User login
Evaluation of Interventions by Clinical Pharmacy Specialists in Cardiology at a VA Ambulatory Cardiology Clinic
Integration of CPSs into an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for other cardiology health care providers.
Health care providers face many challenges in utilizing cardiovascular therapies, such as anticipated shortages in physicians, patients with more complicated conditions, shifting medication regimens, management needs, and increased accountability for quality and performance measures.1 To meet the potential increase in service demand, cardiology practices are embracing cardiovascular team-based care.1 Advanced practice providers, such as advanced practice registered nurses (APRNs), physician assistants (PAs), and clinical pharmacy specialists (CPSs), have education, training, and experience to extend the team’s capability to meet these complex management needs.1
The role of CPSs within a cardiovascular care team includes providing a variety of patient-specific services, such as collaborating with other cardiology providers, to optimize evidence-based pharmacotherapy, preventing medication-related adverse events/errors, improving patient understanding of their medication regimen, and ultimately, improving patient outcomes.2 Health care systems, such as Kaiser Permanente of Colorado, have demonstrated improved clinical outcomes for patients with coronary artery disease (CAD) by implementing a multidisciplinary collaborative cardiac care service, including a clinical pharmacy cardiac risk service, in which CPSs assisted with management of cholesterol-lowering, hypertension, diabetes mellitus (DM), and smoking-cessation therapies, which resulted in a 76% to 89% reduction in all-cause mortality associated with CAD in multiple evaluations.3,4
Pharmacists providing medication therapy management (MTM) services in Minnesota had higher goal attainment for patients with hypertension and hyperlipidemia than did pharmacists who did not provide MTM services.5 MTM services provided by pharmacists led to an improvement in clinical outcomes for patients as well as a reduction in overall health care expenditures compared with that of a control group of patients who did not receive MTM services.5 Furthermore, CPS integration in the heart failure (HF) setting has led to improvements in utilization and optimization of guideline-directed medical therapies, an area in which recent data have suggested deficiencies exist.6-8 A full review of the outcomes associated with CPS involvement in cardiovascular care is beyond the scope of this article; but the recent review by Dunn and colleagues provides more detail.2
With the increasing number of patients with cardiovascular disease,expanding integration of CPSs in the cardiovascular team providing MTM services may reduce the burden of other providers (MD, PA, APRN, etc), thereby increasing access for not only new patients, but also diagnostic and interventional work, while potentially improving clinical and economic outcomes.2 The value of integrating CPSs as members of the cardiovascular care team is recognized in a variety of inpatient and ambulatory practice settings.2-6 However, data are limited on the number and types of interventions made per encounter as direct patient care providers. Expanded granularity regarding the effect of CPSs as active members of the cardiovascular team is an essential component to evaluate the potential benefit of CPS integration into direct patient care.
Methods
The West Palm Beach (WPB) Veteran Affairs Medical Center (VAMC) outpatient cardiology clinic consists of 6 full-time employee (FTE) cardiologists, 4 PAs or APRNs, 10 other cardiology health care staff members (registered/license practical nurses and technicians), and 2 cardiology CPSs providing direct patient care
The cardiology pharmacotherapy clinic is open 20.5 hours per week with 41 appointment slots (30 minutes each), of which 7 appointments are delivered via clinic video telehealth and 34 appointments are traditional face-to-face visits.9 The remaining CPS time is assigned to other clinical care and administrative areas to fit facility need, including oversight of the CPS-run 24-hour ambulatory blood pressure clinic, postgraduate year 2 cardiology pharmacy practice residency program directorship, and other administrative activities for the facility.10
The cardiology CPSs practice under an advanced scope of practice in which they independently manage medications (initiate, modify, discontinue), order diagnostic testing (laboratory, monitoring, imaging, etc) needed for medication management, and create monitoring and treatment plans for patients referred to the cardiology pharmacotherapy clinic by other cardiology providers. The diseases managed within the clinic vary based on patient-specific needs, but may include HF, dyslipidemia, hypertension, anticoagulation, CAD, arrhythmias, cardiovascular risk factor assessment and reduction, and medication reconciliation and teaching. Patients are referred for CPS management directly from facility cardiologist and cardiology clinic PAs and APRNs. Workload and interventions carried out are captured in the Pharmacists Achieve Results with Medications Demonstration (PhARMD) tool and patient care encounter tracking.9
Data Collection
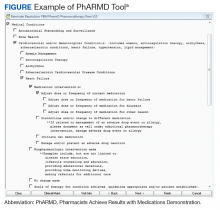
Using local data from workload tracking, the number of CPS encounters was determined from July 6, 2015, to October 1, 2015. Data were collected on the types and volume of interventions made by CPSs in the cardiology pharmacotherapy clinic using the PhARMD tool (Figure).
The PhARMD tool was initially developed and implemented for CPSs in primary care pharmacotherapy clinics and was used to evaluate the types and volume of CPS interventions made in this setting.11 Since this initial evaluation, the tool has been updated, standardized nationally by the Department of Veterans Affairs (VA) Pharmacy Benefits Management Clinical Pharmacy Practice Office, and integrated across numerous VAMCs and associated outpatient clinics. The tool remains embedded within the VA electronic health record (EHR) and allows the capture of specific CPS interventions of several types (ie, both pharmacologic and nonpharmacologic interventions, including adjust dose or frequency; change or discontinue medication; initiate medication; monitor medication; counsel on adherence, contraindications, drug interactions, and drugs not indicated; reconcile medication; and prevent or manage adverse drug events [ADEs]) specific to certain diseases, such as anemia, anticoagulation, HF, type 2 DM (T2DM), hypertension, dyslipidemia, and tobacco cessation.
Given that the interventions captured by the PhARMD tool are based on self-report of the CPS performing the intervention, a quality assurance (QA) measure was taken to audit a random sample of interventions to validate the accuracy of reported data. A Pharmacy Benefits Management PhARMD Project QA report provided the 20% random sample of encounters for each cardiology CPS to be reviewed. This percentage was determined by VAMC Clinical Pharmacy Program Office (CPPO) directives on implementation of the PhARMD tool. During the QA period, the provided sample was reviewed to determine whether the intervention(s) recorded with the PhARMD tool matched the actions documented in the EHR. The QA review was done through a manual chart review by an author not involved in recording the original interventions. Both WPB VAMC cardiology CPSs passed the QA review (> 80% concurrence with tool logged and chart documented interventions as required by VA CPPO directive), with a 90.9% concurrence between the EHR and PhARMD tool documentation.
Statistical Analyses
Data on intervention type and encounter number were evaluated with descriptive statistics. The information was characterized and diagrammed with Excel (Microsoft, Redmond, WA) charts and graphs.
Cost-avoidance calculations were done using previously described methods and are included for exploratory analysis.11,12 Briefly, published estimates of cost avoidance associated with various interventions from the outpatient setting within a VAMC setting were applied as appropriate to the various interventions captured with the PhARMD tool.11,12 These estimates from Lee and colleagues were derived from detailed chart review of interventions made and the potential harm prevented.12 Costs or cost avoidances associated with interventions were calculated from pooled examination of 600 interventions in a VAMC with drug costs before and after the intervention, costs associated with harms prevented by the intervention, as well as the VAMC hourly pharmacist wages associated with making an intervention and processing and filling original vs recommended therapies.
The costs presented represent a “best-case” scenario in which all interventions made are expected to prevent patient harms. The costs related to avoided outcomes, facility overhead, and auxiliary staff cannot be included but highlight the many considerations that must be considered when examining potential cost-avoidance calculations. The estimates and methods at hand were chosen because, to our knowledge, no other consensus model exists that would be more appropriate for use in the situation and health care system at hand. Cost-avoidance estimates were calculated by extrapolating the 88-day study period values to a yearly estimate. All cost estimates were adjusted for inflation using the consumer price index calculator as per convention in previous analyses using the cost-avoidance estimates at hand.11-13
Results
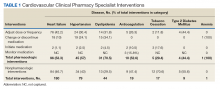
From July 6, 2015, through October 1, 2015, 301 patient encounters occurred, and 529 interventions were documented with the PhARMD tool. The mean number of interventions per encounter was 1.8. Interventions were 65.2% pharmacologic and 34.8% nonpharmacologic. Of pharmacologic interventions, 27.1% were for HF, 12.7% for hypertension, 8.8% for dyslipidemia, 2.8% for anticoagulation, 1.4% for tobacco cessation, 1.1% for T2DM, 0.3% for anemia, and 45.8% for other conditions (Table 1).
The main types of pharmacologic interventions across all diseases were related to adjustments in medication dose or frequency (42.3%) and change or discontinuation of medications (20.0%).
Discussion
Evaluation of the interventions and encounters at the WPB VAMC ambulatory cardiology pharmacotherapy clinic suggests that CPSs are able to contribute to direct patient care independently of interventions performed by other cardiology providers. Specifically, 1.8 interventions per encounter were made by CPSs in this study. In a prior evaluation of CPS interventions recorded with the PhARMD tool in a VAMC primary care setting, 2.3 interventions per encounter were recorded.11 In comparing the present volume of interventions with the volume recorded in the study by Hough and colleagues, the difference in practice setting may account for differences seen.11
The primary care medication management setting would capture a broader array of clinical interventions than would the ambulatory cardiology clinic of the present study, so it is reasonable that more interventions would be captured per encounter in the primary care clinic. The difference in practice settings affecting the character of collected interventions can be seen because most interventions in this study at an ambulatory cardiology clinic were related to HF, whereas in Hough and colleagues 39.2% of the disease-specific interventions were related to DM, and only 2.9% were related to HF.11 The differences inherent in the intervention populations can also be seen by comparing the percentage of interventions related to hypertension and dyslipidemia: 30% and 28% in the study by Hough and colleagues compared with 13% and 9%, respectively, in the present study.11
Comparison of the present evaluation and Hough and colleagues is also hindered by the PhARMD tool used. The PhARMD tool used in the initial evaluation has been modified on a national level to improve the granularity of intervention data collected.
Our cost-avoidance estimate of $433,324.06 per year seems lower than that estimated in the previous evaluation when all applicable interventions were included.11 However, this study had several differences compared with those of previous VAMC studies looking at clinical interventions performed by CPSs. The main differences are the volume and setting in which interventions were being made. For example, in comparison with Hough and colleagues, the studies include different practice settings (primary care vs cardiology specialty clinic) and number of FTEs involved in the study (4.65 vs 1). If the cost avoidance is distributed evenly per FTE in the previous study, the following calculation is observed: $649,551.99 per FTE, which is closer to this study’s estimation. Given that primary care is a broader setting than is ambulatory cardiology, it is not surprising that more types of interventions and the overall volume/absolute number of interventions would be higher. Thus, the lower estimated cost avoidance in our study may be attributed to the lower volume of intervention opportunities availed to the cardiology CPS. Another difference is that detailed types of interventions related to hypertension, DM, dyslipidemia, and HF were not included in Hough and colleagues, whereas our study included all applicable interventions regardless of relation to diseases, which may account for a degree of the variation in intervention breakdown between the 2 studies.11 However, as noted previously, some interventions for these particular diseases may not fully capture the rationale for pharmacotherapy interventions, such as drug dose changes or discontinuations, which may misrepresent the potential cost avoidance associated with them in reality.
Limitations
Of general importance, the PhARMD tool may underestimate the number of interventions made such that multiple interventions for a medical condition may have been completed but only captured as 1 intervention, which may represent a limitation of the tool when multiple interventions are made for the same disease (eg, titration of both β-blocker and angiotensin-converting enzyme inhibitor doses at a single appointment in a patient with HF with reduced left ventricular ejection fraction). Improved clarity about interventions made would require laborious chart review, which was not feasible. The evaluation at hand included a preliminary QA review, adding confidence that overdocumentation was not being done and the values represented at worst an underestimation of actual CPS intervention impact. Because this study was an initial evaluation of interventions made by CPSs in an ambulatory cardiology pharmacotherapy setting, whether these same outcomes would exist in other patient cohorts is unclear. However, these data do provide a foundational understanding of what may be expected from CPS integration into a cardiovascular care team.
These findings may be limited in generalizability to other health care systems and situations in which CPSs are afforded the regulatory opportunity to practice independently within an established scope of practice or collaborative practice agreements. The Veterans Health Administration system has been a leader in integrating CPSs into direct patient care roles and serves as a potential model for application by other groups. This evaluation’s data support continued efforts to create such independent practice environments as they allow for qualified CPSs to practice to their full clinical potential and have the fullest possible effect on cardiovascular outcomes.
Previous studies looking at cost savings in MTM programs have established a substantial return in economic investment with patients being managed by pharmacists.5,14 Given that the interventions made in this study were not tied to attainment of clinical outcomes, a limitation to our study, the cost-avoidance estimates should be interpreted cautiously. However, we know of no such tool that is available to allow accurate capture of clinical event reduction in a single center with consistent CPS involvement in care. A clear opportunity exists regarding design of a model that measures clinical, economic, and humanistic outcomes related to the interventions performed by cardiology CPSs, but developing and deploying such a model may be challenging because guideline-directed medical therapies vary significantly based on many patient-specific issues, and identifying optimal or truly optimized medical therapy is at times a subjective task, especially in a single center. Using the types and volumes of interventions made by CPSs as a surrogate for these higher-level outcomes is still of value in order to understand the effect and role of CPSs in cardiovascular care. At present, the cost-avoidance estimates presented in this evaluation are based on the most appropriate system-specific data at hand, with the realization that actual cost avoidance in practice may vary widely and should be the topic of future research.
Conclusion
As cardiovascular team-based care continues to expand with the support of large organizations, such as the American College of Cardiology Foundation, Heart Failure Society of America, and American College of Clinical Pharmacy Cardiology Practice and Research Network, the need for understanding the effect of CPSs on patient care measures and health care costs becomes more pronounced.2,15 The results of this study demonstrate how integration of CPSs in an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for cardiology physicians and providers, allowing more availability for diagnostic testing and care.
Interventions made by CPSs functioning as independent providers
1. Brush JE Jr, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65(19):2118-2136.
2. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66(19):2129-2139.
3. Sandoff BG, Kuca S, Rasmussen J, Merenich JA. Collaborative cardiac care service: a multidisciplinary approach to caring for patients with coronary artery disease. Perm J. 2008;12(3):4-11.
4. Merenich JA, Olson KL, Delate T, Rasmussen J, Helling DK, Ward DG; Clinical Pharmacy Cardiac Risk Service Study Group. Mortality reduction benefits of a comprehensive cardiac care program for patients with occlusive coronary disease. Pharmacotherapy. 2007;27(10):1370-1378.
5. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience.
6. Martinez AS, Saef J, Paszcuzuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
7. Roth GA, Poole JE, Zaha R, Zhou W, Skinner J, Morden NE. Use of guideline-directed medications for heart failure before cardioverter-defibrillator implantation. J Am Coll Cardiol. 2016;67(9):1062-1069.
8. Noschese LA, Bergman CL, Brar CK, Kansal MM. The pharmacist’s role in medication optimization for patients with chronic heart failure. Fed Pract. 2017;34(suppl 10):S10-S15.
9. Coakley C, Hough A, Dwyer D, Parra D. Clinical video telehealth in a cardiology pharmacotherapy clinic. Am J Health Syst Pharm. 2013;70(22):1974-1975.
10. Khazan E, Anastasia E, Hough A, Parra D. Pharmacist-managed ambulatory blood pressure monitoring service. Am J Health Syst Pharm. 2017;74(4):190-195.
11. Hough A, Vartan CM, Groppi JA, Reyes S, Beckey NP. Evaluation of clinical pharmacy interventions in a Veterans Affairs medical center primary care clinic. Am J Health Syst Pharm. 2013;70(13):1168-1172.
12. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077.
13. US Department of Labor. CPI inflation calculator. www.bls.gov/data/inflation_calculator.htm. Accessed January 18, 2019.
14. Perez A, Doloresco F, Hoffman JM, et al. Economic evaluations of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2008;29(1):128.
15. Milfred-LaForest SK, Chow SL, DiDomenico RJ, et al. Clinical pharmacy services in heart failure: an opinion paper from the Heart Failure Society of America and American College of Clinical Pharmacy Cardiology Practice and Research Network. Pharmacotherapy. 2013;33(5):529-548.
Integration of CPSs into an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for other cardiology health care providers.
Integration of CPSs into an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for other cardiology health care providers.
