User login
Adenovirus: More than just another viral illness
The mother of three looked tired and little worried. She wasn’t one to bring her kids to the pediatrician’s office with every minor illness, but her youngest had 3 days of fever, runny nose, cough, and little of her normal energy.
The pediatrician entered the room and smiled sympathetically.
“We ran tests for flu and RSV [respiratory syncytial virus] and it’s neither of those so. ...”
“So it’s just a virus that we don’t routinely test for and it’s going to need to run its course,” the mother finished his sentence. She knew the drill.
Before the doctor could leave the room though, the mother had one more question. “You don’t think it could be adenovirus do you?”
Most years, influenza and RSV command center stage, and adenovirus is relegated to the wings. It is not so much lack of disease or morbidity, but rather lack of recognition. Yes, we all learned in medical school that it is a cause of epidemic keratoconjunctivitis, but many adenoviral infections are clinically indistinguishable from infections caused by other viruses. Common symptoms – fever, cough, sore throat, and malaise – overlap with those caused by influenza. Like rhinovirus, adenovirus can cause common cold symptoms. Like RSV, it can cause bronchiolitis. Just like parainfluenza, it can cause croup. It can cause a pertussislike syndrome with prolonged cough, and enteric adenoviruses, especially types 40 and 41, cause gastroenteritis that mimics norovirus or rotavirus infection.
Testing for adenovirus is not readily available or routine in most pediatricians’ offices, and while many hospitals and reference labs offer adenovirus polymerase chain reaction testing as part of a comprehensive respiratory virus panel, the test can be expensive and unlikely to change management in most ambulatory patients. This makes it difficult to count the number of adenoviruses annually.
This winter though, adenovirus was in the news ... repeatedly. In November 2018, CBS News reported that a University of Maryland freshman had died of an adenovirus-related illness. The family of Olivia Paregol told reporters that she was being treated for Crohn’s disease. Immune suppression is one recognized risk factor for more severe adenoviral disease; underlying heart and lung disease are others. Testing at the Centers for Disease Control and Prevention revealed that the student and several others on campus were infected with adenovirus type 7, a strain that has been associated with outbreaks of acute, severe respiratory illness in military recruits. As of Jan. 24, 2019, university officials reported 42 confirmed cases of adenovirus in University of Maryland students, 13 of which were confirmed as adenovirus 7.
Adenovirus type 7 also caused an outbreak at a pediatric long-term care facility in New Jersey late last year. Between Sept. 26 and Nov. 11, 2018, 36 residents and 1 staff member became ill. Eleven individuals died. In an unrelated outbreak at a second pediatric long-term care facility, 17 residents were affected between Oct. 20 and Dec. 10, 2018. Adenovirus 3 was identified and all children recovered.
Between October 2013 and July 2014, public health officials in Oregon identified an increase in adenoviral infections in people with respiratory illness. Sixty-nine percent were hospitalized (136/198), 31% needed intensive care, and 18% were mechanically ventilated. Multiple types of adenovirus were recovered but the most common was adenovirus 7 (Emerg Infect Dis. 2016. doi: 10.3201/eid2206.151898).
Depending on your perspective, measures to prevent the spread of adenovirus are elegantly simple, evidence-based, public health intervention or maddeningly little more than common sense. Wash your hands often with soap and water. Avoid touching your eyes, mouth, and nose with unwashed hands. Avoid close contact with people who are sick. The latter is easier if those who are sick stay home. Prior to the start of the most recent academic semester at the University of Maryland, university officials urged students who were sick not to return to campus but to stay at home to rest and recover. Those who fell ill on campus were urged to return home via nonpublic transportation if possible. Those who stayed on campus were advised to stay in their living spaces and clean high-touch surfaces with bleach. Like other nonenveloped viruses, adenovirus is not easily destroyed by many commonly used disinfectants. Under ideal conditions, it can survive on surfaces – remaining infectious – for up to 3 months.
Back at the pediatrician’s office, “We need an adenovirus vaccine,” the mother said as she picked up her child and headed for the door.
There is, in fact, a live oral vaccine that protects against adenovirus types 4 and 7. It is only approved for use in United States military personnel aged 17-50 years and it is given to all recruits as soon as they enter basic training. It works too. Before vaccine was available, up to 80% of recruits became infected during their initial training, half of those developing significant illness and a quarter being hospitalized. When the current vaccine was introduced in 2011, there was a 100-fold decrease in adenovirus-related disease burden (from 5.8 to 0.02 cases per 1,000 person-weeks, P less than .0001). That translates to 1 death, 1,100-2,700 hospitalizations and 13,000 febrile illnesses prevented each year (Clin Infect Dis. 2014 Oct 1. doi: 10.1093/cid/ciu507).
Some experts have suggested that adenovirus vaccine could be useful in civilian populations, too, but I question what the public reception would be. We have safe influenza vaccines that reduce the need for hospitalization and reduce mortality from influenza, but we still can’t convince some people to immunize themselves and their children. In the last 4 years, flu vaccination rates among children have remained just shy of 60% and adult rates are even lower. Collectively, we don’t seem to be ready to relinquish – or at least diminish – the annual suffering that goes with flu. I have to wonder if the same would be true for adenovirus.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The mother of three looked tired and little worried. She wasn’t one to bring her kids to the pediatrician’s office with every minor illness, but her youngest had 3 days of fever, runny nose, cough, and little of her normal energy.
The pediatrician entered the room and smiled sympathetically.
“We ran tests for flu and RSV [respiratory syncytial virus] and it’s neither of those so. ...”
“So it’s just a virus that we don’t routinely test for and it’s going to need to run its course,” the mother finished his sentence. She knew the drill.
Before the doctor could leave the room though, the mother had one more question. “You don’t think it could be adenovirus do you?”
Most years, influenza and RSV command center stage, and adenovirus is relegated to the wings. It is not so much lack of disease or morbidity, but rather lack of recognition. Yes, we all learned in medical school that it is a cause of epidemic keratoconjunctivitis, but many adenoviral infections are clinically indistinguishable from infections caused by other viruses. Common symptoms – fever, cough, sore throat, and malaise – overlap with those caused by influenza. Like rhinovirus, adenovirus can cause common cold symptoms. Like RSV, it can cause bronchiolitis. Just like parainfluenza, it can cause croup. It can cause a pertussislike syndrome with prolonged cough, and enteric adenoviruses, especially types 40 and 41, cause gastroenteritis that mimics norovirus or rotavirus infection.
Testing for adenovirus is not readily available or routine in most pediatricians’ offices, and while many hospitals and reference labs offer adenovirus polymerase chain reaction testing as part of a comprehensive respiratory virus panel, the test can be expensive and unlikely to change management in most ambulatory patients. This makes it difficult to count the number of adenoviruses annually.
This winter though, adenovirus was in the news ... repeatedly. In November 2018, CBS News reported that a University of Maryland freshman had died of an adenovirus-related illness. The family of Olivia Paregol told reporters that she was being treated for Crohn’s disease. Immune suppression is one recognized risk factor for more severe adenoviral disease; underlying heart and lung disease are others. Testing at the Centers for Disease Control and Prevention revealed that the student and several others on campus were infected with adenovirus type 7, a strain that has been associated with outbreaks of acute, severe respiratory illness in military recruits. As of Jan. 24, 2019, university officials reported 42 confirmed cases of adenovirus in University of Maryland students, 13 of which were confirmed as adenovirus 7.
Adenovirus type 7 also caused an outbreak at a pediatric long-term care facility in New Jersey late last year. Between Sept. 26 and Nov. 11, 2018, 36 residents and 1 staff member became ill. Eleven individuals died. In an unrelated outbreak at a second pediatric long-term care facility, 17 residents were affected between Oct. 20 and Dec. 10, 2018. Adenovirus 3 was identified and all children recovered.
Between October 2013 and July 2014, public health officials in Oregon identified an increase in adenoviral infections in people with respiratory illness. Sixty-nine percent were hospitalized (136/198), 31% needed intensive care, and 18% were mechanically ventilated. Multiple types of adenovirus were recovered but the most common was adenovirus 7 (Emerg Infect Dis. 2016. doi: 10.3201/eid2206.151898).
Depending on your perspective, measures to prevent the spread of adenovirus are elegantly simple, evidence-based, public health intervention or maddeningly little more than common sense. Wash your hands often with soap and water. Avoid touching your eyes, mouth, and nose with unwashed hands. Avoid close contact with people who are sick. The latter is easier if those who are sick stay home. Prior to the start of the most recent academic semester at the University of Maryland, university officials urged students who were sick not to return to campus but to stay at home to rest and recover. Those who fell ill on campus were urged to return home via nonpublic transportation if possible. Those who stayed on campus were advised to stay in their living spaces and clean high-touch surfaces with bleach. Like other nonenveloped viruses, adenovirus is not easily destroyed by many commonly used disinfectants. Under ideal conditions, it can survive on surfaces – remaining infectious – for up to 3 months.
Back at the pediatrician’s office, “We need an adenovirus vaccine,” the mother said as she picked up her child and headed for the door.
There is, in fact, a live oral vaccine that protects against adenovirus types 4 and 7. It is only approved for use in United States military personnel aged 17-50 years and it is given to all recruits as soon as they enter basic training. It works too. Before vaccine was available, up to 80% of recruits became infected during their initial training, half of those developing significant illness and a quarter being hospitalized. When the current vaccine was introduced in 2011, there was a 100-fold decrease in adenovirus-related disease burden (from 5.8 to 0.02 cases per 1,000 person-weeks, P less than .0001). That translates to 1 death, 1,100-2,700 hospitalizations and 13,000 febrile illnesses prevented each year (Clin Infect Dis. 2014 Oct 1. doi: 10.1093/cid/ciu507).
Some experts have suggested that adenovirus vaccine could be useful in civilian populations, too, but I question what the public reception would be. We have safe influenza vaccines that reduce the need for hospitalization and reduce mortality from influenza, but we still can’t convince some people to immunize themselves and their children. In the last 4 years, flu vaccination rates among children have remained just shy of 60% and adult rates are even lower. Collectively, we don’t seem to be ready to relinquish – or at least diminish – the annual suffering that goes with flu. I have to wonder if the same would be true for adenovirus.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The mother of three looked tired and little worried. She wasn’t one to bring her kids to the pediatrician’s office with every minor illness, but her youngest had 3 days of fever, runny nose, cough, and little of her normal energy.
The pediatrician entered the room and smiled sympathetically.
“We ran tests for flu and RSV [respiratory syncytial virus] and it’s neither of those so. ...”
“So it’s just a virus that we don’t routinely test for and it’s going to need to run its course,” the mother finished his sentence. She knew the drill.
Before the doctor could leave the room though, the mother had one more question. “You don’t think it could be adenovirus do you?”
Most years, influenza and RSV command center stage, and adenovirus is relegated to the wings. It is not so much lack of disease or morbidity, but rather lack of recognition. Yes, we all learned in medical school that it is a cause of epidemic keratoconjunctivitis, but many adenoviral infections are clinically indistinguishable from infections caused by other viruses. Common symptoms – fever, cough, sore throat, and malaise – overlap with those caused by influenza. Like rhinovirus, adenovirus can cause common cold symptoms. Like RSV, it can cause bronchiolitis. Just like parainfluenza, it can cause croup. It can cause a pertussislike syndrome with prolonged cough, and enteric adenoviruses, especially types 40 and 41, cause gastroenteritis that mimics norovirus or rotavirus infection.
Testing for adenovirus is not readily available or routine in most pediatricians’ offices, and while many hospitals and reference labs offer adenovirus polymerase chain reaction testing as part of a comprehensive respiratory virus panel, the test can be expensive and unlikely to change management in most ambulatory patients. This makes it difficult to count the number of adenoviruses annually.
This winter though, adenovirus was in the news ... repeatedly. In November 2018, CBS News reported that a University of Maryland freshman had died of an adenovirus-related illness. The family of Olivia Paregol told reporters that she was being treated for Crohn’s disease. Immune suppression is one recognized risk factor for more severe adenoviral disease; underlying heart and lung disease are others. Testing at the Centers for Disease Control and Prevention revealed that the student and several others on campus were infected with adenovirus type 7, a strain that has been associated with outbreaks of acute, severe respiratory illness in military recruits. As of Jan. 24, 2019, university officials reported 42 confirmed cases of adenovirus in University of Maryland students, 13 of which were confirmed as adenovirus 7.
Adenovirus type 7 also caused an outbreak at a pediatric long-term care facility in New Jersey late last year. Between Sept. 26 and Nov. 11, 2018, 36 residents and 1 staff member became ill. Eleven individuals died. In an unrelated outbreak at a second pediatric long-term care facility, 17 residents were affected between Oct. 20 and Dec. 10, 2018. Adenovirus 3 was identified and all children recovered.
Between October 2013 and July 2014, public health officials in Oregon identified an increase in adenoviral infections in people with respiratory illness. Sixty-nine percent were hospitalized (136/198), 31% needed intensive care, and 18% were mechanically ventilated. Multiple types of adenovirus were recovered but the most common was adenovirus 7 (Emerg Infect Dis. 2016. doi: 10.3201/eid2206.151898).
Depending on your perspective, measures to prevent the spread of adenovirus are elegantly simple, evidence-based, public health intervention or maddeningly little more than common sense. Wash your hands often with soap and water. Avoid touching your eyes, mouth, and nose with unwashed hands. Avoid close contact with people who are sick. The latter is easier if those who are sick stay home. Prior to the start of the most recent academic semester at the University of Maryland, university officials urged students who were sick not to return to campus but to stay at home to rest and recover. Those who fell ill on campus were urged to return home via nonpublic transportation if possible. Those who stayed on campus were advised to stay in their living spaces and clean high-touch surfaces with bleach. Like other nonenveloped viruses, adenovirus is not easily destroyed by many commonly used disinfectants. Under ideal conditions, it can survive on surfaces – remaining infectious – for up to 3 months.
Back at the pediatrician’s office, “We need an adenovirus vaccine,” the mother said as she picked up her child and headed for the door.
There is, in fact, a live oral vaccine that protects against adenovirus types 4 and 7. It is only approved for use in United States military personnel aged 17-50 years and it is given to all recruits as soon as they enter basic training. It works too. Before vaccine was available, up to 80% of recruits became infected during their initial training, half of those developing significant illness and a quarter being hospitalized. When the current vaccine was introduced in 2011, there was a 100-fold decrease in adenovirus-related disease burden (from 5.8 to 0.02 cases per 1,000 person-weeks, P less than .0001). That translates to 1 death, 1,100-2,700 hospitalizations and 13,000 febrile illnesses prevented each year (Clin Infect Dis. 2014 Oct 1. doi: 10.1093/cid/ciu507).
Some experts have suggested that adenovirus vaccine could be useful in civilian populations, too, but I question what the public reception would be. We have safe influenza vaccines that reduce the need for hospitalization and reduce mortality from influenza, but we still can’t convince some people to immunize themselves and their children. In the last 4 years, flu vaccination rates among children have remained just shy of 60% and adult rates are even lower. Collectively, we don’t seem to be ready to relinquish – or at least diminish – the annual suffering that goes with flu. I have to wonder if the same would be true for adenovirus.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Improving interview skills for hospitalists
Standardized prep courses are helpful
Are residents generally prepared for fellowship interviews? Applications to the Fellowship Match through the National Residency Matching Program (NRMP) Specialties Matching Service (SMS) are at an all-time high, but there is limited data regarding the preparedness of residents who go through the fellowship interview process, said Kelvin Wong, MD, a coauthor of research describing a new approach to fellowship interview preparation, which may be generalizable to hospitalists in applying for other positions.
“Applicants receive little to no feedback after their interviews and are thus likely to repeat the same mistakes throughout the process. Verbal feedback from our own fellowship directors indicated that residents as a whole are unprepared to interview,” according to an abstract written by Dr. Wong and his colleagues.
