User login
Cognition Concern in MS and Changes in the Brain
Subjective cognitive concern in multiple sclerosis (MS) is associated with reduced thalamic and cortical gray matter volumes, areas of the brain that have been implicated in objective cognitive impairment, according to a recent study. These findings may lend neuroanatomical significance to subjective cognitive concerns and patient-reported outcomes as measured by the Quality of Life in Neurologic Disorders Measures (Neuro-QoL). A total of 158 patients with MS completed the Neuro-QoL short forms to assess subjective cognitive concerns and underwent brain magnetic resonance imaging. Regional brain volumes from regions of interest implicated in cognitive dysfunction were measured using NeuroQuant automated volumetric quantitation. Linear regression was used to analyze the relationship between subjective cognitive concerns and brain volume. Researchers found:
- Controlling for age, disease duration, gender, depression and fatigue, increased subjective cognitive concerns were associated with reduced thalamic volume (standardized β = 0.223, t150 =2.406) and reduced cortical gray matter volume (standardized β = 0.240, t150 = 2.777).
- Increased subjective cognitive concerns were not associated with any other regions of interest that were analyzed.
Kletenik I, Alvarez E, Honce JM, Valdez B, Vollmer TL. Subjective cognitive concern in multiple sclerosis is associated with reduced thalamic and cortical gray matter volumes. Mult Scler J Exp Transl Clin. 2019;5(1); doi:10.1177/2055217319827618.
Subjective cognitive concern in multiple sclerosis (MS) is associated with reduced thalamic and cortical gray matter volumes, areas of the brain that have been implicated in objective cognitive impairment, according to a recent study. These findings may lend neuroanatomical significance to subjective cognitive concerns and patient-reported outcomes as measured by the Quality of Life in Neurologic Disorders Measures (Neuro-QoL). A total of 158 patients with MS completed the Neuro-QoL short forms to assess subjective cognitive concerns and underwent brain magnetic resonance imaging. Regional brain volumes from regions of interest implicated in cognitive dysfunction were measured using NeuroQuant automated volumetric quantitation. Linear regression was used to analyze the relationship between subjective cognitive concerns and brain volume. Researchers found:
- Controlling for age, disease duration, gender, depression and fatigue, increased subjective cognitive concerns were associated with reduced thalamic volume (standardized β = 0.223, t150 =2.406) and reduced cortical gray matter volume (standardized β = 0.240, t150 = 2.777).
- Increased subjective cognitive concerns were not associated with any other regions of interest that were analyzed.
Kletenik I, Alvarez E, Honce JM, Valdez B, Vollmer TL. Subjective cognitive concern in multiple sclerosis is associated with reduced thalamic and cortical gray matter volumes. Mult Scler J Exp Transl Clin. 2019;5(1); doi:10.1177/2055217319827618.
Subjective cognitive concern in multiple sclerosis (MS) is associated with reduced thalamic and cortical gray matter volumes, areas of the brain that have been implicated in objective cognitive impairment, according to a recent study. These findings may lend neuroanatomical significance to subjective cognitive concerns and patient-reported outcomes as measured by the Quality of Life in Neurologic Disorders Measures (Neuro-QoL). A total of 158 patients with MS completed the Neuro-QoL short forms to assess subjective cognitive concerns and underwent brain magnetic resonance imaging. Regional brain volumes from regions of interest implicated in cognitive dysfunction were measured using NeuroQuant automated volumetric quantitation. Linear regression was used to analyze the relationship between subjective cognitive concerns and brain volume. Researchers found:
- Controlling for age, disease duration, gender, depression and fatigue, increased subjective cognitive concerns were associated with reduced thalamic volume (standardized β = 0.223, t150 =2.406) and reduced cortical gray matter volume (standardized β = 0.240, t150 = 2.777).
- Increased subjective cognitive concerns were not associated with any other regions of interest that were analyzed.
Kletenik I, Alvarez E, Honce JM, Valdez B, Vollmer TL. Subjective cognitive concern in multiple sclerosis is associated with reduced thalamic and cortical gray matter volumes. Mult Scler J Exp Transl Clin. 2019;5(1); doi:10.1177/2055217319827618.
Advance care planning codes not being used
Starting in 2016, the Centers for Medicare & Medicaid Services began paying physicians for advance care planning discussions with the approval of two new codes: 99497 and 99498. The codes pay about $86 for the first 30 minutes of a face-to-face conversation with a patient, family member, and/or surrogate and about $75 for additional sessions. Services can be furnished in both inpatient and ambulatory settings, and payment is not limited to particular physician specialties.
In 2016, health care professionals in New England (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont) billed Medicare 26,522 times for the advance care planning (ACP) codes for a total of 24,536 patients, which represented less than 1% of Medicare beneficiaries in New England at the time, according to Kimberly Pelland, MPH, of Healthcentric Advisors, Providence, R.I., and her colleagues. Most claims were billed in the office, followed by in nursing homes, and in hospitals; 40% of conversations occurred during an annual wellness visit (JAMA Intern Med. 2019 March 11. doi:10.1001/jamainternmed.2018.8107).
Internists billed Medicare the most for ACP claims (65%), followed by family physicians (22%) gerontologists (5%), and oncologist/hematologists (0.3%), according to the analysis based on 2016 Medicare claims data and Census Bureau data. A greater proportion of patients with ACP claims were female, aged 85 years or older, enrolled in hospice, and died in the study year. Patients had higher odds of having an ACP claim if they were older and had lower income, and if they had cancer, heart failure, stroke, chronic kidney disease, or dementia. Male patients who were Asian, black, and Hispanic had lower chances of having an ACP claim.
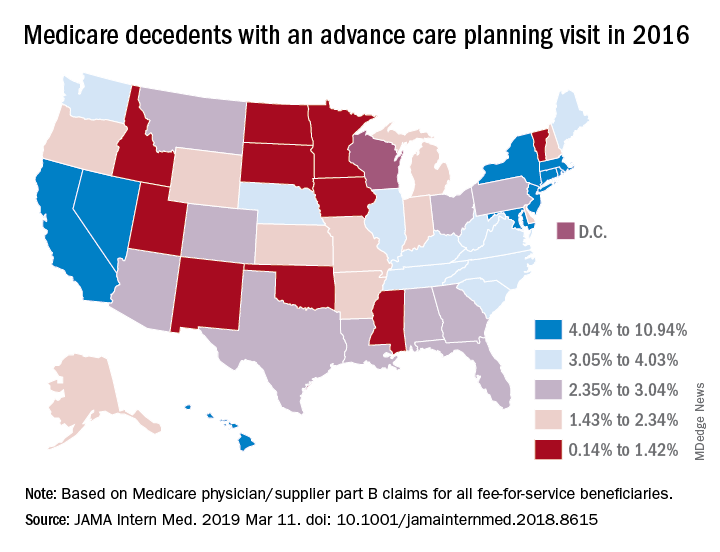
In a related study, Emmanuelle Belanger, PhD, of Brown University, Providence, R.I., and her colleagues examined national Medicare data from 2016 to the third quarter of 2017. Across the United States, 2% of Medicare patients aged 65 years and older received advance care planning services that were billed under the ACP codes (JAMA Intern Med. 2019 March 11. doi: 10.1001/jamainternmed.2018.8615). Visits billed under the ACP codes increased from 538,275 to 633,214 during the same time period. Claim rates were higher among patients who died within the study period, reaching 3% in 2016 and 6% in 2017. The percentage of decedents with an ACP billed visit varied strongly across states, with states such as North Dakota, South Dakota, and Wyoming having the fewest ACP visits billed and states such as California and Nevada having the most. ACP billed visits increased in all settings in 2017, but primarily in hospitals and nursing homes. Nationally, internists billed the codes most (48%), followed by family physicians (28%).
While the two studies indicate low usage of the ACP codes, many physicians are discussing advance care planning with their patients, said Mary M. Newman, MD, an internist based in Lutherville, Md., and former American College of Physicians adviser to the American Medical Association Relative Scale Value Update Committee (RUC).
“What cannot be captured by tracking under Medicare claims data are those shorter conversations that we have frequently,” Dr. Newman said in an interview. “If we have a short conversation about advance care planning, it gets folded into our evaluation and management visit. It’s not going to be separately billed.”
At the same time, some patients are not ready to discuss end-of-life options and decline the discussions when asked, Dr. Newman said. Particularly for healthier patients, end of life care is not a primary focus, she noted.
“Not everybody’s ready to have an advance care planning [discussion] that lasts 16-45 minutes,” she said. “Many people over age 65 are not ready to deal with advance care planning in their day-to-day lives, and it may not be what they wish to discuss. I offer the option to patients and some say, ‘Yes, I’d love to,’ and others decline or postpone.”
Low usage of the ACP codes may be associated with lack of awareness, uncertainty about appropriate code use, or associated billing that is not part of the standard workflow, Ankita Mehta, MD, of Mount Sinai in New York wrote an editorial accompanying the studies (JAMA Intern Med. 2019 March 11. doi:10.1001/jamainternmed.2018.8105).
“Regardless, the low rates of utilization of ACP codes is alarming and highlights the need to create strategies to integrate ACP discussions into standard practice and build ACP documentation and billing in clinical workflow,” Dr. Mehta said.
Dr. Newman agreed that more education among physicians is needed.
“The amount of education clinicians have received varies tremendously across the geography of the country,” she said. “I think the codes are going to be slowly adopted. The challenge to us is to make sure we’re all better educated on palliative care as people age and get sick and that we are sensitive to our patients explicit and implicit needs for these discussions.”

Starting in 2016, the Centers for Medicare & Medicaid Services began paying physicians for advance care planning discussions with the approval of two new codes: 99497 and 99498. The codes pay about $86 for the first 30 minutes of a face-to-face conversation with a patient, family member, and/or surrogate and about $75 for additional sessions. Services can be furnished in both inpatient and ambulatory settings, and payment is not limited to particular physician specialties.
