User login
Does the preterm birth racial disparity persist among black and white IVF users?
Investigators from the National Institutes of Health and Shady Grove Fertility found that among women having a singleton live birth resulting from in vitro fertilization (IVF) that black women are at higher risk for lower gestational age and preterm delivery than white women.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Kate Devine, MD, coinvestigator of the retrospective cohort study said in an interview with OBG Management that “It’s been well documented that African Americans have a higher preterm birth rate in the United States compared to Caucasians and the overall population. While the exact mechanism of preterm birth is unknown and likely varied, and while the mechanism for the preterm birth rate being higher in African Americans is not well understood, it has been hypothesized that socioeconomic factors are responsible at least in part.”2 She added that the investigators used a population of women receiving IVF for the study because “access to reproductive care and IVF is in some way a leveling factor in terms of socioeconomics.”
Details of the study. The investigators reviewed all singleton IVF pregnancies ending in live birth among women self-identifying as white, black, Asian, or Hispanic from 2004 to 2016 at a private IVF practice (N=10,371). The primary outcome was gestational age at birth, calculated as the number of days from oocyte retrieval to birth, plus 14, among white, black, Asian, and Hispanic women receiving IVF.
Births among black women occurred more than 6 days earlier than births among white women. The researchers noted that some of the shorter gestations among the black women could be explained by the higher average body mass index of the group (P<.0001). Dr. Devine explained that another contributing factor was the higher incidence of fibroid uterus among the black women (P<.0001). But after adjusting for these and other demographic variables, the black women still delivered 5.5 days earlier than the white women, and they were more than 3 times as likely to have either very preterm or extremely preterm deliveries (TABLE).1
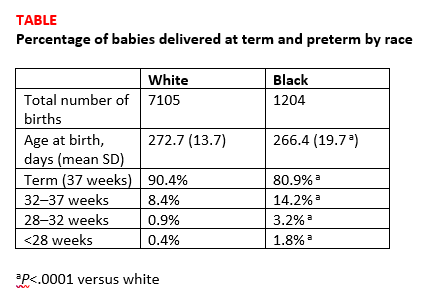
Research implications. Dr. Devine said that black pregnant patients “perhaps should be monitored more closely” for signs or symptoms suggestive of preterm labor and would like to see more research into understanding the mechanisms of preterm birth that are resulting in greater rates of preterm birth among black women. She mentioned that research into how fibroids impact obstetric outcomes is also important.
Share your thoughts! Send your Letters to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bishop LA, Devine K, Sasson I, et al. Lower gestational age and increased risk of preterm birth associated with singleton live birth resulting from in vitro fertilization (IVF) among African American versus comparable Caucasian women. Fertil Steril. 2018;110(45 suppl):e7.
Investigators from the National Institutes of Health and Shady Grove Fertility found that among women having a singleton live birth resulting from in vitro fertilization (IVF) that black women are at higher risk for lower gestational age and preterm delivery than white women.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Kate Devine, MD, coinvestigator of the retrospective cohort study said in an interview with OBG Management that “It’s been well documented that African Americans have a higher preterm birth rate in the United States compared to Caucasians and the overall population. While the exact mechanism of preterm birth is unknown and likely varied, and while the mechanism for the preterm birth rate being higher in African Americans is not well understood, it has been hypothesized that socioeconomic factors are responsible at least in part.”2 She added that the investigators used a population of women receiving IVF for the study because “access to reproductive care and IVF is in some way a leveling factor in terms of socioeconomics.”
Details of the study. The investigators reviewed all singleton IVF pregnancies ending in live birth among women self-identifying as white, black, Asian, or Hispanic from 2004 to 2016 at a private IVF practice (N=10,371). The primary outcome was gestational age at birth, calculated as the number of days from oocyte retrieval to birth, plus 14, among white, black, Asian, and Hispanic women receiving IVF.
Births among black women occurred more than 6 days earlier than births among white women. The researchers noted that some of the shorter gestations among the black women could be explained by the higher average body mass index of the group (P<.0001). Dr. Devine explained that another contributing factor was the higher incidence of fibroid uterus among the black women (P<.0001). But after adjusting for these and other demographic variables, the black women still delivered 5.5 days earlier than the white women, and they were more than 3 times as likely to have either very preterm or extremely preterm deliveries (TABLE).1

Research implications. Dr. Devine said that black pregnant patients “perhaps should be monitored more closely” for signs or symptoms suggestive of preterm labor and would like to see more research into understanding the mechanisms of preterm birth that are resulting in greater rates of preterm birth among black women. She mentioned that research into how fibroids impact obstetric outcomes is also important.
Share your thoughts! Send your Letters to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Investigators from the National Institutes of Health and Shady Grove Fertility found that among women having a singleton live birth resulting from in vitro fertilization (IVF) that black women are at higher risk for lower gestational age and preterm delivery than white women.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Kate Devine, MD, coinvestigator of the retrospective cohort study said in an interview with OBG Management that “It’s been well documented that African Americans have a higher preterm birth rate in the United States compared to Caucasians and the overall population. While the exact mechanism of preterm birth is unknown and likely varied, and while the mechanism for the preterm birth rate being higher in African Americans is not well understood, it has been hypothesized that socioeconomic factors are responsible at least in part.”2 She added that the investigators used a population of women receiving IVF for the study because “access to reproductive care and IVF is in some way a leveling factor in terms of socioeconomics.”
Details of the study. The investigators reviewed all singleton IVF pregnancies ending in live birth among women self-identifying as white, black, Asian, or Hispanic from 2004 to 2016 at a private IVF practice (N=10,371). The primary outcome was gestational age at birth, calculated as the number of days from oocyte retrieval to birth, plus 14, among white, black, Asian, and Hispanic women receiving IVF.
Births among black women occurred more than 6 days earlier than births among white women. The researchers noted that some of the shorter gestations among the black women could be explained by the higher average body mass index of the group (P<.0001). Dr. Devine explained that another contributing factor was the higher incidence of fibroid uterus among the black women (P<.0001). But after adjusting for these and other demographic variables, the black women still delivered 5.5 days earlier than the white women, and they were more than 3 times as likely to have either very preterm or extremely preterm deliveries (TABLE).1

Research implications. Dr. Devine said that black pregnant patients “perhaps should be monitored more closely” for signs or symptoms suggestive of preterm labor and would like to see more research into understanding the mechanisms of preterm birth that are resulting in greater rates of preterm birth among black women. She mentioned that research into how fibroids impact obstetric outcomes is also important.
Share your thoughts! Send your Letters to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bishop LA, Devine K, Sasson I, et al. Lower gestational age and increased risk of preterm birth associated with singleton live birth resulting from in vitro fertilization (IVF) among African American versus comparable Caucasian women. Fertil Steril. 2018;110(45 suppl):e7.
- Bishop LA, Devine K, Sasson I, et al. Lower gestational age and increased risk of preterm birth associated with singleton live birth resulting from in vitro fertilization (IVF) among African American versus comparable Caucasian women. Fertil Steril. 2018;110(45 suppl):e7.
Aspirin cuts risk of ovarian and liver cancer
Regular long-term aspirin use may lower the risk of hepatocellular carcinoma (HCC) and ovarian cancer, adding to the growing evidence that aspirin may play a role as a chemopreventive agent, according to two new studies published in JAMA Oncology.
In the first study, led by Tracey G. Simon, MD, of Massachusetts General Hospital, Boston, the authors evaluated the associations between aspirin dose and duration of use and the risk of developing HCC. They conducted a population-based study, with a pooled analysis of two large prospective U.S. cohort studies: the Nurses’ Health Study and the Health Professionals Follow-up Study. The cohort included a total of 133,371 health care professionals who reported long-term data on aspirin use, frequency, dosage, and duration of use.
For the 87,507 female participants, reporting began in 1980, and for the 45,864 men, reporting began in 1986. The mean age for women was 62 years and was 64 years for men at the midpoint of follow-up (1996). Compared with nonaspirin users, those who used aspirin regularly tended to be older, former smokers, and regularly used statins and multivitamins. During the follow-up period, which was more than 26 years, there were 108 incident cases of HCC (65 women, 43 men; 47 with noncirrhotic HCC).
The investigators found that regular aspirin use was associated with a significantly lower HCC risk versus nonregular use (multivariable hazard ratio, 0.51; 95% confidence interval, 0.34-0.77), and estimates were similar for both sexes. Adjustments for regular NSAID use (for example, at least two tablets per week) did not change the data, and results were similar after further adjustment for coffee consumption and adherence to a healthy diet. The benefit also appeared to be dose related, as compared with nonuse, the multivariable-adjusted HR for HCC was 0.87 (95% CI, 0.51-1.48) for up to 1.5 tablets of standard-dose aspirin per week and 0.51 (95% CI, 0.30-0.86) for 1.5-5 tablets per week. The most benefit was for at least five tablets per week (HR, 0.49; 95% CI, 0.28-0.96; P = .006).
“Our findings add to the growing literature suggesting that the chemopreventive effects of aspirin may extend beyond colorectal cancer,” they wrote.
In the second study, Mollie E. Barnard, ScD, of the Harvard School of Public Health, Boston, and her colleagues looked at whether regular aspirin or NSAID use, as well as the patterns of use, were associated with a lower risk of ovarian cancer.
The data used were obtained from 93,664 women in the Nurses’ Health Study (NHS), who were followed up from 1980 to 2014, and 111,834 people in the Nurses’ Health Study II (NHSII), who were followed up from 1989 to 2015. For each type of agent, including aspirin, low-dose aspirin, nonaspirin NSAIDs, and acetaminophen, they evaluated the timing, duration, frequency, and number of tablets that were used. The mean age of participants in the NHS at baseline was 45.9 years and 34.2 years in the NHSII.
There were 1,054 incident cases of epithelial ovarian cancer identified during the study period. The authors did not detect any significant associations between aspirin and ovarian cancer risk when current users and nonusers were compared, regardless of dose (HR, 0.99; 95% CI, 0.83-1.19). But when low-dose (less than or equal to 100 mg) and standard-dose (325 mg) aspirin were analyzed separately, an inverse association for low-dose aspirin (HR, 0.77; 95% CI, 0.61-0.96) was observed. However, there was no association for standard-dose aspirin (HR, 1.17; 95% CI, 0.92-1.49).
In contrast, use of nonaspirin NSAIDs was positively associated with a higher risk of ovarian cancer when compared with nonuse (HR, 1.19; 95% CI, 1.00-1.41), and there were significant positive trends for duration of use (P = .02) and cumulative average tablets per week (P = .03). No clear associations were identified for acetaminophen use.
“Our results also suggest an increased risk of ovarian cancer among long-term, high-quantity users of nonaspirin analgesics, although this finding may reflect unmeasured confounding,” wrote Dr. Barnard and her coauthors. “Further exploration is warranted to evaluate the mechanisms by which heavy use of aspirin, nonaspirin NSAIDs, and acetaminophen may contribute to the development of ovarian cancer and to replicate our findings.”
The ovarian cancer study was supported by awards from the National Institutes of Health. Dr. Barnard was supported by awards from the National Cancer Institute, and her coauthors had no disclosures to report. The HCC study was funded by an infrastructure grant from the Nurses’ Health Study, an infrastructure grant from the Health Professionals Follow-up Study, and NIH grants to several of the authors. Dr. Chan has previously served as a consultant for Bayer on work unrelated to this article. No other disclosures were reported.
SOURCES: Barnard ME et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4149; Simon TG et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4154.
In an accompanying editorial published in JAMA Oncology, Victoria L. Seewaldt, MD, of the City of Hope Comprehensive Cancer Center in Duarte, Calif., asked if we “have arrived,” as these two studies are a critical step in realizing the potential of aspirin for cancer chemoprevention beyond colorectal cancer.
Aspirin use is very common in the United States, with almost half of adults aged between 45 and 75 years taking it regularly. Many regular users also believe that aspirin has potential to protect against cancer, and in a 2015 study – which was conducted prior to any formal cancer prevention guidelines – 18% of those taking aspirin on a regular basis reported doing so to prevent cancer.
Based on the strength of the association between aspirin use and colorectal cancer risk reduction, the U.S. Preventive Services Task Force recommended in 2015 that, among individuals aged between 50 and 69 years who have specific cardiovascular risk profiles, colorectal cancer prevention be included as part of the rationale for regular aspirin prophylaxis, Dr. Seewaldt noted. Aspirin became the first drug to be included in USPSTF recommendations for cancer chemoprevention in a “population not characterized as having a high risk of developing cancer.”
But it now appears aspirin may be able to go beyond colorectal cancer for chemoprevention. Ovarian cancer and hepatocellular carcinoma are in need of new prevention strategies and these findings provide important information that can help guide chemoprevention with aspirin.
These two studies “have the power to start to change clinical practice,” Dr. Seewaldt wrote, but more research is needed to better understand the underlying mechanism behind the appropriate dose and duration of use. Importantly, the authors of both studies cautioned that the potential benefits of aspirin must be weighed against the risk of bleeding, which is particularly important in patients with chronic liver disease.
“To reach the full promise of aspirin’s ability to prevent cancer, there needs to be better understanding of dose, duration, and mechanism,” she emphasized.
Dr. Seewaldt reported receiving grants from the National Institutes of Health/National Cancer Institute and is supported by the Prevent Cancer Foundation.
In an accompanying editorial published in JAMA Oncology, Victoria L. Seewaldt, MD, of the City of Hope Comprehensive Cancer Center in Duarte, Calif., asked if we “have arrived,” as these two studies are a critical step in realizing the potential of aspirin for cancer chemoprevention beyond colorectal cancer.
Aspirin use is very common in the United States, with almost half of adults aged between 45 and 75 years taking it regularly. Many regular users also believe that aspirin has potential to protect against cancer, and in a 2015 study – which was conducted prior to any formal cancer prevention guidelines – 18% of those taking aspirin on a regular basis reported doing so to prevent cancer.
