User login
Insurance-related barriers impede L-glutamine access
FORT LAUDERDALE, FLA. – When the Food and Drug Administration in 2017 approved L-glutamine (Endari) to treat the symptoms of sickle cell disease (SCD), it was the first new drug indicated for the condition in nearly two decades. But a small study of sickle cell patients in New York has found that patients are having difficulty obtaining the drug and sticking to the regimen.
“We found out that there are multiple barriers, mostly insurance related, and that after 10 months only one-fifth of the patients were still actively taking this medication,” said Ugochi Ogu, MD, assistant director of the Sickle Cell Center for Adults at Montefiore Medical Center in New York. She presented preliminary study results at the annual meeting of the Foundation for Sickle Cell Disease Research.
L-glutamine oral powder is taken twice a day to treat the symptoms of SCD. GoodRx reports that the average cash price for a 60-day supply of L-glutamine is $2,773.
The study followed 101 patients prescribed L-glutamine at the Montefiore Medical Center. When they returned to the clinic, patients were asked about barriers to obtaining the medication and adherence to the twice-a-day dosing. The center used a nearby local specialty pharmacy to fill the prescriptions.
The study also evaluated adherence by calculating the mean possession ratio (MPR) utilizing pharmacy records. The average age of the patient population was 36 years, and 56% were women.
It’s the first study of L-glutamine barriers and adherence in SCD patients in the real-world setting, Dr. Ogu said.
At the end of the 10-month study period, 21% of the patients were actively taking the medication, she said. “Forty-three percent had discontinued the medication, and 33% never filled the prescriptions; 4% had received but never started Endari,” Dr. Ogu said.
Of the patients who never filled the prescriptions, Dr. Ogu reported that 27% said their insurer denied prior authorization, 19% said their deductible was too high, and 16% cited other insurance issues.
“So we can see that insurance alone accounted for over 60% of why patients did not receive or could not start the medication,” she said.
Most patients – 94% – either had Medicare or Medicaid; the remainder had private insurance.
Among the 43% of all study patients who stopped taking the medication, reasons given include poor adherence (47%), side effects (9%), pregnancy and breast feeding (5%), and no perceived benefit (5%), Dr. Ogu said. At the outset, pharmacy records estimated adherence at 74% by using the average MPR, a rate similar to the phase 3 trial adherence rate of 77.4%.
Patient education is important to eliminate these barriers to treatment for SCD, Dr. Ogu said. “The patients need to understand why they’re taking whatever medication you prescribe. We need to educate them about the side effects, and we need to make them understand why it’s important to take certain medications or how they’re going to help them,” she said.
But even more important, she added, is a systems-based method to deal with insurance barriers. “If 62% of the patients did not get the medication due to insurance issues, I don’t think we’re doing a good job of making it accessible to them.”
Dr. Ogu reported a financial relationship with Vertex.
SOURCE: Ogu U et al. FSCDR 2019, Abstract JSCDH-D-19-00041.
FORT LAUDERDALE, FLA. – When the Food and Drug Administration in 2017 approved L-glutamine (Endari) to treat the symptoms of sickle cell disease (SCD), it was the first new drug indicated for the condition in nearly two decades. But a small study of sickle cell patients in New York has found that patients are having difficulty obtaining the drug and sticking to the regimen.
“We found out that there are multiple barriers, mostly insurance related, and that after 10 months only one-fifth of the patients were still actively taking this medication,” said Ugochi Ogu, MD, assistant director of the Sickle Cell Center for Adults at Montefiore Medical Center in New York. She presented preliminary study results at the annual meeting of the Foundation for Sickle Cell Disease Research.
L-glutamine oral powder is taken twice a day to treat the symptoms of SCD. GoodRx reports that the average cash price for a 60-day supply of L-glutamine is $2,773.
The study followed 101 patients prescribed L-glutamine at the Montefiore Medical Center. When they returned to the clinic, patients were asked about barriers to obtaining the medication and adherence to the twice-a-day dosing. The center used a nearby local specialty pharmacy to fill the prescriptions.
The study also evaluated adherence by calculating the mean possession ratio (MPR) utilizing pharmacy records. The average age of the patient population was 36 years, and 56% were women.
It’s the first study of L-glutamine barriers and adherence in SCD patients in the real-world setting, Dr. Ogu said.
At the end of the 10-month study period, 21% of the patients were actively taking the medication, she said. “Forty-three percent had discontinued the medication, and 33% never filled the prescriptions; 4% had received but never started Endari,” Dr. Ogu said.
Of the patients who never filled the prescriptions, Dr. Ogu reported that 27% said their insurer denied prior authorization, 19% said their deductible was too high, and 16% cited other insurance issues.
“So we can see that insurance alone accounted for over 60% of why patients did not receive or could not start the medication,” she said.
Most patients – 94% – either had Medicare or Medicaid; the remainder had private insurance.
Among the 43% of all study patients who stopped taking the medication, reasons given include poor adherence (47%), side effects (9%), pregnancy and breast feeding (5%), and no perceived benefit (5%), Dr. Ogu said. At the outset, pharmacy records estimated adherence at 74% by using the average MPR, a rate similar to the phase 3 trial adherence rate of 77.4%.
Patient education is important to eliminate these barriers to treatment for SCD, Dr. Ogu said. “The patients need to understand why they’re taking whatever medication you prescribe. We need to educate them about the side effects, and we need to make them understand why it’s important to take certain medications or how they’re going to help them,” she said.
But even more important, she added, is a systems-based method to deal with insurance barriers. “If 62% of the patients did not get the medication due to insurance issues, I don’t think we’re doing a good job of making it accessible to them.”
Dr. Ogu reported a financial relationship with Vertex.
SOURCE: Ogu U et al. FSCDR 2019, Abstract JSCDH-D-19-00041.
FORT LAUDERDALE, FLA. – When the Food and Drug Administration in 2017 approved L-glutamine (Endari) to treat the symptoms of sickle cell disease (SCD), it was the first new drug indicated for the condition in nearly two decades. But a small study of sickle cell patients in New York has found that patients are having difficulty obtaining the drug and sticking to the regimen.
“We found out that there are multiple barriers, mostly insurance related, and that after 10 months only one-fifth of the patients were still actively taking this medication,” said Ugochi Ogu, MD, assistant director of the Sickle Cell Center for Adults at Montefiore Medical Center in New York. She presented preliminary study results at the annual meeting of the Foundation for Sickle Cell Disease Research.
L-glutamine oral powder is taken twice a day to treat the symptoms of SCD. GoodRx reports that the average cash price for a 60-day supply of L-glutamine is $2,773.
The study followed 101 patients prescribed L-glutamine at the Montefiore Medical Center. When they returned to the clinic, patients were asked about barriers to obtaining the medication and adherence to the twice-a-day dosing. The center used a nearby local specialty pharmacy to fill the prescriptions.
The study also evaluated adherence by calculating the mean possession ratio (MPR) utilizing pharmacy records. The average age of the patient population was 36 years, and 56% were women.
It’s the first study of L-glutamine barriers and adherence in SCD patients in the real-world setting, Dr. Ogu said.
At the end of the 10-month study period, 21% of the patients were actively taking the medication, she said. “Forty-three percent had discontinued the medication, and 33% never filled the prescriptions; 4% had received but never started Endari,” Dr. Ogu said.
Of the patients who never filled the prescriptions, Dr. Ogu reported that 27% said their insurer denied prior authorization, 19% said their deductible was too high, and 16% cited other insurance issues.
“So we can see that insurance alone accounted for over 60% of why patients did not receive or could not start the medication,” she said.
Most patients – 94% – either had Medicare or Medicaid; the remainder had private insurance.
Among the 43% of all study patients who stopped taking the medication, reasons given include poor adherence (47%), side effects (9%), pregnancy and breast feeding (5%), and no perceived benefit (5%), Dr. Ogu said. At the outset, pharmacy records estimated adherence at 74% by using the average MPR, a rate similar to the phase 3 trial adherence rate of 77.4%.
Patient education is important to eliminate these barriers to treatment for SCD, Dr. Ogu said. “The patients need to understand why they’re taking whatever medication you prescribe. We need to educate them about the side effects, and we need to make them understand why it’s important to take certain medications or how they’re going to help them,” she said.
But even more important, she added, is a systems-based method to deal with insurance barriers. “If 62% of the patients did not get the medication due to insurance issues, I don’t think we’re doing a good job of making it accessible to them.”
Dr. Ogu reported a financial relationship with Vertex.
SOURCE: Ogu U et al. FSCDR 2019, Abstract JSCDH-D-19-00041.
REPORTING FROM FSCDR 2019
Sarcoma—rare, but not insignificant
This year, progress in treating rare cancers has been named the advance of the year by the American Society of Clinical Oncology (ASCO). Advancements in treating desmoid tumors, a subtype of sarcoma, was highlighted as one of the prominent breakthroughs for a rare cancer. While sarcoma is statistically rare, the impact of the disease is great, particularly on patients and families. ASCO’s recognition of rare cancer advancements demonstrates what the sarcoma community has long known: that “rare” shouldn’t mean unimportant or overlooked. In fact, the contributions of families, patients, caregivers, clinicians, researchers, foundations, organizations, and agencies in bringing sarcoma to the forefront and giving it prominence—spending time, effort, and energy in finding effective treatments—is of utmost importance, despite the disease’s rarity.
The Sarcoma Foundation of America (SFA) is leading the race to cure sarcoma, and it is doing so through research, advocacy, and education. Since its founding in 2001, donors to the foundation have funded over $9 million in research, with almost $2 million to be invested in research projects this year alone. The SFA supports research focused on discovering and developing new and effective therapies to treat and eradicate sarcoma—often highrisk, high-reward projects that would not likely be funded by the government or commercial interests. Driving the research agenda are members of its Medical Advisory Board—some of the brightest scientific minds in the world today, several of whom also serve on the Editorial Advisory Board of this, the SFA’s official journal. We are thankful for their dedication. Together, their efforts will continue to make a difference in the lives of those impacted by sarcoma.
The Sarcoma Foundation of America
CureSarcoma.org
This year, progress in treating rare cancers has been named the advance of the year by the American Society of Clinical Oncology (ASCO). Advancements in treating desmoid tumors, a subtype of sarcoma, was highlighted as one of the prominent breakthroughs for a rare cancer. While sarcoma is statistically rare, the impact of the disease is great, particularly on patients and families. ASCO’s recognition of rare cancer advancements demonstrates what the sarcoma community has long known: that “rare” shouldn’t mean unimportant or overlooked. In fact, the contributions of families, patients, caregivers, clinicians, researchers, foundations, organizations, and agencies in bringing sarcoma to the forefront and giving it prominence—spending time, effort, and energy in finding effective treatments—is of utmost importance, despite the disease’s rarity.
The Sarcoma Foundation of America (SFA) is leading the race to cure sarcoma, and it is doing so through research, advocacy, and education. Since its founding in 2001, donors to the foundation have funded over $9 million in research, with almost $2 million to be invested in research projects this year alone. The SFA supports research focused on discovering and developing new and effective therapies to treat and eradicate sarcoma—often highrisk, high-reward projects that would not likely be funded by the government or commercial interests. Driving the research agenda are members of its Medical Advisory Board—some of the brightest scientific minds in the world today, several of whom also serve on the Editorial Advisory Board of this, the SFA’s official journal. We are thankful for their dedication. Together, their efforts will continue to make a difference in the lives of those impacted by sarcoma.
The Sarcoma Foundation of America
CureSarcoma.org
This year, progress in treating rare cancers has been named the advance of the year by the American Society of Clinical Oncology (ASCO). Advancements in treating desmoid tumors, a subtype of sarcoma, was highlighted as one of the prominent breakthroughs for a rare cancer. While sarcoma is statistically rare, the impact of the disease is great, particularly on patients and families. ASCO’s recognition of rare cancer advancements demonstrates what the sarcoma community has long known: that “rare” shouldn’t mean unimportant or overlooked. In fact, the contributions of families, patients, caregivers, clinicians, researchers, foundations, organizations, and agencies in bringing sarcoma to the forefront and giving it prominence—spending time, effort, and energy in finding effective treatments—is of utmost importance, despite the disease’s rarity.
The Sarcoma Foundation of America (SFA) is leading the race to cure sarcoma, and it is doing so through research, advocacy, and education. Since its founding in 2001, donors to the foundation have funded over $9 million in research, with almost $2 million to be invested in research projects this year alone. The SFA supports research focused on discovering and developing new and effective therapies to treat and eradicate sarcoma—often highrisk, high-reward projects that would not likely be funded by the government or commercial interests. Driving the research agenda are members of its Medical Advisory Board—some of the brightest scientific minds in the world today, several of whom also serve on the Editorial Advisory Board of this, the SFA’s official journal. We are thankful for their dedication. Together, their efforts will continue to make a difference in the lives of those impacted by sarcoma.
The Sarcoma Foundation of America
CureSarcoma.org
Physical activity prevalence shows urban/rural divide
according to the Centers for Disease Control and Prevention.
