User login
Combine these screening tools to detect bipolar depression
THE CASE
A 35-year-old police officer visited his family physician (FP) with complaints of low energy, trouble sleeping, a lack of enjoyment in life, and feelings of hopelessness that have persisted for several months. He was worried about the impact they were having on his marriage and work. He had not experienced suicidal thoughts. His Patient Health Questionnaire (PHQ9) score was 18 (moderately severe depression). He had been seen intermittently for similar complaints and had tried several medications (fluoxetine, bupropion, and citalopram) without much effect. He was taking no medications now other than an over-the-counter multivitamin. He had one brother with anxiety and depression. He said his marriage counselor expressed concerns that he might have bipolar disorder or borderline personality disorder.
How would you proceed with this patient?
The prevalence of a spectrum of bipolarity in the community has been shown to be 6.4%.1 Depressive episodes predominate in bipolar disorder (BPD),2 with patients spending less time in manic or hypomanic states.3 Not surprisingly, then, depressive episodes are the most common presentation of BPD.
The depressive symptoms of BPD and unipolar depression, or major depressive disorder (MDD), are similar, making it difficult to distinguish between the disorders.3 As a result, BPD is often misdiagnosed as MDD.4,5 Zimmerman et al point out that “bipolar disorder is prone to being overlooked because its diagnosis is more often based on retrospective report rather than presenting symptoms of mania or hypomania assessment.”6
Accurately recognizing BPD is essential in selecting effective treatment. It’s estimated that approximately one-third of patients given antidepressants for major depression show no treatment response,7 possibly due in part to undiagnosed BPD being more prevalent than previously thought.4,8 Failure to distinguish between depressive episodes of BPD and MDD before prescribing medication introduces the risk of ineffective or suboptimal treatment. Inappropriate treatment can worsen or destabilize the course of bipolar illness by, for instance, inducing rapid cycling or, less commonly, manic symptoms.
Screen for BPD when depressive symptoms are present
Identifying BPD in a patient with current or past depressive symptoms requires screening for manic, hypomanic, and mixed episodes (TABLE 19). Two brief, complementary screening tools — the Mood Disorder Questionnaire (MDQ) and the 9-item PHQ9—are helpful in this assessment. Both questionnaires (TABLE 28,10-14) can be conveniently completed by the patient in the waiting room or with staff assistance before the physician encounter.
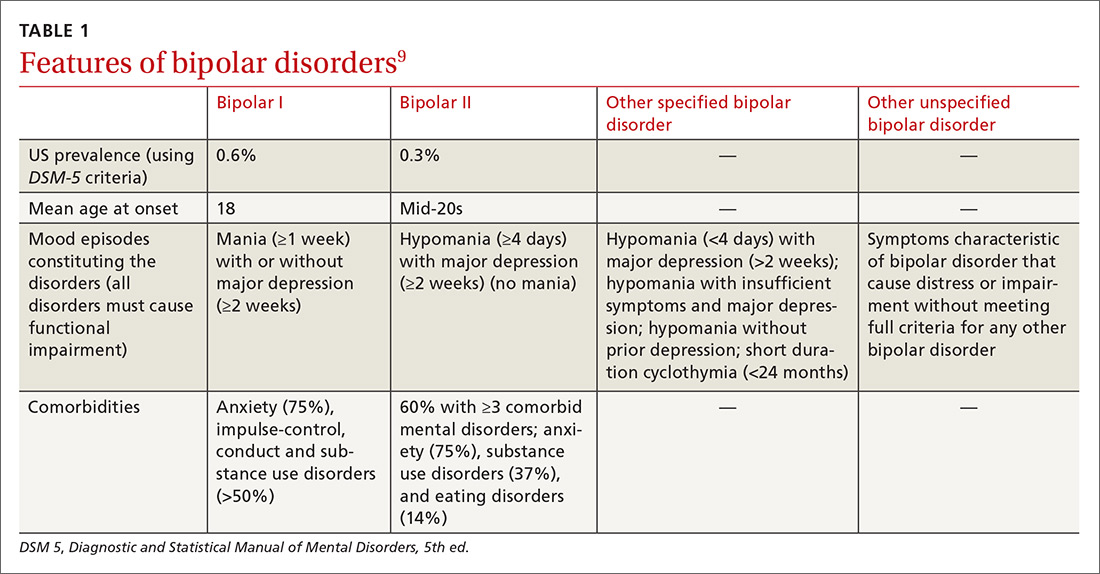
The MDQ screen is for past/lifetime or current manic/hypomanic symptoms (https://www.integration.samhsa.gov/images/res/MDQ.pdf). A positive screen requires answering “Yes” to at least 7 of the 13 items on question 1, answering “yes” on question 2, and answering “moderate problems” or “serious problems” on question 3.

Continue to: The PHQ9 screens for...
The PHQ9 screens for current depressive symptoms/episodes (https://www.uspreventiveservicestaskforce.org/Home/GetFileByID/218).
The value of combining the MDQ and PHQ9. The PHQ9 screens for and assesses the severity of depressive episodes along with clinician assessment, but it cannot distinguish between depressive episodes of MDD or BPD. A brief instrument, such as MDQ, screens for current or past manic or hypomanic symptoms, which, when combined with the clinical interview and patient history, enables detection of BPD if present and avoids erroneously assigning depressive symptoms to MDD.
One cross-sectional study found that the combined MDQ and PHQ9 questionnaires have a higher sensitivity in detecting mood disorder than does routine assessment by general practitioners (0.8 [95% confidence interval (CI), 0.71-0.81] vs 0.2 [95% CI, 0.12- 0.25]) and without loss of specificity (0.9 [95% CI, 0.86-0.96] vs 0.9 [95% CI, 0.88-0.97]).15 In this same study, using a structured clinical interview for DSM-III-R Axis I Disorders (SCID-I) as the gold standard, researchers also found the screening tools to be more accurate (Cohen’s Kappa 0.7 [SE=0.05; 95% CI, 0.5-0.7]) than the general practitioner assessment (Cohen’s Kappa 0.2 [SE=0.07 (95% CI, 0.12-0.27]).15
Delve deeper with a patient interview
Use targeted questions and laboratory tests to rule out other possible causes of depressed mood, such as substance abuse or medical conditions (eg, hypothyroidism). Keep in mind that even when MDD or BPD is present, other medical disorders or substance abuse could be coexistent. Also ask about a personal or family psychiatric history and assess for suicidality. If family members are available, they may be able to help in identifying the patient’s age when symptoms first appeared or in adding information about the affective episode or behavior that the patient may not recollect.
Beyond a history of manic, hypomanic, or mixed episodes, other symptoms and features may assist in distinguishing between bipolar and unipolar depression or in helping the clinician identify depressed patients who may be at higher risk for, or have, BPD. One meta-analysis of 3 multicenter clinical trials assessed sociodemographic factors and clinical features of BPD compared with unipolar depression. The average age of onset of mood symptoms in individuals with BPD was significantly younger (21.2 years) than that of patients with MDD (29.7 years).16 Another study found that patients with either bipolar I or bipolar II similarly experienced their first mood disorder episode 10 years earlier than those with MDD.17
Continue to: BPD is often associated with...
BPD is often associated with more frequent depressive episodes and a higher number of depressive symptoms per episode than is MDD, as well as more frequent family psychiatric histories (especially of mood disorders), anxiety disorders, alcohol and drug use disorders, and personality disorders.17 Other factors more closely associated with BPD than MDD include atypical features such as hypersomnia and psychomotor retardation, psychotic symptoms during the depressive episode, and more frequent recurrences of depressive episodes.18-22 Also, depressive episodes during the postpartum period indicate a higher risk of BPD than do episodes in women outside the postpartum period, with a hazard ratio (HR) of 1.66 (95% CI, 1.12-2.48).23 The risk is much greater when postpartum depressive episodes are associated with anxiety symptoms (HR=10.15; 95% CI, 7.13-14.46).23
Final thoughts
Increased awareness and screening for BPD in primary care—where most individuals with depressive symptoms are first encountered—should lead to more accurate diagnoses and decrease the years-long gaps between symptom onset and detection of BPD,4,5 thereby improving treatment and patient outcomes. Still, some cases of BPD may be difficult to recognize—particularly patients who present predominantly with depression with past irritability and other hypomanic symptoms (but not euphoria).
A positive MDQ screen should also prompt, if possible, a more detailed clinical interview by a mental health care professional, particularly if there is uncertainty about the diagnosis. Complex cases of BPD may require the expertise of a psychiatrist.
THE CASE
The patient’s FP referred him to a psychiatrist colleague, whose inquiry also revealed low mood, anhedonia, hopelessness, difficulty sleeping, low energy, poor appetite, guilt, poor concentration, and psychomotor retardation. The patient had experienced multiple depressive episodes over the past 20 years. Significant interpersonal conflicts frequently triggered his depressive episodes, which were accompanied by mood irritability, racing thoughts, distractibility, increased libido, excessive spending, increased energy, and engagement in risky behaviors.
The patient’s score on the MDQ administered by the psychiatrist was positive, with 7 points on question 1. He also had posttraumatic symptoms related to his police work, which were not the main reason for the visit. He had been divorced 3 times. In prior manic episodes, he had not displayed euphoria, grandiosity, psychotic symptoms, or anxiety, but rather irritability with other manic symptoms.
Continue to: Based on his MDQ results...
Based on his MDQ results, the clinical interview, and current episode with mixed features, the patient was given a diagnosis of bipolar II disorder. The psychiatrist prescribed divalproex 500 mg at bedtime and scheduled a return visit with a plan for further laboratory monitoring and up-titration if needed. He was also encouraged to follow up with his FP.
CORRESPONDENCE
Nagy A. Youssef, MD, Medical College of Georgia at Augusta University, 997 St. Sebastian Way, Augusta, GA 30912; [email protected].
SUPPORT AND ACKNOWLEDGMENT
Dr. Youssef’s work on this paper was supported by the Office of Academic Affairs, Medical College of Georgia at Augusta University. We thank Mark Yassa, BS, for his assistance in editing.
1. Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord. 2003;73:123-131.
2. Yatham LN, Lecrubier Y, Fieve RR, et al. Quality of life in patients with bipolar I depression: data from 920 patients. Bipolar Disord. 2004;6:379-385.
3. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530-537.
4. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52:135-144.
5. Cha B, Kim JH, Ha TH, et al. Polarity of the first episode and time to diagnosis of bipolar I disorder. Psychiatry Investig. 2009;6:96-101. Available at: http://psychiatryinvestigation.org/journal/view.php?doi=10.4306/pi.2009.6.2.96. Accessed June 25, 2018.
6. Zimmerman M, Galione JN, Chelminski I, et al. Psychiatric diagnoses in patients who screen positive on the Mood Disorder Questionnaire: implications for using the scale as a case-finding instrument for bipolar disorder. Psychiatry Res. 2011;185:444-449.
7. Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369-388.
8. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18:233-239.
9. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, D.C.: American Psychiatric Publishing. 2013.
10. Poon Y, Chung KF, Tso KC, et al. The use of Mood Disorder Questionnaire, Hypomania Checklist-32 and clinical predictors for screening previously unrecognised bipolar disorder in a general psychiatric setting. Psychiatry Res. 2012;195:111-117.
11. Gilbody S, Richards D, Brealey S, et al. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22:1596-1602.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
13. Miller CJ, Klugman J, Berv DA, et al. Sensitivity and specificity of the Mood Disorder Questionnaire for detecting bipolar disorder. J Affect Disord. 2004;81:167-171.
14. Sasdelli A, Lia L, Luciano CC, et al. Screening for bipolar disorder symptoms in depressed primary care attenders: comparison between Mood Disorder Questionnaire and Hypomania Checklist (HCL-32). Psychiatry J. 2013;2013:548349.
15. Vohringer PA, Jimenez MI, Igor MA, et al. Detecting mood disorder in resource-limited primary care settings: comparison of a self-administered screening tool to general practitioner assessment. J Med Screen. 2013;20:118-124.
16. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163:225-231.
17. Moreno C, Hasin DS, Arango C, et al. Depression in bipolar disorder versus major depressive disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Bipolar Disord. 2012;14:271-282.
18. Mitchell PB, Malhi GS. Bipolar depression: phenomenological overview and clinical characteristics. Bipolar Disord. 2004;6:530-539.
19. Solomon DA, Leon AC, Maser JD, et al. Distinguishing bipolar major depression from unipolar major depression with the screening assessment of depression-polarity (SAD-P). J Clin Psychiatry. 2006;67:434-442.
20. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84:117-125.
21. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9:901-906.
22. Buzuk G, Lojko D, Owecki M, et al. Depression with atypical features in various kinds of affective disorders. Psychiatr Pol. 2016;50:827-838.
23. Liu X, Agerbo E, Li J, et al. Depression and anxiety in the postpartum period and risk of bipolar disorder: a Danish Nationwide Register-Based Cohort Study. J Clin Psychiatry. 2017;78:e469-e476.
THE CASE
A 35-year-old police officer visited his family physician (FP) with complaints of low energy, trouble sleeping, a lack of enjoyment in life, and feelings of hopelessness that have persisted for several months. He was worried about the impact they were having on his marriage and work. He had not experienced suicidal thoughts. His Patient Health Questionnaire (PHQ9) score was 18 (moderately severe depression). He had been seen intermittently for similar complaints and had tried several medications (fluoxetine, bupropion, and citalopram) without much effect. He was taking no medications now other than an over-the-counter multivitamin. He had one brother with anxiety and depression. He said his marriage counselor expressed concerns that he might have bipolar disorder or borderline personality disorder.
How would you proceed with this patient?
The prevalence of a spectrum of bipolarity in the community has been shown to be 6.4%.1 Depressive episodes predominate in bipolar disorder (BPD),2 with patients spending less time in manic or hypomanic states.3 Not surprisingly, then, depressive episodes are the most common presentation of BPD.
The depressive symptoms of BPD and unipolar depression, or major depressive disorder (MDD), are similar, making it difficult to distinguish between the disorders.3 As a result, BPD is often misdiagnosed as MDD.4,5 Zimmerman et al point out that “bipolar disorder is prone to being overlooked because its diagnosis is more often based on retrospective report rather than presenting symptoms of mania or hypomania assessment.”6
Accurately recognizing BPD is essential in selecting effective treatment. It’s estimated that approximately one-third of patients given antidepressants for major depression show no treatment response,7 possibly due in part to undiagnosed BPD being more prevalent than previously thought.4,8 Failure to distinguish between depressive episodes of BPD and MDD before prescribing medication introduces the risk of ineffective or suboptimal treatment. Inappropriate treatment can worsen or destabilize the course of bipolar illness by, for instance, inducing rapid cycling or, less commonly, manic symptoms.
Screen for BPD when depressive symptoms are present
Identifying BPD in a patient with current or past depressive symptoms requires screening for manic, hypomanic, and mixed episodes (TABLE 19). Two brief, complementary screening tools — the Mood Disorder Questionnaire (MDQ) and the 9-item PHQ9—are helpful in this assessment. Both questionnaires (TABLE 28,10-14) can be conveniently completed by the patient in the waiting room or with staff assistance before the physician encounter.

The MDQ screen is for past/lifetime or current manic/hypomanic symptoms (https://www.integration.samhsa.gov/images/res/MDQ.pdf). A positive screen requires answering “Yes” to at least 7 of the 13 items on question 1, answering “yes” on question 2, and answering “moderate problems” or “serious problems” on question 3.

Continue to: The PHQ9 screens for...
The PHQ9 screens for current depressive symptoms/episodes (https://www.uspreventiveservicestaskforce.org/Home/GetFileByID/218).
The value of combining the MDQ and PHQ9. The PHQ9 screens for and assesses the severity of depressive episodes along with clinician assessment, but it cannot distinguish between depressive episodes of MDD or BPD. A brief instrument, such as MDQ, screens for current or past manic or hypomanic symptoms, which, when combined with the clinical interview and patient history, enables detection of BPD if present and avoids erroneously assigning depressive symptoms to MDD.
One cross-sectional study found that the combined MDQ and PHQ9 questionnaires have a higher sensitivity in detecting mood disorder than does routine assessment by general practitioners (0.8 [95% confidence interval (CI), 0.71-0.81] vs 0.2 [95% CI, 0.12- 0.25]) and without loss of specificity (0.9 [95% CI, 0.86-0.96] vs 0.9 [95% CI, 0.88-0.97]).15 In this same study, using a structured clinical interview for DSM-III-R Axis I Disorders (SCID-I) as the gold standard, researchers also found the screening tools to be more accurate (Cohen’s Kappa 0.7 [SE=0.05; 95% CI, 0.5-0.7]) than the general practitioner assessment (Cohen’s Kappa 0.2 [SE=0.07 (95% CI, 0.12-0.27]).15
Delve deeper with a patient interview
Use targeted questions and laboratory tests to rule out other possible causes of depressed mood, such as substance abuse or medical conditions (eg, hypothyroidism). Keep in mind that even when MDD or BPD is present, other medical disorders or substance abuse could be coexistent. Also ask about a personal or family psychiatric history and assess for suicidality. If family members are available, they may be able to help in identifying the patient’s age when symptoms first appeared or in adding information about the affective episode or behavior that the patient may not recollect.
Beyond a history of manic, hypomanic, or mixed episodes, other symptoms and features may assist in distinguishing between bipolar and unipolar depression or in helping the clinician identify depressed patients who may be at higher risk for, or have, BPD. One meta-analysis of 3 multicenter clinical trials assessed sociodemographic factors and clinical features of BPD compared with unipolar depression. The average age of onset of mood symptoms in individuals with BPD was significantly younger (21.2 years) than that of patients with MDD (29.7 years).16 Another study found that patients with either bipolar I or bipolar II similarly experienced their first mood disorder episode 10 years earlier than those with MDD.17
Continue to: BPD is often associated with...
BPD is often associated with more frequent depressive episodes and a higher number of depressive symptoms per episode than is MDD, as well as more frequent family psychiatric histories (especially of mood disorders), anxiety disorders, alcohol and drug use disorders, and personality disorders.17 Other factors more closely associated with BPD than MDD include atypical features such as hypersomnia and psychomotor retardation, psychotic symptoms during the depressive episode, and more frequent recurrences of depressive episodes.18-22 Also, depressive episodes during the postpartum period indicate a higher risk of BPD than do episodes in women outside the postpartum period, with a hazard ratio (HR) of 1.66 (95% CI, 1.12-2.48).23 The risk is much greater when postpartum depressive episodes are associated with anxiety symptoms (HR=10.15; 95% CI, 7.13-14.46).23
Final thoughts
Increased awareness and screening for BPD in primary care—where most individuals with depressive symptoms are first encountered—should lead to more accurate diagnoses and decrease the years-long gaps between symptom onset and detection of BPD,4,5 thereby improving treatment and patient outcomes. Still, some cases of BPD may be difficult to recognize—particularly patients who present predominantly with depression with past irritability and other hypomanic symptoms (but not euphoria).
A positive MDQ screen should also prompt, if possible, a more detailed clinical interview by a mental health care professional, particularly if there is uncertainty about the diagnosis. Complex cases of BPD may require the expertise of a psychiatrist.
THE CASE
The patient’s FP referred him to a psychiatrist colleague, whose inquiry also revealed low mood, anhedonia, hopelessness, difficulty sleeping, low energy, poor appetite, guilt, poor concentration, and psychomotor retardation. The patient had experienced multiple depressive episodes over the past 20 years. Significant interpersonal conflicts frequently triggered his depressive episodes, which were accompanied by mood irritability, racing thoughts, distractibility, increased libido, excessive spending, increased energy, and engagement in risky behaviors.
The patient’s score on the MDQ administered by the psychiatrist was positive, with 7 points on question 1. He also had posttraumatic symptoms related to his police work, which were not the main reason for the visit. He had been divorced 3 times. In prior manic episodes, he had not displayed euphoria, grandiosity, psychotic symptoms, or anxiety, but rather irritability with other manic symptoms.
Continue to: Based on his MDQ results...
Based on his MDQ results, the clinical interview, and current episode with mixed features, the patient was given a diagnosis of bipolar II disorder. The psychiatrist prescribed divalproex 500 mg at bedtime and scheduled a return visit with a plan for further laboratory monitoring and up-titration if needed. He was also encouraged to follow up with his FP.
CORRESPONDENCE
Nagy A. Youssef, MD, Medical College of Georgia at Augusta University, 997 St. Sebastian Way, Augusta, GA 30912; [email protected].
SUPPORT AND ACKNOWLEDGMENT
Dr. Youssef’s work on this paper was supported by the Office of Academic Affairs, Medical College of Georgia at Augusta University. We thank Mark Yassa, BS, for his assistance in editing.
THE CASE
A 35-year-old police officer visited his family physician (FP) with complaints of low energy, trouble sleeping, a lack of enjoyment in life, and feelings of hopelessness that have persisted for several months. He was worried about the impact they were having on his marriage and work. He had not experienced suicidal thoughts. His Patient Health Questionnaire (PHQ9) score was 18 (moderately severe depression). He had been seen intermittently for similar complaints and had tried several medications (fluoxetine, bupropion, and citalopram) without much effect. He was taking no medications now other than an over-the-counter multivitamin. He had one brother with anxiety and depression. He said his marriage counselor expressed concerns that he might have bipolar disorder or borderline personality disorder.
How would you proceed with this patient?
The prevalence of a spectrum of bipolarity in the community has been shown to be 6.4%.1 Depressive episodes predominate in bipolar disorder (BPD),2 with patients spending less time in manic or hypomanic states.3 Not surprisingly, then, depressive episodes are the most common presentation of BPD.
The depressive symptoms of BPD and unipolar depression, or major depressive disorder (MDD), are similar, making it difficult to distinguish between the disorders.3 As a result, BPD is often misdiagnosed as MDD.4,5 Zimmerman et al point out that “bipolar disorder is prone to being overlooked because its diagnosis is more often based on retrospective report rather than presenting symptoms of mania or hypomania assessment.”6
Accurately recognizing BPD is essential in selecting effective treatment. It’s estimated that approximately one-third of patients given antidepressants for major depression show no treatment response,7 possibly due in part to undiagnosed BPD being more prevalent than previously thought.4,8 Failure to distinguish between depressive episodes of BPD and MDD before prescribing medication introduces the risk of ineffective or suboptimal treatment. Inappropriate treatment can worsen or destabilize the course of bipolar illness by, for instance, inducing rapid cycling or, less commonly, manic symptoms.
Screen for BPD when depressive symptoms are present
Identifying BPD in a patient with current or past depressive symptoms requires screening for manic, hypomanic, and mixed episodes (TABLE 19). Two brief, complementary screening tools — the Mood Disorder Questionnaire (MDQ) and the 9-item PHQ9—are helpful in this assessment. Both questionnaires (TABLE 28,10-14) can be conveniently completed by the patient in the waiting room or with staff assistance before the physician encounter.

The MDQ screen is for past/lifetime or current manic/hypomanic symptoms (https://www.integration.samhsa.gov/images/res/MDQ.pdf). A positive screen requires answering “Yes” to at least 7 of the 13 items on question 1, answering “yes” on question 2, and answering “moderate problems” or “serious problems” on question 3.

Continue to: The PHQ9 screens for...
The PHQ9 screens for current depressive symptoms/episodes (https://www.uspreventiveservicestaskforce.org/Home/GetFileByID/218).
The value of combining the MDQ and PHQ9. The PHQ9 screens for and assesses the severity of depressive episodes along with clinician assessment, but it cannot distinguish between depressive episodes of MDD or BPD. A brief instrument, such as MDQ, screens for current or past manic or hypomanic symptoms, which, when combined with the clinical interview and patient history, enables detection of BPD if present and avoids erroneously assigning depressive symptoms to MDD.
One cross-sectional study found that the combined MDQ and PHQ9 questionnaires have a higher sensitivity in detecting mood disorder than does routine assessment by general practitioners (0.8 [95% confidence interval (CI), 0.71-0.81] vs 0.2 [95% CI, 0.12- 0.25]) and without loss of specificity (0.9 [95% CI, 0.86-0.96] vs 0.9 [95% CI, 0.88-0.97]).15 In this same study, using a structured clinical interview for DSM-III-R Axis I Disorders (SCID-I) as the gold standard, researchers also found the screening tools to be more accurate (Cohen’s Kappa 0.7 [SE=0.05; 95% CI, 0.5-0.7]) than the general practitioner assessment (Cohen’s Kappa 0.2 [SE=0.07 (95% CI, 0.12-0.27]).15
Delve deeper with a patient interview
Use targeted questions and laboratory tests to rule out other possible causes of depressed mood, such as substance abuse or medical conditions (eg, hypothyroidism). Keep in mind that even when MDD or BPD is present, other medical disorders or substance abuse could be coexistent. Also ask about a personal or family psychiatric history and assess for suicidality. If family members are available, they may be able to help in identifying the patient’s age when symptoms first appeared or in adding information about the affective episode or behavior that the patient may not recollect.
Beyond a history of manic, hypomanic, or mixed episodes, other symptoms and features may assist in distinguishing between bipolar and unipolar depression or in helping the clinician identify depressed patients who may be at higher risk for, or have, BPD. One meta-analysis of 3 multicenter clinical trials assessed sociodemographic factors and clinical features of BPD compared with unipolar depression. The average age of onset of mood symptoms in individuals with BPD was significantly younger (21.2 years) than that of patients with MDD (29.7 years).16 Another study found that patients with either bipolar I or bipolar II similarly experienced their first mood disorder episode 10 years earlier than those with MDD.17
Continue to: BPD is often associated with...
BPD is often associated with more frequent depressive episodes and a higher number of depressive symptoms per episode than is MDD, as well as more frequent family psychiatric histories (especially of mood disorders), anxiety disorders, alcohol and drug use disorders, and personality disorders.17 Other factors more closely associated with BPD than MDD include atypical features such as hypersomnia and psychomotor retardation, psychotic symptoms during the depressive episode, and more frequent recurrences of depressive episodes.18-22 Also, depressive episodes during the postpartum period indicate a higher risk of BPD than do episodes in women outside the postpartum period, with a hazard ratio (HR) of 1.66 (95% CI, 1.12-2.48).23 The risk is much greater when postpartum depressive episodes are associated with anxiety symptoms (HR=10.15; 95% CI, 7.13-14.46).23
Final thoughts
Increased awareness and screening for BPD in primary care—where most individuals with depressive symptoms are first encountered—should lead to more accurate diagnoses and decrease the years-long gaps between symptom onset and detection of BPD,4,5 thereby improving treatment and patient outcomes. Still, some cases of BPD may be difficult to recognize—particularly patients who present predominantly with depression with past irritability and other hypomanic symptoms (but not euphoria).
A positive MDQ screen should also prompt, if possible, a more detailed clinical interview by a mental health care professional, particularly if there is uncertainty about the diagnosis. Complex cases of BPD may require the expertise of a psychiatrist.
THE CASE
The patient’s FP referred him to a psychiatrist colleague, whose inquiry also revealed low mood, anhedonia, hopelessness, difficulty sleeping, low energy, poor appetite, guilt, poor concentration, and psychomotor retardation. The patient had experienced multiple depressive episodes over the past 20 years. Significant interpersonal conflicts frequently triggered his depressive episodes, which were accompanied by mood irritability, racing thoughts, distractibility, increased libido, excessive spending, increased energy, and engagement in risky behaviors.
The patient’s score on the MDQ administered by the psychiatrist was positive, with 7 points on question 1. He also had posttraumatic symptoms related to his police work, which were not the main reason for the visit. He had been divorced 3 times. In prior manic episodes, he had not displayed euphoria, grandiosity, psychotic symptoms, or anxiety, but rather irritability with other manic symptoms.
Continue to: Based on his MDQ results...
Based on his MDQ results, the clinical interview, and current episode with mixed features, the patient was given a diagnosis of bipolar II disorder. The psychiatrist prescribed divalproex 500 mg at bedtime and scheduled a return visit with a plan for further laboratory monitoring and up-titration if needed. He was also encouraged to follow up with his FP.
CORRESPONDENCE
Nagy A. Youssef, MD, Medical College of Georgia at Augusta University, 997 St. Sebastian Way, Augusta, GA 30912; [email protected].
SUPPORT AND ACKNOWLEDGMENT
Dr. Youssef’s work on this paper was supported by the Office of Academic Affairs, Medical College of Georgia at Augusta University. We thank Mark Yassa, BS, for his assistance in editing.
1. Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord. 2003;73:123-131.
2. Yatham LN, Lecrubier Y, Fieve RR, et al. Quality of life in patients with bipolar I depression: data from 920 patients. Bipolar Disord. 2004;6:379-385.
3. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530-537.
4. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52:135-144.
5. Cha B, Kim JH, Ha TH, et al. Polarity of the first episode and time to diagnosis of bipolar I disorder. Psychiatry Investig. 2009;6:96-101. Available at: http://psychiatryinvestigation.org/journal/view.php?doi=10.4306/pi.2009.6.2.96. Accessed June 25, 2018.
6. Zimmerman M, Galione JN, Chelminski I, et al. Psychiatric diagnoses in patients who screen positive on the Mood Disorder Questionnaire: implications for using the scale as a case-finding instrument for bipolar disorder. Psychiatry Res. 2011;185:444-449.
7. Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369-388.
8. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18:233-239.
9. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, D.C.: American Psychiatric Publishing. 2013.
10. Poon Y, Chung KF, Tso KC, et al. The use of Mood Disorder Questionnaire, Hypomania Checklist-32 and clinical predictors for screening previously unrecognised bipolar disorder in a general psychiatric setting. Psychiatry Res. 2012;195:111-117.
11. Gilbody S, Richards D, Brealey S, et al. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22:1596-1602.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
13. Miller CJ, Klugman J, Berv DA, et al. Sensitivity and specificity of the Mood Disorder Questionnaire for detecting bipolar disorder. J Affect Disord. 2004;81:167-171.
14. Sasdelli A, Lia L, Luciano CC, et al. Screening for bipolar disorder symptoms in depressed primary care attenders: comparison between Mood Disorder Questionnaire and Hypomania Checklist (HCL-32). Psychiatry J. 2013;2013:548349.
15. Vohringer PA, Jimenez MI, Igor MA, et al. Detecting mood disorder in resource-limited primary care settings: comparison of a self-administered screening tool to general practitioner assessment. J Med Screen. 2013;20:118-124.
16. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163:225-231.
17. Moreno C, Hasin DS, Arango C, et al. Depression in bipolar disorder versus major depressive disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Bipolar Disord. 2012;14:271-282.
18. Mitchell PB, Malhi GS. Bipolar depression: phenomenological overview and clinical characteristics. Bipolar Disord. 2004;6:530-539.
19. Solomon DA, Leon AC, Maser JD, et al. Distinguishing bipolar major depression from unipolar major depression with the screening assessment of depression-polarity (SAD-P). J Clin Psychiatry. 2006;67:434-442.
20. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84:117-125.
21. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9:901-906.
22. Buzuk G, Lojko D, Owecki M, et al. Depression with atypical features in various kinds of affective disorders. Psychiatr Pol. 2016;50:827-838.
23. Liu X, Agerbo E, Li J, et al. Depression and anxiety in the postpartum period and risk of bipolar disorder: a Danish Nationwide Register-Based Cohort Study. J Clin Psychiatry. 2017;78:e469-e476.
1. Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord. 2003;73:123-131.
2. Yatham LN, Lecrubier Y, Fieve RR, et al. Quality of life in patients with bipolar I depression: data from 920 patients. Bipolar Disord. 2004;6:379-385.
3. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530-537.
4. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52:135-144.
5. Cha B, Kim JH, Ha TH, et al. Polarity of the first episode and time to diagnosis of bipolar I disorder. Psychiatry Investig. 2009;6:96-101. Available at: http://psychiatryinvestigation.org/journal/view.php?doi=10.4306/pi.2009.6.2.96. Accessed June 25, 2018.
6. Zimmerman M, Galione JN, Chelminski I, et al. Psychiatric diagnoses in patients who screen positive on the Mood Disorder Questionnaire: implications for using the scale as a case-finding instrument for bipolar disorder. Psychiatry Res. 2011;185:444-449.
7. Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369-388.
8. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18:233-239.
9. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, D.C.: American Psychiatric Publishing. 2013.
10. Poon Y, Chung KF, Tso KC, et al. The use of Mood Disorder Questionnaire, Hypomania Checklist-32 and clinical predictors for screening previously unrecognised bipolar disorder in a general psychiatric setting. Psychiatry Res. 2012;195:111-117.
11. Gilbody S, Richards D, Brealey S, et al. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22:1596-1602.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
13. Miller CJ, Klugman J, Berv DA, et al. Sensitivity and specificity of the Mood Disorder Questionnaire for detecting bipolar disorder. J Affect Disord. 2004;81:167-171.
14. Sasdelli A, Lia L, Luciano CC, et al. Screening for bipolar disorder symptoms in depressed primary care attenders: comparison between Mood Disorder Questionnaire and Hypomania Checklist (HCL-32). Psychiatry J. 2013;2013:548349.
15. Vohringer PA, Jimenez MI, Igor MA, et al. Detecting mood disorder in resource-limited primary care settings: comparison of a self-administered screening tool to general practitioner assessment. J Med Screen. 2013;20:118-124.
16. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163:225-231.
17. Moreno C, Hasin DS, Arango C, et al. Depression in bipolar disorder versus major depressive disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Bipolar Disord. 2012;14:271-282.
18. Mitchell PB, Malhi GS. Bipolar depression: phenomenological overview and clinical characteristics. Bipolar Disord. 2004;6:530-539.
19. Solomon DA, Leon AC, Maser JD, et al. Distinguishing bipolar major depression from unipolar major depression with the screening assessment of depression-polarity (SAD-P). J Clin Psychiatry. 2006;67:434-442.
20. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84:117-125.
21. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9:901-906.
22. Buzuk G, Lojko D, Owecki M, et al. Depression with atypical features in various kinds of affective disorders. Psychiatr Pol. 2016;50:827-838.
23. Liu X, Agerbo E, Li J, et al. Depression and anxiety in the postpartum period and risk of bipolar disorder: a Danish Nationwide Register-Based Cohort Study. J Clin Psychiatry. 2017;78:e469-e476.
TEAM approach reduced wait time, improved “face” time
ABSTRACT
Purpose In 2013-14, 2 clinics in the Watertown Regional Medical Center (WRMC; in southern Wisconsin) launched a new delivery model, “TEAM (Together Each person Achieves More) Primary Care,” as part of a quality improvement project to enhance the delivery experience for the patient, physician, and medical assistant (MA). New work flows, roles, and responsibilities were designed to reduce cycle time, increase patient time with physicians and staff, and reduce patient wait times.
Methods The new model increased the ratio of MAs to physicians from a baseline MA:MD ratio of 1:1 to 3:2, and trained MAs to assume expanded roles during exam-room entry and discharge, including assisting with documentation during the patient visit. A process engineer timed patient visits. The process engineer and a human resources associate conducted surveys to assess the level of satisfaction for patients, physicians, and MAs.
Results Cycle time decreased by a mean of 6 minutes, from 44 to 38 minutes per patient; time with staff increased a mean of 2 minutes, from 24 to 26 minutes per patient; and waiting time decreased from 9 to 2 minutes per patient. Qualitative interviews with patients, physicians, and MAs identified a high level of satisfaction with the new model.
Conclusion The higher staffing ratios and expanded roles for MAs in the new model improved workflow, increased the face time between patients and their physician and MA, and decreased patient wait times. The TEAM model also appeared to improve patient, physician, and MA satisfaction. We faced many challenges while implementing the new model, which could be further evaluated during wide adoption.
In recent years, we observed that our physicians, nurses, and medical assistants (MAs) appeared to be spending more time on administrative and clerical tasks—including tasks in the exam room with the patient—and less time engaged in direct patient care.1,2 We recognized these factors contribute to burnout and threaten staff retention and anticipated that a new model would improve physician time spent in direct patient care, decrease the demands of administrative tasks, and increase patient, physician, and MA satisfaction.3-6 Burnout, known to affect more than half of US physicians, has a negative impact on quality of care and patient safety and satisfaction.7-11 Improving workflow has been shown to reduce burnout.12
Watertown Regional Medical Center (WRMC) is a small, financially stable integrated delivery system in rural southern Wisconsin, composed of a 90-bed hospital, 10 primary care clinics (7 owned and 3 affiliated), and 24 employed physicians in 9 specialties. Two clinics within WRMC launched a new delivery model, “TEAM (Together Each person Achieves More) Primary Care,” to improve the delivery experience for the entire team, defined as the patient, physician, and MA. New workflows, roles, and responsibilities were designed to reduce cycle time (the total amount of time patients spent in the clinic from check-in to check-out), increase the total time a patient spent with staff (physician and MA or in point-of-care testing and radiology), and reduce the total time a patient spent waiting.13
We describe here WRMC’s experience in developing and implementing workflow improvements as a means of reducing burnout and improving satisfaction.
Continue to: METHODS
METHODS
We selected 2 WRMC sites for TEAM re-engineering based on their experience with quality-improvement projects and perceived likelihood of success with a new transformation initiative. In early 2013, WRMC charged one physician (JM), 2 MAs, the clinic scheduler, and the clinic administrator with designing the details of the model including evaluation metrics. WRMC provided a .5 FTE process engineer (MS) to assist with the design and implementation of the model at no extra expense to the clinics. The model was implemented in late 2013 and into 2014 after regular TEAM planning meetings and observational visits to non-WRMC sites identified as examples of best practices in improving outpatient primary care patient satisfaction: Bellin Health (Green Bay, Wis); ThedaCare (Appleton, Wis); the University of Utah (Salt Lake City); and the University of Wisconsin Health Yahara Clinic (Madison, Wis).
TEAM model
The TEAM model—so named to create top-of-mind awareness of its benefits—increased the MA:MD ratio, maintained consistent team composition so that physician/MA teams learned to work together and become more efficient, and added new MA responsibilities. We trained MAs to assist with documentation in the exam room to ensure that physician time was spent in face-to-face direct patient care.14-20 In these ways, we sought not only to increase patient satisfaction but also to enhance our own “joy in practice,” defined primarily by a high level of work-life satisfaction, a low level of burnout, and a feeling that the medical practice is fulfilling.21
In our traditional model, an MA retrieved the patient from the waiting room, conducted initial assessment in the exam room, and then left the patient to wait for the physician to enter. Once the physician entered and conducted the exam, the patient would be left alone again to wait for the MA to return. In our revised model (TABLE 1), we assigned one MA to each patient from arrival to discharge. After greeting the patient in the waiting room, the MA conducted an initial patient interview in the exam room, then remained in the room with the physician to document the visit. After the physician exited the exam room, the MA completed follow-up orders and provided the patient with a visit summary.
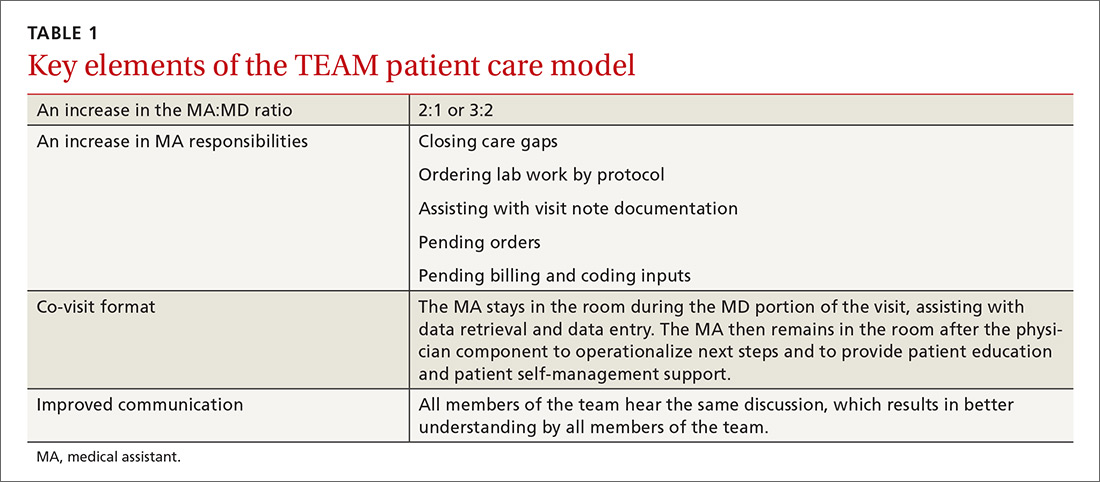
To facilitate consistency throughout the day, we designed a workflow similar to those created in lean models originally designed to increase efficiency in the manufacturing industry (TABLE 2). Visual and electronic cues triggered each step of the process and coordinated the movement of MAs and MDs. Cues included the conventional flag system outside each exam room, an electronic messaging system within the electronic health record (EHR) to indicate when a patient was ready to be seen, and a whiteboard in an area visible to all team members on which we wrote lab and radiology requests.
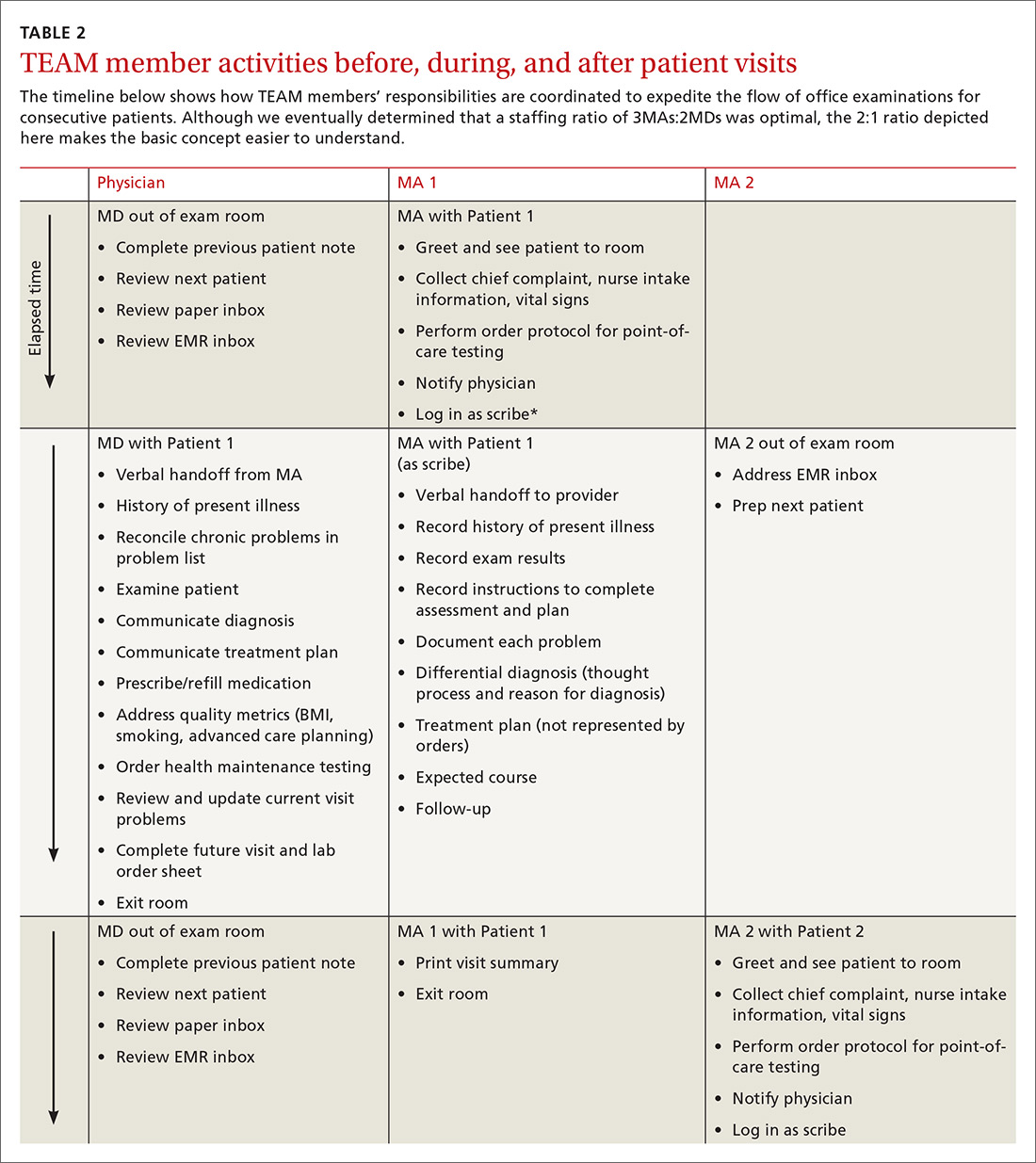
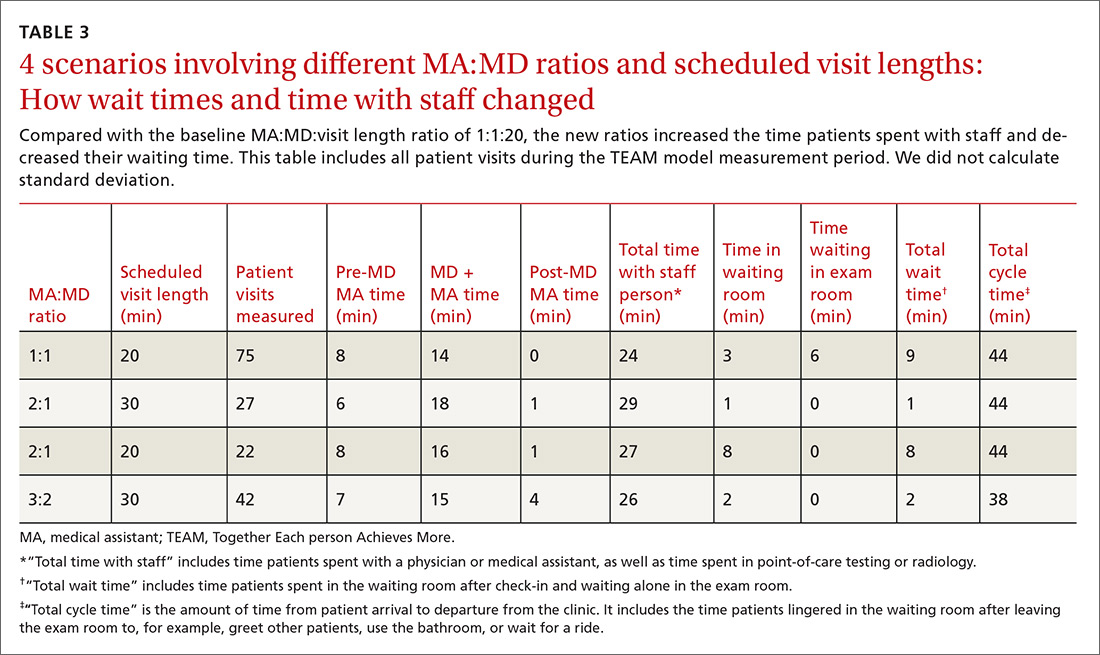
We experimented with the MA:MD ratio to identify the most effective and efficient team composition. On alternating weeks, we assigned one MA to one MD, 2 MAs to one MD, or 3 MAs to 2 MDs. Additionally, with the 2:1 MA:MD ratio, we varied the visit length in 2 tests; one spanning 30 minutes and the other 20 minutes. The MDs and MAs were seated at side-by-side workstations to make communication easier. We developed protocols and checklists that allowed MAs to enter health maintenance orders and conduct point-of-care testing before the physician entered the room. Such details included immunization management, strep screens, urine analyses, diabetic foot exams, extremity x-ray films, and mammogram and colonoscopy referrals.
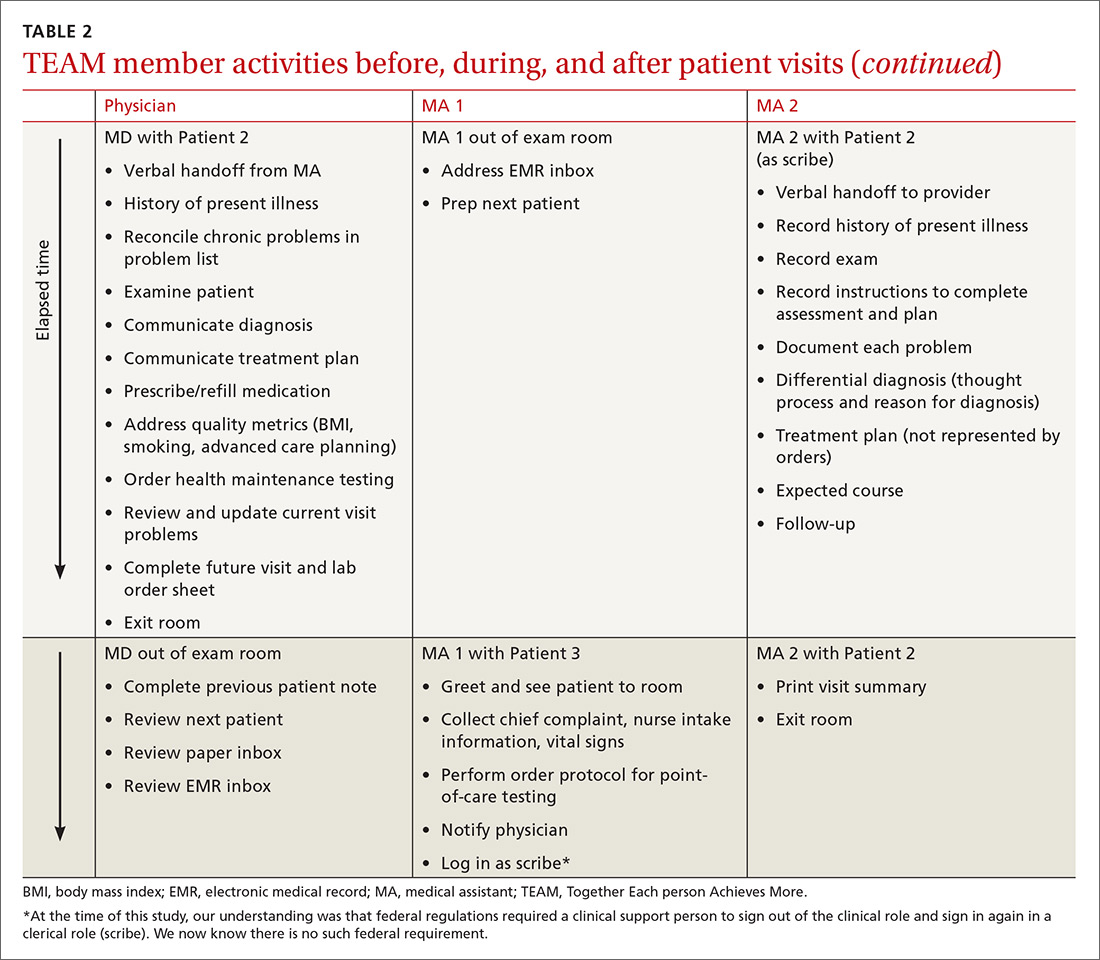
Continue to: To prepare MAs...
To prepare MAs, we obtained special permission for team documentation from our Chief Information Officer and developed associated policies and procedures. A physician assistant (PA) trained each MA, introducing the structure and content of subjective, objective, assessment, and plan (SOAP) notes. Training was continuous, as PAs provided feedback when MAs began team documentation. The MAs documented visits using templates, free form, and quick text. We measured visit cycle-time, face time with staff, and patient waiting times. A process engineer with a stopwatch observed and timed the flow (but did not enter the exam room). We also conducted patient interviews immediately post-visit and administered anonymous questionnaires to clinic staff at different phases of the model. Physicians and MAs met weekly to evaluate the design.
We used qualitative interviews of patients, physicians, and MAs to identify the level of satisfaction with the new model. During the first week of implementation, a nurse and our process engineer conducted brief in-person surveys with approximately 20 post-visit patients. Patients, chosen by convenience, were asked if the visit addressed their concerns, whether they left with a thorough understanding of next steps, and if their wait time was acceptable. Twice during the implementation phase, a human resources associate distributed 9-item anonymous questionnaires to staff members during scheduled department meetings.
RESULTS
Times per activity with different MA:MD ratios and visit lengths are shown in TABLE 3. After 6 months, cycle time decreased by a mean of 6 minutes, from 44 to 38 minutes per patient; time with staff increased by a mean of 2 minutes, from 24 to 26 minutes per patient; and wait time decreased by a mean of 7 minutes, from 9 to 2 minutes per patient. We concluded the MA:MD ratio of 3:2 was most efficient because the 2:1 model left MAs with excess non-patient time.
Our delivery model received consistently positive comments from patients. Many expressed gratitude for the extra set of ears and eyes guiding them through the process. One recalled the “old days” when a nurse joined the doctor in the exam room. Another appreciated that both the MA and physician could answer follow-up questions over the phone.
Employee satisfaction
Surveys to assess satisfaction were distributed to all employees whether they were involved in the new model or not. Sixteen employees responded to the pre-implementation questionnaire and 18 responded to the post-implementation one distributed 7 months later. The questionnaires showed an increase in employee satisfaction scores from 3.70 to 3.89 on a 5-point Likert scale, with 5 ranking highest. “I am learning from [Dr. Milford] and understanding things more fully,” wrote one respondent. Another said, “Dr. Milford and his clinical support staff are less stressed.” Individual observations such as, “I can leave sooner with less work left to do,” and “All documentation is done before [the] patient leaves,” reflect the reduction in time that patient records remained open or incomplete. Some physicians reported a reduction in at-home or after-hours work, from about 2 to 4 hours per day to approximately one hour per day.
Continue to: Additional outcomes
Additional outcomes
The TEAM model allowed us to more easily integrate new initiatives into our practice and meet quality metrics by placing needed components within our workflow and checklist. For example, achieving Stage II Meaningful Use measures required that we print and furnish patients with a visit summary and a reminder to access our portal; something we easily incorporated into the MAs’ expanded responsibilities. We also met specific predetermined quality metrics that were part of a payment-withhold program. During the study period, we achieved scores at the 90th percentile and earned back our total withhold.
Finally, more of our patients completed advanced care planning discussions than the other 7 sites in our Honoring Choices Wisconsin cohort. This was achieved not only by integrating the process into our checklist, but because the MAs observed the MD-led patient conversations which they then emulated, presenting the advanced care planning information to patients before or after MD time with the patient.
Errors and defects in care
With ongoing provider guidance and reinforcement, MAs became integral members of the primary care team. They were empowered through protocols to manage and order health maintenance testing and provide needed immunizations. They also began to identify potentially overlooked aspects of care. For example, MAs prompted physicians to retake vital signs, adjust medications, order labs, discuss previous lab results, and pursue specialty referrals or follow-up care.
Billing
Although we tracked billing, the TEAM model was not designed to influence revenue. We noted no significant change in level of evaluation and management billed regardless of staffing ratio. While our panel size increased as we implemented the new process, this change could have been due to normal variation. We do see opportunity to affect future billing by having coders train MAs, which could enhance documentation and increase revenue.
DISCUSSION
The TEAM Primary Care model reduced the time our patients sat unattended, increased our opportunities to meaningfully interact with them, and seemed to reduce our administrative load. By identifying and implementing ways to work as a more cohesive, interconnected unit, we began to address our work as a team rather than as individuals. After implementing the model, we noted several instances where the MAs caught potential errors in care, although we did not consistently track or measure changes in the rate of these occurrences.
Continue to: Achieving these results also came with...
Achieving these results also came with challenges. Investing in and maintaining a new model opened our eyes to unforeseen inconsistencies in our staff profile and to the cost and administrative support needed for implementation. Moreover, our entire team (patients, MAs, and physicians) had to undergo a major cultural shift to adopt a new model.
Personnel variation
We discovered that implementing and sustaining organization change is highly dependent on constancy in human resources. When one team member was on vacation, sick, or leaving the practice, the process tended to fall apart. Hiring replacements and training employees well enough to fill in at a moment’s notice proved difficult. Bringing new employees into this process was also labor intensive. Despite team members being very engaged in change, these staffing inconsistencies caused significant stress and necessitated pauses in the implementation of the new model (reflected in the timeline of our measures). Larger organizational buy-in and support would allow us to hire and train a larger pool of MAs in anticipation of these fluctuations.
Cost
Our small, rural family practice took advantage of WRMC’s Primary Care Transformation project and the half-time process engineer and additional MA they provided. We question whether this model could be implemented without such support. While a process engineer might not prove necessary, expertise in process improvement is vital to help design and measure workflow and to identify opportunities for improvement.
Cultural change
Adopting a new model required asking every member of the team (patient, MA, and physician) to accommodate change and tolerate disruption. We anticipated patients might resist having an additional person in the room. All patients were informed of our new model at the beginning of the visit and told they could opt out. While we did not document patient resistance, JM recalled only 2 patients who expressed a desire not to have the MA present because of personal and sensitive issues. It’s possible some patients did not feel comfortable opting out. But many patients expressed gratitude for having an extra set of ears and eyes to guide them through the visit.
It was more challenging to support MAs as they stepped out of their comfort zone to assist with documentation. It took time for MAs to grow accustomed to the protocols and checklists essential to our workflow. Without protocols, any point-of-care testing that could have been completed at the beginning of the appointment had to be done at the end. This disrupted our workflow and increased patient wait times.
Continue to: We correctly predicted MAs would have...
We correctly predicted MAs would have difficulty documenting the assessment, plan, and medical decision making. We discovered that MAs more easily categorized and articulated information when we reframed the assessment and plan in first-person and placed it under “Patient instructions.” For this to occur, physicians had to learn to accurately articulate their thought process and instructions to the patient.
When training was provided, as previously described, MAs grasped the subjective section quickly. Surprisingly, they had most difficulty understanding terminology within the objective section. In the future, we would avert this problem by working closely with the human resource department. We believe there should be a newly defined position and additional training for MAs in these roles, since duties such as patient-coaching and documentation assistance may warrant separate certification.
Limitations
Our findings should be interpreted in light of several limitations. Implementing the new model was carried out in a single organization. The patients who were selected and agreed to be interviewed may have differed from the patient population as a whole. We did not measure some important outcomes, such as cost effectiveness and patient morbidity. We did not analyze the data to determine whether the apparent improvements in wait time and cycle time were statistically significant. In addition, measurement of any adverse effects was beyond the scope of this study.
Looking forward
The traditional model of physicians working individually with minimal support staff is no longer viable. To echo our co-author (CAS)’s recent statements on physician dissatisfaction, “The days of hero medicine, with the doctor doing it all, belong in the past.”21 The new model appeared to alleviate some administrative burdens and increase physician time with patients. Pressures to achieve quality measures and growing administrative tasks have altered the role and responsibilities of each member of the team.
Any sustainable system must address the larger crisis of physician dissatisfaction.7,22 We cannot focus on a single perspective—patient, physician, or MA—at the expense of the system as a whole. If the health care system is to resolve the epidemic of burnout and physician dissatisfaction, new approaches to patient care must be imagined and realized. Although we faced many challenges in implementing and evaluating the TEAM model, attempts to overcome these challenges appear justified because of our overall favorable impression of it. Innovations like the TEAM Primary Care model may help us improve the well-being of not just our patients but also our health professionals and the health care industry as a whole.
CORRESPONDENCE
James Milford, MD, Three Oaks Health, S.C., 480 Village Walk Lane, Suite F, Johnson Creek, WI 53038; [email protected].
SUPPORT
Although the Watertown Regional Medical Center has provided general funding for its Primary Care Transformation project, no dollars were specifically earmarked for the TEAM Primary Care process. Support for editorial services in preparing this article was provided by Dr. James Milford.
PRIOR PRESENTATIONS
Co-author Michael R. Strasser, MPA, presented this project at the 2015 i-PrACTISE conference in Madison, Wis, April 12-14, 2015. http://www.fammed.wisc.edu/i-practise/. The proceedings were not published or recorded.
ACKNOWLEDGMENT
We thank Annalynn Skipper and Masarah Van Eyck for their valuable edits.
1. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760.
2. McDonald CJ, Callaghan FM, Weissman A, et al. Use of internist’s free time by ambulatory care electronic medical record systems. JAMA Intern Med. 2014;174:1860-1863.
3. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848.
4. Friedberg MW, Chen PG, Van Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. Available at: http://www.rand.org/pubs/research_reports/RR439.html#key-findings. Accessed October 25, 2016.
5. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO study. J Am Med Inform Assoc. 2014;21:e100-e106.
6. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press. 2001.
7. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clinic Proc. 2015;90:1600-1613.
8. DeMatteo MR, Sherbourne CD, Hays RD, et al. Physicians’ characteristics influence patients’ adherence to medical treatment: Results from the Medical Outcomes Study. Health Psychol. 1993;12:93-102.
9. Shanafelt TD, Bradley KA, Wipf JE, et al. Burnout and self-reported patient care in an internal medicine residency program. Ann Intern Med. 2002;136:358-367.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251:995-1000.
11. Haas JS, Cook EF, Puopolo AL, et al. Is the professional satisfaction of general internists associated with patient satisfaction? J Gen Intern Med. 2000;15:122-128.
12. Linzer M, Poplau S, Grossman E, et al. A cluster randomized trial of interventions to improve work conditions and clinician burnout in primary care: results from the Healthy Work Place (HWP) Study. J Gen Intern Med. 2015;30:1105-1011.
13. Ferrer RL, Mody-Bailey P, Jaén CR, et al. A medical assistant-based program to promote healthy behaviors in primary care. Ann Fam Med. 2009;7:504-512.
14. Sinsky CA, Williard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
15. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
16. Hopkins K, Sinsky CA. Team-based care: saving time and improving efficiency. Fam Pract Manag. 2014;21:23-29.
17. Yan C, Rose S, Rothberg MB, et al. Physician, scribe, and patient perspectives on clinical scribes in primary care. J Gen Intern Med. 2016;31:990-995.
18. Misra-Hebert AD, Rabovsky A, Yan C, et al. A team-based model of primary care delivery and physician-patient interaction. Am J Med. 2015;128:1025-1028.
19. Anderson RJ. Optimizing the role of nursing staff to enhance physician productivity: one physician’s journey. Fam Pract Manag. 2013;20:18-22.
20. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008:15:35-40.
21. Sinsky CA. Dissatisfaction among Wisconsin physicians is part of a serious national trend. Wis Med J. 2015;114:132-133.
22. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
ABSTRACT
Purpose In 2013-14, 2 clinics in the Watertown Regional Medical Center (WRMC; in southern Wisconsin) launched a new delivery model, “TEAM (Together Each person Achieves More) Primary Care,” as part of a quality improvement project to enhance the delivery experience for the patient, physician, and medical assistant (MA). New work flows, roles, and responsibilities were designed to reduce cycle time, increase patient time with physicians and staff, and reduce patient wait times.
Methods The new model increased the ratio of MAs to physicians from a baseline MA:MD ratio of 1:1 to 3:2, and trained MAs to assume expanded roles during exam-room entry and discharge, including assisting with documentation during the patient visit. A process engineer timed patient visits. The process engineer and a human resources associate conducted surveys to assess the level of satisfaction for patients, physicians, and MAs.
Results Cycle time decreased by a mean of 6 minutes, from 44 to 38 minutes per patient; time with staff increased a mean of 2 minutes, from 24 to 26 minutes per patient; and waiting time decreased from 9 to 2 minutes per patient. Qualitative interviews with patients, physicians, and MAs identified a high level of satisfaction with the new model.
Conclusion The higher staffing ratios and expanded roles for MAs in the new model improved workflow, increased the face time between patients and their physician and MA, and decreased patient wait times. The TEAM model also appeared to improve patient, physician, and MA satisfaction. We faced many challenges while implementing the new model, which could be further evaluated during wide adoption.
In recent years, we observed that our physicians, nurses, and medical assistants (MAs) appeared to be spending more time on administrative and clerical tasks—including tasks in the exam room with the patient—and less time engaged in direct patient care.1,2 We recognized these factors contribute to burnout and threaten staff retention and anticipated that a new model would improve physician time spent in direct patient care, decrease the demands of administrative tasks, and increase patient, physician, and MA satisfaction.3-6 Burnout, known to affect more than half of US physicians, has a negative impact on quality of care and patient safety and satisfaction.7-11 Improving workflow has been shown to reduce burnout.12
Watertown Regional Medical Center (WRMC) is a small, financially stable integrated delivery system in rural southern Wisconsin, composed of a 90-bed hospital, 10 primary care clinics (7 owned and 3 affiliated), and 24 employed physicians in 9 specialties. Two clinics within WRMC launched a new delivery model, “TEAM (Together Each person Achieves More) Primary Care,” to improve the delivery experience for the entire team, defined as the patient, physician, and MA. New workflows, roles, and responsibilities were designed to reduce cycle time (the total amount of time patients spent in the clinic from check-in to check-out), increase the total time a patient spent with staff (physician and MA or in point-of-care testing and radiology), and reduce the total time a patient spent waiting.13
We describe here WRMC’s experience in developing and implementing workflow improvements as a means of reducing burnout and improving satisfaction.
Continue to: METHODS
METHODS
We selected 2 WRMC sites for TEAM re-engineering based on their experience with quality-improvement projects and perceived likelihood of success with a new transformation initiative. In early 2013, WRMC charged one physician (JM), 2 MAs, the clinic scheduler, and the clinic administrator with designing the details of the model including evaluation metrics. WRMC provided a .5 FTE process engineer (MS) to assist with the design and implementation of the model at no extra expense to the clinics. The model was implemented in late 2013 and into 2014 after regular TEAM planning meetings and observational visits to non-WRMC sites identified as examples of best practices in improving outpatient primary care patient satisfaction: Bellin Health (Green Bay, Wis); ThedaCare (Appleton, Wis); the University of Utah (Salt Lake City); and the University of Wisconsin Health Yahara Clinic (Madison, Wis).
TEAM model
The TEAM model—so named to create top-of-mind awareness of its benefits—increased the MA:MD ratio, maintained consistent team composition so that physician/MA teams learned to work together and become more efficient, and added new MA responsibilities. We trained MAs to assist with documentation in the exam room to ensure that physician time was spent in face-to-face direct patient care.14-20 In these ways, we sought not only to increase patient satisfaction but also to enhance our own “joy in practice,” defined primarily by a high level of work-life satisfaction, a low level of burnout, and a feeling that the medical practice is fulfilling.21
In our traditional model, an MA retrieved the patient from the waiting room, conducted initial assessment in the exam room, and then left the patient to wait for the physician to enter. Once the physician entered and conducted the exam, the patient would be left alone again to wait for the MA to return. In our revised model (TABLE 1), we assigned one MA to each patient from arrival to discharge. After greeting the patient in the waiting room, the MA conducted an initial patient interview in the exam room, then remained in the room with the physician to document the visit. After the physician exited the exam room, the MA completed follow-up orders and provided the patient with a visit summary.

