User login
DDNA 2019 - Heartburn: Modern Diagnosis of GERD
AT DIGESTIVE DISEASES: NEW ADVANCES
Factors Linked with Migraine in the General Populace
Raising awareness among clinicians that many of the potential variables contributing to the presence of migraine are modifiable (eg, psychological problems and lifestyle behaviors) might intensify resources dedicated to assessing and impacting these factors in order to potentially prevent the frequency and severity of migraine. This according to a recent study that aimed to identify the modifiable and non-modifiable variables that are associated with, and might moderate, the presence of migraine in the general population. Using a nationally representative cross-sectional survey, researchers evaluated responses from individuals aged 15 years and older (n=22,842). There was a secondary analysis of data from the second wave of a health interview survey conducted from 2014 to 2015. They found:
- The 1-year prevalence of migraine was 8%.
- The final multivariate model (Wald χ2=693.00, df=15) retained depression severity, chronic anxiety, exercising several times a month or week, and alcohol use as predictors of migraine (odds ratios=2.1–3.5 for positive associations, odds ratios=0.4–0.9 for negative associations).
Roy R, Sánchez-Rodriguez E, Galán S, et al. Factors associated with migraine in the general population of Spain: Results from the European Health Survey 2014. Pain Med. 2019;20(3):555-563. doi:10.1093/pm/pny093.
Raising awareness among clinicians that many of the potential variables contributing to the presence of migraine are modifiable (eg, psychological problems and lifestyle behaviors) might intensify resources dedicated to assessing and impacting these factors in order to potentially prevent the frequency and severity of migraine. This according to a recent study that aimed to identify the modifiable and non-modifiable variables that are associated with, and might moderate, the presence of migraine in the general population. Using a nationally representative cross-sectional survey, researchers evaluated responses from individuals aged 15 years and older (n=22,842). There was a secondary analysis of data from the second wave of a health interview survey conducted from 2014 to 2015. They found:
- The 1-year prevalence of migraine was 8%.
- The final multivariate model (Wald χ2=693.00, df=15) retained depression severity, chronic anxiety, exercising several times a month or week, and alcohol use as predictors of migraine (odds ratios=2.1–3.5 for positive associations, odds ratios=0.4–0.9 for negative associations).
Roy R, Sánchez-Rodriguez E, Galán S, et al. Factors associated with migraine in the general population of Spain: Results from the European Health Survey 2014. Pain Med. 2019;20(3):555-563. doi:10.1093/pm/pny093.
Raising awareness among clinicians that many of the potential variables contributing to the presence of migraine are modifiable (eg, psychological problems and lifestyle behaviors) might intensify resources dedicated to assessing and impacting these factors in order to potentially prevent the frequency and severity of migraine. This according to a recent study that aimed to identify the modifiable and non-modifiable variables that are associated with, and might moderate, the presence of migraine in the general population. Using a nationally representative cross-sectional survey, researchers evaluated responses from individuals aged 15 years and older (n=22,842). There was a secondary analysis of data from the second wave of a health interview survey conducted from 2014 to 2015. They found:
- The 1-year prevalence of migraine was 8%.
- The final multivariate model (Wald χ2=693.00, df=15) retained depression severity, chronic anxiety, exercising several times a month or week, and alcohol use as predictors of migraine (odds ratios=2.1–3.5 for positive associations, odds ratios=0.4–0.9 for negative associations).
Roy R, Sánchez-Rodriguez E, Galán S, et al. Factors associated with migraine in the general population of Spain: Results from the European Health Survey 2014. Pain Med. 2019;20(3):555-563. doi:10.1093/pm/pny093.
Opioid-Related Adverse Events in Migraineurs
Non-persistence to prophylactic treatment was frequent among migraine patients, a recent study found. Furthermore, opioid use was common in migraine patients and the risk of gastrointestinal-related adverse events and opioid abuse increased with long-term use of opioids. These results suggest a need for more effective prophylactic migraine treatments. This study used the IBM MarketScan databases from 2005 through 2014 to evaluate migraine patients initiating prophylactic medication. In total, 147,832 patients were analyzed. Outcome measures included persistence with prophylactic migraine medications throughout 2 to 5 years. Acute medication use and gastrointestinal-related adverse events and opioid abuse following opioid use were evaluated. Researchers found:
- Non-persistence was observed in 90% of patients; 39% switched, 30% restarted, and 31% discontinued treatment.
- Throughout the follow-up, 59.9% of patients received triptans, 66.6% non-steroidal anti-inflammatory drugs, 77.4% opioids, and 2.6% ergotamines.
- Among opioid users, 16.6% experienced nausea/vomiting, 12.2% had constipation, and 10.4% had diarrhea.
- Opioid abuse was reported in <1% of opioid users.
- Gastrointestinal-related adverse events increased with increasing number of days’ supply of opioids.
Bonafede M, Wilson K, Xue F. Long-term treatment patterns of prophylactic and acute migraine medications and incidence of opioid-related adverse events in patients with migraine. [Published online ahead of print February 28, 2019]. Cephalalgia. doi:10.1177%2F0333102419835465.
Non-persistence to prophylactic treatment was frequent among migraine patients, a recent study found. Furthermore, opioid use was common in migraine patients and the risk of gastrointestinal-related adverse events and opioid abuse increased with long-term use of opioids. These results suggest a need for more effective prophylactic migraine treatments. This study used the IBM MarketScan databases from 2005 through 2014 to evaluate migraine patients initiating prophylactic medication. In total, 147,832 patients were analyzed. Outcome measures included persistence with prophylactic migraine medications throughout 2 to 5 years. Acute medication use and gastrointestinal-related adverse events and opioid abuse following opioid use were evaluated. Researchers found:
- Non-persistence was observed in 90% of patients; 39% switched, 30% restarted, and 31% discontinued treatment.
- Throughout the follow-up, 59.9% of patients received triptans, 66.6% non-steroidal anti-inflammatory drugs, 77.4% opioids, and 2.6% ergotamines.
- Among opioid users, 16.6% experienced nausea/vomiting, 12.2% had constipation, and 10.4% had diarrhea.
- Opioid abuse was reported in <1% of opioid users.
- Gastrointestinal-related adverse events increased with increasing number of days’ supply of opioids.
Bonafede M, Wilson K, Xue F. Long-term treatment patterns of prophylactic and acute migraine medications and incidence of opioid-related adverse events in patients with migraine. [Published online ahead of print February 28, 2019]. Cephalalgia. doi:10.1177%2F0333102419835465.
Non-persistence to prophylactic treatment was frequent among migraine patients, a recent study found. Furthermore, opioid use was common in migraine patients and the risk of gastrointestinal-related adverse events and opioid abuse increased with long-term use of opioids. These results suggest a need for more effective prophylactic migraine treatments. This study used the IBM MarketScan databases from 2005 through 2014 to evaluate migraine patients initiating prophylactic medication. In total, 147,832 patients were analyzed. Outcome measures included persistence with prophylactic migraine medications throughout 2 to 5 years. Acute medication use and gastrointestinal-related adverse events and opioid abuse following opioid use were evaluated. Researchers found:
- Non-persistence was observed in 90% of patients; 39% switched, 30% restarted, and 31% discontinued treatment.
- Throughout the follow-up, 59.9% of patients received triptans, 66.6% non-steroidal anti-inflammatory drugs, 77.4% opioids, and 2.6% ergotamines.
- Among opioid users, 16.6% experienced nausea/vomiting, 12.2% had constipation, and 10.4% had diarrhea.
- Opioid abuse was reported in <1% of opioid users.
- Gastrointestinal-related adverse events increased with increasing number of days’ supply of opioids.
Bonafede M, Wilson K, Xue F. Long-term treatment patterns of prophylactic and acute migraine medications and incidence of opioid-related adverse events in patients with migraine. [Published online ahead of print February 28, 2019]. Cephalalgia. doi:10.1177%2F0333102419835465.
Flu activity levels down, but outpatient visits highest since 1998-99
Influenza activity measures declined for a third consecutive week, but levels are higher than usual at this point in the flu season, according to the Centers for Disease Control and Prevention.
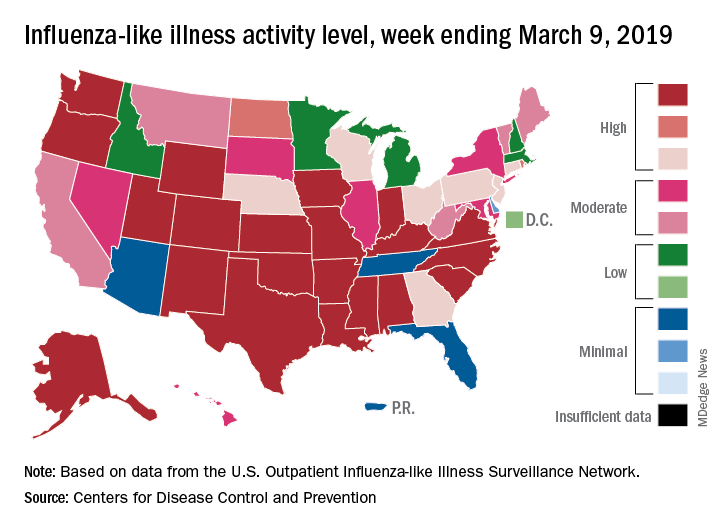
For the week ending March 9, an estimated 4.5% of outpatient visits were for influenza-like illness (ILI), which was down from 4.6% the previous week, the CDC’s influenza division reported March 15, but that is higher than the comparable week for any year since 1998-1999. During last year’s very severe flu season, the outpatient visit rate was just under 3.2% for the week ending March 10.
