User login
Three out of five ain’t bad? Some diabetes measures improved over 17 years
SAN FRANCISCO – New research suggests that.
As the age of statins dawned, cholesterol levels dipped dramatically, while blood pressure levels and smoking prevalence also fell. But hemoglobin A1c levels stubbornly stayed steady, and obesity rates ballooned.
In light of the not entirely impressive numbers, “maybe we need to follow the model of team collaboration we see in the heart care setting,” said study lead author Carla I. Mercado, PhD, an epidemiologist with the Centers for Disease Control & Prevention. She spoke in an interview at the annual scientific sessions of the American Diabetes Association, where she presented the study findings.
For the new study, Dr. Mercado and colleagues used data from the National Health and Nutrition Examination Survey (NHANES) to track the health of adults with diabetes in the United States during 1999-2016. “With all the efforts on diabetes care management, we wanted to see if our efforts are making a difference in the population,” Dr. Mercado said.
The 5,534 participants had self-reported diabetes, were not pregnant, and underwent a physical examination. Throughout the periods examined (1999-2004, 2005-2010, and 2011-2016), the proportion of women remained steady at about one-half. So did the racial makeup, which ranged from 59% to 63% non-Hispanic white, 15% to 18% black, 7% to 10% Mexican-American, and 12% to 15% “other.”
Nearly half were aged 45-64, and 89%-90% had health insurance. There was a significant change in the education levels among those aged 25 and older: The percentage with at least a college degree grew from 14% in 1999-2004 to 21% in 2011-2016, while those with less than a high-school diploma fell from 34% to 23% over that period.
From 1999-2004 to 2011-2016
- Cholesterol: Most notably, the percentage of participants with non-HDL cholesterol levels below 130 mg/dL soared, from 30% to 54%. The CDC considers levels less than 100 mg/dL to be ideal. “I’m sure this is driven by medication use,” Dr. Mercado said.
- Smoking: The percentage of never smokers rose, from 44% to 47% of the subjects, while that of current smokers dropped, from 26% to 21%, a significant difference.
- Hypertension: The percentages with blood pressure levels less than 120/80 mm Hg – considered normal levels by the CDC – rose significantly, from 26% to 30%. “People are a lot more aware of blood pressure,” Dr. Mercado said.
- Glycemic control: HbA1c levels stayed roughly steady. About 10% had levels at or above 10% in both 1999-2004 and 2011-2016, and the number with A1c levels below 6% dipped slightly from 19% to 17%.
- Obesity: The proportion of participants with body mass index levels at or above 30 kg/m2, the line between overweight and obese, rose from 54% to 61%. The percentage of those with BMIs below 25 kg/m2 – considered to have normal weights – fell significantly, from 17% to 12%.
The researchers also looked at the percentage who reached ABCS goals (A1c at or below 9%, blood pressure below 140/90 mm Hg, non-HDL cholesterol under 160 mg/dL, and current nonsmoking status). The percentage who met all of these criteria grew from 26% to 40%, while those who met three of them stayed steady (40%-39%).
The study was funded by the CDC. The study authors report no relevant disclosures.
SAN FRANCISCO – New research suggests that.
As the age of statins dawned, cholesterol levels dipped dramatically, while blood pressure levels and smoking prevalence also fell. But hemoglobin A1c levels stubbornly stayed steady, and obesity rates ballooned.
In light of the not entirely impressive numbers, “maybe we need to follow the model of team collaboration we see in the heart care setting,” said study lead author Carla I. Mercado, PhD, an epidemiologist with the Centers for Disease Control & Prevention. She spoke in an interview at the annual scientific sessions of the American Diabetes Association, where she presented the study findings.
For the new study, Dr. Mercado and colleagues used data from the National Health and Nutrition Examination Survey (NHANES) to track the health of adults with diabetes in the United States during 1999-2016. “With all the efforts on diabetes care management, we wanted to see if our efforts are making a difference in the population,” Dr. Mercado said.
The 5,534 participants had self-reported diabetes, were not pregnant, and underwent a physical examination. Throughout the periods examined (1999-2004, 2005-2010, and 2011-2016), the proportion of women remained steady at about one-half. So did the racial makeup, which ranged from 59% to 63% non-Hispanic white, 15% to 18% black, 7% to 10% Mexican-American, and 12% to 15% “other.”
Nearly half were aged 45-64, and 89%-90% had health insurance. There was a significant change in the education levels among those aged 25 and older: The percentage with at least a college degree grew from 14% in 1999-2004 to 21% in 2011-2016, while those with less than a high-school diploma fell from 34% to 23% over that period.
From 1999-2004 to 2011-2016
- Cholesterol: Most notably, the percentage of participants with non-HDL cholesterol levels below 130 mg/dL soared, from 30% to 54%. The CDC considers levels less than 100 mg/dL to be ideal. “I’m sure this is driven by medication use,” Dr. Mercado said.
- Smoking: The percentage of never smokers rose, from 44% to 47% of the subjects, while that of current smokers dropped, from 26% to 21%, a significant difference.
- Hypertension: The percentages with blood pressure levels less than 120/80 mm Hg – considered normal levels by the CDC – rose significantly, from 26% to 30%. “People are a lot more aware of blood pressure,” Dr. Mercado said.
- Glycemic control: HbA1c levels stayed roughly steady. About 10% had levels at or above 10% in both 1999-2004 and 2011-2016, and the number with A1c levels below 6% dipped slightly from 19% to 17%.
- Obesity: The proportion of participants with body mass index levels at or above 30 kg/m2, the line between overweight and obese, rose from 54% to 61%. The percentage of those with BMIs below 25 kg/m2 – considered to have normal weights – fell significantly, from 17% to 12%.
The researchers also looked at the percentage who reached ABCS goals (A1c at or below 9%, blood pressure below 140/90 mm Hg, non-HDL cholesterol under 160 mg/dL, and current nonsmoking status). The percentage who met all of these criteria grew from 26% to 40%, while those who met three of them stayed steady (40%-39%).
The study was funded by the CDC. The study authors report no relevant disclosures.
SAN FRANCISCO – New research suggests that.
As the age of statins dawned, cholesterol levels dipped dramatically, while blood pressure levels and smoking prevalence also fell. But hemoglobin A1c levels stubbornly stayed steady, and obesity rates ballooned.
In light of the not entirely impressive numbers, “maybe we need to follow the model of team collaboration we see in the heart care setting,” said study lead author Carla I. Mercado, PhD, an epidemiologist with the Centers for Disease Control & Prevention. She spoke in an interview at the annual scientific sessions of the American Diabetes Association, where she presented the study findings.
For the new study, Dr. Mercado and colleagues used data from the National Health and Nutrition Examination Survey (NHANES) to track the health of adults with diabetes in the United States during 1999-2016. “With all the efforts on diabetes care management, we wanted to see if our efforts are making a difference in the population,” Dr. Mercado said.
The 5,534 participants had self-reported diabetes, were not pregnant, and underwent a physical examination. Throughout the periods examined (1999-2004, 2005-2010, and 2011-2016), the proportion of women remained steady at about one-half. So did the racial makeup, which ranged from 59% to 63% non-Hispanic white, 15% to 18% black, 7% to 10% Mexican-American, and 12% to 15% “other.”
Nearly half were aged 45-64, and 89%-90% had health insurance. There was a significant change in the education levels among those aged 25 and older: The percentage with at least a college degree grew from 14% in 1999-2004 to 21% in 2011-2016, while those with less than a high-school diploma fell from 34% to 23% over that period.
From 1999-2004 to 2011-2016
- Cholesterol: Most notably, the percentage of participants with non-HDL cholesterol levels below 130 mg/dL soared, from 30% to 54%. The CDC considers levels less than 100 mg/dL to be ideal. “I’m sure this is driven by medication use,” Dr. Mercado said.
- Smoking: The percentage of never smokers rose, from 44% to 47% of the subjects, while that of current smokers dropped, from 26% to 21%, a significant difference.
- Hypertension: The percentages with blood pressure levels less than 120/80 mm Hg – considered normal levels by the CDC – rose significantly, from 26% to 30%. “People are a lot more aware of blood pressure,” Dr. Mercado said.
- Glycemic control: HbA1c levels stayed roughly steady. About 10% had levels at or above 10% in both 1999-2004 and 2011-2016, and the number with A1c levels below 6% dipped slightly from 19% to 17%.
- Obesity: The proportion of participants with body mass index levels at or above 30 kg/m2, the line between overweight and obese, rose from 54% to 61%. The percentage of those with BMIs below 25 kg/m2 – considered to have normal weights – fell significantly, from 17% to 12%.
The researchers also looked at the percentage who reached ABCS goals (A1c at or below 9%, blood pressure below 140/90 mm Hg, non-HDL cholesterol under 160 mg/dL, and current nonsmoking status). The percentage who met all of these criteria grew from 26% to 40%, while those who met three of them stayed steady (40%-39%).
The study was funded by the CDC. The study authors report no relevant disclosures.
REPORTING FROM ADA 2019
R2 appears active in high-risk FL and MZL
CHICAGO – Lenalidomide plus rituximab (R2) demonstrated activity against relapsed or refractory follicular lymphoma (FL) and marginal zone lymphoma (MZL) in the phase 3b MAGNIFY trial.
R2 produced responses in FL and MZL patients, including those who had previously experienced early relapse and patients who were refractory to rituximab or both lenalidomide and rituximab at baseline.
David Jacob Andorsky, MD, of Rocky Mountain Cancer Centers in Boulder, Colo., and colleagues presented these results in a poster at the annual meeting of the American Society of Clinical Oncology.
The ongoing MAGNIFY trial has enrolled 370 patients with relapsed/refractory FL (grade 1-3a) or MZL.
For induction, patients receive lenalidomide (20 mg per day on days 1-21 for 12 cycles) and rituximab (375 mg/m2 per week in cycle 1 and then on day 1 of cycles 3, 5, 7, 9, and 11). Patients who achieve stable disease or better on R2 induction are randomized to maintenance with R2 or rituximab alone.
Dr. Andorsky and colleagues presented results of R2 induction in 310 evaluable patients – 247 with FL and 63 with MZL.
The patients had a median age of 66 years (range, 35-91 years) at baseline, and they had received a median of two prior therapies (range, one to eight). Some patients had experienced early relapse (37%, n = 115), were refractory to rituximab (36%, n = 113), or were refractory to both rituximab and lenalidomide (20%, n = 63) at baseline.
Results
At a median follow-up of 16.7 months, the overall response rate was 73%, and the complete response rate was 45%. The overall response rate was 74% in FL patients, 65% in MZL patients, 63% in rituximab-refractory patients, 51% in double-refractory patients, and 68% in patients with an early relapse.
The median duration of response was 36.8 months in all patients, 35.8 months in MZL patients, and not reached in FL patients. The median duration of response was 35.8 months in patients who were rituximab refractory and was not reached in patients who were not refractory to rituximab.
The median progression-free survival was 36 months overall, 30 months in FL patients, 38 months in MZL patients, 23 months in patients with early relapse, and 15.5 months in double-refractory patients.
“While these [subgroup analyses of efficacy] were exploratory endpoints, I think this suggests that [R2] is a promising regimen for patients that are in the high-risk subgroup,” said Carla Casulo, MD, of the University of Rochester (N.Y.), who reviewed this study in a poster discussion session.
The most common adverse events in this trial were fatigue (48%), neutropenia (40%), diarrhea (35%), nausea (30%), and constipation (29%). The most common grade 3/4 adverse event was neutropenia (34%).
The MAGNIFY trial is sponsored by Celgene. Dr. Andorsky reported financial relationships with Celgene, CTI BioPharma, and Gilead Sciences. Dr. Casulo reported financial relationships with Gilead Sciences, Celgene, and Roche.
SOURCE: Andorsky DJ et al. ASCO 2019, Abstract 7513.
CHICAGO – Lenalidomide plus rituximab (R2) demonstrated activity against relapsed or refractory follicular lymphoma (FL) and marginal zone lymphoma (MZL) in the phase 3b MAGNIFY trial.
R2 produced responses in FL and MZL patients, including those who had previously experienced early relapse and patients who were refractory to rituximab or both lenalidomide and rituximab at baseline.
David Jacob Andorsky, MD, of Rocky Mountain Cancer Centers in Boulder, Colo., and colleagues presented these results in a poster at the annual meeting of the American Society of Clinical Oncology.
The ongoing MAGNIFY trial has enrolled 370 patients with relapsed/refractory FL (grade 1-3a) or MZL.
For induction, patients receive lenalidomide (20 mg per day on days 1-21 for 12 cycles) and rituximab (375 mg/m2 per week in cycle 1 and then on day 1 of cycles 3, 5, 7, 9, and 11). Patients who achieve stable disease or better on R2 induction are randomized to maintenance with R2 or rituximab alone.
