User login
There’s No Doubt: Blame the Drug
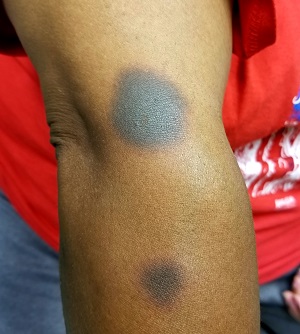
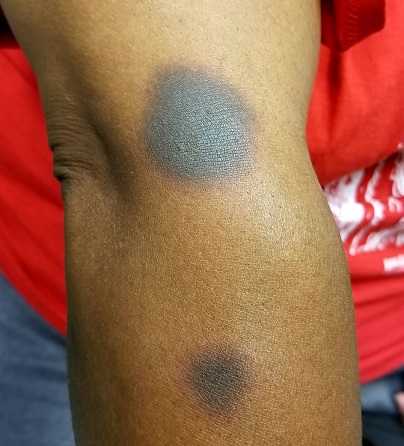
It’s taken 2 years for family members to convince this 50-year-old black woman to consult dermatology about dark circles on her arms and trunk. The asymptomatic lesions were only faintly discolored at manifestation but have darkened with each recurrence. The 6 lesions she currently has are in the exact locations they originally manifested in.
Convinced she had “ringworm,” the patient tried several different antifungal creams, to no avail. Then her primary care provider prescribed a 2-month course of oral antifungal medication (terbinafine)—again, with no change to the lesions.
The patient is otherwise healthy except for osteoarthritis, for which she takes ibuprofen (800 mg 2 or 3 times a day). She denies any history of seizure, chronic urinary tract infection, or other chronic infection.
EXAMINATION
The 6 perfectly round, uniformly pigmented, dark brown macular lesions on the patient’s arms and trunk range from 2 to 5 cm in diameter. A faint, rusty brown halo can be seen around the periphery of the lesions, giving them a targetoid look. The surfaces of the lesions are completely smooth.
What’s the diagnosis?
DISCUSSION
The list of dark brown, round macules that come and go in the exact same locations is a short one—in fact, it only includes fixed drug eruption (FDE). This is a unique adverse reaction to one of several medications; common culprits are ibuprofen or other NSAIDs, aspirin, members of the sulfa family, penicillin, and several antiseizure medications.
The exact mechanism for this reaction is unknown, but it does appear to involve the localized production of cytokines. There is a range of morphologic presentations for FDE: some lesions are more targetoid than others; some are darker (especially in patients with darker skin) while others are more pink.
Furthermore, many patients develop additional lesions over time. A few, on the other hand, will reach a point at which they stop reacting to the offending product.
Of all the drugs causing FDE, sulfa-based products are among the more consistent offenders. Thus, we always ask about chronic urinary tract infections.
TAKE-HOME LEARNING POINTS
- Fixed drug eruptions (FDE) usually manifest with round, targetoid macules that are occasionally blistery.
- The lesions recur or darken with each “challenge” from the drug, which can be one of several potential offenders.
- The fact that the lesions keep recurring in the same location (ie, fixed) is pathognomic for FDE.
- FDE lesions can occur almost anywhere on the body—even on palms or soles.
It’s taken 2 years for family members to convince this 50-year-old black woman to consult dermatology about dark circles on her arms and trunk. The asymptomatic lesions were only faintly discolored at manifestation but have darkened with each recurrence. The 6 lesions she currently has are in the exact locations they originally manifested in.
Convinced she had “ringworm,” the patient tried several different antifungal creams, to no avail. Then her primary care provider prescribed a 2-month course of oral antifungal medication (terbinafine)—again, with no change to the lesions.
The patient is otherwise healthy except for osteoarthritis, for which she takes ibuprofen (800 mg 2 or 3 times a day). She denies any history of seizure, chronic urinary tract infection, or other chronic infection.

EXAMINATION
The 6 perfectly round, uniformly pigmented, dark brown macular lesions on the patient’s arms and trunk range from 2 to 5 cm in diameter. A faint, rusty brown halo can be seen around the periphery of the lesions, giving them a targetoid look. The surfaces of the lesions are completely smooth.
What’s the diagnosis?
DISCUSSION
The list of dark brown, round macules that come and go in the exact same locations is a short one—in fact, it only includes fixed drug eruption (FDE). This is a unique adverse reaction to one of several medications; common culprits are ibuprofen or other NSAIDs, aspirin, members of the sulfa family, penicillin, and several antiseizure medications.
The exact mechanism for this reaction is unknown, but it does appear to involve the localized production of cytokines. There is a range of morphologic presentations for FDE: some lesions are more targetoid than others; some are darker (especially in patients with darker skin) while others are more pink.
Furthermore, many patients develop additional lesions over time. A few, on the other hand, will reach a point at which they stop reacting to the offending product.
Of all the drugs causing FDE, sulfa-based products are among the more consistent offenders. Thus, we always ask about chronic urinary tract infections.
TAKE-HOME LEARNING POINTS
- Fixed drug eruptions (FDE) usually manifest with round, targetoid macules that are occasionally blistery.
- The lesions recur or darken with each “challenge” from the drug, which can be one of several potential offenders.
- The fact that the lesions keep recurring in the same location (ie, fixed) is pathognomic for FDE.
- FDE lesions can occur almost anywhere on the body—even on palms or soles.
It’s taken 2 years for family members to convince this 50-year-old black woman to consult dermatology about dark circles on her arms and trunk. The asymptomatic lesions were only faintly discolored at manifestation but have darkened with each recurrence. The 6 lesions she currently has are in the exact locations they originally manifested in.
Convinced she had “ringworm,” the patient tried several different antifungal creams, to no avail. Then her primary care provider prescribed a 2-month course of oral antifungal medication (terbinafine)—again, with no change to the lesions.
The patient is otherwise healthy except for osteoarthritis, for which she takes ibuprofen (800 mg 2 or 3 times a day). She denies any history of seizure, chronic urinary tract infection, or other chronic infection.

EXAMINATION
The 6 perfectly round, uniformly pigmented, dark brown macular lesions on the patient’s arms and trunk range from 2 to 5 cm in diameter. A faint, rusty brown halo can be seen around the periphery of the lesions, giving them a targetoid look. The surfaces of the lesions are completely smooth.
What’s the diagnosis?
DISCUSSION
The list of dark brown, round macules that come and go in the exact same locations is a short one—in fact, it only includes fixed drug eruption (FDE). This is a unique adverse reaction to one of several medications; common culprits are ibuprofen or other NSAIDs, aspirin, members of the sulfa family, penicillin, and several antiseizure medications.
The exact mechanism for this reaction is unknown, but it does appear to involve the localized production of cytokines. There is a range of morphologic presentations for FDE: some lesions are more targetoid than others; some are darker (especially in patients with darker skin) while others are more pink.
Furthermore, many patients develop additional lesions over time. A few, on the other hand, will reach a point at which they stop reacting to the offending product.
Of all the drugs causing FDE, sulfa-based products are among the more consistent offenders. Thus, we always ask about chronic urinary tract infections.
TAKE-HOME LEARNING POINTS
- Fixed drug eruptions (FDE) usually manifest with round, targetoid macules that are occasionally blistery.
- The lesions recur or darken with each “challenge” from the drug, which can be one of several potential offenders.
- The fact that the lesions keep recurring in the same location (ie, fixed) is pathognomic for FDE.
- FDE lesions can occur almost anywhere on the body—even on palms or soles.
Hospital-onset sepsis twice as lethal as community-onset disease
SAN DIEGO – Patients who develop sepsis in the hospital appear to be in greater risk for mortality than those who bring it with them, a new study suggests. Patients with hospital-onset sepsis were twice as likely to die as those infected in the outside world.
“There could be some differences in quality of care that explains the difference in mortality,” said study lead author Chanu Rhee, MD, assistant professor of population medicine at Harvard Medical School, Boston, in a presentation about the findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
In an interview, Dr. Rhee said researchers launched the study to gain a greater understanding of the epidemiology of sepsis in the hospital. They relied on a new Centers for Disease Control and Prevention definition of sepsis that is “enhancing the consistency of surveillance across hospitals and allowing more precise differentiation between hospital-onset versus community-onset sepsis.”
The study authors retrospectively tracked more than 2.2 million patients who were treated at 136 U.S. hospitals from 2009 to 2015. In general, hospital-onset sepsis was defined as patients who had a blood culture, initial antibiotic therapy, and organ dysfunction on their third day in the hospital or later.*
Of the patients, 83,600 had community-onset sepsis and 11,500 had hospital-onset sepsis. Those with sepsis were more likely to be men and have comorbidities such as cancer, congestive heart failure, diabetes, and renal disease.
Patients with hospital-onset sepsis had longer median lengths of stay (19 days) than the community-onset group (8 days) and the no-sepsis group (4 days). The hospital-onset group also had a greater likelihood of ICU admission (61%) than the community-onset (44%) and no-sepsis (9%) groups.
About 34% of those with hospital-onset sepsis died, compared with 17% of the community-onset group and 2% of the patients who didn’t have sepsis. After adjustment, those with hospital-onset sepsis were still more likely to have died (odds ratio, 2.1; 95% confidence interval, 2.0-2.2).
“Other studies have suggested that there may be delays in the recognition and care of patients who develop sepsis in the hospital as opposed to presenting to the hospital with sepsis,” Dr. Rhee said. “It is also possible that hospital-onset sepsis tends to be caused by organisms that are more virulent and resistant to antibiotics.”
Overall, he said, “our findings underscore the importance of targeting hospital-onset sepsis with surveillance, prevention, and quality improvement efforts.”
The study was funded by the CDC and the Agency for Healthcare Research and Quality. The authors reported no relevant disclosures.
SOURCE: Rhee C et al. CCC48, Abstract 29.
*Correction, 3/19/19: An earlier version of this article misstated the definition of sepsis.
SAN DIEGO – Patients who develop sepsis in the hospital appear to be in greater risk for mortality than those who bring it with them, a new study suggests. Patients with hospital-onset sepsis were twice as likely to die as those infected in the outside world.
“There could be some differences in quality of care that explains the difference in mortality,” said study lead author Chanu Rhee, MD, assistant professor of population medicine at Harvard Medical School, Boston, in a presentation about the findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
In an interview, Dr. Rhee said researchers launched the study to gain a greater understanding of the epidemiology of sepsis in the hospital. They relied on a new Centers for Disease Control and Prevention definition of sepsis that is “enhancing the consistency of surveillance across hospitals and allowing more precise differentiation between hospital-onset versus community-onset sepsis.”
