User login
Diagnosing and treating bipolar disorder in primary care
Patients presenting with depression commonly have undiagnosed bipolar depression,1–7 that is, depression with shifts to periods of mania. During manic or hypomanic episodes, people feel energetic, need little sleep, and are often happy and charming.8 But too much of a good thing can also wreak havoc on their life.
Bipolar depression (ie, depression in patients with a diagnosis of bipolar disorder) is treated differently from unipolar depression,3,9–13 making it especially important that clinicians recognize if a patient who presents with depression has a history of (hypo)manic symptoms.
CASE 1: THE IMPULSIVE NURSE
A 32-year-old nurse presents to her primary care provider with depressed mood. She reports having had a single depressive episode when she was a college freshman. Her family history includes depression, bipolar disorder, and schizophrenia, and her paternal grandfather and a maternal aunt committed suicide. Upon questioning, she reveals that in the past, she has had 3 episodes lasting several weeks and characterized by insubordinate behavior at work, irritability, high energy, and decreased need for sleep. She regrets impulsive sexual and financial decisions that she made during these episodes and recently filed for personal bankruptcy. For the past month, her mood has been persistently low, with reduced sleep, appetite, energy, and concentration, and with passive thoughts of suicide.
A CAREFUL HISTORY IS CRITICAL
This case illustrates many typical features of bipolar depression that are revealed only by taking a thorough history. Although the patient is high-functioning, having attained a professional career, she has serious problems with sexual and financial impulsivity and at her job. She has a strong family history of mood disorder. And she describes episodes of depression and mania in the past.
Starts in young adulthood, strong heritability
Bipolar disorder can be a devastating condition with lifelong consequences,14–20 especially as it typically starts when patients are getting an education or embarking on a career. It usually first manifests in the late teenage years and progresses in the patient’s early 20s.21,22 The first hospitalization can occur soon thereafter.23,24
Bipolar disorder is one of the most heritable conditions in psychiatry, and about 13% of children who have an afflicted parent develop it.25 In identical twins, the concordance is about 50% to 75%, indicating the importance of genetics and environmental factors.26,27
Associated with migraine, other conditions
The disorder is associated with a variety of conditions (Table 1).28,29 Some conditions (eg, thyroid disease) can cause mood cycling, and some (eg, sexually transmitted infections, accidents) are the consequences of the lifestyle that may accompany mania. For unknown reasons, migraine is highly associated with bipolar disorder.
DEPRESSION AND MANIA: TWO SIDES OF THE SAME COIN
Symptoms of depression and mania are frequently viewed as opposite mood states, though many times patients report a mixture of them.17,30–35 For both states, the features of a distinct change from the patient’s normal condition and the sustained nature of the symptoms are important diagnostically and indicate a likely underlying biological cause.
Major depressive disorder: Slowing down
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),8 defines major depressive disorder as having either depressed mood or markedly diminished pleasure in most activities for most days during at least 2 weeks.
In addition, at least 4 of the following must be present during the same period:
- Appetite disturbance
- Sleep disturbance
- Motor retardation or agitation
- Lack of energy
- Feelings of worthlessness or excessive guilt
- Decreased concentration
- Recurrent thoughts of death or suicide.
An estimated 20% of the population experience a major depressive episode over their lifetime. A surprisingly high proportion of people with depression—30% to 40%—also have had subthreshold symptoms of mania (symptoms not meeting the criteria for hypomania or mania in terms of number of symptoms or duration).21,22 Because of these odds, it is important to suspect bipolar disorder even in patients who present with depression but who may not yet have manifested episodes of mania or hypomania.
Mood disorders can be regarded as falling into a spectrum, ranging from unipolar or “pure” major depression without any features of hypomania to major depression and severe mania.17,31–36
Mania: Speeding up
The DSM-5 defines mania as the presence of persistently elevated, expansive, or irritable mood with increased activity for more than 1 week. In addition, at least 3 of the following features must be present, with impaired functioning (4 features are required if mood is only irritable)8:
- Inflated self-esteem or grandiosity
- Decreased need for sleep
- Pressured speech
- Racing thoughts
- Distractibility
- Excessive involvement in pleasurable, high-risk activities.
Hypomania: No functional impairment
Hypomania is a less severe condition, in which the abnormally elevated mood is of shorter duration (4–7 days) and meets the other criteria for mania but without significant functional impairment. People may, in fact, be very functional and productive during hypomanic episodes.8
CLASSIFYING BIPOLAR DISORDER
Bipolar disorder is categorized according to severity.24,37,38 The most severe form, bipolar I disorder, is marked by major depression and manic episodes. It affects up to 1.5% of the US population, with equal proportions of men and women.39 Bipolar II disorder is less severe. It affects 0.8% to 1.6% of the US population, predominantly women.21,40 In bipolar II disorder, depression is more prominent, with episodes of hypomania.
Subthreshold bipolar disorders are characterized by episodic symptoms that do not meet the threshold for depression or hypomania; the symptoms are fewer or of shorter duration. These minor types of bipolar disorder affect up to 6% of the US population.17
Other conditions within the spectrum of bipolar and depressive disorders include medication- and substance-induced mania, agitated or anxious depression, and mixed states.31,34–36
DISTINGUISHING UNIPOLAR FROM BIPOLAR DEPRESSION
Considerable research has focused on finding a clear-cut clinical or biological feature to differentiate unipolar from bipolar depression, but so far none has been discovered. Distinguishing the two conditions still depends on clinical judgment. There are important reasons to identify the distinction between unipolar depression and bipolar disorder.
Prognosis differs. Bipolar disorder tends to be a more severe condition. Young people, who may initially present with only mild symptoms of mania, may develop serious episodes over the years. People may lose their savings, their marriage, and their career during a manic episode. The more critical the occupation (eg, doctor, pilot), the greater the potential consequences of impaired judgment brought on by even mild hypomania.14–20
Treatment differs. Typical antidepressants given for depression can trigger a manic episode in patients with bipolar depression, with devastating consequences. Atypical neuroleptic drugs used to treat bipolar disorder can also have serious effects (eg, metabolic and neurologic effects, including irreversible tardive dyskinesia).3,13,40–43
Despite the good reasons to do so, many doctors (including some psychiatrists) do not ask their patients about a propensity to mania or hypomania.4–6 More stigma is attached to the diagnosis of bipolar disorder than to depression44–47; once it is in the medical record, the patient may have problems with employment and obtaining medical insurance.17,44 The old term “manic-depressive” is often associated in the public mind with a person on the streets displaying severely psychotic behavior; the condition is now understood to consist of a spectrum from mild to more severe illness.
Clinical indicators of bipolarity
There are many indicators that a person who presents with depression may be on the bipolar spectrum, but this is not always easily identified.48–53
History of hypomanic symptoms or subthreshold manic symptoms. Although directly asking the patient about the defining symptoms (eg, “Have you ever had episodes of being ‘hyper’ or too happy?”) may help elicit the diagnosis, many patients with bipolar disorder only report depression, as it is psychically painful. In contrast, hypomania and even mania can be perceived as positive, as patients may have less insight into the abnormality of the condition and feel that they are functioning extremely well.
Early age of onset of a mood disorder, such as severe depression in childhood or early adulthood, points toward bipolar disorder. Diagnosing mood disorders in childhood is difficult, as children are less able to recognize or verbalize many of their symptoms.
Postpartum mood disorder, particularly with psychotic symptoms, indicates a strong possibility of a diagnosis of bipolar disorder.
Drug-induced mania, hypomania, and periods of hyperactivity are key features of bipolar disorder. If asked, patients may report feeling a “buzz” when taking an antidepressant.
Erratic patterns in work and relationships are a red flag and are viewed as “soft signs” of bipolar depression. Akiskal54 described the “rule of three” that should make one consider bipolar disorder: for example, three failed marriages, three current jobs or frequent job changes, three distinct professions practiced at the same time, and simultaneously dating three people. Such features indicate both the hyperfunctioning and the disruptive aspects of mania.
Family history of bipolar disorder or severe psychiatric illness is a very important clue. A more subtle clue described by Akiskal54 may be that several members of the family are very high-functioning in several different fields: eg, one may be a highly accomplished doctor, another a famous lawyer, and another a prominent politician. Or several members of the family may have erratic patterns of work and relationships. However, these subtle clues have been derived from clinical experiences and have not been validated in large-scale studies.
CASE 2: THE FRIENDLY SURGEON
Dr. Z is a prominent surgical subspecialist who is part of a small group practice. His wife has become increasingly worried about his behavior changes at home, including sleeping only a few hours a night, spending sprees, and binge drinking. He reluctantly agrees to an outpatient psychiatric evaluation if she attends with him. He creates a disturbance in the waiting room by shaking everyone’s hands and trying to hug all the women. During his examination, he is loud and expansive, denying he has any problems and describing himself as “the greatest doctor in the world.” The psychiatrist recommends hospitalization, but Dr. Z refuses and becomes belligerent. He announces that he just needs a career change and that he will fly to Mexico to open a bar.
This case, from the Texas Medical Association Archives,55 is not unusual. In addition to many characteristics discussed above, this case is typical in that the spouse brought the patient in, reflecting that the patient lacked insight that his behavior was abnormal. The disinhibition (hugging women), grandiosity, and unrealistic plans are also typical.
DIFFERENTIAL DIAGNOSIS OF BIPOLAR DEPRESSION
Anxiety disorders may be associated with dissociative speech or racing thoughts, which can be confused with bipolar illness. Personality disorders (eg, borderline, narcissistic, sociopathic) can involve a tumultuous and impulsive lifestyle resembling episodes of depression and mania. Schizoaffective illness has features of schizophrenia and bipolar disorder.
It is also possible that, despite what may look like mild features of bipolar disorder, there is no psychiatric condition. Some people with mild mania—often successful professionals or politicians—have high energy and can function very well with only a few hours of sleep. Similarly, depressive symptoms for short periods of time can be adaptive, such as in the face of a serious setback when extreme reflection and a period of inactivity can be useful, leading to subsequent reorganization.
A psychiatric diagnosis is usually made only when there is an abnormality, ie, the behavior is beyond normal limits, the person cannot control his or her symptoms, or social or occupational functioning is impaired.
SCREENING INSTRUMENTS
A few tools help determine the likelihood of bipolar disorder.
The Patient Health Questionnaire (PHQ-9)59,60 is a good 9-item screening tool for depression.
The Mood Disorder Questionnaire60 is specific for bipolar disorder, and like the PHQ-9, it is a patient-reported, short questionnaire that is available free online. The Mood Disorder Questionnaire asks about the symptoms of mania in a yes-no format. The result is positive if all of the following are present:
- A “yes” response to 7 of the 13 features
- Several features occur simultaneously
- The features are a moderate or serious problem.
Unlike most screening instruments, the Mood Disorder Questionnaire is more specific than sensitive. It is 93% specific for bipolar disorder in patients treated for depression in a primary care setting, but only 58% sensitive.61–63
WHEN TO REFER TO PSYCHIATRY
Patients suspected of having bipolar disorder or who have been previously diagnosed with it should be referred to a psychiatrist if they have certain features, including:
- Bipolar I disorder
- Psychotic symptoms
- Suicide risk or in danger of harming others
- Significantly impaired functioning
- Unclear diagnosis.
CASE 3: A TELEVISION ANCHOR’S DREAM TURNS TO NIGHTMARE
According to a famous news anchor’s autobiography,64 the steroids prescribed for her hives “revved her up.” The next course left her depressed. Antidepressant medications propelled her into a manic state, and she was soon planning a book, a television show, and a magazine all at once. During that time, she bought a cottage online. Her shyness evaporated at parties. “I was suddenly the equal of my high-energy friends who move fast and talk fast and loud,” she wrote. “I told everyone that I could understand why men felt like they could run the world, because I felt like that. This was a new me, and I liked her!”64 She was soon diagnosed with bipolar disorder and admitted to a psychiatric clinic.
TREAT WITH ANTIDEPRESSANTS, MOOD STABILIZERS
In general, acute bipolar disorder should be treated with a combination of an antidepressant and a mood stabilizer, and possibly an antipsychotic drug. An antidepressant should not be used alone, particularly with patients with a diagnosis of bipolar I disorder, because of the risk of triggering mania or the risk of faster cycling between mania and depression.13
Mood stabilizers include lithium, lamotrigine, and valproate. Each can prevent episodes of depression and mania. Lithium, which has been used as a mood stabilizer for 60 years, is specific for bipolar disorder, and it remains the best mood stabilizer treatment.
Antidepressants. The first-line antidepressant medication is bupropion, which is thought to be less likely to precipitate a manic episode,65 though all antidepressants have been associated with this side effect in patients with bipolar disorder. Other antidepressants—for example, selective serotonin reuptake inhibitors such as fluoxetine and dual reuptake inhibitors such as venlafaxine and duloxetine—can also be used. The precipitation of mania and possible increased mood cycling was first described with tricylic antidepressants, so drugs of this class should be used with caution.
Neuroleptic drugs such as aripiprazole, quetiapine, and lurasidone may be the easiest drugs to use, as they have antidepressant effects and can also prevent the occurrence of mania. These medications are frequently classified as mood stabilizers. However, they may not have true mood stabilizing properties such as that of lithium. Importantly, their use tends to entail significant metabolic problems and can lead to hyperlipidemia and diabetes. In addition, Parkinson disease-like symptoms— and in some cases irreversible involuntary movements of the mouth and tongue, as well as the body (tardive dyskinesia)—are important possible side effects.
All psychiatric medications have potential side effects (Table 3). Newer antidepressants and neuroleptics may have fewer side effects than older medications but are not more effective.
Should milder forms of bipolar depression be treated?
A dilemma is whether we should treat milder forms of bipolar depression, such as bipolar II depression, depression with subthreshold hypomania symptoms, or depression in persons with a strong family history of bipolar disorder.
Many doctors are justifiably reluctant to prescribe antidepressants for depression because of the risk of triggering mania. Although mood stabilizers such as lithium would counteract possible mania emergence, physicians often do not prescribe them because of inexperience and fear of risks and possible side effects. Patients are likewise resistant because they feel that use of mood stabilizers is tantamount to being told they are “manic-depressive,” with its associated stigma.
Overuse of atypical neuroleptics such as aripiprazole, quetiapine, and olanzapine has led to an awareness of metabolic syndrome and tardive dyskinesia, also making doctors cautious about using these drugs.
Answer: Yes, but treat with caution
Not treating depression consigns a patient to suffer with untreated depression, sometimes for years. Outcomes for patients with depression and bipolar disorder are often poor because the conditions are not recognized, and even when the conditions are recognized, doctors and patients may be reluctant to medicate appropriately. Medications should be used as needed to treat depression, but with an awareness of the possible side effects and with close patient monitoring.
A truly sustained manic state (unlike the brief euphoria brought on by some drugs) is not actually so easy to induce. In an unpublished Cleveland Clinic study, we monitored peaks of hypomanic symptoms in young patients (ages 15–30) during antidepressant treatment without mood stabilizers. About 30% to 40% of patients had subthreshold manic symptoms or a family history of bipolar disorder; 3 patients out of 51 developed hypomania leading to a change of diagnosis to bipolar disorder. Even in patients who had no risk factors for bipolar disorder, 2 out of 53 converted to a bipolar diagnosis. So conversion rates in patients with subthreshold bipolar disorder seem to be low, and the disorder can be identified early by monitoring the patient closely.
NONPHARMACOLOGIC TREATMENTS FOR DEPRESSION
Psychotherapy is indicated for all patients on medications for depression, as both pharmacologic and nonpharmacologic treatments are more effective when combined.66 Other treatments include transcranial magnetic stimulation, electroconvulsive therapy, light therapy, and exercise. Having a consistent daily routine, particularly regarding the sleep-wake schedule, is also helpful, and patients should be educated about its importance.
- Cicchetti D, Toth SL. The development of depression in children and adolescents. Am Psychol 1998; 53(2):221–241. doi:10.1037/0003-066X.53.2.221
- Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry 2004;6(1):27–33. pmid:15486598
- Ghaemi SN, Boiman EE, Goodwin FK. Diagnosing bipolar disorder and the effect of antidepressants: a naturalistic study. J Clin Psychiatry 2000; 61(10):804–808. pmid:11078046
- Singh T, Rajput M. Misdiagnosis of bipolar disorder. Psychiatry (Edgmont) 2006; 3(10):57–63. pmid: 20877548
- Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RM. The National Depressive and Manic-depressive Association (DMDA) survey of bipolar members. J Affect Disord 1994; 31(4):281–294. pmid:7989643
- Howes OD, Falkenberg I. Early detection and intervention in bipolar affective disorder: targeting the development of the disorder. Curr Psychiatry Rep 2011; 13(6):493–499. pmid:21850462
- Ghaemi SN, Sachs GS, Chiou AM, Pandurangi AK, Goodwin K. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord 1999; 52(1–3):135–144. pmid:10357026
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Publishing, 2013.
- Hlastala SA, Frank E, Mallinger AG, Thase ME, Ritenour AM, Kupfer DJ. Bipolar depression: an underestimated treatment challenge. Depress Anxiety 1997; 5(2):73–83. pmid:9262937
- Smith DJ, Craddock N. Unipolar and bipolar depression: different or the same? Br J Psychiatry 2011; 199(4):272–274. doi:10.1192/bjp.bp.111.092726
- Viktorin A, Lichtenstein P, Thase ME, et al. The risk of switch to mania in patients with bipolar disorder during treatment with an antidepressant alone and in combination with a mood stabilizer. Am J Psychiatry 2014; 171(10):1067–1073. doi:10.1176/appi.ajp.2014.13111501
- Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007; 356(17):1711–1722. doi:10.1056/NEJMoa064135
- American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002; 159(4 suppl):1–50. pmid:11958165
- Leonpacher AK, Liebers D, Pirooznia M, et al. Distinguishing bipolar from unipolar depression: the importance of clinical symptoms and illness features. Psychol Med 2015; 45(11):2437–2446. doi:10.1017/S0033291715000446
- Angst J, Gamma A, Benazzi F, Ajdacic V, Eich D, Rössler W. Toward a re-definition of subthreshold bipolarity: epidemiology and proposed criteria for bipolar-II, minor bipolar disorders and hypomania. J Affect Disord 2003; 73(1–2):133–146. pmid:12507746
- Faravelli C, Rosi S, Alessandra Scarpato M, Lampronti L, Amedei SG, Rana N. Threshold and subthreshold bipolar disorders in the Sesto Fiorentino Study. J Affect Disord 2006; 94(1–3):111–119. pmid:16701902
- Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord 2003; 73(1–2):123–131. pmid:12507745
- Park Y-M, Lee B-H. Treatment response in relation to subthreshold bipolarity in patients with major depressive disorder receiving antidepressant monotherapy: a post hoc data analysis (KOMDD study). Neuropsychiatr Dis Treat 2016; 12:1221–1227. doi:10.2147/NDT.S104188
- Perlis RH, Uher R, Ostacher M, Goldberg JF, et al. Association between bipolar spectrum features and treatment outcomes in outpatients with major depressive disorder. Arch Gen Psychiatry 2011; 68(4):351–360. doi:10.1001/archgenpsychiatry.2010.179
- Dudek D, Siwek M, Zielin´ska D, Jaeschke R, Rybakowski J. Diagnostic conversions from major depressive disorder into bipolar disorder in an outpatient setting: results of a retrospective chart review. J Affect Disord 2013; 144(1–2):112–115. doi:10.1016/j.jad.2012.06.014
- Angst J, Cui L, Swendsen J, et al. Major depressive disorder with subthreshold bipolarity in the National Comorbidity Survey Replication. Am J Psychiatry 2010; 167(10):1194–1201. doi:10.1176/appi.ajp.2010.09071011
- Zimmermann P, Brückl T, Nocon A, et al. Heterogeneity of DSM-IV major depressive disorder as a consequence of subthreshold bipolarity. Arch Gen Psychiatry 2009; 66(12):1341–1352. doi:10.1001/archgenpsychiatry.2009.158
- Patel NC, DelBello MP, Keck PE, Strakowski SM. Phenomenology associated with age at onset in patients with bipolar disorder at their first psychiatric hospitalization. Bipolar Disord 2006; 8(1):91–94. pmid:16411986
- Hirschfeld RMA, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the National Depressive and Manic-Depressive Association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry 2003; 64(2):161–174. pmid:12633125
- Craddock N, Jones I. Genetics of bipolar disorder. J Med Genet 1999; 36(8):585–594. pmid:10465107
- Griswold KS, Pessar LF. Management of bipolar disorder. Am Fam Physician 2000; 62(6):1343–1358. pmid:11011863
- Kerner B. Genetics of bipolar disorder. Appl Clin Genet 2014; 7:33–42. doi:10.2147/TACG.S39297
- Scheffer RE, Linden S. Concurrent medical conditions with pediatric bipolar disorder. Curr Opin Psychiatry 2007; 20(4):398–401. doi:10.1097/YCO.0b013e3281a305c3
- Carney CP, Jones LE. Medical comorbidity in women and men with bipolar disorders: a population-based controlled study. Psychosom Med 2006;68(5):684–691. doi:10.1097/01.psy.0000237316.09601.88
- Cassano GB, Akiskal HS, Savino M, Musetti L, Perugi G. Proposed subtypes of bipolar II and related disorders: with hypomanic episodes (or cyclothymia) and with hyperthymic temperament. J Affect Disord 1992; 26(2):127–140. pmid:1447430
- Akiskal HS, Mallya G. Criteria for the “soft” bipolar spectrum: treatment implications. Psychopharmacol Bull 1987; 23(1):68–73. pmid:3602332
- Fiedorowicz JG, Endicott J, Leon AC, Solomon DA, Keller MB, Coryell WH. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry 2011; 168(1):40–48. doi:10.1176/appi.ajp.2010.10030328
- Maj M, Pirozzi R, Magliano L, Fiorillo A, Bartoli L. Agitated “unipolar” major depression: prevalence, phenomenology, and outcome. J Clin Psychiatry 2006; 67(5):712–719. pmid:16841620
- Akiskal HS. The bipolar spectrum: new concepts in classification and diagnosis. In: Psychiatry Update: the American Psychiatric Association Annual Review. Washington, DC: American Psychiatric Press; 1983:271–292.
- Akiskal HS, Pinto O. The evolving bipolar spectrum. Prototypes I, II, III, and IV. Psychiatr Clin North Am 1999; 22(3):517–534. pmid:10550853
- Cassano GB, Savino M, Perugi G, Musetti L, Akiskal HS. Major depressive episode: unipolar and bipolar II. Encephale 1992 Jan;18 Spec No:15–18. pmid:1600898
- Müller-Oerlinghausen B, Berghöfer A, Bauer M. Bipolar disorder. Lancet 2002; 359(9302):241–247. pmid:11812578
- Hirschfeld RMA, Calabrese JR, Weissman MM, et al. Screening for bipolar disorder in the community. J Clin Psychiatry 2003; 64(1):53–59. pmid:12590624
- Blanco C, Compton WM, Saha TD, et al. Epidemiology of DSM-5 bipolar I disorder: results from the National Epidemiologic survey on Alcohol and Related Conditions—III. J Psychiatr Res 2017; 84:310–317. doi:10.1016/j.jpsychires.2016.10.003
- McGirr A, Vöhringer PA, Ghaemi SN, Lam RW, Yatham LN. Safety and efficacy of adjunctive second-generation antidepressant therapy with a mood stabiliser or an atypical antipsychotic in acute bipolar depression: a systematic review and meta-analysis of randomised placebo-controlled trials. Lancet Psychiatry 2016; 3(12):1138–1146. doi:10.1016/S2215-0366(16)30264-4
- Gijsman HJ, Geddes JR, Rendell JM, Nolen WA, Goodwin GM. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 2004; 161(9):1537–1547. doi:10.1176/appi.ajp.161.9.1537
- Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry 2011; 72(2):156–167. doi:10.4088/JCP.09r05385gre
- Liu B, Zhang Y, Fang H, Liu J, Liu T, Li L. Efficacy and safety of long-term antidepressant treatment for bipolar disorders - A meta-analysis of randomized controlled trials. J Affect Disord 2017; 223(139):41–48. doi:10.1016/j.jad.2017.07.023
- Krupa T, Kirsh B, Cockburn L, Gewurtz R. Understanding the stigma of mental illness in employment. Work 2009; 33(4):413–425. doi:10.3233/WOR-2009-0890
- Hawke LD, Parikh SV, Michalak EE. Stigma and bipolar disorder: a review of the literature. J Affect Disord 2013; 150(2):181–191. doi:10.1016/j.jad.2013.05.030
- Cerit C, Filizer A, Tural Ü, Tufan AE. Stigma: a core factor on predicting functionality in bipolar disorder. Compr Psychiatry 2012; 53(5):484–489. doi:10.1016/j.comppsych.2011.08.010
- O’Donnell L, Himle JA, Ryan K, et al. Social aspects of the workplace among individuals with bipolar disorder. J Soc Social Work Res 2017; 8(3):379–398. doi:10.1086/693163
- Akiskal HS, Maser JD, Zeller PJ, et al. Switching from “unipolar” to bipolar II. An 11-year prospective study of clinical and temperamental predictors in 559 patients. Arch Gen Psychiatry 1995; 52(2):114–123. pmid:7848047
- Kroon JS, Wohlfarth TD, Dieleman J, et al. Incidence rates and risk factors of bipolar disorder in the general population: a population-based cohort study. Bipolar Disord 2013; 15(3):306–313. doi:10.1111/bdi.12058
- Fiedorowicz JG, Endicott J, Leon AC, Solomon DA, Keller MB, Coryell WH. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry 2011; 168(1):40–48. doi:10.1176/appi.ajp.2010.10030328
- Akiskal HS, Walker P, Puzantian VR, King D, Rosenthal TL, Dranon M. Bipolar outcome in the course of depressive illness. Phenomenologic, familial, and pharmacologic predictors. J Affect Disord 1983; 5(2):115–128. pmid:6222091
- Strober M, Carlson G. Bipolar illness in adolescents with major depression: clinical, genetic, and psychopharmacologic predictors in a three- to four-year prospective follow-up investigation. Arch Gen Psychiatry 1982; 39(5):549–555. pmid:7092488
- Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry 2001; 158(8):1265–1270. pmid:11481161
- Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: the “red sign,” the “rule of three” and other biographic signs of temperamental extravagance, activation and hypomania. J Affect Disord 2005; 84(2–3):279–290. pmid:15708427
- Texas Medical Association. Mood disorders in physicians. www.texmed.org/Template.aspx?id=6833. Accessed June 7, 2018.
- Hirschfeld RM. Differential diagnosis of bipolar disorder and major depressive disorder. J Affect Disord 2014;169(suppl 1):S12–S16. doi:10.1016/S0165-0327(14)70004-7
- Dunner DL. Differential diagnosis of bipolar disorder. J Clin Psychopharmacol 1992; 12(1suppl):7S–12S. pmid:1541721
- Peet M, Peters S. Drug-induced mania. Drug Saf 1995; 12(2):146–153. pmid:7766338
- Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999; 282(18):1737–1744. pmid:10568646
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16(9):606–613. pmid:11556941
- Hirschfeld RMMA, Williams JBBW, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry 2000; 157(11)1873–1875. doi:10.1176/appi.ajp.157.11.1873
- Hirschfeld RMA. The Mood Disorder Questionnaire: a simple, patient-rated screening instrument for bipolar disorder. Prim Care Companion J Clin Psychiatry 2002; 4(1):9–11. pmid: 15014728
- Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA 2005; 293(8):956–963. doi:10.1001/jama.293.8.956
- Pauley J. Skywriting: A Life Out of the Blue. New York: Random House, 2004.
- Goren JL, Levin GM. Mania with bupropion: a dose-related phenomenon? Ann Pharmacother 2000; 34(5):619–621. doi:10.1345/aph.19313
- Swann AC. Long-term treatment in bipolar disorder. J Clin Psychiatry 2005; 66(suppl 1):7–12. pmid:15693746
Patients presenting with depression commonly have undiagnosed bipolar depression,1–7 that is, depression with shifts to periods of mania. During manic or hypomanic episodes, people feel energetic, need little sleep, and are often happy and charming.8 But too much of a good thing can also wreak havoc on their life.
Bipolar depression (ie, depression in patients with a diagnosis of bipolar disorder) is treated differently from unipolar depression,3,9–13 making it especially important that clinicians recognize if a patient who presents with depression has a history of (hypo)manic symptoms.
CASE 1: THE IMPULSIVE NURSE
A 32-year-old nurse presents to her primary care provider with depressed mood. She reports having had a single depressive episode when she was a college freshman. Her family history includes depression, bipolar disorder, and schizophrenia, and her paternal grandfather and a maternal aunt committed suicide. Upon questioning, she reveals that in the past, she has had 3 episodes lasting several weeks and characterized by insubordinate behavior at work, irritability, high energy, and decreased need for sleep. She regrets impulsive sexual and financial decisions that she made during these episodes and recently filed for personal bankruptcy. For the past month, her mood has been persistently low, with reduced sleep, appetite, energy, and concentration, and with passive thoughts of suicide.
A CAREFUL HISTORY IS CRITICAL
This case illustrates many typical features of bipolar depression that are revealed only by taking a thorough history. Although the patient is high-functioning, having attained a professional career, she has serious problems with sexual and financial impulsivity and at her job. She has a strong family history of mood disorder. And she describes episodes of depression and mania in the past.
Starts in young adulthood, strong heritability
Bipolar disorder can be a devastating condition with lifelong consequences,14–20 especially as it typically starts when patients are getting an education or embarking on a career. It usually first manifests in the late teenage years and progresses in the patient’s early 20s.21,22 The first hospitalization can occur soon thereafter.23,24
Bipolar disorder is one of the most heritable conditions in psychiatry, and about 13% of children who have an afflicted parent develop it.25 In identical twins, the concordance is about 50% to 75%, indicating the importance of genetics and environmental factors.26,27
Associated with migraine, other conditions
The disorder is associated with a variety of conditions (Table 1).28,29 Some conditions (eg, thyroid disease) can cause mood cycling, and some (eg, sexually transmitted infections, accidents) are the consequences of the lifestyle that may accompany mania. For unknown reasons, migraine is highly associated with bipolar disorder.
DEPRESSION AND MANIA: TWO SIDES OF THE SAME COIN
Symptoms of depression and mania are frequently viewed as opposite mood states, though many times patients report a mixture of them.17,30–35 For both states, the features of a distinct change from the patient’s normal condition and the sustained nature of the symptoms are important diagnostically and indicate a likely underlying biological cause.
Major depressive disorder: Slowing down
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),8 defines major depressive disorder as having either depressed mood or markedly diminished pleasure in most activities for most days during at least 2 weeks.
In addition, at least 4 of the following must be present during the same period:
- Appetite disturbance
- Sleep disturbance
- Motor retardation or agitation
- Lack of energy
- Feelings of worthlessness or excessive guilt
- Decreased concentration
- Recurrent thoughts of death or suicide.
An estimated 20% of the population experience a major depressive episode over their lifetime. A surprisingly high proportion of people with depression—30% to 40%—also have had subthreshold symptoms of mania (symptoms not meeting the criteria for hypomania or mania in terms of number of symptoms or duration).21,22 Because of these odds, it is important to suspect bipolar disorder even in patients who present with depression but who may not yet have manifested episodes of mania or hypomania.
Mood disorders can be regarded as falling into a spectrum, ranging from unipolar or “pure” major depression without any features of hypomania to major depression and severe mania.17,31–36
Mania: Speeding up
The DSM-5 defines mania as the presence of persistently elevated, expansive, or irritable mood with increased activity for more than 1 week. In addition, at least 3 of the following features must be present, with impaired functioning (4 features are required if mood is only irritable)8:
- Inflated self-esteem or grandiosity
- Decreased need for sleep
- Pressured speech
- Racing thoughts
- Distractibility
- Excessive involvement in pleasurable, high-risk activities.
Hypomania: No functional impairment
Hypomania is a less severe condition, in which the abnormally elevated mood is of shorter duration (4–7 days) and meets the other criteria for mania but without significant functional impairment. People may, in fact, be very functional and productive during hypomanic episodes.8
CLASSIFYING BIPOLAR DISORDER
Bipolar disorder is categorized according to severity.24,37,38 The most severe form, bipolar I disorder, is marked by major depression and manic episodes. It affects up to 1.5% of the US population, with equal proportions of men and women.39 Bipolar II disorder is less severe. It affects 0.8% to 1.6% of the US population, predominantly women.21,40 In bipolar II disorder, depression is more prominent, with episodes of hypomania.
Subthreshold bipolar disorders are characterized by episodic symptoms that do not meet the threshold for depression or hypomania; the symptoms are fewer or of shorter duration. These minor types of bipolar disorder affect up to 6% of the US population.17
Other conditions within the spectrum of bipolar and depressive disorders include medication- and substance-induced mania, agitated or anxious depression, and mixed states.31,34–36
DISTINGUISHING UNIPOLAR FROM BIPOLAR DEPRESSION
Considerable research has focused on finding a clear-cut clinical or biological feature to differentiate unipolar from bipolar depression, but so far none has been discovered. Distinguishing the two conditions still depends on clinical judgment. There are important reasons to identify the distinction between unipolar depression and bipolar disorder.
Prognosis differs. Bipolar disorder tends to be a more severe condition. Young people, who may initially present with only mild symptoms of mania, may develop serious episodes over the years. People may lose their savings, their marriage, and their career during a manic episode. The more critical the occupation (eg, doctor, pilot), the greater the potential consequences of impaired judgment brought on by even mild hypomania.14–20
Treatment differs. Typical antidepressants given for depression can trigger a manic episode in patients with bipolar depression, with devastating consequences. Atypical neuroleptic drugs used to treat bipolar disorder can also have serious effects (eg, metabolic and neurologic effects, including irreversible tardive dyskinesia).3,13,40–43
Despite the good reasons to do so, many doctors (including some psychiatrists) do not ask their patients about a propensity to mania or hypomania.4–6 More stigma is attached to the diagnosis of bipolar disorder than to depression44–47; once it is in the medical record, the patient may have problems with employment and obtaining medical insurance.17,44 The old term “manic-depressive” is often associated in the public mind with a person on the streets displaying severely psychotic behavior; the condition is now understood to consist of a spectrum from mild to more severe illness.
Clinical indicators of bipolarity
There are many indicators that a person who presents with depression may be on the bipolar spectrum, but this is not always easily identified.48–53
History of hypomanic symptoms or subthreshold manic symptoms. Although directly asking the patient about the defining symptoms (eg, “Have you ever had episodes of being ‘hyper’ or too happy?”) may help elicit the diagnosis, many patients with bipolar disorder only report depression, as it is psychically painful. In contrast, hypomania and even mania can be perceived as positive, as patients may have less insight into the abnormality of the condition and feel that they are functioning extremely well.
Early age of onset of a mood disorder, such as severe depression in childhood or early adulthood, points toward bipolar disorder. Diagnosing mood disorders in childhood is difficult, as children are less able to recognize or verbalize many of their symptoms.
Postpartum mood disorder, particularly with psychotic symptoms, indicates a strong possibility of a diagnosis of bipolar disorder.
Drug-induced mania, hypomania, and periods of hyperactivity are key features of bipolar disorder. If asked, patients may report feeling a “buzz” when taking an antidepressant.
Erratic patterns in work and relationships are a red flag and are viewed as “soft signs” of bipolar depression. Akiskal54 described the “rule of three” that should make one consider bipolar disorder: for example, three failed marriages, three current jobs or frequent job changes, three distinct professions practiced at the same time, and simultaneously dating three people. Such features indicate both the hyperfunctioning and the disruptive aspects of mania.
Family history of bipolar disorder or severe psychiatric illness is a very important clue. A more subtle clue described by Akiskal54 may be that several members of the family are very high-functioning in several different fields: eg, one may be a highly accomplished doctor, another a famous lawyer, and another a prominent politician. Or several members of the family may have erratic patterns of work and relationships. However, these subtle clues have been derived from clinical experiences and have not been validated in large-scale studies.
CASE 2: THE FRIENDLY SURGEON
Dr. Z is a prominent surgical subspecialist who is part of a small group practice. His wife has become increasingly worried about his behavior changes at home, including sleeping only a few hours a night, spending sprees, and binge drinking. He reluctantly agrees to an outpatient psychiatric evaluation if she attends with him. He creates a disturbance in the waiting room by shaking everyone’s hands and trying to hug all the women. During his examination, he is loud and expansive, denying he has any problems and describing himself as “the greatest doctor in the world.” The psychiatrist recommends hospitalization, but Dr. Z refuses and becomes belligerent. He announces that he just needs a career change and that he will fly to Mexico to open a bar.
This case, from the Texas Medical Association Archives,55 is not unusual. In addition to many characteristics discussed above, this case is typical in that the spouse brought the patient in, reflecting that the patient lacked insight that his behavior was abnormal. The disinhibition (hugging women), grandiosity, and unrealistic plans are also typical.
DIFFERENTIAL DIAGNOSIS OF BIPOLAR DEPRESSION
Anxiety disorders may be associated with dissociative speech or racing thoughts, which can be confused with bipolar illness. Personality disorders (eg, borderline, narcissistic, sociopathic) can involve a tumultuous and impulsive lifestyle resembling episodes of depression and mania. Schizoaffective illness has features of schizophrenia and bipolar disorder.
It is also possible that, despite what may look like mild features of bipolar disorder, there is no psychiatric condition. Some people with mild mania—often successful professionals or politicians—have high energy and can function very well with only a few hours of sleep. Similarly, depressive symptoms for short periods of time can be adaptive, such as in the face of a serious setback when extreme reflection and a period of inactivity can be useful, leading to subsequent reorganization.
A psychiatric diagnosis is usually made only when there is an abnormality, ie, the behavior is beyond normal limits, the person cannot control his or her symptoms, or social or occupational functioning is impaired.
SCREENING INSTRUMENTS
A few tools help determine the likelihood of bipolar disorder.
The Patient Health Questionnaire (PHQ-9)59,60 is a good 9-item screening tool for depression.
The Mood Disorder Questionnaire60 is specific for bipolar disorder, and like the PHQ-9, it is a patient-reported, short questionnaire that is available free online. The Mood Disorder Questionnaire asks about the symptoms of mania in a yes-no format. The result is positive if all of the following are present:
- A “yes” response to 7 of the 13 features
- Several features occur simultaneously
- The features are a moderate or serious problem.
Unlike most screening instruments, the Mood Disorder Questionnaire is more specific than sensitive. It is 93% specific for bipolar disorder in patients treated for depression in a primary care setting, but only 58% sensitive.61–63
WHEN TO REFER TO PSYCHIATRY
Patients suspected of having bipolar disorder or who have been previously diagnosed with it should be referred to a psychiatrist if they have certain features, including:
- Bipolar I disorder
- Psychotic symptoms
- Suicide risk or in danger of harming others
- Significantly impaired functioning
- Unclear diagnosis.
CASE 3: A TELEVISION ANCHOR’S DREAM TURNS TO NIGHTMARE
According to a famous news anchor’s autobiography,64 the steroids prescribed for her hives “revved her up.” The next course left her depressed. Antidepressant medications propelled her into a manic state, and she was soon planning a book, a television show, and a magazine all at once. During that time, she bought a cottage online. Her shyness evaporated at parties. “I was suddenly the equal of my high-energy friends who move fast and talk fast and loud,” she wrote. “I told everyone that I could understand why men felt like they could run the world, because I felt like that. This was a new me, and I liked her!”64 She was soon diagnosed with bipolar disorder and admitted to a psychiatric clinic.
TREAT WITH ANTIDEPRESSANTS, MOOD STABILIZERS
In general, acute bipolar disorder should be treated with a combination of an antidepressant and a mood stabilizer, and possibly an antipsychotic drug. An antidepressant should not be used alone, particularly with patients with a diagnosis of bipolar I disorder, because of the risk of triggering mania or the risk of faster cycling between mania and depression.13
Mood stabilizers include lithium, lamotrigine, and valproate. Each can prevent episodes of depression and mania. Lithium, which has been used as a mood stabilizer for 60 years, is specific for bipolar disorder, and it remains the best mood stabilizer treatment.
