User login
Psychiatry residents not getting training in treating chronic pain
SAN FRANCISCO –
Given the unique role of psychiatrists in helping chronic pain patients with coping strategies and managing comorbid psychiatric illness, this void is concerning, said Ali Ahsan Ali, MD, a resident psychiatrist at the Micah School of Medicine at Mount Sinai/Elmhurst Hospital Center in New York, in an interview at the annual meeting of the American Psychiatric Association.
In a video interview, Dr. Ali spoke with Ahmar M. Butt, MD, about how and why Dr. Ali and his colleagues conducted the survey of all 221 U.S. psychiatry residency programs in January 2019. They also discuss the implications of these trends for patients, particularly in light of the country’s opioid crisis.
Dr. Ali had no disclosures. Dr. Butt is board certified in general psychiatry, child and adolescent psychiatry, and preventive medicine, with a subspecialty in addiction medicine. Dr. Butt is interim program director of the psychiatry residency program at Broadlawns UnityPointe Health, Des Moines, Iowa. He had no disclosures.
SAN FRANCISCO –
Given the unique role of psychiatrists in helping chronic pain patients with coping strategies and managing comorbid psychiatric illness, this void is concerning, said Ali Ahsan Ali, MD, a resident psychiatrist at the Micah School of Medicine at Mount Sinai/Elmhurst Hospital Center in New York, in an interview at the annual meeting of the American Psychiatric Association.
In a video interview, Dr. Ali spoke with Ahmar M. Butt, MD, about how and why Dr. Ali and his colleagues conducted the survey of all 221 U.S. psychiatry residency programs in January 2019. They also discuss the implications of these trends for patients, particularly in light of the country’s opioid crisis.
Dr. Ali had no disclosures. Dr. Butt is board certified in general psychiatry, child and adolescent psychiatry, and preventive medicine, with a subspecialty in addiction medicine. Dr. Butt is interim program director of the psychiatry residency program at Broadlawns UnityPointe Health, Des Moines, Iowa. He had no disclosures.
SAN FRANCISCO –
Given the unique role of psychiatrists in helping chronic pain patients with coping strategies and managing comorbid psychiatric illness, this void is concerning, said Ali Ahsan Ali, MD, a resident psychiatrist at the Micah School of Medicine at Mount Sinai/Elmhurst Hospital Center in New York, in an interview at the annual meeting of the American Psychiatric Association.
In a video interview, Dr. Ali spoke with Ahmar M. Butt, MD, about how and why Dr. Ali and his colleagues conducted the survey of all 221 U.S. psychiatry residency programs in January 2019. They also discuss the implications of these trends for patients, particularly in light of the country’s opioid crisis.
Dr. Ali had no disclosures. Dr. Butt is board certified in general psychiatry, child and adolescent psychiatry, and preventive medicine, with a subspecialty in addiction medicine. Dr. Butt is interim program director of the psychiatry residency program at Broadlawns UnityPointe Health, Des Moines, Iowa. He had no disclosures.
REPORTING FROM APA 2019
One hundred thousand reasons to donate to your political action committee (PAC)
Payment policy for physicians is now set at the federal level. The Centers for Medicare and Medicaid Services generates a yearly final rule and a fee schedule, and all the Medicare carriers, AND the Medicare Advantage plans AND the private insurers use the rule and fee schedule as a payment guide.
Sometimes these rules can be at odds with best practices for dermatology patients. That is why lobbying is so critically important for us and for our patients.
Each year, SkinPAC contributes up to $5,000 a year to individual congressional races. The extent of contributions is based on an impartial scorecard that ranks congressional members by leadership position as well as the member’s understanding and history of support on our critical issues. I want to emphasize that the personal political leanings of the SkinPAC board members have no bearing on the level of support. We contribute to campaigns based on the congressional members’ positions on our issues, period. Full disclosure: I am the chair of SkinPAC for 2019-2021. This is an unpaid volunteer position.
To dermatologists who question the effectiveness of lobbying, I can attest that I have seen your political action committee contributions in action.
When Congress planned on tightening the Stark exceptions 5 years ago, our Washington office was able to gain access to key legislators. As a result of our good long-term relationships with these congress members and their staff, our lobbying group was able to explain the importance for dermatologists to be able to read their own slides and the value of global periods. Imagine the disasters of being unable to read our own dermatopathology slides, not performing diagnostic frozen sections before Mohs, and charging patients for suture removals. Lobbying efforts averted those potential catastrophes.
Unfortunately, the same issues are coming back. In the most recent Federal Register proposals, CMS again wanted to eliminate global periods and modifier 25, which allows you to bill for a procedure on the same day as an evaluation and management code. This action has been delayed for 2 years but will come back up for consideration next year.
Global periods are follow-up visits that are embedded in the destruction, excision, and repair codes that you currently use. For example, $42 of the $72 you get for destroying a premalignant lesion or a wart is a prepayment for the follow-up visit. Sure, if the global period is eliminated, you can bill the patient for the follow-up visit, but imagine the difficulty of collecting additional copays and deductibles. And imagine the impact of those additional costs on our patients.
This brings me to your 100,000 reasons to contribute to your PAC. In Medicare alone, elimination of global periods and modifier 25 will shift $1.4 billion dollars per year away from dermatology. Assuming you will be able to recoup some payment from follow-up visits and by rescheduling some procedures, you are still looking at $1 billion or so, per year, cut from about 10,000 dermatologists with the expense shifted to patients. That’s a $100,000 loss per dermatologist per year and a $1 billion per year additional responsibility for Medicare insureds.
Yes, this will require a legislative fix. And unless it is fixed, the results will be viewed as price gouging by patients with disastrous implications for the physician-patient relationship. Imagine what your patient will say when you charge them to remove their sutures.
Your SkinPAC contribution should be viewed as a disaster insurance policy, just like any other insurance you buy. It covers the very real possibility of not a hurricane or a tornado, but a catastrophic blunder that will put you out of business as surely as any natural disaster. Support your SkinPAC! Support your patients and yourself.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Dr. Coldiron is the chair of SkinPAC for 2019-2021; this is an unpaid volunteer position. Write to him at [email protected].
Payment policy for physicians is now set at the federal level. The Centers for Medicare and Medicaid Services generates a yearly final rule and a fee schedule, and all the Medicare carriers, AND the Medicare Advantage plans AND the private insurers use the rule and fee schedule as a payment guide.
Sometimes these rules can be at odds with best practices for dermatology patients. That is why lobbying is so critically important for us and for our patients.
Each year, SkinPAC contributes up to $5,000 a year to individual congressional races. The extent of contributions is based on an impartial scorecard that ranks congressional members by leadership position as well as the member’s understanding and history of support on our critical issues. I want to emphasize that the personal political leanings of the SkinPAC board members have no bearing on the level of support. We contribute to campaigns based on the congressional members’ positions on our issues, period. Full disclosure: I am the chair of SkinPAC for 2019-2021. This is an unpaid volunteer position.
To dermatologists who question the effectiveness of lobbying, I can attest that I have seen your political action committee contributions in action.
When Congress planned on tightening the Stark exceptions 5 years ago, our Washington office was able to gain access to key legislators. As a result of our good long-term relationships with these congress members and their staff, our lobbying group was able to explain the importance for dermatologists to be able to read their own slides and the value of global periods. Imagine the disasters of being unable to read our own dermatopathology slides, not performing diagnostic frozen sections before Mohs, and charging patients for suture removals. Lobbying efforts averted those potential catastrophes.
Unfortunately, the same issues are coming back. In the most recent Federal Register proposals, CMS again wanted to eliminate global periods and modifier 25, which allows you to bill for a procedure on the same day as an evaluation and management code. This action has been delayed for 2 years but will come back up for consideration next year.
Global periods are follow-up visits that are embedded in the destruction, excision, and repair codes that you currently use. For example, $42 of the $72 you get for destroying a premalignant lesion or a wart is a prepayment for the follow-up visit. Sure, if the global period is eliminated, you can bill the patient for the follow-up visit, but imagine the difficulty of collecting additional copays and deductibles. And imagine the impact of those additional costs on our patients.
This brings me to your 100,000 reasons to contribute to your PAC. In Medicare alone, elimination of global periods and modifier 25 will shift $1.4 billion dollars per year away from dermatology. Assuming you will be able to recoup some payment from follow-up visits and by rescheduling some procedures, you are still looking at $1 billion or so, per year, cut from about 10,000 dermatologists with the expense shifted to patients. That’s a $100,000 loss per dermatologist per year and a $1 billion per year additional responsibility for Medicare insureds.
Yes, this will require a legislative fix. And unless it is fixed, the results will be viewed as price gouging by patients with disastrous implications for the physician-patient relationship. Imagine what your patient will say when you charge them to remove their sutures.
Your SkinPAC contribution should be viewed as a disaster insurance policy, just like any other insurance you buy. It covers the very real possibility of not a hurricane or a tornado, but a catastrophic blunder that will put you out of business as surely as any natural disaster. Support your SkinPAC! Support your patients and yourself.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Dr. Coldiron is the chair of SkinPAC for 2019-2021; this is an unpaid volunteer position. Write to him at [email protected].
Payment policy for physicians is now set at the federal level. The Centers for Medicare and Medicaid Services generates a yearly final rule and a fee schedule, and all the Medicare carriers, AND the Medicare Advantage plans AND the private insurers use the rule and fee schedule as a payment guide.
Sometimes these rules can be at odds with best practices for dermatology patients. That is why lobbying is so critically important for us and for our patients.
Each year, SkinPAC contributes up to $5,000 a year to individual congressional races. The extent of contributions is based on an impartial scorecard that ranks congressional members by leadership position as well as the member’s understanding and history of support on our critical issues. I want to emphasize that the personal political leanings of the SkinPAC board members have no bearing on the level of support. We contribute to campaigns based on the congressional members’ positions on our issues, period. Full disclosure: I am the chair of SkinPAC for 2019-2021. This is an unpaid volunteer position.
To dermatologists who question the effectiveness of lobbying, I can attest that I have seen your political action committee contributions in action.
When Congress planned on tightening the Stark exceptions 5 years ago, our Washington office was able to gain access to key legislators. As a result of our good long-term relationships with these congress members and their staff, our lobbying group was able to explain the importance for dermatologists to be able to read their own slides and the value of global periods. Imagine the disasters of being unable to read our own dermatopathology slides, not performing diagnostic frozen sections before Mohs, and charging patients for suture removals. Lobbying efforts averted those potential catastrophes.
Unfortunately, the same issues are coming back. In the most recent Federal Register proposals, CMS again wanted to eliminate global periods and modifier 25, which allows you to bill for a procedure on the same day as an evaluation and management code. This action has been delayed for 2 years but will come back up for consideration next year.
Global periods are follow-up visits that are embedded in the destruction, excision, and repair codes that you currently use. For example, $42 of the $72 you get for destroying a premalignant lesion or a wart is a prepayment for the follow-up visit. Sure, if the global period is eliminated, you can bill the patient for the follow-up visit, but imagine the difficulty of collecting additional copays and deductibles. And imagine the impact of those additional costs on our patients.
This brings me to your 100,000 reasons to contribute to your PAC. In Medicare alone, elimination of global periods and modifier 25 will shift $1.4 billion dollars per year away from dermatology. Assuming you will be able to recoup some payment from follow-up visits and by rescheduling some procedures, you are still looking at $1 billion or so, per year, cut from about 10,000 dermatologists with the expense shifted to patients. That’s a $100,000 loss per dermatologist per year and a $1 billion per year additional responsibility for Medicare insureds.
