User login
Cooperation can drive T-ALL, study shows
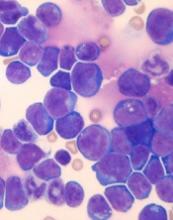
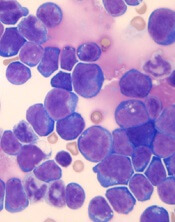
Overexpression of HOXA9 and activated JAK/STAT signaling cooperate to drive the development of T-cell acute lymphoblastic leukemia (ALL), according to researchers.
The team found that JAK3 mutations are significantly associated with elevated HOXA9 expression in T-ALL, and co-expression of HOXA9 and JAK3 mutations prompt “rapid” leukemia development in mice.
In addition, STAT5 and HOXA9 occupy similar genetic loci, which results in increased JAK-STAT signaling in leukemia cells.
These discoveries, and results of subsequent experiments, suggested that PIM1 and JAK1 are potential therapeutic targets for T-ALL.
Jan Cools, PhD, of VIB-KU Leuven Center for Cancer Biology in Leuven, Belgium, and his colleagues described this research in Cancer Discovery.
“JAK3/STAT5 mutations are important in ALL since they stimulate the growth of the cells,” Dr Cools said. “[W]e found that JAK3/STAT5 mutations frequently occur together with HOXA9 mutations.”
In analyzing data from 2 cohorts of T-ALL patients, the researchers found that IL7R/JAK/STAT5 mutations were more frequent in HOXA+ cases, and HOXA9 was “the most important upregulated gene of the HOXA cluster.” (HOXA9 expression levels were significantly higher than HOXA10 or HOXA11 levels.)
“We examined the cooperation between JAK3/STAT5 mutation and HOXA9, [and] we observed that HOXA9 boosts the effects of other genes, leading to tumor development,” said study author Charles de Bock, PhD, of VIB-KU Leuven.
“As a result, when JAK3/STAT5 mutations and HOXA9 are both present, leukemia develops more rapidly and aggressively.”
The researchers found that co-expression of HOXA9 and the JAK3 M511I mutation led to rapid leukemia development in mice. Animals with co-expression of HOXA9 and JAK3 (M511I) developed leukemia that was characterized by an increase in peripheral white blood cell counts that exceeded 10,000 cells/mm3 within 30 days.
In addition, these mice had a significant decrease in disease-free survival compared to mice with JAK3 (M511I) alone. The median disease-free survival was 25 days and 126.5 days, respectively (P<0.0001).
Further analysis revealed co-localization of HOXA9 and STAT5. The researchers also found that HOXA9 enhances transcriptional activity of STAT5 in leukemia cells.
The team said this reconfirms STAT5 as “a major central player” in T-ALL, and it suggests that STAT5 target genes such as PIM1 could be therapeutic targets for T-ALL.
To test this theory, the researchers inhibited both PIM1 and JAK1 in JAK/STAT mutant T-ALL. The PIM1 inhibitor AZD1208 and the JAK1/2 inhibitor ruxolitinib demonstrated synergy and significantly reduced leukemia burden in vivo.
Overexpression of HOXA9 and activated JAK/STAT signaling cooperate to drive the development of T-cell acute lymphoblastic leukemia (ALL), according to researchers.
The team found that JAK3 mutations are significantly associated with elevated HOXA9 expression in T-ALL, and co-expression of HOXA9 and JAK3 mutations prompt “rapid” leukemia development in mice.
In addition, STAT5 and HOXA9 occupy similar genetic loci, which results in increased JAK-STAT signaling in leukemia cells.
These discoveries, and results of subsequent experiments, suggested that PIM1 and JAK1 are potential therapeutic targets for T-ALL.
Jan Cools, PhD, of VIB-KU Leuven Center for Cancer Biology in Leuven, Belgium, and his colleagues described this research in Cancer Discovery.
“JAK3/STAT5 mutations are important in ALL since they stimulate the growth of the cells,” Dr Cools said. “[W]e found that JAK3/STAT5 mutations frequently occur together with HOXA9 mutations.”
In analyzing data from 2 cohorts of T-ALL patients, the researchers found that IL7R/JAK/STAT5 mutations were more frequent in HOXA+ cases, and HOXA9 was “the most important upregulated gene of the HOXA cluster.” (HOXA9 expression levels were significantly higher than HOXA10 or HOXA11 levels.)
“We examined the cooperation between JAK3/STAT5 mutation and HOXA9, [and] we observed that HOXA9 boosts the effects of other genes, leading to tumor development,” said study author Charles de Bock, PhD, of VIB-KU Leuven.
“As a result, when JAK3/STAT5 mutations and HOXA9 are both present, leukemia develops more rapidly and aggressively.”
The researchers found that co-expression of HOXA9 and the JAK3 M511I mutation led to rapid leukemia development in mice. Animals with co-expression of HOXA9 and JAK3 (M511I) developed leukemia that was characterized by an increase in peripheral white blood cell counts that exceeded 10,000 cells/mm3 within 30 days.
In addition, these mice had a significant decrease in disease-free survival compared to mice with JAK3 (M511I) alone. The median disease-free survival was 25 days and 126.5 days, respectively (P<0.0001).
Further analysis revealed co-localization of HOXA9 and STAT5. The researchers also found that HOXA9 enhances transcriptional activity of STAT5 in leukemia cells.
The team said this reconfirms STAT5 as “a major central player” in T-ALL, and it suggests that STAT5 target genes such as PIM1 could be therapeutic targets for T-ALL.
To test this theory, the researchers inhibited both PIM1 and JAK1 in JAK/STAT mutant T-ALL. The PIM1 inhibitor AZD1208 and the JAK1/2 inhibitor ruxolitinib demonstrated synergy and significantly reduced leukemia burden in vivo.
Overexpression of HOXA9 and activated JAK/STAT signaling cooperate to drive the development of T-cell acute lymphoblastic leukemia (ALL), according to researchers.
The team found that JAK3 mutations are significantly associated with elevated HOXA9 expression in T-ALL, and co-expression of HOXA9 and JAK3 mutations prompt “rapid” leukemia development in mice.
In addition, STAT5 and HOXA9 occupy similar genetic loci, which results in increased JAK-STAT signaling in leukemia cells.
These discoveries, and results of subsequent experiments, suggested that PIM1 and JAK1 are potential therapeutic targets for T-ALL.
Jan Cools, PhD, of VIB-KU Leuven Center for Cancer Biology in Leuven, Belgium, and his colleagues described this research in Cancer Discovery.
“JAK3/STAT5 mutations are important in ALL since they stimulate the growth of the cells,” Dr Cools said. “[W]e found that JAK3/STAT5 mutations frequently occur together with HOXA9 mutations.”
In analyzing data from 2 cohorts of T-ALL patients, the researchers found that IL7R/JAK/STAT5 mutations were more frequent in HOXA+ cases, and HOXA9 was “the most important upregulated gene of the HOXA cluster.” (HOXA9 expression levels were significantly higher than HOXA10 or HOXA11 levels.)
“We examined the cooperation between JAK3/STAT5 mutation and HOXA9, [and] we observed that HOXA9 boosts the effects of other genes, leading to tumor development,” said study author Charles de Bock, PhD, of VIB-KU Leuven.
“As a result, when JAK3/STAT5 mutations and HOXA9 are both present, leukemia develops more rapidly and aggressively.”
The researchers found that co-expression of HOXA9 and the JAK3 M511I mutation led to rapid leukemia development in mice. Animals with co-expression of HOXA9 and JAK3 (M511I) developed leukemia that was characterized by an increase in peripheral white blood cell counts that exceeded 10,000 cells/mm3 within 30 days.
In addition, these mice had a significant decrease in disease-free survival compared to mice with JAK3 (M511I) alone. The median disease-free survival was 25 days and 126.5 days, respectively (P<0.0001).
Further analysis revealed co-localization of HOXA9 and STAT5. The researchers also found that HOXA9 enhances transcriptional activity of STAT5 in leukemia cells.
The team said this reconfirms STAT5 as “a major central player” in T-ALL, and it suggests that STAT5 target genes such as PIM1 could be therapeutic targets for T-ALL.
To test this theory, the researchers inhibited both PIM1 and JAK1 in JAK/STAT mutant T-ALL. The PIM1 inhibitor AZD1208 and the JAK1/2 inhibitor ruxolitinib demonstrated synergy and significantly reduced leukemia burden in vivo.
Emotional regulation training lowers risk of adolescents having sex
Emotional regulation (ER) skills training lowers the likelihood that young adolescents with mental health symptoms will have vaginal sex.
“The inclusion of ER training in a small-group behavioral intervention reduced sexual risk behaviors among seventh-graders with suspected mental health symptoms over a 2.5-year follow-up beyond that achieved with more traditional health education,” wrote Chistopher Houck, PhD, and his colleagues at Bradley Hasbro Children’s Research Center, Providence, R.I., in Pediatrics. “This was true across a range of behaviors, such as engaging in fewer condom-less sex acts, being less likely to have multiple partners, and being less likely to use substances before sex.”
Students in the study participated in one of two after school intervention programs, either ER or health promotion (HP). Both programs consisted of 12 twice-weekly, hour-long sessions composed of single-sex groups of 4-8 adolescents. Two follow-up sessions were provided for both groups at 6 and 12 months. Both interventions used identical techniques, such as interactive games, videos, group discussions, and workbook assignments. ER sessions focused more on recognizing feelings, strategies for reducing momentary emotional arousal, and sexual health topics. HP exclusively focused on health topics like sexual risk and substance abuse but did not include emotional education.
During the 30-month study, 63 in the ER group (31%) and 68 students in the HP group (39%) reported having vaginal sex for the first time. This equated to an adjusted hazard ratio that indicated a delay in vaginal sex in the ER group (0.61; 95% confidence interval,0.42-0.89). Overall, students in the ER group were much less likely to endorse risky sexual behaviors than did participants in the HP group: Students in the ER group were less likely to endorse any risky sexual behavior (adjusted odds ratio, 0.52; 95% CI, 0.32-0.84), to support having multiple partners within 6 months (aOR, 0.54; 95% CI, 0.30-0.99), and to support the use of drugs before sex (aOR, 0.42; 95% CI, 0.23-0.75). Students in the ER group also reported fewer condom-less sex acts, compared with students in the HP group (adjusted rate ratio, 0.36; 95% CI, 0.14-0.90).
According to Dr. Houck and his colleagues, this study had several limitations that are common to sexual risk studies. One limitation is the reliance on self-report data, which can be biased. Dr. Houck and his associates utilized computer-assisted self-interviews to minimize biases. Another, and potentially larger, limitation is that the study was powered to assess delay of vaginal sex. Part of the patient sample was not sexually experienced, which provided less power for comparisons to other sexual behaviors.
Dr. Houck and his colleagues also spoke to the potential that ER training has in reducing risky behaviors of adolescents, as well as the issues in implementing it.
“Because ER is a skill that could influence other adolescent risk behaviors, such as substance use, violence, and truancy, addressing ER during this sensitive period in adolescent development promises significant public health benefits,” they wrote. “The challenge is the scale-up and dissemination of ER interventions. Increasing the reach of programs in which health education is enhanced with emotion education may be an important step toward improving the lives of adolescents because they are prone to beginning risk behavior.”
Dr. Houck and his associates have no relevant financial disclosures. This study received funding from the National Institutes of Health and the Providence/Boston Center for AIDS Research.
SOURCE: Houck C et al. Pediatrics. 2018 May 10. doi: 10.1542/peds.2017-2525.
The work of Houck et al. provides an important contribution in understanding strategies to reduce sexually transmitted infections (STIs) and HIV in adolescents by utilizing emotional regulation skills.
By helping young people understand their emotions and how that relates to behavior in the context of a sexual encounter, the after school intervention program helped teens regulate positive and negative emotions. Specifically, it utilized three strategies: get out, let it out, and think it out. Games and role playing gave teens a chance to practice these strategies in scenarios of varying risk.
Teenagers who underwent emotional regulation training, rather than simply being taught about adolescent health topics, fared much better in reducing the transition to vaginal sex over a 30-month period.
Carol Ford, MD, and her colleague James Jaccard, PhD, pointed out the superiority of the emotional training, compared with just sexual health information.
“Together, these findings reveal the importance of gearing more attention toward emotions and the regulation of emotions when developing interventions aimed at influencing adolescent sexual behavior,” they wrote. “Behavioral decision theory implicates the role of adolescent cognitions about engaging in sex, norms and peer pressure, and adolescent image prototypes surrounding sex.”
More broadly, Dr. Ford and Dr. Jaccard, believe that this research is the beginning to designing better interventions.
“As we come to understand the types of cognitions and emotions that dominate working memory in high-risk sexual situations, we can effectively design interventions that help shape cognitive and affective appraisals and how youth process those appraisals when making choices.”
Carol Ford, MD, is the chief of the Craig-Dalsimer Division of Adolescent Medicine at the Children’s Hospital of Philadelphia; she holds the Orton P. Jackson Endowed Chair in Adolescent Medicine. James Jaccard, PhD, is a professor of social work at New York University Silver School of Social Work. This is a summary of their commentary that accompanied the article by Houck et al. (Pediatrics. 2018 May 10. doi: 10.1542/peds.2017-4143). They had no financial disclosures, and there was no external funding.
The work of Houck et al. provides an important contribution in understanding strategies to reduce sexually transmitted infections (STIs) and HIV in adolescents by utilizing emotional regulation skills.
By helping young people understand their emotions and how that relates to behavior in the context of a sexual encounter, the after school intervention program helped teens regulate positive and negative emotions. Specifically, it utilized three strategies: get out, let it out, and think it out. Games and role playing gave teens a chance to practice these strategies in scenarios of varying risk.
Teenagers who underwent emotional regulation training, rather than simply being taught about adolescent health topics, fared much better in reducing the transition to vaginal sex over a 30-month period.
Carol Ford, MD, and her colleague James Jaccard, PhD, pointed out the superiority of the emotional training, compared with just sexual health information.
“Together, these findings reveal the importance of gearing more attention toward emotions and the regulation of emotions when developing interventions aimed at influencing adolescent sexual behavior,” they wrote. “Behavioral decision theory implicates the role of adolescent cognitions about engaging in sex, norms and peer pressure, and adolescent image prototypes surrounding sex.”
More broadly, Dr. Ford and Dr. Jaccard, believe that this research is the beginning to designing better interventions.
“As we come to understand the types of cognitions and emotions that dominate working memory in high-risk sexual situations, we can effectively design interventions that help shape cognitive and affective appraisals and how youth process those appraisals when making choices.”
Carol Ford, MD, is the chief of the Craig-Dalsimer Division of Adolescent Medicine at the Children’s Hospital of Philadelphia; she holds the Orton P. Jackson Endowed Chair in Adolescent Medicine. James Jaccard, PhD, is a professor of social work at New York University Silver School of Social Work. This is a summary of their commentary that accompanied the article by Houck et al. (Pediatrics. 2018 May 10. doi: 10.1542/peds.2017-4143). They had no financial disclosures, and there was no external funding.
The work of Houck et al. provides an important contribution in understanding strategies to reduce sexually transmitted infections (STIs) and HIV in adolescents by utilizing emotional regulation skills.
By helping young people understand their emotions and how that relates to behavior in the context of a sexual encounter, the after school intervention program helped teens regulate positive and negative emotions. Specifically, it utilized three strategies: get out, let it out, and think it out. Games and role playing gave teens a chance to practice these strategies in scenarios of varying risk.
Teenagers who underwent emotional regulation training, rather than simply being taught about adolescent health topics, fared much better in reducing the transition to vaginal sex over a 30-month period.
Carol Ford, MD, and her colleague James Jaccard, PhD, pointed out the superiority of the emotional training, compared with just sexual health information.
“Together, these findings reveal the importance of gearing more attention toward emotions and the regulation of emotions when developing interventions aimed at influencing adolescent sexual behavior,” they wrote. “Behavioral decision theory implicates the role of adolescent cognitions about engaging in sex, norms and peer pressure, and adolescent image prototypes surrounding sex.”
More broadly, Dr. Ford and Dr. Jaccard, believe that this research is the beginning to designing better interventions.
“As we come to understand the types of cognitions and emotions that dominate working memory in high-risk sexual situations, we can effectively design interventions that help shape cognitive and affective appraisals and how youth process those appraisals when making choices.”
Carol Ford, MD, is the chief of the Craig-Dalsimer Division of Adolescent Medicine at the Children’s Hospital of Philadelphia; she holds the Orton P. Jackson Endowed Chair in Adolescent Medicine. James Jaccard, PhD, is a professor of social work at New York University Silver School of Social Work. This is a summary of their commentary that accompanied the article by Houck et al. (Pediatrics. 2018 May 10. doi: 10.1542/peds.2017-4143). They had no financial disclosures, and there was no external funding.
Emotional regulation (ER) skills training lowers the likelihood that young adolescents with mental health symptoms will have vaginal sex.
“The inclusion of ER training in a small-group behavioral intervention reduced sexual risk behaviors among seventh-graders with suspected mental health symptoms over a 2.5-year follow-up beyond that achieved with more traditional health education,” wrote Chistopher Houck, PhD, and his colleagues at Bradley Hasbro Children’s Research Center, Providence, R.I., in Pediatrics. “This was true across a range of behaviors, such as engaging in fewer condom-less sex acts, being less likely to have multiple partners, and being less likely to use substances before sex.”
Students in the study participated in one of two after school intervention programs, either ER or health promotion (HP). Both programs consisted of 12 twice-weekly, hour-long sessions composed of single-sex groups of 4-8 adolescents. Two follow-up sessions were provided for both groups at 6 and 12 months. Both interventions used identical techniques, such as interactive games, videos, group discussions, and workbook assignments. ER sessions focused more on recognizing feelings, strategies for reducing momentary emotional arousal, and sexual health topics. HP exclusively focused on health topics like sexual risk and substance abuse but did not include emotional education.
During the 30-month study, 63 in the ER group (31%) and 68 students in the HP group (39%) reported having vaginal sex for the first time. This equated to an adjusted hazard ratio that indicated a delay in vaginal sex in the ER group (0.61; 95% confidence interval,0.42-0.89). Overall, students in the ER group were much less likely to endorse risky sexual behaviors than did participants in the HP group: Students in the ER group were less likely to endorse any risky sexual behavior (adjusted odds ratio, 0.52; 95% CI, 0.32-0.84), to support having multiple partners within 6 months (aOR, 0.54; 95% CI, 0.30-0.99), and to support the use of drugs before sex (aOR, 0.42; 95% CI, 0.23-0.75). Students in the ER group also reported fewer condom-less sex acts, compared with students in the HP group (adjusted rate ratio, 0.36; 95% CI, 0.14-0.90).
