User login
ERAS adoption for colectomy yielded state-wide outcome improvements
ORLANDO – Widespread implementation of an analysis shows.
The benefits were particularly pronounced in the subset of patients undergoing laparoscopic colectomy in the retrospective analysis, which included data for treated at four institutions in the Virginia Surgical Quality Collaborative Workgroup.
While the benefits of enhanced recovery after surgery (ERAS) are well known, most of the published data has come from single-institution experiences, according to investigator Traci L. Hedrick, MD, FACS, of the University of Virginia, Charlottesville.
At the American College of Surgeons Quality and Safety Conference, Dr. Hedrick presented risk-adjusted National Surgical Quality Improvement Program (NSQIP) data for 2,971 consecutive procedures during 2012-2016 at the University of Virginia, Winchester Medical Center, Carilion Clinic, and Inova Fairfax.
“Institutions came and went from the collaborative during this time period, so we focused on those institutions that maintained in the collaborative throughout the entire study protocol,” Dr. Hedrick said in her presentation.
Of the 2,971 procedures, about half (1,460) were performed after implementation of enhanced recovery protocols. Laparoscopic and open procedures were analyzed separately due to a substantial shift toward laparoscopic procedures, mainly during the 2012-2014 period, Dr. Hedrick said.
Among laparoscopic cases, there was a significant 1-day reduction in median length of stay, dropping from 4 days for pre–enhanced recovery protocol cases to 3 days for post–enhanced recovery protocol cases, Dr. Hedrick reported.
Observed morbidity also dropped significantly from 14.8% to 8.9% for the pre– and post–enhanced recovery cases, and the readmission rate fell significantly from 13% to 8.8%.
For open cases, there was a significant 1-day drop in median length of stay, from 4 to 3 days, but no significant differences in observed morbidity or readmission rates, according to Dr. Hedrick.
“As more of the patients were done laparoscopically, that really selected out the more complicated patients that were undergoing open procedures,” she said.
The protocols implemented by institutions in the Virginia collaborative group were generally uniform in important tenants of enhanced recovery, such as opioid minimization and avoidance of fasting, but specific elements were left up to each institution to improve buy-in, according to Dr. Hedrick.
“A lot of our protocols are very similar, particularly with regards to the order set,” Dr. Hedrick explained, “[but] I really am a firm believer in not being very strict about exactly what to use, because it’s so dependent on preference at the local level.”
The Virginia Surgical Quality Collaborative Workgroup is one of 20 regional ACS NSQIP collaboratives with the objective of improving surgical outcomes through multi-institutional collaboration, Dr. Hedrick said.
Dr. Hedrick and her coinvestigators had no relevant disclosures to report.
ORLANDO – Widespread implementation of an analysis shows.
The benefits were particularly pronounced in the subset of patients undergoing laparoscopic colectomy in the retrospective analysis, which included data for treated at four institutions in the Virginia Surgical Quality Collaborative Workgroup.
While the benefits of enhanced recovery after surgery (ERAS) are well known, most of the published data has come from single-institution experiences, according to investigator Traci L. Hedrick, MD, FACS, of the University of Virginia, Charlottesville.
At the American College of Surgeons Quality and Safety Conference, Dr. Hedrick presented risk-adjusted National Surgical Quality Improvement Program (NSQIP) data for 2,971 consecutive procedures during 2012-2016 at the University of Virginia, Winchester Medical Center, Carilion Clinic, and Inova Fairfax.
“Institutions came and went from the collaborative during this time period, so we focused on those institutions that maintained in the collaborative throughout the entire study protocol,” Dr. Hedrick said in her presentation.
Of the 2,971 procedures, about half (1,460) were performed after implementation of enhanced recovery protocols. Laparoscopic and open procedures were analyzed separately due to a substantial shift toward laparoscopic procedures, mainly during the 2012-2014 period, Dr. Hedrick said.
Among laparoscopic cases, there was a significant 1-day reduction in median length of stay, dropping from 4 days for pre–enhanced recovery protocol cases to 3 days for post–enhanced recovery protocol cases, Dr. Hedrick reported.
Observed morbidity also dropped significantly from 14.8% to 8.9% for the pre– and post–enhanced recovery cases, and the readmission rate fell significantly from 13% to 8.8%.
For open cases, there was a significant 1-day drop in median length of stay, from 4 to 3 days, but no significant differences in observed morbidity or readmission rates, according to Dr. Hedrick.
“As more of the patients were done laparoscopically, that really selected out the more complicated patients that were undergoing open procedures,” she said.
The protocols implemented by institutions in the Virginia collaborative group were generally uniform in important tenants of enhanced recovery, such as opioid minimization and avoidance of fasting, but specific elements were left up to each institution to improve buy-in, according to Dr. Hedrick.
“A lot of our protocols are very similar, particularly with regards to the order set,” Dr. Hedrick explained, “[but] I really am a firm believer in not being very strict about exactly what to use, because it’s so dependent on preference at the local level.”
The Virginia Surgical Quality Collaborative Workgroup is one of 20 regional ACS NSQIP collaboratives with the objective of improving surgical outcomes through multi-institutional collaboration, Dr. Hedrick said.
Dr. Hedrick and her coinvestigators had no relevant disclosures to report.
ORLANDO – Widespread implementation of an analysis shows.
The benefits were particularly pronounced in the subset of patients undergoing laparoscopic colectomy in the retrospective analysis, which included data for treated at four institutions in the Virginia Surgical Quality Collaborative Workgroup.
While the benefits of enhanced recovery after surgery (ERAS) are well known, most of the published data has come from single-institution experiences, according to investigator Traci L. Hedrick, MD, FACS, of the University of Virginia, Charlottesville.
At the American College of Surgeons Quality and Safety Conference, Dr. Hedrick presented risk-adjusted National Surgical Quality Improvement Program (NSQIP) data for 2,971 consecutive procedures during 2012-2016 at the University of Virginia, Winchester Medical Center, Carilion Clinic, and Inova Fairfax.
“Institutions came and went from the collaborative during this time period, so we focused on those institutions that maintained in the collaborative throughout the entire study protocol,” Dr. Hedrick said in her presentation.
Of the 2,971 procedures, about half (1,460) were performed after implementation of enhanced recovery protocols. Laparoscopic and open procedures were analyzed separately due to a substantial shift toward laparoscopic procedures, mainly during the 2012-2014 period, Dr. Hedrick said.
Among laparoscopic cases, there was a significant 1-day reduction in median length of stay, dropping from 4 days for pre–enhanced recovery protocol cases to 3 days for post–enhanced recovery protocol cases, Dr. Hedrick reported.
Observed morbidity also dropped significantly from 14.8% to 8.9% for the pre– and post–enhanced recovery cases, and the readmission rate fell significantly from 13% to 8.8%.
For open cases, there was a significant 1-day drop in median length of stay, from 4 to 3 days, but no significant differences in observed morbidity or readmission rates, according to Dr. Hedrick.
“As more of the patients were done laparoscopically, that really selected out the more complicated patients that were undergoing open procedures,” she said.
The protocols implemented by institutions in the Virginia collaborative group were generally uniform in important tenants of enhanced recovery, such as opioid minimization and avoidance of fasting, but specific elements were left up to each institution to improve buy-in, according to Dr. Hedrick.
“A lot of our protocols are very similar, particularly with regards to the order set,” Dr. Hedrick explained, “[but] I really am a firm believer in not being very strict about exactly what to use, because it’s so dependent on preference at the local level.”
The Virginia Surgical Quality Collaborative Workgroup is one of 20 regional ACS NSQIP collaboratives with the objective of improving surgical outcomes through multi-institutional collaboration, Dr. Hedrick said.
Dr. Hedrick and her coinvestigators had no relevant disclosures to report.
REPORTING FROM ACSQSC 2018
Key clinical point: State-wide adoption of ERAS for colectomy yielded state-wide outcome improvements .
Major finding: Morbidity also dropped significantly from 14.8% to 8.9% and the readmission rate fell significantly from 13% to 8.8%.
Study details: Risk adjusted NSQIP data in 2,971 colectomy patients from four hospitals in the Virginia Surgical Quality Collaborative Workgroup.
Disclosures: Dr. Hedrick and coinvestigators had no relevant disclosures to report.
Higher lymph node harvest could improve right-side colon cancer outcomes
ORLANDO – The inferior outcomes associated with right-sided colon cancers might be mitigated if a higher lymph node harvest is obtained, a retrospective study suggested.
Among patients with right-sided cancers, the rate of survival improved when 22 or more lymph nodes were harvested during operations, according to the study results presented at the American College of Surgeons Quality and Safety Conference.
“These data may provide indirect evidence for complete mesocolic excision to obtain a higher lymph node harvest to improve survival,” said investigator Arman Erkan, MD, of the Center for Colon and Rectal Surgery at Florida Hospital Orlando, in an oral abstract presentation.
This study adds new perspective on recent studies that have also demonstrated worse outcomes for right-sided versus left-sided tumors, which may be related to differences in levels of vascular ligation and nodal harvest. In addition, many studies to date have been limited in their ability to evaluate that hypothesis because of small sample size or other factors, he said in his presentation.
Accordingly, Dr. Erkan and his colleagues queried the National Cancer Database for colectomies for nonmetastatic colon adenocarcinoma occurring between 2004 and 2014, evaluating a total of 504,958 patient records, of which 273,198 were for right-sided tumors. To minimize bias, they used propensity score matching, leaving 148,540 patients in each group for the primary analysis.
Right-sided tumors were associated with inferior 5-year survival for patients with stage II and III disease (P less than .001 for right vs. left in both analyses), the investigators found.
In multivariate analysis, they found a significant interaction between right-sided tumors and a lymph node harvest of greater than 22 nodes toward increased survival, with a hazard ratio of 0.87 (95% confidence interval, 0.84-0.90). “This indicates that survival after right-sided resections can be improved if more than 22 nodes are harvested during the surgery,” Dr. Erkan said.
The difference was most pronounced in stage III of the disease, he added.
Study coauthor Lawrence Lee, MD, a colorectal surgeon at McGill University, said in a related press release that the study findings might prompt surgeons to reevaluate the types of procedures they perform in patients with right-sided tumors. “These patients may need a more extensive resection than is considered to be standard for them.”
Dr. Erkan, Dr. Lee, and other coinvestigators reported no conflicts of interest related to their research.
Help your patients better prepare for their colonoscopy by using AGA patient education materials: https://www.gastro.org/practice-guidance/gi-patient-center/topic/colonoscopy.
ORLANDO – The inferior outcomes associated with right-sided colon cancers might be mitigated if a higher lymph node harvest is obtained, a retrospective study suggested.
Among patients with right-sided cancers, the rate of survival improved when 22 or more lymph nodes were harvested during operations, according to the study results presented at the American College of Surgeons Quality and Safety Conference.
“These data may provide indirect evidence for complete mesocolic excision to obtain a higher lymph node harvest to improve survival,” said investigator Arman Erkan, MD, of the Center for Colon and Rectal Surgery at Florida Hospital Orlando, in an oral abstract presentation.
This study adds new perspective on recent studies that have also demonstrated worse outcomes for right-sided versus left-sided tumors, which may be related to differences in levels of vascular ligation and nodal harvest. In addition, many studies to date have been limited in their ability to evaluate that hypothesis because of small sample size or other factors, he said in his presentation.
Accordingly, Dr. Erkan and his colleagues queried the National Cancer Database for colectomies for nonmetastatic colon adenocarcinoma occurring between 2004 and 2014, evaluating a total of 504,958 patient records, of which 273,198 were for right-sided tumors. To minimize bias, they used propensity score matching, leaving 148,540 patients in each group for the primary analysis.
Right-sided tumors were associated with inferior 5-year survival for patients with stage II and III disease (P less than .001 for right vs. left in both analyses), the investigators found.
In multivariate analysis, they found a significant interaction between right-sided tumors and a lymph node harvest of greater than 22 nodes toward increased survival, with a hazard ratio of 0.87 (95% confidence interval, 0.84-0.90). “This indicates that survival after right-sided resections can be improved if more than 22 nodes are harvested during the surgery,” Dr. Erkan said.
The difference was most pronounced in stage III of the disease, he added.
Study coauthor Lawrence Lee, MD, a colorectal surgeon at McGill University, said in a related press release that the study findings might prompt surgeons to reevaluate the types of procedures they perform in patients with right-sided tumors. “These patients may need a more extensive resection than is considered to be standard for them.”
Dr. Erkan, Dr. Lee, and other coinvestigators reported no conflicts of interest related to their research.
Help your patients better prepare for their colonoscopy by using AGA patient education materials: https://www.gastro.org/practice-guidance/gi-patient-center/topic/colonoscopy.
ORLANDO – The inferior outcomes associated with right-sided colon cancers might be mitigated if a higher lymph node harvest is obtained, a retrospective study suggested.
Among patients with right-sided cancers, the rate of survival improved when 22 or more lymph nodes were harvested during operations, according to the study results presented at the American College of Surgeons Quality and Safety Conference.
“These data may provide indirect evidence for complete mesocolic excision to obtain a higher lymph node harvest to improve survival,” said investigator Arman Erkan, MD, of the Center for Colon and Rectal Surgery at Florida Hospital Orlando, in an oral abstract presentation.
This study adds new perspective on recent studies that have also demonstrated worse outcomes for right-sided versus left-sided tumors, which may be related to differences in levels of vascular ligation and nodal harvest. In addition, many studies to date have been limited in their ability to evaluate that hypothesis because of small sample size or other factors, he said in his presentation.
Accordingly, Dr. Erkan and his colleagues queried the National Cancer Database for colectomies for nonmetastatic colon adenocarcinoma occurring between 2004 and 2014, evaluating a total of 504,958 patient records, of which 273,198 were for right-sided tumors. To minimize bias, they used propensity score matching, leaving 148,540 patients in each group for the primary analysis.
Right-sided tumors were associated with inferior 5-year survival for patients with stage II and III disease (P less than .001 for right vs. left in both analyses), the investigators found.
In multivariate analysis, they found a significant interaction between right-sided tumors and a lymph node harvest of greater than 22 nodes toward increased survival, with a hazard ratio of 0.87 (95% confidence interval, 0.84-0.90). “This indicates that survival after right-sided resections can be improved if more than 22 nodes are harvested during the surgery,” Dr. Erkan said.
The difference was most pronounced in stage III of the disease, he added.
Study coauthor Lawrence Lee, MD, a colorectal surgeon at McGill University, said in a related press release that the study findings might prompt surgeons to reevaluate the types of procedures they perform in patients with right-sided tumors. “These patients may need a more extensive resection than is considered to be standard for them.”
Dr. Erkan, Dr. Lee, and other coinvestigators reported no conflicts of interest related to their research.
Help your patients better prepare for their colonoscopy by using AGA patient education materials: https://www.gastro.org/practice-guidance/gi-patient-center/topic/colonoscopy.
REPORTING FROM ACSQSC 2018
Phase 2 trial: Dendritic cell vaccine maintenance prolongs PFS in EOC
CHICAGO – who have undergone primary debulking surgery, according to interim findings from a randomized, phase 2, open-label trial.
Of 99 patients enrolled in the international, multicenter trial between November 2013 and March 2016, 92 received at least one dose of therapy and had a postbaseline endpoint assessment (the modified intention-to-treat population). Median progression-free survival was 18.3 months in the 31 patients who received autologous dendritic cell vaccine (DCVAC) given concomitantly with chemotherapy (group A), 24.3 months in the 30 patients who received DCVAC given sequentially as maintenance therapy after chemotherapy (group B), and 18.6 months in the 31 controls who received chemotherapy alone (group C), Lukas Rob, MD, reported at the annual meeting of the American Society of Clinical Oncology.
“When DCVAC was administered as maintenance [therapy] after the chemotherapy, the time to disease progression was prolonged by almost 6 months. Based on the hazard ratio, there was a 57% decrease in the hazard of progression in favor of arm B,” said Dr. Rob, of University Hospital Kralovske Vinohrady, Prague.
Compared with group C, progression-free survival hazard ratios were 0.64 and 0.43 in the modified intention-to-treat population in groups A and B, respectively, he said.
“These results are statistically significant and the clinical benefit is even more significant in patients who received more doses of DCVAC,” he added, explaining that the hazard ratios for patients in groups A and B who received at least eight doses of DCVAC and/or three cycles of chemotherapy (the per protocol population, including 87 patients) were 1.01 and 0.32, respectively.
Although the overall survival data in this study is immature, there is “a strong trend in favor of DCVAC in the maintenance treatment arm,” he said.
Study participants had FIGO stage III epithelial ovarian cancer (EOC) and a performance status score of 0-2. All had undergone primary debulking surgery and had less than 1 cm maximal residuum and no prior systemic therapy. Patients were randomized up to 6 weeks after surgery. Chemotherapy in all three groups included six cycles of carboplatin and paclitaxel, and DCVAC was given at doses of 1 x 107 dendritic cells per dose for a planned 10 doses.
Most patients with EOC – about 70% of those with stage III/IV disease – relapse after optimal debulking surgery and chemotherapy.
“There is no doubt that we need a new treatment modality,” he said. Because autologous DCVAC can present tumor antigens to elicit a durable immune response, he and his colleagues hypothesized that adding DCVAC to chemotherapy could improve outcomes – either when used concomitantly to target tumor-induced immune suppression and allowing for partial recovery of the immune system after each cycle, or when used sequentially as maintenance therapy, as minimal tumor burden after chemotherapy would provide optimal conditions for immune stimulation and the immune system would be fully recovered after completing cytotoxic therapy.
The treatments in this study were well tolerated; no grade 3 or greater adverse events were related solely to DCVAC, and the vaccine did not worsen the side effects of chemotherapy, Dr. Rob said.