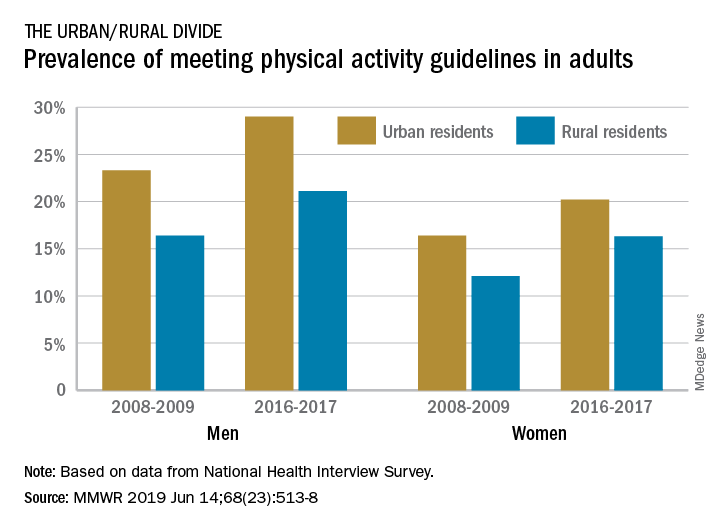
The prevalence of meeting the aerobic and muscle-strengthening recommendations in the 2008 Physical Activity Guidelines for Americans rose from 18.2% of adults in 2008 to 24.3% in 2017, but despite that increase, “insufficient participation in physical activity remains a public health concern,” Geoffrey P. Whitfield, PhD, and his associates said in the Morbidity and Mortality Weekly Report.
There was progress among both urban and rural residents, but those in rural areas were behind at the start of the study period in 2008 and remained behind in 2017. The prevalence of meeting the activity guideline started at 13.3% for rural residents and 19.4% for urbanites and rose to 19.6% and 25.3%, respectively, in 2017 – that’s an annual percentage point change of 0.5% for each population, the investigators reported. Rates among women were well below those of men in both populations.
Rural communities may lack the infrastructure, such as sidewalks, schoolyards, and parks, to support physical activities, or rural residents may get more exercise through occupational and domestic tasks, rather than through the leisure-time activities that are the focus of the National Health Interview Survey, which was the source of the study data, Dr. Whitfield and his associates suggested.
The 2008 federal guidelines recommend that adults get 150-300 minutes of moderate-intensity or 75-150 minutes of vigorous-intensity aerobic physical activity per week, along with muscle-strengthening activities of at least moderate intensity involving all major muscle groups on 2 or more days each week.
SOURCE: Whitfield GP et al. MMWR. 2019 Jun 14;68(23):514-8.
according to the Centers for Disease Control and Prevention.

The prevalence of meeting the aerobic and muscle-strengthening recommendations in the 2008 Physical Activity Guidelines for Americans rose from 18.2% of adults in 2008 to 24.3% in 2017, but despite that increase, “insufficient participation in physical activity remains a public health concern,” Geoffrey P. Whitfield, PhD, and his associates said in the Morbidity and Mortality Weekly Report.
There was progress among both urban and rural residents, but those in rural areas were behind at the start of the study period in 2008 and remained behind in 2017. The prevalence of meeting the activity guideline started at 13.3% for rural residents and 19.4% for urbanites and rose to 19.6% and 25.3%, respectively, in 2017 – that’s an annual percentage point change of 0.5% for each population, the investigators reported. Rates among women were well below those of men in both populations.
Rural communities may lack the infrastructure, such as sidewalks, schoolyards, and parks, to support physical activities, or rural residents may get more exercise through occupational and domestic tasks, rather than through the leisure-time activities that are the focus of the National Health Interview Survey, which was the source of the study data, Dr. Whitfield and his associates suggested.
The 2008 federal guidelines recommend that adults get 150-300 minutes of moderate-intensity or 75-150 minutes of vigorous-intensity aerobic physical activity per week, along with muscle-strengthening activities of at least moderate intensity involving all major muscle groups on 2 or more days each week.
SOURCE: Whitfield GP et al. MMWR. 2019 Jun 14;68(23):514-8.
according to the Centers for Disease Control and Prevention.

The prevalence of meeting the aerobic and muscle-strengthening recommendations in the 2008 Physical Activity Guidelines for Americans rose from 18.2% of adults in 2008 to 24.3% in 2017, but despite that increase, “insufficient participation in physical activity remains a public health concern,” Geoffrey P. Whitfield, PhD, and his associates said in the Morbidity and Mortality Weekly Report.
There was progress among both urban and rural residents, but those in rural areas were behind at the start of the study period in 2008 and remained behind in 2017. The prevalence of meeting the activity guideline started at 13.3% for rural residents and 19.4% for urbanites and rose to 19.6% and 25.3%, respectively, in 2017 – that’s an annual percentage point change of 0.5% for each population, the investigators reported. Rates among women were well below those of men in both populations.
Rural communities may lack the infrastructure, such as sidewalks, schoolyards, and parks, to support physical activities, or rural residents may get more exercise through occupational and domestic tasks, rather than through the leisure-time activities that are the focus of the National Health Interview Survey, which was the source of the study data, Dr. Whitfield and his associates suggested.
The 2008 federal guidelines recommend that adults get 150-300 minutes of moderate-intensity or 75-150 minutes of vigorous-intensity aerobic physical activity per week, along with muscle-strengthening activities of at least moderate intensity involving all major muscle groups on 2 or more days each week.
SOURCE: Whitfield GP et al. MMWR. 2019 Jun 14;68(23):514-8.
FROM MMWR
‘Encouraging’ responses seen with durvalumab plus R-CHOP in DLBCL
CHICAGO – A six-drug combination produced complete responses in previously untreated, high-risk diffuse large B-cell lymphoma (DLBCL) patients in a phase 2 trial.
Induction with durvalumab and R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) produced complete response rates of 54% in the entire cohort and 41% in patients with double- or triple-hit lymphoma. Immune-related adverse events (AEs) were common with this regimen, but no unexpected AEs occurred, according to researchers.
Grzegorz S. Nowakowski, MD, of the Mayo Clinic in Rochester, Minn., and colleagues presented these results in a poster at the annual meeting of the American Society of Clinical Oncology.
Treatment
The phase 2 trial (NCT03003520) was designed to assess durvalumab plus R-CHOP as well as durvalumab plus R-CHOP and lenalidomide (R2-CHOP) in patients with previously untreated, high-risk DLBCL. However, the R2-CHOP arm was closed early.
In cycle one, all patients received durvalumab plus R-CHOP. For subsequent cycles, patients with activated B-cell (ABC) DLBCL were assigned to durvalumab plus R2-CHOP, while patients with non-ABC DLBCL continued on durvalumab plus R-CHOP.
The R2-CHOP arm was closed early due to safety issues observed in trials combining checkpoint inhibitors with immunomodulatory agents. A partial clinical hold was placed on the R2-CHOP arm, but patients could continue on the regimen if they experienced a clinical benefit. Any patients with ABC DLBCL who were enrolled after the partial hold received treatment with durvalumab plus R-CHOP.
Induction was given for up to eight cycles and was followed by consolidation with durvalumab alone for up to 12 months from the start of induction.
Patient characteristics
The researchers presented data on 43 patients in the durvalumab plus R-CHOP arm. The patients’ median age was 62 years, and 61% were men.
“I think it’s worth noting that 46% of patients in the durvalumab plus R-CHOP group had very high-risk features, including double-hit or triple-hit genetic features,” said Justin Kline, MD, of the University of Chicago Medicine who reviewed this study in a poster discussion session.
Specifically, 30% of patients had double-hit lymphoma, and 16% had triple-hit lymphoma. Most patients had a high-intermediate-risk (49%) or high-risk (21%) International Prognostic Index score, and 79% of patients had Ann Arbor stage IV disease.
Efficacy
As of Aug. 2, 2018, 70% of patients had completed induction, 2% had completed consolidation, 44% remained on treatment, and 54% had discontinued therapy. The most common reasons for stopping treatment were progression (16%), AEs (14%), and consent withdrawal (12%).
“The combination of durvalumab plus R-CHOP demonstrated encouraging response rates … in subjects with high-risk DLBCL, including double- and triple-hit lymphomas,” Dr. Kline said.
The complete response rate was 54% (20/37) at the end of induction and 68% (n = 25) at the end of consolidation. The partial response rate at the end of consolidation was 30% (n = 11).
In patients with double- or triple-hit lymphoma, the complete response rate at the end of induction was 41% (7/17). The overall response rate in this group was 88% (n = 15).
Safety
“The safety profile was as expected for the components of the combination, and no new safety signals were observed,” Dr. Kline said.
He noted that AEs of special interest, or immune-related AEs, occurred in 61% of patients, but most of these events were grade 1 or 2.
AEs of special interest included diarrhea (28%), rash (23%), infusion-related reactions (16%), dermatitis (12%), hypothyroidism (5%), myocarditis (5%), adrenal insufficiency (2%), and hepatitis (2%).
Grade 3 or 4 AEs of special interest included infusion-related reactions (5%), rash (2%), diarrhea (2%), and hepatitis (2%).
The safety and efficacy results support further evaluation of durvalumab plus R-CHOP, although it will be important to identify DLBCL patients who are more likely to derive a clinical benefit from PD-1 or PD-L1 blockade, Dr. Kline said.
“This early study showed that the combination is feasible,” Dr. Nowakowski added. “I think, down the road, we’ll need to identify patients who can actually benefit from this combination. We definitely have clinical evidence of exceptional responses to PD-1 blockade.”
The trial was sponsored by Celgene. Dr. Nowakowski reported relationships with Celgene, Genentech, MorphoSys, and NanoString Technologies. Dr. Kline reported relationships with Cardinal Health, Merck, Seattle Genetics, Kite/Gilead, ITeos Therapeutics, and Bristol-Myers Squibb.
SOURCE: Nowakowski GS et al. ASCO 2019, Abstract 7520.
CHICAGO – A six-drug combination produced complete responses in previously untreated, high-risk diffuse large B-cell lymphoma (DLBCL) patients in a phase 2 trial.
Induction with durvalumab and R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) produced complete response rates of 54% in the entire cohort and 41% in patients with double- or triple-hit lymphoma. Immune-related adverse events (AEs) were common with this regimen, but no unexpected AEs occurred, according to researchers.
Grzegorz S. Nowakowski, MD, of the Mayo Clinic in Rochester, Minn., and colleagues presented these results in a poster at the annual meeting of the American Society of Clinical Oncology.
Treatment
The phase 2 trial (NCT03003520) was designed to assess durvalumab plus R-CHOP as well as durvalumab plus R-CHOP and lenalidomide (R2-CHOP) in patients with previously untreated, high-risk DLBCL. However, the R2-CHOP arm was closed early.
In cycle one, all patients received durvalumab plus R-CHOP. For subsequent cycles, patients with activated B-cell (ABC) DLBCL were assigned to durvalumab plus R2-CHOP, while patients with non-ABC DLBCL continued on durvalumab plus R-CHOP.
The R2-CHOP arm was closed early due to safety issues observed in trials combining checkpoint inhibitors with immunomodulatory agents. A partial clinical hold was placed on the R2-CHOP arm, but patients could continue on the regimen if they experienced a clinical benefit. Any patients with ABC DLBCL who were enrolled after the partial hold received treatment with durvalumab plus R-CHOP.
Induction was given for up to eight cycles and was followed by consolidation with durvalumab alone for up to 12 months from the start of induction.
Patient characteristics
The researchers presented data on 43 patients in the durvalumab plus R-CHOP arm. The patients’ median age was 62 years, and 61% were men.
“I think it’s worth noting that 46% of patients in the durvalumab plus R-CHOP group had very high-risk features, including double-hit or triple-hit genetic features,” said Justin Kline, MD, of the University of Chicago Medicine who reviewed this study in a poster discussion session.
Specifically, 30% of patients had double-hit lymphoma, and 16% had triple-hit lymphoma. Most patients had a high-intermediate-risk (49%) or high-risk (21%) International Prognostic Index score, and 79% of patients had Ann Arbor stage IV disease.
Efficacy
As of Aug. 2, 2018, 70% of patients had completed induction, 2% had completed consolidation, 44% remained on treatment, and 54% had discontinued therapy. The most common reasons for stopping treatment were progression (16%), AEs (14%), and consent withdrawal (12%).
“The combination of durvalumab plus R-CHOP demonstrated encouraging response rates … in subjects with high-risk DLBCL, including double- and triple-hit lymphomas,” Dr. Kline said.
The complete response rate was 54% (20/37) at the end of induction and 68% (n = 25) at the end of consolidation. The partial response rate at the end of consolidation was 30% (n = 11).
In patients with double- or triple-hit lymphoma, the complete response rate at the end of induction was 41% (7/17). The overall response rate in this group was 88% (n = 15).
Safety
“The safety profile was as expected for the components of the combination, and no new safety signals were observed,” Dr. Kline said.
He noted that AEs of special interest, or immune-related AEs, occurred in 61% of patients, but most of these events were grade 1 or 2.
AEs of special interest included diarrhea (28%), rash (23%), infusion-related reactions (16%), dermatitis (12%), hypothyroidism (5%), myocarditis (5%), adrenal insufficiency (2%), and hepatitis (2%).
Grade 3 or 4 AEs of special interest included infusion-related reactions (5%), rash (2%), diarrhea (2%), and hepatitis (2%).
The safety and efficacy results support further evaluation of durvalumab plus R-CHOP, although it will be important to identify DLBCL patients who are more likely to derive a clinical benefit from PD-1 or PD-L1 blockade, Dr. Kline said.
“This early study showed that the combination is feasible,” Dr. Nowakowski added. “I think, down the road, we’ll need to identify patients who can actually benefit from this combination. We definitely have clinical evidence of exceptional responses to PD-1 blockade.”
The trial was sponsored by Celgene. Dr. Nowakowski reported relationships with Celgene, Genentech, MorphoSys, and NanoString Technologies. Dr. Kline reported relationships with Cardinal Health, Merck, Seattle Genetics, Kite/Gilead, ITeos Therapeutics, and Bristol-Myers Squibb.
SOURCE: Nowakowski GS et al. ASCO 2019, Abstract 7520.
CHICAGO – A six-drug combination produced complete responses in previously untreated, high-risk diffuse large B-cell lymphoma (DLBCL) patients in a phase 2 trial.