Yes, this will require a legislative fix. And unless it is fixed, the results will be viewed as price gouging by patients with disastrous implications for the physician-patient relationship. Imagine what your patient will say when you charge them to remove their sutures.
Your SkinPAC contribution should be viewed as a disaster insurance policy, just like any other insurance you buy. It covers the very real possibility of not a hurricane or a tornado, but a catastrophic blunder that will put you out of business as surely as any natural disaster. Support your SkinPAC! Support your patients and yourself.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Dr. Coldiron is the chair of SkinPAC for 2019-2021; this is an unpaid volunteer position. Write to him at [email protected].
Pap screen gaps abound in SLE population
SAN FRANCISCO – Ann Igoe, MD, said at an international congress on systemic lupus erythematosus.
Why is this of relevance to rheumatologists?
“The rheumatologist is probably the main physician that lupus patients see. They may see their rheumatologist every couple of months. The question is, how often do rheumatologists say, ‘Hey, you need your Pap smear!’ I don’t think many of them address it,” said Dr. Igoe, a rheumatology fellow at Case Western Reserve University in Cleveland.
She presented a retrospective, cross-sectional, single-center study utilizing the EHRs of 604 women with SLE and 3,337 female controls who had asthma but not SLE. Sixty percent of the SLE patients were overdue for a Pap smear, compared with 51% of controls.
“We also looked at race,” Dr. Igoe said in an interview. “We were able to show that, at our institution, racial disparities do exist, that the black lupus patients had a much higher rate of HPV [human papillomavirus] positivity, compared to the white lupus patients, and they also were more behind on their Pap screening.”
Indeed, 56% of the black lupus patients were overdue for a Pap test, compared with 43% of the white SLE patients, and 46% of black women without SLE. Among the subgroup composed of black HPV-positive SLE patients, the overdue status rate soared to 70%, versus 30% in white HPV-positive SLE patients.
Dr. Igoe noted that in October 2018, the Food and Drug Administration approved an expanded indication for the quadrivalent HPV vaccine known as Gardasil 9 for women through 45 years of age. The prior upper age limit was age 26. This is an especially important development for unvaccinated women with SLE. Women with SLE have been shown to have higher rates of cervical neoplasia than in the general population, and being on potent immunosuppressive agents such as mycophenolate mofetil, azathioprine, and methotrexate further boosts that risk.
“We and others have shown that women with lupus who receive the vaccine mount a good response. So regardless of whether you’ve had HPV in the past, that doesn’t preclude you from getting the vaccine,” she noted.
The Advisory Committee on Immunization Practices and Centers for Disease Control and Prevention have yet to adopt the expanded age limit recommendation. That needs to happen, Dr. Igoe stressed.
“I’d like to see this study as a little stepping stone towards having women get their Pap screen addressed and making the Gardasil vaccine available to women who are not vaccinated,” she said.
She reported having no financial conflicts regarding her study, conducted free of commercial support.
SAN FRANCISCO – Ann Igoe, MD, said at an international congress on systemic lupus erythematosus.
Why is this of relevance to rheumatologists?
“The rheumatologist is probably the main physician that lupus patients see. They may see their rheumatologist every couple of months. The question is, how often do rheumatologists say, ‘Hey, you need your Pap smear!’ I don’t think many of them address it,” said Dr. Igoe, a rheumatology fellow at Case Western Reserve University in Cleveland.
She presented a retrospective, cross-sectional, single-center study utilizing the EHRs of 604 women with SLE and 3,337 female controls who had asthma but not SLE. Sixty percent of the SLE patients were overdue for a Pap smear, compared with 51% of controls.
“We also looked at race,” Dr. Igoe said in an interview. “We were able to show that, at our institution, racial disparities do exist, that the black lupus patients had a much higher rate of HPV [human papillomavirus] positivity, compared to the white lupus patients, and they also were more behind on their Pap screening.”
Indeed, 56% of the black lupus patients were overdue for a Pap test, compared with 43% of the white SLE patients, and 46% of black women without SLE. Among the subgroup composed of black HPV-positive SLE patients, the overdue status rate soared to 70%, versus 30% in white HPV-positive SLE patients.
Dr. Igoe noted that in October 2018, the Food and Drug Administration approved an expanded indication for the quadrivalent HPV vaccine known as Gardasil 9 for women through 45 years of age. The prior upper age limit was age 26. This is an especially important development for unvaccinated women with SLE. Women with SLE have been shown to have higher rates of cervical neoplasia than in the general population, and being on potent immunosuppressive agents such as mycophenolate mofetil, azathioprine, and methotrexate further boosts that risk.
“We and others have shown that women with lupus who receive the vaccine mount a good response. So regardless of whether you’ve had HPV in the past, that doesn’t preclude you from getting the vaccine,” she noted.
The Advisory Committee on Immunization Practices and Centers for Disease Control and Prevention have yet to adopt the expanded age limit recommendation. That needs to happen, Dr. Igoe stressed.
“I’d like to see this study as a little stepping stone towards having women get their Pap screen addressed and making the Gardasil vaccine available to women who are not vaccinated,” she said.
She reported having no financial conflicts regarding her study, conducted free of commercial support.
SAN FRANCISCO – Ann Igoe, MD, said at an international congress on systemic lupus erythematosus.
Why is this of relevance to rheumatologists?
“The rheumatologist is probably the main physician that lupus patients see. They may see their rheumatologist every couple of months. The question is, how often do rheumatologists say, ‘Hey, you need your Pap smear!’ I don’t think many of them address it,” said Dr. Igoe, a rheumatology fellow at Case Western Reserve University in Cleveland.
She presented a retrospective, cross-sectional, single-center study utilizing the EHRs of 604 women with SLE and 3,337 female controls who had asthma but not SLE. Sixty percent of the SLE patients were overdue for a Pap smear, compared with 51% of controls.
“We also looked at race,” Dr. Igoe said in an interview. “We were able to show that, at our institution, racial disparities do exist, that the black lupus patients had a much higher rate of HPV [human papillomavirus] positivity, compared to the white lupus patients, and they also were more behind on their Pap screening.”
Indeed, 56% of the black lupus patients were overdue for a Pap test, compared with 43% of the white SLE patients, and 46% of black women without SLE. Among the subgroup composed of black HPV-positive SLE patients, the overdue status rate soared to 70%, versus 30% in white HPV-positive SLE patients.
Dr. Igoe noted that in October 2018, the Food and Drug Administration approved an expanded indication for the quadrivalent HPV vaccine known as Gardasil 9 for women through 45 years of age. The prior upper age limit was age 26. This is an especially important development for unvaccinated women with SLE. Women with SLE have been shown to have higher rates of cervical neoplasia than in the general population, and being on potent immunosuppressive agents such as mycophenolate mofetil, azathioprine, and methotrexate further boosts that risk.
“We and others have shown that women with lupus who receive the vaccine mount a good response. So regardless of whether you’ve had HPV in the past, that doesn’t preclude you from getting the vaccine,” she noted.
The Advisory Committee on Immunization Practices and Centers for Disease Control and Prevention have yet to adopt the expanded age limit recommendation. That needs to happen, Dr. Igoe stressed.
“I’d like to see this study as a little stepping stone towards having women get their Pap screen addressed and making the Gardasil vaccine available to women who are not vaccinated,” she said.
She reported having no financial conflicts regarding her study, conducted free of commercial support.
REPORTING FROM LUPUS 2019
Which antidiabetic for elderly patients? It depends on their CV risk
SAN FRANCISCO – SGLT2 inhibitors did a better job than GLP-1 receptor agonists at preventing heart failure hospitalizations in elderly patients with type 2 diabetes, but at the cost of more strokes, myocardial infarctions, and deaths among those without preexisting cardiovascular disease, according to Harvard University investigators.
Using Medicare claims data and propensity scoring, they matched 43,609 elderly patients who started a sodium-glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes, 77% of whom were taking canagliflozin (Invokana), to 43,609 who started a glucagonlike peptide–1 (GLP-1)–receptor agonist, 60% of whom were taking liraglutide (Victoza).
Patients were paired by age, comorbidities, diabetes severity, and dozens of other variables, more than 120 in all. The data window ran from April 2013 through December 2016.
The idea was to compare the drugs directly in order to help clinicians decide which class to choose for older patients as second-line therapy, an important consideration at a time when there’s not much guidance specifically for the elderly, and manufacturers are issuing dueling placebo-controlled trials.
Both classes have shown cardiovascular benefits, but studies were mostly in younger people with preexisting cardiovascular disease (CVD). “The comparative impact of these agents in the older population has not yet been established,” lead investigator Elisabetta Patorno, MD, DrPH, of Harvard University, Boston, said at the annual scientific sessions of the American Diabetes Association.
General themes are emerging from Dr. Patorno’s work; it seems that deciding between the two classes has a lot to do with whether the main concern is heart failure or cardiovascular events. Even so, she said, it’s too early to incorporate the observations into guidelines. The analysis is ongoing, and there are plans to compare impacts on renal disease and other problems.
In the meantime, she and her colleagues found that initiating an SGLT2 inhibitor versus a GLP-1 receptor agonist in the elderly was associated with a 34% decreased risk of heart failure hospitalization (2.5 fewer hospitalizations per 1,000 patient years), with an even larger drop among people who had preexisting CVD.
There was, however, a 41% increased risk of lower limb amputations (0.8 more events per 1,000 patient years) and a 62% increase in diabetic ketoacidosis (DKA, 1 more event), problems previously associated with the class.
Results were comparable – fewer heart failure hospitalizations but more amputations and DKA – when SGLT2 initiation was compared to initiation with dipeptidyl peptidase-4 (DPP-4) inhibitors, another second-line option for type 2 diabetes that includes sitagliptin (Januvia), among others.
There was a 25% increased relative risk of the composite primary outcome of myocardial infarction, stroke, and all-cause mortality when patients without baseline CVD were started on an SGLT2 inhibitor instead of a GLP-1 receptor agonist (3.7 more events per 1,000 patient years). There was no increased risk among patients who already had CVD.
SGLT2 initiation actually had a protective effect, compared with dipeptidyl peptidase-4 inhibitors, with a 23% decreased risk of the composite outcome (6.5 fewer events) among patients both with and without baseline CVD. The findings were all statistically significant.
The average age in the study was 71.5 years; 45% of the subjects were men; 40% had a history of cardiovascular disease; and 60% were on metformin and 24% on insulin at study entry.
The work was funded by the National Institutes of Health. Dr. Patorno disclosed research grants form Boehringer Ingelheim and GlaxoSmithKline. Other investigators reported relationships with numerous pharmaceutical companies.
SAN FRANCISCO – SGLT2 inhibitors did a better job than GLP-1 receptor agonists at preventing heart failure hospitalizations in elderly patients with type 2 diabetes, but at the cost of more strokes, myocardial infarctions, and deaths among those without preexisting cardiovascular disease, according to Harvard University investigators.
Using Medicare claims data and propensity scoring, they matched 43,609 elderly patients who started a sodium-glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes, 77% of whom were taking canagliflozin (Invokana), to 43,609 who started a glucagonlike peptide–1 (GLP-1)–receptor agonist, 60% of whom were taking liraglutide (Victoza).