Dr. Wong wanted to investigate the effects of a standardized fellowship interview preparation course on resident preparedness. He and his coauthors developed a formal preparation course for the applicants in the summer of 2017.
Precourse surveys showed that only 17.65% of residents felt prepared to go on interviews; postcourse surveys showed a rise in this number to 82.35%. Immediately after their mock interview, only 27.78% of residents rated their overall interview skills as “very good” or “excellent,” whereas 87.5% of interviewers and 70.59% of observers rated their skills to be “very good” or “excellent.”
A final survey will be given to the applicants and the fellowship program directors once the applicants have completed all of their actual interviews.
“This demonstrates the potential positive impact that mock interviews and standardized interview preparation courses can have, which may be generalizable to hospitalists applying for other positions,” Dr. Wong said. “Specifically for teaching hospitalists in teaching hospitals, the institution of such fellowship interview preparation courses may improve resident preparedness for the fellowship application process.”
Reference
1. Wong K et al. A novel approach to improve fellowship interview skills [abstract]. https://www.shmabstracts.com/abstract/a-novel-approach-to-improve-fellowship-interview-skills/.
Standardized prep courses are helpful
Standardized prep courses are helpful
Are residents generally prepared for fellowship interviews? Applications to the Fellowship Match through the National Residency Matching Program (NRMP) Specialties Matching Service (SMS) are at an all-time high, but there is limited data regarding the preparedness of residents who go through the fellowship interview process, said Kelvin Wong, MD, a coauthor of research describing a new approach to fellowship interview preparation, which may be generalizable to hospitalists in applying for other positions.
“Applicants receive little to no feedback after their interviews and are thus likely to repeat the same mistakes throughout the process. Verbal feedback from our own fellowship directors indicated that residents as a whole are unprepared to interview,” according to an abstract written by Dr. Wong and his colleagues.
Dr. Wong wanted to investigate the effects of a standardized fellowship interview preparation course on resident preparedness. He and his coauthors developed a formal preparation course for the applicants in the summer of 2017.
Precourse surveys showed that only 17.65% of residents felt prepared to go on interviews; postcourse surveys showed a rise in this number to 82.35%. Immediately after their mock interview, only 27.78% of residents rated their overall interview skills as “very good” or “excellent,” whereas 87.5% of interviewers and 70.59% of observers rated their skills to be “very good” or “excellent.”
A final survey will be given to the applicants and the fellowship program directors once the applicants have completed all of their actual interviews.
“This demonstrates the potential positive impact that mock interviews and standardized interview preparation courses can have, which may be generalizable to hospitalists applying for other positions,” Dr. Wong said. “Specifically for teaching hospitalists in teaching hospitals, the institution of such fellowship interview preparation courses may improve resident preparedness for the fellowship application process.”
Reference
1. Wong K et al. A novel approach to improve fellowship interview skills [abstract]. https://www.shmabstracts.com/abstract/a-novel-approach-to-improve-fellowship-interview-skills/.
Are residents generally prepared for fellowship interviews? Applications to the Fellowship Match through the National Residency Matching Program (NRMP) Specialties Matching Service (SMS) are at an all-time high, but there is limited data regarding the preparedness of residents who go through the fellowship interview process, said Kelvin Wong, MD, a coauthor of research describing a new approach to fellowship interview preparation, which may be generalizable to hospitalists in applying for other positions.
“Applicants receive little to no feedback after their interviews and are thus likely to repeat the same mistakes throughout the process. Verbal feedback from our own fellowship directors indicated that residents as a whole are unprepared to interview,” according to an abstract written by Dr. Wong and his colleagues.
Dr. Wong wanted to investigate the effects of a standardized fellowship interview preparation course on resident preparedness. He and his coauthors developed a formal preparation course for the applicants in the summer of 2017.
Precourse surveys showed that only 17.65% of residents felt prepared to go on interviews; postcourse surveys showed a rise in this number to 82.35%. Immediately after their mock interview, only 27.78% of residents rated their overall interview skills as “very good” or “excellent,” whereas 87.5% of interviewers and 70.59% of observers rated their skills to be “very good” or “excellent.”
A final survey will be given to the applicants and the fellowship program directors once the applicants have completed all of their actual interviews.
“This demonstrates the potential positive impact that mock interviews and standardized interview preparation courses can have, which may be generalizable to hospitalists applying for other positions,” Dr. Wong said. “Specifically for teaching hospitalists in teaching hospitals, the institution of such fellowship interview preparation courses may improve resident preparedness for the fellowship application process.”
Reference
1. Wong K et al. A novel approach to improve fellowship interview skills [abstract]. https://www.shmabstracts.com/abstract/a-novel-approach-to-improve-fellowship-interview-skills/.
Radioligand is highly active in metastatic castrate-resistant prostate cancer
SAN FRANCISCO – (LuPSMA), finds a single-center phase 2 trial to be reported at the 2019 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
“Whilst there have been major advances in the last few years with several drugs that prolong survival in these men, the disease remains fatal in a relatively short period of time, and there is an urgent need for new effective therapies,” said lead study author Michael Hofman, MBBS, professor of nuclear medicine at the Peter MacCallum Cancer Centre, Melbourne.
LuPSMA is a radiolabeled small molecule that binds with high affinity to PSMA (prostate-specific membrane antigen), enabling targeted delivery of beta radiation to lesions throughout the body. To be eligible for the trial, patients had to have PSMA-positive disease. Results among the first 30 patients treated showed good activity and acceptable toxicity (Lancet Oncol. 2018;19:825-33), leading to enrollment of an expansion cohort of 20 patients.
With a median follow-up of 23.5 months among all 50 patients, nearly two-thirds achieved a 50% or greater reduction in their PSA level, Dr. Hofman reported in a presscast held before the symposium. Median overall survival exceeded 12 months for the whole cohort and was especially good for the subset achieving that level of PSA reduction, at 18 months.
“This is a single-arm study with no control arm. So whilst my impression is that this is a life-prolonging therapy, this is not a claim that we can make yet because there is no comparator arm with an existing treatment or therapy,” he acknowledged. However, data from the literature suggest that in the absence of this novel radioligand, the patients would likely have survived only about 6-9 months.
“These results in 50 men provide further confidence to our previously published 30-patient cohort, demonstrating high response rates and low toxicity in men with mCRPC who have progressed after multiple conventional therapies,” Dr. Hofman said. The findings also “support a novel mechanism of action for this therapy compared to existing therapies.”
The favorable data have led to initiation of two randomized controlled trials: the phase 2 TheraP trial (NCT03392428) comparing LuPSMA with cabazitaxel (Jevtana), and the phase 3 VISION trial (NCT03511664), comparing LuPSMA with best standard of care. “We really need the larger trials ... to get a confident assessment on whether it improves survival and by how much,” he maintained. “But my impression, seeing these patients come into the clinic very sick and seeing them improve on the therapy, and knowing those averages [for survival without this therapy], is that these trials are likely to be positive.”
“As a clinician, I will tell you that this is a very intriguing agent, and the VISION study, which is a registration trial, is open in the U.S.,” said ASCO expert and presscast moderator Robert Dreicer, MD, MS, MACP, FASCO, deputy director and associate director of clinical research at the University of Virginia Cancer Center and professor of medicine and urology at the University of Virginia, Charlottesville.
Study details
Of the 76 patients screened for the LuPSMA trial, 16 (21%) were ineligible because of insufficient PSMA uptake on a PSMA–FDG PET scan. “The majority of patients with this disease are suitable. Probably somewhere in the range of 20% to 30%, depending on how you measure it, may not be suitable,” Dr. Hofman said.
The 50 patients ultimately enrolled had aggressive disease, as indicated by a median PSA doubling time of 2.6 months. Most had previously received abiraterone (Zytiga), enzalutamide (Xtandi), or both (90%), as well as docetaxel (84%) or cabazitaxel (48%). “Many of these men had no further treatment options, and without this study open, they probably would have received end-of-life palliative care,” he said.
The median number of LuPSMA cycles administered was four, according to data reported in the presscast leading up to the symposium. Eight patients received fewer than four cycles because they had an exceptional response, while 10 patients did not complete all planned cycles because of disease progression.
Main results showed that PSA levels fell by at least 30% in 74% of patients, at least 50% in 64% of patients, and at least 80% in 44% of patients. Only two patients did not see any reduction. “We think this is probably a feature of our careful selection of patients with the PET scanning up front to really enrich the study for patients who are likely to benefit from the treatment,” Dr. Hofman said. The reductions in PSA were accompanied by reductions in positive lesions on PSMA–FDG PET.
For the entire cohort, median overall survival was 13.3 months. But it was significantly longer for those with versus without at least a 50% PSA decline (18.0 vs. 8.7 months; P = .001).
Fourteen patients who experienced progression on LuPSMA were given additional doses after the trial ended, as part of an off-trial expanded-access program. Fully 64% of these patients achieved a PSA reduction of at least 50%, and median overall survival in all of the retreated patients was 33 months.
Dr. Hofman disclosed that he has a consulting or advisory role with Endocyte; receives research funding (institutional) from Endocyte; and receives travel, accommodations, and/or expenses from Ipsen and Sanofi. The trial received research funding from the Peter MacCallum Cancer Centre.
SOURCE: Hofman M et al. GUCS 2019, Abstract 228.
SAN FRANCISCO – (LuPSMA), finds a single-center phase 2 trial to be reported at the 2019 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
“Whilst there have been major advances in the last few years with several drugs that prolong survival in these men, the disease remains fatal in a relatively short period of time, and there is an urgent need for new effective therapies,” said lead study author Michael Hofman, MBBS, professor of nuclear medicine at the Peter MacCallum Cancer Centre, Melbourne.
LuPSMA is a radiolabeled small molecule that binds with high affinity to PSMA (prostate-specific membrane antigen), enabling targeted delivery of beta radiation to lesions throughout the body. To be eligible for the trial, patients had to have PSMA-positive disease. Results among the first 30 patients treated showed good activity and acceptable toxicity (Lancet Oncol. 2018;19:825-33), leading to enrollment of an expansion cohort of 20 patients.
With a median follow-up of 23.5 months among all 50 patients, nearly two-thirds achieved a 50% or greater reduction in their PSA level, Dr. Hofman reported in a presscast held before the symposium. Median overall survival exceeded 12 months for the whole cohort and was especially good for the subset achieving that level of PSA reduction, at 18 months.
“This is a single-arm study with no control arm. So whilst my impression is that this is a life-prolonging therapy, this is not a claim that we can make yet because there is no comparator arm with an existing treatment or therapy,” he acknowledged. However, data from the literature suggest that in the absence of this novel radioligand, the patients would likely have survived only about 6-9 months.
“These results in 50 men provide further confidence to our previously published 30-patient cohort, demonstrating high response rates and low toxicity in men with mCRPC who have progressed after multiple conventional therapies,” Dr. Hofman said. The findings also “support a novel mechanism of action for this therapy compared to existing therapies.”
The favorable data have led to initiation of two randomized controlled trials: the phase 2 TheraP trial (NCT03392428) comparing LuPSMA with cabazitaxel (Jevtana), and the phase 3 VISION trial (NCT03511664), comparing LuPSMA with best standard of care. “We really need the larger trials ... to get a confident assessment on whether it improves survival and by how much,” he maintained. “But my impression, seeing these patients come into the clinic very sick and seeing them improve on the therapy, and knowing those averages [for survival without this therapy], is that these trials are likely to be positive.”
“As a clinician, I will tell you that this is a very intriguing agent, and the VISION study, which is a registration trial, is open in the U.S.,” said ASCO expert and presscast moderator Robert Dreicer, MD, MS, MACP, FASCO, deputy director and associate director of clinical research at the University of Virginia Cancer Center and professor of medicine and urology at the University of Virginia, Charlottesville.
Study details
Of the 76 patients screened for the LuPSMA trial, 16 (21%) were ineligible because of insufficient PSMA uptake on a PSMA–FDG PET scan. “The majority of patients with this disease are suitable. Probably somewhere in the range of 20% to 30%, depending on how you measure it, may not be suitable,” Dr. Hofman said.
The 50 patients ultimately enrolled had aggressive disease, as indicated by a median PSA doubling time of 2.6 months. Most had previously received abiraterone (Zytiga), enzalutamide (Xtandi), or both (90%), as well as docetaxel (84%) or cabazitaxel (48%). “Many of these men had no further treatment options, and without this study open, they probably would have received end-of-life palliative care,” he said.
The median number of LuPSMA cycles administered was four, according to data reported in the presscast leading up to the symposium. Eight patients received fewer than four cycles because they had an exceptional response, while 10 patients did not complete all planned cycles because of disease progression.
Main results showed that PSA levels fell by at least 30% in 74% of patients, at least 50% in 64% of patients, and at least 80% in 44% of patients. Only two patients did not see any reduction. “We think this is probably a feature of our careful selection of patients with the PET scanning up front to really enrich the study for patients who are likely to benefit from the treatment,” Dr. Hofman said. The reductions in PSA were accompanied by reductions in positive lesions on PSMA–FDG PET.
For the entire cohort, median overall survival was 13.3 months. But it was significantly longer for those with versus without at least a 50% PSA decline (18.0 vs. 8.7 months; P = .001).
Fourteen patients who experienced progression on LuPSMA were given additional doses after the trial ended, as part of an off-trial expanded-access program. Fully 64% of these patients achieved a PSA reduction of at least 50%, and median overall survival in all of the retreated patients was 33 months.
Dr. Hofman disclosed that he has a consulting or advisory role with Endocyte; receives research funding (institutional) from Endocyte; and receives travel, accommodations, and/or expenses from Ipsen and Sanofi. The trial received research funding from the Peter MacCallum Cancer Centre.
SOURCE: Hofman M et al. GUCS 2019, Abstract 228.
SAN FRANCISCO – (LuPSMA), finds a single-center phase 2 trial to be reported at the 2019 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
“Whilst there have been major advances in the last few years with several drugs that prolong survival in these men, the disease remains fatal in a relatively short period of time, and there is an urgent need for new effective therapies,” said lead study author Michael Hofman, MBBS, professor of nuclear medicine at the Peter MacCallum Cancer Centre, Melbourne.
LuPSMA is a radiolabeled small molecule that binds with high affinity to PSMA (prostate-specific membrane antigen), enabling targeted delivery of beta radiation to lesions throughout the body. To be eligible for the trial, patients had to have PSMA-positive disease. Results among the first 30 patients treated showed good activity and acceptable toxicity (Lancet Oncol. 2018;19:825-33), leading to enrollment of an expansion cohort of 20 patients.
With a median follow-up of 23.5 months among all 50 patients, nearly two-thirds achieved a 50% or greater reduction in their PSA level, Dr. Hofman reported in a presscast held before the symposium. Median overall survival exceeded 12 months for the whole cohort and was especially good for the subset achieving that level of PSA reduction, at 18 months.
“This is a single-arm study with no control arm. So whilst my impression is that this is a life-prolonging therapy, this is not a claim that we can make yet because there is no comparator arm with an existing treatment or therapy,” he acknowledged. However, data from the literature suggest that in the absence of this novel radioligand, the patients would likely have survived only about 6-9 months.
“These results in 50 men provide further confidence to our previously published 30-patient cohort, demonstrating high response rates and low toxicity in men with mCRPC who have progressed after multiple conventional therapies,” Dr. Hofman said. The findings also “support a novel mechanism of action for this therapy compared to existing therapies.”
The favorable data have led to initiation of two randomized controlled trials: the phase 2 TheraP trial (NCT03392428) comparing LuPSMA with cabazitaxel (Jevtana), and the phase 3 VISION trial (NCT03511664), comparing LuPSMA with best standard of care. “We really need the larger trials ... to get a confident assessment on whether it improves survival and by how much,” he maintained. “But my impression, seeing these patients come into the clinic very sick and seeing them improve on the therapy, and knowing those averages [for survival without this therapy], is that these trials are likely to be positive.”
