User login
Fall Abstract Las Vegas Dermatology Seminar Compendium; Las Vegas, Nevada; November 2-4, 2023
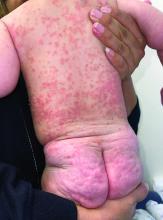
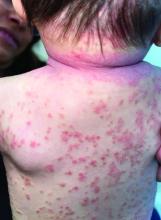
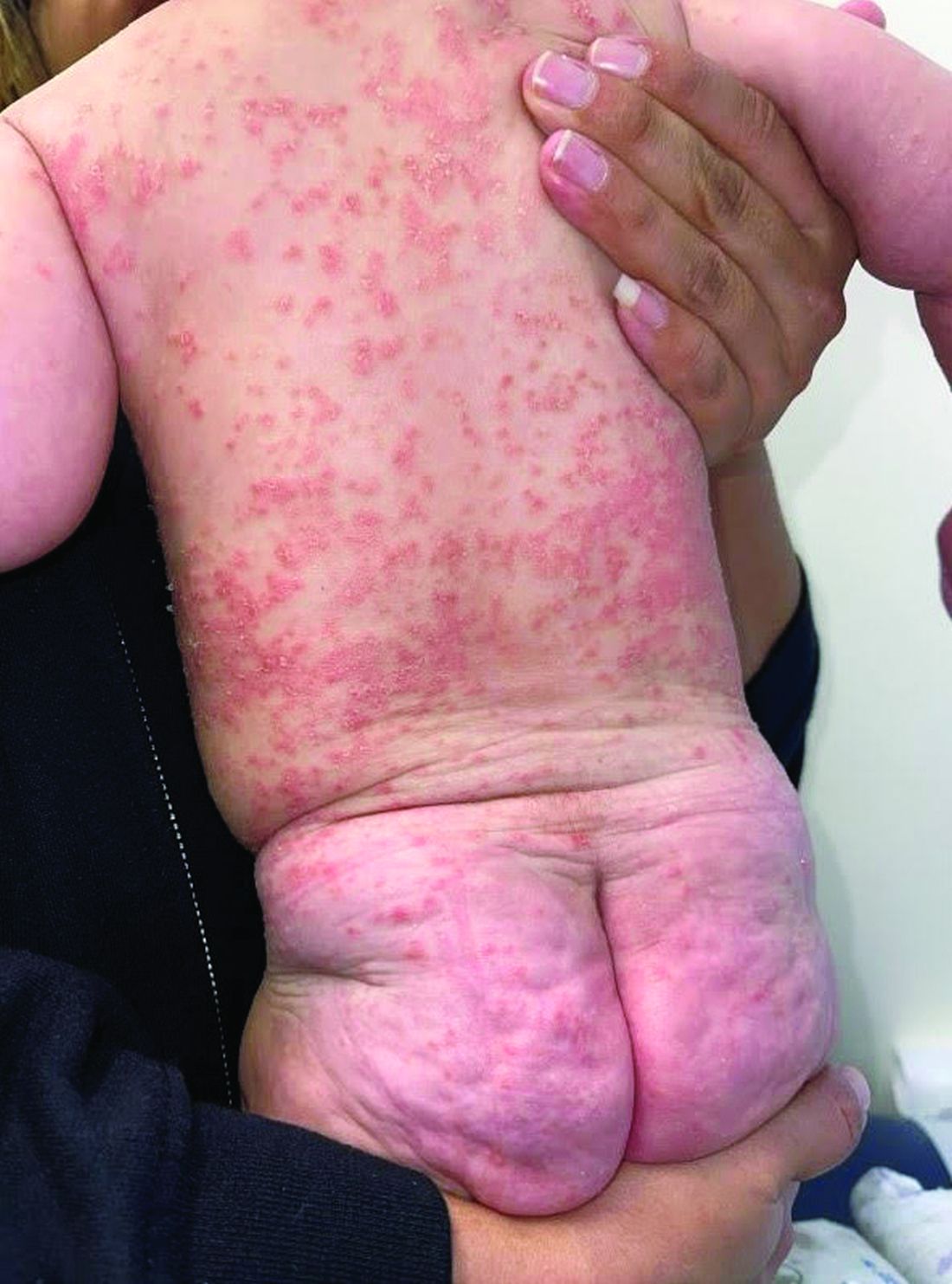
A 4-month-old male was referred for a 3-week history of an itchy generalized rash that started on the neck
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
On physical exam, there was an erythematous patch with overlying areas of macerations on the neck and axilla. The trunk, extremities, and diaper area had multiple psoriasiform erythematous thin plaques with overlying scales.
DSM-5-TR Panel Members Received $14M in Undisclosed Industry Funding
About 60% of US physicians who served as panel and task force members for the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) received more than $14 million in undisclosed industry funding, a new study shows.
Most payments were for food and beverages, travel, and consulting fees. But more than one third of contributors received compensation for services other than consulting, such as serving on a pharmaceutical company’s speakers bureau, which medical ethicists say is particularly problematic.
Often referred to as the bible of psychiatric disorders, the DSM-5-TR was released in 2022 by the American Psychiatric Association (APA) and includes changes that were made online since the DSM-5 was first published in 2013.
An APA spokesperson said that DSM-5-TR decision-makers were unable to participate if they had received more than $5000 in industry payments and that all 186 individuals who worked on the text revision were required to disclose all sources of income prior to their participation.
“The APA implemented and enforced a rigorous process for DSM-5-TR that required transparency by all contributors of their personal and professional interests, followed by an independent review to ensure that personal and professional interests did not bias any results,” the spokesperson said.
However, having industry funding did not preclude contributors’ participation, and investigators note that none of the disclosures were published in the manual or shared publicly.
“The point is not to point fingers at the APA or individual members of the APA but rather to provide hopefully a small piece of research data that would help the APA look at the larger systemic issue of conflicts of interest,” said the study’s lead investigator Lisa Cosgrove, PhD, professor of counseling and faculty fellow in the Applied Ethics Center at the University of Massachusetts Boston.
The findings were published online in The BMJ .
A Deep Dive
The work builds on the investigators’ earlier research into financial conflicts among DSM contributors. The lack of a centralized database of industry payments made the group›s prior studies far more complicated and time-consuming.
For this project, investigators drew on the Open Payments database, which launched in 2014. It collects and publishes data on payments by pharmaceutical and medical device companies to physicians and other healthcare professionals for research, meals, travel, gifts, speaking fees, and other expenses. The program was established as part of the Affordable Care Act and is run by the Centers for Medicare & Medicaid Services.
, just before work on the text revision began. Of the 168 individuals listed as contributors to the manual, 92 met the inclusion criteria of being a US-based physician with industry payments tracked in Open Payments.
Fifty-five of those physicians, or 59.8%, had financial ties to industry. The most common type of payment was for food and beverages (90.9%), travel (69.1%), and consulting (69.1%). Nineteen panel members received $1.8 million for “compensation for services other than consulting, including serving as faculty or as a speaker at a venue other than a continuing education program.”
The greatest proportion of compensation by category of payment was for research funding (71%).
Investigators found that every DSM-5-TR panel included at least one member with industry ties. The panels with the highest number of members with a recent history of industry funding were those for neurodevelopmental disorders; bipolar disorders; obsessive-compulsive disorders; neurocognitive disorders; medication induced movement disorders; and disruptive, impulse control, and conduct disorders. More than 70% of members on those panels had received industry funding.
The total payments received by all contributors was more than $14.2 million, with a range from just under $14 per physician to $2.7 million per physician. The researchers note that the percentage of panel members with industry support was similar between DSM-5-TR and DSM-5.
“What we also see that’s consistent with our 2016 study and 2012 study is the panels for which the members had the most financial ties to industry were those for which pharmaceutical interventions are the first line of therapy,” Dr. Cosgrove said.
No Public Disclosure
For DSM-5, the APA instituted a new disclosure policy for contributors and reported those disclosures on its website.
This time, the association spokesperson said that DSM-5-TR chairs and the DSM Steering Committee who reviewed all proposed changes were required to have no industry-related income above $5000 and that “in fact, many had no industry income.”
Other DSM-5-TR contributors had to submit “extensive” disclosure forms and report “any relationships they or close relations had with industry (very broadly defined) and sources of income,” the spokesperson added. They were also asked to report other nonfinancial interests that they or close relatives had that could potentially bias their work.
The APA’s standing Conflict of Interest Committee reviewed all disclosure forms and flagged those with disclosures that could impact content. Text written by individuals with flagged disclosures received additional review, the spokesperson said.
“If any possible bias was noted in the text content, such as for a potential commercial advantage with a diagnostic instrument, that content was deleted,” the spokesperson said.
However, the real sticking point for medical ethicists is that unlike with the DSM-5, the APA did not share DSM-5-TR contributors’ disclosures publicly.
Commenting on the research, Bernard Lo, MD, professor emeritus of medicine and director emeritus of the Program in Medical Ethics Emeritus at University of California, San Francisco, said that the lack of public disclosure is critical.
“Part of the report should be, ‘Here are the conflicts of interest reported by the members of the panel,’” said Dr. Lo, adding that publishing disclosures is standard in all of APA’s peer-reviewed journals. “Failure to do that in the DSM-5-TR is unacceptable from an ethical and transparency point of view.”
Loss of Public Trust?
In her previous research and in this new study, Dr. Cosgrove recommends the APA follow the 2011 report Clinical Practice Guidelines We Can Trust. Published by the Institute of Medicine (IOM, now called the National Academy of Medicine), that report updated and streamlined a 2009 conflicts of interest guideline, which Dr. Lo coauthored.
“The IOM recommends that the whole guideline development group be free of industry ties,” Dr. Cosgrove said. “At a minimum, the chair should not have ties and the majority of folks should not have ties to industry.”
Some have argued that banning all contributors with industry ties would shrink the expert pool that develops the DSM and other guidelines. Dr. Cosgrove disagrees with that assertion.
“There are hundreds of experts in all medical disciplines that do not have industry ties,” Dr. Cosgrove said. “The ‘most experts have industry ties’ is a spurious and unsupported argument.”
The APA also should ban contributors who receive industry funding as key opinion leaders, known as KOLs, such as members of pharmaceutical companies’ speakers bureaus, Dr. Lo said.
“Certain types of funding relationships with industry are more fraught with ethical problems,” including KOLs, who Dr. Lo said are “basically salespeople trying to increase sales of a product.”
“It really compromises their scientific objectivity and should exclude someone from any practice guideline body,” Dr. Lo said. “This failure to adequately address conflicts of interest doesn’t promote transparency and it doesn’t promote public trust in the diagnostic criteria.”
The Larger Issue
Removing financial conflicts of interest is a start, but it wouldn’t address the larger issue in medicine, said Allen Frances, MD, who chaired the DSM-4 task force and has been an outspoken critic of the DSM-5.
“The financial conflicts of interest may play a role with some people, I’m not denying that,” said Dr. Frances, a professor and chair emeritus of psychiatry at Duke University, Durham, North Carolina. “But that’s a much smaller problem than the fact that any individual from any professional association that has an intense interest in any given diagnosis will always be on the side of expanding that diagnosis and expanding the treatment for it.”
Though financial conflicts of interest can be addressed, Frances believes that professionals’ “intellectual and emotional conflicts” are much harder to overcome.
“People who spend their careers working on any diagnosis are terribly biased by virtue of their attachment to their work,” he said.
The solution is for guidelines in psychiatry and all medical fields to be developed by a truly multidisciplinary “neutral board” that includes broad representation of primary care physicians.
Specialists would be involved in the development of the guidelines but would not have a final say in what diagnoses or treatments are included or excluded.
“80% of psychiatric meds are prescribed by primary care doctors, not psychiatrists,” he said. “So, when you’re making a suggestion for a change in psychiatry, you’re making that suggestion primarily for primary care doctor and have to be thinking about, How will this change play in primary care, which the experts never do.”
The study was unfunded. Dr. Allen reported no relevant disclosures. Dr. Lo served as a paid member of the Takeda Pharmaceuticals Ethics Advisory Committee.
A version of this article appeared on Medscape.com.
About 60% of US physicians who served as panel and task force members for the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) received more than $14 million in undisclosed industry funding, a new study shows.
Most payments were for food and beverages, travel, and consulting fees. But more than one third of contributors received compensation for services other than consulting, such as serving on a pharmaceutical company’s speakers bureau, which medical ethicists say is particularly problematic.
Often referred to as the bible of psychiatric disorders, the DSM-5-TR was released in 2022 by the American Psychiatric Association (APA) and includes changes that were made online since the DSM-5 was first published in 2013.
An APA spokesperson said that DSM-5-TR decision-makers were unable to participate if they had received more than $5000 in industry payments and that all 186 individuals who worked on the text revision were required to disclose all sources of income prior to their participation.
“The APA implemented and enforced a rigorous process for DSM-5-TR that required transparency by all contributors of their personal and professional interests, followed by an independent review to ensure that personal and professional interests did not bias any results,” the spokesperson said.
However, having industry funding did not preclude contributors’ participation, and investigators note that none of the disclosures were published in the manual or shared publicly.
“The point is not to point fingers at the APA or individual members of the APA but rather to provide hopefully a small piece of research data that would help the APA look at the larger systemic issue of conflicts of interest,” said the study’s lead investigator Lisa Cosgrove, PhD, professor of counseling and faculty fellow in the Applied Ethics Center at the University of Massachusetts Boston.
The findings were published online in The BMJ .
A Deep Dive
The work builds on the investigators’ earlier research into financial conflicts among DSM contributors. The lack of a centralized database of industry payments made the group›s prior studies far more complicated and time-consuming.
For this project, investigators drew on the Open Payments database, which launched in 2014. It collects and publishes data on payments by pharmaceutical and medical device companies to physicians and other healthcare professionals for research, meals, travel, gifts, speaking fees, and other expenses. The program was established as part of the Affordable Care Act and is run by the Centers for Medicare & Medicaid Services.
, just before work on the text revision began. Of the 168 individuals listed as contributors to the manual, 92 met the inclusion criteria of being a US-based physician with industry payments tracked in Open Payments.
Fifty-five of those physicians, or 59.8%, had financial ties to industry. The most common type of payment was for food and beverages (90.9%), travel (69.1%), and consulting (69.1%). Nineteen panel members received $1.8 million for “compensation for services other than consulting, including serving as faculty or as a speaker at a venue other than a continuing education program.”
The greatest proportion of compensation by category of payment was for research funding (71%).
Investigators found that every DSM-5-TR panel included at least one member with industry ties. The panels with the highest number of members with a recent history of industry funding were those for neurodevelopmental disorders; bipolar disorders; obsessive-compulsive disorders; neurocognitive disorders; medication induced movement disorders; and disruptive, impulse control, and conduct disorders. More than 70% of members on those panels had received industry funding.
The total payments received by all contributors was more than $14.2 million, with a range from just under $14 per physician to $2.7 million per physician. The researchers note that the percentage of panel members with industry support was similar between DSM-5-TR and DSM-5.
“What we also see that’s consistent with our 2016 study and 2012 study is the panels for which the members had the most financial ties to industry were those for which pharmaceutical interventions are the first line of therapy,” Dr. Cosgrove said.
No Public Disclosure
For DSM-5, the APA instituted a new disclosure policy for contributors and reported those disclosures on its website.
This time, the association spokesperson said that DSM-5-TR chairs and the DSM Steering Committee who reviewed all proposed changes were required to have no industry-related income above $5000 and that “in fact, many had no industry income.”