According to Dr. Houck and his colleagues, this study had several limitations that are common to sexual risk studies. One limitation is the reliance on self-report data, which can be biased. Dr. Houck and his associates utilized computer-assisted self-interviews to minimize biases. Another, and potentially larger, limitation is that the study was powered to assess delay of vaginal sex. Part of the patient sample was not sexually experienced, which provided less power for comparisons to other sexual behaviors.
Dr. Houck and his colleagues also spoke to the potential that ER training has in reducing risky behaviors of adolescents, as well as the issues in implementing it.
“Because ER is a skill that could influence other adolescent risk behaviors, such as substance use, violence, and truancy, addressing ER during this sensitive period in adolescent development promises significant public health benefits,” they wrote. “The challenge is the scale-up and dissemination of ER interventions. Increasing the reach of programs in which health education is enhanced with emotion education may be an important step toward improving the lives of adolescents because they are prone to beginning risk behavior.”
Dr. Houck and his associates have no relevant financial disclosures. This study received funding from the National Institutes of Health and the Providence/Boston Center for AIDS Research.
SOURCE: Houck C et al. Pediatrics. 2018 May 10. doi: 10.1542/peds.2017-2525.
Emotional regulation (ER) skills training lowers the likelihood that young adolescents with mental health symptoms will have vaginal sex.
“The inclusion of ER training in a small-group behavioral intervention reduced sexual risk behaviors among seventh-graders with suspected mental health symptoms over a 2.5-year follow-up beyond that achieved with more traditional health education,” wrote Chistopher Houck, PhD, and his colleagues at Bradley Hasbro Children’s Research Center, Providence, R.I., in Pediatrics. “This was true across a range of behaviors, such as engaging in fewer condom-less sex acts, being less likely to have multiple partners, and being less likely to use substances before sex.”
Students in the study participated in one of two after school intervention programs, either ER or health promotion (HP). Both programs consisted of 12 twice-weekly, hour-long sessions composed of single-sex groups of 4-8 adolescents. Two follow-up sessions were provided for both groups at 6 and 12 months. Both interventions used identical techniques, such as interactive games, videos, group discussions, and workbook assignments. ER sessions focused more on recognizing feelings, strategies for reducing momentary emotional arousal, and sexual health topics. HP exclusively focused on health topics like sexual risk and substance abuse but did not include emotional education.
During the 30-month study, 63 in the ER group (31%) and 68 students in the HP group (39%) reported having vaginal sex for the first time. This equated to an adjusted hazard ratio that indicated a delay in vaginal sex in the ER group (0.61; 95% confidence interval,0.42-0.89). Overall, students in the ER group were much less likely to endorse risky sexual behaviors than did participants in the HP group: Students in the ER group were less likely to endorse any risky sexual behavior (adjusted odds ratio, 0.52; 95% CI, 0.32-0.84), to support having multiple partners within 6 months (aOR, 0.54; 95% CI, 0.30-0.99), and to support the use of drugs before sex (aOR, 0.42; 95% CI, 0.23-0.75). Students in the ER group also reported fewer condom-less sex acts, compared with students in the HP group (adjusted rate ratio, 0.36; 95% CI, 0.14-0.90).
According to Dr. Houck and his colleagues, this study had several limitations that are common to sexual risk studies. One limitation is the reliance on self-report data, which can be biased. Dr. Houck and his associates utilized computer-assisted self-interviews to minimize biases. Another, and potentially larger, limitation is that the study was powered to assess delay of vaginal sex. Part of the patient sample was not sexually experienced, which provided less power for comparisons to other sexual behaviors.
Dr. Houck and his colleagues also spoke to the potential that ER training has in reducing risky behaviors of adolescents, as well as the issues in implementing it.
“Because ER is a skill that could influence other adolescent risk behaviors, such as substance use, violence, and truancy, addressing ER during this sensitive period in adolescent development promises significant public health benefits,” they wrote. “The challenge is the scale-up and dissemination of ER interventions. Increasing the reach of programs in which health education is enhanced with emotion education may be an important step toward improving the lives of adolescents because they are prone to beginning risk behavior.”
Dr. Houck and his associates have no relevant financial disclosures. This study received funding from the National Institutes of Health and the Providence/Boston Center for AIDS Research.
SOURCE: Houck C et al. Pediatrics. 2018 May 10. doi: 10.1542/peds.2017-2525.
FROM PEDIATRICS
Key clinical point: Emotional regulation (ER) training reduces the likelihood adolescents will have sex.
Major finding: There was a delay in vaginal sex in the ER group (adusted hazard ratio, 0.61; 95% confidence interval, 0.42-0.89), compared with the health promotion group.
Study details: The study included 420 seventh grade students aged 12-14 years from five urban Rhode Island middle schools between September 2009 and February 2012.
Disclosures: Dr. Houck and his associates have no relevant financial disclosures. This study received funding from the National Institutes of Health and the Providence/Boston Center for AIDS Research.
Source: Houck C et al. Pediatrics. 2018 May 10. doi: 10.1542/peds.2017-2525.
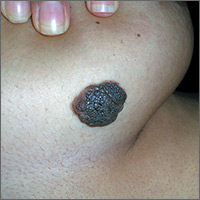
Growing mole on breast
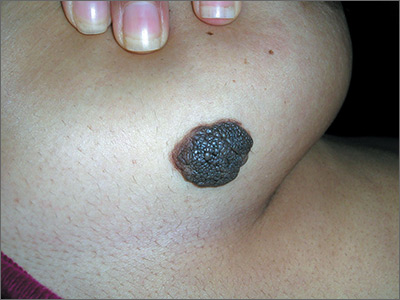
The FP recognized this lesion as a congenital nevus.
He was aware that nevi might become more raised during early adulthood, though this was not necessarily a sign of malignant degeneration. He looked at the nevus carefully and saw that it was relatively symmetrical with one predominant color and a light brown coloration on the left edge. (The patient stated it had always been this way.) The surface texture, which could be described as mamillated, was not unusual for congenital nevi. The FP examined the nevus using a dermatoscope and did not see any melanoma-specific structures.
The FP encouraged the patient to monitor the nevus and return for further evaluation if there were any changes or symptoms. He also offered her the option of a biopsy, but stated that it was not medically required. The patient noted that the changes of increased height of the congenital nevus had been very slow over the past 2 years, and she was willing to keep an eye on it. The patient returned in 6 months, and there were no visible changes to the congenital nevus.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Congenital nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:953-957.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized this lesion as a congenital nevus.
He was aware that nevi might become more raised during early adulthood, though this was not necessarily a sign of malignant degeneration. He looked at the nevus carefully and saw that it was relatively symmetrical with one predominant color and a light brown coloration on the left edge. (The patient stated it had always been this way.) The surface texture, which could be described as mamillated, was not unusual for congenital nevi. The FP examined the nevus using a dermatoscope and did not see any melanoma-specific structures.
The FP encouraged the patient to monitor the nevus and return for further evaluation if there were any changes or symptoms. He also offered her the option of a biopsy, but stated that it was not medically required. The patient noted that the changes of increased height of the congenital nevus had been very slow over the past 2 years, and she was willing to keep an eye on it. The patient returned in 6 months, and there were no visible changes to the congenital nevus.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Congenital nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:953-957.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized this lesion as a congenital nevus.
He was aware that nevi might become more raised during early adulthood, though this was not necessarily a sign of malignant degeneration. He looked at the nevus carefully and saw that it was relatively symmetrical with one predominant color and a light brown coloration on the left edge. (The patient stated it had always been this way.) The surface texture, which could be described as mamillated, was not unusual for congenital nevi. The FP examined the nevus using a dermatoscope and did not see any melanoma-specific structures.
The FP encouraged the patient to monitor the nevus and return for further evaluation if there were any changes or symptoms. He also offered her the option of a biopsy, but stated that it was not medically required. The patient noted that the changes of increased height of the congenital nevus had been very slow over the past 2 years, and she was willing to keep an eye on it. The patient returned in 6 months, and there were no visible changes to the congenital nevus.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Congenital nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:953-957.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
Screening blood donations for Zika proved costly, low yield
Testing donated blood for Zika virus in the United States confirmed just 8 authentic cases and cost nearly $42 million over a period of 15 months, investigators reported online May 10 in the New England Journal of Medicine.
That price did not reflect a commercial price hike, said Paula Saá, PhD, of the American Red Cross in Gaithersburg, MD, and her associates. Furthermore, more than half of the Zika cases had a low viral level that might not be infectious.
Among more than 4 million screened donations, 9% were tested in pools. All pooled tests were negative. The other 3.9 million donations were screened individually. Only 160 were positive, and just 6 were confirmed positive on repeat TMA. Two of these six confirmed positives also were RNA-positive on reverse transcription polymerase chain reaction, while two were equivocal and two were negative. The positive and equivocal donations were negative for Zika immunoglobulin M on enzyme-linked immunosorbent assay, which indicated acute infections. The RNA-negative infections were IgM-positive, indicating prior infections.
Three more donations were initially positive on TMA, were negative on repeat TMA, and were IgM-positive, bringing the total case count to nine. Among these, two donors had been infected in Florida, six had traveled to Zika-endemic areas, and one had received an experimental Zika vaccine, according to the researchers.
For each detection of authentic Zika virus RNA infection in U.S.-donated blood, testing had cost $5.3 million, they concluded. They called the current FDA recommendation to individually screen all U.S. blood donations for Zika virus “low-yield” and “high cost.” Of three acute infections with enough sample left for pooled testing, all were positive, they noted.
The American Red Cross and Grifols Diagnostic Solutions provided funding. Dr. Saá disclosed research support from Grifols, which makes the TMA test used in the study. She had no other conflicts of interest.
SOURCE: Saá P et al. New Engl J Med. 2018;378:1778-88.
Despite these findings, it would be premature to stop testing U.S. blood donations for Zika virus, wrote Evan M. Bloch, MBChB; Paul M. Ness, MD; Aaron A.R. Tobian, MD, PhD; and Jeremy Sugarman, MD, MPH, in an editorial accompanying the study.
Nonetheless, “actual and perceived risks to the blood supply seem to be conflated,” the experts wrote. They noted that the United States currently has no active areas of Zika virus transmission and that confirmed mosquito-borne, locally acquired infections fell from 226 in 2016 to two the following year.
There is no historical precedent for ending a policy of testing blood donations for pathogens, they added. Consequently, ending widespread screening “may actually prove to be far more challenging than the decision to start.” For now, a precautionary, risk-based approach should entail “continuous review” of screening policies and “reassessment as new data emerge.”
The editorialists are with Johns Hopkins University and Johns Hopkins University’s Bergman Institute of Bioethics, both in Baltimore. They reported having no relevant conflicts of interest. These comments are from their editorial (New Engl J Med. 2018;378:19:1837-41).
Despite these findings, it would be premature to stop testing U.S. blood donations for Zika virus, wrote Evan M. Bloch, MBChB; Paul M. Ness, MD; Aaron A.R. Tobian, MD, PhD; and Jeremy Sugarman, MD, MPH, in an editorial accompanying the study.
Nonetheless, “actual and perceived risks to the blood supply seem to be conflated,” the experts wrote. They noted that the United States currently has no active areas of Zika virus transmission and that confirmed mosquito-borne, locally acquired infections fell from 226 in 2016 to two the following year.
There is no historical precedent for ending a policy of testing blood donations for pathogens, they added. Consequently, ending widespread screening “may actually prove to be far more challenging than the decision to start.” For now, a precautionary, risk-based approach should entail “continuous review” of screening policies and “reassessment as new data emerge.”
The editorialists are with Johns Hopkins University and Johns Hopkins University’s Bergman Institute of Bioethics, both in Baltimore. They reported having no relevant conflicts of interest. These comments are from their editorial (New Engl J Med. 2018;378:19:1837-41).
Despite these findings, it would be premature to stop testing U.S. blood donations for Zika virus, wrote Evan M. Bloch, MBChB; Paul M. Ness, MD; Aaron A.R. Tobian, MD, PhD; and Jeremy Sugarman, MD, MPH, in an editorial accompanying the study.
Nonetheless, “actual and perceived risks to the blood supply seem to be conflated,” the experts wrote. They noted that the United States currently has no active areas of Zika virus transmission and that confirmed mosquito-borne, locally acquired infections fell from 226 in 2016 to two the following year.
There is no historical precedent for ending a policy of testing blood donations for pathogens, they added. Consequently, ending widespread screening “may actually prove to be far more challenging than the decision to start.” For now, a precautionary, risk-based approach should entail “continuous review” of screening policies and “reassessment as new data emerge.”
The editorialists are with Johns Hopkins University and Johns Hopkins University’s Bergman Institute of Bioethics, both in Baltimore. They reported having no relevant conflicts of interest. These comments are from their editorial (New Engl J Med. 2018;378:19:1837-41).
Testing donated blood for Zika virus in the United States confirmed just 8 authentic cases and cost nearly $42 million over a period of 15 months, investigators reported online May 10 in the New England Journal of Medicine.
That price did not reflect a commercial price hike, said Paula Saá, PhD, of the American Red Cross in Gaithersburg, MD, and her associates. Furthermore, more than half of the Zika cases had a low viral level that might not be infectious.
Among more than 4 million screened donations, 9% were tested in pools. All pooled tests were negative. The other 3.9 million donations were screened individually. Only 160 were positive, and just 6 were confirmed positive on repeat TMA. Two of these six confirmed positives also were RNA-positive on reverse transcription polymerase chain reaction, while two were equivocal and two were negative. The positive and equivocal donations were negative for Zika immunoglobulin M on enzyme-linked immunosorbent assay, which indicated acute infections. The RNA-negative infections were IgM-positive, indicating prior infections.
Three more donations were initially positive on TMA, were negative on repeat TMA, and were IgM-positive, bringing the total case count to nine. Among these, two donors had been infected in Florida, six had traveled to Zika-endemic areas, and one had received an experimental Zika vaccine, according to the researchers.
For each detection of authentic Zika virus RNA infection in U.S.-donated blood, testing had cost $5.3 million, they concluded. They called the current FDA recommendation to individually screen all U.S. blood donations for Zika virus “low-yield” and “high cost.” Of three acute infections with enough sample left for pooled testing, all were positive, they noted.
The American Red Cross and Grifols Diagnostic Solutions provided funding. Dr. Saá disclosed research support from Grifols, which makes the TMA test used in the study. She had no other conflicts of interest.
SOURCE: Saá P et al. New Engl J Med. 2018;378:1778-88.
Testing donated blood for Zika virus in the United States confirmed just 8 authentic cases and cost nearly $42 million over a period of 15 months, investigators reported online May 10 in the New England Journal of Medicine.
That price did not reflect a commercial price hike, said Paula Saá, PhD, of the American Red Cross in Gaithersburg, MD, and her associates. Furthermore, more than half of the Zika cases had a low viral level that might not be infectious.
Among more than 4 million screened donations, 9% were tested in pools. All pooled tests were negative. The other 3.9 million donations were screened individually. Only 160 were positive, and just 6 were confirmed positive on repeat TMA. Two of these six confirmed positives also were RNA-positive on reverse transcription polymerase chain reaction, while two were equivocal and two were negative. The positive and equivocal donations were negative for Zika immunoglobulin M on enzyme-linked immunosorbent assay, which indicated acute infections. The RNA-negative infections were IgM-positive, indicating prior infections.
Three more donations were initially positive on TMA, were negative on repeat TMA, and were IgM-positive, bringing the total case count to nine. Among these, two donors had been infected in Florida, six had traveled to Zika-endemic areas, and one had received an experimental Zika vaccine, according to the researchers.
For each detection of authentic Zika virus RNA infection in U.S.-donated blood, testing had cost $5.3 million, they concluded. They called the current FDA recommendation to individually screen all U.S. blood donations for Zika virus “low-yield” and “high cost.” Of three acute infections with enough sample left for pooled testing, all were positive, they noted.
The American Red Cross and Grifols Diagnostic Solutions provided funding. Dr. Saá disclosed research support from Grifols, which makes the TMA test used in the study. She had no other conflicts of interest.
SOURCE: Saá P et al. New Engl J Med. 2018;378:1778-88.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Individually screening all blood donations for Zika virus was low yield and costly.
Major finding: Testing confirmed only 8 authentic cases and cost nearly $42 million over 15 months.
Study details: Screening and confirmatory testing of 4,325,889 donations of blood in the United States during 2016-2017.
Disclosures: American Red Cross and Grifols Diagnostic Solutions provided funding. Grifols makes a test used in the study. Dr. Saá disclosed research support from Grifols but had no other conflicts of interest.
Source: Saá P et al. New Engl J Med. 2018;378:1778-88. doi: 10.1056/NEJMoa1714977
Perianal Extramammary Paget Disease Treated With Topical Imiquimod and Oral Cimetidine
Case Report
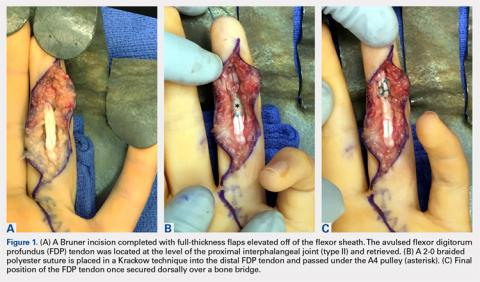
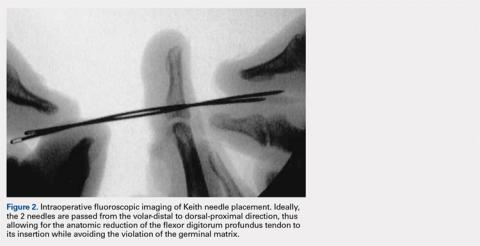
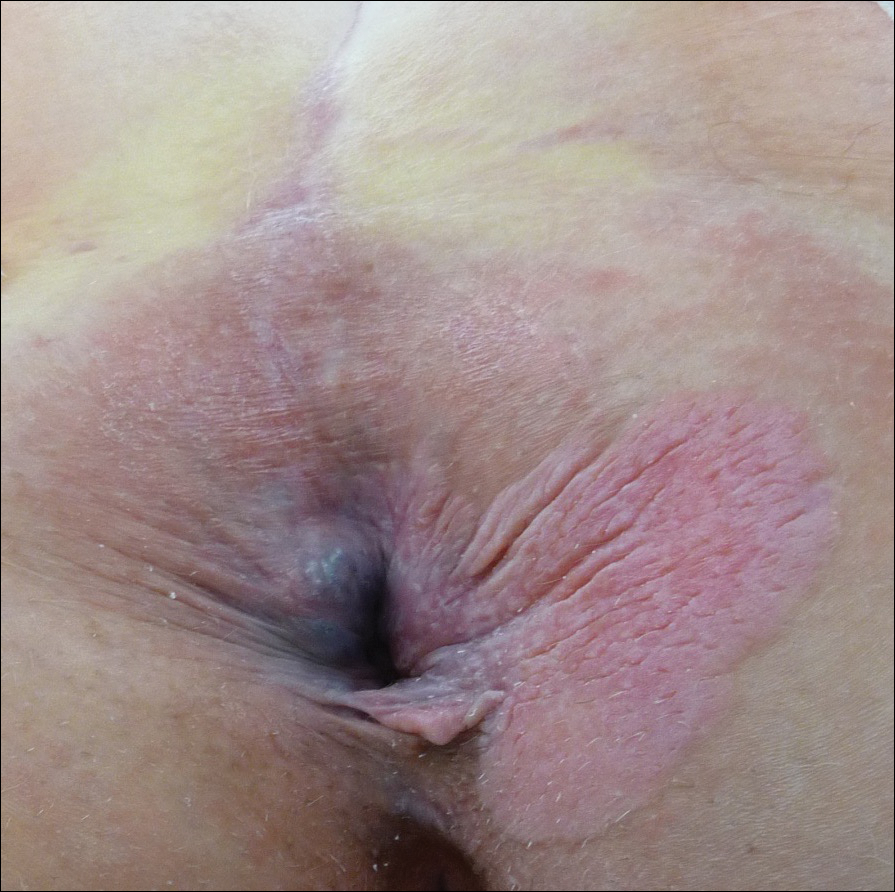
A 56-year-old woman with well-controlled hypertension, hyperlipidemia, and gastroesophageal reflux disease initially presented with itching and a rash in the perianal region of 1 year’s duration. She had been treated intermittently by her primary care physician over the past year for presumed hemorrhoids and a perianal fungal infection without improvement. Physical examination at the time of intitial presentation revealed a single, well-demarcated, scaly, pink plaque on the perianal area on the right buttock extending toward the anal canal (Figure 1).