Dr. Andorsky and colleagues presented results of R2 induction in 310 evaluable patients – 247 with FL and 63 with MZL.
The patients had a median age of 66 years (range, 35-91 years) at baseline, and they had received a median of two prior therapies (range, one to eight). Some patients had experienced early relapse (37%, n = 115), were refractory to rituximab (36%, n = 113), or were refractory to both rituximab and lenalidomide (20%, n = 63) at baseline.
Results
At a median follow-up of 16.7 months, the overall response rate was 73%, and the complete response rate was 45%. The overall response rate was 74% in FL patients, 65% in MZL patients, 63% in rituximab-refractory patients, 51% in double-refractory patients, and 68% in patients with an early relapse.
The median duration of response was 36.8 months in all patients, 35.8 months in MZL patients, and not reached in FL patients. The median duration of response was 35.8 months in patients who were rituximab refractory and was not reached in patients who were not refractory to rituximab.
The median progression-free survival was 36 months overall, 30 months in FL patients, 38 months in MZL patients, 23 months in patients with early relapse, and 15.5 months in double-refractory patients.
“While these [subgroup analyses of efficacy] were exploratory endpoints, I think this suggests that [R2] is a promising regimen for patients that are in the high-risk subgroup,” said Carla Casulo, MD, of the University of Rochester (N.Y.), who reviewed this study in a poster discussion session.
The most common adverse events in this trial were fatigue (48%), neutropenia (40%), diarrhea (35%), nausea (30%), and constipation (29%). The most common grade 3/4 adverse event was neutropenia (34%).
The MAGNIFY trial is sponsored by Celgene. Dr. Andorsky reported financial relationships with Celgene, CTI BioPharma, and Gilead Sciences. Dr. Casulo reported financial relationships with Gilead Sciences, Celgene, and Roche.
SOURCE: Andorsky DJ et al. ASCO 2019, Abstract 7513.
CHICAGO – Lenalidomide plus rituximab (R2) demonstrated activity against relapsed or refractory follicular lymphoma (FL) and marginal zone lymphoma (MZL) in the phase 3b MAGNIFY trial.
R2 produced responses in FL and MZL patients, including those who had previously experienced early relapse and patients who were refractory to rituximab or both lenalidomide and rituximab at baseline.
David Jacob Andorsky, MD, of Rocky Mountain Cancer Centers in Boulder, Colo., and colleagues presented these results in a poster at the annual meeting of the American Society of Clinical Oncology.
The ongoing MAGNIFY trial has enrolled 370 patients with relapsed/refractory FL (grade 1-3a) or MZL.
For induction, patients receive lenalidomide (20 mg per day on days 1-21 for 12 cycles) and rituximab (375 mg/m2 per week in cycle 1 and then on day 1 of cycles 3, 5, 7, 9, and 11). Patients who achieve stable disease or better on R2 induction are randomized to maintenance with R2 or rituximab alone.
Dr. Andorsky and colleagues presented results of R2 induction in 310 evaluable patients – 247 with FL and 63 with MZL.
The patients had a median age of 66 years (range, 35-91 years) at baseline, and they had received a median of two prior therapies (range, one to eight). Some patients had experienced early relapse (37%, n = 115), were refractory to rituximab (36%, n = 113), or were refractory to both rituximab and lenalidomide (20%, n = 63) at baseline.
Results
At a median follow-up of 16.7 months, the overall response rate was 73%, and the complete response rate was 45%. The overall response rate was 74% in FL patients, 65% in MZL patients, 63% in rituximab-refractory patients, 51% in double-refractory patients, and 68% in patients with an early relapse.
The median duration of response was 36.8 months in all patients, 35.8 months in MZL patients, and not reached in FL patients. The median duration of response was 35.8 months in patients who were rituximab refractory and was not reached in patients who were not refractory to rituximab.
The median progression-free survival was 36 months overall, 30 months in FL patients, 38 months in MZL patients, 23 months in patients with early relapse, and 15.5 months in double-refractory patients.
“While these [subgroup analyses of efficacy] were exploratory endpoints, I think this suggests that [R2] is a promising regimen for patients that are in the high-risk subgroup,” said Carla Casulo, MD, of the University of Rochester (N.Y.), who reviewed this study in a poster discussion session.
The most common adverse events in this trial were fatigue (48%), neutropenia (40%), diarrhea (35%), nausea (30%), and constipation (29%). The most common grade 3/4 adverse event was neutropenia (34%).
The MAGNIFY trial is sponsored by Celgene. Dr. Andorsky reported financial relationships with Celgene, CTI BioPharma, and Gilead Sciences. Dr. Casulo reported financial relationships with Gilead Sciences, Celgene, and Roche.
SOURCE: Andorsky DJ et al. ASCO 2019, Abstract 7513.
REPORTING FROM ASCO 2019
Oral voxelotor improves hemoglobin in sickle cell disease
AMSTERDAM – The investigational oral agent voxelotor induced rapid and sustained improvements in hemoglobin and hemolysis in both children and adults with sickle cell disease (SCD), follow-up results from the phase 3 HOPE trial show.
Among 274 patients aged 12-59 years, those who were randomly assigned to receive voxelotor at a dose of 1,500 mg daily had significantly better hemoglobin responses – defined as an increase of more than 1.0 g/dL from baseline – than did patients assigned to placebo, reported Jo Howard, MD, of Guy’s and St. Thomas’ NHS Foundation Trust and King’s College in London.
“. This has the potential to reduce the morbidity in sickle cell disease and to improve the life of our patients,” she said at a briefing prior to her presentation of the data at the annual congress of the European Hematology Association.
There were no new safety signals and patients tolerated voxelotor well, she added.
The study was published simultaneously in the New England Journal of Medicine.
Voxelotor is a novel oral agent that increases hemoglobin’s affinity for oxygen by inhibiting hemoglobin polymerization and sickling of red blood cells, which if unchecked lead to serious consequences, such as chronic anemia and hemolysis, and subsequent organ damage, vaso-occlusion, stroke, or premature death.
In the HOPE (Hemoglobin Oxygen Affinity Modulation to Inhibit HbS Polymerization) trial, investigators enrolled 274 adolescents and adults with SCD and randomized them on a 1:1:1 basis to receive voxelotor at doses of either 1,500 mg or 900 mg daily, or placebo.
Approximately two-thirds of the patients were receiving hydroxyurea at baseline.
In a per-protocol analysis, 59.5% of patients who received the 1,500-mg dose of voxelotor had a hemoglobin response (P less than .001 compared with baseline), as did 38% of patients in the 900-mg group (P less than .001). Among patients assigned to placebo, however, just 9.2% had a hemoglobin response, a difference that was not statistically significant.
In an intention-to-treat analysis, in which patients who did not complete the study were considered to be nonresponders, the respective rates of hemoglobin response were 51.1%, 32.6%, and 6.5%.
The difference between the 1,500-mg dose and placebo was significant (P less than .001). The difference between the 900-mg group and placebo was not statistically significant.
Hemoglobin levels of 10 g/dL or higher at week 24 were seen in 41% of the participants in the 1,500-mg group, 20% in the 900-mg group, and 9% in the placebo group.
Patients on voxelotor had an improvement in hemoglobin, whether or not they were on hydroxyurea, and those with hemoglobin either below or above 7 g/dL at baseline all had an increase in hemoglobin.
The annualized adjusted incidence rate of vaso-occlusive crises was similar in the two voxelotor groups (2.77 for the 1,500-mg dose and 2.76 for the 900-mg group) – both lower than in the placebo group (3.19).
Among patients who had two or more vaso-occlusive crises within the previous year, the respective annualized incidence rates were 2.88, 3.39, and 3.50.
There was a trend toward reduced incidence of crises with voxelotor over time, Dr. Howard said.
Grade 3 or greater adverse events occurred in 26% of patients in the 1,500-mg group, 23% in the 900-mg group, and 26% in the placebo group. The most common adverse events were headache and diarrhea.
“The data presented support the achievement of the stated primary endpoint in the HOPE trial, which was to reduce anemia and hemolysis. The hemoglobin response and reduction in hemolysis observed with an orally administered, once-daily medication with side effects that minimally affect lifestyle may make voxelotor a promising advancement in the management of sickle cell disease if approved by the [Food and Drug Administration],” Alexis Thompson, MD, MPH, of Northwestern University, Chicago, noted in an editorial accompanying the study in the New England Journal of Medicine.
Global Blood Therapeutics funded the study. Dr. Howard reported consultant/advisory board activity for the company. Dr. Thompson reported grants and/or personal fees from other companies.
SOURCE: Vichinsky E et al. EHA Congress, Abstract S147. N Engl J Med. 2019 Jun 14. doi: 10.1056/NEJMoa1903212.
AMSTERDAM – The investigational oral agent voxelotor induced rapid and sustained improvements in hemoglobin and hemolysis in both children and adults with sickle cell disease (SCD), follow-up results from the phase 3 HOPE trial show.
Among 274 patients aged 12-59 years, those who were randomly assigned to receive voxelotor at a dose of 1,500 mg daily had significantly better hemoglobin responses – defined as an increase of more than 1.0 g/dL from baseline – than did patients assigned to placebo, reported Jo Howard, MD, of Guy’s and St. Thomas’ NHS Foundation Trust and King’s College in London.
“. This has the potential to reduce the morbidity in sickle cell disease and to improve the life of our patients,” she said at a briefing prior to her presentation of the data at the annual congress of the European Hematology Association.
There were no new safety signals and patients tolerated voxelotor well, she added.
The study was published simultaneously in the New England Journal of Medicine.
Voxelotor is a novel oral agent that increases hemoglobin’s affinity for oxygen by inhibiting hemoglobin polymerization and sickling of red blood cells, which if unchecked lead to serious consequences, such as chronic anemia and hemolysis, and subsequent organ damage, vaso-occlusion, stroke, or premature death.
In the HOPE (Hemoglobin Oxygen Affinity Modulation to Inhibit HbS Polymerization) trial, investigators enrolled 274 adolescents and adults with SCD and randomized them on a 1:1:1 basis to receive voxelotor at doses of either 1,500 mg or 900 mg daily, or placebo.
Approximately two-thirds of the patients were receiving hydroxyurea at baseline.
In a per-protocol analysis, 59.5% of patients who received the 1,500-mg dose of voxelotor had a hemoglobin response (P less than .001 compared with baseline), as did 38% of patients in the 900-mg group (P less than .001). Among patients assigned to placebo, however, just 9.2% had a hemoglobin response, a difference that was not statistically significant.
In an intention-to-treat analysis, in which patients who did not complete the study were considered to be nonresponders, the respective rates of hemoglobin response were 51.1%, 32.6%, and 6.5%.
The difference between the 1,500-mg dose and placebo was significant (P less than .001). The difference between the 900-mg group and placebo was not statistically significant.
Hemoglobin levels of 10 g/dL or higher at week 24 were seen in 41% of the participants in the 1,500-mg group, 20% in the 900-mg group, and 9% in the placebo group.
Patients on voxelotor had an improvement in hemoglobin, whether or not they were on hydroxyurea, and those with hemoglobin either below or above 7 g/dL at baseline all had an increase in hemoglobin.
The annualized adjusted incidence rate of vaso-occlusive crises was similar in the two voxelotor groups (2.77 for the 1,500-mg dose and 2.76 for the 900-mg group) – both lower than in the placebo group (3.19).
Among patients who had two or more vaso-occlusive crises within the previous year, the respective annualized incidence rates were 2.88, 3.39, and 3.50.
There was a trend toward reduced incidence of crises with voxelotor over time, Dr. Howard said.
Grade 3 or greater adverse events occurred in 26% of patients in the 1,500-mg group, 23% in the 900-mg group, and 26% in the placebo group. The most common adverse events were headache and diarrhea.
“The data presented support the achievement of the stated primary endpoint in the HOPE trial, which was to reduce anemia and hemolysis. The hemoglobin response and reduction in hemolysis observed with an orally administered, once-daily medication with side effects that minimally affect lifestyle may make voxelotor a promising advancement in the management of sickle cell disease if approved by the [Food and Drug Administration],” Alexis Thompson, MD, MPH, of Northwestern University, Chicago, noted in an editorial accompanying the study in the New England Journal of Medicine.
Global Blood Therapeutics funded the study. Dr. Howard reported consultant/advisory board activity for the company. Dr. Thompson reported grants and/or personal fees from other companies.
SOURCE: Vichinsky E et al. EHA Congress, Abstract S147. N Engl J Med. 2019 Jun 14. doi: 10.1056/NEJMoa1903212.
AMSTERDAM – The investigational oral agent voxelotor induced rapid and sustained improvements in hemoglobin and hemolysis in both children and adults with sickle cell disease (SCD), follow-up results from the phase 3 HOPE trial show.
