User login
DTCs in marrow herald worse outcomes for early breast cancer
SAN ANTONIO – When disseminated tumor cells – even a single cell – are detected in the bone marrow of patients with early breast cancer, it’s not a good sign, according to results of pooled international analysis.
The presence of disseminated tumor cells (DTCs) in marrow at the time of primary diagnosis was an independent prognostic factor for overall survival (OS), disease-free survival (DFS), and distant disease-free survival (DDFS), reported Andreas Daniel Hartkopf, MD, MSc, of the University of Tübingen, Germany, on behalf of colleagues in the PADDY study.
“The DTC detection was associated with higher local tumor burden and more aggressive biological subtype,” he said at the San Antonio Breast Cancer Symposium.
The PADDY (Pooled Analysis of DTC Detection in Early Breast Cancer) study is an international cooperative effort designed to evaluate the association between DTC detection in patients with early breast cancer and clinical outcomes.
The investigators collected individual data sets including information on bone marrow samples from 10,307 patients treated at 11 centers in the United States and Europe.
The patients had early invasive breast cancer (T1-4, NO-3, M0) and for inclusion in the study had to have had bone marrow aspiration performed at the time of diagnosis or during primary surgery, with no prior systemic therapy. DTCs were detected by cytokeratin staining.
Patients were defined as being DTC positive if one or more DTCs were detected.
A total of 2,814 of the 10,307 patients (27.3%) were DTC positive. DTC status was not significantly associated with menopausal status or tumor histology (invasive ductal, invasive lobular, or other), but was positively associated with tumor grade, with 21.1% of patients with grade 1 tumors being DTC positive, compared with 27.2% with grade 2 tumors, and 29.9% with grade 3 tumors (P less than .001).
At a median follow-up of 7.6 years from bone marrow sampling DTC positivity was significantly associated with, in addition to higher tumor grade, higher T stage, nodal positivity, estrogen and progesterone receptor negativity, and HER2 positivity (P less than .001 for all).
Univariate analysis showed that DTC-positive patients had significantly shorter OS, breast cancer–specific survival, and DDFS compared with DTC negative patients (P less than .001 for all).
In a multivariate model stratified by center and controlling for age, menopausal status, histology, tumor size, nodal status, and biological subtype, DTC was an independent prognostic marker for OS (hazard ratio 1.23, P = .006), DFS (HR 1.30, P less than .001) and DDFS (HR 1.30, P = .006), but not for locoregional relapse-free survival.
“These results confirm that DTC detection is an independent risk factor for metastatic relapse and poor overall survival in early breast cancer,” Dr. Hartkopf said.
There was a statistically significant interaction between DTCs and subtypes with regard to DDFS, with a HR of 2.34 for patients with luminal B disease (P = .014).
“Future trials must evaluate the predictive value of DTC detection and/or their characterization on adjuvant therapy efficacy,” Dr. Hartkopf concluded.
Dr. Hartkopf did not disclose a study funding source or potential conflicts of interest.
SOURCE: Hartkopf AD et al. SABCS 2018. Abstract GS5-07.
SAN ANTONIO – When disseminated tumor cells – even a single cell – are detected in the bone marrow of patients with early breast cancer, it’s not a good sign, according to results of pooled international analysis.
The presence of disseminated tumor cells (DTCs) in marrow at the time of primary diagnosis was an independent prognostic factor for overall survival (OS), disease-free survival (DFS), and distant disease-free survival (DDFS), reported Andreas Daniel Hartkopf, MD, MSc, of the University of Tübingen, Germany, on behalf of colleagues in the PADDY study.
“The DTC detection was associated with higher local tumor burden and more aggressive biological subtype,” he said at the San Antonio Breast Cancer Symposium.
The PADDY (Pooled Analysis of DTC Detection in Early Breast Cancer) study is an international cooperative effort designed to evaluate the association between DTC detection in patients with early breast cancer and clinical outcomes.
The investigators collected individual data sets including information on bone marrow samples from 10,307 patients treated at 11 centers in the United States and Europe.
The patients had early invasive breast cancer (T1-4, NO-3, M0) and for inclusion in the study had to have had bone marrow aspiration performed at the time of diagnosis or during primary surgery, with no prior systemic therapy. DTCs were detected by cytokeratin staining.
Patients were defined as being DTC positive if one or more DTCs were detected.
A total of 2,814 of the 10,307 patients (27.3%) were DTC positive. DTC status was not significantly associated with menopausal status or tumor histology (invasive ductal, invasive lobular, or other), but was positively associated with tumor grade, with 21.1% of patients with grade 1 tumors being DTC positive, compared with 27.2% with grade 2 tumors, and 29.9% with grade 3 tumors (P less than .001).
At a median follow-up of 7.6 years from bone marrow sampling DTC positivity was significantly associated with, in addition to higher tumor grade, higher T stage, nodal positivity, estrogen and progesterone receptor negativity, and HER2 positivity (P less than .001 for all).
Univariate analysis showed that DTC-positive patients had significantly shorter OS, breast cancer–specific survival, and DDFS compared with DTC negative patients (P less than .001 for all).
In a multivariate model stratified by center and controlling for age, menopausal status, histology, tumor size, nodal status, and biological subtype, DTC was an independent prognostic marker for OS (hazard ratio 1.23, P = .006), DFS (HR 1.30, P less than .001) and DDFS (HR 1.30, P = .006), but not for locoregional relapse-free survival.
“These results confirm that DTC detection is an independent risk factor for metastatic relapse and poor overall survival in early breast cancer,” Dr. Hartkopf said.
There was a statistically significant interaction between DTCs and subtypes with regard to DDFS, with a HR of 2.34 for patients with luminal B disease (P = .014).
“Future trials must evaluate the predictive value of DTC detection and/or their characterization on adjuvant therapy efficacy,” Dr. Hartkopf concluded.
Dr. Hartkopf did not disclose a study funding source or potential conflicts of interest.
SOURCE: Hartkopf AD et al. SABCS 2018. Abstract GS5-07.
SAN ANTONIO – When disseminated tumor cells – even a single cell – are detected in the bone marrow of patients with early breast cancer, it’s not a good sign, according to results of pooled international analysis.
The presence of disseminated tumor cells (DTCs) in marrow at the time of primary diagnosis was an independent prognostic factor for overall survival (OS), disease-free survival (DFS), and distant disease-free survival (DDFS), reported Andreas Daniel Hartkopf, MD, MSc, of the University of Tübingen, Germany, on behalf of colleagues in the PADDY study.
“The DTC detection was associated with higher local tumor burden and more aggressive biological subtype,” he said at the San Antonio Breast Cancer Symposium.
The PADDY (Pooled Analysis of DTC Detection in Early Breast Cancer) study is an international cooperative effort designed to evaluate the association between DTC detection in patients with early breast cancer and clinical outcomes.
The investigators collected individual data sets including information on bone marrow samples from 10,307 patients treated at 11 centers in the United States and Europe.
The patients had early invasive breast cancer (T1-4, NO-3, M0) and for inclusion in the study had to have had bone marrow aspiration performed at the time of diagnosis or during primary surgery, with no prior systemic therapy. DTCs were detected by cytokeratin staining.
Patients were defined as being DTC positive if one or more DTCs were detected.
A total of 2,814 of the 10,307 patients (27.3%) were DTC positive. DTC status was not significantly associated with menopausal status or tumor histology (invasive ductal, invasive lobular, or other), but was positively associated with tumor grade, with 21.1% of patients with grade 1 tumors being DTC positive, compared with 27.2% with grade 2 tumors, and 29.9% with grade 3 tumors (P less than .001).
At a median follow-up of 7.6 years from bone marrow sampling DTC positivity was significantly associated with, in addition to higher tumor grade, higher T stage, nodal positivity, estrogen and progesterone receptor negativity, and HER2 positivity (P less than .001 for all).
Univariate analysis showed that DTC-positive patients had significantly shorter OS, breast cancer–specific survival, and DDFS compared with DTC negative patients (P less than .001 for all).
In a multivariate model stratified by center and controlling for age, menopausal status, histology, tumor size, nodal status, and biological subtype, DTC was an independent prognostic marker for OS (hazard ratio 1.23, P = .006), DFS (HR 1.30, P less than .001) and DDFS (HR 1.30, P = .006), but not for locoregional relapse-free survival.
“These results confirm that DTC detection is an independent risk factor for metastatic relapse and poor overall survival in early breast cancer,” Dr. Hartkopf said.
There was a statistically significant interaction between DTCs and subtypes with regard to DDFS, with a HR of 2.34 for patients with luminal B disease (P = .014).
“Future trials must evaluate the predictive value of DTC detection and/or their characterization on adjuvant therapy efficacy,” Dr. Hartkopf concluded.
Dr. Hartkopf did not disclose a study funding source or potential conflicts of interest.
SOURCE: Hartkopf AD et al. SABCS 2018. Abstract GS5-07.
REPORTING FROM SABCS 2018
Key clinical point: The presence of even a single DTC may be prognostic of metastases and poor survival.
Major finding: DTC was an independent prognostic marker for overall survival, disease-free survival, and distant disease-free survival.
Study details: Retrospective analysis of data on 10,030 patients, including 2,814 with detectable DTCs in bone marrow.
Disclosures: Dr. Hartkopf did not disclose a study funding source or potential conflicts of interest.
Source: Hartkopf AD et al. SABCS 2018. Abstract GS5-07.
Ibrutinib-rituximab ‘new standard of care’ in younger CLL patients
SAN DIEGO – The combination of ibrutinib and rituximab was associated with a two-thirds reduction in chronic lymphocytic leukemia (CLL) progression, compared with standard chemoimmunotherapy in patients younger than 70 years old, interim results of a phase 3 randomized trial showed.
Among 529 patients with previously untreated, symptomatic CLL randomly assigned to ibrutinib-rituximab (IR) or to chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab (FCR), the IR regimen was associated with a 65% reduction in risk for disease progression, which was the trial’s primary endpoint.
The IR regimen was also associated with better overall survival out to 4 years of follow-up, reported Tait D. Shanafelt, MD, of Stanford (Calif.) University.
“This establishes ibrutinib-based therapy as the most effective treatment tested to date in this disease for untreated patients,” he said at a media briefing prior at the annual meeting of the American Society of Hematology.
The study results are likely to dethrone FCR as the most active chemoimmunotherapy regimen against CLL, Dr. Shanafelt said.
In the ECOG-ACRIN Cancer Research Group E1912 trial, 529 patients aged 70 or younger with previously untreated CLL were enrolled and randomly assigned on a 2:1 basis to either standard therapy with six cycles of FCR according to standard protocols (175 patients), or IR, with ibrutinib 420 mg daily for each cycle, and rituximab delivered 50 mg/m2 intravenously on day 1 of cycle 2, and 325 mg/m2 on day 2 of the same cycle, and 500 mg/m2 on day 1 for all remaining cycles (354 patients).
From cycle 8 until progression, patients in the IR arm received daily ibrutinib 420 mg.
Dr. Shanafelt presented results from both an intention-to-treat analysis and a per-protocol analysis excluding 22 patients in the IR arm and 9 patients in the FCR arm who were randomized but later found not to meet eligibility criteria.
After a mean follow-up of 34 months, there were 37 PFS events in the IR arm, compared with 40 events in the FCR arm in an intention-to-treat analysis. The difference translated into a hazard ratio for progression of 0.35 with IR (P less than .00001).
The results were similar in the per-protocol analysis, with an HR of 0.32 favoring IR (P less than .00001).*
There were four deaths in the IR arm, compared with 10 in the FCR arm at the time of the data lock, translating into a hazard ratio (HR) for overall survival of 0.17 (P less than .0003) in the intention-to-treat analysis, and 0.13 in the per-protocol analysis (P less than .0001).
Dr. Shanafelt noted that although the overall number of deaths was relatively small, there were twice as many patients enrolled in the IR arm as in the FCR arm, meaning that the rate of deaths in the FCR arm was fivefold higher than in the IR arm.
In a subgroup analysis of PFS, IR was superior to FCR regardless of patient age, sex, performance status, disease stage, or the presence or absence of the 11q23.3 deletion.
PFS was also significantly better with IR in patients with unmutated immunoglobulin heavy chain variable (IGHV) regions (HR 0.26, P less than .00001), but not in patients with mutated IGHV.*
Grade 3 or greater treatment-related adverse events occurred in 58.5% of patients in the IR arm, compared with 72.1% of patients in the FCR arm. Specific events that occurred significantly less often with IR included neutropenia (22.7% vs. 43.7%), anemia (2.6% vs. 12.0%), thrombocytopenia (2.9% vs. 13.9%), any infection (7.1% vs. 19.0%), and neutropenic fever (2.3% vs. 15.8%; P less than .001 for all comparisons).
Events that occurred more frequently with IR than FCR included atrial fibrillation (2.9% vs. 0%, P = .04), and hypertension (7.4% vs. 1.9%, P = .01).
Dr. Shanafelt acknowledged that one possible barrier to the IR regimen is cost; the monthly cost of ibrutinib maintenance is about $10,000, he said, although he noted that cost considerations were not studied in the trial.
“Future trials testing novel agent combinations to see if we can eliminate the need for chronic therapy should be pursued,” he said.
The trial was sponsored by the National Cancer Institute with additional support from Pharmacyclics. Dr. Shanafelt reported patents and royalties from the Mayo Clinic, and research funding from Celgene, GlaxoSmithKline, Genentech, Abbvie, Pharmacyclics, and Janssen.
SOURCE: Shanafelt TD et al. ASH 2018, Abstract LBA-4.
*Correction, 12/12/2018: An earlier version of this story misstated the P value in two comparisons.
SAN DIEGO – The combination of ibrutinib and rituximab was associated with a two-thirds reduction in chronic lymphocytic leukemia (CLL) progression, compared with standard chemoimmunotherapy in patients younger than 70 years old, interim results of a phase 3 randomized trial showed.
Among 529 patients with previously untreated, symptomatic CLL randomly assigned to ibrutinib-rituximab (IR) or to chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab (FCR), the IR regimen was associated with a 65% reduction in risk for disease progression, which was the trial’s primary endpoint.
The IR regimen was also associated with better overall survival out to 4 years of follow-up, reported Tait D. Shanafelt, MD, of Stanford (Calif.) University.
“This establishes ibrutinib-based therapy as the most effective treatment tested to date in this disease for untreated patients,” he said at a media briefing prior at the annual meeting of the American Society of Hematology.
The study results are likely to dethrone FCR as the most active chemoimmunotherapy regimen against CLL, Dr. Shanafelt said.
In the ECOG-ACRIN Cancer Research Group E1912 trial, 529 patients aged 70 or younger with previously untreated CLL were enrolled and randomly assigned on a 2:1 basis to either standard therapy with six cycles of FCR according to standard protocols (175 patients), or IR, with ibrutinib 420 mg daily for each cycle, and rituximab delivered 50 mg/m2 intravenously on day 1 of cycle 2, and 325 mg/m2 on day 2 of the same cycle, and 500 mg/m2 on day 1 for all remaining cycles (354 patients).
From cycle 8 until progression, patients in the IR arm received daily ibrutinib 420 mg.
Dr. Shanafelt presented results from both an intention-to-treat analysis and a per-protocol analysis excluding 22 patients in the IR arm and 9 patients in the FCR arm who were randomized but later found not to meet eligibility criteria.
After a mean follow-up of 34 months, there were 37 PFS events in the IR arm, compared with 40 events in the FCR arm in an intention-to-treat analysis. The difference translated into a hazard ratio for progression of 0.35 with IR (P less than .00001).
The results were similar in the per-protocol analysis, with an HR of 0.32 favoring IR (P less than .00001).*
There were four deaths in the IR arm, compared with 10 in the FCR arm at the time of the data lock, translating into a hazard ratio (HR) for overall survival of 0.17 (P less than .0003) in the intention-to-treat analysis, and 0.13 in the per-protocol analysis (P less than .0001).
Dr. Shanafelt noted that although the overall number of deaths was relatively small, there were twice as many patients enrolled in the IR arm as in the FCR arm, meaning that the rate of deaths in the FCR arm was fivefold higher than in the IR arm.
In a subgroup analysis of PFS, IR was superior to FCR regardless of patient age, sex, performance status, disease stage, or the presence or absence of the 11q23.3 deletion.
PFS was also significantly better with IR in patients with unmutated immunoglobulin heavy chain variable (IGHV) regions (HR 0.26, P less than .00001), but not in patients with mutated IGHV.*
Grade 3 or greater treatment-related adverse events occurred in 58.5% of patients in the IR arm, compared with 72.1% of patients in the FCR arm. Specific events that occurred significantly less often with IR included neutropenia (22.7% vs. 43.7%), anemia (2.6% vs. 12.0%), thrombocytopenia (2.9% vs. 13.9%), any infection (7.1% vs. 19.0%), and neutropenic fever (2.3% vs. 15.8%; P less than .001 for all comparisons).
Events that occurred more frequently with IR than FCR included atrial fibrillation (2.9% vs. 0%, P = .04), and hypertension (7.4% vs. 1.9%, P = .01).
Dr. Shanafelt acknowledged that one possible barrier to the IR regimen is cost; the monthly cost of ibrutinib maintenance is about $10,000, he said, although he noted that cost considerations were not studied in the trial.
“Future trials testing novel agent combinations to see if we can eliminate the need for chronic therapy should be pursued,” he said.
The trial was sponsored by the National Cancer Institute with additional support from Pharmacyclics. Dr. Shanafelt reported patents and royalties from the Mayo Clinic, and research funding from Celgene, GlaxoSmithKline, Genentech, Abbvie, Pharmacyclics, and Janssen.
SOURCE: Shanafelt TD et al. ASH 2018, Abstract LBA-4.
*Correction, 12/12/2018: An earlier version of this story misstated the P value in two comparisons.
SAN DIEGO – The combination of ibrutinib and rituximab was associated with a two-thirds reduction in chronic lymphocytic leukemia (CLL) progression, compared with standard chemoimmunotherapy in patients younger than 70 years old, interim results of a phase 3 randomized trial showed.
Among 529 patients with previously untreated, symptomatic CLL randomly assigned to ibrutinib-rituximab (IR) or to chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab (FCR), the IR regimen was associated with a 65% reduction in risk for disease progression, which was the trial’s primary endpoint.
The IR regimen was also associated with better overall survival out to 4 years of follow-up, reported Tait D. Shanafelt, MD, of Stanford (Calif.) University.
“This establishes ibrutinib-based therapy as the most effective treatment tested to date in this disease for untreated patients,” he said at a media briefing prior at the annual meeting of the American Society of Hematology.
The study results are likely to dethrone FCR as the most active chemoimmunotherapy regimen against CLL, Dr. Shanafelt said.
In the ECOG-ACRIN Cancer Research Group E1912 trial, 529 patients aged 70 or younger with previously untreated CLL were enrolled and randomly assigned on a 2:1 basis to either standard therapy with six cycles of FCR according to standard protocols (175 patients), or IR, with ibrutinib 420 mg daily for each cycle, and rituximab delivered 50 mg/m2 intravenously on day 1 of cycle 2, and 325 mg/m2 on day 2 of the same cycle, and 500 mg/m2 on day 1 for all remaining cycles (354 patients).
From cycle 8 until progression, patients in the IR arm received daily ibrutinib 420 mg.
Dr. Shanafelt presented results from both an intention-to-treat analysis and a per-protocol analysis excluding 22 patients in the IR arm and 9 patients in the FCR arm who were randomized but later found not to meet eligibility criteria.
After a mean follow-up of 34 months, there were 37 PFS events in the IR arm, compared with 40 events in the FCR arm in an intention-to-treat analysis. The difference translated into a hazard ratio for progression of 0.35 with IR (P less than .00001).
The results were similar in the per-protocol analysis, with an HR of 0.32 favoring IR (P less than .00001).*
There were four deaths in the IR arm, compared with 10 in the FCR arm at the time of the data lock, translating into a hazard ratio (HR) for overall survival of 0.17 (P less than .0003) in the intention-to-treat analysis, and 0.13 in the per-protocol analysis (P less than .0001).
Dr. Shanafelt noted that although the overall number of deaths was relatively small, there were twice as many patients enrolled in the IR arm as in the FCR arm, meaning that the rate of deaths in the FCR arm was fivefold higher than in the IR arm.
