User login
How Does Migraine Change During the Menopausal Transition?
Migraine may worsen or change its pattern for many women approaching menopause.
SAN FRANCISCO—Most women with migraine develop migraine pattern change, worsening migraine, or new-onset migraine at the age of menopause, according to a study presented at the 60th Annual Scientific Meeting of the American Headache Society. These changes most often occur during the perimenopausal or postmenopausal stages.
Previous research indicates that the prevalence and frequency of migraine are higher in perimenopausal women than in other women. Yu-Chen Cheng, MD, MPH, a postdoctoral fellow at Massachusetts General Hospital in Boston, and colleagues investigated patterns of migraine in women at menopausal age (ie, age 40–60) with migraine who presented to the Partners Healthcare Hospitals. The investigators reviewed participants’ medical records, brain image reports, and laboratory data, including levels of estradiol and follicle-stimulating hormone (FSH).
In their retrospective study, Dr. Cheng and colleagues identified 81 patients with concurrent diagnoses of migraine and menopause who had clinical data available. They excluded patients with missing or inaccessible data, as well as patients with organic brain lesions such as those associated with multiple sclerosis or brain tumor. The researchers included 69 patients in the study.
Sixty patients (86.96%) had a history of migraine, and the other nine patients (13.04%) had new-onset migraine. Among participants with a history of migraine, 35 (58.33%) had a change in migraine pattern or worsening of their migraine headaches. The investigators categorized patients in this group as having migraine worsening (60.00%), migraine pattern change (28.57%), worsening related to other cause (8.57%), and not sure (2.86%). Twenty-five patients with migraine history were stable and had no change in the pattern of their headaches.
Dr. Cheng and colleagues also examined the population’s menopausal status when they had migraine change or worsening or new migraine. Among patients with migraine history, nine of 35 (25.71%) were at the perimenopausal stage, 12 (34.29%) were postmenopausal, five (14.29%) were premenopausal, three (8.57%) had worsening because of other causes, and three (8.57%) did not have records on their menopausal status. For patients with new-onset migraine, three of nine (33%) were perimenopausal, three (33%) were postmenopausal, and one (11.11%) was premenopausal.
Among patients with new-onset migraine, brain MRI was normal in 44.44%, showed pituitary abnormality in 22.22%, and showed other brain lesion in 33.33%. In patients with migraine history, brain MRI was normal in 45%, showed pituitary abnormality in 8.3%, showed nonspecific T2 high white matter lesion in 16.67%, and showed other brain lesion in 11.67%.
“Identifying migraine worsening or new-onset migraine during the menopausal transition age may help the diagnosis and treatment optimization of migraine for women during the menopausal age,” said Dr. Cheng.
Migraine may worsen or change its pattern for many women approaching menopause.
Migraine may worsen or change its pattern for many women approaching menopause.
SAN FRANCISCO—Most women with migraine develop migraine pattern change, worsening migraine, or new-onset migraine at the age of menopause, according to a study presented at the 60th Annual Scientific Meeting of the American Headache Society. These changes most often occur during the perimenopausal or postmenopausal stages.
Previous research indicates that the prevalence and frequency of migraine are higher in perimenopausal women than in other women. Yu-Chen Cheng, MD, MPH, a postdoctoral fellow at Massachusetts General Hospital in Boston, and colleagues investigated patterns of migraine in women at menopausal age (ie, age 40–60) with migraine who presented to the Partners Healthcare Hospitals. The investigators reviewed participants’ medical records, brain image reports, and laboratory data, including levels of estradiol and follicle-stimulating hormone (FSH).
In their retrospective study, Dr. Cheng and colleagues identified 81 patients with concurrent diagnoses of migraine and menopause who had clinical data available. They excluded patients with missing or inaccessible data, as well as patients with organic brain lesions such as those associated with multiple sclerosis or brain tumor. The researchers included 69 patients in the study.
Sixty patients (86.96%) had a history of migraine, and the other nine patients (13.04%) had new-onset migraine. Among participants with a history of migraine, 35 (58.33%) had a change in migraine pattern or worsening of their migraine headaches. The investigators categorized patients in this group as having migraine worsening (60.00%), migraine pattern change (28.57%), worsening related to other cause (8.57%), and not sure (2.86%). Twenty-five patients with migraine history were stable and had no change in the pattern of their headaches.
Dr. Cheng and colleagues also examined the population’s menopausal status when they had migraine change or worsening or new migraine. Among patients with migraine history, nine of 35 (25.71%) were at the perimenopausal stage, 12 (34.29%) were postmenopausal, five (14.29%) were premenopausal, three (8.57%) had worsening because of other causes, and three (8.57%) did not have records on their menopausal status. For patients with new-onset migraine, three of nine (33%) were perimenopausal, three (33%) were postmenopausal, and one (11.11%) was premenopausal.
Among patients with new-onset migraine, brain MRI was normal in 44.44%, showed pituitary abnormality in 22.22%, and showed other brain lesion in 33.33%. In patients with migraine history, brain MRI was normal in 45%, showed pituitary abnormality in 8.3%, showed nonspecific T2 high white matter lesion in 16.67%, and showed other brain lesion in 11.67%.
“Identifying migraine worsening or new-onset migraine during the menopausal transition age may help the diagnosis and treatment optimization of migraine for women during the menopausal age,” said Dr. Cheng.
SAN FRANCISCO—Most women with migraine develop migraine pattern change, worsening migraine, or new-onset migraine at the age of menopause, according to a study presented at the 60th Annual Scientific Meeting of the American Headache Society. These changes most often occur during the perimenopausal or postmenopausal stages.
Previous research indicates that the prevalence and frequency of migraine are higher in perimenopausal women than in other women. Yu-Chen Cheng, MD, MPH, a postdoctoral fellow at Massachusetts General Hospital in Boston, and colleagues investigated patterns of migraine in women at menopausal age (ie, age 40–60) with migraine who presented to the Partners Healthcare Hospitals. The investigators reviewed participants’ medical records, brain image reports, and laboratory data, including levels of estradiol and follicle-stimulating hormone (FSH).
In their retrospective study, Dr. Cheng and colleagues identified 81 patients with concurrent diagnoses of migraine and menopause who had clinical data available. They excluded patients with missing or inaccessible data, as well as patients with organic brain lesions such as those associated with multiple sclerosis or brain tumor. The researchers included 69 patients in the study.
Sixty patients (86.96%) had a history of migraine, and the other nine patients (13.04%) had new-onset migraine. Among participants with a history of migraine, 35 (58.33%) had a change in migraine pattern or worsening of their migraine headaches. The investigators categorized patients in this group as having migraine worsening (60.00%), migraine pattern change (28.57%), worsening related to other cause (8.57%), and not sure (2.86%). Twenty-five patients with migraine history were stable and had no change in the pattern of their headaches.
Dr. Cheng and colleagues also examined the population’s menopausal status when they had migraine change or worsening or new migraine. Among patients with migraine history, nine of 35 (25.71%) were at the perimenopausal stage, 12 (34.29%) were postmenopausal, five (14.29%) were premenopausal, three (8.57%) had worsening because of other causes, and three (8.57%) did not have records on their menopausal status. For patients with new-onset migraine, three of nine (33%) were perimenopausal, three (33%) were postmenopausal, and one (11.11%) was premenopausal.
Among patients with new-onset migraine, brain MRI was normal in 44.44%, showed pituitary abnormality in 22.22%, and showed other brain lesion in 33.33%. In patients with migraine history, brain MRI was normal in 45%, showed pituitary abnormality in 8.3%, showed nonspecific T2 high white matter lesion in 16.67%, and showed other brain lesion in 11.67%.
“Identifying migraine worsening or new-onset migraine during the menopausal transition age may help the diagnosis and treatment optimization of migraine for women during the menopausal age,” said Dr. Cheng.
Study supports meningococcal B vaccine in children with rare diseases
A new study, the first of its kind, provided support for guidelines suggesting that the capsular meningococcal B vaccine be given to children with three rare conditions that boost infection risk.
For children with terminal chain complement deficiencies or who are undergoing treatment with eculizumab, “it is important that these patients are identified, receive education about sepsis management plans, and are prescribed prophylactic antibiotics according to local guidelines, along with vaccination, to provide every chance for them to be protected against this deadly disease,” the researchers wrote in Pediatrics.
While some countries suggest that the vaccine be given to all healthy infants, U.S. guidelines advise that the vaccine be given to preteenagers, teenagers, and adults who are considered at special risk. These include those with terminal chain complement deficiencies, who are believed to be up to 10,000 times more likely than healthy children to develop invasive meningococcal disease, and those who take eculizumab (Soliris). The risk groups recommended for vaccinations also include those with asplenia and splenic dysfunction, although their excess risk, if any, is unknown.
The new study of the capsular group meningococcal B vaccine, led by Federico Martinón-Torres, PhD, of the Hospital Clinico Universitario de Santiago de Compostela, Spain, adds to previous research that confirmed the effectiveness of vaccinating complement-deficient patients with capsular group A, C, W, and Y meningococcal vaccines.
For the open-label, phase 3b study, researchers in Italy, Spain, Poland, and Russia gave two doses of the vaccine 2 months apart to 239 children aged 2-17 years with an average age of 10 years. Nearly all were white, and 45% were female.
A total of 40 children had complement deficiency, 112 had asplenia or splenic dysfunction, and 87 children in the control group also received the vaccine.
Following vaccination, the percentages of children with exogenous complement serum bactericidal activity titers greater than or equal to 1:5 to the four test strains were similar in the healthy children and those with asplenia/splenic dysfunction. “It is reasonable to expect that this vaccine will be as effective in children with asplenia or splenic deficiency as in children in the control category,” the researchers wrote.
However, these levels were lower in the complement-deficient children, particularly in those with terminal chain complement deficiency and those who took eculizumab.
The proportions of children with exogenous complement serum bactericidal activity titers greater than or equal to 1:5 against the four test strains were 87% (H44/76), 95% (5/99), 68% (NZ98/254), and 73% (M10713) in complement-deficient children, compared with 98%, 99%, 83%, and 99%, respectively, in the healthy controls.
“Ongoing surveillance for vaccine failures is required to determine the significance of the trend to reduced immune response in children with terminal chain complement deficiencies or undergoing treatment with eculizumab,” the researchers wrote.
Eculizumab’s manufacturer has noted the risk of serious meningococcal infections and warned physicians to “immunize patients with meningococcal vaccines at least 2 weeks prior to administering the first dose of Soliris, unless the risks of delaying Soliris therapy outweigh the risk of developing a meningococcal infection,” according to the website.
The study was funded by Novartis Vaccines and Diagnostics (now GlaxoSmithKline Biologicals). Some of the study authors reported various disclosures, including financial relationships with Novartis and GlaxoSmithKline outside the submitted work. Dr. Kaplan reported no relevant financial disclosures.
SOURCE: Martinón-Torres F et al. Pediatrics. 2018 Aug 1. doi: 10.1542/peds.2017-4250.
The Centers for Disease Control and Prevention reported an annual average of 792 cases of meningococcal disease and 98 deaths in the United States from 2006 to 2015 with serotype B isolates causing the highest numbers of cases. In a recent development, two vaccines against this strain have become available in the United States in the past 3 years for people aged 10-25 years. But officials don’t recommend their routine use, instead, guidelines suggest they be given to those at high risk only.
There’s a gap in knowledge because vaccine researchers didn’t include people with complement deficiency (either congenital or related to eculizumab), asplenia, or splenic dysfunction in studies that led to approval. Now, the new study offers reassuring findings regarding the latter two conditions, as bactericidal antibody responses were equal to those in healthy controls.
The findings regarding complement deficiency aren’t surprising, and suggest that vaccine strength in children with the condition only reached the levels in healthy children when an exogenous complement was added.
The study supports guidelines suggesting antibiotic prophylaxis in patients receiving eculizumab even if they already underwent meningococcal vaccination. It’s not clear if this approach also will be effective in those with congenital complement deficiencies (except for complement component 6 deficiency).
It is hoped that surveillance studies will show that use of serogroup B vaccines will prevent invasive meningococcal infections in these high-risk populations for which they are recommended.
Sheldon L. Kaplan, MD, is a pediatrician at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. These comments are summarized from an editorial accompanying the article by Martinón-Torres et al. (Pediatrics. 2018 Aug 1. doi: 10.1542/peds.2018-0554).
The Centers for Disease Control and Prevention reported an annual average of 792 cases of meningococcal disease and 98 deaths in the United States from 2006 to 2015 with serotype B isolates causing the highest numbers of cases. In a recent development, two vaccines against this strain have become available in the United States in the past 3 years for people aged 10-25 years. But officials don’t recommend their routine use, instead, guidelines suggest they be given to those at high risk only.
There’s a gap in knowledge because vaccine researchers didn’t include people with complement deficiency (either congenital or related to eculizumab), asplenia, or splenic dysfunction in studies that led to approval. Now, the new study offers reassuring findings regarding the latter two conditions, as bactericidal antibody responses were equal to those in healthy controls.
The findings regarding complement deficiency aren’t surprising, and suggest that vaccine strength in children with the condition only reached the levels in healthy children when an exogenous complement was added.
The study supports guidelines suggesting antibiotic prophylaxis in patients receiving eculizumab even if they already underwent meningococcal vaccination. It’s not clear if this approach also will be effective in those with congenital complement deficiencies (except for complement component 6 deficiency).
It is hoped that surveillance studies will show that use of serogroup B vaccines will prevent invasive meningococcal infections in these high-risk populations for which they are recommended.
Sheldon L. Kaplan, MD, is a pediatrician at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. These comments are summarized from an editorial accompanying the article by Martinón-Torres et al. (Pediatrics. 2018 Aug 1. doi: 10.1542/peds.2018-0554).
The Centers for Disease Control and Prevention reported an annual average of 792 cases of meningococcal disease and 98 deaths in the United States from 2006 to 2015 with serotype B isolates causing the highest numbers of cases. In a recent development, two vaccines against this strain have become available in the United States in the past 3 years for people aged 10-25 years. But officials don’t recommend their routine use, instead, guidelines suggest they be given to those at high risk only.
There’s a gap in knowledge because vaccine researchers didn’t include people with complement deficiency (either congenital or related to eculizumab), asplenia, or splenic dysfunction in studies that led to approval. Now, the new study offers reassuring findings regarding the latter two conditions, as bactericidal antibody responses were equal to those in healthy controls.
The findings regarding complement deficiency aren’t surprising, and suggest that vaccine strength in children with the condition only reached the levels in healthy children when an exogenous complement was added.
The study supports guidelines suggesting antibiotic prophylaxis in patients receiving eculizumab even if they already underwent meningococcal vaccination. It’s not clear if this approach also will be effective in those with congenital complement deficiencies (except for complement component 6 deficiency).
It is hoped that surveillance studies will show that use of serogroup B vaccines will prevent invasive meningococcal infections in these high-risk populations for which they are recommended.
Sheldon L. Kaplan, MD, is a pediatrician at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. These comments are summarized from an editorial accompanying the article by Martinón-Torres et al. (Pediatrics. 2018 Aug 1. doi: 10.1542/peds.2018-0554).
A new study, the first of its kind, provided support for guidelines suggesting that the capsular meningococcal B vaccine be given to children with three rare conditions that boost infection risk.
For children with terminal chain complement deficiencies or who are undergoing treatment with eculizumab, “it is important that these patients are identified, receive education about sepsis management plans, and are prescribed prophylactic antibiotics according to local guidelines, along with vaccination, to provide every chance for them to be protected against this deadly disease,” the researchers wrote in Pediatrics.
While some countries suggest that the vaccine be given to all healthy infants, U.S. guidelines advise that the vaccine be given to preteenagers, teenagers, and adults who are considered at special risk. These include those with terminal chain complement deficiencies, who are believed to be up to 10,000 times more likely than healthy children to develop invasive meningococcal disease, and those who take eculizumab (Soliris). The risk groups recommended for vaccinations also include those with asplenia and splenic dysfunction, although their excess risk, if any, is unknown.
The new study of the capsular group meningococcal B vaccine, led by Federico Martinón-Torres, PhD, of the Hospital Clinico Universitario de Santiago de Compostela, Spain, adds to previous research that confirmed the effectiveness of vaccinating complement-deficient patients with capsular group A, C, W, and Y meningococcal vaccines.
For the open-label, phase 3b study, researchers in Italy, Spain, Poland, and Russia gave two doses of the vaccine 2 months apart to 239 children aged 2-17 years with an average age of 10 years. Nearly all were white, and 45% were female.
A total of 40 children had complement deficiency, 112 had asplenia or splenic dysfunction, and 87 children in the control group also received the vaccine.
Following vaccination, the percentages of children with exogenous complement serum bactericidal activity titers greater than or equal to 1:5 to the four test strains were similar in the healthy children and those with asplenia/splenic dysfunction. “It is reasonable to expect that this vaccine will be as effective in children with asplenia or splenic deficiency as in children in the control category,” the researchers wrote.
However, these levels were lower in the complement-deficient children, particularly in those with terminal chain complement deficiency and those who took eculizumab.
The proportions of children with exogenous complement serum bactericidal activity titers greater than or equal to 1:5 against the four test strains were 87% (H44/76), 95% (5/99), 68% (NZ98/254), and 73% (M10713) in complement-deficient children, compared with 98%, 99%, 83%, and 99%, respectively, in the healthy controls.
“Ongoing surveillance for vaccine failures is required to determine the significance of the trend to reduced immune response in children with terminal chain complement deficiencies or undergoing treatment with eculizumab,” the researchers wrote.
Eculizumab’s manufacturer has noted the risk of serious meningococcal infections and warned physicians to “immunize patients with meningococcal vaccines at least 2 weeks prior to administering the first dose of Soliris, unless the risks of delaying Soliris therapy outweigh the risk of developing a meningococcal infection,” according to the website.
The study was funded by Novartis Vaccines and Diagnostics (now GlaxoSmithKline Biologicals). Some of the study authors reported various disclosures, including financial relationships with Novartis and GlaxoSmithKline outside the submitted work. Dr. Kaplan reported no relevant financial disclosures.
SOURCE: Martinón-Torres F et al. Pediatrics. 2018 Aug 1. doi: 10.1542/peds.2017-4250.
A new study, the first of its kind, provided support for guidelines suggesting that the capsular meningococcal B vaccine be given to children with three rare conditions that boost infection risk.
For children with terminal chain complement deficiencies or who are undergoing treatment with eculizumab, “it is important that these patients are identified, receive education about sepsis management plans, and are prescribed prophylactic antibiotics according to local guidelines, along with vaccination, to provide every chance for them to be protected against this deadly disease,” the researchers wrote in Pediatrics.
While some countries suggest that the vaccine be given to all healthy infants, U.S. guidelines advise that the vaccine be given to preteenagers, teenagers, and adults who are considered at special risk. These include those with terminal chain complement deficiencies, who are believed to be up to 10,000 times more likely than healthy children to develop invasive meningococcal disease, and those who take eculizumab (Soliris). The risk groups recommended for vaccinations also include those with asplenia and splenic dysfunction, although their excess risk, if any, is unknown.
The new study of the capsular group meningococcal B vaccine, led by Federico Martinón-Torres, PhD, of the Hospital Clinico Universitario de Santiago de Compostela, Spain, adds to previous research that confirmed the effectiveness of vaccinating complement-deficient patients with capsular group A, C, W, and Y meningococcal vaccines.
For the open-label, phase 3b study, researchers in Italy, Spain, Poland, and Russia gave two doses of the vaccine 2 months apart to 239 children aged 2-17 years with an average age of 10 years. Nearly all were white, and 45% were female.
A total of 40 children had complement deficiency, 112 had asplenia or splenic dysfunction, and 87 children in the control group also received the vaccine.
Following vaccination, the percentages of children with exogenous complement serum bactericidal activity titers greater than or equal to 1:5 to the four test strains were similar in the healthy children and those with asplenia/splenic dysfunction. “It is reasonable to expect that this vaccine will be as effective in children with asplenia or splenic deficiency as in children in the control category,” the researchers wrote.
However, these levels were lower in the complement-deficient children, particularly in those with terminal chain complement deficiency and those who took eculizumab.
The proportions of children with exogenous complement serum bactericidal activity titers greater than or equal to 1:5 against the four test strains were 87% (H44/76), 95% (5/99), 68% (NZ98/254), and 73% (M10713) in complement-deficient children, compared with 98%, 99%, 83%, and 99%, respectively, in the healthy controls.
“Ongoing surveillance for vaccine failures is required to determine the significance of the trend to reduced immune response in children with terminal chain complement deficiencies or undergoing treatment with eculizumab,” the researchers wrote.
Eculizumab’s manufacturer has noted the risk of serious meningococcal infections and warned physicians to “immunize patients with meningococcal vaccines at least 2 weeks prior to administering the first dose of Soliris, unless the risks of delaying Soliris therapy outweigh the risk of developing a meningococcal infection,” according to the website.
The study was funded by Novartis Vaccines and Diagnostics (now GlaxoSmithKline Biologicals). Some of the study authors reported various disclosures, including financial relationships with Novartis and GlaxoSmithKline outside the submitted work. Dr. Kaplan reported no relevant financial disclosures.
SOURCE: Martinón-Torres F et al. Pediatrics. 2018 Aug 1. doi: 10.1542/peds.2017-4250.
FROM PEDIATRICS
Key clinical point: The meningococcal B vaccine retained its strength in kids with two rare immunosuppressive diseases, but may be weaker in a third group.
Major finding: The vaccine’s effectiveness was roughly the same in healthy controls and in those with asplenia and splenic dysfunction, but it dipped in those with complement deficiency.