“As a clinician, I will tell you that this is a very intriguing agent, and the VISION study, which is a registration trial, is open in the U.S.,” said ASCO expert and presscast moderator Robert Dreicer, MD, MS, MACP, FASCO, deputy director and associate director of clinical research at the University of Virginia Cancer Center and professor of medicine and urology at the University of Virginia, Charlottesville.
Study details
Of the 76 patients screened for the LuPSMA trial, 16 (21%) were ineligible because of insufficient PSMA uptake on a PSMA–FDG PET scan. “The majority of patients with this disease are suitable. Probably somewhere in the range of 20% to 30%, depending on how you measure it, may not be suitable,” Dr. Hofman said.
The 50 patients ultimately enrolled had aggressive disease, as indicated by a median PSA doubling time of 2.6 months. Most had previously received abiraterone (Zytiga), enzalutamide (Xtandi), or both (90%), as well as docetaxel (84%) or cabazitaxel (48%). “Many of these men had no further treatment options, and without this study open, they probably would have received end-of-life palliative care,” he said.
The median number of LuPSMA cycles administered was four, according to data reported in the presscast leading up to the symposium. Eight patients received fewer than four cycles because they had an exceptional response, while 10 patients did not complete all planned cycles because of disease progression.
Main results showed that PSA levels fell by at least 30% in 74% of patients, at least 50% in 64% of patients, and at least 80% in 44% of patients. Only two patients did not see any reduction. “We think this is probably a feature of our careful selection of patients with the PET scanning up front to really enrich the study for patients who are likely to benefit from the treatment,” Dr. Hofman said. The reductions in PSA were accompanied by reductions in positive lesions on PSMA–FDG PET.
For the entire cohort, median overall survival was 13.3 months. But it was significantly longer for those with versus without at least a 50% PSA decline (18.0 vs. 8.7 months; P = .001).
Fourteen patients who experienced progression on LuPSMA were given additional doses after the trial ended, as part of an off-trial expanded-access program. Fully 64% of these patients achieved a PSA reduction of at least 50%, and median overall survival in all of the retreated patients was 33 months.
Dr. Hofman disclosed that he has a consulting or advisory role with Endocyte; receives research funding (institutional) from Endocyte; and receives travel, accommodations, and/or expenses from Ipsen and Sanofi. The trial received research funding from the Peter MacCallum Cancer Centre.
SOURCE: Hofman M et al. GUCS 2019, Abstract 228.
REPORTING FROM GUCS 2019
Key clinical point: Lutetium-177 PSMA-617 (LuPSMA) is highly active in PSMA-positive metastatic castrate-resistant prostate cancer.
Major finding: PSA level fell by at least 50% in 64% of men, and median overall survival was 13.3 months.
Study details: A single-center, single-arm phase 2 trial among 50 men with PSMA-positive metastatic castrate-resistant prostate cancer (LuPSMA trial).
Disclosures: Dr. Hofman disclosed that he has a consulting or advisory role with Endocyte; receives research funding (institutional) from Endocyte; and receives travel, accommodations, and/or expenses from Ipsen and Sanofi. The trial received research funding from the Peter MacCallum Cancer Centre.
Source: Hofman M et al. GUCS 2019, Abstract 228.
Dengue antibodies may reduce Zika infection risk
Previous dengue exposure may confer a protective effect against Zika virus infection, according to a paper published in Science.
In a prospective cohort study, researchers followed 1,453 urban residents in Salvador, Brazil, to assess the impact of the 2015 Zika virus outbreak in the region. Data on dengue immunity was available for 642 of these individuals.
Overall, 73% of the cohort were seropositive for Zika virus. However, the frequency of seropositivity varied significantly by location, from 29% in a valley in the northeastern sector of the study area to 83% in the southeast corner; the authors wrote that this was consistent with some form of acquired immunity “blunting the efficiency of transmission.”
When researchers looked at the relationship between prior immunity to the dengue virus and the risk of Zika infection, they found that each doubling of total IgG titers against dengue NS1 was associated with a 9% reduction in the risk of Zika virus infection.
Individuals in the highest tertile of dengue IgG titers showed a 44% reduction in the odds of Zika seropositivity, compared with individuals with no or low dengue IgG titers, while those in the middle tertile of dengue IgG titer had a 38% reduction.
“These findings provide empirical support for the hypothesis that accumulated immunity drove ZIKV [Zika virus] to local extinction by reducing the efficiency of transmission,” wrote Isabel Rodriguez-Barraquer, MD, PhD, from the department of medicine at the University of California, San Francisco, and her coauthors.
Individuals who were infected with the Zika virus but had high dengue IgG titers were significantly less likely to exhibit fever with viral infection, but had the same risk of developing rash as those with low or no IgG titers.
Researchers also examined the link between a subclass of IgG antibodies that are associated with more recent exposure to dengue virus – within the prior 6 months – and the risk of Zika virus infection. In contrast, they found that the levels of this subclass of antibodies, known as IgG3, were positively associated with an increased risk of Zika virus infection. Each doubling in IgG3 levels was associated with a 23% increase in the odds of being positive for Zika.
“This positive association might reflect an immune profile, in individuals who have experienced a recent DENV [dengue virus] infection, that is associated with having a greater risk of a subsequent ZIKV infection,” the authors wrote. “Alternatively, it is also possible that higher levels of IgG3 are a proxy for frequent DENV exposure and thus greater risk of infection by Aedes aegypti–transmitted viruses.”
The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
SOURCE: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
Previous dengue exposure may confer a protective effect against Zika virus infection, according to a paper published in Science.
In a prospective cohort study, researchers followed 1,453 urban residents in Salvador, Brazil, to assess the impact of the 2015 Zika virus outbreak in the region. Data on dengue immunity was available for 642 of these individuals.
Overall, 73% of the cohort were seropositive for Zika virus. However, the frequency of seropositivity varied significantly by location, from 29% in a valley in the northeastern sector of the study area to 83% in the southeast corner; the authors wrote that this was consistent with some form of acquired immunity “blunting the efficiency of transmission.”
When researchers looked at the relationship between prior immunity to the dengue virus and the risk of Zika infection, they found that each doubling of total IgG titers against dengue NS1 was associated with a 9% reduction in the risk of Zika virus infection.
Individuals in the highest tertile of dengue IgG titers showed a 44% reduction in the odds of Zika seropositivity, compared with individuals with no or low dengue IgG titers, while those in the middle tertile of dengue IgG titer had a 38% reduction.
“These findings provide empirical support for the hypothesis that accumulated immunity drove ZIKV [Zika virus] to local extinction by reducing the efficiency of transmission,” wrote Isabel Rodriguez-Barraquer, MD, PhD, from the department of medicine at the University of California, San Francisco, and her coauthors.
Individuals who were infected with the Zika virus but had high dengue IgG titers were significantly less likely to exhibit fever with viral infection, but had the same risk of developing rash as those with low or no IgG titers.
Researchers also examined the link between a subclass of IgG antibodies that are associated with more recent exposure to dengue virus – within the prior 6 months – and the risk of Zika virus infection. In contrast, they found that the levels of this subclass of antibodies, known as IgG3, were positively associated with an increased risk of Zika virus infection. Each doubling in IgG3 levels was associated with a 23% increase in the odds of being positive for Zika.
“This positive association might reflect an immune profile, in individuals who have experienced a recent DENV [dengue virus] infection, that is associated with having a greater risk of a subsequent ZIKV infection,” the authors wrote. “Alternatively, it is also possible that higher levels of IgG3 are a proxy for frequent DENV exposure and thus greater risk of infection by Aedes aegypti–transmitted viruses.”
The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
SOURCE: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
Previous dengue exposure may confer a protective effect against Zika virus infection, according to a paper published in Science.
In a prospective cohort study, researchers followed 1,453 urban residents in Salvador, Brazil, to assess the impact of the 2015 Zika virus outbreak in the region. Data on dengue immunity was available for 642 of these individuals.
Overall, 73% of the cohort were seropositive for Zika virus. However, the frequency of seropositivity varied significantly by location, from 29% in a valley in the northeastern sector of the study area to 83% in the southeast corner; the authors wrote that this was consistent with some form of acquired immunity “blunting the efficiency of transmission.”
When researchers looked at the relationship between prior immunity to the dengue virus and the risk of Zika infection, they found that each doubling of total IgG titers against dengue NS1 was associated with a 9% reduction in the risk of Zika virus infection.
Individuals in the highest tertile of dengue IgG titers showed a 44% reduction in the odds of Zika seropositivity, compared with individuals with no or low dengue IgG titers, while those in the middle tertile of dengue IgG titer had a 38% reduction.
“These findings provide empirical support for the hypothesis that accumulated immunity drove ZIKV [Zika virus] to local extinction by reducing the efficiency of transmission,” wrote Isabel Rodriguez-Barraquer, MD, PhD, from the department of medicine at the University of California, San Francisco, and her coauthors.
Individuals who were infected with the Zika virus but had high dengue IgG titers were significantly less likely to exhibit fever with viral infection, but had the same risk of developing rash as those with low or no IgG titers.
Researchers also examined the link between a subclass of IgG antibodies that are associated with more recent exposure to dengue virus – within the prior 6 months – and the risk of Zika virus infection. In contrast, they found that the levels of this subclass of antibodies, known as IgG3, were positively associated with an increased risk of Zika virus infection. Each doubling in IgG3 levels was associated with a 23% increase in the odds of being positive for Zika.
“This positive association might reflect an immune profile, in individuals who have experienced a recent DENV [dengue virus] infection, that is associated with having a greater risk of a subsequent ZIKV infection,” the authors wrote. “Alternatively, it is also possible that higher levels of IgG3 are a proxy for frequent DENV exposure and thus greater risk of infection by Aedes aegypti–transmitted viruses.”
The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
SOURCE: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
FROM SCIENCE
Key clinical point: Higher dengue antibody titers are associated with a lower risk of Zika virus infection.
Major finding: The highest tertile of dengue antibody titers was associated with a 44% reduction in the risk of Zika seropositivity.
Study details: A prospective cohort study of 1,453 residents in Salvador, Brazil.
Disclosures: The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
Source: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
Acceptance and commitment therapy reduced IBD stress, depression
Eight weeks of a mindfulness intervention known as acceptance and commitment therapy (ACT) significantly improved stress and depression among patients with inflammatory bowel disease, and these improvements persisted for at least 12 weeks after therapy ended, according to the results of a randomized, controlled trial.
Source: The American Gastroenterological Association
In the intention-to-treat analysis, stress symptoms, as measured by the Depression Anxiety and Stress Scales (DASS-21), improved by 39% at week 8 and by 45% at week 20, reported Brona Wynne, PhD, of University College Dublin together with her associates. These improvements were highly significant compared with baseline and treatment as usual (P = .001 for both comparisons). “Post hoc analyses indicated that baseline stress levels were similar in control and treatment groups,” the researchers wrote in Gastroenterology. “The results of the per protocol analysis were comparable, with a 43% and 49% reduction in stress in the treatment group from baseline to 8 and 20 weeks.”
Multiple studies have documented high levels of stress and psychological dysfunction among patients with Crohn’s disease and ulcerative colitis. Studies of various mindfulness therapy, relaxation, stress management, cognitive-behavioral therapy, and hypnotherapy interventions often failed to collect key clinical data or were underpowered, uncontrolled, and unrandomized. Acceptance and commitment therapy uses mindfulness to identify adverse thoughts and experiences, accept these as part of life, and recommit to “move towards values that have been identified and adopted by the individual,” the investigators wrote. “This can be defined as the ability to contact the present moment more fully as a conscious human being and to change, or persist in, behavior when doing so serves valued ends.”
Their single-center study, which they said was the first to evaluate ACT in IBD patients, included 79 individuals with stable or mildly active Crohn’s disease (38 patients) or ulcerative colitis (41 patients) who were randomly assigned to ACT (37 patients) or control treatment as usual (42 patients). The two comparison groups were demographically and clinically similar. The ACT program involved eight 90-minute, weekly sessions of groups of 14-16 individuals, led by a single psychologist who tailored the course material toward IBD with a focus on lowering stress. An independent psychologist observed each session to assess adherence to protocol.
Not only did ACT meet the primary study endpoint, it also produced a 25% decrease in perceived stress (on a 1-10 scale) by week 8 and a 27% decrease in perceived stress by week 20 (P less than .001 versus treatment as usual). Depression scores in the ACT group also fell by 47% by week 8 and by 45% at week 20 (P = .01 versus treatment as usual). Anxiety levels decreased by 29% at week 8 and by 31% at week 20, but these improvements did not significantly differ from those in the control group (P = .39).
Interestingly, ACT did not significantly improve symptom burden, activities of daily living, disease-related worry, general well-being, C-reactive protein (CRP) levels, fecal calprotectin levels, or scores on the version used of the Clinical Assessment of Depression (CAD) or the short Mayo assessment. Hair cortisol levels showed an association with baseline stress and anxiety, but not with treatment response.
Care programs for IBD increasingly emphasize mental health services despite a lack of robust trials to support these interventions, the investigators noted. Thus, their findings highlight “the need for researchers and clinicians to further develop and optimize the content and delivery of psychological programs for IBD patients.”
Tillotts Pharma and Boston Scientific provided partial funding, but had no other role in the study. The researchers reported having no relevant conflicts of interest.
SOURCE: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
Factors that affect stress level and mood symptoms are vast when it comes to living with inflammatory bowel disease (IBD). Comorbid mood symptoms are common in patients with IBD, and psychological interventions are increasingly recommended as part of holistic, multidisciplinary treatment planning. Additionally, patients are open to GI-focused psychology treatments given the recognition that the complexities of living with IBD strongly influence emotional factors.
What must be acknowledged is the importance of long-term adherence to skills learned during the 8 weeks of ACT. Stress and mood symptoms tend to be more prevalent during times of flare. Given the relapsing and remitting nature of IBD, it must be conveyed that patients will need to continue the practice of this mindfulness-based intervention in the long term. Future studies are encouraged to look at longitudinal data assessing the manner in which these patients used their skill set during periods of flare or disease-related stress.
Factors that affect stress level and mood symptoms are vast when it comes to living with inflammatory bowel disease (IBD). Comorbid mood symptoms are common in patients with IBD, and psychological interventions are increasingly recommended as part of holistic, multidisciplinary treatment planning. Additionally, patients are open to GI-focused psychology treatments given the recognition that the complexities of living with IBD strongly influence emotional factors.
What must be acknowledged is the importance of long-term adherence to skills learned during the 8 weeks of ACT. Stress and mood symptoms tend to be more prevalent during times of flare. Given the relapsing and remitting nature of IBD, it must be conveyed that patients will need to continue the practice of this mindfulness-based intervention in the long term. Future studies are encouraged to look at longitudinal data assessing the manner in which these patients used their skill set during periods of flare or disease-related stress.
Factors that affect stress level and mood symptoms are vast when it comes to living with inflammatory bowel disease (IBD). Comorbid mood symptoms are common in patients with IBD, and psychological interventions are increasingly recommended as part of holistic, multidisciplinary treatment planning. Additionally, patients are open to GI-focused psychology treatments given the recognition that the complexities of living with IBD strongly influence emotional factors.
What must be acknowledged is the importance of long-term adherence to skills learned during the 8 weeks of ACT. Stress and mood symptoms tend to be more prevalent during times of flare. Given the relapsing and remitting nature of IBD, it must be conveyed that patients will need to continue the practice of this mindfulness-based intervention in the long term. Future studies are encouraged to look at longitudinal data assessing the manner in which these patients used their skill set during periods of flare or disease-related stress.
