User login
Bleeding disorders don’t carry increased risks for patients undergoing endoscopy
When managed by an experienced team, patients with inherited bleeding disorders undergoing gastrointestinal endoscopy are not at increased bleeding risk, according to researchers reporting the largest series of such patients to date.
The postendoscopy bleeding rate was less than 1% for the patients in this series, many of whom underwent high-risk procedures or had comorbid conditions putting them at risk of bleeding, the researchers reported.
The rate of colonoscopic postpolypectomy bleeding was less than 5%, which is comparable to the general population rate for such high-risk procedures, said investigator Marcel Tomaszewski, MD, of McGill University, Montreal, and colleagues.
Those results favor the use of the “interdisciplinary approach provided by a hemophilia treatment center, communication between services, and the use of periprocedure prophylaxis including tranexamic acid,” Dr. Tomaszewski and coinvestigators said in their report in Haemophilia.
The study cohort included 48 individuals undergoing a total of 104 endoscopies, which is believed to be the largest case series of digestive endoscopy procedures ever reported in patients with congenital bleeding disorders, according to the researchers.
Hemophilia A and von Willebrand disease were the most common bleeding disorders among these patients, accounting for 49 and 40 of the 104 procedures, respectively. The remaining 17 procedures were performed in patients with factor XI deficiency, hemophilia B, or factor VII deficiency.
Before their endoscopies, patients received bleeding prophylaxis, which consisted of combinations of recombinant factor for hemophilia patients, plasma-derived factor for von Willebrand disease patients, desmopressin, and tranexamic acid.
The rate of bleeding within 72 hours of the endoscopic procedure was 0.96% (95% confidence interval, 0.17%-5.25%). The bleeding rate for hemophilia A patients, regardless of severity, was 2.2%, while the rate for hemophilia B, von Willebrand disease, factor VII deficiency and factor XI deficiency was 0%.
The only endoscopic procedures associated with bleeding were colonoscopy, with a bleeding rate of 2%, and colonoscopy with polypectomy, which had a 4.8% bleeding rate, they added, noting that the reported rate of postpolypectomy bleeding in the general population ranges between 0.3% and 10%.
“The very low incidence of bleeding adverse events limited further planned inferential statistical analysis,” the researchers wrote.
These findings stand in contrast to some previous reports, including one series of 19 patients with hemophilia in which 31% experienced gastrointestinal bleeding after colonoscopy polypectomy, despite preprocedure prophylaxis.
“Our approach, particularly with the addition of tranexamic acid, and our patient mix may explain the lower bleeding rate,” they wrote.
The most common indications for endoscopy in the present report were anemia, colorectal cancer screening or polyp surveillance, upper GI symptoms, and screening or surveillance of varices.
Many patients had conditions that might predispose them to bleeding, investigators said. Most commonly, those conditions included hepatitis C virus infection, cirrhosis, esophageal varices, and previous gastrointestinal bleeding.
Dr. Tomaszewski reported that he had no potential conflicts of interest related to the research. Coauthors reported financial disclosures related to AbbVie, Pfizer, Takeda, Janssen, and others.
SOURCE: Tomaszewski M et al. Haemophilia. 2019 Feb 12. doi: 10.1111/hae.13691
When managed by an experienced team, patients with inherited bleeding disorders undergoing gastrointestinal endoscopy are not at increased bleeding risk, according to researchers reporting the largest series of such patients to date.
The postendoscopy bleeding rate was less than 1% for the patients in this series, many of whom underwent high-risk procedures or had comorbid conditions putting them at risk of bleeding, the researchers reported.
The rate of colonoscopic postpolypectomy bleeding was less than 5%, which is comparable to the general population rate for such high-risk procedures, said investigator Marcel Tomaszewski, MD, of McGill University, Montreal, and colleagues.
Those results favor the use of the “interdisciplinary approach provided by a hemophilia treatment center, communication between services, and the use of periprocedure prophylaxis including tranexamic acid,” Dr. Tomaszewski and coinvestigators said in their report in Haemophilia.
The study cohort included 48 individuals undergoing a total of 104 endoscopies, which is believed to be the largest case series of digestive endoscopy procedures ever reported in patients with congenital bleeding disorders, according to the researchers.
Hemophilia A and von Willebrand disease were the most common bleeding disorders among these patients, accounting for 49 and 40 of the 104 procedures, respectively. The remaining 17 procedures were performed in patients with factor XI deficiency, hemophilia B, or factor VII deficiency.
Before their endoscopies, patients received bleeding prophylaxis, which consisted of combinations of recombinant factor for hemophilia patients, plasma-derived factor for von Willebrand disease patients, desmopressin, and tranexamic acid.
The rate of bleeding within 72 hours of the endoscopic procedure was 0.96% (95% confidence interval, 0.17%-5.25%). The bleeding rate for hemophilia A patients, regardless of severity, was 2.2%, while the rate for hemophilia B, von Willebrand disease, factor VII deficiency and factor XI deficiency was 0%.
The only endoscopic procedures associated with bleeding were colonoscopy, with a bleeding rate of 2%, and colonoscopy with polypectomy, which had a 4.8% bleeding rate, they added, noting that the reported rate of postpolypectomy bleeding in the general population ranges between 0.3% and 10%.
“The very low incidence of bleeding adverse events limited further planned inferential statistical analysis,” the researchers wrote.
These findings stand in contrast to some previous reports, including one series of 19 patients with hemophilia in which 31% experienced gastrointestinal bleeding after colonoscopy polypectomy, despite preprocedure prophylaxis.
“Our approach, particularly with the addition of tranexamic acid, and our patient mix may explain the lower bleeding rate,” they wrote.
The most common indications for endoscopy in the present report were anemia, colorectal cancer screening or polyp surveillance, upper GI symptoms, and screening or surveillance of varices.
Many patients had conditions that might predispose them to bleeding, investigators said. Most commonly, those conditions included hepatitis C virus infection, cirrhosis, esophageal varices, and previous gastrointestinal bleeding.
Dr. Tomaszewski reported that he had no potential conflicts of interest related to the research. Coauthors reported financial disclosures related to AbbVie, Pfizer, Takeda, Janssen, and others.
SOURCE: Tomaszewski M et al. Haemophilia. 2019 Feb 12. doi: 10.1111/hae.13691
When managed by an experienced team, patients with inherited bleeding disorders undergoing gastrointestinal endoscopy are not at increased bleeding risk, according to researchers reporting the largest series of such patients to date.
The postendoscopy bleeding rate was less than 1% for the patients in this series, many of whom underwent high-risk procedures or had comorbid conditions putting them at risk of bleeding, the researchers reported.
The rate of colonoscopic postpolypectomy bleeding was less than 5%, which is comparable to the general population rate for such high-risk procedures, said investigator Marcel Tomaszewski, MD, of McGill University, Montreal, and colleagues.
Those results favor the use of the “interdisciplinary approach provided by a hemophilia treatment center, communication between services, and the use of periprocedure prophylaxis including tranexamic acid,” Dr. Tomaszewski and coinvestigators said in their report in Haemophilia.
The study cohort included 48 individuals undergoing a total of 104 endoscopies, which is believed to be the largest case series of digestive endoscopy procedures ever reported in patients with congenital bleeding disorders, according to the researchers.
Hemophilia A and von Willebrand disease were the most common bleeding disorders among these patients, accounting for 49 and 40 of the 104 procedures, respectively. The remaining 17 procedures were performed in patients with factor XI deficiency, hemophilia B, or factor VII deficiency.
Before their endoscopies, patients received bleeding prophylaxis, which consisted of combinations of recombinant factor for hemophilia patients, plasma-derived factor for von Willebrand disease patients, desmopressin, and tranexamic acid.
The rate of bleeding within 72 hours of the endoscopic procedure was 0.96% (95% confidence interval, 0.17%-5.25%). The bleeding rate for hemophilia A patients, regardless of severity, was 2.2%, while the rate for hemophilia B, von Willebrand disease, factor VII deficiency and factor XI deficiency was 0%.
The only endoscopic procedures associated with bleeding were colonoscopy, with a bleeding rate of 2%, and colonoscopy with polypectomy, which had a 4.8% bleeding rate, they added, noting that the reported rate of postpolypectomy bleeding in the general population ranges between 0.3% and 10%.
“The very low incidence of bleeding adverse events limited further planned inferential statistical analysis,” the researchers wrote.
These findings stand in contrast to some previous reports, including one series of 19 patients with hemophilia in which 31% experienced gastrointestinal bleeding after colonoscopy polypectomy, despite preprocedure prophylaxis.
“Our approach, particularly with the addition of tranexamic acid, and our patient mix may explain the lower bleeding rate,” they wrote.
The most common indications for endoscopy in the present report were anemia, colorectal cancer screening or polyp surveillance, upper GI symptoms, and screening or surveillance of varices.
Many patients had conditions that might predispose them to bleeding, investigators said. Most commonly, those conditions included hepatitis C virus infection, cirrhosis, esophageal varices, and previous gastrointestinal bleeding.
Dr. Tomaszewski reported that he had no potential conflicts of interest related to the research. Coauthors reported financial disclosures related to AbbVie, Pfizer, Takeda, Janssen, and others.
SOURCE: Tomaszewski M et al. Haemophilia. 2019 Feb 12. doi: 10.1111/hae.13691
FROM HAEMOPHILIA
Erectile dysfunction appears prevalent with hemophilia
Erectile dysfunction (ED) appears prevalent among men with hemophilia and becomes increasingly common with age, according to results from a recent survey.
Although the findings may not be generalizable because of small sample size (44 respondents), the survey offers a general sense of the sexual health of patients with hemophilia, which is a minimally researched topic, reported lead author Ming Yang, PhD, of the British Columbia Provincial Bleeding Disorders Program at St. Paul’s Hospital in Vancouver and colleagues.
Such data become increasingly important as new hemophilia therapies further extend the lifespan of patients, which thereby exposes them to diseases of old age, the investigators noted in Haemophilia.
Additionally, ED is considered an early sign of endothelial dysfunction in the general population, but data are lacking for patients with hemophilia.
Among the 56 men with hemophilia A or B who were surveyed with the International Index of Erectile Function (IIEF) questionnaire, 44 completed the survey (median age, 49 years). Specifically, respondents completed the “erectile function” component of the questionnaire because this is the only domain that has been validated.
To assess associations between ED and well-established risk factors, the investigators recorded prior surgeries, medications, atherosclerotic diseases, viral infections, and hemophilia-specific factors. To better characterize the population and look for other associations, the investigators had patients undergo baseline laboratory testing and measurements of blood pressure, anthropomorphic indexes, and endothelial function.
According to the IIEF, 38.6% of patients had ED, which was subcategorized as mild and mild to moderate in 4.5% of patients, moderate in 18.2%, and severe in 15.9%.
The investigators found significant associations between ED and coronary artery disease, hypertension, smoking, higher waist/hip ratio, homocysteine level, and age. However, on multivariable analysis, only age was correlated with ED domain score (P = .03). No associations between ED and endothelial dysfunction were reported.
While the findings may offer a rare look at ED rates in patients with hemophilia, they are insufficient for comparisons and generalizations, the investigators cautioned.
“Without comparative studies within the haemophilia population in Canada or elsewhere and due to limited sample size and non‐normalized distribution of our age group, this prevalence cannot reasonably be generalized to the entire haemophilia population,” the investigators wrote.
Instead, the investigators suggested that more studies are needed, especially ones involving customized questionnaires.
The study was funded by Pfizer. Dr. Yang reported having no conflicts of interest. One coauthor reported an unrestricted educational award from the Association of Hemophilia Clinic Directors of Canada/Baxter Canadian Hemostasis Fellowship, and another coauthor reported research funding from Pfizer.
SOURCE: Yang M et al. Haemophilia. 2019 Feb 28. doi: 10.1111/hae.13707.
Erectile dysfunction (ED) appears prevalent among men with hemophilia and becomes increasingly common with age, according to results from a recent survey.
Although the findings may not be generalizable because of small sample size (44 respondents), the survey offers a general sense of the sexual health of patients with hemophilia, which is a minimally researched topic, reported lead author Ming Yang, PhD, of the British Columbia Provincial Bleeding Disorders Program at St. Paul’s Hospital in Vancouver and colleagues.
Such data become increasingly important as new hemophilia therapies further extend the lifespan of patients, which thereby exposes them to diseases of old age, the investigators noted in Haemophilia.
Additionally, ED is considered an early sign of endothelial dysfunction in the general population, but data are lacking for patients with hemophilia.
Among the 56 men with hemophilia A or B who were surveyed with the International Index of Erectile Function (IIEF) questionnaire, 44 completed the survey (median age, 49 years). Specifically, respondents completed the “erectile function” component of the questionnaire because this is the only domain that has been validated.
To assess associations between ED and well-established risk factors, the investigators recorded prior surgeries, medications, atherosclerotic diseases, viral infections, and hemophilia-specific factors. To better characterize the population and look for other associations, the investigators had patients undergo baseline laboratory testing and measurements of blood pressure, anthropomorphic indexes, and endothelial function.
According to the IIEF, 38.6% of patients had ED, which was subcategorized as mild and mild to moderate in 4.5% of patients, moderate in 18.2%, and severe in 15.9%.
The investigators found significant associations between ED and coronary artery disease, hypertension, smoking, higher waist/hip ratio, homocysteine level, and age. However, on multivariable analysis, only age was correlated with ED domain score (P = .03). No associations between ED and endothelial dysfunction were reported.
While the findings may offer a rare look at ED rates in patients with hemophilia, they are insufficient for comparisons and generalizations, the investigators cautioned.
“Without comparative studies within the haemophilia population in Canada or elsewhere and due to limited sample size and non‐normalized distribution of our age group, this prevalence cannot reasonably be generalized to the entire haemophilia population,” the investigators wrote.