Antidepressants. The first-line antidepressant medication is bupropion, which is thought to be less likely to precipitate a manic episode,65 though all antidepressants have been associated with this side effect in patients with bipolar disorder. Other antidepressants—for example, selective serotonin reuptake inhibitors such as fluoxetine and dual reuptake inhibitors such as venlafaxine and duloxetine—can also be used. The precipitation of mania and possible increased mood cycling was first described with tricylic antidepressants, so drugs of this class should be used with caution.
Neuroleptic drugs such as aripiprazole, quetiapine, and lurasidone may be the easiest drugs to use, as they have antidepressant effects and can also prevent the occurrence of mania. These medications are frequently classified as mood stabilizers. However, they may not have true mood stabilizing properties such as that of lithium. Importantly, their use tends to entail significant metabolic problems and can lead to hyperlipidemia and diabetes. In addition, Parkinson disease-like symptoms— and in some cases irreversible involuntary movements of the mouth and tongue, as well as the body (tardive dyskinesia)—are important possible side effects.
All psychiatric medications have potential side effects (Table 3). Newer antidepressants and neuroleptics may have fewer side effects than older medications but are not more effective.
Should milder forms of bipolar depression be treated?
A dilemma is whether we should treat milder forms of bipolar depression, such as bipolar II depression, depression with subthreshold hypomania symptoms, or depression in persons with a strong family history of bipolar disorder.
Many doctors are justifiably reluctant to prescribe antidepressants for depression because of the risk of triggering mania. Although mood stabilizers such as lithium would counteract possible mania emergence, physicians often do not prescribe them because of inexperience and fear of risks and possible side effects. Patients are likewise resistant because they feel that use of mood stabilizers is tantamount to being told they are “manic-depressive,” with its associated stigma.
Overuse of atypical neuroleptics such as aripiprazole, quetiapine, and olanzapine has led to an awareness of metabolic syndrome and tardive dyskinesia, also making doctors cautious about using these drugs.
Answer: Yes, but treat with caution
Not treating depression consigns a patient to suffer with untreated depression, sometimes for years. Outcomes for patients with depression and bipolar disorder are often poor because the conditions are not recognized, and even when the conditions are recognized, doctors and patients may be reluctant to medicate appropriately. Medications should be used as needed to treat depression, but with an awareness of the possible side effects and with close patient monitoring.
A truly sustained manic state (unlike the brief euphoria brought on by some drugs) is not actually so easy to induce. In an unpublished Cleveland Clinic study, we monitored peaks of hypomanic symptoms in young patients (ages 15–30) during antidepressant treatment without mood stabilizers. About 30% to 40% of patients had subthreshold manic symptoms or a family history of bipolar disorder; 3 patients out of 51 developed hypomania leading to a change of diagnosis to bipolar disorder. Even in patients who had no risk factors for bipolar disorder, 2 out of 53 converted to a bipolar diagnosis. So conversion rates in patients with subthreshold bipolar disorder seem to be low, and the disorder can be identified early by monitoring the patient closely.
NONPHARMACOLOGIC TREATMENTS FOR DEPRESSION
Psychotherapy is indicated for all patients on medications for depression, as both pharmacologic and nonpharmacologic treatments are more effective when combined.66 Other treatments include transcranial magnetic stimulation, electroconvulsive therapy, light therapy, and exercise. Having a consistent daily routine, particularly regarding the sleep-wake schedule, is also helpful, and patients should be educated about its importance.
Patients presenting with depression commonly have undiagnosed bipolar depression,1–7 that is, depression with shifts to periods of mania. During manic or hypomanic episodes, people feel energetic, need little sleep, and are often happy and charming.8 But too much of a good thing can also wreak havoc on their life.
Bipolar depression (ie, depression in patients with a diagnosis of bipolar disorder) is treated differently from unipolar depression,3,9–13 making it especially important that clinicians recognize if a patient who presents with depression has a history of (hypo)manic symptoms.
CASE 1: THE IMPULSIVE NURSE
A 32-year-old nurse presents to her primary care provider with depressed mood. She reports having had a single depressive episode when she was a college freshman. Her family history includes depression, bipolar disorder, and schizophrenia, and her paternal grandfather and a maternal aunt committed suicide. Upon questioning, she reveals that in the past, she has had 3 episodes lasting several weeks and characterized by insubordinate behavior at work, irritability, high energy, and decreased need for sleep. She regrets impulsive sexual and financial decisions that she made during these episodes and recently filed for personal bankruptcy. For the past month, her mood has been persistently low, with reduced sleep, appetite, energy, and concentration, and with passive thoughts of suicide.
A CAREFUL HISTORY IS CRITICAL
This case illustrates many typical features of bipolar depression that are revealed only by taking a thorough history. Although the patient is high-functioning, having attained a professional career, she has serious problems with sexual and financial impulsivity and at her job. She has a strong family history of mood disorder. And she describes episodes of depression and mania in the past.
Starts in young adulthood, strong heritability
Bipolar disorder can be a devastating condition with lifelong consequences,14–20 especially as it typically starts when patients are getting an education or embarking on a career. It usually first manifests in the late teenage years and progresses in the patient’s early 20s.21,22 The first hospitalization can occur soon thereafter.23,24
Bipolar disorder is one of the most heritable conditions in psychiatry, and about 13% of children who have an afflicted parent develop it.25 In identical twins, the concordance is about 50% to 75%, indicating the importance of genetics and environmental factors.26,27
Associated with migraine, other conditions
The disorder is associated with a variety of conditions (Table 1).28,29 Some conditions (eg, thyroid disease) can cause mood cycling, and some (eg, sexually transmitted infections, accidents) are the consequences of the lifestyle that may accompany mania. For unknown reasons, migraine is highly associated with bipolar disorder.
DEPRESSION AND MANIA: TWO SIDES OF THE SAME COIN
Symptoms of depression and mania are frequently viewed as opposite mood states, though many times patients report a mixture of them.17,30–35 For both states, the features of a distinct change from the patient’s normal condition and the sustained nature of the symptoms are important diagnostically and indicate a likely underlying biological cause.
Major depressive disorder: Slowing down
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),8 defines major depressive disorder as having either depressed mood or markedly diminished pleasure in most activities for most days during at least 2 weeks.
In addition, at least 4 of the following must be present during the same period:
- Appetite disturbance
- Sleep disturbance
- Motor retardation or agitation
- Lack of energy
- Feelings of worthlessness or excessive guilt
- Decreased concentration
- Recurrent thoughts of death or suicide.
An estimated 20% of the population experience a major depressive episode over their lifetime. A surprisingly high proportion of people with depression—30% to 40%—also have had subthreshold symptoms of mania (symptoms not meeting the criteria for hypomania or mania in terms of number of symptoms or duration).21,22 Because of these odds, it is important to suspect bipolar disorder even in patients who present with depression but who may not yet have manifested episodes of mania or hypomania.
Mood disorders can be regarded as falling into a spectrum, ranging from unipolar or “pure” major depression without any features of hypomania to major depression and severe mania.17,31–36
Mania: Speeding up
The DSM-5 defines mania as the presence of persistently elevated, expansive, or irritable mood with increased activity for more than 1 week. In addition, at least 3 of the following features must be present, with impaired functioning (4 features are required if mood is only irritable)8:
- Inflated self-esteem or grandiosity
- Decreased need for sleep
- Pressured speech
- Racing thoughts
- Distractibility
- Excessive involvement in pleasurable, high-risk activities.
Hypomania: No functional impairment
Hypomania is a less severe condition, in which the abnormally elevated mood is of shorter duration (4–7 days) and meets the other criteria for mania but without significant functional impairment. People may, in fact, be very functional and productive during hypomanic episodes.8
CLASSIFYING BIPOLAR DISORDER
Bipolar disorder is categorized according to severity.24,37,38 The most severe form, bipolar I disorder, is marked by major depression and manic episodes. It affects up to 1.5% of the US population, with equal proportions of men and women.39 Bipolar II disorder is less severe. It affects 0.8% to 1.6% of the US population, predominantly women.21,40 In bipolar II disorder, depression is more prominent, with episodes of hypomania.
Subthreshold bipolar disorders are characterized by episodic symptoms that do not meet the threshold for depression or hypomania; the symptoms are fewer or of shorter duration. These minor types of bipolar disorder affect up to 6% of the US population.17
Other conditions within the spectrum of bipolar and depressive disorders include medication- and substance-induced mania, agitated or anxious depression, and mixed states.31,34–36
DISTINGUISHING UNIPOLAR FROM BIPOLAR DEPRESSION
Considerable research has focused on finding a clear-cut clinical or biological feature to differentiate unipolar from bipolar depression, but so far none has been discovered. Distinguishing the two conditions still depends on clinical judgment. There are important reasons to identify the distinction between unipolar depression and bipolar disorder.
Prognosis differs. Bipolar disorder tends to be a more severe condition. Young people, who may initially present with only mild symptoms of mania, may develop serious episodes over the years. People may lose their savings, their marriage, and their career during a manic episode. The more critical the occupation (eg, doctor, pilot), the greater the potential consequences of impaired judgment brought on by even mild hypomania.14–20
Treatment differs. Typical antidepressants given for depression can trigger a manic episode in patients with bipolar depression, with devastating consequences. Atypical neuroleptic drugs used to treat bipolar disorder can also have serious effects (eg, metabolic and neurologic effects, including irreversible tardive dyskinesia).3,13,40–43
Despite the good reasons to do so, many doctors (including some psychiatrists) do not ask their patients about a propensity to mania or hypomania.4–6 More stigma is attached to the diagnosis of bipolar disorder than to depression44–47; once it is in the medical record, the patient may have problems with employment and obtaining medical insurance.17,44 The old term “manic-depressive” is often associated in the public mind with a person on the streets displaying severely psychotic behavior; the condition is now understood to consist of a spectrum from mild to more severe illness.
Clinical indicators of bipolarity
There are many indicators that a person who presents with depression may be on the bipolar spectrum, but this is not always easily identified.48–53
History of hypomanic symptoms or subthreshold manic symptoms. Although directly asking the patient about the defining symptoms (eg, “Have you ever had episodes of being ‘hyper’ or too happy?”) may help elicit the diagnosis, many patients with bipolar disorder only report depression, as it is psychically painful. In contrast, hypomania and even mania can be perceived as positive, as patients may have less insight into the abnormality of the condition and feel that they are functioning extremely well.
Early age of onset of a mood disorder, such as severe depression in childhood or early adulthood, points toward bipolar disorder. Diagnosing mood disorders in childhood is difficult, as children are less able to recognize or verbalize many of their symptoms.
Postpartum mood disorder, particularly with psychotic symptoms, indicates a strong possibility of a diagnosis of bipolar disorder.
Drug-induced mania, hypomania, and periods of hyperactivity are key features of bipolar disorder. If asked, patients may report feeling a “buzz” when taking an antidepressant.
Erratic patterns in work and relationships are a red flag and are viewed as “soft signs” of bipolar depression. Akiskal54 described the “rule of three” that should make one consider bipolar disorder: for example, three failed marriages, three current jobs or frequent job changes, three distinct professions practiced at the same time, and simultaneously dating three people. Such features indicate both the hyperfunctioning and the disruptive aspects of mania.
Family history of bipolar disorder or severe psychiatric illness is a very important clue. A more subtle clue described by Akiskal54 may be that several members of the family are very high-functioning in several different fields: eg, one may be a highly accomplished doctor, another a famous lawyer, and another a prominent politician. Or several members of the family may have erratic patterns of work and relationships. However, these subtle clues have been derived from clinical experiences and have not been validated in large-scale studies.
CASE 2: THE FRIENDLY SURGEON
Dr. Z is a prominent surgical subspecialist who is part of a small group practice. His wife has become increasingly worried about his behavior changes at home, including sleeping only a few hours a night, spending sprees, and binge drinking. He reluctantly agrees to an outpatient psychiatric evaluation if she attends with him. He creates a disturbance in the waiting room by shaking everyone’s hands and trying to hug all the women. During his examination, he is loud and expansive, denying he has any problems and describing himself as “the greatest doctor in the world.” The psychiatrist recommends hospitalization, but Dr. Z refuses and becomes belligerent. He announces that he just needs a career change and that he will fly to Mexico to open a bar.
This case, from the Texas Medical Association Archives,55 is not unusual. In addition to many characteristics discussed above, this case is typical in that the spouse brought the patient in, reflecting that the patient lacked insight that his behavior was abnormal. The disinhibition (hugging women), grandiosity, and unrealistic plans are also typical.
DIFFERENTIAL DIAGNOSIS OF BIPOLAR DEPRESSION
Anxiety disorders may be associated with dissociative speech or racing thoughts, which can be confused with bipolar illness. Personality disorders (eg, borderline, narcissistic, sociopathic) can involve a tumultuous and impulsive lifestyle resembling episodes of depression and mania. Schizoaffective illness has features of schizophrenia and bipolar disorder.
It is also possible that, despite what may look like mild features of bipolar disorder, there is no psychiatric condition. Some people with mild mania—often successful professionals or politicians—have high energy and can function very well with only a few hours of sleep. Similarly, depressive symptoms for short periods of time can be adaptive, such as in the face of a serious setback when extreme reflection and a period of inactivity can be useful, leading to subsequent reorganization.
A psychiatric diagnosis is usually made only when there is an abnormality, ie, the behavior is beyond normal limits, the person cannot control his or her symptoms, or social or occupational functioning is impaired.
SCREENING INSTRUMENTS
A few tools help determine the likelihood of bipolar disorder.
The Patient Health Questionnaire (PHQ-9)59,60 is a good 9-item screening tool for depression.
The Mood Disorder Questionnaire60 is specific for bipolar disorder, and like the PHQ-9, it is a patient-reported, short questionnaire that is available free online. The Mood Disorder Questionnaire asks about the symptoms of mania in a yes-no format. The result is positive if all of the following are present:
- A “yes” response to 7 of the 13 features
- Several features occur simultaneously
- The features are a moderate or serious problem.
Unlike most screening instruments, the Mood Disorder Questionnaire is more specific than sensitive. It is 93% specific for bipolar disorder in patients treated for depression in a primary care setting, but only 58% sensitive.61–63
WHEN TO REFER TO PSYCHIATRY
Patients suspected of having bipolar disorder or who have been previously diagnosed with it should be referred to a psychiatrist if they have certain features, including:
- Bipolar I disorder
- Psychotic symptoms
- Suicide risk or in danger of harming others
- Significantly impaired functioning
- Unclear diagnosis.
CASE 3: A TELEVISION ANCHOR’S DREAM TURNS TO NIGHTMARE
According to a famous news anchor’s autobiography,64 the steroids prescribed for her hives “revved her up.” The next course left her depressed. Antidepressant medications propelled her into a manic state, and she was soon planning a book, a television show, and a magazine all at once. During that time, she bought a cottage online. Her shyness evaporated at parties. “I was suddenly the equal of my high-energy friends who move fast and talk fast and loud,” she wrote. “I told everyone that I could understand why men felt like they could run the world, because I felt like that. This was a new me, and I liked her!”64 She was soon diagnosed with bipolar disorder and admitted to a psychiatric clinic.
TREAT WITH ANTIDEPRESSANTS, MOOD STABILIZERS
In general, acute bipolar disorder should be treated with a combination of an antidepressant and a mood stabilizer, and possibly an antipsychotic drug. An antidepressant should not be used alone, particularly with patients with a diagnosis of bipolar I disorder, because of the risk of triggering mania or the risk of faster cycling between mania and depression.13
Mood stabilizers include lithium, lamotrigine, and valproate. Each can prevent episodes of depression and mania. Lithium, which has been used as a mood stabilizer for 60 years, is specific for bipolar disorder, and it remains the best mood stabilizer treatment.
Antidepressants. The first-line antidepressant medication is bupropion, which is thought to be less likely to precipitate a manic episode,65 though all antidepressants have been associated with this side effect in patients with bipolar disorder. Other antidepressants—for example, selective serotonin reuptake inhibitors such as fluoxetine and dual reuptake inhibitors such as venlafaxine and duloxetine—can also be used. The precipitation of mania and possible increased mood cycling was first described with tricylic antidepressants, so drugs of this class should be used with caution.
Neuroleptic drugs such as aripiprazole, quetiapine, and lurasidone may be the easiest drugs to use, as they have antidepressant effects and can also prevent the occurrence of mania. These medications are frequently classified as mood stabilizers. However, they may not have true mood stabilizing properties such as that of lithium. Importantly, their use tends to entail significant metabolic problems and can lead to hyperlipidemia and diabetes. In addition, Parkinson disease-like symptoms— and in some cases irreversible involuntary movements of the mouth and tongue, as well as the body (tardive dyskinesia)—are important possible side effects.
All psychiatric medications have potential side effects (Table 3). Newer antidepressants and neuroleptics may have fewer side effects than older medications but are not more effective.
Should milder forms of bipolar depression be treated?
A dilemma is whether we should treat milder forms of bipolar depression, such as bipolar II depression, depression with subthreshold hypomania symptoms, or depression in persons with a strong family history of bipolar disorder.
Many doctors are justifiably reluctant to prescribe antidepressants for depression because of the risk of triggering mania. Although mood stabilizers such as lithium would counteract possible mania emergence, physicians often do not prescribe them because of inexperience and fear of risks and possible side effects. Patients are likewise resistant because they feel that use of mood stabilizers is tantamount to being told they are “manic-depressive,” with its associated stigma.
Overuse of atypical neuroleptics such as aripiprazole, quetiapine, and olanzapine has led to an awareness of metabolic syndrome and tardive dyskinesia, also making doctors cautious about using these drugs.
Answer: Yes, but treat with caution
Not treating depression consigns a patient to suffer with untreated depression, sometimes for years. Outcomes for patients with depression and bipolar disorder are often poor because the conditions are not recognized, and even when the conditions are recognized, doctors and patients may be reluctant to medicate appropriately. Medications should be used as needed to treat depression, but with an awareness of the possible side effects and with close patient monitoring.
A truly sustained manic state (unlike the brief euphoria brought on by some drugs) is not actually so easy to induce. In an unpublished Cleveland Clinic study, we monitored peaks of hypomanic symptoms in young patients (ages 15–30) during antidepressant treatment without mood stabilizers. About 30% to 40% of patients had subthreshold manic symptoms or a family history of bipolar disorder; 3 patients out of 51 developed hypomania leading to a change of diagnosis to bipolar disorder. Even in patients who had no risk factors for bipolar disorder, 2 out of 53 converted to a bipolar diagnosis. So conversion rates in patients with subthreshold bipolar disorder seem to be low, and the disorder can be identified early by monitoring the patient closely.
NONPHARMACOLOGIC TREATMENTS FOR DEPRESSION
Psychotherapy is indicated for all patients on medications for depression, as both pharmacologic and nonpharmacologic treatments are more effective when combined.66 Other treatments include transcranial magnetic stimulation, electroconvulsive therapy, light therapy, and exercise. Having a consistent daily routine, particularly regarding the sleep-wake schedule, is also helpful, and patients should be educated about its importance.
- Cicchetti D, Toth SL. The development of depression in children and adolescents. Am Psychol 1998; 53(2):221–241. doi:10.1037/0003-066X.53.2.221
- Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry 2004;6(1):27–33. pmid:15486598
- Ghaemi SN, Boiman EE, Goodwin FK. Diagnosing bipolar disorder and the effect of antidepressants: a naturalistic study. J Clin Psychiatry 2000; 61(10):804–808. pmid:11078046
- Singh T, Rajput M. Misdiagnosis of bipolar disorder. Psychiatry (Edgmont) 2006; 3(10):57–63. pmid: 20877548
- Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RM. The National Depressive and Manic-depressive Association (DMDA) survey of bipolar members. J Affect Disord 1994; 31(4):281–294. pmid:7989643
- Howes OD, Falkenberg I. Early detection and intervention in bipolar affective disorder: targeting the development of the disorder. Curr Psychiatry Rep 2011; 13(6):493–499. pmid:21850462
- Ghaemi SN, Sachs GS, Chiou AM, Pandurangi AK, Goodwin K. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord 1999; 52(1–3):135–144. pmid:10357026
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Publishing, 2013.
- Hlastala SA, Frank E, Mallinger AG, Thase ME, Ritenour AM, Kupfer DJ. Bipolar depression: an underestimated treatment challenge. Depress Anxiety 1997; 5(2):73–83. pmid:9262937
- Smith DJ, Craddock N. Unipolar and bipolar depression: different or the same? Br J Psychiatry 2011; 199(4):272–274. doi:10.1192/bjp.bp.111.092726
- Viktorin A, Lichtenstein P, Thase ME, et al. The risk of switch to mania in patients with bipolar disorder during treatment with an antidepressant alone and in combination with a mood stabilizer. Am J Psychiatry 2014; 171(10):1067–1073. doi:10.1176/appi.ajp.2014.13111501
- Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007; 356(17):1711–1722. doi:10.1056/NEJMoa064135
- American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002; 159(4 suppl):1–50. pmid:11958165
- Leonpacher AK, Liebers D, Pirooznia M, et al. Distinguishing bipolar from unipolar depression: the importance of clinical symptoms and illness features. Psychol Med 2015; 45(11):2437–2446. doi:10.1017/S0033291715000446
- Angst J, Gamma A, Benazzi F, Ajdacic V, Eich D, Rössler W. Toward a re-definition of subthreshold bipolarity: epidemiology and proposed criteria for bipolar-II, minor bipolar disorders and hypomania. J Affect Disord 2003; 73(1–2):133–146. pmid:12507746
- Faravelli C, Rosi S, Alessandra Scarpato M, Lampronti L, Amedei SG, Rana N. Threshold and subthreshold bipolar disorders in the Sesto Fiorentino Study. J Affect Disord 2006; 94(1–3):111–119. pmid:16701902
- Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord 2003; 73(1–2):123–131. pmid:12507745
- Park Y-M, Lee B-H. Treatment response in relation to subthreshold bipolarity in patients with major depressive disorder receiving antidepressant monotherapy: a post hoc data analysis (KOMDD study). Neuropsychiatr Dis Treat 2016; 12:1221–1227. doi:10.2147/NDT.S104188
- Perlis RH, Uher R, Ostacher M, Goldberg JF, et al. Association between bipolar spectrum features and treatment outcomes in outpatients with major depressive disorder. Arch Gen Psychiatry 2011; 68(4):351–360. doi:10.1001/archgenpsychiatry.2010.179
- Dudek D, Siwek M, Zielin´ska D, Jaeschke R, Rybakowski J. Diagnostic conversions from major depressive disorder into bipolar disorder in an outpatient setting: results of a retrospective chart review. J Affect Disord 2013; 144(1–2):112–115. doi:10.1016/j.jad.2012.06.014
- Angst J, Cui L, Swendsen J, et al. Major depressive disorder with subthreshold bipolarity in the National Comorbidity Survey Replication. Am J Psychiatry 2010; 167(10):1194–1201. doi:10.1176/appi.ajp.2010.09071011
- Zimmermann P, Brückl T, Nocon A, et al. Heterogeneity of DSM-IV major depressive disorder as a consequence of subthreshold bipolarity. Arch Gen Psychiatry 2009; 66(12):1341–1352. doi:10.1001/archgenpsychiatry.2009.158
- Patel NC, DelBello MP, Keck PE, Strakowski SM. Phenomenology associated with age at onset in patients with bipolar disorder at their first psychiatric hospitalization. Bipolar Disord 2006; 8(1):91–94. pmid:16411986
- Hirschfeld RMA, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the National Depressive and Manic-Depressive Association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry 2003; 64(2):161–174. pmid:12633125
- Craddock N, Jones I. Genetics of bipolar disorder. J Med Genet 1999; 36(8):585–594. pmid:10465107
- Griswold KS, Pessar LF. Management of bipolar disorder. Am Fam Physician 2000; 62(6):1343–1358. pmid:11011863
- Kerner B. Genetics of bipolar disorder. Appl Clin Genet 2014; 7:33–42. doi:10.2147/TACG.S39297
- Scheffer RE, Linden S. Concurrent medical conditions with pediatric bipolar disorder. Curr Opin Psychiatry 2007; 20(4):398–401. doi:10.1097/YCO.0b013e3281a305c3
- Carney CP, Jones LE. Medical comorbidity in women and men with bipolar disorders: a population-based controlled study. Psychosom Med 2006;68(5):684–691. doi:10.1097/01.psy.0000237316.09601.88
- Cassano GB, Akiskal HS, Savino M, Musetti L, Perugi G. Proposed subtypes of bipolar II and related disorders: with hypomanic episodes (or cyclothymia) and with hyperthymic temperament. J Affect Disord 1992; 26(2):127–140. pmid:1447430
- Akiskal HS, Mallya G. Criteria for the “soft” bipolar spectrum: treatment implications. Psychopharmacol Bull 1987; 23(1):68–73. pmid:3602332
- Fiedorowicz JG, Endicott J, Leon AC, Solomon DA, Keller MB, Coryell WH. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry 2011; 168(1):40–48. doi:10.1176/appi.ajp.2010.10030328
- Maj M, Pirozzi R, Magliano L, Fiorillo A, Bartoli L. Agitated “unipolar” major depression: prevalence, phenomenology, and outcome. J Clin Psychiatry 2006; 67(5):712–719. pmid:16841620
- Akiskal HS. The bipolar spectrum: new concepts in classification and diagnosis. In: Psychiatry Update: the American Psychiatric Association Annual Review. Washington, DC: American Psychiatric Press; 1983:271–292.
- Akiskal HS, Pinto O. The evolving bipolar spectrum. Prototypes I, II, III, and IV. Psychiatr Clin North Am 1999; 22(3):517–534. pmid:10550853
- Cassano GB, Savino M, Perugi G, Musetti L, Akiskal HS. Major depressive episode: unipolar and bipolar II. Encephale 1992 Jan;18 Spec No:15–18. pmid:1600898
- Müller-Oerlinghausen B, Berghöfer A, Bauer M. Bipolar disorder. Lancet 2002; 359(9302):241–247. pmid:11812578
- Hirschfeld RMA, Calabrese JR, Weissman MM, et al. Screening for bipolar disorder in the community. J Clin Psychiatry 2003; 64(1):53–59. pmid:12590624
- Blanco C, Compton WM, Saha TD, et al. Epidemiology of DSM-5 bipolar I disorder: results from the National Epidemiologic survey on Alcohol and Related Conditions—III. J Psychiatr Res 2017; 84:310–317. doi:10.1016/j.jpsychires.2016.10.003
- McGirr A, Vöhringer PA, Ghaemi SN, Lam RW, Yatham LN. Safety and efficacy of adjunctive second-generation antidepressant therapy with a mood stabiliser or an atypical antipsychotic in acute bipolar depression: a systematic review and meta-analysis of randomised placebo-controlled trials. Lancet Psychiatry 2016; 3(12):1138–1146. doi:10.1016/S2215-0366(16)30264-4
- Gijsman HJ, Geddes JR, Rendell JM, Nolen WA, Goodwin GM. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 2004; 161(9):1537–1547. doi:10.1176/appi.ajp.161.9.1537
- Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry 2011; 72(2):156–167. doi:10.4088/JCP.09r05385gre
- Liu B, Zhang Y, Fang H, Liu J, Liu T, Li L. Efficacy and safety of long-term antidepressant treatment for bipolar disorders - A meta-analysis of randomized controlled trials. J Affect Disord 2017; 223(139):41–48. doi:10.1016/j.jad.2017.07.023
- Krupa T, Kirsh B, Cockburn L, Gewurtz R. Understanding the stigma of mental illness in employment. Work 2009; 33(4):413–425. doi:10.3233/WOR-2009-0890
- Hawke LD, Parikh SV, Michalak EE. Stigma and bipolar disorder: a review of the literature. J Affect Disord 2013; 150(2):181–191. doi:10.1016/j.jad.2013.05.030
- Cerit C, Filizer A, Tural Ü, Tufan AE. Stigma: a core factor on predicting functionality in bipolar disorder. Compr Psychiatry 2012; 53(5):484–489. doi:10.1016/j.comppsych.2011.08.010
- O’Donnell L, Himle JA, Ryan K, et al. Social aspects of the workplace among individuals with bipolar disorder. J Soc Social Work Res 2017; 8(3):379–398. doi:10.1086/693163
- Akiskal HS, Maser JD, Zeller PJ, et al. Switching from “unipolar” to bipolar II. An 11-year prospective study of clinical and temperamental predictors in 559 patients. Arch Gen Psychiatry 1995; 52(2):114–123. pmid:7848047
- Kroon JS, Wohlfarth TD, Dieleman J, et al. Incidence rates and risk factors of bipolar disorder in the general population: a population-based cohort study. Bipolar Disord 2013; 15(3):306–313. doi:10.1111/bdi.12058
- Fiedorowicz JG, Endicott J, Leon AC, Solomon DA, Keller MB, Coryell WH. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry 2011; 168(1):40–48. doi:10.1176/appi.ajp.2010.10030328
- Akiskal HS, Walker P, Puzantian VR, King D, Rosenthal TL, Dranon M. Bipolar outcome in the course of depressive illness. Phenomenologic, familial, and pharmacologic predictors. J Affect Disord 1983; 5(2):115–128. pmid:6222091
- Strober M, Carlson G. Bipolar illness in adolescents with major depression: clinical, genetic, and psychopharmacologic predictors in a three- to four-year prospective follow-up investigation. Arch Gen Psychiatry 1982; 39(5):549–555. pmid:7092488
- Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry 2001; 158(8):1265–1270. pmid:11481161
- Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: the “red sign,” the “rule of three” and other biographic signs of temperamental extravagance, activation and hypomania. J Affect Disord 2005; 84(2–3):279–290. pmid:15708427
- Texas Medical Association. Mood disorders in physicians. www.texmed.org/Template.aspx?id=6833. Accessed June 7, 2018.
- Hirschfeld RM. Differential diagnosis of bipolar disorder and major depressive disorder. J Affect Disord 2014;169(suppl 1):S12–S16. doi:10.1016/S0165-0327(14)70004-7
- Dunner DL. Differential diagnosis of bipolar disorder. J Clin Psychopharmacol 1992; 12(1suppl):7S–12S. pmid:1541721
- Peet M, Peters S. Drug-induced mania. Drug Saf 1995; 12(2):146–153. pmid:7766338
- Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999; 282(18):1737–1744. pmid:10568646
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16(9):606–613. pmid:11556941
- Hirschfeld RMMA, Williams JBBW, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry 2000; 157(11)1873–1875. doi:10.1176/appi.ajp.157.11.1873
- Hirschfeld RMA. The Mood Disorder Questionnaire: a simple, patient-rated screening instrument for bipolar disorder. Prim Care Companion J Clin Psychiatry 2002; 4(1):9–11. pmid: 15014728
- Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA 2005; 293(8):956–963. doi:10.1001/jama.293.8.956
- Pauley J. Skywriting: A Life Out of the Blue. New York: Random House, 2004.
- Goren JL, Levin GM. Mania with bupropion: a dose-related phenomenon? Ann Pharmacother 2000; 34(5):619–621. doi:10.1345/aph.19313
- Swann AC. Long-term treatment in bipolar disorder. J Clin Psychiatry 2005; 66(suppl 1):7–12. pmid:15693746
- Cicchetti D, Toth SL. The development of depression in children and adolescents. Am Psychol 1998; 53(2):221–241. doi:10.1037/0003-066X.53.2.221
- Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry 2004;6(1):27–33. pmid:15486598
- Ghaemi SN, Boiman EE, Goodwin FK. Diagnosing bipolar disorder and the effect of antidepressants: a naturalistic study. J Clin Psychiatry 2000; 61(10):804–808. pmid:11078046
- Singh T, Rajput M. Misdiagnosis of bipolar disorder. Psychiatry (Edgmont) 2006; 3(10):57–63. pmid: 20877548
- Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RM. The National Depressive and Manic-depressive Association (DMDA) survey of bipolar members. J Affect Disord 1994; 31(4):281–294. pmid:7989643
- Howes OD, Falkenberg I. Early detection and intervention in bipolar affective disorder: targeting the development of the disorder. Curr Psychiatry Rep 2011; 13(6):493–499. pmid:21850462
- Ghaemi SN, Sachs GS, Chiou AM, Pandurangi AK, Goodwin K. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord 1999; 52(1–3):135–144. pmid:10357026
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Publishing, 2013.
- Hlastala SA, Frank E, Mallinger AG, Thase ME, Ritenour AM, Kupfer DJ. Bipolar depression: an underestimated treatment challenge. Depress Anxiety 1997; 5(2):73–83. pmid:9262937
- Smith DJ, Craddock N. Unipolar and bipolar depression: different or the same? Br J Psychiatry 2011; 199(4):272–274. doi:10.1192/bjp.bp.111.092726
- Viktorin A, Lichtenstein P, Thase ME, et al. The risk of switch to mania in patients with bipolar disorder during treatment with an antidepressant alone and in combination with a mood stabilizer. Am J Psychiatry 2014; 171(10):1067–1073. doi:10.1176/appi.ajp.2014.13111501
- Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007; 356(17):1711–1722. doi:10.1056/NEJMoa064135
- American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002; 159(4 suppl):1–50. pmid:11958165
- Leonpacher AK, Liebers D, Pirooznia M, et al. Distinguishing bipolar from unipolar depression: the importance of clinical symptoms and illness features. Psychol Med 2015; 45(11):2437–2446. doi:10.1017/S0033291715000446
- Angst J, Gamma A, Benazzi F, Ajdacic V, Eich D, Rössler W. Toward a re-definition of subthreshold bipolarity: epidemiology and proposed criteria for bipolar-II, minor bipolar disorders and hypomania. J Affect Disord 2003; 73(1–2):133–146. pmid:12507746
- Faravelli C, Rosi S, Alessandra Scarpato M, Lampronti L, Amedei SG, Rana N. Threshold and subthreshold bipolar disorders in the Sesto Fiorentino Study. J Affect Disord 2006; 94(1–3):111–119. pmid:16701902
- Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord 2003; 73(1–2):123–131. pmid:12507745
- Park Y-M, Lee B-H. Treatment response in relation to subthreshold bipolarity in patients with major depressive disorder receiving antidepressant monotherapy: a post hoc data analysis (KOMDD study). Neuropsychiatr Dis Treat 2016; 12:1221–1227. doi:10.2147/NDT.S104188
- Perlis RH, Uher R, Ostacher M, Goldberg JF, et al. Association between bipolar spectrum features and treatment outcomes in outpatients with major depressive disorder. Arch Gen Psychiatry 2011; 68(4):351–360. doi:10.1001/archgenpsychiatry.2010.179
- Dudek D, Siwek M, Zielin´ska D, Jaeschke R, Rybakowski J. Diagnostic conversions from major depressive disorder into bipolar disorder in an outpatient setting: results of a retrospective chart review. J Affect Disord 2013; 144(1–2):112–115. doi:10.1016/j.jad.2012.06.014
- Angst J, Cui L, Swendsen J, et al. Major depressive disorder with subthreshold bipolarity in the National Comorbidity Survey Replication. Am J Psychiatry 2010; 167(10):1194–1201. doi:10.1176/appi.ajp.2010.09071011
- Zimmermann P, Brückl T, Nocon A, et al. Heterogeneity of DSM-IV major depressive disorder as a consequence of subthreshold bipolarity. Arch Gen Psychiatry 2009; 66(12):1341–1352. doi:10.1001/archgenpsychiatry.2009.158
- Patel NC, DelBello MP, Keck PE, Strakowski SM. Phenomenology associated with age at onset in patients with bipolar disorder at their first psychiatric hospitalization. Bipolar Disord 2006; 8(1):91–94. pmid:16411986
- Hirschfeld RMA, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the National Depressive and Manic-Depressive Association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry 2003; 64(2):161–174. pmid:12633125
- Craddock N, Jones I. Genetics of bipolar disorder. J Med Genet 1999; 36(8):585–594. pmid:10465107
- Griswold KS, Pessar LF. Management of bipolar disorder. Am Fam Physician 2000; 62(6):1343–1358. pmid:11011863
- Kerner B. Genetics of bipolar disorder. Appl Clin Genet 2014; 7:33–42. doi:10.2147/TACG.S39297
- Scheffer RE, Linden S. Concurrent medical conditions with pediatric bipolar disorder. Curr Opin Psychiatry 2007; 20(4):398–401. doi:10.1097/YCO.0b013e3281a305c3
- Carney CP, Jones LE. Medical comorbidity in women and men with bipolar disorders: a population-based controlled study. Psychosom Med 2006;68(5):684–691. doi:10.1097/01.psy.0000237316.09601.88
- Cassano GB, Akiskal HS, Savino M, Musetti L, Perugi G. Proposed subtypes of bipolar II and related disorders: with hypomanic episodes (or cyclothymia) and with hyperthymic temperament. J Affect Disord 1992; 26(2):127–140. pmid:1447430
- Akiskal HS, Mallya G. Criteria for the “soft” bipolar spectrum: treatment implications. Psychopharmacol Bull 1987; 23(1):68–73. pmid:3602332
- Fiedorowicz JG, Endicott J, Leon AC, Solomon DA, Keller MB, Coryell WH. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry 2011; 168(1):40–48. doi:10.1176/appi.ajp.2010.10030328
- Maj M, Pirozzi R, Magliano L, Fiorillo A, Bartoli L. Agitated “unipolar” major depression: prevalence, phenomenology, and outcome. J Clin Psychiatry 2006; 67(5):712–719. pmid:16841620
- Akiskal HS. The bipolar spectrum: new concepts in classification and diagnosis. In: Psychiatry Update: the American Psychiatric Association Annual Review. Washington, DC: American Psychiatric Press; 1983:271–292.
- Akiskal HS, Pinto O. The evolving bipolar spectrum. Prototypes I, II, III, and IV. Psychiatr Clin North Am 1999; 22(3):517–534. pmid:10550853
- Cassano GB, Savino M, Perugi G, Musetti L, Akiskal HS. Major depressive episode: unipolar and bipolar II. Encephale 1992 Jan;18 Spec No:15–18. pmid:1600898
- Müller-Oerlinghausen B, Berghöfer A, Bauer M. Bipolar disorder. Lancet 2002; 359(9302):241–247. pmid:11812578
- Hirschfeld RMA, Calabrese JR, Weissman MM, et al. Screening for bipolar disorder in the community. J Clin Psychiatry 2003; 64(1):53–59. pmid:12590624
- Blanco C, Compton WM, Saha TD, et al. Epidemiology of DSM-5 bipolar I disorder: results from the National Epidemiologic survey on Alcohol and Related Conditions—III. J Psychiatr Res 2017; 84:310–317. doi:10.1016/j.jpsychires.2016.10.003
- McGirr A, Vöhringer PA, Ghaemi SN, Lam RW, Yatham LN. Safety and efficacy of adjunctive second-generation antidepressant therapy with a mood stabiliser or an atypical antipsychotic in acute bipolar depression: a systematic review and meta-analysis of randomised placebo-controlled trials. Lancet Psychiatry 2016; 3(12):1138–1146. doi:10.1016/S2215-0366(16)30264-4
- Gijsman HJ, Geddes JR, Rendell JM, Nolen WA, Goodwin GM. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 2004; 161(9):1537–1547. doi:10.1176/appi.ajp.161.9.1537
- Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry 2011; 72(2):156–167. doi:10.4088/JCP.09r05385gre
- Liu B, Zhang Y, Fang H, Liu J, Liu T, Li L. Efficacy and safety of long-term antidepressant treatment for bipolar disorders - A meta-analysis of randomized controlled trials. J Affect Disord 2017; 223(139):41–48. doi:10.1016/j.jad.2017.07.023
- Krupa T, Kirsh B, Cockburn L, Gewurtz R. Understanding the stigma of mental illness in employment. Work 2009; 33(4):413–425. doi:10.3233/WOR-2009-0890
- Hawke LD, Parikh SV, Michalak EE. Stigma and bipolar disorder: a review of the literature. J Affect Disord 2013; 150(2):181–191. doi:10.1016/j.jad.2013.05.030
- Cerit C, Filizer A, Tural Ü, Tufan AE. Stigma: a core factor on predicting functionality in bipolar disorder. Compr Psychiatry 2012; 53(5):484–489. doi:10.1016/j.comppsych.2011.08.010
- O’Donnell L, Himle JA, Ryan K, et al. Social aspects of the workplace among individuals with bipolar disorder. J Soc Social Work Res 2017; 8(3):379–398. doi:10.1086/693163
- Akiskal HS, Maser JD, Zeller PJ, et al. Switching from “unipolar” to bipolar II. An 11-year prospective study of clinical and temperamental predictors in 559 patients. Arch Gen Psychiatry 1995; 52(2):114–123. pmid:7848047
- Kroon JS, Wohlfarth TD, Dieleman J, et al. Incidence rates and risk factors of bipolar disorder in the general population: a population-based cohort study. Bipolar Disord 2013; 15(3):306–313. doi:10.1111/bdi.12058
- Fiedorowicz JG, Endicott J, Leon AC, Solomon DA, Keller MB, Coryell WH. Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry 2011; 168(1):40–48. doi:10.1176/appi.ajp.2010.10030328
- Akiskal HS, Walker P, Puzantian VR, King D, Rosenthal TL, Dranon M. Bipolar outcome in the course of depressive illness. Phenomenologic, familial, and pharmacologic predictors. J Affect Disord 1983; 5(2):115–128. pmid:6222091
- Strober M, Carlson G. Bipolar illness in adolescents with major depression: clinical, genetic, and psychopharmacologic predictors in a three- to four-year prospective follow-up investigation. Arch Gen Psychiatry 1982; 39(5):549–555. pmid:7092488
- Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry 2001; 158(8):1265–1270. pmid:11481161
- Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: the “red sign,” the “rule of three” and other biographic signs of temperamental extravagance, activation and hypomania. J Affect Disord 2005; 84(2–3):279–290. pmid:15708427
- Texas Medical Association. Mood disorders in physicians. www.texmed.org/Template.aspx?id=6833. Accessed June 7, 2018.
