User login
Dr. Julie Thompson Discusses Primary Biliary Cholangitis

At Digestive Diseases: New Advances (DDNA 2019), Dr. Julie A. Thompson of the University of Minnesota reviews key issues surrounding primary biliary cholangitis, including difficult symptoms to treat, an update on clinical trials, and patients that take medication and see no improvements.

At Digestive Diseases: New Advances (DDNA 2019), Dr. Julie A. Thompson of the University of Minnesota reviews key issues surrounding primary biliary cholangitis, including difficult symptoms to treat, an update on clinical trials, and patients that take medication and see no improvements.

At Digestive Diseases: New Advances (DDNA 2019), Dr. Julie A. Thompson of the University of Minnesota reviews key issues surrounding primary biliary cholangitis, including difficult symptoms to treat, an update on clinical trials, and patients that take medication and see no improvements.
AT DIGESTIVE DISEASES: NEW ADVANCES
Match Day 2019: Pediatrics up from last year
The pediatrics specialty filled 97.6% of its offered positions, according to the National Resident Matching Program (NRMP). Of these, 60.2% were filled by U.S. seniors, which was down from last year’s 63.1%.
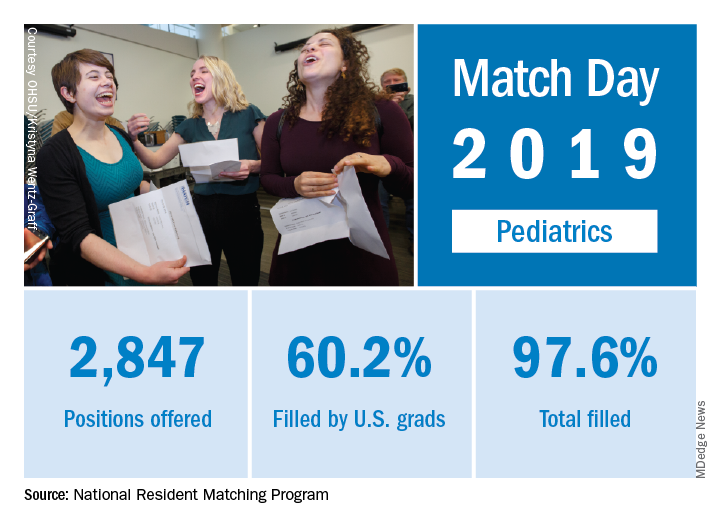
Pediatrics brought 2,847 first-year positions to the 2019 Main Resident Match day, up from 2,768 in 2018. However, a slightly higher proportion (97.9%) of Match Day offerings were filled in 2018 than in 2019. Internal medicine/pediatrics offered 390 positions, more than 2018’s 382 positions. Although a slightly smaller proportion were filled in 2019 (98.2% vs. 98.7% ), a slightly larger proportion of positions in 2019 were filled by U.S. graduates (80.8% in 2019 vs. 80.1% in 2018). For all specialties, U.S. first-year applicants filled 55.2%, and the overall fill rate was 94.9% of the 32,194 available positions, an increase of 6.5% over the number of positions available in 2018, according to the NRMP in its 2019 Main Resident Match report.
Overall, the 2019 Match set a new high for total positions offered at 35,135, for which there were a record-high 38,376 applicants. The increases are likely related, in part, to the greater number of osteopathic programs joining the Match Day offerings because of an ongoing move toward a single accreditation system.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
The pediatrics specialty filled 97.6% of its offered positions, according to the National Resident Matching Program (NRMP). Of these, 60.2% were filled by U.S. seniors, which was down from last year’s 63.1%.

Pediatrics brought 2,847 first-year positions to the 2019 Main Resident Match day, up from 2,768 in 2018. However, a slightly higher proportion (97.9%) of Match Day offerings were filled in 2018 than in 2019. Internal medicine/pediatrics offered 390 positions, more than 2018’s 382 positions. Although a slightly smaller proportion were filled in 2019 (98.2% vs. 98.7% ), a slightly larger proportion of positions in 2019 were filled by U.S. graduates (80.8% in 2019 vs. 80.1% in 2018). For all specialties, U.S. first-year applicants filled 55.2%, and the overall fill rate was 94.9% of the 32,194 available positions, an increase of 6.5% over the number of positions available in 2018, according to the NRMP in its 2019 Main Resident Match report.
Overall, the 2019 Match set a new high for total positions offered at 35,135, for which there were a record-high 38,376 applicants. The increases are likely related, in part, to the greater number of osteopathic programs joining the Match Day offerings because of an ongoing move toward a single accreditation system.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
The pediatrics specialty filled 97.6% of its offered positions, according to the National Resident Matching Program (NRMP). Of these, 60.2% were filled by U.S. seniors, which was down from last year’s 63.1%.

Pediatrics brought 2,847 first-year positions to the 2019 Main Resident Match day, up from 2,768 in 2018. However, a slightly higher proportion (97.9%) of Match Day offerings were filled in 2018 than in 2019. Internal medicine/pediatrics offered 390 positions, more than 2018’s 382 positions. Although a slightly smaller proportion were filled in 2019 (98.2% vs. 98.7% ), a slightly larger proportion of positions in 2019 were filled by U.S. graduates (80.8% in 2019 vs. 80.1% in 2018). For all specialties, U.S. first-year applicants filled 55.2%, and the overall fill rate was 94.9% of the 32,194 available positions, an increase of 6.5% over the number of positions available in 2018, according to the NRMP in its 2019 Main Resident Match report.
Overall, the 2019 Match set a new high for total positions offered at 35,135, for which there were a record-high 38,376 applicants. The increases are likely related, in part, to the greater number of osteopathic programs joining the Match Day offerings because of an ongoing move toward a single accreditation system.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
FDA advises alternatives to paclitaxel-coated devices for PAD, pending review
“Alternative treatment options should generally be used for most patients,” rather than paclitaxel-coated balloons and stents for peripheral arterial disease (PAD), pending an ongoing safety review, according to the Food and Drug Administration.
