User login
Large measles outbreak reported in Michigan
A new measles outbreak in Michigan has already resulted in 39 cases, and four more states reported their first cases of 2019 during the week ending April 4, according to the Centers for Disease Control and Prevention
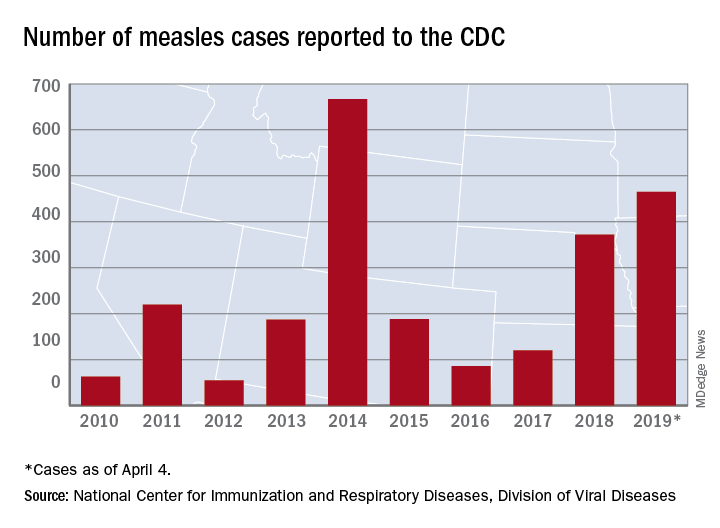
The measles virus has now infected individuals in Florida, Indiana, Massachusetts, and Nevada, which means that 19 states have now reported a total of 465 cases this year, and that is the second-highest total “reported in the U.S. since measles was eliminated in 2000,” the CDC said April 8.
The Michigan outbreak is mostly concentrated in Oakland County, where 38 cases have occurred. The county has posted an up-to-date list of exposure locations.
Not to be outdone, New York reported 45 new cases last week: 44 in Brooklyn and 1 in Queens. There have been 259 confirmed cases in the two boroughs since the outbreak began in October of last year.
Besides Michigan and New York City, there are five other outbreaks ongoing in the United States: Rockland County, N.Y.; Washington State (no new cases since March 22); Butte County, Calif.; Santa Cruz County, Calif.; and New Jersey, the CDC reported.
A judge in New York State temporarily blocked an order banning unimmunized children from public spaces in Rockland County and has set a hearing date of April 19, CNN reported. The ban, ordered by Rockland County Executive Ed Day, went into effect on March 27.
On April 2, the Maine Center for Disease Control & Prevention announced that an out-of-state resident with a confirmed case of measles had visited two health care offices – one in Falmouth and one in Westbrook – on March 27. No cases in Maine residents have been reported yet.
On a vaccine-related note, the Washington State Senate’s Health and Long Term Care Committee approved a proposal on April 1 that would “end the personal exemption for parents who don’t want their children vaccinated against measles,” the Spokane Spokesman-Review said. The bill, which would still allow medical and religious exemptions, has already passed the state’s House of Representatives and goes next to the full senate.
A new measles outbreak in Michigan has already resulted in 39 cases, and four more states reported their first cases of 2019 during the week ending April 4, according to the Centers for Disease Control and Prevention

The measles virus has now infected individuals in Florida, Indiana, Massachusetts, and Nevada, which means that 19 states have now reported a total of 465 cases this year, and that is the second-highest total “reported in the U.S. since measles was eliminated in 2000,” the CDC said April 8.
The Michigan outbreak is mostly concentrated in Oakland County, where 38 cases have occurred. The county has posted an up-to-date list of exposure locations.
Not to be outdone, New York reported 45 new cases last week: 44 in Brooklyn and 1 in Queens. There have been 259 confirmed cases in the two boroughs since the outbreak began in October of last year.
Besides Michigan and New York City, there are five other outbreaks ongoing in the United States: Rockland County, N.Y.; Washington State (no new cases since March 22); Butte County, Calif.; Santa Cruz County, Calif.; and New Jersey, the CDC reported.
A judge in New York State temporarily blocked an order banning unimmunized children from public spaces in Rockland County and has set a hearing date of April 19, CNN reported. The ban, ordered by Rockland County Executive Ed Day, went into effect on March 27.
On April 2, the Maine Center for Disease Control & Prevention announced that an out-of-state resident with a confirmed case of measles had visited two health care offices – one in Falmouth and one in Westbrook – on March 27. No cases in Maine residents have been reported yet.
On a vaccine-related note, the Washington State Senate’s Health and Long Term Care Committee approved a proposal on April 1 that would “end the personal exemption for parents who don’t want their children vaccinated against measles,” the Spokane Spokesman-Review said. The bill, which would still allow medical and religious exemptions, has already passed the state’s House of Representatives and goes next to the full senate.
A new measles outbreak in Michigan has already resulted in 39 cases, and four more states reported their first cases of 2019 during the week ending April 4, according to the Centers for Disease Control and Prevention

The measles virus has now infected individuals in Florida, Indiana, Massachusetts, and Nevada, which means that 19 states have now reported a total of 465 cases this year, and that is the second-highest total “reported in the U.S. since measles was eliminated in 2000,” the CDC said April 8.
The Michigan outbreak is mostly concentrated in Oakland County, where 38 cases have occurred. The county has posted an up-to-date list of exposure locations.
Not to be outdone, New York reported 45 new cases last week: 44 in Brooklyn and 1 in Queens. There have been 259 confirmed cases in the two boroughs since the outbreak began in October of last year.
Besides Michigan and New York City, there are five other outbreaks ongoing in the United States: Rockland County, N.Y.; Washington State (no new cases since March 22); Butte County, Calif.; Santa Cruz County, Calif.; and New Jersey, the CDC reported.
A judge in New York State temporarily blocked an order banning unimmunized children from public spaces in Rockland County and has set a hearing date of April 19, CNN reported. The ban, ordered by Rockland County Executive Ed Day, went into effect on March 27.
On April 2, the Maine Center for Disease Control & Prevention announced that an out-of-state resident with a confirmed case of measles had visited two health care offices – one in Falmouth and one in Westbrook – on March 27. No cases in Maine residents have been reported yet.
On a vaccine-related note, the Washington State Senate’s Health and Long Term Care Committee approved a proposal on April 1 that would “end the personal exemption for parents who don’t want their children vaccinated against measles,” the Spokane Spokesman-Review said. The bill, which would still allow medical and religious exemptions, has already passed the state’s House of Representatives and goes next to the full senate.
Fingernail Abnormalities After a Systemic Illness
A 45-year-old African American woman presented with painless fingernail detachment and cracks on her fingernails that had developed over the previous month. Her medical history was notable for an episode of Stevens-Johnson syndrome 2 months prior that required treatment with prednisone, IV immunoglobulin, etanercept, acetaminophen, and diphenhydramine.
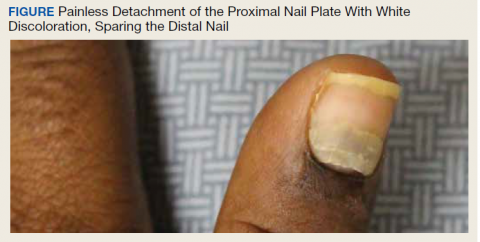
A physical examination revealed multiple fingernails on both hands that exhibited 4 mm of proximal painless nail detachment with cream-colored discoloration, friability, and horizontal splitting (Figure). New, healthy nail was visible beneath the affected areas. Toenails were not affected.
- What is your diagnosis?
- How would you treat this patient?
Diagnosis
Based on the timing and characteristics of her nail detachment, the patient was diagnosed with onychomadesis, which is defined as painless detachment of the proximal nail plate from the nail matrix and nail bed after at least 40 days from an initial insult. Air beneath the detached nail plate causes a characteristic creamy-white discoloration. The severity of onychomadesis ranges from transverse furrows that affect a single nail without shedding, known as Beau lines, to multiple nails that are completely shed.1,2 Nail plate shedding is typical because the nail matrix, the site of stem cells and the most proximal portion of the nail apparatus, is damaged and transiently arrested.
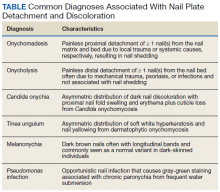
Various etiologies can halt nail plate production abruptly within the matrix. These typically manifest ≥ 40 days after the initial insult (the length of time for a fingernail to emerge from the proximal nail fold).2 The annual incidence of these etiologies ranges from approximately 1 per 1 million people for Stevens-Johnson syndrome, a rare cause of onychomadesis, to 1 per 10 people for onychomycosis, one of the more common causes of onychomadesis.3 The Table compares the characteristics of the diagnoses that are most commonly associated with nail detachment and discoloration.
When a single nail is affected, the etiology of onychomadesis usually is primary and local, including mechanical nail trauma and fungal nail infections (onychomycosis).1,2 Candida onychia is onychomycosis caused by Candida species typically Candida albicans, which result in localized nail darkening, chronic inflammation of the paronychial skin, and cuticle loss. The infection favors immunocompromised people; coinfections are common, and onychomadesis or onycholysis can occur. Unlike onychomadesis, onycholysis is defined by painless detachment of the distal nail plate from the nail bed, but nail shedding typically does not occur because the nail matrix is spared. The preferred treatment for Candida onychia is oral itraconazole, and guided screenings for immunodeficiencies and endocrinopathies, especially diabetes mellitus, should be completed.3,4
Tinea unguium is another form of onychomycosis, but it is caused by dermatophytes, typically Trichophyton rubrum or Trichophyton mentagrophytes, which produce white and yellow nail discoloration followed by distal to proximal nail thickening and softening. Infection usually begins in toenails and demonstrates variable involvement in each nail as well as asymmetric distribution among digits.3 This condition also may eventuate in onychomadesis or onycholysis. Debridement followed by oral terbinafine is the treatment of choice.4
Two other causes of localized nail discoloration with or without nail detachment include melanonychia and nail bed infection by Pseudomonas aeruginosa (P aeruginosa). Melanonychia can be linear or diffuse brown discoloration of 1 or more nails caused by melanin deposition. Either pattern is a common finding in dark-skinned people, especially by age 50 years, but melanocyte hyperplasia should be excluded in all individuals along with drug adverse effects, exogenous pigments, infections, and systemic diseases.3,5 P aeruginosa produces pyocyanin, the green pigment responsible for the discoloration seen in this opportunistic infection often localized to a single nail. Prior maceration of the nail apparatus by repeated water submersion is common among affected individuals. Avoidance of submerging fingernails in liquids followed by nail debridement and oral antipseudomonal antibiotics is the preferred treatment course.3
The etiology is usually secondary and systemic when multiple nails demonstrate onychomadesis, but the exact pathophysiology is poorly understood. One of the most studied infectious etiologies of onychomadesis is hand-foot-and-mouth disease (HFMD), which typically affects children aged < 10 years. Parents often will recall their child being ill 1 to 2 months prior to the nail findings. Scarlet fever and varicella also can result in onychomadesis. Although not common systemic causes, Stevens-Johnson syndrome and toxic epidermal necrolysis can trigger onychomadesis of multiple nails that usually resolves in several months, but other nail deformities often persist.2,6 Onycholysis also can accompany this finding.7 Autoimmune etiologies of onychomadesis include alopecia areata and pemphigus vulgaris. Inciting medications that are toxic to the nail matrix include chemotherapy agents, valproic acid, carbamazepine, lithium, and azithromycin. Rare congenital disorders and birth trauma also can present with onychomadesis of multiple nails during infancy.2
Systemic etiologies typically affect fingernails more than toenails because of the faster growth rate of fingernails. Once the source of onychomadesis is controlled or eradicated, complete regrowth of fingernails can take from 4 to 6 months. Toenails can take twice as long and older age increases all regrowth periods.5
Our patient was treated with analgesics until her mucosal surfaces fully healed, and topical emollients and keratolytics were used to soften eschars from previous blisters and prevent further scar formation. Her affected fingernails shed and regrew after 6 months without additional interventions.
Conclusion
Although Stevens-Johnson syndrome is a rare cause of onychomadesis, and the pathophysiology of this sequela is poorly understood, this case illustrates a common nail abnormality with multiple potential etiologies that are discerned by an accurate history and thorough exam. In the absence of decorative nail polish, nails can be easily examined to provide helpful clues for past injuries or underlying diseases. An understanding of nail growth mechanics and associated terminology reveals the diagnostic and therapeutic implications of proximal vs distal nail detachment, the hue of nail discoloration, as well as single vs multiple affected nails.
Onychomadesis in single nails should prompt questions about nail trauma or risk factors for fungal infections. Depending on the etiology, manual activities need to be adjusted, or antifungals need to be initiated while investigating for an immunocompromised state. Onychomadesis in multiple nails in children should raise suspicion for HFMD or even birth trauma and congenital disorders. Multiple affected nails in adults should prompt guided questions for autoimmune diseases and inciting medications. For onycholysis, trauma, psoriasis, or certain infections should be the target. Green nails are easily recognized and treated with a defined regiment, whereas dark nails should be examined closely to differentiate Candida onychia from melanonychia. Whether from a rare cause in an adult to a common illness in a child, primary care providers have sufficient expertise to diagnose and treat various nail disorders and reassure worried patients and parents with an understanding of nail regrowth.
1. Salgado F, Handler MZ, Schwartz RA. Shedding light on onychomadesis. Cutis. 2017;99(1):33-36.
2. Hardin J, Haber RM. Oncyhomadesis: literature review. Br J Dermatol. 2015;172(3):592-596.
3. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology. 5th ed. New York, NY: McGraw-Hill; 2005.
4. du Vivier A. Atlas of Clinical Dermatology. 4th ed. Philadelphia, PA: Saunders; 2012.
5. Shemer A, Daniel CR III. Common nail disorders. Clin Dermatol. 2013;31(5):578-586.
6. Acharya S, Balachandran C. Onychomadesis in Stevens-Johnson syndrome. Indian J Dermatol Venereol Leprol. 1996;62(4):264-265.
7. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69(2):187.e1-e16.
A 45-year-old African American woman presented with painless fingernail detachment and cracks on her fingernails that had developed over the previous month. Her medical history was notable for an episode of Stevens-Johnson syndrome 2 months prior that required treatment with prednisone, IV immunoglobulin, etanercept, acetaminophen, and diphenhydramine.
A physical examination revealed multiple fingernails on both hands that exhibited 4 mm of proximal painless nail detachment with cream-colored discoloration, friability, and horizontal splitting (Figure). New, healthy nail was visible beneath the affected areas. Toenails were not affected.
- What is your diagnosis?
- How would you treat this patient?
Diagnosis
Based on the timing and characteristics of her nail detachment, the patient was diagnosed with onychomadesis, which is defined as painless detachment of the proximal nail plate from the nail matrix and nail bed after at least 40 days from an initial insult. Air beneath the detached nail plate causes a characteristic creamy-white discoloration. The severity of onychomadesis ranges from transverse furrows that affect a single nail without shedding, known as Beau lines, to multiple nails that are completely shed.1,2 Nail plate shedding is typical because the nail matrix, the site of stem cells and the most proximal portion of the nail apparatus, is damaged and transiently arrested.
Various etiologies can halt nail plate production abruptly within the matrix. These typically manifest ≥ 40 days after the initial insult (the length of time for a fingernail to emerge from the proximal nail fold).2 The annual incidence of these etiologies ranges from approximately 1 per 1 million people for Stevens-Johnson syndrome, a rare cause of onychomadesis, to 1 per 10 people for onychomycosis, one of the more common causes of onychomadesis.3 The Table compares the characteristics of the diagnoses that are most commonly associated with nail detachment and discoloration.
When a single nail is affected, the etiology of onychomadesis usually is primary and local, including mechanical nail trauma and fungal nail infections (onychomycosis).1,2 Candida onychia is onychomycosis caused by Candida species typically Candida albicans, which result in localized nail darkening, chronic inflammation of the paronychial skin, and cuticle loss. The infection favors immunocompromised people; coinfections are common, and onychomadesis or onycholysis can occur. Unlike onychomadesis, onycholysis is defined by painless detachment of the distal nail plate from the nail bed, but nail shedding typically does not occur because the nail matrix is spared. The preferred treatment for Candida onychia is oral itraconazole, and guided screenings for immunodeficiencies and endocrinopathies, especially diabetes mellitus, should be completed.3,4
Tinea unguium is another form of onychomycosis, but it is caused by dermatophytes, typically Trichophyton rubrum or Trichophyton mentagrophytes, which produce white and yellow nail discoloration followed by distal to proximal nail thickening and softening. Infection usually begins in toenails and demonstrates variable involvement in each nail as well as asymmetric distribution among digits.3 This condition also may eventuate in onychomadesis or onycholysis. Debridement followed by oral terbinafine is the treatment of choice.4
Two other causes of localized nail discoloration with or without nail detachment include melanonychia and nail bed infection by Pseudomonas aeruginosa (P aeruginosa). Melanonychia can be linear or diffuse brown discoloration of 1 or more nails caused by melanin deposition. Either pattern is a common finding in dark-skinned people, especially by age 50 years, but melanocyte hyperplasia should be excluded in all individuals along with drug adverse effects, exogenous pigments, infections, and systemic diseases.3,5 P aeruginosa produces pyocyanin, the green pigment responsible for the discoloration seen in this opportunistic infection often localized to a single nail. Prior maceration of the nail apparatus by repeated water submersion is common among affected individuals. Avoidance of submerging fingernails in liquids followed by nail debridement and oral antipseudomonal antibiotics is the preferred treatment course.3
The etiology is usually secondary and systemic when multiple nails demonstrate onychomadesis, but the exact pathophysiology is poorly understood. One of the most studied infectious etiologies of onychomadesis is hand-foot-and-mouth disease (HFMD), which typically affects children aged < 10 years. Parents often will recall their child being ill 1 to 2 months prior to the nail findings. Scarlet fever and varicella also can result in onychomadesis. Although not common systemic causes, Stevens-Johnson syndrome and toxic epidermal necrolysis can trigger onychomadesis of multiple nails that usually resolves in several months, but other nail deformities often persist.2,6 Onycholysis also can accompany this finding.7 Autoimmune etiologies of onychomadesis include alopecia areata and pemphigus vulgaris. Inciting medications that are toxic to the nail matrix include chemotherapy agents, valproic acid, carbamazepine, lithium, and azithromycin. Rare congenital disorders and birth trauma also can present with onychomadesis of multiple nails during infancy.2
Systemic etiologies typically affect fingernails more than toenails because of the faster growth rate of fingernails. Once the source of onychomadesis is controlled or eradicated, complete regrowth of fingernails can take from 4 to 6 months. Toenails can take twice as long and older age increases all regrowth periods.5
Our patient was treated with analgesics until her mucosal surfaces fully healed, and topical emollients and keratolytics were used to soften eschars from previous blisters and prevent further scar formation. Her affected fingernails shed and regrew after 6 months without additional interventions.
Conclusion
Although Stevens-Johnson syndrome is a rare cause of onychomadesis, and the pathophysiology of this sequela is poorly understood, this case illustrates a common nail abnormality with multiple potential etiologies that are discerned by an accurate history and thorough exam. In the absence of decorative nail polish, nails can be easily examined to provide helpful clues for past injuries or underlying diseases. An understanding of nail growth mechanics and associated terminology reveals the diagnostic and therapeutic implications of proximal vs distal nail detachment, the hue of nail discoloration, as well as single vs multiple affected nails.
Onychomadesis in single nails should prompt questions about nail trauma or risk factors for fungal infections. Depending on the etiology, manual activities need to be adjusted, or antifungals need to be initiated while investigating for an immunocompromised state. Onychomadesis in multiple nails in children should raise suspicion for HFMD or even birth trauma and congenital disorders. Multiple affected nails in adults should prompt guided questions for autoimmune diseases and inciting medications. For onycholysis, trauma, psoriasis, or certain infections should be the target. Green nails are easily recognized and treated with a defined regiment, whereas dark nails should be examined closely to differentiate Candida onychia from melanonychia. Whether from a rare cause in an adult to a common illness in a child, primary care providers have sufficient expertise to diagnose and treat various nail disorders and reassure worried patients and parents with an understanding of nail regrowth.
A 45-year-old African American woman presented with painless fingernail detachment and cracks on her fingernails that had developed over the previous month. Her medical history was notable for an episode of Stevens-Johnson syndrome 2 months prior that required treatment with prednisone, IV immunoglobulin, etanercept, acetaminophen, and diphenhydramine.
A physical examination revealed multiple fingernails on both hands that exhibited 4 mm of proximal painless nail detachment with cream-colored discoloration, friability, and horizontal splitting (Figure). New, healthy nail was visible beneath the affected areas. Toenails were not affected.
- What is your diagnosis?
- How would you treat this patient?
Diagnosis
Based on the timing and characteristics of her nail detachment, the patient was diagnosed with onychomadesis, which is defined as painless detachment of the proximal nail plate from the nail matrix and nail bed after at least 40 days from an initial insult. Air beneath the detached nail plate causes a characteristic creamy-white discoloration. The severity of onychomadesis ranges from transverse furrows that affect a single nail without shedding, known as Beau lines, to multiple nails that are completely shed.1,2 Nail plate shedding is typical because the nail matrix, the site of stem cells and the most proximal portion of the nail apparatus, is damaged and transiently arrested.
Various etiologies can halt nail plate production abruptly within the matrix. These typically manifest ≥ 40 days after the initial insult (the length of time for a fingernail to emerge from the proximal nail fold).2 The annual incidence of these etiologies ranges from approximately 1 per 1 million people for Stevens-Johnson syndrome, a rare cause of onychomadesis, to 1 per 10 people for onychomycosis, one of the more common causes of onychomadesis.3 The Table compares the characteristics of the diagnoses that are most commonly associated with nail detachment and discoloration.
When a single nail is affected, the etiology of onychomadesis usually is primary and local, including mechanical nail trauma and fungal nail infections (onychomycosis).1,2 Candida onychia is onychomycosis caused by Candida species typically Candida albicans, which result in localized nail darkening, chronic inflammation of the paronychial skin, and cuticle loss. The infection favors immunocompromised people; coinfections are common, and onychomadesis or onycholysis can occur. Unlike onychomadesis, onycholysis is defined by painless detachment of the distal nail plate from the nail bed, but nail shedding typically does not occur because the nail matrix is spared. The preferred treatment for Candida onychia is oral itraconazole, and guided screenings for immunodeficiencies and endocrinopathies, especially diabetes mellitus, should be completed.3,4
Tinea unguium is another form of onychomycosis, but it is caused by dermatophytes, typically Trichophyton rubrum or Trichophyton mentagrophytes, which produce white and yellow nail discoloration followed by distal to proximal nail thickening and softening. Infection usually begins in toenails and demonstrates variable involvement in each nail as well as asymmetric distribution among digits.3 This condition also may eventuate in onychomadesis or onycholysis. Debridement followed by oral terbinafine is the treatment of choice.4
Two other causes of localized nail discoloration with or without nail detachment include melanonychia and nail bed infection by Pseudomonas aeruginosa (P aeruginosa). Melanonychia can be linear or diffuse brown discoloration of 1 or more nails caused by melanin deposition. Either pattern is a common finding in dark-skinned people, especially by age 50 years, but melanocyte hyperplasia should be excluded in all individuals along with drug adverse effects, exogenous pigments, infections, and systemic diseases.3,5 P aeruginosa produces pyocyanin, the green pigment responsible for the discoloration seen in this opportunistic infection often localized to a single nail. Prior maceration of the nail apparatus by repeated water submersion is common among affected individuals. Avoidance of submerging fingernails in liquids followed by nail debridement and oral antipseudomonal antibiotics is the preferred treatment course.3
The etiology is usually secondary and systemic when multiple nails demonstrate onychomadesis, but the exact pathophysiology is poorly understood. One of the most studied infectious etiologies of onychomadesis is hand-foot-and-mouth disease (HFMD), which typically affects children aged < 10 years. Parents often will recall their child being ill 1 to 2 months prior to the nail findings. Scarlet fever and varicella also can result in onychomadesis. Although not common systemic causes, Stevens-Johnson syndrome and toxic epidermal necrolysis can trigger onychomadesis of multiple nails that usually resolves in several months, but other nail deformities often persist.2,6 Onycholysis also can accompany this finding.7 Autoimmune etiologies of onychomadesis include alopecia areata and pemphigus vulgaris. Inciting medications that are toxic to the nail matrix include chemotherapy agents, valproic acid, carbamazepine, lithium, and azithromycin. Rare congenital disorders and birth trauma also can present with onychomadesis of multiple nails during infancy.2
Systemic etiologies typically affect fingernails more than toenails because of the faster growth rate of fingernails. Once the source of onychomadesis is controlled or eradicated, complete regrowth of fingernails can take from 4 to 6 months. Toenails can take twice as long and older age increases all regrowth periods.5
Our patient was treated with analgesics until her mucosal surfaces fully healed, and topical emollients and keratolytics were used to soften eschars from previous blisters and prevent further scar formation. Her affected fingernails shed and regrew after 6 months without additional interventions.
