User login
New lemborexant efficacy and safety data unveiled
SAN ANTONIO – results from a pooled analysis showed.
A dual orexin receptor antagonist developed by Eisai, lemborexant is being studied as a treatment for insomnia disorder and irregular sleep-wake rhythm disorder. Early in 2019, the Food and Drug Administration accepted for review the New Drug Application for lemborexant for the treatment of insomnia. A target Prescription Drug User Fee Act date is set for Dec. 27, 2019.
“We evaluated early on whether exposure to lemborexant was going to be different between men and women,” lead study author Margaret Moline, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies. “With some drugs, like zolpidem and other so-called Z drugs, because exposure is different, clinical studies could involve different dosing for different sexes. Because we knew the exposure to lemborexant wasn’t different between the sexes, we expected to see similar results in both sexes. That was the case.”
Dr. Moline, executive director of the Neurology Business Group and International Project Team Lead for the lemborexant clinical development program at Eisai, and colleagues presented pooled analyses of subject-reported sleep onset latency (sSOL) and subject-reported wake after sleep onset (sWASO) from lemborexant phase 3 studies, SUNRISE-1 and SUNRISE-2. SUNRISE-1 was a 1-month, double-blind, placebo- and active-controlled, parallel-group study in 1,006 subjects. Participants were females aged 55 years and older and males aged 65 years and older with a primary complaint of sleep maintenance difficulties and an Insomnia Severity Index (ISI) total score of 13 or higher. SUNRISE-2 was a placebo-controlled, 6-month, active treatment, double-blind, parallel-group study in 949 subjects with insomnia disorder. Participants were females and males aged 18 years and older with a primary complaint of sleep onset and/or sleep maintenance difficulties and an ISI total score of 15 or higher. Both analyses included subjects randomized to placebo, lemborexant 5 mg, or lemborexant 10 mg. Each study included a single-blind placebo run-in period prior to randomization.
The pooled analysis of 1,693 subjects included 402 (23.7%) men and 1,291 (76.3%) women. Results on sSOL and sWASO were consistent with the significant results on sleep diary in the individual studies. In both sexes, sSOL for lemborexant 5 mg and lemborexant 10 mg was significantly reduced versus that for placebo during the first 7 days and end of month 1 (P less than .05 for all comparisons). In women, the researchers observed significantly greater reductions in sWASO placebo for both lemborexant doses versus that with placebo (first 7 days and end of month 1; P less than .0001 for all comparisons). In males, sWASO decreased significantly, compared with placebo, for the first 7 days (lemborexant 5 mg and lemborexant 10 mg; P equal to or less than .0001) and at end of month 1 (lemborexant 10 mg only; P = .0032). For placebo, lemborexant 5 mg, and lemborexant 10 mg, the overall incidence of treatment-emergent adverse events was similar across sexes. Incidence of treatment-emergent serious adverse events was low for both sex subgroups; most events occurred in one subject each. Treatment-emergent adverse events leading to study drug withdrawal or interruption were few and similar across sexes for all treatments and was highest in males receiving lemborexant 10 mg. The most frequent treatment-emergent adverse events reported in males were somnolence, fatigue, and headache, while the most common in females were somnolence, headache, and urinary tract infection. About 3% of females (no males) reported a urinary tract infection; the incidence in females was similar across treatment groups.
“Overall, sleep diary outcomes in males and females were consistent with the significant results observed in the total populations of the individual studies,” Dr. Moline concluded. “A dose adjustment based on sex is not anticipated.”
The research was supported by Eisai. Dr. Moline is an employee of the company.
SOURCE: Moline M et al. Sleep 2019, Abstract 0368.
SAN ANTONIO – results from a pooled analysis showed.
A dual orexin receptor antagonist developed by Eisai, lemborexant is being studied as a treatment for insomnia disorder and irregular sleep-wake rhythm disorder. Early in 2019, the Food and Drug Administration accepted for review the New Drug Application for lemborexant for the treatment of insomnia. A target Prescription Drug User Fee Act date is set for Dec. 27, 2019.
“We evaluated early on whether exposure to lemborexant was going to be different between men and women,” lead study author Margaret Moline, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies. “With some drugs, like zolpidem and other so-called Z drugs, because exposure is different, clinical studies could involve different dosing for different sexes. Because we knew the exposure to lemborexant wasn’t different between the sexes, we expected to see similar results in both sexes. That was the case.”
Dr. Moline, executive director of the Neurology Business Group and International Project Team Lead for the lemborexant clinical development program at Eisai, and colleagues presented pooled analyses of subject-reported sleep onset latency (sSOL) and subject-reported wake after sleep onset (sWASO) from lemborexant phase 3 studies, SUNRISE-1 and SUNRISE-2. SUNRISE-1 was a 1-month, double-blind, placebo- and active-controlled, parallel-group study in 1,006 subjects. Participants were females aged 55 years and older and males aged 65 years and older with a primary complaint of sleep maintenance difficulties and an Insomnia Severity Index (ISI) total score of 13 or higher. SUNRISE-2 was a placebo-controlled, 6-month, active treatment, double-blind, parallel-group study in 949 subjects with insomnia disorder. Participants were females and males aged 18 years and older with a primary complaint of sleep onset and/or sleep maintenance difficulties and an ISI total score of 15 or higher. Both analyses included subjects randomized to placebo, lemborexant 5 mg, or lemborexant 10 mg. Each study included a single-blind placebo run-in period prior to randomization.
The pooled analysis of 1,693 subjects included 402 (23.7%) men and 1,291 (76.3%) women. Results on sSOL and sWASO were consistent with the significant results on sleep diary in the individual studies. In both sexes, sSOL for lemborexant 5 mg and lemborexant 10 mg was significantly reduced versus that for placebo during the first 7 days and end of month 1 (P less than .05 for all comparisons). In women, the researchers observed significantly greater reductions in sWASO placebo for both lemborexant doses versus that with placebo (first 7 days and end of month 1; P less than .0001 for all comparisons). In males, sWASO decreased significantly, compared with placebo, for the first 7 days (lemborexant 5 mg and lemborexant 10 mg; P equal to or less than .0001) and at end of month 1 (lemborexant 10 mg only; P = .0032). For placebo, lemborexant 5 mg, and lemborexant 10 mg, the overall incidence of treatment-emergent adverse events was similar across sexes. Incidence of treatment-emergent serious adverse events was low for both sex subgroups; most events occurred in one subject each. Treatment-emergent adverse events leading to study drug withdrawal or interruption were few and similar across sexes for all treatments and was highest in males receiving lemborexant 10 mg. The most frequent treatment-emergent adverse events reported in males were somnolence, fatigue, and headache, while the most common in females were somnolence, headache, and urinary tract infection. About 3% of females (no males) reported a urinary tract infection; the incidence in females was similar across treatment groups.
“Overall, sleep diary outcomes in males and females were consistent with the significant results observed in the total populations of the individual studies,” Dr. Moline concluded. “A dose adjustment based on sex is not anticipated.”
The research was supported by Eisai. Dr. Moline is an employee of the company.
SOURCE: Moline M et al. Sleep 2019, Abstract 0368.
SAN ANTONIO – results from a pooled analysis showed.
A dual orexin receptor antagonist developed by Eisai, lemborexant is being studied as a treatment for insomnia disorder and irregular sleep-wake rhythm disorder. Early in 2019, the Food and Drug Administration accepted for review the New Drug Application for lemborexant for the treatment of insomnia. A target Prescription Drug User Fee Act date is set for Dec. 27, 2019.
“We evaluated early on whether exposure to lemborexant was going to be different between men and women,” lead study author Margaret Moline, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies. “With some drugs, like zolpidem and other so-called Z drugs, because exposure is different, clinical studies could involve different dosing for different sexes. Because we knew the exposure to lemborexant wasn’t different between the sexes, we expected to see similar results in both sexes. That was the case.”
Dr. Moline, executive director of the Neurology Business Group and International Project Team Lead for the lemborexant clinical development program at Eisai, and colleagues presented pooled analyses of subject-reported sleep onset latency (sSOL) and subject-reported wake after sleep onset (sWASO) from lemborexant phase 3 studies, SUNRISE-1 and SUNRISE-2. SUNRISE-1 was a 1-month, double-blind, placebo- and active-controlled, parallel-group study in 1,006 subjects. Participants were females aged 55 years and older and males aged 65 years and older with a primary complaint of sleep maintenance difficulties and an Insomnia Severity Index (ISI) total score of 13 or higher. SUNRISE-2 was a placebo-controlled, 6-month, active treatment, double-blind, parallel-group study in 949 subjects with insomnia disorder. Participants were females and males aged 18 years and older with a primary complaint of sleep onset and/or sleep maintenance difficulties and an ISI total score of 15 or higher. Both analyses included subjects randomized to placebo, lemborexant 5 mg, or lemborexant 10 mg. Each study included a single-blind placebo run-in period prior to randomization.