The FDA conducted a preliminary analysis of long-term follow-up data (up to 5 years in some studies) of the pivotal premarket randomized trials for paclitaxel-coated products indicated for peripheral arterial disease (PAD). In a Letter to Healthcare providers issued March 15, the FDA reported that their preliminary review of these data found “a potentially concerning signal of increased long-term mortality in study subjects treated with paclitaxel-coated products, compared to patients treated with uncoated devices.”
The three trials (totaling 975 patients) that had 5-year follow-up data demonstrated an approximately 50% increased risk of mortality in subjects treated with paclitaxel-coated devices vs. those treated with control devices (20.1% vs. 13.4% crude risk of death at 5 years), according to the agency.
The FDA indicated that these data “should be interpreted with caution for several reasons.” They cited a large variability in the risk estimate of mortality because of the limited amount of long-term data and pointed out that the studies were not designed to be pooled. In addition, the specific cause and mechanism of the increased mortality was unknown.
The FDA also announced that they are planning on convening an Advisory Committee meeting of the Circulatory System Devices Panel to address this issue, including plausible mechanisms for this mortality effect, a re-examination of the benefit-risk profile, modifications of current and future clinical trials regarding these devices, and guidance to any regulatory action, as needed. The timing of this meeting is to be announced within the upcoming weeks.
The FDA letter further stated that the agency intends to conduct additional analyses “to determine whether the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use.”
[email protected]
SOURCE: Food and Drug Administration Letter to Healthcare Providers. 2019 Mar 15.
“Alternative treatment options should generally be used for most patients,” rather than paclitaxel-coated balloons and stents for peripheral arterial disease (PAD), pending an ongoing safety review, according to the Food and Drug Administration.
The FDA conducted a preliminary analysis of long-term follow-up data (up to 5 years in some studies) of the pivotal premarket randomized trials for paclitaxel-coated products indicated for peripheral arterial disease (PAD). In a Letter to Healthcare providers issued March 15, the FDA reported that their preliminary review of these data found “a potentially concerning signal of increased long-term mortality in study subjects treated with paclitaxel-coated products, compared to patients treated with uncoated devices.”
The three trials (totaling 975 patients) that had 5-year follow-up data demonstrated an approximately 50% increased risk of mortality in subjects treated with paclitaxel-coated devices vs. those treated with control devices (20.1% vs. 13.4% crude risk of death at 5 years), according to the agency.
The FDA indicated that these data “should be interpreted with caution for several reasons.” They cited a large variability in the risk estimate of mortality because of the limited amount of long-term data and pointed out that the studies were not designed to be pooled. In addition, the specific cause and mechanism of the increased mortality was unknown.
The FDA also announced that they are planning on convening an Advisory Committee meeting of the Circulatory System Devices Panel to address this issue, including plausible mechanisms for this mortality effect, a re-examination of the benefit-risk profile, modifications of current and future clinical trials regarding these devices, and guidance to any regulatory action, as needed. The timing of this meeting is to be announced within the upcoming weeks.
The FDA letter further stated that the agency intends to conduct additional analyses “to determine whether the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use.”
[email protected]
SOURCE: Food and Drug Administration Letter to Healthcare Providers. 2019 Mar 15.
“Alternative treatment options should generally be used for most patients,” rather than paclitaxel-coated balloons and stents for peripheral arterial disease (PAD), pending an ongoing safety review, according to the Food and Drug Administration.
The FDA conducted a preliminary analysis of long-term follow-up data (up to 5 years in some studies) of the pivotal premarket randomized trials for paclitaxel-coated products indicated for peripheral arterial disease (PAD). In a Letter to Healthcare providers issued March 15, the FDA reported that their preliminary review of these data found “a potentially concerning signal of increased long-term mortality in study subjects treated with paclitaxel-coated products, compared to patients treated with uncoated devices.”
The three trials (totaling 975 patients) that had 5-year follow-up data demonstrated an approximately 50% increased risk of mortality in subjects treated with paclitaxel-coated devices vs. those treated with control devices (20.1% vs. 13.4% crude risk of death at 5 years), according to the agency.
The FDA indicated that these data “should be interpreted with caution for several reasons.” They cited a large variability in the risk estimate of mortality because of the limited amount of long-term data and pointed out that the studies were not designed to be pooled. In addition, the specific cause and mechanism of the increased mortality was unknown.
The FDA also announced that they are planning on convening an Advisory Committee meeting of the Circulatory System Devices Panel to address this issue, including plausible mechanisms for this mortality effect, a re-examination of the benefit-risk profile, modifications of current and future clinical trials regarding these devices, and guidance to any regulatory action, as needed. The timing of this meeting is to be announced within the upcoming weeks.
The FDA letter further stated that the agency intends to conduct additional analyses “to determine whether the benefits continue to outweigh the risks for approved paclitaxel-coated balloons and paclitaxel-eluting stents when used in accordance with their indications for use.”
[email protected]
SOURCE: Food and Drug Administration Letter to Healthcare Providers. 2019 Mar 15.
Key clinical point: FDA advises that alternatives to paclitaxel-coated devices for PAD should be used for most patients.
Major finding: Five-year data demonstrated an approximately 50% increased risk of mortality in patients with paclitaxel-coated devices, compared with uncoated ones.
Study details: Preliminary FDA review of three trials with 975 patients.
Disclosures: Study is funded and performed by the FDA.
Source: Food and Drug Administration. Letter to Healthcare Providers. 2019 Mar 15.
Match Day 2019: Family medicine slots up by 13%
for the first time since 2009, according to the National Resident Matching Program (NRMP).
