User login
AGA Publishes New Pouchitis Management Guideline
The American Gastroenterological Association (AGA) has published a new clinical practice guideline on the management of pouchitis and inflammatory pouch disorders.
The guidance document, authored by Edward L. Barnes, MD, of the University of North Carolina at Chapel Hill and colleagues, includes eleven conditional recommendations that steer usage of probiotics, antibiotics, and immunosuppressive therapies in patients with these conditions, which occur most often after restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) for ulcerative colitis (UC).
“Multiple strategies have been utilized in the treatment and prevention of pouchitis and inflammatory pouch conditions, including antibiotics, probiotics, corticosteroids, and advanced immunosuppressive therapies including biologics and oral small-molecule drugs,” the guideline panelists wrote on the AGA website. “However, most of the evidence base is primarily derived from retrospective observational studies or comparisons of small cohorts. Data on patients’ values and preferences for specific management decisions and treatment choices are also limited. This results in substantial practice variability.”
Still, the area is advancing. Dr. Barnes and colleagues highlighted new scoring systems for characterizing endoscopic findings and patient-reported outcomes, as well as the recent EARNEST trial (N Engl J Med. 2023 Mar 30;388(13):1191-1200), which compared vedolizumab with placebo in patients with chronic refractory pouchitis, and should be considered a “landmark study in the field,” as it could shape future trial design.
Based on all available evidence and clinical experience, the panelists issued the following recommendations, which were approved by the AGA Governing Board.
Probiotics
Because of a knowledge gap, the guideline makes no recommendation for or against use of probiotics for either the primary prevention or treatment of pouchitis.
They offered a similar explanation for the lack of guidance on using probiotics to treat pouchitis, and noted that antibiotics have demonstrated effectiveness where probiotics have not, making them the preferred treatment choice.
“There is potential that delaying therapy or using probiotics when they are not as effective as antibiotics may have significant impact on an individual patient’s quality of life,” Dr. Barnes and colleagues noted.
In contrast with the above statements, the guideline recommends usage of probiotics to prevent recurrent pouchitis in patients with recurrent, antibiotic-responsive pouchitis.
The De Simone formulation of multistrain probiotics is best supported in this scenario, the guideline notes, as this product was used in clinical trials, which collectively showed an 87% reduced risk of relapse over 12 months.
Antibiotics
Although the guideline supports antibiotics for prevention of pouchitis, the panelists noted that only one randomized controlled trial supports this recommendation, and negative effects of long-term usage need to be considered, including promotion of drug-resistant organisms and risk of Clostridioides difficile infection.
Dr. Barnes and colleagues cited more data supporting antibiotics for treatment of pouchitis, and noted that metronidazole and/or ciprofloxacin remain the preferred choices, with a typical duration of 2-4 weeks.
“An approach using a combination of antibiotics may be more effective in patients who do not respond to single-antibiotic therapy,” the panelists wrote, noting that oral vancomycin may also be considered when a patient does not respond to initial therapy.
For patients with recurrent pouchitis that relapses shortly after discontinuing antibiotics, chronic antibiotics should be considered, according to the guideline.
Immunosuppressive therapies
Advanced immunosuppressive therapies are recommended for patients with chronic antibiotic-dependent pouchitis, including those approved for treatment of UC or Crohn’s disease.
“Advanced immunosuppressive therapies may be used in lieu of chronic, continuous antibiotic therapy, particularly in patients who are intolerant to antibiotics or where patients and/or providers are concerned about risks of long-term antibiotic therapy,” the panelists wrote.
For patients with chronic antibiotic-refractory pouchitis, the guideline makes a general recommendation for advanced immunosuppressive therapies while specifically noting that vedolizumab has a greater strength of evidence in this scenario, citing the EARNEST trial.
A separate recommendation for corticosteroids is made for the same patient group, with ileal-release budesonide remaining the preferred formulation. In contrast, mesalamine is not recommended, based on a lack of supporting evidence.
Finally, the panelists recommend using corticosteroids in patients with Crohn’s-like disease of the pouch.
Future directions
“Even though pouchitis is relatively common after IPAA for UC, we observed that most of the evidence informing these guidelines was low to very low quality, derived from case series or small cohort studies, and several knowledge gaps exist,” Dr. Barnes and colleagues wrote. “Several initiatives towards improving management of inflammatory pouch disorders are already underway. However, concerted efforts in key domains are central towards improving patient care.”
They suggested that research should focus on standardizing disease entities, characterizing natural history and risk factors for inflammatory disorders of the pouch, and improving clinical trial design.The guideline was funded by the AGA Institute. The panelists disclosed relationships with Bristol-Myers Squibb, Sandoz, AbbVie, and others.
The American Gastroenterological Association (AGA) has published a new clinical practice guideline on the management of pouchitis and inflammatory pouch disorders.
The guidance document, authored by Edward L. Barnes, MD, of the University of North Carolina at Chapel Hill and colleagues, includes eleven conditional recommendations that steer usage of probiotics, antibiotics, and immunosuppressive therapies in patients with these conditions, which occur most often after restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) for ulcerative colitis (UC).
“Multiple strategies have been utilized in the treatment and prevention of pouchitis and inflammatory pouch conditions, including antibiotics, probiotics, corticosteroids, and advanced immunosuppressive therapies including biologics and oral small-molecule drugs,” the guideline panelists wrote on the AGA website. “However, most of the evidence base is primarily derived from retrospective observational studies or comparisons of small cohorts. Data on patients’ values and preferences for specific management decisions and treatment choices are also limited. This results in substantial practice variability.”
Still, the area is advancing. Dr. Barnes and colleagues highlighted new scoring systems for characterizing endoscopic findings and patient-reported outcomes, as well as the recent EARNEST trial (N Engl J Med. 2023 Mar 30;388(13):1191-1200), which compared vedolizumab with placebo in patients with chronic refractory pouchitis, and should be considered a “landmark study in the field,” as it could shape future trial design.
Based on all available evidence and clinical experience, the panelists issued the following recommendations, which were approved by the AGA Governing Board.
Probiotics
Because of a knowledge gap, the guideline makes no recommendation for or against use of probiotics for either the primary prevention or treatment of pouchitis.
They offered a similar explanation for the lack of guidance on using probiotics to treat pouchitis, and noted that antibiotics have demonstrated effectiveness where probiotics have not, making them the preferred treatment choice.
“There is potential that delaying therapy or using probiotics when they are not as effective as antibiotics may have significant impact on an individual patient’s quality of life,” Dr. Barnes and colleagues noted.
In contrast with the above statements, the guideline recommends usage of probiotics to prevent recurrent pouchitis in patients with recurrent, antibiotic-responsive pouchitis.
The De Simone formulation of multistrain probiotics is best supported in this scenario, the guideline notes, as this product was used in clinical trials, which collectively showed an 87% reduced risk of relapse over 12 months.
Antibiotics
Although the guideline supports antibiotics for prevention of pouchitis, the panelists noted that only one randomized controlled trial supports this recommendation, and negative effects of long-term usage need to be considered, including promotion of drug-resistant organisms and risk of Clostridioides difficile infection.
Dr. Barnes and colleagues cited more data supporting antibiotics for treatment of pouchitis, and noted that metronidazole and/or ciprofloxacin remain the preferred choices, with a typical duration of 2-4 weeks.
“An approach using a combination of antibiotics may be more effective in patients who do not respond to single-antibiotic therapy,” the panelists wrote, noting that oral vancomycin may also be considered when a patient does not respond to initial therapy.
For patients with recurrent pouchitis that relapses shortly after discontinuing antibiotics, chronic antibiotics should be considered, according to the guideline.
Immunosuppressive therapies
Advanced immunosuppressive therapies are recommended for patients with chronic antibiotic-dependent pouchitis, including those approved for treatment of UC or Crohn’s disease.
“Advanced immunosuppressive therapies may be used in lieu of chronic, continuous antibiotic therapy, particularly in patients who are intolerant to antibiotics or where patients and/or providers are concerned about risks of long-term antibiotic therapy,” the panelists wrote.
For patients with chronic antibiotic-refractory pouchitis, the guideline makes a general recommendation for advanced immunosuppressive therapies while specifically noting that vedolizumab has a greater strength of evidence in this scenario, citing the EARNEST trial.
A separate recommendation for corticosteroids is made for the same patient group, with ileal-release budesonide remaining the preferred formulation. In contrast, mesalamine is not recommended, based on a lack of supporting evidence.
Finally, the panelists recommend using corticosteroids in patients with Crohn’s-like disease of the pouch.
Future directions
“Even though pouchitis is relatively common after IPAA for UC, we observed that most of the evidence informing these guidelines was low to very low quality, derived from case series or small cohort studies, and several knowledge gaps exist,” Dr. Barnes and colleagues wrote. “Several initiatives towards improving management of inflammatory pouch disorders are already underway. However, concerted efforts in key domains are central towards improving patient care.”
They suggested that research should focus on standardizing disease entities, characterizing natural history and risk factors for inflammatory disorders of the pouch, and improving clinical trial design.The guideline was funded by the AGA Institute. The panelists disclosed relationships with Bristol-Myers Squibb, Sandoz, AbbVie, and others.
The American Gastroenterological Association (AGA) has published a new clinical practice guideline on the management of pouchitis and inflammatory pouch disorders.
The guidance document, authored by Edward L. Barnes, MD, of the University of North Carolina at Chapel Hill and colleagues, includes eleven conditional recommendations that steer usage of probiotics, antibiotics, and immunosuppressive therapies in patients with these conditions, which occur most often after restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) for ulcerative colitis (UC).
“Multiple strategies have been utilized in the treatment and prevention of pouchitis and inflammatory pouch conditions, including antibiotics, probiotics, corticosteroids, and advanced immunosuppressive therapies including biologics and oral small-molecule drugs,” the guideline panelists wrote on the AGA website. “However, most of the evidence base is primarily derived from retrospective observational studies or comparisons of small cohorts. Data on patients’ values and preferences for specific management decisions and treatment choices are also limited. This results in substantial practice variability.”
Still, the area is advancing. Dr. Barnes and colleagues highlighted new scoring systems for characterizing endoscopic findings and patient-reported outcomes, as well as the recent EARNEST trial (N Engl J Med. 2023 Mar 30;388(13):1191-1200), which compared vedolizumab with placebo in patients with chronic refractory pouchitis, and should be considered a “landmark study in the field,” as it could shape future trial design.
Based on all available evidence and clinical experience, the panelists issued the following recommendations, which were approved by the AGA Governing Board.
Probiotics
Because of a knowledge gap, the guideline makes no recommendation for or against use of probiotics for either the primary prevention or treatment of pouchitis.
They offered a similar explanation for the lack of guidance on using probiotics to treat pouchitis, and noted that antibiotics have demonstrated effectiveness where probiotics have not, making them the preferred treatment choice.
“There is potential that delaying therapy or using probiotics when they are not as effective as antibiotics may have significant impact on an individual patient’s quality of life,” Dr. Barnes and colleagues noted.
In contrast with the above statements, the guideline recommends usage of probiotics to prevent recurrent pouchitis in patients with recurrent, antibiotic-responsive pouchitis.
The De Simone formulation of multistrain probiotics is best supported in this scenario, the guideline notes, as this product was used in clinical trials, which collectively showed an 87% reduced risk of relapse over 12 months.
Antibiotics
Although the guideline supports antibiotics for prevention of pouchitis, the panelists noted that only one randomized controlled trial supports this recommendation, and negative effects of long-term usage need to be considered, including promotion of drug-resistant organisms and risk of Clostridioides difficile infection.
Dr. Barnes and colleagues cited more data supporting antibiotics for treatment of pouchitis, and noted that metronidazole and/or ciprofloxacin remain the preferred choices, with a typical duration of 2-4 weeks.
“An approach using a combination of antibiotics may be more effective in patients who do not respond to single-antibiotic therapy,” the panelists wrote, noting that oral vancomycin may also be considered when a patient does not respond to initial therapy.
For patients with recurrent pouchitis that relapses shortly after discontinuing antibiotics, chronic antibiotics should be considered, according to the guideline.
Immunosuppressive therapies
Advanced immunosuppressive therapies are recommended for patients with chronic antibiotic-dependent pouchitis, including those approved for treatment of UC or Crohn’s disease.
“Advanced immunosuppressive therapies may be used in lieu of chronic, continuous antibiotic therapy, particularly in patients who are intolerant to antibiotics or where patients and/or providers are concerned about risks of long-term antibiotic therapy,” the panelists wrote.