- Hirschfeld RM. Differential diagnosis of bipolar disorder and major depressive disorder. J Affect Disord 2014;169(suppl 1):S12–S16. doi:10.1016/S0165-0327(14)70004-7
- Dunner DL. Differential diagnosis of bipolar disorder. J Clin Psychopharmacol 1992; 12(1suppl):7S–12S. pmid:1541721
- Peet M, Peters S. Drug-induced mania. Drug Saf 1995; 12(2):146–153. pmid:7766338
- Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999; 282(18):1737–1744. pmid:10568646
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16(9):606–613. pmid:11556941
- Hirschfeld RMMA, Williams JBBW, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry 2000; 157(11)1873–1875. doi:10.1176/appi.ajp.157.11.1873
- Hirschfeld RMA. The Mood Disorder Questionnaire: a simple, patient-rated screening instrument for bipolar disorder. Prim Care Companion J Clin Psychiatry 2002; 4(1):9–11. pmid: 15014728
- Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA 2005; 293(8):956–963. doi:10.1001/jama.293.8.956
- Pauley J. Skywriting: A Life Out of the Blue. New York: Random House, 2004.
- Goren JL, Levin GM. Mania with bupropion: a dose-related phenomenon? Ann Pharmacother 2000; 34(5):619–621. doi:10.1345/aph.19313
- Swann AC. Long-term treatment in bipolar disorder. J Clin Psychiatry 2005; 66(suppl 1):7–12. pmid:15693746
KEY POINTS
- Bipolar depression in its manifest and subthreshold forms is nearly as prevalent as unipolar depression and often occurs in successful professionals.
- A manic or hypomanic episode can make a patient highly productive, but it can also be severely disruptive, leading to loss of job, marriage, and financial savings.
- Identifying bipolar depression depends on asking about bipolar symptoms, using screening instruments, and being aware of clues from the patient’s history.
- A major depressive episode in patients with a history of mania or hypomania should be treated with a combination of an antidepressant and a mood stabilizer or a mood stabilizer alone.
Which patients with a parapneumonic effusion need a chest tube?
Hospitalized patients with pneumonia who develop a complicated parapneumonic effusion or empyema need to undergo chest tube placement.
WHAT IS PARAPNEUMONIC EFFUSION?
Parapneumonic effusion is a pleural effusion that forms concurrently with bacterial or viral pneumonia. Up to 40% of patients hospitalized with pneumonia develop a parapneumonic effusion.1 The effusion progresses through a continuum of 3 stages: uncomplicated, complicated, and empyema.
Uncomplicated parapneumonic effusion is an exudative effusion without bacteria or pus that is caused by movement of fluid and neutrophils into the pleural space. Pneumonia itself causes an increase in interstitial fluid and capillary leakage. The effusion becomes complicated as a result of bacteria invading the pleural space, causing a further increase in neutrophils in the pleural fluid. Empyema is defined as the presence of frank pus in the pleural space.
CLINICAL SIGNIFICANCE
According to the US Centers for Disease Control and Prevention, pneumonia accounts for 674,000 emergency department visits each year; of the patients hospitalized, up to 40% develop a parapneumonic effusion.2 The only study done on rates of death associated with parapneumonic effusion showed that, compared with patients with no effusion, the risk of death was 3.7 times higher with a unilateral effusion and 6.5 times higher with bilateral effusions.3
INITIAL EVALUATION
The initial evaluation of suspected parapneumonic effusion should include chest radiography with lateral or decubitus views, followed by thoracentesis if indicated. If thoracentesis is performed, the fluid should be tested as follows:
- Gram stain
- Appropriate cultures based on clinical scenario (eg, aerobic, anaerobic, fungal)
- Total protein in pleural fluid and serum
- Lactate dehydrogenase (LDH) in pleural fluid and serum
- Glucose
- pH.
CLASSIFICATION OF EFFUSIONS
When pleural fluid is obtained, the total protein and LDH levels are used to categorize the effusion as either transudative or exudative based on the Light criteria.4 An effusion is confirmed as exudative when 1 of the following 3 criteria is met:
- The ratio of pleural fluid protein to serum protein is greater than 0.5
- The ratio of pleural fluid LDH to serum LDH is greater than 0.6
- The pleural fluid LDH is greater than two-thirds the upper limit of normal for the serum LDH.
Category 1 effusions are defined as free- flowing fluid with a thickness of less than 10 mm on any imaging modality. Thoracentesis for pleural fluid analysis is not required. The prognosis is very good.
Category 2 effusions are defined as free- flowing fluid with a thickness greater than 10 mm and less than 50% of the hemithorax. Thoracentesis is typically done because of the size of the effusion, but Gram stain and culture of the pleural fluid are usually negative, and the pH is at least 7.2. The prognosis is good.
Category 3 effusions are considered complicated because the anatomy of the pleural space becomes altered or because bacteria have invaded the pleural space. The effusion is larger than 50% of the hemithorax or is loculated, or the parietal pleura is thickened. Since the bacteria have invaded the pleural space, Gram stain or culture of pleural fluid may be positive, the pleural fluid pH may be less than 7.2, or the glucose level of the fluid may be less than 60 mg/dL. The prognosis for category 3 is poor.
Category 4 effusions are defined as empyema. The only characteristic that separates this from category 3 is frank pus in the pleural space. The prognosis is very poor.
TO PLACE A CHEST TUBE OR NOT
For category 1 or 2 effusions, treatment with antibiotics alone is typically enough. Category 3 effusions usually do not respond to antibiotics alone and may require complete drainage of the fluid with or without a chest tube depending on whether loculations are present, as loculations are difficult to drain with a chest tube. Category 4 effusions require both antibiotics and chest tube placement.
WHAT TYPE OF CHEST TUBE?
Studies have shown that small-bore chest tubes (< 20 F) are as efficacious as larger tubes (≥ 20 F) for the treatment of complicated parapneumonic effusion and empyema.6,7 Studies have also shown that the size of the tube makes no difference in the time needed to drain the effusion, the length of hospital stay, or the complication rate.8,9 Based on these studies, a small-bore chest tube should be placed first when clinically appropriate. When a chest tube is placed for empyema, computed tomography should be performed within 24 hours to confirm proper tube placement.
ADVANCED THERAPIES FOR EMPYEMA
Empyema treatment fails when antibiotic coverage is inadequate or when a loculation is not drained appropriately. Options if treatment fails include instillation of fibrinolytics into the pleural space, video-assisted thorascopic surgery, and decortication.
The role of fibrinolytics has not been well-established, but fibrinolytics should be considered in loculated effusions or empyema, or if drainage of the effusion slows.10 Video-assisted thorascopic surgery is reserved for effusions that are incompletely drained with a chest tube with or without fibrinolytics; studies have shown shorter hospital length of stay and higher treatment efficacy when this is performed earlier for loculated effusions.11 Decortication is reserved for symptomatic patients who have a thickened pleura more than 6 months after the initial infection.12 Timing for each of these procedures is not clearly defined and so must be individualized.
TAKE-AWAY POINTS
- Parapneumonic effusion occurs concurrently with pneumonia and with a high frequency.1
- Effusions are associated with an increased risk of death.3
- Categorizing the effusion helps guide treatment.
- Chest tubes should be placed for some cases of complicated effusion and for all cases of empyema.
- A small-bore chest tube (< 20 F) should be tried first.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3(1):75–80. doi:10.1513/pats.200510-113JH
- US Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Health Statistics. National hospital ambulatory medical care survey: 2013 emergency department summary tables. www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf.
- Hasley PB, Albaum MN, Li YH, et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia? Arch Intern Med 1996; 156(19):2206–2212. doi:10.1001/archinte.1996.00440180068008
- Light RW, Macgregor MI, Luchsinger PC, Ball WC. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77(4):507–513. doi:10.7326/0003-4819-77-4-507
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. doi:10.1378/CHEST.118.4.1158
- Ali I, Unruh H. Management of empyema thoracis. Ann Thorac Surg 1990; 50(3):355–359. doi:10.1016/0003-4975(90)90474-K
- Ashbaugh DG. Empyema thoracis. Factors influencing morbidity and mortality. Chest 1991; 99(5):1162–1165. doi:10.1378/CHEST.99.5.1162
- Cooke DT, David EA. Large-bore and small-bore chest tubes: types, function, and placement. Thorac Surg Clin 2013; 23(1):17–24. doi:10.1016/j.thorsurg.2012.10.006
- Halifax RJ, Psallidas I, Rahman NM. Chest drain size: the debate continues. Curr Pulmonol Rep 2017; 6(1):26–29. doi:10.1007/s13665-017-0162-3
- Maskell NA, Davies CW, Nunn AJ, et al; First Multicenter Intrapleural Sepsis Trial (MIST1) Group. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Wait MA, Sharma S, Hohn J, Dal Nogare A. A randomized trial of empyema therapy. Chest 1997; 111(6):1548–1551. doi:10.1378/chest.111.6.1548
- Rzyman W, Skokowski J, Romanowicz G, Lass P, Dziadziuszko R. Decortication in chronic pleural empyema—effect on lung function. Eur J Cardiothorac Surg 2002; 21(3):502–507. doi:10.1016/S1010-7940(01)01167-8
Hospitalized patients with pneumonia who develop a complicated parapneumonic effusion or empyema need to undergo chest tube placement.
WHAT IS PARAPNEUMONIC EFFUSION?
Parapneumonic effusion is a pleural effusion that forms concurrently with bacterial or viral pneumonia. Up to 40% of patients hospitalized with pneumonia develop a parapneumonic effusion.1 The effusion progresses through a continuum of 3 stages: uncomplicated, complicated, and empyema.
Uncomplicated parapneumonic effusion is an exudative effusion without bacteria or pus that is caused by movement of fluid and neutrophils into the pleural space. Pneumonia itself causes an increase in interstitial fluid and capillary leakage. The effusion becomes complicated as a result of bacteria invading the pleural space, causing a further increase in neutrophils in the pleural fluid. Empyema is defined as the presence of frank pus in the pleural space.
CLINICAL SIGNIFICANCE
According to the US Centers for Disease Control and Prevention, pneumonia accounts for 674,000 emergency department visits each year; of the patients hospitalized, up to 40% develop a parapneumonic effusion.2 The only study done on rates of death associated with parapneumonic effusion showed that, compared with patients with no effusion, the risk of death was 3.7 times higher with a unilateral effusion and 6.5 times higher with bilateral effusions.3
INITIAL EVALUATION
The initial evaluation of suspected parapneumonic effusion should include chest radiography with lateral or decubitus views, followed by thoracentesis if indicated. If thoracentesis is performed, the fluid should be tested as follows:
- Gram stain
- Appropriate cultures based on clinical scenario (eg, aerobic, anaerobic, fungal)
- Total protein in pleural fluid and serum
- Lactate dehydrogenase (LDH) in pleural fluid and serum
- Glucose
- pH.
CLASSIFICATION OF EFFUSIONS
When pleural fluid is obtained, the total protein and LDH levels are used to categorize the effusion as either transudative or exudative based on the Light criteria.4 An effusion is confirmed as exudative when 1 of the following 3 criteria is met:
- The ratio of pleural fluid protein to serum protein is greater than 0.5
- The ratio of pleural fluid LDH to serum LDH is greater than 0.6
- The pleural fluid LDH is greater than two-thirds the upper limit of normal for the serum LDH.
Category 1 effusions are defined as free- flowing fluid with a thickness of less than 10 mm on any imaging modality. Thoracentesis for pleural fluid analysis is not required. The prognosis is very good.
Category 2 effusions are defined as free- flowing fluid with a thickness greater than 10 mm and less than 50% of the hemithorax. Thoracentesis is typically done because of the size of the effusion, but Gram stain and culture of the pleural fluid are usually negative, and the pH is at least 7.2. The prognosis is good.
Category 3 effusions are considered complicated because the anatomy of the pleural space becomes altered or because bacteria have invaded the pleural space. The effusion is larger than 50% of the hemithorax or is loculated, or the parietal pleura is thickened. Since the bacteria have invaded the pleural space, Gram stain or culture of pleural fluid may be positive, the pleural fluid pH may be less than 7.2, or the glucose level of the fluid may be less than 60 mg/dL. The prognosis for category 3 is poor.
Category 4 effusions are defined as empyema. The only characteristic that separates this from category 3 is frank pus in the pleural space. The prognosis is very poor.
TO PLACE A CHEST TUBE OR NOT
For category 1 or 2 effusions, treatment with antibiotics alone is typically enough. Category 3 effusions usually do not respond to antibiotics alone and may require complete drainage of the fluid with or without a chest tube depending on whether loculations are present, as loculations are difficult to drain with a chest tube. Category 4 effusions require both antibiotics and chest tube placement.
WHAT TYPE OF CHEST TUBE?
Studies have shown that small-bore chest tubes (< 20 F) are as efficacious as larger tubes (≥ 20 F) for the treatment of complicated parapneumonic effusion and empyema.6,7 Studies have also shown that the size of the tube makes no difference in the time needed to drain the effusion, the length of hospital stay, or the complication rate.8,9 Based on these studies, a small-bore chest tube should be placed first when clinically appropriate. When a chest tube is placed for empyema, computed tomography should be performed within 24 hours to confirm proper tube placement.
ADVANCED THERAPIES FOR EMPYEMA
Empyema treatment fails when antibiotic coverage is inadequate or when a loculation is not drained appropriately. Options if treatment fails include instillation of fibrinolytics into the pleural space, video-assisted thorascopic surgery, and decortication.
The role of fibrinolytics has not been well-established, but fibrinolytics should be considered in loculated effusions or empyema, or if drainage of the effusion slows.10 Video-assisted thorascopic surgery is reserved for effusions that are incompletely drained with a chest tube with or without fibrinolytics; studies have shown shorter hospital length of stay and higher treatment efficacy when this is performed earlier for loculated effusions.11 Decortication is reserved for symptomatic patients who have a thickened pleura more than 6 months after the initial infection.12 Timing for each of these procedures is not clearly defined and so must be individualized.
TAKE-AWAY POINTS
- Parapneumonic effusion occurs concurrently with pneumonia and with a high frequency.1
- Effusions are associated with an increased risk of death.3
- Categorizing the effusion helps guide treatment.
- Chest tubes should be placed for some cases of complicated effusion and for all cases of empyema.
- A small-bore chest tube (< 20 F) should be tried first.
Hospitalized patients with pneumonia who develop a complicated parapneumonic effusion or empyema need to undergo chest tube placement.
WHAT IS PARAPNEUMONIC EFFUSION?
Parapneumonic effusion is a pleural effusion that forms concurrently with bacterial or viral pneumonia. Up to 40% of patients hospitalized with pneumonia develop a parapneumonic effusion.1 The effusion progresses through a continuum of 3 stages: uncomplicated, complicated, and empyema.
Uncomplicated parapneumonic effusion is an exudative effusion without bacteria or pus that is caused by movement of fluid and neutrophils into the pleural space. Pneumonia itself causes an increase in interstitial fluid and capillary leakage. The effusion becomes complicated as a result of bacteria invading the pleural space, causing a further increase in neutrophils in the pleural fluid. Empyema is defined as the presence of frank pus in the pleural space.
CLINICAL SIGNIFICANCE
According to the US Centers for Disease Control and Prevention, pneumonia accounts for 674,000 emergency department visits each year; of the patients hospitalized, up to 40% develop a parapneumonic effusion.2 The only study done on rates of death associated with parapneumonic effusion showed that, compared with patients with no effusion, the risk of death was 3.7 times higher with a unilateral effusion and 6.5 times higher with bilateral effusions.3
INITIAL EVALUATION
The initial evaluation of suspected parapneumonic effusion should include chest radiography with lateral or decubitus views, followed by thoracentesis if indicated. If thoracentesis is performed, the fluid should be tested as follows:
- Gram stain
- Appropriate cultures based on clinical scenario (eg, aerobic, anaerobic, fungal)
- Total protein in pleural fluid and serum
- Lactate dehydrogenase (LDH) in pleural fluid and serum
- Glucose
- pH.
CLASSIFICATION OF EFFUSIONS
When pleural fluid is obtained, the total protein and LDH levels are used to categorize the effusion as either transudative or exudative based on the Light criteria.4 An effusion is confirmed as exudative when 1 of the following 3 criteria is met:
- The ratio of pleural fluid protein to serum protein is greater than 0.5
- The ratio of pleural fluid LDH to serum LDH is greater than 0.6
- The pleural fluid LDH is greater than two-thirds the upper limit of normal for the serum LDH.
Category 1 effusions are defined as free- flowing fluid with a thickness of less than 10 mm on any imaging modality. Thoracentesis for pleural fluid analysis is not required. The prognosis is very good.
Category 2 effusions are defined as free- flowing fluid with a thickness greater than 10 mm and less than 50% of the hemithorax. Thoracentesis is typically done because of the size of the effusion, but Gram stain and culture of the pleural fluid are usually negative, and the pH is at least 7.2. The prognosis is good.
Category 3 effusions are considered complicated because the anatomy of the pleural space becomes altered or because bacteria have invaded the pleural space. The effusion is larger than 50% of the hemithorax or is loculated, or the parietal pleura is thickened. Since the bacteria have invaded the pleural space, Gram stain or culture of pleural fluid may be positive, the pleural fluid pH may be less than 7.2, or the glucose level of the fluid may be less than 60 mg/dL. The prognosis for category 3 is poor.
Category 4 effusions are defined as empyema. The only characteristic that separates this from category 3 is frank pus in the pleural space. The prognosis is very poor.
TO PLACE A CHEST TUBE OR NOT
For category 1 or 2 effusions, treatment with antibiotics alone is typically enough. Category 3 effusions usually do not respond to antibiotics alone and may require complete drainage of the fluid with or without a chest tube depending on whether loculations are present, as loculations are difficult to drain with a chest tube. Category 4 effusions require both antibiotics and chest tube placement.
WHAT TYPE OF CHEST TUBE?
Studies have shown that small-bore chest tubes (< 20 F) are as efficacious as larger tubes (≥ 20 F) for the treatment of complicated parapneumonic effusion and empyema.6,7 Studies have also shown that the size of the tube makes no difference in the time needed to drain the effusion, the length of hospital stay, or the complication rate.8,9 Based on these studies, a small-bore chest tube should be placed first when clinically appropriate. When a chest tube is placed for empyema, computed tomography should be performed within 24 hours to confirm proper tube placement.
ADVANCED THERAPIES FOR EMPYEMA
Empyema treatment fails when antibiotic coverage is inadequate or when a loculation is not drained appropriately. Options if treatment fails include instillation of fibrinolytics into the pleural space, video-assisted thorascopic surgery, and decortication.
The role of fibrinolytics has not been well-established, but fibrinolytics should be considered in loculated effusions or empyema, or if drainage of the effusion slows.10 Video-assisted thorascopic surgery is reserved for effusions that are incompletely drained with a chest tube with or without fibrinolytics; studies have shown shorter hospital length of stay and higher treatment efficacy when this is performed earlier for loculated effusions.11 Decortication is reserved for symptomatic patients who have a thickened pleura more than 6 months after the initial infection.12 Timing for each of these procedures is not clearly defined and so must be individualized.
TAKE-AWAY POINTS
- Parapneumonic effusion occurs concurrently with pneumonia and with a high frequency.1
- Effusions are associated with an increased risk of death.3
- Categorizing the effusion helps guide treatment.
- Chest tubes should be placed for some cases of complicated effusion and for all cases of empyema.
- A small-bore chest tube (< 20 F) should be tried first.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3(1):75–80. doi:10.1513/pats.200510-113JH
- US Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Health Statistics. National hospital ambulatory medical care survey: 2013 emergency department summary tables. www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf.
- Hasley PB, Albaum MN, Li YH, et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia? Arch Intern Med 1996; 156(19):2206–2212. doi:10.1001/archinte.1996.00440180068008
- Light RW, Macgregor MI, Luchsinger PC, Ball WC. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77(4):507–513. doi:10.7326/0003-4819-77-4-507
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. doi:10.1378/CHEST.118.4.1158
- Ali I, Unruh H. Management of empyema thoracis. Ann Thorac Surg 1990; 50(3):355–359. doi:10.1016/0003-4975(90)90474-K
- Ashbaugh DG. Empyema thoracis. Factors influencing morbidity and mortality. Chest 1991; 99(5):1162–1165. doi:10.1378/CHEST.99.5.1162
- Cooke DT, David EA. Large-bore and small-bore chest tubes: types, function, and placement. Thorac Surg Clin 2013; 23(1):17–24. doi:10.1016/j.thorsurg.2012.10.006
- Halifax RJ, Psallidas I, Rahman NM. Chest drain size: the debate continues. Curr Pulmonol Rep 2017; 6(1):26–29. doi:10.1007/s13665-017-0162-3
- Maskell NA, Davies CW, Nunn AJ, et al; First Multicenter Intrapleural Sepsis Trial (MIST1) Group. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Wait MA, Sharma S, Hohn J, Dal Nogare A. A randomized trial of empyema therapy. Chest 1997; 111(6):1548–1551. doi:10.1378/chest.111.6.1548
- Rzyman W, Skokowski J, Romanowicz G, Lass P, Dziadziuszko R. Decortication in chronic pleural empyema—effect on lung function. Eur J Cardiothorac Surg 2002; 21(3):502–507. doi:10.1016/S1010-7940(01)01167-8
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006; 3(1):75–80. doi:10.1513/pats.200510-113JH
- US Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Health Statistics. National hospital ambulatory medical care survey: 2013 emergency department summary tables. www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf.
- Hasley PB, Albaum MN, Li YH, et al. Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia? Arch Intern Med 1996; 156(19):2206–2212. doi:10.1001/archinte.1996.00440180068008
- Light RW, Macgregor MI, Luchsinger PC, Ball WC. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77(4):507–513. doi:10.7326/0003-4819-77-4-507
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. doi:10.1378/CHEST.118.4.1158
- Ali I, Unruh H. Management of empyema thoracis. Ann Thorac Surg 1990; 50(3):355–359. doi:10.1016/0003-4975(90)90474-K
- Ashbaugh DG. Empyema thoracis. Factors influencing morbidity and mortality. Chest 1991; 99(5):1162–1165. doi:10.1378/CHEST.99.5.1162
- Cooke DT, David EA. Large-bore and small-bore chest tubes: types, function, and placement. Thorac Surg Clin 2013; 23(1):17–24. doi:10.1016/j.thorsurg.2012.10.006
- Halifax RJ, Psallidas I, Rahman NM. Chest drain size: the debate continues. Curr Pulmonol Rep 2017; 6(1):26–29. doi:10.1007/s13665-017-0162-3
- Maskell NA, Davies CW, Nunn AJ, et al; First Multicenter Intrapleural Sepsis Trial (MIST1) Group. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Wait MA, Sharma S, Hohn J, Dal Nogare A. A randomized trial of empyema therapy. Chest 1997; 111(6):1548–1551. doi:10.1378/chest.111.6.1548
- Rzyman W, Skokowski J, Romanowicz G, Lass P, Dziadziuszko R. Decortication in chronic pleural empyema—effect on lung function. Eur J Cardiothorac Surg 2002; 21(3):502–507. doi:10.1016/S1010-7940(01)01167-8
Palmoplantar exanthema and liver dysfunction
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
A 51-year-old man with type 2 diabetes was referred to our hospital because of liver dysfunction and nonpruritic exanthema, with papulosquamous, scaly, papular and macular lesions on his trunk and extremities, including his palms (Figure 1) and soles. Also noted were tiny grayish mucus patches on the oral mucosa. Axillary and inguinal superficial lymph nodes were palpable.
Laboratory testing revealed elevated serum levels of markers of liver disease, ie:
- Total bilirubin 9.8 mg/dL (reference range 0.2–1.3)
- Direct bilirubin 8.0 mg/dL (< 0.2)
- Aspartate aminotransferase 57 IU/L (13–35)
- Alanine aminotransferase 90 IU/L (10–54)
- Alkaline phosphatase 4,446 IU/L (36–108).
Possible causes of liver dysfunction such as legal and illicit drugs, alcohol abuse, obstructive biliary tract or liver disease, viral hepatitis, and primary biliary cirrhosis were ruled out by history, serologic testing, abdominal ultrasonography, and computed tomography.
Secondary syphilis was suspected in view of the characteristic distribution of exanthema involving the trunk and extremities, especially the palms and soles. On questioning, the patient admitted to having had unprotected sex with a female sex worker, which also raised the probability of syphilis infection.
The rapid plasma reagin test was positive at a titer of 1:16, and the Treponema pallidum agglutination test was positive at a signal-to-cutoff ratio of 27.02. Antibody testing for human immunodeficiency virus (HIV) was negative.
The patient was started on penicillin G, but 4 hours later, he developed a fever with a temperature of 100.2°F (37.9°C), which was assumed to be a Jarisch-Herxheimer reaction. The fever resolved by the next morning without further treatment.
His course was otherwise uneventful. The exanthema resolved within 3 months, and his liver function returned to normal. Five months later, the rapid plasma reagin test was repeated on an outpatient basis, and the result was normal.
SYPHILIS IS NOT A DISEASE OF THE PAST
Syphilis is caused by T pallidum and is mainly transmitted by sexual contact.1
The incidence of syphilis has substantially increased in recent years in Japan2,3 and worldwide.4 The typical patient is a young man who has sex with men, is infected with HIV, and has a history of syphilis infection.3 However, the rapid increase in syphilis infections in Japan in recent years is largely because of an increase in heterosexual transmission.3
Infectious in its early stages
Syphilis is potentially infectious in its early (primary, secondary, and early latent) stages.1,5 The secondary stage generally begins 6 to 8 weeks after the primary infection1 and presents with diverse symptoms, including arthralgia, condylomata lata, generalized lymphadenopathy, maculopapular and papulosquamous exanthema, myalgia, and pharyngitis.1
Liver dysfunction in secondary syphilis
Liver dysfunction is common in secondary syphilis, occurring in 25% to 50% of cases.5 The liver enzyme pattern in most cases is a disproportionate increase in the alkaline phosphatase level compared with modest elevations of aminotransferases and bilirubin.2,5 However, some cases may show predominant hepatocellular damage (with prominent elevations in aminotransferase levels), and others may present with severe cholestasis (with prominent elevations in alkaline phosphatase and bilirubin) or even fulminant hepatic failure.2,5
The diagnostic criteria for syphilitic hepatitis are abnormal liver enzyme levels, serologic evidence of syphilis in conjunction with acute clinical presentation of secondary syphilis, exclusion of alternative causes of liver dysfunction, and prompt recovery of liver function after antimicrobial therapy.2,5
Pathogenic mechanisms in syphilitic hepatitis include direct portal venous inoculation and immune complex-mediated disease.2 However, direct hepatotoxicity of the microorganism seems unlikely, as spirochetes are rarely detected in liver specimens.2,5
Jarisch-Herxheimer reaction
The Jarisch-Herxheimer reaction is an acute febrile illness during the first 24 hours of antimicrobial treatment.1,6 It is assumed to be due to lysis of large numbers of spirochetes, releasing lipopolysaccharides (endotoxins) that further incite the release of a range of cytokines, resulting in symptoms such as fever, chills, myalgias, headache, tachycardia, hyperventilation, vasodilation with flushing, and mild hypotension.6,7
The frequency of Jarisch-Herxheimer reaction in syphilis and other spirochetal infections has varied widely in different reports.8 It is common in primary and secondary syphilis but usually does not occur in latent syphilis.6
Consider the diagnosis
Physicians should consider secondary syphilis in patients who present with characteristic generalized reddish macules and papules with papulosquamous lesions, including on the palms and soles as in our patient, and also in patients who have had unprotected sexual contact. Syphilis is not a disease of the past.
Acknowledgment: The authors thank Dr. Joel Branch, Shonan Kamakura General Hospital, Japan, for his editorial assistance.
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
- Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. Am Fam Physician 2012; 86(5):433–440. pmid:22963062
- Miura H, Nakano M, Ryu T, Kitamura S, Suzaki A. A case of syphilis presenting with initial syphilitic hepatitis and serological recurrence with cerebrospinal abnormality. Intern Med 2010; 49(14):1377–1381. pmid:20647651
- Nishijima T, Teruya K, Shibata S, et al. Incidence and risk factors for incident syphilis among HIV-1-infected men who have sex with men in a large urban HIV clinic in Tokyo, 2008-2015. PLoS One 2016; 11(12):e0168642. doi:10.1371/journal.pone.0168642
- US Preventive Services Task Force (USPSTF), Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(21):2321–2327. doi:10.1001/jama.2016.5824
- Aggarwal A, Sharma V, Vaiphei K, Duseja A, Chawla YK. An unusual cause of cholestatic hepatitis: syphilis. Dig Dis Sci 2013; 58(10):3049–3051. doi:10.1007/s10620-013-2581-5
- Belum GR, Belum VR, Chaitanya Arudra SK, Reddy BS. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis 2013; 11(4):231–237. doi:10.1016/j.tmaid.2013.04.001
- Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 2002; 15(1):95–110. pmid:11781269
- Butler T. The Jarisch-Herxheimer reaction after antibiotic treatment of spirochetal infections: a review of recent cases and our understanding of pathogenesis. Am J Trop Med Hyg 2017; 96(1):46–52. doi:10.4269/ajtmh.16-0434
Angular cheilitis induced by iron deficiency anemia
A 20-year-old woman had a 4-month history of painful red erosions around the mouth. She had no dysphagia or fatigue and no history of diarrhea, gluten intolerance, or diabetes mellitus. An antifungal-antibacterial ointment prescribed by her dentist had provided no relief.
- Hemoglobin 8.0 g/dL (reference range for females 12.3–15.3)
- Mean corpuscular volume 62 fL (80–100)
- Serum ferritin 1.3 ng/mL (15–200)
- Reticulocyte count 0.86% (0.5–1.5)
- White blood cell count 9.8 × 109/L (4.5–11.0)
- Platelet count 450 × 109/L (150–400).
Vitamin B12 and folate levels were normal, and tests for antitissue transglutaminase and antinuclear antibodies were negative.
Based on these results, the diagnosis was angular cheilitis from iron deficiency anemia. Treatment with oral ferrous gluconate 300 mg twice daily cleared the cheilitis, and after 4 weeks of this treatment, the hemoglobin level increased to 9.8 g/dL, the serum ferritin increased to 7 ng/mL, and the reticulocyte count increased to 2.6%. She was advised to continue taking oral iron tablets for another 3 months until the hemoglobin level reached 12.0 g/dL.
During 2 years of follow-up, she had no recurrence of angular cheilitis, and her hemoglobin and serum ferritin levels remained normal. Ferrous gluconate was her only medication from the time of her diagnosis.
A BROAD DIFFERENTIAL DIAGNOSIS
Angular cheilitis (perlèche) is an inflammatory condition characterized by erosive inflammation at one or both angles of the mouth. It typically presents as erythema, scaling, fissuring, and ulceration. A wide variety of factors, including nutritional deficiencies, local and systemic factors, and drug side effects, may produce cheilitis.1,2
Nutritional deficiencies account for 25% of all cases of angular cheilitis3 and include iron deficiency and deficiencies of the B vitamins riboflavin (B2), niacin (B3), pyridoxine (B6), and cyanocobalamin (B12).1
Local causes include infection with Candida albicans or Staphylococcus aureus and allergic contact dermatitis. Common causes of allergic contact dermatitis include lipstick, toothpaste, mouthwash, cosmetics, sunscreen, fragrance, metals such as nickel, and dental appliances.1
Systemic diseases associated with angular cheilitis include xerostomia, inflammatory bowel disease, Sjögren syndrome, glucagonoma, and human immunodeficiency virus.1
Drugs that cause angular cheilitis include isotretinoin, sorafenib (antineoplastic kinase inhibitor), and ointments or creams such as neomycin sulfate–polymyxin B sulfate, bacitracin, idoxuridine, and steroids.1,4
Conditions that mimic angular cheilitis include herpes simplex type 1 (herpes labialis) and actinic cheilitis. Herpes labialis, characterized by burning sensation, itching, or paresthesia, usually precedes a recurrence of vesicles that eventually ulcerate or form a crust and heal without a crust. Herpes labialis often recurs, affecting the vermilion border and lasting approximately 1 week.
Actinic cheilitis, a premalignant condition that commonly involves the lower lip with sparing of the corners of the mouth, is caused by excessive sun exposure. Patients often have persistent dryness and cracking of the lips.
In our patient, angular cheilitis was the main clinical manifestation of iron deficiency anemia, highlighting the importance of looking for iron deficiency in affected patients without a more obvious cause.
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 2: nutritional, systemic, and drug-related causes and treatment. Cutis 2011; 88(1):27–32. pmid:21877503
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 1: local etiologies. Cutis 2011; 87(6):289–295. pmid:21838086
- Konstantinidis AB, Hatziotis JH. Angular cheilosis: an analysis of 156 cases. J Oral Med 1984; 39(4):199–206. pmid:6594458
- Yang CH, Lin WC, Chuang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 2008; 158(3):592–596. doi:10.1111/j.1365-2133.2007.08357.x
A 20-year-old woman had a 4-month history of painful red erosions around the mouth. She had no dysphagia or fatigue and no history of diarrhea, gluten intolerance, or diabetes mellitus. An antifungal-antibacterial ointment prescribed by her dentist had provided no relief.
- Hemoglobin 8.0 g/dL (reference range for females 12.3–15.3)
- Mean corpuscular volume 62 fL (80–100)
- Serum ferritin 1.3 ng/mL (15–200)
- Reticulocyte count 0.86% (0.5–1.5)
- White blood cell count 9.8 × 109/L (4.5–11.0)
- Platelet count 450 × 109/L (150–400).
Vitamin B12 and folate levels were normal, and tests for antitissue transglutaminase and antinuclear antibodies were negative.
Based on these results, the diagnosis was angular cheilitis from iron deficiency anemia. Treatment with oral ferrous gluconate 300 mg twice daily cleared the cheilitis, and after 4 weeks of this treatment, the hemoglobin level increased to 9.8 g/dL, the serum ferritin increased to 7 ng/mL, and the reticulocyte count increased to 2.6%. She was advised to continue taking oral iron tablets for another 3 months until the hemoglobin level reached 12.0 g/dL.
During 2 years of follow-up, she had no recurrence of angular cheilitis, and her hemoglobin and serum ferritin levels remained normal. Ferrous gluconate was her only medication from the time of her diagnosis.
A BROAD DIFFERENTIAL DIAGNOSIS
Angular cheilitis (perlèche) is an inflammatory condition characterized by erosive inflammation at one or both angles of the mouth. It typically presents as erythema, scaling, fissuring, and ulceration. A wide variety of factors, including nutritional deficiencies, local and systemic factors, and drug side effects, may produce cheilitis.1,2
Nutritional deficiencies account for 25% of all cases of angular cheilitis3 and include iron deficiency and deficiencies of the B vitamins riboflavin (B2), niacin (B3), pyridoxine (B6), and cyanocobalamin (B12).1
Local causes include infection with Candida albicans or Staphylococcus aureus and allergic contact dermatitis. Common causes of allergic contact dermatitis include lipstick, toothpaste, mouthwash, cosmetics, sunscreen, fragrance, metals such as nickel, and dental appliances.1
Systemic diseases associated with angular cheilitis include xerostomia, inflammatory bowel disease, Sjögren syndrome, glucagonoma, and human immunodeficiency virus.1
Drugs that cause angular cheilitis include isotretinoin, sorafenib (antineoplastic kinase inhibitor), and ointments or creams such as neomycin sulfate–polymyxin B sulfate, bacitracin, idoxuridine, and steroids.1,4
Conditions that mimic angular cheilitis include herpes simplex type 1 (herpes labialis) and actinic cheilitis. Herpes labialis, characterized by burning sensation, itching, or paresthesia, usually precedes a recurrence of vesicles that eventually ulcerate or form a crust and heal without a crust. Herpes labialis often recurs, affecting the vermilion border and lasting approximately 1 week.
Actinic cheilitis, a premalignant condition that commonly involves the lower lip with sparing of the corners of the mouth, is caused by excessive sun exposure. Patients often have persistent dryness and cracking of the lips.
In our patient, angular cheilitis was the main clinical manifestation of iron deficiency anemia, highlighting the importance of looking for iron deficiency in affected patients without a more obvious cause.
A 20-year-old woman had a 4-month history of painful red erosions around the mouth. She had no dysphagia or fatigue and no history of diarrhea, gluten intolerance, or diabetes mellitus. An antifungal-antibacterial ointment prescribed by her dentist had provided no relief.
- Hemoglobin 8.0 g/dL (reference range for females 12.3–15.3)
- Mean corpuscular volume 62 fL (80–100)
- Serum ferritin 1.3 ng/mL (15–200)
- Reticulocyte count 0.86% (0.5–1.5)
- White blood cell count 9.8 × 109/L (4.5–11.0)
- Platelet count 450 × 109/L (150–400).
Vitamin B12 and folate levels were normal, and tests for antitissue transglutaminase and antinuclear antibodies were negative.
Based on these results, the diagnosis was angular cheilitis from iron deficiency anemia. Treatment with oral ferrous gluconate 300 mg twice daily cleared the cheilitis, and after 4 weeks of this treatment, the hemoglobin level increased to 9.8 g/dL, the serum ferritin increased to 7 ng/mL, and the reticulocyte count increased to 2.6%. She was advised to continue taking oral iron tablets for another 3 months until the hemoglobin level reached 12.0 g/dL.
During 2 years of follow-up, she had no recurrence of angular cheilitis, and her hemoglobin and serum ferritin levels remained normal. Ferrous gluconate was her only medication from the time of her diagnosis.
A BROAD DIFFERENTIAL DIAGNOSIS
Angular cheilitis (perlèche) is an inflammatory condition characterized by erosive inflammation at one or both angles of the mouth. It typically presents as erythema, scaling, fissuring, and ulceration. A wide variety of factors, including nutritional deficiencies, local and systemic factors, and drug side effects, may produce cheilitis.1,2
Nutritional deficiencies account for 25% of all cases of angular cheilitis3 and include iron deficiency and deficiencies of the B vitamins riboflavin (B2), niacin (B3), pyridoxine (B6), and cyanocobalamin (B12).1
Local causes include infection with Candida albicans or Staphylococcus aureus and allergic contact dermatitis. Common causes of allergic contact dermatitis include lipstick, toothpaste, mouthwash, cosmetics, sunscreen, fragrance, metals such as nickel, and dental appliances.1
Systemic diseases associated with angular cheilitis include xerostomia, inflammatory bowel disease, Sjögren syndrome, glucagonoma, and human immunodeficiency virus.1
Drugs that cause angular cheilitis include isotretinoin, sorafenib (antineoplastic kinase inhibitor), and ointments or creams such as neomycin sulfate–polymyxin B sulfate, bacitracin, idoxuridine, and steroids.1,4
Conditions that mimic angular cheilitis include herpes simplex type 1 (herpes labialis) and actinic cheilitis. Herpes labialis, characterized by burning sensation, itching, or paresthesia, usually precedes a recurrence of vesicles that eventually ulcerate or form a crust and heal without a crust. Herpes labialis often recurs, affecting the vermilion border and lasting approximately 1 week.