Study details: An open-label, multicenter analysis of children aged 2-17 years who received two doses over 2 months.
Disclosures: The study was funded by Novartis Vaccines and Diagnostics (now GlaxoSmithKline Biologicals). Some of the study authors reported various disclosures, including financial relationships with Novartis and GlaxoSmithKline outside the submitted work. Dr. Kaplan reported no relevant financial disclosures.
Source: Martinón-Torres F et al. Pediatrics. 2018 Aug 1. doi: 10.1542/peds.2017-4250.
OnabotulinumtoxinA Versus Topiramate for Prevention of Chronic Migraine: The FORWARD Study
A randomized trial examines discontinuations, efficacy, cognition, and depressive symptoms over 36 weeks of treatment.
SAN FRANCISCO—For the prevention of chronic migraine, onabotulinumtoxinA has a superior tolerability profile versus topiramate based on treatment-related adverse events and overall discontinuations, according to data presented at the 60th Annual Scientific Meeting of the American Headache Society. In addition, “patient-reported outcomes data suggest that changes in cognition, an important adverse event leading to treatment discontinuation with topiramate, may be seen as early as week 12,” said Andrew M. Blumenfeld, MD, Director of the Headache Center of Southern California in Oceanside, and colleagues. Dr. Blumenfeld also reported that onabotulinumtoxinA has a more favorable effect on depressive symptoms than does topiramate.
According to Dr. Blumenfeld and colleagues, many adults with chronic migraine are not receiving appropriate preventive treatment and when prescribed, adherence to treatment is relatively low. To address this problem, he and his colleagues conducted a multicenter, prospective, randomized, parallel-group, open-label study to compare onabotulinumtoxinA and topiramate for headache prevention in adults with chronic migraine (the FORWARD study).
The study assessed the effectiveness of onabotulinumtoxinA 155 U administered to 31 sites across seven head and neck muscles, fixed-site, fixed-dose, every 12 weeks for three cycles versus topiramate 50 to 100 mg/day up to week 36. The primary efficacy measure was the proportion of patients with a 50% or greater reduction in headache days versus baseline in the 28 days before week 32. Safety and tolerability were assessed; adverse events were monitored. Patient-reported outcomes collected from questionnaires at day 1 and weeks 12, 24, and 36 included the Controlled Oral Word Association Test (COWAT) and the nine-item Patient Health Questionnaire (PHQ-9). Baseline observation carried forward (BLOCF) was used to impute missing values at primary time points, followed by questionnaire guidelines for missing questionnaire data.
A total of 282 patients were enrolled—140 in the onabotulinumtoxinA arm and 142 in the topiramate arm. Mean baseline headache days (onabotulinumtoxinA, 22.1; topiramate, 21.8) were similar. Of the patients enrolled, 148 completed randomized treatment (onabotulinumtoxinA, 85.7%; topiramate, 19.7%). Primary reasons for withdrawal were ineffective treatment (onabotulinumtoxinA, 5.0%; topiramate, 19.0%) and adverse events (onabotulinumtoxinA, 3.6%; topiramate, 50.7%). Based on BLOCF, more patients on onabotulinumtoxinA had a 50% or greater reduction in headache frequency compared with baseline versus topiramate (40.0% vs 12.0%). Adverse events were reported by 45.5% of patients who received onabotulinumtoxinA and 76.8% of patients who received topiramate; treatment-related adverse events were reported by 17.3% and 69.0%, respectively. No new safety signals were identified for onabotulinumtoxinA. Adverse events relating to nervous system disorders most commonly led to treatment discontinuation for topiramate. Topiramate reduced mean COWAT scores from as early as week 12, suggesting cognitive changes occurred early in treatment with topiramate. As the study progressed, topiramate’s effect may have been obscured by the BLOCF imputation methodology due to the large proportion of patients withdrawing from topiramate, the investigators said. In contrast, onabotulinumtoxinA resulted in a small increase in COWAT scores from week 12 to week 36. OnabotulinumtoxinA had a significantly greater effect on mean PHQ-9 scores at week 36 (4.4), compared with topiramate (7.1; estimated mean difference, –1.86).
A randomized trial examines discontinuations, efficacy, cognition, and depressive symptoms over 36 weeks of treatment.
A randomized trial examines discontinuations, efficacy, cognition, and depressive symptoms over 36 weeks of treatment.
SAN FRANCISCO—For the prevention of chronic migraine, onabotulinumtoxinA has a superior tolerability profile versus topiramate based on treatment-related adverse events and overall discontinuations, according to data presented at the 60th Annual Scientific Meeting of the American Headache Society. In addition, “patient-reported outcomes data suggest that changes in cognition, an important adverse event leading to treatment discontinuation with topiramate, may be seen as early as week 12,” said Andrew M. Blumenfeld, MD, Director of the Headache Center of Southern California in Oceanside, and colleagues. Dr. Blumenfeld also reported that onabotulinumtoxinA has a more favorable effect on depressive symptoms than does topiramate.
According to Dr. Blumenfeld and colleagues, many adults with chronic migraine are not receiving appropriate preventive treatment and when prescribed, adherence to treatment is relatively low. To address this problem, he and his colleagues conducted a multicenter, prospective, randomized, parallel-group, open-label study to compare onabotulinumtoxinA and topiramate for headache prevention in adults with chronic migraine (the FORWARD study).
The study assessed the effectiveness of onabotulinumtoxinA 155 U administered to 31 sites across seven head and neck muscles, fixed-site, fixed-dose, every 12 weeks for three cycles versus topiramate 50 to 100 mg/day up to week 36. The primary efficacy measure was the proportion of patients with a 50% or greater reduction in headache days versus baseline in the 28 days before week 32. Safety and tolerability were assessed; adverse events were monitored. Patient-reported outcomes collected from questionnaires at day 1 and weeks 12, 24, and 36 included the Controlled Oral Word Association Test (COWAT) and the nine-item Patient Health Questionnaire (PHQ-9). Baseline observation carried forward (BLOCF) was used to impute missing values at primary time points, followed by questionnaire guidelines for missing questionnaire data.
A total of 282 patients were enrolled—140 in the onabotulinumtoxinA arm and 142 in the topiramate arm. Mean baseline headache days (onabotulinumtoxinA, 22.1; topiramate, 21.8) were similar. Of the patients enrolled, 148 completed randomized treatment (onabotulinumtoxinA, 85.7%; topiramate, 19.7%). Primary reasons for withdrawal were ineffective treatment (onabotulinumtoxinA, 5.0%; topiramate, 19.0%) and adverse events (onabotulinumtoxinA, 3.6%; topiramate, 50.7%). Based on BLOCF, more patients on onabotulinumtoxinA had a 50% or greater reduction in headache frequency compared with baseline versus topiramate (40.0% vs 12.0%). Adverse events were reported by 45.5% of patients who received onabotulinumtoxinA and 76.8% of patients who received topiramate; treatment-related adverse events were reported by 17.3% and 69.0%, respectively. No new safety signals were identified for onabotulinumtoxinA. Adverse events relating to nervous system disorders most commonly led to treatment discontinuation for topiramate. Topiramate reduced mean COWAT scores from as early as week 12, suggesting cognitive changes occurred early in treatment with topiramate. As the study progressed, topiramate’s effect may have been obscured by the BLOCF imputation methodology due to the large proportion of patients withdrawing from topiramate, the investigators said. In contrast, onabotulinumtoxinA resulted in a small increase in COWAT scores from week 12 to week 36. OnabotulinumtoxinA had a significantly greater effect on mean PHQ-9 scores at week 36 (4.4), compared with topiramate (7.1; estimated mean difference, –1.86).
SAN FRANCISCO—For the prevention of chronic migraine, onabotulinumtoxinA has a superior tolerability profile versus topiramate based on treatment-related adverse events and overall discontinuations, according to data presented at the 60th Annual Scientific Meeting of the American Headache Society. In addition, “patient-reported outcomes data suggest that changes in cognition, an important adverse event leading to treatment discontinuation with topiramate, may be seen as early as week 12,” said Andrew M. Blumenfeld, MD, Director of the Headache Center of Southern California in Oceanside, and colleagues. Dr. Blumenfeld also reported that onabotulinumtoxinA has a more favorable effect on depressive symptoms than does topiramate.
According to Dr. Blumenfeld and colleagues, many adults with chronic migraine are not receiving appropriate preventive treatment and when prescribed, adherence to treatment is relatively low. To address this problem, he and his colleagues conducted a multicenter, prospective, randomized, parallel-group, open-label study to compare onabotulinumtoxinA and topiramate for headache prevention in adults with chronic migraine (the FORWARD study).
The study assessed the effectiveness of onabotulinumtoxinA 155 U administered to 31 sites across seven head and neck muscles, fixed-site, fixed-dose, every 12 weeks for three cycles versus topiramate 50 to 100 mg/day up to week 36. The primary efficacy measure was the proportion of patients with a 50% or greater reduction in headache days versus baseline in the 28 days before week 32. Safety and tolerability were assessed; adverse events were monitored. Patient-reported outcomes collected from questionnaires at day 1 and weeks 12, 24, and 36 included the Controlled Oral Word Association Test (COWAT) and the nine-item Patient Health Questionnaire (PHQ-9). Baseline observation carried forward (BLOCF) was used to impute missing values at primary time points, followed by questionnaire guidelines for missing questionnaire data.
A total of 282 patients were enrolled—140 in the onabotulinumtoxinA arm and 142 in the topiramate arm. Mean baseline headache days (onabotulinumtoxinA, 22.1; topiramate, 21.8) were similar. Of the patients enrolled, 148 completed randomized treatment (onabotulinumtoxinA, 85.7%; topiramate, 19.7%). Primary reasons for withdrawal were ineffective treatment (onabotulinumtoxinA, 5.0%; topiramate, 19.0%) and adverse events (onabotulinumtoxinA, 3.6%; topiramate, 50.7%). Based on BLOCF, more patients on onabotulinumtoxinA had a 50% or greater reduction in headache frequency compared with baseline versus topiramate (40.0% vs 12.0%). Adverse events were reported by 45.5% of patients who received onabotulinumtoxinA and 76.8% of patients who received topiramate; treatment-related adverse events were reported by 17.3% and 69.0%, respectively. No new safety signals were identified for onabotulinumtoxinA. Adverse events relating to nervous system disorders most commonly led to treatment discontinuation for topiramate. Topiramate reduced mean COWAT scores from as early as week 12, suggesting cognitive changes occurred early in treatment with topiramate. As the study progressed, topiramate’s effect may have been obscured by the BLOCF imputation methodology due to the large proportion of patients withdrawing from topiramate, the investigators said. In contrast, onabotulinumtoxinA resulted in a small increase in COWAT scores from week 12 to week 36. OnabotulinumtoxinA had a significantly greater effect on mean PHQ-9 scores at week 36 (4.4), compared with topiramate (7.1; estimated mean difference, –1.86).
Droxidopa May Reduce Neurogenic Orthostatic Hypotension Symptoms in Patients Taking DDCIs
The number of patients experiencing falls significantly decreased after six months of droxidopa treatment, regardless of whether patients were on dopa decarboxylase inhibitors.
MIAMI—Droxidopa is associated with reductions in fall risk and dizziness or lightheadedness among users and nonusers of dopamine decarboxylase inhibitors (DDCIs), according to research described at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. These findings from an open-label, observational study “support previous data showing the efficacy of droxidopa for neurogenic orthostatic hypotension symptom reduction, even with concomitant DDCI use,” said the researchers.
Neurogenic orthostatic hypotension—a sustained blood pressure drop upon standing due to deficient norepinephrine release—is common among patients with disorders associated with autonomic nervous system dysfunction (eg, Parkinson’s disease, multiple system atrophy, and pure autonomic failure). Symptoms include lightheadedness or dizziness, presyncope, syncope, and falls.
Droxidopa, a prodrug of norepinephrine, is approved to treat symptomatic neurogenic orthostatic hypotension. Droxidopa is converted to norepinephrine by dopamine decarboxylase, which also converts levodopa to dopamine. Patients with Parkinson’s disease are commonly treated with DDCIs in conjunction with levodopa treatment. DDCIs did not appear to interfere with the therapeutic efficacy of droxidopa in clinical studies, but “high doses of DDCIs (8- to 10-fold higher than clinical doses) have been shown to blunt the effects of droxidopa,” said Steven Kymes, PhD, Director of Health Economics and Outcomes Research at Lundbeck in Deerfield, Illinois, and colleagues.
A Post Hoc Analysis
To assess the long-term efficacy of droxidopa for the treatment of neurogenic orthostatic hypotension in patients concomitantly receiving DDCIs, Dr. Kymes and colleagues conducted a post hoc analysis of outcomes related to falls and neurogenic orthostatic hypotension symptoms in patients using DDCIs versus patients not using them. The researchers used data from a six-month open-label, prospective, observational study of patients newly initiating droxidopa.
Eligible participants were 18 and older; had underlying Parkinson’s disease, multiple system atrophy, pure autonomic failure, dopamine beta-hydroxylase deficiency, or nondiabetic autonomic neuropathy; were newly initiating droxidopa; and were able to speak and understand English. The researchers excluded patients with a self-reported diagnosis of dementia, Alzheimer disease, schizophrenia, or other psychiatric disorder, as well as those who were nonambulatory or confined to a wheelchair.
Researchers used a patient falls questionnaire to record the number of falls in the past month at baseline and at one, three, and six months. They also used the Orthostatic Hypotension Symptom Assessment (OHSA) Item I test to assess dizziness or lightheadedness. All outcomes were self-reported.
Investigators then compared baseline differences using chi-square tests for categorical variables and t-tests for continuous variables. “The influence of DDCIs on risk of falling and OHSA Item I scores was compared across time points using generalized linear mixed models (logistic for risk of falling) adjusting for repeated measures within individuals,” said the researchers.
Droxidopa Treatment Was Associated With Reduced Falls
A total of 168 patients were included in this study; 55 were DDCI users, and 113 were non-DDCI users. The mean age in the DDCI group was 75, and the mean age in the non-DDCI group was 57. There were 19 women (34.5%) in the DDCI user group and 68 (60.2%) in non-DDCI user group. Most participants were white in both groups (92.7% in the DDCI group and 81.4% in the non-DDCI group).
“There were significant differences in the primary diagnoses between the groups. Parkinson’s disease was the most frequent diagnosis in the DDCI group (89.1%), and autonomic failure with no cause identified was the most frequent diagnosis in the non-DDCI group (92.9%),” Dr. Kymes and colleagues said. “At baseline, 61.8% of patients receiving DDCIs and 46.9 % of patients not receiving DDCI reported at least one fall in the last month.” The mean OHSA Item I scores at baseline were 5 in the DDCI group and 6 in the non-DDCI group.
The proportion of patients receiving DDCIs who experienced one or more falls in the past month after six months of droxidopa treatment significantly decreased from baseline, with a 36.5% reduction over the course of the study.
Among patients not receiving a DDCI, there was a 6.2% reduction in falls over the course of the study, but the reduction was not significant. Changes in the proportion of patients reporting one or more falls in the past month from baseline to six months did not differ significantly between the groups.
In addition, patients receiving DDCIs and nonusers showed significant improvement in OHSA Item I scores from baseline after six months of droxidopa treatment (change of 1.5 and 1.9 units, respectively). The difference between groups was not statistically significant.
“Specifically designed studies are needed to further examine the impact of DDCIs on droxidopa because the current study sample was not powered for subgroup analyses and all data were self-reported by patients,” the researchers concluded.
—Erica Tricarico
The number of patients experiencing falls significantly decreased after six months of droxidopa treatment, regardless of whether patients were on dopa decarboxylase inhibitors.
The number of patients experiencing falls significantly decreased after six months of droxidopa treatment, regardless of whether patients were on dopa decarboxylase inhibitors.
MIAMI—Droxidopa is associated with reductions in fall risk and dizziness or lightheadedness among users and nonusers of dopamine decarboxylase inhibitors (DDCIs), according to research described at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. These findings from an open-label, observational study “support previous data showing the efficacy of droxidopa for neurogenic orthostatic hypotension symptom reduction, even with concomitant DDCI use,” said the researchers.
Neurogenic orthostatic hypotension—a sustained blood pressure drop upon standing due to deficient norepinephrine release—is common among patients with disorders associated with autonomic nervous system dysfunction (eg, Parkinson’s disease, multiple system atrophy, and pure autonomic failure). Symptoms include lightheadedness or dizziness, presyncope, syncope, and falls.
Droxidopa, a prodrug of norepinephrine, is approved to treat symptomatic neurogenic orthostatic hypotension. Droxidopa is converted to norepinephrine by dopamine decarboxylase, which also converts levodopa to dopamine. Patients with Parkinson’s disease are commonly treated with DDCIs in conjunction with levodopa treatment. DDCIs did not appear to interfere with the therapeutic efficacy of droxidopa in clinical studies, but “high doses of DDCIs (8- to 10-fold higher than clinical doses) have been shown to blunt the effects of droxidopa,” said Steven Kymes, PhD, Director of Health Economics and Outcomes Research at Lundbeck in Deerfield, Illinois, and colleagues.
A Post Hoc Analysis
To assess the long-term efficacy of droxidopa for the treatment of neurogenic orthostatic hypotension in patients concomitantly receiving DDCIs, Dr. Kymes and colleagues conducted a post hoc analysis of outcomes related to falls and neurogenic orthostatic hypotension symptoms in patients using DDCIs versus patients not using them. The researchers used data from a six-month open-label, prospective, observational study of patients newly initiating droxidopa.
Eligible participants were 18 and older; had underlying Parkinson’s disease, multiple system atrophy, pure autonomic failure, dopamine beta-hydroxylase deficiency, or nondiabetic autonomic neuropathy; were newly initiating droxidopa; and were able to speak and understand English. The researchers excluded patients with a self-reported diagnosis of dementia, Alzheimer disease, schizophrenia, or other psychiatric disorder, as well as those who were nonambulatory or confined to a wheelchair.
Researchers used a patient falls questionnaire to record the number of falls in the past month at baseline and at one, three, and six months. They also used the Orthostatic Hypotension Symptom Assessment (OHSA) Item I test to assess dizziness or lightheadedness. All outcomes were self-reported.
Investigators then compared baseline differences using chi-square tests for categorical variables and t-tests for continuous variables. “The influence of DDCIs on risk of falling and OHSA Item I scores was compared across time points using generalized linear mixed models (logistic for risk of falling) adjusting for repeated measures within individuals,” said the researchers.
Droxidopa Treatment Was Associated With Reduced Falls
A total of 168 patients were included in this study; 55 were DDCI users, and 113 were non-DDCI users. The mean age in the DDCI group was 75, and the mean age in the non-DDCI group was 57. There were 19 women (34.5%) in the DDCI user group and 68 (60.2%) in non-DDCI user group. Most participants were white in both groups (92.7% in the DDCI group and 81.4% in the non-DDCI group).
“There were significant differences in the primary diagnoses between the groups. Parkinson’s disease was the most frequent diagnosis in the DDCI group (89.1%), and autonomic failure with no cause identified was the most frequent diagnosis in the non-DDCI group (92.9%),” Dr. Kymes and colleagues said. “At baseline, 61.8% of patients receiving DDCIs and 46.9 % of patients not receiving DDCI reported at least one fall in the last month.” The mean OHSA Item I scores at baseline were 5 in the DDCI group and 6 in the non-DDCI group.
The proportion of patients receiving DDCIs who experienced one or more falls in the past month after six months of droxidopa treatment significantly decreased from baseline, with a 36.5% reduction over the course of the study.
Among patients not receiving a DDCI, there was a 6.2% reduction in falls over the course of the study, but the reduction was not significant. Changes in the proportion of patients reporting one or more falls in the past month from baseline to six months did not differ significantly between the groups.
In addition, patients receiving DDCIs and nonusers showed significant improvement in OHSA Item I scores from baseline after six months of droxidopa treatment (change of 1.5 and 1.9 units, respectively). The difference between groups was not statistically significant.
“Specifically designed studies are needed to further examine the impact of DDCIs on droxidopa because the current study sample was not powered for subgroup analyses and all data were self-reported by patients,” the researchers concluded.
—Erica Tricarico
MIAMI—Droxidopa is associated with reductions in fall risk and dizziness or lightheadedness among users and nonusers of dopamine decarboxylase inhibitors (DDCIs), according to research described at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. These findings from an open-label, observational study “support previous data showing the efficacy of droxidopa for neurogenic orthostatic hypotension symptom reduction, even with concomitant DDCI use,” said the researchers.
Neurogenic orthostatic hypotension—a sustained blood pressure drop upon standing due to deficient norepinephrine release—is common among patients with disorders associated with autonomic nervous system dysfunction (eg, Parkinson’s disease, multiple system atrophy, and pure autonomic failure). Symptoms include lightheadedness or dizziness, presyncope, syncope, and falls.
Droxidopa, a prodrug of norepinephrine, is approved to treat symptomatic neurogenic orthostatic hypotension. Droxidopa is converted to norepinephrine by dopamine decarboxylase, which also converts levodopa to dopamine. Patients with Parkinson’s disease are commonly treated with DDCIs in conjunction with levodopa treatment. DDCIs did not appear to interfere with the therapeutic efficacy of droxidopa in clinical studies, but “high doses of DDCIs (8- to 10-fold higher than clinical doses) have been shown to blunt the effects of droxidopa,” said Steven Kymes, PhD, Director of Health Economics and Outcomes Research at Lundbeck in Deerfield, Illinois, and colleagues.
