User login
Subcutaneous marstacimab appears safe in hemophilia A and B
MELBOURNE – Subcutaneous administration of the monoclonal antibody marstacimab (PF-06741086) provides significant reductions in bleeding rates for patients with hemophilia A and B, with reasonably safety and tolerability, according to research presented at the International Society on Thrombosis and Haemostasis congress.
Johnny Mahlangu, MBBCh, of the University of Witwatersrand in Johannesburg, South Africa, presented data from a multicenter, international phase 1B/2 open-label study involving 26 patients with severe hemophilia, who had experienced at least six acute bleeding episodes in the 6 months prior to enrollment. Twenty-three patients had hemophilia A, the remaining three patients had hemophilia B, and all were receiving on-demand treatment.
Patients were divided into one of four cohorts. Cohort 1 received a weekly 300-mg dose subcutaneously for 30 days, cohort 2 received a 300-mg loading dose followed by 150 mg weekly for 30 days, cohort 3 received 450 mg weekly for 30 days, and cohort 4 also received a 300-mg weekly dose for 30 days but consisted of patients with inhibitors.
With the primary outcome being safety, the researchers reported no treatment-related serious adverse events. There were four grade 3/4 adverse events, including two subjects who reported injection site reactions, and some generalized pruritus and erythematous rash.
Two patients discontinued treatment after reaching prespecified dose-limiting toxicity related to decreasing fibrinogen levels, compared with baseline. However, Dr. Mahlangu pointed out that, in one of these patients, the fibrinogen levels were still within normal levels but protocol required removing the patient from the study.
The study did see a significant 85%-97% reduction in annualized bleed rates across the four cohorts, including among patients with inhibitors.
“Most patients who were exposed to marstacimab actually did not bleed at all when they were receiving marstacimab, compared to when they weren’t receiving marstacimab,” Dr. Mahlangu said.
While three patients developed antidrug antibodies, this did not appear to impact the pharmacokinetics, pharmacodynamics, or safety, he said. No patients developed neutralizing antibodies, and the pharmacodynamics showed no difference between patients with hemophilia A and B.
“I would like to believe that the results of this study are fairly promising in terms of the safety, the efficacy, the pharmoacokinetics, and the pharmacodynamics,” Dr. Mahlangu said.
There is an unmet need for therapies that can be used in patients with either hemophilia A or B, and with or without inhibitors, Dr. Mahlangu said in an interview. Another unmet need that marstacimab could potentially address is for subcutaneous treatment options, he added.
“We are particularly pleased by the fact that injection-site reactions are very low, and they seem not to carry on every time the patients have injected,” Dr. Mahlangu said.
Commenting on the presentation, session cochair Julia Phillips, MD, from PathLab in Waikato, New Zealand, said subcutaneous treatments offered a huge advantage to patients with hemophilia and their families.
“Often more than one member of the family is affected. So [for] a family with children, if they have several sons with hemophilia, then doing IV injections before school on a regular basis can be quite a big burden on the family,” she said in an interview.
The study was sponsored by Pfizer. Dr. Mahlangu reported research support from and scientific advisory board and speakers bureau roles with several pharmaceutical companies, including Pfizer.
SOURCE: Mahlangu J et al. 2019. ISTH CONGRESS, Abstract OC 11.2.
MELBOURNE – Subcutaneous administration of the monoclonal antibody marstacimab (PF-06741086) provides significant reductions in bleeding rates for patients with hemophilia A and B, with reasonably safety and tolerability, according to research presented at the International Society on Thrombosis and Haemostasis congress.
Johnny Mahlangu, MBBCh, of the University of Witwatersrand in Johannesburg, South Africa, presented data from a multicenter, international phase 1B/2 open-label study involving 26 patients with severe hemophilia, who had experienced at least six acute bleeding episodes in the 6 months prior to enrollment. Twenty-three patients had hemophilia A, the remaining three patients had hemophilia B, and all were receiving on-demand treatment.
Patients were divided into one of four cohorts. Cohort 1 received a weekly 300-mg dose subcutaneously for 30 days, cohort 2 received a 300-mg loading dose followed by 150 mg weekly for 30 days, cohort 3 received 450 mg weekly for 30 days, and cohort 4 also received a 300-mg weekly dose for 30 days but consisted of patients with inhibitors.
With the primary outcome being safety, the researchers reported no treatment-related serious adverse events. There were four grade 3/4 adverse events, including two subjects who reported injection site reactions, and some generalized pruritus and erythematous rash.
Two patients discontinued treatment after reaching prespecified dose-limiting toxicity related to decreasing fibrinogen levels, compared with baseline. However, Dr. Mahlangu pointed out that, in one of these patients, the fibrinogen levels were still within normal levels but protocol required removing the patient from the study.
The study did see a significant 85%-97% reduction in annualized bleed rates across the four cohorts, including among patients with inhibitors.
“Most patients who were exposed to marstacimab actually did not bleed at all when they were receiving marstacimab, compared to when they weren’t receiving marstacimab,” Dr. Mahlangu said.
While three patients developed antidrug antibodies, this did not appear to impact the pharmacokinetics, pharmacodynamics, or safety, he said. No patients developed neutralizing antibodies, and the pharmacodynamics showed no difference between patients with hemophilia A and B.
“I would like to believe that the results of this study are fairly promising in terms of the safety, the efficacy, the pharmoacokinetics, and the pharmacodynamics,” Dr. Mahlangu said.
There is an unmet need for therapies that can be used in patients with either hemophilia A or B, and with or without inhibitors, Dr. Mahlangu said in an interview. Another unmet need that marstacimab could potentially address is for subcutaneous treatment options, he added.
“We are particularly pleased by the fact that injection-site reactions are very low, and they seem not to carry on every time the patients have injected,” Dr. Mahlangu said.
Commenting on the presentation, session cochair Julia Phillips, MD, from PathLab in Waikato, New Zealand, said subcutaneous treatments offered a huge advantage to patients with hemophilia and their families.
“Often more than one member of the family is affected. So [for] a family with children, if they have several sons with hemophilia, then doing IV injections before school on a regular basis can be quite a big burden on the family,” she said in an interview.
The study was sponsored by Pfizer. Dr. Mahlangu reported research support from and scientific advisory board and speakers bureau roles with several pharmaceutical companies, including Pfizer.
SOURCE: Mahlangu J et al. 2019. ISTH CONGRESS, Abstract OC 11.2.
MELBOURNE – Subcutaneous administration of the monoclonal antibody marstacimab (PF-06741086) provides significant reductions in bleeding rates for patients with hemophilia A and B, with reasonably safety and tolerability, according to research presented at the International Society on Thrombosis and Haemostasis congress.
Johnny Mahlangu, MBBCh, of the University of Witwatersrand in Johannesburg, South Africa, presented data from a multicenter, international phase 1B/2 open-label study involving 26 patients with severe hemophilia, who had experienced at least six acute bleeding episodes in the 6 months prior to enrollment. Twenty-three patients had hemophilia A, the remaining three patients had hemophilia B, and all were receiving on-demand treatment.
Patients were divided into one of four cohorts. Cohort 1 received a weekly 300-mg dose subcutaneously for 30 days, cohort 2 received a 300-mg loading dose followed by 150 mg weekly for 30 days, cohort 3 received 450 mg weekly for 30 days, and cohort 4 also received a 300-mg weekly dose for 30 days but consisted of patients with inhibitors.
With the primary outcome being safety, the researchers reported no treatment-related serious adverse events. There were four grade 3/4 adverse events, including two subjects who reported injection site reactions, and some generalized pruritus and erythematous rash.
Two patients discontinued treatment after reaching prespecified dose-limiting toxicity related to decreasing fibrinogen levels, compared with baseline. However, Dr. Mahlangu pointed out that, in one of these patients, the fibrinogen levels were still within normal levels but protocol required removing the patient from the study.
The study did see a significant 85%-97% reduction in annualized bleed rates across the four cohorts, including among patients with inhibitors.
“Most patients who were exposed to marstacimab actually did not bleed at all when they were receiving marstacimab, compared to when they weren’t receiving marstacimab,” Dr. Mahlangu said.
While three patients developed antidrug antibodies, this did not appear to impact the pharmacokinetics, pharmacodynamics, or safety, he said. No patients developed neutralizing antibodies, and the pharmacodynamics showed no difference between patients with hemophilia A and B.
“I would like to believe that the results of this study are fairly promising in terms of the safety, the efficacy, the pharmoacokinetics, and the pharmacodynamics,” Dr. Mahlangu said.
There is an unmet need for therapies that can be used in patients with either hemophilia A or B, and with or without inhibitors, Dr. Mahlangu said in an interview. Another unmet need that marstacimab could potentially address is for subcutaneous treatment options, he added.
“We are particularly pleased by the fact that injection-site reactions are very low, and they seem not to carry on every time the patients have injected,” Dr. Mahlangu said.
Commenting on the presentation, session cochair Julia Phillips, MD, from PathLab in Waikato, New Zealand, said subcutaneous treatments offered a huge advantage to patients with hemophilia and their families.
“Often more than one member of the family is affected. So [for] a family with children, if they have several sons with hemophilia, then doing IV injections before school on a regular basis can be quite a big burden on the family,” she said in an interview.
The study was sponsored by Pfizer. Dr. Mahlangu reported research support from and scientific advisory board and speakers bureau roles with several pharmaceutical companies, including Pfizer.
SOURCE: Mahlangu J et al. 2019. ISTH CONGRESS, Abstract OC 11.2.