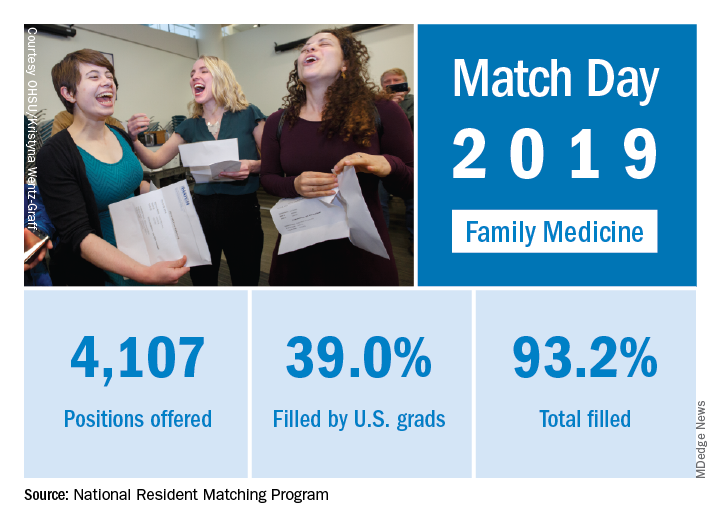
First-year FP slots rose from 3,629 to 4,107 as family medicine doubled the 6.5% increase in all first-year positions over 2018. The numbers of applicants (38,376) and total positions offered (35,185) were both record highs for the Match,although they were affected, in part, by “increased numbers of osteopathic programs that joined the Main Residency Match as a result of the ongoing transition to a single accreditation system for graduate medical education programs,” the NRMP noted.
Family medicine programs filled 39.0% of first-year positions with U.S. allopathic seniors, which was down from 44.9% in 2018 and 45.1% in 2017. In terms of the numbers of U.S. seniors involved, the drop was fairly small: from 1,628 in 2018 to 1,601 in 2019; however, the 986 osteopathic students and graduates who matched set a new record and accounted for almost 26% of all successful FP applicants, the NRMP reported.“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said in a written statement.
for the first time since 2009, according to the National Resident Matching Program (NRMP).

First-year FP slots rose from 3,629 to 4,107 as family medicine doubled the 6.5% increase in all first-year positions over 2018. The numbers of applicants (38,376) and total positions offered (35,185) were both record highs for the Match,although they were affected, in part, by “increased numbers of osteopathic programs that joined the Main Residency Match as a result of the ongoing transition to a single accreditation system for graduate medical education programs,” the NRMP noted.
Family medicine programs filled 39.0% of first-year positions with U.S. allopathic seniors, which was down from 44.9% in 2018 and 45.1% in 2017. In terms of the numbers of U.S. seniors involved, the drop was fairly small: from 1,628 in 2018 to 1,601 in 2019; however, the 986 osteopathic students and graduates who matched set a new record and accounted for almost 26% of all successful FP applicants, the NRMP reported.“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said in a written statement.
for the first time since 2009, according to the National Resident Matching Program (NRMP).

First-year FP slots rose from 3,629 to 4,107 as family medicine doubled the 6.5% increase in all first-year positions over 2018. The numbers of applicants (38,376) and total positions offered (35,185) were both record highs for the Match,although they were affected, in part, by “increased numbers of osteopathic programs that joined the Main Residency Match as a result of the ongoing transition to a single accreditation system for graduate medical education programs,” the NRMP noted.
Family medicine programs filled 39.0% of first-year positions with U.S. allopathic seniors, which was down from 44.9% in 2018 and 45.1% in 2017. In terms of the numbers of U.S. seniors involved, the drop was fairly small: from 1,628 in 2018 to 1,601 in 2019; however, the 986 osteopathic students and graduates who matched set a new record and accounted for almost 26% of all successful FP applicants, the NRMP reported.“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said in a written statement.
Match Day 2019: Dermatology steps up growth after slow 2018
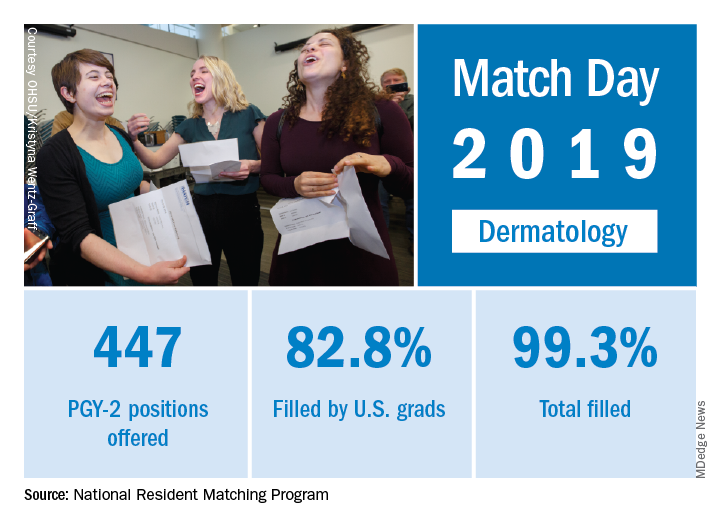
Available dermatology PGY-2 slots rose by 4.9% from 426 in 2018 to 447 in 2019, while slots filled grew by 5.5% from 420 to 443, for an overall fill rate of 99.3%. In addition, the fill rate for U.S. graduates grew for the first time since 2015, rising from 81.7% to 82.8%.
An overall total of 2,756 PGY-2 slots were offered, 97.2% of which were filled; 67.5% were filled by U.S. graduates, the NRMP said in its 2019 Main Residency Match report.
The 2019 Match set a record for most positions offered (35,185; up 6.1%), most positions filled (up 4.8%), most PGY-1 positions offered (32,194; up 6.5%), and total applicants (38,376; up 3.4%).
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” NRMP officials said in a statement.

Available dermatology PGY-2 slots rose by 4.9% from 426 in 2018 to 447 in 2019, while slots filled grew by 5.5% from 420 to 443, for an overall fill rate of 99.3%. In addition, the fill rate for U.S. graduates grew for the first time since 2015, rising from 81.7% to 82.8%.