Conclusion
Although Stevens-Johnson syndrome is a rare cause of onychomadesis, and the pathophysiology of this sequela is poorly understood, this case illustrates a common nail abnormality with multiple potential etiologies that are discerned by an accurate history and thorough exam. In the absence of decorative nail polish, nails can be easily examined to provide helpful clues for past injuries or underlying diseases. An understanding of nail growth mechanics and associated terminology reveals the diagnostic and therapeutic implications of proximal vs distal nail detachment, the hue of nail discoloration, as well as single vs multiple affected nails.
Onychomadesis in single nails should prompt questions about nail trauma or risk factors for fungal infections. Depending on the etiology, manual activities need to be adjusted, or antifungals need to be initiated while investigating for an immunocompromised state. Onychomadesis in multiple nails in children should raise suspicion for HFMD or even birth trauma and congenital disorders. Multiple affected nails in adults should prompt guided questions for autoimmune diseases and inciting medications. For onycholysis, trauma, psoriasis, or certain infections should be the target. Green nails are easily recognized and treated with a defined regiment, whereas dark nails should be examined closely to differentiate Candida onychia from melanonychia. Whether from a rare cause in an adult to a common illness in a child, primary care providers have sufficient expertise to diagnose and treat various nail disorders and reassure worried patients and parents with an understanding of nail regrowth.
1. Salgado F, Handler MZ, Schwartz RA. Shedding light on onychomadesis. Cutis. 2017;99(1):33-36.
2. Hardin J, Haber RM. Oncyhomadesis: literature review. Br J Dermatol. 2015;172(3):592-596.
3. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology. 5th ed. New York, NY: McGraw-Hill; 2005.
4. du Vivier A. Atlas of Clinical Dermatology. 4th ed. Philadelphia, PA: Saunders; 2012.
5. Shemer A, Daniel CR III. Common nail disorders. Clin Dermatol. 2013;31(5):578-586.
6. Acharya S, Balachandran C. Onychomadesis in Stevens-Johnson syndrome. Indian J Dermatol Venereol Leprol. 1996;62(4):264-265.
7. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69(2):187.e1-e16.
1. Salgado F, Handler MZ, Schwartz RA. Shedding light on onychomadesis. Cutis. 2017;99(1):33-36.
2. Hardin J, Haber RM. Oncyhomadesis: literature review. Br J Dermatol. 2015;172(3):592-596.
3. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s Color Atlas & Synopsis of Clinical Dermatology. 5th ed. New York, NY: McGraw-Hill; 2005.
4. du Vivier A. Atlas of Clinical Dermatology. 4th ed. Philadelphia, PA: Saunders; 2012.
5. Shemer A, Daniel CR III. Common nail disorders. Clin Dermatol. 2013;31(5):578-586.
6. Acharya S, Balachandran C. Onychomadesis in Stevens-Johnson syndrome. Indian J Dermatol Venereol Leprol. 1996;62(4):264-265.
7. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69(2):187.e1-e16.
Clinical Pharmacist Credentialing and Privileging: A Process for Ensuring High-Quality Patient Care
The Red Lake Indian Health Service (IHS) health care facility is in north-central Minnesota within the Red Lake Nation. The facility supports primary care, emergency, urgent care, pharmacy, inpatient, optometry, dental, radiology, laboratory, physical therapy, and behavioral health services to about 10,000 Red Lake Band of Chippewa Indian patients. The Red Lake pharmacy provides inpatient and outpatient medication services and pharmacist-managed clinical patient care.
In 2013, the Red Lake IHS medical staff endorsed the implementation of comprehensive clinical pharmacy services to increase health care access and optimize clinical outcomes for patients. During the evolution of pharmacy-based patient-centric care, the clinical programs offered by Red Lake IHS pharmacy expanded from 1 anticoagulation clinic to multiple advanced-practice clinical pharmacy services. This included pharmacy primary care, medication-assisted therapy, naloxone, hepatitis C, and behavioral health medication management clinics.
The immense clinical growth of the pharmacy department demonstrated a need to assess and monitor pharmacist competency to ensure the delivery of quality patient care. Essential quality improvement processes were lacking. To fill these quality improvement gaps, a robust pharmacist credentialing and privileging program was implemented in 2015.
Patient Care
As efforts within health care establishments across the US focus on the delivery of efficient, high-quality, affordable health care, pharmacists have become increasingly instrumental in providing patient care within expanded clinical roles.1-8 Many clinical pharmacy models have evolved into interdisciplinary approaches to care.9 Within these models, abiding by state and federal laws, pharmacists practice under the indirect supervision of licensed independent practitioners (LIPs), such as physicians, nurse practitioners, and physician assistants.8 Under collaborative practice agreements (CPAs), patients are initially diagnosed by LIPs, then referred to clinical pharmacists for therapeutic management.5,7
Clinical pharmacist functions encompass comprehensive medication management (ie, prescribing, monitoring, and adjustment of medications), nonpharmacologic guidance, and coordination of care. Interdisciplinary collaboration allows pharmacists opportunities to provide direct patient care or consultations by telecommunication in many different clinical environments, including disease management, primary care, or specialty care. Pharmacists may manage chronic or acute illnesses associated with endocrine, cardiovascular, respiratory, gastrointestinal, or other systems.
Pharmacists may also provide comprehensive medication review services, such as medication therapy management (MTM), transitions of care, or chronic care management. Examples of specialized areas include psychiatric, opioid use disorder, palliative care, infectious disease, chronic pain, or oncology services. For hospitalized patients, pharmacists may monitor pharmacokinetics and adjust dosing, transition patients from IV to oral medications, or complete medication reconciliation.10 Within these clinical roles, pharmacists assist in providing patient care during shortages of other health care providers (HCPs), improve patient outcomes, decrease health care-associated costs by preventing emergency department and hospital admissions or readmissions, increase access to patient care, and increase revenue through pharmacist-managed clinics and services.11
Pharmacist Credentialing
With the advancement of modern clinical pharmacy practice, many pharmacists have undertaken responsibilities to fulfill the complex duties of clinical care and diverse patient situations, but with few or no requirements to prove initial or ongoing clinical competency.2 Traditionally, pharmacist credentialing is limited to a onetime or periodic review of education and licensure, with little to no involvement in privileging and ongoing monitoring of clinical proficiency.10 These quality assurance disparities can be met and satisfied through credentialing and privileging processes. Credentialing and privileging are systematic, evidence-based processes that provide validation to HCPs, employers, and patients that pharmacists are qualified to practice clinically. 2,9 According to the Council on Credentialing in Pharmacy, clinical pharmacists should be held accountable for demonstrating competency and providing quality care through credentialing and privileging, as required for other HCPs.2,12
Credentialing and recredentialing is a primary source verification process. These processes ensure that there are no license restrictions or revocations; certifications are current; mandatory courses, certificates, and continuing education are complete; training and orientation are satisfactory; and any disciplinary action, malpractice claims, or history of impairment is reported. Privileging is the review of credentials and evaluation of clinical training and competence by the Clinical Director and Medical Executive Committee to determine whether a clinical pharmacist is competent to practice within requested privileges.11
Credentialing and privileging processes are designed not only to initially confirm that a pharmacist is competent to practice clinically, but also monitor ongoing performance.2,13 Participation in professional practice evaluations, which includes peer reviews, ongoing professional practice evaluations, and focused professional practice evaluations, is required for all credentialed and privileged practitioners. These evaluations are used to identify, assess, and correct unsatisfactory trends. Individual practices, documentation, and processes are evaluated against existing department standards (eg, CPAs, policies, processes)11,13 The results of individual professional practice evaluations are reviewed with practitioners on a regular basis and performance improvement plans implemented as needed.
Since 2015, 17 pharmacists at the Red Lake IHS health care facility have been granted membership to the medical staff as credentialed and privileged practitioners. In a retrospective review of professional practice evaluations by the Red Lake IHS pharmacy clinical coordinator, 971 outpatient clinical peer reviews, including the evaluation of 21,526 peer-review elements were completed by pharmacists from fiscal year 2015 through 2018. Peer-review elements assessed
Conclusion
Pharmacists have become increasingly instrumental in providing effective, cost-efficient, and accessible clinical services by continuing to move toward expanding and evolving roles within comprehensive, patient-centered clinical pharmacy practice settings.5,6 Multifaceted clinical responsibilities associated with health care delivery necessitate assessment and monitoring of pharmacist performance. Credentialing and privileging is an established and trusted systematic process that assures HCPs, employers, and patients that pharmacists are qualified and competent to practice clinically.2,4,12 Implementation of professional practice evaluations suggest improved staff compliance with visit documentation, patient care standards, and clinic processes required by CPAs, policies, and department standards to ensure the delivery of safe, high-quality patient care.
1. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed March 15, 2019.
2. Rouse MJ, Vlasses PH, Webb CE; Council on Credentialing in Pharmacy. Credentialing and privileging of pharmacists: a resource paper from the Council on Credentialing in Pharmacy. Am J Health Syst Pharm. 2014;71(21):e109-e118.
3. Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759-769.
4. Blair MM, Carmichael J, Young E, Thrasher K; Qualified Provider Model Ad Hoc Committee. Pharmacist privileging in a health system: report of the Qualified Provider Model Ad Hoc Committee. Am J Health Syst Pharm. 2007;64(22):2373-2381.
5. Claxton KI, Wojtal P. Design and implementation of a credentialing and privileging model for ambulatory care pharmacists. Am J Health Syst Pharm. 2006;63(17):1627-1632.
6. Jordan TA, Hennenfent JA, Lewin JJ III, Nesbit TW, Weber R. Elevating pharmacists’ scope of practice through a health-system clinical privileging process. Am J Health Syst Pharm. 2016;73(18):1395-1405.
7. Centers for Disease Control and Prevention. Collaborative practice agreements and pharmacists’ patient care services: a resource for doctors, nurses, physician assistants, and other providers. https://www.cdc.gov/dhdsp/pubs/docs/Translational_Tools_Providers.pdf. Published October 2013. Accessed March 18, 2019.
8. Council on Credentialing in Pharmacy, Albanese NP, Rouse MJ. Scope of contemporary pharmacy practice: roles, responsibilities, and functions of practitioners and pharmacy technicians. J Am Pharm Assoc (2003). 2010;50(2):e35-e69.
9. Philip B, Weber R. Enhancing pharmacy practice models through pharmacists’ privileging. Hosp Pharm. 2013; 48(2):160-165.
10. Galt KA. Credentialing and privileging of pharmacists. Am J Health Syst Pharm. 2004;61(7):661-670.
11. Smith ML, Gemelas MF; US Public Health Service; Indian Health Service. Indian Health Service medical staff credentialing and privileging guide. https://www.ihs.gov/riskmanagement/includes/themes/newihstheme/display_objects/documents/IHS-Medical-Staff-Credentialing-and-Privileging-Guide.pdf. Published September 2005. Accessed March 15, 2019.
12. US Department of Health and Human Services, Indian Health Service. Indian health manual: medical credentials and privileges review process. https://www.ihs.gov/ihm/pc/part-3/p3c1. Accessed March 15, 2019.
13. Holley SL, Ketel C. Ongoing professional practice evaluation and focused professional practice evaluation: an overview for advanced practice clinicians. J Midwifery Women Health. 2014;59(4):452-459.
The Red Lake Indian Health Service (IHS) health care facility is in north-central Minnesota within the Red Lake Nation. The facility supports primary care, emergency, urgent care, pharmacy, inpatient, optometry, dental, radiology, laboratory, physical therapy, and behavioral health services to about 10,000 Red Lake Band of Chippewa Indian patients. The Red Lake pharmacy provides inpatient and outpatient medication services and pharmacist-managed clinical patient care.
In 2013, the Red Lake IHS medical staff endorsed the implementation of comprehensive clinical pharmacy services to increase health care access and optimize clinical outcomes for patients. During the evolution of pharmacy-based patient-centric care, the clinical programs offered by Red Lake IHS pharmacy expanded from 1 anticoagulation clinic to multiple advanced-practice clinical pharmacy services. This included pharmacy primary care, medication-assisted therapy, naloxone, hepatitis C, and behavioral health medication management clinics.
The immense clinical growth of the pharmacy department demonstrated a need to assess and monitor pharmacist competency to ensure the delivery of quality patient care. Essential quality improvement processes were lacking. To fill these quality improvement gaps, a robust pharmacist credentialing and privileging program was implemented in 2015.
Patient Care
As efforts within health care establishments across the US focus on the delivery of efficient, high-quality, affordable health care, pharmacists have become increasingly instrumental in providing patient care within expanded clinical roles.1-8 Many clinical pharmacy models have evolved into interdisciplinary approaches to care.9 Within these models, abiding by state and federal laws, pharmacists practice under the indirect supervision of licensed independent practitioners (LIPs), such as physicians, nurse practitioners, and physician assistants.8 Under collaborative practice agreements (CPAs), patients are initially diagnosed by LIPs, then referred to clinical pharmacists for therapeutic management.5,7
Clinical pharmacist functions encompass comprehensive medication management (ie, prescribing, monitoring, and adjustment of medications), nonpharmacologic guidance, and coordination of care. Interdisciplinary collaboration allows pharmacists opportunities to provide direct patient care or consultations by telecommunication in many different clinical environments, including disease management, primary care, or specialty care. Pharmacists may manage chronic or acute illnesses associated with endocrine, cardiovascular, respiratory, gastrointestinal, or other systems.
Pharmacists may also provide comprehensive medication review services, such as medication therapy management (MTM), transitions of care, or chronic care management. Examples of specialized areas include psychiatric, opioid use disorder, palliative care, infectious disease, chronic pain, or oncology services. For hospitalized patients, pharmacists may monitor pharmacokinetics and adjust dosing, transition patients from IV to oral medications, or complete medication reconciliation.10 Within these clinical roles, pharmacists assist in providing patient care during shortages of other health care providers (HCPs), improve patient outcomes, decrease health care-associated costs by preventing emergency department and hospital admissions or readmissions, increase access to patient care, and increase revenue through pharmacist-managed clinics and services.11
Pharmacist Credentialing
With the advancement of modern clinical pharmacy practice, many pharmacists have undertaken responsibilities to fulfill the complex duties of clinical care and diverse patient situations, but with few or no requirements to prove initial or ongoing clinical competency.2 Traditionally, pharmacist credentialing is limited to a onetime or periodic review of education and licensure, with little to no involvement in privileging and ongoing monitoring of clinical proficiency.10 These quality assurance disparities can be met and satisfied through credentialing and privileging processes. Credentialing and privileging are systematic, evidence-based processes that provide validation to HCPs, employers, and patients that pharmacists are qualified to practice clinically. 2,9 According to the Council on Credentialing in Pharmacy, clinical pharmacists should be held accountable for demonstrating competency and providing quality care through credentialing and privileging, as required for other HCPs.2,12
Credentialing and recredentialing is a primary source verification process. These processes ensure that there are no license restrictions or revocations; certifications are current; mandatory courses, certificates, and continuing education are complete; training and orientation are satisfactory; and any disciplinary action, malpractice claims, or history of impairment is reported. Privileging is the review of credentials and evaluation of clinical training and competence by the Clinical Director and Medical Executive Committee to determine whether a clinical pharmacist is competent to practice within requested privileges.11
Credentialing and privileging processes are designed not only to initially confirm that a pharmacist is competent to practice clinically, but also monitor ongoing performance.2,13 Participation in professional practice evaluations, which includes peer reviews, ongoing professional practice evaluations, and focused professional practice evaluations, is required for all credentialed and privileged practitioners. These evaluations are used to identify, assess, and correct unsatisfactory trends. Individual practices, documentation, and processes are evaluated against existing department standards (eg, CPAs, policies, processes)11,13 The results of individual professional practice evaluations are reviewed with practitioners on a regular basis and performance improvement plans implemented as needed.
Since 2015, 17 pharmacists at the Red Lake IHS health care facility have been granted membership to the medical staff as credentialed and privileged practitioners. In a retrospective review of professional practice evaluations by the Red Lake IHS pharmacy clinical coordinator, 971 outpatient clinical peer reviews, including the evaluation of 21,526 peer-review elements were completed by pharmacists from fiscal year 2015 through 2018. Peer-review elements assessed
Conclusion
Pharmacists have become increasingly instrumental in providing effective, cost-efficient, and accessible clinical services by continuing to move toward expanding and evolving roles within comprehensive, patient-centered clinical pharmacy practice settings.5,6 Multifaceted clinical responsibilities associated with health care delivery necessitate assessment and monitoring of pharmacist performance. Credentialing and privileging is an established and trusted systematic process that assures HCPs, employers, and patients that pharmacists are qualified and competent to practice clinically.2,4,12 Implementation of professional practice evaluations suggest improved staff compliance with visit documentation, patient care standards, and clinic processes required by CPAs, policies, and department standards to ensure the delivery of safe, high-quality patient care.
The Red Lake Indian Health Service (IHS) health care facility is in north-central Minnesota within the Red Lake Nation. The facility supports primary care, emergency, urgent care, pharmacy, inpatient, optometry, dental, radiology, laboratory, physical therapy, and behavioral health services to about 10,000 Red Lake Band of Chippewa Indian patients. The Red Lake pharmacy provides inpatient and outpatient medication services and pharmacist-managed clinical patient care.
In 2013, the Red Lake IHS medical staff endorsed the implementation of comprehensive clinical pharmacy services to increase health care access and optimize clinical outcomes for patients. During the evolution of pharmacy-based patient-centric care, the clinical programs offered by Red Lake IHS pharmacy expanded from 1 anticoagulation clinic to multiple advanced-practice clinical pharmacy services. This included pharmacy primary care, medication-assisted therapy, naloxone, hepatitis C, and behavioral health medication management clinics.
The immense clinical growth of the pharmacy department demonstrated a need to assess and monitor pharmacist competency to ensure the delivery of quality patient care. Essential quality improvement processes were lacking. To fill these quality improvement gaps, a robust pharmacist credentialing and privileging program was implemented in 2015.
Patient Care
As efforts within health care establishments across the US focus on the delivery of efficient, high-quality, affordable health care, pharmacists have become increasingly instrumental in providing patient care within expanded clinical roles.1-8 Many clinical pharmacy models have evolved into interdisciplinary approaches to care.9 Within these models, abiding by state and federal laws, pharmacists practice under the indirect supervision of licensed independent practitioners (LIPs), such as physicians, nurse practitioners, and physician assistants.8 Under collaborative practice agreements (CPAs), patients are initially diagnosed by LIPs, then referred to clinical pharmacists for therapeutic management.5,7
Clinical pharmacist functions encompass comprehensive medication management (ie, prescribing, monitoring, and adjustment of medications), nonpharmacologic guidance, and coordination of care. Interdisciplinary collaboration allows pharmacists opportunities to provide direct patient care or consultations by telecommunication in many different clinical environments, including disease management, primary care, or specialty care. Pharmacists may manage chronic or acute illnesses associated with endocrine, cardiovascular, respiratory, gastrointestinal, or other systems.
Pharmacists may also provide comprehensive medication review services, such as medication therapy management (MTM), transitions of care, or chronic care management. Examples of specialized areas include psychiatric, opioid use disorder, palliative care, infectious disease, chronic pain, or oncology services. For hospitalized patients, pharmacists may monitor pharmacokinetics and adjust dosing, transition patients from IV to oral medications, or complete medication reconciliation.10 Within these clinical roles, pharmacists assist in providing patient care during shortages of other health care providers (HCPs), improve patient outcomes, decrease health care-associated costs by preventing emergency department and hospital admissions or readmissions, increase access to patient care, and increase revenue through pharmacist-managed clinics and services.11
Pharmacist Credentialing
With the advancement of modern clinical pharmacy practice, many pharmacists have undertaken responsibilities to fulfill the complex duties of clinical care and diverse patient situations, but with few or no requirements to prove initial or ongoing clinical competency.2 Traditionally, pharmacist credentialing is limited to a onetime or periodic review of education and licensure, with little to no involvement in privileging and ongoing monitoring of clinical proficiency.10 These quality assurance disparities can be met and satisfied through credentialing and privileging processes. Credentialing and privileging are systematic, evidence-based processes that provide validation to HCPs, employers, and patients that pharmacists are qualified to practice clinically. 2,9 According to the Council on Credentialing in Pharmacy, clinical pharmacists should be held accountable for demonstrating competency and providing quality care through credentialing and privileging, as required for other HCPs.2,12
Credentialing and recredentialing is a primary source verification process. These processes ensure that there are no license restrictions or revocations; certifications are current; mandatory courses, certificates, and continuing education are complete; training and orientation are satisfactory; and any disciplinary action, malpractice claims, or history of impairment is reported. Privileging is the review of credentials and evaluation of clinical training and competence by the Clinical Director and Medical Executive Committee to determine whether a clinical pharmacist is competent to practice within requested privileges.11
Credentialing and privileging processes are designed not only to initially confirm that a pharmacist is competent to practice clinically, but also monitor ongoing performance.2,13 Participation in professional practice evaluations, which includes peer reviews, ongoing professional practice evaluations, and focused professional practice evaluations, is required for all credentialed and privileged practitioners. These evaluations are used to identify, assess, and correct unsatisfactory trends. Individual practices, documentation, and processes are evaluated against existing department standards (eg, CPAs, policies, processes)11,13 The results of individual professional practice evaluations are reviewed with practitioners on a regular basis and performance improvement plans implemented as needed.
Since 2015, 17 pharmacists at the Red Lake IHS health care facility have been granted membership to the medical staff as credentialed and privileged practitioners. In a retrospective review of professional practice evaluations by the Red Lake IHS pharmacy clinical coordinator, 971 outpatient clinical peer reviews, including the evaluation of 21,526 peer-review elements were completed by pharmacists from fiscal year 2015 through 2018. Peer-review elements assessed
Conclusion
Pharmacists have become increasingly instrumental in providing effective, cost-efficient, and accessible clinical services by continuing to move toward expanding and evolving roles within comprehensive, patient-centered clinical pharmacy practice settings.5,6 Multifaceted clinical responsibilities associated with health care delivery necessitate assessment and monitoring of pharmacist performance. Credentialing and privileging is an established and trusted systematic process that assures HCPs, employers, and patients that pharmacists are qualified and competent to practice clinically.2,4,12 Implementation of professional practice evaluations suggest improved staff compliance with visit documentation, patient care standards, and clinic processes required by CPAs, policies, and department standards to ensure the delivery of safe, high-quality patient care.
1. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed March 15, 2019.
2. Rouse MJ, Vlasses PH, Webb CE; Council on Credentialing in Pharmacy. Credentialing and privileging of pharmacists: a resource paper from the Council on Credentialing in Pharmacy. Am J Health Syst Pharm. 2014;71(21):e109-e118.
3. Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759-769.
4. Blair MM, Carmichael J, Young E, Thrasher K; Qualified Provider Model Ad Hoc Committee. Pharmacist privileging in a health system: report of the Qualified Provider Model Ad Hoc Committee. Am J Health Syst Pharm. 2007;64(22):2373-2381.
5. Claxton KI, Wojtal P. Design and implementation of a credentialing and privileging model for ambulatory care pharmacists. Am J Health Syst Pharm. 2006;63(17):1627-1632.
6. Jordan TA, Hennenfent JA, Lewin JJ III, Nesbit TW, Weber R. Elevating pharmacists’ scope of practice through a health-system clinical privileging process. Am J Health Syst Pharm. 2016;73(18):1395-1405.
7. Centers for Disease Control and Prevention. Collaborative practice agreements and pharmacists’ patient care services: a resource for doctors, nurses, physician assistants, and other providers. https://www.cdc.gov/dhdsp/pubs/docs/Translational_Tools_Providers.pdf. Published October 2013. Accessed March 18, 2019.