Health care providers face many challenges in utilizing cardiovascular therapies, such as anticipated shortages in physicians, patients with more complicated conditions, shifting medication regimens, management needs, and increased accountability for quality and performance measures.1 To meet the potential increase in service demand, cardiology practices are embracing cardiovascular team-based care.1 Advanced practice providers, such as advanced practice registered nurses (APRNs), physician assistants (PAs), and clinical pharmacy specialists (CPSs), have education, training, and experience to extend the team’s capability to meet these complex management needs.1
The role of CPSs within a cardiovascular care team includes providing a variety of patient-specific services, such as collaborating with other cardiology providers, to optimize evidence-based pharmacotherapy, preventing medication-related adverse events/errors, improving patient understanding of their medication regimen, and ultimately, improving patient outcomes.2 Health care systems, such as Kaiser Permanente of Colorado, have demonstrated improved clinical outcomes for patients with coronary artery disease (CAD) by implementing a multidisciplinary collaborative cardiac care service, including a clinical pharmacy cardiac risk service, in which CPSs assisted with management of cholesterol-lowering, hypertension, diabetes mellitus (DM), and smoking-cessation therapies, which resulted in a 76% to 89% reduction in all-cause mortality associated with CAD in multiple evaluations.3,4
Pharmacists providing medication therapy management (MTM) services in Minnesota had higher goal attainment for patients with hypertension and hyperlipidemia than did pharmacists who did not provide MTM services.5 MTM services provided by pharmacists led to an improvement in clinical outcomes for patients as well as a reduction in overall health care expenditures compared with that of a control group of patients who did not receive MTM services.5 Furthermore, CPS integration in the heart failure (HF) setting has led to improvements in utilization and optimization of guideline-directed medical therapies, an area in which recent data have suggested deficiencies exist.6-8 A full review of the outcomes associated with CPS involvement in cardiovascular care is beyond the scope of this article; but the recent review by Dunn and colleagues provides more detail.2
With the increasing number of patients with cardiovascular disease,expanding integration of CPSs in the cardiovascular team providing MTM services may reduce the burden of other providers (MD, PA, APRN, etc), thereby increasing access for not only new patients, but also diagnostic and interventional work, while potentially improving clinical and economic outcomes.2 The value of integrating CPSs as members of the cardiovascular care team is recognized in a variety of inpatient and ambulatory practice settings.2-6 However, data are limited on the number and types of interventions made per encounter as direct patient care providers. Expanded granularity regarding the effect of CPSs as active members of the cardiovascular team is an essential component to evaluate the potential benefit of CPS integration into direct patient care.
Methods
The West Palm Beach (WPB) Veteran Affairs Medical Center (VAMC) outpatient cardiology clinic consists of 6 full-time employee (FTE) cardiologists, 4 PAs or APRNs, 10 other cardiology health care staff members (registered/license practical nurses and technicians), and 2 cardiology CPSs providing direct patient care
The cardiology pharmacotherapy clinic is open 20.5 hours per week with 41 appointment slots (30 minutes each), of which 7 appointments are delivered via clinic video telehealth and 34 appointments are traditional face-to-face visits.9 The remaining CPS time is assigned to other clinical care and administrative areas to fit facility need, including oversight of the CPS-run 24-hour ambulatory blood pressure clinic, postgraduate year 2 cardiology pharmacy practice residency program directorship, and other administrative activities for the facility.10
The cardiology CPSs practice under an advanced scope of practice in which they independently manage medications (initiate, modify, discontinue), order diagnostic testing (laboratory, monitoring, imaging, etc) needed for medication management, and create monitoring and treatment plans for patients referred to the cardiology pharmacotherapy clinic by other cardiology providers. The diseases managed within the clinic vary based on patient-specific needs, but may include HF, dyslipidemia, hypertension, anticoagulation, CAD, arrhythmias, cardiovascular risk factor assessment and reduction, and medication reconciliation and teaching. Patients are referred for CPS management directly from facility cardiologist and cardiology clinic PAs and APRNs. Workload and interventions carried out are captured in the Pharmacists Achieve Results with Medications Demonstration (PhARMD) tool and patient care encounter tracking.9
Data Collection
Using local data from workload tracking, the number of CPS encounters was determined from July 6, 2015, to October 1, 2015. Data were collected on the types and volume of interventions made by CPSs in the cardiology pharmacotherapy clinic using the PhARMD tool (Figure).
The PhARMD tool was initially developed and implemented for CPSs in primary care pharmacotherapy clinics and was used to evaluate the types and volume of CPS interventions made in this setting.11 Since this initial evaluation, the tool has been updated, standardized nationally by the Department of Veterans Affairs (VA) Pharmacy Benefits Management Clinical Pharmacy Practice Office, and integrated across numerous VAMCs and associated outpatient clinics. The tool remains embedded within the VA electronic health record (EHR) and allows the capture of specific CPS interventions of several types (ie, both pharmacologic and nonpharmacologic interventions, including adjust dose or frequency; change or discontinue medication; initiate medication; monitor medication; counsel on adherence, contraindications, drug interactions, and drugs not indicated; reconcile medication; and prevent or manage adverse drug events [ADEs]) specific to certain diseases, such as anemia, anticoagulation, HF, type 2 DM (T2DM), hypertension, dyslipidemia, and tobacco cessation.
Given that the interventions captured by the PhARMD tool are based on self-report of the CPS performing the intervention, a quality assurance (QA) measure was taken to audit a random sample of interventions to validate the accuracy of reported data. A Pharmacy Benefits Management PhARMD Project QA report provided the 20% random sample of encounters for each cardiology CPS to be reviewed. This percentage was determined by VAMC Clinical Pharmacy Program Office (CPPO) directives on implementation of the PhARMD tool. During the QA period, the provided sample was reviewed to determine whether the intervention(s) recorded with the PhARMD tool matched the actions documented in the EHR. The QA review was done through a manual chart review by an author not involved in recording the original interventions. Both WPB VAMC cardiology CPSs passed the QA review (> 80% concurrence with tool logged and chart documented interventions as required by VA CPPO directive), with a 90.9% concurrence between the EHR and PhARMD tool documentation.
Statistical Analyses
Data on intervention type and encounter number were evaluated with descriptive statistics. The information was characterized and diagrammed with Excel (Microsoft, Redmond, WA) charts and graphs.
Cost-avoidance calculations were done using previously described methods and are included for exploratory analysis.11,12 Briefly, published estimates of cost avoidance associated with various interventions from the outpatient setting within a VAMC setting were applied as appropriate to the various interventions captured with the PhARMD tool.11,12 These estimates from Lee and colleagues were derived from detailed chart review of interventions made and the potential harm prevented.12 Costs or cost avoidances associated with interventions were calculated from pooled examination of 600 interventions in a VAMC with drug costs before and after the intervention, costs associated with harms prevented by the intervention, as well as the VAMC hourly pharmacist wages associated with making an intervention and processing and filling original vs recommended therapies.
The costs presented represent a “best-case” scenario in which all interventions made are expected to prevent patient harms. The costs related to avoided outcomes, facility overhead, and auxiliary staff cannot be included but highlight the many considerations that must be considered when examining potential cost-avoidance calculations. The estimates and methods at hand were chosen because, to our knowledge, no other consensus model exists that would be more appropriate for use in the situation and health care system at hand. Cost-avoidance estimates were calculated by extrapolating the 88-day study period values to a yearly estimate. All cost estimates were adjusted for inflation using the consumer price index calculator as per convention in previous analyses using the cost-avoidance estimates at hand.11-13
Results
From July 6, 2015, through October 1, 2015, 301 patient encounters occurred, and 529 interventions were documented with the PhARMD tool. The mean number of interventions per encounter was 1.8. Interventions were 65.2% pharmacologic and 34.8% nonpharmacologic. Of pharmacologic interventions, 27.1% were for HF, 12.7% for hypertension, 8.8% for dyslipidemia, 2.8% for anticoagulation, 1.4% for tobacco cessation, 1.1% for T2DM, 0.3% for anemia, and 45.8% for other conditions (Table 1).
The main types of pharmacologic interventions across all diseases were related to adjustments in medication dose or frequency (42.3%) and change or discontinuation of medications (20.0%).
Discussion
Evaluation of the interventions and encounters at the WPB VAMC ambulatory cardiology pharmacotherapy clinic suggests that CPSs are able to contribute to direct patient care independently of interventions performed by other cardiology providers. Specifically, 1.8 interventions per encounter were made by CPSs in this study. In a prior evaluation of CPS interventions recorded with the PhARMD tool in a VAMC primary care setting, 2.3 interventions per encounter were recorded.11 In comparing the present volume of interventions with the volume recorded in the study by Hough and colleagues, the difference in practice setting may account for differences seen.11
The primary care medication management setting would capture a broader array of clinical interventions than would the ambulatory cardiology clinic of the present study, so it is reasonable that more interventions would be captured per encounter in the primary care clinic. The difference in practice settings affecting the character of collected interventions can be seen because most interventions in this study at an ambulatory cardiology clinic were related to HF, whereas in Hough and colleagues 39.2% of the disease-specific interventions were related to DM, and only 2.9% were related to HF.11 The differences inherent in the intervention populations can also be seen by comparing the percentage of interventions related to hypertension and dyslipidemia: 30% and 28% in the study by Hough and colleagues compared with 13% and 9%, respectively, in the present study.11
Comparison of the present evaluation and Hough and colleagues is also hindered by the PhARMD tool used. The PhARMD tool used in the initial evaluation has been modified on a national level to improve the granularity of intervention data collected.
Our cost-avoidance estimate of $433,324.06 per year seems lower than that estimated in the previous evaluation when all applicable interventions were included.11 However, this study had several differences compared with those of previous VAMC studies looking at clinical interventions performed by CPSs. The main differences are the volume and setting in which interventions were being made. For example, in comparison with Hough and colleagues, the studies include different practice settings (primary care vs cardiology specialty clinic) and number of FTEs involved in the study (4.65 vs 1). If the cost avoidance is distributed evenly per FTE in the previous study, the following calculation is observed: $649,551.99 per FTE, which is closer to this study’s estimation. Given that primary care is a broader setting than is ambulatory cardiology, it is not surprising that more types of interventions and the overall volume/absolute number of interventions would be higher. Thus, the lower estimated cost avoidance in our study may be attributed to the lower volume of intervention opportunities availed to the cardiology CPS. Another difference is that detailed types of interventions related to hypertension, DM, dyslipidemia, and HF were not included in Hough and colleagues, whereas our study included all applicable interventions regardless of relation to diseases, which may account for a degree of the variation in intervention breakdown between the 2 studies.11 However, as noted previously, some interventions for these particular diseases may not fully capture the rationale for pharmacotherapy interventions, such as drug dose changes or discontinuations, which may misrepresent the potential cost avoidance associated with them in reality.
Limitations
Of general importance, the PhARMD tool may underestimate the number of interventions made such that multiple interventions for a medical condition may have been completed but only captured as 1 intervention, which may represent a limitation of the tool when multiple interventions are made for the same disease (eg, titration of both β-blocker and angiotensin-converting enzyme inhibitor doses at a single appointment in a patient with HF with reduced left ventricular ejection fraction). Improved clarity about interventions made would require laborious chart review, which was not feasible. The evaluation at hand included a preliminary QA review, adding confidence that overdocumentation was not being done and the values represented at worst an underestimation of actual CPS intervention impact. Because this study was an initial evaluation of interventions made by CPSs in an ambulatory cardiology pharmacotherapy setting, whether these same outcomes would exist in other patient cohorts is unclear. However, these data do provide a foundational understanding of what may be expected from CPS integration into a cardiovascular care team.
These findings may be limited in generalizability to other health care systems and situations in which CPSs are afforded the regulatory opportunity to practice independently within an established scope of practice or collaborative practice agreements. The Veterans Health Administration system has been a leader in integrating CPSs into direct patient care roles and serves as a potential model for application by other groups. This evaluation’s data support continued efforts to create such independent practice environments as they allow for qualified CPSs to practice to their full clinical potential and have the fullest possible effect on cardiovascular outcomes.
Previous studies looking at cost savings in MTM programs have established a substantial return in economic investment with patients being managed by pharmacists.5,14 Given that the interventions made in this study were not tied to attainment of clinical outcomes, a limitation to our study, the cost-avoidance estimates should be interpreted cautiously. However, we know of no such tool that is available to allow accurate capture of clinical event reduction in a single center with consistent CPS involvement in care. A clear opportunity exists regarding design of a model that measures clinical, economic, and humanistic outcomes related to the interventions performed by cardiology CPSs, but developing and deploying such a model may be challenging because guideline-directed medical therapies vary significantly based on many patient-specific issues, and identifying optimal or truly optimized medical therapy is at times a subjective task, especially in a single center. Using the types and volumes of interventions made by CPSs as a surrogate for these higher-level outcomes is still of value in order to understand the effect and role of CPSs in cardiovascular care. At present, the cost-avoidance estimates presented in this evaluation are based on the most appropriate system-specific data at hand, with the realization that actual cost avoidance in practice may vary widely and should be the topic of future research.
Conclusion
As cardiovascular team-based care continues to expand with the support of large organizations, such as the American College of Cardiology Foundation, Heart Failure Society of America, and American College of Clinical Pharmacy Cardiology Practice and Research Network, the need for understanding the effect of CPSs on patient care measures and health care costs becomes more pronounced.2,15 The results of this study demonstrate how integration of CPSs in an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for cardiology physicians and providers, allowing more availability for diagnostic testing and care.
Interventions made by CPSs functioning as independent providers
Health care providers face many challenges in utilizing cardiovascular therapies, such as anticipated shortages in physicians, patients with more complicated conditions, shifting medication regimens, management needs, and increased accountability for quality and performance measures.1 To meet the potential increase in service demand, cardiology practices are embracing cardiovascular team-based care.1 Advanced practice providers, such as advanced practice registered nurses (APRNs), physician assistants (PAs), and clinical pharmacy specialists (CPSs), have education, training, and experience to extend the team’s capability to meet these complex management needs.1
The role of CPSs within a cardiovascular care team includes providing a variety of patient-specific services, such as collaborating with other cardiology providers, to optimize evidence-based pharmacotherapy, preventing medication-related adverse events/errors, improving patient understanding of their medication regimen, and ultimately, improving patient outcomes.2 Health care systems, such as Kaiser Permanente of Colorado, have demonstrated improved clinical outcomes for patients with coronary artery disease (CAD) by implementing a multidisciplinary collaborative cardiac care service, including a clinical pharmacy cardiac risk service, in which CPSs assisted with management of cholesterol-lowering, hypertension, diabetes mellitus (DM), and smoking-cessation therapies, which resulted in a 76% to 89% reduction in all-cause mortality associated with CAD in multiple evaluations.3,4
Pharmacists providing medication therapy management (MTM) services in Minnesota had higher goal attainment for patients with hypertension and hyperlipidemia than did pharmacists who did not provide MTM services.5 MTM services provided by pharmacists led to an improvement in clinical outcomes for patients as well as a reduction in overall health care expenditures compared with that of a control group of patients who did not receive MTM services.5 Furthermore, CPS integration in the heart failure (HF) setting has led to improvements in utilization and optimization of guideline-directed medical therapies, an area in which recent data have suggested deficiencies exist.6-8 A full review of the outcomes associated with CPS involvement in cardiovascular care is beyond the scope of this article; but the recent review by Dunn and colleagues provides more detail.2
With the increasing number of patients with cardiovascular disease,expanding integration of CPSs in the cardiovascular team providing MTM services may reduce the burden of other providers (MD, PA, APRN, etc), thereby increasing access for not only new patients, but also diagnostic and interventional work, while potentially improving clinical and economic outcomes.2 The value of integrating CPSs as members of the cardiovascular care team is recognized in a variety of inpatient and ambulatory practice settings.2-6 However, data are limited on the number and types of interventions made per encounter as direct patient care providers. Expanded granularity regarding the effect of CPSs as active members of the cardiovascular team is an essential component to evaluate the potential benefit of CPS integration into direct patient care.
Methods
The West Palm Beach (WPB) Veteran Affairs Medical Center (VAMC) outpatient cardiology clinic consists of 6 full-time employee (FTE) cardiologists, 4 PAs or APRNs, 10 other cardiology health care staff members (registered/license practical nurses and technicians), and 2 cardiology CPSs providing direct patient care
The cardiology pharmacotherapy clinic is open 20.5 hours per week with 41 appointment slots (30 minutes each), of which 7 appointments are delivered via clinic video telehealth and 34 appointments are traditional face-to-face visits.9 The remaining CPS time is assigned to other clinical care and administrative areas to fit facility need, including oversight of the CPS-run 24-hour ambulatory blood pressure clinic, postgraduate year 2 cardiology pharmacy practice residency program directorship, and other administrative activities for the facility.10
The cardiology CPSs practice under an advanced scope of practice in which they independently manage medications (initiate, modify, discontinue), order diagnostic testing (laboratory, monitoring, imaging, etc) needed for medication management, and create monitoring and treatment plans for patients referred to the cardiology pharmacotherapy clinic by other cardiology providers. The diseases managed within the clinic vary based on patient-specific needs, but may include HF, dyslipidemia, hypertension, anticoagulation, CAD, arrhythmias, cardiovascular risk factor assessment and reduction, and medication reconciliation and teaching. Patients are referred for CPS management directly from facility cardiologist and cardiology clinic PAs and APRNs. Workload and interventions carried out are captured in the Pharmacists Achieve Results with Medications Demonstration (PhARMD) tool and patient care encounter tracking.9
Data Collection
Using local data from workload tracking, the number of CPS encounters was determined from July 6, 2015, to October 1, 2015. Data were collected on the types and volume of interventions made by CPSs in the cardiology pharmacotherapy clinic using the PhARMD tool (Figure).
The PhARMD tool was initially developed and implemented for CPSs in primary care pharmacotherapy clinics and was used to evaluate the types and volume of CPS interventions made in this setting.11 Since this initial evaluation, the tool has been updated, standardized nationally by the Department of Veterans Affairs (VA) Pharmacy Benefits Management Clinical Pharmacy Practice Office, and integrated across numerous VAMCs and associated outpatient clinics. The tool remains embedded within the VA electronic health record (EHR) and allows the capture of specific CPS interventions of several types (ie, both pharmacologic and nonpharmacologic interventions, including adjust dose or frequency; change or discontinue medication; initiate medication; monitor medication; counsel on adherence, contraindications, drug interactions, and drugs not indicated; reconcile medication; and prevent or manage adverse drug events [ADEs]) specific to certain diseases, such as anemia, anticoagulation, HF, type 2 DM (T2DM), hypertension, dyslipidemia, and tobacco cessation.
