User login
Surprise billing legislation passes Senate committee
A bill aimed at ending the practice of surprise billing, along with a number of other health care cost-containment measures, passed by an overwhelming majority during a mark-up session of the Senate Health, Education, Labor, and Pensions Committee.
S. 1895, the Lower Health Care Costs Act of 2019, passed 20-3; Sens. Rand Paul (R-Ky.), Bernie Sanders (D-Ver.) and Elizabeth Warren (D-Mass.) voted against it.
A summary of the bill’s provisions can be found here.
With Sen. Sanders and Sen. Warren not present, presumably out to prepare for the Democratic presidential nominee debates and voting by proxy, only Sen. Paul was present to speak against the bill. He questioned whether it would have any impact on lowering health care cost.
Among other provisions, the bill would ban all gag clauses that would keep pricing data from being released; all anticompetitive clauses in facility and insurance contracts that would otherwise limit access to higher-quality, lower-cost care; would designate a nongovernment entity focused on price transparency; and would improve the accuracy of directory information.
But government-induced transparency is not the solution, Sen. Paul said.
He called it a “fallacy” that “you can mandate transparency, and you’ll create a marketplace.” Rather, you need to create a marketplace, and transparency will naturally follow, he said.
Sen. Paul noted that just having institutions publishing prices that no one pays and prices that are not freely fluctuating “doesn’t work.”
“The irony here is that, when you have no insurance involved, you actually have a marketplace,” he said. “The people without insurance are the only true marketplace,” he said, adding that those with high deductibles would also fall into that category.
The crux of S. 1895 is protections to end so-called “surprise bills” that occur when patients receive medical services from out-of-network health care professionals at in-network hospitals. These out-of-network services are not constrained by prior agreements and can add up to tens of thousands of dollars.
“There are those who have seen the history of price controls and know that you never get what you intended,” he said, and predicted that this could lead to a shortage of physicians.
The American Medical Association also criticized the surprise billing provisions of the bill.
In a June 25 letter, the AMA noted that “the approach outlined in S. 1895 fails to address some of the fundamental reasons why surprise billing occurs – inadequate provider networks, higher patient-cost sharing requirements for out-of-network services, and noncompetitive local markets that empower plans to offer take-it-or-leave-it contracts.”
AMA also criticized the use of benchmark pricing to settle out-of-network billing issues. “By setting a payment maximum at the individual plans’ median in-network amount, insurers will have even less incentive to negotiate contracts with individual providers,” according to the letter. “They can drive down the median in-network amount by simply dropping from their networks providers who are currently paid above the median. Or, they can simply stop negotiating altogether, knowing that their financial obligation is limited to their own median in-network payment amounts.”
The Physicians Advocacy Institute agreed. In a June 26 statement, the organization stated that it remains “deeply concerned that arbitrary, government-set payment benchmarks championed by the health insurance industry will further undermine provider networks and devastate patients’ access to critical medical services.”
A collective of hospital organizations, including the Federation of American Hospitals and the American Hospital Association also opposed the use of benchmark pricing.
In a June 25 letter to committee leadership, the groups stated that they are “concerned that the rate-setting provision of the legislation is a plan-determined, nontransparent process that will upend private payment negotiation. A default rate will become the payment ceiling and remove incentives for insurers to develop comprehensive networks, as there are already increasing numbers of narrow network products offered that exclude certain types of providers.”
The bill also addresses the cost of prescription drugs, including providing clearer information about patents, ensuring a more timely access to generics, altering exclusivity rules to help get generics to market quicker, reporting requirements for price increases, and a number of other provisions aimed at increasing competition in an effort to lower drug pricing.
Other areas covered by the bill include more oversight of pharmacy benefit managers, strengthening parity in mental health laws, and a number of provisions aimed at public health and health information technology.
A bill aimed at ending the practice of surprise billing, along with a number of other health care cost-containment measures, passed by an overwhelming majority during a mark-up session of the Senate Health, Education, Labor, and Pensions Committee.
S. 1895, the Lower Health Care Costs Act of 2019, passed 20-3; Sens. Rand Paul (R-Ky.), Bernie Sanders (D-Ver.) and Elizabeth Warren (D-Mass.) voted against it.
A summary of the bill’s provisions can be found here.
With Sen. Sanders and Sen. Warren not present, presumably out to prepare for the Democratic presidential nominee debates and voting by proxy, only Sen. Paul was present to speak against the bill. He questioned whether it would have any impact on lowering health care cost.
Among other provisions, the bill would ban all gag clauses that would keep pricing data from being released; all anticompetitive clauses in facility and insurance contracts that would otherwise limit access to higher-quality, lower-cost care; would designate a nongovernment entity focused on price transparency; and would improve the accuracy of directory information.
But government-induced transparency is not the solution, Sen. Paul said.
He called it a “fallacy” that “you can mandate transparency, and you’ll create a marketplace.” Rather, you need to create a marketplace, and transparency will naturally follow, he said.
Sen. Paul noted that just having institutions publishing prices that no one pays and prices that are not freely fluctuating “doesn’t work.”
“The irony here is that, when you have no insurance involved, you actually have a marketplace,” he said. “The people without insurance are the only true marketplace,” he said, adding that those with high deductibles would also fall into that category.
The crux of S. 1895 is protections to end so-called “surprise bills” that occur when patients receive medical services from out-of-network health care professionals at in-network hospitals. These out-of-network services are not constrained by prior agreements and can add up to tens of thousands of dollars.
“There are those who have seen the history of price controls and know that you never get what you intended,” he said, and predicted that this could lead to a shortage of physicians.
The American Medical Association also criticized the surprise billing provisions of the bill.
In a June 25 letter, the AMA noted that “the approach outlined in S. 1895 fails to address some of the fundamental reasons why surprise billing occurs – inadequate provider networks, higher patient-cost sharing requirements for out-of-network services, and noncompetitive local markets that empower plans to offer take-it-or-leave-it contracts.”
AMA also criticized the use of benchmark pricing to settle out-of-network billing issues. “By setting a payment maximum at the individual plans’ median in-network amount, insurers will have even less incentive to negotiate contracts with individual providers,” according to the letter. “They can drive down the median in-network amount by simply dropping from their networks providers who are currently paid above the median. Or, they can simply stop negotiating altogether, knowing that their financial obligation is limited to their own median in-network payment amounts.”
The Physicians Advocacy Institute agreed. In a June 26 statement, the organization stated that it remains “deeply concerned that arbitrary, government-set payment benchmarks championed by the health insurance industry will further undermine provider networks and devastate patients’ access to critical medical services.”
A collective of hospital organizations, including the Federation of American Hospitals and the American Hospital Association also opposed the use of benchmark pricing.
In a June 25 letter to committee leadership, the groups stated that they are “concerned that the rate-setting provision of the legislation is a plan-determined, nontransparent process that will upend private payment negotiation. A default rate will become the payment ceiling and remove incentives for insurers to develop comprehensive networks, as there are already increasing numbers of narrow network products offered that exclude certain types of providers.”
The bill also addresses the cost of prescription drugs, including providing clearer information about patents, ensuring a more timely access to generics, altering exclusivity rules to help get generics to market quicker, reporting requirements for price increases, and a number of other provisions aimed at increasing competition in an effort to lower drug pricing.
Other areas covered by the bill include more oversight of pharmacy benefit managers, strengthening parity in mental health laws, and a number of provisions aimed at public health and health information technology.
A bill aimed at ending the practice of surprise billing, along with a number of other health care cost-containment measures, passed by an overwhelming majority during a mark-up session of the Senate Health, Education, Labor, and Pensions Committee.
S. 1895, the Lower Health Care Costs Act of 2019, passed 20-3; Sens. Rand Paul (R-Ky.), Bernie Sanders (D-Ver.) and Elizabeth Warren (D-Mass.) voted against it.
A summary of the bill’s provisions can be found here.
With Sen. Sanders and Sen. Warren not present, presumably out to prepare for the Democratic presidential nominee debates and voting by proxy, only Sen. Paul was present to speak against the bill. He questioned whether it would have any impact on lowering health care cost.
Among other provisions, the bill would ban all gag clauses that would keep pricing data from being released; all anticompetitive clauses in facility and insurance contracts that would otherwise limit access to higher-quality, lower-cost care; would designate a nongovernment entity focused on price transparency; and would improve the accuracy of directory information.
But government-induced transparency is not the solution, Sen. Paul said.
He called it a “fallacy” that “you can mandate transparency, and you’ll create a marketplace.” Rather, you need to create a marketplace, and transparency will naturally follow, he said.
Sen. Paul noted that just having institutions publishing prices that no one pays and prices that are not freely fluctuating “doesn’t work.”
“The irony here is that, when you have no insurance involved, you actually have a marketplace,” he said. “The people without insurance are the only true marketplace,” he said, adding that those with high deductibles would also fall into that category.
The crux of S. 1895 is protections to end so-called “surprise bills” that occur when patients receive medical services from out-of-network health care professionals at in-network hospitals. These out-of-network services are not constrained by prior agreements and can add up to tens of thousands of dollars.
“There are those who have seen the history of price controls and know that you never get what you intended,” he said, and predicted that this could lead to a shortage of physicians.
The American Medical Association also criticized the surprise billing provisions of the bill.
In a June 25 letter, the AMA noted that “the approach outlined in S. 1895 fails to address some of the fundamental reasons why surprise billing occurs – inadequate provider networks, higher patient-cost sharing requirements for out-of-network services, and noncompetitive local markets that empower plans to offer take-it-or-leave-it contracts.”
AMA also criticized the use of benchmark pricing to settle out-of-network billing issues. “By setting a payment maximum at the individual plans’ median in-network amount, insurers will have even less incentive to negotiate contracts with individual providers,” according to the letter. “They can drive down the median in-network amount by simply dropping from their networks providers who are currently paid above the median. Or, they can simply stop negotiating altogether, knowing that their financial obligation is limited to their own median in-network payment amounts.”
The Physicians Advocacy Institute agreed. In a June 26 statement, the organization stated that it remains “deeply concerned that arbitrary, government-set payment benchmarks championed by the health insurance industry will further undermine provider networks and devastate patients’ access to critical medical services.”
A collective of hospital organizations, including the Federation of American Hospitals and the American Hospital Association also opposed the use of benchmark pricing.
In a June 25 letter to committee leadership, the groups stated that they are “concerned that the rate-setting provision of the legislation is a plan-determined, nontransparent process that will upend private payment negotiation. A default rate will become the payment ceiling and remove incentives for insurers to develop comprehensive networks, as there are already increasing numbers of narrow network products offered that exclude certain types of providers.”
The bill also addresses the cost of prescription drugs, including providing clearer information about patents, ensuring a more timely access to generics, altering exclusivity rules to help get generics to market quicker, reporting requirements for price increases, and a number of other provisions aimed at increasing competition in an effort to lower drug pricing.
Other areas covered by the bill include more oversight of pharmacy benefit managers, strengthening parity in mental health laws, and a number of provisions aimed at public health and health information technology.
REPORTING FROM A SENATE COMMITTEE MEETING
The 21st Century Cures Act: Tearing down fortresses to put patients first
"A fortress not only protects those inside of it, but it also enslaves them to work.”
– Anthony T. Hincks
As physicians, we spend a great deal of time intending to do our best for the people we serve. We believe fundamentally in the idea that our patients come first, and we toil daily to exercise that belief. We also want our patients to feel they are driving their care as active participants along the journey. Yet time and time again, despite our greatest attempts, those efforts are stymied by the state of modern medicine;
Over the past 10 years, we have done a tremendous job of constructing expensive fortresses around patient information known as electronic health records (EHRs). Billions of dollars have been spent implementing, upgrading, and optimizing. In spite of this, physicians are increasingly frustrated by EHRs (and in many cases, long to return to the days of paper). It isn’t surprising, then, that patients are frustrated as well. We use terms such as “patient-centered care,” but patients feel like they are not in the center at all. Instead, they can find themselves feeling like complete outsiders, at the mercy of the medical juggernaut to make sure they have the appropriate information when they need it. There are several issues that contribute to the frustrations of physicians and patients, but two in particular warrant attention. The first is the diversity of Health IT systems and ongoing issues with EHR interoperability. The second is a provincial attitude surrounding transparency and medical record ownership. We will discuss both of these here, as well as recent legislation designed to advance both concerns.
