification with aging, and fracturing and degradation of elastin fibers. As large vessels become less distensible, pulse pressure and pulse wave velocity increases with this drop in diastolic BP, with less forward flow seen in diastole, leading to decreased organ perfusion. Additionally and alternatively, concentric left ventricular hypertrophy develops with aging, leading to reduced cardiac output from decreased stroke volume, which may also contribute to reduced organ perfusion [37,38]. These findings have led many to speculate that hypertension in the elderly may actually serve as a protective mechanism to maintain organ perfusion, and have led to great concern regarding excessive lowering of diastolic blood pressure and increasing of pulse pressure in this population with antihypertensive therapy. This theory was initially corroborated with a sub-analysis of the Systolic Hypertension in the Elderly Program (SHEP) where an increase in pulse pressure by 10 mm Hg was accompanied by increased risk of stroke and congestive heart failure in the treatment arm [39]. Nonetheless, the bulk of evidence continues to support a lower overall risk of cardiovascular events with treatment of hypertension in elderly patients, and general expert consensus recommends treatment with gradual reduction to normal levels of systolic blood pressure accompanied by careful monitoring for adverse effects [40,41].
In addition to these above changes, reduced GFR in elderly likewise results in impaired natriuresis, thereby fostering hypertension via volume expansion. Age-related arteriolosclerosis may result in renal artery stenosis, resulting in decreased renal perfusion and upregulation of the renin-angiotensin-aldosterone cascade. Further challenging treatment decisions is the frequent development of autonomic dysregulation in the elderly, a major risk factor for falls and cardiovascular events [40].
The result of these abnormalities is that roughly 65% of patients greater than the age of 60 have at least isolated systolic hypertension [42]. Similarly corresponding to the underlying physiology highlighted above, rising pulse pressure, rather than systolic or diastolic blood pressure, appears to be the greatest risk factor for cardiovascular events in the elderly population [43,44]. In an interesting analysis of the Framingham Heart Study by Franklin et al, the authors noted that in patients < 50 years of age, diastolic blood pressure was the strongest risk factor for events. However, at age 50 to 59, a change occurred where all 3 blood pressure indexes were comparable risk predictors, and then from age 60 years and on pulse pressure became the superior predictor, with diastolic blood pressure being negatively correlated to cardiovascular risk, highlighting the potential importance for organ perfusion during diastole in this group [45].
Likewise, in the elderly population pulse pressure also appears to be inversely related to GFR, suggesting that vascular stiffness and the reduced forward flow in diastole may contribute to microvascular damage and CKD [46]. In elderly patients with untreated isolated systolic hypertension, increasing systolic blood pressure (a reflection of rising pulse pressure) was associated with the greatest risk of renal decline when compared to diastolic blood pressure, pulse, and mean arterial pressure [47]. In the normal state, high renal blood flow and low renal arterial resistance can contribute to regular large intrarenal pressure variations. Because of vascular stiffness, these pressure variations increase with time, increasing up to 4-fold in the elderly compared with young peers, and likely contribute to renal damage seen in older patients [48].
Treatment
In comparison to the paucity of randomized trials examining CKD progression in the elderly, 4 very large, well designed randomized trials (SHEP, MRC trial, Syst-Eur trial, and HYVET) specifically examining the treatment of hypertension in the elderly have now been conducted [14–17] and confirmed earlier and smaller trials demonstrating the benefits of treatment of hypertension in the elderly [49,50]. In addition to this, several of the other large landmark hypertension trials such as ALLHAT, ACCOMPLISH, and the SPRINT trial included a considerable number of elderly patients [51–53]. Though the primary aim of those trials was not to determine the effects of hypertension treatment in the elderly per se, sub-analysis of this population in these trials has further added to our knowledge of this condition.
In the largest initial trial of hypertension in the elderly (SHEP), the researchers randomized 4376 patients over the age of 60 with an average blood pressure of 170/77 mm Hg into a treatment versus placebo arm. Such a study would be inconceivable today due to the consistent benefit derived from antihypertensive therapy now demonstrated in multiple trials. An achieved systolic blood pressure of 143 mm Hg in the treatment arm versus 155 mm Hg in the placebo arm was obtained. Stroke and nonfatal cardiac events were significantly reduced with treatment. The development of renal dysfunction occurred in 7 patients in the treatment arm and 11 patients in the placebo arm, a nonsignificant difference. As we have noted previously, however, patients with pre-existing kidney disease were excluded from the study [14]. A subsequent analysis of the SHEP trial results by Vaccarino et al, however, showed that in patients on treatment who developed an increase in pulse pressure of 10 mm Hg or more carried a 23% higher risk for developing heart failure and a 24% higher risk for stroke. This effect was not seen in the placebo arm [39].
Shortly following the publication of the SHEP results, the Medical Research Council trial of treatment of hypertension in older adults (MRC) further confirmed the initial findings by demonstrating a 25% reduction in stroke and a 17% reduction in all cardiac events in 4396 patients aged 65 to 74 with a systolic blood pressure greater than 160 mm Hg randomized to treatment of hypertension with either atenolol or a diuretic combination of amiloride and hydrochlorothiazide versus placebo. Like SHEP, however, patients with pre-existing renal disease were excluded, and no report of renal outcomes was published in the initial results [15]. Similarly, the Systolic Hypertension in Europe Trial (Syst-Eur) revealed a 42% reduction in stroke and a 26% reduction in all cardiac endpoints in 4695 patients with a systolic blood pressure of greater than 160 mm Hg randomized to receive nitrendipine with addition of enalapril and hydrochlorothiazide as required. However, CKD patients were likewise excluded in this trial [16].
Finally, the Hypertension in the Very Elderly Trial (HYVET) was unique in that it sought to enroll only patients greater than 80 years of age, a significant departure from the earlier hypertension in elderly trials. This trial randomized 3845 patients, again with a systolic blood pressure of 160 mm Hg or greater, to a placebo arm versus a treatment arm of the thiazide type diuretic indapamide, with addition of the ACE inhibitor perindopril if blood pressure was still greater than 150 mm Hg on monotherapy. Despite the older age of the participants in this trial, patients still benefited from blood pressure reduction with a 30% reduction in rate of stroke, a 21% reduction in the rate of death from any cause, and an impressive 64% reduction in the rate of heart failure [17]. These findings from HYVET, combined with the earlier SHEP, MRC and Syst-Eur trials, confirmed that treatment of hypertension in the elderly of any age should be attempted.
Recomendations for Managing Hypertension in the Elderly with CKD
Though a lack of data exists regarding the treatment of hypertension in elderly patients with the comorbidity of CKD, given the consistent and robust data that exists demonstrating a reduction in cardiovascular risk and mortality in the general elderly population without renal impairment, it is our opinion that elderly patients with CKD and hypertension should receive antihypertensive treatment. This opinion is supported by the fact that in the recently published SPRINT trial, 28.1% of patients in the standard treatment arm (targeting a blood pressure of less than 140 mm Hg), and 28.4% of patients in the intensive treatment arm (blood pressure target less than 120 mm Hg) had CKD, and similarly 28.2% of the trial participants in each group were greater than the age of 75. The percentage of patients with both CKD and age greater than 75 years was not reported in the initial trial results, though it is assumed a significant portion of these patients had both CKD and age greater than 75 years. It is nonetheless reassuring that patients with CKD in the SPRINT trial, as well as those with age > 75 years, both seemed to derive the same benefit in cardiovascular and mortality benefit in the intensive treatment arm compared to the standard treatment arm [53].
It should be noted, however, that though cardiovascular events and mortality were lower in the more intensive treatment arm of the SPRINT trial, CKD progression did not differ between the two treatment groups. Additionally, the risk of acute kidney injury was significantly greater in the intensive treatment arm when compared to the standard treatment arm, with 3.8% of patients in the intensive treatment arm suffering AKI compared to 2.3% in the standard arm [22]. Thus, it should be understood by both the clinician and the elderly patient with hypertension and CKD that the goal of more aggressively lowering blood pressure is to prevent cardiovascular events and not slow renal disease progression.
The recently published 2017 hypertension guidelines by the American College of Cardiology/American Heart Association is the most comprehensive set of hypertension treatment recommendations published to date and includes a section regarding patients with CKD as well as a section on the elderly [54]. Regarding CKD, the guidelines recommend a goal blood pressure of less than 130/80 mm Hg in patients with CKD, and that patients with macroalbuminuria (defined as a daily urine protein excretion of greater than 300 mg/dL or a urine albumin to creatinine ratio of 300 mg/g) be treated with and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB). We feel these are reasonable recommendations for CKD targets and agree with the guideline, with the understanding that the target of 130/80 mm Hg is based largely on the SPRINT data. It is important to recognize that in the Action to Control Cardiovascular Risk in Diabetes trial (ACCORD), a more intensive blood pressure target of 120 mm Hg did not result in further improvement in cardiovascular events compared to a traditional target of 140/90 mm Hg [55]. However, given the larger and more robust sample size from SPRINT, we feel the target of 130/80 mm Hg is warranted and therefore should be the first target for elderly patients with CKD. With this goal in mind, it has been our clinical experience that some elderly patients with CKD have difficulty tolerating this goal, either from the development of worsening of GFR, acute kidney injury events, or due to orthostatic hypotension. Additionally, it should be noted that patients with orthostatic hypotension were excluded from SPRINT, though an increase in falls was not seen in the primary study. Therefore, for patients who are unable to tolerate the SPRINT goal of 130/80 mm Hg, an individualized goal of at least less than 160 mm Hg systolic and ideally less than 140 mm Hg, reflecting achieved blood pressure endpoints from earlier trials, may be a reasonable alternative [55]. The recent hypertension guidelines also recommend that for elderly adults with a high burden of comorbidities or limited life expectancy, “clinical judgement, patient preference, and at team-based approach to risk/benefit is reasonable for decisions regarding intensity of BP lowering and choice of antihypertensive drugs.” We agree that all treatment decisions must be individualized based upon each patient’s clinical scenario, and that a guideline is only a general aid for treatment decisions, not a mandate for care.
Therefore, with the acknowledgement that there is a lack of literature specifically examining blood pressure goals in elderly patients with CKD, it is our opinion based on available evidence that the following suggestions constitute a reasonable approach to this scenario: (1) a blood pressure target of less than 130/80 mm Hg should be sought as the primary blood pressure target; (2) if the patient cannot tolerate this due to rapidly declining GFR, acute kidney injury, orthostatic hypotension and or falls; or in other situations where this is not a practical a goal, individualized goal of at ideally less than 140 mm Hg, though at least less than 160 mm Hg systolic, could be considered; (3) the clinician should attempt careful and gradual reduction of blood pressure, with no more than one agent added or one escalation of medication dose attempted per visit; (4) the patient should have close follow up-after medication changes with an adjustment period of at least 4 weeks before additional medication or dose escalations are made; (5) if CKD is accompanied by albuminuria (daily urine protein excretion of greater than 300 mg/dL or a urine albumin to creatinine ratio of 300 mg/g) an ACEI or ARB should be used in management; (6) a rise in serum creatinine of up to 30% of baseline after addition of an ACEI may be acceptable; however, a rise greater than this amount should prompt discontinuation of the drug and evaluation for renal artery stenosis; (7) frequent monitoring of creatinine is required, with repeat chemistry performed after medication adjustments; (8) patients with a high pulse pressure should be monitored especially closely for symptoms or changes in renal function; and finally (9) individualized treatment and clinical judgement, with the patient being an informed participant, should take priority over all other recommendations and guidelines. We feel that further research in this growing subgroup of elderly patients is needed and will be sought, and we expect recommendations will continue to evolve as future literature becomes available.
Corresponding author: Jonathan G. Owen, MD, MSC04 2785, 1 University of New Mexico, Albuquerque, NM 87131, [email protected].
Financial disclosures: None.
Author contributions: conception and design, JGO; analysis and interpretation of data, KA, FXR, JGO; drafting of article, KA, FXR, JGO; critical revision of the article, KA, FXR, JGO; collection and assembly of data, KA, FXR, JGO.
1. Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 2012;157:471–81.
2. Wetzels JF, Kiemeney LA, Swinkels DW, et al. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int 2007;72:632–7.
3. Minutolo R, Borrelli S, De Nicola L. CKD in the elderly: kidney senescence or blood pressure-related nephropathy? Am J Kidney Dis 2015;66:184–6.
4. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985; 33:278–85.