Actinic cheilitis, a premalignant condition that commonly involves the lower lip with sparing of the corners of the mouth, is caused by excessive sun exposure. Patients often have persistent dryness and cracking of the lips.
In our patient, angular cheilitis was the main clinical manifestation of iron deficiency anemia, highlighting the importance of looking for iron deficiency in affected patients without a more obvious cause.
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 2: nutritional, systemic, and drug-related causes and treatment. Cutis 2011; 88(1):27–32. pmid:21877503
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 1: local etiologies. Cutis 2011; 87(6):289–295. pmid:21838086
- Konstantinidis AB, Hatziotis JH. Angular cheilosis: an analysis of 156 cases. J Oral Med 1984; 39(4):199–206. pmid:6594458
- Yang CH, Lin WC, Chuang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 2008; 158(3):592–596. doi:10.1111/j.1365-2133.2007.08357.x
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 2: nutritional, systemic, and drug-related causes and treatment. Cutis 2011; 88(1):27–32. pmid:21877503
- Park KK, Brodell RT, Helms SE. Angular cheilitis, part 1: local etiologies. Cutis 2011; 87(6):289–295. pmid:21838086
- Konstantinidis AB, Hatziotis JH. Angular cheilosis: an analysis of 156 cases. J Oral Med 1984; 39(4):199–206. pmid:6594458
- Yang CH, Lin WC, Chuang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: a clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol 2008; 158(3):592–596. doi:10.1111/j.1365-2133.2007.08357.x
Diabetes and pregnancy: Risks and opportunities
A 29-year-old nulliparous woman presents for a routine checkup. She has hypertension and type 2 diabetes mellitus. Her current medications are chlorpropamide 500 mg daily, metformin 500 mg twice a day, lisinopril 40 mg daily, simvastatin 40 mg daily, and aspirin 81 mg daily. Her body mass index is 37 kg/m2 and her blood pressure is 130/80 mm Hg. Her hemoglobin A1c level is 7.8% and her low-density lipoprotein cholesterol 90 mg/dL.
She is considering pregnancy. How would you counsel her?
DEFINING DIABETES IN PREGNANCY
Diabetes in pregnant women, both gestational and pregestational, is the most common medical complication associated with pregnancy.1
- Gestational diabetes is defined as diabetes that is diagnosed during the second or third trimester of pregnancy and that is not clearly pregestational.2
- Pregestational diabetes exists before pregnancy and can be either type 1 or type 2.
Most cases of diabetes diagnosed during the first trimester reflect pregestational diabetes, as gestational diabetes occurs when insulin resistance increases in the later trimesters.
Type 1 diabetes involves autoimmune destruction of pancreatic islet cells, leading to insulin deficiency and the need for insulin therapy. Type 2 diabetes is characterized by insulin resistance rather than overall insulin deficiency. Type 2 diabetes tends to be associated with comorbidities such as obesity and hypertension, which are independent risk factors for adverse perinatal outcomes.3,4
Gestational diabetes accounts for most cases of diabetes during pregnancy. Although both pregestational and gestational diabetes increase the risk of maternal and fetal complications, pregestational diabetes is associated with significantly greater risks.1
IMPACT OF DIABETES ON THE MOTHER
Pregnancy increases the risk of maternal hypoglycemia, especially during the first trimester in patients with type 1 diabetes, as insulin sensitivity increases in early pregnancy.1 Pregnant women with diabetes may also have an altered counterregulatory response and less hypoglycemic awareness.1 Insulin resistance rises during the second and early third trimesters, increasing the risk of hyperglycemia in women with diabetes.1
Glycemic control during pregnancy is usually easier to achieve in patients with type 2 diabetes than with type 1, but it may require much higher insulin doses.
Because pregnancy is inherently a ketogenic state, women with type 1 diabetes are at higher risk of diabetic ketoacidosis, particularly during the second and third trimesters.1 There are reports of euglycemic diabetic ketoacidosis in pregnant women with either gestational or pregestational diabetes.5
Diabetes is associated with a risk of preeclampsia 4 times higher than in nondiabetic women.6 Other potential pregnancy-related complications include infections, polyhydramnios, spontaneous abortion, and cesarean delivery.1,7 The risk of pregnancy loss is similar in women with either type 1 or type 2 diabetes (2.6% and 3.7%, respectively), but the causes are different.8 Although preexisting diabetic complications such as retinopathy, nephropathy, and gastroparesis can be exacerbated during pregnancy,1 only severe gastroparesis and advanced renal disease are considered relative contraindications to pregnancy.
IMPACT OF DIABETES ON THE FETUS
Fetal complications of maternal diabetes include embryopathy (fetal malformations) and fetopathy (overgrowth, ie, fetus large for gestational age, and increased risk of fetal death or distress). Maternal hyperglycemia is associated with diabetic embryopathy, resulting in major birth defects in 5% to 25% of pregnancies and spontaneous abortions in 15% to 20%.9,10 There is a 2- to 6-fold increase in risk of congenital malformations.6
The most common diabetes-associated congenital malformations affect the cardiovascular system. Congenital heart disease includes tetralogy of Fallot, transposition of the great vessels, septal defects, and anomalous pulmonary venous return. Other relatively common defects involve the fetal central nervous system, spine, orofacial system, kidneys, urogenital system, gastrointestinal tract, and skeleton.11
The risk of fetopathy is proportional to the degree of maternal hyperglycemia. Excess maternal glucose and fatty acid levels can lead to fetal hyperglycemia and overgrowth, which increases fetal oxygen requirements. Erythropoietin levels rise, causing an increase in red cell mass, with subsequent hyperviscosity within the placenta and higher risk of fetal death.
Other complications include intrauterine growth restriction, prematurity, and preterm delivery. Fetal macrosomia (birth weight > 90th percentile or 4 kg, approximately 8 lb, 13 oz) occurs in 27% to 62% of children born to mothers with diabetes, a rate 10 times higher than in patients without diabetes. It contributes to shoulder dystocia (risk 2 to 4 times higher in diabetic pregnancies) and cesarean delivery.6 Infants born to mothers with diabetes also have higher risks of neonatal hypoglycemia, erythrocytosis, hyperbilirubinemia, hypocalcemia, respiratory distress, cardiomyopathy, and death, as well as for developing diabetes, obesity, and other adverse cardiometabolic outcomes later in life.11
GET GLUCOSE UNDER CONTROL BEFORE PREGNANCY
Nearly half of pregnancies in the general population are unplanned,15 so preconception diabetes assessment needs to be part of routine medical care for all reproductive-age women. Because most organogenesis occurs during the first 5 to 8 weeks after fertilization—potentially before a woman realizes she is pregnant—achieving optimal glycemic control before conception is necessary to improve pregnancy outcomes.1
EVERY VISIT IS AN OPPORTUNITY
Every medical visit with a reproductive-age woman with diabetes is an opportunity for counseling about pregnancy. Topics that need to be discussed include the risks of unplanned pregnancy and of poor metabolic control, and the benefits of improved maternal and fetal outcomes with appropriate pregnancy planning and diabetes management.
Referral to a registered dietitian for individualized counseling about proper nutrition, particularly during pregnancy, has been associated with positive outcomes.16 Patients with diabetes and at high risk of pregnancy complications should be referred to a clinic that specializes in high-risk pregnancies.
Practitioners also should emphasize the importance of regular exercise and encourage patients to maintain or achieve a medically optimal weight before conception. Ideally, this would be a normal body mass index; however, this is not always possible.
In women who are planning pregnancy or are not on effective contraception, medications should be reviewed for potential teratogenicity. If needed, discuss alternative medications or switch to safer ones. However, these changes should not interrupt diabetes treatment.
In addition, ensure that the patient is up to date on age- and disease-appropriate preventive care (eg, immunizations, screening for sexually transmitted disease and malignancy). Counseling and intervention for use of tobacco, alcohol, and recreational drugs are also important. As with any preconception counseling, the patient (and her partner, if possible) should be advised to avoid travel to areas where Zika virus is endemic, and informed about the availability of expanded carrier genetic screening through her obstetric provider.
Finally, pregnant women with diabetes benefit from screening for diabetic complications including hypertension, retinopathy, cardiovascular disease, neuropathy, and nephropathy.
ASSESSING RISKS
Blood pressure
Chronic (preexisting) hypertension is defined as a systolic pressure 140 mm Hg or higher or a diastolic pressure 90 mm Hg or higher, or both, that antedates pregnancy or is present before the 20th week of pregnancy.3 Chronic hypertension has been reported in up to 5% of pregnant women and is associated with increased risk of preterm delivery, superimposed preeclampsia, low birth weight, and perinatal death.3
Reproductive-age women with diabetes and high blood pressure benefit from lifestyle and behavioral modifications.17 If drug therapy is needed, antihypertensive drugs that are safe for the fetus should be used. Treatment of mild or moderate hypertension during pregnancy reduces the risk of progression to severe hypertension but may not improve obstetric outcomes.
Diabetic retinopathy
Diabetic retinopathy can significantly worsen during pregnancy: the risk of progression is double that in the nonpregnant state.18 Women with diabetes who are contemplating pregnancy should have a comprehensive eye examination before conception, and any active proliferative retinopathy needs to be treated. These patients may require ophthalmologic monitoring and treatment during pregnancy. (Note: laser photocoagulation is not contraindicated during pregnancy.)
Cardiovascular disease
Cardiovascular physiology changes dramatically during pregnancy. Cardiovascular disease, especially when superimposed on diabetes, can increase the risk of maternal death. Thus, evaluation for cardiovascular risk factors as well as cardiovascular system integrity before conception is important. Listen for arterial bruits and murmurs, and assess peripheral pulses. Consideration should be given to obtaining a preconception resting electrocardiogram in women with diabetes who are over age 35 or who are suspected of having cardiovascular disease.16
Neurologic disorders
Peripheral neuropathy, the most common neurologic complication of diabetes, is associated with injury and infection.19
Autonomic neuropathy is associated with decreased cardiac responsiveness and orthostatic hypotension.19 Diabetic gastroparesis alone can precipitate serious complications during pregnancy, including extreme hypoglycemia and hyperglycemia, increased risk of diabetic ketoacidosis, weight loss, malnutrition, frequent hospitalizations, and increased requirement for parenteral nutrition.20
Although diabetic neuropathy does not significantly worsen during pregnancy, women with preexisting gastroparesis should be counseled on the substantial risks associated with pregnancy. Screening for neuropathy should be part of all diabetic preconception examinations.
Renal complications
Pregnancy in women with diabetes and preexisting renal dysfunction increases their risk of accelerated progression of diabetic kidney disease.21 Preexisting renal dysfunction also increases the risk of pregnancy-related complications, such as stillbirth, intrauterine growth restriction, gestational hypertension, preeclampsia, and preterm delivery.19,21,22 Further, the risk of pregnancy complications correlates directly with the severity of renal dysfunction.22
Psychiatric disorders
Emotional wellness is essential for optimal diabetes management. It is important to recognize the emotional impact of diabetes in pregnant women and to conduct routine screening for depression, anxiety, stress, and eating disorders.16
LABORATORY TESTS TO CONSIDER
Hemoglobin A1c. The general consensus is to achieve the lowest hemoglobin A1c level possible that does not increase the risk of hypoglycemia. The American Diabetes Association (ADA) recommends that, before attempting to conceive, women should lower their hemoglobin A1c to below 6.5%.1
Thyroid measures. Autoimmune thyroid disease is the most common autoimmune disorder associated with diabetes and has been reported in 35% to 40% of women with type 1 diabetes.23 Recommendations are to check thyroid-stimulating hormone and thyroid peroxidase antibody levels before conception or early in pregnancy in all women with diabetes.1,24 Overt hypothyroidism should be treated before conception, given that early fetal brain development depends on maternal thyroxine.
Renal function testing. Preconception assessment of renal function is important for counseling and risk stratification. This assessment should include serum creatinine level, estimated glomerular filtration rate, and urinary albumin excretion.21
Celiac screening. Because women with type 1 diabetes are more susceptible to autoimmune diseases, they should be screened for celiac disease before conception, with testing for immunoglobulin A (IgA) and tissue transglutaminase antibodies, with or without IgA endomysial antibodies.16,25,26 An estimated 6% of patients with type 1 diabetes have celiac disease vs 1% of the general population.25 Celiac disease is 2 to 3 times more common in women, and asymptomatic people with type 1 diabetes are considered at increased risk for celiac disease.26
The association between type 1 diabetes and celiac disease most likely relates to the overlap in human leukocyte antigens of the diseases. There is no established link between type 2 diabetes and celiac disease.25
Undiagnosed celiac disease increases a woman’s risk of obstetric complications such as preterm birth, low birth weight, and stillbirth.26 The most likely explanation for these adverse effects is nutrient malabsorption, which is characteristic of celiac disease. Adherence to a gluten-free diet before and during gestation may reduce the risk of preterm delivery by as much as 20%.26
Vitamin B12 level. Celiac disease interferes with the absorption of vitamin B12-instrinsic factor in the ileum, which can lead to vitamin B12 deficiency. Therefore, baseline vitamin B12 levels should be checked before conception in women with celiac disease. Levels should also be checked in women taking metformin, which also decreases vitamin B12 absorption. Of note, increased folate levels due to taking supplements can potentially mask vitamin B12 deficiency.
MEDICATIONS TO REVIEW FOR PREGNANCY INTERACTIONS
Diabetic medications
Insulin is the first-line pharmacotherapy for pregnant patients with type 1, type 2, or gestational diabetes. Insulin does not cross the placenta to a measurable extent, and most insulin preparations have been classified as category B,1 meaning no risks to the fetus have been found in humans.
Insulin dosing during pregnancy is not static. Beginning around mid-gestation, insulin requirements increase,28,29 but after 32 weeks the need may decrease. These changes require practitioners to closely monitor blood glucose throughout pregnancy.
Both basal-bolus injections and continuous subcutaneous infusion are reasonable options during pregnancy.30 However, the need for multiple and potentially painful insulin injections daily can lead to poor compliance. This inconvenience has led to studies using oral hypoglycemic medications instead of insulin for patients with gestational and type 2 diabetes.
Metformin is an oral biguanide that decreases hepatic gluconeogenesis and intestinal glucose absorption while peripherally increasing glucose utilization and uptake. Metformin does not pose a risk of hypoglycemia because its mechanism of action does not involve increased insulin production.7
Metformin does cross the placenta, resulting in umbilical cord blood levels higher than maternal levels. Nevertheless, studies support the efficacy and short-term safety of metformin use during a pregnancy complicated by gestational or type 2 diabetes.7,31 Moreover, metformin has been associated with a lower risk of neonatal hypoglycemia and maternal weight gain than insulin.32 However, this agent should be used with caution, as long-term data are not yet available, and it may slightly increase the risk of premature delivery.
Glyburide is another oral hypoglycemic medication that has been used during pregnancy. This second-generation sulfonylurea enhances the release of insulin from the pancreas by binding beta islet cell ATP-calcium channel receptors. Compared with other sulfonylureas, glyburide has the lowest rate of maternal-to-fetal transfer, with umbilical cord plasma concentrations 70% of maternal levels.33 Although some trials support the efficacy and short-term safety of glyburide treatment for gestational diabetes,34 recent studies have associated glyburide use during pregnancy with a higher rate of neonatal hypoglycemia, neonatal respiratory distress, macrosomia, and neonatal intensive care unit admissions than insulin and metformin.1,35
Patients treated with oral agents should be informed that these drugs cross the placenta, and that although no adverse effects on the fetus have been demonstrated, long-term safety data are lacking. In addition, oral agents are ineffective in type 1 diabetes and may be insufficient to overcome the insulin resistance in type 2 diabetes.
Antihypertensive drugs
All antihypertensive drugs cross the placenta, but several have an acceptable safety profile in pregnancy, including methyldopa, labetalol, clonidine, prazosin, and nifedipine. Hydralazine and labetalol are short-acting, come in intravenous formulations, and can be used for urgent blood pressure control during pregnancy. Diltiazem may be used for heart rate control during pregnancy, and it has been shown to lower blood pressure and proteinuria in pregnant patients with underlying renal disease.36,37 The ADA recommends against chronic use of diuretics during pregnancy because of potential reductions in maternal plasma volume and uteroplacental perfusion.1
Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and direct renin inhibitors are contraindicated during pregnancy because of the risk of fetal defects, particularly in the renal system.21,38 Although there is evidence to question the association between first semester exposure and fetotoxicity,39 we avoid these drugs during pregnancy and switch to a different agent in women planning pregnancy.
Other drugs
Statins are contraindicated in pregnancy because they interfere with the development of the fetal nervous system.21 Although preliminary data from a small study did not identify safety risks associated with pravastatin use after 12 weeks of gestation,40 we recommend discontinuing statins in women attempting pregnancy.
Aspirin. The US Preventive Services Task Force41 recommends low-dose aspirin (81 mg/day) after 12 weeks of gestation for women with type 1 or type 2 diabetes, as well as those with renal disease or chronic hypertension, to prevent preeclampsia. Of note, higher doses need to be used with caution during pregnancy because fetal abnormalities have been reported, such as disruption of fetal vasculature (mesenteric vessels), gastroschisis, and small intestinal atresia.16
Folate supplementation (0.6–4 mg/day) is recommended in women with celiac disease to prevent neural tube defects in the offspring, and the US Preventive Services Task Force recommends 0.4 mg daily of folic acid supplementation for all women planning or capable of pregnancy.42–44 Higher doses, ranging from 0.6 to 5 mg/day, have been proposed for patients with diabetes,13 and we recommend at least 1 mg for this group, based on data suggesting that higher doses further reduce the risk of neural tube defects.43
IS BREASTFEEDING AFFECTED?
Maternal diabetes, insulin therapy, and oral hypoglycemic agents are not contraindications to breastfeeding. The US Preventive Services Task Force recommends interventions by primary care physicians to promote and support breastfeeding.45 Breastfeeding is encouraged based on various short- and long-term health benefits for both breastfed infants and breastfeeding mothers. Breastfeeding decreases a woman’s insulin requirements and increases the risk for hypoglycemia, especially in patients with insulin-dependent type 1 diabetes.1
Additionally, insulin sensitivity increases immediately following delivery of the placenta.1 Therefore, it is prudent to adjust insulin doses postpartum, especially while a patient is breastfeeding, or to suggest high-carbohydrate snacks before feeds.9,29
Antihypertensive drugs considered safe to use during lactation include captopril, enalapril, quinapril, labetalol, propranolol, nifedipine, and hydralazine.21,46 Methyldopa is not contraindicated, but it causes fatigue and worsened postpartum depression and should not be used as first-line therapy. Diuretics and ARBs are not recommended during lactation.21 Both metformin and glyburide enter breast milk in small enough amounts that they are not contraindicated during breastfeeding.16 The Lactmed database (www.toxnet.nlm.nih.gov) provides information about drugs and breastfeeding.
WHAT ABOUT CONTRACEPTIVES?
The ADA recommends contraception for women with diabetes because, just as in women without diabetes, the risks of unplanned pregnancy outweigh those of contraceptives.1
We recommend low-dose combination estrogen-progestin oral contraceptives to normotensive women under age 35 with diabetes but without underlying microvascular disease. For women over age 35 or for those with microvascular disease, additional options include intrauterine devices or progestin implants. We prefer not to use injectable depot medroxyprogesterone acetate because of its side effects of insulin resistance and weight gain.47
CASE DISCUSSION: NEXT STEPS
Our patient’s interest in family planning presents an opportunity for preconception counseling. We recommend a prenatal folic acid supplement, diet and regular exercise for weight loss, and screening tests including a comprehensive metabolic panel, hemoglobin A1c, thyroid-stimulating hormone, and dilated eye examination. We make sure she is up to date on her indicated health maintenance (eg, immunizations, disease screening), and we review her medications for potential teratogens. She denies any recreational drug use. Also, she has no plans for long-distance travel.
Our counseling includes discussions of pregnancy risks associated with pregestational diabetes and suboptimal glycemic control. We encourage her to use effective contraception until she is “medically optimized” for pregnancy—ie, until her hemoglobin A1c is lower than 6.5% and she has achieved a medically optimal weight. If feasible, a reduction of weight (7% or so) through lifestyle modification should be attempted, and if her hemoglobin A1c remains elevated, adding insulin would be recommended.
Pregnant patients or patients contemplating pregnancy are usually motivated to modify their behavior, making this a good time to reinforce lifestyle modifications. Many patients benefit from individualized counseling by a registered dietitian to help achieve the recommended weight and glycemic control.
Our physical examination in this patient includes screening for micro- and macrovascular complications of diabetes, and the test results are negative. Patients with active proliferative retinopathy should be referred to an ophthalmologist for assessment and treatment.
We review her medications for potential teratogenic effects and stop her ACE inhibitor (lisinopril) and statin (simvastatin). We switch her from a first-generation sulfonylurea (chlorpropamide) to glyburide, a second-generation sulfonylurea. Second-generation sulfonylureas are considered more “fetus-friendly” because first-generation sulfonylureas cross the placenta more easily and can cause fetal hyperinsulinemia, leading to macrosomia and neonatal hypoglycemia.7
The management of diabetes during pregnancy leans toward insulin use, given the lack of information regarding long-term outcomes with oral agents. If insulin is needed, it is best to initiate it before the patient conceives, and then to stop other diabetes medications. We would not make any changes to her aspirin or metformin use.
Educating the patient and her family about prevention, recognition, and treatment of hypoglycemia is important to prevent and manage the increased risk of hypoglycemia with insulin therapy and in early pregnancy.1 Consideration should be given to providing ketone strips as well as education on diabetic ketoacidosis prevention and detection.1 If the patient conceives, begin prenatal care early to allow adequate planning for care of her disease and evaluation of the fetus. Because of the complexity of insulin management in pregnancy, the ADA recommends referral, if possible, to a center offering team-based care, including an obstetrician specialized in high-risk pregnancies, an endocrinologist, and a dietitian.1
- American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S137–S143. doi:10.2337/dc18-S013
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S13–S27. doi:10.2337/dc18-S002
- Lawler J, Osman M, Shelton JA, Yeh J. Population-based analysis of hypertensive disorders in pregnancy. Hypertens Pregnancy 2007; 26(1):67–76. doi:10.1080/10641950601147945
- Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015; 16(8):621–638. doi:10.1111/obr.12288
- Garrison EA, Jagasia S. Inpatient management of women with gestational and pregestational diabetes in pregnancy. Curr Diab Rep 2014; 14(2):457. doi:10.1007/s11892-013-0457-x
- Ballas J, Moore TR, Ramos GA. Management of diabetes in pregnancy. Curr Diab Rep 2012; 12(1):33–42. doi:10.1007/s11892-011-0249-0
- Ryu RJ, Hays KE, Hebert MF. Gestational diabetes mellitus management with oral hypoglycemic agents. Semin Perinatol 2014; 38(8):508–515. doi:10.1053/j.semperi.2014.08.012
- Cundy T, Gamble G, Neale L, et al. Differing causes of pregnancy loss in type 1 and type 2 diabetes. Diabetes Care 2007; 30(10):2603–2607. doi:10.2337/dc07-0555
- Castorino K, Jovanovic L. Pregnancy and diabetes management: advances and controversies. Clin Chem 2011; 57(2):221–230. doi:10.1373/clinchem.2010.155382
- Hammouda SA, Hakeem R. Role of HbA1c in predicting risk for congenital malformations. Prim Care Diabetes 2015; 9(6):458–464. doi:10.1016/j.pcd.2015.01.004
- Chen CP. Congenital malformations associated with maternal diabetes. Taiwanese J Obstet Gynecol 2005; 44(1):1–7. doi:10.1016/S1028-4559(09)60099-1
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33(3):676–682. doi:10.2337/dc09-1848
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36(5):1384–1395. doi:10.2337/dc12-2480
- HAPO Study Cooperative Research Group; Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358(19):1991–2002. doi:10.1056/NEJMoa0707943
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health 2014; 104(suppl 1):S43–S48. doi:10.2105/AJPH.2013.301416
- Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care 2008; 31(5):1060–1079. doi:10.2337/dc08-9020
- Webster LM, Conti-Ramsden F, Seed PT, Webb AJ, Nelson-Piercy C, Chappell LC. Impact of antihypertensive treatment on maternal and perinatal outcomes in pregnancy complicated by chronic hypertension: a systematic review and meta-analysis. J Am Heart Assoc 2017; 6(5).pii:e005526. doi:10.1161/JAHA.117.005526
- Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy: the Diabetes in Early Pregnancy Study. Diabetes Care 1995; 18(5):631–637. pmid:8586000
- American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care 2016; 39 (suppl 1):S1–S109.
- Hawthorne, G. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):77–90. doi:10.1016/j.bpobgyn.2010.10.015
- Ringholm L, Damm JA, Vestgaard M, Damm P, Mathiesen ER. Diabetic nephropathy in women with preexisting diabetes: from pregnancy planning to breastfeeding. Curr Diab Rep 2016; 16(2):12. doi:10.1007/s11892-015-0705-3
- Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 2015; 10(11):1964–1978. doi:10.2215/CJN.09250914
- Umpierrez GE, Latif KA, Murphy MB, et al. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care 2003; 26(4):1181–1185. pmid:12663594
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Akirov A, Pinhas-Hamiel O. Co-occurrence of type 1 diabetes mellitus and celiac disease. World J Diabetes 2015; 6(5):707–714. doi:10.4239/wjd.v6.i5.707
- Saccone G, Berghella V, Sarno L, et al. Celiac disease and obstetric complications: a systematic review and metaanalysis. Am J Obstet Gynecol 2016; 214(2):225–234. doi:10.1016/j.ajog.2015.09.080
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
- de Valk HW, Visser GH. Insulin during pregnancy, labour and delivery. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):65–76. doi:10.1016/j.bpobgyn.2010.10.002
- Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int J Gen Med 2011; 4:827–835. doi:10.2147/IJGM.S26889
- Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2016; (6):CD005542. doi:10.1002/14651858.CD005542.pub2
- Charles B, Norris R, Xiao X, Hague W. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit 2006; 28(1):67–72. pmid:16418696
- Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 2015; 350:h102. doi:10.1136/bmj.h102
- Hebert MF, Ma X, Naraharisetti SB, et al; Obstetric-Fetal Pharmacology Research Unit Network. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009; 85(6):607–614. doi:10.1038/clpt.2009.5
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000; 343(16):1134–1138. doi:10.1056/NEJM200010193431601
- Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr, Jonsson Funk M. Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr 2015; 169:452–458. doi:10.1001/jamapediatrics.2015.74
- Gowda RM, Khan IA, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac arrhythmias in pregnancy: clinical and therapeutic considerations. Int J Cardiol 2003; 88(2):129–133. pmid:12714190
- Khandelwal M, Kumanova M, Gaughan JP, Reece EA. Role of diltiazem in pregnant women with chronic renal disease. J Matern Fetal Neonatal Med 2002; 12(6):408–412. doi:10.1080/jmf.12.6.408.412
- Magee LA, Abalos E, von Dadelszen P, Sibai B, Easterling T, Walkinshaw S; CHIPS Study Group. How to manage hypertension in pregnancy effectively. Br J Clin Pharmacol 2011; 72(3):394–401. doi:10.1111/j.1365-2125.2011.04002.x
- Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 2006; 354(23):2443–2451. doi:10.1056/NEJMoa055202
- Costantine MM, Cleary K, Hebert MF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 2016; 214(6):720.e1–720.e17. doi:10.1016/j.ajog.2015.12.038
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161(11):819–826. doi:10.7326/M14-1884
- Curry SJ, Grossman DC, Whitlock EP, Cantu A. Behavioral counseling research and evidence-based practice recommendations: US Preventive Services Task Force perspectives. Ann Intern Med 2014; 160(6):407–413. doi:10.7326/M13-2128
- Wald N, Law M, Morris J, Wald D. Quantifying the effect of folic acid. Lancet 2001; 358(9298):2069–2073. pmid:11755633
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. JAMA 2017; 317(2):183–189. doi:10.1001/jama.2016.19438
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Primary care interventions to support breastfeeding: US Preventive Services Task Force recommendation statement. JAMA 2016; 316(16):1688–1693. doi:10.1001/jama.2016.14697
- Newton ER, Hale TW. Drugs in breast milk. Clin Obstet Gynecol 2015; 58(4):868–884. doi:10.1097/GRF.0000000000000142
- Xiang AH, Kawakubo M, Kjos SL, Buchanan TA. Long-acting injectable progestin contraception and risk of type 2 diabetes in Latino women with prior gestational diabetes mellitus. Diabetes Care 2006; 29(3):613–617. pmid:16505515
A 29-year-old nulliparous woman presents for a routine checkup. She has hypertension and type 2 diabetes mellitus. Her current medications are chlorpropamide 500 mg daily, metformin 500 mg twice a day, lisinopril 40 mg daily, simvastatin 40 mg daily, and aspirin 81 mg daily. Her body mass index is 37 kg/m2 and her blood pressure is 130/80 mm Hg. Her hemoglobin A1c level is 7.8% and her low-density lipoprotein cholesterol 90 mg/dL.
She is considering pregnancy. How would you counsel her?
DEFINING DIABETES IN PREGNANCY
Diabetes in pregnant women, both gestational and pregestational, is the most common medical complication associated with pregnancy.1
- Gestational diabetes is defined as diabetes that is diagnosed during the second or third trimester of pregnancy and that is not clearly pregestational.2
- Pregestational diabetes exists before pregnancy and can be either type 1 or type 2.
Most cases of diabetes diagnosed during the first trimester reflect pregestational diabetes, as gestational diabetes occurs when insulin resistance increases in the later trimesters.
Type 1 diabetes involves autoimmune destruction of pancreatic islet cells, leading to insulin deficiency and the need for insulin therapy. Type 2 diabetes is characterized by insulin resistance rather than overall insulin deficiency. Type 2 diabetes tends to be associated with comorbidities such as obesity and hypertension, which are independent risk factors for adverse perinatal outcomes.3,4
Gestational diabetes accounts for most cases of diabetes during pregnancy. Although both pregestational and gestational diabetes increase the risk of maternal and fetal complications, pregestational diabetes is associated with significantly greater risks.1
IMPACT OF DIABETES ON THE MOTHER
Pregnancy increases the risk of maternal hypoglycemia, especially during the first trimester in patients with type 1 diabetes, as insulin sensitivity increases in early pregnancy.1 Pregnant women with diabetes may also have an altered counterregulatory response and less hypoglycemic awareness.1 Insulin resistance rises during the second and early third trimesters, increasing the risk of hyperglycemia in women with diabetes.1
Glycemic control during pregnancy is usually easier to achieve in patients with type 2 diabetes than with type 1, but it may require much higher insulin doses.
Because pregnancy is inherently a ketogenic state, women with type 1 diabetes are at higher risk of diabetic ketoacidosis, particularly during the second and third trimesters.1 There are reports of euglycemic diabetic ketoacidosis in pregnant women with either gestational or pregestational diabetes.5
Diabetes is associated with a risk of preeclampsia 4 times higher than in nondiabetic women.6 Other potential pregnancy-related complications include infections, polyhydramnios, spontaneous abortion, and cesarean delivery.1,7 The risk of pregnancy loss is similar in women with either type 1 or type 2 diabetes (2.6% and 3.7%, respectively), but the causes are different.8 Although preexisting diabetic complications such as retinopathy, nephropathy, and gastroparesis can be exacerbated during pregnancy,1 only severe gastroparesis and advanced renal disease are considered relative contraindications to pregnancy.
IMPACT OF DIABETES ON THE FETUS
Fetal complications of maternal diabetes include embryopathy (fetal malformations) and fetopathy (overgrowth, ie, fetus large for gestational age, and increased risk of fetal death or distress). Maternal hyperglycemia is associated with diabetic embryopathy, resulting in major birth defects in 5% to 25% of pregnancies and spontaneous abortions in 15% to 20%.9,10 There is a 2- to 6-fold increase in risk of congenital malformations.6
The most common diabetes-associated congenital malformations affect the cardiovascular system. Congenital heart disease includes tetralogy of Fallot, transposition of the great vessels, septal defects, and anomalous pulmonary venous return. Other relatively common defects involve the fetal central nervous system, spine, orofacial system, kidneys, urogenital system, gastrointestinal tract, and skeleton.11
The risk of fetopathy is proportional to the degree of maternal hyperglycemia. Excess maternal glucose and fatty acid levels can lead to fetal hyperglycemia and overgrowth, which increases fetal oxygen requirements. Erythropoietin levels rise, causing an increase in red cell mass, with subsequent hyperviscosity within the placenta and higher risk of fetal death.
Other complications include intrauterine growth restriction, prematurity, and preterm delivery. Fetal macrosomia (birth weight > 90th percentile or 4 kg, approximately 8 lb, 13 oz) occurs in 27% to 62% of children born to mothers with diabetes, a rate 10 times higher than in patients without diabetes. It contributes to shoulder dystocia (risk 2 to 4 times higher in diabetic pregnancies) and cesarean delivery.6 Infants born to mothers with diabetes also have higher risks of neonatal hypoglycemia, erythrocytosis, hyperbilirubinemia, hypocalcemia, respiratory distress, cardiomyopathy, and death, as well as for developing diabetes, obesity, and other adverse cardiometabolic outcomes later in life.11
GET GLUCOSE UNDER CONTROL BEFORE PREGNANCY
Nearly half of pregnancies in the general population are unplanned,15 so preconception diabetes assessment needs to be part of routine medical care for all reproductive-age women. Because most organogenesis occurs during the first 5 to 8 weeks after fertilization—potentially before a woman realizes she is pregnant—achieving optimal glycemic control before conception is necessary to improve pregnancy outcomes.1
EVERY VISIT IS AN OPPORTUNITY
Every medical visit with a reproductive-age woman with diabetes is an opportunity for counseling about pregnancy. Topics that need to be discussed include the risks of unplanned pregnancy and of poor metabolic control, and the benefits of improved maternal and fetal outcomes with appropriate pregnancy planning and diabetes management.
Referral to a registered dietitian for individualized counseling about proper nutrition, particularly during pregnancy, has been associated with positive outcomes.16 Patients with diabetes and at high risk of pregnancy complications should be referred to a clinic that specializes in high-risk pregnancies.
Practitioners also should emphasize the importance of regular exercise and encourage patients to maintain or achieve a medically optimal weight before conception. Ideally, this would be a normal body mass index; however, this is not always possible.
In women who are planning pregnancy or are not on effective contraception, medications should be reviewed for potential teratogenicity. If needed, discuss alternative medications or switch to safer ones. However, these changes should not interrupt diabetes treatment.
In addition, ensure that the patient is up to date on age- and disease-appropriate preventive care (eg, immunizations, screening for sexually transmitted disease and malignancy). Counseling and intervention for use of tobacco, alcohol, and recreational drugs are also important. As with any preconception counseling, the patient (and her partner, if possible) should be advised to avoid travel to areas where Zika virus is endemic, and informed about the availability of expanded carrier genetic screening through her obstetric provider.
Finally, pregnant women with diabetes benefit from screening for diabetic complications including hypertension, retinopathy, cardiovascular disease, neuropathy, and nephropathy.
ASSESSING RISKS
Blood pressure
Chronic (preexisting) hypertension is defined as a systolic pressure 140 mm Hg or higher or a diastolic pressure 90 mm Hg or higher, or both, that antedates pregnancy or is present before the 20th week of pregnancy.3 Chronic hypertension has been reported in up to 5% of pregnant women and is associated with increased risk of preterm delivery, superimposed preeclampsia, low birth weight, and perinatal death.3
Reproductive-age women with diabetes and high blood pressure benefit from lifestyle and behavioral modifications.17 If drug therapy is needed, antihypertensive drugs that are safe for the fetus should be used. Treatment of mild or moderate hypertension during pregnancy reduces the risk of progression to severe hypertension but may not improve obstetric outcomes.
Diabetic retinopathy
Diabetic retinopathy can significantly worsen during pregnancy: the risk of progression is double that in the nonpregnant state.18 Women with diabetes who are contemplating pregnancy should have a comprehensive eye examination before conception, and any active proliferative retinopathy needs to be treated. These patients may require ophthalmologic monitoring and treatment during pregnancy. (Note: laser photocoagulation is not contraindicated during pregnancy.)
Cardiovascular disease
Cardiovascular physiology changes dramatically during pregnancy. Cardiovascular disease, especially when superimposed on diabetes, can increase the risk of maternal death. Thus, evaluation for cardiovascular risk factors as well as cardiovascular system integrity before conception is important. Listen for arterial bruits and murmurs, and assess peripheral pulses. Consideration should be given to obtaining a preconception resting electrocardiogram in women with diabetes who are over age 35 or who are suspected of having cardiovascular disease.16
Neurologic disorders
Peripheral neuropathy, the most common neurologic complication of diabetes, is associated with injury and infection.19
Autonomic neuropathy is associated with decreased cardiac responsiveness and orthostatic hypotension.19 Diabetic gastroparesis alone can precipitate serious complications during pregnancy, including extreme hypoglycemia and hyperglycemia, increased risk of diabetic ketoacidosis, weight loss, malnutrition, frequent hospitalizations, and increased requirement for parenteral nutrition.20
Although diabetic neuropathy does not significantly worsen during pregnancy, women with preexisting gastroparesis should be counseled on the substantial risks associated with pregnancy. Screening for neuropathy should be part of all diabetic preconception examinations.
Renal complications
Pregnancy in women with diabetes and preexisting renal dysfunction increases their risk of accelerated progression of diabetic kidney disease.21 Preexisting renal dysfunction also increases the risk of pregnancy-related complications, such as stillbirth, intrauterine growth restriction, gestational hypertension, preeclampsia, and preterm delivery.19,21,22 Further, the risk of pregnancy complications correlates directly with the severity of renal dysfunction.22
Psychiatric disorders
Emotional wellness is essential for optimal diabetes management. It is important to recognize the emotional impact of diabetes in pregnant women and to conduct routine screening for depression, anxiety, stress, and eating disorders.16
LABORATORY TESTS TO CONSIDER
Hemoglobin A1c. The general consensus is to achieve the lowest hemoglobin A1c level possible that does not increase the risk of hypoglycemia. The American Diabetes Association (ADA) recommends that, before attempting to conceive, women should lower their hemoglobin A1c to below 6.5%.1
Thyroid measures. Autoimmune thyroid disease is the most common autoimmune disorder associated with diabetes and has been reported in 35% to 40% of women with type 1 diabetes.23 Recommendations are to check thyroid-stimulating hormone and thyroid peroxidase antibody levels before conception or early in pregnancy in all women with diabetes.1,24 Overt hypothyroidism should be treated before conception, given that early fetal brain development depends on maternal thyroxine.
Renal function testing. Preconception assessment of renal function is important for counseling and risk stratification. This assessment should include serum creatinine level, estimated glomerular filtration rate, and urinary albumin excretion.21
Celiac screening. Because women with type 1 diabetes are more susceptible to autoimmune diseases, they should be screened for celiac disease before conception, with testing for immunoglobulin A (IgA) and tissue transglutaminase antibodies, with or without IgA endomysial antibodies.16,25,26 An estimated 6% of patients with type 1 diabetes have celiac disease vs 1% of the general population.25 Celiac disease is 2 to 3 times more common in women, and asymptomatic people with type 1 diabetes are considered at increased risk for celiac disease.26
The association between type 1 diabetes and celiac disease most likely relates to the overlap in human leukocyte antigens of the diseases. There is no established link between type 2 diabetes and celiac disease.25
Undiagnosed celiac disease increases a woman’s risk of obstetric complications such as preterm birth, low birth weight, and stillbirth.26 The most likely explanation for these adverse effects is nutrient malabsorption, which is characteristic of celiac disease. Adherence to a gluten-free diet before and during gestation may reduce the risk of preterm delivery by as much as 20%.26
Vitamin B12 level. Celiac disease interferes with the absorption of vitamin B12-instrinsic factor in the ileum, which can lead to vitamin B12 deficiency. Therefore, baseline vitamin B12 levels should be checked before conception in women with celiac disease. Levels should also be checked in women taking metformin, which also decreases vitamin B12 absorption. Of note, increased folate levels due to taking supplements can potentially mask vitamin B12 deficiency.