In a subgroup analysis of PFS, IR was superior to FCR regardless of patient age, sex, performance status, disease stage, or the presence or absence of the 11q23.3 deletion.
PFS was also significantly better with IR in patients with unmutated immunoglobulin heavy chain variable (IGHV) regions (HR 0.26, P less than .00001), but not in patients with mutated IGHV.*
Grade 3 or greater treatment-related adverse events occurred in 58.5% of patients in the IR arm, compared with 72.1% of patients in the FCR arm. Specific events that occurred significantly less often with IR included neutropenia (22.7% vs. 43.7%), anemia (2.6% vs. 12.0%), thrombocytopenia (2.9% vs. 13.9%), any infection (7.1% vs. 19.0%), and neutropenic fever (2.3% vs. 15.8%; P less than .001 for all comparisons).
Events that occurred more frequently with IR than FCR included atrial fibrillation (2.9% vs. 0%, P = .04), and hypertension (7.4% vs. 1.9%, P = .01).
Dr. Shanafelt acknowledged that one possible barrier to the IR regimen is cost; the monthly cost of ibrutinib maintenance is about $10,000, he said, although he noted that cost considerations were not studied in the trial.
“Future trials testing novel agent combinations to see if we can eliminate the need for chronic therapy should be pursued,” he said.
The trial was sponsored by the National Cancer Institute with additional support from Pharmacyclics. Dr. Shanafelt reported patents and royalties from the Mayo Clinic, and research funding from Celgene, GlaxoSmithKline, Genentech, Abbvie, Pharmacyclics, and Janssen.
SOURCE: Shanafelt TD et al. ASH 2018, Abstract LBA-4.
*Correction, 12/12/2018: An earlier version of this story misstated the P value in two comparisons.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: The hazard ratio for disease progression with IR versus FCR was 0.35 (P less than .00001).
Study details: Interim analysis of a phase 3 trial in 529 patients aged 70 or younger with newly diagnosed CLL.
Disclosures: The trial was sponsored by the National Cancer Institute with additional support from Pharmacyclics. Dr. Shanafelt reported patents and royalties from the Mayo Clinic, and research funding from Celgene, GlaxoSmithKline, Genentech, Abbvie, Pharmacyclics, and Janssen.
Source: Shanafelt TD et al. ASH 2018, Abstract LBA-4.
Tom Brokaw opens up on surviving multiple myeloma
SAN DIEGO – Tom Brokaw has devoted his life to openness and transparency. But he kept mum about a big story that only he could fully tell – his diagnosis of multiple myeloma. He alerted his bosses and a few loved ones but otherwise kept his condition secret even as he struggled to walk and navigate stairs.
“I didn’t want to be Tom Brokaw, cancer victim,” he said at the annual meeting of the American Society of Hematology. But he did decide to go public in a big way and he said he doesn’t regret it. “I’m kind of the multiple myeloma poster boy.”
Since opening up about myeloma, “I have learned more about life and medicine, and kindness and the extraordinary strength of this country, than I have in all my other experiences,” he said. “I can say, oddly enough, at age 78 about to be 79, that having multiple myeloma has been a kind of privilege for me.”
Mr. Brokaw is best known as the longtime anchor of “NBC Nightly News” and author of “The Greatest Generation,” about the American experience in World War II. He was diagnosed with multiple myeloma in 2013 and revealed his condition publicly in 2014.
In 2016, he described his treatment in a New York Times commentary: “...three years of chemotherapy, a spinal operation that cost me three inches of height, monthly infusions of bone supplements, and drugs to prevent respiratory infection.” He also described fatigue, bone damage, and a 24-pill-a-day regimen.
In his presentation at ASH, Mr. Brokaw detailed the adjustment of having to slow down after an active life as a cyclist and outdoorsman. “I’m not going to go down the street with a cane. My birth certificate says I’m 78 years old, but I still think I’m 38, anchoring the news.”
“There was so much concentration on the disease itself that I don’t think I got as much as I needed regarding the radiant effects.”
At one point, he fell while running with his dog, and developed an infection in a cavity in his elbow. Still, he refused to cancel a flight to Washington, D.C., for an interview with the secretary of defense. The infection got worse, soaking his shirt with leakage, and when he returned “they slammed me into intensive care.”
He got a stern instruction that “you can’t do this anymore,” and he responded with an “ohh-kay.”
“It’s the anchorman in me. You get used to doing what you want to do. But I have to be much more careful about what I do and when I do it,” he said.
Now, Mr. Brokaw still struggles to follow advice about risks such as flying. But he remains active as a speaker, a special correspondent for NBC, and an author. “By and large,” he said, “I’m getting along OK. I’m grateful for that.”
SAN DIEGO – Tom Brokaw has devoted his life to openness and transparency. But he kept mum about a big story that only he could fully tell – his diagnosis of multiple myeloma. He alerted his bosses and a few loved ones but otherwise kept his condition secret even as he struggled to walk and navigate stairs.
“I didn’t want to be Tom Brokaw, cancer victim,” he said at the annual meeting of the American Society of Hematology. But he did decide to go public in a big way and he said he doesn’t regret it. “I’m kind of the multiple myeloma poster boy.”
Since opening up about myeloma, “I have learned more about life and medicine, and kindness and the extraordinary strength of this country, than I have in all my other experiences,” he said. “I can say, oddly enough, at age 78 about to be 79, that having multiple myeloma has been a kind of privilege for me.”
Mr. Brokaw is best known as the longtime anchor of “NBC Nightly News” and author of “The Greatest Generation,” about the American experience in World War II. He was diagnosed with multiple myeloma in 2013 and revealed his condition publicly in 2014.
In 2016, he described his treatment in a New York Times commentary: “...three years of chemotherapy, a spinal operation that cost me three inches of height, monthly infusions of bone supplements, and drugs to prevent respiratory infection.” He also described fatigue, bone damage, and a 24-pill-a-day regimen.
In his presentation at ASH, Mr. Brokaw detailed the adjustment of having to slow down after an active life as a cyclist and outdoorsman. “I’m not going to go down the street with a cane. My birth certificate says I’m 78 years old, but I still think I’m 38, anchoring the news.”
“There was so much concentration on the disease itself that I don’t think I got as much as I needed regarding the radiant effects.”
At one point, he fell while running with his dog, and developed an infection in a cavity in his elbow. Still, he refused to cancel a flight to Washington, D.C., for an interview with the secretary of defense. The infection got worse, soaking his shirt with leakage, and when he returned “they slammed me into intensive care.”
He got a stern instruction that “you can’t do this anymore,” and he responded with an “ohh-kay.”
“It’s the anchorman in me. You get used to doing what you want to do. But I have to be much more careful about what I do and when I do it,” he said.
Now, Mr. Brokaw still struggles to follow advice about risks such as flying. But he remains active as a speaker, a special correspondent for NBC, and an author. “By and large,” he said, “I’m getting along OK. I’m grateful for that.”
SAN DIEGO – Tom Brokaw has devoted his life to openness and transparency. But he kept mum about a big story that only he could fully tell – his diagnosis of multiple myeloma. He alerted his bosses and a few loved ones but otherwise kept his condition secret even as he struggled to walk and navigate stairs.
“I didn’t want to be Tom Brokaw, cancer victim,” he said at the annual meeting of the American Society of Hematology. But he did decide to go public in a big way and he said he doesn’t regret it. “I’m kind of the multiple myeloma poster boy.”
Since opening up about myeloma, “I have learned more about life and medicine, and kindness and the extraordinary strength of this country, than I have in all my other experiences,” he said. “I can say, oddly enough, at age 78 about to be 79, that having multiple myeloma has been a kind of privilege for me.”
Mr. Brokaw is best known as the longtime anchor of “NBC Nightly News” and author of “The Greatest Generation,” about the American experience in World War II. He was diagnosed with multiple myeloma in 2013 and revealed his condition publicly in 2014.
In 2016, he described his treatment in a New York Times commentary: “...three years of chemotherapy, a spinal operation that cost me three inches of height, monthly infusions of bone supplements, and drugs to prevent respiratory infection.” He also described fatigue, bone damage, and a 24-pill-a-day regimen.
In his presentation at ASH, Mr. Brokaw detailed the adjustment of having to slow down after an active life as a cyclist and outdoorsman. “I’m not going to go down the street with a cane. My birth certificate says I’m 78 years old, but I still think I’m 38, anchoring the news.”
“There was so much concentration on the disease itself that I don’t think I got as much as I needed regarding the radiant effects.”
At one point, he fell while running with his dog, and developed an infection in a cavity in his elbow. Still, he refused to cancel a flight to Washington, D.C., for an interview with the secretary of defense. The infection got worse, soaking his shirt with leakage, and when he returned “they slammed me into intensive care.”
He got a stern instruction that “you can’t do this anymore,” and he responded with an “ohh-kay.”
“It’s the anchorman in me. You get used to doing what you want to do. But I have to be much more careful about what I do and when I do it,” he said.
Now, Mr. Brokaw still struggles to follow advice about risks such as flying. But he remains active as a speaker, a special correspondent for NBC, and an author. “By and large,” he said, “I’m getting along OK. I’m grateful for that.”
EXPERT ANALYSIS FROM ASH 2018
Don’t push women into preterm delivery after myomectomy
LAS VEGAS –
The American College of Obstetricians and Gynecologists lists prior myomectomy as a medically-indicated reason for delivery before 39 weeks. The advice reflects a traditional concern that uterine scars will rupture during labor, with potentially devastating consequences for both mother and infant.
Reviews have put the risk at less than 1%, so ob.gyns. have shied away from ACOG’s blanket advice and now use uterine-cavity entry during myomectomy as their talisman for deciding whether or not to offer women vaginal delivery. The assumption is that uterine entry makes rupture more likely, but there’s not much evidence to support that idea, and it’s become clear in recent years that women who have a significant full-thickness insult to uterine integrity – a prior C-section – can usually deliver vaginally with no problem. In short, the uterus seems to have a remarkable ability to heal itself.
Even so, there are still ob.gyns. who pressure women into having premature babies if they’ve had a fibroid removed even without cavity entry. Barring additional indications, that doesn’t happen anymore at Northwestern University, said lead investigator Nathan King, MD, an ob.gyn. resident at the university.
The Northwestern team wanted to clear the fog. What they found adds to “literature that demonstrates the overall low risk of undergoing VTOL [vaginal trial of labor] after a prior myomectomy. We hope providers will feel more comfortable talking to their patients about delivery [options] and the success of VTOL after myomectomy,” Dr. King said at a meeting sponsored by AAGL.*
He and his team analyzed pregnancy outcomes in 112 women who had a live birth after non–cavity-entering myomectomies. Forty-nine women (44%) were allowed to undergo VTOL; 63 others had C-sections, most at term.
Thirty-two VTOL women (65%) had vaginal deliveries, a success rate similar to that of labor after C-section. There was just one uterine rupture in the VTOL group, for an incidence of 2%, which also was comparable to the rupture risk after a low-transverse C-section.
The rupture was discovered after spontaneous vaginal delivery, and an addressed by laparotomy. Both mother and infant were fine.
Adverse events were less likely in the VTOL group, regardless if they ultimately delivered vaginally or by C-section. The lower adverse event rate was driven by fewer postpartum hemorrhages (odds ratio, 0.441, 95% confidence interval, 0.2002-0.9722, P = .042).
There were no demographic difference between women who were allowed to undergo VTOL and those who were not. For most, it was their first delivery.
Women who had their uterine cavities entered during myomectomy weren’t allowed to undergo VTOL at Northwestern, and were not included in the analysis. Also, the study did not include women who became pregnant after myomectomy, but did not have a live delivery. The incidence of uterine rupture among them, if any, was not reported.
There was no external funding for the work, and Dr. King didn’t have any disclosures.
SOURCE: King N et al. 2018 AAGL Global Congress, Abstract 162.
*Correction, 12/11/2018: An earlier version of this story misstated the name of the meeting sponsor. It is AAGL.
LAS VEGAS –
The American College of Obstetricians and Gynecologists lists prior myomectomy as a medically-indicated reason for delivery before 39 weeks. The advice reflects a traditional concern that uterine scars will rupture during labor, with potentially devastating consequences for both mother and infant.
Reviews have put the risk at less than 1%, so ob.gyns. have shied away from ACOG’s blanket advice and now use uterine-cavity entry during myomectomy as their talisman for deciding whether or not to offer women vaginal delivery. The assumption is that uterine entry makes rupture more likely, but there’s not much evidence to support that idea, and it’s become clear in recent years that women who have a significant full-thickness insult to uterine integrity – a prior C-section – can usually deliver vaginally with no problem. In short, the uterus seems to have a remarkable ability to heal itself.
Even so, there are still ob.gyns. who pressure women into having premature babies if they’ve had a fibroid removed even without cavity entry. Barring additional indications, that doesn’t happen anymore at Northwestern University, said lead investigator Nathan King, MD, an ob.gyn. resident at the university.
The Northwestern team wanted to clear the fog. What they found adds to “literature that demonstrates the overall low risk of undergoing VTOL [vaginal trial of labor] after a prior myomectomy. We hope providers will feel more comfortable talking to their patients about delivery [options] and the success of VTOL after myomectomy,” Dr. King said at a meeting sponsored by AAGL.*
He and his team analyzed pregnancy outcomes in 112 women who had a live birth after non–cavity-entering myomectomies. Forty-nine women (44%) were allowed to undergo VTOL; 63 others had C-sections, most at term.
Thirty-two VTOL women (65%) had vaginal deliveries, a success rate similar to that of labor after C-section. There was just one uterine rupture in the VTOL group, for an incidence of 2%, which also was comparable to the rupture risk after a low-transverse C-section.
The rupture was discovered after spontaneous vaginal delivery, and an addressed by laparotomy. Both mother and infant were fine.
Adverse events were less likely in the VTOL group, regardless if they ultimately delivered vaginally or by C-section. The lower adverse event rate was driven by fewer postpartum hemorrhages (odds ratio, 0.441, 95% confidence interval, 0.2002-0.9722, P = .042).
There were no demographic difference between women who were allowed to undergo VTOL and those who were not. For most, it was their first delivery.
Women who had their uterine cavities entered during myomectomy weren’t allowed to undergo VTOL at Northwestern, and were not included in the analysis. Also, the study did not include women who became pregnant after myomectomy, but did not have a live delivery. The incidence of uterine rupture among them, if any, was not reported.
There was no external funding for the work, and Dr. King didn’t have any disclosures.
SOURCE: King N et al. 2018 AAGL Global Congress, Abstract 162.
*Correction, 12/11/2018: An earlier version of this story misstated the name of the meeting sponsor. It is AAGL.
LAS VEGAS –
The American College of Obstetricians and Gynecologists lists prior myomectomy as a medically-indicated reason for delivery before 39 weeks. The advice reflects a traditional concern that uterine scars will rupture during labor, with potentially devastating consequences for both mother and infant.
Reviews have put the risk at less than 1%, so ob.gyns. have shied away from ACOG’s blanket advice and now use uterine-cavity entry during myomectomy as their talisman for deciding whether or not to offer women vaginal delivery. The assumption is that uterine entry makes rupture more likely, but there’s not much evidence to support that idea, and it’s become clear in recent years that women who have a significant full-thickness insult to uterine integrity – a prior C-section – can usually deliver vaginally with no problem. In short, the uterus seems to have a remarkable ability to heal itself.
Even so, there are still ob.gyns. who pressure women into having premature babies if they’ve had a fibroid removed even without cavity entry. Barring additional indications, that doesn’t happen anymore at Northwestern University, said lead investigator Nathan King, MD, an ob.gyn. resident at the university.
The Northwestern team wanted to clear the fog. What they found adds to “literature that demonstrates the overall low risk of undergoing VTOL [vaginal trial of labor] after a prior myomectomy. We hope providers will feel more comfortable talking to their patients about delivery [options] and the success of VTOL after myomectomy,” Dr. King said at a meeting sponsored by AAGL.*
He and his team analyzed pregnancy outcomes in 112 women who had a live birth after non–cavity-entering myomectomies. Forty-nine women (44%) were allowed to undergo VTOL; 63 others had C-sections, most at term.
Thirty-two VTOL women (65%) had vaginal deliveries, a success rate similar to that of labor after C-section. There was just one uterine rupture in the VTOL group, for an incidence of 2%, which also was comparable to the rupture risk after a low-transverse C-section.
The rupture was discovered after spontaneous vaginal delivery, and an addressed by laparotomy. Both mother and infant were fine.
Adverse events were less likely in the VTOL group, regardless if they ultimately delivered vaginally or by C-section. The lower adverse event rate was driven by fewer postpartum hemorrhages (odds ratio, 0.441, 95% confidence interval, 0.2002-0.9722, P = .042).
There were no demographic difference between women who were allowed to undergo VTOL and those who were not. For most, it was their first delivery.
Women who had their uterine cavities entered during myomectomy weren’t allowed to undergo VTOL at Northwestern, and were not included in the analysis. Also, the study did not include women who became pregnant after myomectomy, but did not have a live delivery. The incidence of uterine rupture among them, if any, was not reported.
There was no external funding for the work, and Dr. King didn’t have any disclosures.
SOURCE: King N et al. 2018 AAGL Global Congress, Abstract 162.
*Correction, 12/11/2018: An earlier version of this story misstated the name of the meeting sponsor. It is AAGL.
REPORTING FROM AAGL GLOBAL CONGRESS
Key clinical point: Vaginal trial of labor is safe after myomectomy, at least if the uterine cavity wasn’t entered.
Major finding: Sixty-five percent of women who didn’t have their uterine cavities entered had vaginal deliveries, a success rate similar to labor after C-section.
Study details: Review of 102 pregnancies with live births after myomectomy at Northwestern University, Chicago
Disclosures: There was no external funding, and the lead investigator didn’t have any disclosures.
Source: King N et al. 2018 AAGL Global Congress, Abstract 162.
The Role of the Medical Consultant in 2018: Putting It All Together
Whenever the principles of effective medical consultation are discussed, a classic article published in 1983 by Lee Goldman et al. is invariably referenced. In the “Ten Commandments for Effective Consultation,” Goldman argued that internists should “determine the question, establish urgency, look for yourself, be as brief as appropriate, be specific, provide contingency plans, honor thy turf, teach with tact, provide direct personal contact, and follow up.”1 If these Ten Commandments were followed, then the consultation would be more effective and satisfactory for both the consultant and the referring provider. However, with the advent of comanagement in 1994 where internists and surgeons have a “shared responsibility and accountability,”2 there has been a shift, and the once-concrete definitions of a specific reason for consult and the nature of “turf” have become blurred. Since 1994, the use of medical consultation and comanagement has skyrocketed, and today, more than 50% of surgical patients have a medical consultation or comanagement.3 This may be due to increased time pressures on surgeons and better outcomes of comanaged patients (eg, fewer postoperative complications, fewer transfers to an intensive care unit for acute medical deterioration, and increased likelihood to discharge to home).4
Medical management of surgical patients in the hospital involves a different skill set than that required to manage general medical patients. Accordingly, in 2012, the Accreditation Council for Graduate Medical Education (ACGME) made medical consultation and perioperative care an End of Training Entrustable Professional Activities and ACGME subcompetency. Earlier this year, a nationwide perioperative curriculum for graduate medical education was consisting of eight objective and core topic modules and pretest/posttest questions selected from SHMConsults.com, including assessment and management of perioperative cardiac and pulmonary risk and management of diabetes, perioperative fever, and anticoagulants. Trainees were assessed using the multiple-choice questions, observed mini-cex, and written evaluation of a consultation report. Despite this encouraging development of curricula and competencies for trainees, there are still important gaps in our knowledge of basic patterns for consultation practices. For example, the type of patients and medical conditions currently encountered on our medical consultation and comanagement services had been previously unknown.
In the December issue of the Journal of Hospital Medicine, Wang et al. answer this question through the first cross-sectional multicenter prospective survey to examine medical consultation/comanagement practices since observational studies in the 1970-1990s.6 In a sample of 1,264 consultation requests from 11 academic medical centers over four two-week periods from July 2014 through July 2015, they found that the most common requests for consultation were medical management/comanagement, preoperative evaluation, blood pressure management, and other common postoperative complications, including postoperative atrial fibrillation, heart failure, renal failure, hyponatremia, anemia, hypoxia, and altered mental status.9 The majority of referrals were from orthopedic surgery and neurosurgery. They also found that medical consultants and comanagers provided comprehensive evaluations where more than a third of encounters addressed issues that were not stated in the initial reason for consult (RFC) and that consultants addressed more than two RFCs per encounter.9
These findings illustrate the paradigm shift of medical consultation focusing on a single specific question to addressing and optimizing the entire patient. This shift toward a broader, more open-ended reason for consultation may present some challenges such as “dumping” where referring surgeons and other specialists signoff their patients after surgery is completed, with internists processing the surgeons’ patients through the hospitalization. These challenges can be mitigated with predefined comanagement agreements with clearly defined roles and collaborative professional relationships.