We have written in previous columns about the many challenges of interoperability. Electronic health records, sold by different vendors, typically won’t “talk” to each other. In spite of years of maturation, issues of compatibility remain. Patient data locked inside of one EHR is not easily accessible by a physician using a different EHR. While efforts have been made to streamline information sharing, there are still many fortresses that cannot be breached.
Bridging the moat
The 21st Century Cures Act, enacted by Congress in December of 2016, seeks to define and require interoperability while addressing many other significant problems in health care. According to the legislation, true interoperability means that health IT should enable the secure exchange of electronic health information with other electronic record systems without special effort on the part of the user; the process should be seamless and shouldn’t be cumbersome for physicians or patients. It also must be fully supported by EHR vendors, but those vendors have been expressing significant concerns with the ways in which the act is being interpreted.
In a recent blog post, the HIMSS Electronic Health Record Association – a consortium of vendors including Epic, Allscripts, eClinicalWorks, as well as several others – expressed “significant concerns regarding timelines, ambiguous language, disincentives for innovation, and definitions related to information blocking.”1 This is not surprising, as the onus for improving interoperability falls squarely on their shoulders, and the work to get there is arduous. Regardless of one’s interpretation, the goal of the Cures act is clear: Arrive at true interoperability in the shortest period of time, while eliminating barriers that prevent patients from accessing their health records. In other words, it asks for the avoidance of “information blocking.”
Breaching the gate
Information blocking, as defined by the Cures Act, is “a practice by a health care provider, health IT developer, health information exchange, or health information network that … is likely to interfere with, prevent, or materially discourage access, exchange, or use of electronic health information.”2 This practice is explicitly prohibited by the legislation – and is ethically wrong – yet it continues to occur implicitly every day as it has for many years. Even if unintentional and solely because of the growing complexity of our information systems, it makes accessing health information incredibly cumbersome for patients. Even worse, attempts to improve patients’ ability to access their health records have only created additional obstacles.
HIPAA (the Health Insurance Portability and Accountability Act of 1996) was designed to protect patient confidentiality and create security around protected health information. While noble in purpose, many have found it burdensome to work within the parameters set forth in the law. Physicians and patients needing legitimate access to clinical data discover endless release forms and convoluted processes standing in their way. Access to the information eventually comes in the form of reams of printed paper or faxed notes that cannot be easily consumed by or integrated into other systems.
The Meaningful Use initiative, while envisioned to improve data exchange and enhance population health, did little to help. Instead of enabling documentation efficiency and improving patient access, it promoted the proliferation of incompatible EHRs and poorly conceived patient portals. It also created heavy costs for both the federal government and physicians and was largely ineffective at producing systems whose use could be considered meaningful. The federal government paid out as much as $44,000 per physician to incentivize them to purchase medical records, while physicians often spent more than the $44,000 and, in many cases, wound up with EHRs that didn’t work well and had to be replaced.
Authors and supporters of the 21st Century Cures Act are hoping to avoid the shortcomings of prior legislation by attaching financial penalties to health care providers or IT vendors who engage in information blocking. While allowing for exceptions in appropriate cases, the law is clear: Patients deserve complete access to their medical records. While this goes against tradition, it has been proven to result in better outcomes.
Initiatives such as the OpenNotes movement have been pushing the value of full transparency for some time, and their website includes a long list of numerous examples to prove it. Indeed, several studies have demonstrated increased physician and patient satisfaction when both parties have ready access to health information. We believe that we, as physicians, should fully support the idea and lobby our EHR vendors to do the same.
It is time to tear down the impenetrable fortresses of traditional medicine, then work diligently to rebuild them with our patients safely inside.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
References
1. The Electronic Health Record Association blog
"A fortress not only protects those inside of it, but it also enslaves them to work.”
– Anthony T. Hincks
As physicians, we spend a great deal of time intending to do our best for the people we serve. We believe fundamentally in the idea that our patients come first, and we toil daily to exercise that belief. We also want our patients to feel they are driving their care as active participants along the journey. Yet time and time again, despite our greatest attempts, those efforts are stymied by the state of modern medicine;
Over the past 10 years, we have done a tremendous job of constructing expensive fortresses around patient information known as electronic health records (EHRs). Billions of dollars have been spent implementing, upgrading, and optimizing. In spite of this, physicians are increasingly frustrated by EHRs (and in many cases, long to return to the days of paper). It isn’t surprising, then, that patients are frustrated as well. We use terms such as “patient-centered care,” but patients feel like they are not in the center at all. Instead, they can find themselves feeling like complete outsiders, at the mercy of the medical juggernaut to make sure they have the appropriate information when they need it. There are several issues that contribute to the frustrations of physicians and patients, but two in particular warrant attention. The first is the diversity of Health IT systems and ongoing issues with EHR interoperability. The second is a provincial attitude surrounding transparency and medical record ownership. We will discuss both of these here, as well as recent legislation designed to advance both concerns.
We have written in previous columns about the many challenges of interoperability. Electronic health records, sold by different vendors, typically won’t “talk” to each other. In spite of years of maturation, issues of compatibility remain. Patient data locked inside of one EHR is not easily accessible by a physician using a different EHR. While efforts have been made to streamline information sharing, there are still many fortresses that cannot be breached.
Bridging the moat
The 21st Century Cures Act, enacted by Congress in December of 2016, seeks to define and require interoperability while addressing many other significant problems in health care. According to the legislation, true interoperability means that health IT should enable the secure exchange of electronic health information with other electronic record systems without special effort on the part of the user; the process should be seamless and shouldn’t be cumbersome for physicians or patients. It also must be fully supported by EHR vendors, but those vendors have been expressing significant concerns with the ways in which the act is being interpreted.
In a recent blog post, the HIMSS Electronic Health Record Association – a consortium of vendors including Epic, Allscripts, eClinicalWorks, as well as several others – expressed “significant concerns regarding timelines, ambiguous language, disincentives for innovation, and definitions related to information blocking.”1 This is not surprising, as the onus for improving interoperability falls squarely on their shoulders, and the work to get there is arduous. Regardless of one’s interpretation, the goal of the Cures act is clear: Arrive at true interoperability in the shortest period of time, while eliminating barriers that prevent patients from accessing their health records. In other words, it asks for the avoidance of “information blocking.”
Breaching the gate
Information blocking, as defined by the Cures Act, is “a practice by a health care provider, health IT developer, health information exchange, or health information network that … is likely to interfere with, prevent, or materially discourage access, exchange, or use of electronic health information.”2 This practice is explicitly prohibited by the legislation – and is ethically wrong – yet it continues to occur implicitly every day as it has for many years. Even if unintentional and solely because of the growing complexity of our information systems, it makes accessing health information incredibly cumbersome for patients. Even worse, attempts to improve patients’ ability to access their health records have only created additional obstacles.
HIPAA (the Health Insurance Portability and Accountability Act of 1996) was designed to protect patient confidentiality and create security around protected health information. While noble in purpose, many have found it burdensome to work within the parameters set forth in the law. Physicians and patients needing legitimate access to clinical data discover endless release forms and convoluted processes standing in their way. Access to the information eventually comes in the form of reams of printed paper or faxed notes that cannot be easily consumed by or integrated into other systems.
The Meaningful Use initiative, while envisioned to improve data exchange and enhance population health, did little to help. Instead of enabling documentation efficiency and improving patient access, it promoted the proliferation of incompatible EHRs and poorly conceived patient portals. It also created heavy costs for both the federal government and physicians and was largely ineffective at producing systems whose use could be considered meaningful. The federal government paid out as much as $44,000 per physician to incentivize them to purchase medical records, while physicians often spent more than the $44,000 and, in many cases, wound up with EHRs that didn’t work well and had to be replaced.
Authors and supporters of the 21st Century Cures Act are hoping to avoid the shortcomings of prior legislation by attaching financial penalties to health care providers or IT vendors who engage in information blocking. While allowing for exceptions in appropriate cases, the law is clear: Patients deserve complete access to their medical records. While this goes against tradition, it has been proven to result in better outcomes.
Initiatives such as the OpenNotes movement have been pushing the value of full transparency for some time, and their website includes a long list of numerous examples to prove it. Indeed, several studies have demonstrated increased physician and patient satisfaction when both parties have ready access to health information. We believe that we, as physicians, should fully support the idea and lobby our EHR vendors to do the same.
It is time to tear down the impenetrable fortresses of traditional medicine, then work diligently to rebuild them with our patients safely inside.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
References
1. The Electronic Health Record Association blog
"A fortress not only protects those inside of it, but it also enslaves them to work.”
– Anthony T. Hincks
As physicians, we spend a great deal of time intending to do our best for the people we serve. We believe fundamentally in the idea that our patients come first, and we toil daily to exercise that belief. We also want our patients to feel they are driving their care as active participants along the journey. Yet time and time again, despite our greatest attempts, those efforts are stymied by the state of modern medicine;
Over the past 10 years, we have done a tremendous job of constructing expensive fortresses around patient information known as electronic health records (EHRs). Billions of dollars have been spent implementing, upgrading, and optimizing. In spite of this, physicians are increasingly frustrated by EHRs (and in many cases, long to return to the days of paper). It isn’t surprising, then, that patients are frustrated as well. We use terms such as “patient-centered care,” but patients feel like they are not in the center at all. Instead, they can find themselves feeling like complete outsiders, at the mercy of the medical juggernaut to make sure they have the appropriate information when they need it. There are several issues that contribute to the frustrations of physicians and patients, but two in particular warrant attention. The first is the diversity of Health IT systems and ongoing issues with EHR interoperability. The second is a provincial attitude surrounding transparency and medical record ownership. We will discuss both of these here, as well as recent legislation designed to advance both concerns.
We have written in previous columns about the many challenges of interoperability. Electronic health records, sold by different vendors, typically won’t “talk” to each other. In spite of years of maturation, issues of compatibility remain. Patient data locked inside of one EHR is not easily accessible by a physician using a different EHR. While efforts have been made to streamline information sharing, there are still many fortresses that cannot be breached.
Bridging the moat
The 21st Century Cures Act, enacted by Congress in December of 2016, seeks to define and require interoperability while addressing many other significant problems in health care. According to the legislation, true interoperability means that health IT should enable the secure exchange of electronic health information with other electronic record systems without special effort on the part of the user; the process should be seamless and shouldn’t be cumbersome for physicians or patients. It also must be fully supported by EHR vendors, but those vendors have been expressing significant concerns with the ways in which the act is being interpreted.
In a recent blog post, the HIMSS Electronic Health Record Association – a consortium of vendors including Epic, Allscripts, eClinicalWorks, as well as several others – expressed “significant concerns regarding timelines, ambiguous language, disincentives for innovation, and definitions related to information blocking.”1 This is not surprising, as the onus for improving interoperability falls squarely on their shoulders, and the work to get there is arduous. Regardless of one’s interpretation, the goal of the Cures act is clear: Arrive at true interoperability in the shortest period of time, while eliminating barriers that prevent patients from accessing their health records. In other words, it asks for the avoidance of “information blocking.”
Breaching the gate
Information blocking, as defined by the Cures Act, is “a practice by a health care provider, health IT developer, health information exchange, or health information network that … is likely to interfere with, prevent, or materially discourage access, exchange, or use of electronic health information.”2 This practice is explicitly prohibited by the legislation – and is ethically wrong – yet it continues to occur implicitly every day as it has for many years. Even if unintentional and solely because of the growing complexity of our information systems, it makes accessing health information incredibly cumbersome for patients. Even worse, attempts to improve patients’ ability to access their health records have only created additional obstacles.