Given that the interventions captured by the PhARMD tool are based on self-report of the CPS performing the intervention, a quality assurance (QA) measure was taken to audit a random sample of interventions to validate the accuracy of reported data. A Pharmacy Benefits Management PhARMD Project QA report provided the 20% random sample of encounters for each cardiology CPS to be reviewed. This percentage was determined by VAMC Clinical Pharmacy Program Office (CPPO) directives on implementation of the PhARMD tool. During the QA period, the provided sample was reviewed to determine whether the intervention(s) recorded with the PhARMD tool matched the actions documented in the EHR. The QA review was done through a manual chart review by an author not involved in recording the original interventions. Both WPB VAMC cardiology CPSs passed the QA review (> 80% concurrence with tool logged and chart documented interventions as required by VA CPPO directive), with a 90.9% concurrence between the EHR and PhARMD tool documentation.
Statistical Analyses
Data on intervention type and encounter number were evaluated with descriptive statistics. The information was characterized and diagrammed with Excel (Microsoft, Redmond, WA) charts and graphs.
Cost-avoidance calculations were done using previously described methods and are included for exploratory analysis.11,12 Briefly, published estimates of cost avoidance associated with various interventions from the outpatient setting within a VAMC setting were applied as appropriate to the various interventions captured with the PhARMD tool.11,12 These estimates from Lee and colleagues were derived from detailed chart review of interventions made and the potential harm prevented.12 Costs or cost avoidances associated with interventions were calculated from pooled examination of 600 interventions in a VAMC with drug costs before and after the intervention, costs associated with harms prevented by the intervention, as well as the VAMC hourly pharmacist wages associated with making an intervention and processing and filling original vs recommended therapies.
The costs presented represent a “best-case” scenario in which all interventions made are expected to prevent patient harms. The costs related to avoided outcomes, facility overhead, and auxiliary staff cannot be included but highlight the many considerations that must be considered when examining potential cost-avoidance calculations. The estimates and methods at hand were chosen because, to our knowledge, no other consensus model exists that would be more appropriate for use in the situation and health care system at hand. Cost-avoidance estimates were calculated by extrapolating the 88-day study period values to a yearly estimate. All cost estimates were adjusted for inflation using the consumer price index calculator as per convention in previous analyses using the cost-avoidance estimates at hand.11-13
Results
From July 6, 2015, through October 1, 2015, 301 patient encounters occurred, and 529 interventions were documented with the PhARMD tool. The mean number of interventions per encounter was 1.8. Interventions were 65.2% pharmacologic and 34.8% nonpharmacologic. Of pharmacologic interventions, 27.1% were for HF, 12.7% for hypertension, 8.8% for dyslipidemia, 2.8% for anticoagulation, 1.4% for tobacco cessation, 1.1% for T2DM, 0.3% for anemia, and 45.8% for other conditions (Table 1).
The main types of pharmacologic interventions across all diseases were related to adjustments in medication dose or frequency (42.3%) and change or discontinuation of medications (20.0%).
Discussion
Evaluation of the interventions and encounters at the WPB VAMC ambulatory cardiology pharmacotherapy clinic suggests that CPSs are able to contribute to direct patient care independently of interventions performed by other cardiology providers. Specifically, 1.8 interventions per encounter were made by CPSs in this study. In a prior evaluation of CPS interventions recorded with the PhARMD tool in a VAMC primary care setting, 2.3 interventions per encounter were recorded.11 In comparing the present volume of interventions with the volume recorded in the study by Hough and colleagues, the difference in practice setting may account for differences seen.11
The primary care medication management setting would capture a broader array of clinical interventions than would the ambulatory cardiology clinic of the present study, so it is reasonable that more interventions would be captured per encounter in the primary care clinic. The difference in practice settings affecting the character of collected interventions can be seen because most interventions in this study at an ambulatory cardiology clinic were related to HF, whereas in Hough and colleagues 39.2% of the disease-specific interventions were related to DM, and only 2.9% were related to HF.11 The differences inherent in the intervention populations can also be seen by comparing the percentage of interventions related to hypertension and dyslipidemia: 30% and 28% in the study by Hough and colleagues compared with 13% and 9%, respectively, in the present study.11
Comparison of the present evaluation and Hough and colleagues is also hindered by the PhARMD tool used. The PhARMD tool used in the initial evaluation has been modified on a national level to improve the granularity of intervention data collected.
Our cost-avoidance estimate of $433,324.06 per year seems lower than that estimated in the previous evaluation when all applicable interventions were included.11 However, this study had several differences compared with those of previous VAMC studies looking at clinical interventions performed by CPSs. The main differences are the volume and setting in which interventions were being made. For example, in comparison with Hough and colleagues, the studies include different practice settings (primary care vs cardiology specialty clinic) and number of FTEs involved in the study (4.65 vs 1). If the cost avoidance is distributed evenly per FTE in the previous study, the following calculation is observed: $649,551.99 per FTE, which is closer to this study’s estimation. Given that primary care is a broader setting than is ambulatory cardiology, it is not surprising that more types of interventions and the overall volume/absolute number of interventions would be higher. Thus, the lower estimated cost avoidance in our study may be attributed to the lower volume of intervention opportunities availed to the cardiology CPS. Another difference is that detailed types of interventions related to hypertension, DM, dyslipidemia, and HF were not included in Hough and colleagues, whereas our study included all applicable interventions regardless of relation to diseases, which may account for a degree of the variation in intervention breakdown between the 2 studies.11 However, as noted previously, some interventions for these particular diseases may not fully capture the rationale for pharmacotherapy interventions, such as drug dose changes or discontinuations, which may misrepresent the potential cost avoidance associated with them in reality.
Limitations
Of general importance, the PhARMD tool may underestimate the number of interventions made such that multiple interventions for a medical condition may have been completed but only captured as 1 intervention, which may represent a limitation of the tool when multiple interventions are made for the same disease (eg, titration of both β-blocker and angiotensin-converting enzyme inhibitor doses at a single appointment in a patient with HF with reduced left ventricular ejection fraction). Improved clarity about interventions made would require laborious chart review, which was not feasible. The evaluation at hand included a preliminary QA review, adding confidence that overdocumentation was not being done and the values represented at worst an underestimation of actual CPS intervention impact. Because this study was an initial evaluation of interventions made by CPSs in an ambulatory cardiology pharmacotherapy setting, whether these same outcomes would exist in other patient cohorts is unclear. However, these data do provide a foundational understanding of what may be expected from CPS integration into a cardiovascular care team.
These findings may be limited in generalizability to other health care systems and situations in which CPSs are afforded the regulatory opportunity to practice independently within an established scope of practice or collaborative practice agreements. The Veterans Health Administration system has been a leader in integrating CPSs into direct patient care roles and serves as a potential model for application by other groups. This evaluation’s data support continued efforts to create such independent practice environments as they allow for qualified CPSs to practice to their full clinical potential and have the fullest possible effect on cardiovascular outcomes.
Previous studies looking at cost savings in MTM programs have established a substantial return in economic investment with patients being managed by pharmacists.5,14 Given that the interventions made in this study were not tied to attainment of clinical outcomes, a limitation to our study, the cost-avoidance estimates should be interpreted cautiously. However, we know of no such tool that is available to allow accurate capture of clinical event reduction in a single center with consistent CPS involvement in care. A clear opportunity exists regarding design of a model that measures clinical, economic, and humanistic outcomes related to the interventions performed by cardiology CPSs, but developing and deploying such a model may be challenging because guideline-directed medical therapies vary significantly based on many patient-specific issues, and identifying optimal or truly optimized medical therapy is at times a subjective task, especially in a single center. Using the types and volumes of interventions made by CPSs as a surrogate for these higher-level outcomes is still of value in order to understand the effect and role of CPSs in cardiovascular care. At present, the cost-avoidance estimates presented in this evaluation are based on the most appropriate system-specific data at hand, with the realization that actual cost avoidance in practice may vary widely and should be the topic of future research.
Conclusion
As cardiovascular team-based care continues to expand with the support of large organizations, such as the American College of Cardiology Foundation, Heart Failure Society of America, and American College of Clinical Pharmacy Cardiology Practice and Research Network, the need for understanding the effect of CPSs on patient care measures and health care costs becomes more pronounced.2,15 The results of this study demonstrate how integration of CPSs in an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for cardiology physicians and providers, allowing more availability for diagnostic testing and care.
Interventions made by CPSs functioning as independent providers
1. Brush JE Jr, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65(19):2118-2136.
2. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66(19):2129-2139.
3. Sandoff BG, Kuca S, Rasmussen J, Merenich JA. Collaborative cardiac care service: a multidisciplinary approach to caring for patients with coronary artery disease. Perm J. 2008;12(3):4-11.
4. Merenich JA, Olson KL, Delate T, Rasmussen J, Helling DK, Ward DG; Clinical Pharmacy Cardiac Risk Service Study Group. Mortality reduction benefits of a comprehensive cardiac care program for patients with occlusive coronary disease. Pharmacotherapy. 2007;27(10):1370-1378.
5. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience.
6. Martinez AS, Saef J, Paszcuzuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
7. Roth GA, Poole JE, Zaha R, Zhou W, Skinner J, Morden NE. Use of guideline-directed medications for heart failure before cardioverter-defibrillator implantation. J Am Coll Cardiol. 2016;67(9):1062-1069.
8. Noschese LA, Bergman CL, Brar CK, Kansal MM. The pharmacist’s role in medication optimization for patients with chronic heart failure. Fed Pract. 2017;34(suppl 10):S10-S15.
9. Coakley C, Hough A, Dwyer D, Parra D. Clinical video telehealth in a cardiology pharmacotherapy clinic. Am J Health Syst Pharm. 2013;70(22):1974-1975.
10. Khazan E, Anastasia E, Hough A, Parra D. Pharmacist-managed ambulatory blood pressure monitoring service. Am J Health Syst Pharm. 2017;74(4):190-195.
11. Hough A, Vartan CM, Groppi JA, Reyes S, Beckey NP. Evaluation of clinical pharmacy interventions in a Veterans Affairs medical center primary care clinic. Am J Health Syst Pharm. 2013;70(13):1168-1172.
12. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077.
13. US Department of Labor. CPI inflation calculator. www.bls.gov/data/inflation_calculator.htm. Accessed January 18, 2019.
14. Perez A, Doloresco F, Hoffman JM, et al. Economic evaluations of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2008;29(1):128.
15. Milfred-LaForest SK, Chow SL, DiDomenico RJ, et al. Clinical pharmacy services in heart failure: an opinion paper from the Heart Failure Society of America and American College of Clinical Pharmacy Cardiology Practice and Research Network. Pharmacotherapy. 2013;33(5):529-548.
1. Brush JE Jr, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65(19):2118-2136.
2. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66(19):2129-2139.
3. Sandoff BG, Kuca S, Rasmussen J, Merenich JA. Collaborative cardiac care service: a multidisciplinary approach to caring for patients with coronary artery disease. Perm J. 2008;12(3):4-11.
4. Merenich JA, Olson KL, Delate T, Rasmussen J, Helling DK, Ward DG; Clinical Pharmacy Cardiac Risk Service Study Group. Mortality reduction benefits of a comprehensive cardiac care program for patients with occlusive coronary disease. Pharmacotherapy. 2007;27(10):1370-1378.
5. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience.
6. Martinez AS, Saef J, Paszcuzuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
7. Roth GA, Poole JE, Zaha R, Zhou W, Skinner J, Morden NE. Use of guideline-directed medications for heart failure before cardioverter-defibrillator implantation. J Am Coll Cardiol. 2016;67(9):1062-1069.
8. Noschese LA, Bergman CL, Brar CK, Kansal MM. The pharmacist’s role in medication optimization for patients with chronic heart failure. Fed Pract. 2017;34(suppl 10):S10-S15.
9. Coakley C, Hough A, Dwyer D, Parra D. Clinical video telehealth in a cardiology pharmacotherapy clinic. Am J Health Syst Pharm. 2013;70(22):1974-1975.
10. Khazan E, Anastasia E, Hough A, Parra D. Pharmacist-managed ambulatory blood pressure monitoring service. Am J Health Syst Pharm. 2017;74(4):190-195.
11. Hough A, Vartan CM, Groppi JA, Reyes S, Beckey NP. Evaluation of clinical pharmacy interventions in a Veterans Affairs medical center primary care clinic. Am J Health Syst Pharm. 2013;70(13):1168-1172.
12. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077.
13. US Department of Labor. CPI inflation calculator. www.bls.gov/data/inflation_calculator.htm. Accessed January 18, 2019.
14. Perez A, Doloresco F, Hoffman JM, et al. Economic evaluations of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2008;29(1):128.
15. Milfred-LaForest SK, Chow SL, DiDomenico RJ, et al. Clinical pharmacy services in heart failure: an opinion paper from the Heart Failure Society of America and American College of Clinical Pharmacy Cardiology Practice and Research Network. Pharmacotherapy. 2013;33(5):529-548.
The Best of 2018 Is Also the Worst
I am a doctor, not an engineer.Dr. McCoy, Star Trek “Mirror, Mirror” episode
Last year in my annual wrap-up, I wrote back-to-back editorials (December 2017 and January 2018) on the worst and best of 2017 from a federal health care perspective, emphasizing ethics or the lack thereof. I featured the altruism of federal health care providers (HCPs) responding to natural disasters and the terrible outcome of seemingly banal moral lapses.
This year the best and worst are one and the same, and I am not sure how it could be otherwise: the Department of Veterans Affairs (VA) and Department of Defense (DoD) electronic health record (EHR) contract with Cerner (North Kansas City, MO). Former VA Secretary David Shulkin, MD, announced the deal in 2017 shortly before his departure, and it was signed under then Acting VA Secretary Robert Wilkie in May of 2018.1 But the reason the Cerner contract is the most impactful and momentous ethical event of the year is perhaps not what readers expect. Search engines will efficiently unearth plentiful drama with ethical import about the contract. There were conspiracy charges that the shadow regime improperly engineered the selection.2 The usual Congressional hearings on the VA leadership mismanagement of the EHR culminated in Sen Jon Tester’s (D-MO) martial declaration in a letter to the newly sworn-in VA Chief Information Officer James Paul Gfrerer that “EHR modernization cannot fail.”3
While all this is obviously important, it is not why the annual awards for ethical and unethical behaviors are bestowed on what is essentially an information technology acquisition. The Cerner contract is chosen because of its enormous potential to change the human practice of health care for good or ill; hence, the dual nomination. This column is not about Cerner qua Cerner but about how the EHR has transformed—or deformed—the humanistic aspects of medical practice.
I am old enough to remember the original transition from paper charts to VistA EHR. As an intern with illegible handwriting, I can remember breathing a sigh of relief when the blue screen appeared for the first time. The commands were cumbersome and the code laborious, but it was a technologic marvel to see the clean, organized progress notes and be able to print your medication list or discharge summary. However, it also was the first stuttering waves of a tsunami that would alter medical practice forever. The human cost of the revolution could be seen almost immediately as older clinicians or those who could not type struggled to complete work that with paper and pen would have been easily accomplished.
For many years there was a steady stream of updates to VistA, including the Computerized Patient Record System (CPRS). For a relatively long time in technology terms, VistA and CPRS were the envy of the medical world, which rushed to catch up. Gradually though, VA fell behind; the wizard IT guys could not patch and fix new versions fast enough, and eventually, like all things created, VistA and CPRS became obsolete.4 Attitudes toward this microcosm of the modernization of an aging organization were intense and diverse. Some of us held onto CPRS as though it was a transitional object that we had personalized and became attached to with all its quirks and problems. Others could not wait to get rid of it, believing anything new and streamlined had to be better.
Yet the opposite also is true. EHRs have been, and could be again, incredible time-savers, enabling HCPs to deliver more evidence-based, patient-centered care in a more accurate, integrated, timely, and comprehensive manner. For example, Cerner finally could discover the Holy Grail of VA-DoD interoperability and even—dare we dream—integrate with the community. Yet as science fiction aficionados know, the machine designed to free humankind of drudgery may also end up controlling us.
The other commonplace year-end practice is for ersatz prophets to predict the future. I have no idea whether the Cerner EHR will be good or bad for VA and DoD. According to the insightful critic of medical culture, Atul Gawande, MD, who has examined the practitioner-computer interface, what we must guard against is that it does not replace the practitioner-patient relationship.5 The most common complaint I hear from patients in VA mental health care is: “They never listen to me, they just sit there typing.” Similarly, clinicians complain: “I spend all my time looking at a screen not at a patient.” As an ethicist, I cannot tell you how many times the blight of copy and paste has thwarted or damaged a patient’s care. And the direct correlation between medical computing and burnout has been well documented as all health care systems struggle with a doctor shortage particularly in primary care—arguably where computer fatigue hits hardest.6
What will decide whether EHR modernization will be a positive or negative development for VA and DoD patients? And is there anything we as federal HCPs can do to tip the scales in favor of the what is best for patients and clinicians? The most encouraging step has already been taken: VA and Cerner have set up EHR Councils composed of 60% practicing VA HCPs to provide the clinical perspective and 40% from VA Central Office to encourage synchronization of the top-down and bottom-up processes.7
Many experts have pointed out the inherent tension between how computers and human beings work, which I will simplify as the battle between the 3 S’s and the 3 F’s.5 The optimal operation of EHRs requires systems, structure, stability; to function successfully human beings need flexibility, freedom, and fragmentation. VistA had more than 100 versions according to a report from the Federal News Network (FNN), which is a striking example of the challenge EHR modernization faces in bridging the 2 orientations. As former VA Chief Information Officer Roger Baker told FNN, replacing this approach of EHR tinkering with a locked-down commercial system will require “a culture change that is orders of magnitude bigger than expected.”8
Think of the 2 domains as a Venn diagram. Where the circles overlap is all the things we and patients want and need in health care: empathic listening, strong enduring relationships, accurate diagnosis, accessibility, personalized treatment, continuity of care, mutual respect, patient safety, room to exercise professional judgment, and the data needed to promote shared decision making. Our contribution and duty are to make that inner circle where we all dwell together as wide and full as possible and the overlap between the 2 outer circles as seamless as human imperfection and artificial intelligence permit.