5. Gill J, Malyk R, Djurdiev O, Levin A. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group – a cautionary tale. Nephrol Dial Transplant 2007;22:2894–9.
6. Spruill WJ, Wade WE, Cobb HH 3rd. Comparison of estimated glomerular filtration rate with estimated creatinine clearance in the dosing of drugs requiring adjustments in elderly patients with declining renal function. Am J Geriatr Pharmacother 2008;6:153–60.
7. Dowling TC, Wang ES, Ferruci L, Sorkin JD. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: impact on renal drug dosing. Pharmacotherapy 2013;33:912–21.
8. Ballew SH, Chen Y, Daya NR, Godino JG, Windham BG, McAdams-DeMarco M, Coresh J, Selvin E, Grams ME. Frailty, kidney function, and polypharmacy: the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis 2017;69:228–36.
9. Grams ME, Sang Y, Ballew SH, et al; CKD Prognosis Consortium. A meta-analysis of the association of estimated GFR, albuminuria, race, and sex with acute kidney injury. Am J Kidney Dis 2015;66:591–601.
10. Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011;80:93–104.
11. Masson P, Webster AC, Hong M, et al. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant 2015;30:1162–9.
12. Tellez-Plaza M, Orozco-Beltran D, Gil-Guillen V, et al; ESCARVAL STUDY GROUP. Renal function and attributable risk of death and cardiovascular hospitalization in patients with cardiovascular risk factors from a registry-based cohort: the Estudio Cardiovascular Valencia-risk study. J Hypertens 2016;34:2266–73.
13. Smink PA, Lambers-Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012;60:804–11.
14. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the systolic hypertension in the elderly program (SHEP). JAMA 1991;265:3255–64.
15. Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ 1992;304:405–12.
16. Staessen JA, Faggard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997;350:757–64.
17. Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–98.
18. Fesler P, Safar ME, du Cailar G, et al. Pulse pressure is an independent determinant of renal function decline during treatment of essential hypertension. J Hypertens 2007;25:1915–20.
19. Weir MR, Dworkin LD. Antihypertensive drugs, dietary salt, and renal protection: how low should you go and with which therapy? Am J Kidney Dis 1998;32:1–22.
20. Obi Y, Kalantar-Zadeh K, Shintani A, et al. Estimated glomerular filtration rate and the risk-benefit profile of intensive blood pressure control amongst nondiabetic patients: a post hoc analysis of a randomized clinical trial. J Intern Med 2017;283:314–27.
21. Peralta CA, McClure LA, Scherzer R, et al. Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: a post hoc analysis of the secondary prevention of small subcortical strokes (SPS3) randomized trial. Circulation 2016;133:584–91.
22. Rocco MV, Sink KM, Lovato LC, et al; SPRINT Research Group. Effects of intensive blood pressure treatment on acute kidney injury events in the systolic blood pressure intervention trial (SPRINT). Am J Kidney Dis 2018;71:352–61.
23. Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013;3:19–62.
24. Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 2004;141:929–37.
25. Stevens LA, Schmid CH, Green T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 2009;75:652–60.
26. Biork J, Grubb A, Gudnason V, et al. Comparison of glomerular filtration rate estimating equations derived from creatinine and cystatin C: validation in the age, gene/environment susceptibility-Reykjavik elderly cohort. Nephrol Dial Transplant 2017.
27. Bevc S, Hojs N, Hois R, et al. Estimation of glomerular filtration rate in elderly chronic kidney disease patients: comparison of three novel sophisticated equations and simple cystatin C equation. Therapeutic Apheresis and Dialysis 2017;21:126–32.
28. Pottel H, Delanaye P, Schaeffner E, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant 2017;32:497–507.
29. Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis 2016;23:19–28.
30. US Department of Health and Human Services, Centers for Disease Control and Prevention. The state of aging and health in America 2013. Centers for Disease Control and Prevention website. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf. Published 2013. Accessed April 5, 2018.
31. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47.
32. United Stated Renal Data System. USRD 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. United States Renal Data System website. https://www.usrds.org/atlas13.aspx . Published 2013. Accessed April 5, 2018.
33. Rule AD, Amer H, Cornell LD, et al. The association between age and nephroclerosis on renal biopsy among healthy adults. Ann Intern Med 2010;152:561–7.
34. Inker LA, Okparavero A, Tighiouart H, et al. Midlife blood pressure and late-life GFR and albuminuria: an elderly general population cohort. Am J Kidney Dis 2015;66:240–8.
35. Vikse BE, Irgens LM, Leivestad T, et al. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 2008;19:151–7.
36. Luyckx VA, Bertram JF, Brenner BM, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013;382:273–83.
37. Franklin SS, Gustin W 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96:308–15.
38. Messerli FH, Sundgaard-Riise K, Ventura HO, et al. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983;2:983–6.
39. Vaccarino V, Berger AK, Abramson J, et al. Pulse pressure and risk of cardiovascular events in the systolic hypertension in the elderly program. Am J Cardiol 2001;88:980–6.
40. Aronow WS, Harrington RA, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Forciea MA, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents. Circulation 2011;123:2434–506.
41. Chaudhry SI, Krumholz HM, Foody JM. Systolic hypertension in older persons. JAMA 2004;292:1074–80.
42. Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension 1995;25: 305–13.
43. Blacher J, Staessen JA, Girerd X, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med 2000;160:1085–90.
44. Franklin SS, Lopez VA, Wong ND, et al. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation 2009;119:243–50.
45. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001;103:1245–9.
46. Verhave JC, Fesler P, du Cailar G, et al. Elevated pulse pressure is associated with low renal function in elderly patients with isolated systolic hypertension. Hypertension 2005;45:586–91.
47. Young JH, Klaq MJ, Muntner P, et al. Blood pressure and decline in kidney function: findings from the systolic hypertension in elderly program (SHEP). J Am Soc Nephrol 2002;13:2776–82.
48. O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–4.
49. Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986;293:1145–8.
50. Dahlof B, Lindholm LH, Hansson L, et al. Morbidity and mortality in the Swedish trial in old patients with hypertension (STOP-Hypertension). Lancet 1991;338:1281–5.
51. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2002;288:2981–97.
52. Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators. Benazapril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008;359:2417–28.
53. SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373: 2103–16.
54. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 2017.
55. ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85.
1. Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 2012;157:471–81.
2. Wetzels JF, Kiemeney LA, Swinkels DW, et al. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int 2007;72:632–7.
3. Minutolo R, Borrelli S, De Nicola L. CKD in the elderly: kidney senescence or blood pressure-related nephropathy? Am J Kidney Dis 2015;66:184–6.
4. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985; 33:278–85.
5. Gill J, Malyk R, Djurdiev O, Levin A. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group – a cautionary tale. Nephrol Dial Transplant 2007;22:2894–9.
6. Spruill WJ, Wade WE, Cobb HH 3rd. Comparison of estimated glomerular filtration rate with estimated creatinine clearance in the dosing of drugs requiring adjustments in elderly patients with declining renal function. Am J Geriatr Pharmacother 2008;6:153–60.
7. Dowling TC, Wang ES, Ferruci L, Sorkin JD. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: impact on renal drug dosing. Pharmacotherapy 2013;33:912–21.
8. Ballew SH, Chen Y, Daya NR, Godino JG, Windham BG, McAdams-DeMarco M, Coresh J, Selvin E, Grams ME. Frailty, kidney function, and polypharmacy: the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis 2017;69:228–36.
9. Grams ME, Sang Y, Ballew SH, et al; CKD Prognosis Consortium. A meta-analysis of the association of estimated GFR, albuminuria, race, and sex with acute kidney injury. Am J Kidney Dis 2015;66:591–601.
10. Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011;80:93–104.
11. Masson P, Webster AC, Hong M, et al. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant 2015;30:1162–9.
12. Tellez-Plaza M, Orozco-Beltran D, Gil-Guillen V, et al; ESCARVAL STUDY GROUP. Renal function and attributable risk of death and cardiovascular hospitalization in patients with cardiovascular risk factors from a registry-based cohort: the Estudio Cardiovascular Valencia-risk study. J Hypertens 2016;34:2266–73.
13. Smink PA, Lambers-Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012;60:804–11.
14. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the systolic hypertension in the elderly program (SHEP). JAMA 1991;265:3255–64.
15. Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ 1992;304:405–12.
16. Staessen JA, Faggard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997;350:757–64.
17. Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–98.
18. Fesler P, Safar ME, du Cailar G, et al. Pulse pressure is an independent determinant of renal function decline during treatment of essential hypertension. J Hypertens 2007;25:1915–20.
19. Weir MR, Dworkin LD. Antihypertensive drugs, dietary salt, and renal protection: how low should you go and with which therapy? Am J Kidney Dis 1998;32:1–22.
20. Obi Y, Kalantar-Zadeh K, Shintani A, et al. Estimated glomerular filtration rate and the risk-benefit profile of intensive blood pressure control amongst nondiabetic patients: a post hoc analysis of a randomized clinical trial. J Intern Med 2017;283:314–27.
21. Peralta CA, McClure LA, Scherzer R, et al. Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: a post hoc analysis of the secondary prevention of small subcortical strokes (SPS3) randomized trial. Circulation 2016;133:584–91.
22. Rocco MV, Sink KM, Lovato LC, et al; SPRINT Research Group. Effects of intensive blood pressure treatment on acute kidney injury events in the systolic blood pressure intervention trial (SPRINT). Am J Kidney Dis 2018;71:352–61.
23. Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013;3:19–62.
24. Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 2004;141:929–37.
25. Stevens LA, Schmid CH, Green T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 2009;75:652–60.
26. Biork J, Grubb A, Gudnason V, et al. Comparison of glomerular filtration rate estimating equations derived from creatinine and cystatin C: validation in the age, gene/environment susceptibility-Reykjavik elderly cohort. Nephrol Dial Transplant 2017.
27. Bevc S, Hojs N, Hois R, et al. Estimation of glomerular filtration rate in elderly chronic kidney disease patients: comparison of three novel sophisticated equations and simple cystatin C equation. Therapeutic Apheresis and Dialysis 2017;21:126–32.
28. Pottel H, Delanaye P, Schaeffner E, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant 2017;32:497–507.
29. Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis 2016;23:19–28.
30. US Department of Health and Human Services, Centers for Disease Control and Prevention. The state of aging and health in America 2013. Centers for Disease Control and Prevention website. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf. Published 2013. Accessed April 5, 2018.
31. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47.
32. United Stated Renal Data System. USRD 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. United States Renal Data System website. https://www.usrds.org/atlas13.aspx . Published 2013. Accessed April 5, 2018.
33. Rule AD, Amer H, Cornell LD, et al. The association between age and nephroclerosis on renal biopsy among healthy adults. Ann Intern Med 2010;152:561–7.
34. Inker LA, Okparavero A, Tighiouart H, et al. Midlife blood pressure and late-life GFR and albuminuria: an elderly general population cohort. Am J Kidney Dis 2015;66:240–8.
35. Vikse BE, Irgens LM, Leivestad T, et al. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 2008;19:151–7.
36. Luyckx VA, Bertram JF, Brenner BM, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013;382:273–83.
37. Franklin SS, Gustin W 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96:308–15.
38. Messerli FH, Sundgaard-Riise K, Ventura HO, et al. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983;2:983–6.
39. Vaccarino V, Berger AK, Abramson J, et al. Pulse pressure and risk of cardiovascular events in the systolic hypertension in the elderly program. Am J Cardiol 2001;88:980–6.
40. Aronow WS, Harrington RA, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Forciea MA, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents. Circulation 2011;123:2434–506.
41. Chaudhry SI, Krumholz HM, Foody JM. Systolic hypertension in older persons. JAMA 2004;292:1074–80.
42. Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension 1995;25: 305–13.
43. Blacher J, Staessen JA, Girerd X, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med 2000;160:1085–90.
44. Franklin SS, Lopez VA, Wong ND, et al. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation 2009;119:243–50.
45. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001;103:1245–9.
46. Verhave JC, Fesler P, du Cailar G, et al. Elevated pulse pressure is associated with low renal function in elderly patients with isolated systolic hypertension. Hypertension 2005;45:586–91.
47. Young JH, Klaq MJ, Muntner P, et al. Blood pressure and decline in kidney function: findings from the systolic hypertension in elderly program (SHEP). J Am Soc Nephrol 2002;13:2776–82.
48. O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–4.
49. Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986;293:1145–8.