Although the number of states at level 10 on the CDC’s 1-10 scale remained at 21, the activity map actually looks more red than last week since Rhode Island and West Virgina were replaced by the much larger states of Iowa and Washington. The number of states in the high range (8-10), did go down from 32 to 30, data from the CDC’s Outpatient ILI Surveillance Network show.
Of those four deaths, only one occurred during the most recent reporting week, the CDC said.
Influenza activity measures declined for a third consecutive week, but levels are higher than usual at this point in the flu season, according to the Centers for Disease Control and Prevention.

For the week ending March 9, an estimated 4.5% of outpatient visits were for influenza-like illness (ILI), which was down from 4.6% the previous week, the CDC’s influenza division reported March 15, but that is higher than the comparable week for any year since 1998-1999. During last year’s very severe flu season, the outpatient visit rate was just under 3.2% for the week ending March 10.
Although the number of states at level 10 on the CDC’s 1-10 scale remained at 21, the activity map actually looks more red than last week since Rhode Island and West Virgina were replaced by the much larger states of Iowa and Washington. The number of states in the high range (8-10), did go down from 32 to 30, data from the CDC’s Outpatient ILI Surveillance Network show.
Of those four deaths, only one occurred during the most recent reporting week, the CDC said.
Influenza activity measures declined for a third consecutive week, but levels are higher than usual at this point in the flu season, according to the Centers for Disease Control and Prevention.

For the week ending March 9, an estimated 4.5% of outpatient visits were for influenza-like illness (ILI), which was down from 4.6% the previous week, the CDC’s influenza division reported March 15, but that is higher than the comparable week for any year since 1998-1999. During last year’s very severe flu season, the outpatient visit rate was just under 3.2% for the week ending March 10.
Although the number of states at level 10 on the CDC’s 1-10 scale remained at 21, the activity map actually looks more red than last week since Rhode Island and West Virgina were replaced by the much larger states of Iowa and Washington. The number of states in the high range (8-10), did go down from 32 to 30, data from the CDC’s Outpatient ILI Surveillance Network show.
Of those four deaths, only one occurred during the most recent reporting week, the CDC said.
Access to abortion care: Facts matter
In 1973, the Supreme Court of the United States recognized a constitutional right to abortion in the landmark case of Roe v Wade. The Court held that states may regulate, but not ban, abortion after the first trimester, for the purpose of protecting the woman’s health. The Court further indicated that states’ interest in “potential life” could be the basis for abortion regulations only after the point of viability, at which point states may ban abortion except when necessary to preserve the life or health of the woman.1 In 1992, the Court decided Planned Parenthood v Casey and eliminated the trimester framework while upholding women’s right to abortion.2 As with Roe v Wade, the Casey decision held that there must be an exception for the woman’s health and life.
Fast forward to 2019
New York passed a law in 2019,3 and Virginia had a proposed law that was recently tabled by the House of Delegates,4 both related to abortions performed past the first trimester.
New York. The New York law supports legal abortion by a licensed practitioner within 24 weeks of pregnancy commencement. After 24 weeks’ gestation, if there is “an absence of fetal viability, or the abortion is necessary to protect the patient’s life or health” then termination is permissible.3
Virginia. Previously, Virginia had abortion laws that required significant measures to approve a third-trimester abortion, including certification by 3 physicians that the procedure is necessary to “save mother’s life or [prevent] substantial and irremediable impairment of mental or physical health of the mother.”5 Violation included potential for jail time and a significant monetary fine.
The proposed bill, now tabled, was introduced by delegate Kathy Tran (House Bill 2491) and would have rolled back many requirements of the old law, including the 24-hour waiting period and mandate for second-trimester abortions to occur in a hospital.
The controversy centered on a provision concerning third-trimester abortions. Specifically, the proposed bill would only have required 1 doctor to deem the abortion necessary and would have removed the “substantially and irremediably” qualifier. Thus, abortions would be allowed in cases in which the woman’s mental or physical health was threatened, even in cases in which the potential damage may be reversible.5
The facts
Misconceptions about abortion care can be dangerous and work to further stigmatize our patients who may need an abortion or who have had an abortion in the past. The American College of Obstetricians and Gynecologists (ACOG) recently published a document discussing facts regarding abortion care later in pregnancy. The document (aptly named “Facts are Important”) enforces that policy be based on medical science and facts, and not simply driven by political beliefs.6
Fact. The majority of abortions occur prior to 21 weeks, before viability:
- 91.1% of abortions occur at or before 13 weeks’ gestation7
- only 1.3% of abortions occur at or after 21 weeks’ gestation7
- abortions occurring later in the second trimester or in the third trimester are very uncommon.
Fact. The language “late-term abortion” has no medical definition, is not used in a clinical setting or to describe the delivery of abortion care later in pregnancy in any medical institution.6
Fact. Many of the abortions occurring later in pregnancy are due to fetal anomalies incompatible with life. Anomalies can include lack of a major portion of the brain (anencephaly), bilateral renal agenesis, some skeletal dysplasias, and other chromosomal abnormalities. These are cases in which death is likely before or shortly after birth, with great potential for suffering of both the fetus and the family.
Fact. The need for abortion also may be due to serious complications that will likely cause significant morbidity or mortality to the woman. These complications, in turn, reduce the likelihood of survival of the fetus.
It is thus vital for women to have the freedom to evaluate their medical circumstance with their provider and, using evidence, make informed health care decisions—which may include abortion, induction of labor, or cesarean delivery in some circumstances. Access to accurate, complete information and care is a right bestowed amongst all women and “must never be constrained by politicians.”6 We must focus on medically appropriate and compassionate care for both the family and the fetus.
Use your voice
As clinicians, we are trusted members of our communities. The New York law and the prior proposed Virginia law emphasize important access to care for women and their families. Abortions at a later gestational age are a rare event but are most often performed when the health or life of the mother is at risk or the fetus has an anomaly incompatible with life.
We urge you to use your voice to correct misconceptions, whether in your office with your patients or colleagues or in your communities, locally and nationally. Email your friends and colleagues about ACOG’s “Facts are Important” document, organize a grand rounds on the topic, and utilize social media to share facts about abortion care. These actions support our patients and can make an impact by spreading factual information.
For more facts and figures about abortion laws, visit the website of the Guttmacher Institute.
- Roe v Wade, 410 US 113 (1973).
- Planned Parenthood v Casey, 505 US 833 (1992).
- New York abortion laws. FindLaw website. https://statelaws.findlaw.com/new-york-law/new-york-abortion-laws.html. Accessed March 7, 2019.
- North A. The controversy around Virginia’s new abortion bill, explained. https://www.vox.com/2019/2/1/18205428/virginia-abortion-bill-kathy-tran-ralph-northam Accessed March 13, 2019.
- Virginia abortion laws. FindLaw website. https://statelaws.findlaw.com/virginia-law/virginia-abortion-laws.html. Accessed March 7, 2019.
- Facts are important. The American College of Obstetricians and Gynecologists website. https://www.acog.org/-/media/Departments/Government-Relations-and-Outreach/Facts-Are-Important_Abortion-Care-Later-In-Pregnancy-February-2019-College.pdf?dmc=1&ts=20190214T2242210541. Accessed March 7, 2019.
- Jatlaoui TC, Boutot ME, Mandel MG, et al. Abortion surveillance—United States, 2015. MMWR Surveill Summ. 2018;67(13):1-45.
In 1973, the Supreme Court of the United States recognized a constitutional right to abortion in the landmark case of Roe v Wade. The Court held that states may regulate, but not ban, abortion after the first trimester, for the purpose of protecting the woman’s health. The Court further indicated that states’ interest in “potential life” could be the basis for abortion regulations only after the point of viability, at which point states may ban abortion except when necessary to preserve the life or health of the woman.1 In 1992, the Court decided Planned Parenthood v Casey and eliminated the trimester framework while upholding women’s right to abortion.2 As with Roe v Wade, the Casey decision held that there must be an exception for the woman’s health and life.
Fast forward to 2019
New York passed a law in 2019,3 and Virginia had a proposed law that was recently tabled by the House of Delegates,4 both related to abortions performed past the first trimester.
New York. The New York law supports legal abortion by a licensed practitioner within 24 weeks of pregnancy commencement. After 24 weeks’ gestation, if there is “an absence of fetal viability, or the abortion is necessary to protect the patient’s life or health” then termination is permissible.3
Virginia. Previously, Virginia had abortion laws that required significant measures to approve a third-trimester abortion, including certification by 3 physicians that the procedure is necessary to “save mother’s life or [prevent] substantial and irremediable impairment of mental or physical health of the mother.”5 Violation included potential for jail time and a significant monetary fine.
The proposed bill, now tabled, was introduced by delegate Kathy Tran (House Bill 2491) and would have rolled back many requirements of the old law, including the 24-hour waiting period and mandate for second-trimester abortions to occur in a hospital.
The controversy centered on a provision concerning third-trimester abortions. Specifically, the proposed bill would only have required 1 doctor to deem the abortion necessary and would have removed the “substantially and irremediably” qualifier. Thus, abortions would be allowed in cases in which the woman’s mental or physical health was threatened, even in cases in which the potential damage may be reversible.5
The facts
Misconceptions about abortion care can be dangerous and work to further stigmatize our patients who may need an abortion or who have had an abortion in the past. The American College of Obstetricians and Gynecologists (ACOG) recently published a document discussing facts regarding abortion care later in pregnancy. The document (aptly named “Facts are Important”) enforces that policy be based on medical science and facts, and not simply driven by political beliefs.6
Fact. The majority of abortions occur prior to 21 weeks, before viability:
- 91.1% of abortions occur at or before 13 weeks’ gestation7
- only 1.3% of abortions occur at or after 21 weeks’ gestation7
- abortions occurring later in the second trimester or in the third trimester are very uncommon.