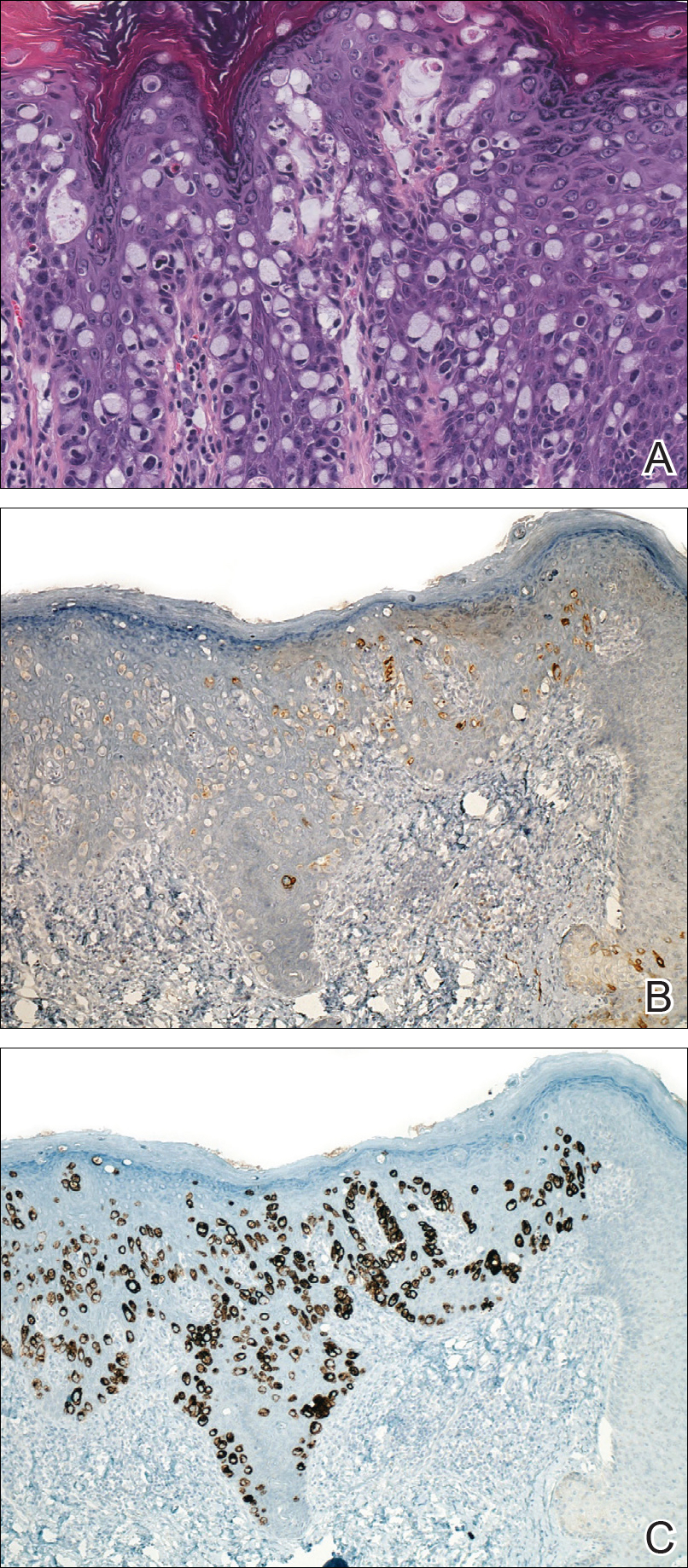
Four years later, the patient returned with new symptoms of bleeding when wiping the perianal region, pruritus, and fecal urgency of 3 to 4 months’ duration. Physical examination revealed scaly patches on the anus that were suspicious for recurrence of EMPD. Biopsies from the anal margin and anal canal confirmed recurrent EMPD involving the anal canal. Repeat evaluation for internal malignancy was negative.
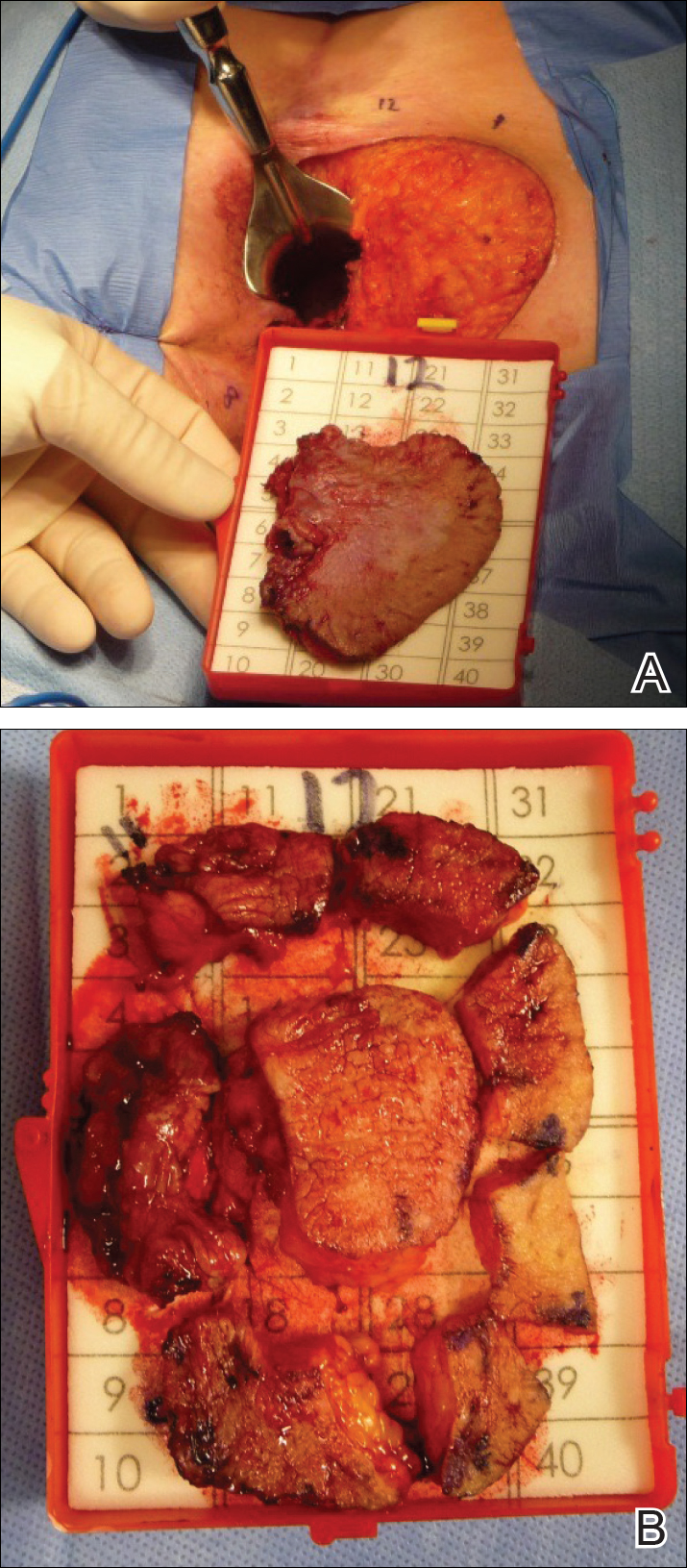
Given the involvement of the anal canal, repeat wide local excision would have required anal resection and would therefore have been functionally impairing. The patient refused further surgical intervention as well as radiotherapy. Rather, a novel 16-week immunomodulatory regimen involving imiquimod cream 5% cream and low-dose oral cimetidine was started. To address the anal involvement, the patient was instructed to lubricate glycerin suppositories with the imiquimod cream and insert intra-anally once weekly. Dosing was adjusted based on the patient’s inflammatory response and tolerability, as she did initially report some flulike symptoms with the first few weeks of treatment. For most of the 16-week course, she applied 250 mg of imiquimod cream 5% to the perianal area 3 times weekly and 250 mg into the anal canal once weekly. Oral cimetidine initially was dosed at 800 mg twice daily as tolerated, but due to stomach irritation, the patient self-reduced her intake to 800 mg 3 times weekly.
To determine treatment response, scouting biopsies of the anal margin and anal canal were obtained 4 weeks after treatment cessation and demonstrated no evidence of residual disease. The patient resumed topical imiquimod applied once weekly into the anal canal and around the anus for a planned prolonged course of at least 1 year. To reduce the risk of recurrence, the patient continued taking oral cimetidine 800 mg 3 times weekly. Recommended follow-up included annual anoscopy or colonoscopy, serum carcinoembryonic antigen evaluation, and regular clinical monitoring by the dermatology and colorectal surgery teams.
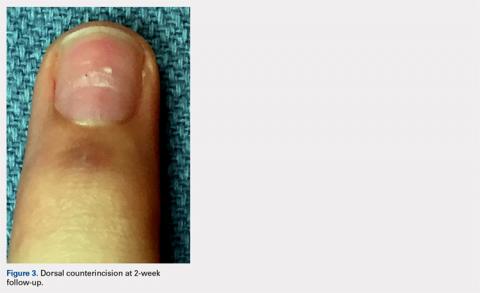
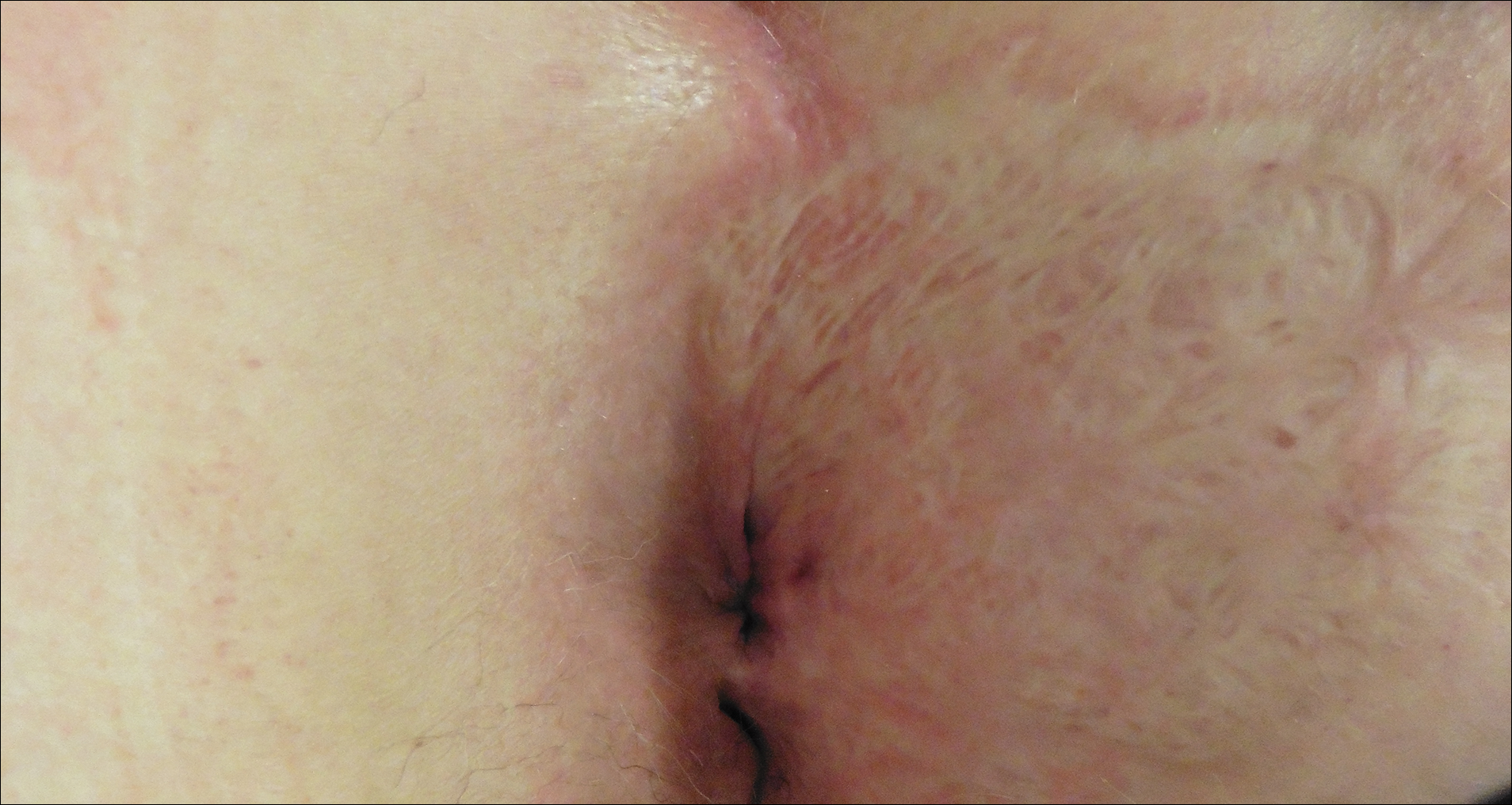
Six months after completing the combination therapy, she was seen by the dermatology department and remained clinically free of disease (Figure 4). Anoscopy examination by the colorectal surgery department 4 months later showed no clinical evidence of malignancy.
Comment
Extramammary Paget disease is a rare intraepithelial adenocarcinoma with a predilection for white females and an average age of onset of 50 to 80 years.1-3 The vulva, perianal region, scrotum, penis, and perineum are the most commonly affected sites.1-3 Clinically, EMPD presents as a chronic, well-demarcated, scaly, and often expanding plaque. The incidence of EMPD is unknown, as there are only a few hundred cases reported in the literature.2
Extramammary Paget disease can occur primarily, arising in the epidermis at the sweat-gland level or from primitive epidermal basal cells, or secondarily due to pagetoid spread of malignant cells from an adjacent or contiguous underlying adnexal adenocarcinoma or visceral malignancy.2 While primary EMPD is not associated with an underlying adenocarcinoma, it may become invasive, infiltrate the dermis, or metastasize via the lymphatics.2 Secondary EMPD is associated with underlying malignancy most often originating in the gastrointestinal or genitourinary tracts.1,2
Currently, treatment of primary EMPD typically is surgical with wide local excision or Mohs micrographic surgery.1,2 However, margins often are positive, and the local recurrence rate is high (ie, 33%–66%).2,3 There are a variety of other therapies that have been reported in the literature, including radiation, topical chemotherapeutics (eg, imiquimod, 5-fluorouracil, bleomycin), photodynamic therapy, and CO2 laser ablation.1,3 To our knowledge, there are no randomized controlled trials that compare surgery with other treatment options for EMPD.
Despite recurrence of EMPD with involvement of the anal canal, our patient refused further surgical intervention, as it would have required anal resection and radiotherapy due to the potentially negative impact on sphincter function. While investigating minimally invasive treatment options, we found several citations in the literature highlighting positive response with imiquimod cream 5% in patients with vulvar and periscrotal EMPD.4,5 A large, systematic review that analyzed 63 cases of vulvar EMPD—nearly half of which were recurrences of a prior malignancy—reported a response rate of 52% to 80% following treatment with imiquimod.5 Almost 70% of patients achieved complete clearance while applying imiquimod 3 to 4 times weekly for a median of 4 months; however, little has been written about the effectiveness of topical imiquimod in EMPD. Knight et al6 reported the case of a 40-year-old woman with perianal EMPD who was treated with imiquimod 3 times weekly for 16 weeks. At the end of treatment, the patient was completely clear of disease both clinically and histologically on random biopsies of the perianal skin; however, the EMPD later recurred with lymph node metastasis 18 months after stopping treatment.6
Given the growing evidence demonstrating disease control of EMPD with topical imiquimod, we elected to utilize this agent in combination with oral cimetidine in our patient. Cimetidine, an H2 receptor antagonist, has been shown to have antineoplastic properties in a broad range of preclinical and clinical studies for a number of different malignancies.7 Four distinct mechanisms of action have been shown. Cimetidine, which blocks the histamine pathway, has been shown to have a direct antiproliferative action on cancer cells.7 Histamine has been associated with increased regulatory T-cell activity, decreased antigen-presenting activity of dendritic cells, reduced natural killer cell activity, and increased myeloid-derived suppressor cell activity, which create an immunosuppressive tumor microenvironment in the setting of cancer. By blocking histamine and thus reversing this immunosuppressive environment, cimetidine demonstrates immunomodulatory effects.7 Cimetidine also has demonstrated an inhibitory effect on cancer cell adhesion to endothelial cells, which is noted to be independent of histamine-blocking activity.7 Finally, an antiangiogenic action is attributed to blocking of the upregulation of vascular endothelial growth factor that is normally induced by histamine.7
Cimetidine’s antineoplastic properties, specifically in the setting of colorectal cancer,8 were particularly compelling given our patient’s EMPD involvement of the anal canal. The most impressive clinical trial data showed a dramatically increased survival rate for colorectal cancer patients treated with oral cimetidine (800 mg once daily) and oral 5-fluorouracil (200 mg once daily) for 1 year following curative resection. The cimetidine-treated group had a 10-year survival rate of 84.6% versus 49.8% for the 5-fluorouracil–only group.8
Conclusion
We present this case of recurrent perianal and anal EMPD treated successfully with imiquimod cream 5% and oral cimetidine to highlight a potential alternative treatment regimen for poor surgical candidates with EMPD.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. London, England: Elsevier Saunders; 2012.