The Gawande article is titled “Why Doctors Hate Their Computers.” Of course, his piece shows that we also love them. None of the proposed liberations from our EHR domination—be they medical scribes or dictation programs—has solved the problem, probably because they are all technologic and just move the slavery downstream. We have come too far, and medicine is too complex, to go back to the age of paper. If we can no longer do the good work of healing and caring without computers, then we have to learn to live with them as our allies not our enemies. After all, even Dr. McCoy had a tricorder.
1. VA Office of Public and Intergovernmental Affairs. Statement by Acting Secretary Robert Wilkie—VA signs contract with Cerner for an electronic health record system. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4061. Published May 17, 2018. Accessed January 15, 2019.
2. Arnsdorf I. The VA shadow ruler’s signature program is “trending towards red.” https://www.propublica.org/article/va-shadow-rulers-program-is-trending-towards-red. Published November 1, 2018. Accessed January 15, 2019.
3. Murphy K. Senate committee says EHR modernization cannot be allowed to fail. https://ehrintelligence.com/news/senate-committee-says-ehr-modernization-cannot-be-allowed-to-fail. Published January 14, 2019. Accessed January 15, 2019.
4. US Department of Veterans Affairs. A history of the electronic health record. https://www.ehrm.va.gov/about/history. Updated September 28, 2018. Accessed January 16, 2019.
5. Gawande A. Why doctors hate their computers. https://www.newyorker.com/magazine/2018/11/12/why-doctors-hate-their-computers. Published November 12, 2018. Accessed January 16, 2019.
6. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care results from the MEMO study. J Am Med Inform Assoc. 2014;21(e1):100-106.
7. US Department of Veterans Affairs. EHRM councils. https://www.ehrm.va.gov/deployment/councils. Updated July 17, 2018. Accessed January 15, 2019.
8. Ogrysko N. In abandoning VistA, VA faces culture change that’s ‘orders of magnitude bigger’ than expected. https://federalnewsnetwork.com/veterans-affairs/2017/06/in-abandoning-vista-va-faces-culture-change-thats-orders-of-magnitude-bigger-than-expected. Published June 26, 2017. Accessed January 16, 2018.
I am a doctor, not an engineer.Dr. McCoy, Star Trek “Mirror, Mirror” episode
Last year in my annual wrap-up, I wrote back-to-back editorials (December 2017 and January 2018) on the worst and best of 2017 from a federal health care perspective, emphasizing ethics or the lack thereof. I featured the altruism of federal health care providers (HCPs) responding to natural disasters and the terrible outcome of seemingly banal moral lapses.
This year the best and worst are one and the same, and I am not sure how it could be otherwise: the Department of Veterans Affairs (VA) and Department of Defense (DoD) electronic health record (EHR) contract with Cerner (North Kansas City, MO). Former VA Secretary David Shulkin, MD, announced the deal in 2017 shortly before his departure, and it was signed under then Acting VA Secretary Robert Wilkie in May of 2018.1 But the reason the Cerner contract is the most impactful and momentous ethical event of the year is perhaps not what readers expect. Search engines will efficiently unearth plentiful drama with ethical import about the contract. There were conspiracy charges that the shadow regime improperly engineered the selection.2 The usual Congressional hearings on the VA leadership mismanagement of the EHR culminated in Sen Jon Tester’s (D-MO) martial declaration in a letter to the newly sworn-in VA Chief Information Officer James Paul Gfrerer that “EHR modernization cannot fail.”3
While all this is obviously important, it is not why the annual awards for ethical and unethical behaviors are bestowed on what is essentially an information technology acquisition. The Cerner contract is chosen because of its enormous potential to change the human practice of health care for good or ill; hence, the dual nomination. This column is not about Cerner qua Cerner but about how the EHR has transformed—or deformed—the humanistic aspects of medical practice.
I am old enough to remember the original transition from paper charts to VistA EHR. As an intern with illegible handwriting, I can remember breathing a sigh of relief when the blue screen appeared for the first time. The commands were cumbersome and the code laborious, but it was a technologic marvel to see the clean, organized progress notes and be able to print your medication list or discharge summary. However, it also was the first stuttering waves of a tsunami that would alter medical practice forever. The human cost of the revolution could be seen almost immediately as older clinicians or those who could not type struggled to complete work that with paper and pen would have been easily accomplished.
For many years there was a steady stream of updates to VistA, including the Computerized Patient Record System (CPRS). For a relatively long time in technology terms, VistA and CPRS were the envy of the medical world, which rushed to catch up. Gradually though, VA fell behind; the wizard IT guys could not patch and fix new versions fast enough, and eventually, like all things created, VistA and CPRS became obsolete.4 Attitudes toward this microcosm of the modernization of an aging organization were intense and diverse. Some of us held onto CPRS as though it was a transitional object that we had personalized and became attached to with all its quirks and problems. Others could not wait to get rid of it, believing anything new and streamlined had to be better.
Yet the opposite also is true. EHRs have been, and could be again, incredible time-savers, enabling HCPs to deliver more evidence-based, patient-centered care in a more accurate, integrated, timely, and comprehensive manner. For example, Cerner finally could discover the Holy Grail of VA-DoD interoperability and even—dare we dream—integrate with the community. Yet as science fiction aficionados know, the machine designed to free humankind of drudgery may also end up controlling us.
The other commonplace year-end practice is for ersatz prophets to predict the future. I have no idea whether the Cerner EHR will be good or bad for VA and DoD. According to the insightful critic of medical culture, Atul Gawande, MD, who has examined the practitioner-computer interface, what we must guard against is that it does not replace the practitioner-patient relationship.5 The most common complaint I hear from patients in VA mental health care is: “They never listen to me, they just sit there typing.” Similarly, clinicians complain: “I spend all my time looking at a screen not at a patient.” As an ethicist, I cannot tell you how many times the blight of copy and paste has thwarted or damaged a patient’s care. And the direct correlation between medical computing and burnout has been well documented as all health care systems struggle with a doctor shortage particularly in primary care—arguably where computer fatigue hits hardest.6
What will decide whether EHR modernization will be a positive or negative development for VA and DoD patients? And is there anything we as federal HCPs can do to tip the scales in favor of the what is best for patients and clinicians? The most encouraging step has already been taken: VA and Cerner have set up EHR Councils composed of 60% practicing VA HCPs to provide the clinical perspective and 40% from VA Central Office to encourage synchronization of the top-down and bottom-up processes.7
Many experts have pointed out the inherent tension between how computers and human beings work, which I will simplify as the battle between the 3 S’s and the 3 F’s.5 The optimal operation of EHRs requires systems, structure, stability; to function successfully human beings need flexibility, freedom, and fragmentation. VistA had more than 100 versions according to a report from the Federal News Network (FNN), which is a striking example of the challenge EHR modernization faces in bridging the 2 orientations. As former VA Chief Information Officer Roger Baker told FNN, replacing this approach of EHR tinkering with a locked-down commercial system will require “a culture change that is orders of magnitude bigger than expected.”8
Think of the 2 domains as a Venn diagram. Where the circles overlap is all the things we and patients want and need in health care: empathic listening, strong enduring relationships, accurate diagnosis, accessibility, personalized treatment, continuity of care, mutual respect, patient safety, room to exercise professional judgment, and the data needed to promote shared decision making. Our contribution and duty are to make that inner circle where we all dwell together as wide and full as possible and the overlap between the 2 outer circles as seamless as human imperfection and artificial intelligence permit.
The Gawande article is titled “Why Doctors Hate Their Computers.” Of course, his piece shows that we also love them. None of the proposed liberations from our EHR domination—be they medical scribes or dictation programs—has solved the problem, probably because they are all technologic and just move the slavery downstream. We have come too far, and medicine is too complex, to go back to the age of paper. If we can no longer do the good work of healing and caring without computers, then we have to learn to live with them as our allies not our enemies. After all, even Dr. McCoy had a tricorder.
I am a doctor, not an engineer.Dr. McCoy, Star Trek “Mirror, Mirror” episode
Last year in my annual wrap-up, I wrote back-to-back editorials (December 2017 and January 2018) on the worst and best of 2017 from a federal health care perspective, emphasizing ethics or the lack thereof. I featured the altruism of federal health care providers (HCPs) responding to natural disasters and the terrible outcome of seemingly banal moral lapses.
This year the best and worst are one and the same, and I am not sure how it could be otherwise: the Department of Veterans Affairs (VA) and Department of Defense (DoD) electronic health record (EHR) contract with Cerner (North Kansas City, MO). Former VA Secretary David Shulkin, MD, announced the deal in 2017 shortly before his departure, and it was signed under then Acting VA Secretary Robert Wilkie in May of 2018.1 But the reason the Cerner contract is the most impactful and momentous ethical event of the year is perhaps not what readers expect. Search engines will efficiently unearth plentiful drama with ethical import about the contract. There were conspiracy charges that the shadow regime improperly engineered the selection.2 The usual Congressional hearings on the VA leadership mismanagement of the EHR culminated in Sen Jon Tester’s (D-MO) martial declaration in a letter to the newly sworn-in VA Chief Information Officer James Paul Gfrerer that “EHR modernization cannot fail.”3
While all this is obviously important, it is not why the annual awards for ethical and unethical behaviors are bestowed on what is essentially an information technology acquisition. The Cerner contract is chosen because of its enormous potential to change the human practice of health care for good or ill; hence, the dual nomination. This column is not about Cerner qua Cerner but about how the EHR has transformed—or deformed—the humanistic aspects of medical practice.
I am old enough to remember the original transition from paper charts to VistA EHR. As an intern with illegible handwriting, I can remember breathing a sigh of relief when the blue screen appeared for the first time. The commands were cumbersome and the code laborious, but it was a technologic marvel to see the clean, organized progress notes and be able to print your medication list or discharge summary. However, it also was the first stuttering waves of a tsunami that would alter medical practice forever. The human cost of the revolution could be seen almost immediately as older clinicians or those who could not type struggled to complete work that with paper and pen would have been easily accomplished.
For many years there was a steady stream of updates to VistA, including the Computerized Patient Record System (CPRS). For a relatively long time in technology terms, VistA and CPRS were the envy of the medical world, which rushed to catch up. Gradually though, VA fell behind; the wizard IT guys could not patch and fix new versions fast enough, and eventually, like all things created, VistA and CPRS became obsolete.4 Attitudes toward this microcosm of the modernization of an aging organization were intense and diverse. Some of us held onto CPRS as though it was a transitional object that we had personalized and became attached to with all its quirks and problems. Others could not wait to get rid of it, believing anything new and streamlined had to be better.
Yet the opposite also is true. EHRs have been, and could be again, incredible time-savers, enabling HCPs to deliver more evidence-based, patient-centered care in a more accurate, integrated, timely, and comprehensive manner. For example, Cerner finally could discover the Holy Grail of VA-DoD interoperability and even—dare we dream—integrate with the community. Yet as science fiction aficionados know, the machine designed to free humankind of drudgery may also end up controlling us.
The other commonplace year-end practice is for ersatz prophets to predict the future. I have no idea whether the Cerner EHR will be good or bad for VA and DoD. According to the insightful critic of medical culture, Atul Gawande, MD, who has examined the practitioner-computer interface, what we must guard against is that it does not replace the practitioner-patient relationship.5 The most common complaint I hear from patients in VA mental health care is: “They never listen to me, they just sit there typing.” Similarly, clinicians complain: “I spend all my time looking at a screen not at a patient.” As an ethicist, I cannot tell you how many times the blight of copy and paste has thwarted or damaged a patient’s care. And the direct correlation between medical computing and burnout has been well documented as all health care systems struggle with a doctor shortage particularly in primary care—arguably where computer fatigue hits hardest.6
What will decide whether EHR modernization will be a positive or negative development for VA and DoD patients? And is there anything we as federal HCPs can do to tip the scales in favor of the what is best for patients and clinicians? The most encouraging step has already been taken: VA and Cerner have set up EHR Councils composed of 60% practicing VA HCPs to provide the clinical perspective and 40% from VA Central Office to encourage synchronization of the top-down and bottom-up processes.7
Many experts have pointed out the inherent tension between how computers and human beings work, which I will simplify as the battle between the 3 S’s and the 3 F’s.5 The optimal operation of EHRs requires systems, structure, stability; to function successfully human beings need flexibility, freedom, and fragmentation. VistA had more than 100 versions according to a report from the Federal News Network (FNN), which is a striking example of the challenge EHR modernization faces in bridging the 2 orientations. As former VA Chief Information Officer Roger Baker told FNN, replacing this approach of EHR tinkering with a locked-down commercial system will require “a culture change that is orders of magnitude bigger than expected.”8
Think of the 2 domains as a Venn diagram. Where the circles overlap is all the things we and patients want and need in health care: empathic listening, strong enduring relationships, accurate diagnosis, accessibility, personalized treatment, continuity of care, mutual respect, patient safety, room to exercise professional judgment, and the data needed to promote shared decision making. Our contribution and duty are to make that inner circle where we all dwell together as wide and full as possible and the overlap between the 2 outer circles as seamless as human imperfection and artificial intelligence permit.
The Gawande article is titled “Why Doctors Hate Their Computers.” Of course, his piece shows that we also love them. None of the proposed liberations from our EHR domination—be they medical scribes or dictation programs—has solved the problem, probably because they are all technologic and just move the slavery downstream. We have come too far, and medicine is too complex, to go back to the age of paper. If we can no longer do the good work of healing and caring without computers, then we have to learn to live with them as our allies not our enemies. After all, even Dr. McCoy had a tricorder.
1. VA Office of Public and Intergovernmental Affairs. Statement by Acting Secretary Robert Wilkie—VA signs contract with Cerner for an electronic health record system. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4061. Published May 17, 2018. Accessed January 15, 2019.
2. Arnsdorf I. The VA shadow ruler’s signature program is “trending towards red.” https://www.propublica.org/article/va-shadow-rulers-program-is-trending-towards-red. Published November 1, 2018. Accessed January 15, 2019.
3. Murphy K. Senate committee says EHR modernization cannot be allowed to fail. https://ehrintelligence.com/news/senate-committee-says-ehr-modernization-cannot-be-allowed-to-fail. Published January 14, 2019. Accessed January 15, 2019.
4. US Department of Veterans Affairs. A history of the electronic health record. https://www.ehrm.va.gov/about/history. Updated September 28, 2018. Accessed January 16, 2019.
5. Gawande A. Why doctors hate their computers. https://www.newyorker.com/magazine/2018/11/12/why-doctors-hate-their-computers. Published November 12, 2018. Accessed January 16, 2019.
6. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care results from the MEMO study. J Am Med Inform Assoc. 2014;21(e1):100-106.
7. US Department of Veterans Affairs. EHRM councils. https://www.ehrm.va.gov/deployment/councils. Updated July 17, 2018. Accessed January 15, 2019.
8. Ogrysko N. In abandoning VistA, VA faces culture change that’s ‘orders of magnitude bigger’ than expected. https://federalnewsnetwork.com/veterans-affairs/2017/06/in-abandoning-vista-va-faces-culture-change-thats-orders-of-magnitude-bigger-than-expected. Published June 26, 2017. Accessed January 16, 2018.
1. VA Office of Public and Intergovernmental Affairs. Statement by Acting Secretary Robert Wilkie—VA signs contract with Cerner for an electronic health record system. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4061. Published May 17, 2018. Accessed January 15, 2019.
2. Arnsdorf I. The VA shadow ruler’s signature program is “trending towards red.” https://www.propublica.org/article/va-shadow-rulers-program-is-trending-towards-red. Published November 1, 2018. Accessed January 15, 2019.
3. Murphy K. Senate committee says EHR modernization cannot be allowed to fail. https://ehrintelligence.com/news/senate-committee-says-ehr-modernization-cannot-be-allowed-to-fail. Published January 14, 2019. Accessed January 15, 2019.
4. US Department of Veterans Affairs. A history of the electronic health record. https://www.ehrm.va.gov/about/history. Updated September 28, 2018. Accessed January 16, 2019.
5. Gawande A. Why doctors hate their computers. https://www.newyorker.com/magazine/2018/11/12/why-doctors-hate-their-computers. Published November 12, 2018. Accessed January 16, 2019.
6. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care results from the MEMO study. J Am Med Inform Assoc. 2014;21(e1):100-106.
7. US Department of Veterans Affairs. EHRM councils. https://www.ehrm.va.gov/deployment/councils. Updated July 17, 2018. Accessed January 15, 2019.