HIPAA (the Health Insurance Portability and Accountability Act of 1996) was designed to protect patient confidentiality and create security around protected health information. While noble in purpose, many have found it burdensome to work within the parameters set forth in the law. Physicians and patients needing legitimate access to clinical data discover endless release forms and convoluted processes standing in their way. Access to the information eventually comes in the form of reams of printed paper or faxed notes that cannot be easily consumed by or integrated into other systems.
The Meaningful Use initiative, while envisioned to improve data exchange and enhance population health, did little to help. Instead of enabling documentation efficiency and improving patient access, it promoted the proliferation of incompatible EHRs and poorly conceived patient portals. It also created heavy costs for both the federal government and physicians and was largely ineffective at producing systems whose use could be considered meaningful. The federal government paid out as much as $44,000 per physician to incentivize them to purchase medical records, while physicians often spent more than the $44,000 and, in many cases, wound up with EHRs that didn’t work well and had to be replaced.
Authors and supporters of the 21st Century Cures Act are hoping to avoid the shortcomings of prior legislation by attaching financial penalties to health care providers or IT vendors who engage in information blocking. While allowing for exceptions in appropriate cases, the law is clear: Patients deserve complete access to their medical records. While this goes against tradition, it has been proven to result in better outcomes.
Initiatives such as the OpenNotes movement have been pushing the value of full transparency for some time, and their website includes a long list of numerous examples to prove it. Indeed, several studies have demonstrated increased physician and patient satisfaction when both parties have ready access to health information. We believe that we, as physicians, should fully support the idea and lobby our EHR vendors to do the same.
It is time to tear down the impenetrable fortresses of traditional medicine, then work diligently to rebuild them with our patients safely inside.
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
References
1. The Electronic Health Record Association blog
Video roundtable–Fibroids: Patient considerations in medical and surgical management
Read the article: Fibroids: Patient considerations in medical and surgical management
Read the article: Fibroids: Patient considerations in medical and surgical management
Read the article: Fibroids: Patient considerations in medical and surgical management
FDA approves dupilumab for chronic rhinosinusitis with nasal polyps
The Food and Drug Administration has approved dupilumab (Dupixent) for the treatment of chronic rhinosinusitis accompanied by nasal polyps in adults.
FDA approval is based on results from a pair of studies involving 724 patients aged 18 years or older with chronic rhinosinusitis with nasal polyps who were symptomatic despite undergoing treatment with intranasal corticosteroids and who received either dupilumab or a placebo. Compared with the placebo group, patients receiving dupilumab had statistically significant reductions in nasal polyp size and nasal congestion; they also had an increased ability to smell and required less nasal polyp surgery and oral steroids.
The most commonly reported adverse events were injection-site reactions and eye and eyelid inflammation, which included redness, swelling, and itching. The drug can cause severe allergic reactions and eye problems, such as conjunctivitis or keratitis; patients should also not receive live vaccines while taking dupilumab.
“Nasal polyps can lead to loss of smell, and often patients require surgery to remove the polyps. Dupixent provides an important treatment option for patients whose nasal polyps are not adequately controlled with intranasal steroids. It also reduces the need for nasal polyp surgery and oral steroids,” said Sally Seymour, MD, director of the Division of Pulmonary, Allergy, and Rheumatology Products in the FDA’s Center for Drug Evaluation and Research.
Find the full press release on the FDA website.
The Food and Drug Administration has approved dupilumab (Dupixent) for the treatment of chronic rhinosinusitis accompanied by nasal polyps in adults.
FDA approval is based on results from a pair of studies involving 724 patients aged 18 years or older with chronic rhinosinusitis with nasal polyps who were symptomatic despite undergoing treatment with intranasal corticosteroids and who received either dupilumab or a placebo. Compared with the placebo group, patients receiving dupilumab had statistically significant reductions in nasal polyp size and nasal congestion; they also had an increased ability to smell and required less nasal polyp surgery and oral steroids.
The most commonly reported adverse events were injection-site reactions and eye and eyelid inflammation, which included redness, swelling, and itching. The drug can cause severe allergic reactions and eye problems, such as conjunctivitis or keratitis; patients should also not receive live vaccines while taking dupilumab.
“Nasal polyps can lead to loss of smell, and often patients require surgery to remove the polyps. Dupixent provides an important treatment option for patients whose nasal polyps are not adequately controlled with intranasal steroids. It also reduces the need for nasal polyp surgery and oral steroids,” said Sally Seymour, MD, director of the Division of Pulmonary, Allergy, and Rheumatology Products in the FDA’s Center for Drug Evaluation and Research.
Find the full press release on the FDA website.
The Food and Drug Administration has approved dupilumab (Dupixent) for the treatment of chronic rhinosinusitis accompanied by nasal polyps in adults.
FDA approval is based on results from a pair of studies involving 724 patients aged 18 years or older with chronic rhinosinusitis with nasal polyps who were symptomatic despite undergoing treatment with intranasal corticosteroids and who received either dupilumab or a placebo. Compared with the placebo group, patients receiving dupilumab had statistically significant reductions in nasal polyp size and nasal congestion; they also had an increased ability to smell and required less nasal polyp surgery and oral steroids.
The most commonly reported adverse events were injection-site reactions and eye and eyelid inflammation, which included redness, swelling, and itching. The drug can cause severe allergic reactions and eye problems, such as conjunctivitis or keratitis; patients should also not receive live vaccines while taking dupilumab.
“Nasal polyps can lead to loss of smell, and often patients require surgery to remove the polyps. Dupixent provides an important treatment option for patients whose nasal polyps are not adequately controlled with intranasal steroids. It also reduces the need for nasal polyp surgery and oral steroids,” said Sally Seymour, MD, director of the Division of Pulmonary, Allergy, and Rheumatology Products in the FDA’s Center for Drug Evaluation and Research.
Find the full press release on the FDA website.
Almost half of Americans express doubts about vaccines
according to the American Osteopathic Association.
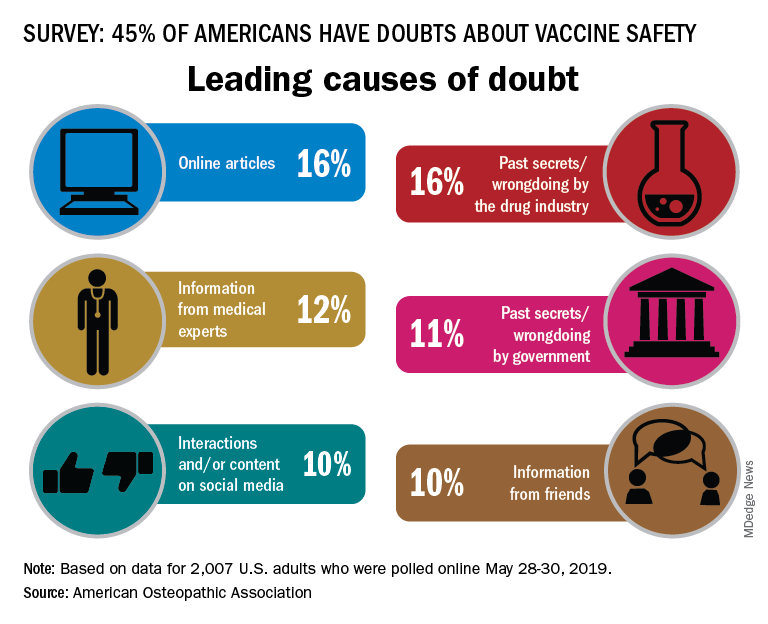
In a survey conducted by the Harris Poll on behalf of the AOA, 45% of the 2,007 respondents expressed a negative attitude towards vaccine safety, with online articles (16%) and past secrets/wrongdoings by the pharmaceutical industry (16%) cited as the leading causes, the AOA said.
There was no difference in negative attitude between men and women, but age, region, and parental status each had a notable effect. Doubts of vaccine safety were highest in those aged 18-34 years (55%) and lowest in those aged 65 and older (29%). Those living in the West had the highest rate at 50%, while residents of the Midwest were lowest at 39%, and the negative attitude rate was 55% for adults who had children under age 18 years and 40% for those who did not, the AOA reported.
Respondents to the survey, conducted May 28-30, 2019, also were asked to choose one of five statements that best expressed their view of vaccines, and those data paint a somewhat different picture:
- 2% said vaccines are unsafe and ineffective.
- 6% said that the side-effect risks outweigh the benefits.
- 9% said they were not sure if vaccines are safe and effective.
- 31% said that the benefits outweigh the risks.
- 51% said that vaccines are safe and effective.
Social media were another important source of doubt among respondents, but they have not been effective at countering the spread of vaccine misinformation, said psychiatrist Rachel Shmuts, DO, of Cherry Hill, N.J.
Confirmation bias makes it difficult to convince someone vaccines are safe, effective, and necessary once they believe they are not. “The number of people who believe vaccines are dangerous and refuse to get them is still relatively small. However, online support groups seem to solidify their beliefs, making them less susceptible to influence from their neighbors and real-world communities,” she said in the AOA statement.
Osteopathic family physician Paul Ehrmann, DO, said in the statement, “People know that a lot of practices won’t accept patients who don’t vaccinate, so when they find one that will, they spread the word to their community that it’s a safe place. Whether intentional or not, those doctors are often seen as endorsing anti-vaxxer beliefs.”
In 2017, his home state of Michigan, with other partners, put on a public information campaign. It has “significantly improved vaccination rates across demographics,” according to the statement.
“Beliefs are hard to change especially when they’re based in fear,” Dr. Ehrmann, of Royal Oak, Mich., said in the statement. “But, being responsible for our patients’ health and the public’s health, we can’t afford to give in to those fears. We must insist on evidence-based medicine.”
according to the American Osteopathic Association.

In a survey conducted by the Harris Poll on behalf of the AOA, 45% of the 2,007 respondents expressed a negative attitude towards vaccine safety, with online articles (16%) and past secrets/wrongdoings by the pharmaceutical industry (16%) cited as the leading causes, the AOA said.
There was no difference in negative attitude between men and women, but age, region, and parental status each had a notable effect. Doubts of vaccine safety were highest in those aged 18-34 years (55%) and lowest in those aged 65 and older (29%). Those living in the West had the highest rate at 50%, while residents of the Midwest were lowest at 39%, and the negative attitude rate was 55% for adults who had children under age 18 years and 40% for those who did not, the AOA reported.
Respondents to the survey, conducted May 28-30, 2019, also were asked to choose one of five statements that best expressed their view of vaccines, and those data paint a somewhat different picture:
- 2% said vaccines are unsafe and ineffective.
- 6% said that the side-effect risks outweigh the benefits.
- 9% said they were not sure if vaccines are safe and effective.
- 31% said that the benefits outweigh the risks.
- 51% said that vaccines are safe and effective.
Social media were another important source of doubt among respondents, but they have not been effective at countering the spread of vaccine misinformation, said psychiatrist Rachel Shmuts, DO, of Cherry Hill, N.J.
Confirmation bias makes it difficult to convince someone vaccines are safe, effective, and necessary once they believe they are not. “The number of people who believe vaccines are dangerous and refuse to get them is still relatively small. However, online support groups seem to solidify their beliefs, making them less susceptible to influence from their neighbors and real-world communities,” she said in the AOA statement.
Osteopathic family physician Paul Ehrmann, DO, said in the statement, “People know that a lot of practices won’t accept patients who don’t vaccinate, so when they find one that will, they spread the word to their community that it’s a safe place. Whether intentional or not, those doctors are often seen as endorsing anti-vaxxer beliefs.”
In 2017, his home state of Michigan, with other partners, put on a public information campaign. It has “significantly improved vaccination rates across demographics,” according to the statement.
“Beliefs are hard to change especially when they’re based in fear,” Dr. Ehrmann, of Royal Oak, Mich., said in the statement. “But, being responsible for our patients’ health and the public’s health, we can’t afford to give in to those fears. We must insist on evidence-based medicine.”
according to the American Osteopathic Association.