- Lam C, Funaro D. Extramammary Paget’s disease: summary of current knowledge. Dermatol Clin. 2010;28:807-826.
- Vergati M, Filingeri V, Palmieri G, et al. Perianal Paget’s disease: a case report and literature review. Anticancer Res. 2012;32:4461-4465.
- Liau MM, Yang SS, Tan KB, et al. Topical imiquimod in the treatment of extramammary Paget’s disease: a 10 year retrospective analysis in an Asian tertiary centre. Dermatol Ther. 2016;29:459-462.
- Machida H, Moeini A, Roman LD, et al. Effects of imiquimod on vulvar Paget’s disease: a systematic review of literature. Gynecol Oncol. 2015;139:165-171.
- Knight SR, Proby C, Ziyaie D, et al. Extramammary Paget disease of the perianal region: the potential role of imiquimod in achieving disease control. J Surg Case Rep. 2016;8:1-3.
- Pantziarka P, Bouche G, Meheus L, et al. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485.
- Matsumoto S, Imaeda Y, Umemoto S, et al. Cimetidine increases survival of colorectal cancer patients with high levels of sialyl Lewis-X and sialyl Lewis-A epitope expression on tumour cells. Br J Cancer. 2002;86:161-167.
Case Report
A 56-year-old woman with well-controlled hypertension, hyperlipidemia, and gastroesophageal reflux disease initially presented with itching and a rash in the perianal region of 1 year’s duration. She had been treated intermittently by her primary care physician over the past year for presumed hemorrhoids and a perianal fungal infection without improvement. Physical examination at the time of intitial presentation revealed a single, well-demarcated, scaly, pink plaque on the perianal area on the right buttock extending toward the anal canal (Figure 1).



Four years later, the patient returned with new symptoms of bleeding when wiping the perianal region, pruritus, and fecal urgency of 3 to 4 months’ duration. Physical examination revealed scaly patches on the anus that were suspicious for recurrence of EMPD. Biopsies from the anal margin and anal canal confirmed recurrent EMPD involving the anal canal. Repeat evaluation for internal malignancy was negative.
Given the involvement of the anal canal, repeat wide local excision would have required anal resection and would therefore have been functionally impairing. The patient refused further surgical intervention as well as radiotherapy. Rather, a novel 16-week immunomodulatory regimen involving imiquimod cream 5% cream and low-dose oral cimetidine was started. To address the anal involvement, the patient was instructed to lubricate glycerin suppositories with the imiquimod cream and insert intra-anally once weekly. Dosing was adjusted based on the patient’s inflammatory response and tolerability, as she did initially report some flulike symptoms with the first few weeks of treatment. For most of the 16-week course, she applied 250 mg of imiquimod cream 5% to the perianal area 3 times weekly and 250 mg into the anal canal once weekly. Oral cimetidine initially was dosed at 800 mg twice daily as tolerated, but due to stomach irritation, the patient self-reduced her intake to 800 mg 3 times weekly.
To determine treatment response, scouting biopsies of the anal margin and anal canal were obtained 4 weeks after treatment cessation and demonstrated no evidence of residual disease. The patient resumed topical imiquimod applied once weekly into the anal canal and around the anus for a planned prolonged course of at least 1 year. To reduce the risk of recurrence, the patient continued taking oral cimetidine 800 mg 3 times weekly. Recommended follow-up included annual anoscopy or colonoscopy, serum carcinoembryonic antigen evaluation, and regular clinical monitoring by the dermatology and colorectal surgery teams.
Six months after completing the combination therapy, she was seen by the dermatology department and remained clinically free of disease (Figure 4). Anoscopy examination by the colorectal surgery department 4 months later showed no clinical evidence of malignancy.

Comment
Extramammary Paget disease is a rare intraepithelial adenocarcinoma with a predilection for white females and an average age of onset of 50 to 80 years.1-3 The vulva, perianal region, scrotum, penis, and perineum are the most commonly affected sites.1-3 Clinically, EMPD presents as a chronic, well-demarcated, scaly, and often expanding plaque. The incidence of EMPD is unknown, as there are only a few hundred cases reported in the literature.2
Extramammary Paget disease can occur primarily, arising in the epidermis at the sweat-gland level or from primitive epidermal basal cells, or secondarily due to pagetoid spread of malignant cells from an adjacent or contiguous underlying adnexal adenocarcinoma or visceral malignancy.2 While primary EMPD is not associated with an underlying adenocarcinoma, it may become invasive, infiltrate the dermis, or metastasize via the lymphatics.2 Secondary EMPD is associated with underlying malignancy most often originating in the gastrointestinal or genitourinary tracts.1,2
Currently, treatment of primary EMPD typically is surgical with wide local excision or Mohs micrographic surgery.1,2 However, margins often are positive, and the local recurrence rate is high (ie, 33%–66%).2,3 There are a variety of other therapies that have been reported in the literature, including radiation, topical chemotherapeutics (eg, imiquimod, 5-fluorouracil, bleomycin), photodynamic therapy, and CO2 laser ablation.1,3 To our knowledge, there are no randomized controlled trials that compare surgery with other treatment options for EMPD.
Despite recurrence of EMPD with involvement of the anal canal, our patient refused further surgical intervention, as it would have required anal resection and radiotherapy due to the potentially negative impact on sphincter function. While investigating minimally invasive treatment options, we found several citations in the literature highlighting positive response with imiquimod cream 5% in patients with vulvar and periscrotal EMPD.4,5 A large, systematic review that analyzed 63 cases of vulvar EMPD—nearly half of which were recurrences of a prior malignancy—reported a response rate of 52% to 80% following treatment with imiquimod.5 Almost 70% of patients achieved complete clearance while applying imiquimod 3 to 4 times weekly for a median of 4 months; however, little has been written about the effectiveness of topical imiquimod in EMPD. Knight et al6 reported the case of a 40-year-old woman with perianal EMPD who was treated with imiquimod 3 times weekly for 16 weeks. At the end of treatment, the patient was completely clear of disease both clinically and histologically on random biopsies of the perianal skin; however, the EMPD later recurred with lymph node metastasis 18 months after stopping treatment.6
Given the growing evidence demonstrating disease control of EMPD with topical imiquimod, we elected to utilize this agent in combination with oral cimetidine in our patient. Cimetidine, an H2 receptor antagonist, has been shown to have antineoplastic properties in a broad range of preclinical and clinical studies for a number of different malignancies.7 Four distinct mechanisms of action have been shown. Cimetidine, which blocks the histamine pathway, has been shown to have a direct antiproliferative action on cancer cells.7 Histamine has been associated with increased regulatory T-cell activity, decreased antigen-presenting activity of dendritic cells, reduced natural killer cell activity, and increased myeloid-derived suppressor cell activity, which create an immunosuppressive tumor microenvironment in the setting of cancer. By blocking histamine and thus reversing this immunosuppressive environment, cimetidine demonstrates immunomodulatory effects.7 Cimetidine also has demonstrated an inhibitory effect on cancer cell adhesion to endothelial cells, which is noted to be independent of histamine-blocking activity.7 Finally, an antiangiogenic action is attributed to blocking of the upregulation of vascular endothelial growth factor that is normally induced by histamine.7
Cimetidine’s antineoplastic properties, specifically in the setting of colorectal cancer,8 were particularly compelling given our patient’s EMPD involvement of the anal canal. The most impressive clinical trial data showed a dramatically increased survival rate for colorectal cancer patients treated with oral cimetidine (800 mg once daily) and oral 5-fluorouracil (200 mg once daily) for 1 year following curative resection. The cimetidine-treated group had a 10-year survival rate of 84.6% versus 49.8% for the 5-fluorouracil–only group.8
Conclusion
We present this case of recurrent perianal and anal EMPD treated successfully with imiquimod cream 5% and oral cimetidine to highlight a potential alternative treatment regimen for poor surgical candidates with EMPD.
Case Report
A 56-year-old woman with well-controlled hypertension, hyperlipidemia, and gastroesophageal reflux disease initially presented with itching and a rash in the perianal region of 1 year’s duration. She had been treated intermittently by her primary care physician over the past year for presumed hemorrhoids and a perianal fungal infection without improvement. Physical examination at the time of intitial presentation revealed a single, well-demarcated, scaly, pink plaque on the perianal area on the right buttock extending toward the anal canal (Figure 1).



Four years later, the patient returned with new symptoms of bleeding when wiping the perianal region, pruritus, and fecal urgency of 3 to 4 months’ duration. Physical examination revealed scaly patches on the anus that were suspicious for recurrence of EMPD. Biopsies from the anal margin and anal canal confirmed recurrent EMPD involving the anal canal. Repeat evaluation for internal malignancy was negative.
Given the involvement of the anal canal, repeat wide local excision would have required anal resection and would therefore have been functionally impairing. The patient refused further surgical intervention as well as radiotherapy. Rather, a novel 16-week immunomodulatory regimen involving imiquimod cream 5% cream and low-dose oral cimetidine was started. To address the anal involvement, the patient was instructed to lubricate glycerin suppositories with the imiquimod cream and insert intra-anally once weekly. Dosing was adjusted based on the patient’s inflammatory response and tolerability, as she did initially report some flulike symptoms with the first few weeks of treatment. For most of the 16-week course, she applied 250 mg of imiquimod cream 5% to the perianal area 3 times weekly and 250 mg into the anal canal once weekly. Oral cimetidine initially was dosed at 800 mg twice daily as tolerated, but due to stomach irritation, the patient self-reduced her intake to 800 mg 3 times weekly.
To determine treatment response, scouting biopsies of the anal margin and anal canal were obtained 4 weeks after treatment cessation and demonstrated no evidence of residual disease. The patient resumed topical imiquimod applied once weekly into the anal canal and around the anus for a planned prolonged course of at least 1 year. To reduce the risk of recurrence, the patient continued taking oral cimetidine 800 mg 3 times weekly. Recommended follow-up included annual anoscopy or colonoscopy, serum carcinoembryonic antigen evaluation, and regular clinical monitoring by the dermatology and colorectal surgery teams.
Six months after completing the combination therapy, she was seen by the dermatology department and remained clinically free of disease (Figure 4). Anoscopy examination by the colorectal surgery department 4 months later showed no clinical evidence of malignancy.

Comment
Extramammary Paget disease is a rare intraepithelial adenocarcinoma with a predilection for white females and an average age of onset of 50 to 80 years.1-3 The vulva, perianal region, scrotum, penis, and perineum are the most commonly affected sites.1-3 Clinically, EMPD presents as a chronic, well-demarcated, scaly, and often expanding plaque. The incidence of EMPD is unknown, as there are only a few hundred cases reported in the literature.2
Extramammary Paget disease can occur primarily, arising in the epidermis at the sweat-gland level or from primitive epidermal basal cells, or secondarily due to pagetoid spread of malignant cells from an adjacent or contiguous underlying adnexal adenocarcinoma or visceral malignancy.2 While primary EMPD is not associated with an underlying adenocarcinoma, it may become invasive, infiltrate the dermis, or metastasize via the lymphatics.2 Secondary EMPD is associated with underlying malignancy most often originating in the gastrointestinal or genitourinary tracts.1,2
Currently, treatment of primary EMPD typically is surgical with wide local excision or Mohs micrographic surgery.1,2 However, margins often are positive, and the local recurrence rate is high (ie, 33%–66%).2,3 There are a variety of other therapies that have been reported in the literature, including radiation, topical chemotherapeutics (eg, imiquimod, 5-fluorouracil, bleomycin), photodynamic therapy, and CO2 laser ablation.1,3 To our knowledge, there are no randomized controlled trials that compare surgery with other treatment options for EMPD.
Despite recurrence of EMPD with involvement of the anal canal, our patient refused further surgical intervention, as it would have required anal resection and radiotherapy due to the potentially negative impact on sphincter function. While investigating minimally invasive treatment options, we found several citations in the literature highlighting positive response with imiquimod cream 5% in patients with vulvar and periscrotal EMPD.4,5 A large, systematic review that analyzed 63 cases of vulvar EMPD—nearly half of which were recurrences of a prior malignancy—reported a response rate of 52% to 80% following treatment with imiquimod.5 Almost 70% of patients achieved complete clearance while applying imiquimod 3 to 4 times weekly for a median of 4 months; however, little has been written about the effectiveness of topical imiquimod in EMPD. Knight et al6 reported the case of a 40-year-old woman with perianal EMPD who was treated with imiquimod 3 times weekly for 16 weeks. At the end of treatment, the patient was completely clear of disease both clinically and histologically on random biopsies of the perianal skin; however, the EMPD later recurred with lymph node metastasis 18 months after stopping treatment.6
Given the growing evidence demonstrating disease control of EMPD with topical imiquimod, we elected to utilize this agent in combination with oral cimetidine in our patient. Cimetidine, an H2 receptor antagonist, has been shown to have antineoplastic properties in a broad range of preclinical and clinical studies for a number of different malignancies.7 Four distinct mechanisms of action have been shown. Cimetidine, which blocks the histamine pathway, has been shown to have a direct antiproliferative action on cancer cells.7 Histamine has been associated with increased regulatory T-cell activity, decreased antigen-presenting activity of dendritic cells, reduced natural killer cell activity, and increased myeloid-derived suppressor cell activity, which create an immunosuppressive tumor microenvironment in the setting of cancer. By blocking histamine and thus reversing this immunosuppressive environment, cimetidine demonstrates immunomodulatory effects.7 Cimetidine also has demonstrated an inhibitory effect on cancer cell adhesion to endothelial cells, which is noted to be independent of histamine-blocking activity.7 Finally, an antiangiogenic action is attributed to blocking of the upregulation of vascular endothelial growth factor that is normally induced by histamine.7
Cimetidine’s antineoplastic properties, specifically in the setting of colorectal cancer,8 were particularly compelling given our patient’s EMPD involvement of the anal canal. The most impressive clinical trial data showed a dramatically increased survival rate for colorectal cancer patients treated with oral cimetidine (800 mg once daily) and oral 5-fluorouracil (200 mg once daily) for 1 year following curative resection. The cimetidine-treated group had a 10-year survival rate of 84.6% versus 49.8% for the 5-fluorouracil–only group.8
Conclusion
We present this case of recurrent perianal and anal EMPD treated successfully with imiquimod cream 5% and oral cimetidine to highlight a potential alternative treatment regimen for poor surgical candidates with EMPD.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. London, England: Elsevier Saunders; 2012.
- Lam C, Funaro D. Extramammary Paget’s disease: summary of current knowledge. Dermatol Clin. 2010;28:807-826.
- Vergati M, Filingeri V, Palmieri G, et al. Perianal Paget’s disease: a case report and literature review. Anticancer Res. 2012;32:4461-4465.
- Liau MM, Yang SS, Tan KB, et al. Topical imiquimod in the treatment of extramammary Paget’s disease: a 10 year retrospective analysis in an Asian tertiary centre. Dermatol Ther. 2016;29:459-462.
- Machida H, Moeini A, Roman LD, et al. Effects of imiquimod on vulvar Paget’s disease: a systematic review of literature. Gynecol Oncol. 2015;139:165-171.
- Knight SR, Proby C, Ziyaie D, et al. Extramammary Paget disease of the perianal region: the potential role of imiquimod in achieving disease control. J Surg Case Rep. 2016;8:1-3.
- Pantziarka P, Bouche G, Meheus L, et al. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485.
- Matsumoto S, Imaeda Y, Umemoto S, et al. Cimetidine increases survival of colorectal cancer patients with high levels of sialyl Lewis-X and sialyl Lewis-A epitope expression on tumour cells. Br J Cancer. 2002;86:161-167.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. London, England: Elsevier Saunders; 2012.
- Lam C, Funaro D. Extramammary Paget’s disease: summary of current knowledge. Dermatol Clin. 2010;28:807-826.
- Vergati M, Filingeri V, Palmieri G, et al. Perianal Paget’s disease: a case report and literature review. Anticancer Res. 2012;32:4461-4465.
- Liau MM, Yang SS, Tan KB, et al. Topical imiquimod in the treatment of extramammary Paget’s disease: a 10 year retrospective analysis in an Asian tertiary centre. Dermatol Ther. 2016;29:459-462.
- Machida H, Moeini A, Roman LD, et al. Effects of imiquimod on vulvar Paget’s disease: a systematic review of literature. Gynecol Oncol. 2015;139:165-171.
- Knight SR, Proby C, Ziyaie D, et al. Extramammary Paget disease of the perianal region: the potential role of imiquimod in achieving disease control. J Surg Case Rep. 2016;8:1-3.
- Pantziarka P, Bouche G, Meheus L, et al. Repurposing drugs in oncology (ReDO)—cimetidine as an anti-cancer agent. Ecancermedicalscience. 2014;8:485.
- Matsumoto S, Imaeda Y, Umemoto S, et al. Cimetidine increases survival of colorectal cancer patients with high levels of sialyl Lewis-X and sialyl Lewis-A epitope expression on tumour cells. Br J Cancer. 2002;86:161-167.
Resident Pearls
- Topical imiquimod cream 5% and oral cimetidine can be a potential alternative treatment regimen for poor surgical candidates with perianal extramammary Paget disease (EMPD).
- Its antineoplastic and immunomodulatory properties may suggest a role for oral cimetidine as an adjuvant therapy in the treatment of perianal EMPD.
Female physicians face enduring wage gap
Male physicians make more money than female physicians, and that seems to be a rule with few exceptions. Among the 50 largest metro areas, there were none where women earn as much as men, according to a new survey by the medical social network Doximity.