The findings suggested that sequential DCVAC after chemotherapy in patients with EOC is a promising maintenance treatment option that can delay disease progression, he concluded, noting that “due to the excellent result in the maintenance arm we decided ... to enroll more patients in arms B and C to increase the power of this study.”
Additionally, a phase 3 trial is planned and enrollment should begin in early 2019, he said.
Dr. Rob reported having no disclosures.
SOURCE: Rob L et al. ASCO 2018, Abstract 5509.
CHICAGO – who have undergone primary debulking surgery, according to interim findings from a randomized, phase 2, open-label trial.
Of 99 patients enrolled in the international, multicenter trial between November 2013 and March 2016, 92 received at least one dose of therapy and had a postbaseline endpoint assessment (the modified intention-to-treat population). Median progression-free survival was 18.3 months in the 31 patients who received autologous dendritic cell vaccine (DCVAC) given concomitantly with chemotherapy (group A), 24.3 months in the 30 patients who received DCVAC given sequentially as maintenance therapy after chemotherapy (group B), and 18.6 months in the 31 controls who received chemotherapy alone (group C), Lukas Rob, MD, reported at the annual meeting of the American Society of Clinical Oncology.
“When DCVAC was administered as maintenance [therapy] after the chemotherapy, the time to disease progression was prolonged by almost 6 months. Based on the hazard ratio, there was a 57% decrease in the hazard of progression in favor of arm B,” said Dr. Rob, of University Hospital Kralovske Vinohrady, Prague.
Compared with group C, progression-free survival hazard ratios were 0.64 and 0.43 in the modified intention-to-treat population in groups A and B, respectively, he said.
“These results are statistically significant and the clinical benefit is even more significant in patients who received more doses of DCVAC,” he added, explaining that the hazard ratios for patients in groups A and B who received at least eight doses of DCVAC and/or three cycles of chemotherapy (the per protocol population, including 87 patients) were 1.01 and 0.32, respectively.
Although the overall survival data in this study is immature, there is “a strong trend in favor of DCVAC in the maintenance treatment arm,” he said.
Study participants had FIGO stage III epithelial ovarian cancer (EOC) and a performance status score of 0-2. All had undergone primary debulking surgery and had less than 1 cm maximal residuum and no prior systemic therapy. Patients were randomized up to 6 weeks after surgery. Chemotherapy in all three groups included six cycles of carboplatin and paclitaxel, and DCVAC was given at doses of 1 x 107 dendritic cells per dose for a planned 10 doses.
Most patients with EOC – about 70% of those with stage III/IV disease – relapse after optimal debulking surgery and chemotherapy.
“There is no doubt that we need a new treatment modality,” he said. Because autologous DCVAC can present tumor antigens to elicit a durable immune response, he and his colleagues hypothesized that adding DCVAC to chemotherapy could improve outcomes – either when used concomitantly to target tumor-induced immune suppression and allowing for partial recovery of the immune system after each cycle, or when used sequentially as maintenance therapy, as minimal tumor burden after chemotherapy would provide optimal conditions for immune stimulation and the immune system would be fully recovered after completing cytotoxic therapy.
The treatments in this study were well tolerated; no grade 3 or greater adverse events were related solely to DCVAC, and the vaccine did not worsen the side effects of chemotherapy, Dr. Rob said.
The findings suggested that sequential DCVAC after chemotherapy in patients with EOC is a promising maintenance treatment option that can delay disease progression, he concluded, noting that “due to the excellent result in the maintenance arm we decided ... to enroll more patients in arms B and C to increase the power of this study.”
Additionally, a phase 3 trial is planned and enrollment should begin in early 2019, he said.
Dr. Rob reported having no disclosures.
SOURCE: Rob L et al. ASCO 2018, Abstract 5509.
CHICAGO – who have undergone primary debulking surgery, according to interim findings from a randomized, phase 2, open-label trial.
Of 99 patients enrolled in the international, multicenter trial between November 2013 and March 2016, 92 received at least one dose of therapy and had a postbaseline endpoint assessment (the modified intention-to-treat population). Median progression-free survival was 18.3 months in the 31 patients who received autologous dendritic cell vaccine (DCVAC) given concomitantly with chemotherapy (group A), 24.3 months in the 30 patients who received DCVAC given sequentially as maintenance therapy after chemotherapy (group B), and 18.6 months in the 31 controls who received chemotherapy alone (group C), Lukas Rob, MD, reported at the annual meeting of the American Society of Clinical Oncology.
“When DCVAC was administered as maintenance [therapy] after the chemotherapy, the time to disease progression was prolonged by almost 6 months. Based on the hazard ratio, there was a 57% decrease in the hazard of progression in favor of arm B,” said Dr. Rob, of University Hospital Kralovske Vinohrady, Prague.
Compared with group C, progression-free survival hazard ratios were 0.64 and 0.43 in the modified intention-to-treat population in groups A and B, respectively, he said.
“These results are statistically significant and the clinical benefit is even more significant in patients who received more doses of DCVAC,” he added, explaining that the hazard ratios for patients in groups A and B who received at least eight doses of DCVAC and/or three cycles of chemotherapy (the per protocol population, including 87 patients) were 1.01 and 0.32, respectively.
Although the overall survival data in this study is immature, there is “a strong trend in favor of DCVAC in the maintenance treatment arm,” he said.
Study participants had FIGO stage III epithelial ovarian cancer (EOC) and a performance status score of 0-2. All had undergone primary debulking surgery and had less than 1 cm maximal residuum and no prior systemic therapy. Patients were randomized up to 6 weeks after surgery. Chemotherapy in all three groups included six cycles of carboplatin and paclitaxel, and DCVAC was given at doses of 1 x 107 dendritic cells per dose for a planned 10 doses.
Most patients with EOC – about 70% of those with stage III/IV disease – relapse after optimal debulking surgery and chemotherapy.
“There is no doubt that we need a new treatment modality,” he said. Because autologous DCVAC can present tumor antigens to elicit a durable immune response, he and his colleagues hypothesized that adding DCVAC to chemotherapy could improve outcomes – either when used concomitantly to target tumor-induced immune suppression and allowing for partial recovery of the immune system after each cycle, or when used sequentially as maintenance therapy, as minimal tumor burden after chemotherapy would provide optimal conditions for immune stimulation and the immune system would be fully recovered after completing cytotoxic therapy.
The treatments in this study were well tolerated; no grade 3 or greater adverse events were related solely to DCVAC, and the vaccine did not worsen the side effects of chemotherapy, Dr. Rob said.
The findings suggested that sequential DCVAC after chemotherapy in patients with EOC is a promising maintenance treatment option that can delay disease progression, he concluded, noting that “due to the excellent result in the maintenance arm we decided ... to enroll more patients in arms B and C to increase the power of this study.”
Additionally, a phase 3 trial is planned and enrollment should begin in early 2019, he said.
Dr. Rob reported having no disclosures.
SOURCE: Rob L et al. ASCO 2018, Abstract 5509.
REPORTING FROM ASCO 2018
Key clinical point: Dendritic cell vaccine maintenance improves progression-free survival in patients with epithelial ovarian carcinoma who have undergone primary debulking surgery.
Major finding: DCVAC maintenance after chemotherapy prolonged progression-free survival by almost 6 months (hazard ratio, 0.43 vs. chemotherapy alone).
Study details: A randomized, phase 2, open-label trial including 99 patients.
Disclosures: Dr. Rob reported having no disclosures.
Source: Rob L et al. ASCO 2018, Abstract 5509.
What is your diagnosis?
Laboratory work revealed a normal CBC and differential, an elevated C-reactive protein (CRP) and sedimentation rate (ESR), negative antistreptolysin O (ASO) titers, negative pregnancy test, a normal urinalysis, and negative blood, throat, and urine cultures. A chest x-ray also was negative as well as angiotensin-converting enzyme (ACE) levels. Tuberculosis interferon-gamma release essay was negative.
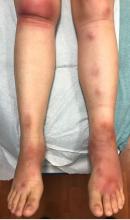
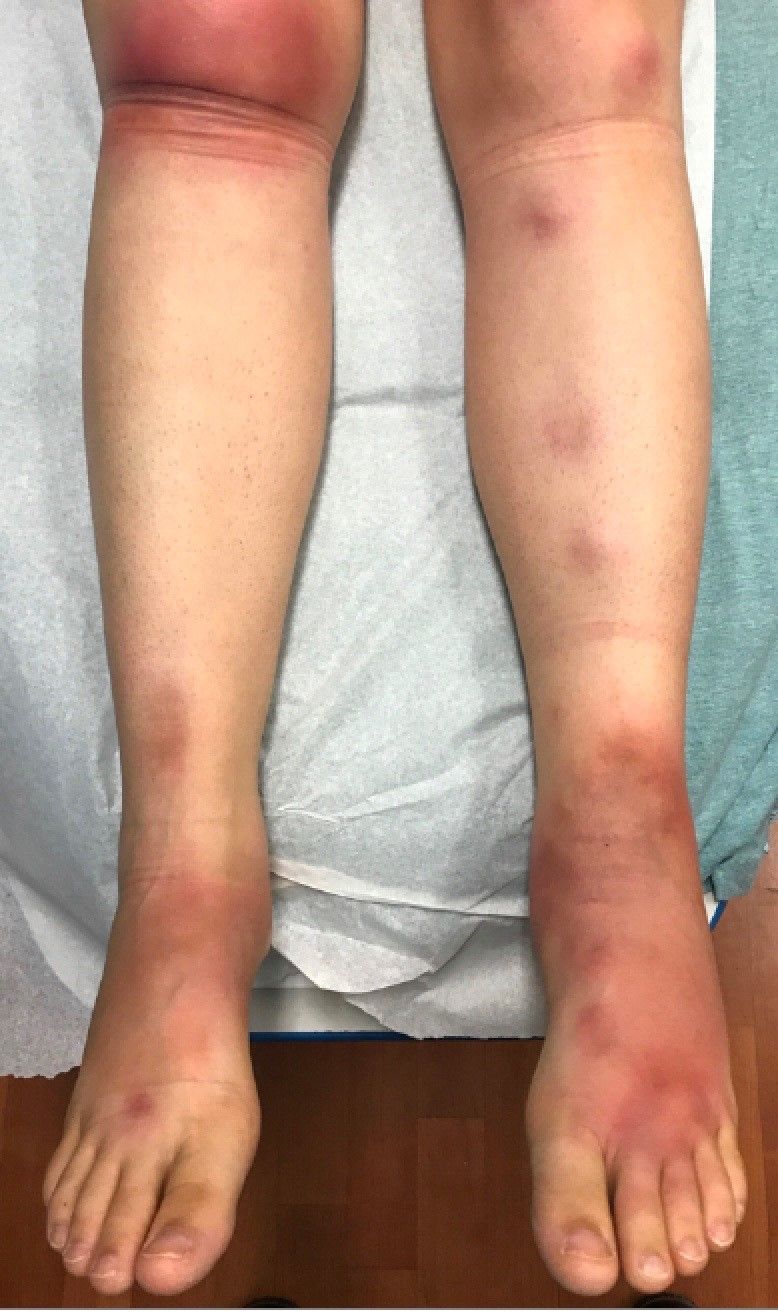
The patient was diagnosed with erythema nodosum (EN), based on physical exam and history of the lesions. In her particular case, infectious causes including streptococcus infection, tuberculosis, and coccidioidomycosis were ruled out. There were no x-ray findings that suggested sarcoidosis and her ACE level was within normal limits. The pregnancy test also was negative. Given her recent start on OCs, this was thought to be the cause of the lesions.
She was treated with elevation, compression stockings, and NSAIDs and discontinuation of OCs. The lesions resolved after 6 weeks leaving bruiselike patches (erythema contusiformis).
EN is a delayed-type hypersensitivity reaction, causing inflammation on the fat (panniculitis) most commonly on the shins, but it can also occur on the arms, face, neck, and thighs. It is the most common type of panniculitis and is usually seen more often in women from the second to fourth decade of life. Erythematous tender nodules in crops commonly located on the shins are the characteristic physical finding. Systemic symptoms can occur including fever, malaise, and joint pain. The lesions usually last up to 6-8 weeks and may leave bruiselike patches or postinflammatory hyperpigmentation that can take months to improve.1
The diagnosis of EN usually is made by physical examination and natural history. In unusual severe cases or lesions in atypical locations, a skin biopsy is indicated. Histologic examination of one of the lesions reveals a septal panniculitis without vasculitis. Miescher’s radial granulomas (grouped macrophages around neutrophils or septa-like spaces) often are present and are a characteristic feature of EN.
EN can be triggered by different types of infections such as streptococcus, mycoplasma, tuberculosis, or bacterial gastroenteritis; medications such as OCs, sulfonamides, iodides, penicillin, or bromides; medical conditions that include inflammatory bowel disease, pregnancy, or sarcoidosis; or neutrophilic dermatosis and malignancy such as leukemia and Hodgkin disease.2,3 A third of the cases are idiopathic. In children, streptococcal infections are responsible for most cases of EN.4
Recommended work-up to investigate possible triggers includes a CBC with differential, sedimentation rate, CRP, ASO titers or anti-DNase B titers, tuberculin skin test or interferon-gamma TB test and a chest X ray. If there are any other symptoms, physical signs, or risk factors are present for the other not so common causes, further ancillary testing may be warranted.
Erythematous nodules and papules on the shin in children are commonly caused by arthropod bites also known as papular urticaria. These lesions are pruritic rather than tender and usually respond to topical corticosteroids and oral antihistamines. Subcutaneous bacterial, fungal, or atypical mycobacterial infections can present with tender nodules that can ulcerate and drain on the shins, feet, or any other body part. These patients may have a history of immunodeficiency and usually systemic symptoms of infection are present. Cutaneous polyarteritis nodosa (PAN) also can present with tender nodules on the legs but these lesions usually necrose and ulcerate and may be associated with livedo racemosa, a transient or persistent, blotchy, reddish-blue to purple, netlike cyanotic pattern. On pathology, PAN presents with necrotizing medium vessel vasculitis. Malignant nodules also can occur on the shin. Pathology will show atypical cells. Other forms of panniculitis, such as erythema induratum and pancreatic panniculitis, can present with tender nodules but these lesions usually occur on the calves and ulcerate.
Management of EN starts with treating the underlying infection or stopping the causative medication. Initial measures include bed rest, leg elevation, compression bandages, and NSAIDs. Potassium iodide is a very effective therapy as it may control the symptoms within 24 hours. When there is no response to the above, or the patient has severe symptoms, a short course of systemic glucocorticoids can be started. Other medications for recalcitrant or recurrent lesions include colchicine, dapsone, or hydroxychloroquine.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Panniculitis, in “Dermatology,” 3rd ed. (Philadelphia: Elsevier Saunders, 2012, p. 1641).
2. Arthritis Rheum. 2000 Mar;43(3):584-92.
3. J Clin Oncol. 2007 Sep 1;25(25):4011-2.
4. Turk J Pediatr. 2014 Mar-Apr;56(2):144-9.
Laboratory work revealed a normal CBC and differential, an elevated C-reactive protein (CRP) and sedimentation rate (ESR), negative antistreptolysin O (ASO) titers, negative pregnancy test, a normal urinalysis, and negative blood, throat, and urine cultures. A chest x-ray also was negative as well as angiotensin-converting enzyme (ACE) levels. Tuberculosis interferon-gamma release essay was negative.
The patient was diagnosed with erythema nodosum (EN), based on physical exam and history of the lesions. In her particular case, infectious causes including streptococcus infection, tuberculosis, and coccidioidomycosis were ruled out. There were no x-ray findings that suggested sarcoidosis and her ACE level was within normal limits. The pregnancy test also was negative. Given her recent start on OCs, this was thought to be the cause of the lesions.
She was treated with elevation, compression stockings, and NSAIDs and discontinuation of OCs. The lesions resolved after 6 weeks leaving bruiselike patches (erythema contusiformis).
EN is a delayed-type hypersensitivity reaction, causing inflammation on the fat (panniculitis) most commonly on the shins, but it can also occur on the arms, face, neck, and thighs. It is the most common type of panniculitis and is usually seen more often in women from the second to fourth decade of life. Erythematous tender nodules in crops commonly located on the shins are the characteristic physical finding. Systemic symptoms can occur including fever, malaise, and joint pain. The lesions usually last up to 6-8 weeks and may leave bruiselike patches or postinflammatory hyperpigmentation that can take months to improve.1
The diagnosis of EN usually is made by physical examination and natural history. In unusual severe cases or lesions in atypical locations, a skin biopsy is indicated. Histologic examination of one of the lesions reveals a septal panniculitis without vasculitis. Miescher’s radial granulomas (grouped macrophages around neutrophils or septa-like spaces) often are present and are a characteristic feature of EN.
EN can be triggered by different types of infections such as streptococcus, mycoplasma, tuberculosis, or bacterial gastroenteritis; medications such as OCs, sulfonamides, iodides, penicillin, or bromides; medical conditions that include inflammatory bowel disease, pregnancy, or sarcoidosis; or neutrophilic dermatosis and malignancy such as leukemia and Hodgkin disease.2,3 A third of the cases are idiopathic. In children, streptococcal infections are responsible for most cases of EN.4
Recommended work-up to investigate possible triggers includes a CBC with differential, sedimentation rate, CRP, ASO titers or anti-DNase B titers, tuberculin skin test or interferon-gamma TB test and a chest X ray. If there are any other symptoms, physical signs, or risk factors are present for the other not so common causes, further ancillary testing may be warranted.
