User login
Trends in Utilization of Total Hip Arthroplasty for Femoral Neck Fractures in the United States
ABSTRACT
The ideal mode of fixation for patients with femoral neck fractures is not well defined in the current literature. This study describes the recent trends in surgical management of femoral neck fractures with an analysis on perioperative outcomes.
The National Hospital Discharge Survey was used to identify femoral neck fractures in the United States between 1990 and 2007 (n = 1,155,960) treated with open reduction and internal fixation (ORIF), total hip arthroplasty (THA), or hemiarthroplasty (HA). Trends were examined over the following 3 time periods: 1990 to 1995 (group 1), 1996 to 2001 (group 2), and 2002 to 2007 (group 3). Elixhauser Comorbidity Index and perioperative complications were calculated.
Use of HA increased (74.4% to 84.6%), whereas that of THA (7.3% to 4.9%) and ORIF (18.3% to 10.6%) decreased, from group 1 to group 3 in the age group of >80 years. The use of ORIF increased (63.9% to 81.4%), whereas the use of both HA and THA decreased, from group 1 to group 3 in the age group of <50 years. The rate of adverse events increased across all fixation types but was greatest among THA (32.2% to 48.3%).
The femoral neck patient population is now older and has more medical comorbidities. We observed a trend toward performing HA in older patients and ORIF in younger patients. Despite superior functional outcomes reported in THA, this study found a decreased utilization of THA in all age groups along with an increase in adverse events and nonroutine discharges for patients with femoral neck fractures treated with THA.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Continue to: Femoral neck fractures...
Femoral neck fractures are a common occurrence in the United States. A recent study estimated an incidence of >63 per 100,000 population.1-8 Although the incidence appears to have decreased over recent decades, there is a projected exponential increase in the incidence of hip fractures over the next 30 years in the baby boomer population.8,9 Given that these fractures have a significant impact on patient morbidity, mortality, and quality of life, research efforts have been directed toward optimizing the treatment of affected patients and improving the outcomes.4,9-24
The treatment of choice for femoral neck fractures and the use of total hip arthroplasty (THA)11 have been a topic of debate.4,9,10,15-17,22,25 Total hip arthroplasty has been advocated for younger, more active patients, whereas hemiarthroplasty (HA) has been reserved for patients who are older and less active. Although several studies have demonstrated that arthroplasty outperforms open reduction and internal fixation (ORIF) in the elderly population with displaced femoral neck fractures, ORIF is still commonly performed in the United States for nondisplaced fractures and in patients aged <50 years.26-29
In an attempt to quantify the use of THA in the treatment of femoral neck fractures and demonstrate the national trends, Miller and colleagues5 pooled the American Board of Orthopaedic Surgery (ABOS) database and analyzed the treatment trends of surgeons taking part II of the ABOS examination from 1999 to 2011. The authors found an increased utilization of THA by recently graduated orthopedic surgeons. In contrast, Jain and colleagues30 found different national trends when they analyzed data from the National Inpatient Sample containing data between 1990 and 2001 and further found decreased utilization of THA procedures by orthopedic surgeons of all levels of training nationwide. However, neither of these studies reported about the trends in demographics, comorbidities, risk factors, or outcomes in this patient population following surgery.
The purpose of this study was to help clarify the findings of these authors using the largest dataset to date and also report on the perioperative complications associated with each mode of fixation in patients who undergo operative treatment for femoral neck fractures in the United States. Our hypotheses were that the femoral neck fracture patient population has become older and has more medical comorbidities. We also hypothesized that there has been a trend toward performing fewer THA procedures in the United States and that THA is associated with increased perioperative complications compared to those with HA and ORIF.
MATERIALS AND METHODS
We conducted a retrospective epidemiological study using the National Hospital Discharge Survey (NHDS) on surgical trends in the management of femoral neck fractures. The NHDS is a publicly available survey that is conducted annually to provide data of nonfederal, short-stay hospitals to the public. The sample data are weighted to provide nationwide estimates of annual inpatient care. The NHDS includes up to 7 medical diagnoses and 4 procedural codes per case, which are categorized using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, that were collected along with patient demographic information, length of stay (LOS), and discharge disposition. The diagnostic and procedural codes used for this study are presented in the Appendix. The year 2007 was chosen as the endpoint of this study due to the fact that the relative standard error of the NHDS doubled in 2008 as a result of a decrease in its survey size. As this is a publicly available database, our study was exempt from institutional review board approval.
Continue to: All pateints admitted...
All patients admitted with a primary diagnosis of closed transcervical fracture of the femoral neck (ICD-9-CM 820.0x) were selected. This resulted in 1,674,160 fractures. All patients with fractures with a concurrent primary procedural code of ORIF (79.35), HA (81.52), or THA (81.51) were identified, resulting in a total sample size of 1,155,960 surgical fractures. Analysis of the fractures based on additional specificity,ie subcapital versus midcervical versus basicervical, was not carried out because >90% of femoral neck fractures in the database were coded as “unspecified” or “other” (ICD9 CM 820.00 and 820.09, respectively).
Comorbidity burden was quantified using Elixhauser coding algorithms as previously described.31 The Elixhauser comorbidity measure is a model consisting of 31 conditions and has recently been identified as a better predictor of mortality in patients undergoing orthopedic procedures when compared with the Charlson Comorbidity Index.31 Dichotomous variables for each Elixhauser comorbidity were created, and χ2 tests were utilized to assess the association between each comorbidity and mortality. The weighted Elixhauser score for each statistically significant comorbidity was calculated as described by van Walraven and colleagues.32 The Elixhauser comorbidity score was then calculated for each patient by summing the individual weights of all comorbidities. Postoperative adverse events were determined using the complication-screening-package as previously described.33
All adverse events were categorized into 3 categories, including general medical complications, mechanical complications, and surgical complications. All adverse events recorded in the NHDS database are events that occurred during a single hospitalization. Therefore, it does not take into account adverse events that occurred after discharge, and, for example, mortality refers to postoperative mortality that occurs prior to discharge. The study period comprised data captured from 1990 to 2007, and 3 groups were generated from this time period to better characterize patients throughout the large study time frame. Group 1 comprised patients who underwent surgical management of femoral neck fractures from 1990 to 1995, group 2 consisted of patients treated from 1996 to 2001, and group 3 included patients treated from 2002 to 2007.
Categorical data were analyzed using the χ2 test, and continuous data were analyzed by the independent-samples t test and ANOVA. Multivariable binary logistic regression analyses were performed to assess the contributions of individual comorbidities to mortality, adverse events, and nonroutine discharge. Elixhauser comorbidities with a P value of < .10 in the bivariate analysis and presenting in at least 0.2% of the population were included in the logistic regression.31 Odds ratios and confidence intervals were calculated to assess the association between comorbidities and our dichotomous variables. A P value of < .001 defined statistical significance.33 Statistical analysis was conducted using SPSS version 21 (IBM).
RESULTS
Patient Demographics
Our query demonstrated a total of 1,155,960 patients who underwent surgical fixation of femoral neck fractures (Table 1). The most commonly used treatment modality was HA (75%), followed by ORIF (18%) and later by THA (7%). The majority of patients were females in each treatment group. Patients’ age varied according to treatment group, with patients undergoing HA having a mean age of 81.0 ± 9.0 years, patients undergoing ORIF having a mean age of 75.0 ± 17.0 years, and those undergoing THA having a mean age of 79.0 ± 10.0 years (P < .001). The majority of patients were ≥80 years in all treatment groups, but the ORIF group had the greatest proportion of patients <65 years (P < .001). Among patients undergoing HA, 62.4% were ≥80 years, while the ORIF and HA groups consisted of 48.6% and 51.5% of patients in that same age group, respectively.
Continue to: TRENDS ANALYSIS
TRENDS ANALYSIS
There was a significant change in the distributions of the procedures performed according to age group over time. Patients >80 years continued to undergo primarily HA, with an increase from 74.4% during 1990 to 1995 up to 84.6% during the 2002 to 2007 period and a concomitant decrease in ORIF from 18.3% to 10.6% during the same time period in this age group. Surgical trends in patients 65 to 79 years demonstrated a significant decrease in management with ORIF from 19.1% in 1990 to 1995 to 16.8% in the 2002 to 2007 cohort (P < .001 for all, Table 2). There was an increase in the use of HA from 71.9% during the 1990 to 1995 period to 75.5% during the final study period (Table 2, Figure 1). The use of THA for all age groups decreased between 1990 and 2007, except for the 50- to 64-year-old group where THA utilization remained constant.
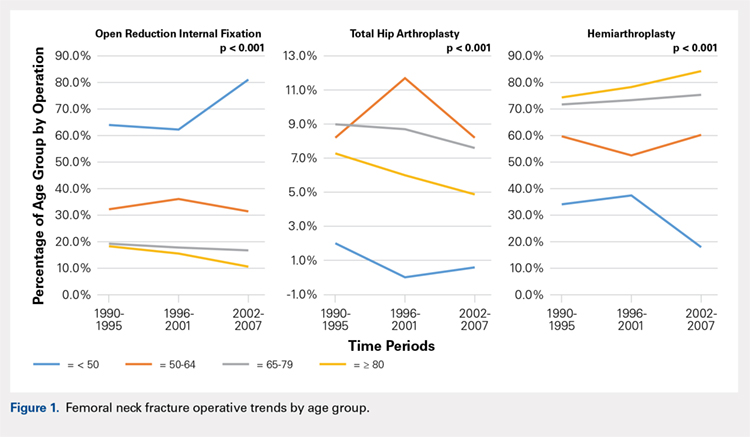
Management patterns in patients 50 to 64 years varied throughout the analysis and demonstrated the following trend: treatment with HA remained the most common technique used but varied slightly from 59.7% during 1990 to 1995 to 60.3% during 2002 to2007 (P < .001, Table 2). The second most common treatment used was ORIF, which decreased from 32.2% to 31.5% (P < .001, Table 2). The use of THA varied significantly from 8.2% among those managed during 1990 to 1995 to 11.7% during 1996 to 2001 but later declined to the initial 8.2% (P < .001, Table 2).
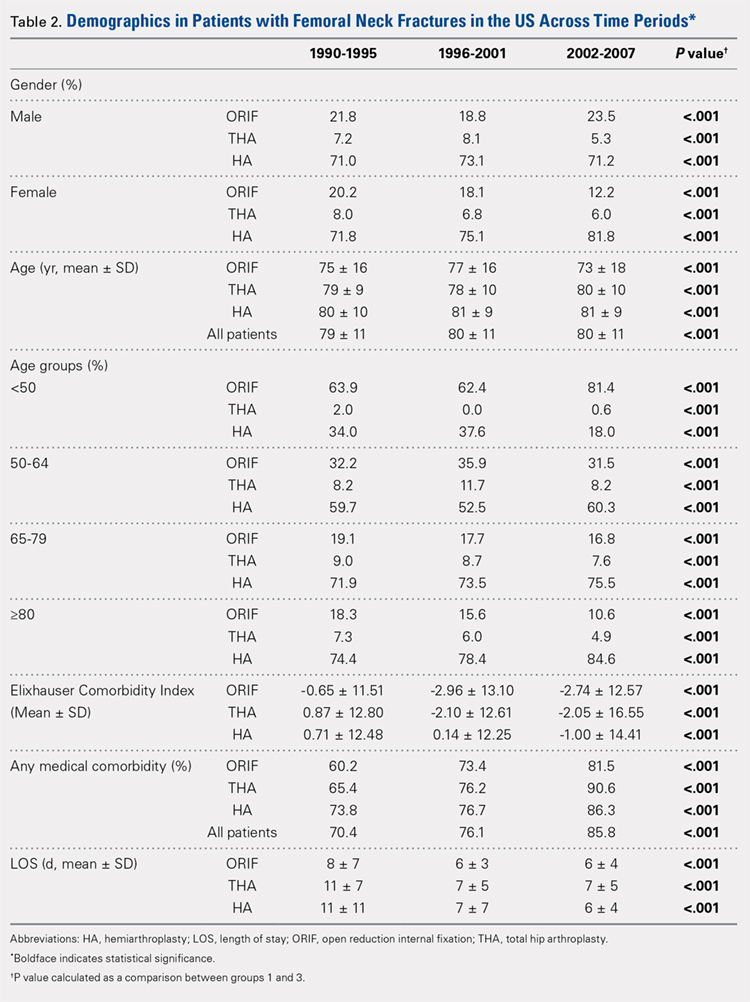
Analysis of patients ≤49 years demonstrated that ORIF was the preferred technique, which experienced a growth from 63.9% during 1990 to 1995 to 81.4% during the 2002 to 2007 period (P < .001, Table 2). A decreased use in THA was observed from 2.0% in the initial period to 0.6% in the final period (P < .001, Table 2). Use of HA decreased from 34.0% in 1990 to 1995 to 18.0% in 2002 to 2007 (P < .001, Table 2).
LENGTH OF STAY
Mean number of in-hospital days decreased throughout the study period for all treatment techniques. During the 1990 to 1995 study period, patients who underwent ORIF had a mean LOS of 8 ± 7 days, which decreased (P < .001, Table 2) to 6 ± 3 days in 1996 to 2001 and remained constant during 2002 to 2007 (mean 6 ± 4 days). This decrease in LOS was also observed in patients who underwent THA (P < .001, Table 2), who initially had a mean LOS of 11 ± 7 days during 1990 to 1995, which later decreased to 7 ± 5 days for the remainder of the study. The LOS for patients who underwent HA also decreased (P < .001, Table 2), which initially was reported to be 11 ± 11 days during 1990 to 1995, decreasing to 7 ±7 days in 1996–2001 and later to 6 ± 4 days in 2002 to 2007.
COMORBIDITIY ANALYSIS
The Elixhauser Comorbidity Index varied significantly among groups over time (P < .001, Table 2). Overall mean Elixhauser Comorbidity Index score per procedure type is provided in Table 1, with HA patients having the highest score (-0.15 ± 13.09, p<.001).
Continue to: Analysis of the preoperative comorbidities...
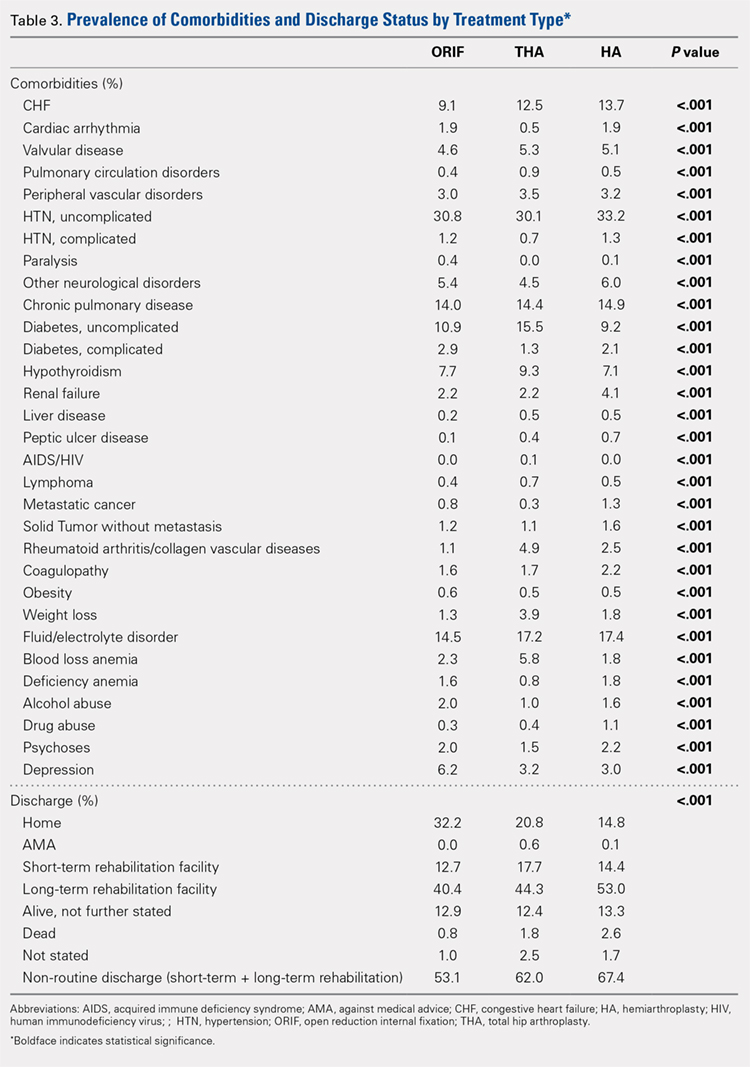
Analysis of the preoperative comorbidities demonstrated significant differences among each surgical treatment group (P < .001 for all, Table 3). The most common comorbidities in patients who underwent HA were uncomplicated hypertension (33.2%), fluid/electrolyte disorders (17.4%), chronic pulmonary disease (14.9%), and congestive heart failure (13.7%). The most common comorbidities in the ORIF group were uncomplicated hypertension (30.8%), fluid/electrolyte disorders (14.5%), chronic pulmonary disease (14.0%), and uncomplicated diabetes (10.9%). Patients treated with THA had most commonly uncomplicated hypertension (30.1%), fluid/electrolyte disorders (17.2%), uncomplicated diabetes (15.5%), and chronic pulmonary disease (14.4%). The prevalence of comorbidities is displayed in Table 3.
DISCHARGE STATUS
Mortality varied significantly, being lowest in those who underwent ORIF (0.8%), followed those who underwent THA (1.8%), and HA (2.6%) (P < .001, Table 1).
The majority of patients in each group were discharged to long-term rehabilitation facilities, including 53.0% of those treated with HA, 40.4% of those treated with ORIF, and 44.3% of patients treated with THA. The second most common discharge location was home, which included 14.8% of patients who underwent HA, 32.2% of patients treated with ORIF, and 20.8% of those who underwent THA. Table 3 demonstrates the details of the discharge settings.
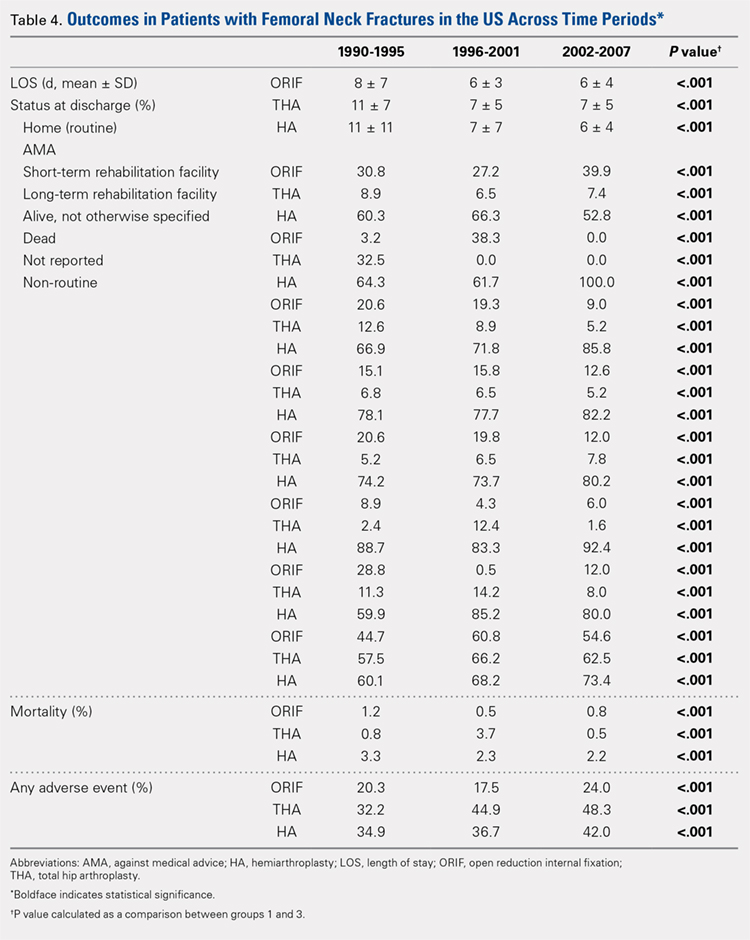
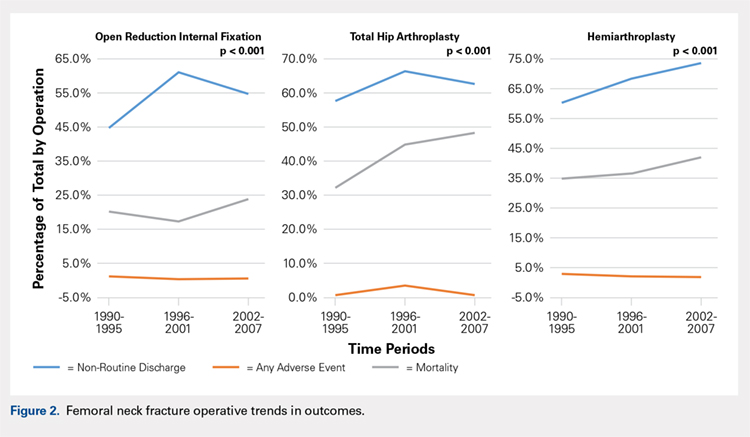
Mortality analysis over time demonstrated a significant decrease in each treatment group (P < .001). Mortality in the ORIF group decreased from 1.2% during 1990 to 1995 to 0.8% in 2002 to 2007. Mortality in the THA group also decreased significantly from 0.8% during 1990 to 1995 to 0.5% during the 2002 to 2007 time period. Patients who underwent HA also exhibited a decrease in mortality rate from 3.3% during 1990 to 1995 to 2.2% during 2002 to 2007 (P < .001, Table 4, Figure 2).
GENERAL ADVERSE EVENTS
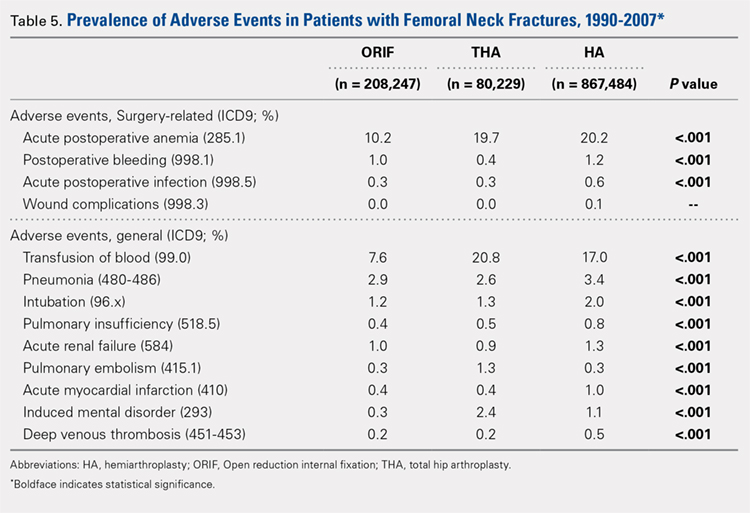
There was a significant difference (P < .001) in the percentage of adverse events experienced, the maximum being observed in the THA group (41.0%), followed by the HA group (37.9%) and trailed by the ORIF group (20.3%, (P < .001, Table 1). The prevalence of adverse events is detailed in Table 5.
Continue to: Patients who underwent THA...
Patients who underwent THA had the highest rate of any adverse event, LOS, and transfusion rate (Table 1 and Table 5).
The prevalence of postoperative pneumonia was highest in the HA group (3.4%), followed by the ORIF group (2.9%), and the THA group (2.6%) (P < .001, Table 5). There was also a significant difference in rates of intubation, pulmonary insufficiency, acute renal failure, pulmonary embolism, acute myocardial infarction, induced mental disorder, and deep venous thrombosis (P < .001 for all, Table 5).
SURGERY-RELATED ADVERSE EVENTS
Surgery-related outcomes over the entire study period were significantly different according to the type of procedure performed (P < .001, Table 5). Patients who underwent HA had the highest rate of acute postoperative anemia (20.2%), followed by those who underwent THA (19.7%), and ORIF (10.2%). Postoperative bleeding rates also varied significantly, with 1.2% in the HA group, followed by 1.0% in the ORIF group and 0.4% in the THA group (P < .001, Table 5). Acute postoperative infection rates also varied significantly, with the highest rate being observed in the HA group (0.6%) compared to that in the THA and ORIF groups (both 0.3%) (P < .001, Table 5).
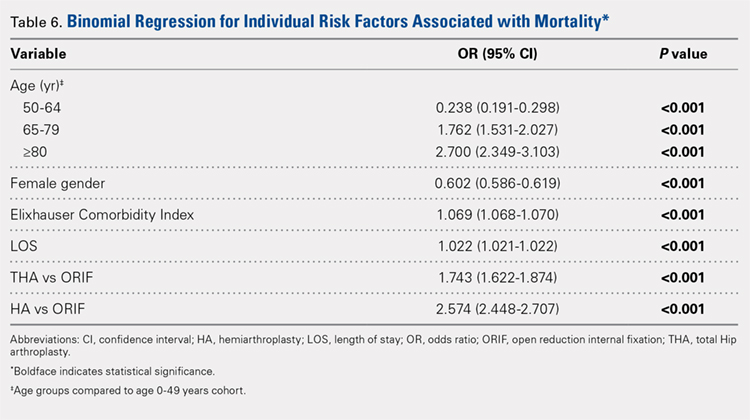
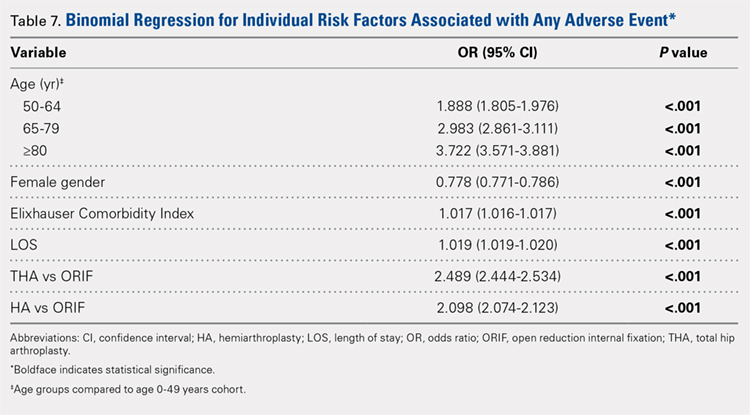
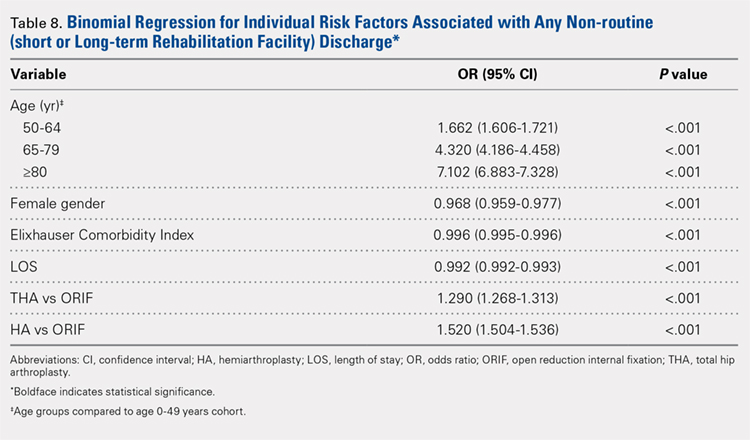
Table 6, Table 7, and Table 8 detail the results of regression analyses in patients with femoral neck fractures for individual risk factors associated with mortality, any adverse event, and nonroutine discharge to a short- or long-term rehabilitation facility, respectively. Increasing age (50–64 years, OR: 0.238; 65–79 years, OR: 1.762; and ≥80 years, OR: 2.700), THA (OR: 1.743), and HA (OR: 2.574) were found to be independent risk factors for mortality in the perioperative period (P < .001 for each, Table 6). Increasing age (50–64 years, OR: 1.888; 65–79 years, OR: 2.983; and ≥80 years, OR: 3.722), THA (OR: 2.489), and HA (OR: 2.098) were also found to be independent risk factors for any adverse event in the perioperative period (P < .001, Table 7). Age (50–64 years, OR: 1.662; 65–79 years, OR: 4.320; and ≥80 years, OR: 7.102) was the best predictor for nonroutine discharge to a short- or long-term rehabilitation facility (P < .001, Table 8).
DISCUSSION
Femoral neck fractures in the elderly population present a significant financial burden to the healthcare system.1-3,24,25 Consistent with previous epidemiological studies, our results show that the femoral neck fracture population has become older and has more medical comorbidities over the last 3 decades.27,28. Similarly, we also found that the rate of medical, surgical, and mechanical perioperative complications has increased in the same time period. Interestingly, the mortality rate has remained relatively similar.
Continue to: Although patients undergoing HA...
Although patients undergoing HA for femoral neck fractures are older and have more medical comorbidities, we found that the rate of adverse events in the perioperative period for patients undergoing THA was higher than that in the HA group. Consistent with prior studies, patients who underwent THA had higher rates of blood transfusion, pulmonary embolism, and induced mental disorders.34 Multivariable regression analysis demonstrated that after controlling for age, medical comorbidity, and type of surgery performed, THA emerged as an independent risk factor for any adverse event in the perioperative period. Increased anesthesia time, reaming of the acetabulum, and increased complexity of surgery probably account for these changes.
Our study results are consistent with those of Jain and colleagues,30 which showed a decrease in utilization of THA for femoral neck fractures between 1990 and 2001. Since THA is generally indicated for younger, more active patients in relatively good health, this would explain why changes in baseline health in this cohort over the last 20 years would lead to fewer THA procedures being performed. Surgeons in the US may be finding there are fewer patients who are candidates for THA. Miller and colleagues5 reported conflicting results and showed an increase in THA utilization in this patient population. However, their study evaluated treatment trends based on data from the ABOS part II of recently graduated orthopedic surgeons and may not be an accurate representation of national practice trends in the US. The trend toward increased subspecialization may explain their findings. As the authors noted, although they found an increase in the use of THA for femoral neck fractures by new adult reconstruction surgeons, the percentage of new surgeons treating femoral neck fractures has declined.5
Our analysis showed very concrete trends in treatment management at the extremes of the age ranges. There were substantial increases in the use of ORIF for patients <50 years (from 63.9% in 1990–1995 to 81.4% in 2002–2007, P < .001) and in the use of HA for patients >80 years (from 74.4% in 1990–1995 to 84.6% in 2002–2007, P < .001). This trend parallels recent studies that purport better outcomes for young patients undergoing ORIF and elderly patients undergoing HA.30 Our analysis did not demonstrate a large shift in surgeon preference for treatment of patients between 50 and 80 years, although there was a statistically significant decrease in ORIF and THA usage and a reflective increase in HA usage in this population as well. The fact that there has not been as substantial a shift in treatment trends for this large age group is potentially due to the wide variations in comorbid conditions and the functionality that abounds in this age group.1
The limitations of the current study are those inherent with a retrospective database analysis. The reliance on accurate coding brings up a potential for error; however, it is unlikely that comorbidities and outcomes are undercoded as hospitals are incentivized to input values that increase the acuity and thus reimbursement for each hospital stay.35 The database also relies on the ICD-9 procedural and diagnostic codes, which are not as specific as the currently adopted ICD-10 codes; hence, we are unable to distinguish between different forms of internal fixation, for example intramedullary nailing versus dynamic hip screw. This also precludes us from including other critical data such as degree of fracture displacement, cemented versus uncemented implantation, surgical approach for arthroplasty, and functional outcomes of individual patients. Moreover, the database used, although the largest inpatient sample available for analysis, represents only approximately 20% of hospitals nationwide. In addition, as patients cannot be tracked over time within the database, we are limited to outcomes in the perioperative period captured in a single hospital stay and cannot identify readmissions. Finally, our analysis is limited to the years 1990 to 2007 because of an increase in the relative standard error of the database in more recent years. Although this results in data that are not the most current, we believe that this study provides valuable insight regarding the trends in surgical treatment and acute postoperative outcomes of these injuries that have hitherto not been reported. To limit the inherent biases and the limitations within this study, prospective, randomized studies with long-term follow-up comparing outcomes across modes of treatment are needed to definitively determine the optimum form of treatment for this fracture type.
CONCLUSION
This is the largest study to date reporting on national trends in the surgical treatment and outcomes of the femoral neck fracture population. Orthopedic surgeons performing THA should be aware that the femoral neck fracture population is changing and at higher risk for perioperative complications. The advent of bisphosphonate therapy has been suggested as a possible reason for the decrease in fragility fractures and why a larger proportion of the femoral neck fracture population is now >80 years.36,37 With an aging population at a higher risk for perioperative complications, clinicians must take special care in choosing the appropriate surgical intervention that will give their patients the best functional outcome while minimizing the risk of surgical complications. Orthopedic surgeons should weigh the added risk associated with THA in this population.
1. Bishop J, Yang A, Githens M, Sox AH. Evaluation of contemporary trends in femoral neck fracture management reveals discrepancies in treatment. Geriatr Orthop Surg Rehabil. 2016;7(3):135. doi:10.1177/2151458516658328.
2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res Off J Am Soc Bone Miner Res. 2007;22(3):465. doi:10.1359/jbmr.061113.
3. Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl.):57s. doi:10.1016/8756-3282(95)00381-9.
4. Koval KJ, Zuckerman JD. Hip fractures: I. Overview and evaluation and treatment of femoral-neck fractures. J Am Acad Orthop Surg. 1994;2(3):141. doi:10.5435/00124635-199405000-00002.
5. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg. (American) 2014;96(17):e149. doi:10.2106/JBJS.M.01122.
6. Miller BJ, Lu X, Cram P. The trends in treatment of femoral neck fractures in the Medicare population from 1991 to 2008. J Bone Joint Surg. (American) 2013;95(18):e132. doi:10.2106/JBJS.L.01163.
7. Nwachukwu BU, McCormick F, Provencher MT, Roche M, Rubash HE. A comprehensive analysis of Medicare trends in utilization and hospital economics for total knee and hip arthroplasty from 2005 to 2011. J Arthroplast. 2015;30(1):15. doi:10.1016/j.arth.2014.08.025.
8. Su EP, Su SL. Femoral neck fractures: a changing paradigm. Bone Joint J. 2014;96-b(11) Supple A):43. doi:10.1302/0301-620X.96B11.34334.
9. Ahn J, Man LX, Park S, Sodl JF, Esterhai JL. Systematic review of cemented and uncemented hemiarthroplasty outcomes for femoral neck fractures. Clin Orthop Relat Res. 2008;466(10):2513. doi:10.1007/s11999-008-0368-3.
10. Alolabi B, Bajammal S, Shirali J, Karanicolas PJ, Gafni A, Bhandari M. Treatment of displaced femoral neck fractures in the elderly: a cost-benefit analysis. J Orthop Trauma. 2009;23(6):442. doi:10.1097/BOT.0b013e31817614dd.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290. doi:10.1093/aje/kwp266.
12. Brox WT, Chan PH, Cafri G, Inacio MC. Similar mortality with general or regional anesthesia in elderly hip fracture patients. Acta Orthop. 2016;87(2):152. doi:10.3109/17453674.2015.1128781.
13. Catal B, Sener M. Treatment and displacement affect the reoperation rate for femoral neck fracture. Clin Orthop Relat Res. 2013;471(12):4096. doi:10.1007/s11999-013-3295-x.
14. Dailiana Z, Papakostidou I, Varitimidis S, Michalitsis S, Veloni A, Malizos K. Surgical treatment of hip fractures: factors influencing mortality. Hippokratia. 2013;17(3):252.
15. Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012;26(3):135. doi:10.1097/BOT.0b013e318238b7a5.
16. Hedbeck CJ, Inngul C, Blomfeldt R, Ponzer S, Tornkvist H, Enocson A. Internal fixation versus cemented hemiarthroplasty for displaced femoral neck fractures in patients with severe cognitive dysfunction: a randomized controlled trial. J Orthop Trauma. 2013;27(12):690. doi:10.1097/BOT.0b013e318291f544.
17. Jia Z, Ding F, Wu Y, et al. Unipolar versus bipolar hemiarthroplasty for displaced femoral neck fractures: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10:8. doi:10.1186/s13018-015-0165-0.
18. Lapidus LJ, Charalampidis A, Rundgren J, Enocson A. Internal fixation of garden I and II femoral neck fractures: posterior tilt did not influence the reoperation rate in 382 consecutive hips followed for a minimum of 5 years. J Orthop Trauma. 2013;27(7):386. doi:10.1097/BOT.0b013e318281da6e.
19. Mariconda M, Costa GG, Cerbasi S, et al. Factors predicting mobility and the change in Activities of Daily Living After hip fracture: A 1-year prospective cohort study. J Orthop Trauma. 2016;30(2):71. doi:10.1097/BOT.0000000000000448.
20. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality After proximal femoral fracture: A retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg. (American) 2015;97(16):1333. doi:10.2106/JBJS.O.00029.
21. Samuel AM, Russo GS, Lukasiewicz AM, et al. Surgical treatment of femoral neck fractures after 24 hours in patients between the ages of 18 and 49 is associated with poor inpatient outcomes: an analysis of 1361 patients in the National Trauma Data Bank. J Orthop Trauma. 2016;30(2):89. doi:10.1097/BOT.0000000000000456.
22. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235. doi:10.1007/s11999-012-2293-8.
23. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplast. 2012;27(4):583. doi:10.1016/j.arth.2011.07.009.
24. Zielinski SM, Keijsers NL, Praet SF, et al. Functional outcome after successful internal fixation versus salvage arthroplasty of patients with a femoral neck fracture. J Orthop Trauma. 2014;28(12):e273. doi:10.1097/BOT.0000000000000123.
25. Gu Q, Koenig L, Mather RC, 3rd, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536. doi:10.1007/s11999-014-3820-6.
26. Forsh DA, Ferguson TA. Contemporary management of femoral neck fractures: the young and the old. Curr Rev Musculoskelet Med. 2012;5(3):214. doi:10.1007/s12178-012-9127-x.
27. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287. doi:10.5435/00124635-200605000-00004.
28. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: Femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596. doi:10.5435/00124635-200810000-00005.
29. Probe R, Ward R. Internal fixation of femoral neck fractures. J Am Acad Orthop Surg. 2006;14(9):565. doi:10.5435/00124635-200609000-00006.
30. Jain NB, Losina E, Ward DM, Harris MB, Katz JN. Trends in surgical management of femoral neck fractures in the United States. Clin Orthop Relat Res. 2008;466(12):3116. doi:10.1007/s11999-008-0392-3.
31. Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878. doi:10.1007/s11999-014-3686-7.
32. Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser Comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633.
33. Best MJ, Buller LT, Falakassa J, Vecchione D. Risk factors for nonroutine discharge in patients undergoing spinal fusion for intervertebral disc disorders. Iowa Orthop J. 2015;35:147.
34. Schairer WW, Lane JM, Halsey DA, Iorio R, Padgett DE, McLawhorn AS. The Frank Stinchfield award: total hip arthroplasty for femoral neck fracture is not a typical DRG 470: A propensity-matched cohort study. Clin Orthop Relat Res. 2017;475(2):353-360. doi:10.1007/s11999-016-4868-2.
35. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9. doi:10.2106/JBJS.J.01077.
36. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl.):S14. doi:10.1016/j.amjmed.2008.12.003.
37. Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001-2008. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(1):3. doi:10.1002/jbmr.189.
ABSTRACT
The ideal mode of fixation for patients with femoral neck fractures is not well defined in the current literature. This study describes the recent trends in surgical management of femoral neck fractures with an analysis on perioperative outcomes.
The National Hospital Discharge Survey was used to identify femoral neck fractures in the United States between 1990 and 2007 (n = 1,155,960) treated with open reduction and internal fixation (ORIF), total hip arthroplasty (THA), or hemiarthroplasty (HA). Trends were examined over the following 3 time periods: 1990 to 1995 (group 1), 1996 to 2001 (group 2), and 2002 to 2007 (group 3). Elixhauser Comorbidity Index and perioperative complications were calculated.
Use of HA increased (74.4% to 84.6%), whereas that of THA (7.3% to 4.9%) and ORIF (18.3% to 10.6%) decreased, from group 1 to group 3 in the age group of >80 years. The use of ORIF increased (63.9% to 81.4%), whereas the use of both HA and THA decreased, from group 1 to group 3 in the age group of <50 years. The rate of adverse events increased across all fixation types but was greatest among THA (32.2% to 48.3%).
The femoral neck patient population is now older and has more medical comorbidities. We observed a trend toward performing HA in older patients and ORIF in younger patients. Despite superior functional outcomes reported in THA, this study found a decreased utilization of THA in all age groups along with an increase in adverse events and nonroutine discharges for patients with femoral neck fractures treated with THA.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Continue to: Femoral neck fractures...
Femoral neck fractures are a common occurrence in the United States. A recent study estimated an incidence of >63 per 100,000 population.1-8 Although the incidence appears to have decreased over recent decades, there is a projected exponential increase in the incidence of hip fractures over the next 30 years in the baby boomer population.8,9 Given that these fractures have a significant impact on patient morbidity, mortality, and quality of life, research efforts have been directed toward optimizing the treatment of affected patients and improving the outcomes.4,9-24
The treatment of choice for femoral neck fractures and the use of total hip arthroplasty (THA)11 have been a topic of debate.4,9,10,15-17,22,25 Total hip arthroplasty has been advocated for younger, more active patients, whereas hemiarthroplasty (HA) has been reserved for patients who are older and less active. Although several studies have demonstrated that arthroplasty outperforms open reduction and internal fixation (ORIF) in the elderly population with displaced femoral neck fractures, ORIF is still commonly performed in the United States for nondisplaced fractures and in patients aged <50 years.26-29
In an attempt to quantify the use of THA in the treatment of femoral neck fractures and demonstrate the national trends, Miller and colleagues5 pooled the American Board of Orthopaedic Surgery (ABOS) database and analyzed the treatment trends of surgeons taking part II of the ABOS examination from 1999 to 2011. The authors found an increased utilization of THA by recently graduated orthopedic surgeons. In contrast, Jain and colleagues30 found different national trends when they analyzed data from the National Inpatient Sample containing data between 1990 and 2001 and further found decreased utilization of THA procedures by orthopedic surgeons of all levels of training nationwide. However, neither of these studies reported about the trends in demographics, comorbidities, risk factors, or outcomes in this patient population following surgery.
The purpose of this study was to help clarify the findings of these authors using the largest dataset to date and also report on the perioperative complications associated with each mode of fixation in patients who undergo operative treatment for femoral neck fractures in the United States. Our hypotheses were that the femoral neck fracture patient population has become older and has more medical comorbidities. We also hypothesized that there has been a trend toward performing fewer THA procedures in the United States and that THA is associated with increased perioperative complications compared to those with HA and ORIF.
MATERIALS AND METHODS
We conducted a retrospective epidemiological study using the National Hospital Discharge Survey (NHDS) on surgical trends in the management of femoral neck fractures. The NHDS is a publicly available survey that is conducted annually to provide data of nonfederal, short-stay hospitals to the public. The sample data are weighted to provide nationwide estimates of annual inpatient care. The NHDS includes up to 7 medical diagnoses and 4 procedural codes per case, which are categorized using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, that were collected along with patient demographic information, length of stay (LOS), and discharge disposition. The diagnostic and procedural codes used for this study are presented in the Appendix. The year 2007 was chosen as the endpoint of this study due to the fact that the relative standard error of the NHDS doubled in 2008 as a result of a decrease in its survey size. As this is a publicly available database, our study was exempt from institutional review board approval.