Instead, the investigators suggested that more studies are needed, especially ones involving customized questionnaires.
The study was funded by Pfizer. Dr. Yang reported having no conflicts of interest. One coauthor reported an unrestricted educational award from the Association of Hemophilia Clinic Directors of Canada/Baxter Canadian Hemostasis Fellowship, and another coauthor reported research funding from Pfizer.
SOURCE: Yang M et al. Haemophilia. 2019 Feb 28. doi: 10.1111/hae.13707.
Erectile dysfunction (ED) appears prevalent among men with hemophilia and becomes increasingly common with age, according to results from a recent survey.
Although the findings may not be generalizable because of small sample size (44 respondents), the survey offers a general sense of the sexual health of patients with hemophilia, which is a minimally researched topic, reported lead author Ming Yang, PhD, of the British Columbia Provincial Bleeding Disorders Program at St. Paul’s Hospital in Vancouver and colleagues.
Such data become increasingly important as new hemophilia therapies further extend the lifespan of patients, which thereby exposes them to diseases of old age, the investigators noted in Haemophilia.
Additionally, ED is considered an early sign of endothelial dysfunction in the general population, but data are lacking for patients with hemophilia.
Among the 56 men with hemophilia A or B who were surveyed with the International Index of Erectile Function (IIEF) questionnaire, 44 completed the survey (median age, 49 years). Specifically, respondents completed the “erectile function” component of the questionnaire because this is the only domain that has been validated.
To assess associations between ED and well-established risk factors, the investigators recorded prior surgeries, medications, atherosclerotic diseases, viral infections, and hemophilia-specific factors. To better characterize the population and look for other associations, the investigators had patients undergo baseline laboratory testing and measurements of blood pressure, anthropomorphic indexes, and endothelial function.
According to the IIEF, 38.6% of patients had ED, which was subcategorized as mild and mild to moderate in 4.5% of patients, moderate in 18.2%, and severe in 15.9%.
The investigators found significant associations between ED and coronary artery disease, hypertension, smoking, higher waist/hip ratio, homocysteine level, and age. However, on multivariable analysis, only age was correlated with ED domain score (P = .03). No associations between ED and endothelial dysfunction were reported.
While the findings may offer a rare look at ED rates in patients with hemophilia, they are insufficient for comparisons and generalizations, the investigators cautioned.
“Without comparative studies within the haemophilia population in Canada or elsewhere and due to limited sample size and non‐normalized distribution of our age group, this prevalence cannot reasonably be generalized to the entire haemophilia population,” the investigators wrote.
Instead, the investigators suggested that more studies are needed, especially ones involving customized questionnaires.
The study was funded by Pfizer. Dr. Yang reported having no conflicts of interest. One coauthor reported an unrestricted educational award from the Association of Hemophilia Clinic Directors of Canada/Baxter Canadian Hemostasis Fellowship, and another coauthor reported research funding from Pfizer.
SOURCE: Yang M et al. Haemophilia. 2019 Feb 28. doi: 10.1111/hae.13707.
FROM HAEMOPHILIA
Haplo-HSCT bests chemotherapy for MRD-positive adult ALL
HOUSTON – Haploidentical stem cell transplantation (Haplo-HSCT) outperforms chemotherapy for the treatment of adults with acute lymphoblastic leukemia (ALL) in first complete remission, findings from a prospective multicenter trial suggest.
The 2-year leukemia-free survival (LFS) was about 70% in 49 patients in first remission who received haplo-HSCT vs. 40% in 40 patients who received chemotherapy, and 2-year overall survival (OS) was about 80% vs. 50% in the groups, respectively, Meng Lv, MD, PhD, of Peking University People’s Hospital in Beijing reported at the Transplantation & Cellular Therapy Meetings.
“This result is comparable to results of our previous reports,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
He noted that the findings also support those from other institutions.
Study subjects initially included 112 newly diagnosed standard-risk ALL patients aged 18-39 years without high-risk features who achieved complete remission (CR) after one or two cycles of induction. They were consecutively enrolled at five centers in China, including high-volume centers, between July 2014 and June 2017 and were followed for a median of 24.6 months.
Subjects without a suitable HLA-matched sibling donor (MSD) or HLA-matched unrelated donor after two cycles of consolidation with hyper-CVAD chemotherapy were eligible for haplo-HSCT or further hyper-CVAD chemotherapy.
The final analysis included 89 patients after 23 were excluded because of early relapse (6 patients) or a decision to undergo MSD HSCT (16 patients), or unrelated donor-HSCT (1 patient), Dr. Lv said, noting that landmark analysis was used when comparing the outcomes of patients receiving haplo-HSCT with those receiving chemotherapy.
Multivariate analysis with adjustment for a propensity score calculated for each patient showed that treatment (haplo-HSCT vs. chemotherapy) independently predicted LFS (hazard ratio, 0.388), OS (HR, 0.346), and cumulative incidence of relapse (CIR; HR, 0.247). Minimal residual disease (MRD) positivity after the first consolidation was an independent risk factor for LFS (HR, 2.162) and CIR (HR, 3.667). Additionally, diagnosis (T- vs. B-cell) was an independent risk factor for OS (HR, 2.267), Dr. Lv said, adding that nonrelapse mortality was similar in the groups in the propensity score–adjusted analysis.
The findings overall show that haplo-HSCT has variable impact on survival in standard-risk ALL, when compared with traditional chemotherapy, with subgroup analyses showing MRD-positive patients deriving the greatest benefit, he said. Future studies are planned to look more closely at MRD-positive disease and the possible benefits of postponing transplant until the second CR.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Lv reported having no financial disclosures.
SOURCE: Lv M et al. TCT 2019, Abstract 8.
HOUSTON – Haploidentical stem cell transplantation (Haplo-HSCT) outperforms chemotherapy for the treatment of adults with acute lymphoblastic leukemia (ALL) in first complete remission, findings from a prospective multicenter trial suggest.
The 2-year leukemia-free survival (LFS) was about 70% in 49 patients in first remission who received haplo-HSCT vs. 40% in 40 patients who received chemotherapy, and 2-year overall survival (OS) was about 80% vs. 50% in the groups, respectively, Meng Lv, MD, PhD, of Peking University People’s Hospital in Beijing reported at the Transplantation & Cellular Therapy Meetings.
“This result is comparable to results of our previous reports,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
He noted that the findings also support those from other institutions.
Study subjects initially included 112 newly diagnosed standard-risk ALL patients aged 18-39 years without high-risk features who achieved complete remission (CR) after one or two cycles of induction. They were consecutively enrolled at five centers in China, including high-volume centers, between July 2014 and June 2017 and were followed for a median of 24.6 months.
Subjects without a suitable HLA-matched sibling donor (MSD) or HLA-matched unrelated donor after two cycles of consolidation with hyper-CVAD chemotherapy were eligible for haplo-HSCT or further hyper-CVAD chemotherapy.
The final analysis included 89 patients after 23 were excluded because of early relapse (6 patients) or a decision to undergo MSD HSCT (16 patients), or unrelated donor-HSCT (1 patient), Dr. Lv said, noting that landmark analysis was used when comparing the outcomes of patients receiving haplo-HSCT with those receiving chemotherapy.
Multivariate analysis with adjustment for a propensity score calculated for each patient showed that treatment (haplo-HSCT vs. chemotherapy) independently predicted LFS (hazard ratio, 0.388), OS (HR, 0.346), and cumulative incidence of relapse (CIR; HR, 0.247). Minimal residual disease (MRD) positivity after the first consolidation was an independent risk factor for LFS (HR, 2.162) and CIR (HR, 3.667). Additionally, diagnosis (T- vs. B-cell) was an independent risk factor for OS (HR, 2.267), Dr. Lv said, adding that nonrelapse mortality was similar in the groups in the propensity score–adjusted analysis.
The findings overall show that haplo-HSCT has variable impact on survival in standard-risk ALL, when compared with traditional chemotherapy, with subgroup analyses showing MRD-positive patients deriving the greatest benefit, he said. Future studies are planned to look more closely at MRD-positive disease and the possible benefits of postponing transplant until the second CR.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Lv reported having no financial disclosures.
SOURCE: Lv M et al. TCT 2019, Abstract 8.
HOUSTON – Haploidentical stem cell transplantation (Haplo-HSCT) outperforms chemotherapy for the treatment of adults with acute lymphoblastic leukemia (ALL) in first complete remission, findings from a prospective multicenter trial suggest.
The 2-year leukemia-free survival (LFS) was about 70% in 49 patients in first remission who received haplo-HSCT vs. 40% in 40 patients who received chemotherapy, and 2-year overall survival (OS) was about 80% vs. 50% in the groups, respectively, Meng Lv, MD, PhD, of Peking University People’s Hospital in Beijing reported at the Transplantation & Cellular Therapy Meetings.
“This result is comparable to results of our previous reports,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
He noted that the findings also support those from other institutions.
Study subjects initially included 112 newly diagnosed standard-risk ALL patients aged 18-39 years without high-risk features who achieved complete remission (CR) after one or two cycles of induction. They were consecutively enrolled at five centers in China, including high-volume centers, between July 2014 and June 2017 and were followed for a median of 24.6 months.
Subjects without a suitable HLA-matched sibling donor (MSD) or HLA-matched unrelated donor after two cycles of consolidation with hyper-CVAD chemotherapy were eligible for haplo-HSCT or further hyper-CVAD chemotherapy.
The final analysis included 89 patients after 23 were excluded because of early relapse (6 patients) or a decision to undergo MSD HSCT (16 patients), or unrelated donor-HSCT (1 patient), Dr. Lv said, noting that landmark analysis was used when comparing the outcomes of patients receiving haplo-HSCT with those receiving chemotherapy.
Multivariate analysis with adjustment for a propensity score calculated for each patient showed that treatment (haplo-HSCT vs. chemotherapy) independently predicted LFS (hazard ratio, 0.388), OS (HR, 0.346), and cumulative incidence of relapse (CIR; HR, 0.247). Minimal residual disease (MRD) positivity after the first consolidation was an independent risk factor for LFS (HR, 2.162) and CIR (HR, 3.667). Additionally, diagnosis (T- vs. B-cell) was an independent risk factor for OS (HR, 2.267), Dr. Lv said, adding that nonrelapse mortality was similar in the groups in the propensity score–adjusted analysis.
The findings overall show that haplo-HSCT has variable impact on survival in standard-risk ALL, when compared with traditional chemotherapy, with subgroup analyses showing MRD-positive patients deriving the greatest benefit, he said. Future studies are planned to look more closely at MRD-positive disease and the possible benefits of postponing transplant until the second CR.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Lv reported having no financial disclosures.
SOURCE: Lv M et al. TCT 2019, Abstract 8.
REPORTING FROM TCT 2019
FXIII replacement may improve hemostasis in acquired hemophilia A
Measurement of factor XIII levels, followed by replacing deficiencies if found, may help to achieve sustained hemostasis in the management of bleeding in patients with acquired hemophilia A.
Jameel Abdulrehman, MD, of the University of Toronto in Ontario and his colleagues retrospectively reviewed cases of acquired hemophilia A with bleeding at a large hospital from 2015-2016. The findings were published in a letter to the editor in Haemophilia.
The researchers reported that depletion of Factor XIII (FXIII) in the setting of acquired hemophilia A has only been reported in a single case. In the present analysis, the researchers identified a total of seven patients with acquired hemophilia A from 2015-2016. FXIII antigen levels were measured in five cases and were found to be low in four cases, and three patients required FXIII replacement to secure hemostasis.
The relationship between acquired hemophilia A and FXIII deficiency was evaluated using descriptive case analysis.
After analysis, the team reported that measuring FXIII levels may be useful in refractory patients, in whom bleeding remains uncontrolled despite the use of standard therapy. Additionally, the results supported FXIII supplementation if a deficiency is detected.
“FXIII replacement was not complicated by any infusion reactions or thromboembolic events,” they wrote.
The replaced FXIII levels dropped more rapidly than expected, the researchers noted. The established half-life of pd-XIII is 6.6 days plus or minus 2.29 days; however, in the most severe of the cases reported, it was depleted “within a few days,” they wrote. “This is compatible with either consumption of FXIII or inhibition/clearance by an autoantibody.”
Dr. Abdulrehman and his colleagues acknowledged that FXIII deficiency can be challenging to identify because the majority of coagulation assays do not measure FXIII levels.
“As we gain a greater appreciation for the role of FXIII in various pathological states, access to accurate FXIII testing will become increasingly important,” they added.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Abdulrehman J et al. Haemophilia. 2019 Mar 7. doi: 10.1111/hae.13690.
Measurement of factor XIII levels, followed by replacing deficiencies if found, may help to achieve sustained hemostasis in the management of bleeding in patients with acquired hemophilia A.
Jameel Abdulrehman, MD, of the University of Toronto in Ontario and his colleagues retrospectively reviewed cases of acquired hemophilia A with bleeding at a large hospital from 2015-2016. The findings were published in a letter to the editor in Haemophilia.
The researchers reported that depletion of Factor XIII (FXIII) in the setting of acquired hemophilia A has only been reported in a single case. In the present analysis, the researchers identified a total of seven patients with acquired hemophilia A from 2015-2016. FXIII antigen levels were measured in five cases and were found to be low in four cases, and three patients required FXIII replacement to secure hemostasis.
The relationship between acquired hemophilia A and FXIII deficiency was evaluated using descriptive case analysis.
After analysis, the team reported that measuring FXIII levels may be useful in refractory patients, in whom bleeding remains uncontrolled despite the use of standard therapy. Additionally, the results supported FXIII supplementation if a deficiency is detected.
“FXIII replacement was not complicated by any infusion reactions or thromboembolic events,” they wrote.