Nonetheless, given the recent developments in curricula and training competencies mentioned above, internists are better equipped than ever before to put everything together and take care of the medical conditions of the increasingly complex and older surgical patient. For example, if one is consulted to see a patient for postoperative hypertension, it is difficult to not address the patient’s blood sugars in the 300s, lack of venous thromboembolism prophylaxis, delirium, acute renal failure, and acute blood loss anemia. The authors are correct to assert it is critically important to ensure that this input is desired by the referring physician either via verbal communication or comanagement agreements.
The findings of Wang et al. suggest some important future steps in medical consultation to ensure that our trainees and colleagues are prepared to take care of the entire patient regardless of whether the patient is on a consultant or comanagement agreement. This study shows that trainees are exposed to a diverse clinical experience on our medical consultation and comanagement services, which is in accordance with the objectives, assessment tools, and modules of the nationwide curriculum. It is likely that comanagement services will continue to expand as more of our medically complex patients will need either elective or emergency surgeries and surgeons have become less comfortable managing these patients on their own. We also may be asked to participate in quality improvement initiatives in the management of surgical patients, including the “perioperative surgical home programs,” where physicians work on a patient-centered approach to the surgical patient using evidence-based standard clinical care pathways and transitions from before surgery to postdischarge.7 We should share our experiences in quality improvement and the patient-centered medical home to ensure that our patients are optimized for surgery and beyond. As Lee Goldman et al. stated in the “Ten Commandments for Effective Consultations,1” consultative medicine is an important part of an internal medicine practice. Today, more than ever, the consultant or comanagement role or roles
1. Goldman L, Lee T, Rudd P. Ten commandments for effective consultations. Arch Intern Med. 1983;143(9):1753-1755. PubMed
2. Macpherson DS, Parenti C, Nee J, et al. An internist joins the surgery service: does comanagement make a difference? J Gen Intern Med 1994;9:440-446. PubMed
3. Chen, LM, Wilk, AS, Thumma, JR et al. Use of medical consultants for hospitalized surgical patients. An observational cohort study. JAMA Intern Med. 2014;174(9):1470-1477. doi: 10.1001/jamainternmed.2014.3376. PubMed
4. Kammerlander C, Roth T, Friedman SM, et al. Ortho-geriatric service–a literature review comparing different models. Osteoporos Int. 2010;21(Suppl 4):S637-S646. doi: 10.1007/s00198-010-1396-x. PubMed
5. Fang M, O’Glasser A, Sahai S, Pfeifer K, Johnson KM, Kuperman E. Development of a nationwide consensus curriculum of perioperative medicine: a modified Delphi method. Periop Care Oper Room Manag. 2018;12:31-34. doi: 10.1016/j.pcorm.2018.09.002.
6. Wang ES, Moreland C, Shoffeitt M, Leykum LK. Who consults us and why? An evaluation of medicine consult/co-management services at academic medical centers. J Hosp Med. 2018;12(4):840-843. doi: 10.12788/jhm.3010. PubMed
7. Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118(5):1126-1130. doi: 10.1213/ANE.0000000000000190. PubMed
Whenever the principles of effective medical consultation are discussed, a classic article published in 1983 by Lee Goldman et al. is invariably referenced. In the “Ten Commandments for Effective Consultation,” Goldman argued that internists should “determine the question, establish urgency, look for yourself, be as brief as appropriate, be specific, provide contingency plans, honor thy turf, teach with tact, provide direct personal contact, and follow up.”1 If these Ten Commandments were followed, then the consultation would be more effective and satisfactory for both the consultant and the referring provider. However, with the advent of comanagement in 1994 where internists and surgeons have a “shared responsibility and accountability,”2 there has been a shift, and the once-concrete definitions of a specific reason for consult and the nature of “turf” have become blurred. Since 1994, the use of medical consultation and comanagement has skyrocketed, and today, more than 50% of surgical patients have a medical consultation or comanagement.3 This may be due to increased time pressures on surgeons and better outcomes of comanaged patients (eg, fewer postoperative complications, fewer transfers to an intensive care unit for acute medical deterioration, and increased likelihood to discharge to home).4
Medical management of surgical patients in the hospital involves a different skill set than that required to manage general medical patients. Accordingly, in 2012, the Accreditation Council for Graduate Medical Education (ACGME) made medical consultation and perioperative care an End of Training Entrustable Professional Activities and ACGME subcompetency. Earlier this year, a nationwide perioperative curriculum for graduate medical education was consisting of eight objective and core topic modules and pretest/posttest questions selected from SHMConsults.com, including assessment and management of perioperative cardiac and pulmonary risk and management of diabetes, perioperative fever, and anticoagulants. Trainees were assessed using the multiple-choice questions, observed mini-cex, and written evaluation of a consultation report. Despite this encouraging development of curricula and competencies for trainees, there are still important gaps in our knowledge of basic patterns for consultation practices. For example, the type of patients and medical conditions currently encountered on our medical consultation and comanagement services had been previously unknown.
In the December issue of the Journal of Hospital Medicine, Wang et al. answer this question through the first cross-sectional multicenter prospective survey to examine medical consultation/comanagement practices since observational studies in the 1970-1990s.6 In a sample of 1,264 consultation requests from 11 academic medical centers over four two-week periods from July 2014 through July 2015, they found that the most common requests for consultation were medical management/comanagement, preoperative evaluation, blood pressure management, and other common postoperative complications, including postoperative atrial fibrillation, heart failure, renal failure, hyponatremia, anemia, hypoxia, and altered mental status.9 The majority of referrals were from orthopedic surgery and neurosurgery. They also found that medical consultants and comanagers provided comprehensive evaluations where more than a third of encounters addressed issues that were not stated in the initial reason for consult (RFC) and that consultants addressed more than two RFCs per encounter.9
These findings illustrate the paradigm shift of medical consultation focusing on a single specific question to addressing and optimizing the entire patient. This shift toward a broader, more open-ended reason for consultation may present some challenges such as “dumping” where referring surgeons and other specialists signoff their patients after surgery is completed, with internists processing the surgeons’ patients through the hospitalization. These challenges can be mitigated with predefined comanagement agreements with clearly defined roles and collaborative professional relationships.
Nonetheless, given the recent developments in curricula and training competencies mentioned above, internists are better equipped than ever before to put everything together and take care of the medical conditions of the increasingly complex and older surgical patient. For example, if one is consulted to see a patient for postoperative hypertension, it is difficult to not address the patient’s blood sugars in the 300s, lack of venous thromboembolism prophylaxis, delirium, acute renal failure, and acute blood loss anemia. The authors are correct to assert it is critically important to ensure that this input is desired by the referring physician either via verbal communication or comanagement agreements.
The findings of Wang et al. suggest some important future steps in medical consultation to ensure that our trainees and colleagues are prepared to take care of the entire patient regardless of whether the patient is on a consultant or comanagement agreement. This study shows that trainees are exposed to a diverse clinical experience on our medical consultation and comanagement services, which is in accordance with the objectives, assessment tools, and modules of the nationwide curriculum. It is likely that comanagement services will continue to expand as more of our medically complex patients will need either elective or emergency surgeries and surgeons have become less comfortable managing these patients on their own. We also may be asked to participate in quality improvement initiatives in the management of surgical patients, including the “perioperative surgical home programs,” where physicians work on a patient-centered approach to the surgical patient using evidence-based standard clinical care pathways and transitions from before surgery to postdischarge.7 We should share our experiences in quality improvement and the patient-centered medical home to ensure that our patients are optimized for surgery and beyond. As Lee Goldman et al. stated in the “Ten Commandments for Effective Consultations,1” consultative medicine is an important part of an internal medicine practice. Today, more than ever, the consultant or comanagement role or roles
Whenever the principles of effective medical consultation are discussed, a classic article published in 1983 by Lee Goldman et al. is invariably referenced. In the “Ten Commandments for Effective Consultation,” Goldman argued that internists should “determine the question, establish urgency, look for yourself, be as brief as appropriate, be specific, provide contingency plans, honor thy turf, teach with tact, provide direct personal contact, and follow up.”1 If these Ten Commandments were followed, then the consultation would be more effective and satisfactory for both the consultant and the referring provider. However, with the advent of comanagement in 1994 where internists and surgeons have a “shared responsibility and accountability,”2 there has been a shift, and the once-concrete definitions of a specific reason for consult and the nature of “turf” have become blurred. Since 1994, the use of medical consultation and comanagement has skyrocketed, and today, more than 50% of surgical patients have a medical consultation or comanagement.3 This may be due to increased time pressures on surgeons and better outcomes of comanaged patients (eg, fewer postoperative complications, fewer transfers to an intensive care unit for acute medical deterioration, and increased likelihood to discharge to home).4
Medical management of surgical patients in the hospital involves a different skill set than that required to manage general medical patients. Accordingly, in 2012, the Accreditation Council for Graduate Medical Education (ACGME) made medical consultation and perioperative care an End of Training Entrustable Professional Activities and ACGME subcompetency. Earlier this year, a nationwide perioperative curriculum for graduate medical education was consisting of eight objective and core topic modules and pretest/posttest questions selected from SHMConsults.com, including assessment and management of perioperative cardiac and pulmonary risk and management of diabetes, perioperative fever, and anticoagulants. Trainees were assessed using the multiple-choice questions, observed mini-cex, and written evaluation of a consultation report. Despite this encouraging development of curricula and competencies for trainees, there are still important gaps in our knowledge of basic patterns for consultation practices. For example, the type of patients and medical conditions currently encountered on our medical consultation and comanagement services had been previously unknown.
In the December issue of the Journal of Hospital Medicine, Wang et al. answer this question through the first cross-sectional multicenter prospective survey to examine medical consultation/comanagement practices since observational studies in the 1970-1990s.6 In a sample of 1,264 consultation requests from 11 academic medical centers over four two-week periods from July 2014 through July 2015, they found that the most common requests for consultation were medical management/comanagement, preoperative evaluation, blood pressure management, and other common postoperative complications, including postoperative atrial fibrillation, heart failure, renal failure, hyponatremia, anemia, hypoxia, and altered mental status.9 The majority of referrals were from orthopedic surgery and neurosurgery. They also found that medical consultants and comanagers provided comprehensive evaluations where more than a third of encounters addressed issues that were not stated in the initial reason for consult (RFC) and that consultants addressed more than two RFCs per encounter.9
These findings illustrate the paradigm shift of medical consultation focusing on a single specific question to addressing and optimizing the entire patient. This shift toward a broader, more open-ended reason for consultation may present some challenges such as “dumping” where referring surgeons and other specialists signoff their patients after surgery is completed, with internists processing the surgeons’ patients through the hospitalization. These challenges can be mitigated with predefined comanagement agreements with clearly defined roles and collaborative professional relationships.
Nonetheless, given the recent developments in curricula and training competencies mentioned above, internists are better equipped than ever before to put everything together and take care of the medical conditions of the increasingly complex and older surgical patient. For example, if one is consulted to see a patient for postoperative hypertension, it is difficult to not address the patient’s blood sugars in the 300s, lack of venous thromboembolism prophylaxis, delirium, acute renal failure, and acute blood loss anemia. The authors are correct to assert it is critically important to ensure that this input is desired by the referring physician either via verbal communication or comanagement agreements.
The findings of Wang et al. suggest some important future steps in medical consultation to ensure that our trainees and colleagues are prepared to take care of the entire patient regardless of whether the patient is on a consultant or comanagement agreement. This study shows that trainees are exposed to a diverse clinical experience on our medical consultation and comanagement services, which is in accordance with the objectives, assessment tools, and modules of the nationwide curriculum. It is likely that comanagement services will continue to expand as more of our medically complex patients will need either elective or emergency surgeries and surgeons have become less comfortable managing these patients on their own. We also may be asked to participate in quality improvement initiatives in the management of surgical patients, including the “perioperative surgical home programs,” where physicians work on a patient-centered approach to the surgical patient using evidence-based standard clinical care pathways and transitions from before surgery to postdischarge.7 We should share our experiences in quality improvement and the patient-centered medical home to ensure that our patients are optimized for surgery and beyond. As Lee Goldman et al. stated in the “Ten Commandments for Effective Consultations,1” consultative medicine is an important part of an internal medicine practice. Today, more than ever, the consultant or comanagement role or roles
1. Goldman L, Lee T, Rudd P. Ten commandments for effective consultations. Arch Intern Med. 1983;143(9):1753-1755. PubMed
2. Macpherson DS, Parenti C, Nee J, et al. An internist joins the surgery service: does comanagement make a difference? J Gen Intern Med 1994;9:440-446. PubMed
3. Chen, LM, Wilk, AS, Thumma, JR et al. Use of medical consultants for hospitalized surgical patients. An observational cohort study. JAMA Intern Med. 2014;174(9):1470-1477. doi: 10.1001/jamainternmed.2014.3376. PubMed
4. Kammerlander C, Roth T, Friedman SM, et al. Ortho-geriatric service–a literature review comparing different models. Osteoporos Int. 2010;21(Suppl 4):S637-S646. doi: 10.1007/s00198-010-1396-x. PubMed
5. Fang M, O’Glasser A, Sahai S, Pfeifer K, Johnson KM, Kuperman E. Development of a nationwide consensus curriculum of perioperative medicine: a modified Delphi method. Periop Care Oper Room Manag. 2018;12:31-34. doi: 10.1016/j.pcorm.2018.09.002.
6. Wang ES, Moreland C, Shoffeitt M, Leykum LK. Who consults us and why? An evaluation of medicine consult/co-management services at academic medical centers. J Hosp Med. 2018;12(4):840-843. doi: 10.12788/jhm.3010. PubMed
7. Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118(5):1126-1130. doi: 10.1213/ANE.0000000000000190. PubMed
1. Goldman L, Lee T, Rudd P. Ten commandments for effective consultations. Arch Intern Med. 1983;143(9):1753-1755. PubMed
2. Macpherson DS, Parenti C, Nee J, et al. An internist joins the surgery service: does comanagement make a difference? J Gen Intern Med 1994;9:440-446. PubMed
3. Chen, LM, Wilk, AS, Thumma, JR et al. Use of medical consultants for hospitalized surgical patients. An observational cohort study. JAMA Intern Med. 2014;174(9):1470-1477. doi: 10.1001/jamainternmed.2014.3376. PubMed
4. Kammerlander C, Roth T, Friedman SM, et al. Ortho-geriatric service–a literature review comparing different models. Osteoporos Int. 2010;21(Suppl 4):S637-S646. doi: 10.1007/s00198-010-1396-x. PubMed
5. Fang M, O’Glasser A, Sahai S, Pfeifer K, Johnson KM, Kuperman E. Development of a nationwide consensus curriculum of perioperative medicine: a modified Delphi method. Periop Care Oper Room Manag. 2018;12:31-34. doi: 10.1016/j.pcorm.2018.09.002.
6. Wang ES, Moreland C, Shoffeitt M, Leykum LK. Who consults us and why? An evaluation of medicine consult/co-management services at academic medical centers. J Hosp Med. 2018;12(4):840-843. doi: 10.12788/jhm.3010. PubMed
7. Kain ZN, Vakharia S, Garson L, et al. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118(5):1126-1130. doi: 10.1213/ANE.0000000000000190. PubMed
© 2019 Society of Hospital Medicine
Decreased insulin clearance and insulin resistance
Also today, tender joint count may confound assessment of RA inflammation, common AEDs confer moderately increased risk of major congenital malformations, and the ACR and the NPF unveil new treatment guidelines for psoriatic arthritis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, tender joint count may confound assessment of RA inflammation, common AEDs confer moderately increased risk of major congenital malformations, and the ACR and the NPF unveil new treatment guidelines for psoriatic arthritis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, tender joint count may confound assessment of RA inflammation, common AEDs confer moderately increased risk of major congenital malformations, and the ACR and the NPF unveil new treatment guidelines for psoriatic arthritis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Lymphodepletion improves efficacy of CAR T cells in HL
SAN DIEGO—A phase 1 study suggests lymphodepletion can improve the efficacy of CD30-directed chimeric antigen receptor (CAR) T-cell therapy in patients with Hodgkin lymphoma (HL).
Researchers observed improved responses in HL patients treated with fludarabine and cyclophosphamide prior to CD30.CAR T-cell therapy.
This lymphodepleting regimen was also associated with increased toxicity, compared to no lymphodepletion. However, researchers consider the regimen safe.
Carlos A. Ramos, MD, of Baylor College of Medicine in Houston, Texas, presented these results at the 2018 ASH Annual Meeting (abstract 680*).
Without lymphodepletion
Dr. Ramos first discussed a previous phase 1 trial (NCT01316146), which was published in The Journal of Clinical Investigation in 2017.
In this trial, he and his colleagues had tested CD30.CAR T-cell therapy in patients with relapsed/refractory, CD30+ HL or T-cell non-Hodgkin lymphoma. None of these patients underwent lymphodepletion.
There were no dose-limiting toxicities in this trial—including no neurotoxicity or cytokine release syndrome—but responses were “limited,” according to Dr. Ramos.
Three patients achieved a complete response (CR), three had stable disease, and three progressed.
“Although we saw no significant toxicities and some good clinical responses . . ., the bottom line is that the responses were still quite limited, with several patients having, at most, stable disease or progressive disease,” Dr. Ramos said.
With lymphodepletion
Results from the previous trial prompted Dr. Ramos and his colleagues to conduct the RELY-30 trial (NCT02917083) and investigate whether lymphodepletion would improve responses to CD30.CAR T-cell therapy.
Thus far, 11 patients have been treated on this trial. All had relapsed, CD30+ HL at baseline. Six patients are male, and their median age at baseline was 30 (range, 17-69).
The patients had a median of 5 prior treatments (range, 2-9). This included PD-1 inhibitors (n=10), brentuximab vedotin (n=8), and transplant (n=6).
All patients received lymphodepletion with cyclophosphamide at 500 mg/m2 and fludarabine at 30 mg/m2 daily for 3 days. They then received CD30.CAR T-cell therapy at 2×107 cells/m2 or 1×108 cells/m2.
Dr. Ramos noted that CD30.CAR T-cell expansion was dose-dependent and increased by lymphodepleting chemotherapy.
“The peak expansion is much higher [with lymphodepletion], probably in the order of two to three logs higher than what we see without lymphodepleting chemotherapy,” he said. “So chemotherapy makes a difference.”
Increased CD30.CAR T-cell expansion was associated with improved response. Of the nine evaluable patients, six achieved a CR, and three progressed.
Four complete responders were still in CR at last follow-up, one of them for more than a year. However, two complete responders ultimately progressed.
In addition to improved responses, the researchers observed increased toxicity in this trial. Dr. Ramos said some of these toxicities are “probably attributable” to the lymphodepleting chemotherapy.
Toxicities included grade 1 cytokine release syndrome (no tocilizumab required), maculopapular rash, transient cytopenias, nausea, vomiting, and alopecia.
Dr. Ramos said these results suggest adoptive transfer of CD30.CAR T cells is “safe, even with chemotherapy.”
He noted that the duration of response with this treatment is unknown, but trial enrollment and follow-up are ongoing.
RELY-30 was sponsored by Baylor College of Medicine. Dr. Ramos reported relationships with Novartis, Celgene, Bluebird Bio, and Tessa Therapeutics.
*Data in the abstract differ from the presentation.
SAN DIEGO—A phase 1 study suggests lymphodepletion can improve the efficacy of CD30-directed chimeric antigen receptor (CAR) T-cell therapy in patients with Hodgkin lymphoma (HL).
Researchers observed improved responses in HL patients treated with fludarabine and cyclophosphamide prior to CD30.CAR T-cell therapy.
This lymphodepleting regimen was also associated with increased toxicity, compared to no lymphodepletion. However, researchers consider the regimen safe.