In a survey conducted by the Harris Poll on behalf of the AOA, 45% of the 2,007 respondents expressed a negative attitude towards vaccine safety, with online articles (16%) and past secrets/wrongdoings by the pharmaceutical industry (16%) cited as the leading causes, the AOA said.
There was no difference in negative attitude between men and women, but age, region, and parental status each had a notable effect. Doubts of vaccine safety were highest in those aged 18-34 years (55%) and lowest in those aged 65 and older (29%). Those living in the West had the highest rate at 50%, while residents of the Midwest were lowest at 39%, and the negative attitude rate was 55% for adults who had children under age 18 years and 40% for those who did not, the AOA reported.
Respondents to the survey, conducted May 28-30, 2019, also were asked to choose one of five statements that best expressed their view of vaccines, and those data paint a somewhat different picture:
- 2% said vaccines are unsafe and ineffective.
- 6% said that the side-effect risks outweigh the benefits.
- 9% said they were not sure if vaccines are safe and effective.
- 31% said that the benefits outweigh the risks.
- 51% said that vaccines are safe and effective.
Social media were another important source of doubt among respondents, but they have not been effective at countering the spread of vaccine misinformation, said psychiatrist Rachel Shmuts, DO, of Cherry Hill, N.J.
Confirmation bias makes it difficult to convince someone vaccines are safe, effective, and necessary once they believe they are not. “The number of people who believe vaccines are dangerous and refuse to get them is still relatively small. However, online support groups seem to solidify their beliefs, making them less susceptible to influence from their neighbors and real-world communities,” she said in the AOA statement.
Osteopathic family physician Paul Ehrmann, DO, said in the statement, “People know that a lot of practices won’t accept patients who don’t vaccinate, so when they find one that will, they spread the word to their community that it’s a safe place. Whether intentional or not, those doctors are often seen as endorsing anti-vaxxer beliefs.”
In 2017, his home state of Michigan, with other partners, put on a public information campaign. It has “significantly improved vaccination rates across demographics,” according to the statement.
“Beliefs are hard to change especially when they’re based in fear,” Dr. Ehrmann, of Royal Oak, Mich., said in the statement. “But, being responsible for our patients’ health and the public’s health, we can’t afford to give in to those fears. We must insist on evidence-based medicine.”
Guadecitabine offers limited advantage over other standards for high-risk AML
AMSTERDAM – For treatment-naive patients with acute myeloid leukemia (AML) who are ineligible for chemotherapy, guadecitabine offers similar efficacy to other standard treatment options until four cycles are administered, after which guadecitabine offers a slight survival advantage, based on results from the phase 3 ASTRAL-1 trial.
Complete responders also derived greater benefit from guadecitabine, a new hypomethylating agent, reported lead author Pierre Fenaux, MD, PhD, of the Hôpital Saint Louis, Paris.
With 815 patients, ASTRAL-1 was the largest global, randomized trial to compare low-intensity therapy options in this elderly, unfit population – specifically, patients who were at least 75 years old or had an Eastern Cooperative Oncology Group (ECOG) performance status of 3 or more, Dr. Fenaux said at the annual congress of the European Hematology Association.
They were randomized in a 1:1 ratio to receive guadecitabine or one of three other treatment options: azacitidine, decitabine, or low-dose cytarabine. The coprimary endpoints of the trial were complete response rate and median overall survival. Safety measures were also investigated.
A demographic analysis showed that almost two-thirds of patients were at least 75 years old (62%), and about half had an ECOG status of 2 or 3, or bone marrow blasts. Approximately one-third of patients had poor-risk cytogenetics and a slightly higher proportion had secondary AML.
After a median follow-up of 25.5 months, patients had received, on average, five cycles of therapy. However, many patients (42%) received three or fewer cycles because of early death or disease progression. This therapy cessation rate was similar between the guadecitabine group (42.4%) and the other treatment group (40.8%).
The study failed to meet either coprimary endpoint across the entire patient population. Median overall survival was 7.10 months for guadecitabine versus 8.47 months for the other treatments, but this difference was not statistically significant (P = .73). Similarly, the complete response rate was slightly higher for guadecitabine (19.4% vs. 17.4%), but again, this finding carried a nonsignificant P value (P = .48).
The benefit offered by guadecitabine was realized only with extended treatment and in complete responders.
Patients who received a minimum of four cycles of guadecitabine had a median overall survival of 15.6 months, compared with 13.0 months for other treatments (P = .02). This benefit became more pronounced in those who received at least six cycles, which was associated with median overall survival of 19.5 months versus 14.9 months (P = .002). Complete responders also had extended survival when treated with guadecitabine, although this benefit was of a lesser magnitude (22.6 vs. 20.6 months; P = .07).
Most subgroup analyses, accounting for various clinical and genetic factors, showed no significant differences in primary outcomes between treatment arms, with one exception: TP53 mutations were associated with poor responses to guadecitabine, and a lack of the TP53 mutation predicted better responses to guadecitabine.
Adverse events were common, although most measures were not significantly different between treatment arms. For example, serious adverse events occurred in 81% and 75.5% of patients treated with guadecitabine and other options, respectively, while grade 3 or higher adverse events occurred in 91.5% of guadecitabine patients and 87.5% of patients treated with other options, but neither difference was statistically significant.
Adverse events leading to death occurred in 28.7% of patients treated with guadecitabine versus 29.8% of other patients, a nonsignificant difference. In contrast, Dr. Fenaux noted that patients treated with guadecitabine were significantly more likely to develop febrile neutropenia (33.9% vs. 26.5%), neutropenia (27.4% vs. 20.7%), and pneumonia (29.4% vs. 19.6%).
“In those patients [that received at least four cycles], there seemed to be some advantage of guadecitabine, which needs to be further explored,” Dr. Fenaux said. “But at least [this finding] suggests once more that for a hypomethylating agent to be efficacious, it requires a certain number of cycles, and whenever possible, at least 6 cycles to have full efficacy.”
The study was funded by Astex and Otsuka. The investigators reported additional relationships with Celgene, Janssen, and other companies.
SOURCE: Fenaux P et al. EHA Congress, Abstract S879.
AMSTERDAM – For treatment-naive patients with acute myeloid leukemia (AML) who are ineligible for chemotherapy, guadecitabine offers similar efficacy to other standard treatment options until four cycles are administered, after which guadecitabine offers a slight survival advantage, based on results from the phase 3 ASTRAL-1 trial.
Complete responders also derived greater benefit from guadecitabine, a new hypomethylating agent, reported lead author Pierre Fenaux, MD, PhD, of the Hôpital Saint Louis, Paris.
With 815 patients, ASTRAL-1 was the largest global, randomized trial to compare low-intensity therapy options in this elderly, unfit population – specifically, patients who were at least 75 years old or had an Eastern Cooperative Oncology Group (ECOG) performance status of 3 or more, Dr. Fenaux said at the annual congress of the European Hematology Association.
They were randomized in a 1:1 ratio to receive guadecitabine or one of three other treatment options: azacitidine, decitabine, or low-dose cytarabine. The coprimary endpoints of the trial were complete response rate and median overall survival. Safety measures were also investigated.
A demographic analysis showed that almost two-thirds of patients were at least 75 years old (62%), and about half had an ECOG status of 2 or 3, or bone marrow blasts. Approximately one-third of patients had poor-risk cytogenetics and a slightly higher proportion had secondary AML.
After a median follow-up of 25.5 months, patients had received, on average, five cycles of therapy. However, many patients (42%) received three or fewer cycles because of early death or disease progression. This therapy cessation rate was similar between the guadecitabine group (42.4%) and the other treatment group (40.8%).
The study failed to meet either coprimary endpoint across the entire patient population. Median overall survival was 7.10 months for guadecitabine versus 8.47 months for the other treatments, but this difference was not statistically significant (P = .73). Similarly, the complete response rate was slightly higher for guadecitabine (19.4% vs. 17.4%), but again, this finding carried a nonsignificant P value (P = .48).
The benefit offered by guadecitabine was realized only with extended treatment and in complete responders.
Patients who received a minimum of four cycles of guadecitabine had a median overall survival of 15.6 months, compared with 13.0 months for other treatments (P = .02). This benefit became more pronounced in those who received at least six cycles, which was associated with median overall survival of 19.5 months versus 14.9 months (P = .002). Complete responders also had extended survival when treated with guadecitabine, although this benefit was of a lesser magnitude (22.6 vs. 20.6 months; P = .07).
Most subgroup analyses, accounting for various clinical and genetic factors, showed no significant differences in primary outcomes between treatment arms, with one exception: TP53 mutations were associated with poor responses to guadecitabine, and a lack of the TP53 mutation predicted better responses to guadecitabine.
Adverse events were common, although most measures were not significantly different between treatment arms. For example, serious adverse events occurred in 81% and 75.5% of patients treated with guadecitabine and other options, respectively, while grade 3 or higher adverse events occurred in 91.5% of guadecitabine patients and 87.5% of patients treated with other options, but neither difference was statistically significant.
Adverse events leading to death occurred in 28.7% of patients treated with guadecitabine versus 29.8% of other patients, a nonsignificant difference. In contrast, Dr. Fenaux noted that patients treated with guadecitabine were significantly more likely to develop febrile neutropenia (33.9% vs. 26.5%), neutropenia (27.4% vs. 20.7%), and pneumonia (29.4% vs. 19.6%).
“In those patients [that received at least four cycles], there seemed to be some advantage of guadecitabine, which needs to be further explored,” Dr. Fenaux said. “But at least [this finding] suggests once more that for a hypomethylating agent to be efficacious, it requires a certain number of cycles, and whenever possible, at least 6 cycles to have full efficacy.”
The study was funded by Astex and Otsuka. The investigators reported additional relationships with Celgene, Janssen, and other companies.
SOURCE: Fenaux P et al. EHA Congress, Abstract S879.
AMSTERDAM – For treatment-naive patients with acute myeloid leukemia (AML) who are ineligible for chemotherapy, guadecitabine offers similar efficacy to other standard treatment options until four cycles are administered, after which guadecitabine offers a slight survival advantage, based on results from the phase 3 ASTRAL-1 trial.
Complete responders also derived greater benefit from guadecitabine, a new hypomethylating agent, reported lead author Pierre Fenaux, MD, PhD, of the Hôpital Saint Louis, Paris.
With 815 patients, ASTRAL-1 was the largest global, randomized trial to compare low-intensity therapy options in this elderly, unfit population – specifically, patients who were at least 75 years old or had an Eastern Cooperative Oncology Group (ECOG) performance status of 3 or more, Dr. Fenaux said at the annual congress of the European Hematology Association.
They were randomized in a 1:1 ratio to receive guadecitabine or one of three other treatment options: azacitidine, decitabine, or low-dose cytarabine. The coprimary endpoints of the trial were complete response rate and median overall survival. Safety measures were also investigated.
A demographic analysis showed that almost two-thirds of patients were at least 75 years old (62%), and about half had an ECOG status of 2 or 3, or bone marrow blasts. Approximately one-third of patients had poor-risk cytogenetics and a slightly higher proportion had secondary AML.
After a median follow-up of 25.5 months, patients had received, on average, five cycles of therapy. However, many patients (42%) received three or fewer cycles because of early death or disease progression. This therapy cessation rate was similar between the guadecitabine group (42.4%) and the other treatment group (40.8%).
The study failed to meet either coprimary endpoint across the entire patient population. Median overall survival was 7.10 months for guadecitabine versus 8.47 months for the other treatments, but this difference was not statistically significant (P = .73). Similarly, the complete response rate was slightly higher for guadecitabine (19.4% vs. 17.4%), but again, this finding carried a nonsignificant P value (P = .48).
The benefit offered by guadecitabine was realized only with extended treatment and in complete responders.