Induction with durvalumab and R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) produced complete response rates of 54% in the entire cohort and 41% in patients with double- or triple-hit lymphoma. Immune-related adverse events (AEs) were common with this regimen, but no unexpected AEs occurred, according to researchers.
Grzegorz S. Nowakowski, MD, of the Mayo Clinic in Rochester, Minn., and colleagues presented these results in a poster at the annual meeting of the American Society of Clinical Oncology.
Treatment
The phase 2 trial (NCT03003520) was designed to assess durvalumab plus R-CHOP as well as durvalumab plus R-CHOP and lenalidomide (R2-CHOP) in patients with previously untreated, high-risk DLBCL. However, the R2-CHOP arm was closed early.
In cycle one, all patients received durvalumab plus R-CHOP. For subsequent cycles, patients with activated B-cell (ABC) DLBCL were assigned to durvalumab plus R2-CHOP, while patients with non-ABC DLBCL continued on durvalumab plus R-CHOP.
The R2-CHOP arm was closed early due to safety issues observed in trials combining checkpoint inhibitors with immunomodulatory agents. A partial clinical hold was placed on the R2-CHOP arm, but patients could continue on the regimen if they experienced a clinical benefit. Any patients with ABC DLBCL who were enrolled after the partial hold received treatment with durvalumab plus R-CHOP.
Induction was given for up to eight cycles and was followed by consolidation with durvalumab alone for up to 12 months from the start of induction.
Patient characteristics
The researchers presented data on 43 patients in the durvalumab plus R-CHOP arm. The patients’ median age was 62 years, and 61% were men.
“I think it’s worth noting that 46% of patients in the durvalumab plus R-CHOP group had very high-risk features, including double-hit or triple-hit genetic features,” said Justin Kline, MD, of the University of Chicago Medicine who reviewed this study in a poster discussion session.
Specifically, 30% of patients had double-hit lymphoma, and 16% had triple-hit lymphoma. Most patients had a high-intermediate-risk (49%) or high-risk (21%) International Prognostic Index score, and 79% of patients had Ann Arbor stage IV disease.
Efficacy
As of Aug. 2, 2018, 70% of patients had completed induction, 2% had completed consolidation, 44% remained on treatment, and 54% had discontinued therapy. The most common reasons for stopping treatment were progression (16%), AEs (14%), and consent withdrawal (12%).
“The combination of durvalumab plus R-CHOP demonstrated encouraging response rates … in subjects with high-risk DLBCL, including double- and triple-hit lymphomas,” Dr. Kline said.
The complete response rate was 54% (20/37) at the end of induction and 68% (n = 25) at the end of consolidation. The partial response rate at the end of consolidation was 30% (n = 11).
In patients with double- or triple-hit lymphoma, the complete response rate at the end of induction was 41% (7/17). The overall response rate in this group was 88% (n = 15).
Safety
“The safety profile was as expected for the components of the combination, and no new safety signals were observed,” Dr. Kline said.
He noted that AEs of special interest, or immune-related AEs, occurred in 61% of patients, but most of these events were grade 1 or 2.
AEs of special interest included diarrhea (28%), rash (23%), infusion-related reactions (16%), dermatitis (12%), hypothyroidism (5%), myocarditis (5%), adrenal insufficiency (2%), and hepatitis (2%).
Grade 3 or 4 AEs of special interest included infusion-related reactions (5%), rash (2%), diarrhea (2%), and hepatitis (2%).
The safety and efficacy results support further evaluation of durvalumab plus R-CHOP, although it will be important to identify DLBCL patients who are more likely to derive a clinical benefit from PD-1 or PD-L1 blockade, Dr. Kline said.
“This early study showed that the combination is feasible,” Dr. Nowakowski added. “I think, down the road, we’ll need to identify patients who can actually benefit from this combination. We definitely have clinical evidence of exceptional responses to PD-1 blockade.”
The trial was sponsored by Celgene. Dr. Nowakowski reported relationships with Celgene, Genentech, MorphoSys, and NanoString Technologies. Dr. Kline reported relationships with Cardinal Health, Merck, Seattle Genetics, Kite/Gilead, ITeos Therapeutics, and Bristol-Myers Squibb.
SOURCE: Nowakowski GS et al. ASCO 2019, Abstract 7520.
REPORTING FROM ASCO 2019
COPD exacerbations associated with poor sleep quality
in an 18-month prospective study of 480 patients.
“Poor sleep quality in COPD has previously been associated with reduced health-related quality of life and reduced physical activity during the day,” wrote Matthew Shorofsky, MD, of McGill University, Montreal, and associates. Their report is in CHEST. “However, to our knowledge, this is the first population-based longitudinal study evaluating exacerbation risk in relation to subjective sleep disturbances and assessing previously diagnosed and undiagnosed COPD.”
The study included participants enrolled in the Canadian Respiratory Research Network and the Canadian Cohort Obstructive Lung Disease (CanCOLD) study who had COPD, available baseline PSQI scores, and 18 months of follow-up data. The PSQI includes 19 questions on sleep quality, latency, duration, efficiency, disturbances, use of sleep medications, and daytime dysfunction. Total score ranges between 0 and 21, and a score above 5 is considered poor sleep. Online patient surveys and quarterly phone interviews were used to track symptom-based exacerbations (at least 48 hours of increased dyspnea, sputum volume, or sputum purulence) and event-based exacerbations (a symptom-based exacerbation plus the use antibiotics or corticosteroids or health services).
At baseline, 203 patients met the PSQI threshold for poor sleep quality. During follow-up, 185 patients had at least one COPD exacerbation. Poor sleep at baseline was significantly more prevalent among patients with symptoms-based COPD exacerbations (50.3%) than among patients without symptoms-based exacerbations (37.3%; P = .01). Poor baseline sleep quality remained a significant risk factor for symptom-based exacerbations of COPD even after the researchers accounted for the effect of age, gender, body mass index, smoking, depression, angina, baseline inhaled respiratory medications, forced expiratory volume in 1 second %predicted, and modified Medical Research Council (mMRC) dyspnea scale (adjusted risk ratio, 1.09; 95% confidence interval, 1.01-1.18; P =.02).
Patients with at least one symptomatic exacerbation of COPD were significantly more likely to meet the threshold for poor sleep quality on the Pittsburgh Sleep Quality Index and have significantly higher median PSQI scores compared with patients without exacerbations (6.0 [interquartile range, 3.0 to 8.0] vs. 5.0 [2.0 to 7.0]; P = .01). Poor baseline sleep quality also was associated with event-based exacerbations and with a shorter time to symptoms-based exacerbations. Sleep disturbances, such as rising to void or experiencing respiratory issues or pain during sleep, correlated most strongly with symptoms-based exacerbations.
Several factors could explain the link between poor sleep quality and COPD exacerbations, the investigators wrote. Patients with inadequately controlled COPD have more frequent and unstable respiratory symptoms, which could disrupt sleep either directly or indirectly (secondary to medication use or anxiety, for example). Conversely, sleep disruption can impede immune function and increase systemic inflammation, which might worsen COPD control and increase exacerbation risk. Poor sleep can impair memory and cognition, “potentially fostering medication nonadherence and symptom flare-up, especially in the older COPD population.” Although the link is poorly understood, patients with COPD often have comorbid obstructive sleep apnea (OSA), which is associated with COPD exacerbations, the researchers wrote. Treating OSA is associated with improved COPD morbidity and fewer exacerbations and hospitalizations.
The researchers acknowledged limitations to their study design. “Individuals with asthma or other obstructive lung diseases could not be definitively excluded; methacholine challenges were not performed. However, analyses excluding self-reported asthma were consistent with our main results. Second, because definitions of COPD exacerbation vary among studies, comparison may be limited, but CanCOLD used a standard definition, as recommended by GOLD.”
The CanCOLD study has received funding from the Canadian Respiratory Research Network, Astra Zeneca Canada, Boehringer Ingelheim Canada, GlaxoSmithKline Canada, Novartis, Merck Nycomed, Pfizer Canada, and Theratechnologies. Dr. Shorofsky had no disclosures. Several coinvestigators reported ties to GlaxoSmithKline, Novartis, Boehringer Ingelheim, Merck, Almirall, and Theratechnologies.
SOURCE: Shorofsky M et al. CHEST. 2019 May 28. doi: 10.1016/j.chest.2019.04.132.
in an 18-month prospective study of 480 patients.
“Poor sleep quality in COPD has previously been associated with reduced health-related quality of life and reduced physical activity during the day,” wrote Matthew Shorofsky, MD, of McGill University, Montreal, and associates. Their report is in CHEST. “However, to our knowledge, this is the first population-based longitudinal study evaluating exacerbation risk in relation to subjective sleep disturbances and assessing previously diagnosed and undiagnosed COPD.”
The study included participants enrolled in the Canadian Respiratory Research Network and the Canadian Cohort Obstructive Lung Disease (CanCOLD) study who had COPD, available baseline PSQI scores, and 18 months of follow-up data. The PSQI includes 19 questions on sleep quality, latency, duration, efficiency, disturbances, use of sleep medications, and daytime dysfunction. Total score ranges between 0 and 21, and a score above 5 is considered poor sleep. Online patient surveys and quarterly phone interviews were used to track symptom-based exacerbations (at least 48 hours of increased dyspnea, sputum volume, or sputum purulence) and event-based exacerbations (a symptom-based exacerbation plus the use antibiotics or corticosteroids or health services).
At baseline, 203 patients met the PSQI threshold for poor sleep quality. During follow-up, 185 patients had at least one COPD exacerbation. Poor sleep at baseline was significantly more prevalent among patients with symptoms-based COPD exacerbations (50.3%) than among patients without symptoms-based exacerbations (37.3%; P = .01). Poor baseline sleep quality remained a significant risk factor for symptom-based exacerbations of COPD even after the researchers accounted for the effect of age, gender, body mass index, smoking, depression, angina, baseline inhaled respiratory medications, forced expiratory volume in 1 second %predicted, and modified Medical Research Council (mMRC) dyspnea scale (adjusted risk ratio, 1.09; 95% confidence interval, 1.01-1.18; P =.02).
Patients with at least one symptomatic exacerbation of COPD were significantly more likely to meet the threshold for poor sleep quality on the Pittsburgh Sleep Quality Index and have significantly higher median PSQI scores compared with patients without exacerbations (6.0 [interquartile range, 3.0 to 8.0] vs. 5.0 [2.0 to 7.0]; P = .01). Poor baseline sleep quality also was associated with event-based exacerbations and with a shorter time to symptoms-based exacerbations. Sleep disturbances, such as rising to void or experiencing respiratory issues or pain during sleep, correlated most strongly with symptoms-based exacerbations.
Several factors could explain the link between poor sleep quality and COPD exacerbations, the investigators wrote. Patients with inadequately controlled COPD have more frequent and unstable respiratory symptoms, which could disrupt sleep either directly or indirectly (secondary to medication use or anxiety, for example). Conversely, sleep disruption can impede immune function and increase systemic inflammation, which might worsen COPD control and increase exacerbation risk. Poor sleep can impair memory and cognition, “potentially fostering medication nonadherence and symptom flare-up, especially in the older COPD population.” Although the link is poorly understood, patients with COPD often have comorbid obstructive sleep apnea (OSA), which is associated with COPD exacerbations, the researchers wrote. Treating OSA is associated with improved COPD morbidity and fewer exacerbations and hospitalizations.
The researchers acknowledged limitations to their study design. “Individuals with asthma or other obstructive lung diseases could not be definitively excluded; methacholine challenges were not performed. However, analyses excluding self-reported asthma were consistent with our main results. Second, because definitions of COPD exacerbation vary among studies, comparison may be limited, but CanCOLD used a standard definition, as recommended by GOLD.”
The CanCOLD study has received funding from the Canadian Respiratory Research Network, Astra Zeneca Canada, Boehringer Ingelheim Canada, GlaxoSmithKline Canada, Novartis, Merck Nycomed, Pfizer Canada, and Theratechnologies. Dr. Shorofsky had no disclosures. Several coinvestigators reported ties to GlaxoSmithKline, Novartis, Boehringer Ingelheim, Merck, Almirall, and Theratechnologies.
SOURCE: Shorofsky M et al. CHEST. 2019 May 28. doi: 10.1016/j.chest.2019.04.132.
in an 18-month prospective study of 480 patients.
“Poor sleep quality in COPD has previously been associated with reduced health-related quality of life and reduced physical activity during the day,” wrote Matthew Shorofsky, MD, of McGill University, Montreal, and associates. Their report is in CHEST. “However, to our knowledge, this is the first population-based longitudinal study evaluating exacerbation risk in relation to subjective sleep disturbances and assessing previously diagnosed and undiagnosed COPD.”
The study included participants enrolled in the Canadian Respiratory Research Network and the Canadian Cohort Obstructive Lung Disease (CanCOLD) study who had COPD, available baseline PSQI scores, and 18 months of follow-up data. The PSQI includes 19 questions on sleep quality, latency, duration, efficiency, disturbances, use of sleep medications, and daytime dysfunction. Total score ranges between 0 and 21, and a score above 5 is considered poor sleep. Online patient surveys and quarterly phone interviews were used to track symptom-based exacerbations (at least 48 hours of increased dyspnea, sputum volume, or sputum purulence) and event-based exacerbations (a symptom-based exacerbation plus the use antibiotics or corticosteroids or health services).
At baseline, 203 patients met the PSQI threshold for poor sleep quality. During follow-up, 185 patients had at least one COPD exacerbation. Poor sleep at baseline was significantly more prevalent among patients with symptoms-based COPD exacerbations (50.3%) than among patients without symptoms-based exacerbations (37.3%; P = .01). Poor baseline sleep quality remained a significant risk factor for symptom-based exacerbations of COPD even after the researchers accounted for the effect of age, gender, body mass index, smoking, depression, angina, baseline inhaled respiratory medications, forced expiratory volume in 1 second %predicted, and modified Medical Research Council (mMRC) dyspnea scale (adjusted risk ratio, 1.09; 95% confidence interval, 1.01-1.18; P =.02).