REPORTING FROM 2019 ISTH CONGRESS
Bilateral salpingectomy gains favor for sterilization
NASHVILLE, TENN. –
“[It is] probably the newest thing on the block ... this is becoming super widespread,” Eve Espey, MD, said of the procedure during a contraceptive update at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Although evidence directly supporting bilateral salpingectomy for sterilization is lacking, there are good reasons to consider it, she said.
For example, the procedure is likely more effective than tubal ligation with no increased risk for complications, and is probably more likely to cut ovarian cancer risk than is tubal ligation, explained Dr. Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque.
“So we don’t actually have good [randomized controlled trials] on effectiveness for [bilateral] salpingectomy, but it is most like a partial salpingectomy, which is highly effective, so there is reason to believe that it might be more effective,” she added. The downsides are that the procedure may take longer, it may impair ovarian blood supply, and long-term population-level data on outcomes are lacking.
ACOG said in a 2015 committee opinion that when counseling women, bilateral salpingectomy can be discussed and considered “a method that provides effective contraception,” but also stressed the need for randomized controlled trials to support any related reduction in ovarian cancer risk. That opinion (#620) was replaced in April 2019 by Committee Opinion #774, which addresses opportunistic salpingectomy for epithelial ovarian cancer prevention, and which states that “the risks and benefits of salpingectomy should be discussed with patients who desire permanent sterilization.”
“[The Society of Gynecologic Oncology] is much, much more emphatic,” Dr. Espey said, citing a 2013 Clinical Practice Statement calling for discussion and consideration of risk-reducing salpingectomy in lieu of tubal ligation for women at average risk of ovarian cancer (after childbearing).
Dr. Espey also noted that during a recent grand rounds on sterilization, about 90% of participants said they were doing bilateral salpingectomy in the setting of vaginal delivery. “So I think we’re going to see this coming not just with C-section, but also with vaginal delivery.”
Dr. Espey reported having no relevant financial disclosures.
NASHVILLE, TENN. –
“[It is] probably the newest thing on the block ... this is becoming super widespread,” Eve Espey, MD, said of the procedure during a contraceptive update at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Although evidence directly supporting bilateral salpingectomy for sterilization is lacking, there are good reasons to consider it, she said.
For example, the procedure is likely more effective than tubal ligation with no increased risk for complications, and is probably more likely to cut ovarian cancer risk than is tubal ligation, explained Dr. Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque.
“So we don’t actually have good [randomized controlled trials] on effectiveness for [bilateral] salpingectomy, but it is most like a partial salpingectomy, which is highly effective, so there is reason to believe that it might be more effective,” she added. The downsides are that the procedure may take longer, it may impair ovarian blood supply, and long-term population-level data on outcomes are lacking.
ACOG said in a 2015 committee opinion that when counseling women, bilateral salpingectomy can be discussed and considered “a method that provides effective contraception,” but also stressed the need for randomized controlled trials to support any related reduction in ovarian cancer risk. That opinion (#620) was replaced in April 2019 by Committee Opinion #774, which addresses opportunistic salpingectomy for epithelial ovarian cancer prevention, and which states that “the risks and benefits of salpingectomy should be discussed with patients who desire permanent sterilization.”
“[The Society of Gynecologic Oncology] is much, much more emphatic,” Dr. Espey said, citing a 2013 Clinical Practice Statement calling for discussion and consideration of risk-reducing salpingectomy in lieu of tubal ligation for women at average risk of ovarian cancer (after childbearing).
Dr. Espey also noted that during a recent grand rounds on sterilization, about 90% of participants said they were doing bilateral salpingectomy in the setting of vaginal delivery. “So I think we’re going to see this coming not just with C-section, but also with vaginal delivery.”
Dr. Espey reported having no relevant financial disclosures.
NASHVILLE, TENN. –
“[It is] probably the newest thing on the block ... this is becoming super widespread,” Eve Espey, MD, said of the procedure during a contraceptive update at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Although evidence directly supporting bilateral salpingectomy for sterilization is lacking, there are good reasons to consider it, she said.
For example, the procedure is likely more effective than tubal ligation with no increased risk for complications, and is probably more likely to cut ovarian cancer risk than is tubal ligation, explained Dr. Espey, professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship at the University of New Mexico, Albuquerque.
“So we don’t actually have good [randomized controlled trials] on effectiveness for [bilateral] salpingectomy, but it is most like a partial salpingectomy, which is highly effective, so there is reason to believe that it might be more effective,” she added. The downsides are that the procedure may take longer, it may impair ovarian blood supply, and long-term population-level data on outcomes are lacking.
ACOG said in a 2015 committee opinion that when counseling women, bilateral salpingectomy can be discussed and considered “a method that provides effective contraception,” but also stressed the need for randomized controlled trials to support any related reduction in ovarian cancer risk. That opinion (#620) was replaced in April 2019 by Committee Opinion #774, which addresses opportunistic salpingectomy for epithelial ovarian cancer prevention, and which states that “the risks and benefits of salpingectomy should be discussed with patients who desire permanent sterilization.”
“[The Society of Gynecologic Oncology] is much, much more emphatic,” Dr. Espey said, citing a 2013 Clinical Practice Statement calling for discussion and consideration of risk-reducing salpingectomy in lieu of tubal ligation for women at average risk of ovarian cancer (after childbearing).
Dr. Espey also noted that during a recent grand rounds on sterilization, about 90% of participants said they were doing bilateral salpingectomy in the setting of vaginal delivery. “So I think we’re going to see this coming not just with C-section, but also with vaginal delivery.”
Dr. Espey reported having no relevant financial disclosures.
EXPERT COMMENTARY FROM ACOG 2019
Update on Diet and Acne
Acne is a common condition that most often affects adolescents but is not uncommon in adults. It can result in considerable anxiety, depression, and medical and pharmaceutical costs. Additionally, oral antibiotics, the standard treatment for acne, are increasingly under suspicion for causing bacterial resistance as well as disruption of the cutaneous and gut microbiomes.1,2 These factors are among those that often drive patients and physicians to search for alternative and complementary treatments, including dietary modification.
Over the last few decades, the interaction between diet and acne has been one of the most fluid areas of research in dermatology. The role of diet in acne incidence and presentation has evolved from the general view in the 1970s that there was no connection to today’s more data-driven understanding that the acne disease course likely is modified by specific dietary components. Better designed and more rigorous studies have supported a link between acne severity and glycemic index (GI)/glycemic load (GL) and possibly dairy consumption. The ability to use data-driven evidence to counsel patients regarding dietary treatment of acne is increasingly important to counteract the pseudoadvice that patients can easily find on the Internet.
This article summarizes the history of beliefs about diet and acne, reviews more recent published data regarding dietary components that can modify acne severity, and outlines the current American Academy of Dermatology (AAD) guidelines and recommendations for diet and acne.
History of Diet and Acne
In most of the current literature, acne frequently is referred to as a disease of modern civilization or a consequence of the typical Western diet.3 For clarity, the Western diet is most commonly described as “a dietary regimen characterized by high amounts of sugary desserts, refined grains, high protein, high-fat dairy products, and high-sugar drinks.”4 The role of dairy in the etiology of acne typically is discussed separately from the Western diet. It has been reported that acne is not found in nonwesternized populations where a Paleolithic diet, which does not include consumption of high-GI carbohydrates, milk, or other dairy products, is common.5
Extending this line of argument, acne vulgaris has been called a metabolic syndrome of the sebaceous follicle and one of the mammalian target of rapamycin complex 1–driven diseases of civilization, along with cancer, obesity, and diabetes mellitus.3 This view seems somewhat extreme and discounts other drivers of acne incidence and severity. Twin studies have shown that acne is highly heritable, with 81% of the population variance attributed to genetic factors.6 Similar incidence numbers for acne vulgaris have been reported worldwide, and global incidence in late adolescence is rising; however, it is unknown whether this increase is a result of the adoption of the Western diet, which is thought to encourage early onset of puberty; genetic drift; changes in regional and cultural understanding and reporting of acne; or a byproduct of unknown environmental factors.4 More nuanced views acknowledge that acne is a multifactorial disease,7 and therefore genetic and possibly epigenetic factors as well as the cutaneous and gut microbiomes also must be taken into account. An interesting historical perspective on acne by Mahmood and Shipman8 outlined acne descriptions, diagnoses, topical treatments, and dietary advice going back to ancient Greek and Egyptian civilizations. They also cited recommendations from the 1930s that suggested avoiding “starchy foods, bread rolls, noodles, spaghetti, potatoes, oily nuts, chop suey, chow mein, and waffles” and listed the following foods as suitable to cure acne: “cooked and raw fruit, farina, rice, wheat, oatmeal, green vegetables, boiled or broiled meat and poultry, clear soup, vegetable soup, and an abundance of water.”8
More Recent Evidence of Dietary Influence on Acne
Importantly, the available research does not demonstrate that diet causes acne but rather that it may influence or aggravate existing acne. Data collection for acne studies also can be confounded by the interplay of many factors, such as increased access to health care, socioeconomic status, and shifting cultural perceptions of skin care and beauty.4 An important facet of any therapeutic recommendation is that it should be supported by confirmable mechanistic pathways.