A Post Hoc Analysis
To assess the long-term efficacy of droxidopa for the treatment of neurogenic orthostatic hypotension in patients concomitantly receiving DDCIs, Dr. Kymes and colleagues conducted a post hoc analysis of outcomes related to falls and neurogenic orthostatic hypotension symptoms in patients using DDCIs versus patients not using them. The researchers used data from a six-month open-label, prospective, observational study of patients newly initiating droxidopa.
Eligible participants were 18 and older; had underlying Parkinson’s disease, multiple system atrophy, pure autonomic failure, dopamine beta-hydroxylase deficiency, or nondiabetic autonomic neuropathy; were newly initiating droxidopa; and were able to speak and understand English. The researchers excluded patients with a self-reported diagnosis of dementia, Alzheimer disease, schizophrenia, or other psychiatric disorder, as well as those who were nonambulatory or confined to a wheelchair.
Researchers used a patient falls questionnaire to record the number of falls in the past month at baseline and at one, three, and six months. They also used the Orthostatic Hypotension Symptom Assessment (OHSA) Item I test to assess dizziness or lightheadedness. All outcomes were self-reported.
Investigators then compared baseline differences using chi-square tests for categorical variables and t-tests for continuous variables. “The influence of DDCIs on risk of falling and OHSA Item I scores was compared across time points using generalized linear mixed models (logistic for risk of falling) adjusting for repeated measures within individuals,” said the researchers.
Droxidopa Treatment Was Associated With Reduced Falls
A total of 168 patients were included in this study; 55 were DDCI users, and 113 were non-DDCI users. The mean age in the DDCI group was 75, and the mean age in the non-DDCI group was 57. There were 19 women (34.5%) in the DDCI user group and 68 (60.2%) in non-DDCI user group. Most participants were white in both groups (92.7% in the DDCI group and 81.4% in the non-DDCI group).
“There were significant differences in the primary diagnoses between the groups. Parkinson’s disease was the most frequent diagnosis in the DDCI group (89.1%), and autonomic failure with no cause identified was the most frequent diagnosis in the non-DDCI group (92.9%),” Dr. Kymes and colleagues said. “At baseline, 61.8% of patients receiving DDCIs and 46.9 % of patients not receiving DDCI reported at least one fall in the last month.” The mean OHSA Item I scores at baseline were 5 in the DDCI group and 6 in the non-DDCI group.
The proportion of patients receiving DDCIs who experienced one or more falls in the past month after six months of droxidopa treatment significantly decreased from baseline, with a 36.5% reduction over the course of the study.
Among patients not receiving a DDCI, there was a 6.2% reduction in falls over the course of the study, but the reduction was not significant. Changes in the proportion of patients reporting one or more falls in the past month from baseline to six months did not differ significantly between the groups.
In addition, patients receiving DDCIs and nonusers showed significant improvement in OHSA Item I scores from baseline after six months of droxidopa treatment (change of 1.5 and 1.9 units, respectively). The difference between groups was not statistically significant.
“Specifically designed studies are needed to further examine the impact of DDCIs on droxidopa because the current study sample was not powered for subgroup analyses and all data were self-reported by patients,” the researchers concluded.
—Erica Tricarico
Ch4 Density Is a Potential Imaging Biomarker of Cognition in Early Parkinson’s Disease
Increasing Ch4 density is associated with higher scores on various cognitive measurements.
MIAMI—Reduced cholinergic nucleus 4 (Ch4) density in Parkinson’s disease, as measured with MRI, is associated with deficits in attention, processing speed, and visuospatial function, according to research described at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. Ch4 density may serve as a surrogate imaging biomarker of cognition in early Parkinson’s disease, said the researchers.
Degeneration of the nucleus basalis of Meynert (NBM) contributes to dementia in Parkinson’s disease through a loss of cholinergic innervation to the neocortex. Cholinergic neurons of the NBM are in Ch4, a structure that can be measured with MRI techniques using cytoarchitectonic maps.
Evaluating Ch4 Density and Cognitive Performance
To determine whether Ch4 density, a proxy measure for NBM volume, is associated with cognitive test performance in de novo Parkinson’s disease, Cody S. Freeman, MD, a fellow at the University of Virginia School of Medicine in Charlottesville, and colleagues analyzed baseline brain MRIs and neuropsychologic test scores for 228 patients with Parkinson’s disease and 101 healthy controls from the Parkinson’s Progression Markers Initiative (PPMI). They also analyzed brain MRIs and neuropsychologic test scores at four years for a subset of 92 participants with Parkinson’s disease in the PPMI.
Neuropsychologic testing included the Montreal Cognitive Assessment (MoCA), Hopkins Verbal Learning Test (HVLT), Judgment of Line Orientation (JLO), Letter Number Sequencing (LNS), Symbol Digit Modalities Test (SDMT), and semantic fluency (animals).
The researchers used MP-RAGE T1 sequences and a probabilistic atlas from the reference Montreal Neurological Institute single subject brain to apply voxel-based morphometry methods to determine Ch4 density. In addition, they used correlation coefficients and linear regression models to analyze relationships between Ch4 density and cognitive scores.
Ch4 Density Was Significantly Associated With Higher MoCA Scores
At baseline, 33.7% of healthy controls and 38.2% of patients with Parkinson’s disease were female. The mean age at neurologic testing was 59.5 among healthy controls and 61.0 in the Parkinson’s disease cohort. The median MoCA score was 28 for controls and patients with Parkinson’s disease at baseline. The mean Ch4 density was 87.9 in the control group and 86.4 in the Parkinson’s disease cohort.
At baseline, Ch4 density was significantly correlated with MoCA, JLO, LNS, and SDMT scores. In a linear regression model adjusted for age and sex, Ch4 density was significantly associated with higher MoCA scores in patients with Parkinson’s disease. In linear regression models adjusted for sex, increasing Ch4 density was associated with higher JLO, LNS, and SDMT scores. The researchers observed no associations between Ch4 density and JLO and semantic fluency in linear regression models adjusted for sex.
For the subset of participants with Parkinson’s disease with brain MRI and neuropsychologic testing available at four years, Ch4 density was significantly correlated with MoCA, JLO, LNS, and SDMT. In a linear regression model adjusted for age and sex, increasing Ch4 density was associated with higher MoCA scores in patients with Parkinson’s disease. In linear regression models adjusted for sex, increasing Ch4 density was associated with higher JLO, LNS, and SDMT scores.
Increasing Ch4 density is associated with higher scores on various cognitive measurements.
Increasing Ch4 density is associated with higher scores on various cognitive measurements.
MIAMI—Reduced cholinergic nucleus 4 (Ch4) density in Parkinson’s disease, as measured with MRI, is associated with deficits in attention, processing speed, and visuospatial function, according to research described at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. Ch4 density may serve as a surrogate imaging biomarker of cognition in early Parkinson’s disease, said the researchers.
Degeneration of the nucleus basalis of Meynert (NBM) contributes to dementia in Parkinson’s disease through a loss of cholinergic innervation to the neocortex. Cholinergic neurons of the NBM are in Ch4, a structure that can be measured with MRI techniques using cytoarchitectonic maps.
Evaluating Ch4 Density and Cognitive Performance
To determine whether Ch4 density, a proxy measure for NBM volume, is associated with cognitive test performance in de novo Parkinson’s disease, Cody S. Freeman, MD, a fellow at the University of Virginia School of Medicine in Charlottesville, and colleagues analyzed baseline brain MRIs and neuropsychologic test scores for 228 patients with Parkinson’s disease and 101 healthy controls from the Parkinson’s Progression Markers Initiative (PPMI). They also analyzed brain MRIs and neuropsychologic test scores at four years for a subset of 92 participants with Parkinson’s disease in the PPMI.
Neuropsychologic testing included the Montreal Cognitive Assessment (MoCA), Hopkins Verbal Learning Test (HVLT), Judgment of Line Orientation (JLO), Letter Number Sequencing (LNS), Symbol Digit Modalities Test (SDMT), and semantic fluency (animals).
The researchers used MP-RAGE T1 sequences and a probabilistic atlas from the reference Montreal Neurological Institute single subject brain to apply voxel-based morphometry methods to determine Ch4 density. In addition, they used correlation coefficients and linear regression models to analyze relationships between Ch4 density and cognitive scores.
Ch4 Density Was Significantly Associated With Higher MoCA Scores
At baseline, 33.7% of healthy controls and 38.2% of patients with Parkinson’s disease were female. The mean age at neurologic testing was 59.5 among healthy controls and 61.0 in the Parkinson’s disease cohort. The median MoCA score was 28 for controls and patients with Parkinson’s disease at baseline. The mean Ch4 density was 87.9 in the control group and 86.4 in the Parkinson’s disease cohort.
At baseline, Ch4 density was significantly correlated with MoCA, JLO, LNS, and SDMT scores. In a linear regression model adjusted for age and sex, Ch4 density was significantly associated with higher MoCA scores in patients with Parkinson’s disease. In linear regression models adjusted for sex, increasing Ch4 density was associated with higher JLO, LNS, and SDMT scores. The researchers observed no associations between Ch4 density and JLO and semantic fluency in linear regression models adjusted for sex.
For the subset of participants with Parkinson’s disease with brain MRI and neuropsychologic testing available at four years, Ch4 density was significantly correlated with MoCA, JLO, LNS, and SDMT. In a linear regression model adjusted for age and sex, increasing Ch4 density was associated with higher MoCA scores in patients with Parkinson’s disease. In linear regression models adjusted for sex, increasing Ch4 density was associated with higher JLO, LNS, and SDMT scores.
MIAMI—Reduced cholinergic nucleus 4 (Ch4) density in Parkinson’s disease, as measured with MRI, is associated with deficits in attention, processing speed, and visuospatial function, according to research described at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. Ch4 density may serve as a surrogate imaging biomarker of cognition in early Parkinson’s disease, said the researchers.
Degeneration of the nucleus basalis of Meynert (NBM) contributes to dementia in Parkinson’s disease through a loss of cholinergic innervation to the neocortex. Cholinergic neurons of the NBM are in Ch4, a structure that can be measured with MRI techniques using cytoarchitectonic maps.
Evaluating Ch4 Density and Cognitive Performance
To determine whether Ch4 density, a proxy measure for NBM volume, is associated with cognitive test performance in de novo Parkinson’s disease, Cody S. Freeman, MD, a fellow at the University of Virginia School of Medicine in Charlottesville, and colleagues analyzed baseline brain MRIs and neuropsychologic test scores for 228 patients with Parkinson’s disease and 101 healthy controls from the Parkinson’s Progression Markers Initiative (PPMI). They also analyzed brain MRIs and neuropsychologic test scores at four years for a subset of 92 participants with Parkinson’s disease in the PPMI.
Neuropsychologic testing included the Montreal Cognitive Assessment (MoCA), Hopkins Verbal Learning Test (HVLT), Judgment of Line Orientation (JLO), Letter Number Sequencing (LNS), Symbol Digit Modalities Test (SDMT), and semantic fluency (animals).
The researchers used MP-RAGE T1 sequences and a probabilistic atlas from the reference Montreal Neurological Institute single subject brain to apply voxel-based morphometry methods to determine Ch4 density. In addition, they used correlation coefficients and linear regression models to analyze relationships between Ch4 density and cognitive scores.
Ch4 Density Was Significantly Associated With Higher MoCA Scores
At baseline, 33.7% of healthy controls and 38.2% of patients with Parkinson’s disease were female. The mean age at neurologic testing was 59.5 among healthy controls and 61.0 in the Parkinson’s disease cohort. The median MoCA score was 28 for controls and patients with Parkinson’s disease at baseline. The mean Ch4 density was 87.9 in the control group and 86.4 in the Parkinson’s disease cohort.
At baseline, Ch4 density was significantly correlated with MoCA, JLO, LNS, and SDMT scores. In a linear regression model adjusted for age and sex, Ch4 density was significantly associated with higher MoCA scores in patients with Parkinson’s disease. In linear regression models adjusted for sex, increasing Ch4 density was associated with higher JLO, LNS, and SDMT scores. The researchers observed no associations between Ch4 density and JLO and semantic fluency in linear regression models adjusted for sex.
For the subset of participants with Parkinson’s disease with brain MRI and neuropsychologic testing available at four years, Ch4 density was significantly correlated with MoCA, JLO, LNS, and SDMT. In a linear regression model adjusted for age and sex, increasing Ch4 density was associated with higher MoCA scores in patients with Parkinson’s disease. In linear regression models adjusted for sex, increasing Ch4 density was associated with higher JLO, LNS, and SDMT scores.
Strategies for Treating Motor Fluctuations in Parkinson’s Disease
Improved delivery of levodopa and new therapies may help to reduce off time.
MIAMI—Motor fluctuations in Parkinson’s disease can arise from more than one cause, and a clinician needs to consider a range of possibilities. Most commonly, motor fluctuations arise as a consequence of chronic levodopa therapy, though the progression of parkinsonism is a contributing factor, according to an overview presented at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. The pharmacokinetics of levodopa provide the basis for studying most clinical patterns of motor fluctuations, and new pharmacologic strategies are under development to improve upon existing treatment options.
“In recent years, there have been some exciting and novel directions of Parkinson’s disease therapeutics for motor fluctuations,” said Peter A. LeWitt, MD, Director of the Parkinson’s Disease and Movement Disorder Program at Henry Ford Hospital in West Bloomfield, Michigan.
A Need to Improve Levodopa Delivery
Beyond irregular effects of levodopa, motor fluctuations may be intrinsic to Parkinson’s disease, said Dr. LeWitt. One problem experienced by some patients is freezing of gait, immobility that is often situation-specific irrespective of medication dosing, he added. The sleep-benefit phenomenon, stress-exacerbated tremors and dyskinesias, and end-of-day medication unresponsiveness are further examples. “But for the most part, most motor fluctuations tend to be closely linked to the variable delivery of levodopa to the brain, where, after a short delay, it undergoes conversion to dopamine. This neurotransmitter does not have long to carry out its intended signaling because enzymes and re-uptake mechanisms quickly dispose of it. So, consistent delivery is the key for averting dose-by-dose motor fluctuations.”
During its 50 years of service to the Parkinson’s disease patient, levodopa has revolutionized the identity of this disorder. It has improved longevity, disability, and overall quality of life, and it inspired
Because the short-duration response pattern is associated with benefits as brief as two to three hours per oral immediate-release dose, the focus for improving levodopa has been the use of extension therapies. Blocking the breakdown of peripheral levodopa metabolism (the mechanism for catechol-O-methyltransferase inhibition) or slowing the central metabolism of dopamine (by inhibiting monoamine oxidase-type B) join extended-release carbidopa-levodopa preparations as ways to improve upon the immediate-release product. “While these strategies do provide some level of effectiveness, the problems of irregular responsiveness and up to several hours of daily ‘off’ time haven’t been solved. ‘Off’ time still imposes a major burden on many patients living with Parkinson’s disease,” said Dr. LeWitt. Like delayed onset of effect and rapid wearing-off, levodopa-induced dyskinesias present another challenge for understanding their origin and optimal control. While new mechanisms of blocking dyskinesia are being sought, a simpler solution can be more continuous levodopa delivery so that drug concentration peaks causing involuntary movements are averted.
Future Therapies Undergoing Trials Today
Several new therapeutic approaches have been developed for dealing with the shortcomings of current therapies, especially levodopa. “The first of these options was a tube inserted through the stomach into the upper small intestine for continuous pumping of a carbidopa-levodopa microsuspension gel –quite effective but not an easy choice for most patients,” said Dr. LeWitt. Less cumbersome ways to extend levodopa effects have been the several sustained-release formulations now under development. One is a gastric-retention product, termed the “Accordion Pill,” which slowly leaches carbidopa and levodopa to enhance their pharmacokinetic absorption profile. Another treatment strategy for motor fluctuations that, like the Accordion Pill, is also in worldwide clinical trials, involves continuous subcutaneous infusion of solubilized levodopa and carbidopa. With the latter approach, the drug is administered by a small pump adjusted to optimized rate of delivery. Dr. LeWitt also described another novel way for administering levodopa for rapid entry into the bloodstream for treating “off” states. This involves an inhalation device for pulmonary uptake of a micro-particulate levodopa formulation. In a recently completed study, “off” states were reversed rapidly with this approach.
Subcutaneous apomorphine infusion has already been used for more than 30 years in treating motor fluctuations. However, just recently, a more complete story of what this adjunctive therapy offers was reported from a large-scale randomized clinical trial in Europe. A similar study is underway in the United States and might lead to availability of apomorphine infusion in the near future, said Dr. LeWitt. Another approach to motor fluctuations can be found in a drug for motor fluctuations that does not act on dopaminergic pathways. This medication is istradefylline, a selective inhibitor of adenosine A2a receptors (which are located in the same pathway targeted by deep brain stimulation). In Japan, istradefylline is marketed for reducing “off” time, and studies with this drug are planned for review in the US, said Dr. LeWitt.
For a nonpharmacologic approach to managing motor fluctuations, neurosurgical targeting of brain circuitry with deep brain electrical stimulation has had several decades of experience. Another direction of neurosurgical intervention is under investigation; this involves gene therapy to improve the efficacy of oral levodopa therapy. “Inserting into the putamen a gene for producing an increase of L-aromatic amino acid decarboxylase appears to offer a way for enhancing dopamine formation. The clinical investigation currently underway is testing whether producing this localized alteration of brain neurochemistry might succeed at attenuating motor fluctuations,” said Dr. LeWitt
“In talking to patients about their experiences with motor fluctuations, my advice is to think both about levodopa pharmacokinetics and how the patient uses levodopa (since schedule compliance, the interaction of meals, and drinking sufficient water with medications commonly contribute to these problems). Fortunately, new treatment options are on their way to help in fighting back against the limitations of levodopa therapy,” Dr. LeWitt concluded.
—Erica Tricarico
Suggested Reading
Anderson E, Nutt J. The long-duration response to levodopa: phenomenology, potential mechanisms and clinical implications. Parkinsonism Relat Disord. 2011;17:587-592.
Cilia R, Akpalu A, Sarfo FS, et al. The modern pre-levodopa era of Parkinson’s disease: insights into motor complications from sub-Saharan Africa. Brain. 2014;137(10);2731-2742.
LeWitt PA. Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics. Mov Disord. 2015;30(1):64-72.
Improved delivery of levodopa and new therapies may help to reduce off time.
Improved delivery of levodopa and new therapies may help to reduce off time.
MIAMI—Motor fluctuations in Parkinson’s disease can arise from more than one cause, and a clinician needs to consider a range of possibilities. Most commonly, motor fluctuations arise as a consequence of chronic levodopa therapy, though the progression of parkinsonism is a contributing factor, according to an overview presented at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. The pharmacokinetics of levodopa provide the basis for studying most clinical patterns of motor fluctuations, and new pharmacologic strategies are under development to improve upon existing treatment options.
“In recent years, there have been some exciting and novel directions of Parkinson’s disease therapeutics for motor fluctuations,” said Peter A. LeWitt, MD, Director of the Parkinson’s Disease and Movement Disorder Program at Henry Ford Hospital in West Bloomfield, Michigan.
A Need to Improve Levodopa Delivery
Beyond irregular effects of levodopa, motor fluctuations may be intrinsic to Parkinson’s disease, said Dr. LeWitt. One problem experienced by some patients is freezing of gait, immobility that is often situation-specific irrespective of medication dosing, he added. The sleep-benefit phenomenon, stress-exacerbated tremors and dyskinesias, and end-of-day medication unresponsiveness are further examples. “But for the most part, most motor fluctuations tend to be closely linked to the variable delivery of levodopa to the brain, where, after a short delay, it undergoes conversion to dopamine. This neurotransmitter does not have long to carry out its intended signaling because enzymes and re-uptake mechanisms quickly dispose of it. So, consistent delivery is the key for averting dose-by-dose motor fluctuations.”
During its 50 years of service to the Parkinson’s disease patient, levodopa has revolutionized the identity of this disorder. It has improved longevity, disability, and overall quality of life, and it inspired
Because the short-duration response pattern is associated with benefits as brief as two to three hours per oral immediate-release dose, the focus for improving levodopa has been the use of extension therapies. Blocking the breakdown of peripheral levodopa metabolism (the mechanism for catechol-O-methyltransferase inhibition) or slowing the central metabolism of dopamine (by inhibiting monoamine oxidase-type B) join extended-release carbidopa-levodopa preparations as ways to improve upon the immediate-release product. “While these strategies do provide some level of effectiveness, the problems of irregular responsiveness and up to several hours of daily ‘off’ time haven’t been solved. ‘Off’ time still imposes a major burden on many patients living with Parkinson’s disease,” said Dr. LeWitt. Like delayed onset of effect and rapid wearing-off, levodopa-induced dyskinesias present another challenge for understanding their origin and optimal control. While new mechanisms of blocking dyskinesia are being sought, a simpler solution can be more continuous levodopa delivery so that drug concentration peaks causing involuntary movements are averted.
Future Therapies Undergoing Trials Today
Several new therapeutic approaches have been developed for dealing with the shortcomings of current therapies, especially levodopa. “The first of these options was a tube inserted through the stomach into the upper small intestine for continuous pumping of a carbidopa-levodopa microsuspension gel –quite effective but not an easy choice for most patients,” said Dr. LeWitt. Less cumbersome ways to extend levodopa effects have been the several sustained-release formulations now under development. One is a gastric-retention product, termed the “Accordion Pill,” which slowly leaches carbidopa and levodopa to enhance their pharmacokinetic absorption profile. Another treatment strategy for motor fluctuations that, like the Accordion Pill, is also in worldwide clinical trials, involves continuous subcutaneous infusion of solubilized levodopa and carbidopa. With the latter approach, the drug is administered by a small pump adjusted to optimized rate of delivery. Dr. LeWitt also described another novel way for administering levodopa for rapid entry into the bloodstream for treating “off” states. This involves an inhalation device for pulmonary uptake of a micro-particulate levodopa formulation. In a recently completed study, “off” states were reversed rapidly with this approach.