For patients with chronic antibiotic-refractory pouchitis, the guideline makes a general recommendation for advanced immunosuppressive therapies while specifically noting that vedolizumab has a greater strength of evidence in this scenario, citing the EARNEST trial.
A separate recommendation for corticosteroids is made for the same patient group, with ileal-release budesonide remaining the preferred formulation. In contrast, mesalamine is not recommended, based on a lack of supporting evidence.
Finally, the panelists recommend using corticosteroids in patients with Crohn’s-like disease of the pouch.
Future directions
“Even though pouchitis is relatively common after IPAA for UC, we observed that most of the evidence informing these guidelines was low to very low quality, derived from case series or small cohort studies, and several knowledge gaps exist,” Dr. Barnes and colleagues wrote. “Several initiatives towards improving management of inflammatory pouch disorders are already underway. However, concerted efforts in key domains are central towards improving patient care.”
They suggested that research should focus on standardizing disease entities, characterizing natural history and risk factors for inflammatory disorders of the pouch, and improving clinical trial design.The guideline was funded by the AGA Institute. The panelists disclosed relationships with Bristol-Myers Squibb, Sandoz, AbbVie, and others.
FROM THE AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Chest pain and shortness of breath
In a lifelong smoker, a tumor in the periphery of the lung and histology showing glandular cells with some neuroendocrine differentiation is most likely large cell carcinoma, a type of non–small cell lung cancer (NSCLC). Although small cell lung cancer is also associated with smoking, histology typically demonstrates highly cellular aspirates with small blue cells with very scant or null cytoplasm, loosely arranged or in a syncytial pattern. Bronchial adenoma is unlikely, given the patient's unintentional weight loss and fatigue over the past few months. Mesothelioma is most associated with asbestos exposure and is found in the lung pleura, which typically presents with pleural effusion.
Lung cancer is the top cause of cancer deaths in the US, second only to prostate cancer in men and breast cancer in women; approximately 85% of all lung cancers are classified as NSCLC. Histologically, NSCLC is further categorized into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma (LCC). When a patient presents with intrathoracic symptoms (including cough, chest pain, wheezing, or dyspnea) and a pulmonary nodule on chest radiography, NSCLC is typically suspected as a possible diagnosis. Smoking is the most common cause of this lung cancer (78% in men, 90% in women).
Several methods confirm the diagnosis of NSCLC, including bronchoscopy, sputum cytology, mediastinoscopy, thoracentesis, thoracoscopy, and transthoracic needle biopsy. Which method is chosen depends on the primary lesion location and accessibility. Histologic evaluation helps differentiate between the various subtypes of NSCLC. LCC is a subset of NSCLC that is a diagnosis of exclusion. Histologically, LCC is poorly differentiated, and 90% of cases will show squamous, glandular, or neuroendocrine differentiation.
When first diagnosed with NSCLC, 20% of patients have cancer confined to a specific area, 25% of patients have cancer that has spread to nearby areas, and 55% of patients have cancer that has spread to distant body parts. The specific symptoms experienced by patients will vary depending on the location of the cancer. The prognosis for NSCLC depends on the staging of the tumor, nodes, and metastases, the patient's performance status, and any existing health conditions. In the US, the 5-year relative survival rate is 61.2% for localized disease, 33.5% for regional disease, and 7.0% for disease with distant metastases.
Treatment of NSCLC also varies according to the patient's functional status, tumor stage, molecular characteristics, and comorbidities. Generally, patients with stage I, II, or III NSCLC are treated with the intent to cure, which can include surgery, chemotherapy, radiation therapy, or a combined approach. Lobectomy or resection is generally accepted as an approach for surgical intervention on early-stage NSCLC; however, for stages higher than IB (including stage II/III), patients are recommended to undergo adjuvant chemotherapy. Patients with stage IV disease (or recurrence after initial management) are typically treated with systemic therapy or should be considered for palliative treatment to improve quality of life and overall survival.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
In a lifelong smoker, a tumor in the periphery of the lung and histology showing glandular cells with some neuroendocrine differentiation is most likely large cell carcinoma, a type of non–small cell lung cancer (NSCLC). Although small cell lung cancer is also associated with smoking, histology typically demonstrates highly cellular aspirates with small blue cells with very scant or null cytoplasm, loosely arranged or in a syncytial pattern. Bronchial adenoma is unlikely, given the patient's unintentional weight loss and fatigue over the past few months. Mesothelioma is most associated with asbestos exposure and is found in the lung pleura, which typically presents with pleural effusion.
Lung cancer is the top cause of cancer deaths in the US, second only to prostate cancer in men and breast cancer in women; approximately 85% of all lung cancers are classified as NSCLC. Histologically, NSCLC is further categorized into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma (LCC). When a patient presents with intrathoracic symptoms (including cough, chest pain, wheezing, or dyspnea) and a pulmonary nodule on chest radiography, NSCLC is typically suspected as a possible diagnosis. Smoking is the most common cause of this lung cancer (78% in men, 90% in women).
Several methods confirm the diagnosis of NSCLC, including bronchoscopy, sputum cytology, mediastinoscopy, thoracentesis, thoracoscopy, and transthoracic needle biopsy. Which method is chosen depends on the primary lesion location and accessibility. Histologic evaluation helps differentiate between the various subtypes of NSCLC. LCC is a subset of NSCLC that is a diagnosis of exclusion. Histologically, LCC is poorly differentiated, and 90% of cases will show squamous, glandular, or neuroendocrine differentiation.
When first diagnosed with NSCLC, 20% of patients have cancer confined to a specific area, 25% of patients have cancer that has spread to nearby areas, and 55% of patients have cancer that has spread to distant body parts. The specific symptoms experienced by patients will vary depending on the location of the cancer. The prognosis for NSCLC depends on the staging of the tumor, nodes, and metastases, the patient's performance status, and any existing health conditions. In the US, the 5-year relative survival rate is 61.2% for localized disease, 33.5% for regional disease, and 7.0% for disease with distant metastases.
Treatment of NSCLC also varies according to the patient's functional status, tumor stage, molecular characteristics, and comorbidities. Generally, patients with stage I, II, or III NSCLC are treated with the intent to cure, which can include surgery, chemotherapy, radiation therapy, or a combined approach. Lobectomy or resection is generally accepted as an approach for surgical intervention on early-stage NSCLC; however, for stages higher than IB (including stage II/III), patients are recommended to undergo adjuvant chemotherapy. Patients with stage IV disease (or recurrence after initial management) are typically treated with systemic therapy or should be considered for palliative treatment to improve quality of life and overall survival.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
In a lifelong smoker, a tumor in the periphery of the lung and histology showing glandular cells with some neuroendocrine differentiation is most likely large cell carcinoma, a type of non–small cell lung cancer (NSCLC). Although small cell lung cancer is also associated with smoking, histology typically demonstrates highly cellular aspirates with small blue cells with very scant or null cytoplasm, loosely arranged or in a syncytial pattern. Bronchial adenoma is unlikely, given the patient's unintentional weight loss and fatigue over the past few months. Mesothelioma is most associated with asbestos exposure and is found in the lung pleura, which typically presents with pleural effusion.
Lung cancer is the top cause of cancer deaths in the US, second only to prostate cancer in men and breast cancer in women; approximately 85% of all lung cancers are classified as NSCLC. Histologically, NSCLC is further categorized into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma (LCC). When a patient presents with intrathoracic symptoms (including cough, chest pain, wheezing, or dyspnea) and a pulmonary nodule on chest radiography, NSCLC is typically suspected as a possible diagnosis. Smoking is the most common cause of this lung cancer (78% in men, 90% in women).
Several methods confirm the diagnosis of NSCLC, including bronchoscopy, sputum cytology, mediastinoscopy, thoracentesis, thoracoscopy, and transthoracic needle biopsy. Which method is chosen depends on the primary lesion location and accessibility. Histologic evaluation helps differentiate between the various subtypes of NSCLC. LCC is a subset of NSCLC that is a diagnosis of exclusion. Histologically, LCC is poorly differentiated, and 90% of cases will show squamous, glandular, or neuroendocrine differentiation.
When first diagnosed with NSCLC, 20% of patients have cancer confined to a specific area, 25% of patients have cancer that has spread to nearby areas, and 55% of patients have cancer that has spread to distant body parts. The specific symptoms experienced by patients will vary depending on the location of the cancer. The prognosis for NSCLC depends on the staging of the tumor, nodes, and metastases, the patient's performance status, and any existing health conditions. In the US, the 5-year relative survival rate is 61.2% for localized disease, 33.5% for regional disease, and 7.0% for disease with distant metastases.
Treatment of NSCLC also varies according to the patient's functional status, tumor stage, molecular characteristics, and comorbidities. Generally, patients with stage I, II, or III NSCLC are treated with the intent to cure, which can include surgery, chemotherapy, radiation therapy, or a combined approach. Lobectomy or resection is generally accepted as an approach for surgical intervention on early-stage NSCLC; however, for stages higher than IB (including stage II/III), patients are recommended to undergo adjuvant chemotherapy. Patients with stage IV disease (or recurrence after initial management) are typically treated with systemic therapy or should be considered for palliative treatment to improve quality of life and overall survival.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
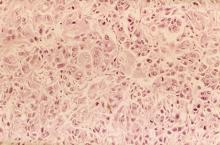
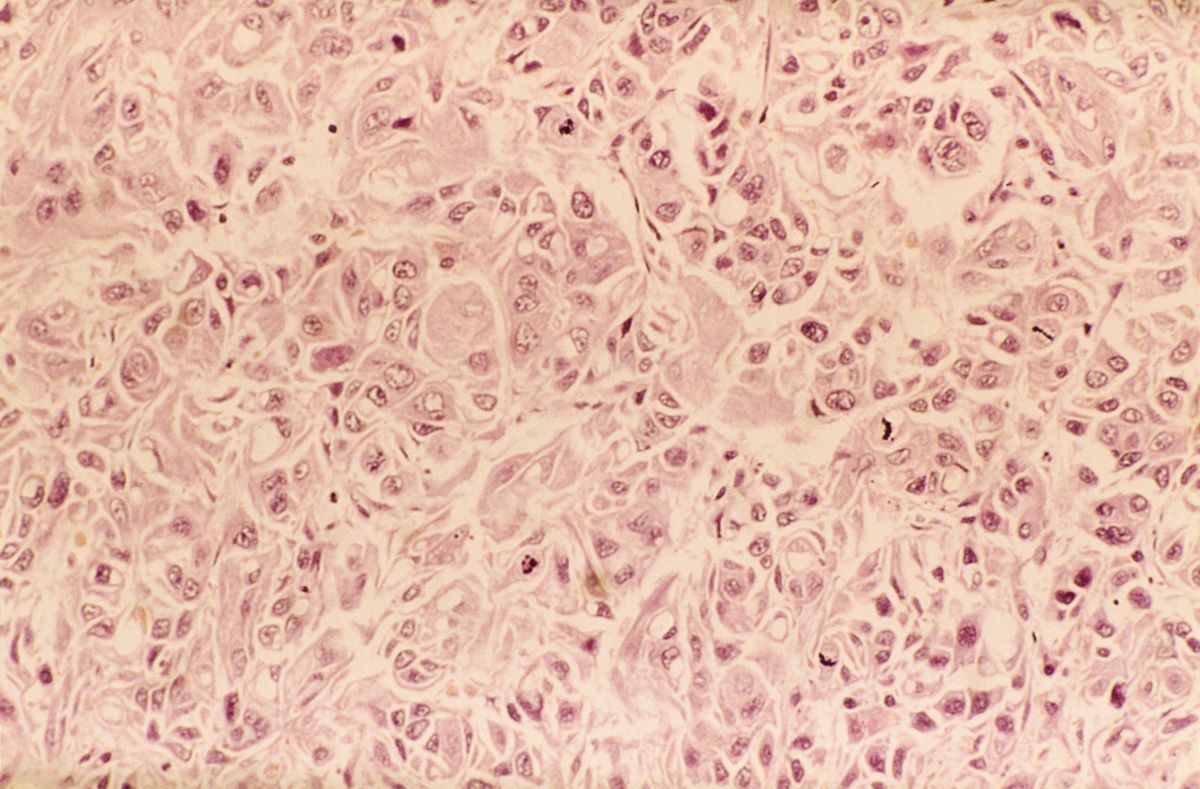
A 62-year-old man presents to his primary care physician with a persistent cough, dyspnea, unintentional weight loss, and fatigue over the past few months. He has a history of smoking for 30 years but quit 5 years ago. He also reports occasional chest pain and shortness of breath during physical activities. Physical examination reveals crackles in the middle lobe of the right lung. The patient occasionally coughs up blood. Chest radiography shows a large mass in the right lung, and a subsequent CT scan confirms a large peripheral mass of solid attenuation with an irregular margin. The patient undergoes thoracoscopy to obtain a biopsy sample from the tumor for further analysis. The biopsy reveals glandular cells with some neuroendocrine differentiation.