In 2016, health care professionals in New England (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont) billed Medicare 26,522 times for the advance care planning (ACP) codes for a total of 24,536 patients, which represented less than 1% of Medicare beneficiaries in New England at the time, according to Kimberly Pelland, MPH, of Healthcentric Advisors, Providence, R.I., and her colleagues. Most claims were billed in the office, followed by in nursing homes, and in hospitals; 40% of conversations occurred during an annual wellness visit (JAMA Intern Med. 2019 March 11. doi:10.1001/jamainternmed.2018.8107).
Internists billed Medicare the most for ACP claims (65%), followed by family physicians (22%) gerontologists (5%), and oncologist/hematologists (0.3%), according to the analysis based on 2016 Medicare claims data and Census Bureau data. A greater proportion of patients with ACP claims were female, aged 85 years or older, enrolled in hospice, and died in the study year. Patients had higher odds of having an ACP claim if they were older and had lower income, and if they had cancer, heart failure, stroke, chronic kidney disease, or dementia. Male patients who were Asian, black, and Hispanic had lower chances of having an ACP claim.
In a related study, Emmanuelle Belanger, PhD, of Brown University, Providence, R.I., and her colleagues examined national Medicare data from 2016 to the third quarter of 2017. Across the United States, 2% of Medicare patients aged 65 years and older received advance care planning services that were billed under the ACP codes (JAMA Intern Med. 2019 March 11. doi: 10.1001/jamainternmed.2018.8615). Visits billed under the ACP codes increased from 538,275 to 633,214 during the same time period. Claim rates were higher among patients who died within the study period, reaching 3% in 2016 and 6% in 2017. The percentage of decedents with an ACP billed visit varied strongly across states, with states such as North Dakota, South Dakota, and Wyoming having the fewest ACP visits billed and states such as California and Nevada having the most. ACP billed visits increased in all settings in 2017, but primarily in hospitals and nursing homes. Nationally, internists billed the codes most (48%), followed by family physicians (28%).
While the two studies indicate low usage of the ACP codes, many physicians are discussing advance care planning with their patients, said Mary M. Newman, MD, an internist based in Lutherville, Md., and former American College of Physicians adviser to the American Medical Association Relative Scale Value Update Committee (RUC).
“What cannot be captured by tracking under Medicare claims data are those shorter conversations that we have frequently,” Dr. Newman said in an interview. “If we have a short conversation about advance care planning, it gets folded into our evaluation and management visit. It’s not going to be separately billed.”
At the same time, some patients are not ready to discuss end-of-life options and decline the discussions when asked, Dr. Newman said. Particularly for healthier patients, end of life care is not a primary focus, she noted.
“Not everybody’s ready to have an advance care planning [discussion] that lasts 16-45 minutes,” she said. “Many people over age 65 are not ready to deal with advance care planning in their day-to-day lives, and it may not be what they wish to discuss. I offer the option to patients and some say, ‘Yes, I’d love to,’ and others decline or postpone.”
Low usage of the ACP codes may be associated with lack of awareness, uncertainty about appropriate code use, or associated billing that is not part of the standard workflow, Ankita Mehta, MD, of Mount Sinai in New York wrote an editorial accompanying the studies (JAMA Intern Med. 2019 March 11. doi:10.1001/jamainternmed.2018.8105).
“Regardless, the low rates of utilization of ACP codes is alarming and highlights the need to create strategies to integrate ACP discussions into standard practice and build ACP documentation and billing in clinical workflow,” Dr. Mehta said.
Dr. Newman agreed that more education among physicians is needed.
“The amount of education clinicians have received varies tremendously across the geography of the country,” she said. “I think the codes are going to be slowly adopted. The challenge to us is to make sure we’re all better educated on palliative care as people age and get sick and that we are sensitive to our patients explicit and implicit needs for these discussions.”

Starting in 2016, the Centers for Medicare & Medicaid Services began paying physicians for advance care planning discussions with the approval of two new codes: 99497 and 99498. The codes pay about $86 for the first 30 minutes of a face-to-face conversation with a patient, family member, and/or surrogate and about $75 for additional sessions. Services can be furnished in both inpatient and ambulatory settings, and payment is not limited to particular physician specialties.
In 2016, health care professionals in New England (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont) billed Medicare 26,522 times for the advance care planning (ACP) codes for a total of 24,536 patients, which represented less than 1% of Medicare beneficiaries in New England at the time, according to Kimberly Pelland, MPH, of Healthcentric Advisors, Providence, R.I., and her colleagues. Most claims were billed in the office, followed by in nursing homes, and in hospitals; 40% of conversations occurred during an annual wellness visit (JAMA Intern Med. 2019 March 11. doi:10.1001/jamainternmed.2018.8107).
Internists billed Medicare the most for ACP claims (65%), followed by family physicians (22%) gerontologists (5%), and oncologist/hematologists (0.3%), according to the analysis based on 2016 Medicare claims data and Census Bureau data. A greater proportion of patients with ACP claims were female, aged 85 years or older, enrolled in hospice, and died in the study year. Patients had higher odds of having an ACP claim if they were older and had lower income, and if they had cancer, heart failure, stroke, chronic kidney disease, or dementia. Male patients who were Asian, black, and Hispanic had lower chances of having an ACP claim.
In a related study, Emmanuelle Belanger, PhD, of Brown University, Providence, R.I., and her colleagues examined national Medicare data from 2016 to the third quarter of 2017. Across the United States, 2% of Medicare patients aged 65 years and older received advance care planning services that were billed under the ACP codes (JAMA Intern Med. 2019 March 11. doi: 10.1001/jamainternmed.2018.8615). Visits billed under the ACP codes increased from 538,275 to 633,214 during the same time period. Claim rates were higher among patients who died within the study period, reaching 3% in 2016 and 6% in 2017. The percentage of decedents with an ACP billed visit varied strongly across states, with states such as North Dakota, South Dakota, and Wyoming having the fewest ACP visits billed and states such as California and Nevada having the most. ACP billed visits increased in all settings in 2017, but primarily in hospitals and nursing homes. Nationally, internists billed the codes most (48%), followed by family physicians (28%).
While the two studies indicate low usage of the ACP codes, many physicians are discussing advance care planning with their patients, said Mary M. Newman, MD, an internist based in Lutherville, Md., and former American College of Physicians adviser to the American Medical Association Relative Scale Value Update Committee (RUC).
“What cannot be captured by tracking under Medicare claims data are those shorter conversations that we have frequently,” Dr. Newman said in an interview. “If we have a short conversation about advance care planning, it gets folded into our evaluation and management visit. It’s not going to be separately billed.”
At the same time, some patients are not ready to discuss end-of-life options and decline the discussions when asked, Dr. Newman said. Particularly for healthier patients, end of life care is not a primary focus, she noted.
“Not everybody’s ready to have an advance care planning [discussion] that lasts 16-45 minutes,” she said. “Many people over age 65 are not ready to deal with advance care planning in their day-to-day lives, and it may not be what they wish to discuss. I offer the option to patients and some say, ‘Yes, I’d love to,’ and others decline or postpone.”
Low usage of the ACP codes may be associated with lack of awareness, uncertainty about appropriate code use, or associated billing that is not part of the standard workflow, Ankita Mehta, MD, of Mount Sinai in New York wrote an editorial accompanying the studies (JAMA Intern Med. 2019 March 11. doi:10.1001/jamainternmed.2018.8105).
“Regardless, the low rates of utilization of ACP codes is alarming and highlights the need to create strategies to integrate ACP discussions into standard practice and build ACP documentation and billing in clinical workflow,” Dr. Mehta said.
Dr. Newman agreed that more education among physicians is needed.
“The amount of education clinicians have received varies tremendously across the geography of the country,” she said. “I think the codes are going to be slowly adopted. The challenge to us is to make sure we’re all better educated on palliative care as people age and get sick and that we are sensitive to our patients explicit and implicit needs for these discussions.”
Friendly gut bugs, MCI-battling mushrooms, and remembering to forget
Friend or foe, how do we know?
That’s the question immune cells ask all the time, especially about gut bacteria. A study published March 7 seeks to explain how immune systems can distinguish between happy-go-lucky gut microbes and deadly pathogens. Turns out, the friendly microbes simply high five!
Well, not really. But they do have a hook-like arm, called a holdfast, which latches onto the gut lining. The holdfast is lined with vesicles that carry antigens into the gut. While antigens normally cause immune cells to attack, something about these antigens are telling T cells to hold their fire.
The authors of the study hypothesized that the packaging of the antigens – the vesicles – might be the reason for the friendliness between microbes and T cells. It’s like the immune system expects a cannonball, but is pleasantly surprised by an Amazon Prime package full of goodies showing up on their doorstep instead. Yay for presents!
Don’t skimp on the ’shrooms
While you’re piling onions onto your plate to reduce cancer and cheese for your heart, make sure you add mushrooms for extra brain power. Researchers conducting a 6-year study in Singapore observed cognitive decline in 600 Chinese people aged at least 60 years, and they found that those who eat more than two portions of cooked mushrooms per week have up to 50% reduced odds of mild cognitive impairment.
Researchers here at the LOTME Lab have harnessed the power of these food studies to determine that a Philly cheesesteak with mushrooms and onions is the healthiest meal out there. Chow down!
As far as we know, all the mushrooms were standard edible fungi, and none were magic mushrooms (although, that might help, too; try that on your own time). Researchers believe that the compound ergothioneine, an antioxidant and anti-inflammatory that cannot be synthesized by humans, might be reason for the reduced risk of mild cognitive impairment Maybe it’s time to add a cup of cooked shiitake mushrooms to your morning routine.
Fuggedaboutit!
We all have unwanted memories that we’d rather forget about. An embarrassing incident, a painful experience – everyone has moments they’d rather not think about. So, the question is: How do you get rid of these bad memories?
The obvious solution is to stop thinking about it. But if you’re a regular reader of Livin’ on the MDedge, you can probably guess that the answer isn’t that simple.
And, in fact, it isn’t! A group of researchers at the University of Texas at Austin, has performed a study on intentional forgetting, and they found that the best way to forget something is ... to think about it. Study subjects were shown a series of images and told to either remember or forget those images while their ventral temporal cortex was monitored for activity. Not only were participants successfully able to forget images by thinking about it, but activity in the brain was higher when forgetting than while remembering.