Based on the strength of the association between aspirin use and colorectal cancer risk reduction, the U.S. Preventive Services Task Force recommended in 2015 that, among individuals aged between 50 and 69 years who have specific cardiovascular risk profiles, colorectal cancer prevention be included as part of the rationale for regular aspirin prophylaxis, Dr. Seewaldt noted. Aspirin became the first drug to be included in USPSTF recommendations for cancer chemoprevention in a “population not characterized as having a high risk of developing cancer.”
But it now appears aspirin may be able to go beyond colorectal cancer for chemoprevention. Ovarian cancer and hepatocellular carcinoma are in need of new prevention strategies and these findings provide important information that can help guide chemoprevention with aspirin.
These two studies “have the power to start to change clinical practice,” Dr. Seewaldt wrote, but more research is needed to better understand the underlying mechanism behind the appropriate dose and duration of use. Importantly, the authors of both studies cautioned that the potential benefits of aspirin must be weighed against the risk of bleeding, which is particularly important in patients with chronic liver disease.
“To reach the full promise of aspirin’s ability to prevent cancer, there needs to be better understanding of dose, duration, and mechanism,” she emphasized.
Dr. Seewaldt reported receiving grants from the National Institutes of Health/National Cancer Institute and is supported by the Prevent Cancer Foundation.
In an accompanying editorial published in JAMA Oncology, Victoria L. Seewaldt, MD, of the City of Hope Comprehensive Cancer Center in Duarte, Calif., asked if we “have arrived,” as these two studies are a critical step in realizing the potential of aspirin for cancer chemoprevention beyond colorectal cancer.
Aspirin use is very common in the United States, with almost half of adults aged between 45 and 75 years taking it regularly. Many regular users also believe that aspirin has potential to protect against cancer, and in a 2015 study – which was conducted prior to any formal cancer prevention guidelines – 18% of those taking aspirin on a regular basis reported doing so to prevent cancer.
Based on the strength of the association between aspirin use and colorectal cancer risk reduction, the U.S. Preventive Services Task Force recommended in 2015 that, among individuals aged between 50 and 69 years who have specific cardiovascular risk profiles, colorectal cancer prevention be included as part of the rationale for regular aspirin prophylaxis, Dr. Seewaldt noted. Aspirin became the first drug to be included in USPSTF recommendations for cancer chemoprevention in a “population not characterized as having a high risk of developing cancer.”
But it now appears aspirin may be able to go beyond colorectal cancer for chemoprevention. Ovarian cancer and hepatocellular carcinoma are in need of new prevention strategies and these findings provide important information that can help guide chemoprevention with aspirin.
These two studies “have the power to start to change clinical practice,” Dr. Seewaldt wrote, but more research is needed to better understand the underlying mechanism behind the appropriate dose and duration of use. Importantly, the authors of both studies cautioned that the potential benefits of aspirin must be weighed against the risk of bleeding, which is particularly important in patients with chronic liver disease.
“To reach the full promise of aspirin’s ability to prevent cancer, there needs to be better understanding of dose, duration, and mechanism,” she emphasized.
Dr. Seewaldt reported receiving grants from the National Institutes of Health/National Cancer Institute and is supported by the Prevent Cancer Foundation.
Regular long-term aspirin use may lower the risk of hepatocellular carcinoma (HCC) and ovarian cancer, adding to the growing evidence that aspirin may play a role as a chemopreventive agent, according to two new studies published in JAMA Oncology.
In the first study, led by Tracey G. Simon, MD, of Massachusetts General Hospital, Boston, the authors evaluated the associations between aspirin dose and duration of use and the risk of developing HCC. They conducted a population-based study, with a pooled analysis of two large prospective U.S. cohort studies: the Nurses’ Health Study and the Health Professionals Follow-up Study. The cohort included a total of 133,371 health care professionals who reported long-term data on aspirin use, frequency, dosage, and duration of use.
For the 87,507 female participants, reporting began in 1980, and for the 45,864 men, reporting began in 1986. The mean age for women was 62 years and was 64 years for men at the midpoint of follow-up (1996). Compared with nonaspirin users, those who used aspirin regularly tended to be older, former smokers, and regularly used statins and multivitamins. During the follow-up period, which was more than 26 years, there were 108 incident cases of HCC (65 women, 43 men; 47 with noncirrhotic HCC).
The investigators found that regular aspirin use was associated with a significantly lower HCC risk versus nonregular use (multivariable hazard ratio, 0.51; 95% confidence interval, 0.34-0.77), and estimates were similar for both sexes. Adjustments for regular NSAID use (for example, at least two tablets per week) did not change the data, and results were similar after further adjustment for coffee consumption and adherence to a healthy diet. The benefit also appeared to be dose related, as compared with nonuse, the multivariable-adjusted HR for HCC was 0.87 (95% CI, 0.51-1.48) for up to 1.5 tablets of standard-dose aspirin per week and 0.51 (95% CI, 0.30-0.86) for 1.5-5 tablets per week. The most benefit was for at least five tablets per week (HR, 0.49; 95% CI, 0.28-0.96; P = .006).
“Our findings add to the growing literature suggesting that the chemopreventive effects of aspirin may extend beyond colorectal cancer,” they wrote.
In the second study, Mollie E. Barnard, ScD, of the Harvard School of Public Health, Boston, and her colleagues looked at whether regular aspirin or NSAID use, as well as the patterns of use, were associated with a lower risk of ovarian cancer.
The data used were obtained from 93,664 women in the Nurses’ Health Study (NHS), who were followed up from 1980 to 2014, and 111,834 people in the Nurses’ Health Study II (NHSII), who were followed up from 1989 to 2015. For each type of agent, including aspirin, low-dose aspirin, nonaspirin NSAIDs, and acetaminophen, they evaluated the timing, duration, frequency, and number of tablets that were used. The mean age of participants in the NHS at baseline was 45.9 years and 34.2 years in the NHSII.
There were 1,054 incident cases of epithelial ovarian cancer identified during the study period. The authors did not detect any significant associations between aspirin and ovarian cancer risk when current users and nonusers were compared, regardless of dose (HR, 0.99; 95% CI, 0.83-1.19). But when low-dose (less than or equal to 100 mg) and standard-dose (325 mg) aspirin were analyzed separately, an inverse association for low-dose aspirin (HR, 0.77; 95% CI, 0.61-0.96) was observed. However, there was no association for standard-dose aspirin (HR, 1.17; 95% CI, 0.92-1.49).
In contrast, use of nonaspirin NSAIDs was positively associated with a higher risk of ovarian cancer when compared with nonuse (HR, 1.19; 95% CI, 1.00-1.41), and there were significant positive trends for duration of use (P = .02) and cumulative average tablets per week (P = .03). No clear associations were identified for acetaminophen use.
“Our results also suggest an increased risk of ovarian cancer among long-term, high-quantity users of nonaspirin analgesics, although this finding may reflect unmeasured confounding,” wrote Dr. Barnard and her coauthors. “Further exploration is warranted to evaluate the mechanisms by which heavy use of aspirin, nonaspirin NSAIDs, and acetaminophen may contribute to the development of ovarian cancer and to replicate our findings.”
The ovarian cancer study was supported by awards from the National Institutes of Health. Dr. Barnard was supported by awards from the National Cancer Institute, and her coauthors had no disclosures to report. The HCC study was funded by an infrastructure grant from the Nurses’ Health Study, an infrastructure grant from the Health Professionals Follow-up Study, and NIH grants to several of the authors. Dr. Chan has previously served as a consultant for Bayer on work unrelated to this article. No other disclosures were reported.
SOURCES: Barnard ME et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4149; Simon TG et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4154.
Regular long-term aspirin use may lower the risk of hepatocellular carcinoma (HCC) and ovarian cancer, adding to the growing evidence that aspirin may play a role as a chemopreventive agent, according to two new studies published in JAMA Oncology.
In the first study, led by Tracey G. Simon, MD, of Massachusetts General Hospital, Boston, the authors evaluated the associations between aspirin dose and duration of use and the risk of developing HCC. They conducted a population-based study, with a pooled analysis of two large prospective U.S. cohort studies: the Nurses’ Health Study and the Health Professionals Follow-up Study. The cohort included a total of 133,371 health care professionals who reported long-term data on aspirin use, frequency, dosage, and duration of use.
For the 87,507 female participants, reporting began in 1980, and for the 45,864 men, reporting began in 1986. The mean age for women was 62 years and was 64 years for men at the midpoint of follow-up (1996). Compared with nonaspirin users, those who used aspirin regularly tended to be older, former smokers, and regularly used statins and multivitamins. During the follow-up period, which was more than 26 years, there were 108 incident cases of HCC (65 women, 43 men; 47 with noncirrhotic HCC).
The investigators found that regular aspirin use was associated with a significantly lower HCC risk versus nonregular use (multivariable hazard ratio, 0.51; 95% confidence interval, 0.34-0.77), and estimates were similar for both sexes. Adjustments for regular NSAID use (for example, at least two tablets per week) did not change the data, and results were similar after further adjustment for coffee consumption and adherence to a healthy diet. The benefit also appeared to be dose related, as compared with nonuse, the multivariable-adjusted HR for HCC was 0.87 (95% CI, 0.51-1.48) for up to 1.5 tablets of standard-dose aspirin per week and 0.51 (95% CI, 0.30-0.86) for 1.5-5 tablets per week. The most benefit was for at least five tablets per week (HR, 0.49; 95% CI, 0.28-0.96; P = .006).
“Our findings add to the growing literature suggesting that the chemopreventive effects of aspirin may extend beyond colorectal cancer,” they wrote.
In the second study, Mollie E. Barnard, ScD, of the Harvard School of Public Health, Boston, and her colleagues looked at whether regular aspirin or NSAID use, as well as the patterns of use, were associated with a lower risk of ovarian cancer.
The data used were obtained from 93,664 women in the Nurses’ Health Study (NHS), who were followed up from 1980 to 2014, and 111,834 people in the Nurses’ Health Study II (NHSII), who were followed up from 1989 to 2015. For each type of agent, including aspirin, low-dose aspirin, nonaspirin NSAIDs, and acetaminophen, they evaluated the timing, duration, frequency, and number of tablets that were used. The mean age of participants in the NHS at baseline was 45.9 years and 34.2 years in the NHSII.
There were 1,054 incident cases of epithelial ovarian cancer identified during the study period. The authors did not detect any significant associations between aspirin and ovarian cancer risk when current users and nonusers were compared, regardless of dose (HR, 0.99; 95% CI, 0.83-1.19). But when low-dose (less than or equal to 100 mg) and standard-dose (325 mg) aspirin were analyzed separately, an inverse association for low-dose aspirin (HR, 0.77; 95% CI, 0.61-0.96) was observed. However, there was no association for standard-dose aspirin (HR, 1.17; 95% CI, 0.92-1.49).
In contrast, use of nonaspirin NSAIDs was positively associated with a higher risk of ovarian cancer when compared with nonuse (HR, 1.19; 95% CI, 1.00-1.41), and there were significant positive trends for duration of use (P = .02) and cumulative average tablets per week (P = .03). No clear associations were identified for acetaminophen use.
“Our results also suggest an increased risk of ovarian cancer among long-term, high-quantity users of nonaspirin analgesics, although this finding may reflect unmeasured confounding,” wrote Dr. Barnard and her coauthors. “Further exploration is warranted to evaluate the mechanisms by which heavy use of aspirin, nonaspirin NSAIDs, and acetaminophen may contribute to the development of ovarian cancer and to replicate our findings.”
The ovarian cancer study was supported by awards from the National Institutes of Health. Dr. Barnard was supported by awards from the National Cancer Institute, and her coauthors had no disclosures to report. The HCC study was funded by an infrastructure grant from the Nurses’ Health Study, an infrastructure grant from the Health Professionals Follow-up Study, and NIH grants to several of the authors. Dr. Chan has previously served as a consultant for Bayer on work unrelated to this article. No other disclosures were reported.
SOURCES: Barnard ME et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4149; Simon TG et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4154.
FROM JAMA ONCOLOGY
Key clinical point: Regular aspirin use was associated with a decreased risk of ovarian cancer and hepatocellular carcinoma.
Major finding: Low-dose aspirin was associated with a 23% lower risk of ovarian cancer and a 49% reduced risk of developing hepatocellular carcinoma.
Study details: The hepatocellular carcinoma study was a population-based study of two nationwide, prospective cohorts of 87,507 men and 45,864 women; the ovarian cancer study was a cohort study using data from two prospective cohorts, with 93,664 people in one and 111,834 in the other.
Disclosures: The ovarian cancer study was supported by awards from the National Institutes of Health. Dr. Barnard was supported by awards from the National Cancer Institute, and her coauthors had no disclosures to report. The hepatocellular carcinoma study was funded by an infrastructure grant from the Nurses’ Health Study, an infrastructure grant from the Health Professionals Follow-up Study, and NIH grants to several of the authors. Dr. Chan has previously served as a consultant for Bayer on work unrelated to this article. No other disclosures were reported.
Sources: Barnard ME et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4149; Simon TG et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4154.
Two-thirds of COPD patients not using inhalers correctly
SAN ANTONIO – Two-thirds of U.S. adults with (MDIs), according to new research. About half of patients failed to inhale slowly and deeply to ensure they received the appropriate dose, and about 40% of patients failed to hold their breath for 5-10 seconds afterward so that the medication made its way to their lungs, the findings show.
“There’s a need to educate patients on proper inhalation technique to optimize the appropriate delivery of medication,” Maryam Navaie, DrPH, of Advance Health Solutions in New York told attendees at the annual meeting of the American College of Chest Physicians. She also urged practitioners to think more carefully about what devices to prescribe to patients based on their own personal attributes.