The study authors retrospectively tracked more than 2.2 million patients who were treated at 136 U.S. hospitals from 2009 to 2015. In general, hospital-onset sepsis was defined as patients who had a blood culture, initial antibiotic therapy, and organ dysfunction on their third day in the hospital or later.*
Of the patients, 83,600 had community-onset sepsis and 11,500 had hospital-onset sepsis. Those with sepsis were more likely to be men and have comorbidities such as cancer, congestive heart failure, diabetes, and renal disease.
Patients with hospital-onset sepsis had longer median lengths of stay (19 days) than the community-onset group (8 days) and the no-sepsis group (4 days). The hospital-onset group also had a greater likelihood of ICU admission (61%) than the community-onset (44%) and no-sepsis (9%) groups.
About 34% of those with hospital-onset sepsis died, compared with 17% of the community-onset group and 2% of the patients who didn’t have sepsis. After adjustment, those with hospital-onset sepsis were still more likely to have died (odds ratio, 2.1; 95% confidence interval, 2.0-2.2).
“Other studies have suggested that there may be delays in the recognition and care of patients who develop sepsis in the hospital as opposed to presenting to the hospital with sepsis,” Dr. Rhee said. “It is also possible that hospital-onset sepsis tends to be caused by organisms that are more virulent and resistant to antibiotics.”
Overall, he said, “our findings underscore the importance of targeting hospital-onset sepsis with surveillance, prevention, and quality improvement efforts.”
The study was funded by the CDC and the Agency for Healthcare Research and Quality. The authors reported no relevant disclosures.
SOURCE: Rhee C et al. CCC48, Abstract 29.
*Correction, 3/19/19: An earlier version of this article misstated the definition of sepsis.
SAN DIEGO – Patients who develop sepsis in the hospital appear to be in greater risk for mortality than those who bring it with them, a new study suggests. Patients with hospital-onset sepsis were twice as likely to die as those infected in the outside world.
“There could be some differences in quality of care that explains the difference in mortality,” said study lead author Chanu Rhee, MD, assistant professor of population medicine at Harvard Medical School, Boston, in a presentation about the findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
In an interview, Dr. Rhee said researchers launched the study to gain a greater understanding of the epidemiology of sepsis in the hospital. They relied on a new Centers for Disease Control and Prevention definition of sepsis that is “enhancing the consistency of surveillance across hospitals and allowing more precise differentiation between hospital-onset versus community-onset sepsis.”
The study authors retrospectively tracked more than 2.2 million patients who were treated at 136 U.S. hospitals from 2009 to 2015. In general, hospital-onset sepsis was defined as patients who had a blood culture, initial antibiotic therapy, and organ dysfunction on their third day in the hospital or later.*
Of the patients, 83,600 had community-onset sepsis and 11,500 had hospital-onset sepsis. Those with sepsis were more likely to be men and have comorbidities such as cancer, congestive heart failure, diabetes, and renal disease.
Patients with hospital-onset sepsis had longer median lengths of stay (19 days) than the community-onset group (8 days) and the no-sepsis group (4 days). The hospital-onset group also had a greater likelihood of ICU admission (61%) than the community-onset (44%) and no-sepsis (9%) groups.
About 34% of those with hospital-onset sepsis died, compared with 17% of the community-onset group and 2% of the patients who didn’t have sepsis. After adjustment, those with hospital-onset sepsis were still more likely to have died (odds ratio, 2.1; 95% confidence interval, 2.0-2.2).
“Other studies have suggested that there may be delays in the recognition and care of patients who develop sepsis in the hospital as opposed to presenting to the hospital with sepsis,” Dr. Rhee said. “It is also possible that hospital-onset sepsis tends to be caused by organisms that are more virulent and resistant to antibiotics.”
Overall, he said, “our findings underscore the importance of targeting hospital-onset sepsis with surveillance, prevention, and quality improvement efforts.”
The study was funded by the CDC and the Agency for Healthcare Research and Quality. The authors reported no relevant disclosures.
SOURCE: Rhee C et al. CCC48, Abstract 29.
*Correction, 3/19/19: An earlier version of this article misstated the definition of sepsis.
REPORTING FROM CCC48
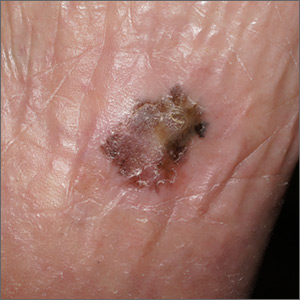
Pigmentation on foot
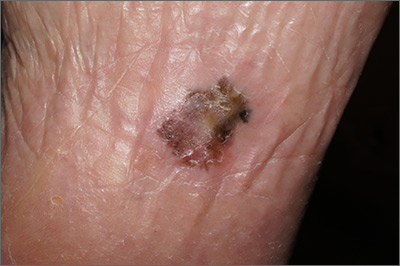
The FP suspected that this was an acral lentiginous melanoma (ALM).
The FP considered the various ways to biopsy this lesion. Doing a biopsy of the whole lesion was neither practical, nor desirable, given that the lesion was >2 cm and located on the bottom of the patient’s foot. And while incomplete sampling could result in a false negative result, this lesion was so suspicious for melanoma that any well-performed biopsy would likely produce a diagnosis of melanoma. (Of course if melanoma was not diagnosable with partial sampling, a full excisional biopsy would be needed.)
The FP also debated doing a 6-mm punch biopsy vs a shave biopsy; his intent was to get below some of the deeper pigment. Incisional biopsy done as a small ellipse with suturing was also an option. It would also be the most time consuming.
The FP presented the biopsy options to the patient and explained that the shave biopsy would require a dressing and the punch biopsy would require sutures, which would need to be removed at the next visit. Both biopsy methods would likely cause the foot to be uncomfortable to walk on for several days.
The FP and patient decided to do a deep shave biopsy (saucerization). After administering local anesthesia with lidocaine and epinephrine, the FP performed a deep shave biopsy that included about 1 cm of the darkest and thickest portion of the suspected melanoma.
The biopsy results indicated that the patient had an acral lentiginous melanoma, which measured 0.7 mm at its deepest point in the biopsied portion. The patient was referred to Orthopedics for a wide excision and repair. A sentinel node biopsy was not needed because the lesion was less than 1 mm in depth and there was no ulceration. The surgery and healing time were challenging for the patient, but she did not lose her foot and has remained cancer free.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that this was an acral lentiginous melanoma (ALM).
The FP considered the various ways to biopsy this lesion. Doing a biopsy of the whole lesion was neither practical, nor desirable, given that the lesion was >2 cm and located on the bottom of the patient’s foot. And while incomplete sampling could result in a false negative result, this lesion was so suspicious for melanoma that any well-performed biopsy would likely produce a diagnosis of melanoma. (Of course if melanoma was not diagnosable with partial sampling, a full excisional biopsy would be needed.)
The FP also debated doing a 6-mm punch biopsy vs a shave biopsy; his intent was to get below some of the deeper pigment. Incisional biopsy done as a small ellipse with suturing was also an option. It would also be the most time consuming.
The FP presented the biopsy options to the patient and explained that the shave biopsy would require a dressing and the punch biopsy would require sutures, which would need to be removed at the next visit. Both biopsy methods would likely cause the foot to be uncomfortable to walk on for several days.
The FP and patient decided to do a deep shave biopsy (saucerization). After administering local anesthesia with lidocaine and epinephrine, the FP performed a deep shave biopsy that included about 1 cm of the darkest and thickest portion of the suspected melanoma.
The biopsy results indicated that the patient had an acral lentiginous melanoma, which measured 0.7 mm at its deepest point in the biopsied portion. The patient was referred to Orthopedics for a wide excision and repair. A sentinel node biopsy was not needed because the lesion was less than 1 mm in depth and there was no ulceration. The surgery and healing time were challenging for the patient, but she did not lose her foot and has remained cancer free.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that this was an acral lentiginous melanoma (ALM).
The FP considered the various ways to biopsy this lesion. Doing a biopsy of the whole lesion was neither practical, nor desirable, given that the lesion was >2 cm and located on the bottom of the patient’s foot. And while incomplete sampling could result in a false negative result, this lesion was so suspicious for melanoma that any well-performed biopsy would likely produce a diagnosis of melanoma. (Of course if melanoma was not diagnosable with partial sampling, a full excisional biopsy would be needed.)
The FP also debated doing a 6-mm punch biopsy vs a shave biopsy; his intent was to get below some of the deeper pigment. Incisional biopsy done as a small ellipse with suturing was also an option. It would also be the most time consuming.
The FP presented the biopsy options to the patient and explained that the shave biopsy would require a dressing and the punch biopsy would require sutures, which would need to be removed at the next visit. Both biopsy methods would likely cause the foot to be uncomfortable to walk on for several days.
The FP and patient decided to do a deep shave biopsy (saucerization). After administering local anesthesia with lidocaine and epinephrine, the FP performed a deep shave biopsy that included about 1 cm of the darkest and thickest portion of the suspected melanoma.
The biopsy results indicated that the patient had an acral lentiginous melanoma, which measured 0.7 mm at its deepest point in the biopsied portion. The patient was referred to Orthopedics for a wide excision and repair. A sentinel node biopsy was not needed because the lesion was less than 1 mm in depth and there was no ulceration. The surgery and healing time were challenging for the patient, but she did not lose her foot and has remained cancer free.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Firing patients
After last month’s
One might assume that, just as patients are free to choose or reject their doctors, physicians have an equal right to reject their patients; and to a certain extent, that’s true. There are no specific laws prohibiting a provider from terminating a patient relationship for any reason, other than a discriminatory one – race, nationality, religion, age, sex, sexual orientation, and so on. However, our ethical obligations to “do no harm” and to place our patients’ welfare above our own self-interests dictate that dismissing a patient should be the absolute last resort, after all other options have been exhausted.
First, to avoid charges of arbitrary termination, you should draw up a specific list of situations that could merit a dismissal from your office, and add it to your office manual. Every list will probably differ in some respects, but for the sake of example, here is mine:
- Threats or violence toward physicians or staff.
- Inappropriate sexual advances toward physicians or staff.
- Providing false or misleading medical history.
- Repeated rude or disruptive behavior.
- Demands for unapproved, unindicated, or inappropriate treatments or medications (particularly controlled substances).
- Refusal to adhere to agreed-upon treatment plans.