Fact. The language “late-term abortion” has no medical definition, is not used in a clinical setting or to describe the delivery of abortion care later in pregnancy in any medical institution.6
Fact. Many of the abortions occurring later in pregnancy are due to fetal anomalies incompatible with life. Anomalies can include lack of a major portion of the brain (anencephaly), bilateral renal agenesis, some skeletal dysplasias, and other chromosomal abnormalities. These are cases in which death is likely before or shortly after birth, with great potential for suffering of both the fetus and the family.
Fact. The need for abortion also may be due to serious complications that will likely cause significant morbidity or mortality to the woman. These complications, in turn, reduce the likelihood of survival of the fetus.
It is thus vital for women to have the freedom to evaluate their medical circumstance with their provider and, using evidence, make informed health care decisions—which may include abortion, induction of labor, or cesarean delivery in some circumstances. Access to accurate, complete information and care is a right bestowed amongst all women and “must never be constrained by politicians.”6 We must focus on medically appropriate and compassionate care for both the family and the fetus.
Use your voice
As clinicians, we are trusted members of our communities. The New York law and the prior proposed Virginia law emphasize important access to care for women and their families. Abortions at a later gestational age are a rare event but are most often performed when the health or life of the mother is at risk or the fetus has an anomaly incompatible with life.
We urge you to use your voice to correct misconceptions, whether in your office with your patients or colleagues or in your communities, locally and nationally. Email your friends and colleagues about ACOG’s “Facts are Important” document, organize a grand rounds on the topic, and utilize social media to share facts about abortion care. These actions support our patients and can make an impact by spreading factual information.
For more facts and figures about abortion laws, visit the website of the Guttmacher Institute.
In 1973, the Supreme Court of the United States recognized a constitutional right to abortion in the landmark case of Roe v Wade. The Court held that states may regulate, but not ban, abortion after the first trimester, for the purpose of protecting the woman’s health. The Court further indicated that states’ interest in “potential life” could be the basis for abortion regulations only after the point of viability, at which point states may ban abortion except when necessary to preserve the life or health of the woman.1 In 1992, the Court decided Planned Parenthood v Casey and eliminated the trimester framework while upholding women’s right to abortion.2 As with Roe v Wade, the Casey decision held that there must be an exception for the woman’s health and life.
Fast forward to 2019
New York passed a law in 2019,3 and Virginia had a proposed law that was recently tabled by the House of Delegates,4 both related to abortions performed past the first trimester.
New York. The New York law supports legal abortion by a licensed practitioner within 24 weeks of pregnancy commencement. After 24 weeks’ gestation, if there is “an absence of fetal viability, or the abortion is necessary to protect the patient’s life or health” then termination is permissible.3
Virginia. Previously, Virginia had abortion laws that required significant measures to approve a third-trimester abortion, including certification by 3 physicians that the procedure is necessary to “save mother’s life or [prevent] substantial and irremediable impairment of mental or physical health of the mother.”5 Violation included potential for jail time and a significant monetary fine.
The proposed bill, now tabled, was introduced by delegate Kathy Tran (House Bill 2491) and would have rolled back many requirements of the old law, including the 24-hour waiting period and mandate for second-trimester abortions to occur in a hospital.
The controversy centered on a provision concerning third-trimester abortions. Specifically, the proposed bill would only have required 1 doctor to deem the abortion necessary and would have removed the “substantially and irremediably” qualifier. Thus, abortions would be allowed in cases in which the woman’s mental or physical health was threatened, even in cases in which the potential damage may be reversible.5
The facts
Misconceptions about abortion care can be dangerous and work to further stigmatize our patients who may need an abortion or who have had an abortion in the past. The American College of Obstetricians and Gynecologists (ACOG) recently published a document discussing facts regarding abortion care later in pregnancy. The document (aptly named “Facts are Important”) enforces that policy be based on medical science and facts, and not simply driven by political beliefs.6
Fact. The majority of abortions occur prior to 21 weeks, before viability:
- 91.1% of abortions occur at or before 13 weeks’ gestation7
- only 1.3% of abortions occur at or after 21 weeks’ gestation7
- abortions occurring later in the second trimester or in the third trimester are very uncommon.
Fact. The language “late-term abortion” has no medical definition, is not used in a clinical setting or to describe the delivery of abortion care later in pregnancy in any medical institution.6
Fact. Many of the abortions occurring later in pregnancy are due to fetal anomalies incompatible with life. Anomalies can include lack of a major portion of the brain (anencephaly), bilateral renal agenesis, some skeletal dysplasias, and other chromosomal abnormalities. These are cases in which death is likely before or shortly after birth, with great potential for suffering of both the fetus and the family.
Fact. The need for abortion also may be due to serious complications that will likely cause significant morbidity or mortality to the woman. These complications, in turn, reduce the likelihood of survival of the fetus.
It is thus vital for women to have the freedom to evaluate their medical circumstance with their provider and, using evidence, make informed health care decisions—which may include abortion, induction of labor, or cesarean delivery in some circumstances. Access to accurate, complete information and care is a right bestowed amongst all women and “must never be constrained by politicians.”6 We must focus on medically appropriate and compassionate care for both the family and the fetus.
Use your voice
As clinicians, we are trusted members of our communities. The New York law and the prior proposed Virginia law emphasize important access to care for women and their families. Abortions at a later gestational age are a rare event but are most often performed when the health or life of the mother is at risk or the fetus has an anomaly incompatible with life.
We urge you to use your voice to correct misconceptions, whether in your office with your patients or colleagues or in your communities, locally and nationally. Email your friends and colleagues about ACOG’s “Facts are Important” document, organize a grand rounds on the topic, and utilize social media to share facts about abortion care. These actions support our patients and can make an impact by spreading factual information.
For more facts and figures about abortion laws, visit the website of the Guttmacher Institute.
- Roe v Wade, 410 US 113 (1973).
- Planned Parenthood v Casey, 505 US 833 (1992).
- New York abortion laws. FindLaw website. https://statelaws.findlaw.com/new-york-law/new-york-abortion-laws.html. Accessed March 7, 2019.
- North A. The controversy around Virginia’s new abortion bill, explained. https://www.vox.com/2019/2/1/18205428/virginia-abortion-bill-kathy-tran-ralph-northam Accessed March 13, 2019.
- Virginia abortion laws. FindLaw website. https://statelaws.findlaw.com/virginia-law/virginia-abortion-laws.html. Accessed March 7, 2019.
- Facts are important. The American College of Obstetricians and Gynecologists website. https://www.acog.org/-/media/Departments/Government-Relations-and-Outreach/Facts-Are-Important_Abortion-Care-Later-In-Pregnancy-February-2019-College.pdf?dmc=1&ts=20190214T2242210541. Accessed March 7, 2019.
- Jatlaoui TC, Boutot ME, Mandel MG, et al. Abortion surveillance—United States, 2015. MMWR Surveill Summ. 2018;67(13):1-45.
- Roe v Wade, 410 US 113 (1973).
- Planned Parenthood v Casey, 505 US 833 (1992).
- New York abortion laws. FindLaw website. https://statelaws.findlaw.com/new-york-law/new-york-abortion-laws.html. Accessed March 7, 2019.
- North A. The controversy around Virginia’s new abortion bill, explained. https://www.vox.com/2019/2/1/18205428/virginia-abortion-bill-kathy-tran-ralph-northam Accessed March 13, 2019.
- Virginia abortion laws. FindLaw website. https://statelaws.findlaw.com/virginia-law/virginia-abortion-laws.html. Accessed March 7, 2019.
- Facts are important. The American College of Obstetricians and Gynecologists website. https://www.acog.org/-/media/Departments/Government-Relations-and-Outreach/Facts-Are-Important_Abortion-Care-Later-In-Pregnancy-February-2019-College.pdf?dmc=1&ts=20190214T2242210541. Accessed March 7, 2019.
- Jatlaoui TC, Boutot ME, Mandel MG, et al. Abortion surveillance—United States, 2015. MMWR Surveill Summ. 2018;67(13):1-45.
The power of policy at HM19
Mini-track features CMS insights
Due to the steadily growing interest of SHM members in health care policy and advocacy issues, the 2019 Annual Conference will include a mini-track dedicated to policy issues.
To be held on Monday, March 25th at HM19 in Orlando, the health care policy mini-track will update conference attendees on some of the Washington developments that affect hospitalists, said Josh Boswell, director of government relations at SHM.
“Many of the policy developments in D.C. are directly impacting our members’ practices,” he said. “A couple of years ago, it was decided to add a specific track at the annual conference to cover some of these policy issues, and we’ve generally had positive feedback on the sessions.”
This year, the mini-track will consist of two separate sessions, held back to back. “Both sessions are designed to give attendees an entrée into health policy and explain developments that are happening right now in Washington that impact their practice,” said Joshua Lapps, government relations manager at SHM.
The first session – “CMS Policy Update: An Overview of Meaningful Measures and the Quality Payment Program” – will take place from 2:00 to 3:30 p.m., and will feature Reena Duseja, MD, MS, the acting director for Quality Measurement and Value-Based Incentives Group in the Centers for Clinical Standards and Quality at the Centers for Medicare & Medicaid Services. Dr. Duseja oversees the development of measures and analyses for a variety of CMS quality reporting and value-based purchasing programs. She is also an emergency medicine physician and was an associate professor at the University of California, San Francisco, in the department of emergency medicine, where she led quality improvement activities.
“The session with Dr. Duseja will be an inside look into the approach that CMS is taking for quality measurement and pay-for-performance programs, specifically looking at the quality payment program which came out of the Medicare Access and Chip Reauthorization Act,” Mr. Lapps said. “It will be a high-level discussion about how the programs affect hospitalists, and how hospitalists participate in the programs. It’s also a chance for attendees to hear some of the thinking inside CMS.”