The pooled analysis of 1,693 subjects included 402 (23.7%) men and 1,291 (76.3%) women. Results on sSOL and sWASO were consistent with the significant results on sleep diary in the individual studies. In both sexes, sSOL for lemborexant 5 mg and lemborexant 10 mg was significantly reduced versus that for placebo during the first 7 days and end of month 1 (P less than .05 for all comparisons). In women, the researchers observed significantly greater reductions in sWASO placebo for both lemborexant doses versus that with placebo (first 7 days and end of month 1; P less than .0001 for all comparisons). In males, sWASO decreased significantly, compared with placebo, for the first 7 days (lemborexant 5 mg and lemborexant 10 mg; P equal to or less than .0001) and at end of month 1 (lemborexant 10 mg only; P = .0032). For placebo, lemborexant 5 mg, and lemborexant 10 mg, the overall incidence of treatment-emergent adverse events was similar across sexes. Incidence of treatment-emergent serious adverse events was low for both sex subgroups; most events occurred in one subject each. Treatment-emergent adverse events leading to study drug withdrawal or interruption were few and similar across sexes for all treatments and was highest in males receiving lemborexant 10 mg. The most frequent treatment-emergent adverse events reported in males were somnolence, fatigue, and headache, while the most common in females were somnolence, headache, and urinary tract infection. About 3% of females (no males) reported a urinary tract infection; the incidence in females was similar across treatment groups.
“Overall, sleep diary outcomes in males and females were consistent with the significant results observed in the total populations of the individual studies,” Dr. Moline concluded. “A dose adjustment based on sex is not anticipated.”
The research was supported by Eisai. Dr. Moline is an employee of the company.
SOURCE: Moline M et al. Sleep 2019, Abstract 0368.
REPORTING FROM SLEEP 2019
An unusual presentation of low-grade clavicle osteosarcoma: a case report and literature review
Osteosarcoma (OS) is a rare disease with approximately 800- 900 newly diagnosed cases each year in the United States. Of those, the majority occur about the knee. The distal femur is the most common site, followed by the proximal tibia, with the proximal humerus being a distant third. OS of the clavicle has been reported, with the earliest case report dating from 1975.1 Since then, additional case reports of high-grade OS of the clavicle have been published.2,3 We describe the case of a 16-year-old female who presented with a mass on her right medial clavicle, which was confirmed to be a low-grade central OS.
Case Presentation
The patient is a 16-year-old female who presented to the Emergency Department (ED) for evaluation of a mass on her right clavicle, after being evaluated by her primary care physician (PCP). She noted an enlarging mass over the previous 2 months but stated that it had been asymptomatic until 4 days prior to presentation to her PCP, at which time she had developed tenderness to palpation and pain with range of motion of the right arm. X-rays were obtained at the PCP’s office and she was referred to the ED for further evaluation. She denied constitutional symptoms.
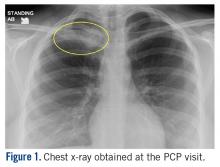
At the ED visit, she was noted to have an area of erythema and tenderness over the medial aspect of the right clavicle with increased bony prominence. A chest x-ray demonstrated medial clavicle enlargement with periosteal reaction and sclerosis (Figure 1).
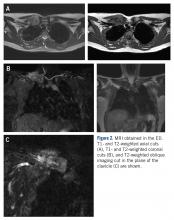
MRI demonstrated a 6-cm x 3.8-cm x 4.1-cm mass arising from the right medial clavicle with cortical destruction and concomitant displacement of the right subclavian and brachiocephalic veins (Figure 2). A CT-guided biopsy was performed 1 week later and demonstrated low-grade OS. The pathologist was concerned about the possibility of sampling error and the presence of a higher-grade component, as low-grade OS of the clavicle had not been reported.
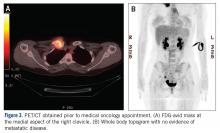
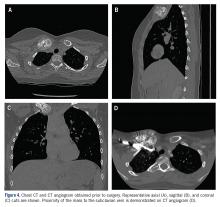
The patient was evaluated by a pediatric hematologist/oncologist 2 weeks later after having obtained the biopsy and a PET/CT scan. At that time, the PET/CT showed an FDG-avid mass at the clavicle without evidence of pulmonary metastatic disease (Figure 3). She was subsequently evaluated by orthopedic oncology, at which time a discussion was had regarding further treatment. There was essentially no literature to guide the surgical and medical teams, as low-grade clavicular OS is unknown. Based on the evidence of localized, low-grade disease, the determination was made to proceedwith surgical resection. In the event that high-grade disease was identified at the time of final pathological evaluation, the pediatric hematology/oncology team felt that administering all of the patient’s chemotherapy postoperatively would be acceptable and not affect her long-term prognosis. CT and CT angiogram were obtained for further operative planning (Figure 4).
Given the intimacy of the mass to the subclavian vessels, she was also seen preoperatively by pediatric general and cardiothoracic surgeons. The plan was formulated to have them in the operating room for mobilization of the subclavian vessels and in the event that a sternotomy was required for proximal control of the vessels. Following this visit, the case was discussed at the multidisciplinary pediatric tumor board and the consensus was to proceed with surgical resection.
Surgical Technique
General endotracheal anesthesia was administered without complication. The patient was positioned supine with a soft bump under her shoulders to place her neck in slight extension and thus facilitate access to the clavicle and great vessels. A 14-cm oblique incision was made over the subcutaneous clavicle extending to the contralateral sternoclavicular joint. Dissection was carried down to the fascia and the biopsy site was excised with the skin paddle. Dissection was carried through the sternocleidomastoid superiorly and the pectoralis major inferiorly, to 8 cm lateral from the right sternoclavicular joint. The clavicle was osteotomized well lateral of the palpable tumor and a marrow margin was sent for frozen section, which was found to be negative.
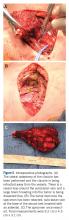
Dissection was continued circumferentially. Assistance from pediatric general and cardiothoracic surgery was required at the inferior aspect of the mass to assist with exposure and control of the subclavian vein (Figure 5A). A large branch of the subclavian vein near its junction with the internal jugular vein was found to be involved with the tumor and thus required suture ligation. The subclavian vein was noted to be intimate with the mass and somewhat friable. With the vein mobilized, a cuff of normal tissue was obtained inferiorly and superiorly to the mass. Medially, the sternoclavicular joint was disarticulated (Figure 5B). At this point, the specimen was delivered from the operative field and tagged in the usual fashion (Figure 5C). A medial soft tissue margin from the sternal side of the sternoclavicular joint was also sent and found to be negative for tumor. The wound was closed in layered fashion over a ¼” Penrose drain. A soft dressing was placed, and the patient was successfully extubated and transferred to the post-anesthesia care unit in stable condition.
Postoperative Course
The patient was found to be neurologically and vascularly intact on postoperative exam and was discharged on postoperative day 1.
She was seen 14 days postoperatively and was doing well at that time, with full range of motion of the shoulder, elbow, wrist, and hand. Final pathology confirmed a low-grade OS with extraosseous extension. All margins were negative except the medial (sternoclavicular joint) margin and the inferior margin adjacent to the subclavian vein. The intraoperative frozen section from the medial margin was negative for tumor.
The pediatric hematology/oncology team determined that, as no high-grade areas were identified, chemotherapy should be deferred. The positive margins were also discussed with the patient and her family specifically regarding further possible treatments. The findings from the pathology were discussed in a multidisciplinary tumor board and it was felt that, given the low-grade nature of the lesion as well as the high morbidity and risk of mortality with further surgery, additional surgery would be potentially more harmful than helpful. Additionally, low-grade OS is extremely resistant to radiotherapy. The plan remains to monitor her for local recurrence as well as metastases with serial imaging.
Discussion
The clavicle is one of the first bones in the body to ossify but one of the last to have final physeal closure. Its unique characteristics have led to various descriptions, such as a “short tubular bone” versus a “flat bone.”4,5 Of note are its paucity of a true intramedullary space and scanty red marrow, which make it an unlikely site for a primarily intramedullary- based neoplasm to arise.4 However, it has also been noted that malignant lesions are more common in the clavicle than benign lesions, and special attention should be paid to aggressiveappearing lesions in the clavicle.