Among 274 patients aged 12-59 years, those who were randomly assigned to receive voxelotor at a dose of 1,500 mg daily had significantly better hemoglobin responses – defined as an increase of more than 1.0 g/dL from baseline – than did patients assigned to placebo, reported Jo Howard, MD, of Guy’s and St. Thomas’ NHS Foundation Trust and King’s College in London.
“. This has the potential to reduce the morbidity in sickle cell disease and to improve the life of our patients,” she said at a briefing prior to her presentation of the data at the annual congress of the European Hematology Association.
There were no new safety signals and patients tolerated voxelotor well, she added.
The study was published simultaneously in the New England Journal of Medicine.
Voxelotor is a novel oral agent that increases hemoglobin’s affinity for oxygen by inhibiting hemoglobin polymerization and sickling of red blood cells, which if unchecked lead to serious consequences, such as chronic anemia and hemolysis, and subsequent organ damage, vaso-occlusion, stroke, or premature death.
In the HOPE (Hemoglobin Oxygen Affinity Modulation to Inhibit HbS Polymerization) trial, investigators enrolled 274 adolescents and adults with SCD and randomized them on a 1:1:1 basis to receive voxelotor at doses of either 1,500 mg or 900 mg daily, or placebo.
Approximately two-thirds of the patients were receiving hydroxyurea at baseline.
In a per-protocol analysis, 59.5% of patients who received the 1,500-mg dose of voxelotor had a hemoglobin response (P less than .001 compared with baseline), as did 38% of patients in the 900-mg group (P less than .001). Among patients assigned to placebo, however, just 9.2% had a hemoglobin response, a difference that was not statistically significant.
In an intention-to-treat analysis, in which patients who did not complete the study were considered to be nonresponders, the respective rates of hemoglobin response were 51.1%, 32.6%, and 6.5%.
The difference between the 1,500-mg dose and placebo was significant (P less than .001). The difference between the 900-mg group and placebo was not statistically significant.
Hemoglobin levels of 10 g/dL or higher at week 24 were seen in 41% of the participants in the 1,500-mg group, 20% in the 900-mg group, and 9% in the placebo group.
Patients on voxelotor had an improvement in hemoglobin, whether or not they were on hydroxyurea, and those with hemoglobin either below or above 7 g/dL at baseline all had an increase in hemoglobin.
The annualized adjusted incidence rate of vaso-occlusive crises was similar in the two voxelotor groups (2.77 for the 1,500-mg dose and 2.76 for the 900-mg group) – both lower than in the placebo group (3.19).
Among patients who had two or more vaso-occlusive crises within the previous year, the respective annualized incidence rates were 2.88, 3.39, and 3.50.
There was a trend toward reduced incidence of crises with voxelotor over time, Dr. Howard said.
Grade 3 or greater adverse events occurred in 26% of patients in the 1,500-mg group, 23% in the 900-mg group, and 26% in the placebo group. The most common adverse events were headache and diarrhea.
“The data presented support the achievement of the stated primary endpoint in the HOPE trial, which was to reduce anemia and hemolysis. The hemoglobin response and reduction in hemolysis observed with an orally administered, once-daily medication with side effects that minimally affect lifestyle may make voxelotor a promising advancement in the management of sickle cell disease if approved by the [Food and Drug Administration],” Alexis Thompson, MD, MPH, of Northwestern University, Chicago, noted in an editorial accompanying the study in the New England Journal of Medicine.
Global Blood Therapeutics funded the study. Dr. Howard reported consultant/advisory board activity for the company. Dr. Thompson reported grants and/or personal fees from other companies.
SOURCE: Vichinsky E et al. EHA Congress, Abstract S147. N Engl J Med. 2019 Jun 14. doi: 10.1056/NEJMoa1903212.
REPORTING FROM EHA CONGRESS
Bone Health in Kidney Disease
Q) What are the current recommendations for the use of DXA and bisphosphonates in patients with chronic kidney disease and end-stage renal disease?
For patients with kidney disease, mineral and bone disorder (MBD) is a common complication, affecting the majority of those with moderate to severe chronic kidney disease (CKD; see Table 1).1,2 CKD-MBD is a systemic disorder that encompasses abnormalities in mineral metabolism, skeletal health, and soft-tissue calcifications.1,2 It manifests as one or more of the following:
- Abnormalities of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism
- Abnormalities in bone turnover, mineralization, volume, linear growth, or strength
- Vascular or other soft-tissue calcification.2

The Figure provides an illustration of the effect of CKD on bone health: In the general population, risk for hip fracture increases with age; risk is further exacerbated in those who have CKD.3
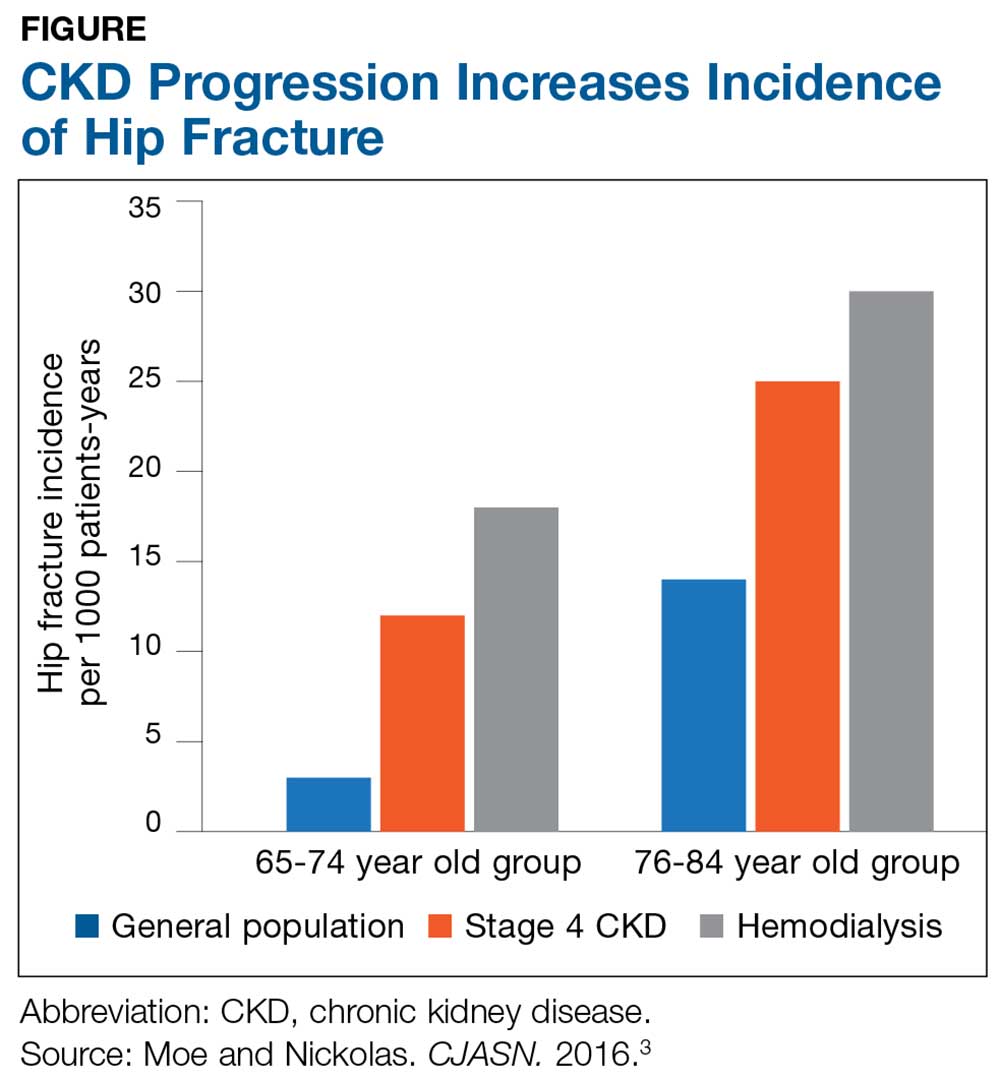
To assess for fracture risk in patients with advanced stages of CKD (3-5) who have evidence of CKD-MBD and/or risk factors for osteoporosis, the Kidney Disease: Improving Global Outcomes (KDIGO) group recommends bone mineral density testing with dual-energy X-ray absorptiometry (DXA).2 Bone biopsy—the gold standard for diagnosis of renal osteodystrophy, a form of osteoporosis and one type of bone abnormality seen in CKD-MBD—is “reasonable” to perform in cases in which knowing the type of renal osteodystrophy would inform treatment choices.2 KDIGO also recognizes limitations in the ability to perform a bone biopsy and therefore recommends monitoring serial PTH and bone-specific alkaline phosphatase to evaluate for bone disease.2
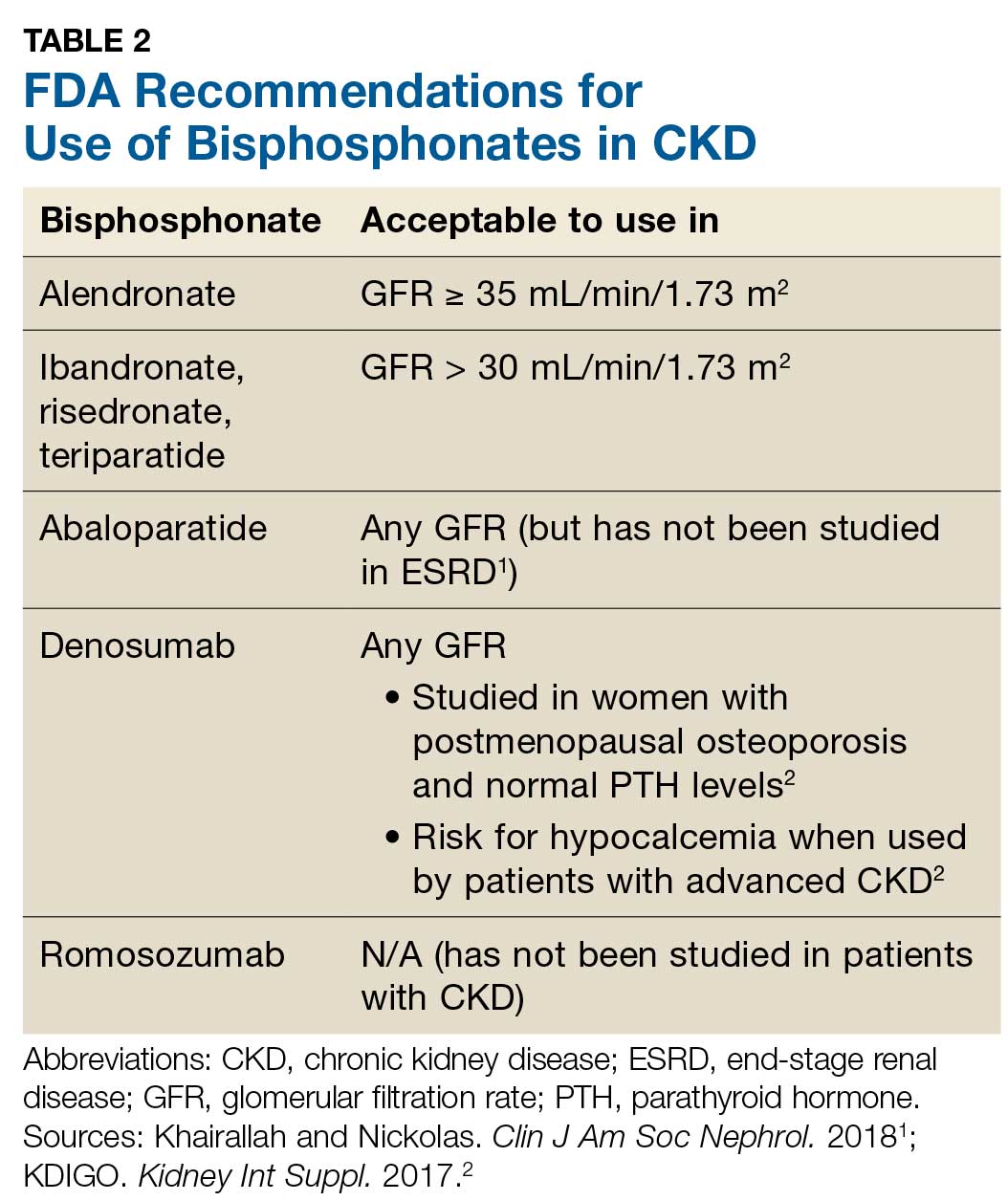
Prevention of fractures and treatment of patients with CKD-MDB has historically been challenging, since many of the available pharmacologic agents have not been developed for or studied in patients with CKD.1 According to KDIGO, it is acceptable for patients with CKD stages 1 and 2 to receive the same osteoporosis/fracture risk management as recommended for the general population.2 Patients with CKD stages 3a and 3b can also receive treatment as recommended for the general population, as long as the patient’s PTH level is in normal range.2 Table 2 outlines the FDA-approved glomerular filtration rate cutoffs for some bisphosphonates commonly used to treat osteoporosis.