Erythematous nodules and papules on the shin in children are commonly caused by arthropod bites also known as papular urticaria. These lesions are pruritic rather than tender and usually respond to topical corticosteroids and oral antihistamines. Subcutaneous bacterial, fungal, or atypical mycobacterial infections can present with tender nodules that can ulcerate and drain on the shins, feet, or any other body part. These patients may have a history of immunodeficiency and usually systemic symptoms of infection are present. Cutaneous polyarteritis nodosa (PAN) also can present with tender nodules on the legs but these lesions usually necrose and ulcerate and may be associated with livedo racemosa, a transient or persistent, blotchy, reddish-blue to purple, netlike cyanotic pattern. On pathology, PAN presents with necrotizing medium vessel vasculitis. Malignant nodules also can occur on the shin. Pathology will show atypical cells. Other forms of panniculitis, such as erythema induratum and pancreatic panniculitis, can present with tender nodules but these lesions usually occur on the calves and ulcerate.
Management of EN starts with treating the underlying infection or stopping the causative medication. Initial measures include bed rest, leg elevation, compression bandages, and NSAIDs. Potassium iodide is a very effective therapy as it may control the symptoms within 24 hours. When there is no response to the above, or the patient has severe symptoms, a short course of systemic glucocorticoids can be started. Other medications for recalcitrant or recurrent lesions include colchicine, dapsone, or hydroxychloroquine.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Panniculitis, in “Dermatology,” 3rd ed. (Philadelphia: Elsevier Saunders, 2012, p. 1641).
2. Arthritis Rheum. 2000 Mar;43(3):584-92.
3. J Clin Oncol. 2007 Sep 1;25(25):4011-2.
4. Turk J Pediatr. 2014 Mar-Apr;56(2):144-9.
Laboratory work revealed a normal CBC and differential, an elevated C-reactive protein (CRP) and sedimentation rate (ESR), negative antistreptolysin O (ASO) titers, negative pregnancy test, a normal urinalysis, and negative blood, throat, and urine cultures. A chest x-ray also was negative as well as angiotensin-converting enzyme (ACE) levels. Tuberculosis interferon-gamma release essay was negative.
The patient was diagnosed with erythema nodosum (EN), based on physical exam and history of the lesions. In her particular case, infectious causes including streptococcus infection, tuberculosis, and coccidioidomycosis were ruled out. There were no x-ray findings that suggested sarcoidosis and her ACE level was within normal limits. The pregnancy test also was negative. Given her recent start on OCs, this was thought to be the cause of the lesions.
She was treated with elevation, compression stockings, and NSAIDs and discontinuation of OCs. The lesions resolved after 6 weeks leaving bruiselike patches (erythema contusiformis).
EN is a delayed-type hypersensitivity reaction, causing inflammation on the fat (panniculitis) most commonly on the shins, but it can also occur on the arms, face, neck, and thighs. It is the most common type of panniculitis and is usually seen more often in women from the second to fourth decade of life. Erythematous tender nodules in crops commonly located on the shins are the characteristic physical finding. Systemic symptoms can occur including fever, malaise, and joint pain. The lesions usually last up to 6-8 weeks and may leave bruiselike patches or postinflammatory hyperpigmentation that can take months to improve.1
The diagnosis of EN usually is made by physical examination and natural history. In unusual severe cases or lesions in atypical locations, a skin biopsy is indicated. Histologic examination of one of the lesions reveals a septal panniculitis without vasculitis. Miescher’s radial granulomas (grouped macrophages around neutrophils or septa-like spaces) often are present and are a characteristic feature of EN.
EN can be triggered by different types of infections such as streptococcus, mycoplasma, tuberculosis, or bacterial gastroenteritis; medications such as OCs, sulfonamides, iodides, penicillin, or bromides; medical conditions that include inflammatory bowel disease, pregnancy, or sarcoidosis; or neutrophilic dermatosis and malignancy such as leukemia and Hodgkin disease.2,3 A third of the cases are idiopathic. In children, streptococcal infections are responsible for most cases of EN.4
Recommended work-up to investigate possible triggers includes a CBC with differential, sedimentation rate, CRP, ASO titers or anti-DNase B titers, tuberculin skin test or interferon-gamma TB test and a chest X ray. If there are any other symptoms, physical signs, or risk factors are present for the other not so common causes, further ancillary testing may be warranted.
Erythematous nodules and papules on the shin in children are commonly caused by arthropod bites also known as papular urticaria. These lesions are pruritic rather than tender and usually respond to topical corticosteroids and oral antihistamines. Subcutaneous bacterial, fungal, or atypical mycobacterial infections can present with tender nodules that can ulcerate and drain on the shins, feet, or any other body part. These patients may have a history of immunodeficiency and usually systemic symptoms of infection are present. Cutaneous polyarteritis nodosa (PAN) also can present with tender nodules on the legs but these lesions usually necrose and ulcerate and may be associated with livedo racemosa, a transient or persistent, blotchy, reddish-blue to purple, netlike cyanotic pattern. On pathology, PAN presents with necrotizing medium vessel vasculitis. Malignant nodules also can occur on the shin. Pathology will show atypical cells. Other forms of panniculitis, such as erythema induratum and pancreatic panniculitis, can present with tender nodules but these lesions usually occur on the calves and ulcerate.
Management of EN starts with treating the underlying infection or stopping the causative medication. Initial measures include bed rest, leg elevation, compression bandages, and NSAIDs. Potassium iodide is a very effective therapy as it may control the symptoms within 24 hours. When there is no response to the above, or the patient has severe symptoms, a short course of systemic glucocorticoids can be started. Other medications for recalcitrant or recurrent lesions include colchicine, dapsone, or hydroxychloroquine.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Panniculitis, in “Dermatology,” 3rd ed. (Philadelphia: Elsevier Saunders, 2012, p. 1641).
2. Arthritis Rheum. 2000 Mar;43(3):584-92.
3. J Clin Oncol. 2007 Sep 1;25(25):4011-2.
4. Turk J Pediatr. 2014 Mar-Apr;56(2):144-9.
A 16-year-old female came to the dermatology clinic for acne follow-up. She reported some improvement on her acne since she started taking OCs. She also had been using benzoyl peroxide and tretinoin on her face. In addition to the acne, she also wanted us to check some tender bumps she had been getting on her shins after she came back from a camping trip. Initially she thought they were bug bites, but the lesions were getting larger, more tender, and not improving with diphenhydramine.
The physical exam did not reveal acute distress. She was afebrile. On skin examination, she had comedones, papules and scars on her face, chest, and back. On her shins there were several erythematous tender nodules and plaques. There was no edema on her legs and pulses were present.
August 2018 - What's your diagnosis?
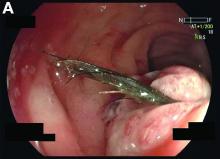
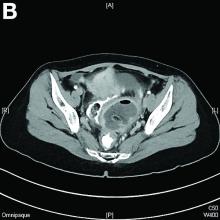
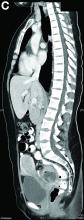
Mature cystic teratoma of the ovary invading the rectum
The patient was diagnosed with an ovarian dermoid cyst that was fistulating into the rectum. In view of these findings, a decision was made for surgical intervention to remove this lesion. A diagnostic laparoscopy, robotic-assisted left salpingo-oopherectomy, excision of cystorectal fistula, proctotomy, and primary repair of the rectal defect was performed. Intraoperative findings include a large left ovarian dermoid cyst with sebaceous content and hair fistulating into the upper rectum just above the rectovaginal pouch, and was adherent to surrounding structures. After excision of the fistula, the anterior rectal wall defect measured 1.5 cm and was closed primarily. Histology revealed a mature cystic teratoma and a fistula tract in the rectum; no malignant features were found. The patient had an uneventful recovery and was well at follow-up.
Mature cystic teratomas of the ovary, also referred to as ovarian dermoid cysts, are benign germ cell tumors of the ovary. These account for 10%-20% of all ovarian neoplasms.1 These are commonly asymptomatic and are found incidentally on imaging studies performed for other indications.2 Complications of these tumors include torsion (16%), rupture (0.5%), and malignant degeneration (2%-6%).3 Rectal invasion via fistulation by these lesions are rare; to date, six cases of colorectal involvement by mature cystic teratomas of the ovary are reported in the literature. These fistulas are a result of rupture of the cyst into the pouch of Douglas, which leads to an intense inflammatory response that results in fistulation, rather than freely into the peritoneum.3 These are therefore undetected until symptoms such as diarrhea or passage of cystic contents (mucus, hair, teeth) develop. The management of mature cystic teratomas of the ovary with symptoms or complications commonly involves surgical intervention to resect the involved ovary and address other pathology.
References
1. Rajaganeshan R., Wang H., Abouleid A., et al. Conservative surgery in the management of a benign ovarian cystic teratoma presenting as a rectal mass: a case report. Ann R Coll Surg Engl. 2001;93 e46-8.
2. Wichremasinghe D., Samarasekera D. A benign teratoma of the ovary fistulating into the rectum. Ceylon Med J. 2010;55:133.
3. Stern J.L., Buscema J., Rosenshein N.B., et al. Spontaneous rupture of benign cystic teratomas. Obstet Gynecol. 1981;57:363-6.
Mature cystic teratoma of the ovary invading the rectum
The patient was diagnosed with an ovarian dermoid cyst that was fistulating into the rectum. In view of these findings, a decision was made for surgical intervention to remove this lesion. A diagnostic laparoscopy, robotic-assisted left salpingo-oopherectomy, excision of cystorectal fistula, proctotomy, and primary repair of the rectal defect was performed. Intraoperative findings include a large left ovarian dermoid cyst with sebaceous content and hair fistulating into the upper rectum just above the rectovaginal pouch, and was adherent to surrounding structures. After excision of the fistula, the anterior rectal wall defect measured 1.5 cm and was closed primarily. Histology revealed a mature cystic teratoma and a fistula tract in the rectum; no malignant features were found. The patient had an uneventful recovery and was well at follow-up.
Mature cystic teratomas of the ovary, also referred to as ovarian dermoid cysts, are benign germ cell tumors of the ovary. These account for 10%-20% of all ovarian neoplasms.1 These are commonly asymptomatic and are found incidentally on imaging studies performed for other indications.2 Complications of these tumors include torsion (16%), rupture (0.5%), and malignant degeneration (2%-6%).3 Rectal invasion via fistulation by these lesions are rare; to date, six cases of colorectal involvement by mature cystic teratomas of the ovary are reported in the literature. These fistulas are a result of rupture of the cyst into the pouch of Douglas, which leads to an intense inflammatory response that results in fistulation, rather than freely into the peritoneum.3 These are therefore undetected until symptoms such as diarrhea or passage of cystic contents (mucus, hair, teeth) develop. The management of mature cystic teratomas of the ovary with symptoms or complications commonly involves surgical intervention to resect the involved ovary and address other pathology.
References
1. Rajaganeshan R., Wang H., Abouleid A., et al. Conservative surgery in the management of a benign ovarian cystic teratoma presenting as a rectal mass: a case report. Ann R Coll Surg Engl. 2001;93 e46-8.
2. Wichremasinghe D., Samarasekera D. A benign teratoma of the ovary fistulating into the rectum. Ceylon Med J. 2010;55:133.
3. Stern J.L., Buscema J., Rosenshein N.B., et al. Spontaneous rupture of benign cystic teratomas. Obstet Gynecol. 1981;57:363-6.
Mature cystic teratoma of the ovary invading the rectum
The patient was diagnosed with an ovarian dermoid cyst that was fistulating into the rectum. In view of these findings, a decision was made for surgical intervention to remove this lesion. A diagnostic laparoscopy, robotic-assisted left salpingo-oopherectomy, excision of cystorectal fistula, proctotomy, and primary repair of the rectal defect was performed. Intraoperative findings include a large left ovarian dermoid cyst with sebaceous content and hair fistulating into the upper rectum just above the rectovaginal pouch, and was adherent to surrounding structures. After excision of the fistula, the anterior rectal wall defect measured 1.5 cm and was closed primarily. Histology revealed a mature cystic teratoma and a fistula tract in the rectum; no malignant features were found. The patient had an uneventful recovery and was well at follow-up.
Mature cystic teratomas of the ovary, also referred to as ovarian dermoid cysts, are benign germ cell tumors of the ovary. These account for 10%-20% of all ovarian neoplasms.1 These are commonly asymptomatic and are found incidentally on imaging studies performed for other indications.2 Complications of these tumors include torsion (16%), rupture (0.5%), and malignant degeneration (2%-6%).3 Rectal invasion via fistulation by these lesions are rare; to date, six cases of colorectal involvement by mature cystic teratomas of the ovary are reported in the literature. These fistulas are a result of rupture of the cyst into the pouch of Douglas, which leads to an intense inflammatory response that results in fistulation, rather than freely into the peritoneum.3 These are therefore undetected until symptoms such as diarrhea or passage of cystic contents (mucus, hair, teeth) develop. The management of mature cystic teratomas of the ovary with symptoms or complications commonly involves surgical intervention to resect the involved ovary and address other pathology.
References
1. Rajaganeshan R., Wang H., Abouleid A., et al. Conservative surgery in the management of a benign ovarian cystic teratoma presenting as a rectal mass: a case report. Ann R Coll Surg Engl. 2001;93 e46-8.
2. Wichremasinghe D., Samarasekera D. A benign teratoma of the ovary fistulating into the rectum. Ceylon Med J. 2010;55:133.
3. Stern J.L., Buscema J., Rosenshein N.B., et al. Spontaneous rupture of benign cystic teratomas. Obstet Gynecol. 1981;57:363-6.
RA seroconversion not associated with sustained drug-free remission
In patients with seropositive rheumatoid arthritis, seroconversion within the first year of treatment is not associated with long-term sustained drug-free remission (SDFR), according to results of a randomized, treat-to-target study of patients with early RA.
“The clinical significance of seroconversion in RA and especially its relationship with long-term SDFR, an approximation of disease ‘cure’ of RA, is a topic of major interest,” wrote Emma C. de Moel, of the department of rheumatology at Leiden (Netherlands) University Medical Center, and her coauthors.
“Previous studies found no association of seroconversion with remission or radiographic damage,” the investigators wrote in Annals of the Rheumatic Diseases. “We here investigated the association between seroconversion and ... SDFR and found no association.”
The study involved 381 patients with early RA (less than 2 years) from the IMPROVED trial who were treated with methotrexate and high-dose prednisone. At baseline and 12 months, 14 RA-associated autoantibodies were measured by ELISA, including anti-CCP2 and rheumatoid factor. Patients were monitored for long-term SDFR, defined as remission lasting longer than 1 year, beginning at any time, and persisting until the maximum follow-up of 5 years.
An association between seroconversion and SDFR was not found. At 12 months, 6 of 170 patients (3.5%) had seroconverted all autoantibodies to negative, and 2 of these 6 patients achieved SDFR, compared with 19 of 164 seropositive patients (11.6%) who achieved SDFR without seroconversion (P = .11). Additionally, neither the proportion of autoantibodies converted to negative nor relative decreases in autoantibody levels were associated with SDFR.
“It appears that seroconversion (as measured by current standards) does not identify a group of patients in ... true immunological remission, and is not superior to signals of low inflammatory load (e.g. by DAS 6) for predicting successful drug tapering,” the investigators concluded. “Future studies are needed to identify whether other immunological parameters such as the numbers or phenotype of circulating autoreactive B or T cells might be a better reflection of disease persistence and markers for immunological remission.”
The study was funded by ZonMw (the Netherlands Organization for Health Research and Development)-consortium Molecular Diagnostics in RA (MODIRA).
SOURCE: de Moel EC et al. Ann Rheum Dis. 2018 Jul 25. doi: 10.1136/annrheumdis-2018-213823.
In patients with seropositive rheumatoid arthritis, seroconversion within the first year of treatment is not associated with long-term sustained drug-free remission (SDFR), according to results of a randomized, treat-to-target study of patients with early RA.
“The clinical significance of seroconversion in RA and especially its relationship with long-term SDFR, an approximation of disease ‘cure’ of RA, is a topic of major interest,” wrote Emma C. de Moel, of the department of rheumatology at Leiden (Netherlands) University Medical Center, and her coauthors.
“Previous studies found no association of seroconversion with remission or radiographic damage,” the investigators wrote in Annals of the Rheumatic Diseases. “We here investigated the association between seroconversion and ... SDFR and found no association.”
The study involved 381 patients with early RA (less than 2 years) from the IMPROVED trial who were treated with methotrexate and high-dose prednisone. At baseline and 12 months, 14 RA-associated autoantibodies were measured by ELISA, including anti-CCP2 and rheumatoid factor. Patients were monitored for long-term SDFR, defined as remission lasting longer than 1 year, beginning at any time, and persisting until the maximum follow-up of 5 years.
An association between seroconversion and SDFR was not found. At 12 months, 6 of 170 patients (3.5%) had seroconverted all autoantibodies to negative, and 2 of these 6 patients achieved SDFR, compared with 19 of 164 seropositive patients (11.6%) who achieved SDFR without seroconversion (P = .11). Additionally, neither the proportion of autoantibodies converted to negative nor relative decreases in autoantibody levels were associated with SDFR.
“It appears that seroconversion (as measured by current standards) does not identify a group of patients in ... true immunological remission, and is not superior to signals of low inflammatory load (e.g. by DAS 6) for predicting successful drug tapering,” the investigators concluded. “Future studies are needed to identify whether other immunological parameters such as the numbers or phenotype of circulating autoreactive B or T cells might be a better reflection of disease persistence and markers for immunological remission.”
The study was funded by ZonMw (the Netherlands Organization for Health Research and Development)-consortium Molecular Diagnostics in RA (MODIRA).