“Nebulizer devices may be a better consideration for patients who have difficulty performing the necessary steps required by handheld inhalers,” Dr. Navaie said.
She and fellow researchers conducted a systematic review to gain more insights into the errors and difficulties experienced by U.S. adults using MDIs for COPD or asthma. They combed through PubMed, EMBASE, PsycINFO, Cochrane, and Google Scholar databases for English language studies about MDI-related errors in U.S. adult COPD or asthma patients published between January 2003 and February 2017.
The researchers included only randomized controlled trials and cross-sectional and observational studies, and they excluded studies with combined error rates across multiple devices so they could better parse out the data. They also used baseline rates only in studies that involved an intervention to reduce errors.
The researchers defined the proportion of overall MDI errors as “the percentage of patients who made errors in equal to or greater than 20% of inhalation steps.” They computed pooled estimates and created forest plots for both overall errors and for errors according to each step in using an MDI.
The eight studies they identified involved 1,221 patients, with ages ranging from a mean 48 to 82 years, 53% of whom were female. Nearly two-thirds of the patients had COPD (63.6%) while 36.4% had asthma. Most of the devices studied were MDIs alone (68.8%), while 31.2% included a spacer.
The pooled weighted average revealed a 66.5% error rate, that is, two-thirds of all the patients were making at least two errors during the 10 steps involved in using their device. The researchers then used individual error rates data in five studies to calculate the overall error rate for each step in using MDIs. The most common error, made by 73.8% of people in those five studies, was failing to attach the inhaler to the spacer. In addition, 68.7% of patients were failing to exhale fully and away from the inhaler before inhaling, and 47.8% were inhaling too fast instead of inhaling deeply.
“So these [findings] actually give you [some specific] ideas of how we could help improve patients’ ability to use the device properly,” Dr. Navaie told attendees, adding that these data can inform patient education needs and interventions.
Based on the data from those five studies, the error rates for all 10 steps to using an MDI were as follows:
- Failed to shake inhaler before use (37.9%).
- Failed to attach inhaler to spacer (73.8%).
- Failed to exhale fully and away from inhaler before inhalation (68.7%).
- Failed to place mouthpiece between teeth and sealed lips (7.4%).
- Failed to actuate once during inhalation (24.4%).
- Inhalation too fast, not deep (47.8%).
- Failed to hold breath for 5-10 seconds (40.1%).
- Failed to remove the inhaler/spacer from mouth (11.3%).
- Failed to exhale after inhalation (33.2%).
- Failed to repeat steps for second puff (36.7%).
Dr. Navaie also noted the investigators were surprised to learn that physicians themselves sometimes make several of these errors in explaining to patients how to use their devices.
“I think for the reps and other people who go out and visit doctors, it’s important to think about making sure the clinicians are using the devices properly,” Dr. Navaie said. She pointed out the potential for patients to forget steps between visits.
“One of the things a lot of our clinicians and key opinion leaders told us during the course of this study is that you shouldn’t just educate the patient at the time you are scripting the device but repeatedly because patients forget,” she said. She recommended having patients demonstrate their use of the device at each visit. If patients continue to struggle, it may be worth considering other therapies, such as a nebulizer, for patients unable to regularly use their devices correctly.
The meta-analysis was limited by the sparse research available in general on MDI errors in the U.S. adult population, so the data on error rates for each individual step may not be broadly generalizable. The studies also did not distinguish between rates among users with asthma vs. users with COPD. Further, too few data exist on associations between MDI errors and health outcomes to have a clear picture of the clinical implications of regularly making multiple errors in MDI use.
Dr. Navaie is employed by Advance Health Solutions, which received Sunovion Pharmaceuticals funding for the study.
SOURCE: Navaie M et al. CHEST 2018. doi: 10.1016/j.chest.2018.08.705.
SAN ANTONIO – Two-thirds of U.S. adults with (MDIs), according to new research. About half of patients failed to inhale slowly and deeply to ensure they received the appropriate dose, and about 40% of patients failed to hold their breath for 5-10 seconds afterward so that the medication made its way to their lungs, the findings show.
“There’s a need to educate patients on proper inhalation technique to optimize the appropriate delivery of medication,” Maryam Navaie, DrPH, of Advance Health Solutions in New York told attendees at the annual meeting of the American College of Chest Physicians. She also urged practitioners to think more carefully about what devices to prescribe to patients based on their own personal attributes.
“Nebulizer devices may be a better consideration for patients who have difficulty performing the necessary steps required by handheld inhalers,” Dr. Navaie said.
She and fellow researchers conducted a systematic review to gain more insights into the errors and difficulties experienced by U.S. adults using MDIs for COPD or asthma. They combed through PubMed, EMBASE, PsycINFO, Cochrane, and Google Scholar databases for English language studies about MDI-related errors in U.S. adult COPD or asthma patients published between January 2003 and February 2017.
The researchers included only randomized controlled trials and cross-sectional and observational studies, and they excluded studies with combined error rates across multiple devices so they could better parse out the data. They also used baseline rates only in studies that involved an intervention to reduce errors.
The researchers defined the proportion of overall MDI errors as “the percentage of patients who made errors in equal to or greater than 20% of inhalation steps.” They computed pooled estimates and created forest plots for both overall errors and for errors according to each step in using an MDI.
The eight studies they identified involved 1,221 patients, with ages ranging from a mean 48 to 82 years, 53% of whom were female. Nearly two-thirds of the patients had COPD (63.6%) while 36.4% had asthma. Most of the devices studied were MDIs alone (68.8%), while 31.2% included a spacer.
The pooled weighted average revealed a 66.5% error rate, that is, two-thirds of all the patients were making at least two errors during the 10 steps involved in using their device. The researchers then used individual error rates data in five studies to calculate the overall error rate for each step in using MDIs. The most common error, made by 73.8% of people in those five studies, was failing to attach the inhaler to the spacer. In addition, 68.7% of patients were failing to exhale fully and away from the inhaler before inhaling, and 47.8% were inhaling too fast instead of inhaling deeply.
“So these [findings] actually give you [some specific] ideas of how we could help improve patients’ ability to use the device properly,” Dr. Navaie told attendees, adding that these data can inform patient education needs and interventions.
Based on the data from those five studies, the error rates for all 10 steps to using an MDI were as follows:
- Failed to shake inhaler before use (37.9%).
- Failed to attach inhaler to spacer (73.8%).
- Failed to exhale fully and away from inhaler before inhalation (68.7%).
- Failed to place mouthpiece between teeth and sealed lips (7.4%).
- Failed to actuate once during inhalation (24.4%).
- Inhalation too fast, not deep (47.8%).
- Failed to hold breath for 5-10 seconds (40.1%).
- Failed to remove the inhaler/spacer from mouth (11.3%).
- Failed to exhale after inhalation (33.2%).
- Failed to repeat steps for second puff (36.7%).
Dr. Navaie also noted the investigators were surprised to learn that physicians themselves sometimes make several of these errors in explaining to patients how to use their devices.
“I think for the reps and other people who go out and visit doctors, it’s important to think about making sure the clinicians are using the devices properly,” Dr. Navaie said. She pointed out the potential for patients to forget steps between visits.
“One of the things a lot of our clinicians and key opinion leaders told us during the course of this study is that you shouldn’t just educate the patient at the time you are scripting the device but repeatedly because patients forget,” she said. She recommended having patients demonstrate their use of the device at each visit. If patients continue to struggle, it may be worth considering other therapies, such as a nebulizer, for patients unable to regularly use their devices correctly.
The meta-analysis was limited by the sparse research available in general on MDI errors in the U.S. adult population, so the data on error rates for each individual step may not be broadly generalizable. The studies also did not distinguish between rates among users with asthma vs. users with COPD. Further, too few data exist on associations between MDI errors and health outcomes to have a clear picture of the clinical implications of regularly making multiple errors in MDI use.
Dr. Navaie is employed by Advance Health Solutions, which received Sunovion Pharmaceuticals funding for the study.
SOURCE: Navaie M et al. CHEST 2018. doi: 10.1016/j.chest.2018.08.705.
SAN ANTONIO – Two-thirds of U.S. adults with (MDIs), according to new research. About half of patients failed to inhale slowly and deeply to ensure they received the appropriate dose, and about 40% of patients failed to hold their breath for 5-10 seconds afterward so that the medication made its way to their lungs, the findings show.
“There’s a need to educate patients on proper inhalation technique to optimize the appropriate delivery of medication,” Maryam Navaie, DrPH, of Advance Health Solutions in New York told attendees at the annual meeting of the American College of Chest Physicians. She also urged practitioners to think more carefully about what devices to prescribe to patients based on their own personal attributes.
“Nebulizer devices may be a better consideration for patients who have difficulty performing the necessary steps required by handheld inhalers,” Dr. Navaie said.
She and fellow researchers conducted a systematic review to gain more insights into the errors and difficulties experienced by U.S. adults using MDIs for COPD or asthma. They combed through PubMed, EMBASE, PsycINFO, Cochrane, and Google Scholar databases for English language studies about MDI-related errors in U.S. adult COPD or asthma patients published between January 2003 and February 2017.
The researchers included only randomized controlled trials and cross-sectional and observational studies, and they excluded studies with combined error rates across multiple devices so they could better parse out the data. They also used baseline rates only in studies that involved an intervention to reduce errors.
The researchers defined the proportion of overall MDI errors as “the percentage of patients who made errors in equal to or greater than 20% of inhalation steps.” They computed pooled estimates and created forest plots for both overall errors and for errors according to each step in using an MDI.
The eight studies they identified involved 1,221 patients, with ages ranging from a mean 48 to 82 years, 53% of whom were female. Nearly two-thirds of the patients had COPD (63.6%) while 36.4% had asthma. Most of the devices studied were MDIs alone (68.8%), while 31.2% included a spacer.
The pooled weighted average revealed a 66.5% error rate, that is, two-thirds of all the patients were making at least two errors during the 10 steps involved in using their device. The researchers then used individual error rates data in five studies to calculate the overall error rate for each step in using MDIs. The most common error, made by 73.8% of people in those five studies, was failing to attach the inhaler to the spacer. In addition, 68.7% of patients were failing to exhale fully and away from the inhaler before inhaling, and 47.8% were inhaling too fast instead of inhaling deeply.
“So these [findings] actually give you [some specific] ideas of how we could help improve patients’ ability to use the device properly,” Dr. Navaie told attendees, adding that these data can inform patient education needs and interventions.
Based on the data from those five studies, the error rates for all 10 steps to using an MDI were as follows:
- Failed to shake inhaler before use (37.9%).
- Failed to attach inhaler to spacer (73.8%).
- Failed to exhale fully and away from inhaler before inhalation (68.7%).
- Failed to place mouthpiece between teeth and sealed lips (7.4%).
- Failed to actuate once during inhalation (24.4%).
- Inhalation too fast, not deep (47.8%).
- Failed to hold breath for 5-10 seconds (40.1%).
- Failed to remove the inhaler/spacer from mouth (11.3%).
- Failed to exhale after inhalation (33.2%).
- Failed to repeat steps for second puff (36.7%).
Dr. Navaie also noted the investigators were surprised to learn that physicians themselves sometimes make several of these errors in explaining to patients how to use their devices.
“I think for the reps and other people who go out and visit doctors, it’s important to think about making sure the clinicians are using the devices properly,” Dr. Navaie said. She pointed out the potential for patients to forget steps between visits.
“One of the things a lot of our clinicians and key opinion leaders told us during the course of this study is that you shouldn’t just educate the patient at the time you are scripting the device but repeatedly because patients forget,” she said. She recommended having patients demonstrate their use of the device at each visit. If patients continue to struggle, it may be worth considering other therapies, such as a nebulizer, for patients unable to regularly use their devices correctly.
The meta-analysis was limited by the sparse research available in general on MDI errors in the U.S. adult population, so the data on error rates for each individual step may not be broadly generalizable. The studies also did not distinguish between rates among users with asthma vs. users with COPD. Further, too few data exist on associations between MDI errors and health outcomes to have a clear picture of the clinical implications of regularly making multiple errors in MDI use.
Dr. Navaie is employed by Advance Health Solutions, which received Sunovion Pharmaceuticals funding for the study.
SOURCE: Navaie M et al. CHEST 2018. doi: 10.1016/j.chest.2018.08.705.
REPORTING FROM CHEST 2018
Key clinical point: 67% of US adult patients with COPD or asthma report making errors in using metered-dose inhalers.
Major finding: 69% of patients do not exhale fully and away from the inhaler before inhalation; 50% do not inhale slowly and deeply.
Study details: Meta-analysis of eight studies involving 1,221 U.S. adult patients with COPD or asthma who use metered-dose inhalers.
Disclosures: Dr. Navaie is employed by Advance Health Solutions, which received Sunovion Pharmaceuticals funding for the study.
Source: Navaie M et al. CHEST 2018. doi: 10.1016/j.chest.2018.08.705.
No ADT-dementia link in large VA prostate cancer cohort study
In contrast to other recent studies, androgen deprivation therapy (ADT) had no link to dementia in a observational cohort study of more than 45,000 men with prostate cancer who received definitive radiotherapy, investigators have reported.
No significant associations were found between ADT and Alzheimer’s disease or vascular dementia, or between shorter or longer courses of ADT and any dementia studied, according to Rishi Deka, PhD, of Veterans Affairs San Diego Health Care System, La Jolla, Calif., and coinvestigators.
“These results may mitigate concerns regarding the long-term risks of ADT on cognitive health in the treatment of prostate cancer,” Dr. Deka and colleagues wrote in JAMA Oncology.
Two other recent studies showed strong, statistically significant associations between ADT and dementia in prostate cancer. However, those studies combined patients with local and metastatic disease, receiving ADT in the upfront or recurrent settings, while the present study looked specifically at men with nonmetastatic prostate cancer who received radiotherapy.