Radiographs can be misleading as well. Prior studies have demonstrated that low-grade central OS can be readily misdiagnosed as fibrous dysplasia, desmoplastic fibroma, nonossifying fibroma, osteoblastoma, and aneurysmal bone cyst.6 Findings found in low-grade OS can include evidence of cortical interruption, local soft tissue mass development, intramedullary involvement, cortical destruction, and poor margination; however, low-grade OS is typically sclerotic and highly trabeculated. Cross-sectional imaging can help differentiate between OS and other more benign pathologies and should be considered in the clavicle where biopsy may be perilous.5
The difficulty of clavicular biopsy has been reported. Not only does clavicular anatomy make biopsy hazardous, but also the potential for sampling error does exist. In a case report of one patient with a highgrade lesion, fine needle aspiration biopsy was initially diagnosed as an aneurysmal bone cyst but was ultimately found to be osteosarcoma.2 Histology of low-grade lesions usually demonstrates minimal cytological atypia, rare mitotic activity, and variable osteoid production.5 Lower mitotic indices typically make wide resection curative for these patients, without the need for chemotherapy.
In this case, wide resection was carried out with the subclavian vein as the posterior-inferior margin and the sternoclavicular joint as the medial margin. Though the intra-operative medial margin was clear of disease, final pathology demonstrated focal (microscopic) involvement of the posterior and medial margins. A study of soft tissue sarcoma evaluated positive margins and concluded that the imperative of preservation of vital structures supersedes the need for negative margins.7,8 The rate of metastasis and overall survival was similar to surgical resections with positive margins. In the case of our patient, further resection would have carried significant morbidity and possibly mortality, including sacrifice of the major vessels to the arm below and entering into the sternum and thoracic cavity. The likely disability as well as the hazards of surgery were deemed to be too great to justify further excision. Frequent cross-sectional imaging will be necessary to evaluate the presence of recurrent or metastatic disease. To our knowledge, this is the first documented case of low-grade clavicle OS. This report demonstrates the need for multidisciplinary sarcoma care at a center of excellence, particularly in instances of unusual diagnoses.
1. Zinghi G. Osteosarcoma of the clavicle (description of a case) [in Italian]. Chir Organi Mov. 1975;62(6):671-674.
2. Cundy WJ, Carter C, Dhatrak D, Clayer M. Primary osteosarcoma of the clavicle and the perils of bone biopsy. BMJ Case Rep. 2015;2015:bcr2014208859.
3. Greenspan A, Unni KK, Mann J. Case report 804: Chondroblastic osteosarcoma grade 3 of the left clavicle. Skeletal Radiol. 1993;22(6):469-471.
4. Rossi B, Fabbriciani C, Chalidis BE, Visci F, Maccauro G. Primary malignant clavicular tumours: a clinicopathological analysis of six cases and evaluation of surgical management. Arch Orthop Trauma Surg. 2011;131(7):935-939.
5. Andresen KJ, Sundaram M, Unni KK, Sim FH. Imaging features of low-grade central osteosarcoma of the long bones and pelvis. Skeletal Radiol. 2004;33(7):373-379.
6. Malhas AM, Sumathi VP, James SL, et al. Low-grade central osteosarcoma: A difficult condition to diagnose. Sarcoma. 2012; 2012:764796.
7. O’Donnell PW, Griffin AM, Eward WC, et al. The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer. 2014;120(18):2866-2875.
8. Kawaguchi N, Ahmed AR, Matsumoto S, Manabe J, Matsushita Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:165-172.
Osteosarcoma (OS) is a rare disease with approximately 800- 900 newly diagnosed cases each year in the United States. Of those, the majority occur about the knee. The distal femur is the most common site, followed by the proximal tibia, with the proximal humerus being a distant third. OS of the clavicle has been reported, with the earliest case report dating from 1975.1 Since then, additional case reports of high-grade OS of the clavicle have been published.2,3 We describe the case of a 16-year-old female who presented with a mass on her right medial clavicle, which was confirmed to be a low-grade central OS.
Case Presentation
The patient is a 16-year-old female who presented to the Emergency Department (ED) for evaluation of a mass on her right clavicle, after being evaluated by her primary care physician (PCP). She noted an enlarging mass over the previous 2 months but stated that it had been asymptomatic until 4 days prior to presentation to her PCP, at which time she had developed tenderness to palpation and pain with range of motion of the right arm. X-rays were obtained at the PCP’s office and she was referred to the ED for further evaluation. She denied constitutional symptoms.
At the ED visit, she was noted to have an area of erythema and tenderness over the medial aspect of the right clavicle with increased bony prominence. A chest x-ray demonstrated medial clavicle enlargement with periosteal reaction and sclerosis (Figure 1).
MRI demonstrated a 6-cm x 3.8-cm x 4.1-cm mass arising from the right medial clavicle with cortical destruction and concomitant displacement of the right subclavian and brachiocephalic veins (Figure 2). A CT-guided biopsy was performed 1 week later and demonstrated low-grade OS. The pathologist was concerned about the possibility of sampling error and the presence of a higher-grade component, as low-grade OS of the clavicle had not been reported.
The patient was evaluated by a pediatric hematologist/oncologist 2 weeks later after having obtained the biopsy and a PET/CT scan. At that time, the PET/CT showed an FDG-avid mass at the clavicle without evidence of pulmonary metastatic disease (Figure 3). She was subsequently evaluated by orthopedic oncology, at which time a discussion was had regarding further treatment. There was essentially no literature to guide the surgical and medical teams, as low-grade clavicular OS is unknown. Based on the evidence of localized, low-grade disease, the determination was made to proceedwith surgical resection. In the event that high-grade disease was identified at the time of final pathological evaluation, the pediatric hematology/oncology team felt that administering all of the patient’s chemotherapy postoperatively would be acceptable and not affect her long-term prognosis. CT and CT angiogram were obtained for further operative planning (Figure 4).
Given the intimacy of the mass to the subclavian vessels, she was also seen preoperatively by pediatric general and cardiothoracic surgeons. The plan was formulated to have them in the operating room for mobilization of the subclavian vessels and in the event that a sternotomy was required for proximal control of the vessels. Following this visit, the case was discussed at the multidisciplinary pediatric tumor board and the consensus was to proceed with surgical resection.
Surgical Technique
General endotracheal anesthesia was administered without complication. The patient was positioned supine with a soft bump under her shoulders to place her neck in slight extension and thus facilitate access to the clavicle and great vessels. A 14-cm oblique incision was made over the subcutaneous clavicle extending to the contralateral sternoclavicular joint. Dissection was carried down to the fascia and the biopsy site was excised with the skin paddle. Dissection was carried through the sternocleidomastoid superiorly and the pectoralis major inferiorly, to 8 cm lateral from the right sternoclavicular joint. The clavicle was osteotomized well lateral of the palpable tumor and a marrow margin was sent for frozen section, which was found to be negative.
Dissection was continued circumferentially. Assistance from pediatric general and cardiothoracic surgery was required at the inferior aspect of the mass to assist with exposure and control of the subclavian vein (Figure 5A). A large branch of the subclavian vein near its junction with the internal jugular vein was found to be involved with the tumor and thus required suture ligation. The subclavian vein was noted to be intimate with the mass and somewhat friable. With the vein mobilized, a cuff of normal tissue was obtained inferiorly and superiorly to the mass. Medially, the sternoclavicular joint was disarticulated (Figure 5B). At this point, the specimen was delivered from the operative field and tagged in the usual fashion (Figure 5C). A medial soft tissue margin from the sternal side of the sternoclavicular joint was also sent and found to be negative for tumor. The wound was closed in layered fashion over a ¼” Penrose drain. A soft dressing was placed, and the patient was successfully extubated and transferred to the post-anesthesia care unit in stable condition.
Postoperative Course
The patient was found to be neurologically and vascularly intact on postoperative exam and was discharged on postoperative day 1.
She was seen 14 days postoperatively and was doing well at that time, with full range of motion of the shoulder, elbow, wrist, and hand. Final pathology confirmed a low-grade OS with extraosseous extension. All margins were negative except the medial (sternoclavicular joint) margin and the inferior margin adjacent to the subclavian vein. The intraoperative frozen section from the medial margin was negative for tumor.
The pediatric hematology/oncology team determined that, as no high-grade areas were identified, chemotherapy should be deferred. The positive margins were also discussed with the patient and her family specifically regarding further possible treatments. The findings from the pathology were discussed in a multidisciplinary tumor board and it was felt that, given the low-grade nature of the lesion as well as the high morbidity and risk of mortality with further surgery, additional surgery would be potentially more harmful than helpful. Additionally, low-grade OS is extremely resistant to radiotherapy. The plan remains to monitor her for local recurrence as well as metastases with serial imaging.
Discussion
The clavicle is one of the first bones in the body to ossify but one of the last to have final physeal closure. Its unique characteristics have led to various descriptions, such as a “short tubular bone” versus a “flat bone.”4,5 Of note are its paucity of a true intramedullary space and scanty red marrow, which make it an unlikely site for a primarily intramedullary- based neoplasm to arise.4 However, it has also been noted that malignant lesions are more common in the clavicle than benign lesions, and special attention should be paid to aggressiveappearing lesions in the clavicle.