8. Council on Credentialing in Pharmacy, Albanese NP, Rouse MJ. Scope of contemporary pharmacy practice: roles, responsibilities, and functions of practitioners and pharmacy technicians. J Am Pharm Assoc (2003). 2010;50(2):e35-e69.
9. Philip B, Weber R. Enhancing pharmacy practice models through pharmacists’ privileging. Hosp Pharm. 2013; 48(2):160-165.
10. Galt KA. Credentialing and privileging of pharmacists. Am J Health Syst Pharm. 2004;61(7):661-670.
11. Smith ML, Gemelas MF; US Public Health Service; Indian Health Service. Indian Health Service medical staff credentialing and privileging guide. https://www.ihs.gov/riskmanagement/includes/themes/newihstheme/display_objects/documents/IHS-Medical-Staff-Credentialing-and-Privileging-Guide.pdf. Published September 2005. Accessed March 15, 2019.
12. US Department of Health and Human Services, Indian Health Service. Indian health manual: medical credentials and privileges review process. https://www.ihs.gov/ihm/pc/part-3/p3c1. Accessed March 15, 2019.
13. Holley SL, Ketel C. Ongoing professional practice evaluation and focused professional practice evaluation: an overview for advanced practice clinicians. J Midwifery Women Health. 2014;59(4):452-459.
1. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed March 15, 2019.
2. Rouse MJ, Vlasses PH, Webb CE; Council on Credentialing in Pharmacy. Credentialing and privileging of pharmacists: a resource paper from the Council on Credentialing in Pharmacy. Am J Health Syst Pharm. 2014;71(21):e109-e118.
3. Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759-769.
4. Blair MM, Carmichael J, Young E, Thrasher K; Qualified Provider Model Ad Hoc Committee. Pharmacist privileging in a health system: report of the Qualified Provider Model Ad Hoc Committee. Am J Health Syst Pharm. 2007;64(22):2373-2381.
5. Claxton KI, Wojtal P. Design and implementation of a credentialing and privileging model for ambulatory care pharmacists. Am J Health Syst Pharm. 2006;63(17):1627-1632.
6. Jordan TA, Hennenfent JA, Lewin JJ III, Nesbit TW, Weber R. Elevating pharmacists’ scope of practice through a health-system clinical privileging process. Am J Health Syst Pharm. 2016;73(18):1395-1405.
7. Centers for Disease Control and Prevention. Collaborative practice agreements and pharmacists’ patient care services: a resource for doctors, nurses, physician assistants, and other providers. https://www.cdc.gov/dhdsp/pubs/docs/Translational_Tools_Providers.pdf. Published October 2013. Accessed March 18, 2019.
8. Council on Credentialing in Pharmacy, Albanese NP, Rouse MJ. Scope of contemporary pharmacy practice: roles, responsibilities, and functions of practitioners and pharmacy technicians. J Am Pharm Assoc (2003). 2010;50(2):e35-e69.
9. Philip B, Weber R. Enhancing pharmacy practice models through pharmacists’ privileging. Hosp Pharm. 2013; 48(2):160-165.
10. Galt KA. Credentialing and privileging of pharmacists. Am J Health Syst Pharm. 2004;61(7):661-670.
11. Smith ML, Gemelas MF; US Public Health Service; Indian Health Service. Indian Health Service medical staff credentialing and privileging guide. https://www.ihs.gov/riskmanagement/includes/themes/newihstheme/display_objects/documents/IHS-Medical-Staff-Credentialing-and-Privileging-Guide.pdf. Published September 2005. Accessed March 15, 2019.
12. US Department of Health and Human Services, Indian Health Service. Indian health manual: medical credentials and privileges review process. https://www.ihs.gov/ihm/pc/part-3/p3c1. Accessed March 15, 2019.
13. Holley SL, Ketel C. Ongoing professional practice evaluation and focused professional practice evaluation: an overview for advanced practice clinicians. J Midwifery Women Health. 2014;59(4):452-459.
Don’t delay palliative care for IPF patients
and indicates that early, integrated palliative care should be a priority, according to the finding of a survey study.
“Patients with IPF suffer from exceptionally low [health-related quality of life] together with severe breathlessness and fatigue already two years before death. In addition, physical and emotional well-being further deteriorates near death concurrently with escalating overall symptom burden,” wrote Kaisa Rajala, MD, and her colleagues at Helsinki University Hospital.
They conducted a substudy of patients in the larger FinnishIPF study to assess health-related quality of life (HRQOL) and symptom burden in the period before death. Among 300 patients invited to participate, 247 agreed. Patient disease and sociodemographic data were collected from the FinnishIPF records and the study group completed questionnaires five times at 6 month intervals. The study began in April 2015 and continued until August 2017, by which time 92 (37%) of the patients had died (BMC Pulmonary Medicine 2018;18:172; doi: 0.1186/s12890-018-0738-x).
The investigators used self-reporting tools to look at HRQOL and symptom burden: RAND 36-item Health Survey (RAND-36), the Modified Medical Research and Council Dyspnea Scale (MMRC), the Modified Edmonton Symptom Assessment Scale (ESAS), and the Numeric Rating Scale (NRS).
About 35% of these patients were being treated with antifibrotic medication. Most of the patients had comorbidities, with cardiovascular disease being the most common.
The dimensions of HRQOL studied were physical function, general health, vitality, mental health, social function, and bodily pain. These patients experienced a gradual impairment in HRQOL similar to that of patients with chronic obstructive pulmonary disease, but with a pronounced, rapid deterioration beginning in the last 2 years of life.
The symptom burden also intensified in the last 2 years of life and ramped up significantly in the last 6 months before death. NRS scores are on a scale of 0-10, from no symptoms to worst symptoms. In most clinical situations, NRS scores equal to greater than 4 trigger more comprehensive symptom assessment. The scores for symptoms for these patients during the last 6 months were dyspnea, 7.1 (standard deviation 2.8); tiredness, 6.0 (SD 2.5), cough, 5.0 (SD 3.5), pain with movement, 3.9 (SD 3.1), insomnia, 3.9 (SD 2.9), anxiety, 3.9 (SD 2.9), and depression, 3.6 (SD 3.1).
Investigators noted the steep change in the proportion of patients with MMRC scores greater than or equal to 3 (needing to stop walking after approximately 100 m or a few minutes because of breathlessness) beginning in the last 2 years of life.
The study limitations are its relatively small size, the self-reported data, and the lack of lung function measurements in most patients in the last 6 months of life.
The findings point to the urgent need for early palliative care in IPF patients, the investigators concluded. They noted that the sharp decline in HRQOL is similar to that seen in lung cancer patients, in contrast to the more gradual trend seen in COPD patients.
But there are common benefits of an early palliative program for all of these patients, they stressed. “Early integrated palliative care for patients with lung cancer has shown substantial benefits, such as lower depression scores, higher HRQOL, better communication of end-of-life care preferences, less aggressive care at the end of life, and longer overall survival. Similarly, a randomized trial demonstrated better control of dyspnea and a survival benefit with integrated palliative care in patients with COPD and interstitial lung disease. In addition to cancer patients, early integrated palliative care may reduce end-of-life acute care utilization, and allow patients with IPF to die in their preferred locations. Integrated palliative care in IPF patients seems to lower respiratory-related emergency room visits and hospitalizations and may allow more patients to die at home.”
The study was funded by The Academy of Finland and various Finnish nonprofit organizations funded the study.
SOURCE: Rajala K et al. BMC Pulm Med. 2018;18:172. doi: 0.1186/s12890-018-0738-x.
and indicates that early, integrated palliative care should be a priority, according to the finding of a survey study.
“Patients with IPF suffer from exceptionally low [health-related quality of life] together with severe breathlessness and fatigue already two years before death. In addition, physical and emotional well-being further deteriorates near death concurrently with escalating overall symptom burden,” wrote Kaisa Rajala, MD, and her colleagues at Helsinki University Hospital.
They conducted a substudy of patients in the larger FinnishIPF study to assess health-related quality of life (HRQOL) and symptom burden in the period before death. Among 300 patients invited to participate, 247 agreed. Patient disease and sociodemographic data were collected from the FinnishIPF records and the study group completed questionnaires five times at 6 month intervals. The study began in April 2015 and continued until August 2017, by which time 92 (37%) of the patients had died (BMC Pulmonary Medicine 2018;18:172; doi: 0.1186/s12890-018-0738-x).
The investigators used self-reporting tools to look at HRQOL and symptom burden: RAND 36-item Health Survey (RAND-36), the Modified Medical Research and Council Dyspnea Scale (MMRC), the Modified Edmonton Symptom Assessment Scale (ESAS), and the Numeric Rating Scale (NRS).
About 35% of these patients were being treated with antifibrotic medication. Most of the patients had comorbidities, with cardiovascular disease being the most common.
The dimensions of HRQOL studied were physical function, general health, vitality, mental health, social function, and bodily pain. These patients experienced a gradual impairment in HRQOL similar to that of patients with chronic obstructive pulmonary disease, but with a pronounced, rapid deterioration beginning in the last 2 years of life.
The symptom burden also intensified in the last 2 years of life and ramped up significantly in the last 6 months before death. NRS scores are on a scale of 0-10, from no symptoms to worst symptoms. In most clinical situations, NRS scores equal to greater than 4 trigger more comprehensive symptom assessment. The scores for symptoms for these patients during the last 6 months were dyspnea, 7.1 (standard deviation 2.8); tiredness, 6.0 (SD 2.5), cough, 5.0 (SD 3.5), pain with movement, 3.9 (SD 3.1), insomnia, 3.9 (SD 2.9), anxiety, 3.9 (SD 2.9), and depression, 3.6 (SD 3.1).
Investigators noted the steep change in the proportion of patients with MMRC scores greater than or equal to 3 (needing to stop walking after approximately 100 m or a few minutes because of breathlessness) beginning in the last 2 years of life.
The study limitations are its relatively small size, the self-reported data, and the lack of lung function measurements in most patients in the last 6 months of life.
The findings point to the urgent need for early palliative care in IPF patients, the investigators concluded. They noted that the sharp decline in HRQOL is similar to that seen in lung cancer patients, in contrast to the more gradual trend seen in COPD patients.
But there are common benefits of an early palliative program for all of these patients, they stressed. “Early integrated palliative care for patients with lung cancer has shown substantial benefits, such as lower depression scores, higher HRQOL, better communication of end-of-life care preferences, less aggressive care at the end of life, and longer overall survival. Similarly, a randomized trial demonstrated better control of dyspnea and a survival benefit with integrated palliative care in patients with COPD and interstitial lung disease. In addition to cancer patients, early integrated palliative care may reduce end-of-life acute care utilization, and allow patients with IPF to die in their preferred locations. Integrated palliative care in IPF patients seems to lower respiratory-related emergency room visits and hospitalizations and may allow more patients to die at home.”
The study was funded by The Academy of Finland and various Finnish nonprofit organizations funded the study.
SOURCE: Rajala K et al. BMC Pulm Med. 2018;18:172. doi: 0.1186/s12890-018-0738-x.
and indicates that early, integrated palliative care should be a priority, according to the finding of a survey study.
“Patients with IPF suffer from exceptionally low [health-related quality of life] together with severe breathlessness and fatigue already two years before death. In addition, physical and emotional well-being further deteriorates near death concurrently with escalating overall symptom burden,” wrote Kaisa Rajala, MD, and her colleagues at Helsinki University Hospital.
They conducted a substudy of patients in the larger FinnishIPF study to assess health-related quality of life (HRQOL) and symptom burden in the period before death. Among 300 patients invited to participate, 247 agreed. Patient disease and sociodemographic data were collected from the FinnishIPF records and the study group completed questionnaires five times at 6 month intervals. The study began in April 2015 and continued until August 2017, by which time 92 (37%) of the patients had died (BMC Pulmonary Medicine 2018;18:172; doi: 0.1186/s12890-018-0738-x).
The investigators used self-reporting tools to look at HRQOL and symptom burden: RAND 36-item Health Survey (RAND-36), the Modified Medical Research and Council Dyspnea Scale (MMRC), the Modified Edmonton Symptom Assessment Scale (ESAS), and the Numeric Rating Scale (NRS).
About 35% of these patients were being treated with antifibrotic medication. Most of the patients had comorbidities, with cardiovascular disease being the most common.
The dimensions of HRQOL studied were physical function, general health, vitality, mental health, social function, and bodily pain. These patients experienced a gradual impairment in HRQOL similar to that of patients with chronic obstructive pulmonary disease, but with a pronounced, rapid deterioration beginning in the last 2 years of life.
The symptom burden also intensified in the last 2 years of life and ramped up significantly in the last 6 months before death. NRS scores are on a scale of 0-10, from no symptoms to worst symptoms. In most clinical situations, NRS scores equal to greater than 4 trigger more comprehensive symptom assessment. The scores for symptoms for these patients during the last 6 months were dyspnea, 7.1 (standard deviation 2.8); tiredness, 6.0 (SD 2.5), cough, 5.0 (SD 3.5), pain with movement, 3.9 (SD 3.1), insomnia, 3.9 (SD 2.9), anxiety, 3.9 (SD 2.9), and depression, 3.6 (SD 3.1).
Investigators noted the steep change in the proportion of patients with MMRC scores greater than or equal to 3 (needing to stop walking after approximately 100 m or a few minutes because of breathlessness) beginning in the last 2 years of life.
The study limitations are its relatively small size, the self-reported data, and the lack of lung function measurements in most patients in the last 6 months of life.
The findings point to the urgent need for early palliative care in IPF patients, the investigators concluded. They noted that the sharp decline in HRQOL is similar to that seen in lung cancer patients, in contrast to the more gradual trend seen in COPD patients.
But there are common benefits of an early palliative program for all of these patients, they stressed. “Early integrated palliative care for patients with lung cancer has shown substantial benefits, such as lower depression scores, higher HRQOL, better communication of end-of-life care preferences, less aggressive care at the end of life, and longer overall survival. Similarly, a randomized trial demonstrated better control of dyspnea and a survival benefit with integrated palliative care in patients with COPD and interstitial lung disease. In addition to cancer patients, early integrated palliative care may reduce end-of-life acute care utilization, and allow patients with IPF to die in their preferred locations. Integrated palliative care in IPF patients seems to lower respiratory-related emergency room visits and hospitalizations and may allow more patients to die at home.”
The study was funded by The Academy of Finland and various Finnish nonprofit organizations funded the study.
SOURCE: Rajala K et al. BMC Pulm Med. 2018;18:172. doi: 0.1186/s12890-018-0738-x.
FROM BMC PULMONARY MEDICINE
New Diagnostic Procedure Codes and Reimbursement
As the US population continues to grow and patients become more aware of their health needs, payers are beginning to recognize the benefits of more efficient and cost-effective health care. With the implementation of the new Medicare Physician Fee Schedule on January 1, 2019, some old billing codes were revalued while others were replaced entirely with new codes.1 The restructuring of the standard biopsy codes now takes the complexity of different sampling techniques into consideration. Furthermore, Current Procedural Terminology (CPT) Category III tracking codes for some imaging devices (eg, optical coherence tomography) added in 2017 require more data before obtaining a Category I reimbursable code, while codes for other imaging devices such as reflectance confocal microscopy (RCM) remain relatively the same.2-4 Notably, the majority of the new 2019 telemedicine codes are applicable to dermatology.2,3 In this article, we discuss the new CPT codes for reporting diagnostic procedures, including biopsy, noninvasive imaging, and telemedicine services. We also provide a summary of the national average reimbursement rates for these procedures.
Background on Reimbursement
To better understand how reimbursement works, it is important to know that all billing codes are provided a relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.5 The total RVU consists of the work RVU (wRVU), practice expense RVU (peRVU), and malpractice expense RVU (mRVU). The wRVU represents the time, effort, and complexity involved in performing the service. The peRVU reflects the direct cost of supplies, personnel, and durable equipment involved in providing the service, excluding typical office overhead costs such as rent, utilities, and administrative staff. The mRVU is to cover the cost of malpractice insurance.5 The peRVU can be further specified as facility versus nonfacility services depending on where the service is performed.6 A facility peRVU is for services completed in a facility such as a hospital, outpatient hospital setting, or nursing home. The facility provides some of the involved supplies, personnel, and equipment for which they can recapture costs by separate reporting, resulting in a lower total RVU for the provider charges compared with nonfacility locations where the physician must provide these items.6 Many physicians may not be aware of how critical their role is in determining their own reimbursement rates by understanding RVUs and properly filling out Relative Value Scale Update Committee (RUC) surveys. If surveys sent to practitioners are accurately completed, RVUs have the potential to be fairly valued; however, if respondents are unaware of all of the components that are inherent to a procedure, they may end up minimizing the effort or time involved, which would skew the results and hurt those who perform the procedure. Rather than inputting appropriate preoperative and postoperative service times, many respondents often put 0s and 1s throughout the survey, which misrepresents the amount of time involved for a procedure. For example, inputting a preoperative time as 0 or 1 minute may severely underestimate the work involved for a procedure if the true preoperative time is 5 minutes. Such survey responses affect whether or not RVUs are valued appropriately.
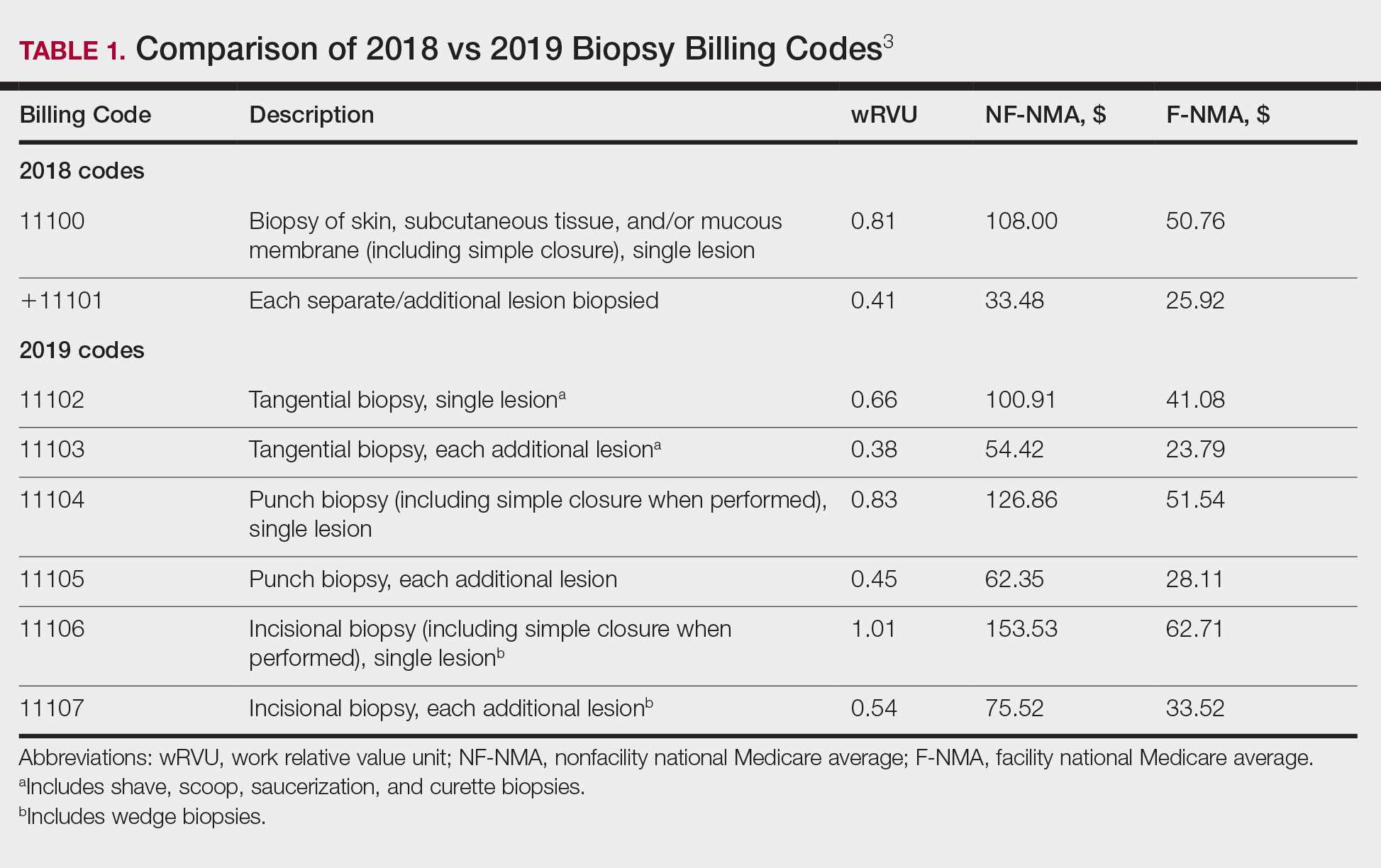
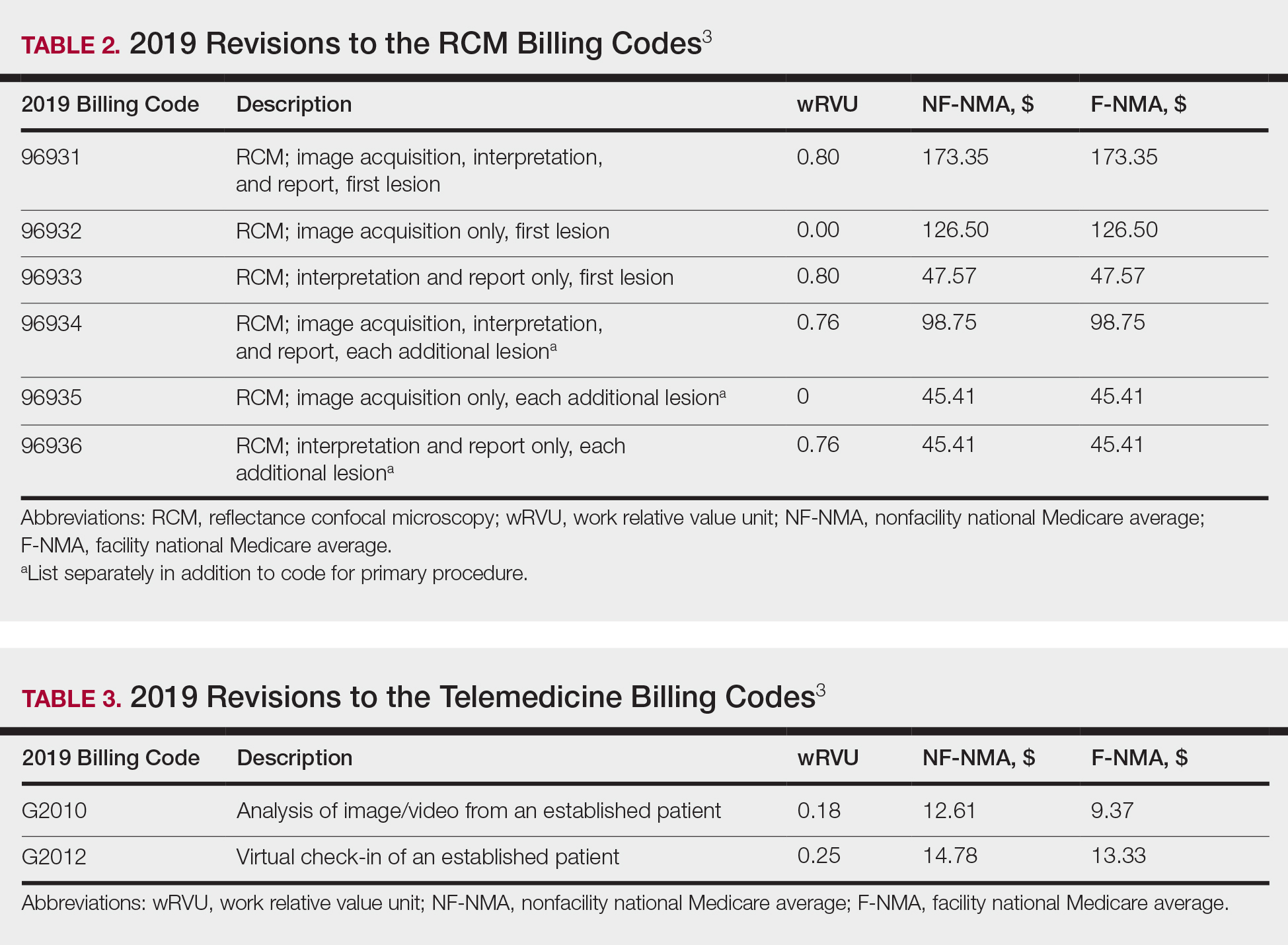
The billing codes and their RVUs as well as Medicare payment values in your area can be found on the Centers for Medicare & Medicaid Services website.2,3 Table 1 provides a comparison of the old and new biopsy codes, and Table 2 shows the new RCM codes.