Before initiating treatment for CKD-associated osteoporosis, no matter what the stage, it is important to manage vitamin D deficiency, hyperphosphatemia, and hyperparathyroidism.1 In CKD patients with abnormalities of calcium, phosphorus, PTH, and/or vitamin D, involve the nephrology team to assist in providing MBD care. Different approaches to treatment may include, but are not limited to, adjusting phosphorus binders; using vitamin D supplements or analogs; using calcimimetics; prescribing dialysis; providing dietary education; and addressing medication costs.
1. Khairallah P, Nickolas TL. Management of osteoporosis in CKD. Clin J Am Soc Nephrol. 2018;13(6):962-969.
2. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1-59.
3. Moe SM, Nickolas TL. Fractures in patients with CKD: time for action. Clin J Am Soc Nephrol. 2016;11(11):1929-1931.
Q) What are the current recommendations for the use of DXA and bisphosphonates in patients with chronic kidney disease and end-stage renal disease?
For patients with kidney disease, mineral and bone disorder (MBD) is a common complication, affecting the majority of those with moderate to severe chronic kidney disease (CKD; see Table 1).1,2 CKD-MBD is a systemic disorder that encompasses abnormalities in mineral metabolism, skeletal health, and soft-tissue calcifications.1,2 It manifests as one or more of the following:
- Abnormalities of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism
- Abnormalities in bone turnover, mineralization, volume, linear growth, or strength
- Vascular or other soft-tissue calcification.2

The Figure provides an illustration of the effect of CKD on bone health: In the general population, risk for hip fracture increases with age; risk is further exacerbated in those who have CKD.3

To assess for fracture risk in patients with advanced stages of CKD (3-5) who have evidence of CKD-MBD and/or risk factors for osteoporosis, the Kidney Disease: Improving Global Outcomes (KDIGO) group recommends bone mineral density testing with dual-energy X-ray absorptiometry (DXA).2 Bone biopsy—the gold standard for diagnosis of renal osteodystrophy, a form of osteoporosis and one type of bone abnormality seen in CKD-MBD—is “reasonable” to perform in cases in which knowing the type of renal osteodystrophy would inform treatment choices.2 KDIGO also recognizes limitations in the ability to perform a bone biopsy and therefore recommends monitoring serial PTH and bone-specific alkaline phosphatase to evaluate for bone disease.2
Prevention of fractures and treatment of patients with CKD-MDB has historically been challenging, since many of the available pharmacologic agents have not been developed for or studied in patients with CKD.1 According to KDIGO, it is acceptable for patients with CKD stages 1 and 2 to receive the same osteoporosis/fracture risk management as recommended for the general population.2 Patients with CKD stages 3a and 3b can also receive treatment as recommended for the general population, as long as the patient’s PTH level is in normal range.2 Table 2 outlines the FDA-approved glomerular filtration rate cutoffs for some bisphosphonates commonly used to treat osteoporosis.

Before initiating treatment for CKD-associated osteoporosis, no matter what the stage, it is important to manage vitamin D deficiency, hyperphosphatemia, and hyperparathyroidism.1 In CKD patients with abnormalities of calcium, phosphorus, PTH, and/or vitamin D, involve the nephrology team to assist in providing MBD care. Different approaches to treatment may include, but are not limited to, adjusting phosphorus binders; using vitamin D supplements or analogs; using calcimimetics; prescribing dialysis; providing dietary education; and addressing medication costs.
Q) What are the current recommendations for the use of DXA and bisphosphonates in patients with chronic kidney disease and end-stage renal disease?
For patients with kidney disease, mineral and bone disorder (MBD) is a common complication, affecting the majority of those with moderate to severe chronic kidney disease (CKD; see Table 1).1,2 CKD-MBD is a systemic disorder that encompasses abnormalities in mineral metabolism, skeletal health, and soft-tissue calcifications.1,2 It manifests as one or more of the following:
- Abnormalities of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism
- Abnormalities in bone turnover, mineralization, volume, linear growth, or strength
- Vascular or other soft-tissue calcification.2

The Figure provides an illustration of the effect of CKD on bone health: In the general population, risk for hip fracture increases with age; risk is further exacerbated in those who have CKD.3

To assess for fracture risk in patients with advanced stages of CKD (3-5) who have evidence of CKD-MBD and/or risk factors for osteoporosis, the Kidney Disease: Improving Global Outcomes (KDIGO) group recommends bone mineral density testing with dual-energy X-ray absorptiometry (DXA).2 Bone biopsy—the gold standard for diagnosis of renal osteodystrophy, a form of osteoporosis and one type of bone abnormality seen in CKD-MBD—is “reasonable” to perform in cases in which knowing the type of renal osteodystrophy would inform treatment choices.2 KDIGO also recognizes limitations in the ability to perform a bone biopsy and therefore recommends monitoring serial PTH and bone-specific alkaline phosphatase to evaluate for bone disease.2
Prevention of fractures and treatment of patients with CKD-MDB has historically been challenging, since many of the available pharmacologic agents have not been developed for or studied in patients with CKD.1 According to KDIGO, it is acceptable for patients with CKD stages 1 and 2 to receive the same osteoporosis/fracture risk management as recommended for the general population.2 Patients with CKD stages 3a and 3b can also receive treatment as recommended for the general population, as long as the patient’s PTH level is in normal range.2 Table 2 outlines the FDA-approved glomerular filtration rate cutoffs for some bisphosphonates commonly used to treat osteoporosis.

Before initiating treatment for CKD-associated osteoporosis, no matter what the stage, it is important to manage vitamin D deficiency, hyperphosphatemia, and hyperparathyroidism.1 In CKD patients with abnormalities of calcium, phosphorus, PTH, and/or vitamin D, involve the nephrology team to assist in providing MBD care. Different approaches to treatment may include, but are not limited to, adjusting phosphorus binders; using vitamin D supplements or analogs; using calcimimetics; prescribing dialysis; providing dietary education; and addressing medication costs.
1. Khairallah P, Nickolas TL. Management of osteoporosis in CKD. Clin J Am Soc Nephrol. 2018;13(6):962-969.
2. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1-59.
3. Moe SM, Nickolas TL. Fractures in patients with CKD: time for action. Clin J Am Soc Nephrol. 2016;11(11):1929-1931.
1. Khairallah P, Nickolas TL. Management of osteoporosis in CKD. Clin J Am Soc Nephrol. 2018;13(6):962-969.
2. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1-59.
3. Moe SM, Nickolas TL. Fractures in patients with CKD: time for action. Clin J Am Soc Nephrol. 2016;11(11):1929-1931.
Antibody hierarchy may drive development of SLE vs. antiphospholipid syndrome
according to study findings presented at the European Congress of Rheumatology.
Spanish researchers found that the number of antiphospholipid (aPL) antibodies present was important for the development of antiphospholipid syndrome (APS) and that lupus anticoagulant (LA) was the major aPL antibody linked to systemic lupus erythematosus (SLE)–related organ involvement.
“aPL [antibodies] has been extensively associated with an increased risk of thrombosis and poor pregnancy outcomes, mainly in patients with primary APS,” study investigator Leyre Riancho-Zarrabeitia, MD, PhD, explained in an interview ahead of the congress.
“Moreover, aPL [antibody] positivity in SLE has been proposed to be associated with higher damage accrual and with certain manifestations such as valvular heart disease, pulmonary hypertension, and neuropsychiatric manifestations,” she added.
Anticardiolipin antibodies – notably IgG rather than IgM isotypes – also seemed to play an important role in APS and SLE manifestations, Dr. Riancho-Zarrabeitia, of Hospital Sierrallana, Instituto De Investigación Marqués De Valdecilla, and the University of Cantabria (Spain), noted during her oral presentation.
She reported data on 3,651 patients included in the RELESSER registry between October 2011 and August 2012. This large, multicenter, hospital-based registry retrospectively collects immunologic, clinical and demographic data from unselected adult patients with SLE who are attending 45 Spanish rheumatology services within the country’s national health system.
Over one-third (37.5%) of patients, who had a mean age of 47 years and were mostly (90%) women, were positive for aPL. The most frequent aPL detected was IgG anticardiolipin (aCL) antibodies, seen in 25% of patients, followed by LA in 24%, and IgM aCL in 20%.
Of the aPL-positive patients, 20.6% were positive for only one antibody, 12.1% were positive for two antibodies, and 4.8% were positive for three antibodies.
“All types of aPL were associated with classic APS manifestations,” Dr. Riancho-Zarrabeitia said. The associations were strongest for thrombotic events, such as arterial and venous small-vessel thrombosis and recurrent early pregnancy losses.
aCL antibodies conferred the highest risk for arterial thrombosis, she noted (odds ratio, 5.7), whereas LA conferred the highest risk for venous thrombosis (OR, 4.7). Both IgG and IgM isotypes were associated with thrombotic events, fetal death and recurrent pregnancy loss, but the association was stronger with the IgG isotypes.
Having more than one aPL was particularly associated with a higher risk of these APS manifestations. For example, when one antibody was present the OR for arterial thrombosis was 4.45, but when two or more aPL were detected, the ORs rose to 9.23 and 15.6, respectively.
aCL and LA also were associated with thrombocytopenia and hemolytic anemia, with ORs of around 1-2 and 2-3 respectively. There also were antibody associations with cognitive impairments.
Similar results were seen in patients with SLE. “aPL [antibody] positivity in SLE patients influenced the risk for thrombotic and obstetric manifestations,” Dr. Riancho-Zarrabeitia said. LA and aCL were associated with an increased risk of neuropsychiatric manifestations, and LA was linked to an increased risk for renal disease.
The risk for specific SLE manifestations was again higher with IgG isotypes of aCL, notably an increased risk for cardiac and respiratory events.
While increased antibody numbers generally led to a higher risk of complications, the risk for cutaneous manifestations decreased.
“The load of aPL [antibodies] confers a higher risk for APS,” Dr. Riancho-Zarrabeitia said during her conclusion. “Regarding systemic lupus erythematosus, the number of positive antibodies is directly associated with neurological and ophthalmological manifestations, and inversely associated with cutaneous manifestations.”
What these findings show, said Dr. Riancho-Zarrabeitia in the precongress interview, is that individuals who test positive for aPL antibodies need careful monitoring to prevent and treat severe manifestations. “The next step would be to confirm our findings with a prospective study.”
Dr. Riancho-Zarrabeitia has received travel grants from AbbVie, Pfizer, UCB, Merck, GlaxoSmithKline, Amgen, and Roche.
SOURCE: Riancho-Zarrabeitia L et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):136-7. Abstract OP0124. doi: 10.1136/annrheumdis-2019-eular.2485.
according to study findings presented at the European Congress of Rheumatology.
Spanish researchers found that the number of antiphospholipid (aPL) antibodies present was important for the development of antiphospholipid syndrome (APS) and that lupus anticoagulant (LA) was the major aPL antibody linked to systemic lupus erythematosus (SLE)–related organ involvement.
“aPL [antibodies] has been extensively associated with an increased risk of thrombosis and poor pregnancy outcomes, mainly in patients with primary APS,” study investigator Leyre Riancho-Zarrabeitia, MD, PhD, explained in an interview ahead of the congress.
“Moreover, aPL [antibody] positivity in SLE has been proposed to be associated with higher damage accrual and with certain manifestations such as valvular heart disease, pulmonary hypertension, and neuropsychiatric manifestations,” she added.
Anticardiolipin antibodies – notably IgG rather than IgM isotypes – also seemed to play an important role in APS and SLE manifestations, Dr. Riancho-Zarrabeitia, of Hospital Sierrallana, Instituto De Investigación Marqués De Valdecilla, and the University of Cantabria (Spain), noted during her oral presentation.
She reported data on 3,651 patients included in the RELESSER registry between October 2011 and August 2012. This large, multicenter, hospital-based registry retrospectively collects immunologic, clinical and demographic data from unselected adult patients with SLE who are attending 45 Spanish rheumatology services within the country’s national health system.
Over one-third (37.5%) of patients, who had a mean age of 47 years and were mostly (90%) women, were positive for aPL. The most frequent aPL detected was IgG anticardiolipin (aCL) antibodies, seen in 25% of patients, followed by LA in 24%, and IgM aCL in 20%.
Of the aPL-positive patients, 20.6% were positive for only one antibody, 12.1% were positive for two antibodies, and 4.8% were positive for three antibodies.
“All types of aPL were associated with classic APS manifestations,” Dr. Riancho-Zarrabeitia said. The associations were strongest for thrombotic events, such as arterial and venous small-vessel thrombosis and recurrent early pregnancy losses.
aCL antibodies conferred the highest risk for arterial thrombosis, she noted (odds ratio, 5.7), whereas LA conferred the highest risk for venous thrombosis (OR, 4.7). Both IgG and IgM isotypes were associated with thrombotic events, fetal death and recurrent pregnancy loss, but the association was stronger with the IgG isotypes.