“Different treatment modalities and disease stages are associated with substantial selection bias that may predispose results to false associations,” noted Dr. Deka and coauthors.
Their observational cohort study comprised 45,218 men diagnosed with nonmetastatic prostate cancer at the U.S. Department of Veterans Affairs who underwent radiotherapy with or without ADT. The investigators excluded men who had a diagnosis of dementia within 1 year of the prostate cancer diagnosis or who had prior diagnoses of dementia, stroke, or cognitive impairment.
A total of 1,497 patients were diagnosed with dementia over a median of 6.8 years of follow-up: 404 with Alzheimer disease, 335 with vascular dementia, and 758 with other types or unclassified dementias.
The investigators found no significant association between use of ADT and development of any dementia, the primary outcome of the analysis (subdistribution hazard ratio [SHR], 1.04; 95% confidence interval, 0.94-1.16; P = .43).
Likewise, there was no association between ADT and vascular dementia, specifically, with an SHR of 1.20 (95% CI, 0.97-1.50; P = .10) or Alzheimer’s disease, with an SHR of 1.11 (95% CI, 0.91-1.36; P = .29).
Duration of ADT longer than 1 year was not significantly associated with dementia, nor was duration shorter than 1 year, with SHRs, of 1.08 and 1.01 respectively, the analysis shows.
The SHRs in these and other analysis reported ranged from 1.00 to 1.21. That is substantially lower than hazard ratios of 1.66 to 2.32 in one previous study linking ADT to dementia, according to the investigators, suggesting that the results of the current analysis were not due to inadequate power to detect differences.
Nevertheless, the findings may not be generalizable to some other populations, they cautioned, since it was focused demographically on veterans, and was limited to radiotherapy-treated patients.
Dr. Deka and coauthors reported no conflict of interest. Their study was funded by grants from the University of California San Diego Center for Precision Radiation Medicine.
SOURCE: Deka R et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4423.
In contrast to other recent studies, androgen deprivation therapy (ADT) had no link to dementia in a observational cohort study of more than 45,000 men with prostate cancer who received definitive radiotherapy, investigators have reported.
No significant associations were found between ADT and Alzheimer’s disease or vascular dementia, or between shorter or longer courses of ADT and any dementia studied, according to Rishi Deka, PhD, of Veterans Affairs San Diego Health Care System, La Jolla, Calif., and coinvestigators.
“These results may mitigate concerns regarding the long-term risks of ADT on cognitive health in the treatment of prostate cancer,” Dr. Deka and colleagues wrote in JAMA Oncology.
Two other recent studies showed strong, statistically significant associations between ADT and dementia in prostate cancer. However, those studies combined patients with local and metastatic disease, receiving ADT in the upfront or recurrent settings, while the present study looked specifically at men with nonmetastatic prostate cancer who received radiotherapy.
“Different treatment modalities and disease stages are associated with substantial selection bias that may predispose results to false associations,” noted Dr. Deka and coauthors.
Their observational cohort study comprised 45,218 men diagnosed with nonmetastatic prostate cancer at the U.S. Department of Veterans Affairs who underwent radiotherapy with or without ADT. The investigators excluded men who had a diagnosis of dementia within 1 year of the prostate cancer diagnosis or who had prior diagnoses of dementia, stroke, or cognitive impairment.
A total of 1,497 patients were diagnosed with dementia over a median of 6.8 years of follow-up: 404 with Alzheimer disease, 335 with vascular dementia, and 758 with other types or unclassified dementias.
The investigators found no significant association between use of ADT and development of any dementia, the primary outcome of the analysis (subdistribution hazard ratio [SHR], 1.04; 95% confidence interval, 0.94-1.16; P = .43).
Likewise, there was no association between ADT and vascular dementia, specifically, with an SHR of 1.20 (95% CI, 0.97-1.50; P = .10) or Alzheimer’s disease, with an SHR of 1.11 (95% CI, 0.91-1.36; P = .29).
Duration of ADT longer than 1 year was not significantly associated with dementia, nor was duration shorter than 1 year, with SHRs, of 1.08 and 1.01 respectively, the analysis shows.
The SHRs in these and other analysis reported ranged from 1.00 to 1.21. That is substantially lower than hazard ratios of 1.66 to 2.32 in one previous study linking ADT to dementia, according to the investigators, suggesting that the results of the current analysis were not due to inadequate power to detect differences.
Nevertheless, the findings may not be generalizable to some other populations, they cautioned, since it was focused demographically on veterans, and was limited to radiotherapy-treated patients.
Dr. Deka and coauthors reported no conflict of interest. Their study was funded by grants from the University of California San Diego Center for Precision Radiation Medicine.
SOURCE: Deka R et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4423.
In contrast to other recent studies, androgen deprivation therapy (ADT) had no link to dementia in a observational cohort study of more than 45,000 men with prostate cancer who received definitive radiotherapy, investigators have reported.
No significant associations were found between ADT and Alzheimer’s disease or vascular dementia, or between shorter or longer courses of ADT and any dementia studied, according to Rishi Deka, PhD, of Veterans Affairs San Diego Health Care System, La Jolla, Calif., and coinvestigators.
“These results may mitigate concerns regarding the long-term risks of ADT on cognitive health in the treatment of prostate cancer,” Dr. Deka and colleagues wrote in JAMA Oncology.
Two other recent studies showed strong, statistically significant associations between ADT and dementia in prostate cancer. However, those studies combined patients with local and metastatic disease, receiving ADT in the upfront or recurrent settings, while the present study looked specifically at men with nonmetastatic prostate cancer who received radiotherapy.
“Different treatment modalities and disease stages are associated with substantial selection bias that may predispose results to false associations,” noted Dr. Deka and coauthors.
Their observational cohort study comprised 45,218 men diagnosed with nonmetastatic prostate cancer at the U.S. Department of Veterans Affairs who underwent radiotherapy with or without ADT. The investigators excluded men who had a diagnosis of dementia within 1 year of the prostate cancer diagnosis or who had prior diagnoses of dementia, stroke, or cognitive impairment.
A total of 1,497 patients were diagnosed with dementia over a median of 6.8 years of follow-up: 404 with Alzheimer disease, 335 with vascular dementia, and 758 with other types or unclassified dementias.
The investigators found no significant association between use of ADT and development of any dementia, the primary outcome of the analysis (subdistribution hazard ratio [SHR], 1.04; 95% confidence interval, 0.94-1.16; P = .43).
Likewise, there was no association between ADT and vascular dementia, specifically, with an SHR of 1.20 (95% CI, 0.97-1.50; P = .10) or Alzheimer’s disease, with an SHR of 1.11 (95% CI, 0.91-1.36; P = .29).
Duration of ADT longer than 1 year was not significantly associated with dementia, nor was duration shorter than 1 year, with SHRs, of 1.08 and 1.01 respectively, the analysis shows.
The SHRs in these and other analysis reported ranged from 1.00 to 1.21. That is substantially lower than hazard ratios of 1.66 to 2.32 in one previous study linking ADT to dementia, according to the investigators, suggesting that the results of the current analysis were not due to inadequate power to detect differences.
Nevertheless, the findings may not be generalizable to some other populations, they cautioned, since it was focused demographically on veterans, and was limited to radiotherapy-treated patients.
Dr. Deka and coauthors reported no conflict of interest. Their study was funded by grants from the University of California San Diego Center for Precision Radiation Medicine.
SOURCE: Deka R et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4423.
FROM JAMA ONCOLOGY
Key clinical point: In contrast with other recent investigations in prostate cancer, researchers found no link between androgen deprivation therapy (ADT) and development of dementia.
Major finding: No significant association was found between use of ADT and development of any dementia (subdistribution hazard ratio [SHR], 1.04; 95% CI, 0.94-1.16; P = .43).
Study details: Observational cohort study of more than 45,000 veterans with nonmetastatic prostate cancer treated with radiotherapy with or without ADT.
Disclosures: This study was funded by grants from the University of California San Diego Center for Precision Radiation Medicine. Dr. Deka and coauthors reported no conflict of interest disclosures related to the work.
Source: Deka R et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4423.
Prior fertility treatment is associated with higher maternal morbidity during delivery
Investigators from the Stanford Hospital and Clinics in California found that while absolute risk is low, women who have received an infertility diagnosis or who have received fertility treatment are at higher risk of several markers of severe maternal morbidity than women who never received an infertility diagnosis or fertility treatment.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Gaya Murugappan, MD, lead investigator on the study, explained in an interview with OBG Management, “We know that in the last decade or so the rate of maternal morbidity has been rising gradually in the US, and we know that the utilization of fertility technology and the incidence of infertility are also rising.” The retrospective analysis set out to determine if a connection exists.
Methods. The investigators used a large insurance claims database to look at data from 2003 to 2016. They identified a group of infertile women who later conceived without fertility treatment (n=1822 deliveries) and a group of women who received fertility treatment (n=782 deliveries) and compared them with a control group of women who never received an infertility diagnosis or fertility treatment (n=37,944 deliveries). Women who currently or previously had cancer were excluded from the study.
The primary outcome was the number of indicators of severe maternal morbidity that occurred during the 6 months prior to or following delivery.
Findings. Compared with the control group, the women diagnosed with infertility were almost 4 times as likely to experience severe anesthesia complications (0.38% vs 0.11%; odds ratio [OR], 3.83; 95% confidence interval [CI], 1.69–8.70), about twice as likely to experience intraoperative heart failure (0.71% vs 0.31%; OR, 1.88; 95% CI, 1.05–3.34), and more than 3 times as likely to receive a hysterectomy (1.04% vs 0.28%; OR, 3.30; 95% CI, 2.02–5.40).
Similarly, compared with controls, women who had received fertility treatment had an OR of 2.66 for disseminated intravascular coagulation (2.81% vs 0.91%; 95% CI, 1.66–4.24), an OR of 5.17 for shock (0.90% vs 0.15%; 95% CI, 2.21–12.06), an OR of 1.61 for blood transfusions (3.71% vs 1.64%; 95% CI, 1.07–2.42), and an OR of 1.43 for cardiac monitoring (13.17% vs 8.14%; 95% CI, 1.14–1.79).
More research is needed. Dr. Murugappan noted, “I hope that these data help us identify high-risk populations of women so that we can minimize the occurrence of these potentially devastating health outcomes. Women need to be telling their ObGyns that they have a history of infertility and/or fertility treatment. Some women may not want to say that they conceived with donor egg, for example, but that could be a critical element of a patient’s history that an ObGyn should be aware of.”
More study is necessary, she added. For instance, “a study in the future looking at risk of maternal morbidity in patients who are infertile but then who go on to conceive spontaneously. Then we can tease out what is the effect of infertility versus the effect of fertility treatment.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Murugappan G, Li S, Lathi RB, Baker VL, Eisenberg ML. Increased risk of maternal morbidity in infertile women: analysis of US claims data. Fertil Steril. 2018;110(45 suppl):e9.
Investigators from the Stanford Hospital and Clinics in California found that while absolute risk is low, women who have received an infertility diagnosis or who have received fertility treatment are at higher risk of several markers of severe maternal morbidity than women who never received an infertility diagnosis or fertility treatment.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Gaya Murugappan, MD, lead investigator on the study, explained in an interview with OBG Management, “We know that in the last decade or so the rate of maternal morbidity has been rising gradually in the US, and we know that the utilization of fertility technology and the incidence of infertility are also rising.” The retrospective analysis set out to determine if a connection exists.
Methods. The investigators used a large insurance claims database to look at data from 2003 to 2016. They identified a group of infertile women who later conceived without fertility treatment (n=1822 deliveries) and a group of women who received fertility treatment (n=782 deliveries) and compared them with a control group of women who never received an infertility diagnosis or fertility treatment (n=37,944 deliveries). Women who currently or previously had cancer were excluded from the study.
The primary outcome was the number of indicators of severe maternal morbidity that occurred during the 6 months prior to or following delivery.
Findings. Compared with the control group, the women diagnosed with infertility were almost 4 times as likely to experience severe anesthesia complications (0.38% vs 0.11%; odds ratio [OR], 3.83; 95% confidence interval [CI], 1.69–8.70), about twice as likely to experience intraoperative heart failure (0.71% vs 0.31%; OR, 1.88; 95% CI, 1.05–3.34), and more than 3 times as likely to receive a hysterectomy (1.04% vs 0.28%; OR, 3.30; 95% CI, 2.02–5.40).
Similarly, compared with controls, women who had received fertility treatment had an OR of 2.66 for disseminated intravascular coagulation (2.81% vs 0.91%; 95% CI, 1.66–4.24), an OR of 5.17 for shock (0.90% vs 0.15%; 95% CI, 2.21–12.06), an OR of 1.61 for blood transfusions (3.71% vs 1.64%; 95% CI, 1.07–2.42), and an OR of 1.43 for cardiac monitoring (13.17% vs 8.14%; 95% CI, 1.14–1.79).
More research is needed. Dr. Murugappan noted, “I hope that these data help us identify high-risk populations of women so that we can minimize the occurrence of these potentially devastating health outcomes. Women need to be telling their ObGyns that they have a history of infertility and/or fertility treatment. Some women may not want to say that they conceived with donor egg, for example, but that could be a critical element of a patient’s history that an ObGyn should be aware of.”
More study is necessary, she added. For instance, “a study in the future looking at risk of maternal morbidity in patients who are infertile but then who go on to conceive spontaneously. Then we can tease out what is the effect of infertility versus the effect of fertility treatment.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Investigators from the Stanford Hospital and Clinics in California found that while absolute risk is low, women who have received an infertility diagnosis or who have received fertility treatment are at higher risk of several markers of severe maternal morbidity than women who never received an infertility diagnosis or fertility treatment.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Gaya Murugappan, MD, lead investigator on the study, explained in an interview with OBG Management, “We know that in the last decade or so the rate of maternal morbidity has been rising gradually in the US, and we know that the utilization of fertility technology and the incidence of infertility are also rising.” The retrospective analysis set out to determine if a connection exists.