GI and GL
Over the last few decades, a number of observational and intervention studies have focused on the possible influence of the GI/GL of foods on acne incidence and/or severity. A high GI diet is characterized by a relatively high intake of carbohydrate-containing foods that are quickly digested and absorbed, increasing blood glucose and insulin concentrations. Glycemic load takes the portion size of dietary carbohydrates into consideration and therefore is a measure of both the quality and quantity of carbohydrate-containing foods.9 TheGI/GL values of more than 2480 food items are available in the literature.10
Evidence from several studies supports the role of high GI/GL diets in exacerbating acne and suggests that transitioning to low GI/GL diets may lead to decreased lesion counts after 12 weeks.11-13 In one randomized controlled trial, male participants aged 15 to 25 years with mild to moderate facial acne were instructed either to eat a high protein/low GI diet or a conventional high GL control diet.13 After 12 weeks, total lesion counts had decreased more in the low GI diet group than the control. As partial confirmation of a mechanistic pathway for a high GI diet and acne, the low GI group demonstrated lower free androgen index and insulin levels than the control group.13 In a Korean study, a 10-week low GL regimen led to a reduction in acne lesion count, a decrease in sebaceous gland size, decreased inflammation, and reduced expression of sterol regulatory element-binding protein 1 and IL-8.14
More recent studies have further solidified the role of high GI/GL diets in acne severity.9,15,16 High GI/GL diets are believed to stimulate acne pathways by stimulating insulinlike growth factor 1 (IGF-1), which induces proliferation of both keratinocytes and sebocytes and simulates androgen production.17 An excellent diagram showing the connection between high GI diets (and dairy) and IGF-1, insulin and its receptors, androgen and its receptors, mammalian target of rapamycin, and the pilosebaceous unit was published in the literature in 2016.4 Interestingly, metformin has been shown to be an effective adjunctive therapy in the treatment of moderate to severe acne vulgaris.18,19
Milk and Dairy Consumption
Milk consumption also has been examined for its potential role in the pathogenesis of acne, including its ability to increase insulin and IGF-1 levels and bind to the human IGF-1 receptor as well as the fact that it contains bovine IGF-1 and dihydrotestosterone precursors.20 Although not studied quite as extensively or rigorously as GI/GL, consumption of milk and dairy products does appear to have the potential to exacerbate acne lesions. Beginning with a series of retrospective and prospective epidemiologic studies published from 2005 to 2008,21-23 a link between clinical acne and milk or dairy consumption in adolescent subjects was reported. A recent meta-analysis found a positive relationship between dairy, total milk, whole milk, low-fat milk, and skim milk consumption and acne occurrence but no significant association between yogurt/cheese consumption and acne development.24
AAD Guidelines
In their public forum, the AAD has advised that a low-glycemic diet may reduce the number of lesions in acne patients and highlighted data from around the world that support the concept that a high-glycemic diet and dairy are correlated with acne severity. They stated that consumption of milk—whole, low fat, and skim—may be linked to an increase in acne breakouts but that no studies have found that products made from milk, such as yogurt or cheese, lead to more breakouts.25
Other Considerations
Acne can be a serious quality-of-life issue with considerable psychological distress, physical morbidity, and social prejudice.9 Consequently, acne patients may be more willing to accept nonprofessional treatment advice, and there is no shortage of non–health care “experts” willing to provide an array of unfounded and fantastical advice. Dietary recommendations found online range from specific “miracle” foods to the more data-driven suggestions to “avoid dairy” or “eat low GI foods.” An important study recently published in Cutis concluded that most of the information found online regarding diet and acne is unfounded and/or misleading.26
Two additional reasons for recommending that acne patients consider dietary modification are not directly related to the disease: (1) the general health benefits of a lower GI/GL diet, and (2) the potential for decreasing the use of antibiotics. Antibiotic resistance is a growing problem across medicine, and dermatologists prescribe more antibiotics per provider than any other specialty.17 Dietary modification, where appropriate, could provide an approach to limiting the use of antibiotics in acne.
Final Thoughts
When advising acne patients, dermatologists can refer to the Table for general guidelines that incorporate the most current data-driven information on the relationship between diet and acne. Dietary modification, of course, will not work for all but can be safely recommended in cases of mild to moderate acne.

- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016 [published online January 16, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2018.4944.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle [published online September 8, 2017]. Clin Dermatol. 2018;36:29-40.
- Lynn DD, Umari T, Dunnick CA, et al. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13-25.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris [published online February 17, 2016]. J Am Acad Dermatol. 2016;74:945.e33-973.e33.
- Rezakovic´ S, Bukvic´ Mokos Z, Basta-Juzbašic´ A. Acne and diet: facts and controversies. Acta Dermatovenerol Croat. 2012;20:170-174.
- Mahmood NF, Shipman AR. The age-old problem of acne. Int J Womens Dermatol. 2017;3:71-76.
- Burris J, Shikany JM, Rietkerk W, et al. A low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008 [published online October 3, 2008]. Diabetes Care. 2008;31:2281-2283.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52
- Smith RN, Braue A, Varigos GA, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Burris J, Rietkerk W, Woolf K. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Burris J, Rietkerk W, Woolf K. Relationships of self-reported dietary factors and perceived acne severity in a cohort of New York young adults [published online January 9, 2014]. J Acad Nutr Diet. 2014;114:384-392.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016 [published online January 16, 2019]. JAMA Dermatol. 2019. doi:10.1001/jamadermatol.2018.4944.
- Lee JK, Smith AD. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris [published online November 15, 2017]. Dermatol Online J. 2017;23. pii:13030/qt53m2q13s.
- Robinson S, Kwan Z, Tang MM. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris: a randomized open-labeled study [published online May 1, 2019]. Dermatol Ther. 2019. doi:10.1111/dth.12953.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limitsystemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments [published online October 5, 2018]. J Am Acad Dermatol. 2019;80:538-549.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Can the right diet get rid of acne? American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/can-the-right-diet-get-rid-of-acne. Accessed June 13, 2019.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44-46, 48.
Acne is a common condition that most often affects adolescents but is not uncommon in adults. It can result in considerable anxiety, depression, and medical and pharmaceutical costs. Additionally, oral antibiotics, the standard treatment for acne, are increasingly under suspicion for causing bacterial resistance as well as disruption of the cutaneous and gut microbiomes.1,2 These factors are among those that often drive patients and physicians to search for alternative and complementary treatments, including dietary modification.
Over the last few decades, the interaction between diet and acne has been one of the most fluid areas of research in dermatology. The role of diet in acne incidence and presentation has evolved from the general view in the 1970s that there was no connection to today’s more data-driven understanding that the acne disease course likely is modified by specific dietary components. Better designed and more rigorous studies have supported a link between acne severity and glycemic index (GI)/glycemic load (GL) and possibly dairy consumption. The ability to use data-driven evidence to counsel patients regarding dietary treatment of acne is increasingly important to counteract the pseudoadvice that patients can easily find on the Internet.
This article summarizes the history of beliefs about diet and acne, reviews more recent published data regarding dietary components that can modify acne severity, and outlines the current American Academy of Dermatology (AAD) guidelines and recommendations for diet and acne.
History of Diet and Acne
In most of the current literature, acne frequently is referred to as a disease of modern civilization or a consequence of the typical Western diet.3 For clarity, the Western diet is most commonly described as “a dietary regimen characterized by high amounts of sugary desserts, refined grains, high protein, high-fat dairy products, and high-sugar drinks.”4 The role of dairy in the etiology of acne typically is discussed separately from the Western diet. It has been reported that acne is not found in nonwesternized populations where a Paleolithic diet, which does not include consumption of high-GI carbohydrates, milk, or other dairy products, is common.5
Extending this line of argument, acne vulgaris has been called a metabolic syndrome of the sebaceous follicle and one of the mammalian target of rapamycin complex 1–driven diseases of civilization, along with cancer, obesity, and diabetes mellitus.3 This view seems somewhat extreme and discounts other drivers of acne incidence and severity. Twin studies have shown that acne is highly heritable, with 81% of the population variance attributed to genetic factors.6 Similar incidence numbers for acne vulgaris have been reported worldwide, and global incidence in late adolescence is rising; however, it is unknown whether this increase is a result of the adoption of the Western diet, which is thought to encourage early onset of puberty; genetic drift; changes in regional and cultural understanding and reporting of acne; or a byproduct of unknown environmental factors.4 More nuanced views acknowledge that acne is a multifactorial disease,7 and therefore genetic and possibly epigenetic factors as well as the cutaneous and gut microbiomes also must be taken into account. An interesting historical perspective on acne by Mahmood and Shipman8 outlined acne descriptions, diagnoses, topical treatments, and dietary advice going back to ancient Greek and Egyptian civilizations. They also cited recommendations from the 1930s that suggested avoiding “starchy foods, bread rolls, noodles, spaghetti, potatoes, oily nuts, chop suey, chow mein, and waffles” and listed the following foods as suitable to cure acne: “cooked and raw fruit, farina, rice, wheat, oatmeal, green vegetables, boiled or broiled meat and poultry, clear soup, vegetable soup, and an abundance of water.”8
More Recent Evidence of Dietary Influence on Acne
Importantly, the available research does not demonstrate that diet causes acne but rather that it may influence or aggravate existing acne. Data collection for acne studies also can be confounded by the interplay of many factors, such as increased access to health care, socioeconomic status, and shifting cultural perceptions of skin care and beauty.4 An important facet of any therapeutic recommendation is that it should be supported by confirmable mechanistic pathways.