The metro area that comes the closest is Las Vegas, where female physicians earned 20% less – that works out to $73,654 – than their male counterparts in 2017. Rochester, N.Y., had the smallest gap in terms of dollars ($68,758) and the second-smallest percent difference (21%), Doximity said in its 2018 Physician Compensation Report.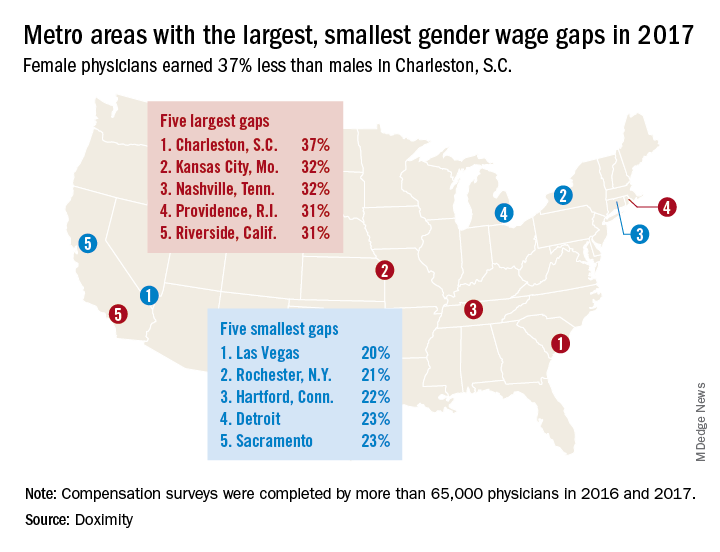
The largest wage gap on both measures can be found in Charleston, S.C., where women earned 37%, or $134,499, less than men in 2017. The other members of the largest-wage-gap club are as follows: Kansas City, Mo., and Nashville, Tenn., both had differences of 32%, and Providence, R.I., and Riverside, Calif., had differences of 31%, Doximity said in the report, which was based on data from “compensation surveys completed in 2016 and 2017 by more than 65,000 full-time, licensed U.S. physicians who practice at least 40 hours per week.”
A quick look at the 2016 data shows that the wage gap between female and male physicians increased from 26.5% to 27.7% in 2017, going from more than $92,000 to $105,000. “Medicine is a highly trained field, and as such, one might expect the gender wage gap to be less prominent here than in other industries. However, the gap endures, despite the level of education required to practice medicine and market forces suggesting that this gap should shrink,” Doximity said.
In a recent issue of AGA Perspectives, Ellen M. Zimmermann, MD, AGAF, chair of the AGA Women’s Committee, wrote about the need for transparent policies at institutions to help close the gender gap. Read more at http://ow.ly/43If30jTlEc.
Male physicians make more money than female physicians, and that seems to be a rule with few exceptions. Among the 50 largest metro areas, there were none where women earn as much as men, according to a new survey by the medical social network Doximity.
The metro area that comes the closest is Las Vegas, where female physicians earned 20% less – that works out to $73,654 – than their male counterparts in 2017. Rochester, N.Y., had the smallest gap in terms of dollars ($68,758) and the second-smallest percent difference (21%), Doximity said in its 2018 Physician Compensation Report.
The largest wage gap on both measures can be found in Charleston, S.C., where women earned 37%, or $134,499, less than men in 2017. The other members of the largest-wage-gap club are as follows: Kansas City, Mo., and Nashville, Tenn., both had differences of 32%, and Providence, R.I., and Riverside, Calif., had differences of 31%, Doximity said in the report, which was based on data from “compensation surveys completed in 2016 and 2017 by more than 65,000 full-time, licensed U.S. physicians who practice at least 40 hours per week.”
A quick look at the 2016 data shows that the wage gap between female and male physicians increased from 26.5% to 27.7% in 2017, going from more than $92,000 to $105,000. “Medicine is a highly trained field, and as such, one might expect the gender wage gap to be less prominent here than in other industries. However, the gap endures, despite the level of education required to practice medicine and market forces suggesting that this gap should shrink,” Doximity said.
In a recent issue of AGA Perspectives, Ellen M. Zimmermann, MD, AGAF, chair of the AGA Women’s Committee, wrote about the need for transparent policies at institutions to help close the gender gap. Read more at http://ow.ly/43If30jTlEc.
Male physicians make more money than female physicians, and that seems to be a rule with few exceptions. Among the 50 largest metro areas, there were none where women earn as much as men, according to a new survey by the medical social network Doximity.
The metro area that comes the closest is Las Vegas, where female physicians earned 20% less – that works out to $73,654 – than their male counterparts in 2017. Rochester, N.Y., had the smallest gap in terms of dollars ($68,758) and the second-smallest percent difference (21%), Doximity said in its 2018 Physician Compensation Report.
The largest wage gap on both measures can be found in Charleston, S.C., where women earned 37%, or $134,499, less than men in 2017. The other members of the largest-wage-gap club are as follows: Kansas City, Mo., and Nashville, Tenn., both had differences of 32%, and Providence, R.I., and Riverside, Calif., had differences of 31%, Doximity said in the report, which was based on data from “compensation surveys completed in 2016 and 2017 by more than 65,000 full-time, licensed U.S. physicians who practice at least 40 hours per week.”
A quick look at the 2016 data shows that the wage gap between female and male physicians increased from 26.5% to 27.7% in 2017, going from more than $92,000 to $105,000. “Medicine is a highly trained field, and as such, one might expect the gender wage gap to be less prominent here than in other industries. However, the gap endures, despite the level of education required to practice medicine and market forces suggesting that this gap should shrink,” Doximity said.
In a recent issue of AGA Perspectives, Ellen M. Zimmermann, MD, AGAF, chair of the AGA Women’s Committee, wrote about the need for transparent policies at institutions to help close the gender gap. Read more at http://ow.ly/43If30jTlEc.
Responsive parenting intervention slows weight gain in infancy
TORONTO – Teaching parents of newborns to respond to eating and satiety cues in ways that promote self-regulation was associated with improvements in some weight outcomes at 3 years in a randomized clinical trial.
For the primary outcome of body mass index (BMI) z score at 3 years, a significant difference favoring the responsive parenting (RP) intervention was seen (–0.13 vs. 0.15 for controls; absolute difference, –0.28; P = .04). A longitudinal analysis examining the entire intervention period confirmed that the mean BMI group differences across seven study visits confirmed the effect of the RP intervention on BMI (P less than .001).
“We felt that the BMI z score and longitudinal growth analysis are probably the most sustained effects for an early-life intervention that have been recorded to date,” reported Ian M. Paul, MD, MSc, of Penn State University, Hershey. “While the differences between study groups were modest and not all achieved statistical significance, all favored the responsive-parenting intervention.”
Mean BMI percentile, a secondary outcome, was 47th for the RP group and 54th for controls, narrowly missing statistical significance (P = .07). Similarly, the percent of children deemed overweight at 3 years was 11.2% for the RP group and 19.8% for controls (P = .07), while 2.6% and 7.8%, respectively, were obese (P = .08).
No significant differences were seen in growth-related adverse events, such a weight-for-age less than the 5th percentile. The issue of “inducing” failure-to-thrive with a feeding intervention is a concern, said Dr. Paul, but there was no evidence for it in their study.
“One could question whether [the small differences seen between groups] are clinically significant, but if we look at how small differences have changed in the population over time and how those equate as far as longitudinal risk for cardiovascular outcomes and metabolic syndrome, etc., the small differences [we saw] might be important on a population level,” said Dr. Paul at the Pediatric Academic Societies meeting.
Study details
With upwards of one-quarter of U.S. children aged 2-5 years being overweight or obese, interventions to prevent rapid weight gain and reduce risk for overweight status in infancy are needed, noted Dr. Paul. Another reason to consider very early intervention, he added, is that infancy is a time of both “metabolic and behavioral plasticity.” However, most efforts to intervene early have, thus far, had limited success.
“Our responses to a baby crying are to feed that baby,” said Dr. Paul. This urge, along with others (such as “clear your plate”), evolved during times of food scarcity but persist now that we have inexpensive and palatable food, and promote rapid infant weight gain and increased obesity risk.
An alternative to those traditional parenting practices are responsive feeding and responsive parenting, he explained. “Responsive feeding and parenting requires prompt, developmentally appropriate responses to a child’s behaviors including hunger and satiety cues.”
In other studies, RP has been shown to foster cognitive, social, and emotional development. “The question we had was: Can responsive parenting reduce obesity risk?” he said.
The INSIGHT (Intervention Nurses Start Infants Growing on Healthy Trajectories) study is an ongoing, randomized clinical trial started in January 2012 comparing an RP intervention designed to prevent childhood obesity with a safety control, with the interventions matched on intensity and length.
Parent-child dyads were randomized 2 weeks after birth and were told that the purpose of the study was “to see if nurse visits to your home during your baby’s infancy can improve your ability to either respond to your child’s cues related to feeding and fussiness or improve your ability to provide a safe environment for your child and prevent injuries.”
A total of 279 primiparous mother-newborn dyads were studied. Most were white (89%) and non-Hispanic (94%), and the majority were married (75%). Mean prepregnancy BMI was 25.5 kg/m2.
“We chose first-time mothers because we thought they were more likely to listen to the parenting advice that we had to offer,” said Dr. Paul.
INSIGHT’s curriculum focused on RP in domains of infant feeding, sleep, interactive play, and emotion regulation. “We tried to promote self-regulation by setting limits but still being responsive in a variety of behavior domains,” Dr. Paul said. “So, for example…, for feeding we talked about exposure to healthy foods, shared feeding responsibility, for those that were bottle feeding we gave tips on size of bottle appropriate for the child and also not using bottle finishing practices. In the emotional and social regulation domain, we talked about alternatives to food to soothe, and emphasized embracing each child’s temperament and how to respond to different temperaments.”
Dr. Paul reported no conflicts of interest. INSIGHT is supported by National Institute of Diabetes and Digestive and Kidney Diseases research grants, with additional support from the Children’s Miracle Network at Penn State Children’s Hospital.
TORONTO – Teaching parents of newborns to respond to eating and satiety cues in ways that promote self-regulation was associated with improvements in some weight outcomes at 3 years in a randomized clinical trial.
For the primary outcome of body mass index (BMI) z score at 3 years, a significant difference favoring the responsive parenting (RP) intervention was seen (–0.13 vs. 0.15 for controls; absolute difference, –0.28; P = .04). A longitudinal analysis examining the entire intervention period confirmed that the mean BMI group differences across seven study visits confirmed the effect of the RP intervention on BMI (P less than .001).
“We felt that the BMI z score and longitudinal growth analysis are probably the most sustained effects for an early-life intervention that have been recorded to date,” reported Ian M. Paul, MD, MSc, of Penn State University, Hershey. “While the differences between study groups were modest and not all achieved statistical significance, all favored the responsive-parenting intervention.”
Mean BMI percentile, a secondary outcome, was 47th for the RP group and 54th for controls, narrowly missing statistical significance (P = .07). Similarly, the percent of children deemed overweight at 3 years was 11.2% for the RP group and 19.8% for controls (P = .07), while 2.6% and 7.8%, respectively, were obese (P = .08).
No significant differences were seen in growth-related adverse events, such a weight-for-age less than the 5th percentile. The issue of “inducing” failure-to-thrive with a feeding intervention is a concern, said Dr. Paul, but there was no evidence for it in their study.
“One could question whether [the small differences seen between groups] are clinically significant, but if we look at how small differences have changed in the population over time and how those equate as far as longitudinal risk for cardiovascular outcomes and metabolic syndrome, etc., the small differences [we saw] might be important on a population level,” said Dr. Paul at the Pediatric Academic Societies meeting.
Study details
With upwards of one-quarter of U.S. children aged 2-5 years being overweight or obese, interventions to prevent rapid weight gain and reduce risk for overweight status in infancy are needed, noted Dr. Paul. Another reason to consider very early intervention, he added, is that infancy is a time of both “metabolic and behavioral plasticity.” However, most efforts to intervene early have, thus far, had limited success.
“Our responses to a baby crying are to feed that baby,” said Dr. Paul. This urge, along with others (such as “clear your plate”), evolved during times of food scarcity but persist now that we have inexpensive and palatable food, and promote rapid infant weight gain and increased obesity risk.
An alternative to those traditional parenting practices are responsive feeding and responsive parenting, he explained. “Responsive feeding and parenting requires prompt, developmentally appropriate responses to a child’s behaviors including hunger and satiety cues.”
In other studies, RP has been shown to foster cognitive, social, and emotional development. “The question we had was: Can responsive parenting reduce obesity risk?” he said.
The INSIGHT (Intervention Nurses Start Infants Growing on Healthy Trajectories) study is an ongoing, randomized clinical trial started in January 2012 comparing an RP intervention designed to prevent childhood obesity with a safety control, with the interventions matched on intensity and length.
Parent-child dyads were randomized 2 weeks after birth and were told that the purpose of the study was “to see if nurse visits to your home during your baby’s infancy can improve your ability to either respond to your child’s cues related to feeding and fussiness or improve your ability to provide a safe environment for your child and prevent injuries.”
A total of 279 primiparous mother-newborn dyads were studied. Most were white (89%) and non-Hispanic (94%), and the majority were married (75%). Mean prepregnancy BMI was 25.5 kg/m2.
“We chose first-time mothers because we thought they were more likely to listen to the parenting advice that we had to offer,” said Dr. Paul.
INSIGHT’s curriculum focused on RP in domains of infant feeding, sleep, interactive play, and emotion regulation. “We tried to promote self-regulation by setting limits but still being responsive in a variety of behavior domains,” Dr. Paul said. “So, for example…, for feeding we talked about exposure to healthy foods, shared feeding responsibility, for those that were bottle feeding we gave tips on size of bottle appropriate for the child and also not using bottle finishing practices. In the emotional and social regulation domain, we talked about alternatives to food to soothe, and emphasized embracing each child’s temperament and how to respond to different temperaments.”
Dr. Paul reported no conflicts of interest. INSIGHT is supported by National Institute of Diabetes and Digestive and Kidney Diseases research grants, with additional support from the Children’s Miracle Network at Penn State Children’s Hospital.
TORONTO – Teaching parents of newborns to respond to eating and satiety cues in ways that promote self-regulation was associated with improvements in some weight outcomes at 3 years in a randomized clinical trial.
For the primary outcome of body mass index (BMI) z score at 3 years, a significant difference favoring the responsive parenting (RP) intervention was seen (–0.13 vs. 0.15 for controls; absolute difference, –0.28; P = .04). A longitudinal analysis examining the entire intervention period confirmed that the mean BMI group differences across seven study visits confirmed the effect of the RP intervention on BMI (P less than .001).
“We felt that the BMI z score and longitudinal growth analysis are probably the most sustained effects for an early-life intervention that have been recorded to date,” reported Ian M. Paul, MD, MSc, of Penn State University, Hershey. “While the differences between study groups were modest and not all achieved statistical significance, all favored the responsive-parenting intervention.”
Mean BMI percentile, a secondary outcome, was 47th for the RP group and 54th for controls, narrowly missing statistical significance (P = .07). Similarly, the percent of children deemed overweight at 3 years was 11.2% for the RP group and 19.8% for controls (P = .07), while 2.6% and 7.8%, respectively, were obese (P = .08).
No significant differences were seen in growth-related adverse events, such a weight-for-age less than the 5th percentile. The issue of “inducing” failure-to-thrive with a feeding intervention is a concern, said Dr. Paul, but there was no evidence for it in their study.
“One could question whether [the small differences seen between groups] are clinically significant, but if we look at how small differences have changed in the population over time and how those equate as far as longitudinal risk for cardiovascular outcomes and metabolic syndrome, etc., the small differences [we saw] might be important on a population level,” said Dr. Paul at the Pediatric Academic Societies meeting.
Study details
With upwards of one-quarter of U.S. children aged 2-5 years being overweight or obese, interventions to prevent rapid weight gain and reduce risk for overweight status in infancy are needed, noted Dr. Paul. Another reason to consider very early intervention, he added, is that infancy is a time of both “metabolic and behavioral plasticity.” However, most efforts to intervene early have, thus far, had limited success.
“Our responses to a baby crying are to feed that baby,” said Dr. Paul. This urge, along with others (such as “clear your plate”), evolved during times of food scarcity but persist now that we have inexpensive and palatable food, and promote rapid infant weight gain and increased obesity risk.
An alternative to those traditional parenting practices are responsive feeding and responsive parenting, he explained. “Responsive feeding and parenting requires prompt, developmentally appropriate responses to a child’s behaviors including hunger and satiety cues.”
In other studies, RP has been shown to foster cognitive, social, and emotional development. “The question we had was: Can responsive parenting reduce obesity risk?” he said.
The INSIGHT (Intervention Nurses Start Infants Growing on Healthy Trajectories) study is an ongoing, randomized clinical trial started in January 2012 comparing an RP intervention designed to prevent childhood obesity with a safety control, with the interventions matched on intensity and length.
Parent-child dyads were randomized 2 weeks after birth and were told that the purpose of the study was “to see if nurse visits to your home during your baby’s infancy can improve your ability to either respond to your child’s cues related to feeding and fussiness or improve your ability to provide a safe environment for your child and prevent injuries.”
A total of 279 primiparous mother-newborn dyads were studied. Most were white (89%) and non-Hispanic (94%), and the majority were married (75%). Mean prepregnancy BMI was 25.5 kg/m2.
“We chose first-time mothers because we thought they were more likely to listen to the parenting advice that we had to offer,” said Dr. Paul.
INSIGHT’s curriculum focused on RP in domains of infant feeding, sleep, interactive play, and emotion regulation. “We tried to promote self-regulation by setting limits but still being responsive in a variety of behavior domains,” Dr. Paul said. “So, for example…, for feeding we talked about exposure to healthy foods, shared feeding responsibility, for those that were bottle feeding we gave tips on size of bottle appropriate for the child and also not using bottle finishing practices. In the emotional and social regulation domain, we talked about alternatives to food to soothe, and emphasized embracing each child’s temperament and how to respond to different temperaments.”
Dr. Paul reported no conflicts of interest. INSIGHT is supported by National Institute of Diabetes and Digestive and Kidney Diseases research grants, with additional support from the Children’s Miracle Network at Penn State Children’s Hospital.
REPORTING FROM PAS 2018
Key clinical point:
Major finding: For the primary outcome of body mass index z score at 3 years, a significant difference favoring the responsive parenting intervention was seen (–0.13 vs. 0.15 for controls; absolute difference, –0.28; P = .04).
Study details: A randomized clinical trial including 279 mother-newborn dyads.
Disclosures: Dr. Paul reported no conflicts of interest. INSIGHT is supported by National Institute of Diabetes and Digestive and Kidney Diseases research grants, with additional support from the Children’s Miracle Network at Penn State Children’s Hospital.
IV superior to oral treatment for iron deficiency during pregnancy
AUSTIN, TEX. – Utilizing intravenous treatment for iron deficiency in anemic pregnant women was more efficacious than oral iron supplements, according to a study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
With 42% of pregnancies worldwide affected by anemia, according to the World Health Organization, improving treatment beyond the standard oral treatment could have a large effect on decreasing pregnancy complications.
“Women with bariatric surgery and inflammatory bowel disease are at higher risk of failure,” said Shravya Govindappagari, MD, a gynecologist affiliated with New York–Presbyterian Hospital. “Intravenous iron overcomes the limited intestinal absorption of oral formulations, and may increase iron stores more quickly.”