Radiographs can be misleading as well. Prior studies have demonstrated that low-grade central OS can be readily misdiagnosed as fibrous dysplasia, desmoplastic fibroma, nonossifying fibroma, osteoblastoma, and aneurysmal bone cyst.6 Findings found in low-grade OS can include evidence of cortical interruption, local soft tissue mass development, intramedullary involvement, cortical destruction, and poor margination; however, low-grade OS is typically sclerotic and highly trabeculated. Cross-sectional imaging can help differentiate between OS and other more benign pathologies and should be considered in the clavicle where biopsy may be perilous.5
The difficulty of clavicular biopsy has been reported. Not only does clavicular anatomy make biopsy hazardous, but also the potential for sampling error does exist. In a case report of one patient with a highgrade lesion, fine needle aspiration biopsy was initially diagnosed as an aneurysmal bone cyst but was ultimately found to be osteosarcoma.2 Histology of low-grade lesions usually demonstrates minimal cytological atypia, rare mitotic activity, and variable osteoid production.5 Lower mitotic indices typically make wide resection curative for these patients, without the need for chemotherapy.
In this case, wide resection was carried out with the subclavian vein as the posterior-inferior margin and the sternoclavicular joint as the medial margin. Though the intra-operative medial margin was clear of disease, final pathology demonstrated focal (microscopic) involvement of the posterior and medial margins. A study of soft tissue sarcoma evaluated positive margins and concluded that the imperative of preservation of vital structures supersedes the need for negative margins.7,8 The rate of metastasis and overall survival was similar to surgical resections with positive margins. In the case of our patient, further resection would have carried significant morbidity and possibly mortality, including sacrifice of the major vessels to the arm below and entering into the sternum and thoracic cavity. The likely disability as well as the hazards of surgery were deemed to be too great to justify further excision. Frequent cross-sectional imaging will be necessary to evaluate the presence of recurrent or metastatic disease. To our knowledge, this is the first documented case of low-grade clavicle OS. This report demonstrates the need for multidisciplinary sarcoma care at a center of excellence, particularly in instances of unusual diagnoses.
Osteosarcoma (OS) is a rare disease with approximately 800- 900 newly diagnosed cases each year in the United States. Of those, the majority occur about the knee. The distal femur is the most common site, followed by the proximal tibia, with the proximal humerus being a distant third. OS of the clavicle has been reported, with the earliest case report dating from 1975.1 Since then, additional case reports of high-grade OS of the clavicle have been published.2,3 We describe the case of a 16-year-old female who presented with a mass on her right medial clavicle, which was confirmed to be a low-grade central OS.
Case Presentation
The patient is a 16-year-old female who presented to the Emergency Department (ED) for evaluation of a mass on her right clavicle, after being evaluated by her primary care physician (PCP). She noted an enlarging mass over the previous 2 months but stated that it had been asymptomatic until 4 days prior to presentation to her PCP, at which time she had developed tenderness to palpation and pain with range of motion of the right arm. X-rays were obtained at the PCP’s office and she was referred to the ED for further evaluation. She denied constitutional symptoms.
At the ED visit, she was noted to have an area of erythema and tenderness over the medial aspect of the right clavicle with increased bony prominence. A chest x-ray demonstrated medial clavicle enlargement with periosteal reaction and sclerosis (Figure 1).
MRI demonstrated a 6-cm x 3.8-cm x 4.1-cm mass arising from the right medial clavicle with cortical destruction and concomitant displacement of the right subclavian and brachiocephalic veins (Figure 2). A CT-guided biopsy was performed 1 week later and demonstrated low-grade OS. The pathologist was concerned about the possibility of sampling error and the presence of a higher-grade component, as low-grade OS of the clavicle had not been reported.
The patient was evaluated by a pediatric hematologist/oncologist 2 weeks later after having obtained the biopsy and a PET/CT scan. At that time, the PET/CT showed an FDG-avid mass at the clavicle without evidence of pulmonary metastatic disease (Figure 3). She was subsequently evaluated by orthopedic oncology, at which time a discussion was had regarding further treatment. There was essentially no literature to guide the surgical and medical teams, as low-grade clavicular OS is unknown. Based on the evidence of localized, low-grade disease, the determination was made to proceedwith surgical resection. In the event that high-grade disease was identified at the time of final pathological evaluation, the pediatric hematology/oncology team felt that administering all of the patient’s chemotherapy postoperatively would be acceptable and not affect her long-term prognosis. CT and CT angiogram were obtained for further operative planning (Figure 4).
Given the intimacy of the mass to the subclavian vessels, she was also seen preoperatively by pediatric general and cardiothoracic surgeons. The plan was formulated to have them in the operating room for mobilization of the subclavian vessels and in the event that a sternotomy was required for proximal control of the vessels. Following this visit, the case was discussed at the multidisciplinary pediatric tumor board and the consensus was to proceed with surgical resection.
Surgical Technique
General endotracheal anesthesia was administered without complication. The patient was positioned supine with a soft bump under her shoulders to place her neck in slight extension and thus facilitate access to the clavicle and great vessels. A 14-cm oblique incision was made over the subcutaneous clavicle extending to the contralateral sternoclavicular joint. Dissection was carried down to the fascia and the biopsy site was excised with the skin paddle. Dissection was carried through the sternocleidomastoid superiorly and the pectoralis major inferiorly, to 8 cm lateral from the right sternoclavicular joint. The clavicle was osteotomized well lateral of the palpable tumor and a marrow margin was sent for frozen section, which was found to be negative.
Dissection was continued circumferentially. Assistance from pediatric general and cardiothoracic surgery was required at the inferior aspect of the mass to assist with exposure and control of the subclavian vein (Figure 5A). A large branch of the subclavian vein near its junction with the internal jugular vein was found to be involved with the tumor and thus required suture ligation. The subclavian vein was noted to be intimate with the mass and somewhat friable. With the vein mobilized, a cuff of normal tissue was obtained inferiorly and superiorly to the mass. Medially, the sternoclavicular joint was disarticulated (Figure 5B). At this point, the specimen was delivered from the operative field and tagged in the usual fashion (Figure 5C). A medial soft tissue margin from the sternal side of the sternoclavicular joint was also sent and found to be negative for tumor. The wound was closed in layered fashion over a ¼” Penrose drain. A soft dressing was placed, and the patient was successfully extubated and transferred to the post-anesthesia care unit in stable condition.
Postoperative Course
The patient was found to be neurologically and vascularly intact on postoperative exam and was discharged on postoperative day 1.
She was seen 14 days postoperatively and was doing well at that time, with full range of motion of the shoulder, elbow, wrist, and hand. Final pathology confirmed a low-grade OS with extraosseous extension. All margins were negative except the medial (sternoclavicular joint) margin and the inferior margin adjacent to the subclavian vein. The intraoperative frozen section from the medial margin was negative for tumor.
The pediatric hematology/oncology team determined that, as no high-grade areas were identified, chemotherapy should be deferred. The positive margins were also discussed with the patient and her family specifically regarding further possible treatments. The findings from the pathology were discussed in a multidisciplinary tumor board and it was felt that, given the low-grade nature of the lesion as well as the high morbidity and risk of mortality with further surgery, additional surgery would be potentially more harmful than helpful. Additionally, low-grade OS is extremely resistant to radiotherapy. The plan remains to monitor her for local recurrence as well as metastases with serial imaging.
Discussion
The clavicle is one of the first bones in the body to ossify but one of the last to have final physeal closure. Its unique characteristics have led to various descriptions, such as a “short tubular bone” versus a “flat bone.”4,5 Of note are its paucity of a true intramedullary space and scanty red marrow, which make it an unlikely site for a primarily intramedullary- based neoplasm to arise.4 However, it has also been noted that malignant lesions are more common in the clavicle than benign lesions, and special attention should be paid to aggressiveappearing lesions in the clavicle.
Radiographs can be misleading as well. Prior studies have demonstrated that low-grade central OS can be readily misdiagnosed as fibrous dysplasia, desmoplastic fibroma, nonossifying fibroma, osteoblastoma, and aneurysmal bone cyst.6 Findings found in low-grade OS can include evidence of cortical interruption, local soft tissue mass development, intramedullary involvement, cortical destruction, and poor margination; however, low-grade OS is typically sclerotic and highly trabeculated. Cross-sectional imaging can help differentiate between OS and other more benign pathologies and should be considered in the clavicle where biopsy may be perilous.5
The difficulty of clavicular biopsy has been reported. Not only does clavicular anatomy make biopsy hazardous, but also the potential for sampling error does exist. In a case report of one patient with a highgrade lesion, fine needle aspiration biopsy was initially diagnosed as an aneurysmal bone cyst but was ultimately found to be osteosarcoma.2 Histology of low-grade lesions usually demonstrates minimal cytological atypia, rare mitotic activity, and variable osteoid production.5 Lower mitotic indices typically make wide resection curative for these patients, without the need for chemotherapy.