Eight weeks of a mindfulness intervention known as acceptance and commitment therapy (ACT) significantly improved stress and depression among patients with inflammatory bowel disease, and these improvements persisted for at least 12 weeks after therapy ended, according to the results of a randomized, controlled trial.
Source: The American Gastroenterological Association
In the intention-to-treat analysis, stress symptoms, as measured by the Depression Anxiety and Stress Scales (DASS-21), improved by 39% at week 8 and by 45% at week 20, reported Brona Wynne, PhD, of University College Dublin together with her associates. These improvements were highly significant compared with baseline and treatment as usual (P = .001 for both comparisons). “Post hoc analyses indicated that baseline stress levels were similar in control and treatment groups,” the researchers wrote in Gastroenterology. “The results of the per protocol analysis were comparable, with a 43% and 49% reduction in stress in the treatment group from baseline to 8 and 20 weeks.”
Multiple studies have documented high levels of stress and psychological dysfunction among patients with Crohn’s disease and ulcerative colitis. Studies of various mindfulness therapy, relaxation, stress management, cognitive-behavioral therapy, and hypnotherapy interventions often failed to collect key clinical data or were underpowered, uncontrolled, and unrandomized. Acceptance and commitment therapy uses mindfulness to identify adverse thoughts and experiences, accept these as part of life, and recommit to “move towards values that have been identified and adopted by the individual,” the investigators wrote. “This can be defined as the ability to contact the present moment more fully as a conscious human being and to change, or persist in, behavior when doing so serves valued ends.”
Their single-center study, which they said was the first to evaluate ACT in IBD patients, included 79 individuals with stable or mildly active Crohn’s disease (38 patients) or ulcerative colitis (41 patients) who were randomly assigned to ACT (37 patients) or control treatment as usual (42 patients). The two comparison groups were demographically and clinically similar. The ACT program involved eight 90-minute, weekly sessions of groups of 14-16 individuals, led by a single psychologist who tailored the course material toward IBD with a focus on lowering stress. An independent psychologist observed each session to assess adherence to protocol.
Not only did ACT meet the primary study endpoint, it also produced a 25% decrease in perceived stress (on a 1-10 scale) by week 8 and a 27% decrease in perceived stress by week 20 (P less than .001 versus treatment as usual). Depression scores in the ACT group also fell by 47% by week 8 and by 45% at week 20 (P = .01 versus treatment as usual). Anxiety levels decreased by 29% at week 8 and by 31% at week 20, but these improvements did not significantly differ from those in the control group (P = .39).
Interestingly, ACT did not significantly improve symptom burden, activities of daily living, disease-related worry, general well-being, C-reactive protein (CRP) levels, fecal calprotectin levels, or scores on the version used of the Clinical Assessment of Depression (CAD) or the short Mayo assessment. Hair cortisol levels showed an association with baseline stress and anxiety, but not with treatment response.
Care programs for IBD increasingly emphasize mental health services despite a lack of robust trials to support these interventions, the investigators noted. Thus, their findings highlight “the need for researchers and clinicians to further develop and optimize the content and delivery of psychological programs for IBD patients.”
Tillotts Pharma and Boston Scientific provided partial funding, but had no other role in the study. The researchers reported having no relevant conflicts of interest.
SOURCE: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
Eight weeks of a mindfulness intervention known as acceptance and commitment therapy (ACT) significantly improved stress and depression among patients with inflammatory bowel disease, and these improvements persisted for at least 12 weeks after therapy ended, according to the results of a randomized, controlled trial.
Source: The American Gastroenterological Association
In the intention-to-treat analysis, stress symptoms, as measured by the Depression Anxiety and Stress Scales (DASS-21), improved by 39% at week 8 and by 45% at week 20, reported Brona Wynne, PhD, of University College Dublin together with her associates. These improvements were highly significant compared with baseline and treatment as usual (P = .001 for both comparisons). “Post hoc analyses indicated that baseline stress levels were similar in control and treatment groups,” the researchers wrote in Gastroenterology. “The results of the per protocol analysis were comparable, with a 43% and 49% reduction in stress in the treatment group from baseline to 8 and 20 weeks.”
Multiple studies have documented high levels of stress and psychological dysfunction among patients with Crohn’s disease and ulcerative colitis. Studies of various mindfulness therapy, relaxation, stress management, cognitive-behavioral therapy, and hypnotherapy interventions often failed to collect key clinical data or were underpowered, uncontrolled, and unrandomized. Acceptance and commitment therapy uses mindfulness to identify adverse thoughts and experiences, accept these as part of life, and recommit to “move towards values that have been identified and adopted by the individual,” the investigators wrote. “This can be defined as the ability to contact the present moment more fully as a conscious human being and to change, or persist in, behavior when doing so serves valued ends.”
Their single-center study, which they said was the first to evaluate ACT in IBD patients, included 79 individuals with stable or mildly active Crohn’s disease (38 patients) or ulcerative colitis (41 patients) who were randomly assigned to ACT (37 patients) or control treatment as usual (42 patients). The two comparison groups were demographically and clinically similar. The ACT program involved eight 90-minute, weekly sessions of groups of 14-16 individuals, led by a single psychologist who tailored the course material toward IBD with a focus on lowering stress. An independent psychologist observed each session to assess adherence to protocol.
Not only did ACT meet the primary study endpoint, it also produced a 25% decrease in perceived stress (on a 1-10 scale) by week 8 and a 27% decrease in perceived stress by week 20 (P less than .001 versus treatment as usual). Depression scores in the ACT group also fell by 47% by week 8 and by 45% at week 20 (P = .01 versus treatment as usual). Anxiety levels decreased by 29% at week 8 and by 31% at week 20, but these improvements did not significantly differ from those in the control group (P = .39).
Interestingly, ACT did not significantly improve symptom burden, activities of daily living, disease-related worry, general well-being, C-reactive protein (CRP) levels, fecal calprotectin levels, or scores on the version used of the Clinical Assessment of Depression (CAD) or the short Mayo assessment. Hair cortisol levels showed an association with baseline stress and anxiety, but not with treatment response.
Care programs for IBD increasingly emphasize mental health services despite a lack of robust trials to support these interventions, the investigators noted. Thus, their findings highlight “the need for researchers and clinicians to further develop and optimize the content and delivery of psychological programs for IBD patients.”
Tillotts Pharma and Boston Scientific provided partial funding, but had no other role in the study. The researchers reported having no relevant conflicts of interest.
SOURCE: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
FROM GASTROENTEROLOGY
Key clinical point: An 8-week course of acceptance and commitment therapy improved stress and depression in patients with inflammatory bowel disease.
Major finding: Compared with controls, the intervention group experienced significant improvements in stress (P = .001) and depression (P = .01), but not anxiety.
Study details: Randomized controlled trial of 79 patients.
Disclosures: Tillotts Pharma and Boston Scientific provided partial funding but had no other role in the study. The researchers reported having no relevant conflicts of interest.
Source: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
Retrospective Analysis of Liraglutide as Add-On Therapy in Type 2 Diabetes Mellitus: Quantifying the Changes in Insulin Requirements
Clinical pharmacists in VA primary care pharmacy clinics can effectively and safely use liraglutide to reduce hemoglobin A1c and insulin requirements in veterans.
Diabetes mellitus (DM) was the third most common medical diagnosis in 2016.1 Uncontrolled DM can lead to cardiovascular disease, nephropathy, neuropathy, and retinopathy. It is estimated that only 52.5% of patients with DM have achieved their goal hemoglobin A1c (HbA1c) level. The 2018 American Diabetes Association (ADA) clinical guidelines lack strong recommendations on sequential therapy for patients who have received a diagnosis of type 2 diabetes mellitus (T2DM) and have been unable to achieve their goal HbA1c level with lifestyle changes and maximum-dose metformin.2 Although those guidelines support treatment intensification with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), prescribing patterns for T2DM most commonly include adding insulin to try to control blood glucose and reduce long-term comorbidities.2,3
Related:
Insulin therapy is known for its ability to effectively lower blood glucose and HbA1c levels but comes with many limitations. Mealtime insulin has the highest risk of hypoglycemia, causes significant weight gain, requires several additional injections per day, and additional monitoring of blood glucose.4,5 The 2018 ADA guidelines state that hypoglycemia is the major limiting factor in the management of insulin-treated T2DM.2
Compared with mealtime insulin, GLP-1 RAs have the benefit of reducing the risk of hypoglycemia, weight gain, and number of daily injections.5 In addition, compared with insulin alone, GLP-1 RAs have the advantage of reducing glycemic variability.6 These advantages are especially attractive in the treatment of geriatric patients. Given its mechanism of action, liraglutide is expected to have an effect on both fasting and postprandial blood glucose. There are no recommendations on how to empirically reduce the dose of insulin when starting liraglutide.7
Background
GLP-1 is an incretin hormone that is secreted in response to meal ingestion. GLP-1 stimulates insulin release, suppresses elevated glucagon levels, and delays gastric emptying. Patients with a DM diagnosis have impaired secretion of GLP-1.8
The GLP-1 RA liraglutide was approved by the FDA in January 2010 as a once-daily injection for patients with uncontrolled T2DM despite lifestyle changes and metformin monotherapy. Because of its intermediate half-life, liraglutide has an effect on both fasting and postprandial blood glucose.7 GLP-1 RAs are associated with reduced hypoglycemic episodes—an association attributable to the mechanism of action and potentially to improved pancreatic α-cell function.3,4 In July 2016, results of the LEADER trial showed that liraglutide therapy had a cardiovascular benefit in high-risk patients.8 In October 2017, liraglutide was FDAapproved for reducing 3-point major adverse cardiac events.7
Xultophy (Novo Nordisk, Plainsboro, NJ) is a fixed-dose medication combining degludec, a long-acting basal insulin analog, with liraglutide. As seen in the DUAL trials, Xultophy was more beneficial in reducing HbA1c levels than each component alone, and minimized hypoglycemic events, weight gain, and complexity of insulin treatment intensification.9-11 Therapy that combines basal insulin and a GLP-1 RA may be more effective than either agent as monotherapy and may have a significant impact on cardiovascular risk because of the synergistic vasodilatory, anti-inflammatory, and antioxidant properties of insulin and GLP-1 RA.6 In addition, combination therapy offers many benefits over traditional basal and bolus insulin regimens. These benefits include fewer daily injections, additional weight reduction resulting from the reduced insulin requirement, and fewer episodes of hypoglycemia. Reported gastrointestinal adverse effects have been transient and were not augmented when a GLP-1 RA was used in combination with basal insulin.11
Methods
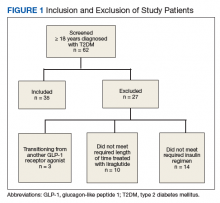
We performed a retrospective chart analysis to quantify the benefit of using liraglutide as an add-on therapy to basal and bolus insulin regimens in veterans treated at VA Boston Healthcare System (VABHS). The analysis evaluated changes in insulin doses and HbA1c levels when liraglutide was added to these regimens. Patients identified for the study had electronic medication orders for concurrent therapy with liraglutide, insulin glargine, and insulin aspart filled through outpatient VABHS campus pharmacies for at least 3 months between January 2010 and December 2016. Sixty-nine patients who were on basal-bolus insulin for T2DM and who were prescribed liraglutide for treatment intensification were screened for inclusion and exclusion criteria. Data were analyzed at baseline and 3 months after liraglutide treatment.
Study Protocol
The inclusion criteria were patients aged ≥ 18 years, T2DM diagnosis, and therapy with insulin glargine and insulin aspart for at least 3 months before treatment intensi fication with liraglutide. Exclusion criteria were diagnosis of type 1 DM. To accurately quantify mean change in number of insulin units used, the study included patients only if they had been prescribed insulin glargine and insulin aspart before starting liraglutide. All other insulin regimens were excluded. To detect the true change that occurs when liraglutide is added to basal-bolus insulin, the study also excluded patients if they had been previously prescribed another GLP-1 RA. Patients with contraindications to liraglutide, insulin aspart, or insulin glargine were excluded as well. In addition, patients were excluded from the exposed arm if they were injecting < 1.2 mg of liraglutide once daily or if they had been on liraglutide for < 3 months.
Study Outcomes
All 35 patients who met the inclusion and exclusion criteria were included in this retrospective chart review. The primary outcome was determined by changes in HbA1c level and number of insulin doses 3 months after treatment with liraglutide. For each patient, a chart review was performed to determine the amount of insulin added or reduced during the study period. Data were collected at baseline and 3 months after initiation of liraglutide.
Statistical Analysis
Statistical analyses were performed with SPSS Version 20.0 (IBM, Armonk, NY). Population characteristics and study outcomes with normal distribution were compared using a paired t test and are reported as means with standard deviations. Nonnormally distributed variables (bolus insulin, HbA1c level) were compared using the nonparametric Wilcoxon rank sum test and are reported as median values with interquartile ranges. Normality was tested with the Shapiro-Wilk test. The primary outcome evaluated was change in number of insulin units used. Secondary outcomes included change in HbA1c level and change in body weight. A Bonferroni correction for multiple comparisons was used to prevent type I error. Significance at the Bonferroni-corrected level of .01 (.05/5 = .01) is indicated.
Results
Patients were included if they were previously on insulin glargine and insulin aspart before starting liraglutide for treatment intensification.
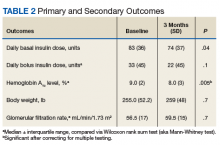
As Table 1 indicates, 100% of patients were male, and mean (SD) age was 65.5 (9.3) years.
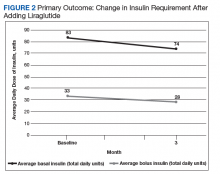
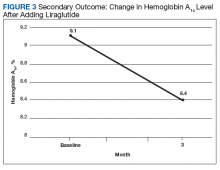
After 3 months of therapy with liraglutide, HbA1c levels were reduced by a mean of 1.0% (P = .005) (Table 2).
Discussion
After 3 months of treatment with liraglutide, patients experienced a significant decrease in HbA1c levels. Insulin doses also decreased, but this finding was not statistically significant after correcting for multiple testing. These results are similar with those in larger studies of the effectiveness of liraglutide and the addition of liraglutide to insulin therapy. 6,8,12,13 Liraglutide has been shown to decrease HbA1c levels, lower rates of progression of kidney failure, decrease weight, and provide cardiovascular benefit.
In a prospective, randomized controlled trial evaluating the effect of adding liraglutide to insulin therapy, 21 of the 37 patients who had T2DM and required more than 100 total units of basal-bolus insulin daily were initiated on liraglutide, and changes in HbA1c level, body weight, and glycemic variability were compared. Results showed statistically significant improvement in all 3 outcomes in the group treated with liraglutide.6 Our findings, in conjunction with those of the larger studies, suggest that many of these results are generalizable to our local veteran population. Importantly, liraglutide was successfully started in pharmacy clinics—an indication that this treatment need not be initiated by an endocrine specialist.
Limitations
Given the lack of gender and racial diversity in this study population, our findings have limited generalizability to other populations. It is possible that, with a larger sample size, these results regarding reduced basal insulin doses would be significant. It has been hypothesized that patients experience fewer episodes of hypoglycemia when insulin doses are reduced, but we were unable to measure the frequency of these episodes. Other study limitations include inability to assess adherence and inability to account for concurrent regimens and/or for lifestyle changes that may have been made during the study period. Further, the study did not collect data on changes made to current DM medication regimens during the study period, and these changes may have influenced outcomes.