Dr. Govindappagari and her colleagues conducted a meta-analysis of 11 randomly controlled trials published between 2002 and 2017 to uncover the possible benefits of intravenous iron over oral treatment.
Studies were conducted in India, Egypt, France, and Turkey, with one additional multicenter study that gathered patients from seven different countries. Participants were given iron sucrose, ferric carboxymaltose, or low molecular weight iron dextran, according to Dr. Govindappagari.
In an overall assessment of subjects who achieved target hemoglobin levels, patients receiving intravenous iron were 2.66 times more likely to reach target levels than those given oral treatment (P less than .001). After 4 weeks of treatment, patients in the intravenous groups had a mean hemoglobin increase of 0.84 g/dl higher than those in the oral group (P less than .001).
Some clinicians may be wary about switching treatment modality from oral to intravenous; however, Dr. Govindappagari and fellow investigators found those taking oral treatment were 35% more likely to experience adverse effects than those receiving intravenous treatment.
While the analysis, according to Dr. Govindappagari, has merit, she and her team did not have access to relevant blinded, randomly controlled trials, which may have affected the findings. Maternal and neonatal outcomes were also not included in any of the studies analyzed, nor was a cost analysis of the financial burden of switching from oral to intravenous treatment.
Despite these limitations, Dr. Govindappagari and her colleagues assert the use of intravenous iron could have a significant effect on this problem.
“Intravenous iron compared to oral iron has a higher number reach target, a greater increase in hemoglobin, and has fewer side effects,” Dr. Govindappagari said to attendees. “This could be particularly useful in women in labor, during the third trimester, and women who are iron deficient and are at risk for postpartum hemorrhage.”
Dr. Govindappagari and her colleagues reported no relevant financial disclosures.
SOURCE: Govindappagari S et al. ACOG 2018, Abstract 10OP.
AUSTIN, TEX. – Utilizing intravenous treatment for iron deficiency in anemic pregnant women was more efficacious than oral iron supplements, according to a study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
With 42% of pregnancies worldwide affected by anemia, according to the World Health Organization, improving treatment beyond the standard oral treatment could have a large effect on decreasing pregnancy complications.
“Women with bariatric surgery and inflammatory bowel disease are at higher risk of failure,” said Shravya Govindappagari, MD, a gynecologist affiliated with New York–Presbyterian Hospital. “Intravenous iron overcomes the limited intestinal absorption of oral formulations, and may increase iron stores more quickly.”
Dr. Govindappagari and her colleagues conducted a meta-analysis of 11 randomly controlled trials published between 2002 and 2017 to uncover the possible benefits of intravenous iron over oral treatment.
Studies were conducted in India, Egypt, France, and Turkey, with one additional multicenter study that gathered patients from seven different countries. Participants were given iron sucrose, ferric carboxymaltose, or low molecular weight iron dextran, according to Dr. Govindappagari.
In an overall assessment of subjects who achieved target hemoglobin levels, patients receiving intravenous iron were 2.66 times more likely to reach target levels than those given oral treatment (P less than .001). After 4 weeks of treatment, patients in the intravenous groups had a mean hemoglobin increase of 0.84 g/dl higher than those in the oral group (P less than .001).
Some clinicians may be wary about switching treatment modality from oral to intravenous; however, Dr. Govindappagari and fellow investigators found those taking oral treatment were 35% more likely to experience adverse effects than those receiving intravenous treatment.
While the analysis, according to Dr. Govindappagari, has merit, she and her team did not have access to relevant blinded, randomly controlled trials, which may have affected the findings. Maternal and neonatal outcomes were also not included in any of the studies analyzed, nor was a cost analysis of the financial burden of switching from oral to intravenous treatment.
Despite these limitations, Dr. Govindappagari and her colleagues assert the use of intravenous iron could have a significant effect on this problem.
“Intravenous iron compared to oral iron has a higher number reach target, a greater increase in hemoglobin, and has fewer side effects,” Dr. Govindappagari said to attendees. “This could be particularly useful in women in labor, during the third trimester, and women who are iron deficient and are at risk for postpartum hemorrhage.”
Dr. Govindappagari and her colleagues reported no relevant financial disclosures.
SOURCE: Govindappagari S et al. ACOG 2018, Abstract 10OP.
AUSTIN, TEX. – Utilizing intravenous treatment for iron deficiency in anemic pregnant women was more efficacious than oral iron supplements, according to a study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
With 42% of pregnancies worldwide affected by anemia, according to the World Health Organization, improving treatment beyond the standard oral treatment could have a large effect on decreasing pregnancy complications.
“Women with bariatric surgery and inflammatory bowel disease are at higher risk of failure,” said Shravya Govindappagari, MD, a gynecologist affiliated with New York–Presbyterian Hospital. “Intravenous iron overcomes the limited intestinal absorption of oral formulations, and may increase iron stores more quickly.”
Dr. Govindappagari and her colleagues conducted a meta-analysis of 11 randomly controlled trials published between 2002 and 2017 to uncover the possible benefits of intravenous iron over oral treatment.
Studies were conducted in India, Egypt, France, and Turkey, with one additional multicenter study that gathered patients from seven different countries. Participants were given iron sucrose, ferric carboxymaltose, or low molecular weight iron dextran, according to Dr. Govindappagari.
In an overall assessment of subjects who achieved target hemoglobin levels, patients receiving intravenous iron were 2.66 times more likely to reach target levels than those given oral treatment (P less than .001). After 4 weeks of treatment, patients in the intravenous groups had a mean hemoglobin increase of 0.84 g/dl higher than those in the oral group (P less than .001).
Some clinicians may be wary about switching treatment modality from oral to intravenous; however, Dr. Govindappagari and fellow investigators found those taking oral treatment were 35% more likely to experience adverse effects than those receiving intravenous treatment.
While the analysis, according to Dr. Govindappagari, has merit, she and her team did not have access to relevant blinded, randomly controlled trials, which may have affected the findings. Maternal and neonatal outcomes were also not included in any of the studies analyzed, nor was a cost analysis of the financial burden of switching from oral to intravenous treatment.
Despite these limitations, Dr. Govindappagari and her colleagues assert the use of intravenous iron could have a significant effect on this problem.
“Intravenous iron compared to oral iron has a higher number reach target, a greater increase in hemoglobin, and has fewer side effects,” Dr. Govindappagari said to attendees. “This could be particularly useful in women in labor, during the third trimester, and women who are iron deficient and are at risk for postpartum hemorrhage.”
Dr. Govindappagari and her colleagues reported no relevant financial disclosures.
SOURCE: Govindappagari S et al. ACOG 2018, Abstract 10OP.
REPORTING FROM ACOG 2018
Key clinical point: Intravenous iron treatment is better for pregnant women with anemia.
Major finding: Hemoglobin levels in women with intravenous iron increased by 1.2 g/dl more than in those using oral supplements after 4 weeks (P less than .001).
Data source: A meta-analysis of 11 randomized, controlled trials comparing intravenous with oral iron treatment.
Disclosures: Dr. Govindappagari and her colleagues reported no relevant financial disclosures.
Source: Govindappagari S et al. ACOG 2018, Abstract 10OP.
SHM and Neurohospitalist Society partner on new program for stroke patients
The Society of Hospital Medicine recently partnered with the Neurohospitalist Society (NHS) to apply the neurology, stroke, and neurohospitalist expertise of NHS to the hospital and mentored implementation expertise of SHM for a uniquely positioned program for hospitals and health care systems: the Optimizing Neurovascular Intervention Care for Stroke Patients Mentored Implementation program.
This program aims to provide the resources and training to equip neurologists and hospitals with the skills to help assure continuous quality in the care of stroke patients with large vessel occlusion. The program will help neurohospitalists and other clinicians identify opportunities to engage multidisciplinary team members to implement evidence-based management practices in their hospital.
Reading Hospital – Tower Health, West Reading, Pa., was one of four hospitals selected to participate in the first wave of this program. Tower Health also recently became SHM’s first health system institutional partner. The Hospitalist spoke with a team from Reading Hospital about their participation in the new program and how they think it could affect their care. Interviewees included Sarah Keller, RN, nurse specialist; Deepam Gokal, MD, an associate director of hospitalist services; and Ruth Bailey, RN, stroke program manager.
What led you to partner with SHM for this program?
Dr. Gokal is an associate director of hospitalist services and comedical director of the stroke program, is a member of SHM, and was a former member of NHS; he received an email regarding the mentored implementation program for continuous quality monitoring and improvement in the care of stroke patients with large vessel occlusions. Karen Hoerst, MD, is a vascular neurologist and stroke program comedical director, and Ruth Bailey, RN, is the stroke program manager; together, we reviewed the introductory webinar with Dr. Gokal and felt this program would be beneficial for our organization, in particular because of Reading Hospital’s recent acquisition of five hospitals to form Tower Health – Brandywine Hospital, Coatesville, Pa.; Chestnut Hill Hospital, Philadelphia; Jennersville Hospital, West Grove, Pa.; Phoenixville (Pa.) Hospital; and Pottstown (Pa.) Hospital – and to help fulfill our vision to become the hub facility and a comprehensive stroke center.
Did you have a history with SHM prior to this program and before Tower Health’s new institutional partnership with SHM?
Reading Hospital participated in Project BOOST, SHM’s care transitions mentored implementation program, from 2012 to 2013. The goal was to optimize the hospital discharge process and to mitigate and prevent known complications and errors that occur during transitions. This was championed by hospitalists Walter R. Bohnenblust Jr., MD, SFHM, former Director of Hospitalist Services, and Binu Pappachen, MD, FHM.
The pain management provider team at Reading Hospital also championed an opioid management mentored implementation program in 2016-2017 that sought to improve safety and reduce adverse events for patients receiving opioids.
How do you anticipate this program will affect outcomes?
Reading Hospital – Tower Health is committed to advancing health care and transforming lives. The aim is to provide better care for individuals, improve health strategies, and reduce health care costs. This mentorship program should support this commitment to value-based care and population health management. It should prove beneficial to Reading Hospital by optimizing neurovascular interventions, which will help it become the intended hub for the Tower Health Teleneurology Program.
What will success look like to you and to members of the hospitalist team?
Future success for hospitalist services at Reading Hospital will include the fruition of a neurohospitalist subspecialty. Participation in this mentored implementation program should provide valuable resources for the development of this subspecialty that are aligned with the vision of Reading Hospital’s Advanced Primary Stroke Center. This vision is to serve as the comprehensive stroke center of choice for the patients both in our community and the surrounding region and to provide them with 24/7 state-of-the-art complex stroke treatment with demonstrated optimization of quality patient outcomes throughout the continuum of care.
For more information about SHM’s mentored implementation programs, visit hospitalmedicine.org/qi.
The Society of Hospital Medicine recently partnered with the Neurohospitalist Society (NHS) to apply the neurology, stroke, and neurohospitalist expertise of NHS to the hospital and mentored implementation expertise of SHM for a uniquely positioned program for hospitals and health care systems: the Optimizing Neurovascular Intervention Care for Stroke Patients Mentored Implementation program.
This program aims to provide the resources and training to equip neurologists and hospitals with the skills to help assure continuous quality in the care of stroke patients with large vessel occlusion. The program will help neurohospitalists and other clinicians identify opportunities to engage multidisciplinary team members to implement evidence-based management practices in their hospital.
Reading Hospital – Tower Health, West Reading, Pa., was one of four hospitals selected to participate in the first wave of this program. Tower Health also recently became SHM’s first health system institutional partner. The Hospitalist spoke with a team from Reading Hospital about their participation in the new program and how they think it could affect their care. Interviewees included Sarah Keller, RN, nurse specialist; Deepam Gokal, MD, an associate director of hospitalist services; and Ruth Bailey, RN, stroke program manager.
What led you to partner with SHM for this program?
Dr. Gokal is an associate director of hospitalist services and comedical director of the stroke program, is a member of SHM, and was a former member of NHS; he received an email regarding the mentored implementation program for continuous quality monitoring and improvement in the care of stroke patients with large vessel occlusions. Karen Hoerst, MD, is a vascular neurologist and stroke program comedical director, and Ruth Bailey, RN, is the stroke program manager; together, we reviewed the introductory webinar with Dr. Gokal and felt this program would be beneficial for our organization, in particular because of Reading Hospital’s recent acquisition of five hospitals to form Tower Health – Brandywine Hospital, Coatesville, Pa.; Chestnut Hill Hospital, Philadelphia; Jennersville Hospital, West Grove, Pa.; Phoenixville (Pa.) Hospital; and Pottstown (Pa.) Hospital – and to help fulfill our vision to become the hub facility and a comprehensive stroke center.
Did you have a history with SHM prior to this program and before Tower Health’s new institutional partnership with SHM?
Reading Hospital participated in Project BOOST, SHM’s care transitions mentored implementation program, from 2012 to 2013. The goal was to optimize the hospital discharge process and to mitigate and prevent known complications and errors that occur during transitions. This was championed by hospitalists Walter R. Bohnenblust Jr., MD, SFHM, former Director of Hospitalist Services, and Binu Pappachen, MD, FHM.
The pain management provider team at Reading Hospital also championed an opioid management mentored implementation program in 2016-2017 that sought to improve safety and reduce adverse events for patients receiving opioids.
How do you anticipate this program will affect outcomes?
Reading Hospital – Tower Health is committed to advancing health care and transforming lives. The aim is to provide better care for individuals, improve health strategies, and reduce health care costs. This mentorship program should support this commitment to value-based care and population health management. It should prove beneficial to Reading Hospital by optimizing neurovascular interventions, which will help it become the intended hub for the Tower Health Teleneurology Program.
What will success look like to you and to members of the hospitalist team?
Future success for hospitalist services at Reading Hospital will include the fruition of a neurohospitalist subspecialty. Participation in this mentored implementation program should provide valuable resources for the development of this subspecialty that are aligned with the vision of Reading Hospital’s Advanced Primary Stroke Center. This vision is to serve as the comprehensive stroke center of choice for the patients both in our community and the surrounding region and to provide them with 24/7 state-of-the-art complex stroke treatment with demonstrated optimization of quality patient outcomes throughout the continuum of care.
For more information about SHM’s mentored implementation programs, visit hospitalmedicine.org/qi.
The Society of Hospital Medicine recently partnered with the Neurohospitalist Society (NHS) to apply the neurology, stroke, and neurohospitalist expertise of NHS to the hospital and mentored implementation expertise of SHM for a uniquely positioned program for hospitals and health care systems: the Optimizing Neurovascular Intervention Care for Stroke Patients Mentored Implementation program.
This program aims to provide the resources and training to equip neurologists and hospitals with the skills to help assure continuous quality in the care of stroke patients with large vessel occlusion. The program will help neurohospitalists and other clinicians identify opportunities to engage multidisciplinary team members to implement evidence-based management practices in their hospital.
Reading Hospital – Tower Health, West Reading, Pa., was one of four hospitals selected to participate in the first wave of this program. Tower Health also recently became SHM’s first health system institutional partner. The Hospitalist spoke with a team from Reading Hospital about their participation in the new program and how they think it could affect their care. Interviewees included Sarah Keller, RN, nurse specialist; Deepam Gokal, MD, an associate director of hospitalist services; and Ruth Bailey, RN, stroke program manager.
What led you to partner with SHM for this program?
Dr. Gokal is an associate director of hospitalist services and comedical director of the stroke program, is a member of SHM, and was a former member of NHS; he received an email regarding the mentored implementation program for continuous quality monitoring and improvement in the care of stroke patients with large vessel occlusions. Karen Hoerst, MD, is a vascular neurologist and stroke program comedical director, and Ruth Bailey, RN, is the stroke program manager; together, we reviewed the introductory webinar with Dr. Gokal and felt this program would be beneficial for our organization, in particular because of Reading Hospital’s recent acquisition of five hospitals to form Tower Health – Brandywine Hospital, Coatesville, Pa.; Chestnut Hill Hospital, Philadelphia; Jennersville Hospital, West Grove, Pa.; Phoenixville (Pa.) Hospital; and Pottstown (Pa.) Hospital – and to help fulfill our vision to become the hub facility and a comprehensive stroke center.
Did you have a history with SHM prior to this program and before Tower Health’s new institutional partnership with SHM?
Reading Hospital participated in Project BOOST, SHM’s care transitions mentored implementation program, from 2012 to 2013. The goal was to optimize the hospital discharge process and to mitigate and prevent known complications and errors that occur during transitions. This was championed by hospitalists Walter R. Bohnenblust Jr., MD, SFHM, former Director of Hospitalist Services, and Binu Pappachen, MD, FHM.
The pain management provider team at Reading Hospital also championed an opioid management mentored implementation program in 2016-2017 that sought to improve safety and reduce adverse events for patients receiving opioids.
How do you anticipate this program will affect outcomes?
Reading Hospital – Tower Health is committed to advancing health care and transforming lives. The aim is to provide better care for individuals, improve health strategies, and reduce health care costs. This mentorship program should support this commitment to value-based care and population health management. It should prove beneficial to Reading Hospital by optimizing neurovascular interventions, which will help it become the intended hub for the Tower Health Teleneurology Program.
What will success look like to you and to members of the hospitalist team?
Future success for hospitalist services at Reading Hospital will include the fruition of a neurohospitalist subspecialty. Participation in this mentored implementation program should provide valuable resources for the development of this subspecialty that are aligned with the vision of Reading Hospital’s Advanced Primary Stroke Center. This vision is to serve as the comprehensive stroke center of choice for the patients both in our community and the surrounding region and to provide them with 24/7 state-of-the-art complex stroke treatment with demonstrated optimization of quality patient outcomes throughout the continuum of care.
For more information about SHM’s mentored implementation programs, visit hospitalmedicine.org/qi.
A Novel Technique for the Treatment of Jersey Fingers
ABSTRACT
The avulsion of the flexor digitorum profundus from its insertion, or “jersey finger,” is a relatively common injury. Numerous modifications have been made to the classification and treatment of this injury since its initial description. We describe a novel variation of the surgical management of jersey finger.