Other DSM-5-TR contributors had to submit “extensive” disclosure forms and report “any relationships they or close relations had with industry (very broadly defined) and sources of income,” the spokesperson added. They were also asked to report other nonfinancial interests that they or close relatives had that could potentially bias their work.
The APA’s standing Conflict of Interest Committee reviewed all disclosure forms and flagged those with disclosures that could impact content. Text written by individuals with flagged disclosures received additional review, the spokesperson said.
“If any possible bias was noted in the text content, such as for a potential commercial advantage with a diagnostic instrument, that content was deleted,” the spokesperson said.
However, the real sticking point for medical ethicists is that unlike with the DSM-5, the APA did not share DSM-5-TR contributors’ disclosures publicly.
Commenting on the research, Bernard Lo, MD, professor emeritus of medicine and director emeritus of the Program in Medical Ethics Emeritus at University of California, San Francisco, said that the lack of public disclosure is critical.
“Part of the report should be, ‘Here are the conflicts of interest reported by the members of the panel,’” said Dr. Lo, adding that publishing disclosures is standard in all of APA’s peer-reviewed journals. “Failure to do that in the DSM-5-TR is unacceptable from an ethical and transparency point of view.”
Loss of Public Trust?
In her previous research and in this new study, Dr. Cosgrove recommends the APA follow the 2011 report Clinical Practice Guidelines We Can Trust. Published by the Institute of Medicine (IOM, now called the National Academy of Medicine), that report updated and streamlined a 2009 conflicts of interest guideline, which Dr. Lo coauthored.
“The IOM recommends that the whole guideline development group be free of industry ties,” Dr. Cosgrove said. “At a minimum, the chair should not have ties and the majority of folks should not have ties to industry.”
Some have argued that banning all contributors with industry ties would shrink the expert pool that develops the DSM and other guidelines. Dr. Cosgrove disagrees with that assertion.
“There are hundreds of experts in all medical disciplines that do not have industry ties,” Dr. Cosgrove said. “The ‘most experts have industry ties’ is a spurious and unsupported argument.”
The APA also should ban contributors who receive industry funding as key opinion leaders, known as KOLs, such as members of pharmaceutical companies’ speakers bureaus, Dr. Lo said.
“Certain types of funding relationships with industry are more fraught with ethical problems,” including KOLs, who Dr. Lo said are “basically salespeople trying to increase sales of a product.”
“It really compromises their scientific objectivity and should exclude someone from any practice guideline body,” Dr. Lo said. “This failure to adequately address conflicts of interest doesn’t promote transparency and it doesn’t promote public trust in the diagnostic criteria.”
The Larger Issue
Removing financial conflicts of interest is a start, but it wouldn’t address the larger issue in medicine, said Allen Frances, MD, who chaired the DSM-4 task force and has been an outspoken critic of the DSM-5.
“The financial conflicts of interest may play a role with some people, I’m not denying that,” said Dr. Frances, a professor and chair emeritus of psychiatry at Duke University, Durham, North Carolina. “But that’s a much smaller problem than the fact that any individual from any professional association that has an intense interest in any given diagnosis will always be on the side of expanding that diagnosis and expanding the treatment for it.”
Though financial conflicts of interest can be addressed, Frances believes that professionals’ “intellectual and emotional conflicts” are much harder to overcome.
“People who spend their careers working on any diagnosis are terribly biased by virtue of their attachment to their work,” he said.
The solution is for guidelines in psychiatry and all medical fields to be developed by a truly multidisciplinary “neutral board” that includes broad representation of primary care physicians.
Specialists would be involved in the development of the guidelines but would not have a final say in what diagnoses or treatments are included or excluded.
“80% of psychiatric meds are prescribed by primary care doctors, not psychiatrists,” he said. “So, when you’re making a suggestion for a change in psychiatry, you’re making that suggestion primarily for primary care doctor and have to be thinking about, How will this change play in primary care, which the experts never do.”
The study was unfunded. Dr. Allen reported no relevant disclosures. Dr. Lo served as a paid member of the Takeda Pharmaceuticals Ethics Advisory Committee.
A version of this article appeared on Medscape.com.
About 60% of US physicians who served as panel and task force members for the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) received more than $14 million in undisclosed industry funding, a new study shows.
Most payments were for food and beverages, travel, and consulting fees. But more than one third of contributors received compensation for services other than consulting, such as serving on a pharmaceutical company’s speakers bureau, which medical ethicists say is particularly problematic.
Often referred to as the bible of psychiatric disorders, the DSM-5-TR was released in 2022 by the American Psychiatric Association (APA) and includes changes that were made online since the DSM-5 was first published in 2013.
An APA spokesperson said that DSM-5-TR decision-makers were unable to participate if they had received more than $5000 in industry payments and that all 186 individuals who worked on the text revision were required to disclose all sources of income prior to their participation.
“The APA implemented and enforced a rigorous process for DSM-5-TR that required transparency by all contributors of their personal and professional interests, followed by an independent review to ensure that personal and professional interests did not bias any results,” the spokesperson said.
However, having industry funding did not preclude contributors’ participation, and investigators note that none of the disclosures were published in the manual or shared publicly.
“The point is not to point fingers at the APA or individual members of the APA but rather to provide hopefully a small piece of research data that would help the APA look at the larger systemic issue of conflicts of interest,” said the study’s lead investigator Lisa Cosgrove, PhD, professor of counseling and faculty fellow in the Applied Ethics Center at the University of Massachusetts Boston.
The findings were published online in The BMJ .
A Deep Dive
The work builds on the investigators’ earlier research into financial conflicts among DSM contributors. The lack of a centralized database of industry payments made the group›s prior studies far more complicated and time-consuming.
For this project, investigators drew on the Open Payments database, which launched in 2014. It collects and publishes data on payments by pharmaceutical and medical device companies to physicians and other healthcare professionals for research, meals, travel, gifts, speaking fees, and other expenses. The program was established as part of the Affordable Care Act and is run by the Centers for Medicare & Medicaid Services.
, just before work on the text revision began. Of the 168 individuals listed as contributors to the manual, 92 met the inclusion criteria of being a US-based physician with industry payments tracked in Open Payments.
Fifty-five of those physicians, or 59.8%, had financial ties to industry. The most common type of payment was for food and beverages (90.9%), travel (69.1%), and consulting (69.1%). Nineteen panel members received $1.8 million for “compensation for services other than consulting, including serving as faculty or as a speaker at a venue other than a continuing education program.”
The greatest proportion of compensation by category of payment was for research funding (71%).
Investigators found that every DSM-5-TR panel included at least one member with industry ties. The panels with the highest number of members with a recent history of industry funding were those for neurodevelopmental disorders; bipolar disorders; obsessive-compulsive disorders; neurocognitive disorders; medication induced movement disorders; and disruptive, impulse control, and conduct disorders. More than 70% of members on those panels had received industry funding.
The total payments received by all contributors was more than $14.2 million, with a range from just under $14 per physician to $2.7 million per physician. The researchers note that the percentage of panel members with industry support was similar between DSM-5-TR and DSM-5.
“What we also see that’s consistent with our 2016 study and 2012 study is the panels for which the members had the most financial ties to industry were those for which pharmaceutical interventions are the first line of therapy,” Dr. Cosgrove said.
No Public Disclosure
For DSM-5, the APA instituted a new disclosure policy for contributors and reported those disclosures on its website.
This time, the association spokesperson said that DSM-5-TR chairs and the DSM Steering Committee who reviewed all proposed changes were required to have no industry-related income above $5000 and that “in fact, many had no industry income.”
Other DSM-5-TR contributors had to submit “extensive” disclosure forms and report “any relationships they or close relations had with industry (very broadly defined) and sources of income,” the spokesperson added. They were also asked to report other nonfinancial interests that they or close relatives had that could potentially bias their work.
The APA’s standing Conflict of Interest Committee reviewed all disclosure forms and flagged those with disclosures that could impact content. Text written by individuals with flagged disclosures received additional review, the spokesperson said.
“If any possible bias was noted in the text content, such as for a potential commercial advantage with a diagnostic instrument, that content was deleted,” the spokesperson said.
However, the real sticking point for medical ethicists is that unlike with the DSM-5, the APA did not share DSM-5-TR contributors’ disclosures publicly.
Commenting on the research, Bernard Lo, MD, professor emeritus of medicine and director emeritus of the Program in Medical Ethics Emeritus at University of California, San Francisco, said that the lack of public disclosure is critical.
“Part of the report should be, ‘Here are the conflicts of interest reported by the members of the panel,’” said Dr. Lo, adding that publishing disclosures is standard in all of APA’s peer-reviewed journals. “Failure to do that in the DSM-5-TR is unacceptable from an ethical and transparency point of view.”
Loss of Public Trust?
In her previous research and in this new study, Dr. Cosgrove recommends the APA follow the 2011 report Clinical Practice Guidelines We Can Trust. Published by the Institute of Medicine (IOM, now called the National Academy of Medicine), that report updated and streamlined a 2009 conflicts of interest guideline, which Dr. Lo coauthored.
“The IOM recommends that the whole guideline development group be free of industry ties,” Dr. Cosgrove said. “At a minimum, the chair should not have ties and the majority of folks should not have ties to industry.”
Some have argued that banning all contributors with industry ties would shrink the expert pool that develops the DSM and other guidelines. Dr. Cosgrove disagrees with that assertion.
“There are hundreds of experts in all medical disciplines that do not have industry ties,” Dr. Cosgrove said. “The ‘most experts have industry ties’ is a spurious and unsupported argument.”
The APA also should ban contributors who receive industry funding as key opinion leaders, known as KOLs, such as members of pharmaceutical companies’ speakers bureaus, Dr. Lo said.
“Certain types of funding relationships with industry are more fraught with ethical problems,” including KOLs, who Dr. Lo said are “basically salespeople trying to increase sales of a product.”
“It really compromises their scientific objectivity and should exclude someone from any practice guideline body,” Dr. Lo said. “This failure to adequately address conflicts of interest doesn’t promote transparency and it doesn’t promote public trust in the diagnostic criteria.”
The Larger Issue
Removing financial conflicts of interest is a start, but it wouldn’t address the larger issue in medicine, said Allen Frances, MD, who chaired the DSM-4 task force and has been an outspoken critic of the DSM-5.
“The financial conflicts of interest may play a role with some people, I’m not denying that,” said Dr. Frances, a professor and chair emeritus of psychiatry at Duke University, Durham, North Carolina. “But that’s a much smaller problem than the fact that any individual from any professional association that has an intense interest in any given diagnosis will always be on the side of expanding that diagnosis and expanding the treatment for it.”
Though financial conflicts of interest can be addressed, Frances believes that professionals’ “intellectual and emotional conflicts” are much harder to overcome.
“People who spend their careers working on any diagnosis are terribly biased by virtue of their attachment to their work,” he said.
The solution is for guidelines in psychiatry and all medical fields to be developed by a truly multidisciplinary “neutral board” that includes broad representation of primary care physicians.
Specialists would be involved in the development of the guidelines but would not have a final say in what diagnoses or treatments are included or excluded.
“80% of psychiatric meds are prescribed by primary care doctors, not psychiatrists,” he said. “So, when you’re making a suggestion for a change in psychiatry, you’re making that suggestion primarily for primary care doctor and have to be thinking about, How will this change play in primary care, which the experts never do.”
The study was unfunded. Dr. Allen reported no relevant disclosures. Dr. Lo served as a paid member of the Takeda Pharmaceuticals Ethics Advisory Committee.
A version of this article appeared on Medscape.com.
FROM THE BMJ
What’s the Disease Burden From Plastic Exposure?
Exposure to endocrine-disrupting chemicals (EDCs) via daily use of plastics is a major contributor to the overall disease burden in the United States and the associated costs to society amount to more than 1% of the gross domestic product, revealed a large-scale analysis.
The research, published in the Journal of the Endocrine Society, indicated that taken together, the disease burden attributable to EDCs used in the manufacture of plastics added up to almost $250 billion in 2018 alone.
“The diseases due to plastics run the entire life course from preterm birth to obesity, heart disease, and cancers,” commented lead author Leonardo Trasande, MD, MPP, Jim G. Hendrick, MD Professor of Pediatrics, Department of Pediatrics, NYU Langone Medical Center, New York, in a release.
“Our study drives home the need to address chemicals used in plastic materials” through global treaties and other policy initiatives, he said, so as to “reduce these costs” in line with reductions in exposure to the chemicals.
Co-author Michael Belliveau, Executive Director at Defend Our Health in Portland, ME, agreed, saying: “We can reduce these health costs and the prevalence of chronic endocrine diseases such as diabetes and obesity if governments and companies enact policies that minimize exposure to EDCs to protect public health and the environment.”
Plastics may contain any one of a number of EDCs, such as polybrominated diphenylethers in flame retardant additives, phthalates in food packaging, bisphenols in can linings, and perfluoroalkyl and polyfluoroalkyl substances (PFAS) in nonstick cooking utensils.
in developing fetuses and children, and even death.
In March 2022, the United Nations Environment Assembly committed to a global plastics treaty to “end plastic pollution and forge an international legally binding agreement by 2024” that “addresses the full life cycle of plastic, including its production, design and disposal.”
Minimizing EDC Exposure
But what can doctors tell their patients today to help them reduce their exposure to EDCs?
“There are safe and simple steps that people can take to limit their exposure to the chemicals of greatest concern,” Dr. Trasande told this news organization.
This can be partly achieved by reducing plastic use down to its essentials. “To use an example, when you are flying, fill up a stainless steel container after clearing security. At home, use glass or stainless steel” rather than plastic bottles or containers.
In particular, “avoiding microwaving plastic is important,” Dr. Trasande said, “even if a container says it’s microwave-safe.”
He warned that “many chemicals used in plastic are not covalently bound, and heat facilitates leaching into food. Microscopic contaminants can also get into food when you microwave plastic.”
Dr. Trasande also suggests limiting canned food consumption and avoiding cleaning plastic food containers in machine dishwashers.
Calculating the Disease Burden
To accurately assess the “the tradeoffs involved in the ongoing reliance on plastic production as a source of economic productivity,” the current researchers calculated the attributable disease burden and cost related to EDCs used in plastic materials in the United States in 2018.
Building on previously published analyses, they used industry reports, publications by national and international governing bodies, and peer-reviewed publications to determine the usage of each type of EDC and its attributable disease and disability burden.
This plastic-related fraction (PRF) of disease burden was then used to calculate an updated cost estimate for each EDC, based on the assumption that the disease burden is directly proportional to its exposure.
They found that for bisphenol A, 97.5% of its use, and therefore its estimated PRF of disease burden, was related to the manufacture of plastics, while this figure was 98%-100% for phthalates. For PDBE, 98% of its use was in plastics vs 93% for PFAS.
The researchers then estimated that the total plastic-attributable disease burden in the United States in 2018 cost the nation $249 billion, or 1.22% of the gross domestic product. Of this, $159 billion was linked to PDBE exposure, which is associated with diseases such as cancer.
Moreover, $1.02 billion plastic-attributable disease burden was associated with bisphenol A exposure, which can have potentially harmful health effects on the immune system; followed by $66.7 billion due to phthalates, which are linked to preterm birth, reduced sperm count, and childhood obesity; and $22.4 billion due to PFAS, which are associated with kidney failure and gestational diabetes.
The study was supported by the National Institutes of Health and the Passport Foundation.
Dr. Trasande declared relationships with Audible, Houghton Mifflin, Paidos, and Kobunsha, none of which relate to the present manuscript.
