User login
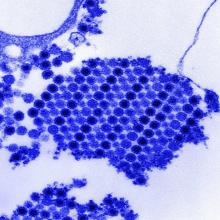
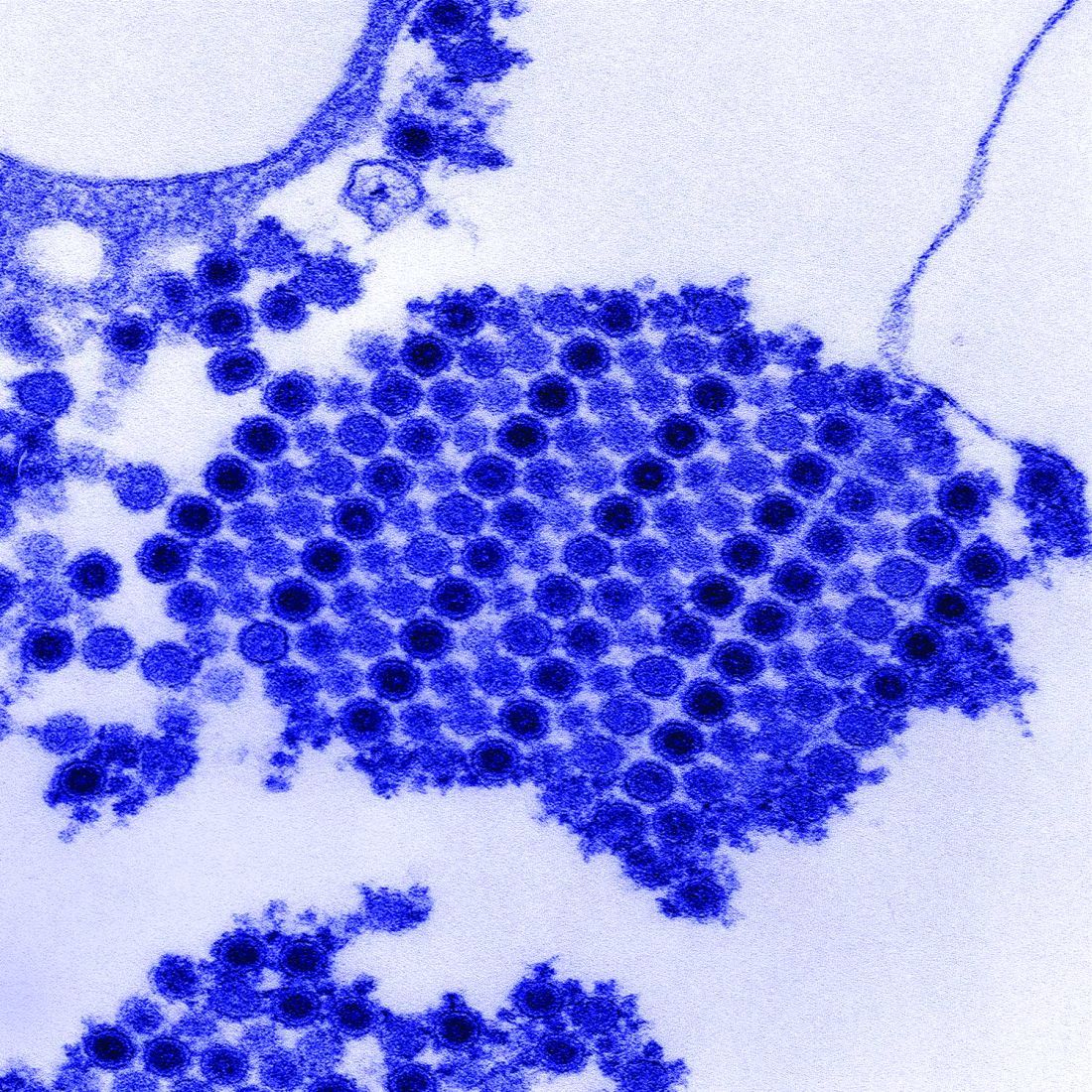
Virus-Specific T-Cell Infusions May Resolve Progressive Multifocal Leukoencephalopathy
Two of three patients cleared JC virus from CSF after infusion.
Infusion of allogeneic BK virus-specific T cells may be an effective treatment for patients with progressive multifocal leukoencephalopathy (PML), according to a report in the October 11 New England Journal of Medicine. The infusion cleared JC virus from the CSF of two patients and reduced viral load in the third, reported lead author Muharrem Muftuoglu, MD, of the Department of Stem Cell Transplantation and Cellular Therapy at the University of Texas MD Anderson Cancer Center in Houston, and colleagues. One of the patients completely recovered and returned to work; this outcome was unprecedented in PML therapy.
“Several approaches for the treatment of PML, including the use of antiviral medications and mirtazapine, have been tested, with poor results,” the investigators said. Although virus-specific T-cell infusion is a novel approach to treating PML, this method has been used for other conditions. “Several groups, including ours, have successfully used viral-specific T cells to treat BK virus infection after stem-cell transplantation,” the investigators said. “Because BK virus and JC virus are genetically similar to one another and share a number of immunogenic proteins with a substantial degree of sequence homology ... we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
This hypothesis proved correct. The investigators infused three patients with PML with “cryopreserved, third-party–produced, viral-specific T cells that had been designed for the treatment of patients with BK virus infection after stem-cell transplantation.” Each patient presented with a different condition and PML-precipitating therapy. The first patient was a 32-year-old woman with high-risk acute myeloid leukemia who had received a cord-blood transplantation, the second a 73-year-old woman with JAK2-positive myeloproliferative neoplasia on ruxolitinib therapy, and the third a 35-year-old man with HIV who had received highly active antiretroviral therapy.
T-cell infusions cleared JC virus from the CSF of the woman with leukemia (three infusions) and the man with HIV (four infusions). These patients recovered to different degrees.The woman had full resolution of symptoms, while the man had slurred speech and walked with a cane. Treatment reduced JC viral load in the elderly woman with myeloproliferative neoplasia (two infusions), but she did not clear the virus and died about eight months later.
No adverse events occurred, but two patients developed immune reconstitution inflammatory syndrome. This outcome was likely caused by the T-cell infusion, since absolute T-cell counts remained steady and white matter enhancement was detected on MRI within four weeks of treatment. Still, the investigators were optimistic about future potential.
“Third-party–produced, ‘off-the-shelf,’ partially HLA-matched, BK virus–specific T cells may serve as therapy for PML,” the investigators concluded. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events associated with this treatment.”
The study was funded by the MD Anderson Cancer Center Moon Shots Program and the National Institutes of Health.
—Will Pass
Suggested Reading
Muftuoglu M, Olson A, Marin D, et al. Allogeneic BK specific T cells for progressive multifocal leukoencephalopathy. N Engl J Med. 2018;379(15):1443-1451.
Two of three patients cleared JC virus from CSF after infusion.
Two of three patients cleared JC virus from CSF after infusion.
Infusion of allogeneic BK virus-specific T cells may be an effective treatment for patients with progressive multifocal leukoencephalopathy (PML), according to a report in the October 11 New England Journal of Medicine. The infusion cleared JC virus from the CSF of two patients and reduced viral load in the third, reported lead author Muharrem Muftuoglu, MD, of the Department of Stem Cell Transplantation and Cellular Therapy at the University of Texas MD Anderson Cancer Center in Houston, and colleagues. One of the patients completely recovered and returned to work; this outcome was unprecedented in PML therapy.
“Several approaches for the treatment of PML, including the use of antiviral medications and mirtazapine, have been tested, with poor results,” the investigators said. Although virus-specific T-cell infusion is a novel approach to treating PML, this method has been used for other conditions. “Several groups, including ours, have successfully used viral-specific T cells to treat BK virus infection after stem-cell transplantation,” the investigators said. “Because BK virus and JC virus are genetically similar to one another and share a number of immunogenic proteins with a substantial degree of sequence homology ... we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
This hypothesis proved correct. The investigators infused three patients with PML with “cryopreserved, third-party–produced, viral-specific T cells that had been designed for the treatment of patients with BK virus infection after stem-cell transplantation.” Each patient presented with a different condition and PML-precipitating therapy. The first patient was a 32-year-old woman with high-risk acute myeloid leukemia who had received a cord-blood transplantation, the second a 73-year-old woman with JAK2-positive myeloproliferative neoplasia on ruxolitinib therapy, and the third a 35-year-old man with HIV who had received highly active antiretroviral therapy.
T-cell infusions cleared JC virus from the CSF of the woman with leukemia (three infusions) and the man with HIV (four infusions). These patients recovered to different degrees.The woman had full resolution of symptoms, while the man had slurred speech and walked with a cane. Treatment reduced JC viral load in the elderly woman with myeloproliferative neoplasia (two infusions), but she did not clear the virus and died about eight months later.
No adverse events occurred, but two patients developed immune reconstitution inflammatory syndrome. This outcome was likely caused by the T-cell infusion, since absolute T-cell counts remained steady and white matter enhancement was detected on MRI within four weeks of treatment. Still, the investigators were optimistic about future potential.
“Third-party–produced, ‘off-the-shelf,’ partially HLA-matched, BK virus–specific T cells may serve as therapy for PML,” the investigators concluded. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events associated with this treatment.”
The study was funded by the MD Anderson Cancer Center Moon Shots Program and the National Institutes of Health.
—Will Pass
Suggested Reading
Muftuoglu M, Olson A, Marin D, et al. Allogeneic BK specific T cells for progressive multifocal leukoencephalopathy. N Engl J Med. 2018;379(15):1443-1451.
Infusion of allogeneic BK virus-specific T cells may be an effective treatment for patients with progressive multifocal leukoencephalopathy (PML), according to a report in the October 11 New England Journal of Medicine. The infusion cleared JC virus from the CSF of two patients and reduced viral load in the third, reported lead author Muharrem Muftuoglu, MD, of the Department of Stem Cell Transplantation and Cellular Therapy at the University of Texas MD Anderson Cancer Center in Houston, and colleagues. One of the patients completely recovered and returned to work; this outcome was unprecedented in PML therapy.
“Several approaches for the treatment of PML, including the use of antiviral medications and mirtazapine, have been tested, with poor results,” the investigators said. Although virus-specific T-cell infusion is a novel approach to treating PML, this method has been used for other conditions. “Several groups, including ours, have successfully used viral-specific T cells to treat BK virus infection after stem-cell transplantation,” the investigators said. “Because BK virus and JC virus are genetically similar to one another and share a number of immunogenic proteins with a substantial degree of sequence homology ... we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
This hypothesis proved correct. The investigators infused three patients with PML with “cryopreserved, third-party–produced, viral-specific T cells that had been designed for the treatment of patients with BK virus infection after stem-cell transplantation.” Each patient presented with a different condition and PML-precipitating therapy. The first patient was a 32-year-old woman with high-risk acute myeloid leukemia who had received a cord-blood transplantation, the second a 73-year-old woman with JAK2-positive myeloproliferative neoplasia on ruxolitinib therapy, and the third a 35-year-old man with HIV who had received highly active antiretroviral therapy.