Patients who received a minimum of four cycles of guadecitabine had a median overall survival of 15.6 months, compared with 13.0 months for other treatments (P = .02). This benefit became more pronounced in those who received at least six cycles, which was associated with median overall survival of 19.5 months versus 14.9 months (P = .002). Complete responders also had extended survival when treated with guadecitabine, although this benefit was of a lesser magnitude (22.6 vs. 20.6 months; P = .07).
Most subgroup analyses, accounting for various clinical and genetic factors, showed no significant differences in primary outcomes between treatment arms, with one exception: TP53 mutations were associated with poor responses to guadecitabine, and a lack of the TP53 mutation predicted better responses to guadecitabine.
Adverse events were common, although most measures were not significantly different between treatment arms. For example, serious adverse events occurred in 81% and 75.5% of patients treated with guadecitabine and other options, respectively, while grade 3 or higher adverse events occurred in 91.5% of guadecitabine patients and 87.5% of patients treated with other options, but neither difference was statistically significant.
Adverse events leading to death occurred in 28.7% of patients treated with guadecitabine versus 29.8% of other patients, a nonsignificant difference. In contrast, Dr. Fenaux noted that patients treated with guadecitabine were significantly more likely to develop febrile neutropenia (33.9% vs. 26.5%), neutropenia (27.4% vs. 20.7%), and pneumonia (29.4% vs. 19.6%).
“In those patients [that received at least four cycles], there seemed to be some advantage of guadecitabine, which needs to be further explored,” Dr. Fenaux said. “But at least [this finding] suggests once more that for a hypomethylating agent to be efficacious, it requires a certain number of cycles, and whenever possible, at least 6 cycles to have full efficacy.”
The study was funded by Astex and Otsuka. The investigators reported additional relationships with Celgene, Janssen, and other companies.
SOURCE: Fenaux P et al. EHA Congress, Abstract S879.
REPORTING FROM EHA CONGRESS
Dr. Google, potty pot, Snoopy smells cancer
Paging Dr. Google
When something hurts, itches, or burns, to whom do you turn? Not Mom or your physician – we all turn first to Google.
Apparently, sharing symptoms with our omniscient virtual overlords is a national pastime. A recent survey found that nearly 90% of people googled their health symptoms well before going to a doctor. Maybe that’s why we keep getting targeted ads for itchy foot cream?
The survey team constructed a map that broke down the most-googled symptoms for each state. While many states’ highest search were related to cold and flu, some places had more intriguing googles.
Californians have issues with sweaty palms, and New Jerseyans are concerned about their lucid dreaming. Wisconsin seems to have an epidemic of “light-colored poop” (must be all the cheese), while South Carolina has the opposite problem – their most googled symptom is “dark green stool.”
Idaho’s biggest health concern was “symptoms of E. coli”; so, if you’re visiting the Gem State, maybe bring your own food.
Find your state here!
Smartphone skull spikes
Ever felt a little down because you’re just a regular Homo sapiens? Thanks to smartphones, you might be the next step on the evolutionary chain!
Humans have started developing external occipital protuberances – actual spikes – at the base of their skull. Spikes on your skull? How metal is that?
The spike was first observed in 1885. But there has been a rapid increase in the appearance of them, and researchers believe it’s because of smartphones.
Before you start panicking, know that the skull spike is not caused by toxic radiation from your phone. David Shahar’s team from the University of the Sunshine Coast in Australia studied thousands of x-rays. They believe that the skull spikes develop because of the constant hunched-neck position we all take as we pore over our devices for hours a day. The spike is most common in younger people – 1 in 4 people aged 18-30 years had it. This calls for a LOT more yoga.
The nose knows cancer
Just when you thought man’s best friend couldn’t get any better, they go and learn how to smell cancer.
According to a study published in the Journal of the American Osteopathic Association, researchers trained a group of beagles – noted for their superior sense of smell – to sniff out differences in blood samples from healthy patients and those with lung cancer. Snoopy and friends correctly identified the cancerous samples 97% of the time and are now learning how to identify lung, breast, and colorectal cancer using breath samples.
The researchers argued that their findings could pave the way for an over-the-counter test, similar to that used for pregnancy – but where the patient breathes into a device, and it tells them whether they’re positive for cancer or not. However, we suspect the researchers just want to give everyone a dog. There are worse ideas.
And that’s not even the only bit of olfactory-related cancer news we’ve got this week. We’re moving from lung cancer to brain tumors, as a group of researchers at Tampere University in Finland have developed an artificial nose to literally sniff out malignant tissue during surgery.
Electrosurgical resection is common during brain operations, and this process gives off smoke. The nose can detect differences in the smoke from malignant tissue and healthy tissue, allowing the surgeons to more precisely remove tumors from the brain.
No word yet as to whether the surgeons actually have to wear the nose on top of their own, but we can only hope.
Legalizing a not-so-straight flush
How many times has this happened to you? You get up early, hoping to be the first one to the sewage treatment plant so you can get the really fresh wastewater samples. But when you get there, all they have is frozen.
Or maybe you’re part of the research team that analyzed the wastewater of Tacoma, Wash., to determine marijuana usage before and after it became legal in the state. In that case, you’re used to the frozen stuff. Those scientists spent 3 years looking for THC-COOH, which is produced when the psychoactive THC in cannabis is metabolized in the human body, to determine if users were switching from the illegal to the legal market.
Turns out they did. THC-COOH in wastewater increased by 9% per quarter from December 2013 to December 2016, while sales increased by nearly 70% per quarter from Aug. 1, 2014, when legal sales went into effect, to December 2016.
“Given that wastewater represents a total population measure, these findings suggest that many established users switched very quickly from the illegal to the legal market,” team leader Dan Burgard, PhD, of the University of Puget Sound, said in a written statement. “This is the strongest statement possible regarding displacement of the illegal market.”
And the frozen samples? Over the course of the study, the investigators made 387 trips to the two sewage treatment plants. We’ll let MyNorthwest.com explain the rest: “The scientists would pick up a cooler full of frozen wastewater samples, thaw them, and analyze them using liquid chromatography and mass spectrometry.”
Mmm, frozen sewage. Who says science isn’t glamorous?
Paging Dr. Google
When something hurts, itches, or burns, to whom do you turn? Not Mom or your physician – we all turn first to Google.
Apparently, sharing symptoms with our omniscient virtual overlords is a national pastime. A recent survey found that nearly 90% of people googled their health symptoms well before going to a doctor. Maybe that’s why we keep getting targeted ads for itchy foot cream?
The survey team constructed a map that broke down the most-googled symptoms for each state. While many states’ highest search were related to cold and flu, some places had more intriguing googles.
Californians have issues with sweaty palms, and New Jerseyans are concerned about their lucid dreaming. Wisconsin seems to have an epidemic of “light-colored poop” (must be all the cheese), while South Carolina has the opposite problem – their most googled symptom is “dark green stool.”
Idaho’s biggest health concern was “symptoms of E. coli”; so, if you’re visiting the Gem State, maybe bring your own food.
Find your state here!
Smartphone skull spikes
Ever felt a little down because you’re just a regular Homo sapiens? Thanks to smartphones, you might be the next step on the evolutionary chain!
Humans have started developing external occipital protuberances – actual spikes – at the base of their skull. Spikes on your skull? How metal is that?
The spike was first observed in 1885. But there has been a rapid increase in the appearance of them, and researchers believe it’s because of smartphones.
Before you start panicking, know that the skull spike is not caused by toxic radiation from your phone. David Shahar’s team from the University of the Sunshine Coast in Australia studied thousands of x-rays. They believe that the skull spikes develop because of the constant hunched-neck position we all take as we pore over our devices for hours a day. The spike is most common in younger people – 1 in 4 people aged 18-30 years had it. This calls for a LOT more yoga.
The nose knows cancer
Just when you thought man’s best friend couldn’t get any better, they go and learn how to smell cancer.
According to a study published in the Journal of the American Osteopathic Association, researchers trained a group of beagles – noted for their superior sense of smell – to sniff out differences in blood samples from healthy patients and those with lung cancer. Snoopy and friends correctly identified the cancerous samples 97% of the time and are now learning how to identify lung, breast, and colorectal cancer using breath samples.
The researchers argued that their findings could pave the way for an over-the-counter test, similar to that used for pregnancy – but where the patient breathes into a device, and it tells them whether they’re positive for cancer or not. However, we suspect the researchers just want to give everyone a dog. There are worse ideas.
And that’s not even the only bit of olfactory-related cancer news we’ve got this week. We’re moving from lung cancer to brain tumors, as a group of researchers at Tampere University in Finland have developed an artificial nose to literally sniff out malignant tissue during surgery.
Electrosurgical resection is common during brain operations, and this process gives off smoke. The nose can detect differences in the smoke from malignant tissue and healthy tissue, allowing the surgeons to more precisely remove tumors from the brain.
No word yet as to whether the surgeons actually have to wear the nose on top of their own, but we can only hope.
Legalizing a not-so-straight flush
How many times has this happened to you? You get up early, hoping to be the first one to the sewage treatment plant so you can get the really fresh wastewater samples. But when you get there, all they have is frozen.
Or maybe you’re part of the research team that analyzed the wastewater of Tacoma, Wash., to determine marijuana usage before and after it became legal in the state. In that case, you’re used to the frozen stuff. Those scientists spent 3 years looking for THC-COOH, which is produced when the psychoactive THC in cannabis is metabolized in the human body, to determine if users were switching from the illegal to the legal market.
Turns out they did. THC-COOH in wastewater increased by 9% per quarter from December 2013 to December 2016, while sales increased by nearly 70% per quarter from Aug. 1, 2014, when legal sales went into effect, to December 2016.
“Given that wastewater represents a total population measure, these findings suggest that many established users switched very quickly from the illegal to the legal market,” team leader Dan Burgard, PhD, of the University of Puget Sound, said in a written statement. “This is the strongest statement possible regarding displacement of the illegal market.”
And the frozen samples? Over the course of the study, the investigators made 387 trips to the two sewage treatment plants. We’ll let MyNorthwest.com explain the rest: “The scientists would pick up a cooler full of frozen wastewater samples, thaw them, and analyze them using liquid chromatography and mass spectrometry.”
Mmm, frozen sewage. Who says science isn’t glamorous?
Paging Dr. Google
When something hurts, itches, or burns, to whom do you turn? Not Mom or your physician – we all turn first to Google.
Apparently, sharing symptoms with our omniscient virtual overlords is a national pastime. A recent survey found that nearly 90% of people googled their health symptoms well before going to a doctor. Maybe that’s why we keep getting targeted ads for itchy foot cream?
The survey team constructed a map that broke down the most-googled symptoms for each state. While many states’ highest search were related to cold and flu, some places had more intriguing googles.
Californians have issues with sweaty palms, and New Jerseyans are concerned about their lucid dreaming. Wisconsin seems to have an epidemic of “light-colored poop” (must be all the cheese), while South Carolina has the opposite problem – their most googled symptom is “dark green stool.”
Idaho’s biggest health concern was “symptoms of E. coli”; so, if you’re visiting the Gem State, maybe bring your own food.
Find your state here!
Smartphone skull spikes
Ever felt a little down because you’re just a regular Homo sapiens? Thanks to smartphones, you might be the next step on the evolutionary chain!
Humans have started developing external occipital protuberances – actual spikes – at the base of their skull. Spikes on your skull? How metal is that?
The spike was first observed in 1885. But there has been a rapid increase in the appearance of them, and researchers believe it’s because of smartphones.
Before you start panicking, know that the skull spike is not caused by toxic radiation from your phone. David Shahar’s team from the University of the Sunshine Coast in Australia studied thousands of x-rays. They believe that the skull spikes develop because of the constant hunched-neck position we all take as we pore over our devices for hours a day. The spike is most common in younger people – 1 in 4 people aged 18-30 years had it. This calls for a LOT more yoga.
The nose knows cancer
Just when you thought man’s best friend couldn’t get any better, they go and learn how to smell cancer.
