User login
Measles cases confirmed in three more states
Forty more cases and three more states were added to the measles count in the last week, bringing the U.S. total to 268 cases in 15 states so far in 2019, according to the Centers for Disease Control and Prevention.
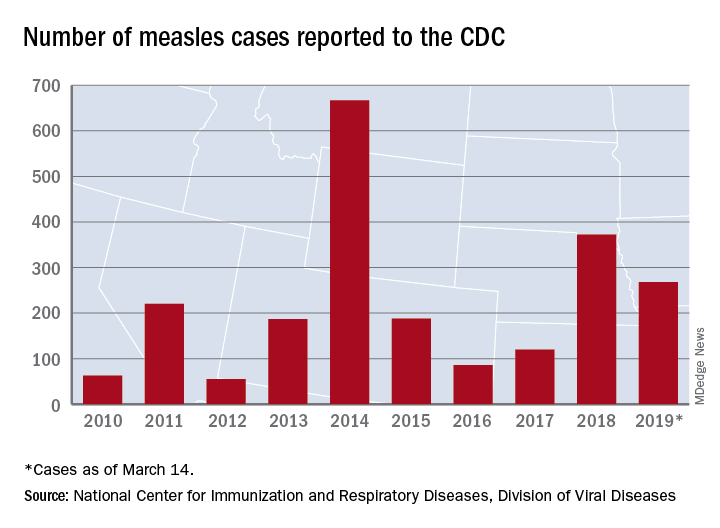
Arizona, Michigan, and Missouri reported their first confirmed cases of the year, joining California (one outbreak), Colorado, Connecticut, Georgia, Illinois (one outbreak), Kentucky, New Hampshire, New Jersey, New York (two outbreaks), Oregon, Texas (one outbreak), and Washington (one outbreak), the CDC reported March 18.
Brooklyn, N.Y., has become the epicenter of measles activity since mid-February, and with 25 of the 40 new cases occurring there, the borough has now led the nation for four consecutive weeks. There have been 157 confirmed cases in Brooklyn and one in Queens since the outbreak began in October of 2018, the New York City Department of Health and Mental Hygiene reported.
Michigan’s first case of measles is travel related and involved an individual visiting from Israel following a stay in New York, the Michigan Department of Health and Human Services and Oakland County Health Division announced March 13.
In Arizona, the state department of health services and the Pima County Public Health Department announced that a 12-month-old infant from Pima County has been diagnosed with measles after traveling to Asia.
A single case of measles, contracted while the person was traveling out of state, has been reported in Jefferson County, Mo., and is being managed by the county health department, according to St. Louis Public Radio.
Forty more cases and three more states were added to the measles count in the last week, bringing the U.S. total to 268 cases in 15 states so far in 2019, according to the Centers for Disease Control and Prevention.

Arizona, Michigan, and Missouri reported their first confirmed cases of the year, joining California (one outbreak), Colorado, Connecticut, Georgia, Illinois (one outbreak), Kentucky, New Hampshire, New Jersey, New York (two outbreaks), Oregon, Texas (one outbreak), and Washington (one outbreak), the CDC reported March 18.
Brooklyn, N.Y., has become the epicenter of measles activity since mid-February, and with 25 of the 40 new cases occurring there, the borough has now led the nation for four consecutive weeks. There have been 157 confirmed cases in Brooklyn and one in Queens since the outbreak began in October of 2018, the New York City Department of Health and Mental Hygiene reported.
Michigan’s first case of measles is travel related and involved an individual visiting from Israel following a stay in New York, the Michigan Department of Health and Human Services and Oakland County Health Division announced March 13.
In Arizona, the state department of health services and the Pima County Public Health Department announced that a 12-month-old infant from Pima County has been diagnosed with measles after traveling to Asia.
A single case of measles, contracted while the person was traveling out of state, has been reported in Jefferson County, Mo., and is being managed by the county health department, according to St. Louis Public Radio.
Forty more cases and three more states were added to the measles count in the last week, bringing the U.S. total to 268 cases in 15 states so far in 2019, according to the Centers for Disease Control and Prevention.

Arizona, Michigan, and Missouri reported their first confirmed cases of the year, joining California (one outbreak), Colorado, Connecticut, Georgia, Illinois (one outbreak), Kentucky, New Hampshire, New Jersey, New York (two outbreaks), Oregon, Texas (one outbreak), and Washington (one outbreak), the CDC reported March 18.
Brooklyn, N.Y., has become the epicenter of measles activity since mid-February, and with 25 of the 40 new cases occurring there, the borough has now led the nation for four consecutive weeks. There have been 157 confirmed cases in Brooklyn and one in Queens since the outbreak began in October of 2018, the New York City Department of Health and Mental Hygiene reported.
Michigan’s first case of measles is travel related and involved an individual visiting from Israel following a stay in New York, the Michigan Department of Health and Human Services and Oakland County Health Division announced March 13.
In Arizona, the state department of health services and the Pima County Public Health Department announced that a 12-month-old infant from Pima County has been diagnosed with measles after traveling to Asia.
A single case of measles, contracted while the person was traveling out of state, has been reported in Jefferson County, Mo., and is being managed by the county health department, according to St. Louis Public Radio.
The New Gastroenterologist seeks its next editor-in-chief
AGA’s cutting-edge, trainee and early-career focused e-newsletter The New Gastroenterologist (TNG) is seeking applications for the position of editor-in-chief (EIC). The role will facilitate the communication of the latest clinical advances among peers and build strong leadership skills managing editorial responsibilities as well as working with reviewers and fellow editors at AGA’s journals.
The term is from Oct. 1, 2019 – Sept. 30, 2022, with a transition period starting July 2019.

About TNG
TNG content covers highly relevant clinical topics, such as endoscopic management of obesity and quality metrics on colonoscopy. Also included in each issue are articles that focus on career pathways, financial and legal matters, perspectives from private practice, and other topics that are relevant to early-career GIs.
Honorarium
The EIC will receive an annual honorarium of $5,000.
Qualifications
• AGA member, between second year of fellowship and five years post-fellowship.
• Experience identifying and promoting newsworthy content that is relevant to the trainee and early-career GI community, as well as excellent judgment that expands the outstanding reputation of TNG and AGA.
• Experience in medical, scientific or news-related publishing is preferred, but not required.
• Familiarity with AGA and its priorities, activities and stances on important issues is ideal, preferably via past volunteer member experience with the association.
• The EIC must be able to devote sufficient time to TNG matters and may not accept editorial appointments to competing publications during their tenure as EIC.
For more information or to apply view the full request for application.
If you have questions, please contact Ryan Farrell, managing editor, The New Gastroenterologist, at [email protected].
AGA’s cutting-edge, trainee and early-career focused e-newsletter The New Gastroenterologist (TNG) is seeking applications for the position of editor-in-chief (EIC). The role will facilitate the communication of the latest clinical advances among peers and build strong leadership skills managing editorial responsibilities as well as working with reviewers and fellow editors at AGA’s journals.
The term is from Oct. 1, 2019 – Sept. 30, 2022, with a transition period starting July 2019.

About TNG
TNG content covers highly relevant clinical topics, such as endoscopic management of obesity and quality metrics on colonoscopy. Also included in each issue are articles that focus on career pathways, financial and legal matters, perspectives from private practice, and other topics that are relevant to early-career GIs.
Honorarium
The EIC will receive an annual honorarium of $5,000.
Qualifications
• AGA member, between second year of fellowship and five years post-fellowship.
• Experience identifying and promoting newsworthy content that is relevant to the trainee and early-career GI community, as well as excellent judgment that expands the outstanding reputation of TNG and AGA.
• Experience in medical, scientific or news-related publishing is preferred, but not required.
• Familiarity with AGA and its priorities, activities and stances on important issues is ideal, preferably via past volunteer member experience with the association.
• The EIC must be able to devote sufficient time to TNG matters and may not accept editorial appointments to competing publications during their tenure as EIC.
For more information or to apply view the full request for application.
If you have questions, please contact Ryan Farrell, managing editor, The New Gastroenterologist, at [email protected].
AGA’s cutting-edge, trainee and early-career focused e-newsletter The New Gastroenterologist (TNG) is seeking applications for the position of editor-in-chief (EIC). The role will facilitate the communication of the latest clinical advances among peers and build strong leadership skills managing editorial responsibilities as well as working with reviewers and fellow editors at AGA’s journals.
The term is from Oct. 1, 2019 – Sept. 30, 2022, with a transition period starting July 2019.

About TNG
TNG content covers highly relevant clinical topics, such as endoscopic management of obesity and quality metrics on colonoscopy. Also included in each issue are articles that focus on career pathways, financial and legal matters, perspectives from private practice, and other topics that are relevant to early-career GIs.
Honorarium
The EIC will receive an annual honorarium of $5,000.
Qualifications
• AGA member, between second year of fellowship and five years post-fellowship.
• Experience identifying and promoting newsworthy content that is relevant to the trainee and early-career GI community, as well as excellent judgment that expands the outstanding reputation of TNG and AGA.
• Experience in medical, scientific or news-related publishing is preferred, but not required.
• Familiarity with AGA and its priorities, activities and stances on important issues is ideal, preferably via past volunteer member experience with the association.
• The EIC must be able to devote sufficient time to TNG matters and may not accept editorial appointments to competing publications during their tenure as EIC.
For more information or to apply view the full request for application.
If you have questions, please contact Ryan Farrell, managing editor, The New Gastroenterologist, at [email protected].
Survey: Americans support regulation of vaping products
Almost 70% of adults believe that the Food and Drug Administration should raise the legal age to purchase e-cigarettes and tobacco, according to a new survey by NORC at the University of Chicago, a nonpartisan research institution.
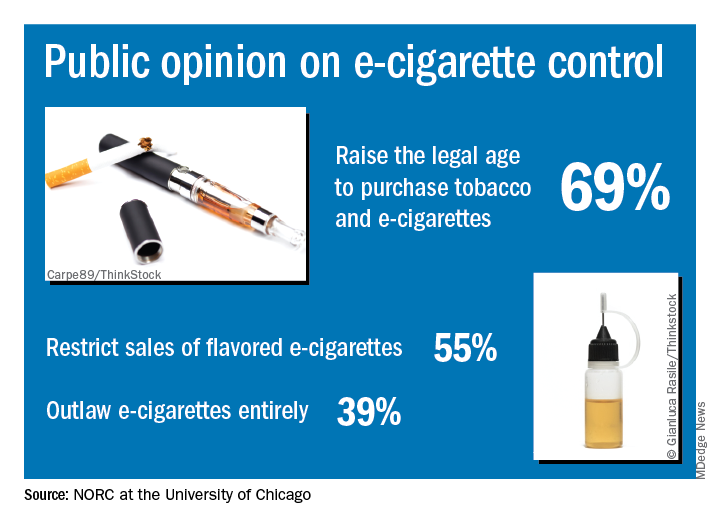
“Americans are particularly concerned about teens becoming newly addicted to e-cigarettes, and they support a range of actions the federal government could take to make vaping products less available, less addictive, and less appealing,” Caroline Pearson, senior vice president at NORC, said in a written statement.
The AmeriSpeak Spotlight on Health Poll, conducted Feb. 14-18, 2019 (margin of error, plus or minus 4.12%), showed that 69% of adults strongly or somewhat support raising the age limit to purchase e-cigarettes and tobacco and 55% support restricting sales of flavored e-cigarettes, NORC reported. Almost 40% of the 1,004 respondents expressed support for a complete ban on e-cigarettes.
Despite FDA efforts under Commissioner Scott Gottlieb, MD, to raise awareness of teen vaping, only 21% of those surveyed correctly responded that e-cigarettes generally contain more nicotine that regular cigarettes. Dr. Gottlieb announced his resignation recently, “but he indicated that the Trump Administration will continue efforts to increase regulation of e-cigarettes,” NORC said.
Almost 70% of adults believe that the Food and Drug Administration should raise the legal age to purchase e-cigarettes and tobacco, according to a new survey by NORC at the University of Chicago, a nonpartisan research institution.

“Americans are particularly concerned about teens becoming newly addicted to e-cigarettes, and they support a range of actions the federal government could take to make vaping products less available, less addictive, and less appealing,” Caroline Pearson, senior vice president at NORC, said in a written statement.
The AmeriSpeak Spotlight on Health Poll, conducted Feb. 14-18, 2019 (margin of error, plus or minus 4.12%), showed that 69% of adults strongly or somewhat support raising the age limit to purchase e-cigarettes and tobacco and 55% support restricting sales of flavored e-cigarettes, NORC reported. Almost 40% of the 1,004 respondents expressed support for a complete ban on e-cigarettes.
Despite FDA efforts under Commissioner Scott Gottlieb, MD, to raise awareness of teen vaping, only 21% of those surveyed correctly responded that e-cigarettes generally contain more nicotine that regular cigarettes. Dr. Gottlieb announced his resignation recently, “but he indicated that the Trump Administration will continue efforts to increase regulation of e-cigarettes,” NORC said.
Almost 70% of adults believe that the Food and Drug Administration should raise the legal age to purchase e-cigarettes and tobacco, according to a new survey by NORC at the University of Chicago, a nonpartisan research institution.

“Americans are particularly concerned about teens becoming newly addicted to e-cigarettes, and they support a range of actions the federal government could take to make vaping products less available, less addictive, and less appealing,” Caroline Pearson, senior vice president at NORC, said in a written statement.
The AmeriSpeak Spotlight on Health Poll, conducted Feb. 14-18, 2019 (margin of error, plus or minus 4.12%), showed that 69% of adults strongly or somewhat support raising the age limit to purchase e-cigarettes and tobacco and 55% support restricting sales of flavored e-cigarettes, NORC reported. Almost 40% of the 1,004 respondents expressed support for a complete ban on e-cigarettes.
Despite FDA efforts under Commissioner Scott Gottlieb, MD, to raise awareness of teen vaping, only 21% of those surveyed correctly responded that e-cigarettes generally contain more nicotine that regular cigarettes. Dr. Gottlieb announced his resignation recently, “but he indicated that the Trump Administration will continue efforts to increase regulation of e-cigarettes,” NORC said.
New data bolster latitude’s association with MS prevalence
DALLAS – Latitude continues to be associated with the prevalence of multiple sclerosis (MS), according to an updated meta-analysis presented at ACTRIMS Forum 2019, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
After integrating data from 94 new studies, “the latitudinal gradient in MS prevalence ... significantly enhanced in magnitude,” said presenting author Steve Simpson Jr., PhD, and his colleagues. Dr. Simpson is a researcher at the Melbourne School of Population & Global Health at the University of Melbourne and the Menzies Institute for Medical Research at the University of Tasmania.
The researchers’ original study, published in 2011, found a slope of 2.68.
Latitudinal variations in MS prevalence have given credence to the theory that sun exposure and vitamin D may play a role in MS onset. The researchers’ original meta-analysis – based on 650 prevalence estimates from 321 studies – “confirmed the existence of a robust latitudinal gradient,” they said. To examine whether the gradient has changed, the researchers identified relevant studies published between 2010 and 2018.
They included complete, peer-reviewed articles in their analysis and extracted data about the study area, prevalence year, MS diagnostic criteria used, sample size, source population, crude prevalence, and age-specific prevalence. They combined the new prevalence estimates with those from their original study. The estimates were log-transformed and weighted with the inverse of the variance. In addition, the researchers used random-effects meta-regression models that adjusted for prevalence year and method of case ascertainment.
Their literature review identified 126 new studies, 94 of which met inclusion criteria. The new studies yielded 230 additional prevalence points, predominantly in Scandinavia, France, and the Middle East.
“Latitude was consistently and significantly associated with MS prevalence in all analyses, increasing in magnitude on adjustment and persisting on age-standardization,” Dr. Simpson and his colleagues reported.
By region, strong and significant positive gradients continue to exist in Australasia, the United Kingdom/Ireland, and North America. Meanwhile, a significant inverse gradient continues to exist in the Italian region, which the researchers have said relates to the frequency of an MS-related HLA-DRB1 allele there. A negative gradient in the Scandinavian/North Atlantic region in the original meta-analysis, considered potentially related to dietary differences, was “markedly reduced” and no longer statistically significant in the updated meta-analysis.
“While there are potential intra-regional effects contributing to the latitudinal variation in MS prevalence, these results and the relative consistency across the whole of the globe continue to provide indirect evidence in support of the role of sun and vitamin D in MS etiology,” Dr. Simpson and his colleagues concluded.
The researchers had no disclosures.
SOURCE: Simpson S Jr et al. ACTRIMS Forum 2019, Abstract 126.
DALLAS – Latitude continues to be associated with the prevalence of multiple sclerosis (MS), according to an updated meta-analysis presented at ACTRIMS Forum 2019, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
After integrating data from 94 new studies, “the latitudinal gradient in MS prevalence ... significantly enhanced in magnitude,” said presenting author Steve Simpson Jr., PhD, and his colleagues. Dr. Simpson is a researcher at the Melbourne School of Population & Global Health at the University of Melbourne and the Menzies Institute for Medical Research at the University of Tasmania.
The researchers’ original study, published in 2011, found a slope of 2.68.
Latitudinal variations in MS prevalence have given credence to the theory that sun exposure and vitamin D may play a role in MS onset. The researchers’ original meta-analysis – based on 650 prevalence estimates from 321 studies – “confirmed the existence of a robust latitudinal gradient,” they said. To examine whether the gradient has changed, the researchers identified relevant studies published between 2010 and 2018.
They included complete, peer-reviewed articles in their analysis and extracted data about the study area, prevalence year, MS diagnostic criteria used, sample size, source population, crude prevalence, and age-specific prevalence. They combined the new prevalence estimates with those from their original study. The estimates were log-transformed and weighted with the inverse of the variance. In addition, the researchers used random-effects meta-regression models that adjusted for prevalence year and method of case ascertainment.
Their literature review identified 126 new studies, 94 of which met inclusion criteria. The new studies yielded 230 additional prevalence points, predominantly in Scandinavia, France, and the Middle East.
“Latitude was consistently and significantly associated with MS prevalence in all analyses, increasing in magnitude on adjustment and persisting on age-standardization,” Dr. Simpson and his colleagues reported.
By region, strong and significant positive gradients continue to exist in Australasia, the United Kingdom/Ireland, and North America. Meanwhile, a significant inverse gradient continues to exist in the Italian region, which the researchers have said relates to the frequency of an MS-related HLA-DRB1 allele there. A negative gradient in the Scandinavian/North Atlantic region in the original meta-analysis, considered potentially related to dietary differences, was “markedly reduced” and no longer statistically significant in the updated meta-analysis.
“While there are potential intra-regional effects contributing to the latitudinal variation in MS prevalence, these results and the relative consistency across the whole of the globe continue to provide indirect evidence in support of the role of sun and vitamin D in MS etiology,” Dr. Simpson and his colleagues concluded.
The researchers had no disclosures.
SOURCE: Simpson S Jr et al. ACTRIMS Forum 2019, Abstract 126.
DALLAS – Latitude continues to be associated with the prevalence of multiple sclerosis (MS), according to an updated meta-analysis presented at ACTRIMS Forum 2019, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
After integrating data from 94 new studies, “the latitudinal gradient in MS prevalence ... significantly enhanced in magnitude,” said presenting author Steve Simpson Jr., PhD, and his colleagues. Dr. Simpson is a researcher at the Melbourne School of Population & Global Health at the University of Melbourne and the Menzies Institute for Medical Research at the University of Tasmania.
The researchers’ original study, published in 2011, found a slope of 2.68.
Latitudinal variations in MS prevalence have given credence to the theory that sun exposure and vitamin D may play a role in MS onset. The researchers’ original meta-analysis – based on 650 prevalence estimates from 321 studies – “confirmed the existence of a robust latitudinal gradient,” they said. To examine whether the gradient has changed, the researchers identified relevant studies published between 2010 and 2018.
They included complete, peer-reviewed articles in their analysis and extracted data about the study area, prevalence year, MS diagnostic criteria used, sample size, source population, crude prevalence, and age-specific prevalence. They combined the new prevalence estimates with those from their original study. The estimates were log-transformed and weighted with the inverse of the variance. In addition, the researchers used random-effects meta-regression models that adjusted for prevalence year and method of case ascertainment.
Their literature review identified 126 new studies, 94 of which met inclusion criteria. The new studies yielded 230 additional prevalence points, predominantly in Scandinavia, France, and the Middle East.
“Latitude was consistently and significantly associated with MS prevalence in all analyses, increasing in magnitude on adjustment and persisting on age-standardization,” Dr. Simpson and his colleagues reported.
By region, strong and significant positive gradients continue to exist in Australasia, the United Kingdom/Ireland, and North America. Meanwhile, a significant inverse gradient continues to exist in the Italian region, which the researchers have said relates to the frequency of an MS-related HLA-DRB1 allele there. A negative gradient in the Scandinavian/North Atlantic region in the original meta-analysis, considered potentially related to dietary differences, was “markedly reduced” and no longer statistically significant in the updated meta-analysis.
“While there are potential intra-regional effects contributing to the latitudinal variation in MS prevalence, these results and the relative consistency across the whole of the globe continue to provide indirect evidence in support of the role of sun and vitamin D in MS etiology,” Dr. Simpson and his colleagues concluded.
The researchers had no disclosures.
SOURCE: Simpson S Jr et al. ACTRIMS Forum 2019, Abstract 126.
REPORTING FROM ACTRIMS FORUM 2019
Key clinical point: Latitude continues to be associated with the prevalence of multiple sclerosis (MS).
Major finding: For every degree of latitude increase, prevalence of MS per 100,000 people increased by 3.64 in a fully adjusted, age-standardized model.
Study details: A meta-analysis of data from more than 400 studies.
Disclosures: The investigators had no disclosures.
Source: Simpson S Jr et al. ACTRIMS Forum 2019, Abstract 126.
Sex differences in MS: It’s the chromosomes, not just the hormones
DALLAS – Hormonal differences are not the only reason that multiple sclerosis (MS) disease progression and severity differ between the sexes, according to Rhonda Voskuhl, MD, who delivered the Kenneth P. Johnson Memorial Lecture at a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis.
“Sex differences in disease are widely prevalent across immunological and neurological diseases. For example, lupus affects women 9:1 more frequently, rheumatoid arthritis is about 3:1, and MS is 3:1,” said Dr. Voskuhl, director of the MS program and Jack H. Skirball Chair of Multiple Sclerosis Research at the University of California, Los Angeles.
However, although women are more likely to experience these diseases, men are often more severely affected by them, Dr. Voskuhl said. “Sometimes in neurodegenerative diseases like MS, we’re seeing that the men, although they get it less frequently, they do worse. ... So these are actually two very important sex differences in disease, one affecting susceptibility and frequency, and the other affecting how they do over the long run with respect to their progression and severity.”
This clinically apparent observation, known for decades, prompted Dr. Voskuhl and others to parse why sex differences exist in this gamut of diseases.
A novel animal model – the four-core genotype mouse model – has allowed Dr. Voskuhl and others to discern the contributions of hormonal versus chromosomal influences on disease susceptibility and progression. The model separates the sex chromosome complement (XX or XY) from gonadal influences, and it’s been extremely helpful in revealing the surprising influence that sex chromosomes play in MS and similar diseases, said Dr. Voskuhl in an interview.
Dr. Voskuhl is also the president-elect of the Organization for the Study of Sex Differences.
DALLAS – Hormonal differences are not the only reason that multiple sclerosis (MS) disease progression and severity differ between the sexes, according to Rhonda Voskuhl, MD, who delivered the Kenneth P. Johnson Memorial Lecture at a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis.
“Sex differences in disease are widely prevalent across immunological and neurological diseases. For example, lupus affects women 9:1 more frequently, rheumatoid arthritis is about 3:1, and MS is 3:1,” said Dr. Voskuhl, director of the MS program and Jack H. Skirball Chair of Multiple Sclerosis Research at the University of California, Los Angeles.