- Repeated failure to keep scheduled appointments.
- Repeated failure to pay medical bills.
As with pretty much everything in a private practice, accurate and written documentation of dismissible behavior is essential. Record all incidents and assemble as much material evidence as possible from all available sources.
In most cases (except the first two infractions on our list, for which we have zero tolerance), we make every effort to resolve the problem amicably. We communicate with the patients in question, explain our concerns, and discuss options for resolution. I also may send a letter, repeating my concerns and proposed solutions, as further documentation of our efforts to achieve an amicable resolution. All verbal and written warnings are, of course, documented as well. If the patient has a managed care policy, we review the managed care contract, which sometimes includes specific requirements for dismissal of its patients.
When such efforts fail, we send the patient two letters – one certified with return receipt, the other by conventional first class, in case the patient refuses the certified copy – explaining the reason for dismissal, and that care will be discontinued in 30 days from the letter’s date. (Most attorneys and medical associations agree that 30 days is sufficient reasonable notice.) We offer to provide care during the interim period, include a list of names and contact information for potential alternate providers, and offer to transfer records after receiving written permission.
Following these precautions will usually protect you from charges of “patient abandonment,” which is generally defined as the unilateral severance by the physician of the physician-patient relationship without giving the patient sufficient advance notice to obtain the services of another practitioner, and at a time when the patient still requires medical attention.
Some states have their own unique definitions of patient abandonment. You should check with your state’s health department, and your attorney, for any unusual requirements in your state, because violating these could lead to intervention by your state licensing board. There also is the risk of civil litigation, which typically is not covered by malpractice policies and may not be covered by your general liability policy either.
Patients who feel that termination was unjustified also may respond with negative reviews on social media, which I’ve discussed in recent columns, and will again, soon.
If something untrue is posted about you on a doctor-rating site, take action. Reputable sites have their own reputations to protect and can usually be persuaded to remove anything that is demonstrably false, although you may need a lawyer’s letter to get their attention. Try to get the error removed entirely or corrected within the original posting. An erratum on some distant page of the website is likely to be ignored, and will leave the false information online, intact.
Unfair comments are unlikely to be removed unless they are blatantly libelous; but many sites allow you to post a response, giving your side of the story. (More on that in the near future.) Also, there is nothing wrong with encouraging happy patients to write favorable reviews on those same sites. Sauce for the goose, and all that.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
After last month’s
One might assume that, just as patients are free to choose or reject their doctors, physicians have an equal right to reject their patients; and to a certain extent, that’s true. There are no specific laws prohibiting a provider from terminating a patient relationship for any reason, other than a discriminatory one – race, nationality, religion, age, sex, sexual orientation, and so on. However, our ethical obligations to “do no harm” and to place our patients’ welfare above our own self-interests dictate that dismissing a patient should be the absolute last resort, after all other options have been exhausted.
First, to avoid charges of arbitrary termination, you should draw up a specific list of situations that could merit a dismissal from your office, and add it to your office manual. Every list will probably differ in some respects, but for the sake of example, here is mine:
- Threats or violence toward physicians or staff.
- Inappropriate sexual advances toward physicians or staff.
- Providing false or misleading medical history.
- Repeated rude or disruptive behavior.
- Demands for unapproved, unindicated, or inappropriate treatments or medications (particularly controlled substances).
- Refusal to adhere to agreed-upon treatment plans.
- Repeated failure to keep scheduled appointments.
- Repeated failure to pay medical bills.
As with pretty much everything in a private practice, accurate and written documentation of dismissible behavior is essential. Record all incidents and assemble as much material evidence as possible from all available sources.
In most cases (except the first two infractions on our list, for which we have zero tolerance), we make every effort to resolve the problem amicably. We communicate with the patients in question, explain our concerns, and discuss options for resolution. I also may send a letter, repeating my concerns and proposed solutions, as further documentation of our efforts to achieve an amicable resolution. All verbal and written warnings are, of course, documented as well. If the patient has a managed care policy, we review the managed care contract, which sometimes includes specific requirements for dismissal of its patients.
When such efforts fail, we send the patient two letters – one certified with return receipt, the other by conventional first class, in case the patient refuses the certified copy – explaining the reason for dismissal, and that care will be discontinued in 30 days from the letter’s date. (Most attorneys and medical associations agree that 30 days is sufficient reasonable notice.) We offer to provide care during the interim period, include a list of names and contact information for potential alternate providers, and offer to transfer records after receiving written permission.
Following these precautions will usually protect you from charges of “patient abandonment,” which is generally defined as the unilateral severance by the physician of the physician-patient relationship without giving the patient sufficient advance notice to obtain the services of another practitioner, and at a time when the patient still requires medical attention.
Some states have their own unique definitions of patient abandonment. You should check with your state’s health department, and your attorney, for any unusual requirements in your state, because violating these could lead to intervention by your state licensing board. There also is the risk of civil litigation, which typically is not covered by malpractice policies and may not be covered by your general liability policy either.
Patients who feel that termination was unjustified also may respond with negative reviews on social media, which I’ve discussed in recent columns, and will again, soon.
If something untrue is posted about you on a doctor-rating site, take action. Reputable sites have their own reputations to protect and can usually be persuaded to remove anything that is demonstrably false, although you may need a lawyer’s letter to get their attention. Try to get the error removed entirely or corrected within the original posting. An erratum on some distant page of the website is likely to be ignored, and will leave the false information online, intact.
Unfair comments are unlikely to be removed unless they are blatantly libelous; but many sites allow you to post a response, giving your side of the story. (More on that in the near future.) Also, there is nothing wrong with encouraging happy patients to write favorable reviews on those same sites. Sauce for the goose, and all that.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
After last month’s
One might assume that, just as patients are free to choose or reject their doctors, physicians have an equal right to reject their patients; and to a certain extent, that’s true. There are no specific laws prohibiting a provider from terminating a patient relationship for any reason, other than a discriminatory one – race, nationality, religion, age, sex, sexual orientation, and so on. However, our ethical obligations to “do no harm” and to place our patients’ welfare above our own self-interests dictate that dismissing a patient should be the absolute last resort, after all other options have been exhausted.
First, to avoid charges of arbitrary termination, you should draw up a specific list of situations that could merit a dismissal from your office, and add it to your office manual. Every list will probably differ in some respects, but for the sake of example, here is mine:
- Threats or violence toward physicians or staff.
- Inappropriate sexual advances toward physicians or staff.
- Providing false or misleading medical history.
- Repeated rude or disruptive behavior.
- Demands for unapproved, unindicated, or inappropriate treatments or medications (particularly controlled substances).
- Refusal to adhere to agreed-upon treatment plans.
- Repeated failure to keep scheduled appointments.
- Repeated failure to pay medical bills.
As with pretty much everything in a private practice, accurate and written documentation of dismissible behavior is essential. Record all incidents and assemble as much material evidence as possible from all available sources.
In most cases (except the first two infractions on our list, for which we have zero tolerance), we make every effort to resolve the problem amicably. We communicate with the patients in question, explain our concerns, and discuss options for resolution. I also may send a letter, repeating my concerns and proposed solutions, as further documentation of our efforts to achieve an amicable resolution. All verbal and written warnings are, of course, documented as well. If the patient has a managed care policy, we review the managed care contract, which sometimes includes specific requirements for dismissal of its patients.
When such efforts fail, we send the patient two letters – one certified with return receipt, the other by conventional first class, in case the patient refuses the certified copy – explaining the reason for dismissal, and that care will be discontinued in 30 days from the letter’s date. (Most attorneys and medical associations agree that 30 days is sufficient reasonable notice.) We offer to provide care during the interim period, include a list of names and contact information for potential alternate providers, and offer to transfer records after receiving written permission.
Following these precautions will usually protect you from charges of “patient abandonment,” which is generally defined as the unilateral severance by the physician of the physician-patient relationship without giving the patient sufficient advance notice to obtain the services of another practitioner, and at a time when the patient still requires medical attention.
Some states have their own unique definitions of patient abandonment. You should check with your state’s health department, and your attorney, for any unusual requirements in your state, because violating these could lead to intervention by your state licensing board. There also is the risk of civil litigation, which typically is not covered by malpractice policies and may not be covered by your general liability policy either.
Patients who feel that termination was unjustified also may respond with negative reviews on social media, which I’ve discussed in recent columns, and will again, soon.
If something untrue is posted about you on a doctor-rating site, take action. Reputable sites have their own reputations to protect and can usually be persuaded to remove anything that is demonstrably false, although you may need a lawyer’s letter to get their attention. Try to get the error removed entirely or corrected within the original posting. An erratum on some distant page of the website is likely to be ignored, and will leave the false information online, intact.
Unfair comments are unlikely to be removed unless they are blatantly libelous; but many sites allow you to post a response, giving your side of the story. (More on that in the near future.) Also, there is nothing wrong with encouraging happy patients to write favorable reviews on those same sites. Sauce for the goose, and all that.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Semaglutide plus SGLT2 inhibitors for type 2 diabetes
Also today, patients with HIV may have a greater risk of opioid overdose, why Eisenmeyer syndrome can be a minefield for unwary physicians, and heart attacks and strokes spike in the month after cancer diagnosis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, patients with HIV may have a greater risk of opioid overdose, why Eisenmeyer syndrome can be a minefield for unwary physicians, and heart attacks and strokes spike in the month after cancer diagnosis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, patients with HIV may have a greater risk of opioid overdose, why Eisenmeyer syndrome can be a minefield for unwary physicians, and heart attacks and strokes spike in the month after cancer diagnosis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Treating lymphoma in patients with HIV
In this episode, Stefan Barta, MD, of the University of Pennsylvania, joins David Henry, MD, to discuss the treatment and diagnosis of lymphoma in patients with HIV.
And in this week’s Clinical Correlation, Ilana Yurkiewicz, MD, has Part 2 of her discussion on informed consent in cancer. Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University and is also a columnist for Hematology News. More from Dr. Yurkiewicz here.
Subscribe to Blood & Cancer here:
Apple Podcasts
Google Podcasts
Show notes
By Emily Bryer, DO
Resident in the department of internal medicine, University of Pennsylvania Health System
Immunosuppression in patients with HIV, especially with low CD4 counts, is associated with the development of lymphomas.
Diffuse large B-cell lymphoma is the most common lymphoma in patients with HIV followed by Burkitt lymphoma and Hodgkin lymphoma.
Extra-nodal manifestations of lymphoma are more common in patients with HIV, especially with lower CD4 counts.