GI and GL
Over the last few decades, a number of observational and intervention studies have focused on the possible influence of the GI/GL of foods on acne incidence and/or severity. A high GI diet is characterized by a relatively high intake of carbohydrate-containing foods that are quickly digested and absorbed, increasing blood glucose and insulin concentrations. Glycemic load takes the portion size of dietary carbohydrates into consideration and therefore is a measure of both the quality and quantity of carbohydrate-containing foods.9 TheGI/GL values of more than 2480 food items are available in the literature.10
Evidence from several studies supports the role of high GI/GL diets in exacerbating acne and suggests that transitioning to low GI/GL diets may lead to decreased lesion counts after 12 weeks.11-13 In one randomized controlled trial, male participants aged 15 to 25 years with mild to moderate facial acne were instructed either to eat a high protein/low GI diet or a conventional high GL control diet.13 After 12 weeks, total lesion counts had decreased more in the low GI diet group than the control. As partial confirmation of a mechanistic pathway for a high GI diet and acne, the low GI group demonstrated lower free androgen index and insulin levels than the control group.13 In a Korean study, a 10-week low GL regimen led to a reduction in acne lesion count, a decrease in sebaceous gland size, decreased inflammation, and reduced expression of sterol regulatory element-binding protein 1 and IL-8.14
More recent studies have further solidified the role of high GI/GL diets in acne severity.9,15,16 High GI/GL diets are believed to stimulate acne pathways by stimulating insulinlike growth factor 1 (IGF-1), which induces proliferation of both keratinocytes and sebocytes and simulates androgen production.17 An excellent diagram showing the connection between high GI diets (and dairy) and IGF-1, insulin and its receptors, androgen and its receptors, mammalian target of rapamycin, and the pilosebaceous unit was published in the literature in 2016.4 Interestingly, metformin has been shown to be an effective adjunctive therapy in the treatment of moderate to severe acne vulgaris.18,19
Milk and Dairy Consumption
Milk consumption also has been examined for its potential role in the pathogenesis of acne, including its ability to increase insulin and IGF-1 levels and bind to the human IGF-1 receptor as well as the fact that it contains bovine IGF-1 and dihydrotestosterone precursors.20 Although not studied quite as extensively or rigorously as GI/GL, consumption of milk and dairy products does appear to have the potential to exacerbate acne lesions. Beginning with a series of retrospective and prospective epidemiologic studies published from 2005 to 2008,21-23 a link between clinical acne and milk or dairy consumption in adolescent subjects was reported. A recent meta-analysis found a positive relationship between dairy, total milk, whole milk, low-fat milk, and skim milk consumption and acne occurrence but no significant association between yogurt/cheese consumption and acne development.24
AAD Guidelines
In their public forum, the AAD has advised that a low-glycemic diet may reduce the number of lesions in acne patients and highlighted data from around the world that support the concept that a high-glycemic diet and dairy are correlated with acne severity. They stated that consumption of milk—whole, low fat, and skim—may be linked to an increase in acne breakouts but that no studies have found that products made from milk, such as yogurt or cheese, lead to more breakouts.25
Other Considerations
Acne can be a serious quality-of-life issue with considerable psychological distress, physical morbidity, and social prejudice.9 Consequently, acne patients may be more willing to accept nonprofessional treatment advice, and there is no shortage of non–health care “experts” willing to provide an array of unfounded and fantastical advice. Dietary recommendations found online range from specific “miracle” foods to the more data-driven suggestions to “avoid dairy” or “eat low GI foods.” An important study recently published in Cutis concluded that most of the information found online regarding diet and acne is unfounded and/or misleading.26
Two additional reasons for recommending that acne patients consider dietary modification are not directly related to the disease: (1) the general health benefits of a lower GI/GL diet, and (2) the potential for decreasing the use of antibiotics. Antibiotic resistance is a growing problem across medicine, and dermatologists prescribe more antibiotics per provider than any other specialty.17 Dietary modification, where appropriate, could provide an approach to limiting the use of antibiotics in acne.
Final Thoughts
When advising acne patients, dermatologists can refer to the Table for general guidelines that incorporate the most current data-driven information on the relationship between diet and acne. Dietary modification, of course, will not work for all but can be safely recommended in cases of mild to moderate acne.

Acne is a common condition that most often affects adolescents but is not uncommon in adults. It can result in considerable anxiety, depression, and medical and pharmaceutical costs. Additionally, oral antibiotics, the standard treatment for acne, are increasingly under suspicion for causing bacterial resistance as well as disruption of the cutaneous and gut microbiomes.1,2 These factors are among those that often drive patients and physicians to search for alternative and complementary treatments, including dietary modification.
Over the last few decades, the interaction between diet and acne has been one of the most fluid areas of research in dermatology. The role of diet in acne incidence and presentation has evolved from the general view in the 1970s that there was no connection to today’s more data-driven understanding that the acne disease course likely is modified by specific dietary components. Better designed and more rigorous studies have supported a link between acne severity and glycemic index (GI)/glycemic load (GL) and possibly dairy consumption. The ability to use data-driven evidence to counsel patients regarding dietary treatment of acne is increasingly important to counteract the pseudoadvice that patients can easily find on the Internet.
This article summarizes the history of beliefs about diet and acne, reviews more recent published data regarding dietary components that can modify acne severity, and outlines the current American Academy of Dermatology (AAD) guidelines and recommendations for diet and acne.
History of Diet and Acne
In most of the current literature, acne frequently is referred to as a disease of modern civilization or a consequence of the typical Western diet.3 For clarity, the Western diet is most commonly described as “a dietary regimen characterized by high amounts of sugary desserts, refined grains, high protein, high-fat dairy products, and high-sugar drinks.”4 The role of dairy in the etiology of acne typically is discussed separately from the Western diet. It has been reported that acne is not found in nonwesternized populations where a Paleolithic diet, which does not include consumption of high-GI carbohydrates, milk, or other dairy products, is common.5
Extending this line of argument, acne vulgaris has been called a metabolic syndrome of the sebaceous follicle and one of the mammalian target of rapamycin complex 1–driven diseases of civilization, along with cancer, obesity, and diabetes mellitus.3 This view seems somewhat extreme and discounts other drivers of acne incidence and severity. Twin studies have shown that acne is highly heritable, with 81% of the population variance attributed to genetic factors.6 Similar incidence numbers for acne vulgaris have been reported worldwide, and global incidence in late adolescence is rising; however, it is unknown whether this increase is a result of the adoption of the Western diet, which is thought to encourage early onset of puberty; genetic drift; changes in regional and cultural understanding and reporting of acne; or a byproduct of unknown environmental factors.4 More nuanced views acknowledge that acne is a multifactorial disease,7 and therefore genetic and possibly epigenetic factors as well as the cutaneous and gut microbiomes also must be taken into account. An interesting historical perspective on acne by Mahmood and Shipman8 outlined acne descriptions, diagnoses, topical treatments, and dietary advice going back to ancient Greek and Egyptian civilizations. They also cited recommendations from the 1930s that suggested avoiding “starchy foods, bread rolls, noodles, spaghetti, potatoes, oily nuts, chop suey, chow mein, and waffles” and listed the following foods as suitable to cure acne: “cooked and raw fruit, farina, rice, wheat, oatmeal, green vegetables, boiled or broiled meat and poultry, clear soup, vegetable soup, and an abundance of water.”8
More Recent Evidence of Dietary Influence on Acne
Importantly, the available research does not demonstrate that diet causes acne but rather that it may influence or aggravate existing acne. Data collection for acne studies also can be confounded by the interplay of many factors, such as increased access to health care, socioeconomic status, and shifting cultural perceptions of skin care and beauty.4 An important facet of any therapeutic recommendation is that it should be supported by confirmable mechanistic pathways.
GI and GL
Over the last few decades, a number of observational and intervention studies have focused on the possible influence of the GI/GL of foods on acne incidence and/or severity. A high GI diet is characterized by a relatively high intake of carbohydrate-containing foods that are quickly digested and absorbed, increasing blood glucose and insulin concentrations. Glycemic load takes the portion size of dietary carbohydrates into consideration and therefore is a measure of both the quality and quantity of carbohydrate-containing foods.9 TheGI/GL values of more than 2480 food items are available in the literature.10
Evidence from several studies supports the role of high GI/GL diets in exacerbating acne and suggests that transitioning to low GI/GL diets may lead to decreased lesion counts after 12 weeks.11-13 In one randomized controlled trial, male participants aged 15 to 25 years with mild to moderate facial acne were instructed either to eat a high protein/low GI diet or a conventional high GL control diet.13 After 12 weeks, total lesion counts had decreased more in the low GI diet group than the control. As partial confirmation of a mechanistic pathway for a high GI diet and acne, the low GI group demonstrated lower free androgen index and insulin levels than the control group.13 In a Korean study, a 10-week low GL regimen led to a reduction in acne lesion count, a decrease in sebaceous gland size, decreased inflammation, and reduced expression of sterol regulatory element-binding protein 1 and IL-8.14
More recent studies have further solidified the role of high GI/GL diets in acne severity.9,15,16 High GI/GL diets are believed to stimulate acne pathways by stimulating insulinlike growth factor 1 (IGF-1), which induces proliferation of both keratinocytes and sebocytes and simulates androgen production.17 An excellent diagram showing the connection between high GI diets (and dairy) and IGF-1, insulin and its receptors, androgen and its receptors, mammalian target of rapamycin, and the pilosebaceous unit was published in the literature in 2016.4 Interestingly, metformin has been shown to be an effective adjunctive therapy in the treatment of moderate to severe acne vulgaris.18,19
Milk and Dairy Consumption
Milk consumption also has been examined for its potential role in the pathogenesis of acne, including its ability to increase insulin and IGF-1 levels and bind to the human IGF-1 receptor as well as the fact that it contains bovine IGF-1 and dihydrotestosterone precursors.20 Although not studied quite as extensively or rigorously as GI/GL, consumption of milk and dairy products does appear to have the potential to exacerbate acne lesions. Beginning with a series of retrospective and prospective epidemiologic studies published from 2005 to 2008,21-23 a link between clinical acne and milk or dairy consumption in adolescent subjects was reported. A recent meta-analysis found a positive relationship between dairy, total milk, whole milk, low-fat milk, and skim milk consumption and acne occurrence but no significant association between yogurt/cheese consumption and acne development.24
AAD Guidelines
In their public forum, the AAD has advised that a low-glycemic diet may reduce the number of lesions in acne patients and highlighted data from around the world that support the concept that a high-glycemic diet and dairy are correlated with acne severity. They stated that consumption of milk—whole, low fat, and skim—may be linked to an increase in acne breakouts but that no studies have found that products made from milk, such as yogurt or cheese, lead to more breakouts.25
Other Considerations
Acne can be a serious quality-of-life issue with considerable psychological distress, physical morbidity, and social prejudice.9 Consequently, acne patients may be more willing to accept nonprofessional treatment advice, and there is no shortage of non–health care “experts” willing to provide an array of unfounded and fantastical advice. Dietary recommendations found online range from specific “miracle” foods to the more data-driven suggestions to “avoid dairy” or “eat low GI foods.” An important study recently published in Cutis concluded that most of the information found online regarding diet and acne is unfounded and/or misleading.26
Two additional reasons for recommending that acne patients consider dietary modification are not directly related to the disease: (1) the general health benefits of a lower GI/GL diet, and (2) the potential for decreasing the use of antibiotics. Antibiotic resistance is a growing problem across medicine, and dermatologists prescribe more antibiotics per provider than any other specialty.17 Dietary modification, where appropriate, could provide an approach to limiting the use of antibiotics in acne.