However, although women are more likely to experience these diseases, men are often more severely affected by them, Dr. Voskuhl said. “Sometimes in neurodegenerative diseases like MS, we’re seeing that the men, although they get it less frequently, they do worse. ... So these are actually two very important sex differences in disease, one affecting susceptibility and frequency, and the other affecting how they do over the long run with respect to their progression and severity.”
This clinically apparent observation, known for decades, prompted Dr. Voskuhl and others to parse why sex differences exist in this gamut of diseases.
A novel animal model – the four-core genotype mouse model – has allowed Dr. Voskuhl and others to discern the contributions of hormonal versus chromosomal influences on disease susceptibility and progression. The model separates the sex chromosome complement (XX or XY) from gonadal influences, and it’s been extremely helpful in revealing the surprising influence that sex chromosomes play in MS and similar diseases, said Dr. Voskuhl in an interview.
Dr. Voskuhl is also the president-elect of the Organization for the Study of Sex Differences.
DALLAS – Hormonal differences are not the only reason that multiple sclerosis (MS) disease progression and severity differ between the sexes, according to Rhonda Voskuhl, MD, who delivered the Kenneth P. Johnson Memorial Lecture at a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis.
“Sex differences in disease are widely prevalent across immunological and neurological diseases. For example, lupus affects women 9:1 more frequently, rheumatoid arthritis is about 3:1, and MS is 3:1,” said Dr. Voskuhl, director of the MS program and Jack H. Skirball Chair of Multiple Sclerosis Research at the University of California, Los Angeles.
However, although women are more likely to experience these diseases, men are often more severely affected by them, Dr. Voskuhl said. “Sometimes in neurodegenerative diseases like MS, we’re seeing that the men, although they get it less frequently, they do worse. ... So these are actually two very important sex differences in disease, one affecting susceptibility and frequency, and the other affecting how they do over the long run with respect to their progression and severity.”
This clinically apparent observation, known for decades, prompted Dr. Voskuhl and others to parse why sex differences exist in this gamut of diseases.
A novel animal model – the four-core genotype mouse model – has allowed Dr. Voskuhl and others to discern the contributions of hormonal versus chromosomal influences on disease susceptibility and progression. The model separates the sex chromosome complement (XX or XY) from gonadal influences, and it’s been extremely helpful in revealing the surprising influence that sex chromosomes play in MS and similar diseases, said Dr. Voskuhl in an interview.
Dr. Voskuhl is also the president-elect of the Organization for the Study of Sex Differences.
REPORTING FROM ACTRIMS FORUM 2019
Venetoclax and obinutuzumab induces deep responses in CLL
The combination of venetoclax and obinutuzumab provided high response rates and deep remissions regardless of cytogenetic risk factors in patients with chronic lymphocytic leukemia, according to recently reported results of a phase 1b study.
The regimen elicited high rates of undetectable minimal residual disease in peripheral blood and had an acceptable safety profile with manageable toxicities in the study reported in Blood, which included patients with previously untreated or relapsed/refractory chronic lymphocytic leukemia (CLL).
“The deep remission rates we observed with venetoclax-obinutuzumab have not been reported with previously available CLL treatments, including FCR [fludarabine, cyclophosphamide, and rituximab], which is currently considered the most efficacious regimen with limited-duration therapy,” wrote the investigators, led by Ian W. Flinn, MD, PhD, of Sarah Cannon Research Institute/Tennessee Oncology, Nashville.
Venetoclax-obinutuzumab combinations are meanwhile being tested in other studies – including the phase 3 CLL13 and CLL14 studies – which have enrolled previously untreated fit or unfit CLL patients, respectively.
“If the primary endpoints of these large-scale trials are met, venetoclax-obinutuzumab may become a new standard treatment option in [first-line] CLL, irrespective of clinical fitness,” Dr. Flinn and his colleagues wrote in their report.
The present phase 1b, dose-escalation study enrolled 32 patients who were previously untreated (median age, 63 years) and 46 patients who were relapsed or refractory to previous treatments (median age, 61 years).
Doses of venetoclax were escalated from 100 mg to 400 mg to determine its maximum tolerated dose when combined with obinutuzumab, the investigators wrote. Some patients received venetoclax first, while others received obinutuzumab first, for a total of 1 year of treatment.
The study confirmed favorable risk-benefit treatment used a dose of 400 mg venetoclax plus the standard dose of obinutuzumab, according to the researchers.
The overall best response rate was 95% for relapsed/refractory patients, including a 37% rate of complete response or complete response with incomplete marrow recovery. In previously untreated patients, the overall best response rate was 100%, including a 78% rate of complete responses by those criteria.
Undetectable minimal residual disease was observed in 64% of relapsed/refractory patients and 91% of previously untreated patients at 3 months after the last obinutuzumab dose, the investigators reported.
There were no dose-limiting toxicities in the study, no clinical tumor lysis syndrome, and no differences between the two schedules (venetoclax first or obinutuzumab first) in terms of adverse events, the investigators wrote.
Neutropenia was the most common serious (grade 3-4) adverse event, occurring in 58% of relapsed/refractory patients and 53% of patients treated in the first line. Grade 3-4 infections were seen in 29% and 13% of the relapsed/refractory and previously untreated patients, respectively.
There were no fatal infections among previously untreated patients, while three relapsed/refractory patients (7%) had fatal adverse events, including one case of acute respiratory failure in a patient with suspected Richter’s transformation, pneumonia in a patient with metastatic squamous cell lung carcinoma, and another case of pneumonia occurring about 3 months after the last dose of venetoclax.
Genentech and AbbVie provided financial support for the study. Dr. Flinn reported receiving research funding for his institution from Genentech, AbbVie, and several other companies.
SOURCE: Flinn IW et al. Blood. 2019 Mar 12. doi: 10.1182/blood-2019-01-896290.
The combination of venetoclax and obinutuzumab provided high response rates and deep remissions regardless of cytogenetic risk factors in patients with chronic lymphocytic leukemia, according to recently reported results of a phase 1b study.
The regimen elicited high rates of undetectable minimal residual disease in peripheral blood and had an acceptable safety profile with manageable toxicities in the study reported in Blood, which included patients with previously untreated or relapsed/refractory chronic lymphocytic leukemia (CLL).
“The deep remission rates we observed with venetoclax-obinutuzumab have not been reported with previously available CLL treatments, including FCR [fludarabine, cyclophosphamide, and rituximab], which is currently considered the most efficacious regimen with limited-duration therapy,” wrote the investigators, led by Ian W. Flinn, MD, PhD, of Sarah Cannon Research Institute/Tennessee Oncology, Nashville.
Venetoclax-obinutuzumab combinations are meanwhile being tested in other studies – including the phase 3 CLL13 and CLL14 studies – which have enrolled previously untreated fit or unfit CLL patients, respectively.
“If the primary endpoints of these large-scale trials are met, venetoclax-obinutuzumab may become a new standard treatment option in [first-line] CLL, irrespective of clinical fitness,” Dr. Flinn and his colleagues wrote in their report.
The present phase 1b, dose-escalation study enrolled 32 patients who were previously untreated (median age, 63 years) and 46 patients who were relapsed or refractory to previous treatments (median age, 61 years).
Doses of venetoclax were escalated from 100 mg to 400 mg to determine its maximum tolerated dose when combined with obinutuzumab, the investigators wrote. Some patients received venetoclax first, while others received obinutuzumab first, for a total of 1 year of treatment.
The study confirmed favorable risk-benefit treatment used a dose of 400 mg venetoclax plus the standard dose of obinutuzumab, according to the researchers.
The overall best response rate was 95% for relapsed/refractory patients, including a 37% rate of complete response or complete response with incomplete marrow recovery. In previously untreated patients, the overall best response rate was 100%, including a 78% rate of complete responses by those criteria.
Undetectable minimal residual disease was observed in 64% of relapsed/refractory patients and 91% of previously untreated patients at 3 months after the last obinutuzumab dose, the investigators reported.
There were no dose-limiting toxicities in the study, no clinical tumor lysis syndrome, and no differences between the two schedules (venetoclax first or obinutuzumab first) in terms of adverse events, the investigators wrote.
Neutropenia was the most common serious (grade 3-4) adverse event, occurring in 58% of relapsed/refractory patients and 53% of patients treated in the first line. Grade 3-4 infections were seen in 29% and 13% of the relapsed/refractory and previously untreated patients, respectively.
There were no fatal infections among previously untreated patients, while three relapsed/refractory patients (7%) had fatal adverse events, including one case of acute respiratory failure in a patient with suspected Richter’s transformation, pneumonia in a patient with metastatic squamous cell lung carcinoma, and another case of pneumonia occurring about 3 months after the last dose of venetoclax.
Genentech and AbbVie provided financial support for the study. Dr. Flinn reported receiving research funding for his institution from Genentech, AbbVie, and several other companies.
SOURCE: Flinn IW et al. Blood. 2019 Mar 12. doi: 10.1182/blood-2019-01-896290.
The combination of venetoclax and obinutuzumab provided high response rates and deep remissions regardless of cytogenetic risk factors in patients with chronic lymphocytic leukemia, according to recently reported results of a phase 1b study.
The regimen elicited high rates of undetectable minimal residual disease in peripheral blood and had an acceptable safety profile with manageable toxicities in the study reported in Blood, which included patients with previously untreated or relapsed/refractory chronic lymphocytic leukemia (CLL).
“The deep remission rates we observed with venetoclax-obinutuzumab have not been reported with previously available CLL treatments, including FCR [fludarabine, cyclophosphamide, and rituximab], which is currently considered the most efficacious regimen with limited-duration therapy,” wrote the investigators, led by Ian W. Flinn, MD, PhD, of Sarah Cannon Research Institute/Tennessee Oncology, Nashville.
Venetoclax-obinutuzumab combinations are meanwhile being tested in other studies – including the phase 3 CLL13 and CLL14 studies – which have enrolled previously untreated fit or unfit CLL patients, respectively.
“If the primary endpoints of these large-scale trials are met, venetoclax-obinutuzumab may become a new standard treatment option in [first-line] CLL, irrespective of clinical fitness,” Dr. Flinn and his colleagues wrote in their report.
The present phase 1b, dose-escalation study enrolled 32 patients who were previously untreated (median age, 63 years) and 46 patients who were relapsed or refractory to previous treatments (median age, 61 years).
Doses of venetoclax were escalated from 100 mg to 400 mg to determine its maximum tolerated dose when combined with obinutuzumab, the investigators wrote. Some patients received venetoclax first, while others received obinutuzumab first, for a total of 1 year of treatment.
The study confirmed favorable risk-benefit treatment used a dose of 400 mg venetoclax plus the standard dose of obinutuzumab, according to the researchers.
The overall best response rate was 95% for relapsed/refractory patients, including a 37% rate of complete response or complete response with incomplete marrow recovery. In previously untreated patients, the overall best response rate was 100%, including a 78% rate of complete responses by those criteria.
Undetectable minimal residual disease was observed in 64% of relapsed/refractory patients and 91% of previously untreated patients at 3 months after the last obinutuzumab dose, the investigators reported.
There were no dose-limiting toxicities in the study, no clinical tumor lysis syndrome, and no differences between the two schedules (venetoclax first or obinutuzumab first) in terms of adverse events, the investigators wrote.
Neutropenia was the most common serious (grade 3-4) adverse event, occurring in 58% of relapsed/refractory patients and 53% of patients treated in the first line. Grade 3-4 infections were seen in 29% and 13% of the relapsed/refractory and previously untreated patients, respectively.
There were no fatal infections among previously untreated patients, while three relapsed/refractory patients (7%) had fatal adverse events, including one case of acute respiratory failure in a patient with suspected Richter’s transformation, pneumonia in a patient with metastatic squamous cell lung carcinoma, and another case of pneumonia occurring about 3 months after the last dose of venetoclax.
Genentech and AbbVie provided financial support for the study. Dr. Flinn reported receiving research funding for his institution from Genentech, AbbVie, and several other companies.
SOURCE: Flinn IW et al. Blood. 2019 Mar 12. doi: 10.1182/blood-2019-01-896290.
FROM BLOOD
Breast cancer survivors offer realistic strategies for easing cost burden
A qualitative study representing the patient perspective provides insight on reducing economic burden after breast cancer, including specific recommendations for changes to insurance, supportive services, financial assistance, and protective policies.
As part of a 6-month observational study conducted in 2015, Lorraine T. Dean, ScD, of Johns Hopkins Schools of Public Health and Medicine, Baltimore, and her associates, interviewed 40 women diagnosed with invasive stage I-III breast cancer who had completed active cancer treatment. All patients, who reported having more than one lymph node removed resided in Pennsylvania or New Jersey. The mean age of the women was 64 years.
Of those interviewed, 53% were white; 42.5% were black. More than half of participants (53%) were college graduates or had received a graduate degree. Annual income for 58% of the patients ranged from $30,000 to $70,000; 11% earned under $30,000. All participants included in the study were insured, including 82.5% who had private insurance. The patients had been diagnosed a mean of 12 years prior. Breast cancer–related lymphedema was reported in 60% of patients, Dr. Dean and her associates reported in a report published in Cancer.
Among the 40 participants, 27 made recommendations for easing economic burden, including nine key recommendations across four significant areas: insurance, supportive services and care, financial assistance, and protective policies. These findings are consistent with previous studies that examined patient recommendations, but they address additional areas where cost-saving services and policies could be offered or improved upon, the investigators noted.
Insurance-related recommendations included offering more complementary and integrative treatments as well as helping patients understand what insurance plans cover and how to adjust to changes under new insurance plans. Providing high-quality plans with low copays, premiums, and deductibles that cover required as well as elective cancer-related services, and covering lymphedema-related materials and treatments also were flagged as important.
Supportive service recommendations included addressing psychosocial costs through expansion of support groups and buddy services, offering extended home health services following cancer treatment, and providing domestic assistance with household chores, child care, and transportation.
Financial assistance that broadens financial aid and social services eligibility to those not classified as being in poverty was considered important.
Protective policy recommendations focused on expanding employment and medical leave policies concerning the amount of time offered off from work.
Patient recommendations offer just one viewpoint concerning potential challenges to the overall system, but “their thoughts on how it can be improved add value to decision-making processes,” noted Dr. Dean and her associates.
They were careful to acknowledge the benefits of the Patient Protection and Affordable Care Act, but they noted that it does not include provisions to address the adverse treatment effects of conditions such as cancer. While some states already have successfully passed legislation requiring private insurance carriers to cover lymphedema treatment, similar legislation should be adopted at a national level through joint efforts of Congress and the Department of Labor, they advised.
Any such efforts to make sweeping changes within the insurance industry would take considerable effort on the part of patients, providers, insurers, and state and federal policy makers, as well as the pharmaceutical industry. Yet, such “top-down and bottom-up strategies that involve all parties are warranted,” they urged.
Several important limitations of the study are worth noting. All participants were from the East Coast, had insurance coverage, and reported an overall low level of economic burden. Responses may have differed had the study been conducted in other regions of the country. The study was voluntary, so it is important to consider that patients with greater financial challenges may not have had time to enroll and participate, which suggests that the level of economic burden affecting this population actually could be understated.
SOURCE: Dean LT et al. Cancer 2019 Mar 6. doi: 10.1002/cncr.32012.
A qualitative study representing the patient perspective provides insight on reducing economic burden after breast cancer, including specific recommendations for changes to insurance, supportive services, financial assistance, and protective policies.
As part of a 6-month observational study conducted in 2015, Lorraine T. Dean, ScD, of Johns Hopkins Schools of Public Health and Medicine, Baltimore, and her associates, interviewed 40 women diagnosed with invasive stage I-III breast cancer who had completed active cancer treatment. All patients, who reported having more than one lymph node removed resided in Pennsylvania or New Jersey. The mean age of the women was 64 years.
Of those interviewed, 53% were white; 42.5% were black. More than half of participants (53%) were college graduates or had received a graduate degree. Annual income for 58% of the patients ranged from $30,000 to $70,000; 11% earned under $30,000. All participants included in the study were insured, including 82.5% who had private insurance. The patients had been diagnosed a mean of 12 years prior. Breast cancer–related lymphedema was reported in 60% of patients, Dr. Dean and her associates reported in a report published in Cancer.
Among the 40 participants, 27 made recommendations for easing economic burden, including nine key recommendations across four significant areas: insurance, supportive services and care, financial assistance, and protective policies. These findings are consistent with previous studies that examined patient recommendations, but they address additional areas where cost-saving services and policies could be offered or improved upon, the investigators noted.
Insurance-related recommendations included offering more complementary and integrative treatments as well as helping patients understand what insurance plans cover and how to adjust to changes under new insurance plans. Providing high-quality plans with low copays, premiums, and deductibles that cover required as well as elective cancer-related services, and covering lymphedema-related materials and treatments also were flagged as important.
Supportive service recommendations included addressing psychosocial costs through expansion of support groups and buddy services, offering extended home health services following cancer treatment, and providing domestic assistance with household chores, child care, and transportation.
Financial assistance that broadens financial aid and social services eligibility to those not classified as being in poverty was considered important.
Protective policy recommendations focused on expanding employment and medical leave policies concerning the amount of time offered off from work.
Patient recommendations offer just one viewpoint concerning potential challenges to the overall system, but “their thoughts on how it can be improved add value to decision-making processes,” noted Dr. Dean and her associates.
They were careful to acknowledge the benefits of the Patient Protection and Affordable Care Act, but they noted that it does not include provisions to address the adverse treatment effects of conditions such as cancer. While some states already have successfully passed legislation requiring private insurance carriers to cover lymphedema treatment, similar legislation should be adopted at a national level through joint efforts of Congress and the Department of Labor, they advised.
Any such efforts to make sweeping changes within the insurance industry would take considerable effort on the part of patients, providers, insurers, and state and federal policy makers, as well as the pharmaceutical industry. Yet, such “top-down and bottom-up strategies that involve all parties are warranted,” they urged.
Several important limitations of the study are worth noting. All participants were from the East Coast, had insurance coverage, and reported an overall low level of economic burden. Responses may have differed had the study been conducted in other regions of the country. The study was voluntary, so it is important to consider that patients with greater financial challenges may not have had time to enroll and participate, which suggests that the level of economic burden affecting this population actually could be understated.
SOURCE: Dean LT et al. Cancer 2019 Mar 6. doi: 10.1002/cncr.32012.
A qualitative study representing the patient perspective provides insight on reducing economic burden after breast cancer, including specific recommendations for changes to insurance, supportive services, financial assistance, and protective policies.
As part of a 6-month observational study conducted in 2015, Lorraine T. Dean, ScD, of Johns Hopkins Schools of Public Health and Medicine, Baltimore, and her associates, interviewed 40 women diagnosed with invasive stage I-III breast cancer who had completed active cancer treatment. All patients, who reported having more than one lymph node removed resided in Pennsylvania or New Jersey. The mean age of the women was 64 years.
Of those interviewed, 53% were white; 42.5% were black. More than half of participants (53%) were college graduates or had received a graduate degree. Annual income for 58% of the patients ranged from $30,000 to $70,000; 11% earned under $30,000. All participants included in the study were insured, including 82.5% who had private insurance. The patients had been diagnosed a mean of 12 years prior. Breast cancer–related lymphedema was reported in 60% of patients, Dr. Dean and her associates reported in a report published in Cancer.
Among the 40 participants, 27 made recommendations for easing economic burden, including nine key recommendations across four significant areas: insurance, supportive services and care, financial assistance, and protective policies. These findings are consistent with previous studies that examined patient recommendations, but they address additional areas where cost-saving services and policies could be offered or improved upon, the investigators noted.
Insurance-related recommendations included offering more complementary and integrative treatments as well as helping patients understand what insurance plans cover and how to adjust to changes under new insurance plans. Providing high-quality plans with low copays, premiums, and deductibles that cover required as well as elective cancer-related services, and covering lymphedema-related materials and treatments also were flagged as important.
Supportive service recommendations included addressing psychosocial costs through expansion of support groups and buddy services, offering extended home health services following cancer treatment, and providing domestic assistance with household chores, child care, and transportation.
Financial assistance that broadens financial aid and social services eligibility to those not classified as being in poverty was considered important.
Protective policy recommendations focused on expanding employment and medical leave policies concerning the amount of time offered off from work.
Patient recommendations offer just one viewpoint concerning potential challenges to the overall system, but “their thoughts on how it can be improved add value to decision-making processes,” noted Dr. Dean and her associates.
They were careful to acknowledge the benefits of the Patient Protection and Affordable Care Act, but they noted that it does not include provisions to address the adverse treatment effects of conditions such as cancer. While some states already have successfully passed legislation requiring private insurance carriers to cover lymphedema treatment, similar legislation should be adopted at a national level through joint efforts of Congress and the Department of Labor, they advised.
Any such efforts to make sweeping changes within the insurance industry would take considerable effort on the part of patients, providers, insurers, and state and federal policy makers, as well as the pharmaceutical industry. Yet, such “top-down and bottom-up strategies that involve all parties are warranted,” they urged.
Several important limitations of the study are worth noting. All participants were from the East Coast, had insurance coverage, and reported an overall low level of economic burden. Responses may have differed had the study been conducted in other regions of the country. The study was voluntary, so it is important to consider that patients with greater financial challenges may not have had time to enroll and participate, which suggests that the level of economic burden affecting this population actually could be understated.
SOURCE: Dean LT et al. Cancer 2019 Mar 6. doi: 10.1002/cncr.32012.
FROM CANCER
Genetic signature helps identify those at risk of MS
DALLAS – in a precision medicine–focused session at the meeting of the Americas Committee on Treatment and Research in Multiple Sclerosis.
“MS remains a diagnosis of exclusion ... But we’re now beginning to understand a lot more about the earliest stages of the disease, and we’re constantly redefining the disease in terms of when it starts, and what it consists of,” said Dr. De Jager, professor of neurology and chief of neuroimmunology at Columbia University, New York, in an interview.
For example, physicians are now starting to treat asymptomatic individuals with radiologically isolated syndrome, he said. “Is that part of the disease? Well, a lot of us think so, and we’re currently doing the studies to see whether treating them has an impact on long-term disability.”
“In this effort to redefine this disease and when it starts, these molecular and cellular studies are becoming very important,” Dr. De Jager said. Both individuals in the general population and high-risk individuals, such as family members of people with MS, will benefit from these research approaches, he said.
Right now, it’s hard to know who could benefit most from future preventive therapies, or who should have the most rigorous surveillance.
Dr. De Jager pointed to a presentation by his collaborator, Nikolaos Patsopoulos, MD, PhD, of Brigham and Women’s Hospital, Boston, who reported on the activities of the International MS Genetics Consortium. The consortium has collected and is nearing publication of data from more than 45,000 people with MS and 65,000 control participants to identify the genetic architecture of MS onset.
“We’re going to be reporting that there are over 234 genetic variations” that contribute to the onset of MS, Dr. De Jager said. “There are more to be found, but that’s a large number,” he said. The data point toward a genetic fingerprint that’s close to lupus, type 1 diabetes, and other inflammatory diseases. This shared genetic architecture means that there’s overlapping susceptibility for many diseases in this spectrum.
DALLAS – in a precision medicine–focused session at the meeting of the Americas Committee on Treatment and Research in Multiple Sclerosis.
“MS remains a diagnosis of exclusion ... But we’re now beginning to understand a lot more about the earliest stages of the disease, and we’re constantly redefining the disease in terms of when it starts, and what it consists of,” said Dr. De Jager, professor of neurology and chief of neuroimmunology at Columbia University, New York, in an interview.
For example, physicians are now starting to treat asymptomatic individuals with radiologically isolated syndrome, he said. “Is that part of the disease? Well, a lot of us think so, and we’re currently doing the studies to see whether treating them has an impact on long-term disability.”