Subcutaneous apomorphine infusion has already been used for more than 30 years in treating motor fluctuations. However, just recently, a more complete story of what this adjunctive therapy offers was reported from a large-scale randomized clinical trial in Europe. A similar study is underway in the United States and might lead to availability of apomorphine infusion in the near future, said Dr. LeWitt. Another approach to motor fluctuations can be found in a drug for motor fluctuations that does not act on dopaminergic pathways. This medication is istradefylline, a selective inhibitor of adenosine A2a receptors (which are located in the same pathway targeted by deep brain stimulation). In Japan, istradefylline is marketed for reducing “off” time, and studies with this drug are planned for review in the US, said Dr. LeWitt.
For a nonpharmacologic approach to managing motor fluctuations, neurosurgical targeting of brain circuitry with deep brain electrical stimulation has had several decades of experience. Another direction of neurosurgical intervention is under investigation; this involves gene therapy to improve the efficacy of oral levodopa therapy. “Inserting into the putamen a gene for producing an increase of L-aromatic amino acid decarboxylase appears to offer a way for enhancing dopamine formation. The clinical investigation currently underway is testing whether producing this localized alteration of brain neurochemistry might succeed at attenuating motor fluctuations,” said Dr. LeWitt
“In talking to patients about their experiences with motor fluctuations, my advice is to think both about levodopa pharmacokinetics and how the patient uses levodopa (since schedule compliance, the interaction of meals, and drinking sufficient water with medications commonly contribute to these problems). Fortunately, new treatment options are on their way to help in fighting back against the limitations of levodopa therapy,” Dr. LeWitt concluded.
—Erica Tricarico
Suggested Reading
Anderson E, Nutt J. The long-duration response to levodopa: phenomenology, potential mechanisms and clinical implications. Parkinsonism Relat Disord. 2011;17:587-592.
Cilia R, Akpalu A, Sarfo FS, et al. The modern pre-levodopa era of Parkinson’s disease: insights into motor complications from sub-Saharan Africa. Brain. 2014;137(10);2731-2742.
LeWitt PA. Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics. Mov Disord. 2015;30(1):64-72.
MIAMI—Motor fluctuations in Parkinson’s disease can arise from more than one cause, and a clinician needs to consider a range of possibilities. Most commonly, motor fluctuations arise as a consequence of chronic levodopa therapy, though the progression of parkinsonism is a contributing factor, according to an overview presented at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. The pharmacokinetics of levodopa provide the basis for studying most clinical patterns of motor fluctuations, and new pharmacologic strategies are under development to improve upon existing treatment options.
“In recent years, there have been some exciting and novel directions of Parkinson’s disease therapeutics for motor fluctuations,” said Peter A. LeWitt, MD, Director of the Parkinson’s Disease and Movement Disorder Program at Henry Ford Hospital in West Bloomfield, Michigan.
A Need to Improve Levodopa Delivery
Beyond irregular effects of levodopa, motor fluctuations may be intrinsic to Parkinson’s disease, said Dr. LeWitt. One problem experienced by some patients is freezing of gait, immobility that is often situation-specific irrespective of medication dosing, he added. The sleep-benefit phenomenon, stress-exacerbated tremors and dyskinesias, and end-of-day medication unresponsiveness are further examples. “But for the most part, most motor fluctuations tend to be closely linked to the variable delivery of levodopa to the brain, where, after a short delay, it undergoes conversion to dopamine. This neurotransmitter does not have long to carry out its intended signaling because enzymes and re-uptake mechanisms quickly dispose of it. So, consistent delivery is the key for averting dose-by-dose motor fluctuations.”
During its 50 years of service to the Parkinson’s disease patient, levodopa has revolutionized the identity of this disorder. It has improved longevity, disability, and overall quality of life, and it inspired
Because the short-duration response pattern is associated with benefits as brief as two to three hours per oral immediate-release dose, the focus for improving levodopa has been the use of extension therapies. Blocking the breakdown of peripheral levodopa metabolism (the mechanism for catechol-O-methyltransferase inhibition) or slowing the central metabolism of dopamine (by inhibiting monoamine oxidase-type B) join extended-release carbidopa-levodopa preparations as ways to improve upon the immediate-release product. “While these strategies do provide some level of effectiveness, the problems of irregular responsiveness and up to several hours of daily ‘off’ time haven’t been solved. ‘Off’ time still imposes a major burden on many patients living with Parkinson’s disease,” said Dr. LeWitt. Like delayed onset of effect and rapid wearing-off, levodopa-induced dyskinesias present another challenge for understanding their origin and optimal control. While new mechanisms of blocking dyskinesia are being sought, a simpler solution can be more continuous levodopa delivery so that drug concentration peaks causing involuntary movements are averted.
Future Therapies Undergoing Trials Today
Several new therapeutic approaches have been developed for dealing with the shortcomings of current therapies, especially levodopa. “The first of these options was a tube inserted through the stomach into the upper small intestine for continuous pumping of a carbidopa-levodopa microsuspension gel –quite effective but not an easy choice for most patients,” said Dr. LeWitt. Less cumbersome ways to extend levodopa effects have been the several sustained-release formulations now under development. One is a gastric-retention product, termed the “Accordion Pill,” which slowly leaches carbidopa and levodopa to enhance their pharmacokinetic absorption profile. Another treatment strategy for motor fluctuations that, like the Accordion Pill, is also in worldwide clinical trials, involves continuous subcutaneous infusion of solubilized levodopa and carbidopa. With the latter approach, the drug is administered by a small pump adjusted to optimized rate of delivery. Dr. LeWitt also described another novel way for administering levodopa for rapid entry into the bloodstream for treating “off” states. This involves an inhalation device for pulmonary uptake of a micro-particulate levodopa formulation. In a recently completed study, “off” states were reversed rapidly with this approach.
Subcutaneous apomorphine infusion has already been used for more than 30 years in treating motor fluctuations. However, just recently, a more complete story of what this adjunctive therapy offers was reported from a large-scale randomized clinical trial in Europe. A similar study is underway in the United States and might lead to availability of apomorphine infusion in the near future, said Dr. LeWitt. Another approach to motor fluctuations can be found in a drug for motor fluctuations that does not act on dopaminergic pathways. This medication is istradefylline, a selective inhibitor of adenosine A2a receptors (which are located in the same pathway targeted by deep brain stimulation). In Japan, istradefylline is marketed for reducing “off” time, and studies with this drug are planned for review in the US, said Dr. LeWitt.
For a nonpharmacologic approach to managing motor fluctuations, neurosurgical targeting of brain circuitry with deep brain electrical stimulation has had several decades of experience. Another direction of neurosurgical intervention is under investigation; this involves gene therapy to improve the efficacy of oral levodopa therapy. “Inserting into the putamen a gene for producing an increase of L-aromatic amino acid decarboxylase appears to offer a way for enhancing dopamine formation. The clinical investigation currently underway is testing whether producing this localized alteration of brain neurochemistry might succeed at attenuating motor fluctuations,” said Dr. LeWitt
“In talking to patients about their experiences with motor fluctuations, my advice is to think both about levodopa pharmacokinetics and how the patient uses levodopa (since schedule compliance, the interaction of meals, and drinking sufficient water with medications commonly contribute to these problems). Fortunately, new treatment options are on their way to help in fighting back against the limitations of levodopa therapy,” Dr. LeWitt concluded.
—Erica Tricarico
Suggested Reading
Anderson E, Nutt J. The long-duration response to levodopa: phenomenology, potential mechanisms and clinical implications. Parkinsonism Relat Disord. 2011;17:587-592.
Cilia R, Akpalu A, Sarfo FS, et al. The modern pre-levodopa era of Parkinson’s disease: insights into motor complications from sub-Saharan Africa. Brain. 2014;137(10);2731-2742.
LeWitt PA. Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics. Mov Disord. 2015;30(1):64-72.
Optimize the medical treatment of endometriosis—Use all available medications
CASE Endometriosis pain increases despite hormonal treatment
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy of a cul-de-sac lesion showed endometriosis on histopathology. The patient was treated with a continuous low-dose estrogen-progestin contraceptive. Initially, the treatment helped relieve her pain symptoms. Over the next year, while on that treatment, her pain gradually increased in severity until it was disabling. At an office visit, the primary clinician renewed the estrogen-progestin contraceptive for another year, even though it was not relieving the patient’s pain. The patient sought a second opinion.
We are the experts in the management of pelvic pain caused by endometriosis
Women’s health clinicians are the specialists best trained to care for patients with severe pain caused by endometriosis. Low-dose continuous estrogen-progestin contraceptives are commonly prescribed as a first-line hormonal treatment for pain caused by endometriosis. My observation is that estrogen-progestincontraceptives are often effective when initially prescribed, but with continued use over years, pain often recurs. Estrogen is known to stimulate endometriosis disease activity. Progestins at high doses suppress endometriosis disease activity. However, endometriosis implants often manifest decreased responsiveness to progestins, permitting the estrogen in the combination contraceptive to exert its disease-stimulating effect.1,2 I frequently see women with pelvic pain caused by endometriosis, who initially had a significant decrease in pain with continuous estrogen-progestin contraceptive treatment but who develop increasing pain with continued use of the medication. In this clinical situation, it is useful to consider stopping the estrogen-progestin therapy and to prescribe a hormone with a different mechanism of action (TABLE).
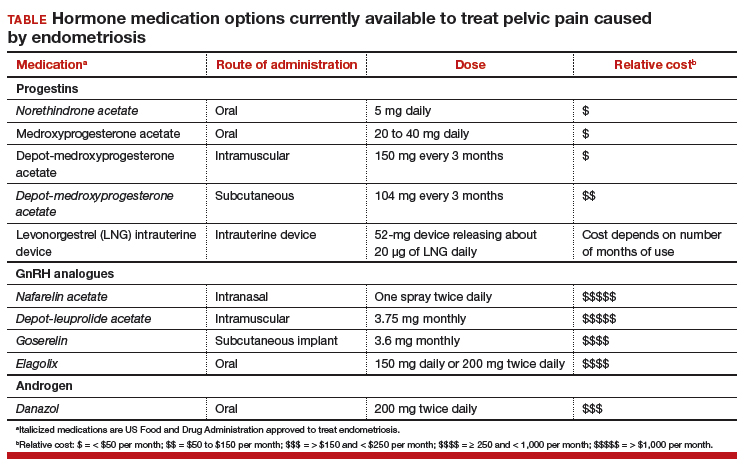
Progestin-only medications
Progestin-only medications are often effective in the treatment of pain caused by endometriosis. High-dose progestin-only medications suppress pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby suppressing ovarian synthesis of estrogen, resulting in low circulating levels of estrogen. This removes the estrogen stimulus that exacerbates endometriosis disease activity. High-dose progestins also directly suppress cellular activity in endometriosis implants. High-dose progestins often overcome the relative resistance of endometriosis lesions to progestin suppression of disease activity. Hence, high-dose progestin-only medications have two mechanisms of action: suppression of estrogen synthesis through pituitary suppression of LH and FSH, and direct inhibition of cellular activity in the endometriosis lesions. High-dose progestin-only treatments include:
- oral norethindrone acetate 5 mg daily
- oral medroxyprogesterone acetate (MPA) 20 to 40 mg daily
- subcutaneous, or depot MPA
- levonorgestrel-releasing intrauterine device (LNG-IUD).
In my practice, I frequently use oral norethindrone acetate 5 mg daily to treat pelvic pain caused by endometriosis. In one randomized trial, 90 women with pelvic pain and rectovaginal endometriosis were randomly assigned to treatment with norethindrone acetate 2.5 mg daily or an estrogen-progestin contraceptive. After 12 months of treatment, satisfaction with treatment was reported by 73% and 62% of the women in the norethindrone acetate and estrogen-progestin groups, respectively.3 The most common adverse effects reported by women taking norethindrone acetate were weight gain (27%) and decreased libido (9%).
Oral MPA at doses of 30 mg to 100 mg daily has been reported to be effective for the treatment of pelvic pain caused by endometriosis. MPA treatment can induce atrophy and pseudodecidualization in endometrium and endometriosis implants. In my practice I typically prescribe doses in the range of 20 mg to 40 mg daily. With oral MPA treatment, continued uterine bleeding may occur in up to 30% of women, somewhat limiting its efficacy.4–7
Subcutaneous and depot MPA have been reported to be effective in the treatment of pelvic pain caused by endometriosis.4,8 In some resource-limited countries, depot MPA may be the most available progestin for the treatment of pelvic pain caused by endometriosis.
The LNG-IUD, inserted after surgery for endometriosis, has been reported to result in decreased pelvic pain in studies with a modest number of participants.9–11
GnRH analogue medications
Gonadotropin-releasing hormone (GnRH) analogues, including both GnRH agonists (nafarelin, leuprolide, and goserelin) and GnRH antagonists (elagolix) reduce pelvic pain caused by endometriosis by suppressing pituitary secretion of LH and FSH, thereby reducing ovarian synthesis of estradiol. In the absence of estradiol stimulation, cellular activity in endometriosis lesions decreases and pain symptoms improve. In my practice, I frequently use either nafarelin12 or leuprolide acetate depot plus norethindrone add-back.13 I generally avoid the use of leuprolide depot monotherapy because in many women it causes severe vasomotor symptoms.
At standard doses, nafarelin therapy generally results in serum estradiol levels in the range of 20 to 30 pg/mL, a “sweet spot” associated with modest vasomotor symptoms and reduced cellular activity in endometriosis implants.12,14 In many women who become amenorrheic on nafarelin two sprays daily, the dose can be reduced with maintenance of pain control and ovarian suppression.15 Leuprolide acetate depot monotherapy results in serum estradiol levels in the range of 5 to 10 pg/mL, causing severe vasomotor symptoms and reduction in cellular activity in endometriosis lesions. To reduce the adverse effects of leuprolide acetate depot monotherapy, I generally initiate concomitant add-back therapy with norethindrone acetate.13 A little recognized pharmacokinetic observation is that a very small amount of norethindrone acetate, generally less than 1%, is metabolized to ethinyl estradiol.16
The oral GnRH antagonist, elagolix, 150 mg daily for up to 24 months or 200 mg twice daily for 6 months, was approved by the US Food and Drug Administration (FDA) in July 2018. It is now available in pharmacies. Elagolix treatment results in significant reduction in pain caused by endometriosis, but only moderately bothersome vasomotor symptoms.17,18 Elagolix likely will become a widely used medication because of the simplicity of oral administration, efficacy against endometriosis, and acceptable adverse-effect profile. A major disadvantage of the GnRH analogue-class of medications is that they are more expensive than the progestin medications mentioned above. Among the GnRH analogue class of medications, elagolix and goserelin are the least expensive.
Androgens
Estrogen stimulates cellular activity in endometriosis lesions. Androgen and high-dose progestins inhibit cellular activity in endometriosis lesions. Danazol, an attenuated androgen and a progestin is effectivein treating pelvic pain caused by endometriosis.19,20 However, many women decline to use danazol because it is often associated with weight gain. As an androgen, danazol can permanently change a woman’s voice pitch and should not be used by professional singers or speech therapists.
Aromatase Inhibitors
Estrogen is a critically important stimulus of cell activity in endometriosis lesions. Aromatase inhibitors, which block the synthesis of estrogen, have been explored in the treatment of endometriosis that has proven to be resistant to other therapies. Although the combination of an aromatase inhibitor plus a high-dose progestin or GnRH analogue may be effective, more data are needed before widely using the aromatase inhibitors in clinical practice.21
Don’t get stuck in a rut
When treating pelvic pain caused by endometriosis, if the patient’s hormone regimen is not working, prescribe a medication from another class of hormones. In the case presented above, a woman with pelvic pain and surgically proven endometriosis reported inadequate control of her pain symptoms with a continuous estrogen-progestin medication. Her physician prescribed another year of the same estrogen-progestin medication. Instead of renewing the medication, the physician could have offered the patient a hormone medication from another drug class: 1) progestin only, 2) GnRH analogue, or 3) danazol. By using every available hormonal agent, physicians will improve the treatment of pelvic pain caused by endometriosis. Millions of women in our country have pelvic pain caused by endometriosis. They are counting on us, women’s health specialists, to effectively treat their disease.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632.
- Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43.
- Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Syst Rev. 2012;(3):CD002122.
- Moghissi KS, Boyce CR. Management of endometriosis with oral medroxyprogesterone acetate. Obstet Gynecol. 1976;47(3):265–267.
- Telimaa S, Puolakka J, Rönnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1(1):13–23.
- Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 pt 1):323–327.
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leu-prolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314–325.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database of Syst Rev. 2013;(1):CD005072.
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-pain: a randomized controlled trial. Obstet Gynecol. 2012;119(3):519–526.
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273–279.
- Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobsen J. Administration of nasal nafarelin as compared with oral danazol for endo-metriosis. A multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318(8):485–489.
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998; 91(1):16–24.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745.
- Hull ME, Barbieri RL. Nafarelin in the treatment of endometriosis. Dose management. Gynecol Obstet Invest. 1994;37(4):263–264.
- Barbieri RL, Petro Z, Canick JA, Ryan KJ. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57(2):299–303.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160.
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(4):CD000068.
- Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;141(4):453–463.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
CASE Endometriosis pain increases despite hormonal treatment
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy of a cul-de-sac lesion showed endometriosis on histopathology. The patient was treated with a continuous low-dose estrogen-progestin contraceptive. Initially, the treatment helped relieve her pain symptoms. Over the next year, while on that treatment, her pain gradually increased in severity until it was disabling. At an office visit, the primary clinician renewed the estrogen-progestin contraceptive for another year, even though it was not relieving the patient’s pain. The patient sought a second opinion.
We are the experts in the management of pelvic pain caused by endometriosis
Women’s health clinicians are the specialists best trained to care for patients with severe pain caused by endometriosis. Low-dose continuous estrogen-progestin contraceptives are commonly prescribed as a first-line hormonal treatment for pain caused by endometriosis. My observation is that estrogen-progestincontraceptives are often effective when initially prescribed, but with continued use over years, pain often recurs. Estrogen is known to stimulate endometriosis disease activity. Progestins at high doses suppress endometriosis disease activity. However, endometriosis implants often manifest decreased responsiveness to progestins, permitting the estrogen in the combination contraceptive to exert its disease-stimulating effect.1,2 I frequently see women with pelvic pain caused by endometriosis, who initially had a significant decrease in pain with continuous estrogen-progestin contraceptive treatment but who develop increasing pain with continued use of the medication. In this clinical situation, it is useful to consider stopping the estrogen-progestin therapy and to prescribe a hormone with a different mechanism of action (TABLE).

Progestin-only medications
Progestin-only medications are often effective in the treatment of pain caused by endometriosis. High-dose progestin-only medications suppress pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby suppressing ovarian synthesis of estrogen, resulting in low circulating levels of estrogen. This removes the estrogen stimulus that exacerbates endometriosis disease activity. High-dose progestins also directly suppress cellular activity in endometriosis implants. High-dose progestins often overcome the relative resistance of endometriosis lesions to progestin suppression of disease activity. Hence, high-dose progestin-only medications have two mechanisms of action: suppression of estrogen synthesis through pituitary suppression of LH and FSH, and direct inhibition of cellular activity in the endometriosis lesions. High-dose progestin-only treatments include:
- oral norethindrone acetate 5 mg daily
- oral medroxyprogesterone acetate (MPA) 20 to 40 mg daily
- subcutaneous, or depot MPA
- levonorgestrel-releasing intrauterine device (LNG-IUD).
In my practice, I frequently use oral norethindrone acetate 5 mg daily to treat pelvic pain caused by endometriosis. In one randomized trial, 90 women with pelvic pain and rectovaginal endometriosis were randomly assigned to treatment with norethindrone acetate 2.5 mg daily or an estrogen-progestin contraceptive. After 12 months of treatment, satisfaction with treatment was reported by 73% and 62% of the women in the norethindrone acetate and estrogen-progestin groups, respectively.3 The most common adverse effects reported by women taking norethindrone acetate were weight gain (27%) and decreased libido (9%).
Oral MPA at doses of 30 mg to 100 mg daily has been reported to be effective for the treatment of pelvic pain caused by endometriosis. MPA treatment can induce atrophy and pseudodecidualization in endometrium and endometriosis implants. In my practice I typically prescribe doses in the range of 20 mg to 40 mg daily. With oral MPA treatment, continued uterine bleeding may occur in up to 30% of women, somewhat limiting its efficacy.4–7
Subcutaneous and depot MPA have been reported to be effective in the treatment of pelvic pain caused by endometriosis.4,8 In some resource-limited countries, depot MPA may be the most available progestin for the treatment of pelvic pain caused by endometriosis.
The LNG-IUD, inserted after surgery for endometriosis, has been reported to result in decreased pelvic pain in studies with a modest number of participants.9–11
GnRH analogue medications
Gonadotropin-releasing hormone (GnRH) analogues, including both GnRH agonists (nafarelin, leuprolide, and goserelin) and GnRH antagonists (elagolix) reduce pelvic pain caused by endometriosis by suppressing pituitary secretion of LH and FSH, thereby reducing ovarian synthesis of estradiol. In the absence of estradiol stimulation, cellular activity in endometriosis lesions decreases and pain symptoms improve. In my practice, I frequently use either nafarelin12 or leuprolide acetate depot plus norethindrone add-back.13 I generally avoid the use of leuprolide depot monotherapy because in many women it causes severe vasomotor symptoms.