Patients with at least one symptomatic exacerbation of COPD were significantly more likely to meet the threshold for poor sleep quality on the Pittsburgh Sleep Quality Index and have significantly higher median PSQI scores compared with patients without exacerbations (6.0 [interquartile range, 3.0 to 8.0] vs. 5.0 [2.0 to 7.0]; P = .01). Poor baseline sleep quality also was associated with event-based exacerbations and with a shorter time to symptoms-based exacerbations. Sleep disturbances, such as rising to void or experiencing respiratory issues or pain during sleep, correlated most strongly with symptoms-based exacerbations.
Several factors could explain the link between poor sleep quality and COPD exacerbations, the investigators wrote. Patients with inadequately controlled COPD have more frequent and unstable respiratory symptoms, which could disrupt sleep either directly or indirectly (secondary to medication use or anxiety, for example). Conversely, sleep disruption can impede immune function and increase systemic inflammation, which might worsen COPD control and increase exacerbation risk. Poor sleep can impair memory and cognition, “potentially fostering medication nonadherence and symptom flare-up, especially in the older COPD population.” Although the link is poorly understood, patients with COPD often have comorbid obstructive sleep apnea (OSA), which is associated with COPD exacerbations, the researchers wrote. Treating OSA is associated with improved COPD morbidity and fewer exacerbations and hospitalizations.
The researchers acknowledged limitations to their study design. “Individuals with asthma or other obstructive lung diseases could not be definitively excluded; methacholine challenges were not performed. However, analyses excluding self-reported asthma were consistent with our main results. Second, because definitions of COPD exacerbation vary among studies, comparison may be limited, but CanCOLD used a standard definition, as recommended by GOLD.”
The CanCOLD study has received funding from the Canadian Respiratory Research Network, Astra Zeneca Canada, Boehringer Ingelheim Canada, GlaxoSmithKline Canada, Novartis, Merck Nycomed, Pfizer Canada, and Theratechnologies. Dr. Shorofsky had no disclosures. Several coinvestigators reported ties to GlaxoSmithKline, Novartis, Boehringer Ingelheim, Merck, Almirall, and Theratechnologies.
SOURCE: Shorofsky M et al. CHEST. 2019 May 28. doi: 10.1016/j.chest.2019.04.132.
FROM CHEST
Niraparib-pembrolizumab combo finds niche in breast, ovarian cancers
The strategy of simultaneously exploiting deficient DNA damage repair and unleashing the immune response could expand treatment options for hard-to-treat breast and ovarian cancers, findings of the TOPACIO/KEYNOTE-162 trial suggest.
Triple-negative breast cancer (TNBC) and high-grade serous ovarian carcinoma share a number of genomic features, including a high frequency of BRCA1 and BRCA2 inactivation (Nature. 2012;490:61-70), as well as potential immunoreactivity (Lancet Oncol. 2018;19:40-50).
The open-label, single-arm phase 1/2 trial therefore tested the combination of niraparib (Zejula), an oral poly (ADP-ribose) polymerase (PARP) inhibitor, and pembrolizumab (Keytruda), an antibody to programmed death 1 (PD-1), among more than 100 patients with advanced or metastatic TNBC or recurrent platinum-resistant ovarian carcinoma. Patients were enrolled irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression.
Main results, reported in JAMA Oncology, showed that the combination was safe, and about a fifth of patients with each type of cancer had an objective response. Median progression-free survival (PFS) was about 2 months in those with TNBC overall (although it exceeded 8 months in the subset with a tumor BRCA mutation) and about 3 months in those with ovarian cancer.
TNBC cohort
Investigators led by Shaveta Vinayak, MD, of the division of oncology at Fred Hutchinson Cancer Research Center, and University of Washington School of Medicine, Seattle Cancer Care Alliance, Seattle, studied 55 patients with TNBC treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 47 patients, the objective response rate (ORR) was 21%, and the disease control rate (DCR) was 49%. With a median duration of follow-up of 14.8 months, the median duration of response was not reached.
Activity of the combination varied by tumor BRCA mutation status. Compared with counterparts having BRCA wild-type tumors, patients having tumors with BRCA mutations had a numerically higher ORR (47% vs. 11%), DCR (80% vs. 33%), and PFS (8.3 vs. 2.1 months).
Some 18% of patients had treatment-related anemia, 15% thrombocytopenia, and 7% fatigue. In addition, 15% of patients had immune-related adverse events, with 4% having grade 3 immune-related adverse events.
“Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations,” Dr. Vinayak and colleagues conclude. “The combination therapy was safe with a tolerable safety profile, warranting further investigation.”
Ovarian cancer cohort
Investigators led by Panagiotis A. Konstantinopoulos, MD, PhD, of the division of gynecologic oncology, department of medical oncology at Dana-Farber Cancer Institute, Harvard Medical School, Boston, studied 62 patients with ovarian carcinoma treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 60 patients, the ORR was 18% and the DCR was 65%. The ORRs were similar regardless of patients’ platinum-based chemotherapy sensitivity, previous bevacizumab treatment, or tumor BRCA or homologous recombination deficiency (HRD) biomarker status.
With a median duration of follow-up of 12.4 months, the median duration of response was not reached, ranging from 4.2 to roughly 14.5 months. Median progression-free survival was 3.4 months.
The leading treatment-related adverse events of grade 3 or higher in this cohort were anemia (21%) and thrombocytopenia (9%). In addition, 19% of patients had immune-related adverse events, with 9% having grade 3 or higher immune-related adverse events.
“Niraparib in combination with pembrolizumab is tolerable, with promising antitumor activity for patients with ovarian carcinoma who have limited treatment options regardless of platinum status, biomarker status, or prior treatment with bevacizumab,” Dr. Konstantinopoulos and colleagues conclude. “Responses in patients without tumor BRCA mutations or non-HRD cancers were higher than expected with either agent as monotherapy.”
Dr. Vinayak disclosed receiving clinical trial funding from TESARO; serving on an advisory board for TESARO; and serving on an advisory board for OncoSec Medical (uncompensated). Dr. Konstantinopoulos disclosed serving on advisory boards for AstraZeneca, Pfizer, and Merck. The trial was supported by TESARO: a GSK company and Merck, and in part by Stand Up to Cancer (a program of the Entertainment Industry Foundation); the Ovarian Cancer Research Fund Alliance; and National Ovarian Cancer Coalition Dream Team Translational Research.
SOURCE: Vinayak A et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1029. Konstantinopoulos PA et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1048.
“Targeting DNA repair and immune checkpoint pathways has emerged as an important concept in cancer therapy, well supported by preclinical and clinical data in ovarian cancer and TNBC. However, there are some limitations to the two studies presented herein,” maintain editorialists Kunle Odunsi, MD, PhD, and Tanja Pejovic, MD, PhD.
Patients varied considerably with respect to number of prior chemotherapy regimens, they elaborate. Also, there may have been some misclassification of patients into DNA damage repair (DDR) groups, and small sample sizes precluded rigorous subgroup analyses.
“Because DDR and, by extension, tumor mutational burden and PD-L1 status do not fully explain the effects of the combination of PARP inhibitors and anti–PD-1 therapy, additional predictive biomarkers based on tumor intrinsic or adaptive mechanisms of resistance are needed for both cancer types,” the editorialists contend. In particular, knowledge of the tumor microenvironment could be used to tailor therapy for individual patients.
“The TOPACIO clinical studies are clearly steps in the right direction for patients with [platinum-resistant ovarian carcinoma] and TNBC,” they conclude. “However, larger confirmatory randomized clinical trials are needed that use panels of integrated biomarkers that would allow identification of patients most likely to respond.”
Dr. Odunsi is the deputy director and chair of the department of gynecologic oncology, executive director of the Center for Immunotherapy, and co-leader of the Tumor Immunology and Immunotherapy Research Program–Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Dr. Pejovic is associate professor, division of gynecologic oncology, department of obstetrics & gynecology, Knight Cancer Institute, Oregon Health & Science University, Portland, Ore. These remarks are adapted from a related editorial (JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1009 ).
“Targeting DNA repair and immune checkpoint pathways has emerged as an important concept in cancer therapy, well supported by preclinical and clinical data in ovarian cancer and TNBC. However, there are some limitations to the two studies presented herein,” maintain editorialists Kunle Odunsi, MD, PhD, and Tanja Pejovic, MD, PhD.
Patients varied considerably with respect to number of prior chemotherapy regimens, they elaborate. Also, there may have been some misclassification of patients into DNA damage repair (DDR) groups, and small sample sizes precluded rigorous subgroup analyses.
“Because DDR and, by extension, tumor mutational burden and PD-L1 status do not fully explain the effects of the combination of PARP inhibitors and anti–PD-1 therapy, additional predictive biomarkers based on tumor intrinsic or adaptive mechanisms of resistance are needed for both cancer types,” the editorialists contend. In particular, knowledge of the tumor microenvironment could be used to tailor therapy for individual patients.
“The TOPACIO clinical studies are clearly steps in the right direction for patients with [platinum-resistant ovarian carcinoma] and TNBC,” they conclude. “However, larger confirmatory randomized clinical trials are needed that use panels of integrated biomarkers that would allow identification of patients most likely to respond.”
Dr. Odunsi is the deputy director and chair of the department of gynecologic oncology, executive director of the Center for Immunotherapy, and co-leader of the Tumor Immunology and Immunotherapy Research Program–Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Dr. Pejovic is associate professor, division of gynecologic oncology, department of obstetrics & gynecology, Knight Cancer Institute, Oregon Health & Science University, Portland, Ore. These remarks are adapted from a related editorial (JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1009 ).
“Targeting DNA repair and immune checkpoint pathways has emerged as an important concept in cancer therapy, well supported by preclinical and clinical data in ovarian cancer and TNBC. However, there are some limitations to the two studies presented herein,” maintain editorialists Kunle Odunsi, MD, PhD, and Tanja Pejovic, MD, PhD.
Patients varied considerably with respect to number of prior chemotherapy regimens, they elaborate. Also, there may have been some misclassification of patients into DNA damage repair (DDR) groups, and small sample sizes precluded rigorous subgroup analyses.
“Because DDR and, by extension, tumor mutational burden and PD-L1 status do not fully explain the effects of the combination of PARP inhibitors and anti–PD-1 therapy, additional predictive biomarkers based on tumor intrinsic or adaptive mechanisms of resistance are needed for both cancer types,” the editorialists contend. In particular, knowledge of the tumor microenvironment could be used to tailor therapy for individual patients.
“The TOPACIO clinical studies are clearly steps in the right direction for patients with [platinum-resistant ovarian carcinoma] and TNBC,” they conclude. “However, larger confirmatory randomized clinical trials are needed that use panels of integrated biomarkers that would allow identification of patients most likely to respond.”
Dr. Odunsi is the deputy director and chair of the department of gynecologic oncology, executive director of the Center for Immunotherapy, and co-leader of the Tumor Immunology and Immunotherapy Research Program–Roswell Park Comprehensive Cancer Center, Buffalo, N.Y. Dr. Pejovic is associate professor, division of gynecologic oncology, department of obstetrics & gynecology, Knight Cancer Institute, Oregon Health & Science University, Portland, Ore. These remarks are adapted from a related editorial (JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1009 ).
The strategy of simultaneously exploiting deficient DNA damage repair and unleashing the immune response could expand treatment options for hard-to-treat breast and ovarian cancers, findings of the TOPACIO/KEYNOTE-162 trial suggest.
Triple-negative breast cancer (TNBC) and high-grade serous ovarian carcinoma share a number of genomic features, including a high frequency of BRCA1 and BRCA2 inactivation (Nature. 2012;490:61-70), as well as potential immunoreactivity (Lancet Oncol. 2018;19:40-50).
The open-label, single-arm phase 1/2 trial therefore tested the combination of niraparib (Zejula), an oral poly (ADP-ribose) polymerase (PARP) inhibitor, and pembrolizumab (Keytruda), an antibody to programmed death 1 (PD-1), among more than 100 patients with advanced or metastatic TNBC or recurrent platinum-resistant ovarian carcinoma. Patients were enrolled irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression.
Main results, reported in JAMA Oncology, showed that the combination was safe, and about a fifth of patients with each type of cancer had an objective response. Median progression-free survival (PFS) was about 2 months in those with TNBC overall (although it exceeded 8 months in the subset with a tumor BRCA mutation) and about 3 months in those with ovarian cancer.
TNBC cohort
Investigators led by Shaveta Vinayak, MD, of the division of oncology at Fred Hutchinson Cancer Research Center, and University of Washington School of Medicine, Seattle Cancer Care Alliance, Seattle, studied 55 patients with TNBC treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 47 patients, the objective response rate (ORR) was 21%, and the disease control rate (DCR) was 49%. With a median duration of follow-up of 14.8 months, the median duration of response was not reached.
Activity of the combination varied by tumor BRCA mutation status. Compared with counterparts having BRCA wild-type tumors, patients having tumors with BRCA mutations had a numerically higher ORR (47% vs. 11%), DCR (80% vs. 33%), and PFS (8.3 vs. 2.1 months).
Some 18% of patients had treatment-related anemia, 15% thrombocytopenia, and 7% fatigue. In addition, 15% of patients had immune-related adverse events, with 4% having grade 3 immune-related adverse events.
“Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations,” Dr. Vinayak and colleagues conclude. “The combination therapy was safe with a tolerable safety profile, warranting further investigation.”