“In this effort to redefine this disease and when it starts, these molecular and cellular studies are becoming very important,” Dr. De Jager said. Both individuals in the general population and high-risk individuals, such as family members of people with MS, will benefit from these research approaches, he said.
Right now, it’s hard to know who could benefit most from future preventive therapies, or who should have the most rigorous surveillance.
Dr. De Jager pointed to a presentation by his collaborator, Nikolaos Patsopoulos, MD, PhD, of Brigham and Women’s Hospital, Boston, who reported on the activities of the International MS Genetics Consortium. The consortium has collected and is nearing publication of data from more than 45,000 people with MS and 65,000 control participants to identify the genetic architecture of MS onset.
“We’re going to be reporting that there are over 234 genetic variations” that contribute to the onset of MS, Dr. De Jager said. “There are more to be found, but that’s a large number,” he said. The data point toward a genetic fingerprint that’s close to lupus, type 1 diabetes, and other inflammatory diseases. This shared genetic architecture means that there’s overlapping susceptibility for many diseases in this spectrum.
DALLAS – in a precision medicine–focused session at the meeting of the Americas Committee on Treatment and Research in Multiple Sclerosis.
“MS remains a diagnosis of exclusion ... But we’re now beginning to understand a lot more about the earliest stages of the disease, and we’re constantly redefining the disease in terms of when it starts, and what it consists of,” said Dr. De Jager, professor of neurology and chief of neuroimmunology at Columbia University, New York, in an interview.
For example, physicians are now starting to treat asymptomatic individuals with radiologically isolated syndrome, he said. “Is that part of the disease? Well, a lot of us think so, and we’re currently doing the studies to see whether treating them has an impact on long-term disability.”
“In this effort to redefine this disease and when it starts, these molecular and cellular studies are becoming very important,” Dr. De Jager said. Both individuals in the general population and high-risk individuals, such as family members of people with MS, will benefit from these research approaches, he said.
Right now, it’s hard to know who could benefit most from future preventive therapies, or who should have the most rigorous surveillance.
Dr. De Jager pointed to a presentation by his collaborator, Nikolaos Patsopoulos, MD, PhD, of Brigham and Women’s Hospital, Boston, who reported on the activities of the International MS Genetics Consortium. The consortium has collected and is nearing publication of data from more than 45,000 people with MS and 65,000 control participants to identify the genetic architecture of MS onset.
“We’re going to be reporting that there are over 234 genetic variations” that contribute to the onset of MS, Dr. De Jager said. “There are more to be found, but that’s a large number,” he said. The data point toward a genetic fingerprint that’s close to lupus, type 1 diabetes, and other inflammatory diseases. This shared genetic architecture means that there’s overlapping susceptibility for many diseases in this spectrum.
REPORTING FROM ACTRIMS FORUM 2019
Andexanet alfa effectively reverses factor Xa inhibition
HONOLULU – according to a study presented at the International Stroke Conference sponsored by the American Heart Association. The medication is associated with a low rate of mortality resulting from intracerebral hemorrhage (ICH), compared with the general population of patients with ICH receiving anticoagulation.
Factor Xa inhibitors such as apixaban and rivaroxaban effectively prevent thromboembolic events but may cause or exacerbate acute major bleeding. Andexanet alfa, a modified, recombinant, inactive form of human factor Xa, was developed and approved as a reversal agent for factor Xa inhibitors. In a 2015 study, andexanet rapidly and safely reversed anti–factor Xa activity in large cohorts of patients without bleeding.
A single-cohort study
Truman John Milling Jr., MD, an emergency medicine physician at Dell Seton Medical Center at the University of Texas in Austin, and his colleagues conducted the Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors (ANNEXA-4) study to evaluate the drug’s safety and efficacy in patients with acute major bleeding associated with treatment with a factor Xa inhibitor. For participants to be eligible, their bleeding had to be life threatening with signs of hemodynamic compromise, be associated with a decrease in hemoglobin level of at least 2 g/dL, or occur in a critical organ such as the brain. An independent academic committee determined whether patients met these criteria.
The trial’s primary efficacy outcomes were change from baseline in anti–factor Xa activity and the percentage of patients with excellent or good hemostatic efficacy at 12 hours. The primary safety endpoints were death, thrombotic events, and the development of neutralizing antibodies to andexanet or to native factor X and factor Xa. The efficacy population included patients with major bleeding and baseline anti–factor Xa activity of at least 75 ng/mL. The safety population included all patients who received a dose of andexanet. The independent committee adjudicated the efficacy and safety outcomes.
Hemostasis was sustained for 12 hours
The investigators enrolled 352 participants into the study, all of whom received andexanet and were followed for at least 30 days or until death. The population’s mean age was 77 years. “These were older and sicker patients with a significant amount of comorbid disease,” said Dr. Milling. The primary indication for anticoagulation was atrial fibrillation in 80% of patients. The primary site of bleeding was intracranial in 64% of patients and gastrointestinal in 26% of patients. The remaining 10% of patients had bleeding affecting other areas (such as pericardial or intramuscular bleeding).
The investigators included 254 patients in the efficacy population. At the end of the administration of the andexanet bolus, the median value for anti–factor Xa activity decreased by 92% among participants receiving apixaban, 92% among participants receiving rivaroxaban, and 75% among patients receiving enoxaparin. Among patients receiving apixaban, the median value for anti–factor Xa activity was decreased by 32% at 4 hours, 34% at 8 hours, and 38% at 12 hours. Among patients receiving rivaroxaban, the median value for anti–factor Xa activity was decreased by 42% at 4 hours, 48% at 8 hours, and 62% at 12 hours.
Dr. Milling and his colleagues assessed hemostatic efficacy in 249 patients. Of this group, 82% achieved good or excellent hemostasis. Among participants with good or excellent hemostasis, 84% had excellent results, and 16% had good results. Subanalysis by factor Xa inhibitor, type of bleed, age, and dose of andexanet did not alter the findings significantly.
To determine whether hemostasis had been sustained sufficiently to prevent clinical deterioration, the investigators examined 71 patients with ICH and a single-compartment bleed. From 1 hour to 12 hours, one patient’s outcome changed from excellent/good to poor/none, and one patient’s outcome changed from excellent to good. For the majority of these patients, however, good hemostasis was sustained from 1 to 12 hours.
The rate of thromboembolic events was 9.7%, which is in the expected range for this population, said Dr. Milling. These events were distributed evenly among the 4 weeks of the study. Stroke and deep vein thrombosis accounted for most of these events, and pulmonary emboli and heart attacks occurred as well. “Once we restarted oral anticoagulation ... there were no more thrombotic events,” said Dr. Milling. No patient developed neutralizing antibodies to factor X or factor Xa, nor did any patient develop neutralizing antibodies to andexanet.
The overall mortality rate was 13.9%. The rate of mortality resulting from ICH was 15%, and the rate of mortality resulting from gastrointestinal bleeding was 11%. These results are impressive, considering that patients had received anticoagulants, said Dr. Milling.
Portola Pharmaceuticals, the maker of andexanet alfa, funded the study. Dr. Milling reported receiving funding and honoraria from the Population Health Research Institute at McMasters University, Janssen, CSL Behring, and Octapharma. He also received a small research payment from Portola Pharmaceuticals. Several of the investigators reported receiving funding from Portola Pharmaceuticals.
SOURCE: Milling TJ et al. ISC 2019, Abstract LB7.
HONOLULU – according to a study presented at the International Stroke Conference sponsored by the American Heart Association. The medication is associated with a low rate of mortality resulting from intracerebral hemorrhage (ICH), compared with the general population of patients with ICH receiving anticoagulation.
Factor Xa inhibitors such as apixaban and rivaroxaban effectively prevent thromboembolic events but may cause or exacerbate acute major bleeding. Andexanet alfa, a modified, recombinant, inactive form of human factor Xa, was developed and approved as a reversal agent for factor Xa inhibitors. In a 2015 study, andexanet rapidly and safely reversed anti–factor Xa activity in large cohorts of patients without bleeding.
A single-cohort study
Truman John Milling Jr., MD, an emergency medicine physician at Dell Seton Medical Center at the University of Texas in Austin, and his colleagues conducted the Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors (ANNEXA-4) study to evaluate the drug’s safety and efficacy in patients with acute major bleeding associated with treatment with a factor Xa inhibitor. For participants to be eligible, their bleeding had to be life threatening with signs of hemodynamic compromise, be associated with a decrease in hemoglobin level of at least 2 g/dL, or occur in a critical organ such as the brain. An independent academic committee determined whether patients met these criteria.
The trial’s primary efficacy outcomes were change from baseline in anti–factor Xa activity and the percentage of patients with excellent or good hemostatic efficacy at 12 hours. The primary safety endpoints were death, thrombotic events, and the development of neutralizing antibodies to andexanet or to native factor X and factor Xa. The efficacy population included patients with major bleeding and baseline anti–factor Xa activity of at least 75 ng/mL. The safety population included all patients who received a dose of andexanet. The independent committee adjudicated the efficacy and safety outcomes.
Hemostasis was sustained for 12 hours
The investigators enrolled 352 participants into the study, all of whom received andexanet and were followed for at least 30 days or until death. The population’s mean age was 77 years. “These were older and sicker patients with a significant amount of comorbid disease,” said Dr. Milling. The primary indication for anticoagulation was atrial fibrillation in 80% of patients. The primary site of bleeding was intracranial in 64% of patients and gastrointestinal in 26% of patients. The remaining 10% of patients had bleeding affecting other areas (such as pericardial or intramuscular bleeding).
The investigators included 254 patients in the efficacy population. At the end of the administration of the andexanet bolus, the median value for anti–factor Xa activity decreased by 92% among participants receiving apixaban, 92% among participants receiving rivaroxaban, and 75% among patients receiving enoxaparin. Among patients receiving apixaban, the median value for anti–factor Xa activity was decreased by 32% at 4 hours, 34% at 8 hours, and 38% at 12 hours. Among patients receiving rivaroxaban, the median value for anti–factor Xa activity was decreased by 42% at 4 hours, 48% at 8 hours, and 62% at 12 hours.
Dr. Milling and his colleagues assessed hemostatic efficacy in 249 patients. Of this group, 82% achieved good or excellent hemostasis. Among participants with good or excellent hemostasis, 84% had excellent results, and 16% had good results. Subanalysis by factor Xa inhibitor, type of bleed, age, and dose of andexanet did not alter the findings significantly.
To determine whether hemostasis had been sustained sufficiently to prevent clinical deterioration, the investigators examined 71 patients with ICH and a single-compartment bleed. From 1 hour to 12 hours, one patient’s outcome changed from excellent/good to poor/none, and one patient’s outcome changed from excellent to good. For the majority of these patients, however, good hemostasis was sustained from 1 to 12 hours.
The rate of thromboembolic events was 9.7%, which is in the expected range for this population, said Dr. Milling. These events were distributed evenly among the 4 weeks of the study. Stroke and deep vein thrombosis accounted for most of these events, and pulmonary emboli and heart attacks occurred as well. “Once we restarted oral anticoagulation ... there were no more thrombotic events,” said Dr. Milling. No patient developed neutralizing antibodies to factor X or factor Xa, nor did any patient develop neutralizing antibodies to andexanet.
The overall mortality rate was 13.9%. The rate of mortality resulting from ICH was 15%, and the rate of mortality resulting from gastrointestinal bleeding was 11%. These results are impressive, considering that patients had received anticoagulants, said Dr. Milling.
Portola Pharmaceuticals, the maker of andexanet alfa, funded the study. Dr. Milling reported receiving funding and honoraria from the Population Health Research Institute at McMasters University, Janssen, CSL Behring, and Octapharma. He also received a small research payment from Portola Pharmaceuticals. Several of the investigators reported receiving funding from Portola Pharmaceuticals.
SOURCE: Milling TJ et al. ISC 2019, Abstract LB7.
HONOLULU – according to a study presented at the International Stroke Conference sponsored by the American Heart Association. The medication is associated with a low rate of mortality resulting from intracerebral hemorrhage (ICH), compared with the general population of patients with ICH receiving anticoagulation.
Factor Xa inhibitors such as apixaban and rivaroxaban effectively prevent thromboembolic events but may cause or exacerbate acute major bleeding. Andexanet alfa, a modified, recombinant, inactive form of human factor Xa, was developed and approved as a reversal agent for factor Xa inhibitors. In a 2015 study, andexanet rapidly and safely reversed anti–factor Xa activity in large cohorts of patients without bleeding.
A single-cohort study
Truman John Milling Jr., MD, an emergency medicine physician at Dell Seton Medical Center at the University of Texas in Austin, and his colleagues conducted the Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors (ANNEXA-4) study to evaluate the drug’s safety and efficacy in patients with acute major bleeding associated with treatment with a factor Xa inhibitor. For participants to be eligible, their bleeding had to be life threatening with signs of hemodynamic compromise, be associated with a decrease in hemoglobin level of at least 2 g/dL, or occur in a critical organ such as the brain. An independent academic committee determined whether patients met these criteria.
The trial’s primary efficacy outcomes were change from baseline in anti–factor Xa activity and the percentage of patients with excellent or good hemostatic efficacy at 12 hours. The primary safety endpoints were death, thrombotic events, and the development of neutralizing antibodies to andexanet or to native factor X and factor Xa. The efficacy population included patients with major bleeding and baseline anti–factor Xa activity of at least 75 ng/mL. The safety population included all patients who received a dose of andexanet. The independent committee adjudicated the efficacy and safety outcomes.
Hemostasis was sustained for 12 hours
The investigators enrolled 352 participants into the study, all of whom received andexanet and were followed for at least 30 days or until death. The population’s mean age was 77 years. “These were older and sicker patients with a significant amount of comorbid disease,” said Dr. Milling. The primary indication for anticoagulation was atrial fibrillation in 80% of patients. The primary site of bleeding was intracranial in 64% of patients and gastrointestinal in 26% of patients. The remaining 10% of patients had bleeding affecting other areas (such as pericardial or intramuscular bleeding).
The investigators included 254 patients in the efficacy population. At the end of the administration of the andexanet bolus, the median value for anti–factor Xa activity decreased by 92% among participants receiving apixaban, 92% among participants receiving rivaroxaban, and 75% among patients receiving enoxaparin. Among patients receiving apixaban, the median value for anti–factor Xa activity was decreased by 32% at 4 hours, 34% at 8 hours, and 38% at 12 hours. Among patients receiving rivaroxaban, the median value for anti–factor Xa activity was decreased by 42% at 4 hours, 48% at 8 hours, and 62% at 12 hours.
Dr. Milling and his colleagues assessed hemostatic efficacy in 249 patients. Of this group, 82% achieved good or excellent hemostasis. Among participants with good or excellent hemostasis, 84% had excellent results, and 16% had good results. Subanalysis by factor Xa inhibitor, type of bleed, age, and dose of andexanet did not alter the findings significantly.
To determine whether hemostasis had been sustained sufficiently to prevent clinical deterioration, the investigators examined 71 patients with ICH and a single-compartment bleed. From 1 hour to 12 hours, one patient’s outcome changed from excellent/good to poor/none, and one patient’s outcome changed from excellent to good. For the majority of these patients, however, good hemostasis was sustained from 1 to 12 hours.
The rate of thromboembolic events was 9.7%, which is in the expected range for this population, said Dr. Milling. These events were distributed evenly among the 4 weeks of the study. Stroke and deep vein thrombosis accounted for most of these events, and pulmonary emboli and heart attacks occurred as well. “Once we restarted oral anticoagulation ... there were no more thrombotic events,” said Dr. Milling. No patient developed neutralizing antibodies to factor X or factor Xa, nor did any patient develop neutralizing antibodies to andexanet.
The overall mortality rate was 13.9%. The rate of mortality resulting from ICH was 15%, and the rate of mortality resulting from gastrointestinal bleeding was 11%. These results are impressive, considering that patients had received anticoagulants, said Dr. Milling.
Portola Pharmaceuticals, the maker of andexanet alfa, funded the study. Dr. Milling reported receiving funding and honoraria from the Population Health Research Institute at McMasters University, Janssen, CSL Behring, and Octapharma. He also received a small research payment from Portola Pharmaceuticals. Several of the investigators reported receiving funding from Portola Pharmaceuticals.
SOURCE: Milling TJ et al. ISC 2019, Abstract LB7.
REPORTING FROM ISC 2019
Death by a thousand clicks
Where electronic health records went wrong.
The pain radiated from the top of Annette Monachelli’s head, and it got worse when she changed positions. It didn’t feel like her usual migraine. The 47-year-old Vermont attorney turned innkeeper visited her local doctor at the Stowe Family Practice twice about the problem in late November 2012, but got little relief.
Two months later, Monachelli was dead of an aneurysm, a condition that, despite the symptoms and the appointments, had never been tested for or diagnosed until she turned up in the emergency room days before her death.
Monachelli’s husband sued Stowe, the federally qualified health center the physician worked for. Owen Foster, a newly hired assistant U.S. attorney with the District of Vermont, was assigned to defend the government. Though it looked to be a standard medical malpractice case, Foster was on the cusp of discovering something much bigger – what his boss, U.S. Attorney Christina Nolan, calls the “frontier of health care fraud” – and prosecuting a first-of-its-kind case that landed the largest-ever financial recovery in Vermont’s history.
Foster began with Monachelli’s medical records, which offered a puzzle. Her doctor had considered the possibility of an aneurysm and, to rule it out, had ordered a head scan through the clinic’s software system, the government alleged in court filings. The test, in theory, would have caught the bleeding in Monachelli’s brain. But the order never made it to the lab; it had never been transmitted.
The software in question was an electronic health records system, or EHR, made by eClinicalWorks (eCW), one of the leading sellers of record-keeping software for physicians in America, currently used by 850,000 health professionals in the U.S. It didn’t take long for Foster to assemble a dossier of troubling reports – Better Business Bureau complaints, issues flagged on an eCW user board, and legal cases filed around the country – suggesting the company’s technology didn’t work quite the way it said it did.
Until this point, Foster, like most Americans, knew next to nothing about electronic medical records, but he was quickly amassing clues that eCW’s software had major problems – some of which put patients, like Annette Monachelli, at risk.
Damning evidence came from a whistleblower claim filed in 2011 against the company. Brendan Delaney, a British cop turned EHR expert, was hired in 2010 by New York City to work on the eCW implementation at Rikers Island, a jail complex that then had more than 100,000 inmates. But soon after he was hired, Delaney noticed scores of troubling problems with the system, which became the basis for his lawsuit. The patient medication lists weren’t reliable; prescribed drugs would not show up, while discontinued drugs would appear as current, according to the complaint. The EHR would sometimes display one patient’s medication profile accompanied by the physician’s note for a different patient, making it easy to misdiagnose or prescribe a drug to the wrong individual. Prescriptions, some 30,000 of them in 2010, lacked proper start and stop dates, introducing the opportunity for under- or overmedication. The eCW system did not reliably track lab results, concluded Delaney, who tallied 1,884 tests for which they had never gotten outcomes.
The District of Vermont launched an official federal investigation in 2015.
The eCW spaghetti code was so buggy that when one glitch got fixed, another would develop, the government found. The user interface offered a few ways to order a lab test or diagnostic image, for example, but not all of them seemed to function. The software would detect and warn users of dangerous drug interactions, but unbeknownst to physicians, the alerts stopped if the drug order was customized. “It would be like if I was driving with the radio on and the windshield wipers going and when I hit the turn signal, the brakes suddenly didn’t work,” said Foster.
The eCW system also failed to use the standard drug codes and, in some instances, lab and diagnosis codes as well, the government alleged.
The case never got to a jury. In May 2017, eCW paid a $155 million settlement to the government over alleged “false claims” and kickbacks – one physician made tens of thousands of dollars – to clients who promoted its product. Despite the record settlement, the company denied wrongdoing; eCW did not respond to numerous requests for comment.
If there is a kicker to this tale, it is this: The U.S. government bankrolled the adoption of this software – and continues to pay for it. Or we should say: You do.
Which brings us to the strange, sad, and aggravating story that unfolds below. It is not about one lawsuit or a piece of sloppy technology. Rather, it’s about a trouble-prone industry that intersects, in the most personal way, with every one of our lives. It’s about a $3.7 trillion health care system idling at the crossroads of progress. And it’s about a slew of unintended consequences – the surprising casualties of a big idea whose time had seemingly come.
The virtual magic bullet
Electronic health records were supposed to do a lot: make medicine safer, bring higher-quality care, empower patients, and yes, even save money. Boosters heralded an age when researchers could harness the big data within to reveal the most effective treatments for disease and sharply reduce medical errors. Patients, in turn, would have truly portable health records, being able to share their medical histories in a flash with doctors and hospitals anywhere in the country – essential when life-and-death decisions are being made in the ER.
But 10 years after President Barack Obama signed a law to accelerate the digitization of medical records – with the federal government, so far, sinking $36 billion into the effort – America has little to show for its investment. KHN and Fortune spoke with more than 100 physicians, patients, IT experts and administrators, health policy leaders, attorneys, top government officials and representatives at more than a half-dozen EHR vendors, including the CEOs of two of the companies. The interviews reveal a tragic missed opportunity: Rather than an electronic ecosystem of information, the nation’s thousands of EHRs largely remain a sprawling, disconnected patchwork. Moreover, the effort has handcuffed health providers to technology they mostly can’t stand and has enriched and empowered the $13-billion-a-year industry that sells it.
By one measure, certainly, the effort has achieved what it set out to do: Today, 96% of hospitals have adopted EHRs, up from just 9% in 2008. But on most other counts, the newly installed technology has fallen well short. Physicians complain about clumsy, unintuitive systems and the number of hours spent clicking, typing and trying to navigate them – which is more than the hours they spend with patients. Unlike, say, with the global network of ATMs, the proprietary EHR systems made by more than 700 vendors routinely don’t talk to one another, meaning that doctors still resort to transferring medical data via fax and CD-ROM. Patients, meanwhile, still struggle to access their own records – and, sometimes, just plain can’t.
Instead of reducing costs, many say, EHRs, which were originally optimized for billing rather than for patient care, have instead made it easier to engage in “upcoding” or bill inflation (though some say the systems also make such fraud easier to catch).
More gravely still, a months-long joint investigation by KHN and Fortune has found that instead of streamlining medicine, the government’s EHR initiative has created a host of largely unacknowledged patient safety risks. Our investigation found that alarming reports of patient deaths, serious injuries and near misses – thousands of them – tied to software glitches, user errors or other flaws have piled up, largely unseen, in various government-funded and private repositories.
Compounding the problem are entrenched secrecy policies that continue to keep software failures out of public view. EHR vendors often impose contractual “gag clauses” that discourage buyers from speaking out about safety issues and disastrous software installations – though some customers have taken to the courts to air their grievances. Plaintiffs, moreover, say hospitals often fight to withhold records from injured patients or their families. Indeed, two doctors who spoke candidly about the problems they faced with EHRs later asked that their names not be used, adding that they were forbidden by their health care organizations to talk. Says Assistant U.S. Attorney Foster, the EHR vendors “are protected by a shield of silence.”
Though the software has reduced some types of clinical mistakes common in the era of handwritten notes, Raj Ratwani, a researcher at MedStar Health in Washington, D.C., has documented new patterns of medical errors tied to EHRs that he believes are both perilous and preventable. “The fact that we’re not able to broadcast that nationally and solve these issues immediately, and that another patient somewhere else may be harmed by the very same issue – that just can’t happen,” he said.
David Blumenthal, who, as Obama’s national coordinator for health information technology, was one of the architects of the EHR initiative, acknowledged to KHN and Fortune that electronic health records “have not fulfilled their potential. I think few would argue they have.”