Methods. The investigators used a large insurance claims database to look at data from 2003 to 2016. They identified a group of infertile women who later conceived without fertility treatment (n=1822 deliveries) and a group of women who received fertility treatment (n=782 deliveries) and compared them with a control group of women who never received an infertility diagnosis or fertility treatment (n=37,944 deliveries). Women who currently or previously had cancer were excluded from the study.
The primary outcome was the number of indicators of severe maternal morbidity that occurred during the 6 months prior to or following delivery.
Findings. Compared with the control group, the women diagnosed with infertility were almost 4 times as likely to experience severe anesthesia complications (0.38% vs 0.11%; odds ratio [OR], 3.83; 95% confidence interval [CI], 1.69–8.70), about twice as likely to experience intraoperative heart failure (0.71% vs 0.31%; OR, 1.88; 95% CI, 1.05–3.34), and more than 3 times as likely to receive a hysterectomy (1.04% vs 0.28%; OR, 3.30; 95% CI, 2.02–5.40).
Similarly, compared with controls, women who had received fertility treatment had an OR of 2.66 for disseminated intravascular coagulation (2.81% vs 0.91%; 95% CI, 1.66–4.24), an OR of 5.17 for shock (0.90% vs 0.15%; 95% CI, 2.21–12.06), an OR of 1.61 for blood transfusions (3.71% vs 1.64%; 95% CI, 1.07–2.42), and an OR of 1.43 for cardiac monitoring (13.17% vs 8.14%; 95% CI, 1.14–1.79).
More research is needed. Dr. Murugappan noted, “I hope that these data help us identify high-risk populations of women so that we can minimize the occurrence of these potentially devastating health outcomes. Women need to be telling their ObGyns that they have a history of infertility and/or fertility treatment. Some women may not want to say that they conceived with donor egg, for example, but that could be a critical element of a patient’s history that an ObGyn should be aware of.”
More study is necessary, she added. For instance, “a study in the future looking at risk of maternal morbidity in patients who are infertile but then who go on to conceive spontaneously. Then we can tease out what is the effect of infertility versus the effect of fertility treatment.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Murugappan G, Li S, Lathi RB, Baker VL, Eisenberg ML. Increased risk of maternal morbidity in infertile women: analysis of US claims data. Fertil Steril. 2018;110(45 suppl):e9.
- Murugappan G, Li S, Lathi RB, Baker VL, Eisenberg ML. Increased risk of maternal morbidity in infertile women: analysis of US claims data. Fertil Steril. 2018;110(45 suppl):e9.
Rock Steady Boxing could prove beneficial for Parkinson’s patients
Exercise classes that focus on boxing movements such as punching a bag may help to improve motor learning patterns in patients with Parkinson’s disease, according to a new pilot study.
When presented with a computerized test in which study participants with Parkinson’s disease had to press buttons corresponding to patterns appearing on a screen, those who had been taking Rock Steady Boxing classes for at least 6 months demonstrated faster reaction time than did those who had never taken the classes, according to Christopher K. McLeod, a second-year medical student at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y. Mr. McLeod, who worked with Adena Leder, DO, director of the Parkinson’s Disease Treatment Center at the college, will present his research findings Oct. 20 at the International Conference on Parkinson’s Disease and Movement Disorders in New York.
While the results were not statistically significant, and the number of participants (n = 28) was relatively small, said Mr. McLeod, “We think this is a good pilot study for research going forward, since there really isn’t anything in the literature right now about how procedural memory and learning could be addressed from a therapeutic standpoint in Parkinson’s disease patients.” Procedural learning is a means of acquiring a new skill through repeating the task, like learning to drive a stick shift by doing it.
The investigators used a serial reaction time test to assess procedural memory in 14 patients diagnosed with Parkinson’s disease who had been regularly attending Rock Steady Boxing classes and in 14 patients who did not go to the classes. The test featured a computer screen with four squares that would light up and a box with four corresponding buttons to push as each square lit up. There were seven time blocks in which patients were presented with a series of 10 stimuli. The first pattern was random to get participants accustomed to the task. The second, third, fourth, and fifth blocks had the same sequence, to assess participants’ ability to learn the pattern and respond more quickly. The sixth block again had a random pattern to see if participants slowed down to learn the new pattern, and the seventh block repeated the familiar sequence from the second to fifth blocks.
Experienced boxers generally demonstrated faster reaction time than did nonboxers, the researchers found. Statistical analysis of the four learning blocks (blocks 2-5) revealed a moderate effect (P = .19), indicating that experienced boxers tended to react faster than nonboxers.
Diminished reaction time is a hallmark symptom of Parkinson’s disease, often resulting in patients having to give up the ability drive as the disease progresses, Mr. McLeod said: “Reaction time is something that could eventually lead to falling or not being able to drive, which are huge lifestyle changes that affect these patients emotionally and impact their quality of life.”
The researchers also observed a visible difference in how the two groups tended to respond to the random sequence following the repetitive blocks. Experienced boxers slowed slightly, with a 27.3-ms increase in reaction time, while nonboxers got faster, with a 93.5-ms decrease in reaction time. One possible explanation is that nonboxers simply got better at reacting to the stimuli over time without actually learning the repeated sequence, Mr. McLeod said.
Rock Steady Boxing was founded in Indianapolis by Scott Newman, a former county prosecutor who was diagnosed with Parkinson’s disease at age 40 and experienced significant improvement in his health and agility by engaging in rigorous workouts, such as boxing. Dr. Leder became certified in Rock Steady Boxing and opened a chapter at the New York college in May 2016. The classes include group activities, games, and boxing exercises designed to improve patients’ physical and mental stamina.
Mr. McLeod and Dr. Leder reported no relevant financial disclosures.
Exercise classes that focus on boxing movements such as punching a bag may help to improve motor learning patterns in patients with Parkinson’s disease, according to a new pilot study.
When presented with a computerized test in which study participants with Parkinson’s disease had to press buttons corresponding to patterns appearing on a screen, those who had been taking Rock Steady Boxing classes for at least 6 months demonstrated faster reaction time than did those who had never taken the classes, according to Christopher K. McLeod, a second-year medical student at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y. Mr. McLeod, who worked with Adena Leder, DO, director of the Parkinson’s Disease Treatment Center at the college, will present his research findings Oct. 20 at the International Conference on Parkinson’s Disease and Movement Disorders in New York.
While the results were not statistically significant, and the number of participants (n = 28) was relatively small, said Mr. McLeod, “We think this is a good pilot study for research going forward, since there really isn’t anything in the literature right now about how procedural memory and learning could be addressed from a therapeutic standpoint in Parkinson’s disease patients.” Procedural learning is a means of acquiring a new skill through repeating the task, like learning to drive a stick shift by doing it.
The investigators used a serial reaction time test to assess procedural memory in 14 patients diagnosed with Parkinson’s disease who had been regularly attending Rock Steady Boxing classes and in 14 patients who did not go to the classes. The test featured a computer screen with four squares that would light up and a box with four corresponding buttons to push as each square lit up. There were seven time blocks in which patients were presented with a series of 10 stimuli. The first pattern was random to get participants accustomed to the task. The second, third, fourth, and fifth blocks had the same sequence, to assess participants’ ability to learn the pattern and respond more quickly. The sixth block again had a random pattern to see if participants slowed down to learn the new pattern, and the seventh block repeated the familiar sequence from the second to fifth blocks.
Experienced boxers generally demonstrated faster reaction time than did nonboxers, the researchers found. Statistical analysis of the four learning blocks (blocks 2-5) revealed a moderate effect (P = .19), indicating that experienced boxers tended to react faster than nonboxers.
Diminished reaction time is a hallmark symptom of Parkinson’s disease, often resulting in patients having to give up the ability drive as the disease progresses, Mr. McLeod said: “Reaction time is something that could eventually lead to falling or not being able to drive, which are huge lifestyle changes that affect these patients emotionally and impact their quality of life.”
The researchers also observed a visible difference in how the two groups tended to respond to the random sequence following the repetitive blocks. Experienced boxers slowed slightly, with a 27.3-ms increase in reaction time, while nonboxers got faster, with a 93.5-ms decrease in reaction time. One possible explanation is that nonboxers simply got better at reacting to the stimuli over time without actually learning the repeated sequence, Mr. McLeod said.
Rock Steady Boxing was founded in Indianapolis by Scott Newman, a former county prosecutor who was diagnosed with Parkinson’s disease at age 40 and experienced significant improvement in his health and agility by engaging in rigorous workouts, such as boxing. Dr. Leder became certified in Rock Steady Boxing and opened a chapter at the New York college in May 2016. The classes include group activities, games, and boxing exercises designed to improve patients’ physical and mental stamina.
Mr. McLeod and Dr. Leder reported no relevant financial disclosures.
Exercise classes that focus on boxing movements such as punching a bag may help to improve motor learning patterns in patients with Parkinson’s disease, according to a new pilot study.
When presented with a computerized test in which study participants with Parkinson’s disease had to press buttons corresponding to patterns appearing on a screen, those who had been taking Rock Steady Boxing classes for at least 6 months demonstrated faster reaction time than did those who had never taken the classes, according to Christopher K. McLeod, a second-year medical student at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y. Mr. McLeod, who worked with Adena Leder, DO, director of the Parkinson’s Disease Treatment Center at the college, will present his research findings Oct. 20 at the International Conference on Parkinson’s Disease and Movement Disorders in New York.
While the results were not statistically significant, and the number of participants (n = 28) was relatively small, said Mr. McLeod, “We think this is a good pilot study for research going forward, since there really isn’t anything in the literature right now about how procedural memory and learning could be addressed from a therapeutic standpoint in Parkinson’s disease patients.” Procedural learning is a means of acquiring a new skill through repeating the task, like learning to drive a stick shift by doing it.
The investigators used a serial reaction time test to assess procedural memory in 14 patients diagnosed with Parkinson’s disease who had been regularly attending Rock Steady Boxing classes and in 14 patients who did not go to the classes. The test featured a computer screen with four squares that would light up and a box with four corresponding buttons to push as each square lit up. There were seven time blocks in which patients were presented with a series of 10 stimuli. The first pattern was random to get participants accustomed to the task. The second, third, fourth, and fifth blocks had the same sequence, to assess participants’ ability to learn the pattern and respond more quickly. The sixth block again had a random pattern to see if participants slowed down to learn the new pattern, and the seventh block repeated the familiar sequence from the second to fifth blocks.
Experienced boxers generally demonstrated faster reaction time than did nonboxers, the researchers found. Statistical analysis of the four learning blocks (blocks 2-5) revealed a moderate effect (P = .19), indicating that experienced boxers tended to react faster than nonboxers.
Diminished reaction time is a hallmark symptom of Parkinson’s disease, often resulting in patients having to give up the ability drive as the disease progresses, Mr. McLeod said: “Reaction time is something that could eventually lead to falling or not being able to drive, which are huge lifestyle changes that affect these patients emotionally and impact their quality of life.”
The researchers also observed a visible difference in how the two groups tended to respond to the random sequence following the repetitive blocks. Experienced boxers slowed slightly, with a 27.3-ms increase in reaction time, while nonboxers got faster, with a 93.5-ms decrease in reaction time. One possible explanation is that nonboxers simply got better at reacting to the stimuli over time without actually learning the repeated sequence, Mr. McLeod said.
Rock Steady Boxing was founded in Indianapolis by Scott Newman, a former county prosecutor who was diagnosed with Parkinson’s disease at age 40 and experienced significant improvement in his health and agility by engaging in rigorous workouts, such as boxing. Dr. Leder became certified in Rock Steady Boxing and opened a chapter at the New York college in May 2016. The classes include group activities, games, and boxing exercises designed to improve patients’ physical and mental stamina.
Mr. McLeod and Dr. Leder reported no relevant financial disclosures.
REPORTING FROM ICPDMD 2018
DETERRED trial: Concurrent atezolizumab, CRT shows promise in LA-NSCLC
TORONTO – (LA-NSCLC) in the phase 2 DETERRED trial.
In part 1 of the single-institution study, 10 patients underwent chemoradiation therapy (CRT) with low-dose carboplatin/paclitaxel followed by high-dose consolidation chemotherapy plus atezolizumab and atezolizumab maintenance for 1 year. Six patients in this group (60%) experienced grade 3 or higher adverse events (AEs). In part 2 of the study, 30 patients received concurrent atezolizumab and CRT followed by the same consolidation and maintenance used in part 1, and 17 (57%) experienced grade 3 or higher AEs, Steven H. Lin, MD, reported at the World Conference on Lung Cancer.
Grade 3 or higher AEs were associated with atezolizumab in 30% and 23% of patients in part 1 and part 2, respectively. In part 1 these included dyspnea, arthralgia, and a grade 5 tracheoesophageal fistula, and in part 2 included diarrhea, pneumonitis, nephritis, fatigue, respiratory failure, and heart failure in one patient each, and fatigue in three patients.
Grade 2 radiation pneumonitis was seen in two patients in each group, Dr. Lin of the University of Texas MD Anderson Cancer Center, Houston, said at the meeting, which was sponsored by the International Association for the Study of Lung Cancer.
Withdrawals caused by toxicity occurred in three and four patients in part 1 and 2, respectively.
Four patients in part 1 progressed with disease during atezolizumab maintenance and five have died from either tracheoesophageal fistula or grade 5 toxicity. In part 2, six have progressed and five have died, most caused by cancer progression, he said.