T-cell infusions cleared JC virus from the CSF of the woman with leukemia (three infusions) and the man with HIV (four infusions). These patients recovered to different degrees.The woman had full resolution of symptoms, while the man had slurred speech and walked with a cane. Treatment reduced JC viral load in the elderly woman with myeloproliferative neoplasia (two infusions), but she did not clear the virus and died about eight months later.
No adverse events occurred, but two patients developed immune reconstitution inflammatory syndrome. This outcome was likely caused by the T-cell infusion, since absolute T-cell counts remained steady and white matter enhancement was detected on MRI within four weeks of treatment. Still, the investigators were optimistic about future potential.
“Third-party–produced, ‘off-the-shelf,’ partially HLA-matched, BK virus–specific T cells may serve as therapy for PML,” the investigators concluded. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events associated with this treatment.”
The study was funded by the MD Anderson Cancer Center Moon Shots Program and the National Institutes of Health.
—Will Pass
Suggested Reading
Muftuoglu M, Olson A, Marin D, et al. Allogeneic BK specific T cells for progressive multifocal leukoencephalopathy. N Engl J Med. 2018;379(15):1443-1451.
Methotrexate relieves pain of Chikungunya-associated arthritis
Methotrexate is effective for the control of pain produced by arthritis associated with Chikungunya virus infection, according to a retrospective review of outcomes in a series of 50 patients.
Joint pain and joint inflammation are commonly seen in the approximately 60% of patients who progress to the chronic phase of Chikungunya virus (CHIKV) infection, but there is no current consensus about how best to manage this complication, according to first author J. Kennedy Amaral, MD, of the department of infectious diseases and tropical medicine at the University of Minas Gerais (Brazil) and his colleagues, who published their experience in 50 patients in the Journal of Clinical Rheumatology.
In this study, the primary measure of efficacy was pain control because not all CHIKV infection patients with rheumatic symptoms demonstrate synovitis on radiological examination. The 50 patients included in this series all had joint symptoms persisting more than 12 weeks after onset of CHIKV infection.
All but four of the patients in this series were women. The mean age was 61.9 years. At baseline, 28 had a musculoskeletal disorder defined by presence of arthralgia, 11 had rheumatoid arthritis, seven had fibromyalgia, and four had undifferentiated polyarthritis.
On a 0-10 visual analog scale (VAS), the mean pain score at baseline was 7.7. All patients were initiated on a 4-week course of 7.5 mg of methotrexate administered with folic acid.
Four patients not examined after 4 weeks of treatment were excluded from analysis. Of those evaluated, 80% had achieved at least a 2-point reduction in VAS score, which is considered clinically meaningful. The mean reduction in VAS pain score at 4 weeks was 4.3 points (P less than .0001 vs. baseline). In 12 patients, symptoms were resolved, and they were not further evaluated.
Those with inadequate pain control at 4 weeks were permitted to begin a higher dose of methotrexate and to receive additional therapies. At 8 weeks, the reduction in VAS pain score was only modestly increased, reaching a mean 4.5-point reduction from baseline on a mean methotrexate dose of 9.2 mg/week. A substantial proportion of patients had added other medications, such as prednisone and hydroxychloroquine.
Only 20 patients had joint swelling and frank arthritis at baseline. In these, the mean swollen joint count decreased from 7.15 to 2.89 (P less than .0001). There was no further reduction at 8 weeks.
Over the course of the study, there was no evidence that methotrexate exacerbated CHIKV infection.
The data were collected retrospectively, and there was no control group, but the findings inform practitioners of the “possible benefit of low-dose methotrexate to treat both arthralgia and arthritis” in chronic CHIK-associated arthritis, according to Dr. Amaral and his coinvestigators.
The authors declared no potential conflicts of interest.
SOURCE: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943.
Methotrexate is effective for the control of pain produced by arthritis associated with Chikungunya virus infection, according to a retrospective review of outcomes in a series of 50 patients.
Joint pain and joint inflammation are commonly seen in the approximately 60% of patients who progress to the chronic phase of Chikungunya virus (CHIKV) infection, but there is no current consensus about how best to manage this complication, according to first author J. Kennedy Amaral, MD, of the department of infectious diseases and tropical medicine at the University of Minas Gerais (Brazil) and his colleagues, who published their experience in 50 patients in the Journal of Clinical Rheumatology.
In this study, the primary measure of efficacy was pain control because not all CHIKV infection patients with rheumatic symptoms demonstrate synovitis on radiological examination. The 50 patients included in this series all had joint symptoms persisting more than 12 weeks after onset of CHIKV infection.
All but four of the patients in this series were women. The mean age was 61.9 years. At baseline, 28 had a musculoskeletal disorder defined by presence of arthralgia, 11 had rheumatoid arthritis, seven had fibromyalgia, and four had undifferentiated polyarthritis.
On a 0-10 visual analog scale (VAS), the mean pain score at baseline was 7.7. All patients were initiated on a 4-week course of 7.5 mg of methotrexate administered with folic acid.
Four patients not examined after 4 weeks of treatment were excluded from analysis. Of those evaluated, 80% had achieved at least a 2-point reduction in VAS score, which is considered clinically meaningful. The mean reduction in VAS pain score at 4 weeks was 4.3 points (P less than .0001 vs. baseline). In 12 patients, symptoms were resolved, and they were not further evaluated.
Those with inadequate pain control at 4 weeks were permitted to begin a higher dose of methotrexate and to receive additional therapies. At 8 weeks, the reduction in VAS pain score was only modestly increased, reaching a mean 4.5-point reduction from baseline on a mean methotrexate dose of 9.2 mg/week. A substantial proportion of patients had added other medications, such as prednisone and hydroxychloroquine.
Only 20 patients had joint swelling and frank arthritis at baseline. In these, the mean swollen joint count decreased from 7.15 to 2.89 (P less than .0001). There was no further reduction at 8 weeks.
Over the course of the study, there was no evidence that methotrexate exacerbated CHIKV infection.
The data were collected retrospectively, and there was no control group, but the findings inform practitioners of the “possible benefit of low-dose methotrexate to treat both arthralgia and arthritis” in chronic CHIK-associated arthritis, according to Dr. Amaral and his coinvestigators.
The authors declared no potential conflicts of interest.
SOURCE: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943.
Methotrexate is effective for the control of pain produced by arthritis associated with Chikungunya virus infection, according to a retrospective review of outcomes in a series of 50 patients.
Joint pain and joint inflammation are commonly seen in the approximately 60% of patients who progress to the chronic phase of Chikungunya virus (CHIKV) infection, but there is no current consensus about how best to manage this complication, according to first author J. Kennedy Amaral, MD, of the department of infectious diseases and tropical medicine at the University of Minas Gerais (Brazil) and his colleagues, who published their experience in 50 patients in the Journal of Clinical Rheumatology.
In this study, the primary measure of efficacy was pain control because not all CHIKV infection patients with rheumatic symptoms demonstrate synovitis on radiological examination. The 50 patients included in this series all had joint symptoms persisting more than 12 weeks after onset of CHIKV infection.
All but four of the patients in this series were women. The mean age was 61.9 years. At baseline, 28 had a musculoskeletal disorder defined by presence of arthralgia, 11 had rheumatoid arthritis, seven had fibromyalgia, and four had undifferentiated polyarthritis.
On a 0-10 visual analog scale (VAS), the mean pain score at baseline was 7.7. All patients were initiated on a 4-week course of 7.5 mg of methotrexate administered with folic acid.
Four patients not examined after 4 weeks of treatment were excluded from analysis. Of those evaluated, 80% had achieved at least a 2-point reduction in VAS score, which is considered clinically meaningful. The mean reduction in VAS pain score at 4 weeks was 4.3 points (P less than .0001 vs. baseline). In 12 patients, symptoms were resolved, and they were not further evaluated.
Those with inadequate pain control at 4 weeks were permitted to begin a higher dose of methotrexate and to receive additional therapies. At 8 weeks, the reduction in VAS pain score was only modestly increased, reaching a mean 4.5-point reduction from baseline on a mean methotrexate dose of 9.2 mg/week. A substantial proportion of patients had added other medications, such as prednisone and hydroxychloroquine.
Only 20 patients had joint swelling and frank arthritis at baseline. In these, the mean swollen joint count decreased from 7.15 to 2.89 (P less than .0001). There was no further reduction at 8 weeks.
Over the course of the study, there was no evidence that methotrexate exacerbated CHIKV infection.
The data were collected retrospectively, and there was no control group, but the findings inform practitioners of the “possible benefit of low-dose methotrexate to treat both arthralgia and arthritis” in chronic CHIK-associated arthritis, according to Dr. Amaral and his coinvestigators.
The authors declared no potential conflicts of interest.
SOURCE: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943.
FROM JOURNAL OF CLINICAL RHEUMATOLOGY
Key clinical point:
Major finding: On a 10-point visual analog scale, the pain reduction from baseline on methotrexate at 8 weeks was 4.5 (P less than .0001).
Study details: Retrospective observational study.
Disclosures: The authors declared no potential conflicts of interest.
Source: Amaral JK et al. J Clin Rheumatol. 2018 Dec 5. doi: 10.1097/RHU.0000000000000943
Debunking Psoriasis Myths: Psoriasis Is More Than Skin Deep
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
Non-TB mycobacteria infections rising in COPD patients
Veterans with chronic obstructive pulmonary disease (COPD) have seen a sharp increase since 2012 in rates of non-TB mycobacteria infections, which carry a significantly higher risk of death in COPD patients, according to findings from a nationwide study.
For their research, published in Frontiers of Medicine, Fahim Pyarali, MD, and colleagues at the University of Miami, reviewed data from Veterans Affairs hospitals to identify non-TB mycobacteria (NTM) infections among more than 2 million COPD patients seen between 2000 and 2015. Incidence of NTM infections was 34.2 per 100,000 COPD patients in 2001, a rate that remained steady until 2012, when it began climbing sharply through 2015 to reach 70.3 per 100,000 (P = .035). Dr. Pyarali and colleagues also found that, during the study period, prevalence of NTM climbed from 93.1 infections per 100,000 population in 2001 to 277.6 per 100,000 in 2015.
Hotspots for NTM infections included Puerto Rico, which had the highest prevalence seen in the study at 370 infections per 100,000 COPD population; Florida, with 351 per 100,000; and Washington, D.C., with 309 per 100,000. Additional hotspots were identified around Lake Michigan, in coastal Louisiana, and in parts of the Southwest.