No other financial relationships were declared.
A version of this article appeared on Medscape.com.
Exposure to endocrine-disrupting chemicals (EDCs) via daily use of plastics is a major contributor to the overall disease burden in the United States and the associated costs to society amount to more than 1% of the gross domestic product, revealed a large-scale analysis.
The research, published in the Journal of the Endocrine Society, indicated that taken together, the disease burden attributable to EDCs used in the manufacture of plastics added up to almost $250 billion in 2018 alone.
“The diseases due to plastics run the entire life course from preterm birth to obesity, heart disease, and cancers,” commented lead author Leonardo Trasande, MD, MPP, Jim G. Hendrick, MD Professor of Pediatrics, Department of Pediatrics, NYU Langone Medical Center, New York, in a release.
“Our study drives home the need to address chemicals used in plastic materials” through global treaties and other policy initiatives, he said, so as to “reduce these costs” in line with reductions in exposure to the chemicals.
Co-author Michael Belliveau, Executive Director at Defend Our Health in Portland, ME, agreed, saying: “We can reduce these health costs and the prevalence of chronic endocrine diseases such as diabetes and obesity if governments and companies enact policies that minimize exposure to EDCs to protect public health and the environment.”
Plastics may contain any one of a number of EDCs, such as polybrominated diphenylethers in flame retardant additives, phthalates in food packaging, bisphenols in can linings, and perfluoroalkyl and polyfluoroalkyl substances (PFAS) in nonstick cooking utensils.
in developing fetuses and children, and even death.
In March 2022, the United Nations Environment Assembly committed to a global plastics treaty to “end plastic pollution and forge an international legally binding agreement by 2024” that “addresses the full life cycle of plastic, including its production, design and disposal.”
Minimizing EDC Exposure
But what can doctors tell their patients today to help them reduce their exposure to EDCs?
“There are safe and simple steps that people can take to limit their exposure to the chemicals of greatest concern,” Dr. Trasande told this news organization.
This can be partly achieved by reducing plastic use down to its essentials. “To use an example, when you are flying, fill up a stainless steel container after clearing security. At home, use glass or stainless steel” rather than plastic bottles or containers.
In particular, “avoiding microwaving plastic is important,” Dr. Trasande said, “even if a container says it’s microwave-safe.”
He warned that “many chemicals used in plastic are not covalently bound, and heat facilitates leaching into food. Microscopic contaminants can also get into food when you microwave plastic.”
Dr. Trasande also suggests limiting canned food consumption and avoiding cleaning plastic food containers in machine dishwashers.
Calculating the Disease Burden
To accurately assess the “the tradeoffs involved in the ongoing reliance on plastic production as a source of economic productivity,” the current researchers calculated the attributable disease burden and cost related to EDCs used in plastic materials in the United States in 2018.
Building on previously published analyses, they used industry reports, publications by national and international governing bodies, and peer-reviewed publications to determine the usage of each type of EDC and its attributable disease and disability burden.
This plastic-related fraction (PRF) of disease burden was then used to calculate an updated cost estimate for each EDC, based on the assumption that the disease burden is directly proportional to its exposure.
They found that for bisphenol A, 97.5% of its use, and therefore its estimated PRF of disease burden, was related to the manufacture of plastics, while this figure was 98%-100% for phthalates. For PDBE, 98% of its use was in plastics vs 93% for PFAS.
The researchers then estimated that the total plastic-attributable disease burden in the United States in 2018 cost the nation $249 billion, or 1.22% of the gross domestic product. Of this, $159 billion was linked to PDBE exposure, which is associated with diseases such as cancer.
Moreover, $1.02 billion plastic-attributable disease burden was associated with bisphenol A exposure, which can have potentially harmful health effects on the immune system; followed by $66.7 billion due to phthalates, which are linked to preterm birth, reduced sperm count, and childhood obesity; and $22.4 billion due to PFAS, which are associated with kidney failure and gestational diabetes.
The study was supported by the National Institutes of Health and the Passport Foundation.
Dr. Trasande declared relationships with Audible, Houghton Mifflin, Paidos, and Kobunsha, none of which relate to the present manuscript.
No other financial relationships were declared.
A version of this article appeared on Medscape.com.
Exposure to endocrine-disrupting chemicals (EDCs) via daily use of plastics is a major contributor to the overall disease burden in the United States and the associated costs to society amount to more than 1% of the gross domestic product, revealed a large-scale analysis.
The research, published in the Journal of the Endocrine Society, indicated that taken together, the disease burden attributable to EDCs used in the manufacture of plastics added up to almost $250 billion in 2018 alone.
“The diseases due to plastics run the entire life course from preterm birth to obesity, heart disease, and cancers,” commented lead author Leonardo Trasande, MD, MPP, Jim G. Hendrick, MD Professor of Pediatrics, Department of Pediatrics, NYU Langone Medical Center, New York, in a release.
“Our study drives home the need to address chemicals used in plastic materials” through global treaties and other policy initiatives, he said, so as to “reduce these costs” in line with reductions in exposure to the chemicals.
Co-author Michael Belliveau, Executive Director at Defend Our Health in Portland, ME, agreed, saying: “We can reduce these health costs and the prevalence of chronic endocrine diseases such as diabetes and obesity if governments and companies enact policies that minimize exposure to EDCs to protect public health and the environment.”
Plastics may contain any one of a number of EDCs, such as polybrominated diphenylethers in flame retardant additives, phthalates in food packaging, bisphenols in can linings, and perfluoroalkyl and polyfluoroalkyl substances (PFAS) in nonstick cooking utensils.
in developing fetuses and children, and even death.
In March 2022, the United Nations Environment Assembly committed to a global plastics treaty to “end plastic pollution and forge an international legally binding agreement by 2024” that “addresses the full life cycle of plastic, including its production, design and disposal.”
Minimizing EDC Exposure
But what can doctors tell their patients today to help them reduce their exposure to EDCs?
“There are safe and simple steps that people can take to limit their exposure to the chemicals of greatest concern,” Dr. Trasande told this news organization.
This can be partly achieved by reducing plastic use down to its essentials. “To use an example, when you are flying, fill up a stainless steel container after clearing security. At home, use glass or stainless steel” rather than plastic bottles or containers.
In particular, “avoiding microwaving plastic is important,” Dr. Trasande said, “even if a container says it’s microwave-safe.”
He warned that “many chemicals used in plastic are not covalently bound, and heat facilitates leaching into food. Microscopic contaminants can also get into food when you microwave plastic.”
Dr. Trasande also suggests limiting canned food consumption and avoiding cleaning plastic food containers in machine dishwashers.
Calculating the Disease Burden
To accurately assess the “the tradeoffs involved in the ongoing reliance on plastic production as a source of economic productivity,” the current researchers calculated the attributable disease burden and cost related to EDCs used in plastic materials in the United States in 2018.
Building on previously published analyses, they used industry reports, publications by national and international governing bodies, and peer-reviewed publications to determine the usage of each type of EDC and its attributable disease and disability burden.
This plastic-related fraction (PRF) of disease burden was then used to calculate an updated cost estimate for each EDC, based on the assumption that the disease burden is directly proportional to its exposure.
They found that for bisphenol A, 97.5% of its use, and therefore its estimated PRF of disease burden, was related to the manufacture of plastics, while this figure was 98%-100% for phthalates. For PDBE, 98% of its use was in plastics vs 93% for PFAS.
The researchers then estimated that the total plastic-attributable disease burden in the United States in 2018 cost the nation $249 billion, or 1.22% of the gross domestic product. Of this, $159 billion was linked to PDBE exposure, which is associated with diseases such as cancer.
Moreover, $1.02 billion plastic-attributable disease burden was associated with bisphenol A exposure, which can have potentially harmful health effects on the immune system; followed by $66.7 billion due to phthalates, which are linked to preterm birth, reduced sperm count, and childhood obesity; and $22.4 billion due to PFAS, which are associated with kidney failure and gestational diabetes.
The study was supported by the National Institutes of Health and the Passport Foundation.
Dr. Trasande declared relationships with Audible, Houghton Mifflin, Paidos, and Kobunsha, none of which relate to the present manuscript.
No other financial relationships were declared.
A version of this article appeared on Medscape.com.
FROM THE JOURNAL OF THE ENDOCRINE SOCIETY
The Knowns and Unknowns About Delivery Timing in Diabetes
FAIRFAX, VIRGINIA — The lack of data on optimal timing of delivery for pregnancies complicated by diabetes remains a major challenge in obstetrics — one with considerable implications given the high and rising prevalence of pregestational and gestational diabetes, Katherine Laughon Grantz, MD, MS, of the National Institute of Child Health and Human Development, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“While 39-40 weeks might be ideal for low-risk pregnancies, the optimal timing for pregnancies with complications [like diabetes] is unknown,” said Dr. Grantz, a senior investigator in the NICHD’s epidemiology branch.
The percentage of mothers with gestational diabetes mellitus (GDM) increased from 6% in 2016 to 8% in 2021, according to the most recent data from the National Vital Statistics System of the Centers for Disease Control and Prevention (MMWR Morb Mortal Wkly Rep. 2023;72:16). Meanwhile, the prevalence of prepregnancy obesity, which raises the risk of gestational and type 2 diabetes, was 29% in 2019; this represents an 11% increase from 2015 (NCHS Data Brief. 2020;392:1-8) and has occurred across all maternal ages, races, ethnic groups, and educational levels, she said.
“The reason clinicians deliver pregnancies with diabetes earlier is because there’s a decreased risk of macrosomia, shoulder dystocia, and stillbirth. And these risks need to be balanced with the increased risk of neonatal morbidity and mortality associated with earlier delivery,” said Dr. Grantz, who noted during her talk that delivery timing also appears to influence long-term neurodevelopmental outcomes. “Yet despite [diabetes in pregnancy] being so common, there is complete uncertainty about when to deliver.”
ACOG Recommendations, Randomized Trials (New And Old)
The American College of Obstetricians and Gynecologists, in a Committee Opinion on Medically Indicated Late-Preterm and Early-Term Deliveries, published in collaboration with the Society of Maternal-Fetal Medicine, offers recommendations based on the type of diabetes and the level of control. For instance, the suggested delivery timing for well-controlled GDM is full term (39 0/7 to 40 6/7 weeks of gestation), while the recommendation for poorly controlled diabetes is individualized late preterm/early term management (Obstet Gynecol. 2021;138:e35-9).
In defining and evaluating control, she noted, “the clinical focus is on glucose, but there are likely other important parameters that are not taken into account ... which [could be] important when considering the timing of delivery.” Potentially important factors include estimated fetal weight, fetal growth velocity, lipids, and amino acids, she said.
ACOG’s recommendations are based mainly on retrospective data, Dr. Grantz said. Only two randomized controlled trials have investigated the timing of delivery in the context of diabetes, and both focused on cesarean section and were “generally underpowered to study neonatal outcomes,” she said.
The first RCT, published in 1993, enrolled 200 women with uncomplicated insulin-requiring diabetes (187 with GDM and 13 with pregestational diabetes) at 38 weeks of gestation, and compared active induction of labor within 5 days to expectant management. There was no significant difference in the cesarean delivery rate (the primary outcome), but rates of macrosomia and large for gestational age were higher in the expectant management group (27% vs. 15%, P = .05, and 23% vs. 10%, P = .02, respectively). Shoulder dystocia occurred in three deliveries, each of which was expectantly managed (Am J Obstet Gynecol. 1993;169[3]:611-5). Notably, the study included “only women with excellent glucose control,” Dr. Grantz said.
The second RCT, published in 2017 by a group in Italy, enrolled 425 patients with GDM (diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria) between week 38 and week 39 of gestation and similarly randomized them to induction of labor or expectant management. No difference in cesarean delivery was found (BJOG. 2017;124[4]:669-77). Induction of labor was associated with a higher risk of hyperbilirubinemia, and there was a trend toward a decreased risk of macrosomia, but again, the study was underpowered to detect differences in most outcomes, she said. (The study also was stopped early because of an inability to recruit, she noted.)
Dr. Grantz is currently recruiting for a randomized trial aimed at determining the optimal time between 37 and 39 weeks to initiate delivery — the time when neonatal morbidity and perinatal mortality risk is the lowest – for uncontrolled GDM-complicated pregnancies. The trial is designed to recruit up to 3,450 pregnant women with uncontrolled GDM and randomize the timing of their delivery (NCT05515744).
Those who are eligible for the study but do not consent to participate in randomization for delivery will be asked about chart review only (an estimated additional 3,000). The SPAN TIME study will also assess newborn development and behavior outcomes, as well as anthropometric measures, as secondary outcomes. An exploratory analysis will look for clinical, nonclinical or biochemical factors that could be helpful in optimizing delivery timing.
What Retrospective Studies Reveal
Factors that may influence the timing of delivery include the duration of neonatal exposure to hyperglycemia/hyperinsulinemia (pregestational vs. gestational diabetes), the level of diabetes control, and comorbidities (e.g. maternal renal disease or chronic hypertension). However, research “investigating how these factors influence morbidity and the timing of delivery is limited,” said Dr. Grantz.
Overall, it has been difficult through retrospective studies, she said, to investigate neonatal morbidity in diabetic pregnancies and tease apart the relative effects of diabetes as a precursor for early delivery and prematurity itself. Among the studies suggesting an independent risk of diabetes is a retrospective study focusing on neonatal respiratory morbidity — “one of the most common adverse outcomes associated with diabetes.”
The study, an analysis of the Consortium on Safe Labor study (an electronic medical record study of more than 220,000 singleton pregnancies), stratified morbidity by the probability of delivering at term (≥ 37 weeks). GDM and pregestational diabetes complicated 5.1% and 1.5% of the pregnancies, respectively, and were found to be associated with increased risks of neonatal respiratory morbidity compared to women without diabetes — regardless of the probability of delivering at term.
However, these associations were stronger with a higher probability of delivering at term, which suggests that the neonatal respiratory morbidity associated with diabetes is not fully explained by a greater propensity for prematurity (Am J Perinatol. 2017;34[11]:1160-8).
In addition, the rates of all neonatal respiratory morbidities and mortality were higher for pregestational diabetes compared with gestational diabetes, said Dr. Grantz, a senior author of the study. (Morbidities included neonatal intensive care unit admission, transient tachypnea of newborn, apnea, respiratory distress syndrome, mechanical ventilation, and stillbirth.)
The pathophysiology of diabetes and neonatal respiratory morbidity is “not fully known,” she said. It is believed that fetal hyperinsulinemia may cause delayed pulmonary maturation and there is evidence from animal studies that insulin decreases the incorporation of glucose and fatty acids into phospholipid phosphatidylglycerol. Indirect effects stem from the physiologic immaturity of earlier delivery and a higher cesarean delivery rate in pregnancies complicated by diabetes, Dr. Grantz said.
Among other retrospective studies was a population-based study from Canada (2004-2014), published in 2020, of large numbers of women with all types of diabetes and a comparison group of over 2.5 million without diabetes. For maternal morbidity/mortality, there were no significant differences by gestational age between iatrogenic delivery and expectant management among any form of diabetes. But for neonatal morbidity and mortality, the study found differences.
In women with gestational diabetes, iatrogenic delivery was associated with increased risk of neonatal morbidity/mortality at 36 and 37 weeks’ gestation and with decreased risk at weeks 38-40. Increased risk with iatrogenic delivery was also found for women with type 1 and type 2 diabetes at weeks 36 and 37 (Acta Obstet Gynecol Scand. 2020;99[3]:341-9).