SOURCE: de Moel EC et al. Ann Rheum Dis. 2018 Jul 25. doi: 10.1136/annrheumdis-2018-213823.
In patients with seropositive rheumatoid arthritis, seroconversion within the first year of treatment is not associated with long-term sustained drug-free remission (SDFR), according to results of a randomized, treat-to-target study of patients with early RA.
“The clinical significance of seroconversion in RA and especially its relationship with long-term SDFR, an approximation of disease ‘cure’ of RA, is a topic of major interest,” wrote Emma C. de Moel, of the department of rheumatology at Leiden (Netherlands) University Medical Center, and her coauthors.
“Previous studies found no association of seroconversion with remission or radiographic damage,” the investigators wrote in Annals of the Rheumatic Diseases. “We here investigated the association between seroconversion and ... SDFR and found no association.”
The study involved 381 patients with early RA (less than 2 years) from the IMPROVED trial who were treated with methotrexate and high-dose prednisone. At baseline and 12 months, 14 RA-associated autoantibodies were measured by ELISA, including anti-CCP2 and rheumatoid factor. Patients were monitored for long-term SDFR, defined as remission lasting longer than 1 year, beginning at any time, and persisting until the maximum follow-up of 5 years.
An association between seroconversion and SDFR was not found. At 12 months, 6 of 170 patients (3.5%) had seroconverted all autoantibodies to negative, and 2 of these 6 patients achieved SDFR, compared with 19 of 164 seropositive patients (11.6%) who achieved SDFR without seroconversion (P = .11). Additionally, neither the proportion of autoantibodies converted to negative nor relative decreases in autoantibody levels were associated with SDFR.
“It appears that seroconversion (as measured by current standards) does not identify a group of patients in ... true immunological remission, and is not superior to signals of low inflammatory load (e.g. by DAS 6) for predicting successful drug tapering,” the investigators concluded. “Future studies are needed to identify whether other immunological parameters such as the numbers or phenotype of circulating autoreactive B or T cells might be a better reflection of disease persistence and markers for immunological remission.”
The study was funded by ZonMw (the Netherlands Organization for Health Research and Development)-consortium Molecular Diagnostics in RA (MODIRA).
SOURCE: de Moel EC et al. Ann Rheum Dis. 2018 Jul 25. doi: 10.1136/annrheumdis-2018-213823.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: At 12 months, 6 of 170 seropositive patients (3.5%) had seroconverted, and 2 of these 6 patients achieved long-term SDFR, compared with 19 of 164 seropositive patients (11.6%) who achieved long-term SDFR without seroconversion (P = .11).
Study details: Baseline and 12-month serum autoantibody analysis of 381 RA patients from the IMPROVED trial; a randomized, treat-to-target study of patients with early RA (less than 2 years).
Disclosures: Funding was provided by ZonMw (the Netherlands Organization for Health Research and Development)-consortium Molecular Diagnostics in RA (MODIRA).
Source: de Moel EC et al. Ann Rheum Dis. 2018 Jul 25. doi: 10.1136/annrheumdis-2018-213823.
Who are the 'high-need, high-cost' patients?
Among patients hospitalized with gastrointestinal and liver diseases, a clearly identifiable subset uses significantly more health care resources, which incurs significantly greater costs, according to the results of a national database analysis published in the August issue of Clinical Gastroenterology and Hepatology.
Compared with otherwise similar inpatients, these “high-need, high-cost” individuals are significantly more likely to be enrolled in Medicare or Medicaid, to have lower income, to initially be admitted to a large, rural hospital, to have multiple comorbidities, to be obese, or to be hospitalized for infection, said Nghia Nguyen, MD, and his associates. “[A] small fraction of high-need, high-cost patients contribute disproportionately to hospitalization costs,” they wrote. “Population health management directed toward these patients would facilitate high-value care.”
Gastrointestinal and liver diseases incur more than $100 billion in health care expenses annually in the United States, of which more than 60% is related to inpatient care, the researchers noted. However, few studies have comprehensively evaluated the annual burden and costs of hospitalization in patients with chronic gastrointestinal and liver diseases. Therefore, using the Nationwide Readmissions Database, the investigators studied patients with inflammatory bowel disease (IBD), chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases who were hospitalized at least once during the first 6 months of 2013. All patients were diagnosed with IBD, chronic liver diseases, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases and followed for at least 6 months. The researchers stratified hospital days and costs and characterized the subset of patients who fell into the highest decile of days spent in the hospital per month.
The most common reason for hospitalization was chronic liver disease (nearly 377,000 patients), followed by functional gastrointestinal disorders (more than 351,000 patients), gastrointestinal hemorrhage (nearly 191,000 patients), pancreatic diseases (more than 98,000 patients), and IBD (more than 47,000 patients). Patients spent a median of 6-7 days in the hospital per year, with an interquartile range of 3-14 days. Compared with patients in the lowest decile for annual hospital stay (median, 0.13-0.14 days per month), patients in the highest decile spent a median of 3.7-5.1 days in the hospital per month. In this high-cost, high-need subset of patients, the costs of each hospitalization ranged from $7,438 per month to $11,425 per month, and they were typically hospitalized once every 2 months.
“Gastrointestinal diseases, infections, and cardiopulmonary causes were leading reasons for hospitalization of these patients,” the researchers wrote. “At a patient level, modifiable risk factors may include tackling the obesity epidemic and mental health issues and minimizing risk of iatrogenic or health care–associated infections, whereas at a health system level, interventions may include better access to care and connectivity between rural and specialty hospitals.”
Funders included the American College of Gastroenterology, the Crohn’s and Colitis Foundation, and the National Institutes of Health. Senior author Siddharth Singh disclosed unrelated grant funding from Pifzer and AbbVie. The other investigators reported having no conflicts of interest.
SOURCE: Nguyen NH et al. Clin Gastroenterol Hepatol. 2018 Feb 20. doi: 10.1016/j.cgh.2018.02.015.
Understanding the reasons underlying variations in health care utilization is central to any plan to reduce costs at the population level. To this end, Nguyen et al. provide crucial data for the patients for whom we care as gastroenterologists. Studying a longitudinal database of hospitalizations in 2013, the authors provide comprehensive demographic data for the top decile of inpatient health care utilizers (defined by hospital-days/month) with inflammatory bowel disease, chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, and pancreatic diseases. Although constrained by the limits of administrative data and the lack of outpatient/pharmaceutical data linkage, these findings are strengthened by their consistency across conditions. Indeed, despite the heterogeneous disorders surveyed, a remarkably consistent high-need/high-cost "phenotype" emerges: publicly insured, low-income, rural, obese but malnourished, and beset by infections and the complications of diabetes.
What are the next steps?
When a minority of the patients are responsible for a substantial portion of the costs (i.e., the 80/20 rule), one strategy for cost containment is "hot-spotting." Hot-spotting is a two-step process: Identify high-need, high-cost patients, and then deploy interventions tailored to their needs. Nguyen and colleague's work is a landmark for the first step. However, before these findings may be translated into policy or intervention, we need granular data to explain these associations and suggest clear action items. Solutions will likely be multifactorial including early, intensified care for obesity and diabetes (before end-stage complications arise), novel care delivery methods for gastroenterology specialty care in rural hospitals, and intensified outpatient resources for high-need patients in order to coordinate alternatives to hospitalization.
Elliot B. Tapper, MD, is assistant professor, division of gastroenterology and hepatology, University of Michigan, Ann Arbor. He reports consulting for Novartis and receiving unrestricted research grants from Valeant and Gilead, all unrelated to this work.
Understanding the reasons underlying variations in health care utilization is central to any plan to reduce costs at the population level. To this end, Nguyen et al. provide crucial data for the patients for whom we care as gastroenterologists. Studying a longitudinal database of hospitalizations in 2013, the authors provide comprehensive demographic data for the top decile of inpatient health care utilizers (defined by hospital-days/month) with inflammatory bowel disease, chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, and pancreatic diseases. Although constrained by the limits of administrative data and the lack of outpatient/pharmaceutical data linkage, these findings are strengthened by their consistency across conditions. Indeed, despite the heterogeneous disorders surveyed, a remarkably consistent high-need/high-cost "phenotype" emerges: publicly insured, low-income, rural, obese but malnourished, and beset by infections and the complications of diabetes.
What are the next steps?
When a minority of the patients are responsible for a substantial portion of the costs (i.e., the 80/20 rule), one strategy for cost containment is "hot-spotting." Hot-spotting is a two-step process: Identify high-need, high-cost patients, and then deploy interventions tailored to their needs. Nguyen and colleague's work is a landmark for the first step. However, before these findings may be translated into policy or intervention, we need granular data to explain these associations and suggest clear action items. Solutions will likely be multifactorial including early, intensified care for obesity and diabetes (before end-stage complications arise), novel care delivery methods for gastroenterology specialty care in rural hospitals, and intensified outpatient resources for high-need patients in order to coordinate alternatives to hospitalization.
Elliot B. Tapper, MD, is assistant professor, division of gastroenterology and hepatology, University of Michigan, Ann Arbor. He reports consulting for Novartis and receiving unrestricted research grants from Valeant and Gilead, all unrelated to this work.
Understanding the reasons underlying variations in health care utilization is central to any plan to reduce costs at the population level. To this end, Nguyen et al. provide crucial data for the patients for whom we care as gastroenterologists. Studying a longitudinal database of hospitalizations in 2013, the authors provide comprehensive demographic data for the top decile of inpatient health care utilizers (defined by hospital-days/month) with inflammatory bowel disease, chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, and pancreatic diseases. Although constrained by the limits of administrative data and the lack of outpatient/pharmaceutical data linkage, these findings are strengthened by their consistency across conditions. Indeed, despite the heterogeneous disorders surveyed, a remarkably consistent high-need/high-cost "phenotype" emerges: publicly insured, low-income, rural, obese but malnourished, and beset by infections and the complications of diabetes.
What are the next steps?
When a minority of the patients are responsible for a substantial portion of the costs (i.e., the 80/20 rule), one strategy for cost containment is "hot-spotting." Hot-spotting is a two-step process: Identify high-need, high-cost patients, and then deploy interventions tailored to their needs. Nguyen and colleague's work is a landmark for the first step. However, before these findings may be translated into policy or intervention, we need granular data to explain these associations and suggest clear action items. Solutions will likely be multifactorial including early, intensified care for obesity and diabetes (before end-stage complications arise), novel care delivery methods for gastroenterology specialty care in rural hospitals, and intensified outpatient resources for high-need patients in order to coordinate alternatives to hospitalization.
Elliot B. Tapper, MD, is assistant professor, division of gastroenterology and hepatology, University of Michigan, Ann Arbor. He reports consulting for Novartis and receiving unrestricted research grants from Valeant and Gilead, all unrelated to this work.
Among patients hospitalized with gastrointestinal and liver diseases, a clearly identifiable subset uses significantly more health care resources, which incurs significantly greater costs, according to the results of a national database analysis published in the August issue of Clinical Gastroenterology and Hepatology.
Compared with otherwise similar inpatients, these “high-need, high-cost” individuals are significantly more likely to be enrolled in Medicare or Medicaid, to have lower income, to initially be admitted to a large, rural hospital, to have multiple comorbidities, to be obese, or to be hospitalized for infection, said Nghia Nguyen, MD, and his associates. “[A] small fraction of high-need, high-cost patients contribute disproportionately to hospitalization costs,” they wrote. “Population health management directed toward these patients would facilitate high-value care.”
Gastrointestinal and liver diseases incur more than $100 billion in health care expenses annually in the United States, of which more than 60% is related to inpatient care, the researchers noted. However, few studies have comprehensively evaluated the annual burden and costs of hospitalization in patients with chronic gastrointestinal and liver diseases. Therefore, using the Nationwide Readmissions Database, the investigators studied patients with inflammatory bowel disease (IBD), chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases who were hospitalized at least once during the first 6 months of 2013. All patients were diagnosed with IBD, chronic liver diseases, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases and followed for at least 6 months. The researchers stratified hospital days and costs and characterized the subset of patients who fell into the highest decile of days spent in the hospital per month.
The most common reason for hospitalization was chronic liver disease (nearly 377,000 patients), followed by functional gastrointestinal disorders (more than 351,000 patients), gastrointestinal hemorrhage (nearly 191,000 patients), pancreatic diseases (more than 98,000 patients), and IBD (more than 47,000 patients). Patients spent a median of 6-7 days in the hospital per year, with an interquartile range of 3-14 days. Compared with patients in the lowest decile for annual hospital stay (median, 0.13-0.14 days per month), patients in the highest decile spent a median of 3.7-5.1 days in the hospital per month. In this high-cost, high-need subset of patients, the costs of each hospitalization ranged from $7,438 per month to $11,425 per month, and they were typically hospitalized once every 2 months.
“Gastrointestinal diseases, infections, and cardiopulmonary causes were leading reasons for hospitalization of these patients,” the researchers wrote. “At a patient level, modifiable risk factors may include tackling the obesity epidemic and mental health issues and minimizing risk of iatrogenic or health care–associated infections, whereas at a health system level, interventions may include better access to care and connectivity between rural and specialty hospitals.”
Funders included the American College of Gastroenterology, the Crohn’s and Colitis Foundation, and the National Institutes of Health. Senior author Siddharth Singh disclosed unrelated grant funding from Pifzer and AbbVie. The other investigators reported having no conflicts of interest.
SOURCE: Nguyen NH et al. Clin Gastroenterol Hepatol. 2018 Feb 20. doi: 10.1016/j.cgh.2018.02.015.
Among patients hospitalized with gastrointestinal and liver diseases, a clearly identifiable subset uses significantly more health care resources, which incurs significantly greater costs, according to the results of a national database analysis published in the August issue of Clinical Gastroenterology and Hepatology.
Compared with otherwise similar inpatients, these “high-need, high-cost” individuals are significantly more likely to be enrolled in Medicare or Medicaid, to have lower income, to initially be admitted to a large, rural hospital, to have multiple comorbidities, to be obese, or to be hospitalized for infection, said Nghia Nguyen, MD, and his associates. “[A] small fraction of high-need, high-cost patients contribute disproportionately to hospitalization costs,” they wrote. “Population health management directed toward these patients would facilitate high-value care.”
Gastrointestinal and liver diseases incur more than $100 billion in health care expenses annually in the United States, of which more than 60% is related to inpatient care, the researchers noted. However, few studies have comprehensively evaluated the annual burden and costs of hospitalization in patients with chronic gastrointestinal and liver diseases. Therefore, using the Nationwide Readmissions Database, the investigators studied patients with inflammatory bowel disease (IBD), chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases who were hospitalized at least once during the first 6 months of 2013. All patients were diagnosed with IBD, chronic liver diseases, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases and followed for at least 6 months. The researchers stratified hospital days and costs and characterized the subset of patients who fell into the highest decile of days spent in the hospital per month.
The most common reason for hospitalization was chronic liver disease (nearly 377,000 patients), followed by functional gastrointestinal disorders (more than 351,000 patients), gastrointestinal hemorrhage (nearly 191,000 patients), pancreatic diseases (more than 98,000 patients), and IBD (more than 47,000 patients). Patients spent a median of 6-7 days in the hospital per year, with an interquartile range of 3-14 days. Compared with patients in the lowest decile for annual hospital stay (median, 0.13-0.14 days per month), patients in the highest decile spent a median of 3.7-5.1 days in the hospital per month. In this high-cost, high-need subset of patients, the costs of each hospitalization ranged from $7,438 per month to $11,425 per month, and they were typically hospitalized once every 2 months.
“Gastrointestinal diseases, infections, and cardiopulmonary causes were leading reasons for hospitalization of these patients,” the researchers wrote. “At a patient level, modifiable risk factors may include tackling the obesity epidemic and mental health issues and minimizing risk of iatrogenic or health care–associated infections, whereas at a health system level, interventions may include better access to care and connectivity between rural and specialty hospitals.”
Funders included the American College of Gastroenterology, the Crohn’s and Colitis Foundation, and the National Institutes of Health. Senior author Siddharth Singh disclosed unrelated grant funding from Pifzer and AbbVie. The other investigators reported having no conflicts of interest.
SOURCE: Nguyen NH et al. Clin Gastroenterol Hepatol. 2018 Feb 20. doi: 10.1016/j.cgh.2018.02.015.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: For patients with gastrointestinal or liver disease, significant predictors of high need and cost during hospitalization included Medicare or Medicaid insurance, lower income, first hospitalization in a large rural hospital, high comorbidity burden, obesity, and hospitalization for infection.
Major finding: Patients in the highest decile spent a median of 3.7-4.1 days in the hospital per month for all causes. Gastrointestinal disease, infections, and cardiopulmonary morbidity were the most common reasons for hospitalization.
Study details: Analysis of patients with inflammatory bowel disease, chronic liver disease, functional gastrointestinal disorders, gastrointestinal hemorrhage, or pancreatic diseases hospitalized at least once during 2013.
Disclosures: Funders included the American College of Gastroenterology, the Crohn’s and Colitis Foundation, and the National Institutes of Health. Senior author Siddharth Singh disclosed unrelated grant funding from Pifzer and AbbVie. The other investigators reported having no conflicts of interest.
Source: Nguyen NH et al. Clin Gastroenterol Hepatol. 2018 Feb 20. doi: 10.1016/j.cgh.2018.02.015.
Physician Burnout in Dermatology
Many articles about physician burnout and more alarmingly depression and suicide include chilling statistics; however, the data are limited. The same study from Medscape about burnout broken down by medical specialty often is cited.1 Although dermatology fares better than many specialties in this research, the percentages are still abysmal.