Continue to: All pateints admitted...
All patients admitted with a primary diagnosis of closed transcervical fracture of the femoral neck (ICD-9-CM 820.0x) were selected. This resulted in 1,674,160 fractures. All patients with fractures with a concurrent primary procedural code of ORIF (79.35), HA (81.52), or THA (81.51) were identified, resulting in a total sample size of 1,155,960 surgical fractures. Analysis of the fractures based on additional specificity,ie subcapital versus midcervical versus basicervical, was not carried out because >90% of femoral neck fractures in the database were coded as “unspecified” or “other” (ICD9 CM 820.00 and 820.09, respectively).
Comorbidity burden was quantified using Elixhauser coding algorithms as previously described.31 The Elixhauser comorbidity measure is a model consisting of 31 conditions and has recently been identified as a better predictor of mortality in patients undergoing orthopedic procedures when compared with the Charlson Comorbidity Index.31 Dichotomous variables for each Elixhauser comorbidity were created, and χ2 tests were utilized to assess the association between each comorbidity and mortality. The weighted Elixhauser score for each statistically significant comorbidity was calculated as described by van Walraven and colleagues.32 The Elixhauser comorbidity score was then calculated for each patient by summing the individual weights of all comorbidities. Postoperative adverse events were determined using the complication-screening-package as previously described.33
All adverse events were categorized into 3 categories, including general medical complications, mechanical complications, and surgical complications. All adverse events recorded in the NHDS database are events that occurred during a single hospitalization. Therefore, it does not take into account adverse events that occurred after discharge, and, for example, mortality refers to postoperative mortality that occurs prior to discharge. The study period comprised data captured from 1990 to 2007, and 3 groups were generated from this time period to better characterize patients throughout the large study time frame. Group 1 comprised patients who underwent surgical management of femoral neck fractures from 1990 to 1995, group 2 consisted of patients treated from 1996 to 2001, and group 3 included patients treated from 2002 to 2007.
Categorical data were analyzed using the χ2 test, and continuous data were analyzed by the independent-samples t test and ANOVA. Multivariable binary logistic regression analyses were performed to assess the contributions of individual comorbidities to mortality, adverse events, and nonroutine discharge. Elixhauser comorbidities with a P value of < .10 in the bivariate analysis and presenting in at least 0.2% of the population were included in the logistic regression.31 Odds ratios and confidence intervals were calculated to assess the association between comorbidities and our dichotomous variables. A P value of < .001 defined statistical significance.33 Statistical analysis was conducted using SPSS version 21 (IBM).
RESULTS
Patient Demographics
Our query demonstrated a total of 1,155,960 patients who underwent surgical fixation of femoral neck fractures (Table 1). The most commonly used treatment modality was HA (75%), followed by ORIF (18%) and later by THA (7%). The majority of patients were females in each treatment group. Patients’ age varied according to treatment group, with patients undergoing HA having a mean age of 81.0 ± 9.0 years, patients undergoing ORIF having a mean age of 75.0 ± 17.0 years, and those undergoing THA having a mean age of 79.0 ± 10.0 years (P < .001). The majority of patients were ≥80 years in all treatment groups, but the ORIF group had the greatest proportion of patients <65 years (P < .001). Among patients undergoing HA, 62.4% were ≥80 years, while the ORIF and HA groups consisted of 48.6% and 51.5% of patients in that same age group, respectively.