An overall total of 2,756 PGY-2 slots were offered, 97.2% of which were filled; 67.5% were filled by U.S. graduates, the NRMP said in its 2019 Main Residency Match report.
The 2019 Match set a record for most positions offered (35,185; up 6.1%), most positions filled (up 4.8%), most PGY-1 positions offered (32,194; up 6.5%), and total applicants (38,376; up 3.4%).
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” NRMP officials said in a statement.

Available dermatology PGY-2 slots rose by 4.9% from 426 in 2018 to 447 in 2019, while slots filled grew by 5.5% from 420 to 443, for an overall fill rate of 99.3%. In addition, the fill rate for U.S. graduates grew for the first time since 2015, rising from 81.7% to 82.8%.
An overall total of 2,756 PGY-2 slots were offered, 97.2% of which were filled; 67.5% were filled by U.S. graduates, the NRMP said in its 2019 Main Residency Match report.
The 2019 Match set a record for most positions offered (35,185; up 6.1%), most positions filled (up 4.8%), most PGY-1 positions offered (32,194; up 6.5%), and total applicants (38,376; up 3.4%).
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” NRMP officials said in a statement.
Match Day 2019: Ob.gyn. up from last year
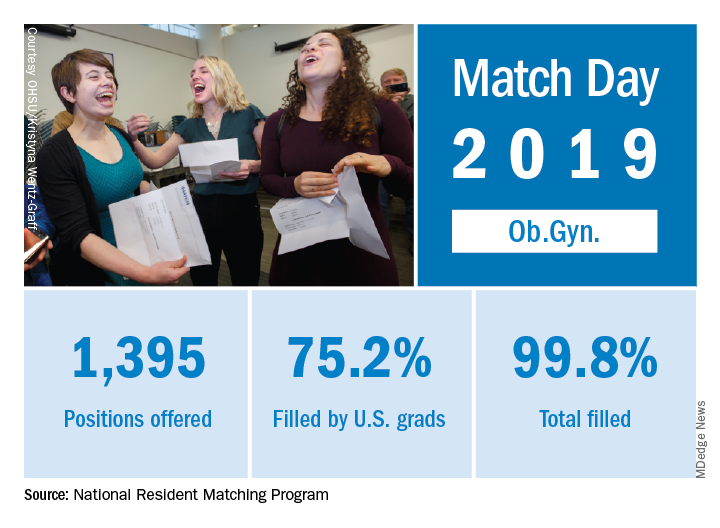
Ob.gyn. brought 1,395 first-year positions to the 2019 Main Resident Match, up from 1,336 in 2018. Also up from last year is the fill rate, with 2018’s Match Day filling 99.6% of available positions.
For all specialties in the main Match, U.S. first-year applicants filled 55.2%, and the overall fill rate was 94.9% of the 32,194 available positions, an increase of 6.5% over the number of positions available in 2018, according to the NRMP report.
The 2019 Match also set a new high for total positions offered at 35,135, for which there was a record-high 38,376 applicants. The increases are likely related, in part, to the greater number of osteopathic programs joining the Match Day offerings because of an ongoing move toward a single accreditation system.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.

Ob.gyn. brought 1,395 first-year positions to the 2019 Main Resident Match, up from 1,336 in 2018. Also up from last year is the fill rate, with 2018’s Match Day filling 99.6% of available positions.
For all specialties in the main Match, U.S. first-year applicants filled 55.2%, and the overall fill rate was 94.9% of the 32,194 available positions, an increase of 6.5% over the number of positions available in 2018, according to the NRMP report.
The 2019 Match also set a new high for total positions offered at 35,135, for which there was a record-high 38,376 applicants. The increases are likely related, in part, to the greater number of osteopathic programs joining the Match Day offerings because of an ongoing move toward a single accreditation system.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.

Ob.gyn. brought 1,395 first-year positions to the 2019 Main Resident Match, up from 1,336 in 2018. Also up from last year is the fill rate, with 2018’s Match Day filling 99.6% of available positions.
For all specialties in the main Match, U.S. first-year applicants filled 55.2%, and the overall fill rate was 94.9% of the 32,194 available positions, an increase of 6.5% over the number of positions available in 2018, according to the NRMP report.
The 2019 Match also set a new high for total positions offered at 35,135, for which there was a record-high 38,376 applicants. The increases are likely related, in part, to the greater number of osteopathic programs joining the Match Day offerings because of an ongoing move toward a single accreditation system.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Match Day 2019: Psychiatry sees double-digit growth
Match Day 2019 was another record breaker, and psychiatry helped play a big role by offering nearly 200 more residency slots and matches than in 2018, according to the National Resident Matching Program (NRMP).

There were a total of 1,740 PGY-1 psychiatry slots offered in 2019, up from 1,556 in 2018, for an increase of 11.8%. The 184-position increase was the fourth-largest overall increase among all measured specialties, behind only internal medicine, family medicine, and emergency medicine.
in a press release. However, while the fill rate increased slightly to 99%, leaving only 20 slots unfilled, the fill rate by U.S. graduates dropped from 63.1% to 60.6%.
Overall, a total of 32,194 PGY-1 slots were offered, a new record, and 94.9% were filled, with U.S. graduates filling 55.2%, the NRMP said in its 2019 Main Residency Match report.
The 2019 Match set a record for most positions offered (35,185; up 6.1%), most positions filled (33,426; up 4.8%), most PGY-1 positions offered (32,194; up 6.5%), and total applicants (38,376; up 3.4%).
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Match Day 2019 was another record breaker, and psychiatry helped play a big role by offering nearly 200 more residency slots and matches than in 2018, according to the National Resident Matching Program (NRMP).

There were a total of 1,740 PGY-1 psychiatry slots offered in 2019, up from 1,556 in 2018, for an increase of 11.8%. The 184-position increase was the fourth-largest overall increase among all measured specialties, behind only internal medicine, family medicine, and emergency medicine.
in a press release. However, while the fill rate increased slightly to 99%, leaving only 20 slots unfilled, the fill rate by U.S. graduates dropped from 63.1% to 60.6%.