Ovarian cancer cohort
Investigators led by Panagiotis A. Konstantinopoulos, MD, PhD, of the division of gynecologic oncology, department of medical oncology at Dana-Farber Cancer Institute, Harvard Medical School, Boston, studied 62 patients with ovarian carcinoma treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 60 patients, the ORR was 18% and the DCR was 65%. The ORRs were similar regardless of patients’ platinum-based chemotherapy sensitivity, previous bevacizumab treatment, or tumor BRCA or homologous recombination deficiency (HRD) biomarker status.
With a median duration of follow-up of 12.4 months, the median duration of response was not reached, ranging from 4.2 to roughly 14.5 months. Median progression-free survival was 3.4 months.
The leading treatment-related adverse events of grade 3 or higher in this cohort were anemia (21%) and thrombocytopenia (9%). In addition, 19% of patients had immune-related adverse events, with 9% having grade 3 or higher immune-related adverse events.
“Niraparib in combination with pembrolizumab is tolerable, with promising antitumor activity for patients with ovarian carcinoma who have limited treatment options regardless of platinum status, biomarker status, or prior treatment with bevacizumab,” Dr. Konstantinopoulos and colleagues conclude. “Responses in patients without tumor BRCA mutations or non-HRD cancers were higher than expected with either agent as monotherapy.”
Dr. Vinayak disclosed receiving clinical trial funding from TESARO; serving on an advisory board for TESARO; and serving on an advisory board for OncoSec Medical (uncompensated). Dr. Konstantinopoulos disclosed serving on advisory boards for AstraZeneca, Pfizer, and Merck. The trial was supported by TESARO: a GSK company and Merck, and in part by Stand Up to Cancer (a program of the Entertainment Industry Foundation); the Ovarian Cancer Research Fund Alliance; and National Ovarian Cancer Coalition Dream Team Translational Research.
SOURCE: Vinayak A et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1029. Konstantinopoulos PA et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1048.
The strategy of simultaneously exploiting deficient DNA damage repair and unleashing the immune response could expand treatment options for hard-to-treat breast and ovarian cancers, findings of the TOPACIO/KEYNOTE-162 trial suggest.
Triple-negative breast cancer (TNBC) and high-grade serous ovarian carcinoma share a number of genomic features, including a high frequency of BRCA1 and BRCA2 inactivation (Nature. 2012;490:61-70), as well as potential immunoreactivity (Lancet Oncol. 2018;19:40-50).
The open-label, single-arm phase 1/2 trial therefore tested the combination of niraparib (Zejula), an oral poly (ADP-ribose) polymerase (PARP) inhibitor, and pembrolizumab (Keytruda), an antibody to programmed death 1 (PD-1), among more than 100 patients with advanced or metastatic TNBC or recurrent platinum-resistant ovarian carcinoma. Patients were enrolled irrespective of BRCA mutation status or programmed death-ligand 1 (PD-L1) expression.
Main results, reported in JAMA Oncology, showed that the combination was safe, and about a fifth of patients with each type of cancer had an objective response. Median progression-free survival (PFS) was about 2 months in those with TNBC overall (although it exceeded 8 months in the subset with a tumor BRCA mutation) and about 3 months in those with ovarian cancer.
TNBC cohort
Investigators led by Shaveta Vinayak, MD, of the division of oncology at Fred Hutchinson Cancer Research Center, and University of Washington School of Medicine, Seattle Cancer Care Alliance, Seattle, studied 55 patients with TNBC treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 47 patients, the objective response rate (ORR) was 21%, and the disease control rate (DCR) was 49%. With a median duration of follow-up of 14.8 months, the median duration of response was not reached.
Activity of the combination varied by tumor BRCA mutation status. Compared with counterparts having BRCA wild-type tumors, patients having tumors with BRCA mutations had a numerically higher ORR (47% vs. 11%), DCR (80% vs. 33%), and PFS (8.3 vs. 2.1 months).
Some 18% of patients had treatment-related anemia, 15% thrombocytopenia, and 7% fatigue. In addition, 15% of patients had immune-related adverse events, with 4% having grade 3 immune-related adverse events.
“Combination niraparib plus pembrolizumab provides promising antitumor activity in patients with advanced or metastatic TNBC, with numerically higher response rates in those with tumor BRCA mutations,” Dr. Vinayak and colleagues conclude. “The combination therapy was safe with a tolerable safety profile, warranting further investigation.”
Ovarian cancer cohort
Investigators led by Panagiotis A. Konstantinopoulos, MD, PhD, of the division of gynecologic oncology, department of medical oncology at Dana-Farber Cancer Institute, Harvard Medical School, Boston, studied 62 patients with ovarian carcinoma treated with niraparib-pembrolizumab in the trial.
In the efficacy-evaluable population of 60 patients, the ORR was 18% and the DCR was 65%. The ORRs were similar regardless of patients’ platinum-based chemotherapy sensitivity, previous bevacizumab treatment, or tumor BRCA or homologous recombination deficiency (HRD) biomarker status.
With a median duration of follow-up of 12.4 months, the median duration of response was not reached, ranging from 4.2 to roughly 14.5 months. Median progression-free survival was 3.4 months.
The leading treatment-related adverse events of grade 3 or higher in this cohort were anemia (21%) and thrombocytopenia (9%). In addition, 19% of patients had immune-related adverse events, with 9% having grade 3 or higher immune-related adverse events.
“Niraparib in combination with pembrolizumab is tolerable, with promising antitumor activity for patients with ovarian carcinoma who have limited treatment options regardless of platinum status, biomarker status, or prior treatment with bevacizumab,” Dr. Konstantinopoulos and colleagues conclude. “Responses in patients without tumor BRCA mutations or non-HRD cancers were higher than expected with either agent as monotherapy.”
Dr. Vinayak disclosed receiving clinical trial funding from TESARO; serving on an advisory board for TESARO; and serving on an advisory board for OncoSec Medical (uncompensated). Dr. Konstantinopoulos disclosed serving on advisory boards for AstraZeneca, Pfizer, and Merck. The trial was supported by TESARO: a GSK company and Merck, and in part by Stand Up to Cancer (a program of the Entertainment Industry Foundation); the Ovarian Cancer Research Fund Alliance; and National Ovarian Cancer Coalition Dream Team Translational Research.
SOURCE: Vinayak A et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1029. Konstantinopoulos PA et al. JAMA Oncol. 2019 Jun 13. doi: 10.1001/jamaoncol.2019.1048.
FROM JAMA ONCOLOGY
From the journals: sarcoma around the world
EWING SARCOMA IN NEPAL: Investigators reported what they believe to be the first prospective clinical trial providing state-of-the-art chemotherapy to patients with Ewing sarcoma in Nepal. They treated 20 newly diagnosed patients with combination chemotherapy, including a course of etoposide and ifosfamide during external-beam radiotherapy. Radiotherapy was the only available treatment modality for local tumor control because advanced tumor-orthopedic services are not available in Nepal.
The 11 females and 9 males enrolled ranged in age from 6 to 37 years.
The treatment protocol—based on the Nepali-Norwegian Ewing Sarcoma Study treatment initiative— consisted of:
- Cyclophosphamide (1,200 mg/m2 as a 30-minute intravenous [IV] infusion)
- Doxorubicin (40 mg/m2/d as a 4-hour IV infusion on days 1 and 2; total dose, 80 mg/m2 in 2 days; total cumulative dose, 400 mg/m2)
- Etoposide (150 mg/m2/d as a 2-hour IV infusion; total dose, 450 mg/m2 in 3 days)
- Ifosfamide (3,000 mg/m2 over 21 to 24 hours as a 3-day continuous IV infusion; total dose, 9,000 mg/m2 in 3 days)
- Vincristine (1.5 mg/m2 IV push; maximum, 2 mg)
Patients received 5 courses of chemotherapy, then radiotherapy twice daily for 4 weeks for a total accumulated 54-Gy dose with a course of etoposide and ifosfamide, followed by 6 additional courses of chemotherapy.
Patients had primary tumors in the following sites: femur (n = 4), pubic bone (n = 1), fibula (n = 1), thoracic wall or costae (n = 4), clavicle (n = 1), craniofacial bone (n = 3), humerus (n = 3), forearm (n = 1), musculus sartorius with invasion into adjacent femur (n = 1), and uterine cervix (n = 1).
Eleven patients completed the entire treatment regimen, 6 of whom had no evidence of disease at a median follow-up of 2.3 years (range, 1.3 to 3.1 years). Four of them died of metastatic disease, and 1 experienced a recurrence 6 months later.
Three patients died due to chemotherapy- related toxicity, and 6 patients did not complete the treatment protocol, 4 of whom experienced progressive disease, were lost to follow-up, and presumed dead.
The investigators concluded that radiotherapy as the sole local treatment modality in combination with chemotherapy is feasible. They observed no fractures among the 15 patients who received radiotherapy.
SOURCE: Jha AK, Neupane P, Pradhan M, et al. Ewing sarcoma in Nepal treated with combined chemotherapy and definitive radiotherapy. J Glob Oncol. 2019;5:1-10.
PEDIATRIC SOFT TISSUE AND BONE SARCOMAS IN TANZANIA: In this retrospective review, investigators documented the epidemiologic and clinical features of pediatric sarcomas in the largest pediatric oncology center in Tanzania—Muhimbili National Hospital. Their objective in collecting the data was to compare the results with those of other countries and ultimately prioritize treatment protocols and resources for the more common pediatric sarcomas in Tanzania. Prior to this study, no data existed on the frequency and types most commonly seen in the country.
Between 2011 and 2016, the investigators collected information on 135 pediatric cases seen at the hospital. Eighty-nine cases (66%) were soft tissue sarcomas (STS) and 46 (34%) were bone sarcomas. Most patients, they reported, presented with a painless swelling.
Investigators found that, as in other countries, embryonal rhabdomyosarcoma accounted for the majority (75%) of all sarcomas seen in this study and osteosarcoma accounted for most (87%) bone sarcomas. However, unlike pediatric sarcomas in other countries, few cases of Ewing sarcoma were diagnosed during the study period.
An important disparity between Tanzania and other countries is that most patients in Tanzania present with advanced- stage disease, when the possibility of curative therapy is vastly reduced. Investigators found the lung to be the most common site of distant metastasis.
Other clinical and tumor characteristics reported in this study included:
- Slight female predominance (51%)
- Mean age, 6.3 years
- 42% of STS patients were younger than 5 years (n = 37)
- 46% of bone sarcoma patients were 10 to 15 years old (n = 21)
- Head and neck were the most common sites for STS
- Extremities were the most common sites for bone sarcomas
- Most patients presented with large tumors (>5 cm for STS and >8 cm for bone sarcomas).
The investigators believe these findings and others they reported will help them adapt treatment protocols used in Europe and America so that they will be most appropriate for their patients.
SOURCE: Siwillis EM, Dharse NJ, Scanlan T, et al. Pediatric soft tissue and bone sarcomas in Tanzania: Epidemiology and clinical features. J Glob Oncol. 2019;5:1-6.
PEDIATRIC OSTEOSARCOMA IN LEBANON: Investigators at a single institution in Lebanon reported a similar survival rate for newly diagnosed patients with pediatric osteosarcoma treated at their center as for those treated in more developed countries. In a retrospective review of the medical records of 38 patients treated at the American University of Beirut Medical Center between August 2001 and May 2012, they determined the 5-year overall survival (OS) for all patients to be 74% and the event-free survival (EFS), 62%. Patients with localized disease had a 5-year OS of 81% and an EFS of 68%. Patients with metastatic disease had OS and EFS rates of about 42%.
All patients with localized disease received chemotherapy according to the Pediatric Oncology Group 9351 protocol, which consisted of cisplatin, doxorubicin, and methotrexate. If patients had metastatic disease or tumor necrosis less than 90%, they also received ifosfamide and etoposide.
Patients were a mean age of 12.9 years at diagnosis and there were an equal number of male and female patients. Most patients (n=34) had a primary tumor site affecting the long bones around the knee.
Six patients had metastatic disease to the lungs, and 3 patients had multifocal bone disease with lung metastases.
Thirty-three patients (86.8%) underwent surgical resection after 2 courses of induction chemotherapy. Twenty-two (66.7%) of these patients had a delay in local tumor control of more than 4 weeks. And 12 patients (31.5%) had tumor necrosis of less than 90%.
The investigators analyzed the prognostic importance of age, sex, metastatic disease, tumor site, delay in local control, and degree of tumor necrosis. Bivariate analysis revealed that only the degree of tumor necrosis was a statistically significant adverse prognostic factor for EFS (P=.001) and OS (P=.002).
SOURCE: Abou Ali B, Salman M, Ghanem KM, et al. Clinical prognostic factors and outcome in pediatric osteosarcoma: Effect of delay in local control and degree of necrosis in a multidisciplinary setting in Lebanon. J Glob Oncol. 2019;5:1-8.
EWING SARCOMA IN NEPAL: Investigators reported what they believe to be the first prospective clinical trial providing state-of-the-art chemotherapy to patients with Ewing sarcoma in Nepal. They treated 20 newly diagnosed patients with combination chemotherapy, including a course of etoposide and ifosfamide during external-beam radiotherapy. Radiotherapy was the only available treatment modality for local tumor control because advanced tumor-orthopedic services are not available in Nepal.
The 11 females and 9 males enrolled ranged in age from 6 to 37 years.