Dr. Duseja is also hoping to get feedback from HM19 attendees. “She wants the session to be educational for our members, as well as an opportunity for her to learn from hospitalists,” Mr. Lapps said.
According to Dr. Duseja, her presentation will provide attendees with an overview of the Quality Payment Program under the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), specifically highlighting policy changes from 2018 to 2019 to the Merit-based Incentive Payment System (MIPS) and Meaningful Measures Initiative. Attendees will learn more about CMS’s approach to quality and quality measurement, as well as the future of quality reporting programs.
Following Dr. Duseja’s presentation, the second mini-track session will take place from 3:40 to 4:25 p.m. It will focus more intently on the processes around health care policy making.
“We heard from our members who attended this mini-track at the past two annual conferences that they would like us to explain how policy making works: the play-by-play in D.C. on how we get to where we are,” Mr. Boswell said.
The second session will feature a presentation by Jennifer Bell, founding partner at Chamber Hill Strategies, who represents SHM in Washington. “Jennifer will be discussing how Washington works, the policy process and the pressure points at which SHM and its members can exert influence,” Mr. Lapps said.
Attendees can expect to learn a lot from either session, Mr. Lapps said. “Attendees will learn about the basic contours of the Quality Payment Program that Medicare oversees, and some of the specific new elements of that program this year that were designed with hospitalists in mind. For example, Dr. Duseja will be talking about a facility-based reporting option under the Merit-Based Incentive Payment System. I think our members should gain a concrete understanding of some of the new directions that CMS is heading this year. Overall, they’ll have a better sense of the vision behind quality measures and quality measurement. This is a really exciting opportunity to hear from someone who is both a clinician and works on policy at CMS.”
The policy mini-track offers hospitalists a chance to get a look “behind the curtain” at policy making from someone who is helping to write the rules.
“Attendees will gain insight on where they fit in these programs – and also have the opportunity to tell Dr. Duseja if they don’t feel these programs are a good fit for them,” Mr. Boswell said. “Oftentimes these programs are not structured ideally for hospitalists. So, hearing directly from hospitalists who are experiencing problems would be extraordinarily helpful to a CMS official. I think attendees should view the policy track not only as an opportunity to learn from CMS, but as an opportunity to educate CMS about our issues.”
Mini-track features CMS insights
Mini-track features CMS insights
Due to the steadily growing interest of SHM members in health care policy and advocacy issues, the 2019 Annual Conference will include a mini-track dedicated to policy issues.
To be held on Monday, March 25th at HM19 in Orlando, the health care policy mini-track will update conference attendees on some of the Washington developments that affect hospitalists, said Josh Boswell, director of government relations at SHM.
“Many of the policy developments in D.C. are directly impacting our members’ practices,” he said. “A couple of years ago, it was decided to add a specific track at the annual conference to cover some of these policy issues, and we’ve generally had positive feedback on the sessions.”
This year, the mini-track will consist of two separate sessions, held back to back. “Both sessions are designed to give attendees an entrée into health policy and explain developments that are happening right now in Washington that impact their practice,” said Joshua Lapps, government relations manager at SHM.
The first session – “CMS Policy Update: An Overview of Meaningful Measures and the Quality Payment Program” – will take place from 2:00 to 3:30 p.m., and will feature Reena Duseja, MD, MS, the acting director for Quality Measurement and Value-Based Incentives Group in the Centers for Clinical Standards and Quality at the Centers for Medicare & Medicaid Services. Dr. Duseja oversees the development of measures and analyses for a variety of CMS quality reporting and value-based purchasing programs. She is also an emergency medicine physician and was an associate professor at the University of California, San Francisco, in the department of emergency medicine, where she led quality improvement activities.
“The session with Dr. Duseja will be an inside look into the approach that CMS is taking for quality measurement and pay-for-performance programs, specifically looking at the quality payment program which came out of the Medicare Access and Chip Reauthorization Act,” Mr. Lapps said. “It will be a high-level discussion about how the programs affect hospitalists, and how hospitalists participate in the programs. It’s also a chance for attendees to hear some of the thinking inside CMS.”
Dr. Duseja is also hoping to get feedback from HM19 attendees. “She wants the session to be educational for our members, as well as an opportunity for her to learn from hospitalists,” Mr. Lapps said.
According to Dr. Duseja, her presentation will provide attendees with an overview of the Quality Payment Program under the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), specifically highlighting policy changes from 2018 to 2019 to the Merit-based Incentive Payment System (MIPS) and Meaningful Measures Initiative. Attendees will learn more about CMS’s approach to quality and quality measurement, as well as the future of quality reporting programs.
Following Dr. Duseja’s presentation, the second mini-track session will take place from 3:40 to 4:25 p.m. It will focus more intently on the processes around health care policy making.
“We heard from our members who attended this mini-track at the past two annual conferences that they would like us to explain how policy making works: the play-by-play in D.C. on how we get to where we are,” Mr. Boswell said.
The second session will feature a presentation by Jennifer Bell, founding partner at Chamber Hill Strategies, who represents SHM in Washington. “Jennifer will be discussing how Washington works, the policy process and the pressure points at which SHM and its members can exert influence,” Mr. Lapps said.
Attendees can expect to learn a lot from either session, Mr. Lapps said. “Attendees will learn about the basic contours of the Quality Payment Program that Medicare oversees, and some of the specific new elements of that program this year that were designed with hospitalists in mind. For example, Dr. Duseja will be talking about a facility-based reporting option under the Merit-Based Incentive Payment System. I think our members should gain a concrete understanding of some of the new directions that CMS is heading this year. Overall, they’ll have a better sense of the vision behind quality measures and quality measurement. This is a really exciting opportunity to hear from someone who is both a clinician and works on policy at CMS.”
The policy mini-track offers hospitalists a chance to get a look “behind the curtain” at policy making from someone who is helping to write the rules.
“Attendees will gain insight on where they fit in these programs – and also have the opportunity to tell Dr. Duseja if they don’t feel these programs are a good fit for them,” Mr. Boswell said. “Oftentimes these programs are not structured ideally for hospitalists. So, hearing directly from hospitalists who are experiencing problems would be extraordinarily helpful to a CMS official. I think attendees should view the policy track not only as an opportunity to learn from CMS, but as an opportunity to educate CMS about our issues.”
Due to the steadily growing interest of SHM members in health care policy and advocacy issues, the 2019 Annual Conference will include a mini-track dedicated to policy issues.
To be held on Monday, March 25th at HM19 in Orlando, the health care policy mini-track will update conference attendees on some of the Washington developments that affect hospitalists, said Josh Boswell, director of government relations at SHM.
“Many of the policy developments in D.C. are directly impacting our members’ practices,” he said. “A couple of years ago, it was decided to add a specific track at the annual conference to cover some of these policy issues, and we’ve generally had positive feedback on the sessions.”
This year, the mini-track will consist of two separate sessions, held back to back. “Both sessions are designed to give attendees an entrée into health policy and explain developments that are happening right now in Washington that impact their practice,” said Joshua Lapps, government relations manager at SHM.
The first session – “CMS Policy Update: An Overview of Meaningful Measures and the Quality Payment Program” – will take place from 2:00 to 3:30 p.m., and will feature Reena Duseja, MD, MS, the acting director for Quality Measurement and Value-Based Incentives Group in the Centers for Clinical Standards and Quality at the Centers for Medicare & Medicaid Services. Dr. Duseja oversees the development of measures and analyses for a variety of CMS quality reporting and value-based purchasing programs. She is also an emergency medicine physician and was an associate professor at the University of California, San Francisco, in the department of emergency medicine, where she led quality improvement activities.
“The session with Dr. Duseja will be an inside look into the approach that CMS is taking for quality measurement and pay-for-performance programs, specifically looking at the quality payment program which came out of the Medicare Access and Chip Reauthorization Act,” Mr. Lapps said. “It will be a high-level discussion about how the programs affect hospitalists, and how hospitalists participate in the programs. It’s also a chance for attendees to hear some of the thinking inside CMS.”
Dr. Duseja is also hoping to get feedback from HM19 attendees. “She wants the session to be educational for our members, as well as an opportunity for her to learn from hospitalists,” Mr. Lapps said.
According to Dr. Duseja, her presentation will provide attendees with an overview of the Quality Payment Program under the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), specifically highlighting policy changes from 2018 to 2019 to the Merit-based Incentive Payment System (MIPS) and Meaningful Measures Initiative. Attendees will learn more about CMS’s approach to quality and quality measurement, as well as the future of quality reporting programs.
Following Dr. Duseja’s presentation, the second mini-track session will take place from 3:40 to 4:25 p.m. It will focus more intently on the processes around health care policy making.
“We heard from our members who attended this mini-track at the past two annual conferences that they would like us to explain how policy making works: the play-by-play in D.C. on how we get to where we are,” Mr. Boswell said.
The second session will feature a presentation by Jennifer Bell, founding partner at Chamber Hill Strategies, who represents SHM in Washington. “Jennifer will be discussing how Washington works, the policy process and the pressure points at which SHM and its members can exert influence,” Mr. Lapps said.
Attendees can expect to learn a lot from either session, Mr. Lapps said. “Attendees will learn about the basic contours of the Quality Payment Program that Medicare oversees, and some of the specific new elements of that program this year that were designed with hospitalists in mind. For example, Dr. Duseja will be talking about a facility-based reporting option under the Merit-Based Incentive Payment System. I think our members should gain a concrete understanding of some of the new directions that CMS is heading this year. Overall, they’ll have a better sense of the vision behind quality measures and quality measurement. This is a really exciting opportunity to hear from someone who is both a clinician and works on policy at CMS.”