Obviously, this research would be helpful for anyone dealing with trauma, and we hope doctors who have to treat such patients keep it in mind. Just don’t think about it too much, or you’ll forget about it.
The Golden Lobbyist
If you need health care in your neighborhood
Who you gonna call? Jack Nicklaus!
You need 20 mill to make it good
Who you gonna call? Jack Nicklaus!
Health care in general didn’t do very well in President Trump’s 2020 budget proposal; Medicare, Medicaid, and the National Cancer Institute were all targeted for cuts. But it did include one particular $20-million initiative for a mobile children’s hospital.
Politico reports that the nation’s golfer in chief “personally directed the Department of Health and Human Services to earmark the funds” after playing a couple of rounds with the Golden Bear himself, Jack Nicklaus. The mobile unit would be part of the Nicklaus Children’s Hospital in Miami. The golf legend turned lobbyist also had meetings off the course with HHS Secretary Alex Azar and then-OMB Director Mick Mulvaney.
Are health care ideas running through your head?
Who you gonna call? Jack Nicklaus!
He’ll golf with the prez, and get your bread
Who you gonna call? Jack Nicklaus!
He ain’t afraid of no tweets

Friend or foe, how do we know?
That’s the question immune cells ask all the time, especially about gut bacteria. A study published March 7 seeks to explain how immune systems can distinguish between happy-go-lucky gut microbes and deadly pathogens. Turns out, the friendly microbes simply high five!
Well, not really. But they do have a hook-like arm, called a holdfast, which latches onto the gut lining. The holdfast is lined with vesicles that carry antigens into the gut. While antigens normally cause immune cells to attack, something about these antigens are telling T cells to hold their fire.
The authors of the study hypothesized that the packaging of the antigens – the vesicles – might be the reason for the friendliness between microbes and T cells. It’s like the immune system expects a cannonball, but is pleasantly surprised by an Amazon Prime package full of goodies showing up on their doorstep instead. Yay for presents!
Don’t skimp on the ’shrooms
While you’re piling onions onto your plate to reduce cancer and cheese for your heart, make sure you add mushrooms for extra brain power. Researchers conducting a 6-year study in Singapore observed cognitive decline in 600 Chinese people aged at least 60 years, and they found that those who eat more than two portions of cooked mushrooms per week have up to 50% reduced odds of mild cognitive impairment.
Researchers here at the LOTME Lab have harnessed the power of these food studies to determine that a Philly cheesesteak with mushrooms and onions is the healthiest meal out there. Chow down!
As far as we know, all the mushrooms were standard edible fungi, and none were magic mushrooms (although, that might help, too; try that on your own time). Researchers believe that the compound ergothioneine, an antioxidant and anti-inflammatory that cannot be synthesized by humans, might be reason for the reduced risk of mild cognitive impairment Maybe it’s time to add a cup of cooked shiitake mushrooms to your morning routine.
Fuggedaboutit!
We all have unwanted memories that we’d rather forget about. An embarrassing incident, a painful experience – everyone has moments they’d rather not think about. So, the question is: How do you get rid of these bad memories?
The obvious solution is to stop thinking about it. But if you’re a regular reader of Livin’ on the MDedge, you can probably guess that the answer isn’t that simple.
And, in fact, it isn’t! A group of researchers at the University of Texas at Austin, has performed a study on intentional forgetting, and they found that the best way to forget something is ... to think about it. Study subjects were shown a series of images and told to either remember or forget those images while their ventral temporal cortex was monitored for activity. Not only were participants successfully able to forget images by thinking about it, but activity in the brain was higher when forgetting than while remembering.
Obviously, this research would be helpful for anyone dealing with trauma, and we hope doctors who have to treat such patients keep it in mind. Just don’t think about it too much, or you’ll forget about it.
The Golden Lobbyist
If you need health care in your neighborhood
Who you gonna call? Jack Nicklaus!
You need 20 mill to make it good
Who you gonna call? Jack Nicklaus!
Health care in general didn’t do very well in President Trump’s 2020 budget proposal; Medicare, Medicaid, and the National Cancer Institute were all targeted for cuts. But it did include one particular $20-million initiative for a mobile children’s hospital.
Politico reports that the nation’s golfer in chief “personally directed the Department of Health and Human Services to earmark the funds” after playing a couple of rounds with the Golden Bear himself, Jack Nicklaus. The mobile unit would be part of the Nicklaus Children’s Hospital in Miami. The golf legend turned lobbyist also had meetings off the course with HHS Secretary Alex Azar and then-OMB Director Mick Mulvaney.
Are health care ideas running through your head?
Who you gonna call? Jack Nicklaus!
He’ll golf with the prez, and get your bread
Who you gonna call? Jack Nicklaus!
He ain’t afraid of no tweets

Friend or foe, how do we know?
That’s the question immune cells ask all the time, especially about gut bacteria. A study published March 7 seeks to explain how immune systems can distinguish between happy-go-lucky gut microbes and deadly pathogens. Turns out, the friendly microbes simply high five!
Well, not really. But they do have a hook-like arm, called a holdfast, which latches onto the gut lining. The holdfast is lined with vesicles that carry antigens into the gut. While antigens normally cause immune cells to attack, something about these antigens are telling T cells to hold their fire.
The authors of the study hypothesized that the packaging of the antigens – the vesicles – might be the reason for the friendliness between microbes and T cells. It’s like the immune system expects a cannonball, but is pleasantly surprised by an Amazon Prime package full of goodies showing up on their doorstep instead. Yay for presents!
Don’t skimp on the ’shrooms
While you’re piling onions onto your plate to reduce cancer and cheese for your heart, make sure you add mushrooms for extra brain power. Researchers conducting a 6-year study in Singapore observed cognitive decline in 600 Chinese people aged at least 60 years, and they found that those who eat more than two portions of cooked mushrooms per week have up to 50% reduced odds of mild cognitive impairment.
Researchers here at the LOTME Lab have harnessed the power of these food studies to determine that a Philly cheesesteak with mushrooms and onions is the healthiest meal out there. Chow down!
As far as we know, all the mushrooms were standard edible fungi, and none were magic mushrooms (although, that might help, too; try that on your own time). Researchers believe that the compound ergothioneine, an antioxidant and anti-inflammatory that cannot be synthesized by humans, might be reason for the reduced risk of mild cognitive impairment Maybe it’s time to add a cup of cooked shiitake mushrooms to your morning routine.
Fuggedaboutit!
We all have unwanted memories that we’d rather forget about. An embarrassing incident, a painful experience – everyone has moments they’d rather not think about. So, the question is: How do you get rid of these bad memories?
The obvious solution is to stop thinking about it. But if you’re a regular reader of Livin’ on the MDedge, you can probably guess that the answer isn’t that simple.
And, in fact, it isn’t! A group of researchers at the University of Texas at Austin, has performed a study on intentional forgetting, and they found that the best way to forget something is ... to think about it. Study subjects were shown a series of images and told to either remember or forget those images while their ventral temporal cortex was monitored for activity. Not only were participants successfully able to forget images by thinking about it, but activity in the brain was higher when forgetting than while remembering.
Obviously, this research would be helpful for anyone dealing with trauma, and we hope doctors who have to treat such patients keep it in mind. Just don’t think about it too much, or you’ll forget about it.
The Golden Lobbyist
If you need health care in your neighborhood
Who you gonna call? Jack Nicklaus!
You need 20 mill to make it good
Who you gonna call? Jack Nicklaus!
Health care in general didn’t do very well in President Trump’s 2020 budget proposal; Medicare, Medicaid, and the National Cancer Institute were all targeted for cuts. But it did include one particular $20-million initiative for a mobile children’s hospital.
Politico reports that the nation’s golfer in chief “personally directed the Department of Health and Human Services to earmark the funds” after playing a couple of rounds with the Golden Bear himself, Jack Nicklaus. The mobile unit would be part of the Nicklaus Children’s Hospital in Miami. The golf legend turned lobbyist also had meetings off the course with HHS Secretary Alex Azar and then-OMB Director Mick Mulvaney.
Are health care ideas running through your head?
Who you gonna call? Jack Nicklaus!
He’ll golf with the prez, and get your bread
Who you gonna call? Jack Nicklaus!
He ain’t afraid of no tweets

What is your diagnosis?