Delayed Meals Tied to Increased CVD Risk
TOPLINE:
(CVDs), especially in women, results of a large prospective study suggested.
METHODOLOGY:
- The study included 103,389 participants, mean baseline age 42.6 years and 79% women, who were volunteers in the ongoing NutriNet-Santé, a cohort study launched in France to better understand the relationship between nutrition and health.
- Participants completed questionnaires that in addition to data on socio-demographics, lifestyle, and physical activity provided information on when foods and beverages were consumed during each day, and they self-reported major health events, including CVDs.
- Researchers assessed associations between time of first meal of the day (before 8 am, 8-9 am, after 9 am) and last meal (before 8 pm, 8-9 pm, after 9 pm), number of eating occasions, and duration of nighttime fasting (12 h or less, 12-13 h, more than 13 h) and the risk for CVD, controlling for a large number of potential confounders, among them age, sex, education, income, smoking, and physical activity level.
- During a median follow-up of 7.2 years, 2036 cases of overall CVD, 988 cases of cerebrovascular disease (stroke, transient ischemic attack), and 1071 cases of coronary heart diseases (myocardial infraction, angina pectoris, acute coronary syndrome, angioplasty) were reported.
TAKEAWAY:
- Each additional hour delaying the time of the first meal of the day was associated with a higher risk for overall CVD (hazard ratio [HR], 1.06; 95% CI, 1.01-1.12; P = .02), with the association stronger in women than in men.
- Each additional hour in delaying the time of the last meal was associated with an increased risk for cerebrovascular disease; here, a last meal after 9 pm was associated with a 28% higher risk than a meal before 8 pm (HR, 1.28; 95% CI, 1.05-1.55; P < .01).
- There was no association between number of eating occasions and either overall CVD or cerebrovascular disease and no association between meal timing or number of eating occasions and risk for coronary heart disease.
- Each hour increase in nighttime fasting was associated with a 7% lower risk for cerebrovascular disease (HR, 0.93; 95% CI, 0.87-0.99; P = .02) but not with a risk for overall CVD or coronary heart disease.
IN PRACTICE:
“Our results suggest a potential benefit of adopting earlier eating timing patterns and coupling a longer nighttime fasting period with an early last meal, rather than breakfast skipping, in CVD prevention,” the authors wrote.
SOURCE:
The study was conducted by Anna Palomar-Cros, Barcelona Institute for Global Health and Department of Experimental and Health Sciences, Universitat Pompeu Fabra, Barcelona, Spain, and colleagues. It was published online on December 14, 2023, in Nature Communications.
LIMITATIONS:
Information on shift work, exposure to night light, use of recreational drugs, and timing of physical activity, medication or alcohol consumption, all of which are potential disruptors of circadian rhythms, was not available, and sleep time and duration were available for only a subgroup of patients. Unknown or unmeasured potential confounders (eg, being awakened by children) could have contributed to residual confounding. Reverse causation bias linked to change in behaviors in people with poor health having difficulty getting out of bed in the mornings can’t be ruled out. Participants in the NutriNet-Santé cohort are more likely to be women, have a higher socioeconomic status, and healthier behavior patterns than the general population, perhaps limiting extrapolation of results.
DISCLOSURES:
The NutriNet-Santé study is supported by Ministère de la Santé, Santé Publique France, Institut National de la Santé et de la Recherche Médicale (INSERM), Institut national de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), Conservatoire National des Arts et Métiers (CNAM), and Université Sorbonne Paris Nord. The authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
(CVDs), especially in women, results of a large prospective study suggested.
METHODOLOGY:
- The study included 103,389 participants, mean baseline age 42.6 years and 79% women, who were volunteers in the ongoing NutriNet-Santé, a cohort study launched in France to better understand the relationship between nutrition and health.
- Participants completed questionnaires that in addition to data on socio-demographics, lifestyle, and physical activity provided information on when foods and beverages were consumed during each day, and they self-reported major health events, including CVDs.
- Researchers assessed associations between time of first meal of the day (before 8 am, 8-9 am, after 9 am) and last meal (before 8 pm, 8-9 pm, after 9 pm), number of eating occasions, and duration of nighttime fasting (12 h or less, 12-13 h, more than 13 h) and the risk for CVD, controlling for a large number of potential confounders, among them age, sex, education, income, smoking, and physical activity level.
- During a median follow-up of 7.2 years, 2036 cases of overall CVD, 988 cases of cerebrovascular disease (stroke, transient ischemic attack), and 1071 cases of coronary heart diseases (myocardial infraction, angina pectoris, acute coronary syndrome, angioplasty) were reported.
TAKEAWAY:
- Each additional hour delaying the time of the first meal of the day was associated with a higher risk for overall CVD (hazard ratio [HR], 1.06; 95% CI, 1.01-1.12; P = .02), with the association stronger in women than in men.
- Each additional hour in delaying the time of the last meal was associated with an increased risk for cerebrovascular disease; here, a last meal after 9 pm was associated with a 28% higher risk than a meal before 8 pm (HR, 1.28; 95% CI, 1.05-1.55; P < .01).
- There was no association between number of eating occasions and either overall CVD or cerebrovascular disease and no association between meal timing or number of eating occasions and risk for coronary heart disease.
- Each hour increase in nighttime fasting was associated with a 7% lower risk for cerebrovascular disease (HR, 0.93; 95% CI, 0.87-0.99; P = .02) but not with a risk for overall CVD or coronary heart disease.
IN PRACTICE:
“Our results suggest a potential benefit of adopting earlier eating timing patterns and coupling a longer nighttime fasting period with an early last meal, rather than breakfast skipping, in CVD prevention,” the authors wrote.
SOURCE:
The study was conducted by Anna Palomar-Cros, Barcelona Institute for Global Health and Department of Experimental and Health Sciences, Universitat Pompeu Fabra, Barcelona, Spain, and colleagues. It was published online on December 14, 2023, in Nature Communications.
LIMITATIONS:
Information on shift work, exposure to night light, use of recreational drugs, and timing of physical activity, medication or alcohol consumption, all of which are potential disruptors of circadian rhythms, was not available, and sleep time and duration were available for only a subgroup of patients. Unknown or unmeasured potential confounders (eg, being awakened by children) could have contributed to residual confounding. Reverse causation bias linked to change in behaviors in people with poor health having difficulty getting out of bed in the mornings can’t be ruled out. Participants in the NutriNet-Santé cohort are more likely to be women, have a higher socioeconomic status, and healthier behavior patterns than the general population, perhaps limiting extrapolation of results.
DISCLOSURES:
The NutriNet-Santé study is supported by Ministère de la Santé, Santé Publique France, Institut National de la Santé et de la Recherche Médicale (INSERM), Institut national de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), Conservatoire National des Arts et Métiers (CNAM), and Université Sorbonne Paris Nord. The authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
(CVDs), especially in women, results of a large prospective study suggested.
METHODOLOGY:
- The study included 103,389 participants, mean baseline age 42.6 years and 79% women, who were volunteers in the ongoing NutriNet-Santé, a cohort study launched in France to better understand the relationship between nutrition and health.
- Participants completed questionnaires that in addition to data on socio-demographics, lifestyle, and physical activity provided information on when foods and beverages were consumed during each day, and they self-reported major health events, including CVDs.
- Researchers assessed associations between time of first meal of the day (before 8 am, 8-9 am, after 9 am) and last meal (before 8 pm, 8-9 pm, after 9 pm), number of eating occasions, and duration of nighttime fasting (12 h or less, 12-13 h, more than 13 h) and the risk for CVD, controlling for a large number of potential confounders, among them age, sex, education, income, smoking, and physical activity level.
- During a median follow-up of 7.2 years, 2036 cases of overall CVD, 988 cases of cerebrovascular disease (stroke, transient ischemic attack), and 1071 cases of coronary heart diseases (myocardial infraction, angina pectoris, acute coronary syndrome, angioplasty) were reported.
TAKEAWAY:
- Each additional hour delaying the time of the first meal of the day was associated with a higher risk for overall CVD (hazard ratio [HR], 1.06; 95% CI, 1.01-1.12; P = .02), with the association stronger in women than in men.
- Each additional hour in delaying the time of the last meal was associated with an increased risk for cerebrovascular disease; here, a last meal after 9 pm was associated with a 28% higher risk than a meal before 8 pm (HR, 1.28; 95% CI, 1.05-1.55; P < .01).
- There was no association between number of eating occasions and either overall CVD or cerebrovascular disease and no association between meal timing or number of eating occasions and risk for coronary heart disease.
- Each hour increase in nighttime fasting was associated with a 7% lower risk for cerebrovascular disease (HR, 0.93; 95% CI, 0.87-0.99; P = .02) but not with a risk for overall CVD or coronary heart disease.
IN PRACTICE:
“Our results suggest a potential benefit of adopting earlier eating timing patterns and coupling a longer nighttime fasting period with an early last meal, rather than breakfast skipping, in CVD prevention,” the authors wrote.
SOURCE:
The study was conducted by Anna Palomar-Cros, Barcelona Institute for Global Health and Department of Experimental and Health Sciences, Universitat Pompeu Fabra, Barcelona, Spain, and colleagues. It was published online on December 14, 2023, in Nature Communications.
LIMITATIONS:
Information on shift work, exposure to night light, use of recreational drugs, and timing of physical activity, medication or alcohol consumption, all of which are potential disruptors of circadian rhythms, was not available, and sleep time and duration were available for only a subgroup of patients. Unknown or unmeasured potential confounders (eg, being awakened by children) could have contributed to residual confounding. Reverse causation bias linked to change in behaviors in people with poor health having difficulty getting out of bed in the mornings can’t be ruled out. Participants in the NutriNet-Santé cohort are more likely to be women, have a higher socioeconomic status, and healthier behavior patterns than the general population, perhaps limiting extrapolation of results.
DISCLOSURES:
The NutriNet-Santé study is supported by Ministère de la Santé, Santé Publique France, Institut National de la Santé et de la Recherche Médicale (INSERM), Institut national de recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), Conservatoire National des Arts et Métiers (CNAM), and Université Sorbonne Paris Nord. The authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Common Diabetes Pills Also Protect Kidneys
, according to a study in JAMA Network Open.
These pills, known as sodium-glucose cotransport protein 2 (SGLT2) inhibitors, reduce the amount of blood sugar in a kidney by causing more glucose to be excreted in urine.
Chronic kidney disease (CKD) cannot be cured and often leads to renal failure. SGLT2 inhibitor drugs can help stave off this possibility. Acute kidney disease (AKD), on the other hand, is potentially reversible. It typically occurs after an acute kidney injury, lasts for up to 90 days, and can progress to CKD if left unchecked.
“There has been a notable absence of targeted pharmacotherapy to offer protection to these patients,” said Vin-Cent Wu, MD, PhD, a nephrologist at National Taiwan University Hospital in Taipei, and an author of the study.
For the retrospective analysis, Dr. Wu and his colleagues looked at data from more than 230,000 adults with type 2 diabetes whose health records were gathered into a research tool called the TriNetX, a global research database. Patients had been treated for AKD between 2002 and 2022. Major adverse kidney events were noted for 5 years after discharge, which were defined as events which required regular dialysis, major adverse cardiovascular events such as a heart attack or stroke, or death.
To determine the effects of SGLT2 inhibitors, Dr. Wu and colleagues compared outcomes among 5317 patients with AKD who received the drugs with 5317 similar patients who did not. Members of both groups had lived for at least 90 days after being discharged from the hospital and did not require dialysis during that period.
After a median follow-up of 2.3 years, more patients who did not receive an SGLT2 inhibitor had died (994 compared with 481) or had endured major stress to their kidneys (1119 compared with 504) or heart (612 compared with 295). The relative reduction in mortality risk for people in the SGLT2-inhibitor arm was 31% (adjusted hazard ratio, 0.69; 95% CI, 0.62-0.77).
Only 2.3% of patients with AKD in the study were prescribed an SGLT2 inhibitor.
In the United States, approximately 20% of people with type 2 diabetes and CKD receive a SGLT2 inhibitor, according to 2023 research.