According to a study published in the Journal of the American Osteopathic Association, researchers trained a group of beagles – noted for their superior sense of smell – to sniff out differences in blood samples from healthy patients and those with lung cancer. Snoopy and friends correctly identified the cancerous samples 97% of the time and are now learning how to identify lung, breast, and colorectal cancer using breath samples.
The researchers argued that their findings could pave the way for an over-the-counter test, similar to that used for pregnancy – but where the patient breathes into a device, and it tells them whether they’re positive for cancer or not. However, we suspect the researchers just want to give everyone a dog. There are worse ideas.
And that’s not even the only bit of olfactory-related cancer news we’ve got this week. We’re moving from lung cancer to brain tumors, as a group of researchers at Tampere University in Finland have developed an artificial nose to literally sniff out malignant tissue during surgery.
Electrosurgical resection is common during brain operations, and this process gives off smoke. The nose can detect differences in the smoke from malignant tissue and healthy tissue, allowing the surgeons to more precisely remove tumors from the brain.
No word yet as to whether the surgeons actually have to wear the nose on top of their own, but we can only hope.
Legalizing a not-so-straight flush
How many times has this happened to you? You get up early, hoping to be the first one to the sewage treatment plant so you can get the really fresh wastewater samples. But when you get there, all they have is frozen.
Or maybe you’re part of the research team that analyzed the wastewater of Tacoma, Wash., to determine marijuana usage before and after it became legal in the state. In that case, you’re used to the frozen stuff. Those scientists spent 3 years looking for THC-COOH, which is produced when the psychoactive THC in cannabis is metabolized in the human body, to determine if users were switching from the illegal to the legal market.
Turns out they did. THC-COOH in wastewater increased by 9% per quarter from December 2013 to December 2016, while sales increased by nearly 70% per quarter from Aug. 1, 2014, when legal sales went into effect, to December 2016.
“Given that wastewater represents a total population measure, these findings suggest that many established users switched very quickly from the illegal to the legal market,” team leader Dan Burgard, PhD, of the University of Puget Sound, said in a written statement. “This is the strongest statement possible regarding displacement of the illegal market.”
And the frozen samples? Over the course of the study, the investigators made 387 trips to the two sewage treatment plants. We’ll let MyNorthwest.com explain the rest: “The scientists would pick up a cooler full of frozen wastewater samples, thaw them, and analyze them using liquid chromatography and mass spectrometry.”
Mmm, frozen sewage. Who says science isn’t glamorous?
Rituximab and vemurafenib could challenge frontline chemotherapy for HCL
AMSTERDAM – A combination of rituximab and the BRAF inhibitor vemurafenib could be the one-two punch needed for relapsed or refractory hairy cell leukemia (HCL), according to investigators.
Among evaluable patients treated with this combination, 96% achieved complete remission, reported lead author, Enrico Tiacci, MD, of the University and Hospital of Perugia, Italy.
This level of efficacy is “clearly superior to historical results with either agent alone,” Dr. Tiacci said during a presentation at the annual congress of the European Hematology Association, citing previous complete response rates with vemurafenib alone of 35%-40%. “[This combination] has potential for challenging chemotherapy in the frontline setting,” he said.
The phase 2 trial involved 31 patients with relapsed or refractory HCL who had received a median of three previous therapies. Eight of the patients (26%) had primary refractory disease. Patients received vemurafenib 960 mg, twice daily for 8 weeks and rituximab 375 mg/m2, every 2 weeks. After finishing vemurafenib, patients received rituximab four more times, keeping the interval of 2 weeks. Complete remission was defined as a normal blood count, no leukemic cells in bone marrow biopsies and blood smears, and no palpable splenomegaly.
Out of 31 patients, 27 were evaluable at data cutoff. Of these, 26 (96%) achieved complete remission. The investigators noted that two complete responders had incomplete platelet recovery at the end of treatment that resolved soon after, and two patients had persistent splenomegaly, but were considered to be in complete remission at 22.5 and 25 months after finishing therapy.
All of the complete responders had previously received purine analogs, while a few had been refractory to a prior BRAF inhibitor (n = 7) and/or rituximab (n = 5).
The investigators also pointed out that 15 out of 24 evaluable patients (63%) achieved complete remission just 4 weeks after starting the trial regimen. Almost two-thirds of patients (65%) were negative for minimal residual disease (MRD). The rate of progression-free survival at a median follow-up of 29.5 months was 83%. Disease progression occurred exclusively in patients who were MRD positive.
The combination was well tolerated; most adverse events were of grade 1 or 2, overlapping with the safety profile of each agent alone.
Reflecting on the study findings, Dr. Tiacci suggested that the combination could be most effective if delivered immediately, instead of after BRAF failure.
“Interestingly,” he said, “the relapse-free survival in patients naive to a BRAF inhibitor remained significantly longer than the relapse-free interval that patients previously exposed to a BRAF inhibitor enjoyed, both following monotherapy with a BRAF inhibitor and following subsequent combination with rituximab, potentially suggesting that vemurafenib should be used directly in combination with rituximab rather than being delivered first as a monotherapy and then added to rituximab at relapse.”
Randomized testing of the combination against the chemotherapy-based standard of care in the frontline setting is warranted, the investigators concluded.
Dr. Tiacci reported financial relationships with Roche, AbbVie, and Shire.
SOURCE: Tiacci E et al. EHA Congress, Abstract S104.
AMSTERDAM – A combination of rituximab and the BRAF inhibitor vemurafenib could be the one-two punch needed for relapsed or refractory hairy cell leukemia (HCL), according to investigators.
Among evaluable patients treated with this combination, 96% achieved complete remission, reported lead author, Enrico Tiacci, MD, of the University and Hospital of Perugia, Italy.
This level of efficacy is “clearly superior to historical results with either agent alone,” Dr. Tiacci said during a presentation at the annual congress of the European Hematology Association, citing previous complete response rates with vemurafenib alone of 35%-40%. “[This combination] has potential for challenging chemotherapy in the frontline setting,” he said.
The phase 2 trial involved 31 patients with relapsed or refractory HCL who had received a median of three previous therapies. Eight of the patients (26%) had primary refractory disease. Patients received vemurafenib 960 mg, twice daily for 8 weeks and rituximab 375 mg/m2, every 2 weeks. After finishing vemurafenib, patients received rituximab four more times, keeping the interval of 2 weeks. Complete remission was defined as a normal blood count, no leukemic cells in bone marrow biopsies and blood smears, and no palpable splenomegaly.
Out of 31 patients, 27 were evaluable at data cutoff. Of these, 26 (96%) achieved complete remission. The investigators noted that two complete responders had incomplete platelet recovery at the end of treatment that resolved soon after, and two patients had persistent splenomegaly, but were considered to be in complete remission at 22.5 and 25 months after finishing therapy.
All of the complete responders had previously received purine analogs, while a few had been refractory to a prior BRAF inhibitor (n = 7) and/or rituximab (n = 5).
The investigators also pointed out that 15 out of 24 evaluable patients (63%) achieved complete remission just 4 weeks after starting the trial regimen. Almost two-thirds of patients (65%) were negative for minimal residual disease (MRD). The rate of progression-free survival at a median follow-up of 29.5 months was 83%. Disease progression occurred exclusively in patients who were MRD positive.
The combination was well tolerated; most adverse events were of grade 1 or 2, overlapping with the safety profile of each agent alone.
Reflecting on the study findings, Dr. Tiacci suggested that the combination could be most effective if delivered immediately, instead of after BRAF failure.
“Interestingly,” he said, “the relapse-free survival in patients naive to a BRAF inhibitor remained significantly longer than the relapse-free interval that patients previously exposed to a BRAF inhibitor enjoyed, both following monotherapy with a BRAF inhibitor and following subsequent combination with rituximab, potentially suggesting that vemurafenib should be used directly in combination with rituximab rather than being delivered first as a monotherapy and then added to rituximab at relapse.”
Randomized testing of the combination against the chemotherapy-based standard of care in the frontline setting is warranted, the investigators concluded.
Dr. Tiacci reported financial relationships with Roche, AbbVie, and Shire.
SOURCE: Tiacci E et al. EHA Congress, Abstract S104.
AMSTERDAM – A combination of rituximab and the BRAF inhibitor vemurafenib could be the one-two punch needed for relapsed or refractory hairy cell leukemia (HCL), according to investigators.
Among evaluable patients treated with this combination, 96% achieved complete remission, reported lead author, Enrico Tiacci, MD, of the University and Hospital of Perugia, Italy.
This level of efficacy is “clearly superior to historical results with either agent alone,” Dr. Tiacci said during a presentation at the annual congress of the European Hematology Association, citing previous complete response rates with vemurafenib alone of 35%-40%. “[This combination] has potential for challenging chemotherapy in the frontline setting,” he said.
The phase 2 trial involved 31 patients with relapsed or refractory HCL who had received a median of three previous therapies. Eight of the patients (26%) had primary refractory disease. Patients received vemurafenib 960 mg, twice daily for 8 weeks and rituximab 375 mg/m2, every 2 weeks. After finishing vemurafenib, patients received rituximab four more times, keeping the interval of 2 weeks. Complete remission was defined as a normal blood count, no leukemic cells in bone marrow biopsies and blood smears, and no palpable splenomegaly.
Out of 31 patients, 27 were evaluable at data cutoff. Of these, 26 (96%) achieved complete remission. The investigators noted that two complete responders had incomplete platelet recovery at the end of treatment that resolved soon after, and two patients had persistent splenomegaly, but were considered to be in complete remission at 22.5 and 25 months after finishing therapy.
All of the complete responders had previously received purine analogs, while a few had been refractory to a prior BRAF inhibitor (n = 7) and/or rituximab (n = 5).
The investigators also pointed out that 15 out of 24 evaluable patients (63%) achieved complete remission just 4 weeks after starting the trial regimen. Almost two-thirds of patients (65%) were negative for minimal residual disease (MRD). The rate of progression-free survival at a median follow-up of 29.5 months was 83%. Disease progression occurred exclusively in patients who were MRD positive.
The combination was well tolerated; most adverse events were of grade 1 or 2, overlapping with the safety profile of each agent alone.
Reflecting on the study findings, Dr. Tiacci suggested that the combination could be most effective if delivered immediately, instead of after BRAF failure.
“Interestingly,” he said, “the relapse-free survival in patients naive to a BRAF inhibitor remained significantly longer than the relapse-free interval that patients previously exposed to a BRAF inhibitor enjoyed, both following monotherapy with a BRAF inhibitor and following subsequent combination with rituximab, potentially suggesting that vemurafenib should be used directly in combination with rituximab rather than being delivered first as a monotherapy and then added to rituximab at relapse.”
Randomized testing of the combination against the chemotherapy-based standard of care in the frontline setting is warranted, the investigators concluded.
Dr. Tiacci reported financial relationships with Roche, AbbVie, and Shire.
SOURCE: Tiacci E et al. EHA Congress, Abstract S104.
REPORTING FROM EHA CONGRESS
Subset of patients benefits from in-hospital sleep apnea screening
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
EXPERT ANALYSIS FROM SLEEP 2019
Affiliate cancer centers: What’s in a name?
Daniel Boffa, MD, was never able to shake the unfortunate incident from his mind. An acquaintance receiving cancer care at an affiliate center of a top-ranked cancer hospital had experienced a poor, and possibly preventable, outcome.
“As I learned more about the outcome, it became clear to me that the affiliate wasn’t prepared to handle a complication that was not unexpected,” said Dr. Boffa, a thoracic surgeon at Yale Cancer Center in New Haven, Conn. “When I talked to this individual and the people who helped make the decision for where care was going to be given, they kept saying over and over, ‘It says the name of a top-ranked hospital on the sign [of the affiliate], therefore it’s the same.’ ”
The incident compelled Dr. Boffa and colleagues to learn more about the safety at affiliate cancer centers. The result is an analysis that found that patients who underwent complex cancer surgery at affiliate hospitals were significantly more likely to die within 90 days, compared with patients receiving the same surgery at the flagship hospital. When the relative safety of each top-ranked cancer hospital was compared with its collective affiliates, the top-ranked hospital was safer than affiliates in 41 of 49 networks studied (JAMA Netw Open. 2019 Apr 12. doi: 10.1001/jamanetworkopen.2019.1912).