50. Dahlof B, Lindholm LH, Hansson L, et al. Morbidity and mortality in the Swedish trial in old patients with hypertension (STOP-Hypertension). Lancet 1991;338:1281–5.
51. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2002;288:2981–97.
52. Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators. Benazapril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008;359:2417–28.
53. SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373: 2103–16.
54. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 2017.
55. ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85.
Multidisciplinary Diabetes Care in a Safety Net Clinic: Lessons Learned from a Quality Improvement Initiative
From the Department of Family and Community Medicine, McGovern Medical School at the University of Texas Health Science Center, Houston, TX (Dr. Zare, Ms Klawans, Dr. Moreno), Department of Family Medicine and Community Medicine, Baylor College of Medicine, Houston, TX (Dr. Mejia de Grubb, Dr. Juneja, Dr. Zoorob), Department of Psychiatry, McGovern Medical School at the University of Texas Health Science Center, Houston, TX (Dr. Suchting), andHarris Health System, Houston TX (Ms. Mathis).
Abstract
- Objective: To describe a pilot project to improve care for patients with uncontrolled diabetes in a safety net clinic.
- Methods: One of 3 clinical teams was designated the intervention team. Changes implemented by the intervention team included patient referral to a dietician and/or clinical pharmacist, provision of patient education, and assignment of a case manager. We compared outcomes of patients in the intervention group (n = 71), vs those receiving care from the other 2 teams (usual care) (n = 188).
- Results: HbA1c significantly decreased over time for patients in the intervention group as well as the usual care group. Within the intervention group, visits to clinical pharmacist (P = 0.034) and education (P = 0.004) predicted significantly greater decreases in HbA1c over time.
- Conclusions: Diffusion of treatment may account for the overall HbA1c reduction regardless of treatment group. Results support the need for further pragmatic research to weigh the impact of unblinded designs, outcome measurement, and real-world behaviors on evidence-based interventions.
Key words: diabetes; safety net; multidisciplinary diabetes care; primary care; diffusion of treatment.
The prevalence of type 2 diabetes in the United States is significantly higher among Hispanics and African Americans than in the general population (13% vs. 9.3%) [1]. Similarly, diabetes is highly prevalent among the uninsured, and many patients delay or forgo treatment due to cost [2]. Subsequently, the rates of comorbidities, including stroke, hypertension, and CVD, are elevated in these groups [3].
Association between elevated HbA1c and morbidity and mortality is well-documented, and an HbA1c reduction of just 1% has been shown to reduce mortality and improve quality of life [4]. Uncontrolled diabetes also results in increased medical costs. Reducing HbA1c from 9.0 to 7.5 reduces annual expenditures by as much as 73% [5].
Metropolitan Houston and Harris County, Texas, has one of the largest uninsured metro populations in the United States (over 3.6 million) [6]. Harris Health System serves this uninsured population and is the fourth largest safety net health system in the nation. Approximately 40,000 patients with diabetes receive care within the health system, and 34% of them have an HbA1c value greater than 9.
Developing novel, cost-efficient treatment and management models is crucial when providing care for patients with uncontrolled diabetes. However, the study of implementation strategies to successfully integrate evidence-based interventions in primary care using pragmatic approaches that aim to determine the effectiveness of interventions in “the real world” remain a challenge [7,8]. To address this issue, a quality improvement project was instituted at one of the system’s health centers to improve the care of patients with uncontrolled diabetes (known HbA1c above 9).
Methods
Setting
The pilot project was conducted from 1 Oct 2015 to 31 Dec 2015 in a primary care community health center within Harris Health System in Houston, Texas. This pilot was the first step of an institutional effort to introduce a multidisciplinary model of care across all clinics [9]. Our health center has 6 family medicine providers and 1 advanced practice nurse practitioner, organized into 3 pods with 2 physicians each. We randomly selected 1 pod (team) and designated it the intervention group.
The Standards for Quality Improvement Reporting Excellence guidelines [10] were followed and institutional review board approval was obtained.
Intervention
Practice changes introduced in the intervention team were assignment of a case manager to all patients, referral to a dietician and clinical pharmacist as needed, and patient education sessions. The team’s nurse assumed the role of case manager. The case manager was responsible for reviewing a patient checklist based on the America Diabetes Association guideline for comprehensive diabetes medical evaluation at initial and follow-up visits. Referrals were based on ADA guideline recommendations. Onsite brief patient education was provided to all patients. In addition, patients were enrolled in a “Diabetes 101” class, which follows an evidence-based curriculum that includes participation in at least 2 monthly sessions. Patients were asked to return to the clinic for a follow-up visit after 3 months in order to monitor medication compliance, re-evaluate their care plan, and measure HbA1c The usual care group patients were managed based on the current Standards of Medical Care in Diabetes [11]. The usual care group included patients from the same clinic under the care of providers in the teams that were not included in the multidisciplinary intervention.
Analysis
Data abstracted from de-identified patient records included HbA1c values, interventions received, and sociodemographic data. Generalized linear mixed modeling (GLMM) was used to examine changes in patient HbA1c levels over time [12]. All models included a random intercept to account for correlated observations within patient. All analyses were performed using Proc GLIMMIX in SAS v. 9.3 [13].
Results
A total 271 patients with HbA1c above 9 were included in the analysis: 71 in the intervention group and 188 in the usual care group. The intervention group was further differentiated by month of enrollment: October (n = 37), November (n = 27), and December (n = 9). Mean patient age in the overall sample was 51.6 years.
In the intervention group, most patients received patient education 56% (n = 40), almost half had a clinical pharmacy visit, but only 17% (n = 12) received a dietitian consultation. Overall, there was a 1.4% decrease in HbA1c in the intervention group, compared to a 1.3% HbA1c decrease in the usual care group.
GLMM was used to examine differences in HbA1c levels according to month of intervention enrollment (October vs. November vs. December) in the intervention group over time. Figure 1 shows predicted HbA1c values over time with trend lines fit for each of the three subgroups.
Preliminary analysis showed that potential contamination (diffusion of the treatment) would be likely to attenuate differences in the outcomes between the intervention and usual care conditions. Further analysis by subgroups were conducted to describe the intervention potential “spillover” to the usual care group participants not intended to receive the intervention. GLMM also examined differences in HbA1c levels between the intervention and usual care groups over time. The interaction between each treatment group and time was not statistically significant (F(2,268) = 1.34, P = 0.26), indicating that changes in HbA1c over time were not related to treatment group. A statistically reliable main effect for time (F(1,268) = 44.33, P < 0.001) indicated that in all groups, HbA1c values significantly decreased over time.
Follow-up analyses utilized GLMM to examine differences in HbA1c levels among patients in both groups who received at least one of the interventions (visiting a dietician, clinical pharmacist, education session, and clinical case manager). The interaction between intervention and time was not statistically significant for visiting the dietician, receiving education, or being assigned a case manager. The interaction between time and visiting a clinical pharmacist was statistically significant (F(1,204)= 7.78, P = 0.01) such that patients visiting the clinical pharmacist had lower HbA1c values over time relative to those that did not (Figure 2).
Discussion
HbA1c decreased significantly among intervention patients with uncontrolled diabetes over a 3-month period, regardless of which month they entered the study. However, there was no significant difference in HbA1c reduction between patients who received all 4 multidisciplinary interventions, one intervention, or those who received usual care. Patients in the intervention who attended clinical pharmacist visits had significantly greater HbA1c reduction than patients who did not, as did patients who attended a diabetes education session by a patient educator.
Diffusion of treatment may account for the overall HbA1c reduction regardless of treatment group. Diffusion refers to the unintended spread of a treatment effect when participants receive some or all treatments from an intervention to which they were not assigned, making outcomes descriptions of all study groups more challenging [14]. During the implementation period, other physicians and nurses in the clinic were aware of the multidisciplinary care model being piloted, and may have taken the initiative to connect their patients with clinical pharmacists, dieticians, certified diabetes educators, and clinical case managers. Pragmatic interventions are intended to maintain the internal validity of randomized control trials, yet they are meant to be implemented as close as possible to real-world settings in order to help patients, clinicians, and payers making informed health care decisions [8]. In this regard, participants in the control group could be exposed to the intervention through staff contact between the assigned groups implementing some of the intervention under study. In that case, the diffusion of treatment would be likely to attenuate differences in the outcomes between treatment and control groups [15].
This study has several limitations. We studied a small sample of patients that reflected the primary care population in one clinic in a safety net system with minority, underserved, and high-risk patients. Although attempts were made to keep the intervention limited to the intervention pod, diffusion of treatment might have impacted the internal validity of this intervention.
In summary, our results support the need for further systematic research work to weigh the impact of unblinded designs, simplified recruitment and outcome measurement, and real-world behaviors (such as noncompliance, cross over, and dropout) on evidence-based and multidisciplinary clinical interventions.
Acknowledgements: The authors would like to thank Krystal Gamarra, MSW, LCSW, and Hope Galvan, MS, RN, CVRN-BC, CDE for assistance with project implementation and data collection throughout this process.
Corresponding author: Maria C. Mejia de Grubb, MD, MPH, 3751 Kirby Dr, Suite 600, Houston, TX 77098, [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. Accessed at www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
2. Casagrande SS, Cowie CC. Health insurance coverage among people with and without diabetes in the US adult population. Diabetes Care 2012;35:2243–9.
3. American Diabetes Association. Statistics about diabetes. Arlington, VA; 2017.
4. Eeg-Olofsson K, Eliasson B, Zethelius B, et al. HbA1c reduction and risk of cardiovascular diseases in type 2 diabetes: an observational study from the Swedish NDR. Diabetes 2012.
5. Baxter M, Hudson R, Mahon J, et al. Estimating the impact of better management of glycaemic control in adults with type 1 and type 2 diabetes on the number of clinical complications and the associated financial benefit. Diabet Med 2016;33:1575–81.
6. Harris County Healthcare Alliance. The State of Health in Houston/Harris County 2015-2016. Accessed 17 Mar 2015 at http://houstonstateofhealth.org/soh_doc/.
7. Chalkidou K, Tunis S, Whicher D, et al. The role for pragmatic randomized controlled trials (pRCTs) in comparative effectiveness research. Clin Trials (London, England) 2012;9:436–46.
8. Tricoci P, Allen JM, Kramer JM, et al. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009;301:831–41.
9. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Qty 1996:511–44.
10. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Safety 2016;25:986–92.
11. Standards of Medical Care in Diabetes—2015 Abridged for Primary Care Providers. American Diabetes Association. Clin Diabetes 2015;33:97–111.
12. Gelman A, Hill J. Data analysis using regression and multilevelhierarchical models. New York: Cambridge University Press; 2007.
13. SAS Institute I. Base SAS Procedures Guide: Statistical procedures. In: SAS Institute I, editor. Cary, NC; 2011.
14. Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for general causal inference. Boston: Houghton Mifflin; 2002.
15. Kane R. Understanding health care outcomes research. 2nd ed. In: Learning JB, editor. Burlington, MA: Jones and Bartlett; 2006: 44–6.
From the Department of Family and Community Medicine, McGovern Medical School at the University of Texas Health Science Center, Houston, TX (Dr. Zare, Ms Klawans, Dr. Moreno), Department of Family Medicine and Community Medicine, Baylor College of Medicine, Houston, TX (Dr. Mejia de Grubb, Dr. Juneja, Dr. Zoorob), Department of Psychiatry, McGovern Medical School at the University of Texas Health Science Center, Houston, TX (Dr. Suchting), andHarris Health System, Houston TX (Ms. Mathis).
Abstract
- Objective: To describe a pilot project to improve care for patients with uncontrolled diabetes in a safety net clinic.
- Methods: One of 3 clinical teams was designated the intervention team. Changes implemented by the intervention team included patient referral to a dietician and/or clinical pharmacist, provision of patient education, and assignment of a case manager. We compared outcomes of patients in the intervention group (n = 71), vs those receiving care from the other 2 teams (usual care) (n = 188).
- Results: HbA1c significantly decreased over time for patients in the intervention group as well as the usual care group. Within the intervention group, visits to clinical pharmacist (P = 0.034) and education (P = 0.004) predicted significantly greater decreases in HbA1c over time.
- Conclusions: Diffusion of treatment may account for the overall HbA1c reduction regardless of treatment group. Results support the need for further pragmatic research to weigh the impact of unblinded designs, outcome measurement, and real-world behaviors on evidence-based interventions.
Key words: diabetes; safety net; multidisciplinary diabetes care; primary care; diffusion of treatment.
