User login
Hydroxyurea in infancy yields better SCD outcomes
PITTSBURGH – Children with sickle cell disease (SCD) who were started on hydroxyurea in infancy had significantly better outcomes than do children started on the drug as toddlers, researchers report.
Among 65 children with SCD, those who started on hydroxyurea before age 1 year had significantly fewer hospitalizations, pain crises, and transfusions in the first 2 years of life, compared with patients started on the drug during 1-2 years of age or after age 2 years, found Sarah B. Schuchard, PharmD, from the SSM Health Cardinal Glennon Children’s Hospital in St. Louis and her colleagues.
At the Children’s Hospital and Clinics of Minnesota in Minneapolis, where Dr. Schuchard recently completed her training and conducted the research, the goal is to initiate all infants with sickle cell anemia and sickle-beta0-thalassemia on hydroxyurea within this first year of life.
To evaluate outcomes associated with this practice, the investigators conducted a retrospective review of all children with SCD who began hydroxyurea therapy at their center during 2008-2016.
They divided the population into three cohorts. Patients in cohort 1 were started on hydroxyurea before age 1 year (35 patients; mean age, 7.2 months), those in cohort 2 started between 1 and 2 years of age (13 patients; mean age, 19.5 months) and those in cohort 3 were started after 2 years of age (three patients; mean age, 35.5 months).
All patients had been diagnosed with either sickle cell anemia or sickle-beta0-thalassemia, and all had at least two laboratory assessments at 6 months, 12 months, 18 months, or 24 months of age.
For the coprimary endpoint of laboratory data, the investigators found that patients in cohort 1, the early starters, had significantly higher hemoglobin (P = .0003), lower absolute reticulocyte counts (P = .0304), and mean corpuscular volume (P = .0199) than did the patients in cohort 3.
Infants in cohort 1 had significantly lower white blood cell counts than did the patients in either cohorts 2 or 3 (P = .0007 and P less than .0001, respectively) and lower absolute neutrophil counts (P = .0364 and .0025, respectively), although no patients required hydroxyurea therapy to be held because of low ANC.
Clinical events, the other coprimary endpoint, were also significantly better among patients in cohort 1, who had significantly fewer hospitalizations (P = .0025), a trend toward fewer painful events (P = .0618), and significantly fewer transfusions (P = .0426) than did patients in the other two cohorts.
“Early hydroxyurea also appears to contribute to fewer pain crises requiring admission,” the investigators noted.
They noted that in their study, the hematologic response was greater than that seen in the BABY HUG study, which studied the protective effects of hydroxyurea in children aged 9-18 months. The mean age of hydroxyurea initiation was 13.6 months in that study, compared with 7.2 months in the study by Dr. Schuchard and her colleagues.
“It would be interesting to see if the splenic and renal function (the unmet primary endpoints of BABY HUG) are preserved in patients starting hydroxyurea at this younger age,” they wrote.
The study was internally funded. Dr. Schuchard reported having no conflicts of interest.
SOURCE: Schuchard S et al. ASPHO 2018, Poster 342.
PITTSBURGH – Children with sickle cell disease (SCD) who were started on hydroxyurea in infancy had significantly better outcomes than do children started on the drug as toddlers, researchers report.
Among 65 children with SCD, those who started on hydroxyurea before age 1 year had significantly fewer hospitalizations, pain crises, and transfusions in the first 2 years of life, compared with patients started on the drug during 1-2 years of age or after age 2 years, found Sarah B. Schuchard, PharmD, from the SSM Health Cardinal Glennon Children’s Hospital in St. Louis and her colleagues.
At the Children’s Hospital and Clinics of Minnesota in Minneapolis, where Dr. Schuchard recently completed her training and conducted the research, the goal is to initiate all infants with sickle cell anemia and sickle-beta0-thalassemia on hydroxyurea within this first year of life.
To evaluate outcomes associated with this practice, the investigators conducted a retrospective review of all children with SCD who began hydroxyurea therapy at their center during 2008-2016.
They divided the population into three cohorts. Patients in cohort 1 were started on hydroxyurea before age 1 year (35 patients; mean age, 7.2 months), those in cohort 2 started between 1 and 2 years of age (13 patients; mean age, 19.5 months) and those in cohort 3 were started after 2 years of age (three patients; mean age, 35.5 months).
All patients had been diagnosed with either sickle cell anemia or sickle-beta0-thalassemia, and all had at least two laboratory assessments at 6 months, 12 months, 18 months, or 24 months of age.
For the coprimary endpoint of laboratory data, the investigators found that patients in cohort 1, the early starters, had significantly higher hemoglobin (P = .0003), lower absolute reticulocyte counts (P = .0304), and mean corpuscular volume (P = .0199) than did the patients in cohort 3.
Infants in cohort 1 had significantly lower white blood cell counts than did the patients in either cohorts 2 or 3 (P = .0007 and P less than .0001, respectively) and lower absolute neutrophil counts (P = .0364 and .0025, respectively), although no patients required hydroxyurea therapy to be held because of low ANC.
Clinical events, the other coprimary endpoint, were also significantly better among patients in cohort 1, who had significantly fewer hospitalizations (P = .0025), a trend toward fewer painful events (P = .0618), and significantly fewer transfusions (P = .0426) than did patients in the other two cohorts.
“Early hydroxyurea also appears to contribute to fewer pain crises requiring admission,” the investigators noted.
They noted that in their study, the hematologic response was greater than that seen in the BABY HUG study, which studied the protective effects of hydroxyurea in children aged 9-18 months. The mean age of hydroxyurea initiation was 13.6 months in that study, compared with 7.2 months in the study by Dr. Schuchard and her colleagues.
“It would be interesting to see if the splenic and renal function (the unmet primary endpoints of BABY HUG) are preserved in patients starting hydroxyurea at this younger age,” they wrote.
The study was internally funded. Dr. Schuchard reported having no conflicts of interest.
SOURCE: Schuchard S et al. ASPHO 2018, Poster 342.
PITTSBURGH – Children with sickle cell disease (SCD) who were started on hydroxyurea in infancy had significantly better outcomes than do children started on the drug as toddlers, researchers report.
Among 65 children with SCD, those who started on hydroxyurea before age 1 year had significantly fewer hospitalizations, pain crises, and transfusions in the first 2 years of life, compared with patients started on the drug during 1-2 years of age or after age 2 years, found Sarah B. Schuchard, PharmD, from the SSM Health Cardinal Glennon Children’s Hospital in St. Louis and her colleagues.
At the Children’s Hospital and Clinics of Minnesota in Minneapolis, where Dr. Schuchard recently completed her training and conducted the research, the goal is to initiate all infants with sickle cell anemia and sickle-beta0-thalassemia on hydroxyurea within this first year of life.
To evaluate outcomes associated with this practice, the investigators conducted a retrospective review of all children with SCD who began hydroxyurea therapy at their center during 2008-2016.
They divided the population into three cohorts. Patients in cohort 1 were started on hydroxyurea before age 1 year (35 patients; mean age, 7.2 months), those in cohort 2 started between 1 and 2 years of age (13 patients; mean age, 19.5 months) and those in cohort 3 were started after 2 years of age (three patients; mean age, 35.5 months).
All patients had been diagnosed with either sickle cell anemia or sickle-beta0-thalassemia, and all had at least two laboratory assessments at 6 months, 12 months, 18 months, or 24 months of age.
For the coprimary endpoint of laboratory data, the investigators found that patients in cohort 1, the early starters, had significantly higher hemoglobin (P = .0003), lower absolute reticulocyte counts (P = .0304), and mean corpuscular volume (P = .0199) than did the patients in cohort 3.
Infants in cohort 1 had significantly lower white blood cell counts than did the patients in either cohorts 2 or 3 (P = .0007 and P less than .0001, respectively) and lower absolute neutrophil counts (P = .0364 and .0025, respectively), although no patients required hydroxyurea therapy to be held because of low ANC.
Clinical events, the other coprimary endpoint, were also significantly better among patients in cohort 1, who had significantly fewer hospitalizations (P = .0025), a trend toward fewer painful events (P = .0618), and significantly fewer transfusions (P = .0426) than did patients in the other two cohorts.
“Early hydroxyurea also appears to contribute to fewer pain crises requiring admission,” the investigators noted.
They noted that in their study, the hematologic response was greater than that seen in the BABY HUG study, which studied the protective effects of hydroxyurea in children aged 9-18 months. The mean age of hydroxyurea initiation was 13.6 months in that study, compared with 7.2 months in the study by Dr. Schuchard and her colleagues.
“It would be interesting to see if the splenic and renal function (the unmet primary endpoints of BABY HUG) are preserved in patients starting hydroxyurea at this younger age,” they wrote.
The study was internally funded. Dr. Schuchard reported having no conflicts of interest.
SOURCE: Schuchard S et al. ASPHO 2018, Poster 342.
REPORTING FROM ASPHO 2018
Key clinical point: Early initiation of hydroxyurea is associated with better SCD outcomes.
Major finding: Patients started on hydroxyurea at a mean of 7.2 months had significantly fewer admissions, pain crises, and transfusions than did patients started after age 1 year.
Study details: Retrospective review of data on 65 children with SCD in a single center.
Disclosures: The study was internally funded. Dr. Schuchard reported having no conflicts of interest.
Source: Schuchard S et al. ASPHO 2018, Poster 342.
Abstract: Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Poole, R., et al, BMJ 359:J5024, November 22, 2017
BACKGROUND: Studies examining the benefits versus harms of coffee consumption have yielded conflicting results.
METHODS: The authors, from the United Kingdom, present an umbrella review of meta-analyses published up to 2017 to determine the associations between coffee consumption and any health outcome in adults.