The policy mini-track offers hospitalists a chance to get a look “behind the curtain” at policy making from someone who is helping to write the rules.
“Attendees will gain insight on where they fit in these programs – and also have the opportunity to tell Dr. Duseja if they don’t feel these programs are a good fit for them,” Mr. Boswell said. “Oftentimes these programs are not structured ideally for hospitalists. So, hearing directly from hospitalists who are experiencing problems would be extraordinarily helpful to a CMS official. I think attendees should view the policy track not only as an opportunity to learn from CMS, but as an opportunity to educate CMS about our issues.”
Very early ART may benefit infants with HIV
SEATTLE – Antiretroviral therapy (ART) in the earliest weeks of life is associated with reduced time to suppression, according to a new study. Each week that treatment was delayed, patients had a 35% reduction in odds of achieving earlier viral load (VL) suppression.
Previous research had already shown that patients treated in the first year of life have better outcomes, including shortened time to viral suppression, and a lower reservoir size. Those studies looked at median age of ART start by month, and a review of the literature revealed that no research had been done on the first month.
The research was presented at the Conference on Retroviruses & Opportunistic Infections by Sara Domínguez Rodríguez, biostatistician and data manager in the pediatric infectious disease service at Hospital 12 Octubre, Madrid.
The researchers retrospectively analyzed 44 patients who were treated in the first 28 days of life, who had uninterrupted ART for at least 2 years, and who had VL measured at least twice during follow-up. Among these, 25 patients received ART in the first week, 19 in weeks 2-4. Patients treated prophylactically with AZT + 3TC (lamivudine) + NVP (nevirapine) any time in the first 15 days of life were considered treated at day 1.
Five of the patients were from the United Kingdom, 23 from Spain, 3 from Italy, and 13 from Thailand. Fifty-seven percent were girls; 35% were preterm. Patients treated in the first week had a higher log10 HIV viral load at ART initiation (P = .02). There was no significant difference between the two groups with respect to CD4 count at ART initiation.
The time to suppression was not significantly different between the two groups, nor was the percentage of patients suppressed at various time points. Patients treated in the first week had reached suppression more often at 3 months and 6 months, but neither result reached statistical significance.
The small sample size of the study produced a challenge, and that led the team to consider suppression time and age as continuous variables. That revealed a curve that favored treatment in the first week of life: Each week of delay reduced the probability of achieving early viral suppression by 35% (hazard ratio, 0.65; 95% confidence interval, 0.46-0.92). “This means that if you delay the age at ART in terms of weeks, the probability of achieving suppression (over) time decreases, and this effect is particularly seen in the first year of follow-up,” Dr. Domínguez Rodríguez said in an interview.
Some might have concerns that treating children too early could have adverse effects. This study, though small, showed promising results. “You might think if you treat very early, maybe the child will not tolerate the medicine or keep on with the treatment, and we saw no difference. So that supports treating early,” senior author Pablo Rojo Conejo, PhD, an infectious disease specialist at Hospital 12 de Octubre, said in an interview.
Others in attendance found the results encouraging. “It’s very good news to see that early starting impacts the life of these children,” Filipe de Barros Perini, MD, head of care and treatment of the HIV program at the Brazilian Ministry of Health, said in an interview.
SOURCE: Domínguez Rodríguez S et al. CROI 2019, Abstract 44.
SEATTLE – Antiretroviral therapy (ART) in the earliest weeks of life is associated with reduced time to suppression, according to a new study. Each week that treatment was delayed, patients had a 35% reduction in odds of achieving earlier viral load (VL) suppression.
Previous research had already shown that patients treated in the first year of life have better outcomes, including shortened time to viral suppression, and a lower reservoir size. Those studies looked at median age of ART start by month, and a review of the literature revealed that no research had been done on the first month.
The research was presented at the Conference on Retroviruses & Opportunistic Infections by Sara Domínguez Rodríguez, biostatistician and data manager in the pediatric infectious disease service at Hospital 12 Octubre, Madrid.
The researchers retrospectively analyzed 44 patients who were treated in the first 28 days of life, who had uninterrupted ART for at least 2 years, and who had VL measured at least twice during follow-up. Among these, 25 patients received ART in the first week, 19 in weeks 2-4. Patients treated prophylactically with AZT + 3TC (lamivudine) + NVP (nevirapine) any time in the first 15 days of life were considered treated at day 1.
Five of the patients were from the United Kingdom, 23 from Spain, 3 from Italy, and 13 from Thailand. Fifty-seven percent were girls; 35% were preterm. Patients treated in the first week had a higher log10 HIV viral load at ART initiation (P = .02). There was no significant difference between the two groups with respect to CD4 count at ART initiation.
The time to suppression was not significantly different between the two groups, nor was the percentage of patients suppressed at various time points. Patients treated in the first week had reached suppression more often at 3 months and 6 months, but neither result reached statistical significance.
The small sample size of the study produced a challenge, and that led the team to consider suppression time and age as continuous variables. That revealed a curve that favored treatment in the first week of life: Each week of delay reduced the probability of achieving early viral suppression by 35% (hazard ratio, 0.65; 95% confidence interval, 0.46-0.92). “This means that if you delay the age at ART in terms of weeks, the probability of achieving suppression (over) time decreases, and this effect is particularly seen in the first year of follow-up,” Dr. Domínguez Rodríguez said in an interview.
Some might have concerns that treating children too early could have adverse effects. This study, though small, showed promising results. “You might think if you treat very early, maybe the child will not tolerate the medicine or keep on with the treatment, and we saw no difference. So that supports treating early,” senior author Pablo Rojo Conejo, PhD, an infectious disease specialist at Hospital 12 de Octubre, said in an interview.
Others in attendance found the results encouraging. “It’s very good news to see that early starting impacts the life of these children,” Filipe de Barros Perini, MD, head of care and treatment of the HIV program at the Brazilian Ministry of Health, said in an interview.
SOURCE: Domínguez Rodríguez S et al. CROI 2019, Abstract 44.
SEATTLE – Antiretroviral therapy (ART) in the earliest weeks of life is associated with reduced time to suppression, according to a new study. Each week that treatment was delayed, patients had a 35% reduction in odds of achieving earlier viral load (VL) suppression.
Previous research had already shown that patients treated in the first year of life have better outcomes, including shortened time to viral suppression, and a lower reservoir size. Those studies looked at median age of ART start by month, and a review of the literature revealed that no research had been done on the first month.
The research was presented at the Conference on Retroviruses & Opportunistic Infections by Sara Domínguez Rodríguez, biostatistician and data manager in the pediatric infectious disease service at Hospital 12 Octubre, Madrid.
The researchers retrospectively analyzed 44 patients who were treated in the first 28 days of life, who had uninterrupted ART for at least 2 years, and who had VL measured at least twice during follow-up. Among these, 25 patients received ART in the first week, 19 in weeks 2-4. Patients treated prophylactically with AZT + 3TC (lamivudine) + NVP (nevirapine) any time in the first 15 days of life were considered treated at day 1.
Five of the patients were from the United Kingdom, 23 from Spain, 3 from Italy, and 13 from Thailand. Fifty-seven percent were girls; 35% were preterm. Patients treated in the first week had a higher log10 HIV viral load at ART initiation (P = .02). There was no significant difference between the two groups with respect to CD4 count at ART initiation.
The time to suppression was not significantly different between the two groups, nor was the percentage of patients suppressed at various time points. Patients treated in the first week had reached suppression more often at 3 months and 6 months, but neither result reached statistical significance.
The small sample size of the study produced a challenge, and that led the team to consider suppression time and age as continuous variables. That revealed a curve that favored treatment in the first week of life: Each week of delay reduced the probability of achieving early viral suppression by 35% (hazard ratio, 0.65; 95% confidence interval, 0.46-0.92). “This means that if you delay the age at ART in terms of weeks, the probability of achieving suppression (over) time decreases, and this effect is particularly seen in the first year of follow-up,” Dr. Domínguez Rodríguez said in an interview.
Some might have concerns that treating children too early could have adverse effects. This study, though small, showed promising results. “You might think if you treat very early, maybe the child will not tolerate the medicine or keep on with the treatment, and we saw no difference. So that supports treating early,” senior author Pablo Rojo Conejo, PhD, an infectious disease specialist at Hospital 12 de Octubre, said in an interview.
Others in attendance found the results encouraging. “It’s very good news to see that early starting impacts the life of these children,” Filipe de Barros Perini, MD, head of care and treatment of the HIV program at the Brazilian Ministry of Health, said in an interview.
SOURCE: Domínguez Rodríguez S et al. CROI 2019, Abstract 44.
REPORTING FROM CROI 2019
Bempedoic acid: funny name, serious LDL lowering
Recent trials advance axial spondyloarthritis therapy. Newer antihyperglycemic drugs have distinctive cardiovascular, kidney benefits. And doctors’ prior authorization burden is increasing.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Recent trials advance axial spondyloarthritis therapy. Newer antihyperglycemic drugs have distinctive cardiovascular, kidney benefits. And doctors’ prior authorization burden is increasing.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Recent trials advance axial spondyloarthritis therapy. Newer antihyperglycemic drugs have distinctive cardiovascular, kidney benefits. And doctors’ prior authorization burden is increasing.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Light physical activity lowers CVD risk in older women
Even light physical activity can significantly reduce the risks of acquiring coronary heart disease specifically and the broad range of cardiovascular diseases in older women, new data suggests.
A paper published in JAMA Network Open reported the outcome of a prospective cohort study in 5,861 women, with a mean age of 78.5 years, who wore accelerometers for 7 days to measure physical activity.
than those in the lowest quartile of activity, who engaged in less than 3.9 hours per day, after adjusting for factors such as comorbidities, lifestyle, and cardiovascular risk.