Continue to: TRENDS ANALYSIS
TRENDS ANALYSIS
There was a significant change in the distributions of the procedures performed according to age group over time. Patients >80 years continued to undergo primarily HA, with an increase from 74.4% during 1990 to 1995 up to 84.6% during the 2002 to 2007 period and a concomitant decrease in ORIF from 18.3% to 10.6% during the same time period in this age group. Surgical trends in patients 65 to 79 years demonstrated a significant decrease in management with ORIF from 19.1% in 1990 to 1995 to 16.8% in the 2002 to 2007 cohort (P < .001 for all, Table 2). There was an increase in the use of HA from 71.9% during the 1990 to 1995 period to 75.5% during the final study period (Table 2, Figure 1). The use of THA for all age groups decreased between 1990 and 2007, except for the 50- to 64-year-old group where THA utilization remained constant.

Management patterns in patients 50 to 64 years varied throughout the analysis and demonstrated the following trend: treatment with HA remained the most common technique used but varied slightly from 59.7% during 1990 to 1995 to 60.3% during 2002 to2007 (P < .001, Table 2). The second most common treatment used was ORIF, which decreased from 32.2% to 31.5% (P < .001, Table 2). The use of THA varied significantly from 8.2% among those managed during 1990 to 1995 to 11.7% during 1996 to 2001 but later declined to the initial 8.2% (P < .001, Table 2).

Analysis of patients ≤49 years demonstrated that ORIF was the preferred technique, which experienced a growth from 63.9% during 1990 to 1995 to 81.4% during the 2002 to 2007 period (P < .001, Table 2). A decreased use in THA was observed from 2.0% in the initial period to 0.6% in the final period (P < .001, Table 2). Use of HA decreased from 34.0% in 1990 to 1995 to 18.0% in 2002 to 2007 (P < .001, Table 2).
LENGTH OF STAY
Mean number of in-hospital days decreased throughout the study period for all treatment techniques. During the 1990 to 1995 study period, patients who underwent ORIF had a mean LOS of 8 ± 7 days, which decreased (P < .001, Table 2) to 6 ± 3 days in 1996 to 2001 and remained constant during 2002 to 2007 (mean 6 ± 4 days). This decrease in LOS was also observed in patients who underwent THA (P < .001, Table 2), who initially had a mean LOS of 11 ± 7 days during 1990 to 1995, which later decreased to 7 ± 5 days for the remainder of the study. The LOS for patients who underwent HA also decreased (P < .001, Table 2), which initially was reported to be 11 ± 11 days during 1990 to 1995, decreasing to 7 ±7 days in 1996–2001 and later to 6 ± 4 days in 2002 to 2007.
COMORBIDITIY ANALYSIS
The Elixhauser Comorbidity Index varied significantly among groups over time (P < .001, Table 2). Overall mean Elixhauser Comorbidity Index score per procedure type is provided in Table 1, with HA patients having the highest score (-0.15 ± 13.09, p<.001).
Continue to: Analysis of the preoperative comorbidities...
Analysis of the preoperative comorbidities demonstrated significant differences among each surgical treatment group (P < .001 for all, Table 3). The most common comorbidities in patients who underwent HA were uncomplicated hypertension (33.2%), fluid/electrolyte disorders (17.4%), chronic pulmonary disease (14.9%), and congestive heart failure (13.7%). The most common comorbidities in the ORIF group were uncomplicated hypertension (30.8%), fluid/electrolyte disorders (14.5%), chronic pulmonary disease (14.0%), and uncomplicated diabetes (10.9%). Patients treated with THA had most commonly uncomplicated hypertension (30.1%), fluid/electrolyte disorders (17.2%), uncomplicated diabetes (15.5%), and chronic pulmonary disease (14.4%). The prevalence of comorbidities is displayed in Table 3.

DISCHARGE STATUS
Mortality varied significantly, being lowest in those who underwent ORIF (0.8%), followed those who underwent THA (1.8%), and HA (2.6%) (P < .001, Table 1).
The majority of patients in each group were discharged to long-term rehabilitation facilities, including 53.0% of those treated with HA, 40.4% of those treated with ORIF, and 44.3% of patients treated with THA. The second most common discharge location was home, which included 14.8% of patients who underwent HA, 32.2% of patients treated with ORIF, and 20.8% of those who underwent THA. Table 3 demonstrates the details of the discharge settings.

Mortality analysis over time demonstrated a significant decrease in each treatment group (P < .001). Mortality in the ORIF group decreased from 1.2% during 1990 to 1995 to 0.8% in 2002 to 2007. Mortality in the THA group also decreased significantly from 0.8% during 1990 to 1995 to 0.5% during the 2002 to 2007 time period. Patients who underwent HA also exhibited a decrease in mortality rate from 3.3% during 1990 to 1995 to 2.2% during 2002 to 2007 (P < .001, Table 4, Figure 2).

GENERAL ADVERSE EVENTS
There was a significant difference (P < .001) in the percentage of adverse events experienced, the maximum being observed in the THA group (41.0%), followed by the HA group (37.9%) and trailed by the ORIF group (20.3%, (P < .001, Table 1). The prevalence of adverse events is detailed in Table 5.

Continue to: Patients who underwent THA...
Patients who underwent THA had the highest rate of any adverse event, LOS, and transfusion rate (Table 1 and Table 5).
The prevalence of postoperative pneumonia was highest in the HA group (3.4%), followed by the ORIF group (2.9%), and the THA group (2.6%) (P < .001, Table 5). There was also a significant difference in rates of intubation, pulmonary insufficiency, acute renal failure, pulmonary embolism, acute myocardial infarction, induced mental disorder, and deep venous thrombosis (P < .001 for all, Table 5).
SURGERY-RELATED ADVERSE EVENTS
Surgery-related outcomes over the entire study period were significantly different according to the type of procedure performed (P < .001, Table 5). Patients who underwent HA had the highest rate of acute postoperative anemia (20.2%), followed by those who underwent THA (19.7%), and ORIF (10.2%). Postoperative bleeding rates also varied significantly, with 1.2% in the HA group, followed by 1.0% in the ORIF group and 0.4% in the THA group (P < .001, Table 5). Acute postoperative infection rates also varied significantly, with the highest rate being observed in the HA group (0.6%) compared to that in the THA and ORIF groups (both 0.3%) (P < .001, Table 5).

Table 6, Table 7, and Table 8 detail the results of regression analyses in patients with femoral neck fractures for individual risk factors associated with mortality, any adverse event, and nonroutine discharge to a short- or long-term rehabilitation facility, respectively. Increasing age (50–64 years, OR: 0.238; 65–79 years, OR: 1.762; and ≥80 years, OR: 2.700), THA (OR: 1.743), and HA (OR: 2.574) were found to be independent risk factors for mortality in the perioperative period (P < .001 for each, Table 6). Increasing age (50–64 years, OR: 1.888; 65–79 years, OR: 2.983; and ≥80 years, OR: 3.722), THA (OR: 2.489), and HA (OR: 2.098) were also found to be independent risk factors for any adverse event in the perioperative period (P < .001, Table 7). Age (50–64 years, OR: 1.662; 65–79 years, OR: 4.320; and ≥80 years, OR: 7.102) was the best predictor for nonroutine discharge to a short- or long-term rehabilitation facility (P < .001, Table 8).

DISCUSSION
Femoral neck fractures in the elderly population present a significant financial burden to the healthcare system.1-3,24,25 Consistent with previous epidemiological studies, our results show that the femoral neck fracture population has become older and has more medical comorbidities over the last 3 decades.27,28. Similarly, we also found that the rate of medical, surgical, and mechanical perioperative complications has increased in the same time period. Interestingly, the mortality rate has remained relatively similar.

Continue to: Although patients undergoing HA...
Although patients undergoing HA for femoral neck fractures are older and have more medical comorbidities, we found that the rate of adverse events in the perioperative period for patients undergoing THA was higher than that in the HA group. Consistent with prior studies, patients who underwent THA had higher rates of blood transfusion, pulmonary embolism, and induced mental disorders.34 Multivariable regression analysis demonstrated that after controlling for age, medical comorbidity, and type of surgery performed, THA emerged as an independent risk factor for any adverse event in the perioperative period. Increased anesthesia time, reaming of the acetabulum, and increased complexity of surgery probably account for these changes.
Our study results are consistent with those of Jain and colleagues,30 which showed a decrease in utilization of THA for femoral neck fractures between 1990 and 2001. Since THA is generally indicated for younger, more active patients in relatively good health, this would explain why changes in baseline health in this cohort over the last 20 years would lead to fewer THA procedures being performed. Surgeons in the US may be finding there are fewer patients who are candidates for THA. Miller and colleagues5 reported conflicting results and showed an increase in THA utilization in this patient population. However, their study evaluated treatment trends based on data from the ABOS part II of recently graduated orthopedic surgeons and may not be an accurate representation of national practice trends in the US. The trend toward increased subspecialization may explain their findings. As the authors noted, although they found an increase in the use of THA for femoral neck fractures by new adult reconstruction surgeons, the percentage of new surgeons treating femoral neck fractures has declined.5
Our analysis showed very concrete trends in treatment management at the extremes of the age ranges. There were substantial increases in the use of ORIF for patients <50 years (from 63.9% in 1990–1995 to 81.4% in 2002–2007, P < .001) and in the use of HA for patients >80 years (from 74.4% in 1990–1995 to 84.6% in 2002–2007, P < .001). This trend parallels recent studies that purport better outcomes for young patients undergoing ORIF and elderly patients undergoing HA.30 Our analysis did not demonstrate a large shift in surgeon preference for treatment of patients between 50 and 80 years, although there was a statistically significant decrease in ORIF and THA usage and a reflective increase in HA usage in this population as well. The fact that there has not been as substantial a shift in treatment trends for this large age group is potentially due to the wide variations in comorbid conditions and the functionality that abounds in this age group.1
The limitations of the current study are those inherent with a retrospective database analysis. The reliance on accurate coding brings up a potential for error; however, it is unlikely that comorbidities and outcomes are undercoded as hospitals are incentivized to input values that increase the acuity and thus reimbursement for each hospital stay.35 The database also relies on the ICD-9 procedural and diagnostic codes, which are not as specific as the currently adopted ICD-10 codes; hence, we are unable to distinguish between different forms of internal fixation, for example intramedullary nailing versus dynamic hip screw. This also precludes us from including other critical data such as degree of fracture displacement, cemented versus uncemented implantation, surgical approach for arthroplasty, and functional outcomes of individual patients. Moreover, the database used, although the largest inpatient sample available for analysis, represents only approximately 20% of hospitals nationwide. In addition, as patients cannot be tracked over time within the database, we are limited to outcomes in the perioperative period captured in a single hospital stay and cannot identify readmissions. Finally, our analysis is limited to the years 1990 to 2007 because of an increase in the relative standard error of the database in more recent years. Although this results in data that are not the most current, we believe that this study provides valuable insight regarding the trends in surgical treatment and acute postoperative outcomes of these injuries that have hitherto not been reported. To limit the inherent biases and the limitations within this study, prospective, randomized studies with long-term follow-up comparing outcomes across modes of treatment are needed to definitively determine the optimum form of treatment for this fracture type.
CONCLUSION
This is the largest study to date reporting on national trends in the surgical treatment and outcomes of the femoral neck fracture population. Orthopedic surgeons performing THA should be aware that the femoral neck fracture population is changing and at higher risk for perioperative complications. The advent of bisphosphonate therapy has been suggested as a possible reason for the decrease in fragility fractures and why a larger proportion of the femoral neck fracture population is now >80 years.36,37 With an aging population at a higher risk for perioperative complications, clinicians must take special care in choosing the appropriate surgical intervention that will give their patients the best functional outcome while minimizing the risk of surgical complications. Orthopedic surgeons should weigh the added risk associated with THA in this population.
ABSTRACT
The ideal mode of fixation for patients with femoral neck fractures is not well defined in the current literature. This study describes the recent trends in surgical management of femoral neck fractures with an analysis on perioperative outcomes.
The National Hospital Discharge Survey was used to identify femoral neck fractures in the United States between 1990 and 2007 (n = 1,155,960) treated with open reduction and internal fixation (ORIF), total hip arthroplasty (THA), or hemiarthroplasty (HA). Trends were examined over the following 3 time periods: 1990 to 1995 (group 1), 1996 to 2001 (group 2), and 2002 to 2007 (group 3). Elixhauser Comorbidity Index and perioperative complications were calculated.
Use of HA increased (74.4% to 84.6%), whereas that of THA (7.3% to 4.9%) and ORIF (18.3% to 10.6%) decreased, from group 1 to group 3 in the age group of >80 years. The use of ORIF increased (63.9% to 81.4%), whereas the use of both HA and THA decreased, from group 1 to group 3 in the age group of <50 years. The rate of adverse events increased across all fixation types but was greatest among THA (32.2% to 48.3%).
The femoral neck patient population is now older and has more medical comorbidities. We observed a trend toward performing HA in older patients and ORIF in younger patients. Despite superior functional outcomes reported in THA, this study found a decreased utilization of THA in all age groups along with an increase in adverse events and nonroutine discharges for patients with femoral neck fractures treated with THA.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Continue to: Femoral neck fractures...
Femoral neck fractures are a common occurrence in the United States. A recent study estimated an incidence of >63 per 100,000 population.1-8 Although the incidence appears to have decreased over recent decades, there is a projected exponential increase in the incidence of hip fractures over the next 30 years in the baby boomer population.8,9 Given that these fractures have a significant impact on patient morbidity, mortality, and quality of life, research efforts have been directed toward optimizing the treatment of affected patients and improving the outcomes.4,9-24
The treatment of choice for femoral neck fractures and the use of total hip arthroplasty (THA)11 have been a topic of debate.4,9,10,15-17,22,25 Total hip arthroplasty has been advocated for younger, more active patients, whereas hemiarthroplasty (HA) has been reserved for patients who are older and less active. Although several studies have demonstrated that arthroplasty outperforms open reduction and internal fixation (ORIF) in the elderly population with displaced femoral neck fractures, ORIF is still commonly performed in the United States for nondisplaced fractures and in patients aged <50 years.26-29
In an attempt to quantify the use of THA in the treatment of femoral neck fractures and demonstrate the national trends, Miller and colleagues5 pooled the American Board of Orthopaedic Surgery (ABOS) database and analyzed the treatment trends of surgeons taking part II of the ABOS examination from 1999 to 2011. The authors found an increased utilization of THA by recently graduated orthopedic surgeons. In contrast, Jain and colleagues30 found different national trends when they analyzed data from the National Inpatient Sample containing data between 1990 and 2001 and further found decreased utilization of THA procedures by orthopedic surgeons of all levels of training nationwide. However, neither of these studies reported about the trends in demographics, comorbidities, risk factors, or outcomes in this patient population following surgery.
The purpose of this study was to help clarify the findings of these authors using the largest dataset to date and also report on the perioperative complications associated with each mode of fixation in patients who undergo operative treatment for femoral neck fractures in the United States. Our hypotheses were that the femoral neck fracture patient population has become older and has more medical comorbidities. We also hypothesized that there has been a trend toward performing fewer THA procedures in the United States and that THA is associated with increased perioperative complications compared to those with HA and ORIF.
MATERIALS AND METHODS
We conducted a retrospective epidemiological study using the National Hospital Discharge Survey (NHDS) on surgical trends in the management of femoral neck fractures. The NHDS is a publicly available survey that is conducted annually to provide data of nonfederal, short-stay hospitals to the public. The sample data are weighted to provide nationwide estimates of annual inpatient care. The NHDS includes up to 7 medical diagnoses and 4 procedural codes per case, which are categorized using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, that were collected along with patient demographic information, length of stay (LOS), and discharge disposition. The diagnostic and procedural codes used for this study are presented in the Appendix. The year 2007 was chosen as the endpoint of this study due to the fact that the relative standard error of the NHDS doubled in 2008 as a result of a decrease in its survey size. As this is a publicly available database, our study was exempt from institutional review board approval.

Continue to: All pateints admitted...
All patients admitted with a primary diagnosis of closed transcervical fracture of the femoral neck (ICD-9-CM 820.0x) were selected. This resulted in 1,674,160 fractures. All patients with fractures with a concurrent primary procedural code of ORIF (79.35), HA (81.52), or THA (81.51) were identified, resulting in a total sample size of 1,155,960 surgical fractures. Analysis of the fractures based on additional specificity,ie subcapital versus midcervical versus basicervical, was not carried out because >90% of femoral neck fractures in the database were coded as “unspecified” or “other” (ICD9 CM 820.00 and 820.09, respectively).
Comorbidity burden was quantified using Elixhauser coding algorithms as previously described.31 The Elixhauser comorbidity measure is a model consisting of 31 conditions and has recently been identified as a better predictor of mortality in patients undergoing orthopedic procedures when compared with the Charlson Comorbidity Index.31 Dichotomous variables for each Elixhauser comorbidity were created, and χ2 tests were utilized to assess the association between each comorbidity and mortality. The weighted Elixhauser score for each statistically significant comorbidity was calculated as described by van Walraven and colleagues.32 The Elixhauser comorbidity score was then calculated for each patient by summing the individual weights of all comorbidities. Postoperative adverse events were determined using the complication-screening-package as previously described.33
All adverse events were categorized into 3 categories, including general medical complications, mechanical complications, and surgical complications. All adverse events recorded in the NHDS database are events that occurred during a single hospitalization. Therefore, it does not take into account adverse events that occurred after discharge, and, for example, mortality refers to postoperative mortality that occurs prior to discharge. The study period comprised data captured from 1990 to 2007, and 3 groups were generated from this time period to better characterize patients throughout the large study time frame. Group 1 comprised patients who underwent surgical management of femoral neck fractures from 1990 to 1995, group 2 consisted of patients treated from 1996 to 2001, and group 3 included patients treated from 2002 to 2007.
Categorical data were analyzed using the χ2 test, and continuous data were analyzed by the independent-samples t test and ANOVA. Multivariable binary logistic regression analyses were performed to assess the contributions of individual comorbidities to mortality, adverse events, and nonroutine discharge. Elixhauser comorbidities with a P value of < .10 in the bivariate analysis and presenting in at least 0.2% of the population were included in the logistic regression.31 Odds ratios and confidence intervals were calculated to assess the association between comorbidities and our dichotomous variables. A P value of < .001 defined statistical significance.33 Statistical analysis was conducted using SPSS version 21 (IBM).
RESULTS
Patient Demographics
Our query demonstrated a total of 1,155,960 patients who underwent surgical fixation of femoral neck fractures (Table 1). The most commonly used treatment modality was HA (75%), followed by ORIF (18%) and later by THA (7%). The majority of patients were females in each treatment group. Patients’ age varied according to treatment group, with patients undergoing HA having a mean age of 81.0 ± 9.0 years, patients undergoing ORIF having a mean age of 75.0 ± 17.0 years, and those undergoing THA having a mean age of 79.0 ± 10.0 years (P < .001). The majority of patients were ≥80 years in all treatment groups, but the ORIF group had the greatest proportion of patients <65 years (P < .001). Among patients undergoing HA, 62.4% were ≥80 years, while the ORIF and HA groups consisted of 48.6% and 51.5% of patients in that same age group, respectively.