RESULTS: This review includes 201 meta-analyses of observational studies with 67 health outcomes and 17 meta-analyses of randomized trials with 9 health outcomes according to coffee consumption defined as high versus low, any versus none, or per each extra cup per day. Coffee was associated with statistically significant protective effects against several diseases including type 2 diabetes, renal stones, Parkinson’s disease, Alzheimer’s disease, depression, leukemia, gout, colorectal cancer, liver cancer, chronic liver disease, cirrhosis, and endometrial cancer (odds ratios of 0.35-0.94). For some outcomes including all-cause mortality, cardiovascular mortality and cardiovascular disease, a nonlinear association was found whereby 3-4 cups per day (versus 0) conferred the greatest risk reduction (by 17%, 19% and 15%, respectively). High versus low consumption reduced the risk of incident cancers by 18%. Significant harms included lung cancer, urinary tract cancer, pregnancy loss, low birth weight, preterm birth, acute leukemia in childhood, and fracture risk in women (odds ratios of 1.03-1.57). Many of the harms were mitigated after adjustment for smoking, except the risks during pregnancy. This analysis of observational trials cannot prove causality.
CONCLUSIONS: Moderate levels of coffee consumption appear to be safe or even beneficial, except during pregnancy and in women at high fracture risk. 132 references ([email protected] – no reprints)
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Poole, R., et al, BMJ 359:J5024, November 22, 2017
BACKGROUND: Studies examining the benefits versus harms of coffee consumption have yielded conflicting results.
METHODS: The authors, from the United Kingdom, present an umbrella review of meta-analyses published up to 2017 to determine the associations between coffee consumption and any health outcome in adults.
RESULTS: This review includes 201 meta-analyses of observational studies with 67 health outcomes and 17 meta-analyses of randomized trials with 9 health outcomes according to coffee consumption defined as high versus low, any versus none, or per each extra cup per day. Coffee was associated with statistically significant protective effects against several diseases including type 2 diabetes, renal stones, Parkinson’s disease, Alzheimer’s disease, depression, leukemia, gout, colorectal cancer, liver cancer, chronic liver disease, cirrhosis, and endometrial cancer (odds ratios of 0.35-0.94). For some outcomes including all-cause mortality, cardiovascular mortality and cardiovascular disease, a nonlinear association was found whereby 3-4 cups per day (versus 0) conferred the greatest risk reduction (by 17%, 19% and 15%, respectively). High versus low consumption reduced the risk of incident cancers by 18%. Significant harms included lung cancer, urinary tract cancer, pregnancy loss, low birth weight, preterm birth, acute leukemia in childhood, and fracture risk in women (odds ratios of 1.03-1.57). Many of the harms were mitigated after adjustment for smoking, except the risks during pregnancy. This analysis of observational trials cannot prove causality.
CONCLUSIONS: Moderate levels of coffee consumption appear to be safe or even beneficial, except during pregnancy and in women at high fracture risk. 132 references ([email protected] – no reprints)
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Poole, R., et al, BMJ 359:J5024, November 22, 2017
BACKGROUND: Studies examining the benefits versus harms of coffee consumption have yielded conflicting results.
METHODS: The authors, from the United Kingdom, present an umbrella review of meta-analyses published up to 2017 to determine the associations between coffee consumption and any health outcome in adults.
RESULTS: This review includes 201 meta-analyses of observational studies with 67 health outcomes and 17 meta-analyses of randomized trials with 9 health outcomes according to coffee consumption defined as high versus low, any versus none, or per each extra cup per day. Coffee was associated with statistically significant protective effects against several diseases including type 2 diabetes, renal stones, Parkinson’s disease, Alzheimer’s disease, depression, leukemia, gout, colorectal cancer, liver cancer, chronic liver disease, cirrhosis, and endometrial cancer (odds ratios of 0.35-0.94). For some outcomes including all-cause mortality, cardiovascular mortality and cardiovascular disease, a nonlinear association was found whereby 3-4 cups per day (versus 0) conferred the greatest risk reduction (by 17%, 19% and 15%, respectively). High versus low consumption reduced the risk of incident cancers by 18%. Significant harms included lung cancer, urinary tract cancer, pregnancy loss, low birth weight, preterm birth, acute leukemia in childhood, and fracture risk in women (odds ratios of 1.03-1.57). Many of the harms were mitigated after adjustment for smoking, except the risks during pregnancy. This analysis of observational trials cannot prove causality.
CONCLUSIONS: Moderate levels of coffee consumption appear to be safe or even beneficial, except during pregnancy and in women at high fracture risk. 132 references ([email protected] – no reprints)
Learn more about the Primary Care Medical Abstracts and podcasts, for which you can earn up to 9 CME credits per month.
Copyright © The Center for Medical Education
FDA approves marketing of device to control GI bleeding
The Food and Drug Administration announced May 7 that it has permitted marketing of .
Hemospray is an aerosolized spray device that delivers a mineral blend to the bleeding site in the GI tract and is applied during endoscopic procedures and can cover large ulcers or tumors.
The FDA evaluated data from clinical studies consisting of 228 patients with upper and lower GI bleeding, supplemented with evidence from medical literature, including an additional 522 patients. The studies found that Hemospray stopped GI bleeding in 95% of patients within 5 minutes of device usage. Results also found that bleeding recurred, usually within 72 hours, and up to 30 days following device usage, in 20% of patients. Bowel perforation was observed as a serious side effect in approximately 1% of patients.
“The device provides an additional, nonsurgical option for treating upper and lower GI bleeding in certain patients, and may help reduce the risk of death from a GI bleed for many patients,” said Binita Ashar, MD, director, division of surgical devices, in the FDA’s Center for Devices and Radiological Health in a press release.
Hemospray is not intended for patients who have a gastrointestinal fistula or are at high risk for GI perforation. The device is not intended for use in patients with variceal bleeding. The FDA permitted the marketing of the Hemospray device to Wilson-Cook Medical.*
Read the full press release here.
Correction, 5/10/18: An earlier version of this article incorrectly described the patient population that should not be treated with Hemospray.
The Food and Drug Administration announced May 7 that it has permitted marketing of .
Hemospray is an aerosolized spray device that delivers a mineral blend to the bleeding site in the GI tract and is applied during endoscopic procedures and can cover large ulcers or tumors.
The FDA evaluated data from clinical studies consisting of 228 patients with upper and lower GI bleeding, supplemented with evidence from medical literature, including an additional 522 patients. The studies found that Hemospray stopped GI bleeding in 95% of patients within 5 minutes of device usage. Results also found that bleeding recurred, usually within 72 hours, and up to 30 days following device usage, in 20% of patients. Bowel perforation was observed as a serious side effect in approximately 1% of patients.
“The device provides an additional, nonsurgical option for treating upper and lower GI bleeding in certain patients, and may help reduce the risk of death from a GI bleed for many patients,” said Binita Ashar, MD, director, division of surgical devices, in the FDA’s Center for Devices and Radiological Health in a press release.
Hemospray is not intended for patients who have a gastrointestinal fistula or are at high risk for GI perforation. The device is not intended for use in patients with variceal bleeding. The FDA permitted the marketing of the Hemospray device to Wilson-Cook Medical.*
Read the full press release here.
Correction, 5/10/18: An earlier version of this article incorrectly described the patient population that should not be treated with Hemospray.
The Food and Drug Administration announced May 7 that it has permitted marketing of .
Hemospray is an aerosolized spray device that delivers a mineral blend to the bleeding site in the GI tract and is applied during endoscopic procedures and can cover large ulcers or tumors.
The FDA evaluated data from clinical studies consisting of 228 patients with upper and lower GI bleeding, supplemented with evidence from medical literature, including an additional 522 patients. The studies found that Hemospray stopped GI bleeding in 95% of patients within 5 minutes of device usage. Results also found that bleeding recurred, usually within 72 hours, and up to 30 days following device usage, in 20% of patients. Bowel perforation was observed as a serious side effect in approximately 1% of patients.
“The device provides an additional, nonsurgical option for treating upper and lower GI bleeding in certain patients, and may help reduce the risk of death from a GI bleed for many patients,” said Binita Ashar, MD, director, division of surgical devices, in the FDA’s Center for Devices and Radiological Health in a press release.
Hemospray is not intended for patients who have a gastrointestinal fistula or are at high risk for GI perforation. The device is not intended for use in patients with variceal bleeding. The FDA permitted the marketing of the Hemospray device to Wilson-Cook Medical.*
Read the full press release here.
Correction, 5/10/18: An earlier version of this article incorrectly described the patient population that should not be treated with Hemospray.