To facilitate consistency throughout the day, we designed a workflow similar to those created in lean models originally designed to increase efficiency in the manufacturing industry (TABLE 2). Visual and electronic cues triggered each step of the process and coordinated the movement of MAs and MDs. Cues included the conventional flag system outside each exam room, an electronic messaging system within the electronic health record (EHR) to indicate when a patient was ready to be seen, and a whiteboard in an area visible to all team members on which we wrote lab and radiology requests.

We experimented with the MA:MD ratio to identify the most effective and efficient team composition. On alternating weeks, we assigned one MA to one MD, 2 MAs to one MD, or 3 MAs to 2 MDs. Additionally, with the 2:1 MA:MD ratio, we varied the visit length in 2 tests; one spanning 30 minutes and the other 20 minutes. The MDs and MAs were seated at side-by-side workstations to make communication easier. We developed protocols and checklists that allowed MAs to enter health maintenance orders and conduct point-of-care testing before the physician entered the room. Such details included immunization management, strep screens, urine analyses, diabetic foot exams, extremity x-ray films, and mammogram and colonoscopy referrals.

Continue to: To prepare MAs...
To prepare MAs, we obtained special permission for team documentation from our Chief Information Officer and developed associated policies and procedures. A physician assistant (PA) trained each MA, introducing the structure and content of subjective, objective, assessment, and plan (SOAP) notes. Training was continuous, as PAs provided feedback when MAs began team documentation. The MAs documented visits using templates, free form, and quick text. We measured visit cycle-time, face time with staff, and patient waiting times. A process engineer with a stopwatch observed and timed the flow (but did not enter the exam room). We also conducted patient interviews immediately post-visit and administered anonymous questionnaires to clinic staff at different phases of the model. Physicians and MAs met weekly to evaluate the design.
We used qualitative interviews of patients, physicians, and MAs to identify the level of satisfaction with the new model. During the first week of implementation, a nurse and our process engineer conducted brief in-person surveys with approximately 20 post-visit patients. Patients, chosen by convenience, were asked if the visit addressed their concerns, whether they left with a thorough understanding of next steps, and if their wait time was acceptable. Twice during the implementation phase, a human resources associate distributed 9-item anonymous questionnaires to staff members during scheduled department meetings.
RESULTS
Times per activity with different MA:MD ratios and visit lengths are shown in TABLE 3. After 6 months, cycle time decreased by a mean of 6 minutes, from 44 to 38 minutes per patient; time with staff increased by a mean of 2 minutes, from 24 to 26 minutes per patient; and wait time decreased by a mean of 7 minutes, from 9 to 2 minutes per patient. We concluded the MA:MD ratio of 3:2 was most efficient because the 2:1 model left MAs with excess non-patient time.

Our delivery model received consistently positive comments from patients. Many expressed gratitude for the extra set of ears and eyes guiding them through the process. One recalled the “old days” when a nurse joined the doctor in the exam room. Another appreciated that both the MA and physician could answer follow-up questions over the phone.
Employee satisfaction
Surveys to assess satisfaction were distributed to all employees whether they were involved in the new model or not. Sixteen employees responded to the pre-implementation questionnaire and 18 responded to the post-implementation one distributed 7 months later. The questionnaires showed an increase in employee satisfaction scores from 3.70 to 3.89 on a 5-point Likert scale, with 5 ranking highest. “I am learning from [Dr. Milford] and understanding things more fully,” wrote one respondent. Another said, “Dr. Milford and his clinical support staff are less stressed.” Individual observations such as, “I can leave sooner with less work left to do,” and “All documentation is done before [the] patient leaves,” reflect the reduction in time that patient records remained open or incomplete. Some physicians reported a reduction in at-home or after-hours work, from about 2 to 4 hours per day to approximately one hour per day.
Continue to: Additional outcomes
Additional outcomes
The TEAM model allowed us to more easily integrate new initiatives into our practice and meet quality metrics by placing needed components within our workflow and checklist. For example, achieving Stage II Meaningful Use measures required that we print and furnish patients with a visit summary and a reminder to access our portal; something we easily incorporated into the MAs’ expanded responsibilities. We also met specific predetermined quality metrics that were part of a payment-withhold program. During the study period, we achieved scores at the 90th percentile and earned back our total withhold.
Finally, more of our patients completed advanced care planning discussions than the other 7 sites in our Honoring Choices Wisconsin cohort. This was achieved not only by integrating the process into our checklist, but because the MAs observed the MD-led patient conversations which they then emulated, presenting the advanced care planning information to patients before or after MD time with the patient.
Errors and defects in care
With ongoing provider guidance and reinforcement, MAs became integral members of the primary care team. They were empowered through protocols to manage and order health maintenance testing and provide needed immunizations. They also began to identify potentially overlooked aspects of care. For example, MAs prompted physicians to retake vital signs, adjust medications, order labs, discuss previous lab results, and pursue specialty referrals or follow-up care.
Billing
Although we tracked billing, the TEAM model was not designed to influence revenue. We noted no significant change in level of evaluation and management billed regardless of staffing ratio. While our panel size increased as we implemented the new process, this change could have been due to normal variation. We do see opportunity to affect future billing by having coders train MAs, which could enhance documentation and increase revenue.
DISCUSSION
The TEAM Primary Care model reduced the time our patients sat unattended, increased our opportunities to meaningfully interact with them, and seemed to reduce our administrative load. By identifying and implementing ways to work as a more cohesive, interconnected unit, we began to address our work as a team rather than as individuals. After implementing the model, we noted several instances where the MAs caught potential errors in care, although we did not consistently track or measure changes in the rate of these occurrences.
Continue to: Achieving these results also came with...
Achieving these results also came with challenges. Investing in and maintaining a new model opened our eyes to unforeseen inconsistencies in our staff profile and to the cost and administrative support needed for implementation. Moreover, our entire team (patients, MAs, and physicians) had to undergo a major cultural shift to adopt a new model.
Personnel variation
We discovered that implementing and sustaining organization change is highly dependent on constancy in human resources. When one team member was on vacation, sick, or leaving the practice, the process tended to fall apart. Hiring replacements and training employees well enough to fill in at a moment’s notice proved difficult. Bringing new employees into this process was also labor intensive. Despite team members being very engaged in change, these staffing inconsistencies caused significant stress and necessitated pauses in the implementation of the new model (reflected in the timeline of our measures). Larger organizational buy-in and support would allow us to hire and train a larger pool of MAs in anticipation of these fluctuations.
Cost
Our small, rural family practice took advantage of WRMC’s Primary Care Transformation project and the half-time process engineer and additional MA they provided. We question whether this model could be implemented without such support. While a process engineer might not prove necessary, expertise in process improvement is vital to help design and measure workflow and to identify opportunities for improvement.
Cultural change
Adopting a new model required asking every member of the team (patient, MA, and physician) to accommodate change and tolerate disruption. We anticipated patients might resist having an additional person in the room. All patients were informed of our new model at the beginning of the visit and told they could opt out. While we did not document patient resistance, JM recalled only 2 patients who expressed a desire not to have the MA present because of personal and sensitive issues. It’s possible some patients did not feel comfortable opting out. But many patients expressed gratitude for having an extra set of ears and eyes to guide them through the visit.
It was more challenging to support MAs as they stepped out of their comfort zone to assist with documentation. It took time for MAs to grow accustomed to the protocols and checklists essential to our workflow. Without protocols, any point-of-care testing that could have been completed at the beginning of the appointment had to be done at the end. This disrupted our workflow and increased patient wait times.
Continue to: We correctly predicted MAs would have...
We correctly predicted MAs would have difficulty documenting the assessment, plan, and medical decision making. We discovered that MAs more easily categorized and articulated information when we reframed the assessment and plan in first-person and placed it under “Patient instructions.” For this to occur, physicians had to learn to accurately articulate their thought process and instructions to the patient.
When training was provided, as previously described, MAs grasped the subjective section quickly. Surprisingly, they had most difficulty understanding terminology within the objective section. In the future, we would avert this problem by working closely with the human resource department. We believe there should be a newly defined position and additional training for MAs in these roles, since duties such as patient-coaching and documentation assistance may warrant separate certification.
Limitations
Our findings should be interpreted in light of several limitations. Implementing the new model was carried out in a single organization. The patients who were selected and agreed to be interviewed may have differed from the patient population as a whole. We did not measure some important outcomes, such as cost effectiveness and patient morbidity. We did not analyze the data to determine whether the apparent improvements in wait time and cycle time were statistically significant. In addition, measurement of any adverse effects was beyond the scope of this study.
Looking forward
The traditional model of physicians working individually with minimal support staff is no longer viable. To echo our co-author (CAS)’s recent statements on physician dissatisfaction, “The days of hero medicine, with the doctor doing it all, belong in the past.”21 The new model appeared to alleviate some administrative burdens and increase physician time with patients. Pressures to achieve quality measures and growing administrative tasks have altered the role and responsibilities of each member of the team.
Any sustainable system must address the larger crisis of physician dissatisfaction.7,22 We cannot focus on a single perspective—patient, physician, or MA—at the expense of the system as a whole. If the health care system is to resolve the epidemic of burnout and physician dissatisfaction, new approaches to patient care must be imagined and realized. Although we faced many challenges in implementing and evaluating the TEAM model, attempts to overcome these challenges appear justified because of our overall favorable impression of it. Innovations like the TEAM Primary Care model may help us improve the well-being of not just our patients but also our health professionals and the health care industry as a whole.
CORRESPONDENCE
James Milford, MD, Three Oaks Health, S.C., 480 Village Walk Lane, Suite F, Johnson Creek, WI 53038; [email protected].
SUPPORT
Although the Watertown Regional Medical Center has provided general funding for its Primary Care Transformation project, no dollars were specifically earmarked for the TEAM Primary Care process. Support for editorial services in preparing this article was provided by Dr. James Milford.
PRIOR PRESENTATIONS
Co-author Michael R. Strasser, MPA, presented this project at the 2015 i-PrACTISE conference in Madison, Wis, April 12-14, 2015. http://www.fammed.wisc.edu/i-practise/. The proceedings were not published or recorded.
ACKNOWLEDGMENT
We thank Annalynn Skipper and Masarah Van Eyck for their valuable edits.
ABSTRACT
Purpose In 2013-14, 2 clinics in the Watertown Regional Medical Center (WRMC; in southern Wisconsin) launched a new delivery model, “TEAM (Together Each person Achieves More) Primary Care,” as part of a quality improvement project to enhance the delivery experience for the patient, physician, and medical assistant (MA). New work flows, roles, and responsibilities were designed to reduce cycle time, increase patient time with physicians and staff, and reduce patient wait times.
Methods The new model increased the ratio of MAs to physicians from a baseline MA:MD ratio of 1:1 to 3:2, and trained MAs to assume expanded roles during exam-room entry and discharge, including assisting with documentation during the patient visit. A process engineer timed patient visits. The process engineer and a human resources associate conducted surveys to assess the level of satisfaction for patients, physicians, and MAs.
Results Cycle time decreased by a mean of 6 minutes, from 44 to 38 minutes per patient; time with staff increased a mean of 2 minutes, from 24 to 26 minutes per patient; and waiting time decreased from 9 to 2 minutes per patient. Qualitative interviews with patients, physicians, and MAs identified a high level of satisfaction with the new model.
Conclusion The higher staffing ratios and expanded roles for MAs in the new model improved workflow, increased the face time between patients and their physician and MA, and decreased patient wait times. The TEAM model also appeared to improve patient, physician, and MA satisfaction. We faced many challenges while implementing the new model, which could be further evaluated during wide adoption.
In recent years, we observed that our physicians, nurses, and medical assistants (MAs) appeared to be spending more time on administrative and clerical tasks—including tasks in the exam room with the patient—and less time engaged in direct patient care.1,2 We recognized these factors contribute to burnout and threaten staff retention and anticipated that a new model would improve physician time spent in direct patient care, decrease the demands of administrative tasks, and increase patient, physician, and MA satisfaction.3-6 Burnout, known to affect more than half of US physicians, has a negative impact on quality of care and patient safety and satisfaction.7-11 Improving workflow has been shown to reduce burnout.12
Watertown Regional Medical Center (WRMC) is a small, financially stable integrated delivery system in rural southern Wisconsin, composed of a 90-bed hospital, 10 primary care clinics (7 owned and 3 affiliated), and 24 employed physicians in 9 specialties. Two clinics within WRMC launched a new delivery model, “TEAM (Together Each person Achieves More) Primary Care,” to improve the delivery experience for the entire team, defined as the patient, physician, and MA. New workflows, roles, and responsibilities were designed to reduce cycle time (the total amount of time patients spent in the clinic from check-in to check-out), increase the total time a patient spent with staff (physician and MA or in point-of-care testing and radiology), and reduce the total time a patient spent waiting.13
We describe here WRMC’s experience in developing and implementing workflow improvements as a means of reducing burnout and improving satisfaction.
Continue to: METHODS
METHODS
We selected 2 WRMC sites for TEAM re-engineering based on their experience with quality-improvement projects and perceived likelihood of success with a new transformation initiative. In early 2013, WRMC charged one physician (JM), 2 MAs, the clinic scheduler, and the clinic administrator with designing the details of the model including evaluation metrics. WRMC provided a .5 FTE process engineer (MS) to assist with the design and implementation of the model at no extra expense to the clinics. The model was implemented in late 2013 and into 2014 after regular TEAM planning meetings and observational visits to non-WRMC sites identified as examples of best practices in improving outpatient primary care patient satisfaction: Bellin Health (Green Bay, Wis); ThedaCare (Appleton, Wis); the University of Utah (Salt Lake City); and the University of Wisconsin Health Yahara Clinic (Madison, Wis).
TEAM model
The TEAM model—so named to create top-of-mind awareness of its benefits—increased the MA:MD ratio, maintained consistent team composition so that physician/MA teams learned to work together and become more efficient, and added new MA responsibilities. We trained MAs to assist with documentation in the exam room to ensure that physician time was spent in face-to-face direct patient care.14-20 In these ways, we sought not only to increase patient satisfaction but also to enhance our own “joy in practice,” defined primarily by a high level of work-life satisfaction, a low level of burnout, and a feeling that the medical practice is fulfilling.21
In our traditional model, an MA retrieved the patient from the waiting room, conducted initial assessment in the exam room, and then left the patient to wait for the physician to enter. Once the physician entered and conducted the exam, the patient would be left alone again to wait for the MA to return. In our revised model (TABLE 1), we assigned one MA to each patient from arrival to discharge. After greeting the patient in the waiting room, the MA conducted an initial patient interview in the exam room, then remained in the room with the physician to document the visit. After the physician exited the exam room, the MA completed follow-up orders and provided the patient with a visit summary.

To facilitate consistency throughout the day, we designed a workflow similar to those created in lean models originally designed to increase efficiency in the manufacturing industry (TABLE 2). Visual and electronic cues triggered each step of the process and coordinated the movement of MAs and MDs. Cues included the conventional flag system outside each exam room, an electronic messaging system within the electronic health record (EHR) to indicate when a patient was ready to be seen, and a whiteboard in an area visible to all team members on which we wrote lab and radiology requests.

We experimented with the MA:MD ratio to identify the most effective and efficient team composition. On alternating weeks, we assigned one MA to one MD, 2 MAs to one MD, or 3 MAs to 2 MDs. Additionally, with the 2:1 MA:MD ratio, we varied the visit length in 2 tests; one spanning 30 minutes and the other 20 minutes. The MDs and MAs were seated at side-by-side workstations to make communication easier. We developed protocols and checklists that allowed MAs to enter health maintenance orders and conduct point-of-care testing before the physician entered the room. Such details included immunization management, strep screens, urine analyses, diabetic foot exams, extremity x-ray films, and mammogram and colonoscopy referrals.

Continue to: To prepare MAs...
To prepare MAs, we obtained special permission for team documentation from our Chief Information Officer and developed associated policies and procedures. A physician assistant (PA) trained each MA, introducing the structure and content of subjective, objective, assessment, and plan (SOAP) notes. Training was continuous, as PAs provided feedback when MAs began team documentation. The MAs documented visits using templates, free form, and quick text. We measured visit cycle-time, face time with staff, and patient waiting times. A process engineer with a stopwatch observed and timed the flow (but did not enter the exam room). We also conducted patient interviews immediately post-visit and administered anonymous questionnaires to clinic staff at different phases of the model. Physicians and MAs met weekly to evaluate the design.
We used qualitative interviews of patients, physicians, and MAs to identify the level of satisfaction with the new model. During the first week of implementation, a nurse and our process engineer conducted brief in-person surveys with approximately 20 post-visit patients. Patients, chosen by convenience, were asked if the visit addressed their concerns, whether they left with a thorough understanding of next steps, and if their wait time was acceptable. Twice during the implementation phase, a human resources associate distributed 9-item anonymous questionnaires to staff members during scheduled department meetings.
RESULTS
Times per activity with different MA:MD ratios and visit lengths are shown in TABLE 3. After 6 months, cycle time decreased by a mean of 6 minutes, from 44 to 38 minutes per patient; time with staff increased by a mean of 2 minutes, from 24 to 26 minutes per patient; and wait time decreased by a mean of 7 minutes, from 9 to 2 minutes per patient. We concluded the MA:MD ratio of 3:2 was most efficient because the 2:1 model left MAs with excess non-patient time.