Having more than one aPL was particularly associated with a higher risk of these APS manifestations. For example, when one antibody was present the OR for arterial thrombosis was 4.45, but when two or more aPL were detected, the ORs rose to 9.23 and 15.6, respectively.
aCL and LA also were associated with thrombocytopenia and hemolytic anemia, with ORs of around 1-2 and 2-3 respectively. There also were antibody associations with cognitive impairments.
Similar results were seen in patients with SLE. “aPL [antibody] positivity in SLE patients influenced the risk for thrombotic and obstetric manifestations,” Dr. Riancho-Zarrabeitia said. LA and aCL were associated with an increased risk of neuropsychiatric manifestations, and LA was linked to an increased risk for renal disease.
The risk for specific SLE manifestations was again higher with IgG isotypes of aCL, notably an increased risk for cardiac and respiratory events.
While increased antibody numbers generally led to a higher risk of complications, the risk for cutaneous manifestations decreased.
“The load of aPL [antibodies] confers a higher risk for APS,” Dr. Riancho-Zarrabeitia said during her conclusion. “Regarding systemic lupus erythematosus, the number of positive antibodies is directly associated with neurological and ophthalmological manifestations, and inversely associated with cutaneous manifestations.”
What these findings show, said Dr. Riancho-Zarrabeitia in the precongress interview, is that individuals who test positive for aPL antibodies need careful monitoring to prevent and treat severe manifestations. “The next step would be to confirm our findings with a prospective study.”
Dr. Riancho-Zarrabeitia has received travel grants from AbbVie, Pfizer, UCB, Merck, GlaxoSmithKline, Amgen, and Roche.
SOURCE: Riancho-Zarrabeitia L et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):136-7. Abstract OP0124. doi: 10.1136/annrheumdis-2019-eular.2485.
according to study findings presented at the European Congress of Rheumatology.
Spanish researchers found that the number of antiphospholipid (aPL) antibodies present was important for the development of antiphospholipid syndrome (APS) and that lupus anticoagulant (LA) was the major aPL antibody linked to systemic lupus erythematosus (SLE)–related organ involvement.
“aPL [antibodies] has been extensively associated with an increased risk of thrombosis and poor pregnancy outcomes, mainly in patients with primary APS,” study investigator Leyre Riancho-Zarrabeitia, MD, PhD, explained in an interview ahead of the congress.
“Moreover, aPL [antibody] positivity in SLE has been proposed to be associated with higher damage accrual and with certain manifestations such as valvular heart disease, pulmonary hypertension, and neuropsychiatric manifestations,” she added.
Anticardiolipin antibodies – notably IgG rather than IgM isotypes – also seemed to play an important role in APS and SLE manifestations, Dr. Riancho-Zarrabeitia, of Hospital Sierrallana, Instituto De Investigación Marqués De Valdecilla, and the University of Cantabria (Spain), noted during her oral presentation.
She reported data on 3,651 patients included in the RELESSER registry between October 2011 and August 2012. This large, multicenter, hospital-based registry retrospectively collects immunologic, clinical and demographic data from unselected adult patients with SLE who are attending 45 Spanish rheumatology services within the country’s national health system.
Over one-third (37.5%) of patients, who had a mean age of 47 years and were mostly (90%) women, were positive for aPL. The most frequent aPL detected was IgG anticardiolipin (aCL) antibodies, seen in 25% of patients, followed by LA in 24%, and IgM aCL in 20%.
Of the aPL-positive patients, 20.6% were positive for only one antibody, 12.1% were positive for two antibodies, and 4.8% were positive for three antibodies.
“All types of aPL were associated with classic APS manifestations,” Dr. Riancho-Zarrabeitia said. The associations were strongest for thrombotic events, such as arterial and venous small-vessel thrombosis and recurrent early pregnancy losses.
aCL antibodies conferred the highest risk for arterial thrombosis, she noted (odds ratio, 5.7), whereas LA conferred the highest risk for venous thrombosis (OR, 4.7). Both IgG and IgM isotypes were associated with thrombotic events, fetal death and recurrent pregnancy loss, but the association was stronger with the IgG isotypes.
Having more than one aPL was particularly associated with a higher risk of these APS manifestations. For example, when one antibody was present the OR for arterial thrombosis was 4.45, but when two or more aPL were detected, the ORs rose to 9.23 and 15.6, respectively.
aCL and LA also were associated with thrombocytopenia and hemolytic anemia, with ORs of around 1-2 and 2-3 respectively. There also were antibody associations with cognitive impairments.
Similar results were seen in patients with SLE. “aPL [antibody] positivity in SLE patients influenced the risk for thrombotic and obstetric manifestations,” Dr. Riancho-Zarrabeitia said. LA and aCL were associated with an increased risk of neuropsychiatric manifestations, and LA was linked to an increased risk for renal disease.
The risk for specific SLE manifestations was again higher with IgG isotypes of aCL, notably an increased risk for cardiac and respiratory events.
While increased antibody numbers generally led to a higher risk of complications, the risk for cutaneous manifestations decreased.
“The load of aPL [antibodies] confers a higher risk for APS,” Dr. Riancho-Zarrabeitia said during her conclusion. “Regarding systemic lupus erythematosus, the number of positive antibodies is directly associated with neurological and ophthalmological manifestations, and inversely associated with cutaneous manifestations.”
What these findings show, said Dr. Riancho-Zarrabeitia in the precongress interview, is that individuals who test positive for aPL antibodies need careful monitoring to prevent and treat severe manifestations. “The next step would be to confirm our findings with a prospective study.”
Dr. Riancho-Zarrabeitia has received travel grants from AbbVie, Pfizer, UCB, Merck, GlaxoSmithKline, Amgen, and Roche.
SOURCE: Riancho-Zarrabeitia L et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):136-7. Abstract OP0124. doi: 10.1136/annrheumdis-2019-eular.2485.
REPORTING FROM EULAR 2019 CONGRESS
TAILORx: Clinical data add value to recurrence score
CHICAGO – , according to a secondary analysis of data from the practice-changing TAILORx study.
Specifically, tumor size and histology-based risk stratification improves the prediction of disease-free survival and distant recurrence, and – for some patient groups – chemotherapy benefit, Joseph A. Sparano, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Combining these tools could help determine whether endocrine therapy (ET) alone or ET with adjuvant chemotherapy is the best treatment approach for a given patient, said Dr. Sparano, professor of medicine and obstetrics, gynecology, and women’s health at Albert Einstein College of Medicine, New York.
The phase 3 TAILORx study established that ET alone is noninferior to adjuvant chemotherapy (CT) plus ET in patients with early breast cancer and RS of 11-25, and that ET alone has some benefit over ET+CT in women aged 50 years and younger with RS of 16-25, he explained.
Those findings were presented at the 2018 ASCO annual meeting and subsequently published in the New England Journal of Medicine.
The current analysis focused on the integration of clinical and genomic features for prognosis, and the results were published online June 3 in a corresponding article in the New England Journal of Medicine.
“The totality of the data, including TAILORx and the prior prospective validation studies, indicate that assessment of genomic risk with the 21-gene recurrence score provides complementary prognostic information to pathologic features, and is also predictive of a large chemotherapy benefit if the recurrence score is greater than 25, or lack thereof if 25 or lower,” he said.
However, there is a three-way interaction between age, RS, and CT use, which results in an absolute CT benefit in women aged 50 or younger of about 2% for RS of 16-20, and about 7% for RS of 21-25, he added.
“Assessment of clinical risk using pathological features also provides prognostic information that doesn’t correlate well with the recurrence score, therefore it stands to reason that integration of clinical and genomic risk offers the potential for greater precision in prognosis and, ultimately, guiding the use of adjuvant therapy,” he said.
Clinical risk for this analysis was assessed using a binary clinical risk categorization employed in the MINDACT trial and calibrated to greater than 92% 10-year breast cancer-specific survival for ET alone based on Adjuvant! version 8.0. Low-grade tumors up to 3 cm, intermediate-grade tumors up to 2 cm, and high-grade tumors up to 1 cm were categorized as low clinical risk (LCR), and all others not meeting these criteria were categorized as high clinical risk (HCR), he explained.
Of 9,427 patients included in the analysis, 70% had LCR and 30% had HCR.
“For distant recurrence, high clinical risk was associated with a 2.5- to 3-fold higher recurrence rate for those with a recurrence score of 11 or higher, and in a multivariate model for distant recurrence in the [group with a] recurrence score of 11-25, high clinical risk was independently associated with a 2.4-fold higher recurrence risk,” he said. “Continuous recurrence score also provided significant prognostic information, with each 1-unit increase associated with an 8% higher distant recurrence risk.”
For the overall population, clinical risk added significant prognostic information to the RS for both distant recurrence and disease-free survival, and stratification by age showed that among women over age 50 years, the hazard ratios for distant recurrence ranged from 2.20 to 2.36, and did not substantially vary by age or RS, he said.
However, for the overall population, adding clinical risk to the RS did not improve prediction of chemotherapy benefit.
“This was also true for the two-thirds of women who were over 50 years of age. For the remaining women 50 or younger, there was a trend favoring chemo, irrespective of clinical risk, though not significant – a finding consistent with the treatment interaction previously described,” he said.
Finally, the absolute differences in 9-year distant recurrence rates by clinical risk stratified by age, RS, and CT use showed an absolute 4%-6% higher distant recurrence risk for HCR vs. LCR among those over age 50 with RS of 0-25 irrespective of CT use, and a 13% difference for those with RS of 26-100 who were treated with CT.
“For those 50 or younger, clinical risk had no impact on recurrence if the RS was 0-10. For RS of 11-25, the difference was about 9% with endocrine therapy alone, and 2% with chemo plus ET, reflecting absolute chemo benefit in younger women who had high clinical risk,” he said, adding that for those with RS of 26-100, there was a 9% higher absolute recurrence rate in the HCR vs. LCR population.
“We therefore further evaluated absolute differences in distance recurrence rates associated with chemotherapy use in women 50 and younger with RS of 16-25, further stratified by RS and clinical risk,” he said, noting that when not stratified by clinical risk, as reported in the primary analysis, the absolute CT benefit was 1.6% for RS of 16-20, and 6.5% for RS of 21-25.
When stratified by clinical risk, the absolute CT benefit ranged from 6% to 9% in those with RS of 21-25, irrespective of clinical risk, and in those with RS of 16-20 and HCR.
“This accounted for 51% of patients with RS of 16-25,” he said. “However, there was no demonstrable chemo benefit for those with LCR and RS of 16-20, who accounted for the remaining 49%.”
Additional analysis looking at age at diagnosis and CT benefit showed a benefit in premenopausal women aged 46-50 years (but not postmenopausal women), a trend toward benefit in those aged 41-45 years, and no benefit in those aged 40 years and younger, who are less likely to develop premature menopause as a consequence of cytotoxic CT.
“In addition, we saw no consistent effect favoring chemotherapy in older women. Taken together, these findings suggest the chemo benefit observed for the RS 16-25 group may, in fact, be due to a castration effect associated with cytotoxic therapy rather than an effect in eradicating micrometastatic disease,” Dr. Sparano said.
Applying this framework to the TAILORx study population categorized 68% of those aged 50 years and younger into a low integrated risk group with less than 5% risk of distant recurrence. This included all patients with RS of 0-10 irrespective of clinical risk (14% of the patient population; distant recurrence rate 1.8% or less), and all with RS of 11-25 and LCR (54% of the patient population, 4.7% distant recurrence rate).
In contrast, 25% fell into the high integrated risk group (greater than 10% distant recurrence risk), including those with RS of 11-25 and HCR (17% of the patient population; distant recurrence rate 12.3%), and RS of 26-100 and HCR (8% of the patient population; distant recurrence rate 15.2%).
“This framework encompasses 93% of all TAILORx subjects, with the remaining 7% having a distant recurrence risk of between 5% and 10%,” he said.
Overall, the primary results of TAILORx remain unchanged based on this secondary analysis as the addition of clinical risk did not predict CT benefit in the RS 11-25 group, he noted.
“However, for women 50 and under and RS 16-25, integrated risk distinguished 50% who derived no chemo benefit from the 50% who derived an absolute benefit of approximately 6%-9% – a level that is higher than an unselected population,” he said, reiterating that the absolute CT benefit was greater in premenopausal women aged 45-50 with RS 16-25, suggesting that the absolute CR benefit seen in younger women in TAILORx may be due to an endocrine effect.
“Integrated risk clearly provides greater prognostic precision and may have clinical utility; the prognostic precision afforded by the integrated risk model is superior to that by the use of clinical or genomic features alone, and in addition, the genomic assay also provides predictive information for chemo benefit that is not captured by clinical features alone,” he concluded.