Following pathologic diagnosis, staging of lymphoma should include:
- CT scan, PET scan, evaluation of CNS (MRI brain and LP), bone marrow biopsy, and evaluation for hepatitis B and C co-infection.
- Fluorescence in situ hybridization (FISH) is a molecular technique that identifies portions of DNA and helps to identify translocations and rearrangements.
- cMYC, BCL2, and BCL6 are all pro-proliferative genes and commonly implicated in lymphoma.
- cMYC rearrangement poses higher risk of CNS involvement and CNS relapse.
- cMYC rearrangement (as opposed to cMYC translocation) requires therapy that is more aggressive therapy than R-CHOP.
Treatment of high grade diffuse large B-cell lymphoma:
- R-EPOCH
- Ibrutinib plus R-EPOCH
Resources:AIDS Malignancy ConsortiumBlood. 2004;103:275-82.
Blood. 2010 Apr 15; 115(15): 3008–16.
NCT03220022: Ibrutinib, Rituximab, Etoposide, Prednisone, Vincristine Sulfate, Cyclophosphamide, and Doxorubicin Hydrochloride in Treating Patients With HIV-Positive Stage II-IV Diffuse Large B-Cell Lymphomas
In this episode, Stefan Barta, MD, of the University of Pennsylvania, joins David Henry, MD, to discuss the treatment and diagnosis of lymphoma in patients with HIV.
And in this week’s Clinical Correlation, Ilana Yurkiewicz, MD, has Part 2 of her discussion on informed consent in cancer. Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University and is also a columnist for Hematology News. More from Dr. Yurkiewicz here.
Subscribe to Blood & Cancer here:
Apple Podcasts
Google Podcasts
Show notes
By Emily Bryer, DO
Resident in the department of internal medicine, University of Pennsylvania Health System
Immunosuppression in patients with HIV, especially with low CD4 counts, is associated with the development of lymphomas.
Diffuse large B-cell lymphoma is the most common lymphoma in patients with HIV followed by Burkitt lymphoma and Hodgkin lymphoma.
Extra-nodal manifestations of lymphoma are more common in patients with HIV, especially with lower CD4 counts.
Following pathologic diagnosis, staging of lymphoma should include:
- CT scan, PET scan, evaluation of CNS (MRI brain and LP), bone marrow biopsy, and evaluation for hepatitis B and C co-infection.
- Fluorescence in situ hybridization (FISH) is a molecular technique that identifies portions of DNA and helps to identify translocations and rearrangements.
- cMYC, BCL2, and BCL6 are all pro-proliferative genes and commonly implicated in lymphoma.
- cMYC rearrangement poses higher risk of CNS involvement and CNS relapse.
- cMYC rearrangement (as opposed to cMYC translocation) requires therapy that is more aggressive therapy than R-CHOP.
Treatment of high grade diffuse large B-cell lymphoma:
- R-EPOCH
- Ibrutinib plus R-EPOCH
Resources:AIDS Malignancy ConsortiumBlood. 2004;103:275-82.
Blood. 2010 Apr 15; 115(15): 3008–16.
NCT03220022: Ibrutinib, Rituximab, Etoposide, Prednisone, Vincristine Sulfate, Cyclophosphamide, and Doxorubicin Hydrochloride in Treating Patients With HIV-Positive Stage II-IV Diffuse Large B-Cell Lymphomas
In this episode, Stefan Barta, MD, of the University of Pennsylvania, joins David Henry, MD, to discuss the treatment and diagnosis of lymphoma in patients with HIV.
And in this week’s Clinical Correlation, Ilana Yurkiewicz, MD, has Part 2 of her discussion on informed consent in cancer. Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University and is also a columnist for Hematology News. More from Dr. Yurkiewicz here.
Subscribe to Blood & Cancer here:
Apple Podcasts
Google Podcasts
Show notes
By Emily Bryer, DO
Resident in the department of internal medicine, University of Pennsylvania Health System
Immunosuppression in patients with HIV, especially with low CD4 counts, is associated with the development of lymphomas.
Diffuse large B-cell lymphoma is the most common lymphoma in patients with HIV followed by Burkitt lymphoma and Hodgkin lymphoma.
Extra-nodal manifestations of lymphoma are more common in patients with HIV, especially with lower CD4 counts.
Following pathologic diagnosis, staging of lymphoma should include:
- CT scan, PET scan, evaluation of CNS (MRI brain and LP), bone marrow biopsy, and evaluation for hepatitis B and C co-infection.
- Fluorescence in situ hybridization (FISH) is a molecular technique that identifies portions of DNA and helps to identify translocations and rearrangements.
- cMYC, BCL2, and BCL6 are all pro-proliferative genes and commonly implicated in lymphoma.
- cMYC rearrangement poses higher risk of CNS involvement and CNS relapse.
- cMYC rearrangement (as opposed to cMYC translocation) requires therapy that is more aggressive therapy than R-CHOP.
Treatment of high grade diffuse large B-cell lymphoma:
- R-EPOCH
- Ibrutinib plus R-EPOCH
Resources:AIDS Malignancy ConsortiumBlood. 2004;103:275-82.
Blood. 2010 Apr 15; 115(15): 3008–16.
NCT03220022: Ibrutinib, Rituximab, Etoposide, Prednisone, Vincristine Sulfate, Cyclophosphamide, and Doxorubicin Hydrochloride in Treating Patients With HIV-Positive Stage II-IV Diffuse Large B-Cell Lymphomas
ICYMI: Noninferior tuberculosis prevention in HIV has shorter duration
The primary endpoint – first case of tuberculosis or death from tuberculosis or unknown cause among patients with HIV – was reported in 2% of both arms in the open-label, phase 3, noninferiority trial BRIEF TB (NCT01404312).
In the New England Journal of Medicine (2019 Mar 14;380[11]:1001-11), 3,000 patients with HIV were randomized to receive either 1 month of rifapentine/isoniazid or 9 months of isoniazid monotherapy for prevention of tuberculosis. Although safety was also similar between arms, the completion rate was significantly higher in the combination treatment arm, compared with the monotherapy arm (97% vs. 90%; P less than .001).
We covered this story at the annual Conference on Retroviruses & Opportunistic Infections. See our coverage at the link below.
The primary endpoint – first case of tuberculosis or death from tuberculosis or unknown cause among patients with HIV – was reported in 2% of both arms in the open-label, phase 3, noninferiority trial BRIEF TB (NCT01404312).
In the New England Journal of Medicine (2019 Mar 14;380[11]:1001-11), 3,000 patients with HIV were randomized to receive either 1 month of rifapentine/isoniazid or 9 months of isoniazid monotherapy for prevention of tuberculosis. Although safety was also similar between arms, the completion rate was significantly higher in the combination treatment arm, compared with the monotherapy arm (97% vs. 90%; P less than .001).
We covered this story at the annual Conference on Retroviruses & Opportunistic Infections. See our coverage at the link below.
The primary endpoint – first case of tuberculosis or death from tuberculosis or unknown cause among patients with HIV – was reported in 2% of both arms in the open-label, phase 3, noninferiority trial BRIEF TB (NCT01404312).
In the New England Journal of Medicine (2019 Mar 14;380[11]:1001-11), 3,000 patients with HIV were randomized to receive either 1 month of rifapentine/isoniazid or 9 months of isoniazid monotherapy for prevention of tuberculosis. Although safety was also similar between arms, the completion rate was significantly higher in the combination treatment arm, compared with the monotherapy arm (97% vs. 90%; P less than .001).
We covered this story at the annual Conference on Retroviruses & Opportunistic Infections. See our coverage at the link below.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Bempedoic acid safely dropped LDL, now seeks FDA approval
Based on its performance, bempedoic acid seems on track for agency approval.
Aside from demonstrating safety, the efficacy goal of the CLEAR Harmony (Evaluation of Long-Term Safety and Tolerability of ETC-1002 in High-Risk Patients With Hyperlipidemia and High CV Risk) trial was to cut LDL cholesterol as an add-on to maximally tolerated statin treatment, as well as discretionary use of other lipid-lowering agents in patients with atherosclerotic cardiovascular disease.
That goal appeared to be met by the CLEAR Harmony results, as well as the results from four other studies that contributed data to the approval application filed for bempedoic acid. A clinical outcomes study is underway with more than 12,000 patients aimed at showing incremental clinical benefit from bempedoic acid on top of approved treatments for lowering LDL cholesterol, but those results are not expected until 2022. Until then, the application’s data focus on the evidence that the new drug safely lowers LDL cholesterol when used on top of existing treatments.
“Bempedoic acid provides an additional therapeutic option to safely lower LDL cholesterol in patients with high risk for atherosclerotic cardiovascular disease already treated with a statin,” Kausik K. Ray, MD, said when he first reported these findings during the annual meeting of the European Society of Cardiology last August in Munich. The same results appeared in a newly released article (N Engl J Med. 2019 Mar 14;380[11]:1022-32).
Maximum recommended statin dosages exist because, for every doubling of the statin dosage, LDL cholesterol levels drop by about another 6%, but adverse events grow more common. Because bempedoic acid and other ATP citrate lyase inhibitors work via the same metabolic pathway as that of statins – HMG CoA reductase inhibitors – “we did not know whether squeezing another 20% of LDL cholesterol lowering would be tolerated. What we have clearly and reassuringly shown is it is well tolerated,” said Dr. Ray, a cardiologist and professor of public health at Imperial College, London.
The CLEAR Harmony results showed in 2,230 randomized patients that treatment with bempedoic acid cut LDL cholesterol levels by an average of 18% more compared with placebo in the intention-to-treat analysis, and by 20% in an on-treatment analysis.
By seeking U.S. marketing approval for bempedoic acid based on LDL-lowering and safety only, but without data on clinical endpoints, the company developing the drug is following a path already established by several other lipid-lowering drug classes.
“Statins, ezetimibe and the PCSK9 inhibitors were all approved based on clinical trial results that showed LDL-cholesterol reductions with what the FDA judged to be acceptable safety, but without results from completed outcomes trials,” Christie M. Ballantyne, MD, a coauthor of the CLEAR Harmony study and professor and chief of cardiology at Baylor College of Medicine in Houston, said in an interview.
Stephen Nicholls, MD, professor of cardiology at Monash University in Melbourne, added in an interview that “the precedent has been to conditionally approve LDL cholesterol–lowering drugs at this stage of development, based on the large body of evidence demonstrating that LDL cholesterol–lowering consistently produces clinical benefit.”