Overall, a total of 32,194 PGY-1 slots were offered, a new record, and 94.9% were filled, with U.S. graduates filling 55.2%, the NRMP said in its 2019 Main Residency Match report.
The 2019 Match set a record for most positions offered (35,185; up 6.1%), most positions filled (33,426; up 4.8%), most PGY-1 positions offered (32,194; up 6.5%), and total applicants (38,376; up 3.4%).
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
Match Day 2019 was another record breaker, and psychiatry helped play a big role by offering nearly 200 more residency slots and matches than in 2018, according to the National Resident Matching Program (NRMP).

There were a total of 1,740 PGY-1 psychiatry slots offered in 2019, up from 1,556 in 2018, for an increase of 11.8%. The 184-position increase was the fourth-largest overall increase among all measured specialties, behind only internal medicine, family medicine, and emergency medicine.
in a press release. However, while the fill rate increased slightly to 99%, leaving only 20 slots unfilled, the fill rate by U.S. graduates dropped from 63.1% to 60.6%.
Overall, a total of 32,194 PGY-1 slots were offered, a new record, and 94.9% were filled, with U.S. graduates filling 55.2%, the NRMP said in its 2019 Main Residency Match report.
The 2019 Match set a record for most positions offered (35,185; up 6.1%), most positions filled (33,426; up 4.8%), most PGY-1 positions offered (32,194; up 6.5%), and total applicants (38,376; up 3.4%).
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
New study a reprieve for isoniazid during HIV pregnancy?
SEATTLE – At the 2018 Conference on Retroviruses and Opportunistic Infections, investigators reported that the rate of adverse pregnancy outcomes was 23% among HIV-positive women who took isoniazid to prevent tuberculosis during pregnancy, but just 17% among women who waited until after delivery.
It was an international, randomized trial that included 156 women, and outcomes included in utero demise and low birth weight. The investigators concluded that the World Health Organization needed to reconsider its recommendation for isoniazid preventative treatment (IPT) during HIV pregnancy in areas where tuberculosis (TB) is common.
The news was in the exact opposite direction at this year’s conference: An observational study of 151 pregnant HIV-positive women in Soweto, South Africa, found no evidence of poor maternal or infant outcomes with isoniazid prophylaxis, according to investigators led by Nicole Salazar-Austin, MD, a pediatric instructor at Johns Hopkins University, Baltimore.
The team was alarmed by the 2018 findings, so it decided to take a closer look with their own data. They compared outcomes in 69 HIV-positive women (46%) who reported initiating IPT during pregnancy with 82 who did not from January 2011 through January 2014. There was higher use of combination antiretroviral therapy in the IPT group, and viral loads below 400 copies/mL were more common.
The proportion of neonates born prematurely was lower in the IPT group than in the group not using it, but this did not reach significance (10% vs. 22%; P = 0.06). There was no difference in fetal demise (1% in both groups); low birth weight (9% vs. 12%; P = 0.51); or congenital anomalies (1% vs. 2%, P = 1.0).
A composite of the four outcomes actually showed fewer events among infants exposed to IPT (16% vs. 28%; P = 0.08). The outcomes held when stratified by viral load suppression and when other causes of poor infant outcomes – advanced HIV disease, advanced maternal age, and low weight gain during pregnancy – were taken into account.
As an observational study based on self-report, the new work was much less rigorous than the 2018 randomized trial, Dr. Salazar-Austin noted. Also, the work was part of a larger project that was not designed specifically to study pregnancy outcomes. IPT was initiated by public antenatal and HIV clinics, not by the study team.
Still, “we hope that these results provide some reassurance that IPT can be used in the second or third trimester among pregnant women with HIV,” Dr. Salazar-Austin said.
So, how to mesh the two studies? Like everything in medicine, it comes down to a risk/benefit analysis.
“For pregnant women living in a household with an active TB case, maybe the benefit outweighs the risk, especially if they are living with HIV. Whether all women living with HIV in a high [TB] burden [area] should receive isoniazid is still somewhat in question. I think we need to weigh” IPT during HIV pregnancy on an “individual basis moving forward,” she said.
Women in the observational trial were a median of about 30 years old. The median CD4 T-cell count at enrollment was about 370 cells/mm3.
The work was funded by the National Institutes of Health. Dr. Salazar-Austin didn’t have any disclosures.
SOURCE: Salazar-Austin NM et al. CROI 2019, Abstract 77.
SEATTLE – At the 2018 Conference on Retroviruses and Opportunistic Infections, investigators reported that the rate of adverse pregnancy outcomes was 23% among HIV-positive women who took isoniazid to prevent tuberculosis during pregnancy, but just 17% among women who waited until after delivery.
It was an international, randomized trial that included 156 women, and outcomes included in utero demise and low birth weight. The investigators concluded that the World Health Organization needed to reconsider its recommendation for isoniazid preventative treatment (IPT) during HIV pregnancy in areas where tuberculosis (TB) is common.
The news was in the exact opposite direction at this year’s conference: An observational study of 151 pregnant HIV-positive women in Soweto, South Africa, found no evidence of poor maternal or infant outcomes with isoniazid prophylaxis, according to investigators led by Nicole Salazar-Austin, MD, a pediatric instructor at Johns Hopkins University, Baltimore.
The team was alarmed by the 2018 findings, so it decided to take a closer look with their own data. They compared outcomes in 69 HIV-positive women (46%) who reported initiating IPT during pregnancy with 82 who did not from January 2011 through January 2014. There was higher use of combination antiretroviral therapy in the IPT group, and viral loads below 400 copies/mL were more common.
The proportion of neonates born prematurely was lower in the IPT group than in the group not using it, but this did not reach significance (10% vs. 22%; P = 0.06). There was no difference in fetal demise (1% in both groups); low birth weight (9% vs. 12%; P = 0.51); or congenital anomalies (1% vs. 2%, P = 1.0).