The replaced FXIII levels dropped more rapidly than expected, the researchers noted. The established half-life of pd-XIII is 6.6 days plus or minus 2.29 days; however, in the most severe of the cases reported, it was depleted “within a few days,” they wrote. “This is compatible with either consumption of FXIII or inhibition/clearance by an autoantibody.”
Dr. Abdulrehman and his colleagues acknowledged that FXIII deficiency can be challenging to identify because the majority of coagulation assays do not measure FXIII levels.
“As we gain a greater appreciation for the role of FXIII in various pathological states, access to accurate FXIII testing will become increasingly important,” they added.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Abdulrehman J et al. Haemophilia. 2019 Mar 7. doi: 10.1111/hae.13690.
Measurement of factor XIII levels, followed by replacing deficiencies if found, may help to achieve sustained hemostasis in the management of bleeding in patients with acquired hemophilia A.
Jameel Abdulrehman, MD, of the University of Toronto in Ontario and his colleagues retrospectively reviewed cases of acquired hemophilia A with bleeding at a large hospital from 2015-2016. The findings were published in a letter to the editor in Haemophilia.
The researchers reported that depletion of Factor XIII (FXIII) in the setting of acquired hemophilia A has only been reported in a single case. In the present analysis, the researchers identified a total of seven patients with acquired hemophilia A from 2015-2016. FXIII antigen levels were measured in five cases and were found to be low in four cases, and three patients required FXIII replacement to secure hemostasis.
The relationship between acquired hemophilia A and FXIII deficiency was evaluated using descriptive case analysis.
After analysis, the team reported that measuring FXIII levels may be useful in refractory patients, in whom bleeding remains uncontrolled despite the use of standard therapy. Additionally, the results supported FXIII supplementation if a deficiency is detected.
“FXIII replacement was not complicated by any infusion reactions or thromboembolic events,” they wrote.
The replaced FXIII levels dropped more rapidly than expected, the researchers noted. The established half-life of pd-XIII is 6.6 days plus or minus 2.29 days; however, in the most severe of the cases reported, it was depleted “within a few days,” they wrote. “This is compatible with either consumption of FXIII or inhibition/clearance by an autoantibody.”
Dr. Abdulrehman and his colleagues acknowledged that FXIII deficiency can be challenging to identify because the majority of coagulation assays do not measure FXIII levels.
“As we gain a greater appreciation for the role of FXIII in various pathological states, access to accurate FXIII testing will become increasingly important,” they added.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Abdulrehman J et al. Haemophilia. 2019 Mar 7. doi: 10.1111/hae.13690.
FROM HAEMOPHILIA
Injectable nimodipine does not improve outcomes of subarachnoid hemorrhage
HONOLULU – according to a study presented at the International Stroke Conference sponsored by the American Heart Association. However, the treatment does reduce the rate of angiographic vasospasm significantly and does not raise significant safety concerns.
Approximately 70% of patients with subarachnoid hemorrhage develop vasospasm, which can in turn cause delayed cerebral ischemia. The only evidence-based treatment for ischemia of the brain resulting from vasospasm is nimodipine, which has been the standard of care for more than a decade.
EG-1962 was developed to deliver higher amounts of nimodipine to the CNS and spastic vessels than oral nimodipine. The formulation contains 50-mcm particles of nimodipine combined with a biodegradable polymer. Pharmacokinetic studies indicate that EG-1962 successfully delivers higher amounts of nimodipine to the CNS than the oral formulation does.
A multisite, phase 3 trial
Stephan A. Mayer, MD, William T. Gossett Endowed Chair of Neurology at the Henry Ford Health System in Detroit, presented the trial results. He and his colleagues conducted NEWTON2, a phase 3 trial to evaluate the safety and efficacy of a single 600-mg intraventricular dose of EG-1962 in patients with aneurysmal subarachnoid hemorrhage, compared with those of oral nimodipine. Eligible participants had a World Federation of Neurosurgeons Score (WFNS) of 2-4, Glasgow Coma Scale scores of 7-14, and an indication for placement of an external ventricular drain (such as hydrocephalus). All patients had their aneurysms clipped or coiled.
On the first day after the operation, the investigators randomized patients in equal groups to EG-1962 plus oral placebo or oral nimodipine plus placebo injection. Patients in the EG-1962 arm received a 600-mg injection of nimodipine into the ventricular system. The primary endpoint was the proportion of subjects with an extended Glasgow Outcome Scale (eGOS) score of 6-8 (that is, minimal or mild disability) at day 90. The main secondary endpoint was the proportion of subjects with a Montreal Cognitive Assessment (MOCA) score of 26 or greater (indicating no important cognitive disability) at day 90. Safety outcomes included cerebral infarction, hypotension, and ventriculitis.
Dr. Mayer and his colleagues conducted the study at 65 centers in 11 countries. They planned to enroll 374 patients and conduct an interim analysis after the first 210 participants had their 90-day follow-up. An independent data monitoring committee reviewed the safety data as it was collected. The investigators stopped the study for futility after the preplanned interim analysis was completed.
The challenge of identifying responders
The two study arms were well matched on age and gender. The proportion of patients with poor WFNS was 45% in the EG-1962 arm and 53% in the oral nimodipine arm.
The rate of any vasospasm (such as symptomatic or by imaging) was 56% in the intervention group and 70% in the oral nimodipine group, a statistically significant difference. Follow-up angiography showed vasospasm in 50% of the intervention group, compared with 63% of the oral nimodipine group, which was also statistically significant.
When the investigators examined the primary endpoint, they found that 46% of patients who received EG-1962 had a favorable outcome, compared with 43% of patients who received oral nimodipine, a nonsignificant difference. If the investigators had defined a favorable outcome as an eGOS score of 4 or greater, “there would have been a more favorable effect,” said Dr. Mayer. The investigators found no difference between groups in MOCA score at day 90.
Subgroup analyses produced “puzzling” results, said Dr. Mayer. For example, more patients with poor-grade WFNS had a favorable outcome at 3 months in the EG-1962 group than in the nimodipine group, but fewer patients with good-grade WFNS had a favorable outcome at 3 months in the EG-1962 group than in the nimodipine group. In addition, the treatment effect was more evident in the United States than abroad.
The safety analysis indicated a trend toward less rescue therapy – defined as hypertensive therapy or any interventional approach – in the EG-1962 group (27%), compared with the oral nimodipine group (35%). The rate of hypotension was slightly lower in the EG-1962 group. The rates of treatment-emergent serious adverse events and hydrocephalus were not significantly different between groups.
The results may encourage neurologists to treat “extremely sick patients with highly refractory vasospasms,” said Dr. Mayer. “We have a biologically active agent. The problem is, though, that it’s very hard ... to prove efficacy in trials, because we do not fully understand who the responders were going to be.”
Dr. Mayer reported receiving consulting fees from Edge Therapeutics, the company that developed EG-1962, in the past for activities unrelated to this study. Other investigators are employees of Edge Therapeutics.
SOURCE: Mayer SA et al. ISC 2019, Abstract LB15.
HONOLULU – according to a study presented at the International Stroke Conference sponsored by the American Heart Association. However, the treatment does reduce the rate of angiographic vasospasm significantly and does not raise significant safety concerns.
Approximately 70% of patients with subarachnoid hemorrhage develop vasospasm, which can in turn cause delayed cerebral ischemia. The only evidence-based treatment for ischemia of the brain resulting from vasospasm is nimodipine, which has been the standard of care for more than a decade.
EG-1962 was developed to deliver higher amounts of nimodipine to the CNS and spastic vessels than oral nimodipine. The formulation contains 50-mcm particles of nimodipine combined with a biodegradable polymer. Pharmacokinetic studies indicate that EG-1962 successfully delivers higher amounts of nimodipine to the CNS than the oral formulation does.
A multisite, phase 3 trial
Stephan A. Mayer, MD, William T. Gossett Endowed Chair of Neurology at the Henry Ford Health System in Detroit, presented the trial results. He and his colleagues conducted NEWTON2, a phase 3 trial to evaluate the safety and efficacy of a single 600-mg intraventricular dose of EG-1962 in patients with aneurysmal subarachnoid hemorrhage, compared with those of oral nimodipine. Eligible participants had a World Federation of Neurosurgeons Score (WFNS) of 2-4, Glasgow Coma Scale scores of 7-14, and an indication for placement of an external ventricular drain (such as hydrocephalus). All patients had their aneurysms clipped or coiled.
On the first day after the operation, the investigators randomized patients in equal groups to EG-1962 plus oral placebo or oral nimodipine plus placebo injection. Patients in the EG-1962 arm received a 600-mg injection of nimodipine into the ventricular system. The primary endpoint was the proportion of subjects with an extended Glasgow Outcome Scale (eGOS) score of 6-8 (that is, minimal or mild disability) at day 90. The main secondary endpoint was the proportion of subjects with a Montreal Cognitive Assessment (MOCA) score of 26 or greater (indicating no important cognitive disability) at day 90. Safety outcomes included cerebral infarction, hypotension, and ventriculitis.
Dr. Mayer and his colleagues conducted the study at 65 centers in 11 countries. They planned to enroll 374 patients and conduct an interim analysis after the first 210 participants had their 90-day follow-up. An independent data monitoring committee reviewed the safety data as it was collected. The investigators stopped the study for futility after the preplanned interim analysis was completed.
The challenge of identifying responders
The two study arms were well matched on age and gender. The proportion of patients with poor WFNS was 45% in the EG-1962 arm and 53% in the oral nimodipine arm.
The rate of any vasospasm (such as symptomatic or by imaging) was 56% in the intervention group and 70% in the oral nimodipine group, a statistically significant difference. Follow-up angiography showed vasospasm in 50% of the intervention group, compared with 63% of the oral nimodipine group, which was also statistically significant.
When the investigators examined the primary endpoint, they found that 46% of patients who received EG-1962 had a favorable outcome, compared with 43% of patients who received oral nimodipine, a nonsignificant difference. If the investigators had defined a favorable outcome as an eGOS score of 4 or greater, “there would have been a more favorable effect,” said Dr. Mayer. The investigators found no difference between groups in MOCA score at day 90.
Subgroup analyses produced “puzzling” results, said Dr. Mayer. For example, more patients with poor-grade WFNS had a favorable outcome at 3 months in the EG-1962 group than in the nimodipine group, but fewer patients with good-grade WFNS had a favorable outcome at 3 months in the EG-1962 group than in the nimodipine group. In addition, the treatment effect was more evident in the United States than abroad.
The safety analysis indicated a trend toward less rescue therapy – defined as hypertensive therapy or any interventional approach – in the EG-1962 group (27%), compared with the oral nimodipine group (35%). The rate of hypotension was slightly lower in the EG-1962 group. The rates of treatment-emergent serious adverse events and hydrocephalus were not significantly different between groups.
The results may encourage neurologists to treat “extremely sick patients with highly refractory vasospasms,” said Dr. Mayer. “We have a biologically active agent. The problem is, though, that it’s very hard ... to prove efficacy in trials, because we do not fully understand who the responders were going to be.”
Dr. Mayer reported receiving consulting fees from Edge Therapeutics, the company that developed EG-1962, in the past for activities unrelated to this study. Other investigators are employees of Edge Therapeutics.
SOURCE: Mayer SA et al. ISC 2019, Abstract LB15.
HONOLULU – according to a study presented at the International Stroke Conference sponsored by the American Heart Association. However, the treatment does reduce the rate of angiographic vasospasm significantly and does not raise significant safety concerns.
Approximately 70% of patients with subarachnoid hemorrhage develop vasospasm, which can in turn cause delayed cerebral ischemia. The only evidence-based treatment for ischemia of the brain resulting from vasospasm is nimodipine, which has been the standard of care for more than a decade.
EG-1962 was developed to deliver higher amounts of nimodipine to the CNS and spastic vessels than oral nimodipine. The formulation contains 50-mcm particles of nimodipine combined with a biodegradable polymer. Pharmacokinetic studies indicate that EG-1962 successfully delivers higher amounts of nimodipine to the CNS than the oral formulation does.
A multisite, phase 3 trial
Stephan A. Mayer, MD, William T. Gossett Endowed Chair of Neurology at the Henry Ford Health System in Detroit, presented the trial results. He and his colleagues conducted NEWTON2, a phase 3 trial to evaluate the safety and efficacy of a single 600-mg intraventricular dose of EG-1962 in patients with aneurysmal subarachnoid hemorrhage, compared with those of oral nimodipine. Eligible participants had a World Federation of Neurosurgeons Score (WFNS) of 2-4, Glasgow Coma Scale scores of 7-14, and an indication for placement of an external ventricular drain (such as hydrocephalus). All patients had their aneurysms clipped or coiled.
On the first day after the operation, the investigators randomized patients in equal groups to EG-1962 plus oral placebo or oral nimodipine plus placebo injection. Patients in the EG-1962 arm received a 600-mg injection of nimodipine into the ventricular system. The primary endpoint was the proportion of subjects with an extended Glasgow Outcome Scale (eGOS) score of 6-8 (that is, minimal or mild disability) at day 90. The main secondary endpoint was the proportion of subjects with a Montreal Cognitive Assessment (MOCA) score of 26 or greater (indicating no important cognitive disability) at day 90. Safety outcomes included cerebral infarction, hypotension, and ventriculitis.
Dr. Mayer and his colleagues conducted the study at 65 centers in 11 countries. They planned to enroll 374 patients and conduct an interim analysis after the first 210 participants had their 90-day follow-up. An independent data monitoring committee reviewed the safety data as it was collected. The investigators stopped the study for futility after the preplanned interim analysis was completed.
The challenge of identifying responders
The two study arms were well matched on age and gender. The proportion of patients with poor WFNS was 45% in the EG-1962 arm and 53% in the oral nimodipine arm.