At standard doses, nafarelin therapy generally results in serum estradiol levels in the range of 20 to 30 pg/mL, a “sweet spot” associated with modest vasomotor symptoms and reduced cellular activity in endometriosis implants.12,14 In many women who become amenorrheic on nafarelin two sprays daily, the dose can be reduced with maintenance of pain control and ovarian suppression.15 Leuprolide acetate depot monotherapy results in serum estradiol levels in the range of 5 to 10 pg/mL, causing severe vasomotor symptoms and reduction in cellular activity in endometriosis lesions. To reduce the adverse effects of leuprolide acetate depot monotherapy, I generally initiate concomitant add-back therapy with norethindrone acetate.13 A little recognized pharmacokinetic observation is that a very small amount of norethindrone acetate, generally less than 1%, is metabolized to ethinyl estradiol.16
The oral GnRH antagonist, elagolix, 150 mg daily for up to 24 months or 200 mg twice daily for 6 months, was approved by the US Food and Drug Administration (FDA) in July 2018. It is now available in pharmacies. Elagolix treatment results in significant reduction in pain caused by endometriosis, but only moderately bothersome vasomotor symptoms.17,18 Elagolix likely will become a widely used medication because of the simplicity of oral administration, efficacy against endometriosis, and acceptable adverse-effect profile. A major disadvantage of the GnRH analogue-class of medications is that they are more expensive than the progestin medications mentioned above. Among the GnRH analogue class of medications, elagolix and goserelin are the least expensive.
Androgens
Estrogen stimulates cellular activity in endometriosis lesions. Androgen and high-dose progestins inhibit cellular activity in endometriosis lesions. Danazol, an attenuated androgen and a progestin is effectivein treating pelvic pain caused by endometriosis.19,20 However, many women decline to use danazol because it is often associated with weight gain. As an androgen, danazol can permanently change a woman’s voice pitch and should not be used by professional singers or speech therapists.
Aromatase Inhibitors
Estrogen is a critically important stimulus of cell activity in endometriosis lesions. Aromatase inhibitors, which block the synthesis of estrogen, have been explored in the treatment of endometriosis that has proven to be resistant to other therapies. Although the combination of an aromatase inhibitor plus a high-dose progestin or GnRH analogue may be effective, more data are needed before widely using the aromatase inhibitors in clinical practice.21
Don’t get stuck in a rut
When treating pelvic pain caused by endometriosis, if the patient’s hormone regimen is not working, prescribe a medication from another class of hormones. In the case presented above, a woman with pelvic pain and surgically proven endometriosis reported inadequate control of her pain symptoms with a continuous estrogen-progestin medication. Her physician prescribed another year of the same estrogen-progestin medication. Instead of renewing the medication, the physician could have offered the patient a hormone medication from another drug class: 1) progestin only, 2) GnRH analogue, or 3) danazol. By using every available hormonal agent, physicians will improve the treatment of pelvic pain caused by endometriosis. Millions of women in our country have pelvic pain caused by endometriosis. They are counting on us, women’s health specialists, to effectively treat their disease.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Endometriosis pain increases despite hormonal treatment
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy of a cul-de-sac lesion showed endometriosis on histopathology. The patient was treated with a continuous low-dose estrogen-progestin contraceptive. Initially, the treatment helped relieve her pain symptoms. Over the next year, while on that treatment, her pain gradually increased in severity until it was disabling. At an office visit, the primary clinician renewed the estrogen-progestin contraceptive for another year, even though it was not relieving the patient’s pain. The patient sought a second opinion.
We are the experts in the management of pelvic pain caused by endometriosis
Women’s health clinicians are the specialists best trained to care for patients with severe pain caused by endometriosis. Low-dose continuous estrogen-progestin contraceptives are commonly prescribed as a first-line hormonal treatment for pain caused by endometriosis. My observation is that estrogen-progestincontraceptives are often effective when initially prescribed, but with continued use over years, pain often recurs. Estrogen is known to stimulate endometriosis disease activity. Progestins at high doses suppress endometriosis disease activity. However, endometriosis implants often manifest decreased responsiveness to progestins, permitting the estrogen in the combination contraceptive to exert its disease-stimulating effect.1,2 I frequently see women with pelvic pain caused by endometriosis, who initially had a significant decrease in pain with continuous estrogen-progestin contraceptive treatment but who develop increasing pain with continued use of the medication. In this clinical situation, it is useful to consider stopping the estrogen-progestin therapy and to prescribe a hormone with a different mechanism of action (TABLE).

Progestin-only medications
Progestin-only medications are often effective in the treatment of pain caused by endometriosis. High-dose progestin-only medications suppress pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby suppressing ovarian synthesis of estrogen, resulting in low circulating levels of estrogen. This removes the estrogen stimulus that exacerbates endometriosis disease activity. High-dose progestins also directly suppress cellular activity in endometriosis implants. High-dose progestins often overcome the relative resistance of endometriosis lesions to progestin suppression of disease activity. Hence, high-dose progestin-only medications have two mechanisms of action: suppression of estrogen synthesis through pituitary suppression of LH and FSH, and direct inhibition of cellular activity in the endometriosis lesions. High-dose progestin-only treatments include:
- oral norethindrone acetate 5 mg daily
- oral medroxyprogesterone acetate (MPA) 20 to 40 mg daily
- subcutaneous, or depot MPA
- levonorgestrel-releasing intrauterine device (LNG-IUD).
In my practice, I frequently use oral norethindrone acetate 5 mg daily to treat pelvic pain caused by endometriosis. In one randomized trial, 90 women with pelvic pain and rectovaginal endometriosis were randomly assigned to treatment with norethindrone acetate 2.5 mg daily or an estrogen-progestin contraceptive. After 12 months of treatment, satisfaction with treatment was reported by 73% and 62% of the women in the norethindrone acetate and estrogen-progestin groups, respectively.3 The most common adverse effects reported by women taking norethindrone acetate were weight gain (27%) and decreased libido (9%).
Oral MPA at doses of 30 mg to 100 mg daily has been reported to be effective for the treatment of pelvic pain caused by endometriosis. MPA treatment can induce atrophy and pseudodecidualization in endometrium and endometriosis implants. In my practice I typically prescribe doses in the range of 20 mg to 40 mg daily. With oral MPA treatment, continued uterine bleeding may occur in up to 30% of women, somewhat limiting its efficacy.4–7
Subcutaneous and depot MPA have been reported to be effective in the treatment of pelvic pain caused by endometriosis.4,8 In some resource-limited countries, depot MPA may be the most available progestin for the treatment of pelvic pain caused by endometriosis.
The LNG-IUD, inserted after surgery for endometriosis, has been reported to result in decreased pelvic pain in studies with a modest number of participants.9–11
GnRH analogue medications
Gonadotropin-releasing hormone (GnRH) analogues, including both GnRH agonists (nafarelin, leuprolide, and goserelin) and GnRH antagonists (elagolix) reduce pelvic pain caused by endometriosis by suppressing pituitary secretion of LH and FSH, thereby reducing ovarian synthesis of estradiol. In the absence of estradiol stimulation, cellular activity in endometriosis lesions decreases and pain symptoms improve. In my practice, I frequently use either nafarelin12 or leuprolide acetate depot plus norethindrone add-back.13 I generally avoid the use of leuprolide depot monotherapy because in many women it causes severe vasomotor symptoms.
At standard doses, nafarelin therapy generally results in serum estradiol levels in the range of 20 to 30 pg/mL, a “sweet spot” associated with modest vasomotor symptoms and reduced cellular activity in endometriosis implants.12,14 In many women who become amenorrheic on nafarelin two sprays daily, the dose can be reduced with maintenance of pain control and ovarian suppression.15 Leuprolide acetate depot monotherapy results in serum estradiol levels in the range of 5 to 10 pg/mL, causing severe vasomotor symptoms and reduction in cellular activity in endometriosis lesions. To reduce the adverse effects of leuprolide acetate depot monotherapy, I generally initiate concomitant add-back therapy with norethindrone acetate.13 A little recognized pharmacokinetic observation is that a very small amount of norethindrone acetate, generally less than 1%, is metabolized to ethinyl estradiol.16
The oral GnRH antagonist, elagolix, 150 mg daily for up to 24 months or 200 mg twice daily for 6 months, was approved by the US Food and Drug Administration (FDA) in July 2018. It is now available in pharmacies. Elagolix treatment results in significant reduction in pain caused by endometriosis, but only moderately bothersome vasomotor symptoms.17,18 Elagolix likely will become a widely used medication because of the simplicity of oral administration, efficacy against endometriosis, and acceptable adverse-effect profile. A major disadvantage of the GnRH analogue-class of medications is that they are more expensive than the progestin medications mentioned above. Among the GnRH analogue class of medications, elagolix and goserelin are the least expensive.
Androgens
Estrogen stimulates cellular activity in endometriosis lesions. Androgen and high-dose progestins inhibit cellular activity in endometriosis lesions. Danazol, an attenuated androgen and a progestin is effectivein treating pelvic pain caused by endometriosis.19,20 However, many women decline to use danazol because it is often associated with weight gain. As an androgen, danazol can permanently change a woman’s voice pitch and should not be used by professional singers or speech therapists.
Aromatase Inhibitors
Estrogen is a critically important stimulus of cell activity in endometriosis lesions. Aromatase inhibitors, which block the synthesis of estrogen, have been explored in the treatment of endometriosis that has proven to be resistant to other therapies. Although the combination of an aromatase inhibitor plus a high-dose progestin or GnRH analogue may be effective, more data are needed before widely using the aromatase inhibitors in clinical practice.21
Don’t get stuck in a rut
When treating pelvic pain caused by endometriosis, if the patient’s hormone regimen is not working, prescribe a medication from another class of hormones. In the case presented above, a woman with pelvic pain and surgically proven endometriosis reported inadequate control of her pain symptoms with a continuous estrogen-progestin medication. Her physician prescribed another year of the same estrogen-progestin medication. Instead of renewing the medication, the physician could have offered the patient a hormone medication from another drug class: 1) progestin only, 2) GnRH analogue, or 3) danazol. By using every available hormonal agent, physicians will improve the treatment of pelvic pain caused by endometriosis. Millions of women in our country have pelvic pain caused by endometriosis. They are counting on us, women’s health specialists, to effectively treat their disease.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632.
- Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43.
- Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Syst Rev. 2012;(3):CD002122.
- Moghissi KS, Boyce CR. Management of endometriosis with oral medroxyprogesterone acetate. Obstet Gynecol. 1976;47(3):265–267.
- Telimaa S, Puolakka J, Rönnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1(1):13–23.
- Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 pt 1):323–327.
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leu-prolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314–325.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database of Syst Rev. 2013;(1):CD005072.
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-pain: a randomized controlled trial. Obstet Gynecol. 2012;119(3):519–526.
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273–279.
- Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobsen J. Administration of nasal nafarelin as compared with oral danazol for endo-metriosis. A multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318(8):485–489.
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998; 91(1):16–24.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745.
- Hull ME, Barbieri RL. Nafarelin in the treatment of endometriosis. Dose management. Gynecol Obstet Invest. 1994;37(4):263–264.
- Barbieri RL, Petro Z, Canick JA, Ryan KJ. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57(2):299–303.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160.
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(4):CD000068.
- Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;141(4):453–463.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96(6):623–632.
- Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43.
- Vercellini P, Pietropaolo G, De Giorgi O, Pasin R, Chiodini A, Crosignani PG. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84(5):1375-1387.
- Brown J, Kives S, Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database of Syst Rev. 2012;(3):CD002122.
- Moghissi KS, Boyce CR. Management of endometriosis with oral medroxyprogesterone acetate. Obstet Gynecol. 1976;47(3):265–267.
- Telimaa S, Puolakka J, Rönnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis. Gynecol Endocrinol. 1987;1(1):13–23.
- Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstet Gynecol. 1988;72(3 pt 1):323–327.
- Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leu-prolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314–325.
- Abou-Setta AM, Houston B, Al-Inany HG, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database of Syst Rev. 2013;(1):CD005072.
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-pain: a randomized controlled trial. Obstet Gynecol. 2012;119(3):519–526.
- Wong AY, Tang LC, Chin RK. Levonorgestrel-releasing intrauterine system (Mirena) and Depot medroxyprogesterone acetate (Depoprovera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2010;50(3):273–279.
- Henzl MR, Corson SL, Moghissi K, Buttram VC, Berqvist C, Jacobsen J. Administration of nasal nafarelin as compared with oral danazol for endo-metriosis. A multicenter double-blind comparative clinical trial. N Engl J Med. 1988;318(8):485–489.
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998; 91(1):16–24.
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745.
- Hull ME, Barbieri RL. Nafarelin in the treatment of endometriosis. Dose management. Gynecol Obstet Invest. 1994;37(4):263–264.
- Barbieri RL, Petro Z, Canick JA, Ryan KJ. Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab. 1983;57(2):299–303.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377(1):28–40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132(1):147–160.
- Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;(4):CD000068.
- Barbieri RL, Ryan KJ. Danazol: endocrine pharmacology and therapeutic applications. Am J Obstet Gynecol. 1981;141(4):453–463.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412.
Occipital Nerve Blocks May Be an Effective Option for Acute Migraine in the Emergency Room
When IV metoclopramide fails to relieve acute migraine in the emergency department, greater occipital nerve block may be an effective treatment.
SAN FRANCISCO—Greater occipital nerve blocks with bupivacaine may be an effective treatment for patients with acute migraine in the emergency department who continue to experience moderate or severe headache after administration of intravenous metoclopramide, according to a presentation at the 60th Annual Scientific Meeting of the American Headache Society.
Greater occipital nerve block is thought to be an effective treatment for acute migraine, although no randomized efficacy data have been published for this indication. Benjamin W. Friedman, MD, Professor of Emergency Medicine, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York, and colleagues hypothesized that bilateral greater occipital nerve block with bupivacaine would provide greater rates of headache freedom than a sham injection among a population of emergency department patients who reported persistence of moderate or severe headache despite standard treatment with intravenous metoclopramide.
Dr. Friedman and colleagues conducted a randomized, sham-controlled trial of bilateral greater occipital nerve blocks with bupivacaine in two urban emergency departments. Patients with acute migraine who reported persistence of a moderate or severe headache for at least one hour or longer after treatment with 10 mg of intravenous metoclopramide were randomized to bilateral greater occipital nerve block with a total of 6 cc of 0.5% bupivacaine or bilateral intradermal scalp injection with a total of 1 cc of 0.5% bupivacaine. The primary outcome was complete headache freedom 30 minutes after the injection. An important secondary outcome was sustained headache relief, defined as achieving a headache level of mild or none in the emergency department and maintaining a level of mild or no headache without the use of any additional medication for 48 hours.
Over a 32-month period, 76 patients were screened for participation and 28 were enrolled, of whom 15 received sham injection and 13 received greater occipital nerve block. The primary outcome, headache freedom at 30 minutes, was achieved by none of the patients in the sham arm and by four patients (31%) in the nerve block arm. The secondary outcome, sustained headache relief for 48 hours, was reported by none of the patients who received sham and by three of the patients (23%) who received greater occipital nerve blocks. Reported side effects did not differ substantially between the two groups.
When IV metoclopramide fails to relieve acute migraine in the emergency department, greater occipital nerve block may be an effective treatment.
When IV metoclopramide fails to relieve acute migraine in the emergency department, greater occipital nerve block may be an effective treatment.
SAN FRANCISCO—Greater occipital nerve blocks with bupivacaine may be an effective treatment for patients with acute migraine in the emergency department who continue to experience moderate or severe headache after administration of intravenous metoclopramide, according to a presentation at the 60th Annual Scientific Meeting of the American Headache Society.
Greater occipital nerve block is thought to be an effective treatment for acute migraine, although no randomized efficacy data have been published for this indication. Benjamin W. Friedman, MD, Professor of Emergency Medicine, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York, and colleagues hypothesized that bilateral greater occipital nerve block with bupivacaine would provide greater rates of headache freedom than a sham injection among a population of emergency department patients who reported persistence of moderate or severe headache despite standard treatment with intravenous metoclopramide.
Dr. Friedman and colleagues conducted a randomized, sham-controlled trial of bilateral greater occipital nerve blocks with bupivacaine in two urban emergency departments. Patients with acute migraine who reported persistence of a moderate or severe headache for at least one hour or longer after treatment with 10 mg of intravenous metoclopramide were randomized to bilateral greater occipital nerve block with a total of 6 cc of 0.5% bupivacaine or bilateral intradermal scalp injection with a total of 1 cc of 0.5% bupivacaine. The primary outcome was complete headache freedom 30 minutes after the injection. An important secondary outcome was sustained headache relief, defined as achieving a headache level of mild or none in the emergency department and maintaining a level of mild or no headache without the use of any additional medication for 48 hours.
Over a 32-month period, 76 patients were screened for participation and 28 were enrolled, of whom 15 received sham injection and 13 received greater occipital nerve block. The primary outcome, headache freedom at 30 minutes, was achieved by none of the patients in the sham arm and by four patients (31%) in the nerve block arm. The secondary outcome, sustained headache relief for 48 hours, was reported by none of the patients who received sham and by three of the patients (23%) who received greater occipital nerve blocks. Reported side effects did not differ substantially between the two groups.
SAN FRANCISCO—Greater occipital nerve blocks with bupivacaine may be an effective treatment for patients with acute migraine in the emergency department who continue to experience moderate or severe headache after administration of intravenous metoclopramide, according to a presentation at the 60th Annual Scientific Meeting of the American Headache Society.
Greater occipital nerve block is thought to be an effective treatment for acute migraine, although no randomized efficacy data have been published for this indication. Benjamin W. Friedman, MD, Professor of Emergency Medicine, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, New York, and colleagues hypothesized that bilateral greater occipital nerve block with bupivacaine would provide greater rates of headache freedom than a sham injection among a population of emergency department patients who reported persistence of moderate or severe headache despite standard treatment with intravenous metoclopramide.
Dr. Friedman and colleagues conducted a randomized, sham-controlled trial of bilateral greater occipital nerve blocks with bupivacaine in two urban emergency departments. Patients with acute migraine who reported persistence of a moderate or severe headache for at least one hour or longer after treatment with 10 mg of intravenous metoclopramide were randomized to bilateral greater occipital nerve block with a total of 6 cc of 0.5% bupivacaine or bilateral intradermal scalp injection with a total of 1 cc of 0.5% bupivacaine. The primary outcome was complete headache freedom 30 minutes after the injection. An important secondary outcome was sustained headache relief, defined as achieving a headache level of mild or none in the emergency department and maintaining a level of mild or no headache without the use of any additional medication for 48 hours.
Over a 32-month period, 76 patients were screened for participation and 28 were enrolled, of whom 15 received sham injection and 13 received greater occipital nerve block. The primary outcome, headache freedom at 30 minutes, was achieved by none of the patients in the sham arm and by four patients (31%) in the nerve block arm. The secondary outcome, sustained headache relief for 48 hours, was reported by none of the patients who received sham and by three of the patients (23%) who received greater occipital nerve blocks. Reported side effects did not differ substantially between the two groups.
Fremanezumab May Reduce Medication Overuse in Migraineurs
A reduction in medication overuse is apparent after four weeks of treatment.
SAN FRANCISCO—Treatment with fremanezumab is associated with reduced overuse of acute medications and a corresponding decrease in days on which a patient uses acute medications, according to a phase III study described at the 60th Annual Scientific Meeting of the American Headache Society.
The overuse of acute or symptomatic headache medications (eg, triptans, ergot derivatives, opioids, and combination analgesics) can cause medication overuse headache (MOH). Chronic migraine is often accompanied by MOH, and the prevention of MOH is one of the main goals in the preventive treatment of migraine.
Fremanezumab, a fully humanized monoclonal antibody that selectively targets calcitonin gene-related peptide, reduced the frequency and severity of headaches in patients with chronic migraine who participated in clinical trials. Stephen D. Silberstein, MD, Director of the Headache Center at Thomas Jefferson University Hospital in Philadelphia, and colleagues assessed the effect of fremanezumab, compared with placebo, on medication overuse and acute headache medication use in patients with chronic migraine.
Comparing Two Fremanezumab Doses With Placebo
The investigators conducted a multicenter, randomized, double-blind, placebo-controlled, phase III study, during which they randomized eligible patients with chronic migraine in equal groups to receive subcutaneous injections of fremanezumab quarterly dosing (ie, 675 mg at baseline and placebo at Weeks 4 and 8), fremanezumab monthly dosing (ie, 675 mg at baseline and 225 mg at Weeks 4 and 8), or placebo at each time point over a 12-week treatment period. Dr. Silberstein’s group defined medication overuse as the use of acute headache medication on 15 or more days, the use of migraine-specific acute medication on 10 or more days, or the use of combination medications for headache on 10 or more days during the 28-day baseline period.
In a post hoc analysis, the researchers assessed the proportion of patients who reverted from overusing medications at baseline to not overusing medications at Week 12, as well as the change from baseline in the number of days of acute headache medication use among these patients. Analyses were performed using data for all randomized patients who received at least one dose of study drug and had at least 10 days of postbaseline efficacy assessments on the primary end point.