As an example of the potential clinical utility of this integrated approach for guiding treatment in women aged 50 years or younger, he presented “a highly stratified integrated risk assessment model” separating TAILORx patients into low integrated risk (58% of the study population) and high integrated risk (31% of the study population).
In the low integrated risk patients with RS of 0-10 and any clinical risk level, or with RS of 11-25 and LCR, tamoxifen alone appears adequate, he said.
In those with high integrated risk and RS of 16-25 with HCR, ovarian function suppression plus an aromatase inhibitor (OFS/AI) could be considered as an alternative to chemo, and in those with high integrated risk, RS of 26-100, and HCR who have not developed chemotherapy-induced menopause, ovarian function suppression and an AI could be added to chemotherapy.
“Indeed, data from the SOFT and TEXT trials indicate that patients with a high RS risk experienced an absolute improvement of up to 10%-15% in 5-year breast cancer–free interval with an OFS/AI, compared with tamoxifen, whereas improvement was minimal in those at lowest risk, supporting the strategy of using integrated clinical and genomic risk to select for ovarian function suppression plus an AI,” he said.
During a discussion of the findings and how they might impact practice, Vered Stearns, MD, an oncology professor and codirector of the Breast Cancer Program at Johns Hopkins University, Baltimore, noted that in her practice she will “carefully select women for whom genomic assay [use] is appropriate.
“I will also assess clinical risk and RS to inform recommendations for chemotherapy use, and possibly appropriate endocrine agents in select populations,” she said.
Dr. Stearns further noted that the interaction between RS and age as reported by Dr. Sparano is exploratory and should be interpreted with caution as the majority of those aged 50 and younger received tamoxifen alone and the question remains as to whether they would have received similar benefits from ovarian suppression and tamoxifen/AI instead of chemo-endocrine therapy.
“Indeed, indirect hypotheses from other studies suggest that may be the case,” she said, adding that these women may be offered ovarian suppression and tamoxifen or AI based on the SOFT and TEXT results.
“TAILORx remains a rich resource for new explorations, new biomarkers, new models, and new machine learning opportunities,” she said.
TAILORx was funded by the National Institutes of Health. Dr. Sparano reported stock ownership, a consulting role, and research funding from several pharmaceutical companies. Dr. Stearns reported consulting or advisory roles with Iridium Therapeutics; research funding from Abbvie, Biocept, MedImmune, Novartis, Pfizer, and Puma Biotechnology; and an “other relationship” with Immunomedics.
SOURCE: Sparano JA et al. ASCO 2019. Abstract 503.
CHICAGO – , according to a secondary analysis of data from the practice-changing TAILORx study.
Specifically, tumor size and histology-based risk stratification improves the prediction of disease-free survival and distant recurrence, and – for some patient groups – chemotherapy benefit, Joseph A. Sparano, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Combining these tools could help determine whether endocrine therapy (ET) alone or ET with adjuvant chemotherapy is the best treatment approach for a given patient, said Dr. Sparano, professor of medicine and obstetrics, gynecology, and women’s health at Albert Einstein College of Medicine, New York.
The phase 3 TAILORx study established that ET alone is noninferior to adjuvant chemotherapy (CT) plus ET in patients with early breast cancer and RS of 11-25, and that ET alone has some benefit over ET+CT in women aged 50 years and younger with RS of 16-25, he explained.
Those findings were presented at the 2018 ASCO annual meeting and subsequently published in the New England Journal of Medicine.
The current analysis focused on the integration of clinical and genomic features for prognosis, and the results were published online June 3 in a corresponding article in the New England Journal of Medicine.
“The totality of the data, including TAILORx and the prior prospective validation studies, indicate that assessment of genomic risk with the 21-gene recurrence score provides complementary prognostic information to pathologic features, and is also predictive of a large chemotherapy benefit if the recurrence score is greater than 25, or lack thereof if 25 or lower,” he said.
However, there is a three-way interaction between age, RS, and CT use, which results in an absolute CT benefit in women aged 50 or younger of about 2% for RS of 16-20, and about 7% for RS of 21-25, he added.
“Assessment of clinical risk using pathological features also provides prognostic information that doesn’t correlate well with the recurrence score, therefore it stands to reason that integration of clinical and genomic risk offers the potential for greater precision in prognosis and, ultimately, guiding the use of adjuvant therapy,” he said.
Clinical risk for this analysis was assessed using a binary clinical risk categorization employed in the MINDACT trial and calibrated to greater than 92% 10-year breast cancer-specific survival for ET alone based on Adjuvant! version 8.0. Low-grade tumors up to 3 cm, intermediate-grade tumors up to 2 cm, and high-grade tumors up to 1 cm were categorized as low clinical risk (LCR), and all others not meeting these criteria were categorized as high clinical risk (HCR), he explained.
Of 9,427 patients included in the analysis, 70% had LCR and 30% had HCR.
“For distant recurrence, high clinical risk was associated with a 2.5- to 3-fold higher recurrence rate for those with a recurrence score of 11 or higher, and in a multivariate model for distant recurrence in the [group with a] recurrence score of 11-25, high clinical risk was independently associated with a 2.4-fold higher recurrence risk,” he said. “Continuous recurrence score also provided significant prognostic information, with each 1-unit increase associated with an 8% higher distant recurrence risk.”
For the overall population, clinical risk added significant prognostic information to the RS for both distant recurrence and disease-free survival, and stratification by age showed that among women over age 50 years, the hazard ratios for distant recurrence ranged from 2.20 to 2.36, and did not substantially vary by age or RS, he said.
However, for the overall population, adding clinical risk to the RS did not improve prediction of chemotherapy benefit.
“This was also true for the two-thirds of women who were over 50 years of age. For the remaining women 50 or younger, there was a trend favoring chemo, irrespective of clinical risk, though not significant – a finding consistent with the treatment interaction previously described,” he said.
Finally, the absolute differences in 9-year distant recurrence rates by clinical risk stratified by age, RS, and CT use showed an absolute 4%-6% higher distant recurrence risk for HCR vs. LCR among those over age 50 with RS of 0-25 irrespective of CT use, and a 13% difference for those with RS of 26-100 who were treated with CT.
“For those 50 or younger, clinical risk had no impact on recurrence if the RS was 0-10. For RS of 11-25, the difference was about 9% with endocrine therapy alone, and 2% with chemo plus ET, reflecting absolute chemo benefit in younger women who had high clinical risk,” he said, adding that for those with RS of 26-100, there was a 9% higher absolute recurrence rate in the HCR vs. LCR population.
“We therefore further evaluated absolute differences in distance recurrence rates associated with chemotherapy use in women 50 and younger with RS of 16-25, further stratified by RS and clinical risk,” he said, noting that when not stratified by clinical risk, as reported in the primary analysis, the absolute CT benefit was 1.6% for RS of 16-20, and 6.5% for RS of 21-25.
When stratified by clinical risk, the absolute CT benefit ranged from 6% to 9% in those with RS of 21-25, irrespective of clinical risk, and in those with RS of 16-20 and HCR.
“This accounted for 51% of patients with RS of 16-25,” he said. “However, there was no demonstrable chemo benefit for those with LCR and RS of 16-20, who accounted for the remaining 49%.”
Additional analysis looking at age at diagnosis and CT benefit showed a benefit in premenopausal women aged 46-50 years (but not postmenopausal women), a trend toward benefit in those aged 41-45 years, and no benefit in those aged 40 years and younger, who are less likely to develop premature menopause as a consequence of cytotoxic CT.
“In addition, we saw no consistent effect favoring chemotherapy in older women. Taken together, these findings suggest the chemo benefit observed for the RS 16-25 group may, in fact, be due to a castration effect associated with cytotoxic therapy rather than an effect in eradicating micrometastatic disease,” Dr. Sparano said.
Applying this framework to the TAILORx study population categorized 68% of those aged 50 years and younger into a low integrated risk group with less than 5% risk of distant recurrence. This included all patients with RS of 0-10 irrespective of clinical risk (14% of the patient population; distant recurrence rate 1.8% or less), and all with RS of 11-25 and LCR (54% of the patient population, 4.7% distant recurrence rate).
In contrast, 25% fell into the high integrated risk group (greater than 10% distant recurrence risk), including those with RS of 11-25 and HCR (17% of the patient population; distant recurrence rate 12.3%), and RS of 26-100 and HCR (8% of the patient population; distant recurrence rate 15.2%).
“This framework encompasses 93% of all TAILORx subjects, with the remaining 7% having a distant recurrence risk of between 5% and 10%,” he said.
Overall, the primary results of TAILORx remain unchanged based on this secondary analysis as the addition of clinical risk did not predict CT benefit in the RS 11-25 group, he noted.
“However, for women 50 and under and RS 16-25, integrated risk distinguished 50% who derived no chemo benefit from the 50% who derived an absolute benefit of approximately 6%-9% – a level that is higher than an unselected population,” he said, reiterating that the absolute CT benefit was greater in premenopausal women aged 45-50 with RS 16-25, suggesting that the absolute CR benefit seen in younger women in TAILORx may be due to an endocrine effect.
“Integrated risk clearly provides greater prognostic precision and may have clinical utility; the prognostic precision afforded by the integrated risk model is superior to that by the use of clinical or genomic features alone, and in addition, the genomic assay also provides predictive information for chemo benefit that is not captured by clinical features alone,” he concluded.
As an example of the potential clinical utility of this integrated approach for guiding treatment in women aged 50 years or younger, he presented “a highly stratified integrated risk assessment model” separating TAILORx patients into low integrated risk (58% of the study population) and high integrated risk (31% of the study population).
In the low integrated risk patients with RS of 0-10 and any clinical risk level, or with RS of 11-25 and LCR, tamoxifen alone appears adequate, he said.
In those with high integrated risk and RS of 16-25 with HCR, ovarian function suppression plus an aromatase inhibitor (OFS/AI) could be considered as an alternative to chemo, and in those with high integrated risk, RS of 26-100, and HCR who have not developed chemotherapy-induced menopause, ovarian function suppression and an AI could be added to chemotherapy.
“Indeed, data from the SOFT and TEXT trials indicate that patients with a high RS risk experienced an absolute improvement of up to 10%-15% in 5-year breast cancer–free interval with an OFS/AI, compared with tamoxifen, whereas improvement was minimal in those at lowest risk, supporting the strategy of using integrated clinical and genomic risk to select for ovarian function suppression plus an AI,” he said.
During a discussion of the findings and how they might impact practice, Vered Stearns, MD, an oncology professor and codirector of the Breast Cancer Program at Johns Hopkins University, Baltimore, noted that in her practice she will “carefully select women for whom genomic assay [use] is appropriate.
“I will also assess clinical risk and RS to inform recommendations for chemotherapy use, and possibly appropriate endocrine agents in select populations,” she said.
Dr. Stearns further noted that the interaction between RS and age as reported by Dr. Sparano is exploratory and should be interpreted with caution as the majority of those aged 50 and younger received tamoxifen alone and the question remains as to whether they would have received similar benefits from ovarian suppression and tamoxifen/AI instead of chemo-endocrine therapy.
“Indeed, indirect hypotheses from other studies suggest that may be the case,” she said, adding that these women may be offered ovarian suppression and tamoxifen or AI based on the SOFT and TEXT results.
“TAILORx remains a rich resource for new explorations, new biomarkers, new models, and new machine learning opportunities,” she said.
TAILORx was funded by the National Institutes of Health. Dr. Sparano reported stock ownership, a consulting role, and research funding from several pharmaceutical companies. Dr. Stearns reported consulting or advisory roles with Iridium Therapeutics; research funding from Abbvie, Biocept, MedImmune, Novartis, Pfizer, and Puma Biotechnology; and an “other relationship” with Immunomedics.
SOURCE: Sparano JA et al. ASCO 2019. Abstract 503.
CHICAGO – , according to a secondary analysis of data from the practice-changing TAILORx study.
Specifically, tumor size and histology-based risk stratification improves the prediction of disease-free survival and distant recurrence, and – for some patient groups – chemotherapy benefit, Joseph A. Sparano, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Combining these tools could help determine whether endocrine therapy (ET) alone or ET with adjuvant chemotherapy is the best treatment approach for a given patient, said Dr. Sparano, professor of medicine and obstetrics, gynecology, and women’s health at Albert Einstein College of Medicine, New York.
The phase 3 TAILORx study established that ET alone is noninferior to adjuvant chemotherapy (CT) plus ET in patients with early breast cancer and RS of 11-25, and that ET alone has some benefit over ET+CT in women aged 50 years and younger with RS of 16-25, he explained.
Those findings were presented at the 2018 ASCO annual meeting and subsequently published in the New England Journal of Medicine.