To gain more confidence that treatment with bempedoic acid exerts a benefit to patients prior to availability of clinical-outcome results, the developing company sponsored a genetic and epidemiologic study that looked at the association between naturally occurring mutations that dampen the activity of ATP citrate lyase, an enzyme that metabolically sits just upstream from HMG CoA reductase, the enzyme that statins target. Dr. Nicholls, Dr. Ray, and several other collaborators devised “genetic scores” that took into account variants of the gene that codes for ATP citrate lyase, and a separate score for HMG CoA reductase variants. Each score estimated a person’s activity for each of these enzymes. The researchers then used the scores to classify enzymatic activity in more than 650,000 people in several different databases, including more than 100,000 people with a history of major cardiovascular events.
The results showed that “genetic variants that mimic the effect of ATP citrate lyase inhibitors and statins appeared to lower plasma LDL cholesterol levels by the same mechanism of action. They were associated with nearly identical effects on the risk of cardiovascular disease and cancer per unit decrease in the LDL cholesterol level,” the investigators said in an article published concurrently with the CLEAR Harmony results (N Engl J Med. 2019 March 14;380[11]:1033-42).
This finding “strengthens the likelihood that an outcome trial will show benefits,” Steven E. Nissen, MD, chairman of cardiovascular medicine at the Cleveland Clinic Foundation, and a lead investigator in the bempedoic acid outcomes trial, CLEAR Outcomes, said in an interview.
Dr. Nicholls noted that, on the basis of the genetic study’s results “it would seem very reasonable to extrapolate and expect that bempedoic acid should reduce cardiovascular events.”
The data reported by Dr. Ray and his associates from the CLEAR Harmony trial showed that overall, the adverse-event rates associated with bempedoic acid treatment were similar to the rates seen in patients who received placebo. But Michael V. Holmes, MD, who wrote an editorial that accompanied both reports, highlighted a few differences in the adverse event rates that concerned him. These were a statistically significant higher rate of gout and blood uric acid levels on bempedoic acid treatment compared with controls, a significantly higher rate of discontinuations because of adverse events, and nominally higher rates of death, cardiovascular death, and heart failure hospitalization, said Dr. Holmes, an epidemiologist at the University of Oxford, England.
But Dr. Nissen said that “the safety ‘signals’ involve very small numbers of patients and do not raise major concerns. The increases in gout and uric acid are worth noting, but are less weighty than the disease the drug is intended to treat: major cardiovascular events.”
Dr. Nichols added that “the large cardiovascular outcome trial will provide the opportunity to further study safety and potential side effects. I’m hesitant to overinterpret event data from a relatively small clinical trial that primarily focused on the lipid effects.”
Despite recent success of the PCSK9 inhibitors as an add-on or substitute for statin treatment of elevated LDL cholesterol, the cost of the drugs in this class has made the cardiovascular disease community eager for another alternative to statins.
“The PCSK9 inhibitors are extremely effective, but uptake has been slow due to their cost and formulary restrictions,” said Dr. Ballantyne. “Many high-risk patients continue to have persistently elevated levels of LDL cholesterol.” Bempedoic acid is “a new therapy that can help people who continue to have elevations of LDL cholesterol despite current therapies.”
CLEAR Harmony and the genetic study were sponsored by Esperion, the company developing bempedoic acid. Dr. Ray has been a consultant to several drug companies but had no other financial relationship to Esperion. Dr. Ballantyne and Dr. Nicholls have been consultants to and have received research funding from Esperion and from several other companies. Dr. Nissen is leading an Esperion-sponsored study but has no financial relationships with the company or with other companies.
SOURCEs: N Engl J Med. 2019 Mar 14;380[11]:1022-32 and 1033-42.
The primary endpoint of bempedoic acid’s safety, assessed by means of the incidence of adverse events and changes in safety laboratory variables during the CLEAR Harmony trial, did not differ meaningfully between the bempedoic acid group and the placebo group. However, patients in the bempedoic acid group had an excess risk of adverse events leading to discontinuation of the blinded trial regimen, an excess risk of gout, and higher blood concentrations of uric acid than those in the placebo group.
In further analyses of nonprimary and secondary endpoints, there were additional potentially troubling signals, although the 95% confidence intervals were wide: 0.9% of the patients treated with bempedoic acid died, compared with 0.3% of those in the placebo group; 0.4% and 0.1%, respectively, died from adjudicated cardiovascular disease; and 0.6% and 0.1%, respectively, were hospitalized for heart failure. Although the findings are potentially alarming, the imprecision that is reflected in the 95% confidence intervals renders the relative risk values virtually meaningless, but can these effect estimates be unseen?
The cumulative data from randomized treatment trials and from studies of human genetics say that, for a given reduction in the LDL cholesterol level, drugs that inhibit ATP citrate lyase and do not have off-target effects ought to yield reductions in the risk of cardiovascular disease that are similar to those achieved with statins. However, analysis of endpoints in treatment trials for which there is marginal statistical power is best avoided. Fortunately for bempedoic acid, an ongoing phase 3, cardiovascular outcome trial will provide additional information.
More broadly, the genetic characterization of drug targets is set to revolutionize how we develop medicines.
Michael V. Holmes, MD, is an epidemiologist at the University of Oxford, England. He made these comments in a published editorial (N Engl J Med. 2019 Mar 14;380[11]:1076-9). He had no disclosures.
The primary endpoint of bempedoic acid’s safety, assessed by means of the incidence of adverse events and changes in safety laboratory variables during the CLEAR Harmony trial, did not differ meaningfully between the bempedoic acid group and the placebo group. However, patients in the bempedoic acid group had an excess risk of adverse events leading to discontinuation of the blinded trial regimen, an excess risk of gout, and higher blood concentrations of uric acid than those in the placebo group.
In further analyses of nonprimary and secondary endpoints, there were additional potentially troubling signals, although the 95% confidence intervals were wide: 0.9% of the patients treated with bempedoic acid died, compared with 0.3% of those in the placebo group; 0.4% and 0.1%, respectively, died from adjudicated cardiovascular disease; and 0.6% and 0.1%, respectively, were hospitalized for heart failure. Although the findings are potentially alarming, the imprecision that is reflected in the 95% confidence intervals renders the relative risk values virtually meaningless, but can these effect estimates be unseen?
The cumulative data from randomized treatment trials and from studies of human genetics say that, for a given reduction in the LDL cholesterol level, drugs that inhibit ATP citrate lyase and do not have off-target effects ought to yield reductions in the risk of cardiovascular disease that are similar to those achieved with statins. However, analysis of endpoints in treatment trials for which there is marginal statistical power is best avoided. Fortunately for bempedoic acid, an ongoing phase 3, cardiovascular outcome trial will provide additional information.
More broadly, the genetic characterization of drug targets is set to revolutionize how we develop medicines.
Michael V. Holmes, MD, is an epidemiologist at the University of Oxford, England. He made these comments in a published editorial (N Engl J Med. 2019 Mar 14;380[11]:1076-9). He had no disclosures.
The primary endpoint of bempedoic acid’s safety, assessed by means of the incidence of adverse events and changes in safety laboratory variables during the CLEAR Harmony trial, did not differ meaningfully between the bempedoic acid group and the placebo group. However, patients in the bempedoic acid group had an excess risk of adverse events leading to discontinuation of the blinded trial regimen, an excess risk of gout, and higher blood concentrations of uric acid than those in the placebo group.
In further analyses of nonprimary and secondary endpoints, there were additional potentially troubling signals, although the 95% confidence intervals were wide: 0.9% of the patients treated with bempedoic acid died, compared with 0.3% of those in the placebo group; 0.4% and 0.1%, respectively, died from adjudicated cardiovascular disease; and 0.6% and 0.1%, respectively, were hospitalized for heart failure. Although the findings are potentially alarming, the imprecision that is reflected in the 95% confidence intervals renders the relative risk values virtually meaningless, but can these effect estimates be unseen?
The cumulative data from randomized treatment trials and from studies of human genetics say that, for a given reduction in the LDL cholesterol level, drugs that inhibit ATP citrate lyase and do not have off-target effects ought to yield reductions in the risk of cardiovascular disease that are similar to those achieved with statins. However, analysis of endpoints in treatment trials for which there is marginal statistical power is best avoided. Fortunately for bempedoic acid, an ongoing phase 3, cardiovascular outcome trial will provide additional information.
More broadly, the genetic characterization of drug targets is set to revolutionize how we develop medicines.
Michael V. Holmes, MD, is an epidemiologist at the University of Oxford, England. He made these comments in a published editorial (N Engl J Med. 2019 Mar 14;380[11]:1076-9). He had no disclosures.
Based on its performance, bempedoic acid seems on track for agency approval.
Aside from demonstrating safety, the efficacy goal of the CLEAR Harmony (Evaluation of Long-Term Safety and Tolerability of ETC-1002 in High-Risk Patients With Hyperlipidemia and High CV Risk) trial was to cut LDL cholesterol as an add-on to maximally tolerated statin treatment, as well as discretionary use of other lipid-lowering agents in patients with atherosclerotic cardiovascular disease.
That goal appeared to be met by the CLEAR Harmony results, as well as the results from four other studies that contributed data to the approval application filed for bempedoic acid. A clinical outcomes study is underway with more than 12,000 patients aimed at showing incremental clinical benefit from bempedoic acid on top of approved treatments for lowering LDL cholesterol, but those results are not expected until 2022. Until then, the application’s data focus on the evidence that the new drug safely lowers LDL cholesterol when used on top of existing treatments.
“Bempedoic acid provides an additional therapeutic option to safely lower LDL cholesterol in patients with high risk for atherosclerotic cardiovascular disease already treated with a statin,” Kausik K. Ray, MD, said when he first reported these findings during the annual meeting of the European Society of Cardiology last August in Munich. The same results appeared in a newly released article (N Engl J Med. 2019 Mar 14;380[11]:1022-32).
Maximum recommended statin dosages exist because, for every doubling of the statin dosage, LDL cholesterol levels drop by about another 6%, but adverse events grow more common. Because bempedoic acid and other ATP citrate lyase inhibitors work via the same metabolic pathway as that of statins – HMG CoA reductase inhibitors – “we did not know whether squeezing another 20% of LDL cholesterol lowering would be tolerated. What we have clearly and reassuringly shown is it is well tolerated,” said Dr. Ray, a cardiologist and professor of public health at Imperial College, London.