The former president has likewise singled out the effort as one of his most disappointing, bemoaning in a January 2017 interview with Vox “the fact that there are still just mountains of paperwork ... and the doctors still have to input stuff, and the nurses are spending all their time on all this administrative work. We put a big slug of money into trying to encourage everyone to digitalize, to catch up with the rest of the world ... that’s been harder than we expected.”
Seema Verma, the current chief of the Centers for Medicare & Medicaid Services (CMS), which oversees the EHR effort today, shudders at the billions of dollars spent building software that doesn’t share data – an electronic bridge to nowhere. “Providers developed their own systems that may or may not even have worked well for them,” she told KHN and Fortune in an interview last month, “but we didn’t think about how all these systems connect with one another. That was the real missing piece.”
Perhaps none of the initiative’s former boosters is quite as frustrated as former Vice President Joe Biden. At a 2017 meeting with health care leaders in Washington, he railed against the infuriating challenge of getting his son Beau’s medical records from one hospital to another. “I was stunned when my son for a year was battling stage 4 glioblastoma,” said Biden. “I couldn’t get his records. I’m the vice president of the United States of America. ... It was an absolute nightmare. It was ridiculous, absolutely ridiculous, that we’re in that circumstance.”
A bridge to nowhere
As Biden would tell you, the original concept was a smart one. The wave of digitization had swept up virtually every industry, bringing both disruption and, in most cases, greater efficiency. And perhaps none of these industries was more deserving of digital liberation than medicine, where life-measuring and potentially lifesaving data was locked away in paper crypts – stack upon stack of file folders at doctors’ offices across the country.
Stowed in steel cabinets, the records were next to useless. Nobody – particularly at the dawn of the age of the iPhone – thought it was a good idea to leave them that way. The problem, say critics, was in the way that policymakers set about to transform them.
“Every single idea was well-meaning and potentially of societal benefit, but the combined burden of all of them hitting clinicians simultaneously made office practice basically impossible,” said John Halamka, chief information officer at Beth Israel Deaconess Medical Center, who served on the EHR standards committees under both President George W. Bush and President Obama. “In America, we have 11 minutes to see a patient, and, you know, you’re going to be empathetic, make eye contact, enter about 100 pieces of data, and never commit malpractice. It’s not possible!”
KHN and Fortune examined more than two dozen medical negligence cases that have alleged that EHRs either contributed to injuries, had been improperly altered, or were withheld from patients to conceal substandard care. In such cases, the suits typically settle prior to trial with strict confidentiality pledges, so it’s often not possible to determine the merits of the allegations. EHR vendors also frequently have contract stipulations, known as “hold harmless clauses,” that protect them from liability if hospitals are later sued for medical errors – even if they relate to an issue with the technology.
But lawsuits, like that filed by Fabian Ronisky, which do emerge from this veil, are quite telling.
Ronisky, according to his complaint, arrived by ambulance at Providence Saint John’s Health Center in Santa Monica on the afternoon of March 2, 2015. For two days, the young lawyer had been suffering from severe headaches while a disorienting fever left him struggling to tell the 911 operator his address.
Suspecting meningitis, a doctor at the hospital performed a spinal tap, and the next day an infectious disease specialist typed in an order for a critical lab test – a check of the spinal fluid for viruses, including herpes simplex – into the hospital’s EHR.
The multimillion-dollar system, manufactured by Epic Systems Corp. and considered by some to be the Cadillac of medical software, had been installed at the hospital about four months earlier. Although the order appeared on Epic’s screen, it was not sent to the lab. It turned out, Epic’s software didn’t fully “interface” with the lab’s software, according to a lawsuit Ronisky filed in February 2017 in Los Angeles County Superior Court. His results and diagnosis were delayed – by days, he claimed – during which time he suffered irreversible brain damage from herpes encephalitis. The suit alleged the mishap delayed doctors from giving Ronisky a drug called acyclovir that might have minimized damage to his brain.
Epic denied any liability or defects in its software; the company said the doctor failed to push the right button to send the order and that the hospital, not Epic, had configured the interface with the lab. Epic, among the nation’s largest manufacturers of computerized health records and the leading provider to most of America’s most elite medical centers, quietly paid $1 million to settle the suit in July 2018, according to court records. The hospital and two doctors paid a total of $7.5 million, and a case against a third doctor is pending trial. Ronisky, 34, who is fighting to rebuild his life, declined to comment.
Incidents like that which happened to Ronisky – or to Annette Monachelli, for that matter – are surprisingly common, data show. And the back-and-forth about where the fault lies in such cases is actually part of the problem: The systems are often so confusing (and training on them seldom sufficient) that errors frequently fall into a nether zone of responsibility. It can be hard to tell where human error begins and the technological shortcomings end.
EHRs promised to put all of a patient’s records in one place, but often that’s the problem. Critical or time-sensitive information routinely gets buried in an endless scroll of data, where in the rush of medical decision-making – and amid the maze of pulldown menus – it can be missed.
Thirteen-year-old Brooke Dilliplaine, who was severely allergic to dairy, was given a probiotic containing milk. The two doses sent her into “complete respiratory distress” and resulted in a collapsed lung, according to a lawsuit filed by her mother. Rory Staunton, 12, scraped his arm in gym class and then died of sepsis after ER doctors discharged the boy on the basis of lab results in the EHR that weren’t complete. And then there’s the case of Thomas Eric Duncan. The 42-year-old man was sent home in 2014 from a Dallas hospital infected with Ebola virus. Though a nurse had entered in the EHR his recent travel to Liberia, where an Ebola epidemic was then in full swing, the doctor never saw it. Duncan died a week later.
Many such cases end up in court. Typically, doctors and nurses blame faulty technology in the medical-records systems. The EHR vendors blame human error. And meanwhile, the cases mount.
Quantros, a private health care analytics firm, said it has logged 18,000 EHR-related safety events from 2007 through 2018, 3 percent of which resulted in patient harm, including seven deaths – a figure that a Quantros director said is “drastically underreported.”
A 2016 study by The Leapfrog Group, a patient-safety watchdog based in Washington, D.C., found that the medication-ordering function of hospital EHRs – a feature required by the government for certification but often configured differently in each system – failed to flag potentially harmful drug orders in 39 percent of cases in a test simulation. In 13 percent of those cases, the mistake could have been fatal
The Pew Charitable Trusts has, for the past few years, run an EHR safety project, taking aim at issues like usability and patient matching – the process of linking the correct medical record to the correct patient – a seemingly basic task at which the systems, even when made by the same EHR vendor, often fail. At some institutions, according to Pew, such matching was accurate only 50 percent of the time. Patients have discovered mistakes as well: A January survey by the Kaiser Family Foundation found that 1 in 5 patients spotted an error in their electronic medical records. (Kaiser Health News is an editorially independent program of the foundation.)
The Joint Commission, which certifies hospitals, has sounded alarms about a number of issues, including false alarms – which account for between 85 and 99 percent of EHR and medical device alerts. (One study by researchers at Oregon Health & Science University estimated that the average clinician working in the intensive care unit may be exposed to up to 7,000 passive alerts per day.) Such over-warning can be dangerous. From 2014 to 2018, the commission tallied 170 mostly voluntary reports of patient harm related to alarm management and alert fatigue – the phenomenon in which health workers, so overloaded with unnecessary warnings, ignore the occasional meaningful one. Of those 170 incidents, 101 resulted in patient deaths.
The Pennsylvania Patient Safety Authority, an independent state agency that collects information about adverse events and incidents, counted 775 “laboratory-test problems” related to health IT from January 2016 to December 2017.
To be sure, medical errors happened en masse in the age of paper medicine, when hospital staffers misinterpreted a physician’s scrawl or read the wrong chart to deadly consequence, for instance. But what is perhaps telling is how many doctors today opt for manual workarounds to their EHRs. Aaron Zachary Hettinger, an emergency medicine physician with MedStar Health in Washington, D.C., said that when he and fellow clinicians need to share critical patient information, they write it on a whiteboard or on a paper towel and leave it on their colleagues’ computer keyboards.
While the Food and Drug Administration doesn’t mandate reporting of EHR safety events – as it does for regulated medical devices – concerned posts have nonetheless proliferated in the FDA MAUDE database of adverse events, which now serves as an ad hoc bulletin board of warnings about the various systems.
Further complicating the picture is that health providers nearly always tailor their one-size-fits-all EHR systems to their own specifications. Such customization makes every one unique and often hard to compare with others – which, in turn, makes the source of mistakes difficult to determine.
Dr. Martin Makary, a surgical oncologist at Johns Hopkins and the co-author of a much-cited 2016 study that identified medical errors as the third-leading cause of death in America, credits EHRs for some safety improvements – including recent changes that have helped put electronic brakes on the opioid epidemic. But, he said, “we’ve swapped one set of problems for another. We used to struggle with handwriting and missing information. We now struggle with a lack of visual cues to know we’re writing and ordering on the correct patient.”
Dr. Joseph Schneider, a pediatrician at UT Southwestern Medical Center, compares the transition we’ve made, from paper records to electronic ones, to moving from horses to automobiles. But in this analogy, he added, “our cars have advanced to about the 1960s. They still don’t have seat belts or air bags.”
Schneider recalled one episode when his colleagues couldn’t understand why chunks of their notes would inexplicably disappear. They figured out the problem weeks later after intense study: Physicians had been inputting squiggly brackets – {} – the use of which, unbeknownst to even vendor representatives, deleted the text between them. (The EHR maker initially blamed the doctors, said Schneider.)
A broad coalition of actors, from National Nurses United to the Texas Medical Association to leaders within the FDA, has long called for oversight on electronic-record safety issues. Among the most outspoken is Ratwani, who directs MedStar Health’s National Center on Human Factors in Healthcare, a 30-person institute focused on optimizing the safety and usability of medical technology. Ratwani spent his early career in the defense industry, studying things like the intuitiveness of information displays. When he got to MedStar in 2012, he was stunned by “the types of [digital] interfaces being used” in health care, he said.
In a study published last year in the journal Health Affairs, Ratwani and colleagues studied medication errors at three pediatric hospitals from 2012 to 2017. They discovered that 3,243 of them were owing in part to EHR “usability issues.” Roughly 1 in 5 of these could have resulted in patient harm, the researchers found. “Poor interface design and poor implementations can lead to errors and sometimes death, and that is just unbelievably bad as well as completely fixable,” he said. “We should not have patients harmed this way.”
Using eye-tracking technology, Ratwani has demonstrated on video just how easy it is to make mistakes when performing basic tasks on the nation’s two leading EHR systems. When emergency room doctors went to order Tylenol, for example, they saw a drop-down menu listing 86 options, many of which were irrelevant for the specified patient. They had to read the list carefully, so as not to click the wrong dosage or form – though many do that too: In roughly 1 out of 1,000 orders, physicians accidentally select the suppository (designated “PR”) rather than the tablet dose (“OR”), according to one estimate. That’s not an error that will harm a patient – though other medication mix-ups can and do.
Earlier this year, MedStar’s human-factors center launched a website and public awareness campaign with the American Medical Association to draw attention to such rampant mistakes – they use the letters “EHR” as an initialism for “Errors Happen Regularly” – and to petition Congress for action. Ratwani is pushing for a central database to track such errors and adverse events.
Others have turned to social media to vent. Dr. Mark Friedberg, a health-policy researcher with the Rand Corp. who is also a practicing primary care physician, champions the Twitter hashtag #EHRbuglist to encourage fellow health care workers to air their pain points. And last month, a scathing Epic parody account cropped up on Twitter, earning more than 8,000 followers in its first five days. Its maiden tweet, written in the mock voice of an Epic overlord, read: “I once saw a doctor make eye contact with a patient. This horror must stop.”
As much as EHR systems are blamed for sins of commission, it is often the sins of omission that trip up users even more.
Consider the case of Lynne Chauvin, who worked as a medical assistant at Ochsner Health System, in Louisiana. In a still-pending 2015 lawsuit, Chauvin alleges that Epic’s software failed to fire a critical medication warning; Chauvin suffered from conditions that heightened her risk for blood clots, and though that history was documented in her records, she was treated with drugs that restricted blood flow after a heart procedure at the hospital. She developed gangrene, which led to the amputation of her lower legs and forearm. (Ochsner Health System said that while it cannot comment on ongoing litigation, it “remains committed to patient safety which we strongly believe is optimized through the use of electronic health record technology.” Epic declined to comment.)
Echoing the complaints of many doctors, the suit argues that Epic software “is extremely complicated to view and understand,” owing to “significant repetition of data.” Chauvin said that her medical bills have topped $1 million and that she is permanently disabled. Her husband, Richard, has become her primary caregiver and had to retire early from his job with the city of Kenner to care for his wife, according to the suit. Each party declined to comment.
An epidemic of burnout
The numbing repetition, the box-ticking and the endless searching on pulldown menus are all part of what Ratwani called the “cognitive burden” that’s wearing out today’s physicians and driving increasing numbers into early retirement.
In recent years, “physician burnout” has skyrocketed to the top of the agenda in medicine. A 2018 Merritt Hawkins survey found a staggering 78 percent of doctors suffered symptoms of burnout, and in January the Harvard School of Public Health and other institutions deemed it a “public health crisis.”
One of the co-authors of the Harvard study, Ashish Jha, pinned much of the blame on “the growth in poorly designed digital health records ... that [have] required that physicians spend more and more time on tasks that don’t directly benefit patients.”
Few would deny that the swift digitization of America’s medical system has been transformative. With EHRs now nearly universal, the face and feel of medicine has changed. The doctor is now typing away, making more eye contact with the computer screen, perhaps, than with the patient. Patients don’t like that dynamic; for doctors, whose days increasingly begin and end with such fleeting encounters, the effect can be downright deadening.
“You’re sitting in front of a patient, and there are so many things you have to do, and you only have so much time to do it in – seven to 11 minutes, probably – so when do you really listen?” asked John-Henry Pfifferling, a medical anthropologist who counsels physicians suffering from burnout. “If you go into medicine because you care about interacting, and then you’re just a tool, it’s dehumanizing,” said Pfifferling, who has seen many physicians leave medicine over the shift to electronic records. “It’s a disaster,” he said.
Beyond complicating the physician-patient relationship, EHRs have in some ways made practicing medicine harder, said Dr. Hal Baker, a physician and the chief information officer at WellSpan, a Pennsylvania hospital system. “Physicians have to cognitively switch between focusing on the record and focusing on the patient,” he said. He points out how unusual – and potentially dangerous – this is: “Texting while you’re driving is not a good idea. And I have yet to see the CEO who, while running a board meeting, takes minutes, and certainly I’ve never heard of a judge who, during the trial, would also be the court stenographer. But in medicine ... we’ve asked the physician to move from writing in pen to [entering a computer] record, and it’s a pretty complicated interface.”
Even if docs may be at the keyboard during visits, they report having to spend hours more outside that time – at lunch, late at night – in order to finish notes and keep up with electronic paperwork (sending referrals, corresponding with patients, resolving coding issues). That’s right. EHRs didn’t take away paperwork; the systems just moved it online. And there’s a lot of it: 44 percent of the roughly six hours a physician spends on the EHR each day is focused on clerical and administrative tasks, like billing and coding, according to a 2017 Annals of Family Medicine study.
For all that so-called pajama time – the average physician logs 1.4 hours per day on the EHR after work – they don’t get a cent.
Many doctors do recognize the value in the technology: 60 percent of participants in Stanford Medicine’s 2018 National Physician Poll said EHRs had led to improved patient care. At the same time, about as many (59 percent) said EHRs needed a “complete overhaul” and that the systems had detracted from their professional satisfaction (54 percent) as well as from their clinical effectiveness (49 percent).
In preliminary studies, Ratwani has found that doctors have a typical physiological reaction to using an EHR: stress. When he and his team shadow clinicians on the job, they use a range of sensors to monitor the doctors’ heart rate and other vital signs over the course of their shift. The physicians’ heart rates will spike – as high as 160 beats per minute – on two sorts of occasions: when they are interacting with patients and when they’re using the EHR.
4,000
Approximate number of computer clicks an ER doctor makes over the course of a single shift, according to an American Journal of Emergency Medicine study
“Everything is so cumbersome,” said Dr. Karla Dick, a family medicine physician in Arlington, Texas. “It’s slow compared to a paper chart. You’re having to click and zoom in and zoom out to look for stuff.” With all the zooming in and out, she explained, it’s easy to end up in the wrong record. “I can’t tell you how many times I’ve had to cancel an order because I was in the wrong chart.”
Among the daily frustrations for one emergency room physician in Rhode Island is ordering ibuprofen, a seemingly simple task that now requires many rounds of mouse clicking. Every time she prescribes the basic painkiller for a female patient, whether that patient is 9 or 68 years old, the prescription is blocked by a pop-up alert warning her that it may be dangerous to give the drug to a pregnant woman. The physician, whose institution does not allow her to comment on the systems, must then override the warning with yet more clicks. “That’s just the tiniest tip of the iceberg,” she said.
What worries the doctor most is the ease with which diligent, well-meaning physicians can make serious medical errors. She noted that the average ER doc will make 4,000 mouse clicks over the course of a shift, and that the odds of doing anything 4,000 times without an error is small. “The interfaces are just so confusing and clunky,” she added. “They invite error ... it’s not a negligence issue. This is a poor tool issue.”
Many of the EHR makers acknowledge physician burnout is real and say they’re doing what they can to lessen the burden and enhance user experience. Dr. Sam Butler, a pulmonary critical care specialist who started working at Epic in 2001, leads those efforts at the Wisconsin-based company. When doctors get more than 100 messages per week in their in-basket (akin to an email inbox), there’s a higher likelihood of burnout. Butler’s team has also analyzed doctors’ electronic notes – they’re twice as long as they were nine years ago, and three to four times as long as notes in the rest of the world. He said Epic uses such insights to improve the client experience. But coming up with fixes is difficult because doctors “have different viewpoints on everything,” he said. (KHN and Fortune made multiple requests to interview Epic CEO Judy Faulkner, but the company declined to make her available. In a trade interview in February, however, Faulkner said that EHRs were unfairly blamed for physician burnout and cited a study suggesting that there’s little correlation between burnout and EHR satisfaction. Executives at other vendors noted that they’re aware of usability issues and that they’re working on addressing them.)
“It’s not that we’re a bunch of Luddites who don’t know how to use technology,” said the Rhode Island ER doctor. “I have an iPhone and a computer and they work the way they’re supposed to work, and then we’re given these incredibly cumbersome and error-prone tools. This is something the government mandated. There really wasn’t the time to let the cream rise to the top; everyone had to jump in and pick something that worked and spend tens of millions of dollars on a system that is slowly killing us.”
$36 billion and change
The effort to digitize America’s health records got its biggest push in a very low moment: the financial crisis of 2008. In early December of that year, Obama, barely four weeks after his election, pitched an ambitious economic recovery plan. “We will make sure that every doctor’s office and hospital in this country is using cutting-edge technology and electronic medical records so that we can cut red tape, prevent medical mistakes and help save billions of dollars each year,” he said in a radio address.
The idea had already been a fashionable one in Washington. Former House Speaker Newt Gingrich was fond of saying it was easier to track a FedEx package than one’s medical records. Obama’s predecessor, President George W. Bush, had also pursued the idea of wiring up the country’s health system. He didn’t commit much money, but Bush did create an agency to do the job: the Office of the National Coordinator (ONC).
In the depths of recession, the EHR conceit looked like a shovel-ready project that only the paper lobby could hate. In February 2009, legislators passed the HITECH Act, which carved out a hefty chunk of the massive stimulus package for health information technology. The goal was not just to get hospitals and doctors to buy EHRs, but rather to get them using them in a way that would drive better care. So lawmakers devised a carrot-and-stick approach: Physicians would qualify for federal subsidies (a sum of up to nearly $64,000 over a period of years) only if they were “meaningful users” of a government-certified system. Vendors, for their part, had to develop systems that met the government’s requirements.
They didn’t have much time, though. The need to stimulate the economy, which meant getting providers to adopt EHRs quickly, “presented a tremendous conundrum,” said Farzad Mostashari, who joined the ONC as deputy director in 2009 and became its leader in 2011: The ideal – creating a useful, interoperable, nationwide records system – was “utterly infeasible to get to in a short time frame.”
That didn’t stop the federal planners from pursuing their grand ambitions. Everyone had big ideas for the EHRs. The FDA wanted the systems to track unique device identifiers for medical implants, the Centers for Disease Control and Prevention wanted them to support disease surveillance, CMS wanted them to include quality metrics and so on. “We had all the right ideas that were discussed and hashed out by the committee,” said Mostashari, “but they were all of the right ideas.”
Not everyone agreed, though, that they were the right ideas. Before long, “meaningful use” became pejorative shorthand to many for a burdensome government program – making doctors do things like check a box indicating a patient’s smoking status each and every visit.
The EHR vendor community, then a scrappy $2 billion industry, griped at the litany of requirements but stood to gain so much from the government’s $36 billion injection that it jumped in line. As Rusty Frantz, CEO of EHR vendor NextGen Healthcare, put it: “The industry was like, ‘I’ve got this check dangling in front of me, and I have to check these boxes to get there, and so I’m going to do that.’”
Halamka, who was an enthusiastic backer of the initiative in both the Bush and Obama administrations, blames the pressure for a speedy launch as much as the excessive wish list. “To go from a regulation to a highly usable product that is in the hands of doctors in 18 months, that’s too fast,” he said. “It’s like asking nine women to have a baby in a month.”
Several of those who worked on the project admit the rollout was not as easy or seamless as they’d anticipated, but they contend that was never the point. Aneesh Chopra, appointed by Obama in 2009 as the nation’s first chief technology officer, called the spending a “down payment” on a vision to fundamentally change American medicine – creating a digital infrastructure to support new ways to pay for health services based on their quality and outcomes.
Dr. Bob Kocher, a physician and star investor with venture capital firm Venrock, who served in the Obama administration from 2009 to 2011 as a health and economic policy adviser, not only defends the rollout then but also disputes the notion that the government initiative has been a failure at all. “EHRs have totally lived up to the hype and expectations,” he said, emphasizing that they also serve as a technology foundation to support innovation on everything from patients accessing their medical records on a smartphone to AI-driven medical sleuthing. Others note the systems’ value in aggregating medical data in ways that were never possible with paper – helping, for example, to figure out that contaminated water was poisoning children in Flint, Mich.
But Rusty Frantz heard a far different message about EHRs – and, more important, it was coming from his own customers.
The Stanford-trained engineer, who in 2015 became CEO of NextGen, a $500-million-a-year EHR heavyweight in the physician-office market, learned the hard way about how his product was being viewed. As he stood at the podium at his first meeting with thousands of NextGen customers at Las Vegas’ Mandalay Bay Resort, just four months after getting the job, he told KHN and Fortune, “People were lining up at the microphones to yell at us: ‘We weren’t delivering stable software! The executive team was inaccessible! The service experience was terrible!’ ” (He now refers to the event as “Festivus: the airing of the grievances.”)
Frantz had bounced around the health care industry for much of his career, and from the nearby perch of a medical device company, he watched the EHR incentive bonanza with a mix of envy and slack-jawed awe. “The industry was moving along in a natural Darwinist way, and then along came the stimulus,” said Frantz, who blames the government’s ham-handed approach to regulation. “The software got slammed in, and the software wasn’t implemented in a way that supported care,” he said. “It was installed in a way that supported stimulus. This company, we were complicit in it, too.”
Even that may be a generous description. KHN and Fortune found a trail of lawsuits against the company, stretching from White Sulphur Springs, Mont., to Neillsville, Wis. Mary Rutan Hospital in Bellefontaine, Ohio, sued NextGen (formerly called Quality Systems) in federal court in 2013, arguing that it experienced hundreds of problems with the “materially defective” software the company had installed in 2011.