Dr. Pyarali and colleagues noted that the geographical concentration of cases near oceans and lakes was “supported by previous findings that warmer temperatures, lower dissolved oxygen, and lower pH in the soils and waters provide a major environmental source for NTM organisms;” however, the study is the first to identify Puerto Rico as having exceptionally high prevalence. The reasons for this should be extensively investigated, the investigators argued.
The mortality risk was 43% higher among NTM-infected patients than in COPD patients without an NTM diagnosis (95% confidence interval, 1.31-1.58; P less than .001), independent of other comorbidities.
Though rates of NTM infection were seen rising steeply in men and women alike, Dr. Pyarali and colleagues noted as a limitation of their study its use of an overwhelmingly male population, writing that this may obscure “the true reach of NTM disease and mortality” in the general population. The average age of NTM diagnosis remained steady throughout the study period, suggesting that rising incidence is not attributable to earlier diagnosis.
Dr. Pyarali and colleagues reported no outside sources of funding or financial conflicts of interest.
SOURCE: Pyarali F et al. Front Med. 2018 Nov 6. doi: 10.3389/fmed2018.00311.
Veterans with chronic obstructive pulmonary disease (COPD) have seen a sharp increase since 2012 in rates of non-TB mycobacteria infections, which carry a significantly higher risk of death in COPD patients, according to findings from a nationwide study.
For their research, published in Frontiers of Medicine, Fahim Pyarali, MD, and colleagues at the University of Miami, reviewed data from Veterans Affairs hospitals to identify non-TB mycobacteria (NTM) infections among more than 2 million COPD patients seen between 2000 and 2015. Incidence of NTM infections was 34.2 per 100,000 COPD patients in 2001, a rate that remained steady until 2012, when it began climbing sharply through 2015 to reach 70.3 per 100,000 (P = .035). Dr. Pyarali and colleagues also found that, during the study period, prevalence of NTM climbed from 93.1 infections per 100,000 population in 2001 to 277.6 per 100,000 in 2015.
Hotspots for NTM infections included Puerto Rico, which had the highest prevalence seen in the study at 370 infections per 100,000 COPD population; Florida, with 351 per 100,000; and Washington, D.C., with 309 per 100,000. Additional hotspots were identified around Lake Michigan, in coastal Louisiana, and in parts of the Southwest.
Dr. Pyarali and colleagues noted that the geographical concentration of cases near oceans and lakes was “supported by previous findings that warmer temperatures, lower dissolved oxygen, and lower pH in the soils and waters provide a major environmental source for NTM organisms;” however, the study is the first to identify Puerto Rico as having exceptionally high prevalence. The reasons for this should be extensively investigated, the investigators argued.
The mortality risk was 43% higher among NTM-infected patients than in COPD patients without an NTM diagnosis (95% confidence interval, 1.31-1.58; P less than .001), independent of other comorbidities.
Though rates of NTM infection were seen rising steeply in men and women alike, Dr. Pyarali and colleagues noted as a limitation of their study its use of an overwhelmingly male population, writing that this may obscure “the true reach of NTM disease and mortality” in the general population. The average age of NTM diagnosis remained steady throughout the study period, suggesting that rising incidence is not attributable to earlier diagnosis.
Dr. Pyarali and colleagues reported no outside sources of funding or financial conflicts of interest.
SOURCE: Pyarali F et al. Front Med. 2018 Nov 6. doi: 10.3389/fmed2018.00311.
Veterans with chronic obstructive pulmonary disease (COPD) have seen a sharp increase since 2012 in rates of non-TB mycobacteria infections, which carry a significantly higher risk of death in COPD patients, according to findings from a nationwide study.
For their research, published in Frontiers of Medicine, Fahim Pyarali, MD, and colleagues at the University of Miami, reviewed data from Veterans Affairs hospitals to identify non-TB mycobacteria (NTM) infections among more than 2 million COPD patients seen between 2000 and 2015. Incidence of NTM infections was 34.2 per 100,000 COPD patients in 2001, a rate that remained steady until 2012, when it began climbing sharply through 2015 to reach 70.3 per 100,000 (P = .035). Dr. Pyarali and colleagues also found that, during the study period, prevalence of NTM climbed from 93.1 infections per 100,000 population in 2001 to 277.6 per 100,000 in 2015.
Hotspots for NTM infections included Puerto Rico, which had the highest prevalence seen in the study at 370 infections per 100,000 COPD population; Florida, with 351 per 100,000; and Washington, D.C., with 309 per 100,000. Additional hotspots were identified around Lake Michigan, in coastal Louisiana, and in parts of the Southwest.
Dr. Pyarali and colleagues noted that the geographical concentration of cases near oceans and lakes was “supported by previous findings that warmer temperatures, lower dissolved oxygen, and lower pH in the soils and waters provide a major environmental source for NTM organisms;” however, the study is the first to identify Puerto Rico as having exceptionally high prevalence. The reasons for this should be extensively investigated, the investigators argued.
The mortality risk was 43% higher among NTM-infected patients than in COPD patients without an NTM diagnosis (95% confidence interval, 1.31-1.58; P less than .001), independent of other comorbidities.
Though rates of NTM infection were seen rising steeply in men and women alike, Dr. Pyarali and colleagues noted as a limitation of their study its use of an overwhelmingly male population, writing that this may obscure “the true reach of NTM disease and mortality” in the general population. The average age of NTM diagnosis remained steady throughout the study period, suggesting that rising incidence is not attributable to earlier diagnosis.
Dr. Pyarali and colleagues reported no outside sources of funding or financial conflicts of interest.
SOURCE: Pyarali F et al. Front Med. 2018 Nov 6. doi: 10.3389/fmed2018.00311.
FROM FRONTIERS IN MEDICINE
Key clinical point: Incidence and prevalence of non-TB mycobacteria infections rose sharply in a national veterans population with chronic obstructive pulmonary disease after 2012.
Major finding: Incidence of non-TB mycobacteria infections doubled in chronic obstructive pulmonary disease patients between 2001 and 2015, with most of the increase seen after 2012
Study details: A retrospective, cross-sectional study using records from over 2 million, mostly male chronic obstructive pulmonary disease patients in a Veterans Affairs database.
Disclosures: The study authors reported no outside sources of funding or financial conflicts of interest.
Source: Pyarali F et al. Front Med. 2018 Nov 6. doi: 10.3389/fmed2018.00311.
NSAIDs can play major role in pre- and postoperative hysterectomy pain
LAS VEGAS – An ob.gyn. has some handy hysterectomy-related pain management tips for her colleagues: Don’t assume patients know how to titrate between NSAIDs and opioids after surgery. Consider neuropathic medications alone in patients undergoing minimally invasive hysterectomies. And
Sawsan As-Sanie, MD, MPH, director of the University of Michigan Endometriosis Center, Ann Arbor, offered these and other recommendations about hysterectomy-related pain at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Try acetaminophen and an NSAID
In the preoperative period, a combination of acetaminophen (Tylenol) and an NSAID can provide significant postop relief, Dr. As-Sanie said.
She highlighted a 2010 systematic review of 21 studies that included 1,909 patients and found acetaminophen/NSAID combinations improved pain intensity by about 35% in positive studies when compared with either acetaminophen or NSAID alone. The painkiller combination was positive – more effective than a solo agent – in 85% of studies of combo versus acetaminophen alone and 64% of studies of combo versus NSAID alone (Anesth Analg. 2010 Apr 1;110[4]:1170-9).
Another study, she said, found that there’s no clear advantage to IV administration for acetaminophen if patients can take the drug orally (Can J Hosp Pharm. 2015 May-Jun;68[3]:238-47).
Consider gabapentin, but not postoperatively
Dr. As-Sanie pointed to a 2014 systematic review and meta-analysis that suggested the use of preoperative gabapentin in abdominal hysterectomy reduces pain and opioid use. However, adding postoperative doses of gabapentin, she said, don’t appear to produce a greater effect (Obstet Gynecol. 2014 Jun;123[6]:1221-9).
Consider neuropathics for minimally invasive hysterectomy
Two studies, one in 2004 and the other in 2008, suggest that gabapentin (on a postop basis) and pregabalin (perioperatively) can reduce postop opioid use. (Pregabalin also was linked to more adverse effects.) “Even if they’re having a little bit of pain, they’re using fewer opioids,” she said (Pain. 2004 Jul;110[1-2]:175-81; Pain. 2008 Jan;134[1-2]:106-12).
Educate patients about postop painkiller use
Don’t assume that patients know how to adjust their over-the-counter painkiller use after surgery, Dr. As-Sanie said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company. “While we as physicians think that knowledge about the use of ibuprofen and Tylenol is something everyone should be born with, it’s not obvious to most patients and families.”
It’s important to teach patients to start with NSAIDs or Tylenol postoperatively, and if that doesn’t control pain, “you add opioids and use medications to control constipation as needed. As you recover, you reduce the amount of opioids first and then reduce the NSAIDs or Tylenol,” she said. “That education can be very helpful for the vast majority of patients, and it’s one of the most important things we can provide.”
Don’t over-prescribe opioids
For a 2017 study, Dr. As-Sanie and colleagues tracked hysterectomy patients and surveyed them about their postop opioid use. “When asked 2 weeks after surgery, most used far less than half of what they prescribed,” Dr. As-Sanie said. “If we gave them about 40 pills, they had between 13-15 pills left after the surgery on average. Nearly 50% didn’t use any of their medication” (Obstet Gynecol. 2017 Dec;130[6]:1261-8).
Dr. As-Sanie urged colleagues to remember the lesson of the rise of super-sized portions at fast-food restaurants: Give people more of something and they’ll eat (or use) more of it. And the reverse is true: “If you give people fewer pills, they will use fewer pills.”
Dr. As-Sanie highlighted the recommendations about opioid prescription levels for various surgical procedures, including different types of hysterectomies, at www.opioidprescribing.info. The recommendations are provided by the Michigan Opioid Prescribing Engagement Network. They’re designed for opioid-naive patients and suggest the lowest doses for vaginal hysterectomy and the highest for abdominal hysterectomy, with recommended doses for laparoscopic and robotic hysterectomy in between.
Dr. As-Sanie disclosed she is a consultant for AbbVie and Myovant.
LAS VEGAS – An ob.gyn. has some handy hysterectomy-related pain management tips for her colleagues: Don’t assume patients know how to titrate between NSAIDs and opioids after surgery. Consider neuropathic medications alone in patients undergoing minimally invasive hysterectomies. And
Sawsan As-Sanie, MD, MPH, director of the University of Michigan Endometriosis Center, Ann Arbor, offered these and other recommendations about hysterectomy-related pain at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Try acetaminophen and an NSAID
In the preoperative period, a combination of acetaminophen (Tylenol) and an NSAID can provide significant postop relief, Dr. As-Sanie said.