“Our study reveals that the prescription rate of SGLT2 inhibitors remains relatively low in clinical practice among patients with type 2 diabetes and AKD,” Dr. Wu told this news organization. “This underscores the need for increased awareness and greater consideration of this critical issue in clinical decision-making.”
Dr. Wu said that AKD management tends to be conservative and relies on symptom monitoring. He acknowledged that confounders may have influenced the results, and that the use of SGLT2 inhibitors might only be correlated with better results instead of producing a causation effect.
This point was raised by Ayodele Odutayo, MD, DPhil, a nephrologist at the University of Toronto, who was not involved in the study. But despite that caution, Dr. Odutayo said that he found the study to be encouraging overall and broadly in line with known benefits of SGLT2 inhibitors in CKD.
“The findings are reassuring that the medications work even in people who’ve already had some kidney injury beforehand,” but who are not yet diagnosed with CKD, Dr. Odutayo said.
“There is vast underuse of these medications in people for whom they are indicated,” perhaps due to clinician concern that the drugs will cause side effects such as low blood pressure or loss of salt and fluid, Dr. Odutayo said. Though those concerns are valid, the benefits of these drugs exceed the risks for most patients with CKD.
Dr. Wu and Dr. Odutayo report no relevant financial relationships.
A version of this article appeared on Medscape.com.
, according to a study in JAMA Network Open.
These pills, known as sodium-glucose cotransport protein 2 (SGLT2) inhibitors, reduce the amount of blood sugar in a kidney by causing more glucose to be excreted in urine.
Chronic kidney disease (CKD) cannot be cured and often leads to renal failure. SGLT2 inhibitor drugs can help stave off this possibility. Acute kidney disease (AKD), on the other hand, is potentially reversible. It typically occurs after an acute kidney injury, lasts for up to 90 days, and can progress to CKD if left unchecked.
“There has been a notable absence of targeted pharmacotherapy to offer protection to these patients,” said Vin-Cent Wu, MD, PhD, a nephrologist at National Taiwan University Hospital in Taipei, and an author of the study.
For the retrospective analysis, Dr. Wu and his colleagues looked at data from more than 230,000 adults with type 2 diabetes whose health records were gathered into a research tool called the TriNetX, a global research database. Patients had been treated for AKD between 2002 and 2022. Major adverse kidney events were noted for 5 years after discharge, which were defined as events which required regular dialysis, major adverse cardiovascular events such as a heart attack or stroke, or death.
To determine the effects of SGLT2 inhibitors, Dr. Wu and colleagues compared outcomes among 5317 patients with AKD who received the drugs with 5317 similar patients who did not. Members of both groups had lived for at least 90 days after being discharged from the hospital and did not require dialysis during that period.
After a median follow-up of 2.3 years, more patients who did not receive an SGLT2 inhibitor had died (994 compared with 481) or had endured major stress to their kidneys (1119 compared with 504) or heart (612 compared with 295). The relative reduction in mortality risk for people in the SGLT2-inhibitor arm was 31% (adjusted hazard ratio, 0.69; 95% CI, 0.62-0.77).
Only 2.3% of patients with AKD in the study were prescribed an SGLT2 inhibitor.
In the United States, approximately 20% of people with type 2 diabetes and CKD receive a SGLT2 inhibitor, according to 2023 research.
“Our study reveals that the prescription rate of SGLT2 inhibitors remains relatively low in clinical practice among patients with type 2 diabetes and AKD,” Dr. Wu told this news organization. “This underscores the need for increased awareness and greater consideration of this critical issue in clinical decision-making.”
Dr. Wu said that AKD management tends to be conservative and relies on symptom monitoring. He acknowledged that confounders may have influenced the results, and that the use of SGLT2 inhibitors might only be correlated with better results instead of producing a causation effect.
This point was raised by Ayodele Odutayo, MD, DPhil, a nephrologist at the University of Toronto, who was not involved in the study. But despite that caution, Dr. Odutayo said that he found the study to be encouraging overall and broadly in line with known benefits of SGLT2 inhibitors in CKD.
“The findings are reassuring that the medications work even in people who’ve already had some kidney injury beforehand,” but who are not yet diagnosed with CKD, Dr. Odutayo said.
“There is vast underuse of these medications in people for whom they are indicated,” perhaps due to clinician concern that the drugs will cause side effects such as low blood pressure or loss of salt and fluid, Dr. Odutayo said. Though those concerns are valid, the benefits of these drugs exceed the risks for most patients with CKD.
Dr. Wu and Dr. Odutayo report no relevant financial relationships.
A version of this article appeared on Medscape.com.
, according to a study in JAMA Network Open.
These pills, known as sodium-glucose cotransport protein 2 (SGLT2) inhibitors, reduce the amount of blood sugar in a kidney by causing more glucose to be excreted in urine.
Chronic kidney disease (CKD) cannot be cured and often leads to renal failure. SGLT2 inhibitor drugs can help stave off this possibility. Acute kidney disease (AKD), on the other hand, is potentially reversible. It typically occurs after an acute kidney injury, lasts for up to 90 days, and can progress to CKD if left unchecked.
“There has been a notable absence of targeted pharmacotherapy to offer protection to these patients,” said Vin-Cent Wu, MD, PhD, a nephrologist at National Taiwan University Hospital in Taipei, and an author of the study.
For the retrospective analysis, Dr. Wu and his colleagues looked at data from more than 230,000 adults with type 2 diabetes whose health records were gathered into a research tool called the TriNetX, a global research database. Patients had been treated for AKD between 2002 and 2022. Major adverse kidney events were noted for 5 years after discharge, which were defined as events which required regular dialysis, major adverse cardiovascular events such as a heart attack or stroke, or death.
To determine the effects of SGLT2 inhibitors, Dr. Wu and colleagues compared outcomes among 5317 patients with AKD who received the drugs with 5317 similar patients who did not. Members of both groups had lived for at least 90 days after being discharged from the hospital and did not require dialysis during that period.
After a median follow-up of 2.3 years, more patients who did not receive an SGLT2 inhibitor had died (994 compared with 481) or had endured major stress to their kidneys (1119 compared with 504) or heart (612 compared with 295). The relative reduction in mortality risk for people in the SGLT2-inhibitor arm was 31% (adjusted hazard ratio, 0.69; 95% CI, 0.62-0.77).
Only 2.3% of patients with AKD in the study were prescribed an SGLT2 inhibitor.
In the United States, approximately 20% of people with type 2 diabetes and CKD receive a SGLT2 inhibitor, according to 2023 research.
“Our study reveals that the prescription rate of SGLT2 inhibitors remains relatively low in clinical practice among patients with type 2 diabetes and AKD,” Dr. Wu told this news organization. “This underscores the need for increased awareness and greater consideration of this critical issue in clinical decision-making.”
Dr. Wu said that AKD management tends to be conservative and relies on symptom monitoring. He acknowledged that confounders may have influenced the results, and that the use of SGLT2 inhibitors might only be correlated with better results instead of producing a causation effect.
This point was raised by Ayodele Odutayo, MD, DPhil, a nephrologist at the University of Toronto, who was not involved in the study. But despite that caution, Dr. Odutayo said that he found the study to be encouraging overall and broadly in line with known benefits of SGLT2 inhibitors in CKD.
“The findings are reassuring that the medications work even in people who’ve already had some kidney injury beforehand,” but who are not yet diagnosed with CKD, Dr. Odutayo said.
“There is vast underuse of these medications in people for whom they are indicated,” perhaps due to clinician concern that the drugs will cause side effects such as low blood pressure or loss of salt and fluid, Dr. Odutayo said. Though those concerns are valid, the benefits of these drugs exceed the risks for most patients with CKD.
Dr. Wu and Dr. Odutayo report no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Myo-inositol is one of the components of an integrative approach to acne
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
FROM IDS 2023
Appetite loss and unusual agitation
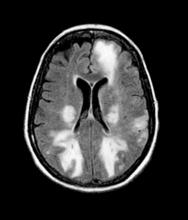
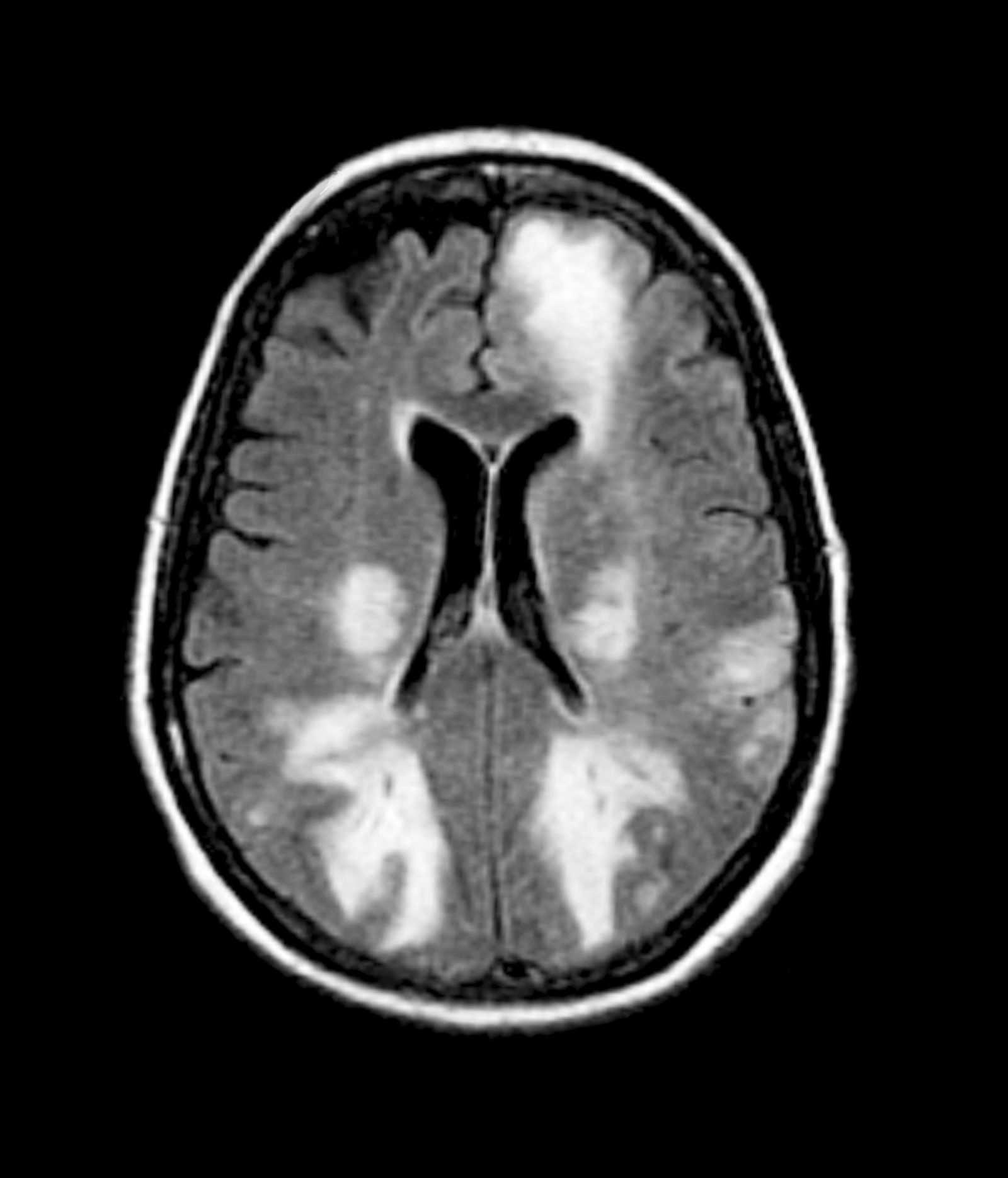
Given the patient's results on the genetic panel and MRI, as well as the noted cognitive decline and increased aggression, this patient is suspected of having limbic-predominant age-related TDP-43 encephalopathy (LATE) secondary to AD and is referred to the neurologist on her multidisciplinary care team for further consultation and testing.
AD is one of the most common forms of dementia. More than 6 million people in the United States have clinical AD or mild cognitive impairment because of AD. LATE is a new classification of dementia, identified in 2019, that mimics AD but is a unique disease entity driven by the misfolding of the protein TDP-43, which regulates gene expression in the brain. Misfolded TDP-43 protein is common among older adults aged ≥ 85 years, and about a quarter of this population has enough misfolded TDP-43 protein to affect their memory and cognition.