Our delivery model received consistently positive comments from patients. Many expressed gratitude for the extra set of ears and eyes guiding them through the process. One recalled the “old days” when a nurse joined the doctor in the exam room. Another appreciated that both the MA and physician could answer follow-up questions over the phone.
Employee satisfaction
Surveys to assess satisfaction were distributed to all employees whether they were involved in the new model or not. Sixteen employees responded to the pre-implementation questionnaire and 18 responded to the post-implementation one distributed 7 months later. The questionnaires showed an increase in employee satisfaction scores from 3.70 to 3.89 on a 5-point Likert scale, with 5 ranking highest. “I am learning from [Dr. Milford] and understanding things more fully,” wrote one respondent. Another said, “Dr. Milford and his clinical support staff are less stressed.” Individual observations such as, “I can leave sooner with less work left to do,” and “All documentation is done before [the] patient leaves,” reflect the reduction in time that patient records remained open or incomplete. Some physicians reported a reduction in at-home or after-hours work, from about 2 to 4 hours per day to approximately one hour per day.
Continue to: Additional outcomes
Additional outcomes
The TEAM model allowed us to more easily integrate new initiatives into our practice and meet quality metrics by placing needed components within our workflow and checklist. For example, achieving Stage II Meaningful Use measures required that we print and furnish patients with a visit summary and a reminder to access our portal; something we easily incorporated into the MAs’ expanded responsibilities. We also met specific predetermined quality metrics that were part of a payment-withhold program. During the study period, we achieved scores at the 90th percentile and earned back our total withhold.
Finally, more of our patients completed advanced care planning discussions than the other 7 sites in our Honoring Choices Wisconsin cohort. This was achieved not only by integrating the process into our checklist, but because the MAs observed the MD-led patient conversations which they then emulated, presenting the advanced care planning information to patients before or after MD time with the patient.
Errors and defects in care
With ongoing provider guidance and reinforcement, MAs became integral members of the primary care team. They were empowered through protocols to manage and order health maintenance testing and provide needed immunizations. They also began to identify potentially overlooked aspects of care. For example, MAs prompted physicians to retake vital signs, adjust medications, order labs, discuss previous lab results, and pursue specialty referrals or follow-up care.
Billing
Although we tracked billing, the TEAM model was not designed to influence revenue. We noted no significant change in level of evaluation and management billed regardless of staffing ratio. While our panel size increased as we implemented the new process, this change could have been due to normal variation. We do see opportunity to affect future billing by having coders train MAs, which could enhance documentation and increase revenue.
DISCUSSION
The TEAM Primary Care model reduced the time our patients sat unattended, increased our opportunities to meaningfully interact with them, and seemed to reduce our administrative load. By identifying and implementing ways to work as a more cohesive, interconnected unit, we began to address our work as a team rather than as individuals. After implementing the model, we noted several instances where the MAs caught potential errors in care, although we did not consistently track or measure changes in the rate of these occurrences.
Continue to: Achieving these results also came with...
Achieving these results also came with challenges. Investing in and maintaining a new model opened our eyes to unforeseen inconsistencies in our staff profile and to the cost and administrative support needed for implementation. Moreover, our entire team (patients, MAs, and physicians) had to undergo a major cultural shift to adopt a new model.
Personnel variation
We discovered that implementing and sustaining organization change is highly dependent on constancy in human resources. When one team member was on vacation, sick, or leaving the practice, the process tended to fall apart. Hiring replacements and training employees well enough to fill in at a moment’s notice proved difficult. Bringing new employees into this process was also labor intensive. Despite team members being very engaged in change, these staffing inconsistencies caused significant stress and necessitated pauses in the implementation of the new model (reflected in the timeline of our measures). Larger organizational buy-in and support would allow us to hire and train a larger pool of MAs in anticipation of these fluctuations.
Cost
Our small, rural family practice took advantage of WRMC’s Primary Care Transformation project and the half-time process engineer and additional MA they provided. We question whether this model could be implemented without such support. While a process engineer might not prove necessary, expertise in process improvement is vital to help design and measure workflow and to identify opportunities for improvement.
Cultural change
Adopting a new model required asking every member of the team (patient, MA, and physician) to accommodate change and tolerate disruption. We anticipated patients might resist having an additional person in the room. All patients were informed of our new model at the beginning of the visit and told they could opt out. While we did not document patient resistance, JM recalled only 2 patients who expressed a desire not to have the MA present because of personal and sensitive issues. It’s possible some patients did not feel comfortable opting out. But many patients expressed gratitude for having an extra set of ears and eyes to guide them through the visit.
It was more challenging to support MAs as they stepped out of their comfort zone to assist with documentation. It took time for MAs to grow accustomed to the protocols and checklists essential to our workflow. Without protocols, any point-of-care testing that could have been completed at the beginning of the appointment had to be done at the end. This disrupted our workflow and increased patient wait times.
Continue to: We correctly predicted MAs would have...
We correctly predicted MAs would have difficulty documenting the assessment, plan, and medical decision making. We discovered that MAs more easily categorized and articulated information when we reframed the assessment and plan in first-person and placed it under “Patient instructions.” For this to occur, physicians had to learn to accurately articulate their thought process and instructions to the patient.
When training was provided, as previously described, MAs grasped the subjective section quickly. Surprisingly, they had most difficulty understanding terminology within the objective section. In the future, we would avert this problem by working closely with the human resource department. We believe there should be a newly defined position and additional training for MAs in these roles, since duties such as patient-coaching and documentation assistance may warrant separate certification.
Limitations
Our findings should be interpreted in light of several limitations. Implementing the new model was carried out in a single organization. The patients who were selected and agreed to be interviewed may have differed from the patient population as a whole. We did not measure some important outcomes, such as cost effectiveness and patient morbidity. We did not analyze the data to determine whether the apparent improvements in wait time and cycle time were statistically significant. In addition, measurement of any adverse effects was beyond the scope of this study.
Looking forward
The traditional model of physicians working individually with minimal support staff is no longer viable. To echo our co-author (CAS)’s recent statements on physician dissatisfaction, “The days of hero medicine, with the doctor doing it all, belong in the past.”21 The new model appeared to alleviate some administrative burdens and increase physician time with patients. Pressures to achieve quality measures and growing administrative tasks have altered the role and responsibilities of each member of the team.
Any sustainable system must address the larger crisis of physician dissatisfaction.7,22 We cannot focus on a single perspective—patient, physician, or MA—at the expense of the system as a whole. If the health care system is to resolve the epidemic of burnout and physician dissatisfaction, new approaches to patient care must be imagined and realized. Although we faced many challenges in implementing and evaluating the TEAM model, attempts to overcome these challenges appear justified because of our overall favorable impression of it. Innovations like the TEAM Primary Care model may help us improve the well-being of not just our patients but also our health professionals and the health care industry as a whole.
CORRESPONDENCE
James Milford, MD, Three Oaks Health, S.C., 480 Village Walk Lane, Suite F, Johnson Creek, WI 53038; [email protected].
SUPPORT
Although the Watertown Regional Medical Center has provided general funding for its Primary Care Transformation project, no dollars were specifically earmarked for the TEAM Primary Care process. Support for editorial services in preparing this article was provided by Dr. James Milford.
PRIOR PRESENTATIONS
Co-author Michael R. Strasser, MPA, presented this project at the 2015 i-PrACTISE conference in Madison, Wis, April 12-14, 2015. http://www.fammed.wisc.edu/i-practise/. The proceedings were not published or recorded.
ACKNOWLEDGMENT
We thank Annalynn Skipper and Masarah Van Eyck for their valuable edits.
1. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760.
2. McDonald CJ, Callaghan FM, Weissman A, et al. Use of internist’s free time by ambulatory care electronic medical record systems. JAMA Intern Med. 2014;174:1860-1863.
3. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848.
4. Friedberg MW, Chen PG, Van Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. Available at: http://www.rand.org/pubs/research_reports/RR439.html#key-findings. Accessed October 25, 2016.
5. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO study. J Am Med Inform Assoc. 2014;21:e100-e106.
6. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press. 2001.
7. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clinic Proc. 2015;90:1600-1613.
8. DeMatteo MR, Sherbourne CD, Hays RD, et al. Physicians’ characteristics influence patients’ adherence to medical treatment: Results from the Medical Outcomes Study. Health Psychol. 1993;12:93-102.
9. Shanafelt TD, Bradley KA, Wipf JE, et al. Burnout and self-reported patient care in an internal medicine residency program. Ann Intern Med. 2002;136:358-367.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251:995-1000.
11. Haas JS, Cook EF, Puopolo AL, et al. Is the professional satisfaction of general internists associated with patient satisfaction? J Gen Intern Med. 2000;15:122-128.
12. Linzer M, Poplau S, Grossman E, et al. A cluster randomized trial of interventions to improve work conditions and clinician burnout in primary care: results from the Healthy Work Place (HWP) Study. J Gen Intern Med. 2015;30:1105-1011.
13. Ferrer RL, Mody-Bailey P, Jaén CR, et al. A medical assistant-based program to promote healthy behaviors in primary care. Ann Fam Med. 2009;7:504-512.
14. Sinsky CA, Williard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
15. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
16. Hopkins K, Sinsky CA. Team-based care: saving time and improving efficiency. Fam Pract Manag. 2014;21:23-29.
17. Yan C, Rose S, Rothberg MB, et al. Physician, scribe, and patient perspectives on clinical scribes in primary care. J Gen Intern Med. 2016;31:990-995.
18. Misra-Hebert AD, Rabovsky A, Yan C, et al. A team-based model of primary care delivery and physician-patient interaction. Am J Med. 2015;128:1025-1028.
19. Anderson RJ. Optimizing the role of nursing staff to enhance physician productivity: one physician’s journey. Fam Pract Manag. 2013;20:18-22.
20. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008:15:35-40.
21. Sinsky CA. Dissatisfaction among Wisconsin physicians is part of a serious national trend. Wis Med J. 2015;114:132-133.
22. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
1. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760.
2. McDonald CJ, Callaghan FM, Weissman A, et al. Use of internist’s free time by ambulatory care electronic medical record systems. JAMA Intern Med. 2014;174:1860-1863.
3. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848.
4. Friedberg MW, Chen PG, Van Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. Available at: http://www.rand.org/pubs/research_reports/RR439.html#key-findings. Accessed October 25, 2016.
5. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO study. J Am Med Inform Assoc. 2014;21:e100-e106.
6. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press. 2001.
7. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clinic Proc. 2015;90:1600-1613.
8. DeMatteo MR, Sherbourne CD, Hays RD, et al. Physicians’ characteristics influence patients’ adherence to medical treatment: Results from the Medical Outcomes Study. Health Psychol. 1993;12:93-102.
9. Shanafelt TD, Bradley KA, Wipf JE, et al. Burnout and self-reported patient care in an internal medicine residency program. Ann Intern Med. 2002;136:358-367.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251:995-1000.
11. Haas JS, Cook EF, Puopolo AL, et al. Is the professional satisfaction of general internists associated with patient satisfaction? J Gen Intern Med. 2000;15:122-128.
12. Linzer M, Poplau S, Grossman E, et al. A cluster randomized trial of interventions to improve work conditions and clinician burnout in primary care: results from the Healthy Work Place (HWP) Study. J Gen Intern Med. 2015;30:1105-1011.
13. Ferrer RL, Mody-Bailey P, Jaén CR, et al. A medical assistant-based program to promote healthy behaviors in primary care. Ann Fam Med. 2009;7:504-512.
14. Sinsky CA, Williard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
15. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
16. Hopkins K, Sinsky CA. Team-based care: saving time and improving efficiency. Fam Pract Manag. 2014;21:23-29.
17. Yan C, Rose S, Rothberg MB, et al. Physician, scribe, and patient perspectives on clinical scribes in primary care. J Gen Intern Med. 2016;31:990-995.
18. Misra-Hebert AD, Rabovsky A, Yan C, et al. A team-based model of primary care delivery and physician-patient interaction. Am J Med. 2015;128:1025-1028.
19. Anderson RJ. Optimizing the role of nursing staff to enhance physician productivity: one physician’s journey. Fam Pract Manag. 2013;20:18-22.
20. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008:15:35-40.
21. Sinsky CA. Dissatisfaction among Wisconsin physicians is part of a serious national trend. Wis Med J. 2015;114:132-133.
22. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
Standardizing your approach to dizziness and vertigo
Dizziness. Vertigo. These 2 terms are often used interchangeably by patients, with the sensations described as imbalance, lightheadedness, disorientation, presyncope, confusion—among others. While dizziness is a broad term that is often used to describe all the aforementioned sensations, including vertigo, true vertigo (a specific type of dizziness) is defined as the perception of movement within one’s visual field while stationary.1 Because patients are not usually aware of the distinction, their reports of signs and symptoms can cause much confusion for health care providers, thereby delaying a diagnosis.
International studies have reported the prevalence of both dizziness and vertigo to be between 15% and 36%.2,3 Over half of all patients with dizziness and vertigo are cared for by the family physician (FP), and the sensations combined account for approximately 5% of all family medicine visits.4,5 Additionally, between 2.5% and 4% of all emergency department (ED) visits stem from complaints of dizziness and vertigo, with an incidence of up to 25% in those >65 years of age.6,7
Causes of dizziness and vertigo are broad, ranging from the benign to the life-threatening. It has been reported that upwards of 50% of patients presenting to the FP’s office for dizziness leave without a diagnosis.8 Given the confusion surrounding the terms and their broad differential, this review aims to provide FPs with the tools to accurately discern benign from ominous causes.
Nonvestibular benign causes vastly outnumber life-threatening ones
Causes of dizziness are classified as either vestibular (these cause true vertigo) or nonvestibular in origin, with nonvestibular causes being more common.7
Nonvestibular etiologies: Numerous and varied
Nonvestibular causes are broad, spanning many different body systems. Cardiovascular causes of dizziness may include orthostatic hypotension, cardiac arrhythmia, myocardial infarction, and carotid artery stenosis.4,9 Metabolic causes include complications of diabetes such as hypoglycemia and peripheral neuropathy.4,9 Psychiatric conditions such as anxiety, depression, and bipolar disorder can manifest as dizziness, disorientation, or psychogenic vertigo.4,10 Medications including nonsteroidal anti-inflammatory drugs, anticonvulsants, antipsychotics, and sedatives can all contribute to dizziness.11 Other causes of dizziness include Parkinson’s disease, musculoskeletal disorders, and gait disorders.4,9 Especially in the elderly, sensory deficit (peripheral neuropathy), poor vision, and polypharmacy (≥5 medications) are common causes of dizziness.12
Vestibular etiologies of dizziness = true vertigo
Vestibular causes of a patient’s feelings of dizziness manifest as true vertigo and can be categorized as either central (a dysfunction of one or more parts of the central nervous system that help process balance and spatial information or along the pathway where these sensations are interpreted) or peripheral (a dysfunction of the balance organs of the inner ear) in origin.
Central vestibular causes include vertebrobasilar ischemic stroke, vertebrobasilar insufficiency (transient ischemic attack), vestibular migraines, and meningioma of the cerebellopontine angle and posterior fossa.13
Continue to: Peripheral vestibular causes
Peripheral vestibular causes. Benign paroxysmal positional vertigo (BPPV) represents the most common peripheral diagnosis. It is caused by dislodged otoliths in the posterior semicircular canal. While the majority of BPPV cases are idiopathic in nature, up to 15% may result from previous head injury.14 Other peripheral vestibular causes include vestibular neuronitis, viral labyrinthitis, Meniere’s disease, vestibular schwannoma, perilymphatic fistula, superior semicircular canal dehiscence (SSCD), and head trauma (basilar skull fracture).13
Start with a history: Is it dizziness or true vertigo?
The clinical history typically guides the differential diagnosis (FIGURE). Identifying true vertigo from among other sensations helps to limit the differential because true vertigo is caused by vestibular etiologies only. True vertigo is often reported by patients as “seeing the room spin;” this stems from the perception of motion.1 A notable exception is that patients with orthostatic hypotension will often describe spinning sensations lasting seconds to minutes when they rise from a seated or supine position.
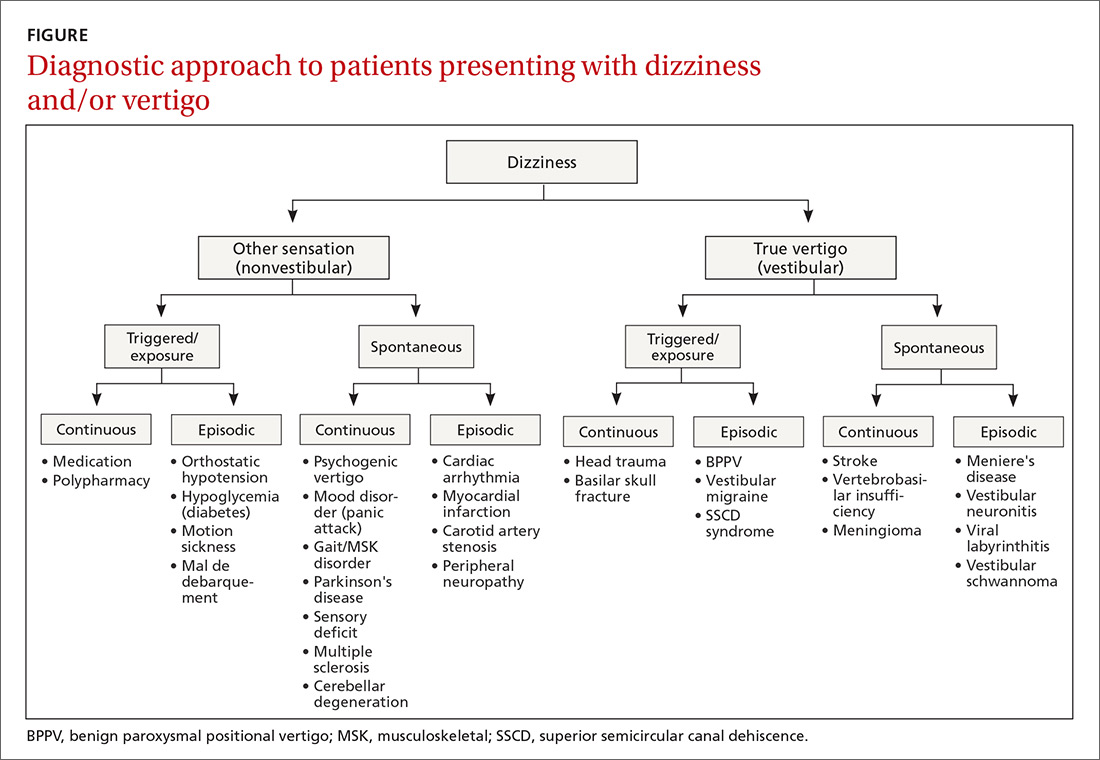
Never depend solely, however, on patient-reported sensations, as not all patients with true vertigo report spinning, and some patients with nonvestibular causes interpret their dizziness as a spinning sensation.15 Therefore, it is important to tease out specifics about the timing, triggers, and associated symptoms in order to further delineate possible causes (TABLE).16
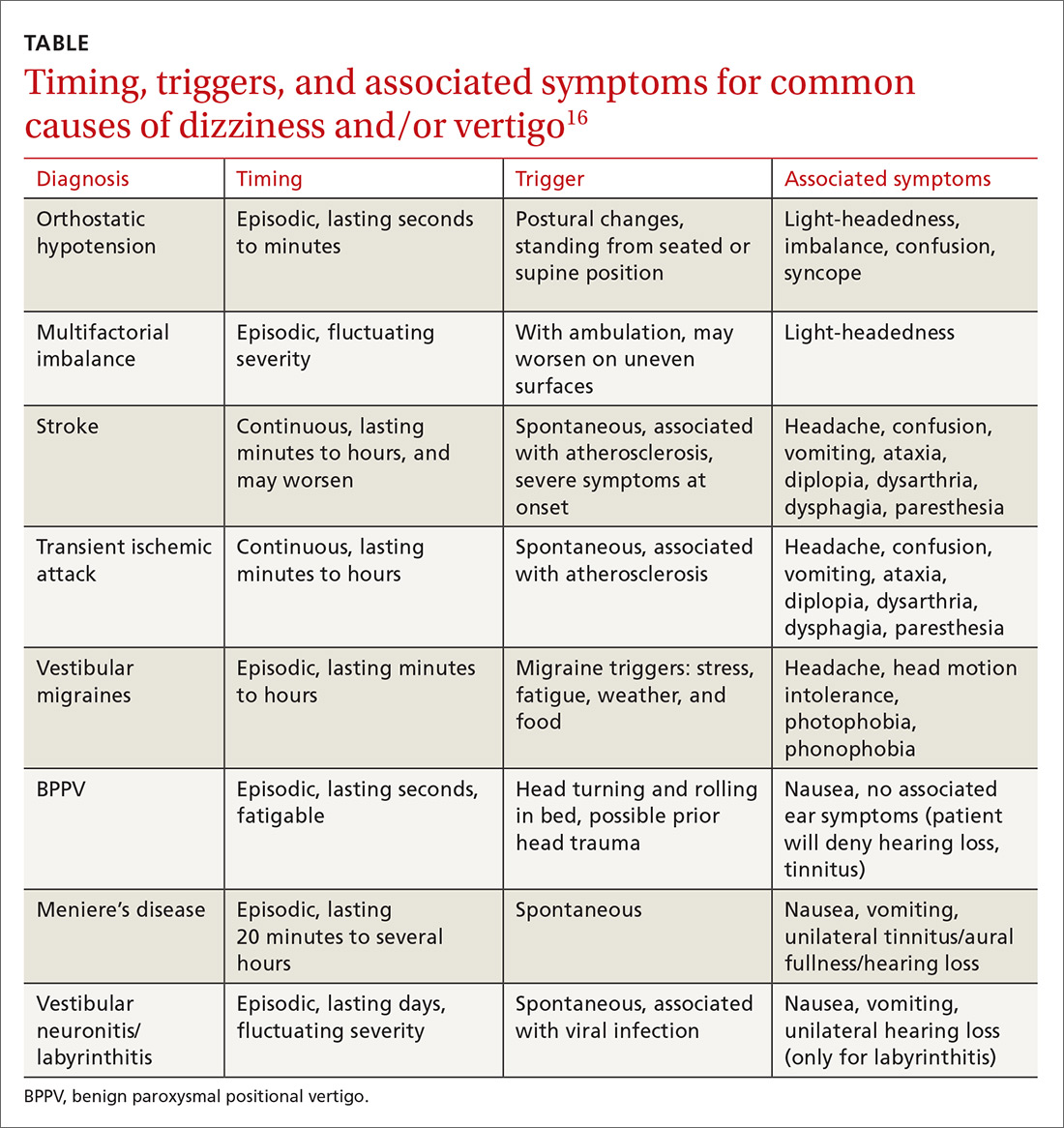
Make a list of current medications. Gather a comprehensive list of current medications, especially from elderly patients, because polypharmacy is a major contributor to dizziness in this population.12 Keep in mind that elderly patients presenting with dizziness/vertigo may have multifactorial balance difficulties, which can be revealed by a detailed history.
Physical exam: May be broad or focused
Given the broad range of causes for dizziness, cardiovascular, head/neck, and neurologic examinations may be performed as part of the work-up, as the clinical history warrants. More typically, time is spent ruling out the following common causes.
Continue to: Orthostatic hypotension
Orthostatic hypotension. Orthostatic vitals are recommended initially in all patients with dizziness, although these may be normal in patients with orthostatic hypotension.17 A diagnosis of orthostatic hypotension can be made with systolic blood pressure decreases of 20 mm Hg or diastolic pressure decreases of ≥10 mm Hg within 3 minutes of standing.18 An increase in heart rate >30 beats per minute after rising from a supine position may indicate autonomic disturbances such as postural orthostatic tachycardia syndrome.19 However, physical examination findings alone are insufficient to make the diagnosis of orthostatic hypotension, and determining the underlying cause of the orthostatic hypotension (dehydration, cardiac dysfunction, pure-autonomic failure, medication adverse effect) is vital.18
BPPV. Perform the Dix-Hallpike maneuver (see https://collections.lib.utah.edu/details?id=177177 for a demonstration of the maneuver) on patients presenting with dizziness with features suggestive of BPPV (eg, attacks of dizziness triggered by head movements).20,21
As BPPV is the most common cause of vestibular dizziness, a negative Dix-Hallpike can be helpful in refining the differential diagnosis.20,21 The maneuver begins with the patient seated, looking directly ahead. To test the left side, ask the patient to turn his/her head 45 degrees to the left. Then direct the patient to lie back, so that the patient’s head is off the edge of the examination table and hyperextended, while maintaining the same head orientation. To test the right side, repeat the procedure with the patient turning his/her head to the right.
Torsional nystagmus is necessary for a positive Dix-Hallpike, which is diagnostic for BPPV. The laterality of BPPV can be determined by paying attention to the fast phase of the torsional nystagmus; the superior pole of the eye beats toward the affected side.14 The patient may report severe dizziness or vertigo during the Dix-Hallpike, but without torsional nystagmus, the test is negative, and the patient does not have BPPV.14
Neurologic causes. Perform a complete neurologic examination in patients who clearly do not have a history of orthostatic hypotension and who have a Dix-Hallpike test that is negative or not indicated.4 Also perform cerebellar testing including rapid-alternating movements, a finger-to-nose test, and a heel-to-shin test. Round out the neurologic exam with an assessment of gait and a Romberg’s test (see https://www.youtube.com/watch?v=U5a4lbmwmOw for a demonstration of Romberg’s test). Romberg’s test is performed by having the patient place his/her feet together with hands at sides and eyes closed. The patient is observed for up to a minute, with a positive test denoted by a loss of balance.
Continue to: Abnormal gait may indicate...
Abnormal gait may indicate peripheral neuropathy, while a positive Romberg’s test suggests involvement of the proprioceptive receptors and/or their pathway.
Central/peripheral vestibular causes. The head impulse, nystagmus, test of skew (HINTS) examination can differentiate between central and peripheral vestibular causes of dizziness and rule out stroke (a central vestibular cause).22 (See https://collections.lib.utah.edu/details?id=177180 for a video demonstrating the steps involved in performing the HINTS examination.) The head impulse (HI) portion of the exam is performed by moving the patient’s head side to side, while having the patient focus on the examiner’s nose. Rapid movements of both eyes (“abnormal” HI) suggest a peripheral etiology, while no eye movement with gaze fixated on the examiner’s nose (“normal” HI) is concerning for stroke or another central cause of vertigo.22
Nystagmus is assessed by having the patient follow the examiner’s finger as it moves in a horizontal direction. Spontaneous horizontal unidirectional nystagmus suggests a peripheral cause, while vertical or torsional bidirectional (direction-changing) nystagmus points to a central cause.22
The test of skew is executed by covering and uncovering each of the patient’s eyes, while asking the patient to look ahead. Vertical deviation of the eye after uncovering suggests a central etiology, more specifically one involving the brainstem.22
Diagnostic testing/imaging has a limited, but pivotal role
There is a limited role for routine laboratory testing in patients with dizziness. However, for those patients with underlying medical conditions (eg, diabetes), which may contribute to the symptoms, routine blood work can be ordered (ie, finger-stick blood glucose test).22
Continue to: More worrisome suspicions
More worrisome suspicions. Patients suspected of cardiac causes should have a full cardiac work-up performed.22 For suspected stroke, brain tumor, or head trauma, specific computed tomography or magnetic resonance imaging can be arranged.22 Carotid doppler can be used if dizziness is suspected to be caused by orthostatic hypotension or a vascular cause.23
Audiologic and vestibular testing. Audiologic testing is not routinely recommended and is only warranted in instances when patients report hearing loss or changes. Referral to an otolaryngologist for vestibular testing is warranted once life-threatening and alternate etiologies have been ruled out, and a vestibular disorder remains at the top of the differential.24
Treatment hinges on cause and may be multifaceted
Treatment hinges on the specific cause of the patient’s dizziness and may involve useful maneuvers, medication, physiotherapy, or perhaps even surgery.
Employ a particle repositioning maneuver for BPPV
A positive Dix-Hallpike test should prompt the use of a particle repositioning maneuver (PRM) to treat BPPV.21 The goal of PRMs, such as the Epley maneuver (see https://www.youtube.com/watch?v=9SLm76jQg3g for a demonstration of this maneuver), is to move the head in such a way as to return displaced otoliths in the semicircular canal back to the utricle. The Epley maneuver is specific for treating posterior semicircular canal BPPV, which is the most common variant.
Performing the Epley maneuver. To perform the Epley PRM for correction of an otolith in the left posterior semicircular canal, ask the patient to sit and look straight ahead. Lay the patient back, while asking the patient to turn his/her head 45 degrees to the left side. Then ask the patient to turn his/her head 45 degrees to the right side. Instruct the patient to maintain the same 45-degree head orientation, while rolling over to his/her right shoulder, ending in the right decubitus position. Conclude the maneuver by having the patient sit up.
Continue to: Performing the barbecue roll maneuver
Performing the barbecue roll maneuver. Different PRMs exist to treat less common variants of BPPV, including the “barbecue roll” maneuver for horizontal BPPV (see https://www.youtube.com/watch?v=mwTmM6uF5yA for a demonstration of this maneuver).25 The barbecue roll maneuver is initiated with the patient looking ahead and lying back. For a left-sided horizontal canal otolith, the patient first turns to the left decubitus position, then moves clockwise to the right decubitus position, stopping at each position for approximately 20 seconds, all while maintaining a straight head position. The patient then turns clockwise into a prone position, pausing, and finally turning into the left decubitus position again. The maneuver is completed with the patient sitting up.
Medications are used to treat symptoms and/or underlying causes
Adjustments in antihypertensives can be made in cases of orthostatic hypotension.17 Antiemetics (ondansetron, promethazine, metoclopramide), antihistamines (meclizine, dimenhydrinate, diphenhydramine), and benzodiazepines (lorazepam, diazepam) may be used during acute and brief vertiginous episodes to decrease symptom severity after central causes have been ruled out.26,27 However, patients with BPPV should avoid these medications as they may blunt central compensation and increase the risk of falls.27 Research has shown betahistine to improve vertigo control only in patients with Meniere’s disease and only when taken regularly and prophylactically.28 Therefore, do not prescribe betahistine for all other causes of dizziness/vertigo.28
Consider physiotherapy
All patients with dizziness/vertigo, and particularly those presenting with primary balance concerns, may benefit from vestibular rehabilitation therapy (VRT). This is an exercise-based program focusing on habituation of dizziness and improvement of postural stability.29 VRT can improve dizziness associated with central and peripheral vestibular lesions, vertigo of uncertain etiology, and psychogenic vertigo.30 Typically, the vestibular physiotherapist will provide home exercises for the patient, reducing the cost and inconvenience of attending multiple sessions.
Surgery and referrals
Referrals for surgery are rare and are typically reserved for refractory causes of vestibular disease, such as Meniere’s disease, BPPV, SSCD syndrome.31
Referral to the ED is warranted for symptom control if an acute vertiginous episode is refractory to initial management. Emergent or urgent neurology consultation is indicated for suspected or confirmed central disorders. Urgent cardiology referral is recommended for patients with symptoms of presyncope/syncope, arrhythmia, or persistent orthostatic hypotension after conservative management. Outpatient referral to an otolaryngologist is warranted if the patient has failed a course of balance physiotherapy, has a persistently positive Dix-Hallpike test after a PRM and vestibular/balance physiotherapy, or has asymmetric hearing loss.
Continue to: Management starts with primary and secondary prevention
Management starts with primary and secondary prevention
Patient education is essential for avoiding potential triggers of dizziness. Patients with orthostatic hypotension should be educated about the need to correct the underlying mechanism, including the need for adequate hydration and recognition of offending medications and contributory conditions/situations (caffeine, heat, standing quickly).17 Encouraging balance maintenance through exercise and physiotherapy can help with gait and musculoskeletal disorders, and reducing harmful habits (smoking, poor diet, no exercise) can lead to overall improved cardiovascular health.32 Advise those with Meniere’s disease to avoid potential triggers such as caffeine, high sodium foods, and alcohol.33
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Otology/Neurotology, Assistant Professor, Department of Otolaryngology, Queen's University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected].
1. Bisdorff A, Von Brevern M, Lempert T, et al. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19:1-13.
2. Mendel B, Bergenius J, Langius-Eklöf A. Dizziness: a common, troublesome symptom but often treatable. J Vestib Res. 2010;20:391-398.
3. Gopinath B, McMahon CM, Rochtchina E, et al. Dizziness and vertigo in an older population: the Blue Mountains prospective cross‐sectional study. Clin Otolaryngol. 2009;34:552-556.
4. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-368.
5. Sloan PD. Dizziness in primary care. Results from the National Ambulatory Care Survey. Fam Pract. 1989;29:33-38.
6. Kerber KA, Meurer WJ, West BT, et al. Dizziness presentations in US emergency departments, 1995–2004. Acad Emerg Med. 2008;15:744-750.
7. Newman-Toker DE, Hsieh YH, Camargo CA Jr, et al. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. 2008;83:765-775.
8. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: vertigo and dizziness. Can Fam Physician. 2007;53:1959.
9. Chan Y. Differential diagnosis of dizziness. Curr Opin in Otolaryngol Head Neck Surg. 2009;17:200-203.
10. Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of chronic dizziness. Arch Otolaryngol Head Neck Surg. 2007;133:170-176.
11. Kutz JW Jr. The dizzy patient. Med Clin North Am. 2010;94:989-1002.
12. Jahn K, Kressig RW, Bridenbaugh SA, et al. Dizziness and unstable gait in old age: etiology, diagnosis and treatment. Dtsch Ärztebl Int. 2015;112:387-393.
13. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
14. Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). CMAJ. 2003;169:681-693.
15. Newman-Toker DE, Dy FJ, Stanton VA, et al. How often is dizziness from primary cardiovascular disease true vertigo? A systematic review. J Gen Intern Med. 2008;23:2087-2094.
16. Newman-Toker DE, Edlow JA. TiTrATE: a novel, evidence-based approach to diagnosing acute dizziness and vertigo. Neurol Clin. 2015;33:577-599.
17. Shibao C, Lipsitz LA, Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. J Clin Hypertens (Greenwich). 2013;15:147-153.
18. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125-126.
19. Agarwal AK, Garg R, Ritch A, et al. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478-480.
20. Halker RB, Barrs DM, Wellik KE, et al. Establishing a diagnosis of benign paroxysmal positional vertigo through the dix-hallpike and side-lying maneuvers: a critically appraised topic. Neurologist. 2008;14:201-204.
21. Hilton MP, Pinder DK. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2014;(12):CD003162.
22. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome. Stroke. 2009;40:3504-3510.
23. Hamaguchi T, Iwasa K, Okino S, et al. Carotid duplex ultrasonography during head-up tilt in patients with orthostatic hypotension. Eur Neurol. 2007;57:219-222.
24. Canadian Society of Otolaryngology - Head & Neck Surgery. Five Things Physicians and Patients Should Question [Internet]. Choosing Wisely Canada. 2016 [cited 2017 August 17]. Available at: https://choosingwiselycanada.org/wp-content/uploads/2017/02/Hospital-medicine.pdf. Accessed August 30, 2017.
25. Lee SH, Kim JS. Benign paroxysmal positional vertigo. J Clin Neurol. 2010;6:51-63.
26. Zatonski T, Temporale H, Holanowska J, et al. Current views of treatment of vertigo and dizziness. J Med Diagn Meth. 2014;2:150.
27. Wipperman J. Dizziness and vertigo. Prim Care Clin Office Pract. 2014;41:115-131
28. Murdin L, Hussain K, Schilder AG. Betahistine for symptoms of vertigo. Cochrane Database Syst Rev. 2016;(6):CD010696.
29. Han BI, Song HS, Kim JS. Vestibular rehabilitation therapy: review of indications, mechanisms, and key exercises. J Clin Neurol. 2011;7:184-196.
30. Jung JY, Kim JS, Chung PS, et al. Effect of vestibular rehabilitation on dizziness in the elderly. Am J Otolaryngol. 2009;30:295-299.
31. Semaan MT, Megerian CA. Meniere’s disease: a challenging and relentless disorder. Otolaryngol Clin North Am. 2011;44:383-403.
32. Pirker W, Katzenschlager R. Gait disorders in adults and the elderly. Wien Klin Wochenschr. 2017;129:81-95.
33. Kirby SE, Yardley L. Physical and psychological triggers for attacks in Ménière’s disease: the patient perspective. Psychother Psychosom. 2012;81:396-398.
Dizziness. Vertigo. These 2 terms are often used interchangeably by patients, with the sensations described as imbalance, lightheadedness, disorientation, presyncope, confusion—among others. While dizziness is a broad term that is often used to describe all the aforementioned sensations, including vertigo, true vertigo (a specific type of dizziness) is defined as the perception of movement within one’s visual field while stationary.1 Because patients are not usually aware of the distinction, their reports of signs and symptoms can cause much confusion for health care providers, thereby delaying a diagnosis.
International studies have reported the prevalence of both dizziness and vertigo to be between 15% and 36%.2,3 Over half of all patients with dizziness and vertigo are cared for by the family physician (FP), and the sensations combined account for approximately 5% of all family medicine visits.4,5 Additionally, between 2.5% and 4% of all emergency department (ED) visits stem from complaints of dizziness and vertigo, with an incidence of up to 25% in those >65 years of age.6,7
Causes of dizziness and vertigo are broad, ranging from the benign to the life-threatening. It has been reported that upwards of 50% of patients presenting to the FP’s office for dizziness leave without a diagnosis.8 Given the confusion surrounding the terms and their broad differential, this review aims to provide FPs with the tools to accurately discern benign from ominous causes.
Nonvestibular benign causes vastly outnumber life-threatening ones
Causes of dizziness are classified as either vestibular (these cause true vertigo) or nonvestibular in origin, with nonvestibular causes being more common.7
Nonvestibular etiologies: Numerous and varied
Nonvestibular causes are broad, spanning many different body systems. Cardiovascular causes of dizziness may include orthostatic hypotension, cardiac arrhythmia, myocardial infarction, and carotid artery stenosis.4,9 Metabolic causes include complications of diabetes such as hypoglycemia and peripheral neuropathy.4,9 Psychiatric conditions such as anxiety, depression, and bipolar disorder can manifest as dizziness, disorientation, or psychogenic vertigo.4,10 Medications including nonsteroidal anti-inflammatory drugs, anticonvulsants, antipsychotics, and sedatives can all contribute to dizziness.11 Other causes of dizziness include Parkinson’s disease, musculoskeletal disorders, and gait disorders.4,9 Especially in the elderly, sensory deficit (peripheral neuropathy), poor vision, and polypharmacy (≥5 medications) are common causes of dizziness.12
Vestibular etiologies of dizziness = true vertigo
Vestibular causes of a patient’s feelings of dizziness manifest as true vertigo and can be categorized as either central (a dysfunction of one or more parts of the central nervous system that help process balance and spatial information or along the pathway where these sensations are interpreted) or peripheral (a dysfunction of the balance organs of the inner ear) in origin.
Central vestibular causes include vertebrobasilar ischemic stroke, vertebrobasilar insufficiency (transient ischemic attack), vestibular migraines, and meningioma of the cerebellopontine angle and posterior fossa.13
Continue to: Peripheral vestibular causes
Peripheral vestibular causes. Benign paroxysmal positional vertigo (BPPV) represents the most common peripheral diagnosis. It is caused by dislodged otoliths in the posterior semicircular canal. While the majority of BPPV cases are idiopathic in nature, up to 15% may result from previous head injury.14 Other peripheral vestibular causes include vestibular neuronitis, viral labyrinthitis, Meniere’s disease, vestibular schwannoma, perilymphatic fistula, superior semicircular canal dehiscence (SSCD), and head trauma (basilar skull fracture).13
Start with a history: Is it dizziness or true vertigo?
The clinical history typically guides the differential diagnosis (FIGURE). Identifying true vertigo from among other sensations helps to limit the differential because true vertigo is caused by vestibular etiologies only. True vertigo is often reported by patients as “seeing the room spin;” this stems from the perception of motion.1 A notable exception is that patients with orthostatic hypotension will often describe spinning sensations lasting seconds to minutes when they rise from a seated or supine position.

Never depend solely, however, on patient-reported sensations, as not all patients with true vertigo report spinning, and some patients with nonvestibular causes interpret their dizziness as a spinning sensation.15 Therefore, it is important to tease out specifics about the timing, triggers, and associated symptoms in order to further delineate possible causes (TABLE).16

Make a list of current medications. Gather a comprehensive list of current medications, especially from elderly patients, because polypharmacy is a major contributor to dizziness in this population.12 Keep in mind that elderly patients presenting with dizziness/vertigo may have multifactorial balance difficulties, which can be revealed by a detailed history.
Physical exam: May be broad or focused
Given the broad range of causes for dizziness, cardiovascular, head/neck, and neurologic examinations may be performed as part of the work-up, as the clinical history warrants. More typically, time is spent ruling out the following common causes.
Continue to: Orthostatic hypotension
Orthostatic hypotension. Orthostatic vitals are recommended initially in all patients with dizziness, although these may be normal in patients with orthostatic hypotension.17 A diagnosis of orthostatic hypotension can be made with systolic blood pressure decreases of 20 mm Hg or diastolic pressure decreases of ≥10 mm Hg within 3 minutes of standing.18 An increase in heart rate >30 beats per minute after rising from a supine position may indicate autonomic disturbances such as postural orthostatic tachycardia syndrome.19 However, physical examination findings alone are insufficient to make the diagnosis of orthostatic hypotension, and determining the underlying cause of the orthostatic hypotension (dehydration, cardiac dysfunction, pure-autonomic failure, medication adverse effect) is vital.18
BPPV. Perform the Dix-Hallpike maneuver (see https://collections.lib.utah.edu/details?id=177177 for a demonstration of the maneuver) on patients presenting with dizziness with features suggestive of BPPV (eg, attacks of dizziness triggered by head movements).20,21
As BPPV is the most common cause of vestibular dizziness, a negative Dix-Hallpike can be helpful in refining the differential diagnosis.20,21 The maneuver begins with the patient seated, looking directly ahead. To test the left side, ask the patient to turn his/her head 45 degrees to the left. Then direct the patient to lie back, so that the patient’s head is off the edge of the examination table and hyperextended, while maintaining the same head orientation. To test the right side, repeat the procedure with the patient turning his/her head to the right.
Torsional nystagmus is necessary for a positive Dix-Hallpike, which is diagnostic for BPPV. The laterality of BPPV can be determined by paying attention to the fast phase of the torsional nystagmus; the superior pole of the eye beats toward the affected side.14 The patient may report severe dizziness or vertigo during the Dix-Hallpike, but without torsional nystagmus, the test is negative, and the patient does not have BPPV.14
Neurologic causes. Perform a complete neurologic examination in patients who clearly do not have a history of orthostatic hypotension and who have a Dix-Hallpike test that is negative or not indicated.4 Also perform cerebellar testing including rapid-alternating movements, a finger-to-nose test, and a heel-to-shin test. Round out the neurologic exam with an assessment of gait and a Romberg’s test (see https://www.youtube.com/watch?v=U5a4lbmwmOw for a demonstration of Romberg’s test). Romberg’s test is performed by having the patient place his/her feet together with hands at sides and eyes closed. The patient is observed for up to a minute, with a positive test denoted by a loss of balance.
Continue to: Abnormal gait may indicate...
Abnormal gait may indicate peripheral neuropathy, while a positive Romberg’s test suggests involvement of the proprioceptive receptors and/or their pathway.
Central/peripheral vestibular causes. The head impulse, nystagmus, test of skew (HINTS) examination can differentiate between central and peripheral vestibular causes of dizziness and rule out stroke (a central vestibular cause).22 (See https://collections.lib.utah.edu/details?id=177180 for a video demonstrating the steps involved in performing the HINTS examination.) The head impulse (HI) portion of the exam is performed by moving the patient’s head side to side, while having the patient focus on the examiner’s nose. Rapid movements of both eyes (“abnormal” HI) suggest a peripheral etiology, while no eye movement with gaze fixated on the examiner’s nose (“normal” HI) is concerning for stroke or another central cause of vertigo.22
Nystagmus is assessed by having the patient follow the examiner’s finger as it moves in a horizontal direction. Spontaneous horizontal unidirectional nystagmus suggests a peripheral cause, while vertical or torsional bidirectional (direction-changing) nystagmus points to a central cause.22
The test of skew is executed by covering and uncovering each of the patient’s eyes, while asking the patient to look ahead. Vertical deviation of the eye after uncovering suggests a central etiology, more specifically one involving the brainstem.22
Diagnostic testing/imaging has a limited, but pivotal role
There is a limited role for routine laboratory testing in patients with dizziness. However, for those patients with underlying medical conditions (eg, diabetes), which may contribute to the symptoms, routine blood work can be ordered (ie, finger-stick blood glucose test).22
Continue to: More worrisome suspicions
More worrisome suspicions. Patients suspected of cardiac causes should have a full cardiac work-up performed.22 For suspected stroke, brain tumor, or head trauma, specific computed tomography or magnetic resonance imaging can be arranged.22 Carotid doppler can be used if dizziness is suspected to be caused by orthostatic hypotension or a vascular cause.23
Audiologic and vestibular testing. Audiologic testing is not routinely recommended and is only warranted in instances when patients report hearing loss or changes. Referral to an otolaryngologist for vestibular testing is warranted once life-threatening and alternate etiologies have been ruled out, and a vestibular disorder remains at the top of the differential.24
Treatment hinges on cause and may be multifaceted
Treatment hinges on the specific cause of the patient’s dizziness and may involve useful maneuvers, medication, physiotherapy, or perhaps even surgery.
Employ a particle repositioning maneuver for BPPV
A positive Dix-Hallpike test should prompt the use of a particle repositioning maneuver (PRM) to treat BPPV.21 The goal of PRMs, such as the Epley maneuver (see https://www.youtube.com/watch?v=9SLm76jQg3g for a demonstration of this maneuver), is to move the head in such a way as to return displaced otoliths in the semicircular canal back to the utricle. The Epley maneuver is specific for treating posterior semicircular canal BPPV, which is the most common variant.
Performing the Epley maneuver. To perform the Epley PRM for correction of an otolith in the left posterior semicircular canal, ask the patient to sit and look straight ahead. Lay the patient back, while asking the patient to turn his/her head 45 degrees to the left side. Then ask the patient to turn his/her head 45 degrees to the right side. Instruct the patient to maintain the same 45-degree head orientation, while rolling over to his/her right shoulder, ending in the right decubitus position. Conclude the maneuver by having the patient sit up.
Continue to: Performing the barbecue roll maneuver
Performing the barbecue roll maneuver. Different PRMs exist to treat less common variants of BPPV, including the “barbecue roll” maneuver for horizontal BPPV (see https://www.youtube.com/watch?v=mwTmM6uF5yA for a demonstration of this maneuver).25 The barbecue roll maneuver is initiated with the patient looking ahead and lying back. For a left-sided horizontal canal otolith, the patient first turns to the left decubitus position, then moves clockwise to the right decubitus position, stopping at each position for approximately 20 seconds, all while maintaining a straight head position. The patient then turns clockwise into a prone position, pausing, and finally turning into the left decubitus position again. The maneuver is completed with the patient sitting up.
Medications are used to treat symptoms and/or underlying causes
Adjustments in antihypertensives can be made in cases of orthostatic hypotension.17 Antiemetics (ondansetron, promethazine, metoclopramide), antihistamines (meclizine, dimenhydrinate, diphenhydramine), and benzodiazepines (lorazepam, diazepam) may be used during acute and brief vertiginous episodes to decrease symptom severity after central causes have been ruled out.26,27 However, patients with BPPV should avoid these medications as they may blunt central compensation and increase the risk of falls.27 Research has shown betahistine to improve vertigo control only in patients with Meniere’s disease and only when taken regularly and prophylactically.28 Therefore, do not prescribe betahistine for all other causes of dizziness/vertigo.28
Consider physiotherapy
All patients with dizziness/vertigo, and particularly those presenting with primary balance concerns, may benefit from vestibular rehabilitation therapy (VRT). This is an exercise-based program focusing on habituation of dizziness and improvement of postural stability.29 VRT can improve dizziness associated with central and peripheral vestibular lesions, vertigo of uncertain etiology, and psychogenic vertigo.30 Typically, the vestibular physiotherapist will provide home exercises for the patient, reducing the cost and inconvenience of attending multiple sessions.
Surgery and referrals
Referrals for surgery are rare and are typically reserved for refractory causes of vestibular disease, such as Meniere’s disease, BPPV, SSCD syndrome.31
Referral to the ED is warranted for symptom control if an acute vertiginous episode is refractory to initial management. Emergent or urgent neurology consultation is indicated for suspected or confirmed central disorders. Urgent cardiology referral is recommended for patients with symptoms of presyncope/syncope, arrhythmia, or persistent orthostatic hypotension after conservative management. Outpatient referral to an otolaryngologist is warranted if the patient has failed a course of balance physiotherapy, has a persistently positive Dix-Hallpike test after a PRM and vestibular/balance physiotherapy, or has asymmetric hearing loss.
Continue to: Management starts with primary and secondary prevention
Management starts with primary and secondary prevention
Patient education is essential for avoiding potential triggers of dizziness. Patients with orthostatic hypotension should be educated about the need to correct the underlying mechanism, including the need for adequate hydration and recognition of offending medications and contributory conditions/situations (caffeine, heat, standing quickly).17 Encouraging balance maintenance through exercise and physiotherapy can help with gait and musculoskeletal disorders, and reducing harmful habits (smoking, poor diet, no exercise) can lead to overall improved cardiovascular health.32 Advise those with Meniere’s disease to avoid potential triggers such as caffeine, high sodium foods, and alcohol.33
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Otology/Neurotology, Assistant Professor, Department of Otolaryngology, Queen's University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected].
Dizziness. Vertigo. These 2 terms are often used interchangeably by patients, with the sensations described as imbalance, lightheadedness, disorientation, presyncope, confusion—among others. While dizziness is a broad term that is often used to describe all the aforementioned sensations, including vertigo, true vertigo (a specific type of dizziness) is defined as the perception of movement within one’s visual field while stationary.1 Because patients are not usually aware of the distinction, their reports of signs and symptoms can cause much confusion for health care providers, thereby delaying a diagnosis.
International studies have reported the prevalence of both dizziness and vertigo to be between 15% and 36%.2,3 Over half of all patients with dizziness and vertigo are cared for by the family physician (FP), and the sensations combined account for approximately 5% of all family medicine visits.4,5 Additionally, between 2.5% and 4% of all emergency department (ED) visits stem from complaints of dizziness and vertigo, with an incidence of up to 25% in those >65 years of age.6,7
Causes of dizziness and vertigo are broad, ranging from the benign to the life-threatening. It has been reported that upwards of 50% of patients presenting to the FP’s office for dizziness leave without a diagnosis.8 Given the confusion surrounding the terms and their broad differential, this review aims to provide FPs with the tools to accurately discern benign from ominous causes.
Nonvestibular benign causes vastly outnumber life-threatening ones
Causes of dizziness are classified as either vestibular (these cause true vertigo) or nonvestibular in origin, with nonvestibular causes being more common.7
Nonvestibular etiologies: Numerous and varied
Nonvestibular causes are broad, spanning many different body systems. Cardiovascular causes of dizziness may include orthostatic hypotension, cardiac arrhythmia, myocardial infarction, and carotid artery stenosis.4,9 Metabolic causes include complications of diabetes such as hypoglycemia and peripheral neuropathy.4,9 Psychiatric conditions such as anxiety, depression, and bipolar disorder can manifest as dizziness, disorientation, or psychogenic vertigo.4,10 Medications including nonsteroidal anti-inflammatory drugs, anticonvulsants, antipsychotics, and sedatives can all contribute to dizziness.11 Other causes of dizziness include Parkinson’s disease, musculoskeletal disorders, and gait disorders.4,9 Especially in the elderly, sensory deficit (peripheral neuropathy), poor vision, and polypharmacy (≥5 medications) are common causes of dizziness.12
Vestibular etiologies of dizziness = true vertigo
Vestibular causes of a patient’s feelings of dizziness manifest as true vertigo and can be categorized as either central (a dysfunction of one or more parts of the central nervous system that help process balance and spatial information or along the pathway where these sensations are interpreted) or peripheral (a dysfunction of the balance organs of the inner ear) in origin.
Central vestibular causes include vertebrobasilar ischemic stroke, vertebrobasilar insufficiency (transient ischemic attack), vestibular migraines, and meningioma of the cerebellopontine angle and posterior fossa.13
Continue to: Peripheral vestibular causes
Peripheral vestibular causes. Benign paroxysmal positional vertigo (BPPV) represents the most common peripheral diagnosis. It is caused by dislodged otoliths in the posterior semicircular canal. While the majority of BPPV cases are idiopathic in nature, up to 15% may result from previous head injury.14 Other peripheral vestibular causes include vestibular neuronitis, viral labyrinthitis, Meniere’s disease, vestibular schwannoma, perilymphatic fistula, superior semicircular canal dehiscence (SSCD), and head trauma (basilar skull fracture).13
Start with a history: Is it dizziness or true vertigo?
The clinical history typically guides the differential diagnosis (FIGURE). Identifying true vertigo from among other sensations helps to limit the differential because true vertigo is caused by vestibular etiologies only. True vertigo is often reported by patients as “seeing the room spin;” this stems from the perception of motion.1 A notable exception is that patients with orthostatic hypotension will often describe spinning sensations lasting seconds to minutes when they rise from a seated or supine position.