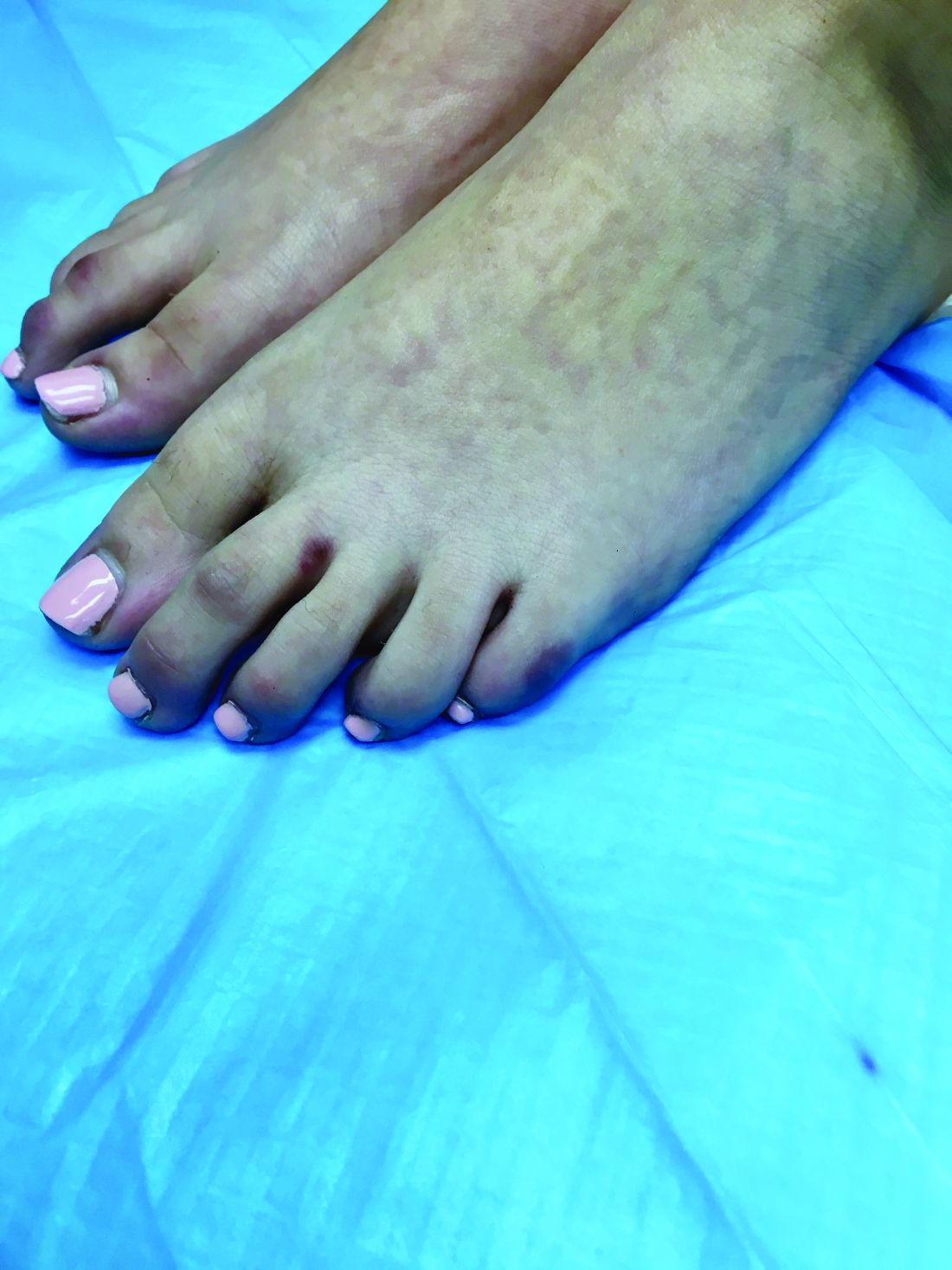
A skin biopsy of one of the lesions on the right toe showed dermal edema with an associated lymphohistiocytic infiltrate. There are scattered areas of perieccrine involvement and areas of vasculitis. Laboratory work up showed a normal complete blood count, a negative antinuclear antibodies (ANA) titer, a negative double-stranded DNA, normal levels of inflammatory markers, and negative cryoglobulins and cold agglutinins. The patient was diagnosed with pernio. The lesions improved within several weeks. She now wears thicker socks when she is ice skating.
Children, women, and the elderly are at a higher risk.1 This condition is frequently described in Northwestern Europe and the United Kingdom, especially in those living in houses without central heating.2
Clinically, the lesions appear a few hours or days after cold exposure on the toes, fingers, and in some unusual cases on the nose and the ears. The lesions present as erythematous to violaceous macules, papules, or nodules that in severe cases may blister and ulcerate. The lesions may be asymptomatic, pruritic, or tender. In children, pernio can be associated with the presence of cryoglobulins, cold agglutinins, anorexia nervosa, and genetic interferonopathy; it may precede the diagnosis of chronic myelomonocytic leukemia and may occur as a presenting sign of a blast crisis in acute lymphoblastic leukemia.3,4 The skin lesions usually resolve within days to a few weeks. Histopathologic analysis shows dermal edema with associated superficial and deep lymphohistiocytic infiltrate and perieccrine involvement.
The differential diagnosis of pernio includes other cold-induced syndromes such as Raynaud’s syndrome, cold panniculitis, cold urticaria, livedo reticularis, acrocyanosis, and chilblain lupus. In chilblain lupus (a form of chronic cutaneous lupus), the lesions may be very similar to pernio but the histopathology is consistent with changes of discoid lupus. Lesions of idiopathic palmoplantar hidradenitis present as erythematous tender nodules on the palms and the soles.5 The lesions can be triggered by vigorous physical activity, exposure to moisture, and excessive sweating. White, blue, and red discoloration of the fingers is seen in Raynaud’s phenomenon rather than the fixed erythematous to violaceous macules, papules, or nodules seen in pernio. Patients with erythromelalgia present with red painful palms and soles triggered by heat and, in contrast to pernio, relieved by cooling. Sweet syndrome, a febrile neutrophilic dermatoses, is characterized by tender erythematous papules and plaques with associated systemic symptoms. These patients may have an associated internal malignancy or infection, or the disorder may be triggered by medications or pregnancy.
Our patient had no systemic symptoms, and the pathology didn’t show any neutrophils. When the diagnosis is in doubt, a skin biopsy may help elucidate the diagnosis.
Once the diagnosis of pernio is made, it is recommended to order a complete blood count to rule out blood malignancies and cryoproteins.
Treatment of this condition consists of rewarming the extremity. If rewarming does not improve the patient’s symptoms, systemic treatment with nifedipine may be warranted.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Matiz said she had no relevant financial disclosures. Email her at [email protected].
References
1. Pediatrics. 2005 Sep;116(3):e472-5.
2. Mayo Clin Proc. 2014 Feb;89(2):207-15.
3. Pediatr Dermatol. 2018 Jan;35(1):e74-5.
4. Pediatr Dermatol. 2000 Mar-Apr;17(2):97-9.
5. Eur J Pediatr. 2001 Mar;160(3):189-91.
A skin biopsy of one of the lesions on the right toe showed dermal edema with an associated lymphohistiocytic infiltrate. There are scattered areas of perieccrine involvement and areas of vasculitis. Laboratory work up showed a normal complete blood count, a negative antinuclear antibodies (ANA) titer, a negative double-stranded DNA, normal levels of inflammatory markers, and negative cryoglobulins and cold agglutinins. The patient was diagnosed with pernio. The lesions improved within several weeks. She now wears thicker socks when she is ice skating.
Children, women, and the elderly are at a higher risk.1 This condition is frequently described in Northwestern Europe and the United Kingdom, especially in those living in houses without central heating.2
Clinically, the lesions appear a few hours or days after cold exposure on the toes, fingers, and in some unusual cases on the nose and the ears. The lesions present as erythematous to violaceous macules, papules, or nodules that in severe cases may blister and ulcerate. The lesions may be asymptomatic, pruritic, or tender. In children, pernio can be associated with the presence of cryoglobulins, cold agglutinins, anorexia nervosa, and genetic interferonopathy; it may precede the diagnosis of chronic myelomonocytic leukemia and may occur as a presenting sign of a blast crisis in acute lymphoblastic leukemia.3,4 The skin lesions usually resolve within days to a few weeks. Histopathologic analysis shows dermal edema with associated superficial and deep lymphohistiocytic infiltrate and perieccrine involvement.
The differential diagnosis of pernio includes other cold-induced syndromes such as Raynaud’s syndrome, cold panniculitis, cold urticaria, livedo reticularis, acrocyanosis, and chilblain lupus. In chilblain lupus (a form of chronic cutaneous lupus), the lesions may be very similar to pernio but the histopathology is consistent with changes of discoid lupus. Lesions of idiopathic palmoplantar hidradenitis present as erythematous tender nodules on the palms and the soles.5 The lesions can be triggered by vigorous physical activity, exposure to moisture, and excessive sweating. White, blue, and red discoloration of the fingers is seen in Raynaud’s phenomenon rather than the fixed erythematous to violaceous macules, papules, or nodules seen in pernio. Patients with erythromelalgia present with red painful palms and soles triggered by heat and, in contrast to pernio, relieved by cooling. Sweet syndrome, a febrile neutrophilic dermatoses, is characterized by tender erythematous papules and plaques with associated systemic symptoms. These patients may have an associated internal malignancy or infection, or the disorder may be triggered by medications or pregnancy.
Our patient had no systemic symptoms, and the pathology didn’t show any neutrophils. When the diagnosis is in doubt, a skin biopsy may help elucidate the diagnosis.
Once the diagnosis of pernio is made, it is recommended to order a complete blood count to rule out blood malignancies and cryoproteins.
Treatment of this condition consists of rewarming the extremity. If rewarming does not improve the patient’s symptoms, systemic treatment with nifedipine may be warranted.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Matiz said she had no relevant financial disclosures. Email her at [email protected].
References
1. Pediatrics. 2005 Sep;116(3):e472-5.
2. Mayo Clin Proc. 2014 Feb;89(2):207-15.
3. Pediatr Dermatol. 2018 Jan;35(1):e74-5.
4. Pediatr Dermatol. 2000 Mar-Apr;17(2):97-9.
5. Eur J Pediatr. 2001 Mar;160(3):189-91.
A skin biopsy of one of the lesions on the right toe showed dermal edema with an associated lymphohistiocytic infiltrate. There are scattered areas of perieccrine involvement and areas of vasculitis. Laboratory work up showed a normal complete blood count, a negative antinuclear antibodies (ANA) titer, a negative double-stranded DNA, normal levels of inflammatory markers, and negative cryoglobulins and cold agglutinins. The patient was diagnosed with pernio. The lesions improved within several weeks. She now wears thicker socks when she is ice skating.
Children, women, and the elderly are at a higher risk.1 This condition is frequently described in Northwestern Europe and the United Kingdom, especially in those living in houses without central heating.2
Clinically, the lesions appear a few hours or days after cold exposure on the toes, fingers, and in some unusual cases on the nose and the ears. The lesions present as erythematous to violaceous macules, papules, or nodules that in severe cases may blister and ulcerate. The lesions may be asymptomatic, pruritic, or tender. In children, pernio can be associated with the presence of cryoglobulins, cold agglutinins, anorexia nervosa, and genetic interferonopathy; it may precede the diagnosis of chronic myelomonocytic leukemia and may occur as a presenting sign of a blast crisis in acute lymphoblastic leukemia.3,4 The skin lesions usually resolve within days to a few weeks. Histopathologic analysis shows dermal edema with associated superficial and deep lymphohistiocytic infiltrate and perieccrine involvement.