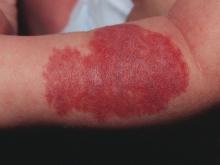
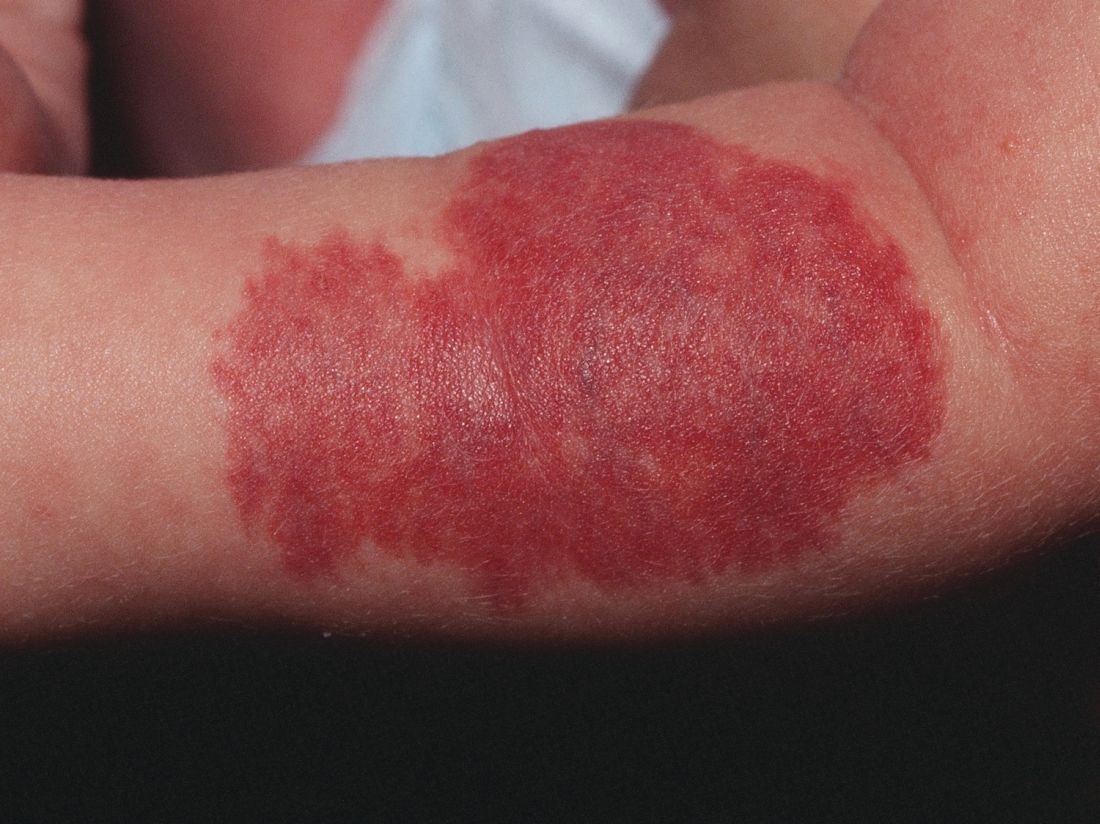
Pretreatment ECG unwarranted for most infantile hemangioma patients starting propranolol
reported Emily B. Lund, MD, of the University of Chicago, and her associates.
This finding supports previously published studies that pretreatment ECG is not necessary, despite consensus guidelines published in 2013 that recommend ECG screening of high-risk infants presenting with below-normal heart rate, arrhythmia, or family history of either arrhythmia or congenital heart disease.
Among the 6% of patients included in the study who had a positive personal cardiac history, congenital heart disease was most common; coronary artery disease was most prevalent among the 41% with a positive family cardiac history. Baseline vital signs revealed no hypotension or bradycardia.
All patients prescribed propranolol were routinely screened with ECG prior to therapy during the study period. Baseline heart rate and blood pressure were observed for abnormalities; patients also were observed during follow-up for possible propranolol side effects.
A total of 43% of ECG screenings performed were found to be abnormal; left ventricular hypertrophy was the most common abnormality. Despite further cardiac evaluation of all but one patient with abnormal ECG, no contraindications to treatment were identified, Dr. Lund and her colleagues reported in Pediatric Dermatology.
Ultimately, 96% of patients observed started treatment with propranolol; of the remaining 4% who did not, the authors cited parental preference and lack of follow-up as the primary reasons for nontreatment.
The researchers found no association between reported side effects and abnormal ECG, a positive personal history of cardiac problems, or a positive family history of cardiac problems.
Dr. Lund and her associates suggested that future revision of the guidelines should emphasize the absence of significant positive predictive value of ECG abnormalities for treatment-related side effects.
The researchers reported no relevant financial disclosures.
SOURCE: Lund EB et al. Ped Dermatol. 2018. doi: 10.1111/pde.13508.
reported Emily B. Lund, MD, of the University of Chicago, and her associates.
This finding supports previously published studies that pretreatment ECG is not necessary, despite consensus guidelines published in 2013 that recommend ECG screening of high-risk infants presenting with below-normal heart rate, arrhythmia, or family history of either arrhythmia or congenital heart disease.
Among the 6% of patients included in the study who had a positive personal cardiac history, congenital heart disease was most common; coronary artery disease was most prevalent among the 41% with a positive family cardiac history. Baseline vital signs revealed no hypotension or bradycardia.
All patients prescribed propranolol were routinely screened with ECG prior to therapy during the study period. Baseline heart rate and blood pressure were observed for abnormalities; patients also were observed during follow-up for possible propranolol side effects.
A total of 43% of ECG screenings performed were found to be abnormal; left ventricular hypertrophy was the most common abnormality. Despite further cardiac evaluation of all but one patient with abnormal ECG, no contraindications to treatment were identified, Dr. Lund and her colleagues reported in Pediatric Dermatology.
Ultimately, 96% of patients observed started treatment with propranolol; of the remaining 4% who did not, the authors cited parental preference and lack of follow-up as the primary reasons for nontreatment.
The researchers found no association between reported side effects and abnormal ECG, a positive personal history of cardiac problems, or a positive family history of cardiac problems.
Dr. Lund and her associates suggested that future revision of the guidelines should emphasize the absence of significant positive predictive value of ECG abnormalities for treatment-related side effects.
The researchers reported no relevant financial disclosures.
SOURCE: Lund EB et al. Ped Dermatol. 2018. doi: 10.1111/pde.13508.
reported Emily B. Lund, MD, of the University of Chicago, and her associates.
This finding supports previously published studies that pretreatment ECG is not necessary, despite consensus guidelines published in 2013 that recommend ECG screening of high-risk infants presenting with below-normal heart rate, arrhythmia, or family history of either arrhythmia or congenital heart disease.
Among the 6% of patients included in the study who had a positive personal cardiac history, congenital heart disease was most common; coronary artery disease was most prevalent among the 41% with a positive family cardiac history. Baseline vital signs revealed no hypotension or bradycardia.
All patients prescribed propranolol were routinely screened with ECG prior to therapy during the study period. Baseline heart rate and blood pressure were observed for abnormalities; patients also were observed during follow-up for possible propranolol side effects.
A total of 43% of ECG screenings performed were found to be abnormal; left ventricular hypertrophy was the most common abnormality. Despite further cardiac evaluation of all but one patient with abnormal ECG, no contraindications to treatment were identified, Dr. Lund and her colleagues reported in Pediatric Dermatology.
Ultimately, 96% of patients observed started treatment with propranolol; of the remaining 4% who did not, the authors cited parental preference and lack of follow-up as the primary reasons for nontreatment.
The researchers found no association between reported side effects and abnormal ECG, a positive personal history of cardiac problems, or a positive family history of cardiac problems.
Dr. Lund and her associates suggested that future revision of the guidelines should emphasize the absence of significant positive predictive value of ECG abnormalities for treatment-related side effects.
The researchers reported no relevant financial disclosures.
SOURCE: Lund EB et al. Ped Dermatol. 2018. doi: 10.1111/pde.13508.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: There was no association between side effects, abnormal ECG, and personal or family history of cardiac problems in children with infantile hemangioma who underwent propranolol therapy.
Major finding: Despite the fact that 43% of ECG screenings were abnormal, 96% of patients started treatment with propranolol, with no side effects related to abnormal ECG or to personal or family history of cardiac problems.
Study details: A retrospective chart review of 272 patients with infantile hemangioma.
Disclosures: The researchers reported no relevant financial disclosures.
Source: Lund EB et al. Ped Dermatol. 2018. doi: 10.1111/pde.13508.
Analysis finds inconsistent uptake of meningococcal B vaccines
TORONTO – results from a large analysis showed.
“In 2015, two meningococcal B (MenB) vaccines were given a Category B recommendation by the Advisory Committee on Immunization Practices with a preferred vaccination window of 16-18 years,” researchers led by Kristen A. Feemster, MD, MPH, wrote in an abstract presented at the Pediatric Academic Societies meeting. “Factors that may influence provider recommendation and subsequent uptake of a Category B vaccine are unknown.”
In an effort to identify sociodemographic and provider factors associated with MenB vaccine receipt, Dr. Feemster and her associates conducted a cross-sectional study of 85,789 Philadelphia youth aged 16-18 years who had a record in the KIDS Plus II Philadelphia database between Oct. 31, 2015 and July 31, 2017. They acquired neighborhood-level data from the 2016 U.S. Census American Community Survey. Next, the researchers used multivariate logistic regression to assess the association between MenB series initiation and individual- and neighborhood-level sociodemographic, clinical, and provider characteristics.
Of the 85,789 youth, only 16% received at least one MenB dose, while just 5% completed the series, reported Dr. Feemster, who is medical director of the Immunization Program and Acute Communicable Diseases at the Philadelphia department of public health in the division of disease control. Nearly half of youth (49%) were black or African-American, 25% were white, 5.5% were Asian, while the remainder were from “other” or “unknown” races. A private pediatrician was listed as the provider for 70% of the youth, followed by a community health center (11%), the Philadelphia District Center (7%), and hospitals (2%), while the remaining providers were “other” or “unknown.” The proportion of MenB recipients varied significantly by provider type, from 0.67% to 20%.
On multivariate logistic regression, MenB recipients were more likely to be female (adjusted odds ratio, 1.07; P = .0006); they were also more likely to be up-to-date on human papillomavirus vaccines (AOR, 1.65; P less than .0001) and measles-containing vaccines (AOR, 9.90; P less than .0001).
MenB recipients were more likely to be of “unknown” or “other” reported race, compared with those who were Black/African-American (AOR, 1.36 and 1.24, respectively; P less than .0001) or non-Hispanic/Latino (AOR, 1.21; P less than .0001); they were also more likely to reside in a neighborhood with median household income of greater than $100,000, compared with those who lived in a neighborhood where the median household income is less than $20,000 (AOR, 1.63; P less than .0001). Asian teens (AOR, 0.87; P = .0062) and teens who received care in community (AOR, 0.52; P less than .0001) or district health centers (AOR, 0.03; P less than .0001) also were less likely to receive the MenB vaccine, reported Dr. Feemster, who is also director of research for Children’s Hospital of Philadelphia’s Vaccine Education Center, and her colleagues.