Fremanezumab Was More Likely to Reduce Overuse
At baseline, the number of patients with medication overuse was 201 in the quarterly arm, 198 in the monthly arm, and 188 in the placebo arm. Among these participants, significantly more fremanezumab-treated patients reported no medication overuse during the 12-week treatment period. The number of patients reporting no medication overuse was 111 (55%) in the quarterly arm, 120 (61%) in the monthly arm, and 87 (46%) in the placebo arm. The investigators observed a response to treatment as early as Week 4 (102 [51%] quarterly patients, 107 [54%] monthly patients, and 73 [39%] controls).
Among the patients who responded to treatment over the 12-week treatment period, the baseline number of days with medication overuse was similar across treatment groups (approximately 16.6). Within this population, fremanezumab treatment significantly reduced the number of days of acute headache medication use over the 12-week treatment period by nine in the quarterly arm and 8.9 in the monthly arm, compared with 7.1 among controls.
A reduction in medication overuse is apparent after four weeks of treatment.
A reduction in medication overuse is apparent after four weeks of treatment.
SAN FRANCISCO—Treatment with fremanezumab is associated with reduced overuse of acute medications and a corresponding decrease in days on which a patient uses acute medications, according to a phase III study described at the 60th Annual Scientific Meeting of the American Headache Society.
The overuse of acute or symptomatic headache medications (eg, triptans, ergot derivatives, opioids, and combination analgesics) can cause medication overuse headache (MOH). Chronic migraine is often accompanied by MOH, and the prevention of MOH is one of the main goals in the preventive treatment of migraine.
Fremanezumab, a fully humanized monoclonal antibody that selectively targets calcitonin gene-related peptide, reduced the frequency and severity of headaches in patients with chronic migraine who participated in clinical trials. Stephen D. Silberstein, MD, Director of the Headache Center at Thomas Jefferson University Hospital in Philadelphia, and colleagues assessed the effect of fremanezumab, compared with placebo, on medication overuse and acute headache medication use in patients with chronic migraine.
Comparing Two Fremanezumab Doses With Placebo
The investigators conducted a multicenter, randomized, double-blind, placebo-controlled, phase III study, during which they randomized eligible patients with chronic migraine in equal groups to receive subcutaneous injections of fremanezumab quarterly dosing (ie, 675 mg at baseline and placebo at Weeks 4 and 8), fremanezumab monthly dosing (ie, 675 mg at baseline and 225 mg at Weeks 4 and 8), or placebo at each time point over a 12-week treatment period. Dr. Silberstein’s group defined medication overuse as the use of acute headache medication on 15 or more days, the use of migraine-specific acute medication on 10 or more days, or the use of combination medications for headache on 10 or more days during the 28-day baseline period.
In a post hoc analysis, the researchers assessed the proportion of patients who reverted from overusing medications at baseline to not overusing medications at Week 12, as well as the change from baseline in the number of days of acute headache medication use among these patients. Analyses were performed using data for all randomized patients who received at least one dose of study drug and had at least 10 days of postbaseline efficacy assessments on the primary end point.
Fremanezumab Was More Likely to Reduce Overuse
At baseline, the number of patients with medication overuse was 201 in the quarterly arm, 198 in the monthly arm, and 188 in the placebo arm. Among these participants, significantly more fremanezumab-treated patients reported no medication overuse during the 12-week treatment period. The number of patients reporting no medication overuse was 111 (55%) in the quarterly arm, 120 (61%) in the monthly arm, and 87 (46%) in the placebo arm. The investigators observed a response to treatment as early as Week 4 (102 [51%] quarterly patients, 107 [54%] monthly patients, and 73 [39%] controls).
Among the patients who responded to treatment over the 12-week treatment period, the baseline number of days with medication overuse was similar across treatment groups (approximately 16.6). Within this population, fremanezumab treatment significantly reduced the number of days of acute headache medication use over the 12-week treatment period by nine in the quarterly arm and 8.9 in the monthly arm, compared with 7.1 among controls.
SAN FRANCISCO—Treatment with fremanezumab is associated with reduced overuse of acute medications and a corresponding decrease in days on which a patient uses acute medications, according to a phase III study described at the 60th Annual Scientific Meeting of the American Headache Society.
The overuse of acute or symptomatic headache medications (eg, triptans, ergot derivatives, opioids, and combination analgesics) can cause medication overuse headache (MOH). Chronic migraine is often accompanied by MOH, and the prevention of MOH is one of the main goals in the preventive treatment of migraine.
Fremanezumab, a fully humanized monoclonal antibody that selectively targets calcitonin gene-related peptide, reduced the frequency and severity of headaches in patients with chronic migraine who participated in clinical trials. Stephen D. Silberstein, MD, Director of the Headache Center at Thomas Jefferson University Hospital in Philadelphia, and colleagues assessed the effect of fremanezumab, compared with placebo, on medication overuse and acute headache medication use in patients with chronic migraine.
Comparing Two Fremanezumab Doses With Placebo
The investigators conducted a multicenter, randomized, double-blind, placebo-controlled, phase III study, during which they randomized eligible patients with chronic migraine in equal groups to receive subcutaneous injections of fremanezumab quarterly dosing (ie, 675 mg at baseline and placebo at Weeks 4 and 8), fremanezumab monthly dosing (ie, 675 mg at baseline and 225 mg at Weeks 4 and 8), or placebo at each time point over a 12-week treatment period. Dr. Silberstein’s group defined medication overuse as the use of acute headache medication on 15 or more days, the use of migraine-specific acute medication on 10 or more days, or the use of combination medications for headache on 10 or more days during the 28-day baseline period.
In a post hoc analysis, the researchers assessed the proportion of patients who reverted from overusing medications at baseline to not overusing medications at Week 12, as well as the change from baseline in the number of days of acute headache medication use among these patients. Analyses were performed using data for all randomized patients who received at least one dose of study drug and had at least 10 days of postbaseline efficacy assessments on the primary end point.
Fremanezumab Was More Likely to Reduce Overuse
At baseline, the number of patients with medication overuse was 201 in the quarterly arm, 198 in the monthly arm, and 188 in the placebo arm. Among these participants, significantly more fremanezumab-treated patients reported no medication overuse during the 12-week treatment period. The number of patients reporting no medication overuse was 111 (55%) in the quarterly arm, 120 (61%) in the monthly arm, and 87 (46%) in the placebo arm. The investigators observed a response to treatment as early as Week 4 (102 [51%] quarterly patients, 107 [54%] monthly patients, and 73 [39%] controls).
Among the patients who responded to treatment over the 12-week treatment period, the baseline number of days with medication overuse was similar across treatment groups (approximately 16.6). Within this population, fremanezumab treatment significantly reduced the number of days of acute headache medication use over the 12-week treatment period by nine in the quarterly arm and 8.9 in the monthly arm, compared with 7.1 among controls.
Obesity: When to consider medication
Modest weight loss of 5% to 10% among patients who are overweight or obese can result in a clinically relevant reduction in cardiovascular (CV) disease risk.1 This amount of weight loss can increase insulin sensitivity in adipose tissue, liver, and muscle, and have a positive impact on blood sugar, blood pressure, triglycerides, and high-density lipoprotein cholesterol.1,2
All patients who are obese or overweight with increased CV risk should be counseled on diet, exercise, and other behavioral interventions.3 Weight loss secondary to lifestyle modification alone, however, leads to adaptive physiologic responses, which increase appetite and reduce energy expenditure.4–6
Pharmacotherapy can counteract this metabolic adaptation and lead to sustained weight loss. Antiobesity medication can be considered if a patient has a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with obesity-related comorbidities such as hypertension, type 2 diabetes, dyslipidemia, or obstructive sleep apnea.3,7
For patients with a body mass index (BMI) ≥30 kg/m2 or BMI ≥27 kg/m2 with weight-related comorbidities:
- Consider antiobesity pharmacotherapy when diet, exercise, and behavior modification do not produce sufficient weight loss. A
- Continue an antiobesity medication if it is deemed effective and well tolerated.A
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Until recently, there were few pharmacologic options approved by the US Food and Drug Administration (FDA) for the management of obesity. The mainstays of treatment were phentermine (Adipex-P, Ionamin, Suprenza) and orlistat (Alli, Xenical). Since 2012, however, 4 agents have been approved as adjuncts to a reduced-calorie diet and increased physical activity for long-term weight management.8,9 Phentermine/topiramate extended-release (ER) (Qsymia) and lorcaserin (Belviq) were approved in 2012,10,11 and naltrexone sustained release (SR)/bupropion SR (Contrave) and liraglutide 3 mg (Saxenda) were approved in 201412,13 (TABLE9,14–39). These medications have the potential to not only limit weight gain but also promote weight loss and, thus, improve blood pressure, cholesterol, glucose, and insulin.40
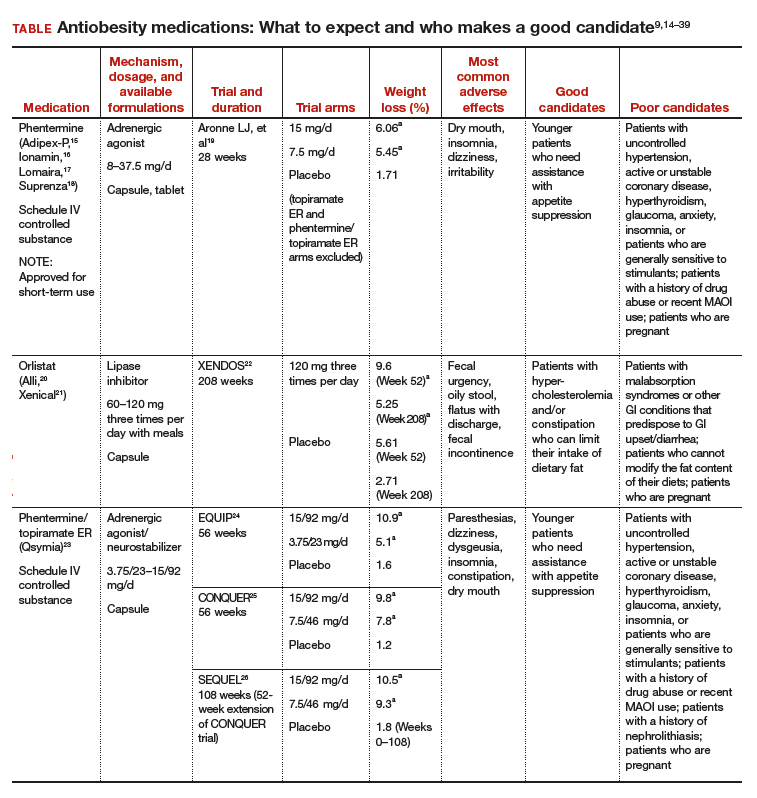
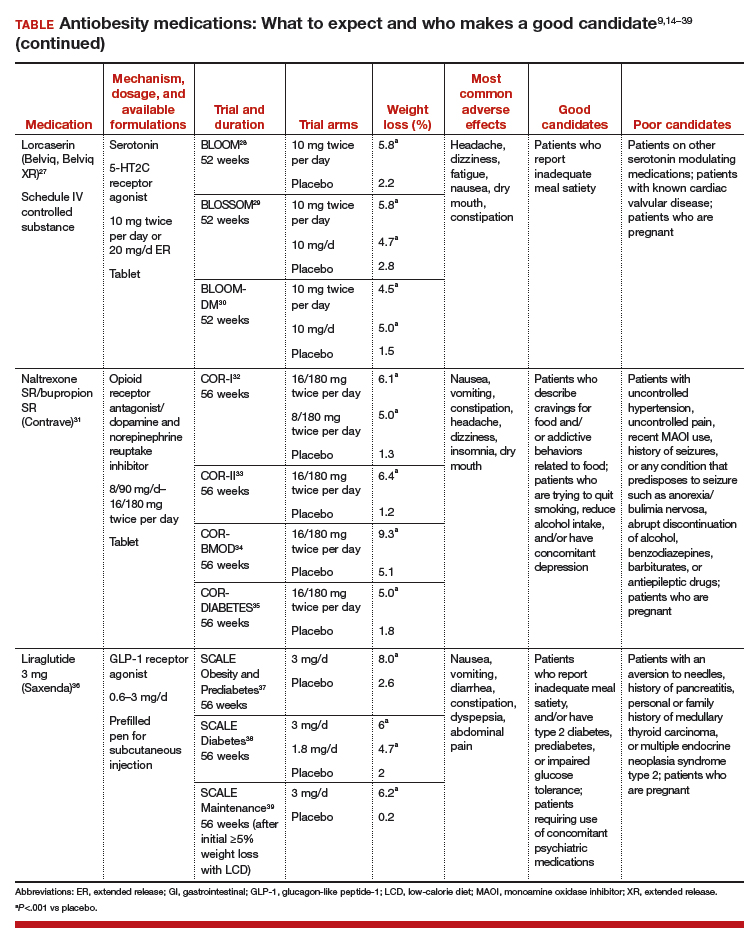
Despite the growing obesity epidemic and the availability of several additional medications for chronic weight management, use of antiobesity pharmacotherapy has been limited. Barriers to use include inadequate training of health care professionals, poor insurance coverage for new agents, and low reimbursement for office visits to address weight.41
In addition, the number of obesity medicine specialists, while increasing, is still not sufficient. Therefore, it is imperative for other health care professionals—including ObGyns—to be aware of the treatment options available to patients who are overweight or obese and to be adept at using them.
In this review, we present 4 cases that depict patients who could benefit from the addition of antiobesity pharmacotherapy to a comprehen‑sive treatment plan that includes diet, physical activity, and behavioral modification.
CASE 1 Young obese woman is unable to lose weight
A 27-year-old woman with obesity (BMI 33 kg/m2),hyperlipidemia, and migraine headaches, pre‑sents for weight management. Despite a calorie-reduced diet and 200 minutes per week of exercise for the past 6 months, she has been unable to lose weight. The only medications she is taking are oral contraceptive pills and sumatriptan, as needed. She suffers from migraines 3 times a month and has no anxiety. Laboratory test results are normal with the exception of an elevated low-density lipoprotein (LDL) level.
Which medication is an appropriate next step for this patient?
Ask 2 important questions
When considering an antiobesity agent for any patient, there are 2 important questions to ask:
- Are there contraindications, drug-drug interactions, or undesirable adverse effects associated with this medication that could be problematic for the patient?
- Can this medication improve other symptoms or conditions the patient has?
In addition, see “Before prescribing antiobesity medication . . .” below.
Have a frank discussion with the patient and be sure to cover the following points:
The rationale for pharmacologic treatment is to counteract adaptive physiologic responses, which increase appetite and reduce energy expenditure, in response to diet-induced weight loss.
Antiobesity medication is only one component of a comprehensive treatment plan, which also includes diet, physical activity, and behavior modification.
Antiobesity agents are intended for long-term use, as obesity is a chronic disease. If/when you stop the medication, there may be some weight regain, similar to an increase in blood pressure after discontinuing an antihypertensive agent.
Because antiobesity medications improve many parameters including glucose/hemoglobin A1c, lipids, blood pressure, and waist circumference, it is possible that the addition of one antiobesity medication can reduce, or even eliminate, the need for several other medications.
Remember that many patients who present for obesity management have experienced weight bias. It is important to not be judgmental, but rather explain why obesity is a chronic disease. If patients understand the physiology of their condition, they will understand that their limited success with weight loss in the past is not just a matter of willpower. Lifestyle change and weight loss are extremely difficult, so it is important to provide encouragement and support for ongoing behavioral modification.
Phentermine/topiramate ER
Phentermine/topiramate ER is a good first choice for this young patient with class I (BMI 30–34.9 kg/m2) obesity and migraines, as she can likely tolerate a stimulant and her migraines might improve with topiramate. Before starting the medication, ask about insomnia and nephrolithiasis in addition to anxiety and other contraindications (ie, glaucoma, hyperthyroidism, recent monoamine oxidase inhibitor use, or a known hypersensitivity or idiosyncrasy to sympathomimetic amines).23 The most common adverse events reported in phase III trials were dry mouth, paresthesia, and constipation.24–26
Not for pregnant women. Women of childbearing age must have a negative pregnancy test before starting phentermine/topiramate ER and every month while taking the medication. The FDA requires a Risk Evaluation and Mitigation Strategy (REMS) to inform prescribers and patients about the increased risk of congenital malformation, specifically orofacial clefts, in infants exposed to topiramate during the first trimester of pregnancy.42 REMS focuses on the importance of pregnancy prevention, the consistent use of birth control, and the need to discontinue phentermine/topiramate ER immediately if pregnancy occurs.
Flexible dosing. Phentermine/topiramate ER is available in 4 dosages: phentermine 3.75 mg/topiramate 23 mg ER; phentermine 7.5 mg/topiramate 46 mg ER; phentermine 11.25 mg/topiramate 69 mg ER; and phentermine 15 mg/topiramate 92 mg ER. Gradual dose escalation minimizes risks and adverse events.23
Monitor patients frequently to evaluate for adverse effects and ensure adherence to diet, exercise, and lifestyle modifications. If weight loss is slower or less robust than expected, check for dietary indiscretion, as medications have limited efficacy without appropriate behavioral changes.
Discontinue phentermine/topiramate ER if the patient does not achieve 5% weight loss after 12 weeks on the maximum dose, as it is unlikely that she will achieve and sustain clinically meaningful weight loss with continued treatment.23 In this case, consider another agent with a different mechanism of action. Any of the other antiobesity medications could be appropriate for this patient.
CASE 2 Overweight woman with comorbidities
A 52-year-old overweight woman (BMI 29 kg/m2)with type 2 diabetes, hyperlipidemia, osteoarthritis, and glaucoma has recently hit a plateau with her weight loss. She lost 45 lb secondary to diet and exercise, but hasn’t been able to lose any more. She also struggles with constant hunger. Her medications include metformin 1,000 mg twice per day, atorvastatin 10 mg/d, and occasional acetaminophen/oxycodone for knee pain until she undergoes a left knee replacement. Laboratory values are normal except for a hemoglobin A1c of 7.2%.
The patient is afraid of needles and cannot tolerate stimulants due to anxiety. Which medication is an appropriate next step for this patient?
What are good choices for this patient?
Lorcaserin is a good choice for this patient who is overweight and has several weight-related comorbidities. She has worked hard to lose a significant number of pounds and is now at high risk of regaining them. That’s because her appetite has increased with her new exercise regimen, but her energy expenditure has decreased secondary to metabolic adaptation.
Narrowing the field. Naltrexone SR/bupropion SR cannot be used because of her opioid use. Phentermine/topiramate ER is contraindicated for patients with glaucoma, and liraglutide 3 mg is not appropriate given the patient’s fear of needles.
She could try orlistat, especially if she struggles with constipation, but the gastrointestinal adverse effects are difficult for many patients to tolerate. While not an antiobesity medication, a sodium-glucose co-transporter 2 (SGLT2) inhibitor could be prescribed for her diabetes and also may promote weight loss.43
An appealing choice. The glucose-lowering effect of lorcaserin could provide an added benefit for the patient. The BLOOMDM (Behavioral modification and lorcaserin for overweight and obesity management in diabetes mellitus) study reported a mean reduction in hemoglobin A1c of 0.9% in the treatment group compared with a 0.4% reduction in the placebo group,30 and the effect of lorcaserin on A1c appeared to be independent of weight loss.
Mechanism of action: Cause for concern? Although lorcaserin selectively binds to serotonin 5-HT2C receptors, the theoretical risk of cardiac valvulopathy was evaluated in phase III studies, as fenfluramine, a 5-HT2B-receptor agonist, was withdrawn from the US market in 1997 for this reason.44 Both the BLOOM (Behavioral modification and lorcaserin for overweight and obesity management) and BLOSSOM (Behavioral modification and lorcaserin second study for obesity management) studies found that lorcaserin did not increase the incidence of FDA-defined cardiac valvulopathy.28,29
Formulations/adverse effects. Lorcaserin is available in 2 formulations: 10-mg tablets, which are taken twice daily, or 20-mg XR tablets, which are taken once daily. Both are generally well tolerated.27,45 The most common adverse event reported in phase III trials was headache.28,30,43 Discontinue lorcaserin if the patient does not lose 5% of her initial weight after 12 weeks, as weight loss at this stage is a good predictor of longer-term success.46
Some patients don’t respond. Interestingly, a subset of patients do not respond to lorcaserin. The most likely explanation for different responses to the medication is that there are many causes of obesity, only some of which respond to 5-HT2C agonism. Currently, we do not perform pharmacogenomics testing before prescribing lorcaserin, but perhaps an inexpensive test to identify responders will be available in the future.
CASE 3 A preoccupation with food
A 38-year-old woman with obesity (BMI 42 kg/m2),obstructive sleep apnea, gastroesophageal reflux disease, and depression is eager to get better control over her weight. Her medications include lansoprazole 30 mg/d and a multivitamin. She reports constantly thinking about food and not being able to control her impulses to buy large quantities of unhealthy snacks. She is so preoccupied by thoughts of food that she has difficulty concentrating at work.
The patient smokes a quarter of a pack of cigarettes daily, but she is ready to quit. She views bariatric surgery as a “last resort” and has no anxiety, pain, or history of seizures. Which medication is appropriate for this patient?
Discuss all options
This patient with class III obesity (BMI ≥40 kg/m2) is eligible for bariatric surgery; however, she is not interested in pursuing it at this time. It is important to discuss all of her options before deciding on a treatment plan. For patients like this, who would benefit from more than modest weight loss, consider a multidisciplinary approach including lifestyle modifications, pharmacotherapy, devices (eg, an intragastric balloon), and/or surgery. You would need to make clear to the patient that she may still be eligible for insurance coverage for surgery if she changes her mind after pursuing other treatments as long as her BMI remains ≥35 kg/m2 with obesity-related comorbidities.