Diagnosing AD currently relies on a clinical approach. A complete physical examination, with a detailed neurologic examination and a mental status examination, is used to evaluate disease stage. Initial mental status testing evaluates attention and concentration, recent and remote memory, language, praxis, executive function, and visuospatial function. Because LATE is a newly discovered form of dementia, there are no set guidelines on diagnosing LATE and no robust biomarker for TDP-43. What is known about LATE has been gleaned mostly from retrospective clinicopathologic studies.
The LATE consensus working group reports that the clinical course of disease, as studied by autopsy-proven LATE neuropathologic change (LATE-NC), is described as an "amnestic cognitive syndrome that can evolve to incorporate multiple cognitive domains and ultimately to impair activities of daily living." Researchers are currently analyzing different clinical assessments and neuroimaging with MRI to characterize LATE. A group of international researchers recently published a set of clinical criteria for limbic-predominant amnestic neurodegenerative syndrome (LANS), which is associated with LATE-NC. Their criteria include "core, standard and advanced features that are measurable in vivo, including older age at evaluation, mild clinical syndrome, disproportionate hippocampal atrophy, impaired semantic memory, limbic hypometabolism, absence of neocortical degenerative patterns and low likelihood of neocortical tau, with degrees of certainty (highest, high, moderate, low)." Other neuroimaging studies of autopsy-confirmed LATE-NC have shown that atrophy is mostly focused in the medial temporal lobe with marked reduced hippocampal volume.
The group reports that LATE and AD probably share pathophysiologic mechanisms. One of the universally accepted hallmarks of AD is the formation of beta-amyloid plaques, which are dense, mostly insoluble deposits of beta-amyloid protein that develop around neurons in the hippocampus and other regions in the cerebral cortex used for decision-making. These plaques disrupt brain function and lead to brain atrophy. The LATE group also reports that this same pathology has been noted with LATE: "Many subjects with LATE-NC have comorbid brain pathologies, often including amyloid-beta plaques and tauopathy." That said, genetic studies have helped identify five genes with risk alleles for LATE (GRN, TMEM106B, ABCC9, KCNMB2, and APOE), suggesting disease-specific underlying mechanisms compared to AD.
Patient and caregiver education and guidance is vital with a dementia diagnosis. If LATE and/or AD are suspected, physicians should encourage the involvement of family and friends who agree to become more involved in the patient's care as the disease progresses. These individuals need to understand the patient's wishes around care, especially for the future when the patient is no longer able to make decisions. The patient may also consider establishing medical advance directives and durable power of attorney for medical and financial decision-making. Caregivers supporting the patient are encouraged to help balance the physical needs of the patient while maintaining respect for them as a competent adult to the extent allowed by the progression of their disease.
Because LATE is a new classification of dementia, there are no known effective treatments. One ongoing study is testing the use of autologous bone marrow–derived stem cells to help improve cognitive impairment among patients with LATE, AD, and other dementias. Current AD treatments are focused on symptomatic therapies that modulate neurotransmitters — either acetylcholine or glutamate. The standard medical treatment includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Two amyloid-directed antibodies (aducanumab, lecanemab) are currently available in the United States for individuals with AD exhibiting mild cognitive impairment or mild dementia. A third agent currently in clinical trials (donanemab) has shown significantly slowed clinical progression after 1.5 years among clinical trial participants with early symptomatic AD and amyloid and tau pathology.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's results on the genetic panel and MRI, as well as the noted cognitive decline and increased aggression, this patient is suspected of having limbic-predominant age-related TDP-43 encephalopathy (LATE) secondary to AD and is referred to the neurologist on her multidisciplinary care team for further consultation and testing.
AD is one of the most common forms of dementia. More than 6 million people in the United States have clinical AD or mild cognitive impairment because of AD. LATE is a new classification of dementia, identified in 2019, that mimics AD but is a unique disease entity driven by the misfolding of the protein TDP-43, which regulates gene expression in the brain. Misfolded TDP-43 protein is common among older adults aged ≥ 85 years, and about a quarter of this population has enough misfolded TDP-43 protein to affect their memory and cognition.
Diagnosing AD currently relies on a clinical approach. A complete physical examination, with a detailed neurologic examination and a mental status examination, is used to evaluate disease stage. Initial mental status testing evaluates attention and concentration, recent and remote memory, language, praxis, executive function, and visuospatial function. Because LATE is a newly discovered form of dementia, there are no set guidelines on diagnosing LATE and no robust biomarker for TDP-43. What is known about LATE has been gleaned mostly from retrospective clinicopathologic studies.
The LATE consensus working group reports that the clinical course of disease, as studied by autopsy-proven LATE neuropathologic change (LATE-NC), is described as an "amnestic cognitive syndrome that can evolve to incorporate multiple cognitive domains and ultimately to impair activities of daily living." Researchers are currently analyzing different clinical assessments and neuroimaging with MRI to characterize LATE. A group of international researchers recently published a set of clinical criteria for limbic-predominant amnestic neurodegenerative syndrome (LANS), which is associated with LATE-NC. Their criteria include "core, standard and advanced features that are measurable in vivo, including older age at evaluation, mild clinical syndrome, disproportionate hippocampal atrophy, impaired semantic memory, limbic hypometabolism, absence of neocortical degenerative patterns and low likelihood of neocortical tau, with degrees of certainty (highest, high, moderate, low)." Other neuroimaging studies of autopsy-confirmed LATE-NC have shown that atrophy is mostly focused in the medial temporal lobe with marked reduced hippocampal volume.
The group reports that LATE and AD probably share pathophysiologic mechanisms. One of the universally accepted hallmarks of AD is the formation of beta-amyloid plaques, which are dense, mostly insoluble deposits of beta-amyloid protein that develop around neurons in the hippocampus and other regions in the cerebral cortex used for decision-making. These plaques disrupt brain function and lead to brain atrophy. The LATE group also reports that this same pathology has been noted with LATE: "Many subjects with LATE-NC have comorbid brain pathologies, often including amyloid-beta plaques and tauopathy." That said, genetic studies have helped identify five genes with risk alleles for LATE (GRN, TMEM106B, ABCC9, KCNMB2, and APOE), suggesting disease-specific underlying mechanisms compared to AD.
Patient and caregiver education and guidance is vital with a dementia diagnosis. If LATE and/or AD are suspected, physicians should encourage the involvement of family and friends who agree to become more involved in the patient's care as the disease progresses. These individuals need to understand the patient's wishes around care, especially for the future when the patient is no longer able to make decisions. The patient may also consider establishing medical advance directives and durable power of attorney for medical and financial decision-making. Caregivers supporting the patient are encouraged to help balance the physical needs of the patient while maintaining respect for them as a competent adult to the extent allowed by the progression of their disease.
Because LATE is a new classification of dementia, there are no known effective treatments. One ongoing study is testing the use of autologous bone marrow–derived stem cells to help improve cognitive impairment among patients with LATE, AD, and other dementias. Current AD treatments are focused on symptomatic therapies that modulate neurotransmitters — either acetylcholine or glutamate. The standard medical treatment includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Two amyloid-directed antibodies (aducanumab, lecanemab) are currently available in the United States for individuals with AD exhibiting mild cognitive impairment or mild dementia. A third agent currently in clinical trials (donanemab) has shown significantly slowed clinical progression after 1.5 years among clinical trial participants with early symptomatic AD and amyloid and tau pathology.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's results on the genetic panel and MRI, as well as the noted cognitive decline and increased aggression, this patient is suspected of having limbic-predominant age-related TDP-43 encephalopathy (LATE) secondary to AD and is referred to the neurologist on her multidisciplinary care team for further consultation and testing.
AD is one of the most common forms of dementia. More than 6 million people in the United States have clinical AD or mild cognitive impairment because of AD. LATE is a new classification of dementia, identified in 2019, that mimics AD but is a unique disease entity driven by the misfolding of the protein TDP-43, which regulates gene expression in the brain. Misfolded TDP-43 protein is common among older adults aged ≥ 85 years, and about a quarter of this population has enough misfolded TDP-43 protein to affect their memory and cognition.
Diagnosing AD currently relies on a clinical approach. A complete physical examination, with a detailed neurologic examination and a mental status examination, is used to evaluate disease stage. Initial mental status testing evaluates attention and concentration, recent and remote memory, language, praxis, executive function, and visuospatial function. Because LATE is a newly discovered form of dementia, there are no set guidelines on diagnosing LATE and no robust biomarker for TDP-43. What is known about LATE has been gleaned mostly from retrospective clinicopathologic studies.
The LATE consensus working group reports that the clinical course of disease, as studied by autopsy-proven LATE neuropathologic change (LATE-NC), is described as an "amnestic cognitive syndrome that can evolve to incorporate multiple cognitive domains and ultimately to impair activities of daily living." Researchers are currently analyzing different clinical assessments and neuroimaging with MRI to characterize LATE. A group of international researchers recently published a set of clinical criteria for limbic-predominant amnestic neurodegenerative syndrome (LANS), which is associated with LATE-NC. Their criteria include "core, standard and advanced features that are measurable in vivo, including older age at evaluation, mild clinical syndrome, disproportionate hippocampal atrophy, impaired semantic memory, limbic hypometabolism, absence of neocortical degenerative patterns and low likelihood of neocortical tau, with degrees of certainty (highest, high, moderate, low)." Other neuroimaging studies of autopsy-confirmed LATE-NC have shown that atrophy is mostly focused in the medial temporal lobe with marked reduced hippocampal volume.
The group reports that LATE and AD probably share pathophysiologic mechanisms. One of the universally accepted hallmarks of AD is the formation of beta-amyloid plaques, which are dense, mostly insoluble deposits of beta-amyloid protein that develop around neurons in the hippocampus and other regions in the cerebral cortex used for decision-making. These plaques disrupt brain function and lead to brain atrophy. The LATE group also reports that this same pathology has been noted with LATE: "Many subjects with LATE-NC have comorbid brain pathologies, often including amyloid-beta plaques and tauopathy." That said, genetic studies have helped identify five genes with risk alleles for LATE (GRN, TMEM106B, ABCC9, KCNMB2, and APOE), suggesting disease-specific underlying mechanisms compared to AD.
Patient and caregiver education and guidance is vital with a dementia diagnosis. If LATE and/or AD are suspected, physicians should encourage the involvement of family and friends who agree to become more involved in the patient's care as the disease progresses. These individuals need to understand the patient's wishes around care, especially for the future when the patient is no longer able to make decisions. The patient may also consider establishing medical advance directives and durable power of attorney for medical and financial decision-making. Caregivers supporting the patient are encouraged to help balance the physical needs of the patient while maintaining respect for them as a competent adult to the extent allowed by the progression of their disease.
Because LATE is a new classification of dementia, there are no known effective treatments. One ongoing study is testing the use of autologous bone marrow–derived stem cells to help improve cognitive impairment among patients with LATE, AD, and other dementias. Current AD treatments are focused on symptomatic therapies that modulate neurotransmitters — either acetylcholine or glutamate. The standard medical treatment includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Two amyloid-directed antibodies (aducanumab, lecanemab) are currently available in the United States for individuals with AD exhibiting mild cognitive impairment or mild dementia. A third agent currently in clinical trials (donanemab) has shown significantly slowed clinical progression after 1.5 years among clinical trial participants with early symptomatic AD and amyloid and tau pathology.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
An 85-year-old woman presents to her geriatrician with her daughter, who is her primary caregiver. Seven years ago, the patient was diagnosed with mild Alzheimer's disease (AD). Her symptoms at diagnosis were irritability, forgetfulness, and panic attacks. Cognitive, behavioral, and functional assessments showed levels of decline; neurologic examination revealed mild hyposmia. The patient has been living with her daughter ever since her AD diagnosis.
At today's visit, the daughter reports that her mother has been experiencing loss of appetite and wide mood fluctuations with moments of unusual agitation. In addition, she tells the geriatrician that her mother has had trouble maintaining her balance and seems to have lost her sense of time. The patient has difficulty remembering what month and day it is, and how long it's been since her brother came to visit — which has been every Sunday like clockwork since the patient moved in with her daughter. The daughter also notes that her mother loses track of the story line when she is watching movie and TV shows lately.
The physician orders a brain MRI and genetic panel. MRI reveals atrophy in the frontal cortex as well as the medial temporal lobe, with hippocampal sclerosis. The genetic panel shows APOE and TMEM106 mutations.