MEDICATIONS TO REVIEW FOR PREGNANCY INTERACTIONS
Diabetic medications
Insulin is the first-line pharmacotherapy for pregnant patients with type 1, type 2, or gestational diabetes. Insulin does not cross the placenta to a measurable extent, and most insulin preparations have been classified as category B,1 meaning no risks to the fetus have been found in humans.
Insulin dosing during pregnancy is not static. Beginning around mid-gestation, insulin requirements increase,28,29 but after 32 weeks the need may decrease. These changes require practitioners to closely monitor blood glucose throughout pregnancy.
Both basal-bolus injections and continuous subcutaneous infusion are reasonable options during pregnancy.30 However, the need for multiple and potentially painful insulin injections daily can lead to poor compliance. This inconvenience has led to studies using oral hypoglycemic medications instead of insulin for patients with gestational and type 2 diabetes.
Metformin is an oral biguanide that decreases hepatic gluconeogenesis and intestinal glucose absorption while peripherally increasing glucose utilization and uptake. Metformin does not pose a risk of hypoglycemia because its mechanism of action does not involve increased insulin production.7
Metformin does cross the placenta, resulting in umbilical cord blood levels higher than maternal levels. Nevertheless, studies support the efficacy and short-term safety of metformin use during a pregnancy complicated by gestational or type 2 diabetes.7,31 Moreover, metformin has been associated with a lower risk of neonatal hypoglycemia and maternal weight gain than insulin.32 However, this agent should be used with caution, as long-term data are not yet available, and it may slightly increase the risk of premature delivery.
Glyburide is another oral hypoglycemic medication that has been used during pregnancy. This second-generation sulfonylurea enhances the release of insulin from the pancreas by binding beta islet cell ATP-calcium channel receptors. Compared with other sulfonylureas, glyburide has the lowest rate of maternal-to-fetal transfer, with umbilical cord plasma concentrations 70% of maternal levels.33 Although some trials support the efficacy and short-term safety of glyburide treatment for gestational diabetes,34 recent studies have associated glyburide use during pregnancy with a higher rate of neonatal hypoglycemia, neonatal respiratory distress, macrosomia, and neonatal intensive care unit admissions than insulin and metformin.1,35
Patients treated with oral agents should be informed that these drugs cross the placenta, and that although no adverse effects on the fetus have been demonstrated, long-term safety data are lacking. In addition, oral agents are ineffective in type 1 diabetes and may be insufficient to overcome the insulin resistance in type 2 diabetes.
Antihypertensive drugs
All antihypertensive drugs cross the placenta, but several have an acceptable safety profile in pregnancy, including methyldopa, labetalol, clonidine, prazosin, and nifedipine. Hydralazine and labetalol are short-acting, come in intravenous formulations, and can be used for urgent blood pressure control during pregnancy. Diltiazem may be used for heart rate control during pregnancy, and it has been shown to lower blood pressure and proteinuria in pregnant patients with underlying renal disease.36,37 The ADA recommends against chronic use of diuretics during pregnancy because of potential reductions in maternal plasma volume and uteroplacental perfusion.1
Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and direct renin inhibitors are contraindicated during pregnancy because of the risk of fetal defects, particularly in the renal system.21,38 Although there is evidence to question the association between first semester exposure and fetotoxicity,39 we avoid these drugs during pregnancy and switch to a different agent in women planning pregnancy.
Other drugs
Statins are contraindicated in pregnancy because they interfere with the development of the fetal nervous system.21 Although preliminary data from a small study did not identify safety risks associated with pravastatin use after 12 weeks of gestation,40 we recommend discontinuing statins in women attempting pregnancy.
Aspirin. The US Preventive Services Task Force41 recommends low-dose aspirin (81 mg/day) after 12 weeks of gestation for women with type 1 or type 2 diabetes, as well as those with renal disease or chronic hypertension, to prevent preeclampsia. Of note, higher doses need to be used with caution during pregnancy because fetal abnormalities have been reported, such as disruption of fetal vasculature (mesenteric vessels), gastroschisis, and small intestinal atresia.16
Folate supplementation (0.6–4 mg/day) is recommended in women with celiac disease to prevent neural tube defects in the offspring, and the US Preventive Services Task Force recommends 0.4 mg daily of folic acid supplementation for all women planning or capable of pregnancy.42–44 Higher doses, ranging from 0.6 to 5 mg/day, have been proposed for patients with diabetes,13 and we recommend at least 1 mg for this group, based on data suggesting that higher doses further reduce the risk of neural tube defects.43
IS BREASTFEEDING AFFECTED?
Maternal diabetes, insulin therapy, and oral hypoglycemic agents are not contraindications to breastfeeding. The US Preventive Services Task Force recommends interventions by primary care physicians to promote and support breastfeeding.45 Breastfeeding is encouraged based on various short- and long-term health benefits for both breastfed infants and breastfeeding mothers. Breastfeeding decreases a woman’s insulin requirements and increases the risk for hypoglycemia, especially in patients with insulin-dependent type 1 diabetes.1
Additionally, insulin sensitivity increases immediately following delivery of the placenta.1 Therefore, it is prudent to adjust insulin doses postpartum, especially while a patient is breastfeeding, or to suggest high-carbohydrate snacks before feeds.9,29
Antihypertensive drugs considered safe to use during lactation include captopril, enalapril, quinapril, labetalol, propranolol, nifedipine, and hydralazine.21,46 Methyldopa is not contraindicated, but it causes fatigue and worsened postpartum depression and should not be used as first-line therapy. Diuretics and ARBs are not recommended during lactation.21 Both metformin and glyburide enter breast milk in small enough amounts that they are not contraindicated during breastfeeding.16 The Lactmed database (www.toxnet.nlm.nih.gov) provides information about drugs and breastfeeding.
WHAT ABOUT CONTRACEPTIVES?
The ADA recommends contraception for women with diabetes because, just as in women without diabetes, the risks of unplanned pregnancy outweigh those of contraceptives.1
We recommend low-dose combination estrogen-progestin oral contraceptives to normotensive women under age 35 with diabetes but without underlying microvascular disease. For women over age 35 or for those with microvascular disease, additional options include intrauterine devices or progestin implants. We prefer not to use injectable depot medroxyprogesterone acetate because of its side effects of insulin resistance and weight gain.47
CASE DISCUSSION: NEXT STEPS
Our patient’s interest in family planning presents an opportunity for preconception counseling. We recommend a prenatal folic acid supplement, diet and regular exercise for weight loss, and screening tests including a comprehensive metabolic panel, hemoglobin A1c, thyroid-stimulating hormone, and dilated eye examination. We make sure she is up to date on her indicated health maintenance (eg, immunizations, disease screening), and we review her medications for potential teratogens. She denies any recreational drug use. Also, she has no plans for long-distance travel.
Our counseling includes discussions of pregnancy risks associated with pregestational diabetes and suboptimal glycemic control. We encourage her to use effective contraception until she is “medically optimized” for pregnancy—ie, until her hemoglobin A1c is lower than 6.5% and she has achieved a medically optimal weight. If feasible, a reduction of weight (7% or so) through lifestyle modification should be attempted, and if her hemoglobin A1c remains elevated, adding insulin would be recommended.
Pregnant patients or patients contemplating pregnancy are usually motivated to modify their behavior, making this a good time to reinforce lifestyle modifications. Many patients benefit from individualized counseling by a registered dietitian to help achieve the recommended weight and glycemic control.
Our physical examination in this patient includes screening for micro- and macrovascular complications of diabetes, and the test results are negative. Patients with active proliferative retinopathy should be referred to an ophthalmologist for assessment and treatment.
We review her medications for potential teratogenic effects and stop her ACE inhibitor (lisinopril) and statin (simvastatin). We switch her from a first-generation sulfonylurea (chlorpropamide) to glyburide, a second-generation sulfonylurea. Second-generation sulfonylureas are considered more “fetus-friendly” because first-generation sulfonylureas cross the placenta more easily and can cause fetal hyperinsulinemia, leading to macrosomia and neonatal hypoglycemia.7
The management of diabetes during pregnancy leans toward insulin use, given the lack of information regarding long-term outcomes with oral agents. If insulin is needed, it is best to initiate it before the patient conceives, and then to stop other diabetes medications. We would not make any changes to her aspirin or metformin use.
Educating the patient and her family about prevention, recognition, and treatment of hypoglycemia is important to prevent and manage the increased risk of hypoglycemia with insulin therapy and in early pregnancy.1 Consideration should be given to providing ketone strips as well as education on diabetic ketoacidosis prevention and detection.1 If the patient conceives, begin prenatal care early to allow adequate planning for care of her disease and evaluation of the fetus. Because of the complexity of insulin management in pregnancy, the ADA recommends referral, if possible, to a center offering team-based care, including an obstetrician specialized in high-risk pregnancies, an endocrinologist, and a dietitian.1
A 29-year-old nulliparous woman presents for a routine checkup. She has hypertension and type 2 diabetes mellitus. Her current medications are chlorpropamide 500 mg daily, metformin 500 mg twice a day, lisinopril 40 mg daily, simvastatin 40 mg daily, and aspirin 81 mg daily. Her body mass index is 37 kg/m2 and her blood pressure is 130/80 mm Hg. Her hemoglobin A1c level is 7.8% and her low-density lipoprotein cholesterol 90 mg/dL.
She is considering pregnancy. How would you counsel her?
DEFINING DIABETES IN PREGNANCY
Diabetes in pregnant women, both gestational and pregestational, is the most common medical complication associated with pregnancy.1
- Gestational diabetes is defined as diabetes that is diagnosed during the second or third trimester of pregnancy and that is not clearly pregestational.2
- Pregestational diabetes exists before pregnancy and can be either type 1 or type 2.
Most cases of diabetes diagnosed during the first trimester reflect pregestational diabetes, as gestational diabetes occurs when insulin resistance increases in the later trimesters.
Type 1 diabetes involves autoimmune destruction of pancreatic islet cells, leading to insulin deficiency and the need for insulin therapy. Type 2 diabetes is characterized by insulin resistance rather than overall insulin deficiency. Type 2 diabetes tends to be associated with comorbidities such as obesity and hypertension, which are independent risk factors for adverse perinatal outcomes.3,4
Gestational diabetes accounts for most cases of diabetes during pregnancy. Although both pregestational and gestational diabetes increase the risk of maternal and fetal complications, pregestational diabetes is associated with significantly greater risks.1
IMPACT OF DIABETES ON THE MOTHER
Pregnancy increases the risk of maternal hypoglycemia, especially during the first trimester in patients with type 1 diabetes, as insulin sensitivity increases in early pregnancy.1 Pregnant women with diabetes may also have an altered counterregulatory response and less hypoglycemic awareness.1 Insulin resistance rises during the second and early third trimesters, increasing the risk of hyperglycemia in women with diabetes.1
Glycemic control during pregnancy is usually easier to achieve in patients with type 2 diabetes than with type 1, but it may require much higher insulin doses.
Because pregnancy is inherently a ketogenic state, women with type 1 diabetes are at higher risk of diabetic ketoacidosis, particularly during the second and third trimesters.1 There are reports of euglycemic diabetic ketoacidosis in pregnant women with either gestational or pregestational diabetes.5
Diabetes is associated with a risk of preeclampsia 4 times higher than in nondiabetic women.6 Other potential pregnancy-related complications include infections, polyhydramnios, spontaneous abortion, and cesarean delivery.1,7 The risk of pregnancy loss is similar in women with either type 1 or type 2 diabetes (2.6% and 3.7%, respectively), but the causes are different.8 Although preexisting diabetic complications such as retinopathy, nephropathy, and gastroparesis can be exacerbated during pregnancy,1 only severe gastroparesis and advanced renal disease are considered relative contraindications to pregnancy.
IMPACT OF DIABETES ON THE FETUS
Fetal complications of maternal diabetes include embryopathy (fetal malformations) and fetopathy (overgrowth, ie, fetus large for gestational age, and increased risk of fetal death or distress). Maternal hyperglycemia is associated with diabetic embryopathy, resulting in major birth defects in 5% to 25% of pregnancies and spontaneous abortions in 15% to 20%.9,10 There is a 2- to 6-fold increase in risk of congenital malformations.6
The most common diabetes-associated congenital malformations affect the cardiovascular system. Congenital heart disease includes tetralogy of Fallot, transposition of the great vessels, septal defects, and anomalous pulmonary venous return. Other relatively common defects involve the fetal central nervous system, spine, orofacial system, kidneys, urogenital system, gastrointestinal tract, and skeleton.11
The risk of fetopathy is proportional to the degree of maternal hyperglycemia. Excess maternal glucose and fatty acid levels can lead to fetal hyperglycemia and overgrowth, which increases fetal oxygen requirements. Erythropoietin levels rise, causing an increase in red cell mass, with subsequent hyperviscosity within the placenta and higher risk of fetal death.
Other complications include intrauterine growth restriction, prematurity, and preterm delivery. Fetal macrosomia (birth weight > 90th percentile or 4 kg, approximately 8 lb, 13 oz) occurs in 27% to 62% of children born to mothers with diabetes, a rate 10 times higher than in patients without diabetes. It contributes to shoulder dystocia (risk 2 to 4 times higher in diabetic pregnancies) and cesarean delivery.6 Infants born to mothers with diabetes also have higher risks of neonatal hypoglycemia, erythrocytosis, hyperbilirubinemia, hypocalcemia, respiratory distress, cardiomyopathy, and death, as well as for developing diabetes, obesity, and other adverse cardiometabolic outcomes later in life.11
GET GLUCOSE UNDER CONTROL BEFORE PREGNANCY
Nearly half of pregnancies in the general population are unplanned,15 so preconception diabetes assessment needs to be part of routine medical care for all reproductive-age women. Because most organogenesis occurs during the first 5 to 8 weeks after fertilization—potentially before a woman realizes she is pregnant—achieving optimal glycemic control before conception is necessary to improve pregnancy outcomes.1
EVERY VISIT IS AN OPPORTUNITY
Every medical visit with a reproductive-age woman with diabetes is an opportunity for counseling about pregnancy. Topics that need to be discussed include the risks of unplanned pregnancy and of poor metabolic control, and the benefits of improved maternal and fetal outcomes with appropriate pregnancy planning and diabetes management.
Referral to a registered dietitian for individualized counseling about proper nutrition, particularly during pregnancy, has been associated with positive outcomes.16 Patients with diabetes and at high risk of pregnancy complications should be referred to a clinic that specializes in high-risk pregnancies.
Practitioners also should emphasize the importance of regular exercise and encourage patients to maintain or achieve a medically optimal weight before conception. Ideally, this would be a normal body mass index; however, this is not always possible.
In women who are planning pregnancy or are not on effective contraception, medications should be reviewed for potential teratogenicity. If needed, discuss alternative medications or switch to safer ones. However, these changes should not interrupt diabetes treatment.
In addition, ensure that the patient is up to date on age- and disease-appropriate preventive care (eg, immunizations, screening for sexually transmitted disease and malignancy). Counseling and intervention for use of tobacco, alcohol, and recreational drugs are also important. As with any preconception counseling, the patient (and her partner, if possible) should be advised to avoid travel to areas where Zika virus is endemic, and informed about the availability of expanded carrier genetic screening through her obstetric provider.
Finally, pregnant women with diabetes benefit from screening for diabetic complications including hypertension, retinopathy, cardiovascular disease, neuropathy, and nephropathy.
ASSESSING RISKS
Blood pressure
Chronic (preexisting) hypertension is defined as a systolic pressure 140 mm Hg or higher or a diastolic pressure 90 mm Hg or higher, or both, that antedates pregnancy or is present before the 20th week of pregnancy.3 Chronic hypertension has been reported in up to 5% of pregnant women and is associated with increased risk of preterm delivery, superimposed preeclampsia, low birth weight, and perinatal death.3
Reproductive-age women with diabetes and high blood pressure benefit from lifestyle and behavioral modifications.17 If drug therapy is needed, antihypertensive drugs that are safe for the fetus should be used. Treatment of mild or moderate hypertension during pregnancy reduces the risk of progression to severe hypertension but may not improve obstetric outcomes.
Diabetic retinopathy
Diabetic retinopathy can significantly worsen during pregnancy: the risk of progression is double that in the nonpregnant state.18 Women with diabetes who are contemplating pregnancy should have a comprehensive eye examination before conception, and any active proliferative retinopathy needs to be treated. These patients may require ophthalmologic monitoring and treatment during pregnancy. (Note: laser photocoagulation is not contraindicated during pregnancy.)
Cardiovascular disease
Cardiovascular physiology changes dramatically during pregnancy. Cardiovascular disease, especially when superimposed on diabetes, can increase the risk of maternal death. Thus, evaluation for cardiovascular risk factors as well as cardiovascular system integrity before conception is important. Listen for arterial bruits and murmurs, and assess peripheral pulses. Consideration should be given to obtaining a preconception resting electrocardiogram in women with diabetes who are over age 35 or who are suspected of having cardiovascular disease.16
Neurologic disorders
Peripheral neuropathy, the most common neurologic complication of diabetes, is associated with injury and infection.19
Autonomic neuropathy is associated with decreased cardiac responsiveness and orthostatic hypotension.19 Diabetic gastroparesis alone can precipitate serious complications during pregnancy, including extreme hypoglycemia and hyperglycemia, increased risk of diabetic ketoacidosis, weight loss, malnutrition, frequent hospitalizations, and increased requirement for parenteral nutrition.20
Although diabetic neuropathy does not significantly worsen during pregnancy, women with preexisting gastroparesis should be counseled on the substantial risks associated with pregnancy. Screening for neuropathy should be part of all diabetic preconception examinations.
Renal complications
Pregnancy in women with diabetes and preexisting renal dysfunction increases their risk of accelerated progression of diabetic kidney disease.21 Preexisting renal dysfunction also increases the risk of pregnancy-related complications, such as stillbirth, intrauterine growth restriction, gestational hypertension, preeclampsia, and preterm delivery.19,21,22 Further, the risk of pregnancy complications correlates directly with the severity of renal dysfunction.22
Psychiatric disorders
Emotional wellness is essential for optimal diabetes management. It is important to recognize the emotional impact of diabetes in pregnant women and to conduct routine screening for depression, anxiety, stress, and eating disorders.16
LABORATORY TESTS TO CONSIDER
Hemoglobin A1c. The general consensus is to achieve the lowest hemoglobin A1c level possible that does not increase the risk of hypoglycemia. The American Diabetes Association (ADA) recommends that, before attempting to conceive, women should lower their hemoglobin A1c to below 6.5%.1
Thyroid measures. Autoimmune thyroid disease is the most common autoimmune disorder associated with diabetes and has been reported in 35% to 40% of women with type 1 diabetes.23 Recommendations are to check thyroid-stimulating hormone and thyroid peroxidase antibody levels before conception or early in pregnancy in all women with diabetes.1,24 Overt hypothyroidism should be treated before conception, given that early fetal brain development depends on maternal thyroxine.
Renal function testing. Preconception assessment of renal function is important for counseling and risk stratification. This assessment should include serum creatinine level, estimated glomerular filtration rate, and urinary albumin excretion.21
Celiac screening. Because women with type 1 diabetes are more susceptible to autoimmune diseases, they should be screened for celiac disease before conception, with testing for immunoglobulin A (IgA) and tissue transglutaminase antibodies, with or without IgA endomysial antibodies.16,25,26 An estimated 6% of patients with type 1 diabetes have celiac disease vs 1% of the general population.25 Celiac disease is 2 to 3 times more common in women, and asymptomatic people with type 1 diabetes are considered at increased risk for celiac disease.26
The association between type 1 diabetes and celiac disease most likely relates to the overlap in human leukocyte antigens of the diseases. There is no established link between type 2 diabetes and celiac disease.25
Undiagnosed celiac disease increases a woman’s risk of obstetric complications such as preterm birth, low birth weight, and stillbirth.26 The most likely explanation for these adverse effects is nutrient malabsorption, which is characteristic of celiac disease. Adherence to a gluten-free diet before and during gestation may reduce the risk of preterm delivery by as much as 20%.26
Vitamin B12 level. Celiac disease interferes with the absorption of vitamin B12-instrinsic factor in the ileum, which can lead to vitamin B12 deficiency. Therefore, baseline vitamin B12 levels should be checked before conception in women with celiac disease. Levels should also be checked in women taking metformin, which also decreases vitamin B12 absorption. Of note, increased folate levels due to taking supplements can potentially mask vitamin B12 deficiency.
MEDICATIONS TO REVIEW FOR PREGNANCY INTERACTIONS
Diabetic medications
Insulin is the first-line pharmacotherapy for pregnant patients with type 1, type 2, or gestational diabetes. Insulin does not cross the placenta to a measurable extent, and most insulin preparations have been classified as category B,1 meaning no risks to the fetus have been found in humans.
Insulin dosing during pregnancy is not static. Beginning around mid-gestation, insulin requirements increase,28,29 but after 32 weeks the need may decrease. These changes require practitioners to closely monitor blood glucose throughout pregnancy.
Both basal-bolus injections and continuous subcutaneous infusion are reasonable options during pregnancy.30 However, the need for multiple and potentially painful insulin injections daily can lead to poor compliance. This inconvenience has led to studies using oral hypoglycemic medications instead of insulin for patients with gestational and type 2 diabetes.
Metformin is an oral biguanide that decreases hepatic gluconeogenesis and intestinal glucose absorption while peripherally increasing glucose utilization and uptake. Metformin does not pose a risk of hypoglycemia because its mechanism of action does not involve increased insulin production.7
Metformin does cross the placenta, resulting in umbilical cord blood levels higher than maternal levels. Nevertheless, studies support the efficacy and short-term safety of metformin use during a pregnancy complicated by gestational or type 2 diabetes.7,31 Moreover, metformin has been associated with a lower risk of neonatal hypoglycemia and maternal weight gain than insulin.32 However, this agent should be used with caution, as long-term data are not yet available, and it may slightly increase the risk of premature delivery.
Glyburide is another oral hypoglycemic medication that has been used during pregnancy. This second-generation sulfonylurea enhances the release of insulin from the pancreas by binding beta islet cell ATP-calcium channel receptors. Compared with other sulfonylureas, glyburide has the lowest rate of maternal-to-fetal transfer, with umbilical cord plasma concentrations 70% of maternal levels.33 Although some trials support the efficacy and short-term safety of glyburide treatment for gestational diabetes,34 recent studies have associated glyburide use during pregnancy with a higher rate of neonatal hypoglycemia, neonatal respiratory distress, macrosomia, and neonatal intensive care unit admissions than insulin and metformin.1,35
Patients treated with oral agents should be informed that these drugs cross the placenta, and that although no adverse effects on the fetus have been demonstrated, long-term safety data are lacking. In addition, oral agents are ineffective in type 1 diabetes and may be insufficient to overcome the insulin resistance in type 2 diabetes.
Antihypertensive drugs
All antihypertensive drugs cross the placenta, but several have an acceptable safety profile in pregnancy, including methyldopa, labetalol, clonidine, prazosin, and nifedipine. Hydralazine and labetalol are short-acting, come in intravenous formulations, and can be used for urgent blood pressure control during pregnancy. Diltiazem may be used for heart rate control during pregnancy, and it has been shown to lower blood pressure and proteinuria in pregnant patients with underlying renal disease.36,37 The ADA recommends against chronic use of diuretics during pregnancy because of potential reductions in maternal plasma volume and uteroplacental perfusion.1
Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and direct renin inhibitors are contraindicated during pregnancy because of the risk of fetal defects, particularly in the renal system.21,38 Although there is evidence to question the association between first semester exposure and fetotoxicity,39 we avoid these drugs during pregnancy and switch to a different agent in women planning pregnancy.
Other drugs
Statins are contraindicated in pregnancy because they interfere with the development of the fetal nervous system.21 Although preliminary data from a small study did not identify safety risks associated with pravastatin use after 12 weeks of gestation,40 we recommend discontinuing statins in women attempting pregnancy.
Aspirin. The US Preventive Services Task Force41 recommends low-dose aspirin (81 mg/day) after 12 weeks of gestation for women with type 1 or type 2 diabetes, as well as those with renal disease or chronic hypertension, to prevent preeclampsia. Of note, higher doses need to be used with caution during pregnancy because fetal abnormalities have been reported, such as disruption of fetal vasculature (mesenteric vessels), gastroschisis, and small intestinal atresia.16
Folate supplementation (0.6–4 mg/day) is recommended in women with celiac disease to prevent neural tube defects in the offspring, and the US Preventive Services Task Force recommends 0.4 mg daily of folic acid supplementation for all women planning or capable of pregnancy.42–44 Higher doses, ranging from 0.6 to 5 mg/day, have been proposed for patients with diabetes,13 and we recommend at least 1 mg for this group, based on data suggesting that higher doses further reduce the risk of neural tube defects.43
IS BREASTFEEDING AFFECTED?
Maternal diabetes, insulin therapy, and oral hypoglycemic agents are not contraindications to breastfeeding. The US Preventive Services Task Force recommends interventions by primary care physicians to promote and support breastfeeding.45 Breastfeeding is encouraged based on various short- and long-term health benefits for both breastfed infants and breastfeeding mothers. Breastfeeding decreases a woman’s insulin requirements and increases the risk for hypoglycemia, especially in patients with insulin-dependent type 1 diabetes.1
Additionally, insulin sensitivity increases immediately following delivery of the placenta.1 Therefore, it is prudent to adjust insulin doses postpartum, especially while a patient is breastfeeding, or to suggest high-carbohydrate snacks before feeds.9,29
Antihypertensive drugs considered safe to use during lactation include captopril, enalapril, quinapril, labetalol, propranolol, nifedipine, and hydralazine.21,46 Methyldopa is not contraindicated, but it causes fatigue and worsened postpartum depression and should not be used as first-line therapy. Diuretics and ARBs are not recommended during lactation.21 Both metformin and glyburide enter breast milk in small enough amounts that they are not contraindicated during breastfeeding.16 The Lactmed database (www.toxnet.nlm.nih.gov) provides information about drugs and breastfeeding.
WHAT ABOUT CONTRACEPTIVES?
The ADA recommends contraception for women with diabetes because, just as in women without diabetes, the risks of unplanned pregnancy outweigh those of contraceptives.1
We recommend low-dose combination estrogen-progestin oral contraceptives to normotensive women under age 35 with diabetes but without underlying microvascular disease. For women over age 35 or for those with microvascular disease, additional options include intrauterine devices or progestin implants. We prefer not to use injectable depot medroxyprogesterone acetate because of its side effects of insulin resistance and weight gain.47
CASE DISCUSSION: NEXT STEPS
Our patient’s interest in family planning presents an opportunity for preconception counseling. We recommend a prenatal folic acid supplement, diet and regular exercise for weight loss, and screening tests including a comprehensive metabolic panel, hemoglobin A1c, thyroid-stimulating hormone, and dilated eye examination. We make sure she is up to date on her indicated health maintenance (eg, immunizations, disease screening), and we review her medications for potential teratogens. She denies any recreational drug use. Also, she has no plans for long-distance travel.
Our counseling includes discussions of pregnancy risks associated with pregestational diabetes and suboptimal glycemic control. We encourage her to use effective contraception until she is “medically optimized” for pregnancy—ie, until her hemoglobin A1c is lower than 6.5% and she has achieved a medically optimal weight. If feasible, a reduction of weight (7% or so) through lifestyle modification should be attempted, and if her hemoglobin A1c remains elevated, adding insulin would be recommended.
Pregnant patients or patients contemplating pregnancy are usually motivated to modify their behavior, making this a good time to reinforce lifestyle modifications. Many patients benefit from individualized counseling by a registered dietitian to help achieve the recommended weight and glycemic control.
Our physical examination in this patient includes screening for micro- and macrovascular complications of diabetes, and the test results are negative. Patients with active proliferative retinopathy should be referred to an ophthalmologist for assessment and treatment.
We review her medications for potential teratogenic effects and stop her ACE inhibitor (lisinopril) and statin (simvastatin). We switch her from a first-generation sulfonylurea (chlorpropamide) to glyburide, a second-generation sulfonylurea. Second-generation sulfonylureas are considered more “fetus-friendly” because first-generation sulfonylureas cross the placenta more easily and can cause fetal hyperinsulinemia, leading to macrosomia and neonatal hypoglycemia.7
The management of diabetes during pregnancy leans toward insulin use, given the lack of information regarding long-term outcomes with oral agents. If insulin is needed, it is best to initiate it before the patient conceives, and then to stop other diabetes medications. We would not make any changes to her aspirin or metformin use.
Educating the patient and her family about prevention, recognition, and treatment of hypoglycemia is important to prevent and manage the increased risk of hypoglycemia with insulin therapy and in early pregnancy.1 Consideration should be given to providing ketone strips as well as education on diabetic ketoacidosis prevention and detection.1 If the patient conceives, begin prenatal care early to allow adequate planning for care of her disease and evaluation of the fetus. Because of the complexity of insulin management in pregnancy, the ADA recommends referral, if possible, to a center offering team-based care, including an obstetrician specialized in high-risk pregnancies, an endocrinologist, and a dietitian.1
- American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S137–S143. doi:10.2337/dc18-S013
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S13–S27. doi:10.2337/dc18-S002
- Lawler J, Osman M, Shelton JA, Yeh J. Population-based analysis of hypertensive disorders in pregnancy. Hypertens Pregnancy 2007; 26(1):67–76. doi:10.1080/10641950601147945
- Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015; 16(8):621–638. doi:10.1111/obr.12288
- Garrison EA, Jagasia S. Inpatient management of women with gestational and pregestational diabetes in pregnancy. Curr Diab Rep 2014; 14(2):457. doi:10.1007/s11892-013-0457-x
- Ballas J, Moore TR, Ramos GA. Management of diabetes in pregnancy. Curr Diab Rep 2012; 12(1):33–42. doi:10.1007/s11892-011-0249-0
- Ryu RJ, Hays KE, Hebert MF. Gestational diabetes mellitus management with oral hypoglycemic agents. Semin Perinatol 2014; 38(8):508–515. doi:10.1053/j.semperi.2014.08.012
- Cundy T, Gamble G, Neale L, et al. Differing causes of pregnancy loss in type 1 and type 2 diabetes. Diabetes Care 2007; 30(10):2603–2607. doi:10.2337/dc07-0555
- Castorino K, Jovanovic L. Pregnancy and diabetes management: advances and controversies. Clin Chem 2011; 57(2):221–230. doi:10.1373/clinchem.2010.155382
- Hammouda SA, Hakeem R. Role of HbA1c in predicting risk for congenital malformations. Prim Care Diabetes 2015; 9(6):458–464. doi:10.1016/j.pcd.2015.01.004
- Chen CP. Congenital malformations associated with maternal diabetes. Taiwanese J Obstet Gynecol 2005; 44(1):1–7. doi:10.1016/S1028-4559(09)60099-1
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33(3):676–682. doi:10.2337/dc09-1848
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36(5):1384–1395. doi:10.2337/dc12-2480
- HAPO Study Cooperative Research Group; Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358(19):1991–2002. doi:10.1056/NEJMoa0707943
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health 2014; 104(suppl 1):S43–S48. doi:10.2105/AJPH.2013.301416
- Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care 2008; 31(5):1060–1079. doi:10.2337/dc08-9020
- Webster LM, Conti-Ramsden F, Seed PT, Webb AJ, Nelson-Piercy C, Chappell LC. Impact of antihypertensive treatment on maternal and perinatal outcomes in pregnancy complicated by chronic hypertension: a systematic review and meta-analysis. J Am Heart Assoc 2017; 6(5).pii:e005526. doi:10.1161/JAHA.117.005526
- Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy: the Diabetes in Early Pregnancy Study. Diabetes Care 1995; 18(5):631–637. pmid:8586000
- American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care 2016; 39 (suppl 1):S1–S109.
- Hawthorne, G. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):77–90. doi:10.1016/j.bpobgyn.2010.10.015
- Ringholm L, Damm JA, Vestgaard M, Damm P, Mathiesen ER. Diabetic nephropathy in women with preexisting diabetes: from pregnancy planning to breastfeeding. Curr Diab Rep 2016; 16(2):12. doi:10.1007/s11892-015-0705-3
- Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 2015; 10(11):1964–1978. doi:10.2215/CJN.09250914
- Umpierrez GE, Latif KA, Murphy MB, et al. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care 2003; 26(4):1181–1185. pmid:12663594
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Akirov A, Pinhas-Hamiel O. Co-occurrence of type 1 diabetes mellitus and celiac disease. World J Diabetes 2015; 6(5):707–714. doi:10.4239/wjd.v6.i5.707
- Saccone G, Berghella V, Sarno L, et al. Celiac disease and obstetric complications: a systematic review and metaanalysis. Am J Obstet Gynecol 2016; 214(2):225–234. doi:10.1016/j.ajog.2015.09.080
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
- de Valk HW, Visser GH. Insulin during pregnancy, labour and delivery. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):65–76. doi:10.1016/j.bpobgyn.2010.10.002
- Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int J Gen Med 2011; 4:827–835. doi:10.2147/IJGM.S26889
- Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2016; (6):CD005542. doi:10.1002/14651858.CD005542.pub2
- Charles B, Norris R, Xiao X, Hague W. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit 2006; 28(1):67–72. pmid:16418696
- Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 2015; 350:h102. doi:10.1136/bmj.h102
- Hebert MF, Ma X, Naraharisetti SB, et al; Obstetric-Fetal Pharmacology Research Unit Network. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009; 85(6):607–614. doi:10.1038/clpt.2009.5
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000; 343(16):1134–1138. doi:10.1056/NEJM200010193431601
- Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr, Jonsson Funk M. Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr 2015; 169:452–458. doi:10.1001/jamapediatrics.2015.74
- Gowda RM, Khan IA, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac arrhythmias in pregnancy: clinical and therapeutic considerations. Int J Cardiol 2003; 88(2):129–133. pmid:12714190
- Khandelwal M, Kumanova M, Gaughan JP, Reece EA. Role of diltiazem in pregnant women with chronic renal disease. J Matern Fetal Neonatal Med 2002; 12(6):408–412. doi:10.1080/jmf.12.6.408.412
- Magee LA, Abalos E, von Dadelszen P, Sibai B, Easterling T, Walkinshaw S; CHIPS Study Group. How to manage hypertension in pregnancy effectively. Br J Clin Pharmacol 2011; 72(3):394–401. doi:10.1111/j.1365-2125.2011.04002.x
- Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 2006; 354(23):2443–2451. doi:10.1056/NEJMoa055202
- Costantine MM, Cleary K, Hebert MF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 2016; 214(6):720.e1–720.e17. doi:10.1016/j.ajog.2015.12.038
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161(11):819–826. doi:10.7326/M14-1884
- Curry SJ, Grossman DC, Whitlock EP, Cantu A. Behavioral counseling research and evidence-based practice recommendations: US Preventive Services Task Force perspectives. Ann Intern Med 2014; 160(6):407–413. doi:10.7326/M13-2128
- Wald N, Law M, Morris J, Wald D. Quantifying the effect of folic acid. Lancet 2001; 358(9298):2069–2073. pmid:11755633
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. JAMA 2017; 317(2):183–189. doi:10.1001/jama.2016.19438
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Primary care interventions to support breastfeeding: US Preventive Services Task Force recommendation statement. JAMA 2016; 316(16):1688–1693. doi:10.1001/jama.2016.14697
- Newton ER, Hale TW. Drugs in breast milk. Clin Obstet Gynecol 2015; 58(4):868–884. doi:10.1097/GRF.0000000000000142
- Xiang AH, Kawakubo M, Kjos SL, Buchanan TA. Long-acting injectable progestin contraception and risk of type 2 diabetes in Latino women with prior gestational diabetes mellitus. Diabetes Care 2006; 29(3):613–617. pmid:16505515
- American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S137–S143. doi:10.2337/dc18-S013
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S13–S27. doi:10.2337/dc18-S002
- Lawler J, Osman M, Shelton JA, Yeh J. Population-based analysis of hypertensive disorders in pregnancy. Hypertens Pregnancy 2007; 26(1):67–76. doi:10.1080/10641950601147945
- Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015; 16(8):621–638. doi:10.1111/obr.12288
- Garrison EA, Jagasia S. Inpatient management of women with gestational and pregestational diabetes in pregnancy. Curr Diab Rep 2014; 14(2):457. doi:10.1007/s11892-013-0457-x
- Ballas J, Moore TR, Ramos GA. Management of diabetes in pregnancy. Curr Diab Rep 2012; 12(1):33–42. doi:10.1007/s11892-011-0249-0
- Ryu RJ, Hays KE, Hebert MF. Gestational diabetes mellitus management with oral hypoglycemic agents. Semin Perinatol 2014; 38(8):508–515. doi:10.1053/j.semperi.2014.08.012
- Cundy T, Gamble G, Neale L, et al. Differing causes of pregnancy loss in type 1 and type 2 diabetes. Diabetes Care 2007; 30(10):2603–2607. doi:10.2337/dc07-0555
- Castorino K, Jovanovic L. Pregnancy and diabetes management: advances and controversies. Clin Chem 2011; 57(2):221–230. doi:10.1373/clinchem.2010.155382
- Hammouda SA, Hakeem R. Role of HbA1c in predicting risk for congenital malformations. Prim Care Diabetes 2015; 9(6):458–464. doi:10.1016/j.pcd.2015.01.004
- Chen CP. Congenital malformations associated with maternal diabetes. Taiwanese J Obstet Gynecol 2005; 44(1):1–7. doi:10.1016/S1028-4559(09)60099-1
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, et al. International Association of Diabetes and Pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33(3):676–682. doi:10.2337/dc09-1848
- Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36(5):1384–1395. doi:10.2337/dc12-2480
- HAPO Study Cooperative Research Group; Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358(19):1991–2002. doi:10.1056/NEJMoa0707943
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health 2014; 104(suppl 1):S43–S48. doi:10.2105/AJPH.2013.301416
- Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care 2008; 31(5):1060–1079. doi:10.2337/dc08-9020
- Webster LM, Conti-Ramsden F, Seed PT, Webb AJ, Nelson-Piercy C, Chappell LC. Impact of antihypertensive treatment on maternal and perinatal outcomes in pregnancy complicated by chronic hypertension: a systematic review and meta-analysis. J Am Heart Assoc 2017; 6(5).pii:e005526. doi:10.1161/JAHA.117.005526
- Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy: the Diabetes in Early Pregnancy Study. Diabetes Care 1995; 18(5):631–637. pmid:8586000
- American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care 2016; 39 (suppl 1):S1–S109.