Naltrexone SR/bupropion SR is a good choice for this patient because she describes debilitating cravings and addictive behavior surrounding food. Patients taking naltrexone SR/bupropion SR in the Contrave Obesity Research (COR)-I and COR-II phase III trials experienced a reduced frequency of food cravings, reduced difficulty in resisting food cravings, and an increased ability to control eating compared with those assigned to placebo.32,33
Added benefits. Bupropion also could help this patient quit smoking and improve her mood, as it is FDA-approved for smoking cessation and depression. She denies anxiety and seizures, so bupropion is not contraindicated. Even if a patient denies a history of seizure, ask about any conditions that predispose to seizures, such as anorexia nervosa or bulimia or the abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs.
Opioid use. Although the patient denies pain, ask about potential opioid use, as naltrexone is an opioid receptor antagonist. Patients should be informed that opioids may be ineffective if they are required unexpectedly (eg, for trauma) and that naltrexone SR/bupropion SR should be withheld for any planned surgical procedure potentially requiring opioid use.
Other options. While naltrexone SR/bupropion SR is the most appropriate choice for this patient because it addresses her problematic eating behaviors while potentially improving mood and assisting with smoking cessation, phentermine/topiramate ER, lorcaserin, and liraglutide 3 mg also could be used and certainly should be tried if naltrexone SR/bupropion SR does not produce the desired weight loss.
Adverse effects. Titrate naltrexone SR/bupropion SR slowly to the treatment dose to minimize risks and adverse events.31 The most common adverse effects reported in phase III trials were nausea, constipation, and headache.34,35,45,46 Discontinue naltrexone SR/bupropion SR if the patient does not achieve 5% weight loss at 16 weeks (after 12 weeks at the maintenance dose).31
CASE 4 Regaining weight after gastric bypass
A 65-year-old woman with obesity (BMI 39 kg/m2)who underwent Roux-en-Y gastric bypass surgery and who has type 2 diabetes, congestive heart failure, coronary artery disease, hypertension, and hyperlipidemia, remains concerned about her weight. She lost 100 lb following surgery and maintained her weight for 3 years, but then regained 30 lb. She comes in for an office visit because she is concerned about her increasing blood sugar and wants to prevent further weight gain. Her medications include metformin 1,000 mg twice per day, lisinopril 5 mg/d, carvedilol 12.5 mg twice per day, simvastatin 20 mg/d, and aspirin 81 mg/d. Laboratory test results are normal except for a hemoglobin A1c of 8%. She denies pancreatitis and a personal or family history of thyroid cancer.
Which medication is an appropriate next step for this patient?
Pharmacotherapy is an option
Pharmacotherapy is a great option for this patient, who is regaining weight following bariatric surgery. Phentermine/topiramate ER is the only medication that would be contraindicated because of her heart disease. Lorcaserin and naltrexone SR/bupropion SR could be considered, but liraglutide 3 mg is the most appropriate option, given her need for further glucose control.
Furthermore, the recent LEADER (Liraglutide effect and action in diabetes: evaluation of CV outcome results) trial reported a significant mortality benefit with liraglutide 1.8 mg/d among patients with type 2 diabetes and high CV risk.47 The study found that liraglutide was superior to placebo in reducing CV events.
Contraindications. Ask patients about a history of pancreatitis before starting liraglutide 3 mg, given the possible increased risk. In addition, liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2. Thyroid C-cell tumors have been found in rodents given supratherapeutic doses of liraglutide48; however, there is no evidence of liraglutide causing C-cell tumors in humans.
For patients taking a medication that can cause hypoglycemia, such as insulin or a sulfonylurea, monitor blood sugar and consider reducing the dose of that medication when starting liraglutide.
Administration and titration. Liraglutide is injected subcutaneously once daily. The dose is titrated up weekly to reduce gastrointestinal symptoms.36 The most common adverse effects reported in phase III trials were nausea, diarrhea, and constipation.37–39Discontinue liraglutide 3 mg if the patient does not lose at least 4% of baseline body weight after 16 weeks.49
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
- Magkos F, Fraterrigo G, Yoshino J. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 pt B):2985-3023.
- Sumithran P, Predergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604.
- Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196.
- Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring). 2016;24:1612-1619.
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
- Saunders KH, Shukla AP, Igel LI, et al. Pharmacotherapy for obesity. Endocrinol Metab Clin North Am. 2016;45:521-538.
- Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
- US Food and Drug Administration. Drug approval package. Qsymia. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000_qsymia_toc.cfm. Accessed August 28, 2017.
- Arena Pharmaceuticals. Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQR (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182. Accessed August 28, 2017.
- Drugs.com. Contrave approval history. https://www.drugs.com/history/contrave.html. Accessed August 28, 2017.
- US Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206321. Accessed August 28, 2017.
- Igel LI, Kumar RB, Saunders KH, et al. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152:1765-1779.
- Adipex-P package insert. http://www.iodine.com/drug/phentermine/fda-package-insert. Accessed August 28, 2017.
- Ionamin package insert. http://druginserts.com/lib/rx/meds/ionamin/. Accessed August 28, 2017.
- Lomaira package insert. https://www.lomaira.com/Prescribing_Information.pdf. Accessed August 28, 2017.
- Suprenza package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202088s001lbl.pdf. Accessed August 28, 2017.
- Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended release in obese adults. Obesity (Silver Spring). 2013;21:2163-2171.
- Alli package labeling. http://druginserts.com/lib/otc/meds/alli-1/. Accessed August 28, 2017.
- Xenical package insert. https://www.gene.com/download/pdf/xenical_prescribing.pdf. Accessed August 28,2017.
- Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161.
- Qsymia package insert. https://www.qsymia.com/pdf/prescribing-information.pdf. Accessed August 28, 2017.
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352.
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308.
- Belviq package insert. https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/Belviq_Prescribing_information-pdf.PDF?la=en. Accessed August 28, 2017.
- Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256.
- Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067-3077.
- O'Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20:1426-1436.
- Contrave package insert. https://contrave.com/wpcontent/uploads/2017/05/Contrave_PI.pdf. Accessed August 28, 2017.
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595-605.
- Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935-943.
- Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110-120.
- Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022-4029.
- Saxenda package insert. http://www.novo-pi.com/saxenda.pdf. Accessed August 28, 2017.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015;314:687-699.
- Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451.
- Saunders KH, Igel LI, Aronne LJ. An update on naltrexone/bupropion extended-release in the treatment of obesity. Expert Opin Pharmacother. 2016. 10.1080/14656566.2016.1244527.
- Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring). 2016;24:1955-1961.
- Qsymia Risk Evaluation and Mitigation Strategy (REMS). VIVUS, Inc. http://www.qsymiarems.com. Accessed January 16, 2017.
- Zinman B, Wanner C, Lachin JM, et al. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
- US Food and Drug Administration. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafety-InformationforPatientsandProviders/ucm179871.htm. Accessed August 28, 2017.
- Belviq XR package insert. https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/belviqxr_prescribing_information-pdf.PDF?la=en. Accessed January 16, 2017.
- Smith SR, O'Neil PM, Astrup A. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity (Silver Spring). 2014;22:2137-2146.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
- Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012;153:1538-1547.
- Fujioka K, O'Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24:2278-2288.

Modest weight loss of 5% to 10% among patients who are overweight or obese can result in a clinically relevant reduction in cardiovascular (CV) disease risk.1 This amount of weight loss can increase insulin sensitivity in adipose tissue, liver, and muscle, and have a positive impact on blood sugar, blood pressure, triglycerides, and high-density lipoprotein cholesterol.1,2
All patients who are obese or overweight with increased CV risk should be counseled on diet, exercise, and other behavioral interventions.3 Weight loss secondary to lifestyle modification alone, however, leads to adaptive physiologic responses, which increase appetite and reduce energy expenditure.4–6
Pharmacotherapy can counteract this metabolic adaptation and lead to sustained weight loss. Antiobesity medication can be considered if a patient has a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with obesity-related comorbidities such as hypertension, type 2 diabetes, dyslipidemia, or obstructive sleep apnea.3,7
For patients with a body mass index (BMI) ≥30 kg/m2 or BMI ≥27 kg/m2 with weight-related comorbidities:
- Consider antiobesity pharmacotherapy when diet, exercise, and behavior modification do not produce sufficient weight loss. A
- Continue an antiobesity medication if it is deemed effective and well tolerated.A
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Until recently, there were few pharmacologic options approved by the US Food and Drug Administration (FDA) for the management of obesity. The mainstays of treatment were phentermine (Adipex-P, Ionamin, Suprenza) and orlistat (Alli, Xenical). Since 2012, however, 4 agents have been approved as adjuncts to a reduced-calorie diet and increased physical activity for long-term weight management.8,9 Phentermine/topiramate extended-release (ER) (Qsymia) and lorcaserin (Belviq) were approved in 2012,10,11 and naltrexone sustained release (SR)/bupropion SR (Contrave) and liraglutide 3 mg (Saxenda) were approved in 201412,13 (TABLE9,14–39). These medications have the potential to not only limit weight gain but also promote weight loss and, thus, improve blood pressure, cholesterol, glucose, and insulin.40


Despite the growing obesity epidemic and the availability of several additional medications for chronic weight management, use of antiobesity pharmacotherapy has been limited. Barriers to use include inadequate training of health care professionals, poor insurance coverage for new agents, and low reimbursement for office visits to address weight.41
In addition, the number of obesity medicine specialists, while increasing, is still not sufficient. Therefore, it is imperative for other health care professionals—including ObGyns—to be aware of the treatment options available to patients who are overweight or obese and to be adept at using them.
In this review, we present 4 cases that depict patients who could benefit from the addition of antiobesity pharmacotherapy to a comprehen‑sive treatment plan that includes diet, physical activity, and behavioral modification.
CASE 1 Young obese woman is unable to lose weight
A 27-year-old woman with obesity (BMI 33 kg/m2),hyperlipidemia, and migraine headaches, pre‑sents for weight management. Despite a calorie-reduced diet and 200 minutes per week of exercise for the past 6 months, she has been unable to lose weight. The only medications she is taking are oral contraceptive pills and sumatriptan, as needed. She suffers from migraines 3 times a month and has no anxiety. Laboratory test results are normal with the exception of an elevated low-density lipoprotein (LDL) level.
Which medication is an appropriate next step for this patient?
Ask 2 important questions
When considering an antiobesity agent for any patient, there are 2 important questions to ask:
- Are there contraindications, drug-drug interactions, or undesirable adverse effects associated with this medication that could be problematic for the patient?
- Can this medication improve other symptoms or conditions the patient has?
In addition, see “Before prescribing antiobesity medication . . .” below.
Have a frank discussion with the patient and be sure to cover the following points:
The rationale for pharmacologic treatment is to counteract adaptive physiologic responses, which increase appetite and reduce energy expenditure, in response to diet-induced weight loss.
Antiobesity medication is only one component of a comprehensive treatment plan, which also includes diet, physical activity, and behavior modification.
Antiobesity agents are intended for long-term use, as obesity is a chronic disease. If/when you stop the medication, there may be some weight regain, similar to an increase in blood pressure after discontinuing an antihypertensive agent.
Because antiobesity medications improve many parameters including glucose/hemoglobin A1c, lipids, blood pressure, and waist circumference, it is possible that the addition of one antiobesity medication can reduce, or even eliminate, the need for several other medications.
Remember that many patients who present for obesity management have experienced weight bias. It is important to not be judgmental, but rather explain why obesity is a chronic disease. If patients understand the physiology of their condition, they will understand that their limited success with weight loss in the past is not just a matter of willpower. Lifestyle change and weight loss are extremely difficult, so it is important to provide encouragement and support for ongoing behavioral modification.
Phentermine/topiramate ER
Phentermine/topiramate ER is a good first choice for this young patient with class I (BMI 30–34.9 kg/m2) obesity and migraines, as she can likely tolerate a stimulant and her migraines might improve with topiramate. Before starting the medication, ask about insomnia and nephrolithiasis in addition to anxiety and other contraindications (ie, glaucoma, hyperthyroidism, recent monoamine oxidase inhibitor use, or a known hypersensitivity or idiosyncrasy to sympathomimetic amines).23 The most common adverse events reported in phase III trials were dry mouth, paresthesia, and constipation.24–26
Not for pregnant women. Women of childbearing age must have a negative pregnancy test before starting phentermine/topiramate ER and every month while taking the medication. The FDA requires a Risk Evaluation and Mitigation Strategy (REMS) to inform prescribers and patients about the increased risk of congenital malformation, specifically orofacial clefts, in infants exposed to topiramate during the first trimester of pregnancy.42 REMS focuses on the importance of pregnancy prevention, the consistent use of birth control, and the need to discontinue phentermine/topiramate ER immediately if pregnancy occurs.
Flexible dosing. Phentermine/topiramate ER is available in 4 dosages: phentermine 3.75 mg/topiramate 23 mg ER; phentermine 7.5 mg/topiramate 46 mg ER; phentermine 11.25 mg/topiramate 69 mg ER; and phentermine 15 mg/topiramate 92 mg ER. Gradual dose escalation minimizes risks and adverse events.23
Monitor patients frequently to evaluate for adverse effects and ensure adherence to diet, exercise, and lifestyle modifications. If weight loss is slower or less robust than expected, check for dietary indiscretion, as medications have limited efficacy without appropriate behavioral changes.
Discontinue phentermine/topiramate ER if the patient does not achieve 5% weight loss after 12 weeks on the maximum dose, as it is unlikely that she will achieve and sustain clinically meaningful weight loss with continued treatment.23 In this case, consider another agent with a different mechanism of action. Any of the other antiobesity medications could be appropriate for this patient.
CASE 2 Overweight woman with comorbidities
A 52-year-old overweight woman (BMI 29 kg/m2)with type 2 diabetes, hyperlipidemia, osteoarthritis, and glaucoma has recently hit a plateau with her weight loss. She lost 45 lb secondary to diet and exercise, but hasn’t been able to lose any more. She also struggles with constant hunger. Her medications include metformin 1,000 mg twice per day, atorvastatin 10 mg/d, and occasional acetaminophen/oxycodone for knee pain until she undergoes a left knee replacement. Laboratory values are normal except for a hemoglobin A1c of 7.2%.
The patient is afraid of needles and cannot tolerate stimulants due to anxiety. Which medication is an appropriate next step for this patient?
What are good choices for this patient?
Lorcaserin is a good choice for this patient who is overweight and has several weight-related comorbidities. She has worked hard to lose a significant number of pounds and is now at high risk of regaining them. That’s because her appetite has increased with her new exercise regimen, but her energy expenditure has decreased secondary to metabolic adaptation.
Narrowing the field. Naltrexone SR/bupropion SR cannot be used because of her opioid use. Phentermine/topiramate ER is contraindicated for patients with glaucoma, and liraglutide 3 mg is not appropriate given the patient’s fear of needles.
She could try orlistat, especially if she struggles with constipation, but the gastrointestinal adverse effects are difficult for many patients to tolerate. While not an antiobesity medication, a sodium-glucose co-transporter 2 (SGLT2) inhibitor could be prescribed for her diabetes and also may promote weight loss.43
An appealing choice. The glucose-lowering effect of lorcaserin could provide an added benefit for the patient. The BLOOMDM (Behavioral modification and lorcaserin for overweight and obesity management in diabetes mellitus) study reported a mean reduction in hemoglobin A1c of 0.9% in the treatment group compared with a 0.4% reduction in the placebo group,30 and the effect of lorcaserin on A1c appeared to be independent of weight loss.
Mechanism of action: Cause for concern? Although lorcaserin selectively binds to serotonin 5-HT2C receptors, the theoretical risk of cardiac valvulopathy was evaluated in phase III studies, as fenfluramine, a 5-HT2B-receptor agonist, was withdrawn from the US market in 1997 for this reason.44 Both the BLOOM (Behavioral modification and lorcaserin for overweight and obesity management) and BLOSSOM (Behavioral modification and lorcaserin second study for obesity management) studies found that lorcaserin did not increase the incidence of FDA-defined cardiac valvulopathy.28,29
Formulations/adverse effects. Lorcaserin is available in 2 formulations: 10-mg tablets, which are taken twice daily, or 20-mg XR tablets, which are taken once daily. Both are generally well tolerated.27,45 The most common adverse event reported in phase III trials was headache.28,30,43 Discontinue lorcaserin if the patient does not lose 5% of her initial weight after 12 weeks, as weight loss at this stage is a good predictor of longer-term success.46
Some patients don’t respond. Interestingly, a subset of patients do not respond to lorcaserin. The most likely explanation for different responses to the medication is that there are many causes of obesity, only some of which respond to 5-HT2C agonism. Currently, we do not perform pharmacogenomics testing before prescribing lorcaserin, but perhaps an inexpensive test to identify responders will be available in the future.
CASE 3 A preoccupation with food
A 38-year-old woman with obesity (BMI 42 kg/m2),obstructive sleep apnea, gastroesophageal reflux disease, and depression is eager to get better control over her weight. Her medications include lansoprazole 30 mg/d and a multivitamin. She reports constantly thinking about food and not being able to control her impulses to buy large quantities of unhealthy snacks. She is so preoccupied by thoughts of food that she has difficulty concentrating at work.
The patient smokes a quarter of a pack of cigarettes daily, but she is ready to quit. She views bariatric surgery as a “last resort” and has no anxiety, pain, or history of seizures. Which medication is appropriate for this patient?
Discuss all options
This patient with class III obesity (BMI ≥40 kg/m2) is eligible for bariatric surgery; however, she is not interested in pursuing it at this time. It is important to discuss all of her options before deciding on a treatment plan. For patients like this, who would benefit from more than modest weight loss, consider a multidisciplinary approach including lifestyle modifications, pharmacotherapy, devices (eg, an intragastric balloon), and/or surgery. You would need to make clear to the patient that she may still be eligible for insurance coverage for surgery if she changes her mind after pursuing other treatments as long as her BMI remains ≥35 kg/m2 with obesity-related comorbidities.
Naltrexone SR/bupropion SR is a good choice for this patient because she describes debilitating cravings and addictive behavior surrounding food. Patients taking naltrexone SR/bupropion SR in the Contrave Obesity Research (COR)-I and COR-II phase III trials experienced a reduced frequency of food cravings, reduced difficulty in resisting food cravings, and an increased ability to control eating compared with those assigned to placebo.32,33
Added benefits. Bupropion also could help this patient quit smoking and improve her mood, as it is FDA-approved for smoking cessation and depression. She denies anxiety and seizures, so bupropion is not contraindicated. Even if a patient denies a history of seizure, ask about any conditions that predispose to seizures, such as anorexia nervosa or bulimia or the abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs.
Opioid use. Although the patient denies pain, ask about potential opioid use, as naltrexone is an opioid receptor antagonist. Patients should be informed that opioids may be ineffective if they are required unexpectedly (eg, for trauma) and that naltrexone SR/bupropion SR should be withheld for any planned surgical procedure potentially requiring opioid use.
Other options. While naltrexone SR/bupropion SR is the most appropriate choice for this patient because it addresses her problematic eating behaviors while potentially improving mood and assisting with smoking cessation, phentermine/topiramate ER, lorcaserin, and liraglutide 3 mg also could be used and certainly should be tried if naltrexone SR/bupropion SR does not produce the desired weight loss.
Adverse effects. Titrate naltrexone SR/bupropion SR slowly to the treatment dose to minimize risks and adverse events.31 The most common adverse effects reported in phase III trials were nausea, constipation, and headache.34,35,45,46 Discontinue naltrexone SR/bupropion SR if the patient does not achieve 5% weight loss at 16 weeks (after 12 weeks at the maintenance dose).31
CASE 4 Regaining weight after gastric bypass
A 65-year-old woman with obesity (BMI 39 kg/m2)who underwent Roux-en-Y gastric bypass surgery and who has type 2 diabetes, congestive heart failure, coronary artery disease, hypertension, and hyperlipidemia, remains concerned about her weight. She lost 100 lb following surgery and maintained her weight for 3 years, but then regained 30 lb. She comes in for an office visit because she is concerned about her increasing blood sugar and wants to prevent further weight gain. Her medications include metformin 1,000 mg twice per day, lisinopril 5 mg/d, carvedilol 12.5 mg twice per day, simvastatin 20 mg/d, and aspirin 81 mg/d. Laboratory test results are normal except for a hemoglobin A1c of 8%. She denies pancreatitis and a personal or family history of thyroid cancer.
Which medication is an appropriate next step for this patient?
Pharmacotherapy is an option
Pharmacotherapy is a great option for this patient, who is regaining weight following bariatric surgery. Phentermine/topiramate ER is the only medication that would be contraindicated because of her heart disease. Lorcaserin and naltrexone SR/bupropion SR could be considered, but liraglutide 3 mg is the most appropriate option, given her need for further glucose control.
Furthermore, the recent LEADER (Liraglutide effect and action in diabetes: evaluation of CV outcome results) trial reported a significant mortality benefit with liraglutide 1.8 mg/d among patients with type 2 diabetes and high CV risk.47 The study found that liraglutide was superior to placebo in reducing CV events.
Contraindications. Ask patients about a history of pancreatitis before starting liraglutide 3 mg, given the possible increased risk. In addition, liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2. Thyroid C-cell tumors have been found in rodents given supratherapeutic doses of liraglutide48; however, there is no evidence of liraglutide causing C-cell tumors in humans.