8. Ogrysko N. In abandoning VistA, VA faces culture change that’s ‘orders of magnitude bigger’ than expected. https://federalnewsnetwork.com/veterans-affairs/2017/06/in-abandoning-vista-va-faces-culture-change-thats-orders-of-magnitude-bigger-than-expected. Published June 26, 2017. Accessed January 16, 2018.
Breast cancer recurrence lower, survival better with dose-intensified regimens
Dose-intense adjuvant chemotherapy is associated with significant if modest improvements in recurrence-free, breast cancer–specific, and overall survival among women with early breast cancer, results of a meta-analysis of data on individual patients showed.
Among more than 37,000 patients treated in 26 clinical trials with a median follow-up of 7.4 years, there was a 14% reduction in relative risk and 3.4% reduction in absolute 10-year risk of breast cancer recurrence for women who were treated either with accelerated-schedule or sequential chemotherapy, reported members of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG).
There were no differences in deaths from cardiovascular disease, acute myeloid leukemia, or other cancers between patients treated with dose-intense regimens or schedules and those treated with standard chemotherapy, although patients on dose-intense regimens had higher incidence of grade 3 or 4 anemia, and more did not complete the prescribed courses compared with standard chemotherapy, the investigators noted.
“The balance of benefit versus toxicity, therefore, appears to favor more dose-intense chemotherapy. A further advantage of 2-weekly versus 3-weekly chemotherapy – but not of sequential versus concurrent chemotherapy – is treatment is completed sooner,” they wrote in The Lancet.
The investigators examined individual patient data for 26 of 33 trials comparing either 2-weekly chemotherapy with 3-weekly therapy, or sequential vs. concurrent anthracycline and taxane-based chemotherapy.
The trials comprised a total cohort of 37,297 women randomized, most of whom were younger than 70 years at the time of diagnose and had node-positive disease.
The 10 year-risk for breast cancer recurrence, one of two primary endpoints, was 28% with dose intensification vs. 31.4% with standard dosing, translating into a first-event rate ratio (RR) for recurrence of 0.86 (P less than .0001).
Ten-year breast-cancer mortality, the other primary endpoint, was 18.9% among patients treated with dose-intensified regimens or schedules, compared with 21.3% for patients treated under standard protocols.
All-cause mortality was lower with dose intensification (22.1% vs. 24.8%, P less than .0001), and death without recurrence was also slightly but significantly lower (4.1% vs. 4.6%, respectively, P = .034).
The reductions in recurrence rates were similar among trials comparing 2-week vs. 3-week chemotherapy cycles, sequential vs. concurrent schedules, and both strategies together.
“The proportional reductions in recurrence with dose-intense chemotherapy were similar and highly significant [P less than .0001) in estrogen receptor (ER)-positive and ER-negative disease and did not differ significantly by other patient or tumor characteristics,” the investigators wrote.
“The present findings are of limited relevance to the question of which women with early breast cancer should be offered chemotherapy, although they do indicate that chemotherapy can reduce breast cancer mortality rates by 40% rather than a third. The absolute gain from this proportional reduction in recurrence depends chiefly on what the risk of distant recurrence would be without chemotherapy, which varies greatly from one woman to another, and is the subject of much ongoing research,” the investigators wrote.
“The findings are, however, directly relevant to selection of what regimen to use, and they show that, if chemotherapy is to be given, a dose-intense regimen should at least be considered,” they wrote.
The meta-analysis was funded by Cancer Research UK and the Medical Research Council. All authors reported having no relevant disclosures.
SOURCE: EBCTCG. The Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736(18)33137-4.
Although these results are meaningful, several limitations should be recognized as we translate these findings into practice. First, the benefits of dose intensification have not been established in the era of targeted therapy. Given that these studies enrolled women from 1985 to 2011, HER2 status was known for only 50% of tumors. Of those tested, 16% (n = 2,994) were HER2 positive. Use of trastuzumab was not reported but was probably uncommon since adjuvant trastuzumab was not approved until 2006. The remaining 18,625 patients did not have HER2 testing; thus, no HER2-directed therapy would have been given. Therefore, the majority of patients with HER2- positive breast cancer did not receive targeted therapy.
Although the authors report that women with HER2-positive and HER2-negative disease benefit similarly from dose intensification, it is impossible to know whether dose intensification benefits trastuzumab-treated patients or those who receive more than one HER2-targeted therapy (pertuzumab, neratinib, or trastuzumab-emtansine). Similarly, if other targeted therapies such as CDK4/6 inhibitors and PARP inhibitors show significant benefit in the curative setting for high-risk estrogen receptor (ER)-positive or BRCA-mutated breast cancer, prospective studies will be required to establish whether dose-intensive chemotherapy is better than standard chemotherapy in those settings.
Second, it is premature to conclude that patients older than 70 years or those with node-negative disease benefit from dose intensification, given the small number of patients in those groups and the fact that no significant benefit was observed for these patients. Moreover, gene-expression profiling was not used in these studies; thus, the benefit, if any, of a dose-intense approach for women with lymph-node-negative, high-risk, ER-positive disease is impossible to know. Finally, the use of dose intensification has not been studied in non-anthracycline, taxane-based regimens, which are being increasingly evaluated and used in women with node-negative, ER-positive disease.
With these caveats in mind, the results of this meta-analysis are undoubtedly clinically important. In modern practice, if anthracycline-based chemotherapy is warranted, these data provide convincing evidence that a dose-intense approach should be considered.
Sara A Hurvitz, MD, is from the David Geffen School of Medicine at UCLA, Santa Monica, Calif. Her remarks are excerpted from an editorial accompanying the study. She reports institutional research funding and fees for abstract and manuscript writing from several pharmaceutical companies outside of the submitted work, and travel reimbursement from Lilly outside of the submitted work.
Although these results are meaningful, several limitations should be recognized as we translate these findings into practice. First, the benefits of dose intensification have not been established in the era of targeted therapy. Given that these studies enrolled women from 1985 to 2011, HER2 status was known for only 50% of tumors. Of those tested, 16% (n = 2,994) were HER2 positive. Use of trastuzumab was not reported but was probably uncommon since adjuvant trastuzumab was not approved until 2006. The remaining 18,625 patients did not have HER2 testing; thus, no HER2-directed therapy would have been given. Therefore, the majority of patients with HER2- positive breast cancer did not receive targeted therapy.
Although the authors report that women with HER2-positive and HER2-negative disease benefit similarly from dose intensification, it is impossible to know whether dose intensification benefits trastuzumab-treated patients or those who receive more than one HER2-targeted therapy (pertuzumab, neratinib, or trastuzumab-emtansine). Similarly, if other targeted therapies such as CDK4/6 inhibitors and PARP inhibitors show significant benefit in the curative setting for high-risk estrogen receptor (ER)-positive or BRCA-mutated breast cancer, prospective studies will be required to establish whether dose-intensive chemotherapy is better than standard chemotherapy in those settings.
Second, it is premature to conclude that patients older than 70 years or those with node-negative disease benefit from dose intensification, given the small number of patients in those groups and the fact that no significant benefit was observed for these patients. Moreover, gene-expression profiling was not used in these studies; thus, the benefit, if any, of a dose-intense approach for women with lymph-node-negative, high-risk, ER-positive disease is impossible to know. Finally, the use of dose intensification has not been studied in non-anthracycline, taxane-based regimens, which are being increasingly evaluated and used in women with node-negative, ER-positive disease.
With these caveats in mind, the results of this meta-analysis are undoubtedly clinically important. In modern practice, if anthracycline-based chemotherapy is warranted, these data provide convincing evidence that a dose-intense approach should be considered.
Sara A Hurvitz, MD, is from the David Geffen School of Medicine at UCLA, Santa Monica, Calif. Her remarks are excerpted from an editorial accompanying the study. She reports institutional research funding and fees for abstract and manuscript writing from several pharmaceutical companies outside of the submitted work, and travel reimbursement from Lilly outside of the submitted work.
Although these results are meaningful, several limitations should be recognized as we translate these findings into practice. First, the benefits of dose intensification have not been established in the era of targeted therapy. Given that these studies enrolled women from 1985 to 2011, HER2 status was known for only 50% of tumors. Of those tested, 16% (n = 2,994) were HER2 positive. Use of trastuzumab was not reported but was probably uncommon since adjuvant trastuzumab was not approved until 2006. The remaining 18,625 patients did not have HER2 testing; thus, no HER2-directed therapy would have been given. Therefore, the majority of patients with HER2- positive breast cancer did not receive targeted therapy.
Although the authors report that women with HER2-positive and HER2-negative disease benefit similarly from dose intensification, it is impossible to know whether dose intensification benefits trastuzumab-treated patients or those who receive more than one HER2-targeted therapy (pertuzumab, neratinib, or trastuzumab-emtansine). Similarly, if other targeted therapies such as CDK4/6 inhibitors and PARP inhibitors show significant benefit in the curative setting for high-risk estrogen receptor (ER)-positive or BRCA-mutated breast cancer, prospective studies will be required to establish whether dose-intensive chemotherapy is better than standard chemotherapy in those settings.
Second, it is premature to conclude that patients older than 70 years or those with node-negative disease benefit from dose intensification, given the small number of patients in those groups and the fact that no significant benefit was observed for these patients. Moreover, gene-expression profiling was not used in these studies; thus, the benefit, if any, of a dose-intense approach for women with lymph-node-negative, high-risk, ER-positive disease is impossible to know. Finally, the use of dose intensification has not been studied in non-anthracycline, taxane-based regimens, which are being increasingly evaluated and used in women with node-negative, ER-positive disease.
With these caveats in mind, the results of this meta-analysis are undoubtedly clinically important. In modern practice, if anthracycline-based chemotherapy is warranted, these data provide convincing evidence that a dose-intense approach should be considered.
Sara A Hurvitz, MD, is from the David Geffen School of Medicine at UCLA, Santa Monica, Calif. Her remarks are excerpted from an editorial accompanying the study. She reports institutional research funding and fees for abstract and manuscript writing from several pharmaceutical companies outside of the submitted work, and travel reimbursement from Lilly outside of the submitted work.
Dose-intense adjuvant chemotherapy is associated with significant if modest improvements in recurrence-free, breast cancer–specific, and overall survival among women with early breast cancer, results of a meta-analysis of data on individual patients showed.
Among more than 37,000 patients treated in 26 clinical trials with a median follow-up of 7.4 years, there was a 14% reduction in relative risk and 3.4% reduction in absolute 10-year risk of breast cancer recurrence for women who were treated either with accelerated-schedule or sequential chemotherapy, reported members of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG).
There were no differences in deaths from cardiovascular disease, acute myeloid leukemia, or other cancers between patients treated with dose-intense regimens or schedules and those treated with standard chemotherapy, although patients on dose-intense regimens had higher incidence of grade 3 or 4 anemia, and more did not complete the prescribed courses compared with standard chemotherapy, the investigators noted.
“The balance of benefit versus toxicity, therefore, appears to favor more dose-intense chemotherapy. A further advantage of 2-weekly versus 3-weekly chemotherapy – but not of sequential versus concurrent chemotherapy – is treatment is completed sooner,” they wrote in The Lancet.
The investigators examined individual patient data for 26 of 33 trials comparing either 2-weekly chemotherapy with 3-weekly therapy, or sequential vs. concurrent anthracycline and taxane-based chemotherapy.
The trials comprised a total cohort of 37,297 women randomized, most of whom were younger than 70 years at the time of diagnose and had node-positive disease.
The 10 year-risk for breast cancer recurrence, one of two primary endpoints, was 28% with dose intensification vs. 31.4% with standard dosing, translating into a first-event rate ratio (RR) for recurrence of 0.86 (P less than .0001).
Ten-year breast-cancer mortality, the other primary endpoint, was 18.9% among patients treated with dose-intensified regimens or schedules, compared with 21.3% for patients treated under standard protocols.
All-cause mortality was lower with dose intensification (22.1% vs. 24.8%, P less than .0001), and death without recurrence was also slightly but significantly lower (4.1% vs. 4.6%, respectively, P = .034).
The reductions in recurrence rates were similar among trials comparing 2-week vs. 3-week chemotherapy cycles, sequential vs. concurrent schedules, and both strategies together.
“The proportional reductions in recurrence with dose-intense chemotherapy were similar and highly significant [P less than .0001) in estrogen receptor (ER)-positive and ER-negative disease and did not differ significantly by other patient or tumor characteristics,” the investigators wrote.
“The present findings are of limited relevance to the question of which women with early breast cancer should be offered chemotherapy, although they do indicate that chemotherapy can reduce breast cancer mortality rates by 40% rather than a third. The absolute gain from this proportional reduction in recurrence depends chiefly on what the risk of distant recurrence would be without chemotherapy, which varies greatly from one woman to another, and is the subject of much ongoing research,” the investigators wrote.
“The findings are, however, directly relevant to selection of what regimen to use, and they show that, if chemotherapy is to be given, a dose-intense regimen should at least be considered,” they wrote.
The meta-analysis was funded by Cancer Research UK and the Medical Research Council. All authors reported having no relevant disclosures.
SOURCE: EBCTCG. The Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736(18)33137-4.
Dose-intense adjuvant chemotherapy is associated with significant if modest improvements in recurrence-free, breast cancer–specific, and overall survival among women with early breast cancer, results of a meta-analysis of data on individual patients showed.
Among more than 37,000 patients treated in 26 clinical trials with a median follow-up of 7.4 years, there was a 14% reduction in relative risk and 3.4% reduction in absolute 10-year risk of breast cancer recurrence for women who were treated either with accelerated-schedule or sequential chemotherapy, reported members of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG).
There were no differences in deaths from cardiovascular disease, acute myeloid leukemia, or other cancers between patients treated with dose-intense regimens or schedules and those treated with standard chemotherapy, although patients on dose-intense regimens had higher incidence of grade 3 or 4 anemia, and more did not complete the prescribed courses compared with standard chemotherapy, the investigators noted.
“The balance of benefit versus toxicity, therefore, appears to favor more dose-intense chemotherapy. A further advantage of 2-weekly versus 3-weekly chemotherapy – but not of sequential versus concurrent chemotherapy – is treatment is completed sooner,” they wrote in The Lancet.
The investigators examined individual patient data for 26 of 33 trials comparing either 2-weekly chemotherapy with 3-weekly therapy, or sequential vs. concurrent anthracycline and taxane-based chemotherapy.
The trials comprised a total cohort of 37,297 women randomized, most of whom were younger than 70 years at the time of diagnose and had node-positive disease.
The 10 year-risk for breast cancer recurrence, one of two primary endpoints, was 28% with dose intensification vs. 31.4% with standard dosing, translating into a first-event rate ratio (RR) for recurrence of 0.86 (P less than .0001).
Ten-year breast-cancer mortality, the other primary endpoint, was 18.9% among patients treated with dose-intensified regimens or schedules, compared with 21.3% for patients treated under standard protocols.
All-cause mortality was lower with dose intensification (22.1% vs. 24.8%, P less than .0001), and death without recurrence was also slightly but significantly lower (4.1% vs. 4.6%, respectively, P = .034).
The reductions in recurrence rates were similar among trials comparing 2-week vs. 3-week chemotherapy cycles, sequential vs. concurrent schedules, and both strategies together.
“The proportional reductions in recurrence with dose-intense chemotherapy were similar and highly significant [P less than .0001) in estrogen receptor (ER)-positive and ER-negative disease and did not differ significantly by other patient or tumor characteristics,” the investigators wrote.
“The present findings are of limited relevance to the question of which women with early breast cancer should be offered chemotherapy, although they do indicate that chemotherapy can reduce breast cancer mortality rates by 40% rather than a third. The absolute gain from this proportional reduction in recurrence depends chiefly on what the risk of distant recurrence would be without chemotherapy, which varies greatly from one woman to another, and is the subject of much ongoing research,” the investigators wrote.
“The findings are, however, directly relevant to selection of what regimen to use, and they show that, if chemotherapy is to be given, a dose-intense regimen should at least be considered,” they wrote.
The meta-analysis was funded by Cancer Research UK and the Medical Research Council. All authors reported having no relevant disclosures.
SOURCE: EBCTCG. The Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736(18)33137-4.
FROM THE LANCET
Key clinical point: Consider dose-intensification or sequential therapy for patients undergoing chemotherapy.
Major finding: Ten-year recurrence rates were 28% with dose intensification vs. 31.4% for standard dosing.
Study details: Meta-analysis of individual data on 37,298 women enrolled in 26 randomized trials.
Disclosures: The meta-analysis was funded by Cancer Research UK and the Medical Research Council. All authors reported having no relevant disclosures.
Source: EBCTCG. The Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736(18)33137-4.
Terminating an employee
I’ve written more than once about the private practitioner’s least favorite task. Now, new federal worker protection laws are making terminations even more difficult, even when they’re justified; however, that’s still no excuse for keeping an employee that should be replaced.
Once you make the decision to replace an employee, be sure that you have legitimate grounds and assemble as much documentation as you can. Record all terminable transgressions in the employee’s permanent record and document all verbal and written warnings. This is essential; you must be prepared to prove that your reasons for termination were legal.
Former employees will sometimes charge that any of a number of their civil rights were violated. For example, federal law prohibits you from firing anyone because of race, gender, national origin, disability, religion, or age – if the employee is over 40. You cannot fire a woman because she is pregnant or recently gave birth. Other illegal reasons include assertion of antidiscrimination rights, refusing to take a lie detector test, and reporting Occupational Safety and Health Administration violations.