Preliminary survival data show a median progression-free survival of 20.1 months in part 1, whereas progression-free survival was not reached in part 2. Median overall survival at 1 year was 60% versus 77% in parts 1 and 2, respectively.
Consolidation immunotherapy with durvalumab after CRT has been the standard of care for LA-NSCLC as established by the phase 3 PACIFIC trial, but evidence from that trial also suggested timing of the start of immunotherapy may be important.
“If patients were randomized less than 14 days after starting durvalumab there was a trend, or suggestion, that there was potentially an improvement in progression-free survival, compared with patients who started durvalumab outside of this window,” Dr. Lin said, noting that this is also supported by some preclinical evidence showing that the effectiveness of immunotherapies may be enhanced when combined with concurrent CRT.
The DETERRED trial evaluated the safety and preliminary efficacy of this approach followed by consolidation full-dose carboplatin/paclitaxel with atezolizumab and maintenance atezolizumab for up to 1 year for LA-NSCLC.
Patients, who had a median age of 66.5 years, were enrolled between February 2016 and April 2018; 15% had stage II disease, 50% had stage IIIA, and 35% had stage IIIB. Most (58%) had adenocarcinoma.
In part 1, standard chemoradiation including low-dose carboplatin/paclitaxel was given for 6 weeks. After CRT completion, patients were given consolidated high-dose chemotherapy with carboplatin/paclitaxel and intravenous atezolizumab was started at that point at a dose of 1,200 mg every 3 weeks for up to 1 year from the first dose. Part 2 was initiated based on the safety data in part 1 showing no concerning toxicities. In part 2, atezolizumab was given concurrently with CRT followed by the same consolidated regimen and maintenance.
“So the take-home message from this study is that the concurrent immunotherapy with atezolizumab and chemoradiation therapy can be administered safely,” Dr. Lin said, adding that grade 3+ pneumonitis is low and not significantly increased with the addition of concurrent atezolizumab with CRT.
Early efficacy analyses also show promising results, but further follow-up is needed, he said.
Trials now being planned include a phase 3 trial comparing the DETERRED and PACIFIC regimens, a phase 1 study comparing durvalumab with radiation to replace chemotherapy in programmed death–ligand 1–high locoregionally advanced NSCLC, and a phase 1 study of nivolumab with radiation to replace chemotherapy in locoregionally advanced NSCLC, he noted.
The DETERRED trial was supported by Genentech. Dr. Lin has received research grants from STCube Pharmaceuticals, Hitachi Chemical Diagnostics, Genentech, New River Labs, and BeyondSpring Pharmaceuticals, and is an advisory board member for AstraZeneca and New River Labs.
SOURCE: Lin SH et al. WCLC 2018, Abstract OA01.06.
TORONTO – (LA-NSCLC) in the phase 2 DETERRED trial.
In part 1 of the single-institution study, 10 patients underwent chemoradiation therapy (CRT) with low-dose carboplatin/paclitaxel followed by high-dose consolidation chemotherapy plus atezolizumab and atezolizumab maintenance for 1 year. Six patients in this group (60%) experienced grade 3 or higher adverse events (AEs). In part 2 of the study, 30 patients received concurrent atezolizumab and CRT followed by the same consolidation and maintenance used in part 1, and 17 (57%) experienced grade 3 or higher AEs, Steven H. Lin, MD, reported at the World Conference on Lung Cancer.
Grade 3 or higher AEs were associated with atezolizumab in 30% and 23% of patients in part 1 and part 2, respectively. In part 1 these included dyspnea, arthralgia, and a grade 5 tracheoesophageal fistula, and in part 2 included diarrhea, pneumonitis, nephritis, fatigue, respiratory failure, and heart failure in one patient each, and fatigue in three patients.
Grade 2 radiation pneumonitis was seen in two patients in each group, Dr. Lin of the University of Texas MD Anderson Cancer Center, Houston, said at the meeting, which was sponsored by the International Association for the Study of Lung Cancer.
Withdrawals caused by toxicity occurred in three and four patients in part 1 and 2, respectively.
Four patients in part 1 progressed with disease during atezolizumab maintenance and five have died from either tracheoesophageal fistula or grade 5 toxicity. In part 2, six have progressed and five have died, most caused by cancer progression, he said.
Preliminary survival data show a median progression-free survival of 20.1 months in part 1, whereas progression-free survival was not reached in part 2. Median overall survival at 1 year was 60% versus 77% in parts 1 and 2, respectively.
Consolidation immunotherapy with durvalumab after CRT has been the standard of care for LA-NSCLC as established by the phase 3 PACIFIC trial, but evidence from that trial also suggested timing of the start of immunotherapy may be important.
“If patients were randomized less than 14 days after starting durvalumab there was a trend, or suggestion, that there was potentially an improvement in progression-free survival, compared with patients who started durvalumab outside of this window,” Dr. Lin said, noting that this is also supported by some preclinical evidence showing that the effectiveness of immunotherapies may be enhanced when combined with concurrent CRT.
The DETERRED trial evaluated the safety and preliminary efficacy of this approach followed by consolidation full-dose carboplatin/paclitaxel with atezolizumab and maintenance atezolizumab for up to 1 year for LA-NSCLC.
Patients, who had a median age of 66.5 years, were enrolled between February 2016 and April 2018; 15% had stage II disease, 50% had stage IIIA, and 35% had stage IIIB. Most (58%) had adenocarcinoma.
In part 1, standard chemoradiation including low-dose carboplatin/paclitaxel was given for 6 weeks. After CRT completion, patients were given consolidated high-dose chemotherapy with carboplatin/paclitaxel and intravenous atezolizumab was started at that point at a dose of 1,200 mg every 3 weeks for up to 1 year from the first dose. Part 2 was initiated based on the safety data in part 1 showing no concerning toxicities. In part 2, atezolizumab was given concurrently with CRT followed by the same consolidated regimen and maintenance.
“So the take-home message from this study is that the concurrent immunotherapy with atezolizumab and chemoradiation therapy can be administered safely,” Dr. Lin said, adding that grade 3+ pneumonitis is low and not significantly increased with the addition of concurrent atezolizumab with CRT.
Early efficacy analyses also show promising results, but further follow-up is needed, he said.
Trials now being planned include a phase 3 trial comparing the DETERRED and PACIFIC regimens, a phase 1 study comparing durvalumab with radiation to replace chemotherapy in programmed death–ligand 1–high locoregionally advanced NSCLC, and a phase 1 study of nivolumab with radiation to replace chemotherapy in locoregionally advanced NSCLC, he noted.
The DETERRED trial was supported by Genentech. Dr. Lin has received research grants from STCube Pharmaceuticals, Hitachi Chemical Diagnostics, Genentech, New River Labs, and BeyondSpring Pharmaceuticals, and is an advisory board member for AstraZeneca and New River Labs.
SOURCE: Lin SH et al. WCLC 2018, Abstract OA01.06.
TORONTO – (LA-NSCLC) in the phase 2 DETERRED trial.
In part 1 of the single-institution study, 10 patients underwent chemoradiation therapy (CRT) with low-dose carboplatin/paclitaxel followed by high-dose consolidation chemotherapy plus atezolizumab and atezolizumab maintenance for 1 year. Six patients in this group (60%) experienced grade 3 or higher adverse events (AEs). In part 2 of the study, 30 patients received concurrent atezolizumab and CRT followed by the same consolidation and maintenance used in part 1, and 17 (57%) experienced grade 3 or higher AEs, Steven H. Lin, MD, reported at the World Conference on Lung Cancer.
Grade 3 or higher AEs were associated with atezolizumab in 30% and 23% of patients in part 1 and part 2, respectively. In part 1 these included dyspnea, arthralgia, and a grade 5 tracheoesophageal fistula, and in part 2 included diarrhea, pneumonitis, nephritis, fatigue, respiratory failure, and heart failure in one patient each, and fatigue in three patients.
Grade 2 radiation pneumonitis was seen in two patients in each group, Dr. Lin of the University of Texas MD Anderson Cancer Center, Houston, said at the meeting, which was sponsored by the International Association for the Study of Lung Cancer.
Withdrawals caused by toxicity occurred in three and four patients in part 1 and 2, respectively.
Four patients in part 1 progressed with disease during atezolizumab maintenance and five have died from either tracheoesophageal fistula or grade 5 toxicity. In part 2, six have progressed and five have died, most caused by cancer progression, he said.
Preliminary survival data show a median progression-free survival of 20.1 months in part 1, whereas progression-free survival was not reached in part 2. Median overall survival at 1 year was 60% versus 77% in parts 1 and 2, respectively.
Consolidation immunotherapy with durvalumab after CRT has been the standard of care for LA-NSCLC as established by the phase 3 PACIFIC trial, but evidence from that trial also suggested timing of the start of immunotherapy may be important.
“If patients were randomized less than 14 days after starting durvalumab there was a trend, or suggestion, that there was potentially an improvement in progression-free survival, compared with patients who started durvalumab outside of this window,” Dr. Lin said, noting that this is also supported by some preclinical evidence showing that the effectiveness of immunotherapies may be enhanced when combined with concurrent CRT.
The DETERRED trial evaluated the safety and preliminary efficacy of this approach followed by consolidation full-dose carboplatin/paclitaxel with atezolizumab and maintenance atezolizumab for up to 1 year for LA-NSCLC.
Patients, who had a median age of 66.5 years, were enrolled between February 2016 and April 2018; 15% had stage II disease, 50% had stage IIIA, and 35% had stage IIIB. Most (58%) had adenocarcinoma.
In part 1, standard chemoradiation including low-dose carboplatin/paclitaxel was given for 6 weeks. After CRT completion, patients were given consolidated high-dose chemotherapy with carboplatin/paclitaxel and intravenous atezolizumab was started at that point at a dose of 1,200 mg every 3 weeks for up to 1 year from the first dose. Part 2 was initiated based on the safety data in part 1 showing no concerning toxicities. In part 2, atezolizumab was given concurrently with CRT followed by the same consolidated regimen and maintenance.
“So the take-home message from this study is that the concurrent immunotherapy with atezolizumab and chemoradiation therapy can be administered safely,” Dr. Lin said, adding that grade 3+ pneumonitis is low and not significantly increased with the addition of concurrent atezolizumab with CRT.
Early efficacy analyses also show promising results, but further follow-up is needed, he said.
Trials now being planned include a phase 3 trial comparing the DETERRED and PACIFIC regimens, a phase 1 study comparing durvalumab with radiation to replace chemotherapy in programmed death–ligand 1–high locoregionally advanced NSCLC, and a phase 1 study of nivolumab with radiation to replace chemotherapy in locoregionally advanced NSCLC, he noted.
The DETERRED trial was supported by Genentech. Dr. Lin has received research grants from STCube Pharmaceuticals, Hitachi Chemical Diagnostics, Genentech, New River Labs, and BeyondSpring Pharmaceuticals, and is an advisory board member for AstraZeneca and New River Labs.
SOURCE: Lin SH et al. WCLC 2018, Abstract OA01.06.
REPORTING FROM WCLC 2018
Key clinical point: Concurrent atezolizumab and chemoradiation therapy is safe and shows promising efficacy in locally advanced non–small cell lung cancer.
Major finding: A total of 60% and 57% of part 1 and 2 patients, respectively, experienced grade 3 or higher adverse events.
Study details: The phase 2 DETERRED trial of 40 patients.
Disclosures: The DETERRED trial was supported by Genentech. Dr. Lin has received research grants from STCube Pharmaceuticals, Hitachi Chemical Diagnostics, Genentech, New River Labs, and BeyondSpring Pharmaceuticals, and is an advisory board member for AstraZeneca and New River Labs.
Source: Lin SH et al. WCLC 2018, Abstract OA01.06.
Higher rate of loss seen in unplanned pregnancies for women with epilepsy
when compared against women with epilepsy who planned their pregnancy, according to recent results from a retrospective study published in JAMA Neurology.
“This analysis adds the finding that unplanned pregnancy may increase the risk of [spontaneous fetal loss] in women with epilepsy and identifies pregnancy planning, maternal age, and interpregnancy interval as significant modifiable variables,” Andrew G. Herzog, MD, of the Harvard Neuroendocrine Unit at Beth Israel Deaconess Medical Center in Wellesley, Mass., and colleagues wrote in their study.
The researchers examined results from a web-based survey completed by 1,144 women in the Epilepsy Birth Control Registry (EBCR) between 2010 and 2014 with data on contraception use, pregnancy history, and antiepileptic drug (AED) treatment. Patients were aged 18-47 years (mean 28.5 years) with 8.7% of the cohort consisting of minority women and 39.8% having household incomes of $25,000 or less.
Pregnancy history data included number of pregnancies, number of planned or unplanned pregnancies, AED type used during pregnancies, pregnancy outcomes such as live birth, induced abortion, and spontaneous fetal loss (SFL), while AED data included categorizing patients into no therapy, monotherapy, and polytherapy groups. AED use was further subdivided into no AED, enzyme-inducing AED, non–enzyme-inducing AED, enzyme-inhibiting AED, glucuronidated AED, and mixed category groups.
Of 794 pregnancies, 530 pregnancies (66.8%) were unplanned and 264 (33.2%) were planned, with 473 live births (59.6%), 141 induced abortions (17.8%), and 180 SFL (22.7%). Among patients who did not have an induced abortion, SFL risk was higher if the pregnancy was unplanned (137 patients, 35.0%), compared with those who planned (43 patients, 16.4%) their pregnancy (risk ratio = 2.14; 95% confidence interval, 1.59-2.90; P less than .001). According to a regression analysis, SFL risk was higher for patients where “planning was entered alone” in unplanned pregnancies (odds ratio = 2.75; 95% CI, 1.87-4.05; P less than .001) as well as when adjusted for AED category, maternal age, and interpregnancy interval (OR = 3.57; 95% CI, 1.54-8.78; P = .003).