Carlos A. Ramos, MD, of Baylor College of Medicine in Houston, Texas, presented these results at the 2018 ASH Annual Meeting (abstract 680*).
Without lymphodepletion
Dr. Ramos first discussed a previous phase 1 trial (NCT01316146), which was published in The Journal of Clinical Investigation in 2017.
In this trial, he and his colleagues had tested CD30.CAR T-cell therapy in patients with relapsed/refractory, CD30+ HL or T-cell non-Hodgkin lymphoma. None of these patients underwent lymphodepletion.
There were no dose-limiting toxicities in this trial—including no neurotoxicity or cytokine release syndrome—but responses were “limited,” according to Dr. Ramos.
Three patients achieved a complete response (CR), three had stable disease, and three progressed.
“Although we saw no significant toxicities and some good clinical responses . . ., the bottom line is that the responses were still quite limited, with several patients having, at most, stable disease or progressive disease,” Dr. Ramos said.
With lymphodepletion
Results from the previous trial prompted Dr. Ramos and his colleagues to conduct the RELY-30 trial (NCT02917083) and investigate whether lymphodepletion would improve responses to CD30.CAR T-cell therapy.
Thus far, 11 patients have been treated on this trial. All had relapsed, CD30+ HL at baseline. Six patients are male, and their median age at baseline was 30 (range, 17-69).
The patients had a median of 5 prior treatments (range, 2-9). This included PD-1 inhibitors (n=10), brentuximab vedotin (n=8), and transplant (n=6).
All patients received lymphodepletion with cyclophosphamide at 500 mg/m2 and fludarabine at 30 mg/m2 daily for 3 days. They then received CD30.CAR T-cell therapy at 2×107 cells/m2 or 1×108 cells/m2.
Dr. Ramos noted that CD30.CAR T-cell expansion was dose-dependent and increased by lymphodepleting chemotherapy.
“The peak expansion is much higher [with lymphodepletion], probably in the order of two to three logs higher than what we see without lymphodepleting chemotherapy,” he said. “So chemotherapy makes a difference.”
Increased CD30.CAR T-cell expansion was associated with improved response. Of the nine evaluable patients, six achieved a CR, and three progressed.
Four complete responders were still in CR at last follow-up, one of them for more than a year. However, two complete responders ultimately progressed.
In addition to improved responses, the researchers observed increased toxicity in this trial. Dr. Ramos said some of these toxicities are “probably attributable” to the lymphodepleting chemotherapy.
Toxicities included grade 1 cytokine release syndrome (no tocilizumab required), maculopapular rash, transient cytopenias, nausea, vomiting, and alopecia.
Dr. Ramos said these results suggest adoptive transfer of CD30.CAR T cells is “safe, even with chemotherapy.”
He noted that the duration of response with this treatment is unknown, but trial enrollment and follow-up are ongoing.
RELY-30 was sponsored by Baylor College of Medicine. Dr. Ramos reported relationships with Novartis, Celgene, Bluebird Bio, and Tessa Therapeutics.
*Data in the abstract differ from the presentation.
SAN DIEGO—A phase 1 study suggests lymphodepletion can improve the efficacy of CD30-directed chimeric antigen receptor (CAR) T-cell therapy in patients with Hodgkin lymphoma (HL).
Researchers observed improved responses in HL patients treated with fludarabine and cyclophosphamide prior to CD30.CAR T-cell therapy.
This lymphodepleting regimen was also associated with increased toxicity, compared to no lymphodepletion. However, researchers consider the regimen safe.
Carlos A. Ramos, MD, of Baylor College of Medicine in Houston, Texas, presented these results at the 2018 ASH Annual Meeting (abstract 680*).
Without lymphodepletion
Dr. Ramos first discussed a previous phase 1 trial (NCT01316146), which was published in The Journal of Clinical Investigation in 2017.
In this trial, he and his colleagues had tested CD30.CAR T-cell therapy in patients with relapsed/refractory, CD30+ HL or T-cell non-Hodgkin lymphoma. None of these patients underwent lymphodepletion.
There were no dose-limiting toxicities in this trial—including no neurotoxicity or cytokine release syndrome—but responses were “limited,” according to Dr. Ramos.
Three patients achieved a complete response (CR), three had stable disease, and three progressed.
“Although we saw no significant toxicities and some good clinical responses . . ., the bottom line is that the responses were still quite limited, with several patients having, at most, stable disease or progressive disease,” Dr. Ramos said.
With lymphodepletion
Results from the previous trial prompted Dr. Ramos and his colleagues to conduct the RELY-30 trial (NCT02917083) and investigate whether lymphodepletion would improve responses to CD30.CAR T-cell therapy.
Thus far, 11 patients have been treated on this trial. All had relapsed, CD30+ HL at baseline. Six patients are male, and their median age at baseline was 30 (range, 17-69).
The patients had a median of 5 prior treatments (range, 2-9). This included PD-1 inhibitors (n=10), brentuximab vedotin (n=8), and transplant (n=6).
All patients received lymphodepletion with cyclophosphamide at 500 mg/m2 and fludarabine at 30 mg/m2 daily for 3 days. They then received CD30.CAR T-cell therapy at 2×107 cells/m2 or 1×108 cells/m2.
Dr. Ramos noted that CD30.CAR T-cell expansion was dose-dependent and increased by lymphodepleting chemotherapy.
“The peak expansion is much higher [with lymphodepletion], probably in the order of two to three logs higher than what we see without lymphodepleting chemotherapy,” he said. “So chemotherapy makes a difference.”
Increased CD30.CAR T-cell expansion was associated with improved response. Of the nine evaluable patients, six achieved a CR, and three progressed.
Four complete responders were still in CR at last follow-up, one of them for more than a year. However, two complete responders ultimately progressed.
In addition to improved responses, the researchers observed increased toxicity in this trial. Dr. Ramos said some of these toxicities are “probably attributable” to the lymphodepleting chemotherapy.
Toxicities included grade 1 cytokine release syndrome (no tocilizumab required), maculopapular rash, transient cytopenias, nausea, vomiting, and alopecia.
Dr. Ramos said these results suggest adoptive transfer of CD30.CAR T cells is “safe, even with chemotherapy.”
He noted that the duration of response with this treatment is unknown, but trial enrollment and follow-up are ongoing.
RELY-30 was sponsored by Baylor College of Medicine. Dr. Ramos reported relationships with Novartis, Celgene, Bluebird Bio, and Tessa Therapeutics.
*Data in the abstract differ from the presentation.
Fludarabine deemed important for CD30.CAR T-cell therapy
SAN DIEGO—Fludarabine is “very important” for lymphodepletion prior to CD30-directed chimeric antigen receptor (CAR) T-cell therapy, according to a presentation at the 2018 ASH Annual Meeting.
A phase 1/2 study showed that bendamustine alone was not sufficient as lymphodepletion.
However, adding fludarabine to bendamustine could enhance responses to CD30.CAR T-cell therapy and improve progression-free survival (PFS) in patients with Hodgkin or non-Hodgkin lymphoma.
Natalie S. Grover, MD, of the University of North Carolina in Chapel Hill, presented these results as abstract 681.*
This trial (NCT02690545) included patients with relapsed/refractory, CD30+ Hodgkin lymphoma or T-cell non-Hodgkin lymphoma.
Twenty-four adult patients have been treated thus far. Twenty-two had classical Hodgkin lymphoma, one had Sézary syndrome, and one had enteropathy-associated T-cell lymphoma.
The patients’ median age at baseline was 34.5 years (range, 23-69), and they had received a median of 7.5 prior lines of therapy (range, 3-17).
Prior treatments included brentuximab vedotin (n=23), checkpoint inhibitors (n=16), autologous transplant (n=17), and allogeneic transplant (n=7).
In this trial, patients could receive bridging therapy while their T cells were being processed. They then underwent lymphodepletion and received CAR T-cell therapy at one of two doses.
Bendamustine alone
Eight patients received lymphodepletion with 2 days of bendamustine at 90 mg/m2. Three of these patients received CD30.CAR T-cell therapy at 1×108 cells/m2, and all three progressed.
Of the five patients who received CAR T-cell therapy at a dose of 2×108 cells/m2, one progressed, one had stable disease, and three had a complete response (CR).
However, all three complete responders were in CR prior to lymphodepletion as a result of bridging therapy.
“Responses were more modest than what we were hoping for with lymphodepletion,” Dr. Grover noted. “We looked at the cytokine levels in patients getting bendamustine lymphodepletion and saw that bendamustine wasn’t supporting an ideal cytokine milieu. IL-7 and IL-15 are important for T-cell expansion, and these levels were not increased in patients post-bendamustine.”
When the researchers added fludarabine to the lymphodepleting regimen, they observed an increase in T-cell expansion.
Bendamustine plus fludarabine
Sixteen patients received bendamustine plus fludarabine prior to CAR T-cell therapy. The regimen consisted of 3 days of bendamustine at 70 mg/m2 and fludarabine at 30 mg/m2.
All 16 patients received CAR T cells at 2×108 cells/m2, which was the recommended phase 2 dose.
“Responses were more impressive in the bendamustine-fludarabine cohort,” Dr. Grover noted.
Twelve of the 16 patients achieved a CR, although two patients were already in CR prior to lymphodepletion.
Two patients had a partial response, one had stable disease, and one progressed.
PFS and toxicity
Dr. Grover and her colleagues also assessed PFS. At a median follow-up of 100 days, the median PFS was 164 days for the entire cohort, excluding patients who were in CR prior to lymphodepletion.
The median PFS was 396 days for the bendamustine-fludarabine cohort and 55 days for patients in the bendamustine-alone cohort (P=0.001).
There was no neurotoxicity in this trial.
Three patients developed cytokine release syndrome (CRS). Two patients had grade 1 CRS that resolved spontaneously, and one patient had grade 2 CRS, which responded to tocilizumab. Two of the patients with CRS had T-cell lymphoma. The Sézary patient had grade 2 CRS.
Eight patients had a mild rash, one of whom had a rash at baseline.
“CAR T cells against CD30 preceded by lymphodepletion with bendamustine and fludarabine have promising efficacy and a good safety profile in treating patients with relapsed/refractory, CD30+ lymphomas,” Dr. Grover said in closing.
“Fludarabine is very important in enhancing cytokines for improved growth and persistence of CAR T cells.”
This trial was sponsored by UNC Lineberger Comprehensive Cancer Center. Dr. Grover reported consulting for Seattle Genetics.
*Data in the abstract differ from the presentation.
SAN DIEGO—Fludarabine is “very important” for lymphodepletion prior to CD30-directed chimeric antigen receptor (CAR) T-cell therapy, according to a presentation at the 2018 ASH Annual Meeting.
A phase 1/2 study showed that bendamustine alone was not sufficient as lymphodepletion.
However, adding fludarabine to bendamustine could enhance responses to CD30.CAR T-cell therapy and improve progression-free survival (PFS) in patients with Hodgkin or non-Hodgkin lymphoma.
Natalie S. Grover, MD, of the University of North Carolina in Chapel Hill, presented these results as abstract 681.*
This trial (NCT02690545) included patients with relapsed/refractory, CD30+ Hodgkin lymphoma or T-cell non-Hodgkin lymphoma.
Twenty-four adult patients have been treated thus far. Twenty-two had classical Hodgkin lymphoma, one had Sézary syndrome, and one had enteropathy-associated T-cell lymphoma.
The patients’ median age at baseline was 34.5 years (range, 23-69), and they had received a median of 7.5 prior lines of therapy (range, 3-17).
Prior treatments included brentuximab vedotin (n=23), checkpoint inhibitors (n=16), autologous transplant (n=17), and allogeneic transplant (n=7).
In this trial, patients could receive bridging therapy while their T cells were being processed. They then underwent lymphodepletion and received CAR T-cell therapy at one of two doses.
Bendamustine alone
Eight patients received lymphodepletion with 2 days of bendamustine at 90 mg/m2. Three of these patients received CD30.CAR T-cell therapy at 1×108 cells/m2, and all three progressed.
Of the five patients who received CAR T-cell therapy at a dose of 2×108 cells/m2, one progressed, one had stable disease, and three had a complete response (CR).
However, all three complete responders were in CR prior to lymphodepletion as a result of bridging therapy.
“Responses were more modest than what we were hoping for with lymphodepletion,” Dr. Grover noted. “We looked at the cytokine levels in patients getting bendamustine lymphodepletion and saw that bendamustine wasn’t supporting an ideal cytokine milieu. IL-7 and IL-15 are important for T-cell expansion, and these levels were not increased in patients post-bendamustine.”
When the researchers added fludarabine to the lymphodepleting regimen, they observed an increase in T-cell expansion.
Bendamustine plus fludarabine
Sixteen patients received bendamustine plus fludarabine prior to CAR T-cell therapy. The regimen consisted of 3 days of bendamustine at 70 mg/m2 and fludarabine at 30 mg/m2.
All 16 patients received CAR T cells at 2×108 cells/m2, which was the recommended phase 2 dose.
“Responses were more impressive in the bendamustine-fludarabine cohort,” Dr. Grover noted.
Twelve of the 16 patients achieved a CR, although two patients were already in CR prior to lymphodepletion.
Two patients had a partial response, one had stable disease, and one progressed.
PFS and toxicity
Dr. Grover and her colleagues also assessed PFS. At a median follow-up of 100 days, the median PFS was 164 days for the entire cohort, excluding patients who were in CR prior to lymphodepletion.
The median PFS was 396 days for the bendamustine-fludarabine cohort and 55 days for patients in the bendamustine-alone cohort (P=0.001).
There was no neurotoxicity in this trial.
Three patients developed cytokine release syndrome (CRS). Two patients had grade 1 CRS that resolved spontaneously, and one patient had grade 2 CRS, which responded to tocilizumab. Two of the patients with CRS had T-cell lymphoma. The Sézary patient had grade 2 CRS.
Eight patients had a mild rash, one of whom had a rash at baseline.
“CAR T cells against CD30 preceded by lymphodepletion with bendamustine and fludarabine have promising efficacy and a good safety profile in treating patients with relapsed/refractory, CD30+ lymphomas,” Dr. Grover said in closing.
“Fludarabine is very important in enhancing cytokines for improved growth and persistence of CAR T cells.”
This trial was sponsored by UNC Lineberger Comprehensive Cancer Center. Dr. Grover reported consulting for Seattle Genetics.
*Data in the abstract differ from the presentation.
SAN DIEGO—Fludarabine is “very important” for lymphodepletion prior to CD30-directed chimeric antigen receptor (CAR) T-cell therapy, according to a presentation at the 2018 ASH Annual Meeting.
A phase 1/2 study showed that bendamustine alone was not sufficient as lymphodepletion.
However, adding fludarabine to bendamustine could enhance responses to CD30.CAR T-cell therapy and improve progression-free survival (PFS) in patients with Hodgkin or non-Hodgkin lymphoma.
Natalie S. Grover, MD, of the University of North Carolina in Chapel Hill, presented these results as abstract 681.*
This trial (NCT02690545) included patients with relapsed/refractory, CD30+ Hodgkin lymphoma or T-cell non-Hodgkin lymphoma.
Twenty-four adult patients have been treated thus far. Twenty-two had classical Hodgkin lymphoma, one had Sézary syndrome, and one had enteropathy-associated T-cell lymphoma.
The patients’ median age at baseline was 34.5 years (range, 23-69), and they had received a median of 7.5 prior lines of therapy (range, 3-17).
Prior treatments included brentuximab vedotin (n=23), checkpoint inhibitors (n=16), autologous transplant (n=17), and allogeneic transplant (n=7).
In this trial, patients could receive bridging therapy while their T cells were being processed. They then underwent lymphodepletion and received CAR T-cell therapy at one of two doses.
Bendamustine alone
Eight patients received lymphodepletion with 2 days of bendamustine at 90 mg/m2. Three of these patients received CD30.CAR T-cell therapy at 1×108 cells/m2, and all three progressed.
Of the five patients who received CAR T-cell therapy at a dose of 2×108 cells/m2, one progressed, one had stable disease, and three had a complete response (CR).
However, all three complete responders were in CR prior to lymphodepletion as a result of bridging therapy.
“Responses were more modest than what we were hoping for with lymphodepletion,” Dr. Grover noted. “We looked at the cytokine levels in patients getting bendamustine lymphodepletion and saw that bendamustine wasn’t supporting an ideal cytokine milieu. IL-7 and IL-15 are important for T-cell expansion, and these levels were not increased in patients post-bendamustine.”
When the researchers added fludarabine to the lymphodepleting regimen, they observed an increase in T-cell expansion.
Bendamustine plus fludarabine
Sixteen patients received bendamustine plus fludarabine prior to CAR T-cell therapy. The regimen consisted of 3 days of bendamustine at 70 mg/m2 and fludarabine at 30 mg/m2.
All 16 patients received CAR T cells at 2×108 cells/m2, which was the recommended phase 2 dose.
“Responses were more impressive in the bendamustine-fludarabine cohort,” Dr. Grover noted.
Twelve of the 16 patients achieved a CR, although two patients were already in CR prior to lymphodepletion.
Two patients had a partial response, one had stable disease, and one progressed.
PFS and toxicity
Dr. Grover and her colleagues also assessed PFS. At a median follow-up of 100 days, the median PFS was 164 days for the entire cohort, excluding patients who were in CR prior to lymphodepletion.
The median PFS was 396 days for the bendamustine-fludarabine cohort and 55 days for patients in the bendamustine-alone cohort (P=0.001).
There was no neurotoxicity in this trial.
Three patients developed cytokine release syndrome (CRS). Two patients had grade 1 CRS that resolved spontaneously, and one patient had grade 2 CRS, which responded to tocilizumab. Two of the patients with CRS had T-cell lymphoma. The Sézary patient had grade 2 CRS.
Eight patients had a mild rash, one of whom had a rash at baseline.
“CAR T cells against CD30 preceded by lymphodepletion with bendamustine and fludarabine have promising efficacy and a good safety profile in treating patients with relapsed/refractory, CD30+ lymphomas,” Dr. Grover said in closing.
“Fludarabine is very important in enhancing cytokines for improved growth and persistence of CAR T cells.”
This trial was sponsored by UNC Lineberger Comprehensive Cancer Center. Dr. Grover reported consulting for Seattle Genetics.
*Data in the abstract differ from the presentation.
MetS after HSCT linked to CV events, second cancers
SAN DIEGO—Patients who develop metabolic syndrome (MetS) after hematopoietic stem cell transplant (HSCT) have an increased risk of cardiovascular (CV) events and second malignancies, according to research presented at the 2018 ASH Annual Meeting.
Researchers found that HSCT recipients had a higher prevalence of MetS than the general population, and the incidence of MetS increased with age.
In addition, MetS was a predictor of CV events, and these events were associated with second malignancy.
“Our data support metabolic syndrome being an age-related late effect of transplant that is strongly associated with not only cardiovascular events but also the occurrence of second cancers, and this is a novel finding,” said Diana M. Greenfield, RN, PhD, of Sheffield Teaching Hospitals NHS Foundation Trust in Sheffield, United Kingdom.
Dr. Greenfield presented the data at ASH as abstract 251.
She noted that previous studies have reported rates of MetS after HSCT ranging from 31% to 49%. Intensive chemotherapy and radiation have been associated with MetS, and proposed mechanisms of MetS after HSCT include:
- Conditioning regimen-mediated damage to the neuro-hormonal system and vascular endothelium
- Immunological and inflammatory effects of allo-grafting, including graft-vs-host disease (GVHD) and treatment.
“Those with metabolic syndrome in the general population are already known to be twice as likely to develop cardiovascular disease as those without metabolic syndrome,” Dr. Greenfield noted. “The risk of cardiovascular hospitalizations and mortality is 3.6-fold higher in transplant patients vs the general population.”
With all this in mind, she and her colleagues set out to determine the prevalence of MetS and associated complications among HSCT recipients treated at 9 European centers in Belgium, France, Germany, Turkey, and the United Kingdom.
The researchers analyzed 462 patients—375 who had received an allogeneic HSCT and 87 who had undergone autologous HSCT. All patients were at least 18 years of age and at least 2 years post-HSCT.
The median age at transplant was 43 (interquartile range, 32-53), and 57.4% of patients were male.
Patients’ diagnoses included acute leukemia (37.9%), lymphoma (27.3%), myelodysplastic syndromes/myeloproliferative neoplasms (13%), chronic leukemia (12.3%), plasma cell disorders (5%), bone marrow failure (4.1%), solid tumors (0.2%), and inherited disorders (0.2%).