You also can’t terminate someone for refusing to commit an illegal act – such as filing false insurance claims – or for exercising a legal right – such as voting or participating in a political demonstration.
While you cannot fire an alcohol abuser unless he or she is caught drinking at work, many forms of illegal drug use are legitimate causes for termination. Other laws may apply, depending on where you live. When in doubt, contact your attorney, state labor department, or fair employment office.
If a fired employee alleges that he or she was fired for any of these illegal reasons and you do not have convincing documentation to counter the charge, you may find yourself defending your actions in court. If you anticipate such problems, you can ask the employee to sign a waiver of future litigation in exchange for a concession from you – such as extra severance pay or a promise not to contest an unemployment application. Also, consider adding employment practices liability insurance – which I covered in detail a few months ago – to your umbrella policy, since lawsuits are always a possibility, despite all efforts to prevent them.
Once you have all your legal ducks in a row, don’t procrastinate. Get it over with first thing on Monday morning. If you wait until Friday afternoon, you will worry about the dreaded task all week long, and the fired employee will stew about it all weekend. Ask your manager or another trusted employee to be present to reduce the risk of subsequent disputes over what was discussed.
I’ve been asked to share exactly what I say; so for what it’s worth, here it is: “We have called you in to discuss a difficult issue. You know that we have not been happy with your performance. We are still not happy with it, despite all the discussions we have had, and we feel that you can do better elsewhere. So today we will part company, and I wish you the best of luck in your future endeavors. Here is your severance check. I hope there are no hard feelings.”
There will, of course, be hard feelings, despite all your “hopes,” but that cannot be helped. The point is to be quick, firm, and decisive. Get it over with and allow everyone to move on. Make it clear, when necessary, that the decision has already been made, so arguing or pleading will change nothing.
Be sure to get all your office keys back – or change the locks if you cannot. Back up all important computer files and change all your passwords. Most employees know more of them than you would ever suspect.
Finally, call the staff together and explain everything. They should hear it from you, not some distorted version via the rumor mill. You don’t have to divulge every detail, but do explain how the termination will affect everyone else. Responsibilities will need to be shifted until a replacement can be hired, and all employees should understand that.
If you are asked in the future to give a reference or write a letter of recommendation for the terminated employee, be sure that everything you say is truthful and well documented.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I’ve written more than once about the private practitioner’s least favorite task. Now, new federal worker protection laws are making terminations even more difficult, even when they’re justified; however, that’s still no excuse for keeping an employee that should be replaced.
Once you make the decision to replace an employee, be sure that you have legitimate grounds and assemble as much documentation as you can. Record all terminable transgressions in the employee’s permanent record and document all verbal and written warnings. This is essential; you must be prepared to prove that your reasons for termination were legal.
Former employees will sometimes charge that any of a number of their civil rights were violated. For example, federal law prohibits you from firing anyone because of race, gender, national origin, disability, religion, or age – if the employee is over 40. You cannot fire a woman because she is pregnant or recently gave birth. Other illegal reasons include assertion of antidiscrimination rights, refusing to take a lie detector test, and reporting Occupational Safety and Health Administration violations.
You also can’t terminate someone for refusing to commit an illegal act – such as filing false insurance claims – or for exercising a legal right – such as voting or participating in a political demonstration.
While you cannot fire an alcohol abuser unless he or she is caught drinking at work, many forms of illegal drug use are legitimate causes for termination. Other laws may apply, depending on where you live. When in doubt, contact your attorney, state labor department, or fair employment office.
If a fired employee alleges that he or she was fired for any of these illegal reasons and you do not have convincing documentation to counter the charge, you may find yourself defending your actions in court. If you anticipate such problems, you can ask the employee to sign a waiver of future litigation in exchange for a concession from you – such as extra severance pay or a promise not to contest an unemployment application. Also, consider adding employment practices liability insurance – which I covered in detail a few months ago – to your umbrella policy, since lawsuits are always a possibility, despite all efforts to prevent them.
Once you have all your legal ducks in a row, don’t procrastinate. Get it over with first thing on Monday morning. If you wait until Friday afternoon, you will worry about the dreaded task all week long, and the fired employee will stew about it all weekend. Ask your manager or another trusted employee to be present to reduce the risk of subsequent disputes over what was discussed.
I’ve been asked to share exactly what I say; so for what it’s worth, here it is: “We have called you in to discuss a difficult issue. You know that we have not been happy with your performance. We are still not happy with it, despite all the discussions we have had, and we feel that you can do better elsewhere. So today we will part company, and I wish you the best of luck in your future endeavors. Here is your severance check. I hope there are no hard feelings.”
There will, of course, be hard feelings, despite all your “hopes,” but that cannot be helped. The point is to be quick, firm, and decisive. Get it over with and allow everyone to move on. Make it clear, when necessary, that the decision has already been made, so arguing or pleading will change nothing.
Be sure to get all your office keys back – or change the locks if you cannot. Back up all important computer files and change all your passwords. Most employees know more of them than you would ever suspect.
Finally, call the staff together and explain everything. They should hear it from you, not some distorted version via the rumor mill. You don’t have to divulge every detail, but do explain how the termination will affect everyone else. Responsibilities will need to be shifted until a replacement can be hired, and all employees should understand that.
If you are asked in the future to give a reference or write a letter of recommendation for the terminated employee, be sure that everything you say is truthful and well documented.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I’ve written more than once about the private practitioner’s least favorite task. Now, new federal worker protection laws are making terminations even more difficult, even when they’re justified; however, that’s still no excuse for keeping an employee that should be replaced.
Once you make the decision to replace an employee, be sure that you have legitimate grounds and assemble as much documentation as you can. Record all terminable transgressions in the employee’s permanent record and document all verbal and written warnings. This is essential; you must be prepared to prove that your reasons for termination were legal.
Former employees will sometimes charge that any of a number of their civil rights were violated. For example, federal law prohibits you from firing anyone because of race, gender, national origin, disability, religion, or age – if the employee is over 40. You cannot fire a woman because she is pregnant or recently gave birth. Other illegal reasons include assertion of antidiscrimination rights, refusing to take a lie detector test, and reporting Occupational Safety and Health Administration violations.
You also can’t terminate someone for refusing to commit an illegal act – such as filing false insurance claims – or for exercising a legal right – such as voting or participating in a political demonstration.
While you cannot fire an alcohol abuser unless he or she is caught drinking at work, many forms of illegal drug use are legitimate causes for termination. Other laws may apply, depending on where you live. When in doubt, contact your attorney, state labor department, or fair employment office.
If a fired employee alleges that he or she was fired for any of these illegal reasons and you do not have convincing documentation to counter the charge, you may find yourself defending your actions in court. If you anticipate such problems, you can ask the employee to sign a waiver of future litigation in exchange for a concession from you – such as extra severance pay or a promise not to contest an unemployment application. Also, consider adding employment practices liability insurance – which I covered in detail a few months ago – to your umbrella policy, since lawsuits are always a possibility, despite all efforts to prevent them.
Once you have all your legal ducks in a row, don’t procrastinate. Get it over with first thing on Monday morning. If you wait until Friday afternoon, you will worry about the dreaded task all week long, and the fired employee will stew about it all weekend. Ask your manager or another trusted employee to be present to reduce the risk of subsequent disputes over what was discussed.
I’ve been asked to share exactly what I say; so for what it’s worth, here it is: “We have called you in to discuss a difficult issue. You know that we have not been happy with your performance. We are still not happy with it, despite all the discussions we have had, and we feel that you can do better elsewhere. So today we will part company, and I wish you the best of luck in your future endeavors. Here is your severance check. I hope there are no hard feelings.”
There will, of course, be hard feelings, despite all your “hopes,” but that cannot be helped. The point is to be quick, firm, and decisive. Get it over with and allow everyone to move on. Make it clear, when necessary, that the decision has already been made, so arguing or pleading will change nothing.
Be sure to get all your office keys back – or change the locks if you cannot. Back up all important computer files and change all your passwords. Most employees know more of them than you would ever suspect.
Finally, call the staff together and explain everything. They should hear it from you, not some distorted version via the rumor mill. You don’t have to divulge every detail, but do explain how the termination will affect everyone else. Responsibilities will need to be shifted until a replacement can be hired, and all employees should understand that.
If you are asked in the future to give a reference or write a letter of recommendation for the terminated employee, be sure that everything you say is truthful and well documented.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Exercise type matters for fall prevention among elderly
according to a Cochrane Review meta-analysis of 108 randomized controlled trials.
Exercise has been shown to prevent falls in older people, but given the potential consequences, the investigators thought an up-to-date synthesis of the evidence was in order. The analysis focused on people living independently who had not recently been discharged from a hospital. The trials involved 23,407 subjects from 25 countries. The review was exhaustive; the final report is almost 600 pages long (Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2).
The type of exercise matters. The researchers cited “high-certainty evidence” that exercise involving balance and functional training reduces falls. “Tai chi may also prevent falls,” they noted, adding that they were uncertain of the effect of dance, walking, and resistance training by itself. There was no evidence to determine the effects of flexibility or endurance exercises, added the researchers, led by Cathie Sherrington, PhD, of the University of Sydney Institute for Musculoskeletal Health.
Functional exercise mimics everyday movement, with the goal of improving performance. Multidirectional lunges are an example, helping the body prepare for vacuuming, yard work, and other common activities.
“Exercise [programs] carried out in group classes or done at home prescribed by a health professional ... or a trained exercise leader were effective. Exercises were mostly done while standing as this better enhances balance and the ability to do daily activities such as standing up from a low chair or climbing stairs,” according to a Cochrane press release regarding the study.
Overall, exercise reduced the number of falls by 23%, and the number of fallers by 15%, with high-certainty evidence.
Exercise also brought down the number of people facing fall fractures by over 27%, the number of people requiring medical attention for a fall by 39%, and the number ending up in the hospital for a fall by 22%.
Balance and functional exercises reduced the rate of falls by 24%, and the number of fallers by 13%. The effects were even greater when resistance exercises were added to the mix; drops in fall rates and the number of people experiencing falls were 34% and 22%, respectively. There was low-certainty evidence that tai chi reduces the rate of falls by 19% and the number of people experiencing falls by 20%.
Despite fall prevention, “exercise may make little important difference to health-related quality of life;” when results were converted to EQ-5D and 36-Item Short Form Survey scores, “the respective 95% [confidence intervals] were much smaller than minimally important differences,” the investigators said.
Serious adverse events occurred in participants in one of the 27 trials that reported adverse events. These two serious adverse events were a pelvic stress fracture and an inguinal hernia surgery. Most of the other adverse events reported, all non-serious, were musculoskeletal.
On average, participants were 76 years old, and 77% were women. Disease specific trials – such as exercise for stroke rehabilitation – were excluded.
The work was supported primarily by the Cochrane Bone, Joint and Muscle Trauma Group, based at the University of Manchester, England, and Cochrane’s Acute and Emergency Care Network. There were no industry disclosures.
SOURCE: Sherrington C et al. Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2.
according to a Cochrane Review meta-analysis of 108 randomized controlled trials.
Exercise has been shown to prevent falls in older people, but given the potential consequences, the investigators thought an up-to-date synthesis of the evidence was in order. The analysis focused on people living independently who had not recently been discharged from a hospital. The trials involved 23,407 subjects from 25 countries. The review was exhaustive; the final report is almost 600 pages long (Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2).
The type of exercise matters. The researchers cited “high-certainty evidence” that exercise involving balance and functional training reduces falls. “Tai chi may also prevent falls,” they noted, adding that they were uncertain of the effect of dance, walking, and resistance training by itself. There was no evidence to determine the effects of flexibility or endurance exercises, added the researchers, led by Cathie Sherrington, PhD, of the University of Sydney Institute for Musculoskeletal Health.
Functional exercise mimics everyday movement, with the goal of improving performance. Multidirectional lunges are an example, helping the body prepare for vacuuming, yard work, and other common activities.
“Exercise [programs] carried out in group classes or done at home prescribed by a health professional ... or a trained exercise leader were effective. Exercises were mostly done while standing as this better enhances balance and the ability to do daily activities such as standing up from a low chair or climbing stairs,” according to a Cochrane press release regarding the study.
Overall, exercise reduced the number of falls by 23%, and the number of fallers by 15%, with high-certainty evidence.
Exercise also brought down the number of people facing fall fractures by over 27%, the number of people requiring medical attention for a fall by 39%, and the number ending up in the hospital for a fall by 22%.
Balance and functional exercises reduced the rate of falls by 24%, and the number of fallers by 13%. The effects were even greater when resistance exercises were added to the mix; drops in fall rates and the number of people experiencing falls were 34% and 22%, respectively. There was low-certainty evidence that tai chi reduces the rate of falls by 19% and the number of people experiencing falls by 20%.
Despite fall prevention, “exercise may make little important difference to health-related quality of life;” when results were converted to EQ-5D and 36-Item Short Form Survey scores, “the respective 95% [confidence intervals] were much smaller than minimally important differences,” the investigators said.
Serious adverse events occurred in participants in one of the 27 trials that reported adverse events. These two serious adverse events were a pelvic stress fracture and an inguinal hernia surgery. Most of the other adverse events reported, all non-serious, were musculoskeletal.
On average, participants were 76 years old, and 77% were women. Disease specific trials – such as exercise for stroke rehabilitation – were excluded.
The work was supported primarily by the Cochrane Bone, Joint and Muscle Trauma Group, based at the University of Manchester, England, and Cochrane’s Acute and Emergency Care Network. There were no industry disclosures.
SOURCE: Sherrington C et al. Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2.
according to a Cochrane Review meta-analysis of 108 randomized controlled trials.
Exercise has been shown to prevent falls in older people, but given the potential consequences, the investigators thought an up-to-date synthesis of the evidence was in order. The analysis focused on people living independently who had not recently been discharged from a hospital. The trials involved 23,407 subjects from 25 countries. The review was exhaustive; the final report is almost 600 pages long (Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2).
The type of exercise matters. The researchers cited “high-certainty evidence” that exercise involving balance and functional training reduces falls. “Tai chi may also prevent falls,” they noted, adding that they were uncertain of the effect of dance, walking, and resistance training by itself. There was no evidence to determine the effects of flexibility or endurance exercises, added the researchers, led by Cathie Sherrington, PhD, of the University of Sydney Institute for Musculoskeletal Health.
Functional exercise mimics everyday movement, with the goal of improving performance. Multidirectional lunges are an example, helping the body prepare for vacuuming, yard work, and other common activities.
“Exercise [programs] carried out in group classes or done at home prescribed by a health professional ... or a trained exercise leader were effective. Exercises were mostly done while standing as this better enhances balance and the ability to do daily activities such as standing up from a low chair or climbing stairs,” according to a Cochrane press release regarding the study.
Overall, exercise reduced the number of falls by 23%, and the number of fallers by 15%, with high-certainty evidence.
Exercise also brought down the number of people facing fall fractures by over 27%, the number of people requiring medical attention for a fall by 39%, and the number ending up in the hospital for a fall by 22%.
Balance and functional exercises reduced the rate of falls by 24%, and the number of fallers by 13%. The effects were even greater when resistance exercises were added to the mix; drops in fall rates and the number of people experiencing falls were 34% and 22%, respectively. There was low-certainty evidence that tai chi reduces the rate of falls by 19% and the number of people experiencing falls by 20%.
Despite fall prevention, “exercise may make little important difference to health-related quality of life;” when results were converted to EQ-5D and 36-Item Short Form Survey scores, “the respective 95% [confidence intervals] were much smaller than minimally important differences,” the investigators said.
Serious adverse events occurred in participants in one of the 27 trials that reported adverse events. These two serious adverse events were a pelvic stress fracture and an inguinal hernia surgery. Most of the other adverse events reported, all non-serious, were musculoskeletal.
On average, participants were 76 years old, and 77% were women. Disease specific trials – such as exercise for stroke rehabilitation – were excluded.
The work was supported primarily by the Cochrane Bone, Joint and Muscle Trauma Group, based at the University of Manchester, England, and Cochrane’s Acute and Emergency Care Network. There were no industry disclosures.
SOURCE: Sherrington C et al. Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2.
FROM COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Key clinical point: Exercise helps elderly people avoid falls, especially if it focuses on balance and mimics daily activities.
Major finding: Balance and functional exercises reduce the rate of falls by 24%, and the number of fallers by 13%. The effects were even greater when resistance exercises were added to the mix; drops in fall rates and the number of people experiencing falls were 34% and 22%, respectively.
Study details: A meta-analysis of 108 randomized, controlled trials.
Disclosures: The work was supported by Cochrane. There were no industry disclosures.
Source: Sherrington C et al. Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2.
She Won’t Quit—But Will Her Heart?
ANSWER
The correct interpretation is sinus rhythm with a first-degree atrioventricular (AV) block and possible left atrial enlargement. Criteria for sinus rhythm include a P wave for every QRS complex and a QRS complex for every P wave with a consistent PR interval.
Criteria for a first-degree AV block include a consistent PR interval > 200 ms in all leads. Criteria for left atrial enlargement include a P-wave duration > 120 ms in lead II or a downward deflection of the P wave in lead V1 > 40 ms in length with > 1-mm negative deflection.
The P wave in leads II and V1, along with a murmur, is consistent with mitral regurgitation and a history of palpitations and paroxysmal atrial fibrillation. Left atrial enlargement should be considered. An echocardiogram was ordered and confirmed the diagnosis of left atrial enlargement in this patient.