There was an association between maternal age (OR = 0.957; 95% CI, 0.928-0.986; P = .02) and risk of SFL, with lower risk seen in the 18- to 27-year-old group (118 patients, 29.5%; RR = 0.57; 95% CI, 0.39-0.84; P less than .004) and 28- to 37-year-old group (44 patients, 20.8%; RR = 0.40; 95% CI, 0.26-0.62; P less than .001), compared with the under-18 group (15 patients, 51.7%). There was also a higher risk of SFL with regard to interpregnancy interval (OR = 2.878; 95% CI, 1.8094-4.5801; P = .008), with a greater risk seen if the interpregnancy interval was under 1 year (56 patients, 45.9%), compared with 1 year (56 patients, 22.8%) or higher (RR = 2.02; 95% CI, 1.49-2.72; P less than .001).
“In view of the finding of increased risk for SFL in unplanned pregnancies in women with epilepsy, and because a history of SFL in women with epilepsy may increase the risk that subsequent live-born offspring will develop epilepsy, the finding warrants prospective investigation with medical record verification of pregnancy outcomes,” Dr. Herzog and his colleagues wrote.
The Epilepsy Foundation and Lundbeck funded the study. Dr. Herzog reports support by grants, and two coauthors received salary support from grants, from the two organizations.
SOURCE: Herzog AG et al. JAMA Neurol. 2018 Oct 15. doi: 10.1001/jamaneurol.2018.3089.
when compared against women with epilepsy who planned their pregnancy, according to recent results from a retrospective study published in JAMA Neurology.
“This analysis adds the finding that unplanned pregnancy may increase the risk of [spontaneous fetal loss] in women with epilepsy and identifies pregnancy planning, maternal age, and interpregnancy interval as significant modifiable variables,” Andrew G. Herzog, MD, of the Harvard Neuroendocrine Unit at Beth Israel Deaconess Medical Center in Wellesley, Mass., and colleagues wrote in their study.
The researchers examined results from a web-based survey completed by 1,144 women in the Epilepsy Birth Control Registry (EBCR) between 2010 and 2014 with data on contraception use, pregnancy history, and antiepileptic drug (AED) treatment. Patients were aged 18-47 years (mean 28.5 years) with 8.7% of the cohort consisting of minority women and 39.8% having household incomes of $25,000 or less.
Pregnancy history data included number of pregnancies, number of planned or unplanned pregnancies, AED type used during pregnancies, pregnancy outcomes such as live birth, induced abortion, and spontaneous fetal loss (SFL), while AED data included categorizing patients into no therapy, monotherapy, and polytherapy groups. AED use was further subdivided into no AED, enzyme-inducing AED, non–enzyme-inducing AED, enzyme-inhibiting AED, glucuronidated AED, and mixed category groups.
Of 794 pregnancies, 530 pregnancies (66.8%) were unplanned and 264 (33.2%) were planned, with 473 live births (59.6%), 141 induced abortions (17.8%), and 180 SFL (22.7%). Among patients who did not have an induced abortion, SFL risk was higher if the pregnancy was unplanned (137 patients, 35.0%), compared with those who planned (43 patients, 16.4%) their pregnancy (risk ratio = 2.14; 95% confidence interval, 1.59-2.90; P less than .001). According to a regression analysis, SFL risk was higher for patients where “planning was entered alone” in unplanned pregnancies (odds ratio = 2.75; 95% CI, 1.87-4.05; P less than .001) as well as when adjusted for AED category, maternal age, and interpregnancy interval (OR = 3.57; 95% CI, 1.54-8.78; P = .003).
There was an association between maternal age (OR = 0.957; 95% CI, 0.928-0.986; P = .02) and risk of SFL, with lower risk seen in the 18- to 27-year-old group (118 patients, 29.5%; RR = 0.57; 95% CI, 0.39-0.84; P less than .004) and 28- to 37-year-old group (44 patients, 20.8%; RR = 0.40; 95% CI, 0.26-0.62; P less than .001), compared with the under-18 group (15 patients, 51.7%). There was also a higher risk of SFL with regard to interpregnancy interval (OR = 2.878; 95% CI, 1.8094-4.5801; P = .008), with a greater risk seen if the interpregnancy interval was under 1 year (56 patients, 45.9%), compared with 1 year (56 patients, 22.8%) or higher (RR = 2.02; 95% CI, 1.49-2.72; P less than .001).
“In view of the finding of increased risk for SFL in unplanned pregnancies in women with epilepsy, and because a history of SFL in women with epilepsy may increase the risk that subsequent live-born offspring will develop epilepsy, the finding warrants prospective investigation with medical record verification of pregnancy outcomes,” Dr. Herzog and his colleagues wrote.
The Epilepsy Foundation and Lundbeck funded the study. Dr. Herzog reports support by grants, and two coauthors received salary support from grants, from the two organizations.
SOURCE: Herzog AG et al. JAMA Neurol. 2018 Oct 15. doi: 10.1001/jamaneurol.2018.3089.
when compared against women with epilepsy who planned their pregnancy, according to recent results from a retrospective study published in JAMA Neurology.
“This analysis adds the finding that unplanned pregnancy may increase the risk of [spontaneous fetal loss] in women with epilepsy and identifies pregnancy planning, maternal age, and interpregnancy interval as significant modifiable variables,” Andrew G. Herzog, MD, of the Harvard Neuroendocrine Unit at Beth Israel Deaconess Medical Center in Wellesley, Mass., and colleagues wrote in their study.
The researchers examined results from a web-based survey completed by 1,144 women in the Epilepsy Birth Control Registry (EBCR) between 2010 and 2014 with data on contraception use, pregnancy history, and antiepileptic drug (AED) treatment. Patients were aged 18-47 years (mean 28.5 years) with 8.7% of the cohort consisting of minority women and 39.8% having household incomes of $25,000 or less.
Pregnancy history data included number of pregnancies, number of planned or unplanned pregnancies, AED type used during pregnancies, pregnancy outcomes such as live birth, induced abortion, and spontaneous fetal loss (SFL), while AED data included categorizing patients into no therapy, monotherapy, and polytherapy groups. AED use was further subdivided into no AED, enzyme-inducing AED, non–enzyme-inducing AED, enzyme-inhibiting AED, glucuronidated AED, and mixed category groups.
Of 794 pregnancies, 530 pregnancies (66.8%) were unplanned and 264 (33.2%) were planned, with 473 live births (59.6%), 141 induced abortions (17.8%), and 180 SFL (22.7%). Among patients who did not have an induced abortion, SFL risk was higher if the pregnancy was unplanned (137 patients, 35.0%), compared with those who planned (43 patients, 16.4%) their pregnancy (risk ratio = 2.14; 95% confidence interval, 1.59-2.90; P less than .001). According to a regression analysis, SFL risk was higher for patients where “planning was entered alone” in unplanned pregnancies (odds ratio = 2.75; 95% CI, 1.87-4.05; P less than .001) as well as when adjusted for AED category, maternal age, and interpregnancy interval (OR = 3.57; 95% CI, 1.54-8.78; P = .003).
There was an association between maternal age (OR = 0.957; 95% CI, 0.928-0.986; P = .02) and risk of SFL, with lower risk seen in the 18- to 27-year-old group (118 patients, 29.5%; RR = 0.57; 95% CI, 0.39-0.84; P less than .004) and 28- to 37-year-old group (44 patients, 20.8%; RR = 0.40; 95% CI, 0.26-0.62; P less than .001), compared with the under-18 group (15 patients, 51.7%). There was also a higher risk of SFL with regard to interpregnancy interval (OR = 2.878; 95% CI, 1.8094-4.5801; P = .008), with a greater risk seen if the interpregnancy interval was under 1 year (56 patients, 45.9%), compared with 1 year (56 patients, 22.8%) or higher (RR = 2.02; 95% CI, 1.49-2.72; P less than .001).
“In view of the finding of increased risk for SFL in unplanned pregnancies in women with epilepsy, and because a history of SFL in women with epilepsy may increase the risk that subsequent live-born offspring will develop epilepsy, the finding warrants prospective investigation with medical record verification of pregnancy outcomes,” Dr. Herzog and his colleagues wrote.
The Epilepsy Foundation and Lundbeck funded the study. Dr. Herzog reports support by grants, and two coauthors received salary support from grants, from the two organizations.
SOURCE: Herzog AG et al. JAMA Neurol. 2018 Oct 15. doi: 10.1001/jamaneurol.2018.3089.
FROM JAMA NEUROLOGY
Key clinical point: Women with epilepsy who experience unplanned pregnancies have a higher rate of spontaneous fetal loss, compared with those with epilepsy who plan their pregnancies.
Major finding: Thirty-five percent of women with unplanned pregnancies experienced spontaneous fetal loss, compared with 16.4% of women in the planned pregnancy group.
Study details: A retrospective analysis of results from a web-based survey of 1,144 women from the Epilepsy Birth Control Registry.
Disclosures: The Epilepsy Foundation and Lundbeck funded the study. Dr. Herzog reports support by grants, and two coauthors received salary support from grants, from the two organizations.
Source: Herzog AG et al. JAMA Neurol. 2018 Oct 15. doi: 10.1001/jamaneurol.2018.3089.
Blood test outreach, navigation could close colorectal cancer screening gap
Fecal blood test outreach and patient navigation have robust evidence to show they improve colorectal cancer screening rates, results of a meta-analysis show.
Both strategies increased screening rates by about 20 percentage points, according to results of the systematic review and meta-analysis reported in JAMA Internal Medicine.
“This finding suggests that broad implementation of either of these interventions could bring the current national screening rate of 63% close to the national goal of 80%,” wrote Michael K. Dougherty, MD, of the University of North Carolina at Chapel Hill, and his coauthors in the report.
Active distribution of fecal blood tests (FBTs) was tested in 17 of the studies in the main meta-analysis, which was limited to 73 trials that the researchers said were at low to medium risk of bias.
Most of those studies looked at mailing FBTs, though a few studies tied distribution to a patient encounter. Compared with usual care, FBT outreach was associated with increased screening, with a risk ratio of 2.26, Dr. Dougherty and his coauthors reported.
Patient navigation interventions, tested in 16 studies of low to medium bias risk, were usually done by health care professionals, though in a few cases, they involved lay or peer navigators.
Navigation was also associated with increased screening when compared with usual care, the investigators found. The risk ratio was 2.01, which increased to 2.33 for interventions that included some additional component more than a standardized reminder or mailing, such as a video decision aid or intensive automated reminders.
Patient reminders and patient education strategies were also associated with increased screening in the meta-analysis, both with risk ratios of 1.20.
Incorporating clinician reminders or academic detailing appeared to improve the net benefit of interventions, according to the investigators.
“Clinicians, health administrators, and policy makers should consider how to incorporate patient navigation, FBT outreach, and/or clinician prompts into their health care settings and sociocultural contexts,” Dr. Dougherty and his colleagues wrote in their report.
Multicomponent interventions appeared to have an edge over single-component interventions, they added.
In general, the aim of FBT outreach is to improve test distribution and thus overcome structural barriers to screening, the study authors wrote. Similarly, patient navigation is designed to help guide patients through the complex health care system and avoid barriers to care, which may be sociocultural, logistical, or educational.
Major funding for the study came from the University Cancer Research Fund of the University of North Carolina Lineberger Comprehensive Cancer Center. One coinvestigator reported institutional grant funding from Pfizer unrelated to the current study.
SOURCE: Dougherty MK et al. JAMA Intern Med. 2018 Oct 15. doi: 10.1001/jamainternmed.2018.4637.
This systematic review and meta-analysis is an “ambitious project” that should inform decision makers, according to Beverly B. Green, MD, MPH.
“We can now safely say that, in general, no more studies are needed to demonstrate that outreach with fecal blood tests and patient navigation increase colorectal cancer screening,” Dr. Green wrote in an associated commentary.
Now, research should turn to other areas of colorectal cancer control, such as how to actually implement screening strategies and potentially adapt them for specific populations, she added. The effects of these interventions may be different in disadvantaged populations that have low screening rates and worse outcomes of colorectal cancer.
“Knowing that an intervention is effective in a highly controlled research setting does not guarantee local or widespread adoption,” she wrote. The interventions need to be “feasible, acceptable, and compatible” with existing fast-paced and complicated processes that provide day-to-day health care, and with the resources available to clinicians and organizations.
Dr. Green is with Kaiser Permanente Washington Health Research Institute in Seattle. Her comments appeared in an editorial in JAMA Internal Medicine (doi:10.1001/jamainternmed.2018.4627) . She reported no conflicts of interest.
This systematic review and meta-analysis is an “ambitious project” that should inform decision makers, according to Beverly B. Green, MD, MPH.
“We can now safely say that, in general, no more studies are needed to demonstrate that outreach with fecal blood tests and patient navigation increase colorectal cancer screening,” Dr. Green wrote in an associated commentary.
Now, research should turn to other areas of colorectal cancer control, such as how to actually implement screening strategies and potentially adapt them for specific populations, she added. The effects of these interventions may be different in disadvantaged populations that have low screening rates and worse outcomes of colorectal cancer.
“Knowing that an intervention is effective in a highly controlled research setting does not guarantee local or widespread adoption,” she wrote. The interventions need to be “feasible, acceptable, and compatible” with existing fast-paced and complicated processes that provide day-to-day health care, and with the resources available to clinicians and organizations.
Dr. Green is with Kaiser Permanente Washington Health Research Institute in Seattle. Her comments appeared in an editorial in JAMA Internal Medicine (doi:10.1001/jamainternmed.2018.4627) . She reported no conflicts of interest.
This systematic review and meta-analysis is an “ambitious project” that should inform decision makers, according to Beverly B. Green, MD, MPH.