The treatment protocol—based on the Nepali-Norwegian Ewing Sarcoma Study treatment initiative— consisted of:
- Cyclophosphamide (1,200 mg/m2 as a 30-minute intravenous [IV] infusion)
- Doxorubicin (40 mg/m2/d as a 4-hour IV infusion on days 1 and 2; total dose, 80 mg/m2 in 2 days; total cumulative dose, 400 mg/m2)
- Etoposide (150 mg/m2/d as a 2-hour IV infusion; total dose, 450 mg/m2 in 3 days)
- Ifosfamide (3,000 mg/m2 over 21 to 24 hours as a 3-day continuous IV infusion; total dose, 9,000 mg/m2 in 3 days)
- Vincristine (1.5 mg/m2 IV push; maximum, 2 mg)
Patients received 5 courses of chemotherapy, then radiotherapy twice daily for 4 weeks for a total accumulated 54-Gy dose with a course of etoposide and ifosfamide, followed by 6 additional courses of chemotherapy.
Patients had primary tumors in the following sites: femur (n = 4), pubic bone (n = 1), fibula (n = 1), thoracic wall or costae (n = 4), clavicle (n = 1), craniofacial bone (n = 3), humerus (n = 3), forearm (n = 1), musculus sartorius with invasion into adjacent femur (n = 1), and uterine cervix (n = 1).
Eleven patients completed the entire treatment regimen, 6 of whom had no evidence of disease at a median follow-up of 2.3 years (range, 1.3 to 3.1 years). Four of them died of metastatic disease, and 1 experienced a recurrence 6 months later.
Three patients died due to chemotherapy- related toxicity, and 6 patients did not complete the treatment protocol, 4 of whom experienced progressive disease, were lost to follow-up, and presumed dead.
The investigators concluded that radiotherapy as the sole local treatment modality in combination with chemotherapy is feasible. They observed no fractures among the 15 patients who received radiotherapy.
SOURCE: Jha AK, Neupane P, Pradhan M, et al. Ewing sarcoma in Nepal treated with combined chemotherapy and definitive radiotherapy. J Glob Oncol. 2019;5:1-10.
PEDIATRIC SOFT TISSUE AND BONE SARCOMAS IN TANZANIA: In this retrospective review, investigators documented the epidemiologic and clinical features of pediatric sarcomas in the largest pediatric oncology center in Tanzania—Muhimbili National Hospital. Their objective in collecting the data was to compare the results with those of other countries and ultimately prioritize treatment protocols and resources for the more common pediatric sarcomas in Tanzania. Prior to this study, no data existed on the frequency and types most commonly seen in the country.
Between 2011 and 2016, the investigators collected information on 135 pediatric cases seen at the hospital. Eighty-nine cases (66%) were soft tissue sarcomas (STS) and 46 (34%) were bone sarcomas. Most patients, they reported, presented with a painless swelling.
Investigators found that, as in other countries, embryonal rhabdomyosarcoma accounted for the majority (75%) of all sarcomas seen in this study and osteosarcoma accounted for most (87%) bone sarcomas. However, unlike pediatric sarcomas in other countries, few cases of Ewing sarcoma were diagnosed during the study period.
An important disparity between Tanzania and other countries is that most patients in Tanzania present with advanced- stage disease, when the possibility of curative therapy is vastly reduced. Investigators found the lung to be the most common site of distant metastasis.
Other clinical and tumor characteristics reported in this study included:
- Slight female predominance (51%)
- Mean age, 6.3 years
- 42% of STS patients were younger than 5 years (n = 37)
- 46% of bone sarcoma patients were 10 to 15 years old (n = 21)
- Head and neck were the most common sites for STS
- Extremities were the most common sites for bone sarcomas
- Most patients presented with large tumors (>5 cm for STS and >8 cm for bone sarcomas).
The investigators believe these findings and others they reported will help them adapt treatment protocols used in Europe and America so that they will be most appropriate for their patients.
SOURCE: Siwillis EM, Dharse NJ, Scanlan T, et al. Pediatric soft tissue and bone sarcomas in Tanzania: Epidemiology and clinical features. J Glob Oncol. 2019;5:1-6.
PEDIATRIC OSTEOSARCOMA IN LEBANON: Investigators at a single institution in Lebanon reported a similar survival rate for newly diagnosed patients with pediatric osteosarcoma treated at their center as for those treated in more developed countries. In a retrospective review of the medical records of 38 patients treated at the American University of Beirut Medical Center between August 2001 and May 2012, they determined the 5-year overall survival (OS) for all patients to be 74% and the event-free survival (EFS), 62%. Patients with localized disease had a 5-year OS of 81% and an EFS of 68%. Patients with metastatic disease had OS and EFS rates of about 42%.
All patients with localized disease received chemotherapy according to the Pediatric Oncology Group 9351 protocol, which consisted of cisplatin, doxorubicin, and methotrexate. If patients had metastatic disease or tumor necrosis less than 90%, they also received ifosfamide and etoposide.
Patients were a mean age of 12.9 years at diagnosis and there were an equal number of male and female patients. Most patients (n=34) had a primary tumor site affecting the long bones around the knee.
Six patients had metastatic disease to the lungs, and 3 patients had multifocal bone disease with lung metastases.
Thirty-three patients (86.8%) underwent surgical resection after 2 courses of induction chemotherapy. Twenty-two (66.7%) of these patients had a delay in local tumor control of more than 4 weeks. And 12 patients (31.5%) had tumor necrosis of less than 90%.
The investigators analyzed the prognostic importance of age, sex, metastatic disease, tumor site, delay in local control, and degree of tumor necrosis. Bivariate analysis revealed that only the degree of tumor necrosis was a statistically significant adverse prognostic factor for EFS (P=.001) and OS (P=.002).
SOURCE: Abou Ali B, Salman M, Ghanem KM, et al. Clinical prognostic factors and outcome in pediatric osteosarcoma: Effect of delay in local control and degree of necrosis in a multidisciplinary setting in Lebanon. J Glob Oncol. 2019;5:1-8.
EWING SARCOMA IN NEPAL: Investigators reported what they believe to be the first prospective clinical trial providing state-of-the-art chemotherapy to patients with Ewing sarcoma in Nepal. They treated 20 newly diagnosed patients with combination chemotherapy, including a course of etoposide and ifosfamide during external-beam radiotherapy. Radiotherapy was the only available treatment modality for local tumor control because advanced tumor-orthopedic services are not available in Nepal.
The 11 females and 9 males enrolled ranged in age from 6 to 37 years.
The treatment protocol—based on the Nepali-Norwegian Ewing Sarcoma Study treatment initiative— consisted of:
- Cyclophosphamide (1,200 mg/m2 as a 30-minute intravenous [IV] infusion)
- Doxorubicin (40 mg/m2/d as a 4-hour IV infusion on days 1 and 2; total dose, 80 mg/m2 in 2 days; total cumulative dose, 400 mg/m2)
- Etoposide (150 mg/m2/d as a 2-hour IV infusion; total dose, 450 mg/m2 in 3 days)
- Ifosfamide (3,000 mg/m2 over 21 to 24 hours as a 3-day continuous IV infusion; total dose, 9,000 mg/m2 in 3 days)
- Vincristine (1.5 mg/m2 IV push; maximum, 2 mg)
Patients received 5 courses of chemotherapy, then radiotherapy twice daily for 4 weeks for a total accumulated 54-Gy dose with a course of etoposide and ifosfamide, followed by 6 additional courses of chemotherapy.
Patients had primary tumors in the following sites: femur (n = 4), pubic bone (n = 1), fibula (n = 1), thoracic wall or costae (n = 4), clavicle (n = 1), craniofacial bone (n = 3), humerus (n = 3), forearm (n = 1), musculus sartorius with invasion into adjacent femur (n = 1), and uterine cervix (n = 1).
Eleven patients completed the entire treatment regimen, 6 of whom had no evidence of disease at a median follow-up of 2.3 years (range, 1.3 to 3.1 years). Four of them died of metastatic disease, and 1 experienced a recurrence 6 months later.
Three patients died due to chemotherapy- related toxicity, and 6 patients did not complete the treatment protocol, 4 of whom experienced progressive disease, were lost to follow-up, and presumed dead.
The investigators concluded that radiotherapy as the sole local treatment modality in combination with chemotherapy is feasible. They observed no fractures among the 15 patients who received radiotherapy.
SOURCE: Jha AK, Neupane P, Pradhan M, et al. Ewing sarcoma in Nepal treated with combined chemotherapy and definitive radiotherapy. J Glob Oncol. 2019;5:1-10.
PEDIATRIC SOFT TISSUE AND BONE SARCOMAS IN TANZANIA: In this retrospective review, investigators documented the epidemiologic and clinical features of pediatric sarcomas in the largest pediatric oncology center in Tanzania—Muhimbili National Hospital. Their objective in collecting the data was to compare the results with those of other countries and ultimately prioritize treatment protocols and resources for the more common pediatric sarcomas in Tanzania. Prior to this study, no data existed on the frequency and types most commonly seen in the country.
Between 2011 and 2016, the investigators collected information on 135 pediatric cases seen at the hospital. Eighty-nine cases (66%) were soft tissue sarcomas (STS) and 46 (34%) were bone sarcomas. Most patients, they reported, presented with a painless swelling.
Investigators found that, as in other countries, embryonal rhabdomyosarcoma accounted for the majority (75%) of all sarcomas seen in this study and osteosarcoma accounted for most (87%) bone sarcomas. However, unlike pediatric sarcomas in other countries, few cases of Ewing sarcoma were diagnosed during the study period.
An important disparity between Tanzania and other countries is that most patients in Tanzania present with advanced- stage disease, when the possibility of curative therapy is vastly reduced. Investigators found the lung to be the most common site of distant metastasis.
Other clinical and tumor characteristics reported in this study included:
- Slight female predominance (51%)
- Mean age, 6.3 years
- 42% of STS patients were younger than 5 years (n = 37)
- 46% of bone sarcoma patients were 10 to 15 years old (n = 21)
- Head and neck were the most common sites for STS
- Extremities were the most common sites for bone sarcomas
- Most patients presented with large tumors (>5 cm for STS and >8 cm for bone sarcomas).
The investigators believe these findings and others they reported will help them adapt treatment protocols used in Europe and America so that they will be most appropriate for their patients.
SOURCE: Siwillis EM, Dharse NJ, Scanlan T, et al. Pediatric soft tissue and bone sarcomas in Tanzania: Epidemiology and clinical features. J Glob Oncol. 2019;5:1-6.
PEDIATRIC OSTEOSARCOMA IN LEBANON: Investigators at a single institution in Lebanon reported a similar survival rate for newly diagnosed patients with pediatric osteosarcoma treated at their center as for those treated in more developed countries. In a retrospective review of the medical records of 38 patients treated at the American University of Beirut Medical Center between August 2001 and May 2012, they determined the 5-year overall survival (OS) for all patients to be 74% and the event-free survival (EFS), 62%. Patients with localized disease had a 5-year OS of 81% and an EFS of 68%. Patients with metastatic disease had OS and EFS rates of about 42%.
All patients with localized disease received chemotherapy according to the Pediatric Oncology Group 9351 protocol, which consisted of cisplatin, doxorubicin, and methotrexate. If patients had metastatic disease or tumor necrosis less than 90%, they also received ifosfamide and etoposide.
Patients were a mean age of 12.9 years at diagnosis and there were an equal number of male and female patients. Most patients (n=34) had a primary tumor site affecting the long bones around the knee.
Six patients had metastatic disease to the lungs, and 3 patients had multifocal bone disease with lung metastases.
Thirty-three patients (86.8%) underwent surgical resection after 2 courses of induction chemotherapy. Twenty-two (66.7%) of these patients had a delay in local tumor control of more than 4 weeks. And 12 patients (31.5%) had tumor necrosis of less than 90%.
The investigators analyzed the prognostic importance of age, sex, metastatic disease, tumor site, delay in local control, and degree of tumor necrosis. Bivariate analysis revealed that only the degree of tumor necrosis was a statistically significant adverse prognostic factor for EFS (P=.001) and OS (P=.002).
SOURCE: Abou Ali B, Salman M, Ghanem KM, et al. Clinical prognostic factors and outcome in pediatric osteosarcoma: Effect of delay in local control and degree of necrosis in a multidisciplinary setting in Lebanon. J Glob Oncol. 2019;5:1-8.
Cognitive decline sped up after CHD
according to the results of a large prospective study with a median of 12 years of follow-up.
“We found that incident CHD was significantly associated with faster post–CHD-diagnosis cognitive decline, but not pre–CHD-diagnosis or short-term cognitive decline after the event,” Wuxiang Xie, PhD, of Peking University Health Science Center, Beijing, and associates wrote in the Journal of the American College of Cardiology. Linear mixed models showed that cognitive decline sped up during the year after incident CHD.
Past research had suggested a link between accelerated cognitive decline and CHD, but the temporal pattern of the relationship was unclear. For the study, Dr. Xie and associates followed 7,888 adults from the English Longitudinal Study of Aging who were an average of 62 years old and had no history of stroke, MI, angina, or dementia (Alzheimer’s disease or otherwise). All participants underwent a baseline cognitive assessment for verbal memory, semantic fluency, and temporal orientation, plus a median of six follow-up assessments.
In all, 480 (6%) participants developed CHD during follow-up. Their rate of cognitive decline remained constant before and immediately after their CHD diagnosis, but in subsequent years, they experienced significant accelerations in loss of global cognitive function, verbal memory, and temporal orientation even after accounting for time and many demographic and clinical variables. For example, the slope representing temporal change in global cognitive score decreased by a mean of 0.039 per year, compared with the pre-CHD slope (slope difference, –0.039; 95% confidence interval, –0.063 to –0.015; P =. 002). Semantic fluency also declined faster after CHD, but the difference, compared with before CHD, did not reach statistical significance (P = .11).
Individuals without CHD showed no such accelerations in cognitive decline throughout follow-up in adjusted models, the researchers wrote. “Based on repeated cognitive measurements over a long follow-up period, this study revealed a reliable and robust trajectory of cognitive decline [after CHD]. Future studies are warranted to determine the precise mechanisms linking incident CHD to cognitive decline.”