ANSWER
The correct interpretation is sinus rhythm with a first-degree atrioventricular (AV) block and possible left atrial enlargement. Criteria for sinus rhythm include a P wave for every QRS complex and a QRS complex for every P wave with a consistent PR interval.
Criteria for a first-degree AV block include a consistent PR interval > 200 ms in all leads. Criteria for left atrial enlargement include a P-wave duration > 120 ms in lead II or a downward deflection of the P wave in lead V1 > 40 ms in length with > 1-mm negative deflection.
The P wave in leads II and V1, along with a murmur, is consistent with mitral regurgitation and a history of palpitations and paroxysmal atrial fibrillation. Left atrial enlargement should be considered. An echocardiogram was ordered and confirmed the diagnosis of left atrial enlargement in this patient.
ANSWER
The correct interpretation is sinus rhythm with a first-degree atrioventricular (AV) block and possible left atrial enlargement. Criteria for sinus rhythm include a P wave for every QRS complex and a QRS complex for every P wave with a consistent PR interval.
Criteria for a first-degree AV block include a consistent PR interval > 200 ms in all leads. Criteria for left atrial enlargement include a P-wave duration > 120 ms in lead II or a downward deflection of the P wave in lead V1 > 40 ms in length with > 1-mm negative deflection.
The P wave in leads II and V1, along with a murmur, is consistent with mitral regurgitation and a history of palpitations and paroxysmal atrial fibrillation. Left atrial enlargement should be considered. An echocardiogram was ordered and confirmed the diagnosis of left atrial enlargement in this patient.
A 58-year-old woman presents for preoperative workup for surgical repair of a distal left tibial fracture sustained while snowshoeing. She had been descending a side slope when she lost her footing. Her left snowshoe became entangled in a large granite rock, which defined the lateral wall of the path she was traversing. She has no prior orthopedic injuries.
Cardiac history is remarkable for hypertension, palpitations, and two episodes of paroxysmal atrial fibrillation. A Holter monitor, worn to help determine the etiology of her palpitations, captured the atrial fibrillation episodes, each of which was cardioverted within 48 hours of onset without complication. Following the second cardioversion, about 6 months ago, a novel oral anticoagulation agent was recommended, but she refused to take it because she felt such medication would interfere with her active lifestyle.
The patient is otherwise quite healthy and has been personally active in her preventive health maintenance.
Her current medications include lisinopril (5 mg/d) and aspirin (81 mg/d). She also takes a multivitamin daily. In the past 24 hours, she has taken two doses of acetaminophen/oxycodone (325/5 mg) for left ankle pain. She has no drug allergies.
The patient, an immigration attorney for a prominent law firm, is divorced and has no children. She works as a Zumba instructor on the weekends and has run 3 marathons within the past 2 years. She has never smoked or used recreational drugs, but she does partake in one or two glasses of wine with friends on weekends.
Family history is remarkable for hypertension in both parents and two of her three siblings. All are alive and otherwise healthy.
Review of systems reveals no current problems. She states she went through menopause about 10 years ago and was recommended to start estrogen therapy but refused this treatment.
Vital signs include a blood pressure of 118/88 mm Hg; pulse, 90 beats/min; temperature, 98.4°F; and O2 saturation, 98% on room air. Her weight is 129 lb, and her height, 64 in.
Physical exam reveals a healthy, athletic woman in no distress. She wears an orthopedic boot on her left foot, but it isn’t removed to examine the affected ankle. She wears contact lenses and has a posterior lingual brace on her lower teeth.
The HEENT exam is normal. The neck is supple without masses. There is no thyromegaly, carotid bruits, or jugular venous distention. The lungs are clear in all fields. The breasts are symmetrical without palpable nodules.
Cardiac exam is remarkable for a regular rate and rhythm at 90 beats/min. There is a soft end-systolic murmur consistent with mild mitral regurgitation. S1 and S2 are of normal intensity, and there are no extra heart sounds.
The abdomen is nontender with no organomegaly; the patient proudly shows her core strength and “a hint of a six-pack.” The genitourinary exam is deferred. Peripheral pulses are strong and equal bilaterally. The neurologic exam is grossly intact.
A preoperative chest x-ray is performed; results are pending.
The ECG shows a ventricular rate of 89 beats/min; PR interval, 232 ms; QRS duration, 82 ms; QT/QTc interval, 364/442 ms; P axis, 86°; R axis, 23°
Pregnant women want genome guidance
Also today, a look at asthma, obesity, and the risk for severe sleep apnea in children, new questions about the role that antinuclear antibodies are playing in systemic lupus erythematosus, and the lifetime cost of tobacco is nearly $2 million per smoker.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, a look at asthma, obesity, and the risk for severe sleep apnea in children, new questions about the role that antinuclear antibodies are playing in systemic lupus erythematosus, and the lifetime cost of tobacco is nearly $2 million per smoker.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, a look at asthma, obesity, and the risk for severe sleep apnea in children, new questions about the role that antinuclear antibodies are playing in systemic lupus erythematosus, and the lifetime cost of tobacco is nearly $2 million per smoker.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Interactive parenting, life skill intervention improves self-esteem in teen mothers
than did those who received standard care, according to Joanne E. Cox, MD, director of primary care at Boston Children’s Hospital and Harvard Medical School, and her associates.
A total of 140 mothers who were aged less than 19 years when they delivered and whose child was aged less than 12 months were included in the study published in Pediatrics. Of this group, 72 received the intervention, which included a series of five 1-hour, one-on-one, interactive modules adapted from the Nurturing and Ansell-Casey Life Skills curricula, delivered over the infant’s first 15 months, in addition to standard teen-tot clinic care. The remaining 68 mothers received teen-tot care alone.
While overall maternal self-esteem decreased in both the intervention and control groups when measured at 36 months, the intervention group experienced a significantly smaller decrease from baseline (P = .011). Similarly, the intervention group had higher scores regarding preparedness for motherhood (P = .011), acceptance of infant (P = .008), and expected relationship with infant (P = .029).
Of the 52 mothers in the intervention group and 48 mothers in the control group for whom pregnancy data was available at 36 months, 42% in the intervention group had a repeat pregnancy, compared with 67% in the control group (adjusted odds ratio, 0.20; 95% confidence interval, 0.06-0.75; P = .017).
The study findings “highlight the positive impact of pairing medical services with comprehensive social services and parenting education and can inform future policy and services for teen parents. These positive effects also have potential to improve long-term outcomes for teens and their children,” Dr. Cox and her associates concluded.
The study authors reported no conflicts of interest. The study was supported in part by a grant from the Office of Adolescent Pregnancy Programs, the Edgerley Family Endowment, a Leadership Education in Adolescent Health training grant, the Maternal and Child Health Bureau, and the Health Resources and Services Administration.
[email protected]
SOURCE: Cox JE et al. Pediatrics. 2019 Feb 12. doi: 10.1542/peds.2018-2303.
than did those who received standard care, according to Joanne E. Cox, MD, director of primary care at Boston Children’s Hospital and Harvard Medical School, and her associates.
A total of 140 mothers who were aged less than 19 years when they delivered and whose child was aged less than 12 months were included in the study published in Pediatrics. Of this group, 72 received the intervention, which included a series of five 1-hour, one-on-one, interactive modules adapted from the Nurturing and Ansell-Casey Life Skills curricula, delivered over the infant’s first 15 months, in addition to standard teen-tot clinic care. The remaining 68 mothers received teen-tot care alone.
While overall maternal self-esteem decreased in both the intervention and control groups when measured at 36 months, the intervention group experienced a significantly smaller decrease from baseline (P = .011). Similarly, the intervention group had higher scores regarding preparedness for motherhood (P = .011), acceptance of infant (P = .008), and expected relationship with infant (P = .029).
Of the 52 mothers in the intervention group and 48 mothers in the control group for whom pregnancy data was available at 36 months, 42% in the intervention group had a repeat pregnancy, compared with 67% in the control group (adjusted odds ratio, 0.20; 95% confidence interval, 0.06-0.75; P = .017).
The study findings “highlight the positive impact of pairing medical services with comprehensive social services and parenting education and can inform future policy and services for teen parents. These positive effects also have potential to improve long-term outcomes for teens and their children,” Dr. Cox and her associates concluded.
The study authors reported no conflicts of interest. The study was supported in part by a grant from the Office of Adolescent Pregnancy Programs, the Edgerley Family Endowment, a Leadership Education in Adolescent Health training grant, the Maternal and Child Health Bureau, and the Health Resources and Services Administration.
[email protected]
SOURCE: Cox JE et al. Pediatrics. 2019 Feb 12. doi: 10.1542/peds.2018-2303.
than did those who received standard care, according to Joanne E. Cox, MD, director of primary care at Boston Children’s Hospital and Harvard Medical School, and her associates.
A total of 140 mothers who were aged less than 19 years when they delivered and whose child was aged less than 12 months were included in the study published in Pediatrics. Of this group, 72 received the intervention, which included a series of five 1-hour, one-on-one, interactive modules adapted from the Nurturing and Ansell-Casey Life Skills curricula, delivered over the infant’s first 15 months, in addition to standard teen-tot clinic care. The remaining 68 mothers received teen-tot care alone.
While overall maternal self-esteem decreased in both the intervention and control groups when measured at 36 months, the intervention group experienced a significantly smaller decrease from baseline (P = .011). Similarly, the intervention group had higher scores regarding preparedness for motherhood (P = .011), acceptance of infant (P = .008), and expected relationship with infant (P = .029).
Of the 52 mothers in the intervention group and 48 mothers in the control group for whom pregnancy data was available at 36 months, 42% in the intervention group had a repeat pregnancy, compared with 67% in the control group (adjusted odds ratio, 0.20; 95% confidence interval, 0.06-0.75; P = .017).
The study findings “highlight the positive impact of pairing medical services with comprehensive social services and parenting education and can inform future policy and services for teen parents. These positive effects also have potential to improve long-term outcomes for teens and their children,” Dr. Cox and her associates concluded.
The study authors reported no conflicts of interest. The study was supported in part by a grant from the Office of Adolescent Pregnancy Programs, the Edgerley Family Endowment, a Leadership Education in Adolescent Health training grant, the Maternal and Child Health Bureau, and the Health Resources and Services Administration.
[email protected]
SOURCE: Cox JE et al. Pediatrics. 2019 Feb 12. doi: 10.1542/peds.2018-2303.
FROM PEDIATRICS
DAAs reduce mortality, cancer risk in HCV study
Direct-acting antivirals significantly decrease risk of hepatocellular carcinoma and mortality in persons with hepatitis C, according to results of the first prospective, longitudinal study to evaluate the effect of the drugs on complications related to the infection.
Compared with no treatment, DAA therapy cut risk of hepatocellular carcinoma by about one-third and all-cause mortality by about half in the study, which included about 10,000 adult patients with chronic hepatitis C virus (HCV) infection treated at 1 of 32 hepatology centers in France (NCT01953458).
There were no signs of increased risk of hepatocellular carcinoma during treatment with DAAs, providing more evidence refuting earlier, single-center reports that had suggested an increased incidence early after treatment. These findings also counterbalance a recent Cochrane review that could not confirm or reject a potential benefit of drugs on long-term morbidity and mortality.
Results of the study, published in the Lancet, are based on analysis of 9,895 patients, including 7,344 who started DAA treatment and 2,551 who remained untreated at a median follow-up of more than 31 months. The median patient age was 56 years, and 53% were men.
Treatment with DAAs reduced risk of hepatocellular carcinoma when compared with no DAA treatment, with a hazard ratio of 0.66 (95% confidence interval, 0.46-0.93), and reduced risk of all-cause mortality, with an HR of 0.48 (95% CI, 0.33-0.70), investigators reported in a multivariable analysis that adjusted for variables including age, sex, fibrosis score, HCV genotype, alcohol use, and more.
“These inverse associations persisted in the subgroup of patients who achieved a sustained virological response, whereas those who did not achieve a sustained virological response were a higher risk for hepatocellular carcinoma,” said the investigators, led by Fabrice Carrat, PhD, of Sorbonne Université, Institut National de la Santé et de la Recherche Médicale (INSERM), Paris.
Sustained virologic response was observed in 94% of patients who had known response status and sufficient follow-up, investigators said.
In patients with cirrhosis at baseline, DAA treatment had a similarly strong association with reduced hepatocellular carcinoma and mortality, with a sustained virologic response rate of 92% in those for whom sufficient data was available, they said.
There was no evidence for an increased risk of hepatocellular carcinoma on treatment, with an adjusted HR of 0.74 (95% CI, 0.49-1.13; P = 0.17), they added.
“Our results support urgent treatment of patients with advanced liver disease and extension of the follow-up of treated patients with less severe disease to assess the long-term clinical effect of direct-acting antiviral treatment,” Dr. Carrat and colleagues said in a commentary on their results.
However, the long-term effect of DAAs on liver decompensation has yet to be clarified, they added, noting that their study excluded patients with decompensated cirrhosis or a history of hepatocellular carcinoma.
Funding for the study came from INSERM, Agence Nationale de la Recherche, DGS (Direction Générale de la Santé), MSD, Janssen, Gilead, AbbVie, Bristol-Myers Squibb, and Roche. Dr. Carrat reported personal fees from Imaxio not related to the present study. Coauthors provided additional disclosures related to Gilead, AbbVie, Bristol-Myers Squibb, MSD, and Janssen, among others.
SOURCE: Carrat F et al. Lancet. 2019 Feb 11. doi: 10.1016/S0140-6736(18)32111-1
This study provides “substantive evidence” that curing hepatitis C virus with all-oral direct-acting antiviral regimens provides clinical benefits, according to Raymond T. Chung, MD, and his coauthors of a related editorial.
Investigators in this study provide the best evidence so far in support of guidelines that advise direct-acting antiviral (DAA) treatment for all patients with chronic hepatitis C virus (HCV) infection, the editorial’s authors stated.
Results of the French study provide a strong counterpoint to the findings of a recent Cochrane review of DAA trials that could not confirm or reject whether DAAs had effects on long-term morbidity and mortality related to HCV, added Dr. Chung and his coauthors. “Finally, they provide credence to the achievability of the goals set out by the World Health Organization (WHO), not only to eliminate HCV but also to substantially reduce its complications.”
The WHO targets were established in light of earlier evidence that sustained virologic responses are linked to reductions in hepatocellular carcinoma, liver transplantation, and mortality, they said.
“In view of the high sustained virological response and excellent tolerability achieved with DAAs, it seemed highly plausible to envision reductions in chronic HCV infection–related complications with these drugs,” they said in reference to the study by Carrat and colleagues.
This editorial appearing in the Lancet was authored by Jacinta A. Holmes, Stephanie M. Rutledge, and Raymond T. Chung of the Liver Center, Gastrointestinal Division, Massachusetts General Hospital, Boston. Dr. Chung provided disclosures related to AbbVie, Gilead, Merck, Bristol-Myers Squibb, Roche, Janssen, and Boehringer Ingelheim.
This study provides “substantive evidence” that curing hepatitis C virus with all-oral direct-acting antiviral regimens provides clinical benefits, according to Raymond T. Chung, MD, and his coauthors of a related editorial.
Investigators in this study provide the best evidence so far in support of guidelines that advise direct-acting antiviral (DAA) treatment for all patients with chronic hepatitis C virus (HCV) infection, the editorial’s authors stated.
Results of the French study provide a strong counterpoint to the findings of a recent Cochrane review of DAA trials that could not confirm or reject whether DAAs had effects on long-term morbidity and mortality related to HCV, added Dr. Chung and his coauthors. “Finally, they provide credence to the achievability of the goals set out by the World Health Organization (WHO), not only to eliminate HCV but also to substantially reduce its complications.”
The WHO targets were established in light of earlier evidence that sustained virologic responses are linked to reductions in hepatocellular carcinoma, liver transplantation, and mortality, they said.
“In view of the high sustained virological response and excellent tolerability achieved with DAAs, it seemed highly plausible to envision reductions in chronic HCV infection–related complications with these drugs,” they said in reference to the study by Carrat and colleagues.
This editorial appearing in the Lancet was authored by Jacinta A. Holmes, Stephanie M. Rutledge, and Raymond T. Chung of the Liver Center, Gastrointestinal Division, Massachusetts General Hospital, Boston. Dr. Chung provided disclosures related to AbbVie, Gilead, Merck, Bristol-Myers Squibb, Roche, Janssen, and Boehringer Ingelheim.
This study provides “substantive evidence” that curing hepatitis C virus with all-oral direct-acting antiviral regimens provides clinical benefits, according to Raymond T. Chung, MD, and his coauthors of a related editorial.
Investigators in this study provide the best evidence so far in support of guidelines that advise direct-acting antiviral (DAA) treatment for all patients with chronic hepatitis C virus (HCV) infection, the editorial’s authors stated.
Results of the French study provide a strong counterpoint to the findings of a recent Cochrane review of DAA trials that could not confirm or reject whether DAAs had effects on long-term morbidity and mortality related to HCV, added Dr. Chung and his coauthors. “Finally, they provide credence to the achievability of the goals set out by the World Health Organization (WHO), not only to eliminate HCV but also to substantially reduce its complications.”
The WHO targets were established in light of earlier evidence that sustained virologic responses are linked to reductions in hepatocellular carcinoma, liver transplantation, and mortality, they said.
“In view of the high sustained virological response and excellent tolerability achieved with DAAs, it seemed highly plausible to envision reductions in chronic HCV infection–related complications with these drugs,” they said in reference to the study by Carrat and colleagues.