In this case, wide resection was carried out with the subclavian vein as the posterior-inferior margin and the sternoclavicular joint as the medial margin. Though the intra-operative medial margin was clear of disease, final pathology demonstrated focal (microscopic) involvement of the posterior and medial margins. A study of soft tissue sarcoma evaluated positive margins and concluded that the imperative of preservation of vital structures supersedes the need for negative margins.7,8 The rate of metastasis and overall survival was similar to surgical resections with positive margins. In the case of our patient, further resection would have carried significant morbidity and possibly mortality, including sacrifice of the major vessels to the arm below and entering into the sternum and thoracic cavity. The likely disability as well as the hazards of surgery were deemed to be too great to justify further excision. Frequent cross-sectional imaging will be necessary to evaluate the presence of recurrent or metastatic disease. To our knowledge, this is the first documented case of low-grade clavicle OS. This report demonstrates the need for multidisciplinary sarcoma care at a center of excellence, particularly in instances of unusual diagnoses.
1. Zinghi G. Osteosarcoma of the clavicle (description of a case) [in Italian]. Chir Organi Mov. 1975;62(6):671-674.
2. Cundy WJ, Carter C, Dhatrak D, Clayer M. Primary osteosarcoma of the clavicle and the perils of bone biopsy. BMJ Case Rep. 2015;2015:bcr2014208859.
3. Greenspan A, Unni KK, Mann J. Case report 804: Chondroblastic osteosarcoma grade 3 of the left clavicle. Skeletal Radiol. 1993;22(6):469-471.
4. Rossi B, Fabbriciani C, Chalidis BE, Visci F, Maccauro G. Primary malignant clavicular tumours: a clinicopathological analysis of six cases and evaluation of surgical management. Arch Orthop Trauma Surg. 2011;131(7):935-939.
5. Andresen KJ, Sundaram M, Unni KK, Sim FH. Imaging features of low-grade central osteosarcoma of the long bones and pelvis. Skeletal Radiol. 2004;33(7):373-379.
6. Malhas AM, Sumathi VP, James SL, et al. Low-grade central osteosarcoma: A difficult condition to diagnose. Sarcoma. 2012; 2012:764796.
7. O’Donnell PW, Griffin AM, Eward WC, et al. The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer. 2014;120(18):2866-2875.
8. Kawaguchi N, Ahmed AR, Matsumoto S, Manabe J, Matsushita Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:165-172.
1. Zinghi G. Osteosarcoma of the clavicle (description of a case) [in Italian]. Chir Organi Mov. 1975;62(6):671-674.
2. Cundy WJ, Carter C, Dhatrak D, Clayer M. Primary osteosarcoma of the clavicle and the perils of bone biopsy. BMJ Case Rep. 2015;2015:bcr2014208859.
3. Greenspan A, Unni KK, Mann J. Case report 804: Chondroblastic osteosarcoma grade 3 of the left clavicle. Skeletal Radiol. 1993;22(6):469-471.
4. Rossi B, Fabbriciani C, Chalidis BE, Visci F, Maccauro G. Primary malignant clavicular tumours: a clinicopathological analysis of six cases and evaluation of surgical management. Arch Orthop Trauma Surg. 2011;131(7):935-939.
5. Andresen KJ, Sundaram M, Unni KK, Sim FH. Imaging features of low-grade central osteosarcoma of the long bones and pelvis. Skeletal Radiol. 2004;33(7):373-379.
6. Malhas AM, Sumathi VP, James SL, et al. Low-grade central osteosarcoma: A difficult condition to diagnose. Sarcoma. 2012; 2012:764796.
7. O’Donnell PW, Griffin AM, Eward WC, et al. The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer. 2014;120(18):2866-2875.
8. Kawaguchi N, Ahmed AR, Matsumoto S, Manabe J, Matsushita Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:165-172.
Remain Connected on Connect
How have you managed a possible infected aortitis, a severe focal stenosis or a fractured carotid stent? Give your input in discussions about these topics, and more, on your online community, SVSConnect. If you attended VAM last week, continue the discussions with other attendees. All SVS members can participate in discussions – log in here with your SVS credentials. Reach out to [email protected] or call 312-334-2300 with questions.
How have you managed a possible infected aortitis, a severe focal stenosis or a fractured carotid stent? Give your input in discussions about these topics, and more, on your online community, SVSConnect. If you attended VAM last week, continue the discussions with other attendees. All SVS members can participate in discussions – log in here with your SVS credentials. Reach out to [email protected] or call 312-334-2300 with questions.
How have you managed a possible infected aortitis, a severe focal stenosis or a fractured carotid stent? Give your input in discussions about these topics, and more, on your online community, SVSConnect. If you attended VAM last week, continue the discussions with other attendees. All SVS members can participate in discussions – log in here with your SVS credentials. Reach out to [email protected] or call 312-334-2300 with questions.
Subscribe to SVS Student Newsletters
The SVS has recently re-vamped its newsletters geared towards future vascular surgeons. These provide residents, students and vascular trainees with up-to-date information on upcoming events, awards and scholarships, open positions and more. These are sent on a bi-weekly and monthly basis, depending on what content you are interested in. Learn more and subscribe here. They will also be posted on the SVS future vascular surgeon’s Twitter and Facebook.
The SVS has recently re-vamped its newsletters geared towards future vascular surgeons. These provide residents, students and vascular trainees with up-to-date information on upcoming events, awards and scholarships, open positions and more. These are sent on a bi-weekly and monthly basis, depending on what content you are interested in. Learn more and subscribe here. They will also be posted on the SVS future vascular surgeon’s Twitter and Facebook.
The SVS has recently re-vamped its newsletters geared towards future vascular surgeons. These provide residents, students and vascular trainees with up-to-date information on upcoming events, awards and scholarships, open positions and more. These are sent on a bi-weekly and monthly basis, depending on what content you are interested in. Learn more and subscribe here. They will also be posted on the SVS future vascular surgeon’s Twitter and Facebook.
Apply for the Research Career Development Travel Award
The SVS Foundation developed the Research Career Development Travel Awards program to develop strong leaders in vascular surgery research. Recipients of the award will be assigned SVS research mentors who will provide guidance and discuss academic career advancement. They’ll also receive financial support to be used for travel, hotel accommodations and registration expenses for a research course. Applicants must be an SVS Candidate or Active Member who’s completed postgraduate clinical training in vascular surgery and has been in practice no more than seven years. Apply before August 15 to be considered.
The SVS Foundation developed the Research Career Development Travel Awards program to develop strong leaders in vascular surgery research. Recipients of the award will be assigned SVS research mentors who will provide guidance and discuss academic career advancement. They’ll also receive financial support to be used for travel, hotel accommodations and registration expenses for a research course. Applicants must be an SVS Candidate or Active Member who’s completed postgraduate clinical training in vascular surgery and has been in practice no more than seven years. Apply before August 15 to be considered.
The SVS Foundation developed the Research Career Development Travel Awards program to develop strong leaders in vascular surgery research. Recipients of the award will be assigned SVS research mentors who will provide guidance and discuss academic career advancement. They’ll also receive financial support to be used for travel, hotel accommodations and registration expenses for a research course. Applicants must be an SVS Candidate or Active Member who’s completed postgraduate clinical training in vascular surgery and has been in practice no more than seven years. Apply before August 15 to be considered.
Ask patients about worst example of suicidal ideation
CRYSTAL CITY, VA. – Some patients experience consistent suicidal ideation – but most do not, an expert said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“In most patients, the ideation tends to go up and down – which means you ask the patient about the most severe example of suicidal ideation … in the last week or 2,” J. John Mann, MD, said. Getting a handle on patients’ worst suicidal ideation also can provide clues into the range of suicidal behavior they might be subject to, he added.
U.S. suicide rates have increased dramatically since 2000, and most people who die by suicide had depression, said Dr. Mann, the Paul Janssen Professor of Translational Neuroscience (in psychiatry and in radiology) at Columbia University, New York. However, those patients who are depressed tend to attempt suicide early in their depression.
“Most people with a major depressive episode never attempt suicide,” said Dr. Mann, who also is affiliated with the New York State Psychiatric Institute. “Suicidal behavior is not a ‘wear and tear’ phenomenon.”
When assessing risk of suicide clinically, patients most at risk include those with past history of suicide attempts, a family history of suicide, and those who have the worst suicidal ideation.