Conclusion
Patients who require treatment intensification for insulin-dependent T2DM may benefit from having liraglutide added to their basal-bolus insulin regimen. Liraglutide may prove to be more favorable than bolus insulin when choosing add-on therapy to basal insulin. Benefits include reductions in insulin doses, HbA1c levels, number of daily injections, and body weight. Therefore, we suggest that empirically reducing basal insulin by 10% to 25% and bolus insulin by 25% to 50% will avoid relative hypoglycemia. Prescribers must keep in mind patient-specific factors when adjusting insulin doses, if these doses are adjusted at all. Follow-up of 2 to 4 weeks is recommended for review of home monitoring of glucose for further insulin adjustments.
This study has important clinical implications. First, the finding of a reduction in HbA1c levels supports use of liraglutide therapy for HbA1c reduction in veterans. Second, the number of veterans who were successfully initiated on liraglutide therapy by nonphysician providers indicates that liraglutide can be effectively and safely started in primary care pharmacy clinics, increasing access to the medication.
1. Centers for Medicare & Medicaid Services. ICD-10. https://www.cms.gov/medicare/coding/icd10. Accessed July 26, 2018.
2. American Diabetes Association. Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S2.
3. Combination therapy with insulins and GLP-1 receptor agonists. http://www.powerpak.com/course/content/113275. Updated 2018. Accessed July 26, 2018.
4. Carris NW, Taylor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74(18):2141-2152.
5. Young LA, Buse JB, Weaver MA, et al; Monitor Trial Group. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017;177(7):920-929.
6. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832.
7. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; August 2017.
8. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
9. Buse JB, Vilsbøll T, Thurman J, et al; NN9068-3912 (DUAL-II) Trial Investigators. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-2933
10. Glough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its component given alone: results of a phase 3, open-label, randomized, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
11. Lingvay I, Pérez Manghi F, García-Hernández P, et al; DUAL V Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized controlled trial. JAMA. 2016;315(9):898-907.
12. Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, anti-inflammatory and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37(7):1938-1943.
13. Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Torffvit O, Pehrsson NG. Design and methods of a randomised double-blind trial of adding liraglutide to control HbA1c in patients with type 2 diabetes with impaired glycaemic control treated with multiple daily insulin injections (MDI-Liraglutide trial). Prim Care Diabetes. 2015;9(1):15-22.
Clinical pharmacists in VA primary care pharmacy clinics can effectively and safely use liraglutide to reduce hemoglobin A1c and insulin requirements in veterans.
Clinical pharmacists in VA primary care pharmacy clinics can effectively and safely use liraglutide to reduce hemoglobin A1c and insulin requirements in veterans.
Diabetes mellitus (DM) was the third most common medical diagnosis in 2016.1 Uncontrolled DM can lead to cardiovascular disease, nephropathy, neuropathy, and retinopathy. It is estimated that only 52.5% of patients with DM have achieved their goal hemoglobin A1c (HbA1c) level. The 2018 American Diabetes Association (ADA) clinical guidelines lack strong recommendations on sequential therapy for patients who have received a diagnosis of type 2 diabetes mellitus (T2DM) and have been unable to achieve their goal HbA1c level with lifestyle changes and maximum-dose metformin.2 Although those guidelines support treatment intensification with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), prescribing patterns for T2DM most commonly include adding insulin to try to control blood glucose and reduce long-term comorbidities.2,3
Related:
Insulin therapy is known for its ability to effectively lower blood glucose and HbA1c levels but comes with many limitations. Mealtime insulin has the highest risk of hypoglycemia, causes significant weight gain, requires several additional injections per day, and additional monitoring of blood glucose.4,5 The 2018 ADA guidelines state that hypoglycemia is the major limiting factor in the management of insulin-treated T2DM.2
Compared with mealtime insulin, GLP-1 RAs have the benefit of reducing the risk of hypoglycemia, weight gain, and number of daily injections.5 In addition, compared with insulin alone, GLP-1 RAs have the advantage of reducing glycemic variability.6 These advantages are especially attractive in the treatment of geriatric patients. Given its mechanism of action, liraglutide is expected to have an effect on both fasting and postprandial blood glucose. There are no recommendations on how to empirically reduce the dose of insulin when starting liraglutide.7
Background
GLP-1 is an incretin hormone that is secreted in response to meal ingestion. GLP-1 stimulates insulin release, suppresses elevated glucagon levels, and delays gastric emptying. Patients with a DM diagnosis have impaired secretion of GLP-1.8
The GLP-1 RA liraglutide was approved by the FDA in January 2010 as a once-daily injection for patients with uncontrolled T2DM despite lifestyle changes and metformin monotherapy. Because of its intermediate half-life, liraglutide has an effect on both fasting and postprandial blood glucose.7 GLP-1 RAs are associated with reduced hypoglycemic episodes—an association attributable to the mechanism of action and potentially to improved pancreatic α-cell function.3,4 In July 2016, results of the LEADER trial showed that liraglutide therapy had a cardiovascular benefit in high-risk patients.8 In October 2017, liraglutide was FDAapproved for reducing 3-point major adverse cardiac events.7
Xultophy (Novo Nordisk, Plainsboro, NJ) is a fixed-dose medication combining degludec, a long-acting basal insulin analog, with liraglutide. As seen in the DUAL trials, Xultophy was more beneficial in reducing HbA1c levels than each component alone, and minimized hypoglycemic events, weight gain, and complexity of insulin treatment intensification.9-11 Therapy that combines basal insulin and a GLP-1 RA may be more effective than either agent as monotherapy and may have a significant impact on cardiovascular risk because of the synergistic vasodilatory, anti-inflammatory, and antioxidant properties of insulin and GLP-1 RA.6 In addition, combination therapy offers many benefits over traditional basal and bolus insulin regimens. These benefits include fewer daily injections, additional weight reduction resulting from the reduced insulin requirement, and fewer episodes of hypoglycemia. Reported gastrointestinal adverse effects have been transient and were not augmented when a GLP-1 RA was used in combination with basal insulin.11
Methods
We performed a retrospective chart analysis to quantify the benefit of using liraglutide as an add-on therapy to basal and bolus insulin regimens in veterans treated at VA Boston Healthcare System (VABHS). The analysis evaluated changes in insulin doses and HbA1c levels when liraglutide was added to these regimens. Patients identified for the study had electronic medication orders for concurrent therapy with liraglutide, insulin glargine, and insulin aspart filled through outpatient VABHS campus pharmacies for at least 3 months between January 2010 and December 2016. Sixty-nine patients who were on basal-bolus insulin for T2DM and who were prescribed liraglutide for treatment intensification were screened for inclusion and exclusion criteria. Data were analyzed at baseline and 3 months after liraglutide treatment.
Study Protocol
The inclusion criteria were patients aged ≥ 18 years, T2DM diagnosis, and therapy with insulin glargine and insulin aspart for at least 3 months before treatment intensi fication with liraglutide. Exclusion criteria were diagnosis of type 1 DM. To accurately quantify mean change in number of insulin units used, the study included patients only if they had been prescribed insulin glargine and insulin aspart before starting liraglutide. All other insulin regimens were excluded. To detect the true change that occurs when liraglutide is added to basal-bolus insulin, the study also excluded patients if they had been previously prescribed another GLP-1 RA. Patients with contraindications to liraglutide, insulin aspart, or insulin glargine were excluded as well. In addition, patients were excluded from the exposed arm if they were injecting < 1.2 mg of liraglutide once daily or if they had been on liraglutide for < 3 months.
Study Outcomes
All 35 patients who met the inclusion and exclusion criteria were included in this retrospective chart review. The primary outcome was determined by changes in HbA1c level and number of insulin doses 3 months after treatment with liraglutide. For each patient, a chart review was performed to determine the amount of insulin added or reduced during the study period. Data were collected at baseline and 3 months after initiation of liraglutide.
Statistical Analysis
Statistical analyses were performed with SPSS Version 20.0 (IBM, Armonk, NY). Population characteristics and study outcomes with normal distribution were compared using a paired t test and are reported as means with standard deviations. Nonnormally distributed variables (bolus insulin, HbA1c level) were compared using the nonparametric Wilcoxon rank sum test and are reported as median values with interquartile ranges. Normality was tested with the Shapiro-Wilk test. The primary outcome evaluated was change in number of insulin units used. Secondary outcomes included change in HbA1c level and change in body weight. A Bonferroni correction for multiple comparisons was used to prevent type I error. Significance at the Bonferroni-corrected level of .01 (.05/5 = .01) is indicated.
Results
Patients were included if they were previously on insulin glargine and insulin aspart before starting liraglutide for treatment intensification.
As Table 1 indicates, 100% of patients were male, and mean (SD) age was 65.5 (9.3) years.
After 3 months of therapy with liraglutide, HbA1c levels were reduced by a mean of 1.0% (P = .005) (Table 2).
Discussion
After 3 months of treatment with liraglutide, patients experienced a significant decrease in HbA1c levels. Insulin doses also decreased, but this finding was not statistically significant after correcting for multiple testing. These results are similar with those in larger studies of the effectiveness of liraglutide and the addition of liraglutide to insulin therapy. 6,8,12,13 Liraglutide has been shown to decrease HbA1c levels, lower rates of progression of kidney failure, decrease weight, and provide cardiovascular benefit.
In a prospective, randomized controlled trial evaluating the effect of adding liraglutide to insulin therapy, 21 of the 37 patients who had T2DM and required more than 100 total units of basal-bolus insulin daily were initiated on liraglutide, and changes in HbA1c level, body weight, and glycemic variability were compared. Results showed statistically significant improvement in all 3 outcomes in the group treated with liraglutide.6 Our findings, in conjunction with those of the larger studies, suggest that many of these results are generalizable to our local veteran population. Importantly, liraglutide was successfully started in pharmacy clinics—an indication that this treatment need not be initiated by an endocrine specialist.
Limitations
Given the lack of gender and racial diversity in this study population, our findings have limited generalizability to other populations. It is possible that, with a larger sample size, these results regarding reduced basal insulin doses would be significant. It has been hypothesized that patients experience fewer episodes of hypoglycemia when insulin doses are reduced, but we were unable to measure the frequency of these episodes. Other study limitations include inability to assess adherence and inability to account for concurrent regimens and/or for lifestyle changes that may have been made during the study period. Further, the study did not collect data on changes made to current DM medication regimens during the study period, and these changes may have influenced outcomes.
Conclusion
Patients who require treatment intensification for insulin-dependent T2DM may benefit from having liraglutide added to their basal-bolus insulin regimen. Liraglutide may prove to be more favorable than bolus insulin when choosing add-on therapy to basal insulin. Benefits include reductions in insulin doses, HbA1c levels, number of daily injections, and body weight. Therefore, we suggest that empirically reducing basal insulin by 10% to 25% and bolus insulin by 25% to 50% will avoid relative hypoglycemia. Prescribers must keep in mind patient-specific factors when adjusting insulin doses, if these doses are adjusted at all. Follow-up of 2 to 4 weeks is recommended for review of home monitoring of glucose for further insulin adjustments.
This study has important clinical implications. First, the finding of a reduction in HbA1c levels supports use of liraglutide therapy for HbA1c reduction in veterans. Second, the number of veterans who were successfully initiated on liraglutide therapy by nonphysician providers indicates that liraglutide can be effectively and safely started in primary care pharmacy clinics, increasing access to the medication.
Diabetes mellitus (DM) was the third most common medical diagnosis in 2016.1 Uncontrolled DM can lead to cardiovascular disease, nephropathy, neuropathy, and retinopathy. It is estimated that only 52.5% of patients with DM have achieved their goal hemoglobin A1c (HbA1c) level. The 2018 American Diabetes Association (ADA) clinical guidelines lack strong recommendations on sequential therapy for patients who have received a diagnosis of type 2 diabetes mellitus (T2DM) and have been unable to achieve their goal HbA1c level with lifestyle changes and maximum-dose metformin.2 Although those guidelines support treatment intensification with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), prescribing patterns for T2DM most commonly include adding insulin to try to control blood glucose and reduce long-term comorbidities.2,3
Related:
Insulin therapy is known for its ability to effectively lower blood glucose and HbA1c levels but comes with many limitations. Mealtime insulin has the highest risk of hypoglycemia, causes significant weight gain, requires several additional injections per day, and additional monitoring of blood glucose.4,5 The 2018 ADA guidelines state that hypoglycemia is the major limiting factor in the management of insulin-treated T2DM.2
Compared with mealtime insulin, GLP-1 RAs have the benefit of reducing the risk of hypoglycemia, weight gain, and number of daily injections.5 In addition, compared with insulin alone, GLP-1 RAs have the advantage of reducing glycemic variability.6 These advantages are especially attractive in the treatment of geriatric patients. Given its mechanism of action, liraglutide is expected to have an effect on both fasting and postprandial blood glucose. There are no recommendations on how to empirically reduce the dose of insulin when starting liraglutide.7
Background
GLP-1 is an incretin hormone that is secreted in response to meal ingestion. GLP-1 stimulates insulin release, suppresses elevated glucagon levels, and delays gastric emptying. Patients with a DM diagnosis have impaired secretion of GLP-1.8
The GLP-1 RA liraglutide was approved by the FDA in January 2010 as a once-daily injection for patients with uncontrolled T2DM despite lifestyle changes and metformin monotherapy. Because of its intermediate half-life, liraglutide has an effect on both fasting and postprandial blood glucose.7 GLP-1 RAs are associated with reduced hypoglycemic episodes—an association attributable to the mechanism of action and potentially to improved pancreatic α-cell function.3,4 In July 2016, results of the LEADER trial showed that liraglutide therapy had a cardiovascular benefit in high-risk patients.8 In October 2017, liraglutide was FDAapproved for reducing 3-point major adverse cardiac events.7
Xultophy (Novo Nordisk, Plainsboro, NJ) is a fixed-dose medication combining degludec, a long-acting basal insulin analog, with liraglutide. As seen in the DUAL trials, Xultophy was more beneficial in reducing HbA1c levels than each component alone, and minimized hypoglycemic events, weight gain, and complexity of insulin treatment intensification.9-11 Therapy that combines basal insulin and a GLP-1 RA may be more effective than either agent as monotherapy and may have a significant impact on cardiovascular risk because of the synergistic vasodilatory, anti-inflammatory, and antioxidant properties of insulin and GLP-1 RA.6 In addition, combination therapy offers many benefits over traditional basal and bolus insulin regimens. These benefits include fewer daily injections, additional weight reduction resulting from the reduced insulin requirement, and fewer episodes of hypoglycemia. Reported gastrointestinal adverse effects have been transient and were not augmented when a GLP-1 RA was used in combination with basal insulin.11
Methods
We performed a retrospective chart analysis to quantify the benefit of using liraglutide as an add-on therapy to basal and bolus insulin regimens in veterans treated at VA Boston Healthcare System (VABHS). The analysis evaluated changes in insulin doses and HbA1c levels when liraglutide was added to these regimens. Patients identified for the study had electronic medication orders for concurrent therapy with liraglutide, insulin glargine, and insulin aspart filled through outpatient VABHS campus pharmacies for at least 3 months between January 2010 and December 2016. Sixty-nine patients who were on basal-bolus insulin for T2DM and who were prescribed liraglutide for treatment intensification were screened for inclusion and exclusion criteria. Data were analyzed at baseline and 3 months after liraglutide treatment.
Study Protocol
The inclusion criteria were patients aged ≥ 18 years, T2DM diagnosis, and therapy with insulin glargine and insulin aspart for at least 3 months before treatment intensi fication with liraglutide. Exclusion criteria were diagnosis of type 1 DM. To accurately quantify mean change in number of insulin units used, the study included patients only if they had been prescribed insulin glargine and insulin aspart before starting liraglutide. All other insulin regimens were excluded. To detect the true change that occurs when liraglutide is added to basal-bolus insulin, the study also excluded patients if they had been previously prescribed another GLP-1 RA. Patients with contraindications to liraglutide, insulin aspart, or insulin glargine were excluded as well. In addition, patients were excluded from the exposed arm if they were injecting < 1.2 mg of liraglutide once daily or if they had been on liraglutide for < 3 months.