The prevalence of type 2 diabetes in the United States is significantly higher among Hispanics and African Americans than in the general population (13% vs. 9.3%) [1]. Similarly, diabetes is highly prevalent among the uninsured, and many patients delay or forgo treatment due to cost [2]. Subsequently, the rates of comorbidities, including stroke, hypertension, and CVD, are elevated in these groups [3].
Association between elevated HbA1c and morbidity and mortality is well-documented, and an HbA1c reduction of just 1% has been shown to reduce mortality and improve quality of life [4]. Uncontrolled diabetes also results in increased medical costs. Reducing HbA1c from 9.0 to 7.5 reduces annual expenditures by as much as 73% [5].
Metropolitan Houston and Harris County, Texas, has one of the largest uninsured metro populations in the United States (over 3.6 million) [6]. Harris Health System serves this uninsured population and is the fourth largest safety net health system in the nation. Approximately 40,000 patients with diabetes receive care within the health system, and 34% of them have an HbA1c value greater than 9.
Developing novel, cost-efficient treatment and management models is crucial when providing care for patients with uncontrolled diabetes. However, the study of implementation strategies to successfully integrate evidence-based interventions in primary care using pragmatic approaches that aim to determine the effectiveness of interventions in “the real world” remain a challenge [7,8]. To address this issue, a quality improvement project was instituted at one of the system’s health centers to improve the care of patients with uncontrolled diabetes (known HbA1c above 9).
Methods
Setting
The pilot project was conducted from 1 Oct 2015 to 31 Dec 2015 in a primary care community health center within Harris Health System in Houston, Texas. This pilot was the first step of an institutional effort to introduce a multidisciplinary model of care across all clinics [9]. Our health center has 6 family medicine providers and 1 advanced practice nurse practitioner, organized into 3 pods with 2 physicians each. We randomly selected 1 pod (team) and designated it the intervention group.
The Standards for Quality Improvement Reporting Excellence guidelines [10] were followed and institutional review board approval was obtained.
Intervention
Practice changes introduced in the intervention team were assignment of a case manager to all patients, referral to a dietician and clinical pharmacist as needed, and patient education sessions. The team’s nurse assumed the role of case manager. The case manager was responsible for reviewing a patient checklist based on the America Diabetes Association guideline for comprehensive diabetes medical evaluation at initial and follow-up visits. Referrals were based on ADA guideline recommendations. Onsite brief patient education was provided to all patients. In addition, patients were enrolled in a “Diabetes 101” class, which follows an evidence-based curriculum that includes participation in at least 2 monthly sessions. Patients were asked to return to the clinic for a follow-up visit after 3 months in order to monitor medication compliance, re-evaluate their care plan, and measure HbA1c The usual care group patients were managed based on the current Standards of Medical Care in Diabetes [11]. The usual care group included patients from the same clinic under the care of providers in the teams that were not included in the multidisciplinary intervention.
Analysis
Data abstracted from de-identified patient records included HbA1c values, interventions received, and sociodemographic data. Generalized linear mixed modeling (GLMM) was used to examine changes in patient HbA1c levels over time [12]. All models included a random intercept to account for correlated observations within patient. All analyses were performed using Proc GLIMMIX in SAS v. 9.3 [13].
Results
A total 271 patients with HbA1c above 9 were included in the analysis: 71 in the intervention group and 188 in the usual care group. The intervention group was further differentiated by month of enrollment: October (n = 37), November (n = 27), and December (n = 9). Mean patient age in the overall sample was 51.6 years.
In the intervention group, most patients received patient education 56% (n = 40), almost half had a clinical pharmacy visit, but only 17% (n = 12) received a dietitian consultation. Overall, there was a 1.4% decrease in HbA1c in the intervention group, compared to a 1.3% HbA1c decrease in the usual care group.
GLMM was used to examine differences in HbA1c levels according to month of intervention enrollment (October vs. November vs. December) in the intervention group over time. Figure 1 shows predicted HbA1c values over time with trend lines fit for each of the three subgroups.
Preliminary analysis showed that potential contamination (diffusion of the treatment) would be likely to attenuate differences in the outcomes between the intervention and usual care conditions. Further analysis by subgroups were conducted to describe the intervention potential “spillover” to the usual care group participants not intended to receive the intervention. GLMM also examined differences in HbA1c levels between the intervention and usual care groups over time. The interaction between each treatment group and time was not statistically significant (F(2,268) = 1.34, P = 0.26), indicating that changes in HbA1c over time were not related to treatment group. A statistically reliable main effect for time (F(1,268) = 44.33, P < 0.001) indicated that in all groups, HbA1c values significantly decreased over time.
Follow-up analyses utilized GLMM to examine differences in HbA1c levels among patients in both groups who received at least one of the interventions (visiting a dietician, clinical pharmacist, education session, and clinical case manager). The interaction between intervention and time was not statistically significant for visiting the dietician, receiving education, or being assigned a case manager. The interaction between time and visiting a clinical pharmacist was statistically significant (F(1,204)= 7.78, P = 0.01) such that patients visiting the clinical pharmacist had lower HbA1c values over time relative to those that did not (Figure 2).
Discussion
HbA1c decreased significantly among intervention patients with uncontrolled diabetes over a 3-month period, regardless of which month they entered the study. However, there was no significant difference in HbA1c reduction between patients who received all 4 multidisciplinary interventions, one intervention, or those who received usual care. Patients in the intervention who attended clinical pharmacist visits had significantly greater HbA1c reduction than patients who did not, as did patients who attended a diabetes education session by a patient educator.
Diffusion of treatment may account for the overall HbA1c reduction regardless of treatment group. Diffusion refers to the unintended spread of a treatment effect when participants receive some or all treatments from an intervention to which they were not assigned, making outcomes descriptions of all study groups more challenging [14]. During the implementation period, other physicians and nurses in the clinic were aware of the multidisciplinary care model being piloted, and may have taken the initiative to connect their patients with clinical pharmacists, dieticians, certified diabetes educators, and clinical case managers. Pragmatic interventions are intended to maintain the internal validity of randomized control trials, yet they are meant to be implemented as close as possible to real-world settings in order to help patients, clinicians, and payers making informed health care decisions [8]. In this regard, participants in the control group could be exposed to the intervention through staff contact between the assigned groups implementing some of the intervention under study. In that case, the diffusion of treatment would be likely to attenuate differences in the outcomes between treatment and control groups [15].
This study has several limitations. We studied a small sample of patients that reflected the primary care population in one clinic in a safety net system with minority, underserved, and high-risk patients. Although attempts were made to keep the intervention limited to the intervention pod, diffusion of treatment might have impacted the internal validity of this intervention.
In summary, our results support the need for further systematic research work to weigh the impact of unblinded designs, simplified recruitment and outcome measurement, and real-world behaviors (such as noncompliance, cross over, and dropout) on evidence-based and multidisciplinary clinical interventions.
Acknowledgements: The authors would like to thank Krystal Gamarra, MSW, LCSW, and Hope Galvan, MS, RN, CVRN-BC, CDE for assistance with project implementation and data collection throughout this process.
Corresponding author: Maria C. Mejia de Grubb, MD, MPH, 3751 Kirby Dr, Suite 600, Houston, TX 77098, [email protected].
Financial disclosures: None.
From the Department of Family and Community Medicine, McGovern Medical School at the University of Texas Health Science Center, Houston, TX (Dr. Zare, Ms Klawans, Dr. Moreno), Department of Family Medicine and Community Medicine, Baylor College of Medicine, Houston, TX (Dr. Mejia de Grubb, Dr. Juneja, Dr. Zoorob), Department of Psychiatry, McGovern Medical School at the University of Texas Health Science Center, Houston, TX (Dr. Suchting), andHarris Health System, Houston TX (Ms. Mathis).
Abstract
- Objective: To describe a pilot project to improve care for patients with uncontrolled diabetes in a safety net clinic.
- Methods: One of 3 clinical teams was designated the intervention team. Changes implemented by the intervention team included patient referral to a dietician and/or clinical pharmacist, provision of patient education, and assignment of a case manager. We compared outcomes of patients in the intervention group (n = 71), vs those receiving care from the other 2 teams (usual care) (n = 188).
- Results: HbA1c significantly decreased over time for patients in the intervention group as well as the usual care group. Within the intervention group, visits to clinical pharmacist (P = 0.034) and education (P = 0.004) predicted significantly greater decreases in HbA1c over time.
- Conclusions: Diffusion of treatment may account for the overall HbA1c reduction regardless of treatment group. Results support the need for further pragmatic research to weigh the impact of unblinded designs, outcome measurement, and real-world behaviors on evidence-based interventions.
Key words: diabetes; safety net; multidisciplinary diabetes care; primary care; diffusion of treatment.
The prevalence of type 2 diabetes in the United States is significantly higher among Hispanics and African Americans than in the general population (13% vs. 9.3%) [1]. Similarly, diabetes is highly prevalent among the uninsured, and many patients delay or forgo treatment due to cost [2]. Subsequently, the rates of comorbidities, including stroke, hypertension, and CVD, are elevated in these groups [3].
Association between elevated HbA1c and morbidity and mortality is well-documented, and an HbA1c reduction of just 1% has been shown to reduce mortality and improve quality of life [4]. Uncontrolled diabetes also results in increased medical costs. Reducing HbA1c from 9.0 to 7.5 reduces annual expenditures by as much as 73% [5].
Metropolitan Houston and Harris County, Texas, has one of the largest uninsured metro populations in the United States (over 3.6 million) [6]. Harris Health System serves this uninsured population and is the fourth largest safety net health system in the nation. Approximately 40,000 patients with diabetes receive care within the health system, and 34% of them have an HbA1c value greater than 9.
Developing novel, cost-efficient treatment and management models is crucial when providing care for patients with uncontrolled diabetes. However, the study of implementation strategies to successfully integrate evidence-based interventions in primary care using pragmatic approaches that aim to determine the effectiveness of interventions in “the real world” remain a challenge [7,8]. To address this issue, a quality improvement project was instituted at one of the system’s health centers to improve the care of patients with uncontrolled diabetes (known HbA1c above 9).
Methods
Setting
The pilot project was conducted from 1 Oct 2015 to 31 Dec 2015 in a primary care community health center within Harris Health System in Houston, Texas. This pilot was the first step of an institutional effort to introduce a multidisciplinary model of care across all clinics [9]. Our health center has 6 family medicine providers and 1 advanced practice nurse practitioner, organized into 3 pods with 2 physicians each. We randomly selected 1 pod (team) and designated it the intervention group.
The Standards for Quality Improvement Reporting Excellence guidelines [10] were followed and institutional review board approval was obtained.
Intervention
Practice changes introduced in the intervention team were assignment of a case manager to all patients, referral to a dietician and clinical pharmacist as needed, and patient education sessions. The team’s nurse assumed the role of case manager. The case manager was responsible for reviewing a patient checklist based on the America Diabetes Association guideline for comprehensive diabetes medical evaluation at initial and follow-up visits. Referrals were based on ADA guideline recommendations. Onsite brief patient education was provided to all patients. In addition, patients were enrolled in a “Diabetes 101” class, which follows an evidence-based curriculum that includes participation in at least 2 monthly sessions. Patients were asked to return to the clinic for a follow-up visit after 3 months in order to monitor medication compliance, re-evaluate their care plan, and measure HbA1c The usual care group patients were managed based on the current Standards of Medical Care in Diabetes [11]. The usual care group included patients from the same clinic under the care of providers in the teams that were not included in the multidisciplinary intervention.
Analysis
Data abstracted from de-identified patient records included HbA1c values, interventions received, and sociodemographic data. Generalized linear mixed modeling (GLMM) was used to examine changes in patient HbA1c levels over time [12]. All models included a random intercept to account for correlated observations within patient. All analyses were performed using Proc GLIMMIX in SAS v. 9.3 [13].
Results
A total 271 patients with HbA1c above 9 were included in the analysis: 71 in the intervention group and 188 in the usual care group. The intervention group was further differentiated by month of enrollment: October (n = 37), November (n = 27), and December (n = 9). Mean patient age in the overall sample was 51.6 years.
In the intervention group, most patients received patient education 56% (n = 40), almost half had a clinical pharmacy visit, but only 17% (n = 12) received a dietitian consultation. Overall, there was a 1.4% decrease in HbA1c in the intervention group, compared to a 1.3% HbA1c decrease in the usual care group.