Continue to: TRENDS ANALYSIS
TRENDS ANALYSIS
There was a significant change in the distributions of the procedures performed according to age group over time. Patients >80 years continued to undergo primarily HA, with an increase from 74.4% during 1990 to 1995 up to 84.6% during the 2002 to 2007 period and a concomitant decrease in ORIF from 18.3% to 10.6% during the same time period in this age group. Surgical trends in patients 65 to 79 years demonstrated a significant decrease in management with ORIF from 19.1% in 1990 to 1995 to 16.8% in the 2002 to 2007 cohort (P < .001 for all, Table 2). There was an increase in the use of HA from 71.9% during the 1990 to 1995 period to 75.5% during the final study period (Table 2, Figure 1). The use of THA for all age groups decreased between 1990 and 2007, except for the 50- to 64-year-old group where THA utilization remained constant.

Management patterns in patients 50 to 64 years varied throughout the analysis and demonstrated the following trend: treatment with HA remained the most common technique used but varied slightly from 59.7% during 1990 to 1995 to 60.3% during 2002 to2007 (P < .001, Table 2). The second most common treatment used was ORIF, which decreased from 32.2% to 31.5% (P < .001, Table 2). The use of THA varied significantly from 8.2% among those managed during 1990 to 1995 to 11.7% during 1996 to 2001 but later declined to the initial 8.2% (P < .001, Table 2).

Analysis of patients ≤49 years demonstrated that ORIF was the preferred technique, which experienced a growth from 63.9% during 1990 to 1995 to 81.4% during the 2002 to 2007 period (P < .001, Table 2). A decreased use in THA was observed from 2.0% in the initial period to 0.6% in the final period (P < .001, Table 2). Use of HA decreased from 34.0% in 1990 to 1995 to 18.0% in 2002 to 2007 (P < .001, Table 2).
LENGTH OF STAY
Mean number of in-hospital days decreased throughout the study period for all treatment techniques. During the 1990 to 1995 study period, patients who underwent ORIF had a mean LOS of 8 ± 7 days, which decreased (P < .001, Table 2) to 6 ± 3 days in 1996 to 2001 and remained constant during 2002 to 2007 (mean 6 ± 4 days). This decrease in LOS was also observed in patients who underwent THA (P < .001, Table 2), who initially had a mean LOS of 11 ± 7 days during 1990 to 1995, which later decreased to 7 ± 5 days for the remainder of the study. The LOS for patients who underwent HA also decreased (P < .001, Table 2), which initially was reported to be 11 ± 11 days during 1990 to 1995, decreasing to 7 ±7 days in 1996–2001 and later to 6 ± 4 days in 2002 to 2007.
COMORBIDITIY ANALYSIS
The Elixhauser Comorbidity Index varied significantly among groups over time (P < .001, Table 2). Overall mean Elixhauser Comorbidity Index score per procedure type is provided in Table 1, with HA patients having the highest score (-0.15 ± 13.09, p<.001).
Continue to: Analysis of the preoperative comorbidities...
Analysis of the preoperative comorbidities demonstrated significant differences among each surgical treatment group (P < .001 for all, Table 3). The most common comorbidities in patients who underwent HA were uncomplicated hypertension (33.2%), fluid/electrolyte disorders (17.4%), chronic pulmonary disease (14.9%), and congestive heart failure (13.7%). The most common comorbidities in the ORIF group were uncomplicated hypertension (30.8%), fluid/electrolyte disorders (14.5%), chronic pulmonary disease (14.0%), and uncomplicated diabetes (10.9%). Patients treated with THA had most commonly uncomplicated hypertension (30.1%), fluid/electrolyte disorders (17.2%), uncomplicated diabetes (15.5%), and chronic pulmonary disease (14.4%). The prevalence of comorbidities is displayed in Table 3.

DISCHARGE STATUS
Mortality varied significantly, being lowest in those who underwent ORIF (0.8%), followed those who underwent THA (1.8%), and HA (2.6%) (P < .001, Table 1).
The majority of patients in each group were discharged to long-term rehabilitation facilities, including 53.0% of those treated with HA, 40.4% of those treated with ORIF, and 44.3% of patients treated with THA. The second most common discharge location was home, which included 14.8% of patients who underwent HA, 32.2% of patients treated with ORIF, and 20.8% of those who underwent THA. Table 3 demonstrates the details of the discharge settings.

Mortality analysis over time demonstrated a significant decrease in each treatment group (P < .001). Mortality in the ORIF group decreased from 1.2% during 1990 to 1995 to 0.8% in 2002 to 2007. Mortality in the THA group also decreased significantly from 0.8% during 1990 to 1995 to 0.5% during the 2002 to 2007 time period. Patients who underwent HA also exhibited a decrease in mortality rate from 3.3% during 1990 to 1995 to 2.2% during 2002 to 2007 (P < .001, Table 4, Figure 2).

GENERAL ADVERSE EVENTS
There was a significant difference (P < .001) in the percentage of adverse events experienced, the maximum being observed in the THA group (41.0%), followed by the HA group (37.9%) and trailed by the ORIF group (20.3%, (P < .001, Table 1). The prevalence of adverse events is detailed in Table 5.

Continue to: Patients who underwent THA...
Patients who underwent THA had the highest rate of any adverse event, LOS, and transfusion rate (Table 1 and Table 5).
The prevalence of postoperative pneumonia was highest in the HA group (3.4%), followed by the ORIF group (2.9%), and the THA group (2.6%) (P < .001, Table 5). There was also a significant difference in rates of intubation, pulmonary insufficiency, acute renal failure, pulmonary embolism, acute myocardial infarction, induced mental disorder, and deep venous thrombosis (P < .001 for all, Table 5).
SURGERY-RELATED ADVERSE EVENTS
Surgery-related outcomes over the entire study period were significantly different according to the type of procedure performed (P < .001, Table 5). Patients who underwent HA had the highest rate of acute postoperative anemia (20.2%), followed by those who underwent THA (19.7%), and ORIF (10.2%). Postoperative bleeding rates also varied significantly, with 1.2% in the HA group, followed by 1.0% in the ORIF group and 0.4% in the THA group (P < .001, Table 5). Acute postoperative infection rates also varied significantly, with the highest rate being observed in the HA group (0.6%) compared to that in the THA and ORIF groups (both 0.3%) (P < .001, Table 5).

Table 6, Table 7, and Table 8 detail the results of regression analyses in patients with femoral neck fractures for individual risk factors associated with mortality, any adverse event, and nonroutine discharge to a short- or long-term rehabilitation facility, respectively. Increasing age (50–64 years, OR: 0.238; 65–79 years, OR: 1.762; and ≥80 years, OR: 2.700), THA (OR: 1.743), and HA (OR: 2.574) were found to be independent risk factors for mortality in the perioperative period (P < .001 for each, Table 6). Increasing age (50–64 years, OR: 1.888; 65–79 years, OR: 2.983; and ≥80 years, OR: 3.722), THA (OR: 2.489), and HA (OR: 2.098) were also found to be independent risk factors for any adverse event in the perioperative period (P < .001, Table 7). Age (50–64 years, OR: 1.662; 65–79 years, OR: 4.320; and ≥80 years, OR: 7.102) was the best predictor for nonroutine discharge to a short- or long-term rehabilitation facility (P < .001, Table 8).

DISCUSSION
Femoral neck fractures in the elderly population present a significant financial burden to the healthcare system.1-3,24,25 Consistent with previous epidemiological studies, our results show that the femoral neck fracture population has become older and has more medical comorbidities over the last 3 decades.27,28. Similarly, we also found that the rate of medical, surgical, and mechanical perioperative complications has increased in the same time period. Interestingly, the mortality rate has remained relatively similar.