I am writing as a physician, for physicians. I do not want to quote the data to you. If you are reading this article, you have probably felt some burnout, even transiently. Maybe you even feel it now, at this very moment. Physicians are competitive capable people. I do not want to present numbers and statistics that make you question the validity of your feelings, whether you fit with the average statistics, or make you try to calculate how many of your friends or colleagues match these statistics. The numbers are terrible, no matter how you look at them, and all trends show them worsening with time.
What is burnout?
To simply define burnout as fatigue or high workload would be to undervalue the term. Physicians are trained through college, medical school, and countless hours of residency to cope with both challenges. Maslach et al2 defined burnout as “a psychological syndrome in response to chronic interpersonal stressors on the job” leading to “overwhelming exhaustion, feelings of cynicism and detachment from the job, and a sense of ineffectiveness and lack of accomplishment.”
Who does burnout affect?
Physician burnout affects both the patient and the physician. It has been demonstrated that physician burnout leads to lower patient satisfaction and care as well as higher risk for medical errors. There are the more obvious and direct effects on the physician, with affected physicians having much higher employment turnover and risk for addiction and suicide.3 One could argue that there are even more downstream effects of burnout, including physicians’ families who may be directly affected and even societal effects when fully trained physicians leave the clinical arena to pursue other careers.
How do you recognize when you are burnt out?
The first time I recognized that I was burnt out was in medical school. I understood my burnout through the lens of my undergraduate training in anthropology as compassion fatigue, a term that has been used to describe the lack of empathy that can develop when any individual is presented with an overwhelming tragedy or horror. When you are in survival mode—waking up just to survive the next day or clinic shift or call—you are surviving but hardly thriving as a physician.3 I believe that humans have a tremendous capacity for survival, but when we are in survival mode we have little energy leftover for the pleasures of life, from family to hobbies. I would similarly argue that in survival mode we have limited ability to appreciate the pain and suffering our patients are experiencing. Survival mode limits our ability as physicians to connect with our patients and to engage in the full spectrum of emotion in our time outside of our job.
What are the causes of burnout in dermatology?
As dermatologists, we often have milder on-call schedules and fewer critically ill patients than many of our medical colleagues. For this reason, we may be afraid to address the real role of physician burnout in our field. Fellow dermatologist Jeffrey Benabio, MD (San Diego, California), notes that the phrase dermatologist burnout may even seem oxymoronic, but we face many of the same daily frustrations with electronic medical records, increasing patient volume, and insurance struggles.4 The electronic medical record looms large in many physicians’ complaints these days. A recent article in the New York Times described the physician as “the highest-paid clerical worker in the hospital,”5 which is not wrong. For every hour of patient time, we have nearly double that spent on paperwork.5
Dike Drummond, MD, a family practice physician who focuses on physician burnout, notes that physicians are taught very early to put the patient first, but it is never discussed when or how to turn this switch off.3 However, there is little written about dermatology-specific burnout. A problem that is not studied or even considered will be harder to fix.
Why does it matter?
I believe that addressing physician burnout is critical for 2 reasons: (1) we can improve patient care by improving patient satisfaction and decreasing medical error, and (2) we can find greater satisfaction and professional fulfillment while caring for our patients. Ultimately, patient care and physician care are intimately linked; as stated by Thomas et al,6 “[p]hysicians who are well can best serve their patients.”
As a resident in 2018, I recognize that my coresidents and I are training as physicians in the time of “duty hours” and an ongoing discussion of burnout. However, I sense a burnout fatigue setting in among residents, many who do not want to discuss wellness anymore. The newer data suggest that work hour restrictions do not improve patient safety, negating one of the driving reasons for the change.7 At the same time, residency programs are initiating wellness programs in response to the growing literature on physician burnout. These wellness programs vary in the types of activities included, from individual coping techniques such as mindfulness training to social gatherings for the residents. In general, these wellness initiatives focus on burnout at the individual level, but they do not take into account systemic or structural challenges that might contribute to this worsening epidemic.
Final Thoughts
As a profession, I believe that physicians have internalized the concept of burnout to equate with a personal individual failing. At various times in my training, I have felt that if I could just practice mediation, study more, or shift my perspective, I personally could overcome burnout. I have intermittently felt my burnout as proof that I should never have become a physician. As a woman and the first physician in my family, fighting the sense of burnout so early in my career seemed demoralizing and nearly drove me to change my career path. It exacerbated my sense of imposter syndrome: that I never truly belonged in medicine at all. After much soul-searching, I have concluded that burnout is a concept propagated by administrators and businesspeople to stigmatize the reaction by many physicians to the growing trends in medicine and cast it as a personal failure rather than as the symptom of a broken medical system.
If we continue to identify burnout as an individual failing and treat it as such, I believe that we will fail to stem the growing trend within dermatology and within medicine more broadly. We need to consider the driving factors behind dermatology burnout so that we can begin to address them at a structural level.
- Peckham C. Medscape national physician burnout & depression report 2018. Medscape website. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Published January 17, 2018. Accessed June 21, 2018.
- Maslach C, Schaufeli WB, Leiter MP. Job
burnout. Annu Rev Psychol. 2001;52:397-422. - Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
- Benabio J. Burnout. Dermatology News. November 14, 2017. https://www.mdedge.com/edermatologynews/article/152098/business-medicine/burnout. Accessed June 30, 2018.
- Verghese A. How tech can turn doctors into clerical workers. New York Times. May 16, 2018. https://www.nytimes.com/interactive/2018/05/16/magazine/health-issue-what-we-lose-with-data-driven-medicine.html. Accessed July 3, 2018.
- Thomas LR, Ripp JA, West CP. Charter on physician well-being. JAMA. 2018;319:1541-1542.
- Osborne R, Parshuram CS. Delinking resident duty hours from patient safety [published online December 11, 2014]. BMC Med Educ. 2014;14(suppl 1):S2.
Many articles about physician burnout and more alarmingly depression and suicide include chilling statistics; however, the data are limited. The same study from Medscape about burnout broken down by medical specialty often is cited.1 Although dermatology fares better than many specialties in this research, the percentages are still abysmal.
I am writing as a physician, for physicians. I do not want to quote the data to you. If you are reading this article, you have probably felt some burnout, even transiently. Maybe you even feel it now, at this very moment. Physicians are competitive capable people. I do not want to present numbers and statistics that make you question the validity of your feelings, whether you fit with the average statistics, or make you try to calculate how many of your friends or colleagues match these statistics. The numbers are terrible, no matter how you look at them, and all trends show them worsening with time.
What is burnout?
To simply define burnout as fatigue or high workload would be to undervalue the term. Physicians are trained through college, medical school, and countless hours of residency to cope with both challenges. Maslach et al2 defined burnout as “a psychological syndrome in response to chronic interpersonal stressors on the job” leading to “overwhelming exhaustion, feelings of cynicism and detachment from the job, and a sense of ineffectiveness and lack of accomplishment.”
Who does burnout affect?
Physician burnout affects both the patient and the physician. It has been demonstrated that physician burnout leads to lower patient satisfaction and care as well as higher risk for medical errors. There are the more obvious and direct effects on the physician, with affected physicians having much higher employment turnover and risk for addiction and suicide.3 One could argue that there are even more downstream effects of burnout, including physicians’ families who may be directly affected and even societal effects when fully trained physicians leave the clinical arena to pursue other careers.
How do you recognize when you are burnt out?
The first time I recognized that I was burnt out was in medical school. I understood my burnout through the lens of my undergraduate training in anthropology as compassion fatigue, a term that has been used to describe the lack of empathy that can develop when any individual is presented with an overwhelming tragedy or horror. When you are in survival mode—waking up just to survive the next day or clinic shift or call—you are surviving but hardly thriving as a physician.3 I believe that humans have a tremendous capacity for survival, but when we are in survival mode we have little energy leftover for the pleasures of life, from family to hobbies. I would similarly argue that in survival mode we have limited ability to appreciate the pain and suffering our patients are experiencing. Survival mode limits our ability as physicians to connect with our patients and to engage in the full spectrum of emotion in our time outside of our job.
What are the causes of burnout in dermatology?
As dermatologists, we often have milder on-call schedules and fewer critically ill patients than many of our medical colleagues. For this reason, we may be afraid to address the real role of physician burnout in our field. Fellow dermatologist Jeffrey Benabio, MD (San Diego, California), notes that the phrase dermatologist burnout may even seem oxymoronic, but we face many of the same daily frustrations with electronic medical records, increasing patient volume, and insurance struggles.4 The electronic medical record looms large in many physicians’ complaints these days. A recent article in the New York Times described the physician as “the highest-paid clerical worker in the hospital,”5 which is not wrong. For every hour of patient time, we have nearly double that spent on paperwork.5
Dike Drummond, MD, a family practice physician who focuses on physician burnout, notes that physicians are taught very early to put the patient first, but it is never discussed when or how to turn this switch off.3 However, there is little written about dermatology-specific burnout. A problem that is not studied or even considered will be harder to fix.
Why does it matter?
I believe that addressing physician burnout is critical for 2 reasons: (1) we can improve patient care by improving patient satisfaction and decreasing medical error, and (2) we can find greater satisfaction and professional fulfillment while caring for our patients. Ultimately, patient care and physician care are intimately linked; as stated by Thomas et al,6 “[p]hysicians who are well can best serve their patients.”
As a resident in 2018, I recognize that my coresidents and I are training as physicians in the time of “duty hours” and an ongoing discussion of burnout. However, I sense a burnout fatigue setting in among residents, many who do not want to discuss wellness anymore. The newer data suggest that work hour restrictions do not improve patient safety, negating one of the driving reasons for the change.7 At the same time, residency programs are initiating wellness programs in response to the growing literature on physician burnout. These wellness programs vary in the types of activities included, from individual coping techniques such as mindfulness training to social gatherings for the residents. In general, these wellness initiatives focus on burnout at the individual level, but they do not take into account systemic or structural challenges that might contribute to this worsening epidemic.
Final Thoughts
As a profession, I believe that physicians have internalized the concept of burnout to equate with a personal individual failing. At various times in my training, I have felt that if I could just practice mediation, study more, or shift my perspective, I personally could overcome burnout. I have intermittently felt my burnout as proof that I should never have become a physician. As a woman and the first physician in my family, fighting the sense of burnout so early in my career seemed demoralizing and nearly drove me to change my career path. It exacerbated my sense of imposter syndrome: that I never truly belonged in medicine at all. After much soul-searching, I have concluded that burnout is a concept propagated by administrators and businesspeople to stigmatize the reaction by many physicians to the growing trends in medicine and cast it as a personal failure rather than as the symptom of a broken medical system.
If we continue to identify burnout as an individual failing and treat it as such, I believe that we will fail to stem the growing trend within dermatology and within medicine more broadly. We need to consider the driving factors behind dermatology burnout so that we can begin to address them at a structural level.
Many articles about physician burnout and more alarmingly depression and suicide include chilling statistics; however, the data are limited. The same study from Medscape about burnout broken down by medical specialty often is cited.1 Although dermatology fares better than many specialties in this research, the percentages are still abysmal.
I am writing as a physician, for physicians. I do not want to quote the data to you. If you are reading this article, you have probably felt some burnout, even transiently. Maybe you even feel it now, at this very moment. Physicians are competitive capable people. I do not want to present numbers and statistics that make you question the validity of your feelings, whether you fit with the average statistics, or make you try to calculate how many of your friends or colleagues match these statistics. The numbers are terrible, no matter how you look at them, and all trends show them worsening with time.
What is burnout?
To simply define burnout as fatigue or high workload would be to undervalue the term. Physicians are trained through college, medical school, and countless hours of residency to cope with both challenges. Maslach et al2 defined burnout as “a psychological syndrome in response to chronic interpersonal stressors on the job” leading to “overwhelming exhaustion, feelings of cynicism and detachment from the job, and a sense of ineffectiveness and lack of accomplishment.”
Who does burnout affect?
Physician burnout affects both the patient and the physician. It has been demonstrated that physician burnout leads to lower patient satisfaction and care as well as higher risk for medical errors. There are the more obvious and direct effects on the physician, with affected physicians having much higher employment turnover and risk for addiction and suicide.3 One could argue that there are even more downstream effects of burnout, including physicians’ families who may be directly affected and even societal effects when fully trained physicians leave the clinical arena to pursue other careers.
How do you recognize when you are burnt out?
The first time I recognized that I was burnt out was in medical school. I understood my burnout through the lens of my undergraduate training in anthropology as compassion fatigue, a term that has been used to describe the lack of empathy that can develop when any individual is presented with an overwhelming tragedy or horror. When you are in survival mode—waking up just to survive the next day or clinic shift or call—you are surviving but hardly thriving as a physician.3 I believe that humans have a tremendous capacity for survival, but when we are in survival mode we have little energy leftover for the pleasures of life, from family to hobbies. I would similarly argue that in survival mode we have limited ability to appreciate the pain and suffering our patients are experiencing. Survival mode limits our ability as physicians to connect with our patients and to engage in the full spectrum of emotion in our time outside of our job.
What are the causes of burnout in dermatology?
As dermatologists, we often have milder on-call schedules and fewer critically ill patients than many of our medical colleagues. For this reason, we may be afraid to address the real role of physician burnout in our field. Fellow dermatologist Jeffrey Benabio, MD (San Diego, California), notes that the phrase dermatologist burnout may even seem oxymoronic, but we face many of the same daily frustrations with electronic medical records, increasing patient volume, and insurance struggles.4 The electronic medical record looms large in many physicians’ complaints these days. A recent article in the New York Times described the physician as “the highest-paid clerical worker in the hospital,”5 which is not wrong. For every hour of patient time, we have nearly double that spent on paperwork.5
Dike Drummond, MD, a family practice physician who focuses on physician burnout, notes that physicians are taught very early to put the patient first, but it is never discussed when or how to turn this switch off.3 However, there is little written about dermatology-specific burnout. A problem that is not studied or even considered will be harder to fix.
Why does it matter?
I believe that addressing physician burnout is critical for 2 reasons: (1) we can improve patient care by improving patient satisfaction and decreasing medical error, and (2) we can find greater satisfaction and professional fulfillment while caring for our patients. Ultimately, patient care and physician care are intimately linked; as stated by Thomas et al,6 “[p]hysicians who are well can best serve their patients.”
As a resident in 2018, I recognize that my coresidents and I are training as physicians in the time of “duty hours” and an ongoing discussion of burnout. However, I sense a burnout fatigue setting in among residents, many who do not want to discuss wellness anymore. The newer data suggest that work hour restrictions do not improve patient safety, negating one of the driving reasons for the change.7 At the same time, residency programs are initiating wellness programs in response to the growing literature on physician burnout. These wellness programs vary in the types of activities included, from individual coping techniques such as mindfulness training to social gatherings for the residents. In general, these wellness initiatives focus on burnout at the individual level, but they do not take into account systemic or structural challenges that might contribute to this worsening epidemic.
Final Thoughts
As a profession, I believe that physicians have internalized the concept of burnout to equate with a personal individual failing. At various times in my training, I have felt that if I could just practice mediation, study more, or shift my perspective, I personally could overcome burnout. I have intermittently felt my burnout as proof that I should never have become a physician. As a woman and the first physician in my family, fighting the sense of burnout so early in my career seemed demoralizing and nearly drove me to change my career path. It exacerbated my sense of imposter syndrome: that I never truly belonged in medicine at all. After much soul-searching, I have concluded that burnout is a concept propagated by administrators and businesspeople to stigmatize the reaction by many physicians to the growing trends in medicine and cast it as a personal failure rather than as the symptom of a broken medical system.
If we continue to identify burnout as an individual failing and treat it as such, I believe that we will fail to stem the growing trend within dermatology and within medicine more broadly. We need to consider the driving factors behind dermatology burnout so that we can begin to address them at a structural level.
- Peckham C. Medscape national physician burnout & depression report 2018. Medscape website. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Published January 17, 2018. Accessed June 21, 2018.
- Maslach C, Schaufeli WB, Leiter MP. Job
burnout. Annu Rev Psychol. 2001;52:397-422. - Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
- Benabio J. Burnout. Dermatology News. November 14, 2017. https://www.mdedge.com/edermatologynews/article/152098/business-medicine/burnout. Accessed June 30, 2018.
- Verghese A. How tech can turn doctors into clerical workers. New York Times. May 16, 2018. https://www.nytimes.com/interactive/2018/05/16/magazine/health-issue-what-we-lose-with-data-driven-medicine.html. Accessed July 3, 2018.
- Thomas LR, Ripp JA, West CP. Charter on physician well-being. JAMA. 2018;319:1541-1542.
- Osborne R, Parshuram CS. Delinking resident duty hours from patient safety [published online December 11, 2014]. BMC Med Educ. 2014;14(suppl 1):S2.
- Peckham C. Medscape national physician burnout & depression report 2018. Medscape website. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Published January 17, 2018. Accessed June 21, 2018.
- Maslach C, Schaufeli WB, Leiter MP. Job
burnout. Annu Rev Psychol. 2001;52:397-422. - Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
- Benabio J. Burnout. Dermatology News. November 14, 2017. https://www.mdedge.com/edermatologynews/article/152098/business-medicine/burnout. Accessed June 30, 2018.
- Verghese A. How tech can turn doctors into clerical workers. New York Times. May 16, 2018. https://www.nytimes.com/interactive/2018/05/16/magazine/health-issue-what-we-lose-with-data-driven-medicine.html. Accessed July 3, 2018.
- Thomas LR, Ripp JA, West CP. Charter on physician well-being. JAMA. 2018;319:1541-1542.
- Osborne R, Parshuram CS. Delinking resident duty hours from patient safety [published online December 11, 2014]. BMC Med Educ. 2014;14(suppl 1):S2.