The rate of any vasospasm (such as symptomatic or by imaging) was 56% in the intervention group and 70% in the oral nimodipine group, a statistically significant difference. Follow-up angiography showed vasospasm in 50% of the intervention group, compared with 63% of the oral nimodipine group, which was also statistically significant.
When the investigators examined the primary endpoint, they found that 46% of patients who received EG-1962 had a favorable outcome, compared with 43% of patients who received oral nimodipine, a nonsignificant difference. If the investigators had defined a favorable outcome as an eGOS score of 4 or greater, “there would have been a more favorable effect,” said Dr. Mayer. The investigators found no difference between groups in MOCA score at day 90.
Subgroup analyses produced “puzzling” results, said Dr. Mayer. For example, more patients with poor-grade WFNS had a favorable outcome at 3 months in the EG-1962 group than in the nimodipine group, but fewer patients with good-grade WFNS had a favorable outcome at 3 months in the EG-1962 group than in the nimodipine group. In addition, the treatment effect was more evident in the United States than abroad.
The safety analysis indicated a trend toward less rescue therapy – defined as hypertensive therapy or any interventional approach – in the EG-1962 group (27%), compared with the oral nimodipine group (35%). The rate of hypotension was slightly lower in the EG-1962 group. The rates of treatment-emergent serious adverse events and hydrocephalus were not significantly different between groups.
The results may encourage neurologists to treat “extremely sick patients with highly refractory vasospasms,” said Dr. Mayer. “We have a biologically active agent. The problem is, though, that it’s very hard ... to prove efficacy in trials, because we do not fully understand who the responders were going to be.”
Dr. Mayer reported receiving consulting fees from Edge Therapeutics, the company that developed EG-1962, in the past for activities unrelated to this study. Other investigators are employees of Edge Therapeutics.
SOURCE: Mayer SA et al. ISC 2019, Abstract LB15.
REPORTING FROM ISC 2019
Descovy noninferior to Truvada for PrEP
SEATTLE – Descovy [emtricitabine/tenofovir alafenamide (F/TAF]) was noninferior to Truvada [emtricitabine/tenofovir disoproxil fumarate (F/TDF)] for preexposure HIV prophylaxis in a blinded, randomized trial involving more than 5,000 men at high risk for the infection.
Both nucleotide reverse transcriptase inhibitors are made by Gilead, and the company funded the trial.
F/TDF (Truvada) has been a blockbuster for the company, both for HIV treatment and, since 2012, for preexposure prophylaxis (PrEP); it’s the only medication to carry the indication. However, F/TDF is set to go off patent in 2021, so the company has turned its development efforts to a successor, F/TAF (Descovy), a prodrug of tenofovir that is already approved for HIV treatment.
The new study builds a case for F/TAF for PrEP, but whether the results are strong enough to persuade people to opt for it over a much less expensive generic version of F/TDF remains to be seen.
The trial randomized 2,694 men who have sex with men to F/TAF, and 2,693 to F/TDF for up to 96 weeks. Entrance criteria included at least two episodes of unprotected anal sex in the previous 12 weeks, or a diagnosis of rectal gonorrhea, chlamydia, or syphilis in the previous 6 months.
More than half the men contracted at least one sexually transmitted infection during the trial, “which indicated to us that these were the right patients to be enrolled in the study,” lead investigator Charles Hare, MD, an infectious disease specialist at the University of California, San Francisco, said at the Conference on Retroviruses and Opportunistic Infections.
There were 15 new HIV infections in the F/TDF group (0.34 per 100 person-years), versus 7 in the F/TAF group (0.16 per 100 person-years). Almost all of the new infections were due to poor adherence – as proven by blood levels and dry blood spot testing – and most of the rest were in men who probably entered the trial with newly acquired HIV.
When those subjects were excluded, there were just two new onset HIV cases in subjects adherent to dosing, one in each arm. Infection rates were far lower than would have been expected had the subjects not been on PrEP.
One of Gilead’s main selling points for F/TAF over F/TDF is that the newer drug has better bone and renal safety, and there were slight biomarker differences in the trial that supported the assertion.
For instance, spine bone mineral density decreased 3% or greater from baseline in 10 F/TAF patients, but 27 men on F/TDF (P less than .001). Results were similar with hip bone density.
On the renal front, estimated glomerular filtration rates fell a median of 2.3 mL/min per 1.73 m2 in the F/TDF arm, but rose 1.8 mL/min per 1.73 m2 in men on F/TAF (P less than .001). Proximal tubular protein to creatinine ratios were largely unchanged from baseline with F/TAF, but slightly higher in the F/TDF group, at 48 weeks.
There were no statistically significant differences on actual safety outcomes – as opposed to biomarkers – between the two drugs or discontinuations due to side effects, which were rare and most often due to gastrointestinal issues. F/TAF patients gained about 1.1 kg in the trial, while weight held steady in the F/TDF arm. The study team plans to analyze lipid profile differences between the groups, since concern has been raised about F/TAF’s effect on them.
In a press conference at the conference, there was quite a bit of discussion about whether the results would justify using F/TAF for PrEP when less expensive generic versions of F/TDF become available.
“That’s a great question,” Dr. Hare said. “Both drugs actually performed quite well,” and both “do pretty well in terms of safety in this population.”
It’s not known at this point if the biomarker differences will prove to be clinically relevant. Hip fractures, kidney failure, and other problems are so rare in young, relatively healthy PrEP users that a trial to demonstrate clinical relevance would have to be huge, with years-long follow-up, Dr. Hare noted.
The average age of the men in this study was 36 years. Most were white, and about 60% lived in the United States. Other participants were from Canada or Europe.
The work was funded by Gilead; five investigators, including the senior investigator, were employees. Dr. Hare is an investigator for the company.
SOURCE: Hare CB et al. CROI 2019, Abstract 104 LB.
SEATTLE – Descovy [emtricitabine/tenofovir alafenamide (F/TAF]) was noninferior to Truvada [emtricitabine/tenofovir disoproxil fumarate (F/TDF)] for preexposure HIV prophylaxis in a blinded, randomized trial involving more than 5,000 men at high risk for the infection.
Both nucleotide reverse transcriptase inhibitors are made by Gilead, and the company funded the trial.
F/TDF (Truvada) has been a blockbuster for the company, both for HIV treatment and, since 2012, for preexposure prophylaxis (PrEP); it’s the only medication to carry the indication. However, F/TDF is set to go off patent in 2021, so the company has turned its development efforts to a successor, F/TAF (Descovy), a prodrug of tenofovir that is already approved for HIV treatment.
The new study builds a case for F/TAF for PrEP, but whether the results are strong enough to persuade people to opt for it over a much less expensive generic version of F/TDF remains to be seen.
The trial randomized 2,694 men who have sex with men to F/TAF, and 2,693 to F/TDF for up to 96 weeks. Entrance criteria included at least two episodes of unprotected anal sex in the previous 12 weeks, or a diagnosis of rectal gonorrhea, chlamydia, or syphilis in the previous 6 months.
More than half the men contracted at least one sexually transmitted infection during the trial, “which indicated to us that these were the right patients to be enrolled in the study,” lead investigator Charles Hare, MD, an infectious disease specialist at the University of California, San Francisco, said at the Conference on Retroviruses and Opportunistic Infections.
There were 15 new HIV infections in the F/TDF group (0.34 per 100 person-years), versus 7 in the F/TAF group (0.16 per 100 person-years). Almost all of the new infections were due to poor adherence – as proven by blood levels and dry blood spot testing – and most of the rest were in men who probably entered the trial with newly acquired HIV.
When those subjects were excluded, there were just two new onset HIV cases in subjects adherent to dosing, one in each arm. Infection rates were far lower than would have been expected had the subjects not been on PrEP.
One of Gilead’s main selling points for F/TAF over F/TDF is that the newer drug has better bone and renal safety, and there were slight biomarker differences in the trial that supported the assertion.
For instance, spine bone mineral density decreased 3% or greater from baseline in 10 F/TAF patients, but 27 men on F/TDF (P less than .001). Results were similar with hip bone density.
On the renal front, estimated glomerular filtration rates fell a median of 2.3 mL/min per 1.73 m2 in the F/TDF arm, but rose 1.8 mL/min per 1.73 m2 in men on F/TAF (P less than .001). Proximal tubular protein to creatinine ratios were largely unchanged from baseline with F/TAF, but slightly higher in the F/TDF group, at 48 weeks.
There were no statistically significant differences on actual safety outcomes – as opposed to biomarkers – between the two drugs or discontinuations due to side effects, which were rare and most often due to gastrointestinal issues. F/TAF patients gained about 1.1 kg in the trial, while weight held steady in the F/TDF arm. The study team plans to analyze lipid profile differences between the groups, since concern has been raised about F/TAF’s effect on them.
In a press conference at the conference, there was quite a bit of discussion about whether the results would justify using F/TAF for PrEP when less expensive generic versions of F/TDF become available.
“That’s a great question,” Dr. Hare said. “Both drugs actually performed quite well,” and both “do pretty well in terms of safety in this population.”
It’s not known at this point if the biomarker differences will prove to be clinically relevant. Hip fractures, kidney failure, and other problems are so rare in young, relatively healthy PrEP users that a trial to demonstrate clinical relevance would have to be huge, with years-long follow-up, Dr. Hare noted.
The average age of the men in this study was 36 years. Most were white, and about 60% lived in the United States. Other participants were from Canada or Europe.
The work was funded by Gilead; five investigators, including the senior investigator, were employees. Dr. Hare is an investigator for the company.
SOURCE: Hare CB et al. CROI 2019, Abstract 104 LB.
SEATTLE – Descovy [emtricitabine/tenofovir alafenamide (F/TAF]) was noninferior to Truvada [emtricitabine/tenofovir disoproxil fumarate (F/TDF)] for preexposure HIV prophylaxis in a blinded, randomized trial involving more than 5,000 men at high risk for the infection.
Both nucleotide reverse transcriptase inhibitors are made by Gilead, and the company funded the trial.
F/TDF (Truvada) has been a blockbuster for the company, both for HIV treatment and, since 2012, for preexposure prophylaxis (PrEP); it’s the only medication to carry the indication. However, F/TDF is set to go off patent in 2021, so the company has turned its development efforts to a successor, F/TAF (Descovy), a prodrug of tenofovir that is already approved for HIV treatment.
The new study builds a case for F/TAF for PrEP, but whether the results are strong enough to persuade people to opt for it over a much less expensive generic version of F/TDF remains to be seen.
The trial randomized 2,694 men who have sex with men to F/TAF, and 2,693 to F/TDF for up to 96 weeks. Entrance criteria included at least two episodes of unprotected anal sex in the previous 12 weeks, or a diagnosis of rectal gonorrhea, chlamydia, or syphilis in the previous 6 months.
More than half the men contracted at least one sexually transmitted infection during the trial, “which indicated to us that these were the right patients to be enrolled in the study,” lead investigator Charles Hare, MD, an infectious disease specialist at the University of California, San Francisco, said at the Conference on Retroviruses and Opportunistic Infections.
There were 15 new HIV infections in the F/TDF group (0.34 per 100 person-years), versus 7 in the F/TAF group (0.16 per 100 person-years). Almost all of the new infections were due to poor adherence – as proven by blood levels and dry blood spot testing – and most of the rest were in men who probably entered the trial with newly acquired HIV.
When those subjects were excluded, there were just two new onset HIV cases in subjects adherent to dosing, one in each arm. Infection rates were far lower than would have been expected had the subjects not been on PrEP.
One of Gilead’s main selling points for F/TAF over F/TDF is that the newer drug has better bone and renal safety, and there were slight biomarker differences in the trial that supported the assertion.
For instance, spine bone mineral density decreased 3% or greater from baseline in 10 F/TAF patients, but 27 men on F/TDF (P less than .001). Results were similar with hip bone density.
On the renal front, estimated glomerular filtration rates fell a median of 2.3 mL/min per 1.73 m2 in the F/TDF arm, but rose 1.8 mL/min per 1.73 m2 in men on F/TAF (P less than .001). Proximal tubular protein to creatinine ratios were largely unchanged from baseline with F/TAF, but slightly higher in the F/TDF group, at 48 weeks.
There were no statistically significant differences on actual safety outcomes – as opposed to biomarkers – between the two drugs or discontinuations due to side effects, which were rare and most often due to gastrointestinal issues. F/TAF patients gained about 1.1 kg in the trial, while weight held steady in the F/TDF arm. The study team plans to analyze lipid profile differences between the groups, since concern has been raised about F/TAF’s effect on them.
In a press conference at the conference, there was quite a bit of discussion about whether the results would justify using F/TAF for PrEP when less expensive generic versions of F/TDF become available.
“That’s a great question,” Dr. Hare said. “Both drugs actually performed quite well,” and both “do pretty well in terms of safety in this population.”
It’s not known at this point if the biomarker differences will prove to be clinically relevant. Hip fractures, kidney failure, and other problems are so rare in young, relatively healthy PrEP users that a trial to demonstrate clinical relevance would have to be huge, with years-long follow-up, Dr. Hare noted.
The average age of the men in this study was 36 years. Most were white, and about 60% lived in the United States. Other participants were from Canada or Europe.
The work was funded by Gilead; five investigators, including the senior investigator, were employees. Dr. Hare is an investigator for the company.
SOURCE: Hare CB et al. CROI 2019, Abstract 104 LB.
REPORTING FROM CROI 2019
Registry supports efficacy of coated balloon for peripheral artery stenosis
WASHINGTON – After patients with symptomatic peripheral arterial disease (PAD) were treated with a paclitaxel-coated balloon for 1 year, 89.5% remain free of target lesion restenosis (TLR), according to real-world registry data presented as a late-breaker at CRT 2019 sponsored by MedStar Heart & Vascular Institute.