Another retrospective study using California vital statistics (1997-2006) examined rates of stillbirth and infant death in women with GDM by gestational age at delivery (Am J Obstet Gynecol. 2012;206[4]:309.e1-e7). The 190,000-plus women with GDM had elevated risk of stillbirth at each gestational age compared to those without GDM, but “the [excess] risk for GDM was lowest at 38 weeks and again at 40 weeks,” Dr. Grantz said. The investigators concluded, she said, “that the risk of expectant management exceeded that of delivery at 38 weeks and beyond.”
Dr. Grantz reported no disclosures.
FAIRFAX, VIRGINIA — The lack of data on optimal timing of delivery for pregnancies complicated by diabetes remains a major challenge in obstetrics — one with considerable implications given the high and rising prevalence of pregestational and gestational diabetes, Katherine Laughon Grantz, MD, MS, of the National Institute of Child Health and Human Development, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“While 39-40 weeks might be ideal for low-risk pregnancies, the optimal timing for pregnancies with complications [like diabetes] is unknown,” said Dr. Grantz, a senior investigator in the NICHD’s epidemiology branch.
The percentage of mothers with gestational diabetes mellitus (GDM) increased from 6% in 2016 to 8% in 2021, according to the most recent data from the National Vital Statistics System of the Centers for Disease Control and Prevention (MMWR Morb Mortal Wkly Rep. 2023;72:16). Meanwhile, the prevalence of prepregnancy obesity, which raises the risk of gestational and type 2 diabetes, was 29% in 2019; this represents an 11% increase from 2015 (NCHS Data Brief. 2020;392:1-8) and has occurred across all maternal ages, races, ethnic groups, and educational levels, she said.
“The reason clinicians deliver pregnancies with diabetes earlier is because there’s a decreased risk of macrosomia, shoulder dystocia, and stillbirth. And these risks need to be balanced with the increased risk of neonatal morbidity and mortality associated with earlier delivery,” said Dr. Grantz, who noted during her talk that delivery timing also appears to influence long-term neurodevelopmental outcomes. “Yet despite [diabetes in pregnancy] being so common, there is complete uncertainty about when to deliver.”
ACOG Recommendations, Randomized Trials (New And Old)
The American College of Obstetricians and Gynecologists, in a Committee Opinion on Medically Indicated Late-Preterm and Early-Term Deliveries, published in collaboration with the Society of Maternal-Fetal Medicine, offers recommendations based on the type of diabetes and the level of control. For instance, the suggested delivery timing for well-controlled GDM is full term (39 0/7 to 40 6/7 weeks of gestation), while the recommendation for poorly controlled diabetes is individualized late preterm/early term management (Obstet Gynecol. 2021;138:e35-9).
In defining and evaluating control, she noted, “the clinical focus is on glucose, but there are likely other important parameters that are not taken into account ... which [could be] important when considering the timing of delivery.” Potentially important factors include estimated fetal weight, fetal growth velocity, lipids, and amino acids, she said.
ACOG’s recommendations are based mainly on retrospective data, Dr. Grantz said. Only two randomized controlled trials have investigated the timing of delivery in the context of diabetes, and both focused on cesarean section and were “generally underpowered to study neonatal outcomes,” she said.
The first RCT, published in 1993, enrolled 200 women with uncomplicated insulin-requiring diabetes (187 with GDM and 13 with pregestational diabetes) at 38 weeks of gestation, and compared active induction of labor within 5 days to expectant management. There was no significant difference in the cesarean delivery rate (the primary outcome), but rates of macrosomia and large for gestational age were higher in the expectant management group (27% vs. 15%, P = .05, and 23% vs. 10%, P = .02, respectively). Shoulder dystocia occurred in three deliveries, each of which was expectantly managed (Am J Obstet Gynecol. 1993;169[3]:611-5). Notably, the study included “only women with excellent glucose control,” Dr. Grantz said.
The second RCT, published in 2017 by a group in Italy, enrolled 425 patients with GDM (diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria) between week 38 and week 39 of gestation and similarly randomized them to induction of labor or expectant management. No difference in cesarean delivery was found (BJOG. 2017;124[4]:669-77). Induction of labor was associated with a higher risk of hyperbilirubinemia, and there was a trend toward a decreased risk of macrosomia, but again, the study was underpowered to detect differences in most outcomes, she said. (The study also was stopped early because of an inability to recruit, she noted.)
Dr. Grantz is currently recruiting for a randomized trial aimed at determining the optimal time between 37 and 39 weeks to initiate delivery — the time when neonatal morbidity and perinatal mortality risk is the lowest – for uncontrolled GDM-complicated pregnancies. The trial is designed to recruit up to 3,450 pregnant women with uncontrolled GDM and randomize the timing of their delivery (NCT05515744).
Those who are eligible for the study but do not consent to participate in randomization for delivery will be asked about chart review only (an estimated additional 3,000). The SPAN TIME study will also assess newborn development and behavior outcomes, as well as anthropometric measures, as secondary outcomes. An exploratory analysis will look for clinical, nonclinical or biochemical factors that could be helpful in optimizing delivery timing.
What Retrospective Studies Reveal
Factors that may influence the timing of delivery include the duration of neonatal exposure to hyperglycemia/hyperinsulinemia (pregestational vs. gestational diabetes), the level of diabetes control, and comorbidities (e.g. maternal renal disease or chronic hypertension). However, research “investigating how these factors influence morbidity and the timing of delivery is limited,” said Dr. Grantz.
Overall, it has been difficult through retrospective studies, she said, to investigate neonatal morbidity in diabetic pregnancies and tease apart the relative effects of diabetes as a precursor for early delivery and prematurity itself. Among the studies suggesting an independent risk of diabetes is a retrospective study focusing on neonatal respiratory morbidity — “one of the most common adverse outcomes associated with diabetes.”
The study, an analysis of the Consortium on Safe Labor study (an electronic medical record study of more than 220,000 singleton pregnancies), stratified morbidity by the probability of delivering at term (≥ 37 weeks). GDM and pregestational diabetes complicated 5.1% and 1.5% of the pregnancies, respectively, and were found to be associated with increased risks of neonatal respiratory morbidity compared to women without diabetes — regardless of the probability of delivering at term.
However, these associations were stronger with a higher probability of delivering at term, which suggests that the neonatal respiratory morbidity associated with diabetes is not fully explained by a greater propensity for prematurity (Am J Perinatol. 2017;34[11]:1160-8).
In addition, the rates of all neonatal respiratory morbidities and mortality were higher for pregestational diabetes compared with gestational diabetes, said Dr. Grantz, a senior author of the study. (Morbidities included neonatal intensive care unit admission, transient tachypnea of newborn, apnea, respiratory distress syndrome, mechanical ventilation, and stillbirth.)
The pathophysiology of diabetes and neonatal respiratory morbidity is “not fully known,” she said. It is believed that fetal hyperinsulinemia may cause delayed pulmonary maturation and there is evidence from animal studies that insulin decreases the incorporation of glucose and fatty acids into phospholipid phosphatidylglycerol. Indirect effects stem from the physiologic immaturity of earlier delivery and a higher cesarean delivery rate in pregnancies complicated by diabetes, Dr. Grantz said.
Among other retrospective studies was a population-based study from Canada (2004-2014), published in 2020, of large numbers of women with all types of diabetes and a comparison group of over 2.5 million without diabetes. For maternal morbidity/mortality, there were no significant differences by gestational age between iatrogenic delivery and expectant management among any form of diabetes. But for neonatal morbidity and mortality, the study found differences.
In women with gestational diabetes, iatrogenic delivery was associated with increased risk of neonatal morbidity/mortality at 36 and 37 weeks’ gestation and with decreased risk at weeks 38-40. Increased risk with iatrogenic delivery was also found for women with type 1 and type 2 diabetes at weeks 36 and 37 (Acta Obstet Gynecol Scand. 2020;99[3]:341-9).
Another retrospective study using California vital statistics (1997-2006) examined rates of stillbirth and infant death in women with GDM by gestational age at delivery (Am J Obstet Gynecol. 2012;206[4]:309.e1-e7). The 190,000-plus women with GDM had elevated risk of stillbirth at each gestational age compared to those without GDM, but “the [excess] risk for GDM was lowest at 38 weeks and again at 40 weeks,” Dr. Grantz said. The investigators concluded, she said, “that the risk of expectant management exceeded that of delivery at 38 weeks and beyond.”
Dr. Grantz reported no disclosures.
FAIRFAX, VIRGINIA — The lack of data on optimal timing of delivery for pregnancies complicated by diabetes remains a major challenge in obstetrics — one with considerable implications given the high and rising prevalence of pregestational and gestational diabetes, Katherine Laughon Grantz, MD, MS, of the National Institute of Child Health and Human Development, said at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“While 39-40 weeks might be ideal for low-risk pregnancies, the optimal timing for pregnancies with complications [like diabetes] is unknown,” said Dr. Grantz, a senior investigator in the NICHD’s epidemiology branch.
The percentage of mothers with gestational diabetes mellitus (GDM) increased from 6% in 2016 to 8% in 2021, according to the most recent data from the National Vital Statistics System of the Centers for Disease Control and Prevention (MMWR Morb Mortal Wkly Rep. 2023;72:16). Meanwhile, the prevalence of prepregnancy obesity, which raises the risk of gestational and type 2 diabetes, was 29% in 2019; this represents an 11% increase from 2015 (NCHS Data Brief. 2020;392:1-8) and has occurred across all maternal ages, races, ethnic groups, and educational levels, she said.
“The reason clinicians deliver pregnancies with diabetes earlier is because there’s a decreased risk of macrosomia, shoulder dystocia, and stillbirth. And these risks need to be balanced with the increased risk of neonatal morbidity and mortality associated with earlier delivery,” said Dr. Grantz, who noted during her talk that delivery timing also appears to influence long-term neurodevelopmental outcomes. “Yet despite [diabetes in pregnancy] being so common, there is complete uncertainty about when to deliver.”
ACOG Recommendations, Randomized Trials (New And Old)
The American College of Obstetricians and Gynecologists, in a Committee Opinion on Medically Indicated Late-Preterm and Early-Term Deliveries, published in collaboration with the Society of Maternal-Fetal Medicine, offers recommendations based on the type of diabetes and the level of control. For instance, the suggested delivery timing for well-controlled GDM is full term (39 0/7 to 40 6/7 weeks of gestation), while the recommendation for poorly controlled diabetes is individualized late preterm/early term management (Obstet Gynecol. 2021;138:e35-9).
In defining and evaluating control, she noted, “the clinical focus is on glucose, but there are likely other important parameters that are not taken into account ... which [could be] important when considering the timing of delivery.” Potentially important factors include estimated fetal weight, fetal growth velocity, lipids, and amino acids, she said.
ACOG’s recommendations are based mainly on retrospective data, Dr. Grantz said. Only two randomized controlled trials have investigated the timing of delivery in the context of diabetes, and both focused on cesarean section and were “generally underpowered to study neonatal outcomes,” she said.
The first RCT, published in 1993, enrolled 200 women with uncomplicated insulin-requiring diabetes (187 with GDM and 13 with pregestational diabetes) at 38 weeks of gestation, and compared active induction of labor within 5 days to expectant management. There was no significant difference in the cesarean delivery rate (the primary outcome), but rates of macrosomia and large for gestational age were higher in the expectant management group (27% vs. 15%, P = .05, and 23% vs. 10%, P = .02, respectively). Shoulder dystocia occurred in three deliveries, each of which was expectantly managed (Am J Obstet Gynecol. 1993;169[3]:611-5). Notably, the study included “only women with excellent glucose control,” Dr. Grantz said.
The second RCT, published in 2017 by a group in Italy, enrolled 425 patients with GDM (diagnosed by the International Association of Diabetes and Pregnancy Study Groups criteria) between week 38 and week 39 of gestation and similarly randomized them to induction of labor or expectant management. No difference in cesarean delivery was found (BJOG. 2017;124[4]:669-77). Induction of labor was associated with a higher risk of hyperbilirubinemia, and there was a trend toward a decreased risk of macrosomia, but again, the study was underpowered to detect differences in most outcomes, she said. (The study also was stopped early because of an inability to recruit, she noted.)
Dr. Grantz is currently recruiting for a randomized trial aimed at determining the optimal time between 37 and 39 weeks to initiate delivery — the time when neonatal morbidity and perinatal mortality risk is the lowest – for uncontrolled GDM-complicated pregnancies. The trial is designed to recruit up to 3,450 pregnant women with uncontrolled GDM and randomize the timing of their delivery (NCT05515744).
Those who are eligible for the study but do not consent to participate in randomization for delivery will be asked about chart review only (an estimated additional 3,000). The SPAN TIME study will also assess newborn development and behavior outcomes, as well as anthropometric measures, as secondary outcomes. An exploratory analysis will look for clinical, nonclinical or biochemical factors that could be helpful in optimizing delivery timing.
What Retrospective Studies Reveal
Factors that may influence the timing of delivery include the duration of neonatal exposure to hyperglycemia/hyperinsulinemia (pregestational vs. gestational diabetes), the level of diabetes control, and comorbidities (e.g. maternal renal disease or chronic hypertension). However, research “investigating how these factors influence morbidity and the timing of delivery is limited,” said Dr. Grantz.
Overall, it has been difficult through retrospective studies, she said, to investigate neonatal morbidity in diabetic pregnancies and tease apart the relative effects of diabetes as a precursor for early delivery and prematurity itself. Among the studies suggesting an independent risk of diabetes is a retrospective study focusing on neonatal respiratory morbidity — “one of the most common adverse outcomes associated with diabetes.”
The study, an analysis of the Consortium on Safe Labor study (an electronic medical record study of more than 220,000 singleton pregnancies), stratified morbidity by the probability of delivering at term (≥ 37 weeks). GDM and pregestational diabetes complicated 5.1% and 1.5% of the pregnancies, respectively, and were found to be associated with increased risks of neonatal respiratory morbidity compared to women without diabetes — regardless of the probability of delivering at term.
However, these associations were stronger with a higher probability of delivering at term, which suggests that the neonatal respiratory morbidity associated with diabetes is not fully explained by a greater propensity for prematurity (Am J Perinatol. 2017;34[11]:1160-8).
In addition, the rates of all neonatal respiratory morbidities and mortality were higher for pregestational diabetes compared with gestational diabetes, said Dr. Grantz, a senior author of the study. (Morbidities included neonatal intensive care unit admission, transient tachypnea of newborn, apnea, respiratory distress syndrome, mechanical ventilation, and stillbirth.)
The pathophysiology of diabetes and neonatal respiratory morbidity is “not fully known,” she said. It is believed that fetal hyperinsulinemia may cause delayed pulmonary maturation and there is evidence from animal studies that insulin decreases the incorporation of glucose and fatty acids into phospholipid phosphatidylglycerol. Indirect effects stem from the physiologic immaturity of earlier delivery and a higher cesarean delivery rate in pregnancies complicated by diabetes, Dr. Grantz said.
Among other retrospective studies was a population-based study from Canada (2004-2014), published in 2020, of large numbers of women with all types of diabetes and a comparison group of over 2.5 million without diabetes. For maternal morbidity/mortality, there were no significant differences by gestational age between iatrogenic delivery and expectant management among any form of diabetes. But for neonatal morbidity and mortality, the study found differences.
In women with gestational diabetes, iatrogenic delivery was associated with increased risk of neonatal morbidity/mortality at 36 and 37 weeks’ gestation and with decreased risk at weeks 38-40. Increased risk with iatrogenic delivery was also found for women with type 1 and type 2 diabetes at weeks 36 and 37 (Acta Obstet Gynecol Scand. 2020;99[3]:341-9).