This editorial appearing in the Lancet was authored by Jacinta A. Holmes, Stephanie M. Rutledge, and Raymond T. Chung of the Liver Center, Gastrointestinal Division, Massachusetts General Hospital, Boston. Dr. Chung provided disclosures related to AbbVie, Gilead, Merck, Bristol-Myers Squibb, Roche, Janssen, and Boehringer Ingelheim.
Direct-acting antivirals significantly decrease risk of hepatocellular carcinoma and mortality in persons with hepatitis C, according to results of the first prospective, longitudinal study to evaluate the effect of the drugs on complications related to the infection.
Compared with no treatment, DAA therapy cut risk of hepatocellular carcinoma by about one-third and all-cause mortality by about half in the study, which included about 10,000 adult patients with chronic hepatitis C virus (HCV) infection treated at 1 of 32 hepatology centers in France (NCT01953458).
There were no signs of increased risk of hepatocellular carcinoma during treatment with DAAs, providing more evidence refuting earlier, single-center reports that had suggested an increased incidence early after treatment. These findings also counterbalance a recent Cochrane review that could not confirm or reject a potential benefit of drugs on long-term morbidity and mortality.
Results of the study, published in the Lancet, are based on analysis of 9,895 patients, including 7,344 who started DAA treatment and 2,551 who remained untreated at a median follow-up of more than 31 months. The median patient age was 56 years, and 53% were men.
Treatment with DAAs reduced risk of hepatocellular carcinoma when compared with no DAA treatment, with a hazard ratio of 0.66 (95% confidence interval, 0.46-0.93), and reduced risk of all-cause mortality, with an HR of 0.48 (95% CI, 0.33-0.70), investigators reported in a multivariable analysis that adjusted for variables including age, sex, fibrosis score, HCV genotype, alcohol use, and more.
“These inverse associations persisted in the subgroup of patients who achieved a sustained virological response, whereas those who did not achieve a sustained virological response were a higher risk for hepatocellular carcinoma,” said the investigators, led by Fabrice Carrat, PhD, of Sorbonne Université, Institut National de la Santé et de la Recherche Médicale (INSERM), Paris.
Sustained virologic response was observed in 94% of patients who had known response status and sufficient follow-up, investigators said.
In patients with cirrhosis at baseline, DAA treatment had a similarly strong association with reduced hepatocellular carcinoma and mortality, with a sustained virologic response rate of 92% in those for whom sufficient data was available, they said.
There was no evidence for an increased risk of hepatocellular carcinoma on treatment, with an adjusted HR of 0.74 (95% CI, 0.49-1.13; P = 0.17), they added.
“Our results support urgent treatment of patients with advanced liver disease and extension of the follow-up of treated patients with less severe disease to assess the long-term clinical effect of direct-acting antiviral treatment,” Dr. Carrat and colleagues said in a commentary on their results.
However, the long-term effect of DAAs on liver decompensation has yet to be clarified, they added, noting that their study excluded patients with decompensated cirrhosis or a history of hepatocellular carcinoma.
Funding for the study came from INSERM, Agence Nationale de la Recherche, DGS (Direction Générale de la Santé), MSD, Janssen, Gilead, AbbVie, Bristol-Myers Squibb, and Roche. Dr. Carrat reported personal fees from Imaxio not related to the present study. Coauthors provided additional disclosures related to Gilead, AbbVie, Bristol-Myers Squibb, MSD, and Janssen, among others.
SOURCE: Carrat F et al. Lancet. 2019 Feb 11. doi: 10.1016/S0140-6736(18)32111-1
Direct-acting antivirals significantly decrease risk of hepatocellular carcinoma and mortality in persons with hepatitis C, according to results of the first prospective, longitudinal study to evaluate the effect of the drugs on complications related to the infection.
Compared with no treatment, DAA therapy cut risk of hepatocellular carcinoma by about one-third and all-cause mortality by about half in the study, which included about 10,000 adult patients with chronic hepatitis C virus (HCV) infection treated at 1 of 32 hepatology centers in France (NCT01953458).
There were no signs of increased risk of hepatocellular carcinoma during treatment with DAAs, providing more evidence refuting earlier, single-center reports that had suggested an increased incidence early after treatment. These findings also counterbalance a recent Cochrane review that could not confirm or reject a potential benefit of drugs on long-term morbidity and mortality.
Results of the study, published in the Lancet, are based on analysis of 9,895 patients, including 7,344 who started DAA treatment and 2,551 who remained untreated at a median follow-up of more than 31 months. The median patient age was 56 years, and 53% were men.
Treatment with DAAs reduced risk of hepatocellular carcinoma when compared with no DAA treatment, with a hazard ratio of 0.66 (95% confidence interval, 0.46-0.93), and reduced risk of all-cause mortality, with an HR of 0.48 (95% CI, 0.33-0.70), investigators reported in a multivariable analysis that adjusted for variables including age, sex, fibrosis score, HCV genotype, alcohol use, and more.
“These inverse associations persisted in the subgroup of patients who achieved a sustained virological response, whereas those who did not achieve a sustained virological response were a higher risk for hepatocellular carcinoma,” said the investigators, led by Fabrice Carrat, PhD, of Sorbonne Université, Institut National de la Santé et de la Recherche Médicale (INSERM), Paris.
Sustained virologic response was observed in 94% of patients who had known response status and sufficient follow-up, investigators said.
In patients with cirrhosis at baseline, DAA treatment had a similarly strong association with reduced hepatocellular carcinoma and mortality, with a sustained virologic response rate of 92% in those for whom sufficient data was available, they said.
There was no evidence for an increased risk of hepatocellular carcinoma on treatment, with an adjusted HR of 0.74 (95% CI, 0.49-1.13; P = 0.17), they added.
“Our results support urgent treatment of patients with advanced liver disease and extension of the follow-up of treated patients with less severe disease to assess the long-term clinical effect of direct-acting antiviral treatment,” Dr. Carrat and colleagues said in a commentary on their results.
However, the long-term effect of DAAs on liver decompensation has yet to be clarified, they added, noting that their study excluded patients with decompensated cirrhosis or a history of hepatocellular carcinoma.
Funding for the study came from INSERM, Agence Nationale de la Recherche, DGS (Direction Générale de la Santé), MSD, Janssen, Gilead, AbbVie, Bristol-Myers Squibb, and Roche. Dr. Carrat reported personal fees from Imaxio not related to the present study. Coauthors provided additional disclosures related to Gilead, AbbVie, Bristol-Myers Squibb, MSD, and Janssen, among others.
SOURCE: Carrat F et al. Lancet. 2019 Feb 11. doi: 10.1016/S0140-6736(18)32111-1
FROM THE LANCET
Key clinical point:
Major finding: DAAs reduced risk of hepatocellular carcinoma (HR, 0.66; 95% confidence interval, 0.46-0.93) and all-cause mortality (HR, 0.48; 95% CI, 0.33-0.70).
Study details: A prospective study including about 10,000 adults with chronic HCV infection enrolled at 1 of 32 centers in France.
Disclosures: Funding for the study came from INSERM, Agence Nationale de la Recherche, DGS (Direction Générale de la Santé), MSD, Janssen, Gilead, AbbVie, Bristol-Myers Squibb, and Roche. Dr. Carrat reported personal fees from Imaxio not related to the present study. Coauthors provided additional disclosures related to the study pharma sponsors among others.
Source: Carrat F et al. Lancet. 2019 Feb 11. doi: 10.1016/20S0140-6736(18)32111-1.
Watchful waiting up for low-risk prostate cancer
Conservative management for low-risk localized prostate cancer is up recently, in line with clinical practice guideline changes, while in high-risk disease, use of radical prostatectomy has increased despite a lack of new high-level evidence supporting the approach, according to researchers.
Active surveillance or watchful waiting surpassed both radical prostatectomy and radiotherapy to become the most common management strategy in low-risk disease over the 2010-2015 time period, according to their analysis of a Surveillance, Epidemiology, and End Results (SEER) database.
Meanwhile, radical prostatectomy use declined in low-risk patients, but increased in those with higher-risk disease at the expense of radiotherapy, said authors of the analysis, led by Brandon A. Mahal, MD, and Paul L. Nguyen, MD, of Dana-Farber Cancer Institute, Boston.
“Although increasing use of active surveillance or watchful waiting for low-risk disease has been supported by high-level evidence and guidelines since 2010, shifting management patterns toward more radical prostatectomy in higher-risk disease and away from radiotherapy does not coincide with any new level 1 evidence or guideline changes,” Dr. Mahal, Dr. Nguyen, and coauthors said in JAMA.
The analysis included 164,760 men with a diagnosis of localized prostate cancer between 2010 and 2015 in the SEER Prostate Active Surveillance/Watchful Waiting database. Of that group, 12.7% were managed by active surveillance or watchful waiting, while 41.5% underwent radiotherapy and 45.8% had a radical prostatectomy.
For men with low-risk disease, active surveillance or watchful waiting increased from just 14.5% in 2010 to 42.1% in 2015, investigators found. Radical prostatectomy decreased from 47.4% to 31.3% over that 5-year period, while radiotherapy likewise decreased from 38.0% to 26.6% (P less than .001 for all three trends).
By contrast, in men with high-risk disease, use of radical prostatectomy increased from 38.0% to 42.8%, while radiotherapy decreased from 60.1% to 55.0% (P less than .001 for both trends), and use of active surveillance remained low and steady at 1.9% in 2010 to 2.2% in 2015.
Intermediate-risk disease saw a significant increase in active surveillance, from 5.8% to 9.6% over the time period, with commensurate decreases in both radical prostatectomy and radiotherapy, according to the report.
While low-risk prostate cancer was traditionally managed with radical prostatectomy, national clinical practice guidelines starting in 2010 began recommending conservative management with active surveillance or watchful waiting, researchers noted in their report.
These epidemiologic data don’t provide any insights on clinical outcomes related to the management changes, investigators acknowledged. They said further study is needed to determine the “downstream effects” of increased active surveillance or watchful waiting in low-risk prostate cancer.
Dr. Mahal reported no conflicts of interest, while Dr. Nguyen provided disclosures related to Ferring, Augmenix, Bayer, Janssen, Astellas, Dendreon, Genome DX, Blue Earth Diagnostics, Cota, Nanobiotix, Janssen, and Astellas. Coauthors had disclosures relate to Janssen, Blue Earth, and the National Institutes of Health.
SOURCE: Mahal BA et al. JAMA. 2019 Feb 11. doi: 10.1001/jama.2018.19941.
Conservative management for low-risk localized prostate cancer is up recently, in line with clinical practice guideline changes, while in high-risk disease, use of radical prostatectomy has increased despite a lack of new high-level evidence supporting the approach, according to researchers.
Active surveillance or watchful waiting surpassed both radical prostatectomy and radiotherapy to become the most common management strategy in low-risk disease over the 2010-2015 time period, according to their analysis of a Surveillance, Epidemiology, and End Results (SEER) database.
Meanwhile, radical prostatectomy use declined in low-risk patients, but increased in those with higher-risk disease at the expense of radiotherapy, said authors of the analysis, led by Brandon A. Mahal, MD, and Paul L. Nguyen, MD, of Dana-Farber Cancer Institute, Boston.
“Although increasing use of active surveillance or watchful waiting for low-risk disease has been supported by high-level evidence and guidelines since 2010, shifting management patterns toward more radical prostatectomy in higher-risk disease and away from radiotherapy does not coincide with any new level 1 evidence or guideline changes,” Dr. Mahal, Dr. Nguyen, and coauthors said in JAMA.
The analysis included 164,760 men with a diagnosis of localized prostate cancer between 2010 and 2015 in the SEER Prostate Active Surveillance/Watchful Waiting database. Of that group, 12.7% were managed by active surveillance or watchful waiting, while 41.5% underwent radiotherapy and 45.8% had a radical prostatectomy.
For men with low-risk disease, active surveillance or watchful waiting increased from just 14.5% in 2010 to 42.1% in 2015, investigators found. Radical prostatectomy decreased from 47.4% to 31.3% over that 5-year period, while radiotherapy likewise decreased from 38.0% to 26.6% (P less than .001 for all three trends).
By contrast, in men with high-risk disease, use of radical prostatectomy increased from 38.0% to 42.8%, while radiotherapy decreased from 60.1% to 55.0% (P less than .001 for both trends), and use of active surveillance remained low and steady at 1.9% in 2010 to 2.2% in 2015.
Intermediate-risk disease saw a significant increase in active surveillance, from 5.8% to 9.6% over the time period, with commensurate decreases in both radical prostatectomy and radiotherapy, according to the report.
While low-risk prostate cancer was traditionally managed with radical prostatectomy, national clinical practice guidelines starting in 2010 began recommending conservative management with active surveillance or watchful waiting, researchers noted in their report.
These epidemiologic data don’t provide any insights on clinical outcomes related to the management changes, investigators acknowledged. They said further study is needed to determine the “downstream effects” of increased active surveillance or watchful waiting in low-risk prostate cancer.
Dr. Mahal reported no conflicts of interest, while Dr. Nguyen provided disclosures related to Ferring, Augmenix, Bayer, Janssen, Astellas, Dendreon, Genome DX, Blue Earth Diagnostics, Cota, Nanobiotix, Janssen, and Astellas. Coauthors had disclosures relate to Janssen, Blue Earth, and the National Institutes of Health.
SOURCE: Mahal BA et al. JAMA. 2019 Feb 11. doi: 10.1001/jama.2018.19941.
Conservative management for low-risk localized prostate cancer is up recently, in line with clinical practice guideline changes, while in high-risk disease, use of radical prostatectomy has increased despite a lack of new high-level evidence supporting the approach, according to researchers.
Active surveillance or watchful waiting surpassed both radical prostatectomy and radiotherapy to become the most common management strategy in low-risk disease over the 2010-2015 time period, according to their analysis of a Surveillance, Epidemiology, and End Results (SEER) database.
Meanwhile, radical prostatectomy use declined in low-risk patients, but increased in those with higher-risk disease at the expense of radiotherapy, said authors of the analysis, led by Brandon A. Mahal, MD, and Paul L. Nguyen, MD, of Dana-Farber Cancer Institute, Boston.
“Although increasing use of active surveillance or watchful waiting for low-risk disease has been supported by high-level evidence and guidelines since 2010, shifting management patterns toward more radical prostatectomy in higher-risk disease and away from radiotherapy does not coincide with any new level 1 evidence or guideline changes,” Dr. Mahal, Dr. Nguyen, and coauthors said in JAMA.
The analysis included 164,760 men with a diagnosis of localized prostate cancer between 2010 and 2015 in the SEER Prostate Active Surveillance/Watchful Waiting database. Of that group, 12.7% were managed by active surveillance or watchful waiting, while 41.5% underwent radiotherapy and 45.8% had a radical prostatectomy.
For men with low-risk disease, active surveillance or watchful waiting increased from just 14.5% in 2010 to 42.1% in 2015, investigators found. Radical prostatectomy decreased from 47.4% to 31.3% over that 5-year period, while radiotherapy likewise decreased from 38.0% to 26.6% (P less than .001 for all three trends).
By contrast, in men with high-risk disease, use of radical prostatectomy increased from 38.0% to 42.8%, while radiotherapy decreased from 60.1% to 55.0% (P less than .001 for both trends), and use of active surveillance remained low and steady at 1.9% in 2010 to 2.2% in 2015.
Intermediate-risk disease saw a significant increase in active surveillance, from 5.8% to 9.6% over the time period, with commensurate decreases in both radical prostatectomy and radiotherapy, according to the report.
While low-risk prostate cancer was traditionally managed with radical prostatectomy, national clinical practice guidelines starting in 2010 began recommending conservative management with active surveillance or watchful waiting, researchers noted in their report.
These epidemiologic data don’t provide any insights on clinical outcomes related to the management changes, investigators acknowledged. They said further study is needed to determine the “downstream effects” of increased active surveillance or watchful waiting in low-risk prostate cancer.
Dr. Mahal reported no conflicts of interest, while Dr. Nguyen provided disclosures related to Ferring, Augmenix, Bayer, Janssen, Astellas, Dendreon, Genome DX, Blue Earth Diagnostics, Cota, Nanobiotix, Janssen, and Astellas. Coauthors had disclosures relate to Janssen, Blue Earth, and the National Institutes of Health.
SOURCE: Mahal BA et al. JAMA. 2019 Feb 11. doi: 10.1001/jama.2018.19941.
FROM JAMA
Key clinical point: Conservative management for low-risk localized prostate cancer is up, in line with guidelines, while radical prostatectomy use has increased in high-risk disease despite a lack of new high-level evidence to support that approach.
Major finding: In men with low-risk disease, active surveillance or watchful waiting increased from 14.5% in 2010 to 42.1% in 2015, while in high-risk disease, radical prostatectomy increased from 38.0% to 42.8%
Study details: Analysis including 164,760 men with a diagnosis of localized prostate cancer between 2010 and 2015 in the SEER Prostate Active Surveillance/Watchful Waiting database.
Disclosures: Study authors reported disclosures related to Ferring, Augmenix, Bayer, Janssen, Astellas, Dendreon, Genome DX, Blue Earth Diagnostics, Cota, Nanobiotix, Janssen, Astellas, and the National Institutes of Health.
Source: Mahal BA et al. JAMA. 2019 Feb 11. doi: 10.1001/jama.2018.19941.