Never depend solely, however, on patient-reported sensations, as not all patients with true vertigo report spinning, and some patients with nonvestibular causes interpret their dizziness as a spinning sensation.15 Therefore, it is important to tease out specifics about the timing, triggers, and associated symptoms in order to further delineate possible causes (TABLE).16

Make a list of current medications. Gather a comprehensive list of current medications, especially from elderly patients, because polypharmacy is a major contributor to dizziness in this population.12 Keep in mind that elderly patients presenting with dizziness/vertigo may have multifactorial balance difficulties, which can be revealed by a detailed history.
Physical exam: May be broad or focused
Given the broad range of causes for dizziness, cardiovascular, head/neck, and neurologic examinations may be performed as part of the work-up, as the clinical history warrants. More typically, time is spent ruling out the following common causes.
Continue to: Orthostatic hypotension
Orthostatic hypotension. Orthostatic vitals are recommended initially in all patients with dizziness, although these may be normal in patients with orthostatic hypotension.17 A diagnosis of orthostatic hypotension can be made with systolic blood pressure decreases of 20 mm Hg or diastolic pressure decreases of ≥10 mm Hg within 3 minutes of standing.18 An increase in heart rate >30 beats per minute after rising from a supine position may indicate autonomic disturbances such as postural orthostatic tachycardia syndrome.19 However, physical examination findings alone are insufficient to make the diagnosis of orthostatic hypotension, and determining the underlying cause of the orthostatic hypotension (dehydration, cardiac dysfunction, pure-autonomic failure, medication adverse effect) is vital.18
BPPV. Perform the Dix-Hallpike maneuver (see https://collections.lib.utah.edu/details?id=177177 for a demonstration of the maneuver) on patients presenting with dizziness with features suggestive of BPPV (eg, attacks of dizziness triggered by head movements).20,21
As BPPV is the most common cause of vestibular dizziness, a negative Dix-Hallpike can be helpful in refining the differential diagnosis.20,21 The maneuver begins with the patient seated, looking directly ahead. To test the left side, ask the patient to turn his/her head 45 degrees to the left. Then direct the patient to lie back, so that the patient’s head is off the edge of the examination table and hyperextended, while maintaining the same head orientation. To test the right side, repeat the procedure with the patient turning his/her head to the right.
Torsional nystagmus is necessary for a positive Dix-Hallpike, which is diagnostic for BPPV. The laterality of BPPV can be determined by paying attention to the fast phase of the torsional nystagmus; the superior pole of the eye beats toward the affected side.14 The patient may report severe dizziness or vertigo during the Dix-Hallpike, but without torsional nystagmus, the test is negative, and the patient does not have BPPV.14
Neurologic causes. Perform a complete neurologic examination in patients who clearly do not have a history of orthostatic hypotension and who have a Dix-Hallpike test that is negative or not indicated.4 Also perform cerebellar testing including rapid-alternating movements, a finger-to-nose test, and a heel-to-shin test. Round out the neurologic exam with an assessment of gait and a Romberg’s test (see https://www.youtube.com/watch?v=U5a4lbmwmOw for a demonstration of Romberg’s test). Romberg’s test is performed by having the patient place his/her feet together with hands at sides and eyes closed. The patient is observed for up to a minute, with a positive test denoted by a loss of balance.
Continue to: Abnormal gait may indicate...
Abnormal gait may indicate peripheral neuropathy, while a positive Romberg’s test suggests involvement of the proprioceptive receptors and/or their pathway.
Central/peripheral vestibular causes. The head impulse, nystagmus, test of skew (HINTS) examination can differentiate between central and peripheral vestibular causes of dizziness and rule out stroke (a central vestibular cause).22 (See https://collections.lib.utah.edu/details?id=177180 for a video demonstrating the steps involved in performing the HINTS examination.) The head impulse (HI) portion of the exam is performed by moving the patient’s head side to side, while having the patient focus on the examiner’s nose. Rapid movements of both eyes (“abnormal” HI) suggest a peripheral etiology, while no eye movement with gaze fixated on the examiner’s nose (“normal” HI) is concerning for stroke or another central cause of vertigo.22
Nystagmus is assessed by having the patient follow the examiner’s finger as it moves in a horizontal direction. Spontaneous horizontal unidirectional nystagmus suggests a peripheral cause, while vertical or torsional bidirectional (direction-changing) nystagmus points to a central cause.22
The test of skew is executed by covering and uncovering each of the patient’s eyes, while asking the patient to look ahead. Vertical deviation of the eye after uncovering suggests a central etiology, more specifically one involving the brainstem.22
Diagnostic testing/imaging has a limited, but pivotal role
There is a limited role for routine laboratory testing in patients with dizziness. However, for those patients with underlying medical conditions (eg, diabetes), which may contribute to the symptoms, routine blood work can be ordered (ie, finger-stick blood glucose test).22
Continue to: More worrisome suspicions
More worrisome suspicions. Patients suspected of cardiac causes should have a full cardiac work-up performed.22 For suspected stroke, brain tumor, or head trauma, specific computed tomography or magnetic resonance imaging can be arranged.22 Carotid doppler can be used if dizziness is suspected to be caused by orthostatic hypotension or a vascular cause.23
Audiologic and vestibular testing. Audiologic testing is not routinely recommended and is only warranted in instances when patients report hearing loss or changes. Referral to an otolaryngologist for vestibular testing is warranted once life-threatening and alternate etiologies have been ruled out, and a vestibular disorder remains at the top of the differential.24
Treatment hinges on cause and may be multifaceted
Treatment hinges on the specific cause of the patient’s dizziness and may involve useful maneuvers, medication, physiotherapy, or perhaps even surgery.
Employ a particle repositioning maneuver for BPPV
A positive Dix-Hallpike test should prompt the use of a particle repositioning maneuver (PRM) to treat BPPV.21 The goal of PRMs, such as the Epley maneuver (see https://www.youtube.com/watch?v=9SLm76jQg3g for a demonstration of this maneuver), is to move the head in such a way as to return displaced otoliths in the semicircular canal back to the utricle. The Epley maneuver is specific for treating posterior semicircular canal BPPV, which is the most common variant.
Performing the Epley maneuver. To perform the Epley PRM for correction of an otolith in the left posterior semicircular canal, ask the patient to sit and look straight ahead. Lay the patient back, while asking the patient to turn his/her head 45 degrees to the left side. Then ask the patient to turn his/her head 45 degrees to the right side. Instruct the patient to maintain the same 45-degree head orientation, while rolling over to his/her right shoulder, ending in the right decubitus position. Conclude the maneuver by having the patient sit up.
Continue to: Performing the barbecue roll maneuver
Performing the barbecue roll maneuver. Different PRMs exist to treat less common variants of BPPV, including the “barbecue roll” maneuver for horizontal BPPV (see https://www.youtube.com/watch?v=mwTmM6uF5yA for a demonstration of this maneuver).25 The barbecue roll maneuver is initiated with the patient looking ahead and lying back. For a left-sided horizontal canal otolith, the patient first turns to the left decubitus position, then moves clockwise to the right decubitus position, stopping at each position for approximately 20 seconds, all while maintaining a straight head position. The patient then turns clockwise into a prone position, pausing, and finally turning into the left decubitus position again. The maneuver is completed with the patient sitting up.
Medications are used to treat symptoms and/or underlying causes
Adjustments in antihypertensives can be made in cases of orthostatic hypotension.17 Antiemetics (ondansetron, promethazine, metoclopramide), antihistamines (meclizine, dimenhydrinate, diphenhydramine), and benzodiazepines (lorazepam, diazepam) may be used during acute and brief vertiginous episodes to decrease symptom severity after central causes have been ruled out.26,27 However, patients with BPPV should avoid these medications as they may blunt central compensation and increase the risk of falls.27 Research has shown betahistine to improve vertigo control only in patients with Meniere’s disease and only when taken regularly and prophylactically.28 Therefore, do not prescribe betahistine for all other causes of dizziness/vertigo.28
Consider physiotherapy
All patients with dizziness/vertigo, and particularly those presenting with primary balance concerns, may benefit from vestibular rehabilitation therapy (VRT). This is an exercise-based program focusing on habituation of dizziness and improvement of postural stability.29 VRT can improve dizziness associated with central and peripheral vestibular lesions, vertigo of uncertain etiology, and psychogenic vertigo.30 Typically, the vestibular physiotherapist will provide home exercises for the patient, reducing the cost and inconvenience of attending multiple sessions.
Surgery and referrals
Referrals for surgery are rare and are typically reserved for refractory causes of vestibular disease, such as Meniere’s disease, BPPV, SSCD syndrome.31
Referral to the ED is warranted for symptom control if an acute vertiginous episode is refractory to initial management. Emergent or urgent neurology consultation is indicated for suspected or confirmed central disorders. Urgent cardiology referral is recommended for patients with symptoms of presyncope/syncope, arrhythmia, or persistent orthostatic hypotension after conservative management. Outpatient referral to an otolaryngologist is warranted if the patient has failed a course of balance physiotherapy, has a persistently positive Dix-Hallpike test after a PRM and vestibular/balance physiotherapy, or has asymmetric hearing loss.
Continue to: Management starts with primary and secondary prevention
Management starts with primary and secondary prevention
Patient education is essential for avoiding potential triggers of dizziness. Patients with orthostatic hypotension should be educated about the need to correct the underlying mechanism, including the need for adequate hydration and recognition of offending medications and contributory conditions/situations (caffeine, heat, standing quickly).17 Encouraging balance maintenance through exercise and physiotherapy can help with gait and musculoskeletal disorders, and reducing harmful habits (smoking, poor diet, no exercise) can lead to overall improved cardiovascular health.32 Advise those with Meniere’s disease to avoid potential triggers such as caffeine, high sodium foods, and alcohol.33
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Otology/Neurotology, Assistant Professor, Department of Otolaryngology, Queen's University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected].
1. Bisdorff A, Von Brevern M, Lempert T, et al. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19:1-13.
2. Mendel B, Bergenius J, Langius-Eklöf A. Dizziness: a common, troublesome symptom but often treatable. J Vestib Res. 2010;20:391-398.
3. Gopinath B, McMahon CM, Rochtchina E, et al. Dizziness and vertigo in an older population: the Blue Mountains prospective cross‐sectional study. Clin Otolaryngol. 2009;34:552-556.
4. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-368.
5. Sloan PD. Dizziness in primary care. Results from the National Ambulatory Care Survey. Fam Pract. 1989;29:33-38.
6. Kerber KA, Meurer WJ, West BT, et al. Dizziness presentations in US emergency departments, 1995–2004. Acad Emerg Med. 2008;15:744-750.
7. Newman-Toker DE, Hsieh YH, Camargo CA Jr, et al. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. 2008;83:765-775.
8. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: vertigo and dizziness. Can Fam Physician. 2007;53:1959.
9. Chan Y. Differential diagnosis of dizziness. Curr Opin in Otolaryngol Head Neck Surg. 2009;17:200-203.
10. Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of chronic dizziness. Arch Otolaryngol Head Neck Surg. 2007;133:170-176.
11. Kutz JW Jr. The dizzy patient. Med Clin North Am. 2010;94:989-1002.
12. Jahn K, Kressig RW, Bridenbaugh SA, et al. Dizziness and unstable gait in old age: etiology, diagnosis and treatment. Dtsch Ärztebl Int. 2015;112:387-393.
13. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
14. Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). CMAJ. 2003;169:681-693.
15. Newman-Toker DE, Dy FJ, Stanton VA, et al. How often is dizziness from primary cardiovascular disease true vertigo? A systematic review. J Gen Intern Med. 2008;23:2087-2094.
16. Newman-Toker DE, Edlow JA. TiTrATE: a novel, evidence-based approach to diagnosing acute dizziness and vertigo. Neurol Clin. 2015;33:577-599.
17. Shibao C, Lipsitz LA, Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. J Clin Hypertens (Greenwich). 2013;15:147-153.
18. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125-126.
19. Agarwal AK, Garg R, Ritch A, et al. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478-480.
20. Halker RB, Barrs DM, Wellik KE, et al. Establishing a diagnosis of benign paroxysmal positional vertigo through the dix-hallpike and side-lying maneuvers: a critically appraised topic. Neurologist. 2008;14:201-204.
21. Hilton MP, Pinder DK. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2014;(12):CD003162.
22. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome. Stroke. 2009;40:3504-3510.
23. Hamaguchi T, Iwasa K, Okino S, et al. Carotid duplex ultrasonography during head-up tilt in patients with orthostatic hypotension. Eur Neurol. 2007;57:219-222.
24. Canadian Society of Otolaryngology - Head & Neck Surgery. Five Things Physicians and Patients Should Question [Internet]. Choosing Wisely Canada. 2016 [cited 2017 August 17]. Available at: https://choosingwiselycanada.org/wp-content/uploads/2017/02/Hospital-medicine.pdf. Accessed August 30, 2017.
25. Lee SH, Kim JS. Benign paroxysmal positional vertigo. J Clin Neurol. 2010;6:51-63.
26. Zatonski T, Temporale H, Holanowska J, et al. Current views of treatment of vertigo and dizziness. J Med Diagn Meth. 2014;2:150.
27. Wipperman J. Dizziness and vertigo. Prim Care Clin Office Pract. 2014;41:115-131
28. Murdin L, Hussain K, Schilder AG. Betahistine for symptoms of vertigo. Cochrane Database Syst Rev. 2016;(6):CD010696.
29. Han BI, Song HS, Kim JS. Vestibular rehabilitation therapy: review of indications, mechanisms, and key exercises. J Clin Neurol. 2011;7:184-196.
30. Jung JY, Kim JS, Chung PS, et al. Effect of vestibular rehabilitation on dizziness in the elderly. Am J Otolaryngol. 2009;30:295-299.
31. Semaan MT, Megerian CA. Meniere’s disease: a challenging and relentless disorder. Otolaryngol Clin North Am. 2011;44:383-403.
32. Pirker W, Katzenschlager R. Gait disorders in adults and the elderly. Wien Klin Wochenschr. 2017;129:81-95.
33. Kirby SE, Yardley L. Physical and psychological triggers for attacks in Ménière’s disease: the patient perspective. Psychother Psychosom. 2012;81:396-398.
1. Bisdorff A, Von Brevern M, Lempert T, et al. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19:1-13.
2. Mendel B, Bergenius J, Langius-Eklöf A. Dizziness: a common, troublesome symptom but often treatable. J Vestib Res. 2010;20:391-398.
3. Gopinath B, McMahon CM, Rochtchina E, et al. Dizziness and vertigo in an older population: the Blue Mountains prospective cross‐sectional study. Clin Otolaryngol. 2009;34:552-556.
4. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82:361-368.
5. Sloan PD. Dizziness in primary care. Results from the National Ambulatory Care Survey. Fam Pract. 1989;29:33-38.
6. Kerber KA, Meurer WJ, West BT, et al. Dizziness presentations in US emergency departments, 1995–2004. Acad Emerg Med. 2008;15:744-750.
7. Newman-Toker DE, Hsieh YH, Camargo CA Jr, et al. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. 2008;83:765-775.
8. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: vertigo and dizziness. Can Fam Physician. 2007;53:1959.
9. Chan Y. Differential diagnosis of dizziness. Curr Opin in Otolaryngol Head Neck Surg. 2009;17:200-203.
10. Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of chronic dizziness. Arch Otolaryngol Head Neck Surg. 2007;133:170-176.
11. Kutz JW Jr. The dizzy patient. Med Clin North Am. 2010;94:989-1002.
12. Jahn K, Kressig RW, Bridenbaugh SA, et al. Dizziness and unstable gait in old age: etiology, diagnosis and treatment. Dtsch Ärztebl Int. 2015;112:387-393.
13. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
14. Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV). CMAJ. 2003;169:681-693.
15. Newman-Toker DE, Dy FJ, Stanton VA, et al. How often is dizziness from primary cardiovascular disease true vertigo? A systematic review. J Gen Intern Med. 2008;23:2087-2094.
16. Newman-Toker DE, Edlow JA. TiTrATE: a novel, evidence-based approach to diagnosing acute dizziness and vertigo. Neurol Clin. 2015;33:577-599.
17. Shibao C, Lipsitz LA, Biaggioni I. ASH position paper: evaluation and treatment of orthostatic hypotension. J Clin Hypertens (Greenwich). 2013;15:147-153.
18. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125-126.
19. Agarwal AK, Garg R, Ritch A, et al. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83:478-480.
20. Halker RB, Barrs DM, Wellik KE, et al. Establishing a diagnosis of benign paroxysmal positional vertigo through the dix-hallpike and side-lying maneuvers: a critically appraised topic. Neurologist. 2008;14:201-204.
21. Hilton MP, Pinder DK. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2014;(12):CD003162.
22. Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome. Stroke. 2009;40:3504-3510.
23. Hamaguchi T, Iwasa K, Okino S, et al. Carotid duplex ultrasonography during head-up tilt in patients with orthostatic hypotension. Eur Neurol. 2007;57:219-222.
24. Canadian Society of Otolaryngology - Head & Neck Surgery. Five Things Physicians and Patients Should Question [Internet]. Choosing Wisely Canada. 2016 [cited 2017 August 17]. Available at: https://choosingwiselycanada.org/wp-content/uploads/2017/02/Hospital-medicine.pdf. Accessed August 30, 2017.
25. Lee SH, Kim JS. Benign paroxysmal positional vertigo. J Clin Neurol. 2010;6:51-63.
26. Zatonski T, Temporale H, Holanowska J, et al. Current views of treatment of vertigo and dizziness. J Med Diagn Meth. 2014;2:150.
27. Wipperman J. Dizziness and vertigo. Prim Care Clin Office Pract. 2014;41:115-131
28. Murdin L, Hussain K, Schilder AG. Betahistine for symptoms of vertigo. Cochrane Database Syst Rev. 2016;(6):CD010696.
29. Han BI, Song HS, Kim JS. Vestibular rehabilitation therapy: review of indications, mechanisms, and key exercises. J Clin Neurol. 2011;7:184-196.
30. Jung JY, Kim JS, Chung PS, et al. Effect of vestibular rehabilitation on dizziness in the elderly. Am J Otolaryngol. 2009;30:295-299.
31. Semaan MT, Megerian CA. Meniere’s disease: a challenging and relentless disorder. Otolaryngol Clin North Am. 2011;44:383-403.
32. Pirker W, Katzenschlager R. Gait disorders in adults and the elderly. Wien Klin Wochenschr. 2017;129:81-95.
33. Kirby SE, Yardley L. Physical and psychological triggers for attacks in Ménière’s disease: the patient perspective. Psychother Psychosom. 2012;81:396-398.
From The Journal of Family Practice | 2018;67(8):490-492,495-498.
PRACTICE RECOMMENDATIONS
› Employ the Dix-Hallpike maneuver to diagnose patients presenting with dizziness with features suggestive of benign paroxysmal positional vertigo (BPPV). A
› Use the head impulse, nystagmus, test of skew (HINTS) examination to differentiate between central and peripheral vestibular causes of dizziness and rule out stroke. B
› Prescribe betahistine only for patients with Meniere’s disease and not for patients with other causes of dizziness and/or vertigo. B
› Rely on antiemetics, antihistamines, and benzodiazepines to manage acute and brief episodes of vertigo, but not to treat BPPV because they blunt central compensation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Beat the heat: Identification and Tx of heat-related illness
Heat-related illnesses can affect people of any age who are subjected to extreme heat and humidity regardless of physical fitness level or baseline health status. The most serious of the heat-related illnesses is heat stroke. Prompt identification, early initiation of cooling measures (including cold-water immersion [CWI]), and transport to a higher level of care, when appropriate, are imperative. This article reviews heat-related illness identification, as well as management strategies.
Heat-related illnesses: From the benign to the severe
Some of the less severe forms of heat-related illness include heat cramps (which are due to dehydration and salt loss), heat rash, and heat edema. Heat rash and heat edema are benign. Heat rash typically resolves with cooler clothing and a cooler environment. Heat edema tends to improve after sleeping in a cooler environment with legs elevated. Heat syncope is the result of decreased cerebral perfusion due to fluid loss and vasodilation that results in a distributive hypovolemia. It commonly occurs after vigorous exercise when the athlete is standing still.
Heat exhaustion requires a more careful clinical assessment. It is the inability to continue activity in the heat, often with weakness and collapse. Also due to salt and water losses, it results in cardiovascular output that is insufficient to meet the circulatory and metabolic demands of the body. The body temperature is often elevated but <40° C (104° F), vomiting can occur, and mild central nervous system (CNS) dysfunction may be present.
Heat stroke is the most severe form of heat-related illness and can be life-threatening.1
It is important to understand that these heat-related illnesses do not progress along a continuum. Patients develop heat stroke without having had milder forms of heat illness, and patients with a milder type of heat illness usually do not progress to heat stroke.
Heat stroke: Definition, types, risk factors
Heat stroke is defined as a core body temperature ≥40° C (104° F) with CNS dysfunction in the setting of environmental heat stress. The mortality rate can reach over 50%.2-6
There are 2 main types of heat stroke: exertional heat stroke and nonexertional (classic) heat stroke. Exertional heat stroke more commonly affects healthy, young people, such as athletes or military personnel. Classic (nonexertional) heat stroke patients are typically elderly and/or have a chronic illness, although occasionally it involves children who are unable to escape from a hot environment.5,7 While exertional heat stroke typically develops over a period of a few hours in participants of prolonged activities, such as marathons, classic heat stroke in the elderly typically develops over a period of days in the setting of high environmental temperatures. In both conditions, there is an inability to maintain a normal body temperature leading to CNS dysregulation with altered mental status and often multisystem organ dysfunction.7
Continue to: Risk factors
Risk factors. Heat-related illness can affect patients of all ages and levels of physical fitness; however, certain factors place patients at increased risk. These include physical deconditioning, dehydration, high levels of exercise intensity, obesity, elevated environmental temperatures, sleep deprivation, certain medications, alcohol and drug abuse, concurrent illness, and wearing excessive clothing or equipment. It is imperative that severe cases of heat illness be identified early and treatment be initiated rapidly, as delays in cooling can significantly increase the fatality rate.5
Management: First suspect the diagnosis
Health care providers must first suspect heat-related illness and then accurately diagnose it. It is important to differentiate heat-related illness from syncope, cardiac abnormalities, gastroenteritis, hypoglycemia, and other entities that require alternate management. For cases of collapse, syncope or near-syncope, or altered mental status during exertion, heat stroke should be the default diagnosis until proven otherwise.
Obtain a core body temperature. While attending to airway, breathing, and circulation, obtain a core body temperature. Rectal (or esophageal) core temperatures provide a reliable reading that can assist in determining the severity of the heat illness. Axillary, tympanic, temporal, oral, and skin temperatures are affected by environmental factors and are not accurate determinants of core body temperature.8
Once heat stroke is diagnosed, the physician must immediately initiate cooling by removing clothing, placing the patient in the shade or an air-conditioned area, and beginning aggressive cooling measures (more on this in a bit). While field management requires an accurate diagnosis of the severity of a patient’s heat-related illness, one should not delay treatment in order to obtain a rectal temperature.
When treating the milder forms of heat illness, administer oral or intravenous (IV) isotonic fluids. For heat cramps, stretching the affected muscle can help. For heat syncope, lying the patient down and elevating the legs restores perfusion. Patients with heat exhaustion will require some cooling measures such as relocation to a shaded area, removal of excess clothing, and the use of cold towels, along with hydration and elevation of the feet.
Continue to: Cooling techniques for heat stroke
Cooling techniques for heat stroke
In order to adequately cool a patient suffering from heat stroke, health care providers must create a gradient for heat to escape the body through the skin into the environment by conduction, convection, or evaporation.3 Cooling heat stroke patients to less than 40° C (104° F) within 30 minutes after collapse decreases the fatality rate to almost zero.8
CWI comes out on top. CWI, also called an ice-bath, is typically performed in the field. The patient is submerged up to the neck in a tub containing ice and water. Circulating the water and ice mixture helps accelerate cooling.
There have been differences in opinion regarding which cooling method is superior3 (TABLE 13,8,9). Traditionally, there were some concerns that CWI might actually increase body temperature via peripheral vasoconstriction and shivering. But current research suggests that for exertional heat stroke, CWI to promote conductive cooling is the most effective strategy.3,8,10,11 A review of cooling rates in healthy hyperthermic athletes and heat stroke victims showed that ice-water immersion or CWI at 1° to 14° C (35.6°-57.2° F) is superior to all other types of cooling, including ice packs, fans, and partial-body ice-water immersion.10
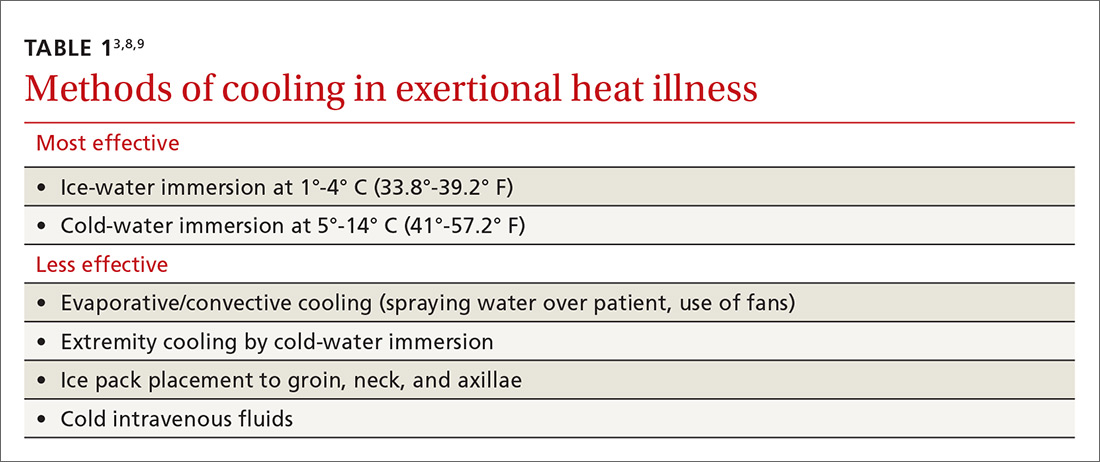
Furthermore, a 2015 meta-analysis looking at optimal procedures for cooling found that CWI cooled patients twice as fast as passive cooling (without any treatment).11 When cooling with CWI, core temperature drops about 0.2° C/min (0.36° F/min).10 Therefore, the temperature can be expected to drop about 1° C (1.8° F) for every 5 minutes of immersion. When unable to monitor a rectal temperature continuously, 10 to 15 minutes of immersion should get most patients below 40° C (104° F).
Extremity cooling. While CWI is the standard for cases of exertional heat illness, whole-body immersion is not always possible. In such cases, extremity cooling can be an effective body cooling method for exertional heat-related illness.12 Research has shown evaporative and convective cooling methods to have benefits for nonexertional heat-related illnesses.3,8,9 These methods usually involve directing air currents over exposed skin and spraying water on the affected individual.3
Contine to: Guidlines for transport
Guidelines for transport: Cool first, transport second
Most patients suspected of suffering from heat stroke should be transported to a hospital for further evaluation because of the high morbidity and mortality rates associated with it. However, cooling techniques should be implemented while awaiting transport. The current standard is “cool first, transport second.”7 Cooling interventions should continue in the ambulance if the core body temperature is still elevated. Techniques that can be used include the use of air conditioning, convective methods, and administration of IV fluids. As previously discussed, core body temperature should be continuously monitored. Cooling measures should be discontinued only when the patient’s rectal temperature reaches 38.9° C (102° F). Overly aggressive prehospital cooling beyond this point can result in prolonged hypothermia as well as cardiac arrhythmias.6
Monitoring and further evaluation
Monitoring patients with heat-related illness can be difficult, especially when utilizing CWI, as this may limit the ability to use devices such as a cardiac monitor or to continuously monitor rectal temperature. Beyond lowering core body temperature to below 39° C (102.2° F), early evaluation and treatment of other organ systems is vital, keeping in mind that these patients may develop multisystem organ failure. The initial work-up is listed in TABLE 2.
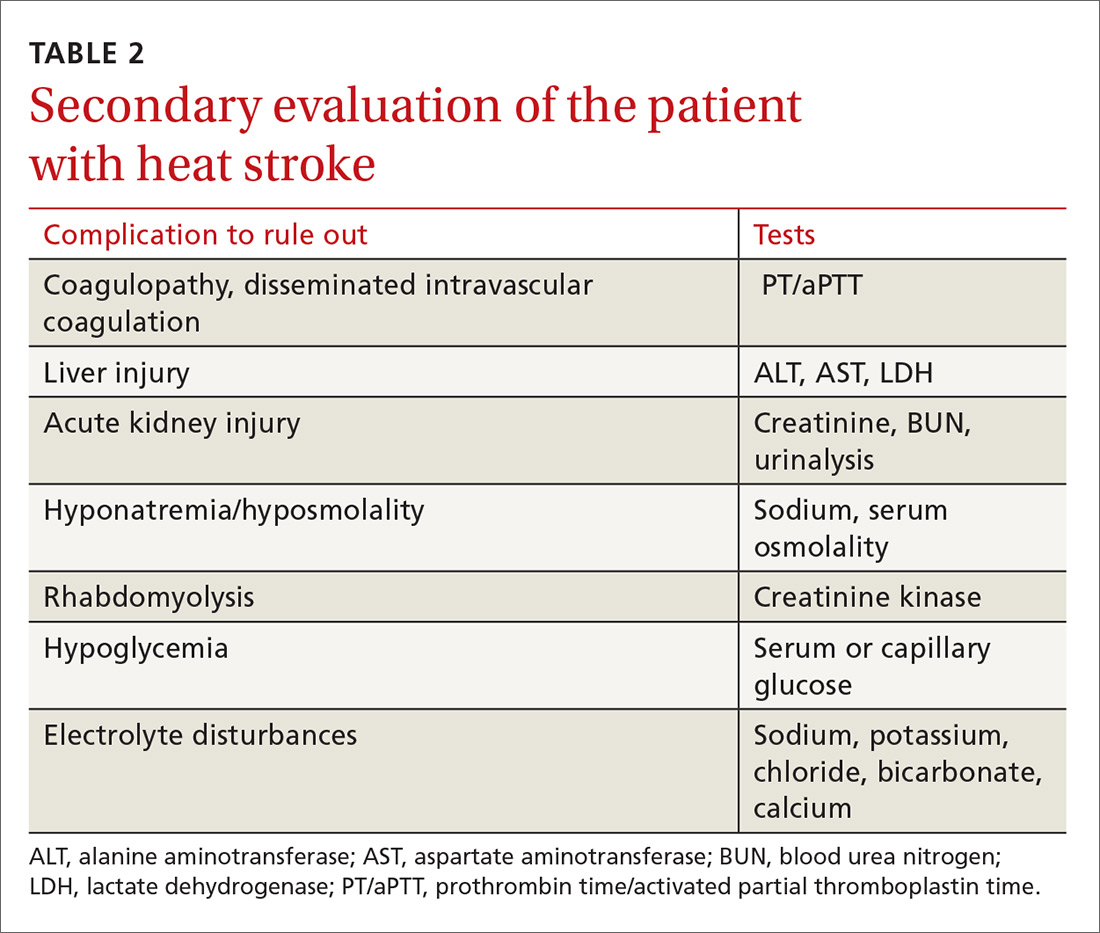
Depending on the severity of the injury and whether you suspect another diagnosis at work, additional studies may include urine output monitoring with a Foley catheter, electrocardiogram, chest radiograph, toxicology screen, a serum lactate level, and cardiac biomarkers.
Imaging. When evaluating for heat stroke, it usually isn’t necessary to obtain head imaging initially, as there are rarely abnormal findings in the early stages. Imaging may be obtained, however, if there is concern about a head injury or if neurologic abnormalities persist into later stages of treatment.5
Pharmacologic agents have not been shown to be of benefit in the treatment of heat-related illness. While dantrolene is commonly used in the treatment of neuroleptic malignant syndrome and malignant hyperthermia, the literature has not described any benefit associated with this agent in relation to heat-related illness. The same goes for antipyretics. Researchers have hypothesized that the reason these agents are ineffective is because body temperature is raised via a different mechanism in these conditions vs heat stroke.3
Continue to: Prevention
Prevention: Modifications and acclimatization are key
People who know they will be exposed to extreme heat should attempt to modify activities. There are many predisposing risk factors ranging from fever and illness to fatigue and dehydration. Risks can be minimized with physiologic adaptation through acclimatization, as well as making various behavioral changes such as adjusting activities, ensuring adequate hydration, and wearing appropriate clothing.13
Certain types of equipment, such as football helmets, can increase the risk of heat-related illness because they prevent heat exchange; however, the benefits sometimes outweigh the risks. With this in mind, consider modification of clothing and equipment if possible.1
In order to prevent heat-related illness, individuals should prehydrate prior to an event and replace fluids orally in order to prevent a >2% loss in body weight. Greater than a 2% loss directly correlates with increased core temperatures during exercise.1
Care should also be taken to perform regular physical activity prior to extreme heat exposure.1 Heat acclimatization takes place when a person’s body adapts to a hotter climate than they are accustomed to. This process can take up to 2 weeks, but once heat acclimation is accomplished, the person will have undergone physical changes, such as reduced metabolic heat production, which will decrease the risk of heat-related illness.13
Return to activity: Customize the approach
Each heat-related injury case is different; thus, return to activity should be individualized. In patients whose heat injury was believed to be secondary to a modifiable risk factor, efforts should be made to correct the predisposing factors that placed the patient at increased risk in the first place.
Additionally, the patient should allow sufficient time to recover. Guidelines recommend at least 1 to 2 weeks recovery before return to activity after heat stroke.8 Moreover, a graded return to activity, starting in a cool environment, is recommended. Gradual introduction of activity in the heat with close monitoring can help with acclimatization and help identify participants who continue to have cooling dysregulation. In the military and among athletes, tools such as heat-tolerance testing can be used to gauge the person’s readiness to return to play or duty.8 Heat tolerance testing is performed in a lab using continuous core temperature monitoring while having the subject exercise in a heated room.
CORRESPONDENCE
Scott Kinkade, MD, EdD, MA303 Medical Sciences Building, DC032.00, Columbia, MO 65212; [email protected].
1. Lipman GS, Eifling KP, Ellis MA, et al. Wilderness Medical Society practice guidelines for the prevention and treatment of heat-related illness: 2014 update. Wilderness Environ Med. 2014;25(4 Suppl):S55-S65.
2. Update: Heat injuries, active component, U.S. Armed Forces, 2014. MSMR. 2015;22:17-20.
3. Gaudio FG, Grissom CK. Cooling methods in heat stroke. J Emerg Med. 2016;50:607-616.
4. Hess JJ, Saha S, Luber G. Summertime acute heat illness in U.S. emergency departments from 2006 through 2010: analysis of a nationally representative sample. Environ Health Perspect. 2014;122:1209-1215.
5. People’s Liberation Army Professional Committee of Critical Care Medicine. Expert consensus on standardized diagnosis and treatment for heat stroke. Mil Med Res. 2016;3:1.
6. Stewart TE, Whitford AC. Dangers of prehospital cooling: a case report of afterdrop in a patient with exertional heat stroke. J Emerg Med. 2015;49:630-633.
7. Chan YK, Mamat M. Management of heat stroke. Trends Anaesthesia Crit Care. 2015;5:65-69.
8. Casa DJ, Armstrong LE, Kenny GP, et al. Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012;11:115-123.
9. Demartini JK, Casa DJ, Stearns R, et al. Effectiveness of cold water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc. 2015;47:240-245.
10. Casa DJ, McDermott BP, Lee EC, et al. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35:141-149.
11. Zhang Y, Davis JK, Casa DJ, et al. Optimizing cold water immersion for exercise-induced hyperthermia: a meta-analysis. Med Sci Sports Exerc. 2015;47:2464-2472.
12. DeGroot DW, Kenefick RW, Sawka MN. Impact of arm immersion cooling during ranger training on exertional heat illness and treatment costs. Mil Med. 2015;180:1178-1183.
13. Epstein Y, Druyan A, Heled Y. Heat injury prevention—a military perspective. J Strength Cond Res. 2012;26 (suppl 2):S82-S86.
Heat-related illnesses can affect people of any age who are subjected to extreme heat and humidity regardless of physical fitness level or baseline health status. The most serious of the heat-related illnesses is heat stroke. Prompt identification, early initiation of cooling measures (including cold-water immersion [CWI]), and transport to a higher level of care, when appropriate, are imperative. This article reviews heat-related illness identification, as well as management strategies.
Heat-related illnesses: From the benign to the severe
Some of the less severe forms of heat-related illness include heat cramps (which are due to dehydration and salt loss), heat rash, and heat edema. Heat rash and heat edema are benign. Heat rash typically resolves with cooler clothing and a cooler environment. Heat edema tends to improve after sleeping in a cooler environment with legs elevated. Heat syncope is the result of decreased cerebral perfusion due to fluid loss and vasodilation that results in a distributive hypovolemia. It commonly occurs after vigorous exercise when the athlete is standing still.
Heat exhaustion requires a more careful clinical assessment. It is the inability to continue activity in the heat, often with weakness and collapse. Also due to salt and water losses, it results in cardiovascular output that is insufficient to meet the circulatory and metabolic demands of the body. The body temperature is often elevated but <40° C (104° F), vomiting can occur, and mild central nervous system (CNS) dysfunction may be present.
Heat stroke is the most severe form of heat-related illness and can be life-threatening.1
It is important to understand that these heat-related illnesses do not progress along a continuum. Patients develop heat stroke without having had milder forms of heat illness, and patients with a milder type of heat illness usually do not progress to heat stroke.
Heat stroke: Definition, types, risk factors
Heat stroke is defined as a core body temperature ≥40° C (104° F) with CNS dysfunction in the setting of environmental heat stress. The mortality rate can reach over 50%.2-6
There are 2 main types of heat stroke: exertional heat stroke and nonexertional (classic) heat stroke. Exertional heat stroke more commonly affects healthy, young people, such as athletes or military personnel. Classic (nonexertional) heat stroke patients are typically elderly and/or have a chronic illness, although occasionally it involves children who are unable to escape from a hot environment.5,7 While exertional heat stroke typically develops over a period of a few hours in participants of prolonged activities, such as marathons, classic heat stroke in the elderly typically develops over a period of days in the setting of high environmental temperatures. In both conditions, there is an inability to maintain a normal body temperature leading to CNS dysregulation with altered mental status and often multisystem organ dysfunction.7
Continue to: Risk factors
Risk factors. Heat-related illness can affect patients of all ages and levels of physical fitness; however, certain factors place patients at increased risk. These include physical deconditioning, dehydration, high levels of exercise intensity, obesity, elevated environmental temperatures, sleep deprivation, certain medications, alcohol and drug abuse, concurrent illness, and wearing excessive clothing or equipment. It is imperative that severe cases of heat illness be identified early and treatment be initiated rapidly, as delays in cooling can significantly increase the fatality rate.5
Management: First suspect the diagnosis
Health care providers must first suspect heat-related illness and then accurately diagnose it. It is important to differentiate heat-related illness from syncope, cardiac abnormalities, gastroenteritis, hypoglycemia, and other entities that require alternate management. For cases of collapse, syncope or near-syncope, or altered mental status during exertion, heat stroke should be the default diagnosis until proven otherwise.
Obtain a core body temperature. While attending to airway, breathing, and circulation, obtain a core body temperature. Rectal (or esophageal) core temperatures provide a reliable reading that can assist in determining the severity of the heat illness. Axillary, tympanic, temporal, oral, and skin temperatures are affected by environmental factors and are not accurate determinants of core body temperature.8
Once heat stroke is diagnosed, the physician must immediately initiate cooling by removing clothing, placing the patient in the shade or an air-conditioned area, and beginning aggressive cooling measures (more on this in a bit). While field management requires an accurate diagnosis of the severity of a patient’s heat-related illness, one should not delay treatment in order to obtain a rectal temperature.
When treating the milder forms of heat illness, administer oral or intravenous (IV) isotonic fluids. For heat cramps, stretching the affected muscle can help. For heat syncope, lying the patient down and elevating the legs restores perfusion. Patients with heat exhaustion will require some cooling measures such as relocation to a shaded area, removal of excess clothing, and the use of cold towels, along with hydration and elevation of the feet.
Continue to: Cooling techniques for heat stroke
Cooling techniques for heat stroke
In order to adequately cool a patient suffering from heat stroke, health care providers must create a gradient for heat to escape the body through the skin into the environment by conduction, convection, or evaporation.3 Cooling heat stroke patients to less than 40° C (104° F) within 30 minutes after collapse decreases the fatality rate to almost zero.8
CWI comes out on top. CWI, also called an ice-bath, is typically performed in the field. The patient is submerged up to the neck in a tub containing ice and water. Circulating the water and ice mixture helps accelerate cooling.
There have been differences in opinion regarding which cooling method is superior3 (TABLE 13,8,9). Traditionally, there were some concerns that CWI might actually increase body temperature via peripheral vasoconstriction and shivering. But current research suggests that for exertional heat stroke, CWI to promote conductive cooling is the most effective strategy.3,8,10,11 A review of cooling rates in healthy hyperthermic athletes and heat stroke victims showed that ice-water immersion or CWI at 1° to 14° C (35.6°-57.2° F) is superior to all other types of cooling, including ice packs, fans, and partial-body ice-water immersion.10

Furthermore, a 2015 meta-analysis looking at optimal procedures for cooling found that CWI cooled patients twice as fast as passive cooling (without any treatment).11 When cooling with CWI, core temperature drops about 0.2° C/min (0.36° F/min).10 Therefore, the temperature can be expected to drop about 1° C (1.8° F) for every 5 minutes of immersion. When unable to monitor a rectal temperature continuously, 10 to 15 minutes of immersion should get most patients below 40° C (104° F).
Extremity cooling. While CWI is the standard for cases of exertional heat illness, whole-body immersion is not always possible. In such cases, extremity cooling can be an effective body cooling method for exertional heat-related illness.12 Research has shown evaporative and convective cooling methods to have benefits for nonexertional heat-related illnesses.3,8,9 These methods usually involve directing air currents over exposed skin and spraying water on the affected individual.3
Contine to: Guidlines for transport
Guidelines for transport: Cool first, transport second
Most patients suspected of suffering from heat stroke should be transported to a hospital for further evaluation because of the high morbidity and mortality rates associated with it. However, cooling techniques should be implemented while awaiting transport. The current standard is “cool first, transport second.”7 Cooling interventions should continue in the ambulance if the core body temperature is still elevated. Techniques that can be used include the use of air conditioning, convective methods, and administration of IV fluids. As previously discussed, core body temperature should be continuously monitored. Cooling measures should be discontinued only when the patient’s rectal temperature reaches 38.9° C (102° F). Overly aggressive prehospital cooling beyond this point can result in prolonged hypothermia as well as cardiac arrhythmias.6
Monitoring and further evaluation
Monitoring patients with heat-related illness can be difficult, especially when utilizing CWI, as this may limit the ability to use devices such as a cardiac monitor or to continuously monitor rectal temperature. Beyond lowering core body temperature to below 39° C (102.2° F), early evaluation and treatment of other organ systems is vital, keeping in mind that these patients may develop multisystem organ failure. The initial work-up is listed in TABLE 2.