“Variation in uptake by race, ethnicity, and neighborhood socioeconomic status suggest potential sociodemographic disparities in MenB receipt, [while] variation by neighborhood socioeconomic status may also suggest financial barriers related to access to care,” the researchers wrote in their abstract. They also speculated that variation in MenB receipt across different providers “may reflect different recommendation practices, perceived need for MenB vaccines in a provider’s patient population, or clinic-level purchasing decisions.”
The next steps in their research, they wrote, are to “investigate factors associated with provider recommendation of MenB vaccine to identify targets for initiatives to ensure equitable vaccine access.”
The researchers reported having no financial disclosures.
TORONTO – results from a large analysis showed.
“In 2015, two meningococcal B (MenB) vaccines were given a Category B recommendation by the Advisory Committee on Immunization Practices with a preferred vaccination window of 16-18 years,” researchers led by Kristen A. Feemster, MD, MPH, wrote in an abstract presented at the Pediatric Academic Societies meeting. “Factors that may influence provider recommendation and subsequent uptake of a Category B vaccine are unknown.”
In an effort to identify sociodemographic and provider factors associated with MenB vaccine receipt, Dr. Feemster and her associates conducted a cross-sectional study of 85,789 Philadelphia youth aged 16-18 years who had a record in the KIDS Plus II Philadelphia database between Oct. 31, 2015 and July 31, 2017. They acquired neighborhood-level data from the 2016 U.S. Census American Community Survey. Next, the researchers used multivariate logistic regression to assess the association between MenB series initiation and individual- and neighborhood-level sociodemographic, clinical, and provider characteristics.
Of the 85,789 youth, only 16% received at least one MenB dose, while just 5% completed the series, reported Dr. Feemster, who is medical director of the Immunization Program and Acute Communicable Diseases at the Philadelphia department of public health in the division of disease control. Nearly half of youth (49%) were black or African-American, 25% were white, 5.5% were Asian, while the remainder were from “other” or “unknown” races. A private pediatrician was listed as the provider for 70% of the youth, followed by a community health center (11%), the Philadelphia District Center (7%), and hospitals (2%), while the remaining providers were “other” or “unknown.” The proportion of MenB recipients varied significantly by provider type, from 0.67% to 20%.
On multivariate logistic regression, MenB recipients were more likely to be female (adjusted odds ratio, 1.07; P = .0006); they were also more likely to be up-to-date on human papillomavirus vaccines (AOR, 1.65; P less than .0001) and measles-containing vaccines (AOR, 9.90; P less than .0001).
MenB recipients were more likely to be of “unknown” or “other” reported race, compared with those who were Black/African-American (AOR, 1.36 and 1.24, respectively; P less than .0001) or non-Hispanic/Latino (AOR, 1.21; P less than .0001); they were also more likely to reside in a neighborhood with median household income of greater than $100,000, compared with those who lived in a neighborhood where the median household income is less than $20,000 (AOR, 1.63; P less than .0001). Asian teens (AOR, 0.87; P = .0062) and teens who received care in community (AOR, 0.52; P less than .0001) or district health centers (AOR, 0.03; P less than .0001) also were less likely to receive the MenB vaccine, reported Dr. Feemster, who is also director of research for Children’s Hospital of Philadelphia’s Vaccine Education Center, and her colleagues.
“Variation in uptake by race, ethnicity, and neighborhood socioeconomic status suggest potential sociodemographic disparities in MenB receipt, [while] variation by neighborhood socioeconomic status may also suggest financial barriers related to access to care,” the researchers wrote in their abstract. They also speculated that variation in MenB receipt across different providers “may reflect different recommendation practices, perceived need for MenB vaccines in a provider’s patient population, or clinic-level purchasing decisions.”
The next steps in their research, they wrote, are to “investigate factors associated with provider recommendation of MenB vaccine to identify targets for initiatives to ensure equitable vaccine access.”
The researchers reported having no financial disclosures.
TORONTO – results from a large analysis showed.
“In 2015, two meningococcal B (MenB) vaccines were given a Category B recommendation by the Advisory Committee on Immunization Practices with a preferred vaccination window of 16-18 years,” researchers led by Kristen A. Feemster, MD, MPH, wrote in an abstract presented at the Pediatric Academic Societies meeting. “Factors that may influence provider recommendation and subsequent uptake of a Category B vaccine are unknown.”
In an effort to identify sociodemographic and provider factors associated with MenB vaccine receipt, Dr. Feemster and her associates conducted a cross-sectional study of 85,789 Philadelphia youth aged 16-18 years who had a record in the KIDS Plus II Philadelphia database between Oct. 31, 2015 and July 31, 2017. They acquired neighborhood-level data from the 2016 U.S. Census American Community Survey. Next, the researchers used multivariate logistic regression to assess the association between MenB series initiation and individual- and neighborhood-level sociodemographic, clinical, and provider characteristics.
Of the 85,789 youth, only 16% received at least one MenB dose, while just 5% completed the series, reported Dr. Feemster, who is medical director of the Immunization Program and Acute Communicable Diseases at the Philadelphia department of public health in the division of disease control. Nearly half of youth (49%) were black or African-American, 25% were white, 5.5% were Asian, while the remainder were from “other” or “unknown” races. A private pediatrician was listed as the provider for 70% of the youth, followed by a community health center (11%), the Philadelphia District Center (7%), and hospitals (2%), while the remaining providers were “other” or “unknown.” The proportion of MenB recipients varied significantly by provider type, from 0.67% to 20%.
On multivariate logistic regression, MenB recipients were more likely to be female (adjusted odds ratio, 1.07; P = .0006); they were also more likely to be up-to-date on human papillomavirus vaccines (AOR, 1.65; P less than .0001) and measles-containing vaccines (AOR, 9.90; P less than .0001).
MenB recipients were more likely to be of “unknown” or “other” reported race, compared with those who were Black/African-American (AOR, 1.36 and 1.24, respectively; P less than .0001) or non-Hispanic/Latino (AOR, 1.21; P less than .0001); they were also more likely to reside in a neighborhood with median household income of greater than $100,000, compared with those who lived in a neighborhood where the median household income is less than $20,000 (AOR, 1.63; P less than .0001). Asian teens (AOR, 0.87; P = .0062) and teens who received care in community (AOR, 0.52; P less than .0001) or district health centers (AOR, 0.03; P less than .0001) also were less likely to receive the MenB vaccine, reported Dr. Feemster, who is also director of research for Children’s Hospital of Philadelphia’s Vaccine Education Center, and her colleagues.
“Variation in uptake by race, ethnicity, and neighborhood socioeconomic status suggest potential sociodemographic disparities in MenB receipt, [while] variation by neighborhood socioeconomic status may also suggest financial barriers related to access to care,” the researchers wrote in their abstract. They also speculated that variation in MenB receipt across different providers “may reflect different recommendation practices, perceived need for MenB vaccines in a provider’s patient population, or clinic-level purchasing decisions.”
The next steps in their research, they wrote, are to “investigate factors associated with provider recommendation of MenB vaccine to identify targets for initiatives to ensure equitable vaccine access.”
The researchers reported having no financial disclosures.
AT PAS 2018
Key clinical point: Significant variation in the likelihood of MenB vaccine receipt correlated with sociodemographic, clinical, and provider factors.
Major finding: Only 16% received at least one MenB dose while just 5% completed the series.
Study details: A cross-sectional study of 85,789 Philadelphia youth aged 16-18 years.
Disclosures: The researchers reported having no financial disclosures.
Annual ob.gyn. visit a ‘powerful opportunity’ to talk heart health
Clinicians in the cardiology, obstetrics, and gynecology specialties should collaborate and use a woman’s annual visit to her ob.gyn. to promote healthy lifestyle choices, screen for signs of cardiovascular disease and risk factors, and improve her overall cardiovascular health, according to a joint advisory released by the presidents of the American Heart Association and the American College of Obstetricians and Gynecologists.
“Ob.gyns. are primary care providers for many women, and the annual ‘well woman’ visit provides a powerful opportunity to counsel patients about achieving and maintaining a heart-healthy lifestyle, which is a cornerstone of maintaining heart health,” John Warner, MD, president of the AHA and executive vice president for Health System Affairs at University of Texas Southwestern Medical Center in Dallas, said in a press release.
“Traditional atherosclerotic cardiovascular disease (ASCVD) risk factors, such as hypertension, diabetes mellitus, hypercholesterolemia, and obesity, affect both sexes, but some may affect women differently and are considered to be more potent,” the authors wrote in their advisory.
The advisory also stated clinicians should be aware of ASCVD risk factors that are not specific to but are more prevalent in women, such as systemic lupus erythematosus, scleroderma, and rheumatoid arthritis.
“These disorders are highly prevalent among women who have an increased risk of coronary artery disease and other cardiovascular disease,” the authors wrote.
Gender-specific ASCVD risk factors for women include pregnancy complications such as gestational diabetes mellitus and low birth weight as estimated during gestation. Hypertension during gestation and preeclampsia carries a threefold to sixfold increased risk for subsequent hypertension while also carrying a twofold increased risk of stroke and ischemic heart disease. Non–pregnancy related risk factors included menopausal status, hormone use, polycystic ovarian syndrome, and functional hypothalamic amenorrhea.
“Pregnancy is essentially a ‘stress test’ for women, and these adverse pregnancy outcomes can be used to identify women who are at an increased risk for ASCVD, even in those for whom the conditions resolve after delivery,” the authors said.
To reduce or prevent these issues, the authors recommended ob.gyn. clinicians perform a “thorough family history, screening for and targeted review of cardiovascular risk factors (including those unique to women), and lifestyle counseling to improve cardiovascular risk factors with the goal of preventing future cardiovascular events.”
“The American College of Cardiology/American Heart Association guidelines for the assessment of cardiovascular risk find it reasonable to screen adults free of cardiovascular disease for risk factors such as smoking, hypertension, diabetes mellitus, total cholesterol, and high-density lipoprotein cholesterol every 4-6 years between the ages of 20 and 79 years to calculate their 10-year cardiovascular risk,” they wrote.