Another retrospective study using California vital statistics (1997-2006) examined rates of stillbirth and infant death in women with GDM by gestational age at delivery (Am J Obstet Gynecol. 2012;206[4]:309.e1-e7). The 190,000-plus women with GDM had elevated risk of stillbirth at each gestational age compared to those without GDM, but “the [excess] risk for GDM was lowest at 38 weeks and again at 40 weeks,” Dr. Grantz said. The investigators concluded, she said, “that the risk of expectant management exceeded that of delivery at 38 weeks and beyond.”
Dr. Grantz reported no disclosures.
FROM DPSG-NA 2023
Shingles Vaccine Offers 4 Years of Protection
Two doses of the recombinant zoster vaccine (RZV) are effective against herpes zoster (HZ) for 4 years after vaccination, according to a new study published in Annals of Internal Medicine.
Findings from the prospective cohort study showed that people who received two doses of the vaccine, regardless of when they received their second dose, experienced 79% vaccine effectiveness (VE) during the first year, with effectiveness decreasing to 73% by year 4. By contrast, the rate of effectiveness during the first year was 70% for people who received a single dose, falling to 52% effectiveness by year 4.
The findings also showed that the rate of effectiveness was 65% for those taking corticosteroids.
The study was conducted between 2018 and 2022 using data from the Vaccine Safety Datalink, a collaboration between the US Centers for Disease Control and Prevention (CDC) and nine healthcare systems across the country.
Researchers evaluated the incidence of HZ, as determined by a diagnosis and prescription for antiviral medication within 7 days of diagnosis, and monitored RZV status over time.
The findings may quell fears that waiting too long for the second dose reduces the effectiveness of the herpes vaccine, according to Nicola Klein, MD, PhD, director of the Vaccine Study Center at Kaiser Permanente in Oakland, California, who led the study.
The long-term efficacy of the vaccine is especially important because older adults are now living much longer than in previous years, according to Alexandra Tien, MD, a family physician at Medical Associates of Rhode Island in Providence.
“People live these days into their 80s and even 90s,” Dr. Tien said. “That’s a large number of years to need protection for, so it’s really important to have a long-lasting vaccine.”
The CDC currently recommends two doses of RZV separated by 2-6 months for patients aged 50 years and older. Adults older than 19 years who are immunocompromised should receive two doses of RZV separated by 1-2 months, the agency said.
According to Dr. Klein, research does not show whether VE for RZV wanes after 4 years. But interim findings from another study following people in clinical trials found VE levels remained high after 7 years.
The risk for HZ increases with age, reaching a lifetime risk of 50% among adults aged 85 years. Complications like postherpetic neuralgia (PHN) — characterized by long-term tingling, numbness, and disabling pain at the site of the rash — can interfere with the quality of life and ability to function in older adults. The CDC estimates that up to 18% of people with shingles experience PHN, and the risk increases with age.
Just like with any other vaccine, patients sometimes have concerns about the potential side effects of RZV, said Dr. Tien. But those effects, such as muscle pain, nausea, and fever, are mild compared to shingles.
“I always tell patients, with any vaccine, immunization is one of the biggest bangs for your buck in healthcare because you’re preventing a problem,” Dr. Tien said.
This study was funded by the CDC through contracts with participating sites. Study authors reported no disclosures. Dr. Tien reported no disclosures.
A version of this article appeared on Medscape.com.
Two doses of the recombinant zoster vaccine (RZV) are effective against herpes zoster (HZ) for 4 years after vaccination, according to a new study published in Annals of Internal Medicine.
Findings from the prospective cohort study showed that people who received two doses of the vaccine, regardless of when they received their second dose, experienced 79% vaccine effectiveness (VE) during the first year, with effectiveness decreasing to 73% by year 4. By contrast, the rate of effectiveness during the first year was 70% for people who received a single dose, falling to 52% effectiveness by year 4.
The findings also showed that the rate of effectiveness was 65% for those taking corticosteroids.
The study was conducted between 2018 and 2022 using data from the Vaccine Safety Datalink, a collaboration between the US Centers for Disease Control and Prevention (CDC) and nine healthcare systems across the country.
Researchers evaluated the incidence of HZ, as determined by a diagnosis and prescription for antiviral medication within 7 days of diagnosis, and monitored RZV status over time.
The findings may quell fears that waiting too long for the second dose reduces the effectiveness of the herpes vaccine, according to Nicola Klein, MD, PhD, director of the Vaccine Study Center at Kaiser Permanente in Oakland, California, who led the study.
The long-term efficacy of the vaccine is especially important because older adults are now living much longer than in previous years, according to Alexandra Tien, MD, a family physician at Medical Associates of Rhode Island in Providence.
“People live these days into their 80s and even 90s,” Dr. Tien said. “That’s a large number of years to need protection for, so it’s really important to have a long-lasting vaccine.”
The CDC currently recommends two doses of RZV separated by 2-6 months for patients aged 50 years and older. Adults older than 19 years who are immunocompromised should receive two doses of RZV separated by 1-2 months, the agency said.
According to Dr. Klein, research does not show whether VE for RZV wanes after 4 years. But interim findings from another study following people in clinical trials found VE levels remained high after 7 years.
The risk for HZ increases with age, reaching a lifetime risk of 50% among adults aged 85 years. Complications like postherpetic neuralgia (PHN) — characterized by long-term tingling, numbness, and disabling pain at the site of the rash — can interfere with the quality of life and ability to function in older adults. The CDC estimates that up to 18% of people with shingles experience PHN, and the risk increases with age.
Just like with any other vaccine, patients sometimes have concerns about the potential side effects of RZV, said Dr. Tien. But those effects, such as muscle pain, nausea, and fever, are mild compared to shingles.
“I always tell patients, with any vaccine, immunization is one of the biggest bangs for your buck in healthcare because you’re preventing a problem,” Dr. Tien said.
This study was funded by the CDC through contracts with participating sites. Study authors reported no disclosures. Dr. Tien reported no disclosures.
A version of this article appeared on Medscape.com.
Two doses of the recombinant zoster vaccine (RZV) are effective against herpes zoster (HZ) for 4 years after vaccination, according to a new study published in Annals of Internal Medicine.
Findings from the prospective cohort study showed that people who received two doses of the vaccine, regardless of when they received their second dose, experienced 79% vaccine effectiveness (VE) during the first year, with effectiveness decreasing to 73% by year 4. By contrast, the rate of effectiveness during the first year was 70% for people who received a single dose, falling to 52% effectiveness by year 4.
The findings also showed that the rate of effectiveness was 65% for those taking corticosteroids.
The study was conducted between 2018 and 2022 using data from the Vaccine Safety Datalink, a collaboration between the US Centers for Disease Control and Prevention (CDC) and nine healthcare systems across the country.
Researchers evaluated the incidence of HZ, as determined by a diagnosis and prescription for antiviral medication within 7 days of diagnosis, and monitored RZV status over time.
The findings may quell fears that waiting too long for the second dose reduces the effectiveness of the herpes vaccine, according to Nicola Klein, MD, PhD, director of the Vaccine Study Center at Kaiser Permanente in Oakland, California, who led the study.
The long-term efficacy of the vaccine is especially important because older adults are now living much longer than in previous years, according to Alexandra Tien, MD, a family physician at Medical Associates of Rhode Island in Providence.
“People live these days into their 80s and even 90s,” Dr. Tien said. “That’s a large number of years to need protection for, so it’s really important to have a long-lasting vaccine.”
The CDC currently recommends two doses of RZV separated by 2-6 months for patients aged 50 years and older. Adults older than 19 years who are immunocompromised should receive two doses of RZV separated by 1-2 months, the agency said.
According to Dr. Klein, research does not show whether VE for RZV wanes after 4 years. But interim findings from another study following people in clinical trials found VE levels remained high after 7 years.
The risk for HZ increases with age, reaching a lifetime risk of 50% among adults aged 85 years. Complications like postherpetic neuralgia (PHN) — characterized by long-term tingling, numbness, and disabling pain at the site of the rash — can interfere with the quality of life and ability to function in older adults. The CDC estimates that up to 18% of people with shingles experience PHN, and the risk increases with age.
Just like with any other vaccine, patients sometimes have concerns about the potential side effects of RZV, said Dr. Tien. But those effects, such as muscle pain, nausea, and fever, are mild compared to shingles.
“I always tell patients, with any vaccine, immunization is one of the biggest bangs for your buck in healthcare because you’re preventing a problem,” Dr. Tien said.
This study was funded by the CDC through contracts with participating sites. Study authors reported no disclosures. Dr. Tien reported no disclosures.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
More Evidence Suggests That ‘Long Flu’ Is a Thing
You may have never heard of it, but you may have had it. More evidence points to “long flu” being a real phenomenon, with a large study showing symptoms persist at least 4 weeks or more after some people are hospitalized for the flu.
Researchers compared long flu to long COVID-19 and found long flu happened less often and was less severe overall. This difference could be because the flu mostly affects the lungs whereas COVID can affect any number of organ systems in the body.
The investigators were surprised that both long flu and long COVID were linked to a greater burden of health loss, compared to either initial infection.
“I think COVID and long COVID made us realize that infections have long-term consequences, and often the toll of those long-term consequences is much larger than the toll of acute disease,” said Ziyad Al-Aly, MD, senior author of the study and chief of research and development at the VA St. Louis Health Care System.
“I know, having studied long COVID for the past 4 years, I should not be surprised. But I am in awe of what these infections can do to the long-term health of affected individuals,” said Dr. Al-Aly, who is also a clinical epidemiologist at Washington University in St. Louis.
Dr. Al-Aly and colleagues Yan Xie, PhD, and Taeyoung Choi, MS, analyzed US Department of Veterans Affairs medical records. They compared 81,280 people hospitalized with COVID to 10,985 people hospitalized with the flu before the COVID pandemic. They checked up to 18 months after initial infections to see who developed long flu or long COVID symptoms.
The study was published online in The Lancet Infectious Diseases.
It’s an interesting study, said Aaron E. Glatt, MD, chairman of the Department of Medicine and a hospital epidemiologist at Mount Sinai South Nassau in Oceanside, NY, who was not part of the research.
“There is a concern with many viruses that you can have long-term consequences,” said Dr. Glatt, who is also a fellow of the Infectious Diseases Society of America. He said the possibility of long-term symptoms with the flu is not new, “but it’s nice to have more data.”
People hospitalized with COVID had a 50% higher risk of death during the study period than people hospitalized with the flu. Put another way, for every 100 people admitted to the hospital with COVID, about eight more died than those hospitalized with the flu over the following 18 months. Hospital admissions and admissions to the intensive care unit were also higher in the long COVID group — 20 more people and nine more people, respectively, for every 100 people admitted to the hospital with COVID.
More research is needed, Dr. Glatt said. “With many of these viruses, we don’t understand what they do to the body.” A prospective study to see if antiviral treatments make a difference, for example, would be useful, he noted.
Dr. Al-Aly and colleagues would like to do more studies.
“We need to more deeply understand how and why acute infections cause long-term illness,” he said, noting that he also wants to investigate ways to prevent and treat the long-term effects.
“Much remains to be done, and we are deeply committed to doing our best to develop those answers.”
A version of this article first appeared on WebMD.com.
You may have never heard of it, but you may have had it. More evidence points to “long flu” being a real phenomenon, with a large study showing symptoms persist at least 4 weeks or more after some people are hospitalized for the flu.
Researchers compared long flu to long COVID-19 and found long flu happened less often and was less severe overall. This difference could be because the flu mostly affects the lungs whereas COVID can affect any number of organ systems in the body.
The investigators were surprised that both long flu and long COVID were linked to a greater burden of health loss, compared to either initial infection.
“I think COVID and long COVID made us realize that infections have long-term consequences, and often the toll of those long-term consequences is much larger than the toll of acute disease,” said Ziyad Al-Aly, MD, senior author of the study and chief of research and development at the VA St. Louis Health Care System.
“I know, having studied long COVID for the past 4 years, I should not be surprised. But I am in awe of what these infections can do to the long-term health of affected individuals,” said Dr. Al-Aly, who is also a clinical epidemiologist at Washington University in St. Louis.
Dr. Al-Aly and colleagues Yan Xie, PhD, and Taeyoung Choi, MS, analyzed US Department of Veterans Affairs medical records. They compared 81,280 people hospitalized with COVID to 10,985 people hospitalized with the flu before the COVID pandemic. They checked up to 18 months after initial infections to see who developed long flu or long COVID symptoms.
The study was published online in The Lancet Infectious Diseases.
It’s an interesting study, said Aaron E. Glatt, MD, chairman of the Department of Medicine and a hospital epidemiologist at Mount Sinai South Nassau in Oceanside, NY, who was not part of the research.
“There is a concern with many viruses that you can have long-term consequences,” said Dr. Glatt, who is also a fellow of the Infectious Diseases Society of America. He said the possibility of long-term symptoms with the flu is not new, “but it’s nice to have more data.”
People hospitalized with COVID had a 50% higher risk of death during the study period than people hospitalized with the flu. Put another way, for every 100 people admitted to the hospital with COVID, about eight more died than those hospitalized with the flu over the following 18 months. Hospital admissions and admissions to the intensive care unit were also higher in the long COVID group — 20 more people and nine more people, respectively, for every 100 people admitted to the hospital with COVID.
More research is needed, Dr. Glatt said. “With many of these viruses, we don’t understand what they do to the body.” A prospective study to see if antiviral treatments make a difference, for example, would be useful, he noted.
Dr. Al-Aly and colleagues would like to do more studies.
“We need to more deeply understand how and why acute infections cause long-term illness,” he said, noting that he also wants to investigate ways to prevent and treat the long-term effects.
“Much remains to be done, and we are deeply committed to doing our best to develop those answers.”
A version of this article first appeared on WebMD.com.
You may have never heard of it, but you may have had it. More evidence points to “long flu” being a real phenomenon, with a large study showing symptoms persist at least 4 weeks or more after some people are hospitalized for the flu.
Researchers compared long flu to long COVID-19 and found long flu happened less often and was less severe overall. This difference could be because the flu mostly affects the lungs whereas COVID can affect any number of organ systems in the body.
The investigators were surprised that both long flu and long COVID were linked to a greater burden of health loss, compared to either initial infection.
“I think COVID and long COVID made us realize that infections have long-term consequences, and often the toll of those long-term consequences is much larger than the toll of acute disease,” said Ziyad Al-Aly, MD, senior author of the study and chief of research and development at the VA St. Louis Health Care System.
“I know, having studied long COVID for the past 4 years, I should not be surprised. But I am in awe of what these infections can do to the long-term health of affected individuals,” said Dr. Al-Aly, who is also a clinical epidemiologist at Washington University in St. Louis.
Dr. Al-Aly and colleagues Yan Xie, PhD, and Taeyoung Choi, MS, analyzed US Department of Veterans Affairs medical records. They compared 81,280 people hospitalized with COVID to 10,985 people hospitalized with the flu before the COVID pandemic. They checked up to 18 months after initial infections to see who developed long flu or long COVID symptoms.
The study was published online in The Lancet Infectious Diseases.
It’s an interesting study, said Aaron E. Glatt, MD, chairman of the Department of Medicine and a hospital epidemiologist at Mount Sinai South Nassau in Oceanside, NY, who was not part of the research.