Depending on the severity of the injury and whether you suspect another diagnosis at work, additional studies may include urine output monitoring with a Foley catheter, electrocardiogram, chest radiograph, toxicology screen, a serum lactate level, and cardiac biomarkers.
Imaging. When evaluating for heat stroke, it usually isn’t necessary to obtain head imaging initially, as there are rarely abnormal findings in the early stages. Imaging may be obtained, however, if there is concern about a head injury or if neurologic abnormalities persist into later stages of treatment.5
Pharmacologic agents have not been shown to be of benefit in the treatment of heat-related illness. While dantrolene is commonly used in the treatment of neuroleptic malignant syndrome and malignant hyperthermia, the literature has not described any benefit associated with this agent in relation to heat-related illness. The same goes for antipyretics. Researchers have hypothesized that the reason these agents are ineffective is because body temperature is raised via a different mechanism in these conditions vs heat stroke.3
Continue to: Prevention
Prevention: Modifications and acclimatization are key
People who know they will be exposed to extreme heat should attempt to modify activities. There are many predisposing risk factors ranging from fever and illness to fatigue and dehydration. Risks can be minimized with physiologic adaptation through acclimatization, as well as making various behavioral changes such as adjusting activities, ensuring adequate hydration, and wearing appropriate clothing.13
Certain types of equipment, such as football helmets, can increase the risk of heat-related illness because they prevent heat exchange; however, the benefits sometimes outweigh the risks. With this in mind, consider modification of clothing and equipment if possible.1
In order to prevent heat-related illness, individuals should prehydrate prior to an event and replace fluids orally in order to prevent a >2% loss in body weight. Greater than a 2% loss directly correlates with increased core temperatures during exercise.1
Care should also be taken to perform regular physical activity prior to extreme heat exposure.1 Heat acclimatization takes place when a person’s body adapts to a hotter climate than they are accustomed to. This process can take up to 2 weeks, but once heat acclimation is accomplished, the person will have undergone physical changes, such as reduced metabolic heat production, which will decrease the risk of heat-related illness.13
Return to activity: Customize the approach
Each heat-related injury case is different; thus, return to activity should be individualized. In patients whose heat injury was believed to be secondary to a modifiable risk factor, efforts should be made to correct the predisposing factors that placed the patient at increased risk in the first place.
Additionally, the patient should allow sufficient time to recover. Guidelines recommend at least 1 to 2 weeks recovery before return to activity after heat stroke.8 Moreover, a graded return to activity, starting in a cool environment, is recommended. Gradual introduction of activity in the heat with close monitoring can help with acclimatization and help identify participants who continue to have cooling dysregulation. In the military and among athletes, tools such as heat-tolerance testing can be used to gauge the person’s readiness to return to play or duty.8 Heat tolerance testing is performed in a lab using continuous core temperature monitoring while having the subject exercise in a heated room.
CORRESPONDENCE
Scott Kinkade, MD, EdD, MA303 Medical Sciences Building, DC032.00, Columbia, MO 65212; [email protected].
Heat-related illnesses can affect people of any age who are subjected to extreme heat and humidity regardless of physical fitness level or baseline health status. The most serious of the heat-related illnesses is heat stroke. Prompt identification, early initiation of cooling measures (including cold-water immersion [CWI]), and transport to a higher level of care, when appropriate, are imperative. This article reviews heat-related illness identification, as well as management strategies.
Heat-related illnesses: From the benign to the severe
Some of the less severe forms of heat-related illness include heat cramps (which are due to dehydration and salt loss), heat rash, and heat edema. Heat rash and heat edema are benign. Heat rash typically resolves with cooler clothing and a cooler environment. Heat edema tends to improve after sleeping in a cooler environment with legs elevated. Heat syncope is the result of decreased cerebral perfusion due to fluid loss and vasodilation that results in a distributive hypovolemia. It commonly occurs after vigorous exercise when the athlete is standing still.
Heat exhaustion requires a more careful clinical assessment. It is the inability to continue activity in the heat, often with weakness and collapse. Also due to salt and water losses, it results in cardiovascular output that is insufficient to meet the circulatory and metabolic demands of the body. The body temperature is often elevated but <40° C (104° F), vomiting can occur, and mild central nervous system (CNS) dysfunction may be present.
Heat stroke is the most severe form of heat-related illness and can be life-threatening.1
It is important to understand that these heat-related illnesses do not progress along a continuum. Patients develop heat stroke without having had milder forms of heat illness, and patients with a milder type of heat illness usually do not progress to heat stroke.
Heat stroke: Definition, types, risk factors
Heat stroke is defined as a core body temperature ≥40° C (104° F) with CNS dysfunction in the setting of environmental heat stress. The mortality rate can reach over 50%.2-6
There are 2 main types of heat stroke: exertional heat stroke and nonexertional (classic) heat stroke. Exertional heat stroke more commonly affects healthy, young people, such as athletes or military personnel. Classic (nonexertional) heat stroke patients are typically elderly and/or have a chronic illness, although occasionally it involves children who are unable to escape from a hot environment.5,7 While exertional heat stroke typically develops over a period of a few hours in participants of prolonged activities, such as marathons, classic heat stroke in the elderly typically develops over a period of days in the setting of high environmental temperatures. In both conditions, there is an inability to maintain a normal body temperature leading to CNS dysregulation with altered mental status and often multisystem organ dysfunction.7
Continue to: Risk factors
Risk factors. Heat-related illness can affect patients of all ages and levels of physical fitness; however, certain factors place patients at increased risk. These include physical deconditioning, dehydration, high levels of exercise intensity, obesity, elevated environmental temperatures, sleep deprivation, certain medications, alcohol and drug abuse, concurrent illness, and wearing excessive clothing or equipment. It is imperative that severe cases of heat illness be identified early and treatment be initiated rapidly, as delays in cooling can significantly increase the fatality rate.5
Management: First suspect the diagnosis
Health care providers must first suspect heat-related illness and then accurately diagnose it. It is important to differentiate heat-related illness from syncope, cardiac abnormalities, gastroenteritis, hypoglycemia, and other entities that require alternate management. For cases of collapse, syncope or near-syncope, or altered mental status during exertion, heat stroke should be the default diagnosis until proven otherwise.
Obtain a core body temperature. While attending to airway, breathing, and circulation, obtain a core body temperature. Rectal (or esophageal) core temperatures provide a reliable reading that can assist in determining the severity of the heat illness. Axillary, tympanic, temporal, oral, and skin temperatures are affected by environmental factors and are not accurate determinants of core body temperature.8
Once heat stroke is diagnosed, the physician must immediately initiate cooling by removing clothing, placing the patient in the shade or an air-conditioned area, and beginning aggressive cooling measures (more on this in a bit). While field management requires an accurate diagnosis of the severity of a patient’s heat-related illness, one should not delay treatment in order to obtain a rectal temperature.
When treating the milder forms of heat illness, administer oral or intravenous (IV) isotonic fluids. For heat cramps, stretching the affected muscle can help. For heat syncope, lying the patient down and elevating the legs restores perfusion. Patients with heat exhaustion will require some cooling measures such as relocation to a shaded area, removal of excess clothing, and the use of cold towels, along with hydration and elevation of the feet.
Continue to: Cooling techniques for heat stroke
Cooling techniques for heat stroke
In order to adequately cool a patient suffering from heat stroke, health care providers must create a gradient for heat to escape the body through the skin into the environment by conduction, convection, or evaporation.3 Cooling heat stroke patients to less than 40° C (104° F) within 30 minutes after collapse decreases the fatality rate to almost zero.8
CWI comes out on top. CWI, also called an ice-bath, is typically performed in the field. The patient is submerged up to the neck in a tub containing ice and water. Circulating the water and ice mixture helps accelerate cooling.
There have been differences in opinion regarding which cooling method is superior3 (TABLE 13,8,9). Traditionally, there were some concerns that CWI might actually increase body temperature via peripheral vasoconstriction and shivering. But current research suggests that for exertional heat stroke, CWI to promote conductive cooling is the most effective strategy.3,8,10,11 A review of cooling rates in healthy hyperthermic athletes and heat stroke victims showed that ice-water immersion or CWI at 1° to 14° C (35.6°-57.2° F) is superior to all other types of cooling, including ice packs, fans, and partial-body ice-water immersion.10

Furthermore, a 2015 meta-analysis looking at optimal procedures for cooling found that CWI cooled patients twice as fast as passive cooling (without any treatment).11 When cooling with CWI, core temperature drops about 0.2° C/min (0.36° F/min).10 Therefore, the temperature can be expected to drop about 1° C (1.8° F) for every 5 minutes of immersion. When unable to monitor a rectal temperature continuously, 10 to 15 minutes of immersion should get most patients below 40° C (104° F).
Extremity cooling. While CWI is the standard for cases of exertional heat illness, whole-body immersion is not always possible. In such cases, extremity cooling can be an effective body cooling method for exertional heat-related illness.12 Research has shown evaporative and convective cooling methods to have benefits for nonexertional heat-related illnesses.3,8,9 These methods usually involve directing air currents over exposed skin and spraying water on the affected individual.3
Contine to: Guidlines for transport
Guidelines for transport: Cool first, transport second
Most patients suspected of suffering from heat stroke should be transported to a hospital for further evaluation because of the high morbidity and mortality rates associated with it. However, cooling techniques should be implemented while awaiting transport. The current standard is “cool first, transport second.”7 Cooling interventions should continue in the ambulance if the core body temperature is still elevated. Techniques that can be used include the use of air conditioning, convective methods, and administration of IV fluids. As previously discussed, core body temperature should be continuously monitored. Cooling measures should be discontinued only when the patient’s rectal temperature reaches 38.9° C (102° F). Overly aggressive prehospital cooling beyond this point can result in prolonged hypothermia as well as cardiac arrhythmias.6
Monitoring and further evaluation
Monitoring patients with heat-related illness can be difficult, especially when utilizing CWI, as this may limit the ability to use devices such as a cardiac monitor or to continuously monitor rectal temperature. Beyond lowering core body temperature to below 39° C (102.2° F), early evaluation and treatment of other organ systems is vital, keeping in mind that these patients may develop multisystem organ failure. The initial work-up is listed in TABLE 2.

Depending on the severity of the injury and whether you suspect another diagnosis at work, additional studies may include urine output monitoring with a Foley catheter, electrocardiogram, chest radiograph, toxicology screen, a serum lactate level, and cardiac biomarkers.
Imaging. When evaluating for heat stroke, it usually isn’t necessary to obtain head imaging initially, as there are rarely abnormal findings in the early stages. Imaging may be obtained, however, if there is concern about a head injury or if neurologic abnormalities persist into later stages of treatment.5
Pharmacologic agents have not been shown to be of benefit in the treatment of heat-related illness. While dantrolene is commonly used in the treatment of neuroleptic malignant syndrome and malignant hyperthermia, the literature has not described any benefit associated with this agent in relation to heat-related illness. The same goes for antipyretics. Researchers have hypothesized that the reason these agents are ineffective is because body temperature is raised via a different mechanism in these conditions vs heat stroke.3
Continue to: Prevention
Prevention: Modifications and acclimatization are key
People who know they will be exposed to extreme heat should attempt to modify activities. There are many predisposing risk factors ranging from fever and illness to fatigue and dehydration. Risks can be minimized with physiologic adaptation through acclimatization, as well as making various behavioral changes such as adjusting activities, ensuring adequate hydration, and wearing appropriate clothing.13
Certain types of equipment, such as football helmets, can increase the risk of heat-related illness because they prevent heat exchange; however, the benefits sometimes outweigh the risks. With this in mind, consider modification of clothing and equipment if possible.1
In order to prevent heat-related illness, individuals should prehydrate prior to an event and replace fluids orally in order to prevent a >2% loss in body weight. Greater than a 2% loss directly correlates with increased core temperatures during exercise.1
Care should also be taken to perform regular physical activity prior to extreme heat exposure.1 Heat acclimatization takes place when a person’s body adapts to a hotter climate than they are accustomed to. This process can take up to 2 weeks, but once heat acclimation is accomplished, the person will have undergone physical changes, such as reduced metabolic heat production, which will decrease the risk of heat-related illness.13
Return to activity: Customize the approach
Each heat-related injury case is different; thus, return to activity should be individualized. In patients whose heat injury was believed to be secondary to a modifiable risk factor, efforts should be made to correct the predisposing factors that placed the patient at increased risk in the first place.
Additionally, the patient should allow sufficient time to recover. Guidelines recommend at least 1 to 2 weeks recovery before return to activity after heat stroke.8 Moreover, a graded return to activity, starting in a cool environment, is recommended. Gradual introduction of activity in the heat with close monitoring can help with acclimatization and help identify participants who continue to have cooling dysregulation. In the military and among athletes, tools such as heat-tolerance testing can be used to gauge the person’s readiness to return to play or duty.8 Heat tolerance testing is performed in a lab using continuous core temperature monitoring while having the subject exercise in a heated room.
CORRESPONDENCE
Scott Kinkade, MD, EdD, MA303 Medical Sciences Building, DC032.00, Columbia, MO 65212; [email protected].
1. Lipman GS, Eifling KP, Ellis MA, et al. Wilderness Medical Society practice guidelines for the prevention and treatment of heat-related illness: 2014 update. Wilderness Environ Med. 2014;25(4 Suppl):S55-S65.
2. Update: Heat injuries, active component, U.S. Armed Forces, 2014. MSMR. 2015;22:17-20.
3. Gaudio FG, Grissom CK. Cooling methods in heat stroke. J Emerg Med. 2016;50:607-616.
4. Hess JJ, Saha S, Luber G. Summertime acute heat illness in U.S. emergency departments from 2006 through 2010: analysis of a nationally representative sample. Environ Health Perspect. 2014;122:1209-1215.
5. People’s Liberation Army Professional Committee of Critical Care Medicine. Expert consensus on standardized diagnosis and treatment for heat stroke. Mil Med Res. 2016;3:1.
6. Stewart TE, Whitford AC. Dangers of prehospital cooling: a case report of afterdrop in a patient with exertional heat stroke. J Emerg Med. 2015;49:630-633.
7. Chan YK, Mamat M. Management of heat stroke. Trends Anaesthesia Crit Care. 2015;5:65-69.
8. Casa DJ, Armstrong LE, Kenny GP, et al. Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012;11:115-123.
9. Demartini JK, Casa DJ, Stearns R, et al. Effectiveness of cold water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc. 2015;47:240-245.
10. Casa DJ, McDermott BP, Lee EC, et al. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35:141-149.
11. Zhang Y, Davis JK, Casa DJ, et al. Optimizing cold water immersion for exercise-induced hyperthermia: a meta-analysis. Med Sci Sports Exerc. 2015;47:2464-2472.
12. DeGroot DW, Kenefick RW, Sawka MN. Impact of arm immersion cooling during ranger training on exertional heat illness and treatment costs. Mil Med. 2015;180:1178-1183.
13. Epstein Y, Druyan A, Heled Y. Heat injury prevention—a military perspective. J Strength Cond Res. 2012;26 (suppl 2):S82-S86.
1. Lipman GS, Eifling KP, Ellis MA, et al. Wilderness Medical Society practice guidelines for the prevention and treatment of heat-related illness: 2014 update. Wilderness Environ Med. 2014;25(4 Suppl):S55-S65.
2. Update: Heat injuries, active component, U.S. Armed Forces, 2014. MSMR. 2015;22:17-20.
3. Gaudio FG, Grissom CK. Cooling methods in heat stroke. J Emerg Med. 2016;50:607-616.
4. Hess JJ, Saha S, Luber G. Summertime acute heat illness in U.S. emergency departments from 2006 through 2010: analysis of a nationally representative sample. Environ Health Perspect. 2014;122:1209-1215.
5. People’s Liberation Army Professional Committee of Critical Care Medicine. Expert consensus on standardized diagnosis and treatment for heat stroke. Mil Med Res. 2016;3:1.
6. Stewart TE, Whitford AC. Dangers of prehospital cooling: a case report of afterdrop in a patient with exertional heat stroke. J Emerg Med. 2015;49:630-633.
7. Chan YK, Mamat M. Management of heat stroke. Trends Anaesthesia Crit Care. 2015;5:65-69.
8. Casa DJ, Armstrong LE, Kenny GP, et al. Exertional heat stroke: new concepts regarding cause and care. Curr Sports Med Rep. 2012;11:115-123.
9. Demartini JK, Casa DJ, Stearns R, et al. Effectiveness of cold water immersion in the treatment of exertional heat stroke at the Falmouth Road Race. Med Sci Sports Exerc. 2015;47:240-245.
10. Casa DJ, McDermott BP, Lee EC, et al. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35:141-149.
11. Zhang Y, Davis JK, Casa DJ, et al. Optimizing cold water immersion for exercise-induced hyperthermia: a meta-analysis. Med Sci Sports Exerc. 2015;47:2464-2472.
12. DeGroot DW, Kenefick RW, Sawka MN. Impact of arm immersion cooling during ranger training on exertional heat illness and treatment costs. Mil Med. 2015;180:1178-1183.
13. Epstein Y, Druyan A, Heled Y. Heat injury prevention—a military perspective. J Strength Cond Res. 2012;26 (suppl 2):S82-S86.
Nonpharmacologic treatment of chronic pain: What works?
In 2017, the American College of Physicians (ACP) published a clinical practice guideline on the management of low back pain (LBP) that states: “For patients with chronic low back pain, clinicians and patients should initially select nonpharmacologic treatment…”1
This represents a significant shift in clinical practice, as treatment of pain syndromes often starts with analgesics and other medication therapy. This recommendation highlights the need for physicians to place nonpharmacologic therapies front and center in the management of chronic pain syndromes. But recommending nonpharmacologic therapies often represents a daunting task for physicians, as this category encompasses a broad range of treatments, some of which are considered “alternative” and others that are less familiar to physicians.

This article discusses 3 categories of nonpharmacologic therapies in detail: exercise-based therapies, mind-body therapies, and complementary modalities, and answers the question: Which nonpharmacologic treatments should you recommend for specific pain conditions?
In answering the question, we will provide a brief synopsis of several treatments within these 3 broad categories to allow a framework to discuss them with your patients, and we will summarize the evidence for these therapies when used for 3 common pain conditions: chronic LBP, osteoarthritis (OA), and fibromyalgia. Finally, we will offer suggestions on how to utilize these therapies within the context of a patient’s treatment plan.
This review is not without limitations. The quality of evidence is sometimes difficult to evaluate when considering nonpharmacologic therapies and can vary significantly among modalities. We sought to include the highest quality systematic reviews available to best reflect the current state of the evidence. We included Cochrane-based reviews when possible and provided evidence ratings using the Strength of Recommendation Taxonomy (SORT) system2 in the hope of helping you best counsel your patients on the appropriate use of available options.
Exercise-based therapies: Options to get patients moving
Therapeutic exercise is broadly defined as physical activity that contributes to enhanced aerobic capacity, strength, and/or flexibility, although health benefits are derived from lower-intensity physical activity even when these parameters do not change. Therapeutic exercise has well-documented salubrious effects including decreased all-cause mortality, improved physical fitness, and improvement in a variety of chronic pain conditions. In a 2017 Cochrane review of aerobic exercise for fibromyalgia, pain scores improved by 18%, compared with controls, although the quality of evidence was low (6 trials; n=351).3
Yoga is a system of physical postures and breathing and meditation practices based in Hindu philosophy. Most yoga classes and research protocols involve some combination of these elements.
Continue to: There is a growing body of research demonstrating...
There is a growing body of research demonstrating the benefits and safety of yoga for the treatment of chronic pain. Multiple reviews have evaluated the effectiveness of yoga in the treatment of chronic LBP with fairly consistent results. A 2017 Cochrane review (12 trials; n=1080) found moderate evidence of improvement in functional outcomes, although the magnitude of benefit was small.4 Chou et al found low-quality evidence of improvement in pain and function with yoga compared with usual care, education, and other exercise therapy (14 trials; n=1431).5
Tai chi is a centuries-old system of slow, deliberate, flowing movements based in the Chinese martial arts. The gentle movements make this a particularly appealing treatment for those who may have difficulty with other forms of exercise, such as the elderly and patients with OA. Tai chi is effective for treating a variety of conditions such as back pain, knee pain, and fibromyalgia. Multiple reviews have shown effectiveness in the treatment of OA.6,7
A 2016 randomized controlled trial (RCT) compared a 12-week course of tai chi to standard physical therapy (PT) for knee OA (n=204).8 The authors found that both strategies yielded similar improvement in pain and function, but that the tai chi group had better outcomes in secondary measures of depression and quality of life.8 Chou et al also found tai chi effective for chronic LBP (2 trials; n=480)5 (TABLE 13-5,7,9-13).

Counsel patients seeking to learn tai chi that it takes time to learn all the postures. Beginner classes typically offer the most detailed instruction and are best suited to patients new to the activity.
Mind-body/behavioral therapies: Taking on a greater role
Mind-body therapies are becoming increasingly important in the management of chronic pain syndromes because of an improved understanding of chronic pain pathophysiology. Studies have shown chronic pain can induce changes in the cortex, which can affect pain processing and perpetuate the experience of pain. Mind-body therapies have the potential to directly address brain centers affected by chronic pain.14 In addition, mind-body therapies can improve coexisting psychological symptoms and coping skills.
Continue to: Psychological therapies
Psychological therapies for the treatment of chronic pain are generally based on a cognitive-behavioral theoretical platform. Cognitive processes surrounding the experience (or avoidance) of pain are thought to exacerbate pain symptoms. Patients are encouraged to shift their mental framework away from a pain-oriented focus and toward a personal goal-oriented focus.15
Overall, research has found cognitive behavioral therapies (CBT) to be effective in the management of chronic pain. A 2012 Cochrane review of psychological therapies used in the treatment of nonspecific chronic pain found CBT particularly effective at pain reduction and improvement in disability and pain-related coping skills (35 trials; n=4788).15
Psychological therapy is generally delivered in a face-to-face encounter, either individually or in a group setting; however, a 2014 Cochrane review suggests that Web-based interventions are efficacious as well.16 Low-quality evidence in a 2013 Cochrane review of CBT for fibromyalgia demonstrated a medium-sized effect of CBT on pain at long-term follow-up (23 trials; n=2031)17 (TABLE 25,17-25).
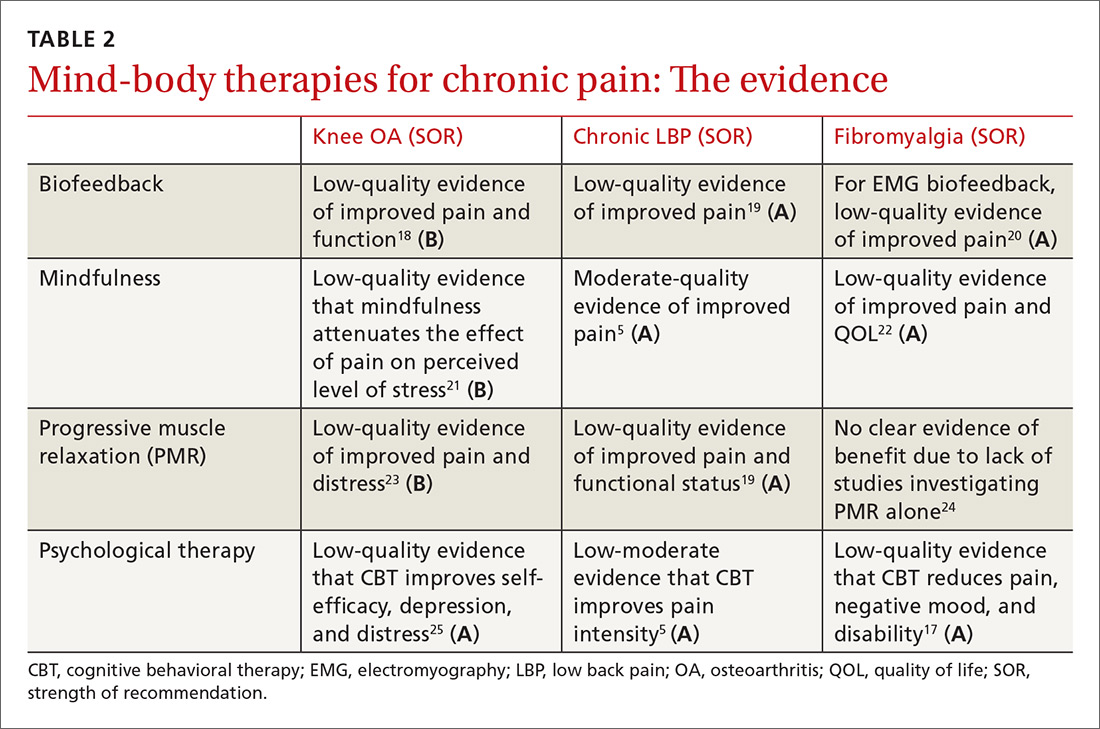
Biofeedback therapy gives patients real-time information about body processes to help bring those processes under voluntary control. Biofeedback devices measure parameters such as heart rate, blood pressure, and muscle tension and give patients visual or auditory cues to help bring those parameters into desired ranges. There is evidence of benefit in a variety of pain conditions including fibromyalgia, arthritis, LBP, and headache.18,19,26
Many psychologists are trained in biofeedback. A trained therapist usually guides biofeedback interventions initially, but patients can then utilize the skills independently. Devices can be purchased for home use. Phone-based applications are available and can be used, as well.
Continue to: Mindfulness
Mindfulness. Based on Eastern meditative traditions, mindfulness interventions focus on breathing and other body sensations as a means of bringing attention to the felt experience of the present moment. Mindfulness encourages a practice of detached observation with openness and curiosity, which allows for a reframing of experience. The growing body of mindfulness literature points to its effectiveness in a variety of pain conditions. A 2017 meta-analysis of mindfulness for pain conditions found a medium-sized effect on pain based on low-quality evidence (30 trials; n=2292).27
Participants can be taught in a series of group sessions (instruct interested patients to look for classes in their geographic area) or individually through a number of resources such as online audios, books, and smartphone applications.
Progressive muscle relaxation is a relaxation technique consisting of serially tightening and releasing different muscle groups to induce relaxation. Careful attention is paid to the somatic experience of tensing and releasing. Researchers have studied this technique for a variety of pain conditions, with the strongest effects observed in those with arthritis and those with LBP.19,28A variety of health care professionals can administer this therapy in office-based settings, and Internet-based audio recordings are available for home practice.
Complementary modalities for chronic pain
Complementary modalities are frequent additions to pain treatment plans. Spinal manipulative therapy (SMT) and massage therapy are regarded as biomechanical interventions, while acupuncture is categorized as a bio-energetic intervention. As a group, these treatments can address structural issues that may be contributing to pain conditions.
SMT is practiced by chiropractors, osteopathic physicians, and physical therapists. SMT improves function through the use of thrust techniques—quick, high-velocity, low-amplitude force applied to a joint, as well as other manual non-thrust techniques sometimes referred to as “mobilization” techniques. Experts have proposed multiple mechanisms of action for spinal manipulation and mobilization techniques, but ultimately SMT attempts to improve joint range of motion.
Continue to: SMT is most often studied for...
SMT is most often studied for the management of spinal pain. The authors of a 2017 systematic review and meta-analysis of 15 RCTs (n=1711) found moderate-quality evidence that SMT improves pain and function in chronic LBP at up to 6 weeks of follow-up.29 A 2017 systematic review performed for an ACP clinical practice guideline on the management of LBP found low-quality evidence of improvement in pain with SMT compared with an inactive treatment, although the magnitude of benefit was small.5 The authors also noted moderate-quality evidence that the benefits of SMT are comparable to other active treatments.5
Massage therapy is commonly used for a variety of pain conditions, but is most studied for LBP. A 2017 systematic review found low-quality evidence of short-term pain relief with massage therapy compared with other active interventions, although the effects were small.5 A 2015 Cochrane review of 25 RCTs (n=3096) found low-quality evidence of benefit for massage in chronic LBP when compared with both active and inactive controls.30
There was a small functional difference when compared with inactive controls. This review highlights the likely short-lived benefit of massage therapy. Although some studies have hinted at longer-term relief with massage therapy, the majority of the literature suggests the benefit is limited to immediate and short-term relief. Massage therapy is safe, although patients with central sensitization should be cautioned that more aggressive massage treatments may cause a flare of myofascial pain.
Acupuncture is one element of traditional Chinese medicine (TCM). And while the holistic system of TCM also includes herbal medicine, nutrition, meditative practices, and movement, acupuncture is often practiced as an independent therapy. In the United States, licensed acupuncturists and physicians provide the therapy. Training and licensing laws vary by state, as does insurance coverage.
Pain is the most common reason that people in the United States seek acupuncture therapy. It is not surprising then that the majority of research surrounding acupuncture involves its use for pain conditions. Chou et al reviewed acupuncture for chronic LBP in 2017 (32 trials; n=5931).5 Acupuncture improved both pain and function compared to inactive controls. In addition, 3 trials compared acupuncture to standard medications and found acupuncture to be superior at providing pain relief.
Continue to: In the management of headache pain...
In the management of headache pain, the literature has consistently found acupuncture to be beneficial in the prevention of migraine headaches. A 2016 Cochrane review found acupuncture beneficial compared to no treatment (4 trials; n=2199) or sham acupuncture (10 trials; n=1534), with benefit similar to prophylactic medications but with fewer adverse effects (3 trials; n=744).31
Evidence for benefit in OA pain has been mixed, but a 2016 meta-analysis evaluating 10 trials (n=2007) found acupuncture improved both short-term pain and functional outcome measures when compared with either no treatment or a sham control.32 There have also been reviews showing short-term benefit in fibromyalgia pain (TABLE 35,33-38).33

Building an effective treatment plan
When creating a treatment plan for chronic pain, it’s helpful to keep the following points in mind:
- Emphasize active treatments. Most traditional medical treatments and many complementary therapies are passive, meaning a patient receives a treatment with little agency in its implementation. Active therapies, such as exercise or relaxation practices, engage patients and improve pain-related coping skills. Active treatments promote self-efficacy, which is associated with improved outcomes in chronic pain.39
- Use treatments from different categories. Just as it is uncommon to choose multiple medications from the same pharmaceutical class, avoid recommending more than one nonpharmacologic treatment from each category. For example, adding chiropractic therapy to a treatment plan of PT, osteopathic manipulation, and massage isn’t likely to add significant benefit because all of these are structural therapies. Addition of a mind-body therapy would likely be a better choice. Consider the template provided when putting together a pain management plan (FIGURE).
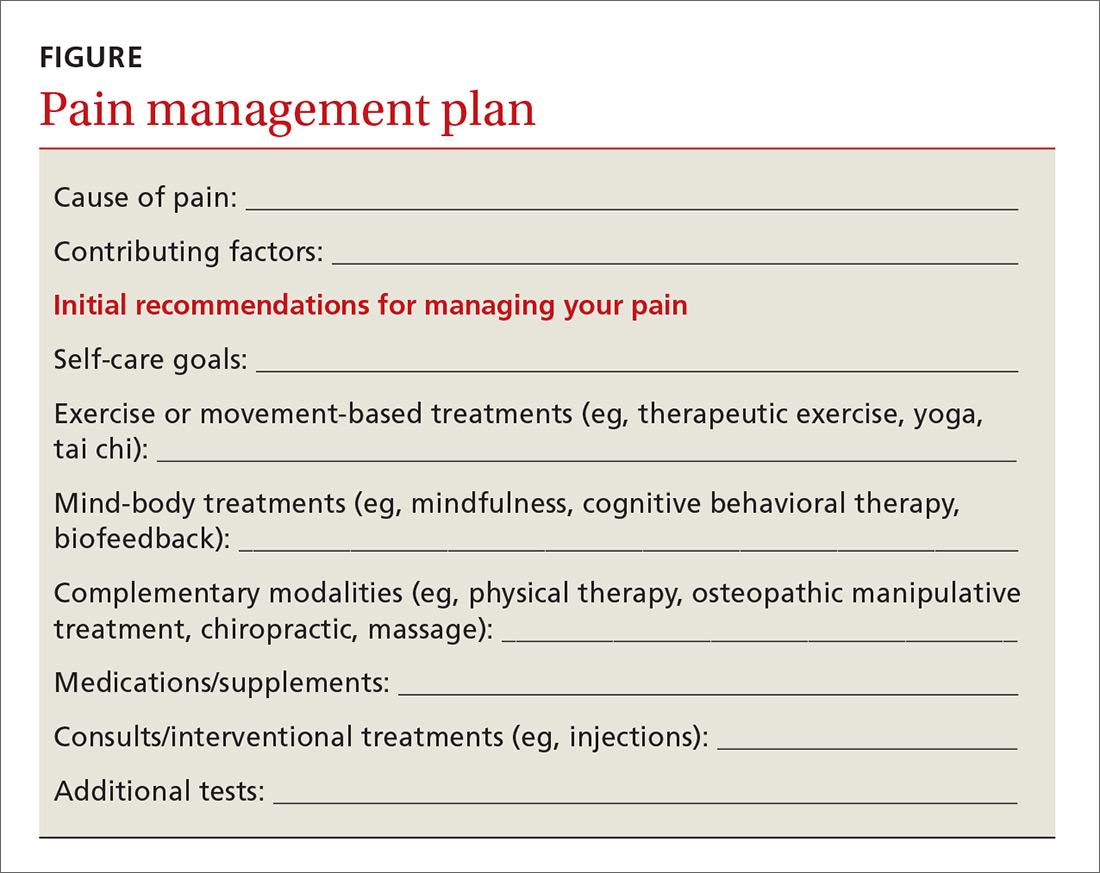
Continue to: Good plan, but how did the office visit go?
Good plan, but how did the office visit go?
A 2006 study by Laerum et al provided unique insights into the best ways to manage chronic pain.40 The authors asked patients a simple question: “What makes a good back consult?” The answers were deceptively simple, but serve as an excellent resource when working with patients to address their pain.
Patients indicated that taking their pain seriously was key to a good back consult. Other factors that were important to patients included: receiving an explanation of what is causing the pain, addressing psychosocial factors, and discussing what could be done.40 The following tips can help you address these patient priorities:
- Explain the underlying cause of the pain. Explaining the complex interplay of factors affecting pain helps patients understand why nonpharmacologic therapies are important. As an example, patients may accept mindfulness meditation as a treatment option if they understand that their chronic LBP is modulated in the brain.
- Address lifestyle and psychosocial issues. Pain syndromes cause far-reaching problems ranging from sleep dysfunction and weight gain to disrupted relationships and loss of employment. Explicitly addressing these issues helps patients cope better with these realities and gives clinicians more therapeutic targets.
The Veterans Affairs Health System offers a self-administered personal health inventory that can facilitate a patient-driven discussion about self-care. (See the Personal Health Inventory form available at: https://www.va.gov/PATIENTCENTEREDCARE/docs/PHI_Short_508.pdf.) In addition to identifying areas for growth, the inventory can highlight what is going well for a patient, adding an element of optimism that is often lacking in office visits for pain problems.
- Discuss what can be done in a way that empowers patients. Moving past medications when discussing pain treatment plans can be challenging. The goal of such discussions is to be as comprehensive as possible by including self-management aspects and nonpharmacologic approaches, in addition to appropriate medications. But this doesn’t all have to be done at once. Help patients set realistic goals for lifestyle-related change, and start with 1 or 2 nonpharmacologic therapies first. This approach both empowers patients and provides them with new treatment options that offer the hope of improved function.
CORRESPONDENCE
Russell Lemmon, DO, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Qaseem A, Wilt TJ, McLean RM, et al, for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Int Med. 2017;166:514-530.
2. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69:548-556.
3. Bidonde J, Busch AJ, Schachter CL, et al. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. 2017;(6):CD012700.
4. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev: 2017;(1):CD010671.
5. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166:493-505.
6. Hall A, Copsey B, Richmond H, et al. Effectiveness of tai chi for chronic musculoskeletal pain conditions: updated systematic review and meta-analysis. Phys Ther. 2017;97:227-238.
7. Ye J, Cai S, Zhong W, et al. Effects of tai chi for patients with knee osteoarthritis: a systematic review. J Phys Ther Sci. 2014;26:1133-1137.
8. Wang C, Schmid CH, Iversen MD, et al. Comparative effectiveness of tai chi versus physical therapy for knee osteoarthritis. Ann Int Med. 2016;165:77-86.
9. Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil. 2017;31:596-611.
10. Busch AJ, Webber SC, Richards RS, et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev. 2013;(12):CD010884.
11. Bidonde J, Busch AJ, Webber SC, et al. Aquatic exercise training for fibromyalgia. Cochrane Database Syst Rev. 2014;(10):CD011336.
12. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
13. Langhorst J, Klose P, Dobos GJ, et al. Efficacy and safety of meditative movement therapies in fibromyalgia syndrome: a systematic review and meta-analysis of randomized controlled trials. Rheumatol Int. 2013;33:193-207.
14. Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;(41 Suppl):66-72.
15. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;(11):CD007407.
16. Eccleston C, Fisher E, Craig L, et al. Psychological therapies (internet-delivered) for the management of chronic pain in adults. Cochrane Database Syst Rev. 2014;(2):CD010152.
17. Bernardy K, Klose P, Busch AJ, et al. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev. 2013;(9):CD009796.
18. Shull PB, Silder A, Shultz R, et al. Six-week gait retraining program reduces knee adduction moment, reduces pain, and improves function for individuals with medial compartment knee osteoarthritis. J Orthop Res. 2013;31:1020-1025.
19. Henschke N, Ostelo RW, van Tulder MW, et al. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev. 2010;(7):CD002014.
20. Glombiewski JA, Sawyer AT, Gutermann J, et al. Psychological treatments for fibromyalgia: a meta-analysis. Pain. 2010;151:280-295.
21. Lee AC, Harvey WF, Price LL, et al. Mindfulness is associated with psychological health and moderates pain in knee osteoarthritis. Osteoarthritis Cartilage. 2017;25:824-831.
22. Lauche R, Cramer H, Dobos G, et al. A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. J Psychosom Res. 2013;75:500-510.
23. Gay MC, Philippot P, Luminet O. Differential effectiveness of psychological interventions for reducing osteoarthritis pain: a comparison of Erickson hypnosis and Jacobson relaxation. Eur J Pain. 2002;6:1-16.
24. Meeus M, Nijs J, Vanderheiden T, et al. The effect of relaxation therapy on autonomic functioning, symptoms and daily functioning, in patients with chronic fatigue syndrome or fibromyalgia: a systematic review. Clin Rehabil. 2015;29:221-233.
25. Briani RV, Ferreira AS, Pazzinatto MF, et al. What interventions can improve quality of life or psychosocial factors of individuals with knee osteoarthritis? A systematic review with meta-analysis of primary outcomes from randomised controlled trials. Br J Sports Med. 2018. doi: 10.1136/bjsports-2017-098099.
26. Glombiewski JA, Bernardy K, Häuser W. Efficacy of EMG- and EEG-biofeedback in fibromyalgia syndrome: a meta-analysis and a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:962741.
27. Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51:199-213.
28. Kwekkeboom KL, Gretarsdottir E. Systematic review of relaxation interventions for pain. J Nurs Scholarsh. 2006;38:269-277.
29. Paige NM, Miake-Lye IM, Booth MS, et al. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain. Systematic review and meta-analysis. JAMA. 2017;317:1451-1460.
30. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015;(9):CD001929.
31. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;(6):CD001218.
32. Lin X, Huang K, Zhu G, et al. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J Bone Joint Surg Am. 2016;98:1578-1585.
33. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
34. Salamh P, Cook C, Reiman MP, et al. Treatment effectiveness and fidelity of manual therapy to the knee: a systematic review and meta-analysis. Musculoskeletal Care. 2017;15:238-248.
35. Posadzki P. Is spinal manipulation effective for pain? An overview of systematic reviews. Pain Med. 2012;13:754-761.
36. Perlman AI, Ali A, Njike VY, et al. Massage therapy for osteoarthritis of the knee: a randomized dose-finding trial. PLoS One. 2012;7:e30248.
37. Kalichman L. Massage therapy for fibromyalgia symptoms. Rheumatol Int. 2010;30:1151-1157.
38. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
39. Somers TJ, Wren AA, Shelby RA. The context of pain in arthritis: self-efficacy for managing pain and other symptoms. Curr Pain Headache Rep. 2012;16:502-508.
40. Laerum E, Indahl A, Skouen JS. What is “the good back-consultation”? A combined qualitative and quantitative study of chronic low back pain patients’ interaction with and perceptions of consultations with specialists. J Rehabil Med. 2006;38:255-262.
In 2017, the American College of Physicians (ACP) published a clinical practice guideline on the management of low back pain (LBP) that states: “For patients with chronic low back pain, clinicians and patients should initially select nonpharmacologic treatment…”1
This represents a significant shift in clinical practice, as treatment of pain syndromes often starts with analgesics and other medication therapy. This recommendation highlights the need for physicians to place nonpharmacologic therapies front and center in the management of chronic pain syndromes. But recommending nonpharmacologic therapies often represents a daunting task for physicians, as this category encompasses a broad range of treatments, some of which are considered “alternative” and others that are less familiar to physicians.

This article discusses 3 categories of nonpharmacologic therapies in detail: exercise-based therapies, mind-body therapies, and complementary modalities, and answers the question: Which nonpharmacologic treatments should you recommend for specific pain conditions?
In answering the question, we will provide a brief synopsis of several treatments within these 3 broad categories to allow a framework to discuss them with your patients, and we will summarize the evidence for these therapies when used for 3 common pain conditions: chronic LBP, osteoarthritis (OA), and fibromyalgia. Finally, we will offer suggestions on how to utilize these therapies within the context of a patient’s treatment plan.
This review is not without limitations. The quality of evidence is sometimes difficult to evaluate when considering nonpharmacologic therapies and can vary significantly among modalities. We sought to include the highest quality systematic reviews available to best reflect the current state of the evidence. We included Cochrane-based reviews when possible and provided evidence ratings using the Strength of Recommendation Taxonomy (SORT) system2 in the hope of helping you best counsel your patients on the appropriate use of available options.
Exercise-based therapies: Options to get patients moving
Therapeutic exercise is broadly defined as physical activity that contributes to enhanced aerobic capacity, strength, and/or flexibility, although health benefits are derived from lower-intensity physical activity even when these parameters do not change. Therapeutic exercise has well-documented salubrious effects including decreased all-cause mortality, improved physical fitness, and improvement in a variety of chronic pain conditions. In a 2017 Cochrane review of aerobic exercise for fibromyalgia, pain scores improved by 18%, compared with controls, although the quality of evidence was low (6 trials; n=351).3
Yoga is a system of physical postures and breathing and meditation practices based in Hindu philosophy. Most yoga classes and research protocols involve some combination of these elements.
Continue to: There is a growing body of research demonstrating...
There is a growing body of research demonstrating the benefits and safety of yoga for the treatment of chronic pain. Multiple reviews have evaluated the effectiveness of yoga in the treatment of chronic LBP with fairly consistent results. A 2017 Cochrane review (12 trials; n=1080) found moderate evidence of improvement in functional outcomes, although the magnitude of benefit was small.4 Chou et al found low-quality evidence of improvement in pain and function with yoga compared with usual care, education, and other exercise therapy (14 trials; n=1431).5
Tai chi is a centuries-old system of slow, deliberate, flowing movements based in the Chinese martial arts. The gentle movements make this a particularly appealing treatment for those who may have difficulty with other forms of exercise, such as the elderly and patients with OA. Tai chi is effective for treating a variety of conditions such as back pain, knee pain, and fibromyalgia. Multiple reviews have shown effectiveness in the treatment of OA.6,7
A 2016 randomized controlled trial (RCT) compared a 12-week course of tai chi to standard physical therapy (PT) for knee OA (n=204).8 The authors found that both strategies yielded similar improvement in pain and function, but that the tai chi group had better outcomes in secondary measures of depression and quality of life.8 Chou et al also found tai chi effective for chronic LBP (2 trials; n=480)5 (TABLE 13-5,7,9-13).

Counsel patients seeking to learn tai chi that it takes time to learn all the postures. Beginner classes typically offer the most detailed instruction and are best suited to patients new to the activity.
Mind-body/behavioral therapies: Taking on a greater role
Mind-body therapies are becoming increasingly important in the management of chronic pain syndromes because of an improved understanding of chronic pain pathophysiology. Studies have shown chronic pain can induce changes in the cortex, which can affect pain processing and perpetuate the experience of pain. Mind-body therapies have the potential to directly address brain centers affected by chronic pain.14 In addition, mind-body therapies can improve coexisting psychological symptoms and coping skills.
Continue to: Psychological therapies
Psychological therapies for the treatment of chronic pain are generally based on a cognitive-behavioral theoretical platform. Cognitive processes surrounding the experience (or avoidance) of pain are thought to exacerbate pain symptoms. Patients are encouraged to shift their mental framework away from a pain-oriented focus and toward a personal goal-oriented focus.15
Overall, research has found cognitive behavioral therapies (CBT) to be effective in the management of chronic pain. A 2012 Cochrane review of psychological therapies used in the treatment of nonspecific chronic pain found CBT particularly effective at pain reduction and improvement in disability and pain-related coping skills (35 trials; n=4788).15
Psychological therapy is generally delivered in a face-to-face encounter, either individually or in a group setting; however, a 2014 Cochrane review suggests that Web-based interventions are efficacious as well.16 Low-quality evidence in a 2013 Cochrane review of CBT for fibromyalgia demonstrated a medium-sized effect of CBT on pain at long-term follow-up (23 trials; n=2031)17 (TABLE 25,17-25).

Biofeedback therapy gives patients real-time information about body processes to help bring those processes under voluntary control. Biofeedback devices measure parameters such as heart rate, blood pressure, and muscle tension and give patients visual or auditory cues to help bring those parameters into desired ranges. There is evidence of benefit in a variety of pain conditions including fibromyalgia, arthritis, LBP, and headache.18,19,26
Many psychologists are trained in biofeedback. A trained therapist usually guides biofeedback interventions initially, but patients can then utilize the skills independently. Devices can be purchased for home use. Phone-based applications are available and can be used, as well.
Continue to: Mindfulness
Mindfulness. Based on Eastern meditative traditions, mindfulness interventions focus on breathing and other body sensations as a means of bringing attention to the felt experience of the present moment. Mindfulness encourages a practice of detached observation with openness and curiosity, which allows for a reframing of experience. The growing body of mindfulness literature points to its effectiveness in a variety of pain conditions. A 2017 meta-analysis of mindfulness for pain conditions found a medium-sized effect on pain based on low-quality evidence (30 trials; n=2292).27
Participants can be taught in a series of group sessions (instruct interested patients to look for classes in their geographic area) or individually through a number of resources such as online audios, books, and smartphone applications.
Progressive muscle relaxation is a relaxation technique consisting of serially tightening and releasing different muscle groups to induce relaxation. Careful attention is paid to the somatic experience of tensing and releasing. Researchers have studied this technique for a variety of pain conditions, with the strongest effects observed in those with arthritis and those with LBP.19,28A variety of health care professionals can administer this therapy in office-based settings, and Internet-based audio recordings are available for home practice.
Complementary modalities for chronic pain
Complementary modalities are frequent additions to pain treatment plans. Spinal manipulative therapy (SMT) and massage therapy are regarded as biomechanical interventions, while acupuncture is categorized as a bio-energetic intervention. As a group, these treatments can address structural issues that may be contributing to pain conditions.
SMT is practiced by chiropractors, osteopathic physicians, and physical therapists. SMT improves function through the use of thrust techniques—quick, high-velocity, low-amplitude force applied to a joint, as well as other manual non-thrust techniques sometimes referred to as “mobilization” techniques. Experts have proposed multiple mechanisms of action for spinal manipulation and mobilization techniques, but ultimately SMT attempts to improve joint range of motion.
Continue to: SMT is most often studied for...
SMT is most often studied for the management of spinal pain. The authors of a 2017 systematic review and meta-analysis of 15 RCTs (n=1711) found moderate-quality evidence that SMT improves pain and function in chronic LBP at up to 6 weeks of follow-up.29 A 2017 systematic review performed for an ACP clinical practice guideline on the management of LBP found low-quality evidence of improvement in pain with SMT compared with an inactive treatment, although the magnitude of benefit was small.5 The authors also noted moderate-quality evidence that the benefits of SMT are comparable to other active treatments.5
Massage therapy is commonly used for a variety of pain conditions, but is most studied for LBP. A 2017 systematic review found low-quality evidence of short-term pain relief with massage therapy compared with other active interventions, although the effects were small.5 A 2015 Cochrane review of 25 RCTs (n=3096) found low-quality evidence of benefit for massage in chronic LBP when compared with both active and inactive controls.30
There was a small functional difference when compared with inactive controls. This review highlights the likely short-lived benefit of massage therapy. Although some studies have hinted at longer-term relief with massage therapy, the majority of the literature suggests the benefit is limited to immediate and short-term relief. Massage therapy is safe, although patients with central sensitization should be cautioned that more aggressive massage treatments may cause a flare of myofascial pain.
Acupuncture is one element of traditional Chinese medicine (TCM). And while the holistic system of TCM also includes herbal medicine, nutrition, meditative practices, and movement, acupuncture is often practiced as an independent therapy. In the United States, licensed acupuncturists and physicians provide the therapy. Training and licensing laws vary by state, as does insurance coverage.
Pain is the most common reason that people in the United States seek acupuncture therapy. It is not surprising then that the majority of research surrounding acupuncture involves its use for pain conditions. Chou et al reviewed acupuncture for chronic LBP in 2017 (32 trials; n=5931).5 Acupuncture improved both pain and function compared to inactive controls. In addition, 3 trials compared acupuncture to standard medications and found acupuncture to be superior at providing pain relief.
Continue to: In the management of headache pain...
In the management of headache pain, the literature has consistently found acupuncture to be beneficial in the prevention of migraine headaches. A 2016 Cochrane review found acupuncture beneficial compared to no treatment (4 trials; n=2199) or sham acupuncture (10 trials; n=1534), with benefit similar to prophylactic medications but with fewer adverse effects (3 trials; n=744).31
Evidence for benefit in OA pain has been mixed, but a 2016 meta-analysis evaluating 10 trials (n=2007) found acupuncture improved both short-term pain and functional outcome measures when compared with either no treatment or a sham control.32 There have also been reviews showing short-term benefit in fibromyalgia pain (TABLE 35,33-38).33

Building an effective treatment plan
When creating a treatment plan for chronic pain, it’s helpful to keep the following points in mind:
- Emphasize active treatments. Most traditional medical treatments and many complementary therapies are passive, meaning a patient receives a treatment with little agency in its implementation. Active therapies, such as exercise or relaxation practices, engage patients and improve pain-related coping skills. Active treatments promote self-efficacy, which is associated with improved outcomes in chronic pain.39
- Use treatments from different categories. Just as it is uncommon to choose multiple medications from the same pharmaceutical class, avoid recommending more than one nonpharmacologic treatment from each category. For example, adding chiropractic therapy to a treatment plan of PT, osteopathic manipulation, and massage isn’t likely to add significant benefit because all of these are structural therapies. Addition of a mind-body therapy would likely be a better choice. Consider the template provided when putting together a pain management plan (FIGURE).