Patients were paired by age, comorbidities, diabetes severity, and dozens of other variables, more than 120 in all. The data window ran from April 2013 through December 2016.
The idea was to compare the drugs directly in order to help clinicians decide which class to choose for older patients as second-line therapy, an important consideration at a time when there’s not much guidance specifically for the elderly, and manufacturers are issuing dueling placebo-controlled trials.
Both classes have shown cardiovascular benefits, but studies were mostly in younger people with preexisting cardiovascular disease (CVD). “The comparative impact of these agents in the older population has not yet been established,” lead investigator Elisabetta Patorno, MD, DrPH, of Harvard University, Boston, said at the annual scientific sessions of the American Diabetes Association.
General themes are emerging from Dr. Patorno’s work; it seems that deciding between the two classes has a lot to do with whether the main concern is heart failure or cardiovascular events. Even so, she said, it’s too early to incorporate the observations into guidelines. The analysis is ongoing, and there are plans to compare impacts on renal disease and other problems.
In the meantime, she and her colleagues found that initiating an SGLT2 inhibitor versus a GLP-1 receptor agonist in the elderly was associated with a 34% decreased risk of heart failure hospitalization (2.5 fewer hospitalizations per 1,000 patient years), with an even larger drop among people who had preexisting CVD.
There was, however, a 41% increased risk of lower limb amputations (0.8 more events per 1,000 patient years) and a 62% increase in diabetic ketoacidosis (DKA, 1 more event), problems previously associated with the class.
Results were comparable – fewer heart failure hospitalizations but more amputations and DKA – when SGLT2 initiation was compared to initiation with dipeptidyl peptidase-4 (DPP-4) inhibitors, another second-line option for type 2 diabetes that includes sitagliptin (Januvia), among others.
There was a 25% increased relative risk of the composite primary outcome of myocardial infarction, stroke, and all-cause mortality when patients without baseline CVD were started on an SGLT2 inhibitor instead of a GLP-1 receptor agonist (3.7 more events per 1,000 patient years). There was no increased risk among patients who already had CVD.
SGLT2 initiation actually had a protective effect, compared with dipeptidyl peptidase-4 inhibitors, with a 23% decreased risk of the composite outcome (6.5 fewer events) among patients both with and without baseline CVD. The findings were all statistically significant.
The average age in the study was 71.5 years; 45% of the subjects were men; 40% had a history of cardiovascular disease; and 60% were on metformin and 24% on insulin at study entry.
The work was funded by the National Institutes of Health. Dr. Patorno disclosed research grants form Boehringer Ingelheim and GlaxoSmithKline. Other investigators reported relationships with numerous pharmaceutical companies.
SAN FRANCISCO – SGLT2 inhibitors did a better job than GLP-1 receptor agonists at preventing heart failure hospitalizations in elderly patients with type 2 diabetes, but at the cost of more strokes, myocardial infarctions, and deaths among those without preexisting cardiovascular disease, according to Harvard University investigators.
Using Medicare claims data and propensity scoring, they matched 43,609 elderly patients who started a sodium-glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes, 77% of whom were taking canagliflozin (Invokana), to 43,609 who started a glucagonlike peptide–1 (GLP-1)–receptor agonist, 60% of whom were taking liraglutide (Victoza).
Patients were paired by age, comorbidities, diabetes severity, and dozens of other variables, more than 120 in all. The data window ran from April 2013 through December 2016.
The idea was to compare the drugs directly in order to help clinicians decide which class to choose for older patients as second-line therapy, an important consideration at a time when there’s not much guidance specifically for the elderly, and manufacturers are issuing dueling placebo-controlled trials.
Both classes have shown cardiovascular benefits, but studies were mostly in younger people with preexisting cardiovascular disease (CVD). “The comparative impact of these agents in the older population has not yet been established,” lead investigator Elisabetta Patorno, MD, DrPH, of Harvard University, Boston, said at the annual scientific sessions of the American Diabetes Association.
General themes are emerging from Dr. Patorno’s work; it seems that deciding between the two classes has a lot to do with whether the main concern is heart failure or cardiovascular events. Even so, she said, it’s too early to incorporate the observations into guidelines. The analysis is ongoing, and there are plans to compare impacts on renal disease and other problems.
In the meantime, she and her colleagues found that initiating an SGLT2 inhibitor versus a GLP-1 receptor agonist in the elderly was associated with a 34% decreased risk of heart failure hospitalization (2.5 fewer hospitalizations per 1,000 patient years), with an even larger drop among people who had preexisting CVD.
There was, however, a 41% increased risk of lower limb amputations (0.8 more events per 1,000 patient years) and a 62% increase in diabetic ketoacidosis (DKA, 1 more event), problems previously associated with the class.
Results were comparable – fewer heart failure hospitalizations but more amputations and DKA – when SGLT2 initiation was compared to initiation with dipeptidyl peptidase-4 (DPP-4) inhibitors, another second-line option for type 2 diabetes that includes sitagliptin (Januvia), among others.
There was a 25% increased relative risk of the composite primary outcome of myocardial infarction, stroke, and all-cause mortality when patients without baseline CVD were started on an SGLT2 inhibitor instead of a GLP-1 receptor agonist (3.7 more events per 1,000 patient years). There was no increased risk among patients who already had CVD.
SGLT2 initiation actually had a protective effect, compared with dipeptidyl peptidase-4 inhibitors, with a 23% decreased risk of the composite outcome (6.5 fewer events) among patients both with and without baseline CVD. The findings were all statistically significant.
The average age in the study was 71.5 years; 45% of the subjects were men; 40% had a history of cardiovascular disease; and 60% were on metformin and 24% on insulin at study entry.
The work was funded by the National Institutes of Health. Dr. Patorno disclosed research grants form Boehringer Ingelheim and GlaxoSmithKline. Other investigators reported relationships with numerous pharmaceutical companies.
REPORTING FROM ADA 2019
Are hospitalists being more highly valued?
An uptrend in financial support
Since the inception of hospital medicine more than 2 decades ago, the total number of hospitalists has rapidly increased to more than 60,000. The Society of Hospital Medicine’s State of Hospital Medicine Report (SoHM), published biennially, captures new changes in our growing field and sheds light on current practice trends.
Among its findings, the 2018 SoHM Report reassuringly reveals that financial support from hospitals to hospital medicine groups (HMGs) continues to climb, even in the setting of rising health care costs and ongoing budget pressure.
The median amount of financial support per full-time equivalent (FTE) physician for HMGs serving adults was $176,658, according to the 2018 SoHM Report, which is up 12% from the 2016 median of $157,535. While there is no correlation between group sizes and the amount of financial support per FTE physician, there are significant differences across regions, with HMGs in the Midwest garnering the highest median support, at $193,121 per FTE physician.
The report also reveals big differences by employment model. For example, private multispecialty and primary care medical groups receive much less financial support ($58,396 per FTE physician) than HMGs employed by hospitals. This likely signifies that their main source of revenue is from professional service fees. Regardless of the types of employment models, past surveys have reported more than 95% of HMGs receive support from their hospitals to help cover expenses.
The median amount of financial support per FTE provider (including nurse practitioners, physician assistants, and locum tenens) was $134,300, which represents a 3.3% decrease, compared with the 2016 SoHM Report. For the first time, the 2018 SoHM also collected data on financial support per “work relative value unit” (wRVU) in addition to support per FTE physician and support per FTE provider. HMGs and their hospitals can use support per wRVU data to evaluate the support per unit of work, regardless of who (whether it is a physician, an advanced practice provider, and/or others) performed that work.
The median amount of financial support per wRVU for HMGs serving adults in 2018 was $41.92, with academic HMGs reporting a higher amount ($45.81) than nonacademic HMGs ($41.28). It will be interesting to track these numbers over time.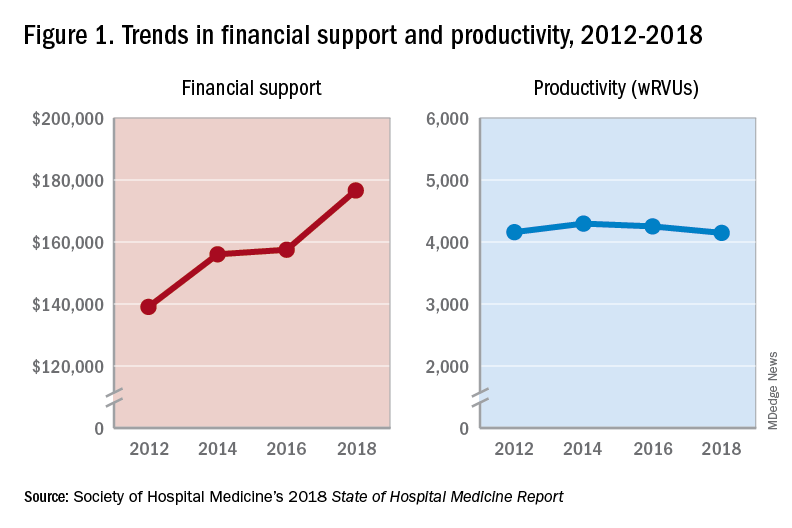
One of the most intriguing findings from the SHM’s 2018 SoHM Report is that financial support has risen despite relatively flat professional fee productivity (see Figure 1). Productivity, calculated as work relative value units (wRVUs) per physician declined slightly from 4,252 in 2016 to 4,147 in 2018.
There may be a few reasons why wRVUs per physician has remained relatively unchanged over the years. Many hospitals emphasize quality of care above provider productivity. The volume-to-value shift in theory serves as a means to reduce hospital-associated complications, length of stay, and readmission rates, thereby avoiding penalties and saving the overall costs for the hospitals in the long run.
Hospitalists involved in quality improvement projects and other essential nonclinical work perform tasks that are rarely captured in the wRVU metric. Improving patient experience, one of the Triple Aim components, necessitates extra time and effort, which also are nonbillable. In addition, increasing productivity can be challenging, a double-edged sword that may further escalate burnout and turnover rates. The static productivity may portend that it has leveled off or hit the ceiling in spite of ongoing efforts to improve efficacy.
In my view, the decision to invest in hospitalists for their contributions and dedications should not be determined based on a single metric such as wRVUs per physician. Hospitalist work on quality improvements; patient safety; efficiency, from direct bedside patient care to nonclinical efforts; teaching; research; involvements in various committees; administrative tasks; and leadership roles in improving health care systems are immeasurable. These are the reasons that most hospitals chose to adopt the hospitalist model and continue to support it. In fact, demand for hospitalists still outstrips supply, as evidenced by more than half of the hospital medicine groups with unfilled positions and an overall high turnover rate per 2018 SoHM data.
Although hospitalists are needed for the value that they provide, they should not take the status quo for granted. Instead, in return for the favorable financial support and in appreciation of being valued, hospitalists have a responsibility to prove that they are the right group chosen to do the work and help achieve their hospital’s mission and goals.
Dr. Vuong is a hospitalist at HealthPartners Medical Group in St Paul, Minn., and an assistant professor of medicine at the University of Minnesota. He is a member of SHM’s Practice Analysis Committee.
References
Afsar N. Looking into the Future and Making History. Hospitalist. 2019;23(1):31.
Beresford L. The State of Hospital Medicine in 2018. Hospitalist. 2019;23(1):1-11.