‘Fake Xanax’ Tied to Seizures, Coma Is Resistant to Naloxone
Bromazolam, a street drug that has been detected with increasing frequency in the United States, has reportedly caused protracted seizures, myocardial injury, comas, and multiday intensive care stays in three individuals, new data from the US Centers for Disease Control and Prevention (CDC) showed.
The substance is one of at least a dozen designer benzodiazepines created in the lab but not approved for any therapeutic use. The Center for Forensic Science Research and Education (CFSRE) reported that bromazolam was first detected in 2016 in recreational drugs in Europe and subsequently appeared in the United States.
It is sold under names such as “XLI-268,” “Xanax,” “Fake Xanax,” and “Dope.” Bromazolam may be sold in tablet or powder form, or sometimes as gummies, and is often taken with fentanyl by users.
The CDC report, published in the Morbidity and Mortality Weekly Report (MMWR), described three cases of “previously healthy young adults,” two 25-year-old men and a 20-year-old woman, who took tablets believing it was alprazolam, when it was actually bromazolam and were found unresponsive.
They could not be revived with naloxone and continued to be unresponsive upon arrival at the emergency department. One of the men was hypertensive (152/100 mmHg), tachycardic (heart rate of 124 beats per minute), and hyperthermic (101.7 °F [38.7 °C]) and experienced multiple generalized seizures. He was intubated and admitted to intensive care.
The other man also had an elevated temperature (100.4 °F) and was intubated and admitted to the ICU because of unresponsiveness and multiple generalized seizures.
The woman was also intubated and nonresponsive with focal seizures. All three had elevated troponin levels and had urine tests positive for benzodiazepines.
The first man was intubated for 5 days and discharged after 11 days, while the second man was discharged on the fourth day with mild hearing difficulty.
The woman progressed to status epilepticus despite administration of multiple antiepileptic medications and was in a persistent coma. She was transferred to a second hospital after 11 days and was subsequently lost to follow-up.
Toxicology testing by the Drug Enforcement Administration confirmed the presence of bromazolam (range = 31.1-207 ng/mL), without the presence of fentanyl or any other opioid.
The CDC said that “the constellation of findings reported should prompt close involvement with public health officials and regional poison centers, given the more severe findings in these reported cases compared with those expected from routine benzodiazepine overdoses.” In addition, it noted that clinicians and first responders should “consider bromazolam in cases of patients requiring treatment for seizures, myocardial injury, or hyperthermia after illicit drug use.”
Surging Supply, Increased Warnings
In 2022, the CDC warned that the drug was surging in the United States, noting that as of mid-2022, bromazolam was identified in more than 250 toxicology cases submitted to NMS Labs, and that it had been identified in more than 190 toxicology samples tested at CFSRE.
In early 2021, only 1% of samples were positive for bromazolam. By mid-2022, 13% of samples were positive for bromazolam, and 75% of the bromazolam samples were positive for fentanyl.
The combination is sold on the street as benzo-dope.
Health authorities across the globe have been warning about the dangers of designer benzodiazepines, and bromazolam in particular. They’ve noted that the overdose reversal agent naloxone does not combat the effects of a benzodiazepine overdose.
In December 2022, the Canadian province of New Brunswick said that bromazolam had been detected in nine sudden death investigations, and that fentanyl was detected in some of those cases. The provincial government of the Northwest Territories warned in May 2023 that bromazolam had been detected in the region’s drug supply and cautioned against combining it with opioids.
The Indiana Department of Health notified the public, first responders, law enforcement, and clinicians in August 2023 that the drug was increasingly being detected in the state. In the first half of the year, 35 people who had overdosed in Indiana tested positive for bromazolam. The state did not test for the presence of bromazolam before 2023.
According to the MMWR, the law enforcement seizures in the United States of bromazolam increased from no more than three per year during 2016-2018 to 2142 in 2022 and 2913 in 2023.
Illinois has been an area of increased use. Bromazolam-involved deaths increased from 10 in 2021 to 51 in 2022, the CDC researchers reported.
A version of this article appeared on Medscape.com.
Bromazolam, a street drug that has been detected with increasing frequency in the United States, has reportedly caused protracted seizures, myocardial injury, comas, and multiday intensive care stays in three individuals, new data from the US Centers for Disease Control and Prevention (CDC) showed.
The substance is one of at least a dozen designer benzodiazepines created in the lab but not approved for any therapeutic use. The Center for Forensic Science Research and Education (CFSRE) reported that bromazolam was first detected in 2016 in recreational drugs in Europe and subsequently appeared in the United States.
It is sold under names such as “XLI-268,” “Xanax,” “Fake Xanax,” and “Dope.” Bromazolam may be sold in tablet or powder form, or sometimes as gummies, and is often taken with fentanyl by users.
The CDC report, published in the Morbidity and Mortality Weekly Report (MMWR), described three cases of “previously healthy young adults,” two 25-year-old men and a 20-year-old woman, who took tablets believing it was alprazolam, when it was actually bromazolam and were found unresponsive.
They could not be revived with naloxone and continued to be unresponsive upon arrival at the emergency department. One of the men was hypertensive (152/100 mmHg), tachycardic (heart rate of 124 beats per minute), and hyperthermic (101.7 °F [38.7 °C]) and experienced multiple generalized seizures. He was intubated and admitted to intensive care.
The other man also had an elevated temperature (100.4 °F) and was intubated and admitted to the ICU because of unresponsiveness and multiple generalized seizures.
The woman was also intubated and nonresponsive with focal seizures. All three had elevated troponin levels and had urine tests positive for benzodiazepines.
The first man was intubated for 5 days and discharged after 11 days, while the second man was discharged on the fourth day with mild hearing difficulty.
The woman progressed to status epilepticus despite administration of multiple antiepileptic medications and was in a persistent coma. She was transferred to a second hospital after 11 days and was subsequently lost to follow-up.
Toxicology testing by the Drug Enforcement Administration confirmed the presence of bromazolam (range = 31.1-207 ng/mL), without the presence of fentanyl or any other opioid.
The CDC said that “the constellation of findings reported should prompt close involvement with public health officials and regional poison centers, given the more severe findings in these reported cases compared with those expected from routine benzodiazepine overdoses.” In addition, it noted that clinicians and first responders should “consider bromazolam in cases of patients requiring treatment for seizures, myocardial injury, or hyperthermia after illicit drug use.”
Surging Supply, Increased Warnings
In 2022, the CDC warned that the drug was surging in the United States, noting that as of mid-2022, bromazolam was identified in more than 250 toxicology cases submitted to NMS Labs, and that it had been identified in more than 190 toxicology samples tested at CFSRE.
In early 2021, only 1% of samples were positive for bromazolam. By mid-2022, 13% of samples were positive for bromazolam, and 75% of the bromazolam samples were positive for fentanyl.
The combination is sold on the street as benzo-dope.
Health authorities across the globe have been warning about the dangers of designer benzodiazepines, and bromazolam in particular. They’ve noted that the overdose reversal agent naloxone does not combat the effects of a benzodiazepine overdose.
In December 2022, the Canadian province of New Brunswick said that bromazolam had been detected in nine sudden death investigations, and that fentanyl was detected in some of those cases. The provincial government of the Northwest Territories warned in May 2023 that bromazolam had been detected in the region’s drug supply and cautioned against combining it with opioids.
The Indiana Department of Health notified the public, first responders, law enforcement, and clinicians in August 2023 that the drug was increasingly being detected in the state. In the first half of the year, 35 people who had overdosed in Indiana tested positive for bromazolam. The state did not test for the presence of bromazolam before 2023.
According to the MMWR, the law enforcement seizures in the United States of bromazolam increased from no more than three per year during 2016-2018 to 2142 in 2022 and 2913 in 2023.
Illinois has been an area of increased use. Bromazolam-involved deaths increased from 10 in 2021 to 51 in 2022, the CDC researchers reported.
A version of this article appeared on Medscape.com.
Bromazolam, a street drug that has been detected with increasing frequency in the United States, has reportedly caused protracted seizures, myocardial injury, comas, and multiday intensive care stays in three individuals, new data from the US Centers for Disease Control and Prevention (CDC) showed.
The substance is one of at least a dozen designer benzodiazepines created in the lab but not approved for any therapeutic use. The Center for Forensic Science Research and Education (CFSRE) reported that bromazolam was first detected in 2016 in recreational drugs in Europe and subsequently appeared in the United States.
It is sold under names such as “XLI-268,” “Xanax,” “Fake Xanax,” and “Dope.” Bromazolam may be sold in tablet or powder form, or sometimes as gummies, and is often taken with fentanyl by users.
The CDC report, published in the Morbidity and Mortality Weekly Report (MMWR), described three cases of “previously healthy young adults,” two 25-year-old men and a 20-year-old woman, who took tablets believing it was alprazolam, when it was actually bromazolam and were found unresponsive.
They could not be revived with naloxone and continued to be unresponsive upon arrival at the emergency department. One of the men was hypertensive (152/100 mmHg), tachycardic (heart rate of 124 beats per minute), and hyperthermic (101.7 °F [38.7 °C]) and experienced multiple generalized seizures. He was intubated and admitted to intensive care.
The other man also had an elevated temperature (100.4 °F) and was intubated and admitted to the ICU because of unresponsiveness and multiple generalized seizures.
The woman was also intubated and nonresponsive with focal seizures. All three had elevated troponin levels and had urine tests positive for benzodiazepines.
The first man was intubated for 5 days and discharged after 11 days, while the second man was discharged on the fourth day with mild hearing difficulty.
The woman progressed to status epilepticus despite administration of multiple antiepileptic medications and was in a persistent coma. She was transferred to a second hospital after 11 days and was subsequently lost to follow-up.
Toxicology testing by the Drug Enforcement Administration confirmed the presence of bromazolam (range = 31.1-207 ng/mL), without the presence of fentanyl or any other opioid.
The CDC said that “the constellation of findings reported should prompt close involvement with public health officials and regional poison centers, given the more severe findings in these reported cases compared with those expected from routine benzodiazepine overdoses.” In addition, it noted that clinicians and first responders should “consider bromazolam in cases of patients requiring treatment for seizures, myocardial injury, or hyperthermia after illicit drug use.”
Surging Supply, Increased Warnings
In 2022, the CDC warned that the drug was surging in the United States, noting that as of mid-2022, bromazolam was identified in more than 250 toxicology cases submitted to NMS Labs, and that it had been identified in more than 190 toxicology samples tested at CFSRE.
In early 2021, only 1% of samples were positive for bromazolam. By mid-2022, 13% of samples were positive for bromazolam, and 75% of the bromazolam samples were positive for fentanyl.
The combination is sold on the street as benzo-dope.
Health authorities across the globe have been warning about the dangers of designer benzodiazepines, and bromazolam in particular. They’ve noted that the overdose reversal agent naloxone does not combat the effects of a benzodiazepine overdose.
In December 2022, the Canadian province of New Brunswick said that bromazolam had been detected in nine sudden death investigations, and that fentanyl was detected in some of those cases. The provincial government of the Northwest Territories warned in May 2023 that bromazolam had been detected in the region’s drug supply and cautioned against combining it with opioids.
The Indiana Department of Health notified the public, first responders, law enforcement, and clinicians in August 2023 that the drug was increasingly being detected in the state. In the first half of the year, 35 people who had overdosed in Indiana tested positive for bromazolam. The state did not test for the presence of bromazolam before 2023.
According to the MMWR, the law enforcement seizures in the United States of bromazolam increased from no more than three per year during 2016-2018 to 2142 in 2022 and 2913 in 2023.
Illinois has been an area of increased use. Bromazolam-involved deaths increased from 10 in 2021 to 51 in 2022, the CDC researchers reported.
A version of this article appeared on Medscape.com.
FROM THE MORBIDITY AND MORTALITY WEEKLY REPORT
Side Effects of Local Treatment for Advanced Prostate Cancer May Linger for Years
TOPLINE:
METHODOLOGY:
Recent evidence suggested that in men with advanced prostate cancer, local therapy with radical prostatectomy or radiation may improve survival outcomes; however, data on the long-term side effects from these local options were limited.
The retrospective cohort included 5502 men (mean age, 68 years) diagnosed with advanced (T4, N1, and/or M1) prostate cancer.
A total of 1705 men (31%) received initial local treatment, consisting of radical prostatectomy, (55%), radiation (39%), or both (5.6%), while 3797 (69%) opted for initial nonlocal treatment (hormone therapy, chemotherapy, or both).
The main outcomes were treatment-related adverse effects, including GI, chronic pain, sexual dysfunction, and urinary symptoms, assessed at three timepoints after initial treatment — up to 1 year, between 1 and 2 years, and between 2 and 5 years.