The avulsion-type injury of the flexor digitorum profundus (FDP) from its insertion on the distal phalanx is relatively common. FDP avulsions are seen in athletes and nonathletes, and are the result of the sudden hyperextension of the distal interphalangeal joint during active flexion. These injuries usually occur while grasping the jersey of an opposing player and are thus commonly referred to as “jersey finger.” Initially described in 1977 by Leddy and Packer1, FDP avulsions are classified on the basis of the proximal extent of the retraction of the FDP and the presence or absence of a bony avulsion fracture fragment. Type I injuries are defined by tendon retraction to the level of the palm, where it is tethered by the lumbricals. At this level, the vinculum longus profundus (VLP) and vinculum brevis profundus (VBP) are ruptured, resulting in the substantial loss of intrinsic and extrinsic vascular supply to the tendon. In type II injuries, which are the most common type of FDP avulsions, the FDP tendon retracts to the level of the proximal interphalangeal (PIP) joint. Although the VBP is disrupted in this scenario, the VLP remains preserved because it arises at the level of the volar plate of the PIP joint. Type III lesions involve tendon avulsions with an associated bony fragment that is typically sufficiently large to not pass through the flexor sheath, thus limiting retraction to the level of the A4 pulley. Both vincula remain intact, given that the VBP originates at the distal portion of the middle phalanx. The Leddy and Packer classification was later expanded to include type IV and V injury patterns, which are less common than other injury patterns. Similar to type III injuries, type IV injuries involve a bony avulsion; however, the FDP subsequently ruptures from this fragment and the tendon subsequently retracts into the finger or palm.2,3 Type V injuries are more complex than other injury types because they involve a concomitant distal phalanx fracture with the FDP avulsion.4 Al-Qattan5 subclassified type V injuries into extra-articular (type Va) and intra-articular (type Vb) distal phalanx fractures on the basis of the distinct management of these 2 entities.
Numerous techniques have been proposed and described for the repair of FDP avulsion injuries. The pullout suture-dorsal button combination is the most widely described technique and was initially described by Bunnell.6 Unfortunately, this technique is accompanied by numerous potential postoperative complications.6 Nail plate deformity is the most commonly described complication. Other complications include local wound irritation, pain, button snagging, and repair failure. Additionally, the presence of external sutures creates a potential route of ingress for bacterial infection.
Continue to: Bone suture anchor techniques...
Bone suture anchor techniques were later utilized to repair FDP avulsions in an attempt to decrease complications associated with the external suture-button construct.7 The use of a transosseous suture without external button fixation has also been proposed. Sood and Elliot8 described a technique where the suture is passed through a hole, drilled transversely through the tuft of the distal phalanx, and affixed to the other limb. In 1999, Schultz and colleagues9 described a technique where transosseous tunnels are placed in the distal phalanx in a dorsal-to-volar direction. The suture is then passed through and tied on the dorsal surface. In this article, we propose a transosseous suture technique that may provide advantages over previously described methods.
SURGICAL TECHNIQUE
TYPES I, II, AND III
A Bruner incision is performed on the volar aspect of the affected finger, and full thickness flaps are elevated off the flexor sheath (Figures 1A-1C).
TYPES IV AND V
In cases of type IV or V injury (Figure 4A), a screw or plate construct is first used to allow for the successful reduction and fixation of the fracture (Figure 4B).
DISCUSSION
The avulsion of the FDP tendon from its insertion (zone I) on the distal phalanx is commonly called “jersey finger” and is a well-described injury that occurs most commonly in the ring finger.10 These injuries can be difficult to treat and are associated with a complication rate of as high as 60%.11,12 Bunnell’s initial description of a suture passed through the fingernail and then tied over a polypropylene button has been associated with multiple complications. Kang and colleagues13 reported abnormal nail growth, nail fold necrosis, fingertip deformity, stiffness, infection, and amputation, 43% of all complications were directly related to the button. As an alternative to the button, sutures may be tied directly over the nail plate itself via 2 separate holes.14 While this technique eliminates the complications directly associated with the button, the potential for infection remains. Additionally, increased direct pressure is placed on the nail plate and nail bed, thus potentially increasing the risk of nail deformity.
In 1994, Hallock7 initially described the use of bone anchors as an “internal fixation” alternative and cited the “expense of the apparatus” as the major drawback of this technique. McCallister and colleagues15 compared the clinical outcome of suture anchor fixation with that of the button-over-nail technique. Although they ultimately demonstrated that the clinical outcomes of the 2 techniques are not significantly different, they noted that suture anchor fixation is associated with decreased infection rate (7% vs 0%) and time to return to work. Poor bone mineral density and low cortical thickness are correlated with anchor pull-out, thus limiting its universal use.16 Furthermore, the universal use of many commonly available anchors is limited given that they are too large to be accommodated within many phalanges, particularly in women and in the small and ring fingers.17 The use of microanchors rather than mini anchors not only decreases this risk but also decreases construct strength, thus necessitating the use of 2 anchors to restore adequate fixation strength. Anchor use is associated with specific risks, including the dorsal migration of the anchor, the osteolysis of the surrounding bone, as well as the perforation of the dorsal cortex and the possible extrusion of the anchor through the phalanx and into the nail bed.18,19 Additionally, in the wake of a changing healthcare system, the cost of suture anchors, as initially noted by Hallock,7 must be considered. This consideration is particularly relevant to the use of a 2 microanchor construct, which has been advocated given its biomechanical advantage.20,21
Continue to: Transosseous tendon repair...
Transosseous tendon repair is a cost-effective option that obviates many complications commonly observed with other fixation methods. By keeping the suture within the body, the complications inherent in external sutures and buttons are eliminated, including the loss of fixation as a result of button or suture damage and facilitating hand hygiene maintenance. The rate of infection is also reduced. Moreover, the risk of nail deformities is decreased because the suture is not passed through the nail bed and nail plate in the described technique. Occasionally, some patients do note irritation from the dorsal suture knot under the thin skin proximal to the germinal matrix. This can be easily addressed in the clinic by removing the knot under local anesthesia following sufficient tendon healing. Additionally, the described technique can be used safely in pediatric patients with open physes because the needles can be placed to prevent violating the physis. This technique can be performed in conjunction with the skeletal fixation of type III, IV, and V jersey fingers. In our experience, the transosseous suture repair is more secure than the limited screw fixation, which can be accomplished in many type III jersey fingers, and in at least 1 case, has maintained flexor function when the skeletal fixation of the jersey finger has failed (Figures 5A, 5B).
All internal fixation techniques have been described previously by Sood and Elliot8 and, later, by Schultz and colleagues.9 In contrast to Sood and Elliott’s8 technique, which requires the creation of transverse tunnels, a volar-to-dorsal tunnel is technically easy to create and creates a direct repair to tendon insertion. Our technique is similar to that of Schultz and colleagues'9 but has the following differences and potential improvements:
- Keith needles are passed in a volar-to-dorsal fashion, thus allowing for the direct visualization of the transosseous tunnel origin, minimizing the size of the transosseous tunnels, and allowing for the anatomic reduction of the tendon.
- Fluoroscopy is used to confirm wire placement prior to skin incision, thus enabling precise placement and potentially allowing the needles to be placed so as to avoid physeal injury in pediatric jersey fingers.
- By using Keith needles, sutures can be passed with the same instrument that created the tunnel, thus simplifying surgical technique.
- A Krakow suture technique is used. This technique results in less gapping and higher load-to-failure than other suturing techniques.22
- A 2-0 braided suture is used, therefore strengthening repair.
This paper will be judged for the Resident Writer’s Award.
1. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69. doi:https://doi.org/10.1016/S0363-5023(77)80012-9.
2. Langa V, Posner MA. Unusual rupture of a flexor profundus tendon. J Hand Surg Am. 1986;11(2):227-229. doi:https://doi.org/10.1016/S0363-5023(86)80056-9.
3. Ehlert KJ, Gould JS, Black KP. A simultaneous distal phalanx avulsion fracture with profundus tendon avulsion: A case report and review of the literature. Clin Orthop Relat Res. 1992;(283):265-269.
4. Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx–case report. J Hand Surg Am. 1981;6(6):600-601. doi:10.1097/00006534-198305000-00081.
5. Al-Qattan MM. Type 5 avulsion of the insertion of the flexor digitorum profundus tendon. J Hand Surg Br. 2001;26(5):427-431. doi:10.1054/jhsb.2001.0619.
6. Bunnell S. Surgery of the hand, 2nd edition. Philadelphia, PA: JB Lippincott; 1948:381-466.
7. Hallock GG. The Mitek Mini GII anchor introduced for tendon reinsertion in the hand. Ann Plast Surg. 1994;33(2):211-213.
8. Sood MK, Elliot D. A new technique of attachment of flexor tendons to the distal phalanx without a button tie-over. J Hand Surg Br. 1996;21(5):629-632. doi:https://doi.org/10.1016/S0266-7681(96)80146-X.
9. Schultz RO, Drake DB, Morgan RF. A new technique for the treatment of flexor digitorum profundus tendon avulsion. Ann Plast Surg. 1999;42(1):46-48. doi:10.1097/00000637-199901000-00008.
10. Manske PR, Lesker PA. Avulsion of the ring finger flexor digitorum profundus tendon: An experimental study. Hand 1978;10(1):52-55. doi:https://doi.org/10.1016/S0072-968X(78)80025-4.
11. Gerbino PG, Saldana MJ, Westerbeck P, Schacherer TG. Complications experienced in the rehabilitation of zone I flexor tendon injuries with dynamic traction splinting. J Hand Surg Am. 1991;16(4):680-686. doi:https://doi.org/10.1016/0363-5023(91)90194-G
12. Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. J Hand Ther. 2005;18(2):128-140. doi:10.1197/j.jht.2005.02.001
13. Kang N, Marsh D, Dewar D. The morbidity of the button-over-nail technique for zone 1 flexor tendon repairs. Should we still be using this technique? J Hand Surg Eur Vol. 2008;33(5):566-570. doi:10.1177/1753193408090118
14. Taras JS. Flexor tendon reconstruction: Single stage flexor tendon grafting: FDP, FDS disrupted. In: Green DP, Hotchkiss RN, Pederson WL, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:248-249.
15. McCallister WV, Ambrose HC, Katolik LI, Trumble TE. Comparison of pullout button versus suture anchor for zone I flexor tendon repair. J Hand Surg Am. 2006;31:246-251. doi:10.1016/j.jhsa.2005.10.020
16. Matzsuzaki H, Zaegel MA, Gelberman RH, Silva MJ. Effect of suture material and bone quality on the mechanical properties of zone 1 flexor tendon-bone reattachment with bone anchors. J Hand Surg Am. 2008;33(5):709-717. doi:10.1016/j.jhsa.2008.01.025
17. Singh R, Kakarala G, Persaud I, Roberts M, Strandring S, Compson J. The optimal length of tissue anchors for distal phalanges. A study in 395 cadaver digits. J Bone Joint Surg Br. 2006;88-B(SUPP I):37.
18. Giannikas D, Athanaselis E, Matzaroglou C, Saridis A, Tyllianakis M. An unusual complication of Mitek suture anchor use in primary treatment of flexor digitorum profundus tendon laceration: a case report. Cases J. 2009;2:9319. doi:10.1186/1757-1626-2-9319
19. Tiong WH, O'Sullivan ST. Extrusion of bone anchor suture following flexor digitorum profundus tendon avulsion injury repair. J Plast Reconstr Aesthet Surg. 2011;64(9):1242-1244. doi:10.1016/j.bjps.2011.01.016
20. Silva MJ, Hollstien SB, Brodt MD, Boyer MI, Tetro AM, Gelberman RH. Flexor digitorum profundus tendon-to-bone repair: An ex vivo biomechanical analysis of 3 pullout suture techniques. J Hand Surg Am. 1998;23(1):120-126. doi:10.1016/S0363-5023(98)80099-3
21. Latendresse K, Dona E, Scougall PJ, Schreuder FB, Puchert E, Walsh WR. Cyclic testing of pullout sutures and micro-mitek suture anchors in flexor digitorum profundus tendon distal fixation. J Hand Surg Am. 2005;30(3):471-478. doi:10.1016/j.jhsa.2004.10.014
22. Lee SK, Fajardo M, Kardashian G, Klein J, Tsai P, Christoforou D. Repair of flexor digitorum profundus to distal phalanx: a biomechanical evaluation of four techniques. J Hand Surg Am. 2011;36(10):1604-1609. doi:10.1016/j.jhsa.2011.07.017
ABSTRACT
The avulsion of the flexor digitorum profundus from its insertion, or “jersey finger,” is a relatively common injury. Numerous modifications have been made to the classification and treatment of this injury since its initial description. We describe a novel variation of the surgical management of jersey finger.
The avulsion-type injury of the flexor digitorum profundus (FDP) from its insertion on the distal phalanx is relatively common. FDP avulsions are seen in athletes and nonathletes, and are the result of the sudden hyperextension of the distal interphalangeal joint during active flexion. These injuries usually occur while grasping the jersey of an opposing player and are thus commonly referred to as “jersey finger.” Initially described in 1977 by Leddy and Packer1, FDP avulsions are classified on the basis of the proximal extent of the retraction of the FDP and the presence or absence of a bony avulsion fracture fragment. Type I injuries are defined by tendon retraction to the level of the palm, where it is tethered by the lumbricals. At this level, the vinculum longus profundus (VLP) and vinculum brevis profundus (VBP) are ruptured, resulting in the substantial loss of intrinsic and extrinsic vascular supply to the tendon. In type II injuries, which are the most common type of FDP avulsions, the FDP tendon retracts to the level of the proximal interphalangeal (PIP) joint. Although the VBP is disrupted in this scenario, the VLP remains preserved because it arises at the level of the volar plate of the PIP joint. Type III lesions involve tendon avulsions with an associated bony fragment that is typically sufficiently large to not pass through the flexor sheath, thus limiting retraction to the level of the A4 pulley. Both vincula remain intact, given that the VBP originates at the distal portion of the middle phalanx. The Leddy and Packer classification was later expanded to include type IV and V injury patterns, which are less common than other injury patterns. Similar to type III injuries, type IV injuries involve a bony avulsion; however, the FDP subsequently ruptures from this fragment and the tendon subsequently retracts into the finger or palm.2,3 Type V injuries are more complex than other injury types because they involve a concomitant distal phalanx fracture with the FDP avulsion.4 Al-Qattan5 subclassified type V injuries into extra-articular (type Va) and intra-articular (type Vb) distal phalanx fractures on the basis of the distinct management of these 2 entities.
Numerous techniques have been proposed and described for the repair of FDP avulsion injuries. The pullout suture-dorsal button combination is the most widely described technique and was initially described by Bunnell.6 Unfortunately, this technique is accompanied by numerous potential postoperative complications.6 Nail plate deformity is the most commonly described complication. Other complications include local wound irritation, pain, button snagging, and repair failure. Additionally, the presence of external sutures creates a potential route of ingress for bacterial infection.
Continue to: Bone suture anchor techniques...
Bone suture anchor techniques were later utilized to repair FDP avulsions in an attempt to decrease complications associated with the external suture-button construct.7 The use of a transosseous suture without external button fixation has also been proposed. Sood and Elliot8 described a technique where the suture is passed through a hole, drilled transversely through the tuft of the distal phalanx, and affixed to the other limb. In 1999, Schultz and colleagues9 described a technique where transosseous tunnels are placed in the distal phalanx in a dorsal-to-volar direction. The suture is then passed through and tied on the dorsal surface. In this article, we propose a transosseous suture technique that may provide advantages over previously described methods.
SURGICAL TECHNIQUE
TYPES I, II, AND III
A Bruner incision is performed on the volar aspect of the affected finger, and full thickness flaps are elevated off the flexor sheath (Figures 1A-1C).
TYPES IV AND V
In cases of type IV or V injury (Figure 4A), a screw or plate construct is first used to allow for the successful reduction and fixation of the fracture (Figure 4B).
DISCUSSION
The avulsion of the FDP tendon from its insertion (zone I) on the distal phalanx is commonly called “jersey finger” and is a well-described injury that occurs most commonly in the ring finger.10 These injuries can be difficult to treat and are associated with a complication rate of as high as 60%.11,12 Bunnell’s initial description of a suture passed through the fingernail and then tied over a polypropylene button has been associated with multiple complications. Kang and colleagues13 reported abnormal nail growth, nail fold necrosis, fingertip deformity, stiffness, infection, and amputation, 43% of all complications were directly related to the button. As an alternative to the button, sutures may be tied directly over the nail plate itself via 2 separate holes.14 While this technique eliminates the complications directly associated with the button, the potential for infection remains. Additionally, increased direct pressure is placed on the nail plate and nail bed, thus potentially increasing the risk of nail deformity.
In 1994, Hallock7 initially described the use of bone anchors as an “internal fixation” alternative and cited the “expense of the apparatus” as the major drawback of this technique. McCallister and colleagues15 compared the clinical outcome of suture anchor fixation with that of the button-over-nail technique. Although they ultimately demonstrated that the clinical outcomes of the 2 techniques are not significantly different, they noted that suture anchor fixation is associated with decreased infection rate (7% vs 0%) and time to return to work. Poor bone mineral density and low cortical thickness are correlated with anchor pull-out, thus limiting its universal use.16 Furthermore, the universal use of many commonly available anchors is limited given that they are too large to be accommodated within many phalanges, particularly in women and in the small and ring fingers.17 The use of microanchors rather than mini anchors not only decreases this risk but also decreases construct strength, thus necessitating the use of 2 anchors to restore adequate fixation strength. Anchor use is associated with specific risks, including the dorsal migration of the anchor, the osteolysis of the surrounding bone, as well as the perforation of the dorsal cortex and the possible extrusion of the anchor through the phalanx and into the nail bed.18,19 Additionally, in the wake of a changing healthcare system, the cost of suture anchors, as initially noted by Hallock,7 must be considered. This consideration is particularly relevant to the use of a 2 microanchor construct, which has been advocated given its biomechanical advantage.20,21
Continue to: Transosseous tendon repair...
Transosseous tendon repair is a cost-effective option that obviates many complications commonly observed with other fixation methods. By keeping the suture within the body, the complications inherent in external sutures and buttons are eliminated, including the loss of fixation as a result of button or suture damage and facilitating hand hygiene maintenance. The rate of infection is also reduced. Moreover, the risk of nail deformities is decreased because the suture is not passed through the nail bed and nail plate in the described technique. Occasionally, some patients do note irritation from the dorsal suture knot under the thin skin proximal to the germinal matrix. This can be easily addressed in the clinic by removing the knot under local anesthesia following sufficient tendon healing. Additionally, the described technique can be used safely in pediatric patients with open physes because the needles can be placed to prevent violating the physis. This technique can be performed in conjunction with the skeletal fixation of type III, IV, and V jersey fingers. In our experience, the transosseous suture repair is more secure than the limited screw fixation, which can be accomplished in many type III jersey fingers, and in at least 1 case, has maintained flexor function when the skeletal fixation of the jersey finger has failed (Figures 5A, 5B).
All internal fixation techniques have been described previously by Sood and Elliot8 and, later, by Schultz and colleagues.9 In contrast to Sood and Elliott’s8 technique, which requires the creation of transverse tunnels, a volar-to-dorsal tunnel is technically easy to create and creates a direct repair to tendon insertion. Our technique is similar to that of Schultz and colleagues'9 but has the following differences and potential improvements:
- Keith needles are passed in a volar-to-dorsal fashion, thus allowing for the direct visualization of the transosseous tunnel origin, minimizing the size of the transosseous tunnels, and allowing for the anatomic reduction of the tendon.