“We can now safely say that, in general, no more studies are needed to demonstrate that outreach with fecal blood tests and patient navigation increase colorectal cancer screening,” Dr. Green wrote in an associated commentary.
Now, research should turn to other areas of colorectal cancer control, such as how to actually implement screening strategies and potentially adapt them for specific populations, she added. The effects of these interventions may be different in disadvantaged populations that have low screening rates and worse outcomes of colorectal cancer.
“Knowing that an intervention is effective in a highly controlled research setting does not guarantee local or widespread adoption,” she wrote. The interventions need to be “feasible, acceptable, and compatible” with existing fast-paced and complicated processes that provide day-to-day health care, and with the resources available to clinicians and organizations.
Dr. Green is with Kaiser Permanente Washington Health Research Institute in Seattle. Her comments appeared in an editorial in JAMA Internal Medicine (doi:10.1001/jamainternmed.2018.4627) . She reported no conflicts of interest.
Fecal blood test outreach and patient navigation have robust evidence to show they improve colorectal cancer screening rates, results of a meta-analysis show.
Both strategies increased screening rates by about 20 percentage points, according to results of the systematic review and meta-analysis reported in JAMA Internal Medicine.
“This finding suggests that broad implementation of either of these interventions could bring the current national screening rate of 63% close to the national goal of 80%,” wrote Michael K. Dougherty, MD, of the University of North Carolina at Chapel Hill, and his coauthors in the report.
Active distribution of fecal blood tests (FBTs) was tested in 17 of the studies in the main meta-analysis, which was limited to 73 trials that the researchers said were at low to medium risk of bias.
Most of those studies looked at mailing FBTs, though a few studies tied distribution to a patient encounter. Compared with usual care, FBT outreach was associated with increased screening, with a risk ratio of 2.26, Dr. Dougherty and his coauthors reported.
Patient navigation interventions, tested in 16 studies of low to medium bias risk, were usually done by health care professionals, though in a few cases, they involved lay or peer navigators.
Navigation was also associated with increased screening when compared with usual care, the investigators found. The risk ratio was 2.01, which increased to 2.33 for interventions that included some additional component more than a standardized reminder or mailing, such as a video decision aid or intensive automated reminders.
Patient reminders and patient education strategies were also associated with increased screening in the meta-analysis, both with risk ratios of 1.20.
Incorporating clinician reminders or academic detailing appeared to improve the net benefit of interventions, according to the investigators.
“Clinicians, health administrators, and policy makers should consider how to incorporate patient navigation, FBT outreach, and/or clinician prompts into their health care settings and sociocultural contexts,” Dr. Dougherty and his colleagues wrote in their report.
Multicomponent interventions appeared to have an edge over single-component interventions, they added.
In general, the aim of FBT outreach is to improve test distribution and thus overcome structural barriers to screening, the study authors wrote. Similarly, patient navigation is designed to help guide patients through the complex health care system and avoid barriers to care, which may be sociocultural, logistical, or educational.
Major funding for the study came from the University Cancer Research Fund of the University of North Carolina Lineberger Comprehensive Cancer Center. One coinvestigator reported institutional grant funding from Pfizer unrelated to the current study.
SOURCE: Dougherty MK et al. JAMA Intern Med. 2018 Oct 15. doi: 10.1001/jamainternmed.2018.4637.
Fecal blood test outreach and patient navigation have robust evidence to show they improve colorectal cancer screening rates, results of a meta-analysis show.
Both strategies increased screening rates by about 20 percentage points, according to results of the systematic review and meta-analysis reported in JAMA Internal Medicine.
“This finding suggests that broad implementation of either of these interventions could bring the current national screening rate of 63% close to the national goal of 80%,” wrote Michael K. Dougherty, MD, of the University of North Carolina at Chapel Hill, and his coauthors in the report.
Active distribution of fecal blood tests (FBTs) was tested in 17 of the studies in the main meta-analysis, which was limited to 73 trials that the researchers said were at low to medium risk of bias.
Most of those studies looked at mailing FBTs, though a few studies tied distribution to a patient encounter. Compared with usual care, FBT outreach was associated with increased screening, with a risk ratio of 2.26, Dr. Dougherty and his coauthors reported.
Patient navigation interventions, tested in 16 studies of low to medium bias risk, were usually done by health care professionals, though in a few cases, they involved lay or peer navigators.
Navigation was also associated with increased screening when compared with usual care, the investigators found. The risk ratio was 2.01, which increased to 2.33 for interventions that included some additional component more than a standardized reminder or mailing, such as a video decision aid or intensive automated reminders.
Patient reminders and patient education strategies were also associated with increased screening in the meta-analysis, both with risk ratios of 1.20.
Incorporating clinician reminders or academic detailing appeared to improve the net benefit of interventions, according to the investigators.
“Clinicians, health administrators, and policy makers should consider how to incorporate patient navigation, FBT outreach, and/or clinician prompts into their health care settings and sociocultural contexts,” Dr. Dougherty and his colleagues wrote in their report.
Multicomponent interventions appeared to have an edge over single-component interventions, they added.
In general, the aim of FBT outreach is to improve test distribution and thus overcome structural barriers to screening, the study authors wrote. Similarly, patient navigation is designed to help guide patients through the complex health care system and avoid barriers to care, which may be sociocultural, logistical, or educational.
Major funding for the study came from the University Cancer Research Fund of the University of North Carolina Lineberger Comprehensive Cancer Center. One coinvestigator reported institutional grant funding from Pfizer unrelated to the current study.
SOURCE: Dougherty MK et al. JAMA Intern Med. 2018 Oct 15. doi: 10.1001/jamainternmed.2018.4637.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Fecal blood test outreach and patient navigation improved colorectal cancer screening rates.
Major finding: Compared with usual care, fecal blood test outreach and navigation were associated with increased screening, with risk ratios of 2.26 and 2.01, respectively.
Study details: A systematic review and meta-analysis including 73 randomized trials at low to medium risk of bias.
Disclosures: Major funding was provided by the University of North Carolina Lineberger Comprehensive Cancer Center. One study author reported institutional grant funding unrelated to the study.
Source: Dougherty MK et al. JAMA Intern Med. 2018 Oct 15. doi: 10.1001/jamainternmed.2018.4637.
Is a Unidimensional Cognitive Screen Sufficient for Patients With MS?
A unidimensional approach to evaluating cognitive deficits misses the variability and impact of the disease.
BERLIN—Cognitive impairment in patients with multiple sclerosis (MS) varies in presence and degree in a manner that is neither identified nor quantified by the Symbol Digit Modalities Test (SDMT), according to a study presented at ECTRIMS 2018. “A unidimensional score or measure is insufficient to adequately identify and appreciate the richness and variation of the combinations and degrees of cognitive impairment that occur in patients with MS and impact the appearance of meaningful cognitive-related disability,” said Mark Gudesblatt, MD, Medical Director of the Comprehensive MS Care Center at South Shore Neurologic Associates in Islip, New York, and colleagues. “Better screening tools are required for evaluation of cognitive impairment in patients with MS.”
Cognitive impairment is common in people with MS. This impairment impacts economically important milestones and patient quality of life. Clinician and patient perceptions of the presence and degree of cognitive impairment are insufficiently sensitive measures, according to Dr. Gudesblatt. An increasing number of cognitive domains impaired greater than 1 standard deviation below age- and education-matched persons with MS has been shown to progressively impact self-reported driving, employment, and fall risk in patients with an EDSS score less than 6. Important cognitive disability in patients with MS can be unrelated to visible physical disability. Variability in the location and degree of MS plaque burden may differentially affect cognitive and physical ability, and many other factors may influence cognitive impairment in patients with MS. Routine cognitive screening in MS care is uncommon, Dr. Gudesblatt said. The SDMT, although frequently recommended, provides a single screening score that does not provide information about individual cognitive domains or the presence and degree of impairment across multiple cognitive domains or the accumulation of cognitive impairment.
Dr. Gudesblatt and colleagues conducted a retrospective review of consecutive patients with MS referred for screening with a multidomain computerized screening cognitive assessment battery (CAB) in the course of routine care who also underwent testing with the oral version of the SDMT on the same day. Their study included 113 patients with MS. The cohort had a mean age of 48.9, and 85% were female.
Within this patient sample, the SDMT defined cognitive function as follows: 68% normal classification, 14% low, 5% moderately low, and 12% very low. In this same patient group, the multidomain screening CAB identified the following domains of cognitive impairment greater than 1 standard deviation below normal values: memory (32%), executive function (25%), attention (28%), information processing speed (30%), visuospatial processing (20%), verbal function (23%), motor skills (20%), and a global summary screening score (24%). The multidimensional screening CAB in this same patient population further identified the number of cognitive domains impaired: zero domains, 36%; one domain, 24%; two domains, 11.5%; and three or more domains, 28%.
A unidimensional approach to evaluating cognitive deficits misses the variability and impact of the disease.
A unidimensional approach to evaluating cognitive deficits misses the variability and impact of the disease.
BERLIN—Cognitive impairment in patients with multiple sclerosis (MS) varies in presence and degree in a manner that is neither identified nor quantified by the Symbol Digit Modalities Test (SDMT), according to a study presented at ECTRIMS 2018. “A unidimensional score or measure is insufficient to adequately identify and appreciate the richness and variation of the combinations and degrees of cognitive impairment that occur in patients with MS and impact the appearance of meaningful cognitive-related disability,” said Mark Gudesblatt, MD, Medical Director of the Comprehensive MS Care Center at South Shore Neurologic Associates in Islip, New York, and colleagues. “Better screening tools are required for evaluation of cognitive impairment in patients with MS.”
Cognitive impairment is common in people with MS. This impairment impacts economically important milestones and patient quality of life. Clinician and patient perceptions of the presence and degree of cognitive impairment are insufficiently sensitive measures, according to Dr. Gudesblatt. An increasing number of cognitive domains impaired greater than 1 standard deviation below age- and education-matched persons with MS has been shown to progressively impact self-reported driving, employment, and fall risk in patients with an EDSS score less than 6. Important cognitive disability in patients with MS can be unrelated to visible physical disability. Variability in the location and degree of MS plaque burden may differentially affect cognitive and physical ability, and many other factors may influence cognitive impairment in patients with MS. Routine cognitive screening in MS care is uncommon, Dr. Gudesblatt said. The SDMT, although frequently recommended, provides a single screening score that does not provide information about individual cognitive domains or the presence and degree of impairment across multiple cognitive domains or the accumulation of cognitive impairment.
Dr. Gudesblatt and colleagues conducted a retrospective review of consecutive patients with MS referred for screening with a multidomain computerized screening cognitive assessment battery (CAB) in the course of routine care who also underwent testing with the oral version of the SDMT on the same day. Their study included 113 patients with MS. The cohort had a mean age of 48.9, and 85% were female.
Within this patient sample, the SDMT defined cognitive function as follows: 68% normal classification, 14% low, 5% moderately low, and 12% very low. In this same patient group, the multidomain screening CAB identified the following domains of cognitive impairment greater than 1 standard deviation below normal values: memory (32%), executive function (25%), attention (28%), information processing speed (30%), visuospatial processing (20%), verbal function (23%), motor skills (20%), and a global summary screening score (24%). The multidimensional screening CAB in this same patient population further identified the number of cognitive domains impaired: zero domains, 36%; one domain, 24%; two domains, 11.5%; and three or more domains, 28%.
BERLIN—Cognitive impairment in patients with multiple sclerosis (MS) varies in presence and degree in a manner that is neither identified nor quantified by the Symbol Digit Modalities Test (SDMT), according to a study presented at ECTRIMS 2018. “A unidimensional score or measure is insufficient to adequately identify and appreciate the richness and variation of the combinations and degrees of cognitive impairment that occur in patients with MS and impact the appearance of meaningful cognitive-related disability,” said Mark Gudesblatt, MD, Medical Director of the Comprehensive MS Care Center at South Shore Neurologic Associates in Islip, New York, and colleagues. “Better screening tools are required for evaluation of cognitive impairment in patients with MS.”
Cognitive impairment is common in people with MS. This impairment impacts economically important milestones and patient quality of life. Clinician and patient perceptions of the presence and degree of cognitive impairment are insufficiently sensitive measures, according to Dr. Gudesblatt. An increasing number of cognitive domains impaired greater than 1 standard deviation below age- and education-matched persons with MS has been shown to progressively impact self-reported driving, employment, and fall risk in patients with an EDSS score less than 6. Important cognitive disability in patients with MS can be unrelated to visible physical disability. Variability in the location and degree of MS plaque burden may differentially affect cognitive and physical ability, and many other factors may influence cognitive impairment in patients with MS. Routine cognitive screening in MS care is uncommon, Dr. Gudesblatt said. The SDMT, although frequently recommended, provides a single screening score that does not provide information about individual cognitive domains or the presence and degree of impairment across multiple cognitive domains or the accumulation of cognitive impairment.
Dr. Gudesblatt and colleagues conducted a retrospective review of consecutive patients with MS referred for screening with a multidomain computerized screening cognitive assessment battery (CAB) in the course of routine care who also underwent testing with the oral version of the SDMT on the same day. Their study included 113 patients with MS. The cohort had a mean age of 48.9, and 85% were female.
Within this patient sample, the SDMT defined cognitive function as follows: 68% normal classification, 14% low, 5% moderately low, and 12% very low. In this same patient group, the multidomain screening CAB identified the following domains of cognitive impairment greater than 1 standard deviation below normal values: memory (32%), executive function (25%), attention (28%), information processing speed (30%), visuospatial processing (20%), verbal function (23%), motor skills (20%), and a global summary screening score (24%). The multidimensional screening CAB in this same patient population further identified the number of cognitive domains impaired: zero domains, 36%; one domain, 24%; two domains, 11.5%; and three or more domains, 28%.