Study Outcomes
All 35 patients who met the inclusion and exclusion criteria were included in this retrospective chart review. The primary outcome was determined by changes in HbA1c level and number of insulin doses 3 months after treatment with liraglutide. For each patient, a chart review was performed to determine the amount of insulin added or reduced during the study period. Data were collected at baseline and 3 months after initiation of liraglutide.
Statistical Analysis
Statistical analyses were performed with SPSS Version 20.0 (IBM, Armonk, NY). Population characteristics and study outcomes with normal distribution were compared using a paired t test and are reported as means with standard deviations. Nonnormally distributed variables (bolus insulin, HbA1c level) were compared using the nonparametric Wilcoxon rank sum test and are reported as median values with interquartile ranges. Normality was tested with the Shapiro-Wilk test. The primary outcome evaluated was change in number of insulin units used. Secondary outcomes included change in HbA1c level and change in body weight. A Bonferroni correction for multiple comparisons was used to prevent type I error. Significance at the Bonferroni-corrected level of .01 (.05/5 = .01) is indicated.
Results
Patients were included if they were previously on insulin glargine and insulin aspart before starting liraglutide for treatment intensification.
As Table 1 indicates, 100% of patients were male, and mean (SD) age was 65.5 (9.3) years.
After 3 months of therapy with liraglutide, HbA1c levels were reduced by a mean of 1.0% (P = .005) (Table 2).
Discussion
After 3 months of treatment with liraglutide, patients experienced a significant decrease in HbA1c levels. Insulin doses also decreased, but this finding was not statistically significant after correcting for multiple testing. These results are similar with those in larger studies of the effectiveness of liraglutide and the addition of liraglutide to insulin therapy. 6,8,12,13 Liraglutide has been shown to decrease HbA1c levels, lower rates of progression of kidney failure, decrease weight, and provide cardiovascular benefit.
In a prospective, randomized controlled trial evaluating the effect of adding liraglutide to insulin therapy, 21 of the 37 patients who had T2DM and required more than 100 total units of basal-bolus insulin daily were initiated on liraglutide, and changes in HbA1c level, body weight, and glycemic variability were compared. Results showed statistically significant improvement in all 3 outcomes in the group treated with liraglutide.6 Our findings, in conjunction with those of the larger studies, suggest that many of these results are generalizable to our local veteran population. Importantly, liraglutide was successfully started in pharmacy clinics—an indication that this treatment need not be initiated by an endocrine specialist.
Limitations
Given the lack of gender and racial diversity in this study population, our findings have limited generalizability to other populations. It is possible that, with a larger sample size, these results regarding reduced basal insulin doses would be significant. It has been hypothesized that patients experience fewer episodes of hypoglycemia when insulin doses are reduced, but we were unable to measure the frequency of these episodes. Other study limitations include inability to assess adherence and inability to account for concurrent regimens and/or for lifestyle changes that may have been made during the study period. Further, the study did not collect data on changes made to current DM medication regimens during the study period, and these changes may have influenced outcomes.
Conclusion
Patients who require treatment intensification for insulin-dependent T2DM may benefit from having liraglutide added to their basal-bolus insulin regimen. Liraglutide may prove to be more favorable than bolus insulin when choosing add-on therapy to basal insulin. Benefits include reductions in insulin doses, HbA1c levels, number of daily injections, and body weight. Therefore, we suggest that empirically reducing basal insulin by 10% to 25% and bolus insulin by 25% to 50% will avoid relative hypoglycemia. Prescribers must keep in mind patient-specific factors when adjusting insulin doses, if these doses are adjusted at all. Follow-up of 2 to 4 weeks is recommended for review of home monitoring of glucose for further insulin adjustments.
This study has important clinical implications. First, the finding of a reduction in HbA1c levels supports use of liraglutide therapy for HbA1c reduction in veterans. Second, the number of veterans who were successfully initiated on liraglutide therapy by nonphysician providers indicates that liraglutide can be effectively and safely started in primary care pharmacy clinics, increasing access to the medication.
1. Centers for Medicare & Medicaid Services. ICD-10. https://www.cms.gov/medicare/coding/icd10. Accessed July 26, 2018.
2. American Diabetes Association. Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S2.
3. Combination therapy with insulins and GLP-1 receptor agonists. http://www.powerpak.com/course/content/113275. Updated 2018. Accessed July 26, 2018.
4. Carris NW, Taylor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74(18):2141-2152.
5. Young LA, Buse JB, Weaver MA, et al; Monitor Trial Group. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017;177(7):920-929.
6. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832.
7. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; August 2017.
8. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
9. Buse JB, Vilsbøll T, Thurman J, et al; NN9068-3912 (DUAL-II) Trial Investigators. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-2933
10. Glough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its component given alone: results of a phase 3, open-label, randomized, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
11. Lingvay I, Pérez Manghi F, García-Hernández P, et al; DUAL V Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized controlled trial. JAMA. 2016;315(9):898-907.
12. Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, anti-inflammatory and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37(7):1938-1943.
13. Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Torffvit O, Pehrsson NG. Design and methods of a randomised double-blind trial of adding liraglutide to control HbA1c in patients with type 2 diabetes with impaired glycaemic control treated with multiple daily insulin injections (MDI-Liraglutide trial). Prim Care Diabetes. 2015;9(1):15-22.
1. Centers for Medicare & Medicaid Services. ICD-10. https://www.cms.gov/medicare/coding/icd10. Accessed July 26, 2018.
2. American Diabetes Association. Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S2.
3. Combination therapy with insulins and GLP-1 receptor agonists. http://www.powerpak.com/course/content/113275. Updated 2018. Accessed July 26, 2018.
4. Carris NW, Taylor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74(18):2141-2152.
5. Young LA, Buse JB, Weaver MA, et al; Monitor Trial Group. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017;177(7):920-929.
6. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832.
7. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; August 2017.
8. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
9. Buse JB, Vilsbøll T, Thurman J, et al; NN9068-3912 (DUAL-II) Trial Investigators. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-2933
10. Glough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its component given alone: results of a phase 3, open-label, randomized, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
11. Lingvay I, Pérez Manghi F, García-Hernández P, et al; DUAL V Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized controlled trial. JAMA. 2016;315(9):898-907.
12. Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, anti-inflammatory and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37(7):1938-1943.
13. Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Torffvit O, Pehrsson NG. Design and methods of a randomised double-blind trial of adding liraglutide to control HbA1c in patients with type 2 diabetes with impaired glycaemic control treated with multiple daily insulin injections (MDI-Liraglutide trial). Prim Care Diabetes. 2015;9(1):15-22.
FDA issues warnings to companies selling illegal Alzheimer’s treatments
The Food and Drug Administration has issued warning letters to 12 companies and advisory letters to 5 companies illegally selling more than 58 products claiming to treat Alzheimer’s disease.
The products, many of which are marketed as dietary supplements, are being sold in a variety of forms, including tablets, capsules, and oils. These drugs are either unapproved or mislabeled and claim to prevent, treat, or cure Alzheimer’s disease, as well as a number of other serious diseases and health conditions, in violation of the Federal Food, Drug, and Cosmetic Act.
“Alzheimer’s is a challenging disease that, unfortunately, has no cure. Any products making unproven drug claims could mislead consumers to believe that such therapies exist and keep them from accessing therapies that are known to help support the symptoms of the disease, or worse, as some fraudulent treatments can cause serious or even fatal injuries,” FDA Commissioner Scott Gottlieb, MD, said in a press release.
In an additional statement, Dr. Gottlieb detailed several new strategies for improving the safety and accuracy of dietary supplements, including efforts to more rapidly communicate to the public potential safety issues with dietary supplement products and to establish a flexible regulatory framework that promotes innovation and upholds product safety.
The Food and Drug Administration has issued warning letters to 12 companies and advisory letters to 5 companies illegally selling more than 58 products claiming to treat Alzheimer’s disease.
The products, many of which are marketed as dietary supplements, are being sold in a variety of forms, including tablets, capsules, and oils. These drugs are either unapproved or mislabeled and claim to prevent, treat, or cure Alzheimer’s disease, as well as a number of other serious diseases and health conditions, in violation of the Federal Food, Drug, and Cosmetic Act.
“Alzheimer’s is a challenging disease that, unfortunately, has no cure. Any products making unproven drug claims could mislead consumers to believe that such therapies exist and keep them from accessing therapies that are known to help support the symptoms of the disease, or worse, as some fraudulent treatments can cause serious or even fatal injuries,” FDA Commissioner Scott Gottlieb, MD, said in a press release.
In an additional statement, Dr. Gottlieb detailed several new strategies for improving the safety and accuracy of dietary supplements, including efforts to more rapidly communicate to the public potential safety issues with dietary supplement products and to establish a flexible regulatory framework that promotes innovation and upholds product safety.
The Food and Drug Administration has issued warning letters to 12 companies and advisory letters to 5 companies illegally selling more than 58 products claiming to treat Alzheimer’s disease.
The products, many of which are marketed as dietary supplements, are being sold in a variety of forms, including tablets, capsules, and oils. These drugs are either unapproved or mislabeled and claim to prevent, treat, or cure Alzheimer’s disease, as well as a number of other serious diseases and health conditions, in violation of the Federal Food, Drug, and Cosmetic Act.
“Alzheimer’s is a challenging disease that, unfortunately, has no cure. Any products making unproven drug claims could mislead consumers to believe that such therapies exist and keep them from accessing therapies that are known to help support the symptoms of the disease, or worse, as some fraudulent treatments can cause serious or even fatal injuries,” FDA Commissioner Scott Gottlieb, MD, said in a press release.
In an additional statement, Dr. Gottlieb detailed several new strategies for improving the safety and accuracy of dietary supplements, including efforts to more rapidly communicate to the public potential safety issues with dietary supplement products and to establish a flexible regulatory framework that promotes innovation and upholds product safety.
Miscommunication With Dermatology Patients: Are We Speaking the Same Language?
I was a third-year medical student, dutifully reviewing discharge instructions with a patient and her family. The patient’s adult daughter asked, “What about that diet you put her on?” As they looked at me quizzically, I looked back equally confused, until it clicked: We needed to talk about the word diet. In everyday conversation, diet generally is understood to mean restriction of food to lose weight, which is what the family hoped would be prescribed for their obese family member. I needed to tell them that I was sorry for the misunderstanding. If they overheard us “ordering a diet,” we simply meant providing trays of hospital food.
We become so familiar with the language of our profession that we do not remember it may be foreign to our patients. In dermatology, we are aware that our specialty is full of esoteric jargon and complex concepts that need to be carefully explained to our patients in simpler terms. But since that incident in medical school, I have been interested in the more insidious potential misunderstandings that can arise from words as seemingly simple as diet. There are many examples in dermatology, particularly in the way we prescribe topical therapy and use trade names.
Topical Therapy
Instructions for systemic medications may be as simple as “take 1 pill twice daily.” Prescriptions for topical medications can be written with an equally simple patient signature such as “apply twice daily to affected area,” but the simplicity is deceptive. The direction to “apply” may seem intuitive to the prescriber, but we do not always specify the amount. Sunscreen, for example, is notoriously underapplied when the actual amount of product needed for protection is not demonstrated.1 One study of new dermatology patients given a prescription for a new topical medication found that the majority of patients underdosed.2
Determination of an “affected area,” regardless of whether the site is indicated, can be even less straightforward. In acne treatment, the affected area is the whole region prone to acne breakouts, whereas in psoriasis it may be discrete psoriatic plaques. We may believe our explanations are perfectly clear, but we have all seen patients spot treating their acne or psoriasis patients covering entire territories of normal skin with topical steroids, despite our education. One study of eczema action plans found that there was considerable variability in the way different providers described disease flares that require treatment. For example, redness was only used as a descriptor of an eczema flare in 68.2% of eczema action plans studied.3 Ensuring our patients understand our criteria for skin requiring topical treatment may mean the difference between treatment success and failure and also may help to avoid unnecessary side effects.
Adherence to topical medication regimens is poor, and inadequate patient education is only one factor.4,5 One study found that more than one-third of new prescriptions for topical medications were never even filled.6 However, improving our communication about application of topical drugs is one way we must address the complicated issue of adherence.
Trade Names
In dermatology, we often use trade names to refer to our medications, even if we do not intend to reference the brand name of the drug specifically. We may tell a patient to use Lidex (Medicis Pharmaceutical Corporation) for her hands but then send an escript to her pharmacy for fluocinonide. Trade names are designed to roll off the tongue, in contrast to the unwieldy, clumsily long generic names assigned to many of our medications.
Substituting trade names may facilitate more natural conversation to promote patient understanding in some cases; however, there are pitfalls associated with this habit. First, we may be doing our patients a disservice if we do not clarify when it would be acceptable to substitute with the generic when the medication is available over-the-counter. If we decide to treat with Rogaine (Johnson & Johnson Consumer Inc) but do not suggest the option of purchasing the generic minoxidil, the patient could be unnecessarily overpaying for a brand name by following our instructions.
Conversely, there are scenarios in which the use of a brand name is actually not specific enough. A patient once told me she was using Differin (Galderma Laboratories, LP) as discussed at her prior visit, but she revealed she was washing it off after application. I initially assumed she misunderstood that adapalene was a gel to be applied and left on. After additional questioning, however, it became clear that she purchased the Differin gentle cleanser, a nonmedicated facial wash, rather than the retinoid we had intended for her. I had not considered that Differin would market an entire line of skin care products but now realize we must be cautious using Differin and adapalene interchangeably. Other examples include popular over-the-counter antihistamine brands such as Allegra (Chattem, a Sanofi company) or Benadryl (Johnson & Johnson Consumer Inc) that market multiple products with different active ingredients.
Final Thoughts
The smooth transfer of information between physician and patient is key to a healthy therapeutic relationship. In residency and throughout our careers, we will continue to develop and refine our communication skills to best serve our patients. We should pay particular attention to the unexpected and surprising ways in which we fail to adequately communicate, make note of these patterns, and share with our colleagues so that we can all learn from our collective experiences.
- Schneider, J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Storm A, Benfeldt E, Andersen SE, et al. A prospective study of patient adherence to topical treatments: 95% of patients underdose. J Am Acad Dermatol. 2008;59:975-980.
- Stringer T, Yin HS, Gittler J, et al. The readability, suitability, and content features of eczema action plans in the United States. Pediatr Dermatol. 2018;35:800-807.
- Hougeir FG, Cook-Bolden FE, Rodriguez D, et al. Critical considerations on optimizing topical corticosteroid therapy. J Clin Aesthet Dermatol. 2015;8(suppl 1):S2-S14.
- Savary J, Ortonne JP, Aractingi S. The right dose in the right place: an overview of current prescription, instruction and application modalities for topical psoriasis treatments. J Eur Acad Dermatol Venereol. 2005;19:14-17.
- Storm A, Anderson SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
I was a third-year medical student, dutifully reviewing discharge instructions with a patient and her family. The patient’s adult daughter asked, “What about that diet you put her on?” As they looked at me quizzically, I looked back equally confused, until it clicked: We needed to talk about the word diet. In everyday conversation, diet generally is understood to mean restriction of food to lose weight, which is what the family hoped would be prescribed for their obese family member. I needed to tell them that I was sorry for the misunderstanding. If they overheard us “ordering a diet,” we simply meant providing trays of hospital food.
We become so familiar with the language of our profession that we do not remember it may be foreign to our patients. In dermatology, we are aware that our specialty is full of esoteric jargon and complex concepts that need to be carefully explained to our patients in simpler terms. But since that incident in medical school, I have been interested in the more insidious potential misunderstandings that can arise from words as seemingly simple as diet. There are many examples in dermatology, particularly in the way we prescribe topical therapy and use trade names.