The current analysis focused on the integration of clinical and genomic features for prognosis, and the results were published online June 3 in a corresponding article in the New England Journal of Medicine.
“The totality of the data, including TAILORx and the prior prospective validation studies, indicate that assessment of genomic risk with the 21-gene recurrence score provides complementary prognostic information to pathologic features, and is also predictive of a large chemotherapy benefit if the recurrence score is greater than 25, or lack thereof if 25 or lower,” he said.
However, there is a three-way interaction between age, RS, and CT use, which results in an absolute CT benefit in women aged 50 or younger of about 2% for RS of 16-20, and about 7% for RS of 21-25, he added.
“Assessment of clinical risk using pathological features also provides prognostic information that doesn’t correlate well with the recurrence score, therefore it stands to reason that integration of clinical and genomic risk offers the potential for greater precision in prognosis and, ultimately, guiding the use of adjuvant therapy,” he said.
Clinical risk for this analysis was assessed using a binary clinical risk categorization employed in the MINDACT trial and calibrated to greater than 92% 10-year breast cancer-specific survival for ET alone based on Adjuvant! version 8.0. Low-grade tumors up to 3 cm, intermediate-grade tumors up to 2 cm, and high-grade tumors up to 1 cm were categorized as low clinical risk (LCR), and all others not meeting these criteria were categorized as high clinical risk (HCR), he explained.
Of 9,427 patients included in the analysis, 70% had LCR and 30% had HCR.
“For distant recurrence, high clinical risk was associated with a 2.5- to 3-fold higher recurrence rate for those with a recurrence score of 11 or higher, and in a multivariate model for distant recurrence in the [group with a] recurrence score of 11-25, high clinical risk was independently associated with a 2.4-fold higher recurrence risk,” he said. “Continuous recurrence score also provided significant prognostic information, with each 1-unit increase associated with an 8% higher distant recurrence risk.”
For the overall population, clinical risk added significant prognostic information to the RS for both distant recurrence and disease-free survival, and stratification by age showed that among women over age 50 years, the hazard ratios for distant recurrence ranged from 2.20 to 2.36, and did not substantially vary by age or RS, he said.
However, for the overall population, adding clinical risk to the RS did not improve prediction of chemotherapy benefit.
“This was also true for the two-thirds of women who were over 50 years of age. For the remaining women 50 or younger, there was a trend favoring chemo, irrespective of clinical risk, though not significant – a finding consistent with the treatment interaction previously described,” he said.
Finally, the absolute differences in 9-year distant recurrence rates by clinical risk stratified by age, RS, and CT use showed an absolute 4%-6% higher distant recurrence risk for HCR vs. LCR among those over age 50 with RS of 0-25 irrespective of CT use, and a 13% difference for those with RS of 26-100 who were treated with CT.
“For those 50 or younger, clinical risk had no impact on recurrence if the RS was 0-10. For RS of 11-25, the difference was about 9% with endocrine therapy alone, and 2% with chemo plus ET, reflecting absolute chemo benefit in younger women who had high clinical risk,” he said, adding that for those with RS of 26-100, there was a 9% higher absolute recurrence rate in the HCR vs. LCR population.
“We therefore further evaluated absolute differences in distance recurrence rates associated with chemotherapy use in women 50 and younger with RS of 16-25, further stratified by RS and clinical risk,” he said, noting that when not stratified by clinical risk, as reported in the primary analysis, the absolute CT benefit was 1.6% for RS of 16-20, and 6.5% for RS of 21-25.
When stratified by clinical risk, the absolute CT benefit ranged from 6% to 9% in those with RS of 21-25, irrespective of clinical risk, and in those with RS of 16-20 and HCR.
“This accounted for 51% of patients with RS of 16-25,” he said. “However, there was no demonstrable chemo benefit for those with LCR and RS of 16-20, who accounted for the remaining 49%.”
Additional analysis looking at age at diagnosis and CT benefit showed a benefit in premenopausal women aged 46-50 years (but not postmenopausal women), a trend toward benefit in those aged 41-45 years, and no benefit in those aged 40 years and younger, who are less likely to develop premature menopause as a consequence of cytotoxic CT.
“In addition, we saw no consistent effect favoring chemotherapy in older women. Taken together, these findings suggest the chemo benefit observed for the RS 16-25 group may, in fact, be due to a castration effect associated with cytotoxic therapy rather than an effect in eradicating micrometastatic disease,” Dr. Sparano said.
Applying this framework to the TAILORx study population categorized 68% of those aged 50 years and younger into a low integrated risk group with less than 5% risk of distant recurrence. This included all patients with RS of 0-10 irrespective of clinical risk (14% of the patient population; distant recurrence rate 1.8% or less), and all with RS of 11-25 and LCR (54% of the patient population, 4.7% distant recurrence rate).
In contrast, 25% fell into the high integrated risk group (greater than 10% distant recurrence risk), including those with RS of 11-25 and HCR (17% of the patient population; distant recurrence rate 12.3%), and RS of 26-100 and HCR (8% of the patient population; distant recurrence rate 15.2%).
“This framework encompasses 93% of all TAILORx subjects, with the remaining 7% having a distant recurrence risk of between 5% and 10%,” he said.
Overall, the primary results of TAILORx remain unchanged based on this secondary analysis as the addition of clinical risk did not predict CT benefit in the RS 11-25 group, he noted.
“However, for women 50 and under and RS 16-25, integrated risk distinguished 50% who derived no chemo benefit from the 50% who derived an absolute benefit of approximately 6%-9% – a level that is higher than an unselected population,” he said, reiterating that the absolute CT benefit was greater in premenopausal women aged 45-50 with RS 16-25, suggesting that the absolute CR benefit seen in younger women in TAILORx may be due to an endocrine effect.
“Integrated risk clearly provides greater prognostic precision and may have clinical utility; the prognostic precision afforded by the integrated risk model is superior to that by the use of clinical or genomic features alone, and in addition, the genomic assay also provides predictive information for chemo benefit that is not captured by clinical features alone,” he concluded.
As an example of the potential clinical utility of this integrated approach for guiding treatment in women aged 50 years or younger, he presented “a highly stratified integrated risk assessment model” separating TAILORx patients into low integrated risk (58% of the study population) and high integrated risk (31% of the study population).
In the low integrated risk patients with RS of 0-10 and any clinical risk level, or with RS of 11-25 and LCR, tamoxifen alone appears adequate, he said.
In those with high integrated risk and RS of 16-25 with HCR, ovarian function suppression plus an aromatase inhibitor (OFS/AI) could be considered as an alternative to chemo, and in those with high integrated risk, RS of 26-100, and HCR who have not developed chemotherapy-induced menopause, ovarian function suppression and an AI could be added to chemotherapy.
“Indeed, data from the SOFT and TEXT trials indicate that patients with a high RS risk experienced an absolute improvement of up to 10%-15% in 5-year breast cancer–free interval with an OFS/AI, compared with tamoxifen, whereas improvement was minimal in those at lowest risk, supporting the strategy of using integrated clinical and genomic risk to select for ovarian function suppression plus an AI,” he said.
During a discussion of the findings and how they might impact practice, Vered Stearns, MD, an oncology professor and codirector of the Breast Cancer Program at Johns Hopkins University, Baltimore, noted that in her practice she will “carefully select women for whom genomic assay [use] is appropriate.
“I will also assess clinical risk and RS to inform recommendations for chemotherapy use, and possibly appropriate endocrine agents in select populations,” she said.
Dr. Stearns further noted that the interaction between RS and age as reported by Dr. Sparano is exploratory and should be interpreted with caution as the majority of those aged 50 and younger received tamoxifen alone and the question remains as to whether they would have received similar benefits from ovarian suppression and tamoxifen/AI instead of chemo-endocrine therapy.
“Indeed, indirect hypotheses from other studies suggest that may be the case,” she said, adding that these women may be offered ovarian suppression and tamoxifen or AI based on the SOFT and TEXT results.
“TAILORx remains a rich resource for new explorations, new biomarkers, new models, and new machine learning opportunities,” she said.
TAILORx was funded by the National Institutes of Health. Dr. Sparano reported stock ownership, a consulting role, and research funding from several pharmaceutical companies. Dr. Stearns reported consulting or advisory roles with Iridium Therapeutics; research funding from Abbvie, Biocept, MedImmune, Novartis, Pfizer, and Puma Biotechnology; and an “other relationship” with Immunomedics.
SOURCE: Sparano JA et al. ASCO 2019. Abstract 503.
REPORTING FROM ASCO 2019
Cannabis Misuse in MS Linked to Anxiety, Depression
Key clinical point: Providers should consider screening for hazardous cannabis use, especially if patients report depression, anxiety, or difficulty sleeping.
Major finding: Patients with MS who are depressed, anxious, or have poor sleep may be more likely to misuse or abuse cannabis.
Study details: 100 patients with a confirmed MS diagnosis who were receiving outpatient care in a university-affiliated MS center.
Disclosures: No study funding was reported and the authors report no relevant disclosures.
Citation: REPORTING FROM CMSC 2019
Key clinical point: Providers should consider screening for hazardous cannabis use, especially if patients report depression, anxiety, or difficulty sleeping.
Major finding: Patients with MS who are depressed, anxious, or have poor sleep may be more likely to misuse or abuse cannabis.
Study details: 100 patients with a confirmed MS diagnosis who were receiving outpatient care in a university-affiliated MS center.
Disclosures: No study funding was reported and the authors report no relevant disclosures.
Citation: REPORTING FROM CMSC 2019
Key clinical point: Providers should consider screening for hazardous cannabis use, especially if patients report depression, anxiety, or difficulty sleeping.
Major finding: Patients with MS who are depressed, anxious, or have poor sleep may be more likely to misuse or abuse cannabis.
Study details: 100 patients with a confirmed MS diagnosis who were receiving outpatient care in a university-affiliated MS center.
Disclosures: No study funding was reported and the authors report no relevant disclosures.
Citation: REPORTING FROM CMSC 2019
Lapses in DMT Increases Risk of Relapse in MS Patients
Key clinical point: Lapses in the use of MS disease-modifying oral therapy increases the risk for relapse, hospitalization, emergency department visits, and outpatient visits, and leads to higher healthcare costs.
Major finding: Over an 18-month follow-up period, those with drug lapses of more than 60 days had 28% more relapses than did the other subjects (mean 1.2 vs. 0.8; P less than .0001).
Study details: A claims database study of 8,779 patients with MS during 2011-2015
Disclosures: EMD Serono, a division of Merck KGaA, provided funding for the study. Dr. Nicholas disclosed grant support from EMD Serono, and two other study authors are employees of the company. Another two authors worked for a consulting firm that received funding from EMD Serono to conduct the study.
Citation: REPORTING FROM CMSC 2019
Key clinical point: Lapses in the use of MS disease-modifying oral therapy increases the risk for relapse, hospitalization, emergency department visits, and outpatient visits, and leads to higher healthcare costs.
Major finding: Over an 18-month follow-up period, those with drug lapses of more than 60 days had 28% more relapses than did the other subjects (mean 1.2 vs. 0.8; P less than .0001).
Study details: A claims database study of 8,779 patients with MS during 2011-2015
Disclosures: EMD Serono, a division of Merck KGaA, provided funding for the study. Dr. Nicholas disclosed grant support from EMD Serono, and two other study authors are employees of the company. Another two authors worked for a consulting firm that received funding from EMD Serono to conduct the study.
Citation: REPORTING FROM CMSC 2019
Key clinical point: Lapses in the use of MS disease-modifying oral therapy increases the risk for relapse, hospitalization, emergency department visits, and outpatient visits, and leads to higher healthcare costs.
Major finding: Over an 18-month follow-up period, those with drug lapses of more than 60 days had 28% more relapses than did the other subjects (mean 1.2 vs. 0.8; P less than .0001).
Study details: A claims database study of 8,779 patients with MS during 2011-2015
Disclosures: EMD Serono, a division of Merck KGaA, provided funding for the study. Dr. Nicholas disclosed grant support from EMD Serono, and two other study authors are employees of the company. Another two authors worked for a consulting firm that received funding from EMD Serono to conduct the study.
Citation: REPORTING FROM CMSC 2019
Adherence to Oral Treatments for MS is Poor
Key clinical point: Adherence to current oral therapies for multiple sclerosis (MS) is poor.
Major finding: Almost half of patients with MS discontinue their initial oral therapy.
Study details: A retrospective administrative claims study of 8,251 patients with MS.
Disclosures: The authors received no financial support for this study. Dr. Nicholas reported receiving grant support from EMD Serono.
Citation: Nicholas J et al. CMSC 2019. Abstract DXT34.
Key clinical point: Adherence to current oral therapies for multiple sclerosis (MS) is poor.
Major finding: Almost half of patients with MS discontinue their initial oral therapy.
Study details: A retrospective administrative claims study of 8,251 patients with MS.