Results
The prevalence of MetS in this population was 30.4%. This is higher than the prevalence in the general population in Europe, which is 25%, according to the World Health Organization.
There was no significant difference in MetS prevalence between allogeneic and autologous HSCT recipients—29% and 35.6%, respectively.
However, there was a significant difference in MetS prevalence by age (P<0.001 with increasing age).
Among allogeneic HSCT recipients, there was no relationship between MetS prevalence and the presence or degree of acute or chronic GVHD, current use of immunosuppressive therapy, or conditioning intensity.
There was a significantly higher prevalence of CV events in patients with MetS than in patients without the syndrome—22.6% and 10.7%, respectively (P=0.006).
And logistic regression analysis confirmed that MetS is a predictor of CV events (odds ratio [OR]=4.72; 95% confidence interval [CI], 2.11-10.57).
The researchers also found that increasing age influenced the prevalence of MetS (OR=7.3; 95% CI, 3.2-16.8) and CV events (OR=3; 95% CI, 0.8-11.32) for patients older than 50, compared to patients in the 18-29 age group.
And CV events were associated with second malignancy (OR=7.93; 95% CI, 2.91-21.61).
“Early intervention of reversible features of metabolic syndrome with lifestyle and medical management may reduce the risk of cardiovascular events,” Dr. Greenfield said. “Meanwhile, screening and management should be robustly integrated within routine transplant long-term follow-up care.”
She added that the CIBMTR and EBMT guidelines on MetS and CV disease after HSCT should help in that regard.
Dr. Greenfield reported no conflicts of interest. However, her fellow researchers reported relationships with a range of pharmaceutical companies.
SAN DIEGO—Patients who develop metabolic syndrome (MetS) after hematopoietic stem cell transplant (HSCT) have an increased risk of cardiovascular (CV) events and second malignancies, according to research presented at the 2018 ASH Annual Meeting.
Researchers found that HSCT recipients had a higher prevalence of MetS than the general population, and the incidence of MetS increased with age.
In addition, MetS was a predictor of CV events, and these events were associated with second malignancy.
“Our data support metabolic syndrome being an age-related late effect of transplant that is strongly associated with not only cardiovascular events but also the occurrence of second cancers, and this is a novel finding,” said Diana M. Greenfield, RN, PhD, of Sheffield Teaching Hospitals NHS Foundation Trust in Sheffield, United Kingdom.
Dr. Greenfield presented the data at ASH as abstract 251.
She noted that previous studies have reported rates of MetS after HSCT ranging from 31% to 49%. Intensive chemotherapy and radiation have been associated with MetS, and proposed mechanisms of MetS after HSCT include:
- Conditioning regimen-mediated damage to the neuro-hormonal system and vascular endothelium
- Immunological and inflammatory effects of allo-grafting, including graft-vs-host disease (GVHD) and treatment.
“Those with metabolic syndrome in the general population are already known to be twice as likely to develop cardiovascular disease as those without metabolic syndrome,” Dr. Greenfield noted. “The risk of cardiovascular hospitalizations and mortality is 3.6-fold higher in transplant patients vs the general population.”
With all this in mind, she and her colleagues set out to determine the prevalence of MetS and associated complications among HSCT recipients treated at 9 European centers in Belgium, France, Germany, Turkey, and the United Kingdom.
The researchers analyzed 462 patients—375 who had received an allogeneic HSCT and 87 who had undergone autologous HSCT. All patients were at least 18 years of age and at least 2 years post-HSCT.
The median age at transplant was 43 (interquartile range, 32-53), and 57.4% of patients were male.
Patients’ diagnoses included acute leukemia (37.9%), lymphoma (27.3%), myelodysplastic syndromes/myeloproliferative neoplasms (13%), chronic leukemia (12.3%), plasma cell disorders (5%), bone marrow failure (4.1%), solid tumors (0.2%), and inherited disorders (0.2%).
Results
The prevalence of MetS in this population was 30.4%. This is higher than the prevalence in the general population in Europe, which is 25%, according to the World Health Organization.
There was no significant difference in MetS prevalence between allogeneic and autologous HSCT recipients—29% and 35.6%, respectively.
However, there was a significant difference in MetS prevalence by age (P<0.001 with increasing age).
Among allogeneic HSCT recipients, there was no relationship between MetS prevalence and the presence or degree of acute or chronic GVHD, current use of immunosuppressive therapy, or conditioning intensity.
There was a significantly higher prevalence of CV events in patients with MetS than in patients without the syndrome—22.6% and 10.7%, respectively (P=0.006).
And logistic regression analysis confirmed that MetS is a predictor of CV events (odds ratio [OR]=4.72; 95% confidence interval [CI], 2.11-10.57).
The researchers also found that increasing age influenced the prevalence of MetS (OR=7.3; 95% CI, 3.2-16.8) and CV events (OR=3; 95% CI, 0.8-11.32) for patients older than 50, compared to patients in the 18-29 age group.
And CV events were associated with second malignancy (OR=7.93; 95% CI, 2.91-21.61).
“Early intervention of reversible features of metabolic syndrome with lifestyle and medical management may reduce the risk of cardiovascular events,” Dr. Greenfield said. “Meanwhile, screening and management should be robustly integrated within routine transplant long-term follow-up care.”
She added that the CIBMTR and EBMT guidelines on MetS and CV disease after HSCT should help in that regard.
Dr. Greenfield reported no conflicts of interest. However, her fellow researchers reported relationships with a range of pharmaceutical companies.
SAN DIEGO—Patients who develop metabolic syndrome (MetS) after hematopoietic stem cell transplant (HSCT) have an increased risk of cardiovascular (CV) events and second malignancies, according to research presented at the 2018 ASH Annual Meeting.
Researchers found that HSCT recipients had a higher prevalence of MetS than the general population, and the incidence of MetS increased with age.
In addition, MetS was a predictor of CV events, and these events were associated with second malignancy.
“Our data support metabolic syndrome being an age-related late effect of transplant that is strongly associated with not only cardiovascular events but also the occurrence of second cancers, and this is a novel finding,” said Diana M. Greenfield, RN, PhD, of Sheffield Teaching Hospitals NHS Foundation Trust in Sheffield, United Kingdom.
Dr. Greenfield presented the data at ASH as abstract 251.
She noted that previous studies have reported rates of MetS after HSCT ranging from 31% to 49%. Intensive chemotherapy and radiation have been associated with MetS, and proposed mechanisms of MetS after HSCT include:
- Conditioning regimen-mediated damage to the neuro-hormonal system and vascular endothelium
- Immunological and inflammatory effects of allo-grafting, including graft-vs-host disease (GVHD) and treatment.
“Those with metabolic syndrome in the general population are already known to be twice as likely to develop cardiovascular disease as those without metabolic syndrome,” Dr. Greenfield noted. “The risk of cardiovascular hospitalizations and mortality is 3.6-fold higher in transplant patients vs the general population.”
With all this in mind, she and her colleagues set out to determine the prevalence of MetS and associated complications among HSCT recipients treated at 9 European centers in Belgium, France, Germany, Turkey, and the United Kingdom.
The researchers analyzed 462 patients—375 who had received an allogeneic HSCT and 87 who had undergone autologous HSCT. All patients were at least 18 years of age and at least 2 years post-HSCT.
The median age at transplant was 43 (interquartile range, 32-53), and 57.4% of patients were male.
Patients’ diagnoses included acute leukemia (37.9%), lymphoma (27.3%), myelodysplastic syndromes/myeloproliferative neoplasms (13%), chronic leukemia (12.3%), plasma cell disorders (5%), bone marrow failure (4.1%), solid tumors (0.2%), and inherited disorders (0.2%).
Results
The prevalence of MetS in this population was 30.4%. This is higher than the prevalence in the general population in Europe, which is 25%, according to the World Health Organization.
There was no significant difference in MetS prevalence between allogeneic and autologous HSCT recipients—29% and 35.6%, respectively.
However, there was a significant difference in MetS prevalence by age (P<0.001 with increasing age).
Among allogeneic HSCT recipients, there was no relationship between MetS prevalence and the presence or degree of acute or chronic GVHD, current use of immunosuppressive therapy, or conditioning intensity.
There was a significantly higher prevalence of CV events in patients with MetS than in patients without the syndrome—22.6% and 10.7%, respectively (P=0.006).
And logistic regression analysis confirmed that MetS is a predictor of CV events (odds ratio [OR]=4.72; 95% confidence interval [CI], 2.11-10.57).
The researchers also found that increasing age influenced the prevalence of MetS (OR=7.3; 95% CI, 3.2-16.8) and CV events (OR=3; 95% CI, 0.8-11.32) for patients older than 50, compared to patients in the 18-29 age group.
And CV events were associated with second malignancy (OR=7.93; 95% CI, 2.91-21.61).
“Early intervention of reversible features of metabolic syndrome with lifestyle and medical management may reduce the risk of cardiovascular events,” Dr. Greenfield said. “Meanwhile, screening and management should be robustly integrated within routine transplant long-term follow-up care.”
She added that the CIBMTR and EBMT guidelines on MetS and CV disease after HSCT should help in that regard.
Dr. Greenfield reported no conflicts of interest. However, her fellow researchers reported relationships with a range of pharmaceutical companies.
Preoperative Corticosteroid Use for Medical Conditions is Associated with Increased Postoperative Infectious Complications and Readmissions After Total Hip Arthroplasty: A Propensity-Matched Study
ABSTRACT
Systemic corticosteroids are used to treat a number of medical conditions; however, they are associated with numerous adverse effects. The impact of preoperative chronic corticosteroid use on postoperative outcomes following total hip arthroplasty (THA) is unclear. The purpose of this study was to assess the independent effect of chronic systemic preoperative steroid use on short-term perioperative complications and readmissions after THA.
All patients undergoing primary THA in the American College of Surgeons National Surgical Quality Improvement Program registry from 2005 to -–2015 were identified. Patients were considered chronic steroid users if they used any dosage of oral or parenteral steroids for >10 of the preceding 30 days before THA. Two equally sized propensity-matched groups based on preoperative steroid use were generated to account for differences in operative and baseline characteristics between the groups. Thirty-day complications and hospital readmissions rates were compared using bivariate analysis.
Of 101,532 THA patients who underwent primary THA, 3714 (3.7%) were identified as chronic corticosteroid users. Comparison of propensity-matched cohorts identified an increased rate of any complication (odds ratio [OR] 1.30, P = .003), sepsis (OR 2.07, P = .022), urinary tract infection (OR 1.61, P = .020), superficial surgical site infection (OR 1.73, P = .038), and hospital readmission (OR 1.50, P < .001) in patients who used systemic steroids preoperatively. Readmissions in preoperative steroid users were most commonly for infectious reasons.
Patients prescribed chronic corticosteroids are at a significantly increased risk of both 30-day periopative complications and hospital readmissions. This finding has important implications for pre- and postoperative patient counseling as well as preoperative risk stratification.
Continue to: Corticosteroids are powerful...
Corticosteroids are powerful anti-inflammatory steroid hormones that have many indications in the treatment of medical diseases, including advanced or poorly controlled asthma, chronic obstructive pulmonary disease (COPD), inflammatory bowel disease, allergic conditions, among other indications.1-4 In orthopedics and rheumatology, systemic steroids are, at times, used in patients with rheumatoid arthritis, systemic lupus erythematosus, and vasculitides.5-7 Overman and colleagues,8 using data from the National Health and Nutrition Examination Survey between 1999 and 2008 identified both a 1.2% prevalence of chronic corticosteroid usage in the United States across all age groups and a positive correlation between steroid use prevalence and increasing age. In that study, nearly two-thirds of survey respondents reported using corticosteroids chronically for >90 days. Another observational study in the United Kingdom found that long-term steroid prescriptions increased between 1989 to 2008 and that 13.6% of patients with rheumatoid arthritis and 66.5% of patients with polymyalgia rheumatica or giant cell arteritis used long-term steroids.9
Enterally- or parenterally-administered corticosteroids have numerous systemic effects that are of particular relevance to orthopedic surgeons. Corticosteroids induce osteoporosis by preferentially inducing osteoclastic activity while inhibiting the differentiation of osteoblasts, ultimately leading to decreased bone quality and mass.10 As a consequence, patients who have previously used corticosteroids are more than twice as likely to have a hip fracture.11 Steroids also increase the risk of both osteonecrosis and myopathy, among other musculoskeletal effects.12 In addition to orthopedic complications, steroids have broad inhibitory effects on both acquired and innate immunity, which significantly increases the risk of infections.13 This increased risk of infection is dose-dependent14 and synergistic with other immunosuppressive drugs.15
Patients with hip pain may receive localized corticosteroid hip joint injections during the nonoperative management of various hip pathologies, including arthritis, bursitis, and labral tears.16,17 Outcomes of patients who received intra-articular corticosteroid injections before total hip arthroplasty (THA) were evaluated in a systematic review of 9 studies by Pereira and colleagues.17 These authors found that the infection rate (both superficial and deep surgical site infections [SSI]) after THA in patients who received local steroid injection into the hip before surgery was between 0% and 30%.17 However, similar studies assessing the impact that systemic steroids have on outcomes after THA are lacking. Patients who undergo THA for conditions associated with higher lifetime steroid usage have worse outcomes than those who do not. For instance, in patients undergoing THA for rheumatoid arthritis, the rates of both postoperative periprosthetic joint infection and hip dislocation are higher, when compared with osteoarthritis.18,19 However, it is unclear how much of this difference in outcomes is due to the underlying disease, adverse effects of steroids, or both. Given the high prevalence of chronic systemic steroid use, it is essential to elucidate more clearly the impact that these medications have on perioperative outcomes after THA.
Therefore, the purpose of this study was to characterize short-term perioperative outcomes, including complication and readmission rates in patients undergoing THA while taking chronic preoperative corticosteroids. We also sought to identify the most common reasons for hospital readmission in patients who did and did not use long-term steroids.
MATERIALS AND METHODS
STUDY DESIGN AND SETTING
This investigation was a retrospective cohort study that utilized the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) registry.20 The ACS-NSQIP is a prospectively collected, multi-institutional database that collects demographical information, operative variables, and both postoperative complications and hospital readmission data. Data is collected for up to 30 days after the index procedure, and patients are contacted by telephone if they are discharged before 30 days. Patient data is entered by specially trained surgical clinical reviewers and is routinely audited by the ACS-NSQIP, leading to more accurate data when compared with administrative research databases.21,22 The ACS-NSQIP has been used in orthopedic surgery outcomes-based studies.23-25
Continue to: All patients undergoing...
All patients undergoing THA between 2005 and 2015 were identified in the registry using primary Current Procedural Terminology code 27130. Patients were split into 2 groups based on whether or not they chronically used corticosteroids preoperatively for a medical condition. A patient was considered a chronic corticosteroid user if he/she used oral or parenteral corticosteroids within 30 days before the index procedure for >10 of the preceding 30 days. Those who received a 1-time steroid pulse or those who used topical or inhaled steroids were not considered as steroid users in this study.
BASELINE CHARACTERISTICS AND PERIOPERATIVE OUTCOMES
Baseline patient and operative characteristics, including patient age, gender, body mass index (BMI), functional status, American Society of Anesthesiologists (ASA) class, anesthesia type, operative duration, and medical comorbidities including hypertension, COPD, diabetes mellitus, and smoking history, were compared between both groups. Perioperative outcomes that were assessed in this study include death, renal, respiratory, and cardiac complications, deep vein thrombosis or pulmonary embolism, stroke, sepsis, return to the operating room, urinary tract infection (UTI), wound dehiscence, superficial and deep SSI, need for a blood transfusion within 72 hours of index surgical procedure, and hospital readmissions. Renal complications were defined as acute or progressive renal insufficiency; respiratory complications were defined as failure to wean from the ventilator, need for intubation after the index procedure, and the occurrence of pneumonia; and cardiac complications were defined as myocardial infarction or cardiac arrest requiring cardiopulmonary resuscitation. Patients were excluded if they had missing baseline or operative characteristic data, an unclean wound classification at the time of admission, or if their THA was considered emergent.
STATISTICAL ANALYSIS
A propensity score-matched comparison was performed to adjust for differences in baseline and operative characteristics between the 2 cohorts in this study. In the current study, the propensity score was defined as the conditional probability that a patient chronically used preoperative corticosteroids for a medical condition, as a function of age, BMI, gender, ASA class, functional status, medical comorbidities, anesthesia type, and operative duration. A 1:1 matching with tight calipers (0.0001), and nearest-neighbor matching was used to generate 2 equally-sized, propensity-matched cohorts based on steroid status.26 Nearest-neighbor matching identifies patients in both cohorts with the closest propensity scores for inclusion in propensity-matched cohorts. This matching is continued until 1 group runs out of patients to match. Baseline patient and operative characteristics for the unadjusted and propensity-matched groups were compared using Pearson’s χ2 analysis. Outcomes after THA by steroid status were also compared in both unadjusted and propensity-matched groups. Finally, all patients who were readmitted were identified, and the reason for readmission was determined using the International Classification of Disease Ninth (ICD-9) and Tenth (ICD-10) edition codes. Patients were classified as having an infectious readmission only if the ICD code clearly stated an infectious etiology. For instance, a patient with an intestinal infection due to Clostridium difficile (ICD-9 008.45) was counted as a gastrointestinal infection, whereas diarrhea without a distinctly specified etiology (ICD-9 787.91, ICD-10 R19.7) was counted as a gastrointestinal medical complication. Readmission data was only available in ACS-NSQIP from 2011 to 2015, constituting 92.5% of all patients included in this study. We used SPSS version 23 (IBM Corporation) for all statistical analyses, and defined a significant P value as <.05.
RESULTS
BASELINE PATIENTS AND OPERATIVE CHARACTERISTICS
In total, we identified 101,532 patients who underwent THA (Table 1). O these, 3714 (3.7%) chronically used corticosteroids preoperatively, whereas 97,818 (96.3%) did not.
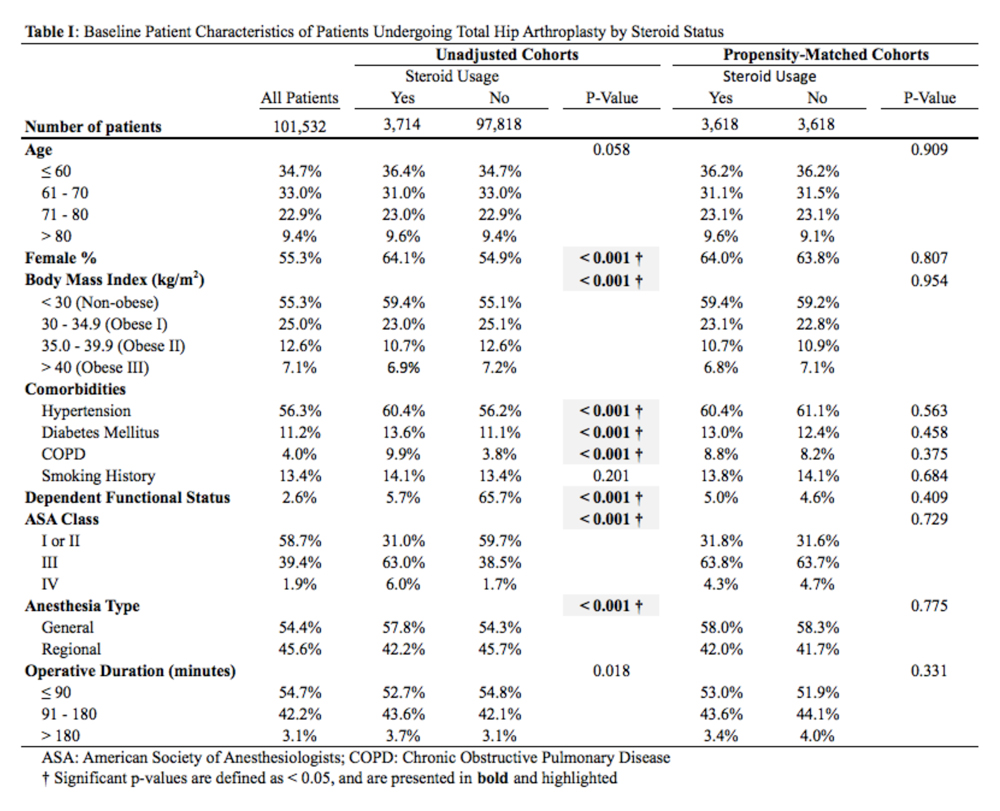
When the unadjusted cohorts were compared, patients using corticosteroids were more likely to be female, less likely to obese, more likely to have hypertension, diabetes mellitus, COPD, higher ASA class, undergone THA with general anesthesia, and have a dependent functional status (P < .001 for all comparisons). After propensity matching, 2 equally sized cohorts of 3618 patients each were generated based on steroid status and no differences in baseline and operative characteristics were identified between the 2 groups.