Continue to: Although patients undergoing HA...
Although patients undergoing HA for femoral neck fractures are older and have more medical comorbidities, we found that the rate of adverse events in the perioperative period for patients undergoing THA was higher than that in the HA group. Consistent with prior studies, patients who underwent THA had higher rates of blood transfusion, pulmonary embolism, and induced mental disorders.34 Multivariable regression analysis demonstrated that after controlling for age, medical comorbidity, and type of surgery performed, THA emerged as an independent risk factor for any adverse event in the perioperative period. Increased anesthesia time, reaming of the acetabulum, and increased complexity of surgery probably account for these changes.
Our study results are consistent with those of Jain and colleagues,30 which showed a decrease in utilization of THA for femoral neck fractures between 1990 and 2001. Since THA is generally indicated for younger, more active patients in relatively good health, this would explain why changes in baseline health in this cohort over the last 20 years would lead to fewer THA procedures being performed. Surgeons in the US may be finding there are fewer patients who are candidates for THA. Miller and colleagues5 reported conflicting results and showed an increase in THA utilization in this patient population. However, their study evaluated treatment trends based on data from the ABOS part II of recently graduated orthopedic surgeons and may not be an accurate representation of national practice trends in the US. The trend toward increased subspecialization may explain their findings. As the authors noted, although they found an increase in the use of THA for femoral neck fractures by new adult reconstruction surgeons, the percentage of new surgeons treating femoral neck fractures has declined.5
Our analysis showed very concrete trends in treatment management at the extremes of the age ranges. There were substantial increases in the use of ORIF for patients <50 years (from 63.9% in 1990–1995 to 81.4% in 2002–2007, P < .001) and in the use of HA for patients >80 years (from 74.4% in 1990–1995 to 84.6% in 2002–2007, P < .001). This trend parallels recent studies that purport better outcomes for young patients undergoing ORIF and elderly patients undergoing HA.30 Our analysis did not demonstrate a large shift in surgeon preference for treatment of patients between 50 and 80 years, although there was a statistically significant decrease in ORIF and THA usage and a reflective increase in HA usage in this population as well. The fact that there has not been as substantial a shift in treatment trends for this large age group is potentially due to the wide variations in comorbid conditions and the functionality that abounds in this age group.1
The limitations of the current study are those inherent with a retrospective database analysis. The reliance on accurate coding brings up a potential for error; however, it is unlikely that comorbidities and outcomes are undercoded as hospitals are incentivized to input values that increase the acuity and thus reimbursement for each hospital stay.35 The database also relies on the ICD-9 procedural and diagnostic codes, which are not as specific as the currently adopted ICD-10 codes; hence, we are unable to distinguish between different forms of internal fixation, for example intramedullary nailing versus dynamic hip screw. This also precludes us from including other critical data such as degree of fracture displacement, cemented versus uncemented implantation, surgical approach for arthroplasty, and functional outcomes of individual patients. Moreover, the database used, although the largest inpatient sample available for analysis, represents only approximately 20% of hospitals nationwide. In addition, as patients cannot be tracked over time within the database, we are limited to outcomes in the perioperative period captured in a single hospital stay and cannot identify readmissions. Finally, our analysis is limited to the years 1990 to 2007 because of an increase in the relative standard error of the database in more recent years. Although this results in data that are not the most current, we believe that this study provides valuable insight regarding the trends in surgical treatment and acute postoperative outcomes of these injuries that have hitherto not been reported. To limit the inherent biases and the limitations within this study, prospective, randomized studies with long-term follow-up comparing outcomes across modes of treatment are needed to definitively determine the optimum form of treatment for this fracture type.
CONCLUSION
This is the largest study to date reporting on national trends in the surgical treatment and outcomes of the femoral neck fracture population. Orthopedic surgeons performing THA should be aware that the femoral neck fracture population is changing and at higher risk for perioperative complications. The advent of bisphosphonate therapy has been suggested as a possible reason for the decrease in fragility fractures and why a larger proportion of the femoral neck fracture population is now >80 years.36,37 With an aging population at a higher risk for perioperative complications, clinicians must take special care in choosing the appropriate surgical intervention that will give their patients the best functional outcome while minimizing the risk of surgical complications. Orthopedic surgeons should weigh the added risk associated with THA in this population.
1. Bishop J, Yang A, Githens M, Sox AH. Evaluation of contemporary trends in femoral neck fracture management reveals discrepancies in treatment. Geriatr Orthop Surg Rehabil. 2016;7(3):135. doi:10.1177/2151458516658328.
2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res Off J Am Soc Bone Miner Res. 2007;22(3):465. doi:10.1359/jbmr.061113.
3. Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl.):57s. doi:10.1016/8756-3282(95)00381-9.
4. Koval KJ, Zuckerman JD. Hip fractures: I. Overview and evaluation and treatment of femoral-neck fractures. J Am Acad Orthop Surg. 1994;2(3):141. doi:10.5435/00124635-199405000-00002.
5. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg. (American) 2014;96(17):e149. doi:10.2106/JBJS.M.01122.
6. Miller BJ, Lu X, Cram P. The trends in treatment of femoral neck fractures in the Medicare population from 1991 to 2008. J Bone Joint Surg. (American) 2013;95(18):e132. doi:10.2106/JBJS.L.01163.
7. Nwachukwu BU, McCormick F, Provencher MT, Roche M, Rubash HE. A comprehensive analysis of Medicare trends in utilization and hospital economics for total knee and hip arthroplasty from 2005 to 2011. J Arthroplast. 2015;30(1):15. doi:10.1016/j.arth.2014.08.025.
8. Su EP, Su SL. Femoral neck fractures: a changing paradigm. Bone Joint J. 2014;96-b(11) Supple A):43. doi:10.1302/0301-620X.96B11.34334.
9. Ahn J, Man LX, Park S, Sodl JF, Esterhai JL. Systematic review of cemented and uncemented hemiarthroplasty outcomes for femoral neck fractures. Clin Orthop Relat Res. 2008;466(10):2513. doi:10.1007/s11999-008-0368-3.
10. Alolabi B, Bajammal S, Shirali J, Karanicolas PJ, Gafni A, Bhandari M. Treatment of displaced femoral neck fractures in the elderly: a cost-benefit analysis. J Orthop Trauma. 2009;23(6):442. doi:10.1097/BOT.0b013e31817614dd.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290. doi:10.1093/aje/kwp266.
12. Brox WT, Chan PH, Cafri G, Inacio MC. Similar mortality with general or regional anesthesia in elderly hip fracture patients. Acta Orthop. 2016;87(2):152. doi:10.3109/17453674.2015.1128781.
13. Catal B, Sener M. Treatment and displacement affect the reoperation rate for femoral neck fracture. Clin Orthop Relat Res. 2013;471(12):4096. doi:10.1007/s11999-013-3295-x.
14. Dailiana Z, Papakostidou I, Varitimidis S, Michalitsis S, Veloni A, Malizos K. Surgical treatment of hip fractures: factors influencing mortality. Hippokratia. 2013;17(3):252.
15. Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012;26(3):135. doi:10.1097/BOT.0b013e318238b7a5.
16. Hedbeck CJ, Inngul C, Blomfeldt R, Ponzer S, Tornkvist H, Enocson A. Internal fixation versus cemented hemiarthroplasty for displaced femoral neck fractures in patients with severe cognitive dysfunction: a randomized controlled trial. J Orthop Trauma. 2013;27(12):690. doi:10.1097/BOT.0b013e318291f544.
17. Jia Z, Ding F, Wu Y, et al. Unipolar versus bipolar hemiarthroplasty for displaced femoral neck fractures: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10:8. doi:10.1186/s13018-015-0165-0.
18. Lapidus LJ, Charalampidis A, Rundgren J, Enocson A. Internal fixation of garden I and II femoral neck fractures: posterior tilt did not influence the reoperation rate in 382 consecutive hips followed for a minimum of 5 years. J Orthop Trauma. 2013;27(7):386. doi:10.1097/BOT.0b013e318281da6e.
19. Mariconda M, Costa GG, Cerbasi S, et al. Factors predicting mobility and the change in Activities of Daily Living After hip fracture: A 1-year prospective cohort study. J Orthop Trauma. 2016;30(2):71. doi:10.1097/BOT.0000000000000448.
20. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality After proximal femoral fracture: A retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg. (American) 2015;97(16):1333. doi:10.2106/JBJS.O.00029.
21. Samuel AM, Russo GS, Lukasiewicz AM, et al. Surgical treatment of femoral neck fractures after 24 hours in patients between the ages of 18 and 49 is associated with poor inpatient outcomes: an analysis of 1361 patients in the National Trauma Data Bank. J Orthop Trauma. 2016;30(2):89. doi:10.1097/BOT.0000000000000456.
22. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235. doi:10.1007/s11999-012-2293-8.
23. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplast. 2012;27(4):583. doi:10.1016/j.arth.2011.07.009.
24. Zielinski SM, Keijsers NL, Praet SF, et al. Functional outcome after successful internal fixation versus salvage arthroplasty of patients with a femoral neck fracture. J Orthop Trauma. 2014;28(12):e273. doi:10.1097/BOT.0000000000000123.
25. Gu Q, Koenig L, Mather RC, 3rd, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536. doi:10.1007/s11999-014-3820-6.
26. Forsh DA, Ferguson TA. Contemporary management of femoral neck fractures: the young and the old. Curr Rev Musculoskelet Med. 2012;5(3):214. doi:10.1007/s12178-012-9127-x.
27. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287. doi:10.5435/00124635-200605000-00004.
28. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: Femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596. doi:10.5435/00124635-200810000-00005.
29. Probe R, Ward R. Internal fixation of femoral neck fractures. J Am Acad Orthop Surg. 2006;14(9):565. doi:10.5435/00124635-200609000-00006.
30. Jain NB, Losina E, Ward DM, Harris MB, Katz JN. Trends in surgical management of femoral neck fractures in the United States. Clin Orthop Relat Res. 2008;466(12):3116. doi:10.1007/s11999-008-0392-3.
31. Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878. doi:10.1007/s11999-014-3686-7.
32. Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser Comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633.
33. Best MJ, Buller LT, Falakassa J, Vecchione D. Risk factors for nonroutine discharge in patients undergoing spinal fusion for intervertebral disc disorders. Iowa Orthop J. 2015;35:147.
34. Schairer WW, Lane JM, Halsey DA, Iorio R, Padgett DE, McLawhorn AS. The Frank Stinchfield award: total hip arthroplasty for femoral neck fracture is not a typical DRG 470: A propensity-matched cohort study. Clin Orthop Relat Res. 2017;475(2):353-360. doi:10.1007/s11999-016-4868-2.
35. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9. doi:10.2106/JBJS.J.01077.
36. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl.):S14. doi:10.1016/j.amjmed.2008.12.003.
37. Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001-2008. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(1):3. doi:10.1002/jbmr.189.
1. Bishop J, Yang A, Githens M, Sox AH. Evaluation of contemporary trends in femoral neck fracture management reveals discrepancies in treatment. Geriatr Orthop Surg Rehabil. 2016;7(3):135. doi:10.1177/2151458516658328.
2. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res Off J Am Soc Bone Miner Res. 2007;22(3):465. doi:10.1359/jbmr.061113.
3. Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 Suppl.):57s. doi:10.1016/8756-3282(95)00381-9.
4. Koval KJ, Zuckerman JD. Hip fractures: I. Overview and evaluation and treatment of femoral-neck fractures. J Am Acad Orthop Surg. 1994;2(3):141. doi:10.5435/00124635-199405000-00002.
5. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg. (American) 2014;96(17):e149. doi:10.2106/JBJS.M.01122.
6. Miller BJ, Lu X, Cram P. The trends in treatment of femoral neck fractures in the Medicare population from 1991 to 2008. J Bone Joint Surg. (American) 2013;95(18):e132. doi:10.2106/JBJS.L.01163.
7. Nwachukwu BU, McCormick F, Provencher MT, Roche M, Rubash HE. A comprehensive analysis of Medicare trends in utilization and hospital economics for total knee and hip arthroplasty from 2005 to 2011. J Arthroplast. 2015;30(1):15. doi:10.1016/j.arth.2014.08.025.
8. Su EP, Su SL. Femoral neck fractures: a changing paradigm. Bone Joint J. 2014;96-b(11) Supple A):43. doi:10.1302/0301-620X.96B11.34334.
9. Ahn J, Man LX, Park S, Sodl JF, Esterhai JL. Systematic review of cemented and uncemented hemiarthroplasty outcomes for femoral neck fractures. Clin Orthop Relat Res. 2008;466(10):2513. doi:10.1007/s11999-008-0368-3.
10. Alolabi B, Bajammal S, Shirali J, Karanicolas PJ, Gafni A, Bhandari M. Treatment of displaced femoral neck fractures in the elderly: a cost-benefit analysis. J Orthop Trauma. 2009;23(6):442. doi:10.1097/BOT.0b013e31817614dd.
11. Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170(10):1290. doi:10.1093/aje/kwp266.
12. Brox WT, Chan PH, Cafri G, Inacio MC. Similar mortality with general or regional anesthesia in elderly hip fracture patients. Acta Orthop. 2016;87(2):152. doi:10.3109/17453674.2015.1128781.
13. Catal B, Sener M. Treatment and displacement affect the reoperation rate for femoral neck fracture. Clin Orthop Relat Res. 2013;471(12):4096. doi:10.1007/s11999-013-3295-x.
14. Dailiana Z, Papakostidou I, Varitimidis S, Michalitsis S, Veloni A, Malizos K. Surgical treatment of hip fractures: factors influencing mortality. Hippokratia. 2013;17(3):252.
15. Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012;26(3):135. doi:10.1097/BOT.0b013e318238b7a5.
16. Hedbeck CJ, Inngul C, Blomfeldt R, Ponzer S, Tornkvist H, Enocson A. Internal fixation versus cemented hemiarthroplasty for displaced femoral neck fractures in patients with severe cognitive dysfunction: a randomized controlled trial. J Orthop Trauma. 2013;27(12):690. doi:10.1097/BOT.0b013e318291f544.
17. Jia Z, Ding F, Wu Y, et al. Unipolar versus bipolar hemiarthroplasty for displaced femoral neck fractures: a systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10:8. doi:10.1186/s13018-015-0165-0.
18. Lapidus LJ, Charalampidis A, Rundgren J, Enocson A. Internal fixation of garden I and II femoral neck fractures: posterior tilt did not influence the reoperation rate in 382 consecutive hips followed for a minimum of 5 years. J Orthop Trauma. 2013;27(7):386. doi:10.1097/BOT.0b013e318281da6e.
19. Mariconda M, Costa GG, Cerbasi S, et al. Factors predicting mobility and the change in Activities of Daily Living After hip fracture: A 1-year prospective cohort study. J Orthop Trauma. 2016;30(2):71. doi:10.1097/BOT.0000000000000448.
20. Nyholm AM, Gromov K, Palm H, et al. Time to surgery is associated with thirty-day and ninety-day mortality After proximal femoral fracture: A retrospective observational study on prospectively collected data from the Danish Fracture Database Collaborators. J Bone Joint Surg. (American) 2015;97(16):1333. doi:10.2106/JBJS.O.00029.
21. Samuel AM, Russo GS, Lukasiewicz AM, et al. Surgical treatment of femoral neck fractures after 24 hours in patients between the ages of 18 and 49 is associated with poor inpatient outcomes: an analysis of 1361 patients in the National Trauma Data Bank. J Orthop Trauma. 2016;30(2):89. doi:10.1097/BOT.0000000000000456.
22. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235. doi:10.1007/s11999-012-2293-8.
23. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplast. 2012;27(4):583. doi:10.1016/j.arth.2011.07.009.
24. Zielinski SM, Keijsers NL, Praet SF, et al. Functional outcome after successful internal fixation versus salvage arthroplasty of patients with a femoral neck fracture. J Orthop Trauma. 2014;28(12):e273. doi:10.1097/BOT.0000000000000123.
25. Gu Q, Koenig L, Mather RC, 3rd, Tongue J. Surgery for hip fracture yields societal benefits that exceed the direct medical costs. Clin Orthop Relat Res. 2014;472(11):3536. doi:10.1007/s11999-014-3820-6.
26. Forsh DA, Ferguson TA. Contemporary management of femoral neck fractures: the young and the old. Curr Rev Musculoskelet Med. 2012;5(3):214. doi:10.1007/s12178-012-9127-x.
27. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287. doi:10.5435/00124635-200605000-00004.
28. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: Femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596. doi:10.5435/00124635-200810000-00005.
29. Probe R, Ward R. Internal fixation of femoral neck fractures. J Am Acad Orthop Surg. 2006;14(9):565. doi:10.5435/00124635-200609000-00006.
30. Jain NB, Losina E, Ward DM, Harris MB, Katz JN. Trends in surgical management of femoral neck fractures in the United States. Clin Orthop Relat Res. 2008;466(12):3116. doi:10.1007/s11999-008-0392-3.
31. Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878. doi:10.1007/s11999-014-3686-7.
32. Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser Comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633.
33. Best MJ, Buller LT, Falakassa J, Vecchione D. Risk factors for nonroutine discharge in patients undergoing spinal fusion for intervertebral disc disorders. Iowa Orthop J. 2015;35:147.
34. Schairer WW, Lane JM, Halsey DA, Iorio R, Padgett DE, McLawhorn AS. The Frank Stinchfield award: total hip arthroplasty for femoral neck fracture is not a typical DRG 470: A propensity-matched cohort study. Clin Orthop Relat Res. 2017;475(2):353-360. doi:10.1007/s11999-016-4868-2.
35. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9. doi:10.2106/JBJS.J.01077.
36. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl.):S14. doi:10.1016/j.amjmed.2008.12.003.
37. Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001-2008. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(1):3. doi:10.1002/jbmr.189.
TAKE-HOME POINTS
- The femoral neck patient population is older and has more medical comorbidities.
- Hemiarthroplasty (HA) is being performed more commonly in patients > 50 years old for femoral neck fractures.
- Open reduction and internal fixation is being performed more commonly in patients > 80 years old for femoral neck fractures.
- The rate of adverse events following femoral neck fracture is higher in the total hip arthroplasty (THA) group than in the HA group.
- THA is an independent risk factor for adverse events following femoral neck fracture.
Bring Schwartz Rounds to your hospital
A more emotional approach to rounds
If you are not doing Schwartz Rounds, get them started. ASAP.
I recently completed a 4-year tenure as physician moderator for our hospital’s Schwartz Rounds. An amazing team at my hospital helped pull the bimonthly sessions together. These compassionate care rounds are a national initiative to help foster empathy and compassion in the health care setting.
We gather a panel of two to three people involved in our patient presentation who share and move quickly through the clinical details, and head on toward the thornier ethical issues, emotional triggers, and responses. The best sessions are when the audience’s voice is heard for the bulk of the time.
The emotional cadence flows from boiling in frustration, drowning in tears, followed by comfort, and ending in thoughts for the next session. It is a more powerful arc than an episode of the television program “This is Us.” Largely, because this was us. This was real life. Real-time catharsis in the hospital.
In the daily grind, we often skip the step of processing our frustration, sadness, and anger, moving right on to the next patient and walking into the next room with that stoic layer of equanimity. I walk the hallways and find I grab my phone to catch up on emails, walking to the wrong floor because I’m not paying attention. Always something to do, someone to talk to, a family to call, pagers going off, phone calls. When do we sit and reflect?
These Schwartz Rounds are those moments of reflection – a slowdown in the day to think more deeply about the case. We talk about everything and anything. We have discussions with opposing views:
“Everything should have been done!”
“How did you not stop care?!”
“I agree with the doctors.”
“I can see the patient’s view more clearly now.”
Our first Schwartz Rounds tended to be end-of-life stories, particularly regarding the family mantra of “Do everything.” The health care team watches the suffering of a patient, a family, in a seemingly futile situation. Conversations around the end of life, choices, and quality of life are cut short daily by family members who simply recite, “Do everything.”
After several of these sessions, a case swings us in the other direction. The elderly gentleman with treatable cancer, who could easily survive another 20 years, declines treatment. “I’m fine, doc; I’ve lived long enough.” His wife at his bedside, shaking her head, tells us, “I don’t know why he wants to give up. He’s been as stubborn as a mule since the day I met him.” I spend 30 minutes convincing him to stay. The nurse does the same. Now we have a patient with a “Do nothing.” The patient’s decisions conflict with the family and the health care team.
Every day in the hospital provides a new ethical dilemma, a frustrating case, a challenging patient. Fodder for rounds.
Read the full post at hospitalleader.org.
Dr. Messler is a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. He previously chaired SHM’s Quality and Patient Safety Committee and has been active in several SHM mentoring programs, most recently with Project BOOST and Glycemic Control.
Also on The Hospital Leader
- Incubating Success: How We Used Structured Feedback to Reduce A Dangerous Practice by Rich Bottner, PA-C & Victoria Valencia, MPH
- New SoHM Report Provides Unique Window into Hospital Medicine Practice Trends by Leslie Flores, MHA, SFHM
- IGNITE Change: Improving Care via Interprofessional Clinical Learning Environments by Vineet Arora, MD, MAPP, MHM
A more emotional approach to rounds
A more emotional approach to rounds
If you are not doing Schwartz Rounds, get them started. ASAP.
I recently completed a 4-year tenure as physician moderator for our hospital’s Schwartz Rounds. An amazing team at my hospital helped pull the bimonthly sessions together. These compassionate care rounds are a national initiative to help foster empathy and compassion in the health care setting.
We gather a panel of two to three people involved in our patient presentation who share and move quickly through the clinical details, and head on toward the thornier ethical issues, emotional triggers, and responses. The best sessions are when the audience’s voice is heard for the bulk of the time.
The emotional cadence flows from boiling in frustration, drowning in tears, followed by comfort, and ending in thoughts for the next session. It is a more powerful arc than an episode of the television program “This is Us.” Largely, because this was us. This was real life. Real-time catharsis in the hospital.
In the daily grind, we often skip the step of processing our frustration, sadness, and anger, moving right on to the next patient and walking into the next room with that stoic layer of equanimity. I walk the hallways and find I grab my phone to catch up on emails, walking to the wrong floor because I’m not paying attention. Always something to do, someone to talk to, a family to call, pagers going off, phone calls. When do we sit and reflect?
These Schwartz Rounds are those moments of reflection – a slowdown in the day to think more deeply about the case. We talk about everything and anything. We have discussions with opposing views:
“Everything should have been done!”
“How did you not stop care?!”
“I agree with the doctors.”
“I can see the patient’s view more clearly now.”
Our first Schwartz Rounds tended to be end-of-life stories, particularly regarding the family mantra of “Do everything.” The health care team watches the suffering of a patient, a family, in a seemingly futile situation. Conversations around the end of life, choices, and quality of life are cut short daily by family members who simply recite, “Do everything.”
After several of these sessions, a case swings us in the other direction. The elderly gentleman with treatable cancer, who could easily survive another 20 years, declines treatment. “I’m fine, doc; I’ve lived long enough.” His wife at his bedside, shaking her head, tells us, “I don’t know why he wants to give up. He’s been as stubborn as a mule since the day I met him.” I spend 30 minutes convincing him to stay. The nurse does the same. Now we have a patient with a “Do nothing.” The patient’s decisions conflict with the family and the health care team.
Every day in the hospital provides a new ethical dilemma, a frustrating case, a challenging patient. Fodder for rounds.
Read the full post at hospitalleader.org.
Dr. Messler is a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. He previously chaired SHM’s Quality and Patient Safety Committee and has been active in several SHM mentoring programs, most recently with Project BOOST and Glycemic Control.
Also on The Hospital Leader
- Incubating Success: How We Used Structured Feedback to Reduce A Dangerous Practice by Rich Bottner, PA-C & Victoria Valencia, MPH
- New SoHM Report Provides Unique Window into Hospital Medicine Practice Trends by Leslie Flores, MHA, SFHM
- IGNITE Change: Improving Care via Interprofessional Clinical Learning Environments by Vineet Arora, MD, MAPP, MHM
If you are not doing Schwartz Rounds, get them started. ASAP.
I recently completed a 4-year tenure as physician moderator for our hospital’s Schwartz Rounds. An amazing team at my hospital helped pull the bimonthly sessions together. These compassionate care rounds are a national initiative to help foster empathy and compassion in the health care setting.
We gather a panel of two to three people involved in our patient presentation who share and move quickly through the clinical details, and head on toward the thornier ethical issues, emotional triggers, and responses. The best sessions are when the audience’s voice is heard for the bulk of the time.
The emotional cadence flows from boiling in frustration, drowning in tears, followed by comfort, and ending in thoughts for the next session. It is a more powerful arc than an episode of the television program “This is Us.” Largely, because this was us. This was real life. Real-time catharsis in the hospital.
In the daily grind, we often skip the step of processing our frustration, sadness, and anger, moving right on to the next patient and walking into the next room with that stoic layer of equanimity. I walk the hallways and find I grab my phone to catch up on emails, walking to the wrong floor because I’m not paying attention. Always something to do, someone to talk to, a family to call, pagers going off, phone calls. When do we sit and reflect?
These Schwartz Rounds are those moments of reflection – a slowdown in the day to think more deeply about the case. We talk about everything and anything. We have discussions with opposing views:
“Everything should have been done!”
“How did you not stop care?!”
“I agree with the doctors.”
“I can see the patient’s view more clearly now.”
Our first Schwartz Rounds tended to be end-of-life stories, particularly regarding the family mantra of “Do everything.” The health care team watches the suffering of a patient, a family, in a seemingly futile situation. Conversations around the end of life, choices, and quality of life are cut short daily by family members who simply recite, “Do everything.”
After several of these sessions, a case swings us in the other direction. The elderly gentleman with treatable cancer, who could easily survive another 20 years, declines treatment. “I’m fine, doc; I’ve lived long enough.” His wife at his bedside, shaking her head, tells us, “I don’t know why he wants to give up. He’s been as stubborn as a mule since the day I met him.” I spend 30 minutes convincing him to stay. The nurse does the same. Now we have a patient with a “Do nothing.” The patient’s decisions conflict with the family and the health care team.
Every day in the hospital provides a new ethical dilemma, a frustrating case, a challenging patient. Fodder for rounds.
Read the full post at hospitalleader.org.