Freedom from TLR is the primary endpoint of this registry, which will continue to accrue data for 2 more years, according to Nicolas W. Shammas, MD, medical director of Midwest Cardiovascular Research Foundation, Davenport, Iowa.
The nearly 90% rate of freedom from TLR at 1 year was achieved “despite the fact that over 50% of the patients had diabetes, 29% had severe calcification, 35% had critical limb ischemia, and 25% had complete total occlusions,” said Dr. Shammas, an interventional cardiologist.
The registry, called SAFE-DCB, was created to evaluate long-term outcomes after treatment with the Lutonix (Bard Medical) paclitaxel-coated balloon catheter, which is employed in percutaneous angioplasty to treat stenotic lesions in the peripheral vasculature. Over an 18-month period, 1,005 patients were enrolled at 74 treatment centers. Dr. Shammas presented data on 766 of these patients, who have completed 12-months of follow-up. There are 835 patients enrolled in the ongoing study.
In a review of characteristics prior to treatment, Dr. Shammas reported that the average target lesion stenosis was 86.7% and the average target lesion length was 75 mm. Endovascular treatments prior to angioplasty were permitted in the registry protocol. Half of the patients underwent directional atherectomy.
After treatment, the residual stenosis was 11.54%. Even though the recommended protocol called for balloon inflations of 30 seconds each at a pressure of 7 atmospheres, the mean balloon inflation times were 35 seconds at 8 atmospheres. The mean total time for balloon inflations per patient was 152 seconds against the protocol recommendation of 140 seconds.
The primary safety endpoint was freedom from periprocedural mortality, limb amputation, and TLR at 30 days, which was achieved in 98.2% of patients.
Mortality at 1 year was 7.1%. Cardiovascular deaths, such as those due to myocardial infarction, were the most common, but there were noncardiovascular deaths, including those due to sepsis, respiratory failure, and kidney disease.
Women represented 43% of the study population. When compared with men, women achieved the primary outcome at a numerically lower rate, but the difference was not statistically significant. Dr. Shammas reported similar findings for those without complete total occlusions relative to those with complete total occlusions and those treated within the study protocol relative to those who were not. In each case, the differences in the proportion that achieved the primary outcome did not reach statistical significance.
Following his presentation, Dr. Shammas was asked to respond to the criticism that TLR is a soft endpoint. Since some proportion of patients might have had a return of symptoms due to restenosis but elected not to have a second procedure, TLR at 1 year is not equivalent to patency at 1 year.
While acknowledging the accuracy of this criticism, Dr. Shammas reported that TLR was a practical surrogate in the absence of imaging or another objective method of target lesion assessment. Noting that this endpoint has been employed before for long-term follow-up in trials of percutaneous therapies, he said that the TLR rates in this SAFE-DCB registry “are well within previously reported data” for 1-year outcomes with other treatments of symptomatic PAD.
SOURCE: Shammas N. CRT 2019 Mar 5.
WASHINGTON – After patients with symptomatic peripheral arterial disease (PAD) were treated with a paclitaxel-coated balloon for 1 year, 89.5% remain free of target lesion restenosis (TLR), according to real-world registry data presented as a late-breaker at CRT 2019 sponsored by MedStar Heart & Vascular Institute.
Freedom from TLR is the primary endpoint of this registry, which will continue to accrue data for 2 more years, according to Nicolas W. Shammas, MD, medical director of Midwest Cardiovascular Research Foundation, Davenport, Iowa.
The nearly 90% rate of freedom from TLR at 1 year was achieved “despite the fact that over 50% of the patients had diabetes, 29% had severe calcification, 35% had critical limb ischemia, and 25% had complete total occlusions,” said Dr. Shammas, an interventional cardiologist.
The registry, called SAFE-DCB, was created to evaluate long-term outcomes after treatment with the Lutonix (Bard Medical) paclitaxel-coated balloon catheter, which is employed in percutaneous angioplasty to treat stenotic lesions in the peripheral vasculature. Over an 18-month period, 1,005 patients were enrolled at 74 treatment centers. Dr. Shammas presented data on 766 of these patients, who have completed 12-months of follow-up. There are 835 patients enrolled in the ongoing study.
In a review of characteristics prior to treatment, Dr. Shammas reported that the average target lesion stenosis was 86.7% and the average target lesion length was 75 mm. Endovascular treatments prior to angioplasty were permitted in the registry protocol. Half of the patients underwent directional atherectomy.
After treatment, the residual stenosis was 11.54%. Even though the recommended protocol called for balloon inflations of 30 seconds each at a pressure of 7 atmospheres, the mean balloon inflation times were 35 seconds at 8 atmospheres. The mean total time for balloon inflations per patient was 152 seconds against the protocol recommendation of 140 seconds.
The primary safety endpoint was freedom from periprocedural mortality, limb amputation, and TLR at 30 days, which was achieved in 98.2% of patients.
Mortality at 1 year was 7.1%. Cardiovascular deaths, such as those due to myocardial infarction, were the most common, but there were noncardiovascular deaths, including those due to sepsis, respiratory failure, and kidney disease.
Women represented 43% of the study population. When compared with men, women achieved the primary outcome at a numerically lower rate, but the difference was not statistically significant. Dr. Shammas reported similar findings for those without complete total occlusions relative to those with complete total occlusions and those treated within the study protocol relative to those who were not. In each case, the differences in the proportion that achieved the primary outcome did not reach statistical significance.
Following his presentation, Dr. Shammas was asked to respond to the criticism that TLR is a soft endpoint. Since some proportion of patients might have had a return of symptoms due to restenosis but elected not to have a second procedure, TLR at 1 year is not equivalent to patency at 1 year.
While acknowledging the accuracy of this criticism, Dr. Shammas reported that TLR was a practical surrogate in the absence of imaging or another objective method of target lesion assessment. Noting that this endpoint has been employed before for long-term follow-up in trials of percutaneous therapies, he said that the TLR rates in this SAFE-DCB registry “are well within previously reported data” for 1-year outcomes with other treatments of symptomatic PAD.
SOURCE: Shammas N. CRT 2019 Mar 5.
WASHINGTON – After patients with symptomatic peripheral arterial disease (PAD) were treated with a paclitaxel-coated balloon for 1 year, 89.5% remain free of target lesion restenosis (TLR), according to real-world registry data presented as a late-breaker at CRT 2019 sponsored by MedStar Heart & Vascular Institute.
Freedom from TLR is the primary endpoint of this registry, which will continue to accrue data for 2 more years, according to Nicolas W. Shammas, MD, medical director of Midwest Cardiovascular Research Foundation, Davenport, Iowa.
The nearly 90% rate of freedom from TLR at 1 year was achieved “despite the fact that over 50% of the patients had diabetes, 29% had severe calcification, 35% had critical limb ischemia, and 25% had complete total occlusions,” said Dr. Shammas, an interventional cardiologist.
The registry, called SAFE-DCB, was created to evaluate long-term outcomes after treatment with the Lutonix (Bard Medical) paclitaxel-coated balloon catheter, which is employed in percutaneous angioplasty to treat stenotic lesions in the peripheral vasculature. Over an 18-month period, 1,005 patients were enrolled at 74 treatment centers. Dr. Shammas presented data on 766 of these patients, who have completed 12-months of follow-up. There are 835 patients enrolled in the ongoing study.
In a review of characteristics prior to treatment, Dr. Shammas reported that the average target lesion stenosis was 86.7% and the average target lesion length was 75 mm. Endovascular treatments prior to angioplasty were permitted in the registry protocol. Half of the patients underwent directional atherectomy.
After treatment, the residual stenosis was 11.54%. Even though the recommended protocol called for balloon inflations of 30 seconds each at a pressure of 7 atmospheres, the mean balloon inflation times were 35 seconds at 8 atmospheres. The mean total time for balloon inflations per patient was 152 seconds against the protocol recommendation of 140 seconds.
The primary safety endpoint was freedom from periprocedural mortality, limb amputation, and TLR at 30 days, which was achieved in 98.2% of patients.
Mortality at 1 year was 7.1%. Cardiovascular deaths, such as those due to myocardial infarction, were the most common, but there were noncardiovascular deaths, including those due to sepsis, respiratory failure, and kidney disease.
Women represented 43% of the study population. When compared with men, women achieved the primary outcome at a numerically lower rate, but the difference was not statistically significant. Dr. Shammas reported similar findings for those without complete total occlusions relative to those with complete total occlusions and those treated within the study protocol relative to those who were not. In each case, the differences in the proportion that achieved the primary outcome did not reach statistical significance.
Following his presentation, Dr. Shammas was asked to respond to the criticism that TLR is a soft endpoint. Since some proportion of patients might have had a return of symptoms due to restenosis but elected not to have a second procedure, TLR at 1 year is not equivalent to patency at 1 year.
While acknowledging the accuracy of this criticism, Dr. Shammas reported that TLR was a practical surrogate in the absence of imaging or another objective method of target lesion assessment. Noting that this endpoint has been employed before for long-term follow-up in trials of percutaneous therapies, he said that the TLR rates in this SAFE-DCB registry “are well within previously reported data” for 1-year outcomes with other treatments of symptomatic PAD.
SOURCE: Shammas N. CRT 2019 Mar 5.
REPORTING FROM CRT 2019
Pruritic Nodules on the Breast
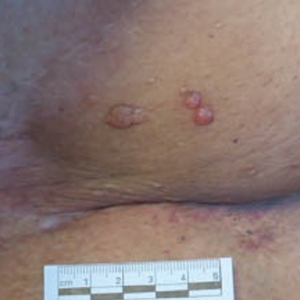
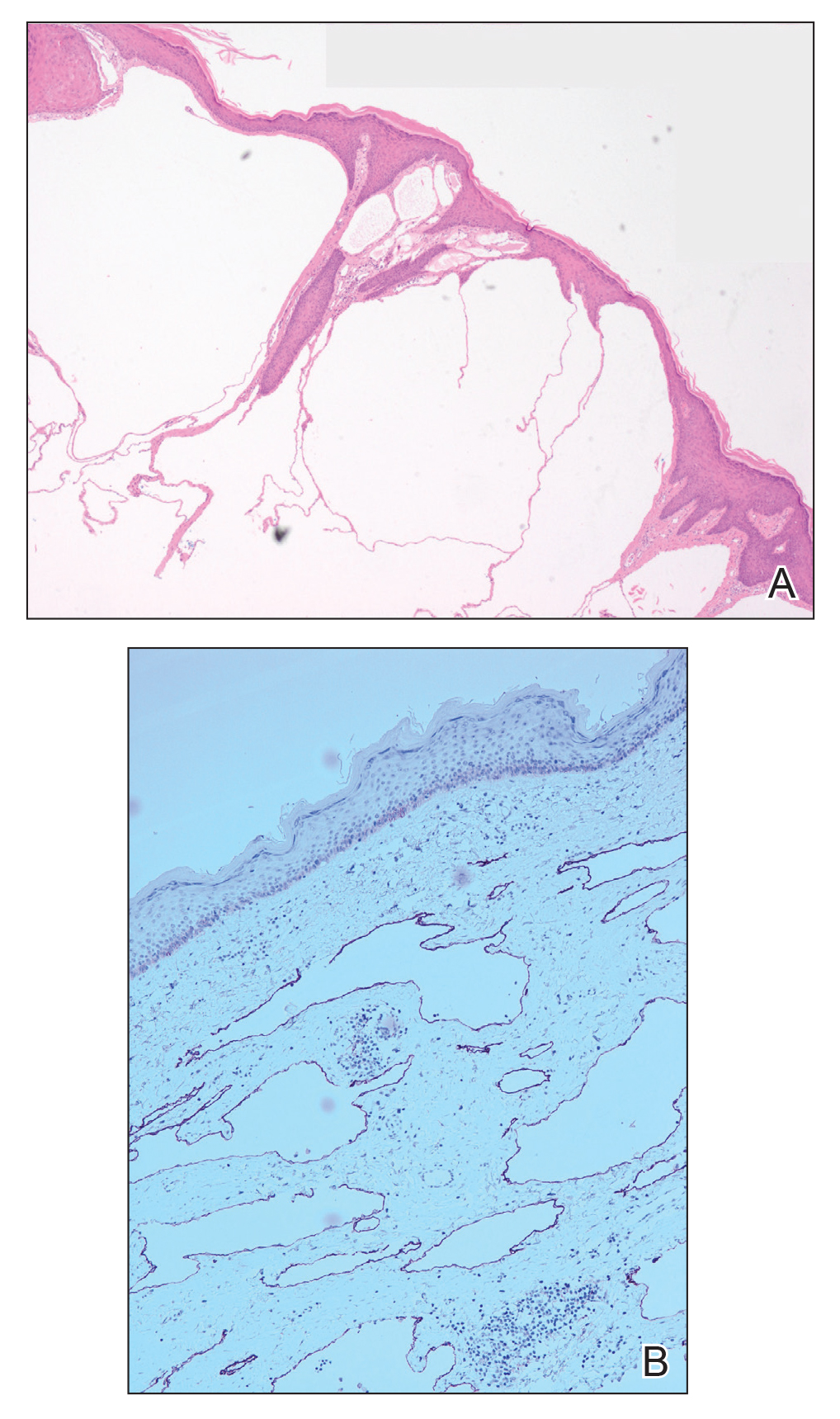
Microcystic lymphatic malformations, also known as lymphangioma circumscriptum, are rare hamartomatous lesions comprised of dilated lymphatic channels that can be both congenital and acquired.1 They often present as translucent or hemorrhagic vesicles of varying sizes that may contain lymphatic fluid and often can cluster together and appear verrucous (Figure 1). The differential diagnosis for microcystic lymphatic malformations commonly includes molluscum contagiosum, squamous cell carcinoma, verruca vulgaris, or condylomas, as well as atypical vascular lesions. They most often are found in children as congenital lesions but also may be acquired. Most acquired cases are due to chronic inflammatory and scarring processes that damage lymphatic structures, including surgery, radiation, infections, and even Crohn disease.2,3 Because the differential diagnosis is so broad and the disease can clinically mimic other common disease processes, biopsies often are performed to determine the diagnosis. On biopsy, pathologic examination revealed well-circumscribed nodular lesions with large lymphatic channels often in a background of connective tissue stroma. Increased eosinophilic material, including mast cells, also was seen (Figure 2A). On immunohistochemistry, staining showed D2-40 positivity (Figure 2B).