“There is a concern with many viruses that you can have long-term consequences,” said Dr. Glatt, who is also a fellow of the Infectious Diseases Society of America. He said the possibility of long-term symptoms with the flu is not new, “but it’s nice to have more data.”
People hospitalized with COVID had a 50% higher risk of death during the study period than people hospitalized with the flu. Put another way, for every 100 people admitted to the hospital with COVID, about eight more died than those hospitalized with the flu over the following 18 months. Hospital admissions and admissions to the intensive care unit were also higher in the long COVID group — 20 more people and nine more people, respectively, for every 100 people admitted to the hospital with COVID.
More research is needed, Dr. Glatt said. “With many of these viruses, we don’t understand what they do to the body.” A prospective study to see if antiviral treatments make a difference, for example, would be useful, he noted.
Dr. Al-Aly and colleagues would like to do more studies.
“We need to more deeply understand how and why acute infections cause long-term illness,” he said, noting that he also wants to investigate ways to prevent and treat the long-term effects.
“Much remains to be done, and we are deeply committed to doing our best to develop those answers.”
A version of this article first appeared on WebMD.com.
FROM THE LANCET INFECTIOUS DISEASES
Is This the Cure for Restless Legs?
I don’t rightly remember when I first learned of restless legs syndrome (RLS). It was many decades ago, and I recognized that once in a while, I would be restless during sleep, tossing and turning, seeking a favorable sleeping position. I felt like I just needed to move my legs around; my gastrocnemii and hamstrings might cramp; and my torso skin might strangely “crawl” a bit, but then normal sleep would return. I never sought medical care for it and used no treatment, except moving my legs when indicated.
My trusty LLM (large language model), Bard, tells me that there are about 53,000 articles about RLS in English, of which, some 20,000 are in the primary source, peer reviewed literature. Count this as one more article. Will it make a difference? Read on and see.
For many centuries (since Sir Thomas Willis in 1672), the symptoms now grouped and categorized as RLS have been recognized and reported but were often dismissed as bizarre and unexplained. The name was applied in 1948 by Dr Karl-Axel Ekborn.
In the 1960s, in sleep labs, RLS became better studied and characterized.
Mayo Clinic describes RLS as “… compelling, unpleasant sensations in the legs or feet ... both sides of the body ... within the limb rather than on the skin ... crawling, creeping, pulling, throbbing, aching, itching, electric ... difficult to explain …” Not numbness, but a consistent desire to move the legs.
When I read about it many decades ago, I realized that I may have RLS. But then many months would pass with no recurrence, so I dismissed it as just another of those “symptoms of unknown origin” that my late friend Clifton Meador has written about so eloquently.
I am sure that a lot of people experience this, don’t understand it, and don’t consider it important enough to do anything about. The cause is unknown, but it seems to run in families. It may be autosomal dominant, but no causative genes have been confirmed.
Treatment of RLS
Many pharmacologic and physical treatments have been tried with some success for some patients, but over time, these treatments have mostly failed.
We know how Big Pharma often operates. A company owns a drug, preferably under patent protection, but without an apparent profitable indication. They need to find a medical condition, ideally one with troublesome symptoms, that the drug might ameliorate to some degree. Armed with a plausible candidate symptom, the company embarks upon a campaign to find people who might want to take the drug. Mass communications, such as direct-to-consumer advertising, can identify large numbers of people who match to pretty much any symptoms, although many of these people never suspected they had a disease, much less a treatable one.
I figured long ago that RLS was just another of those nonspecific entities experienced by many people, making them good candidates for disease mongering.
In 2005, the marketing of GlaxoSmithKline’s (GSK’s) dopamine agonist drug Requip (ropinirole) was approved by the FDA. GSK had already undertaken an intensive promotional campaign for Requip, issuing press releases, advertising to doctors in medical journals, and advertising directly to consumers. To increase general awareness of RLS, GSK’s campaign told consumers that a “new survey reveals that a common yet underrecognized disorder-restless legs syndrome—is keeping Americans awake at night.” GSK was accused of “disease mongering,” trying to turn ordinary people into patients who needed specific drugs.
Within a year, sales of the drug had doubled, climbing from $165 million in 2005 to nearly $330 million in 2006. Soon, 4.4 million prescriptions were written annually for the drug, with sales reported to be nearly $491 million. However, the focus on RLS faded rapidly as the Requip television commercials were pulled from the airwaves following approval of generic ropinirole.
And Requip had competition. Boehringer Ingelheim manufactures pramipexole (brand name Mirapex) another dopamine agonist. Gabapentin enacarbil (marketed as Horizant by UCB Pharma) is also approved for RLS, and Pfizer’s pregabalin (brand name Lyrica) is used off-label to manage symptoms of RLS. Janssen Pharmaceuticals manufactures rotigotine, (brand name Neupro), a dopamine agonist delivered via a transdermal patch.
It is safe to say that RLS is a real clinical entity composed of clearly recognizable symptoms, with no cure and no ending, unless it is associated with iron-deficiency anemia. However, as a disease, it seems to lack etiology, pathology, pathogenesis, pathophysiology, diagnostic findings on physical examination, laboratory tests, or imaging, and any clear strategy for prevention.
Pharmacologic treatments include dopaminergic agents, benzodiazepines, opioids, anticonvulsants, alpha 2–adrenergic agonists and iron salts. Yes, you read that right; RLS is treated with a broad array of different drugs, which is usually a sign that nothing works very well. Some agents work for a while, but none seem to be the definitive solution.
Same for the physical interventions: sleep hygiene, exercise, hot or cold bathing, limb massage, vibratory or electrical stimulation of the feet, stopping caffeine before bedtime. Try everything and see if something works.
Taking the Sugar Challenge
Could the culprit be sugar?
Lacking clarity of scientific understanding of RLS or its treatment from an extensive clinical literature, after ascertaining that RLS is real, one might look for real-world evidence, including well-performed N-of-1 trials.
I am an antisugar guy. Read my prior Medscape columns. I practice what I preach, but sugar does taste good.
Early in November 2023, after a healthy, conservative dinner at home with some wine, I enjoyed a mini Dove bar for dessert. But I didn’t stop there.
Mini Dove bars contain 11 grams sugar. It was also just a few days after Halloween. Having had fewer trick-or-treaters than expected, we had leftovers. Snickers, Milky Ways, Twix mini bars, each with at least 20 grams of sugar.
I ate several of these not long before bedtime. Lo and behold, in the dark of that night, and continuing off and on for a few fitful hours, I had bad RLS. Shifting, tossing, turning, compulsively seeking a new sleeping position only to have to soon move again. Plus, I had repetitive leg cramps and that creepy-crawly skin sensation. An altogether unpleasant experience. Sound sleep eventually arrived, and there were no recurrences over subsequent weeks.
The classic way to determine whether a drug is causing a reaction, condition, or disease is to apply the challenge-dechallenge-rechallenge testing method.
Give the drug, the patient demonstrates the disease finding. Remove the drug, the problem disappears. Rinse and repeat three times. We pathologists first worked this out for drug-induced liver disease, such as steatosis, in the late 1960s. Blinding or double blinding in these N-of-1 situations would be nice but often not practical.
Siwert de Groot, in the Netherlands, published a very convincing use of this technique in 2023: Big-time sugar consumption for a week, then low intake of sugar for the following week, repeated three times on one patient.
Very elaborate RLS symptom reporting. I’m pretty convinced from my unintentional challenge and single dechallenge that my unusually high sugar intake resulted in RLS. I will not undergo a rechallenge, although it might be fun to binge on sucrose and see what happens.
If you are serious about identifying or treating RLS, I suggest that you incorporate the International Restless Legs Study Group Severity Rating Scale into your practice, and begin the systematic use of the dechallenge-rechallenge exclusion process for your patients with RLS. Start with sugar and see what happens. Keep records and let the world know what you discover. Be your own clinical investigator. Social media offers you abundant opportunity to share your results, whatever they may be.
How many millions of dollars would Big Pharma lose if patients with RLS just said no to sugar and it worked? Of course, humans being humans, many would probably prefer to continue to gorge on sugar, gain weight, develop diabetes, and then take medications to control their RLS symptoms. But patients ought to at least be given an informed choice.
I will be watching for your reports.
Dr. Lundberg had no disclosures.
A version of this article appeared on Medscape.com.
I don’t rightly remember when I first learned of restless legs syndrome (RLS). It was many decades ago, and I recognized that once in a while, I would be restless during sleep, tossing and turning, seeking a favorable sleeping position. I felt like I just needed to move my legs around; my gastrocnemii and hamstrings might cramp; and my torso skin might strangely “crawl” a bit, but then normal sleep would return. I never sought medical care for it and used no treatment, except moving my legs when indicated.
My trusty LLM (large language model), Bard, tells me that there are about 53,000 articles about RLS in English, of which, some 20,000 are in the primary source, peer reviewed literature. Count this as one more article. Will it make a difference? Read on and see.
For many centuries (since Sir Thomas Willis in 1672), the symptoms now grouped and categorized as RLS have been recognized and reported but were often dismissed as bizarre and unexplained. The name was applied in 1948 by Dr Karl-Axel Ekborn.
In the 1960s, in sleep labs, RLS became better studied and characterized.
Mayo Clinic describes RLS as “… compelling, unpleasant sensations in the legs or feet ... both sides of the body ... within the limb rather than on the skin ... crawling, creeping, pulling, throbbing, aching, itching, electric ... difficult to explain …” Not numbness, but a consistent desire to move the legs.
When I read about it many decades ago, I realized that I may have RLS. But then many months would pass with no recurrence, so I dismissed it as just another of those “symptoms of unknown origin” that my late friend Clifton Meador has written about so eloquently.
I am sure that a lot of people experience this, don’t understand it, and don’t consider it important enough to do anything about. The cause is unknown, but it seems to run in families. It may be autosomal dominant, but no causative genes have been confirmed.
Treatment of RLS
Many pharmacologic and physical treatments have been tried with some success for some patients, but over time, these treatments have mostly failed.
We know how Big Pharma often operates. A company owns a drug, preferably under patent protection, but without an apparent profitable indication. They need to find a medical condition, ideally one with troublesome symptoms, that the drug might ameliorate to some degree. Armed with a plausible candidate symptom, the company embarks upon a campaign to find people who might want to take the drug. Mass communications, such as direct-to-consumer advertising, can identify large numbers of people who match to pretty much any symptoms, although many of these people never suspected they had a disease, much less a treatable one.
I figured long ago that RLS was just another of those nonspecific entities experienced by many people, making them good candidates for disease mongering.
In 2005, the marketing of GlaxoSmithKline’s (GSK’s) dopamine agonist drug Requip (ropinirole) was approved by the FDA. GSK had already undertaken an intensive promotional campaign for Requip, issuing press releases, advertising to doctors in medical journals, and advertising directly to consumers. To increase general awareness of RLS, GSK’s campaign told consumers that a “new survey reveals that a common yet underrecognized disorder-restless legs syndrome—is keeping Americans awake at night.” GSK was accused of “disease mongering,” trying to turn ordinary people into patients who needed specific drugs.
Within a year, sales of the drug had doubled, climbing from $165 million in 2005 to nearly $330 million in 2006. Soon, 4.4 million prescriptions were written annually for the drug, with sales reported to be nearly $491 million. However, the focus on RLS faded rapidly as the Requip television commercials were pulled from the airwaves following approval of generic ropinirole.
And Requip had competition. Boehringer Ingelheim manufactures pramipexole (brand name Mirapex) another dopamine agonist. Gabapentin enacarbil (marketed as Horizant by UCB Pharma) is also approved for RLS, and Pfizer’s pregabalin (brand name Lyrica) is used off-label to manage symptoms of RLS. Janssen Pharmaceuticals manufactures rotigotine, (brand name Neupro), a dopamine agonist delivered via a transdermal patch.
It is safe to say that RLS is a real clinical entity composed of clearly recognizable symptoms, with no cure and no ending, unless it is associated with iron-deficiency anemia. However, as a disease, it seems to lack etiology, pathology, pathogenesis, pathophysiology, diagnostic findings on physical examination, laboratory tests, or imaging, and any clear strategy for prevention.
Pharmacologic treatments include dopaminergic agents, benzodiazepines, opioids, anticonvulsants, alpha 2–adrenergic agonists and iron salts. Yes, you read that right; RLS is treated with a broad array of different drugs, which is usually a sign that nothing works very well. Some agents work for a while, but none seem to be the definitive solution.
Same for the physical interventions: sleep hygiene, exercise, hot or cold bathing, limb massage, vibratory or electrical stimulation of the feet, stopping caffeine before bedtime. Try everything and see if something works.
Taking the Sugar Challenge
Could the culprit be sugar?
Lacking clarity of scientific understanding of RLS or its treatment from an extensive clinical literature, after ascertaining that RLS is real, one might look for real-world evidence, including well-performed N-of-1 trials.
I am an antisugar guy. Read my prior Medscape columns. I practice what I preach, but sugar does taste good.
Early in November 2023, after a healthy, conservative dinner at home with some wine, I enjoyed a mini Dove bar for dessert. But I didn’t stop there.
Mini Dove bars contain 11 grams sugar. It was also just a few days after Halloween. Having had fewer trick-or-treaters than expected, we had leftovers. Snickers, Milky Ways, Twix mini bars, each with at least 20 grams of sugar.
I ate several of these not long before bedtime. Lo and behold, in the dark of that night, and continuing off and on for a few fitful hours, I had bad RLS. Shifting, tossing, turning, compulsively seeking a new sleeping position only to have to soon move again. Plus, I had repetitive leg cramps and that creepy-crawly skin sensation. An altogether unpleasant experience. Sound sleep eventually arrived, and there were no recurrences over subsequent weeks.
The classic way to determine whether a drug is causing a reaction, condition, or disease is to apply the challenge-dechallenge-rechallenge testing method.
Give the drug, the patient demonstrates the disease finding. Remove the drug, the problem disappears. Rinse and repeat three times. We pathologists first worked this out for drug-induced liver disease, such as steatosis, in the late 1960s. Blinding or double blinding in these N-of-1 situations would be nice but often not practical.
Siwert de Groot, in the Netherlands, published a very convincing use of this technique in 2023: Big-time sugar consumption for a week, then low intake of sugar for the following week, repeated three times on one patient.
Very elaborate RLS symptom reporting. I’m pretty convinced from my unintentional challenge and single dechallenge that my unusually high sugar intake resulted in RLS. I will not undergo a rechallenge, although it might be fun to binge on sucrose and see what happens.
If you are serious about identifying or treating RLS, I suggest that you incorporate the International Restless Legs Study Group Severity Rating Scale into your practice, and begin the systematic use of the dechallenge-rechallenge exclusion process for your patients with RLS. Start with sugar and see what happens. Keep records and let the world know what you discover. Be your own clinical investigator. Social media offers you abundant opportunity to share your results, whatever they may be.
How many millions of dollars would Big Pharma lose if patients with RLS just said no to sugar and it worked? Of course, humans being humans, many would probably prefer to continue to gorge on sugar, gain weight, develop diabetes, and then take medications to control their RLS symptoms. But patients ought to at least be given an informed choice.
I will be watching for your reports.
Dr. Lundberg had no disclosures.
A version of this article appeared on Medscape.com.
I don’t rightly remember when I first learned of restless legs syndrome (RLS). It was many decades ago, and I recognized that once in a while, I would be restless during sleep, tossing and turning, seeking a favorable sleeping position. I felt like I just needed to move my legs around; my gastrocnemii and hamstrings might cramp; and my torso skin might strangely “crawl” a bit, but then normal sleep would return. I never sought medical care for it and used no treatment, except moving my legs when indicated.