FitzGerald S. Not a Time for Modesty. Oct. 2009. Retrieved from https://acphospitalist.org/archives/2009/10/value.htm.
Watcher RM et al. Zero to 50,000 – The 20th Anniversary of the Hospitalist. N Eng J Med. 2016. 375(11):1009-11.
An uptrend in financial support
An uptrend in financial support
Since the inception of hospital medicine more than 2 decades ago, the total number of hospitalists has rapidly increased to more than 60,000. The Society of Hospital Medicine’s State of Hospital Medicine Report (SoHM), published biennially, captures new changes in our growing field and sheds light on current practice trends.
Among its findings, the 2018 SoHM Report reassuringly reveals that financial support from hospitals to hospital medicine groups (HMGs) continues to climb, even in the setting of rising health care costs and ongoing budget pressure.
The median amount of financial support per full-time equivalent (FTE) physician for HMGs serving adults was $176,658, according to the 2018 SoHM Report, which is up 12% from the 2016 median of $157,535. While there is no correlation between group sizes and the amount of financial support per FTE physician, there are significant differences across regions, with HMGs in the Midwest garnering the highest median support, at $193,121 per FTE physician.
The report also reveals big differences by employment model. For example, private multispecialty and primary care medical groups receive much less financial support ($58,396 per FTE physician) than HMGs employed by hospitals. This likely signifies that their main source of revenue is from professional service fees. Regardless of the types of employment models, past surveys have reported more than 95% of HMGs receive support from their hospitals to help cover expenses.
The median amount of financial support per FTE provider (including nurse practitioners, physician assistants, and locum tenens) was $134,300, which represents a 3.3% decrease, compared with the 2016 SoHM Report. For the first time, the 2018 SoHM also collected data on financial support per “work relative value unit” (wRVU) in addition to support per FTE physician and support per FTE provider. HMGs and their hospitals can use support per wRVU data to evaluate the support per unit of work, regardless of who (whether it is a physician, an advanced practice provider, and/or others) performed that work.
The median amount of financial support per wRVU for HMGs serving adults in 2018 was $41.92, with academic HMGs reporting a higher amount ($45.81) than nonacademic HMGs ($41.28). It will be interesting to track these numbers over time.
One of the most intriguing findings from the SHM’s 2018 SoHM Report is that financial support has risen despite relatively flat professional fee productivity (see Figure 1). Productivity, calculated as work relative value units (wRVUs) per physician declined slightly from 4,252 in 2016 to 4,147 in 2018.
There may be a few reasons why wRVUs per physician has remained relatively unchanged over the years. Many hospitals emphasize quality of care above provider productivity. The volume-to-value shift in theory serves as a means to reduce hospital-associated complications, length of stay, and readmission rates, thereby avoiding penalties and saving the overall costs for the hospitals in the long run.
Hospitalists involved in quality improvement projects and other essential nonclinical work perform tasks that are rarely captured in the wRVU metric. Improving patient experience, one of the Triple Aim components, necessitates extra time and effort, which also are nonbillable. In addition, increasing productivity can be challenging, a double-edged sword that may further escalate burnout and turnover rates. The static productivity may portend that it has leveled off or hit the ceiling in spite of ongoing efforts to improve efficacy.
In my view, the decision to invest in hospitalists for their contributions and dedications should not be determined based on a single metric such as wRVUs per physician. Hospitalist work on quality improvements; patient safety; efficiency, from direct bedside patient care to nonclinical efforts; teaching; research; involvements in various committees; administrative tasks; and leadership roles in improving health care systems are immeasurable. These are the reasons that most hospitals chose to adopt the hospitalist model and continue to support it. In fact, demand for hospitalists still outstrips supply, as evidenced by more than half of the hospital medicine groups with unfilled positions and an overall high turnover rate per 2018 SoHM data.
Although hospitalists are needed for the value that they provide, they should not take the status quo for granted. Instead, in return for the favorable financial support and in appreciation of being valued, hospitalists have a responsibility to prove that they are the right group chosen to do the work and help achieve their hospital’s mission and goals.
Dr. Vuong is a hospitalist at HealthPartners Medical Group in St Paul, Minn., and an assistant professor of medicine at the University of Minnesota. He is a member of SHM’s Practice Analysis Committee.
References
Afsar N. Looking into the Future and Making History. Hospitalist. 2019;23(1):31.
Beresford L. The State of Hospital Medicine in 2018. Hospitalist. 2019;23(1):1-11.
FitzGerald S. Not a Time for Modesty. Oct. 2009. Retrieved from https://acphospitalist.org/archives/2009/10/value.htm.
Watcher RM et al. Zero to 50,000 – The 20th Anniversary of the Hospitalist. N Eng J Med. 2016. 375(11):1009-11.
Since the inception of hospital medicine more than 2 decades ago, the total number of hospitalists has rapidly increased to more than 60,000. The Society of Hospital Medicine’s State of Hospital Medicine Report (SoHM), published biennially, captures new changes in our growing field and sheds light on current practice trends.
Among its findings, the 2018 SoHM Report reassuringly reveals that financial support from hospitals to hospital medicine groups (HMGs) continues to climb, even in the setting of rising health care costs and ongoing budget pressure.
The median amount of financial support per full-time equivalent (FTE) physician for HMGs serving adults was $176,658, according to the 2018 SoHM Report, which is up 12% from the 2016 median of $157,535. While there is no correlation between group sizes and the amount of financial support per FTE physician, there are significant differences across regions, with HMGs in the Midwest garnering the highest median support, at $193,121 per FTE physician.
The report also reveals big differences by employment model. For example, private multispecialty and primary care medical groups receive much less financial support ($58,396 per FTE physician) than HMGs employed by hospitals. This likely signifies that their main source of revenue is from professional service fees. Regardless of the types of employment models, past surveys have reported more than 95% of HMGs receive support from their hospitals to help cover expenses.
The median amount of financial support per FTE provider (including nurse practitioners, physician assistants, and locum tenens) was $134,300, which represents a 3.3% decrease, compared with the 2016 SoHM Report. For the first time, the 2018 SoHM also collected data on financial support per “work relative value unit” (wRVU) in addition to support per FTE physician and support per FTE provider. HMGs and their hospitals can use support per wRVU data to evaluate the support per unit of work, regardless of who (whether it is a physician, an advanced practice provider, and/or others) performed that work.
The median amount of financial support per wRVU for HMGs serving adults in 2018 was $41.92, with academic HMGs reporting a higher amount ($45.81) than nonacademic HMGs ($41.28). It will be interesting to track these numbers over time.
One of the most intriguing findings from the SHM’s 2018 SoHM Report is that financial support has risen despite relatively flat professional fee productivity (see Figure 1). Productivity, calculated as work relative value units (wRVUs) per physician declined slightly from 4,252 in 2016 to 4,147 in 2018.
There may be a few reasons why wRVUs per physician has remained relatively unchanged over the years. Many hospitals emphasize quality of care above provider productivity. The volume-to-value shift in theory serves as a means to reduce hospital-associated complications, length of stay, and readmission rates, thereby avoiding penalties and saving the overall costs for the hospitals in the long run.
Hospitalists involved in quality improvement projects and other essential nonclinical work perform tasks that are rarely captured in the wRVU metric. Improving patient experience, one of the Triple Aim components, necessitates extra time and effort, which also are nonbillable. In addition, increasing productivity can be challenging, a double-edged sword that may further escalate burnout and turnover rates. The static productivity may portend that it has leveled off or hit the ceiling in spite of ongoing efforts to improve efficacy.
In my view, the decision to invest in hospitalists for their contributions and dedications should not be determined based on a single metric such as wRVUs per physician. Hospitalist work on quality improvements; patient safety; efficiency, from direct bedside patient care to nonclinical efforts; teaching; research; involvements in various committees; administrative tasks; and leadership roles in improving health care systems are immeasurable. These are the reasons that most hospitals chose to adopt the hospitalist model and continue to support it. In fact, demand for hospitalists still outstrips supply, as evidenced by more than half of the hospital medicine groups with unfilled positions and an overall high turnover rate per 2018 SoHM data.
Although hospitalists are needed for the value that they provide, they should not take the status quo for granted. Instead, in return for the favorable financial support and in appreciation of being valued, hospitalists have a responsibility to prove that they are the right group chosen to do the work and help achieve their hospital’s mission and goals.
Dr. Vuong is a hospitalist at HealthPartners Medical Group in St Paul, Minn., and an assistant professor of medicine at the University of Minnesota. He is a member of SHM’s Practice Analysis Committee.
References
Afsar N. Looking into the Future and Making History. Hospitalist. 2019;23(1):31.
Beresford L. The State of Hospital Medicine in 2018. Hospitalist. 2019;23(1):1-11.
FitzGerald S. Not a Time for Modesty. Oct. 2009. Retrieved from https://acphospitalist.org/archives/2009/10/value.htm.
Watcher RM et al. Zero to 50,000 – The 20th Anniversary of the Hospitalist. N Eng J Med. 2016. 375(11):1009-11.
Universal health care hearing: GOP hears what it wants to
While most Republicans used their time at the House Ways & Means Committee hearing on Medicare-for-all to trash the concept, one rogue member criticized the hearing as simply misguided.
“We are living a time of disruption,” Rep. David Schweikert (R-Ariz) said. “There is incredible technology that is about to crash the price of health care if this committee particularly is willing to challenge and do something that’s incredibly uncomfortable for those of us in elective office, and that is look incumbent providers, business, insurers, systems in the face and say ‘it’s time for the revolution.’
“Are we willing to talk to our hospitals, talk to our providers, talk to technology, talk to the FDA [Food and Drug Administration], and have the honest discussion that this is about to become your primary care physician,” he said, raising a smartphone in his hand. “We will be healthier because it is individualized to us instead of what is going here in the discussion of a collectivization of a system that is already pretty crappy.”
He cited the recent Medicare Trustees Report showing that the hospital insurance trust fund (Medicare Part A) is 6.5 years from insolvency. “I don’t know why this hearing isn’t about Medicare itself and protecting Medicare itself instead of nationalization of health care. ... So defending the current system is absurd for all of us.”
The partisan nature of the June 12 hearing was clear.
Republican committee members focused their questioning on Grace-Marie Turner, president of the conservative Galen Institute and an outspoken opponent of Medicare-for-all. Less attention was paid to witnesses who offered alternatives to achieving greater health care coverage for the population.
Ranking member Kevin Brady (R-Texas) set the tone in his opening statement: “While our American health care system does have real problems, we should focus on improving what’s working and to fix what’s broken, rather than starting over with a massive new socialized medicine scheme that will leave many families worse off,” he said.
He noted that the federal government is on the cusp of yet another shutdown after three shutdowns in 2018. “The federal government can’t even keep its doors open. Can you really trust Washington with your life-and-death health care decisions? Make no mistake, Medicare-for-all guts quality health care in favor of delays and long waiting lines. It gives Washington politicians unlimited control over your health care. It cancels good quality health care plans for millions of workers, children, and the elderly and is so costly – tens of trillions of dollars – it will bankrupt America.”
Ms. Turner was regularly called upon to back up these talking points, using an analysis of a specific legislative proposal (H.R. 1384), in which she noted that under that specific Medicare-for-all bill, “Washington would be deciding what benefits people are eligible to receive. It will be deciding how much providers will be paid, so yes, it significantly limits choices of individuals and we see this, of course, in other countries as well.”