Dr. Messler is a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. He previously chaired SHM’s Quality and Patient Safety Committee and has been active in several SHM mentoring programs, most recently with Project BOOST and Glycemic Control.
Also on The Hospital Leader
- Incubating Success: How We Used Structured Feedback to Reduce A Dangerous Practice by Rich Bottner, PA-C & Victoria Valencia, MPH
- New SoHM Report Provides Unique Window into Hospital Medicine Practice Trends by Leslie Flores, MHA, SFHM
- IGNITE Change: Improving Care via Interprofessional Clinical Learning Environments by Vineet Arora, MD, MAPP, MHM
Part D proposal includes prior authorization, step therapy
Rules governing the six protected medication classes covered by Medicare Part D could change under a proposal that would allow for utilization management or potential formulary exclusion of a drug for price increases.
Currently, Medicare Part D prescription drug benefit plans must cover “all or substantially all” approved drugs in six classes (antidepressants, antipsychotics, anticonvulsants, antiretrovirals, and antineoplastics). The proposed rule would allow three exceptions aimed at giving plans more negotiating leverage to help lower prices.
Plans would be allowed to implement prior authorization and step therapy for protected-class drugs, “including to determine use for a protected class indication,” according to a fact sheet. They also could exclude a protected-class drug from their formulary “if the drug represents only a new formulation of an existing single-source drug or biological product, regardless of whether the older formulation remains on the market.”
This does not change requirements that at least two drugs per class be covered, Seema Verma, administrator of the Centers for Medicare & Medicaid Services, said at a briefing. “In some classes, there are lots of competitors. For example, for antidepressants, there are lots of new generics available, so we see plans being in a very strengthened negotiating position. But in other classes, where there may not be as many drugs that are available, you might not see the same type of step therapy and prior authorization because there are just not that many options. It is really going to depend on the class of drugs and what’s available and the plans’ ability to negotiate discounts with manufacturers.”
Plans could exclude a protected-class drug if its price had increased greater than inflation, Ms. Verma said, but they could not use this to not cover any drugs in a class if available options are limited to one or two drugs.
“Foremost in our minds was the impact on patients and ensuring affordability and access to prescription drugs,” Ms. Verma said.
Oncologists don’t seem to agree.
“For the first time ever, Medicare patients with cancer and other serious diseases [who] rely on drugs in these protected therapeutic categories, will no longer have guaranteed access to potentially life-saving drugs. Instead, they will be subjected to ‘fail first’ step therapy and formulary restrictions that potentially restrict them from receiving the evidence-based therapies that their trained physicians prescribe as first-line cancer treatment,” Jeff Vacirca, MD, president of the Community Oncology Alliance, said in a statement. “Step therapy requirements are driven by financial interests to save money and not by what is in the best medical interest of patients. Treatment decisions are made by nameless and faceless corporate bureaucrats who are often not board certified in the diseases they are making coverage decisions over.”
AGA is concerned that these proposed changes will limit access for current and future beneficiaries and will add to the growing regulatory burden that physicians already face.
The proposal also would codify a policy implemented for 2019 that allows Medicare Advantage to implement step therapy tools for Part B drugs. And like the 2019 policy, the proposal would apply to new medication starts only, must be reviewed by a plan’s pharmacy and therapeutics committee, and must have an expedited exceptions process.
The proposal also specifically allows pharmacists to advise Part D beneficiaries on lower-cost options – something current regulations prohibit – and would require Part D explanation of benefits forms to include drug pricing information and lower-cost therapeutic alternatives.
The proposal is part of a broader update for Medicare Parts C and D in 2020 issued by CMS. It was published online Nov. 26 and is scheduled for publication in the Federal Register on Nov. 30. Comments can be made at www.regulations.gov through Jan. 25, 2019.
Rules governing the six protected medication classes covered by Medicare Part D could change under a proposal that would allow for utilization management or potential formulary exclusion of a drug for price increases.
Currently, Medicare Part D prescription drug benefit plans must cover “all or substantially all” approved drugs in six classes (antidepressants, antipsychotics, anticonvulsants, antiretrovirals, and antineoplastics). The proposed rule would allow three exceptions aimed at giving plans more negotiating leverage to help lower prices.
Plans would be allowed to implement prior authorization and step therapy for protected-class drugs, “including to determine use for a protected class indication,” according to a fact sheet. They also could exclude a protected-class drug from their formulary “if the drug represents only a new formulation of an existing single-source drug or biological product, regardless of whether the older formulation remains on the market.”
This does not change requirements that at least two drugs per class be covered, Seema Verma, administrator of the Centers for Medicare & Medicaid Services, said at a briefing. “In some classes, there are lots of competitors. For example, for antidepressants, there are lots of new generics available, so we see plans being in a very strengthened negotiating position. But in other classes, where there may not be as many drugs that are available, you might not see the same type of step therapy and prior authorization because there are just not that many options. It is really going to depend on the class of drugs and what’s available and the plans’ ability to negotiate discounts with manufacturers.”
Plans could exclude a protected-class drug if its price had increased greater than inflation, Ms. Verma said, but they could not use this to not cover any drugs in a class if available options are limited to one or two drugs.
“Foremost in our minds was the impact on patients and ensuring affordability and access to prescription drugs,” Ms. Verma said.
Oncologists don’t seem to agree.
“For the first time ever, Medicare patients with cancer and other serious diseases [who] rely on drugs in these protected therapeutic categories, will no longer have guaranteed access to potentially life-saving drugs. Instead, they will be subjected to ‘fail first’ step therapy and formulary restrictions that potentially restrict them from receiving the evidence-based therapies that their trained physicians prescribe as first-line cancer treatment,” Jeff Vacirca, MD, president of the Community Oncology Alliance, said in a statement. “Step therapy requirements are driven by financial interests to save money and not by what is in the best medical interest of patients. Treatment decisions are made by nameless and faceless corporate bureaucrats who are often not board certified in the diseases they are making coverage decisions over.”
AGA is concerned that these proposed changes will limit access for current and future beneficiaries and will add to the growing regulatory burden that physicians already face.
The proposal also would codify a policy implemented for 2019 that allows Medicare Advantage to implement step therapy tools for Part B drugs. And like the 2019 policy, the proposal would apply to new medication starts only, must be reviewed by a plan’s pharmacy and therapeutics committee, and must have an expedited exceptions process.
The proposal also specifically allows pharmacists to advise Part D beneficiaries on lower-cost options – something current regulations prohibit – and would require Part D explanation of benefits forms to include drug pricing information and lower-cost therapeutic alternatives.
The proposal is part of a broader update for Medicare Parts C and D in 2020 issued by CMS. It was published online Nov. 26 and is scheduled for publication in the Federal Register on Nov. 30. Comments can be made at www.regulations.gov through Jan. 25, 2019.
Rules governing the six protected medication classes covered by Medicare Part D could change under a proposal that would allow for utilization management or potential formulary exclusion of a drug for price increases.
Currently, Medicare Part D prescription drug benefit plans must cover “all or substantially all” approved drugs in six classes (antidepressants, antipsychotics, anticonvulsants, antiretrovirals, and antineoplastics). The proposed rule would allow three exceptions aimed at giving plans more negotiating leverage to help lower prices.
Plans would be allowed to implement prior authorization and step therapy for protected-class drugs, “including to determine use for a protected class indication,” according to a fact sheet. They also could exclude a protected-class drug from their formulary “if the drug represents only a new formulation of an existing single-source drug or biological product, regardless of whether the older formulation remains on the market.”
This does not change requirements that at least two drugs per class be covered, Seema Verma, administrator of the Centers for Medicare & Medicaid Services, said at a briefing. “In some classes, there are lots of competitors. For example, for antidepressants, there are lots of new generics available, so we see plans being in a very strengthened negotiating position. But in other classes, where there may not be as many drugs that are available, you might not see the same type of step therapy and prior authorization because there are just not that many options. It is really going to depend on the class of drugs and what’s available and the plans’ ability to negotiate discounts with manufacturers.”
Plans could exclude a protected-class drug if its price had increased greater than inflation, Ms. Verma said, but they could not use this to not cover any drugs in a class if available options are limited to one or two drugs.
“Foremost in our minds was the impact on patients and ensuring affordability and access to prescription drugs,” Ms. Verma said.
Oncologists don’t seem to agree.
“For the first time ever, Medicare patients with cancer and other serious diseases [who] rely on drugs in these protected therapeutic categories, will no longer have guaranteed access to potentially life-saving drugs. Instead, they will be subjected to ‘fail first’ step therapy and formulary restrictions that potentially restrict them from receiving the evidence-based therapies that their trained physicians prescribe as first-line cancer treatment,” Jeff Vacirca, MD, president of the Community Oncology Alliance, said in a statement. “Step therapy requirements are driven by financial interests to save money and not by what is in the best medical interest of patients. Treatment decisions are made by nameless and faceless corporate bureaucrats who are often not board certified in the diseases they are making coverage decisions over.”
AGA is concerned that these proposed changes will limit access for current and future beneficiaries and will add to the growing regulatory burden that physicians already face.
The proposal also would codify a policy implemented for 2019 that allows Medicare Advantage to implement step therapy tools for Part B drugs. And like the 2019 policy, the proposal would apply to new medication starts only, must be reviewed by a plan’s pharmacy and therapeutics committee, and must have an expedited exceptions process.
The proposal also specifically allows pharmacists to advise Part D beneficiaries on lower-cost options – something current regulations prohibit – and would require Part D explanation of benefits forms to include drug pricing information and lower-cost therapeutic alternatives.
The proposal is part of a broader update for Medicare Parts C and D in 2020 issued by CMS. It was published online Nov. 26 and is scheduled for publication in the Federal Register on Nov. 30. Comments can be made at www.regulations.gov through Jan. 25, 2019.
Nicotine patch may be an effective precision therapy for select epilepsies
NEW ORLEANS – according to research presented at the annual meeting of the American Epilepsy Society. Of four epilepsy patients at one center who received nicotine-patch treatment, three had a good clinical response, one of whom became seizure free.
“We confirm that, in select patients, treatment with a nicotine patch ... can be an effective precision therapy for epilepsy. We propose consideration of nicotine-patch treatment in refractory patients with known cholinergic nicotine receptor subunit variants, especially those with a clinical history consistent with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE),” said Jordana Fox, DO, and Alison Dolce, MD, both with the University of Texas Southwestern Medical Center in Dallas.
Gene variants in CHRAn4,CHRNA2, and CHRNB2 can cause ADNFLE. Preclinical and n-of-1 studies have suggested that nicotine may be a precision treatment for ADNFLE.
Dr. Fox and Dr. Dolce reviewed next-generation sequencing epilepsy panels from patients seen at Children’s Medical Center, Dallas, during 2011-2015 to identify patients with nAChR gene variants (CHNRA4, CHRNA2, CHRNB2, and CHRNA7). They reviewed patients’ medical and laboratory records, including genetic variant details and treatment history, and focused on patients who underwent a trial of nicotine-patch treatment.
Of the 21 patients who had nAChR gene variants, 4 tried treatment with a nicotine patch, either 7 mg or 14 mg. The patients who received nicotine-patch treatment had genetic variants in CHRNA4, CHRNB2, and CHRNA2. Three of the patients who tried nicotine-patch treatment had a greater than 50% reduction in seizures, whereas one had no treatment response.
“One patient became seizure free and is now treated with the nicotine patch as monotherapy,” Dr. Fox said.
The patient with complete resolution of seizures had a heterozygous disease–causing mutation in CHRNB2. This patient had nocturnal focal seizures, normal neuroimaging, and had been receiving treatment with oxcarbazepine and zonisamide.
The review identified four patients with nAChR gene variants and the ADNFLE phenotype who have not been treated with nicotine. Further phenotype-genotype characterizations and preclinical studies will help neurologists understand the mechanisms of these complex gene variants.
The researchers received no funding for the study and had no relevant financial disclosures.
SOURCE: Fox J et al. AES 2018, Abstract 1.230.
NEW ORLEANS – according to research presented at the annual meeting of the American Epilepsy Society. Of four epilepsy patients at one center who received nicotine-patch treatment, three had a good clinical response, one of whom became seizure free.
“We confirm that, in select patients, treatment with a nicotine patch ... can be an effective precision therapy for epilepsy. We propose consideration of nicotine-patch treatment in refractory patients with known cholinergic nicotine receptor subunit variants, especially those with a clinical history consistent with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE),” said Jordana Fox, DO, and Alison Dolce, MD, both with the University of Texas Southwestern Medical Center in Dallas.
Gene variants in CHRAn4,CHRNA2, and CHRNB2 can cause ADNFLE. Preclinical and n-of-1 studies have suggested that nicotine may be a precision treatment for ADNFLE.
Dr. Fox and Dr. Dolce reviewed next-generation sequencing epilepsy panels from patients seen at Children’s Medical Center, Dallas, during 2011-2015 to identify patients with nAChR gene variants (CHNRA4, CHRNA2, CHRNB2, and CHRNA7). They reviewed patients’ medical and laboratory records, including genetic variant details and treatment history, and focused on patients who underwent a trial of nicotine-patch treatment.
Of the 21 patients who had nAChR gene variants, 4 tried treatment with a nicotine patch, either 7 mg or 14 mg. The patients who received nicotine-patch treatment had genetic variants in CHRNA4, CHRNB2, and CHRNA2. Three of the patients who tried nicotine-patch treatment had a greater than 50% reduction in seizures, whereas one had no treatment response.
“One patient became seizure free and is now treated with the nicotine patch as monotherapy,” Dr. Fox said.
The patient with complete resolution of seizures had a heterozygous disease–causing mutation in CHRNB2. This patient had nocturnal focal seizures, normal neuroimaging, and had been receiving treatment with oxcarbazepine and zonisamide.
The review identified four patients with nAChR gene variants and the ADNFLE phenotype who have not been treated with nicotine. Further phenotype-genotype characterizations and preclinical studies will help neurologists understand the mechanisms of these complex gene variants.
The researchers received no funding for the study and had no relevant financial disclosures.
SOURCE: Fox J et al. AES 2018, Abstract 1.230.
NEW ORLEANS – according to research presented at the annual meeting of the American Epilepsy Society. Of four epilepsy patients at one center who received nicotine-patch treatment, three had a good clinical response, one of whom became seizure free.
“We confirm that, in select patients, treatment with a nicotine patch ... can be an effective precision therapy for epilepsy. We propose consideration of nicotine-patch treatment in refractory patients with known cholinergic nicotine receptor subunit variants, especially those with a clinical history consistent with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE),” said Jordana Fox, DO, and Alison Dolce, MD, both with the University of Texas Southwestern Medical Center in Dallas.
Gene variants in CHRAn4,CHRNA2, and CHRNB2 can cause ADNFLE. Preclinical and n-of-1 studies have suggested that nicotine may be a precision treatment for ADNFLE.
Dr. Fox and Dr. Dolce reviewed next-generation sequencing epilepsy panels from patients seen at Children’s Medical Center, Dallas, during 2011-2015 to identify patients with nAChR gene variants (CHNRA4, CHRNA2, CHRNB2, and CHRNA7). They reviewed patients’ medical and laboratory records, including genetic variant details and treatment history, and focused on patients who underwent a trial of nicotine-patch treatment.
Of the 21 patients who had nAChR gene variants, 4 tried treatment with a nicotine patch, either 7 mg or 14 mg. The patients who received nicotine-patch treatment had genetic variants in CHRNA4, CHRNB2, and CHRNA2. Three of the patients who tried nicotine-patch treatment had a greater than 50% reduction in seizures, whereas one had no treatment response.
“One patient became seizure free and is now treated with the nicotine patch as monotherapy,” Dr. Fox said.
The patient with complete resolution of seizures had a heterozygous disease–causing mutation in CHRNB2. This patient had nocturnal focal seizures, normal neuroimaging, and had been receiving treatment with oxcarbazepine and zonisamide.
The review identified four patients with nAChR gene variants and the ADNFLE phenotype who have not been treated with nicotine. Further phenotype-genotype characterizations and preclinical studies will help neurologists understand the mechanisms of these complex gene variants.
The researchers received no funding for the study and had no relevant financial disclosures.
SOURCE: Fox J et al. AES 2018, Abstract 1.230.
REPORTING FROM AES 2018
Key clinical point: In select patients with epilepsy, nicotine may be an effective precision therapy.
Major finding: Of four patients who received nicotine-patch treatment at one center, three had a good clinical response, one of whom became seizure free.
Study details: Single-center chart review of 21 patients with gene variants in subunits of the nicotinic acetylcholine receptor.
Disclosures: The researchers received no funding for the study and had no relevant financial disclosures.
Source: Fox J et al. AES 2018, Abstract 1.230.
Rapid test could solve Africa’s sickle cell screening problem
SAN DIEGO – An inexpensive, rapid, and easy-to-use blood test was more than 99% accurate in detecting sickle cell disease in young children in sub-Saharan Africa, according to research reported at the annual meeting of the American Society of Hematology.
The test, called HemoTypeSC, uses monoclonal antibodies to detect hemoglobins A, S, and C in a drop of whole blood, said investigator Erik Serrao, PhD, of Silver Lake Research in Azusa, Calif.
Findings from the diagnostic accuracy trial, which included 1,000 children in Uganda, suggest that the immunoassay is a promising tool to enable newborn and general population screening in resource-constrained regions of high prevalence, such as Africa and India.
“Early screening plus treatment plus counseling equals saving millions of lives over the coming decades, and we believe HemoTypeSC can form an integral part of the initial part of this equation,” Dr. Serrao said during a late-breaking abstract session at the meeting.
Each test kit costs less than $2 to the end user; requires no electricity, special equipment, or training; and delivers results in about 10 minutes, he added.
Of all the late-breaking abstracts at ASH this year, the study by Dr. Serrao and his colleagues is the one with the potential to save the most lives, said Mark Crowther, MD, of McMaster University, Hamilton, Ont.
“The ability to diagnose sickle cell disease early and intervene early will result in potentially thousands of infants, who would otherwise die in infancy or early childhood, surviving into adulthood,” Dr. Crowther said during a press briefing.
Using current gold standard methods for diagnosing sickle cell disease is, at minimum, challenging and “frankly impossible” in many low-resource settings, because of the cost and the requirement for sophisticated equipment and reliable electricity, Dr. Crowther added.
In the study, investigators compared results of the HemoTypeSC test with hemoglobin electrophoresis for detection of the phenotypes HbAA (normal), HbAS (sickle cell trait), and HbSS (sickle cell disease). They compared these two testing methods in 1,000 children between the ages of 1 month and 5 years who were prospectively recruited from hospital wards and outpatient clinics in Uganda.
The immunoassay had an overall accuracy of 99.8%, correctly identifying 998 of 1,000 phenotypes as initially determined by electrophoresis. Specifically, the test correctly identified 100% of the 720 HbAA specimens, 100% of 182 HbAS specimens, and 98% of HbSS, or 96 of 98 specimens, leaving just 2 discordant samples, both of which HemoTypeSC identified as HbAS.
Investigators subsequently discovered that both of the individuals with the discordant samples had previously been diagnosed with sickle cell disease and had received recent transfusions. Both cases were subsequently confirmed as HbSS in review of previous diagnostic result reports, bringing the accuracy rate up to 100% in a secondary analysis also reported at the meeting.
Although this particular study excluded newborns, a different study of the immunoassay, recently published in the American Journal of Hematology, demonstrated 100% accuracy across multiple phenotypes in the setting of newborn screening (2018 Oct 5. doi: 10.1002/ajh.25305).
Sickle cell disease screening programs have been projected to be cost effective in Africa, Dr. Serrano said, and could even save money for governments over time as budgets are reallocated toward screening, with less money needed for treatment of patients presenting with severe complications in hospitals.
Dr. Serrao reported that he is an employee of Silver Lake Research, which funded the study, approved the study design, and donated HemoTypeSC tests.
On March 11, 2019, the editors of Blood, an ASH journal, retracted the abstract for this study. The second listed author on the abstract said that it was submitted without his consent or approval. The retraction makes no statement on the underlying science of the study, the editors noted.
This article was updated on 3/14/2019.
SOURCE: Serrao E et al. ASH 2018, Abstract LBA-3.
SAN DIEGO – An inexpensive, rapid, and easy-to-use blood test was more than 99% accurate in detecting sickle cell disease in young children in sub-Saharan Africa, according to research reported at the annual meeting of the American Society of Hematology.
The test, called HemoTypeSC, uses monoclonal antibodies to detect hemoglobins A, S, and C in a drop of whole blood, said investigator Erik Serrao, PhD, of Silver Lake Research in Azusa, Calif.
Findings from the diagnostic accuracy trial, which included 1,000 children in Uganda, suggest that the immunoassay is a promising tool to enable newborn and general population screening in resource-constrained regions of high prevalence, such as Africa and India.
“Early screening plus treatment plus counseling equals saving millions of lives over the coming decades, and we believe HemoTypeSC can form an integral part of the initial part of this equation,” Dr. Serrao said during a late-breaking abstract session at the meeting.
Each test kit costs less than $2 to the end user; requires no electricity, special equipment, or training; and delivers results in about 10 minutes, he added.
Of all the late-breaking abstracts at ASH this year, the study by Dr. Serrao and his colleagues is the one with the potential to save the most lives, said Mark Crowther, MD, of McMaster University, Hamilton, Ont.
“The ability to diagnose sickle cell disease early and intervene early will result in potentially thousands of infants, who would otherwise die in infancy or early childhood, surviving into adulthood,” Dr. Crowther said during a press briefing.
Using current gold standard methods for diagnosing sickle cell disease is, at minimum, challenging and “frankly impossible” in many low-resource settings, because of the cost and the requirement for sophisticated equipment and reliable electricity, Dr. Crowther added.
In the study, investigators compared results of the HemoTypeSC test with hemoglobin electrophoresis for detection of the phenotypes HbAA (normal), HbAS (sickle cell trait), and HbSS (sickle cell disease). They compared these two testing methods in 1,000 children between the ages of 1 month and 5 years who were prospectively recruited from hospital wards and outpatient clinics in Uganda.
The immunoassay had an overall accuracy of 99.8%, correctly identifying 998 of 1,000 phenotypes as initially determined by electrophoresis. Specifically, the test correctly identified 100% of the 720 HbAA specimens, 100% of 182 HbAS specimens, and 98% of HbSS, or 96 of 98 specimens, leaving just 2 discordant samples, both of which HemoTypeSC identified as HbAS.
Investigators subsequently discovered that both of the individuals with the discordant samples had previously been diagnosed with sickle cell disease and had received recent transfusions. Both cases were subsequently confirmed as HbSS in review of previous diagnostic result reports, bringing the accuracy rate up to 100% in a secondary analysis also reported at the meeting.
Although this particular study excluded newborns, a different study of the immunoassay, recently published in the American Journal of Hematology, demonstrated 100% accuracy across multiple phenotypes in the setting of newborn screening (2018 Oct 5. doi: 10.1002/ajh.25305).
Sickle cell disease screening programs have been projected to be cost effective in Africa, Dr. Serrano said, and could even save money for governments over time as budgets are reallocated toward screening, with less money needed for treatment of patients presenting with severe complications in hospitals.
Dr. Serrao reported that he is an employee of Silver Lake Research, which funded the study, approved the study design, and donated HemoTypeSC tests.
On March 11, 2019, the editors of Blood, an ASH journal, retracted the abstract for this study. The second listed author on the abstract said that it was submitted without his consent or approval. The retraction makes no statement on the underlying science of the study, the editors noted.
This article was updated on 3/14/2019.
SOURCE: Serrao E et al. ASH 2018, Abstract LBA-3.
SAN DIEGO – An inexpensive, rapid, and easy-to-use blood test was more than 99% accurate in detecting sickle cell disease in young children in sub-Saharan Africa, according to research reported at the annual meeting of the American Society of Hematology.
The test, called HemoTypeSC, uses monoclonal antibodies to detect hemoglobins A, S, and C in a drop of whole blood, said investigator Erik Serrao, PhD, of Silver Lake Research in Azusa, Calif.