A consultant hired by the hospital to evaluate the NextGen system, whose 60-page report was submitted to the court, identified “many functional defects” that he said rendered the software “unfit for its intended purpose.” Some patient information was not accurately recorded, which had the potential, the consultant wrote, “to create major patient care risk which could lead to, at a minimum, inconvenience, and at worst, malpractice or even death.” Glitches at Mary Rutan included incidents in which the software would apparently change a patient’s gender at random or lose a doctor’s observations after an exam, the consultant reported. The company, he found, sometimes took months to address issues: One IT ticket, which related to a physician’s notes inexplicably deleting themselves, reportedly took 10 months to resolve. (The consultant also noted that similar problems appeared to be occurring at as many as a dozen other hospitals that had installed NextGen software.)
The Ohio hospital, which paid more than $1.5 million for its EHR system, claimed breach of contract. NextGen responded that it disputed the claims made in the lawsuit and that the matter was resolved in 2015 “with no findings of fact by a court related to the allegations.” The hospital declined to comment.
At the time, as it has been since then, NextGen’s software was certified by the government as meeting the requirements of the stimulus program. By 2016, NextGen had more than 19,000 customers who had received federal subsidies.
NextGen was subpoenaed by the Department of Justice in December 2017, months after becoming the subject of a federal investigation led by the District of Vermont. Frantz tells KHN and Fortune that NextGen is cooperating with the investigation. “This company was not dishonest, but it was not effective four years ago,” he said. Frantz also emphasized that NextGen has “rapidly evolved” during his tenure, earning five industry awards since 2017, and that customers have “responded very positively.”
Glen Tullman, who until 2012 led Allscripts, another leading EHR vendor that benefited royally from the stimulus and that has been sued by numerous unhappy customers, admitted that the industry’s race to market took priority over all else.
“It was a big distraction. That was an unintended consequence of that,” Tullman said. “All the companies were saying, This is a one-time opportunity to expand our share, focus everything there, and then we’ll go back and fix it.” The Justice Department has opened a civil investigation into the company, Securities and Exchange Commission filings show. Allscripts said in an email that it cannot comment on an ongoing investigation, but that the civil investigations by the Department of Justice relate to businesses it acquired after the investigations were opened.
Much of the marketing mayhem occurred because federal officials imposed few controls over firms scrambling to cash in on the stimulus. It was a gold rush – and any system, it seemed, could be marketed as “federally approved.” Doctors could shop for bargain-price software packages at Costco and Walmart’s Sam’s Club – where eClinicalWorks sold a “turnkey” system for $11,925 – and cash in on the government’s adoption incentives.
The top-shelf vendors in 2009 crisscrossed the country on a “stimulus tour” like rock groups, gigging at some 30 cities, where they offered doctors who showed up to hear the pitch “a customized analysis” of how much money they could earn off the government incentives. Following the same playbook used by pharmaceutical companies, EHR sellers courted doctors at fancy dinners in ritzy hotels. One enterprising software firm advertised a “cash for clunkers” deal that paid $3,000 to doctors willing to trade in their current records system for a new one. Athenahealth held “invitation only” dinners at luxury hotels to advise doctors, among other things, how to use the stimulus to get paid more and capture available incentives. Allscripts offered a no-money-down purchase plan to help doctors “maximize the return on your EHR investment.” (An Athenahealth spokesperson said the company’s “dinners were educational in nature and aimed at helping physicians navigate the government program.” Allscripts did not respond directly to questions about its marketing practices, but said it “is proud of the software and services [it provides] to hundreds of thousands of caregivers across the globe.”)
EHRs were supposed to reduce health care costs, at least in part by preventing duplicative tests. But as the federal government opened the stimulus tap, many raised doubts about the promised savings. Advocates bandied about a figure of $80 billion in cost savings even as congressional auditors were debunking it. While the jury’s still out, there’s growing suspicion the digital revolution may potentially raise health care costs by encouraging overbilling and new strains of fraud and abuse.
In September 2012, following press reports suggesting that some doctors and hospitals were using the new technology to improperly boost their fees, a practice known as “upcoding,” then-Health and Human Services chief Kathleen Sebelius and Attorney General Eric Holder warned the industry not to try to “game the system.”
There’s also growing evidence that some doctors and health systems may have overstated their use of the new technology to secure stimulus funds, a potentially enormous fraud against Medicare and Medicaid that likely will take many years to unravel. In June 2017, the HHS inspector general estimated that Medicare officials made more than $729 million in subsidy payments to hospitals and doctors that didn’t deserve them.
Individual states, which administer the Medicaid portion of the program, haven’t fared much better. Audits have uncovered overpayments in 14 of 17 state programs reviewed, totaling more than $66 million, according to inspector general reports.
Last month, Sen. Chuck Grassley, an Iowa Republican who chairs the Senate Finance Committee, sharply criticized CMS for recovering only a tiny fraction of these bogus payments, or what he termed a “spit in the ocean.”
EHR vendors have also been accused of egregious and patient-endangering acts of fraud as they raced to cash in on the stimulus money grab. In addition to the U.S. government’s $155 million False Claims Act settlement with eClinicalWorks noted above, the federal government has reached a second settlement over similar charges against another large vendor, Tampa-based Greenway Health. In February, that company settled with the government for just over $57 million without denying or admitting wrongdoing. “These are cases of corporate greed, companies that prioritized profits over everything else,” said Christina Nolan, the U.S. attorney for the District of Vermont, whose office led the cases. (In a response, Greenway Health did not address the charges or the settlement but said it was “committing itself to being the standard-bearer for quality, compliance, and transparency.”)
Tower of Babel
In early 2017, Seema Verma, then the country’s newly appointed CMS administrator, went on a listening tour. She visited doctors around the country, at big urban practices and tiny rural clinics, and from those front-line physicians she consistently heard one thing: They hated their electronic health records. “Physician burnout is real,” she told KHN and Fortune. The doctors spoke of the difficulty in getting information from other systems and providers, and they complained about the government’s reporting requirements, which they perceived as burdensome and not meaningful.
What she heard then became suddenly personal one summer day in 2017, when her husband, himself a physician, collapsed in the airport on his way home to Indianapolis after a family vacation. For a frantic few hours, the CMS administrator fielded phone calls from first responders and physicians – Did she know his medical history? Did she have information that could save his life? – and made calls to his doctors in Indiana, scrambling to piece together his record, which should have been there in one piece. Her husband survived the episode, but it laid bare the dysfunction and danger inherent in the existing health information ecosystem.
The notion that one EHR should talk to another was a key part of the original vision for the HITECH Act, with the government calling for systems to be eventually interoperable.
What the framers of that vision didn’t count on were the business incentives working against it. A free exchange of information means that patients can be treated anywhere. And though they may not admit it, many health providers are loath to lose their patients to a competing doctor’s office or hospital. There’s a term for that lost revenue: “leakage.” And keeping a tight hold on patients’ medical records is one way to prevent it.
There’s a ton of proprietary value in that data, said Blumenthal, who now heads the Commonwealth Fund, a philanthropy that does health research. Asking hospitals to give it up is “like asking Amazon to share their data with Walmart,” he said.
Blumenthal acknowledged that he failed to grasp these perverse business dynamics and foresee what a challenge getting the systems to talk to one another would be. He added that forcing interoperability goals early on, when 90 percent of the nation’s providers still didn’t have systems or data to exchange, seemed unrealistic. “We had an expression: They had to operate before they could interoperate,” he said.
In the absence of true incentives for systems to communicate, the industry limped along; some providers wired up directly to other select providers or through regional exchanges, but the efforts were spotty. A Cerner-backed interoperability network called CommonWell formed in 2013, but some companies, including dominant Epic, didn’t join. (“Initially, Epic was neither invited nor allowed to join,” said Sumit Rana, senior vice president of R&D at Epic. Jitin Asnaani, executive director of CommonWell countered, “We made repeated invitations to every major EHR ... and numerous public and private invitations to Epic.”)
Epic then supported a separate effort to do much the same.
Last spring, Verma attempted to kick-start the sharing effort and later pledged a war on “information blocking,” threatening penalties for bad actors. She has promised to reduce the documentation burden on physicians and end the gag clauses that protect the EHR industry. Regarding the first effort at least, “there was consensus that this needed to happen and that it would take the government to push this forward,” she said. In one sign of progress last summer, the dueling sharing initiatives of Epic and Cerner, the two largest players in the industry, began to share with each other – though the effort is fledgling.
When it comes to patients, though, the real sharing too often stops. Despite federal requirements that providers give patients their medical records in a timely fashion, in their chosen format and at low cost (the government recommends a flat fee of $6.50 or less), patients struggle mightily to get them. A 2017 study by researchers at Yale found that of America’s 83 top-rated hospitals, only 53 percent offer forms that provide patients with the option to receive their entire medical record. Fewer than half would share records via email. One hospital charged more than $500 to release them.
Sometimes the mere effort to access records leads to court. Jennifer De Angelis, a Tulsa attorney, has frequently sparred with hospitals over releasing her clients’ records. She said they either attempt to charge huge sums for them or force her to obtain a court order before releasing them. De Angelis added that she sometimes suspects the records have been overwritten to cover up medical mistakes.
Consider the case of 5-year-old Uriah R. Roach, who fractured and cut his finger on Oct. 2, 2014, when it was accidentally slammed in a door at school. Five days later, an operation to repair the damage went awry, and he suffered permanent brain damage, apparently owing to an anesthesia problem. The Epic electronic medical file had been accessed more than 76,000 times during the 22 days the boy was in the hospital, and a lawsuit brought by his parents contended that numerous entries had been “corrected, altered, modified and possibly deleted after an unexpected outcome during the induction of anesthesia.” The hospital denied wrongdoing. The case settled in November 2016, and the terms are confidential.
More than a dozen other attorneys interviewed cited similar problems, especially with gaining access to computerized “audit trails.” In several cases, court records show, government lawyers resisted turning over electronic files from federally run hospitals. That happened to Russell Uselton, an Oklahoma lawyer who represented a pregnant teen admitted to the Choctaw Nation Health Care Center in Talihina, Okla. Shelby Carshall, 18, was more than 40 weeks pregnant at the time. Doctors failed to perform a cesarean section, and her baby was born brain-damaged as a result, she alleged in a lawsuit filed in 2017 against the U.S. government. The baby began having seizures at 10 hours old and will “likely never walk, talk, eat, or otherwise live normally,” according to pleadings in the suit. Though the federal government requires hospitals to produce electronic health records to patients and their families, Uselton had to obtain a court order to get the baby’s complete medical files. Government lawyers denied any negligence in the case, which is pending.
“They try to hide anything from you that they can hide from you,” said Uselton. “They make it extremely difficult to get records, so expensive and hard that most lawyers can’t take it on,” he said.
Nor, it seems, can high-ranking federal officials. When Seema Verma’s husband was discharged from the hospital after his summer health scare, he was handed a few papers and a CD-ROM containing some medical images – but missing key tests and monitoring data. Said Verma, “We left that hospital and we still don’t have his information today.” That was nearly two years ago
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Where electronic health records went wrong.
Where electronic health records went wrong.
The pain radiated from the top of Annette Monachelli’s head, and it got worse when she changed positions. It didn’t feel like her usual migraine. The 47-year-old Vermont attorney turned innkeeper visited her local doctor at the Stowe Family Practice twice about the problem in late November 2012, but got little relief.
Two months later, Monachelli was dead of an aneurysm, a condition that, despite the symptoms and the appointments, had never been tested for or diagnosed until she turned up in the emergency room days before her death.
Monachelli’s husband sued Stowe, the federally qualified health center the physician worked for. Owen Foster, a newly hired assistant U.S. attorney with the District of Vermont, was assigned to defend the government. Though it looked to be a standard medical malpractice case, Foster was on the cusp of discovering something much bigger – what his boss, U.S. Attorney Christina Nolan, calls the “frontier of health care fraud” – and prosecuting a first-of-its-kind case that landed the largest-ever financial recovery in Vermont’s history.
Foster began with Monachelli’s medical records, which offered a puzzle. Her doctor had considered the possibility of an aneurysm and, to rule it out, had ordered a head scan through the clinic’s software system, the government alleged in court filings. The test, in theory, would have caught the bleeding in Monachelli’s brain. But the order never made it to the lab; it had never been transmitted.
The software in question was an electronic health records system, or EHR, made by eClinicalWorks (eCW), one of the leading sellers of record-keeping software for physicians in America, currently used by 850,000 health professionals in the U.S. It didn’t take long for Foster to assemble a dossier of troubling reports – Better Business Bureau complaints, issues flagged on an eCW user board, and legal cases filed around the country – suggesting the company’s technology didn’t work quite the way it said it did.
Until this point, Foster, like most Americans, knew next to nothing about electronic medical records, but he was quickly amassing clues that eCW’s software had major problems – some of which put patients, like Annette Monachelli, at risk.
Damning evidence came from a whistleblower claim filed in 2011 against the company. Brendan Delaney, a British cop turned EHR expert, was hired in 2010 by New York City to work on the eCW implementation at Rikers Island, a jail complex that then had more than 100,000 inmates. But soon after he was hired, Delaney noticed scores of troubling problems with the system, which became the basis for his lawsuit. The patient medication lists weren’t reliable; prescribed drugs would not show up, while discontinued drugs would appear as current, according to the complaint. The EHR would sometimes display one patient’s medication profile accompanied by the physician’s note for a different patient, making it easy to misdiagnose or prescribe a drug to the wrong individual. Prescriptions, some 30,000 of them in 2010, lacked proper start and stop dates, introducing the opportunity for under- or overmedication. The eCW system did not reliably track lab results, concluded Delaney, who tallied 1,884 tests for which they had never gotten outcomes.
The District of Vermont launched an official federal investigation in 2015.
The eCW spaghetti code was so buggy that when one glitch got fixed, another would develop, the government found. The user interface offered a few ways to order a lab test or diagnostic image, for example, but not all of them seemed to function. The software would detect and warn users of dangerous drug interactions, but unbeknownst to physicians, the alerts stopped if the drug order was customized. “It would be like if I was driving with the radio on and the windshield wipers going and when I hit the turn signal, the brakes suddenly didn’t work,” said Foster.
The eCW system also failed to use the standard drug codes and, in some instances, lab and diagnosis codes as well, the government alleged.
The case never got to a jury. In May 2017, eCW paid a $155 million settlement to the government over alleged “false claims” and kickbacks – one physician made tens of thousands of dollars – to clients who promoted its product. Despite the record settlement, the company denied wrongdoing; eCW did not respond to numerous requests for comment.
If there is a kicker to this tale, it is this: The U.S. government bankrolled the adoption of this software – and continues to pay for it. Or we should say: You do.
Which brings us to the strange, sad, and aggravating story that unfolds below. It is not about one lawsuit or a piece of sloppy technology. Rather, it’s about a trouble-prone industry that intersects, in the most personal way, with every one of our lives. It’s about a $3.7 trillion health care system idling at the crossroads of progress. And it’s about a slew of unintended consequences – the surprising casualties of a big idea whose time had seemingly come.
The virtual magic bullet
Electronic health records were supposed to do a lot: make medicine safer, bring higher-quality care, empower patients, and yes, even save money. Boosters heralded an age when researchers could harness the big data within to reveal the most effective treatments for disease and sharply reduce medical errors. Patients, in turn, would have truly portable health records, being able to share their medical histories in a flash with doctors and hospitals anywhere in the country – essential when life-and-death decisions are being made in the ER.
But 10 years after President Barack Obama signed a law to accelerate the digitization of medical records – with the federal government, so far, sinking $36 billion into the effort – America has little to show for its investment. KHN and Fortune spoke with more than 100 physicians, patients, IT experts and administrators, health policy leaders, attorneys, top government officials and representatives at more than a half-dozen EHR vendors, including the CEOs of two of the companies. The interviews reveal a tragic missed opportunity: Rather than an electronic ecosystem of information, the nation’s thousands of EHRs largely remain a sprawling, disconnected patchwork. Moreover, the effort has handcuffed health providers to technology they mostly can’t stand and has enriched and empowered the $13-billion-a-year industry that sells it.
By one measure, certainly, the effort has achieved what it set out to do: Today, 96% of hospitals have adopted EHRs, up from just 9% in 2008. But on most other counts, the newly installed technology has fallen well short. Physicians complain about clumsy, unintuitive systems and the number of hours spent clicking, typing and trying to navigate them – which is more than the hours they spend with patients. Unlike, say, with the global network of ATMs, the proprietary EHR systems made by more than 700 vendors routinely don’t talk to one another, meaning that doctors still resort to transferring medical data via fax and CD-ROM. Patients, meanwhile, still struggle to access their own records – and, sometimes, just plain can’t.
Instead of reducing costs, many say, EHRs, which were originally optimized for billing rather than for patient care, have instead made it easier to engage in “upcoding” or bill inflation (though some say the systems also make such fraud easier to catch).
More gravely still, a months-long joint investigation by KHN and Fortune has found that instead of streamlining medicine, the government’s EHR initiative has created a host of largely unacknowledged patient safety risks. Our investigation found that alarming reports of patient deaths, serious injuries and near misses – thousands of them – tied to software glitches, user errors or other flaws have piled up, largely unseen, in various government-funded and private repositories.
Compounding the problem are entrenched secrecy policies that continue to keep software failures out of public view. EHR vendors often impose contractual “gag clauses” that discourage buyers from speaking out about safety issues and disastrous software installations – though some customers have taken to the courts to air their grievances. Plaintiffs, moreover, say hospitals often fight to withhold records from injured patients or their families. Indeed, two doctors who spoke candidly about the problems they faced with EHRs later asked that their names not be used, adding that they were forbidden by their health care organizations to talk. Says Assistant U.S. Attorney Foster, the EHR vendors “are protected by a shield of silence.”
Though the software has reduced some types of clinical mistakes common in the era of handwritten notes, Raj Ratwani, a researcher at MedStar Health in Washington, D.C., has documented new patterns of medical errors tied to EHRs that he believes are both perilous and preventable. “The fact that we’re not able to broadcast that nationally and solve these issues immediately, and that another patient somewhere else may be harmed by the very same issue – that just can’t happen,” he said.
David Blumenthal, who, as Obama’s national coordinator for health information technology, was one of the architects of the EHR initiative, acknowledged to KHN and Fortune that electronic health records “have not fulfilled their potential. I think few would argue they have.”
The former president has likewise singled out the effort as one of his most disappointing, bemoaning in a January 2017 interview with Vox “the fact that there are still just mountains of paperwork ... and the doctors still have to input stuff, and the nurses are spending all their time on all this administrative work. We put a big slug of money into trying to encourage everyone to digitalize, to catch up with the rest of the world ... that’s been harder than we expected.”
Seema Verma, the current chief of the Centers for Medicare & Medicaid Services (CMS), which oversees the EHR effort today, shudders at the billions of dollars spent building software that doesn’t share data – an electronic bridge to nowhere. “Providers developed their own systems that may or may not even have worked well for them,” she told KHN and Fortune in an interview last month, “but we didn’t think about how all these systems connect with one another. That was the real missing piece.”
Perhaps none of the initiative’s former boosters is quite as frustrated as former Vice President Joe Biden. At a 2017 meeting with health care leaders in Washington, he railed against the infuriating challenge of getting his son Beau’s medical records from one hospital to another. “I was stunned when my son for a year was battling stage 4 glioblastoma,” said Biden. “I couldn’t get his records. I’m the vice president of the United States of America. ... It was an absolute nightmare. It was ridiculous, absolutely ridiculous, that we’re in that circumstance.”
A bridge to nowhere
As Biden would tell you, the original concept was a smart one. The wave of digitization had swept up virtually every industry, bringing both disruption and, in most cases, greater efficiency. And perhaps none of these industries was more deserving of digital liberation than medicine, where life-measuring and potentially lifesaving data was locked away in paper crypts – stack upon stack of file folders at doctors’ offices across the country.
Stowed in steel cabinets, the records were next to useless. Nobody – particularly at the dawn of the age of the iPhone – thought it was a good idea to leave them that way. The problem, say critics, was in the way that policymakers set about to transform them.
“Every single idea was well-meaning and potentially of societal benefit, but the combined burden of all of them hitting clinicians simultaneously made office practice basically impossible,” said John Halamka, chief information officer at Beth Israel Deaconess Medical Center, who served on the EHR standards committees under both President George W. Bush and President Obama. “In America, we have 11 minutes to see a patient, and, you know, you’re going to be empathetic, make eye contact, enter about 100 pieces of data, and never commit malpractice. It’s not possible!”
KHN and Fortune examined more than two dozen medical negligence cases that have alleged that EHRs either contributed to injuries, had been improperly altered, or were withheld from patients to conceal substandard care. In such cases, the suits typically settle prior to trial with strict confidentiality pledges, so it’s often not possible to determine the merits of the allegations. EHR vendors also frequently have contract stipulations, known as “hold harmless clauses,” that protect them from liability if hospitals are later sued for medical errors – even if they relate to an issue with the technology.
But lawsuits, like that filed by Fabian Ronisky, which do emerge from this veil, are quite telling.
Ronisky, according to his complaint, arrived by ambulance at Providence Saint John’s Health Center in Santa Monica on the afternoon of March 2, 2015. For two days, the young lawyer had been suffering from severe headaches while a disorienting fever left him struggling to tell the 911 operator his address.
Suspecting meningitis, a doctor at the hospital performed a spinal tap, and the next day an infectious disease specialist typed in an order for a critical lab test – a check of the spinal fluid for viruses, including herpes simplex – into the hospital’s EHR.
The multimillion-dollar system, manufactured by Epic Systems Corp. and considered by some to be the Cadillac of medical software, had been installed at the hospital about four months earlier. Although the order appeared on Epic’s screen, it was not sent to the lab. It turned out, Epic’s software didn’t fully “interface” with the lab’s software, according to a lawsuit Ronisky filed in February 2017 in Los Angeles County Superior Court. His results and diagnosis were delayed – by days, he claimed – during which time he suffered irreversible brain damage from herpes encephalitis. The suit alleged the mishap delayed doctors from giving Ronisky a drug called acyclovir that might have minimized damage to his brain.
Epic denied any liability or defects in its software; the company said the doctor failed to push the right button to send the order and that the hospital, not Epic, had configured the interface with the lab. Epic, among the nation’s largest manufacturers of computerized health records and the leading provider to most of America’s most elite medical centers, quietly paid $1 million to settle the suit in July 2018, according to court records. The hospital and two doctors paid a total of $7.5 million, and a case against a third doctor is pending trial. Ronisky, 34, who is fighting to rebuild his life, declined to comment.
Incidents like that which happened to Ronisky – or to Annette Monachelli, for that matter – are surprisingly common, data show. And the back-and-forth about where the fault lies in such cases is actually part of the problem: The systems are often so confusing (and training on them seldom sufficient) that errors frequently fall into a nether zone of responsibility. It can be hard to tell where human error begins and the technological shortcomings end.
EHRs promised to put all of a patient’s records in one place, but often that’s the problem. Critical or time-sensitive information routinely gets buried in an endless scroll of data, where in the rush of medical decision-making – and amid the maze of pulldown menus – it can be missed.
Thirteen-year-old Brooke Dilliplaine, who was severely allergic to dairy, was given a probiotic containing milk. The two doses sent her into “complete respiratory distress” and resulted in a collapsed lung, according to a lawsuit filed by her mother. Rory Staunton, 12, scraped his arm in gym class and then died of sepsis after ER doctors discharged the boy on the basis of lab results in the EHR that weren’t complete. And then there’s the case of Thomas Eric Duncan. The 42-year-old man was sent home in 2014 from a Dallas hospital infected with Ebola virus. Though a nurse had entered in the EHR his recent travel to Liberia, where an Ebola epidemic was then in full swing, the doctor never saw it. Duncan died a week later.