The CLEAR Harmony results showed in 2,230 randomized patients that treatment with bempedoic acid cut LDL cholesterol levels by an average of 18% more compared with placebo in the intention-to-treat analysis, and by 20% in an on-treatment analysis.
By seeking U.S. marketing approval for bempedoic acid based on LDL-lowering and safety only, but without data on clinical endpoints, the company developing the drug is following a path already established by several other lipid-lowering drug classes.
“Statins, ezetimibe and the PCSK9 inhibitors were all approved based on clinical trial results that showed LDL-cholesterol reductions with what the FDA judged to be acceptable safety, but without results from completed outcomes trials,” Christie M. Ballantyne, MD, a coauthor of the CLEAR Harmony study and professor and chief of cardiology at Baylor College of Medicine in Houston, said in an interview.
Stephen Nicholls, MD, professor of cardiology at Monash University in Melbourne, added in an interview that “the precedent has been to conditionally approve LDL cholesterol–lowering drugs at this stage of development, based on the large body of evidence demonstrating that LDL cholesterol–lowering consistently produces clinical benefit.”
To gain more confidence that treatment with bempedoic acid exerts a benefit to patients prior to availability of clinical-outcome results, the developing company sponsored a genetic and epidemiologic study that looked at the association between naturally occurring mutations that dampen the activity of ATP citrate lyase, an enzyme that metabolically sits just upstream from HMG CoA reductase, the enzyme that statins target. Dr. Nicholls, Dr. Ray, and several other collaborators devised “genetic scores” that took into account variants of the gene that codes for ATP citrate lyase, and a separate score for HMG CoA reductase variants. Each score estimated a person’s activity for each of these enzymes. The researchers then used the scores to classify enzymatic activity in more than 650,000 people in several different databases, including more than 100,000 people with a history of major cardiovascular events.
The results showed that “genetic variants that mimic the effect of ATP citrate lyase inhibitors and statins appeared to lower plasma LDL cholesterol levels by the same mechanism of action. They were associated with nearly identical effects on the risk of cardiovascular disease and cancer per unit decrease in the LDL cholesterol level,” the investigators said in an article published concurrently with the CLEAR Harmony results (N Engl J Med. 2019 March 14;380[11]:1033-42).
This finding “strengthens the likelihood that an outcome trial will show benefits,” Steven E. Nissen, MD, chairman of cardiovascular medicine at the Cleveland Clinic Foundation, and a lead investigator in the bempedoic acid outcomes trial, CLEAR Outcomes, said in an interview.
Dr. Nicholls noted that, on the basis of the genetic study’s results “it would seem very reasonable to extrapolate and expect that bempedoic acid should reduce cardiovascular events.”
The data reported by Dr. Ray and his associates from the CLEAR Harmony trial showed that overall, the adverse-event rates associated with bempedoic acid treatment were similar to the rates seen in patients who received placebo. But Michael V. Holmes, MD, who wrote an editorial that accompanied both reports, highlighted a few differences in the adverse event rates that concerned him. These were a statistically significant higher rate of gout and blood uric acid levels on bempedoic acid treatment compared with controls, a significantly higher rate of discontinuations because of adverse events, and nominally higher rates of death, cardiovascular death, and heart failure hospitalization, said Dr. Holmes, an epidemiologist at the University of Oxford, England.
But Dr. Nissen said that “the safety ‘signals’ involve very small numbers of patients and do not raise major concerns. The increases in gout and uric acid are worth noting, but are less weighty than the disease the drug is intended to treat: major cardiovascular events.”
Dr. Nichols added that “the large cardiovascular outcome trial will provide the opportunity to further study safety and potential side effects. I’m hesitant to overinterpret event data from a relatively small clinical trial that primarily focused on the lipid effects.”
Despite recent success of the PCSK9 inhibitors as an add-on or substitute for statin treatment of elevated LDL cholesterol, the cost of the drugs in this class has made the cardiovascular disease community eager for another alternative to statins.
“The PCSK9 inhibitors are extremely effective, but uptake has been slow due to their cost and formulary restrictions,” said Dr. Ballantyne. “Many high-risk patients continue to have persistently elevated levels of LDL cholesterol.” Bempedoic acid is “a new therapy that can help people who continue to have elevations of LDL cholesterol despite current therapies.”
CLEAR Harmony and the genetic study were sponsored by Esperion, the company developing bempedoic acid. Dr. Ray has been a consultant to several drug companies but had no other financial relationship to Esperion. Dr. Ballantyne and Dr. Nicholls have been consultants to and have received research funding from Esperion and from several other companies. Dr. Nissen is leading an Esperion-sponsored study but has no financial relationships with the company or with other companies.
SOURCEs: N Engl J Med. 2019 Mar 14;380[11]:1022-32 and 1033-42.
Based on its performance, bempedoic acid seems on track for agency approval.
Aside from demonstrating safety, the efficacy goal of the CLEAR Harmony (Evaluation of Long-Term Safety and Tolerability of ETC-1002 in High-Risk Patients With Hyperlipidemia and High CV Risk) trial was to cut LDL cholesterol as an add-on to maximally tolerated statin treatment, as well as discretionary use of other lipid-lowering agents in patients with atherosclerotic cardiovascular disease.
That goal appeared to be met by the CLEAR Harmony results, as well as the results from four other studies that contributed data to the approval application filed for bempedoic acid. A clinical outcomes study is underway with more than 12,000 patients aimed at showing incremental clinical benefit from bempedoic acid on top of approved treatments for lowering LDL cholesterol, but those results are not expected until 2022. Until then, the application’s data focus on the evidence that the new drug safely lowers LDL cholesterol when used on top of existing treatments.
“Bempedoic acid provides an additional therapeutic option to safely lower LDL cholesterol in patients with high risk for atherosclerotic cardiovascular disease already treated with a statin,” Kausik K. Ray, MD, said when he first reported these findings during the annual meeting of the European Society of Cardiology last August in Munich. The same results appeared in a newly released article (N Engl J Med. 2019 Mar 14;380[11]:1022-32).
Maximum recommended statin dosages exist because, for every doubling of the statin dosage, LDL cholesterol levels drop by about another 6%, but adverse events grow more common. Because bempedoic acid and other ATP citrate lyase inhibitors work via the same metabolic pathway as that of statins – HMG CoA reductase inhibitors – “we did not know whether squeezing another 20% of LDL cholesterol lowering would be tolerated. What we have clearly and reassuringly shown is it is well tolerated,” said Dr. Ray, a cardiologist and professor of public health at Imperial College, London.
The CLEAR Harmony results showed in 2,230 randomized patients that treatment with bempedoic acid cut LDL cholesterol levels by an average of 18% more compared with placebo in the intention-to-treat analysis, and by 20% in an on-treatment analysis.
By seeking U.S. marketing approval for bempedoic acid based on LDL-lowering and safety only, but without data on clinical endpoints, the company developing the drug is following a path already established by several other lipid-lowering drug classes.
“Statins, ezetimibe and the PCSK9 inhibitors were all approved based on clinical trial results that showed LDL-cholesterol reductions with what the FDA judged to be acceptable safety, but without results from completed outcomes trials,” Christie M. Ballantyne, MD, a coauthor of the CLEAR Harmony study and professor and chief of cardiology at Baylor College of Medicine in Houston, said in an interview.
Stephen Nicholls, MD, professor of cardiology at Monash University in Melbourne, added in an interview that “the precedent has been to conditionally approve LDL cholesterol–lowering drugs at this stage of development, based on the large body of evidence demonstrating that LDL cholesterol–lowering consistently produces clinical benefit.”
To gain more confidence that treatment with bempedoic acid exerts a benefit to patients prior to availability of clinical-outcome results, the developing company sponsored a genetic and epidemiologic study that looked at the association between naturally occurring mutations that dampen the activity of ATP citrate lyase, an enzyme that metabolically sits just upstream from HMG CoA reductase, the enzyme that statins target. Dr. Nicholls, Dr. Ray, and several other collaborators devised “genetic scores” that took into account variants of the gene that codes for ATP citrate lyase, and a separate score for HMG CoA reductase variants. Each score estimated a person’s activity for each of these enzymes. The researchers then used the scores to classify enzymatic activity in more than 650,000 people in several different databases, including more than 100,000 people with a history of major cardiovascular events.
The results showed that “genetic variants that mimic the effect of ATP citrate lyase inhibitors and statins appeared to lower plasma LDL cholesterol levels by the same mechanism of action. They were associated with nearly identical effects on the risk of cardiovascular disease and cancer per unit decrease in the LDL cholesterol level,” the investigators said in an article published concurrently with the CLEAR Harmony results (N Engl J Med. 2019 March 14;380[11]:1033-42).
This finding “strengthens the likelihood that an outcome trial will show benefits,” Steven E. Nissen, MD, chairman of cardiovascular medicine at the Cleveland Clinic Foundation, and a lead investigator in the bempedoic acid outcomes trial, CLEAR Outcomes, said in an interview.
Dr. Nicholls noted that, on the basis of the genetic study’s results “it would seem very reasonable to extrapolate and expect that bempedoic acid should reduce cardiovascular events.”
The data reported by Dr. Ray and his associates from the CLEAR Harmony trial showed that overall, the adverse-event rates associated with bempedoic acid treatment were similar to the rates seen in patients who received placebo. But Michael V. Holmes, MD, who wrote an editorial that accompanied both reports, highlighted a few differences in the adverse event rates that concerned him. These were a statistically significant higher rate of gout and blood uric acid levels on bempedoic acid treatment compared with controls, a significantly higher rate of discontinuations because of adverse events, and nominally higher rates of death, cardiovascular death, and heart failure hospitalization, said Dr. Holmes, an epidemiologist at the University of Oxford, England.
But Dr. Nissen said that “the safety ‘signals’ involve very small numbers of patients and do not raise major concerns. The increases in gout and uric acid are worth noting, but are less weighty than the disease the drug is intended to treat: major cardiovascular events.”
Dr. Nichols added that “the large cardiovascular outcome trial will provide the opportunity to further study safety and potential side effects. I’m hesitant to overinterpret event data from a relatively small clinical trial that primarily focused on the lipid effects.”
Despite recent success of the PCSK9 inhibitors as an add-on or substitute for statin treatment of elevated LDL cholesterol, the cost of the drugs in this class has made the cardiovascular disease community eager for another alternative to statins.
“The PCSK9 inhibitors are extremely effective, but uptake has been slow due to their cost and formulary restrictions,” said Dr. Ballantyne. “Many high-risk patients continue to have persistently elevated levels of LDL cholesterol.” Bempedoic acid is “a new therapy that can help people who continue to have elevations of LDL cholesterol despite current therapies.”