- Hawthorne, G. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):77–90. doi:10.1016/j.bpobgyn.2010.10.015
- Ringholm L, Damm JA, Vestgaard M, Damm P, Mathiesen ER. Diabetic nephropathy in women with preexisting diabetes: from pregnancy planning to breastfeeding. Curr Diab Rep 2016; 16(2):12. doi:10.1007/s11892-015-0705-3
- Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 2015; 10(11):1964–1978. doi:10.2215/CJN.09250914
- Umpierrez GE, Latif KA, Murphy MB, et al. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care 2003; 26(4):1181–1185. pmid:12663594
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Akirov A, Pinhas-Hamiel O. Co-occurrence of type 1 diabetes mellitus and celiac disease. World J Diabetes 2015; 6(5):707–714. doi:10.4239/wjd.v6.i5.707
- Saccone G, Berghella V, Sarno L, et al. Celiac disease and obstetric complications: a systematic review and metaanalysis. Am J Obstet Gynecol 2016; 214(2):225–234. doi:10.1016/j.ajog.2015.09.080
- Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol 2015; 39(7):512–519. doi:10.1053/j.semperi.2015.08.003
- de Valk HW, Visser GH. Insulin during pregnancy, labour and delivery. Best Pract Res Clin Obstet Gynaecol 2011; 25(1):65–76. doi:10.1016/j.bpobgyn.2010.10.002
- Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int J Gen Med 2011; 4:827–835. doi:10.2147/IJGM.S26889
- Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2016; (6):CD005542. doi:10.1002/14651858.CD005542.pub2
- Charles B, Norris R, Xiao X, Hague W. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit 2006; 28(1):67–72. pmid:16418696
- Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ 2015; 350:h102. doi:10.1136/bmj.h102
- Hebert MF, Ma X, Naraharisetti SB, et al; Obstetric-Fetal Pharmacology Research Unit Network. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther 2009; 85(6):607–614. doi:10.1038/clpt.2009.5
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med 2000; 343(16):1134–1138. doi:10.1056/NEJM200010193431601
- Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr, Jonsson Funk M. Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr 2015; 169:452–458. doi:10.1001/jamapediatrics.2015.74
- Gowda RM, Khan IA, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac arrhythmias in pregnancy: clinical and therapeutic considerations. Int J Cardiol 2003; 88(2):129–133. pmid:12714190
- Khandelwal M, Kumanova M, Gaughan JP, Reece EA. Role of diltiazem in pregnant women with chronic renal disease. J Matern Fetal Neonatal Med 2002; 12(6):408–412. doi:10.1080/jmf.12.6.408.412
- Magee LA, Abalos E, von Dadelszen P, Sibai B, Easterling T, Walkinshaw S; CHIPS Study Group. How to manage hypertension in pregnancy effectively. Br J Clin Pharmacol 2011; 72(3):394–401. doi:10.1111/j.1365-2125.2011.04002.x
- Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 2006; 354(23):2443–2451. doi:10.1056/NEJMoa055202
- Costantine MM, Cleary K, Hebert MF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol 2016; 214(6):720.e1–720.e17. doi:10.1016/j.ajog.2015.12.038
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161(11):819–826. doi:10.7326/M14-1884
- Curry SJ, Grossman DC, Whitlock EP, Cantu A. Behavioral counseling research and evidence-based practice recommendations: US Preventive Services Task Force perspectives. Ann Intern Med 2014; 160(6):407–413. doi:10.7326/M13-2128
- Wald N, Law M, Morris J, Wald D. Quantifying the effect of folic acid. Lancet 2001; 358(9298):2069–2073. pmid:11755633
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. JAMA 2017; 317(2):183–189. doi:10.1001/jama.2016.19438
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Primary care interventions to support breastfeeding: US Preventive Services Task Force recommendation statement. JAMA 2016; 316(16):1688–1693. doi:10.1001/jama.2016.14697
- Newton ER, Hale TW. Drugs in breast milk. Clin Obstet Gynecol 2015; 58(4):868–884. doi:10.1097/GRF.0000000000000142
- Xiang AH, Kawakubo M, Kjos SL, Buchanan TA. Long-acting injectable progestin contraception and risk of type 2 diabetes in Latino women with prior gestational diabetes mellitus. Diabetes Care 2006; 29(3):613–617. pmid:16505515
KEY POINTS
- Aim for a hemoglobin A1c of 6.5% or lower, if it is attainable without increasing the risk of hypoglycemia.
- Avoid teratogenic drugs in sexually active women of childbearing age unless the patient uses effective contraception.
- Because about half of pregnancies are unplanned, it is important to routinely discuss family planning and provide preconception counseling that includes reducing risks associated with pregnancy.
- Screen for diabetic end-organ damage, especially retinopathy and nephropathy.
What works best for genitourinary syndrome of menopause: vaginal estrogen, vaginal laser, or combined laser and estrogen therapy?
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.
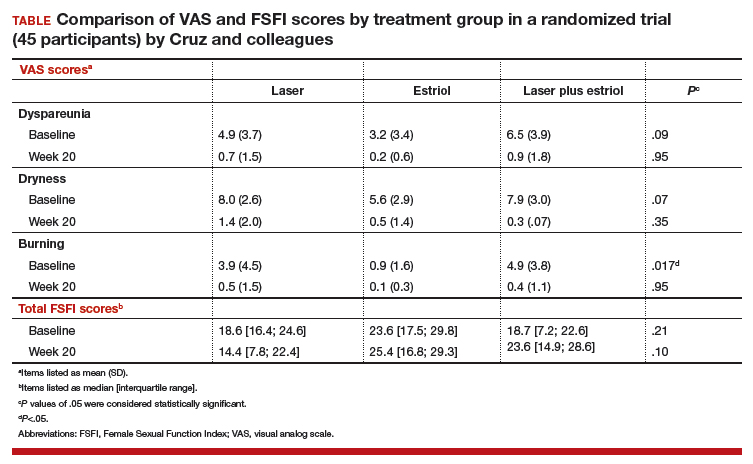
While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.

While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.

While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
August 2018 Digital Edition
Click here to access the August 2018 Digital Edition.
Table of Contents
- Access to Transplant Care and Services Within the Veterans Health Administration
- VA Boston Medical Forum: A Veteran With Fibromyalgia Presenting With Dyspnea
- Expanding the Scope of Telemedicine in Gastroenterology
- Acute Aortic Occlusion With Spinal Cord Infarction
- Cognitive Behavioral Therapy for Veterans With Tinnitus
- Am I My Brother’s/Sister’s Keeper?
- Louis Johnson: First Cold War Secretary of Defense

Click here to access the August 2018 Digital Edition.
Table of Contents
- Access to Transplant Care and Services Within the Veterans Health Administration
- VA Boston Medical Forum: A Veteran With Fibromyalgia Presenting With Dyspnea
- Expanding the Scope of Telemedicine in Gastroenterology
- Acute Aortic Occlusion With Spinal Cord Infarction
- Cognitive Behavioral Therapy for Veterans With Tinnitus
- Am I My Brother’s/Sister’s Keeper?
- Louis Johnson: First Cold War Secretary of Defense

Click here to access the August 2018 Digital Edition.
Table of Contents
- Access to Transplant Care and Services Within the Veterans Health Administration
- VA Boston Medical Forum: A Veteran With Fibromyalgia Presenting With Dyspnea
- Expanding the Scope of Telemedicine in Gastroenterology
- Acute Aortic Occlusion With Spinal Cord Infarction
- Cognitive Behavioral Therapy for Veterans With Tinnitus
- Am I My Brother’s/Sister’s Keeper?
- Louis Johnson: First Cold War Secretary of Defense

Cervical screening recommendations do not cover all circumstances
Cervical screening recommendations do not cover all circumstances
Starting cervical cancer screening at age 21 does not necessarily take into account the fact that we are seeing youngsters initiating sexual activity as young as age 9. We obviously see pregnancies early as well. Waiting to screen until age 21, therefore, may cause us to miss the development of high-grade lesions and cervical cancer. As you know, cases in the literature report instances of invasive cancer with first Pap test at age 21. Also, human papillomavirus (HPV) is spread by sexual activity, with the squamous columnar junction more susceptible to infection at a young age.
Recommendations regarding cervical cancer screening for older women also should take into account new sexual partners. Currently, both men and women are living longer and are remarrying or are sexually active with multiple partners. The fact that older women are desiring hormone replacement for vaginal lubrication and dyspareunia shows that they are sexually active even in their late 70s. I believe that the incidence of HPV infection to cervical, vaginal, and vulvar tissue will be increasing as a result.
In an age in which primary care physicians do not have time to perform Pap tests or vaginal, cervical, and vulvar exams because they are overwhelmed with keeping up with patients’ major medical issues is a misunderstanding regarding current recommendations for Pap test screening.
Elizabeth Reinoehl-McClaskey, DO
Onley, Virginia
Dr. Einstein responds
Sexual behavior can start early, but this does not lead to cancer. When we screen, we are looking for cancer, not HPV infection, which is quite common in women and men younger than age 21. Also, one might question whether current screening techniques pick up early-onset tumors. Regarding older women, sexual activity and the rate of older women getting cervical cancer should be considered in future guidelines.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Cervical screening recommendations do not cover all circumstances
Starting cervical cancer screening at age 21 does not necessarily take into account the fact that we are seeing youngsters initiating sexual activity as young as age 9. We obviously see pregnancies early as well. Waiting to screen until age 21, therefore, may cause us to miss the development of high-grade lesions and cervical cancer. As you know, cases in the literature report instances of invasive cancer with first Pap test at age 21. Also, human papillomavirus (HPV) is spread by sexual activity, with the squamous columnar junction more susceptible to infection at a young age.
Recommendations regarding cervical cancer screening for older women also should take into account new sexual partners. Currently, both men and women are living longer and are remarrying or are sexually active with multiple partners. The fact that older women are desiring hormone replacement for vaginal lubrication and dyspareunia shows that they are sexually active even in their late 70s. I believe that the incidence of HPV infection to cervical, vaginal, and vulvar tissue will be increasing as a result.
In an age in which primary care physicians do not have time to perform Pap tests or vaginal, cervical, and vulvar exams because they are overwhelmed with keeping up with patients’ major medical issues is a misunderstanding regarding current recommendations for Pap test screening.
Elizabeth Reinoehl-McClaskey, DO
Onley, Virginia
Dr. Einstein responds
Sexual behavior can start early, but this does not lead to cancer. When we screen, we are looking for cancer, not HPV infection, which is quite common in women and men younger than age 21. Also, one might question whether current screening techniques pick up early-onset tumors. Regarding older women, sexual activity and the rate of older women getting cervical cancer should be considered in future guidelines.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Cervical screening recommendations do not cover all circumstances
Starting cervical cancer screening at age 21 does not necessarily take into account the fact that we are seeing youngsters initiating sexual activity as young as age 9. We obviously see pregnancies early as well. Waiting to screen until age 21, therefore, may cause us to miss the development of high-grade lesions and cervical cancer. As you know, cases in the literature report instances of invasive cancer with first Pap test at age 21. Also, human papillomavirus (HPV) is spread by sexual activity, with the squamous columnar junction more susceptible to infection at a young age.
Recommendations regarding cervical cancer screening for older women also should take into account new sexual partners. Currently, both men and women are living longer and are remarrying or are sexually active with multiple partners. The fact that older women are desiring hormone replacement for vaginal lubrication and dyspareunia shows that they are sexually active even in their late 70s. I believe that the incidence of HPV infection to cervical, vaginal, and vulvar tissue will be increasing as a result.
In an age in which primary care physicians do not have time to perform Pap tests or vaginal, cervical, and vulvar exams because they are overwhelmed with keeping up with patients’ major medical issues is a misunderstanding regarding current recommendations for Pap test screening.
Elizabeth Reinoehl-McClaskey, DO
Onley, Virginia
Dr. Einstein responds
Sexual behavior can start early, but this does not lead to cancer. When we screen, we are looking for cancer, not HPV infection, which is quite common in women and men younger than age 21. Also, one might question whether current screening techniques pick up early-onset tumors. Regarding older women, sexual activity and the rate of older women getting cervical cancer should be considered in future guidelines.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
How Does Your PICCOMPARE? A Pilot Randomized Controlled Trial Comparing Various PICC Materials in Pediatrics
Peripherally inserted central catheters (PICCs) have evolved since their inception in the early 1970s and are used with increasing frequency for pediatric inpatients and outpatients.1-3 Emerging literature, including a meta-analysis of international observational studies,4 reports PICC failure (complications necessitating premature removal) occurs in up to 30% of PICCs, most commonly due to infection, thrombosis, occlusion, and fracture.4-7 Raffini et al.7 report the increasing incidence of pediatric PICC-related thrombosis increases morbidity and mortality8 and negatively impacts future vessel health and preservation.9
PICCs have progressed from relatively simple, silicone-based catheters with an external clamp to chemically engineered polyurethane with pressure-activated valves placed at the proximal or distal catheter hub with the intent to reduce occlusion.10 Further modernization of PICC material occurred with the incorporation of antithrombogenic (AT) material (Endexo®). These PICCs are designed to contain a nonstick polymer, which is designed to reduce the adherence of blood components (platelets and clotting factors) and inhibit thrombus formation (and hence prevent deep vein thrombosis andocclusion, as well as inhibit microbial biofilm attachment [and subsequent infection]).11
In addition to new materials, other aspects of this PICC design have been the addition of a pressure-activated safety valve (PASV®) built into the proximal hub. Pressure-activated valve technology promises to prevent catheter occlusion by reducing blood reflux into the PICC; the valve opens with pressure during infusion and aspiration and remains closed with normal venous pressure, circumventing the need for clinicians to manually clamp the PICC and reducing human error and the potential for thrombosis, occlusion, and fracture development.12 Hoffer et al.13 reported half as many occlusions of valved PICCs (3.3%) compared with nonvalved or clamped PICCs (7.1%); although not statistically significant (P = .10), perhaps due to the small sample, overall complications, including occlusion and infection, were significantly lessened with the valved PICC (35% vs 79%; P = .02). Comparatively, Pittiruti et al.14 conducted a trial of 2 types of valved PICCs with an open-ended, nonvalved PICC and found no reduction in PICC occlusion or catheter malfunction.
Today, PICC use is common for patients who require short-to-medium intravenous therapy. PICCs are increasingly recognized for their significant complications, including thrombosis and infection.15 Novel PICC technology, including the incorporation of AT material such as Endexo® and PASV®, may reduce complications; however, the clinical efficacy, cost-effectiveness, and acceptability of these innovations have not been tested through randomized trials in pediatric patients. In accordance with Medical Research Council guidelines16 for developing interventions, we pilot tested the feasibility of the BioFlo® PICC, including intervention acceptability, compliance, recruitment, and initial estimates of effect, in anticipation of a subsequent full-scale efficacy randomized controlled trial. Our secondary aim was to compare the effectiveness of the BioFlo® PICC with Endexo® and PASV® technology in reducing PICC complications and failure.
METHODS
Design
We undertook a pilot randomized controlled trial comparing the standard polyurethane PICC (with external clamp) with the BioFlo® PICC (with internal valve) in preventing catheter failure in pediatric patients. The study was prospectively registered with the Australian Clinical Trials Registry (ACTRN12615001290583), and the research protocol was published.17
Study Setting
The study commenced in March 2016 at the Lady Cilento Children’s Hospital in South Brisbane, Australia, a tertiary-level, specialist, pediatric teaching hospital in Queensland, Australia, providing full-spectrum health services to children and young people from birth to 18 years of age. Recruitment, including data collection, was completed in November 2016.
Sample
The target sample size was 110 participants, 50 participants per group plus 10% for potential attrition, as determined by standard pilot trial sample size recommendations.18 With ethics approval, the sample size was later increased to 150 participants in order to adequately pilot a microbiological substudy method (published separately).17 Participants were consecutively recruited if they met the inclusion criteria: PICC insertion, age <18 years, predicted hospital stay >24 hours, single-lumen PICC, and written informed consent by an English-speaking, legal parent or guardian. Patients were excluded if they had a current (<48 hours) blood stream infection (BSI), vessel size <2 mm, could not speak English without an interpreter, required a multilumen PICC, or were previously enrolled in the study.
Interventions
Participants were randomized to receive either of the following PICCs: (1) standard care: Cook™ polyurethane, turbo-ject, power-injectable PICC (Cook Medical, Bloomington, IN) or (2) comparison: BioFlo® polyurethane with Endexo® technology (AngioDynamics Inc, Queensbury, NY).
Outcomes
The primary outcome was feasibility of a full-efficacy trial established by composite analysis of the elements of eligibility (>70% of patients will be eligible), recruitment (>70% of patients will agree to enroll), retention and attrition (<15% of participants are lost to follow-up or withdraw from the study), protocol adherence (>80% of participants receive their allocated, randomly assigned study product), missing data (<10% of data are missed during data collection), parent and healthcare staff satisfaction, and PICC failure effect size estimates to allow sample size calculations.18,19 PICC failure was defined as the following complications associated with PICC removal: (1) catheter-associated BSI,8,20-22 (2) local site infection,22 (3) venous thrombosis,23 (4) occlusion,24,25 (5) PICC fracture, or (6) PICC dislodgement.25,26 Parents (or caregivers) and healthcare staff were asked to rate their level of confidence with the study product and ease of PICC removal by using a 0 to 100 numeric rating scale (NRS) of increasing confidence and/or ease. These data were collected at the time of PICC removal. Operators were also asked to rate their levels of satisfaction with the insertion equipment and ease of PICC insertion immediately upon completion of the insertion procedure (both 0-100 NRS of increasing satisfaction and/or ease). Secondary outcomes included individual PICC complications (eg, occlusion) occurring at any time point during the PICC dwell (including at removal), adverse events, pain, redness at the insertion site, and overall PICC dwell.
Study Procedures
The research nurse (ReN) screened operating theater lists for patients, obtained written informed consent, and initiated the randomization. Randomization was computer generated, and web based via Griffith University (https://www151.griffith.edu.au/random) to ensure allocation concealment until study entry. Patients were randomly assigned in a 1:1 ratio with computer-generated and randomly varied block sizes of 2 and 4. Data were collected by the ReN on the day of insertion, at day 1 postinsertion, then every 2 to 3 days thereafter so that PICCs were checked at least twice per week until study completion. Participants were included in the trial until 12 weeks post-PICC insertion, study withdrawal or PICC removal (whichever came first), with an additional 48 hours follow-up for infection outcomes. Patient review was face to face during the inpatient stay, with discharged patients’ follow-up occurring via outpatient clinics, hospital-in-the-home service, or telephone.
Data collection was via Research Electronic Data Capture (http://project-redcap.org/). The ReN collected data on primary and secondary outcomes by using the predefined criteria. Demographic and clinical data were collected to assess the success of randomization, describe the participant group, and display characteristics known to increase the risk of PICC complication and thrombosis. A blinded radiologist and infectious disease specialist reviewed and diagnosed thrombosis of deep veins and catheter-associated BSI outcomes, respectively.
PICC Procedures
Extensive prestudy education for 2 months prior to trial commencement was provided to all clinicians involved with the insertion and care of PICCs, including the study products. PICCs were inserted in an operating theater environment by a qualified consultant pediatric anesthetist, a senior anesthetic registrar or fellow in an approved anesthetic training program, or pediatric vascular access nurse practitioner. Ultrasound guidance was used to assess a patient’s vasculature and puncture the vessel. The operator chose the PICC size on the basis of clinical judgment of vessel size and patient needs and then inserted the allocated PICC.27 Preferred PICC tip location was the cavoatrial junction. All PICC tip positions were confirmed with a chest x-ray before use.
Postinsertion, PICCs were managed by local interdisciplinary clinicians in accordance with local practice guidelines.27-31 PICC care and management includes the use of 2% chlorhexidine gluconate in 70% alcohol for site antisepsis and neutral displacement needleless connectors (TUTA Pulse; Medical Australia Limited, Lidcombe, New South Wales, Australia); normal saline was used to flush after medication administration, and if the device was not in use for 6 hours or longer, heparin is instilled with securement via bordered polyurethane dressing (Tegaderm 1616; 3M, St Paul, Minnesota) and a sutureless securement device (Statlock VPPCSP; Bard, Georgia).
Statistical Analyses
Data were exported to Stata 1532 for cleaning and analysis. Data cleaning of outlying figures and missing and implausible data was undertaken prior to analysis. Missing data were not imputed. The PICC was the unit of measurement, and all randomly assigned patients were analyzed on an intention-to-treat basis.33 Descriptive statistics (frequencies and percentages) were used to ascertain the primary outcome of feasibility for the larger trial. Incidence rates (per 1,000 catheter days) and rate ratios, including 95% confidence intervals (CIs), were calculated. The comparability of groups at baseline was described across demographic, clinical, and device characteristics. Kaplan-Meier survival curves (with log-rank tests) were used to compare PICC failure between study groups over time. Associations between baseline characteristics and failure were described by calculating hazard ratios (HRs). Univariable Cox regression was performed only due to the relatively low number of outcomes. P values of <.05 were considered statistically significant.
Ethics
The Children’s Health Service District, Queensland (Human Research Ethics Committee/15/QRCH/164), and Griffith University (2016/077) Human Research Ethics Committees provided ethics and governance approval. Informed consent was obtained from parents or legal guardians, with children providing youth assent if they were 7 years or older, dependent upon cognitive ability.
RESULTS
Participant and PICC Characteristics
Feasibility Outcomes
PICC Failure and Complications
As per supplementary Table 1, univariate Cox regression identified PICC failure as significantly associated with tip placement in the proximal superior vena cava (SVC) compared to the SVC–right atrium junction (HR 2.61; 95% CI, 1.17-5.82; P = .024). Reduced risk of PICC failure was significantly associated with any infusion during the dwell (continuous fluid infusion, P = .007; continuous antibiotic, P = .042; or intermittent infusion, P = .046) compared to no infusion. Other variables potentially influencing the risk of failure included PICC insertion by nurse specialist compared to consultant anesthetist (HR 2.61; 95% CI, 0.85-5.44) or registrar (HR 1.97; 95% CI, 0.57-6.77). These differences were not statistically significant; however, baseline imbalance between study groups for this variable and the feasibility design preclude absolute conclusions.
DISCUSSION
This is the first pilot feasibility trial of new PICC materials and valve design incorporated in the BioFlo® PICC in the pediatric population. The trial incorporated best practice for randomized trials, including using a concurrent control group, centralized and concealed randomization, predetermined feasibility criteria, and a registered and published trial protocol.17 As in other studies,15,24,34 PICC failure and complication prevalence was unacceptably high for this essential device. Standard care PICCs failed twice as often as the new BioFlo® PICCs (22% vs 11%), which is a clinically important difference. As researchers in a pilot study, we did not expect to detect statistically significant differences; however, we found that overall complications during the dwell occurred significantly more with the standard care than BioFlo® PICCs (P = .009).
BioFlo® PICC material offers a major advancement in PICC material through the incorporation of AT technologies into catheter materials, such as PICCs. Endexo® is a low molecular–weight, fluoro-oligomeric additive that self-locates to the top few nanometers of the material surface. When added to power-injectable polyurethane, the additive results in a strong but passive, nonstick, fluorinated surface in the base PICC material. This inhibits platelet adhesion, suppresses protein procoagulant conformation, and thereby reduces thrombus formation in medical devices. Additionally, Endexo® is not a catheter coating; rather, it is incorporated within the polyurethane of the PICC, thereby ensuring these AT properties are present on the internal, external, and cut surfaces of the PICC. If this technology can reduce complication during treatment and reduce failure from infection, thrombosis, occlusion, fracture, and dislodgement, it will improve patient outcomes considerably and lower health system costs. Previous studies investigating valve technology in PICC design to reduce occlusion have been inconclusive.12-14,35,36 Occlusion (both partial and complete) was less frequent in our study with the BioFlo® group (n = 3; 4%) compared to the standard care group (n = 6; 8%). The results of this pilot study suggest that either the Endexo® material or PASV® technology has a positive association with occlusion reduction during PICC treatment.
Thrombosis was the primary failure type for the standard care PICCs, comprising one-third of failures. All but one patient with radiologically confirmed thrombosis required the removal of the PICC prior to completion of treatment. The decision to remove the PICC or retain and treat conservatively remained with the treating team. Raffini et al.7 found thrombosis to increase in patients with one or more coexisting chronic medical condition. Slightly more standard care than BioFlo® patients were free of such comorbidities (25% vs 16%), yet standard care patients still had the higher number of thromboses (7% vs 3%). Morgenthaler and Rodriguez37 reported vascular access-associated thrombosis in pediatrics to be less common than in adults but higher in medically complex children. Worryingly, Menendez et al.38 reported pediatric thrombosis to be largely asymptomatic, so the true incidence in our study is likely higher because only radiologically confirmed thromboses were recorded.
Occlusion (partial or complete) was the predominant complication across the study, being associated with one-third of all failures. When occlusion complications during the dwell (some of which were resolved with treatment), in addition to those causing failure, were considered, this number was even greater. Occlusion complications are prevalent and costly. Smith et al.24 reported that occlusion was the most common reason for PICC removal and the most likely complication to delay treatment. Both the BioFlo® and standard care PICCs are pressure rated with good tensile strength; however, fracture occurred in 4% (n = 3) of standard care PICCs compared to no fractures in BioFlo® PICCs. Although the numbers are small, it may suggest a superior tensile strength of the BioFlo® material.
This study reinforces previously published results24,38 that PICC tip position is important and can influence complications, such as occlusion and thrombosis. In addition, we found a significant association with failure when PICCs did not have a continuous infusion. These findings reinforce the need for optimal tip location at insertion and ongoing flushing and maintenance of PICCs not used for infusions.
Limitations of this study include the small sample size, which was not designed to detect statistical differences in the primary outcome between groups. Despite randomization, there were slight imbalances at baseline for inserter type and leukocyte count, although these were not significantly associated with PICC failure in the Cox regression (data not shown), and thus were unlikely to influence findings. Additionally, a difference of <10% was associated with PICC tip position, favoring the BioFlo® group. PICC tip position outside the cavoatrial junction was positively associated with failure; therefore, the effect of tip positioning on outcomes is difficult to ascertain given the small sample size and feasibility nature of the study. Further study is warranted to further explore this effect. The population sampled was pediatric medical and surgical inpatients with a vessel size >2 mm attending the operating theater suite for PICC insertion, thereby limiting the study’s generalizability to adults and other populations, including neonates and those with PICCs inserted in the pediatric intensive care unit. The study could not be blinded because study products had to be visible to the clinical and research staff. However, it is unlikely that staff would intentionally sabotage PICCs to bias the study. Blinding was possible for the assessment of blood culture and ultrasound reports to diagnose infection and thrombosis. Strengths of this study included 100% protocol adherence, and no patients were lost to follow-up.
CONCLUSION
These results confirm that PICC failure is unacceptably high and suggest that the innovative BioFlo® PICC material and design holds promise to improve PICC outcomes by reducing complications and overall PICC failure. Trials of this technology are feasible, safe, and acceptable to healthcare staff and parents. Further trials are required, including in other patient populations, to definitively identify clinical, cost-effective methods to prevent PICC failure and improve reliability during treatment.
Acknowledgments
The authors thank the children and parents of Lady Cilento Children’s Hospital for participating in this important research. A special thank you goes to the nurses within the Vascular Assessment and Management Service and to Karen Turner, Julieta Woosley, and Anna Dean for their efforts in data collecting and ensuring protocol adherence.
Disclosure
Griffith University has received unrestricted, investigator-initiated research or educational grants to support the research of T. K., A. J. U., and C. R. M. from product manufacturers 3M, Adhezion Inc, AngioDynamics, Bard Medical, Baxter, B. Braun Medical Inc, Becton Dickinson, CareFusion, Centurion Medical Products, Cook Medical, Entrotech, FloMedical, ICU Medical Inc, Medical Australia Limited, Medtronic, Smiths Medical, and Teleflex. Griffith University has received consultancy payments on behalf of C. R. M., A. J. U., and T. K. from manufacturers 3M, AngioDynamics, Bard Medical, B. Braun Medical Inc, Becton Dickinson, CareFusion, Mayo Healthcare Inc, ResQDevices, and Smiths Medical. AngioDynamics (the BioFlo® PICC manufacturer) provided partial funds to undertake this research via an unrestricted donation to Griffith University (but not the study authors). Queensland Health provided in-kind support to fund the remainder of the trial. The funders had no role in the study design, collection, analysis, or interpretation of the data, writing of the report, or decision to submit the article for publication.
1. Chopra V, Flanders SA, Saint S. The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527-1528. PubMed
2. Gibson C, Connolly BL, Moineddin R, Mahant S, Filipescu D, Amaral JG. Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323-1331. PubMed
3. Ullman AJ, Cooke M, Kleidon T, Rickard CM. Road map for improvement: point prevalence audit and survey of central venous access devices in paediatric acute care. J Paediatr Child Health. 2017;53(2):123-130. PubMed
4. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. PubMed
5. Barrier A, Williams DJ, Connelly M, Creech CB. Frequency of peripherally inserted central catheter complications in children. Pediatr Infect Dis J. 2012;31(5):519-521. PubMed
6. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. PubMed
7. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001-1008. PubMed
8. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. PubMed
9. Moureau NL, Trick N, Nifong T, et al. Vessel health and preservation (part 1): a new evidence-based approach to vascular access selection and management. J Vasc Access. 2012;13(3):351-356. PubMed
10. Poli P, Scocca A, Di Puccio F, Gallone G, Angelini L, Calabro EM. A comparative study on the mechanical behavior of polyurethane PICCs. J Vasc Access. 2016;17(2):175-181. PubMed
11. Interface Biologics. Surface modification technology platform. 2017. http://www.interfacebiologics.com/endexo.htm. Accessed April 5, 2017.
12. Hoffer EK, Bloch RD, Borsa JJ, Santulli P, Fontaine AB, Francoeur N. Peripherally inserted central catheters with distal versus proximal valves: prospective randomized trial. J Vasc Interv Radiol. 2001;12(10):1173-1177. PubMed
13. Hoffer EK, Borsa J, Santulli P, Bloch R, Fontaine AB. Prospective randomized comparison of valved versus nonvalved peripherally inserted central vein catheters. AJR Am J Roentgenol. 1999;173(5):1393-1398. PubMed
14. Pittiruti M, Emoli A, Porta P, Marche B, DeAngelis R, Scoppettuolo G. A prospective, randomized comparison of three different types of valved and nonvalved peripherally inserted central catheters. J Vasc Access. 2014;15(6):519-523.
15. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
16. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337. PubMed
17. Kleidon TM, Ullman AJ, Zhang L, Mihala G, Rickard CM. How does your PICCOMPARE? A pilot randomized controlled trial comparing PICC materials in pediatrics. J Hosp Med. 2017;(under review). PubMed
18. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180-191. PubMed
19. Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. PubMed
20. Chopra V, O’Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013;34(9):908-918. PubMed
21. Kramer RD, Rogers MA, Conte M, Mann J, Saint S, Chopra V. Are antimicrobial peripherally inserted central catheters associated with reduction in central line-associated bloodstream infection? A systematic review and meta-analysis. Am J Infect Control. 2017;45(2):108-114. PubMed
22. Centers for Disease Control and Prevention. National Healthcare Safety Network Device Associated Module: CLABSI. 2014.
23. Lobo BL, Vaidean G, Broyles J, Reaves AB, Shorr RI. Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417-422. PubMed
24. Smith SN, Moureau N, Vaughn VM, et al. Patterns and Predictors of Peripherally Inserted Central Catheter Occlusion: The 3P-O Study. J Vasc Interv Radiol. 28(5):749.e742-756.e742. PubMed
25. Chow LML, Friedman JN, MacArthur C, et al. Peripherally inserted central catheter (PICC) fracture and embolozation in the pediatric population. Pediatrics. 2003;142(2):141-144. PubMed
26. Chopra V, Kuhn L, Ratz D, Flanders SA, Krein SL. Vascular nursing experience, practice knowledge, and beliefs: Results from the Michigan PICC1 survey. J Hosp Med. 2016;11(4):269-275. PubMed
27. Frasca D, Dahyot-Fizelier C, Mimoz O. Prevention of central venous catheter-related infection in the intensive care unit. Crit Care. 2010;14(2):212. PubMed
28. Centre for Healthcare Related Infection Surveilance and Prevention and Tuberculosis Control. Guideline: Peripherally inserted central catheter (PICC). 2013.
29. Services Children’s Health Service. Central venous catheters: nursing care and management of peripherally inserted central catheter (PICC) in paediatric patients. 2011. http://qheps.health.qld.gov.au/childrenshealth/resources/nursestand/docs/ns_03452.pdf. Accessed Februrary 1, 2016.
30. Services CsH. Central Venous Access Device Insertion and Management. 2014.
31. Central venous access device insertion and management. Queensland Government; 2014. http://qheps.health.qld.gov.au/childrenshealth/resources/proc/docs/proc_03450.pdf Accessed March 13, 2014.
32. StatCorp. Stata Statistical Software: Release 12.1 College Station. 2006.
33. Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials. 2006;1(1):e9. PubMed
34. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. PubMed
35. Alport B, Burbridge B, Lim H. Bard PowerPICC Solo2 vs Cook Turbo-Ject: A Tale of Two PICCs. Can Assoc Radiol J. 2012;63(4):323-328. PubMed
36. Johnston AJ, Streater CT, Noorani R, Crofts JL, Del Mundo AB, Parker RA. The effect of peripherally inserted central catheter (PICC) valve technology on catheter occlusion rates—the ‘ELeCTRiC’ study. J Vasc Access. 2012;13(4):421-425. PubMed
37. Morgenthaler TI, Rodriguez V. Preventing acute care-associated venous thromboembolism in adult and pediatric patients across a large healthcare system. J Hosp Med. 2016;11(Suppl 2):S15-S21. PubMed
38. Menendez JJ, Verdu C, Calderon B, et al. Incidence and risk factors of superficial and deep vein thrombosis associated with peripherally inserted central catheters in children. J Thromb Haemost. 2016;14(11):2158-2168. PubMed
Peripherally inserted central catheters (PICCs) have evolved since their inception in the early 1970s and are used with increasing frequency for pediatric inpatients and outpatients.1-3 Emerging literature, including a meta-analysis of international observational studies,4 reports PICC failure (complications necessitating premature removal) occurs in up to 30% of PICCs, most commonly due to infection, thrombosis, occlusion, and fracture.4-7 Raffini et al.7 report the increasing incidence of pediatric PICC-related thrombosis increases morbidity and mortality8 and negatively impacts future vessel health and preservation.9
PICCs have progressed from relatively simple, silicone-based catheters with an external clamp to chemically engineered polyurethane with pressure-activated valves placed at the proximal or distal catheter hub with the intent to reduce occlusion.10 Further modernization of PICC material occurred with the incorporation of antithrombogenic (AT) material (Endexo®). These PICCs are designed to contain a nonstick polymer, which is designed to reduce the adherence of blood components (platelets and clotting factors) and inhibit thrombus formation (and hence prevent deep vein thrombosis andocclusion, as well as inhibit microbial biofilm attachment [and subsequent infection]).11
In addition to new materials, other aspects of this PICC design have been the addition of a pressure-activated safety valve (PASV®) built into the proximal hub. Pressure-activated valve technology promises to prevent catheter occlusion by reducing blood reflux into the PICC; the valve opens with pressure during infusion and aspiration and remains closed with normal venous pressure, circumventing the need for clinicians to manually clamp the PICC and reducing human error and the potential for thrombosis, occlusion, and fracture development.12 Hoffer et al.13 reported half as many occlusions of valved PICCs (3.3%) compared with nonvalved or clamped PICCs (7.1%); although not statistically significant (P = .10), perhaps due to the small sample, overall complications, including occlusion and infection, were significantly lessened with the valved PICC (35% vs 79%; P = .02). Comparatively, Pittiruti et al.14 conducted a trial of 2 types of valved PICCs with an open-ended, nonvalved PICC and found no reduction in PICC occlusion or catheter malfunction.
Today, PICC use is common for patients who require short-to-medium intravenous therapy. PICCs are increasingly recognized for their significant complications, including thrombosis and infection.15 Novel PICC technology, including the incorporation of AT material such as Endexo® and PASV®, may reduce complications; however, the clinical efficacy, cost-effectiveness, and acceptability of these innovations have not been tested through randomized trials in pediatric patients. In accordance with Medical Research Council guidelines16 for developing interventions, we pilot tested the feasibility of the BioFlo® PICC, including intervention acceptability, compliance, recruitment, and initial estimates of effect, in anticipation of a subsequent full-scale efficacy randomized controlled trial. Our secondary aim was to compare the effectiveness of the BioFlo® PICC with Endexo® and PASV® technology in reducing PICC complications and failure.
METHODS
Design
We undertook a pilot randomized controlled trial comparing the standard polyurethane PICC (with external clamp) with the BioFlo® PICC (with internal valve) in preventing catheter failure in pediatric patients. The study was prospectively registered with the Australian Clinical Trials Registry (ACTRN12615001290583), and the research protocol was published.17
Study Setting
The study commenced in March 2016 at the Lady Cilento Children’s Hospital in South Brisbane, Australia, a tertiary-level, specialist, pediatric teaching hospital in Queensland, Australia, providing full-spectrum health services to children and young people from birth to 18 years of age. Recruitment, including data collection, was completed in November 2016.
Sample
The target sample size was 110 participants, 50 participants per group plus 10% for potential attrition, as determined by standard pilot trial sample size recommendations.18 With ethics approval, the sample size was later increased to 150 participants in order to adequately pilot a microbiological substudy method (published separately).17 Participants were consecutively recruited if they met the inclusion criteria: PICC insertion, age <18 years, predicted hospital stay >24 hours, single-lumen PICC, and written informed consent by an English-speaking, legal parent or guardian. Patients were excluded if they had a current (<48 hours) blood stream infection (BSI), vessel size <2 mm, could not speak English without an interpreter, required a multilumen PICC, or were previously enrolled in the study.
Interventions
Participants were randomized to receive either of the following PICCs: (1) standard care: Cook™ polyurethane, turbo-ject, power-injectable PICC (Cook Medical, Bloomington, IN) or (2) comparison: BioFlo® polyurethane with Endexo® technology (AngioDynamics Inc, Queensbury, NY).
Outcomes
The primary outcome was feasibility of a full-efficacy trial established by composite analysis of the elements of eligibility (>70% of patients will be eligible), recruitment (>70% of patients will agree to enroll), retention and attrition (<15% of participants are lost to follow-up or withdraw from the study), protocol adherence (>80% of participants receive their allocated, randomly assigned study product), missing data (<10% of data are missed during data collection), parent and healthcare staff satisfaction, and PICC failure effect size estimates to allow sample size calculations.18,19 PICC failure was defined as the following complications associated with PICC removal: (1) catheter-associated BSI,8,20-22 (2) local site infection,22 (3) venous thrombosis,23 (4) occlusion,24,25 (5) PICC fracture, or (6) PICC dislodgement.25,26 Parents (or caregivers) and healthcare staff were asked to rate their level of confidence with the study product and ease of PICC removal by using a 0 to 100 numeric rating scale (NRS) of increasing confidence and/or ease. These data were collected at the time of PICC removal. Operators were also asked to rate their levels of satisfaction with the insertion equipment and ease of PICC insertion immediately upon completion of the insertion procedure (both 0-100 NRS of increasing satisfaction and/or ease). Secondary outcomes included individual PICC complications (eg, occlusion) occurring at any time point during the PICC dwell (including at removal), adverse events, pain, redness at the insertion site, and overall PICC dwell.
Study Procedures
The research nurse (ReN) screened operating theater lists for patients, obtained written informed consent, and initiated the randomization. Randomization was computer generated, and web based via Griffith University (https://www151.griffith.edu.au/random) to ensure allocation concealment until study entry. Patients were randomly assigned in a 1:1 ratio with computer-generated and randomly varied block sizes of 2 and 4. Data were collected by the ReN on the day of insertion, at day 1 postinsertion, then every 2 to 3 days thereafter so that PICCs were checked at least twice per week until study completion. Participants were included in the trial until 12 weeks post-PICC insertion, study withdrawal or PICC removal (whichever came first), with an additional 48 hours follow-up for infection outcomes. Patient review was face to face during the inpatient stay, with discharged patients’ follow-up occurring via outpatient clinics, hospital-in-the-home service, or telephone.
Data collection was via Research Electronic Data Capture (http://project-redcap.org/). The ReN collected data on primary and secondary outcomes by using the predefined criteria. Demographic and clinical data were collected to assess the success of randomization, describe the participant group, and display characteristics known to increase the risk of PICC complication and thrombosis. A blinded radiologist and infectious disease specialist reviewed and diagnosed thrombosis of deep veins and catheter-associated BSI outcomes, respectively.
PICC Procedures
Extensive prestudy education for 2 months prior to trial commencement was provided to all clinicians involved with the insertion and care of PICCs, including the study products. PICCs were inserted in an operating theater environment by a qualified consultant pediatric anesthetist, a senior anesthetic registrar or fellow in an approved anesthetic training program, or pediatric vascular access nurse practitioner. Ultrasound guidance was used to assess a patient’s vasculature and puncture the vessel. The operator chose the PICC size on the basis of clinical judgment of vessel size and patient needs and then inserted the allocated PICC.27 Preferred PICC tip location was the cavoatrial junction. All PICC tip positions were confirmed with a chest x-ray before use.