Similarly, those in the highest quartile had an 18% lower risk of cardiovascular disease than those in the lowest quartile, after adjusting for potential confounders.
Researchers saw a significant dose-dependent decrease in the risk for incident coronary heart disease and cardiovascular disease with increasing light physical activity, such that each 1-hour increment of activity was associated with a 20% decrease in coronary heart disease risk and 10% decrease in cardiovascular disease risk.
Andrea Z. LaCroix, PhD, from the University of California, San Diego, and her coauthors noted that physical activity guidelines for aerobic activity suggest 75 minutes of vigorous physical activity or 150 minutes of moderate activity each day, but only around 25% of U.S. women aged over 75 years are estimated to meet this requirement.
“These guidelines may have discouraged PA [physical activity] when perceived to be unattainable by large segments of the population,” they wrote.
While the majority of active time in older adults is spent doing light physical activity, little is known about the cardiovascular effects of participating in this level of activity. “A major barrier has been that self-reported questionnaires measuring leisure-time PA do not adequately capture light PA that is acquired throughout the day in activities of daily living,” they wrote.
The study also looked at the impact of moderate to vigorous physical activity, finding a significant 46% reduction between the highest to lowest quartiles of activity in coronary heart disease risk and a 31% reduction in cardiovascular disease risk.
Even after adjusting for the use of lipid-lowering medication, antihypertensive medication or healthy eating scores, the results remained unchanged. The researchers also saw no change when women with angina and heart failure at baseline were excluded or when they excluded cardiovascular events that occurred during the first 6 months of follow-up.
The study was supported by the National Heart, Lung, and Blood Institute; the National Institutes of Health; and the Department of Health & Human Services. Six authors reported receiving funding from the study supporters and other research institutions, and one reported membership on the advisory committee for physical activity guidelines. No other conflicts of interest were reported.
SOURCE: LaCroix AZ et al. JAMA Netw Open. 2019 Mar 15. doi: 10.1001/jamanetworkopen.2019.0419.
Older women do not get enough physical activity, so this finding that light physical activity is associated with improved coronary heart disease and cardiovascular disease outcomes supports the recent scientific report by the 2018 Physical Activity Guidelines Advisory Committee. It is also helpful in extending the evidence about the benefits of physical activity in reducing incident coronary heart disease to older women, as previous studies on this topic showed such benefits in men.
These findings should remind health care professionals, systems, and agencies to promote the 2018 Physical Activity Guidelines for Americans to all patients. Otherwise, the future health and well-being of older women is likely to suffer from the consequences of sedentary behavior and inadequate physical activity.
Gregory W. Heath, DHSc, MPH, is from the department of health and human performance at the University of Tennessee, Chattanooga. These comments are adapted from an accompanying editorial (JAMA Netw Open. 2019 Mar 15. doi: 10.1001/jamanetworkopen.2019.0405). No conflicts of interest were reported.
Older women do not get enough physical activity, so this finding that light physical activity is associated with improved coronary heart disease and cardiovascular disease outcomes supports the recent scientific report by the 2018 Physical Activity Guidelines Advisory Committee. It is also helpful in extending the evidence about the benefits of physical activity in reducing incident coronary heart disease to older women, as previous studies on this topic showed such benefits in men.
These findings should remind health care professionals, systems, and agencies to promote the 2018 Physical Activity Guidelines for Americans to all patients. Otherwise, the future health and well-being of older women is likely to suffer from the consequences of sedentary behavior and inadequate physical activity.
Gregory W. Heath, DHSc, MPH, is from the department of health and human performance at the University of Tennessee, Chattanooga. These comments are adapted from an accompanying editorial (JAMA Netw Open. 2019 Mar 15. doi: 10.1001/jamanetworkopen.2019.0405). No conflicts of interest were reported.
Older women do not get enough physical activity, so this finding that light physical activity is associated with improved coronary heart disease and cardiovascular disease outcomes supports the recent scientific report by the 2018 Physical Activity Guidelines Advisory Committee. It is also helpful in extending the evidence about the benefits of physical activity in reducing incident coronary heart disease to older women, as previous studies on this topic showed such benefits in men.
These findings should remind health care professionals, systems, and agencies to promote the 2018 Physical Activity Guidelines for Americans to all patients. Otherwise, the future health and well-being of older women is likely to suffer from the consequences of sedentary behavior and inadequate physical activity.
Gregory W. Heath, DHSc, MPH, is from the department of health and human performance at the University of Tennessee, Chattanooga. These comments are adapted from an accompanying editorial (JAMA Netw Open. 2019 Mar 15. doi: 10.1001/jamanetworkopen.2019.0405). No conflicts of interest were reported.
Even light physical activity can significantly reduce the risks of acquiring coronary heart disease specifically and the broad range of cardiovascular diseases in older women, new data suggests.
A paper published in JAMA Network Open reported the outcome of a prospective cohort study in 5,861 women, with a mean age of 78.5 years, who wore accelerometers for 7 days to measure physical activity.
than those in the lowest quartile of activity, who engaged in less than 3.9 hours per day, after adjusting for factors such as comorbidities, lifestyle, and cardiovascular risk.
Similarly, those in the highest quartile had an 18% lower risk of cardiovascular disease than those in the lowest quartile, after adjusting for potential confounders.
Researchers saw a significant dose-dependent decrease in the risk for incident coronary heart disease and cardiovascular disease with increasing light physical activity, such that each 1-hour increment of activity was associated with a 20% decrease in coronary heart disease risk and 10% decrease in cardiovascular disease risk.
Andrea Z. LaCroix, PhD, from the University of California, San Diego, and her coauthors noted that physical activity guidelines for aerobic activity suggest 75 minutes of vigorous physical activity or 150 minutes of moderate activity each day, but only around 25% of U.S. women aged over 75 years are estimated to meet this requirement.
“These guidelines may have discouraged PA [physical activity] when perceived to be unattainable by large segments of the population,” they wrote.
While the majority of active time in older adults is spent doing light physical activity, little is known about the cardiovascular effects of participating in this level of activity. “A major barrier has been that self-reported questionnaires measuring leisure-time PA do not adequately capture light PA that is acquired throughout the day in activities of daily living,” they wrote.
The study also looked at the impact of moderate to vigorous physical activity, finding a significant 46% reduction between the highest to lowest quartiles of activity in coronary heart disease risk and a 31% reduction in cardiovascular disease risk.
Even after adjusting for the use of lipid-lowering medication, antihypertensive medication or healthy eating scores, the results remained unchanged. The researchers also saw no change when women with angina and heart failure at baseline were excluded or when they excluded cardiovascular events that occurred during the first 6 months of follow-up.
The study was supported by the National Heart, Lung, and Blood Institute; the National Institutes of Health; and the Department of Health & Human Services. Six authors reported receiving funding from the study supporters and other research institutions, and one reported membership on the advisory committee for physical activity guidelines. No other conflicts of interest were reported.
SOURCE: LaCroix AZ et al. JAMA Netw Open. 2019 Mar 15. doi: 10.1001/jamanetworkopen.2019.0419.
Even light physical activity can significantly reduce the risks of acquiring coronary heart disease specifically and the broad range of cardiovascular diseases in older women, new data suggests.
A paper published in JAMA Network Open reported the outcome of a prospective cohort study in 5,861 women, with a mean age of 78.5 years, who wore accelerometers for 7 days to measure physical activity.
than those in the lowest quartile of activity, who engaged in less than 3.9 hours per day, after adjusting for factors such as comorbidities, lifestyle, and cardiovascular risk.
Similarly, those in the highest quartile had an 18% lower risk of cardiovascular disease than those in the lowest quartile, after adjusting for potential confounders.
Researchers saw a significant dose-dependent decrease in the risk for incident coronary heart disease and cardiovascular disease with increasing light physical activity, such that each 1-hour increment of activity was associated with a 20% decrease in coronary heart disease risk and 10% decrease in cardiovascular disease risk.
Andrea Z. LaCroix, PhD, from the University of California, San Diego, and her coauthors noted that physical activity guidelines for aerobic activity suggest 75 minutes of vigorous physical activity or 150 minutes of moderate activity each day, but only around 25% of U.S. women aged over 75 years are estimated to meet this requirement.
“These guidelines may have discouraged PA [physical activity] when perceived to be unattainable by large segments of the population,” they wrote.
While the majority of active time in older adults is spent doing light physical activity, little is known about the cardiovascular effects of participating in this level of activity. “A major barrier has been that self-reported questionnaires measuring leisure-time PA do not adequately capture light PA that is acquired throughout the day in activities of daily living,” they wrote.
The study also looked at the impact of moderate to vigorous physical activity, finding a significant 46% reduction between the highest to lowest quartiles of activity in coronary heart disease risk and a 31% reduction in cardiovascular disease risk.
Even after adjusting for the use of lipid-lowering medication, antihypertensive medication or healthy eating scores, the results remained unchanged. The researchers also saw no change when women with angina and heart failure at baseline were excluded or when they excluded cardiovascular events that occurred during the first 6 months of follow-up.
The study was supported by the National Heart, Lung, and Blood Institute; the National Institutes of Health; and the Department of Health & Human Services. Six authors reported receiving funding from the study supporters and other research institutions, and one reported membership on the advisory committee for physical activity guidelines. No other conflicts of interest were reported.
SOURCE: LaCroix AZ et al. JAMA Netw Open. 2019 Mar 15. doi: 10.1001/jamanetworkopen.2019.0419.