- Fluoroscopy is used to confirm wire placement prior to skin incision, thus enabling precise placement and potentially allowing the needles to be placed so as to avoid physeal injury in pediatric jersey fingers.
- By using Keith needles, sutures can be passed with the same instrument that created the tunnel, thus simplifying surgical technique.
- A Krakow suture technique is used. This technique results in less gapping and higher load-to-failure than other suturing techniques.22
- A 2-0 braided suture is used, therefore strengthening repair.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The avulsion of the flexor digitorum profundus from its insertion, or “jersey finger,” is a relatively common injury. Numerous modifications have been made to the classification and treatment of this injury since its initial description. We describe a novel variation of the surgical management of jersey finger.
The avulsion-type injury of the flexor digitorum profundus (FDP) from its insertion on the distal phalanx is relatively common. FDP avulsions are seen in athletes and nonathletes, and are the result of the sudden hyperextension of the distal interphalangeal joint during active flexion. These injuries usually occur while grasping the jersey of an opposing player and are thus commonly referred to as “jersey finger.” Initially described in 1977 by Leddy and Packer1, FDP avulsions are classified on the basis of the proximal extent of the retraction of the FDP and the presence or absence of a bony avulsion fracture fragment. Type I injuries are defined by tendon retraction to the level of the palm, where it is tethered by the lumbricals. At this level, the vinculum longus profundus (VLP) and vinculum brevis profundus (VBP) are ruptured, resulting in the substantial loss of intrinsic and extrinsic vascular supply to the tendon. In type II injuries, which are the most common type of FDP avulsions, the FDP tendon retracts to the level of the proximal interphalangeal (PIP) joint. Although the VBP is disrupted in this scenario, the VLP remains preserved because it arises at the level of the volar plate of the PIP joint. Type III lesions involve tendon avulsions with an associated bony fragment that is typically sufficiently large to not pass through the flexor sheath, thus limiting retraction to the level of the A4 pulley. Both vincula remain intact, given that the VBP originates at the distal portion of the middle phalanx. The Leddy and Packer classification was later expanded to include type IV and V injury patterns, which are less common than other injury patterns. Similar to type III injuries, type IV injuries involve a bony avulsion; however, the FDP subsequently ruptures from this fragment and the tendon subsequently retracts into the finger or palm.2,3 Type V injuries are more complex than other injury types because they involve a concomitant distal phalanx fracture with the FDP avulsion.4 Al-Qattan5 subclassified type V injuries into extra-articular (type Va) and intra-articular (type Vb) distal phalanx fractures on the basis of the distinct management of these 2 entities.
Numerous techniques have been proposed and described for the repair of FDP avulsion injuries. The pullout suture-dorsal button combination is the most widely described technique and was initially described by Bunnell.6 Unfortunately, this technique is accompanied by numerous potential postoperative complications.6 Nail plate deformity is the most commonly described complication. Other complications include local wound irritation, pain, button snagging, and repair failure. Additionally, the presence of external sutures creates a potential route of ingress for bacterial infection.
Continue to: Bone suture anchor techniques...
Bone suture anchor techniques were later utilized to repair FDP avulsions in an attempt to decrease complications associated with the external suture-button construct.7 The use of a transosseous suture without external button fixation has also been proposed. Sood and Elliot8 described a technique where the suture is passed through a hole, drilled transversely through the tuft of the distal phalanx, and affixed to the other limb. In 1999, Schultz and colleagues9 described a technique where transosseous tunnels are placed in the distal phalanx in a dorsal-to-volar direction. The suture is then passed through and tied on the dorsal surface. In this article, we propose a transosseous suture technique that may provide advantages over previously described methods.
SURGICAL TECHNIQUE
TYPES I, II, AND III
A Bruner incision is performed on the volar aspect of the affected finger, and full thickness flaps are elevated off the flexor sheath (Figures 1A-1C).
TYPES IV AND V
In cases of type IV or V injury (Figure 4A), a screw or plate construct is first used to allow for the successful reduction and fixation of the fracture (Figure 4B).
DISCUSSION
The avulsion of the FDP tendon from its insertion (zone I) on the distal phalanx is commonly called “jersey finger” and is a well-described injury that occurs most commonly in the ring finger.10 These injuries can be difficult to treat and are associated with a complication rate of as high as 60%.11,12 Bunnell’s initial description of a suture passed through the fingernail and then tied over a polypropylene button has been associated with multiple complications. Kang and colleagues13 reported abnormal nail growth, nail fold necrosis, fingertip deformity, stiffness, infection, and amputation, 43% of all complications were directly related to the button. As an alternative to the button, sutures may be tied directly over the nail plate itself via 2 separate holes.14 While this technique eliminates the complications directly associated with the button, the potential for infection remains. Additionally, increased direct pressure is placed on the nail plate and nail bed, thus potentially increasing the risk of nail deformity.
In 1994, Hallock7 initially described the use of bone anchors as an “internal fixation” alternative and cited the “expense of the apparatus” as the major drawback of this technique. McCallister and colleagues15 compared the clinical outcome of suture anchor fixation with that of the button-over-nail technique. Although they ultimately demonstrated that the clinical outcomes of the 2 techniques are not significantly different, they noted that suture anchor fixation is associated with decreased infection rate (7% vs 0%) and time to return to work. Poor bone mineral density and low cortical thickness are correlated with anchor pull-out, thus limiting its universal use.16 Furthermore, the universal use of many commonly available anchors is limited given that they are too large to be accommodated within many phalanges, particularly in women and in the small and ring fingers.17 The use of microanchors rather than mini anchors not only decreases this risk but also decreases construct strength, thus necessitating the use of 2 anchors to restore adequate fixation strength. Anchor use is associated with specific risks, including the dorsal migration of the anchor, the osteolysis of the surrounding bone, as well as the perforation of the dorsal cortex and the possible extrusion of the anchor through the phalanx and into the nail bed.18,19 Additionally, in the wake of a changing healthcare system, the cost of suture anchors, as initially noted by Hallock,7 must be considered. This consideration is particularly relevant to the use of a 2 microanchor construct, which has been advocated given its biomechanical advantage.20,21
Continue to: Transosseous tendon repair...
Transosseous tendon repair is a cost-effective option that obviates many complications commonly observed with other fixation methods. By keeping the suture within the body, the complications inherent in external sutures and buttons are eliminated, including the loss of fixation as a result of button or suture damage and facilitating hand hygiene maintenance. The rate of infection is also reduced. Moreover, the risk of nail deformities is decreased because the suture is not passed through the nail bed and nail plate in the described technique. Occasionally, some patients do note irritation from the dorsal suture knot under the thin skin proximal to the germinal matrix. This can be easily addressed in the clinic by removing the knot under local anesthesia following sufficient tendon healing. Additionally, the described technique can be used safely in pediatric patients with open physes because the needles can be placed to prevent violating the physis. This technique can be performed in conjunction with the skeletal fixation of type III, IV, and V jersey fingers. In our experience, the transosseous suture repair is more secure than the limited screw fixation, which can be accomplished in many type III jersey fingers, and in at least 1 case, has maintained flexor function when the skeletal fixation of the jersey finger has failed (Figures 5A, 5B).
All internal fixation techniques have been described previously by Sood and Elliot8 and, later, by Schultz and colleagues.9 In contrast to Sood and Elliott’s8 technique, which requires the creation of transverse tunnels, a volar-to-dorsal tunnel is technically easy to create and creates a direct repair to tendon insertion. Our technique is similar to that of Schultz and colleagues'9 but has the following differences and potential improvements:
- Keith needles are passed in a volar-to-dorsal fashion, thus allowing for the direct visualization of the transosseous tunnel origin, minimizing the size of the transosseous tunnels, and allowing for the anatomic reduction of the tendon.
- Fluoroscopy is used to confirm wire placement prior to skin incision, thus enabling precise placement and potentially allowing the needles to be placed so as to avoid physeal injury in pediatric jersey fingers.
- By using Keith needles, sutures can be passed with the same instrument that created the tunnel, thus simplifying surgical technique.
- A Krakow suture technique is used. This technique results in less gapping and higher load-to-failure than other suturing techniques.22
- A 2-0 braided suture is used, therefore strengthening repair.
This paper will be judged for the Resident Writer’s Award.
1. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69. doi:https://doi.org/10.1016/S0363-5023(77)80012-9.
2. Langa V, Posner MA. Unusual rupture of a flexor profundus tendon. J Hand Surg Am. 1986;11(2):227-229. doi:https://doi.org/10.1016/S0363-5023(86)80056-9.
3. Ehlert KJ, Gould JS, Black KP. A simultaneous distal phalanx avulsion fracture with profundus tendon avulsion: A case report and review of the literature. Clin Orthop Relat Res. 1992;(283):265-269.
4. Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx–case report. J Hand Surg Am. 1981;6(6):600-601. doi:10.1097/00006534-198305000-00081.
5. Al-Qattan MM. Type 5 avulsion of the insertion of the flexor digitorum profundus tendon. J Hand Surg Br. 2001;26(5):427-431. doi:10.1054/jhsb.2001.0619.
6. Bunnell S. Surgery of the hand, 2nd edition. Philadelphia, PA: JB Lippincott; 1948:381-466.
7. Hallock GG. The Mitek Mini GII anchor introduced for tendon reinsertion in the hand. Ann Plast Surg. 1994;33(2):211-213.
8. Sood MK, Elliot D. A new technique of attachment of flexor tendons to the distal phalanx without a button tie-over. J Hand Surg Br. 1996;21(5):629-632. doi:https://doi.org/10.1016/S0266-7681(96)80146-X.
9. Schultz RO, Drake DB, Morgan RF. A new technique for the treatment of flexor digitorum profundus tendon avulsion. Ann Plast Surg. 1999;42(1):46-48. doi:10.1097/00000637-199901000-00008.
10. Manske PR, Lesker PA. Avulsion of the ring finger flexor digitorum profundus tendon: An experimental study. Hand 1978;10(1):52-55. doi:https://doi.org/10.1016/S0072-968X(78)80025-4.
11. Gerbino PG, Saldana MJ, Westerbeck P, Schacherer TG. Complications experienced in the rehabilitation of zone I flexor tendon injuries with dynamic traction splinting. J Hand Surg Am. 1991;16(4):680-686. doi:https://doi.org/10.1016/0363-5023(91)90194-G
12. Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. J Hand Ther. 2005;18(2):128-140. doi:10.1197/j.jht.2005.02.001
13. Kang N, Marsh D, Dewar D. The morbidity of the button-over-nail technique for zone 1 flexor tendon repairs. Should we still be using this technique? J Hand Surg Eur Vol. 2008;33(5):566-570. doi:10.1177/1753193408090118
14. Taras JS. Flexor tendon reconstruction: Single stage flexor tendon grafting: FDP, FDS disrupted. In: Green DP, Hotchkiss RN, Pederson WL, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:248-249.
15. McCallister WV, Ambrose HC, Katolik LI, Trumble TE. Comparison of pullout button versus suture anchor for zone I flexor tendon repair. J Hand Surg Am. 2006;31:246-251. doi:10.1016/j.jhsa.2005.10.020
16. Matzsuzaki H, Zaegel MA, Gelberman RH, Silva MJ. Effect of suture material and bone quality on the mechanical properties of zone 1 flexor tendon-bone reattachment with bone anchors. J Hand Surg Am. 2008;33(5):709-717. doi:10.1016/j.jhsa.2008.01.025
17. Singh R, Kakarala G, Persaud I, Roberts M, Strandring S, Compson J. The optimal length of tissue anchors for distal phalanges. A study in 395 cadaver digits. J Bone Joint Surg Br. 2006;88-B(SUPP I):37.
18. Giannikas D, Athanaselis E, Matzaroglou C, Saridis A, Tyllianakis M. An unusual complication of Mitek suture anchor use in primary treatment of flexor digitorum profundus tendon laceration: a case report. Cases J. 2009;2:9319. doi:10.1186/1757-1626-2-9319
19. Tiong WH, O'Sullivan ST. Extrusion of bone anchor suture following flexor digitorum profundus tendon avulsion injury repair. J Plast Reconstr Aesthet Surg. 2011;64(9):1242-1244. doi:10.1016/j.bjps.2011.01.016
20. Silva MJ, Hollstien SB, Brodt MD, Boyer MI, Tetro AM, Gelberman RH. Flexor digitorum profundus tendon-to-bone repair: An ex vivo biomechanical analysis of 3 pullout suture techniques. J Hand Surg Am. 1998;23(1):120-126. doi:10.1016/S0363-5023(98)80099-3
21. Latendresse K, Dona E, Scougall PJ, Schreuder FB, Puchert E, Walsh WR. Cyclic testing of pullout sutures and micro-mitek suture anchors in flexor digitorum profundus tendon distal fixation. J Hand Surg Am. 2005;30(3):471-478. doi:10.1016/j.jhsa.2004.10.014
22. Lee SK, Fajardo M, Kardashian G, Klein J, Tsai P, Christoforou D. Repair of flexor digitorum profundus to distal phalanx: a biomechanical evaluation of four techniques. J Hand Surg Am. 2011;36(10):1604-1609. doi:10.1016/j.jhsa.2011.07.017
1. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69. doi:https://doi.org/10.1016/S0363-5023(77)80012-9.
2. Langa V, Posner MA. Unusual rupture of a flexor profundus tendon. J Hand Surg Am. 1986;11(2):227-229. doi:https://doi.org/10.1016/S0363-5023(86)80056-9.
3. Ehlert KJ, Gould JS, Black KP. A simultaneous distal phalanx avulsion fracture with profundus tendon avulsion: A case report and review of the literature. Clin Orthop Relat Res. 1992;(283):265-269.
4. Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx–case report. J Hand Surg Am. 1981;6(6):600-601. doi:10.1097/00006534-198305000-00081.
5. Al-Qattan MM. Type 5 avulsion of the insertion of the flexor digitorum profundus tendon. J Hand Surg Br. 2001;26(5):427-431. doi:10.1054/jhsb.2001.0619.
6. Bunnell S. Surgery of the hand, 2nd edition. Philadelphia, PA: JB Lippincott; 1948:381-466.
7. Hallock GG. The Mitek Mini GII anchor introduced for tendon reinsertion in the hand. Ann Plast Surg. 1994;33(2):211-213.
8. Sood MK, Elliot D. A new technique of attachment of flexor tendons to the distal phalanx without a button tie-over. J Hand Surg Br. 1996;21(5):629-632. doi:https://doi.org/10.1016/S0266-7681(96)80146-X.
9. Schultz RO, Drake DB, Morgan RF. A new technique for the treatment of flexor digitorum profundus tendon avulsion. Ann Plast Surg. 1999;42(1):46-48. doi:10.1097/00000637-199901000-00008.
10. Manske PR, Lesker PA. Avulsion of the ring finger flexor digitorum profundus tendon: An experimental study. Hand 1978;10(1):52-55. doi:https://doi.org/10.1016/S0072-968X(78)80025-4.
11. Gerbino PG, Saldana MJ, Westerbeck P, Schacherer TG. Complications experienced in the rehabilitation of zone I flexor tendon injuries with dynamic traction splinting. J Hand Surg Am. 1991;16(4):680-686. doi:https://doi.org/10.1016/0363-5023(91)90194-G
12. Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. J Hand Ther. 2005;18(2):128-140. doi:10.1197/j.jht.2005.02.001
13. Kang N, Marsh D, Dewar D. The morbidity of the button-over-nail technique for zone 1 flexor tendon repairs. Should we still be using this technique? J Hand Surg Eur Vol. 2008;33(5):566-570. doi:10.1177/1753193408090118
14. Taras JS. Flexor tendon reconstruction: Single stage flexor tendon grafting: FDP, FDS disrupted. In: Green DP, Hotchkiss RN, Pederson WL, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:248-249.
15. McCallister WV, Ambrose HC, Katolik LI, Trumble TE. Comparison of pullout button versus suture anchor for zone I flexor tendon repair. J Hand Surg Am. 2006;31:246-251. doi:10.1016/j.jhsa.2005.10.020
16. Matzsuzaki H, Zaegel MA, Gelberman RH, Silva MJ. Effect of suture material and bone quality on the mechanical properties of zone 1 flexor tendon-bone reattachment with bone anchors. J Hand Surg Am. 2008;33(5):709-717. doi:10.1016/j.jhsa.2008.01.025
17. Singh R, Kakarala G, Persaud I, Roberts M, Strandring S, Compson J. The optimal length of tissue anchors for distal phalanges. A study in 395 cadaver digits. J Bone Joint Surg Br. 2006;88-B(SUPP I):37.
18. Giannikas D, Athanaselis E, Matzaroglou C, Saridis A, Tyllianakis M. An unusual complication of Mitek suture anchor use in primary treatment of flexor digitorum profundus tendon laceration: a case report. Cases J. 2009;2:9319. doi:10.1186/1757-1626-2-9319
19. Tiong WH, O'Sullivan ST. Extrusion of bone anchor suture following flexor digitorum profundus tendon avulsion injury repair. J Plast Reconstr Aesthet Surg. 2011;64(9):1242-1244. doi:10.1016/j.bjps.2011.01.016
20. Silva MJ, Hollstien SB, Brodt MD, Boyer MI, Tetro AM, Gelberman RH. Flexor digitorum profundus tendon-to-bone repair: An ex vivo biomechanical analysis of 3 pullout suture techniques. J Hand Surg Am. 1998;23(1):120-126. doi:10.1016/S0363-5023(98)80099-3
21. Latendresse K, Dona E, Scougall PJ, Schreuder FB, Puchert E, Walsh WR. Cyclic testing of pullout sutures and micro-mitek suture anchors in flexor digitorum profundus tendon distal fixation. J Hand Surg Am. 2005;30(3):471-478. doi:10.1016/j.jhsa.2004.10.014
22. Lee SK, Fajardo M, Kardashian G, Klein J, Tsai P, Christoforou D. Repair of flexor digitorum profundus to distal phalanx: a biomechanical evaluation of four techniques. J Hand Surg Am. 2011;36(10):1604-1609. doi:10.1016/j.jhsa.2011.07.017
TAKE-HOME POINTS
- Transosseous repair of FDP has been long utilized, tying the sutures over a polyethylene button at the nail plate, which is associated with significant complications.
- Avoiding use of a button decreases these complications, eliminating damage to the nailbed and eliminating external sutures, thus decreasing infection risk.
- Keith needles can be utilized to pass the sutures from volar to dorsal, and can be inserted using a wire drive; their position can be checked fluoroscopically prior to suture passage.
- This technique can be used in conjunction with skeletal fixation of associated fractures.
- This technique can be utilized in pediatric patients, placing the sutures distal to the physis.