Many such cases end up in court. Typically, doctors and nurses blame faulty technology in the medical-records systems. The EHR vendors blame human error. And meanwhile, the cases mount.
Quantros, a private health care analytics firm, said it has logged 18,000 EHR-related safety events from 2007 through 2018, 3 percent of which resulted in patient harm, including seven deaths – a figure that a Quantros director said is “drastically underreported.”
A 2016 study by The Leapfrog Group, a patient-safety watchdog based in Washington, D.C., found that the medication-ordering function of hospital EHRs – a feature required by the government for certification but often configured differently in each system – failed to flag potentially harmful drug orders in 39 percent of cases in a test simulation. In 13 percent of those cases, the mistake could have been fatal
The Pew Charitable Trusts has, for the past few years, run an EHR safety project, taking aim at issues like usability and patient matching – the process of linking the correct medical record to the correct patient – a seemingly basic task at which the systems, even when made by the same EHR vendor, often fail. At some institutions, according to Pew, such matching was accurate only 50 percent of the time. Patients have discovered mistakes as well: A January survey by the Kaiser Family Foundation found that 1 in 5 patients spotted an error in their electronic medical records. (Kaiser Health News is an editorially independent program of the foundation.)
The Joint Commission, which certifies hospitals, has sounded alarms about a number of issues, including false alarms – which account for between 85 and 99 percent of EHR and medical device alerts. (One study by researchers at Oregon Health & Science University estimated that the average clinician working in the intensive care unit may be exposed to up to 7,000 passive alerts per day.) Such over-warning can be dangerous. From 2014 to 2018, the commission tallied 170 mostly voluntary reports of patient harm related to alarm management and alert fatigue – the phenomenon in which health workers, so overloaded with unnecessary warnings, ignore the occasional meaningful one. Of those 170 incidents, 101 resulted in patient deaths.
The Pennsylvania Patient Safety Authority, an independent state agency that collects information about adverse events and incidents, counted 775 “laboratory-test problems” related to health IT from January 2016 to December 2017.
To be sure, medical errors happened en masse in the age of paper medicine, when hospital staffers misinterpreted a physician’s scrawl or read the wrong chart to deadly consequence, for instance. But what is perhaps telling is how many doctors today opt for manual workarounds to their EHRs. Aaron Zachary Hettinger, an emergency medicine physician with MedStar Health in Washington, D.C., said that when he and fellow clinicians need to share critical patient information, they write it on a whiteboard or on a paper towel and leave it on their colleagues’ computer keyboards.
While the Food and Drug Administration doesn’t mandate reporting of EHR safety events – as it does for regulated medical devices – concerned posts have nonetheless proliferated in the FDA MAUDE database of adverse events, which now serves as an ad hoc bulletin board of warnings about the various systems.
Further complicating the picture is that health providers nearly always tailor their one-size-fits-all EHR systems to their own specifications. Such customization makes every one unique and often hard to compare with others – which, in turn, makes the source of mistakes difficult to determine.
Dr. Martin Makary, a surgical oncologist at Johns Hopkins and the co-author of a much-cited 2016 study that identified medical errors as the third-leading cause of death in America, credits EHRs for some safety improvements – including recent changes that have helped put electronic brakes on the opioid epidemic. But, he said, “we’ve swapped one set of problems for another. We used to struggle with handwriting and missing information. We now struggle with a lack of visual cues to know we’re writing and ordering on the correct patient.”
Dr. Joseph Schneider, a pediatrician at UT Southwestern Medical Center, compares the transition we’ve made, from paper records to electronic ones, to moving from horses to automobiles. But in this analogy, he added, “our cars have advanced to about the 1960s. They still don’t have seat belts or air bags.”
Schneider recalled one episode when his colleagues couldn’t understand why chunks of their notes would inexplicably disappear. They figured out the problem weeks later after intense study: Physicians had been inputting squiggly brackets – {} – the use of which, unbeknownst to even vendor representatives, deleted the text between them. (The EHR maker initially blamed the doctors, said Schneider.)
A broad coalition of actors, from National Nurses United to the Texas Medical Association to leaders within the FDA, has long called for oversight on electronic-record safety issues. Among the most outspoken is Ratwani, who directs MedStar Health’s National Center on Human Factors in Healthcare, a 30-person institute focused on optimizing the safety and usability of medical technology. Ratwani spent his early career in the defense industry, studying things like the intuitiveness of information displays. When he got to MedStar in 2012, he was stunned by “the types of [digital] interfaces being used” in health care, he said.
In a study published last year in the journal Health Affairs, Ratwani and colleagues studied medication errors at three pediatric hospitals from 2012 to 2017. They discovered that 3,243 of them were owing in part to EHR “usability issues.” Roughly 1 in 5 of these could have resulted in patient harm, the researchers found. “Poor interface design and poor implementations can lead to errors and sometimes death, and that is just unbelievably bad as well as completely fixable,” he said. “We should not have patients harmed this way.”
Using eye-tracking technology, Ratwani has demonstrated on video just how easy it is to make mistakes when performing basic tasks on the nation’s two leading EHR systems. When emergency room doctors went to order Tylenol, for example, they saw a drop-down menu listing 86 options, many of which were irrelevant for the specified patient. They had to read the list carefully, so as not to click the wrong dosage or form – though many do that too: In roughly 1 out of 1,000 orders, physicians accidentally select the suppository (designated “PR”) rather than the tablet dose (“OR”), according to one estimate. That’s not an error that will harm a patient – though other medication mix-ups can and do.
Earlier this year, MedStar’s human-factors center launched a website and public awareness campaign with the American Medical Association to draw attention to such rampant mistakes – they use the letters “EHR” as an initialism for “Errors Happen Regularly” – and to petition Congress for action. Ratwani is pushing for a central database to track such errors and adverse events.
Others have turned to social media to vent. Dr. Mark Friedberg, a health-policy researcher with the Rand Corp. who is also a practicing primary care physician, champions the Twitter hashtag #EHRbuglist to encourage fellow health care workers to air their pain points. And last month, a scathing Epic parody account cropped up on Twitter, earning more than 8,000 followers in its first five days. Its maiden tweet, written in the mock voice of an Epic overlord, read: “I once saw a doctor make eye contact with a patient. This horror must stop.”
As much as EHR systems are blamed for sins of commission, it is often the sins of omission that trip up users even more.
Consider the case of Lynne Chauvin, who worked as a medical assistant at Ochsner Health System, in Louisiana. In a still-pending 2015 lawsuit, Chauvin alleges that Epic’s software failed to fire a critical medication warning; Chauvin suffered from conditions that heightened her risk for blood clots, and though that history was documented in her records, she was treated with drugs that restricted blood flow after a heart procedure at the hospital. She developed gangrene, which led to the amputation of her lower legs and forearm. (Ochsner Health System said that while it cannot comment on ongoing litigation, it “remains committed to patient safety which we strongly believe is optimized through the use of electronic health record technology.” Epic declined to comment.)
Echoing the complaints of many doctors, the suit argues that Epic software “is extremely complicated to view and understand,” owing to “significant repetition of data.” Chauvin said that her medical bills have topped $1 million and that she is permanently disabled. Her husband, Richard, has become her primary caregiver and had to retire early from his job with the city of Kenner to care for his wife, according to the suit. Each party declined to comment.
An epidemic of burnout
The numbing repetition, the box-ticking and the endless searching on pulldown menus are all part of what Ratwani called the “cognitive burden” that’s wearing out today’s physicians and driving increasing numbers into early retirement.
In recent years, “physician burnout” has skyrocketed to the top of the agenda in medicine. A 2018 Merritt Hawkins survey found a staggering 78 percent of doctors suffered symptoms of burnout, and in January the Harvard School of Public Health and other institutions deemed it a “public health crisis.”
One of the co-authors of the Harvard study, Ashish Jha, pinned much of the blame on “the growth in poorly designed digital health records ... that [have] required that physicians spend more and more time on tasks that don’t directly benefit patients.”
Few would deny that the swift digitization of America’s medical system has been transformative. With EHRs now nearly universal, the face and feel of medicine has changed. The doctor is now typing away, making more eye contact with the computer screen, perhaps, than with the patient. Patients don’t like that dynamic; for doctors, whose days increasingly begin and end with such fleeting encounters, the effect can be downright deadening.
“You’re sitting in front of a patient, and there are so many things you have to do, and you only have so much time to do it in – seven to 11 minutes, probably – so when do you really listen?” asked John-Henry Pfifferling, a medical anthropologist who counsels physicians suffering from burnout. “If you go into medicine because you care about interacting, and then you’re just a tool, it’s dehumanizing,” said Pfifferling, who has seen many physicians leave medicine over the shift to electronic records. “It’s a disaster,” he said.
Beyond complicating the physician-patient relationship, EHRs have in some ways made practicing medicine harder, said Dr. Hal Baker, a physician and the chief information officer at WellSpan, a Pennsylvania hospital system. “Physicians have to cognitively switch between focusing on the record and focusing on the patient,” he said. He points out how unusual – and potentially dangerous – this is: “Texting while you’re driving is not a good idea. And I have yet to see the CEO who, while running a board meeting, takes minutes, and certainly I’ve never heard of a judge who, during the trial, would also be the court stenographer. But in medicine ... we’ve asked the physician to move from writing in pen to [entering a computer] record, and it’s a pretty complicated interface.”
Even if docs may be at the keyboard during visits, they report having to spend hours more outside that time – at lunch, late at night – in order to finish notes and keep up with electronic paperwork (sending referrals, corresponding with patients, resolving coding issues). That’s right. EHRs didn’t take away paperwork; the systems just moved it online. And there’s a lot of it: 44 percent of the roughly six hours a physician spends on the EHR each day is focused on clerical and administrative tasks, like billing and coding, according to a 2017 Annals of Family Medicine study.
For all that so-called pajama time – the average physician logs 1.4 hours per day on the EHR after work – they don’t get a cent.
Many doctors do recognize the value in the technology: 60 percent of participants in Stanford Medicine’s 2018 National Physician Poll said EHRs had led to improved patient care. At the same time, about as many (59 percent) said EHRs needed a “complete overhaul” and that the systems had detracted from their professional satisfaction (54 percent) as well as from their clinical effectiveness (49 percent).
In preliminary studies, Ratwani has found that doctors have a typical physiological reaction to using an EHR: stress. When he and his team shadow clinicians on the job, they use a range of sensors to monitor the doctors’ heart rate and other vital signs over the course of their shift. The physicians’ heart rates will spike – as high as 160 beats per minute – on two sorts of occasions: when they are interacting with patients and when they’re using the EHR.
4,000
Approximate number of computer clicks an ER doctor makes over the course of a single shift, according to an American Journal of Emergency Medicine study
“Everything is so cumbersome,” said Dr. Karla Dick, a family medicine physician in Arlington, Texas. “It’s slow compared to a paper chart. You’re having to click and zoom in and zoom out to look for stuff.” With all the zooming in and out, she explained, it’s easy to end up in the wrong record. “I can’t tell you how many times I’ve had to cancel an order because I was in the wrong chart.”
Among the daily frustrations for one emergency room physician in Rhode Island is ordering ibuprofen, a seemingly simple task that now requires many rounds of mouse clicking. Every time she prescribes the basic painkiller for a female patient, whether that patient is 9 or 68 years old, the prescription is blocked by a pop-up alert warning her that it may be dangerous to give the drug to a pregnant woman. The physician, whose institution does not allow her to comment on the systems, must then override the warning with yet more clicks. “That’s just the tiniest tip of the iceberg,” she said.
What worries the doctor most is the ease with which diligent, well-meaning physicians can make serious medical errors. She noted that the average ER doc will make 4,000 mouse clicks over the course of a shift, and that the odds of doing anything 4,000 times without an error is small. “The interfaces are just so confusing and clunky,” she added. “They invite error ... it’s not a negligence issue. This is a poor tool issue.”
Many of the EHR makers acknowledge physician burnout is real and say they’re doing what they can to lessen the burden and enhance user experience. Dr. Sam Butler, a pulmonary critical care specialist who started working at Epic in 2001, leads those efforts at the Wisconsin-based company. When doctors get more than 100 messages per week in their in-basket (akin to an email inbox), there’s a higher likelihood of burnout. Butler’s team has also analyzed doctors’ electronic notes – they’re twice as long as they were nine years ago, and three to four times as long as notes in the rest of the world. He said Epic uses such insights to improve the client experience. But coming up with fixes is difficult because doctors “have different viewpoints on everything,” he said. (KHN and Fortune made multiple requests to interview Epic CEO Judy Faulkner, but the company declined to make her available. In a trade interview in February, however, Faulkner said that EHRs were unfairly blamed for physician burnout and cited a study suggesting that there’s little correlation between burnout and EHR satisfaction. Executives at other vendors noted that they’re aware of usability issues and that they’re working on addressing them.)
“It’s not that we’re a bunch of Luddites who don’t know how to use technology,” said the Rhode Island ER doctor. “I have an iPhone and a computer and they work the way they’re supposed to work, and then we’re given these incredibly cumbersome and error-prone tools. This is something the government mandated. There really wasn’t the time to let the cream rise to the top; everyone had to jump in and pick something that worked and spend tens of millions of dollars on a system that is slowly killing us.”
$36 billion and change
The effort to digitize America’s health records got its biggest push in a very low moment: the financial crisis of 2008. In early December of that year, Obama, barely four weeks after his election, pitched an ambitious economic recovery plan. “We will make sure that every doctor’s office and hospital in this country is using cutting-edge technology and electronic medical records so that we can cut red tape, prevent medical mistakes and help save billions of dollars each year,” he said in a radio address.
The idea had already been a fashionable one in Washington. Former House Speaker Newt Gingrich was fond of saying it was easier to track a FedEx package than one’s medical records. Obama’s predecessor, President George W. Bush, had also pursued the idea of wiring up the country’s health system. He didn’t commit much money, but Bush did create an agency to do the job: the Office of the National Coordinator (ONC).
In the depths of recession, the EHR conceit looked like a shovel-ready project that only the paper lobby could hate. In February 2009, legislators passed the HITECH Act, which carved out a hefty chunk of the massive stimulus package for health information technology. The goal was not just to get hospitals and doctors to buy EHRs, but rather to get them using them in a way that would drive better care. So lawmakers devised a carrot-and-stick approach: Physicians would qualify for federal subsidies (a sum of up to nearly $64,000 over a period of years) only if they were “meaningful users” of a government-certified system. Vendors, for their part, had to develop systems that met the government’s requirements.
They didn’t have much time, though. The need to stimulate the economy, which meant getting providers to adopt EHRs quickly, “presented a tremendous conundrum,” said Farzad Mostashari, who joined the ONC as deputy director in 2009 and became its leader in 2011: The ideal – creating a useful, interoperable, nationwide records system – was “utterly infeasible to get to in a short time frame.”
That didn’t stop the federal planners from pursuing their grand ambitions. Everyone had big ideas for the EHRs. The FDA wanted the systems to track unique device identifiers for medical implants, the Centers for Disease Control and Prevention wanted them to support disease surveillance, CMS wanted them to include quality metrics and so on. “We had all the right ideas that were discussed and hashed out by the committee,” said Mostashari, “but they were all of the right ideas.”
Not everyone agreed, though, that they were the right ideas. Before long, “meaningful use” became pejorative shorthand to many for a burdensome government program – making doctors do things like check a box indicating a patient’s smoking status each and every visit.
The EHR vendor community, then a scrappy $2 billion industry, griped at the litany of requirements but stood to gain so much from the government’s $36 billion injection that it jumped in line. As Rusty Frantz, CEO of EHR vendor NextGen Healthcare, put it: “The industry was like, ‘I’ve got this check dangling in front of me, and I have to check these boxes to get there, and so I’m going to do that.’”
Halamka, who was an enthusiastic backer of the initiative in both the Bush and Obama administrations, blames the pressure for a speedy launch as much as the excessive wish list. “To go from a regulation to a highly usable product that is in the hands of doctors in 18 months, that’s too fast,” he said. “It’s like asking nine women to have a baby in a month.”
Several of those who worked on the project admit the rollout was not as easy or seamless as they’d anticipated, but they contend that was never the point. Aneesh Chopra, appointed by Obama in 2009 as the nation’s first chief technology officer, called the spending a “down payment” on a vision to fundamentally change American medicine – creating a digital infrastructure to support new ways to pay for health services based on their quality and outcomes.
Dr. Bob Kocher, a physician and star investor with venture capital firm Venrock, who served in the Obama administration from 2009 to 2011 as a health and economic policy adviser, not only defends the rollout then but also disputes the notion that the government initiative has been a failure at all. “EHRs have totally lived up to the hype and expectations,” he said, emphasizing that they also serve as a technology foundation to support innovation on everything from patients accessing their medical records on a smartphone to AI-driven medical sleuthing. Others note the systems’ value in aggregating medical data in ways that were never possible with paper – helping, for example, to figure out that contaminated water was poisoning children in Flint, Mich.
But Rusty Frantz heard a far different message about EHRs – and, more important, it was coming from his own customers.
The Stanford-trained engineer, who in 2015 became CEO of NextGen, a $500-million-a-year EHR heavyweight in the physician-office market, learned the hard way about how his product was being viewed. As he stood at the podium at his first meeting with thousands of NextGen customers at Las Vegas’ Mandalay Bay Resort, just four months after getting the job, he told KHN and Fortune, “People were lining up at the microphones to yell at us: ‘We weren’t delivering stable software! The executive team was inaccessible! The service experience was terrible!’ ” (He now refers to the event as “Festivus: the airing of the grievances.”)
Frantz had bounced around the health care industry for much of his career, and from the nearby perch of a medical device company, he watched the EHR incentive bonanza with a mix of envy and slack-jawed awe. “The industry was moving along in a natural Darwinist way, and then along came the stimulus,” said Frantz, who blames the government’s ham-handed approach to regulation. “The software got slammed in, and the software wasn’t implemented in a way that supported care,” he said. “It was installed in a way that supported stimulus. This company, we were complicit in it, too.”
Even that may be a generous description. KHN and Fortune found a trail of lawsuits against the company, stretching from White Sulphur Springs, Mont., to Neillsville, Wis. Mary Rutan Hospital in Bellefontaine, Ohio, sued NextGen (formerly called Quality Systems) in federal court in 2013, arguing that it experienced hundreds of problems with the “materially defective” software the company had installed in 2011.
A consultant hired by the hospital to evaluate the NextGen system, whose 60-page report was submitted to the court, identified “many functional defects” that he said rendered the software “unfit for its intended purpose.” Some patient information was not accurately recorded, which had the potential, the consultant wrote, “to create major patient care risk which could lead to, at a minimum, inconvenience, and at worst, malpractice or even death.” Glitches at Mary Rutan included incidents in which the software would apparently change a patient’s gender at random or lose a doctor’s observations after an exam, the consultant reported. The company, he found, sometimes took months to address issues: One IT ticket, which related to a physician’s notes inexplicably deleting themselves, reportedly took 10 months to resolve. (The consultant also noted that similar problems appeared to be occurring at as many as a dozen other hospitals that had installed NextGen software.)
The Ohio hospital, which paid more than $1.5 million for its EHR system, claimed breach of contract. NextGen responded that it disputed the claims made in the lawsuit and that the matter was resolved in 2015 “with no findings of fact by a court related to the allegations.” The hospital declined to comment.
At the time, as it has been since then, NextGen’s software was certified by the government as meeting the requirements of the stimulus program. By 2016, NextGen had more than 19,000 customers who had received federal subsidies.
NextGen was subpoenaed by the Department of Justice in December 2017, months after becoming the subject of a federal investigation led by the District of Vermont. Frantz tells KHN and Fortune that NextGen is cooperating with the investigation. “This company was not dishonest, but it was not effective four years ago,” he said. Frantz also emphasized that NextGen has “rapidly evolved” during his tenure, earning five industry awards since 2017, and that customers have “responded very positively.”
Glen Tullman, who until 2012 led Allscripts, another leading EHR vendor that benefited royally from the stimulus and that has been sued by numerous unhappy customers, admitted that the industry’s race to market took priority over all else.
“It was a big distraction. That was an unintended consequence of that,” Tullman said. “All the companies were saying, This is a one-time opportunity to expand our share, focus everything there, and then we’ll go back and fix it.” The Justice Department has opened a civil investigation into the company, Securities and Exchange Commission filings show. Allscripts said in an email that it cannot comment on an ongoing investigation, but that the civil investigations by the Department of Justice relate to businesses it acquired after the investigations were opened.
Much of the marketing mayhem occurred because federal officials imposed few controls over firms scrambling to cash in on the stimulus. It was a gold rush – and any system, it seemed, could be marketed as “federally approved.” Doctors could shop for bargain-price software packages at Costco and Walmart’s Sam’s Club – where eClinicalWorks sold a “turnkey” system for $11,925 – and cash in on the government’s adoption incentives.
The top-shelf vendors in 2009 crisscrossed the country on a “stimulus tour” like rock groups, gigging at some 30 cities, where they offered doctors who showed up to hear the pitch “a customized analysis” of how much money they could earn off the government incentives. Following the same playbook used by pharmaceutical companies, EHR sellers courted doctors at fancy dinners in ritzy hotels. One enterprising software firm advertised a “cash for clunkers” deal that paid $3,000 to doctors willing to trade in their current records system for a new one. Athenahealth held “invitation only” dinners at luxury hotels to advise doctors, among other things, how to use the stimulus to get paid more and capture available incentives. Allscripts offered a no-money-down purchase plan to help doctors “maximize the return on your EHR investment.” (An Athenahealth spokesperson said the company’s “dinners were educational in nature and aimed at helping physicians navigate the government program.” Allscripts did not respond directly to questions about its marketing practices, but said it “is proud of the software and services [it provides] to hundreds of thousands of caregivers across the globe.”)
EHRs were supposed to reduce health care costs, at least in part by preventing duplicative tests. But as the federal government opened the stimulus tap, many raised doubts about the promised savings. Advocates bandied about a figure of $80 billion in cost savings even as congressional auditors were debunking it. While the jury’s still out, there’s growing suspicion the digital revolution may potentially raise health care costs by encouraging overbilling and new strains of fraud and abuse.
In September 2012, following press reports suggesting that some doctors and hospitals were using the new technology to improperly boost their fees, a practice known as “upcoding,” then-Health and Human Services chief Kathleen Sebelius and Attorney General Eric Holder warned the industry not to try to “game the system.”
There’s also growing evidence that some doctors and health systems may have overstated their use of the new technology to secure stimulus funds, a potentially enormous fraud against Medicare and Medicaid that likely will take many years to unravel. In June 2017, the HHS inspector general estimated that Medicare officials made more than $729 million in subsidy payments to hospitals and doctors that didn’t deserve them.
Individual states, which administer the Medicaid portion of the program, haven’t fared much better. Audits have uncovered overpayments in 14 of 17 state programs reviewed, totaling more than $66 million, according to inspector general reports.
Last month, Sen. Chuck Grassley, an Iowa Republican who chairs the Senate Finance Committee, sharply criticized CMS for recovering only a tiny fraction of these bogus payments, or what he termed a “spit in the ocean.”
EHR vendors have also been accused of egregious and patient-endangering acts of fraud as they raced to cash in on the stimulus money grab. In addition to the U.S. government’s $155 million False Claims Act settlement with eClinicalWorks noted above, the federal government has reached a second settlement over similar charges against another large vendor, Tampa-based Greenway Health. In February, that company settled with the government for just over $57 million without denying or admitting wrongdoing. “These are cases of corporate greed, companies that prioritized profits over everything else,” said Christina Nolan, the U.S. attorney for the District of Vermont, whose office led the cases. (In a response, Greenway Health did not address the charges or the settlement but said it was “committing itself to being the standard-bearer for quality, compliance, and transparency.”)