About half of the predisposition to suicidal behavior is genetic and independent of genetic risk associated with major psychiatric disorders. This genetic risk affects the diathesis each patient has for suicidal behavior. In the stress-diathesis model for suicidal behavior, stress from major depressive episodes and life events contributes to the patient’s perception of stress, which in turn contributes to that patient’s response to stress. Rather than depression itself being a suicidal trigger, these stressors in the form of adverse life events appear to be the trigger for suicide attempts, Dr. Mann noted.
“All of the risk is pretty much accounted for by whether the patient was in or out of an episode of major depression,” said Dr. Mann. “If they were in an episode of major depression, all the risk was accounted for by the major depression, and the stressors counted for enough. When they’re out of an episode of major depression, the risk fell right away and the stressors didn’t matter much.”
In the stress-diathesis model, trait components of suicidal behavior include mood and emotion dysregulation and perception; misreading social signals; reactive or impulsive aggressive traits of decision making or delayed discounting; and altered learning, memory, and problem solving. However, clinicians should look to the patients for whom depression appears more painful in subjective scores, because going by these trait components alone will not distinguish between patients at risk for suicide and those who will not make an attempt.
According to the Columbia Classification Algorithm of Suicide Assessment, suicide is distinguished by whether a patient wished to die, if an attempt is stopped by themselves or another person before harm has begun, and whether a patient prepared for the act beyond verbalizing or thinking of suicide but before harm has begun.
In addition to prescribing antidepressants, treatments with evidence for preventing suicide include means restriction and cognitive-behavioral therapy. For patients with borderline personality disorder, dialectical behavior therapy has proven effective. School interventions that educate students about mental health also have shown effectiveness. Other strategies include educating reporters about media guidelines on writing about suicide. Internet outreach interventions are promising, he said, but more evidence is needed to determine whether they work.
Among antidepressant options for patients with suicidal ideation, fluoxetine appears best for adolescents, and data show that venlafaxine is effective in adults. The Food and Drug Administration originally put a black box warning on selective serotonin reuptake inhibitors in 2004; however, recent data have shown that the increased risk of suicidal ideation brought on by those medications tapers off after the first week on the medication. Meanwhile, in the case of ketamine, there is “rapid and robust improvement” in depressive symptoms and suicidal ideation, which targets the diathesis, Dr. Mann said at the meeting presented by Global Academy for Medical Education.
“We need to identify rapidly acting antisuicidal medications, and we now see there’s a clear path forward to do that” with treatments like ketamine, he said.
Dr. Mann’s presentation was based on research funded by the National Institute of Mental Health and the Brain & Behavior Research Foundation. He reported receiving royalties from the Research Foundation for Mental Hygiene for commercial use of the Columbia-Suicide Severity Rating Scale.
Global Academy for Medical Education, Current Psychiatry, and this publication are owned by the same company.
CRYSTAL CITY, VA. – Some patients experience consistent suicidal ideation – but most do not, an expert said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“In most patients, the ideation tends to go up and down – which means you ask the patient about the most severe example of suicidal ideation … in the last week or 2,” J. John Mann, MD, said. Getting a handle on patients’ worst suicidal ideation also can provide clues into the range of suicidal behavior they might be subject to, he added.
U.S. suicide rates have increased dramatically since 2000, and most people who die by suicide had depression, said Dr. Mann, the Paul Janssen Professor of Translational Neuroscience (in psychiatry and in radiology) at Columbia University, New York. However, those patients who are depressed tend to attempt suicide early in their depression.
“Most people with a major depressive episode never attempt suicide,” said Dr. Mann, who also is affiliated with the New York State Psychiatric Institute. “Suicidal behavior is not a ‘wear and tear’ phenomenon.”
When assessing risk of suicide clinically, patients most at risk include those with past history of suicide attempts, a family history of suicide, and those who have the worst suicidal ideation.
About half of the predisposition to suicidal behavior is genetic and independent of genetic risk associated with major psychiatric disorders. This genetic risk affects the diathesis each patient has for suicidal behavior. In the stress-diathesis model for suicidal behavior, stress from major depressive episodes and life events contributes to the patient’s perception of stress, which in turn contributes to that patient’s response to stress. Rather than depression itself being a suicidal trigger, these stressors in the form of adverse life events appear to be the trigger for suicide attempts, Dr. Mann noted.
“All of the risk is pretty much accounted for by whether the patient was in or out of an episode of major depression,” said Dr. Mann. “If they were in an episode of major depression, all the risk was accounted for by the major depression, and the stressors counted for enough. When they’re out of an episode of major depression, the risk fell right away and the stressors didn’t matter much.”
In the stress-diathesis model, trait components of suicidal behavior include mood and emotion dysregulation and perception; misreading social signals; reactive or impulsive aggressive traits of decision making or delayed discounting; and altered learning, memory, and problem solving. However, clinicians should look to the patients for whom depression appears more painful in subjective scores, because going by these trait components alone will not distinguish between patients at risk for suicide and those who will not make an attempt.
According to the Columbia Classification Algorithm of Suicide Assessment, suicide is distinguished by whether a patient wished to die, if an attempt is stopped by themselves or another person before harm has begun, and whether a patient prepared for the act beyond verbalizing or thinking of suicide but before harm has begun.
In addition to prescribing antidepressants, treatments with evidence for preventing suicide include means restriction and cognitive-behavioral therapy. For patients with borderline personality disorder, dialectical behavior therapy has proven effective. School interventions that educate students about mental health also have shown effectiveness. Other strategies include educating reporters about media guidelines on writing about suicide. Internet outreach interventions are promising, he said, but more evidence is needed to determine whether they work.
Among antidepressant options for patients with suicidal ideation, fluoxetine appears best for adolescents, and data show that venlafaxine is effective in adults. The Food and Drug Administration originally put a black box warning on selective serotonin reuptake inhibitors in 2004; however, recent data have shown that the increased risk of suicidal ideation brought on by those medications tapers off after the first week on the medication. Meanwhile, in the case of ketamine, there is “rapid and robust improvement” in depressive symptoms and suicidal ideation, which targets the diathesis, Dr. Mann said at the meeting presented by Global Academy for Medical Education.
“We need to identify rapidly acting antisuicidal medications, and we now see there’s a clear path forward to do that” with treatments like ketamine, he said.
Dr. Mann’s presentation was based on research funded by the National Institute of Mental Health and the Brain & Behavior Research Foundation. He reported receiving royalties from the Research Foundation for Mental Hygiene for commercial use of the Columbia-Suicide Severity Rating Scale.
Global Academy for Medical Education, Current Psychiatry, and this publication are owned by the same company.
CRYSTAL CITY, VA. – Some patients experience consistent suicidal ideation – but most do not, an expert said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“In most patients, the ideation tends to go up and down – which means you ask the patient about the most severe example of suicidal ideation … in the last week or 2,” J. John Mann, MD, said. Getting a handle on patients’ worst suicidal ideation also can provide clues into the range of suicidal behavior they might be subject to, he added.
U.S. suicide rates have increased dramatically since 2000, and most people who die by suicide had depression, said Dr. Mann, the Paul Janssen Professor of Translational Neuroscience (in psychiatry and in radiology) at Columbia University, New York. However, those patients who are depressed tend to attempt suicide early in their depression.
“Most people with a major depressive episode never attempt suicide,” said Dr. Mann, who also is affiliated with the New York State Psychiatric Institute. “Suicidal behavior is not a ‘wear and tear’ phenomenon.”
When assessing risk of suicide clinically, patients most at risk include those with past history of suicide attempts, a family history of suicide, and those who have the worst suicidal ideation.
About half of the predisposition to suicidal behavior is genetic and independent of genetic risk associated with major psychiatric disorders. This genetic risk affects the diathesis each patient has for suicidal behavior. In the stress-diathesis model for suicidal behavior, stress from major depressive episodes and life events contributes to the patient’s perception of stress, which in turn contributes to that patient’s response to stress. Rather than depression itself being a suicidal trigger, these stressors in the form of adverse life events appear to be the trigger for suicide attempts, Dr. Mann noted.
“All of the risk is pretty much accounted for by whether the patient was in or out of an episode of major depression,” said Dr. Mann. “If they were in an episode of major depression, all the risk was accounted for by the major depression, and the stressors counted for enough. When they’re out of an episode of major depression, the risk fell right away and the stressors didn’t matter much.”
In the stress-diathesis model, trait components of suicidal behavior include mood and emotion dysregulation and perception; misreading social signals; reactive or impulsive aggressive traits of decision making or delayed discounting; and altered learning, memory, and problem solving. However, clinicians should look to the patients for whom depression appears more painful in subjective scores, because going by these trait components alone will not distinguish between patients at risk for suicide and those who will not make an attempt.
According to the Columbia Classification Algorithm of Suicide Assessment, suicide is distinguished by whether a patient wished to die, if an attempt is stopped by themselves or another person before harm has begun, and whether a patient prepared for the act beyond verbalizing or thinking of suicide but before harm has begun.