My trusty LLM (large language model), Bard, tells me that there are about 53,000 articles about RLS in English, of which, some 20,000 are in the primary source, peer reviewed literature. Count this as one more article. Will it make a difference? Read on and see.
For many centuries (since Sir Thomas Willis in 1672), the symptoms now grouped and categorized as RLS have been recognized and reported but were often dismissed as bizarre and unexplained. The name was applied in 1948 by Dr Karl-Axel Ekborn.
In the 1960s, in sleep labs, RLS became better studied and characterized.
Mayo Clinic describes RLS as “… compelling, unpleasant sensations in the legs or feet ... both sides of the body ... within the limb rather than on the skin ... crawling, creeping, pulling, throbbing, aching, itching, electric ... difficult to explain …” Not numbness, but a consistent desire to move the legs.
When I read about it many decades ago, I realized that I may have RLS. But then many months would pass with no recurrence, so I dismissed it as just another of those “symptoms of unknown origin” that my late friend Clifton Meador has written about so eloquently.
I am sure that a lot of people experience this, don’t understand it, and don’t consider it important enough to do anything about. The cause is unknown, but it seems to run in families. It may be autosomal dominant, but no causative genes have been confirmed.
Treatment of RLS
Many pharmacologic and physical treatments have been tried with some success for some patients, but over time, these treatments have mostly failed.
We know how Big Pharma often operates. A company owns a drug, preferably under patent protection, but without an apparent profitable indication. They need to find a medical condition, ideally one with troublesome symptoms, that the drug might ameliorate to some degree. Armed with a plausible candidate symptom, the company embarks upon a campaign to find people who might want to take the drug. Mass communications, such as direct-to-consumer advertising, can identify large numbers of people who match to pretty much any symptoms, although many of these people never suspected they had a disease, much less a treatable one.
I figured long ago that RLS was just another of those nonspecific entities experienced by many people, making them good candidates for disease mongering.
In 2005, the marketing of GlaxoSmithKline’s (GSK’s) dopamine agonist drug Requip (ropinirole) was approved by the FDA. GSK had already undertaken an intensive promotional campaign for Requip, issuing press releases, advertising to doctors in medical journals, and advertising directly to consumers. To increase general awareness of RLS, GSK’s campaign told consumers that a “new survey reveals that a common yet underrecognized disorder-restless legs syndrome—is keeping Americans awake at night.” GSK was accused of “disease mongering,” trying to turn ordinary people into patients who needed specific drugs.
Within a year, sales of the drug had doubled, climbing from $165 million in 2005 to nearly $330 million in 2006. Soon, 4.4 million prescriptions were written annually for the drug, with sales reported to be nearly $491 million. However, the focus on RLS faded rapidly as the Requip television commercials were pulled from the airwaves following approval of generic ropinirole.
And Requip had competition. Boehringer Ingelheim manufactures pramipexole (brand name Mirapex) another dopamine agonist. Gabapentin enacarbil (marketed as Horizant by UCB Pharma) is also approved for RLS, and Pfizer’s pregabalin (brand name Lyrica) is used off-label to manage symptoms of RLS. Janssen Pharmaceuticals manufactures rotigotine, (brand name Neupro), a dopamine agonist delivered via a transdermal patch.
It is safe to say that RLS is a real clinical entity composed of clearly recognizable symptoms, with no cure and no ending, unless it is associated with iron-deficiency anemia. However, as a disease, it seems to lack etiology, pathology, pathogenesis, pathophysiology, diagnostic findings on physical examination, laboratory tests, or imaging, and any clear strategy for prevention.
Pharmacologic treatments include dopaminergic agents, benzodiazepines, opioids, anticonvulsants, alpha 2–adrenergic agonists and iron salts. Yes, you read that right; RLS is treated with a broad array of different drugs, which is usually a sign that nothing works very well. Some agents work for a while, but none seem to be the definitive solution.
Same for the physical interventions: sleep hygiene, exercise, hot or cold bathing, limb massage, vibratory or electrical stimulation of the feet, stopping caffeine before bedtime. Try everything and see if something works.
Taking the Sugar Challenge
Could the culprit be sugar?
Lacking clarity of scientific understanding of RLS or its treatment from an extensive clinical literature, after ascertaining that RLS is real, one might look for real-world evidence, including well-performed N-of-1 trials.
I am an antisugar guy. Read my prior Medscape columns. I practice what I preach, but sugar does taste good.
Early in November 2023, after a healthy, conservative dinner at home with some wine, I enjoyed a mini Dove bar for dessert. But I didn’t stop there.
Mini Dove bars contain 11 grams sugar. It was also just a few days after Halloween. Having had fewer trick-or-treaters than expected, we had leftovers. Snickers, Milky Ways, Twix mini bars, each with at least 20 grams of sugar.
I ate several of these not long before bedtime. Lo and behold, in the dark of that night, and continuing off and on for a few fitful hours, I had bad RLS. Shifting, tossing, turning, compulsively seeking a new sleeping position only to have to soon move again. Plus, I had repetitive leg cramps and that creepy-crawly skin sensation. An altogether unpleasant experience. Sound sleep eventually arrived, and there were no recurrences over subsequent weeks.
The classic way to determine whether a drug is causing a reaction, condition, or disease is to apply the challenge-dechallenge-rechallenge testing method.
Give the drug, the patient demonstrates the disease finding. Remove the drug, the problem disappears. Rinse and repeat three times. We pathologists first worked this out for drug-induced liver disease, such as steatosis, in the late 1960s. Blinding or double blinding in these N-of-1 situations would be nice but often not practical.
Siwert de Groot, in the Netherlands, published a very convincing use of this technique in 2023: Big-time sugar consumption for a week, then low intake of sugar for the following week, repeated three times on one patient.
Very elaborate RLS symptom reporting. I’m pretty convinced from my unintentional challenge and single dechallenge that my unusually high sugar intake resulted in RLS. I will not undergo a rechallenge, although it might be fun to binge on sucrose and see what happens.
If you are serious about identifying or treating RLS, I suggest that you incorporate the International Restless Legs Study Group Severity Rating Scale into your practice, and begin the systematic use of the dechallenge-rechallenge exclusion process for your patients with RLS. Start with sugar and see what happens. Keep records and let the world know what you discover. Be your own clinical investigator. Social media offers you abundant opportunity to share your results, whatever they may be.
How many millions of dollars would Big Pharma lose if patients with RLS just said no to sugar and it worked? Of course, humans being humans, many would probably prefer to continue to gorge on sugar, gain weight, develop diabetes, and then take medications to control their RLS symptoms. But patients ought to at least be given an informed choice.
I will be watching for your reports.
Dr. Lundberg had no disclosures.
A version of this article appeared on Medscape.com.
IBS Meta-Analysis Offers Some Support for Mesalamine
Certain patients with irritable bowel syndrome (IBS) may benefit from treatment with mesalamine, although the quality of evidence supporting this strategy remains low, according to a recent systematic literature review and meta-analysis.
Global IBS symptoms improved significantly across the entire population, however, a subgroup analysis suggested that mesalamine may be most beneficial for patients who present with diarrhea, providing support for a large clinical trial in this patient population, reported lead author Vivek C. Goodoory, MBChB, of St. James’s University Hospital, Leeds, United Kingdom, and colleagues.
Some patients with IBS may present with low-grade inflammation in the intestine, offering theoretical grounds for prescribing mesalamine, which is typically used for treating ulcerative colitis, the investigators wrote in Clinical Gastroenterology and Hepatology. Yet previous randomized controlled trials (RCTs) evaluating mesalamine for IBS have yielded mixed results, and a meta-analysis showed that mesalamine offered no benefit.
According to Dr. Goodoory and colleagues, however, that meta-analysis fell short since it “only pooled mean symptom scores, rather than the proportion of patients in each trial experiencing an improvement in symptoms, and did not appear to include data from all available RCTs.” Furthermore, they noted that this prior study lacked subgroup analyses conducted based on IBS subtype or postinfection status.
“We, therefore, conducted a contemporaneous meta-analysis to examine the efficacy and safety of mesalamine in IBS addressing these deficits in knowledge,” the investigators wrote.
Their meta-analysis included 820 patients from 8 RCTs published between 2009 and 2022. Efficacy and safety were evaluated via dichotomous assessments of global IBS symptoms, bowel habit or stool frequency, abdominal pain, and adverse events. Two subgroup analyses were planned to evaluate responses based on post-infection status and predominant stool pattern.
Unlike the previous meta-analysis, Dr. Goodoory and colleagues detected a potential signal for efficacy.
Across all patients, mesalamine was associated with significant improvement in global IBS symptoms, compared with placebo (relative risk [RR], 0.86; 95% CI, 0.79-0.95). However, no significant improvements were detected for abdominal pain or bowel habit/stool frequency.
A subgroup analysis of patients exhibiting IBS with diarrhea showed significantly greater improvements in global IBS symptoms for mesalamine versus placebo (RR, 0.88; 95% CI, 0.79-0.99). This subgroup showed no improvements in abdominal pain or bowel habit/stool frequency.
Subgroup analyses for patients with constipation or mixed bowel habits, or based on postinfection status, revealed no significant differences, although the investigators noted that relevant data were limited.
Mesalamine appeared to be well tolerated. Across five studies reporting adverse events, 43.5% of patients receiving mesalamine reported any adverse event, compared with 41.4% of patients on placebo. The RR of experiencing an adverse event in those taking mesalamine was 1.20 (95% CI, 0.89-1.63), which was not statistically significant.
“There was no evidence of heterogeneity between studies in most of our analyses, but only one trial was at low risk of bias across all domains, and there were insufficient studies to assess for funnel plot asymmetry,” Dr. Goodoory and colleagues wrote. “Based on these limitations of the evidence,” they continued, “our confidence in the results of the meta-analysis would be low, and further large trials at low risk of bias would be informative.”
Specifically, the investigators suggested an RCT recruiting only patients with IBS with diarrhea, and reporting efficacy according to postinfection status.
One coauthor reported research funding from Tillotts Pharma and Dr Falk Pharma UK. The remaining authors reported no conflicts.
Advancements in the understanding of irritable bowel syndrome (IBS) pathophysiology have led to new pharmacological agents and guidelines on the delivery of patient-specific IBS care. However, treatments targeting specific IBS mechanisms including altered immune responses, barrier dysfunction, and low-grade inflammation are lacking.
In a systematic review and meta-analysis, Goodoory et al. find that pooled results from six randomized controlled trials suggest efficacy with mesalamine, an anti-inflammatory agent, for global IBS symptoms with subgroup analyses further suggesting efficacy in IBS with diarrhea. However, results are tempered by the overall low quality of evidence and lack of benefit for abdominal pain or bowel habits. Notably, the only study rated as low risk of bias did not find mesalamine to be effective. Thus, these findings cannot yet be used to inform clinical decision-makers.
Still, further study is warranted. Such future work will benefit from including well-phenotyped patients and novel biomarkers with the ability to identify individuals in whom inflammatory mechanisms contribute to IBS symptoms.
Dr. Andrea Shin is based in the Vatche and Tamar Manoukian Division of Digestive Diseases at the University of California Los Angeles. She is a member of the editorial boards of Clinical Gastroenterology and Hepatology, and Alimentary Pharmacology & Therapeutics. She is associate clinical editor of Neurogastroenterology & Motility, and a member of the Scientific Communications Advisory Board for IBS-C. As an AGA member, she sits on the Research Awards Panel, and is a section councilor for neurogastroenterology & motility, as well as a patient education advisor. Dr. Shin has received funding from the NIH R03 Limited Competiation Small Grant Program, an ANMS Diversity Development Award, and is an NIH Loan Repayment Program Awardee. She has no other relevant disclosures.
Advancements in the understanding of irritable bowel syndrome (IBS) pathophysiology have led to new pharmacological agents and guidelines on the delivery of patient-specific IBS care. However, treatments targeting specific IBS mechanisms including altered immune responses, barrier dysfunction, and low-grade inflammation are lacking.
In a systematic review and meta-analysis, Goodoory et al. find that pooled results from six randomized controlled trials suggest efficacy with mesalamine, an anti-inflammatory agent, for global IBS symptoms with subgroup analyses further suggesting efficacy in IBS with diarrhea. However, results are tempered by the overall low quality of evidence and lack of benefit for abdominal pain or bowel habits. Notably, the only study rated as low risk of bias did not find mesalamine to be effective. Thus, these findings cannot yet be used to inform clinical decision-makers.
Still, further study is warranted. Such future work will benefit from including well-phenotyped patients and novel biomarkers with the ability to identify individuals in whom inflammatory mechanisms contribute to IBS symptoms.
Dr. Andrea Shin is based in the Vatche and Tamar Manoukian Division of Digestive Diseases at the University of California Los Angeles. She is a member of the editorial boards of Clinical Gastroenterology and Hepatology, and Alimentary Pharmacology & Therapeutics. She is associate clinical editor of Neurogastroenterology & Motility, and a member of the Scientific Communications Advisory Board for IBS-C. As an AGA member, she sits on the Research Awards Panel, and is a section councilor for neurogastroenterology & motility, as well as a patient education advisor. Dr. Shin has received funding from the NIH R03 Limited Competiation Small Grant Program, an ANMS Diversity Development Award, and is an NIH Loan Repayment Program Awardee. She has no other relevant disclosures.
Advancements in the understanding of irritable bowel syndrome (IBS) pathophysiology have led to new pharmacological agents and guidelines on the delivery of patient-specific IBS care. However, treatments targeting specific IBS mechanisms including altered immune responses, barrier dysfunction, and low-grade inflammation are lacking.
In a systematic review and meta-analysis, Goodoory et al. find that pooled results from six randomized controlled trials suggest efficacy with mesalamine, an anti-inflammatory agent, for global IBS symptoms with subgroup analyses further suggesting efficacy in IBS with diarrhea. However, results are tempered by the overall low quality of evidence and lack of benefit for abdominal pain or bowel habits. Notably, the only study rated as low risk of bias did not find mesalamine to be effective. Thus, these findings cannot yet be used to inform clinical decision-makers.
Still, further study is warranted. Such future work will benefit from including well-phenotyped patients and novel biomarkers with the ability to identify individuals in whom inflammatory mechanisms contribute to IBS symptoms.
Dr. Andrea Shin is based in the Vatche and Tamar Manoukian Division of Digestive Diseases at the University of California Los Angeles. She is a member of the editorial boards of Clinical Gastroenterology and Hepatology, and Alimentary Pharmacology & Therapeutics. She is associate clinical editor of Neurogastroenterology & Motility, and a member of the Scientific Communications Advisory Board for IBS-C. As an AGA member, she sits on the Research Awards Panel, and is a section councilor for neurogastroenterology & motility, as well as a patient education advisor. Dr. Shin has received funding from the NIH R03 Limited Competiation Small Grant Program, an ANMS Diversity Development Award, and is an NIH Loan Repayment Program Awardee. She has no other relevant disclosures.
Certain patients with irritable bowel syndrome (IBS) may benefit from treatment with mesalamine, although the quality of evidence supporting this strategy remains low, according to a recent systematic literature review and meta-analysis.
Global IBS symptoms improved significantly across the entire population, however, a subgroup analysis suggested that mesalamine may be most beneficial for patients who present with diarrhea, providing support for a large clinical trial in this patient population, reported lead author Vivek C. Goodoory, MBChB, of St. James’s University Hospital, Leeds, United Kingdom, and colleagues.