The differential diagnosis of pernio includes other cold-induced syndromes such as Raynaud’s syndrome, cold panniculitis, cold urticaria, livedo reticularis, acrocyanosis, and chilblain lupus. In chilblain lupus (a form of chronic cutaneous lupus), the lesions may be very similar to pernio but the histopathology is consistent with changes of discoid lupus. Lesions of idiopathic palmoplantar hidradenitis present as erythematous tender nodules on the palms and the soles.5 The lesions can be triggered by vigorous physical activity, exposure to moisture, and excessive sweating. White, blue, and red discoloration of the fingers is seen in Raynaud’s phenomenon rather than the fixed erythematous to violaceous macules, papules, or nodules seen in pernio. Patients with erythromelalgia present with red painful palms and soles triggered by heat and, in contrast to pernio, relieved by cooling. Sweet syndrome, a febrile neutrophilic dermatoses, is characterized by tender erythematous papules and plaques with associated systemic symptoms. These patients may have an associated internal malignancy or infection, or the disorder may be triggered by medications or pregnancy.
Our patient had no systemic symptoms, and the pathology didn’t show any neutrophils. When the diagnosis is in doubt, a skin biopsy may help elucidate the diagnosis.
Once the diagnosis of pernio is made, it is recommended to order a complete blood count to rule out blood malignancies and cryoproteins.
Treatment of this condition consists of rewarming the extremity. If rewarming does not improve the patient’s symptoms, systemic treatment with nifedipine may be warranted.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Matiz said she had no relevant financial disclosures. Email her at [email protected].
References
1. Pediatrics. 2005 Sep;116(3):e472-5.
2. Mayo Clin Proc. 2014 Feb;89(2):207-15.
3. Pediatr Dermatol. 2018 Jan;35(1):e74-5.
4. Pediatr Dermatol. 2000 Mar-Apr;17(2):97-9.
5. Eur J Pediatr. 2001 Mar;160(3):189-91.
An 8-year-old girl comes to our pediatric dermatology clinic in the company of her mother for evaluation of painless purple spots on her toes. The lesions have been present for about 2 weeks. She has not been treated with any medications or creams. She denies any fevers, weight loss, mouth ulcers, sun sensitivity, joint pain, or any other symptoms. The patient has been a very healthy girl with occasional colds and no recent illnesses. The girl has never been admitted to the hospital. All her vaccinations are up to date. She takes no chronic medications. She lives in San Diego with her parents and two siblings. The girl recently started practicing ice-skating several times a week. There is no family history of any chronic medical conditions. She has no pets.
Continuers vs Discontinuers of DMT in MS Aged >60
Most patients with multiple sclerosis (MS) aged >60 who discontinued disease modifying therapy (DMT) remained off treatment, a recent study found. Among the outcomes, only the European Quality of Life 5 Dimensions (EQ-5D) index demonstrated significant differences over time, with continuers having lower quality of life scores compared to discontinuers before discontinuation (DBD). Researchers conducted a retrospective, observational study in which they identified patients from MS clinics aged ≥60 years who had been on DMT ≥2 years. They compared outcome evolution over time among treatment groups (continuers, DBD), and discontinuers after discontinuation [DAD]), by creating separate mixed-effects linear regression models that included an interaction term between time from age 60 and treatment group to study outcome trajectories. They found:
- 178 of 600 patients discontinued DMT, and 89.3% (n=159) of those who discontinued remained off DMT.
- Only the EQ-5D mixed-effects linear regression model with the interaction term was statistically significant.
- The slope relating time to EQ-5D was significantly different when comparing continuers to DBD.
- The slopes were not significantly different when comparing continuers to DAD, or when comparing the before and after discontinuation slopes among the discontinuers.
Hua LH, Harris H, Conway D, Thompson NR. Changes in patient-reported outcomes between continuers and discontinuers of disease modifying therapy in patients with multiple sclerosis over age 60. [Published online ahead of print March 1, 2019]. Mult Scler Relat Disord. doi:10.1016/j.msard.2019.02.028.
Most patients with multiple sclerosis (MS) aged >60 who discontinued disease modifying therapy (DMT) remained off treatment, a recent study found. Among the outcomes, only the European Quality of Life 5 Dimensions (EQ-5D) index demonstrated significant differences over time, with continuers having lower quality of life scores compared to discontinuers before discontinuation (DBD). Researchers conducted a retrospective, observational study in which they identified patients from MS clinics aged ≥60 years who had been on DMT ≥2 years. They compared outcome evolution over time among treatment groups (continuers, DBD), and discontinuers after discontinuation [DAD]), by creating separate mixed-effects linear regression models that included an interaction term between time from age 60 and treatment group to study outcome trajectories. They found:
- 178 of 600 patients discontinued DMT, and 89.3% (n=159) of those who discontinued remained off DMT.
- Only the EQ-5D mixed-effects linear regression model with the interaction term was statistically significant.
- The slope relating time to EQ-5D was significantly different when comparing continuers to DBD.
- The slopes were not significantly different when comparing continuers to DAD, or when comparing the before and after discontinuation slopes among the discontinuers.
Hua LH, Harris H, Conway D, Thompson NR. Changes in patient-reported outcomes between continuers and discontinuers of disease modifying therapy in patients with multiple sclerosis over age 60. [Published online ahead of print March 1, 2019]. Mult Scler Relat Disord. doi:10.1016/j.msard.2019.02.028.
Most patients with multiple sclerosis (MS) aged >60 who discontinued disease modifying therapy (DMT) remained off treatment, a recent study found. Among the outcomes, only the European Quality of Life 5 Dimensions (EQ-5D) index demonstrated significant differences over time, with continuers having lower quality of life scores compared to discontinuers before discontinuation (DBD). Researchers conducted a retrospective, observational study in which they identified patients from MS clinics aged ≥60 years who had been on DMT ≥2 years. They compared outcome evolution over time among treatment groups (continuers, DBD), and discontinuers after discontinuation [DAD]), by creating separate mixed-effects linear regression models that included an interaction term between time from age 60 and treatment group to study outcome trajectories. They found:
- 178 of 600 patients discontinued DMT, and 89.3% (n=159) of those who discontinued remained off DMT.
- Only the EQ-5D mixed-effects linear regression model with the interaction term was statistically significant.
- The slope relating time to EQ-5D was significantly different when comparing continuers to DBD.
- The slopes were not significantly different when comparing continuers to DAD, or when comparing the before and after discontinuation slopes among the discontinuers.
Hua LH, Harris H, Conway D, Thompson NR. Changes in patient-reported outcomes between continuers and discontinuers of disease modifying therapy in patients with multiple sclerosis over age 60. [Published online ahead of print March 1, 2019]. Mult Scler Relat Disord. doi:10.1016/j.msard.2019.02.028.
Aerobic Fitness and Activities of Daily Living in MS
Recent findings support previous studies on the activities of daily living in people with multiple sclerosis (MS) and the effect of aerobic exercise on independence regarding instrumental activities of daily living (IADLs) in this population. 62 adults with MS completed an incremental exercise test as a measure of aerobic fitness (peak oxygen consumption), a demographic questionnaire, and an IADL scale and underwent a neurologic examination for characterization of disability level (ie, Expanded Disability Status Scale) in a single session. Researchers found:
- The analysis revealed a weak but significant association between aerobic fitness and total IADL score (r=0.28).
- Those reporting dependence in different IADL categories (eg, shopping, food preparation, housekeeping, laundry, and responsibility for own medication) presented with lower aerobic fitness compared with those reporting independence, although the difference was not statistically significant.
Sebastião E, Pilutti LA, Motl RW. Aerobic fitness and instrumental activities of daily living in people with multiple sclerosis. A cross-sectional study. Int J MS Care. 2019;21(1):23-28. doi:10.7224/1537-2073.2017-078.
Recent findings support previous studies on the activities of daily living in people with multiple sclerosis (MS) and the effect of aerobic exercise on independence regarding instrumental activities of daily living (IADLs) in this population. 62 adults with MS completed an incremental exercise test as a measure of aerobic fitness (peak oxygen consumption), a demographic questionnaire, and an IADL scale and underwent a neurologic examination for characterization of disability level (ie, Expanded Disability Status Scale) in a single session. Researchers found:
- The analysis revealed a weak but significant association between aerobic fitness and total IADL score (r=0.28).
- Those reporting dependence in different IADL categories (eg, shopping, food preparation, housekeeping, laundry, and responsibility for own medication) presented with lower aerobic fitness compared with those reporting independence, although the difference was not statistically significant.
Sebastião E, Pilutti LA, Motl RW. Aerobic fitness and instrumental activities of daily living in people with multiple sclerosis. A cross-sectional study. Int J MS Care. 2019;21(1):23-28. doi:10.7224/1537-2073.2017-078.
Recent findings support previous studies on the activities of daily living in people with multiple sclerosis (MS) and the effect of aerobic exercise on independence regarding instrumental activities of daily living (IADLs) in this population. 62 adults with MS completed an incremental exercise test as a measure of aerobic fitness (peak oxygen consumption), a demographic questionnaire, and an IADL scale and underwent a neurologic examination for characterization of disability level (ie, Expanded Disability Status Scale) in a single session. Researchers found:
- The analysis revealed a weak but significant association between aerobic fitness and total IADL score (r=0.28).
- Those reporting dependence in different IADL categories (eg, shopping, food preparation, housekeeping, laundry, and responsibility for own medication) presented with lower aerobic fitness compared with those reporting independence, although the difference was not statistically significant.
Sebastião E, Pilutti LA, Motl RW. Aerobic fitness and instrumental activities of daily living in people with multiple sclerosis. A cross-sectional study. Int J MS Care. 2019;21(1):23-28. doi:10.7224/1537-2073.2017-078.
Immunomodulators for pediatric skin diseases
WASHINGTON – At the annual meeting of the American Academy of Dermatology, colleagues A. Yasmine Kirkorian, MD, a pediatric dermatologist at George Washington University, Washington, and interim chief of pediatric dermatology at Children’s National Health System, and Adam Friedman, MD, professor and interim chair of dermatology at the university, sat down with Dermatology News and discussed their presentations at a session on the use of immunomodulators for inflammatory and neoplastic skin diseases.