Biopsy Codes
Prior to 2019, biopsies were reimbursed using CPT code 11100 for the initial biopsy and 11101 for each additional biopsy.2 Called up for refinement in the RUC process, initial data from the Physician Practice Expense Information Survey pointed to the likelihood of different sampling techniques having different amounts of work being supplied by different techniques.1 Imaging modalities such as dermoscopy or RCM could help minimize the need for surgical biopsies. Dermoscopy, which has been proven to allow for more efficient and accurate diagnoses in dermatology, is reimbursed in Europe but not in the United States.7-9 In 2016, CPT codes 96931 through 96936 were created for RCM and are covered by most insurances.10 Optical coherence tomography, another noninvasive imaging technology, currently is not reimbursed but did receive Category III codes (0470T-0471T), also known as a tracking codes, in 2017.4 Category III codes are used for emerging technologies that have future potential but do not have enough US-based evidence to support receiving Category I CPT codes. The use of Category III codes allows for data collection on emerging technologies and services, with the potential to convert the Category III codes to Category I codes once certain criteria are met.11
Beginning in 2019, the standard biopsy codes 11100 and 11101 were replaced with 6 new codes to represent primary (11102, 11104, 11106) and add-on biopsies (11103, 11105, 11107) based on the sampling technique utilized and the thickness of the sample (Table 1). Previously, the biopsy codes did not reflect the complexity of the different biopsy techniques, whereas the new codes provide differentiation of the method of removal (ie, tangential, punch, incisional).2,3 The base code is dependent on whichever biopsy performed has the highest complexity, with incisional biopsy--a partial excision--being considered the most complex.3 Punch biopsy is considered the next level of complexity, followed by tangential biopsy. Each of the 6 new biopsy codes also received a new wRVU, which determines reimbursement under Medicare and most other insurers when combined with direct peRVU and mRVU. Additional biopsies, reported using the add-on codes, are reimbursed at a lower level than the base codes because of removal of duplicate inputs for preservice and postservice care.3
Telehealth Codes
Telemedicine services offer another form of imaging that providers can use to communicate remotely with patients through a live interactive video stream (with audio), a store-and-forward system with photographs or videos shared asynchronously, or remote patient monitoring.12 Although live video streaming uses a webcam, store-and-forward services involve sending photographs or videos electronically for later evaluation.12,13 Remote patient monitoring allows the collection of health-related data and transmission to a physician without the need for an office visit.13 Most states require physicians to have a license in the state in which the patient is located at the time of the encounter. Given the difficulty of applying for licensure in multiple states, several states started creating their own special licenses to allow out-of-state providers to offer services through telemedicine.14 The Federation of State Medical Boards then created the Interstate Medical Licensure Compact (IMLC) for an expedited process to apply for medical licensure in other states. The IMLC was formed to increase access to health care in underserved or rural areas including but not limited to the use of telemedicine.15 To qualify for IMLC, a physician must have a medical license in a state registered with the IMLC (ie, state of principal license) and have at least one of the following in their state of principal license: primary residence, 25% of their medical practice, a current employer, or US federal income taxes filed.15 The remaining states that do not have a licensing process for telemedicine allow practice in contiguous states or may provide temporary licenses dependent on the situation.14
Since 2017, billing codes for telemedicine have been the same as those used for in-person evaluation and management services with modifiers -95 or GQ added to the end of the code. Modifier -95 has been used for real-time telemedicine services, while modifier GQ has been used for store-and-forward services.16 For example, the code 99201, which is used to bill for new patients at outpatient visits, would become 99201-95 if performed using a live audio and video feed or 99201-GQ if information was sent electronically for later analysis. To receive reimbursement from Medicare, modifier -95 requires real-time communication using both audio and video; however, modifier GQ is only reimbursable in federal telemedicine demonstration programs in Alaska or Hawaii.12 Note that reimbursement is up to the discretion of private providers, and even Medicare reimbursement can vary from state to state.
In 2019, new Healthcare Common Procedure Coding System telemedicine codes were introduced to include virtual check-ins (G2012) and evaluation of patient-transmitted images and videos (G2010). G2010 is the first store-and-forward code that has the potential to be reimbursed outside of Alaska or Hawaii.3,12 G2012 allows providers to monitor the patients' well-being outside of the office setting, a cost-effective alternative if patients do not require a full visit. More detailed descriptions of the new codes can be found in Table 3.1
Final Thoughts
As insurance providers continue to better monitor health care costs, it is of utmost importance that physicians become more involved in accurately assessing their services and procedures, given that the changes in RVUs mirror the Centers for Medicare & Medicaid Services' utilization of the RUC's interpretation of our survey responses.1 The current billing codes attempt to better represent the work involved for each service, one example being the modification to more specific biopsy codes in 2019.
With the growth of technology, CPT and Healthcare Common Procedure Coding System codes also reflect a push toward more efficient health care delivery and broader coverage for provider services, as demonstrated by the introduction of new telemedicine codes as well as recent additions of noninvasive imaging codes. Although technology makes health care more cost-effective for patients, clinicians can still maintain their overall reimbursements by efficiently seeing an increasing number of patients; for example, a patient diagnosed noninvasively using RCM can then receive same-day care, which impacts patients' quality of life by minimizing travel time, number of office visits, and time taken off from work, while allowing providers to manage a higher patient volume more productively. The new CPT codes discussed here reflect the growth of medical technology potential, which increases our diagnostic capability, making it even more critical for physicians to engage with these developments.
- Centers for Medicare & Medicaid Services. Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program--Extreme and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the Medicare Shared Savings Program-- Accountable Care Organizations--Pathways to Success; and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. Fed Registr. 2018;83(226):59452-60303. To be codified at 42 CFR §405, 410, 411, 414, 415, 425, and 495.
- Centers for Medicare & Medicaid Services. CY 2018 PFS Final Rule Addenda. https://www.cms.gov/Medicare/Medicare-Fee-for-Service Payment/PhysicianFeeSched/Downloads/CY2018-PFS-FR-Addenda.zip. Published 2018. Accessed March 28, 2019.
- Overview: Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed March 28, 2019.
- Medicare Learning Network. July 2017 update of the hospital outpatient prospective payment system (OPPS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10122.pdf. Published 2017. Accessed March 21, 2019.
- Medicare Learning Network. Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/medcrephysfeeschedfctsht.pdf. Published February 2017. Accessed March 19, 2019.
- Medicare Learning Network. How to use the searchable Medicare Physician Fee Schedule (MPFS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/How_to_MPFS_Booklet_ICN901344.pdf. Published September 2017. Accessed March 19, 2019.
- Fox GN. Dermoscopy: an invaluable tool for evaluating skin lesions. Am Fam Physician. 2008;78:704, 706.
- Soyer HP, Argenziano G, Talamini R, et al. Is dermoscopy useful for the diagnosis of melanoma? Arch Dermatol. 2001;137:1361-1363.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists' perspective. Dermatology. 2012;225:289-293.
- American Academy of Dermatology Association. New CPT coding updates for 2016. Derm Coding Consult. 2015;19:1-2. https://www.aad.org/File Library/Main navigation/Member resources and programs/Publications/DCC/DCC_Winter_2015.pdf. Published 2014. Accessed March 21, 2019.
- American Medical Association. CPT Category III codes. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/physicians/cpt/cpt-category3-codes-long-descriptors.pdf. Updated July 26, 2018. Accessed March 21, 2019.
- Medicare Learning Network. Telehealth services. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf. Accessed March 19, 2019.
- Final policy, payment, and quality provisions in the Medicare Physician Fee Schedule for calendar year 2018. Centers for Medicare & Medicaid Services website. https://www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-medicare-physician-fee-schedule-calendar-year-2018. Published November 2, 2017. Accessed March 19, 2019.
- State Telehealth Laws & Reimbursement Policies. Sacramento, CA: Center for Connected Health Policy; 2018. https://www.cchpca.org/sites/default/files/2018-10/CCHP_50_State_Report_Fall_2018.pdf. Accessed March 19, 2019.
- The IMLC. Interstate Medical Licensure Compact website. https://imlcc.org/. Accessed March 19, 2019.
- Current Procedural Terminology 2018, Professional Edition. Chicago, IL: American Medical Association; 2018.
As the US population continues to grow and patients become more aware of their health needs, payers are beginning to recognize the benefits of more efficient and cost-effective health care. With the implementation of the new Medicare Physician Fee Schedule on January 1, 2019, some old billing codes were revalued while others were replaced entirely with new codes.1 The restructuring of the standard biopsy codes now takes the complexity of different sampling techniques into consideration. Furthermore, Current Procedural Terminology (CPT) Category III tracking codes for some imaging devices (eg, optical coherence tomography) added in 2017 require more data before obtaining a Category I reimbursable code, while codes for other imaging devices such as reflectance confocal microscopy (RCM) remain relatively the same.2-4 Notably, the majority of the new 2019 telemedicine codes are applicable to dermatology.2,3 In this article, we discuss the new CPT codes for reporting diagnostic procedures, including biopsy, noninvasive imaging, and telemedicine services. We also provide a summary of the national average reimbursement rates for these procedures.
Background on Reimbursement
To better understand how reimbursement works, it is important to know that all billing codes are provided a relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.5 The total RVU consists of the work RVU (wRVU), practice expense RVU (peRVU), and malpractice expense RVU (mRVU). The wRVU represents the time, effort, and complexity involved in performing the service. The peRVU reflects the direct cost of supplies, personnel, and durable equipment involved in providing the service, excluding typical office overhead costs such as rent, utilities, and administrative staff. The mRVU is to cover the cost of malpractice insurance.5 The peRVU can be further specified as facility versus nonfacility services depending on where the service is performed.6 A facility peRVU is for services completed in a facility such as a hospital, outpatient hospital setting, or nursing home. The facility provides some of the involved supplies, personnel, and equipment for which they can recapture costs by separate reporting, resulting in a lower total RVU for the provider charges compared with nonfacility locations where the physician must provide these items.6 Many physicians may not be aware of how critical their role is in determining their own reimbursement rates by understanding RVUs and properly filling out Relative Value Scale Update Committee (RUC) surveys. If surveys sent to practitioners are accurately completed, RVUs have the potential to be fairly valued; however, if respondents are unaware of all of the components that are inherent to a procedure, they may end up minimizing the effort or time involved, which would skew the results and hurt those who perform the procedure. Rather than inputting appropriate preoperative and postoperative service times, many respondents often put 0s and 1s throughout the survey, which misrepresents the amount of time involved for a procedure. For example, inputting a preoperative time as 0 or 1 minute may severely underestimate the work involved for a procedure if the true preoperative time is 5 minutes. Such survey responses affect whether or not RVUs are valued appropriately.
The billing codes and their RVUs as well as Medicare payment values in your area can be found on the Centers for Medicare & Medicaid Services website.2,3 Table 1 provides a comparison of the old and new biopsy codes, and Table 2 shows the new RCM codes.


Biopsy Codes
Prior to 2019, biopsies were reimbursed using CPT code 11100 for the initial biopsy and 11101 for each additional biopsy.2 Called up for refinement in the RUC process, initial data from the Physician Practice Expense Information Survey pointed to the likelihood of different sampling techniques having different amounts of work being supplied by different techniques.1 Imaging modalities such as dermoscopy or RCM could help minimize the need for surgical biopsies. Dermoscopy, which has been proven to allow for more efficient and accurate diagnoses in dermatology, is reimbursed in Europe but not in the United States.7-9 In 2016, CPT codes 96931 through 96936 were created for RCM and are covered by most insurances.10 Optical coherence tomography, another noninvasive imaging technology, currently is not reimbursed but did receive Category III codes (0470T-0471T), also known as a tracking codes, in 2017.4 Category III codes are used for emerging technologies that have future potential but do not have enough US-based evidence to support receiving Category I CPT codes. The use of Category III codes allows for data collection on emerging technologies and services, with the potential to convert the Category III codes to Category I codes once certain criteria are met.11
Beginning in 2019, the standard biopsy codes 11100 and 11101 were replaced with 6 new codes to represent primary (11102, 11104, 11106) and add-on biopsies (11103, 11105, 11107) based on the sampling technique utilized and the thickness of the sample (Table 1). Previously, the biopsy codes did not reflect the complexity of the different biopsy techniques, whereas the new codes provide differentiation of the method of removal (ie, tangential, punch, incisional).2,3 The base code is dependent on whichever biopsy performed has the highest complexity, with incisional biopsy--a partial excision--being considered the most complex.3 Punch biopsy is considered the next level of complexity, followed by tangential biopsy. Each of the 6 new biopsy codes also received a new wRVU, which determines reimbursement under Medicare and most other insurers when combined with direct peRVU and mRVU. Additional biopsies, reported using the add-on codes, are reimbursed at a lower level than the base codes because of removal of duplicate inputs for preservice and postservice care.3
Telehealth Codes
Telemedicine services offer another form of imaging that providers can use to communicate remotely with patients through a live interactive video stream (with audio), a store-and-forward system with photographs or videos shared asynchronously, or remote patient monitoring.12 Although live video streaming uses a webcam, store-and-forward services involve sending photographs or videos electronically for later evaluation.12,13 Remote patient monitoring allows the collection of health-related data and transmission to a physician without the need for an office visit.13 Most states require physicians to have a license in the state in which the patient is located at the time of the encounter. Given the difficulty of applying for licensure in multiple states, several states started creating their own special licenses to allow out-of-state providers to offer services through telemedicine.14 The Federation of State Medical Boards then created the Interstate Medical Licensure Compact (IMLC) for an expedited process to apply for medical licensure in other states. The IMLC was formed to increase access to health care in underserved or rural areas including but not limited to the use of telemedicine.15 To qualify for IMLC, a physician must have a medical license in a state registered with the IMLC (ie, state of principal license) and have at least one of the following in their state of principal license: primary residence, 25% of their medical practice, a current employer, or US federal income taxes filed.15 The remaining states that do not have a licensing process for telemedicine allow practice in contiguous states or may provide temporary licenses dependent on the situation.14
Since 2017, billing codes for telemedicine have been the same as those used for in-person evaluation and management services with modifiers -95 or GQ added to the end of the code. Modifier -95 has been used for real-time telemedicine services, while modifier GQ has been used for store-and-forward services.16 For example, the code 99201, which is used to bill for new patients at outpatient visits, would become 99201-95 if performed using a live audio and video feed or 99201-GQ if information was sent electronically for later analysis. To receive reimbursement from Medicare, modifier -95 requires real-time communication using both audio and video; however, modifier GQ is only reimbursable in federal telemedicine demonstration programs in Alaska or Hawaii.12 Note that reimbursement is up to the discretion of private providers, and even Medicare reimbursement can vary from state to state.
In 2019, new Healthcare Common Procedure Coding System telemedicine codes were introduced to include virtual check-ins (G2012) and evaluation of patient-transmitted images and videos (G2010). G2010 is the first store-and-forward code that has the potential to be reimbursed outside of Alaska or Hawaii.3,12 G2012 allows providers to monitor the patients' well-being outside of the office setting, a cost-effective alternative if patients do not require a full visit. More detailed descriptions of the new codes can be found in Table 3.1
Final Thoughts
As insurance providers continue to better monitor health care costs, it is of utmost importance that physicians become more involved in accurately assessing their services and procedures, given that the changes in RVUs mirror the Centers for Medicare & Medicaid Services' utilization of the RUC's interpretation of our survey responses.1 The current billing codes attempt to better represent the work involved for each service, one example being the modification to more specific biopsy codes in 2019.
With the growth of technology, CPT and Healthcare Common Procedure Coding System codes also reflect a push toward more efficient health care delivery and broader coverage for provider services, as demonstrated by the introduction of new telemedicine codes as well as recent additions of noninvasive imaging codes. Although technology makes health care more cost-effective for patients, clinicians can still maintain their overall reimbursements by efficiently seeing an increasing number of patients; for example, a patient diagnosed noninvasively using RCM can then receive same-day care, which impacts patients' quality of life by minimizing travel time, number of office visits, and time taken off from work, while allowing providers to manage a higher patient volume more productively. The new CPT codes discussed here reflect the growth of medical technology potential, which increases our diagnostic capability, making it even more critical for physicians to engage with these developments.
As the US population continues to grow and patients become more aware of their health needs, payers are beginning to recognize the benefits of more efficient and cost-effective health care. With the implementation of the new Medicare Physician Fee Schedule on January 1, 2019, some old billing codes were revalued while others were replaced entirely with new codes.1 The restructuring of the standard biopsy codes now takes the complexity of different sampling techniques into consideration. Furthermore, Current Procedural Terminology (CPT) Category III tracking codes for some imaging devices (eg, optical coherence tomography) added in 2017 require more data before obtaining a Category I reimbursable code, while codes for other imaging devices such as reflectance confocal microscopy (RCM) remain relatively the same.2-4 Notably, the majority of the new 2019 telemedicine codes are applicable to dermatology.2,3 In this article, we discuss the new CPT codes for reporting diagnostic procedures, including biopsy, noninvasive imaging, and telemedicine services. We also provide a summary of the national average reimbursement rates for these procedures.
Background on Reimbursement
To better understand how reimbursement works, it is important to know that all billing codes are provided a relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.5 The total RVU consists of the work RVU (wRVU), practice expense RVU (peRVU), and malpractice expense RVU (mRVU). The wRVU represents the time, effort, and complexity involved in performing the service. The peRVU reflects the direct cost of supplies, personnel, and durable equipment involved in providing the service, excluding typical office overhead costs such as rent, utilities, and administrative staff. The mRVU is to cover the cost of malpractice insurance.5 The peRVU can be further specified as facility versus nonfacility services depending on where the service is performed.6 A facility peRVU is for services completed in a facility such as a hospital, outpatient hospital setting, or nursing home. The facility provides some of the involved supplies, personnel, and equipment for which they can recapture costs by separate reporting, resulting in a lower total RVU for the provider charges compared with nonfacility locations where the physician must provide these items.6 Many physicians may not be aware of how critical their role is in determining their own reimbursement rates by understanding RVUs and properly filling out Relative Value Scale Update Committee (RUC) surveys. If surveys sent to practitioners are accurately completed, RVUs have the potential to be fairly valued; however, if respondents are unaware of all of the components that are inherent to a procedure, they may end up minimizing the effort or time involved, which would skew the results and hurt those who perform the procedure. Rather than inputting appropriate preoperative and postoperative service times, many respondents often put 0s and 1s throughout the survey, which misrepresents the amount of time involved for a procedure. For example, inputting a preoperative time as 0 or 1 minute may severely underestimate the work involved for a procedure if the true preoperative time is 5 minutes. Such survey responses affect whether or not RVUs are valued appropriately.
The billing codes and their RVUs as well as Medicare payment values in your area can be found on the Centers for Medicare & Medicaid Services website.2,3 Table 1 provides a comparison of the old and new biopsy codes, and Table 2 shows the new RCM codes.


Biopsy Codes
Prior to 2019, biopsies were reimbursed using CPT code 11100 for the initial biopsy and 11101 for each additional biopsy.2 Called up for refinement in the RUC process, initial data from the Physician Practice Expense Information Survey pointed to the likelihood of different sampling techniques having different amounts of work being supplied by different techniques.1 Imaging modalities such as dermoscopy or RCM could help minimize the need for surgical biopsies. Dermoscopy, which has been proven to allow for more efficient and accurate diagnoses in dermatology, is reimbursed in Europe but not in the United States.7-9 In 2016, CPT codes 96931 through 96936 were created for RCM and are covered by most insurances.10 Optical coherence tomography, another noninvasive imaging technology, currently is not reimbursed but did receive Category III codes (0470T-0471T), also known as a tracking codes, in 2017.4 Category III codes are used for emerging technologies that have future potential but do not have enough US-based evidence to support receiving Category I CPT codes. The use of Category III codes allows for data collection on emerging technologies and services, with the potential to convert the Category III codes to Category I codes once certain criteria are met.11
Beginning in 2019, the standard biopsy codes 11100 and 11101 were replaced with 6 new codes to represent primary (11102, 11104, 11106) and add-on biopsies (11103, 11105, 11107) based on the sampling technique utilized and the thickness of the sample (Table 1). Previously, the biopsy codes did not reflect the complexity of the different biopsy techniques, whereas the new codes provide differentiation of the method of removal (ie, tangential, punch, incisional).2,3 The base code is dependent on whichever biopsy performed has the highest complexity, with incisional biopsy--a partial excision--being considered the most complex.3 Punch biopsy is considered the next level of complexity, followed by tangential biopsy. Each of the 6 new biopsy codes also received a new wRVU, which determines reimbursement under Medicare and most other insurers when combined with direct peRVU and mRVU. Additional biopsies, reported using the add-on codes, are reimbursed at a lower level than the base codes because of removal of duplicate inputs for preservice and postservice care.3
Telehealth Codes
Telemedicine services offer another form of imaging that providers can use to communicate remotely with patients through a live interactive video stream (with audio), a store-and-forward system with photographs or videos shared asynchronously, or remote patient monitoring.12 Although live video streaming uses a webcam, store-and-forward services involve sending photographs or videos electronically for later evaluation.12,13 Remote patient monitoring allows the collection of health-related data and transmission to a physician without the need for an office visit.13 Most states require physicians to have a license in the state in which the patient is located at the time of the encounter. Given the difficulty of applying for licensure in multiple states, several states started creating their own special licenses to allow out-of-state providers to offer services through telemedicine.14 The Federation of State Medical Boards then created the Interstate Medical Licensure Compact (IMLC) for an expedited process to apply for medical licensure in other states. The IMLC was formed to increase access to health care in underserved or rural areas including but not limited to the use of telemedicine.15 To qualify for IMLC, a physician must have a medical license in a state registered with the IMLC (ie, state of principal license) and have at least one of the following in their state of principal license: primary residence, 25% of their medical practice, a current employer, or US federal income taxes filed.15 The remaining states that do not have a licensing process for telemedicine allow practice in contiguous states or may provide temporary licenses dependent on the situation.14
Since 2017, billing codes for telemedicine have been the same as those used for in-person evaluation and management services with modifiers -95 or GQ added to the end of the code. Modifier -95 has been used for real-time telemedicine services, while modifier GQ has been used for store-and-forward services.16 For example, the code 99201, which is used to bill for new patients at outpatient visits, would become 99201-95 if performed using a live audio and video feed or 99201-GQ if information was sent electronically for later analysis. To receive reimbursement from Medicare, modifier -95 requires real-time communication using both audio and video; however, modifier GQ is only reimbursable in federal telemedicine demonstration programs in Alaska or Hawaii.12 Note that reimbursement is up to the discretion of private providers, and even Medicare reimbursement can vary from state to state.
In 2019, new Healthcare Common Procedure Coding System telemedicine codes were introduced to include virtual check-ins (G2012) and evaluation of patient-transmitted images and videos (G2010). G2010 is the first store-and-forward code that has the potential to be reimbursed outside of Alaska or Hawaii.3,12 G2012 allows providers to monitor the patients' well-being outside of the office setting, a cost-effective alternative if patients do not require a full visit. More detailed descriptions of the new codes can be found in Table 3.1
Final Thoughts
As insurance providers continue to better monitor health care costs, it is of utmost importance that physicians become more involved in accurately assessing their services and procedures, given that the changes in RVUs mirror the Centers for Medicare & Medicaid Services' utilization of the RUC's interpretation of our survey responses.1 The current billing codes attempt to better represent the work involved for each service, one example being the modification to more specific biopsy codes in 2019.