She highlighted a 2010 systematic review of 21 studies that included 1,909 patients and found acetaminophen/NSAID combinations improved pain intensity by about 35% in positive studies when compared with either acetaminophen or NSAID alone. The painkiller combination was positive – more effective than a solo agent – in 85% of studies of combo versus acetaminophen alone and 64% of studies of combo versus NSAID alone (Anesth Analg. 2010 Apr 1;110[4]:1170-9).
Another study, she said, found that there’s no clear advantage to IV administration for acetaminophen if patients can take the drug orally (Can J Hosp Pharm. 2015 May-Jun;68[3]:238-47).
Consider gabapentin, but not postoperatively
Dr. As-Sanie pointed to a 2014 systematic review and meta-analysis that suggested the use of preoperative gabapentin in abdominal hysterectomy reduces pain and opioid use. However, adding postoperative doses of gabapentin, she said, don’t appear to produce a greater effect (Obstet Gynecol. 2014 Jun;123[6]:1221-9).
Consider neuropathics for minimally invasive hysterectomy
Two studies, one in 2004 and the other in 2008, suggest that gabapentin (on a postop basis) and pregabalin (perioperatively) can reduce postop opioid use. (Pregabalin also was linked to more adverse effects.) “Even if they’re having a little bit of pain, they’re using fewer opioids,” she said (Pain. 2004 Jul;110[1-2]:175-81; Pain. 2008 Jan;134[1-2]:106-12).
Educate patients about postop painkiller use
Don’t assume that patients know how to adjust their over-the-counter painkiller use after surgery, Dr. As-Sanie said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company. “While we as physicians think that knowledge about the use of ibuprofen and Tylenol is something everyone should be born with, it’s not obvious to most patients and families.”
It’s important to teach patients to start with NSAIDs or Tylenol postoperatively, and if that doesn’t control pain, “you add opioids and use medications to control constipation as needed. As you recover, you reduce the amount of opioids first and then reduce the NSAIDs or Tylenol,” she said. “That education can be very helpful for the vast majority of patients, and it’s one of the most important things we can provide.”
Don’t over-prescribe opioids
For a 2017 study, Dr. As-Sanie and colleagues tracked hysterectomy patients and surveyed them about their postop opioid use. “When asked 2 weeks after surgery, most used far less than half of what they prescribed,” Dr. As-Sanie said. “If we gave them about 40 pills, they had between 13-15 pills left after the surgery on average. Nearly 50% didn’t use any of their medication” (Obstet Gynecol. 2017 Dec;130[6]:1261-8).
Dr. As-Sanie urged colleagues to remember the lesson of the rise of super-sized portions at fast-food restaurants: Give people more of something and they’ll eat (or use) more of it. And the reverse is true: “If you give people fewer pills, they will use fewer pills.”
Dr. As-Sanie highlighted the recommendations about opioid prescription levels for various surgical procedures, including different types of hysterectomies, at www.opioidprescribing.info. The recommendations are provided by the Michigan Opioid Prescribing Engagement Network. They’re designed for opioid-naive patients and suggest the lowest doses for vaginal hysterectomy and the highest for abdominal hysterectomy, with recommended doses for laparoscopic and robotic hysterectomy in between.
Dr. As-Sanie disclosed she is a consultant for AbbVie and Myovant.
LAS VEGAS – An ob.gyn. has some handy hysterectomy-related pain management tips for her colleagues: Don’t assume patients know how to titrate between NSAIDs and opioids after surgery. Consider neuropathic medications alone in patients undergoing minimally invasive hysterectomies. And
Sawsan As-Sanie, MD, MPH, director of the University of Michigan Endometriosis Center, Ann Arbor, offered these and other recommendations about hysterectomy-related pain at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Try acetaminophen and an NSAID
In the preoperative period, a combination of acetaminophen (Tylenol) and an NSAID can provide significant postop relief, Dr. As-Sanie said.
She highlighted a 2010 systematic review of 21 studies that included 1,909 patients and found acetaminophen/NSAID combinations improved pain intensity by about 35% in positive studies when compared with either acetaminophen or NSAID alone. The painkiller combination was positive – more effective than a solo agent – in 85% of studies of combo versus acetaminophen alone and 64% of studies of combo versus NSAID alone (Anesth Analg. 2010 Apr 1;110[4]:1170-9).
Another study, she said, found that there’s no clear advantage to IV administration for acetaminophen if patients can take the drug orally (Can J Hosp Pharm. 2015 May-Jun;68[3]:238-47).
Consider gabapentin, but not postoperatively
Dr. As-Sanie pointed to a 2014 systematic review and meta-analysis that suggested the use of preoperative gabapentin in abdominal hysterectomy reduces pain and opioid use. However, adding postoperative doses of gabapentin, she said, don’t appear to produce a greater effect (Obstet Gynecol. 2014 Jun;123[6]:1221-9).
Consider neuropathics for minimally invasive hysterectomy
Two studies, one in 2004 and the other in 2008, suggest that gabapentin (on a postop basis) and pregabalin (perioperatively) can reduce postop opioid use. (Pregabalin also was linked to more adverse effects.) “Even if they’re having a little bit of pain, they’re using fewer opioids,” she said (Pain. 2004 Jul;110[1-2]:175-81; Pain. 2008 Jan;134[1-2]:106-12).
Educate patients about postop painkiller use
Don’t assume that patients know how to adjust their over-the-counter painkiller use after surgery, Dr. As-Sanie said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company. “While we as physicians think that knowledge about the use of ibuprofen and Tylenol is something everyone should be born with, it’s not obvious to most patients and families.”
It’s important to teach patients to start with NSAIDs or Tylenol postoperatively, and if that doesn’t control pain, “you add opioids and use medications to control constipation as needed. As you recover, you reduce the amount of opioids first and then reduce the NSAIDs or Tylenol,” she said. “That education can be very helpful for the vast majority of patients, and it’s one of the most important things we can provide.”
Don’t over-prescribe opioids
For a 2017 study, Dr. As-Sanie and colleagues tracked hysterectomy patients and surveyed them about their postop opioid use. “When asked 2 weeks after surgery, most used far less than half of what they prescribed,” Dr. As-Sanie said. “If we gave them about 40 pills, they had between 13-15 pills left after the surgery on average. Nearly 50% didn’t use any of their medication” (Obstet Gynecol. 2017 Dec;130[6]:1261-8).
Dr. As-Sanie urged colleagues to remember the lesson of the rise of super-sized portions at fast-food restaurants: Give people more of something and they’ll eat (or use) more of it. And the reverse is true: “If you give people fewer pills, they will use fewer pills.”
Dr. As-Sanie highlighted the recommendations about opioid prescription levels for various surgical procedures, including different types of hysterectomies, at www.opioidprescribing.info. The recommendations are provided by the Michigan Opioid Prescribing Engagement Network. They’re designed for opioid-naive patients and suggest the lowest doses for vaginal hysterectomy and the highest for abdominal hysterectomy, with recommended doses for laparoscopic and robotic hysterectomy in between.
Dr. As-Sanie disclosed she is a consultant for AbbVie and Myovant.
EXPERT ANALYSIS FROM PAGS
Uterine volume, fibroid diameter predict robotic myomectomy duration
LAS VEGAS – It would be nice if surgeons could know beforehand how long robotic laparoscopic myomectomies will take, according to Peter Movilla, MD, a minimally invasive gynecologic surgery fellow at Newton (Mass.) Wellesley Hospital.
Best guesses are sometimes wrong, and it’s not uncommon for robotic cases to go longer than expected, especially when they have to be converted to an open approach.
Among other problems, going long backs up operating room (OR)scheduling and makes families impatient. Also, if it was known beforehand that a robotic case might take 5 hours, patients could be offered a quicker open procedure, especially if they are not good candidates for prolonged pneumoperitoneum.
After a case went past 6 hours at the University of California, San Francisco (UCSF), when Dr. Movilla was an ob.gyn. resident, he wanted to find a better way.
“I saw that we were not the best at guessing how long these surgeries were going to take, and thought maybe we could make prediction a little better by [incorporating] preoperative factors” in a structured way. “I wanted to create something that would give us an answer of how long it will take,” he said at a meeting sponsored by AAGL.
So he and his colleagues reviewed 126 robot-assisted laparoscopic myomectomies at UCSF. The mean operative time from skin incision to closure was 213 minutes, mean specimen weight 264.4 g, mean dominant fibroid diameter 8.5 cm, and mean number of fibroids removed 2.5. Four cases (3%) were converted to open laparotomy.
The team divided the cases by how long they took; 20% were under 3 hours, 70% took 3-5 hours; and 10% went over 5 hours. “Five hours is a long time to be in the OR,” especially when a case could have been done open, Dr. Movilla said.
Length of surgery correlated with 7 of the 21 preoperative factors considered on multivariate logistic regression. Cases tended to be longer in younger women and in women with diabetes, and when surgeons had less experience. There was a trend toward longer cases with higher body mass indices, but it was not statistically significant.
Having three or more fibroids on preoperative imaging and a larger number of fibroids over 3 cm were predictive of operations longer than 3 hours. However, the strongest predictors of long cases were uterine volume and the diameter of the largest fibroid, a mean of 532.4 cm3 and 8.8 cm, respectively, in cases over 5 hours. Posterior and intramural fibroids also increased operative time, but, again, the trends were not statistically significant.
The team put it all together in a risk calculator they tested against their subjects’ actual surgery times. The model tended to underestimate very short and very long cases at either end of the curve, but overall the fit was “not too bad,” and the more cases that are added to the model, the more accurate it will get, Dr. Movilla said.
There was no external funding for the work, and Dr. Movilla had no disclosures.
SOURCE: Movilla P et al. 2018 AAGL Global Congress, Abstract 69.
LAS VEGAS – It would be nice if surgeons could know beforehand how long robotic laparoscopic myomectomies will take, according to Peter Movilla, MD, a minimally invasive gynecologic surgery fellow at Newton (Mass.) Wellesley Hospital.
Best guesses are sometimes wrong, and it’s not uncommon for robotic cases to go longer than expected, especially when they have to be converted to an open approach.
Among other problems, going long backs up operating room (OR)scheduling and makes families impatient. Also, if it was known beforehand that a robotic case might take 5 hours, patients could be offered a quicker open procedure, especially if they are not good candidates for prolonged pneumoperitoneum.
After a case went past 6 hours at the University of California, San Francisco (UCSF), when Dr. Movilla was an ob.gyn. resident, he wanted to find a better way.
“I saw that we were not the best at guessing how long these surgeries were going to take, and thought maybe we could make prediction a little better by [incorporating] preoperative factors” in a structured way. “I wanted to create something that would give us an answer of how long it will take,” he said at a meeting sponsored by AAGL.