Final Thoughts
When advising acne patients, dermatologists can refer to the Table for general guidelines that incorporate the most current data-driven information on the relationship between diet and acne. Dietary modification, of course, will not work for all but can be safely recommended in cases of mild to moderate acne.

- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016 [published online January 16, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2018.4944.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle [published online September 8, 2017]. Clin Dermatol. 2018;36:29-40.
- Lynn DD, Umari T, Dunnick CA, et al. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13-25.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris [published online February 17, 2016]. J Am Acad Dermatol. 2016;74:945.e33-973.e33.
- Rezakovic´ S, Bukvic´ Mokos Z, Basta-Juzbašic´ A. Acne and diet: facts and controversies. Acta Dermatovenerol Croat. 2012;20:170-174.
- Mahmood NF, Shipman AR. The age-old problem of acne. Int J Womens Dermatol. 2017;3:71-76.
- Burris J, Shikany JM, Rietkerk W, et al. A low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008 [published online October 3, 2008]. Diabetes Care. 2008;31:2281-2283.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52
- Smith RN, Braue A, Varigos GA, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Burris J, Rietkerk W, Woolf K. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Burris J, Rietkerk W, Woolf K. Relationships of self-reported dietary factors and perceived acne severity in a cohort of New York young adults [published online January 9, 2014]. J Acad Nutr Diet. 2014;114:384-392.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016 [published online January 16, 2019]. JAMA Dermatol. 2019. doi:10.1001/jamadermatol.2018.4944.
- Lee JK, Smith AD. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris [published online November 15, 2017]. Dermatol Online J. 2017;23. pii:13030/qt53m2q13s.
- Robinson S, Kwan Z, Tang MM. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris: a randomized open-labeled study [published online May 1, 2019]. Dermatol Ther. 2019. doi:10.1111/dth.12953.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limitsystemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments [published online October 5, 2018]. J Am Acad Dermatol. 2019;80:538-549.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Can the right diet get rid of acne? American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/can-the-right-diet-get-rid-of-acne. Accessed June 13, 2019.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44-46, 48.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016 [published online January 16, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2018.4944.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle [published online September 8, 2017]. Clin Dermatol. 2018;36:29-40.
- Lynn DD, Umari T, Dunnick CA, et al. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13-25.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris [published online February 17, 2016]. J Am Acad Dermatol. 2016;74:945.e33-973.e33.
- Rezakovic´ S, Bukvic´ Mokos Z, Basta-Juzbašic´ A. Acne and diet: facts and controversies. Acta Dermatovenerol Croat. 2012;20:170-174.
- Mahmood NF, Shipman AR. The age-old problem of acne. Int J Womens Dermatol. 2017;3:71-76.
- Burris J, Shikany JM, Rietkerk W, et al. A low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008 [published online October 3, 2008]. Diabetes Care. 2008;31:2281-2283.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52
- Smith RN, Braue A, Varigos GA, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Burris J, Rietkerk W, Woolf K. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Burris J, Rietkerk W, Woolf K. Relationships of self-reported dietary factors and perceived acne severity in a cohort of New York young adults [published online January 9, 2014]. J Acad Nutr Diet. 2014;114:384-392.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016 [published online January 16, 2019]. JAMA Dermatol. 2019. doi:10.1001/jamadermatol.2018.4944.
- Lee JK, Smith AD. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris [published online November 15, 2017]. Dermatol Online J. 2017;23. pii:13030/qt53m2q13s.
- Robinson S, Kwan Z, Tang MM. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris: a randomized open-labeled study [published online May 1, 2019]. Dermatol Ther. 2019. doi:10.1111/dth.12953.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limitsystemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments [published online October 5, 2018]. J Am Acad Dermatol. 2019;80:538-549.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Can the right diet get rid of acne? American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/can-the-right-diet-get-rid-of-acne. Accessed June 13, 2019.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44-46, 48.
No reduction in PE risk with vena cava filters after severe injury
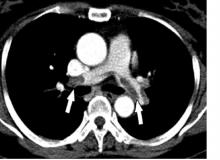
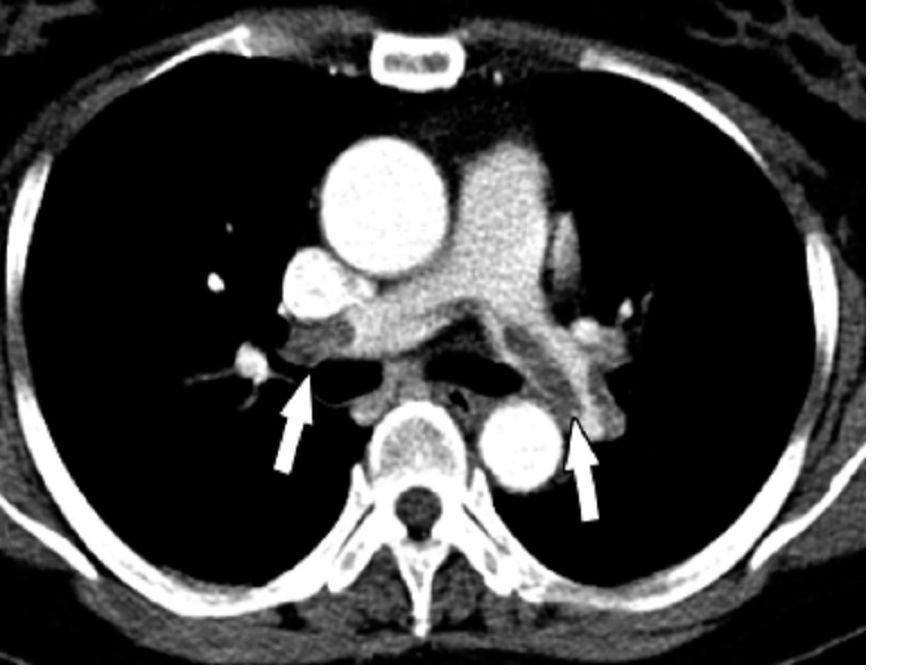
MELBOURNE – Use of a prophylactic vena cava filter to trap blood clots in severely injured patients does not appear to reduce the risk of pulmonary embolism or death, according to data presented at the International Society on Thrombosis and Haemostasis congress.
The researchers reported the outcomes of a multicenter, controlled trial in which 240 severely injured patients with a contraindication to anticoagulants were randomized to receive a vena cava filter within 72 hours of admission, or no filter. The findings were published simultaneously in the New England Journal of Medicine.
The study showed no significant differences between the filter and no-filter groups in the primary outcome of a composite of symptomatic pulmonary embolism or death from any cause at 90 days after enrollment (13.9% vs. 14.4% respectively, P = .98).
In a prespecified subgroup analysis, researchers examined patients who survived 7 days after injury and did not receive prophylactic anticoagulation in those 7 days. Among this group of patients, none of those who received the vena cava filter experienced a symptomatic pulmonary embolism between day 8 and day 90, but five patients (14.7%) in the no-filter group did.
Filters were left in place for a median duration of 27 days (11-90 days). Among the 122 patients who received a filter – which included two patients in the control group – researchers found trapped thrombi in the filter in six patients.
Transfusion requirements, and the incidence of major and nonmajor bleeding and leg deep vein thrombosis, were similar between the filter and no-filter groups. Seven patients in the filter group (5.7%) required more than one attempt to remove the filter, and in one patient the filter had to be removed surgically.
Kwok M. Ho, PhD, of the department of intensive care medicine at Royal Perth Hospital, Australia, and coauthors wrote that while vena cava filters are widely used in trauma centers to prevent pulmonary embolism in patients at high risk of bleeding, there are conflicting recommendations regarding their use, and most studies so far have been observational.
“Given the cost and risks associated with a vena cava filter, our data suggest that there is no urgency to insert the filter in patients who can be treated with prophylactic anticoagulation within 7 days after injury,” they wrote. “Unnecessary insertion of a vena cava filter has the potential to cause harm.”
However, they noted that patients with multiple, large intracranial hematomas were particularly at risk from bleeding with anticoagulant therapy, and therefore may benefit from the use of a vena cava filter.
The Medical Research Foundation of Royal Perth Hospital and the Western Australian Department of Health funded the study. Dr. Ho reported funding from the Western Australian Department of Health and the Raine Medical Research Foundation to conduct the study, as well as serving as an adviser to Medtronic and Cardinal Health.