Findings from the diagnostic accuracy trial, which included 1,000 children in Uganda, suggest that the immunoassay is a promising tool to enable newborn and general population screening in resource-constrained regions of high prevalence, such as Africa and India.
“Early screening plus treatment plus counseling equals saving millions of lives over the coming decades, and we believe HemoTypeSC can form an integral part of the initial part of this equation,” Dr. Serrao said during a late-breaking abstract session at the meeting.
Each test kit costs less than $2 to the end user; requires no electricity, special equipment, or training; and delivers results in about 10 minutes, he added.
Of all the late-breaking abstracts at ASH this year, the study by Dr. Serrao and his colleagues is the one with the potential to save the most lives, said Mark Crowther, MD, of McMaster University, Hamilton, Ont.
“The ability to diagnose sickle cell disease early and intervene early will result in potentially thousands of infants, who would otherwise die in infancy or early childhood, surviving into adulthood,” Dr. Crowther said during a press briefing.
Using current gold standard methods for diagnosing sickle cell disease is, at minimum, challenging and “frankly impossible” in many low-resource settings, because of the cost and the requirement for sophisticated equipment and reliable electricity, Dr. Crowther added.
In the study, investigators compared results of the HemoTypeSC test with hemoglobin electrophoresis for detection of the phenotypes HbAA (normal), HbAS (sickle cell trait), and HbSS (sickle cell disease). They compared these two testing methods in 1,000 children between the ages of 1 month and 5 years who were prospectively recruited from hospital wards and outpatient clinics in Uganda.
The immunoassay had an overall accuracy of 99.8%, correctly identifying 998 of 1,000 phenotypes as initially determined by electrophoresis. Specifically, the test correctly identified 100% of the 720 HbAA specimens, 100% of 182 HbAS specimens, and 98% of HbSS, or 96 of 98 specimens, leaving just 2 discordant samples, both of which HemoTypeSC identified as HbAS.
Investigators subsequently discovered that both of the individuals with the discordant samples had previously been diagnosed with sickle cell disease and had received recent transfusions. Both cases were subsequently confirmed as HbSS in review of previous diagnostic result reports, bringing the accuracy rate up to 100% in a secondary analysis also reported at the meeting.
Although this particular study excluded newborns, a different study of the immunoassay, recently published in the American Journal of Hematology, demonstrated 100% accuracy across multiple phenotypes in the setting of newborn screening (2018 Oct 5. doi: 10.1002/ajh.25305).
Sickle cell disease screening programs have been projected to be cost effective in Africa, Dr. Serrano said, and could even save money for governments over time as budgets are reallocated toward screening, with less money needed for treatment of patients presenting with severe complications in hospitals.
Dr. Serrao reported that he is an employee of Silver Lake Research, which funded the study, approved the study design, and donated HemoTypeSC tests.
On March 11, 2019, the editors of Blood, an ASH journal, retracted the abstract for this study. The second listed author on the abstract said that it was submitted without his consent or approval. The retraction makes no statement on the underlying science of the study, the editors noted.
This article was updated on 3/14/2019.
SOURCE: Serrao E et al. ASH 2018, Abstract LBA-3.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: The assay had an overall accuracy of 99.8% in correctly identifying phenotypes as initially determined by hemoglobin electrophoresis.
Study details: A diagnostic accuracy study including 1,000 children aged 5 years and younger were prospectively recruited from hospitals and outpatient clinics in Uganda.
Disclosures: Dr. Serrao reported that he is an employee of Silver Lake Research, which funded the study and makes the test.
Source: Serrao E et al. ASH 2018, Abstract LBA-3.
Common AEDs confer modestly increased risk of major congenital malformations
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
REPORTING FROM AES 2018
Key clinical point:
Major finding: The malformation rate was 5% in exposed pregnancies.
Study details: The MONEAD study comprised 351 pregnant women with epilepsy, 109 nonpregnant women with epilepsy, and 105 healthy pregnant women.
Disclosures: The National Institutes of Health funded the study; Dr. Meador reported no financial disclosures.
Source: Meador KJ et al. AES 2018, Abstract 3.231.
Tender joint count may confound assessment of RA inflammation
In RA, patients with predominantly tender joint counts were found to have lower levels of inflammation defined by ultrasound than those with predominantly swollen joints, according to an observational study that has implications for management decisions.
When patients do not achieve a remission defined by a composite disease activity score (CDAS) that includes tender joint counts, it suggests that inflammation persists, but this may be a “misinterpretation,” according to a report in Arthritis Care & Research by Hilde B. Hammer, MD, PhD, of the department of rheumatology at Diakonhjemmet Hospital, Oslo, and her associates.
In this observational study, 209 RA patients were evaluated at baseline and again at months 1, 2, 3, 6, and 12. The researchers compared 84 patients with predominantly tender joints, defined as tender-swollen joint count difference (TSJD) score of greater than zero, against 125 patients with predominantly swollen joints, defined as TSJD of zero or less.
Scores on specific CDAS measures, such as the Disease Activity Score based on 28 joints, the Clinical Disease Activity Index, and the Simplified Disease Activity Index, were significantly higher (P less than .001) at all visits in patients with predominantly tender joints, compared with those with predominantly swollen joints. Although laboratory markers, such as C-reactive protein and rheumatoid factor, were similar between groups, synovitis as scored with ultrasound assessment was significantly lower (P less than .001) among patients with predominantly tender joints than in those with predominantly swollen joints.
This disparity is important, according to the authors, who suggested that failure to reach a CDAS-defined remission solely on the basis of tender joints might complicate clinical assessment.
“Taking the high impact of CDAS levels in the treat-to-target management of RA into account, our results suggest a careful interpretation of CDAS levels in patients who have predominantly tender joints,” the authors reported.
Based on the evidence from this study, which found tender joints to be a positive predictor of CDAS and patient-reported outcomes but a negative predictor of ultrasound scoring of inflammation, “these results indicate that tender joints do not reflect the same pathology as found with ultrasound,” they wrote.
The research was supported by AbbVie, Pfizer, and Roche in the form of study grants awarded to the department of rheumatology at Diakonhjemmet Hospital via Dr. Hammer and two other authors. Dr. Hammer and the two other authors also reported financial relationships with those companies and others.
SOURCE: Hammer HB et al. Arthritis Care Res. 2018 Nov 26. doi: 10.1002/acr.23815.
In RA, patients with predominantly tender joint counts were found to have lower levels of inflammation defined by ultrasound than those with predominantly swollen joints, according to an observational study that has implications for management decisions.
When patients do not achieve a remission defined by a composite disease activity score (CDAS) that includes tender joint counts, it suggests that inflammation persists, but this may be a “misinterpretation,” according to a report in Arthritis Care & Research by Hilde B. Hammer, MD, PhD, of the department of rheumatology at Diakonhjemmet Hospital, Oslo, and her associates.
In this observational study, 209 RA patients were evaluated at baseline and again at months 1, 2, 3, 6, and 12. The researchers compared 84 patients with predominantly tender joints, defined as tender-swollen joint count difference (TSJD) score of greater than zero, against 125 patients with predominantly swollen joints, defined as TSJD of zero or less.
Scores on specific CDAS measures, such as the Disease Activity Score based on 28 joints, the Clinical Disease Activity Index, and the Simplified Disease Activity Index, were significantly higher (P less than .001) at all visits in patients with predominantly tender joints, compared with those with predominantly swollen joints. Although laboratory markers, such as C-reactive protein and rheumatoid factor, were similar between groups, synovitis as scored with ultrasound assessment was significantly lower (P less than .001) among patients with predominantly tender joints than in those with predominantly swollen joints.
This disparity is important, according to the authors, who suggested that failure to reach a CDAS-defined remission solely on the basis of tender joints might complicate clinical assessment.
“Taking the high impact of CDAS levels in the treat-to-target management of RA into account, our results suggest a careful interpretation of CDAS levels in patients who have predominantly tender joints,” the authors reported.
Based on the evidence from this study, which found tender joints to be a positive predictor of CDAS and patient-reported outcomes but a negative predictor of ultrasound scoring of inflammation, “these results indicate that tender joints do not reflect the same pathology as found with ultrasound,” they wrote.
The research was supported by AbbVie, Pfizer, and Roche in the form of study grants awarded to the department of rheumatology at Diakonhjemmet Hospital via Dr. Hammer and two other authors. Dr. Hammer and the two other authors also reported financial relationships with those companies and others.
SOURCE: Hammer HB et al. Arthritis Care Res. 2018 Nov 26. doi: 10.1002/acr.23815.
In RA, patients with predominantly tender joint counts were found to have lower levels of inflammation defined by ultrasound than those with predominantly swollen joints, according to an observational study that has implications for management decisions.
When patients do not achieve a remission defined by a composite disease activity score (CDAS) that includes tender joint counts, it suggests that inflammation persists, but this may be a “misinterpretation,” according to a report in Arthritis Care & Research by Hilde B. Hammer, MD, PhD, of the department of rheumatology at Diakonhjemmet Hospital, Oslo, and her associates.
In this observational study, 209 RA patients were evaluated at baseline and again at months 1, 2, 3, 6, and 12. The researchers compared 84 patients with predominantly tender joints, defined as tender-swollen joint count difference (TSJD) score of greater than zero, against 125 patients with predominantly swollen joints, defined as TSJD of zero or less.
Scores on specific CDAS measures, such as the Disease Activity Score based on 28 joints, the Clinical Disease Activity Index, and the Simplified Disease Activity Index, were significantly higher (P less than .001) at all visits in patients with predominantly tender joints, compared with those with predominantly swollen joints. Although laboratory markers, such as C-reactive protein and rheumatoid factor, were similar between groups, synovitis as scored with ultrasound assessment was significantly lower (P less than .001) among patients with predominantly tender joints than in those with predominantly swollen joints.
This disparity is important, according to the authors, who suggested that failure to reach a CDAS-defined remission solely on the basis of tender joints might complicate clinical assessment.
“Taking the high impact of CDAS levels in the treat-to-target management of RA into account, our results suggest a careful interpretation of CDAS levels in patients who have predominantly tender joints,” the authors reported.
Based on the evidence from this study, which found tender joints to be a positive predictor of CDAS and patient-reported outcomes but a negative predictor of ultrasound scoring of inflammation, “these results indicate that tender joints do not reflect the same pathology as found with ultrasound,” they wrote.
The research was supported by AbbVie, Pfizer, and Roche in the form of study grants awarded to the department of rheumatology at Diakonhjemmet Hospital via Dr. Hammer and two other authors. Dr. Hammer and the two other authors also reported financial relationships with those companies and others.
SOURCE: Hammer HB et al. Arthritis Care Res. 2018 Nov 26. doi: 10.1002/acr.23815.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: Baseline tender joint count correlates with patient-reported outcomes (P less than .001) but not with ultrasound-assessed inflammation.
Study details: An observational study of 209 RA patients.
Disclosures: The research was supported by AbbVie, Pfizer, and Roche in the form of study grants awarded to the department of rheumatology at Diakonhjemmet Hospital via Dr. Hammer and two other authors. Dr. Hammer and the two other authors also reported financial relationships with those companies and others.
Source: Hammer HB et al. Arthritis Care Res. 2018 Nov 26. doi: 10.1002/acr.23815.
Breastfeeding with MS: Good for mom, too
BERLIN – In the changing multiple sclerosis landscape, more women are having babies, and more are asking questions. With these women, what’s the best way to address the complicated interplay among pregnancy, relapse risk, breastfeeding, and medication resumption? A starting point is to recognize that “women with MS are very different today than they were 25 years ago,” said Annette Langer-Gould, MD, PhD. Not only have diagnostic criteria changed but also highly effective treatments now exist that were not available when the first pregnancy cohorts were studied, she pointed out, speaking at the annual congress of the European Committee on Treatment and Research in Multiple Sclerosis.
The existing literature, said Dr. Langer-Gould, has addressed one controversy: “Most women with MS can have normal pregnancies – and breastfeed – without incurring harm,” though it’s true that severe rebound relapses are possible if natalizumab (Tysabri) or fingolimod (Gilenya) are stopped before pregnancy. In any case, new small-molecule MS medications need to be stopped during pregnancy and breastfeeding, she pointed out. “We didn’t have to worry about that too much when we only had injectables and monoclonal antibodies because they were larger and didn’t cross the placenta.”
Since the 1980s, the conversation about pregnancy and MS has moved from asking “Is pregnancy bad for women with MS?” to the current MS landscape, in which sicker women are able to become pregnant, Dr. Langer-Gould said, adding that how women with MS fare through pregnancy and in the postpartum period is changing over time as well. She and her colleagues’ experience with pregnancy in a cohort of women with MS in the Kaiser Permanente care system, where she is a clinical neurologist and regional research lead, revealed a relapse rate of 8.4%. “So it was pretty rare for a woman to have a relapse during pregnancy,” Dr. Langer-Gould said.
Most women with MS who become pregnant, whether their care is received in a referral center or is community based, are now doing so while on a disease-modifying therapy (DMT), Dr. Langer-Gould said. On these highly effective treatments, “women who were too sick to get pregnant are now well controlled and having babies.”
As more women with MS become pregnant, more conversations about breastfeeding will inevitably crop up, she said. And the discussion about breastfeeding has now begun to acknowledge the “strong benefits to mom and the baby of not just breastfeeding, but longer breastfeeding,” as well.
“Because of this baby-friendly push in a lot of hospitals in the United States, where they’re trying to encourage all women to breastfeed,” a full 87% of women breastfed their infants at least some of the time, and over a third of women (35%) breastfed exclusively for at least 2 months, Dr. Langer-Gould said.
“There’s no one clear explanation of why the women seem to be healthier and doing better through pregnancy as a group, but it’s probably a combination of having milder disease, breastfeeding more, and they’ve got better controlled disease before pregnancy,” she said.
At least eight studies to date have examined the relationship between postpartum MS relapses and breastfeeding, Dr. Langer-Gould said.
“The thing to take away ... is that, even though we’ve studied this many, many times, no one can show that it’s harmful,” she said. For mothers who want to breastfeed, “you can support them in the breastfeeding choice, because they are not going to have more severe disease because of that.”
Whether breastfeeding is exclusive or not has not always been tracked in studies of childbearing women with MS, but when it was captured in the data, exclusive breastfeeding has exerted a protective effect, with about a 50% reduction in risk for postpartum relapse seen in one study (JAMA Neurol. 2015 Oct;72[10]:1132-8).
There is a hormonal rationale for exclusive breastfeeding exerting a protective effect on MS: With exclusive breastfeeding comes more frequent, intense suckling, with more profound elevations in prolactin, and larger drops in follicle-stimulating hormone, luteinizing hormone, progesterone, and estradiol. All these hormonal changes work together to produce more prolonged amenorrhea and anovulation, Dr. Langer-Gould said, with potentially beneficial immunologic effects.
When other, more general maternal and infant health benefits of breastfeeding also are taken into account, there’s strong evidence for the benefits of breastfeeding for women with MS whose medication profile allows them to breastfeed, she said.
However, the “treatment” effect of exclusive breastfeeding is only effective until the infant starts taking regular supplemental feedings, including the introduction of table food at around 6 months of age. “Once regular supplemental feedings are introduced, relapses return,” Dr. Langer-Gould said.
There is some suggestion that, in women without MS, prolonged breastfeeding may be associated with reduced risk of MS. In the MS Sunshine study, breastfeeding for 15 months or longer decreased the risk of later MS by 23%-53% (Nutrients. 2018 Feb 27;10[3]:268). The investigators, led by Dr. Langer-Gould, summed the total months of breastfeeding across all children, so that the 15-month threshold could be reached by breastfeeding one child for 15 months, or three children for 5 months each. “It’s a single study; I wouldn’t make too much out of it,” Dr. Langer-Gould said.
Open questions still remain, she said: “So far, no one has been able to demonstrate a clear beneficial effect in reducing the risk of postpartum relapse if they resume their DMT early in the postpartum period.” Dr. Langer-Gould noted that the literature in this area is hampered by heterogeneity and by the fact that newer, more highly active DMTs have not been well studied.
Also, the link between postpartum relapses and long-term prognosis is not completely delineated. Indirect evidence, she said, points to a postpartum relapse as being “overall, a low-impact event.”
Dr. Langer-Gould reported that she has been the site principal investigator for clinical trials sponsored by Roche and Biogen.
SOURCE: Langer-Gould A. ECTRIMS 2018, Abstract 5.
BERLIN – In the changing multiple sclerosis landscape, more women are having babies, and more are asking questions. With these women, what’s the best way to address the complicated interplay among pregnancy, relapse risk, breastfeeding, and medication resumption? A starting point is to recognize that “women with MS are very different today than they were 25 years ago,” said Annette Langer-Gould, MD, PhD. Not only have diagnostic criteria changed but also highly effective treatments now exist that were not available when the first pregnancy cohorts were studied, she pointed out, speaking at the annual congress of the European Committee on Treatment and Research in Multiple Sclerosis.
The existing literature, said Dr. Langer-Gould, has addressed one controversy: “Most women with MS can have normal pregnancies – and breastfeed – without incurring harm,” though it’s true that severe rebound relapses are possible if natalizumab (Tysabri) or fingolimod (Gilenya) are stopped before pregnancy. In any case, new small-molecule MS medications need to be stopped during pregnancy and breastfeeding, she pointed out. “We didn’t have to worry about that too much when we only had injectables and monoclonal antibodies because they were larger and didn’t cross the placenta.”
Since the 1980s, the conversation about pregnancy and MS has moved from asking “Is pregnancy bad for women with MS?” to the current MS landscape, in which sicker women are able to become pregnant, Dr. Langer-Gould said, adding that how women with MS fare through pregnancy and in the postpartum period is changing over time as well. She and her colleagues’ experience with pregnancy in a cohort of women with MS in the Kaiser Permanente care system, where she is a clinical neurologist and regional research lead, revealed a relapse rate of 8.4%. “So it was pretty rare for a woman to have a relapse during pregnancy,” Dr. Langer-Gould said.
Most women with MS who become pregnant, whether their care is received in a referral center or is community based, are now doing so while on a disease-modifying therapy (DMT), Dr. Langer-Gould said. On these highly effective treatments, “women who were too sick to get pregnant are now well controlled and having babies.”
As more women with MS become pregnant, more conversations about breastfeeding will inevitably crop up, she said. And the discussion about breastfeeding has now begun to acknowledge the “strong benefits to mom and the baby of not just breastfeeding, but longer breastfeeding,” as well.
“Because of this baby-friendly push in a lot of hospitals in the United States, where they’re trying to encourage all women to breastfeed,” a full 87% of women breastfed their infants at least some of the time, and over a third of women (35%) breastfed exclusively for at least 2 months, Dr. Langer-Gould said.
“There’s no one clear explanation of why the women seem to be healthier and doing better through pregnancy as a group, but it’s probably a combination of having milder disease, breastfeeding more, and they’ve got better controlled disease before pregnancy,” she said.
At least eight studies to date have examined the relationship between postpartum MS relapses and breastfeeding, Dr. Langer-Gould said.
“The thing to take away ... is that, even though we’ve studied this many, many times, no one can show that it’s harmful,” she said. For mothers who want to breastfeed, “you can support them in the breastfeeding choice, because they are not going to have more severe disease because of that.”
Whether breastfeeding is exclusive or not has not always been tracked in studies of childbearing women with MS, but when it was captured in the data, exclusive breastfeeding has exerted a protective effect, with about a 50% reduction in risk for postpartum relapse seen in one study (JAMA Neurol. 2015 Oct;72[10]:1132-8).
There is a hormonal rationale for exclusive breastfeeding exerting a protective effect on MS: With exclusive breastfeeding comes more frequent, intense suckling, with more profound elevations in prolactin, and larger drops in follicle-stimulating hormone, luteinizing hormone, progesterone, and estradiol. All these hormonal changes work together to produce more prolonged amenorrhea and anovulation, Dr. Langer-Gould said, with potentially beneficial immunologic effects.
When other, more general maternal and infant health benefits of breastfeeding also are taken into account, there’s strong evidence for the benefits of breastfeeding for women with MS whose medication profile allows them to breastfeed, she said.
However, the “treatment” effect of exclusive breastfeeding is only effective until the infant starts taking regular supplemental feedings, including the introduction of table food at around 6 months of age. “Once regular supplemental feedings are introduced, relapses return,” Dr. Langer-Gould said.
There is some suggestion that, in women without MS, prolonged breastfeeding may be associated with reduced risk of MS. In the MS Sunshine study, breastfeeding for 15 months or longer decreased the risk of later MS by 23%-53% (Nutrients. 2018 Feb 27;10[3]:268). The investigators, led by Dr. Langer-Gould, summed the total months of breastfeeding across all children, so that the 15-month threshold could be reached by breastfeeding one child for 15 months, or three children for 5 months each. “It’s a single study; I wouldn’t make too much out of it,” Dr. Langer-Gould said.
Open questions still remain, she said: “So far, no one has been able to demonstrate a clear beneficial effect in reducing the risk of postpartum relapse if they resume their DMT early in the postpartum period.” Dr. Langer-Gould noted that the literature in this area is hampered by heterogeneity and by the fact that newer, more highly active DMTs have not been well studied.
Also, the link between postpartum relapses and long-term prognosis is not completely delineated. Indirect evidence, she said, points to a postpartum relapse as being “overall, a low-impact event.”
Dr. Langer-Gould reported that she has been the site principal investigator for clinical trials sponsored by Roche and Biogen.
SOURCE: Langer-Gould A. ECTRIMS 2018, Abstract 5.
BERLIN – In the changing multiple sclerosis landscape, more women are having babies, and more are asking questions. With these women, what’s the best way to address the complicated interplay among pregnancy, relapse risk, breastfeeding, and medication resumption? A starting point is to recognize that “women with MS are very different today than they were 25 years ago,” said Annette Langer-Gould, MD, PhD. Not only have diagnostic criteria changed but also highly effective treatments now exist that were not available when the first pregnancy cohorts were studied, she pointed out, speaking at the annual congress of the European Committee on Treatment and Research in Multiple Sclerosis.
The existing literature, said Dr. Langer-Gould, has addressed one controversy: “Most women with MS can have normal pregnancies – and breastfeed – without incurring harm,” though it’s true that severe rebound relapses are possible if natalizumab (Tysabri) or fingolimod (Gilenya) are stopped before pregnancy. In any case, new small-molecule MS medications need to be stopped during pregnancy and breastfeeding, she pointed out. “We didn’t have to worry about that too much when we only had injectables and monoclonal antibodies because they were larger and didn’t cross the placenta.”
Since the 1980s, the conversation about pregnancy and MS has moved from asking “Is pregnancy bad for women with MS?” to the current MS landscape, in which sicker women are able to become pregnant, Dr. Langer-Gould said, adding that how women with MS fare through pregnancy and in the postpartum period is changing over time as well. She and her colleagues’ experience with pregnancy in a cohort of women with MS in the Kaiser Permanente care system, where she is a clinical neurologist and regional research lead, revealed a relapse rate of 8.4%. “So it was pretty rare for a woman to have a relapse during pregnancy,” Dr. Langer-Gould said.
Most women with MS who become pregnant, whether their care is received in a referral center or is community based, are now doing so while on a disease-modifying therapy (DMT), Dr. Langer-Gould said. On these highly effective treatments, “women who were too sick to get pregnant are now well controlled and having babies.”
As more women with MS become pregnant, more conversations about breastfeeding will inevitably crop up, she said. And the discussion about breastfeeding has now begun to acknowledge the “strong benefits to mom and the baby of not just breastfeeding, but longer breastfeeding,” as well.
“Because of this baby-friendly push in a lot of hospitals in the United States, where they’re trying to encourage all women to breastfeed,” a full 87% of women breastfed their infants at least some of the time, and over a third of women (35%) breastfed exclusively for at least 2 months, Dr. Langer-Gould said.
“There’s no one clear explanation of why the women seem to be healthier and doing better through pregnancy as a group, but it’s probably a combination of having milder disease, breastfeeding more, and they’ve got better controlled disease before pregnancy,” she said.
At least eight studies to date have examined the relationship between postpartum MS relapses and breastfeeding, Dr. Langer-Gould said.