So he and his colleagues reviewed 126 robot-assisted laparoscopic myomectomies at UCSF. The mean operative time from skin incision to closure was 213 minutes, mean specimen weight 264.4 g, mean dominant fibroid diameter 8.5 cm, and mean number of fibroids removed 2.5. Four cases (3%) were converted to open laparotomy.
The team divided the cases by how long they took; 20% were under 3 hours, 70% took 3-5 hours; and 10% went over 5 hours. “Five hours is a long time to be in the OR,” especially when a case could have been done open, Dr. Movilla said.
Length of surgery correlated with 7 of the 21 preoperative factors considered on multivariate logistic regression. Cases tended to be longer in younger women and in women with diabetes, and when surgeons had less experience. There was a trend toward longer cases with higher body mass indices, but it was not statistically significant.
Having three or more fibroids on preoperative imaging and a larger number of fibroids over 3 cm were predictive of operations longer than 3 hours. However, the strongest predictors of long cases were uterine volume and the diameter of the largest fibroid, a mean of 532.4 cm3 and 8.8 cm, respectively, in cases over 5 hours. Posterior and intramural fibroids also increased operative time, but, again, the trends were not statistically significant.
The team put it all together in a risk calculator they tested against their subjects’ actual surgery times. The model tended to underestimate very short and very long cases at either end of the curve, but overall the fit was “not too bad,” and the more cases that are added to the model, the more accurate it will get, Dr. Movilla said.
There was no external funding for the work, and Dr. Movilla had no disclosures.
SOURCE: Movilla P et al. 2018 AAGL Global Congress, Abstract 69.
LAS VEGAS – It would be nice if surgeons could know beforehand how long robotic laparoscopic myomectomies will take, according to Peter Movilla, MD, a minimally invasive gynecologic surgery fellow at Newton (Mass.) Wellesley Hospital.
Best guesses are sometimes wrong, and it’s not uncommon for robotic cases to go longer than expected, especially when they have to be converted to an open approach.
Among other problems, going long backs up operating room (OR)scheduling and makes families impatient. Also, if it was known beforehand that a robotic case might take 5 hours, patients could be offered a quicker open procedure, especially if they are not good candidates for prolonged pneumoperitoneum.
After a case went past 6 hours at the University of California, San Francisco (UCSF), when Dr. Movilla was an ob.gyn. resident, he wanted to find a better way.
“I saw that we were not the best at guessing how long these surgeries were going to take, and thought maybe we could make prediction a little better by [incorporating] preoperative factors” in a structured way. “I wanted to create something that would give us an answer of how long it will take,” he said at a meeting sponsored by AAGL.
So he and his colleagues reviewed 126 robot-assisted laparoscopic myomectomies at UCSF. The mean operative time from skin incision to closure was 213 minutes, mean specimen weight 264.4 g, mean dominant fibroid diameter 8.5 cm, and mean number of fibroids removed 2.5. Four cases (3%) were converted to open laparotomy.
The team divided the cases by how long they took; 20% were under 3 hours, 70% took 3-5 hours; and 10% went over 5 hours. “Five hours is a long time to be in the OR,” especially when a case could have been done open, Dr. Movilla said.
Length of surgery correlated with 7 of the 21 preoperative factors considered on multivariate logistic regression. Cases tended to be longer in younger women and in women with diabetes, and when surgeons had less experience. There was a trend toward longer cases with higher body mass indices, but it was not statistically significant.
Having three or more fibroids on preoperative imaging and a larger number of fibroids over 3 cm were predictive of operations longer than 3 hours. However, the strongest predictors of long cases were uterine volume and the diameter of the largest fibroid, a mean of 532.4 cm3 and 8.8 cm, respectively, in cases over 5 hours. Posterior and intramural fibroids also increased operative time, but, again, the trends were not statistically significant.
The team put it all together in a risk calculator they tested against their subjects’ actual surgery times. The model tended to underestimate very short and very long cases at either end of the curve, but overall the fit was “not too bad,” and the more cases that are added to the model, the more accurate it will get, Dr. Movilla said.
There was no external funding for the work, and Dr. Movilla had no disclosures.
SOURCE: Movilla P et al. 2018 AAGL Global Congress, Abstract 69.
REPORTING FROM AAGL GLOBAL CONGRESS
Key clinical point: A calculator is in the works to predict exactly how long robotic myomectomies will take.
Major finding: a mean of 532.4 cm3 and 8.8 cm, respectively, in cases over 5 hours.
Study details: Review of 126 cases.
Disclosures: There was no external funding, and Dr. Movilla had no disclosures.
Source: Movilla P et al. 2018 AAGL Global Congress, Abstract 69.
Gynecologic surgery insufflation pressure: Less is more
LAS VEGAS – performed at a single center by the same surgeon, said researchers at New York University (NYU) Medical Center.
Each incremental drop in abdominal insufflation pressure “improved intraoperative and postoperative clinical outcomes” with “faster postoperative recovery times, decreased immediate postoperative pain, and improved intraoperative respiratory parameters, without increasing duration of surgery or blood loss,” said investigator Christine Foley, MD, formerly at NYU, and now a minimally-invasive gynecologic surgery fellow at the University of Pittsburgh.
An abdominal insufflation pressure of 10 mm Hg or less was the sweet spot, she said at the meeting sponsored by AAGL.
The general surgery literature recommends operating at the lowest possible abdominal insufflation pressure to reduce postoperative pain, and that recommendation has been incorporated into enhanced recovery after surgery (ERAS) protocols. Gynecologic surgeons have not routinely followed suit, she noted. “Surgeons should consider operating at lower insufflation pressures to improve patient outcomes and PACU [postanesthesia care unit] utilization. Further research is warranted to determine if lower pressures ... should be included in ERAS protocols” for gynecologic surgery.
There’s not much in the way of data on insufflation pressures in robotic gynecologic surgery. What has been published suggests, as in general surgery, less postop pain, but at the cost of impaired visualization and greater blood loss. At the moment, robotic cases are often done at insufflation pressures above 12 mm Hg.
To get a better grasp of the issue, Dr. Foley and her team reviewed 196 hysterectomies, 275 myomectomies, and 127 endometriosis surgeries at NYU, all performed robotically by the same surgeon for benign indications. Ninety-nine cases were at 15 mm Hg; 100 at 12 mm Hg; 99 at 10 mm Hg, and 300 at 8 mm Hg.
The study did not address why the surgeon opted for different pressures in different cases. The body mass index was a mean of 27 kg/m2, and patient age was about 40 years, in all four pressure groups. There were trends for higher pressures with hysterectomies and lower pressures for endometriosis, but also considerable crossover, with more than 40% of the hysterectomies performed at 8 mm Hg, and almost 10% of the endometriosis cases done at 15 mm Hg.
Across the board, patients did better at lower pressures. Each drop in insufflation pressure correlated with a significant decrease in the initial pain score in the PACU (5.9 out of 10 points at 15 mm Hg, 5.4 at 12 mm Hg, 4.4 at 10 mm Hg, and 3.8 at 8 mm Hg, P less than .0001); lower pressures also correlated with shorter PACU stays (449 minutes, 467 minutes, 351 minutes, and 317 minutes, P less than .0001).
Surgery duration was a mean of 70 minutes across all four groups. Estimated blood loss was 114 mL at 15 mm Hg, 97.4 mL at 12 mm Hg, 127 mL 10 mm Hg, and 78.4 mL at 8 mm HG; the differences were not statistically significant. Maximum PACU pain levels favored lower pressures, and lower pressures correlated with significantly lower peak inspiratory pressures and tidal volumes.
The results argue for operating at the lowest possible pressure, Dr. Foley said, but she and her team did not address how their outcomes might have been influenced by the type of surgery the women had.
There was no external funding for the study. Dr. Foley had no relevant financial disclosures.
SOURCE: Foley C et al. 2018 AAGL Global Congress, Abstract 23.
LAS VEGAS – performed at a single center by the same surgeon, said researchers at New York University (NYU) Medical Center.
Each incremental drop in abdominal insufflation pressure “improved intraoperative and postoperative clinical outcomes” with “faster postoperative recovery times, decreased immediate postoperative pain, and improved intraoperative respiratory parameters, without increasing duration of surgery or blood loss,” said investigator Christine Foley, MD, formerly at NYU, and now a minimally-invasive gynecologic surgery fellow at the University of Pittsburgh.
An abdominal insufflation pressure of 10 mm Hg or less was the sweet spot, she said at the meeting sponsored by AAGL.
The general surgery literature recommends operating at the lowest possible abdominal insufflation pressure to reduce postoperative pain, and that recommendation has been incorporated into enhanced recovery after surgery (ERAS) protocols. Gynecologic surgeons have not routinely followed suit, she noted. “Surgeons should consider operating at lower insufflation pressures to improve patient outcomes and PACU [postanesthesia care unit] utilization. Further research is warranted to determine if lower pressures ... should be included in ERAS protocols” for gynecologic surgery.
There’s not much in the way of data on insufflation pressures in robotic gynecologic surgery. What has been published suggests, as in general surgery, less postop pain, but at the cost of impaired visualization and greater blood loss. At the moment, robotic cases are often done at insufflation pressures above 12 mm Hg.
To get a better grasp of the issue, Dr. Foley and her team reviewed 196 hysterectomies, 275 myomectomies, and 127 endometriosis surgeries at NYU, all performed robotically by the same surgeon for benign indications. Ninety-nine cases were at 15 mm Hg; 100 at 12 mm Hg; 99 at 10 mm Hg, and 300 at 8 mm Hg.
The study did not address why the surgeon opted for different pressures in different cases. The body mass index was a mean of 27 kg/m2, and patient age was about 40 years, in all four pressure groups. There were trends for higher pressures with hysterectomies and lower pressures for endometriosis, but also considerable crossover, with more than 40% of the hysterectomies performed at 8 mm Hg, and almost 10% of the endometriosis cases done at 15 mm Hg.
Across the board, patients did better at lower pressures. Each drop in insufflation pressure correlated with a significant decrease in the initial pain score in the PACU (5.9 out of 10 points at 15 mm Hg, 5.4 at 12 mm Hg, 4.4 at 10 mm Hg, and 3.8 at 8 mm Hg, P less than .0001); lower pressures also correlated with shorter PACU stays (449 minutes, 467 minutes, 351 minutes, and 317 minutes, P less than .0001).
Surgery duration was a mean of 70 minutes across all four groups. Estimated blood loss was 114 mL at 15 mm Hg, 97.4 mL at 12 mm Hg, 127 mL 10 mm Hg, and 78.4 mL at 8 mm HG; the differences were not statistically significant. Maximum PACU pain levels favored lower pressures, and lower pressures correlated with significantly lower peak inspiratory pressures and tidal volumes.