TAKEAWAY:
Overall, 916 men (75%) who had initial local treatment and 897 men (67%) with initial nonlocal therapy reported at least one adverse condition up to 5 years after initial treatment.
In the first year after initial treatment, local therapy was associated with a higher prevalence of GI (9% vs 3%), pain (60% vs 38%), sexual (37% vs 8%), and urinary (46.5% vs 18%) conditions. Men receiving local therapy were more likely to experience GI (adjusted odds ratio [aOR], 4.08), pain (aOR, 1.57), sexual (aOR, 2.96), and urinary (aOR, 2.25) conditions.
Between 2 and 5 years after local therapy, certain conditions remained more prevalent — 7.8% vs 4.2% for GI, 40% vs 13% for sexual, and 40.5% vs 26% for urinary issues. Men receiving local vs nonlocal therapy were more likely to experience GI (aOR, 2.39), sexual (aOR, 3.36), and urinary (aOR, 1.39) issues over the long term.
The researchers found no difference in the prevalence of constitutional conditions such as hot flashes (36.5% vs 34.4%) in the first year following initial local or nonlocal therapy. However, local treatment followed by any secondary treatment was associated with a higher likelihood of developing constitutional conditions at 1-2 years (aOR, 1.50) and 2-5 years (aOR, 1.78) after initial treatment.
IN PRACTICE:
“These results suggest that patients and clinicians should consider the adverse effects of local treatment” alongside the potential for enhanced survival when making treatment decisions in the setting of advanced prostate cancer, the authors explained. Careful informed decision-making by both patients and practitioners is especially important because “there are currently no established guidelines regarding the use of local treatment among men with advanced prostate cancer.”
SOURCE:
The study, with first author Saira Khan, PhD, MPH, Washington University School of Medicine in St. Louis, Missouri, was published online in JAMA Network Open.
LIMITATIONS:
The authors noted that the study was limited by its retrospective design. Men who received local treatment were, on average, younger; older or lesser healthy patients who received local treatment may experience worse adverse effects than observed in the study. The study was limited to US veterans.
DISCLOSURES:
The study was supported by a grant from the US Department of Defense. The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
Recent evidence suggested that in men with advanced prostate cancer, local therapy with radical prostatectomy or radiation may improve survival outcomes; however, data on the long-term side effects from these local options were limited.
The retrospective cohort included 5502 men (mean age, 68 years) diagnosed with advanced (T4, N1, and/or M1) prostate cancer.
A total of 1705 men (31%) received initial local treatment, consisting of radical prostatectomy, (55%), radiation (39%), or both (5.6%), while 3797 (69%) opted for initial nonlocal treatment (hormone therapy, chemotherapy, or both).
The main outcomes were treatment-related adverse effects, including GI, chronic pain, sexual dysfunction, and urinary symptoms, assessed at three timepoints after initial treatment — up to 1 year, between 1 and 2 years, and between 2 and 5 years.
TAKEAWAY:
Overall, 916 men (75%) who had initial local treatment and 897 men (67%) with initial nonlocal therapy reported at least one adverse condition up to 5 years after initial treatment.
In the first year after initial treatment, local therapy was associated with a higher prevalence of GI (9% vs 3%), pain (60% vs 38%), sexual (37% vs 8%), and urinary (46.5% vs 18%) conditions. Men receiving local therapy were more likely to experience GI (adjusted odds ratio [aOR], 4.08), pain (aOR, 1.57), sexual (aOR, 2.96), and urinary (aOR, 2.25) conditions.
Between 2 and 5 years after local therapy, certain conditions remained more prevalent — 7.8% vs 4.2% for GI, 40% vs 13% for sexual, and 40.5% vs 26% for urinary issues. Men receiving local vs nonlocal therapy were more likely to experience GI (aOR, 2.39), sexual (aOR, 3.36), and urinary (aOR, 1.39) issues over the long term.
The researchers found no difference in the prevalence of constitutional conditions such as hot flashes (36.5% vs 34.4%) in the first year following initial local or nonlocal therapy. However, local treatment followed by any secondary treatment was associated with a higher likelihood of developing constitutional conditions at 1-2 years (aOR, 1.50) and 2-5 years (aOR, 1.78) after initial treatment.
IN PRACTICE:
“These results suggest that patients and clinicians should consider the adverse effects of local treatment” alongside the potential for enhanced survival when making treatment decisions in the setting of advanced prostate cancer, the authors explained. Careful informed decision-making by both patients and practitioners is especially important because “there are currently no established guidelines regarding the use of local treatment among men with advanced prostate cancer.”
SOURCE:
The study, with first author Saira Khan, PhD, MPH, Washington University School of Medicine in St. Louis, Missouri, was published online in JAMA Network Open.
LIMITATIONS:
The authors noted that the study was limited by its retrospective design. Men who received local treatment were, on average, younger; older or lesser healthy patients who received local treatment may experience worse adverse effects than observed in the study. The study was limited to US veterans.
DISCLOSURES:
The study was supported by a grant from the US Department of Defense. The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
Recent evidence suggested that in men with advanced prostate cancer, local therapy with radical prostatectomy or radiation may improve survival outcomes; however, data on the long-term side effects from these local options were limited.
The retrospective cohort included 5502 men (mean age, 68 years) diagnosed with advanced (T4, N1, and/or M1) prostate cancer.
A total of 1705 men (31%) received initial local treatment, consisting of radical prostatectomy, (55%), radiation (39%), or both (5.6%), while 3797 (69%) opted for initial nonlocal treatment (hormone therapy, chemotherapy, or both).
The main outcomes were treatment-related adverse effects, including GI, chronic pain, sexual dysfunction, and urinary symptoms, assessed at three timepoints after initial treatment — up to 1 year, between 1 and 2 years, and between 2 and 5 years.
TAKEAWAY:
Overall, 916 men (75%) who had initial local treatment and 897 men (67%) with initial nonlocal therapy reported at least one adverse condition up to 5 years after initial treatment.
In the first year after initial treatment, local therapy was associated with a higher prevalence of GI (9% vs 3%), pain (60% vs 38%), sexual (37% vs 8%), and urinary (46.5% vs 18%) conditions. Men receiving local therapy were more likely to experience GI (adjusted odds ratio [aOR], 4.08), pain (aOR, 1.57), sexual (aOR, 2.96), and urinary (aOR, 2.25) conditions.
Between 2 and 5 years after local therapy, certain conditions remained more prevalent — 7.8% vs 4.2% for GI, 40% vs 13% for sexual, and 40.5% vs 26% for urinary issues. Men receiving local vs nonlocal therapy were more likely to experience GI (aOR, 2.39), sexual (aOR, 3.36), and urinary (aOR, 1.39) issues over the long term.
The researchers found no difference in the prevalence of constitutional conditions such as hot flashes (36.5% vs 34.4%) in the first year following initial local or nonlocal therapy. However, local treatment followed by any secondary treatment was associated with a higher likelihood of developing constitutional conditions at 1-2 years (aOR, 1.50) and 2-5 years (aOR, 1.78) after initial treatment.
IN PRACTICE:
“These results suggest that patients and clinicians should consider the adverse effects of local treatment” alongside the potential for enhanced survival when making treatment decisions in the setting of advanced prostate cancer, the authors explained. Careful informed decision-making by both patients and practitioners is especially important because “there are currently no established guidelines regarding the use of local treatment among men with advanced prostate cancer.”
SOURCE:
The study, with first author Saira Khan, PhD, MPH, Washington University School of Medicine in St. Louis, Missouri, was published online in JAMA Network Open.
LIMITATIONS:
The authors noted that the study was limited by its retrospective design. Men who received local treatment were, on average, younger; older or lesser healthy patients who received local treatment may experience worse adverse effects than observed in the study. The study was limited to US veterans.
DISCLOSURES:
The study was supported by a grant from the US Department of Defense. The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
Rebranding NAFLD: Correcting ‘Flawed’ Conventions
Nonalcoholic fatty liver disease (NAFLD) should now be referred to as metabolic dysfunction–associated steatotic liver disease (MASLD), according to a recent commentary by leading hepatologists.
This update, which was determined by a panel of 236 panelists from 56 countries, is part of a broader effort to rebrand “fatty liver disease” as “steatotic liver disease” (SLD), reported lead author Alina M. Allen, MD, of Mayo Clinic, Rochester, Minnesota, and colleagues.
Writing in Gastroenterology, they described a range of reasons for the nomenclature changes, from the need for better characterization of disease subtypes, to the concern that the term “fatty” may be perceived as stigmatizing by some patients.
“The scientific community and stakeholder organizations associated with liver diseases determined there was a need for new terminology to cover liver disease related to alcohol alone, metabolic risk factors (until recently termed NAFLD/nonalcoholic steatohepatitis [NASH]) alone, the combination of alcohol and metabolic risk factors, and hepatic steatosis due to other specific etiologies,” the authors wrote.
Naming conventions in this area have been flawed since inception, Dr. Allen and colleagues wrote, noting that “nonalcoholic” is exclusionary rather than descriptive, and is particularly misplaced in the pediatric setting. These shortcomings could explain why the term “NASH” took more than a decade to enter common usage, they suggested, and why the present effort is not the first of its kind.
“There have been several movements to change the nomenclature [of NAFLD], including most recently to ‘metabolic dysfunction–associated fatty liver disease’ (MAFLD), a term that received limited traction,” the authors wrote.
Still, a change is needed, they added, as metabolic dysfunction is becoming increasingly common on a global scale, driving up rates of liver disease. Furthermore, in some patients, alcohol consumption and metabolic factors concurrently drive steatosis, suggesting an intermediate condition between alcohol-related liver disease (ALD) and NAFLD that is indescribable via current naming conventions.
SLD (determined by imaging or biopsy) now comprises five disease subtypes that can be determined via an algorithm provided in the present publication.
If at least one metabolic criterion is present, but no other causes of steatosis, then that patient has MASLD. The three other metabolic subtypes include MetALD (2-3 drinks per day for women and 3-4 drinks per day for men), ALD (more than 3 drinks per day for women and more than 4 drinks per day for men), and monogenic miscellaneous drug-induced liver injury (DILI).
Patients without metabolic criteria can also be classified with monogenic miscellaneous DILI with no caveat, whereas patients with metabolic criteria need only consume 2 or 3 drinks per day for women or 3-4 drinks per day for men, respectively, to be diagnosed with ALD.
Finally, patients with no metabolic criteria or other cause of steatosis should be characterized by cryptogenic SLD.
“While renaming and redefining the disease was needed, the implementation is not without challenges,” Dr. Allen and colleagues wrote. “A more complex classification may add confusion in the mind of nonhepatology providers when awareness and understanding of the implications of SLD are already suboptimal.”
Still, they predicted that the new naming system could lead to several positive outcomes, including improved SLD screening among individuals with metabolic risk factors, more accurate phenotyping of patients with moderate alcohol consumption, increased disease awareness in nonhepatology practices, and improved multidisciplinary collaboration.
Only time will tell whether these benefits come to fruition, Dr. Allen and colleagues noted, before closing with a quote: “In the words of Jean Piaget, the developmental psychologist of the 20th century, who coincidentally died the year the term NASH was coined, ‘Scientific knowledge is in perpetual evolution; it finds itself changed from one day to the next.’”
The authors disclosed no conflicts of interest.
Nonalcoholic fatty liver disease (NAFLD) should now be referred to as metabolic dysfunction–associated steatotic liver disease (MASLD), according to a recent commentary by leading hepatologists.
This update, which was determined by a panel of 236 panelists from 56 countries, is part of a broader effort to rebrand “fatty liver disease” as “steatotic liver disease” (SLD), reported lead author Alina M. Allen, MD, of Mayo Clinic, Rochester, Minnesota, and colleagues.
Writing in Gastroenterology, they described a range of reasons for the nomenclature changes, from the need for better characterization of disease subtypes, to the concern that the term “fatty” may be perceived as stigmatizing by some patients.
“The scientific community and stakeholder organizations associated with liver diseases determined there was a need for new terminology to cover liver disease related to alcohol alone, metabolic risk factors (until recently termed NAFLD/nonalcoholic steatohepatitis [NASH]) alone, the combination of alcohol and metabolic risk factors, and hepatic steatosis due to other specific etiologies,” the authors wrote.
Naming conventions in this area have been flawed since inception, Dr. Allen and colleagues wrote, noting that “nonalcoholic” is exclusionary rather than descriptive, and is particularly misplaced in the pediatric setting. These shortcomings could explain why the term “NASH” took more than a decade to enter common usage, they suggested, and why the present effort is not the first of its kind.