Postinsertion, PICCs were managed by local interdisciplinary clinicians in accordance with local practice guidelines.27-31 PICC care and management includes the use of 2% chlorhexidine gluconate in 70% alcohol for site antisepsis and neutral displacement needleless connectors (TUTA Pulse; Medical Australia Limited, Lidcombe, New South Wales, Australia); normal saline was used to flush after medication administration, and if the device was not in use for 6 hours or longer, heparin is instilled with securement via bordered polyurethane dressing (Tegaderm 1616; 3M, St Paul, Minnesota) and a sutureless securement device (Statlock VPPCSP; Bard, Georgia).
Statistical Analyses
Data were exported to Stata 1532 for cleaning and analysis. Data cleaning of outlying figures and missing and implausible data was undertaken prior to analysis. Missing data were not imputed. The PICC was the unit of measurement, and all randomly assigned patients were analyzed on an intention-to-treat basis.33 Descriptive statistics (frequencies and percentages) were used to ascertain the primary outcome of feasibility for the larger trial. Incidence rates (per 1,000 catheter days) and rate ratios, including 95% confidence intervals (CIs), were calculated. The comparability of groups at baseline was described across demographic, clinical, and device characteristics. Kaplan-Meier survival curves (with log-rank tests) were used to compare PICC failure between study groups over time. Associations between baseline characteristics and failure were described by calculating hazard ratios (HRs). Univariable Cox regression was performed only due to the relatively low number of outcomes. P values of <.05 were considered statistically significant.
Ethics
The Children’s Health Service District, Queensland (Human Research Ethics Committee/15/QRCH/164), and Griffith University (2016/077) Human Research Ethics Committees provided ethics and governance approval. Informed consent was obtained from parents or legal guardians, with children providing youth assent if they were 7 years or older, dependent upon cognitive ability.
RESULTS
Participant and PICC Characteristics
Feasibility Outcomes
PICC Failure and Complications
As per supplementary Table 1, univariate Cox regression identified PICC failure as significantly associated with tip placement in the proximal superior vena cava (SVC) compared to the SVC–right atrium junction (HR 2.61; 95% CI, 1.17-5.82; P = .024). Reduced risk of PICC failure was significantly associated with any infusion during the dwell (continuous fluid infusion, P = .007; continuous antibiotic, P = .042; or intermittent infusion, P = .046) compared to no infusion. Other variables potentially influencing the risk of failure included PICC insertion by nurse specialist compared to consultant anesthetist (HR 2.61; 95% CI, 0.85-5.44) or registrar (HR 1.97; 95% CI, 0.57-6.77). These differences were not statistically significant; however, baseline imbalance between study groups for this variable and the feasibility design preclude absolute conclusions.
DISCUSSION
This is the first pilot feasibility trial of new PICC materials and valve design incorporated in the BioFlo® PICC in the pediatric population. The trial incorporated best practice for randomized trials, including using a concurrent control group, centralized and concealed randomization, predetermined feasibility criteria, and a registered and published trial protocol.17 As in other studies,15,24,34 PICC failure and complication prevalence was unacceptably high for this essential device. Standard care PICCs failed twice as often as the new BioFlo® PICCs (22% vs 11%), which is a clinically important difference. As researchers in a pilot study, we did not expect to detect statistically significant differences; however, we found that overall complications during the dwell occurred significantly more with the standard care than BioFlo® PICCs (P = .009).
BioFlo® PICC material offers a major advancement in PICC material through the incorporation of AT technologies into catheter materials, such as PICCs. Endexo® is a low molecular–weight, fluoro-oligomeric additive that self-locates to the top few nanometers of the material surface. When added to power-injectable polyurethane, the additive results in a strong but passive, nonstick, fluorinated surface in the base PICC material. This inhibits platelet adhesion, suppresses protein procoagulant conformation, and thereby reduces thrombus formation in medical devices. Additionally, Endexo® is not a catheter coating; rather, it is incorporated within the polyurethane of the PICC, thereby ensuring these AT properties are present on the internal, external, and cut surfaces of the PICC. If this technology can reduce complication during treatment and reduce failure from infection, thrombosis, occlusion, fracture, and dislodgement, it will improve patient outcomes considerably and lower health system costs. Previous studies investigating valve technology in PICC design to reduce occlusion have been inconclusive.12-14,35,36 Occlusion (both partial and complete) was less frequent in our study with the BioFlo® group (n = 3; 4%) compared to the standard care group (n = 6; 8%). The results of this pilot study suggest that either the Endexo® material or PASV® technology has a positive association with occlusion reduction during PICC treatment.
Thrombosis was the primary failure type for the standard care PICCs, comprising one-third of failures. All but one patient with radiologically confirmed thrombosis required the removal of the PICC prior to completion of treatment. The decision to remove the PICC or retain and treat conservatively remained with the treating team. Raffini et al.7 found thrombosis to increase in patients with one or more coexisting chronic medical condition. Slightly more standard care than BioFlo® patients were free of such comorbidities (25% vs 16%), yet standard care patients still had the higher number of thromboses (7% vs 3%). Morgenthaler and Rodriguez37 reported vascular access-associated thrombosis in pediatrics to be less common than in adults but higher in medically complex children. Worryingly, Menendez et al.38 reported pediatric thrombosis to be largely asymptomatic, so the true incidence in our study is likely higher because only radiologically confirmed thromboses were recorded.
Occlusion (partial or complete) was the predominant complication across the study, being associated with one-third of all failures. When occlusion complications during the dwell (some of which were resolved with treatment), in addition to those causing failure, were considered, this number was even greater. Occlusion complications are prevalent and costly. Smith et al.24 reported that occlusion was the most common reason for PICC removal and the most likely complication to delay treatment. Both the BioFlo® and standard care PICCs are pressure rated with good tensile strength; however, fracture occurred in 4% (n = 3) of standard care PICCs compared to no fractures in BioFlo® PICCs. Although the numbers are small, it may suggest a superior tensile strength of the BioFlo® material.
This study reinforces previously published results24,38 that PICC tip position is important and can influence complications, such as occlusion and thrombosis. In addition, we found a significant association with failure when PICCs did not have a continuous infusion. These findings reinforce the need for optimal tip location at insertion and ongoing flushing and maintenance of PICCs not used for infusions.
Limitations of this study include the small sample size, which was not designed to detect statistical differences in the primary outcome between groups. Despite randomization, there were slight imbalances at baseline for inserter type and leukocyte count, although these were not significantly associated with PICC failure in the Cox regression (data not shown), and thus were unlikely to influence findings. Additionally, a difference of <10% was associated with PICC tip position, favoring the BioFlo® group. PICC tip position outside the cavoatrial junction was positively associated with failure; therefore, the effect of tip positioning on outcomes is difficult to ascertain given the small sample size and feasibility nature of the study. Further study is warranted to further explore this effect. The population sampled was pediatric medical and surgical inpatients with a vessel size >2 mm attending the operating theater suite for PICC insertion, thereby limiting the study’s generalizability to adults and other populations, including neonates and those with PICCs inserted in the pediatric intensive care unit. The study could not be blinded because study products had to be visible to the clinical and research staff. However, it is unlikely that staff would intentionally sabotage PICCs to bias the study. Blinding was possible for the assessment of blood culture and ultrasound reports to diagnose infection and thrombosis. Strengths of this study included 100% protocol adherence, and no patients were lost to follow-up.
CONCLUSION
These results confirm that PICC failure is unacceptably high and suggest that the innovative BioFlo® PICC material and design holds promise to improve PICC outcomes by reducing complications and overall PICC failure. Trials of this technology are feasible, safe, and acceptable to healthcare staff and parents. Further trials are required, including in other patient populations, to definitively identify clinical, cost-effective methods to prevent PICC failure and improve reliability during treatment.
Acknowledgments
The authors thank the children and parents of Lady Cilento Children’s Hospital for participating in this important research. A special thank you goes to the nurses within the Vascular Assessment and Management Service and to Karen Turner, Julieta Woosley, and Anna Dean for their efforts in data collecting and ensuring protocol adherence.
Disclosure
Griffith University has received unrestricted, investigator-initiated research or educational grants to support the research of T. K., A. J. U., and C. R. M. from product manufacturers 3M, Adhezion Inc, AngioDynamics, Bard Medical, Baxter, B. Braun Medical Inc, Becton Dickinson, CareFusion, Centurion Medical Products, Cook Medical, Entrotech, FloMedical, ICU Medical Inc, Medical Australia Limited, Medtronic, Smiths Medical, and Teleflex. Griffith University has received consultancy payments on behalf of C. R. M., A. J. U., and T. K. from manufacturers 3M, AngioDynamics, Bard Medical, B. Braun Medical Inc, Becton Dickinson, CareFusion, Mayo Healthcare Inc, ResQDevices, and Smiths Medical. AngioDynamics (the BioFlo® PICC manufacturer) provided partial funds to undertake this research via an unrestricted donation to Griffith University (but not the study authors). Queensland Health provided in-kind support to fund the remainder of the trial. The funders had no role in the study design, collection, analysis, or interpretation of the data, writing of the report, or decision to submit the article for publication.
Peripherally inserted central catheters (PICCs) have evolved since their inception in the early 1970s and are used with increasing frequency for pediatric inpatients and outpatients.1-3 Emerging literature, including a meta-analysis of international observational studies,4 reports PICC failure (complications necessitating premature removal) occurs in up to 30% of PICCs, most commonly due to infection, thrombosis, occlusion, and fracture.4-7 Raffini et al.7 report the increasing incidence of pediatric PICC-related thrombosis increases morbidity and mortality8 and negatively impacts future vessel health and preservation.9
PICCs have progressed from relatively simple, silicone-based catheters with an external clamp to chemically engineered polyurethane with pressure-activated valves placed at the proximal or distal catheter hub with the intent to reduce occlusion.10 Further modernization of PICC material occurred with the incorporation of antithrombogenic (AT) material (Endexo®). These PICCs are designed to contain a nonstick polymer, which is designed to reduce the adherence of blood components (platelets and clotting factors) and inhibit thrombus formation (and hence prevent deep vein thrombosis andocclusion, as well as inhibit microbial biofilm attachment [and subsequent infection]).11
In addition to new materials, other aspects of this PICC design have been the addition of a pressure-activated safety valve (PASV®) built into the proximal hub. Pressure-activated valve technology promises to prevent catheter occlusion by reducing blood reflux into the PICC; the valve opens with pressure during infusion and aspiration and remains closed with normal venous pressure, circumventing the need for clinicians to manually clamp the PICC and reducing human error and the potential for thrombosis, occlusion, and fracture development.12 Hoffer et al.13 reported half as many occlusions of valved PICCs (3.3%) compared with nonvalved or clamped PICCs (7.1%); although not statistically significant (P = .10), perhaps due to the small sample, overall complications, including occlusion and infection, were significantly lessened with the valved PICC (35% vs 79%; P = .02). Comparatively, Pittiruti et al.14 conducted a trial of 2 types of valved PICCs with an open-ended, nonvalved PICC and found no reduction in PICC occlusion or catheter malfunction.
Today, PICC use is common for patients who require short-to-medium intravenous therapy. PICCs are increasingly recognized for their significant complications, including thrombosis and infection.15 Novel PICC technology, including the incorporation of AT material such as Endexo® and PASV®, may reduce complications; however, the clinical efficacy, cost-effectiveness, and acceptability of these innovations have not been tested through randomized trials in pediatric patients. In accordance with Medical Research Council guidelines16 for developing interventions, we pilot tested the feasibility of the BioFlo® PICC, including intervention acceptability, compliance, recruitment, and initial estimates of effect, in anticipation of a subsequent full-scale efficacy randomized controlled trial. Our secondary aim was to compare the effectiveness of the BioFlo® PICC with Endexo® and PASV® technology in reducing PICC complications and failure.
METHODS
Design
We undertook a pilot randomized controlled trial comparing the standard polyurethane PICC (with external clamp) with the BioFlo® PICC (with internal valve) in preventing catheter failure in pediatric patients. The study was prospectively registered with the Australian Clinical Trials Registry (ACTRN12615001290583), and the research protocol was published.17
Study Setting
The study commenced in March 2016 at the Lady Cilento Children’s Hospital in South Brisbane, Australia, a tertiary-level, specialist, pediatric teaching hospital in Queensland, Australia, providing full-spectrum health services to children and young people from birth to 18 years of age. Recruitment, including data collection, was completed in November 2016.
Sample
The target sample size was 110 participants, 50 participants per group plus 10% for potential attrition, as determined by standard pilot trial sample size recommendations.18 With ethics approval, the sample size was later increased to 150 participants in order to adequately pilot a microbiological substudy method (published separately).17 Participants were consecutively recruited if they met the inclusion criteria: PICC insertion, age <18 years, predicted hospital stay >24 hours, single-lumen PICC, and written informed consent by an English-speaking, legal parent or guardian. Patients were excluded if they had a current (<48 hours) blood stream infection (BSI), vessel size <2 mm, could not speak English without an interpreter, required a multilumen PICC, or were previously enrolled in the study.
Interventions
Participants were randomized to receive either of the following PICCs: (1) standard care: Cook™ polyurethane, turbo-ject, power-injectable PICC (Cook Medical, Bloomington, IN) or (2) comparison: BioFlo® polyurethane with Endexo® technology (AngioDynamics Inc, Queensbury, NY).
Outcomes
The primary outcome was feasibility of a full-efficacy trial established by composite analysis of the elements of eligibility (>70% of patients will be eligible), recruitment (>70% of patients will agree to enroll), retention and attrition (<15% of participants are lost to follow-up or withdraw from the study), protocol adherence (>80% of participants receive their allocated, randomly assigned study product), missing data (<10% of data are missed during data collection), parent and healthcare staff satisfaction, and PICC failure effect size estimates to allow sample size calculations.18,19 PICC failure was defined as the following complications associated with PICC removal: (1) catheter-associated BSI,8,20-22 (2) local site infection,22 (3) venous thrombosis,23 (4) occlusion,24,25 (5) PICC fracture, or (6) PICC dislodgement.25,26 Parents (or caregivers) and healthcare staff were asked to rate their level of confidence with the study product and ease of PICC removal by using a 0 to 100 numeric rating scale (NRS) of increasing confidence and/or ease. These data were collected at the time of PICC removal. Operators were also asked to rate their levels of satisfaction with the insertion equipment and ease of PICC insertion immediately upon completion of the insertion procedure (both 0-100 NRS of increasing satisfaction and/or ease). Secondary outcomes included individual PICC complications (eg, occlusion) occurring at any time point during the PICC dwell (including at removal), adverse events, pain, redness at the insertion site, and overall PICC dwell.
Study Procedures
The research nurse (ReN) screened operating theater lists for patients, obtained written informed consent, and initiated the randomization. Randomization was computer generated, and web based via Griffith University (https://www151.griffith.edu.au/random) to ensure allocation concealment until study entry. Patients were randomly assigned in a 1:1 ratio with computer-generated and randomly varied block sizes of 2 and 4. Data were collected by the ReN on the day of insertion, at day 1 postinsertion, then every 2 to 3 days thereafter so that PICCs were checked at least twice per week until study completion. Participants were included in the trial until 12 weeks post-PICC insertion, study withdrawal or PICC removal (whichever came first), with an additional 48 hours follow-up for infection outcomes. Patient review was face to face during the inpatient stay, with discharged patients’ follow-up occurring via outpatient clinics, hospital-in-the-home service, or telephone.
Data collection was via Research Electronic Data Capture (http://project-redcap.org/). The ReN collected data on primary and secondary outcomes by using the predefined criteria. Demographic and clinical data were collected to assess the success of randomization, describe the participant group, and display characteristics known to increase the risk of PICC complication and thrombosis. A blinded radiologist and infectious disease specialist reviewed and diagnosed thrombosis of deep veins and catheter-associated BSI outcomes, respectively.
PICC Procedures
Extensive prestudy education for 2 months prior to trial commencement was provided to all clinicians involved with the insertion and care of PICCs, including the study products. PICCs were inserted in an operating theater environment by a qualified consultant pediatric anesthetist, a senior anesthetic registrar or fellow in an approved anesthetic training program, or pediatric vascular access nurse practitioner. Ultrasound guidance was used to assess a patient’s vasculature and puncture the vessel. The operator chose the PICC size on the basis of clinical judgment of vessel size and patient needs and then inserted the allocated PICC.27 Preferred PICC tip location was the cavoatrial junction. All PICC tip positions were confirmed with a chest x-ray before use.
Postinsertion, PICCs were managed by local interdisciplinary clinicians in accordance with local practice guidelines.27-31 PICC care and management includes the use of 2% chlorhexidine gluconate in 70% alcohol for site antisepsis and neutral displacement needleless connectors (TUTA Pulse; Medical Australia Limited, Lidcombe, New South Wales, Australia); normal saline was used to flush after medication administration, and if the device was not in use for 6 hours or longer, heparin is instilled with securement via bordered polyurethane dressing (Tegaderm 1616; 3M, St Paul, Minnesota) and a sutureless securement device (Statlock VPPCSP; Bard, Georgia).
Statistical Analyses
Data were exported to Stata 1532 for cleaning and analysis. Data cleaning of outlying figures and missing and implausible data was undertaken prior to analysis. Missing data were not imputed. The PICC was the unit of measurement, and all randomly assigned patients were analyzed on an intention-to-treat basis.33 Descriptive statistics (frequencies and percentages) were used to ascertain the primary outcome of feasibility for the larger trial. Incidence rates (per 1,000 catheter days) and rate ratios, including 95% confidence intervals (CIs), were calculated. The comparability of groups at baseline was described across demographic, clinical, and device characteristics. Kaplan-Meier survival curves (with log-rank tests) were used to compare PICC failure between study groups over time. Associations between baseline characteristics and failure were described by calculating hazard ratios (HRs). Univariable Cox regression was performed only due to the relatively low number of outcomes. P values of <.05 were considered statistically significant.
Ethics
The Children’s Health Service District, Queensland (Human Research Ethics Committee/15/QRCH/164), and Griffith University (2016/077) Human Research Ethics Committees provided ethics and governance approval. Informed consent was obtained from parents or legal guardians, with children providing youth assent if they were 7 years or older, dependent upon cognitive ability.
RESULTS
Participant and PICC Characteristics
Feasibility Outcomes
PICC Failure and Complications
As per supplementary Table 1, univariate Cox regression identified PICC failure as significantly associated with tip placement in the proximal superior vena cava (SVC) compared to the SVC–right atrium junction (HR 2.61; 95% CI, 1.17-5.82; P = .024). Reduced risk of PICC failure was significantly associated with any infusion during the dwell (continuous fluid infusion, P = .007; continuous antibiotic, P = .042; or intermittent infusion, P = .046) compared to no infusion. Other variables potentially influencing the risk of failure included PICC insertion by nurse specialist compared to consultant anesthetist (HR 2.61; 95% CI, 0.85-5.44) or registrar (HR 1.97; 95% CI, 0.57-6.77). These differences were not statistically significant; however, baseline imbalance between study groups for this variable and the feasibility design preclude absolute conclusions.
DISCUSSION
This is the first pilot feasibility trial of new PICC materials and valve design incorporated in the BioFlo® PICC in the pediatric population. The trial incorporated best practice for randomized trials, including using a concurrent control group, centralized and concealed randomization, predetermined feasibility criteria, and a registered and published trial protocol.17 As in other studies,15,24,34 PICC failure and complication prevalence was unacceptably high for this essential device. Standard care PICCs failed twice as often as the new BioFlo® PICCs (22% vs 11%), which is a clinically important difference. As researchers in a pilot study, we did not expect to detect statistically significant differences; however, we found that overall complications during the dwell occurred significantly more with the standard care than BioFlo® PICCs (P = .009).
BioFlo® PICC material offers a major advancement in PICC material through the incorporation of AT technologies into catheter materials, such as PICCs. Endexo® is a low molecular–weight, fluoro-oligomeric additive that self-locates to the top few nanometers of the material surface. When added to power-injectable polyurethane, the additive results in a strong but passive, nonstick, fluorinated surface in the base PICC material. This inhibits platelet adhesion, suppresses protein procoagulant conformation, and thereby reduces thrombus formation in medical devices. Additionally, Endexo® is not a catheter coating; rather, it is incorporated within the polyurethane of the PICC, thereby ensuring these AT properties are present on the internal, external, and cut surfaces of the PICC. If this technology can reduce complication during treatment and reduce failure from infection, thrombosis, occlusion, fracture, and dislodgement, it will improve patient outcomes considerably and lower health system costs. Previous studies investigating valve technology in PICC design to reduce occlusion have been inconclusive.12-14,35,36 Occlusion (both partial and complete) was less frequent in our study with the BioFlo® group (n = 3; 4%) compared to the standard care group (n = 6; 8%). The results of this pilot study suggest that either the Endexo® material or PASV® technology has a positive association with occlusion reduction during PICC treatment.
Thrombosis was the primary failure type for the standard care PICCs, comprising one-third of failures. All but one patient with radiologically confirmed thrombosis required the removal of the PICC prior to completion of treatment. The decision to remove the PICC or retain and treat conservatively remained with the treating team. Raffini et al.7 found thrombosis to increase in patients with one or more coexisting chronic medical condition. Slightly more standard care than BioFlo® patients were free of such comorbidities (25% vs 16%), yet standard care patients still had the higher number of thromboses (7% vs 3%). Morgenthaler and Rodriguez37 reported vascular access-associated thrombosis in pediatrics to be less common than in adults but higher in medically complex children. Worryingly, Menendez et al.38 reported pediatric thrombosis to be largely asymptomatic, so the true incidence in our study is likely higher because only radiologically confirmed thromboses were recorded.
Occlusion (partial or complete) was the predominant complication across the study, being associated with one-third of all failures. When occlusion complications during the dwell (some of which were resolved with treatment), in addition to those causing failure, were considered, this number was even greater. Occlusion complications are prevalent and costly. Smith et al.24 reported that occlusion was the most common reason for PICC removal and the most likely complication to delay treatment. Both the BioFlo® and standard care PICCs are pressure rated with good tensile strength; however, fracture occurred in 4% (n = 3) of standard care PICCs compared to no fractures in BioFlo® PICCs. Although the numbers are small, it may suggest a superior tensile strength of the BioFlo® material.
This study reinforces previously published results24,38 that PICC tip position is important and can influence complications, such as occlusion and thrombosis. In addition, we found a significant association with failure when PICCs did not have a continuous infusion. These findings reinforce the need for optimal tip location at insertion and ongoing flushing and maintenance of PICCs not used for infusions.
Limitations of this study include the small sample size, which was not designed to detect statistical differences in the primary outcome between groups. Despite randomization, there were slight imbalances at baseline for inserter type and leukocyte count, although these were not significantly associated with PICC failure in the Cox regression (data not shown), and thus were unlikely to influence findings. Additionally, a difference of <10% was associated with PICC tip position, favoring the BioFlo® group. PICC tip position outside the cavoatrial junction was positively associated with failure; therefore, the effect of tip positioning on outcomes is difficult to ascertain given the small sample size and feasibility nature of the study. Further study is warranted to further explore this effect. The population sampled was pediatric medical and surgical inpatients with a vessel size >2 mm attending the operating theater suite for PICC insertion, thereby limiting the study’s generalizability to adults and other populations, including neonates and those with PICCs inserted in the pediatric intensive care unit. The study could not be blinded because study products had to be visible to the clinical and research staff. However, it is unlikely that staff would intentionally sabotage PICCs to bias the study. Blinding was possible for the assessment of blood culture and ultrasound reports to diagnose infection and thrombosis. Strengths of this study included 100% protocol adherence, and no patients were lost to follow-up.
CONCLUSION
These results confirm that PICC failure is unacceptably high and suggest that the innovative BioFlo® PICC material and design holds promise to improve PICC outcomes by reducing complications and overall PICC failure. Trials of this technology are feasible, safe, and acceptable to healthcare staff and parents. Further trials are required, including in other patient populations, to definitively identify clinical, cost-effective methods to prevent PICC failure and improve reliability during treatment.
Acknowledgments
The authors thank the children and parents of Lady Cilento Children’s Hospital for participating in this important research. A special thank you goes to the nurses within the Vascular Assessment and Management Service and to Karen Turner, Julieta Woosley, and Anna Dean for their efforts in data collecting and ensuring protocol adherence.
Disclosure
Griffith University has received unrestricted, investigator-initiated research or educational grants to support the research of T. K., A. J. U., and C. R. M. from product manufacturers 3M, Adhezion Inc, AngioDynamics, Bard Medical, Baxter, B. Braun Medical Inc, Becton Dickinson, CareFusion, Centurion Medical Products, Cook Medical, Entrotech, FloMedical, ICU Medical Inc, Medical Australia Limited, Medtronic, Smiths Medical, and Teleflex. Griffith University has received consultancy payments on behalf of C. R. M., A. J. U., and T. K. from manufacturers 3M, AngioDynamics, Bard Medical, B. Braun Medical Inc, Becton Dickinson, CareFusion, Mayo Healthcare Inc, ResQDevices, and Smiths Medical. AngioDynamics (the BioFlo® PICC manufacturer) provided partial funds to undertake this research via an unrestricted donation to Griffith University (but not the study authors). Queensland Health provided in-kind support to fund the remainder of the trial. The funders had no role in the study design, collection, analysis, or interpretation of the data, writing of the report, or decision to submit the article for publication.
1. Chopra V, Flanders SA, Saint S. The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527-1528. PubMed
2. Gibson C, Connolly BL, Moineddin R, Mahant S, Filipescu D, Amaral JG. Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323-1331. PubMed
3. Ullman AJ, Cooke M, Kleidon T, Rickard CM. Road map for improvement: point prevalence audit and survey of central venous access devices in paediatric acute care. J Paediatr Child Health. 2017;53(2):123-130. PubMed
4. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. PubMed
5. Barrier A, Williams DJ, Connelly M, Creech CB. Frequency of peripherally inserted central catheter complications in children. Pediatr Infect Dis J. 2012;31(5):519-521. PubMed
6. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. PubMed
7. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001-1008. PubMed
8. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. PubMed
9. Moureau NL, Trick N, Nifong T, et al. Vessel health and preservation (part 1): a new evidence-based approach to vascular access selection and management. J Vasc Access. 2012;13(3):351-356. PubMed
10. Poli P, Scocca A, Di Puccio F, Gallone G, Angelini L, Calabro EM. A comparative study on the mechanical behavior of polyurethane PICCs. J Vasc Access. 2016;17(2):175-181. PubMed
11. Interface Biologics. Surface modification technology platform. 2017. http://www.interfacebiologics.com/endexo.htm. Accessed April 5, 2017.
12. Hoffer EK, Bloch RD, Borsa JJ, Santulli P, Fontaine AB, Francoeur N. Peripherally inserted central catheters with distal versus proximal valves: prospective randomized trial. J Vasc Interv Radiol. 2001;12(10):1173-1177. PubMed
13. Hoffer EK, Borsa J, Santulli P, Bloch R, Fontaine AB. Prospective randomized comparison of valved versus nonvalved peripherally inserted central vein catheters. AJR Am J Roentgenol. 1999;173(5):1393-1398. PubMed
14. Pittiruti M, Emoli A, Porta P, Marche B, DeAngelis R, Scoppettuolo G. A prospective, randomized comparison of three different types of valved and nonvalved peripherally inserted central catheters. J Vasc Access. 2014;15(6):519-523.
15. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
16. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337. PubMed
17. Kleidon TM, Ullman AJ, Zhang L, Mihala G, Rickard CM. How does your PICCOMPARE? A pilot randomized controlled trial comparing PICC materials in pediatrics. J Hosp Med. 2017;(under review). PubMed
18. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180-191. PubMed
19. Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. PubMed
20. Chopra V, O’Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013;34(9):908-918. PubMed
21. Kramer RD, Rogers MA, Conte M, Mann J, Saint S, Chopra V. Are antimicrobial peripherally inserted central catheters associated with reduction in central line-associated bloodstream infection? A systematic review and meta-analysis. Am J Infect Control. 2017;45(2):108-114. PubMed
22. Centers for Disease Control and Prevention. National Healthcare Safety Network Device Associated Module: CLABSI. 2014.
23. Lobo BL, Vaidean G, Broyles J, Reaves AB, Shorr RI. Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417-422. PubMed
24. Smith SN, Moureau N, Vaughn VM, et al. Patterns and Predictors of Peripherally Inserted Central Catheter Occlusion: The 3P-O Study. J Vasc Interv Radiol. 28(5):749.e742-756.e742. PubMed
25. Chow LML, Friedman JN, MacArthur C, et al. Peripherally inserted central catheter (PICC) fracture and embolozation in the pediatric population. Pediatrics. 2003;142(2):141-144. PubMed
26. Chopra V, Kuhn L, Ratz D, Flanders SA, Krein SL. Vascular nursing experience, practice knowledge, and beliefs: Results from the Michigan PICC1 survey. J Hosp Med. 2016;11(4):269-275. PubMed
27. Frasca D, Dahyot-Fizelier C, Mimoz O. Prevention of central venous catheter-related infection in the intensive care unit. Crit Care. 2010;14(2):212. PubMed
28. Centre for Healthcare Related Infection Surveilance and Prevention and Tuberculosis Control. Guideline: Peripherally inserted central catheter (PICC). 2013.
29. Services Children’s Health Service. Central venous catheters: nursing care and management of peripherally inserted central catheter (PICC) in paediatric patients. 2011. http://qheps.health.qld.gov.au/childrenshealth/resources/nursestand/docs/ns_03452.pdf. Accessed Februrary 1, 2016.
30. Services CsH. Central Venous Access Device Insertion and Management. 2014.
31. Central venous access device insertion and management. Queensland Government; 2014. http://qheps.health.qld.gov.au/childrenshealth/resources/proc/docs/proc_03450.pdf Accessed March 13, 2014.
32. StatCorp. Stata Statistical Software: Release 12.1 College Station. 2006.
33. Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials. 2006;1(1):e9. PubMed
34. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. PubMed
35. Alport B, Burbridge B, Lim H. Bard PowerPICC Solo2 vs Cook Turbo-Ject: A Tale of Two PICCs. Can Assoc Radiol J. 2012;63(4):323-328. PubMed
36. Johnston AJ, Streater CT, Noorani R, Crofts JL, Del Mundo AB, Parker RA. The effect of peripherally inserted central catheter (PICC) valve technology on catheter occlusion rates—the ‘ELeCTRiC’ study. J Vasc Access. 2012;13(4):421-425. PubMed
37. Morgenthaler TI, Rodriguez V. Preventing acute care-associated venous thromboembolism in adult and pediatric patients across a large healthcare system. J Hosp Med. 2016;11(Suppl 2):S15-S21. PubMed
38. Menendez JJ, Verdu C, Calderon B, et al. Incidence and risk factors of superficial and deep vein thrombosis associated with peripherally inserted central catheters in children. J Thromb Haemost. 2016;14(11):2158-2168. PubMed
1. Chopra V, Flanders SA, Saint S. The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527-1528. PubMed
2. Gibson C, Connolly BL, Moineddin R, Mahant S, Filipescu D, Amaral JG. Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323-1331. PubMed
3. Ullman AJ, Cooke M, Kleidon T, Rickard CM. Road map for improvement: point prevalence audit and survey of central venous access devices in paediatric acute care. J Paediatr Child Health. 2017;53(2):123-130. PubMed
4. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. PubMed
5. Barrier A, Williams DJ, Connelly M, Creech CB. Frequency of peripherally inserted central catheter complications in children. Pediatr Infect Dis J. 2012;31(5):519-521. PubMed
6. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. PubMed
7. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001-1008. PubMed
8. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. PubMed
9. Moureau NL, Trick N, Nifong T, et al. Vessel health and preservation (part 1): a new evidence-based approach to vascular access selection and management. J Vasc Access. 2012;13(3):351-356. PubMed
10. Poli P, Scocca A, Di Puccio F, Gallone G, Angelini L, Calabro EM. A comparative study on the mechanical behavior of polyurethane PICCs. J Vasc Access. 2016;17(2):175-181. PubMed
11. Interface Biologics. Surface modification technology platform. 2017. http://www.interfacebiologics.com/endexo.htm. Accessed April 5, 2017.
12. Hoffer EK, Bloch RD, Borsa JJ, Santulli P, Fontaine AB, Francoeur N. Peripherally inserted central catheters with distal versus proximal valves: prospective randomized trial. J Vasc Interv Radiol. 2001;12(10):1173-1177. PubMed
13. Hoffer EK, Borsa J, Santulli P, Bloch R, Fontaine AB. Prospective randomized comparison of valved versus nonvalved peripherally inserted central vein catheters. AJR Am J Roentgenol. 1999;173(5):1393-1398. PubMed
14. Pittiruti M, Emoli A, Porta P, Marche B, DeAngelis R, Scoppettuolo G. A prospective, randomized comparison of three different types of valved and nonvalved peripherally inserted central catheters. J Vasc Access. 2014;15(6):519-523.
15. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
16. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337. PubMed
17. Kleidon TM, Ullman AJ, Zhang L, Mihala G, Rickard CM. How does your PICCOMPARE? A pilot randomized controlled trial comparing PICC materials in pediatrics. J Hosp Med. 2017;(under review). PubMed
18. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180-191. PubMed
19. Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. PubMed
20. Chopra V, O’Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013;34(9):908-918. PubMed
21. Kramer RD, Rogers MA, Conte M, Mann J, Saint S, Chopra V. Are antimicrobial peripherally inserted central catheters associated with reduction in central line-associated bloodstream infection? A systematic review and meta-analysis. Am J Infect Control. 2017;45(2):108-114. PubMed
22. Centers for Disease Control and Prevention. National Healthcare Safety Network Device Associated Module: CLABSI. 2014.
23. Lobo BL, Vaidean G, Broyles J, Reaves AB, Shorr RI. Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417-422. PubMed
24. Smith SN, Moureau N, Vaughn VM, et al. Patterns and Predictors of Peripherally Inserted Central Catheter Occlusion: The 3P-O Study. J Vasc Interv Radiol. 28(5):749.e742-756.e742. PubMed
25. Chow LML, Friedman JN, MacArthur C, et al. Peripherally inserted central catheter (PICC) fracture and embolozation in the pediatric population. Pediatrics. 2003;142(2):141-144. PubMed
26. Chopra V, Kuhn L, Ratz D, Flanders SA, Krein SL. Vascular nursing experience, practice knowledge, and beliefs: Results from the Michigan PICC1 survey. J Hosp Med. 2016;11(4):269-275. PubMed
27. Frasca D, Dahyot-Fizelier C, Mimoz O. Prevention of central venous catheter-related infection in the intensive care unit. Crit Care. 2010;14(2):212. PubMed
28. Centre for Healthcare Related Infection Surveilance and Prevention and Tuberculosis Control. Guideline: Peripherally inserted central catheter (PICC). 2013.
29. Services Children’s Health Service. Central venous catheters: nursing care and management of peripherally inserted central catheter (PICC) in paediatric patients. 2011. http://qheps.health.qld.gov.au/childrenshealth/resources/nursestand/docs/ns_03452.pdf. Accessed Februrary 1, 2016.
30. Services CsH. Central Venous Access Device Insertion and Management. 2014.
31. Central venous access device insertion and management. Queensland Government; 2014. http://qheps.health.qld.gov.au/childrenshealth/resources/proc/docs/proc_03450.pdf Accessed March 13, 2014.
32. StatCorp. Stata Statistical Software: Release 12.1 College Station. 2006.
33. Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials. 2006;1(1):e9. PubMed
34. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. PubMed
35. Alport B, Burbridge B, Lim H. Bard PowerPICC Solo2 vs Cook Turbo-Ject: A Tale of Two PICCs. Can Assoc Radiol J. 2012;63(4):323-328. PubMed
36. Johnston AJ, Streater CT, Noorani R, Crofts JL, Del Mundo AB, Parker RA. The effect of peripherally inserted central catheter (PICC) valve technology on catheter occlusion rates—the ‘ELeCTRiC’ study. J Vasc Access. 2012;13(4):421-425. PubMed
37. Morgenthaler TI, Rodriguez V. Preventing acute care-associated venous thromboembolism in adult and pediatric patients across a large healthcare system. J Hosp Med. 2016;11(Suppl 2):S15-S21. PubMed
38. Menendez JJ, Verdu C, Calderon B, et al. Incidence and risk factors of superficial and deep vein thrombosis associated with peripherally inserted central catheters in children. J Thromb Haemost. 2016;14(11):2158-2168. PubMed
© 2018 Society of Hospital Medicine
A Single, Post-ACTH Cortisol Measurement to Screen for Adrenal Insufficiency in the Hospitalized Patient
Testing for adrenal insufficiency (AI) is common in the hospital setting. The gold standard remains the insulin tolerance test (ITT), in which cortisol concentration is measured after the induction of hypoglycemia to <35 mg/dL.1 Alternatively, metyrapone testing works by blocking cortisol synthesis. If pretest adrenocorticotropic hormone (ACTH) concentrations are low and ACTH concentrations do not rise after the administration of metyrapone, the patient is given a diagnosis of AI. Both assays pose some risk to patients with AI and are typically only performed as confirmatory tests. Morning random cortisol concentrations can be used to suggest AI if concentrations are <3 mcg/dL, but they often provide indeterminate results if concentrations are between 3 and 15 mcg/dL.2 Thus, morning cortisol concentrations in isolation are not diagnostic of AI. For these reasons, most experts recommend a dynamic, high-dose cosyntropin stimulation testing (CST) with 250 mcg of intravenous cosyntropin to screen for AI. The test can be done any time of day.3 Historically, an incremental response to cosyntropin, or “delta,” was also required to indicate a normal response to stimulation.4 However, the baseline cortisol concentration is dependent on circadian rhythm and level of stress. For this reason, a delta, whether large or small, has been abandoned as a requisite for the diagnosis of AI.5-7 A normal CST is widely accepted to be identified by any cortisol concentration >18 mcg/dL during the test (basal or poststimulation).8
The seminal studies by Lindholm, Kehlet, and coauthors9-11 validated the CST against the gold standard ITT and utilized only 0- and 30-minute cortisol concentrations. A later study in patients with pituitary disease demonstrated that 30-minute concentrations had a stronger correlation with the ITT than 60-minute concentrations (false-negative rate: 10% vs 27%).12 However, in that study, a higher threshold was used for the 60-minute concentration than for what was obtained at 30 minutes (25.4 vs 21.8 mcg/dL, respectively). Multiple studies have shown that the 60-minute concentration is higher than the 30-minute concentration after cosyntropin stimulation.4,5,13 Subsequent, small studies of patients who were known to have AI have shown that 60-minute concentrations are as useful as 30-minute concentrations.5,14,15 Because 30-minute cortisol concentrations are often lower than 60-minute concentrations, a single 30-minute result may lead to a falsely abnormal test.16,17 As such, the use of a single 60-minute test may be more appropriate. Indeed, some authors have suggested that measuring only 30-minute concentrations may lead to overdiagnosis of AI by missing an appropriate response, serum cortisol >18 mcg/dL, at 60 minutes.17-19 Peak cortisol concentrations after low-dose cosyntropin stimulation (1 mcg) are seen at 60 minutes, and low-dose stimulation has been shown to be more variable than in the high-dose test (250 mg).19,20
There is a lack of consensus to guide clinicians as to when cortisol concentrations should be measured after stimulation, and standard references lack uniformity. Commonly accessed medical resources—such as UpToDate and Jameson’s Endocrinology—recommend basal, 30-minute, and 60-minute cortisol concentrations, while Williams Textbook of Endocrinology recommends basal and 30-minute concentrations, and the Washington Manual recommends only a single 30-minute concentration.7,21,22 Goldman-Cecil Medicine8 recommends checking a cortisol concentration between 30 and 60 minutes and recommends the same 18 mcg/dL cutoff for any test obtained in this time period. As a result of these variable recommendations, all 3 time points are often obtained. Prominent review articles continue to recommend checking all 3 concentrations while presenting evidence of peak cortisol response at 60 minutes poststimulation.13
In this study, we retrospectively examined CSTs in hospitalized, adult patients both in the intensive care unit (ICU) and hospital ward and/or floor settings to evaluate for significant differences in 30- and 60-minute cortisol concentrations and compare the concordance of screening at each time point alone with traditional CST at all 3 time points. By using these results, we discuss the utility of obtaining 3 cortisol samples.