Continue to: Good plan, but how did the office visit go?
Good plan, but how did the office visit go?
A 2006 study by Laerum et al provided unique insights into the best ways to manage chronic pain.40 The authors asked patients a simple question: “What makes a good back consult?” The answers were deceptively simple, but serve as an excellent resource when working with patients to address their pain.
Patients indicated that taking their pain seriously was key to a good back consult. Other factors that were important to patients included: receiving an explanation of what is causing the pain, addressing psychosocial factors, and discussing what could be done.40 The following tips can help you address these patient priorities:
- Explain the underlying cause of the pain. Explaining the complex interplay of factors affecting pain helps patients understand why nonpharmacologic therapies are important. As an example, patients may accept mindfulness meditation as a treatment option if they understand that their chronic LBP is modulated in the brain.
- Address lifestyle and psychosocial issues. Pain syndromes cause far-reaching problems ranging from sleep dysfunction and weight gain to disrupted relationships and loss of employment. Explicitly addressing these issues helps patients cope better with these realities and gives clinicians more therapeutic targets.
The Veterans Affairs Health System offers a self-administered personal health inventory that can facilitate a patient-driven discussion about self-care. (See the Personal Health Inventory form available at: https://www.va.gov/PATIENTCENTEREDCARE/docs/PHI_Short_508.pdf.) In addition to identifying areas for growth, the inventory can highlight what is going well for a patient, adding an element of optimism that is often lacking in office visits for pain problems.
- Discuss what can be done in a way that empowers patients. Moving past medications when discussing pain treatment plans can be challenging. The goal of such discussions is to be as comprehensive as possible by including self-management aspects and nonpharmacologic approaches, in addition to appropriate medications. But this doesn’t all have to be done at once. Help patients set realistic goals for lifestyle-related change, and start with 1 or 2 nonpharmacologic therapies first. This approach both empowers patients and provides them with new treatment options that offer the hope of improved function.
CORRESPONDENCE
Russell Lemmon, DO, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
In 2017, the American College of Physicians (ACP) published a clinical practice guideline on the management of low back pain (LBP) that states: “For patients with chronic low back pain, clinicians and patients should initially select nonpharmacologic treatment…”1
This represents a significant shift in clinical practice, as treatment of pain syndromes often starts with analgesics and other medication therapy. This recommendation highlights the need for physicians to place nonpharmacologic therapies front and center in the management of chronic pain syndromes. But recommending nonpharmacologic therapies often represents a daunting task for physicians, as this category encompasses a broad range of treatments, some of which are considered “alternative” and others that are less familiar to physicians.

This article discusses 3 categories of nonpharmacologic therapies in detail: exercise-based therapies, mind-body therapies, and complementary modalities, and answers the question: Which nonpharmacologic treatments should you recommend for specific pain conditions?
In answering the question, we will provide a brief synopsis of several treatments within these 3 broad categories to allow a framework to discuss them with your patients, and we will summarize the evidence for these therapies when used for 3 common pain conditions: chronic LBP, osteoarthritis (OA), and fibromyalgia. Finally, we will offer suggestions on how to utilize these therapies within the context of a patient’s treatment plan.
This review is not without limitations. The quality of evidence is sometimes difficult to evaluate when considering nonpharmacologic therapies and can vary significantly among modalities. We sought to include the highest quality systematic reviews available to best reflect the current state of the evidence. We included Cochrane-based reviews when possible and provided evidence ratings using the Strength of Recommendation Taxonomy (SORT) system2 in the hope of helping you best counsel your patients on the appropriate use of available options.
Exercise-based therapies: Options to get patients moving
Therapeutic exercise is broadly defined as physical activity that contributes to enhanced aerobic capacity, strength, and/or flexibility, although health benefits are derived from lower-intensity physical activity even when these parameters do not change. Therapeutic exercise has well-documented salubrious effects including decreased all-cause mortality, improved physical fitness, and improvement in a variety of chronic pain conditions. In a 2017 Cochrane review of aerobic exercise for fibromyalgia, pain scores improved by 18%, compared with controls, although the quality of evidence was low (6 trials; n=351).3
Yoga is a system of physical postures and breathing and meditation practices based in Hindu philosophy. Most yoga classes and research protocols involve some combination of these elements.
Continue to: There is a growing body of research demonstrating...
There is a growing body of research demonstrating the benefits and safety of yoga for the treatment of chronic pain. Multiple reviews have evaluated the effectiveness of yoga in the treatment of chronic LBP with fairly consistent results. A 2017 Cochrane review (12 trials; n=1080) found moderate evidence of improvement in functional outcomes, although the magnitude of benefit was small.4 Chou et al found low-quality evidence of improvement in pain and function with yoga compared with usual care, education, and other exercise therapy (14 trials; n=1431).5
Tai chi is a centuries-old system of slow, deliberate, flowing movements based in the Chinese martial arts. The gentle movements make this a particularly appealing treatment for those who may have difficulty with other forms of exercise, such as the elderly and patients with OA. Tai chi is effective for treating a variety of conditions such as back pain, knee pain, and fibromyalgia. Multiple reviews have shown effectiveness in the treatment of OA.6,7
A 2016 randomized controlled trial (RCT) compared a 12-week course of tai chi to standard physical therapy (PT) for knee OA (n=204).8 The authors found that both strategies yielded similar improvement in pain and function, but that the tai chi group had better outcomes in secondary measures of depression and quality of life.8 Chou et al also found tai chi effective for chronic LBP (2 trials; n=480)5 (TABLE 13-5,7,9-13).

Counsel patients seeking to learn tai chi that it takes time to learn all the postures. Beginner classes typically offer the most detailed instruction and are best suited to patients new to the activity.
Mind-body/behavioral therapies: Taking on a greater role
Mind-body therapies are becoming increasingly important in the management of chronic pain syndromes because of an improved understanding of chronic pain pathophysiology. Studies have shown chronic pain can induce changes in the cortex, which can affect pain processing and perpetuate the experience of pain. Mind-body therapies have the potential to directly address brain centers affected by chronic pain.14 In addition, mind-body therapies can improve coexisting psychological symptoms and coping skills.
Continue to: Psychological therapies
Psychological therapies for the treatment of chronic pain are generally based on a cognitive-behavioral theoretical platform. Cognitive processes surrounding the experience (or avoidance) of pain are thought to exacerbate pain symptoms. Patients are encouraged to shift their mental framework away from a pain-oriented focus and toward a personal goal-oriented focus.15
Overall, research has found cognitive behavioral therapies (CBT) to be effective in the management of chronic pain. A 2012 Cochrane review of psychological therapies used in the treatment of nonspecific chronic pain found CBT particularly effective at pain reduction and improvement in disability and pain-related coping skills (35 trials; n=4788).15
Psychological therapy is generally delivered in a face-to-face encounter, either individually or in a group setting; however, a 2014 Cochrane review suggests that Web-based interventions are efficacious as well.16 Low-quality evidence in a 2013 Cochrane review of CBT for fibromyalgia demonstrated a medium-sized effect of CBT on pain at long-term follow-up (23 trials; n=2031)17 (TABLE 25,17-25).

Biofeedback therapy gives patients real-time information about body processes to help bring those processes under voluntary control. Biofeedback devices measure parameters such as heart rate, blood pressure, and muscle tension and give patients visual or auditory cues to help bring those parameters into desired ranges. There is evidence of benefit in a variety of pain conditions including fibromyalgia, arthritis, LBP, and headache.18,19,26
Many psychologists are trained in biofeedback. A trained therapist usually guides biofeedback interventions initially, but patients can then utilize the skills independently. Devices can be purchased for home use. Phone-based applications are available and can be used, as well.
Continue to: Mindfulness
Mindfulness. Based on Eastern meditative traditions, mindfulness interventions focus on breathing and other body sensations as a means of bringing attention to the felt experience of the present moment. Mindfulness encourages a practice of detached observation with openness and curiosity, which allows for a reframing of experience. The growing body of mindfulness literature points to its effectiveness in a variety of pain conditions. A 2017 meta-analysis of mindfulness for pain conditions found a medium-sized effect on pain based on low-quality evidence (30 trials; n=2292).27
Participants can be taught in a series of group sessions (instruct interested patients to look for classes in their geographic area) or individually through a number of resources such as online audios, books, and smartphone applications.
Progressive muscle relaxation is a relaxation technique consisting of serially tightening and releasing different muscle groups to induce relaxation. Careful attention is paid to the somatic experience of tensing and releasing. Researchers have studied this technique for a variety of pain conditions, with the strongest effects observed in those with arthritis and those with LBP.19,28A variety of health care professionals can administer this therapy in office-based settings, and Internet-based audio recordings are available for home practice.
Complementary modalities for chronic pain
Complementary modalities are frequent additions to pain treatment plans. Spinal manipulative therapy (SMT) and massage therapy are regarded as biomechanical interventions, while acupuncture is categorized as a bio-energetic intervention. As a group, these treatments can address structural issues that may be contributing to pain conditions.
SMT is practiced by chiropractors, osteopathic physicians, and physical therapists. SMT improves function through the use of thrust techniques—quick, high-velocity, low-amplitude force applied to a joint, as well as other manual non-thrust techniques sometimes referred to as “mobilization” techniques. Experts have proposed multiple mechanisms of action for spinal manipulation and mobilization techniques, but ultimately SMT attempts to improve joint range of motion.
Continue to: SMT is most often studied for...
SMT is most often studied for the management of spinal pain. The authors of a 2017 systematic review and meta-analysis of 15 RCTs (n=1711) found moderate-quality evidence that SMT improves pain and function in chronic LBP at up to 6 weeks of follow-up.29 A 2017 systematic review performed for an ACP clinical practice guideline on the management of LBP found low-quality evidence of improvement in pain with SMT compared with an inactive treatment, although the magnitude of benefit was small.5 The authors also noted moderate-quality evidence that the benefits of SMT are comparable to other active treatments.5
Massage therapy is commonly used for a variety of pain conditions, but is most studied for LBP. A 2017 systematic review found low-quality evidence of short-term pain relief with massage therapy compared with other active interventions, although the effects were small.5 A 2015 Cochrane review of 25 RCTs (n=3096) found low-quality evidence of benefit for massage in chronic LBP when compared with both active and inactive controls.30
There was a small functional difference when compared with inactive controls. This review highlights the likely short-lived benefit of massage therapy. Although some studies have hinted at longer-term relief with massage therapy, the majority of the literature suggests the benefit is limited to immediate and short-term relief. Massage therapy is safe, although patients with central sensitization should be cautioned that more aggressive massage treatments may cause a flare of myofascial pain.
Acupuncture is one element of traditional Chinese medicine (TCM). And while the holistic system of TCM also includes herbal medicine, nutrition, meditative practices, and movement, acupuncture is often practiced as an independent therapy. In the United States, licensed acupuncturists and physicians provide the therapy. Training and licensing laws vary by state, as does insurance coverage.
Pain is the most common reason that people in the United States seek acupuncture therapy. It is not surprising then that the majority of research surrounding acupuncture involves its use for pain conditions. Chou et al reviewed acupuncture for chronic LBP in 2017 (32 trials; n=5931).5 Acupuncture improved both pain and function compared to inactive controls. In addition, 3 trials compared acupuncture to standard medications and found acupuncture to be superior at providing pain relief.
Continue to: In the management of headache pain...
In the management of headache pain, the literature has consistently found acupuncture to be beneficial in the prevention of migraine headaches. A 2016 Cochrane review found acupuncture beneficial compared to no treatment (4 trials; n=2199) or sham acupuncture (10 trials; n=1534), with benefit similar to prophylactic medications but with fewer adverse effects (3 trials; n=744).31
Evidence for benefit in OA pain has been mixed, but a 2016 meta-analysis evaluating 10 trials (n=2007) found acupuncture improved both short-term pain and functional outcome measures when compared with either no treatment or a sham control.32 There have also been reviews showing short-term benefit in fibromyalgia pain (TABLE 35,33-38).33

Building an effective treatment plan
When creating a treatment plan for chronic pain, it’s helpful to keep the following points in mind:
- Emphasize active treatments. Most traditional medical treatments and many complementary therapies are passive, meaning a patient receives a treatment with little agency in its implementation. Active therapies, such as exercise or relaxation practices, engage patients and improve pain-related coping skills. Active treatments promote self-efficacy, which is associated with improved outcomes in chronic pain.39
- Use treatments from different categories. Just as it is uncommon to choose multiple medications from the same pharmaceutical class, avoid recommending more than one nonpharmacologic treatment from each category. For example, adding chiropractic therapy to a treatment plan of PT, osteopathic manipulation, and massage isn’t likely to add significant benefit because all of these are structural therapies. Addition of a mind-body therapy would likely be a better choice. Consider the template provided when putting together a pain management plan (FIGURE).

Continue to: Good plan, but how did the office visit go?
Good plan, but how did the office visit go?
A 2006 study by Laerum et al provided unique insights into the best ways to manage chronic pain.40 The authors asked patients a simple question: “What makes a good back consult?” The answers were deceptively simple, but serve as an excellent resource when working with patients to address their pain.
Patients indicated that taking their pain seriously was key to a good back consult. Other factors that were important to patients included: receiving an explanation of what is causing the pain, addressing psychosocial factors, and discussing what could be done.40 The following tips can help you address these patient priorities:
- Explain the underlying cause of the pain. Explaining the complex interplay of factors affecting pain helps patients understand why nonpharmacologic therapies are important. As an example, patients may accept mindfulness meditation as a treatment option if they understand that their chronic LBP is modulated in the brain.
- Address lifestyle and psychosocial issues. Pain syndromes cause far-reaching problems ranging from sleep dysfunction and weight gain to disrupted relationships and loss of employment. Explicitly addressing these issues helps patients cope better with these realities and gives clinicians more therapeutic targets.
The Veterans Affairs Health System offers a self-administered personal health inventory that can facilitate a patient-driven discussion about self-care. (See the Personal Health Inventory form available at: https://www.va.gov/PATIENTCENTEREDCARE/docs/PHI_Short_508.pdf.) In addition to identifying areas for growth, the inventory can highlight what is going well for a patient, adding an element of optimism that is often lacking in office visits for pain problems.
- Discuss what can be done in a way that empowers patients. Moving past medications when discussing pain treatment plans can be challenging. The goal of such discussions is to be as comprehensive as possible by including self-management aspects and nonpharmacologic approaches, in addition to appropriate medications. But this doesn’t all have to be done at once. Help patients set realistic goals for lifestyle-related change, and start with 1 or 2 nonpharmacologic therapies first. This approach both empowers patients and provides them with new treatment options that offer the hope of improved function.
CORRESPONDENCE
Russell Lemmon, DO, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Qaseem A, Wilt TJ, McLean RM, et al, for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Int Med. 2017;166:514-530.
2. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69:548-556.
3. Bidonde J, Busch AJ, Schachter CL, et al. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. 2017;(6):CD012700.
4. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev: 2017;(1):CD010671.
5. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166:493-505.
6. Hall A, Copsey B, Richmond H, et al. Effectiveness of tai chi for chronic musculoskeletal pain conditions: updated systematic review and meta-analysis. Phys Ther. 2017;97:227-238.
7. Ye J, Cai S, Zhong W, et al. Effects of tai chi for patients with knee osteoarthritis: a systematic review. J Phys Ther Sci. 2014;26:1133-1137.
8. Wang C, Schmid CH, Iversen MD, et al. Comparative effectiveness of tai chi versus physical therapy for knee osteoarthritis. Ann Int Med. 2016;165:77-86.
9. Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil. 2017;31:596-611.
10. Busch AJ, Webber SC, Richards RS, et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev. 2013;(12):CD010884.
11. Bidonde J, Busch AJ, Webber SC, et al. Aquatic exercise training for fibromyalgia. Cochrane Database Syst Rev. 2014;(10):CD011336.
12. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
13. Langhorst J, Klose P, Dobos GJ, et al. Efficacy and safety of meditative movement therapies in fibromyalgia syndrome: a systematic review and meta-analysis of randomized controlled trials. Rheumatol Int. 2013;33:193-207.
14. Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;(41 Suppl):66-72.
15. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;(11):CD007407.
16. Eccleston C, Fisher E, Craig L, et al. Psychological therapies (internet-delivered) for the management of chronic pain in adults. Cochrane Database Syst Rev. 2014;(2):CD010152.
17. Bernardy K, Klose P, Busch AJ, et al. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev. 2013;(9):CD009796.
18. Shull PB, Silder A, Shultz R, et al. Six-week gait retraining program reduces knee adduction moment, reduces pain, and improves function for individuals with medial compartment knee osteoarthritis. J Orthop Res. 2013;31:1020-1025.
19. Henschke N, Ostelo RW, van Tulder MW, et al. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev. 2010;(7):CD002014.
20. Glombiewski JA, Sawyer AT, Gutermann J, et al. Psychological treatments for fibromyalgia: a meta-analysis. Pain. 2010;151:280-295.
21. Lee AC, Harvey WF, Price LL, et al. Mindfulness is associated with psychological health and moderates pain in knee osteoarthritis. Osteoarthritis Cartilage. 2017;25:824-831.
22. Lauche R, Cramer H, Dobos G, et al. A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. J Psychosom Res. 2013;75:500-510.
23. Gay MC, Philippot P, Luminet O. Differential effectiveness of psychological interventions for reducing osteoarthritis pain: a comparison of Erickson hypnosis and Jacobson relaxation. Eur J Pain. 2002;6:1-16.
24. Meeus M, Nijs J, Vanderheiden T, et al. The effect of relaxation therapy on autonomic functioning, symptoms and daily functioning, in patients with chronic fatigue syndrome or fibromyalgia: a systematic review. Clin Rehabil. 2015;29:221-233.
25. Briani RV, Ferreira AS, Pazzinatto MF, et al. What interventions can improve quality of life or psychosocial factors of individuals with knee osteoarthritis? A systematic review with meta-analysis of primary outcomes from randomised controlled trials. Br J Sports Med. 2018. doi: 10.1136/bjsports-2017-098099.
26. Glombiewski JA, Bernardy K, Häuser W. Efficacy of EMG- and EEG-biofeedback in fibromyalgia syndrome: a meta-analysis and a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:962741.
27. Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51:199-213.
28. Kwekkeboom KL, Gretarsdottir E. Systematic review of relaxation interventions for pain. J Nurs Scholarsh. 2006;38:269-277.
29. Paige NM, Miake-Lye IM, Booth MS, et al. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain. Systematic review and meta-analysis. JAMA. 2017;317:1451-1460.
30. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015;(9):CD001929.
31. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;(6):CD001218.
32. Lin X, Huang K, Zhu G, et al. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J Bone Joint Surg Am. 2016;98:1578-1585.
33. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
34. Salamh P, Cook C, Reiman MP, et al. Treatment effectiveness and fidelity of manual therapy to the knee: a systematic review and meta-analysis. Musculoskeletal Care. 2017;15:238-248.
35. Posadzki P. Is spinal manipulation effective for pain? An overview of systematic reviews. Pain Med. 2012;13:754-761.
36. Perlman AI, Ali A, Njike VY, et al. Massage therapy for osteoarthritis of the knee: a randomized dose-finding trial. PLoS One. 2012;7:e30248.
37. Kalichman L. Massage therapy for fibromyalgia symptoms. Rheumatol Int. 2010;30:1151-1157.
38. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
39. Somers TJ, Wren AA, Shelby RA. The context of pain in arthritis: self-efficacy for managing pain and other symptoms. Curr Pain Headache Rep. 2012;16:502-508.
40. Laerum E, Indahl A, Skouen JS. What is “the good back-consultation”? A combined qualitative and quantitative study of chronic low back pain patients’ interaction with and perceptions of consultations with specialists. J Rehabil Med. 2006;38:255-262.
1. Qaseem A, Wilt TJ, McLean RM, et al, for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Int Med. 2017;166:514-530.
2. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69:548-556.
3. Bidonde J, Busch AJ, Schachter CL, et al. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev. 2017;(6):CD012700.
4. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain. Cochrane Database Syst Rev: 2017;(1):CD010671.
5. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166:493-505.
6. Hall A, Copsey B, Richmond H, et al. Effectiveness of tai chi for chronic musculoskeletal pain conditions: updated systematic review and meta-analysis. Phys Ther. 2017;97:227-238.
7. Ye J, Cai S, Zhong W, et al. Effects of tai chi for patients with knee osteoarthritis: a systematic review. J Phys Ther Sci. 2014;26:1133-1137.
8. Wang C, Schmid CH, Iversen MD, et al. Comparative effectiveness of tai chi versus physical therapy for knee osteoarthritis. Ann Int Med. 2016;165:77-86.
9. Brosseau L, Taki J, Desjardins B, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil. 2017;31:596-611.
10. Busch AJ, Webber SC, Richards RS, et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev. 2013;(12):CD010884.
11. Bidonde J, Busch AJ, Webber SC, et al. Aquatic exercise training for fibromyalgia. Cochrane Database Syst Rev. 2014;(10):CD011336.
12. Kan L, Zhang J, Yang Y, et al. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid Based Complement Alternat Med. 2016;2016:6016532.
13. Langhorst J, Klose P, Dobos GJ, et al. Efficacy and safety of meditative movement therapies in fibromyalgia syndrome: a systematic review and meta-analysis of randomized controlled trials. Rheumatol Int. 2013;33:193-207.
14. Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;(41 Suppl):66-72.
15. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;(11):CD007407.
16. Eccleston C, Fisher E, Craig L, et al. Psychological therapies (internet-delivered) for the management of chronic pain in adults. Cochrane Database Syst Rev. 2014;(2):CD010152.
17. Bernardy K, Klose P, Busch AJ, et al. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev. 2013;(9):CD009796.
18. Shull PB, Silder A, Shultz R, et al. Six-week gait retraining program reduces knee adduction moment, reduces pain, and improves function for individuals with medial compartment knee osteoarthritis. J Orthop Res. 2013;31:1020-1025.
19. Henschke N, Ostelo RW, van Tulder MW, et al. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev. 2010;(7):CD002014.
20. Glombiewski JA, Sawyer AT, Gutermann J, et al. Psychological treatments for fibromyalgia: a meta-analysis. Pain. 2010;151:280-295.
21. Lee AC, Harvey WF, Price LL, et al. Mindfulness is associated with psychological health and moderates pain in knee osteoarthritis. Osteoarthritis Cartilage. 2017;25:824-831.
22. Lauche R, Cramer H, Dobos G, et al. A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. J Psychosom Res. 2013;75:500-510.
23. Gay MC, Philippot P, Luminet O. Differential effectiveness of psychological interventions for reducing osteoarthritis pain: a comparison of Erickson hypnosis and Jacobson relaxation. Eur J Pain. 2002;6:1-16.
24. Meeus M, Nijs J, Vanderheiden T, et al. The effect of relaxation therapy on autonomic functioning, symptoms and daily functioning, in patients with chronic fatigue syndrome or fibromyalgia: a systematic review. Clin Rehabil. 2015;29:221-233.
25. Briani RV, Ferreira AS, Pazzinatto MF, et al. What interventions can improve quality of life or psychosocial factors of individuals with knee osteoarthritis? A systematic review with meta-analysis of primary outcomes from randomised controlled trials. Br J Sports Med. 2018. doi: 10.1136/bjsports-2017-098099.
26. Glombiewski JA, Bernardy K, Häuser W. Efficacy of EMG- and EEG-biofeedback in fibromyalgia syndrome: a meta-analysis and a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:962741.
27. Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51:199-213.
28. Kwekkeboom KL, Gretarsdottir E. Systematic review of relaxation interventions for pain. J Nurs Scholarsh. 2006;38:269-277.
29. Paige NM, Miake-Lye IM, Booth MS, et al. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain. Systematic review and meta-analysis. JAMA. 2017;317:1451-1460.
30. Furlan AD, Giraldo M, Baskwill A, et al. Massage for low-back pain. Cochrane Database Syst Rev. 2015;(9):CD001929.
31. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;(6):CD001218.
32. Lin X, Huang K, Zhu G, et al. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J Bone Joint Surg Am. 2016;98:1578-1585.
33. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;(5):CD007070.
34. Salamh P, Cook C, Reiman MP, et al. Treatment effectiveness and fidelity of manual therapy to the knee: a systematic review and meta-analysis. Musculoskeletal Care. 2017;15:238-248.
35. Posadzki P. Is spinal manipulation effective for pain? An overview of systematic reviews. Pain Med. 2012;13:754-761.
36. Perlman AI, Ali A, Njike VY, et al. Massage therapy for osteoarthritis of the knee: a randomized dose-finding trial. PLoS One. 2012;7:e30248.
37. Kalichman L. Massage therapy for fibromyalgia symptoms. Rheumatol Int. 2010;30:1151-1157.
38. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;(1):CD001977.
39. Somers TJ, Wren AA, Shelby RA. The context of pain in arthritis: self-efficacy for managing pain and other symptoms. Curr Pain Headache Rep. 2012;16:502-508.
40. Laerum E, Indahl A, Skouen JS. What is “the good back-consultation”? A combined qualitative and quantitative study of chronic low back pain patients’ interaction with and perceptions of consultations with specialists. J Rehabil Med. 2006;38:255-262.
From The Journal of Family Practice | 2018;67(8):474-477,480-483.
PRACTICE RECOMMENDATIONS
› Recommend tai chi as an exercise modality for patients with osteoarthritis. A
› Recommend mindfulness training for patients with chronic low back pain (LBP). A
› Recommend a trial of either acupuncture or spinal manipulation for patients with chronic LBP. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Diverticulitis: A Primer for Primary Care Providers
CE/CME No: CR-1808
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the pathophysiology of diverticulitis.
• Describe the spectrum of clinical presentations of diverticulitis.
• Understand the diagnostic evaluation of diverticulitis.
• Differentiate the management of uncomplicated and complicated diverticulitis.
FACULTY
Priscilla Marsicovetere is Assistant Professor of Medical Education and Surgery, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire, and Program Director for the Franklin Pierece University, PA Program, Lebanon, New Hampshire. She practices with Emergency Services of New England, Springfield Hospital, Springfield, Vermont.
The author has no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through July 31, 2019.
Article begins on next page >>
Treatment of this common complication of diverticular disease is predicated on whether the presentation signals uncomplicated or complicated disease. While some uncomplicated cases require hospitalization, many are amenable to primary care outpatient, and often conservative, management. The longstanding practice of antibiotic treatment of uncomplicated cases is now considered a selective, rather than a routine, option.
Diverticular disease is one of the most common conditions in the Western world and one of the most frequent indications for gastrointestinal-related hospitalization.1 It is among the 10 most common diagnoses in patients presenting to the clinic or emergency department with acute abdominal pain.2 Prevalence increases with age: Up to 60% of persons older than 60 are affected.3 The most common complication of diverticular disease is diverticulitis, which occurs in up to 25% of patients.4
The spectrum of clinical presentations of diverticular disease ranges from mild, uncomplicated disease that can be treated in the outpatient setting to complicated disease with sepsis and possible emergent surgical intervention. The traditional approach to diverticulitis has been management with antibiotics and likely sigmoid colectomy, but recent studies support a paradigm shift toward more conservative, nonsurgical treatment.
This article highlights current trends in diagnosis and management of acute diverticulitis.
DEFINITION AND EPIDEMIOLOGY
Diverticular disease is marked by sac-like outpouchings, called diverticula, that form at structurally weak points of the colon wall, predominantly in the descending and sigmoid colon.5 The prevalence of diverticular disease is increasing globally, affecting more than 10% of people older than 40, as many as 60% of those older than 60, and more than 70% of people older than 80.1,3 The mean age for hospital admission for acute diverticulitis is 63.3
Worldwide, males and females are affected equally.3 In Western society, the presence of diverticula, also called diverticulosis, is more often left-sided; right-sided disease is more prevalent in Asia.3,5
The most common complication of diverticular disease is diverticulitis—inflammation of a diverticulum—which affects 10% to 25% of patients with diverticular disease during their lifetime.4,5 Diverticulitis can be classified as uncomplicated (characterized by colonic wall thickening or pericolic inflammatory changes) or complicated (characterized by abscesses, fistulae, obstruction, or localized or free perforations).1,6 As many as 25% of diverticulitis cases are considered complicated.4,5 The severity of diverticulitis is commonly graded using the Hinchey Classification (Table 1).1,7
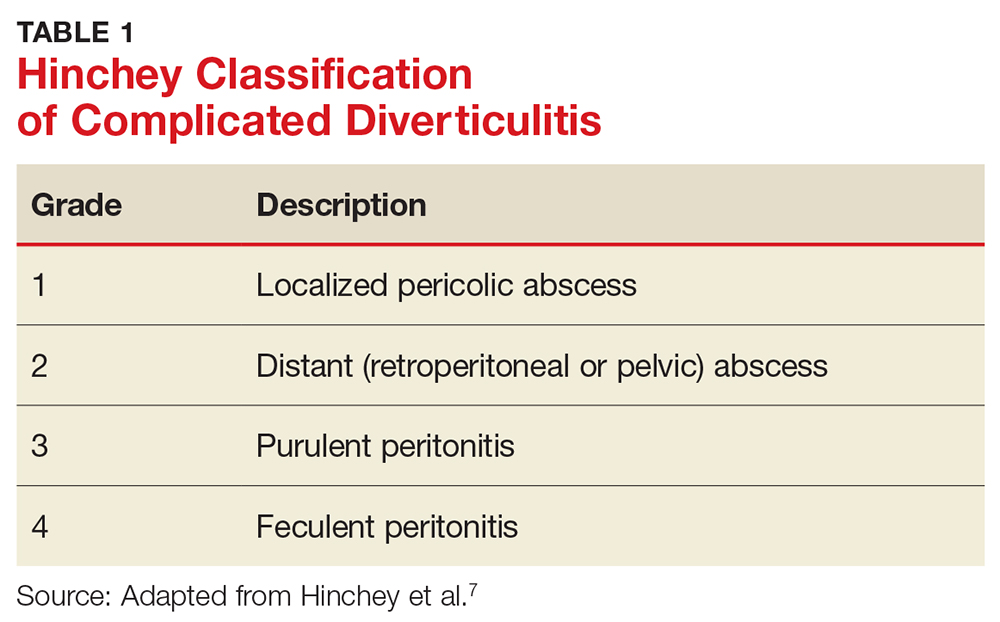
Continue to: PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Diverticula tend to occur in areas where the colonic wall is weak: namely, between the mesenteric and antimesenteric taeniae, where the vasa recta penetrate the muscle—points of entry of blood vessels through the colonic wall.1,4 The exact pathogenesis of diverticular disease is not completely understood but is thought to be multifactorial. Microscopic studies have shown muscular atrophy at the sites of diverticula, making them more susceptible to mucosal herniation in the setting of increased intraluminal pressure.1 Additional potential contributing factors include alterations in colonic microbiota, muscular dysfunction or dysmotility, lifestyle, and genetics.
Diverticulitis is the result of microscopic and macroscopic perforation of diverticula. Historically, the perforations were thought to result from obstruction of a diverticulum by a fecalith, leading to increased pressure within the outpouching, followed by perforation.3 Such obstruction is now thought to be rare. A more recent theory suggests that increased intraluminal pressure is due to inspissated food particles that lead to erosion of the diverticular wall, causing focal inflammation and necrosis and resulting in perforation.3 Microperforations can easily be contained by surrounding mesenteric fat; however, progression to abscess, fistulization, or intestinal obstruction can occur. Frank bowel wall perforation is not contained by mesenteric fat and can lead quickly to peritonitis and death if not treated emergently.
RISK FACTORS
Dietary fiber
In 1971, Burkitt was the first to posit that diverticular disease developed due to small quantities of fiber in the diet that led to increased intracolonic pressures.8 His theory was based on the observation that residents of several African countries, who ate a high-fiber diet, had a low incidence of diverticular disease. Burkitt hypothesized that this was due to shorter colonic transit time induced by high dietary fiber.
Several studies conducted since Burkitt made his observations have examined the association of dietary fiber and diverticular disease, with conflicting results. In 1998, Aldoori et al found that a low-fiber diet increases the incidence of symptomatic diverticular disease.9 However, in 2012, a large cohort study of patients undergoing colonoscopy found that those who reported the highest fiber intake were at highest risk for diverticulosis.10 In 2013, Peery et al examined the relationship among bowel habits, dietary fiber, and asymptomatic diverticulosis and found that less-frequent bowel movements and hard stools were associated with a decreased risk for diverticulosis.11 In 2017, a prospective cohort study of nearly 50,000 men without a known history of diverticulosis showed that diets high in red meat were associated with a higher incidence of diverticulitis over nearly three decades of follow-up, whereas a diet high in fiber was associated with a decreased incidence of diverticulitis.12
Although no definitive association has been found between dietary fiber intake and risk for diverticulosis, some studies have demonstrated an association between dietary fiber and diverticular complications. In 2014, Crowe et al found that consumption of a high-fiber diet was associated with a lower risk for hospital admission and death from diverticular disease.13 Recent guidelines from the American Gastroenterological Association (AGA) on diverticulitis recommend high dietary fiber intake in patients with a history of acute diverticulitis.14 However, no study has shown a reversal of the process or a reduction in the number of episodes of diverticulitis after adoption of a high-fiber diet.
Continue to: Historically, patients with diverticulitis...
Historically, patients with diverticulitis were advised to avoid eating nuts, corn, popcorn, and seeds to reduce the risk for complications. But studies have found no support for this caution. In a 2008 large, prospective study of men without known diverticular disease, the researchers found no association between nut, corn, or popcorn ingestion and diverticulitis; in fact, increased nut intake was specifically associated with a lower risk for diverticulitis.15
Smoking
Smoking has been linked to diverticulitis and has been associated with a threefold risk for complications, including severe diverticulitis.16,17 An increased risk for recurrent episodes has also been found in smokers following surgical intervention.17
Medications
NSAIDs, corticosteroids, and opioids have been associated with an increased risk for perforated diverticulitis.18,19 A significant association has been found between NSAID use and severity of diverticulitis, including perforation; one study reported a relative risk of 1.25 (95% confidence interval, 10.5 to 1.47) for diverticulitis with regular use of aspirin (≥ 2x/wk).20,21
More frequent steroid use has been found in patients with complicated diverticulitis, compared to patients with uncomplicated disease (7.3% vs 3.3%; P = .015).22 A systematic review of five studies comparing patients with and without steroid use showed significantly higher odds of diverticular perforation in patients taking a steroid.23 Pooled data showed significantly increased odds of perforation and abscess formation with use of an NSAID (odds ratio [OR], 2.49), steroid (OR, 9.08), or opioid (OR, 2.52).22
Continue to: Vitamin D
Vitamin D
In a 2013 retrospective cohort study of 9,116 patients with uncomplicated diverticulosis and 922 patients who developed diverticulitis that required hospitalization, Maguire et al examined the association of prediagnostic serum levels of vitamin D and diverticulitis.24 Among patients with diverticulosis, higher prediagnostic levels of 25-hydroxyvitamin D were significantly associated with a lower risk for diverticulitis—indicating that vitamin D deficiency could be involved in the pathogenesis of diverticulitis.
The association between diverticulitis and vitamin D levels was supported by an additional study in 2015, in which the authors investigated the association between ultraviolet (UV) light and diverticulitis.25 They identified nonelective diverticulitis admissions in the Nationwide Inpatient Sample database and linked hospital locations to geographic UV data. They examined UV exposure in relation to risk for admission for diverticulitis and found that, compared with high-UV (UV4) areas, low-UV (UV1) areas had a higher rate of diverticulitis (751.8/100,000 admissions, compared with 668.1/100,000 admissions, respectively [P < .001]), diverticular abscess (12.0% compared with 9.7% [P < .001]), and colectomy (13.5% compared with 11.5% [P < .001]). They also observed significant seasonal variation, with a lower rate of diverticulitis in winter (645/100,000 admissions) compared with summer (748/100,000 admissions [P < .001]). Because UV exposure largely determines vitamin D status, these findings are thought to support a role for vitamin D in the pathogenesis of diverticulitis.
Genetics
Two studies found an association between genetics and diverticular disease. A 2012 study using The Swedish Twin Registry found that if one twin is affected with the disease, the odds that the other will be affected was 7.15 in monozygotic (identical) twins and 3.20 in dizygotic (fraternal) twins.26 A 2013 Danish twin study found a relative risk of 2.92 in twin siblings compared to the general population.27 Both studies estimated the genetic contribution to diverticular disease to be 40% to 50%.26,27
Obesity
Several large prospective studies have shown a positive association between high BMI, waist circumference, and waist-to-hip ratio and risk for diverticulitis.4 A BMI > 30 was found to increase the relative risk of acute diverticulitis by 1.78, compared with a normal BMI.17 In a large, prospective, population-based cohort study in 2016, Jamal Talabani et al found that obese persons had twice the risk for admission for acute colonic diverticulitis than normal-weight persons did.28 Waist circumference and waist-to-hip ratio were also independently associated with risk for diverticulitis. The pathophysiology of the associations is not clearly understood but may involve pro-inflammatory changes of adipose tissue, which secrete cytokines that promote an inflammatory response, and changes in gut microbiota.4,12
Physical activity
Data on the association of physical activity and diverticulitis is inconsistent. Some studies have found as much as a 25% decrease in the risk for diverticulitis with increased physical activity; more recent studies (2013 and 2016), on the other hand, found no association between diverticulosis and physical activity.11,17,19,28
Continue to: CLINICAL PRESENTATION
CLINICAL PRESENTATION
The clinical presentation of diverticulitis typically depends on the severity of inflammation and the presence (or absence) of complications. The most common presenting symptom is left lower-quadrant abdominal pain, which occurs in approximately 70% of cases and lasts for longer than 24 hours.29 Fever (usually < 102°F), leukocytosis, nausea, vomiting, and changes in bowel function may also be present.1,30,31 Approximately 50% of patients report constipation in diverticular disease; 20% to 35% report diarrhea.5
Patients may also report dysuria, secondary to irritation of the bladder by an inflamed segment of colon.3,17 Patients may report fecaluria, pneumaturia, or pyuria, which indicate a colovesical fistula.1 Passage of feces or flatus through the vagina indicates a colovaginal fistula.
The differential diagnosis of diverticulitis is listed in Table 2.17
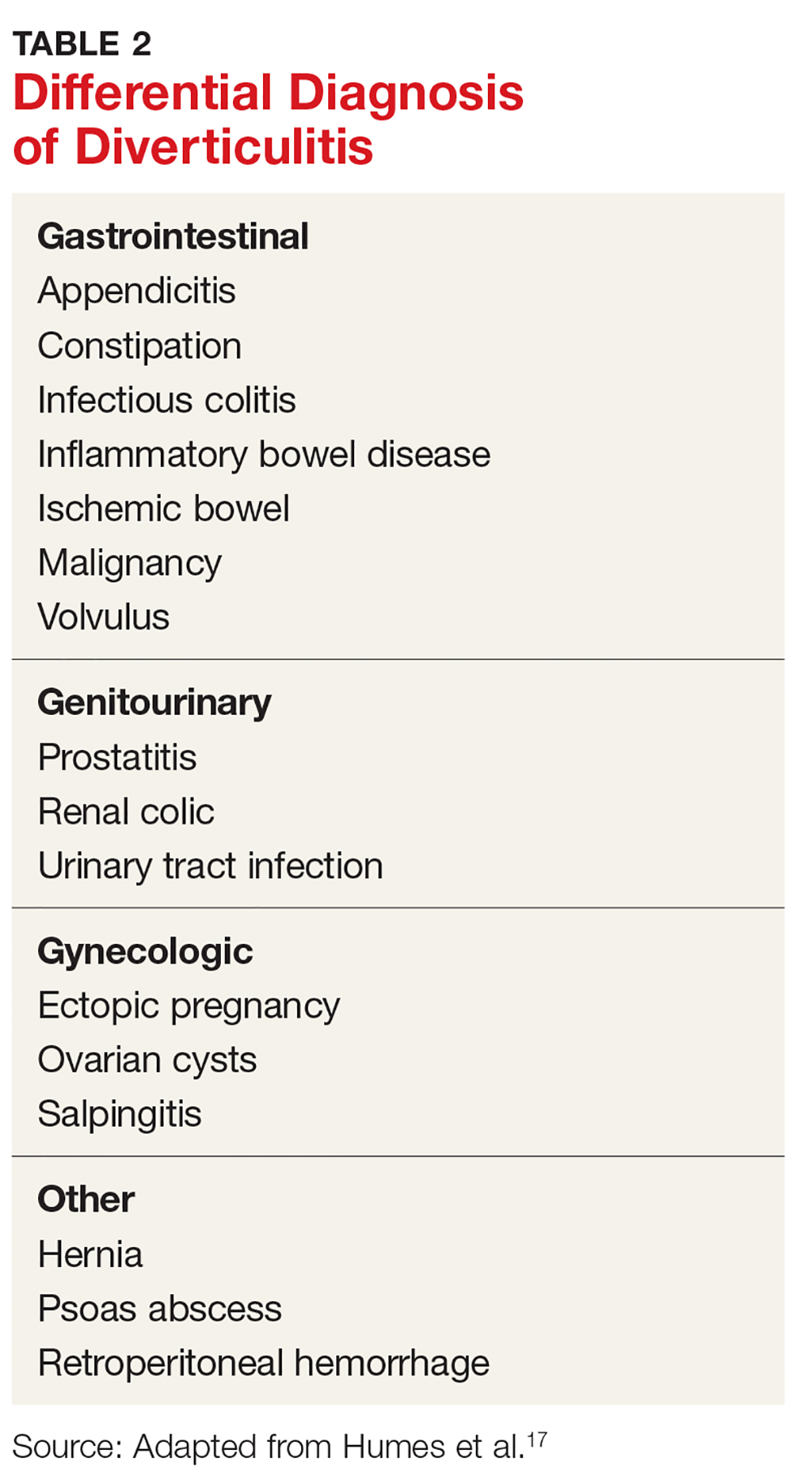
PHYSICAL EXAMINATION
Physical examination in diverticulitis will almost always elicit tenderness to palpation over the area of inflammation, typically in the left lower quadrant. This is due to irritation of the peritoneum.3 A palpable mass may be present in as many as 20% of patients if an abscess is present. Bowel sounds may be hypoactive or hyperactive if there is a bowel obstruction.17 In cases of frank bowel-wall perforation, patients can present with peritoneal signs of rigidity, guarding, and rebound tenderness.3,31 Tachycardia, hypotension, and shock are rare but possible findings. Digital rectal examination may reveal tenderness or a mass if a pelvic abscess is present.17,31
DIAGNOSTICS
The diagnosis of acute diverticulitis can often be made clinically, based on the history and physical examination. Because clinical diagnosis can be inaccurate in as many as 68% of cases, however, laboratory testing and imaging play an important role in diagnosis.3
Continue to: Clinical laboratory studies
Clinical laboratory studies
Because leukocytosis is present in approximately one-half of patients with diverticulitis, a complete blood count (CBC) should be obtained; that recommendation notwithstanding, approximately one-half of patients with diverticulitis have a normal white blood cell count.29,30 A urine test of human chorionic gonadotropin should be ordered to exclude pregnancy in all premenopausal and perimenopausal women, particularly if antibiotics, imaging, or surgery are being considered.31 Urinalysis can assess for urinary tract infection.
Multiple studies have demonstrated the utility of C-reactive protein (CRP) in the workup of acute diverticulitis. In general, patients with a complicated episode will present with a significantly higher CRP level than that of uncomplicated disease.32 Kechagias et al found that the CRP level at initial evaluation may be helpful in predicting the clinical severity of the attack. A CRP level > 170 mg/L has been found to have a greater probability of severe disease, warranting CT and referral for hospitalization.33 A low CRP level was more likely to herald a mild course of disease that is amenable to outpatient antibiotic management or supportive care. This finding is consistent with previous reports of the association between CRP levels of 90 to 200 mg/L and the severity of diverticulitis.32,34
Imaging
Abdominopelvic CT with intravenous (IV) contrast. This imaging study is the gold standard diagnostic tool for diverticulitis, with sensitivity as high as 97%.3 CT can distinguish diverticulitis from other conditions, such as irritable bowel syndrome (based on a history of symptoms and the absence of CT findings), gastroenteritis, and gynecologic disease. It can also distinguish between uncomplicated and complicated diverticulitis and therefore guide therapeutic interventions, such as percutaneous drainage of an intra-abdominal abscess. CT findings associated with uncomplicated diverticulitis include colonic wall thickening and pericolonic fluid and inflammatory changes, such as fat stranding. CT findings associated with complicated disease include abscess (paracolonic or pelvic), peritonitis (purulent or feculent), phlegmon, perforation, fistula, and obstruction.1,3
Ultrasonography (US) can also be used in the assessment of diverticulitis, although it has lower sensitivity (approximately 61% to 84%) than CT and is inferior to CT for showing the extent of large abscesses or free air.3,18,30 A typical US finding in acute diverticulitis is a thickened loop of bowel with a target-like appearance.17 Findings are highly operator-dependent, however, and accuracy is diminished in obese patients. US may be a good option for pregnant women to avoid ionizing radiation.
Magnetic resonance imaging (MRI) is another option for imaging in diverticulitis but is not routinely recommended. It provides excellent soft-tissue detail and does not deliver ionizing radiation, but it is not as sensitive as CT for identifying free air.18,31 Furthermore, MRI requires prolonged examination time, which may not be tolerated by acutely ill patients, and is not an option for patients with certain types of surgical clips, metallic fragments, or a cardiac pacemaker.
Continue to: Abdominal radiography...
Abdominal radiography is useful to show free air, which would indicate perforation, and to show nonspecific abnormalities, such as bowel-gas patterns.31
MANAGEMENT
For decades, patients with diverticulitis were managed with antibiotics to cover colonic flora; many underwent urgent or emergent surgery to remove the affected segment of colon. Over the years, however, the treatment paradigm has shifted from such invasive management toward a nonsurgical approach—often, with equivalent or superior outcomes. More and more, management of diverticulitis is dictated by disease presentation: namely, whether disease is uncomplicated or complicated.1
Current guidelines recommend hospitalization, with possible surgical intervention, in complicated disease (free perforation, large abscesses, fistula, obstruction, stricture) and in patients who cannot tolerate oral hydration, who have a relevant comorbidity, or who do not have adequate support at home.35 Uncomplicated cases may also require hospitalization if certain criteria for admission are met: immunosuppression, severe or persistent abdominal pain, inability to tolerate oral intake, and significant comorbidity.5
Absent these criteria, outpatient management of uncomplicated diverticulitis is appropriate. After the treatment setting is determined, choice of intervention and length of treatment should be addressed.
Nonpharmacotherapeutic management
Dietary restrictions, from a full liquid diet to complete bowel rest, have been recommended for the management of acute diverticulitis. This recommendation is not supported by the literature, however. At least two studies have shown no association between an unrestricted diet and an increase in diverticular complications. In a 2013 retrospective cohort study, no increase in diverticular perforation or abscess was found with a diet of solid food compared to a liquid diet, a clear liquid diet, or no food by mouth.36 In a more recent (2017) prospective cohort study of 86 patients with uncomplicated diverticulitis, all of whom were on an unrestricted diet, only 8% developed complications.37
Continue to: There is no high-quality evidence for...
There is no high-quality evidence for instituting dietary restrictions in acute uncomplicated diverticulitis. As such, permitting oral intake as tolerated is a reasonable option.
Pharmacotherapy
Antibiotics have long been the cornerstone of pharmacotherapy for acute diverticulitis, covering gram-negative rods and anaerobes. The rationale for such management is the long-held belief that diverticulitis is caused by an infectious process.38 Common outpatient regimens include
- Ciprofloxacin (500 mg every 12 h) plus metronidazole (500 mg every 8 h)
- Trimethoprim–sulfamethoxazole (1 double-strength tablet every 12 h) plus metronidazole (500 mg every 8 h)
- Amoxicillin (875 mg)–clavulanate (1 tablet every 8 h) or extended-release amoxicillin–clavulanate (2 tablets every 12 h)
- Moxifloxacin (400 mg/d; for patients who cannot tolerate metronidazole or ß-lactam antibiotics).
Providers should always consult their local antibiogram to avoid prescribing antibiotics to which bacterial resistance exceeds 10%.
Despite widespread use of antibiotics for diverticulitis, multiple studies in recent years have shown no benefit to their use for uncomplicated cases. In 2012, Chabok et al investigated the need for antibiotic therapy to treat acute uncomplicated diverticulitis and found no statistically significant difference in outcome among patients treated with antibiotics and those managed conservatively.39 In 2014, Isacson et al performed a retrospective population-based cohort study to assess the applicability of a selective “no antibiotic” policy and its consequences in terms of complications and recurrence; the authors found that withholding antibiotics was safe and did not result in a higher complication or recurrence rate.40 Furthermore, in a 2017 multicenter study, Daniels et al conducted a randomized controlled trial comparing observation and antibiotic treatment for a first episode of uncomplicated acute diverticulitis in 528 patients and found no prolongation of recovery time, no increased rate of complications, and no need for surgical intervention in patients who were not treated with antibiotics.41
These studies are in agreement with the most recent AGA guidelines, which recommend selective, rather than routine, use of antibiotics for acute diverticulitis.14 This shift in approach may be due, in part, to a change in understanding of the etiology of the disease—from an infectious process to more of an inflammatory process.38
Continue to: For patients who require inpatient management of diverticulitis...
For patients who require inpatient management of diverticulitis, treatment typically involves IV antibiotics, fluids, and analgesics. Surgical treatment may be appropriate (see “Surgical treatment”).
Other agents used to manage diverticulitis include three that lack either strong or any data at all showing efficacy. The most recent AGA guidelines recommend against their use for this indication14:
Rifaximin. Two recent observational cohort studies, one from 2013 and the other from 2017, compared this poorly absorbed oral antibiotic with mesalamine to placebo or no treatment at all.42 Neither provided evidence that rifaximin treats or prevents diverticulitis.
Mesalamine. This anti-inflammatory has also been studied to prevent recurrence of diverticulitis. In a randomized, double-blind, placebo-controlled multicenter trial of 1,182 patients, Raskin et al found that mesalamine did not reduce the rate of recurrence of diverticulitis, time to recurrence, or the number of patients requiring surgery.43 This conclusion was reiterated by a 2016 meta-analysis that found no evidence to support use of mesalamine in the prevention of diverticulitis recurrence.44
Probiotics. Despite multiple studies undertaken to assess the efficacy of probiotics in the prevention and treatment of diverticular disease, strong data supporting their use are sparse. In 2016, Lahner et al examined 11 studies in which various probiotics were used to treat diverticular disease and found that, although there was a weak positive trend in the reduction and remission of abdominal symptoms, the evidence was not strong enough to recommend their routine use in managing the disease.45
Continue to: Surgical treatment
Surgical treatment
Acute uncomplicated diverticulitis can be treated nonsurgically in nearly all patients, regardless of whether treatment occurs in the inpatient or outpatient setting. For complicated disease, however, approximately 15% to 25% of patients require surgery. The main indication for emergent or urgent surgical intervention is colonic perforation, which can lead to acute peritonitis, sepsis, and associated morbidity and mortality.29
The decision to perform elective surgery should be made case by case, not routinely—such as after a recurrent episode of diverticulitis, when there has been a complication, or in young patients (< 50 years).1,11 Immunocompromised patients (transplant recipients, patients taking steroids chronically, and patients with HIV infection who have a CD4 count < 200 cells/μL) can present with more virulent episodes of diverticulitis, have a higher incidence of perforation and fecal peritonitis, and have a greater likelihood of failure of nonsurgical management.1 Surgical intervention after the first episode of diverticulitis in these patients should therefore be considered.
In 2014, the American Society of Colon and Rectal Surgeons (ASCRS) recommended the laparoscopic Hartmann procedure (primary resection of the affected segment of colon, with end colostomy, followed by colostomy closure) as the gold standard for the treatment of acute perforated diverticular disease when surgery is required.46
COLONOSCOPY AFTER DIVERTICULITIS
Although endoscopy is to be avoided during acute diverticulitis because of the risk for perforation, it is recommended six to eight weeks after the acute episode has resolved to rule out malignancy, inflammatory bowel disease, and colitis.1,3 Interestingly, in 2015, Daniels et al compared the colonoscopic detection rate of advanced colonic neoplasia in patients with a first episode of acute diverticulitis and in patients undergoing initial screening for colorectal cancer, and found no significant difference in the detection rate between the two groups.47 The authors concluded that routine colonoscopic follow-up after an episode of acute uncomplicated diverticulitis could be eliminated and that those patients could be screened according to routine guidelines.
Lau et al found a number of cancers and other significant lesions on colonoscopy performed after an episode of acute diverticulitis, with a 2.1% prevalence of colorectal cancer within one year after CT-proven diverticulitis, and an increase in the prevalence of abscess, local perforation, and fistula.48 Their study excluded patients who had had a colonoscopy within one year, however. They therefore recommended performing colonoscopy only for patients who have not had a recent colonoscopic exam. This recommendation is in accord with the most recent AGA and ASCRS guidelines. If a patient has had a recent colonoscopy prior to an acute episode of diverticulitis, the value of repeating the study after the episode resolves is unclear.
Continue to: CONCLUSION
CONCLUSION
As this article shows, the spectrum of clinical presentations for diverticulitis is broad, and management most often requires a case-by-case approach. Treatment is dictated by whether disease presentation is uncomplicated or complicated; outpatient management is appropriate for uncomplicated cases in the absence of specific criteria for hospitalization. Recent evidence supports a paradigm shift away from mandatory dietary restriction and routine antibiotic use.
1. Deery SE, Hodin RA. Management of diverticulitis in 2017. J Gastrointest Surg. 2017;21(10):1732-1741.
2. Boermeester M, Humes D, Velmahos G, et al. Contemporary review of risk-stratified management in acute uncomplicated and complicated diverticulitis. World J Surg. 2016;40(10):2537-2545.
3. Linzay C, Pandit S. Diverticulitis, acute. [Updated 2017 Nov 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018- Jan.
4. Rezapour M, Ali S, Stollman N. Diverticular disease: an update on pathogenesis and management. Gut Liver. 2018;12(2):125-132.
5. Mayl J, Marchenko M, Frierson E. Management of acute uncomplicated diverticulitis may exclude antibiotic therapy. Cureus. 2017;9(5):e1250.
6. Chung BH, Ha GW, Lee MR, Kim JH. Management of colonic diverticulitis tailored to location and severity: comparison of the right and the left colon. Ann Coloproctol. 2016;32(6):228-233.
7. Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg. 1978;12:85-109.
8. Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer. 1971;28(1):3-13.
9. Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. 1998;128(4):714-719.
10. Peery AF, Barrett PR, Park D, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012;142(2):266-272.
11. Peery AF, Sandler RS, Ahnen DJ, et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol. 2013;11(12):1622-1627.
12. Strate LL, Keeley BR, Cao Y, et al. Western dietary pattern increases, whereas prudent dietary pattern decreases, risk of incident diverticulitis in a prospective cohort study. Gastroenterology. 2017;152(5):1023-1030.
13. Crowe FL, Balkwill A, Cairns BJ, et al; Million Women Study Collaborators. Source of dietary fibre and diverticular disease incidence: a prospective study of UK women. Gut. 2014;63(9):1450-1456.
14. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology 2015;149(7):1944-1949.
15. Strate LL, Liu YL, Syngal S, et al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300(8):907-914.
16. Hjern F, Wolk A, Håkansson N. Smoking and the risk of diverticular disease in women. Br J Surg. 2011;98(7):997-1002.
17. Humes DJ, Spiller RC. Review article: The pathogenesis and management of acute colonic diverticulitis. Aliment Pharmacol Ther. 2014;39(4):359-370.
18. Moubax K, Urbain D. Diverticulitis: new insights on the traditional point of view. Acta Gastroenterol Belg. 2015;78(1):38-48.
19. Morris AM, Regenbogen SE, Hardiman KM, Hendren S. Sigmoid diverticulitis: a systematic review. JAMA. 2014; 311(3):287-297.
20. Tan JP, Barazanchi AW, Singh PP, et al. Predictors of acute diverticulitis severity: a systematic review. Int J Surg. 2016;26:43-52.
21. Strate LL, Liu YL, Huang ES, et al. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology. 2011;140(5):1427-1433.
22. Nizri E, Spring S, Ben-Yehuda A, et al. C-reactive protein as a marker of complicated diverticulitis in patients on anti-inflammatory medications. Tech Coloproctol. 2014; 18(2):145-149.
23. Kvasnovsky CL, Papagrigoriadis S, Bjarnason I. Increased diverticular complications with nonsteroidal anti-inflammatory drugs and other medications: a systematic review and meta-analysis. Colorectal Dis. 2014; 16(6):O189-O196.
24. Maguire LH, Song M, Strate LL, et al. Higher serum levels of vitamin D are associated with a reduced risk of diverticulitis. Clin Gastroenterol Hepatol. 2013;11(12):1631-1635.
25. Maguire LH, Song M, Strate LL, et al. Association of geographic and seasonal variation with diverticulitis admissions. JAMA Surg. 2015;150(1):74-77.
26. Granlund J, Svensson T, Olén O, et al. The genetic influence on diverticular disease—a twin study. Aliment Pharmacol Ther. 2012;35(9):1103-1107.
27. Strate LL, Erichsen R, Baron JA, et al. Heritability and familial aggregation of diverticular disease: a population-based study of twins and siblings. Gastroenterology. 2013;144(4):736-742.
28. Jamal Talabani A, Lydersen S, Ness-Jensen E, et al. Risk factors of admission for acute colonic diverticulitis in a population-based cohort study: The North Trondelag Health Study, Norway. World J Gastroenterol. 2016; 22(48):10663-10672.
29. Horesh N, Wasserberg N, Zbar AP, et al. Changing paradigms in the management of diverticulitis. Int J Surg. 2016(33 pt A):146-150.
30. McSweeney W, Srinath H. Diverticular disease practice points. Aust Fam Physician. 2017;46(11):829-832.
31. Wilkins T, Embry K, George R. Diagnosis and management of acute diverticulitis. Am Fam Physician. 2013; 87(9):612-620.
32. van de Wall BJ, Draaisma WA, van der Kaaij RT, et al. The value of inflammation markers and body temperature in acute diverticulitis. Colorectal Dis. 2013;15(5):621-626.
33. Kechagias A, Rautio T, Kechagias G, Mäkelä J. The role of C-reactive protein in the prediction of the clinical severity of acute diverticulitis. Am Surg. 2014;80(4):391-395.
34. Bolkenstein HE, van de Wall BJM, Consten ECJ, et al. Risk factors for complicated diverticulitis: systematic review and meta-analysis. Int J Colorectal Dis. 2017; 32(10):1375-1383.
35. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57(3):284-294.
36. van de Wall BJ, Draaisma WA, van Iersel JJ, et al. Dietary restrictions for acute diverticulitis: evidence-based or expert opinion? Int J Colorectal Dis. 2013;28(9):1287-1293.
37. Stam MA, Draaisma WA, van de Wall BJ, et al. An unrestricted diet for uncomplicated diverticulitis is safe: results of a prospective diverticulitis diet study. Colorectal Dis. 2017;19(4):372-377.
38. Khan DZ, Kelly ME, O’Reilly J, et al. A national evaluation of the management practices of acute diverticulitis. Surgeon. 2017;15(4):206-210.
39. Chabok A, Påhlman L, Hjern F, et al; AVOD Study Group. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99(4):532-539.
40. Isacson D, Andreasson K, Nikberg M, et al. No antibiotics in acute uncomplicated diverticulitis: does it work? Scand J Gastroenterol. 2014;49(12):1441-1446.
41. Daniels L, Ünlü Ç, de Korte N, et al; Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104(1):52-61.
42. van Dijk S, Rottier SJ, van Geloven AAW, Boermeester MA. Conservative treatment of acute colonic diverticulitis. Curr Infect Dis Rep. 2017;19(11):44.
43. Raskin J, Kamm M, Jamal M, Howden CW. Mesalamine did not prevent recurrent diverticulitis in phase 3 controlled trials. Gastroenterology. 2014;147:793-802.
44. Kahn M, Ali B, Lee W, et al. Mesalamine does not help prevent recurrent acute colonic diverticulitis: meta-analysis of randomized, placebo-controlled trials. Am J Gastroenterol. 2016;111(4):579-581.
45. Lahner E, Bellisario C, Hassan C, et al. Probiotics in the treatment of diverticular disease. A systematic review. J Gastrointestin Liver Dis. 2016;25(1):79-86.
46. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57(3):284-294.
47. Daniels I, Ünlü Ç, de Wijkerslooth TR, et al. Yield of colonoscopy after recent CT-proven uncomplicated acute diverticulitis: a comparative cohort study. Surg Endosc. 2015;29(9):2605-2613.
48. Lau KC, Spilsbury K, Farooque Y, et al. Is colonoscopy still mandatory after a CT diagnosis of left-sided diverticulitis: can colorectal cancer be confidently excluded? Dis Colon Rectum. 2011;54(10):1265-1270.
CE/CME No: CR-1808
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the pathophysiology of diverticulitis.
• Describe the spectrum of clinical presentations of diverticulitis.
• Understand the diagnostic evaluation of diverticulitis.
• Differentiate the management of uncomplicated and complicated diverticulitis.
FACULTY
Priscilla Marsicovetere is Assistant Professor of Medical Education and Surgery, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire, and Program Director for the Franklin Pierece University, PA Program, Lebanon, New Hampshire. She practices with Emergency Services of New England, Springfield Hospital, Springfield, Vermont.
The author has no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through July 31, 2019.
Article begins on next page >>
Treatment of this common complication of diverticular disease is predicated on whether the presentation signals uncomplicated or complicated disease. While some uncomplicated cases require hospitalization, many are amenable to primary care outpatient, and often conservative, management. The longstanding practice of antibiotic treatment of uncomplicated cases is now considered a selective, rather than a routine, option.
Diverticular disease is one of the most common conditions in the Western world and one of the most frequent indications for gastrointestinal-related hospitalization.1 It is among the 10 most common diagnoses in patients presenting to the clinic or emergency department with acute abdominal pain.2 Prevalence increases with age: Up to 60% of persons older than 60 are affected.3 The most common complication of diverticular disease is diverticulitis, which occurs in up to 25% of patients.4
The spectrum of clinical presentations of diverticular disease ranges from mild, uncomplicated disease that can be treated in the outpatient setting to complicated disease with sepsis and possible emergent surgical intervention. The traditional approach to diverticulitis has been management with antibiotics and likely sigmoid colectomy, but recent studies support a paradigm shift toward more conservative, nonsurgical treatment.
This article highlights current trends in diagnosis and management of acute diverticulitis.
DEFINITION AND EPIDEMIOLOGY
Diverticular disease is marked by sac-like outpouchings, called diverticula, that form at structurally weak points of the colon wall, predominantly in the descending and sigmoid colon.5 The prevalence of diverticular disease is increasing globally, affecting more than 10% of people older than 40, as many as 60% of those older than 60, and more than 70% of people older than 80.1,3 The mean age for hospital admission for acute diverticulitis is 63.3
Worldwide, males and females are affected equally.3 In Western society, the presence of diverticula, also called diverticulosis, is more often left-sided; right-sided disease is more prevalent in Asia.3,5
The most common complication of diverticular disease is diverticulitis—inflammation of a diverticulum—which affects 10% to 25% of patients with diverticular disease during their lifetime.4,5 Diverticulitis can be classified as uncomplicated (characterized by colonic wall thickening or pericolic inflammatory changes) or complicated (characterized by abscesses, fistulae, obstruction, or localized or free perforations).1,6 As many as 25% of diverticulitis cases are considered complicated.4,5 The severity of diverticulitis is commonly graded using the Hinchey Classification (Table 1).1,7