A composite of the four outcomes actually showed fewer events among infants exposed to IPT (16% vs. 28%; P = 0.08). The outcomes held when stratified by viral load suppression and when other causes of poor infant outcomes – advanced HIV disease, advanced maternal age, and low weight gain during pregnancy – were taken into account.
As an observational study based on self-report, the new work was much less rigorous than the 2018 randomized trial, Dr. Salazar-Austin noted. Also, the work was part of a larger project that was not designed specifically to study pregnancy outcomes. IPT was initiated by public antenatal and HIV clinics, not by the study team.
Still, “we hope that these results provide some reassurance that IPT can be used in the second or third trimester among pregnant women with HIV,” Dr. Salazar-Austin said.
So, how to mesh the two studies? Like everything in medicine, it comes down to a risk/benefit analysis.
“For pregnant women living in a household with an active TB case, maybe the benefit outweighs the risk, especially if they are living with HIV. Whether all women living with HIV in a high [TB] burden [area] should receive isoniazid is still somewhat in question. I think we need to weigh” IPT during HIV pregnancy on an “individual basis moving forward,” she said.
Women in the observational trial were a median of about 30 years old. The median CD4 T-cell count at enrollment was about 370 cells/mm3.
The work was funded by the National Institutes of Health. Dr. Salazar-Austin didn’t have any disclosures.
SOURCE: Salazar-Austin NM et al. CROI 2019, Abstract 77.
SEATTLE – At the 2018 Conference on Retroviruses and Opportunistic Infections, investigators reported that the rate of adverse pregnancy outcomes was 23% among HIV-positive women who took isoniazid to prevent tuberculosis during pregnancy, but just 17% among women who waited until after delivery.
It was an international, randomized trial that included 156 women, and outcomes included in utero demise and low birth weight. The investigators concluded that the World Health Organization needed to reconsider its recommendation for isoniazid preventative treatment (IPT) during HIV pregnancy in areas where tuberculosis (TB) is common.
The news was in the exact opposite direction at this year’s conference: An observational study of 151 pregnant HIV-positive women in Soweto, South Africa, found no evidence of poor maternal or infant outcomes with isoniazid prophylaxis, according to investigators led by Nicole Salazar-Austin, MD, a pediatric instructor at Johns Hopkins University, Baltimore.
The team was alarmed by the 2018 findings, so it decided to take a closer look with their own data. They compared outcomes in 69 HIV-positive women (46%) who reported initiating IPT during pregnancy with 82 who did not from January 2011 through January 2014. There was higher use of combination antiretroviral therapy in the IPT group, and viral loads below 400 copies/mL were more common.
The proportion of neonates born prematurely was lower in the IPT group than in the group not using it, but this did not reach significance (10% vs. 22%; P = 0.06). There was no difference in fetal demise (1% in both groups); low birth weight (9% vs. 12%; P = 0.51); or congenital anomalies (1% vs. 2%, P = 1.0).
A composite of the four outcomes actually showed fewer events among infants exposed to IPT (16% vs. 28%; P = 0.08). The outcomes held when stratified by viral load suppression and when other causes of poor infant outcomes – advanced HIV disease, advanced maternal age, and low weight gain during pregnancy – were taken into account.
As an observational study based on self-report, the new work was much less rigorous than the 2018 randomized trial, Dr. Salazar-Austin noted. Also, the work was part of a larger project that was not designed specifically to study pregnancy outcomes. IPT was initiated by public antenatal and HIV clinics, not by the study team.
Still, “we hope that these results provide some reassurance that IPT can be used in the second or third trimester among pregnant women with HIV,” Dr. Salazar-Austin said.
So, how to mesh the two studies? Like everything in medicine, it comes down to a risk/benefit analysis.
“For pregnant women living in a household with an active TB case, maybe the benefit outweighs the risk, especially if they are living with HIV. Whether all women living with HIV in a high [TB] burden [area] should receive isoniazid is still somewhat in question. I think we need to weigh” IPT during HIV pregnancy on an “individual basis moving forward,” she said.
Women in the observational trial were a median of about 30 years old. The median CD4 T-cell count at enrollment was about 370 cells/mm3.
The work was funded by the National Institutes of Health. Dr. Salazar-Austin didn’t have any disclosures.
SOURCE: Salazar-Austin NM et al. CROI 2019, Abstract 77.
REPORTING FROM CROI 2019
Repeat VTE risk heightened in HIV patients
SEATTLE – HIV infection is associated with increased risk of recurrent venous thromboembolism, especially within 1 year of the initial episode. The finding, presented during a poster session at the Conference on Retroviruses & Opportunistic Infections, follows up on an earlier study that found that first-time VTE risk also is higher among HIV-positive individuals than in the general population.
The conclusion about first-time VTE risk, published earlier this year in Lancet HIV, came from a comparison between the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort and European population-level of studies of VTE. It found a crude incidence of 2.33 VTE events per 1,000 person-years In HIV patients, with heightened odds when CD4 cell counts were below 200 cells/mcL (adjusted hazard ratio, 3.40).
The new work represents a follow-up and compared results from ATHENA (153 patients with HIV and first VTE) and the Dutch MEGA cohort (4,005 patients without HIV, with first VTE), which includes the general population. Overall, 26% of patients in the ATHENA cohort experienced a second VTE event, compared with 16% of the general population. At 1 year after anticoagulation withdrawal, HIV-positive individuals were at 67% increased risk (HR, 1.67). At 6-years after withdrawal, the relationship was not statistically significant (HR, 1.22).
Researchers also found that CD4 cell-count recovery was associated with lowered risk, with every 100 cell-count increase between initial VTE diagnosis and anticoagulant withdrawal linked to a 20% reduction in risk (HR, 0.80).