And while Republicans were using the testimony of Ms. Turner to back up their agenda, no one queried Donald Berwick, MD, former administrator of the Centers for Medicare & Medicaid Services and president emeritus and senior fellow at the Institute for Healthcare Improvement, who testified in support of a Medicare-for-all program – that the true impact of any universal coverage plan is dependent upon the program’s design.
One common GOP criticism throughout the hearing was that there would be a 40% reduction in pay to physicians and hospitals because the higher payments rates from private insurers that currently help offset lower payments from Medicare and Medicaid would be lost.
“The rhetoric we are hearing about 40% cuts is not necessary,” Dr. Berwick testified. “We can have sensible payment under an expanded Medicare system. That’s rhetoric, not fact. That’s in the design.”
While most Republicans used their time at the House Ways & Means Committee hearing on Medicare-for-all to trash the concept, one rogue member criticized the hearing as simply misguided.
“We are living a time of disruption,” Rep. David Schweikert (R-Ariz) said. “There is incredible technology that is about to crash the price of health care if this committee particularly is willing to challenge and do something that’s incredibly uncomfortable for those of us in elective office, and that is look incumbent providers, business, insurers, systems in the face and say ‘it’s time for the revolution.’
“Are we willing to talk to our hospitals, talk to our providers, talk to technology, talk to the FDA [Food and Drug Administration], and have the honest discussion that this is about to become your primary care physician,” he said, raising a smartphone in his hand. “We will be healthier because it is individualized to us instead of what is going here in the discussion of a collectivization of a system that is already pretty crappy.”
He cited the recent Medicare Trustees Report showing that the hospital insurance trust fund (Medicare Part A) is 6.5 years from insolvency. “I don’t know why this hearing isn’t about Medicare itself and protecting Medicare itself instead of nationalization of health care. ... So defending the current system is absurd for all of us.”
The partisan nature of the June 12 hearing was clear.
Republican committee members focused their questioning on Grace-Marie Turner, president of the conservative Galen Institute and an outspoken opponent of Medicare-for-all. Less attention was paid to witnesses who offered alternatives to achieving greater health care coverage for the population.
Ranking member Kevin Brady (R-Texas) set the tone in his opening statement: “While our American health care system does have real problems, we should focus on improving what’s working and to fix what’s broken, rather than starting over with a massive new socialized medicine scheme that will leave many families worse off,” he said.
He noted that the federal government is on the cusp of yet another shutdown after three shutdowns in 2018. “The federal government can’t even keep its doors open. Can you really trust Washington with your life-and-death health care decisions? Make no mistake, Medicare-for-all guts quality health care in favor of delays and long waiting lines. It gives Washington politicians unlimited control over your health care. It cancels good quality health care plans for millions of workers, children, and the elderly and is so costly – tens of trillions of dollars – it will bankrupt America.”
Ms. Turner was regularly called upon to back up these talking points, using an analysis of a specific legislative proposal (H.R. 1384), in which she noted that under that specific Medicare-for-all bill, “Washington would be deciding what benefits people are eligible to receive. It will be deciding how much providers will be paid, so yes, it significantly limits choices of individuals and we see this, of course, in other countries as well.”
And while Republicans were using the testimony of Ms. Turner to back up their agenda, no one queried Donald Berwick, MD, former administrator of the Centers for Medicare & Medicaid Services and president emeritus and senior fellow at the Institute for Healthcare Improvement, who testified in support of a Medicare-for-all program – that the true impact of any universal coverage plan is dependent upon the program’s design.
One common GOP criticism throughout the hearing was that there would be a 40% reduction in pay to physicians and hospitals because the higher payments rates from private insurers that currently help offset lower payments from Medicare and Medicaid would be lost.
“The rhetoric we are hearing about 40% cuts is not necessary,” Dr. Berwick testified. “We can have sensible payment under an expanded Medicare system. That’s rhetoric, not fact. That’s in the design.”
While most Republicans used their time at the House Ways & Means Committee hearing on Medicare-for-all to trash the concept, one rogue member criticized the hearing as simply misguided.
“We are living a time of disruption,” Rep. David Schweikert (R-Ariz) said. “There is incredible technology that is about to crash the price of health care if this committee particularly is willing to challenge and do something that’s incredibly uncomfortable for those of us in elective office, and that is look incumbent providers, business, insurers, systems in the face and say ‘it’s time for the revolution.’
“Are we willing to talk to our hospitals, talk to our providers, talk to technology, talk to the FDA [Food and Drug Administration], and have the honest discussion that this is about to become your primary care physician,” he said, raising a smartphone in his hand. “We will be healthier because it is individualized to us instead of what is going here in the discussion of a collectivization of a system that is already pretty crappy.”
He cited the recent Medicare Trustees Report showing that the hospital insurance trust fund (Medicare Part A) is 6.5 years from insolvency. “I don’t know why this hearing isn’t about Medicare itself and protecting Medicare itself instead of nationalization of health care. ... So defending the current system is absurd for all of us.”
The partisan nature of the June 12 hearing was clear.
Republican committee members focused their questioning on Grace-Marie Turner, president of the conservative Galen Institute and an outspoken opponent of Medicare-for-all. Less attention was paid to witnesses who offered alternatives to achieving greater health care coverage for the population.
Ranking member Kevin Brady (R-Texas) set the tone in his opening statement: “While our American health care system does have real problems, we should focus on improving what’s working and to fix what’s broken, rather than starting over with a massive new socialized medicine scheme that will leave many families worse off,” he said.
He noted that the federal government is on the cusp of yet another shutdown after three shutdowns in 2018. “The federal government can’t even keep its doors open. Can you really trust Washington with your life-and-death health care decisions? Make no mistake, Medicare-for-all guts quality health care in favor of delays and long waiting lines. It gives Washington politicians unlimited control over your health care. It cancels good quality health care plans for millions of workers, children, and the elderly and is so costly – tens of trillions of dollars – it will bankrupt America.”
Ms. Turner was regularly called upon to back up these talking points, using an analysis of a specific legislative proposal (H.R. 1384), in which she noted that under that specific Medicare-for-all bill, “Washington would be deciding what benefits people are eligible to receive. It will be deciding how much providers will be paid, so yes, it significantly limits choices of individuals and we see this, of course, in other countries as well.”
And while Republicans were using the testimony of Ms. Turner to back up their agenda, no one queried Donald Berwick, MD, former administrator of the Centers for Medicare & Medicaid Services and president emeritus and senior fellow at the Institute for Healthcare Improvement, who testified in support of a Medicare-for-all program – that the true impact of any universal coverage plan is dependent upon the program’s design.
One common GOP criticism throughout the hearing was that there would be a 40% reduction in pay to physicians and hospitals because the higher payments rates from private insurers that currently help offset lower payments from Medicare and Medicaid would be lost.
“The rhetoric we are hearing about 40% cuts is not necessary,” Dr. Berwick testified. “We can have sensible payment under an expanded Medicare system. That’s rhetoric, not fact. That’s in the design.”
REPORTING FROM A HOUSE WAYS & MEANS COMMITTEE HEARING
Teletriage connects uninsured with timely dermatologist care
MILAN – and optimized primary care physicians’ care of nonreferred patients, Cory Simpson, MD, PhD, reported at the World Congress of Dermatology.
With implementation of teledermatology, patient wait times for specialist input dropped from 13.9 days to 1.6 days (P less than .00001).
By allowing dermatologists to evaluate photographs of lesions and perform their own triage of referrals from primary care physicians (PCPs), the teletriage pilot program reduced the number of patients for whom dermatology consults were deemed necessary and also allowed optimal management for the nonreferred patients, said Dr. Simpson, of the University of Pennsylvania, Philadelphia.
“Teledermatology has the potential to increase access to dermatologist-level care, especially for underserved patients,” he commented. “It allows us to educate primary care physicians in resource-limited settings, and it also allows us to avoid suboptimal care of skin disease by nonspecialists – especially the more judicious use of antimicrobial agents and corticosteroids.”
Dr. Simpson explained to the international audience that, for many in the United States, access to a dermatologist requires a lengthy wait that can extend to months.
In Philadelphia, University of Pennsylvania dermatology residents and attending physicians volunteer in an outreach program that serves an uninsured population of primarily Latino immigrants. Operating 1 or 2 evenings a month, the medical and surgical dermatology clinics can accommodate from 8-12 appointments per clinic.
The clinic had been overwhelmed with referrals from PCPs, but Dr. Simpson and his colleagues realized that many of the conditions they were seeing – verruca vulgaris, hand dermatitis, and psoriasis, for example – did not necessarily need a face-to-face dermatologic evaluation.
The AccessDerm app, available at no cost by the American Academy of Dermatology, allows PCPs and dermatologists to communicate and collaborate. “This is a store-and-forward program, meaning the primary physician takes the photos and sends them to an off-site dermatologist who can then review them at his or her convenience,” Dr. Simpson said. “It’s a smartphone-based app, so actually, while I was at this conference, even though I’m thousands of miles from Philadelphia, I got through three consults this morning on my smartphone. It’s a very convenient way to be a volunteer.”
The consultation is between the PCP and the dermatologist, he added. “It’s the dermatologist talking to the PCP, and the patient receives the care recommendations from their primary doctor – so there’s no direct communication with the patient.”
Using the app, PCPs photographed skin lesions and completed simple history and physical exam modules within the app. Then, Dr. Simpson and his dermatology colleagues reviewed the photos and pertinent information.
If diagnostic uncertainty persisted after the teledermatology review, or if Dr. Simpson and his colleagues judged that a procedure such as a biopsy or lesion destruction was required, then the patient was scheduled for an appointment, with an interim plan put in place. Otherwise, patients were managed by teledermatology alone.
Of the 131 patients involved in the pilot study, 48 (37%) were female; the average patient age was 31.7 years (range, 1-92 years).
About 40% of patients were seen for inflammatory conditions, and another 20% for nonpigmented neoplasms. Almost 18% were seen for infectious reasons, with the remainder divided between pigmented neoplasms, hair disorders, and other conditions.
It turned out, said Dr. Simpson, that about two-thirds (65%) of the teletriage consultations ended in a definitive plan not requiring a face-to-face dermatology appointment. About a quarter (23%) were deferred to an in-person dermatology appointment, and the remaining 12% had an interim plan while more information was gathered.
Of the 32 neoplasms addressed by the teletriage strategy, 21 (66%) were deferred to an in-person visit. By contrast, 24 of the 95 non–neoplastic teletriage encounters were deferred to an in-person visit (P less than .001).
Overall, the strategy opened up 18% more appointment slots for new patients, Dr. Simpson said.
As part of the teletriage process, PCPs provided their proposed plan of care before receiving a dermatologist’s advice. When comparing the PCP’s plan to the dermatologist’s final plan, he and his colleagues found that there was a complete change of plan for three-quarters of visits (76%). A partial change happened 14% of the time, and only one in ten patients had no change in treatment plan as a result of the teledermatology consult. “This indicates again that specialist input matters,” he noted.
“This also gives us an opportunity to educate primary care physicians,” Dr. Simpson said, pointing out that in replies, he and his dermatologist colleagues included information about common diagnoses, including first-line treatments and “worrisome features they should be thinking about.”