GLMM was used to examine differences in HbA1c levels according to month of intervention enrollment (October vs. November vs. December) in the intervention group over time. Figure 1 shows predicted HbA1c values over time with trend lines fit for each of the three subgroups.
Preliminary analysis showed that potential contamination (diffusion of the treatment) would be likely to attenuate differences in the outcomes between the intervention and usual care conditions. Further analysis by subgroups were conducted to describe the intervention potential “spillover” to the usual care group participants not intended to receive the intervention. GLMM also examined differences in HbA1c levels between the intervention and usual care groups over time. The interaction between each treatment group and time was not statistically significant (F(2,268) = 1.34, P = 0.26), indicating that changes in HbA1c over time were not related to treatment group. A statistically reliable main effect for time (F(1,268) = 44.33, P < 0.001) indicated that in all groups, HbA1c values significantly decreased over time.
Follow-up analyses utilized GLMM to examine differences in HbA1c levels among patients in both groups who received at least one of the interventions (visiting a dietician, clinical pharmacist, education session, and clinical case manager). The interaction between intervention and time was not statistically significant for visiting the dietician, receiving education, or being assigned a case manager. The interaction between time and visiting a clinical pharmacist was statistically significant (F(1,204)= 7.78, P = 0.01) such that patients visiting the clinical pharmacist had lower HbA1c values over time relative to those that did not (Figure 2).
Discussion
HbA1c decreased significantly among intervention patients with uncontrolled diabetes over a 3-month period, regardless of which month they entered the study. However, there was no significant difference in HbA1c reduction between patients who received all 4 multidisciplinary interventions, one intervention, or those who received usual care. Patients in the intervention who attended clinical pharmacist visits had significantly greater HbA1c reduction than patients who did not, as did patients who attended a diabetes education session by a patient educator.
Diffusion of treatment may account for the overall HbA1c reduction regardless of treatment group. Diffusion refers to the unintended spread of a treatment effect when participants receive some or all treatments from an intervention to which they were not assigned, making outcomes descriptions of all study groups more challenging [14]. During the implementation period, other physicians and nurses in the clinic were aware of the multidisciplinary care model being piloted, and may have taken the initiative to connect their patients with clinical pharmacists, dieticians, certified diabetes educators, and clinical case managers. Pragmatic interventions are intended to maintain the internal validity of randomized control trials, yet they are meant to be implemented as close as possible to real-world settings in order to help patients, clinicians, and payers making informed health care decisions [8]. In this regard, participants in the control group could be exposed to the intervention through staff contact between the assigned groups implementing some of the intervention under study. In that case, the diffusion of treatment would be likely to attenuate differences in the outcomes between treatment and control groups [15].
This study has several limitations. We studied a small sample of patients that reflected the primary care population in one clinic in a safety net system with minority, underserved, and high-risk patients. Although attempts were made to keep the intervention limited to the intervention pod, diffusion of treatment might have impacted the internal validity of this intervention.
In summary, our results support the need for further systematic research work to weigh the impact of unblinded designs, simplified recruitment and outcome measurement, and real-world behaviors (such as noncompliance, cross over, and dropout) on evidence-based and multidisciplinary clinical interventions.
Acknowledgements: The authors would like to thank Krystal Gamarra, MSW, LCSW, and Hope Galvan, MS, RN, CVRN-BC, CDE for assistance with project implementation and data collection throughout this process.
Corresponding author: Maria C. Mejia de Grubb, MD, MPH, 3751 Kirby Dr, Suite 600, Houston, TX 77098, [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. Accessed at www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
2. Casagrande SS, Cowie CC. Health insurance coverage among people with and without diabetes in the US adult population. Diabetes Care 2012;35:2243–9.
3. American Diabetes Association. Statistics about diabetes. Arlington, VA; 2017.
4. Eeg-Olofsson K, Eliasson B, Zethelius B, et al. HbA1c reduction and risk of cardiovascular diseases in type 2 diabetes: an observational study from the Swedish NDR. Diabetes 2012.
5. Baxter M, Hudson R, Mahon J, et al. Estimating the impact of better management of glycaemic control in adults with type 1 and type 2 diabetes on the number of clinical complications and the associated financial benefit. Diabet Med 2016;33:1575–81.
6. Harris County Healthcare Alliance. The State of Health in Houston/Harris County 2015-2016. Accessed 17 Mar 2015 at http://houstonstateofhealth.org/soh_doc/.
7. Chalkidou K, Tunis S, Whicher D, et al. The role for pragmatic randomized controlled trials (pRCTs) in comparative effectiveness research. Clin Trials (London, England) 2012;9:436–46.
8. Tricoci P, Allen JM, Kramer JM, et al. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009;301:831–41.
9. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Qty 1996:511–44.
10. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Safety 2016;25:986–92.
11. Standards of Medical Care in Diabetes—2015 Abridged for Primary Care Providers. American Diabetes Association. Clin Diabetes 2015;33:97–111.
12. Gelman A, Hill J. Data analysis using regression and multilevelhierarchical models. New York: Cambridge University Press; 2007.
13. SAS Institute I. Base SAS Procedures Guide: Statistical procedures. In: SAS Institute I, editor. Cary, NC; 2011.
14. Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for general causal inference. Boston: Houghton Mifflin; 2002.
15. Kane R. Understanding health care outcomes research. 2nd ed. In: Learning JB, editor. Burlington, MA: Jones and Bartlett; 2006: 44–6.
1. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. Accessed at www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
2. Casagrande SS, Cowie CC. Health insurance coverage among people with and without diabetes in the US adult population. Diabetes Care 2012;35:2243–9.
3. American Diabetes Association. Statistics about diabetes. Arlington, VA; 2017.
4. Eeg-Olofsson K, Eliasson B, Zethelius B, et al. HbA1c reduction and risk of cardiovascular diseases in type 2 diabetes: an observational study from the Swedish NDR. Diabetes 2012.
5. Baxter M, Hudson R, Mahon J, et al. Estimating the impact of better management of glycaemic control in adults with type 1 and type 2 diabetes on the number of clinical complications and the associated financial benefit. Diabet Med 2016;33:1575–81.
6. Harris County Healthcare Alliance. The State of Health in Houston/Harris County 2015-2016. Accessed 17 Mar 2015 at http://houstonstateofhealth.org/soh_doc/.
7. Chalkidou K, Tunis S, Whicher D, et al. The role for pragmatic randomized controlled trials (pRCTs) in comparative effectiveness research. Clin Trials (London, England) 2012;9:436–46.
8. Tricoci P, Allen JM, Kramer JM, et al. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009;301:831–41.
9. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Qty 1996:511–44.
10. Ogrinc G, Davies L, Goodman D, et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Safety 2016;25:986–92.
11. Standards of Medical Care in Diabetes—2015 Abridged for Primary Care Providers. American Diabetes Association. Clin Diabetes 2015;33:97–111.
12. Gelman A, Hill J. Data analysis using regression and multilevelhierarchical models. New York: Cambridge University Press; 2007.
13. SAS Institute I. Base SAS Procedures Guide: Statistical procedures. In: SAS Institute I, editor. Cary, NC; 2011.
14. Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for general causal inference. Boston: Houghton Mifflin; 2002.
15. Kane R. Understanding health care outcomes research. 2nd ed. In: Learning JB, editor. Burlington, MA: Jones and Bartlett; 2006: 44–6.
Low-Intensity PSA-Based Screening Did Not Reduce Prostate Cancer Mortality
Study Overview
Objective. To determine the effect of a single prostate-specific antigen (PSA) screening and standardized diagnostic pathway on prostate cancer–specific mortality when compared with no screening.
Design. Cluster randomized controlled trial.
Setting and participants. The study was conducted at 573 primary care clinics in the United Kingdom. 419,582 men, 50 to 69 years of age, were recruited between 2001 and 2009 and follow-up ended in 2016. Primary care clinics were randomized to intervention or control. Men in intervention group primary care clinics received an invitation to a single PSA test followed by standardized prostate biopsy in men with PSA levels of 3 ng/mL or greater. A trial that compared radical prostatectomy, radiotherapy, and androgen deprivation therapy and active monitoring was embedded within the screening trial [1]. The control group practices provided standard treatment and PSA testing was provided only to men who requested it. The majority of primary practices were in urban areas (88%–90%) and with multiple partners within the practice (88%–89%). Cases of prostate cancer that were detected in the intervention or control groups during the course of the study were managed by the same clinicians.
Main outcome measures. Main study outcome measures were definite, probable, or intervention-related prostate cancer mortality at a median follow-up of 10 years. An independent cause of death evaluation committee that was blinded to group assignment determined the cause of death in each case. The secondary outcomes included all-cause mortality and prostate cancer stage and Gleason grade at cancer diagnosis. The analysis was an intention-to-screen analysis. Survival analysis using Kaplan-Meier plots were done to demonstrate cumulative incidence of outcomes discussed above. Mixed effects Poisson regression models were used to compare prostate cancer incidence and mortality in intervention vs. control practices accounting for clustering.
Main results. A total of 189,386 men were in the intervention group, 40% attended the PSA testing clinic, and 67,313 (36%) had a blood sample taken for PSA testing, resulting in 64,436 valid PSA test result. 6857 (11%) had elevated PSA levels, of which 85% had a prostate biopsy. In the control group, it was estimated that contamination (PSA testing in the control group) occurred at a rate of approximately 10%–15% over 10 years. After a median follow-up of 10 years, 549 men died of prostate cancer–related causes in the intervention group, at a rate of 0.3 per 1000 person-years, and 647 men died of prostate cancer–related causes in the control group, at a rate of 0.31 per 1000 person-years. The rate difference was 0.013 per 1000 person-years with a risk ratio (RR) of 0.96 (95% confidence interval [CI], 0.85–1.08), P = 0.50), which was not statistically significant. The number of men diagnosed with prostate cancer was higher in the intervention group than in the control group (4.3% vs. 3.6%, RR 1.19 (95% CI 1.14–1.25), P < 0.001). The incidence rate was 4.45 per 1000 person-years in the intervention group and 3.80 per 1000 person-years in the control group. The prostate cancer tumors in the intervention group were less likely to be high grade or advanced stage when compared to the control group. There were 25,459 deaths in the intervention group and 28,306 deaths in the control group. There was no significant difference in the rates of all-cause mortality between the two groups.
Conclusion. The study found that a single PSA screening among men aged 50–69 did not reduce prostate cancer mortality at 10 years follow-up, but led to the increase in the detection of low-risk prostate cancer cases. This result does not support the screening strategy of a single PSA testing for population-based screening for prostate cancer.
Commentary
The use of a PSA test for population-based screening for prostate cancer is controversial; the United States Preventive Services Task Force (USPSTF) recommended against the routine use of PSA test for screening for prostate cancer because the evidence of its benefit is weak and because of the potential risks of unintended consequences of PSA screening [2]. This study is the largest study to date on PSA screening and it found that a low-intensity screening approach—a single PSA test—was not effective in reducing prostate cancer deaths, but rather identified early-stage prostate cancer cases. This result contrasts with previous large scale studies that found that screening led to an increased rate of prostate cancer diagnosis and reduced prostate cancer mortality in one trial [3] and no effect on diagnosis or mortality in another [4].
The rationale for US
Applications for Clinical Practice
PSA test as a diagnostic tool for prostate cancer has significant drawbacks, and population screening strategies using this test will need to grapple with issues of misdiagnosis, overdiagnosis, and treatment that can have potential harmful consequences. The alternative of not screening is that prostate cancer may be diagnosed at later stages and more men may suffer morbidity and mortality from the disease. A better test and screening strategy are needed to balance the benefits and harms of screening so that older men may benefit from early diagnosis of prostate cancer.
1. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415–24.
2. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:120–34.
3. Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320–8.
4. Andriole GL, Grubb RL III, Buys SS, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009;360:1310–9.
5. Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425–37.
Study Overview
Objective. To determine the effect of a single prostate-specific antigen (PSA) screening and standardized diagnostic pathway on prostate cancer–specific mortality when compared with no screening.
Design. Cluster randomized controlled trial.