“The clinical question is: If it’s true you have an increased risk of recurrence, should you be continuing anticoagulant therapy longer in people with HIV? This poster doesn’t answer that question and you probably need a randomized, controlled trial to look at that,” Peter Reiss, MD, professor of medicine at Amsterdam University Medical Center, said in an interview during the conference.
In the absence of a clear answer, it’s sensible for clinicians to be aware of the potential increased risk, much as clinicians have internalized the increased risk of atherosclerotic vascular disease in HIV patients. “I think the publication [in Lancet HIV] as well as this poster suggest that on the venous side of things there may also be an accentuated risk,” said Dr. Reiss.
Heidi Crane, MD, a professor of medicine at the University of Washington, Seattle, presented a poster examining the underlying factors that may predispose HIV patients to first-time VTE events. Her team performed an adjudicated review of VTE cases among HIV patients at six institutions and found that the risk factors appeared to be distinct from those seen in the general population.
The traditional long plane ride was less common in this population, while factors such as injected drug use and pneumonia were more common. The VTE events occurred at a median age of 49 years; 30% of the patients had a detectable viral load. “We’re seeing a little more (VTE) than you might expect, and in a younger population than you might have guessed,” said Dr. Crane in an interview.
The most frequent predisposing risk factors were recent hospitalization (40%), infection (40%), or immobilization/bed rest (24%) within the past 90 days, and injectable drug use (22%). “It’s not just the traditional risk factors. Some HIV-specific risk factors are driving this,” said Dr. Crane.
She also aims to learn more about the specifics of risk factors, such as catheter-associated thromboses. The team is working to increase the sample size in order to parse out the relationships with specific outcomes.
In the meantime, the data further characterize the health challenges facing people living with HIV. “This is another example demonstrating that comorbid conditions among patients with HIV that are often considered age related occur at much younger ages in our population,” said Dr. Crane.
SOURCE: Rokx C et al. CROI 2019, Abstract 636; and Tenforde MW et al. CROI 2019, Abstract 637.
.
SEATTLE – HIV infection is associated with increased risk of recurrent venous thromboembolism, especially within 1 year of the initial episode. The finding, presented during a poster session at the Conference on Retroviruses & Opportunistic Infections, follows up on an earlier study that found that first-time VTE risk also is higher among HIV-positive individuals than in the general population.
The conclusion about first-time VTE risk, published earlier this year in Lancet HIV, came from a comparison between the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort and European population-level of studies of VTE. It found a crude incidence of 2.33 VTE events per 1,000 person-years In HIV patients, with heightened odds when CD4 cell counts were below 200 cells/mcL (adjusted hazard ratio, 3.40).
The new work represents a follow-up and compared results from ATHENA (153 patients with HIV and first VTE) and the Dutch MEGA cohort (4,005 patients without HIV, with first VTE), which includes the general population. Overall, 26% of patients in the ATHENA cohort experienced a second VTE event, compared with 16% of the general population. At 1 year after anticoagulation withdrawal, HIV-positive individuals were at 67% increased risk (HR, 1.67). At 6-years after withdrawal, the relationship was not statistically significant (HR, 1.22).
Researchers also found that CD4 cell-count recovery was associated with lowered risk, with every 100 cell-count increase between initial VTE diagnosis and anticoagulant withdrawal linked to a 20% reduction in risk (HR, 0.80).
“The clinical question is: If it’s true you have an increased risk of recurrence, should you be continuing anticoagulant therapy longer in people with HIV? This poster doesn’t answer that question and you probably need a randomized, controlled trial to look at that,” Peter Reiss, MD, professor of medicine at Amsterdam University Medical Center, said in an interview during the conference.
In the absence of a clear answer, it’s sensible for clinicians to be aware of the potential increased risk, much as clinicians have internalized the increased risk of atherosclerotic vascular disease in HIV patients. “I think the publication [in Lancet HIV] as well as this poster suggest that on the venous side of things there may also be an accentuated risk,” said Dr. Reiss.
Heidi Crane, MD, a professor of medicine at the University of Washington, Seattle, presented a poster examining the underlying factors that may predispose HIV patients to first-time VTE events. Her team performed an adjudicated review of VTE cases among HIV patients at six institutions and found that the risk factors appeared to be distinct from those seen in the general population.
The traditional long plane ride was less common in this population, while factors such as injected drug use and pneumonia were more common. The VTE events occurred at a median age of 49 years; 30% of the patients had a detectable viral load. “We’re seeing a little more (VTE) than you might expect, and in a younger population than you might have guessed,” said Dr. Crane in an interview.
The most frequent predisposing risk factors were recent hospitalization (40%), infection (40%), or immobilization/bed rest (24%) within the past 90 days, and injectable drug use (22%). “It’s not just the traditional risk factors. Some HIV-specific risk factors are driving this,” said Dr. Crane.
She also aims to learn more about the specifics of risk factors, such as catheter-associated thromboses. The team is working to increase the sample size in order to parse out the relationships with specific outcomes.
In the meantime, the data further characterize the health challenges facing people living with HIV. “This is another example demonstrating that comorbid conditions among patients with HIV that are often considered age related occur at much younger ages in our population,” said Dr. Crane.
SOURCE: Rokx C et al. CROI 2019, Abstract 636; and Tenforde MW et al. CROI 2019, Abstract 637.
.
SEATTLE – HIV infection is associated with increased risk of recurrent venous thromboembolism, especially within 1 year of the initial episode. The finding, presented during a poster session at the Conference on Retroviruses & Opportunistic Infections, follows up on an earlier study that found that first-time VTE risk also is higher among HIV-positive individuals than in the general population.
The conclusion about first-time VTE risk, published earlier this year in Lancet HIV, came from a comparison between the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort and European population-level of studies of VTE. It found a crude incidence of 2.33 VTE events per 1,000 person-years In HIV patients, with heightened odds when CD4 cell counts were below 200 cells/mcL (adjusted hazard ratio, 3.40).