SOURCE: Ho KM et al. N Engl J Med. 2019 Jul 7. doi: 10.156/NEJMoa1806515.
MELBOURNE – Use of a prophylactic vena cava filter to trap blood clots in severely injured patients does not appear to reduce the risk of pulmonary embolism or death, according to data presented at the International Society on Thrombosis and Haemostasis congress.
The researchers reported the outcomes of a multicenter, controlled trial in which 240 severely injured patients with a contraindication to anticoagulants were randomized to receive a vena cava filter within 72 hours of admission, or no filter. The findings were published simultaneously in the New England Journal of Medicine.
The study showed no significant differences between the filter and no-filter groups in the primary outcome of a composite of symptomatic pulmonary embolism or death from any cause at 90 days after enrollment (13.9% vs. 14.4% respectively, P = .98).
In a prespecified subgroup analysis, researchers examined patients who survived 7 days after injury and did not receive prophylactic anticoagulation in those 7 days. Among this group of patients, none of those who received the vena cava filter experienced a symptomatic pulmonary embolism between day 8 and day 90, but five patients (14.7%) in the no-filter group did.
Filters were left in place for a median duration of 27 days (11-90 days). Among the 122 patients who received a filter – which included two patients in the control group – researchers found trapped thrombi in the filter in six patients.
Transfusion requirements, and the incidence of major and nonmajor bleeding and leg deep vein thrombosis, were similar between the filter and no-filter groups. Seven patients in the filter group (5.7%) required more than one attempt to remove the filter, and in one patient the filter had to be removed surgically.
Kwok M. Ho, PhD, of the department of intensive care medicine at Royal Perth Hospital, Australia, and coauthors wrote that while vena cava filters are widely used in trauma centers to prevent pulmonary embolism in patients at high risk of bleeding, there are conflicting recommendations regarding their use, and most studies so far have been observational.
“Given the cost and risks associated with a vena cava filter, our data suggest that there is no urgency to insert the filter in patients who can be treated with prophylactic anticoagulation within 7 days after injury,” they wrote. “Unnecessary insertion of a vena cava filter has the potential to cause harm.”
However, they noted that patients with multiple, large intracranial hematomas were particularly at risk from bleeding with anticoagulant therapy, and therefore may benefit from the use of a vena cava filter.
The Medical Research Foundation of Royal Perth Hospital and the Western Australian Department of Health funded the study. Dr. Ho reported funding from the Western Australian Department of Health and the Raine Medical Research Foundation to conduct the study, as well as serving as an adviser to Medtronic and Cardinal Health.
SOURCE: Ho KM et al. N Engl J Med. 2019 Jul 7. doi: 10.156/NEJMoa1806515.
MELBOURNE – Use of a prophylactic vena cava filter to trap blood clots in severely injured patients does not appear to reduce the risk of pulmonary embolism or death, according to data presented at the International Society on Thrombosis and Haemostasis congress.
The researchers reported the outcomes of a multicenter, controlled trial in which 240 severely injured patients with a contraindication to anticoagulants were randomized to receive a vena cava filter within 72 hours of admission, or no filter. The findings were published simultaneously in the New England Journal of Medicine.
The study showed no significant differences between the filter and no-filter groups in the primary outcome of a composite of symptomatic pulmonary embolism or death from any cause at 90 days after enrollment (13.9% vs. 14.4% respectively, P = .98).
In a prespecified subgroup analysis, researchers examined patients who survived 7 days after injury and did not receive prophylactic anticoagulation in those 7 days. Among this group of patients, none of those who received the vena cava filter experienced a symptomatic pulmonary embolism between day 8 and day 90, but five patients (14.7%) in the no-filter group did.
Filters were left in place for a median duration of 27 days (11-90 days). Among the 122 patients who received a filter – which included two patients in the control group – researchers found trapped thrombi in the filter in six patients.
Transfusion requirements, and the incidence of major and nonmajor bleeding and leg deep vein thrombosis, were similar between the filter and no-filter groups. Seven patients in the filter group (5.7%) required more than one attempt to remove the filter, and in one patient the filter had to be removed surgically.
Kwok M. Ho, PhD, of the department of intensive care medicine at Royal Perth Hospital, Australia, and coauthors wrote that while vena cava filters are widely used in trauma centers to prevent pulmonary embolism in patients at high risk of bleeding, there are conflicting recommendations regarding their use, and most studies so far have been observational.
“Given the cost and risks associated with a vena cava filter, our data suggest that there is no urgency to insert the filter in patients who can be treated with prophylactic anticoagulation within 7 days after injury,” they wrote. “Unnecessary insertion of a vena cava filter has the potential to cause harm.”
However, they noted that patients with multiple, large intracranial hematomas were particularly at risk from bleeding with anticoagulant therapy, and therefore may benefit from the use of a vena cava filter.
The Medical Research Foundation of Royal Perth Hospital and the Western Australian Department of Health funded the study. Dr. Ho reported funding from the Western Australian Department of Health and the Raine Medical Research Foundation to conduct the study, as well as serving as an adviser to Medtronic and Cardinal Health.
SOURCE: Ho KM et al. N Engl J Med. 2019 Jul 7. doi: 10.156/NEJMoa1806515.
REPORTING FROM 2019 ISTH CONGRESS
Black holes are associated with impaired cognition in MS
SEATTLE – according to an investigation presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. Black holes are not associated with physical function, however. Evaluating black holes as part of routine clinical practice could be a quick method for screening people with MS for referral to a comprehensive cognitive assessment, said the authors.
Black holes (also known as T1-hypointense lesions) can be used as a marker of axonal loss and neuronal tissue destruction in patients with MS. Loss of axons and destruction of neuronal tissue contribute to cognitive and physical disability, but the literature contains few data about whether black holes correlate with cognitive and physical outcomes in MS.
Serkan Özakbas, MD, professor of neurology at Dokuz Eylül University in Izmir, Turkey, and colleagues examined 226 patients with MS to investigate this potential correlation. The population’s median Expanded Disability Status Scale score was 1.5. The researchers categorized participants into two groups according whether they had at least one black hole or not. They assessed patients’ cognitive function by administering the Brief International Cognitive Assessment for MS (BICAMS), which comprises the Symbol Digit Modalities Test (SDMT), California Verbal Learning Test II (CVLT-II), and the Brief Visuospatial Memory Test–Revised (BVMTR). They evaluated participants’ physical function using the Timed 25-Foot Walk (T25FW), Nine-Hole Peg Test (9HPT), 6-Minute Walk Test (6MWT), Timed Up and Go (TUG), and 12-Item MS Walking Scale (MSWS-12).
In all, 116 (43.6%) participants had at least one black hole, and 150 (56.4%) had no black hole. Dr. Özakbas and colleagues found no significant difference between patients with and without black holes on the T25FW, 9HPT, 6MWT, TUG, MSWS-12, and CVLT-II. Patients without a black hole, however, had significantly higher SDMT (49.0 vs 42.9) and BVMTR (26.3 vs 23.3) scores, compared with those with at least one black hole.
“This study suggests that presence of black holes is related to cognitive function, but not to physical function,” the researchers concluded.
The investigators had no disclosures and conducted their study without financial support.
SOURCE: Özakbas S et al. CMSC 2019, Abstract IMG02.
SEATTLE – according to an investigation presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. Black holes are not associated with physical function, however. Evaluating black holes as part of routine clinical practice could be a quick method for screening people with MS for referral to a comprehensive cognitive assessment, said the authors.
Black holes (also known as T1-hypointense lesions) can be used as a marker of axonal loss and neuronal tissue destruction in patients with MS. Loss of axons and destruction of neuronal tissue contribute to cognitive and physical disability, but the literature contains few data about whether black holes correlate with cognitive and physical outcomes in MS.
Serkan Özakbas, MD, professor of neurology at Dokuz Eylül University in Izmir, Turkey, and colleagues examined 226 patients with MS to investigate this potential correlation. The population’s median Expanded Disability Status Scale score was 1.5. The researchers categorized participants into two groups according whether they had at least one black hole or not. They assessed patients’ cognitive function by administering the Brief International Cognitive Assessment for MS (BICAMS), which comprises the Symbol Digit Modalities Test (SDMT), California Verbal Learning Test II (CVLT-II), and the Brief Visuospatial Memory Test–Revised (BVMTR). They evaluated participants’ physical function using the Timed 25-Foot Walk (T25FW), Nine-Hole Peg Test (9HPT), 6-Minute Walk Test (6MWT), Timed Up and Go (TUG), and 12-Item MS Walking Scale (MSWS-12).
In all, 116 (43.6%) participants had at least one black hole, and 150 (56.4%) had no black hole. Dr. Özakbas and colleagues found no significant difference between patients with and without black holes on the T25FW, 9HPT, 6MWT, TUG, MSWS-12, and CVLT-II. Patients without a black hole, however, had significantly higher SDMT (49.0 vs 42.9) and BVMTR (26.3 vs 23.3) scores, compared with those with at least one black hole.
“This study suggests that presence of black holes is related to cognitive function, but not to physical function,” the researchers concluded.
The investigators had no disclosures and conducted their study without financial support.
SOURCE: Özakbas S et al. CMSC 2019, Abstract IMG02.
SEATTLE – according to an investigation presented at the annual meeting of the Consortium of Multiple Sclerosis Centers. Black holes are not associated with physical function, however. Evaluating black holes as part of routine clinical practice could be a quick method for screening people with MS for referral to a comprehensive cognitive assessment, said the authors.
Black holes (also known as T1-hypointense lesions) can be used as a marker of axonal loss and neuronal tissue destruction in patients with MS. Loss of axons and destruction of neuronal tissue contribute to cognitive and physical disability, but the literature contains few data about whether black holes correlate with cognitive and physical outcomes in MS.