In this video, , with her clinical pearls and practical considerations for treating atopic dermatitis, psoriasis, and hidradenitis suppurativa in pediatric patients, covering both on- and off-label treatments.
“Children sometimes require systemic treatment and we shouldn’t hold it back from them because of their age; if they’re severely ill ... they need to be treated,” she said, summing up one of her main points.
During the interview immediately after the AAD meeting, she mentioned dupilumab, which was approved by the Food and Drug Administration for treatment of moderate to severe AD in patients aged 12-17 years.
Dr. Friedman and Dr. Kirkorian reported having no financial disclosures.
WASHINGTON – At the annual meeting of the American Academy of Dermatology, colleagues A. Yasmine Kirkorian, MD, a pediatric dermatologist at George Washington University, Washington, and interim chief of pediatric dermatology at Children’s National Health System, and Adam Friedman, MD, professor and interim chair of dermatology at the university, sat down with Dermatology News and discussed their presentations at a session on the use of immunomodulators for inflammatory and neoplastic skin diseases.
In this video, , with her clinical pearls and practical considerations for treating atopic dermatitis, psoriasis, and hidradenitis suppurativa in pediatric patients, covering both on- and off-label treatments.
“Children sometimes require systemic treatment and we shouldn’t hold it back from them because of their age; if they’re severely ill ... they need to be treated,” she said, summing up one of her main points.
During the interview immediately after the AAD meeting, she mentioned dupilumab, which was approved by the Food and Drug Administration for treatment of moderate to severe AD in patients aged 12-17 years.
Dr. Friedman and Dr. Kirkorian reported having no financial disclosures.
WASHINGTON – At the annual meeting of the American Academy of Dermatology, colleagues A. Yasmine Kirkorian, MD, a pediatric dermatologist at George Washington University, Washington, and interim chief of pediatric dermatology at Children’s National Health System, and Adam Friedman, MD, professor and interim chair of dermatology at the university, sat down with Dermatology News and discussed their presentations at a session on the use of immunomodulators for inflammatory and neoplastic skin diseases.
In this video, , with her clinical pearls and practical considerations for treating atopic dermatitis, psoriasis, and hidradenitis suppurativa in pediatric patients, covering both on- and off-label treatments.
“Children sometimes require systemic treatment and we shouldn’t hold it back from them because of their age; if they’re severely ill ... they need to be treated,” she said, summing up one of her main points.
During the interview immediately after the AAD meeting, she mentioned dupilumab, which was approved by the Food and Drug Administration for treatment of moderate to severe AD in patients aged 12-17 years.
Dr. Friedman and Dr. Kirkorian reported having no financial disclosures.
ACC to offer new certification for transcatheter valve repair, replacement
The American College of Cardiology announced at its ACC Quality Summit in New Orleans that it will offer a Transcatheter Valve Certification to assist hospitals that perform transcatheter valve repair and replacement.
The certification is an external review process that will allow hospitals to meet standards for multidisciplinary teams, formalized training, shared decision making, and registry performance. During the certification process, hospitals will learn best practices for implementing evidence-based medicine in the care of individual patients and identify quality improvement opportunities.
The Transcatheter Valve Certification will be launched in mid-2019. To earn the certification, hospitals must already participate in an established national clinical database.
“. This certification incorporates recent guidelines and expert consensus statements regarding the care of patients requiring transcatheter valve therapies,” Phillip D. Levy, MD, chair of the ACC accreditation management board, said in a press release.
Find the full press release on the ACC website.
The American College of Cardiology announced at its ACC Quality Summit in New Orleans that it will offer a Transcatheter Valve Certification to assist hospitals that perform transcatheter valve repair and replacement.
The certification is an external review process that will allow hospitals to meet standards for multidisciplinary teams, formalized training, shared decision making, and registry performance. During the certification process, hospitals will learn best practices for implementing evidence-based medicine in the care of individual patients and identify quality improvement opportunities.
The Transcatheter Valve Certification will be launched in mid-2019. To earn the certification, hospitals must already participate in an established national clinical database.
“. This certification incorporates recent guidelines and expert consensus statements regarding the care of patients requiring transcatheter valve therapies,” Phillip D. Levy, MD, chair of the ACC accreditation management board, said in a press release.
Find the full press release on the ACC website.
The American College of Cardiology announced at its ACC Quality Summit in New Orleans that it will offer a Transcatheter Valve Certification to assist hospitals that perform transcatheter valve repair and replacement.
The certification is an external review process that will allow hospitals to meet standards for multidisciplinary teams, formalized training, shared decision making, and registry performance. During the certification process, hospitals will learn best practices for implementing evidence-based medicine in the care of individual patients and identify quality improvement opportunities.
The Transcatheter Valve Certification will be launched in mid-2019. To earn the certification, hospitals must already participate in an established national clinical database.
“. This certification incorporates recent guidelines and expert consensus statements regarding the care of patients requiring transcatheter valve therapies,” Phillip D. Levy, MD, chair of the ACC accreditation management board, said in a press release.
Find the full press release on the ACC website.
‘Difficult’ discussions reduced anxiety, depression in life-limiting cancer patients
A program to encourage difficult discussions between seriously ill patients and their oncologists reduced anxiety and depression in a recent randomized trial, but its impact on patient-centered outcomes were uncertain.
Goal-concordant care and peacefulness at the end of life, the coprimary study outcomes, were not significantly different between patients who received the quality improvement intervention and controls in the study, which included 91 clinicians providing care for 278 patients with advanced cancer.
However, it’s not clear whether the intervention, known as the Serious Illness Care Program (SICP), failed to improve those outcomes, or if there simply weren’t enough patients in the trial to detect a meaningful difference, according to investigators led by Rachelle Bernacki, MD, of Brigham and Women’s Hospital and the Harvard School of Public Health, Boston.
“Our challenges reflect the need in our field for patient-centered measures of communication that are agreed upon, validated, and demonstrably sensitive to communication interventions,” wrote Dr. Bernacki and her coinvestigators in a report on the study published in JAMA Internal Medicine.
However, the SICP intervention did clearly result in a larger number of serious-illness conversations that occurred earlier and were of higher quality, the investigators wrote in a separate report published in JAMA Oncology. In medical records reviewed after the patients’ deaths, 96% of those who received the intervention had a documented serious-illness conversation with their oncology clinician, compared with 79% of controls (P = .005), according to that report.
The conversations among SICP recipients occurred a median of 2.4 months earlier than controls, and had a greater focus on values and goals, prognosis and understanding of illness, and treatment preferences.
These outcomes are reassuring, since patients “want, require, and deserve” conversations about serious illness, regardless of their impact on measurable outcomes, the authors of an editorial published in JAMA Oncology wrote.
The SICP intervention included a communication guide for clinicians, who also participated in a 2.5-hour training session designed to improve their serious-illness conversation skills. Other aspects of the program for clinicians included email reminders before outpatient visits, a specialized EMR template, and personal coaching. The program also included patient tools, including a letter introducing the intervention and a guide for continuing the conversation with their family.
The study did not demonstrate a significant difference in peacefulness, as measured by the validated Peace, Equanimity, and Acceptance in the Cancer Experience questionnaire, or in goal-concordant care, which was measured by asking patients to select goals of importance, and then asking caregivers to rate whether those goals had been met at the end of life.
However, patients in the SICP group reported less anxiety and depression 14 weeks into the trial, according to the investigators. The proportion of patients reporting moderate to severe anxiety at that time point was 10.2% for the intervention group versus 5.0% for controls (P = .05), while the proportion reporting depression symptoms was 20.8% for the intervention versus 10.6% for controls (P = .04).
The anxiety reduction was maintained at 24 weeks, though the depression reduction was not, the investigators wrote, adding that there were no differences in survival between arms.
Taken together, these results suggest that oncology clinicians can discuss difficult topics without causing harm, and with potential benefit, the investigators wrote in a discussion of their results.
“Further development of serious illness communication interventions will require more reliable and well-accepted patient-centered outcome measures and additional testing of the effect on patients throughout their illness trajectory,” they concluded.
Dr. Bernacki reported no disclosures. Coauthor Susan D. Block, MD, reported compensation from Up to Date and Atul A. Gawande, MD, MPH, reported receiving compensation from health care writing and media and is employed by a health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
SOURCE: Bernacki R et al. JAMA Intern Med. 2018 Mar 14. doi: 10.1001/jamainternmed.2019.0077.
While results of this rigorous and innovative clinical trial are disappointing because of an apparent lack of impact on the primary outcomes of care, oncologists still must initiate serious illness conversations with advanced cancer patients at risk of dying in the foreseeable future, according to the authors of an editorial.
Doing so is important “not because this will necessarily improve outcomes, but because patients want, require, and deserve to know what is coming,” wrote Belinda E. Kiely, MBBS, PhD, FRACP, and Martin R. Stockler, MBBS, MSc, FRACP.
Those difficult conversations should not stop at discussing the limits of care, but should include a discussion of the patient’s preferences, priorities, and values, Dr. Kiely and Dr. Stockler wrote, adding that they should be documented in the EMR to ensure they are accessible to other health care providers.
“If nothing else, oncologists should be reassured that having these conversations is unlikely to increase anxiety or depression in their patients,” wrote the editorial authors, referencing the significantly reduced incidence of those secondary endpoints in the study.
However, conversations alone may not be enough to improve other patient-centered outcomes, based on the inability of this trial to demonstrate significant improvements in goal-centered care or peacefulness at the end of life.
Moreover, building this Serious Illness Care Program intervention into a health system could be complicated and may require significant resources.
“Simple, pragmatic, and effective tactics are needed to ensure greater generalizability and widespread applicability of such programs,” the authors concluded.