The new work represents a follow-up and compared results from ATHENA (153 patients with HIV and first VTE) and the Dutch MEGA cohort (4,005 patients without HIV, with first VTE), which includes the general population. Overall, 26% of patients in the ATHENA cohort experienced a second VTE event, compared with 16% of the general population. At 1 year after anticoagulation withdrawal, HIV-positive individuals were at 67% increased risk (HR, 1.67). At 6-years after withdrawal, the relationship was not statistically significant (HR, 1.22).
Researchers also found that CD4 cell-count recovery was associated with lowered risk, with every 100 cell-count increase between initial VTE diagnosis and anticoagulant withdrawal linked to a 20% reduction in risk (HR, 0.80).
“The clinical question is: If it’s true you have an increased risk of recurrence, should you be continuing anticoagulant therapy longer in people with HIV? This poster doesn’t answer that question and you probably need a randomized, controlled trial to look at that,” Peter Reiss, MD, professor of medicine at Amsterdam University Medical Center, said in an interview during the conference.
In the absence of a clear answer, it’s sensible for clinicians to be aware of the potential increased risk, much as clinicians have internalized the increased risk of atherosclerotic vascular disease in HIV patients. “I think the publication [in Lancet HIV] as well as this poster suggest that on the venous side of things there may also be an accentuated risk,” said Dr. Reiss.
Heidi Crane, MD, a professor of medicine at the University of Washington, Seattle, presented a poster examining the underlying factors that may predispose HIV patients to first-time VTE events. Her team performed an adjudicated review of VTE cases among HIV patients at six institutions and found that the risk factors appeared to be distinct from those seen in the general population.
The traditional long plane ride was less common in this population, while factors such as injected drug use and pneumonia were more common. The VTE events occurred at a median age of 49 years; 30% of the patients had a detectable viral load. “We’re seeing a little more (VTE) than you might expect, and in a younger population than you might have guessed,” said Dr. Crane in an interview.
The most frequent predisposing risk factors were recent hospitalization (40%), infection (40%), or immobilization/bed rest (24%) within the past 90 days, and injectable drug use (22%). “It’s not just the traditional risk factors. Some HIV-specific risk factors are driving this,” said Dr. Crane.
She also aims to learn more about the specifics of risk factors, such as catheter-associated thromboses. The team is working to increase the sample size in order to parse out the relationships with specific outcomes.
In the meantime, the data further characterize the health challenges facing people living with HIV. “This is another example demonstrating that comorbid conditions among patients with HIV that are often considered age related occur at much younger ages in our population,” said Dr. Crane.
SOURCE: Rokx C et al. CROI 2019, Abstract 636; and Tenforde MW et al. CROI 2019, Abstract 637.
.
REPORTING FROM CROI 2019
Distribution of Migraine Attacks During the Week
Persons with migraine show individual attack patterns and weekend migraine can be determined for a subgroup of participants, while others show accumulations of their attacks on other days of the week. This according to a recent analysis of migraine attacks collected online within the project Migraine Radar in respect to the distribution of migraine attacks throughout the week on a single‐participant level. Researchers recorded data using a web app as well as smartphone apps in order to collect data of 44,639 migraine attacks of 1085 participants who reported 7 or more attacks during a period of at least 90 days. They found:
- For 15.9% of the participants, the attacks were not distributed equally throughout the days of the week.
- Instead, participants show different individual patterns for the distribution of their migraine attacks.
- Furthermore, the modes of the individual distributions are not distributed equally throughout the week.
- Saturday seems to be the predominant day for migraine attacks for a greater proportion of participants (195 of 1085).
Drescher J, Wogenstein F, Gaul C, et al. Distribution of migraine attacks over the days of the week: Preliminary results from a web‐based questionnaire. [Published online ahead of print January 12, 2019]. Acta Neurol Scand. doi:10.1111/ane.13065.
Persons with migraine show individual attack patterns and weekend migraine can be determined for a subgroup of participants, while others show accumulations of their attacks on other days of the week. This according to a recent analysis of migraine attacks collected online within the project Migraine Radar in respect to the distribution of migraine attacks throughout the week on a single‐participant level. Researchers recorded data using a web app as well as smartphone apps in order to collect data of 44,639 migraine attacks of 1085 participants who reported 7 or more attacks during a period of at least 90 days. They found:
- For 15.9% of the participants, the attacks were not distributed equally throughout the days of the week.
- Instead, participants show different individual patterns for the distribution of their migraine attacks.
- Furthermore, the modes of the individual distributions are not distributed equally throughout the week.
- Saturday seems to be the predominant day for migraine attacks for a greater proportion of participants (195 of 1085).
Drescher J, Wogenstein F, Gaul C, et al. Distribution of migraine attacks over the days of the week: Preliminary results from a web‐based questionnaire. [Published online ahead of print January 12, 2019]. Acta Neurol Scand. doi:10.1111/ane.13065.
Persons with migraine show individual attack patterns and weekend migraine can be determined for a subgroup of participants, while others show accumulations of their attacks on other days of the week. This according to a recent analysis of migraine attacks collected online within the project Migraine Radar in respect to the distribution of migraine attacks throughout the week on a single‐participant level. Researchers recorded data using a web app as well as smartphone apps in order to collect data of 44,639 migraine attacks of 1085 participants who reported 7 or more attacks during a period of at least 90 days. They found:
- For 15.9% of the participants, the attacks were not distributed equally throughout the days of the week.
- Instead, participants show different individual patterns for the distribution of their migraine attacks.
- Furthermore, the modes of the individual distributions are not distributed equally throughout the week.
- Saturday seems to be the predominant day for migraine attacks for a greater proportion of participants (195 of 1085).
Drescher J, Wogenstein F, Gaul C, et al. Distribution of migraine attacks over the days of the week: Preliminary results from a web‐based questionnaire. [Published online ahead of print January 12, 2019]. Acta Neurol Scand. doi:10.1111/ane.13065.