In addition to prescribing antidepressants, treatments with evidence for preventing suicide include means restriction and cognitive-behavioral therapy. For patients with borderline personality disorder, dialectical behavior therapy has proven effective. School interventions that educate students about mental health also have shown effectiveness. Other strategies include educating reporters about media guidelines on writing about suicide. Internet outreach interventions are promising, he said, but more evidence is needed to determine whether they work.
Among antidepressant options for patients with suicidal ideation, fluoxetine appears best for adolescents, and data show that venlafaxine is effective in adults. The Food and Drug Administration originally put a black box warning on selective serotonin reuptake inhibitors in 2004; however, recent data have shown that the increased risk of suicidal ideation brought on by those medications tapers off after the first week on the medication. Meanwhile, in the case of ketamine, there is “rapid and robust improvement” in depressive symptoms and suicidal ideation, which targets the diathesis, Dr. Mann said at the meeting presented by Global Academy for Medical Education.
“We need to identify rapidly acting antisuicidal medications, and we now see there’s a clear path forward to do that” with treatments like ketamine, he said.
Dr. Mann’s presentation was based on research funded by the National Institute of Mental Health and the Brain & Behavior Research Foundation. He reported receiving royalties from the Research Foundation for Mental Hygiene for commercial use of the Columbia-Suicide Severity Rating Scale.
Global Academy for Medical Education, Current Psychiatry, and this publication are owned by the same company.
EXPERT ANALYSIS FROM FOCUS ON NEUROPSYCHIATRY 2019
FDA warns about fecal microbiota for transplantation
Officials at the Food and Drug Administration have issued a safety alert regarding the use of fecal microbiota for transplantation and the risk of serious adverse reactions because of transmission of multidrug-resistant organisms (MDROs).
According to the alert, which was issued on June 13, 2019, the agency became aware of two immunocompromised adult patients who received investigational fecal microbiota for transplantation (FMT) and developed extended-spectrum beta-lactamase (EBSL)–producing Escherichia coli. One of the patients died.
“This is certainly a theoretical risk that we’ve known about,” Lea Ann Chen, MD, a gastroenterologist at New York University, said in an interview. “This announcement is important, because we probably don’t counsel patients specifically about this risk. We say there is a risk for transmission of infectious agents in general, but I think that probably very few counsel patients about a risk for transmission of MDROs.”
The donor stool and FMT used in the two patients were not tested for ESBL-producing gram-negative organisms prior to use.
As a result of these serious adverse reactions, the FDA has determined that the following donor screening and stool testing protections are needed for any investigational use of FMT.
- Donor screening with questions that specifically address risk factors for colonization with MDROs, and exclusion of individuals at higher risk of colonization with MDROs.
- MDRO testing of donor stool and exclusion of stool that tests positive for MDRO. FDA scientists have determined the specific MDRO testing and frequency that should be implemented.
On June 14, the American Gastroenterological Association sent a communication about the FDA alert to its members, which stated that the AGA “is committed to advancing applications of the gut microbiome. Our top priority is ensuring patient safety from microbiome-based therapeutics, such as FMT. Through the AGA FMT National Registry, AGA is working with physicians and patients to track FMT usage, patient outcomes and adverse events. Associated with the registry is a biorepository of donor and patient stool samples, which will allow further investigation of unexpected events such as those described in FDA’s safety alert.”
Dr. Chen, who received the AGA Research Foundation’s 2016 Research Scholar Award for her work on the gut microbiome and inflammatory bowel disease, pointed out that FMT has also been studied as a way to prevent colonization and infection with certain drug resistant organisms, such as vancomycin-resistant Enterococcus.
“Therefore, it’s not that FMT is ‘bad;’ we just have to be more diligent about optimizing the safety of the procedure by screening for of multidrug-resistant organisms,” she said. “We also need to study the use of FMT more, so that we can fully understand the risks associated with the procedure. It’s an important and potentially lifesaving procedure for some, but it’s important that everyone go into the procedure understanding fully what the risks and benefits are.”
Suspected adverse events related to the administration of FMT products can be reported to the FDA at 1-800-332-1088 or via MedWatch.
Officials at the Food and Drug Administration have issued a safety alert regarding the use of fecal microbiota for transplantation and the risk of serious adverse reactions because of transmission of multidrug-resistant organisms (MDROs).
According to the alert, which was issued on June 13, 2019, the agency became aware of two immunocompromised adult patients who received investigational fecal microbiota for transplantation (FMT) and developed extended-spectrum beta-lactamase (EBSL)–producing Escherichia coli. One of the patients died.
“This is certainly a theoretical risk that we’ve known about,” Lea Ann Chen, MD, a gastroenterologist at New York University, said in an interview. “This announcement is important, because we probably don’t counsel patients specifically about this risk. We say there is a risk for transmission of infectious agents in general, but I think that probably very few counsel patients about a risk for transmission of MDROs.”
The donor stool and FMT used in the two patients were not tested for ESBL-producing gram-negative organisms prior to use.
As a result of these serious adverse reactions, the FDA has determined that the following donor screening and stool testing protections are needed for any investigational use of FMT.
- Donor screening with questions that specifically address risk factors for colonization with MDROs, and exclusion of individuals at higher risk of colonization with MDROs.
- MDRO testing of donor stool and exclusion of stool that tests positive for MDRO. FDA scientists have determined the specific MDRO testing and frequency that should be implemented.
On June 14, the American Gastroenterological Association sent a communication about the FDA alert to its members, which stated that the AGA “is committed to advancing applications of the gut microbiome. Our top priority is ensuring patient safety from microbiome-based therapeutics, such as FMT. Through the AGA FMT National Registry, AGA is working with physicians and patients to track FMT usage, patient outcomes and adverse events. Associated with the registry is a biorepository of donor and patient stool samples, which will allow further investigation of unexpected events such as those described in FDA’s safety alert.”
Dr. Chen, who received the AGA Research Foundation’s 2016 Research Scholar Award for her work on the gut microbiome and inflammatory bowel disease, pointed out that FMT has also been studied as a way to prevent colonization and infection with certain drug resistant organisms, such as vancomycin-resistant Enterococcus.
“Therefore, it’s not that FMT is ‘bad;’ we just have to be more diligent about optimizing the safety of the procedure by screening for of multidrug-resistant organisms,” she said. “We also need to study the use of FMT more, so that we can fully understand the risks associated with the procedure. It’s an important and potentially lifesaving procedure for some, but it’s important that everyone go into the procedure understanding fully what the risks and benefits are.”
Suspected adverse events related to the administration of FMT products can be reported to the FDA at 1-800-332-1088 or via MedWatch.
Officials at the Food and Drug Administration have issued a safety alert regarding the use of fecal microbiota for transplantation and the risk of serious adverse reactions because of transmission of multidrug-resistant organisms (MDROs).
According to the alert, which was issued on June 13, 2019, the agency became aware of two immunocompromised adult patients who received investigational fecal microbiota for transplantation (FMT) and developed extended-spectrum beta-lactamase (EBSL)–producing Escherichia coli. One of the patients died.
“This is certainly a theoretical risk that we’ve known about,” Lea Ann Chen, MD, a gastroenterologist at New York University, said in an interview. “This announcement is important, because we probably don’t counsel patients specifically about this risk. We say there is a risk for transmission of infectious agents in general, but I think that probably very few counsel patients about a risk for transmission of MDROs.”
The donor stool and FMT used in the two patients were not tested for ESBL-producing gram-negative organisms prior to use.
As a result of these serious adverse reactions, the FDA has determined that the following donor screening and stool testing protections are needed for any investigational use of FMT.
- Donor screening with questions that specifically address risk factors for colonization with MDROs, and exclusion of individuals at higher risk of colonization with MDROs.
- MDRO testing of donor stool and exclusion of stool that tests positive for MDRO. FDA scientists have determined the specific MDRO testing and frequency that should be implemented.
On June 14, the American Gastroenterological Association sent a communication about the FDA alert to its members, which stated that the AGA “is committed to advancing applications of the gut microbiome. Our top priority is ensuring patient safety from microbiome-based therapeutics, such as FMT. Through the AGA FMT National Registry, AGA is working with physicians and patients to track FMT usage, patient outcomes and adverse events. Associated with the registry is a biorepository of donor and patient stool samples, which will allow further investigation of unexpected events such as those described in FDA’s safety alert.”
Dr. Chen, who received the AGA Research Foundation’s 2016 Research Scholar Award for her work on the gut microbiome and inflammatory bowel disease, pointed out that FMT has also been studied as a way to prevent colonization and infection with certain drug resistant organisms, such as vancomycin-resistant Enterococcus.