Funders included the National Natural Science Foundation of China, the Beijing Natural Science Foundation, and the Newton International Fellowship from the Academy of Medical Sciences. The researchers reported having no relevant financial disclosures.
SOURCE: Xie W et al. J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.019.
The findings “highlight the role of cardiovascular risk factors and cardiovascular health as crucial determinants of cognitive trajectories in later life,” wrote Suvi P. Rovio, PhD; Katja Pahkala, PhD; and Olli T. Raitakari, MD, PhD. For example, accelerated declines in verbal memory might indicate a specific vulnerability to vascular changes within the medial temporal lobe and hippocampus.
The fact that cognitive decline did not accelerate immediately after coronary heart disease suggests that CHD itself does not acutely alter the brain, such as by causing microinfarcts, they commented. Instead, CHD might induce longer-term shifts in cerebral vascular function by affecting the blood-brain barrier or perfusion and oxidation in the brain. While these complex relationships need further untangling, the study suggests interventions that cut CHD risk also might help prevent cognitive decline itself and slow the rate of cognitive decline if it occurs.
Dr. Rovio, Dr. Pahkala, and Dr. Raitakari are at the University of Turku (Finland) and Turku University Hospital. These comments are adapted from an editorial accompanying the article by Xie et al. (J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.020). They reported having no relevant financial disclosures.
The findings “highlight the role of cardiovascular risk factors and cardiovascular health as crucial determinants of cognitive trajectories in later life,” wrote Suvi P. Rovio, PhD; Katja Pahkala, PhD; and Olli T. Raitakari, MD, PhD. For example, accelerated declines in verbal memory might indicate a specific vulnerability to vascular changes within the medial temporal lobe and hippocampus.
The fact that cognitive decline did not accelerate immediately after coronary heart disease suggests that CHD itself does not acutely alter the brain, such as by causing microinfarcts, they commented. Instead, CHD might induce longer-term shifts in cerebral vascular function by affecting the blood-brain barrier or perfusion and oxidation in the brain. While these complex relationships need further untangling, the study suggests interventions that cut CHD risk also might help prevent cognitive decline itself and slow the rate of cognitive decline if it occurs.
Dr. Rovio, Dr. Pahkala, and Dr. Raitakari are at the University of Turku (Finland) and Turku University Hospital. These comments are adapted from an editorial accompanying the article by Xie et al. (J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.020). They reported having no relevant financial disclosures.
The findings “highlight the role of cardiovascular risk factors and cardiovascular health as crucial determinants of cognitive trajectories in later life,” wrote Suvi P. Rovio, PhD; Katja Pahkala, PhD; and Olli T. Raitakari, MD, PhD. For example, accelerated declines in verbal memory might indicate a specific vulnerability to vascular changes within the medial temporal lobe and hippocampus.
The fact that cognitive decline did not accelerate immediately after coronary heart disease suggests that CHD itself does not acutely alter the brain, such as by causing microinfarcts, they commented. Instead, CHD might induce longer-term shifts in cerebral vascular function by affecting the blood-brain barrier or perfusion and oxidation in the brain. While these complex relationships need further untangling, the study suggests interventions that cut CHD risk also might help prevent cognitive decline itself and slow the rate of cognitive decline if it occurs.
Dr. Rovio, Dr. Pahkala, and Dr. Raitakari are at the University of Turku (Finland) and Turku University Hospital. These comments are adapted from an editorial accompanying the article by Xie et al. (J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.020). They reported having no relevant financial disclosures.
according to the results of a large prospective study with a median of 12 years of follow-up.
“We found that incident CHD was significantly associated with faster post–CHD-diagnosis cognitive decline, but not pre–CHD-diagnosis or short-term cognitive decline after the event,” Wuxiang Xie, PhD, of Peking University Health Science Center, Beijing, and associates wrote in the Journal of the American College of Cardiology. Linear mixed models showed that cognitive decline sped up during the year after incident CHD.
Past research had suggested a link between accelerated cognitive decline and CHD, but the temporal pattern of the relationship was unclear. For the study, Dr. Xie and associates followed 7,888 adults from the English Longitudinal Study of Aging who were an average of 62 years old and had no history of stroke, MI, angina, or dementia (Alzheimer’s disease or otherwise). All participants underwent a baseline cognitive assessment for verbal memory, semantic fluency, and temporal orientation, plus a median of six follow-up assessments.
In all, 480 (6%) participants developed CHD during follow-up. Their rate of cognitive decline remained constant before and immediately after their CHD diagnosis, but in subsequent years, they experienced significant accelerations in loss of global cognitive function, verbal memory, and temporal orientation even after accounting for time and many demographic and clinical variables. For example, the slope representing temporal change in global cognitive score decreased by a mean of 0.039 per year, compared with the pre-CHD slope (slope difference, –0.039; 95% confidence interval, –0.063 to –0.015; P =. 002). Semantic fluency also declined faster after CHD, but the difference, compared with before CHD, did not reach statistical significance (P = .11).
Individuals without CHD showed no such accelerations in cognitive decline throughout follow-up in adjusted models, the researchers wrote. “Based on repeated cognitive measurements over a long follow-up period, this study revealed a reliable and robust trajectory of cognitive decline [after CHD]. Future studies are warranted to determine the precise mechanisms linking incident CHD to cognitive decline.”
Funders included the National Natural Science Foundation of China, the Beijing Natural Science Foundation, and the Newton International Fellowship from the Academy of Medical Sciences. The researchers reported having no relevant financial disclosures.
SOURCE: Xie W et al. J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.019.
according to the results of a large prospective study with a median of 12 years of follow-up.
“We found that incident CHD was significantly associated with faster post–CHD-diagnosis cognitive decline, but not pre–CHD-diagnosis or short-term cognitive decline after the event,” Wuxiang Xie, PhD, of Peking University Health Science Center, Beijing, and associates wrote in the Journal of the American College of Cardiology. Linear mixed models showed that cognitive decline sped up during the year after incident CHD.
Past research had suggested a link between accelerated cognitive decline and CHD, but the temporal pattern of the relationship was unclear. For the study, Dr. Xie and associates followed 7,888 adults from the English Longitudinal Study of Aging who were an average of 62 years old and had no history of stroke, MI, angina, or dementia (Alzheimer’s disease or otherwise). All participants underwent a baseline cognitive assessment for verbal memory, semantic fluency, and temporal orientation, plus a median of six follow-up assessments.
In all, 480 (6%) participants developed CHD during follow-up. Their rate of cognitive decline remained constant before and immediately after their CHD diagnosis, but in subsequent years, they experienced significant accelerations in loss of global cognitive function, verbal memory, and temporal orientation even after accounting for time and many demographic and clinical variables. For example, the slope representing temporal change in global cognitive score decreased by a mean of 0.039 per year, compared with the pre-CHD slope (slope difference, –0.039; 95% confidence interval, –0.063 to –0.015; P =. 002). Semantic fluency also declined faster after CHD, but the difference, compared with before CHD, did not reach statistical significance (P = .11).
Individuals without CHD showed no such accelerations in cognitive decline throughout follow-up in adjusted models, the researchers wrote. “Based on repeated cognitive measurements over a long follow-up period, this study revealed a reliable and robust trajectory of cognitive decline [after CHD]. Future studies are warranted to determine the precise mechanisms linking incident CHD to cognitive decline.”
Funders included the National Natural Science Foundation of China, the Beijing Natural Science Foundation, and the Newton International Fellowship from the Academy of Medical Sciences. The researchers reported having no relevant financial disclosures.
SOURCE: Xie W et al. J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.019.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Genetic analysis links PCSK9 inhibition and CV mortality
, but not all-cause mortality, in a large cohort of individuals.
“We tested the hypothesis that genetically low LDL cholesterol due to PCSK9 [proprotein convertase subtilisin/kexin type 9] variation is causally associated with low cardiovascular and all-cause mortality in a general population of Northern European ancestry,” wrote Marianne Benn, MD, DMSc, and colleagues. The findings were published in the Journal of the American College of Cardiology.
The researchers conducted a large-scale genetic analysis of 109,566 persons from the Copenhagen City Heart Study and Copenhagen General Population Study. In addition, the team included a validation cohort of 431,043 individuals from the UK Biobank.
The median duration of follow-up was 10 years (0-42 years), and the median age at study entry was 57 years.
Study participants were genotyped for several PCSK9 variants and a weighted allele score based the effects of LDL cholesterol, individual allele frequency, and number of variant alleles was calculated for each subject.
Weighted scores were categorized into five stepwise noncontinuous score ranges, with lower levels of LDL cholesterol linked to higher allele scores.
After analysis, the researchers found that a growing number of PCSK9 alleles were associated with lower levels of LDL cholesterol up to 0.61 mmol/L (P for trend less than .001) and reduced CV mortality (P = .001), but not with reduced all-cause mortality (P = .11).
“Our genetic data did not show a reduction in risk of all-cause mortality, and only showed a reduction in risk of all-cause mortality in statin trials and not in the PCSK9-inhibitor trials meta-analyzed,” the researchers wrote. “This may be explained by the low frequency of cardiovascular disease in the 2 populations studied,” they explained.
One key limitation was the homogeneous makeup of the study population. Dr. Benn and colleagues acknowledged this could limit the generalizability of the results.
“Long-term LDL cholesterol treatment (e.g., with PCSK9 inhibitors), may translate into reductions in cardiovascular mortality,” they concluded.
The study was supported by the Danish Council for Independent Research, Medical Sciences, Johan Boserup, and the Lise Boserup’s Fund. The authors reported no conflicts of interest.
SOURCE: Benn M et al. JACC. 2019 Jun 17. doi: 10.1016/j.jacc.2019.03.517
One question that remains from the current study is whether prolonged inhibition of PCSK9 in patients with increased LDL cholesterol levels will reduce cardiovascular mortality in the context of primary and secondary prevention.
The recent development of PCSK9 inhibitors was heavily influenced by genetic analyses showing that person-specific variants in the PCSK9 gene could lower LDL levels and reduce rates of coronary heart disease. Because of the rarity of these gene variants, their impact on mortality on a large-scale basis remains unclear.
Although numerous clinical trials have shown that PCSK9 inhibition can reduce CVD-related events in both chronic and high-risk patients, no study has clearly shown an effect on cardiovascular death. However, the relationship between lipid levels and clinical outcomes is difficult to assess owing to the presence of confounding factors. Certain types of genetic analysis may help eliminate these challenges by analyzing large populations over extended periods of time.
The genetic analysis by Dr. Benn and colleagues showed an association between long-term exposure to lower levels of LDL cholesterol, by means of functional variants in the PCSK9 gene, and reduced cardiovascular mortality. These findings, alongside other studies, provide further support for the relationship between PCSK9 inhibition and prevention of cardiovascular mortality.
Gregory G. Schwartz, MD, PhD , and Matthew R.G. Taylor, MD, PhD , are with the University of Colorado in Aurora. Dr. Schwartz reported having financial affiliations with Resverlogix, Roche, Sanofi, and The Medicines Company. These comments are adapted from their editorial (J Am Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.03.518 ).
One question that remains from the current study is whether prolonged inhibition of PCSK9 in patients with increased LDL cholesterol levels will reduce cardiovascular mortality in the context of primary and secondary prevention.
The recent development of PCSK9 inhibitors was heavily influenced by genetic analyses showing that person-specific variants in the PCSK9 gene could lower LDL levels and reduce rates of coronary heart disease. Because of the rarity of these gene variants, their impact on mortality on a large-scale basis remains unclear.
Although numerous clinical trials have shown that PCSK9 inhibition can reduce CVD-related events in both chronic and high-risk patients, no study has clearly shown an effect on cardiovascular death. However, the relationship between lipid levels and clinical outcomes is difficult to assess owing to the presence of confounding factors. Certain types of genetic analysis may help eliminate these challenges by analyzing large populations over extended periods of time.
The genetic analysis by Dr. Benn and colleagues showed an association between long-term exposure to lower levels of LDL cholesterol, by means of functional variants in the PCSK9 gene, and reduced cardiovascular mortality. These findings, alongside other studies, provide further support for the relationship between PCSK9 inhibition and prevention of cardiovascular mortality.
Gregory G. Schwartz, MD, PhD , and Matthew R.G. Taylor, MD, PhD , are with the University of Colorado in Aurora. Dr. Schwartz reported having financial affiliations with Resverlogix, Roche, Sanofi, and The Medicines Company. These comments are adapted from their editorial (J Am Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.03.518 ).
One question that remains from the current study is whether prolonged inhibition of PCSK9 in patients with increased LDL cholesterol levels will reduce cardiovascular mortality in the context of primary and secondary prevention.
The recent development of PCSK9 inhibitors was heavily influenced by genetic analyses showing that person-specific variants in the PCSK9 gene could lower LDL levels and reduce rates of coronary heart disease. Because of the rarity of these gene variants, their impact on mortality on a large-scale basis remains unclear.
Although numerous clinical trials have shown that PCSK9 inhibition can reduce CVD-related events in both chronic and high-risk patients, no study has clearly shown an effect on cardiovascular death. However, the relationship between lipid levels and clinical outcomes is difficult to assess owing to the presence of confounding factors. Certain types of genetic analysis may help eliminate these challenges by analyzing large populations over extended periods of time.
The genetic analysis by Dr. Benn and colleagues showed an association between long-term exposure to lower levels of LDL cholesterol, by means of functional variants in the PCSK9 gene, and reduced cardiovascular mortality. These findings, alongside other studies, provide further support for the relationship between PCSK9 inhibition and prevention of cardiovascular mortality.