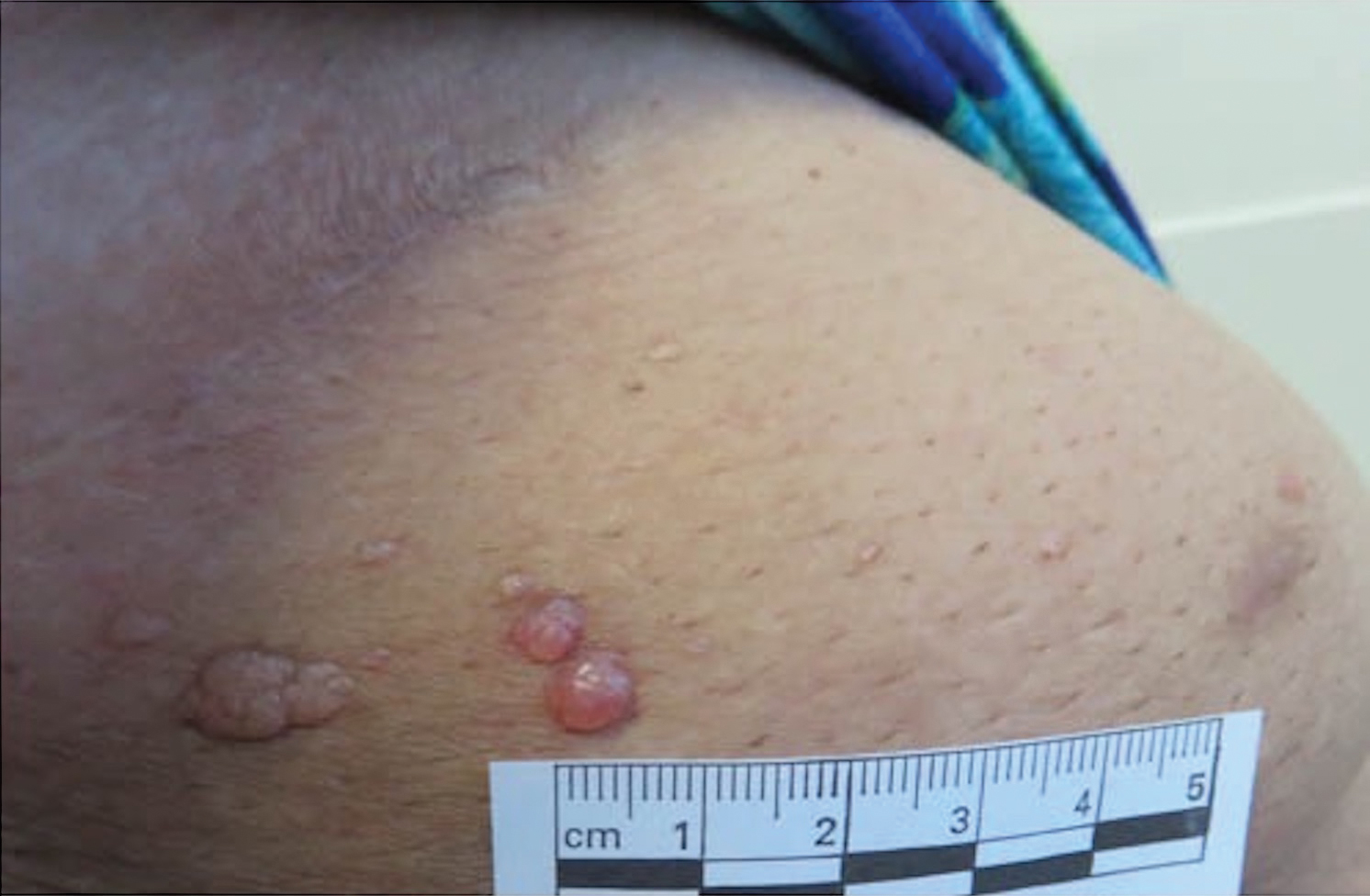
Damage to lymphatics from radiation and postsurgical excision of tumors are well-described causes of microcystic lymphatic malformations, as in our patient, with most instances in the literature occurring secondary to treatment of breast or cervical cancer.4-6 In these acquired cases, the pathogenesis is thought to be due to destruction and fibrosis at the layer of the reticular dermis, which causes lymphatic obstruction and subsequent dilation of superficial lymphatic channels.6
Microcystic lymphatic malformations can be difficult to distinguish from atypical vascular lesions, another common postradiation lesion. Both are benign well-circumscribed lesions that histologically do not extend into surrounding subcutaneous tissues and do not have multilayering of cells, mitosis, or hemorrhage.7 Although lymphatic lesions tend to form vesicles, atypical vascular lesions arising after radiation treatment present as erythematous or flesh-colored patches or papules. They also tend to be fairly superficial and often only involve the superficial to mid dermis. On histology they show thin-walled channels without erythrocytes that are lined by typical endothelial cells.7 Despite these differences, both clinically and histopathologically these lesions can appear similar to acquired microcystic lymphatic malformations. It is important to differentiate between these two entities, as atypical vascular lesions have a slightly higher rate of transformation into malignant tumors such as angiosarcomas.
Although angiosarcomas clinically may present as erythematous patches, plaques, or nodules similar to benign postradiation lesions, they tend to be more edematous than their benign counterparts.7,8 Two other clinical factors that can help determine if a postradiation lesion is benign or malignant are the size and time of onset of the lesion. Angiosarcomas tend to be much larger than benign postradiation lesions (median size, 7.5 cm) and tend to be more multifocal in nature.8,9 They also tend to arise on average 5 to 7 years after the initial radiation treatment, while benign lesions arise sooner.9
Small, asymptomatic, acquired microcystic lymphatic malformations can be followed clinically without treatment, but these lesions do not commonly regress spontaneously. Even when asymptomatic, many clinicians will opt for treatment to prevent secondary complications such as infections, drainage, and pain. Moreover, these lesions can have notable psychosocial impacts on patients due to poor cosmetic appearance. Unfortunately, there is no gold standard of treatment, and recurrence is common, even after treatment. Historically, surgical excision was the treatment of choice, but this option carries a high risk for scarring, invasiveness, and recurrence. Recurrence rates of up to 23.1% have been reported with decreased effectiveness of resection, particularly in areas of deeper involvement.10 For these deeper lesions, CO2 laser therapy is a promising evolving therapy. It can penetrate up to the mid dermis and seems to destroy the lymphatic channels between deep and surface lymphatics, preventing the cutaneous manifestations of the disease. It has the added benefit of minimal invasiveness and fewer side effects than complete excision, with most studies reporting hyperpigmentation and scarring as the most common side effects.11 Additional emerging therapies including sclerotherapy and isotretinoin have shown benefits in case studies. Sclerotherapy causes local tissue destruction and thrombosis leading to destruction of vessel lumens and fibrosis that halts disease progression and clears existing lesions.12 Oral therapy with isotretinoin appears to work by inhibiting certain cytokines and acting as an antiangiogenic factor.13 Given the rarity of microcystic lymphatic malformations, further research must be done to determine definitive treatment.
Acquired microcystic lymphatic malformation is an important sequela of radiation therapy and surgical excision of malignancy. Despite its striking clinical appearance, it is sometimes difficult to diagnose given its rarity. It is important that clinicians are able to recognize it clinically and understand common treatment options to prevent both the mental stigma and complications, including secondary infections, drainage, and pain.
- Whimster IW. The pathology of lymphangioma circumscriptum. Br J Dermatol. 1976;94:473.
- Vlastos AT, Malpica A, Follen M. Lymphangioma circumscriptum of the vulva: a review of the literature. Obstet Gynecol. 2003;101:946-954.
- Papalas JA, Robboy SJ, Burchette JL, et al. Acquired vulvar lymphangioma circumscriptum: a comparison of 12 cases with Crohn’s associated lesions or radiation therapy induced tumors. J Cutan Pathol. 2010;37:958-965.
- Kaya TI, Kokturk A, Polat A, et al. A case of cutaneous lymphangiectasis secondary to breast cancer treatment. Int J Dermatol. 2001;40:760-761.
- Ambrojo P, Cogolluda EF, Aguilar A, et al. Cutaneous lymphangiectases after therapy for carcinoma of the cervix. Clin Exp Dermatol. 1990;15:57-59.
- Tasdelen I, Gokgoz S, Paksoy E, et al. Acquired lymphangiectasis after breast conservation treatment for breast cancer: report of a case. Dermatol Online J. 2004;10:9.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29:983-996.
- Gengler C, Coindre JM, Leroux A. Vascular proliferations of the skin after radiation therapy for breast cancer: clinicopathologic analysis of a series in favor of a benign process: a study from the French Sarcoma Group. Cancer. 2007;109:1584-1598.
- Ghaemmaghami F, Karimi Zarchi M, Mousavi A. Major labiaectomy as surgical management of vulvar lymphangioma circumscriptum: three cases and a review of the literature. Arch Gynecol Obstet. 2008;278:57-60.
- Savas J. Carbon dioxide laser for the treatment of microcystic lymphatic malformations (lymphangioma circumscriptum): a systematic review. Dermatol Surg. 2013;39:1147-1157.
- Al Ghamdi KM, Mubki TF. Treatment of lymphangioma circumscriptum with sclerotherapy: an ignored effective remedy. J Cosmet Dermatol. 2011;10:156-158.
- Ayhan E. Lymphangioma circumscriptum: good clinical response to isotretinoin therapy. Pediatr Dermatol. 2016;33:E208-E209.
Microcystic lymphatic malformations, also known as lymphangioma circumscriptum, are rare hamartomatous lesions comprised of dilated lymphatic channels that can be both congenital and acquired.1 They often present as translucent or hemorrhagic vesicles of varying sizes that may contain lymphatic fluid and often can cluster together and appear verrucous (Figure 1). The differential diagnosis for microcystic lymphatic malformations commonly includes molluscum contagiosum, squamous cell carcinoma, verruca vulgaris, or condylomas, as well as atypical vascular lesions. They most often are found in children as congenital lesions but also may be acquired. Most acquired cases are due to chronic inflammatory and scarring processes that damage lymphatic structures, including surgery, radiation, infections, and even Crohn disease.2,3 Because the differential diagnosis is so broad and the disease can clinically mimic other common disease processes, biopsies often are performed to determine the diagnosis. On biopsy, pathologic examination revealed well-circumscribed nodular lesions with large lymphatic channels often in a background of connective tissue stroma. Increased eosinophilic material, including mast cells, also was seen (Figure 2A). On immunohistochemistry, staining showed D2-40 positivity (Figure 2B).


Damage to lymphatics from radiation and postsurgical excision of tumors are well-described causes of microcystic lymphatic malformations, as in our patient, with most instances in the literature occurring secondary to treatment of breast or cervical cancer.4-6 In these acquired cases, the pathogenesis is thought to be due to destruction and fibrosis at the layer of the reticular dermis, which causes lymphatic obstruction and subsequent dilation of superficial lymphatic channels.6
Microcystic lymphatic malformations can be difficult to distinguish from atypical vascular lesions, another common postradiation lesion. Both are benign well-circumscribed lesions that histologically do not extend into surrounding subcutaneous tissues and do not have multilayering of cells, mitosis, or hemorrhage.7 Although lymphatic lesions tend to form vesicles, atypical vascular lesions arising after radiation treatment present as erythematous or flesh-colored patches or papules. They also tend to be fairly superficial and often only involve the superficial to mid dermis. On histology they show thin-walled channels without erythrocytes that are lined by typical endothelial cells.7 Despite these differences, both clinically and histopathologically these lesions can appear similar to acquired microcystic lymphatic malformations. It is important to differentiate between these two entities, as atypical vascular lesions have a slightly higher rate of transformation into malignant tumors such as angiosarcomas.
Although angiosarcomas clinically may present as erythematous patches, plaques, or nodules similar to benign postradiation lesions, they tend to be more edematous than their benign counterparts.7,8 Two other clinical factors that can help determine if a postradiation lesion is benign or malignant are the size and time of onset of the lesion. Angiosarcomas tend to be much larger than benign postradiation lesions (median size, 7.5 cm) and tend to be more multifocal in nature.8,9 They also tend to arise on average 5 to 7 years after the initial radiation treatment, while benign lesions arise sooner.9
Small, asymptomatic, acquired microcystic lymphatic malformations can be followed clinically without treatment, but these lesions do not commonly regress spontaneously. Even when asymptomatic, many clinicians will opt for treatment to prevent secondary complications such as infections, drainage, and pain. Moreover, these lesions can have notable psychosocial impacts on patients due to poor cosmetic appearance. Unfortunately, there is no gold standard of treatment, and recurrence is common, even after treatment. Historically, surgical excision was the treatment of choice, but this option carries a high risk for scarring, invasiveness, and recurrence. Recurrence rates of up to 23.1% have been reported with decreased effectiveness of resection, particularly in areas of deeper involvement.10 For these deeper lesions, CO2 laser therapy is a promising evolving therapy. It can penetrate up to the mid dermis and seems to destroy the lymphatic channels between deep and surface lymphatics, preventing the cutaneous manifestations of the disease. It has the added benefit of minimal invasiveness and fewer side effects than complete excision, with most studies reporting hyperpigmentation and scarring as the most common side effects.11 Additional emerging therapies including sclerotherapy and isotretinoin have shown benefits in case studies. Sclerotherapy causes local tissue destruction and thrombosis leading to destruction of vessel lumens and fibrosis that halts disease progression and clears existing lesions.12 Oral therapy with isotretinoin appears to work by inhibiting certain cytokines and acting as an antiangiogenic factor.13 Given the rarity of microcystic lymphatic malformations, further research must be done to determine definitive treatment.