“Therefore, it’s not that FMT is ‘bad;’ we just have to be more diligent about optimizing the safety of the procedure by screening for of multidrug-resistant organisms,” she said. “We also need to study the use of FMT more, so that we can fully understand the risks associated with the procedure. It’s an important and potentially lifesaving procedure for some, but it’s important that everyone go into the procedure understanding fully what the risks and benefits are.”
Suspected adverse events related to the administration of FMT products can be reported to the FDA at 1-800-332-1088 or via MedWatch.
Heart Valve Replacement for High-Risk Patients
Left ventricular outflow tract (LVOT) obstruction is a life-threatening complication that can put transcatheter mitral valve replacement (TMVR) out of reach for many patients. But researchers from the National Heart, Lung and Blood Institute (NHLBI) and Emory University in Atlanta, Georgia, have developed a novel technique to essentially slice through the obstacle, increasing treatment options for high-risk patients.
TMVR is a less invasive alternative to open-heart surgery. Physicians replace the mitral valve by inserting an artificial valve via a catheter. In > 50% of patients, though, the heart leaflet is pushed back, blocking blood flow. In surgery, surgeons can cut out the leaflets when they replace valves, because they are looking at the open chest and the heart and can see the problem, says study author Jaffar Khan, MD, clinician at NHLBI.
The researchers describe their new method, LAMPOON, as “a transcatheter mimic of surgical chord-sparing leaflet resection.” LAMPOON involves intentional laceration of the anterior mitral valve leaflet. The operator inserts 2 catheters through the patient’s groin, up to the heart. A thread-sized electrified wire woven through the catheter splits open the leaflet.
In the LAMPOON study, the researchers evaluated the procedure’s results in 30 patients at high risk for surgical valve replacement and prohibitive risk of LVOT obstruction during TMVR.
Survival was 100%, and 30-day survival was 93% (compared with 38% reported with other methods). In all, 73% of patients met the primary outcome: a successful LAMPOON procedure followed by a successful TMVR without reintervention. No one had a stroke.
Every year > 20,000 people in the US die of heart valve disease. The researchers hope their innovative technique will help reduce that number.
Left ventricular outflow tract (LVOT) obstruction is a life-threatening complication that can put transcatheter mitral valve replacement (TMVR) out of reach for many patients. But researchers from the National Heart, Lung and Blood Institute (NHLBI) and Emory University in Atlanta, Georgia, have developed a novel technique to essentially slice through the obstacle, increasing treatment options for high-risk patients.
TMVR is a less invasive alternative to open-heart surgery. Physicians replace the mitral valve by inserting an artificial valve via a catheter. In > 50% of patients, though, the heart leaflet is pushed back, blocking blood flow. In surgery, surgeons can cut out the leaflets when they replace valves, because they are looking at the open chest and the heart and can see the problem, says study author Jaffar Khan, MD, clinician at NHLBI.
The researchers describe their new method, LAMPOON, as “a transcatheter mimic of surgical chord-sparing leaflet resection.” LAMPOON involves intentional laceration of the anterior mitral valve leaflet. The operator inserts 2 catheters through the patient’s groin, up to the heart. A thread-sized electrified wire woven through the catheter splits open the leaflet.
In the LAMPOON study, the researchers evaluated the procedure’s results in 30 patients at high risk for surgical valve replacement and prohibitive risk of LVOT obstruction during TMVR.
Survival was 100%, and 30-day survival was 93% (compared with 38% reported with other methods). In all, 73% of patients met the primary outcome: a successful LAMPOON procedure followed by a successful TMVR without reintervention. No one had a stroke.
Every year > 20,000 people in the US die of heart valve disease. The researchers hope their innovative technique will help reduce that number.
Left ventricular outflow tract (LVOT) obstruction is a life-threatening complication that can put transcatheter mitral valve replacement (TMVR) out of reach for many patients. But researchers from the National Heart, Lung and Blood Institute (NHLBI) and Emory University in Atlanta, Georgia, have developed a novel technique to essentially slice through the obstacle, increasing treatment options for high-risk patients.
TMVR is a less invasive alternative to open-heart surgery. Physicians replace the mitral valve by inserting an artificial valve via a catheter. In > 50% of patients, though, the heart leaflet is pushed back, blocking blood flow. In surgery, surgeons can cut out the leaflets when they replace valves, because they are looking at the open chest and the heart and can see the problem, says study author Jaffar Khan, MD, clinician at NHLBI.
The researchers describe their new method, LAMPOON, as “a transcatheter mimic of surgical chord-sparing leaflet resection.” LAMPOON involves intentional laceration of the anterior mitral valve leaflet. The operator inserts 2 catheters through the patient’s groin, up to the heart. A thread-sized electrified wire woven through the catheter splits open the leaflet.
In the LAMPOON study, the researchers evaluated the procedure’s results in 30 patients at high risk for surgical valve replacement and prohibitive risk of LVOT obstruction during TMVR.
Survival was 100%, and 30-day survival was 93% (compared with 38% reported with other methods). In all, 73% of patients met the primary outcome: a successful LAMPOON procedure followed by a successful TMVR without reintervention. No one had a stroke.
Every year > 20,000 people in the US die of heart valve disease. The researchers hope their innovative technique will help reduce that number.
U.S. travelers to Europe need up to date measles immunization
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
FROM PEDIATRICS
Ovarian reserve markers fall on isotretinoin, but rebound after stopping treatment
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
MILAN – according to data presented at the World Congress of Dermatology.
Although markers for ovarian reserve, including anti-Müllerian hormone (AMH) serum levels, ovarian volume, and antral follicle count, were significantly lower during a period of isotretinoin use than at baseline, these values were were not significantly different from pretreatment levels by 1 month after stopping isotretinoin.
For patients taking isotretinoin at a dose of 0.5 mg/kg/day, AMH levels fell from a baseline level of 5.29 ng/mL to 4.16 ng/mL during treatment, but rebounded to 4.77 ng/mL 1 month after stopping treatment (P less than .001 for difference between baseline and on-drug values), Tuğba Özkök Akbulut, MD, said during a late-breaking abstracts session.
For women taking isotretinoin 1 mg/kg/day, AMH levels went from 5.14 ng/mL at baseline to 4.24 ng/mL on treatment, to 4.65 ng/mL 1 month after treatment (P less than .001 for difference between baseline and on-drug values), reported Dr. Akbulut a dermatologist at the Haseki Training Research Hospital, Istanbul.
Women on the higher dose of isotretinoin had a similar pattern of decline while on treatment and rebound after ceasing isotretinoin for ovarian volume and antral follicle count (P less than .001 for all values). These differences were not statistically significant for women taking 0.5 mg/kg/day of isotretinoin, except for right ovarian volume (P = 0.013).
Although values were numerically lower for many markers of ovarian reserve after ceasing treatment, compared with baseline figures, these differences were not statistically significantly different. Markers of ovarian reserve did not change significantly for a control group of women without acne.
Dr. Akbulut and her colleagues conducted this prospective case-control study of 42 women of reproductive age who sought dermatologist care for severe acne unresponsive to conservative therapy; 26 women who did not have acne constituted the control group. Smokers, patients with thyroid disease, and those with known polycystic ovary syndrome were excluded from participation.
The women with acne received oral isotretinoin dosed either at 0.5 or 1.0 mg/kg/day, with treatment lasting 5-9 months. For each patient, treatment was stopped when the cumulative dose reached 120 mg/kg.
After an initial visit at which blood was collected from all participants to measure serum AMH levels, those receiving isotretinoin were seen every 4 weeks to check serum lipid and liver enzyme levels.
At the 3-month mark during the study period and 1 month after the end of completing isotretinoin treatment, or at the end of the study period for the control group, blood samples also were drawn for AMH levels.
To measure hormone levels, also blood was drawn between days 2 and 5 of the follicular phase of the menstrual cycle. Participants received ultrasounds to measure antral follicle count and ovarian volume between days 2 and 5 of the menstrual cycle at the initial visit, at the 3-month visit, and at the final visit. Results were interpreted by a trained gynecologist.
Patients, who were mostly in their early 20s, had a mean body mass index of about 22 kg/m2. Hormone levels, ovarian volume, and antral follicle count did not differ among study arms at baseline.
“There are contradictory reports in the literature regarding the effect of retinoic acid on ovarian reserve,” noted Dr. Akbulut. Some preclinical studies found that retinoic acid increased fertility and ovarian reserve in rodents; however, some human studies had shown lower serum AMH concentrations in patients using isotretinoin.
This new demonstration of the reversibility of isotretinoin’s negative effect on ovarian reserve helps clarify a confused picture in the medical literature, said Dr. Akbulut. “The results of our study demonstrated that systemic isotretinoin had a reversible effect on ovarian reserve.”
Dr. Akbulut reported no outside sources of funding and that she had no relevant financial disclosures.
REPORTING FROM WCD2019