Serkan Özakbas, MD, professor of neurology at Dokuz Eylül University in Izmir, Turkey, and colleagues examined 226 patients with MS to investigate this potential correlation. The population’s median Expanded Disability Status Scale score was 1.5. The researchers categorized participants into two groups according whether they had at least one black hole or not. They assessed patients’ cognitive function by administering the Brief International Cognitive Assessment for MS (BICAMS), which comprises the Symbol Digit Modalities Test (SDMT), California Verbal Learning Test II (CVLT-II), and the Brief Visuospatial Memory Test–Revised (BVMTR). They evaluated participants’ physical function using the Timed 25-Foot Walk (T25FW), Nine-Hole Peg Test (9HPT), 6-Minute Walk Test (6MWT), Timed Up and Go (TUG), and 12-Item MS Walking Scale (MSWS-12).
In all, 116 (43.6%) participants had at least one black hole, and 150 (56.4%) had no black hole. Dr. Özakbas and colleagues found no significant difference between patients with and without black holes on the T25FW, 9HPT, 6MWT, TUG, MSWS-12, and CVLT-II. Patients without a black hole, however, had significantly higher SDMT (49.0 vs 42.9) and BVMTR (26.3 vs 23.3) scores, compared with those with at least one black hole.
“This study suggests that presence of black holes is related to cognitive function, but not to physical function,” the researchers concluded.
The investigators had no disclosures and conducted their study without financial support.
SOURCE: Özakbas S et al. CMSC 2019, Abstract IMG02.
REPORTING FROM CMSC 2019
Teen mothers using long-acting reversible contraception are least likely to use condoms
such as the birth control pill, vaginal ring, contraceptive patch, or injection, according to research in JAMA Pediatrics.
This highlights a need for education to lower the risk of sexually transmitted infections in this population.
“Our finding that less than 30% of sexually active teenage mothers using LARC or non-LARC hormonal methods also reported using condoms suggests the need for enhanced efforts to increase condom use among teenage mothers,” wrote Katherine Kortsmit, PhD, MPH, of the National Center for Chronic Disease Prevention and Health Promotion at the Centers for Disease Control and Prevention, Atlanta, and colleagues.
The researchers performed a cross-sectional analysis of contraceptive use among 5,480 new teenage mothers between 2012 and 2015 who were aged 19 years or younger in the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were mainly first-time teenage mothers between ages 18 and 19 years (46% non-Hispanic white), current Medicaid users, and reported an unintended pregnancy. Dr. Kortsmit and colleagues monitored use of LARC and non-LARC hormonal methods, including condom use, among participants in PRAMS from 37 different sites.
Among teenage mothers in PRAM, 29% reported using condoms; 18% of mothers using LARC said they also used condoms, compared with 36% of mothers who used non-LARC hormonal methods (adjusted prevalence ratio, 0.50; 95% confidence interval, 0.41-0.60). Participants with IUDs were least likely to report using condoms (15%), compared with participants using implants (22%; aPR, 0.70; 95% CI, 0.51-0.98), participants using the patch, ring, or injection (25%; aPR, 0.61; 95% CI, 0.47-0.79), or the pill (47%; aPR, 0.32; 95% CI, 0.25-0.40).
“These findings can be used to inform clinician counseling that sexually active teenage mothers have low uptake of condom use combined with more effective contraceptive methods and may need additional counseling on the importance of consistent and correct condom use for the prevention of STIs,” Dr. Kortsmit and associates wrote.
Limitations included the self-reported nature of the study, and lack of information on baseline condom use prior to pregnancy, relationship characteristics, and sexual partners during the postpartum period.
Education on contraceptive methods by clinicians is an important part of an adolescent’s contextualization of the benefits and risks of those methods, especially for women of color and marginalized groups, Andrea J. Hoopes, MD, MPH; and Gina S. Sucato, MD, MPH, wrote in an editorial related to the study by Kortsmit and colleagues.
In particular, these groups have higher rates of unplanned pregnancy, may have a history of being coerced to use contraception, and may be reluctant to discuss their sexual history or contraception use. “Many young women, including teenage mothers, remain at risk for coercion from partners, family members, and health care clinicians, so adopting a stance that ensures autonomy while eliciting unique developmental perspectives is paramount,” they said.
It is critically important to give women access to LARCs that are effective and easily used, and patients have a right to choose the contraception method that best fits their situation. It is through integrated programs, made available by Title X funding, that clinicians may be able to monitor their patients’ sexual, reproductive, and psychological health needs, and have conversations about the importance of contraception and prevention of sexually transmitted infections.
“Future studies should examine specific interventions aimed at promoting all adolescents’ motivations to remain safe and healthy by using condoms consistently and by seeking comprehensive sexual health care services, regardless of contraceptive method,” concluded Dr. Hoopes and Dr. Sucato, of the Adolescent Center at Kaiser Permanente Washington in Seattle. “In addition to receiving counseling about, and access to, condoms, adolescents need to develop the skills to negotiate condom use with partners.”
Dr. Kortsmit received support in the form of an appointment to the Research Participation Program at Centers for Disease Control and Prevention through an interagency agreement. The other authors reported no conflicts of interest.
Dr. Hoopes reported previous grant support from Bayer and the North American Society for Pediatric and Adolescent Gynecology. Dr. Sucato reported previous grant and other research support from Teva.
SOURCE: Kortsmit K et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1136; Hoopes AJ et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1133.
such as the birth control pill, vaginal ring, contraceptive patch, or injection, according to research in JAMA Pediatrics.
This highlights a need for education to lower the risk of sexually transmitted infections in this population.
“Our finding that less than 30% of sexually active teenage mothers using LARC or non-LARC hormonal methods also reported using condoms suggests the need for enhanced efforts to increase condom use among teenage mothers,” wrote Katherine Kortsmit, PhD, MPH, of the National Center for Chronic Disease Prevention and Health Promotion at the Centers for Disease Control and Prevention, Atlanta, and colleagues.
The researchers performed a cross-sectional analysis of contraceptive use among 5,480 new teenage mothers between 2012 and 2015 who were aged 19 years or younger in the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were mainly first-time teenage mothers between ages 18 and 19 years (46% non-Hispanic white), current Medicaid users, and reported an unintended pregnancy. Dr. Kortsmit and colleagues monitored use of LARC and non-LARC hormonal methods, including condom use, among participants in PRAMS from 37 different sites.
Among teenage mothers in PRAM, 29% reported using condoms; 18% of mothers using LARC said they also used condoms, compared with 36% of mothers who used non-LARC hormonal methods (adjusted prevalence ratio, 0.50; 95% confidence interval, 0.41-0.60). Participants with IUDs were least likely to report using condoms (15%), compared with participants using implants (22%; aPR, 0.70; 95% CI, 0.51-0.98), participants using the patch, ring, or injection (25%; aPR, 0.61; 95% CI, 0.47-0.79), or the pill (47%; aPR, 0.32; 95% CI, 0.25-0.40).
“These findings can be used to inform clinician counseling that sexually active teenage mothers have low uptake of condom use combined with more effective contraceptive methods and may need additional counseling on the importance of consistent and correct condom use for the prevention of STIs,” Dr. Kortsmit and associates wrote.
Limitations included the self-reported nature of the study, and lack of information on baseline condom use prior to pregnancy, relationship characteristics, and sexual partners during the postpartum period.
Education on contraceptive methods by clinicians is an important part of an adolescent’s contextualization of the benefits and risks of those methods, especially for women of color and marginalized groups, Andrea J. Hoopes, MD, MPH; and Gina S. Sucato, MD, MPH, wrote in an editorial related to the study by Kortsmit and colleagues.
In particular, these groups have higher rates of unplanned pregnancy, may have a history of being coerced to use contraception, and may be reluctant to discuss their sexual history or contraception use. “Many young women, including teenage mothers, remain at risk for coercion from partners, family members, and health care clinicians, so adopting a stance that ensures autonomy while eliciting unique developmental perspectives is paramount,” they said.
It is critically important to give women access to LARCs that are effective and easily used, and patients have a right to choose the contraception method that best fits their situation. It is through integrated programs, made available by Title X funding, that clinicians may be able to monitor their patients’ sexual, reproductive, and psychological health needs, and have conversations about the importance of contraception and prevention of sexually transmitted infections.
“Future studies should examine specific interventions aimed at promoting all adolescents’ motivations to remain safe and healthy by using condoms consistently and by seeking comprehensive sexual health care services, regardless of contraceptive method,” concluded Dr. Hoopes and Dr. Sucato, of the Adolescent Center at Kaiser Permanente Washington in Seattle. “In addition to receiving counseling about, and access to, condoms, adolescents need to develop the skills to negotiate condom use with partners.”
Dr. Kortsmit received support in the form of an appointment to the Research Participation Program at Centers for Disease Control and Prevention through an interagency agreement. The other authors reported no conflicts of interest.
Dr. Hoopes reported previous grant support from Bayer and the North American Society for Pediatric and Adolescent Gynecology. Dr. Sucato reported previous grant and other research support from Teva.
SOURCE: Kortsmit K et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1136; Hoopes AJ et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1133.
such as the birth control pill, vaginal ring, contraceptive patch, or injection, according to research in JAMA Pediatrics.
This highlights a need for education to lower the risk of sexually transmitted infections in this population.
“Our finding that less than 30% of sexually active teenage mothers using LARC or non-LARC hormonal methods also reported using condoms suggests the need for enhanced efforts to increase condom use among teenage mothers,” wrote Katherine Kortsmit, PhD, MPH, of the National Center for Chronic Disease Prevention and Health Promotion at the Centers for Disease Control and Prevention, Atlanta, and colleagues.