He and his collaborators found that proper treatment would have been provided 30% of the time without a teledermatology consult, but that patients would have been undertreated 27% of the time. Overtreatment would have occurred at a rate of 11%, and care would have been unnecessarily delayed for about one in four patients. Unnecessary ED visits were averted for 6% of patients with the teletriage approach.
Examples of undertreatment included use of a weak topical steroid, missing infections or the need for referral, and using a suboptimal acne regimen. On the other hand, Dr. Simpson said, overtreatment with unnecessary antibiotics, antifungals, and antivirals also was averted; on some occasions, the PCP plan for an oral corticosteroid or an overly potent topical steroid was shifted to a more appropriate plan by teledermatology.
In sum, said Dr. Simpson, “teletriage via AccessDerm allowed us to reduce by tenfold the wait time for specialist input in dermatology cases. We were able to remove almost two-thirds of people from the queue ... waiting for dermatology appointments, which was very helpful to our clinic.”
And most importantly, he added, “this allowed us to allocate the limited number of in-person appointments that we had at this volunteer clinic to those that were more complicated cases.”
Dr. Simpson reported that he had no relevant disclosures. The project was funded by Penn Medicine and the American Academy of Dermatology.
MILAN – and optimized primary care physicians’ care of nonreferred patients, Cory Simpson, MD, PhD, reported at the World Congress of Dermatology.
With implementation of teledermatology, patient wait times for specialist input dropped from 13.9 days to 1.6 days (P less than .00001).
By allowing dermatologists to evaluate photographs of lesions and perform their own triage of referrals from primary care physicians (PCPs), the teletriage pilot program reduced the number of patients for whom dermatology consults were deemed necessary and also allowed optimal management for the nonreferred patients, said Dr. Simpson, of the University of Pennsylvania, Philadelphia.
“Teledermatology has the potential to increase access to dermatologist-level care, especially for underserved patients,” he commented. “It allows us to educate primary care physicians in resource-limited settings, and it also allows us to avoid suboptimal care of skin disease by nonspecialists – especially the more judicious use of antimicrobial agents and corticosteroids.”
Dr. Simpson explained to the international audience that, for many in the United States, access to a dermatologist requires a lengthy wait that can extend to months.
In Philadelphia, University of Pennsylvania dermatology residents and attending physicians volunteer in an outreach program that serves an uninsured population of primarily Latino immigrants. Operating 1 or 2 evenings a month, the medical and surgical dermatology clinics can accommodate from 8-12 appointments per clinic.
The clinic had been overwhelmed with referrals from PCPs, but Dr. Simpson and his colleagues realized that many of the conditions they were seeing – verruca vulgaris, hand dermatitis, and psoriasis, for example – did not necessarily need a face-to-face dermatologic evaluation.
The AccessDerm app, available at no cost by the American Academy of Dermatology, allows PCPs and dermatologists to communicate and collaborate. “This is a store-and-forward program, meaning the primary physician takes the photos and sends them to an off-site dermatologist who can then review them at his or her convenience,” Dr. Simpson said. “It’s a smartphone-based app, so actually, while I was at this conference, even though I’m thousands of miles from Philadelphia, I got through three consults this morning on my smartphone. It’s a very convenient way to be a volunteer.”
The consultation is between the PCP and the dermatologist, he added. “It’s the dermatologist talking to the PCP, and the patient receives the care recommendations from their primary doctor – so there’s no direct communication with the patient.”
Using the app, PCPs photographed skin lesions and completed simple history and physical exam modules within the app. Then, Dr. Simpson and his dermatology colleagues reviewed the photos and pertinent information.
If diagnostic uncertainty persisted after the teledermatology review, or if Dr. Simpson and his colleagues judged that a procedure such as a biopsy or lesion destruction was required, then the patient was scheduled for an appointment, with an interim plan put in place. Otherwise, patients were managed by teledermatology alone.
Of the 131 patients involved in the pilot study, 48 (37%) were female; the average patient age was 31.7 years (range, 1-92 years).
About 40% of patients were seen for inflammatory conditions, and another 20% for nonpigmented neoplasms. Almost 18% were seen for infectious reasons, with the remainder divided between pigmented neoplasms, hair disorders, and other conditions.
It turned out, said Dr. Simpson, that about two-thirds (65%) of the teletriage consultations ended in a definitive plan not requiring a face-to-face dermatology appointment. About a quarter (23%) were deferred to an in-person dermatology appointment, and the remaining 12% had an interim plan while more information was gathered.
Of the 32 neoplasms addressed by the teletriage strategy, 21 (66%) were deferred to an in-person visit. By contrast, 24 of the 95 non–neoplastic teletriage encounters were deferred to an in-person visit (P less than .001).
Overall, the strategy opened up 18% more appointment slots for new patients, Dr. Simpson said.
As part of the teletriage process, PCPs provided their proposed plan of care before receiving a dermatologist’s advice. When comparing the PCP’s plan to the dermatologist’s final plan, he and his colleagues found that there was a complete change of plan for three-quarters of visits (76%). A partial change happened 14% of the time, and only one in ten patients had no change in treatment plan as a result of the teledermatology consult. “This indicates again that specialist input matters,” he noted.
“This also gives us an opportunity to educate primary care physicians,” Dr. Simpson said, pointing out that in replies, he and his dermatologist colleagues included information about common diagnoses, including first-line treatments and “worrisome features they should be thinking about.”
He and his collaborators found that proper treatment would have been provided 30% of the time without a teledermatology consult, but that patients would have been undertreated 27% of the time. Overtreatment would have occurred at a rate of 11%, and care would have been unnecessarily delayed for about one in four patients. Unnecessary ED visits were averted for 6% of patients with the teletriage approach.
Examples of undertreatment included use of a weak topical steroid, missing infections or the need for referral, and using a suboptimal acne regimen. On the other hand, Dr. Simpson said, overtreatment with unnecessary antibiotics, antifungals, and antivirals also was averted; on some occasions, the PCP plan for an oral corticosteroid or an overly potent topical steroid was shifted to a more appropriate plan by teledermatology.
In sum, said Dr. Simpson, “teletriage via AccessDerm allowed us to reduce by tenfold the wait time for specialist input in dermatology cases. We were able to remove almost two-thirds of people from the queue ... waiting for dermatology appointments, which was very helpful to our clinic.”
And most importantly, he added, “this allowed us to allocate the limited number of in-person appointments that we had at this volunteer clinic to those that were more complicated cases.”
Dr. Simpson reported that he had no relevant disclosures. The project was funded by Penn Medicine and the American Academy of Dermatology.
MILAN – and optimized primary care physicians’ care of nonreferred patients, Cory Simpson, MD, PhD, reported at the World Congress of Dermatology.
With implementation of teledermatology, patient wait times for specialist input dropped from 13.9 days to 1.6 days (P less than .00001).
By allowing dermatologists to evaluate photographs of lesions and perform their own triage of referrals from primary care physicians (PCPs), the teletriage pilot program reduced the number of patients for whom dermatology consults were deemed necessary and also allowed optimal management for the nonreferred patients, said Dr. Simpson, of the University of Pennsylvania, Philadelphia.
“Teledermatology has the potential to increase access to dermatologist-level care, especially for underserved patients,” he commented. “It allows us to educate primary care physicians in resource-limited settings, and it also allows us to avoid suboptimal care of skin disease by nonspecialists – especially the more judicious use of antimicrobial agents and corticosteroids.”
Dr. Simpson explained to the international audience that, for many in the United States, access to a dermatologist requires a lengthy wait that can extend to months.
In Philadelphia, University of Pennsylvania dermatology residents and attending physicians volunteer in an outreach program that serves an uninsured population of primarily Latino immigrants. Operating 1 or 2 evenings a month, the medical and surgical dermatology clinics can accommodate from 8-12 appointments per clinic.
The clinic had been overwhelmed with referrals from PCPs, but Dr. Simpson and his colleagues realized that many of the conditions they were seeing – verruca vulgaris, hand dermatitis, and psoriasis, for example – did not necessarily need a face-to-face dermatologic evaluation.
The AccessDerm app, available at no cost by the American Academy of Dermatology, allows PCPs and dermatologists to communicate and collaborate. “This is a store-and-forward program, meaning the primary physician takes the photos and sends them to an off-site dermatologist who can then review them at his or her convenience,” Dr. Simpson said. “It’s a smartphone-based app, so actually, while I was at this conference, even though I’m thousands of miles from Philadelphia, I got through three consults this morning on my smartphone. It’s a very convenient way to be a volunteer.”
The consultation is between the PCP and the dermatologist, he added. “It’s the dermatologist talking to the PCP, and the patient receives the care recommendations from their primary doctor – so there’s no direct communication with the patient.”
Using the app, PCPs photographed skin lesions and completed simple history and physical exam modules within the app. Then, Dr. Simpson and his dermatology colleagues reviewed the photos and pertinent information.
If diagnostic uncertainty persisted after the teledermatology review, or if Dr. Simpson and his colleagues judged that a procedure such as a biopsy or lesion destruction was required, then the patient was scheduled for an appointment, with an interim plan put in place. Otherwise, patients were managed by teledermatology alone.
Of the 131 patients involved in the pilot study, 48 (37%) were female; the average patient age was 31.7 years (range, 1-92 years).
About 40% of patients were seen for inflammatory conditions, and another 20% for nonpigmented neoplasms. Almost 18% were seen for infectious reasons, with the remainder divided between pigmented neoplasms, hair disorders, and other conditions.
It turned out, said Dr. Simpson, that about two-thirds (65%) of the teletriage consultations ended in a definitive plan not requiring a face-to-face dermatology appointment. About a quarter (23%) were deferred to an in-person dermatology appointment, and the remaining 12% had an interim plan while more information was gathered.
Of the 32 neoplasms addressed by the teletriage strategy, 21 (66%) were deferred to an in-person visit. By contrast, 24 of the 95 non–neoplastic teletriage encounters were deferred to an in-person visit (P less than .001).
Overall, the strategy opened up 18% more appointment slots for new patients, Dr. Simpson said.
As part of the teletriage process, PCPs provided their proposed plan of care before receiving a dermatologist’s advice. When comparing the PCP’s plan to the dermatologist’s final plan, he and his colleagues found that there was a complete change of plan for three-quarters of visits (76%). A partial change happened 14% of the time, and only one in ten patients had no change in treatment plan as a result of the teledermatology consult. “This indicates again that specialist input matters,” he noted.
“This also gives us an opportunity to educate primary care physicians,” Dr. Simpson said, pointing out that in replies, he and his dermatologist colleagues included information about common diagnoses, including first-line treatments and “worrisome features they should be thinking about.”
He and his collaborators found that proper treatment would have been provided 30% of the time without a teledermatology consult, but that patients would have been undertreated 27% of the time. Overtreatment would have occurred at a rate of 11%, and care would have been unnecessarily delayed for about one in four patients. Unnecessary ED visits were averted for 6% of patients with the teletriage approach.
Examples of undertreatment included use of a weak topical steroid, missing infections or the need for referral, and using a suboptimal acne regimen. On the other hand, Dr. Simpson said, overtreatment with unnecessary antibiotics, antifungals, and antivirals also was averted; on some occasions, the PCP plan for an oral corticosteroid or an overly potent topical steroid was shifted to a more appropriate plan by teledermatology.