Continue to: CLINICAL OUTCOMES BY STEROID STATUS
CLINCIAL OUTCOMES BY STEROID STATUS
A comparison of unadjusted cohorts showed that patients who used preoperative steroids had an increased rate of any complication (7.89%) when compared with those who did not (4.87%) (Table 2).
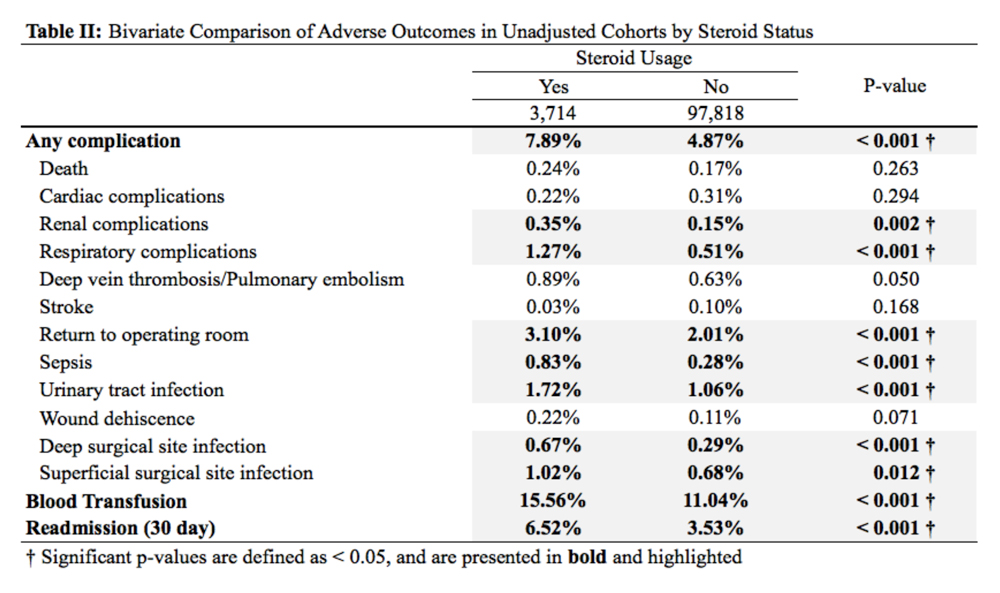
Similarly, those who used corticosteroids preoperatively had an increased rate of renal complications, respiratory complications, return to the operating room, sepsis, UTI, superficial and deep SSI, and perioperative blood transfusions. They also were more likely to have a 30-day hospital readmission (P < .05 for all comparisons).
When propensity-matched cohorts were compared, patients who used steroids preoperatively were found to have higher rates of any complication (odds Ratio [OR] 1.30, P = .003), sepsis (OR 2.07, P = .022), UTI (OR 1.61, P = .020), superficial SSI (OR 1.73, P = .038), and hospital readmission (OR 1.50, P < .001; Table 3).
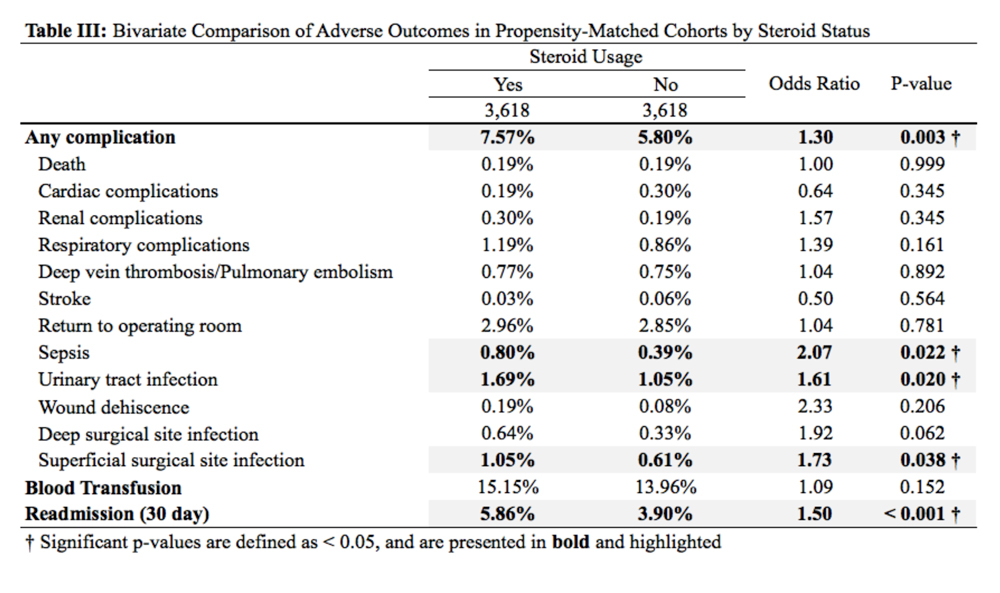
REASONS FOR HOSPITAL READMISSION
In total, 3397 patients were readmitted to the hospital within thirty days. Of these, 226 used steroids preoperatively, and 3171 did not (Table 4).
The most common reason for hospital readmission in patients who used preoperative corticosteroids was infectious complications (72 patients, 31.9% of all readmitted patients in this cohort), followed by medical complications (59 patients, 26.1%), and hip-related complications (48 patients, 21.2%). In those who did not use steroids preoperatively, the most common reason for hospital readmission was medical complications (932 patients, 29.4% of all readmitted patients in this cohort), followed by infectious complications (792 patients, 25.0%), and hip-related complications (763 patients, 24.1%).
Continue to: DISCUSSION
DISCUSSION
Nearly 3% of individuals >80 years in the US population chronically use corticosteroids for a medical condition,8 and this rate is likely higher in specific subsets of patients, such as those with rheumatoid arthritis.9 While some studies have assessed the impact of intra-articular corticosteroid hip injections on perioperative outcomes in THA,17 similar studies assessing systemic corticosteroid usage are lacking. The purpose of this study was to characterize short-term perioperative outcomes in patients undergoing THA who chronically use systemic steroids when compared with those who do not. We found that the prevalence of preoperative chronic steroid use in this cohort of THA patients was 3.7%. We also identified increased rates of infectious complications, including sepsis, UTI, and superficial SSI, in patients who used preoperative corticosteroids. Furthermore, we found an increased rate of hospital readmissions in corticosteroid users and identified the most common reason for hospital readmission as infectious complications in this cohort.
The primary finding of this study was an increase in postoperative infections in patients who use preoperative steroids chronically for medical conditions. Immunosuppression has previously been identified as a risk factor for developing periprosthetic joint infections. Tannenbaum and colleagues27 performed a retrospective study of 19 patients who underwent either a kidney or liver transplant and were maintained on an induction regimen of either prednisone and azathioprine or cyclosporine. These 19 patients also underwent either a THA or total knee arthroplasty, and 5 of these patients (26.3%) developed a periprosthetic joint infection after an average of 3.4 years following the arthroplasty procedure. In another study of 37 renal transplant and dialysis patients who underwent a total of 45 THA procedures, there were 3 instances of superficial SSI and 2 instances of deep SSI.28 However, reported infection rates in transplant patients undergoing THA vary significantly, and studies have been unable to assess the true impact that chronic immunosuppression has on perioperative infection rates.29 In this study, patients who used preoperative corticosteroids chronically were at increased risk of perioperative infections, including sepsis, UTI, and superficial SSI.
Deep vein thrombosis is another postoperative complication that has been associated with chronic steroid use.30 In a case-control study of 38,765 patients who developed a venous thromboembolism and 387,650 control patients who did not, Johannesdottir and colleagues30 found an increased thromboembolic risk in current users of systemic glucocorticoids, but not former users, as well as an increased risk as the dose of glucocorticoids increased. We were not able to identify a similar increase in DVT/PE in chronic corticosteroid users, perhaps due to our sample size, or because we could not do subgroup analyses based on the type or dosage of steroid that a patient was taking. Future studies that identify the highest risk patients among those using systemic corticosteroids are important because parenteral corticosteroids are being increasingly used in THA to alleviate postoperative pain as an opioid-sparing measure.31,32
Finally, we also found that patients who use chronic, systemic corticosteroids are at an increased risk for hospital readmission, when compared with those patients who are not using steroids and are most likely to be readmitted for an infectious complication. Schairer and colleagues33 assessed readmission rates after THA and found 30- and 90-day readmission rate of 4% and 7%, respectively. These authors also found that medical complications accounted for approximately 25% of readmissions, and hip-related complications (eg, dislocation, SSI) accounted for >50%. In our study, we found a 30-day readmission rate in non-steroid users of 3.53% and a rate of 6.52% in chronic steroid users. More than 30% of patients using a steroid were readmitted for infectious complications. As THA is becoming increasingly reimbursed under a bundled payments model by Medicare and Medicaid,34-36 reducing short-term readmissions is imperative. Therefore, discharge counseling that emphasizes how to recognize both the signs and symptoms of infection as well as how to prevent infections, such as reducing SSIs through appropriate wound care, may be warranted in higher risk chronic steroid users.
This study has a number of limitations that are inherent to ACS-NSQIP. First, we lacked specific information on a patient’s steroid history, including which corticosteroid they were using, dosage, frequency, and the indication for corticosteroid therapy. Therefore, we were unable to establish a dose-dependent relationship between steroid exposure and postoperative complications after THA. Second, we were able to assess only 30-day rates of complications and readmissions, and therefore, we were unable to identify intermediate- and long-term effects of systemic corticosteroid use on THA. Finally, we could not determine orthopedic- or hip-specific postoperative outcomes, such as functional scores and range of motion.
Continue to: CONCLUSION
CONCLUSION
In conclusion, this study quantified the increased risk for perioperative complications and hospital readmissions in patients who chronically use corticosteroids and are undergoing THA, when compared with those who do not use corticosteroids. These results suggest that patients who are on long-term steroids are at an increased risk for complications, primarily infectious complications. This finding has important implications for patient counseling, preoperative risk stratification, and suggests that higher risk patients, such as chronic steroid users, may benefit from improved discharge care to decrease complication rates.
1. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;13(5):CD011801. doi: 10.1002/14651858.CD011801.pub2.
2. Walters JA, Tan DJ, White CJ, Wood-Baker R. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(12):CD006897.
3. Nunes T, Barreiro-de Acosta M, Marin-Jimenez I, Nos P, Sans M. Oral locally active steroids in inflammatory bowel disease. J Crohns Colitis. 2013;7(3):183-191. doi: 10.1016/j.crohns.2012.06.010.
4. Karatzanis A, Chatzidakis A, Milioni A, Vlaminck S, Kawauchi H, Velegrakis S, et al. Contemporary use of corticosteroids in rhinology. Curr Allergy Asthm R. 2017;17(2). doi: 10.1007/s11882-017-0679-0.
5. Parker BJ, Bruce IN. High dose methylprednisolone therapy for the treatment of severe systemic lupus erythematosus. Lupus. 2007;16(6):387-393. doi: 10.1177/0961203307079502.
6. Ferreira JF, Ahmed Mohamed AA, Emery P. Glucocorticoids and rheumatoid arthritis. Rheum Dis Clin North Am. 2016;42(1):33-46. doi: 10.1016/j.rdc.2015.08.006.
7. Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA. 2016;315(22):2442-2458. doi: 10.1001/jama.2016.5444.
8. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res. 2013;65(2):294-298. doi: 10.1002/acr.21796.
9. Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology. 2011;50(11):1982-1990. doi: 10.1093/rheumatology/ker017.
10. Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy.Osteoporos Int. 2007;18(10):1319-1328. doi: 10.1007/s00198-007-0394-0.
11. Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton LJ, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19(6):893-899. doi: /10.1359/JBMR.040134.
12. Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76(1):1-9. doi: 10.1016/j.jaad.2016.01.062.
13. Cutolo M, Seriolo B, Pizzorni C, Secchi ME, Soldano S, Paolino S, et al. Use of glucocorticoids and risk of infections. Autoimmun Rev. 2008;8(2):153-155. doi: 10.1016/j.autrev.2008.07.010.
14. Blackwood LL, Pennington JE. Dose-dependent effect of glucocorticosteroids on pulmonary defenses in a steroid-resistant host. Am Rev Respir Dis. 1982;126(6):1045-1049.
15. Toruner M, Loftus EV, Jr., Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929-936. doi: 10.1053/j.gastro.2008.01.012.
16. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51(2):97-104. doi: 10.1136/bjsports-2015-095858.
17. Pereira LC, Kerr J, Jolles BM. Intra-articular steroid injection for osteoarthritis of the hip prior to total hip arthroplasty: is it safe? a systematic review. Bone Joint J. 2016;98-B(8):1027-1035. doi: 10.1302/0301-620X.98B8.37420.
18. Ravi B, Escott B, Shah PS, Jenkinson R, Chahal J, Bogoch E, et al. A systematic review and meta-analysis comparing complications following total joint arthroplasty for rheumatoid arthritis versus for osteoarthritis. Arthritis Rheum. 2012;64(12):3839-3849. doi: 10.1002/art.37690.
19. Ravi B, Croxford R, Hollands S, Paterson JM, Bogoch E, Kreder H, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi: 10.1002/art.38231.
20. ACS NSQIP Participant Use Data Files. https://www.facs.org/quality-programs/acs-nsqip/program-specifics/participant-use. Accessed December 6, 2018.
21. Lawson EH, Louie R, Zingmond DS, Brook RH, Hall BL, Han L, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981. doi: 10.1097/SLA.0b013e31826b4c4f.
22. Weiss A, Anderson JE, Chang DC. Comparing the national surgical quality improvement program with the nationwide inpatient sample database. JAMA Surg. 2015;150(8):815-816. doi: 10.1001/jamasurg.2015.0962.
23. Boddapati V, Fu MC, Mayman DJ, Su EP, Sculco PK, McLawhorn AS. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. J Arthroplasty. 2018;33(2):521-526. doi: 10.1016/j.arth.2017.09.021.
24. Boddapati V, Fu MC, Schairer WW, Gulotta LV, Dines DM, Dines JS. Revision total shoulder arthroplasty is associated with increased thirty-day postoperative complications and wound infections relative to primary total shoulder arthroplasty. HSS J. 2018;14(1):23-28. doi: 10.1007/s11420-017-9573-5.
25. Boddapati V, Fu MC, Schiarer WW, Ranawat AS, Dines DM, Taylor SA, Dines DM. Increased shoulder arthroscopy time is associated with overnight hospital stay and surgical site infection. Arthroscopy. 2018;34(2):363-368. doi: 10.1016/j.arthro.2017.08.243.
26. Lunt M. Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am J Epidemiol. 2014 Jan 15;179(2):226-235. doi: 10.1093/aje/kwt212.
27. Tannenbaum DA, Matthews LS, Grady-Benson JC. Infection around joint replacements in patients who have a renal or liver transplantation. J Bone Joint Surg Am. 1997;79(1):36-43.
28. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329. doi: 10.1016/j.arth.2005.07.008.
29. Nowicki P, Chaudhary H. Total hip replacement in renal transplant patients. J Bone Joint Surg Br. 2007;89(12):1561-1566.
30. Johannesdottir SA, Horváth-Puhó E, Dekkers OM, Cannegieter SC, Jørgensen JO, Ehrenstein V, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173(9):743-752. doi: 10.1001/jamainternmed.2013.122.
31. Hartman J, Khanna V, Habib A, Farrokhyar F, Memon M, Adili A. Perioperative systemic glucocorticoids in total hip and knee arthroplasty: a systematic review of outcomes. J Orthop. 2017;14(2):294-301. doi: 10.1016/j.jor.2017.03.012.
32. Sculco PK, McLawhorn AS, Desai N, Su EP, Padgett DE, Jules-Elysee K. The effect of perioperative corticosteroids in total hip arthroplasty: a prospective double-blind placebo controlled pilot study. J Arthroplasty. 2016;31(6):1208-1212. doi: 10.1016/j.arth.2015.11.011.
33. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470. doi: 10.1007/s11999-013-3121-5.
34. US Department of Health and Human Services. Comprehensive Care for Joint Replacement Model. Centers for Medicare & Medicaid Services. https://innovation.cms.gov/initiatives/cjr. Accessed June 15, 2017.
35. Bozic KJ, Ward L, Vail TP, Maze M. Bundled payments in total joint arthroplasty: targeting opportunities for quality improvement and cost reduction. Clin Orthop Relat Res. 2014;472(1):188-193. doi: 10.1007/s11999-013-3034-3.
36. Bosco JA, 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5): 903-905. doi: 10.1016/j.arth.2013.11.006.
ABSTRACT
Systemic corticosteroids are used to treat a number of medical conditions; however, they are associated with numerous adverse effects. The impact of preoperative chronic corticosteroid use on postoperative outcomes following total hip arthroplasty (THA) is unclear. The purpose of this study was to assess the independent effect of chronic systemic preoperative steroid use on short-term perioperative complications and readmissions after THA.
All patients undergoing primary THA in the American College of Surgeons National Surgical Quality Improvement Program registry from 2005 to -–2015 were identified. Patients were considered chronic steroid users if they used any dosage of oral or parenteral steroids for >10 of the preceding 30 days before THA. Two equally sized propensity-matched groups based on preoperative steroid use were generated to account for differences in operative and baseline characteristics between the groups. Thirty-day complications and hospital readmissions rates were compared using bivariate analysis.
Of 101,532 THA patients who underwent primary THA, 3714 (3.7%) were identified as chronic corticosteroid users. Comparison of propensity-matched cohorts identified an increased rate of any complication (odds ratio [OR] 1.30, P = .003), sepsis (OR 2.07, P = .022), urinary tract infection (OR 1.61, P = .020), superficial surgical site infection (OR 1.73, P = .038), and hospital readmission (OR 1.50, P < .001) in patients who used systemic steroids preoperatively. Readmissions in preoperative steroid users were most commonly for infectious reasons.
Patients prescribed chronic corticosteroids are at a significantly increased risk of both 30-day periopative complications and hospital readmissions. This finding has important implications for pre- and postoperative patient counseling as well as preoperative risk stratification.
Continue to: Corticosteroids are powerful...
Corticosteroids are powerful anti-inflammatory steroid hormones that have many indications in the treatment of medical diseases, including advanced or poorly controlled asthma, chronic obstructive pulmonary disease (COPD), inflammatory bowel disease, allergic conditions, among other indications.1-4 In orthopedics and rheumatology, systemic steroids are, at times, used in patients with rheumatoid arthritis, systemic lupus erythematosus, and vasculitides.5-7 Overman and colleagues,8 using data from the National Health and Nutrition Examination Survey between 1999 and 2008 identified both a 1.2% prevalence of chronic corticosteroid usage in the United States across all age groups and a positive correlation between steroid use prevalence and increasing age. In that study, nearly two-thirds of survey respondents reported using corticosteroids chronically for >90 days. Another observational study in the United Kingdom found that long-term steroid prescriptions increased between 1989 to 2008 and that 13.6% of patients with rheumatoid arthritis and 66.5% of patients with polymyalgia rheumatica or giant cell arteritis used long-term steroids.9
Enterally- or parenterally-administered corticosteroids have numerous systemic effects that are of particular relevance to orthopedic surgeons. Corticosteroids induce osteoporosis by preferentially inducing osteoclastic activity while inhibiting the differentiation of osteoblasts, ultimately leading to decreased bone quality and mass.10 As a consequence, patients who have previously used corticosteroids are more than twice as likely to have a hip fracture.11 Steroids also increase the risk of both osteonecrosis and myopathy, among other musculoskeletal effects.12 In addition to orthopedic complications, steroids have broad inhibitory effects on both acquired and innate immunity, which significantly increases the risk of infections.13 This increased risk of infection is dose-dependent14 and synergistic with other immunosuppressive drugs.15
Patients with hip pain may receive localized corticosteroid hip joint injections during the nonoperative management of various hip pathologies, including arthritis, bursitis, and labral tears.16,17 Outcomes of patients who received intra-articular corticosteroid injections before total hip arthroplasty (THA) were evaluated in a systematic review of 9 studies by Pereira and colleagues.17 These authors found that the infection rate (both superficial and deep surgical site infections [SSI]) after THA in patients who received local steroid injection into the hip before surgery was between 0% and 30%.17 However, similar studies assessing the impact that systemic steroids have on outcomes after THA are lacking. Patients who undergo THA for conditions associated with higher lifetime steroid usage have worse outcomes than those who do not. For instance, in patients undergoing THA for rheumatoid arthritis, the rates of both postoperative periprosthetic joint infection and hip dislocation are higher, when compared with osteoarthritis.18,19 However, it is unclear how much of this difference in outcomes is due to the underlying disease, adverse effects of steroids, or both. Given the high prevalence of chronic systemic steroid use, it is essential to elucidate more clearly the impact that these medications have on perioperative outcomes after THA.