Disclosures: The authors received no financial support for this study. Dr. Nicholas reported receiving grant support from EMD Serono.
Citation: Nicholas J et al. CMSC 2019. Abstract DXT34.
Key clinical point: Adherence to current oral therapies for multiple sclerosis (MS) is poor.
Major finding: Almost half of patients with MS discontinue their initial oral therapy.
Study details: A retrospective administrative claims study of 8,251 patients with MS.
Disclosures: The authors received no financial support for this study. Dr. Nicholas reported receiving grant support from EMD Serono.
Citation: Nicholas J et al. CMSC 2019. Abstract DXT34.
LTC-associated suicide among older adults more common than previously thought
The rate of suicide associated with residential long-term care (LTC) in adults aged 55 years and older may be significantly higher than the injury location coding of the National Violent Death Reporting System (NVDRS) suggests, according to new research.
The Centers for Disease Control and Prevention reports that there are about 16,000 nursing homes and 31,000 assisted living facilities in the United States and they currently house about 25% of all Medicare beneficiaries. “As such, residential LTC may be a potential location for identifying individuals at high risk of self-harm and for implementing interventions to reduce suicide risk, wrote Briana Mezuk, PhD, and associates from the University of Michigan, Ann Arbor. The study was published in JAMA Network Open.
Dr. Mezuk and colleagues conducted a cross-sectional, epidemiologic study using a natural language–processing algorithm to analyze restricted-access data from the NVDRS between 2003 and 2015. A total of 47,759 suicides and undetermined deaths in adults aged 55 years and older from 27 states were included in the analysis (median age, 64 years; 77.6% male; 90.0% non-Hispanic white).
The algorithm identified 1,037 (2.2% of the total) suicide deaths associated with LTC, with 428 occurring in adults living in LTC, 449 occurring during the transition into or out of LTC, and 160 otherwise associated with LTC. Decedents in this group had a median age of 79 years, were 73.8% male, and were 94.3% non-Hispanic white. The number of suicide deaths varied widely from year to year, but no trend was found in the change over the study period.
Deaths while living in LTC were more likely among women, which the investigators noted is to be expected because LTC residents are disproportionately women. Death while transitioning into or out of LTC was more likely among adults who previously had expressed suicide ideation and had a physical health problem cited as a contributing circumstance. Death otherwise associated with LTC was more likely in adults who were married or in a relationship, had a depressed mood, and had a recent crisis cited as a contributing factor.
“Living in LTC or transitioning to LTC is also correlated with a host of characteristics that are established risk factors for suicide,” the investigators noted.
In further analysis, the investigators compared the number of suicide deaths the algorithm identified as occurring within an LTC facility (n = 428) with the injury location code SRF (supervised residential facility; n = 263) and the death location code LTC/nursing home (n = 567) within the NVDRS. Of the 263 SRF injuries, 106 were identified as occurring within a LTC facility by the algorithm. The agreement between the algorithm and the SRF coding was poor (kappa statistic, 0.30; 95% confidence interval, 0.26-0.35).
“Leaders in the field have continued to call for a shift away from a medicalized paradigm of residential LTC toward institutional practices that instead focus on fostering meaningful interactions between residents, promote engagement in care, and enhance quality of life. In addition, existing, scalable programs that support older adults living in the community offer the potential to promote quality of life for older adults who may be considering transitioning into or out of residential LTC,” the investigators concluded. “These findings emphasize the importance of such efforts for the mental health of older adults.”
The study was supported by a grant from the National Institute of Mental Health. The investigators reported no conflicts of interest.
SOURCE: Mezuk B et al. JAMA Netw Open. 2019 Jun 14. doi: 10.1001/jamanetworkopen.2019.5627.
Filling the gap in knowledge about the consequences of transitioning into long-term care is sorely needed, wrote Yoram Barak, MD, MHA, and Chris Gale, MB,ChB, MPH, given that fewer than 20 studies on the subject have been published in the past 30 years. A 2015 systematic review of nursing home suicides included only eight studies and 101 suicide deaths.
Despite the limits of an epidemiologic study on long-term care in elderly adults, such as the significant differences between elderly populations in America and other countries, this study by Mezuk et al. provides useful information on a longer period of time than would be possible with case-control studies and at a more granular level than data that would be available from national case registers, Dr. Barak and Dr. Gale wrote.
Dr. Barak and Dr. Gale are with the department of psychological medicine at the University of Otago in Dunedin, New Zealand. They made these comments in an editorial published in JAMA Network Open (2019 Jun 14. doi: 10.1001/jamanetworkopen.2019.5634). They did not report any conflicts of interest.
Filling the gap in knowledge about the consequences of transitioning into long-term care is sorely needed, wrote Yoram Barak, MD, MHA, and Chris Gale, MB,ChB, MPH, given that fewer than 20 studies on the subject have been published in the past 30 years. A 2015 systematic review of nursing home suicides included only eight studies and 101 suicide deaths.
Despite the limits of an epidemiologic study on long-term care in elderly adults, such as the significant differences between elderly populations in America and other countries, this study by Mezuk et al. provides useful information on a longer period of time than would be possible with case-control studies and at a more granular level than data that would be available from national case registers, Dr. Barak and Dr. Gale wrote.
Dr. Barak and Dr. Gale are with the department of psychological medicine at the University of Otago in Dunedin, New Zealand. They made these comments in an editorial published in JAMA Network Open (2019 Jun 14. doi: 10.1001/jamanetworkopen.2019.5634). They did not report any conflicts of interest.
Filling the gap in knowledge about the consequences of transitioning into long-term care is sorely needed, wrote Yoram Barak, MD, MHA, and Chris Gale, MB,ChB, MPH, given that fewer than 20 studies on the subject have been published in the past 30 years. A 2015 systematic review of nursing home suicides included only eight studies and 101 suicide deaths.
Despite the limits of an epidemiologic study on long-term care in elderly adults, such as the significant differences between elderly populations in America and other countries, this study by Mezuk et al. provides useful information on a longer period of time than would be possible with case-control studies and at a more granular level than data that would be available from national case registers, Dr. Barak and Dr. Gale wrote.
Dr. Barak and Dr. Gale are with the department of psychological medicine at the University of Otago in Dunedin, New Zealand. They made these comments in an editorial published in JAMA Network Open (2019 Jun 14. doi: 10.1001/jamanetworkopen.2019.5634). They did not report any conflicts of interest.
The rate of suicide associated with residential long-term care (LTC) in adults aged 55 years and older may be significantly higher than the injury location coding of the National Violent Death Reporting System (NVDRS) suggests, according to new research.
The Centers for Disease Control and Prevention reports that there are about 16,000 nursing homes and 31,000 assisted living facilities in the United States and they currently house about 25% of all Medicare beneficiaries. “As such, residential LTC may be a potential location for identifying individuals at high risk of self-harm and for implementing interventions to reduce suicide risk, wrote Briana Mezuk, PhD, and associates from the University of Michigan, Ann Arbor. The study was published in JAMA Network Open.
Dr. Mezuk and colleagues conducted a cross-sectional, epidemiologic study using a natural language–processing algorithm to analyze restricted-access data from the NVDRS between 2003 and 2015. A total of 47,759 suicides and undetermined deaths in adults aged 55 years and older from 27 states were included in the analysis (median age, 64 years; 77.6% male; 90.0% non-Hispanic white).
The algorithm identified 1,037 (2.2% of the total) suicide deaths associated with LTC, with 428 occurring in adults living in LTC, 449 occurring during the transition into or out of LTC, and 160 otherwise associated with LTC. Decedents in this group had a median age of 79 years, were 73.8% male, and were 94.3% non-Hispanic white. The number of suicide deaths varied widely from year to year, but no trend was found in the change over the study period.
Deaths while living in LTC were more likely among women, which the investigators noted is to be expected because LTC residents are disproportionately women. Death while transitioning into or out of LTC was more likely among adults who previously had expressed suicide ideation and had a physical health problem cited as a contributing circumstance. Death otherwise associated with LTC was more likely in adults who were married or in a relationship, had a depressed mood, and had a recent crisis cited as a contributing factor.
“Living in LTC or transitioning to LTC is also correlated with a host of characteristics that are established risk factors for suicide,” the investigators noted.
In further analysis, the investigators compared the number of suicide deaths the algorithm identified as occurring within an LTC facility (n = 428) with the injury location code SRF (supervised residential facility; n = 263) and the death location code LTC/nursing home (n = 567) within the NVDRS. Of the 263 SRF injuries, 106 were identified as occurring within a LTC facility by the algorithm. The agreement between the algorithm and the SRF coding was poor (kappa statistic, 0.30; 95% confidence interval, 0.26-0.35).
“Leaders in the field have continued to call for a shift away from a medicalized paradigm of residential LTC toward institutional practices that instead focus on fostering meaningful interactions between residents, promote engagement in care, and enhance quality of life. In addition, existing, scalable programs that support older adults living in the community offer the potential to promote quality of life for older adults who may be considering transitioning into or out of residential LTC,” the investigators concluded. “These findings emphasize the importance of such efforts for the mental health of older adults.”
The study was supported by a grant from the National Institute of Mental Health. The investigators reported no conflicts of interest.
SOURCE: Mezuk B et al. JAMA Netw Open. 2019 Jun 14. doi: 10.1001/jamanetworkopen.2019.5627.
The rate of suicide associated with residential long-term care (LTC) in adults aged 55 years and older may be significantly higher than the injury location coding of the National Violent Death Reporting System (NVDRS) suggests, according to new research.
The Centers for Disease Control and Prevention reports that there are about 16,000 nursing homes and 31,000 assisted living facilities in the United States and they currently house about 25% of all Medicare beneficiaries. “As such, residential LTC may be a potential location for identifying individuals at high risk of self-harm and for implementing interventions to reduce suicide risk, wrote Briana Mezuk, PhD, and associates from the University of Michigan, Ann Arbor. The study was published in JAMA Network Open.
Dr. Mezuk and colleagues conducted a cross-sectional, epidemiologic study using a natural language–processing algorithm to analyze restricted-access data from the NVDRS between 2003 and 2015. A total of 47,759 suicides and undetermined deaths in adults aged 55 years and older from 27 states were included in the analysis (median age, 64 years; 77.6% male; 90.0% non-Hispanic white).
The algorithm identified 1,037 (2.2% of the total) suicide deaths associated with LTC, with 428 occurring in adults living in LTC, 449 occurring during the transition into or out of LTC, and 160 otherwise associated with LTC. Decedents in this group had a median age of 79 years, were 73.8% male, and were 94.3% non-Hispanic white. The number of suicide deaths varied widely from year to year, but no trend was found in the change over the study period.
Deaths while living in LTC were more likely among women, which the investigators noted is to be expected because LTC residents are disproportionately women. Death while transitioning into or out of LTC was more likely among adults who previously had expressed suicide ideation and had a physical health problem cited as a contributing circumstance. Death otherwise associated with LTC was more likely in adults who were married or in a relationship, had a depressed mood, and had a recent crisis cited as a contributing factor.
“Living in LTC or transitioning to LTC is also correlated with a host of characteristics that are established risk factors for suicide,” the investigators noted.
In further analysis, the investigators compared the number of suicide deaths the algorithm identified as occurring within an LTC facility (n = 428) with the injury location code SRF (supervised residential facility; n = 263) and the death location code LTC/nursing home (n = 567) within the NVDRS. Of the 263 SRF injuries, 106 were identified as occurring within a LTC facility by the algorithm. The agreement between the algorithm and the SRF coding was poor (kappa statistic, 0.30; 95% confidence interval, 0.26-0.35).
“Leaders in the field have continued to call for a shift away from a medicalized paradigm of residential LTC toward institutional practices that instead focus on fostering meaningful interactions between residents, promote engagement in care, and enhance quality of life. In addition, existing, scalable programs that support older adults living in the community offer the potential to promote quality of life for older adults who may be considering transitioning into or out of residential LTC,” the investigators concluded. “These findings emphasize the importance of such efforts for the mental health of older adults.”
The study was supported by a grant from the National Institute of Mental Health. The investigators reported no conflicts of interest.
SOURCE: Mezuk B et al. JAMA Netw Open. 2019 Jun 14. doi: 10.1001/jamanetworkopen.2019.5627.
FROM JAMA NETWORK OPEN
Key clinical point: Suicide associated with long-term care may be more common than previous data have indicated.
Major finding: About 2.2% of the suicide deaths reported to the National Violent Death Reporting System occurred or were associated with long-term care.
Study details: An analysis of 47,759 suicides and undetermined deaths in adults aged at least 55 years with data included in the National Violent Death Reporting System.Disclosures: The study was supported by a grant from the National Institute of Mental Health. The investigators reported no conflicts of interest.
Source: Mezuk B et al. JAMA Netw Open. 2019 Jun 14. doi: 10.1001/jamanetworkopen.2019.5627.