METHODS
After receiving approval from the institutional review board, we retrospectively reviewed all standard, high-dose CSTs performed on adult inpatients at the Barnes-Jewish Hospital laboratory from January 1, 2012, to August 31, 2013. All patients received the same standard dose (250 mcg cosyntropin, a synthetic ACTH, at a concentration of 1 mcg/mL administered over 2 minutes) regardless of age or weight. We collected patient gender; age; time of baseline cortisol measurement; cortisol results at baseline, 30, and 60 minutes; and patient location (inpatient floor vs ICU status). Tests were included if results from all 3 time points (0, 30, 60 minute) were available.
Cortisol concentrations were assessed by the laboratory according to the manufacturer’s instructions by using the ADVIA Centaur Cortisol assay (Siemens Healthcare Diagnostics Inc, Tarrytown, NY), a competitive chemiluminescent immunoassay. For the traditional CST, a cortisol concentration ≥18 mcg/dL at any time point during the test was used to define normal (negative). Patients with a positive (no results >18 mcg/mL) CST were defined as “screen positives” for the purposes of this analysis. Patient location data were available that allowed for an ICU vs non-ICU comparison.
Statistical analyses were performed in SAS version 9.4 (SAS Institute Inc, Cary, North Carolina). Continuous variables were compared by using a 2-tailed Student t test. Percentiles and proportions were compared by using χ2 tests or Fisher’s exact tests when appropriate. The concordance of screening at each time point compared with the traditional CST was calculated. Positive percent agreement (PPA) with the traditional CST in each subgroup (ICU and floor) and combined was also evaluated. A P value of .05 was used to determine significance.
RESULTS
Cortisol concentrations obtained at 30 minutes were significantly higher than baseline cortisol concentrations (baseline: 12.8 mcg/dL; 30 minutes: 23.9 mcg/dL; P < .001) for all patients. The average cortisol concentrations obtained at 60 minutes (27.4 mcg/dL) were significantly higher than those at baseline and 30 minutes (P < .001). This trend was seen in each subgroup of patients in the ICU and on the floor (Figure). The average baseline cortisol concentration was higher for ICU patients compared to floor patients (17.6 mcg/dL vs 10.9 mcg/dL, respectively).
By using the traditional CST, there were 26 (13.1%) positive tests for AI in ICU patients and 84 (16.7%) positive tests in floor patients (Table).
Only 13% of CSTs were started in the recommended 3-hour window from 6:00
DISCUSSION
Our investigation of 702 CSTs, the largest retrospective analysis to date, finds that the 60-minute cortisol concentration is significantly higher than the 30-minute concentration in a standard, high-dose CST. Sixty-minute cortisol concentrations are more concordant with traditional CST results than the 30-minute concentrations in both critically ill ICU and noncritically ill floor patients. This suggests that a single 60-minute measurement is sufficient for AI screening. The use of only 30-minute concentrations would lead to a significant increase in false-positive screening tests and significantly lower PPA (98% vs 57%). With peak cortisol concentrations occurring at 60-minutes poststimulation, measuring both 30- and 60-minute poststimulation concentrations does not appear to be of significant clinical benefit. The cost-saving from reduced phlebotomy and laboratory expenses would be significant, especially in locations with limited staff or financial resources. Our findings are similar to other recent results by Chitale et al.,17 Mansoor et al.,16 and Zueger et al.18
Zueger et al.18 evaluated the results of high-dose CST in 73 patients and found 13.7% of patients with inadequate cortisol response (<18 mcg/dL) at 30 minutes had normal concentrations at 60 minutes (>18 mcg/dL). Their study did not identify a single case of normal cortisol concentration at 30 minutes that would have inappropriately screened positive for AI if cortisol concentrations were only checked at 60 minutes. Similarly, they suggested that the 30-minute test did not add any additional diagnostic value; however, no confirmatory testing was performed.
Higher cortisol concentrations at 60 minutes poststimulation may result in improved specificity for AI without reducing sensitivity, but it may also indicate that the cutoff value may need to be raised from 18 mcg/dL at 60 minutes to maintain an appropriate clinical sensitivity. Continued research should resolve this clinical question with gold-standard confirmatory testing. Furthermore, there is debate about an appropriate screening cortisol concentration threshold for critically ill patients. Researchers have compared concentrations of 25 mcg/dL to the traditional 18 mcg/dL to improve sensitivity for AI, but these studies do not involve comparisons to confirmatory testing and often result in reduced specificity.23,24
In our study, only a small fraction of testing was performed in the early-morning hours, when basal cortisol results are of value. There may be indications to perform traditional CSTs with a basal concentration, such as for suspected secondary AI, but testing must be performed in the early morning for interpretable results per current recommendations. However, poststimulation cortisol concentrations may be interpreted regardless of the time of day at which the test was initiated.3
Our study is limited by its scope because it is a retrospective analysis. It is also limited by a lack of gold-standard, clinical confirmatory testing or analysis of other clinical data. Our method of testing and interpretation is considered the screening standard and is often used to plan treatment for AI without confirmatory testing, as ITT is not routinely available for hospitalized patients. The validation of the traditional CST to the ITT has been performed extensively, but a randomized trial comparing a single 60-minute concentration to the ITT may be useful. The exact timing of blood draws may have introduced error in the concentration measurements, and this is critical to screening accuracy. Total serum cortisol is 10% bound to albumin,25 and medications such as steroids or opioids and medical conditions such as obesity or liver disease can affect cortisol concentrations.26 Albumin and free cortisol concentrations that may be used to adjust for these variables were not available.
CONCLUSION
We recommend changes to the standard CST to exclude a basal cortisol concentration unless it is indicated for the evaluation of secondary AI or obtained at the appropriate early-morning hour. A single 60-minute poststimulation cortisol concentration may be an appropriate screening test for AI and demonstrates high concordance with the traditional CST. The use of a 30-minute poststimulation concentration alone may lead to a significantly higher number of false-positive results. Alternatively, the stimulated cortisol threshold used to define a normal test may need to be higher at 60 minutes to maintain the appropriate sensitivity. Further study and comparison with confirmatory testing are needed.
Disclosure
The authors have no relevant conflicts of interest to disclose.
1. Ajala O, Lockett H, Twine G, Flanagan DE. Depth and duration of hypoglycaemia achieved during the insulin tolerance test. Eur J Endocrinol. 2012;167(1):59-65. PubMed
2. Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998;83(7):2350-2354. PubMed
3. Azziz R, Bradley E Jr, Huth J, et al. Acute adrenocorticotropin-(1-24) (ACTH) adrenal stimulation in eumenorrheic women: reproducibility and effect of ACTH dose, subject weight, and sampling time. J Clin Endocrinol Metab. 1990;70(5):1273-1279. PubMed
4. Wood, JB, Frankland AW, James VH, Landon J. A Rapid Test of Adrenocortical Function. Lancet. 1965;1(7379):243-245. PubMed
5. Speckart PF, Nicoloff JT, Bethune JE. Screening for adrenocortical insufficiency with cosyntropin (synthetic ACTH). Arch Intern Med. 1971;128(5):761-763. PubMed
6. Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79(4):923-931. PubMed
7. Melmed S, Polonksy K, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Elsevier: Amsterdam, Netherlands; 2016.
8. Nieman LK. Adrenal Cortex, in Goldman-Cecil Medicine. ed. L. Goldman. 2016, Elsevier: Amsterdam, Netherlands; 2016:1514-1521.
9. Kehlet H, Blichert-Toft M, Lindholm J, Rasmussen P. Short ACTH test in assessing hypothalamic-pituitary-adrenocortical function. Br Med J. 1976;1(6004):249-251. PubMed
10. Lindholm J, Kehlet H. Re-evaluation of the clinical value of the 30 min ACTH test in assessing the hypothalamic-pituitary-adrenocortical function. Clin Endocrinol (Oxf). 1987;26(1):53-59. PubMed
11. Lindholm J, Kehlet H, Blichert-Toft M, Dinesen B, Riishede J. Reliability of the 30-minute ACTH test in assessing hypothalamic-pituitary-adrenal function. J Clin Endocrinol Metab. 1978;47(2):272-274. PubMed
12. Hurel SJ, Thompson CJ, Watson MJ, Harris MM, Baylis PH, Kendall-Taylor P. The short Synacthen and insulin stress tests in the assessment of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf). 1996;44(2):141-146. PubMed
13. Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139(3):194-204. PubMed
14. Oelkers W, Diederich S, Bahr V. Diagnosis and therapy surveillance in Addison’s disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinol Metab. 1992;75(1):259-264. PubMed
15. Gonzalez-Gonzalez JG, De la Garza-Hernandez NE, Mancillas-Adame LG, Montes-Villarreal J, Villarreal-Perez JZ. A high-sensitivity test in the assessment of adrenocortical insufficiency: 10 microg vs 250 microg cosyntropin dose assessment of adrenocortical insufficiency. J Endocrinol. 1998;159(2):275-280. PubMed
16. Mansoor S, Islam N, Siddiqui I, Jabbar A. Sixty-minute post-Synacthen serum cortisol level: a reliable and cost-effective screening test for excluding adrenal insufficiency compared to the conventional short Synacthen test. Singapore Med J. 2007;48(6):519-523. PubMed
17. Chitale A, Musonda P, McGregor AM, Dhatariya KK. Determining the utility of the 60 min cortisol measurement in the short synacthen test. Clin Endocrinol (Oxf). 2013;79(1):14-19. PubMed
18. Zueger T, Jordi M, Laimer M, Stettler C. Utility of 30 and 60 minute cortisol samples after high-dose synthetic ACTH-1-24 injection in the diagnosis of adrenal insufficiency. Swiss Med Wkly. 2014;144:w13987. PubMed
19. Cartaya J, Misra M. The low-dose ACTH stimulation test: is 30 minutes long enough? Endocr Pract. 2015;21(5):508-513. PubMed
20. Gonzálbez, Villabona, Ramon, et al. Establishment of reference values for standard dose short synacthen test (250 μg), low dose short synacthen test (1 μg) and insulin tolerance test for assessment of the hypothalamo–pituitary–adrenal axis in normal subjects. Clin Endocrinol. 2000;53(2):199-204.
21. McGill J, Clutter W, Baranski T. The Washington Manual of Endocrinology Subspecialty Consult. 3rd ed. Washington Manual, ed. Henderson K, De Fer T. Lippincott Williams and Wilkins: Philadelphia, PA; 2012:384.
22. Nieman L. Diagnosis of adrenal insufficiency in adults. In UpToDate, ed. Post T. Wolters Klewer: Waltham, MA; 2017.
23. Marik PE, Kiminyo K, Zaloga GP. Adrenal insufficiency in critically ill patients with human immunodeficiency virus. Crit Care Med. 2002;30(6):1267-1273. PubMed
24. Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003;31(1):141-145. PubMed
25. Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clinica Chemica Acta. 2005;359(1-2):189-194. PubMed
26. Torpy DJ, Ho JT. Value of Free Cortisol Measurement in Systemic Infection. Horm Metab Res. 2007;39(6):439-444. PubMed
Testing for adrenal insufficiency (AI) is common in the hospital setting. The gold standard remains the insulin tolerance test (ITT), in which cortisol concentration is measured after the induction of hypoglycemia to <35 mg/dL.1 Alternatively, metyrapone testing works by blocking cortisol synthesis. If pretest adrenocorticotropic hormone (ACTH) concentrations are low and ACTH concentrations do not rise after the administration of metyrapone, the patient is given a diagnosis of AI. Both assays pose some risk to patients with AI and are typically only performed as confirmatory tests. Morning random cortisol concentrations can be used to suggest AI if concentrations are <3 mcg/dL, but they often provide indeterminate results if concentrations are between 3 and 15 mcg/dL.2 Thus, morning cortisol concentrations in isolation are not diagnostic of AI. For these reasons, most experts recommend a dynamic, high-dose cosyntropin stimulation testing (CST) with 250 mcg of intravenous cosyntropin to screen for AI. The test can be done any time of day.3 Historically, an incremental response to cosyntropin, or “delta,” was also required to indicate a normal response to stimulation.4 However, the baseline cortisol concentration is dependent on circadian rhythm and level of stress. For this reason, a delta, whether large or small, has been abandoned as a requisite for the diagnosis of AI.5-7 A normal CST is widely accepted to be identified by any cortisol concentration >18 mcg/dL during the test (basal or poststimulation).8
The seminal studies by Lindholm, Kehlet, and coauthors9-11 validated the CST against the gold standard ITT and utilized only 0- and 30-minute cortisol concentrations. A later study in patients with pituitary disease demonstrated that 30-minute concentrations had a stronger correlation with the ITT than 60-minute concentrations (false-negative rate: 10% vs 27%).12 However, in that study, a higher threshold was used for the 60-minute concentration than for what was obtained at 30 minutes (25.4 vs 21.8 mcg/dL, respectively). Multiple studies have shown that the 60-minute concentration is higher than the 30-minute concentration after cosyntropin stimulation.4,5,13 Subsequent, small studies of patients who were known to have AI have shown that 60-minute concentrations are as useful as 30-minute concentrations.5,14,15 Because 30-minute cortisol concentrations are often lower than 60-minute concentrations, a single 30-minute result may lead to a falsely abnormal test.16,17 As such, the use of a single 60-minute test may be more appropriate. Indeed, some authors have suggested that measuring only 30-minute concentrations may lead to overdiagnosis of AI by missing an appropriate response, serum cortisol >18 mcg/dL, at 60 minutes.17-19 Peak cortisol concentrations after low-dose cosyntropin stimulation (1 mcg) are seen at 60 minutes, and low-dose stimulation has been shown to be more variable than in the high-dose test (250 mg).19,20
There is a lack of consensus to guide clinicians as to when cortisol concentrations should be measured after stimulation, and standard references lack uniformity. Commonly accessed medical resources—such as UpToDate and Jameson’s Endocrinology—recommend basal, 30-minute, and 60-minute cortisol concentrations, while Williams Textbook of Endocrinology recommends basal and 30-minute concentrations, and the Washington Manual recommends only a single 30-minute concentration.7,21,22 Goldman-Cecil Medicine8 recommends checking a cortisol concentration between 30 and 60 minutes and recommends the same 18 mcg/dL cutoff for any test obtained in this time period. As a result of these variable recommendations, all 3 time points are often obtained. Prominent review articles continue to recommend checking all 3 concentrations while presenting evidence of peak cortisol response at 60 minutes poststimulation.13
In this study, we retrospectively examined CSTs in hospitalized, adult patients both in the intensive care unit (ICU) and hospital ward and/or floor settings to evaluate for significant differences in 30- and 60-minute cortisol concentrations and compare the concordance of screening at each time point alone with traditional CST at all 3 time points. By using these results, we discuss the utility of obtaining 3 cortisol samples.
METHODS
After receiving approval from the institutional review board, we retrospectively reviewed all standard, high-dose CSTs performed on adult inpatients at the Barnes-Jewish Hospital laboratory from January 1, 2012, to August 31, 2013. All patients received the same standard dose (250 mcg cosyntropin, a synthetic ACTH, at a concentration of 1 mcg/mL administered over 2 minutes) regardless of age or weight. We collected patient gender; age; time of baseline cortisol measurement; cortisol results at baseline, 30, and 60 minutes; and patient location (inpatient floor vs ICU status). Tests were included if results from all 3 time points (0, 30, 60 minute) were available.
Cortisol concentrations were assessed by the laboratory according to the manufacturer’s instructions by using the ADVIA Centaur Cortisol assay (Siemens Healthcare Diagnostics Inc, Tarrytown, NY), a competitive chemiluminescent immunoassay. For the traditional CST, a cortisol concentration ≥18 mcg/dL at any time point during the test was used to define normal (negative). Patients with a positive (no results >18 mcg/mL) CST were defined as “screen positives” for the purposes of this analysis. Patient location data were available that allowed for an ICU vs non-ICU comparison.
Statistical analyses were performed in SAS version 9.4 (SAS Institute Inc, Cary, North Carolina). Continuous variables were compared by using a 2-tailed Student t test. Percentiles and proportions were compared by using χ2 tests or Fisher’s exact tests when appropriate. The concordance of screening at each time point compared with the traditional CST was calculated. Positive percent agreement (PPA) with the traditional CST in each subgroup (ICU and floor) and combined was also evaluated. A P value of .05 was used to determine significance.
RESULTS
Cortisol concentrations obtained at 30 minutes were significantly higher than baseline cortisol concentrations (baseline: 12.8 mcg/dL; 30 minutes: 23.9 mcg/dL; P < .001) for all patients. The average cortisol concentrations obtained at 60 minutes (27.4 mcg/dL) were significantly higher than those at baseline and 30 minutes (P < .001). This trend was seen in each subgroup of patients in the ICU and on the floor (Figure). The average baseline cortisol concentration was higher for ICU patients compared to floor patients (17.6 mcg/dL vs 10.9 mcg/dL, respectively).
By using the traditional CST, there were 26 (13.1%) positive tests for AI in ICU patients and 84 (16.7%) positive tests in floor patients (Table).
Only 13% of CSTs were started in the recommended 3-hour window from 6:00
DISCUSSION
Our investigation of 702 CSTs, the largest retrospective analysis to date, finds that the 60-minute cortisol concentration is significantly higher than the 30-minute concentration in a standard, high-dose CST. Sixty-minute cortisol concentrations are more concordant with traditional CST results than the 30-minute concentrations in both critically ill ICU and noncritically ill floor patients. This suggests that a single 60-minute measurement is sufficient for AI screening. The use of only 30-minute concentrations would lead to a significant increase in false-positive screening tests and significantly lower PPA (98% vs 57%). With peak cortisol concentrations occurring at 60-minutes poststimulation, measuring both 30- and 60-minute poststimulation concentrations does not appear to be of significant clinical benefit. The cost-saving from reduced phlebotomy and laboratory expenses would be significant, especially in locations with limited staff or financial resources. Our findings are similar to other recent results by Chitale et al.,17 Mansoor et al.,16 and Zueger et al.18
Zueger et al.18 evaluated the results of high-dose CST in 73 patients and found 13.7% of patients with inadequate cortisol response (<18 mcg/dL) at 30 minutes had normal concentrations at 60 minutes (>18 mcg/dL). Their study did not identify a single case of normal cortisol concentration at 30 minutes that would have inappropriately screened positive for AI if cortisol concentrations were only checked at 60 minutes. Similarly, they suggested that the 30-minute test did not add any additional diagnostic value; however, no confirmatory testing was performed.
Higher cortisol concentrations at 60 minutes poststimulation may result in improved specificity for AI without reducing sensitivity, but it may also indicate that the cutoff value may need to be raised from 18 mcg/dL at 60 minutes to maintain an appropriate clinical sensitivity. Continued research should resolve this clinical question with gold-standard confirmatory testing. Furthermore, there is debate about an appropriate screening cortisol concentration threshold for critically ill patients. Researchers have compared concentrations of 25 mcg/dL to the traditional 18 mcg/dL to improve sensitivity for AI, but these studies do not involve comparisons to confirmatory testing and often result in reduced specificity.23,24
In our study, only a small fraction of testing was performed in the early-morning hours, when basal cortisol results are of value. There may be indications to perform traditional CSTs with a basal concentration, such as for suspected secondary AI, but testing must be performed in the early morning for interpretable results per current recommendations. However, poststimulation cortisol concentrations may be interpreted regardless of the time of day at which the test was initiated.3
Our study is limited by its scope because it is a retrospective analysis. It is also limited by a lack of gold-standard, clinical confirmatory testing or analysis of other clinical data. Our method of testing and interpretation is considered the screening standard and is often used to plan treatment for AI without confirmatory testing, as ITT is not routinely available for hospitalized patients. The validation of the traditional CST to the ITT has been performed extensively, but a randomized trial comparing a single 60-minute concentration to the ITT may be useful. The exact timing of blood draws may have introduced error in the concentration measurements, and this is critical to screening accuracy. Total serum cortisol is 10% bound to albumin,25 and medications such as steroids or opioids and medical conditions such as obesity or liver disease can affect cortisol concentrations.26 Albumin and free cortisol concentrations that may be used to adjust for these variables were not available.
CONCLUSION
We recommend changes to the standard CST to exclude a basal cortisol concentration unless it is indicated for the evaluation of secondary AI or obtained at the appropriate early-morning hour. A single 60-minute poststimulation cortisol concentration may be an appropriate screening test for AI and demonstrates high concordance with the traditional CST. The use of a 30-minute poststimulation concentration alone may lead to a significantly higher number of false-positive results. Alternatively, the stimulated cortisol threshold used to define a normal test may need to be higher at 60 minutes to maintain the appropriate sensitivity. Further study and comparison with confirmatory testing are needed.
Disclosure
The authors have no relevant conflicts of interest to disclose.
Testing for adrenal insufficiency (AI) is common in the hospital setting. The gold standard remains the insulin tolerance test (ITT), in which cortisol concentration is measured after the induction of hypoglycemia to <35 mg/dL.1 Alternatively, metyrapone testing works by blocking cortisol synthesis. If pretest adrenocorticotropic hormone (ACTH) concentrations are low and ACTH concentrations do not rise after the administration of metyrapone, the patient is given a diagnosis of AI. Both assays pose some risk to patients with AI and are typically only performed as confirmatory tests. Morning random cortisol concentrations can be used to suggest AI if concentrations are <3 mcg/dL, but they often provide indeterminate results if concentrations are between 3 and 15 mcg/dL.2 Thus, morning cortisol concentrations in isolation are not diagnostic of AI. For these reasons, most experts recommend a dynamic, high-dose cosyntropin stimulation testing (CST) with 250 mcg of intravenous cosyntropin to screen for AI. The test can be done any time of day.3 Historically, an incremental response to cosyntropin, or “delta,” was also required to indicate a normal response to stimulation.4 However, the baseline cortisol concentration is dependent on circadian rhythm and level of stress. For this reason, a delta, whether large or small, has been abandoned as a requisite for the diagnosis of AI.5-7 A normal CST is widely accepted to be identified by any cortisol concentration >18 mcg/dL during the test (basal or poststimulation).8
The seminal studies by Lindholm, Kehlet, and coauthors9-11 validated the CST against the gold standard ITT and utilized only 0- and 30-minute cortisol concentrations. A later study in patients with pituitary disease demonstrated that 30-minute concentrations had a stronger correlation with the ITT than 60-minute concentrations (false-negative rate: 10% vs 27%).12 However, in that study, a higher threshold was used for the 60-minute concentration than for what was obtained at 30 minutes (25.4 vs 21.8 mcg/dL, respectively). Multiple studies have shown that the 60-minute concentration is higher than the 30-minute concentration after cosyntropin stimulation.4,5,13 Subsequent, small studies of patients who were known to have AI have shown that 60-minute concentrations are as useful as 30-minute concentrations.5,14,15 Because 30-minute cortisol concentrations are often lower than 60-minute concentrations, a single 30-minute result may lead to a falsely abnormal test.16,17 As such, the use of a single 60-minute test may be more appropriate. Indeed, some authors have suggested that measuring only 30-minute concentrations may lead to overdiagnosis of AI by missing an appropriate response, serum cortisol >18 mcg/dL, at 60 minutes.17-19 Peak cortisol concentrations after low-dose cosyntropin stimulation (1 mcg) are seen at 60 minutes, and low-dose stimulation has been shown to be more variable than in the high-dose test (250 mg).19,20
There is a lack of consensus to guide clinicians as to when cortisol concentrations should be measured after stimulation, and standard references lack uniformity. Commonly accessed medical resources—such as UpToDate and Jameson’s Endocrinology—recommend basal, 30-minute, and 60-minute cortisol concentrations, while Williams Textbook of Endocrinology recommends basal and 30-minute concentrations, and the Washington Manual recommends only a single 30-minute concentration.7,21,22 Goldman-Cecil Medicine8 recommends checking a cortisol concentration between 30 and 60 minutes and recommends the same 18 mcg/dL cutoff for any test obtained in this time period. As a result of these variable recommendations, all 3 time points are often obtained. Prominent review articles continue to recommend checking all 3 concentrations while presenting evidence of peak cortisol response at 60 minutes poststimulation.13
In this study, we retrospectively examined CSTs in hospitalized, adult patients both in the intensive care unit (ICU) and hospital ward and/or floor settings to evaluate for significant differences in 30- and 60-minute cortisol concentrations and compare the concordance of screening at each time point alone with traditional CST at all 3 time points. By using these results, we discuss the utility of obtaining 3 cortisol samples.
METHODS
After receiving approval from the institutional review board, we retrospectively reviewed all standard, high-dose CSTs performed on adult inpatients at the Barnes-Jewish Hospital laboratory from January 1, 2012, to August 31, 2013. All patients received the same standard dose (250 mcg cosyntropin, a synthetic ACTH, at a concentration of 1 mcg/mL administered over 2 minutes) regardless of age or weight. We collected patient gender; age; time of baseline cortisol measurement; cortisol results at baseline, 30, and 60 minutes; and patient location (inpatient floor vs ICU status). Tests were included if results from all 3 time points (0, 30, 60 minute) were available.
Cortisol concentrations were assessed by the laboratory according to the manufacturer’s instructions by using the ADVIA Centaur Cortisol assay (Siemens Healthcare Diagnostics Inc, Tarrytown, NY), a competitive chemiluminescent immunoassay. For the traditional CST, a cortisol concentration ≥18 mcg/dL at any time point during the test was used to define normal (negative). Patients with a positive (no results >18 mcg/mL) CST were defined as “screen positives” for the purposes of this analysis. Patient location data were available that allowed for an ICU vs non-ICU comparison.
Statistical analyses were performed in SAS version 9.4 (SAS Institute Inc, Cary, North Carolina). Continuous variables were compared by using a 2-tailed Student t test. Percentiles and proportions were compared by using χ2 tests or Fisher’s exact tests when appropriate. The concordance of screening at each time point compared with the traditional CST was calculated. Positive percent agreement (PPA) with the traditional CST in each subgroup (ICU and floor) and combined was also evaluated. A P value of .05 was used to determine significance.
RESULTS
Cortisol concentrations obtained at 30 minutes were significantly higher than baseline cortisol concentrations (baseline: 12.8 mcg/dL; 30 minutes: 23.9 mcg/dL; P < .001) for all patients. The average cortisol concentrations obtained at 60 minutes (27.4 mcg/dL) were significantly higher than those at baseline and 30 minutes (P < .001). This trend was seen in each subgroup of patients in the ICU and on the floor (Figure). The average baseline cortisol concentration was higher for ICU patients compared to floor patients (17.6 mcg/dL vs 10.9 mcg/dL, respectively).
By using the traditional CST, there were 26 (13.1%) positive tests for AI in ICU patients and 84 (16.7%) positive tests in floor patients (Table).
Only 13% of CSTs were started in the recommended 3-hour window from 6:00
DISCUSSION
Our investigation of 702 CSTs, the largest retrospective analysis to date, finds that the 60-minute cortisol concentration is significantly higher than the 30-minute concentration in a standard, high-dose CST. Sixty-minute cortisol concentrations are more concordant with traditional CST results than the 30-minute concentrations in both critically ill ICU and noncritically ill floor patients. This suggests that a single 60-minute measurement is sufficient for AI screening. The use of only 30-minute concentrations would lead to a significant increase in false-positive screening tests and significantly lower PPA (98% vs 57%). With peak cortisol concentrations occurring at 60-minutes poststimulation, measuring both 30- and 60-minute poststimulation concentrations does not appear to be of significant clinical benefit. The cost-saving from reduced phlebotomy and laboratory expenses would be significant, especially in locations with limited staff or financial resources. Our findings are similar to other recent results by Chitale et al.,17 Mansoor et al.,16 and Zueger et al.18
Zueger et al.18 evaluated the results of high-dose CST in 73 patients and found 13.7% of patients with inadequate cortisol response (<18 mcg/dL) at 30 minutes had normal concentrations at 60 minutes (>18 mcg/dL). Their study did not identify a single case of normal cortisol concentration at 30 minutes that would have inappropriately screened positive for AI if cortisol concentrations were only checked at 60 minutes. Similarly, they suggested that the 30-minute test did not add any additional diagnostic value; however, no confirmatory testing was performed.
Higher cortisol concentrations at 60 minutes poststimulation may result in improved specificity for AI without reducing sensitivity, but it may also indicate that the cutoff value may need to be raised from 18 mcg/dL at 60 minutes to maintain an appropriate clinical sensitivity. Continued research should resolve this clinical question with gold-standard confirmatory testing. Furthermore, there is debate about an appropriate screening cortisol concentration threshold for critically ill patients. Researchers have compared concentrations of 25 mcg/dL to the traditional 18 mcg/dL to improve sensitivity for AI, but these studies do not involve comparisons to confirmatory testing and often result in reduced specificity.23,24
In our study, only a small fraction of testing was performed in the early-morning hours, when basal cortisol results are of value. There may be indications to perform traditional CSTs with a basal concentration, such as for suspected secondary AI, but testing must be performed in the early morning for interpretable results per current recommendations. However, poststimulation cortisol concentrations may be interpreted regardless of the time of day at which the test was initiated.3
Our study is limited by its scope because it is a retrospective analysis. It is also limited by a lack of gold-standard, clinical confirmatory testing or analysis of other clinical data. Our method of testing and interpretation is considered the screening standard and is often used to plan treatment for AI without confirmatory testing, as ITT is not routinely available for hospitalized patients. The validation of the traditional CST to the ITT has been performed extensively, but a randomized trial comparing a single 60-minute concentration to the ITT may be useful. The exact timing of blood draws may have introduced error in the concentration measurements, and this is critical to screening accuracy. Total serum cortisol is 10% bound to albumin,25 and medications such as steroids or opioids and medical conditions such as obesity or liver disease can affect cortisol concentrations.26 Albumin and free cortisol concentrations that may be used to adjust for these variables were not available.
CONCLUSION
We recommend changes to the standard CST to exclude a basal cortisol concentration unless it is indicated for the evaluation of secondary AI or obtained at the appropriate early-morning hour. A single 60-minute poststimulation cortisol concentration may be an appropriate screening test for AI and demonstrates high concordance with the traditional CST. The use of a 30-minute poststimulation concentration alone may lead to a significantly higher number of false-positive results. Alternatively, the stimulated cortisol threshold used to define a normal test may need to be higher at 60 minutes to maintain the appropriate sensitivity. Further study and comparison with confirmatory testing are needed.
Disclosure
The authors have no relevant conflicts of interest to disclose.
1. Ajala O, Lockett H, Twine G, Flanagan DE. Depth and duration of hypoglycaemia achieved during the insulin tolerance test. Eur J Endocrinol. 2012;167(1):59-65. PubMed
2. Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998;83(7):2350-2354. PubMed
3. Azziz R, Bradley E Jr, Huth J, et al. Acute adrenocorticotropin-(1-24) (ACTH) adrenal stimulation in eumenorrheic women: reproducibility and effect of ACTH dose, subject weight, and sampling time. J Clin Endocrinol Metab. 1990;70(5):1273-1279. PubMed
4. Wood, JB, Frankland AW, James VH, Landon J. A Rapid Test of Adrenocortical Function. Lancet. 1965;1(7379):243-245. PubMed
5. Speckart PF, Nicoloff JT, Bethune JE. Screening for adrenocortical insufficiency with cosyntropin (synthetic ACTH). Arch Intern Med. 1971;128(5):761-763. PubMed
6. Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79(4):923-931. PubMed
7. Melmed S, Polonksy K, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Elsevier: Amsterdam, Netherlands; 2016.
8. Nieman LK. Adrenal Cortex, in Goldman-Cecil Medicine. ed. L. Goldman. 2016, Elsevier: Amsterdam, Netherlands; 2016:1514-1521.
9. Kehlet H, Blichert-Toft M, Lindholm J, Rasmussen P. Short ACTH test in assessing hypothalamic-pituitary-adrenocortical function. Br Med J. 1976;1(6004):249-251. PubMed
10. Lindholm J, Kehlet H. Re-evaluation of the clinical value of the 30 min ACTH test in assessing the hypothalamic-pituitary-adrenocortical function. Clin Endocrinol (Oxf). 1987;26(1):53-59. PubMed
11. Lindholm J, Kehlet H, Blichert-Toft M, Dinesen B, Riishede J. Reliability of the 30-minute ACTH test in assessing hypothalamic-pituitary-adrenal function. J Clin Endocrinol Metab. 1978;47(2):272-274. PubMed
12. Hurel SJ, Thompson CJ, Watson MJ, Harris MM, Baylis PH, Kendall-Taylor P. The short Synacthen and insulin stress tests in the assessment of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf). 1996;44(2):141-146. PubMed
13. Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139(3):194-204. PubMed
14. Oelkers W, Diederich S, Bahr V. Diagnosis and therapy surveillance in Addison’s disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinol Metab. 1992;75(1):259-264. PubMed
15. Gonzalez-Gonzalez JG, De la Garza-Hernandez NE, Mancillas-Adame LG, Montes-Villarreal J, Villarreal-Perez JZ. A high-sensitivity test in the assessment of adrenocortical insufficiency: 10 microg vs 250 microg cosyntropin dose assessment of adrenocortical insufficiency. J Endocrinol. 1998;159(2):275-280. PubMed
16. Mansoor S, Islam N, Siddiqui I, Jabbar A. Sixty-minute post-Synacthen serum cortisol level: a reliable and cost-effective screening test for excluding adrenal insufficiency compared to the conventional short Synacthen test. Singapore Med J. 2007;48(6):519-523. PubMed
17. Chitale A, Musonda P, McGregor AM, Dhatariya KK. Determining the utility of the 60 min cortisol measurement in the short synacthen test. Clin Endocrinol (Oxf). 2013;79(1):14-19. PubMed
18. Zueger T, Jordi M, Laimer M, Stettler C. Utility of 30 and 60 minute cortisol samples after high-dose synthetic ACTH-1-24 injection in the diagnosis of adrenal insufficiency. Swiss Med Wkly. 2014;144:w13987. PubMed
19. Cartaya J, Misra M. The low-dose ACTH stimulation test: is 30 minutes long enough? Endocr Pract. 2015;21(5):508-513. PubMed
20. Gonzálbez, Villabona, Ramon, et al. Establishment of reference values for standard dose short synacthen test (250 μg), low dose short synacthen test (1 μg) and insulin tolerance test for assessment of the hypothalamo–pituitary–adrenal axis in normal subjects. Clin Endocrinol. 2000;53(2):199-204.
21. McGill J, Clutter W, Baranski T. The Washington Manual of Endocrinology Subspecialty Consult. 3rd ed. Washington Manual, ed. Henderson K, De Fer T. Lippincott Williams and Wilkins: Philadelphia, PA; 2012:384.
22. Nieman L. Diagnosis of adrenal insufficiency in adults. In UpToDate, ed. Post T. Wolters Klewer: Waltham, MA; 2017.
23. Marik PE, Kiminyo K, Zaloga GP. Adrenal insufficiency in critically ill patients with human immunodeficiency virus. Crit Care Med. 2002;30(6):1267-1273. PubMed
24. Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003;31(1):141-145. PubMed
25. Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clinica Chemica Acta. 2005;359(1-2):189-194. PubMed
26. Torpy DJ, Ho JT. Value of Free Cortisol Measurement in Systemic Infection. Horm Metab Res. 2007;39(6):439-444. PubMed
1. Ajala O, Lockett H, Twine G, Flanagan DE. Depth and duration of hypoglycaemia achieved during the insulin tolerance test. Eur J Endocrinol. 2012;167(1):59-65. PubMed
2. Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998;83(7):2350-2354. PubMed
3. Azziz R, Bradley E Jr, Huth J, et al. Acute adrenocorticotropin-(1-24) (ACTH) adrenal stimulation in eumenorrheic women: reproducibility and effect of ACTH dose, subject weight, and sampling time. J Clin Endocrinol Metab. 1990;70(5):1273-1279. PubMed
4. Wood, JB, Frankland AW, James VH, Landon J. A Rapid Test of Adrenocortical Function. Lancet. 1965;1(7379):243-245. PubMed
5. Speckart PF, Nicoloff JT, Bethune JE. Screening for adrenocortical insufficiency with cosyntropin (synthetic ACTH). Arch Intern Med. 1971;128(5):761-763. PubMed
6. Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79(4):923-931. PubMed
7. Melmed S, Polonksy K, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Elsevier: Amsterdam, Netherlands; 2016.
8. Nieman LK. Adrenal Cortex, in Goldman-Cecil Medicine. ed. L. Goldman. 2016, Elsevier: Amsterdam, Netherlands; 2016:1514-1521.
9. Kehlet H, Blichert-Toft M, Lindholm J, Rasmussen P. Short ACTH test in assessing hypothalamic-pituitary-adrenocortical function. Br Med J. 1976;1(6004):249-251. PubMed
10. Lindholm J, Kehlet H. Re-evaluation of the clinical value of the 30 min ACTH test in assessing the hypothalamic-pituitary-adrenocortical function. Clin Endocrinol (Oxf). 1987;26(1):53-59. PubMed
11. Lindholm J, Kehlet H, Blichert-Toft M, Dinesen B, Riishede J. Reliability of the 30-minute ACTH test in assessing hypothalamic-pituitary-adrenal function. J Clin Endocrinol Metab. 1978;47(2):272-274. PubMed
12. Hurel SJ, Thompson CJ, Watson MJ, Harris MM, Baylis PH, Kendall-Taylor P. The short Synacthen and insulin stress tests in the assessment of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf). 1996;44(2):141-146. PubMed
13. Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139(3):194-204. PubMed
14. Oelkers W, Diederich S, Bahr V. Diagnosis and therapy surveillance in Addison’s disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinol Metab. 1992;75(1):259-264. PubMed
15. Gonzalez-Gonzalez JG, De la Garza-Hernandez NE, Mancillas-Adame LG, Montes-Villarreal J, Villarreal-Perez JZ. A high-sensitivity test in the assessment of adrenocortical insufficiency: 10 microg vs 250 microg cosyntropin dose assessment of adrenocortical insufficiency. J Endocrinol. 1998;159(2):275-280. PubMed
16. Mansoor S, Islam N, Siddiqui I, Jabbar A. Sixty-minute post-Synacthen serum cortisol level: a reliable and cost-effective screening test for excluding adrenal insufficiency compared to the conventional short Synacthen test. Singapore Med J. 2007;48(6):519-523. PubMed
17. Chitale A, Musonda P, McGregor AM, Dhatariya KK. Determining the utility of the 60 min cortisol measurement in the short synacthen test. Clin Endocrinol (Oxf). 2013;79(1):14-19. PubMed
18. Zueger T, Jordi M, Laimer M, Stettler C. Utility of 30 and 60 minute cortisol samples after high-dose synthetic ACTH-1-24 injection in the diagnosis of adrenal insufficiency. Swiss Med Wkly. 2014;144:w13987. PubMed
19. Cartaya J, Misra M. The low-dose ACTH stimulation test: is 30 minutes long enough? Endocr Pract. 2015;21(5):508-513. PubMed
20. Gonzálbez, Villabona, Ramon, et al. Establishment of reference values for standard dose short synacthen test (250 μg), low dose short synacthen test (1 μg) and insulin tolerance test for assessment of the hypothalamo–pituitary–adrenal axis in normal subjects. Clin Endocrinol. 2000;53(2):199-204.
21. McGill J, Clutter W, Baranski T. The Washington Manual of Endocrinology Subspecialty Consult. 3rd ed. Washington Manual, ed. Henderson K, De Fer T. Lippincott Williams and Wilkins: Philadelphia, PA; 2012:384.
22. Nieman L. Diagnosis of adrenal insufficiency in adults. In UpToDate, ed. Post T. Wolters Klewer: Waltham, MA; 2017.
23. Marik PE, Kiminyo K, Zaloga GP. Adrenal insufficiency in critically ill patients with human immunodeficiency virus. Crit Care Med. 2002;30(6):1267-1273. PubMed
24. Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003;31(1):141-145. PubMed
25. Lewis JG, Bagley CJ, Elder PA, Bachmann AW, Torpy DJ. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clinica Chemica Acta. 2005;359(1-2):189-194. PubMed
26. Torpy DJ, Ho JT. Value of Free Cortisol Measurement in Systemic Infection. Horm Metab Res. 2007;39(6):439-444. PubMed
© 2018 Society of Hospital Medicine