Therefore, the purpose of this study was to characterize short-term perioperative outcomes, including complication and readmission rates in patients undergoing THA while taking chronic preoperative corticosteroids. We also sought to identify the most common reasons for hospital readmission in patients who did and did not use long-term steroids.
MATERIALS AND METHODS
STUDY DESIGN AND SETTING
This investigation was a retrospective cohort study that utilized the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) registry.20 The ACS-NSQIP is a prospectively collected, multi-institutional database that collects demographical information, operative variables, and both postoperative complications and hospital readmission data. Data is collected for up to 30 days after the index procedure, and patients are contacted by telephone if they are discharged before 30 days. Patient data is entered by specially trained surgical clinical reviewers and is routinely audited by the ACS-NSQIP, leading to more accurate data when compared with administrative research databases.21,22 The ACS-NSQIP has been used in orthopedic surgery outcomes-based studies.23-25
Continue to: All patients undergoing...
All patients undergoing THA between 2005 and 2015 were identified in the registry using primary Current Procedural Terminology code 27130. Patients were split into 2 groups based on whether or not they chronically used corticosteroids preoperatively for a medical condition. A patient was considered a chronic corticosteroid user if he/she used oral or parenteral corticosteroids within 30 days before the index procedure for >10 of the preceding 30 days. Those who received a 1-time steroid pulse or those who used topical or inhaled steroids were not considered as steroid users in this study.
BASELINE CHARACTERISTICS AND PERIOPERATIVE OUTCOMES
Baseline patient and operative characteristics, including patient age, gender, body mass index (BMI), functional status, American Society of Anesthesiologists (ASA) class, anesthesia type, operative duration, and medical comorbidities including hypertension, COPD, diabetes mellitus, and smoking history, were compared between both groups. Perioperative outcomes that were assessed in this study include death, renal, respiratory, and cardiac complications, deep vein thrombosis or pulmonary embolism, stroke, sepsis, return to the operating room, urinary tract infection (UTI), wound dehiscence, superficial and deep SSI, need for a blood transfusion within 72 hours of index surgical procedure, and hospital readmissions. Renal complications were defined as acute or progressive renal insufficiency; respiratory complications were defined as failure to wean from the ventilator, need for intubation after the index procedure, and the occurrence of pneumonia; and cardiac complications were defined as myocardial infarction or cardiac arrest requiring cardiopulmonary resuscitation. Patients were excluded if they had missing baseline or operative characteristic data, an unclean wound classification at the time of admission, or if their THA was considered emergent.
STATISTICAL ANALYSIS
A propensity score-matched comparison was performed to adjust for differences in baseline and operative characteristics between the 2 cohorts in this study. In the current study, the propensity score was defined as the conditional probability that a patient chronically used preoperative corticosteroids for a medical condition, as a function of age, BMI, gender, ASA class, functional status, medical comorbidities, anesthesia type, and operative duration. A 1:1 matching with tight calipers (0.0001), and nearest-neighbor matching was used to generate 2 equally-sized, propensity-matched cohorts based on steroid status.26 Nearest-neighbor matching identifies patients in both cohorts with the closest propensity scores for inclusion in propensity-matched cohorts. This matching is continued until 1 group runs out of patients to match. Baseline patient and operative characteristics for the unadjusted and propensity-matched groups were compared using Pearson’s χ2 analysis. Outcomes after THA by steroid status were also compared in both unadjusted and propensity-matched groups. Finally, all patients who were readmitted were identified, and the reason for readmission was determined using the International Classification of Disease Ninth (ICD-9) and Tenth (ICD-10) edition codes. Patients were classified as having an infectious readmission only if the ICD code clearly stated an infectious etiology. For instance, a patient with an intestinal infection due to Clostridium difficile (ICD-9 008.45) was counted as a gastrointestinal infection, whereas diarrhea without a distinctly specified etiology (ICD-9 787.91, ICD-10 R19.7) was counted as a gastrointestinal medical complication. Readmission data was only available in ACS-NSQIP from 2011 to 2015, constituting 92.5% of all patients included in this study. We used SPSS version 23 (IBM Corporation) for all statistical analyses, and defined a significant P value as <.05.
RESULTS
BASELINE PATIENTS AND OPERATIVE CHARACTERISTICS
In total, we identified 101,532 patients who underwent THA (Table 1). O these, 3714 (3.7%) chronically used corticosteroids preoperatively, whereas 97,818 (96.3%) did not.

When the unadjusted cohorts were compared, patients using corticosteroids were more likely to be female, less likely to obese, more likely to have hypertension, diabetes mellitus, COPD, higher ASA class, undergone THA with general anesthesia, and have a dependent functional status (P < .001 for all comparisons). After propensity matching, 2 equally sized cohorts of 3618 patients each were generated based on steroid status and no differences in baseline and operative characteristics were identified between the 2 groups.
Continue to: CLINICAL OUTCOMES BY STEROID STATUS
CLINCIAL OUTCOMES BY STEROID STATUS
A comparison of unadjusted cohorts showed that patients who used preoperative steroids had an increased rate of any complication (7.89%) when compared with those who did not (4.87%) (Table 2).

Similarly, those who used corticosteroids preoperatively had an increased rate of renal complications, respiratory complications, return to the operating room, sepsis, UTI, superficial and deep SSI, and perioperative blood transfusions. They also were more likely to have a 30-day hospital readmission (P < .05 for all comparisons).
When propensity-matched cohorts were compared, patients who used steroids preoperatively were found to have higher rates of any complication (odds Ratio [OR] 1.30, P = .003), sepsis (OR 2.07, P = .022), UTI (OR 1.61, P = .020), superficial SSI (OR 1.73, P = .038), and hospital readmission (OR 1.50, P < .001; Table 3).

REASONS FOR HOSPITAL READMISSION
In total, 3397 patients were readmitted to the hospital within thirty days. Of these, 226 used steroids preoperatively, and 3171 did not (Table 4).

The most common reason for hospital readmission in patients who used preoperative corticosteroids was infectious complications (72 patients, 31.9% of all readmitted patients in this cohort), followed by medical complications (59 patients, 26.1%), and hip-related complications (48 patients, 21.2%). In those who did not use steroids preoperatively, the most common reason for hospital readmission was medical complications (932 patients, 29.4% of all readmitted patients in this cohort), followed by infectious complications (792 patients, 25.0%), and hip-related complications (763 patients, 24.1%).
Continue to: DISCUSSION
DISCUSSION
Nearly 3% of individuals >80 years in the US population chronically use corticosteroids for a medical condition,8 and this rate is likely higher in specific subsets of patients, such as those with rheumatoid arthritis.9 While some studies have assessed the impact of intra-articular corticosteroid hip injections on perioperative outcomes in THA,17 similar studies assessing systemic corticosteroid usage are lacking. The purpose of this study was to characterize short-term perioperative outcomes in patients undergoing THA who chronically use systemic steroids when compared with those who do not. We found that the prevalence of preoperative chronic steroid use in this cohort of THA patients was 3.7%. We also identified increased rates of infectious complications, including sepsis, UTI, and superficial SSI, in patients who used preoperative corticosteroids. Furthermore, we found an increased rate of hospital readmissions in corticosteroid users and identified the most common reason for hospital readmission as infectious complications in this cohort.
The primary finding of this study was an increase in postoperative infections in patients who use preoperative steroids chronically for medical conditions. Immunosuppression has previously been identified as a risk factor for developing periprosthetic joint infections. Tannenbaum and colleagues27 performed a retrospective study of 19 patients who underwent either a kidney or liver transplant and were maintained on an induction regimen of either prednisone and azathioprine or cyclosporine. These 19 patients also underwent either a THA or total knee arthroplasty, and 5 of these patients (26.3%) developed a periprosthetic joint infection after an average of 3.4 years following the arthroplasty procedure. In another study of 37 renal transplant and dialysis patients who underwent a total of 45 THA procedures, there were 3 instances of superficial SSI and 2 instances of deep SSI.28 However, reported infection rates in transplant patients undergoing THA vary significantly, and studies have been unable to assess the true impact that chronic immunosuppression has on perioperative infection rates.29 In this study, patients who used preoperative corticosteroids chronically were at increased risk of perioperative infections, including sepsis, UTI, and superficial SSI.
Deep vein thrombosis is another postoperative complication that has been associated with chronic steroid use.30 In a case-control study of 38,765 patients who developed a venous thromboembolism and 387,650 control patients who did not, Johannesdottir and colleagues30 found an increased thromboembolic risk in current users of systemic glucocorticoids, but not former users, as well as an increased risk as the dose of glucocorticoids increased. We were not able to identify a similar increase in DVT/PE in chronic corticosteroid users, perhaps due to our sample size, or because we could not do subgroup analyses based on the type or dosage of steroid that a patient was taking. Future studies that identify the highest risk patients among those using systemic corticosteroids are important because parenteral corticosteroids are being increasingly used in THA to alleviate postoperative pain as an opioid-sparing measure.31,32
Finally, we also found that patients who use chronic, systemic corticosteroids are at an increased risk for hospital readmission, when compared with those patients who are not using steroids and are most likely to be readmitted for an infectious complication. Schairer and colleagues33 assessed readmission rates after THA and found 30- and 90-day readmission rate of 4% and 7%, respectively. These authors also found that medical complications accounted for approximately 25% of readmissions, and hip-related complications (eg, dislocation, SSI) accounted for >50%. In our study, we found a 30-day readmission rate in non-steroid users of 3.53% and a rate of 6.52% in chronic steroid users. More than 30% of patients using a steroid were readmitted for infectious complications. As THA is becoming increasingly reimbursed under a bundled payments model by Medicare and Medicaid,34-36 reducing short-term readmissions is imperative. Therefore, discharge counseling that emphasizes how to recognize both the signs and symptoms of infection as well as how to prevent infections, such as reducing SSIs through appropriate wound care, may be warranted in higher risk chronic steroid users.
This study has a number of limitations that are inherent to ACS-NSQIP. First, we lacked specific information on a patient’s steroid history, including which corticosteroid they were using, dosage, frequency, and the indication for corticosteroid therapy. Therefore, we were unable to establish a dose-dependent relationship between steroid exposure and postoperative complications after THA. Second, we were able to assess only 30-day rates of complications and readmissions, and therefore, we were unable to identify intermediate- and long-term effects of systemic corticosteroid use on THA. Finally, we could not determine orthopedic- or hip-specific postoperative outcomes, such as functional scores and range of motion.
Continue to: CONCLUSION
CONCLUSION
In conclusion, this study quantified the increased risk for perioperative complications and hospital readmissions in patients who chronically use corticosteroids and are undergoing THA, when compared with those who do not use corticosteroids. These results suggest that patients who are on long-term steroids are at an increased risk for complications, primarily infectious complications. This finding has important implications for patient counseling, preoperative risk stratification, and suggests that higher risk patients, such as chronic steroid users, may benefit from improved discharge care to decrease complication rates.
ABSTRACT
Systemic corticosteroids are used to treat a number of medical conditions; however, they are associated with numerous adverse effects. The impact of preoperative chronic corticosteroid use on postoperative outcomes following total hip arthroplasty (THA) is unclear. The purpose of this study was to assess the independent effect of chronic systemic preoperative steroid use on short-term perioperative complications and readmissions after THA.
All patients undergoing primary THA in the American College of Surgeons National Surgical Quality Improvement Program registry from 2005 to -–2015 were identified. Patients were considered chronic steroid users if they used any dosage of oral or parenteral steroids for >10 of the preceding 30 days before THA. Two equally sized propensity-matched groups based on preoperative steroid use were generated to account for differences in operative and baseline characteristics between the groups. Thirty-day complications and hospital readmissions rates were compared using bivariate analysis.
Of 101,532 THA patients who underwent primary THA, 3714 (3.7%) were identified as chronic corticosteroid users. Comparison of propensity-matched cohorts identified an increased rate of any complication (odds ratio [OR] 1.30, P = .003), sepsis (OR 2.07, P = .022), urinary tract infection (OR 1.61, P = .020), superficial surgical site infection (OR 1.73, P = .038), and hospital readmission (OR 1.50, P < .001) in patients who used systemic steroids preoperatively. Readmissions in preoperative steroid users were most commonly for infectious reasons.
Patients prescribed chronic corticosteroids are at a significantly increased risk of both 30-day periopative complications and hospital readmissions. This finding has important implications for pre- and postoperative patient counseling as well as preoperative risk stratification.
Continue to: Corticosteroids are powerful...
Corticosteroids are powerful anti-inflammatory steroid hormones that have many indications in the treatment of medical diseases, including advanced or poorly controlled asthma, chronic obstructive pulmonary disease (COPD), inflammatory bowel disease, allergic conditions, among other indications.1-4 In orthopedics and rheumatology, systemic steroids are, at times, used in patients with rheumatoid arthritis, systemic lupus erythematosus, and vasculitides.5-7 Overman and colleagues,8 using data from the National Health and Nutrition Examination Survey between 1999 and 2008 identified both a 1.2% prevalence of chronic corticosteroid usage in the United States across all age groups and a positive correlation between steroid use prevalence and increasing age. In that study, nearly two-thirds of survey respondents reported using corticosteroids chronically for >90 days. Another observational study in the United Kingdom found that long-term steroid prescriptions increased between 1989 to 2008 and that 13.6% of patients with rheumatoid arthritis and 66.5% of patients with polymyalgia rheumatica or giant cell arteritis used long-term steroids.9
Enterally- or parenterally-administered corticosteroids have numerous systemic effects that are of particular relevance to orthopedic surgeons. Corticosteroids induce osteoporosis by preferentially inducing osteoclastic activity while inhibiting the differentiation of osteoblasts, ultimately leading to decreased bone quality and mass.10 As a consequence, patients who have previously used corticosteroids are more than twice as likely to have a hip fracture.11 Steroids also increase the risk of both osteonecrosis and myopathy, among other musculoskeletal effects.12 In addition to orthopedic complications, steroids have broad inhibitory effects on both acquired and innate immunity, which significantly increases the risk of infections.13 This increased risk of infection is dose-dependent14 and synergistic with other immunosuppressive drugs.15
Patients with hip pain may receive localized corticosteroid hip joint injections during the nonoperative management of various hip pathologies, including arthritis, bursitis, and labral tears.16,17 Outcomes of patients who received intra-articular corticosteroid injections before total hip arthroplasty (THA) were evaluated in a systematic review of 9 studies by Pereira and colleagues.17 These authors found that the infection rate (both superficial and deep surgical site infections [SSI]) after THA in patients who received local steroid injection into the hip before surgery was between 0% and 30%.17 However, similar studies assessing the impact that systemic steroids have on outcomes after THA are lacking. Patients who undergo THA for conditions associated with higher lifetime steroid usage have worse outcomes than those who do not. For instance, in patients undergoing THA for rheumatoid arthritis, the rates of both postoperative periprosthetic joint infection and hip dislocation are higher, when compared with osteoarthritis.18,19 However, it is unclear how much of this difference in outcomes is due to the underlying disease, adverse effects of steroids, or both. Given the high prevalence of chronic systemic steroid use, it is essential to elucidate more clearly the impact that these medications have on perioperative outcomes after THA.
Therefore, the purpose of this study was to characterize short-term perioperative outcomes, including complication and readmission rates in patients undergoing THA while taking chronic preoperative corticosteroids. We also sought to identify the most common reasons for hospital readmission in patients who did and did not use long-term steroids.
MATERIALS AND METHODS
STUDY DESIGN AND SETTING
This investigation was a retrospective cohort study that utilized the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) registry.20 The ACS-NSQIP is a prospectively collected, multi-institutional database that collects demographical information, operative variables, and both postoperative complications and hospital readmission data. Data is collected for up to 30 days after the index procedure, and patients are contacted by telephone if they are discharged before 30 days. Patient data is entered by specially trained surgical clinical reviewers and is routinely audited by the ACS-NSQIP, leading to more accurate data when compared with administrative research databases.21,22 The ACS-NSQIP has been used in orthopedic surgery outcomes-based studies.23-25
Continue to: All patients undergoing...
All patients undergoing THA between 2005 and 2015 were identified in the registry using primary Current Procedural Terminology code 27130. Patients were split into 2 groups based on whether or not they chronically used corticosteroids preoperatively for a medical condition. A patient was considered a chronic corticosteroid user if he/she used oral or parenteral corticosteroids within 30 days before the index procedure for >10 of the preceding 30 days. Those who received a 1-time steroid pulse or those who used topical or inhaled steroids were not considered as steroid users in this study.
BASELINE CHARACTERISTICS AND PERIOPERATIVE OUTCOMES
Baseline patient and operative characteristics, including patient age, gender, body mass index (BMI), functional status, American Society of Anesthesiologists (ASA) class, anesthesia type, operative duration, and medical comorbidities including hypertension, COPD, diabetes mellitus, and smoking history, were compared between both groups. Perioperative outcomes that were assessed in this study include death, renal, respiratory, and cardiac complications, deep vein thrombosis or pulmonary embolism, stroke, sepsis, return to the operating room, urinary tract infection (UTI), wound dehiscence, superficial and deep SSI, need for a blood transfusion within 72 hours of index surgical procedure, and hospital readmissions. Renal complications were defined as acute or progressive renal insufficiency; respiratory complications were defined as failure to wean from the ventilator, need for intubation after the index procedure, and the occurrence of pneumonia; and cardiac complications were defined as myocardial infarction or cardiac arrest requiring cardiopulmonary resuscitation. Patients were excluded if they had missing baseline or operative characteristic data, an unclean wound classification at the time of admission, or if their THA was considered emergent.
STATISTICAL ANALYSIS
A propensity score-matched comparison was performed to adjust for differences in baseline and operative characteristics between the 2 cohorts in this study. In the current study, the propensity score was defined as the conditional probability that a patient chronically used preoperative corticosteroids for a medical condition, as a function of age, BMI, gender, ASA class, functional status, medical comorbidities, anesthesia type, and operative duration. A 1:1 matching with tight calipers (0.0001), and nearest-neighbor matching was used to generate 2 equally-sized, propensity-matched cohorts based on steroid status.26 Nearest-neighbor matching identifies patients in both cohorts with the closest propensity scores for inclusion in propensity-matched cohorts. This matching is continued until 1 group runs out of patients to match. Baseline patient and operative characteristics for the unadjusted and propensity-matched groups were compared using Pearson’s χ2 analysis. Outcomes after THA by steroid status were also compared in both unadjusted and propensity-matched groups. Finally, all patients who were readmitted were identified, and the reason for readmission was determined using the International Classification of Disease Ninth (ICD-9) and Tenth (ICD-10) edition codes. Patients were classified as having an infectious readmission only if the ICD code clearly stated an infectious etiology. For instance, a patient with an intestinal infection due to Clostridium difficile (ICD-9 008.45) was counted as a gastrointestinal infection, whereas diarrhea without a distinctly specified etiology (ICD-9 787.91, ICD-10 R19.7) was counted as a gastrointestinal medical complication. Readmission data was only available in ACS-NSQIP from 2011 to 2015, constituting 92.5% of all patients included in this study. We used SPSS version 23 (IBM Corporation) for all statistical analyses, and defined a significant P value as <.05.
RESULTS
BASELINE PATIENTS AND OPERATIVE CHARACTERISTICS
In total, we identified 101,532 patients who underwent THA (Table 1). O these, 3714 (3.7%) chronically used corticosteroids preoperatively, whereas 97,818 (96.3%) did not.