Dr. Kiely and Dr. Stockler are with the National Health and Medical Research Council Clinical Trials Centre at the University of Sydney. Their editorial appears in JAMA Oncology. Dr. Stockler reported grants outside the submitted work from Astellas, Amgen, AstraZeneca, Cancer Australia, Celgene, Bionomics, Bayer, Medivation, Merck, National Health and Medical Research Council Australia, Pfizer, Roche, Sanofi, and Tilray.
While results of this rigorous and innovative clinical trial are disappointing because of an apparent lack of impact on the primary outcomes of care, oncologists still must initiate serious illness conversations with advanced cancer patients at risk of dying in the foreseeable future, according to the authors of an editorial.
Doing so is important “not because this will necessarily improve outcomes, but because patients want, require, and deserve to know what is coming,” wrote Belinda E. Kiely, MBBS, PhD, FRACP, and Martin R. Stockler, MBBS, MSc, FRACP.
Those difficult conversations should not stop at discussing the limits of care, but should include a discussion of the patient’s preferences, priorities, and values, Dr. Kiely and Dr. Stockler wrote, adding that they should be documented in the EMR to ensure they are accessible to other health care providers.
“If nothing else, oncologists should be reassured that having these conversations is unlikely to increase anxiety or depression in their patients,” wrote the editorial authors, referencing the significantly reduced incidence of those secondary endpoints in the study.
However, conversations alone may not be enough to improve other patient-centered outcomes, based on the inability of this trial to demonstrate significant improvements in goal-centered care or peacefulness at the end of life.
Moreover, building this Serious Illness Care Program intervention into a health system could be complicated and may require significant resources.
“Simple, pragmatic, and effective tactics are needed to ensure greater generalizability and widespread applicability of such programs,” the authors concluded.
Dr. Kiely and Dr. Stockler are with the National Health and Medical Research Council Clinical Trials Centre at the University of Sydney. Their editorial appears in JAMA Oncology. Dr. Stockler reported grants outside the submitted work from Astellas, Amgen, AstraZeneca, Cancer Australia, Celgene, Bionomics, Bayer, Medivation, Merck, National Health and Medical Research Council Australia, Pfizer, Roche, Sanofi, and Tilray.
While results of this rigorous and innovative clinical trial are disappointing because of an apparent lack of impact on the primary outcomes of care, oncologists still must initiate serious illness conversations with advanced cancer patients at risk of dying in the foreseeable future, according to the authors of an editorial.
Doing so is important “not because this will necessarily improve outcomes, but because patients want, require, and deserve to know what is coming,” wrote Belinda E. Kiely, MBBS, PhD, FRACP, and Martin R. Stockler, MBBS, MSc, FRACP.
Those difficult conversations should not stop at discussing the limits of care, but should include a discussion of the patient’s preferences, priorities, and values, Dr. Kiely and Dr. Stockler wrote, adding that they should be documented in the EMR to ensure they are accessible to other health care providers.
“If nothing else, oncologists should be reassured that having these conversations is unlikely to increase anxiety or depression in their patients,” wrote the editorial authors, referencing the significantly reduced incidence of those secondary endpoints in the study.
However, conversations alone may not be enough to improve other patient-centered outcomes, based on the inability of this trial to demonstrate significant improvements in goal-centered care or peacefulness at the end of life.
Moreover, building this Serious Illness Care Program intervention into a health system could be complicated and may require significant resources.
“Simple, pragmatic, and effective tactics are needed to ensure greater generalizability and widespread applicability of such programs,” the authors concluded.
Dr. Kiely and Dr. Stockler are with the National Health and Medical Research Council Clinical Trials Centre at the University of Sydney. Their editorial appears in JAMA Oncology. Dr. Stockler reported grants outside the submitted work from Astellas, Amgen, AstraZeneca, Cancer Australia, Celgene, Bionomics, Bayer, Medivation, Merck, National Health and Medical Research Council Australia, Pfizer, Roche, Sanofi, and Tilray.
A program to encourage difficult discussions between seriously ill patients and their oncologists reduced anxiety and depression in a recent randomized trial, but its impact on patient-centered outcomes were uncertain.
Goal-concordant care and peacefulness at the end of life, the coprimary study outcomes, were not significantly different between patients who received the quality improvement intervention and controls in the study, which included 91 clinicians providing care for 278 patients with advanced cancer.
However, it’s not clear whether the intervention, known as the Serious Illness Care Program (SICP), failed to improve those outcomes, or if there simply weren’t enough patients in the trial to detect a meaningful difference, according to investigators led by Rachelle Bernacki, MD, of Brigham and Women’s Hospital and the Harvard School of Public Health, Boston.
“Our challenges reflect the need in our field for patient-centered measures of communication that are agreed upon, validated, and demonstrably sensitive to communication interventions,” wrote Dr. Bernacki and her coinvestigators in a report on the study published in JAMA Internal Medicine.
However, the SICP intervention did clearly result in a larger number of serious-illness conversations that occurred earlier and were of higher quality, the investigators wrote in a separate report published in JAMA Oncology. In medical records reviewed after the patients’ deaths, 96% of those who received the intervention had a documented serious-illness conversation with their oncology clinician, compared with 79% of controls (P = .005), according to that report.
The conversations among SICP recipients occurred a median of 2.4 months earlier than controls, and had a greater focus on values and goals, prognosis and understanding of illness, and treatment preferences.
These outcomes are reassuring, since patients “want, require, and deserve” conversations about serious illness, regardless of their impact on measurable outcomes, the authors of an editorial published in JAMA Oncology wrote.
The SICP intervention included a communication guide for clinicians, who also participated in a 2.5-hour training session designed to improve their serious-illness conversation skills. Other aspects of the program for clinicians included email reminders before outpatient visits, a specialized EMR template, and personal coaching. The program also included patient tools, including a letter introducing the intervention and a guide for continuing the conversation with their family.
The study did not demonstrate a significant difference in peacefulness, as measured by the validated Peace, Equanimity, and Acceptance in the Cancer Experience questionnaire, or in goal-concordant care, which was measured by asking patients to select goals of importance, and then asking caregivers to rate whether those goals had been met at the end of life.
However, patients in the SICP group reported less anxiety and depression 14 weeks into the trial, according to the investigators. The proportion of patients reporting moderate to severe anxiety at that time point was 10.2% for the intervention group versus 5.0% for controls (P = .05), while the proportion reporting depression symptoms was 20.8% for the intervention versus 10.6% for controls (P = .04).
The anxiety reduction was maintained at 24 weeks, though the depression reduction was not, the investigators wrote, adding that there were no differences in survival between arms.
Taken together, these results suggest that oncology clinicians can discuss difficult topics without causing harm, and with potential benefit, the investigators wrote in a discussion of their results.
“Further development of serious illness communication interventions will require more reliable and well-accepted patient-centered outcome measures and additional testing of the effect on patients throughout their illness trajectory,” they concluded.
Dr. Bernacki reported no disclosures. Coauthor Susan D. Block, MD, reported compensation from Up to Date and Atul A. Gawande, MD, MPH, reported receiving compensation from health care writing and media and is employed by a health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
SOURCE: Bernacki R et al. JAMA Intern Med. 2018 Mar 14. doi: 10.1001/jamainternmed.2019.0077.
A program to encourage difficult discussions between seriously ill patients and their oncologists reduced anxiety and depression in a recent randomized trial, but its impact on patient-centered outcomes were uncertain.
Goal-concordant care and peacefulness at the end of life, the coprimary study outcomes, were not significantly different between patients who received the quality improvement intervention and controls in the study, which included 91 clinicians providing care for 278 patients with advanced cancer.
However, it’s not clear whether the intervention, known as the Serious Illness Care Program (SICP), failed to improve those outcomes, or if there simply weren’t enough patients in the trial to detect a meaningful difference, according to investigators led by Rachelle Bernacki, MD, of Brigham and Women’s Hospital and the Harvard School of Public Health, Boston.
“Our challenges reflect the need in our field for patient-centered measures of communication that are agreed upon, validated, and demonstrably sensitive to communication interventions,” wrote Dr. Bernacki and her coinvestigators in a report on the study published in JAMA Internal Medicine.
However, the SICP intervention did clearly result in a larger number of serious-illness conversations that occurred earlier and were of higher quality, the investigators wrote in a separate report published in JAMA Oncology. In medical records reviewed after the patients’ deaths, 96% of those who received the intervention had a documented serious-illness conversation with their oncology clinician, compared with 79% of controls (P = .005), according to that report.
The conversations among SICP recipients occurred a median of 2.4 months earlier than controls, and had a greater focus on values and goals, prognosis and understanding of illness, and treatment preferences.
These outcomes are reassuring, since patients “want, require, and deserve” conversations about serious illness, regardless of their impact on measurable outcomes, the authors of an editorial published in JAMA Oncology wrote.
The SICP intervention included a communication guide for clinicians, who also participated in a 2.5-hour training session designed to improve their serious-illness conversation skills. Other aspects of the program for clinicians included email reminders before outpatient visits, a specialized EMR template, and personal coaching. The program also included patient tools, including a letter introducing the intervention and a guide for continuing the conversation with their family.
The study did not demonstrate a significant difference in peacefulness, as measured by the validated Peace, Equanimity, and Acceptance in the Cancer Experience questionnaire, or in goal-concordant care, which was measured by asking patients to select goals of importance, and then asking caregivers to rate whether those goals had been met at the end of life.
However, patients in the SICP group reported less anxiety and depression 14 weeks into the trial, according to the investigators. The proportion of patients reporting moderate to severe anxiety at that time point was 10.2% for the intervention group versus 5.0% for controls (P = .05), while the proportion reporting depression symptoms was 20.8% for the intervention versus 10.6% for controls (P = .04).
The anxiety reduction was maintained at 24 weeks, though the depression reduction was not, the investigators wrote, adding that there were no differences in survival between arms.
Taken together, these results suggest that oncology clinicians can discuss difficult topics without causing harm, and with potential benefit, the investigators wrote in a discussion of their results.
“Further development of serious illness communication interventions will require more reliable and well-accepted patient-centered outcome measures and additional testing of the effect on patients throughout their illness trajectory,” they concluded.