FROM JAMA NETWORK OPEN
For Latino patients, mental illness often goes untreated
Intergenerational trauma, attitudes can allow cycles of depression, anxiety to continue
The stigma tied to mental illness can be particularly difficult to overcome for people of Latin American descent, writes Concepción de León in El Espace, a column in the New York Times focused on news and culture relevant to Latinx communities. Sometimes those seeking help run into familiar mantras. “Let me know if any of these sound familiar: 'Boys don’t cry. We don’t air family business. You have to be strong. Turn to God.' These refrains (all of which I’ve heard at least once...) are just some of the responses that people dealing with mental health challenges in Latino communities have come to know well,” Ms. de León wrote. The unequal access to mental health services and health insurance that is a reality for some Latinos compounds the problem. The result is that mental illness can go untreated. Indeed, according to Ms. de León, Latinos, who are just as likely to suffer from a mental illness as non-Hispanic whites, are half as likely to seek treatment. Adriana Alejandre, a Latina who is a licensed marriage and family therapist in Los Angeles, is seeking to change that statistic. Through her podcast, Latinx Therapy, she seeks to spread the word that seeking therapy for mental illness is a positive step. There’s a long way to go, partly because Latino communities tend to value the group over the individual. “The downfall is that people suffer in silence,” said Ms. Alejandre. Therapy is important for some Latinos, according to Ms. Alejandre, because of intergenerational trauma that “allows the cycle to continue – whether it’s trauma, whether it’s depression, anxiety, domestic violence.” Ms. de León said one strategy she used for more than 1 year while she was in therapy was to set boundaries by not sharing what she was doing with family members. Ms. Alejandre said. “But the system will not change if someone does not initiate the change.” The New York Times.
Some state governments are seeking to make mental health services more available. The proposed budget of democratic Gov. Tony Evers of Wisconsin aims to allocate $22 million in mental health funding to school districts in the state to pay for social workers, psychologists, counselors, and nurses. The money would come on top of the $3 million designated by his predecessor and continues the efforts in Wisconsin to give children with mental health problems more access to needed help. The proposed budget also would add $7 million to a state program that works with local health agencies with the goal of providing mental health services for students and would allocate about $2.5 million annually for school staff training. The news is welcome to school districts across Wisconsin. “Schools are struggling to meet all of those [mental health] needs. I think there is an understanding that this is really something we need to be addressing,” said Joanne Juhnke, policy director at Wisconsin Family Ties, which helps families with children who have mental health challenges. Post Crescent, part of the USA Today network.
In Pennsylvania, the state Supreme Court is set to rule on whether those who provide mental health treatment to people addicted to illicit drugs can be free from prosecution. Right now, they are not. As reported in the Legal Intelligence, the case concerns two physicians at a drug addiction treatment facility who treated a man with an opioid addiction. In July 2018, a three-judge Superior Court panel upheld that physicians should not have liability protections under the Mental Health Procedures Act (MHPA). The ruling reversed a lower court decision. The Superior Court judges sympathized with the view that treatment of mental illness in drug treatment facilities be given more legal leeway. Whether that leeway remains in place depends on the Supreme Court. If judges decide no, physicians who recognize signs of mental illness in patients being treated for drug addiction would treat the illness at the risk of subsequent liability. The case has again raised the issue of whether alcoholism and drug dependency should be considered mental illnesses. “We don’t believe it was the intended purpose of the MPHA to include drug addiction. Our concern is we don’t want hospitals or rehab facilities just having patients be seen by psychiatrists in order to invoke the MHPA,” said Patrick Mintzer, the lawyer who will argue the cases before the court. A counter view came from Jack Panella, one of the three Superior Court judges. In his decision, he wrote: “In light of current scientific research, as well as the recent addition of ‘addiction disorders’ to the American Psychiatric Association’s Diagnostic and Statistical Manual–5, we suggest that the Department of Human Services revise this definition.” The Legal Intelligence.
An op-ed in the Des Moines Register applauds republican Gov. Kim Reynolds for introducing two bills that are aimed at expanding mental health services to children and family in Iowa. “After decades of discussion and growing public support, these two bills take a huge step toward establishing a children’s mental health system,” wrote guest columnists Erin Drinnin of the United Way of Central Iowa and Kim Scorza of Seasons Center for Behavioral Health. The two also serve as cochairs of the Coalition to Advance Mental Health in Iowa for Kids (CAMHI4Kids), which includes more than 50 organizations. “Just like building a house requires a sturdy foundation, these bills are an important first step toward creating a structure for children’s mental well-being. In particular, CAMHI4Kids appreciates that these bills establish a voice and a seat for children and families at a regional level, using a system that is already in place,” wrote Ms. Drinnin and Ms. Scorza. The legislation would spell out the core services that would be available regardless of location in Iowa. The services would be geared toward children, rather than adults, reflecting the different mental health needs of children. “These important steps would finally sew together a patchwork of care that families currently must navigate with little direction. If a child is hurt on the playground, a caregiver knows to follow a clear path of care to help that child recover. But for a caregiver who is concerned about a child’s mental health, they often don’t know where to turn for help and must seek out services that might not exist in their community,” wrote Ms. Drinnin and Ms. Scorza. In Iowa, 80,000 children have a diagnosed serious emotional disturbance. About half of children aged 14 years and older with mental illness drop out of high school, and 70% of youth in Iowa’s juvenile justice system have a mental illness. “We are proud that Iowa is working together in a bipartisan way to ensure that our kids have the best start for future success,” wrote Ms. Drinnin and Ms. Scorza. Des Moines Register.
Bill Reilly is the peer support program manager for Bert Nash Community Mental Health Center in Douglas County, Kan. His mental health troubles began in childhood and led to stints in alcohol rehabilitation and mental hospitals, and he tried to end his life several times. But Mr. Reilly now offers his experience to those in trouble. “Those [experiences] can be viewed as a negative until you turn that conversation around and ask, ‘How can this be helpful to another person?’ And to me, that’s where the urgency comes into the work that we’re doing because a clinical relationship is one thing, but a peer support relationship is something different.” He was speaking in support of an initiative that seeks to train and place peer support people in hospital emergency departments in Kansas. The initiative is being spearheaded by Bob Tryanski, Douglas County director of behavioral health projects. “In addition to giving folks the opportunity to have the work experience in an environment where we need peer support, we would wrap around those peers with training, professional development, with coaching and support in an ongoing way,” Mr. Tryanski said, “so that they could become real, robust, huge resources, not just to the emergency department but in our community.” If approved, hiring and training of peers would begin in April, with the goal of having six people in place in emergency rooms by the summer and hiring an additional six people by year end. LJWorld.com.
Intergenerational trauma, attitudes can allow cycles of depression, anxiety to continue
Intergenerational trauma, attitudes can allow cycles of depression, anxiety to continue
The stigma tied to mental illness can be particularly difficult to overcome for people of Latin American descent, writes Concepción de León in El Espace, a column in the New York Times focused on news and culture relevant to Latinx communities. Sometimes those seeking help run into familiar mantras. “Let me know if any of these sound familiar: 'Boys don’t cry. We don’t air family business. You have to be strong. Turn to God.' These refrains (all of which I’ve heard at least once...) are just some of the responses that people dealing with mental health challenges in Latino communities have come to know well,” Ms. de León wrote. The unequal access to mental health services and health insurance that is a reality for some Latinos compounds the problem. The result is that mental illness can go untreated. Indeed, according to Ms. de León, Latinos, who are just as likely to suffer from a mental illness as non-Hispanic whites, are half as likely to seek treatment. Adriana Alejandre, a Latina who is a licensed marriage and family therapist in Los Angeles, is seeking to change that statistic. Through her podcast, Latinx Therapy, she seeks to spread the word that seeking therapy for mental illness is a positive step. There’s a long way to go, partly because Latino communities tend to value the group over the individual. “The downfall is that people suffer in silence,” said Ms. Alejandre. Therapy is important for some Latinos, according to Ms. Alejandre, because of intergenerational trauma that “allows the cycle to continue – whether it’s trauma, whether it’s depression, anxiety, domestic violence.” Ms. de León said one strategy she used for more than 1 year while she was in therapy was to set boundaries by not sharing what she was doing with family members. Ms. Alejandre said. “But the system will not change if someone does not initiate the change.” The New York Times.
Some state governments are seeking to make mental health services more available. The proposed budget of democratic Gov. Tony Evers of Wisconsin aims to allocate $22 million in mental health funding to school districts in the state to pay for social workers, psychologists, counselors, and nurses. The money would come on top of the $3 million designated by his predecessor and continues the efforts in Wisconsin to give children with mental health problems more access to needed help. The proposed budget also would add $7 million to a state program that works with local health agencies with the goal of providing mental health services for students and would allocate about $2.5 million annually for school staff training. The news is welcome to school districts across Wisconsin. “Schools are struggling to meet all of those [mental health] needs. I think there is an understanding that this is really something we need to be addressing,” said Joanne Juhnke, policy director at Wisconsin Family Ties, which helps families with children who have mental health challenges. Post Crescent, part of the USA Today network.
In Pennsylvania, the state Supreme Court is set to rule on whether those who provide mental health treatment to people addicted to illicit drugs can be free from prosecution. Right now, they are not. As reported in the Legal Intelligence, the case concerns two physicians at a drug addiction treatment facility who treated a man with an opioid addiction. In July 2018, a three-judge Superior Court panel upheld that physicians should not have liability protections under the Mental Health Procedures Act (MHPA). The ruling reversed a lower court decision. The Superior Court judges sympathized with the view that treatment of mental illness in drug treatment facilities be given more legal leeway. Whether that leeway remains in place depends on the Supreme Court. If judges decide no, physicians who recognize signs of mental illness in patients being treated for drug addiction would treat the illness at the risk of subsequent liability. The case has again raised the issue of whether alcoholism and drug dependency should be considered mental illnesses. “We don’t believe it was the intended purpose of the MPHA to include drug addiction. Our concern is we don’t want hospitals or rehab facilities just having patients be seen by psychiatrists in order to invoke the MHPA,” said Patrick Mintzer, the lawyer who will argue the cases before the court. A counter view came from Jack Panella, one of the three Superior Court judges. In his decision, he wrote: “In light of current scientific research, as well as the recent addition of ‘addiction disorders’ to the American Psychiatric Association’s Diagnostic and Statistical Manual–5, we suggest that the Department of Human Services revise this definition.” The Legal Intelligence.