With the growth of technology, CPT and Healthcare Common Procedure Coding System codes also reflect a push toward more efficient health care delivery and broader coverage for provider services, as demonstrated by the introduction of new telemedicine codes as well as recent additions of noninvasive imaging codes. Although technology makes health care more cost-effective for patients, clinicians can still maintain their overall reimbursements by efficiently seeing an increasing number of patients; for example, a patient diagnosed noninvasively using RCM can then receive same-day care, which impacts patients' quality of life by minimizing travel time, number of office visits, and time taken off from work, while allowing providers to manage a higher patient volume more productively. The new CPT codes discussed here reflect the growth of medical technology potential, which increases our diagnostic capability, making it even more critical for physicians to engage with these developments.
- Centers for Medicare & Medicaid Services. Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program--Extreme and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the Medicare Shared Savings Program-- Accountable Care Organizations--Pathways to Success; and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. Fed Registr. 2018;83(226):59452-60303. To be codified at 42 CFR §405, 410, 411, 414, 415, 425, and 495.
- Centers for Medicare & Medicaid Services. CY 2018 PFS Final Rule Addenda. https://www.cms.gov/Medicare/Medicare-Fee-for-Service Payment/PhysicianFeeSched/Downloads/CY2018-PFS-FR-Addenda.zip. Published 2018. Accessed March 28, 2019.
- Overview: Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed March 28, 2019.
- Medicare Learning Network. July 2017 update of the hospital outpatient prospective payment system (OPPS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10122.pdf. Published 2017. Accessed March 21, 2019.
- Medicare Learning Network. Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/medcrephysfeeschedfctsht.pdf. Published February 2017. Accessed March 19, 2019.
- Medicare Learning Network. How to use the searchable Medicare Physician Fee Schedule (MPFS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/How_to_MPFS_Booklet_ICN901344.pdf. Published September 2017. Accessed March 19, 2019.
- Fox GN. Dermoscopy: an invaluable tool for evaluating skin lesions. Am Fam Physician. 2008;78:704, 706.
- Soyer HP, Argenziano G, Talamini R, et al. Is dermoscopy useful for the diagnosis of melanoma? Arch Dermatol. 2001;137:1361-1363.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists' perspective. Dermatology. 2012;225:289-293.
- American Academy of Dermatology Association. New CPT coding updates for 2016. Derm Coding Consult. 2015;19:1-2. https://www.aad.org/File Library/Main navigation/Member resources and programs/Publications/DCC/DCC_Winter_2015.pdf. Published 2014. Accessed March 21, 2019.
- American Medical Association. CPT Category III codes. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/physicians/cpt/cpt-category3-codes-long-descriptors.pdf. Updated July 26, 2018. Accessed March 21, 2019.
- Medicare Learning Network. Telehealth services. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf. Accessed March 19, 2019.
- Final policy, payment, and quality provisions in the Medicare Physician Fee Schedule for calendar year 2018. Centers for Medicare & Medicaid Services website. https://www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-medicare-physician-fee-schedule-calendar-year-2018. Published November 2, 2017. Accessed March 19, 2019.
- State Telehealth Laws & Reimbursement Policies. Sacramento, CA: Center for Connected Health Policy; 2018. https://www.cchpca.org/sites/default/files/2018-10/CCHP_50_State_Report_Fall_2018.pdf. Accessed March 19, 2019.
- The IMLC. Interstate Medical Licensure Compact website. https://imlcc.org/. Accessed March 19, 2019.
- Current Procedural Terminology 2018, Professional Edition. Chicago, IL: American Medical Association; 2018.
- Centers for Medicare & Medicaid Services. Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program--Extreme and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the Medicare Shared Savings Program-- Accountable Care Organizations--Pathways to Success; and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. Fed Registr. 2018;83(226):59452-60303. To be codified at 42 CFR §405, 410, 411, 414, 415, 425, and 495.
- Centers for Medicare & Medicaid Services. CY 2018 PFS Final Rule Addenda. https://www.cms.gov/Medicare/Medicare-Fee-for-Service Payment/PhysicianFeeSched/Downloads/CY2018-PFS-FR-Addenda.zip. Published 2018. Accessed March 28, 2019.
- Overview: Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed March 28, 2019.
- Medicare Learning Network. July 2017 update of the hospital outpatient prospective payment system (OPPS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10122.pdf. Published 2017. Accessed March 21, 2019.
- Medicare Learning Network. Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/medcrephysfeeschedfctsht.pdf. Published February 2017. Accessed March 19, 2019.
- Medicare Learning Network. How to use the searchable Medicare Physician Fee Schedule (MPFS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/How_to_MPFS_Booklet_ICN901344.pdf. Published September 2017. Accessed March 19, 2019.
- Fox GN. Dermoscopy: an invaluable tool for evaluating skin lesions. Am Fam Physician. 2008;78:704, 706.
- Soyer HP, Argenziano G, Talamini R, et al. Is dermoscopy useful for the diagnosis of melanoma? Arch Dermatol. 2001;137:1361-1363.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists' perspective. Dermatology. 2012;225:289-293.
- American Academy of Dermatology Association. New CPT coding updates for 2016. Derm Coding Consult. 2015;19:1-2. https://www.aad.org/File Library/Main navigation/Member resources and programs/Publications/DCC/DCC_Winter_2015.pdf. Published 2014. Accessed March 21, 2019.
- American Medical Association. CPT Category III codes. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/physicians/cpt/cpt-category3-codes-long-descriptors.pdf. Updated July 26, 2018. Accessed March 21, 2019.
- Medicare Learning Network. Telehealth services. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf. Accessed March 19, 2019.
- Final policy, payment, and quality provisions in the Medicare Physician Fee Schedule for calendar year 2018. Centers for Medicare & Medicaid Services website. https://www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-medicare-physician-fee-schedule-calendar-year-2018. Published November 2, 2017. Accessed March 19, 2019.
- State Telehealth Laws & Reimbursement Policies. Sacramento, CA: Center for Connected Health Policy; 2018. https://www.cchpca.org/sites/default/files/2018-10/CCHP_50_State_Report_Fall_2018.pdf. Accessed March 19, 2019.
- The IMLC. Interstate Medical Licensure Compact website. https://imlcc.org/. Accessed March 19, 2019.
- Current Procedural Terminology 2018, Professional Edition. Chicago, IL: American Medical Association; 2018.
PRACTICE POINTS
- Reimbursement typically is proportional to the relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.
- The total RVU consists of the work RVU, practice expense RVU, and malpractice expense RVU.
- The new 2019 biopsy codes reflect the complexity of the sampling technique (ie, whether the biopsy is tangential, punch, or incisional).
- Accurate completion of Relative Value Scale Update Committee surveys sent to practitioners will allow RVUs to be valued appropriately.
2019 Legislative Goals: Implementation of VA Mission Act Top Priority
As nurses who are often the first face that a veteran sees, members of NOVA (Nurses Organization of Veterans Affairs) are committed to enhancing access, coordinating care, and improving health care at the US Department of Veterans Affairs (VA). NOVA also is the voice of VA nurses on Capitol Hill. Every year, the leadership and legislative committee members provide a list of critical issues identified in their Legislative Priority Goals. For 2019, the goals are divided into 3 areas of concern that either require legislation, funding, or implementation at the regulatory level within the VA.
At the top of the list is implementation of the VA Mission Act and its community care network. The 2018 VA Mission Act (Section 101 of Public Law 115-182) mandated the VA to consolidate existing community care programs and rewrite eligibility rules. Currently, the VA has at least 7 separate community care programs, including the Veterans Choice Program, which gives veterans who live ≥ 40 miles from a VA facility or have a wait time of ≥ 30 days an option to receive care in the local community with VA picking up the bill. Proposed access standards for the new program—Veterans Community Care Program (VCCP)—were made public in January 2019 and would allow veterans with ≥ 30-minute drive time and/or a wait time of ≥ 20 days for primary care or mental health appointments at a VA facility to use outside care. For specialty care eligibility, the drive time would increase to ≥ 60 minutes and ≥ 28 days for an appointment at a VA facility.
NOVA understands the need for community care partners: They are a crucial part of an integrated network designed to provide care for services that are not readily available within the Veterans Health Administration (VHA), but care that veterans receive in the community must be equal to VHA care. Equal care will require training and strict quality measures and standards verified for the VCCP providers. The VA also must remain the primary provider of care and the coordinator of care for all enrolled veterans.
NOVA identified 6 goals for VA Mission Act implementation. These include the following:
- Require that training, competency, and quality standards for VCCP providers are equal to those of VHA providers;
- Request third-party administrator to verify that providers meet those standards before assigning to VCCP panel;
- Simplify eligibility/access rules for community care without depleting VA funds;
- Ensure that VHA continues to be the first point of access and coordinator of all health care for enrolled veterans;
- Implement a care coordination system allowing veterans to return with ease to the VA when resources are available; and
- Employ mandatory training for VHA personnel and all community providers to improve the coordination of care, understanding of military culture, and health care needs across networks.
Other priorities include staffing/recruitment and retention—a longstanding issue within many VA facilities. Currently, the VHA has > 40,000 unfilled positions. It is no secret that the VA has had difficulty hiring essential staff at many levels. Complexities of job site databases and excessive time required to complete on-boarding, shortages in human resources personnel, and less than competitive salaries all add to the growing backlog. The inability for the VA to hire and train providers negatively impacts the access to VHA care and spurs increases in veterans using private sector care.
The VA modernization must include an electronic health record designed to support VHA’s model of health care delivery. It is crucial that Congress ensure proper IT funding to improve patient safety, software usability, and standardization of patient health care records across VHA.
VA nurses are the largest sector of employees within VHA with > 90,000 currently taking care of veterans. As VA continues its modernization, NOVA asks that nursing leadership be at the forefront of all strategic decision making.
In March, NOVA nurses shared their thoughts and views with members of Congress. They also continued discussions with the administration and VA leadership about how we can work together toward common goals—whether that be educating the next generation of nurses, providing innovative health care solutions, or engaging veterans in how they envision their health care in the future. Visit www.vanurse.org for more information about NOVA, the Legislative Priority Goals, or to become a member.
As nurses who are often the first face that a veteran sees, members of NOVA (Nurses Organization of Veterans Affairs) are committed to enhancing access, coordinating care, and improving health care at the US Department of Veterans Affairs (VA). NOVA also is the voice of VA nurses on Capitol Hill. Every year, the leadership and legislative committee members provide a list of critical issues identified in their Legislative Priority Goals. For 2019, the goals are divided into 3 areas of concern that either require legislation, funding, or implementation at the regulatory level within the VA.
At the top of the list is implementation of the VA Mission Act and its community care network. The 2018 VA Mission Act (Section 101 of Public Law 115-182) mandated the VA to consolidate existing community care programs and rewrite eligibility rules. Currently, the VA has at least 7 separate community care programs, including the Veterans Choice Program, which gives veterans who live ≥ 40 miles from a VA facility or have a wait time of ≥ 30 days an option to receive care in the local community with VA picking up the bill. Proposed access standards for the new program—Veterans Community Care Program (VCCP)—were made public in January 2019 and would allow veterans with ≥ 30-minute drive time and/or a wait time of ≥ 20 days for primary care or mental health appointments at a VA facility to use outside care. For specialty care eligibility, the drive time would increase to ≥ 60 minutes and ≥ 28 days for an appointment at a VA facility.
NOVA understands the need for community care partners: They are a crucial part of an integrated network designed to provide care for services that are not readily available within the Veterans Health Administration (VHA), but care that veterans receive in the community must be equal to VHA care. Equal care will require training and strict quality measures and standards verified for the VCCP providers. The VA also must remain the primary provider of care and the coordinator of care for all enrolled veterans.
NOVA identified 6 goals for VA Mission Act implementation. These include the following:
- Require that training, competency, and quality standards for VCCP providers are equal to those of VHA providers;
- Request third-party administrator to verify that providers meet those standards before assigning to VCCP panel;
- Simplify eligibility/access rules for community care without depleting VA funds;
- Ensure that VHA continues to be the first point of access and coordinator of all health care for enrolled veterans;
- Implement a care coordination system allowing veterans to return with ease to the VA when resources are available; and
- Employ mandatory training for VHA personnel and all community providers to improve the coordination of care, understanding of military culture, and health care needs across networks.
Other priorities include staffing/recruitment and retention—a longstanding issue within many VA facilities. Currently, the VHA has > 40,000 unfilled positions. It is no secret that the VA has had difficulty hiring essential staff at many levels. Complexities of job site databases and excessive time required to complete on-boarding, shortages in human resources personnel, and less than competitive salaries all add to the growing backlog. The inability for the VA to hire and train providers negatively impacts the access to VHA care and spurs increases in veterans using private sector care.
The VA modernization must include an electronic health record designed to support VHA’s model of health care delivery. It is crucial that Congress ensure proper IT funding to improve patient safety, software usability, and standardization of patient health care records across VHA.
VA nurses are the largest sector of employees within VHA with > 90,000 currently taking care of veterans. As VA continues its modernization, NOVA asks that nursing leadership be at the forefront of all strategic decision making.
In March, NOVA nurses shared their thoughts and views with members of Congress. They also continued discussions with the administration and VA leadership about how we can work together toward common goals—whether that be educating the next generation of nurses, providing innovative health care solutions, or engaging veterans in how they envision their health care in the future. Visit www.vanurse.org for more information about NOVA, the Legislative Priority Goals, or to become a member.
As nurses who are often the first face that a veteran sees, members of NOVA (Nurses Organization of Veterans Affairs) are committed to enhancing access, coordinating care, and improving health care at the US Department of Veterans Affairs (VA). NOVA also is the voice of VA nurses on Capitol Hill. Every year, the leadership and legislative committee members provide a list of critical issues identified in their Legislative Priority Goals. For 2019, the goals are divided into 3 areas of concern that either require legislation, funding, or implementation at the regulatory level within the VA.
At the top of the list is implementation of the VA Mission Act and its community care network. The 2018 VA Mission Act (Section 101 of Public Law 115-182) mandated the VA to consolidate existing community care programs and rewrite eligibility rules. Currently, the VA has at least 7 separate community care programs, including the Veterans Choice Program, which gives veterans who live ≥ 40 miles from a VA facility or have a wait time of ≥ 30 days an option to receive care in the local community with VA picking up the bill. Proposed access standards for the new program—Veterans Community Care Program (VCCP)—were made public in January 2019 and would allow veterans with ≥ 30-minute drive time and/or a wait time of ≥ 20 days for primary care or mental health appointments at a VA facility to use outside care. For specialty care eligibility, the drive time would increase to ≥ 60 minutes and ≥ 28 days for an appointment at a VA facility.
NOVA understands the need for community care partners: They are a crucial part of an integrated network designed to provide care for services that are not readily available within the Veterans Health Administration (VHA), but care that veterans receive in the community must be equal to VHA care. Equal care will require training and strict quality measures and standards verified for the VCCP providers. The VA also must remain the primary provider of care and the coordinator of care for all enrolled veterans.
NOVA identified 6 goals for VA Mission Act implementation. These include the following:
- Require that training, competency, and quality standards for VCCP providers are equal to those of VHA providers;
- Request third-party administrator to verify that providers meet those standards before assigning to VCCP panel;
- Simplify eligibility/access rules for community care without depleting VA funds;
- Ensure that VHA continues to be the first point of access and coordinator of all health care for enrolled veterans;
- Implement a care coordination system allowing veterans to return with ease to the VA when resources are available; and
- Employ mandatory training for VHA personnel and all community providers to improve the coordination of care, understanding of military culture, and health care needs across networks.
Other priorities include staffing/recruitment and retention—a longstanding issue within many VA facilities. Currently, the VHA has > 40,000 unfilled positions. It is no secret that the VA has had difficulty hiring essential staff at many levels. Complexities of job site databases and excessive time required to complete on-boarding, shortages in human resources personnel, and less than competitive salaries all add to the growing backlog. The inability for the VA to hire and train providers negatively impacts the access to VHA care and spurs increases in veterans using private sector care.
The VA modernization must include an electronic health record designed to support VHA’s model of health care delivery. It is crucial that Congress ensure proper IT funding to improve patient safety, software usability, and standardization of patient health care records across VHA.
VA nurses are the largest sector of employees within VHA with > 90,000 currently taking care of veterans. As VA continues its modernization, NOVA asks that nursing leadership be at the forefront of all strategic decision making.
In March, NOVA nurses shared their thoughts and views with members of Congress. They also continued discussions with the administration and VA leadership about how we can work together toward common goals—whether that be educating the next generation of nurses, providing innovative health care solutions, or engaging veterans in how they envision their health care in the future. Visit www.vanurse.org for more information about NOVA, the Legislative Priority Goals, or to become a member.
Brexanolone approval ‘marks an important milestone’
In March 2019, the Food and Drug Administration approved a novel medication, Zulresso (brexanolone), for the treatment of postpartum depression. Brexanolone is the first FDA-approved medication for the treatment of postpartum depression, a serious illness that affects nearly one in nine women soon after giving birth.1
Mothers with postpartum depression experience feelings of sadness, irritability, and anxiety, as well as isolation from their loved ones (including their new baby) and exhaustion. The feelings of sadness and anxiety can be extreme, and can interfere with a woman’s ability to care for herself or her family. In some cases, these symptoms can be life threatening. Indeed, the most common cause of maternal death after childbirth in the developed world is suicide.2 Because of the severity of the symptoms and their impact on the family, postpartum depression usually requires treatment.
Until now, there have been no drugs specifically approved to treat postpartum depression. Commonly, postpartum depression is treated with medications that previously were approved for the treatment of major depressive disorder, despite limited evidence documenting their efficacy for postpartum depression. Other putative treatment alternatives include psychotherapy, estrogen therapy, and neuromodulation, such as electroconvulsive therapy and repetitive transcranial magnetic stimulation. Each of these treatments can take weeks or longer to take effect, time that is of elevated importance given the rapidly developing mother-infant relationship in the early postpartum period. Brexanolone addresses both the issue of efficacy and speed of onset, representing a major step forward in the care of women suffering from postpartum depression.
Importantly, the approval of brexanolone marks an important milestone for the psychiatric research community in general and the National Institute of Mental Health in particular, as it represents a compelling example of successful bench-to-bedside translation of basic neuroscience findings to benefit patients. As we have noted elsewhere,3 the research underlying the discovery of endogenous neurosteroids and their role in modulating GABA receptors laid the foundation for the development of brexanolone, an intravenous formulation of the neurosteroid allopregnanolone. The recognition that allopregnanolone was a protective factor induced by stress, that it derived from progesterone, and that its peripheral blood levels were dramatically reduced in the early postpartum period led to the hypothesis that it might be useful as a treatment for postpartum depression.
Sage Therapeutics took on the task of testing this hypothesis, designing a program, in consultation with the FDA, to test the efficacy of allopregnanolone in women with postpartum depression in a series of randomized, placebo-controlled studies assessing brexanolone. 4,5 It is a significant accomplishment of Sage Therapeutics to not only successfully complete the therapeutic program of studies (given past experience with difficulties recruiting these women for placebo-controlled treatment trials) but as well to demonstrate a robust therapeutic effect.
Although the FDA’s approval of a new and novel treatment is exciting for many women, there are still limitations to the broader use of brexanolone. It is delivered intravenously, requires an overnight stay in a certified medical center, and is likely to be considerably expensive, according to early reports – potentially limiting the access to the treatment. There also are potentially serious side effects, such as sedation, dizziness, or sudden loss of consciousness. Nonetheless, this is a promising first step and hopefully will spur further efforts to identify and optimize additional strategies to treat postpartum depression. In fact, other formulations of allopregnanolone and novel analogs to treat postpartum depression already are under study, including some that are orally bioavailable.6,7,8
Several important questions remain to be answered about both brexanolone and postpartum depression: What is the underlying mechanism through which allopregnanolone acts in the brain and reduces depressive symptoms? Is the mechanism unique to postpartum women, or might brexanolone also be effective in nonreproductive depressions in women and men? What causes postpartum depression, and what are the risk factors involved for women who develop this serious condition? Future work will focus on these and other important questions to the benefit of women who have suffered with this condition.
The FDA approval of brexanolone represents the second approval in a month of a new antidepressant treatment targeting different molecules in the brain. In early March 2019, the agency approved Spravato (esketamine) nasal spray as a therapy for treatment-resistant depression. Like brexanolone, esketamine is a fast-acting antidepressant that works through a novel mechanism, completely different from other antidepressants. These new treatment approvals are encouraging, as there has been a paucity for many years in approving new effective treatments for mood disorders.
However, treatment development in psychiatry still has a long way to go and the full underlying neurobiology of mood disorders, including postpartum depression, remains poorly understood. Many challenges are ahead of us in our efforts to develop new treatments and increase our understanding of mental illnesses. Nevertheless, the approval of brexanolone is an important milestone, giving hope to the many women who suffer from postpartum depression, and paving the way for the development of additional novel and effective medications to treat this serious and sometimes life-threatening condition.
Dr. Gordon is the director of the National Institute of Mental Health (NIMH), the lead federal agency for research on mental disorders. He oversees an extensive research portfolio of basic and clinical research that seeks to transform the understanding and treatment of mental illnesses, paving the way for prevention, recovery, and cure. Dr. Hillefors works at the NIMH and oversees the Translational Therapeutics Program in the division of translational research, focusing on the development of novel treatments and biomarkers and early phase clinical trials. She received her MD and PhD in neuroscience at the Karolinska Institute, Sweden. Dr. Schmidt joined the NIMH in 1986 after completing his psychiatric residency at the University of Toronto. He is the chief of the Section on Behavioral Endocrinology, within the Intramural Research Program at the NIMH, where his laboratory studies the relationship between hormones, stress, and mood – particularly in the areas of postpartum depression, severe premenstrual dysphoria, and perimenopausal depression.
References
1. J Psychiatric Res. 2018 Sep;104:235-48.
2. Br J Psychiatry. 2003 Oct;183:279-81.
3. NIMH Director’s Messages. 2019 Mar 20.
4. Lancet. 2017 Jul 29;390(10093):480-9.
5. Lancet. 2018 Sep 22; 392(10152):1058-70.
6. Sage Therapeutics News. 2019 Jan 7.
7. Marinus Pharmaceuticals. 2017 Jun 27.
8. ClinicalTrials.gov Identifier: NCT03460756. 2019 Mar.
In March 2019, the Food and Drug Administration approved a novel medication, Zulresso (brexanolone), for the treatment of postpartum depression. Brexanolone is the first FDA-approved medication for the treatment of postpartum depression, a serious illness that affects nearly one in nine women soon after giving birth.1
Mothers with postpartum depression experience feelings of sadness, irritability, and anxiety, as well as isolation from their loved ones (including their new baby) and exhaustion. The feelings of sadness and anxiety can be extreme, and can interfere with a woman’s ability to care for herself or her family. In some cases, these symptoms can be life threatening. Indeed, the most common cause of maternal death after childbirth in the developed world is suicide.2 Because of the severity of the symptoms and their impact on the family, postpartum depression usually requires treatment.
Until now, there have been no drugs specifically approved to treat postpartum depression. Commonly, postpartum depression is treated with medications that previously were approved for the treatment of major depressive disorder, despite limited evidence documenting their efficacy for postpartum depression. Other putative treatment alternatives include psychotherapy, estrogen therapy, and neuromodulation, such as electroconvulsive therapy and repetitive transcranial magnetic stimulation. Each of these treatments can take weeks or longer to take effect, time that is of elevated importance given the rapidly developing mother-infant relationship in the early postpartum period. Brexanolone addresses both the issue of efficacy and speed of onset, representing a major step forward in the care of women suffering from postpartum depression.
Importantly, the approval of brexanolone marks an important milestone for the psychiatric research community in general and the National Institute of Mental Health in particular, as it represents a compelling example of successful bench-to-bedside translation of basic neuroscience findings to benefit patients. As we have noted elsewhere,3 the research underlying the discovery of endogenous neurosteroids and their role in modulating GABA receptors laid the foundation for the development of brexanolone, an intravenous formulation of the neurosteroid allopregnanolone. The recognition that allopregnanolone was a protective factor induced by stress, that it derived from progesterone, and that its peripheral blood levels were dramatically reduced in the early postpartum period led to the hypothesis that it might be useful as a treatment for postpartum depression.
Sage Therapeutics took on the task of testing this hypothesis, designing a program, in consultation with the FDA, to test the efficacy of allopregnanolone in women with postpartum depression in a series of randomized, placebo-controlled studies assessing brexanolone. 4,5 It is a significant accomplishment of Sage Therapeutics to not only successfully complete the therapeutic program of studies (given past experience with difficulties recruiting these women for placebo-controlled treatment trials) but as well to demonstrate a robust therapeutic effect.