Dr. Bernacki reported no disclosures. Coauthor Susan D. Block, MD, reported compensation from Up to Date and Atul A. Gawande, MD, MPH, reported receiving compensation from health care writing and media and is employed by a health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
SOURCE: Bernacki R et al. JAMA Intern Med. 2018 Mar 14. doi: 10.1001/jamainternmed.2019.0077.
FROM JAMA INTERNAL MEDICINE
Genomic sequencing sheds light on development of pediatric cancer
Genome sequencing technologies are providing a valuable new window into the development and progression of pediatric cancers, according to the authors of a review.
In contrast to adult cancers, which are frequently driven by oncogenic mutations, many pediatric cancers have a low burden of somatic mutations, wrote E. Alejandro Sweet-Cordero, MD, from the University of California, San Francisco, and Jaclyn A. Biegel, MD, from the University of Southern California in Science. Instead, large-scale sequencing studies have found that childhood cancers have a much higher likelihood of being caused by germline mutations in genes that predispose development of cancer.
“Particularly surprising was the observation that even high-risk, highly aggressive cancers in many cases had no identifiable driver gene or pathway,” the authors wrote.
Some pediatric cancers do have identified driver genes, but even these are often different to those seen in adult cancers. The authors gave the example of one study of 1,699 patients and six types of cancer: This study identified 142 likely oncogenes, but only 45% of these matched those seen in the adult cancers.
Many pediatric cancers also have unique genetic features, such as the age-dependent gene fusion events, in which two genes join to form an oncogenic hybrid, and focal areas of gene deletion, which are often seen in pediatric acute myeloid leukemia but less so in adult forms of this cancer.
“In some instances, the fusion events involve genes that are known to be cancer drivers; this raises the intriguing possibility that some pediatric cancers are driven by ‘private’ oncogenic fusions,” the authors wrote, pointing out that this has daunting implications for the development of precision medicine. However they also noted that the presence of common gene fusion events could hold significance for choice of therapies; for example, central nervous system gliomas with the common BRAF V600E mutation may respond to specific BRAF inhibitors.
The authors drew particular attention to the role that genomic analysis could play in studying cancer during treatment and relapse, but they said few studies have explored this in pediatric patients.
“Such studies are critical given what we have learned from adult cancers, which show a capacity to evolve rapidly and acquire new driver mutations,” they wrote. One study found that only one-third of tumors with a potentially targetable genetic mutation had retained that target when analyzed at a later time.
On the issue of targeted therapy, the authors noted that no prospective study has yet looked at the use of sequencing approaches to define new therapies for pediatric cancer. However, they did refer to the Pediatric MATCH clinical trial, which is currently evaluating targeted therapies for relapsed solid tumors in children.
They also identified a need for research on predictors of treatment response in pediatric cancer.
“As the genetic variants that are associated with drug response are, by nature and design, variants present in the normal population, they are typically not included in DNA sequencing panels and are filtered out in WES [whole-exome sequencing] or WGS [whole-genome sequencing] bioinformatics pipelines,” they wrote.
They addressed the question of when to do germline testing in pediatric cancer, saying that, for most pediatric cancer patients, germline testing was indicated by the presence of a pathogenic genetic alternative affecting a gene known to be associated with a predisposition for germline cancer.
The authors suggested that data sharing was important to advancing genomic analysis in pediatric cancers because most of the studies so far had been relatively small. However, they highlighted emerging resources for large-scale analysis of pediatric cancer data, such as public portals for investigating discovery genomic data sets and data repositories of clinical-grade sequencing data.
The review was funded by the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Sweet-Cordero A et al. Science 2019;363:1170-5.
Genome sequencing technologies are providing a valuable new window into the development and progression of pediatric cancers, according to the authors of a review.
In contrast to adult cancers, which are frequently driven by oncogenic mutations, many pediatric cancers have a low burden of somatic mutations, wrote E. Alejandro Sweet-Cordero, MD, from the University of California, San Francisco, and Jaclyn A. Biegel, MD, from the University of Southern California in Science. Instead, large-scale sequencing studies have found that childhood cancers have a much higher likelihood of being caused by germline mutations in genes that predispose development of cancer.
“Particularly surprising was the observation that even high-risk, highly aggressive cancers in many cases had no identifiable driver gene or pathway,” the authors wrote.
Some pediatric cancers do have identified driver genes, but even these are often different to those seen in adult cancers. The authors gave the example of one study of 1,699 patients and six types of cancer: This study identified 142 likely oncogenes, but only 45% of these matched those seen in the adult cancers.
Many pediatric cancers also have unique genetic features, such as the age-dependent gene fusion events, in which two genes join to form an oncogenic hybrid, and focal areas of gene deletion, which are often seen in pediatric acute myeloid leukemia but less so in adult forms of this cancer.
“In some instances, the fusion events involve genes that are known to be cancer drivers; this raises the intriguing possibility that some pediatric cancers are driven by ‘private’ oncogenic fusions,” the authors wrote, pointing out that this has daunting implications for the development of precision medicine. However they also noted that the presence of common gene fusion events could hold significance for choice of therapies; for example, central nervous system gliomas with the common BRAF V600E mutation may respond to specific BRAF inhibitors.
The authors drew particular attention to the role that genomic analysis could play in studying cancer during treatment and relapse, but they said few studies have explored this in pediatric patients.
“Such studies are critical given what we have learned from adult cancers, which show a capacity to evolve rapidly and acquire new driver mutations,” they wrote. One study found that only one-third of tumors with a potentially targetable genetic mutation had retained that target when analyzed at a later time.
On the issue of targeted therapy, the authors noted that no prospective study has yet looked at the use of sequencing approaches to define new therapies for pediatric cancer. However, they did refer to the Pediatric MATCH clinical trial, which is currently evaluating targeted therapies for relapsed solid tumors in children.
They also identified a need for research on predictors of treatment response in pediatric cancer.
“As the genetic variants that are associated with drug response are, by nature and design, variants present in the normal population, they are typically not included in DNA sequencing panels and are filtered out in WES [whole-exome sequencing] or WGS [whole-genome sequencing] bioinformatics pipelines,” they wrote.
They addressed the question of when to do germline testing in pediatric cancer, saying that, for most pediatric cancer patients, germline testing was indicated by the presence of a pathogenic genetic alternative affecting a gene known to be associated with a predisposition for germline cancer.
The authors suggested that data sharing was important to advancing genomic analysis in pediatric cancers because most of the studies so far had been relatively small. However, they highlighted emerging resources for large-scale analysis of pediatric cancer data, such as public portals for investigating discovery genomic data sets and data repositories of clinical-grade sequencing data.
The review was funded by the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Sweet-Cordero A et al. Science 2019;363:1170-5.
Genome sequencing technologies are providing a valuable new window into the development and progression of pediatric cancers, according to the authors of a review.
In contrast to adult cancers, which are frequently driven by oncogenic mutations, many pediatric cancers have a low burden of somatic mutations, wrote E. Alejandro Sweet-Cordero, MD, from the University of California, San Francisco, and Jaclyn A. Biegel, MD, from the University of Southern California in Science. Instead, large-scale sequencing studies have found that childhood cancers have a much higher likelihood of being caused by germline mutations in genes that predispose development of cancer.
“Particularly surprising was the observation that even high-risk, highly aggressive cancers in many cases had no identifiable driver gene or pathway,” the authors wrote.
Some pediatric cancers do have identified driver genes, but even these are often different to those seen in adult cancers. The authors gave the example of one study of 1,699 patients and six types of cancer: This study identified 142 likely oncogenes, but only 45% of these matched those seen in the adult cancers.
Many pediatric cancers also have unique genetic features, such as the age-dependent gene fusion events, in which two genes join to form an oncogenic hybrid, and focal areas of gene deletion, which are often seen in pediatric acute myeloid leukemia but less so in adult forms of this cancer.
“In some instances, the fusion events involve genes that are known to be cancer drivers; this raises the intriguing possibility that some pediatric cancers are driven by ‘private’ oncogenic fusions,” the authors wrote, pointing out that this has daunting implications for the development of precision medicine. However they also noted that the presence of common gene fusion events could hold significance for choice of therapies; for example, central nervous system gliomas with the common BRAF V600E mutation may respond to specific BRAF inhibitors.
The authors drew particular attention to the role that genomic analysis could play in studying cancer during treatment and relapse, but they said few studies have explored this in pediatric patients.
“Such studies are critical given what we have learned from adult cancers, which show a capacity to evolve rapidly and acquire new driver mutations,” they wrote. One study found that only one-third of tumors with a potentially targetable genetic mutation had retained that target when analyzed at a later time.
On the issue of targeted therapy, the authors noted that no prospective study has yet looked at the use of sequencing approaches to define new therapies for pediatric cancer. However, they did refer to the Pediatric MATCH clinical trial, which is currently evaluating targeted therapies for relapsed solid tumors in children.
They also identified a need for research on predictors of treatment response in pediatric cancer.
“As the genetic variants that are associated with drug response are, by nature and design, variants present in the normal population, they are typically not included in DNA sequencing panels and are filtered out in WES [whole-exome sequencing] or WGS [whole-genome sequencing] bioinformatics pipelines,” they wrote.
They addressed the question of when to do germline testing in pediatric cancer, saying that, for most pediatric cancer patients, germline testing was indicated by the presence of a pathogenic genetic alternative affecting a gene known to be associated with a predisposition for germline cancer.
The authors suggested that data sharing was important to advancing genomic analysis in pediatric cancers because most of the studies so far had been relatively small. However, they highlighted emerging resources for large-scale analysis of pediatric cancer data, such as public portals for investigating discovery genomic data sets and data repositories of clinical-grade sequencing data.
The review was funded by the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Sweet-Cordero A et al. Science 2019;363:1170-5.
FROM SCIENCE
Key clinical point: Genome sequencing is providing valuable information on pediatric cancer development and progression.
Major finding: Many pediatric cancers have very different oncogenic drivers to adult cancers.
Study details: Review.
Disclosures: The review was funded by the National Cancer Institute. No conflicts of interest were declared.
Source: Sweet-Cordero EA et al. Science. 2019;363:1170-5.