CLEAR Harmony and the genetic study were sponsored by Esperion, the company developing bempedoic acid. Dr. Ray has been a consultant to several drug companies but had no other financial relationship to Esperion. Dr. Ballantyne and Dr. Nicholls have been consultants to and have received research funding from Esperion and from several other companies. Dr. Nissen is leading an Esperion-sponsored study but has no financial relationships with the company or with other companies.
SOURCEs: N Engl J Med. 2019 Mar 14;380[11]:1022-32 and 1033-42.
Key clinical point: Bempedoic acid safely lowed LDL cholesterol levels in a pivotal trial.
Major finding: In the intention-to-treat analysis, bempedoic acid cut LDL cholesterol by 18%; by 20% in an on-treatment analysis.
Study details: CLEAR Harmony, a multicenter, randomized trial with 2,230 patients.
Disclosures: CLEAR Harmony and the genetic study were sponsored by Esperion, the company developing bempedoic acid. Dr. Ray has been a consultant to several drug companies but had no other financial relationship to Esperion. Dr. Ballantyne and Dr. Nicholls have been consultants to and have received research funding from Esperion and from several other companies. Dr. Nissen is leading an Esperion-sponsored study but has no financial relationships with the company or with other companies.
Sources: N Engl J Med. 2019 Mar 14;380[11]:1022-32 and 1033-42.
Endovascular device sustains blood pressure control after 3 years
WASHINGTON – As a result of remarkably sustained antihypertensive effect, interest is intensifying in the potential for a pivotal trial to associate a novel endovascular device with unprecedented blood pressure control in patients with treatment-resistant hypertension, according to an update presented at CRT 2019, sponsored by MedStar Heart & Vascular Institute.
With up to 3 years of follow-up, “systolic blood pressures have remained persistently reduced by as much as 24 mm Hg,” reported John P. Reilly, MD, an interventional cardiologist in Southampton, N.Y., who presented follow-up data for some of those enrolled in the first-in-human study of this device.
When the stent-like device is placed in the carotid artery, it alters its geometric shape, which increases pulsatile wall strain. The increase on wall strain alters an afferent signaling loop controlled by carotid baroreceptors that inhibits sympathetic outflow to lower blood pressure.
In the proof-of-principle, first-in-human CALM study, 47 patients were implanted with the device (MobiusHD, Vascular Dynamics). The initial study enrolled 30 subjects in Europe and 17 in the United States. Initial findings in the cohort of European patients, which included a mean 21–mm Hg reduction in systolic blood pressure and a 12–mm Hg reduction in diastolic blood pressure measured by ambulatory monitoring at 6 months, were published in the Lancet (2017 Dec 16;390[10113]:2655-661).
The patients enrolled in the proof-of-principle CALM trial were required to have highly-treatment-resistant hypertension, defined as a systolic blood pressure greater than or equal to 160 mm Hg despite at least three antihypertensive medications. The average number of medications was 4.4, according to Dr. Reilly. The mean blood pressure at entry was 165/98 mm Hg. Nearly 20% had previously undergone renal denervation.
The device was successfully deployed in all of the patients who participated in the open-label CALM study. Most of the 10 serious adverse events were related to hypotension, according to Dr. Reilly. Others included a wound infection and a case of intermittent claudication. Two instances of neurologic complaints, such as numbness and weakness, experienced within a day of device placement were considered potential transient ischemic attacks, but these resolved completely and no defects were observed on imaging.
In an update on CALM, Dr. Reilly reported that the large reductions in blood pressure previously reported at 6 months have been sustained. Follow-up is approximately 3 years in most patients, and the reductions previously reported have persisted in responders. When a clinically significant response is defined as a 10–mm Hg or more reduction in office blood pressure or 5–mm Hg or more reduction in ambulatory blood pressure, 75% of patients enrolled are still responding, but the more important point is that there has been no substantial reduction in blood pressure control over time in responders, according to Dr. Reilly.
When patients were stratified by a pulse pressure of greater or less than 70 mm Hg at study entry, response rates have been similar, he added.
The long-term responses are significant because there was concern about tachyphylaxis. In fact, coronary stents also produce a reduction in blood pressure immediately after placement that is likely caused by the same effect, but that effect “peters out in a day or 2,” noted Dr. Reilly. As opposed to the round shape of coronary stents, the rectangular shape of the novel device produces “an increase in the perceived strain on the carotid body” that does not appear to diminish over time.
CALM-2, which is designed to be a pivotal trial to support regulatory approval of the device, began enrolling in September 2018. An enrollment of 300 patients with treatment-resistant hypertension is planned. Participants will be randomized to receive the device or a sham procedure consisting of a carotid artery angiogram, according to Dr. Reilly. Although the initial CALM trial was small, open label, and conducted without a control, the persistent benefit over extended follow-up is driving excitement about the potential of this device.
“These are some of the greatest sustained reductions in ambulatory blood pressure we have ever seen,” according to Vasilios Papademetriou, MD, PhD, a professor of medicine at Georgetown University, Washington. Impressed by undiminished blood pressure control observed so far, he characterized the promise of this device as “very compelling.”
Dr. Reilly disclosed that he was a stockholder in Johnson & Johnson.
WASHINGTON – As a result of remarkably sustained antihypertensive effect, interest is intensifying in the potential for a pivotal trial to associate a novel endovascular device with unprecedented blood pressure control in patients with treatment-resistant hypertension, according to an update presented at CRT 2019, sponsored by MedStar Heart & Vascular Institute.
With up to 3 years of follow-up, “systolic blood pressures have remained persistently reduced by as much as 24 mm Hg,” reported John P. Reilly, MD, an interventional cardiologist in Southampton, N.Y., who presented follow-up data for some of those enrolled in the first-in-human study of this device.
When the stent-like device is placed in the carotid artery, it alters its geometric shape, which increases pulsatile wall strain. The increase on wall strain alters an afferent signaling loop controlled by carotid baroreceptors that inhibits sympathetic outflow to lower blood pressure.
In the proof-of-principle, first-in-human CALM study, 47 patients were implanted with the device (MobiusHD, Vascular Dynamics). The initial study enrolled 30 subjects in Europe and 17 in the United States. Initial findings in the cohort of European patients, which included a mean 21–mm Hg reduction in systolic blood pressure and a 12–mm Hg reduction in diastolic blood pressure measured by ambulatory monitoring at 6 months, were published in the Lancet (2017 Dec 16;390[10113]:2655-661).
The patients enrolled in the proof-of-principle CALM trial were required to have highly-treatment-resistant hypertension, defined as a systolic blood pressure greater than or equal to 160 mm Hg despite at least three antihypertensive medications. The average number of medications was 4.4, according to Dr. Reilly. The mean blood pressure at entry was 165/98 mm Hg. Nearly 20% had previously undergone renal denervation.
The device was successfully deployed in all of the patients who participated in the open-label CALM study. Most of the 10 serious adverse events were related to hypotension, according to Dr. Reilly. Others included a wound infection and a case of intermittent claudication. Two instances of neurologic complaints, such as numbness and weakness, experienced within a day of device placement were considered potential transient ischemic attacks, but these resolved completely and no defects were observed on imaging.
In an update on CALM, Dr. Reilly reported that the large reductions in blood pressure previously reported at 6 months have been sustained. Follow-up is approximately 3 years in most patients, and the reductions previously reported have persisted in responders. When a clinically significant response is defined as a 10–mm Hg or more reduction in office blood pressure or 5–mm Hg or more reduction in ambulatory blood pressure, 75% of patients enrolled are still responding, but the more important point is that there has been no substantial reduction in blood pressure control over time in responders, according to Dr. Reilly.
When patients were stratified by a pulse pressure of greater or less than 70 mm Hg at study entry, response rates have been similar, he added.
The long-term responses are significant because there was concern about tachyphylaxis. In fact, coronary stents also produce a reduction in blood pressure immediately after placement that is likely caused by the same effect, but that effect “peters out in a day or 2,” noted Dr. Reilly. As opposed to the round shape of coronary stents, the rectangular shape of the novel device produces “an increase in the perceived strain on the carotid body” that does not appear to diminish over time.
CALM-2, which is designed to be a pivotal trial to support regulatory approval of the device, began enrolling in September 2018. An enrollment of 300 patients with treatment-resistant hypertension is planned. Participants will be randomized to receive the device or a sham procedure consisting of a carotid artery angiogram, according to Dr. Reilly. Although the initial CALM trial was small, open label, and conducted without a control, the persistent benefit over extended follow-up is driving excitement about the potential of this device.
“These are some of the greatest sustained reductions in ambulatory blood pressure we have ever seen,” according to Vasilios Papademetriou, MD, PhD, a professor of medicine at Georgetown University, Washington. Impressed by undiminished blood pressure control observed so far, he characterized the promise of this device as “very compelling.”
Dr. Reilly disclosed that he was a stockholder in Johnson & Johnson.
WASHINGTON – As a result of remarkably sustained antihypertensive effect, interest is intensifying in the potential for a pivotal trial to associate a novel endovascular device with unprecedented blood pressure control in patients with treatment-resistant hypertension, according to an update presented at CRT 2019, sponsored by MedStar Heart & Vascular Institute.
With up to 3 years of follow-up, “systolic blood pressures have remained persistently reduced by as much as 24 mm Hg,” reported John P. Reilly, MD, an interventional cardiologist in Southampton, N.Y., who presented follow-up data for some of those enrolled in the first-in-human study of this device.
When the stent-like device is placed in the carotid artery, it alters its geometric shape, which increases pulsatile wall strain. The increase on wall strain alters an afferent signaling loop controlled by carotid baroreceptors that inhibits sympathetic outflow to lower blood pressure.
In the proof-of-principle, first-in-human CALM study, 47 patients were implanted with the device (MobiusHD, Vascular Dynamics). The initial study enrolled 30 subjects in Europe and 17 in the United States. Initial findings in the cohort of European patients, which included a mean 21–mm Hg reduction in systolic blood pressure and a 12–mm Hg reduction in diastolic blood pressure measured by ambulatory monitoring at 6 months, were published in the Lancet (2017 Dec 16;390[10113]:2655-661).
The patients enrolled in the proof-of-principle CALM trial were required to have highly-treatment-resistant hypertension, defined as a systolic blood pressure greater than or equal to 160 mm Hg despite at least three antihypertensive medications. The average number of medications was 4.4, according to Dr. Reilly. The mean blood pressure at entry was 165/98 mm Hg. Nearly 20% had previously undergone renal denervation.