Gregory G. Schwartz, MD, PhD , and Matthew R.G. Taylor, MD, PhD , are with the University of Colorado in Aurora. Dr. Schwartz reported having financial affiliations with Resverlogix, Roche, Sanofi, and The Medicines Company. These comments are adapted from their editorial (J Am Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.03.518 ).
, but not all-cause mortality, in a large cohort of individuals.
“We tested the hypothesis that genetically low LDL cholesterol due to PCSK9 [proprotein convertase subtilisin/kexin type 9] variation is causally associated with low cardiovascular and all-cause mortality in a general population of Northern European ancestry,” wrote Marianne Benn, MD, DMSc, and colleagues. The findings were published in the Journal of the American College of Cardiology.
The researchers conducted a large-scale genetic analysis of 109,566 persons from the Copenhagen City Heart Study and Copenhagen General Population Study. In addition, the team included a validation cohort of 431,043 individuals from the UK Biobank.
The median duration of follow-up was 10 years (0-42 years), and the median age at study entry was 57 years.
Study participants were genotyped for several PCSK9 variants and a weighted allele score based the effects of LDL cholesterol, individual allele frequency, and number of variant alleles was calculated for each subject.
Weighted scores were categorized into five stepwise noncontinuous score ranges, with lower levels of LDL cholesterol linked to higher allele scores.
After analysis, the researchers found that a growing number of PCSK9 alleles were associated with lower levels of LDL cholesterol up to 0.61 mmol/L (P for trend less than .001) and reduced CV mortality (P = .001), but not with reduced all-cause mortality (P = .11).
“Our genetic data did not show a reduction in risk of all-cause mortality, and only showed a reduction in risk of all-cause mortality in statin trials and not in the PCSK9-inhibitor trials meta-analyzed,” the researchers wrote. “This may be explained by the low frequency of cardiovascular disease in the 2 populations studied,” they explained.
One key limitation was the homogeneous makeup of the study population. Dr. Benn and colleagues acknowledged this could limit the generalizability of the results.
“Long-term LDL cholesterol treatment (e.g., with PCSK9 inhibitors), may translate into reductions in cardiovascular mortality,” they concluded.
The study was supported by the Danish Council for Independent Research, Medical Sciences, Johan Boserup, and the Lise Boserup’s Fund. The authors reported no conflicts of interest.
SOURCE: Benn M et al. JACC. 2019 Jun 17. doi: 10.1016/j.jacc.2019.03.517
, but not all-cause mortality, in a large cohort of individuals.
“We tested the hypothesis that genetically low LDL cholesterol due to PCSK9 [proprotein convertase subtilisin/kexin type 9] variation is causally associated with low cardiovascular and all-cause mortality in a general population of Northern European ancestry,” wrote Marianne Benn, MD, DMSc, and colleagues. The findings were published in the Journal of the American College of Cardiology.
The researchers conducted a large-scale genetic analysis of 109,566 persons from the Copenhagen City Heart Study and Copenhagen General Population Study. In addition, the team included a validation cohort of 431,043 individuals from the UK Biobank.
The median duration of follow-up was 10 years (0-42 years), and the median age at study entry was 57 years.
Study participants were genotyped for several PCSK9 variants and a weighted allele score based the effects of LDL cholesterol, individual allele frequency, and number of variant alleles was calculated for each subject.
Weighted scores were categorized into five stepwise noncontinuous score ranges, with lower levels of LDL cholesterol linked to higher allele scores.
After analysis, the researchers found that a growing number of PCSK9 alleles were associated with lower levels of LDL cholesterol up to 0.61 mmol/L (P for trend less than .001) and reduced CV mortality (P = .001), but not with reduced all-cause mortality (P = .11).
“Our genetic data did not show a reduction in risk of all-cause mortality, and only showed a reduction in risk of all-cause mortality in statin trials and not in the PCSK9-inhibitor trials meta-analyzed,” the researchers wrote. “This may be explained by the low frequency of cardiovascular disease in the 2 populations studied,” they explained.
One key limitation was the homogeneous makeup of the study population. Dr. Benn and colleagues acknowledged this could limit the generalizability of the results.
“Long-term LDL cholesterol treatment (e.g., with PCSK9 inhibitors), may translate into reductions in cardiovascular mortality,” they concluded.
The study was supported by the Danish Council for Independent Research, Medical Sciences, Johan Boserup, and the Lise Boserup’s Fund. The authors reported no conflicts of interest.
SOURCE: Benn M et al. JACC. 2019 Jun 17. doi: 10.1016/j.jacc.2019.03.517
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Rivaroxaban tied to higher GI bleeding than other NOACs
SAN DIEGO – Patients on rivaroxaban had significantly higher rates of GI bleeding, compared with those taking apixaban or dabigatran, results from a large population-based study showed.
“This may be due to the fact that rivaroxaban is administered as a single daily dose as opposed to the other two non–vitamin K anticoagulants [NOACs], which are given twice daily,” lead study author Arnar B. Ingason said at the annual Digestive Disease Week. “This may lead to a greater variance in plasma drug concentration, making these patients more susceptible to bleeding.”
Mr. Ingason, a medical student at the University of Iceland, Reykjavik, said that although several studies have compared warfarin with NOACs, it remains unclear which NOAC has the most favorable GI profile. In an effort to improve the research in this area, he and his associates performed a nationwide, population-based study during March 2014–Jan. 2018 to compare the GI bleeding risk of patients receiving rivaroxaban to that of a combined pool of patients receiving either apixaban or dabigatran. They drew from the Icelandic Medicine Registry, which contains all outpatient drug prescriptions in the country. Next, the researchers linked the personal identification numbers of patients to the Landspitali University diagnoses registry, which includes more than 90% of all patients hospitalized for GI bleeding. They used 1:1 nearest neighbor propensity score for matching and Kaplan-Meier survival estimates and Cox regression to compare rates of GI bleeding. The study outcome of interest was any clinically relevant GI bleeding.
Mr. Ingason reported that the baseline characteristics were similar between the rivaroxaban group and the apixaban/dabigatran group. They matched for several variables, including age, sex, Charlson score, the proportion being anticoagulant naive, moderate to severe renal disease, moderate to severe liver disease, any prior bleeding, and any prior thrombotic events.
During the study period, 3,473 patients received rivaroxaban, 1,901 received apixaban, and 1,086 received dabigatran. After propensity score matching, the researchers compared 2,635 patients who received rivaroxaban with 2,365 patients who received either apixaban or dabigatran. They found that patients in the rivaroxaban group had significantly higher rates of GI bleeding, compared with in the apixaban/dabigatran group (1.2 and. 0.6 events per 100 patient-years, respectively). This yielded a hazard ratio of 2.02, “which means that patients receiving rivaroxaban are twice as likely to get GI bleeding compared to patients on apixaban or dabigatran,” Mr. Ingason said. When the researchers examined the entire unmatched cohort of patients, the rivaroxaban group also had significantly higher rates of GI bleeding, compared with the apixaban/dabigatran group (1.0 and 0.6 events per 100 patient-years; HR, 1.75).
Mr. Ingason and his colleagues observed that patients in the rivaroxaban group had higher rates of GI bleeding, compared with the apixaban/dabigatran group, during the entire follow-up period. At the end of year 4, the rivaroxaban group had a 4% cumulative event rate of GI bleeding, compared with 1.8% for the apixaban/dabigatran group, a highly significant difference at P = .0057).
When a meeting attendee asked Mr. Ingason why patients taking apixaban or dabigatran were combined into one group, he said that it was done to increase the power of their study. “Our theory was that rivaroxaban was different because it is administered as a single daily dose, while the others are given twice daily,” he said. The researchers reported having no financial disclosures.
SAN DIEGO – Patients on rivaroxaban had significantly higher rates of GI bleeding, compared with those taking apixaban or dabigatran, results from a large population-based study showed.
“This may be due to the fact that rivaroxaban is administered as a single daily dose as opposed to the other two non–vitamin K anticoagulants [NOACs], which are given twice daily,” lead study author Arnar B. Ingason said at the annual Digestive Disease Week. “This may lead to a greater variance in plasma drug concentration, making these patients more susceptible to bleeding.”
Mr. Ingason, a medical student at the University of Iceland, Reykjavik, said that although several studies have compared warfarin with NOACs, it remains unclear which NOAC has the most favorable GI profile. In an effort to improve the research in this area, he and his associates performed a nationwide, population-based study during March 2014–Jan. 2018 to compare the GI bleeding risk of patients receiving rivaroxaban to that of a combined pool of patients receiving either apixaban or dabigatran. They drew from the Icelandic Medicine Registry, which contains all outpatient drug prescriptions in the country. Next, the researchers linked the personal identification numbers of patients to the Landspitali University diagnoses registry, which includes more than 90% of all patients hospitalized for GI bleeding. They used 1:1 nearest neighbor propensity score for matching and Kaplan-Meier survival estimates and Cox regression to compare rates of GI bleeding. The study outcome of interest was any clinically relevant GI bleeding.
Mr. Ingason reported that the baseline characteristics were similar between the rivaroxaban group and the apixaban/dabigatran group. They matched for several variables, including age, sex, Charlson score, the proportion being anticoagulant naive, moderate to severe renal disease, moderate to severe liver disease, any prior bleeding, and any prior thrombotic events.
During the study period, 3,473 patients received rivaroxaban, 1,901 received apixaban, and 1,086 received dabigatran. After propensity score matching, the researchers compared 2,635 patients who received rivaroxaban with 2,365 patients who received either apixaban or dabigatran. They found that patients in the rivaroxaban group had significantly higher rates of GI bleeding, compared with in the apixaban/dabigatran group (1.2 and. 0.6 events per 100 patient-years, respectively). This yielded a hazard ratio of 2.02, “which means that patients receiving rivaroxaban are twice as likely to get GI bleeding compared to patients on apixaban or dabigatran,” Mr. Ingason said. When the researchers examined the entire unmatched cohort of patients, the rivaroxaban group also had significantly higher rates of GI bleeding, compared with the apixaban/dabigatran group (1.0 and 0.6 events per 100 patient-years; HR, 1.75).
Mr. Ingason and his colleagues observed that patients in the rivaroxaban group had higher rates of GI bleeding, compared with the apixaban/dabigatran group, during the entire follow-up period. At the end of year 4, the rivaroxaban group had a 4% cumulative event rate of GI bleeding, compared with 1.8% for the apixaban/dabigatran group, a highly significant difference at P = .0057).
When a meeting attendee asked Mr. Ingason why patients taking apixaban or dabigatran were combined into one group, he said that it was done to increase the power of their study. “Our theory was that rivaroxaban was different because it is administered as a single daily dose, while the others are given twice daily,” he said. The researchers reported having no financial disclosures.
SAN DIEGO – Patients on rivaroxaban had significantly higher rates of GI bleeding, compared with those taking apixaban or dabigatran, results from a large population-based study showed.
“This may be due to the fact that rivaroxaban is administered as a single daily dose as opposed to the other two non–vitamin K anticoagulants [NOACs], which are given twice daily,” lead study author Arnar B. Ingason said at the annual Digestive Disease Week. “This may lead to a greater variance in plasma drug concentration, making these patients more susceptible to bleeding.”
Mr. Ingason, a medical student at the University of Iceland, Reykjavik, said that although several studies have compared warfarin with NOACs, it remains unclear which NOAC has the most favorable GI profile. In an effort to improve the research in this area, he and his associates performed a nationwide, population-based study during March 2014–Jan. 2018 to compare the GI bleeding risk of patients receiving rivaroxaban to that of a combined pool of patients receiving either apixaban or dabigatran. They drew from the Icelandic Medicine Registry, which contains all outpatient drug prescriptions in the country. Next, the researchers linked the personal identification numbers of patients to the Landspitali University diagnoses registry, which includes more than 90% of all patients hospitalized for GI bleeding. They used 1:1 nearest neighbor propensity score for matching and Kaplan-Meier survival estimates and Cox regression to compare rates of GI bleeding. The study outcome of interest was any clinically relevant GI bleeding.
Mr. Ingason reported that the baseline characteristics were similar between the rivaroxaban group and the apixaban/dabigatran group. They matched for several variables, including age, sex, Charlson score, the proportion being anticoagulant naive, moderate to severe renal disease, moderate to severe liver disease, any prior bleeding, and any prior thrombotic events.
During the study period, 3,473 patients received rivaroxaban, 1,901 received apixaban, and 1,086 received dabigatran. After propensity score matching, the researchers compared 2,635 patients who received rivaroxaban with 2,365 patients who received either apixaban or dabigatran. They found that patients in the rivaroxaban group had significantly higher rates of GI bleeding, compared with in the apixaban/dabigatran group (1.2 and. 0.6 events per 100 patient-years, respectively). This yielded a hazard ratio of 2.02, “which means that patients receiving rivaroxaban are twice as likely to get GI bleeding compared to patients on apixaban or dabigatran,” Mr. Ingason said. When the researchers examined the entire unmatched cohort of patients, the rivaroxaban group also had significantly higher rates of GI bleeding, compared with the apixaban/dabigatran group (1.0 and 0.6 events per 100 patient-years; HR, 1.75).
Mr. Ingason and his colleagues observed that patients in the rivaroxaban group had higher rates of GI bleeding, compared with the apixaban/dabigatran group, during the entire follow-up period. At the end of year 4, the rivaroxaban group had a 4% cumulative event rate of GI bleeding, compared with 1.8% for the apixaban/dabigatran group, a highly significant difference at P = .0057).
When a meeting attendee asked Mr. Ingason why patients taking apixaban or dabigatran were combined into one group, he said that it was done to increase the power of their study. “Our theory was that rivaroxaban was different because it is administered as a single daily dose, while the others are given twice daily,” he said. The researchers reported having no financial disclosures.
REPORTING FROM DDW 2019