Regarding diabetes care, clinicians should screen for abnormal glucose levels in overweight or obese patients aged between 40 and 70 years and schedule lifestyle counseling for any patients with prediabetes (HbA1c = 5.7%-6.4%), diabetes mellitus (HbA1c greater than 6.4%), gestational diabetes, or metabolic syndrome.
Cholesterol and other traditional risk factors associated with hyperlipidemia should be checked every 4-6 years and patients with elevated lipids should receive counseling on lowering their saturated fat intake and adding more dietary fiber. Statins are also an option for patients where diet does not lower lipids to appropriate levels, but authors noted “women of childbearing age need to be specifically counseled to not become pregnant while taking a statin.” The advisory also recommended speaking with patients about engaging in “150 min/wk of moderate-intensity physical activity, 75 min/wk of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate- and vigorous-intensity aerobic physical activity” as well as offering counseling and options for women who want to quit smoking.
The authors noted that only 45% of women consider heart disease a “leading cause of death” and most primary care providers do not see cardiovascular disease as a top health concern for women, focusing on risk factors such as weight and breast health.
“As the leading health care providers for women, ob.gyns. provide care that goes far beyond reproductive health and are in a unique position to screen, counsel, and educate patients on heart health. By acknowledging and discussing the risks and communicating steps women can take to reduce their odds of developing heart disease, ob.gyns. have a powerful opportunity to be the secret weapon in the fight against heart disease,” Haywood L. Brown, MD, past president of the ACOG and F. Bayard Carter Professor in the department of obstetrics and gynecology at Duke University, Durham, N.C., said in a press release.
Dr. Gianos is a consultant and/or on the advisory board for Regeneron. Dr. Wenger reports research grants from Gilead Sciences, the National Heart, Lung, and Blood Institute, Pfizer, and the Society for Women’s Health Research and is a consultant and/or on the advisory board for Amgen, AstraZeneca, Gilead Sciences, Janssen, and Merck.
SOURCE: Brown HL et al. Circulation. 2018 May 10. doi: 10.1161/CIR.0000000000000582.
Clinicians in the cardiology, obstetrics, and gynecology specialties should collaborate and use a woman’s annual visit to her ob.gyn. to promote healthy lifestyle choices, screen for signs of cardiovascular disease and risk factors, and improve her overall cardiovascular health, according to a joint advisory released by the presidents of the American Heart Association and the American College of Obstetricians and Gynecologists.
“Ob.gyns. are primary care providers for many women, and the annual ‘well woman’ visit provides a powerful opportunity to counsel patients about achieving and maintaining a heart-healthy lifestyle, which is a cornerstone of maintaining heart health,” John Warner, MD, president of the AHA and executive vice president for Health System Affairs at University of Texas Southwestern Medical Center in Dallas, said in a press release.
“Traditional atherosclerotic cardiovascular disease (ASCVD) risk factors, such as hypertension, diabetes mellitus, hypercholesterolemia, and obesity, affect both sexes, but some may affect women differently and are considered to be more potent,” the authors wrote in their advisory.
The advisory also stated clinicians should be aware of ASCVD risk factors that are not specific to but are more prevalent in women, such as systemic lupus erythematosus, scleroderma, and rheumatoid arthritis.
“These disorders are highly prevalent among women who have an increased risk of coronary artery disease and other cardiovascular disease,” the authors wrote.
Gender-specific ASCVD risk factors for women include pregnancy complications such as gestational diabetes mellitus and low birth weight as estimated during gestation. Hypertension during gestation and preeclampsia carries a threefold to sixfold increased risk for subsequent hypertension while also carrying a twofold increased risk of stroke and ischemic heart disease. Non–pregnancy related risk factors included menopausal status, hormone use, polycystic ovarian syndrome, and functional hypothalamic amenorrhea.
“Pregnancy is essentially a ‘stress test’ for women, and these adverse pregnancy outcomes can be used to identify women who are at an increased risk for ASCVD, even in those for whom the conditions resolve after delivery,” the authors said.
To reduce or prevent these issues, the authors recommended ob.gyn. clinicians perform a “thorough family history, screening for and targeted review of cardiovascular risk factors (including those unique to women), and lifestyle counseling to improve cardiovascular risk factors with the goal of preventing future cardiovascular events.”
“The American College of Cardiology/American Heart Association guidelines for the assessment of cardiovascular risk find it reasonable to screen adults free of cardiovascular disease for risk factors such as smoking, hypertension, diabetes mellitus, total cholesterol, and high-density lipoprotein cholesterol every 4-6 years between the ages of 20 and 79 years to calculate their 10-year cardiovascular risk,” they wrote.
Regarding diabetes care, clinicians should screen for abnormal glucose levels in overweight or obese patients aged between 40 and 70 years and schedule lifestyle counseling for any patients with prediabetes (HbA1c = 5.7%-6.4%), diabetes mellitus (HbA1c greater than 6.4%), gestational diabetes, or metabolic syndrome.
Cholesterol and other traditional risk factors associated with hyperlipidemia should be checked every 4-6 years and patients with elevated lipids should receive counseling on lowering their saturated fat intake and adding more dietary fiber. Statins are also an option for patients where diet does not lower lipids to appropriate levels, but authors noted “women of childbearing age need to be specifically counseled to not become pregnant while taking a statin.” The advisory also recommended speaking with patients about engaging in “150 min/wk of moderate-intensity physical activity, 75 min/wk of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate- and vigorous-intensity aerobic physical activity” as well as offering counseling and options for women who want to quit smoking.
The authors noted that only 45% of women consider heart disease a “leading cause of death” and most primary care providers do not see cardiovascular disease as a top health concern for women, focusing on risk factors such as weight and breast health.
“As the leading health care providers for women, ob.gyns. provide care that goes far beyond reproductive health and are in a unique position to screen, counsel, and educate patients on heart health. By acknowledging and discussing the risks and communicating steps women can take to reduce their odds of developing heart disease, ob.gyns. have a powerful opportunity to be the secret weapon in the fight against heart disease,” Haywood L. Brown, MD, past president of the ACOG and F. Bayard Carter Professor in the department of obstetrics and gynecology at Duke University, Durham, N.C., said in a press release.
Dr. Gianos is a consultant and/or on the advisory board for Regeneron. Dr. Wenger reports research grants from Gilead Sciences, the National Heart, Lung, and Blood Institute, Pfizer, and the Society for Women’s Health Research and is a consultant and/or on the advisory board for Amgen, AstraZeneca, Gilead Sciences, Janssen, and Merck.
SOURCE: Brown HL et al. Circulation. 2018 May 10. doi: 10.1161/CIR.0000000000000582.
Clinicians in the cardiology, obstetrics, and gynecology specialties should collaborate and use a woman’s annual visit to her ob.gyn. to promote healthy lifestyle choices, screen for signs of cardiovascular disease and risk factors, and improve her overall cardiovascular health, according to a joint advisory released by the presidents of the American Heart Association and the American College of Obstetricians and Gynecologists.
“Ob.gyns. are primary care providers for many women, and the annual ‘well woman’ visit provides a powerful opportunity to counsel patients about achieving and maintaining a heart-healthy lifestyle, which is a cornerstone of maintaining heart health,” John Warner, MD, president of the AHA and executive vice president for Health System Affairs at University of Texas Southwestern Medical Center in Dallas, said in a press release.
“Traditional atherosclerotic cardiovascular disease (ASCVD) risk factors, such as hypertension, diabetes mellitus, hypercholesterolemia, and obesity, affect both sexes, but some may affect women differently and are considered to be more potent,” the authors wrote in their advisory.
The advisory also stated clinicians should be aware of ASCVD risk factors that are not specific to but are more prevalent in women, such as systemic lupus erythematosus, scleroderma, and rheumatoid arthritis.
“These disorders are highly prevalent among women who have an increased risk of coronary artery disease and other cardiovascular disease,” the authors wrote.
Gender-specific ASCVD risk factors for women include pregnancy complications such as gestational diabetes mellitus and low birth weight as estimated during gestation. Hypertension during gestation and preeclampsia carries a threefold to sixfold increased risk for subsequent hypertension while also carrying a twofold increased risk of stroke and ischemic heart disease. Non–pregnancy related risk factors included menopausal status, hormone use, polycystic ovarian syndrome, and functional hypothalamic amenorrhea.
“Pregnancy is essentially a ‘stress test’ for women, and these adverse pregnancy outcomes can be used to identify women who are at an increased risk for ASCVD, even in those for whom the conditions resolve after delivery,” the authors said.
To reduce or prevent these issues, the authors recommended ob.gyn. clinicians perform a “thorough family history, screening for and targeted review of cardiovascular risk factors (including those unique to women), and lifestyle counseling to improve cardiovascular risk factors with the goal of preventing future cardiovascular events.”
“The American College of Cardiology/American Heart Association guidelines for the assessment of cardiovascular risk find it reasonable to screen adults free of cardiovascular disease for risk factors such as smoking, hypertension, diabetes mellitus, total cholesterol, and high-density lipoprotein cholesterol every 4-6 years between the ages of 20 and 79 years to calculate their 10-year cardiovascular risk,” they wrote.
Regarding diabetes care, clinicians should screen for abnormal glucose levels in overweight or obese patients aged between 40 and 70 years and schedule lifestyle counseling for any patients with prediabetes (HbA1c = 5.7%-6.4%), diabetes mellitus (HbA1c greater than 6.4%), gestational diabetes, or metabolic syndrome.
Cholesterol and other traditional risk factors associated with hyperlipidemia should be checked every 4-6 years and patients with elevated lipids should receive counseling on lowering their saturated fat intake and adding more dietary fiber. Statins are also an option for patients where diet does not lower lipids to appropriate levels, but authors noted “women of childbearing age need to be specifically counseled to not become pregnant while taking a statin.” The advisory also recommended speaking with patients about engaging in “150 min/wk of moderate-intensity physical activity, 75 min/wk of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate- and vigorous-intensity aerobic physical activity” as well as offering counseling and options for women who want to quit smoking.