Some patients with IBS may present with low-grade inflammation in the intestine, offering theoretical grounds for prescribing mesalamine, which is typically used for treating ulcerative colitis, the investigators wrote in Clinical Gastroenterology and Hepatology. Yet previous randomized controlled trials (RCTs) evaluating mesalamine for IBS have yielded mixed results, and a meta-analysis showed that mesalamine offered no benefit.
According to Dr. Goodoory and colleagues, however, that meta-analysis fell short since it “only pooled mean symptom scores, rather than the proportion of patients in each trial experiencing an improvement in symptoms, and did not appear to include data from all available RCTs.” Furthermore, they noted that this prior study lacked subgroup analyses conducted based on IBS subtype or postinfection status.
“We, therefore, conducted a contemporaneous meta-analysis to examine the efficacy and safety of mesalamine in IBS addressing these deficits in knowledge,” the investigators wrote.
Their meta-analysis included 820 patients from 8 RCTs published between 2009 and 2022. Efficacy and safety were evaluated via dichotomous assessments of global IBS symptoms, bowel habit or stool frequency, abdominal pain, and adverse events. Two subgroup analyses were planned to evaluate responses based on post-infection status and predominant stool pattern.
Unlike the previous meta-analysis, Dr. Goodoory and colleagues detected a potential signal for efficacy.
Across all patients, mesalamine was associated with significant improvement in global IBS symptoms, compared with placebo (relative risk [RR], 0.86; 95% CI, 0.79-0.95). However, no significant improvements were detected for abdominal pain or bowel habit/stool frequency.
A subgroup analysis of patients exhibiting IBS with diarrhea showed significantly greater improvements in global IBS symptoms for mesalamine versus placebo (RR, 0.88; 95% CI, 0.79-0.99). This subgroup showed no improvements in abdominal pain or bowel habit/stool frequency.
Subgroup analyses for patients with constipation or mixed bowel habits, or based on postinfection status, revealed no significant differences, although the investigators noted that relevant data were limited.
Mesalamine appeared to be well tolerated. Across five studies reporting adverse events, 43.5% of patients receiving mesalamine reported any adverse event, compared with 41.4% of patients on placebo. The RR of experiencing an adverse event in those taking mesalamine was 1.20 (95% CI, 0.89-1.63), which was not statistically significant.
“There was no evidence of heterogeneity between studies in most of our analyses, but only one trial was at low risk of bias across all domains, and there were insufficient studies to assess for funnel plot asymmetry,” Dr. Goodoory and colleagues wrote. “Based on these limitations of the evidence,” they continued, “our confidence in the results of the meta-analysis would be low, and further large trials at low risk of bias would be informative.”
Specifically, the investigators suggested an RCT recruiting only patients with IBS with diarrhea, and reporting efficacy according to postinfection status.
One coauthor reported research funding from Tillotts Pharma and Dr Falk Pharma UK. The remaining authors reported no conflicts.
Certain patients with irritable bowel syndrome (IBS) may benefit from treatment with mesalamine, although the quality of evidence supporting this strategy remains low, according to a recent systematic literature review and meta-analysis.
Global IBS symptoms improved significantly across the entire population, however, a subgroup analysis suggested that mesalamine may be most beneficial for patients who present with diarrhea, providing support for a large clinical trial in this patient population, reported lead author Vivek C. Goodoory, MBChB, of St. James’s University Hospital, Leeds, United Kingdom, and colleagues.
Some patients with IBS may present with low-grade inflammation in the intestine, offering theoretical grounds for prescribing mesalamine, which is typically used for treating ulcerative colitis, the investigators wrote in Clinical Gastroenterology and Hepatology. Yet previous randomized controlled trials (RCTs) evaluating mesalamine for IBS have yielded mixed results, and a meta-analysis showed that mesalamine offered no benefit.
According to Dr. Goodoory and colleagues, however, that meta-analysis fell short since it “only pooled mean symptom scores, rather than the proportion of patients in each trial experiencing an improvement in symptoms, and did not appear to include data from all available RCTs.” Furthermore, they noted that this prior study lacked subgroup analyses conducted based on IBS subtype or postinfection status.
“We, therefore, conducted a contemporaneous meta-analysis to examine the efficacy and safety of mesalamine in IBS addressing these deficits in knowledge,” the investigators wrote.
Their meta-analysis included 820 patients from 8 RCTs published between 2009 and 2022. Efficacy and safety were evaluated via dichotomous assessments of global IBS symptoms, bowel habit or stool frequency, abdominal pain, and adverse events. Two subgroup analyses were planned to evaluate responses based on post-infection status and predominant stool pattern.
Unlike the previous meta-analysis, Dr. Goodoory and colleagues detected a potential signal for efficacy.
Across all patients, mesalamine was associated with significant improvement in global IBS symptoms, compared with placebo (relative risk [RR], 0.86; 95% CI, 0.79-0.95). However, no significant improvements were detected for abdominal pain or bowel habit/stool frequency.
A subgroup analysis of patients exhibiting IBS with diarrhea showed significantly greater improvements in global IBS symptoms for mesalamine versus placebo (RR, 0.88; 95% CI, 0.79-0.99). This subgroup showed no improvements in abdominal pain or bowel habit/stool frequency.
Subgroup analyses for patients with constipation or mixed bowel habits, or based on postinfection status, revealed no significant differences, although the investigators noted that relevant data were limited.
Mesalamine appeared to be well tolerated. Across five studies reporting adverse events, 43.5% of patients receiving mesalamine reported any adverse event, compared with 41.4% of patients on placebo. The RR of experiencing an adverse event in those taking mesalamine was 1.20 (95% CI, 0.89-1.63), which was not statistically significant.
“There was no evidence of heterogeneity between studies in most of our analyses, but only one trial was at low risk of bias across all domains, and there were insufficient studies to assess for funnel plot asymmetry,” Dr. Goodoory and colleagues wrote. “Based on these limitations of the evidence,” they continued, “our confidence in the results of the meta-analysis would be low, and further large trials at low risk of bias would be informative.”
Specifically, the investigators suggested an RCT recruiting only patients with IBS with diarrhea, and reporting efficacy according to postinfection status.
One coauthor reported research funding from Tillotts Pharma and Dr Falk Pharma UK. The remaining authors reported no conflicts.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Musculoskeletal Symptoms Often Misattributed to Prior Tick Bites
Non–Lyme disease, tick-borne illnesses — such as spotted fever group rickettsiosis (SFGR), ehrlichiosis, and alpha-gal syndrome (AGS) — are emerging public health threats, but whether prior tick exposures are responsible for long-term complications, such as musculoskeletal symptoms or osteoarthritis, has been unclear.
Many patients attribute their nonspecific long-term symptoms, such as musculoskeletal pain, to previous illnesses from tick bites, note authors of a study published in JAMA Network Open. But the researchers, led by Diana L. Zychowski, MD, MPH, with the Division of Infectious Diseases at the University of North Carolina at Chapel Hill, found that Ehrlichia or Rickettsia seropositivity was not associated with chronic musculoskeletal symptoms, though they write that “further investigation into the pathogenesis of [alpha-gal] syndrome is needed.”
Tick-Borne Illness Cases Multiplying
Cases of tick-borne illness (TBD) in the United States have multiplied in recent years. More than 50,000 cases of TBD in the United States were reported in 2019, which doubled the number of cases over the previous 2 decades, the authors note.
Most of the cases are Lyme disease, but others — including SFGR and ehrlichiosis — represent an important public health threat, especially in southeastern states, the authors write. Cases of ehrlichiosis, for example, transmitted by the lone star tick, soared more than 10-fold since 2000.
The goal of this study was to evaluate whether there was an association between prior exposure to TBDs endemic to the southeastern United States and chronic musculoskeletal symptoms and radiographic measures of osteoarthritis.
Researchers analyzed 488 blood samples from the fourth visit (2017-2018) of the Johnston County Osteoarthritis (JoCo OA) project, an ongoing population-based study in Johnston County, North Carolina. JoCo OA participants include noninstitutionalized White and Black Johnston County residents 45 years old or older with osteoarthritis.
They measured seroprevalence of Rickettsia- and Ehrlichia-specific immunoglobulin G (IgG) as well as alpha-gal immunoglobulin E (IgE) in patient samples. Only alpha-gal IgE was linked in the study with knee pain, aching, or stiffness. Antibodies to Rickettsia, Ehrlichia, and alpha-gal were not associated with radiographic, symptomatic knee osteoarthritis.
“To our knowledge,” the authors write, “this study was the first population-based seroprevalence study of SFGR, Ehrlichia, and [alpha]-gal.”
The study also found a high prevalence of TBD exposure in the cohort. More than a third (36.5%) had either an alpha-gal IgE level greater than 0.1 IU/mL, a positive test for SFGR IgG antibodies, or a positive test for Ehrlichia IgG antibodies.
Given that not every tick carries an infectious pathogen, the findings show human-tick interactions are common, they write.
“These findings suggest that substantial investment is required to examine the pathogenesis of these TBDs and interventions to reduce human-tick interactions,” the authors conclude.
This study was funded by a Creativity Hub Award from the University of North Carolina Office of the Vice Chancellor for Research. The JoCo OA project is supported in part by grants from the Association of Schools of Public Health/Centers for Disease Control and Prevention (CDC); and grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Authors reported grants from the National Institutes of Health, the CDC, and several pharmaceutical companies.
Non–Lyme disease, tick-borne illnesses — such as spotted fever group rickettsiosis (SFGR), ehrlichiosis, and alpha-gal syndrome (AGS) — are emerging public health threats, but whether prior tick exposures are responsible for long-term complications, such as musculoskeletal symptoms or osteoarthritis, has been unclear.
Many patients attribute their nonspecific long-term symptoms, such as musculoskeletal pain, to previous illnesses from tick bites, note authors of a study published in JAMA Network Open. But the researchers, led by Diana L. Zychowski, MD, MPH, with the Division of Infectious Diseases at the University of North Carolina at Chapel Hill, found that Ehrlichia or Rickettsia seropositivity was not associated with chronic musculoskeletal symptoms, though they write that “further investigation into the pathogenesis of [alpha-gal] syndrome is needed.”
Tick-Borne Illness Cases Multiplying
Cases of tick-borne illness (TBD) in the United States have multiplied in recent years. More than 50,000 cases of TBD in the United States were reported in 2019, which doubled the number of cases over the previous 2 decades, the authors note.
Most of the cases are Lyme disease, but others — including SFGR and ehrlichiosis — represent an important public health threat, especially in southeastern states, the authors write. Cases of ehrlichiosis, for example, transmitted by the lone star tick, soared more than 10-fold since 2000.
The goal of this study was to evaluate whether there was an association between prior exposure to TBDs endemic to the southeastern United States and chronic musculoskeletal symptoms and radiographic measures of osteoarthritis.
Researchers analyzed 488 blood samples from the fourth visit (2017-2018) of the Johnston County Osteoarthritis (JoCo OA) project, an ongoing population-based study in Johnston County, North Carolina. JoCo OA participants include noninstitutionalized White and Black Johnston County residents 45 years old or older with osteoarthritis.
They measured seroprevalence of Rickettsia- and Ehrlichia-specific immunoglobulin G (IgG) as well as alpha-gal immunoglobulin E (IgE) in patient samples. Only alpha-gal IgE was linked in the study with knee pain, aching, or stiffness. Antibodies to Rickettsia, Ehrlichia, and alpha-gal were not associated with radiographic, symptomatic knee osteoarthritis.
“To our knowledge,” the authors write, “this study was the first population-based seroprevalence study of SFGR, Ehrlichia, and [alpha]-gal.”
The study also found a high prevalence of TBD exposure in the cohort. More than a third (36.5%) had either an alpha-gal IgE level greater than 0.1 IU/mL, a positive test for SFGR IgG antibodies, or a positive test for Ehrlichia IgG antibodies.
Given that not every tick carries an infectious pathogen, the findings show human-tick interactions are common, they write.
“These findings suggest that substantial investment is required to examine the pathogenesis of these TBDs and interventions to reduce human-tick interactions,” the authors conclude.
This study was funded by a Creativity Hub Award from the University of North Carolina Office of the Vice Chancellor for Research. The JoCo OA project is supported in part by grants from the Association of Schools of Public Health/Centers for Disease Control and Prevention (CDC); and grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Authors reported grants from the National Institutes of Health, the CDC, and several pharmaceutical companies.
Non–Lyme disease, tick-borne illnesses — such as spotted fever group rickettsiosis (SFGR), ehrlichiosis, and alpha-gal syndrome (AGS) — are emerging public health threats, but whether prior tick exposures are responsible for long-term complications, such as musculoskeletal symptoms or osteoarthritis, has been unclear.
Many patients attribute their nonspecific long-term symptoms, such as musculoskeletal pain, to previous illnesses from tick bites, note authors of a study published in JAMA Network Open. But the researchers, led by Diana L. Zychowski, MD, MPH, with the Division of Infectious Diseases at the University of North Carolina at Chapel Hill, found that Ehrlichia or Rickettsia seropositivity was not associated with chronic musculoskeletal symptoms, though they write that “further investigation into the pathogenesis of [alpha-gal] syndrome is needed.”
Tick-Borne Illness Cases Multiplying
Cases of tick-borne illness (TBD) in the United States have multiplied in recent years. More than 50,000 cases of TBD in the United States were reported in 2019, which doubled the number of cases over the previous 2 decades, the authors note.
Most of the cases are Lyme disease, but others — including SFGR and ehrlichiosis — represent an important public health threat, especially in southeastern states, the authors write. Cases of ehrlichiosis, for example, transmitted by the lone star tick, soared more than 10-fold since 2000.
The goal of this study was to evaluate whether there was an association between prior exposure to TBDs endemic to the southeastern United States and chronic musculoskeletal symptoms and radiographic measures of osteoarthritis.
Researchers analyzed 488 blood samples from the fourth visit (2017-2018) of the Johnston County Osteoarthritis (JoCo OA) project, an ongoing population-based study in Johnston County, North Carolina. JoCo OA participants include noninstitutionalized White and Black Johnston County residents 45 years old or older with osteoarthritis.
They measured seroprevalence of Rickettsia- and Ehrlichia-specific immunoglobulin G (IgG) as well as alpha-gal immunoglobulin E (IgE) in patient samples. Only alpha-gal IgE was linked in the study with knee pain, aching, or stiffness. Antibodies to Rickettsia, Ehrlichia, and alpha-gal were not associated with radiographic, symptomatic knee osteoarthritis.
“To our knowledge,” the authors write, “this study was the first population-based seroprevalence study of SFGR, Ehrlichia, and [alpha]-gal.”
The study also found a high prevalence of TBD exposure in the cohort. More than a third (36.5%) had either an alpha-gal IgE level greater than 0.1 IU/mL, a positive test for SFGR IgG antibodies, or a positive test for Ehrlichia IgG antibodies.
Given that not every tick carries an infectious pathogen, the findings show human-tick interactions are common, they write.
“These findings suggest that substantial investment is required to examine the pathogenesis of these TBDs and interventions to reduce human-tick interactions,” the authors conclude.
This study was funded by a Creativity Hub Award from the University of North Carolina Office of the Vice Chancellor for Research. The JoCo OA project is supported in part by grants from the Association of Schools of Public Health/Centers for Disease Control and Prevention (CDC); and grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Authors reported grants from the National Institutes of Health, the CDC, and several pharmaceutical companies.
FROM JAMA NETWORK OPEN