Setting and participants. The study was conducted at 573 primary care clinics in the United Kingdom. 419,582 men, 50 to 69 years of age, were recruited between 2001 and 2009 and follow-up ended in 2016. Primary care clinics were randomized to intervention or control. Men in intervention group primary care clinics received an invitation to a single PSA test followed by standardized prostate biopsy in men with PSA levels of 3 ng/mL or greater. A trial that compared radical prostatectomy, radiotherapy, and androgen deprivation therapy and active monitoring was embedded within the screening trial [1]. The control group practices provided standard treatment and PSA testing was provided only to men who requested it. The majority of primary practices were in urban areas (88%–90%) and with multiple partners within the practice (88%–89%). Cases of prostate cancer that were detected in the intervention or control groups during the course of the study were managed by the same clinicians.
Main outcome measures. Main study outcome measures were definite, probable, or intervention-related prostate cancer mortality at a median follow-up of 10 years. An independent cause of death evaluation committee that was blinded to group assignment determined the cause of death in each case. The secondary outcomes included all-cause mortality and prostate cancer stage and Gleason grade at cancer diagnosis. The analysis was an intention-to-screen analysis. Survival analysis using Kaplan-Meier plots were done to demonstrate cumulative incidence of outcomes discussed above. Mixed effects Poisson regression models were used to compare prostate cancer incidence and mortality in intervention vs. control practices accounting for clustering.
Main results. A total of 189,386 men were in the intervention group, 40% attended the PSA testing clinic, and 67,313 (36%) had a blood sample taken for PSA testing, resulting in 64,436 valid PSA test result. 6857 (11%) had elevated PSA levels, of which 85% had a prostate biopsy. In the control group, it was estimated that contamination (PSA testing in the control group) occurred at a rate of approximately 10%–15% over 10 years. After a median follow-up of 10 years, 549 men died of prostate cancer–related causes in the intervention group, at a rate of 0.3 per 1000 person-years, and 647 men died of prostate cancer–related causes in the control group, at a rate of 0.31 per 1000 person-years. The rate difference was 0.013 per 1000 person-years with a risk ratio (RR) of 0.96 (95% confidence interval [CI], 0.85–1.08), P = 0.50), which was not statistically significant. The number of men diagnosed with prostate cancer was higher in the intervention group than in the control group (4.3% vs. 3.6%, RR 1.19 (95% CI 1.14–1.25), P < 0.001). The incidence rate was 4.45 per 1000 person-years in the intervention group and 3.80 per 1000 person-years in the control group. The prostate cancer tumors in the intervention group were less likely to be high grade or advanced stage when compared to the control group. There were 25,459 deaths in the intervention group and 28,306 deaths in the control group. There was no significant difference in the rates of all-cause mortality between the two groups.
Conclusion. The study found that a single PSA screening among men aged 50–69 did not reduce prostate cancer mortality at 10 years follow-up, but led to the increase in the detection of low-risk prostate cancer cases. This result does not support the screening strategy of a single PSA testing for population-based screening for prostate cancer.
Commentary
The use of a PSA test for population-based screening for prostate cancer is controversial; the United States Preventive Services Task Force (USPSTF) recommended against the routine use of PSA test for screening for prostate cancer because the evidence of its benefit is weak and because of the potential risks of unintended consequences of PSA screening [2]. This study is the largest study to date on PSA screening and it found that a low-intensity screening approach—a single PSA test—was not effective in reducing prostate cancer deaths, but rather identified early-stage prostate cancer cases. This result contrasts with previous large scale studies that found that screening led to an increased rate of prostate cancer diagnosis and reduced prostate cancer mortality in one trial [3] and no effect on diagnosis or mortality in another [4].
The rationale for US
Applications for Clinical Practice
PSA test as a diagnostic tool for prostate cancer has significant drawbacks, and population screening strategies using this test will need to grapple with issues of misdiagnosis, overdiagnosis, and treatment that can have potential harmful consequences. The alternative of not screening is that prostate cancer may be diagnosed at later stages and more men may suffer morbidity and mortality from the disease. A better test and screening strategy are needed to balance the benefits and harms of screening so that older men may benefit from early diagnosis of prostate cancer.
Study Overview
Objective. To determine the effect of a single prostate-specific antigen (PSA) screening and standardized diagnostic pathway on prostate cancer–specific mortality when compared with no screening.
Design. Cluster randomized controlled trial.
Setting and participants. The study was conducted at 573 primary care clinics in the United Kingdom. 419,582 men, 50 to 69 years of age, were recruited between 2001 and 2009 and follow-up ended in 2016. Primary care clinics were randomized to intervention or control. Men in intervention group primary care clinics received an invitation to a single PSA test followed by standardized prostate biopsy in men with PSA levels of 3 ng/mL or greater. A trial that compared radical prostatectomy, radiotherapy, and androgen deprivation therapy and active monitoring was embedded within the screening trial [1]. The control group practices provided standard treatment and PSA testing was provided only to men who requested it. The majority of primary practices were in urban areas (88%–90%) and with multiple partners within the practice (88%–89%). Cases of prostate cancer that were detected in the intervention or control groups during the course of the study were managed by the same clinicians.
Main outcome measures. Main study outcome measures were definite, probable, or intervention-related prostate cancer mortality at a median follow-up of 10 years. An independent cause of death evaluation committee that was blinded to group assignment determined the cause of death in each case. The secondary outcomes included all-cause mortality and prostate cancer stage and Gleason grade at cancer diagnosis. The analysis was an intention-to-screen analysis. Survival analysis using Kaplan-Meier plots were done to demonstrate cumulative incidence of outcomes discussed above. Mixed effects Poisson regression models were used to compare prostate cancer incidence and mortality in intervention vs. control practices accounting for clustering.
Main results. A total of 189,386 men were in the intervention group, 40% attended the PSA testing clinic, and 67,313 (36%) had a blood sample taken for PSA testing, resulting in 64,436 valid PSA test result. 6857 (11%) had elevated PSA levels, of which 85% had a prostate biopsy. In the control group, it was estimated that contamination (PSA testing in the control group) occurred at a rate of approximately 10%–15% over 10 years. After a median follow-up of 10 years, 549 men died of prostate cancer–related causes in the intervention group, at a rate of 0.3 per 1000 person-years, and 647 men died of prostate cancer–related causes in the control group, at a rate of 0.31 per 1000 person-years. The rate difference was 0.013 per 1000 person-years with a risk ratio (RR) of 0.96 (95% confidence interval [CI], 0.85–1.08), P = 0.50), which was not statistically significant. The number of men diagnosed with prostate cancer was higher in the intervention group than in the control group (4.3% vs. 3.6%, RR 1.19 (95% CI 1.14–1.25), P < 0.001). The incidence rate was 4.45 per 1000 person-years in the intervention group and 3.80 per 1000 person-years in the control group. The prostate cancer tumors in the intervention group were less likely to be high grade or advanced stage when compared to the control group. There were 25,459 deaths in the intervention group and 28,306 deaths in the control group. There was no significant difference in the rates of all-cause mortality between the two groups.
Conclusion. The study found that a single PSA screening among men aged 50–69 did not reduce prostate cancer mortality at 10 years follow-up, but led to the increase in the detection of low-risk prostate cancer cases. This result does not support the screening strategy of a single PSA testing for population-based screening for prostate cancer.
Commentary
The use of a PSA test for population-based screening for prostate cancer is controversial; the United States Preventive Services Task Force (USPSTF) recommended against the routine use of PSA test for screening for prostate cancer because the evidence of its benefit is weak and because of the potential risks of unintended consequences of PSA screening [2]. This study is the largest study to date on PSA screening and it found that a low-intensity screening approach—a single PSA test—was not effective in reducing prostate cancer deaths, but rather identified early-stage prostate cancer cases. This result contrasts with previous large scale studies that found that screening led to an increased rate of prostate cancer diagnosis and reduced prostate cancer mortality in one trial [3] and no effect on diagnosis or mortality in another [4].
The rationale for US
Applications for Clinical Practice
PSA test as a diagnostic tool for prostate cancer has significant drawbacks, and population screening strategies using this test will need to grapple with issues of misdiagnosis, overdiagnosis, and treatment that can have potential harmful consequences. The alternative of not screening is that prostate cancer may be diagnosed at later stages and more men may suffer morbidity and mortality from the disease. A better test and screening strategy are needed to balance the benefits and harms of screening so that older men may benefit from early diagnosis of prostate cancer.
1. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415–24.
2. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:120–34.
3. Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320–8.
4. Andriole GL, Grubb RL III, Buys SS, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009;360:1310–9.
5. Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425–37.
1. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415–24.
2. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:120–34.
3. Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320–8.
4. Andriole GL, Grubb RL III, Buys SS, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009;360:1310–9.
5. Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425–37.
CAR T-Cell Therapy Shows High Levels of Durable Response in Refractory Large B-Cell Lymphoma
Study Overview
Objective. To evaluate the efficacy and safety of the anti-CD19 chimeric antigen receptor (CAR) T-cell, axicabtagene ciloleucel (axi-cel), in patients with refractory large B-cell lymphoma.
Design. The ZUMA-1 trial was a phase 1-2 multicenter study. The results of the primary analysis and updated analysis with 1-year follow up of the phase 2 portion of ZUMA-1 are reported here.
Setting and participants. The phase 2 portion of the ZUMA-1 trial enrolled 111 patients from 22 centers in the United States (21) and Israel (1) from November 2015 through September 2016. Eligible patients included those with histologically confirmed large B-cell lymphoma, primary mediastinal B-cell lymphoma or transformed follicular lymphoma. Patients were required to have refractory disease, defined as disease progression or stable disease as the best response to chemotherapy or disease progression within 12 months following autologous stem cell transplantation. All patients were required to have adequate organ function, an absolute neutrophil count > 1000, absolute lymphocyte count > 100 and platelet count > 75,000.
Intervention. Patients first underwent leukapheresis and CAR T-cell manufacturing. Following this patients were admitted to the hospital and received a low-dose conditioning regimen consisting of fludarabine 30 mg/m2 and cyclophosphamide 500 mg/m2 given on days –5, –4 and –3. On day 0 the patient was infused with their manufactured CAR T-cell product at a target dose of 2 x106 CAR T cells per kilogram of body weight. Patients could not receive “bridging chemotherapy” between leukapheresis and infusion of axi-cel product. Patients could be retreated with axi-cel if they experienced disease progression at least 3 months after their first dose.
Main outcome measures. The primary endpoint of this study was objective response rate, which was defined as the combined rate of complete response (CR) and partial response (PR). The secondary endpoints were duration of response, progression-free survival (PFS), overall survival (OS), and adverse events. Blood levels of CAR T cells and serum cytokine levels were followed.
Main results. A total of 111 patients were enrolled. Axi-cel was administered to 101 patients included in the intention to treat analysis. Of these, 77 had diffuse large B-cell lymphoma and 24 had primary mediastinal B-cell lymphoma or transformed follicular lymphoma. The median follow-up was 8.7 months for the primary analysis and updated analysis median follow-up was 15.4 months. The median time from leukapheresis to delivery of the product was 17 days. Only 1 patient had unsuccessful manufacturing. The median age of the treated patients was 58 years. Most of the patients (77%) had disease resistant to second-line or later therapy and 21% had disease relapse after autologous stem cell transplant.
Primary analysis results. The objective response rate was 82% with a 54% CR rate. The median time to response was 1 month and median duration of response was 8.1 months. The response rates were consistent across all subgroups including age, disease stage, IPI score, presence or absence of bulky disease, cell-of-origin subtype, and the use of tocilizumab or glucocorticoids. High response rates were maintained in those with primary refractory disease (response rate 88%) and those with prior autologous stem cell transplant (response rate 76%). The response rate was not influenced by CD19 expression. At the time of the primary analysis 52 patients died from disease progression and 3 died from adverse events during treatment. Forty-four patients remained in remission, 39 of whom maintained a CR.
Updated analysis results. At the time of the updated analysis 108 patients in the phase 1 and phase 2 portions had been followed for at least 12 months. The objective response rate was 82% with a CR rate of 58%. At the data cut-off, 42% remained in response with 40% maintaining a CR. Again, response rates were consistent across all previously mentioned subgroups. The median duration of response was 11.1 months. The median PFS was 5.8 months with PFS rate of 41% at 15 months. The median OS was not reached. A total of 56% of patients remained alive at the time of this analysis.