The authors noted that only 45% of women consider heart disease a “leading cause of death” and most primary care providers do not see cardiovascular disease as a top health concern for women, focusing on risk factors such as weight and breast health.
“As the leading health care providers for women, ob.gyns. provide care that goes far beyond reproductive health and are in a unique position to screen, counsel, and educate patients on heart health. By acknowledging and discussing the risks and communicating steps women can take to reduce their odds of developing heart disease, ob.gyns. have a powerful opportunity to be the secret weapon in the fight against heart disease,” Haywood L. Brown, MD, past president of the ACOG and F. Bayard Carter Professor in the department of obstetrics and gynecology at Duke University, Durham, N.C., said in a press release.
Dr. Gianos is a consultant and/or on the advisory board for Regeneron. Dr. Wenger reports research grants from Gilead Sciences, the National Heart, Lung, and Blood Institute, Pfizer, and the Society for Women’s Health Research and is a consultant and/or on the advisory board for Amgen, AstraZeneca, Gilead Sciences, Janssen, and Merck.
SOURCE: Brown HL et al. Circulation. 2018 May 10. doi: 10.1161/CIR.0000000000000582.
FROM CIRCULATION
Key clinical point: Cardiology, obstetrics, and gynecology providers should use a woman’s annual visit to her ob.gyn. to promote healthy lifestyle choices, screen for signs of cardiovascular disease and risk factors, and improve her overall cardiovascular health.
Major finding: Women are at greater risk than men are of cardiovascular mortality, hypertension, and hypercholesterolemia, and of developing diabetes mellitus–associated cardiovascular risk factors, with factors such as pregnancy, obesity, smoking status, mental health, and genetic risk factors also playing a role in cardiovascular health.
Study details: A joint advisory statement from the presidents of the American Heart Association and the American College of Obstetricians and Gynecologists.
Disclosures: Dr. Gianos is a consultant and/or on the advisory board for Regeneron. Dr. Wenger reports research grants from Gilead Sciences, the National Heart, Lung, and Blood Institute, Pfizer, and the Society for Women’s Health Research and is a consultant and/or on the advisory board for Amgen, AstraZeneca, Gilead Sciences, Janssen, and Merck.
Source: Brown HL et al. Circulation. 2018 May 10. doi: 10.1161/CIR.0000000000000582.
Pulsed-dye laser found effective for erythematotelangiectatic rosacea
DALLAS – in a single-center study of 20 patients.
“Recent advances in pulsed-dye laser technology enable 50% higher output energies and much longer dye life,” lead study author Eric F. Bernstein, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “A 15-mm treatment diameter is now possible, which makes treatment faster and possibly more effective due to increased penetration of laser energy. Compared with a 10-mm spot size, which most of us use, a 15 mm is a 125% larger beam diameter, so it’s a big difference in treatment area.”
Blinded assessment of digital and cross-polarized images taken 8 weeks following the last treatment was performed using an 11-point clearance scale. The Investigator Global Assessment Scale was used to evaluate inflammatory lesions, erythema, and telangiectasia, and safety was assessed by incidence and severity of side effects. The investigators and subjects assessed satisfaction with the treatment outcome on a 5-point Likert scale (–2, very dissatisfied; –1, dissatisfied; 0, no opinion; 1, satisfied; and 2, very satisfied). Dr. Bernstein also evaluated the patients for purpura, petechiae, edema, erythema, blistering, and crusting on a 0-3 point scale (0, absent; 1, mild; 2, moderate; and 3, severe).
Of the 20 subjects, 16 females and 3 males completed the study. All had Fitzpatrick skin types II-IV and 17 of 19 subjects received four treatments, while 2 received three treatments because of scheduling challenges. Between baseline and 8 weeks following the last treatment, mean Investigator Global Assessment scores improved significantly for overall score (from 4.3 to 1.8; P less than .0001), as well as for inflammatory lesions (from 1.7 to 1.0; P less than .005), erythema (from 4.5 to 1.6; P less than .0001), and for telangiectasia (from 4.2 to 1.1; P less than .0001).
Dr. Bernstein also reported that blinded evaluators correctly identified baseline images in 55 of 57 image pairs (96.5%), while the average score improvement in their rosacea was 52.5%, and ranged from 30.0% to 86.7%. In addition, 17 of 19 subjects (89%) showed a score improvement of more than 40%, while 57% of subjects had a score improvement of greater than 50%.
Patients and investigators both recorded a mean satisfaction score of 1.9, and 18 of 19 subjects reported being “very satisfied” with the treatment.
The average pain score was 5.6 on a 0-10 scale, while common side effects that resolved within 1-3 days without intervention included mild edema, mild to moderate erythema, and mild to moderate bruising. No cases of hyperpigmentation, hypopigmentation, blistering, or scarring occurred.
“The newly designed pulsed-dye laser is a major change in a device that has performed for over 10 years with wide acceptance,” Dr. Bernstein said. In addition to a longer-lasting dye kit, he said that new features include 50% more total energy enabling clinically relevant fluences to be delivered using a 15-mm diameter treatment beam, once-daily calibration instead of after every energy or spot size change, “both contact and dynamic spray cooling, and an Nd:YAG wavelength for treating larger vessels.”
Dr. Bernstein reported having received grant funding from Syneron Candela and Zeltiq. He also has received consulting fees from and holds ownership interest in Syneron Candela, and has served on the advisory board for Novoxel, Solta, Syneron Candela, and Zeltiq.
DALLAS – in a single-center study of 20 patients.
“Recent advances in pulsed-dye laser technology enable 50% higher output energies and much longer dye life,” lead study author Eric F. Bernstein, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “A 15-mm treatment diameter is now possible, which makes treatment faster and possibly more effective due to increased penetration of laser energy. Compared with a 10-mm spot size, which most of us use, a 15 mm is a 125% larger beam diameter, so it’s a big difference in treatment area.”
Blinded assessment of digital and cross-polarized images taken 8 weeks following the last treatment was performed using an 11-point clearance scale. The Investigator Global Assessment Scale was used to evaluate inflammatory lesions, erythema, and telangiectasia, and safety was assessed by incidence and severity of side effects. The investigators and subjects assessed satisfaction with the treatment outcome on a 5-point Likert scale (–2, very dissatisfied; –1, dissatisfied; 0, no opinion; 1, satisfied; and 2, very satisfied). Dr. Bernstein also evaluated the patients for purpura, petechiae, edema, erythema, blistering, and crusting on a 0-3 point scale (0, absent; 1, mild; 2, moderate; and 3, severe).
Of the 20 subjects, 16 females and 3 males completed the study. All had Fitzpatrick skin types II-IV and 17 of 19 subjects received four treatments, while 2 received three treatments because of scheduling challenges. Between baseline and 8 weeks following the last treatment, mean Investigator Global Assessment scores improved significantly for overall score (from 4.3 to 1.8; P less than .0001), as well as for inflammatory lesions (from 1.7 to 1.0; P less than .005), erythema (from 4.5 to 1.6; P less than .0001), and for telangiectasia (from 4.2 to 1.1; P less than .0001).
Dr. Bernstein also reported that blinded evaluators correctly identified baseline images in 55 of 57 image pairs (96.5%), while the average score improvement in their rosacea was 52.5%, and ranged from 30.0% to 86.7%. In addition, 17 of 19 subjects (89%) showed a score improvement of more than 40%, while 57% of subjects had a score improvement of greater than 50%.
Patients and investigators both recorded a mean satisfaction score of 1.9, and 18 of 19 subjects reported being “very satisfied” with the treatment.
The average pain score was 5.6 on a 0-10 scale, while common side effects that resolved within 1-3 days without intervention included mild edema, mild to moderate erythema, and mild to moderate bruising. No cases of hyperpigmentation, hypopigmentation, blistering, or scarring occurred.
“The newly designed pulsed-dye laser is a major change in a device that has performed for over 10 years with wide acceptance,” Dr. Bernstein said. In addition to a longer-lasting dye kit, he said that new features include 50% more total energy enabling clinically relevant fluences to be delivered using a 15-mm diameter treatment beam, once-daily calibration instead of after every energy or spot size change, “both contact and dynamic spray cooling, and an Nd:YAG wavelength for treating larger vessels.”
Dr. Bernstein reported having received grant funding from Syneron Candela and Zeltiq. He also has received consulting fees from and holds ownership interest in Syneron Candela, and has served on the advisory board for Novoxel, Solta, Syneron Candela, and Zeltiq.
DALLAS – in a single-center study of 20 patients.
“Recent advances in pulsed-dye laser technology enable 50% higher output energies and much longer dye life,” lead study author Eric F. Bernstein, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “A 15-mm treatment diameter is now possible, which makes treatment faster and possibly more effective due to increased penetration of laser energy. Compared with a 10-mm spot size, which most of us use, a 15 mm is a 125% larger beam diameter, so it’s a big difference in treatment area.”
Blinded assessment of digital and cross-polarized images taken 8 weeks following the last treatment was performed using an 11-point clearance scale. The Investigator Global Assessment Scale was used to evaluate inflammatory lesions, erythema, and telangiectasia, and safety was assessed by incidence and severity of side effects. The investigators and subjects assessed satisfaction with the treatment outcome on a 5-point Likert scale (–2, very dissatisfied; –1, dissatisfied; 0, no opinion; 1, satisfied; and 2, very satisfied). Dr. Bernstein also evaluated the patients for purpura, petechiae, edema, erythema, blistering, and crusting on a 0-3 point scale (0, absent; 1, mild; 2, moderate; and 3, severe).