Topical Therapy
Instructions for systemic medications may be as simple as “take 1 pill twice daily.” Prescriptions for topical medications can be written with an equally simple patient signature such as “apply twice daily to affected area,” but the simplicity is deceptive. The direction to “apply” may seem intuitive to the prescriber, but we do not always specify the amount. Sunscreen, for example, is notoriously underapplied when the actual amount of product needed for protection is not demonstrated.1 One study of new dermatology patients given a prescription for a new topical medication found that the majority of patients underdosed.2
Determination of an “affected area,” regardless of whether the site is indicated, can be even less straightforward. In acne treatment, the affected area is the whole region prone to acne breakouts, whereas in psoriasis it may be discrete psoriatic plaques. We may believe our explanations are perfectly clear, but we have all seen patients spot treating their acne or psoriasis patients covering entire territories of normal skin with topical steroids, despite our education. One study of eczema action plans found that there was considerable variability in the way different providers described disease flares that require treatment. For example, redness was only used as a descriptor of an eczema flare in 68.2% of eczema action plans studied.3 Ensuring our patients understand our criteria for skin requiring topical treatment may mean the difference between treatment success and failure and also may help to avoid unnecessary side effects.
Adherence to topical medication regimens is poor, and inadequate patient education is only one factor.4,5 One study found that more than one-third of new prescriptions for topical medications were never even filled.6 However, improving our communication about application of topical drugs is one way we must address the complicated issue of adherence.
Trade Names
In dermatology, we often use trade names to refer to our medications, even if we do not intend to reference the brand name of the drug specifically. We may tell a patient to use Lidex (Medicis Pharmaceutical Corporation) for her hands but then send an escript to her pharmacy for fluocinonide. Trade names are designed to roll off the tongue, in contrast to the unwieldy, clumsily long generic names assigned to many of our medications.
Substituting trade names may facilitate more natural conversation to promote patient understanding in some cases; however, there are pitfalls associated with this habit. First, we may be doing our patients a disservice if we do not clarify when it would be acceptable to substitute with the generic when the medication is available over-the-counter. If we decide to treat with Rogaine (Johnson & Johnson Consumer Inc) but do not suggest the option of purchasing the generic minoxidil, the patient could be unnecessarily overpaying for a brand name by following our instructions.
Conversely, there are scenarios in which the use of a brand name is actually not specific enough. A patient once told me she was using Differin (Galderma Laboratories, LP) as discussed at her prior visit, but she revealed she was washing it off after application. I initially assumed she misunderstood that adapalene was a gel to be applied and left on. After additional questioning, however, it became clear that she purchased the Differin gentle cleanser, a nonmedicated facial wash, rather than the retinoid we had intended for her. I had not considered that Differin would market an entire line of skin care products but now realize we must be cautious using Differin and adapalene interchangeably. Other examples include popular over-the-counter antihistamine brands such as Allegra (Chattem, a Sanofi company) or Benadryl (Johnson & Johnson Consumer Inc) that market multiple products with different active ingredients.
Final Thoughts
The smooth transfer of information between physician and patient is key to a healthy therapeutic relationship. In residency and throughout our careers, we will continue to develop and refine our communication skills to best serve our patients. We should pay particular attention to the unexpected and surprising ways in which we fail to adequately communicate, make note of these patterns, and share with our colleagues so that we can all learn from our collective experiences.
I was a third-year medical student, dutifully reviewing discharge instructions with a patient and her family. The patient’s adult daughter asked, “What about that diet you put her on?” As they looked at me quizzically, I looked back equally confused, until it clicked: We needed to talk about the word diet. In everyday conversation, diet generally is understood to mean restriction of food to lose weight, which is what the family hoped would be prescribed for their obese family member. I needed to tell them that I was sorry for the misunderstanding. If they overheard us “ordering a diet,” we simply meant providing trays of hospital food.
We become so familiar with the language of our profession that we do not remember it may be foreign to our patients. In dermatology, we are aware that our specialty is full of esoteric jargon and complex concepts that need to be carefully explained to our patients in simpler terms. But since that incident in medical school, I have been interested in the more insidious potential misunderstandings that can arise from words as seemingly simple as diet. There are many examples in dermatology, particularly in the way we prescribe topical therapy and use trade names.
Topical Therapy
Instructions for systemic medications may be as simple as “take 1 pill twice daily.” Prescriptions for topical medications can be written with an equally simple patient signature such as “apply twice daily to affected area,” but the simplicity is deceptive. The direction to “apply” may seem intuitive to the prescriber, but we do not always specify the amount. Sunscreen, for example, is notoriously underapplied when the actual amount of product needed for protection is not demonstrated.1 One study of new dermatology patients given a prescription for a new topical medication found that the majority of patients underdosed.2
Determination of an “affected area,” regardless of whether the site is indicated, can be even less straightforward. In acne treatment, the affected area is the whole region prone to acne breakouts, whereas in psoriasis it may be discrete psoriatic plaques. We may believe our explanations are perfectly clear, but we have all seen patients spot treating their acne or psoriasis patients covering entire territories of normal skin with topical steroids, despite our education. One study of eczema action plans found that there was considerable variability in the way different providers described disease flares that require treatment. For example, redness was only used as a descriptor of an eczema flare in 68.2% of eczema action plans studied.3 Ensuring our patients understand our criteria for skin requiring topical treatment may mean the difference between treatment success and failure and also may help to avoid unnecessary side effects.
Adherence to topical medication regimens is poor, and inadequate patient education is only one factor.4,5 One study found that more than one-third of new prescriptions for topical medications were never even filled.6 However, improving our communication about application of topical drugs is one way we must address the complicated issue of adherence.
Trade Names
In dermatology, we often use trade names to refer to our medications, even if we do not intend to reference the brand name of the drug specifically. We may tell a patient to use Lidex (Medicis Pharmaceutical Corporation) for her hands but then send an escript to her pharmacy for fluocinonide. Trade names are designed to roll off the tongue, in contrast to the unwieldy, clumsily long generic names assigned to many of our medications.
Substituting trade names may facilitate more natural conversation to promote patient understanding in some cases; however, there are pitfalls associated with this habit. First, we may be doing our patients a disservice if we do not clarify when it would be acceptable to substitute with the generic when the medication is available over-the-counter. If we decide to treat with Rogaine (Johnson & Johnson Consumer Inc) but do not suggest the option of purchasing the generic minoxidil, the patient could be unnecessarily overpaying for a brand name by following our instructions.
Conversely, there are scenarios in which the use of a brand name is actually not specific enough. A patient once told me she was using Differin (Galderma Laboratories, LP) as discussed at her prior visit, but she revealed she was washing it off after application. I initially assumed she misunderstood that adapalene was a gel to be applied and left on. After additional questioning, however, it became clear that she purchased the Differin gentle cleanser, a nonmedicated facial wash, rather than the retinoid we had intended for her. I had not considered that Differin would market an entire line of skin care products but now realize we must be cautious using Differin and adapalene interchangeably. Other examples include popular over-the-counter antihistamine brands such as Allegra (Chattem, a Sanofi company) or Benadryl (Johnson & Johnson Consumer Inc) that market multiple products with different active ingredients.
Final Thoughts
The smooth transfer of information between physician and patient is key to a healthy therapeutic relationship. In residency and throughout our careers, we will continue to develop and refine our communication skills to best serve our patients. We should pay particular attention to the unexpected and surprising ways in which we fail to adequately communicate, make note of these patterns, and share with our colleagues so that we can all learn from our collective experiences.
- Schneider, J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Storm A, Benfeldt E, Andersen SE, et al. A prospective study of patient adherence to topical treatments: 95% of patients underdose. J Am Acad Dermatol. 2008;59:975-980.
- Stringer T, Yin HS, Gittler J, et al. The readability, suitability, and content features of eczema action plans in the United States. Pediatr Dermatol. 2018;35:800-807.
- Hougeir FG, Cook-Bolden FE, Rodriguez D, et al. Critical considerations on optimizing topical corticosteroid therapy. J Clin Aesthet Dermatol. 2015;8(suppl 1):S2-S14.
- Savary J, Ortonne JP, Aractingi S. The right dose in the right place: an overview of current prescription, instruction and application modalities for topical psoriasis treatments. J Eur Acad Dermatol Venereol. 2005;19:14-17.
- Storm A, Anderson SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Schneider, J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Storm A, Benfeldt E, Andersen SE, et al. A prospective study of patient adherence to topical treatments: 95% of patients underdose. J Am Acad Dermatol. 2008;59:975-980.
- Stringer T, Yin HS, Gittler J, et al. The readability, suitability, and content features of eczema action plans in the United States. Pediatr Dermatol. 2018;35:800-807.
- Hougeir FG, Cook-Bolden FE, Rodriguez D, et al. Critical considerations on optimizing topical corticosteroid therapy. J Clin Aesthet Dermatol. 2015;8(suppl 1):S2-S14.
- Savary J, Ortonne JP, Aractingi S. The right dose in the right place: an overview of current prescription, instruction and application modalities for topical psoriasis treatments. J Eur Acad Dermatol Venereol. 2005;19:14-17.
- Storm A, Anderson SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
Resident Pearl
- It is not just the esoteric jargon and complex pathophysiologic concepts in dermatology that can challenge effective communication with our patients. We face potential for misunderstanding even in situations that may seem straightforward. Vigilance in avoiding ambiguity in all our exchanges with patients can help foster therapeutic relationships and optimize patient care.
A 60-year-old white woman presented with a 3-month history of a painful, nonhealing ulceration on her left lateral lower leg
It is a vasculopathy rather than a vasculitis as the former is caused by occlusion of blood vessels and the latter results from inflammation of the vessels. Middle-aged women tend to be affected more frequently. Although the exact cause is unclear, systemic diseases, such as hypercoagulable states, may predispose vessels to develop occlusion. Associated disorders include antiphospholipid syndrome, protein C deficiency, factor V mutation, arteriosclerosis, hyperhomocysteinemia, and hepatitis C.
Typically, lesions begin as painful purpura or reticulated macules on the lower extremities that ulcerate and heal very slowly. Ankles, particularly malleoli, are more frequently affected. When they heal, they form painless white stellate scars typical of atrophie blanche. Surrounding erythema, telangiectasias, and sclerosis may be present; livedo reticularis may be seen as well.
Histologically, the epidermis may be atrophic or necrotic. Hyaline thickening of the blood vessel walls is seen. Thrombi may be present. Direct immunofluorescence of perilesional skin may be positive for complement C3 and immunoglobulin (IgM) in dermal blood vessels.
Livedoid vasculopathy can be difficult to treat. Treatment is aimed at reducing clotting and improving blood flow and includes antiplatelet drugs (low-dose aspirin, dipyridamole), anticoagulants, and vasodilating agents (nifedipine). Pentoxifylline two or three times daily may help by altering blood viscosity. A recent literature search reports success in topical dapsone applied to lesions twice daily under occlusion. Leg elevation and compression stockings help healing. Livedoid vasculopathy may have periods of activity and remission.
The case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/edermatologynews.com. To submit a case for possible publication, send an email to [email protected]
It is a vasculopathy rather than a vasculitis as the former is caused by occlusion of blood vessels and the latter results from inflammation of the vessels. Middle-aged women tend to be affected more frequently. Although the exact cause is unclear, systemic diseases, such as hypercoagulable states, may predispose vessels to develop occlusion. Associated disorders include antiphospholipid syndrome, protein C deficiency, factor V mutation, arteriosclerosis, hyperhomocysteinemia, and hepatitis C.
Typically, lesions begin as painful purpura or reticulated macules on the lower extremities that ulcerate and heal very slowly. Ankles, particularly malleoli, are more frequently affected. When they heal, they form painless white stellate scars typical of atrophie blanche. Surrounding erythema, telangiectasias, and sclerosis may be present; livedo reticularis may be seen as well.
Histologically, the epidermis may be atrophic or necrotic. Hyaline thickening of the blood vessel walls is seen. Thrombi may be present. Direct immunofluorescence of perilesional skin may be positive for complement C3 and immunoglobulin (IgM) in dermal blood vessels.
Livedoid vasculopathy can be difficult to treat. Treatment is aimed at reducing clotting and improving blood flow and includes antiplatelet drugs (low-dose aspirin, dipyridamole), anticoagulants, and vasodilating agents (nifedipine). Pentoxifylline two or three times daily may help by altering blood viscosity. A recent literature search reports success in topical dapsone applied to lesions twice daily under occlusion. Leg elevation and compression stockings help healing. Livedoid vasculopathy may have periods of activity and remission.
The case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/edermatologynews.com. To submit a case for possible publication, send an email to [email protected]
It is a vasculopathy rather than a vasculitis as the former is caused by occlusion of blood vessels and the latter results from inflammation of the vessels. Middle-aged women tend to be affected more frequently. Although the exact cause is unclear, systemic diseases, such as hypercoagulable states, may predispose vessels to develop occlusion. Associated disorders include antiphospholipid syndrome, protein C deficiency, factor V mutation, arteriosclerosis, hyperhomocysteinemia, and hepatitis C.
Typically, lesions begin as painful purpura or reticulated macules on the lower extremities that ulcerate and heal very slowly. Ankles, particularly malleoli, are more frequently affected. When they heal, they form painless white stellate scars typical of atrophie blanche. Surrounding erythema, telangiectasias, and sclerosis may be present; livedo reticularis may be seen as well.
Histologically, the epidermis may be atrophic or necrotic. Hyaline thickening of the blood vessel walls is seen. Thrombi may be present. Direct immunofluorescence of perilesional skin may be positive for complement C3 and immunoglobulin (IgM) in dermal blood vessels.
Livedoid vasculopathy can be difficult to treat. Treatment is aimed at reducing clotting and improving blood flow and includes antiplatelet drugs (low-dose aspirin, dipyridamole), anticoagulants, and vasodilating agents (nifedipine). Pentoxifylline two or three times daily may help by altering blood viscosity. A recent literature search reports success in topical dapsone applied to lesions twice daily under occlusion. Leg elevation and compression stockings help healing. Livedoid vasculopathy may have periods of activity and remission.
The case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/edermatologynews.com. To submit a case for possible publication, send an email to [email protected]
USPSTF recommends counseling for perinatal depression prevention
according to a B recommendation from the U.S. Preventive Services Task Force.
The Task Force determined that counseling interventions are effective in preventing perinatal depression, defined as a major or minor depressive episode during pregnancy or within the first year after delivery. The condition affects an estimated 12% of new mothers in the United States each year, according to lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and her colleagues.
The recommendation, published in JAMA, applies to “pregnant persons and persons who are less than 1 year postpartum who do not have a current diagnosis of depression but are at increased risk of developing depression,” according to the authors (JAMA. 2019 Feb 12;321(6):580-7).
Risk factors for development of perinatal depression include:
- Past history of depression.
- Current depressive symptoms (that do not reach a diagnostic threshold).
- History of physical or sexual abuse.
- Unplanned or unwanted pregnancy.
- Stressful life events.
- Lack of social and financial support.
- Intimate partner violence.
- Pregestational or gestational diabetes.
- Complications during pregnancy.
- Adolescent parenthood.
- Low socioeconomic status.
- Lack of social support.
After reviewing the evidence, the USPSTF found a moderate net benefit for counseling interventions, particularly cognitive behavioral therapy and interpersonal therapy, for preventing perinatal depression in women at risk. Counseling sessions reviewed for this recommendation ranged from 4 to 20 meetings (median, 8 meetings).
The USPSTF found inadequate evidence to assess the harms and benefits of other noncounseling interventions, including pharmacologic therapy.
In the evidence review accompanying the recommendations, Elizabeth A. O’Connor, PhD, of Kaiser Permanente, Portland, Ore., and her colleagues analyzed data from 50 studies including 22,385 individuals; 20 of these studies were randomized, controlled trials of counseling interventions (JAMA. 2019 Feb 12;321(6):588-601).