Acquired microcystic lymphatic malformation is an important sequela of radiation therapy and surgical excision of malignancy. Despite its striking clinical appearance, it is sometimes difficult to diagnose given its rarity. It is important that clinicians are able to recognize it clinically and understand common treatment options to prevent both the mental stigma and complications, including secondary infections, drainage, and pain.
Microcystic lymphatic malformations, also known as lymphangioma circumscriptum, are rare hamartomatous lesions comprised of dilated lymphatic channels that can be both congenital and acquired.1 They often present as translucent or hemorrhagic vesicles of varying sizes that may contain lymphatic fluid and often can cluster together and appear verrucous (Figure 1). The differential diagnosis for microcystic lymphatic malformations commonly includes molluscum contagiosum, squamous cell carcinoma, verruca vulgaris, or condylomas, as well as atypical vascular lesions. They most often are found in children as congenital lesions but also may be acquired. Most acquired cases are due to chronic inflammatory and scarring processes that damage lymphatic structures, including surgery, radiation, infections, and even Crohn disease.2,3 Because the differential diagnosis is so broad and the disease can clinically mimic other common disease processes, biopsies often are performed to determine the diagnosis. On biopsy, pathologic examination revealed well-circumscribed nodular lesions with large lymphatic channels often in a background of connective tissue stroma. Increased eosinophilic material, including mast cells, also was seen (Figure 2A). On immunohistochemistry, staining showed D2-40 positivity (Figure 2B).


Damage to lymphatics from radiation and postsurgical excision of tumors are well-described causes of microcystic lymphatic malformations, as in our patient, with most instances in the literature occurring secondary to treatment of breast or cervical cancer.4-6 In these acquired cases, the pathogenesis is thought to be due to destruction and fibrosis at the layer of the reticular dermis, which causes lymphatic obstruction and subsequent dilation of superficial lymphatic channels.6
Microcystic lymphatic malformations can be difficult to distinguish from atypical vascular lesions, another common postradiation lesion. Both are benign well-circumscribed lesions that histologically do not extend into surrounding subcutaneous tissues and do not have multilayering of cells, mitosis, or hemorrhage.7 Although lymphatic lesions tend to form vesicles, atypical vascular lesions arising after radiation treatment present as erythematous or flesh-colored patches or papules. They also tend to be fairly superficial and often only involve the superficial to mid dermis. On histology they show thin-walled channels without erythrocytes that are lined by typical endothelial cells.7 Despite these differences, both clinically and histopathologically these lesions can appear similar to acquired microcystic lymphatic malformations. It is important to differentiate between these two entities, as atypical vascular lesions have a slightly higher rate of transformation into malignant tumors such as angiosarcomas.
Although angiosarcomas clinically may present as erythematous patches, plaques, or nodules similar to benign postradiation lesions, they tend to be more edematous than their benign counterparts.7,8 Two other clinical factors that can help determine if a postradiation lesion is benign or malignant are the size and time of onset of the lesion. Angiosarcomas tend to be much larger than benign postradiation lesions (median size, 7.5 cm) and tend to be more multifocal in nature.8,9 They also tend to arise on average 5 to 7 years after the initial radiation treatment, while benign lesions arise sooner.9
Small, asymptomatic, acquired microcystic lymphatic malformations can be followed clinically without treatment, but these lesions do not commonly regress spontaneously. Even when asymptomatic, many clinicians will opt for treatment to prevent secondary complications such as infections, drainage, and pain. Moreover, these lesions can have notable psychosocial impacts on patients due to poor cosmetic appearance. Unfortunately, there is no gold standard of treatment, and recurrence is common, even after treatment. Historically, surgical excision was the treatment of choice, but this option carries a high risk for scarring, invasiveness, and recurrence. Recurrence rates of up to 23.1% have been reported with decreased effectiveness of resection, particularly in areas of deeper involvement.10 For these deeper lesions, CO2 laser therapy is a promising evolving therapy. It can penetrate up to the mid dermis and seems to destroy the lymphatic channels between deep and surface lymphatics, preventing the cutaneous manifestations of the disease. It has the added benefit of minimal invasiveness and fewer side effects than complete excision, with most studies reporting hyperpigmentation and scarring as the most common side effects.11 Additional emerging therapies including sclerotherapy and isotretinoin have shown benefits in case studies. Sclerotherapy causes local tissue destruction and thrombosis leading to destruction of vessel lumens and fibrosis that halts disease progression and clears existing lesions.12 Oral therapy with isotretinoin appears to work by inhibiting certain cytokines and acting as an antiangiogenic factor.13 Given the rarity of microcystic lymphatic malformations, further research must be done to determine definitive treatment.
Acquired microcystic lymphatic malformation is an important sequela of radiation therapy and surgical excision of malignancy. Despite its striking clinical appearance, it is sometimes difficult to diagnose given its rarity. It is important that clinicians are able to recognize it clinically and understand common treatment options to prevent both the mental stigma and complications, including secondary infections, drainage, and pain.
- Whimster IW. The pathology of lymphangioma circumscriptum. Br J Dermatol. 1976;94:473.
- Vlastos AT, Malpica A, Follen M. Lymphangioma circumscriptum of the vulva: a review of the literature. Obstet Gynecol. 2003;101:946-954.
- Papalas JA, Robboy SJ, Burchette JL, et al. Acquired vulvar lymphangioma circumscriptum: a comparison of 12 cases with Crohn’s associated lesions or radiation therapy induced tumors. J Cutan Pathol. 2010;37:958-965.
- Kaya TI, Kokturk A, Polat A, et al. A case of cutaneous lymphangiectasis secondary to breast cancer treatment. Int J Dermatol. 2001;40:760-761.
- Ambrojo P, Cogolluda EF, Aguilar A, et al. Cutaneous lymphangiectases after therapy for carcinoma of the cervix. Clin Exp Dermatol. 1990;15:57-59.
- Tasdelen I, Gokgoz S, Paksoy E, et al. Acquired lymphangiectasis after breast conservation treatment for breast cancer: report of a case. Dermatol Online J. 2004;10:9.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29:983-996.
- Gengler C, Coindre JM, Leroux A. Vascular proliferations of the skin after radiation therapy for breast cancer: clinicopathologic analysis of a series in favor of a benign process: a study from the French Sarcoma Group. Cancer. 2007;109:1584-1598.
- Ghaemmaghami F, Karimi Zarchi M, Mousavi A. Major labiaectomy as surgical management of vulvar lymphangioma circumscriptum: three cases and a review of the literature. Arch Gynecol Obstet. 2008;278:57-60.
- Savas J. Carbon dioxide laser for the treatment of microcystic lymphatic malformations (lymphangioma circumscriptum): a systematic review. Dermatol Surg. 2013;39:1147-1157.
- Al Ghamdi KM, Mubki TF. Treatment of lymphangioma circumscriptum with sclerotherapy: an ignored effective remedy. J Cosmet Dermatol. 2011;10:156-158.
- Ayhan E. Lymphangioma circumscriptum: good clinical response to isotretinoin therapy. Pediatr Dermatol. 2016;33:E208-E209.
- Whimster IW. The pathology of lymphangioma circumscriptum. Br J Dermatol. 1976;94:473.
- Vlastos AT, Malpica A, Follen M. Lymphangioma circumscriptum of the vulva: a review of the literature. Obstet Gynecol. 2003;101:946-954.
- Papalas JA, Robboy SJ, Burchette JL, et al. Acquired vulvar lymphangioma circumscriptum: a comparison of 12 cases with Crohn’s associated lesions or radiation therapy induced tumors. J Cutan Pathol. 2010;37:958-965.
- Kaya TI, Kokturk A, Polat A, et al. A case of cutaneous lymphangiectasis secondary to breast cancer treatment. Int J Dermatol. 2001;40:760-761.
- Ambrojo P, Cogolluda EF, Aguilar A, et al. Cutaneous lymphangiectases after therapy for carcinoma of the cervix. Clin Exp Dermatol. 1990;15:57-59.
- Tasdelen I, Gokgoz S, Paksoy E, et al. Acquired lymphangiectasis after breast conservation treatment for breast cancer: report of a case. Dermatol Online J. 2004;10:9.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29:983-996.
- Gengler C, Coindre JM, Leroux A. Vascular proliferations of the skin after radiation therapy for breast cancer: clinicopathologic analysis of a series in favor of a benign process: a study from the French Sarcoma Group. Cancer. 2007;109:1584-1598.
- Ghaemmaghami F, Karimi Zarchi M, Mousavi A. Major labiaectomy as surgical management of vulvar lymphangioma circumscriptum: three cases and a review of the literature. Arch Gynecol Obstet. 2008;278:57-60.
- Savas J. Carbon dioxide laser for the treatment of microcystic lymphatic malformations (lymphangioma circumscriptum): a systematic review. Dermatol Surg. 2013;39:1147-1157.
- Al Ghamdi KM, Mubki TF. Treatment of lymphangioma circumscriptum with sclerotherapy: an ignored effective remedy. J Cosmet Dermatol. 2011;10:156-158.
- Ayhan E. Lymphangioma circumscriptum: good clinical response to isotretinoin therapy. Pediatr Dermatol. 2016;33:E208-E209.
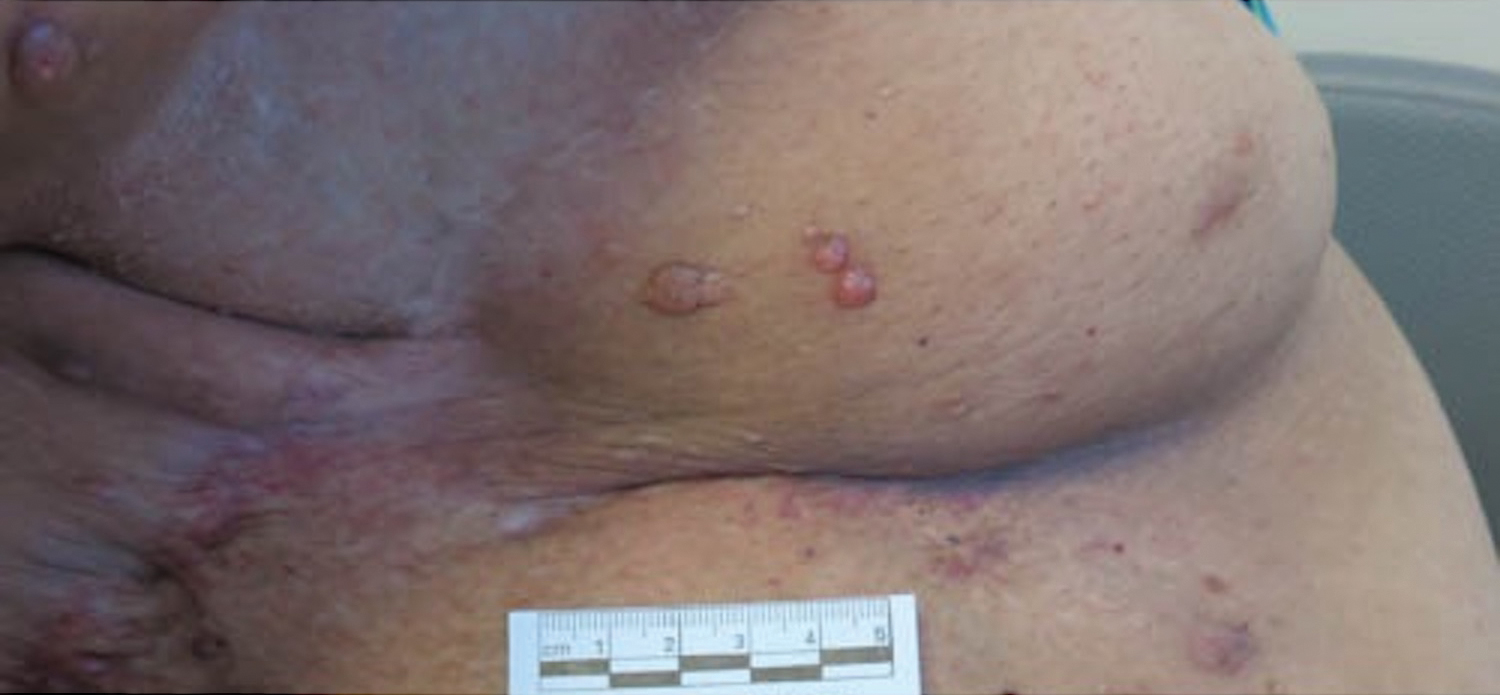
A 51-year-old woman with a history of bilateral breast cancer presented for evaluation of lesions on the underside of the right breast. She was first diagnosed with stage II cancer of the right breast that was subsequently treated with a mastectomy and adjuvant chemotherapy 7 years prior to presentation. One year later, she developed stage IIIC adenocarcinoma of the left breast and was treated with a modified radical mastectomy, adjuvant chemotherapy, and radiation. She had been followed closely by her oncologist with regular surveillance imaging (last at 7 months prior to presentation) that had all been negative for recurrent breast cancer. She presented to our dermatology clinic for evaluation of lesions on the underside of the right breast that were pruritic and occasionally painful with a burning quality. These lesions had recently begun to bleed when scratched but were not otherwise growing or spreading. On physical examination she was afebrile with stable vital signs. Skin examination was notable for numerous violaceous and translucent papules and nodules underneath the right breast and axilla overlying a well-healed mastectomy scar. No lymphadenopathy was present. Shave biopsies were performed and showed well-circumscribed nodular lesions with ectatic vascular channels separated by thin fibrous walls and filled with eosinophilic proteinaceous material and scattered red blood cells. Immunohistochemical staining also showed positivity for D2-40.