For patients taking a medication that can cause hypoglycemia, such as insulin or a sulfonylurea, monitor blood sugar and consider reducing the dose of that medication when starting liraglutide.
Administration and titration. Liraglutide is injected subcutaneously once daily. The dose is titrated up weekly to reduce gastrointestinal symptoms.36 The most common adverse effects reported in phase III trials were nausea, diarrhea, and constipation.37–39Discontinue liraglutide 3 mg if the patient does not lose at least 4% of baseline body weight after 16 weeks.49
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Modest weight loss of 5% to 10% among patients who are overweight or obese can result in a clinically relevant reduction in cardiovascular (CV) disease risk.1 This amount of weight loss can increase insulin sensitivity in adipose tissue, liver, and muscle, and have a positive impact on blood sugar, blood pressure, triglycerides, and high-density lipoprotein cholesterol.1,2
All patients who are obese or overweight with increased CV risk should be counseled on diet, exercise, and other behavioral interventions.3 Weight loss secondary to lifestyle modification alone, however, leads to adaptive physiologic responses, which increase appetite and reduce energy expenditure.4–6
Pharmacotherapy can counteract this metabolic adaptation and lead to sustained weight loss. Antiobesity medication can be considered if a patient has a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with obesity-related comorbidities such as hypertension, type 2 diabetes, dyslipidemia, or obstructive sleep apnea.3,7
For patients with a body mass index (BMI) ≥30 kg/m2 or BMI ≥27 kg/m2 with weight-related comorbidities:
- Consider antiobesity pharmacotherapy when diet, exercise, and behavior modification do not produce sufficient weight loss. A
- Continue an antiobesity medication if it is deemed effective and well tolerated.A
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Until recently, there were few pharmacologic options approved by the US Food and Drug Administration (FDA) for the management of obesity. The mainstays of treatment were phentermine (Adipex-P, Ionamin, Suprenza) and orlistat (Alli, Xenical). Since 2012, however, 4 agents have been approved as adjuncts to a reduced-calorie diet and increased physical activity for long-term weight management.8,9 Phentermine/topiramate extended-release (ER) (Qsymia) and lorcaserin (Belviq) were approved in 2012,10,11 and naltrexone sustained release (SR)/bupropion SR (Contrave) and liraglutide 3 mg (Saxenda) were approved in 201412,13 (TABLE9,14–39). These medications have the potential to not only limit weight gain but also promote weight loss and, thus, improve blood pressure, cholesterol, glucose, and insulin.40


Despite the growing obesity epidemic and the availability of several additional medications for chronic weight management, use of antiobesity pharmacotherapy has been limited. Barriers to use include inadequate training of health care professionals, poor insurance coverage for new agents, and low reimbursement for office visits to address weight.41
In addition, the number of obesity medicine specialists, while increasing, is still not sufficient. Therefore, it is imperative for other health care professionals—including ObGyns—to be aware of the treatment options available to patients who are overweight or obese and to be adept at using them.
In this review, we present 4 cases that depict patients who could benefit from the addition of antiobesity pharmacotherapy to a comprehen‑sive treatment plan that includes diet, physical activity, and behavioral modification.
CASE 1 Young obese woman is unable to lose weight
A 27-year-old woman with obesity (BMI 33 kg/m2),hyperlipidemia, and migraine headaches, pre‑sents for weight management. Despite a calorie-reduced diet and 200 minutes per week of exercise for the past 6 months, she has been unable to lose weight. The only medications she is taking are oral contraceptive pills and sumatriptan, as needed. She suffers from migraines 3 times a month and has no anxiety. Laboratory test results are normal with the exception of an elevated low-density lipoprotein (LDL) level.
Which medication is an appropriate next step for this patient?
Ask 2 important questions
When considering an antiobesity agent for any patient, there are 2 important questions to ask:
- Are there contraindications, drug-drug interactions, or undesirable adverse effects associated with this medication that could be problematic for the patient?
- Can this medication improve other symptoms or conditions the patient has?
In addition, see “Before prescribing antiobesity medication . . .” below.
Have a frank discussion with the patient and be sure to cover the following points:
The rationale for pharmacologic treatment is to counteract adaptive physiologic responses, which increase appetite and reduce energy expenditure, in response to diet-induced weight loss.
Antiobesity medication is only one component of a comprehensive treatment plan, which also includes diet, physical activity, and behavior modification.
Antiobesity agents are intended for long-term use, as obesity is a chronic disease. If/when you stop the medication, there may be some weight regain, similar to an increase in blood pressure after discontinuing an antihypertensive agent.
Because antiobesity medications improve many parameters including glucose/hemoglobin A1c, lipids, blood pressure, and waist circumference, it is possible that the addition of one antiobesity medication can reduce, or even eliminate, the need for several other medications.
Remember that many patients who present for obesity management have experienced weight bias. It is important to not be judgmental, but rather explain why obesity is a chronic disease. If patients understand the physiology of their condition, they will understand that their limited success with weight loss in the past is not just a matter of willpower. Lifestyle change and weight loss are extremely difficult, so it is important to provide encouragement and support for ongoing behavioral modification.
Phentermine/topiramate ER
Phentermine/topiramate ER is a good first choice for this young patient with class I (BMI 30–34.9 kg/m2) obesity and migraines, as she can likely tolerate a stimulant and her migraines might improve with topiramate. Before starting the medication, ask about insomnia and nephrolithiasis in addition to anxiety and other contraindications (ie, glaucoma, hyperthyroidism, recent monoamine oxidase inhibitor use, or a known hypersensitivity or idiosyncrasy to sympathomimetic amines).23 The most common adverse events reported in phase III trials were dry mouth, paresthesia, and constipation.24–26
Not for pregnant women. Women of childbearing age must have a negative pregnancy test before starting phentermine/topiramate ER and every month while taking the medication. The FDA requires a Risk Evaluation and Mitigation Strategy (REMS) to inform prescribers and patients about the increased risk of congenital malformation, specifically orofacial clefts, in infants exposed to topiramate during the first trimester of pregnancy.42 REMS focuses on the importance of pregnancy prevention, the consistent use of birth control, and the need to discontinue phentermine/topiramate ER immediately if pregnancy occurs.
Flexible dosing. Phentermine/topiramate ER is available in 4 dosages: phentermine 3.75 mg/topiramate 23 mg ER; phentermine 7.5 mg/topiramate 46 mg ER; phentermine 11.25 mg/topiramate 69 mg ER; and phentermine 15 mg/topiramate 92 mg ER. Gradual dose escalation minimizes risks and adverse events.23
Monitor patients frequently to evaluate for adverse effects and ensure adherence to diet, exercise, and lifestyle modifications. If weight loss is slower or less robust than expected, check for dietary indiscretion, as medications have limited efficacy without appropriate behavioral changes.
Discontinue phentermine/topiramate ER if the patient does not achieve 5% weight loss after 12 weeks on the maximum dose, as it is unlikely that she will achieve and sustain clinically meaningful weight loss with continued treatment.23 In this case, consider another agent with a different mechanism of action. Any of the other antiobesity medications could be appropriate for this patient.
CASE 2 Overweight woman with comorbidities
A 52-year-old overweight woman (BMI 29 kg/m2)with type 2 diabetes, hyperlipidemia, osteoarthritis, and glaucoma has recently hit a plateau with her weight loss. She lost 45 lb secondary to diet and exercise, but hasn’t been able to lose any more. She also struggles with constant hunger. Her medications include metformin 1,000 mg twice per day, atorvastatin 10 mg/d, and occasional acetaminophen/oxycodone for knee pain until she undergoes a left knee replacement. Laboratory values are normal except for a hemoglobin A1c of 7.2%.
The patient is afraid of needles and cannot tolerate stimulants due to anxiety. Which medication is an appropriate next step for this patient?
What are good choices for this patient?
Lorcaserin is a good choice for this patient who is overweight and has several weight-related comorbidities. She has worked hard to lose a significant number of pounds and is now at high risk of regaining them. That’s because her appetite has increased with her new exercise regimen, but her energy expenditure has decreased secondary to metabolic adaptation.
Narrowing the field. Naltrexone SR/bupropion SR cannot be used because of her opioid use. Phentermine/topiramate ER is contraindicated for patients with glaucoma, and liraglutide 3 mg is not appropriate given the patient’s fear of needles.
She could try orlistat, especially if she struggles with constipation, but the gastrointestinal adverse effects are difficult for many patients to tolerate. While not an antiobesity medication, a sodium-glucose co-transporter 2 (SGLT2) inhibitor could be prescribed for her diabetes and also may promote weight loss.43
An appealing choice. The glucose-lowering effect of lorcaserin could provide an added benefit for the patient. The BLOOMDM (Behavioral modification and lorcaserin for overweight and obesity management in diabetes mellitus) study reported a mean reduction in hemoglobin A1c of 0.9% in the treatment group compared with a 0.4% reduction in the placebo group,30 and the effect of lorcaserin on A1c appeared to be independent of weight loss.
Mechanism of action: Cause for concern? Although lorcaserin selectively binds to serotonin 5-HT2C receptors, the theoretical risk of cardiac valvulopathy was evaluated in phase III studies, as fenfluramine, a 5-HT2B-receptor agonist, was withdrawn from the US market in 1997 for this reason.44 Both the BLOOM (Behavioral modification and lorcaserin for overweight and obesity management) and BLOSSOM (Behavioral modification and lorcaserin second study for obesity management) studies found that lorcaserin did not increase the incidence of FDA-defined cardiac valvulopathy.28,29
Formulations/adverse effects. Lorcaserin is available in 2 formulations: 10-mg tablets, which are taken twice daily, or 20-mg XR tablets, which are taken once daily. Both are generally well tolerated.27,45 The most common adverse event reported in phase III trials was headache.28,30,43 Discontinue lorcaserin if the patient does not lose 5% of her initial weight after 12 weeks, as weight loss at this stage is a good predictor of longer-term success.46
Some patients don’t respond. Interestingly, a subset of patients do not respond to lorcaserin. The most likely explanation for different responses to the medication is that there are many causes of obesity, only some of which respond to 5-HT2C agonism. Currently, we do not perform pharmacogenomics testing before prescribing lorcaserin, but perhaps an inexpensive test to identify responders will be available in the future.
CASE 3 A preoccupation with food
A 38-year-old woman with obesity (BMI 42 kg/m2),obstructive sleep apnea, gastroesophageal reflux disease, and depression is eager to get better control over her weight. Her medications include lansoprazole 30 mg/d and a multivitamin. She reports constantly thinking about food and not being able to control her impulses to buy large quantities of unhealthy snacks. She is so preoccupied by thoughts of food that she has difficulty concentrating at work.
The patient smokes a quarter of a pack of cigarettes daily, but she is ready to quit. She views bariatric surgery as a “last resort” and has no anxiety, pain, or history of seizures. Which medication is appropriate for this patient?
Discuss all options
This patient with class III obesity (BMI ≥40 kg/m2) is eligible for bariatric surgery; however, she is not interested in pursuing it at this time. It is important to discuss all of her options before deciding on a treatment plan. For patients like this, who would benefit from more than modest weight loss, consider a multidisciplinary approach including lifestyle modifications, pharmacotherapy, devices (eg, an intragastric balloon), and/or surgery. You would need to make clear to the patient that she may still be eligible for insurance coverage for surgery if she changes her mind after pursuing other treatments as long as her BMI remains ≥35 kg/m2 with obesity-related comorbidities.
Naltrexone SR/bupropion SR is a good choice for this patient because she describes debilitating cravings and addictive behavior surrounding food. Patients taking naltrexone SR/bupropion SR in the Contrave Obesity Research (COR)-I and COR-II phase III trials experienced a reduced frequency of food cravings, reduced difficulty in resisting food cravings, and an increased ability to control eating compared with those assigned to placebo.32,33
Added benefits. Bupropion also could help this patient quit smoking and improve her mood, as it is FDA-approved for smoking cessation and depression. She denies anxiety and seizures, so bupropion is not contraindicated. Even if a patient denies a history of seizure, ask about any conditions that predispose to seizures, such as anorexia nervosa or bulimia or the abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs.
Opioid use. Although the patient denies pain, ask about potential opioid use, as naltrexone is an opioid receptor antagonist. Patients should be informed that opioids may be ineffective if they are required unexpectedly (eg, for trauma) and that naltrexone SR/bupropion SR should be withheld for any planned surgical procedure potentially requiring opioid use.
Other options. While naltrexone SR/bupropion SR is the most appropriate choice for this patient because it addresses her problematic eating behaviors while potentially improving mood and assisting with smoking cessation, phentermine/topiramate ER, lorcaserin, and liraglutide 3 mg also could be used and certainly should be tried if naltrexone SR/bupropion SR does not produce the desired weight loss.
Adverse effects. Titrate naltrexone SR/bupropion SR slowly to the treatment dose to minimize risks and adverse events.31 The most common adverse effects reported in phase III trials were nausea, constipation, and headache.34,35,45,46 Discontinue naltrexone SR/bupropion SR if the patient does not achieve 5% weight loss at 16 weeks (after 12 weeks at the maintenance dose).31
CASE 4 Regaining weight after gastric bypass
A 65-year-old woman with obesity (BMI 39 kg/m2)who underwent Roux-en-Y gastric bypass surgery and who has type 2 diabetes, congestive heart failure, coronary artery disease, hypertension, and hyperlipidemia, remains concerned about her weight. She lost 100 lb following surgery and maintained her weight for 3 years, but then regained 30 lb. She comes in for an office visit because she is concerned about her increasing blood sugar and wants to prevent further weight gain. Her medications include metformin 1,000 mg twice per day, lisinopril 5 mg/d, carvedilol 12.5 mg twice per day, simvastatin 20 mg/d, and aspirin 81 mg/d. Laboratory test results are normal except for a hemoglobin A1c of 8%. She denies pancreatitis and a personal or family history of thyroid cancer.
Which medication is an appropriate next step for this patient?
Pharmacotherapy is an option
Pharmacotherapy is a great option for this patient, who is regaining weight following bariatric surgery. Phentermine/topiramate ER is the only medication that would be contraindicated because of her heart disease. Lorcaserin and naltrexone SR/bupropion SR could be considered, but liraglutide 3 mg is the most appropriate option, given her need for further glucose control.
Furthermore, the recent LEADER (Liraglutide effect and action in diabetes: evaluation of CV outcome results) trial reported a significant mortality benefit with liraglutide 1.8 mg/d among patients with type 2 diabetes and high CV risk.47 The study found that liraglutide was superior to placebo in reducing CV events.
Contraindications. Ask patients about a history of pancreatitis before starting liraglutide 3 mg, given the possible increased risk. In addition, liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2. Thyroid C-cell tumors have been found in rodents given supratherapeutic doses of liraglutide48; however, there is no evidence of liraglutide causing C-cell tumors in humans.
For patients taking a medication that can cause hypoglycemia, such as insulin or a sulfonylurea, monitor blood sugar and consider reducing the dose of that medication when starting liraglutide.
Administration and titration. Liraglutide is injected subcutaneously once daily. The dose is titrated up weekly to reduce gastrointestinal symptoms.36 The most common adverse effects reported in phase III trials were nausea, diarrhea, and constipation.37–39Discontinue liraglutide 3 mg if the patient does not lose at least 4% of baseline body weight after 16 weeks.49
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
- Magkos F, Fraterrigo G, Yoshino J. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 pt B):2985-3023.
- Sumithran P, Predergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604.
- Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196.
- Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring). 2016;24:1612-1619.
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
- Saunders KH, Shukla AP, Igel LI, et al. Pharmacotherapy for obesity. Endocrinol Metab Clin North Am. 2016;45:521-538.
- Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
- US Food and Drug Administration. Drug approval package. Qsymia. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000_qsymia_toc.cfm. Accessed August 28, 2017.
- Arena Pharmaceuticals. Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQR (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182. Accessed August 28, 2017.
- Drugs.com. Contrave approval history. https://www.drugs.com/history/contrave.html. Accessed August 28, 2017.
- US Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206321. Accessed August 28, 2017.
- Igel LI, Kumar RB, Saunders KH, et al. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152:1765-1779.
- Adipex-P package insert. http://www.iodine.com/drug/phentermine/fda-package-insert. Accessed August 28, 2017.
- Ionamin package insert. http://druginserts.com/lib/rx/meds/ionamin/. Accessed August 28, 2017.
- Lomaira package insert. https://www.lomaira.com/Prescribing_Information.pdf. Accessed August 28, 2017.
- Suprenza package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202088s001lbl.pdf. Accessed August 28, 2017.
- Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended release in obese adults. Obesity (Silver Spring). 2013;21:2163-2171.
- Alli package labeling. http://druginserts.com/lib/otc/meds/alli-1/. Accessed August 28, 2017.
- Xenical package insert. https://www.gene.com/download/pdf/xenical_prescribing.pdf. Accessed August 28,2017.
- Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161.
- Qsymia package insert. https://www.qsymia.com/pdf/prescribing-information.pdf. Accessed August 28, 2017.
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352.
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308.
- Belviq package insert. https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/Belviq_Prescribing_information-pdf.PDF?la=en. Accessed August 28, 2017.
- Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256.
- Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067-3077.
- O'Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20:1426-1436.
- Contrave package insert. https://contrave.com/wpcontent/uploads/2017/05/Contrave_PI.pdf. Accessed August 28, 2017.
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595-605.
- Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935-943.
- Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110-120.
- Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022-4029.
- Saxenda package insert. http://www.novo-pi.com/saxenda.pdf. Accessed August 28, 2017.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015;314:687-699.
- Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451.
- Saunders KH, Igel LI, Aronne LJ. An update on naltrexone/bupropion extended-release in the treatment of obesity. Expert Opin Pharmacother. 2016. 10.1080/14656566.2016.1244527.
- Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring). 2016;24:1955-1961.
- Qsymia Risk Evaluation and Mitigation Strategy (REMS). VIVUS, Inc. http://www.qsymiarems.com. Accessed January 16, 2017.
- Zinman B, Wanner C, Lachin JM, et al. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
- US Food and Drug Administration. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafety-InformationforPatientsandProviders/ucm179871.htm. Accessed August 28, 2017.
- Belviq XR package insert. https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/belviqxr_prescribing_information-pdf.PDF?la=en. Accessed January 16, 2017.
- Smith SR, O'Neil PM, Astrup A. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity (Silver Spring). 2014;22:2137-2146.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
- Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012;153:1538-1547.
- Fujioka K, O'Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24:2278-2288.
- Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
- Magkos F, Fraterrigo G, Yoshino J. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 pt B):2985-3023.
- Sumithran P, Predergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604.
- Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196.
- Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring). 2016;24:1612-1619.
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
- Saunders KH, Shukla AP, Igel LI, et al. Pharmacotherapy for obesity. Endocrinol Metab Clin North Am. 2016;45:521-538.
- Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
- US Food and Drug Administration. Drug approval package. Qsymia. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000_qsymia_toc.cfm. Accessed August 28, 2017.
- Arena Pharmaceuticals. Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQR (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182. Accessed August 28, 2017.
- Drugs.com. Contrave approval history. https://www.drugs.com/history/contrave.html. Accessed August 28, 2017.
- US Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206321. Accessed August 28, 2017.
- Igel LI, Kumar RB, Saunders KH, et al. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152:1765-1779.
- Adipex-P package insert. http://www.iodine.com/drug/phentermine/fda-package-insert. Accessed August 28, 2017.
- Ionamin package insert. http://druginserts.com/lib/rx/meds/ionamin/. Accessed August 28, 2017.
- Lomaira package insert. https://www.lomaira.com/Prescribing_Information.pdf. Accessed August 28, 2017.
- Suprenza package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202088s001lbl.pdf. Accessed August 28, 2017.
- Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended release in obese adults. Obesity (Silver Spring). 2013;21:2163-2171.
- Alli package labeling. http://druginserts.com/lib/otc/meds/alli-1/. Accessed August 28, 2017.
- Xenical package insert. https://www.gene.com/download/pdf/xenical_prescribing.pdf. Accessed August 28,2017.
- Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161.
- Qsymia package insert. https://www.qsymia.com/pdf/prescribing-information.pdf. Accessed August 28, 2017.
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352.
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308.
- Belviq package insert. https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/Belviq_Prescribing_information-pdf.PDF?la=en. Accessed August 28, 2017.
- Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256.
- Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067-3077.
- O'Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20:1426-1436.
- Contrave package insert. https://contrave.com/wpcontent/uploads/2017/05/Contrave_PI.pdf. Accessed August 28, 2017.
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595-605.
- Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935-943.
- Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110-120.
- Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022-4029.
- Saxenda package insert. http://www.novo-pi.com/saxenda.pdf. Accessed August 28, 2017.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015;314:687-699.
- Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451.
- Saunders KH, Igel LI, Aronne LJ. An update on naltrexone/bupropion extended-release in the treatment of obesity. Expert Opin Pharmacother. 2016. 10.1080/14656566.2016.1244527.
- Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring). 2016;24:1955-1961.
- Qsymia Risk Evaluation and Mitigation Strategy (REMS). VIVUS, Inc. http://www.qsymiarems.com. Accessed January 16, 2017.
- Zinman B, Wanner C, Lachin JM, et al. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
- US Food and Drug Administration. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafety-InformationforPatientsandProviders/ucm179871.htm. Accessed August 28, 2017.
- Belviq XR package insert. https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/belviqxr_prescribing_information-pdf.PDF?la=en. Accessed January 16, 2017.
- Smith SR, O'Neil PM, Astrup A. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity (Silver Spring). 2014;22:2137-2146.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
- Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012;153:1538-1547.
- Fujioka K, O'Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24:2278-2288.