The results argue for operating at the lowest possible pressure, Dr. Foley said, but she and her team did not address how their outcomes might have been influenced by the type of surgery the women had.
There was no external funding for the study. Dr. Foley had no relevant financial disclosures.
SOURCE: Foley C et al. 2018 AAGL Global Congress, Abstract 23.
LAS VEGAS – performed at a single center by the same surgeon, said researchers at New York University (NYU) Medical Center.
Each incremental drop in abdominal insufflation pressure “improved intraoperative and postoperative clinical outcomes” with “faster postoperative recovery times, decreased immediate postoperative pain, and improved intraoperative respiratory parameters, without increasing duration of surgery or blood loss,” said investigator Christine Foley, MD, formerly at NYU, and now a minimally-invasive gynecologic surgery fellow at the University of Pittsburgh.
An abdominal insufflation pressure of 10 mm Hg or less was the sweet spot, she said at the meeting sponsored by AAGL.
The general surgery literature recommends operating at the lowest possible abdominal insufflation pressure to reduce postoperative pain, and that recommendation has been incorporated into enhanced recovery after surgery (ERAS) protocols. Gynecologic surgeons have not routinely followed suit, she noted. “Surgeons should consider operating at lower insufflation pressures to improve patient outcomes and PACU [postanesthesia care unit] utilization. Further research is warranted to determine if lower pressures ... should be included in ERAS protocols” for gynecologic surgery.
There’s not much in the way of data on insufflation pressures in robotic gynecologic surgery. What has been published suggests, as in general surgery, less postop pain, but at the cost of impaired visualization and greater blood loss. At the moment, robotic cases are often done at insufflation pressures above 12 mm Hg.
To get a better grasp of the issue, Dr. Foley and her team reviewed 196 hysterectomies, 275 myomectomies, and 127 endometriosis surgeries at NYU, all performed robotically by the same surgeon for benign indications. Ninety-nine cases were at 15 mm Hg; 100 at 12 mm Hg; 99 at 10 mm Hg, and 300 at 8 mm Hg.
The study did not address why the surgeon opted for different pressures in different cases. The body mass index was a mean of 27 kg/m2, and patient age was about 40 years, in all four pressure groups. There were trends for higher pressures with hysterectomies and lower pressures for endometriosis, but also considerable crossover, with more than 40% of the hysterectomies performed at 8 mm Hg, and almost 10% of the endometriosis cases done at 15 mm Hg.
Across the board, patients did better at lower pressures. Each drop in insufflation pressure correlated with a significant decrease in the initial pain score in the PACU (5.9 out of 10 points at 15 mm Hg, 5.4 at 12 mm Hg, 4.4 at 10 mm Hg, and 3.8 at 8 mm Hg, P less than .0001); lower pressures also correlated with shorter PACU stays (449 minutes, 467 minutes, 351 minutes, and 317 minutes, P less than .0001).
Surgery duration was a mean of 70 minutes across all four groups. Estimated blood loss was 114 mL at 15 mm Hg, 97.4 mL at 12 mm Hg, 127 mL 10 mm Hg, and 78.4 mL at 8 mm HG; the differences were not statistically significant. Maximum PACU pain levels favored lower pressures, and lower pressures correlated with significantly lower peak inspiratory pressures and tidal volumes.
The results argue for operating at the lowest possible pressure, Dr. Foley said, but she and her team did not address how their outcomes might have been influenced by the type of surgery the women had.
There was no external funding for the study. Dr. Foley had no relevant financial disclosures.
SOURCE: Foley C et al. 2018 AAGL Global Congress, Abstract 23.
REPORTING FROM AAGL GLOBAL CONGRESS
Key clinical point: Lower insufflation pressures were associated with improved patient outcomes and reduced PACU use.
Major finding: There was a significant decrease in initial postop pain score with each incremental drop in insufflation pressure (5.9 out of 10 points at 15 mm Hg, 5.4 at 12 mm Hg; 4.4 at 10 mm Hg, and 3.8 at 8 mm Hg, P less than .0001).
Study details: Review of 598 robotic gynecologic procedures done at New York University by the same surgeon.
Disclosures: There was no external funding for the study. The presenter did not have any relevant financial disclosures.
Source: Foley C et al. 2018 AAGL Global Congress, Abstract 23.
Gap in care: Female patients with incontinence
LAS VEGAS – A pelvic surgeon brought a bold message to a gathering of gynecologists: There’s a great gap in American care for pelvic floor disorders such as urinary incontinence, and they’re the right physicians to make a difference by treating these common conditions.
“There are never going to be enough specialists to deal with these problems. This is a natural progression for many of you,” said urogynecologist and pelvic surgeon Mickey M. Karram, MD, in a joint presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium. In fact, he said, “there’s so much disease out there to fix that you may become more overwhelmed.”
Dr. Karram, who has offices in Cincinnati, Beverly Hills, and Orange County, Calif., spoke about female urinary incontinence with obstetrician-gynecologist Beri M. Ridgeway, MD, of Cleveland Clinic. They offered these tips:
Test for stress incontinence
Dr. Karram recommends using a “quick and easy” cystometrogram (CMG) test to “corroborate or refute what the patient thinks is going on” in regard to urinary function. “With this simple test, you’ll get a clear understanding of sensation [to urinate] and of what their fullness and capacity numbers are,” he said. And if you have the patient cough or strain during the test, “you should be able to duplicate a sign of stress incontinence 90% of the time.”
If patients don’t leak when they take this test, there may be another problem such as overactive bladder, a condition that can’t be duplicated via the test, he said.
Ask the right questions
When it comes to identifying when they have urinary difficulties, some patients “say yes to every question we ask,” said Dr. Ridgeway, and they may not be able to distinguish between urgency and leakage.
A better approach is to ask women to provide specific examples of when they have continence issues, she said. It’s also useful to ask patients about what bothers them the most if they have multiple symptoms: Is it urgency (“Gotta go; gotta go”)? Leakage during certain situations like coughing and laughing? “That helps me decide how to go about treating them first and foremost,” she said. “It doesn’t mean you won’t treat both [problems], but it really gives you a reference point of where to start.”
Research suggests that women tend to be more bothered by urge incontinence than stress incontinence, she said, because they can regulate their activities or avoid the stress form.
Beware of acute incontinence cases
“If a woman walks in and says ‘Everything was great until a week or two ago, but now I’m living in pads,’ it could be a fecal impaction or a pelvic mass,” Dr. Karram said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Discuss the many treatment options
In some cases of incontinence, Dr. Ridgeway said she’ll mention “the array of treatment options, such as pelvic floor physical therapy, bladder retraining, vaginal estrogen, medications, and Botox.”
She added: “I explain that we’ll work together, and sometimes it will take a couple tries, or we’ll try a couple things at once.”
Dr. Ridgeway disclosed consulting for Coloplast and serving as an independent contractor (legal) for Ethicon. Dr. Karram disclosed speaking for Allergan, Astellas Pharma, Coloplast, and Cynosure/Hologic; consulting for Coloplast and Cynosure/Hologic; and receiving royalties from BihlerMed.
LAS VEGAS – A pelvic surgeon brought a bold message to a gathering of gynecologists: There’s a great gap in American care for pelvic floor disorders such as urinary incontinence, and they’re the right physicians to make a difference by treating these common conditions.
“There are never going to be enough specialists to deal with these problems. This is a natural progression for many of you,” said urogynecologist and pelvic surgeon Mickey M. Karram, MD, in a joint presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium. In fact, he said, “there’s so much disease out there to fix that you may become more overwhelmed.”
Dr. Karram, who has offices in Cincinnati, Beverly Hills, and Orange County, Calif., spoke about female urinary incontinence with obstetrician-gynecologist Beri M. Ridgeway, MD, of Cleveland Clinic. They offered these tips:
Test for stress incontinence
Dr. Karram recommends using a “quick and easy” cystometrogram (CMG) test to “corroborate or refute what the patient thinks is going on” in regard to urinary function. “With this simple test, you’ll get a clear understanding of sensation [to urinate] and of what their fullness and capacity numbers are,” he said. And if you have the patient cough or strain during the test, “you should be able to duplicate a sign of stress incontinence 90% of the time.”
If patients don’t leak when they take this test, there may be another problem such as overactive bladder, a condition that can’t be duplicated via the test, he said.
Ask the right questions
When it comes to identifying when they have urinary difficulties, some patients “say yes to every question we ask,” said Dr. Ridgeway, and they may not be able to distinguish between urgency and leakage.
A better approach is to ask women to provide specific examples of when they have continence issues, she said. It’s also useful to ask patients about what bothers them the most if they have multiple symptoms: Is it urgency (“Gotta go; gotta go”)? Leakage during certain situations like coughing and laughing? “That helps me decide how to go about treating them first and foremost,” she said. “It doesn’t mean you won’t treat both [problems], but it really gives you a reference point of where to start.”
Research suggests that women tend to be more bothered by urge incontinence than stress incontinence, she said, because they can regulate their activities or avoid the stress form.
Beware of acute incontinence cases
“If a woman walks in and says ‘Everything was great until a week or two ago, but now I’m living in pads,’ it could be a fecal impaction or a pelvic mass,” Dr. Karram said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Discuss the many treatment options
In some cases of incontinence, Dr. Ridgeway said she’ll mention “the array of treatment options, such as pelvic floor physical therapy, bladder retraining, vaginal estrogen, medications, and Botox.”
She added: “I explain that we’ll work together, and sometimes it will take a couple tries, or we’ll try a couple things at once.”
Dr. Ridgeway disclosed consulting for Coloplast and serving as an independent contractor (legal) for Ethicon. Dr. Karram disclosed speaking for Allergan, Astellas Pharma, Coloplast, and Cynosure/Hologic; consulting for Coloplast and Cynosure/Hologic; and receiving royalties from BihlerMed.
LAS VEGAS – A pelvic surgeon brought a bold message to a gathering of gynecologists: There’s a great gap in American care for pelvic floor disorders such as urinary incontinence, and they’re the right physicians to make a difference by treating these common conditions.
“There are never going to be enough specialists to deal with these problems. This is a natural progression for many of you,” said urogynecologist and pelvic surgeon Mickey M. Karram, MD, in a joint presentation at the Pelvic Anatomy and Gynecologic Surgery Symposium. In fact, he said, “there’s so much disease out there to fix that you may become more overwhelmed.”