The device was successfully deployed in all of the patients who participated in the open-label CALM study. Most of the 10 serious adverse events were related to hypotension, according to Dr. Reilly. Others included a wound infection and a case of intermittent claudication. Two instances of neurologic complaints, such as numbness and weakness, experienced within a day of device placement were considered potential transient ischemic attacks, but these resolved completely and no defects were observed on imaging.
In an update on CALM, Dr. Reilly reported that the large reductions in blood pressure previously reported at 6 months have been sustained. Follow-up is approximately 3 years in most patients, and the reductions previously reported have persisted in responders. When a clinically significant response is defined as a 10–mm Hg or more reduction in office blood pressure or 5–mm Hg or more reduction in ambulatory blood pressure, 75% of patients enrolled are still responding, but the more important point is that there has been no substantial reduction in blood pressure control over time in responders, according to Dr. Reilly.
When patients were stratified by a pulse pressure of greater or less than 70 mm Hg at study entry, response rates have been similar, he added.
The long-term responses are significant because there was concern about tachyphylaxis. In fact, coronary stents also produce a reduction in blood pressure immediately after placement that is likely caused by the same effect, but that effect “peters out in a day or 2,” noted Dr. Reilly. As opposed to the round shape of coronary stents, the rectangular shape of the novel device produces “an increase in the perceived strain on the carotid body” that does not appear to diminish over time.
CALM-2, which is designed to be a pivotal trial to support regulatory approval of the device, began enrolling in September 2018. An enrollment of 300 patients with treatment-resistant hypertension is planned. Participants will be randomized to receive the device or a sham procedure consisting of a carotid artery angiogram, according to Dr. Reilly. Although the initial CALM trial was small, open label, and conducted without a control, the persistent benefit over extended follow-up is driving excitement about the potential of this device.
“These are some of the greatest sustained reductions in ambulatory blood pressure we have ever seen,” according to Vasilios Papademetriou, MD, PhD, a professor of medicine at Georgetown University, Washington. Impressed by undiminished blood pressure control observed so far, he characterized the promise of this device as “very compelling.”
Dr. Reilly disclosed that he was a stockholder in Johnson & Johnson.
REPORTING FROM CRT 2019
Weekly turoctocog alfa pegol is feasible for hemophilia A
FROM HAEMOPHILIA
Some patients with severe hemophilia A can be managed with weekly injections of the recombinant factor VIII (rFVIII) product turoctocog alfa pegol (N8-GP), based on results from an extension of the pathfinder 2 trial.
In patients who had two or fewer bleeding episodes during the preceding 6 months, N8-GP was effective for prophylaxis and treating bleeding episodes without causing inhibitor development, reported lead author Nicola Curry, MD, of the Oxford (England) Haemophilia and Thrombosis Centre at Churchill Hospital and her colleagues.
The trial exemplifies an ongoing effort to reduce frequency of prophylaxis in hemophilia A.
N8-GP, given every 4 days (or twice weekly in children), was approved by the Food and Drug Administration in February 2019; however, the investigators hypothesized that dose frequency could be stepped down in those who responded well to initial treatment.
“Many patients struggle to integrate prophylactic schedules into their daily lives, leading to missed injections and increased bleeding episodes,” the investigators wrote in a Haemophilia. “Therefore, prophylactic strategies offering effective haemostatic coverage with more convenient administration schedules are needed.”
To this end, the investigators identified 55 patients (at least 12 years old) from the pathfinder 2 trial who had two or fewer bleeding episodes during the preceding 6 months. From this group, 17 patients continued to receive 50 IU/kg of N8-GP every 4 days, as they had done during the main trial, while 38 patients received a higher dose – 75 IU/kg – every 7 days.
When necessary, N8-GP was used to treat bleeding episodes. Primary coendpoints were immunogenicity and efficacy, while all secondary endpoints were safety measures.
For both cohorts, median annualized bleeding rate (ABR) was 0.00 days, and greater than 50% of patients experienced no bleeds (53% every 4 days vs. 58% weekly). No inhibitors developed during the extension phase of the trial.
Along with strong support for weekly prophylaxis, N8-GP demonstrated efficiency in treating bleeding episodes; 87.5% of episodes were controlled with just one injection. This rate increased to 94.7% when including two or fewer injections.
During the study, 108 adverse events were reported in 65.5% of patients, although almost all were mild or moderate in severity, and unrelated to N8-GP. Five adverse events in five patients were related to treatment, including rash, headache, purpura, increased aspartate aminotransferase, and thrombocytopenia. No thromboembolic events were reported.
“These findings suggest that weekly N8-GP may provide effective prophylaxis with a reduced treatment burden for a selected subset of low-bleeding patients with severe haemophilia A,” the investigators wrote.
The study was funded by Novo Nordisk. The investigators reported financial relationships with Bayer, CSL, Novo Nordisk, Octapharma, Genentech, Shire, and others.
SOURCE: Curry N et al. Haemophilia. 2019 Feb 28. doi: 10.1111/hae.13712.
FROM HAEMOPHILIA
Some patients with severe hemophilia A can be managed with weekly injections of the recombinant factor VIII (rFVIII) product turoctocog alfa pegol (N8-GP), based on results from an extension of the pathfinder 2 trial.
In patients who had two or fewer bleeding episodes during the preceding 6 months, N8-GP was effective for prophylaxis and treating bleeding episodes without causing inhibitor development, reported lead author Nicola Curry, MD, of the Oxford (England) Haemophilia and Thrombosis Centre at Churchill Hospital and her colleagues.
The trial exemplifies an ongoing effort to reduce frequency of prophylaxis in hemophilia A.
N8-GP, given every 4 days (or twice weekly in children), was approved by the Food and Drug Administration in February 2019; however, the investigators hypothesized that dose frequency could be stepped down in those who responded well to initial treatment.
“Many patients struggle to integrate prophylactic schedules into their daily lives, leading to missed injections and increased bleeding episodes,” the investigators wrote in a Haemophilia. “Therefore, prophylactic strategies offering effective haemostatic coverage with more convenient administration schedules are needed.”
To this end, the investigators identified 55 patients (at least 12 years old) from the pathfinder 2 trial who had two or fewer bleeding episodes during the preceding 6 months. From this group, 17 patients continued to receive 50 IU/kg of N8-GP every 4 days, as they had done during the main trial, while 38 patients received a higher dose – 75 IU/kg – every 7 days.
When necessary, N8-GP was used to treat bleeding episodes. Primary coendpoints were immunogenicity and efficacy, while all secondary endpoints were safety measures.
For both cohorts, median annualized bleeding rate (ABR) was 0.00 days, and greater than 50% of patients experienced no bleeds (53% every 4 days vs. 58% weekly). No inhibitors developed during the extension phase of the trial.
Along with strong support for weekly prophylaxis, N8-GP demonstrated efficiency in treating bleeding episodes; 87.5% of episodes were controlled with just one injection. This rate increased to 94.7% when including two or fewer injections.
During the study, 108 adverse events were reported in 65.5% of patients, although almost all were mild or moderate in severity, and unrelated to N8-GP. Five adverse events in five patients were related to treatment, including rash, headache, purpura, increased aspartate aminotransferase, and thrombocytopenia. No thromboembolic events were reported.
“These findings suggest that weekly N8-GP may provide effective prophylaxis with a reduced treatment burden for a selected subset of low-bleeding patients with severe haemophilia A,” the investigators wrote.
The study was funded by Novo Nordisk. The investigators reported financial relationships with Bayer, CSL, Novo Nordisk, Octapharma, Genentech, Shire, and others.
SOURCE: Curry N et al. Haemophilia. 2019 Feb 28. doi: 10.1111/hae.13712.
FROM HAEMOPHILIA
Some patients with severe hemophilia A can be managed with weekly injections of the recombinant factor VIII (rFVIII) product turoctocog alfa pegol (N8-GP), based on results from an extension of the pathfinder 2 trial.
In patients who had two or fewer bleeding episodes during the preceding 6 months, N8-GP was effective for prophylaxis and treating bleeding episodes without causing inhibitor development, reported lead author Nicola Curry, MD, of the Oxford (England) Haemophilia and Thrombosis Centre at Churchill Hospital and her colleagues.
The trial exemplifies an ongoing effort to reduce frequency of prophylaxis in hemophilia A.
N8-GP, given every 4 days (or twice weekly in children), was approved by the Food and Drug Administration in February 2019; however, the investigators hypothesized that dose frequency could be stepped down in those who responded well to initial treatment.
“Many patients struggle to integrate prophylactic schedules into their daily lives, leading to missed injections and increased bleeding episodes,” the investigators wrote in a Haemophilia. “Therefore, prophylactic strategies offering effective haemostatic coverage with more convenient administration schedules are needed.”
To this end, the investigators identified 55 patients (at least 12 years old) from the pathfinder 2 trial who had two or fewer bleeding episodes during the preceding 6 months. From this group, 17 patients continued to receive 50 IU/kg of N8-GP every 4 days, as they had done during the main trial, while 38 patients received a higher dose – 75 IU/kg – every 7 days.
When necessary, N8-GP was used to treat bleeding episodes. Primary coendpoints were immunogenicity and efficacy, while all secondary endpoints were safety measures.
For both cohorts, median annualized bleeding rate (ABR) was 0.00 days, and greater than 50% of patients experienced no bleeds (53% every 4 days vs. 58% weekly). No inhibitors developed during the extension phase of the trial.
Along with strong support for weekly prophylaxis, N8-GP demonstrated efficiency in treating bleeding episodes; 87.5% of episodes were controlled with just one injection. This rate increased to 94.7% when including two or fewer injections.
During the study, 108 adverse events were reported in 65.5% of patients, although almost all were mild or moderate in severity, and unrelated to N8-GP. Five adverse events in five patients were related to treatment, including rash, headache, purpura, increased aspartate aminotransferase, and thrombocytopenia. No thromboembolic events were reported.
“These findings suggest that weekly N8-GP may provide effective prophylaxis with a reduced treatment burden for a selected subset of low-bleeding patients with severe haemophilia A,” the investigators wrote.
The study was funded by Novo Nordisk. The investigators reported financial relationships with Bayer, CSL, Novo Nordisk, Octapharma, Genentech, Shire, and others.
SOURCE: Curry N et al. Haemophilia. 2019 Feb 28. doi: 10.1111/hae.13712.