An op-ed in the Des Moines Register applauds republican Gov. Kim Reynolds for introducing two bills that are aimed at expanding mental health services to children and family in Iowa. “After decades of discussion and growing public support, these two bills take a huge step toward establishing a children’s mental health system,” wrote guest columnists Erin Drinnin of the United Way of Central Iowa and Kim Scorza of Seasons Center for Behavioral Health. The two also serve as cochairs of the Coalition to Advance Mental Health in Iowa for Kids (CAMHI4Kids), which includes more than 50 organizations. “Just like building a house requires a sturdy foundation, these bills are an important first step toward creating a structure for children’s mental well-being. In particular, CAMHI4Kids appreciates that these bills establish a voice and a seat for children and families at a regional level, using a system that is already in place,” wrote Ms. Drinnin and Ms. Scorza. The legislation would spell out the core services that would be available regardless of location in Iowa. The services would be geared toward children, rather than adults, reflecting the different mental health needs of children. “These important steps would finally sew together a patchwork of care that families currently must navigate with little direction. If a child is hurt on the playground, a caregiver knows to follow a clear path of care to help that child recover. But for a caregiver who is concerned about a child’s mental health, they often don’t know where to turn for help and must seek out services that might not exist in their community,” wrote Ms. Drinnin and Ms. Scorza. In Iowa, 80,000 children have a diagnosed serious emotional disturbance. About half of children aged 14 years and older with mental illness drop out of high school, and 70% of youth in Iowa’s juvenile justice system have a mental illness. “We are proud that Iowa is working together in a bipartisan way to ensure that our kids have the best start for future success,” wrote Ms. Drinnin and Ms. Scorza. Des Moines Register.
Bill Reilly is the peer support program manager for Bert Nash Community Mental Health Center in Douglas County, Kan. His mental health troubles began in childhood and led to stints in alcohol rehabilitation and mental hospitals, and he tried to end his life several times. But Mr. Reilly now offers his experience to those in trouble. “Those [experiences] can be viewed as a negative until you turn that conversation around and ask, ‘How can this be helpful to another person?’ And to me, that’s where the urgency comes into the work that we’re doing because a clinical relationship is one thing, but a peer support relationship is something different.” He was speaking in support of an initiative that seeks to train and place peer support people in hospital emergency departments in Kansas. The initiative is being spearheaded by Bob Tryanski, Douglas County director of behavioral health projects. “In addition to giving folks the opportunity to have the work experience in an environment where we need peer support, we would wrap around those peers with training, professional development, with coaching and support in an ongoing way,” Mr. Tryanski said, “so that they could become real, robust, huge resources, not just to the emergency department but in our community.” If approved, hiring and training of peers would begin in April, with the goal of having six people in place in emergency rooms by the summer and hiring an additional six people by year end. LJWorld.com.
The stigma tied to mental illness can be particularly difficult to overcome for people of Latin American descent, writes Concepción de León in El Espace, a column in the New York Times focused on news and culture relevant to Latinx communities. Sometimes those seeking help run into familiar mantras. “Let me know if any of these sound familiar: 'Boys don’t cry. We don’t air family business. You have to be strong. Turn to God.' These refrains (all of which I’ve heard at least once...) are just some of the responses that people dealing with mental health challenges in Latino communities have come to know well,” Ms. de León wrote. The unequal access to mental health services and health insurance that is a reality for some Latinos compounds the problem. The result is that mental illness can go untreated. Indeed, according to Ms. de León, Latinos, who are just as likely to suffer from a mental illness as non-Hispanic whites, are half as likely to seek treatment. Adriana Alejandre, a Latina who is a licensed marriage and family therapist in Los Angeles, is seeking to change that statistic. Through her podcast, Latinx Therapy, she seeks to spread the word that seeking therapy for mental illness is a positive step. There’s a long way to go, partly because Latino communities tend to value the group over the individual. “The downfall is that people suffer in silence,” said Ms. Alejandre. Therapy is important for some Latinos, according to Ms. Alejandre, because of intergenerational trauma that “allows the cycle to continue – whether it’s trauma, whether it’s depression, anxiety, domestic violence.” Ms. de León said one strategy she used for more than 1 year while she was in therapy was to set boundaries by not sharing what she was doing with family members. Ms. Alejandre said. “But the system will not change if someone does not initiate the change.” The New York Times.
Some state governments are seeking to make mental health services more available. The proposed budget of democratic Gov. Tony Evers of Wisconsin aims to allocate $22 million in mental health funding to school districts in the state to pay for social workers, psychologists, counselors, and nurses. The money would come on top of the $3 million designated by his predecessor and continues the efforts in Wisconsin to give children with mental health problems more access to needed help. The proposed budget also would add $7 million to a state program that works with local health agencies with the goal of providing mental health services for students and would allocate about $2.5 million annually for school staff training. The news is welcome to school districts across Wisconsin. “Schools are struggling to meet all of those [mental health] needs. I think there is an understanding that this is really something we need to be addressing,” said Joanne Juhnke, policy director at Wisconsin Family Ties, which helps families with children who have mental health challenges. Post Crescent, part of the USA Today network.
In Pennsylvania, the state Supreme Court is set to rule on whether those who provide mental health treatment to people addicted to illicit drugs can be free from prosecution. Right now, they are not. As reported in the Legal Intelligence, the case concerns two physicians at a drug addiction treatment facility who treated a man with an opioid addiction. In July 2018, a three-judge Superior Court panel upheld that physicians should not have liability protections under the Mental Health Procedures Act (MHPA). The ruling reversed a lower court decision. The Superior Court judges sympathized with the view that treatment of mental illness in drug treatment facilities be given more legal leeway. Whether that leeway remains in place depends on the Supreme Court. If judges decide no, physicians who recognize signs of mental illness in patients being treated for drug addiction would treat the illness at the risk of subsequent liability. The case has again raised the issue of whether alcoholism and drug dependency should be considered mental illnesses. “We don’t believe it was the intended purpose of the MPHA to include drug addiction. Our concern is we don’t want hospitals or rehab facilities just having patients be seen by psychiatrists in order to invoke the MHPA,” said Patrick Mintzer, the lawyer who will argue the cases before the court. A counter view came from Jack Panella, one of the three Superior Court judges. In his decision, he wrote: “In light of current scientific research, as well as the recent addition of ‘addiction disorders’ to the American Psychiatric Association’s Diagnostic and Statistical Manual–5, we suggest that the Department of Human Services revise this definition.” The Legal Intelligence.
An op-ed in the Des Moines Register applauds republican Gov. Kim Reynolds for introducing two bills that are aimed at expanding mental health services to children and family in Iowa. “After decades of discussion and growing public support, these two bills take a huge step toward establishing a children’s mental health system,” wrote guest columnists Erin Drinnin of the United Way of Central Iowa and Kim Scorza of Seasons Center for Behavioral Health. The two also serve as cochairs of the Coalition to Advance Mental Health in Iowa for Kids (CAMHI4Kids), which includes more than 50 organizations. “Just like building a house requires a sturdy foundation, these bills are an important first step toward creating a structure for children’s mental well-being. In particular, CAMHI4Kids appreciates that these bills establish a voice and a seat for children and families at a regional level, using a system that is already in place,” wrote Ms. Drinnin and Ms. Scorza. The legislation would spell out the core services that would be available regardless of location in Iowa. The services would be geared toward children, rather than adults, reflecting the different mental health needs of children. “These important steps would finally sew together a patchwork of care that families currently must navigate with little direction. If a child is hurt on the playground, a caregiver knows to follow a clear path of care to help that child recover. But for a caregiver who is concerned about a child’s mental health, they often don’t know where to turn for help and must seek out services that might not exist in their community,” wrote Ms. Drinnin and Ms. Scorza. In Iowa, 80,000 children have a diagnosed serious emotional disturbance. About half of children aged 14 years and older with mental illness drop out of high school, and 70% of youth in Iowa’s juvenile justice system have a mental illness. “We are proud that Iowa is working together in a bipartisan way to ensure that our kids have the best start for future success,” wrote Ms. Drinnin and Ms. Scorza. Des Moines Register.
Bill Reilly is the peer support program manager for Bert Nash Community Mental Health Center in Douglas County, Kan. His mental health troubles began in childhood and led to stints in alcohol rehabilitation and mental hospitals, and he tried to end his life several times. But Mr. Reilly now offers his experience to those in trouble. “Those [experiences] can be viewed as a negative until you turn that conversation around and ask, ‘How can this be helpful to another person?’ And to me, that’s where the urgency comes into the work that we’re doing because a clinical relationship is one thing, but a peer support relationship is something different.” He was speaking in support of an initiative that seeks to train and place peer support people in hospital emergency departments in Kansas. The initiative is being spearheaded by Bob Tryanski, Douglas County director of behavioral health projects. “In addition to giving folks the opportunity to have the work experience in an environment where we need peer support, we would wrap around those peers with training, professional development, with coaching and support in an ongoing way,” Mr. Tryanski said, “so that they could become real, robust, huge resources, not just to the emergency department but in our community.” If approved, hiring and training of peers would begin in April, with the goal of having six people in place in emergency rooms by the summer and hiring an additional six people by year end. LJWorld.com.