Pulmonary Embolism Rule-Out Criteria Strategy is noninferior when clinical probability is low
Background: There is an alarming trend toward overuse of computed tomographic pulmonary angiography (CTPA) for the rule-out of low clinical probability PE. The eight-item Pulmonary Embolism Rule-Out Criteria (PERC) rule was devised to be used in populations of patients with low clinical probability of PE to guide which patients would likely not benefit from CTPA imaging. Recent concerns have been raised that the use of the PERC rule could result in high false-negative rates.
Study design: Crossover cluster–randomized clinical noninferiority trial.
Setting: 14 EDs in France from August 2015 to September 2016.
Synopsis: 1,916 emergency department patients with low clinical probability of PE were cluster-randomized to usual care or to a PERC strategy where, if the PERC score was zero, PE was ruled out without additional testing. The primary outcome was diagnosis of a symptomatic PE within 3 months that had not been diagnosed initially. Primary outcome results met prespecified noninferiority criteria for the PERC group, compared with the usual-care group (0.1% in the PERC group, 0% in the control group). The PERC group had significantly lower median length of ED stay and lower likelihood of admission.
Limitations of this study include its younger average patient age (44 years) and its cluster, as opposed to per-patient, randomization design.
Bottom line: In patients for whom the clinical probability of PE is low, use of the PERC rule is noninferior to a conventional d-dimer and CTPA strategy for ruling out symptomatic PE.
Citation: Freund Y et al. Effect of the pulmonary embolism rule-out criteria on subsequent thromboembolic events among low-risk emergency department patients. JAMA. 2018;319(6):559-66.
Dr. Abdallah is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Background: There is an alarming trend toward overuse of computed tomographic pulmonary angiography (CTPA) for the rule-out of low clinical probability PE. The eight-item Pulmonary Embolism Rule-Out Criteria (PERC) rule was devised to be used in populations of patients with low clinical probability of PE to guide which patients would likely not benefit from CTPA imaging. Recent concerns have been raised that the use of the PERC rule could result in high false-negative rates.
Study design: Crossover cluster–randomized clinical noninferiority trial.
Setting: 14 EDs in France from August 2015 to September 2016.
Synopsis: 1,916 emergency department patients with low clinical probability of PE were cluster-randomized to usual care or to a PERC strategy where, if the PERC score was zero, PE was ruled out without additional testing. The primary outcome was diagnosis of a symptomatic PE within 3 months that had not been diagnosed initially. Primary outcome results met prespecified noninferiority criteria for the PERC group, compared with the usual-care group (0.1% in the PERC group, 0% in the control group). The PERC group had significantly lower median length of ED stay and lower likelihood of admission.
Limitations of this study include its younger average patient age (44 years) and its cluster, as opposed to per-patient, randomization design.
Bottom line: In patients for whom the clinical probability of PE is low, use of the PERC rule is noninferior to a conventional d-dimer and CTPA strategy for ruling out symptomatic PE.
Citation: Freund Y et al. Effect of the pulmonary embolism rule-out criteria on subsequent thromboembolic events among low-risk emergency department patients. JAMA. 2018;319(6):559-66.
Dr. Abdallah is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Background: There is an alarming trend toward overuse of computed tomographic pulmonary angiography (CTPA) for the rule-out of low clinical probability PE. The eight-item Pulmonary Embolism Rule-Out Criteria (PERC) rule was devised to be used in populations of patients with low clinical probability of PE to guide which patients would likely not benefit from CTPA imaging. Recent concerns have been raised that the use of the PERC rule could result in high false-negative rates.
Study design: Crossover cluster–randomized clinical noninferiority trial.
Setting: 14 EDs in France from August 2015 to September 2016.
Synopsis: 1,916 emergency department patients with low clinical probability of PE were cluster-randomized to usual care or to a PERC strategy where, if the PERC score was zero, PE was ruled out without additional testing. The primary outcome was diagnosis of a symptomatic PE within 3 months that had not been diagnosed initially. Primary outcome results met prespecified noninferiority criteria for the PERC group, compared with the usual-care group (0.1% in the PERC group, 0% in the control group). The PERC group had significantly lower median length of ED stay and lower likelihood of admission.
Limitations of this study include its younger average patient age (44 years) and its cluster, as opposed to per-patient, randomization design.
Bottom line: In patients for whom the clinical probability of PE is low, use of the PERC rule is noninferior to a conventional d-dimer and CTPA strategy for ruling out symptomatic PE.
Citation: Freund Y et al. Effect of the pulmonary embolism rule-out criteria on subsequent thromboembolic events among low-risk emergency department patients. JAMA. 2018;319(6):559-66.
Dr. Abdallah is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Ketamine Plus Memantine-Based Multimodality Treatment of Chronic Refractory Migraine
Dr. Charles is Clinical Associate Professor Neurology, Rutgers–New Jersey Medical School, Newark, NJ; Neurology Attending, Holy Name Medical Center, Teaneck, NJ ([email protected]).
Dr. Gallo is Interventional Radiology Attending, Holy Name Medical Center, Teaneck, NJ ([email protected]).
DISCLOSURES
The authors have no financial relationships to disclose relevant to the manuscript. There was no sponsorship of, or funding for, the study.
Dr. Charles designed and conceptualized the study; analyzed study data and performed the statistical analysis; and drafted the manuscript for intellectual content. Dr. Gallo had a major role in the acquisition of interventional sphenopalatine ganglion data.
ABSTRACT
Objective
Chronic refractory migraine patients who failed repetitive dihydroergotamine/dopamine infusion protocols and conventional preventives were treated with repeated low-dose ketamine-based parenteral protocols, followed by memantine-based preventive therapy, and observed for immediate reduction in pain intensity and headache frequency.
Methods
Ten patients were treated at an outpatient infusion center for 2 to 5 sequential days with AM and PM courses of intravenous diphenhydramine, prochlorperazine, and dihydroergotamine. A daily sphenopalatine ganglion block and low-dose intramuscular ketamine were given midday between treatments, with dexamethasone given on the last infusion day. The Numeric Pain Rating Scale was measured after infusion. Carryover effect was assessed 1 month and 2 months after infusion by headache frequency while being treated with memantine and various other preventive and abortive therapies.
Results
Reduction in headache pain of 71% was achieved at the end of the infusion period. Sedation was the only adverse effect. Decreased headache frequency persisted beyond the infusion period, with an 88.6% reduction in headache days per month at 1 month and a 79.4% reduction in headache days per month at 2 months, without adverse effects.
Conclusions
Data indicate that 1) repetitive low-dose, ketamine-based parenteral therapy, followed by memantine-based preventive therapy, reduced refractory headache pain and 2) the decremental effect on headache frequency persisted beyond the infusion period. Our results support the hypothesis that multimechanistic therapies might be better than single-modality treatment. More studies, with a larger patient population, are needed to confirm whether these multimodality ketamine/memantine therapies should become the preferred approach for these extremely disabled patients.
Chronic refractory migraine (CRM) degrades function and quality of life despite elimination of triggers and adequate trials of acute and preventive medicines that have established efficacy. This definition requires that patients with chronic migraine fail adequate trials of preventive drugs, alone or in combination, in at least 2 of 4 drug classes, including beta blockers, anticonvulsants, tricyclic antidepressants, onabotulinumtoxin A, and calcium-channel blockers. Patients must also fail adequate trials of abortive medicines, including both a triptan and dihydroergotamine (DHE), intranasal or injectable formulation, and either a nonsteroidal anti-inflammatory drug or a combination analgesic, unless contraindicated.1-4
In 1986, Raskin published a nonrandomized, nonblinded study of 2 treatments for intractable migraine in which repetitive inpatient intravenous (IV) DHE, administered in the hospital, was statistically more effective than IV diazepam in terminating cycles of intractable migraine.5 Most headache specialists have adopted the so-called Raskin protocol, as originally described or in any of several variations, as cornerstone therapy for CRM, chronic migraine, and prolonged status migrainosus.6 However, DHE-based infusion protocols do not always effectively reset the brain’s pain modulatory pathways in chronic migraine immediately posttreatment and might not induce a meaningful carryover effect.
We present 10 patients with CRM who met criteria for refractory migraine, including failure to terminate their headache with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols, with or without sporadic administration of a sphenopalatine ganglion block. We treated these patients multimechanistically with repetitive IV DHE, a dopamine antagonist, an antihistamine, sphenopalatine ganglion (SPG) block, and low-dose ketamine, plus last-infusion-day dexamethasone, followed by outpatient oral memantine. Subsequently, we observed them for 2 months.
Ketamine is a phencyclidine derivative introduced the early 1960s as an IV anesthetic. Low-dose ketamine has been used successfully in the treatment of chronic pain. Today, increased interest in the application of low-dose ketamine includes cancer pain; treatment and prevention of acute and chronic pain, with and without neuropathic analgesia; fibromyalgia; complex regional pain; and migraine.7,8 The effectiveness of ketamine in different pain disorders may arise through different pathways and/or by way of activity at various receptor systems. Effects arise predominantly by noncompetitive antagonism of the glutamate N-methyl-D-aspartate (NMDA ) receptor.7,8
Memantine also is an NMDA receptor antagonist that is used effectively as an oral agent in CRM.9
METHODS
Patients enrolled in this prospective study had CRM for periods ranging from 1 to 2 years. All had daily headache that could not be terminated with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols with or without sporadic administration of an SPG block. Age ranged from 18 and 68 years; all patients were female. Patients were excluded if they had known coronary artery disease, uncontrolled hypertension, or peripheral arterial disease; a history of stroke, transient ischemic attack, or pregnancy; impaired liver or renal function; smoked a tobacco product; or were taking a protease inhibitor or macrolide antibiotic.
Approval by the institutional review board was unnecessary because all drugs and procedures are FDA-approved and have published evidence-based efficacy for migraine and other diseases.
The Numeric Pain Rating Scale (NPRS; a scale of 0 to 10) was utilized to rate the intensity of pain from the beginning of the infusion to the end of the multiday infusion protocol, when the catheter was removed. All patients but 1 were treated for 5 days; for the 1 exception, treatment was terminated after 48 hours because of a scheduling conflict. The observational follow‐up periods for assessment of outcomes were 1 month and 2 months post-infusion.
Patients started the study with a baseline NPRS of 9 or 10. They were treated at the institution’s headache outpatient infusion center. In the morning, patients received, by sequential IV infusion, diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 1 mg. They then received a midday SPG block under fluoroscopic guidance and ketamine, 0.45 mg/kg intramuscularly (IM), given in the post-anesthesia care unit. In the late afternoon, the patients received diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 0.5 mg, in the Headache Outpatient Infusion Center. Patients were discharged to home by 6 PM. They received IV dexamethasone, 20 mg, on the last day of therapy.
Oral preventive agents were continued and abortives were temporarily discontinued during infusion therapy. Oral memantine was used immediately before, during, and, in all cases, after infusion, at a daily dosage that ranged from 10 mg BID to 28 mg, once-daily extended release.
RESULTS
Therapies were well-tolerated by all patients. On the last day of treatment, the entire cohort (N = 10) demonstrated an average of 71% (mean standard deviation [SD], 10.1%) reduction in pain intensity. The average reduction in headache days per month at 1 month was 88.6% (mean SD, 6.24%) and at 2 months was 79.4% (mean SD, 17.13%) (Table). Adverse effects were mild temporary sedation from ketamine. Pulse oximetry revealed no abnormal decrease in O2 saturation. All patients reported marked overall reduction in headache disability at the end of the infusion protocol. Self-administered abortive therapies posttreatment were more efficacious than they were pretreatment. All patients indicated less headache disability overall by the end of the 2-month observation period.
Table. Chronic Refractory Migraine Baseline Data and Treatment Resultsa
Name | Age (y) | Sex | Treatment Duration (days) | Baseline NPRS | Post-treatment NPRS | One Month Follow-upb | Two Month Follow-upb |
SL | 45 | F | 5 | 10 | 2 | 3 | 3 |
RR | 44 | F | 5 | 9 | 1 | 1 | 3 |
MP | 41 | F | 5 | 10 | 4 | 3 | 6 |
AP | 35 | F | 5 | 10 | 3 | 8 | 15 |
SW | 27 | F | 5 | 10 | 2 | 6 | 12 |
HC | 47 | F | 5 | 10 | 4 | 4 | 6 |
KK | 56 | F | 5 | 10 | 3 | 3 | 8 |
MG | 53 | F | 5 | 9 | 4 | 2 | 3 |
DM | 68 | F | 2 | 9 | 2 | 2 | 4 |
AO | 18 | F | 5 | 9 | 3 | 2 | 2 |
aAll patients had daily headache at initiation of treatment.
bHeadache days/month.
NPRS, Numeric Pain Rating Scale.
DISCUSSION
In our study of 10 patients with CRM who had daily headache treated repetitively in an outpatient infusion center with multimodality therapies, including sub-anesthetic doses of ketamine, all patients experienced marked reduction in headache pain intensity, with a whole-group average reduction of 71% by the end of infusion treatment. During post-infusion observation, all patients continued various preventive therapies, including memantine. At 1 month, the average reduction in headache frequency was 88.6%. Two months post-infusion, the average reduction in headache frequency was 79.4%. Adverse effects were minimal. Overall, the treatment was found to be safe and efficacious. All patients felt less headache disability after 2 months.
Because the protocol was administered comfortably in the Headache Outpatient Infusion Center, the inconvenience and higher cost of inpatient parenteral treatment were avoided. Ketamine, 0.45 mg/kg IM is a sub-anesthetic dose with proven efficacy in treating migraine without adverse effects in an outpatient setting.8 Low-dose ketamine obviated the need for anesthesia personnel and precautions. Temporary sedation was the only adverse effect. Ketamine was administered by a nurse in the post-anesthesia care unit while patients were under observation with conventional measurement of vital signs and pulse oximetry. Memantine, also an NMDA receptor antagonist, is postulated to prolong the NMDA antagonism of ketamine.
Inpatient and outpatient continuous IV DHE and repetitive IV DHE, often combined with dopamine antagonists in controlled and comparator studies, have demonstrated equal effectiveness for the treatment of chronic migraine.5,10,11 Our patients failed these therapies. This raises the question: Should our combined multimodality, ketamine-based approach be standard parenteral therapy for CRM?
In a recent study of continuous inpatient single-modality IV ketamine, a less-impressive carryover effect was obtained, with 23% to 50% 1-month sustained responders.12 Multimechanistic treatment superiority over monotherapy is legendary in the treatment of cancer and human immunodeficiency infection. Sumatriptan plus naproxen sodium as a single tablet for acute treatment of migraine resulted in more favorable clinical benefit compared with either monotherapy, with an acceptable, well-tolerated adverse effect profile. Because multiple pathogenic mechanisms putatively are involved in generation of the migraine symptom complex, multimechanism-targeted therapy may confer advantages over individual monotherapy. Drugs in 2 classes of migraine pharmacotherapy—triptans and nonsteroidal anti-inflammatory drugs —target distinct aspects of the vascular and inflammatory processes hypothesized to underlie migraine.13
Although combination therapy for CRM has not been systematically studied in randomized trials, clinical experience suggests that a rational approach to CRM treatment, utilizing a combination of treatments, may be effective when monotherapy has failed.14 During the infusion protocol, we re-set the trigeminovascular pain pathways 1) by repetitively blocking NMDA receptors (with ketamine), dopamine receptors (with prochlorperazine), and histamine receptors (with diphenhydramine); 2) by lidocaine anesthetic block of the sphenopalatine ganglia; and, on the last day of the protocol, 3) administering 1 large dose of IV dexamethasone to help prevent recurrence.15 NMDA blockade continued with oral outpatient memantine.
Virtually all patients were taking other preventives during the pretreatment period and 2-month observation period, including topiramate, venlafaxine, beta blockers, candesartan, zonisamide, onabotulinumtoxin A, neuromodulation (Cefaly Technology), and transcranial magnetic stimulation (springTMS®). Self-administered abortives were more effective in the 2-month observational period; these included IM/IV DHE; oral, spray, and subcutaneous triptans; IM ketorolac; diclofenac buffered solution; and transcranial magnetic stimulation (springTMS®). The cornerstone strategy of our treatment group that was a constant was the use of low-dose IM sub-anesthetic ketamine at a dosage of 0.45 mg/kg/d and the use of oral memantine during the follow-up observation period, at dosages ranging from 10 mg BID to 28 mg, once-daily extended release.
Limitations of this study design are:
- lack of a control group
- lack of subject randomization for comparative outcomes
- patients remaining on a variety of prophylactic regimens
- patients permitted to take any rescue therapy.
The effect of repetitive SPG block cannot be teased out of the efficacy data, but many of our patients had a poor or temporary response to infrequent sporadic SPG blocks prior to participating in our protocol.
Many migraineurs who seek care in a headache clinic are refractory to treatment, despite advances in headache therapy; refractory migraine was found in 5.1% of these patients.16 In this small series of patients, we demonstrated immediate relief and a significant 2-month carryover effect with our multimodality parenteral protocol. Larger, controlled studies are needed to further explore this protocol with repetitive DHE, diphenhydramine, prochlorperazine, SPG block, and low-dose IM ketamine, followed by outpatient memantine. Such studies would determine whether our protocol should be utilized as a primary treatment, instead of the conventional DHE-based Raskin and modified Raskin protocols.