Tower of Babel
In early 2017, Seema Verma, then the country’s newly appointed CMS administrator, went on a listening tour. She visited doctors around the country, at big urban practices and tiny rural clinics, and from those front-line physicians she consistently heard one thing: They hated their electronic health records. “Physician burnout is real,” she told KHN and Fortune. The doctors spoke of the difficulty in getting information from other systems and providers, and they complained about the government’s reporting requirements, which they perceived as burdensome and not meaningful.
What she heard then became suddenly personal one summer day in 2017, when her husband, himself a physician, collapsed in the airport on his way home to Indianapolis after a family vacation. For a frantic few hours, the CMS administrator fielded phone calls from first responders and physicians – Did she know his medical history? Did she have information that could save his life? – and made calls to his doctors in Indiana, scrambling to piece together his record, which should have been there in one piece. Her husband survived the episode, but it laid bare the dysfunction and danger inherent in the existing health information ecosystem.
The notion that one EHR should talk to another was a key part of the original vision for the HITECH Act, with the government calling for systems to be eventually interoperable.
What the framers of that vision didn’t count on were the business incentives working against it. A free exchange of information means that patients can be treated anywhere. And though they may not admit it, many health providers are loath to lose their patients to a competing doctor’s office or hospital. There’s a term for that lost revenue: “leakage.” And keeping a tight hold on patients’ medical records is one way to prevent it.
There’s a ton of proprietary value in that data, said Blumenthal, who now heads the Commonwealth Fund, a philanthropy that does health research. Asking hospitals to give it up is “like asking Amazon to share their data with Walmart,” he said.
Blumenthal acknowledged that he failed to grasp these perverse business dynamics and foresee what a challenge getting the systems to talk to one another would be. He added that forcing interoperability goals early on, when 90 percent of the nation’s providers still didn’t have systems or data to exchange, seemed unrealistic. “We had an expression: They had to operate before they could interoperate,” he said.
In the absence of true incentives for systems to communicate, the industry limped along; some providers wired up directly to other select providers or through regional exchanges, but the efforts were spotty. A Cerner-backed interoperability network called CommonWell formed in 2013, but some companies, including dominant Epic, didn’t join. (“Initially, Epic was neither invited nor allowed to join,” said Sumit Rana, senior vice president of R&D at Epic. Jitin Asnaani, executive director of CommonWell countered, “We made repeated invitations to every major EHR ... and numerous public and private invitations to Epic.”)
Epic then supported a separate effort to do much the same.
Last spring, Verma attempted to kick-start the sharing effort and later pledged a war on “information blocking,” threatening penalties for bad actors. She has promised to reduce the documentation burden on physicians and end the gag clauses that protect the EHR industry. Regarding the first effort at least, “there was consensus that this needed to happen and that it would take the government to push this forward,” she said. In one sign of progress last summer, the dueling sharing initiatives of Epic and Cerner, the two largest players in the industry, began to share with each other – though the effort is fledgling.
When it comes to patients, though, the real sharing too often stops. Despite federal requirements that providers give patients their medical records in a timely fashion, in their chosen format and at low cost (the government recommends a flat fee of $6.50 or less), patients struggle mightily to get them. A 2017 study by researchers at Yale found that of America’s 83 top-rated hospitals, only 53 percent offer forms that provide patients with the option to receive their entire medical record. Fewer than half would share records via email. One hospital charged more than $500 to release them.
Sometimes the mere effort to access records leads to court. Jennifer De Angelis, a Tulsa attorney, has frequently sparred with hospitals over releasing her clients’ records. She said they either attempt to charge huge sums for them or force her to obtain a court order before releasing them. De Angelis added that she sometimes suspects the records have been overwritten to cover up medical mistakes.
Consider the case of 5-year-old Uriah R. Roach, who fractured and cut his finger on Oct. 2, 2014, when it was accidentally slammed in a door at school. Five days later, an operation to repair the damage went awry, and he suffered permanent brain damage, apparently owing to an anesthesia problem. The Epic electronic medical file had been accessed more than 76,000 times during the 22 days the boy was in the hospital, and a lawsuit brought by his parents contended that numerous entries had been “corrected, altered, modified and possibly deleted after an unexpected outcome during the induction of anesthesia.” The hospital denied wrongdoing. The case settled in November 2016, and the terms are confidential.
More than a dozen other attorneys interviewed cited similar problems, especially with gaining access to computerized “audit trails.” In several cases, court records show, government lawyers resisted turning over electronic files from federally run hospitals. That happened to Russell Uselton, an Oklahoma lawyer who represented a pregnant teen admitted to the Choctaw Nation Health Care Center in Talihina, Okla. Shelby Carshall, 18, was more than 40 weeks pregnant at the time. Doctors failed to perform a cesarean section, and her baby was born brain-damaged as a result, she alleged in a lawsuit filed in 2017 against the U.S. government. The baby began having seizures at 10 hours old and will “likely never walk, talk, eat, or otherwise live normally,” according to pleadings in the suit. Though the federal government requires hospitals to produce electronic health records to patients and their families, Uselton had to obtain a court order to get the baby’s complete medical files. Government lawyers denied any negligence in the case, which is pending.
“They try to hide anything from you that they can hide from you,” said Uselton. “They make it extremely difficult to get records, so expensive and hard that most lawyers can’t take it on,” he said.
Nor, it seems, can high-ranking federal officials. When Seema Verma’s husband was discharged from the hospital after his summer health scare, he was handed a few papers and a CD-ROM containing some medical images – but missing key tests and monitoring data. Said Verma, “We left that hospital and we still don’t have his information today.” That was nearly two years ago
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
The pain radiated from the top of Annette Monachelli’s head, and it got worse when she changed positions. It didn’t feel like her usual migraine. The 47-year-old Vermont attorney turned innkeeper visited her local doctor at the Stowe Family Practice twice about the problem in late November 2012, but got little relief.
Two months later, Monachelli was dead of an aneurysm, a condition that, despite the symptoms and the appointments, had never been tested for or diagnosed until she turned up in the emergency room days before her death.
Monachelli’s husband sued Stowe, the federally qualified health center the physician worked for. Owen Foster, a newly hired assistant U.S. attorney with the District of Vermont, was assigned to defend the government. Though it looked to be a standard medical malpractice case, Foster was on the cusp of discovering something much bigger – what his boss, U.S. Attorney Christina Nolan, calls the “frontier of health care fraud” – and prosecuting a first-of-its-kind case that landed the largest-ever financial recovery in Vermont’s history.
Foster began with Monachelli’s medical records, which offered a puzzle. Her doctor had considered the possibility of an aneurysm and, to rule it out, had ordered a head scan through the clinic’s software system, the government alleged in court filings. The test, in theory, would have caught the bleeding in Monachelli’s brain. But the order never made it to the lab; it had never been transmitted.
The software in question was an electronic health records system, or EHR, made by eClinicalWorks (eCW), one of the leading sellers of record-keeping software for physicians in America, currently used by 850,000 health professionals in the U.S. It didn’t take long for Foster to assemble a dossier of troubling reports – Better Business Bureau complaints, issues flagged on an eCW user board, and legal cases filed around the country – suggesting the company’s technology didn’t work quite the way it said it did.
Until this point, Foster, like most Americans, knew next to nothing about electronic medical records, but he was quickly amassing clues that eCW’s software had major problems – some of which put patients, like Annette Monachelli, at risk.
Damning evidence came from a whistleblower claim filed in 2011 against the company. Brendan Delaney, a British cop turned EHR expert, was hired in 2010 by New York City to work on the eCW implementation at Rikers Island, a jail complex that then had more than 100,000 inmates. But soon after he was hired, Delaney noticed scores of troubling problems with the system, which became the basis for his lawsuit. The patient medication lists weren’t reliable; prescribed drugs would not show up, while discontinued drugs would appear as current, according to the complaint. The EHR would sometimes display one patient’s medication profile accompanied by the physician’s note for a different patient, making it easy to misdiagnose or prescribe a drug to the wrong individual. Prescriptions, some 30,000 of them in 2010, lacked proper start and stop dates, introducing the opportunity for under- or overmedication. The eCW system did not reliably track lab results, concluded Delaney, who tallied 1,884 tests for which they had never gotten outcomes.
The District of Vermont launched an official federal investigation in 2015.
The eCW spaghetti code was so buggy that when one glitch got fixed, another would develop, the government found. The user interface offered a few ways to order a lab test or diagnostic image, for example, but not all of them seemed to function. The software would detect and warn users of dangerous drug interactions, but unbeknownst to physicians, the alerts stopped if the drug order was customized. “It would be like if I was driving with the radio on and the windshield wipers going and when I hit the turn signal, the brakes suddenly didn’t work,” said Foster.
The eCW system also failed to use the standard drug codes and, in some instances, lab and diagnosis codes as well, the government alleged.
The case never got to a jury. In May 2017, eCW paid a $155 million settlement to the government over alleged “false claims” and kickbacks – one physician made tens of thousands of dollars – to clients who promoted its product. Despite the record settlement, the company denied wrongdoing; eCW did not respond to numerous requests for comment.
If there is a kicker to this tale, it is this: The U.S. government bankrolled the adoption of this software – and continues to pay for it. Or we should say: You do.
Which brings us to the strange, sad, and aggravating story that unfolds below. It is not about one lawsuit or a piece of sloppy technology. Rather, it’s about a trouble-prone industry that intersects, in the most personal way, with every one of our lives. It’s about a $3.7 trillion health care system idling at the crossroads of progress. And it’s about a slew of unintended consequences – the surprising casualties of a big idea whose time had seemingly come.
The virtual magic bullet
Electronic health records were supposed to do a lot: make medicine safer, bring higher-quality care, empower patients, and yes, even save money. Boosters heralded an age when researchers could harness the big data within to reveal the most effective treatments for disease and sharply reduce medical errors. Patients, in turn, would have truly portable health records, being able to share their medical histories in a flash with doctors and hospitals anywhere in the country – essential when life-and-death decisions are being made in the ER.
But 10 years after President Barack Obama signed a law to accelerate the digitization of medical records – with the federal government, so far, sinking $36 billion into the effort – America has little to show for its investment. KHN and Fortune spoke with more than 100 physicians, patients, IT experts and administrators, health policy leaders, attorneys, top government officials and representatives at more than a half-dozen EHR vendors, including the CEOs of two of the companies. The interviews reveal a tragic missed opportunity: Rather than an electronic ecosystem of information, the nation’s thousands of EHRs largely remain a sprawling, disconnected patchwork. Moreover, the effort has handcuffed health providers to technology they mostly can’t stand and has enriched and empowered the $13-billion-a-year industry that sells it.
By one measure, certainly, the effort has achieved what it set out to do: Today, 96% of hospitals have adopted EHRs, up from just 9% in 2008. But on most other counts, the newly installed technology has fallen well short. Physicians complain about clumsy, unintuitive systems and the number of hours spent clicking, typing and trying to navigate them – which is more than the hours they spend with patients. Unlike, say, with the global network of ATMs, the proprietary EHR systems made by more than 700 vendors routinely don’t talk to one another, meaning that doctors still resort to transferring medical data via fax and CD-ROM. Patients, meanwhile, still struggle to access their own records – and, sometimes, just plain can’t.
Instead of reducing costs, many say, EHRs, which were originally optimized for billing rather than for patient care, have instead made it easier to engage in “upcoding” or bill inflation (though some say the systems also make such fraud easier to catch).
More gravely still, a months-long joint investigation by KHN and Fortune has found that instead of streamlining medicine, the government’s EHR initiative has created a host of largely unacknowledged patient safety risks. Our investigation found that alarming reports of patient deaths, serious injuries and near misses – thousands of them – tied to software glitches, user errors or other flaws have piled up, largely unseen, in various government-funded and private repositories.
Compounding the problem are entrenched secrecy policies that continue to keep software failures out of public view. EHR vendors often impose contractual “gag clauses” that discourage buyers from speaking out about safety issues and disastrous software installations – though some customers have taken to the courts to air their grievances. Plaintiffs, moreover, say hospitals often fight to withhold records from injured patients or their families. Indeed, two doctors who spoke candidly about the problems they faced with EHRs later asked that their names not be used, adding that they were forbidden by their health care organizations to talk. Says Assistant U.S. Attorney Foster, the EHR vendors “are protected by a shield of silence.”
Though the software has reduced some types of clinical mistakes common in the era of handwritten notes, Raj Ratwani, a researcher at MedStar Health in Washington, D.C., has documented new patterns of medical errors tied to EHRs that he believes are both perilous and preventable. “The fact that we’re not able to broadcast that nationally and solve these issues immediately, and that another patient somewhere else may be harmed by the very same issue – that just can’t happen,” he said.
David Blumenthal, who, as Obama’s national coordinator for health information technology, was one of the architects of the EHR initiative, acknowledged to KHN and Fortune that electronic health records “have not fulfilled their potential. I think few would argue they have.”
The former president has likewise singled out the effort as one of his most disappointing, bemoaning in a January 2017 interview with Vox “the fact that there are still just mountains of paperwork ... and the doctors still have to input stuff, and the nurses are spending all their time on all this administrative work. We put a big slug of money into trying to encourage everyone to digitalize, to catch up with the rest of the world ... that’s been harder than we expected.”
Seema Verma, the current chief of the Centers for Medicare & Medicaid Services (CMS), which oversees the EHR effort today, shudders at the billions of dollars spent building software that doesn’t share data – an electronic bridge to nowhere. “Providers developed their own systems that may or may not even have worked well for them,” she told KHN and Fortune in an interview last month, “but we didn’t think about how all these systems connect with one another. That was the real missing piece.”
Perhaps none of the initiative’s former boosters is quite as frustrated as former Vice President Joe Biden. At a 2017 meeting with health care leaders in Washington, he railed against the infuriating challenge of getting his son Beau’s medical records from one hospital to another. “I was stunned when my son for a year was battling stage 4 glioblastoma,” said Biden. “I couldn’t get his records. I’m the vice president of the United States of America. ... It was an absolute nightmare. It was ridiculous, absolutely ridiculous, that we’re in that circumstance.”
A bridge to nowhere
As Biden would tell you, the original concept was a smart one. The wave of digitization had swept up virtually every industry, bringing both disruption and, in most cases, greater efficiency. And perhaps none of these industries was more deserving of digital liberation than medicine, where life-measuring and potentially lifesaving data was locked away in paper crypts – stack upon stack of file folders at doctors’ offices across the country.
Stowed in steel cabinets, the records were next to useless. Nobody – particularly at the dawn of the age of the iPhone – thought it was a good idea to leave them that way. The problem, say critics, was in the way that policymakers set about to transform them.
“Every single idea was well-meaning and potentially of societal benefit, but the combined burden of all of them hitting clinicians simultaneously made office practice basically impossible,” said John Halamka, chief information officer at Beth Israel Deaconess Medical Center, who served on the EHR standards committees under both President George W. Bush and President Obama. “In America, we have 11 minutes to see a patient, and, you know, you’re going to be empathetic, make eye contact, enter about 100 pieces of data, and never commit malpractice. It’s not possible!”
KHN and Fortune examined more than two dozen medical negligence cases that have alleged that EHRs either contributed to injuries, had been improperly altered, or were withheld from patients to conceal substandard care. In such cases, the suits typically settle prior to trial with strict confidentiality pledges, so it’s often not possible to determine the merits of the allegations. EHR vendors also frequently have contract stipulations, known as “hold harmless clauses,” that protect them from liability if hospitals are later sued for medical errors – even if they relate to an issue with the technology.
But lawsuits, like that filed by Fabian Ronisky, which do emerge from this veil, are quite telling.
Ronisky, according to his complaint, arrived by ambulance at Providence Saint John’s Health Center in Santa Monica on the afternoon of March 2, 2015. For two days, the young lawyer had been suffering from severe headaches while a disorienting fever left him struggling to tell the 911 operator his address.
Suspecting meningitis, a doctor at the hospital performed a spinal tap, and the next day an infectious disease specialist typed in an order for a critical lab test – a check of the spinal fluid for viruses, including herpes simplex – into the hospital’s EHR.
The multimillion-dollar system, manufactured by Epic Systems Corp. and considered by some to be the Cadillac of medical software, had been installed at the hospital about four months earlier. Although the order appeared on Epic’s screen, it was not sent to the lab. It turned out, Epic’s software didn’t fully “interface” with the lab’s software, according to a lawsuit Ronisky filed in February 2017 in Los Angeles County Superior Court. His results and diagnosis were delayed – by days, he claimed – during which time he suffered irreversible brain damage from herpes encephalitis. The suit alleged the mishap delayed doctors from giving Ronisky a drug called acyclovir that might have minimized damage to his brain.
Epic denied any liability or defects in its software; the company said the doctor failed to push the right button to send the order and that the hospital, not Epic, had configured the interface with the lab. Epic, among the nation’s largest manufacturers of computerized health records and the leading provider to most of America’s most elite medical centers, quietly paid $1 million to settle the suit in July 2018, according to court records. The hospital and two doctors paid a total of $7.5 million, and a case against a third doctor is pending trial. Ronisky, 34, who is fighting to rebuild his life, declined to comment.
Incidents like that which happened to Ronisky – or to Annette Monachelli, for that matter – are surprisingly common, data show. And the back-and-forth about where the fault lies in such cases is actually part of the problem: The systems are often so confusing (and training on them seldom sufficient) that errors frequently fall into a nether zone of responsibility. It can be hard to tell where human error begins and the technological shortcomings end.
EHRs promised to put all of a patient’s records in one place, but often that’s the problem. Critical or time-sensitive information routinely gets buried in an endless scroll of data, where in the rush of medical decision-making – and amid the maze of pulldown menus – it can be missed.
Thirteen-year-old Brooke Dilliplaine, who was severely allergic to dairy, was given a probiotic containing milk. The two doses sent her into “complete respiratory distress” and resulted in a collapsed lung, according to a lawsuit filed by her mother. Rory Staunton, 12, scraped his arm in gym class and then died of sepsis after ER doctors discharged the boy on the basis of lab results in the EHR that weren’t complete. And then there’s the case of Thomas Eric Duncan. The 42-year-old man was sent home in 2014 from a Dallas hospital infected with Ebola virus. Though a nurse had entered in the EHR his recent travel to Liberia, where an Ebola epidemic was then in full swing, the doctor never saw it. Duncan died a week later.
Many such cases end up in court. Typically, doctors and nurses blame faulty technology in the medical-records systems. The EHR vendors blame human error. And meanwhile, the cases mount.
Quantros, a private health care analytics firm, said it has logged 18,000 EHR-related safety events from 2007 through 2018, 3 percent of which resulted in patient harm, including seven deaths – a figure that a Quantros director said is “drastically underreported.”
A 2016 study by The Leapfrog Group, a patient-safety watchdog based in Washington, D.C., found that the medication-ordering function of hospital EHRs – a feature required by the government for certification but often configured differently in each system – failed to flag potentially harmful drug orders in 39 percent of cases in a test simulation. In 13 percent of those cases, the mistake could have been fatal
The Pew Charitable Trusts has, for the past few years, run an EHR safety project, taking aim at issues like usability and patient matching – the process of linking the correct medical record to the correct patient – a seemingly basic task at which the systems, even when made by the same EHR vendor, often fail. At some institutions, according to Pew, such matching was accurate only 50 percent of the time. Patients have discovered mistakes as well: A January survey by the Kaiser Family Foundation found that 1 in 5 patients spotted an error in their electronic medical records. (Kaiser Health News is an editorially independent program of the foundation.)
The Joint Commission, which certifies hospitals, has sounded alarms about a number of issues, including false alarms – which account for between 85 and 99 percent of EHR and medical device alerts. (One study by researchers at Oregon Health & Science University estimated that the average clinician working in the intensive care unit may be exposed to up to 7,000 passive alerts per day.) Such over-warning can be dangerous. From 2014 to 2018, the commission tallied 170 mostly voluntary reports of patient harm related to alarm management and alert fatigue – the phenomenon in which health workers, so overloaded with unnecessary warnings, ignore the occasional meaningful one. Of those 170 incidents, 101 resulted in patient deaths.
The Pennsylvania Patient Safety Authority, an independent state agency that collects information about adverse events and incidents, counted 775 “laboratory-test problems” related to health IT from January 2016 to December 2017.
To be sure, medical errors happened en masse in the age of paper medicine, when hospital staffers misinterpreted a physician’s scrawl or read the wrong chart to deadly consequence, for instance. But what is perhaps telling is how many doctors today opt for manual workarounds to their EHRs. Aaron Zachary Hettinger, an emergency medicine physician with MedStar Health in Washington, D.C., said that when he and fellow clinicians need to share critical patient information, they write it on a whiteboard or on a paper towel and leave it on their colleagues’ computer keyboards.
While the Food and Drug Administration doesn’t mandate reporting of EHR safety events – as it does for regulated medical devices – concerned posts have nonetheless proliferated in the FDA MAUDE database of adverse events, which now serves as an ad hoc bulletin board of warnings about the various systems.
Further complicating the picture is that health providers nearly always tailor their one-size-fits-all EHR systems to their own specifications. Such customization makes every one unique and often hard to compare with others – which, in turn, makes the source of mistakes difficult to determine.
Dr. Martin Makary, a surgical oncologist at Johns Hopkins and the co-author of a much-cited 2016 study that identified medical errors as the third-leading cause of death in America, credits EHRs for some safety improvements – including recent changes that have helped put electronic brakes on the opioid epidemic. But, he said, “we’ve swapped one set of problems for another. We used to struggle with handwriting and missing information. We now struggle with a lack of visual cues to know we’re writing and ordering on the correct patient.”
Dr. Joseph Schneider, a pediatrician at UT Southwestern Medical Center, compares the transition we’ve made, from paper records to electronic ones, to moving from horses to automobiles. But in this analogy, he added, “our cars have advanced to about the 1960s. They still don’t have seat belts or air bags.”
Schneider recalled one episode when his colleagues couldn’t understand why chunks of their notes would inexplicably disappear. They figured out the problem weeks later after intense study: Physicians had been inputting squiggly brackets – {} – the use of which, unbeknownst to even vendor representatives, deleted the text between them. (The EHR maker initially blamed the doctors, said Schneider.)
A broad coalition of actors, from National Nurses United to the Texas Medical Association to leaders within the FDA, has long called for oversight on electronic-record safety issues. Among the most outspoken is Ratwani, who directs MedStar Health’s National Center on Human Factors in Healthcare, a 30-person institute focused on optimizing the safety and usability of medical technology. Ratwani spent his early career in the defense industry, studying things like the intuitiveness of information displays. When he got to MedStar in 2012, he was stunned by “the types of [digital] interfaces being used” in health care, he said.
In a study published last year in the journal Health Affairs, Ratwani and colleagues studied medication errors at three pediatric hospitals from 2012 to 2017. They discovered that 3,243 of them were owing in part to EHR “usability issues.” Roughly 1 in 5 of these could have resulted in patient harm, the researchers found. “Poor interface design and poor implementations can lead to errors and sometimes death, and that is just unbelievably bad as well as completely fixable,” he said. “We should not have patients harmed this way.”
Using eye-tracking technology, Ratwani has demonstrated on video just how easy it is to make mistakes when performing basic tasks on the nation’s two leading EHR systems. When emergency room doctors went to order Tylenol, for example, they saw a drop-down menu listing 86 options, many of which were irrelevant for the specified patient. They had to read the list carefully, so as not to click the wrong dosage or form – though many do that too: In roughly 1 out of 1,000 orders, physicians accidentally select the suppository (designated “PR”) rather than the tablet dose (“OR”), according to one estimate. That’s not an error that will harm a patient – though other medication mix-ups can and do.