When the unadjusted cohorts were compared, patients using corticosteroids were more likely to be female, less likely to obese, more likely to have hypertension, diabetes mellitus, COPD, higher ASA class, undergone THA with general anesthesia, and have a dependent functional status (P < .001 for all comparisons). After propensity matching, 2 equally sized cohorts of 3618 patients each were generated based on steroid status and no differences in baseline and operative characteristics were identified between the 2 groups.
Continue to: CLINICAL OUTCOMES BY STEROID STATUS
CLINCIAL OUTCOMES BY STEROID STATUS
A comparison of unadjusted cohorts showed that patients who used preoperative steroids had an increased rate of any complication (7.89%) when compared with those who did not (4.87%) (Table 2).

Similarly, those who used corticosteroids preoperatively had an increased rate of renal complications, respiratory complications, return to the operating room, sepsis, UTI, superficial and deep SSI, and perioperative blood transfusions. They also were more likely to have a 30-day hospital readmission (P < .05 for all comparisons).
When propensity-matched cohorts were compared, patients who used steroids preoperatively were found to have higher rates of any complication (odds Ratio [OR] 1.30, P = .003), sepsis (OR 2.07, P = .022), UTI (OR 1.61, P = .020), superficial SSI (OR 1.73, P = .038), and hospital readmission (OR 1.50, P < .001; Table 3).

REASONS FOR HOSPITAL READMISSION
In total, 3397 patients were readmitted to the hospital within thirty days. Of these, 226 used steroids preoperatively, and 3171 did not (Table 4).

The most common reason for hospital readmission in patients who used preoperative corticosteroids was infectious complications (72 patients, 31.9% of all readmitted patients in this cohort), followed by medical complications (59 patients, 26.1%), and hip-related complications (48 patients, 21.2%). In those who did not use steroids preoperatively, the most common reason for hospital readmission was medical complications (932 patients, 29.4% of all readmitted patients in this cohort), followed by infectious complications (792 patients, 25.0%), and hip-related complications (763 patients, 24.1%).
Continue to: DISCUSSION
DISCUSSION
Nearly 3% of individuals >80 years in the US population chronically use corticosteroids for a medical condition,8 and this rate is likely higher in specific subsets of patients, such as those with rheumatoid arthritis.9 While some studies have assessed the impact of intra-articular corticosteroid hip injections on perioperative outcomes in THA,17 similar studies assessing systemic corticosteroid usage are lacking. The purpose of this study was to characterize short-term perioperative outcomes in patients undergoing THA who chronically use systemic steroids when compared with those who do not. We found that the prevalence of preoperative chronic steroid use in this cohort of THA patients was 3.7%. We also identified increased rates of infectious complications, including sepsis, UTI, and superficial SSI, in patients who used preoperative corticosteroids. Furthermore, we found an increased rate of hospital readmissions in corticosteroid users and identified the most common reason for hospital readmission as infectious complications in this cohort.
The primary finding of this study was an increase in postoperative infections in patients who use preoperative steroids chronically for medical conditions. Immunosuppression has previously been identified as a risk factor for developing periprosthetic joint infections. Tannenbaum and colleagues27 performed a retrospective study of 19 patients who underwent either a kidney or liver transplant and were maintained on an induction regimen of either prednisone and azathioprine or cyclosporine. These 19 patients also underwent either a THA or total knee arthroplasty, and 5 of these patients (26.3%) developed a periprosthetic joint infection after an average of 3.4 years following the arthroplasty procedure. In another study of 37 renal transplant and dialysis patients who underwent a total of 45 THA procedures, there were 3 instances of superficial SSI and 2 instances of deep SSI.28 However, reported infection rates in transplant patients undergoing THA vary significantly, and studies have been unable to assess the true impact that chronic immunosuppression has on perioperative infection rates.29 In this study, patients who used preoperative corticosteroids chronically were at increased risk of perioperative infections, including sepsis, UTI, and superficial SSI.
Deep vein thrombosis is another postoperative complication that has been associated with chronic steroid use.30 In a case-control study of 38,765 patients who developed a venous thromboembolism and 387,650 control patients who did not, Johannesdottir and colleagues30 found an increased thromboembolic risk in current users of systemic glucocorticoids, but not former users, as well as an increased risk as the dose of glucocorticoids increased. We were not able to identify a similar increase in DVT/PE in chronic corticosteroid users, perhaps due to our sample size, or because we could not do subgroup analyses based on the type or dosage of steroid that a patient was taking. Future studies that identify the highest risk patients among those using systemic corticosteroids are important because parenteral corticosteroids are being increasingly used in THA to alleviate postoperative pain as an opioid-sparing measure.31,32
Finally, we also found that patients who use chronic, systemic corticosteroids are at an increased risk for hospital readmission, when compared with those patients who are not using steroids and are most likely to be readmitted for an infectious complication. Schairer and colleagues33 assessed readmission rates after THA and found 30- and 90-day readmission rate of 4% and 7%, respectively. These authors also found that medical complications accounted for approximately 25% of readmissions, and hip-related complications (eg, dislocation, SSI) accounted for >50%. In our study, we found a 30-day readmission rate in non-steroid users of 3.53% and a rate of 6.52% in chronic steroid users. More than 30% of patients using a steroid were readmitted for infectious complications. As THA is becoming increasingly reimbursed under a bundled payments model by Medicare and Medicaid,34-36 reducing short-term readmissions is imperative. Therefore, discharge counseling that emphasizes how to recognize both the signs and symptoms of infection as well as how to prevent infections, such as reducing SSIs through appropriate wound care, may be warranted in higher risk chronic steroid users.
This study has a number of limitations that are inherent to ACS-NSQIP. First, we lacked specific information on a patient’s steroid history, including which corticosteroid they were using, dosage, frequency, and the indication for corticosteroid therapy. Therefore, we were unable to establish a dose-dependent relationship between steroid exposure and postoperative complications after THA. Second, we were able to assess only 30-day rates of complications and readmissions, and therefore, we were unable to identify intermediate- and long-term effects of systemic corticosteroid use on THA. Finally, we could not determine orthopedic- or hip-specific postoperative outcomes, such as functional scores and range of motion.
Continue to: CONCLUSION
CONCLUSION
In conclusion, this study quantified the increased risk for perioperative complications and hospital readmissions in patients who chronically use corticosteroids and are undergoing THA, when compared with those who do not use corticosteroids. These results suggest that patients who are on long-term steroids are at an increased risk for complications, primarily infectious complications. This finding has important implications for patient counseling, preoperative risk stratification, and suggests that higher risk patients, such as chronic steroid users, may benefit from improved discharge care to decrease complication rates.
1. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;13(5):CD011801. doi: 10.1002/14651858.CD011801.pub2.
2. Walters JA, Tan DJ, White CJ, Wood-Baker R. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(12):CD006897.
3. Nunes T, Barreiro-de Acosta M, Marin-Jimenez I, Nos P, Sans M. Oral locally active steroids in inflammatory bowel disease. J Crohns Colitis. 2013;7(3):183-191. doi: 10.1016/j.crohns.2012.06.010.
4. Karatzanis A, Chatzidakis A, Milioni A, Vlaminck S, Kawauchi H, Velegrakis S, et al. Contemporary use of corticosteroids in rhinology. Curr Allergy Asthm R. 2017;17(2). doi: 10.1007/s11882-017-0679-0.
5. Parker BJ, Bruce IN. High dose methylprednisolone therapy for the treatment of severe systemic lupus erythematosus. Lupus. 2007;16(6):387-393. doi: 10.1177/0961203307079502.
6. Ferreira JF, Ahmed Mohamed AA, Emery P. Glucocorticoids and rheumatoid arthritis. Rheum Dis Clin North Am. 2016;42(1):33-46. doi: 10.1016/j.rdc.2015.08.006.
7. Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA. 2016;315(22):2442-2458. doi: 10.1001/jama.2016.5444.
8. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res. 2013;65(2):294-298. doi: 10.1002/acr.21796.
9. Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology. 2011;50(11):1982-1990. doi: 10.1093/rheumatology/ker017.
10. Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy.Osteoporos Int. 2007;18(10):1319-1328. doi: 10.1007/s00198-007-0394-0.
11. Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton LJ, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19(6):893-899. doi: /10.1359/JBMR.040134.
12. Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76(1):1-9. doi: 10.1016/j.jaad.2016.01.062.
13. Cutolo M, Seriolo B, Pizzorni C, Secchi ME, Soldano S, Paolino S, et al. Use of glucocorticoids and risk of infections. Autoimmun Rev. 2008;8(2):153-155. doi: 10.1016/j.autrev.2008.07.010.
14. Blackwood LL, Pennington JE. Dose-dependent effect of glucocorticosteroids on pulmonary defenses in a steroid-resistant host. Am Rev Respir Dis. 1982;126(6):1045-1049.
15. Toruner M, Loftus EV, Jr., Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929-936. doi: 10.1053/j.gastro.2008.01.012.
16. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51(2):97-104. doi: 10.1136/bjsports-2015-095858.
17. Pereira LC, Kerr J, Jolles BM. Intra-articular steroid injection for osteoarthritis of the hip prior to total hip arthroplasty: is it safe? a systematic review. Bone Joint J. 2016;98-B(8):1027-1035. doi: 10.1302/0301-620X.98B8.37420.
18. Ravi B, Escott B, Shah PS, Jenkinson R, Chahal J, Bogoch E, et al. A systematic review and meta-analysis comparing complications following total joint arthroplasty for rheumatoid arthritis versus for osteoarthritis. Arthritis Rheum. 2012;64(12):3839-3849. doi: 10.1002/art.37690.
19. Ravi B, Croxford R, Hollands S, Paterson JM, Bogoch E, Kreder H, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi: 10.1002/art.38231.
20. ACS NSQIP Participant Use Data Files. https://www.facs.org/quality-programs/acs-nsqip/program-specifics/participant-use. Accessed December 6, 2018.
21. Lawson EH, Louie R, Zingmond DS, Brook RH, Hall BL, Han L, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981. doi: 10.1097/SLA.0b013e31826b4c4f.
22. Weiss A, Anderson JE, Chang DC. Comparing the national surgical quality improvement program with the nationwide inpatient sample database. JAMA Surg. 2015;150(8):815-816. doi: 10.1001/jamasurg.2015.0962.
23. Boddapati V, Fu MC, Mayman DJ, Su EP, Sculco PK, McLawhorn AS. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. J Arthroplasty. 2018;33(2):521-526. doi: 10.1016/j.arth.2017.09.021.
24. Boddapati V, Fu MC, Schairer WW, Gulotta LV, Dines DM, Dines JS. Revision total shoulder arthroplasty is associated with increased thirty-day postoperative complications and wound infections relative to primary total shoulder arthroplasty. HSS J. 2018;14(1):23-28. doi: 10.1007/s11420-017-9573-5.
25. Boddapati V, Fu MC, Schiarer WW, Ranawat AS, Dines DM, Taylor SA, Dines DM. Increased shoulder arthroscopy time is associated with overnight hospital stay and surgical site infection. Arthroscopy. 2018;34(2):363-368. doi: 10.1016/j.arthro.2017.08.243.
26. Lunt M. Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am J Epidemiol. 2014 Jan 15;179(2):226-235. doi: 10.1093/aje/kwt212.
27. Tannenbaum DA, Matthews LS, Grady-Benson JC. Infection around joint replacements in patients who have a renal or liver transplantation. J Bone Joint Surg Am. 1997;79(1):36-43.
28. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329. doi: 10.1016/j.arth.2005.07.008.
29. Nowicki P, Chaudhary H. Total hip replacement in renal transplant patients. J Bone Joint Surg Br. 2007;89(12):1561-1566.
30. Johannesdottir SA, Horváth-Puhó E, Dekkers OM, Cannegieter SC, Jørgensen JO, Ehrenstein V, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173(9):743-752. doi: 10.1001/jamainternmed.2013.122.
31. Hartman J, Khanna V, Habib A, Farrokhyar F, Memon M, Adili A. Perioperative systemic glucocorticoids in total hip and knee arthroplasty: a systematic review of outcomes. J Orthop. 2017;14(2):294-301. doi: 10.1016/j.jor.2017.03.012.
32. Sculco PK, McLawhorn AS, Desai N, Su EP, Padgett DE, Jules-Elysee K. The effect of perioperative corticosteroids in total hip arthroplasty: a prospective double-blind placebo controlled pilot study. J Arthroplasty. 2016;31(6):1208-1212. doi: 10.1016/j.arth.2015.11.011.
33. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470. doi: 10.1007/s11999-013-3121-5.
34. US Department of Health and Human Services. Comprehensive Care for Joint Replacement Model. Centers for Medicare & Medicaid Services. https://innovation.cms.gov/initiatives/cjr. Accessed June 15, 2017.
35. Bozic KJ, Ward L, Vail TP, Maze M. Bundled payments in total joint arthroplasty: targeting opportunities for quality improvement and cost reduction. Clin Orthop Relat Res. 2014;472(1):188-193. doi: 10.1007/s11999-013-3034-3.
36. Bosco JA, 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5): 903-905. doi: 10.1016/j.arth.2013.11.006.
1. Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;13(5):CD011801. doi: 10.1002/14651858.CD011801.pub2.
2. Walters JA, Tan DJ, White CJ, Wood-Baker R. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(12):CD006897.
3. Nunes T, Barreiro-de Acosta M, Marin-Jimenez I, Nos P, Sans M. Oral locally active steroids in inflammatory bowel disease. J Crohns Colitis. 2013;7(3):183-191. doi: 10.1016/j.crohns.2012.06.010.
4. Karatzanis A, Chatzidakis A, Milioni A, Vlaminck S, Kawauchi H, Velegrakis S, et al. Contemporary use of corticosteroids in rhinology. Curr Allergy Asthm R. 2017;17(2). doi: 10.1007/s11882-017-0679-0.
5. Parker BJ, Bruce IN. High dose methylprednisolone therapy for the treatment of severe systemic lupus erythematosus. Lupus. 2007;16(6):387-393. doi: 10.1177/0961203307079502.
6. Ferreira JF, Ahmed Mohamed AA, Emery P. Glucocorticoids and rheumatoid arthritis. Rheum Dis Clin North Am. 2016;42(1):33-46. doi: 10.1016/j.rdc.2015.08.006.
7. Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA. 2016;315(22):2442-2458. doi: 10.1001/jama.2016.5444.
8. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res. 2013;65(2):294-298. doi: 10.1002/acr.21796.
9. Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology. 2011;50(11):1982-1990. doi: 10.1093/rheumatology/ker017.
10. Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy.Osteoporos Int. 2007;18(10):1319-1328. doi: 10.1007/s00198-007-0394-0.
11. Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton LJ, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19(6):893-899. doi: /10.1359/JBMR.040134.
12. Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76(1):1-9. doi: 10.1016/j.jaad.2016.01.062.
13. Cutolo M, Seriolo B, Pizzorni C, Secchi ME, Soldano S, Paolino S, et al. Use of glucocorticoids and risk of infections. Autoimmun Rev. 2008;8(2):153-155. doi: 10.1016/j.autrev.2008.07.010.
14. Blackwood LL, Pennington JE. Dose-dependent effect of glucocorticosteroids on pulmonary defenses in a steroid-resistant host. Am Rev Respir Dis. 1982;126(6):1045-1049.
15. Toruner M, Loftus EV, Jr., Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929-936. doi: 10.1053/j.gastro.2008.01.012.
16. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51(2):97-104. doi: 10.1136/bjsports-2015-095858.
17. Pereira LC, Kerr J, Jolles BM. Intra-articular steroid injection for osteoarthritis of the hip prior to total hip arthroplasty: is it safe? a systematic review. Bone Joint J. 2016;98-B(8):1027-1035. doi: 10.1302/0301-620X.98B8.37420.
18. Ravi B, Escott B, Shah PS, Jenkinson R, Chahal J, Bogoch E, et al. A systematic review and meta-analysis comparing complications following total joint arthroplasty for rheumatoid arthritis versus for osteoarthritis. Arthritis Rheum. 2012;64(12):3839-3849. doi: 10.1002/art.37690.
19. Ravi B, Croxford R, Hollands S, Paterson JM, Bogoch E, Kreder H, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi: 10.1002/art.38231.
20. ACS NSQIP Participant Use Data Files. https://www.facs.org/quality-programs/acs-nsqip/program-specifics/participant-use. Accessed December 6, 2018.
21. Lawson EH, Louie R, Zingmond DS, Brook RH, Hall BL, Han L, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981. doi: 10.1097/SLA.0b013e31826b4c4f.
22. Weiss A, Anderson JE, Chang DC. Comparing the national surgical quality improvement program with the nationwide inpatient sample database. JAMA Surg. 2015;150(8):815-816. doi: 10.1001/jamasurg.2015.0962.
23. Boddapati V, Fu MC, Mayman DJ, Su EP, Sculco PK, McLawhorn AS. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. J Arthroplasty. 2018;33(2):521-526. doi: 10.1016/j.arth.2017.09.021.
24. Boddapati V, Fu MC, Schairer WW, Gulotta LV, Dines DM, Dines JS. Revision total shoulder arthroplasty is associated with increased thirty-day postoperative complications and wound infections relative to primary total shoulder arthroplasty. HSS J. 2018;14(1):23-28. doi: 10.1007/s11420-017-9573-5.
25. Boddapati V, Fu MC, Schiarer WW, Ranawat AS, Dines DM, Taylor SA, Dines DM. Increased shoulder arthroscopy time is associated with overnight hospital stay and surgical site infection. Arthroscopy. 2018;34(2):363-368. doi: 10.1016/j.arthro.2017.08.243.
26. Lunt M. Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am J Epidemiol. 2014 Jan 15;179(2):226-235. doi: 10.1093/aje/kwt212.
27. Tannenbaum DA, Matthews LS, Grady-Benson JC. Infection around joint replacements in patients who have a renal or liver transplantation. J Bone Joint Surg Am. 1997;79(1):36-43.
28. Shrader MW, Schall D, Parvizi J, McCarthy JT, Lewallen DG. Total hip arthroplasty in patients with renal failure: a comparison between transplant and dialysis patients. J Arthroplasty. 2006;21(3):324-329. doi: 10.1016/j.arth.2005.07.008.
29. Nowicki P, Chaudhary H. Total hip replacement in renal transplant patients. J Bone Joint Surg Br. 2007;89(12):1561-1566.
30. Johannesdottir SA, Horváth-Puhó E, Dekkers OM, Cannegieter SC, Jørgensen JO, Ehrenstein V, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med. 2013;173(9):743-752. doi: 10.1001/jamainternmed.2013.122.
31. Hartman J, Khanna V, Habib A, Farrokhyar F, Memon M, Adili A. Perioperative systemic glucocorticoids in total hip and knee arthroplasty: a systematic review of outcomes. J Orthop. 2017;14(2):294-301. doi: 10.1016/j.jor.2017.03.012.
32. Sculco PK, McLawhorn AS, Desai N, Su EP, Padgett DE, Jules-Elysee K. The effect of perioperative corticosteroids in total hip arthroplasty: a prospective double-blind placebo controlled pilot study. J Arthroplasty. 2016;31(6):1208-1212. doi: 10.1016/j.arth.2015.11.011.
33. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470. doi: 10.1007/s11999-013-3121-5.
34. US Department of Health and Human Services. Comprehensive Care for Joint Replacement Model. Centers for Medicare & Medicaid Services. https://innovation.cms.gov/initiatives/cjr. Accessed June 15, 2017.
35. Bozic KJ, Ward L, Vail TP, Maze M. Bundled payments in total joint arthroplasty: targeting opportunities for quality improvement and cost reduction. Clin Orthop Relat Res. 2014;472(1):188-193. doi: 10.1007/s11999-013-3034-3.
36. Bosco JA, 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5): 903-905. doi: 10.1016/j.arth.2013.11.006.
TAKE-HOME POINTS
- The rate of preoperative corticosteroid usage is low (3.7%).
- Patients using preoperative corticosteroids had increased rates of total 30-day complications.
- Adverse outcomes that are increased include infectious complications (eg, sepsis, urinary tract infection, surgical site infection).
- Hospital readmissions are also increased in patients taking preoperative corticosteroids, with the most common reason being infection.
- Increased postoperative counseling and surveillance may be warranted in this patient population.