Safety. During treatment 100% of patients had adverse events (AEs), which were grade 3 or higher in 95%. Fevers (85%), neutropenia (84%) and anemia (66%) were the most common AEs. Myelosuppression was the most common grade 3 or higher AE. Cytokine release syndrome occurred in 93% of patients of which 13% were grade 3 or higher (9% grade 3, 3% grade 4 and 1% grade 5). 17% of patients required vasopressor support. The median time from infusion to the onset of cytokine release syndrome was 2 days (range, 1–12). The median time to resolution was 8 days. One grade 5 event of hemophagocytic lymphohistiocytosis and one grade 5 cardiac arrest occurred. Grade 3 or higher neurological events occurred in 28% of patients, with encephalopathy occurring in 21%. Neurological events occurred at a median of 5 days after infusion and lasted for a median of 17 days after infusion. Forty-three percent of patients received tocilizumab and 27% received glucocorticoids.
Biomarkers. CAR T levels peaked within 14 days after infusion. Three patients with a CR at 24 months still had detectable levels in the blood. CAR T cell expansion as significantly associated with disease response. Interleukin -6, -10, -15 and -2Ra levels were significantly associated with neurological events and cytokine release syndrome of grade 3 or higher. Anti-CAR antibodies were not detected in any patient.
Commentary
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma with 5-year survival rates of ~60% following conventional chemoimmunotherapy in the first-line setting. Following relapse, salvage therapy followed by high-dose chemotherapy with autologous stem-cell transplantation can result in long-term remissions; however, those who relapse have a poor prognosis. The recently published SCHOLAR-1 study retrospectively analyzed the outcomes of patients with relapsed or refractory DLBCL and found that for patients with refractory disease the objective response to salvage therapy was only 26% (7% CR) with a median OS of 6.3 months [1]. CAR-engineered T cells offer a novel and revolutionary therapy for these patients, whom otherwise have very poor outcomes.
Early CAR T-cell trials by Bretjens and colleagues first documented a CR in a subset of patients with refractory hematologic malignancies [2]. Since that time there has been tremendous advancement in CAR T development and clinical application. In the December 2017 issue of the New England Journal of Medicine there were 2 studies published validating the efficacy of CD19-targeted CAR T-cell therapy in relapsed/refractory lymphoma, the current ZUMA-1 study as well as another small case-series by Schuster and colleagues. Schuster et al evaluated the CD19-directed CAR, CTL019, in 28 patients with relapsed/refractory DLBCL or follicular lymphoma. The ORR noted in this study was 64% with a CR rate of 57% [3]. Similarly, in the current ZUMA-1 study the CR rate was 54% in 101 patients with relapsed and refractory large B-cell lymphomas. In addition, with a median follow-up of 15.4 months responses were ongoing in 42% of patients including 40% who had a CR. The durability of such responses has been demonstrated in 3 of 7 patients from the phase 1 portion of this study at 24 months. Durable responses have also been reported with anti-CD19 CAR T-cell therapy in 4 of 5 patients who had a CR and remain in remission after 3-4 years of follow-up [4]. While promising, the durability of responses remains unclear. While CAR therapy represents an exciting therapeutic strategy, it should be noted that in this study approximately 50% of patients will not achieve a durable response and the reason for this is not completely understood.
One of the most discussed aspects of CAR therapy has been the unique toxicity profile, which was again noted in the ZUMA-1 study. As noted, 95% of patients in this study experienced a grade 3 or higher AE. Of interest, cytokine release syndrome occurred in 93% of patients with 13% being grade 3 or higher. There were 2 deaths attributed to such. Neurological toxicity was also noted in 64% of patients in this trial. While the vast majority of these AEs were reversible, they clearly represent high treatment-related morbidity.
The results of the ZUMA-1 study lead to the FDA approval of anti-CD19 CAR T-cell therapy for relapsed or refractory large B-cell lymphoma in October 2017 and represents a pivotal advancement in the management of these patients with otherwise limited treatment options and overall poor outcomes. The ZUMA-1 trial not only demonstrates the efficacy of such agents but also demonstrates the feasibility of incorporating them into clinical practice with a 99% manufacturing success rate and short (median 17 days) product delivery time. The economic burden of such therapies warrant particular consideration as the indications for CAR therapy will continue to expand, driving the cost of care higher. Nevertheless, this represents an exciting step forward in personalized medicine.
Applications for Clinical Practice
CAR T-cell therapy with the CD-19 targeted CAR axicabtagene ciloleucel (axi-cel) results in a high rate of objective and durable responses in patients with relapsed or refractory large B-cell lymphomas. While such treatment does carry a high rate of toxicity in regards to cytokine release and neurological complications, this represents an important treatment option in patients with refractory disease with a historically poor prognosis. However, there will be a need to develop policies to address the economic challenges associated with such treatments.
1. Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017;130:1800–8.
2. Brentjens RJ, RIviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011;118:4817–28.
3. Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017;377:2545–54.
4. Kochenderfer JN, Somerville RP, Lu T, et al. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther 2017;25:2245–53.
Study Overview
Objective. To evaluate the efficacy and safety of the anti-CD19 chimeric antigen receptor (CAR) T-cell, axicabtagene ciloleucel (axi-cel), in patients with refractory large B-cell lymphoma.
Design. The ZUMA-1 trial was a phase 1-2 multicenter study. The results of the primary analysis and updated analysis with 1-year follow up of the phase 2 portion of ZUMA-1 are reported here.
Setting and participants. The phase 2 portion of the ZUMA-1 trial enrolled 111 patients from 22 centers in the United States (21) and Israel (1) from November 2015 through September 2016. Eligible patients included those with histologically confirmed large B-cell lymphoma, primary mediastinal B-cell lymphoma or transformed follicular lymphoma. Patients were required to have refractory disease, defined as disease progression or stable disease as the best response to chemotherapy or disease progression within 12 months following autologous stem cell transplantation. All patients were required to have adequate organ function, an absolute neutrophil count > 1000, absolute lymphocyte count > 100 and platelet count > 75,000.
Intervention. Patients first underwent leukapheresis and CAR T-cell manufacturing. Following this patients were admitted to the hospital and received a low-dose conditioning regimen consisting of fludarabine 30 mg/m2 and cyclophosphamide 500 mg/m2 given on days –5, –4 and –3. On day 0 the patient was infused with their manufactured CAR T-cell product at a target dose of 2 x106 CAR T cells per kilogram of body weight. Patients could not receive “bridging chemotherapy” between leukapheresis and infusion of axi-cel product. Patients could be retreated with axi-cel if they experienced disease progression at least 3 months after their first dose.
Main outcome measures. The primary endpoint of this study was objective response rate, which was defined as the combined rate of complete response (CR) and partial response (PR). The secondary endpoints were duration of response, progression-free survival (PFS), overall survival (OS), and adverse events. Blood levels of CAR T cells and serum cytokine levels were followed.
Main results. A total of 111 patients were enrolled. Axi-cel was administered to 101 patients included in the intention to treat analysis. Of these, 77 had diffuse large B-cell lymphoma and 24 had primary mediastinal B-cell lymphoma or transformed follicular lymphoma. The median follow-up was 8.7 months for the primary analysis and updated analysis median follow-up was 15.4 months. The median time from leukapheresis to delivery of the product was 17 days. Only 1 patient had unsuccessful manufacturing. The median age of the treated patients was 58 years. Most of the patients (77%) had disease resistant to second-line or later therapy and 21% had disease relapse after autologous stem cell transplant.
Primary analysis results. The objective response rate was 82% with a 54% CR rate. The median time to response was 1 month and median duration of response was 8.1 months. The response rates were consistent across all subgroups including age, disease stage, IPI score, presence or absence of bulky disease, cell-of-origin subtype, and the use of tocilizumab or glucocorticoids. High response rates were maintained in those with primary refractory disease (response rate 88%) and those with prior autologous stem cell transplant (response rate 76%). The response rate was not influenced by CD19 expression. At the time of the primary analysis 52 patients died from disease progression and 3 died from adverse events during treatment. Forty-four patients remained in remission, 39 of whom maintained a CR.
Updated analysis results. At the time of the updated analysis 108 patients in the phase 1 and phase 2 portions had been followed for at least 12 months. The objective response rate was 82% with a CR rate of 58%. At the data cut-off, 42% remained in response with 40% maintaining a CR. Again, response rates were consistent across all previously mentioned subgroups. The median duration of response was 11.1 months. The median PFS was 5.8 months with PFS rate of 41% at 15 months. The median OS was not reached. A total of 56% of patients remained alive at the time of this analysis.
Safety. During treatment 100% of patients had adverse events (AEs), which were grade 3 or higher in 95%. Fevers (85%), neutropenia (84%) and anemia (66%) were the most common AEs. Myelosuppression was the most common grade 3 or higher AE. Cytokine release syndrome occurred in 93% of patients of which 13% were grade 3 or higher (9% grade 3, 3% grade 4 and 1% grade 5). 17% of patients required vasopressor support. The median time from infusion to the onset of cytokine release syndrome was 2 days (range, 1–12). The median time to resolution was 8 days. One grade 5 event of hemophagocytic lymphohistiocytosis and one grade 5 cardiac arrest occurred. Grade 3 or higher neurological events occurred in 28% of patients, with encephalopathy occurring in 21%. Neurological events occurred at a median of 5 days after infusion and lasted for a median of 17 days after infusion. Forty-three percent of patients received tocilizumab and 27% received glucocorticoids.
Biomarkers. CAR T levels peaked within 14 days after infusion. Three patients with a CR at 24 months still had detectable levels in the blood. CAR T cell expansion as significantly associated with disease response. Interleukin -6, -10, -15 and -2Ra levels were significantly associated with neurological events and cytokine release syndrome of grade 3 or higher. Anti-CAR antibodies were not detected in any patient.
Commentary
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma with 5-year survival rates of ~60% following conventional chemoimmunotherapy in the first-line setting. Following relapse, salvage therapy followed by high-dose chemotherapy with autologous stem-cell transplantation can result in long-term remissions; however, those who relapse have a poor prognosis. The recently published SCHOLAR-1 study retrospectively analyzed the outcomes of patients with relapsed or refractory DLBCL and found that for patients with refractory disease the objective response to salvage therapy was only 26% (7% CR) with a median OS of 6.3 months [1]. CAR-engineered T cells offer a novel and revolutionary therapy for these patients, whom otherwise have very poor outcomes.
Early CAR T-cell trials by Bretjens and colleagues first documented a CR in a subset of patients with refractory hematologic malignancies [2]. Since that time there has been tremendous advancement in CAR T development and clinical application. In the December 2017 issue of the New England Journal of Medicine there were 2 studies published validating the efficacy of CD19-targeted CAR T-cell therapy in relapsed/refractory lymphoma, the current ZUMA-1 study as well as another small case-series by Schuster and colleagues. Schuster et al evaluated the CD19-directed CAR, CTL019, in 28 patients with relapsed/refractory DLBCL or follicular lymphoma. The ORR noted in this study was 64% with a CR rate of 57% [3]. Similarly, in the current ZUMA-1 study the CR rate was 54% in 101 patients with relapsed and refractory large B-cell lymphomas. In addition, with a median follow-up of 15.4 months responses were ongoing in 42% of patients including 40% who had a CR. The durability of such responses has been demonstrated in 3 of 7 patients from the phase 1 portion of this study at 24 months. Durable responses have also been reported with anti-CD19 CAR T-cell therapy in 4 of 5 patients who had a CR and remain in remission after 3-4 years of follow-up [4]. While promising, the durability of responses remains unclear. While CAR therapy represents an exciting therapeutic strategy, it should be noted that in this study approximately 50% of patients will not achieve a durable response and the reason for this is not completely understood.
One of the most discussed aspects of CAR therapy has been the unique toxicity profile, which was again noted in the ZUMA-1 study. As noted, 95% of patients in this study experienced a grade 3 or higher AE. Of interest, cytokine release syndrome occurred in 93% of patients with 13% being grade 3 or higher. There were 2 deaths attributed to such. Neurological toxicity was also noted in 64% of patients in this trial. While the vast majority of these AEs were reversible, they clearly represent high treatment-related morbidity.
The results of the ZUMA-1 study lead to the FDA approval of anti-CD19 CAR T-cell therapy for relapsed or refractory large B-cell lymphoma in October 2017 and represents a pivotal advancement in the management of these patients with otherwise limited treatment options and overall poor outcomes. The ZUMA-1 trial not only demonstrates the efficacy of such agents but also demonstrates the feasibility of incorporating them into clinical practice with a 99% manufacturing success rate and short (median 17 days) product delivery time. The economic burden of such therapies warrant particular consideration as the indications for CAR therapy will continue to expand, driving the cost of care higher. Nevertheless, this represents an exciting step forward in personalized medicine.