The analysis illustrates that a patient’s chances of surviving a complex cancer surgery are markedly lower at affiliates, compared with the hospital whose brand it shares, Dr. Boffa explained.
“Every patient and everybody that is supporting a patient in making these [care] decisions can’t assume the care is the same,” he said. “This is not to say that the affiliates are unsafe, they are just less safe than the top-ranked hospitals.”
The findings come as more top-ranked cancer hospital align with affiliate cancer centers and grow partnerships with smaller, community hospitals.
Leaders at these institutions say the partnerships expand access to care and enable regional centers to draw from the expertise at specialized cancer hospitals. However, in addition to safety concerns, the recent data pose questions about whether marketing by some institutions is creating inaccurate perceptions about the relationship between top hospitals and their affiliates. At the same time, it’s uncertain whether network affiliations really improve cost or quality, said Lesly Dossett, MD, an oncologist and researcher at the University of Michigan, Ann Arbor.
“Affiliation has the theoretical advantage of improving efficiency and quality across hospitals and facilitating regionalization of the most complex patients,” said Dr. Dossett, who wrote a commentary for JAMA on the subject. “An ideal network would provide the patient the most convenient access to the right specialist and service at the right time. Disadvantages are that patients and families can attribute quality and safety outcomes achieved at the flagship hospital to the smaller branded affiliate and decline to travel to the flagship, even though some services, like complex surgery, may be best delivered at the flagship.”
What’s an affiliate?
Part of the problem is the ambiguity surrounding the many different relationships among top-ranked cancer hospitals and associated institutions, said J. Leonard Lichtenfeld, MD, interim chief medical officer for the American Cancer Society. In some cases, the primary institution is closely aligned with an affiliate, sharing staff and collaborating on patient cases. In other instances, a reputable hospital offers its brand to a hospital in a distant location, and the relationship is more marketing and information-sharing based.
“Just because a name shows up on a building, doesn’t necessarily mean the same level of care is being provided at an affiliated institution,” Dr. Lichtenfeld said. “There are factors that neither you nor me can look into to determine how close the affiliation is. Sometimes, it can be a very tight relationship. Sometimes it can be a loose relationship, and it’s very hard to figure that out.”
In a survey of 1,010 patients, 94% of respondents felt that cancer care at a smaller hospital would improve after affiliating with a larger hospital specializing in cancer, and most patients expected physicians at the larger hospital to be involved considerably in the care of patients at the smaller hospital after affiliation (JAMA Oncol. 2018;4[7]:1008-9).
In another survey, 85% of patients said they would rather travel an hour for complex surgery at a larger hospital specializing in cancer, rather than a smaller local hospital. However, if the smaller hospital was affiliated with a top-ranked cancer hospital, 31% of respondents changed their preference to the smaller hospital, according to the analysis (Ann Surg Oncol. 2019 Mar;26[3]:732-8).
When asked to compare leading cancer hospitals and their smaller affiliates, 47% of respondents said they felt that surgical safety would be the same at both hospitals, 66% said that guideline compliance would be the same, and 53% of patients said they believed that cure rates would be the same at both institutions.
“A majority of the public feels when the brand is shared between a top-ranked hospital and a hospital in the community, the quality and safety is the same,” said Dr. Boffa, a coauthor of both survey studies. “The advertising differs for each affiliate, but there are certainly instances where the messaging can be misinterpreted. There are instances where there are billboards and advertisements where the implication is the community hospital to some degree has care that is similar to the main hospital.”
Hospitals: Collaborations beneficial
For the University of Wisconsin–Madison (UW) Carbone Cancer Center, its relationships with several community institutions have enhanced consistency among physician teams and provided new care opportunities for patients, said Dan Mulkerin, MD, regional director of UW’s regional cancer center network. Carbone includes 12 locales of service and four affiliates in various stages of maturity.
In some cases, UW physicians travel to the affiliate to help care for patients but refer surgeries back to the main institution. In other cases, UW surgical specialists operate jointly with local surgeons at the community hospital, according to Dr. Mulkerin.
The surgical safety of affiliates is an issue that UW started to address about 5 years ago, he said.
“We recognized that this was a potential area of concern for our cancer programs and we started incorporating surgical oncology into our affiliate structure,” Dr. Mulkerin said. “We look at the quality outcomes of our partners on a quarterly basis. That drives our approach about which types of surgery gets triaged to come to the main institution and which types of surgeries can appropriately be done in community settings under our brand, and under our guidance.”
At H. Lee Moffitt Cancer Center & Research Institute, physicians travel from the headquarters to its partner hospitals to deliver care and work with doctors at each site. For example, Moffitt leaders are overseeing a program with Memorial Healthcare System in South Florida that focuses on malignant hematology and blood and marrow transplantation, said Louis Harrison, MD, chair for Moffitt’s radiation oncology department and vice president, chief partnership officer.
“These are real partnerships where we put our faculty and our know-how on the ground at these sites,” he said in an interview. “We feel like the only way to deliver care that is representative to Moffitt is to have Moffitt doctors deliver the care.”
The University of Texas MD Anderson Cancer Center, Houston, meanwhile, boasts a robust network of partner members, certified member hospitals, associate members, and affiliates. Partner members are U.S.-based relationships where member health systems integrate their clinical cancer care operations with MD Anderson, said Michael Kupferman, MD, senior vice president of clinical and academic network development at MD Anderson. Certified members receive assessments by MD Anderson and tailored recommendations for clinical quality improvements, while associate members stem from international clinical relationships with MD Anderson. Affiliates have a relationship with MD Anderson in one specialty- or modality-focused area, such as radiation oncology.
“All MD Anderson Cancer Network members operate independently from MD Anderson Cancer Center,” Dr. Kupferman said in an interview. “All cancer network members have access to our expertise and clinical knowledge to improve the level of cancer care in their communities.”
In terms of marketing the different types of network members, Dr. Kupferman said that MD Anderson collaborates with members on internal and external marketing and communications strategies to offer “best practices and guidance on how to best educate the alignment and benefits of the relationship to internal staff and local communities.”
Dr. Kupferman declined to directly comment on how surgical safety at MD Anderson’s member institutions is addressed or respond to the JAMA study findings. “We believe that our cancer network members strive to practice at a level higher than the national safety average, as evidenced by the consistent quality reviews we conduct with our members.”
An opportunity to improve
A key advantage to affiliate cancer centers is the expanded care access they can provide to patients, said Dr. Lichtenfeld. While some patients can bypass their community hospital and travel to a top-ranked cancer hospital for treatment, others do not have that capability.
“Some people will not leave their communities,” he said. “It would be wrong to take this research and point at bad doctors for not sending their patient [to a more specialized hospital]. Sometimes, its patients themselves, by choice or necessity, who can’t go somewhere else.”
Dr. Boffa emphasized that the recent research on safety presents an exciting opportunity for flagship institutions and their affiliates to analyze their structure and make improvements where necessary.
“The fact that [the hospitals] are already connected in some way is a huge advance; that’s half the battle,” Dr. Boffa said. “The next step is how do we distill what elements of care are transferable. How do we leverage this connection to share what makes the safer hospitals safer?”
Daniel Boffa, MD, was never able to shake the unfortunate incident from his mind. An acquaintance receiving cancer care at an affiliate center of a top-ranked cancer hospital had experienced a poor, and possibly preventable, outcome.
“As I learned more about the outcome, it became clear to me that the affiliate wasn’t prepared to handle a complication that was not unexpected,” said Dr. Boffa, a thoracic surgeon at Yale Cancer Center in New Haven, Conn. “When I talked to this individual and the people who helped make the decision for where care was going to be given, they kept saying over and over, ‘It says the name of a top-ranked hospital on the sign [of the affiliate], therefore it’s the same.’ ”
The incident compelled Dr. Boffa and colleagues to learn more about the safety at affiliate cancer centers. The result is an analysis that found that patients who underwent complex cancer surgery at affiliate hospitals were significantly more likely to die within 90 days, compared with patients receiving the same surgery at the flagship hospital. When the relative safety of each top-ranked cancer hospital was compared with its collective affiliates, the top-ranked hospital was safer than affiliates in 41 of 49 networks studied (JAMA Netw Open. 2019 Apr 12. doi: 10.1001/jamanetworkopen.2019.1912).
The analysis illustrates that a patient’s chances of surviving a complex cancer surgery are markedly lower at affiliates, compared with the hospital whose brand it shares, Dr. Boffa explained.
“Every patient and everybody that is supporting a patient in making these [care] decisions can’t assume the care is the same,” he said. “This is not to say that the affiliates are unsafe, they are just less safe than the top-ranked hospitals.”
The findings come as more top-ranked cancer hospital align with affiliate cancer centers and grow partnerships with smaller, community hospitals.
Leaders at these institutions say the partnerships expand access to care and enable regional centers to draw from the expertise at specialized cancer hospitals. However, in addition to safety concerns, the recent data pose questions about whether marketing by some institutions is creating inaccurate perceptions about the relationship between top hospitals and their affiliates. At the same time, it’s uncertain whether network affiliations really improve cost or quality, said Lesly Dossett, MD, an oncologist and researcher at the University of Michigan, Ann Arbor.
“Affiliation has the theoretical advantage of improving efficiency and quality across hospitals and facilitating regionalization of the most complex patients,” said Dr. Dossett, who wrote a commentary for JAMA on the subject. “An ideal network would provide the patient the most convenient access to the right specialist and service at the right time. Disadvantages are that patients and families can attribute quality and safety outcomes achieved at the flagship hospital to the smaller branded affiliate and decline to travel to the flagship, even though some services, like complex surgery, may be best delivered at the flagship.”
What’s an affiliate?
Part of the problem is the ambiguity surrounding the many different relationships among top-ranked cancer hospitals and associated institutions, said J. Leonard Lichtenfeld, MD, interim chief medical officer for the American Cancer Society. In some cases, the primary institution is closely aligned with an affiliate, sharing staff and collaborating on patient cases. In other instances, a reputable hospital offers its brand to a hospital in a distant location, and the relationship is more marketing and information-sharing based.
“Just because a name shows up on a building, doesn’t necessarily mean the same level of care is being provided at an affiliated institution,” Dr. Lichtenfeld said. “There are factors that neither you nor me can look into to determine how close the affiliation is. Sometimes, it can be a very tight relationship. Sometimes it can be a loose relationship, and it’s very hard to figure that out.”
In a survey of 1,010 patients, 94% of respondents felt that cancer care at a smaller hospital would improve after affiliating with a larger hospital specializing in cancer, and most patients expected physicians at the larger hospital to be involved considerably in the care of patients at the smaller hospital after affiliation (JAMA Oncol. 2018;4[7]:1008-9).
In another survey, 85% of patients said they would rather travel an hour for complex surgery at a larger hospital specializing in cancer, rather than a smaller local hospital. However, if the smaller hospital was affiliated with a top-ranked cancer hospital, 31% of respondents changed their preference to the smaller hospital, according to the analysis (Ann Surg Oncol. 2019 Mar;26[3]:732-8).
When asked to compare leading cancer hospitals and their smaller affiliates, 47% of respondents said they felt that surgical safety would be the same at both hospitals, 66% said that guideline compliance would be the same, and 53% of patients said they believed that cure rates would be the same at both institutions.
“A majority of the public feels when the brand is shared between a top-ranked hospital and a hospital in the community, the quality and safety is the same,” said Dr. Boffa, a coauthor of both survey studies. “The advertising differs for each affiliate, but there are certainly instances where the messaging can be misinterpreted. There are instances where there are billboards and advertisements where the implication is the community hospital to some degree has care that is similar to the main hospital.”