Of the 20 subjects, 16 females and 3 males completed the study. All had Fitzpatrick skin types II-IV and 17 of 19 subjects received four treatments, while 2 received three treatments because of scheduling challenges. Between baseline and 8 weeks following the last treatment, mean Investigator Global Assessment scores improved significantly for overall score (from 4.3 to 1.8; P less than .0001), as well as for inflammatory lesions (from 1.7 to 1.0; P less than .005), erythema (from 4.5 to 1.6; P less than .0001), and for telangiectasia (from 4.2 to 1.1; P less than .0001).
Dr. Bernstein also reported that blinded evaluators correctly identified baseline images in 55 of 57 image pairs (96.5%), while the average score improvement in their rosacea was 52.5%, and ranged from 30.0% to 86.7%. In addition, 17 of 19 subjects (89%) showed a score improvement of more than 40%, while 57% of subjects had a score improvement of greater than 50%.
Patients and investigators both recorded a mean satisfaction score of 1.9, and 18 of 19 subjects reported being “very satisfied” with the treatment.
The average pain score was 5.6 on a 0-10 scale, while common side effects that resolved within 1-3 days without intervention included mild edema, mild to moderate erythema, and mild to moderate bruising. No cases of hyperpigmentation, hypopigmentation, blistering, or scarring occurred.
“The newly designed pulsed-dye laser is a major change in a device that has performed for over 10 years with wide acceptance,” Dr. Bernstein said. In addition to a longer-lasting dye kit, he said that new features include 50% more total energy enabling clinically relevant fluences to be delivered using a 15-mm diameter treatment beam, once-daily calibration instead of after every energy or spot size change, “both contact and dynamic spray cooling, and an Nd:YAG wavelength for treating larger vessels.”
Dr. Bernstein reported having received grant funding from Syneron Candela and Zeltiq. He also has received consulting fees from and holds ownership interest in Syneron Candela, and has served on the advisory board for Novoxel, Solta, Syneron Candela, and Zeltiq.
REPORTING FROM ASLMS 2018
Key clinical point: A newly designed pulsed-dye laser allows for fluences to be delivered with a 15-mm diameter treatment beam.
Major finding: Blinded evaluators rated the average score improvement in subjects’ rosacea as 52.5%, and ranged from 30.0% to 86.7%.
Study details: A study of 20 patients with erythematotelangiectatic rosacea who received pulsed-dye laser treatments every 4 weeks.
Disclosures: Dr. Bernstein reported having received grant funding from Syneron Candela and Zeltiq. He also has received consulting fees from and holds ownership interest in Syneron Candela, and has served on the advisory board for Novoxel, Solta, Syneron Candela, and Zeltiq.
Mask provides effective, cheap protection from hazardous electrocautery plumes
CHICAGO – Routine use of an N95 mask during electrocautery is an effective and inexpensive way for dermatologic surgeons to protect themselves from toxic, airborne particulate matter in the smoke generated during the procedure, Emily de Golian, MD, said at the annual meeting of the American College of Mohs Surgery.
“Our data suggest clear as well as superiority to the laser masks that are used in hair removal procedures and ablative procedures in cosmetic clinics,” commented Dr. de Golian, a Mohs micrographic surgery fellow at the University of California, San Diego.
This matter of self-protection from the effects of electrocautery smoke plumes deserves greater attention from the dermatologic community, according to Dr. de Golian. There is solid evidence that these plumes contain high concentrations of known carcinogens, including benzene, acetonitrile, and butadiene – indeed, concentrations far in excess of what’s found in second-hand cigarette smoke. Moreover, many of these airborne carcinogens and other toxins have been linked to leukemia, neurologic disorders, lung cancer, thrombotic disorders, lung disease, and infectious disease transmission, albeit not convincingly so to date in dermatologic surgeons. But why wait for definitive evidence to accrue?
“In light of these hazards – and according to governmental guidelines – dermatologic surgeons would be wise to adopt protective measures during surgical procedures,” Dr. de Golian said.
But they haven’t. She cited a national survey conducted several years ago by a colleague in which 79% of the 316 responding dermatologic surgeons indicated they use no smoke management whatsoever, neither masks nor a local exhaust evacuation system. Only 10% employed smoke management 25%-50% of the time during electrocautery, and a scant 11% of dermatologic surgeons did so at least 75% of the time (Dermatol Surg. 2014 Dec;40[12]:1373-7).
Given the far more substantial expense of installing an office smoke evacuation system, mask filtration becomes an attractive alternative. But the relative efficacy of the various types of masks in blocking fine and ultrafine particulate matter contained in electrocautery plumes hadn’t previously been systematically studied. This created the impetus for Dr. de Golian’s study.
The N95 masks were the clear winner, particularly when it came to filtering the ultrafine particles, which are of greatest concern because they remain suspended in air longer and penetrate deeper into the respiratory tract than larger particles. The N95 masks proved superior to procedural masks, which in turn were significantly more effective than the laser masks. The differences between mask performance for larger particle filtration were smaller, although the N95 remained number one. She noted that the study results probably underestimate the true filtration efficacy of N95 masks, since they form a tighter seal with the face in clinical practice than with the other two mask types.
Mask self-protection “is easily applicable in your own practice, and it meets NIOSH-recommended [National Institute for Occupational Safety and Health] standards for safety in the workplace,” the dermatologist noted.
In the next phase of her research, she plans to evaluate the optimal technology and techniques of smoke evacuation in the surgical suite. That’s an attractive method because it protects everyone in the room, not just the surgeon. And while the practitioner survey indicates this technology isn’t widely used by dermatologic surgeons on a routine basis at present, that could change, particularly in the current era in which patient-reported outcomes and satisfaction surveys have taken on added weight.
“Patients prefer not smelling their own tissue burning,” Dr. de Golian said.
She reported no financial conflicts regarding her study, which was conducted free of commercial support.
CHICAGO – Routine use of an N95 mask during electrocautery is an effective and inexpensive way for dermatologic surgeons to protect themselves from toxic, airborne particulate matter in the smoke generated during the procedure, Emily de Golian, MD, said at the annual meeting of the American College of Mohs Surgery.
“Our data suggest clear as well as superiority to the laser masks that are used in hair removal procedures and ablative procedures in cosmetic clinics,” commented Dr. de Golian, a Mohs micrographic surgery fellow at the University of California, San Diego.
This matter of self-protection from the effects of electrocautery smoke plumes deserves greater attention from the dermatologic community, according to Dr. de Golian. There is solid evidence that these plumes contain high concentrations of known carcinogens, including benzene, acetonitrile, and butadiene – indeed, concentrations far in excess of what’s found in second-hand cigarette smoke. Moreover, many of these airborne carcinogens and other toxins have been linked to leukemia, neurologic disorders, lung cancer, thrombotic disorders, lung disease, and infectious disease transmission, albeit not convincingly so to date in dermatologic surgeons. But why wait for definitive evidence to accrue?
“In light of these hazards – and according to governmental guidelines – dermatologic surgeons would be wise to adopt protective measures during surgical procedures,” Dr. de Golian said.
But they haven’t. She cited a national survey conducted several years ago by a colleague in which 79% of the 316 responding dermatologic surgeons indicated they use no smoke management whatsoever, neither masks nor a local exhaust evacuation system. Only 10% employed smoke management 25%-50% of the time during electrocautery, and a scant 11% of dermatologic surgeons did so at least 75% of the time (Dermatol Surg. 2014 Dec;40[12]:1373-7).
Given the far more substantial expense of installing an office smoke evacuation system, mask filtration becomes an attractive alternative. But the relative efficacy of the various types of masks in blocking fine and ultrafine particulate matter contained in electrocautery plumes hadn’t previously been systematically studied. This created the impetus for Dr. de Golian’s study.
The N95 masks were the clear winner, particularly when it came to filtering the ultrafine particles, which are of greatest concern because they remain suspended in air longer and penetrate deeper into the respiratory tract than larger particles. The N95 masks proved superior to procedural masks, which in turn were significantly more effective than the laser masks. The differences between mask performance for larger particle filtration were smaller, although the N95 remained number one. She noted that the study results probably underestimate the true filtration efficacy of N95 masks, since they form a tighter seal with the face in clinical practice than with the other two mask types.
Mask self-protection “is easily applicable in your own practice, and it meets NIOSH-recommended [National Institute for Occupational Safety and Health] standards for safety in the workplace,” the dermatologist noted.
In the next phase of her research, she plans to evaluate the optimal technology and techniques of smoke evacuation in the surgical suite. That’s an attractive method because it protects everyone in the room, not just the surgeon. And while the practitioner survey indicates this technology isn’t widely used by dermatologic surgeons on a routine basis at present, that could change, particularly in the current era in which patient-reported outcomes and satisfaction surveys have taken on added weight.
“Patients prefer not smelling their own tissue burning,” Dr. de Golian said.
She reported no financial conflicts regarding her study, which was conducted free of commercial support.
CHICAGO – Routine use of an N95 mask during electrocautery is an effective and inexpensive way for dermatologic surgeons to protect themselves from toxic, airborne particulate matter in the smoke generated during the procedure, Emily de Golian, MD, said at the annual meeting of the American College of Mohs Surgery.
“Our data suggest clear as well as superiority to the laser masks that are used in hair removal procedures and ablative procedures in cosmetic clinics,” commented Dr. de Golian, a Mohs micrographic surgery fellow at the University of California, San Diego.
This matter of self-protection from the effects of electrocautery smoke plumes deserves greater attention from the dermatologic community, according to Dr. de Golian. There is solid evidence that these plumes contain high concentrations of known carcinogens, including benzene, acetonitrile, and butadiene – indeed, concentrations far in excess of what’s found in second-hand cigarette smoke. Moreover, many of these airborne carcinogens and other toxins have been linked to leukemia, neurologic disorders, lung cancer, thrombotic disorders, lung disease, and infectious disease transmission, albeit not convincingly so to date in dermatologic surgeons. But why wait for definitive evidence to accrue?
“In light of these hazards – and according to governmental guidelines – dermatologic surgeons would be wise to adopt protective measures during surgical procedures,” Dr. de Golian said.