Although the FDA’s approval of a new and novel treatment is exciting for many women, there are still limitations to the broader use of brexanolone. It is delivered intravenously, requires an overnight stay in a certified medical center, and is likely to be considerably expensive, according to early reports – potentially limiting the access to the treatment. There also are potentially serious side effects, such as sedation, dizziness, or sudden loss of consciousness. Nonetheless, this is a promising first step and hopefully will spur further efforts to identify and optimize additional strategies to treat postpartum depression. In fact, other formulations of allopregnanolone and novel analogs to treat postpartum depression already are under study, including some that are orally bioavailable.6,7,8
Several important questions remain to be answered about both brexanolone and postpartum depression: What is the underlying mechanism through which allopregnanolone acts in the brain and reduces depressive symptoms? Is the mechanism unique to postpartum women, or might brexanolone also be effective in nonreproductive depressions in women and men? What causes postpartum depression, and what are the risk factors involved for women who develop this serious condition? Future work will focus on these and other important questions to the benefit of women who have suffered with this condition.
The FDA approval of brexanolone represents the second approval in a month of a new antidepressant treatment targeting different molecules in the brain. In early March 2019, the agency approved Spravato (esketamine) nasal spray as a therapy for treatment-resistant depression. Like brexanolone, esketamine is a fast-acting antidepressant that works through a novel mechanism, completely different from other antidepressants. These new treatment approvals are encouraging, as there has been a paucity for many years in approving new effective treatments for mood disorders.
However, treatment development in psychiatry still has a long way to go and the full underlying neurobiology of mood disorders, including postpartum depression, remains poorly understood. Many challenges are ahead of us in our efforts to develop new treatments and increase our understanding of mental illnesses. Nevertheless, the approval of brexanolone is an important milestone, giving hope to the many women who suffer from postpartum depression, and paving the way for the development of additional novel and effective medications to treat this serious and sometimes life-threatening condition.
Dr. Gordon is the director of the National Institute of Mental Health (NIMH), the lead federal agency for research on mental disorders. He oversees an extensive research portfolio of basic and clinical research that seeks to transform the understanding and treatment of mental illnesses, paving the way for prevention, recovery, and cure. Dr. Hillefors works at the NIMH and oversees the Translational Therapeutics Program in the division of translational research, focusing on the development of novel treatments and biomarkers and early phase clinical trials. She received her MD and PhD in neuroscience at the Karolinska Institute, Sweden. Dr. Schmidt joined the NIMH in 1986 after completing his psychiatric residency at the University of Toronto. He is the chief of the Section on Behavioral Endocrinology, within the Intramural Research Program at the NIMH, where his laboratory studies the relationship between hormones, stress, and mood – particularly in the areas of postpartum depression, severe premenstrual dysphoria, and perimenopausal depression.
References
1. J Psychiatric Res. 2018 Sep;104:235-48.
2. Br J Psychiatry. 2003 Oct;183:279-81.
3. NIMH Director’s Messages. 2019 Mar 20.
4. Lancet. 2017 Jul 29;390(10093):480-9.
5. Lancet. 2018 Sep 22; 392(10152):1058-70.
6. Sage Therapeutics News. 2019 Jan 7.
7. Marinus Pharmaceuticals. 2017 Jun 27.
8. ClinicalTrials.gov Identifier: NCT03460756. 2019 Mar.
In March 2019, the Food and Drug Administration approved a novel medication, Zulresso (brexanolone), for the treatment of postpartum depression. Brexanolone is the first FDA-approved medication for the treatment of postpartum depression, a serious illness that affects nearly one in nine women soon after giving birth.1
Mothers with postpartum depression experience feelings of sadness, irritability, and anxiety, as well as isolation from their loved ones (including their new baby) and exhaustion. The feelings of sadness and anxiety can be extreme, and can interfere with a woman’s ability to care for herself or her family. In some cases, these symptoms can be life threatening. Indeed, the most common cause of maternal death after childbirth in the developed world is suicide.2 Because of the severity of the symptoms and their impact on the family, postpartum depression usually requires treatment.
Until now, there have been no drugs specifically approved to treat postpartum depression. Commonly, postpartum depression is treated with medications that previously were approved for the treatment of major depressive disorder, despite limited evidence documenting their efficacy for postpartum depression. Other putative treatment alternatives include psychotherapy, estrogen therapy, and neuromodulation, such as electroconvulsive therapy and repetitive transcranial magnetic stimulation. Each of these treatments can take weeks or longer to take effect, time that is of elevated importance given the rapidly developing mother-infant relationship in the early postpartum period. Brexanolone addresses both the issue of efficacy and speed of onset, representing a major step forward in the care of women suffering from postpartum depression.
Importantly, the approval of brexanolone marks an important milestone for the psychiatric research community in general and the National Institute of Mental Health in particular, as it represents a compelling example of successful bench-to-bedside translation of basic neuroscience findings to benefit patients. As we have noted elsewhere,3 the research underlying the discovery of endogenous neurosteroids and their role in modulating GABA receptors laid the foundation for the development of brexanolone, an intravenous formulation of the neurosteroid allopregnanolone. The recognition that allopregnanolone was a protective factor induced by stress, that it derived from progesterone, and that its peripheral blood levels were dramatically reduced in the early postpartum period led to the hypothesis that it might be useful as a treatment for postpartum depression.
Sage Therapeutics took on the task of testing this hypothesis, designing a program, in consultation with the FDA, to test the efficacy of allopregnanolone in women with postpartum depression in a series of randomized, placebo-controlled studies assessing brexanolone. 4,5 It is a significant accomplishment of Sage Therapeutics to not only successfully complete the therapeutic program of studies (given past experience with difficulties recruiting these women for placebo-controlled treatment trials) but as well to demonstrate a robust therapeutic effect.
Although the FDA’s approval of a new and novel treatment is exciting for many women, there are still limitations to the broader use of brexanolone. It is delivered intravenously, requires an overnight stay in a certified medical center, and is likely to be considerably expensive, according to early reports – potentially limiting the access to the treatment. There also are potentially serious side effects, such as sedation, dizziness, or sudden loss of consciousness. Nonetheless, this is a promising first step and hopefully will spur further efforts to identify and optimize additional strategies to treat postpartum depression. In fact, other formulations of allopregnanolone and novel analogs to treat postpartum depression already are under study, including some that are orally bioavailable.6,7,8
Several important questions remain to be answered about both brexanolone and postpartum depression: What is the underlying mechanism through which allopregnanolone acts in the brain and reduces depressive symptoms? Is the mechanism unique to postpartum women, or might brexanolone also be effective in nonreproductive depressions in women and men? What causes postpartum depression, and what are the risk factors involved for women who develop this serious condition? Future work will focus on these and other important questions to the benefit of women who have suffered with this condition.
The FDA approval of brexanolone represents the second approval in a month of a new antidepressant treatment targeting different molecules in the brain. In early March 2019, the agency approved Spravato (esketamine) nasal spray as a therapy for treatment-resistant depression. Like brexanolone, esketamine is a fast-acting antidepressant that works through a novel mechanism, completely different from other antidepressants. These new treatment approvals are encouraging, as there has been a paucity for many years in approving new effective treatments for mood disorders.
However, treatment development in psychiatry still has a long way to go and the full underlying neurobiology of mood disorders, including postpartum depression, remains poorly understood. Many challenges are ahead of us in our efforts to develop new treatments and increase our understanding of mental illnesses. Nevertheless, the approval of brexanolone is an important milestone, giving hope to the many women who suffer from postpartum depression, and paving the way for the development of additional novel and effective medications to treat this serious and sometimes life-threatening condition.
Dr. Gordon is the director of the National Institute of Mental Health (NIMH), the lead federal agency for research on mental disorders. He oversees an extensive research portfolio of basic and clinical research that seeks to transform the understanding and treatment of mental illnesses, paving the way for prevention, recovery, and cure. Dr. Hillefors works at the NIMH and oversees the Translational Therapeutics Program in the division of translational research, focusing on the development of novel treatments and biomarkers and early phase clinical trials. She received her MD and PhD in neuroscience at the Karolinska Institute, Sweden. Dr. Schmidt joined the NIMH in 1986 after completing his psychiatric residency at the University of Toronto. He is the chief of the Section on Behavioral Endocrinology, within the Intramural Research Program at the NIMH, where his laboratory studies the relationship between hormones, stress, and mood – particularly in the areas of postpartum depression, severe premenstrual dysphoria, and perimenopausal depression.
References
1. J Psychiatric Res. 2018 Sep;104:235-48.
2. Br J Psychiatry. 2003 Oct;183:279-81.
3. NIMH Director’s Messages. 2019 Mar 20.
4. Lancet. 2017 Jul 29;390(10093):480-9.
5. Lancet. 2018 Sep 22; 392(10152):1058-70.
6. Sage Therapeutics News. 2019 Jan 7.
7. Marinus Pharmaceuticals. 2017 Jun 27.
8. ClinicalTrials.gov Identifier: NCT03460756. 2019 Mar.
Use of GBCA in MRIs for High-Risk Patients
To the Editor:
We read with interest the case report of nephrogenic systemic fibrosis (NSF) by Chuang, Kaneshiro, and Betancourt in the June 2018 issue of Federal Practitioner.1 It was reported that a 61-year-old Hispanic male patient with a history of IV heroin abuse with end-stage renal disease (ESRD) secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection received 15 mL gadoversetamide, a linear gadolinium-based contrast agent (GBCA) during magnetic resonance imaging (MRI) of the brain. Hemodialysis was performed 18 hours after the contrast administration.
Eight weeks after his initial presentation, the patient developed pyoderma gangrenosum on his right forearm, which was treated with high-dose steroids. He then developed thickening and induration of his bilateral forearm skin with peau d’orange appearance. NSF was confirmed by a skin biopsy. The patient developed contractures of his upper and lower extremities and was finally wheelchair bound.
This case is very concerning since no NSF cases in patients receiving GBCA have been published since 2009. Unfortunately, the authors give no information on the occurrence of this particular case. Thus, it is unclear whether this case was observed before or after the switch to macrocyclic agents in patients with reduced renal function. The reported patient with ESRD was on hemodialysis and received 15 mL gadoversetamide during MRI of the brain. In 2007 the ESUR (European Society of Urogenital Radiology) published guidelines indicating linear GBCA (gadodiamide, gadoversetamide, gadopentetate dimeglumine) as high-risk agents that may not be used in patients with eGFR < 30 mL/min/1.73 m2.2,3
Consequently in 2007, the European Medicines Agency contraindicated these linear GBCA in patients with chronic kidney disease grades 4 and 5. Also in 2007 the US Food and Drug Administration (FDA) requested a revision of the prescribing information for all 5 GBCA approved in the US.4 In response to accumulating more informative data, in 2010 the FDA again used this class labeling approach to more explicitly describe differences in NSF risks among the agents.4 FDA regulation and contraindication of the use of low-stability GBCA in patients with advanced renal impairment and robust local policies on the safe use of these agents have resulted in marked reduction in the prevalence of NSF in the US. This case report needs to clarify why a high-risk linear agent was administered to a patient with ESRD.
In 2006 Grobner and Marckmann and colleagues reported their observations of a previously unrecognized link between exposure to gadodiamide and the development of NSF.5,6 It soon became clear that NSF is a delayed adverse contrast reaction that may cause severe disability and even death. Advanced renal disease and high-risk linear GBCA are the main factors in the pathogenesis of NSF. Additionally, the dose of the agent may play a role. NSF can occur from hours to years after exposure to GBCA. Not all patients with severe kidney disease exposed to high-risk agents developed NSF. Thus, additional factors were proposed to play a role in the pathogenesis of NSF. Among those factors were erythropoietin, metabolic acidosis, anion gap, iron, increased phosphate, zinc loss, proinflammatory conditions/inflammation and angiotensin-converting enzyme (ACE) inhibitors.7 Although there is little proof with these assumptions, special care must be taken as shown by this reported patient with multiple inflammatory disorders.
- Gertraud Heinz, MD, MBA; Aart van der Molen, MD; and Giles Roditi, MD; on behalf of the ESUR Contrast Media Safety Committee
Author affiliations: Gertraud Heinz is former President ESUR and Head of the Department of Radiology, Diagnostics and Intervention University Hospital St. Pölten Karl Landsteiner University of Health Sciences.
Correspondence: Gertraud Heinz (gertraud.heinz@stpoelten .lknoe.at)
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract. 2018;35(6):40-43.
2. Thomsen HS; European Society of Urogenital Radiology (ESUR). ESUR guideline: gadolinium based contrast media and nephrogenic systemic fibrosis. Eur Radiol. 2007;17(10):2692-2696.
3. Thomsen HS, Morcos SK, Almén T, et al; ESUR Contrast Medium Safety Committee. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2013;23(2):307-318
4. Yang L, Krefting I, Gorovets A, et al. Nephrogenic systemic fibrosis and class labeling of gadolinium-based agents by the Food and Drug Administration. Radiology. 2012;265(1):248-253.
5. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
6. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
7. Thomsen HS, Bennett CL. Six years after. Acta Radiol. 2012;53(8):827-829.
To the Editor:
With great interest, I read the case report by Chuang, Kaneshiro, and Betancourt.1 Patients with nephrogenic systemic fibrosis (NSF) are of special interest because the disease is still unclear as mentioned by the authors. Although new cases may occur,2 this case raises some concerns that I would like to address.
First, it would be of great interest to know the date when the patient received the high-risk gadolinium-based contrast agent (GBCA) gadoversetamide. Unfortunately, the authors did not mention the date of the injection of the GBCA that probably caused NSF. Due to the obvious association between the applications of special GBCAs in 2006, the US Food and Drug Administration (FDA) warned physicians not to inject these contrast agents in patients with compromised kidney function.3 Moreover, in 2007 the American College of Radiology (ACR) published guidelines for the safe use of GBCAs in patients with renal failure.4 Also, the European Medicines Agency (EMA) demanded that companies provide warning in product inserts about the acquisition of NSF in patients with severe kidney injury.5
Second, the clinical illustration of the case is inadequate. In the manuscript, we read that the patient acquired NSF-characteristic lesions like peau d’orange skin lesions and contractures of his extremities, but unfortunately, Chuang, Kaneshiro, and Betancourt did not provide figures that show them. On the other hand, Figure 1 shows an uncharacteristic dermal induration around inflammatory and ulcerated skin lesion (pyoderma gangrenosum).1 Such clinical signs are well known and occur perilesional of different conditions independently of NSF.6-8
Third, the histological features described as presence of fibrotic tissue in the deep dermis in Figure 2, and dermal fibrosis with thick collagen deposition in Figure 31 do not confirm the existence of NSF.
Taken together, the case presented by Chuang, Kaneshiro, and Betancourt contains some unclear aspects; therefore, it is questionable whether the published case describes a patient with NSF or not. In the current presentation, the diagnosis NSF seems to be an overestimation.
NSF still is a poorly understood disorder. Therefore, exactly documented new cases could be of clinical value when providing interesting information. Even single cases could shed some light in the darkness of the pathological mechanisms of this entity. On the other hand, we should not mix the existing cohort of published NSF cases with other scleroderma-like diseases, because this will lead to a confusion. Moreover, such a practice could inhibit the discovery of the pathophysiology of NSF.
- Ingrid Böhm, MD
Author affiliations: Ingrid Böhm is a Physician in the Department of Diagnostics, Interventional and Pediatric Radiology at the University Hospital of Bern, Inselspital, University of Bern in Bern, Switzerland.
Correspondence: Ingrid Böhm ([email protected])
Disclosures: The author reports no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract . 2018;35(6):40-43.
2. Larson KN, Gagnon AL, Darling MD, Patterson JW, Cropley TG. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151(10):1117-1120.
3. US Food and Drug Administration. A Public Health Advisory. Gadolinium-containing contrast agents for magnetic resonance imaging (MRI). http://wayback.archive-it.org/7993/20170112033022/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformation forPatientsandProviders/ucm053112.htm. Published June 8, 2006. Accessed March 15, 2019.
4. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
5. European Medicines Agency. Public statement: Vasovist and nephrogenic systemic fibrosis (NSF). https://www.ema.europa.eu/en/news/public-statement-vasovist-nephrogenic-systemic-fibrosis-nsf. Published February 7, 2007. Accessed March 15, 2019.
6. Luke JC. The etiology and modern treatment of varicose ulcer. Can Med Assoc J. 1940;43(3):217-221.
7. Paulsen E, Bygum A. Keratin gel as an adjuvant in the treatment of recalcitrant pyoderma gangrenosum ulcers: a case report. Acta Derm Venereol. 2019;99(2):234-235.
8. Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000; 136(2):167-169.
Response:
We thank Drs. Heinz, van der Molen, and Roditi for their valuable response. The following is the opinion of the authors and is not representative of the views or policies of our institution. The patient in this case received a gadolinium-based contrast agent (GBCA) in 2015 and was diagnosed with nephrogenic systemic fibrosis (NSF) 8 weeks later. We agree with the correspondents that linear GBCAs should not be used in patients with eGFR < 30 mL/min/1.73 m2. To date, a few cases of patients who received GBCA and developed NSF since 2009 have unfortunately continued to be reported in the literature.1-3 Our intention in publishing this case was to provide ongoing education to the medical community regarding this serious condition to ensure prevention of future cases.
We thank Dr. Böhm for her important inquiry. The patient received a histopathologic diagnosis of NSF. The report from the patient’s left dorsal forearm skin punch biopsy was read by our pathologist as “fibrosis and inflammation consistent with nephrogenic systemic fibrosis,” a diagnosis agreed upon by our colleagues in the dermatology and rheumatology departments based on the rapidity of his symptom onset and progression. While we acknowledge that this patient had other inflammatory disorders of the skin that may have coexisted with the diagnosis, after weighing the preponderance of clinical evidence in support of the biopsy results, we believe that this represents a case of NSF, which is associated with high morbidity and mortality. Thankfully, the patient in this case engaged extensively in physical and occupational therapy and is still alive nearly 4 years later. We would like to thank all the letter writers for their correspondence.
Author Affiliations: Kelley Chuang and Casey Kaneshiro are Hospitalists and Jaime Betancourt is a Pulmonologist, all in the Department of Medicine at the VA Greater Los Angeles Healthcare System in California.
Correspondence: Kelley Chuang ([email protected])
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Aggarwal A, Froehlich AA, Essah P, Brinster N, High WA, Downs RW. Complications of nephrogenic systemic fibrosis following repeated exposure to gadolinium in a man with hypothyroidism: a case report. J Med Case Rep. 2011;5:566.
2. Fuah KW, Lim CT. Erythema nodosum masking nephrogenic systemic fibrosis as initial skin manifestation. BMC Nephrol. 2017;18(1):249.
3. Koratala A, Bhatti V. Nephrogenic systemic fibrosis. Clin Case Rep. 2017;5(7):1184-1185.
To the Editor:
We read with interest the case report of nephrogenic systemic fibrosis (NSF) by Chuang, Kaneshiro, and Betancourt in the June 2018 issue of Federal Practitioner.1 It was reported that a 61-year-old Hispanic male patient with a history of IV heroin abuse with end-stage renal disease (ESRD) secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection received 15 mL gadoversetamide, a linear gadolinium-based contrast agent (GBCA) during magnetic resonance imaging (MRI) of the brain. Hemodialysis was performed 18 hours after the contrast administration.
Eight weeks after his initial presentation, the patient developed pyoderma gangrenosum on his right forearm, which was treated with high-dose steroids. He then developed thickening and induration of his bilateral forearm skin with peau d’orange appearance. NSF was confirmed by a skin biopsy. The patient developed contractures of his upper and lower extremities and was finally wheelchair bound.
This case is very concerning since no NSF cases in patients receiving GBCA have been published since 2009. Unfortunately, the authors give no information on the occurrence of this particular case. Thus, it is unclear whether this case was observed before or after the switch to macrocyclic agents in patients with reduced renal function. The reported patient with ESRD was on hemodialysis and received 15 mL gadoversetamide during MRI of the brain. In 2007 the ESUR (European Society of Urogenital Radiology) published guidelines indicating linear GBCA (gadodiamide, gadoversetamide, gadopentetate dimeglumine) as high-risk agents that may not be used in patients with eGFR < 30 mL/min/1.73 m2.2,3
Consequently in 2007, the European Medicines Agency contraindicated these linear GBCA in patients with chronic kidney disease grades 4 and 5. Also in 2007 the US Food and Drug Administration (FDA) requested a revision of the prescribing information for all 5 GBCA approved in the US.4 In response to accumulating more informative data, in 2010 the FDA again used this class labeling approach to more explicitly describe differences in NSF risks among the agents.4 FDA regulation and contraindication of the use of low-stability GBCA in patients with advanced renal impairment and robust local policies on the safe use of these agents have resulted in marked reduction in the prevalence of NSF in the US. This case report needs to clarify why a high-risk linear agent was administered to a patient with ESRD.
In 2006 Grobner and Marckmann and colleagues reported their observations of a previously unrecognized link between exposure to gadodiamide and the development of NSF.5,6 It soon became clear that NSF is a delayed adverse contrast reaction that may cause severe disability and even death. Advanced renal disease and high-risk linear GBCA are the main factors in the pathogenesis of NSF. Additionally, the dose of the agent may play a role. NSF can occur from hours to years after exposure to GBCA. Not all patients with severe kidney disease exposed to high-risk agents developed NSF. Thus, additional factors were proposed to play a role in the pathogenesis of NSF. Among those factors were erythropoietin, metabolic acidosis, anion gap, iron, increased phosphate, zinc loss, proinflammatory conditions/inflammation and angiotensin-converting enzyme (ACE) inhibitors.7 Although there is little proof with these assumptions, special care must be taken as shown by this reported patient with multiple inflammatory disorders.
- Gertraud Heinz, MD, MBA; Aart van der Molen, MD; and Giles Roditi, MD; on behalf of the ESUR Contrast Media Safety Committee
Author affiliations: Gertraud Heinz is former President ESUR and Head of the Department of Radiology, Diagnostics and Intervention University Hospital St. Pölten Karl Landsteiner University of Health Sciences.
Correspondence: Gertraud Heinz (gertraud.heinz@stpoelten .lknoe.at)
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract. 2018;35(6):40-43.
2. Thomsen HS; European Society of Urogenital Radiology (ESUR). ESUR guideline: gadolinium based contrast media and nephrogenic systemic fibrosis. Eur Radiol. 2007;17(10):2692-2696.
3. Thomsen HS, Morcos SK, Almén T, et al; ESUR Contrast Medium Safety Committee. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2013;23(2):307-318
4. Yang L, Krefting I, Gorovets A, et al. Nephrogenic systemic fibrosis and class labeling of gadolinium-based agents by the Food and Drug Administration. Radiology. 2012;265(1):248-253.
5. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
6. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
7. Thomsen HS, Bennett CL. Six years after. Acta Radiol. 2012;53(8):827-829.
To the Editor:
With great interest, I read the case report by Chuang, Kaneshiro, and Betancourt.1 Patients with nephrogenic systemic fibrosis (NSF) are of special interest because the disease is still unclear as mentioned by the authors. Although new cases may occur,2 this case raises some concerns that I would like to address.
First, it would be of great interest to know the date when the patient received the high-risk gadolinium-based contrast agent (GBCA) gadoversetamide. Unfortunately, the authors did not mention the date of the injection of the GBCA that probably caused NSF. Due to the obvious association between the applications of special GBCAs in 2006, the US Food and Drug Administration (FDA) warned physicians not to inject these contrast agents in patients with compromised kidney function.3 Moreover, in 2007 the American College of Radiology (ACR) published guidelines for the safe use of GBCAs in patients with renal failure.4 Also, the European Medicines Agency (EMA) demanded that companies provide warning in product inserts about the acquisition of NSF in patients with severe kidney injury.5
Second, the clinical illustration of the case is inadequate. In the manuscript, we read that the patient acquired NSF-characteristic lesions like peau d’orange skin lesions and contractures of his extremities, but unfortunately, Chuang, Kaneshiro, and Betancourt did not provide figures that show them. On the other hand, Figure 1 shows an uncharacteristic dermal induration around inflammatory and ulcerated skin lesion (pyoderma gangrenosum).1 Such clinical signs are well known and occur perilesional of different conditions independently of NSF.6-8
Third, the histological features described as presence of fibrotic tissue in the deep dermis in Figure 2, and dermal fibrosis with thick collagen deposition in Figure 31 do not confirm the existence of NSF.