Although this is a small series of patients, lack of adverse effects and impressive results should give credence to utilizing our protocol as treatment for this extremely debilitated, often desperate subset of headache patients. Data indicate that, whereas ketamine combined with other therapies immediately reduced refractory headache pain, the ameliorating effect of ketamine on CRM headache frequency and pain in our protocol persisted beyond the infusion period. This phenomenon indicates a disease-modulating role for ketamine in refractory migraine pain, possibly by means of desensitization of NMDA receptors in the trigeminal nucleus caudalis—desensitization that continued with the NMDA receptor antagonist memantine and/or restoration of inhibitory sensory control in the brain.
CONCLUSION
Our results support the hypothesis that multimechanistic therapies, including low-dose IM ketamine and memantine, might be better than single-modality treatment in this debilitated, refractory population. Future studies, with larger patient populations, are needed to confirm whether these multimodality ketamine/memantine-inclusive therapies should become the preferred approach for these extremely disabled patients.
REFERENCES
1. Goadsby PJ, Schoenen J, Ferrari MD, Silberstein SD, Dodick DW. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26(9):1168-1170.
2. Schulman EA, Lipton R, Peterlin BL, Levin M, Grosberg BM. Commentary from the Refractory Headache Special Interest Section on defining the pharmacologically intractable headache for clinical trials and clinical practice. Headache. 2010;50(10):1637-1639.
3. Martelletti P, Jensen RH, Antal A, et al. Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain. 2013;14:86.
4. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921-936.
5. Raskin NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36(7):995‐997.
6. Charles JA, von Dohln P. Outpatient home-based continuous intravenous dihydroergotamine therapy for intractable migraine. Headache. 2010;50(5):852-860.
7. Sigtermans M, Noppers I, Sarton E, et al. An observational study on the effect of S+-ketamine on chronic pain versus experimental acute pain in complex regional pain syndrome type 1 patients. Eur J Pain. 2010;14(3):302-307.
8. Krusz J, Cagle J, Hall S. Intramuscular (IM) ketamine for treating headache and pain flare-ups in the clinic. J Pain. 2008;9(4):30.
9. Bigal M Rapoport A, Sheftell F, Tepper D, Tepper S. Memantine in the preventive treatment of refractory migraine. Headache. 2008;48(9):1337-1342.
10. Ford RG, Ford KT. Continuous intravenous dihydroergotamine for treatment of intractable headache. Headache. 1997;37(3):129‐136.
11. Boudreau G, Aghai E, Marchand L, Langlois M. Outpatient intravenous dihydroergotamine for probable medication overuse headache. Headache Care. 2006;3(1):45‐49.
12. Pomeroy JL, Marmura MJ, Nahas SJ, Viscusi ER. Ketamine infusions for treatment refractory headache. Headache. 2017;57(2):276-282.
13. Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297(13):1443-1454.
14. Peterlin BL, Calhoun AH, Siegel S, Mathew NT. Rational combination therapy in refractory migraine. Headache. 2008;48(6):805-819.
15. Innes G, Macphail I, Dillon EC, Metcalfe C, Gao M. Dexamethasone prevents relapse after emergency department treatment of acute migraine: a randomized clinical trial. CJEM. 2015;1(1):26-33.
16. Irimia P, Palma JA, Fernandez-Torron R, Martinez-Vila E. Refractory migraine in a headache clinic population. BMC Neurol. 2011;11:94.
Dr. Charles is Clinical Associate Professor Neurology, Rutgers–New Jersey Medical School, Newark, NJ; Neurology Attending, Holy Name Medical Center, Teaneck, NJ ([email protected]).
Dr. Gallo is Interventional Radiology Attending, Holy Name Medical Center, Teaneck, NJ ([email protected]).
DISCLOSURES
The authors have no financial relationships to disclose relevant to the manuscript. There was no sponsorship of, or funding for, the study.
Dr. Charles designed and conceptualized the study; analyzed study data and performed the statistical analysis; and drafted the manuscript for intellectual content. Dr. Gallo had a major role in the acquisition of interventional sphenopalatine ganglion data.
ABSTRACT
Objective
Chronic refractory migraine patients who failed repetitive dihydroergotamine/dopamine infusion protocols and conventional preventives were treated with repeated low-dose ketamine-based parenteral protocols, followed by memantine-based preventive therapy, and observed for immediate reduction in pain intensity and headache frequency.
Methods
Ten patients were treated at an outpatient infusion center for 2 to 5 sequential days with AM and PM courses of intravenous diphenhydramine, prochlorperazine, and dihydroergotamine. A daily sphenopalatine ganglion block and low-dose intramuscular ketamine were given midday between treatments, with dexamethasone given on the last infusion day. The Numeric Pain Rating Scale was measured after infusion. Carryover effect was assessed 1 month and 2 months after infusion by headache frequency while being treated with memantine and various other preventive and abortive therapies.
Results
Reduction in headache pain of 71% was achieved at the end of the infusion period. Sedation was the only adverse effect. Decreased headache frequency persisted beyond the infusion period, with an 88.6% reduction in headache days per month at 1 month and a 79.4% reduction in headache days per month at 2 months, without adverse effects.
Conclusions
Data indicate that 1) repetitive low-dose, ketamine-based parenteral therapy, followed by memantine-based preventive therapy, reduced refractory headache pain and 2) the decremental effect on headache frequency persisted beyond the infusion period. Our results support the hypothesis that multimechanistic therapies might be better than single-modality treatment. More studies, with a larger patient population, are needed to confirm whether these multimodality ketamine/memantine therapies should become the preferred approach for these extremely disabled patients.
Chronic refractory migraine (CRM) degrades function and quality of life despite elimination of triggers and adequate trials of acute and preventive medicines that have established efficacy. This definition requires that patients with chronic migraine fail adequate trials of preventive drugs, alone or in combination, in at least 2 of 4 drug classes, including beta blockers, anticonvulsants, tricyclic antidepressants, onabotulinumtoxin A, and calcium-channel blockers. Patients must also fail adequate trials of abortive medicines, including both a triptan and dihydroergotamine (DHE), intranasal or injectable formulation, and either a nonsteroidal anti-inflammatory drug or a combination analgesic, unless contraindicated.1-4
In 1986, Raskin published a nonrandomized, nonblinded study of 2 treatments for intractable migraine in which repetitive inpatient intravenous (IV) DHE, administered in the hospital, was statistically more effective than IV diazepam in terminating cycles of intractable migraine.5 Most headache specialists have adopted the so-called Raskin protocol, as originally described or in any of several variations, as cornerstone therapy for CRM, chronic migraine, and prolonged status migrainosus.6 However, DHE-based infusion protocols do not always effectively reset the brain’s pain modulatory pathways in chronic migraine immediately posttreatment and might not induce a meaningful carryover effect.
We present 10 patients with CRM who met criteria for refractory migraine, including failure to terminate their headache with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols, with or without sporadic administration of a sphenopalatine ganglion block. We treated these patients multimechanistically with repetitive IV DHE, a dopamine antagonist, an antihistamine, sphenopalatine ganglion (SPG) block, and low-dose ketamine, plus last-infusion-day dexamethasone, followed by outpatient oral memantine. Subsequently, we observed them for 2 months.
Ketamine is a phencyclidine derivative introduced the early 1960s as an IV anesthetic. Low-dose ketamine has been used successfully in the treatment of chronic pain. Today, increased interest in the application of low-dose ketamine includes cancer pain; treatment and prevention of acute and chronic pain, with and without neuropathic analgesia; fibromyalgia; complex regional pain; and migraine.7,8 The effectiveness of ketamine in different pain disorders may arise through different pathways and/or by way of activity at various receptor systems. Effects arise predominantly by noncompetitive antagonism of the glutamate N-methyl-D-aspartate (NMDA ) receptor.7,8
Memantine also is an NMDA receptor antagonist that is used effectively as an oral agent in CRM.9
METHODS
Patients enrolled in this prospective study had CRM for periods ranging from 1 to 2 years. All had daily headache that could not be terminated with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols with or without sporadic administration of an SPG block. Age ranged from 18 and 68 years; all patients were female. Patients were excluded if they had known coronary artery disease, uncontrolled hypertension, or peripheral arterial disease; a history of stroke, transient ischemic attack, or pregnancy; impaired liver or renal function; smoked a tobacco product; or were taking a protease inhibitor or macrolide antibiotic.
Approval by the institutional review board was unnecessary because all drugs and procedures are FDA-approved and have published evidence-based efficacy for migraine and other diseases.
The Numeric Pain Rating Scale (NPRS; a scale of 0 to 10) was utilized to rate the intensity of pain from the beginning of the infusion to the end of the multiday infusion protocol, when the catheter was removed. All patients but 1 were treated for 5 days; for the 1 exception, treatment was terminated after 48 hours because of a scheduling conflict. The observational follow‐up periods for assessment of outcomes were 1 month and 2 months post-infusion.
Patients started the study with a baseline NPRS of 9 or 10. They were treated at the institution’s headache outpatient infusion center. In the morning, patients received, by sequential IV infusion, diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 1 mg. They then received a midday SPG block under fluoroscopic guidance and ketamine, 0.45 mg/kg intramuscularly (IM), given in the post-anesthesia care unit. In the late afternoon, the patients received diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 0.5 mg, in the Headache Outpatient Infusion Center. Patients were discharged to home by 6 PM. They received IV dexamethasone, 20 mg, on the last day of therapy.
Oral preventive agents were continued and abortives were temporarily discontinued during infusion therapy. Oral memantine was used immediately before, during, and, in all cases, after infusion, at a daily dosage that ranged from 10 mg BID to 28 mg, once-daily extended release.
RESULTS
Therapies were well-tolerated by all patients. On the last day of treatment, the entire cohort (N = 10) demonstrated an average of 71% (mean standard deviation [SD], 10.1%) reduction in pain intensity. The average reduction in headache days per month at 1 month was 88.6% (mean SD, 6.24%) and at 2 months was 79.4% (mean SD, 17.13%) (Table). Adverse effects were mild temporary sedation from ketamine. Pulse oximetry revealed no abnormal decrease in O2 saturation. All patients reported marked overall reduction in headache disability at the end of the infusion protocol. Self-administered abortive therapies posttreatment were more efficacious than they were pretreatment. All patients indicated less headache disability overall by the end of the 2-month observation period.
Table. Chronic Refractory Migraine Baseline Data and Treatment Resultsa
Name | Age (y) | Sex | Treatment Duration (days) | Baseline NPRS | Post-treatment NPRS | One Month Follow-upb | Two Month Follow-upb |
SL | 45 | F | 5 | 10 | 2 | 3 | 3 |
RR | 44 | F | 5 | 9 | 1 | 1 | 3 |
MP | 41 | F | 5 | 10 | 4 | 3 | 6 |
AP | 35 | F | 5 | 10 | 3 | 8 | 15 |
SW | 27 | F | 5 | 10 | 2 | 6 | 12 |
HC | 47 | F | 5 | 10 | 4 | 4 | 6 |
KK | 56 | F | 5 | 10 | 3 | 3 | 8 |
MG | 53 | F | 5 | 9 | 4 | 2 | 3 |
DM | 68 | F | 2 | 9 | 2 | 2 | 4 |
AO | 18 | F | 5 | 9 | 3 | 2 | 2 |
aAll patients had daily headache at initiation of treatment.
bHeadache days/month.
NPRS, Numeric Pain Rating Scale.
DISCUSSION
In our study of 10 patients with CRM who had daily headache treated repetitively in an outpatient infusion center with multimodality therapies, including sub-anesthetic doses of ketamine, all patients experienced marked reduction in headache pain intensity, with a whole-group average reduction of 71% by the end of infusion treatment. During post-infusion observation, all patients continued various preventive therapies, including memantine. At 1 month, the average reduction in headache frequency was 88.6%. Two months post-infusion, the average reduction in headache frequency was 79.4%. Adverse effects were minimal. Overall, the treatment was found to be safe and efficacious. All patients felt less headache disability after 2 months.
Because the protocol was administered comfortably in the Headache Outpatient Infusion Center, the inconvenience and higher cost of inpatient parenteral treatment were avoided. Ketamine, 0.45 mg/kg IM is a sub-anesthetic dose with proven efficacy in treating migraine without adverse effects in an outpatient setting.8 Low-dose ketamine obviated the need for anesthesia personnel and precautions. Temporary sedation was the only adverse effect. Ketamine was administered by a nurse in the post-anesthesia care unit while patients were under observation with conventional measurement of vital signs and pulse oximetry. Memantine, also an NMDA receptor antagonist, is postulated to prolong the NMDA antagonism of ketamine.
Inpatient and outpatient continuous IV DHE and repetitive IV DHE, often combined with dopamine antagonists in controlled and comparator studies, have demonstrated equal effectiveness for the treatment of chronic migraine.5,10,11 Our patients failed these therapies. This raises the question: Should our combined multimodality, ketamine-based approach be standard parenteral therapy for CRM?
In a recent study of continuous inpatient single-modality IV ketamine, a less-impressive carryover effect was obtained, with 23% to 50% 1-month sustained responders.12 Multimechanistic treatment superiority over monotherapy is legendary in the treatment of cancer and human immunodeficiency infection. Sumatriptan plus naproxen sodium as a single tablet for acute treatment of migraine resulted in more favorable clinical benefit compared with either monotherapy, with an acceptable, well-tolerated adverse effect profile. Because multiple pathogenic mechanisms putatively are involved in generation of the migraine symptom complex, multimechanism-targeted therapy may confer advantages over individual monotherapy. Drugs in 2 classes of migraine pharmacotherapy—triptans and nonsteroidal anti-inflammatory drugs —target distinct aspects of the vascular and inflammatory processes hypothesized to underlie migraine.13
Although combination therapy for CRM has not been systematically studied in randomized trials, clinical experience suggests that a rational approach to CRM treatment, utilizing a combination of treatments, may be effective when monotherapy has failed.14 During the infusion protocol, we re-set the trigeminovascular pain pathways 1) by repetitively blocking NMDA receptors (with ketamine), dopamine receptors (with prochlorperazine), and histamine receptors (with diphenhydramine); 2) by lidocaine anesthetic block of the sphenopalatine ganglia; and, on the last day of the protocol, 3) administering 1 large dose of IV dexamethasone to help prevent recurrence.15 NMDA blockade continued with oral outpatient memantine.
Virtually all patients were taking other preventives during the pretreatment period and 2-month observation period, including topiramate, venlafaxine, beta blockers, candesartan, zonisamide, onabotulinumtoxin A, neuromodulation (Cefaly Technology), and transcranial magnetic stimulation (springTMS®). Self-administered abortives were more effective in the 2-month observational period; these included IM/IV DHE; oral, spray, and subcutaneous triptans; IM ketorolac; diclofenac buffered solution; and transcranial magnetic stimulation (springTMS®). The cornerstone strategy of our treatment group that was a constant was the use of low-dose IM sub-anesthetic ketamine at a dosage of 0.45 mg/kg/d and the use of oral memantine during the follow-up observation period, at dosages ranging from 10 mg BID to 28 mg, once-daily extended release.
Limitations of this study design are:
- lack of a control group
- lack of subject randomization for comparative outcomes
- patients remaining on a variety of prophylactic regimens
- patients permitted to take any rescue therapy.
The effect of repetitive SPG block cannot be teased out of the efficacy data, but many of our patients had a poor or temporary response to infrequent sporadic SPG blocks prior to participating in our protocol.
Many migraineurs who seek care in a headache clinic are refractory to treatment, despite advances in headache therapy; refractory migraine was found in 5.1% of these patients.16 In this small series of patients, we demonstrated immediate relief and a significant 2-month carryover effect with our multimodality parenteral protocol. Larger, controlled studies are needed to further explore this protocol with repetitive DHE, diphenhydramine, prochlorperazine, SPG block, and low-dose IM ketamine, followed by outpatient memantine. Such studies would determine whether our protocol should be utilized as a primary treatment, instead of the conventional DHE-based Raskin and modified Raskin protocols.
Although this is a small series of patients, lack of adverse effects and impressive results should give credence to utilizing our protocol as treatment for this extremely debilitated, often desperate subset of headache patients. Data indicate that, whereas ketamine combined with other therapies immediately reduced refractory headache pain, the ameliorating effect of ketamine on CRM headache frequency and pain in our protocol persisted beyond the infusion period. This phenomenon indicates a disease-modulating role for ketamine in refractory migraine pain, possibly by means of desensitization of NMDA receptors in the trigeminal nucleus caudalis—desensitization that continued with the NMDA receptor antagonist memantine and/or restoration of inhibitory sensory control in the brain.
CONCLUSION
Our results support the hypothesis that multimechanistic therapies, including low-dose IM ketamine and memantine, might be better than single-modality treatment in this debilitated, refractory population. Future studies, with larger patient populations, are needed to confirm whether these multimodality ketamine/memantine-inclusive therapies should become the preferred approach for these extremely disabled patients.
REFERENCES
1. Goadsby PJ, Schoenen J, Ferrari MD, Silberstein SD, Dodick DW. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26(9):1168-1170.
2. Schulman EA, Lipton R, Peterlin BL, Levin M, Grosberg BM. Commentary from the Refractory Headache Special Interest Section on defining the pharmacologically intractable headache for clinical trials and clinical practice. Headache. 2010;50(10):1637-1639.
3. Martelletti P, Jensen RH, Antal A, et al. Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain. 2013;14:86.
4. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921-936.
5. Raskin NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36(7):995‐997.
6. Charles JA, von Dohln P. Outpatient home-based continuous intravenous dihydroergotamine therapy for intractable migraine. Headache. 2010;50(5):852-860.
7. Sigtermans M, Noppers I, Sarton E, et al. An observational study on the effect of S+-ketamine on chronic pain versus experimental acute pain in complex regional pain syndrome type 1 patients. Eur J Pain. 2010;14(3):302-307.