Applications for Clinical Practice
CAR T-cell therapy with the CD-19 targeted CAR axicabtagene ciloleucel (axi-cel) results in a high rate of objective and durable responses in patients with relapsed or refractory large B-cell lymphomas. While such treatment does carry a high rate of toxicity in regards to cytokine release and neurological complications, this represents an important treatment option in patients with refractory disease with a historically poor prognosis. However, there will be a need to develop policies to address the economic challenges associated with such treatments.
Study Overview
Objective. To evaluate the efficacy and safety of the anti-CD19 chimeric antigen receptor (CAR) T-cell, axicabtagene ciloleucel (axi-cel), in patients with refractory large B-cell lymphoma.
Design. The ZUMA-1 trial was a phase 1-2 multicenter study. The results of the primary analysis and updated analysis with 1-year follow up of the phase 2 portion of ZUMA-1 are reported here.
Setting and participants. The phase 2 portion of the ZUMA-1 trial enrolled 111 patients from 22 centers in the United States (21) and Israel (1) from November 2015 through September 2016. Eligible patients included those with histologically confirmed large B-cell lymphoma, primary mediastinal B-cell lymphoma or transformed follicular lymphoma. Patients were required to have refractory disease, defined as disease progression or stable disease as the best response to chemotherapy or disease progression within 12 months following autologous stem cell transplantation. All patients were required to have adequate organ function, an absolute neutrophil count > 1000, absolute lymphocyte count > 100 and platelet count > 75,000.
Intervention. Patients first underwent leukapheresis and CAR T-cell manufacturing. Following this patients were admitted to the hospital and received a low-dose conditioning regimen consisting of fludarabine 30 mg/m2 and cyclophosphamide 500 mg/m2 given on days –5, –4 and –3. On day 0 the patient was infused with their manufactured CAR T-cell product at a target dose of 2 x106 CAR T cells per kilogram of body weight. Patients could not receive “bridging chemotherapy” between leukapheresis and infusion of axi-cel product. Patients could be retreated with axi-cel if they experienced disease progression at least 3 months after their first dose.
Main outcome measures. The primary endpoint of this study was objective response rate, which was defined as the combined rate of complete response (CR) and partial response (PR). The secondary endpoints were duration of response, progression-free survival (PFS), overall survival (OS), and adverse events. Blood levels of CAR T cells and serum cytokine levels were followed.
Main results. A total of 111 patients were enrolled. Axi-cel was administered to 101 patients included in the intention to treat analysis. Of these, 77 had diffuse large B-cell lymphoma and 24 had primary mediastinal B-cell lymphoma or transformed follicular lymphoma. The median follow-up was 8.7 months for the primary analysis and updated analysis median follow-up was 15.4 months. The median time from leukapheresis to delivery of the product was 17 days. Only 1 patient had unsuccessful manufacturing. The median age of the treated patients was 58 years. Most of the patients (77%) had disease resistant to second-line or later therapy and 21% had disease relapse after autologous stem cell transplant.
Primary analysis results. The objective response rate was 82% with a 54% CR rate. The median time to response was 1 month and median duration of response was 8.1 months. The response rates were consistent across all subgroups including age, disease stage, IPI score, presence or absence of bulky disease, cell-of-origin subtype, and the use of tocilizumab or glucocorticoids. High response rates were maintained in those with primary refractory disease (response rate 88%) and those with prior autologous stem cell transplant (response rate 76%). The response rate was not influenced by CD19 expression. At the time of the primary analysis 52 patients died from disease progression and 3 died from adverse events during treatment. Forty-four patients remained in remission, 39 of whom maintained a CR.
Updated analysis results. At the time of the updated analysis 108 patients in the phase 1 and phase 2 portions had been followed for at least 12 months. The objective response rate was 82% with a CR rate of 58%. At the data cut-off, 42% remained in response with 40% maintaining a CR. Again, response rates were consistent across all previously mentioned subgroups. The median duration of response was 11.1 months. The median PFS was 5.8 months with PFS rate of 41% at 15 months. The median OS was not reached. A total of 56% of patients remained alive at the time of this analysis.
Safety. During treatment 100% of patients had adverse events (AEs), which were grade 3 or higher in 95%. Fevers (85%), neutropenia (84%) and anemia (66%) were the most common AEs. Myelosuppression was the most common grade 3 or higher AE. Cytokine release syndrome occurred in 93% of patients of which 13% were grade 3 or higher (9% grade 3, 3% grade 4 and 1% grade 5). 17% of patients required vasopressor support. The median time from infusion to the onset of cytokine release syndrome was 2 days (range, 1–12). The median time to resolution was 8 days. One grade 5 event of hemophagocytic lymphohistiocytosis and one grade 5 cardiac arrest occurred. Grade 3 or higher neurological events occurred in 28% of patients, with encephalopathy occurring in 21%. Neurological events occurred at a median of 5 days after infusion and lasted for a median of 17 days after infusion. Forty-three percent of patients received tocilizumab and 27% received glucocorticoids.
Biomarkers. CAR T levels peaked within 14 days after infusion. Three patients with a CR at 24 months still had detectable levels in the blood. CAR T cell expansion as significantly associated with disease response. Interleukin -6, -10, -15 and -2Ra levels were significantly associated with neurological events and cytokine release syndrome of grade 3 or higher. Anti-CAR antibodies were not detected in any patient.
Commentary
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma with 5-year survival rates of ~60% following conventional chemoimmunotherapy in the first-line setting. Following relapse, salvage therapy followed by high-dose chemotherapy with autologous stem-cell transplantation can result in long-term remissions; however, those who relapse have a poor prognosis. The recently published SCHOLAR-1 study retrospectively analyzed the outcomes of patients with relapsed or refractory DLBCL and found that for patients with refractory disease the objective response to salvage therapy was only 26% (7% CR) with a median OS of 6.3 months [1]. CAR-engineered T cells offer a novel and revolutionary therapy for these patients, whom otherwise have very poor outcomes.
Early CAR T-cell trials by Bretjens and colleagues first documented a CR in a subset of patients with refractory hematologic malignancies [2]. Since that time there has been tremendous advancement in CAR T development and clinical application. In the December 2017 issue of the New England Journal of Medicine there were 2 studies published validating the efficacy of CD19-targeted CAR T-cell therapy in relapsed/refractory lymphoma, the current ZUMA-1 study as well as another small case-series by Schuster and colleagues. Schuster et al evaluated the CD19-directed CAR, CTL019, in 28 patients with relapsed/refractory DLBCL or follicular lymphoma. The ORR noted in this study was 64% with a CR rate of 57% [3]. Similarly, in the current ZUMA-1 study the CR rate was 54% in 101 patients with relapsed and refractory large B-cell lymphomas. In addition, with a median follow-up of 15.4 months responses were ongoing in 42% of patients including 40% who had a CR. The durability of such responses has been demonstrated in 3 of 7 patients from the phase 1 portion of this study at 24 months. Durable responses have also been reported with anti-CD19 CAR T-cell therapy in 4 of 5 patients who had a CR and remain in remission after 3-4 years of follow-up [4]. While promising, the durability of responses remains unclear. While CAR therapy represents an exciting therapeutic strategy, it should be noted that in this study approximately 50% of patients will not achieve a durable response and the reason for this is not completely understood.
One of the most discussed aspects of CAR therapy has been the unique toxicity profile, which was again noted in the ZUMA-1 study. As noted, 95% of patients in this study experienced a grade 3 or higher AE. Of interest, cytokine release syndrome occurred in 93% of patients with 13% being grade 3 or higher. There were 2 deaths attributed to such. Neurological toxicity was also noted in 64% of patients in this trial. While the vast majority of these AEs were reversible, they clearly represent high treatment-related morbidity.
The results of the ZUMA-1 study lead to the FDA approval of anti-CD19 CAR T-cell therapy for relapsed or refractory large B-cell lymphoma in October 2017 and represents a pivotal advancement in the management of these patients with otherwise limited treatment options and overall poor outcomes. The ZUMA-1 trial not only demonstrates the efficacy of such agents but also demonstrates the feasibility of incorporating them into clinical practice with a 99% manufacturing success rate and short (median 17 days) product delivery time. The economic burden of such therapies warrant particular consideration as the indications for CAR therapy will continue to expand, driving the cost of care higher. Nevertheless, this represents an exciting step forward in personalized medicine.
Applications for Clinical Practice
CAR T-cell therapy with the CD-19 targeted CAR axicabtagene ciloleucel (axi-cel) results in a high rate of objective and durable responses in patients with relapsed or refractory large B-cell lymphomas. While such treatment does carry a high rate of toxicity in regards to cytokine release and neurological complications, this represents an important treatment option in patients with refractory disease with a historically poor prognosis. However, there will be a need to develop policies to address the economic challenges associated with such treatments.
1. Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017;130:1800–8.
2. Brentjens RJ, RIviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011;118:4817–28.
3. Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017;377:2545–54.
4. Kochenderfer JN, Somerville RP, Lu T, et al. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther 2017;25:2245–53.
1. Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017;130:1800–8.
2. Brentjens RJ, RIviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011;118:4817–28.
3. Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017;377:2545–54.
4. Kochenderfer JN, Somerville RP, Lu T, et al. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther 2017;25:2245–53.
Help the Heart—Keep the Noise Down
Loud noise is one of the most common workplace hazards in the US. One-quarter of US workers (an estimated 41 million people) report a history of noise exposure at work—and that may put them at risk for heart disease. According to a Centers for Disease Control and Prevention (CDC) study, high blood pressure and high cholesterol are more common among workers exposed to loud noise at work.
The National Institute for Occupational Safety and Health (NIOSH) researchers analyzed data from the 2014 National Health Interview Survey to estimate the prevalence of occupational noise exposure, hearing difficulty, and heart conditions within US industries and occupations. They also looked at the association between workplace noise exposure and heart disease. Their analysis showed:
- 25% of current workers had a history of work-related noise exposure, 14% were exposed in the last year;
- 12% of current workers had hearing difficulty, 24% had high blood pressure, 28% had high cholesterol; and
- Of those cases 58%, 14%, and 9%, respectively, can be attributed to occupational noise exposure.
Study coauthor Liz Masterson, PhD, says, “If noise could be reduced to safer levels in the workplace, more than 5 million cases of hearing difficulty among noise-exposed workers could potentially be prevented.”
Loud noise is one of the most common workplace hazards in the US. One-quarter of US workers (an estimated 41 million people) report a history of noise exposure at work—and that may put them at risk for heart disease. According to a Centers for Disease Control and Prevention (CDC) study, high blood pressure and high cholesterol are more common among workers exposed to loud noise at work.
The National Institute for Occupational Safety and Health (NIOSH) researchers analyzed data from the 2014 National Health Interview Survey to estimate the prevalence of occupational noise exposure, hearing difficulty, and heart conditions within US industries and occupations. They also looked at the association between workplace noise exposure and heart disease. Their analysis showed:
- 25% of current workers had a history of work-related noise exposure, 14% were exposed in the last year;
- 12% of current workers had hearing difficulty, 24% had high blood pressure, 28% had high cholesterol; and
- Of those cases 58%, 14%, and 9%, respectively, can be attributed to occupational noise exposure.
Study coauthor Liz Masterson, PhD, says, “If noise could be reduced to safer levels in the workplace, more than 5 million cases of hearing difficulty among noise-exposed workers could potentially be prevented.”
Loud noise is one of the most common workplace hazards in the US. One-quarter of US workers (an estimated 41 million people) report a history of noise exposure at work—and that may put them at risk for heart disease. According to a Centers for Disease Control and Prevention (CDC) study, high blood pressure and high cholesterol are more common among workers exposed to loud noise at work.
The National Institute for Occupational Safety and Health (NIOSH) researchers analyzed data from the 2014 National Health Interview Survey to estimate the prevalence of occupational noise exposure, hearing difficulty, and heart conditions within US industries and occupations. They also looked at the association between workplace noise exposure and heart disease. Their analysis showed:
- 25% of current workers had a history of work-related noise exposure, 14% were exposed in the last year;
- 12% of current workers had hearing difficulty, 24% had high blood pressure, 28% had high cholesterol; and
- Of those cases 58%, 14%, and 9%, respectively, can be attributed to occupational noise exposure.
Study coauthor Liz Masterson, PhD, says, “If noise could be reduced to safer levels in the workplace, more than 5 million cases of hearing difficulty among noise-exposed workers could potentially be prevented.”