Taken together, the case presented by Chuang, Kaneshiro, and Betancourt contains some unclear aspects; therefore, it is questionable whether the published case describes a patient with NSF or not. In the current presentation, the diagnosis NSF seems to be an overestimation.
NSF still is a poorly understood disorder. Therefore, exactly documented new cases could be of clinical value when providing interesting information. Even single cases could shed some light in the darkness of the pathological mechanisms of this entity. On the other hand, we should not mix the existing cohort of published NSF cases with other scleroderma-like diseases, because this will lead to a confusion. Moreover, such a practice could inhibit the discovery of the pathophysiology of NSF.
- Ingrid Böhm, MD
Author affiliations: Ingrid Böhm is a Physician in the Department of Diagnostics, Interventional and Pediatric Radiology at the University Hospital of Bern, Inselspital, University of Bern in Bern, Switzerland.
Correspondence: Ingrid Böhm ([email protected])
Disclosures: The author reports no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract . 2018;35(6):40-43.
2. Larson KN, Gagnon AL, Darling MD, Patterson JW, Cropley TG. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151(10):1117-1120.
3. US Food and Drug Administration. A Public Health Advisory. Gadolinium-containing contrast agents for magnetic resonance imaging (MRI). http://wayback.archive-it.org/7993/20170112033022/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformation forPatientsandProviders/ucm053112.htm. Published June 8, 2006. Accessed March 15, 2019.
4. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
5. European Medicines Agency. Public statement: Vasovist and nephrogenic systemic fibrosis (NSF). https://www.ema.europa.eu/en/news/public-statement-vasovist-nephrogenic-systemic-fibrosis-nsf. Published February 7, 2007. Accessed March 15, 2019.
6. Luke JC. The etiology and modern treatment of varicose ulcer. Can Med Assoc J. 1940;43(3):217-221.
7. Paulsen E, Bygum A. Keratin gel as an adjuvant in the treatment of recalcitrant pyoderma gangrenosum ulcers: a case report. Acta Derm Venereol. 2019;99(2):234-235.
8. Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000; 136(2):167-169.
Response:
We thank Drs. Heinz, van der Molen, and Roditi for their valuable response. The following is the opinion of the authors and is not representative of the views or policies of our institution. The patient in this case received a gadolinium-based contrast agent (GBCA) in 2015 and was diagnosed with nephrogenic systemic fibrosis (NSF) 8 weeks later. We agree with the correspondents that linear GBCAs should not be used in patients with eGFR < 30 mL/min/1.73 m2. To date, a few cases of patients who received GBCA and developed NSF since 2009 have unfortunately continued to be reported in the literature.1-3 Our intention in publishing this case was to provide ongoing education to the medical community regarding this serious condition to ensure prevention of future cases.
We thank Dr. Böhm for her important inquiry. The patient received a histopathologic diagnosis of NSF. The report from the patient’s left dorsal forearm skin punch biopsy was read by our pathologist as “fibrosis and inflammation consistent with nephrogenic systemic fibrosis,” a diagnosis agreed upon by our colleagues in the dermatology and rheumatology departments based on the rapidity of his symptom onset and progression. While we acknowledge that this patient had other inflammatory disorders of the skin that may have coexisted with the diagnosis, after weighing the preponderance of clinical evidence in support of the biopsy results, we believe that this represents a case of NSF, which is associated with high morbidity and mortality. Thankfully, the patient in this case engaged extensively in physical and occupational therapy and is still alive nearly 4 years later. We would like to thank all the letter writers for their correspondence.
Author Affiliations: Kelley Chuang and Casey Kaneshiro are Hospitalists and Jaime Betancourt is a Pulmonologist, all in the Department of Medicine at the VA Greater Los Angeles Healthcare System in California.
Correspondence: Kelley Chuang ([email protected])
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Aggarwal A, Froehlich AA, Essah P, Brinster N, High WA, Downs RW. Complications of nephrogenic systemic fibrosis following repeated exposure to gadolinium in a man with hypothyroidism: a case report. J Med Case Rep. 2011;5:566.
2. Fuah KW, Lim CT. Erythema nodosum masking nephrogenic systemic fibrosis as initial skin manifestation. BMC Nephrol. 2017;18(1):249.
3. Koratala A, Bhatti V. Nephrogenic systemic fibrosis. Clin Case Rep. 2017;5(7):1184-1185.
To the Editor:
We read with interest the case report of nephrogenic systemic fibrosis (NSF) by Chuang, Kaneshiro, and Betancourt in the June 2018 issue of Federal Practitioner.1 It was reported that a 61-year-old Hispanic male patient with a history of IV heroin abuse with end-stage renal disease (ESRD) secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection received 15 mL gadoversetamide, a linear gadolinium-based contrast agent (GBCA) during magnetic resonance imaging (MRI) of the brain. Hemodialysis was performed 18 hours after the contrast administration.
Eight weeks after his initial presentation, the patient developed pyoderma gangrenosum on his right forearm, which was treated with high-dose steroids. He then developed thickening and induration of his bilateral forearm skin with peau d’orange appearance. NSF was confirmed by a skin biopsy. The patient developed contractures of his upper and lower extremities and was finally wheelchair bound.
This case is very concerning since no NSF cases in patients receiving GBCA have been published since 2009. Unfortunately, the authors give no information on the occurrence of this particular case. Thus, it is unclear whether this case was observed before or after the switch to macrocyclic agents in patients with reduced renal function. The reported patient with ESRD was on hemodialysis and received 15 mL gadoversetamide during MRI of the brain. In 2007 the ESUR (European Society of Urogenital Radiology) published guidelines indicating linear GBCA (gadodiamide, gadoversetamide, gadopentetate dimeglumine) as high-risk agents that may not be used in patients with eGFR < 30 mL/min/1.73 m2.2,3
Consequently in 2007, the European Medicines Agency contraindicated these linear GBCA in patients with chronic kidney disease grades 4 and 5. Also in 2007 the US Food and Drug Administration (FDA) requested a revision of the prescribing information for all 5 GBCA approved in the US.4 In response to accumulating more informative data, in 2010 the FDA again used this class labeling approach to more explicitly describe differences in NSF risks among the agents.4 FDA regulation and contraindication of the use of low-stability GBCA in patients with advanced renal impairment and robust local policies on the safe use of these agents have resulted in marked reduction in the prevalence of NSF in the US. This case report needs to clarify why a high-risk linear agent was administered to a patient with ESRD.
In 2006 Grobner and Marckmann and colleagues reported their observations of a previously unrecognized link between exposure to gadodiamide and the development of NSF.5,6 It soon became clear that NSF is a delayed adverse contrast reaction that may cause severe disability and even death. Advanced renal disease and high-risk linear GBCA are the main factors in the pathogenesis of NSF. Additionally, the dose of the agent may play a role. NSF can occur from hours to years after exposure to GBCA. Not all patients with severe kidney disease exposed to high-risk agents developed NSF. Thus, additional factors were proposed to play a role in the pathogenesis of NSF. Among those factors were erythropoietin, metabolic acidosis, anion gap, iron, increased phosphate, zinc loss, proinflammatory conditions/inflammation and angiotensin-converting enzyme (ACE) inhibitors.7 Although there is little proof with these assumptions, special care must be taken as shown by this reported patient with multiple inflammatory disorders.
- Gertraud Heinz, MD, MBA; Aart van der Molen, MD; and Giles Roditi, MD; on behalf of the ESUR Contrast Media Safety Committee
Author affiliations: Gertraud Heinz is former President ESUR and Head of the Department of Radiology, Diagnostics and Intervention University Hospital St. Pölten Karl Landsteiner University of Health Sciences.
Correspondence: Gertraud Heinz (gertraud.heinz@stpoelten .lknoe.at)
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract. 2018;35(6):40-43.
2. Thomsen HS; European Society of Urogenital Radiology (ESUR). ESUR guideline: gadolinium based contrast media and nephrogenic systemic fibrosis. Eur Radiol. 2007;17(10):2692-2696.
3. Thomsen HS, Morcos SK, Almén T, et al; ESUR Contrast Medium Safety Committee. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2013;23(2):307-318
4. Yang L, Krefting I, Gorovets A, et al. Nephrogenic systemic fibrosis and class labeling of gadolinium-based agents by the Food and Drug Administration. Radiology. 2012;265(1):248-253.
5. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
6. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
7. Thomsen HS, Bennett CL. Six years after. Acta Radiol. 2012;53(8):827-829.
To the Editor:
With great interest, I read the case report by Chuang, Kaneshiro, and Betancourt.1 Patients with nephrogenic systemic fibrosis (NSF) are of special interest because the disease is still unclear as mentioned by the authors. Although new cases may occur,2 this case raises some concerns that I would like to address.
First, it would be of great interest to know the date when the patient received the high-risk gadolinium-based contrast agent (GBCA) gadoversetamide. Unfortunately, the authors did not mention the date of the injection of the GBCA that probably caused NSF. Due to the obvious association between the applications of special GBCAs in 2006, the US Food and Drug Administration (FDA) warned physicians not to inject these contrast agents in patients with compromised kidney function.3 Moreover, in 2007 the American College of Radiology (ACR) published guidelines for the safe use of GBCAs in patients with renal failure.4 Also, the European Medicines Agency (EMA) demanded that companies provide warning in product inserts about the acquisition of NSF in patients with severe kidney injury.5
Second, the clinical illustration of the case is inadequate. In the manuscript, we read that the patient acquired NSF-characteristic lesions like peau d’orange skin lesions and contractures of his extremities, but unfortunately, Chuang, Kaneshiro, and Betancourt did not provide figures that show them. On the other hand, Figure 1 shows an uncharacteristic dermal induration around inflammatory and ulcerated skin lesion (pyoderma gangrenosum).1 Such clinical signs are well known and occur perilesional of different conditions independently of NSF.6-8
Third, the histological features described as presence of fibrotic tissue in the deep dermis in Figure 2, and dermal fibrosis with thick collagen deposition in Figure 31 do not confirm the existence of NSF.
Taken together, the case presented by Chuang, Kaneshiro, and Betancourt contains some unclear aspects; therefore, it is questionable whether the published case describes a patient with NSF or not. In the current presentation, the diagnosis NSF seems to be an overestimation.
NSF still is a poorly understood disorder. Therefore, exactly documented new cases could be of clinical value when providing interesting information. Even single cases could shed some light in the darkness of the pathological mechanisms of this entity. On the other hand, we should not mix the existing cohort of published NSF cases with other scleroderma-like diseases, because this will lead to a confusion. Moreover, such a practice could inhibit the discovery of the pathophysiology of NSF.
- Ingrid Böhm, MD
Author affiliations: Ingrid Böhm is a Physician in the Department of Diagnostics, Interventional and Pediatric Radiology at the University Hospital of Bern, Inselspital, University of Bern in Bern, Switzerland.
Correspondence: Ingrid Böhm ([email protected])
Disclosures: The author reports no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract . 2018;35(6):40-43.
2. Larson KN, Gagnon AL, Darling MD, Patterson JW, Cropley TG. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151(10):1117-1120.
3. US Food and Drug Administration. A Public Health Advisory. Gadolinium-containing contrast agents for magnetic resonance imaging (MRI). http://wayback.archive-it.org/7993/20170112033022/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformation forPatientsandProviders/ucm053112.htm. Published June 8, 2006. Accessed March 15, 2019.
4. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
5. European Medicines Agency. Public statement: Vasovist and nephrogenic systemic fibrosis (NSF). https://www.ema.europa.eu/en/news/public-statement-vasovist-nephrogenic-systemic-fibrosis-nsf. Published February 7, 2007. Accessed March 15, 2019.
6. Luke JC. The etiology and modern treatment of varicose ulcer. Can Med Assoc J. 1940;43(3):217-221.
7. Paulsen E, Bygum A. Keratin gel as an adjuvant in the treatment of recalcitrant pyoderma gangrenosum ulcers: a case report. Acta Derm Venereol. 2019;99(2):234-235.
8. Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000; 136(2):167-169.
Response:
We thank Drs. Heinz, van der Molen, and Roditi for their valuable response. The following is the opinion of the authors and is not representative of the views or policies of our institution. The patient in this case received a gadolinium-based contrast agent (GBCA) in 2015 and was diagnosed with nephrogenic systemic fibrosis (NSF) 8 weeks later. We agree with the correspondents that linear GBCAs should not be used in patients with eGFR < 30 mL/min/1.73 m2. To date, a few cases of patients who received GBCA and developed NSF since 2009 have unfortunately continued to be reported in the literature.1-3 Our intention in publishing this case was to provide ongoing education to the medical community regarding this serious condition to ensure prevention of future cases.
We thank Dr. Böhm for her important inquiry. The patient received a histopathologic diagnosis of NSF. The report from the patient’s left dorsal forearm skin punch biopsy was read by our pathologist as “fibrosis and inflammation consistent with nephrogenic systemic fibrosis,” a diagnosis agreed upon by our colleagues in the dermatology and rheumatology departments based on the rapidity of his symptom onset and progression. While we acknowledge that this patient had other inflammatory disorders of the skin that may have coexisted with the diagnosis, after weighing the preponderance of clinical evidence in support of the biopsy results, we believe that this represents a case of NSF, which is associated with high morbidity and mortality. Thankfully, the patient in this case engaged extensively in physical and occupational therapy and is still alive nearly 4 years later. We would like to thank all the letter writers for their correspondence.
Author Affiliations: Kelley Chuang and Casey Kaneshiro are Hospitalists and Jaime Betancourt is a Pulmonologist, all in the Department of Medicine at the VA Greater Los Angeles Healthcare System in California.
Correspondence: Kelley Chuang ([email protected])
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Aggarwal A, Froehlich AA, Essah P, Brinster N, High WA, Downs RW. Complications of nephrogenic systemic fibrosis following repeated exposure to gadolinium in a man with hypothyroidism: a case report. J Med Case Rep. 2011;5:566.
2. Fuah KW, Lim CT. Erythema nodosum masking nephrogenic systemic fibrosis as initial skin manifestation. BMC Nephrol. 2017;18(1):249.
3. Koratala A, Bhatti V. Nephrogenic systemic fibrosis. Clin Case Rep. 2017;5(7):1184-1185.
Revering Furry Valor
National K9 Veterans Day celebrates the loyalty, bravery, and sacrifice of canine warriors. On March 13, 1942, canines officially became members of the Armed Services, with the Army’s founding of its New War Dog Program, more popularly known as the K9 Corps. The dogs underwent basic training and then entered more specialized preparation just as human soldiers did.2 There had been unofficial dogs of war who served courageously and selflessly in almost all of our armed conflicts.3 Indeed, the title of this column is taken from a wonderful article of the same name narrating the heroism of dogs in the 2 world wars.4
The dedication of canines to those who serve is not confined to combat or even active duty. Thousands of military and veteran men and women have benefited immensely from their relationship with service and emotional support dogs.
Before I continue, let me state 2 important limitations of this column. First, I am a dog person. Of course, veterans have formed healing and caring relationships with many types of companions. Equine therapy is increasingly recognized as a powerful means of helping veterans reduce distress and find purpose.5 Nevertheless, for this column, I will focus exclusively on dogs. Second, there are many worthy organizations, projects, and programs that pair veterans with therapeutic dogs inside and outside the VA. I am in no way an expert and will invariably neglect many of these positive initiatives in this brief review.
The long, proud history of canines in the military and the many moving stories of men and women in and out of uniform for whom dogs have been life changing, if not life-saving, have created 2 ethical dilemmas for the VA that I examine here. Both dilemmas pivot on the terms of official recognition of service dogs, the benefits, and who can qualify for them in the VA.
Under VA regulation and VHA policy, a service companion only can be a dog that is individually trained to do work or perform tasks to assist a person with a disability; dogs whose sole function is to provide emotional support, well-being, comfort, or companionship are not considered service pets.6
Prior to the widespread implementation of VHA Directive 1188, some VA medical centers had, pardon the pun, “gone to the dogs,” in the sense that depending on the facility, emotional support companions were found in almost every area of hospitals and clinics. Their presence enabled many patients to feel comfortable enough to seek medical and mental health care, as the canine companion gave them a sense of security and calm. But some dogs had not received the extensive training that enables a service dog to follow commands and handle the stimulation of a large, busy hospital with all its sights, sounds, and smells. Infectious disease, police, and public health authorities raised legitimate public health and safety risks about the increasing number of dogs on VA grounds who were not formally certified as service dogs. In response to those concerns, in August 2015, VHA declared a uniform policy that restricted service dogs access to VA property.7 This was, as with most health policy, a necessary, albeit utilitarian decision, that the common good outweighed that of individual veterans. Unfortunately, some veterans experienced the decision as a form of psychological rejection, and others no longer felt able mentally or physically to master the stresses of seeking health care without a canine companion.
A valid question to ask is why couldn’t the most vulnerable of these veterans, for instance those with severe mental health conditions, have service dogs that could accompany them into at least most areas of the medical center? Part of the reason is cost: Some training organizations estimate it may cost as much as $27,000 to train service dogs.8 Though there are many wonderful volunteer and not-for-profit organizations that train mostly shelter dogs and their veteran handlers—a double rescue—the lengthy process and expense means that many veterans wait years for a companion.
Congressional representatives, ethicists, veterans advocates, and canine therapy groups claim that this was unjust discrimination against those suffering with the equally, if not more disabling, mental health conditions.9 For many years, the VA has done a very good deed: For those who qualify for a service dog, VA pays for veterinary care and the equipment to handle the dog, but not boarding, grooming, food, and other miscellaneous expenses.10 But until 2016, those veterans approved for service dogs in the main had sensory or physical disabilities.
A partial breakthrough emerged when the Center for Compassionate Care Innovation launched the Mental Health Mobility Service Dogs Program that expanded veterinary health benefits to veterans with a “substantial mobility limitation.” For example, veterans whose hypervigilance and hyperarousal are so severe that they cannot attend medical appointments.11
VA experts argue that at this time there is insufficient evidence to fund service dogs as even adjunctive PTSD therapy for the hundreds of veterans who might potentially qualify. It becomes an ethical question of prudent stewardship of public funds and trust. There is certainly plenty of compelling anecdotal testimony that companion canines are a high-benefit, relatively low-risk form of complementary and integrated therapy for the spectrum of trauma disorders that afflict many of the men and women who served in our conflicts. Demonstrating those positive effects scientifically may be more difficult than it seems, although early evidence is promising, and the VA is intensively researching the question.12 For some veterans and their legislators, the VA has not gone far enough, fast enough in mainstreaming therapy dogs, they are calling for VA to expand veterans’ benefits to include mental health service dogs and to define what benefits would be covered.
National K9 Veterans Day is an important step toward giving dogs of war the homage they have earned, as are increasing efforts to ensure care for military canines throughout their life cycle. But as the seventeenth century poet John Milton wrote when he reflected on his own worth despite his blindness, “Those also serve who only stand and wait.”13 The institutions charged to care for those the battle has most burdened are still trying to discover how to properly and proportionately revere that kind of furry valor.
1. Schweitzer A. Civilization and Ethics. Naish JP, trans. London, England: A. & C. Black; 1923.
2. Bergeron AW Jr. War dogs: the birth of the K-9 Corps. https://www.army.mil/article/7463/war_dogs_the_birth_of_the_k_9_corps. Published February 14, 2008. Accessed March 22, 2019.
3. Nye L. A brief history of dogs in warfare. https://www.military.com/undertheradar/2017/03/brief-history-dogs-warfare. Published March 20, 2017. Accessed March 24, 2019.
4. Liao S. Furry valor: The tactical dogs of WW I and II. Vet Herit. 2016;39(1):24-29.
5. Romaniuk M, Evans J, Kidd C. Evaluation of an equine-assisted therapy program for veterans who identify as ‘wounded, injured, or ill’ and their partners. PLoS One. 2018;13(9):e0203943.
6. US Department of Veterans Affairs. Frequently asked questions: service animals on VA property. https://www.blogs.va.gov/VAntage/wp-content/uploads/2015/08/FAQs_RegulationsAboutAnimalsonVAProperty.pdf. Published Accessed March 24, 2019.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1188: animals on Veterans Health Administration (VHA) property. https://www.boise.va.gov/docs/Service_Animal_Policy.pdf August 26, 2015.
8. Brulliard K. For military veterans suffering from PTSD, are service dogs good therapy? Washington Post. March 27, 2018.
9. Weinmeyer R. Service dogs for veterans with post-traumatic stress disorder. AMA J Ethics. 2015;17(6):547-552.
10. US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Care Services. Guide and service dogs. https://www.prosthetics.va.gov/serviceandguidedogs.asp. Updated August 18, 2016. Accessed March 24, 2019.
11. US Department of Veterans Affairs. VA pilots program to expand veterinary benefits for mental health mobility service dogs. https://www.blogs.va.gov/VAntage/33379/va-pilots-program-to-expand-veterinary-health-benefit-for-mental-health-mobility-service-dogs. Published Accessed March 24, 2019.
12. Yarborough BJH, Stumbo SP, Yarborough MT, Owen-Smith A, Green CA. Benefits and challenges of using service dogs for veterans with posttraumatic stress disorder. Psychiatr Rehabil J. 2018;41(2):118-124.
National K9 Veterans Day celebrates the loyalty, bravery, and sacrifice of canine warriors. On March 13, 1942, canines officially became members of the Armed Services, with the Army’s founding of its New War Dog Program, more popularly known as the K9 Corps. The dogs underwent basic training and then entered more specialized preparation just as human soldiers did.2 There had been unofficial dogs of war who served courageously and selflessly in almost all of our armed conflicts.3 Indeed, the title of this column is taken from a wonderful article of the same name narrating the heroism of dogs in the 2 world wars.4
The dedication of canines to those who serve is not confined to combat or even active duty. Thousands of military and veteran men and women have benefited immensely from their relationship with service and emotional support dogs.
Before I continue, let me state 2 important limitations of this column. First, I am a dog person. Of course, veterans have formed healing and caring relationships with many types of companions. Equine therapy is increasingly recognized as a powerful means of helping veterans reduce distress and find purpose.5 Nevertheless, for this column, I will focus exclusively on dogs. Second, there are many worthy organizations, projects, and programs that pair veterans with therapeutic dogs inside and outside the VA. I am in no way an expert and will invariably neglect many of these positive initiatives in this brief review.
The long, proud history of canines in the military and the many moving stories of men and women in and out of uniform for whom dogs have been life changing, if not life-saving, have created 2 ethical dilemmas for the VA that I examine here. Both dilemmas pivot on the terms of official recognition of service dogs, the benefits, and who can qualify for them in the VA.
Under VA regulation and VHA policy, a service companion only can be a dog that is individually trained to do work or perform tasks to assist a person with a disability; dogs whose sole function is to provide emotional support, well-being, comfort, or companionship are not considered service pets.6
Prior to the widespread implementation of VHA Directive 1188, some VA medical centers had, pardon the pun, “gone to the dogs,” in the sense that depending on the facility, emotional support companions were found in almost every area of hospitals and clinics. Their presence enabled many patients to feel comfortable enough to seek medical and mental health care, as the canine companion gave them a sense of security and calm. But some dogs had not received the extensive training that enables a service dog to follow commands and handle the stimulation of a large, busy hospital with all its sights, sounds, and smells. Infectious disease, police, and public health authorities raised legitimate public health and safety risks about the increasing number of dogs on VA grounds who were not formally certified as service dogs. In response to those concerns, in August 2015, VHA declared a uniform policy that restricted service dogs access to VA property.7 This was, as with most health policy, a necessary, albeit utilitarian decision, that the common good outweighed that of individual veterans. Unfortunately, some veterans experienced the decision as a form of psychological rejection, and others no longer felt able mentally or physically to master the stresses of seeking health care without a canine companion.
A valid question to ask is why couldn’t the most vulnerable of these veterans, for instance those with severe mental health conditions, have service dogs that could accompany them into at least most areas of the medical center? Part of the reason is cost: Some training organizations estimate it may cost as much as $27,000 to train service dogs.8 Though there are many wonderful volunteer and not-for-profit organizations that train mostly shelter dogs and their veteran handlers—a double rescue—the lengthy process and expense means that many veterans wait years for a companion.