In sum, said Dr. Simpson, “teletriage via AccessDerm allowed us to reduce by tenfold the wait time for specialist input in dermatology cases. We were able to remove almost two-thirds of people from the queue ... waiting for dermatology appointments, which was very helpful to our clinic.”
And most importantly, he added, “this allowed us to allocate the limited number of in-person appointments that we had at this volunteer clinic to those that were more complicated cases.”
Dr. Simpson reported that he had no relevant disclosures. The project was funded by Penn Medicine and the American Academy of Dermatology.
REPORTING FROM WCD2019
Advanced basal cell carcinoma responds to pembrolizumab in proof-of-concept study
MILAN – A checkpoint inhibitor given with or without targeted therapy resulted in robust response rates in patients with advanced basal cell carcinoma, according to an investigator in a recent proof-of-concept study.
The side effect profile for the progressive death 1 (PD-1) inhibitor pembrolizumab (Keytruda), plus or minus the hedgehog pathway inhibitor vismodegib (Erivedge), was “not out of range” with what’s been observed in other cancer types, said Anne Lynn S. Chang, MD, a medical dermatologist at Stanford (Calif.) University.
Taken together, these safety and efficacy results suggest pembrolizumab could prove useful for patients with advanced basal cell carcinomas, Dr. Chang said in an oral presentation at the World Congress of Dermatology.
Unexpectedly, dual therapy with pembrolizumab and vismodegib was not clearly superior to pembrolizumab alone, though the two arms are not directly comparable in this nonrandomized, investigator-initiated study, according to Dr. Chang.
“That was a surprise for us, but this is a subjective and a small study,” she told attendees at the conference.
There is currently a multi-institutional clinical trial underway to evaluate another PD-1 inhibitor, cemiplimab, in patients with advanced basal cell carcinoma previously treated with a hedgehog pathway inhibitor, she said.
While a large fraction of basal cell carcinomas are cured by resection, those that are locally advanced or metastatic to lymph nodes or distant organs are challenging to manage. Hedgehog pathway inhibitors, which include vismodegib and sonidegib, are the only Food and Drug Administration–approved drug class in this setting, but more than half of patients do not have a response to treatment, and another 20% or so will initially respond but develop resistance, Dr. Chang said.
Other treatments that have been tried in advanced basal cell carcinomas include taxanes, platinum-based agents, 5-fluorouracil, and everolimus, but their efficacy is unclear, according to Dr. Chang, because of a lack of systematic studies.
Investigators thought PD-1 inhibitors might be promising in basal cell carcinoma for a few reasons, Dr. Chang said. In particular, PD-1 inhibitor treatment response has been linked to mutational burden and PD–ligand 1 expression, and advanced basal cell carcinomas have the largest mutational burden of all human cancers, a large proportion of which express PD-L1.
There are multiple case reports in the literature suggesting that advanced basal cell carcinoma is responsive to PD-1 inhibitors, she added.
In the study, nine patients received 200 mg infusion of pembrolizumab every 3 weeks, and seven received the pembrolizumab infusions plus oral vismodegib 150 mg per day.
The pembrolizumab monotherapy group included patients who had tumor progression on vismodegib, did not tolerate vismodegib, or had contraindications to vismodegib. The pembrolizumab-vismodegib regimen was used in patients who had prior stable or partial responses to hedgehog pathway inhibitors.
The overall response rate was 38%, or 6 of 16 patients, with a 70% probability of 1-year progression-free survival and 94% probability of 1-year overall survival, Dr. Chang reported.
The results did not make the case that pembrolizumab and vismodegib was superior to pembrolizumab alone, though as Dr. Chang noted, the numbers of patients were small. Response rates were 29% (two of seven patients) in the combination arm and 44% (four of nine patients) in the monotherapy arm.
There were 24 immune-related adverse events in the trial; the most serious was hyponatremia, which was reversible, according to Dr. Chang. The overall rates of grade 3-4 adverse events were 22% for pembrolizumab monotherapy and 17% for dual therapy.
A report on the study has also been published in the Journal of the American Academy of Dermatology.
Funding for the study was provided by Merck. Dr. Chang reported serving as a clinical investigator and advisory board member for studies sponsored by Genentech-Roche, Merck, Novartis, and Regeneron.
MILAN – A checkpoint inhibitor given with or without targeted therapy resulted in robust response rates in patients with advanced basal cell carcinoma, according to an investigator in a recent proof-of-concept study.
The side effect profile for the progressive death 1 (PD-1) inhibitor pembrolizumab (Keytruda), plus or minus the hedgehog pathway inhibitor vismodegib (Erivedge), was “not out of range” with what’s been observed in other cancer types, said Anne Lynn S. Chang, MD, a medical dermatologist at Stanford (Calif.) University.
Taken together, these safety and efficacy results suggest pembrolizumab could prove useful for patients with advanced basal cell carcinomas, Dr. Chang said in an oral presentation at the World Congress of Dermatology.
Unexpectedly, dual therapy with pembrolizumab and vismodegib was not clearly superior to pembrolizumab alone, though the two arms are not directly comparable in this nonrandomized, investigator-initiated study, according to Dr. Chang.
“That was a surprise for us, but this is a subjective and a small study,” she told attendees at the conference.
There is currently a multi-institutional clinical trial underway to evaluate another PD-1 inhibitor, cemiplimab, in patients with advanced basal cell carcinoma previously treated with a hedgehog pathway inhibitor, she said.
While a large fraction of basal cell carcinomas are cured by resection, those that are locally advanced or metastatic to lymph nodes or distant organs are challenging to manage. Hedgehog pathway inhibitors, which include vismodegib and sonidegib, are the only Food and Drug Administration–approved drug class in this setting, but more than half of patients do not have a response to treatment, and another 20% or so will initially respond but develop resistance, Dr. Chang said.
Other treatments that have been tried in advanced basal cell carcinomas include taxanes, platinum-based agents, 5-fluorouracil, and everolimus, but their efficacy is unclear, according to Dr. Chang, because of a lack of systematic studies.
Investigators thought PD-1 inhibitors might be promising in basal cell carcinoma for a few reasons, Dr. Chang said. In particular, PD-1 inhibitor treatment response has been linked to mutational burden and PD–ligand 1 expression, and advanced basal cell carcinomas have the largest mutational burden of all human cancers, a large proportion of which express PD-L1.
There are multiple case reports in the literature suggesting that advanced basal cell carcinoma is responsive to PD-1 inhibitors, she added.
In the study, nine patients received 200 mg infusion of pembrolizumab every 3 weeks, and seven received the pembrolizumab infusions plus oral vismodegib 150 mg per day.
The pembrolizumab monotherapy group included patients who had tumor progression on vismodegib, did not tolerate vismodegib, or had contraindications to vismodegib. The pembrolizumab-vismodegib regimen was used in patients who had prior stable or partial responses to hedgehog pathway inhibitors.
The overall response rate was 38%, or 6 of 16 patients, with a 70% probability of 1-year progression-free survival and 94% probability of 1-year overall survival, Dr. Chang reported.
The results did not make the case that pembrolizumab and vismodegib was superior to pembrolizumab alone, though as Dr. Chang noted, the numbers of patients were small. Response rates were 29% (two of seven patients) in the combination arm and 44% (four of nine patients) in the monotherapy arm.
There were 24 immune-related adverse events in the trial; the most serious was hyponatremia, which was reversible, according to Dr. Chang. The overall rates of grade 3-4 adverse events were 22% for pembrolizumab monotherapy and 17% for dual therapy.
A report on the study has also been published in the Journal of the American Academy of Dermatology.
Funding for the study was provided by Merck. Dr. Chang reported serving as a clinical investigator and advisory board member for studies sponsored by Genentech-Roche, Merck, Novartis, and Regeneron.
MILAN – A checkpoint inhibitor given with or without targeted therapy resulted in robust response rates in patients with advanced basal cell carcinoma, according to an investigator in a recent proof-of-concept study.
The side effect profile for the progressive death 1 (PD-1) inhibitor pembrolizumab (Keytruda), plus or minus the hedgehog pathway inhibitor vismodegib (Erivedge), was “not out of range” with what’s been observed in other cancer types, said Anne Lynn S. Chang, MD, a medical dermatologist at Stanford (Calif.) University.
Taken together, these safety and efficacy results suggest pembrolizumab could prove useful for patients with advanced basal cell carcinomas, Dr. Chang said in an oral presentation at the World Congress of Dermatology.
Unexpectedly, dual therapy with pembrolizumab and vismodegib was not clearly superior to pembrolizumab alone, though the two arms are not directly comparable in this nonrandomized, investigator-initiated study, according to Dr. Chang.
“That was a surprise for us, but this is a subjective and a small study,” she told attendees at the conference.
There is currently a multi-institutional clinical trial underway to evaluate another PD-1 inhibitor, cemiplimab, in patients with advanced basal cell carcinoma previously treated with a hedgehog pathway inhibitor, she said.
While a large fraction of basal cell carcinomas are cured by resection, those that are locally advanced or metastatic to lymph nodes or distant organs are challenging to manage. Hedgehog pathway inhibitors, which include vismodegib and sonidegib, are the only Food and Drug Administration–approved drug class in this setting, but more than half of patients do not have a response to treatment, and another 20% or so will initially respond but develop resistance, Dr. Chang said.
Other treatments that have been tried in advanced basal cell carcinomas include taxanes, platinum-based agents, 5-fluorouracil, and everolimus, but their efficacy is unclear, according to Dr. Chang, because of a lack of systematic studies.
Investigators thought PD-1 inhibitors might be promising in basal cell carcinoma for a few reasons, Dr. Chang said. In particular, PD-1 inhibitor treatment response has been linked to mutational burden and PD–ligand 1 expression, and advanced basal cell carcinomas have the largest mutational burden of all human cancers, a large proportion of which express PD-L1.
There are multiple case reports in the literature suggesting that advanced basal cell carcinoma is responsive to PD-1 inhibitors, she added.
In the study, nine patients received 200 mg infusion of pembrolizumab every 3 weeks, and seven received the pembrolizumab infusions plus oral vismodegib 150 mg per day.
The pembrolizumab monotherapy group included patients who had tumor progression on vismodegib, did not tolerate vismodegib, or had contraindications to vismodegib. The pembrolizumab-vismodegib regimen was used in patients who had prior stable or partial responses to hedgehog pathway inhibitors.
The overall response rate was 38%, or 6 of 16 patients, with a 70% probability of 1-year progression-free survival and 94% probability of 1-year overall survival, Dr. Chang reported.
The results did not make the case that pembrolizumab and vismodegib was superior to pembrolizumab alone, though as Dr. Chang noted, the numbers of patients were small. Response rates were 29% (two of seven patients) in the combination arm and 44% (four of nine patients) in the monotherapy arm.
There were 24 immune-related adverse events in the trial; the most serious was hyponatremia, which was reversible, according to Dr. Chang. The overall rates of grade 3-4 adverse events were 22% for pembrolizumab monotherapy and 17% for dual therapy.
A report on the study has also been published in the Journal of the American Academy of Dermatology.
Funding for the study was provided by Merck. Dr. Chang reported serving as a clinical investigator and advisory board member for studies sponsored by Genentech-Roche, Merck, Novartis, and Regeneron.
REPORTING FROM WCD2019
Survey puts a price on health improvement
according to a survey by UnitedHealthcare.