“The thing to take away ... is that, even though we’ve studied this many, many times, no one can show that it’s harmful,” she said. For mothers who want to breastfeed, “you can support them in the breastfeeding choice, because they are not going to have more severe disease because of that.”
Whether breastfeeding is exclusive or not has not always been tracked in studies of childbearing women with MS, but when it was captured in the data, exclusive breastfeeding has exerted a protective effect, with about a 50% reduction in risk for postpartum relapse seen in one study (JAMA Neurol. 2015 Oct;72[10]:1132-8).
There is a hormonal rationale for exclusive breastfeeding exerting a protective effect on MS: With exclusive breastfeeding comes more frequent, intense suckling, with more profound elevations in prolactin, and larger drops in follicle-stimulating hormone, luteinizing hormone, progesterone, and estradiol. All these hormonal changes work together to produce more prolonged amenorrhea and anovulation, Dr. Langer-Gould said, with potentially beneficial immunologic effects.
When other, more general maternal and infant health benefits of breastfeeding also are taken into account, there’s strong evidence for the benefits of breastfeeding for women with MS whose medication profile allows them to breastfeed, she said.
However, the “treatment” effect of exclusive breastfeeding is only effective until the infant starts taking regular supplemental feedings, including the introduction of table food at around 6 months of age. “Once regular supplemental feedings are introduced, relapses return,” Dr. Langer-Gould said.
There is some suggestion that, in women without MS, prolonged breastfeeding may be associated with reduced risk of MS. In the MS Sunshine study, breastfeeding for 15 months or longer decreased the risk of later MS by 23%-53% (Nutrients. 2018 Feb 27;10[3]:268). The investigators, led by Dr. Langer-Gould, summed the total months of breastfeeding across all children, so that the 15-month threshold could be reached by breastfeeding one child for 15 months, or three children for 5 months each. “It’s a single study; I wouldn’t make too much out of it,” Dr. Langer-Gould said.
Open questions still remain, she said: “So far, no one has been able to demonstrate a clear beneficial effect in reducing the risk of postpartum relapse if they resume their DMT early in the postpartum period.” Dr. Langer-Gould noted that the literature in this area is hampered by heterogeneity and by the fact that newer, more highly active DMTs have not been well studied.
Also, the link between postpartum relapses and long-term prognosis is not completely delineated. Indirect evidence, she said, points to a postpartum relapse as being “overall, a low-impact event.”
Dr. Langer-Gould reported that she has been the site principal investigator for clinical trials sponsored by Roche and Biogen.
SOURCE: Langer-Gould A. ECTRIMS 2018, Abstract 5.
REPORTING FROM ECTRIMS 2018
Redo carotid endarterectomy is more risky than previously estimated
NEW YORK – It is well known that reoperative carotid endarterectomy can be technically challenging because of the scarring left from the initial procedure, but an analysis of a large database presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation also revealed that the risk of complications, particularly stroke, is greater.
When “redo” carotid endarterectomies were compared with the index primary procedure collected in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, the odds ratio for stroke was several times greater (odds ratio, 3.71; P = .002) on univariate analysis, reported Jeffrey J. Siracuse, MD, associate professor of surgery and radiology at Boston University.
Previous single-center reports of redo endarterectomies “showed terrific results, really no perioperative stroke or morbidity, but this is older data from a different era,” said Dr. Siracuse, who undertook this study to determine whether “real-world” data would tell a different story.
In this study, 75,943 primary carotid endarterectomies and 140 redo procedures were identified in the ACS NSQIP database and compared. The redo population had a significantly higher incidence of end-stage renal disease (3.6% vs. 1.1%; P = .004), but history of stroke, whether with deficit (20.8% vs. 15.4%) or without (11.5% vs. 9.1%), was numerically higher among those undergoing a primary procedure even though these differences did not reach statistical significance. Baseline demographics and comorbidities were otherwise similar.
Presumably because of the difficulty of recanalizing scarred tissue, the mean procedure time for redos was longer than that for the primary procedures (137 vs. 49 minutes; P less than .001), but there were no significant differences in the rate of surgical site infections (0.7% vs. 0.3%; P = .482), return to the operating room (3.6% vs. 4%; P = .853), or 30-day readmissions (2.1% vs. 6.9%; P = .810) for the redo and index procedures, respectively.
Although perioperative MI rates were higher in the redo group (2.1%) than in the primary endarterectomy group (0.9%), this difference did not reach statistical significance (P = .125). However, a multivariate analysis associated redo carotid endarterectomy procedures with a nearly threefold increase in risk of a composite of major adverse cardiovascular events when compared on a multivariate analysis (OR, 2.76; P = .007), Dr. Siracuse reported.
For the surgeons considering a redo carotid endarterectomy, these data “inform a risk-benefit analysis,” according to Dr. Siracuse, but he also said that redo procedures still should be considered a viable strategy when considered in the context of other options.
Presenting a case he performed just prior to the VEITHsymposium, Dr. Siracuse displayed CT images that showed internal and common carotids with more than 75% stenosis in an 80-year-old women 7 years after a primary carotid endarterectomy. The tight stenoses and the evidence of substantial intra-arterial debris were concerns, but a decision to perform a redo endarterectomy was reached after other options, including stenting, were considered.
“She did great. She went home and has had no more symptoms,” Dr. Siracuse reported. “The point is you still have to take these [potential redo endarterectomies] on a case-by case basis.”
Dr. Siracuse reported he had no financial relationships relevant to this study.
NEW YORK – It is well known that reoperative carotid endarterectomy can be technically challenging because of the scarring left from the initial procedure, but an analysis of a large database presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation also revealed that the risk of complications, particularly stroke, is greater.
When “redo” carotid endarterectomies were compared with the index primary procedure collected in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, the odds ratio for stroke was several times greater (odds ratio, 3.71; P = .002) on univariate analysis, reported Jeffrey J. Siracuse, MD, associate professor of surgery and radiology at Boston University.
Previous single-center reports of redo endarterectomies “showed terrific results, really no perioperative stroke or morbidity, but this is older data from a different era,” said Dr. Siracuse, who undertook this study to determine whether “real-world” data would tell a different story.
In this study, 75,943 primary carotid endarterectomies and 140 redo procedures were identified in the ACS NSQIP database and compared. The redo population had a significantly higher incidence of end-stage renal disease (3.6% vs. 1.1%; P = .004), but history of stroke, whether with deficit (20.8% vs. 15.4%) or without (11.5% vs. 9.1%), was numerically higher among those undergoing a primary procedure even though these differences did not reach statistical significance. Baseline demographics and comorbidities were otherwise similar.
Presumably because of the difficulty of recanalizing scarred tissue, the mean procedure time for redos was longer than that for the primary procedures (137 vs. 49 minutes; P less than .001), but there were no significant differences in the rate of surgical site infections (0.7% vs. 0.3%; P = .482), return to the operating room (3.6% vs. 4%; P = .853), or 30-day readmissions (2.1% vs. 6.9%; P = .810) for the redo and index procedures, respectively.
Although perioperative MI rates were higher in the redo group (2.1%) than in the primary endarterectomy group (0.9%), this difference did not reach statistical significance (P = .125). However, a multivariate analysis associated redo carotid endarterectomy procedures with a nearly threefold increase in risk of a composite of major adverse cardiovascular events when compared on a multivariate analysis (OR, 2.76; P = .007), Dr. Siracuse reported.
For the surgeons considering a redo carotid endarterectomy, these data “inform a risk-benefit analysis,” according to Dr. Siracuse, but he also said that redo procedures still should be considered a viable strategy when considered in the context of other options.
Presenting a case he performed just prior to the VEITHsymposium, Dr. Siracuse displayed CT images that showed internal and common carotids with more than 75% stenosis in an 80-year-old women 7 years after a primary carotid endarterectomy. The tight stenoses and the evidence of substantial intra-arterial debris were concerns, but a decision to perform a redo endarterectomy was reached after other options, including stenting, were considered.
“She did great. She went home and has had no more symptoms,” Dr. Siracuse reported. “The point is you still have to take these [potential redo endarterectomies] on a case-by case basis.”
Dr. Siracuse reported he had no financial relationships relevant to this study.
NEW YORK – It is well known that reoperative carotid endarterectomy can be technically challenging because of the scarring left from the initial procedure, but an analysis of a large database presented at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation also revealed that the risk of complications, particularly stroke, is greater.
When “redo” carotid endarterectomies were compared with the index primary procedure collected in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database, the odds ratio for stroke was several times greater (odds ratio, 3.71; P = .002) on univariate analysis, reported Jeffrey J. Siracuse, MD, associate professor of surgery and radiology at Boston University.
Previous single-center reports of redo endarterectomies “showed terrific results, really no perioperative stroke or morbidity, but this is older data from a different era,” said Dr. Siracuse, who undertook this study to determine whether “real-world” data would tell a different story.
In this study, 75,943 primary carotid endarterectomies and 140 redo procedures were identified in the ACS NSQIP database and compared. The redo population had a significantly higher incidence of end-stage renal disease (3.6% vs. 1.1%; P = .004), but history of stroke, whether with deficit (20.8% vs. 15.4%) or without (11.5% vs. 9.1%), was numerically higher among those undergoing a primary procedure even though these differences did not reach statistical significance. Baseline demographics and comorbidities were otherwise similar.
Presumably because of the difficulty of recanalizing scarred tissue, the mean procedure time for redos was longer than that for the primary procedures (137 vs. 49 minutes; P less than .001), but there were no significant differences in the rate of surgical site infections (0.7% vs. 0.3%; P = .482), return to the operating room (3.6% vs. 4%; P = .853), or 30-day readmissions (2.1% vs. 6.9%; P = .810) for the redo and index procedures, respectively.
Although perioperative MI rates were higher in the redo group (2.1%) than in the primary endarterectomy group (0.9%), this difference did not reach statistical significance (P = .125). However, a multivariate analysis associated redo carotid endarterectomy procedures with a nearly threefold increase in risk of a composite of major adverse cardiovascular events when compared on a multivariate analysis (OR, 2.76; P = .007), Dr. Siracuse reported.
For the surgeons considering a redo carotid endarterectomy, these data “inform a risk-benefit analysis,” according to Dr. Siracuse, but he also said that redo procedures still should be considered a viable strategy when considered in the context of other options.
Presenting a case he performed just prior to the VEITHsymposium, Dr. Siracuse displayed CT images that showed internal and common carotids with more than 75% stenosis in an 80-year-old women 7 years after a primary carotid endarterectomy. The tight stenoses and the evidence of substantial intra-arterial debris were concerns, but a decision to perform a redo endarterectomy was reached after other options, including stenting, were considered.
“She did great. She went home and has had no more symptoms,” Dr. Siracuse reported. “The point is you still have to take these [potential redo endarterectomies] on a case-by case basis.”
Dr. Siracuse reported he had no financial relationships relevant to this study.
REPORTING FROM VEITHSYMPOSIUM
Key clinical point:
Major finding: The odds ratio for stroke is 3.71 times higher (P = .002) with redo than with primary carotid endarterectomy.
Study details: Multivariate retrospective database analysis.
Disclosures: Dr. Siracuse reported he had no financial relationships relevant to this study.
Fewer insured may have helped slow health spending growth in 2017
Health care spending as a percentage of gross domestic product remained relatively stable in 2017, despite a slowdown in the growth of spending.
Total health care spending in the United States was $3.5 trillion in 2017, an increase of 3.9% from 2016, according to data released Dec. 6 by the Centers for Medicare & Medicaid Services.
The growth rate was down from that of 2016 (4.8%) but similar to growth rates experienced during 2008-2013, according to the research article in Health Affairs.
“The slower growth in health care spending in 2017 resulted primarily from slower growth in hospital care, physician and clinical services, and retail prescription drugs, with residual use and intensity of these goods and services contributing substantially to the trend,” Anne B. Martin, an economist in the CMS Office of the Actuary’s National Health Statistics Group, and her colleagues wrote.
The report notes that slower growth in the use and intensity of health care goods and services in 2017 “may have been affected by slower growth in overall health insurance enrollment, as the insured share of the population fell from 91.1% in 2016 to 90.9% in 2017.”
Spending on hospital care increased 4.6% to $1.1 trillion in 2017 and accounted for 33% of total health care spending; however, growth was slower than in the previous year (5.6%). Ms. Martin and her colleagues noted that growth in outpatient visits slowed while growth in inpatient days increased at about the same rate and prices in hospital care grew in 2017 to 1.7% from 1.2% in the previous year.
Spending on physician and clinical services grew 4.2% in 2017 to $694.3 billion and accounted for 20% of total health care spending. The growth rate is down from the previous year (5.6%) and a recent peak of 6% in 2015.
“Although spending growth for both physician services and clinical services slowed in 2017, the growth rate for the latter (5.0%) continued to out pace the rate for the former (3.9%), as spending for most types of outpatient care centers contributed to the stronger growth in spending for clinical services,” Ms. Martin and her colleagues reported.
They attributed the slowdown to non-price factors, such as slower growth in the use and intensity of physician and clinical services, although price growth for physician and clinical services increased 0.4% in 2017, up from 0.2% in 2016.
Spending on retail prescription drugs grew 0.4% in 2017 to $333.4 billion and accounted for 10% of total national health spending. It is the slowest growth rate increase since 2012, a year that saw a number of blockbuster drugs lose patent protection. This was down from a growth rate of 2.3% in 2016 and down from recent rates of 12.4% in 2014 and 8.9% in 2015.
“Slower growth in non-price factors, such as the use and mix of retail prescription drugs – and, to a lesser extent, in retail prescription drug prices – contributed to the slower overall growth in retail prescription drug spending in 2017,” according to the authors. Key factors included slower growth in the number of prescriptions dispensed, the continued shift to lower-cost generics, and slower growth in the volume of high-cost drugs, particularly those used to treat hepatitis C. Price decreases in generics and lower increases for existing brand-name drugs also contributed to the lower spending growth in 2017.
Ms. Martin and her colleagues highlighted the slower growth rate in the number of prescriptions (1.8% in 2017, down from 2.3% in 2016) “resulted in large part from a decline in the number of prescriptions dispensed for drugs used to treat pain.”
Medicare spending, which represents 20% of all national health care spending in 2017 ($705.9 billion), grew 4.2%, a slight decline from the 4.3% growth in 2016. Enrollment growth slowed slightly to 2.5% in 2017 from 2.7% in the previous year, while in the same time frame, per-enrollee expenditures increased slightly to 1.7% from 1.6%. Slower growth in fee-for-service Medicare spending was offset by faster growth in spending by Medicare private health plans.
Medicaid spending reached $581.9 billion (17% of national health care spending), and the growth rate slowed for the third straight year, increasing 2.9% in 2017 versus 4.2% in 2016. The slower growth “was influenced by a deceleration in enrollment growth and a reduction in the Medicaid net cost of health insurance as the federal government recovered payments from managed care organizations based on their favorable prior-period experience,” the authors stated. Enrollment growth has been decelerating following a peak of growth of 11.9% in 2014 because of states that elected to expand Medicaid eligibility, which was followed by 3 years of slower growth rates of 4.9%, 3.0% and 2.0% in 2015, 2016, and 2017, respectively. Per-enrollee spending also slowed to 0.9% growth in 2017 from a rate of 1.2% in 2016, attributed to “the decline in government administration and the net cost of insurance.”
SOURCE: Martin A et al. Health Aff. 2018. doi: 10.1377/hlthaff.2018.05085.
Health care spending as a percentage of gross domestic product remained relatively stable in 2017, despite a slowdown in the growth of spending.
Total health care spending in the United States was $3.5 trillion in 2017, an increase of 3.9% from 2016, according to data released Dec. 6 by the Centers for Medicare & Medicaid Services.
The growth rate was down from that of 2016 (4.8%) but similar to growth rates experienced during 2008-2013, according to the research article in Health Affairs.
“The slower growth in health care spending in 2017 resulted primarily from slower growth in hospital care, physician and clinical services, and retail prescription drugs, with residual use and intensity of these goods and services contributing substantially to the trend,” Anne B. Martin, an economist in the CMS Office of the Actuary’s National Health Statistics Group, and her colleagues wrote.
The report notes that slower growth in the use and intensity of health care goods and services in 2017 “may have been affected by slower growth in overall health insurance enrollment, as the insured share of the population fell from 91.1% in 2016 to 90.9% in 2017.”
Spending on hospital care increased 4.6% to $1.1 trillion in 2017 and accounted for 33% of total health care spending; however, growth was slower than in the previous year (5.6%). Ms. Martin and her colleagues noted that growth in outpatient visits slowed while growth in inpatient days increased at about the same rate and prices in hospital care grew in 2017 to 1.7% from 1.2% in the previous year.
Spending on physician and clinical services grew 4.2% in 2017 to $694.3 billion and accounted for 20% of total health care spending. The growth rate is down from the previous year (5.6%) and a recent peak of 6% in 2015.
“Although spending growth for both physician services and clinical services slowed in 2017, the growth rate for the latter (5.0%) continued to out pace the rate for the former (3.9%), as spending for most types of outpatient care centers contributed to the stronger growth in spending for clinical services,” Ms. Martin and her colleagues reported.
They attributed the slowdown to non-price factors, such as slower growth in the use and intensity of physician and clinical services, although price growth for physician and clinical services increased 0.4% in 2017, up from 0.2% in 2016.
Spending on retail prescription drugs grew 0.4% in 2017 to $333.4 billion and accounted for 10% of total national health spending. It is the slowest growth rate increase since 2012, a year that saw a number of blockbuster drugs lose patent protection. This was down from a growth rate of 2.3% in 2016 and down from recent rates of 12.4% in 2014 and 8.9% in 2015.
“Slower growth in non-price factors, such as the use and mix of retail prescription drugs – and, to a lesser extent, in retail prescription drug prices – contributed to the slower overall growth in retail prescription drug spending in 2017,” according to the authors. Key factors included slower growth in the number of prescriptions dispensed, the continued shift to lower-cost generics, and slower growth in the volume of high-cost drugs, particularly those used to treat hepatitis C. Price decreases in generics and lower increases for existing brand-name drugs also contributed to the lower spending growth in 2017.
Ms. Martin and her colleagues highlighted the slower growth rate in the number of prescriptions (1.8% in 2017, down from 2.3% in 2016) “resulted in large part from a decline in the number of prescriptions dispensed for drugs used to treat pain.”
Medicare spending, which represents 20% of all national health care spending in 2017 ($705.9 billion), grew 4.2%, a slight decline from the 4.3% growth in 2016. Enrollment growth slowed slightly to 2.5% in 2017 from 2.7% in the previous year, while in the same time frame, per-enrollee expenditures increased slightly to 1.7% from 1.6%. Slower growth in fee-for-service Medicare spending was offset by faster growth in spending by Medicare private health plans.
Medicaid spending reached $581.9 billion (17% of national health care spending), and the growth rate slowed for the third straight year, increasing 2.9% in 2017 versus 4.2% in 2016. The slower growth “was influenced by a deceleration in enrollment growth and a reduction in the Medicaid net cost of health insurance as the federal government recovered payments from managed care organizations based on their favorable prior-period experience,” the authors stated. Enrollment growth has been decelerating following a peak of growth of 11.9% in 2014 because of states that elected to expand Medicaid eligibility, which was followed by 3 years of slower growth rates of 4.9%, 3.0% and 2.0% in 2015, 2016, and 2017, respectively. Per-enrollee spending also slowed to 0.9% growth in 2017 from a rate of 1.2% in 2016, attributed to “the decline in government administration and the net cost of insurance.”
SOURCE: Martin A et al. Health Aff. 2018. doi: 10.1377/hlthaff.2018.05085.
Health care spending as a percentage of gross domestic product remained relatively stable in 2017, despite a slowdown in the growth of spending.
Total health care spending in the United States was $3.5 trillion in 2017, an increase of 3.9% from 2016, according to data released Dec. 6 by the Centers for Medicare & Medicaid Services.
The growth rate was down from that of 2016 (4.8%) but similar to growth rates experienced during 2008-2013, according to the research article in Health Affairs.
“The slower growth in health care spending in 2017 resulted primarily from slower growth in hospital care, physician and clinical services, and retail prescription drugs, with residual use and intensity of these goods and services contributing substantially to the trend,” Anne B. Martin, an economist in the CMS Office of the Actuary’s National Health Statistics Group, and her colleagues wrote.
The report notes that slower growth in the use and intensity of health care goods and services in 2017 “may have been affected by slower growth in overall health insurance enrollment, as the insured share of the population fell from 91.1% in 2016 to 90.9% in 2017.”
Spending on hospital care increased 4.6% to $1.1 trillion in 2017 and accounted for 33% of total health care spending; however, growth was slower than in the previous year (5.6%). Ms. Martin and her colleagues noted that growth in outpatient visits slowed while growth in inpatient days increased at about the same rate and prices in hospital care grew in 2017 to 1.7% from 1.2% in the previous year.
Spending on physician and clinical services grew 4.2% in 2017 to $694.3 billion and accounted for 20% of total health care spending. The growth rate is down from the previous year (5.6%) and a recent peak of 6% in 2015.
“Although spending growth for both physician services and clinical services slowed in 2017, the growth rate for the latter (5.0%) continued to out pace the rate for the former (3.9%), as spending for most types of outpatient care centers contributed to the stronger growth in spending for clinical services,” Ms. Martin and her colleagues reported.
They attributed the slowdown to non-price factors, such as slower growth in the use and intensity of physician and clinical services, although price growth for physician and clinical services increased 0.4% in 2017, up from 0.2% in 2016.
Spending on retail prescription drugs grew 0.4% in 2017 to $333.4 billion and accounted for 10% of total national health spending. It is the slowest growth rate increase since 2012, a year that saw a number of blockbuster drugs lose patent protection. This was down from a growth rate of 2.3% in 2016 and down from recent rates of 12.4% in 2014 and 8.9% in 2015.
“Slower growth in non-price factors, such as the use and mix of retail prescription drugs – and, to a lesser extent, in retail prescription drug prices – contributed to the slower overall growth in retail prescription drug spending in 2017,” according to the authors. Key factors included slower growth in the number of prescriptions dispensed, the continued shift to lower-cost generics, and slower growth in the volume of high-cost drugs, particularly those used to treat hepatitis C. Price decreases in generics and lower increases for existing brand-name drugs also contributed to the lower spending growth in 2017.
Ms. Martin and her colleagues highlighted the slower growth rate in the number of prescriptions (1.8% in 2017, down from 2.3% in 2016) “resulted in large part from a decline in the number of prescriptions dispensed for drugs used to treat pain.”
Medicare spending, which represents 20% of all national health care spending in 2017 ($705.9 billion), grew 4.2%, a slight decline from the 4.3% growth in 2016. Enrollment growth slowed slightly to 2.5% in 2017 from 2.7% in the previous year, while in the same time frame, per-enrollee expenditures increased slightly to 1.7% from 1.6%. Slower growth in fee-for-service Medicare spending was offset by faster growth in spending by Medicare private health plans.
Medicaid spending reached $581.9 billion (17% of national health care spending), and the growth rate slowed for the third straight year, increasing 2.9% in 2017 versus 4.2% in 2016. The slower growth “was influenced by a deceleration in enrollment growth and a reduction in the Medicaid net cost of health insurance as the federal government recovered payments from managed care organizations based on their favorable prior-period experience,” the authors stated. Enrollment growth has been decelerating following a peak of growth of 11.9% in 2014 because of states that elected to expand Medicaid eligibility, which was followed by 3 years of slower growth rates of 4.9%, 3.0% and 2.0% in 2015, 2016, and 2017, respectively. Per-enrollee spending also slowed to 0.9% growth in 2017 from a rate of 1.2% in 2016, attributed to “the decline in government administration and the net cost of insurance.”
SOURCE: Martin A et al. Health Aff. 2018. doi: 10.1377/hlthaff.2018.05085.
FROM HEALTH AFFAIRS
Key clinical point: National health care spending growth slowed to 3.9% in 2017.
Major finding: The $3.5 trillion in national health care spending represents 17.9% of GDP.
Study details: Annual analysis of national health expenditures conducted by federal actuaries.
Disclosures: Analysis conducted by the Centers for Medicaid & Medicare Services Office of the Actuary; the authors have no relevant financial conflicts of interest.
Source: Martin A et al. Health Affairs. 2018. doi: 10.1377/hlthaff.2018.05085.