Earlier this year, MedStar’s human-factors center launched a website and public awareness campaign with the American Medical Association to draw attention to such rampant mistakes – they use the letters “EHR” as an initialism for “Errors Happen Regularly” – and to petition Congress for action. Ratwani is pushing for a central database to track such errors and adverse events.
Others have turned to social media to vent. Dr. Mark Friedberg, a health-policy researcher with the Rand Corp. who is also a practicing primary care physician, champions the Twitter hashtag #EHRbuglist to encourage fellow health care workers to air their pain points. And last month, a scathing Epic parody account cropped up on Twitter, earning more than 8,000 followers in its first five days. Its maiden tweet, written in the mock voice of an Epic overlord, read: “I once saw a doctor make eye contact with a patient. This horror must stop.”
As much as EHR systems are blamed for sins of commission, it is often the sins of omission that trip up users even more.
Consider the case of Lynne Chauvin, who worked as a medical assistant at Ochsner Health System, in Louisiana. In a still-pending 2015 lawsuit, Chauvin alleges that Epic’s software failed to fire a critical medication warning; Chauvin suffered from conditions that heightened her risk for blood clots, and though that history was documented in her records, she was treated with drugs that restricted blood flow after a heart procedure at the hospital. She developed gangrene, which led to the amputation of her lower legs and forearm. (Ochsner Health System said that while it cannot comment on ongoing litigation, it “remains committed to patient safety which we strongly believe is optimized through the use of electronic health record technology.” Epic declined to comment.)
Echoing the complaints of many doctors, the suit argues that Epic software “is extremely complicated to view and understand,” owing to “significant repetition of data.” Chauvin said that her medical bills have topped $1 million and that she is permanently disabled. Her husband, Richard, has become her primary caregiver and had to retire early from his job with the city of Kenner to care for his wife, according to the suit. Each party declined to comment.
An epidemic of burnout
The numbing repetition, the box-ticking and the endless searching on pulldown menus are all part of what Ratwani called the “cognitive burden” that’s wearing out today’s physicians and driving increasing numbers into early retirement.
In recent years, “physician burnout” has skyrocketed to the top of the agenda in medicine. A 2018 Merritt Hawkins survey found a staggering 78 percent of doctors suffered symptoms of burnout, and in January the Harvard School of Public Health and other institutions deemed it a “public health crisis.”
One of the co-authors of the Harvard study, Ashish Jha, pinned much of the blame on “the growth in poorly designed digital health records ... that [have] required that physicians spend more and more time on tasks that don’t directly benefit patients.”
Few would deny that the swift digitization of America’s medical system has been transformative. With EHRs now nearly universal, the face and feel of medicine has changed. The doctor is now typing away, making more eye contact with the computer screen, perhaps, than with the patient. Patients don’t like that dynamic; for doctors, whose days increasingly begin and end with such fleeting encounters, the effect can be downright deadening.
“You’re sitting in front of a patient, and there are so many things you have to do, and you only have so much time to do it in – seven to 11 minutes, probably – so when do you really listen?” asked John-Henry Pfifferling, a medical anthropologist who counsels physicians suffering from burnout. “If you go into medicine because you care about interacting, and then you’re just a tool, it’s dehumanizing,” said Pfifferling, who has seen many physicians leave medicine over the shift to electronic records. “It’s a disaster,” he said.
Beyond complicating the physician-patient relationship, EHRs have in some ways made practicing medicine harder, said Dr. Hal Baker, a physician and the chief information officer at WellSpan, a Pennsylvania hospital system. “Physicians have to cognitively switch between focusing on the record and focusing on the patient,” he said. He points out how unusual – and potentially dangerous – this is: “Texting while you’re driving is not a good idea. And I have yet to see the CEO who, while running a board meeting, takes minutes, and certainly I’ve never heard of a judge who, during the trial, would also be the court stenographer. But in medicine ... we’ve asked the physician to move from writing in pen to [entering a computer] record, and it’s a pretty complicated interface.”
Even if docs may be at the keyboard during visits, they report having to spend hours more outside that time – at lunch, late at night – in order to finish notes and keep up with electronic paperwork (sending referrals, corresponding with patients, resolving coding issues). That’s right. EHRs didn’t take away paperwork; the systems just moved it online. And there’s a lot of it: 44 percent of the roughly six hours a physician spends on the EHR each day is focused on clerical and administrative tasks, like billing and coding, according to a 2017 Annals of Family Medicine study.
For all that so-called pajama time – the average physician logs 1.4 hours per day on the EHR after work – they don’t get a cent.
Many doctors do recognize the value in the technology: 60 percent of participants in Stanford Medicine’s 2018 National Physician Poll said EHRs had led to improved patient care. At the same time, about as many (59 percent) said EHRs needed a “complete overhaul” and that the systems had detracted from their professional satisfaction (54 percent) as well as from their clinical effectiveness (49 percent).
In preliminary studies, Ratwani has found that doctors have a typical physiological reaction to using an EHR: stress. When he and his team shadow clinicians on the job, they use a range of sensors to monitor the doctors’ heart rate and other vital signs over the course of their shift. The physicians’ heart rates will spike – as high as 160 beats per minute – on two sorts of occasions: when they are interacting with patients and when they’re using the EHR.
4,000
Approximate number of computer clicks an ER doctor makes over the course of a single shift, according to an American Journal of Emergency Medicine study
“Everything is so cumbersome,” said Dr. Karla Dick, a family medicine physician in Arlington, Texas. “It’s slow compared to a paper chart. You’re having to click and zoom in and zoom out to look for stuff.” With all the zooming in and out, she explained, it’s easy to end up in the wrong record. “I can’t tell you how many times I’ve had to cancel an order because I was in the wrong chart.”
Among the daily frustrations for one emergency room physician in Rhode Island is ordering ibuprofen, a seemingly simple task that now requires many rounds of mouse clicking. Every time she prescribes the basic painkiller for a female patient, whether that patient is 9 or 68 years old, the prescription is blocked by a pop-up alert warning her that it may be dangerous to give the drug to a pregnant woman. The physician, whose institution does not allow her to comment on the systems, must then override the warning with yet more clicks. “That’s just the tiniest tip of the iceberg,” she said.
What worries the doctor most is the ease with which diligent, well-meaning physicians can make serious medical errors. She noted that the average ER doc will make 4,000 mouse clicks over the course of a shift, and that the odds of doing anything 4,000 times without an error is small. “The interfaces are just so confusing and clunky,” she added. “They invite error ... it’s not a negligence issue. This is a poor tool issue.”
Many of the EHR makers acknowledge physician burnout is real and say they’re doing what they can to lessen the burden and enhance user experience. Dr. Sam Butler, a pulmonary critical care specialist who started working at Epic in 2001, leads those efforts at the Wisconsin-based company. When doctors get more than 100 messages per week in their in-basket (akin to an email inbox), there’s a higher likelihood of burnout. Butler’s team has also analyzed doctors’ electronic notes – they’re twice as long as they were nine years ago, and three to four times as long as notes in the rest of the world. He said Epic uses such insights to improve the client experience. But coming up with fixes is difficult because doctors “have different viewpoints on everything,” he said. (KHN and Fortune made multiple requests to interview Epic CEO Judy Faulkner, but the company declined to make her available. In a trade interview in February, however, Faulkner said that EHRs were unfairly blamed for physician burnout and cited a study suggesting that there’s little correlation between burnout and EHR satisfaction. Executives at other vendors noted that they’re aware of usability issues and that they’re working on addressing them.)
“It’s not that we’re a bunch of Luddites who don’t know how to use technology,” said the Rhode Island ER doctor. “I have an iPhone and a computer and they work the way they’re supposed to work, and then we’re given these incredibly cumbersome and error-prone tools. This is something the government mandated. There really wasn’t the time to let the cream rise to the top; everyone had to jump in and pick something that worked and spend tens of millions of dollars on a system that is slowly killing us.”
$36 billion and change
The effort to digitize America’s health records got its biggest push in a very low moment: the financial crisis of 2008. In early December of that year, Obama, barely four weeks after his election, pitched an ambitious economic recovery plan. “We will make sure that every doctor’s office and hospital in this country is using cutting-edge technology and electronic medical records so that we can cut red tape, prevent medical mistakes and help save billions of dollars each year,” he said in a radio address.
The idea had already been a fashionable one in Washington. Former House Speaker Newt Gingrich was fond of saying it was easier to track a FedEx package than one’s medical records. Obama’s predecessor, President George W. Bush, had also pursued the idea of wiring up the country’s health system. He didn’t commit much money, but Bush did create an agency to do the job: the Office of the National Coordinator (ONC).
In the depths of recession, the EHR conceit looked like a shovel-ready project that only the paper lobby could hate. In February 2009, legislators passed the HITECH Act, which carved out a hefty chunk of the massive stimulus package for health information technology. The goal was not just to get hospitals and doctors to buy EHRs, but rather to get them using them in a way that would drive better care. So lawmakers devised a carrot-and-stick approach: Physicians would qualify for federal subsidies (a sum of up to nearly $64,000 over a period of years) only if they were “meaningful users” of a government-certified system. Vendors, for their part, had to develop systems that met the government’s requirements.
They didn’t have much time, though. The need to stimulate the economy, which meant getting providers to adopt EHRs quickly, “presented a tremendous conundrum,” said Farzad Mostashari, who joined the ONC as deputy director in 2009 and became its leader in 2011: The ideal – creating a useful, interoperable, nationwide records system – was “utterly infeasible to get to in a short time frame.”
That didn’t stop the federal planners from pursuing their grand ambitions. Everyone had big ideas for the EHRs. The FDA wanted the systems to track unique device identifiers for medical implants, the Centers for Disease Control and Prevention wanted them to support disease surveillance, CMS wanted them to include quality metrics and so on. “We had all the right ideas that were discussed and hashed out by the committee,” said Mostashari, “but they were all of the right ideas.”
Not everyone agreed, though, that they were the right ideas. Before long, “meaningful use” became pejorative shorthand to many for a burdensome government program – making doctors do things like check a box indicating a patient’s smoking status each and every visit.
The EHR vendor community, then a scrappy $2 billion industry, griped at the litany of requirements but stood to gain so much from the government’s $36 billion injection that it jumped in line. As Rusty Frantz, CEO of EHR vendor NextGen Healthcare, put it: “The industry was like, ‘I’ve got this check dangling in front of me, and I have to check these boxes to get there, and so I’m going to do that.’”
Halamka, who was an enthusiastic backer of the initiative in both the Bush and Obama administrations, blames the pressure for a speedy launch as much as the excessive wish list. “To go from a regulation to a highly usable product that is in the hands of doctors in 18 months, that’s too fast,” he said. “It’s like asking nine women to have a baby in a month.”
Several of those who worked on the project admit the rollout was not as easy or seamless as they’d anticipated, but they contend that was never the point. Aneesh Chopra, appointed by Obama in 2009 as the nation’s first chief technology officer, called the spending a “down payment” on a vision to fundamentally change American medicine – creating a digital infrastructure to support new ways to pay for health services based on their quality and outcomes.
Dr. Bob Kocher, a physician and star investor with venture capital firm Venrock, who served in the Obama administration from 2009 to 2011 as a health and economic policy adviser, not only defends the rollout then but also disputes the notion that the government initiative has been a failure at all. “EHRs have totally lived up to the hype and expectations,” he said, emphasizing that they also serve as a technology foundation to support innovation on everything from patients accessing their medical records on a smartphone to AI-driven medical sleuthing. Others note the systems’ value in aggregating medical data in ways that were never possible with paper – helping, for example, to figure out that contaminated water was poisoning children in Flint, Mich.
But Rusty Frantz heard a far different message about EHRs – and, more important, it was coming from his own customers.
The Stanford-trained engineer, who in 2015 became CEO of NextGen, a $500-million-a-year EHR heavyweight in the physician-office market, learned the hard way about how his product was being viewed. As he stood at the podium at his first meeting with thousands of NextGen customers at Las Vegas’ Mandalay Bay Resort, just four months after getting the job, he told KHN and Fortune, “People were lining up at the microphones to yell at us: ‘We weren’t delivering stable software! The executive team was inaccessible! The service experience was terrible!’ ” (He now refers to the event as “Festivus: the airing of the grievances.”)
Frantz had bounced around the health care industry for much of his career, and from the nearby perch of a medical device company, he watched the EHR incentive bonanza with a mix of envy and slack-jawed awe. “The industry was moving along in a natural Darwinist way, and then along came the stimulus,” said Frantz, who blames the government’s ham-handed approach to regulation. “The software got slammed in, and the software wasn’t implemented in a way that supported care,” he said. “It was installed in a way that supported stimulus. This company, we were complicit in it, too.”
Even that may be a generous description. KHN and Fortune found a trail of lawsuits against the company, stretching from White Sulphur Springs, Mont., to Neillsville, Wis. Mary Rutan Hospital in Bellefontaine, Ohio, sued NextGen (formerly called Quality Systems) in federal court in 2013, arguing that it experienced hundreds of problems with the “materially defective” software the company had installed in 2011.
A consultant hired by the hospital to evaluate the NextGen system, whose 60-page report was submitted to the court, identified “many functional defects” that he said rendered the software “unfit for its intended purpose.” Some patient information was not accurately recorded, which had the potential, the consultant wrote, “to create major patient care risk which could lead to, at a minimum, inconvenience, and at worst, malpractice or even death.” Glitches at Mary Rutan included incidents in which the software would apparently change a patient’s gender at random or lose a doctor’s observations after an exam, the consultant reported. The company, he found, sometimes took months to address issues: One IT ticket, which related to a physician’s notes inexplicably deleting themselves, reportedly took 10 months to resolve. (The consultant also noted that similar problems appeared to be occurring at as many as a dozen other hospitals that had installed NextGen software.)
The Ohio hospital, which paid more than $1.5 million for its EHR system, claimed breach of contract. NextGen responded that it disputed the claims made in the lawsuit and that the matter was resolved in 2015 “with no findings of fact by a court related to the allegations.” The hospital declined to comment.
At the time, as it has been since then, NextGen’s software was certified by the government as meeting the requirements of the stimulus program. By 2016, NextGen had more than 19,000 customers who had received federal subsidies.
NextGen was subpoenaed by the Department of Justice in December 2017, months after becoming the subject of a federal investigation led by the District of Vermont. Frantz tells KHN and Fortune that NextGen is cooperating with the investigation. “This company was not dishonest, but it was not effective four years ago,” he said. Frantz also emphasized that NextGen has “rapidly evolved” during his tenure, earning five industry awards since 2017, and that customers have “responded very positively.”
Glen Tullman, who until 2012 led Allscripts, another leading EHR vendor that benefited royally from the stimulus and that has been sued by numerous unhappy customers, admitted that the industry’s race to market took priority over all else.
“It was a big distraction. That was an unintended consequence of that,” Tullman said. “All the companies were saying, This is a one-time opportunity to expand our share, focus everything there, and then we’ll go back and fix it.” The Justice Department has opened a civil investigation into the company, Securities and Exchange Commission filings show. Allscripts said in an email that it cannot comment on an ongoing investigation, but that the civil investigations by the Department of Justice relate to businesses it acquired after the investigations were opened.
Much of the marketing mayhem occurred because federal officials imposed few controls over firms scrambling to cash in on the stimulus. It was a gold rush – and any system, it seemed, could be marketed as “federally approved.” Doctors could shop for bargain-price software packages at Costco and Walmart’s Sam’s Club – where eClinicalWorks sold a “turnkey” system for $11,925 – and cash in on the government’s adoption incentives.
The top-shelf vendors in 2009 crisscrossed the country on a “stimulus tour” like rock groups, gigging at some 30 cities, where they offered doctors who showed up to hear the pitch “a customized analysis” of how much money they could earn off the government incentives. Following the same playbook used by pharmaceutical companies, EHR sellers courted doctors at fancy dinners in ritzy hotels. One enterprising software firm advertised a “cash for clunkers” deal that paid $3,000 to doctors willing to trade in their current records system for a new one. Athenahealth held “invitation only” dinners at luxury hotels to advise doctors, among other things, how to use the stimulus to get paid more and capture available incentives. Allscripts offered a no-money-down purchase plan to help doctors “maximize the return on your EHR investment.” (An Athenahealth spokesperson said the company’s “dinners were educational in nature and aimed at helping physicians navigate the government program.” Allscripts did not respond directly to questions about its marketing practices, but said it “is proud of the software and services [it provides] to hundreds of thousands of caregivers across the globe.”)
EHRs were supposed to reduce health care costs, at least in part by preventing duplicative tests. But as the federal government opened the stimulus tap, many raised doubts about the promised savings. Advocates bandied about a figure of $80 billion in cost savings even as congressional auditors were debunking it. While the jury’s still out, there’s growing suspicion the digital revolution may potentially raise health care costs by encouraging overbilling and new strains of fraud and abuse.
In September 2012, following press reports suggesting that some doctors and hospitals were using the new technology to improperly boost their fees, a practice known as “upcoding,” then-Health and Human Services chief Kathleen Sebelius and Attorney General Eric Holder warned the industry not to try to “game the system.”
There’s also growing evidence that some doctors and health systems may have overstated their use of the new technology to secure stimulus funds, a potentially enormous fraud against Medicare and Medicaid that likely will take many years to unravel. In June 2017, the HHS inspector general estimated that Medicare officials made more than $729 million in subsidy payments to hospitals and doctors that didn’t deserve them.
Individual states, which administer the Medicaid portion of the program, haven’t fared much better. Audits have uncovered overpayments in 14 of 17 state programs reviewed, totaling more than $66 million, according to inspector general reports.
Last month, Sen. Chuck Grassley, an Iowa Republican who chairs the Senate Finance Committee, sharply criticized CMS for recovering only a tiny fraction of these bogus payments, or what he termed a “spit in the ocean.”
EHR vendors have also been accused of egregious and patient-endangering acts of fraud as they raced to cash in on the stimulus money grab. In addition to the U.S. government’s $155 million False Claims Act settlement with eClinicalWorks noted above, the federal government has reached a second settlement over similar charges against another large vendor, Tampa-based Greenway Health. In February, that company settled with the government for just over $57 million without denying or admitting wrongdoing. “These are cases of corporate greed, companies that prioritized profits over everything else,” said Christina Nolan, the U.S. attorney for the District of Vermont, whose office led the cases. (In a response, Greenway Health did not address the charges or the settlement but said it was “committing itself to being the standard-bearer for quality, compliance, and transparency.”)
Tower of Babel
In early 2017, Seema Verma, then the country’s newly appointed CMS administrator, went on a listening tour. She visited doctors around the country, at big urban practices and tiny rural clinics, and from those front-line physicians she consistently heard one thing: They hated their electronic health records. “Physician burnout is real,” she told KHN and Fortune. The doctors spoke of the difficulty in getting information from other systems and providers, and they complained about the government’s reporting requirements, which they perceived as burdensome and not meaningful.
What she heard then became suddenly personal one summer day in 2017, when her husband, himself a physician, collapsed in the airport on his way home to Indianapolis after a family vacation. For a frantic few hours, the CMS administrator fielded phone calls from first responders and physicians – Did she know his medical history? Did she have information that could save his life? – and made calls to his doctors in Indiana, scrambling to piece together his record, which should have been there in one piece. Her husband survived the episode, but it laid bare the dysfunction and danger inherent in the existing health information ecosystem.
The notion that one EHR should talk to another was a key part of the original vision for the HITECH Act, with the government calling for systems to be eventually interoperable.
What the framers of that vision didn’t count on were the business incentives working against it. A free exchange of information means that patients can be treated anywhere. And though they may not admit it, many health providers are loath to lose their patients to a competing doctor’s office or hospital. There’s a term for that lost revenue: “leakage.” And keeping a tight hold on patients’ medical records is one way to prevent it.
There’s a ton of proprietary value in that data, said Blumenthal, who now heads the Commonwealth Fund, a philanthropy that does health research. Asking hospitals to give it up is “like asking Amazon to share their data with Walmart,” he said.
Blumenthal acknowledged that he failed to grasp these perverse business dynamics and foresee what a challenge getting the systems to talk to one another would be. He added that forcing interoperability goals early on, when 90 percent of the nation’s providers still didn’t have systems or data to exchange, seemed unrealistic. “We had an expression: They had to operate before they could interoperate,” he said.
In the absence of true incentives for systems to communicate, the industry limped along; some providers wired up directly to other select providers or through regional exchanges, but the efforts were spotty. A Cerner-backed interoperability network called CommonWell formed in 2013, but some companies, including dominant Epic, didn’t join. (“Initially, Epic was neither invited nor allowed to join,” said Sumit Rana, senior vice president of R&D at Epic. Jitin Asnaani, executive director of CommonWell countered, “We made repeated invitations to every major EHR ... and numerous public and private invitations to Epic.”)
Epic then supported a separate effort to do much the same.
Last spring, Verma attempted to kick-start the sharing effort and later pledged a war on “information blocking,” threatening penalties for bad actors. She has promised to reduce the documentation burden on physicians and end the gag clauses that protect the EHR industry. Regarding the first effort at least, “there was consensus that this needed to happen and that it would take the government to push this forward,” she said. In one sign of progress last summer, the dueling sharing initiatives of Epic and Cerner, the two largest players in the industry, began to share with each other – though the effort is fledgling.
When it comes to patients, though, the real sharing too often stops. Despite federal requirements that providers give patients their medical records in a timely fashion, in their chosen format and at low cost (the government recommends a flat fee of $6.50 or less), patients struggle mightily to get them. A 2017 study by researchers at Yale found that of America’s 83 top-rated hospitals, only 53 percent offer forms that provide patients with the option to receive their entire medical record. Fewer than half would share records via email. One hospital charged more than $500 to release them.
Sometimes the mere effort to access records leads to court. Jennifer De Angelis, a Tulsa attorney, has frequently sparred with hospitals over releasing her clients’ records. She said they either attempt to charge huge sums for them or force her to obtain a court order before releasing them. De Angelis added that she sometimes suspects the records have been overwritten to cover up medical mistakes.
Consider the case of 5-year-old Uriah R. Roach, who fractured and cut his finger on Oct. 2, 2014, when it was accidentally slammed in a door at school. Five days later, an operation to repair the damage went awry, and he suffered permanent brain damage, apparently owing to an anesthesia problem. The Epic electronic medical file had been accessed more than 76,000 times during the 22 days the boy was in the hospital, and a lawsuit brought by his parents contended that numerous entries had been “corrected, altered, modified and possibly deleted after an unexpected outcome during the induction of anesthesia.” The hospital denied wrongdoing. The case settled in November 2016, and the terms are confidential.
More than a dozen other attorneys interviewed cited similar problems, especially with gaining access to computerized “audit trails.” In several cases, court records show, government lawyers resisted turning over electronic files from federally run hospitals. That happened to Russell Uselton, an Oklahoma lawyer who represented a pregnant teen admitted to the Choctaw Nation Health Care Center in Talihina, Okla. Shelby Carshall, 18, was more than 40 weeks pregnant at the time. Doctors failed to perform a cesarean section, and her baby was born brain-damaged as a result, she alleged in a lawsuit filed in 2017 against the U.S. government. The baby began having seizures at 10 hours old and will “likely never walk, talk, eat, or otherwise live normally,” according to pleadings in the suit. Though the federal government requires hospitals to produce electronic health records to patients and their families, Uselton had to obtain a court order to get the baby’s complete medical files. Government lawyers denied any negligence in the case, which is pending.
“They try to hide anything from you that they can hide from you,” said Uselton. “They make it extremely difficult to get records, so expensive and hard that most lawyers can’t take it on,” he said.
Nor, it seems, can high-ranking federal officials. When Seema Verma’s husband was discharged from the hospital after his summer health scare, he was handed a few papers and a CD-ROM containing some medical images – but missing key tests and monitoring data. Said Verma, “We left that hospital and we still don’t have his information today.” That was nearly two years ago
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.