But they haven’t. She cited a national survey conducted several years ago by a colleague in which 79% of the 316 responding dermatologic surgeons indicated they use no smoke management whatsoever, neither masks nor a local exhaust evacuation system. Only 10% employed smoke management 25%-50% of the time during electrocautery, and a scant 11% of dermatologic surgeons did so at least 75% of the time (Dermatol Surg. 2014 Dec;40[12]:1373-7).
Given the far more substantial expense of installing an office smoke evacuation system, mask filtration becomes an attractive alternative. But the relative efficacy of the various types of masks in blocking fine and ultrafine particulate matter contained in electrocautery plumes hadn’t previously been systematically studied. This created the impetus for Dr. de Golian’s study.
The N95 masks were the clear winner, particularly when it came to filtering the ultrafine particles, which are of greatest concern because they remain suspended in air longer and penetrate deeper into the respiratory tract than larger particles. The N95 masks proved superior to procedural masks, which in turn were significantly more effective than the laser masks. The differences between mask performance for larger particle filtration were smaller, although the N95 remained number one. She noted that the study results probably underestimate the true filtration efficacy of N95 masks, since they form a tighter seal with the face in clinical practice than with the other two mask types.
Mask self-protection “is easily applicable in your own practice, and it meets NIOSH-recommended [National Institute for Occupational Safety and Health] standards for safety in the workplace,” the dermatologist noted.
In the next phase of her research, she plans to evaluate the optimal technology and techniques of smoke evacuation in the surgical suite. That’s an attractive method because it protects everyone in the room, not just the surgeon. And while the practitioner survey indicates this technology isn’t widely used by dermatologic surgeons on a routine basis at present, that could change, particularly in the current era in which patient-reported outcomes and satisfaction surveys have taken on added weight.
“Patients prefer not smelling their own tissue burning,” Dr. de Golian said.
She reported no financial conflicts regarding her study, which was conducted free of commercial support.
REPORTING FROM THE ACMS ANNUAL MEETING
Key clinical point: Electrocautery smoke is bad news, and wearing an N95 mask affords protection.
Major finding: The N95 mask was significantly more effective than basic procedural or laser masks at filtering particulate matter less than 1 mcm in size contained in electrocautery smoke.
Study details: This study utilized highly sensitive airborne particle counting devices to assess the relative protective filtration afforded by three types of masks.
Disclosures: The presenter reported no financial conflicts regarding this study, which was conducted free of commercial support.
It's Just a Growth Spurt
A 38-year-old Latino man self-refers to dermatology for evaluation of a mass on his back that first appeared three years ago. Since then, it has grown steadily. There is no pain or discomfort associated with the lesion, and the patient claims to be quite healthy otherwise. There is no antecedent history for the affected area.
EXAMINATION
There is a subcutaneous, rubbery mass in the left infrascapular area. It measures 11 x 6 cm. Palpation reveals the lesion to be uniformly smooth and readily mobile. The overlying skin is free of abnormalities and increased warmth.

What is the diagnosis?
DISCUSSION
Lipomas are by far the most common soft-tissue tumor to affect humans and are totally benign. They typically measure 2 to 3 cm in diameter, but as this case demonstrates, they can grow much larger. While the rate of growth in this case was unusual, the location—and other features—are typical.
Lipomas are actual tumors, composed completely of adipose tissue contained in a thin, fragile, membranous capsule. Their tendency to develop can be hereditary, though most are spontaneous. They can manifest internally as well.
Superficial lipomas, which often manifest as multiple lesions on the arms and trunk, are usually easy to remove surgically. Lesions that are deeper and older or that appear on the face, however, often require considerable dissection to be freed from surrounding tissue. When excision is attempted, it is essential to remove the entire lesion to prevent recurrence. And, as always, the specimen must be sent for pathologic examination.
Patients often decide against surgery once they understand the issues. This is acceptable, but any deviation from the norm—such as pain, irregular surface texture, change in overlying skin, lack of mobility, or rapid growth—would constitute reasonable grounds for excision.
This man’s lesion likely extended down to the muscle fascia if not into the muscle itself. As a result, surgery would require general anesthesia and placement of a drain in the inferior portion of the wound, since such a large defect would likely invite a collection of blood and serum. For these reasons, he was referred to a general surgeon.
The differential for lipoma includes liposarcoma and angiolipoma. The latter are common and benign but become painful and are often more firm than normal. Histologically, they’re often indistinguishable from ordinary lipomas. Liposarcomas, when superficial, can imitate ordinary lipomas, but their surfaces tend to be more irregular and firm and the lesions themselves less mobile.
TAKE-HOME LEARNING POINTS
- Lipomas are the most common soft-tissue tumor encountered in outpatient practices.
- While the vast majority are benign and easy to remove surgically, most lipomas can be safely left alone.
- When excision is attempted, the entire lesion must be removed lest it regrow.
- Patients with larger, deeper lesions, or those in busy anatomical areas, should be referred to a general surgeon.
A 38-year-old Latino man self-refers to dermatology for evaluation of a mass on his back that first appeared three years ago. Since then, it has grown steadily. There is no pain or discomfort associated with the lesion, and the patient claims to be quite healthy otherwise. There is no antecedent history for the affected area.
EXAMINATION
There is a subcutaneous, rubbery mass in the left infrascapular area. It measures 11 x 6 cm. Palpation reveals the lesion to be uniformly smooth and readily mobile. The overlying skin is free of abnormalities and increased warmth.

What is the diagnosis?
DISCUSSION
Lipomas are by far the most common soft-tissue tumor to affect humans and are totally benign. They typically measure 2 to 3 cm in diameter, but as this case demonstrates, they can grow much larger. While the rate of growth in this case was unusual, the location—and other features—are typical.
Lipomas are actual tumors, composed completely of adipose tissue contained in a thin, fragile, membranous capsule. Their tendency to develop can be hereditary, though most are spontaneous. They can manifest internally as well.
Superficial lipomas, which often manifest as multiple lesions on the arms and trunk, are usually easy to remove surgically. Lesions that are deeper and older or that appear on the face, however, often require considerable dissection to be freed from surrounding tissue. When excision is attempted, it is essential to remove the entire lesion to prevent recurrence. And, as always, the specimen must be sent for pathologic examination.
Patients often decide against surgery once they understand the issues. This is acceptable, but any deviation from the norm—such as pain, irregular surface texture, change in overlying skin, lack of mobility, or rapid growth—would constitute reasonable grounds for excision.
This man’s lesion likely extended down to the muscle fascia if not into the muscle itself. As a result, surgery would require general anesthesia and placement of a drain in the inferior portion of the wound, since such a large defect would likely invite a collection of blood and serum. For these reasons, he was referred to a general surgeon.
The differential for lipoma includes liposarcoma and angiolipoma. The latter are common and benign but become painful and are often more firm than normal. Histologically, they’re often indistinguishable from ordinary lipomas. Liposarcomas, when superficial, can imitate ordinary lipomas, but their surfaces tend to be more irregular and firm and the lesions themselves less mobile.
TAKE-HOME LEARNING POINTS
- Lipomas are the most common soft-tissue tumor encountered in outpatient practices.
- While the vast majority are benign and easy to remove surgically, most lipomas can be safely left alone.
- When excision is attempted, the entire lesion must be removed lest it regrow.
- Patients with larger, deeper lesions, or those in busy anatomical areas, should be referred to a general surgeon.
A 38-year-old Latino man self-refers to dermatology for evaluation of a mass on his back that first appeared three years ago. Since then, it has grown steadily. There is no pain or discomfort associated with the lesion, and the patient claims to be quite healthy otherwise. There is no antecedent history for the affected area.
EXAMINATION
There is a subcutaneous, rubbery mass in the left infrascapular area. It measures 11 x 6 cm. Palpation reveals the lesion to be uniformly smooth and readily mobile. The overlying skin is free of abnormalities and increased warmth.

What is the diagnosis?
DISCUSSION
Lipomas are by far the most common soft-tissue tumor to affect humans and are totally benign. They typically measure 2 to 3 cm in diameter, but as this case demonstrates, they can grow much larger. While the rate of growth in this case was unusual, the location—and other features—are typical.
Lipomas are actual tumors, composed completely of adipose tissue contained in a thin, fragile, membranous capsule. Their tendency to develop can be hereditary, though most are spontaneous. They can manifest internally as well.
Superficial lipomas, which often manifest as multiple lesions on the arms and trunk, are usually easy to remove surgically. Lesions that are deeper and older or that appear on the face, however, often require considerable dissection to be freed from surrounding tissue. When excision is attempted, it is essential to remove the entire lesion to prevent recurrence. And, as always, the specimen must be sent for pathologic examination.
Patients often decide against surgery once they understand the issues. This is acceptable, but any deviation from the norm—such as pain, irregular surface texture, change in overlying skin, lack of mobility, or rapid growth—would constitute reasonable grounds for excision.
This man’s lesion likely extended down to the muscle fascia if not into the muscle itself. As a result, surgery would require general anesthesia and placement of a drain in the inferior portion of the wound, since such a large defect would likely invite a collection of blood and serum. For these reasons, he was referred to a general surgeon.
The differential for lipoma includes liposarcoma and angiolipoma. The latter are common and benign but become painful and are often more firm than normal. Histologically, they’re often indistinguishable from ordinary lipomas. Liposarcomas, when superficial, can imitate ordinary lipomas, but their surfaces tend to be more irregular and firm and the lesions themselves less mobile.
TAKE-HOME LEARNING POINTS
- Lipomas are the most common soft-tissue tumor encountered in outpatient practices.
- While the vast majority are benign and easy to remove surgically, most lipomas can be safely left alone.
- When excision is attempted, the entire lesion must be removed lest it regrow.
- Patients with larger, deeper lesions, or those in busy anatomical areas, should be referred to a general surgeon.