Continue to: PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Diverticula tend to occur in areas where the colonic wall is weak: namely, between the mesenteric and antimesenteric taeniae, where the vasa recta penetrate the muscle—points of entry of blood vessels through the colonic wall.1,4 The exact pathogenesis of diverticular disease is not completely understood but is thought to be multifactorial. Microscopic studies have shown muscular atrophy at the sites of diverticula, making them more susceptible to mucosal herniation in the setting of increased intraluminal pressure.1 Additional potential contributing factors include alterations in colonic microbiota, muscular dysfunction or dysmotility, lifestyle, and genetics.
Diverticulitis is the result of microscopic and macroscopic perforation of diverticula. Historically, the perforations were thought to result from obstruction of a diverticulum by a fecalith, leading to increased pressure within the outpouching, followed by perforation.3 Such obstruction is now thought to be rare. A more recent theory suggests that increased intraluminal pressure is due to inspissated food particles that lead to erosion of the diverticular wall, causing focal inflammation and necrosis and resulting in perforation.3 Microperforations can easily be contained by surrounding mesenteric fat; however, progression to abscess, fistulization, or intestinal obstruction can occur. Frank bowel wall perforation is not contained by mesenteric fat and can lead quickly to peritonitis and death if not treated emergently.

RISK FACTORS
Dietary fiber
In 1971, Burkitt was the first to posit that diverticular disease developed due to small quantities of fiber in the diet that led to increased intracolonic pressures.8 His theory was based on the observation that residents of several African countries, who ate a high-fiber diet, had a low incidence of diverticular disease. Burkitt hypothesized that this was due to shorter colonic transit time induced by high dietary fiber.
Several studies conducted since Burkitt made his observations have examined the association of dietary fiber and diverticular disease, with conflicting results. In 1998, Aldoori et al found that a low-fiber diet increases the incidence of symptomatic diverticular disease.9 However, in 2012, a large cohort study of patients undergoing colonoscopy found that those who reported the highest fiber intake were at highest risk for diverticulosis.10 In 2013, Peery et al examined the relationship among bowel habits, dietary fiber, and asymptomatic diverticulosis and found that less-frequent bowel movements and hard stools were associated with a decreased risk for diverticulosis.11 In 2017, a prospective cohort study of nearly 50,000 men without a known history of diverticulosis showed that diets high in red meat were associated with a higher incidence of diverticulitis over nearly three decades of follow-up, whereas a diet high in fiber was associated with a decreased incidence of diverticulitis.12
Although no definitive association has been found between dietary fiber intake and risk for diverticulosis, some studies have demonstrated an association between dietary fiber and diverticular complications. In 2014, Crowe et al found that consumption of a high-fiber diet was associated with a lower risk for hospital admission and death from diverticular disease.13 Recent guidelines from the American Gastroenterological Association (AGA) on diverticulitis recommend high dietary fiber intake in patients with a history of acute diverticulitis.14 However, no study has shown a reversal of the process or a reduction in the number of episodes of diverticulitis after adoption of a high-fiber diet.
Continue to: Historically, patients with diverticulitis...
Historically, patients with diverticulitis were advised to avoid eating nuts, corn, popcorn, and seeds to reduce the risk for complications. But studies have found no support for this caution. In a 2008 large, prospective study of men without known diverticular disease, the researchers found no association between nut, corn, or popcorn ingestion and diverticulitis; in fact, increased nut intake was specifically associated with a lower risk for diverticulitis.15
Smoking
Smoking has been linked to diverticulitis and has been associated with a threefold risk for complications, including severe diverticulitis.16,17 An increased risk for recurrent episodes has also been found in smokers following surgical intervention.17
Medications
NSAIDs, corticosteroids, and opioids have been associated with an increased risk for perforated diverticulitis.18,19 A significant association has been found between NSAID use and severity of diverticulitis, including perforation; one study reported a relative risk of 1.25 (95% confidence interval, 10.5 to 1.47) for diverticulitis with regular use of aspirin (≥ 2x/wk).20,21
More frequent steroid use has been found in patients with complicated diverticulitis, compared to patients with uncomplicated disease (7.3% vs 3.3%; P = .015).22 A systematic review of five studies comparing patients with and without steroid use showed significantly higher odds of diverticular perforation in patients taking a steroid.23 Pooled data showed significantly increased odds of perforation and abscess formation with use of an NSAID (odds ratio [OR], 2.49), steroid (OR, 9.08), or opioid (OR, 2.52).22
Continue to: Vitamin D
Vitamin D
In a 2013 retrospective cohort study of 9,116 patients with uncomplicated diverticulosis and 922 patients who developed diverticulitis that required hospitalization, Maguire et al examined the association of prediagnostic serum levels of vitamin D and diverticulitis.24 Among patients with diverticulosis, higher prediagnostic levels of 25-hydroxyvitamin D were significantly associated with a lower risk for diverticulitis—indicating that vitamin D deficiency could be involved in the pathogenesis of diverticulitis.
The association between diverticulitis and vitamin D levels was supported by an additional study in 2015, in which the authors investigated the association between ultraviolet (UV) light and diverticulitis.25 They identified nonelective diverticulitis admissions in the Nationwide Inpatient Sample database and linked hospital locations to geographic UV data. They examined UV exposure in relation to risk for admission for diverticulitis and found that, compared with high-UV (UV4) areas, low-UV (UV1) areas had a higher rate of diverticulitis (751.8/100,000 admissions, compared with 668.1/100,000 admissions, respectively [P < .001]), diverticular abscess (12.0% compared with 9.7% [P < .001]), and colectomy (13.5% compared with 11.5% [P < .001]). They also observed significant seasonal variation, with a lower rate of diverticulitis in winter (645/100,000 admissions) compared with summer (748/100,000 admissions [P < .001]). Because UV exposure largely determines vitamin D status, these findings are thought to support a role for vitamin D in the pathogenesis of diverticulitis.
Genetics
Two studies found an association between genetics and diverticular disease. A 2012 study using The Swedish Twin Registry found that if one twin is affected with the disease, the odds that the other will be affected was 7.15 in monozygotic (identical) twins and 3.20 in dizygotic (fraternal) twins.26 A 2013 Danish twin study found a relative risk of 2.92 in twin siblings compared to the general population.27 Both studies estimated the genetic contribution to diverticular disease to be 40% to 50%.26,27
Obesity
Several large prospective studies have shown a positive association between high BMI, waist circumference, and waist-to-hip ratio and risk for diverticulitis.4 A BMI > 30 was found to increase the relative risk of acute diverticulitis by 1.78, compared with a normal BMI.17 In a large, prospective, population-based cohort study in 2016, Jamal Talabani et al found that obese persons had twice the risk for admission for acute colonic diverticulitis than normal-weight persons did.28 Waist circumference and waist-to-hip ratio were also independently associated with risk for diverticulitis. The pathophysiology of the associations is not clearly understood but may involve pro-inflammatory changes of adipose tissue, which secrete cytokines that promote an inflammatory response, and changes in gut microbiota.4,12
Physical activity
Data on the association of physical activity and diverticulitis is inconsistent. Some studies have found as much as a 25% decrease in the risk for diverticulitis with increased physical activity; more recent studies (2013 and 2016), on the other hand, found no association between diverticulosis and physical activity.11,17,19,28
Continue to: CLINICAL PRESENTATION
CLINICAL PRESENTATION
The clinical presentation of diverticulitis typically depends on the severity of inflammation and the presence (or absence) of complications. The most common presenting symptom is left lower-quadrant abdominal pain, which occurs in approximately 70% of cases and lasts for longer than 24 hours.29 Fever (usually < 102°F), leukocytosis, nausea, vomiting, and changes in bowel function may also be present.1,30,31 Approximately 50% of patients report constipation in diverticular disease; 20% to 35% report diarrhea.5
Patients may also report dysuria, secondary to irritation of the bladder by an inflamed segment of colon.3,17 Patients may report fecaluria, pneumaturia, or pyuria, which indicate a colovesical fistula.1 Passage of feces or flatus through the vagina indicates a colovaginal fistula.
The differential diagnosis of diverticulitis is listed in Table 2.17

PHYSICAL EXAMINATION
Physical examination in diverticulitis will almost always elicit tenderness to palpation over the area of inflammation, typically in the left lower quadrant. This is due to irritation of the peritoneum.3 A palpable mass may be present in as many as 20% of patients if an abscess is present. Bowel sounds may be hypoactive or hyperactive if there is a bowel obstruction.17 In cases of frank bowel-wall perforation, patients can present with peritoneal signs of rigidity, guarding, and rebound tenderness.3,31 Tachycardia, hypotension, and shock are rare but possible findings. Digital rectal examination may reveal tenderness or a mass if a pelvic abscess is present.17,31
DIAGNOSTICS
The diagnosis of acute diverticulitis can often be made clinically, based on the history and physical examination. Because clinical diagnosis can be inaccurate in as many as 68% of cases, however, laboratory testing and imaging play an important role in diagnosis.3
Continue to: Clinical laboratory studies
Clinical laboratory studies
Because leukocytosis is present in approximately one-half of patients with diverticulitis, a complete blood count (CBC) should be obtained; that recommendation notwithstanding, approximately one-half of patients with diverticulitis have a normal white blood cell count.29,30 A urine test of human chorionic gonadotropin should be ordered to exclude pregnancy in all premenopausal and perimenopausal women, particularly if antibiotics, imaging, or surgery are being considered.31 Urinalysis can assess for urinary tract infection.
Multiple studies have demonstrated the utility of C-reactive protein (CRP) in the workup of acute diverticulitis. In general, patients with a complicated episode will present with a significantly higher CRP level than that of uncomplicated disease.32 Kechagias et al found that the CRP level at initial evaluation may be helpful in predicting the clinical severity of the attack. A CRP level > 170 mg/L has been found to have a greater probability of severe disease, warranting CT and referral for hospitalization.33 A low CRP level was more likely to herald a mild course of disease that is amenable to outpatient antibiotic management or supportive care. This finding is consistent with previous reports of the association between CRP levels of 90 to 200 mg/L and the severity of diverticulitis.32,34
Imaging
Abdominopelvic CT with intravenous (IV) contrast. This imaging study is the gold standard diagnostic tool for diverticulitis, with sensitivity as high as 97%.3 CT can distinguish diverticulitis from other conditions, such as irritable bowel syndrome (based on a history of symptoms and the absence of CT findings), gastroenteritis, and gynecologic disease. It can also distinguish between uncomplicated and complicated diverticulitis and therefore guide therapeutic interventions, such as percutaneous drainage of an intra-abdominal abscess. CT findings associated with uncomplicated diverticulitis include colonic wall thickening and pericolonic fluid and inflammatory changes, such as fat stranding. CT findings associated with complicated disease include abscess (paracolonic or pelvic), peritonitis (purulent or feculent), phlegmon, perforation, fistula, and obstruction.1,3
Ultrasonography (US) can also be used in the assessment of diverticulitis, although it has lower sensitivity (approximately 61% to 84%) than CT and is inferior to CT for showing the extent of large abscesses or free air.3,18,30 A typical US finding in acute diverticulitis is a thickened loop of bowel with a target-like appearance.17 Findings are highly operator-dependent, however, and accuracy is diminished in obese patients. US may be a good option for pregnant women to avoid ionizing radiation.
Magnetic resonance imaging (MRI) is another option for imaging in diverticulitis but is not routinely recommended. It provides excellent soft-tissue detail and does not deliver ionizing radiation, but it is not as sensitive as CT for identifying free air.18,31 Furthermore, MRI requires prolonged examination time, which may not be tolerated by acutely ill patients, and is not an option for patients with certain types of surgical clips, metallic fragments, or a cardiac pacemaker.
Continue to: Abdominal radiography...
Abdominal radiography is useful to show free air, which would indicate perforation, and to show nonspecific abnormalities, such as bowel-gas patterns.31
MANAGEMENT
For decades, patients with diverticulitis were managed with antibiotics to cover colonic flora; many underwent urgent or emergent surgery to remove the affected segment of colon. Over the years, however, the treatment paradigm has shifted from such invasive management toward a nonsurgical approach—often, with equivalent or superior outcomes. More and more, management of diverticulitis is dictated by disease presentation: namely, whether disease is uncomplicated or complicated.1
Current guidelines recommend hospitalization, with possible surgical intervention, in complicated disease (free perforation, large abscesses, fistula, obstruction, stricture) and in patients who cannot tolerate oral hydration, who have a relevant comorbidity, or who do not have adequate support at home.35 Uncomplicated cases may also require hospitalization if certain criteria for admission are met: immunosuppression, severe or persistent abdominal pain, inability to tolerate oral intake, and significant comorbidity.5
Absent these criteria, outpatient management of uncomplicated diverticulitis is appropriate. After the treatment setting is determined, choice of intervention and length of treatment should be addressed.
Nonpharmacotherapeutic management
Dietary restrictions, from a full liquid diet to complete bowel rest, have been recommended for the management of acute diverticulitis. This recommendation is not supported by the literature, however. At least two studies have shown no association between an unrestricted diet and an increase in diverticular complications. In a 2013 retrospective cohort study, no increase in diverticular perforation or abscess was found with a diet of solid food compared to a liquid diet, a clear liquid diet, or no food by mouth.36 In a more recent (2017) prospective cohort study of 86 patients with uncomplicated diverticulitis, all of whom were on an unrestricted diet, only 8% developed complications.37
Continue to: There is no high-quality evidence for...
There is no high-quality evidence for instituting dietary restrictions in acute uncomplicated diverticulitis. As such, permitting oral intake as tolerated is a reasonable option.
Pharmacotherapy
Antibiotics have long been the cornerstone of pharmacotherapy for acute diverticulitis, covering gram-negative rods and anaerobes. The rationale for such management is the long-held belief that diverticulitis is caused by an infectious process.38 Common outpatient regimens include
- Ciprofloxacin (500 mg every 12 h) plus metronidazole (500 mg every 8 h)
- Trimethoprim–sulfamethoxazole (1 double-strength tablet every 12 h) plus metronidazole (500 mg every 8 h)
- Amoxicillin (875 mg)–clavulanate (1 tablet every 8 h) or extended-release amoxicillin–clavulanate (2 tablets every 12 h)
- Moxifloxacin (400 mg/d; for patients who cannot tolerate metronidazole or ß-lactam antibiotics).
Providers should always consult their local antibiogram to avoid prescribing antibiotics to which bacterial resistance exceeds 10%.
Despite widespread use of antibiotics for diverticulitis, multiple studies in recent years have shown no benefit to their use for uncomplicated cases. In 2012, Chabok et al investigated the need for antibiotic therapy to treat acute uncomplicated diverticulitis and found no statistically significant difference in outcome among patients treated with antibiotics and those managed conservatively.39 In 2014, Isacson et al performed a retrospective population-based cohort study to assess the applicability of a selective “no antibiotic” policy and its consequences in terms of complications and recurrence; the authors found that withholding antibiotics was safe and did not result in a higher complication or recurrence rate.40 Furthermore, in a 2017 multicenter study, Daniels et al conducted a randomized controlled trial comparing observation and antibiotic treatment for a first episode of uncomplicated acute diverticulitis in 528 patients and found no prolongation of recovery time, no increased rate of complications, and no need for surgical intervention in patients who were not treated with antibiotics.41
These studies are in agreement with the most recent AGA guidelines, which recommend selective, rather than routine, use of antibiotics for acute diverticulitis.14 This shift in approach may be due, in part, to a change in understanding of the etiology of the disease—from an infectious process to more of an inflammatory process.38
Continue to: For patients who require inpatient management of diverticulitis...
For patients who require inpatient management of diverticulitis, treatment typically involves IV antibiotics, fluids, and analgesics. Surgical treatment may be appropriate (see “Surgical treatment”).
Other agents used to manage diverticulitis include three that lack either strong or any data at all showing efficacy. The most recent AGA guidelines recommend against their use for this indication14:
Rifaximin. Two recent observational cohort studies, one from 2013 and the other from 2017, compared this poorly absorbed oral antibiotic with mesalamine to placebo or no treatment at all.42 Neither provided evidence that rifaximin treats or prevents diverticulitis.
Mesalamine. This anti-inflammatory has also been studied to prevent recurrence of diverticulitis. In a randomized, double-blind, placebo-controlled multicenter trial of 1,182 patients, Raskin et al found that mesalamine did not reduce the rate of recurrence of diverticulitis, time to recurrence, or the number of patients requiring surgery.43 This conclusion was reiterated by a 2016 meta-analysis that found no evidence to support use of mesalamine in the prevention of diverticulitis recurrence.44
Probiotics. Despite multiple studies undertaken to assess the efficacy of probiotics in the prevention and treatment of diverticular disease, strong data supporting their use are sparse. In 2016, Lahner et al examined 11 studies in which various probiotics were used to treat diverticular disease and found that, although there was a weak positive trend in the reduction and remission of abdominal symptoms, the evidence was not strong enough to recommend their routine use in managing the disease.45
Continue to: Surgical treatment
Surgical treatment
Acute uncomplicated diverticulitis can be treated nonsurgically in nearly all patients, regardless of whether treatment occurs in the inpatient or outpatient setting. For complicated disease, however, approximately 15% to 25% of patients require surgery. The main indication for emergent or urgent surgical intervention is colonic perforation, which can lead to acute peritonitis, sepsis, and associated morbidity and mortality.29
The decision to perform elective surgery should be made case by case, not routinely—such as after a recurrent episode of diverticulitis, when there has been a complication, or in young patients (< 50 years).1,11 Immunocompromised patients (transplant recipients, patients taking steroids chronically, and patients with HIV infection who have a CD4 count < 200 cells/μL) can present with more virulent episodes of diverticulitis, have a higher incidence of perforation and fecal peritonitis, and have a greater likelihood of failure of nonsurgical management.1 Surgical intervention after the first episode of diverticulitis in these patients should therefore be considered.
In 2014, the American Society of Colon and Rectal Surgeons (ASCRS) recommended the laparoscopic Hartmann procedure (primary resection of the affected segment of colon, with end colostomy, followed by colostomy closure) as the gold standard for the treatment of acute perforated diverticular disease when surgery is required.46
COLONOSCOPY AFTER DIVERTICULITIS
Although endoscopy is to be avoided during acute diverticulitis because of the risk for perforation, it is recommended six to eight weeks after the acute episode has resolved to rule out malignancy, inflammatory bowel disease, and colitis.1,3 Interestingly, in 2015, Daniels et al compared the colonoscopic detection rate of advanced colonic neoplasia in patients with a first episode of acute diverticulitis and in patients undergoing initial screening for colorectal cancer, and found no significant difference in the detection rate between the two groups.47 The authors concluded that routine colonoscopic follow-up after an episode of acute uncomplicated diverticulitis could be eliminated and that those patients could be screened according to routine guidelines.
Lau et al found a number of cancers and other significant lesions on colonoscopy performed after an episode of acute diverticulitis, with a 2.1% prevalence of colorectal cancer within one year after CT-proven diverticulitis, and an increase in the prevalence of abscess, local perforation, and fistula.48 Their study excluded patients who had had a colonoscopy within one year, however. They therefore recommended performing colonoscopy only for patients who have not had a recent colonoscopic exam. This recommendation is in accord with the most recent AGA and ASCRS guidelines. If a patient has had a recent colonoscopy prior to an acute episode of diverticulitis, the value of repeating the study after the episode resolves is unclear.
Continue to: CONCLUSION
CONCLUSION
As this article shows, the spectrum of clinical presentations for diverticulitis is broad, and management most often requires a case-by-case approach. Treatment is dictated by whether disease presentation is uncomplicated or complicated; outpatient management is appropriate for uncomplicated cases in the absence of specific criteria for hospitalization. Recent evidence supports a paradigm shift away from mandatory dietary restriction and routine antibiotic use.
CE/CME No: CR-1808
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the pathophysiology of diverticulitis.
• Describe the spectrum of clinical presentations of diverticulitis.
• Understand the diagnostic evaluation of diverticulitis.
• Differentiate the management of uncomplicated and complicated diverticulitis.
FACULTY
Priscilla Marsicovetere is Assistant Professor of Medical Education and Surgery, Geisel School of Medicine at Dartmouth, Hanover, New Hampshire, and Program Director for the Franklin Pierece University, PA Program, Lebanon, New Hampshire. She practices with Emergency Services of New England, Springfield Hospital, Springfield, Vermont.
The author has no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through July 31, 2019.
Article begins on next page >>
Treatment of this common complication of diverticular disease is predicated on whether the presentation signals uncomplicated or complicated disease. While some uncomplicated cases require hospitalization, many are amenable to primary care outpatient, and often conservative, management. The longstanding practice of antibiotic treatment of uncomplicated cases is now considered a selective, rather than a routine, option.
Diverticular disease is one of the most common conditions in the Western world and one of the most frequent indications for gastrointestinal-related hospitalization.1 It is among the 10 most common diagnoses in patients presenting to the clinic or emergency department with acute abdominal pain.2 Prevalence increases with age: Up to 60% of persons older than 60 are affected.3 The most common complication of diverticular disease is diverticulitis, which occurs in up to 25% of patients.4
The spectrum of clinical presentations of diverticular disease ranges from mild, uncomplicated disease that can be treated in the outpatient setting to complicated disease with sepsis and possible emergent surgical intervention. The traditional approach to diverticulitis has been management with antibiotics and likely sigmoid colectomy, but recent studies support a paradigm shift toward more conservative, nonsurgical treatment.
This article highlights current trends in diagnosis and management of acute diverticulitis.
DEFINITION AND EPIDEMIOLOGY
Diverticular disease is marked by sac-like outpouchings, called diverticula, that form at structurally weak points of the colon wall, predominantly in the descending and sigmoid colon.5 The prevalence of diverticular disease is increasing globally, affecting more than 10% of people older than 40, as many as 60% of those older than 60, and more than 70% of people older than 80.1,3 The mean age for hospital admission for acute diverticulitis is 63.3
Worldwide, males and females are affected equally.3 In Western society, the presence of diverticula, also called diverticulosis, is more often left-sided; right-sided disease is more prevalent in Asia.3,5
The most common complication of diverticular disease is diverticulitis—inflammation of a diverticulum—which affects 10% to 25% of patients with diverticular disease during their lifetime.4,5 Diverticulitis can be classified as uncomplicated (characterized by colonic wall thickening or pericolic inflammatory changes) or complicated (characterized by abscesses, fistulae, obstruction, or localized or free perforations).1,6 As many as 25% of diverticulitis cases are considered complicated.4,5 The severity of diverticulitis is commonly graded using the Hinchey Classification (Table 1).1,7

Continue to: PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Diverticula tend to occur in areas where the colonic wall is weak: namely, between the mesenteric and antimesenteric taeniae, where the vasa recta penetrate the muscle—points of entry of blood vessels through the colonic wall.1,4 The exact pathogenesis of diverticular disease is not completely understood but is thought to be multifactorial. Microscopic studies have shown muscular atrophy at the sites of diverticula, making them more susceptible to mucosal herniation in the setting of increased intraluminal pressure.1 Additional potential contributing factors include alterations in colonic microbiota, muscular dysfunction or dysmotility, lifestyle, and genetics.
Diverticulitis is the result of microscopic and macroscopic perforation of diverticula. Historically, the perforations were thought to result from obstruction of a diverticulum by a fecalith, leading to increased pressure within the outpouching, followed by perforation.3 Such obstruction is now thought to be rare. A more recent theory suggests that increased intraluminal pressure is due to inspissated food particles that lead to erosion of the diverticular wall, causing focal inflammation and necrosis and resulting in perforation.3 Microperforations can easily be contained by surrounding mesenteric fat; however, progression to abscess, fistulization, or intestinal obstruction can occur. Frank bowel wall perforation is not contained by mesenteric fat and can lead quickly to peritonitis and death if not treated emergently.