Hospitals: Collaborations beneficial
For the University of Wisconsin–Madison (UW) Carbone Cancer Center, its relationships with several community institutions have enhanced consistency among physician teams and provided new care opportunities for patients, said Dan Mulkerin, MD, regional director of UW’s regional cancer center network. Carbone includes 12 locales of service and four affiliates in various stages of maturity.
In some cases, UW physicians travel to the affiliate to help care for patients but refer surgeries back to the main institution. In other cases, UW surgical specialists operate jointly with local surgeons at the community hospital, according to Dr. Mulkerin.
The surgical safety of affiliates is an issue that UW started to address about 5 years ago, he said.
“We recognized that this was a potential area of concern for our cancer programs and we started incorporating surgical oncology into our affiliate structure,” Dr. Mulkerin said. “We look at the quality outcomes of our partners on a quarterly basis. That drives our approach about which types of surgery gets triaged to come to the main institution and which types of surgeries can appropriately be done in community settings under our brand, and under our guidance.”
At H. Lee Moffitt Cancer Center & Research Institute, physicians travel from the headquarters to its partner hospitals to deliver care and work with doctors at each site. For example, Moffitt leaders are overseeing a program with Memorial Healthcare System in South Florida that focuses on malignant hematology and blood and marrow transplantation, said Louis Harrison, MD, chair for Moffitt’s radiation oncology department and vice president, chief partnership officer.
“These are real partnerships where we put our faculty and our know-how on the ground at these sites,” he said in an interview. “We feel like the only way to deliver care that is representative to Moffitt is to have Moffitt doctors deliver the care.”
The University of Texas MD Anderson Cancer Center, Houston, meanwhile, boasts a robust network of partner members, certified member hospitals, associate members, and affiliates. Partner members are U.S.-based relationships where member health systems integrate their clinical cancer care operations with MD Anderson, said Michael Kupferman, MD, senior vice president of clinical and academic network development at MD Anderson. Certified members receive assessments by MD Anderson and tailored recommendations for clinical quality improvements, while associate members stem from international clinical relationships with MD Anderson. Affiliates have a relationship with MD Anderson in one specialty- or modality-focused area, such as radiation oncology.
“All MD Anderson Cancer Network members operate independently from MD Anderson Cancer Center,” Dr. Kupferman said in an interview. “All cancer network members have access to our expertise and clinical knowledge to improve the level of cancer care in their communities.”
In terms of marketing the different types of network members, Dr. Kupferman said that MD Anderson collaborates with members on internal and external marketing and communications strategies to offer “best practices and guidance on how to best educate the alignment and benefits of the relationship to internal staff and local communities.”
Dr. Kupferman declined to directly comment on how surgical safety at MD Anderson’s member institutions is addressed or respond to the JAMA study findings. “We believe that our cancer network members strive to practice at a level higher than the national safety average, as evidenced by the consistent quality reviews we conduct with our members.”
An opportunity to improve
A key advantage to affiliate cancer centers is the expanded care access they can provide to patients, said Dr. Lichtenfeld. While some patients can bypass their community hospital and travel to a top-ranked cancer hospital for treatment, others do not have that capability.
“Some people will not leave their communities,” he said. “It would be wrong to take this research and point at bad doctors for not sending their patient [to a more specialized hospital]. Sometimes, its patients themselves, by choice or necessity, who can’t go somewhere else.”
Dr. Boffa emphasized that the recent research on safety presents an exciting opportunity for flagship institutions and their affiliates to analyze their structure and make improvements where necessary.
“The fact that [the hospitals] are already connected in some way is a huge advance; that’s half the battle,” Dr. Boffa said. “The next step is how do we distill what elements of care are transferable. How do we leverage this connection to share what makes the safer hospitals safer?”
Daniel Boffa, MD, was never able to shake the unfortunate incident from his mind. An acquaintance receiving cancer care at an affiliate center of a top-ranked cancer hospital had experienced a poor, and possibly preventable, outcome.
“As I learned more about the outcome, it became clear to me that the affiliate wasn’t prepared to handle a complication that was not unexpected,” said Dr. Boffa, a thoracic surgeon at Yale Cancer Center in New Haven, Conn. “When I talked to this individual and the people who helped make the decision for where care was going to be given, they kept saying over and over, ‘It says the name of a top-ranked hospital on the sign [of the affiliate], therefore it’s the same.’ ”
The incident compelled Dr. Boffa and colleagues to learn more about the safety at affiliate cancer centers. The result is an analysis that found that patients who underwent complex cancer surgery at affiliate hospitals were significantly more likely to die within 90 days, compared with patients receiving the same surgery at the flagship hospital. When the relative safety of each top-ranked cancer hospital was compared with its collective affiliates, the top-ranked hospital was safer than affiliates in 41 of 49 networks studied (JAMA Netw Open. 2019 Apr 12. doi: 10.1001/jamanetworkopen.2019.1912).
The analysis illustrates that a patient’s chances of surviving a complex cancer surgery are markedly lower at affiliates, compared with the hospital whose brand it shares, Dr. Boffa explained.
“Every patient and everybody that is supporting a patient in making these [care] decisions can’t assume the care is the same,” he said. “This is not to say that the affiliates are unsafe, they are just less safe than the top-ranked hospitals.”
The findings come as more top-ranked cancer hospital align with affiliate cancer centers and grow partnerships with smaller, community hospitals.
Leaders at these institutions say the partnerships expand access to care and enable regional centers to draw from the expertise at specialized cancer hospitals. However, in addition to safety concerns, the recent data pose questions about whether marketing by some institutions is creating inaccurate perceptions about the relationship between top hospitals and their affiliates. At the same time, it’s uncertain whether network affiliations really improve cost or quality, said Lesly Dossett, MD, an oncologist and researcher at the University of Michigan, Ann Arbor.
“Affiliation has the theoretical advantage of improving efficiency and quality across hospitals and facilitating regionalization of the most complex patients,” said Dr. Dossett, who wrote a commentary for JAMA on the subject. “An ideal network would provide the patient the most convenient access to the right specialist and service at the right time. Disadvantages are that patients and families can attribute quality and safety outcomes achieved at the flagship hospital to the smaller branded affiliate and decline to travel to the flagship, even though some services, like complex surgery, may be best delivered at the flagship.”
What’s an affiliate?
Part of the problem is the ambiguity surrounding the many different relationships among top-ranked cancer hospitals and associated institutions, said J. Leonard Lichtenfeld, MD, interim chief medical officer for the American Cancer Society. In some cases, the primary institution is closely aligned with an affiliate, sharing staff and collaborating on patient cases. In other instances, a reputable hospital offers its brand to a hospital in a distant location, and the relationship is more marketing and information-sharing based.
“Just because a name shows up on a building, doesn’t necessarily mean the same level of care is being provided at an affiliated institution,” Dr. Lichtenfeld said. “There are factors that neither you nor me can look into to determine how close the affiliation is. Sometimes, it can be a very tight relationship. Sometimes it can be a loose relationship, and it’s very hard to figure that out.”
In a survey of 1,010 patients, 94% of respondents felt that cancer care at a smaller hospital would improve after affiliating with a larger hospital specializing in cancer, and most patients expected physicians at the larger hospital to be involved considerably in the care of patients at the smaller hospital after affiliation (JAMA Oncol. 2018;4[7]:1008-9).
In another survey, 85% of patients said they would rather travel an hour for complex surgery at a larger hospital specializing in cancer, rather than a smaller local hospital. However, if the smaller hospital was affiliated with a top-ranked cancer hospital, 31% of respondents changed their preference to the smaller hospital, according to the analysis (Ann Surg Oncol. 2019 Mar;26[3]:732-8).
When asked to compare leading cancer hospitals and their smaller affiliates, 47% of respondents said they felt that surgical safety would be the same at both hospitals, 66% said that guideline compliance would be the same, and 53% of patients said they believed that cure rates would be the same at both institutions.
“A majority of the public feels when the brand is shared between a top-ranked hospital and a hospital in the community, the quality and safety is the same,” said Dr. Boffa, a coauthor of both survey studies. “The advertising differs for each affiliate, but there are certainly instances where the messaging can be misinterpreted. There are instances where there are billboards and advertisements where the implication is the community hospital to some degree has care that is similar to the main hospital.”
Hospitals: Collaborations beneficial
For the University of Wisconsin–Madison (UW) Carbone Cancer Center, its relationships with several community institutions have enhanced consistency among physician teams and provided new care opportunities for patients, said Dan Mulkerin, MD, regional director of UW’s regional cancer center network. Carbone includes 12 locales of service and four affiliates in various stages of maturity.
In some cases, UW physicians travel to the affiliate to help care for patients but refer surgeries back to the main institution. In other cases, UW surgical specialists operate jointly with local surgeons at the community hospital, according to Dr. Mulkerin.
The surgical safety of affiliates is an issue that UW started to address about 5 years ago, he said.
“We recognized that this was a potential area of concern for our cancer programs and we started incorporating surgical oncology into our affiliate structure,” Dr. Mulkerin said. “We look at the quality outcomes of our partners on a quarterly basis. That drives our approach about which types of surgery gets triaged to come to the main institution and which types of surgeries can appropriately be done in community settings under our brand, and under our guidance.”
At H. Lee Moffitt Cancer Center & Research Institute, physicians travel from the headquarters to its partner hospitals to deliver care and work with doctors at each site. For example, Moffitt leaders are overseeing a program with Memorial Healthcare System in South Florida that focuses on malignant hematology and blood and marrow transplantation, said Louis Harrison, MD, chair for Moffitt’s radiation oncology department and vice president, chief partnership officer.
“These are real partnerships where we put our faculty and our know-how on the ground at these sites,” he said in an interview. “We feel like the only way to deliver care that is representative to Moffitt is to have Moffitt doctors deliver the care.”
The University of Texas MD Anderson Cancer Center, Houston, meanwhile, boasts a robust network of partner members, certified member hospitals, associate members, and affiliates. Partner members are U.S.-based relationships where member health systems integrate their clinical cancer care operations with MD Anderson, said Michael Kupferman, MD, senior vice president of clinical and academic network development at MD Anderson. Certified members receive assessments by MD Anderson and tailored recommendations for clinical quality improvements, while associate members stem from international clinical relationships with MD Anderson. Affiliates have a relationship with MD Anderson in one specialty- or modality-focused area, such as radiation oncology.
“All MD Anderson Cancer Network members operate independently from MD Anderson Cancer Center,” Dr. Kupferman said in an interview. “All cancer network members have access to our expertise and clinical knowledge to improve the level of cancer care in their communities.”
In terms of marketing the different types of network members, Dr. Kupferman said that MD Anderson collaborates with members on internal and external marketing and communications strategies to offer “best practices and guidance on how to best educate the alignment and benefits of the relationship to internal staff and local communities.”
Dr. Kupferman declined to directly comment on how surgical safety at MD Anderson’s member institutions is addressed or respond to the JAMA study findings. “We believe that our cancer network members strive to practice at a level higher than the national safety average, as evidenced by the consistent quality reviews we conduct with our members.”
An opportunity to improve
A key advantage to affiliate cancer centers is the expanded care access they can provide to patients, said Dr. Lichtenfeld. While some patients can bypass their community hospital and travel to a top-ranked cancer hospital for treatment, others do not have that capability.
“Some people will not leave their communities,” he said. “It would be wrong to take this research and point at bad doctors for not sending their patient [to a more specialized hospital]. Sometimes, its patients themselves, by choice or necessity, who can’t go somewhere else.”
Dr. Boffa emphasized that the recent research on safety presents an exciting opportunity for flagship institutions and their affiliates to analyze their structure and make improvements where necessary.
“The fact that [the hospitals] are already connected in some way is a huge advance; that’s half the battle,” Dr. Boffa said. “The next step is how do we distill what elements of care are transferable. How do we leverage this connection to share what makes the safer hospitals safer?”