Dr. Karram, who has offices in Cincinnati, Beverly Hills, and Orange County, Calif., spoke about female urinary incontinence with obstetrician-gynecologist Beri M. Ridgeway, MD, of Cleveland Clinic. They offered these tips:
Test for stress incontinence
Dr. Karram recommends using a “quick and easy” cystometrogram (CMG) test to “corroborate or refute what the patient thinks is going on” in regard to urinary function. “With this simple test, you’ll get a clear understanding of sensation [to urinate] and of what their fullness and capacity numbers are,” he said. And if you have the patient cough or strain during the test, “you should be able to duplicate a sign of stress incontinence 90% of the time.”
If patients don’t leak when they take this test, there may be another problem such as overactive bladder, a condition that can’t be duplicated via the test, he said.
Ask the right questions
When it comes to identifying when they have urinary difficulties, some patients “say yes to every question we ask,” said Dr. Ridgeway, and they may not be able to distinguish between urgency and leakage.
A better approach is to ask women to provide specific examples of when they have continence issues, she said. It’s also useful to ask patients about what bothers them the most if they have multiple symptoms: Is it urgency (“Gotta go; gotta go”)? Leakage during certain situations like coughing and laughing? “That helps me decide how to go about treating them first and foremost,” she said. “It doesn’t mean you won’t treat both [problems], but it really gives you a reference point of where to start.”
Research suggests that women tend to be more bothered by urge incontinence than stress incontinence, she said, because they can regulate their activities or avoid the stress form.
Beware of acute incontinence cases
“If a woman walks in and says ‘Everything was great until a week or two ago, but now I’m living in pads,’ it could be a fecal impaction or a pelvic mass,” Dr. Karram said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Discuss the many treatment options
In some cases of incontinence, Dr. Ridgeway said she’ll mention “the array of treatment options, such as pelvic floor physical therapy, bladder retraining, vaginal estrogen, medications, and Botox.”
She added: “I explain that we’ll work together, and sometimes it will take a couple tries, or we’ll try a couple things at once.”
Dr. Ridgeway disclosed consulting for Coloplast and serving as an independent contractor (legal) for Ethicon. Dr. Karram disclosed speaking for Allergan, Astellas Pharma, Coloplast, and Cynosure/Hologic; consulting for Coloplast and Cynosure/Hologic; and receiving royalties from BihlerMed.
EXPERT ANALYSIS FROM PAGS
When is it appropriate to remove ovaries in hysterectomy?
LAS VEGAS – The removal of both ovaries during hysterectomy – bilateral salpingo-oophorectomy (BSO) – has declined sharply in popularity as physicians have become more aware of its risks.
Still, “we’re still seeing a relatively high rate of inappropriate BSO,” Amanda Nickles Fader, MD, said, despite “the many benefits of ovarian conservation. Strong consideration should be made for maintaining normal ovaries in premenopausal women who are not at higher genetic risk of ovarian cancer.”
Dr. Nickles Fader, director of the Kelly gynecologic oncology service and the director of the center for rare gynecologic cancers at Johns Hopkins Hospital, Baltimore, who spoke at the Pelvic Anatomy and Gynecologic Surgery Symposium, urged gynecologists to understand the data about ovarian conservation in hysterectomy and carefully counsel patients.
“We can counsel patients with 100% certainty that BSO absolutely reduces ovarian and fallopian tube cancer rates. That’s a given,” she said. “Women get very excited about that, but you’ve got to be careful to counsel them about the flip side: The overall benefit may not be there when you consider the other morbidity and mortality that may occur because of this removal.”
As she noted, multiple retrospective, prospective, and observational studies have linked ovary removal to a variety of heightened risks, especially on the cardiac front. She highlighted a 2009 study of nearly 30,000 nurses who’d undergone hysterectomy for benign disease, about which the authors wrote that, “compared with ovarian conservation, bilateral oophorectomy at the time of hysterectomy for benign disease is associated with a decreased risk of breast and ovarian cancer but an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.” No age group gained a survival benefit from oophorectomy (Obstet Gynecol. 2009 May;113[5]:1027-37 ).
Meanwhile, over the past decade, the “pendulum has swung” toward ovary conservation, at least in premenopausal women, Dr. Nickles Fader said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
A 2016 analysis of health statistics in five U.S. Eastern and Midwestern states found that, rates of hospital-based, hysterectomy-alone procedures grew by 15% from 2005 to 2013, while rates of oophorectomy alone and hysterectomy/oophorectomy combination procedures declined by 12% and 29%, respectively.
Still, Dr. Nickles Fader said, as many as 60% of hysterectomies are still performed in conjunction with oophorectomy.
Ovary removal, of course, can be appropriate when patients are at risk of ovarian cancer. Hereditary ovarian cancer accounts for up to 25% of epithelial ovarian cancer, she said, and research suggests that risk-reducing surgery is an effective preventative approach when high-penetrance genes are present. However, the value of the surgery is less clear in regard to moderate-penetrance genes.
Dr. Nickles Fader pointed to guidelines from the National Comprehensive Cancer Network that specify genes and syndromes that should trigger risk-reducing salpingo-oophorectomy, hysterectomy, or hysterectomy and risk-reducing salpingo-oophorectomy after childbirth.
Researchers are exploring salpingectomy – fallopian tube removal – as a possible replacement for oophorectomy. Dr. Nickles Fader highlighted a small pilot study published in 2018 that reported “BRCA mutation carriers who underwent bilateral salpingectomy had no intraoperative complications, were satisfied with their procedure choice, and had decreased cancer worry and anxiety after the procedure.”
Moving forward, she said, research will provide more insight into preventative options such as removing fallopian tubes alone instead of ovaries. “We’re starting to learn, and will probably know in the next 10-15 years, whether oophorectomy is necessary for all high-risk and moderate-risk women or if we can get away with removing their tubes and giving them the maximal health benefits of ovarian conservation.”
Dr. Nickles Fader reported consulting for Ethicon Endosurgery.
LAS VEGAS – The removal of both ovaries during hysterectomy – bilateral salpingo-oophorectomy (BSO) – has declined sharply in popularity as physicians have become more aware of its risks.
Still, “we’re still seeing a relatively high rate of inappropriate BSO,” Amanda Nickles Fader, MD, said, despite “the many benefits of ovarian conservation. Strong consideration should be made for maintaining normal ovaries in premenopausal women who are not at higher genetic risk of ovarian cancer.”
Dr. Nickles Fader, director of the Kelly gynecologic oncology service and the director of the center for rare gynecologic cancers at Johns Hopkins Hospital, Baltimore, who spoke at the Pelvic Anatomy and Gynecologic Surgery Symposium, urged gynecologists to understand the data about ovarian conservation in hysterectomy and carefully counsel patients.
“We can counsel patients with 100% certainty that BSO absolutely reduces ovarian and fallopian tube cancer rates. That’s a given,” she said. “Women get very excited about that, but you’ve got to be careful to counsel them about the flip side: The overall benefit may not be there when you consider the other morbidity and mortality that may occur because of this removal.”
As she noted, multiple retrospective, prospective, and observational studies have linked ovary removal to a variety of heightened risks, especially on the cardiac front. She highlighted a 2009 study of nearly 30,000 nurses who’d undergone hysterectomy for benign disease, about which the authors wrote that, “compared with ovarian conservation, bilateral oophorectomy at the time of hysterectomy for benign disease is associated with a decreased risk of breast and ovarian cancer but an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.” No age group gained a survival benefit from oophorectomy (Obstet Gynecol. 2009 May;113[5]:1027-37 ).
Meanwhile, over the past decade, the “pendulum has swung” toward ovary conservation, at least in premenopausal women, Dr. Nickles Fader said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
A 2016 analysis of health statistics in five U.S. Eastern and Midwestern states found that, rates of hospital-based, hysterectomy-alone procedures grew by 15% from 2005 to 2013, while rates of oophorectomy alone and hysterectomy/oophorectomy combination procedures declined by 12% and 29%, respectively.
Still, Dr. Nickles Fader said, as many as 60% of hysterectomies are still performed in conjunction with oophorectomy.
Ovary removal, of course, can be appropriate when patients are at risk of ovarian cancer. Hereditary ovarian cancer accounts for up to 25% of epithelial ovarian cancer, she said, and research suggests that risk-reducing surgery is an effective preventative approach when high-penetrance genes are present. However, the value of the surgery is less clear in regard to moderate-penetrance genes.
Dr. Nickles Fader pointed to guidelines from the National Comprehensive Cancer Network that specify genes and syndromes that should trigger risk-reducing salpingo-oophorectomy, hysterectomy, or hysterectomy and risk-reducing salpingo-oophorectomy after childbirth.
Researchers are exploring salpingectomy – fallopian tube removal – as a possible replacement for oophorectomy. Dr. Nickles Fader highlighted a small pilot study published in 2018 that reported “BRCA mutation carriers who underwent bilateral salpingectomy had no intraoperative complications, were satisfied with their procedure choice, and had decreased cancer worry and anxiety after the procedure.”
Moving forward, she said, research will provide more insight into preventative options such as removing fallopian tubes alone instead of ovaries. “We’re starting to learn, and will probably know in the next 10-15 years, whether oophorectomy is necessary for all high-risk and moderate-risk women or if we can get away with removing their tubes and giving them the maximal health benefits of ovarian conservation.”
Dr. Nickles Fader reported consulting for Ethicon Endosurgery.
LAS VEGAS – The removal of both ovaries during hysterectomy – bilateral salpingo-oophorectomy (BSO) – has declined sharply in popularity as physicians have become more aware of its risks.
Still, “we’re still seeing a relatively high rate of inappropriate BSO,” Amanda Nickles Fader, MD, said, despite “the many benefits of ovarian conservation. Strong consideration should be made for maintaining normal ovaries in premenopausal women who are not at higher genetic risk of ovarian cancer.”
Dr. Nickles Fader, director of the Kelly gynecologic oncology service and the director of the center for rare gynecologic cancers at Johns Hopkins Hospital, Baltimore, who spoke at the Pelvic Anatomy and Gynecologic Surgery Symposium, urged gynecologists to understand the data about ovarian conservation in hysterectomy and carefully counsel patients.
“We can counsel patients with 100% certainty that BSO absolutely reduces ovarian and fallopian tube cancer rates. That’s a given,” she said. “Women get very excited about that, but you’ve got to be careful to counsel them about the flip side: The overall benefit may not be there when you consider the other morbidity and mortality that may occur because of this removal.”
As she noted, multiple retrospective, prospective, and observational studies have linked ovary removal to a variety of heightened risks, especially on the cardiac front. She highlighted a 2009 study of nearly 30,000 nurses who’d undergone hysterectomy for benign disease, about which the authors wrote that, “compared with ovarian conservation, bilateral oophorectomy at the time of hysterectomy for benign disease is associated with a decreased risk of breast and ovarian cancer but an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.” No age group gained a survival benefit from oophorectomy (Obstet Gynecol. 2009 May;113[5]:1027-37 ).