“There have been several movements to change the nomenclature [of NAFLD], including most recently to ‘metabolic dysfunction–associated fatty liver disease’ (MAFLD), a term that received limited traction,” the authors wrote.
Still, a change is needed, they added, as metabolic dysfunction is becoming increasingly common on a global scale, driving up rates of liver disease. Furthermore, in some patients, alcohol consumption and metabolic factors concurrently drive steatosis, suggesting an intermediate condition between alcohol-related liver disease (ALD) and NAFLD that is indescribable via current naming conventions.
SLD (determined by imaging or biopsy) now comprises five disease subtypes that can be determined via an algorithm provided in the present publication.
If at least one metabolic criterion is present, but no other causes of steatosis, then that patient has MASLD. The three other metabolic subtypes include MetALD (2-3 drinks per day for women and 3-4 drinks per day for men), ALD (more than 3 drinks per day for women and more than 4 drinks per day for men), and monogenic miscellaneous drug-induced liver injury (DILI).
Patients without metabolic criteria can also be classified with monogenic miscellaneous DILI with no caveat, whereas patients with metabolic criteria need only consume 2 or 3 drinks per day for women or 3-4 drinks per day for men, respectively, to be diagnosed with ALD.
Finally, patients with no metabolic criteria or other cause of steatosis should be characterized by cryptogenic SLD.
“While renaming and redefining the disease was needed, the implementation is not without challenges,” Dr. Allen and colleagues wrote. “A more complex classification may add confusion in the mind of nonhepatology providers when awareness and understanding of the implications of SLD are already suboptimal.”
Still, they predicted that the new naming system could lead to several positive outcomes, including improved SLD screening among individuals with metabolic risk factors, more accurate phenotyping of patients with moderate alcohol consumption, increased disease awareness in nonhepatology practices, and improved multidisciplinary collaboration.
Only time will tell whether these benefits come to fruition, Dr. Allen and colleagues noted, before closing with a quote: “In the words of Jean Piaget, the developmental psychologist of the 20th century, who coincidentally died the year the term NASH was coined, ‘Scientific knowledge is in perpetual evolution; it finds itself changed from one day to the next.’”
The authors disclosed no conflicts of interest.
Nonalcoholic fatty liver disease (NAFLD) should now be referred to as metabolic dysfunction–associated steatotic liver disease (MASLD), according to a recent commentary by leading hepatologists.
This update, which was determined by a panel of 236 panelists from 56 countries, is part of a broader effort to rebrand “fatty liver disease” as “steatotic liver disease” (SLD), reported lead author Alina M. Allen, MD, of Mayo Clinic, Rochester, Minnesota, and colleagues.
Writing in Gastroenterology, they described a range of reasons for the nomenclature changes, from the need for better characterization of disease subtypes, to the concern that the term “fatty” may be perceived as stigmatizing by some patients.
“The scientific community and stakeholder organizations associated with liver diseases determined there was a need for new terminology to cover liver disease related to alcohol alone, metabolic risk factors (until recently termed NAFLD/nonalcoholic steatohepatitis [NASH]) alone, the combination of alcohol and metabolic risk factors, and hepatic steatosis due to other specific etiologies,” the authors wrote.
Naming conventions in this area have been flawed since inception, Dr. Allen and colleagues wrote, noting that “nonalcoholic” is exclusionary rather than descriptive, and is particularly misplaced in the pediatric setting. These shortcomings could explain why the term “NASH” took more than a decade to enter common usage, they suggested, and why the present effort is not the first of its kind.
“There have been several movements to change the nomenclature [of NAFLD], including most recently to ‘metabolic dysfunction–associated fatty liver disease’ (MAFLD), a term that received limited traction,” the authors wrote.
Still, a change is needed, they added, as metabolic dysfunction is becoming increasingly common on a global scale, driving up rates of liver disease. Furthermore, in some patients, alcohol consumption and metabolic factors concurrently drive steatosis, suggesting an intermediate condition between alcohol-related liver disease (ALD) and NAFLD that is indescribable via current naming conventions.
SLD (determined by imaging or biopsy) now comprises five disease subtypes that can be determined via an algorithm provided in the present publication.
If at least one metabolic criterion is present, but no other causes of steatosis, then that patient has MASLD. The three other metabolic subtypes include MetALD (2-3 drinks per day for women and 3-4 drinks per day for men), ALD (more than 3 drinks per day for women and more than 4 drinks per day for men), and monogenic miscellaneous drug-induced liver injury (DILI).
Patients without metabolic criteria can also be classified with monogenic miscellaneous DILI with no caveat, whereas patients with metabolic criteria need only consume 2 or 3 drinks per day for women or 3-4 drinks per day for men, respectively, to be diagnosed with ALD.
Finally, patients with no metabolic criteria or other cause of steatosis should be characterized by cryptogenic SLD.
“While renaming and redefining the disease was needed, the implementation is not without challenges,” Dr. Allen and colleagues wrote. “A more complex classification may add confusion in the mind of nonhepatology providers when awareness and understanding of the implications of SLD are already suboptimal.”
Still, they predicted that the new naming system could lead to several positive outcomes, including improved SLD screening among individuals with metabolic risk factors, more accurate phenotyping of patients with moderate alcohol consumption, increased disease awareness in nonhepatology practices, and improved multidisciplinary collaboration.
Only time will tell whether these benefits come to fruition, Dr. Allen and colleagues noted, before closing with a quote: “In the words of Jean Piaget, the developmental psychologist of the 20th century, who coincidentally died the year the term NASH was coined, ‘Scientific knowledge is in perpetual evolution; it finds itself changed from one day to the next.’”
The authors disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
Zoom: Convenient and Imperfect
Making eye contact is important in human interactions. It shows attention and comprehension. It also helps us read the nuances of another’s facial expressions when interacting.
Although the idea of video phone calls isn’t new — I remember it from my childhood in “house of the future” TV shows — it certainly didn’t take off until the advent of high-speed Internet, computers, and phones with cameras. Then Facetime, Skype, Zoom, Teams, and others.
Of course, it all still took a back seat to actually seeing people and having meetings in person. Until the pandemic made that the least attractive option. Then the adoption of such things went into hyperdrive and has stayed there ever since.
And ya know, I don’t have too many complaints. Between clinical trials and legal cases, both of which involve A LOT of meetings, it’s made my life easier. I no longer have to leave the office, allow time to drive somewhere and back, fight traffic, burn gas, and find parking. I move from a patient to the meeting and back to a patient from the cozy confines of my office, all without my tea getting cold.
But you can’t really make eye contact on Zoom. Instinctively, we generally look directly at the eyes of the person we’re speaking to, but in the virtual world we really don’t do that. On their end you’re on a screen, your gaze fixed somewhere below the level of your camera.
Try talking directly to the camera on Zoom — or any video platform. It doesn’t work. You feel like Dave addressing HAL’s red light in 2001. Inevitably your eyes are drawn back to the other person’s face, which is what you’re programmed to do. If they’re speaking you look at them, even though the sound is really coming from your speakers.
Interestingly, though, it seems something is lost in there. A recent perspective noted that Zoom meetings seemed to stifle creativity and produced fewer ideas than in person.
An interesting study compared neural response signals of people seeing a presentation on Zoom versus the same talk in person. When looking at a “real” speaker, there was synchronized neural activity, a higher level of engagement, and increased activation of the dorsal-parietal cortex.
Without actual eye contact it’s harder to read subtle facial expressions. Hand gestures and other body language may be out of the camera frame, or absent altogether. The nuances of voice pitch, timbre, and tone may not be the same over the speaker.
Our brains have spent several million of years developing facial recognition and reading, knowing friend from foe, and understanding what’s meant not just in what sounds are used but how they’re conveyed.
I’m not saying we should stop using Zoom altogether — it makes meetings more convenient for most people, including myself. But we also need to keep in mind that what it doesn’t convey is as important as what it does, and that virtual is never a perfect substitute for reality.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Making eye contact is important in human interactions. It shows attention and comprehension. It also helps us read the nuances of another’s facial expressions when interacting.
Although the idea of video phone calls isn’t new — I remember it from my childhood in “house of the future” TV shows — it certainly didn’t take off until the advent of high-speed Internet, computers, and phones with cameras. Then Facetime, Skype, Zoom, Teams, and others.
Of course, it all still took a back seat to actually seeing people and having meetings in person. Until the pandemic made that the least attractive option. Then the adoption of such things went into hyperdrive and has stayed there ever since.
And ya know, I don’t have too many complaints. Between clinical trials and legal cases, both of which involve A LOT of meetings, it’s made my life easier. I no longer have to leave the office, allow time to drive somewhere and back, fight traffic, burn gas, and find parking. I move from a patient to the meeting and back to a patient from the cozy confines of my office, all without my tea getting cold.
But you can’t really make eye contact on Zoom. Instinctively, we generally look directly at the eyes of the person we’re speaking to, but in the virtual world we really don’t do that. On their end you’re on a screen, your gaze fixed somewhere below the level of your camera.
Try talking directly to the camera on Zoom — or any video platform. It doesn’t work. You feel like Dave addressing HAL’s red light in 2001. Inevitably your eyes are drawn back to the other person’s face, which is what you’re programmed to do. If they’re speaking you look at them, even though the sound is really coming from your speakers.
Interestingly, though, it seems something is lost in there. A recent perspective noted that Zoom meetings seemed to stifle creativity and produced fewer ideas than in person.
An interesting study compared neural response signals of people seeing a presentation on Zoom versus the same talk in person. When looking at a “real” speaker, there was synchronized neural activity, a higher level of engagement, and increased activation of the dorsal-parietal cortex.
Without actual eye contact it’s harder to read subtle facial expressions. Hand gestures and other body language may be out of the camera frame, or absent altogether. The nuances of voice pitch, timbre, and tone may not be the same over the speaker.
Our brains have spent several million of years developing facial recognition and reading, knowing friend from foe, and understanding what’s meant not just in what sounds are used but how they’re conveyed.
I’m not saying we should stop using Zoom altogether — it makes meetings more convenient for most people, including myself. But we also need to keep in mind that what it doesn’t convey is as important as what it does, and that virtual is never a perfect substitute for reality.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Making eye contact is important in human interactions. It shows attention and comprehension. It also helps us read the nuances of another’s facial expressions when interacting.
Although the idea of video phone calls isn’t new — I remember it from my childhood in “house of the future” TV shows — it certainly didn’t take off until the advent of high-speed Internet, computers, and phones with cameras. Then Facetime, Skype, Zoom, Teams, and others.
Of course, it all still took a back seat to actually seeing people and having meetings in person. Until the pandemic made that the least attractive option. Then the adoption of such things went into hyperdrive and has stayed there ever since.
And ya know, I don’t have too many complaints. Between clinical trials and legal cases, both of which involve A LOT of meetings, it’s made my life easier. I no longer have to leave the office, allow time to drive somewhere and back, fight traffic, burn gas, and find parking. I move from a patient to the meeting and back to a patient from the cozy confines of my office, all without my tea getting cold.
But you can’t really make eye contact on Zoom. Instinctively, we generally look directly at the eyes of the person we’re speaking to, but in the virtual world we really don’t do that. On their end you’re on a screen, your gaze fixed somewhere below the level of your camera.
Try talking directly to the camera on Zoom — or any video platform. It doesn’t work. You feel like Dave addressing HAL’s red light in 2001. Inevitably your eyes are drawn back to the other person’s face, which is what you’re programmed to do. If they’re speaking you look at them, even though the sound is really coming from your speakers.
Interestingly, though, it seems something is lost in there. A recent perspective noted that Zoom meetings seemed to stifle creativity and produced fewer ideas than in person.
An interesting study compared neural response signals of people seeing a presentation on Zoom versus the same talk in person. When looking at a “real” speaker, there was synchronized neural activity, a higher level of engagement, and increased activation of the dorsal-parietal cortex.
Without actual eye contact it’s harder to read subtle facial expressions. Hand gestures and other body language may be out of the camera frame, or absent altogether. The nuances of voice pitch, timbre, and tone may not be the same over the speaker.
Our brains have spent several million of years developing facial recognition and reading, knowing friend from foe, and understanding what’s meant not just in what sounds are used but how they’re conveyed.
I’m not saying we should stop using Zoom altogether — it makes meetings more convenient for most people, including myself. But we also need to keep in mind that what it doesn’t convey is as important as what it does, and that virtual is never a perfect substitute for reality.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.