Von Willebrand disease screening is low prior to hysterectomy
The number of women being screened for von Willebrand disease (VWD) before undergoing hysterectomy for heavy menstrual bleeding is “very low” and appears not to align with current recommendations, according to a retrospective analysis.
“One essential reason to identify females with VWD prior to hysterectomy or hysterectomy alternative is to avoid peri‐ and postoperative complications,” Amanda E. Jacobson‐Kelly, MD, of Ohio State University in Columbus and her colleagues wrote in a letter to the editor published in Haemophilia.
Current recommendations from the American College of Obstetricians and Gynecologists and other groups call for consideration of underlying bleeding disorders in women with heavy menstrual bleeding and additional risks factors, such as family history of abnormal bleeding.
In this study, researchers retrospectively analyzed 8,998 women under the age of 40 years who had a hysterectomy for heavy menstrual bleeding without a known bleeding disorder. Patient data was collected from a national insurance claims database representative of the entire U.S. population.
“We hypothesized that the overall frequency of VWD screening would be low, despite [more than] 15 years of expert recommendations,” the team wrote.
Study participants were included if they had a diagnosis of anemia or heavy menstrual bleeding before surgery, while women with certain genital malignancies and fibroids were excluded. Other information, such as age, geographical factors, and insurance-related factors were also included to evaluate whether these parameters affected disease screening.
After analysis, the researchers found that just 0.6% (n = 57) of women were screened for VWD in the 12 months leading up to surgery. Additionally, they reported that 14% (n = 1,276) of women underwent other forms of coagulation testing before surgery, such as partial thromboplastin time and prothrombin time.
“We found that greater distance to a [hemophilia treatment center] was strongly predictive of decreased likelihood of undergoing VWD screening,” Dr. Jacobson‐Kelly and her colleagues wrote.
The authors acknowledged that a key limitation of the study was the inability to account for factors not recorded in the database, including drug use, family history of bleeding disorders, and ethnicity.
“This study brings to light the need for the hematology community to improve education and awareness among women’s health providers,” they wrote.
The study was supported by grant funding from the National Heart, Lung, and Blood Institute. The authors reported having no conflicts of interest.
SOURCE: Jacobson-Kelly AE et al. Haemophilia. 2019 Feb 28. doi: 10.1111/hae.13709.
The number of women being screened for von Willebrand disease (VWD) before undergoing hysterectomy for heavy menstrual bleeding is “very low” and appears not to align with current recommendations, according to a retrospective analysis.
“One essential reason to identify females with VWD prior to hysterectomy or hysterectomy alternative is to avoid peri‐ and postoperative complications,” Amanda E. Jacobson‐Kelly, MD, of Ohio State University in Columbus and her colleagues wrote in a letter to the editor published in Haemophilia.
Current recommendations from the American College of Obstetricians and Gynecologists and other groups call for consideration of underlying bleeding disorders in women with heavy menstrual bleeding and additional risks factors, such as family history of abnormal bleeding.
In this study, researchers retrospectively analyzed 8,998 women under the age of 40 years who had a hysterectomy for heavy menstrual bleeding without a known bleeding disorder. Patient data was collected from a national insurance claims database representative of the entire U.S. population.
“We hypothesized that the overall frequency of VWD screening would be low, despite [more than] 15 years of expert recommendations,” the team wrote.
Study participants were included if they had a diagnosis of anemia or heavy menstrual bleeding before surgery, while women with certain genital malignancies and fibroids were excluded. Other information, such as age, geographical factors, and insurance-related factors were also included to evaluate whether these parameters affected disease screening.
After analysis, the researchers found that just 0.6% (n = 57) of women were screened for VWD in the 12 months leading up to surgery. Additionally, they reported that 14% (n = 1,276) of women underwent other forms of coagulation testing before surgery, such as partial thromboplastin time and prothrombin time.
“We found that greater distance to a [hemophilia treatment center] was strongly predictive of decreased likelihood of undergoing VWD screening,” Dr. Jacobson‐Kelly and her colleagues wrote.
The authors acknowledged that a key limitation of the study was the inability to account for factors not recorded in the database, including drug use, family history of bleeding disorders, and ethnicity.
“This study brings to light the need for the hematology community to improve education and awareness among women’s health providers,” they wrote.
The study was supported by grant funding from the National Heart, Lung, and Blood Institute. The authors reported having no conflicts of interest.
SOURCE: Jacobson-Kelly AE et al. Haemophilia. 2019 Feb 28. doi: 10.1111/hae.13709.
The number of women being screened for von Willebrand disease (VWD) before undergoing hysterectomy for heavy menstrual bleeding is “very low” and appears not to align with current recommendations, according to a retrospective analysis.
“One essential reason to identify females with VWD prior to hysterectomy or hysterectomy alternative is to avoid peri‐ and postoperative complications,” Amanda E. Jacobson‐Kelly, MD, of Ohio State University in Columbus and her colleagues wrote in a letter to the editor published in Haemophilia.
Current recommendations from the American College of Obstetricians and Gynecologists and other groups call for consideration of underlying bleeding disorders in women with heavy menstrual bleeding and additional risks factors, such as family history of abnormal bleeding.
In this study, researchers retrospectively analyzed 8,998 women under the age of 40 years who had a hysterectomy for heavy menstrual bleeding without a known bleeding disorder. Patient data was collected from a national insurance claims database representative of the entire U.S. population.
“We hypothesized that the overall frequency of VWD screening would be low, despite [more than] 15 years of expert recommendations,” the team wrote.
Study participants were included if they had a diagnosis of anemia or heavy menstrual bleeding before surgery, while women with certain genital malignancies and fibroids were excluded. Other information, such as age, geographical factors, and insurance-related factors were also included to evaluate whether these parameters affected disease screening.
After analysis, the researchers found that just 0.6% (n = 57) of women were screened for VWD in the 12 months leading up to surgery. Additionally, they reported that 14% (n = 1,276) of women underwent other forms of coagulation testing before surgery, such as partial thromboplastin time and prothrombin time.
“We found that greater distance to a [hemophilia treatment center] was strongly predictive of decreased likelihood of undergoing VWD screening,” Dr. Jacobson‐Kelly and her colleagues wrote.
The authors acknowledged that a key limitation of the study was the inability to account for factors not recorded in the database, including drug use, family history of bleeding disorders, and ethnicity.
“This study brings to light the need for the hematology community to improve education and awareness among women’s health providers,” they wrote.
The study was supported by grant funding from the National Heart, Lung, and Blood Institute. The authors reported having no conflicts of interest.
SOURCE: Jacobson-Kelly AE et al. Haemophilia. 2019 Feb 28. doi: 10.1111/hae.13709.
FROM HAEMOPHILIA
Hypoglycemia in the elderly: Watch for atypical symptoms
We read with interest the review article by Keber and Fiebert, “Diabetes in the elderly: Matching meds to needs” (J Fam Pract. 2018;67:408-410,412-415). The authors have provided a timely overview of antidiabetes medications for elderly people with type 2 diabetes mellitus (T2DM) and their relative risks for hypoglycemia.
We’d like to add to this important conversation.
Aging, per se, modifies the glycemic thresholds for autonomic symptoms and cognitive impairment; in older nondiabetic men (mean + SD: age 65 ± 3 years), autonomic symptoms and cognitive dysfunction commence at identical glycemic thresholds (3 ± 0.2 mmol/L [54 ± 4 mg/dL]). By contrast, in younger men (age 23 ± 2 years), a significant gap is observed between the glycemic threshold for symptom generation (3.6 mmol/L [65 mg/dL]) and the onset of cognitive dysfunction (2.6 mmol/L [47 mg/dL]).1,2 The simultaneous occurrence of symptoms and cognitive impairment in older people may adversely affect their ability to recognize and treat hypoglycemia promptly.
In addition, hypoglycemia in older T2DM patients often presents with atypical neurologic symptoms, including incoordination and ataxia, slurring of speech, and visual disturbances, which either are not identified as hypoglycemia or are misdiagnosed as other medical disorders (eg, transient ischemic attack).3 Knowledge about hypoglycemia symptoms is poor, in both elderly people with diabetes and their relatives and caregivers, which compromises the ability to identify hypoglycemia and provide effective treatment.4 Education about the possible presentations of hypoglycemia and its effective treatment is essential for older patients and their relatives.
Jan Brož, MD
Prague, Czech Republic
1. Meneilly GS, Elahi D. Physiological importance of first-phase insulin release in elderly patients with diabetes. Diabetes Care. 1998;21:1326-1329.
2. Matyka K, Evans M, Lomas J, et al. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care. 1997;20:135-141.
3. Jaap AJ, Jones GC, McCrimmon RJ, et al. Perceived symptoms of hypoglycaemia in elderly type 2 diabetic patients treated with insulin. Diabet Med. 1998;15:398-401.
4. Thomson FJ, Masson EA, Leeming JT, et al. Lack of knowledge of symptoms of hypoglycaemia by elderly diabetic patients. Age Ageing. 1991;20:404-406.
We read with interest the review article by Keber and Fiebert, “Diabetes in the elderly: Matching meds to needs” (J Fam Pract. 2018;67:408-410,412-415). The authors have provided a timely overview of antidiabetes medications for elderly people with type 2 diabetes mellitus (T2DM) and their relative risks for hypoglycemia.
We’d like to add to this important conversation.
Aging, per se, modifies the glycemic thresholds for autonomic symptoms and cognitive impairment; in older nondiabetic men (mean + SD: age 65 ± 3 years), autonomic symptoms and cognitive dysfunction commence at identical glycemic thresholds (3 ± 0.2 mmol/L [54 ± 4 mg/dL]). By contrast, in younger men (age 23 ± 2 years), a significant gap is observed between the glycemic threshold for symptom generation (3.6 mmol/L [65 mg/dL]) and the onset of cognitive dysfunction (2.6 mmol/L [47 mg/dL]).1,2 The simultaneous occurrence of symptoms and cognitive impairment in older people may adversely affect their ability to recognize and treat hypoglycemia promptly.
In addition, hypoglycemia in older T2DM patients often presents with atypical neurologic symptoms, including incoordination and ataxia, slurring of speech, and visual disturbances, which either are not identified as hypoglycemia or are misdiagnosed as other medical disorders (eg, transient ischemic attack).3 Knowledge about hypoglycemia symptoms is poor, in both elderly people with diabetes and their relatives and caregivers, which compromises the ability to identify hypoglycemia and provide effective treatment.4 Education about the possible presentations of hypoglycemia and its effective treatment is essential for older patients and their relatives.
Jan Brož, MD
Prague, Czech Republic
We read with interest the review article by Keber and Fiebert, “Diabetes in the elderly: Matching meds to needs” (J Fam Pract. 2018;67:408-410,412-415). The authors have provided a timely overview of antidiabetes medications for elderly people with type 2 diabetes mellitus (T2DM) and their relative risks for hypoglycemia.
We’d like to add to this important conversation.
Aging, per se, modifies the glycemic thresholds for autonomic symptoms and cognitive impairment; in older nondiabetic men (mean + SD: age 65 ± 3 years), autonomic symptoms and cognitive dysfunction commence at identical glycemic thresholds (3 ± 0.2 mmol/L [54 ± 4 mg/dL]). By contrast, in younger men (age 23 ± 2 years), a significant gap is observed between the glycemic threshold for symptom generation (3.6 mmol/L [65 mg/dL]) and the onset of cognitive dysfunction (2.6 mmol/L [47 mg/dL]).1,2 The simultaneous occurrence of symptoms and cognitive impairment in older people may adversely affect their ability to recognize and treat hypoglycemia promptly.
In addition, hypoglycemia in older T2DM patients often presents with atypical neurologic symptoms, including incoordination and ataxia, slurring of speech, and visual disturbances, which either are not identified as hypoglycemia or are misdiagnosed as other medical disorders (eg, transient ischemic attack).3 Knowledge about hypoglycemia symptoms is poor, in both elderly people with diabetes and their relatives and caregivers, which compromises the ability to identify hypoglycemia and provide effective treatment.4 Education about the possible presentations of hypoglycemia and its effective treatment is essential for older patients and their relatives.
Jan Brož, MD
Prague, Czech Republic
1. Meneilly GS, Elahi D. Physiological importance of first-phase insulin release in elderly patients with diabetes. Diabetes Care. 1998;21:1326-1329.
2. Matyka K, Evans M, Lomas J, et al. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care. 1997;20:135-141.
3. Jaap AJ, Jones GC, McCrimmon RJ, et al. Perceived symptoms of hypoglycaemia in elderly type 2 diabetic patients treated with insulin. Diabet Med. 1998;15:398-401.
4. Thomson FJ, Masson EA, Leeming JT, et al. Lack of knowledge of symptoms of hypoglycaemia by elderly diabetic patients. Age Ageing. 1991;20:404-406.
1. Meneilly GS, Elahi D. Physiological importance of first-phase insulin release in elderly patients with diabetes. Diabetes Care. 1998;21:1326-1329.
2. Matyka K, Evans M, Lomas J, et al. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care. 1997;20:135-141.
3. Jaap AJ, Jones GC, McCrimmon RJ, et al. Perceived symptoms of hypoglycaemia in elderly type 2 diabetic patients treated with insulin. Diabet Med. 1998;15:398-401.
4. Thomson FJ, Masson EA, Leeming JT, et al. Lack of knowledge of symptoms of hypoglycaemia by elderly diabetic patients. Age Ageing. 1991;20:404-406.