Overall, the likelihood of perinatal depression was significantly lower among women who received counseling, compared with controls, among more than 3,000 women in those studies (pooled risk ratio 0.61). Absolute risk differences for perinatal depression ranged from a 1% increased reduction in controls to a 32% increased reduction among women who received counseling. The effects were strongest for cognitive behavioral therapy and interpersonal therapy as interventions. No adverse events were reported in the counseling intervention studies.
In three studies of health system interventions, the researchers found a benefit for interventions vs. controls, but the difference was not statistically significant.
Trials of most other alternative interventions including infant sleep advice, birth-experience postpartum debriefing, omega-3 fatty acid supplementation, expressive writing, antidepressants, and yoga did not show statistical significance in benefit for reducing perinatal depression.
Only one of three randomized controlled trials of physical activity found a statistically significant group difference.
A trial of nortriptyline to prevent perinatal depression showed no benefit, compared with placebo. A sertraline study of found “a smaller percentage of participants taking sertraline had a depression recurrence, compared with those taking placebo,” the investigators wrote. In these two studies, women who took nortriptyline showed no adverse effects, and those in a trial involving sertraline reported significantly more dizziness and drowsiness compared with placebo patients.
The evidence review was limited by the small number of quality studies, especially studies of alternative interventions. More research is needed; however, the findings support data from similar reviews and support the potential for counseling to prevent perinatal depression, particularly in women at increased risk for perinatal depression, Dr. O’Connor and her associates said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. Coauthor Dr. Michelle L. Henninger reported receiving grants from Pfizer IGLC (Independent Grants for Learning & Change) outside the submitted work. Coauthor Dr. Bradley N. Gaynes reported receiving personal fees from LivaNova and Johnson & Johnson outside the submitted work. The remaining researchers had no financial conflicts to disclose.
SOURCE: Curry SJ et al. JAMA. 2019;321(6):580-7; O’Connor E et al. JAMA. 2019;321(6):588-601.
A proactive approach to prevention and management of perinatal depression as recommended by the USPSTF can potentially improve outcomes for new mothers and their babies, Marlene P. Freeman, MD, wrote in an accompanying editorial. She identified three key challenges to implementing the USPSTF recommendations: identifying women at risk, connecting them to evidence-based treatment, and assessing outcomes after treatment.
The development of screening tools would help clinicians identify women at risk for perinatal depression, Dr. Freeman said. No such tool currently exists, but in the meantime, “women at risk may be identified by targeting those with histories of depression, subthreshold depressive symptoms, and certain sociodemographic factors (i.e., economically disadvantaged, single/young, unplanned pregnancy).”
The counseling interventions shown to be effective in preventing perinatal depression – cognitive behavioral therapy and interpersonal psychotherapy – require education and training outside the time limitations and expertise of many clinicians providing obstetric care, she noted.
“The delivery of effective care on a large scale will require creative solutions,” such as the use of telehealth and smartphone platforms, and the involvement of multidisciplinary teams to care for women with severe illness, Dr. Freeman said. “In addition, a substantial number of reproductive-aged women have serious psychiatric disorders and will be identified as at risk for perinatal depression, although their needs may be more comprehensive,” she wrote. “Women who are identified as at risk for perinatal depression may have psychotic disorders, bipolar spectrum disorders, anxiety disorders, and substance use disorders, and there is comorbidity among psychiatric disorders. Therefore, systematic provisions for referral and treatment for other psychiatric disorders should be considered.” Further research is needed to explore treatment options including pharmacotherapy for women with severe psychiatric disorders.
However, she expressed optimism that the recommendations for screening and counseling for perinatal depression are valuable, and they “may return great dividends in the form of enhanced well-being of mothers and their offspring.”
Dr. Freeman is affiliated with the department of psychiatry at Massachusetts General Hospital, Boston. She commented in an editorial accompanying the article by Curry et al. (JAMA. 2019 Feb 12;321[6]:550-2). Dr. Freeman disclosed relationships with companies including Takeda, JayMac, Sage, Otsuka, Alkermes, Janssen, and Sunovion; she also disclosed serving on an independent data safety and monitoring committee for Janssen (Johnson & Johnson); and editing the GOED (Global Organization for EPA & DHA Omega-3) newsletter.
A proactive approach to prevention and management of perinatal depression as recommended by the USPSTF can potentially improve outcomes for new mothers and their babies, Marlene P. Freeman, MD, wrote in an accompanying editorial. She identified three key challenges to implementing the USPSTF recommendations: identifying women at risk, connecting them to evidence-based treatment, and assessing outcomes after treatment.
The development of screening tools would help clinicians identify women at risk for perinatal depression, Dr. Freeman said. No such tool currently exists, but in the meantime, “women at risk may be identified by targeting those with histories of depression, subthreshold depressive symptoms, and certain sociodemographic factors (i.e., economically disadvantaged, single/young, unplanned pregnancy).”
The counseling interventions shown to be effective in preventing perinatal depression – cognitive behavioral therapy and interpersonal psychotherapy – require education and training outside the time limitations and expertise of many clinicians providing obstetric care, she noted.
“The delivery of effective care on a large scale will require creative solutions,” such as the use of telehealth and smartphone platforms, and the involvement of multidisciplinary teams to care for women with severe illness, Dr. Freeman said. “In addition, a substantial number of reproductive-aged women have serious psychiatric disorders and will be identified as at risk for perinatal depression, although their needs may be more comprehensive,” she wrote. “Women who are identified as at risk for perinatal depression may have psychotic disorders, bipolar spectrum disorders, anxiety disorders, and substance use disorders, and there is comorbidity among psychiatric disorders. Therefore, systematic provisions for referral and treatment for other psychiatric disorders should be considered.” Further research is needed to explore treatment options including pharmacotherapy for women with severe psychiatric disorders.
However, she expressed optimism that the recommendations for screening and counseling for perinatal depression are valuable, and they “may return great dividends in the form of enhanced well-being of mothers and their offspring.”
Dr. Freeman is affiliated with the department of psychiatry at Massachusetts General Hospital, Boston. She commented in an editorial accompanying the article by Curry et al. (JAMA. 2019 Feb 12;321[6]:550-2). Dr. Freeman disclosed relationships with companies including Takeda, JayMac, Sage, Otsuka, Alkermes, Janssen, and Sunovion; she also disclosed serving on an independent data safety and monitoring committee for Janssen (Johnson & Johnson); and editing the GOED (Global Organization for EPA & DHA Omega-3) newsletter.
A proactive approach to prevention and management of perinatal depression as recommended by the USPSTF can potentially improve outcomes for new mothers and their babies, Marlene P. Freeman, MD, wrote in an accompanying editorial. She identified three key challenges to implementing the USPSTF recommendations: identifying women at risk, connecting them to evidence-based treatment, and assessing outcomes after treatment.
The development of screening tools would help clinicians identify women at risk for perinatal depression, Dr. Freeman said. No such tool currently exists, but in the meantime, “women at risk may be identified by targeting those with histories of depression, subthreshold depressive symptoms, and certain sociodemographic factors (i.e., economically disadvantaged, single/young, unplanned pregnancy).”
The counseling interventions shown to be effective in preventing perinatal depression – cognitive behavioral therapy and interpersonal psychotherapy – require education and training outside the time limitations and expertise of many clinicians providing obstetric care, she noted.
“The delivery of effective care on a large scale will require creative solutions,” such as the use of telehealth and smartphone platforms, and the involvement of multidisciplinary teams to care for women with severe illness, Dr. Freeman said. “In addition, a substantial number of reproductive-aged women have serious psychiatric disorders and will be identified as at risk for perinatal depression, although their needs may be more comprehensive,” she wrote. “Women who are identified as at risk for perinatal depression may have psychotic disorders, bipolar spectrum disorders, anxiety disorders, and substance use disorders, and there is comorbidity among psychiatric disorders. Therefore, systematic provisions for referral and treatment for other psychiatric disorders should be considered.” Further research is needed to explore treatment options including pharmacotherapy for women with severe psychiatric disorders.
However, she expressed optimism that the recommendations for screening and counseling for perinatal depression are valuable, and they “may return great dividends in the form of enhanced well-being of mothers and their offspring.”
Dr. Freeman is affiliated with the department of psychiatry at Massachusetts General Hospital, Boston. She commented in an editorial accompanying the article by Curry et al. (JAMA. 2019 Feb 12;321[6]:550-2). Dr. Freeman disclosed relationships with companies including Takeda, JayMac, Sage, Otsuka, Alkermes, Janssen, and Sunovion; she also disclosed serving on an independent data safety and monitoring committee for Janssen (Johnson & Johnson); and editing the GOED (Global Organization for EPA & DHA Omega-3) newsletter.
according to a B recommendation from the U.S. Preventive Services Task Force.
The Task Force determined that counseling interventions are effective in preventing perinatal depression, defined as a major or minor depressive episode during pregnancy or within the first year after delivery. The condition affects an estimated 12% of new mothers in the United States each year, according to lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and her colleagues.
The recommendation, published in JAMA, applies to “pregnant persons and persons who are less than 1 year postpartum who do not have a current diagnosis of depression but are at increased risk of developing depression,” according to the authors (JAMA. 2019 Feb 12;321(6):580-7).
Risk factors for development of perinatal depression include:
- Past history of depression.
- Current depressive symptoms (that do not reach a diagnostic threshold).
- History of physical or sexual abuse.
- Unplanned or unwanted pregnancy.
- Stressful life events.
- Lack of social and financial support.
- Intimate partner violence.
- Pregestational or gestational diabetes.
- Complications during pregnancy.
- Adolescent parenthood.
- Low socioeconomic status.
- Lack of social support.
After reviewing the evidence, the USPSTF found a moderate net benefit for counseling interventions, particularly cognitive behavioral therapy and interpersonal therapy, for preventing perinatal depression in women at risk. Counseling sessions reviewed for this recommendation ranged from 4 to 20 meetings (median, 8 meetings).
The USPSTF found inadequate evidence to assess the harms and benefits of other noncounseling interventions, including pharmacologic therapy.
In the evidence review accompanying the recommendations, Elizabeth A. O’Connor, PhD, of Kaiser Permanente, Portland, Ore., and her colleagues analyzed data from 50 studies including 22,385 individuals; 20 of these studies were randomized, controlled trials of counseling interventions (JAMA. 2019 Feb 12;321(6):588-601).
Overall, the likelihood of perinatal depression was significantly lower among women who received counseling, compared with controls, among more than 3,000 women in those studies (pooled risk ratio 0.61). Absolute risk differences for perinatal depression ranged from a 1% increased reduction in controls to a 32% increased reduction among women who received counseling. The effects were strongest for cognitive behavioral therapy and interpersonal therapy as interventions. No adverse events were reported in the counseling intervention studies.
In three studies of health system interventions, the researchers found a benefit for interventions vs. controls, but the difference was not statistically significant.
Trials of most other alternative interventions including infant sleep advice, birth-experience postpartum debriefing, omega-3 fatty acid supplementation, expressive writing, antidepressants, and yoga did not show statistical significance in benefit for reducing perinatal depression.
Only one of three randomized controlled trials of physical activity found a statistically significant group difference.
A trial of nortriptyline to prevent perinatal depression showed no benefit, compared with placebo. A sertraline study of found “a smaller percentage of participants taking sertraline had a depression recurrence, compared with those taking placebo,” the investigators wrote. In these two studies, women who took nortriptyline showed no adverse effects, and those in a trial involving sertraline reported significantly more dizziness and drowsiness compared with placebo patients.
The evidence review was limited by the small number of quality studies, especially studies of alternative interventions. More research is needed; however, the findings support data from similar reviews and support the potential for counseling to prevent perinatal depression, particularly in women at increased risk for perinatal depression, Dr. O’Connor and her associates said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. Coauthor Dr. Michelle L. Henninger reported receiving grants from Pfizer IGLC (Independent Grants for Learning & Change) outside the submitted work. Coauthor Dr. Bradley N. Gaynes reported receiving personal fees from LivaNova and Johnson & Johnson outside the submitted work. The remaining researchers had no financial conflicts to disclose.
SOURCE: Curry SJ et al. JAMA. 2019;321(6):580-7; O’Connor E et al. JAMA. 2019;321(6):588-601.
according to a B recommendation from the U.S. Preventive Services Task Force.
The Task Force determined that counseling interventions are effective in preventing perinatal depression, defined as a major or minor depressive episode during pregnancy or within the first year after delivery. The condition affects an estimated 12% of new mothers in the United States each year, according to lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and her colleagues.
The recommendation, published in JAMA, applies to “pregnant persons and persons who are less than 1 year postpartum who do not have a current diagnosis of depression but are at increased risk of developing depression,” according to the authors (JAMA. 2019 Feb 12;321(6):580-7).
Risk factors for development of perinatal depression include:
- Past history of depression.
- Current depressive symptoms (that do not reach a diagnostic threshold).
- History of physical or sexual abuse.
- Unplanned or unwanted pregnancy.
- Stressful life events.
- Lack of social and financial support.
- Intimate partner violence.
- Pregestational or gestational diabetes.
- Complications during pregnancy.
- Adolescent parenthood.
- Low socioeconomic status.
- Lack of social support.
After reviewing the evidence, the USPSTF found a moderate net benefit for counseling interventions, particularly cognitive behavioral therapy and interpersonal therapy, for preventing perinatal depression in women at risk. Counseling sessions reviewed for this recommendation ranged from 4 to 20 meetings (median, 8 meetings).
The USPSTF found inadequate evidence to assess the harms and benefits of other noncounseling interventions, including pharmacologic therapy.
In the evidence review accompanying the recommendations, Elizabeth A. O’Connor, PhD, of Kaiser Permanente, Portland, Ore., and her colleagues analyzed data from 50 studies including 22,385 individuals; 20 of these studies were randomized, controlled trials of counseling interventions (JAMA. 2019 Feb 12;321(6):588-601).
Overall, the likelihood of perinatal depression was significantly lower among women who received counseling, compared with controls, among more than 3,000 women in those studies (pooled risk ratio 0.61). Absolute risk differences for perinatal depression ranged from a 1% increased reduction in controls to a 32% increased reduction among women who received counseling. The effects were strongest for cognitive behavioral therapy and interpersonal therapy as interventions. No adverse events were reported in the counseling intervention studies.
In three studies of health system interventions, the researchers found a benefit for interventions vs. controls, but the difference was not statistically significant.
Trials of most other alternative interventions including infant sleep advice, birth-experience postpartum debriefing, omega-3 fatty acid supplementation, expressive writing, antidepressants, and yoga did not show statistical significance in benefit for reducing perinatal depression.
Only one of three randomized controlled trials of physical activity found a statistically significant group difference.
A trial of nortriptyline to prevent perinatal depression showed no benefit, compared with placebo. A sertraline study of found “a smaller percentage of participants taking sertraline had a depression recurrence, compared with those taking placebo,” the investigators wrote. In these two studies, women who took nortriptyline showed no adverse effects, and those in a trial involving sertraline reported significantly more dizziness and drowsiness compared with placebo patients.
The evidence review was limited by the small number of quality studies, especially studies of alternative interventions. More research is needed; however, the findings support data from similar reviews and support the potential for counseling to prevent perinatal depression, particularly in women at increased risk for perinatal depression, Dr. O’Connor and her associates said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. Coauthor Dr. Michelle L. Henninger reported receiving grants from Pfizer IGLC (Independent Grants for Learning & Change) outside the submitted work. Coauthor Dr. Bradley N. Gaynes reported receiving personal fees from LivaNova and Johnson & Johnson outside the submitted work. The remaining researchers had no financial conflicts to disclose.
SOURCE: Curry SJ et al. JAMA. 2019;321(6):580-7; O’Connor E et al. JAMA. 2019;321(6):588-601.
FROM JAMA