Congressional representatives, ethicists, veterans advocates, and canine therapy groups claim that this was unjust discrimination against those suffering with the equally, if not more disabling, mental health conditions.9 For many years, the VA has done a very good deed: For those who qualify for a service dog, VA pays for veterinary care and the equipment to handle the dog, but not boarding, grooming, food, and other miscellaneous expenses.10 But until 2016, those veterans approved for service dogs in the main had sensory or physical disabilities.
A partial breakthrough emerged when the Center for Compassionate Care Innovation launched the Mental Health Mobility Service Dogs Program that expanded veterinary health benefits to veterans with a “substantial mobility limitation.” For example, veterans whose hypervigilance and hyperarousal are so severe that they cannot attend medical appointments.11
VA experts argue that at this time there is insufficient evidence to fund service dogs as even adjunctive PTSD therapy for the hundreds of veterans who might potentially qualify. It becomes an ethical question of prudent stewardship of public funds and trust. There is certainly plenty of compelling anecdotal testimony that companion canines are a high-benefit, relatively low-risk form of complementary and integrated therapy for the spectrum of trauma disorders that afflict many of the men and women who served in our conflicts. Demonstrating those positive effects scientifically may be more difficult than it seems, although early evidence is promising, and the VA is intensively researching the question.12 For some veterans and their legislators, the VA has not gone far enough, fast enough in mainstreaming therapy dogs, they are calling for VA to expand veterans’ benefits to include mental health service dogs and to define what benefits would be covered.
National K9 Veterans Day is an important step toward giving dogs of war the homage they have earned, as are increasing efforts to ensure care for military canines throughout their life cycle. But as the seventeenth century poet John Milton wrote when he reflected on his own worth despite his blindness, “Those also serve who only stand and wait.”13 The institutions charged to care for those the battle has most burdened are still trying to discover how to properly and proportionately revere that kind of furry valor.
National K9 Veterans Day celebrates the loyalty, bravery, and sacrifice of canine warriors. On March 13, 1942, canines officially became members of the Armed Services, with the Army’s founding of its New War Dog Program, more popularly known as the K9 Corps. The dogs underwent basic training and then entered more specialized preparation just as human soldiers did.2 There had been unofficial dogs of war who served courageously and selflessly in almost all of our armed conflicts.3 Indeed, the title of this column is taken from a wonderful article of the same name narrating the heroism of dogs in the 2 world wars.4
The dedication of canines to those who serve is not confined to combat or even active duty. Thousands of military and veteran men and women have benefited immensely from their relationship with service and emotional support dogs.
Before I continue, let me state 2 important limitations of this column. First, I am a dog person. Of course, veterans have formed healing and caring relationships with many types of companions. Equine therapy is increasingly recognized as a powerful means of helping veterans reduce distress and find purpose.5 Nevertheless, for this column, I will focus exclusively on dogs. Second, there are many worthy organizations, projects, and programs that pair veterans with therapeutic dogs inside and outside the VA. I am in no way an expert and will invariably neglect many of these positive initiatives in this brief review.
The long, proud history of canines in the military and the many moving stories of men and women in and out of uniform for whom dogs have been life changing, if not life-saving, have created 2 ethical dilemmas for the VA that I examine here. Both dilemmas pivot on the terms of official recognition of service dogs, the benefits, and who can qualify for them in the VA.
Under VA regulation and VHA policy, a service companion only can be a dog that is individually trained to do work or perform tasks to assist a person with a disability; dogs whose sole function is to provide emotional support, well-being, comfort, or companionship are not considered service pets.6
Prior to the widespread implementation of VHA Directive 1188, some VA medical centers had, pardon the pun, “gone to the dogs,” in the sense that depending on the facility, emotional support companions were found in almost every area of hospitals and clinics. Their presence enabled many patients to feel comfortable enough to seek medical and mental health care, as the canine companion gave them a sense of security and calm. But some dogs had not received the extensive training that enables a service dog to follow commands and handle the stimulation of a large, busy hospital with all its sights, sounds, and smells. Infectious disease, police, and public health authorities raised legitimate public health and safety risks about the increasing number of dogs on VA grounds who were not formally certified as service dogs. In response to those concerns, in August 2015, VHA declared a uniform policy that restricted service dogs access to VA property.7 This was, as with most health policy, a necessary, albeit utilitarian decision, that the common good outweighed that of individual veterans. Unfortunately, some veterans experienced the decision as a form of psychological rejection, and others no longer felt able mentally or physically to master the stresses of seeking health care without a canine companion.
A valid question to ask is why couldn’t the most vulnerable of these veterans, for instance those with severe mental health conditions, have service dogs that could accompany them into at least most areas of the medical center? Part of the reason is cost: Some training organizations estimate it may cost as much as $27,000 to train service dogs.8 Though there are many wonderful volunteer and not-for-profit organizations that train mostly shelter dogs and their veteran handlers—a double rescue—the lengthy process and expense means that many veterans wait years for a companion.
Congressional representatives, ethicists, veterans advocates, and canine therapy groups claim that this was unjust discrimination against those suffering with the equally, if not more disabling, mental health conditions.9 For many years, the VA has done a very good deed: For those who qualify for a service dog, VA pays for veterinary care and the equipment to handle the dog, but not boarding, grooming, food, and other miscellaneous expenses.10 But until 2016, those veterans approved for service dogs in the main had sensory or physical disabilities.
A partial breakthrough emerged when the Center for Compassionate Care Innovation launched the Mental Health Mobility Service Dogs Program that expanded veterinary health benefits to veterans with a “substantial mobility limitation.” For example, veterans whose hypervigilance and hyperarousal are so severe that they cannot attend medical appointments.11
VA experts argue that at this time there is insufficient evidence to fund service dogs as even adjunctive PTSD therapy for the hundreds of veterans who might potentially qualify. It becomes an ethical question of prudent stewardship of public funds and trust. There is certainly plenty of compelling anecdotal testimony that companion canines are a high-benefit, relatively low-risk form of complementary and integrated therapy for the spectrum of trauma disorders that afflict many of the men and women who served in our conflicts. Demonstrating those positive effects scientifically may be more difficult than it seems, although early evidence is promising, and the VA is intensively researching the question.12 For some veterans and their legislators, the VA has not gone far enough, fast enough in mainstreaming therapy dogs, they are calling for VA to expand veterans’ benefits to include mental health service dogs and to define what benefits would be covered.
National K9 Veterans Day is an important step toward giving dogs of war the homage they have earned, as are increasing efforts to ensure care for military canines throughout their life cycle. But as the seventeenth century poet John Milton wrote when he reflected on his own worth despite his blindness, “Those also serve who only stand and wait.”13 The institutions charged to care for those the battle has most burdened are still trying to discover how to properly and proportionately revere that kind of furry valor.
1. Schweitzer A. Civilization and Ethics. Naish JP, trans. London, England: A. & C. Black; 1923.
2. Bergeron AW Jr. War dogs: the birth of the K-9 Corps. https://www.army.mil/article/7463/war_dogs_the_birth_of_the_k_9_corps. Published February 14, 2008. Accessed March 22, 2019.
3. Nye L. A brief history of dogs in warfare. https://www.military.com/undertheradar/2017/03/brief-history-dogs-warfare. Published March 20, 2017. Accessed March 24, 2019.
4. Liao S. Furry valor: The tactical dogs of WW I and II. Vet Herit. 2016;39(1):24-29.
5. Romaniuk M, Evans J, Kidd C. Evaluation of an equine-assisted therapy program for veterans who identify as ‘wounded, injured, or ill’ and their partners. PLoS One. 2018;13(9):e0203943.
6. US Department of Veterans Affairs. Frequently asked questions: service animals on VA property. https://www.blogs.va.gov/VAntage/wp-content/uploads/2015/08/FAQs_RegulationsAboutAnimalsonVAProperty.pdf. Published Accessed March 24, 2019.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1188: animals on Veterans Health Administration (VHA) property. https://www.boise.va.gov/docs/Service_Animal_Policy.pdf August 26, 2015.
8. Brulliard K. For military veterans suffering from PTSD, are service dogs good therapy? Washington Post. March 27, 2018.
9. Weinmeyer R. Service dogs for veterans with post-traumatic stress disorder. AMA J Ethics. 2015;17(6):547-552.
10. US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Care Services. Guide and service dogs. https://www.prosthetics.va.gov/serviceandguidedogs.asp. Updated August 18, 2016. Accessed March 24, 2019.
11. US Department of Veterans Affairs. VA pilots program to expand veterinary benefits for mental health mobility service dogs. https://www.blogs.va.gov/VAntage/33379/va-pilots-program-to-expand-veterinary-health-benefit-for-mental-health-mobility-service-dogs. Published Accessed March 24, 2019.
12. Yarborough BJH, Stumbo SP, Yarborough MT, Owen-Smith A, Green CA. Benefits and challenges of using service dogs for veterans with posttraumatic stress disorder. Psychiatr Rehabil J. 2018;41(2):118-124.
1. Schweitzer A. Civilization and Ethics. Naish JP, trans. London, England: A. & C. Black; 1923.
2. Bergeron AW Jr. War dogs: the birth of the K-9 Corps. https://www.army.mil/article/7463/war_dogs_the_birth_of_the_k_9_corps. Published February 14, 2008. Accessed March 22, 2019.
3. Nye L. A brief history of dogs in warfare. https://www.military.com/undertheradar/2017/03/brief-history-dogs-warfare. Published March 20, 2017. Accessed March 24, 2019.
4. Liao S. Furry valor: The tactical dogs of WW I and II. Vet Herit. 2016;39(1):24-29.
5. Romaniuk M, Evans J, Kidd C. Evaluation of an equine-assisted therapy program for veterans who identify as ‘wounded, injured, or ill’ and their partners. PLoS One. 2018;13(9):e0203943.
6. US Department of Veterans Affairs. Frequently asked questions: service animals on VA property. https://www.blogs.va.gov/VAntage/wp-content/uploads/2015/08/FAQs_RegulationsAboutAnimalsonVAProperty.pdf. Published Accessed March 24, 2019.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1188: animals on Veterans Health Administration (VHA) property. https://www.boise.va.gov/docs/Service_Animal_Policy.pdf August 26, 2015.
8. Brulliard K. For military veterans suffering from PTSD, are service dogs good therapy? Washington Post. March 27, 2018.
9. Weinmeyer R. Service dogs for veterans with post-traumatic stress disorder. AMA J Ethics. 2015;17(6):547-552.
10. US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Care Services. Guide and service dogs. https://www.prosthetics.va.gov/serviceandguidedogs.asp. Updated August 18, 2016. Accessed March 24, 2019.
11. US Department of Veterans Affairs. VA pilots program to expand veterinary benefits for mental health mobility service dogs. https://www.blogs.va.gov/VAntage/33379/va-pilots-program-to-expand-veterinary-health-benefit-for-mental-health-mobility-service-dogs. Published Accessed March 24, 2019.
12. Yarborough BJH, Stumbo SP, Yarborough MT, Owen-Smith A, Green CA. Benefits and challenges of using service dogs for veterans with posttraumatic stress disorder. Psychiatr Rehabil J. 2018;41(2):118-124.
Hair Loss in Skin of Color Patients
What does your patient need to know at the first visit?
All patients, regardless of race, gender, or age, are afraid of an alopecia diagnosis. Often, the first thing a patient may say when I enter the examination room is, "Please don't tell me I have alopecia."
The first step to a successful initial visit for hair loss is addressing the angst around the word alopecia, which helps to manage the patient's hair-induced anxiety. The next priority is setting expectations for the journey including what to expect during the diagnosis process, treatment, and beyond.
Next is data collection. An extensive hair care practice investigation can begin with a survey that the patient fills out before the visit. Dive into and expand on hair loss history questions, including medical history as well as hair care practices (eg, history of use, frequency, number of years, maintenance for that particular hairstyle) such as braids (eg, individual braids, cornrow braids, with or without added synthetic or human hair), locs (eg, length of locs), chemical relaxers (eg, number of years, frequency, professionally applied or applied at home), hair color, weaves (eg, glued in, sewn in, combination), and more.1 Include a family history of hair loss, both maternal and paternal.
The hair loss investigation almost always includes a scalp biopsy, hair-pull test, dermoscopy, photographs, and even blood work, if applicable. Scalp biopsies may reveal more than one type of alopecia diagnosis, which may impact the treatment plan.2 Sending the scalp biopsy specimen to a dermatopathologist specializing in alopecia along with clinical information about the patient is preferred.
What are your go-to treatments?
My go-to treatments for patients with skin of color (SOC) and hair loss really depend on the specific diagnosis. Randomized, placebo-controlled clinical trials focusing on treatment are lacking in central centrifugal cicatricial alopecia and traction alopecia, which holds true for many other types of alopecia.
For black patients with central centrifugal cicatricial alopecia, I often address the inflammatory component of the disease with oral doxycycline and either a topical corticosteroid, such as clobetasol, or intralesional triamcinolone. Adding minoxidil-containing products later in the treatment process can be helpful. Various treatment protocols exist but are mainly based on anecdotal evidence.1
For those with traction alopecia, modification of offending hairstyle practices is a must.3 Also, treatment of inflammation is key. Typically, I gravitate to topical or intralesional corticosteroids, followed by minoxidil-containing products. However, a challenge of treating traction alopecia is changing the hair care practices that cause tight pulling, friction, or pressure on the scalp, such as from the band of a tightly fitted wig.
It is important to discuss potential side effects of any treatment with the patient. For the most common side effects, discuss how to best prevent them. For example, because of the photosensitivity potential of doxycycline, I ask patients to wear sunscreen daily. To prevent nausea, I recommend that they avoid taking doxycycline on an empty stomach, drink plenty of fluids, and avoid laying down within a few hours after taking the medication.
How do you keep patients compliant with treatment?
Dermatologists should try to understand their patients' hair. A study of 200 black women demonstrated that 68% of the patients did not think their physician understood their hair,4 which likely impacts patients' perceptions of their physician, confidence in the treatment plan, and even compliance with the plan. Attempting to understand the nuances of tightly coiled hair in those of African descent is the first step in the journey of diagnosing and treating hair loss in partnership with the patient.
Setting the goal is a crucial step toward patient compliance. It may be going out in public without a wig or weave and feeling confident, providing more coverage so affected areas do not show as much, improving scalp tenderness, and/or preventing further progression of the condition. These are all reasonable outcomes and each goal is uniquely tailored to each patient.
Familiarize yourself with various hair types, hairstyles, and preferred medication vehicles by attending continuing medical education lectures on alopecia in patients with SOC and on nuances to diagnosis and treatment, reading textbooks focusing on SOC, or seeking out mentorship from a dermatologist who is a hair expert in the types of alopecia most commonly affecting patients with SOC.
What resources do you recommend to patients for more information
For patients with scarring alopecia, the Cicatricial Alopecia Research Foundation (http://www.carfintl.org/) is a great resource for medical information and support groups. Also, the Skin of Color Society has dermatology patient education information (http://skinofcolorsociety.org/).
For patients who are extremely distressed by hair loss, I encourage them to see a mental health professional. The mental health impact of alopecia, despite the extent of disease, is likely underestimated. Patients sometimes need our permission to seek help, especially in many SOC communities where even seeking mental health care often is frowned upon.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric skin of color patients: bootcamp discussion. Cutis. 2017;100:31-35.
- Wohltmann WE, Sperling L. Histopathologic diagnosis of multifactorial alopecia. J Cutan Pathol. 2016;43:483-491.
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia. J Am Acad Dermatol. 2016;75:606-611.
- Gathers RC, Mahan MG. African American women, hair care and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
What does your patient need to know at the first visit?
All patients, regardless of race, gender, or age, are afraid of an alopecia diagnosis. Often, the first thing a patient may say when I enter the examination room is, "Please don't tell me I have alopecia."
The first step to a successful initial visit for hair loss is addressing the angst around the word alopecia, which helps to manage the patient's hair-induced anxiety. The next priority is setting expectations for the journey including what to expect during the diagnosis process, treatment, and beyond.
Next is data collection. An extensive hair care practice investigation can begin with a survey that the patient fills out before the visit. Dive into and expand on hair loss history questions, including medical history as well as hair care practices (eg, history of use, frequency, number of years, maintenance for that particular hairstyle) such as braids (eg, individual braids, cornrow braids, with or without added synthetic or human hair), locs (eg, length of locs), chemical relaxers (eg, number of years, frequency, professionally applied or applied at home), hair color, weaves (eg, glued in, sewn in, combination), and more.1 Include a family history of hair loss, both maternal and paternal.
The hair loss investigation almost always includes a scalp biopsy, hair-pull test, dermoscopy, photographs, and even blood work, if applicable. Scalp biopsies may reveal more than one type of alopecia diagnosis, which may impact the treatment plan.2 Sending the scalp biopsy specimen to a dermatopathologist specializing in alopecia along with clinical information about the patient is preferred.
What are your go-to treatments?
My go-to treatments for patients with skin of color (SOC) and hair loss really depend on the specific diagnosis. Randomized, placebo-controlled clinical trials focusing on treatment are lacking in central centrifugal cicatricial alopecia and traction alopecia, which holds true for many other types of alopecia.
For black patients with central centrifugal cicatricial alopecia, I often address the inflammatory component of the disease with oral doxycycline and either a topical corticosteroid, such as clobetasol, or intralesional triamcinolone. Adding minoxidil-containing products later in the treatment process can be helpful. Various treatment protocols exist but are mainly based on anecdotal evidence.1
For those with traction alopecia, modification of offending hairstyle practices is a must.3 Also, treatment of inflammation is key. Typically, I gravitate to topical or intralesional corticosteroids, followed by minoxidil-containing products. However, a challenge of treating traction alopecia is changing the hair care practices that cause tight pulling, friction, or pressure on the scalp, such as from the band of a tightly fitted wig.
It is important to discuss potential side effects of any treatment with the patient. For the most common side effects, discuss how to best prevent them. For example, because of the photosensitivity potential of doxycycline, I ask patients to wear sunscreen daily. To prevent nausea, I recommend that they avoid taking doxycycline on an empty stomach, drink plenty of fluids, and avoid laying down within a few hours after taking the medication.
How do you keep patients compliant with treatment?
Dermatologists should try to understand their patients' hair. A study of 200 black women demonstrated that 68% of the patients did not think their physician understood their hair,4 which likely impacts patients' perceptions of their physician, confidence in the treatment plan, and even compliance with the plan. Attempting to understand the nuances of tightly coiled hair in those of African descent is the first step in the journey of diagnosing and treating hair loss in partnership with the patient.
Setting the goal is a crucial step toward patient compliance. It may be going out in public without a wig or weave and feeling confident, providing more coverage so affected areas do not show as much, improving scalp tenderness, and/or preventing further progression of the condition. These are all reasonable outcomes and each goal is uniquely tailored to each patient.
Familiarize yourself with various hair types, hairstyles, and preferred medication vehicles by attending continuing medical education lectures on alopecia in patients with SOC and on nuances to diagnosis and treatment, reading textbooks focusing on SOC, or seeking out mentorship from a dermatologist who is a hair expert in the types of alopecia most commonly affecting patients with SOC.
What resources do you recommend to patients for more information
For patients with scarring alopecia, the Cicatricial Alopecia Research Foundation (http://www.carfintl.org/) is a great resource for medical information and support groups. Also, the Skin of Color Society has dermatology patient education information (http://skinofcolorsociety.org/).
For patients who are extremely distressed by hair loss, I encourage them to see a mental health professional. The mental health impact of alopecia, despite the extent of disease, is likely underestimated. Patients sometimes need our permission to seek help, especially in many SOC communities where even seeking mental health care often is frowned upon.
What does your patient need to know at the first visit?
All patients, regardless of race, gender, or age, are afraid of an alopecia diagnosis. Often, the first thing a patient may say when I enter the examination room is, "Please don't tell me I have alopecia."
The first step to a successful initial visit for hair loss is addressing the angst around the word alopecia, which helps to manage the patient's hair-induced anxiety. The next priority is setting expectations for the journey including what to expect during the diagnosis process, treatment, and beyond.
Next is data collection. An extensive hair care practice investigation can begin with a survey that the patient fills out before the visit. Dive into and expand on hair loss history questions, including medical history as well as hair care practices (eg, history of use, frequency, number of years, maintenance for that particular hairstyle) such as braids (eg, individual braids, cornrow braids, with or without added synthetic or human hair), locs (eg, length of locs), chemical relaxers (eg, number of years, frequency, professionally applied or applied at home), hair color, weaves (eg, glued in, sewn in, combination), and more.1 Include a family history of hair loss, both maternal and paternal.
The hair loss investigation almost always includes a scalp biopsy, hair-pull test, dermoscopy, photographs, and even blood work, if applicable. Scalp biopsies may reveal more than one type of alopecia diagnosis, which may impact the treatment plan.2 Sending the scalp biopsy specimen to a dermatopathologist specializing in alopecia along with clinical information about the patient is preferred.
What are your go-to treatments?
My go-to treatments for patients with skin of color (SOC) and hair loss really depend on the specific diagnosis. Randomized, placebo-controlled clinical trials focusing on treatment are lacking in central centrifugal cicatricial alopecia and traction alopecia, which holds true for many other types of alopecia.
For black patients with central centrifugal cicatricial alopecia, I often address the inflammatory component of the disease with oral doxycycline and either a topical corticosteroid, such as clobetasol, or intralesional triamcinolone. Adding minoxidil-containing products later in the treatment process can be helpful. Various treatment protocols exist but are mainly based on anecdotal evidence.1
For those with traction alopecia, modification of offending hairstyle practices is a must.3 Also, treatment of inflammation is key. Typically, I gravitate to topical or intralesional corticosteroids, followed by minoxidil-containing products. However, a challenge of treating traction alopecia is changing the hair care practices that cause tight pulling, friction, or pressure on the scalp, such as from the band of a tightly fitted wig.
It is important to discuss potential side effects of any treatment with the patient. For the most common side effects, discuss how to best prevent them. For example, because of the photosensitivity potential of doxycycline, I ask patients to wear sunscreen daily. To prevent nausea, I recommend that they avoid taking doxycycline on an empty stomach, drink plenty of fluids, and avoid laying down within a few hours after taking the medication.
How do you keep patients compliant with treatment?
Dermatologists should try to understand their patients' hair. A study of 200 black women demonstrated that 68% of the patients did not think their physician understood their hair,4 which likely impacts patients' perceptions of their physician, confidence in the treatment plan, and even compliance with the plan. Attempting to understand the nuances of tightly coiled hair in those of African descent is the first step in the journey of diagnosing and treating hair loss in partnership with the patient.
Setting the goal is a crucial step toward patient compliance. It may be going out in public without a wig or weave and feeling confident, providing more coverage so affected areas do not show as much, improving scalp tenderness, and/or preventing further progression of the condition. These are all reasonable outcomes and each goal is uniquely tailored to each patient.
Familiarize yourself with various hair types, hairstyles, and preferred medication vehicles by attending continuing medical education lectures on alopecia in patients with SOC and on nuances to diagnosis and treatment, reading textbooks focusing on SOC, or seeking out mentorship from a dermatologist who is a hair expert in the types of alopecia most commonly affecting patients with SOC.
What resources do you recommend to patients for more information
For patients with scarring alopecia, the Cicatricial Alopecia Research Foundation (http://www.carfintl.org/) is a great resource for medical information and support groups. Also, the Skin of Color Society has dermatology patient education information (http://skinofcolorsociety.org/).
For patients who are extremely distressed by hair loss, I encourage them to see a mental health professional. The mental health impact of alopecia, despite the extent of disease, is likely underestimated. Patients sometimes need our permission to seek help, especially in many SOC communities where even seeking mental health care often is frowned upon.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric skin of color patients: bootcamp discussion. Cutis. 2017;100:31-35.
- Wohltmann WE, Sperling L. Histopathologic diagnosis of multifactorial alopecia. J Cutan Pathol. 2016;43:483-491.
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia. J Am Acad Dermatol. 2016;75:606-611.
- Gathers RC, Mahan MG. African American women, hair care and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric skin of color patients: bootcamp discussion. Cutis. 2017;100:31-35.
- Wohltmann WE, Sperling L. Histopathologic diagnosis of multifactorial alopecia. J Cutan Pathol. 2016;43:483-491.
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia. J Am Acad Dermatol. 2016;75:606-611.
- Gathers RC, Mahan MG. African American women, hair care and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.