8. Krusz J, Cagle J, Hall S. Intramuscular (IM) ketamine for treating headache and pain flare-ups in the clinic. J Pain. 2008;9(4):30.
9. Bigal M Rapoport A, Sheftell F, Tepper D, Tepper S. Memantine in the preventive treatment of refractory migraine. Headache. 2008;48(9):1337-1342.
10. Ford RG, Ford KT. Continuous intravenous dihydroergotamine for treatment of intractable headache. Headache. 1997;37(3):129‐136.
11. Boudreau G, Aghai E, Marchand L, Langlois M. Outpatient intravenous dihydroergotamine for probable medication overuse headache. Headache Care. 2006;3(1):45‐49.
12. Pomeroy JL, Marmura MJ, Nahas SJ, Viscusi ER. Ketamine infusions for treatment refractory headache. Headache. 2017;57(2):276-282.
13. Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297(13):1443-1454.
14. Peterlin BL, Calhoun AH, Siegel S, Mathew NT. Rational combination therapy in refractory migraine. Headache. 2008;48(6):805-819.
15. Innes G, Macphail I, Dillon EC, Metcalfe C, Gao M. Dexamethasone prevents relapse after emergency department treatment of acute migraine: a randomized clinical trial. CJEM. 2015;1(1):26-33.
16. Irimia P, Palma JA, Fernandez-Torron R, Martinez-Vila E. Refractory migraine in a headache clinic population. BMC Neurol. 2011;11:94.
Dr. Charles is Clinical Associate Professor Neurology, Rutgers–New Jersey Medical School, Newark, NJ; Neurology Attending, Holy Name Medical Center, Teaneck, NJ ([email protected]).
Dr. Gallo is Interventional Radiology Attending, Holy Name Medical Center, Teaneck, NJ ([email protected]).
DISCLOSURES
The authors have no financial relationships to disclose relevant to the manuscript. There was no sponsorship of, or funding for, the study.
Dr. Charles designed and conceptualized the study; analyzed study data and performed the statistical analysis; and drafted the manuscript for intellectual content. Dr. Gallo had a major role in the acquisition of interventional sphenopalatine ganglion data.
ABSTRACT
Objective
Chronic refractory migraine patients who failed repetitive dihydroergotamine/dopamine infusion protocols and conventional preventives were treated with repeated low-dose ketamine-based parenteral protocols, followed by memantine-based preventive therapy, and observed for immediate reduction in pain intensity and headache frequency.
Methods
Ten patients were treated at an outpatient infusion center for 2 to 5 sequential days with AM and PM courses of intravenous diphenhydramine, prochlorperazine, and dihydroergotamine. A daily sphenopalatine ganglion block and low-dose intramuscular ketamine were given midday between treatments, with dexamethasone given on the last infusion day. The Numeric Pain Rating Scale was measured after infusion. Carryover effect was assessed 1 month and 2 months after infusion by headache frequency while being treated with memantine and various other preventive and abortive therapies.
Results
Reduction in headache pain of 71% was achieved at the end of the infusion period. Sedation was the only adverse effect. Decreased headache frequency persisted beyond the infusion period, with an 88.6% reduction in headache days per month at 1 month and a 79.4% reduction in headache days per month at 2 months, without adverse effects.
Conclusions
Data indicate that 1) repetitive low-dose, ketamine-based parenteral therapy, followed by memantine-based preventive therapy, reduced refractory headache pain and 2) the decremental effect on headache frequency persisted beyond the infusion period. Our results support the hypothesis that multimechanistic therapies might be better than single-modality treatment. More studies, with a larger patient population, are needed to confirm whether these multimodality ketamine/memantine therapies should become the preferred approach for these extremely disabled patients.
Chronic refractory migraine (CRM) degrades function and quality of life despite elimination of triggers and adequate trials of acute and preventive medicines that have established efficacy. This definition requires that patients with chronic migraine fail adequate trials of preventive drugs, alone or in combination, in at least 2 of 4 drug classes, including beta blockers, anticonvulsants, tricyclic antidepressants, onabotulinumtoxin A, and calcium-channel blockers. Patients must also fail adequate trials of abortive medicines, including both a triptan and dihydroergotamine (DHE), intranasal or injectable formulation, and either a nonsteroidal anti-inflammatory drug or a combination analgesic, unless contraindicated.1-4
In 1986, Raskin published a nonrandomized, nonblinded study of 2 treatments for intractable migraine in which repetitive inpatient intravenous (IV) DHE, administered in the hospital, was statistically more effective than IV diazepam in terminating cycles of intractable migraine.5 Most headache specialists have adopted the so-called Raskin protocol, as originally described or in any of several variations, as cornerstone therapy for CRM, chronic migraine, and prolonged status migrainosus.6 However, DHE-based infusion protocols do not always effectively reset the brain’s pain modulatory pathways in chronic migraine immediately posttreatment and might not induce a meaningful carryover effect.
We present 10 patients with CRM who met criteria for refractory migraine, including failure to terminate their headache with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols, with or without sporadic administration of a sphenopalatine ganglion block. We treated these patients multimechanistically with repetitive IV DHE, a dopamine antagonist, an antihistamine, sphenopalatine ganglion (SPG) block, and low-dose ketamine, plus last-infusion-day dexamethasone, followed by outpatient oral memantine. Subsequently, we observed them for 2 months.
Ketamine is a phencyclidine derivative introduced the early 1960s as an IV anesthetic. Low-dose ketamine has been used successfully in the treatment of chronic pain. Today, increased interest in the application of low-dose ketamine includes cancer pain; treatment and prevention of acute and chronic pain, with and without neuropathic analgesia; fibromyalgia; complex regional pain; and migraine.7,8 The effectiveness of ketamine in different pain disorders may arise through different pathways and/or by way of activity at various receptor systems. Effects arise predominantly by noncompetitive antagonism of the glutamate N-methyl-D-aspartate (NMDA ) receptor.7,8
Memantine also is an NMDA receptor antagonist that is used effectively as an oral agent in CRM.9
METHODS
Patients enrolled in this prospective study had CRM for periods ranging from 1 to 2 years. All had daily headache that could not be terminated with repetitive DHE/prochlorperazine/diphenhydramine/ketorolac/dexamethasone IV protocols with or without sporadic administration of an SPG block. Age ranged from 18 and 68 years; all patients were female. Patients were excluded if they had known coronary artery disease, uncontrolled hypertension, or peripheral arterial disease; a history of stroke, transient ischemic attack, or pregnancy; impaired liver or renal function; smoked a tobacco product; or were taking a protease inhibitor or macrolide antibiotic.
Approval by the institutional review board was unnecessary because all drugs and procedures are FDA-approved and have published evidence-based efficacy for migraine and other diseases.
The Numeric Pain Rating Scale (NPRS; a scale of 0 to 10) was utilized to rate the intensity of pain from the beginning of the infusion to the end of the multiday infusion protocol, when the catheter was removed. All patients but 1 were treated for 5 days; for the 1 exception, treatment was terminated after 48 hours because of a scheduling conflict. The observational follow‐up periods for assessment of outcomes were 1 month and 2 months post-infusion.
Patients started the study with a baseline NPRS of 9 or 10. They were treated at the institution’s headache outpatient infusion center. In the morning, patients received, by sequential IV infusion, diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 1 mg. They then received a midday SPG block under fluoroscopic guidance and ketamine, 0.45 mg/kg intramuscularly (IM), given in the post-anesthesia care unit. In the late afternoon, the patients received diphenhydramine, 50 mg; prochlorperazine, 10 mg; and DHE, 0.5 mg, in the Headache Outpatient Infusion Center. Patients were discharged to home by 6 PM. They received IV dexamethasone, 20 mg, on the last day of therapy.
Oral preventive agents were continued and abortives were temporarily discontinued during infusion therapy. Oral memantine was used immediately before, during, and, in all cases, after infusion, at a daily dosage that ranged from 10 mg BID to 28 mg, once-daily extended release.
RESULTS
Therapies were well-tolerated by all patients. On the last day of treatment, the entire cohort (N = 10) demonstrated an average of 71% (mean standard deviation [SD], 10.1%) reduction in pain intensity. The average reduction in headache days per month at 1 month was 88.6% (mean SD, 6.24%) and at 2 months was 79.4% (mean SD, 17.13%) (Table). Adverse effects were mild temporary sedation from ketamine. Pulse oximetry revealed no abnormal decrease in O2 saturation. All patients reported marked overall reduction in headache disability at the end of the infusion protocol. Self-administered abortive therapies posttreatment were more efficacious than they were pretreatment. All patients indicated less headache disability overall by the end of the 2-month observation period.
Table. Chronic Refractory Migraine Baseline Data and Treatment Resultsa
Name | Age (y) | Sex | Treatment Duration (days) | Baseline NPRS | Post-treatment NPRS | One Month Follow-upb | Two Month Follow-upb |
SL | 45 | F | 5 | 10 | 2 | 3 | 3 |
RR | 44 | F | 5 | 9 | 1 | 1 | 3 |
MP | 41 | F | 5 | 10 | 4 | 3 | 6 |
AP | 35 | F | 5 | 10 | 3 | 8 | 15 |
SW | 27 | F | 5 | 10 | 2 | 6 | 12 |
HC | 47 | F | 5 | 10 | 4 | 4 | 6 |
KK | 56 | F | 5 | 10 | 3 | 3 | 8 |
MG | 53 | F | 5 | 9 | 4 | 2 | 3 |
DM | 68 | F | 2 | 9 | 2 | 2 | 4 |
AO | 18 | F | 5 | 9 | 3 | 2 | 2 |
aAll patients had daily headache at initiation of treatment.
bHeadache days/month.
NPRS, Numeric Pain Rating Scale.
DISCUSSION
In our study of 10 patients with CRM who had daily headache treated repetitively in an outpatient infusion center with multimodality therapies, including sub-anesthetic doses of ketamine, all patients experienced marked reduction in headache pain intensity, with a whole-group average reduction of 71% by the end of infusion treatment. During post-infusion observation, all patients continued various preventive therapies, including memantine. At 1 month, the average reduction in headache frequency was 88.6%. Two months post-infusion, the average reduction in headache frequency was 79.4%. Adverse effects were minimal. Overall, the treatment was found to be safe and efficacious. All patients felt less headache disability after 2 months.
Because the protocol was administered comfortably in the Headache Outpatient Infusion Center, the inconvenience and higher cost of inpatient parenteral treatment were avoided. Ketamine, 0.45 mg/kg IM is a sub-anesthetic dose with proven efficacy in treating migraine without adverse effects in an outpatient setting.8 Low-dose ketamine obviated the need for anesthesia personnel and precautions. Temporary sedation was the only adverse effect. Ketamine was administered by a nurse in the post-anesthesia care unit while patients were under observation with conventional measurement of vital signs and pulse oximetry. Memantine, also an NMDA receptor antagonist, is postulated to prolong the NMDA antagonism of ketamine.
Inpatient and outpatient continuous IV DHE and repetitive IV DHE, often combined with dopamine antagonists in controlled and comparator studies, have demonstrated equal effectiveness for the treatment of chronic migraine.5,10,11 Our patients failed these therapies. This raises the question: Should our combined multimodality, ketamine-based approach be standard parenteral therapy for CRM?
In a recent study of continuous inpatient single-modality IV ketamine, a less-impressive carryover effect was obtained, with 23% to 50% 1-month sustained responders.12 Multimechanistic treatment superiority over monotherapy is legendary in the treatment of cancer and human immunodeficiency infection. Sumatriptan plus naproxen sodium as a single tablet for acute treatment of migraine resulted in more favorable clinical benefit compared with either monotherapy, with an acceptable, well-tolerated adverse effect profile. Because multiple pathogenic mechanisms putatively are involved in generation of the migraine symptom complex, multimechanism-targeted therapy may confer advantages over individual monotherapy. Drugs in 2 classes of migraine pharmacotherapy—triptans and nonsteroidal anti-inflammatory drugs —target distinct aspects of the vascular and inflammatory processes hypothesized to underlie migraine.13
Although combination therapy for CRM has not been systematically studied in randomized trials, clinical experience suggests that a rational approach to CRM treatment, utilizing a combination of treatments, may be effective when monotherapy has failed.14 During the infusion protocol, we re-set the trigeminovascular pain pathways 1) by repetitively blocking NMDA receptors (with ketamine), dopamine receptors (with prochlorperazine), and histamine receptors (with diphenhydramine); 2) by lidocaine anesthetic block of the sphenopalatine ganglia; and, on the last day of the protocol, 3) administering 1 large dose of IV dexamethasone to help prevent recurrence.15 NMDA blockade continued with oral outpatient memantine.
Virtually all patients were taking other preventives during the pretreatment period and 2-month observation period, including topiramate, venlafaxine, beta blockers, candesartan, zonisamide, onabotulinumtoxin A, neuromodulation (Cefaly Technology), and transcranial magnetic stimulation (springTMS®). Self-administered abortives were more effective in the 2-month observational period; these included IM/IV DHE; oral, spray, and subcutaneous triptans; IM ketorolac; diclofenac buffered solution; and transcranial magnetic stimulation (springTMS®). The cornerstone strategy of our treatment group that was a constant was the use of low-dose IM sub-anesthetic ketamine at a dosage of 0.45 mg/kg/d and the use of oral memantine during the follow-up observation period, at dosages ranging from 10 mg BID to 28 mg, once-daily extended release.
Limitations of this study design are:
- lack of a control group
- lack of subject randomization for comparative outcomes
- patients remaining on a variety of prophylactic regimens
- patients permitted to take any rescue therapy.
The effect of repetitive SPG block cannot be teased out of the efficacy data, but many of our patients had a poor or temporary response to infrequent sporadic SPG blocks prior to participating in our protocol.
Many migraineurs who seek care in a headache clinic are refractory to treatment, despite advances in headache therapy; refractory migraine was found in 5.1% of these patients.16 In this small series of patients, we demonstrated immediate relief and a significant 2-month carryover effect with our multimodality parenteral protocol. Larger, controlled studies are needed to further explore this protocol with repetitive DHE, diphenhydramine, prochlorperazine, SPG block, and low-dose IM ketamine, followed by outpatient memantine. Such studies would determine whether our protocol should be utilized as a primary treatment, instead of the conventional DHE-based Raskin and modified Raskin protocols.
Although this is a small series of patients, lack of adverse effects and impressive results should give credence to utilizing our protocol as treatment for this extremely debilitated, often desperate subset of headache patients. Data indicate that, whereas ketamine combined with other therapies immediately reduced refractory headache pain, the ameliorating effect of ketamine on CRM headache frequency and pain in our protocol persisted beyond the infusion period. This phenomenon indicates a disease-modulating role for ketamine in refractory migraine pain, possibly by means of desensitization of NMDA receptors in the trigeminal nucleus caudalis—desensitization that continued with the NMDA receptor antagonist memantine and/or restoration of inhibitory sensory control in the brain.
CONCLUSION
Our results support the hypothesis that multimechanistic therapies, including low-dose IM ketamine and memantine, might be better than single-modality treatment in this debilitated, refractory population. Future studies, with larger patient populations, are needed to confirm whether these multimodality ketamine/memantine-inclusive therapies should become the preferred approach for these extremely disabled patients.
REFERENCES
1. Goadsby PJ, Schoenen J, Ferrari MD, Silberstein SD, Dodick DW. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26(9):1168-1170.
2. Schulman EA, Lipton R, Peterlin BL, Levin M, Grosberg BM. Commentary from the Refractory Headache Special Interest Section on defining the pharmacologically intractable headache for clinical trials and clinical practice. Headache. 2010;50(10):1637-1639.
3. Martelletti P, Jensen RH, Antal A, et al. Neuromodulation of chronic headaches: position statement from the European Headache Federation. J Headache Pain. 2013;14:86.
4. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921-936.
5. Raskin NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36(7):995‐997.
6. Charles JA, von Dohln P. Outpatient home-based continuous intravenous dihydroergotamine therapy for intractable migraine. Headache. 2010;50(5):852-860.
7. Sigtermans M, Noppers I, Sarton E, et al. An observational study on the effect of S+-ketamine on chronic pain versus experimental acute pain in complex regional pain syndrome type 1 patients. Eur J Pain. 2010;14(3):302-307.
8. Krusz J, Cagle J, Hall S. Intramuscular (IM) ketamine for treating headache and pain flare-ups in the clinic. J Pain. 2008;9(4):30.
9. Bigal M Rapoport A, Sheftell F, Tepper D, Tepper S. Memantine in the preventive treatment of refractory migraine. Headache. 2008;48(9):1337-1342.
10. Ford RG, Ford KT. Continuous intravenous dihydroergotamine for treatment of intractable headache. Headache. 1997;37(3):129‐136.
11. Boudreau G, Aghai E, Marchand L, Langlois M. Outpatient intravenous dihydroergotamine for probable medication overuse headache. Headache Care. 2006;3(1):45‐49.
12. Pomeroy JL, Marmura MJ, Nahas SJ, Viscusi ER. Ketamine infusions for treatment refractory headache. Headache. 2017;57(2):276-282.
13. Brandes JL, Kudrow D, Stark SR, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA. 2007;297(13):1443-1454.
14. Peterlin BL, Calhoun AH, Siegel S, Mathew NT. Rational combination therapy in refractory migraine. Headache. 2008;48(6):805-819.
15. Innes G, Macphail I, Dillon EC, Metcalfe C, Gao M. Dexamethasone prevents relapse after emergency department treatment of acute migraine: a randomized clinical trial. CJEM. 2015;1(1):26-33.
16. Irimia P, Palma JA, Fernandez-Torron R, Martinez-Vila E. Refractory migraine in a headache clinic population. BMC Neurol. 2011;11:94.