More than half (54%) of the 1,006 respondents said that they didn’t need a financial incentive to get that much exercise, but 21% said that $1-$3 a day would be necessary and 19% said that they would need $4-$5 per day, the company said in its 2019 Wellness Checkup Survey.
Women were more likely than men to say that they needed an incentive (42% vs. 36%). Among age-related subgroups, there was a clear progression from youngest to oldest: The youngest age group (18-34 years, 47%) and youngest generation (Millennials, 46%) in the study were the most likely to require an incentive, while the oldest age group (65 years and older, 30%) and generation (Baby Boomers, 31%) were the least likely, UnitedHealthcare said.
A majority (57%) of respondents said that it was important – either very important (19%) or somewhat important (38%) – for a fitness routine to have a social component, either in-person or virtual. Almost two-thirds of respondents expressed support for wearable fitness-tracking devices: 42% would use one if it was provided by their employer, and 22% said that they already had one, the survey results show.
“This year employers are expected to invest an average of more than $3.6 million on their respective well-being programs, and over 60% of employees are interested in engaging in these initiatives. [This] survey provides insights that we hope can be helpful to enhance the design and implementation of well-being programs, which may help improve employees’ health, reduce absenteeism, and curb care costs,” Rebecca Madsen, UnitedHealthcare’s chief consumer officer, said in the company’s written statement.
The survey was conducted April 11-15, 2019, and the margin of error was plus or minus 3.1%.
according to a survey by UnitedHealthcare.

More than half (54%) of the 1,006 respondents said that they didn’t need a financial incentive to get that much exercise, but 21% said that $1-$3 a day would be necessary and 19% said that they would need $4-$5 per day, the company said in its 2019 Wellness Checkup Survey.
Women were more likely than men to say that they needed an incentive (42% vs. 36%). Among age-related subgroups, there was a clear progression from youngest to oldest: The youngest age group (18-34 years, 47%) and youngest generation (Millennials, 46%) in the study were the most likely to require an incentive, while the oldest age group (65 years and older, 30%) and generation (Baby Boomers, 31%) were the least likely, UnitedHealthcare said.
A majority (57%) of respondents said that it was important – either very important (19%) or somewhat important (38%) – for a fitness routine to have a social component, either in-person or virtual. Almost two-thirds of respondents expressed support for wearable fitness-tracking devices: 42% would use one if it was provided by their employer, and 22% said that they already had one, the survey results show.
“This year employers are expected to invest an average of more than $3.6 million on their respective well-being programs, and over 60% of employees are interested in engaging in these initiatives. [This] survey provides insights that we hope can be helpful to enhance the design and implementation of well-being programs, which may help improve employees’ health, reduce absenteeism, and curb care costs,” Rebecca Madsen, UnitedHealthcare’s chief consumer officer, said in the company’s written statement.
The survey was conducted April 11-15, 2019, and the margin of error was plus or minus 3.1%.
according to a survey by UnitedHealthcare.

More than half (54%) of the 1,006 respondents said that they didn’t need a financial incentive to get that much exercise, but 21% said that $1-$3 a day would be necessary and 19% said that they would need $4-$5 per day, the company said in its 2019 Wellness Checkup Survey.
Women were more likely than men to say that they needed an incentive (42% vs. 36%). Among age-related subgroups, there was a clear progression from youngest to oldest: The youngest age group (18-34 years, 47%) and youngest generation (Millennials, 46%) in the study were the most likely to require an incentive, while the oldest age group (65 years and older, 30%) and generation (Baby Boomers, 31%) were the least likely, UnitedHealthcare said.
A majority (57%) of respondents said that it was important – either very important (19%) or somewhat important (38%) – for a fitness routine to have a social component, either in-person or virtual. Almost two-thirds of respondents expressed support for wearable fitness-tracking devices: 42% would use one if it was provided by their employer, and 22% said that they already had one, the survey results show.
“This year employers are expected to invest an average of more than $3.6 million on their respective well-being programs, and over 60% of employees are interested in engaging in these initiatives. [This] survey provides insights that we hope can be helpful to enhance the design and implementation of well-being programs, which may help improve employees’ health, reduce absenteeism, and curb care costs,” Rebecca Madsen, UnitedHealthcare’s chief consumer officer, said in the company’s written statement.
The survey was conducted April 11-15, 2019, and the margin of error was plus or minus 3.1%.
Eosinophil-guided therapy reduces corticosteroid use in COPD
in terms of the number of days out of hospital and alive, new research has found.
Writing in the Lancet Respiratory Medicine, researchers reported the outcomes of a multicenter, controlled, open-label trial comparing eosinophil-guided and standard therapy with systemic corticosteroids in 318 patients with COPD.
Pradeesh Sivapalan, MD, of the respiratory medicine section of Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors wrote that eosinophilic inflammation had been seen in 20%-40% of patients with acute exacerbations of COPD. Patients with higher eosinophilic blood counts were at increased risk of acute exacerbations but were also more likely to benefit from corticosteroid treatment.
In the eosinophil-guided therapy arm of the study, 159 patients received 80 mg of intravenous methylprednisolone on day 1, then from the second day were treated with 37.5 mg of prednisolone oral tablet daily – up to 4 days – only on days when their blood eosinophil count was at least 0.3 x 10⁹ cells/L. In the control arm, 159 patients also received 80 mg of intravenous methylprednisolone on day 1, followed by 37.5 mg of prednisolone tablets daily for 4 days.
After 14 days, there were no significant differences between the two groups for mean days alive and out of hospital.
There were 12 more cases of readmission with COPD, including three fatalities, in the eosinophil-guided group within the first month. However the authors said these differences were not statistically significant, but “because the study was not powered to detect differences in this absolute risk range, we cannot rule out that this was an actual harm effect from the interventional strategy.”
The eosinophil-guided therapy group did show more than a 50% reduction in the median duration of systemic corticosteroid therapy, which was 2 days in the eosinophil-guided group, compared with 5 days in the control group (P less than .0001), and the differences between the two groups remained significant at days 30 and 90.
“The tested strategy was successful in reducing the exposure to systemic corticosteroids, but we cannot exclude the possibility that a more aggressive algorithm, such as a single dose of systemic corticosteroid, might have been more effective,” the authors wrote.
At the 90-day follow-up, there were no differences in the number of infections requiring antibiotic treatment, nor in dyspepsia, ulcer complications, or initiation of new proton-pump inhibitor treatment.
The study was supported by the Danish Regions Medical Fund and the Danish Council for Independent Research. Two authors declared personal fees from pharmaceutical companies outside the submitted work. No other conflicts were declared.
SOURCE: Sivapalan P et al. Lancet Respir Med. 2019, May 20. doi: 10.1016/S2213-2600(19)30176-6.
in terms of the number of days out of hospital and alive, new research has found.
Writing in the Lancet Respiratory Medicine, researchers reported the outcomes of a multicenter, controlled, open-label trial comparing eosinophil-guided and standard therapy with systemic corticosteroids in 318 patients with COPD.
Pradeesh Sivapalan, MD, of the respiratory medicine section of Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors wrote that eosinophilic inflammation had been seen in 20%-40% of patients with acute exacerbations of COPD. Patients with higher eosinophilic blood counts were at increased risk of acute exacerbations but were also more likely to benefit from corticosteroid treatment.
In the eosinophil-guided therapy arm of the study, 159 patients received 80 mg of intravenous methylprednisolone on day 1, then from the second day were treated with 37.5 mg of prednisolone oral tablet daily – up to 4 days – only on days when their blood eosinophil count was at least 0.3 x 10⁹ cells/L. In the control arm, 159 patients also received 80 mg of intravenous methylprednisolone on day 1, followed by 37.5 mg of prednisolone tablets daily for 4 days.
After 14 days, there were no significant differences between the two groups for mean days alive and out of hospital.
There were 12 more cases of readmission with COPD, including three fatalities, in the eosinophil-guided group within the first month. However the authors said these differences were not statistically significant, but “because the study was not powered to detect differences in this absolute risk range, we cannot rule out that this was an actual harm effect from the interventional strategy.”
The eosinophil-guided therapy group did show more than a 50% reduction in the median duration of systemic corticosteroid therapy, which was 2 days in the eosinophil-guided group, compared with 5 days in the control group (P less than .0001), and the differences between the two groups remained significant at days 30 and 90.
“The tested strategy was successful in reducing the exposure to systemic corticosteroids, but we cannot exclude the possibility that a more aggressive algorithm, such as a single dose of systemic corticosteroid, might have been more effective,” the authors wrote.
At the 90-day follow-up, there were no differences in the number of infections requiring antibiotic treatment, nor in dyspepsia, ulcer complications, or initiation of new proton-pump inhibitor treatment.
The study was supported by the Danish Regions Medical Fund and the Danish Council for Independent Research. Two authors declared personal fees from pharmaceutical companies outside the submitted work. No other conflicts were declared.
SOURCE: Sivapalan P et al. Lancet Respir Med. 2019, May 20. doi: 10.1016/S2213-2600(19)30176-6.
in terms of the number of days out of hospital and alive, new research has found.
Writing in the Lancet Respiratory Medicine, researchers reported the outcomes of a multicenter, controlled, open-label trial comparing eosinophil-guided and standard therapy with systemic corticosteroids in 318 patients with COPD.
Pradeesh Sivapalan, MD, of the respiratory medicine section of Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors wrote that eosinophilic inflammation had been seen in 20%-40% of patients with acute exacerbations of COPD. Patients with higher eosinophilic blood counts were at increased risk of acute exacerbations but were also more likely to benefit from corticosteroid treatment.
In the eosinophil-guided therapy arm of the study, 159 patients received 80 mg of intravenous methylprednisolone on day 1, then from the second day were treated with 37.5 mg of prednisolone oral tablet daily – up to 4 days – only on days when their blood eosinophil count was at least 0.3 x 10⁹ cells/L. In the control arm, 159 patients also received 80 mg of intravenous methylprednisolone on day 1, followed by 37.5 mg of prednisolone tablets daily for 4 days.
After 14 days, there were no significant differences between the two groups for mean days alive and out of hospital.
There were 12 more cases of readmission with COPD, including three fatalities, in the eosinophil-guided group within the first month. However the authors said these differences were not statistically significant, but “because the study was not powered to detect differences in this absolute risk range, we cannot rule out that this was an actual harm effect from the interventional strategy.”
The eosinophil-guided therapy group did show more than a 50% reduction in the median duration of systemic corticosteroid therapy, which was 2 days in the eosinophil-guided group, compared with 5 days in the control group (P less than .0001), and the differences between the two groups remained significant at days 30 and 90.
“The tested strategy was successful in reducing the exposure to systemic corticosteroids, but we cannot exclude the possibility that a more aggressive algorithm, such as a single dose of systemic corticosteroid, might have been more effective,” the authors wrote.
At the 90-day follow-up, there were no differences in the number of infections requiring antibiotic treatment, nor in dyspepsia, ulcer complications, or initiation of new proton-pump inhibitor treatment.
The study was supported by the Danish Regions Medical Fund and the Danish Council for Independent Research. Two authors declared personal fees from pharmaceutical companies outside the submitted work. No other conflicts were declared.
SOURCE: Sivapalan P et al. Lancet Respir Med. 2019, May 20. doi: 10.1016/S2213-2600(19)30176-6.
FROM LANCET RESPIRATORY MEDICINE