Meanwhile, over the past decade, the “pendulum has swung” toward ovary conservation, at least in premenopausal women, Dr. Nickles Fader said at the meeting jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
A 2016 analysis of health statistics in five U.S. Eastern and Midwestern states found that, rates of hospital-based, hysterectomy-alone procedures grew by 15% from 2005 to 2013, while rates of oophorectomy alone and hysterectomy/oophorectomy combination procedures declined by 12% and 29%, respectively.
Still, Dr. Nickles Fader said, as many as 60% of hysterectomies are still performed in conjunction with oophorectomy.
Ovary removal, of course, can be appropriate when patients are at risk of ovarian cancer. Hereditary ovarian cancer accounts for up to 25% of epithelial ovarian cancer, she said, and research suggests that risk-reducing surgery is an effective preventative approach when high-penetrance genes are present. However, the value of the surgery is less clear in regard to moderate-penetrance genes.
Dr. Nickles Fader pointed to guidelines from the National Comprehensive Cancer Network that specify genes and syndromes that should trigger risk-reducing salpingo-oophorectomy, hysterectomy, or hysterectomy and risk-reducing salpingo-oophorectomy after childbirth.
Researchers are exploring salpingectomy – fallopian tube removal – as a possible replacement for oophorectomy. Dr. Nickles Fader highlighted a small pilot study published in 2018 that reported “BRCA mutation carriers who underwent bilateral salpingectomy had no intraoperative complications, were satisfied with their procedure choice, and had decreased cancer worry and anxiety after the procedure.”
Moving forward, she said, research will provide more insight into preventative options such as removing fallopian tubes alone instead of ovaries. “We’re starting to learn, and will probably know in the next 10-15 years, whether oophorectomy is necessary for all high-risk and moderate-risk women or if we can get away with removing their tubes and giving them the maximal health benefits of ovarian conservation.”
Dr. Nickles Fader reported consulting for Ethicon Endosurgery.
EXPERT ANALYSIS FROM PAGS
Composite screening measures advocated for asymptomatic PAH
In patients at risk for pulmonary arterial hypertension (PAH) due to a connective tissue disease, composite novel screening methods are improving early detection when employed in the context of traditional tools, such as transthoracic echocardiography (TTE), according to a systematic review of studies published over the last 5 years.
The review was conducted to prepare for a guideline update, according to the authors of this recently published summary in Seminars in Arthritis and Rheumatism.
In a literature review for 2012-2015, the authors evaluated whether new tools or strategies have improved PAH screening in patients with connective tissue disease since the last review was undertaken (Semin Arthritis Rheum 2014;43:536-41).
The latest review found that although TTE and pulmonary function tests (PFT) remain a mainstay of screening, there is growing evidence that composite measures, such as the DETECT and ASIG algorithms, add sensitivity and specificity, compared with guidelines that rely on TTE and PFT alone.
After a literature search, the systematic review included 16 cohort studies and 6 case-control studies. Most of these evaluated PAH screening strategies for patients with systemic sclerosis specifically despite the potential for other connective tissue disease etiologies to lead to PAH.
“We need more longitudinal observational studies to develop and validate screening algorithms for non–systemic sclerosis connective tissue diseases,” stated the authors, led by senior investigator Dinesh Khanna, MD, medical director of ambulatory and chronic disease in the University of Michigan’s Office of Research, Ann Arbor.
Relative to screening primarily based on TTE and PFT as advocated in 2009 joint guidelines from the European Society of Cardiology and the European Respiratory Society (ESC/ERS), the preponderance of data supported the addition of DIRECT and ASIG algorithms to improve the sensitivity and specificity of traditional screening and diagnostic tools, according to the data reviewed.
Several of the studies evaluating DETECT and ASIG compared their sensitivities and specificities to the screening strategy recommended in the 2009 ESC/ERS guidelines because the 2015 ESC/ERS guidelines were not yet available at the time these studies were taking place.
In fact, the advantage of these algorithms has been acknowledged in a set of subsequent ESC/ERS guidelines issued in 2015. These specifically recommend DETECT in selected populations, such as adults with more than a 3-year history of systemic sclerosis and less than 60% diffusing capacity of the lung for carbon monoxide.
Based on the systematic review, the authors did not conclude that there is a single set of optimal tests for PAH screening whether in patients with systemic sclerosis or another connective tissue disease, but the authors did conclude that there has been progress in strategies for early PAH detection.
They also speculated that more progress might be coming. They noted that several newer tests, such as stress TTE to assess cardiopulmonary function during exercise, might further improve PAH detection at early stages.
According to the authors, this work is important, because better screening that results in earlier PAH detection means earlier treatment, which, in turn, “may improve survival.”
SOURCE: Young A et al. Semin Arthritis Rheum. 2018; Oct 14. doi: 10.1016/j.semarthrit.2018.10.010.
In patients at risk for pulmonary arterial hypertension (PAH) due to a connective tissue disease, composite novel screening methods are improving early detection when employed in the context of traditional tools, such as transthoracic echocardiography (TTE), according to a systematic review of studies published over the last 5 years.
The review was conducted to prepare for a guideline update, according to the authors of this recently published summary in Seminars in Arthritis and Rheumatism.
In a literature review for 2012-2015, the authors evaluated whether new tools or strategies have improved PAH screening in patients with connective tissue disease since the last review was undertaken (Semin Arthritis Rheum 2014;43:536-41).
The latest review found that although TTE and pulmonary function tests (PFT) remain a mainstay of screening, there is growing evidence that composite measures, such as the DETECT and ASIG algorithms, add sensitivity and specificity, compared with guidelines that rely on TTE and PFT alone.
After a literature search, the systematic review included 16 cohort studies and 6 case-control studies. Most of these evaluated PAH screening strategies for patients with systemic sclerosis specifically despite the potential for other connective tissue disease etiologies to lead to PAH.
“We need more longitudinal observational studies to develop and validate screening algorithms for non–systemic sclerosis connective tissue diseases,” stated the authors, led by senior investigator Dinesh Khanna, MD, medical director of ambulatory and chronic disease in the University of Michigan’s Office of Research, Ann Arbor.
Relative to screening primarily based on TTE and PFT as advocated in 2009 joint guidelines from the European Society of Cardiology and the European Respiratory Society (ESC/ERS), the preponderance of data supported the addition of DIRECT and ASIG algorithms to improve the sensitivity and specificity of traditional screening and diagnostic tools, according to the data reviewed.
Several of the studies evaluating DETECT and ASIG compared their sensitivities and specificities to the screening strategy recommended in the 2009 ESC/ERS guidelines because the 2015 ESC/ERS guidelines were not yet available at the time these studies were taking place.
In fact, the advantage of these algorithms has been acknowledged in a set of subsequent ESC/ERS guidelines issued in 2015. These specifically recommend DETECT in selected populations, such as adults with more than a 3-year history of systemic sclerosis and less than 60% diffusing capacity of the lung for carbon monoxide.
Based on the systematic review, the authors did not conclude that there is a single set of optimal tests for PAH screening whether in patients with systemic sclerosis or another connective tissue disease, but the authors did conclude that there has been progress in strategies for early PAH detection.
They also speculated that more progress might be coming. They noted that several newer tests, such as stress TTE to assess cardiopulmonary function during exercise, might further improve PAH detection at early stages.
According to the authors, this work is important, because better screening that results in earlier PAH detection means earlier treatment, which, in turn, “may improve survival.”
SOURCE: Young A et al. Semin Arthritis Rheum. 2018; Oct 14. doi: 10.1016/j.semarthrit.2018.10.010.
In patients at risk for pulmonary arterial hypertension (PAH) due to a connective tissue disease, composite novel screening methods are improving early detection when employed in the context of traditional tools, such as transthoracic echocardiography (TTE), according to a systematic review of studies published over the last 5 years.
The review was conducted to prepare for a guideline update, according to the authors of this recently published summary in Seminars in Arthritis and Rheumatism.
In a literature review for 2012-2015, the authors evaluated whether new tools or strategies have improved PAH screening in patients with connective tissue disease since the last review was undertaken (Semin Arthritis Rheum 2014;43:536-41).
The latest review found that although TTE and pulmonary function tests (PFT) remain a mainstay of screening, there is growing evidence that composite measures, such as the DETECT and ASIG algorithms, add sensitivity and specificity, compared with guidelines that rely on TTE and PFT alone.
After a literature search, the systematic review included 16 cohort studies and 6 case-control studies. Most of these evaluated PAH screening strategies for patients with systemic sclerosis specifically despite the potential for other connective tissue disease etiologies to lead to PAH.
“We need more longitudinal observational studies to develop and validate screening algorithms for non–systemic sclerosis connective tissue diseases,” stated the authors, led by senior investigator Dinesh Khanna, MD, medical director of ambulatory and chronic disease in the University of Michigan’s Office of Research, Ann Arbor.
Relative to screening primarily based on TTE and PFT as advocated in 2009 joint guidelines from the European Society of Cardiology and the European Respiratory Society (ESC/ERS), the preponderance of data supported the addition of DIRECT and ASIG algorithms to improve the sensitivity and specificity of traditional screening and diagnostic tools, according to the data reviewed.
Several of the studies evaluating DETECT and ASIG compared their sensitivities and specificities to the screening strategy recommended in the 2009 ESC/ERS guidelines because the 2015 ESC/ERS guidelines were not yet available at the time these studies were taking place.
In fact, the advantage of these algorithms has been acknowledged in a set of subsequent ESC/ERS guidelines issued in 2015. These specifically recommend DETECT in selected populations, such as adults with more than a 3-year history of systemic sclerosis and less than 60% diffusing capacity of the lung for carbon monoxide.
Based on the systematic review, the authors did not conclude that there is a single set of optimal tests for PAH screening whether in patients with systemic sclerosis or another connective tissue disease, but the authors did conclude that there has been progress in strategies for early PAH detection.
They also speculated that more progress might be coming. They noted that several newer tests, such as stress TTE to assess cardiopulmonary function during exercise, might further improve PAH detection at early stages.
According to the authors, this work is important, because better screening that results in earlier PAH detection means earlier treatment, which, in turn, “may improve survival.”
SOURCE: Young A et al. Semin Arthritis Rheum. 2018; Oct 14. doi: 10.1016/j.semarthrit.2018.10.010.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM
Key clinical point: Strategies for early detection of pulmonary arterial hypertension (PAH) are improving with new tools.
Major finding: Screening algorithms are improving sensitivity and specificity for PAH in patients with connective tissue diseases.
Study details: Systematic literature review.
Disclosures: Dr. Khanna has financial relationships with many companies that produce drugs for PAH but no conflicts relative to this study.
Source: Young A et al. Semin Arthritis Rheum. 2018 Oct 14. doi. 10.1016/j.semarthrit.2018.10.010.