RISK FACTORS
Dietary fiber
In 1971, Burkitt was the first to posit that diverticular disease developed due to small quantities of fiber in the diet that led to increased intracolonic pressures.8 His theory was based on the observation that residents of several African countries, who ate a high-fiber diet, had a low incidence of diverticular disease. Burkitt hypothesized that this was due to shorter colonic transit time induced by high dietary fiber.
Several studies conducted since Burkitt made his observations have examined the association of dietary fiber and diverticular disease, with conflicting results. In 1998, Aldoori et al found that a low-fiber diet increases the incidence of symptomatic diverticular disease.9 However, in 2012, a large cohort study of patients undergoing colonoscopy found that those who reported the highest fiber intake were at highest risk for diverticulosis.10 In 2013, Peery et al examined the relationship among bowel habits, dietary fiber, and asymptomatic diverticulosis and found that less-frequent bowel movements and hard stools were associated with a decreased risk for diverticulosis.11 In 2017, a prospective cohort study of nearly 50,000 men without a known history of diverticulosis showed that diets high in red meat were associated with a higher incidence of diverticulitis over nearly three decades of follow-up, whereas a diet high in fiber was associated with a decreased incidence of diverticulitis.12
Although no definitive association has been found between dietary fiber intake and risk for diverticulosis, some studies have demonstrated an association between dietary fiber and diverticular complications. In 2014, Crowe et al found that consumption of a high-fiber diet was associated with a lower risk for hospital admission and death from diverticular disease.13 Recent guidelines from the American Gastroenterological Association (AGA) on diverticulitis recommend high dietary fiber intake in patients with a history of acute diverticulitis.14 However, no study has shown a reversal of the process or a reduction in the number of episodes of diverticulitis after adoption of a high-fiber diet.
Continue to: Historically, patients with diverticulitis...
Historically, patients with diverticulitis were advised to avoid eating nuts, corn, popcorn, and seeds to reduce the risk for complications. But studies have found no support for this caution. In a 2008 large, prospective study of men without known diverticular disease, the researchers found no association between nut, corn, or popcorn ingestion and diverticulitis; in fact, increased nut intake was specifically associated with a lower risk for diverticulitis.15
Smoking
Smoking has been linked to diverticulitis and has been associated with a threefold risk for complications, including severe diverticulitis.16,17 An increased risk for recurrent episodes has also been found in smokers following surgical intervention.17
Medications
NSAIDs, corticosteroids, and opioids have been associated with an increased risk for perforated diverticulitis.18,19 A significant association has been found between NSAID use and severity of diverticulitis, including perforation; one study reported a relative risk of 1.25 (95% confidence interval, 10.5 to 1.47) for diverticulitis with regular use of aspirin (≥ 2x/wk).20,21
More frequent steroid use has been found in patients with complicated diverticulitis, compared to patients with uncomplicated disease (7.3% vs 3.3%; P = .015).22 A systematic review of five studies comparing patients with and without steroid use showed significantly higher odds of diverticular perforation in patients taking a steroid.23 Pooled data showed significantly increased odds of perforation and abscess formation with use of an NSAID (odds ratio [OR], 2.49), steroid (OR, 9.08), or opioid (OR, 2.52).22
Continue to: Vitamin D
Vitamin D
In a 2013 retrospective cohort study of 9,116 patients with uncomplicated diverticulosis and 922 patients who developed diverticulitis that required hospitalization, Maguire et al examined the association of prediagnostic serum levels of vitamin D and diverticulitis.24 Among patients with diverticulosis, higher prediagnostic levels of 25-hydroxyvitamin D were significantly associated with a lower risk for diverticulitis—indicating that vitamin D deficiency could be involved in the pathogenesis of diverticulitis.
The association between diverticulitis and vitamin D levels was supported by an additional study in 2015, in which the authors investigated the association between ultraviolet (UV) light and diverticulitis.25 They identified nonelective diverticulitis admissions in the Nationwide Inpatient Sample database and linked hospital locations to geographic UV data. They examined UV exposure in relation to risk for admission for diverticulitis and found that, compared with high-UV (UV4) areas, low-UV (UV1) areas had a higher rate of diverticulitis (751.8/100,000 admissions, compared with 668.1/100,000 admissions, respectively [P < .001]), diverticular abscess (12.0% compared with 9.7% [P < .001]), and colectomy (13.5% compared with 11.5% [P < .001]). They also observed significant seasonal variation, with a lower rate of diverticulitis in winter (645/100,000 admissions) compared with summer (748/100,000 admissions [P < .001]). Because UV exposure largely determines vitamin D status, these findings are thought to support a role for vitamin D in the pathogenesis of diverticulitis.
Genetics
Two studies found an association between genetics and diverticular disease. A 2012 study using The Swedish Twin Registry found that if one twin is affected with the disease, the odds that the other will be affected was 7.15 in monozygotic (identical) twins and 3.20 in dizygotic (fraternal) twins.26 A 2013 Danish twin study found a relative risk of 2.92 in twin siblings compared to the general population.27 Both studies estimated the genetic contribution to diverticular disease to be 40% to 50%.26,27
Obesity
Several large prospective studies have shown a positive association between high BMI, waist circumference, and waist-to-hip ratio and risk for diverticulitis.4 A BMI > 30 was found to increase the relative risk of acute diverticulitis by 1.78, compared with a normal BMI.17 In a large, prospective, population-based cohort study in 2016, Jamal Talabani et al found that obese persons had twice the risk for admission for acute colonic diverticulitis than normal-weight persons did.28 Waist circumference and waist-to-hip ratio were also independently associated with risk for diverticulitis. The pathophysiology of the associations is not clearly understood but may involve pro-inflammatory changes of adipose tissue, which secrete cytokines that promote an inflammatory response, and changes in gut microbiota.4,12
Physical activity
Data on the association of physical activity and diverticulitis is inconsistent. Some studies have found as much as a 25% decrease in the risk for diverticulitis with increased physical activity; more recent studies (2013 and 2016), on the other hand, found no association between diverticulosis and physical activity.11,17,19,28
Continue to: CLINICAL PRESENTATION
CLINICAL PRESENTATION
The clinical presentation of diverticulitis typically depends on the severity of inflammation and the presence (or absence) of complications. The most common presenting symptom is left lower-quadrant abdominal pain, which occurs in approximately 70% of cases and lasts for longer than 24 hours.29 Fever (usually < 102°F), leukocytosis, nausea, vomiting, and changes in bowel function may also be present.1,30,31 Approximately 50% of patients report constipation in diverticular disease; 20% to 35% report diarrhea.5
Patients may also report dysuria, secondary to irritation of the bladder by an inflamed segment of colon.3,17 Patients may report fecaluria, pneumaturia, or pyuria, which indicate a colovesical fistula.1 Passage of feces or flatus through the vagina indicates a colovaginal fistula.
The differential diagnosis of diverticulitis is listed in Table 2.17

PHYSICAL EXAMINATION
Physical examination in diverticulitis will almost always elicit tenderness to palpation over the area of inflammation, typically in the left lower quadrant. This is due to irritation of the peritoneum.3 A palpable mass may be present in as many as 20% of patients if an abscess is present. Bowel sounds may be hypoactive or hyperactive if there is a bowel obstruction.17 In cases of frank bowel-wall perforation, patients can present with peritoneal signs of rigidity, guarding, and rebound tenderness.3,31 Tachycardia, hypotension, and shock are rare but possible findings. Digital rectal examination may reveal tenderness or a mass if a pelvic abscess is present.17,31
DIAGNOSTICS
The diagnosis of acute diverticulitis can often be made clinically, based on the history and physical examination. Because clinical diagnosis can be inaccurate in as many as 68% of cases, however, laboratory testing and imaging play an important role in diagnosis.3
Continue to: Clinical laboratory studies
Clinical laboratory studies
Because leukocytosis is present in approximately one-half of patients with diverticulitis, a complete blood count (CBC) should be obtained; that recommendation notwithstanding, approximately one-half of patients with diverticulitis have a normal white blood cell count.29,30 A urine test of human chorionic gonadotropin should be ordered to exclude pregnancy in all premenopausal and perimenopausal women, particularly if antibiotics, imaging, or surgery are being considered.31 Urinalysis can assess for urinary tract infection.
Multiple studies have demonstrated the utility of C-reactive protein (CRP) in the workup of acute diverticulitis. In general, patients with a complicated episode will present with a significantly higher CRP level than that of uncomplicated disease.32 Kechagias et al found that the CRP level at initial evaluation may be helpful in predicting the clinical severity of the attack. A CRP level > 170 mg/L has been found to have a greater probability of severe disease, warranting CT and referral for hospitalization.33 A low CRP level was more likely to herald a mild course of disease that is amenable to outpatient antibiotic management or supportive care. This finding is consistent with previous reports of the association between CRP levels of 90 to 200 mg/L and the severity of diverticulitis.32,34
Imaging
Abdominopelvic CT with intravenous (IV) contrast. This imaging study is the gold standard diagnostic tool for diverticulitis, with sensitivity as high as 97%.3 CT can distinguish diverticulitis from other conditions, such as irritable bowel syndrome (based on a history of symptoms and the absence of CT findings), gastroenteritis, and gynecologic disease. It can also distinguish between uncomplicated and complicated diverticulitis and therefore guide therapeutic interventions, such as percutaneous drainage of an intra-abdominal abscess. CT findings associated with uncomplicated diverticulitis include colonic wall thickening and pericolonic fluid and inflammatory changes, such as fat stranding. CT findings associated with complicated disease include abscess (paracolonic or pelvic), peritonitis (purulent or feculent), phlegmon, perforation, fistula, and obstruction.1,3
Ultrasonography (US) can also be used in the assessment of diverticulitis, although it has lower sensitivity (approximately 61% to 84%) than CT and is inferior to CT for showing the extent of large abscesses or free air.3,18,30 A typical US finding in acute diverticulitis is a thickened loop of bowel with a target-like appearance.17 Findings are highly operator-dependent, however, and accuracy is diminished in obese patients. US may be a good option for pregnant women to avoid ionizing radiation.
Magnetic resonance imaging (MRI) is another option for imaging in diverticulitis but is not routinely recommended. It provides excellent soft-tissue detail and does not deliver ionizing radiation, but it is not as sensitive as CT for identifying free air.18,31 Furthermore, MRI requires prolonged examination time, which may not be tolerated by acutely ill patients, and is not an option for patients with certain types of surgical clips, metallic fragments, or a cardiac pacemaker.
Continue to: Abdominal radiography...
Abdominal radiography is useful to show free air, which would indicate perforation, and to show nonspecific abnormalities, such as bowel-gas patterns.31
MANAGEMENT
For decades, patients with diverticulitis were managed with antibiotics to cover colonic flora; many underwent urgent or emergent surgery to remove the affected segment of colon. Over the years, however, the treatment paradigm has shifted from such invasive management toward a nonsurgical approach—often, with equivalent or superior outcomes. More and more, management of diverticulitis is dictated by disease presentation: namely, whether disease is uncomplicated or complicated.1
Current guidelines recommend hospitalization, with possible surgical intervention, in complicated disease (free perforation, large abscesses, fistula, obstruction, stricture) and in patients who cannot tolerate oral hydration, who have a relevant comorbidity, or who do not have adequate support at home.35 Uncomplicated cases may also require hospitalization if certain criteria for admission are met: immunosuppression, severe or persistent abdominal pain, inability to tolerate oral intake, and significant comorbidity.5
Absent these criteria, outpatient management of uncomplicated diverticulitis is appropriate. After the treatment setting is determined, choice of intervention and length of treatment should be addressed.
Nonpharmacotherapeutic management
Dietary restrictions, from a full liquid diet to complete bowel rest, have been recommended for the management of acute diverticulitis. This recommendation is not supported by the literature, however. At least two studies have shown no association between an unrestricted diet and an increase in diverticular complications. In a 2013 retrospective cohort study, no increase in diverticular perforation or abscess was found with a diet of solid food compared to a liquid diet, a clear liquid diet, or no food by mouth.36 In a more recent (2017) prospective cohort study of 86 patients with uncomplicated diverticulitis, all of whom were on an unrestricted diet, only 8% developed complications.37
Continue to: There is no high-quality evidence for...
There is no high-quality evidence for instituting dietary restrictions in acute uncomplicated diverticulitis. As such, permitting oral intake as tolerated is a reasonable option.
Pharmacotherapy
Antibiotics have long been the cornerstone of pharmacotherapy for acute diverticulitis, covering gram-negative rods and anaerobes. The rationale for such management is the long-held belief that diverticulitis is caused by an infectious process.38 Common outpatient regimens include
- Ciprofloxacin (500 mg every 12 h) plus metronidazole (500 mg every 8 h)
- Trimethoprim–sulfamethoxazole (1 double-strength tablet every 12 h) plus metronidazole (500 mg every 8 h)
- Amoxicillin (875 mg)–clavulanate (1 tablet every 8 h) or extended-release amoxicillin–clavulanate (2 tablets every 12 h)
- Moxifloxacin (400 mg/d; for patients who cannot tolerate metronidazole or ß-lactam antibiotics).
Providers should always consult their local antibiogram to avoid prescribing antibiotics to which bacterial resistance exceeds 10%.
Despite widespread use of antibiotics for diverticulitis, multiple studies in recent years have shown no benefit to their use for uncomplicated cases. In 2012, Chabok et al investigated the need for antibiotic therapy to treat acute uncomplicated diverticulitis and found no statistically significant difference in outcome among patients treated with antibiotics and those managed conservatively.39 In 2014, Isacson et al performed a retrospective population-based cohort study to assess the applicability of a selective “no antibiotic” policy and its consequences in terms of complications and recurrence; the authors found that withholding antibiotics was safe and did not result in a higher complication or recurrence rate.40 Furthermore, in a 2017 multicenter study, Daniels et al conducted a randomized controlled trial comparing observation and antibiotic treatment for a first episode of uncomplicated acute diverticulitis in 528 patients and found no prolongation of recovery time, no increased rate of complications, and no need for surgical intervention in patients who were not treated with antibiotics.41
These studies are in agreement with the most recent AGA guidelines, which recommend selective, rather than routine, use of antibiotics for acute diverticulitis.14 This shift in approach may be due, in part, to a change in understanding of the etiology of the disease—from an infectious process to more of an inflammatory process.38
Continue to: For patients who require inpatient management of diverticulitis...
For patients who require inpatient management of diverticulitis, treatment typically involves IV antibiotics, fluids, and analgesics. Surgical treatment may be appropriate (see “Surgical treatment”).
Other agents used to manage diverticulitis include three that lack either strong or any data at all showing efficacy. The most recent AGA guidelines recommend against their use for this indication14:
Rifaximin. Two recent observational cohort studies, one from 2013 and the other from 2017, compared this poorly absorbed oral antibiotic with mesalamine to placebo or no treatment at all.42 Neither provided evidence that rifaximin treats or prevents diverticulitis.
Mesalamine. This anti-inflammatory has also been studied to prevent recurrence of diverticulitis. In a randomized, double-blind, placebo-controlled multicenter trial of 1,182 patients, Raskin et al found that mesalamine did not reduce the rate of recurrence of diverticulitis, time to recurrence, or the number of patients requiring surgery.43 This conclusion was reiterated by a 2016 meta-analysis that found no evidence to support use of mesalamine in the prevention of diverticulitis recurrence.44
Probiotics. Despite multiple studies undertaken to assess the efficacy of probiotics in the prevention and treatment of diverticular disease, strong data supporting their use are sparse. In 2016, Lahner et al examined 11 studies in which various probiotics were used to treat diverticular disease and found that, although there was a weak positive trend in the reduction and remission of abdominal symptoms, the evidence was not strong enough to recommend their routine use in managing the disease.45
Continue to: Surgical treatment
Surgical treatment
Acute uncomplicated diverticulitis can be treated nonsurgically in nearly all patients, regardless of whether treatment occurs in the inpatient or outpatient setting. For complicated disease, however, approximately 15% to 25% of patients require surgery. The main indication for emergent or urgent surgical intervention is colonic perforation, which can lead to acute peritonitis, sepsis, and associated morbidity and mortality.29
The decision to perform elective surgery should be made case by case, not routinely—such as after a recurrent episode of diverticulitis, when there has been a complication, or in young patients (< 50 years).1,11 Immunocompromised patients (transplant recipients, patients taking steroids chronically, and patients with HIV infection who have a CD4 count < 200 cells/μL) can present with more virulent episodes of diverticulitis, have a higher incidence of perforation and fecal peritonitis, and have a greater likelihood of failure of nonsurgical management.1 Surgical intervention after the first episode of diverticulitis in these patients should therefore be considered.
In 2014, the American Society of Colon and Rectal Surgeons (ASCRS) recommended the laparoscopic Hartmann procedure (primary resection of the affected segment of colon, with end colostomy, followed by colostomy closure) as the gold standard for the treatment of acute perforated diverticular disease when surgery is required.46
COLONOSCOPY AFTER DIVERTICULITIS
Although endoscopy is to be avoided during acute diverticulitis because of the risk for perforation, it is recommended six to eight weeks after the acute episode has resolved to rule out malignancy, inflammatory bowel disease, and colitis.1,3 Interestingly, in 2015, Daniels et al compared the colonoscopic detection rate of advanced colonic neoplasia in patients with a first episode of acute diverticulitis and in patients undergoing initial screening for colorectal cancer, and found no significant difference in the detection rate between the two groups.47 The authors concluded that routine colonoscopic follow-up after an episode of acute uncomplicated diverticulitis could be eliminated and that those patients could be screened according to routine guidelines.
Lau et al found a number of cancers and other significant lesions on colonoscopy performed after an episode of acute diverticulitis, with a 2.1% prevalence of colorectal cancer within one year after CT-proven diverticulitis, and an increase in the prevalence of abscess, local perforation, and fistula.48 Their study excluded patients who had had a colonoscopy within one year, however. They therefore recommended performing colonoscopy only for patients who have not had a recent colonoscopic exam. This recommendation is in accord with the most recent AGA and ASCRS guidelines. If a patient has had a recent colonoscopy prior to an acute episode of diverticulitis, the value of repeating the study after the episode resolves is unclear.
Continue to: CONCLUSION
CONCLUSION
As this article shows, the spectrum of clinical presentations for diverticulitis is broad, and management most often requires a case-by-case approach. Treatment is dictated by whether disease presentation is uncomplicated or complicated; outpatient management is appropriate for uncomplicated cases in the absence of specific criteria for hospitalization. Recent evidence supports a paradigm shift away from mandatory dietary restriction and routine antibiotic use.
1. Deery SE, Hodin RA. Management of diverticulitis in 2017. J Gastrointest Surg. 2017;21(10):1732-1741.
2. Boermeester M, Humes D, Velmahos G, et al. Contemporary review of risk-stratified management in acute uncomplicated and complicated diverticulitis. World J Surg. 2016;40(10):2537-2545.
3. Linzay C, Pandit S. Diverticulitis, acute. [Updated 2017 Nov 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018- Jan.
4. Rezapour M, Ali S, Stollman N. Diverticular disease: an update on pathogenesis and management. Gut Liver. 2018;12(2):125-132.
5. Mayl J, Marchenko M, Frierson E. Management of acute uncomplicated diverticulitis may exclude antibiotic therapy. Cureus. 2017;9(5):e1250.
6. Chung BH, Ha GW, Lee MR, Kim JH. Management of colonic diverticulitis tailored to location and severity: comparison of the right and the left colon. Ann Coloproctol. 2016;32(6):228-233.
7. Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg. 1978;12:85-109.
8. Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer. 1971;28(1):3-13.
9. Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. 1998;128(4):714-719.
10. Peery AF, Barrett PR, Park D, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012;142(2):266-272.
11. Peery AF, Sandler RS, Ahnen DJ, et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol. 2013;11(12):1622-1627.
12. Strate LL, Keeley BR, Cao Y, et al. Western dietary pattern increases, whereas prudent dietary pattern decreases, risk of incident diverticulitis in a prospective cohort study. Gastroenterology. 2017;152(5):1023-1030.
13. Crowe FL, Balkwill A, Cairns BJ, et al; Million Women Study Collaborators. Source of dietary fibre and diverticular disease incidence: a prospective study of UK women. Gut. 2014;63(9):1450-1456.
14. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology 2015;149(7):1944-1949.
15. Strate LL, Liu YL, Syngal S, et al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300(8):907-914.
16. Hjern F, Wolk A, Håkansson N. Smoking and the risk of diverticular disease in women. Br J Surg. 2011;98(7):997-1002.
17. Humes DJ, Spiller RC. Review article: The pathogenesis and management of acute colonic diverticulitis. Aliment Pharmacol Ther. 2014;39(4):359-370.
18. Moubax K, Urbain D. Diverticulitis: new insights on the traditional point of view. Acta Gastroenterol Belg. 2015;78(1):38-48.
19. Morris AM, Regenbogen SE, Hardiman KM, Hendren S. Sigmoid diverticulitis: a systematic review. JAMA. 2014; 311(3):287-297.
20. Tan JP, Barazanchi AW, Singh PP, et al. Predictors of acute diverticulitis severity: a systematic review. Int J Surg. 2016;26:43-52.
21. Strate LL, Liu YL, Huang ES, et al. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology. 2011;140(5):1427-1433.
22. Nizri E, Spring S, Ben-Yehuda A, et al. C-reactive protein as a marker of complicated diverticulitis in patients on anti-inflammatory medications. Tech Coloproctol. 2014; 18(2):145-149.
23. Kvasnovsky CL, Papagrigoriadis S, Bjarnason I. Increased diverticular complications with nonsteroidal anti-inflammatory drugs and other medications: a systematic review and meta-analysis. Colorectal Dis. 2014; 16(6):O189-O196.
24. Maguire LH, Song M, Strate LL, et al. Higher serum levels of vitamin D are associated with a reduced risk of diverticulitis. Clin Gastroenterol Hepatol. 2013;11(12):1631-1635.
25. Maguire LH, Song M, Strate LL, et al. Association of geographic and seasonal variation with diverticulitis admissions. JAMA Surg. 2015;150(1):74-77.
26. Granlund J, Svensson T, Olén O, et al. The genetic influence on diverticular disease—a twin study. Aliment Pharmacol Ther. 2012;35(9):1103-1107.
27. Strate LL, Erichsen R, Baron JA, et al. Heritability and familial aggregation of diverticular disease: a population-based study of twins and siblings. Gastroenterology. 2013;144(4):736-742.
28. Jamal Talabani A, Lydersen S, Ness-Jensen E, et al. Risk factors of admission for acute colonic diverticulitis in a population-based cohort study: The North Trondelag Health Study, Norway. World J Gastroenterol. 2016; 22(48):10663-10672.
29. Horesh N, Wasserberg N, Zbar AP, et al. Changing paradigms in the management of diverticulitis. Int J Surg. 2016(33 pt A):146-150.
30. McSweeney W, Srinath H. Diverticular disease practice points. Aust Fam Physician. 2017;46(11):829-832.
31. Wilkins T, Embry K, George R. Diagnosis and management of acute diverticulitis. Am Fam Physician. 2013; 87(9):612-620.
32. van de Wall BJ, Draaisma WA, van der Kaaij RT, et al. The value of inflammation markers and body temperature in acute diverticulitis. Colorectal Dis. 2013;15(5):621-626.
33. Kechagias A, Rautio T, Kechagias G, Mäkelä J. The role of C-reactive protein in the prediction of the clinical severity of acute diverticulitis. Am Surg. 2014;80(4):391-395.
34. Bolkenstein HE, van de Wall BJM, Consten ECJ, et al. Risk factors for complicated diverticulitis: systematic review and meta-analysis. Int J Colorectal Dis. 2017; 32(10):1375-1383.
35. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57(3):284-294.
36. van de Wall BJ, Draaisma WA, van Iersel JJ, et al. Dietary restrictions for acute diverticulitis: evidence-based or expert opinion? Int J Colorectal Dis. 2013;28(9):1287-1293.
37. Stam MA, Draaisma WA, van de Wall BJ, et al. An unrestricted diet for uncomplicated diverticulitis is safe: results of a prospective diverticulitis diet study. Colorectal Dis. 2017;19(4):372-377.
38. Khan DZ, Kelly ME, O’Reilly J, et al. A national evaluation of the management practices of acute diverticulitis. Surgeon. 2017;15(4):206-210.
39. Chabok A, Påhlman L, Hjern F, et al; AVOD Study Group. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99(4):532-539.
40. Isacson D, Andreasson K, Nikberg M, et al. No antibiotics in acute uncomplicated diverticulitis: does it work? Scand J Gastroenterol. 2014;49(12):1441-1446.
41. Daniels L, Ünlü Ç, de Korte N, et al; Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104(1):52-61.
42. van Dijk S, Rottier SJ, van Geloven AAW, Boermeester MA. Conservative treatment of acute colonic diverticulitis. Curr Infect Dis Rep. 2017;19(11):44.
43. Raskin J, Kamm M, Jamal M, Howden CW. Mesalamine did not prevent recurrent diverticulitis in phase 3 controlled trials. Gastroenterology. 2014;147:793-802.
44. Kahn M, Ali B, Lee W, et al. Mesalamine does not help prevent recurrent acute colonic diverticulitis: meta-analysis of randomized, placebo-controlled trials. Am J Gastroenterol. 2016;111(4):579-581.
45. Lahner E, Bellisario C, Hassan C, et al. Probiotics in the treatment of diverticular disease. A systematic review. J Gastrointestin Liver Dis. 2016;25(1):79-86.
46. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57(3):284-294.
47. Daniels I, Ünlü Ç, de Wijkerslooth TR, et al. Yield of colonoscopy after recent CT-proven uncomplicated acute diverticulitis: a comparative cohort study. Surg Endosc. 2015;29(9):2605-2613.
48. Lau KC, Spilsbury K, Farooque Y, et al. Is colonoscopy still mandatory after a CT diagnosis of left-sided diverticulitis: can colorectal cancer be confidently excluded? Dis Colon Rectum. 2011;54(10):1265-1270.
1. Deery SE, Hodin RA. Management of diverticulitis in 2017. J Gastrointest Surg. 2017;21(10):1732-1741.
2. Boermeester M, Humes D, Velmahos G, et al. Contemporary review of risk-stratified management in acute uncomplicated and complicated diverticulitis. World J Surg. 2016;40(10):2537-2545.
3. Linzay C, Pandit S. Diverticulitis, acute. [Updated 2017 Nov 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018- Jan.
4. Rezapour M, Ali S, Stollman N. Diverticular disease: an update on pathogenesis and management. Gut Liver. 2018;12(2):125-132.
5. Mayl J, Marchenko M, Frierson E. Management of acute uncomplicated diverticulitis may exclude antibiotic therapy. Cureus. 2017;9(5):e1250.
6. Chung BH, Ha GW, Lee MR, Kim JH. Management of colonic diverticulitis tailored to location and severity: comparison of the right and the left colon. Ann Coloproctol. 2016;32(6):228-233.
7. Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg. 1978;12:85-109.
8. Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer. 1971;28(1):3-13.
9. Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. 1998;128(4):714-719.
10. Peery AF, Barrett PR, Park D, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology. 2012;142(2):266-272.
11. Peery AF, Sandler RS, Ahnen DJ, et al. Constipation and a low-fiber diet are not associated with diverticulosis. Clin Gastroenterol Hepatol. 2013;11(12):1622-1627.
12. Strate LL, Keeley BR, Cao Y, et al. Western dietary pattern increases, whereas prudent dietary pattern decreases, risk of incident diverticulitis in a prospective cohort study. Gastroenterology. 2017;152(5):1023-1030.
13. Crowe FL, Balkwill A, Cairns BJ, et al; Million Women Study Collaborators. Source of dietary fibre and diverticular disease incidence: a prospective study of UK women. Gut. 2014;63(9):1450-1456.
14. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology 2015;149(7):1944-1949.
15. Strate LL, Liu YL, Syngal S, et al. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA. 2008;300(8):907-914.
16. Hjern F, Wolk A, Håkansson N. Smoking and the risk of diverticular disease in women. Br J Surg. 2011;98(7):997-1002.
17. Humes DJ, Spiller RC. Review article: The pathogenesis and management of acute colonic diverticulitis. Aliment Pharmacol Ther. 2014;39(4):359-370.
18. Moubax K, Urbain D. Diverticulitis: new insights on the traditional point of view. Acta Gastroenterol Belg. 2015;78(1):38-48.
19. Morris AM, Regenbogen SE, Hardiman KM, Hendren S. Sigmoid diverticulitis: a systematic review. JAMA. 2014; 311(3):287-297.
20. Tan JP, Barazanchi AW, Singh PP, et al. Predictors of acute diverticulitis severity: a systematic review. Int J Surg. 2016;26:43-52.
21. Strate LL, Liu YL, Huang ES, et al. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology. 2011;140(5):1427-1433.
22. Nizri E, Spring S, Ben-Yehuda A, et al. C-reactive protein as a marker of complicated diverticulitis in patients on anti-inflammatory medications. Tech Coloproctol. 2014; 18(2):145-149.
23. Kvasnovsky CL, Papagrigoriadis S, Bjarnason I. Increased diverticular complications with nonsteroidal anti-inflammatory drugs and other medications: a systematic review and meta-analysis. Colorectal Dis. 2014; 16(6):O189-O196.
24. Maguire LH, Song M, Strate LL, et al. Higher serum levels of vitamin D are associated with a reduced risk of diverticulitis. Clin Gastroenterol Hepatol. 2013;11(12):1631-1635.
25. Maguire LH, Song M, Strate LL, et al. Association of geographic and seasonal variation with diverticulitis admissions. JAMA Surg. 2015;150(1):74-77.
26. Granlund J, Svensson T, Olén O, et al. The genetic influence on diverticular disease—a twin study. Aliment Pharmacol Ther. 2012;35(9):1103-1107.
27. Strate LL, Erichsen R, Baron JA, et al. Heritability and familial aggregation of diverticular disease: a population-based study of twins and siblings. Gastroenterology. 2013;144(4):736-742.
28. Jamal Talabani A, Lydersen S, Ness-Jensen E, et al. Risk factors of admission for acute colonic diverticulitis in a population-based cohort study: The North Trondelag Health Study, Norway. World J Gastroenterol. 2016; 22(48):10663-10672.
29. Horesh N, Wasserberg N, Zbar AP, et al. Changing paradigms in the management of diverticulitis. Int J Surg. 2016(33 pt A):146-150.
30. McSweeney W, Srinath H. Diverticular disease practice points. Aust Fam Physician. 2017;46(11):829-832.
31. Wilkins T, Embry K, George R. Diagnosis and management of acute diverticulitis. Am Fam Physician. 2013; 87(9):612-620.
32. van de Wall BJ, Draaisma WA, van der Kaaij RT, et al. The value of inflammation markers and body temperature in acute diverticulitis. Colorectal Dis. 2013;15(5):621-626.
33. Kechagias A, Rautio T, Kechagias G, Mäkelä J. The role of C-reactive protein in the prediction of the clinical severity of acute diverticulitis. Am Surg. 2014;80(4):391-395.
34. Bolkenstein HE, van de Wall BJM, Consten ECJ, et al. Risk factors for complicated diverticulitis: systematic review and meta-analysis. Int J Colorectal Dis. 2017; 32(10):1375-1383.
35. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57(3):284-294.
36. van de Wall BJ, Draaisma WA, van Iersel JJ, et al. Dietary restrictions for acute diverticulitis: evidence-based or expert opinion? Int J Colorectal Dis. 2013;28(9):1287-1293.
37. Stam MA, Draaisma WA, van de Wall BJ, et al. An unrestricted diet for uncomplicated diverticulitis is safe: results of a prospective diverticulitis diet study. Colorectal Dis. 2017;19(4):372-377.
38. Khan DZ, Kelly ME, O’Reilly J, et al. A national evaluation of the management practices of acute diverticulitis. Surgeon. 2017;15(4):206-210.
39. Chabok A, Påhlman L, Hjern F, et al; AVOD Study Group. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99(4):532-539.
40. Isacson D, Andreasson K, Nikberg M, et al. No antibiotics in acute uncomplicated diverticulitis: does it work? Scand J Gastroenterol. 2014;49(12):1441-1446.
41. Daniels L, Ünlü Ç, de Korte N, et al; Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104(1):52-61.
42. van Dijk S, Rottier SJ, van Geloven AAW, Boermeester MA. Conservative treatment of acute colonic diverticulitis. Curr Infect Dis Rep. 2017;19(11):44.
43. Raskin J, Kamm M, Jamal M, Howden CW. Mesalamine did not prevent recurrent diverticulitis in phase 3 controlled trials. Gastroenterology. 2014;147:793-802.
44. Kahn M, Ali B, Lee W, et al. Mesalamine does not help prevent recurrent acute colonic diverticulitis: meta-analysis of randomized, placebo-controlled trials. Am J Gastroenterol. 2016;111(4):579-581.
45. Lahner E, Bellisario C, Hassan C, et al. Probiotics in the treatment of diverticular disease. A systematic review. J Gastrointestin Liver Dis. 2016;25(1):79-86.
46. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57(3):284-294.
47. Daniels I, Ünlü Ç, de Wijkerslooth TR, et al. Yield of colonoscopy after recent CT-proven uncomplicated acute diverticulitis: a comparative cohort study. Surg Endosc. 2015;29(9):2605-2613.
48. Lau KC, Spilsbury K, Farooque Y, et al. Is colonoscopy still mandatory after a CT diagnosis of left-sided diverticulitis: can colorectal cancer be confidently excluded? Dis Colon Rectum. 2011;54(10):1265-1270.
Balanced fluid resuscitation vs. saline does not decrease hospital-free days
Clinical question: Does balanced crystalloid fluid improve outcomes versus saline in noncritically ill patients who are hospitalized?
Background: Prior research has raised concerns about a connection between intravenous saline administration and adverse outcomes. However, this work has been limited to patients in the ICU and operative room settings.
Study design: Single-center, unblinded, multiple crossover (clustered randomization) trial.
Setting: A tertiary-care, academic medical center, from January 2016 to April 2017.
Synopsis: This study enrolled 13,347 adult patients receiving a minimum of 500 cc of intravenous fluid in the emergency department. Participants were randomized to receive either normal saline or balanced crystalloid fluid (lactated Ringer’s solution or Plasma-Lyte A). The study authors found no significant difference between the two groups in the primary outcome of hospital-free days (P = .41), or in several of the secondary outcomes including acute kidney injury stage 2 or higher (P = .14) and in-hospital mortality (P = .36). The balanced crystalloid fluid group did have a significantly lower incidence of a composite secondary outcome of major adverse kidney events (P = .01). However, given the primary and other secondary outcome findings, and concerns that composite outcomes lack patient centeredness, an accompanying editorial urged caution against changing clinical practice based on this finding.
Bottom line: There was no significant difference in hospital-free days for noncritically ill patients receiving IV fluids in the ED between those treated with saline and balanced crystalloid fluid.
Citation: Self WH et al. Balanced crystalloids versus saline in noncritically ill adults. N Eng J Med. 2018;378:819-28.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Does balanced crystalloid fluid improve outcomes versus saline in noncritically ill patients who are hospitalized?
Background: Prior research has raised concerns about a connection between intravenous saline administration and adverse outcomes. However, this work has been limited to patients in the ICU and operative room settings.
Study design: Single-center, unblinded, multiple crossover (clustered randomization) trial.
Setting: A tertiary-care, academic medical center, from January 2016 to April 2017.
Synopsis: This study enrolled 13,347 adult patients receiving a minimum of 500 cc of intravenous fluid in the emergency department. Participants were randomized to receive either normal saline or balanced crystalloid fluid (lactated Ringer’s solution or Plasma-Lyte A). The study authors found no significant difference between the two groups in the primary outcome of hospital-free days (P = .41), or in several of the secondary outcomes including acute kidney injury stage 2 or higher (P = .14) and in-hospital mortality (P = .36). The balanced crystalloid fluid group did have a significantly lower incidence of a composite secondary outcome of major adverse kidney events (P = .01). However, given the primary and other secondary outcome findings, and concerns that composite outcomes lack patient centeredness, an accompanying editorial urged caution against changing clinical practice based on this finding.
Bottom line: There was no significant difference in hospital-free days for noncritically ill patients receiving IV fluids in the ED between those treated with saline and balanced crystalloid fluid.
Citation: Self WH et al. Balanced crystalloids versus saline in noncritically ill adults. N Eng J Med. 2018;378:819-28.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Does balanced crystalloid fluid improve outcomes versus saline in noncritically ill patients who are hospitalized?
Background: Prior research has raised concerns about a connection between intravenous saline administration and adverse outcomes. However, this work has been limited to patients in the ICU and operative room settings.
Study design: Single-center, unblinded, multiple crossover (clustered randomization) trial.
Setting: A tertiary-care, academic medical center, from January 2016 to April 2017.
Synopsis: This study enrolled 13,347 adult patients receiving a minimum of 500 cc of intravenous fluid in the emergency department. Participants were randomized to receive either normal saline or balanced crystalloid fluid (lactated Ringer’s solution or Plasma-Lyte A). The study authors found no significant difference between the two groups in the primary outcome of hospital-free days (P = .41), or in several of the secondary outcomes including acute kidney injury stage 2 or higher (P = .14) and in-hospital mortality (P = .36). The balanced crystalloid fluid group did have a significantly lower incidence of a composite secondary outcome of major adverse kidney events (P = .01). However, given the primary and other secondary outcome findings, and concerns that composite outcomes lack patient centeredness, an accompanying editorial urged caution against changing clinical practice based on this finding.
Bottom line: There was no significant difference in hospital-free days for noncritically ill patients receiving IV fluids in the ED between those treated with saline and balanced crystalloid fluid.
Citation: Self WH et al. Balanced crystalloids versus saline in noncritically ill adults. N Eng J Med. 2018;378:819-28.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Quality metrics, programs for new GIs, and innovation
Dear Colleagues,
In the August issue of The New Gastroenterologist, we have some fantastic articles that I hope you will find both interesting and useful.
First, as quality metrics are becoming increasingly important in all aspects of health care, it is critical that we have a good understanding of quality metrics within our field. This issue’s “In Focus” article, written by Nabiha Shamsi, Ashish Malhotra, and Aasma Shaukat (University of Minnesota/Minnesota VAMC), provides a helpful overview of the currently accepted quality metrics for colonoscopy as well as the data that support their use. This article can be found online as well as in print in the August issue of GI & Hepatology News.
Additionally, in this issue, we have several articles highlighting important AGA programs that are great opportunities for those of us in our early careers. First, Jennifer Weiss (University of Wisconsin) discusses her experience in the Future Leaders Program, which just graduated its second class at DDW this year. Additionally, Sarah Lieber (UNC Chapel Hill) and Ana Maldonado-Contreras (University of Massachusetts) chronicle their experiences at the AGA Academic Skills Workshop which was held in Charlotte earlier this year. Finally, Eric Shah (University of Michigan), who served as this past year’s Gastroenterology Editorial Fellow, provides insights from his experience in this new position designed specifically for those in their early career.
Also in this issue is an article about pursuing a career in the innovation industry, authored by Chang Hee Kim (GoDx) and Wendy Henderson (NINR/NIH), who were winners of this year’s AGA Shark Tank. Finally, as many will be looking for new jobs in the coming year, one of the most important parts of this process will be the contract. Scott Roman, an attorney with significant expertise in contract law, provides an overview highlighting the important points about contracts that should not be overlooked.
As with prior installments of The New Gastroenterologist e-newsletter, please check out the “In Case You Missed It” section to see recent articles published in the AGA journals that have pertinence to those of us in our early careers. If you have any ideas or are interested in contributing to The New Gastroenterologist, please contact me at [email protected] or the managing editor, Ryan Farrell, at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
In the August issue of The New Gastroenterologist, we have some fantastic articles that I hope you will find both interesting and useful.
First, as quality metrics are becoming increasingly important in all aspects of health care, it is critical that we have a good understanding of quality metrics within our field. This issue’s “In Focus” article, written by Nabiha Shamsi, Ashish Malhotra, and Aasma Shaukat (University of Minnesota/Minnesota VAMC), provides a helpful overview of the currently accepted quality metrics for colonoscopy as well as the data that support their use. This article can be found online as well as in print in the August issue of GI & Hepatology News.
Additionally, in this issue, we have several articles highlighting important AGA programs that are great opportunities for those of us in our early careers. First, Jennifer Weiss (University of Wisconsin) discusses her experience in the Future Leaders Program, which just graduated its second class at DDW this year. Additionally, Sarah Lieber (UNC Chapel Hill) and Ana Maldonado-Contreras (University of Massachusetts) chronicle their experiences at the AGA Academic Skills Workshop which was held in Charlotte earlier this year. Finally, Eric Shah (University of Michigan), who served as this past year’s Gastroenterology Editorial Fellow, provides insights from his experience in this new position designed specifically for those in their early career.
Also in this issue is an article about pursuing a career in the innovation industry, authored by Chang Hee Kim (GoDx) and Wendy Henderson (NINR/NIH), who were winners of this year’s AGA Shark Tank. Finally, as many will be looking for new jobs in the coming year, one of the most important parts of this process will be the contract. Scott Roman, an attorney with significant expertise in contract law, provides an overview highlighting the important points about contracts that should not be overlooked.
As with prior installments of The New Gastroenterologist e-newsletter, please check out the “In Case You Missed It” section to see recent articles published in the AGA journals that have pertinence to those of us in our early careers. If you have any ideas or are interested in contributing to The New Gastroenterologist, please contact me at [email protected] or the managing editor, Ryan Farrell, at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
In the August issue of The New Gastroenterologist, we have some fantastic articles that I hope you will find both interesting and useful.
First, as quality metrics are becoming increasingly important in all aspects of health care, it is critical that we have a good understanding of quality metrics within our field. This issue’s “In Focus” article, written by Nabiha Shamsi, Ashish Malhotra, and Aasma Shaukat (University of Minnesota/Minnesota VAMC), provides a helpful overview of the currently accepted quality metrics for colonoscopy as well as the data that support their use. This article can be found online as well as in print in the August issue of GI & Hepatology News.
Additionally, in this issue, we have several articles highlighting important AGA programs that are great opportunities for those of us in our early careers. First, Jennifer Weiss (University of Wisconsin) discusses her experience in the Future Leaders Program, which just graduated its second class at DDW this year. Additionally, Sarah Lieber (UNC Chapel Hill) and Ana Maldonado-Contreras (University of Massachusetts) chronicle their experiences at the AGA Academic Skills Workshop which was held in Charlotte earlier this year. Finally, Eric Shah (University of Michigan), who served as this past year’s Gastroenterology Editorial Fellow, provides insights from his experience in this new position designed specifically for those in their early career.
Also in this issue is an article about pursuing a career in the innovation industry, authored by Chang Hee Kim (GoDx) and Wendy Henderson (NINR/NIH), who were winners of this year’s AGA Shark Tank. Finally, as many will be looking for new jobs in the coming year, one of the most important parts of this process will be the contract. Scott Roman, an attorney with significant expertise in contract law, provides an overview highlighting the important points about contracts that should not be overlooked.
As with prior installments of The New Gastroenterologist e-newsletter, please check out the “In Case You Missed It” section to see recent articles published in the AGA journals that have pertinence to those of us in our early careers. If you have any ideas or are interested in contributing to The New Gastroenterologist, please contact me at [email protected] or the managing editor, Ryan Farrell, at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
FDA approves Perseris for schizophrenia
The Food and Drug Administration has approved Perseris, a once-monthly, long-acting injectable formulation of risperidone, for the treatment of schizophrenia in adults, according to a press release from the drug’s developer, Indivior.
The depot formulation of the atypical antipsychotic, administered subcutaneously, provides sustained levels of risperidone for 1 month; the process of injecting it also releases some of the drug, which helps achieve peak plasma levels within the first 4-6 hours. There is no need for either loading doses or supplemental oral doses with Perseris.
Efficacy was based on a phase 3, randomized, double-blind, placebo-controlled, 8-week study of 354 patients. The primary endpoint was improvement in Positive and Negative Syndrome Scale by day 57. Safety was evaluated in 814 patients who had participated in clinical trials of Perseris and was in line with the known safety profile of risperidone.
The most common adverse reactions, occurring in more than 5% of patients, were increased weight, sedation/somnolence, and musculoskeletal pain. Other risks included neuroleptic malignant syndrome, tardive dyskinesia, and hyperprolactinemia. Full prescribing information can be found on the manufacturer’s website.
The Food and Drug Administration has approved Perseris, a once-monthly, long-acting injectable formulation of risperidone, for the treatment of schizophrenia in adults, according to a press release from the drug’s developer, Indivior.
The depot formulation of the atypical antipsychotic, administered subcutaneously, provides sustained levels of risperidone for 1 month; the process of injecting it also releases some of the drug, which helps achieve peak plasma levels within the first 4-6 hours. There is no need for either loading doses or supplemental oral doses with Perseris.
Efficacy was based on a phase 3, randomized, double-blind, placebo-controlled, 8-week study of 354 patients. The primary endpoint was improvement in Positive and Negative Syndrome Scale by day 57. Safety was evaluated in 814 patients who had participated in clinical trials of Perseris and was in line with the known safety profile of risperidone.
The most common adverse reactions, occurring in more than 5% of patients, were increased weight, sedation/somnolence, and musculoskeletal pain. Other risks included neuroleptic malignant syndrome, tardive dyskinesia, and hyperprolactinemia. Full prescribing information can be found on the manufacturer’s website.
The Food and Drug Administration has approved Perseris, a once-monthly, long-acting injectable formulation of risperidone, for the treatment of schizophrenia in adults, according to a press release from the drug’s developer, Indivior.
The depot formulation of the atypical antipsychotic, administered subcutaneously, provides sustained levels of risperidone for 1 month; the process of injecting it also releases some of the drug, which helps achieve peak plasma levels within the first 4-6 hours. There is no need for either loading doses or supplemental oral doses with Perseris.
Efficacy was based on a phase 3, randomized, double-blind, placebo-controlled, 8-week study of 354 patients. The primary endpoint was improvement in Positive and Negative Syndrome Scale by day 57. Safety was evaluated in 814 patients who had participated in clinical trials of Perseris and was in line with the known safety profile of risperidone.
The most common adverse reactions, occurring in more than 5% of patients, were increased weight, sedation/somnolence, and musculoskeletal pain. Other risks included neuroleptic malignant syndrome, tardive dyskinesia, and hyperprolactinemia. Full prescribing information can be found on the manufacturer’s website.
FDA approves radioactive agent for adrenal tumors
(pheochromocytoma or paraganglioma) that are unresectable, have metastasized, and require systemic therapy.
This is the first FDA-approved drug for this use, the FDA said in a press announcement.
Approval is based on a single-arm, open-label clinical trial that included 68 patients. The primary endpoint was the number or patients with a 50% or greater reduction of antihypertensive medications lasting at least 6 months; the secondary endpoint was overall tumor response according to traditional imaging criteria. The primary endpoint was met by 17 patients, and the secondary endpoint was achieved in 15.
The most common severe side effects were lymphopenia, neutropenia, thrombocytopenia, fatigue, anemia, increased international normalized ratio, nausea, dizziness, hypertension, and vomiting. Furthermore, because this is a radioactive therapeutic agent, there is a warning about radiation exposure for both patients and family members, a risk that is greatest in pediatric patients.
Other warnings and precautions include a risk of myelosuppression, underactive thyroid, elevations in blood pressure, renal failure or kidney injury, and pneumonitis. Myelodysplastic syndrome and acute leukemias were observed in patients who received the radioactive agent, and the magnitude of this risk will continue to be studied, the FDA said.
The approval was granted to Progenics Pharmaceuticals.
(pheochromocytoma or paraganglioma) that are unresectable, have metastasized, and require systemic therapy.
This is the first FDA-approved drug for this use, the FDA said in a press announcement.
Approval is based on a single-arm, open-label clinical trial that included 68 patients. The primary endpoint was the number or patients with a 50% or greater reduction of antihypertensive medications lasting at least 6 months; the secondary endpoint was overall tumor response according to traditional imaging criteria. The primary endpoint was met by 17 patients, and the secondary endpoint was achieved in 15.
The most common severe side effects were lymphopenia, neutropenia, thrombocytopenia, fatigue, anemia, increased international normalized ratio, nausea, dizziness, hypertension, and vomiting. Furthermore, because this is a radioactive therapeutic agent, there is a warning about radiation exposure for both patients and family members, a risk that is greatest in pediatric patients.
Other warnings and precautions include a risk of myelosuppression, underactive thyroid, elevations in blood pressure, renal failure or kidney injury, and pneumonitis. Myelodysplastic syndrome and acute leukemias were observed in patients who received the radioactive agent, and the magnitude of this risk will continue to be studied, the FDA said.
The approval was granted to Progenics Pharmaceuticals.
(pheochromocytoma or paraganglioma) that are unresectable, have metastasized, and require systemic therapy.
This is the first FDA-approved drug for this use, the FDA said in a press announcement.
Approval is based on a single-arm, open-label clinical trial that included 68 patients. The primary endpoint was the number or patients with a 50% or greater reduction of antihypertensive medications lasting at least 6 months; the secondary endpoint was overall tumor response according to traditional imaging criteria. The primary endpoint was met by 17 patients, and the secondary endpoint was achieved in 15.
The most common severe side effects were lymphopenia, neutropenia, thrombocytopenia, fatigue, anemia, increased international normalized ratio, nausea, dizziness, hypertension, and vomiting. Furthermore, because this is a radioactive therapeutic agent, there is a warning about radiation exposure for both patients and family members, a risk that is greatest in pediatric patients.
Other warnings and precautions include a risk of myelosuppression, underactive thyroid, elevations in blood pressure, renal failure or kidney injury, and pneumonitis. Myelodysplastic syndrome and acute leukemias were observed in patients who received the radioactive agent, and the magnitude of this risk will continue to be studied, the FDA said.
The approval was granted to Progenics Pharmaceuticals.