The researchers performed a cross-sectional analysis of contraceptive use among 5,480 new teenage mothers between 2012 and 2015 who were aged 19 years or younger in the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were mainly first-time teenage mothers between ages 18 and 19 years (46% non-Hispanic white), current Medicaid users, and reported an unintended pregnancy. Dr. Kortsmit and colleagues monitored use of LARC and non-LARC hormonal methods, including condom use, among participants in PRAMS from 37 different sites.
Among teenage mothers in PRAM, 29% reported using condoms; 18% of mothers using LARC said they also used condoms, compared with 36% of mothers who used non-LARC hormonal methods (adjusted prevalence ratio, 0.50; 95% confidence interval, 0.41-0.60). Participants with IUDs were least likely to report using condoms (15%), compared with participants using implants (22%; aPR, 0.70; 95% CI, 0.51-0.98), participants using the patch, ring, or injection (25%; aPR, 0.61; 95% CI, 0.47-0.79), or the pill (47%; aPR, 0.32; 95% CI, 0.25-0.40).
“These findings can be used to inform clinician counseling that sexually active teenage mothers have low uptake of condom use combined with more effective contraceptive methods and may need additional counseling on the importance of consistent and correct condom use for the prevention of STIs,” Dr. Kortsmit and associates wrote.
Limitations included the self-reported nature of the study, and lack of information on baseline condom use prior to pregnancy, relationship characteristics, and sexual partners during the postpartum period.
Education on contraceptive methods by clinicians is an important part of an adolescent’s contextualization of the benefits and risks of those methods, especially for women of color and marginalized groups, Andrea J. Hoopes, MD, MPH; and Gina S. Sucato, MD, MPH, wrote in an editorial related to the study by Kortsmit and colleagues.
In particular, these groups have higher rates of unplanned pregnancy, may have a history of being coerced to use contraception, and may be reluctant to discuss their sexual history or contraception use. “Many young women, including teenage mothers, remain at risk for coercion from partners, family members, and health care clinicians, so adopting a stance that ensures autonomy while eliciting unique developmental perspectives is paramount,” they said.
It is critically important to give women access to LARCs that are effective and easily used, and patients have a right to choose the contraception method that best fits their situation. It is through integrated programs, made available by Title X funding, that clinicians may be able to monitor their patients’ sexual, reproductive, and psychological health needs, and have conversations about the importance of contraception and prevention of sexually transmitted infections.
“Future studies should examine specific interventions aimed at promoting all adolescents’ motivations to remain safe and healthy by using condoms consistently and by seeking comprehensive sexual health care services, regardless of contraceptive method,” concluded Dr. Hoopes and Dr. Sucato, of the Adolescent Center at Kaiser Permanente Washington in Seattle. “In addition to receiving counseling about, and access to, condoms, adolescents need to develop the skills to negotiate condom use with partners.”
Dr. Kortsmit received support in the form of an appointment to the Research Participation Program at Centers for Disease Control and Prevention through an interagency agreement. The other authors reported no conflicts of interest.
Dr. Hoopes reported previous grant support from Bayer and the North American Society for Pediatric and Adolescent Gynecology. Dr. Sucato reported previous grant and other research support from Teva.
SOURCE: Kortsmit K et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1136; Hoopes AJ et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1133.
FROM JAMA PEDIATRICS
Court halts rule requiring drug list pricing in advertising
The Department of Health & Human Services lacks the authority to require drug manufacturers to disclose the list price of drugs in television advertising, a district court judge has ruled.
“The court finds that HHS lacks the statutory authority under the Social Security Act to adopt the [Wholesale Acquisition Cost] Disclosure Rule,” Judge Amit P. Mehta of the U.S. District Court for the District of Columbia, said in his July 8 ruling.
“Neither the [Social Security] Act’s text, structure, nor context evince an intent by Congress to empower HHS to issue a rule that compels drug manufacturers to disclose list prices. The rule is therefore invalid,” according to the ruling.
The ruling stems from a case brought against HHS by Merck & Co. Inc., Eli Lilly and Company, and Amgen Inc., along with the National Association of Advertisers Inc., in response to the May 8 final rule that required pharmaceutical manufacturers to include the wholesale acquisition cost (WAC) if above $35, in all television advertising. The final rule also required a disclaimer that if a person had health insurance that covers drugs, “your cost may be different.”
In addition to not having authority, the court took exception to the HHS issuing the rule through the Centers for Medicare & Medicaid Services.
“It has adopted a rule that regulates the conduct of market actors that are not direct participants in the Medicare or Medicaid programs,” the ruling states. “Pharmaceutical manufacturers are not health care providers, private plan carriers, or beneficiaries – each of whom plays a direct role in the public health insurance programs. They do not receive payment for their products from CMS. Their pricing decisions, of course, affect the cost of pharmaceutical benefits offered under the Medicare and Medicaid programs. But those decisions impact the program costs in an indirect way.”
The American Medical Association expressed disappointment that the final rule is not moving forward.
“The AMA supported the Trump Administration’s effort to require pricing information in direct-to-consumer television advertising of prescription drugs,” AMA President Patrice Harris, MD, said in a statement. “Last year, the AMA called for regulations requiring the ads to include the manufacturer’s list price of those drugs, and we have supported similar legislative efforts.”
Dr. Harris noted that having the list price would provide a vital piece of information when patients and their physicians are discussing treatment options, as it would “help patients have a more complete picture when faced with prescription drug ads. While current ads outline the potential benefits and side effects, a crucial factor for patients – the drug’s price – is not included. Patients, especially those who pay a drug’s list price or whose cost-sharing is based on the list price, would benefit by having another tool in their toolbox as they work with their physicians to determine their prescription drug regimens.”
Likewise, AARP also expressed disappointment in the decision, calling it “a step backward in the battle against skyrocketing drug prices and providing more information to consumers. Americans should be trusted to evaluate drug price information and discuss any concerns with their health care providers.”
Judge Mehta noted in his ruling that “the court does not question HHS’ motives in adopting the WAC Disclosure Rule. ... That policy very well could be an effective tool in halting the rising cost of prescription drugs. But no matter how vexing the problem of spiraling drug costs may be, HHS cannot do more than what Congress has authorized. The responsibility rests with Congress to act in the first instance.”
The Department of Health & Human Services lacks the authority to require drug manufacturers to disclose the list price of drugs in television advertising, a district court judge has ruled.
“The court finds that HHS lacks the statutory authority under the Social Security Act to adopt the [Wholesale Acquisition Cost] Disclosure Rule,” Judge Amit P. Mehta of the U.S. District Court for the District of Columbia, said in his July 8 ruling.
“Neither the [Social Security] Act’s text, structure, nor context evince an intent by Congress to empower HHS to issue a rule that compels drug manufacturers to disclose list prices. The rule is therefore invalid,” according to the ruling.
The ruling stems from a case brought against HHS by Merck & Co. Inc., Eli Lilly and Company, and Amgen Inc., along with the National Association of Advertisers Inc., in response to the May 8 final rule that required pharmaceutical manufacturers to include the wholesale acquisition cost (WAC) if above $35, in all television advertising. The final rule also required a disclaimer that if a person had health insurance that covers drugs, “your cost may be different.”
In addition to not having authority, the court took exception to the HHS issuing the rule through the Centers for Medicare & Medicaid Services.
“It has adopted a rule that regulates the conduct of market actors that are not direct participants in the Medicare or Medicaid programs,” the ruling states. “Pharmaceutical manufacturers are not health care providers, private plan carriers, or beneficiaries – each of whom plays a direct role in the public health insurance programs. They do not receive payment for their products from CMS. Their pricing decisions, of course, affect the cost of pharmaceutical benefits offered under the Medicare and Medicaid programs. But those decisions impact the program costs in an indirect way.”
The American Medical Association expressed disappointment that the final rule is not moving forward.
“The AMA supported the Trump Administration’s effort to require pricing information in direct-to-consumer television advertising of prescription drugs,” AMA President Patrice Harris, MD, said in a statement. “Last year, the AMA called for regulations requiring the ads to include the manufacturer’s list price of those drugs, and we have supported similar legislative efforts.”
Dr. Harris noted that having the list price would provide a vital piece of information when patients and their physicians are discussing treatment options, as it would “help patients have a more complete picture when faced with prescription drug ads. While current ads outline the potential benefits and side effects, a crucial factor for patients – the drug’s price – is not included. Patients, especially those who pay a drug’s list price or whose cost-sharing is based on the list price, would benefit by having another tool in their toolbox as they work with their physicians to determine their prescription drug regimens.”
Likewise, AARP also expressed disappointment in the decision, calling it “a step backward in the battle against skyrocketing drug prices and providing more information to consumers. Americans should be trusted to evaluate drug price information and discuss any concerns with their health care providers.”
Judge Mehta noted in his ruling that “the court does not question HHS’ motives in adopting the WAC Disclosure Rule. ... That policy very well could be an effective tool in halting the rising cost of prescription drugs. But no matter how vexing the problem of spiraling drug costs may be, HHS cannot do more than what Congress has authorized. The responsibility rests with Congress to act in the first instance.”
The Department of Health & Human Services lacks the authority to require drug manufacturers to disclose the list price of drugs in television advertising, a district court judge has ruled.
“The court finds that HHS lacks the statutory authority under the Social Security Act to adopt the [Wholesale Acquisition Cost] Disclosure Rule,” Judge Amit P. Mehta of the U.S. District Court for the District of Columbia, said in his July 8 ruling.
“Neither the [Social Security] Act’s text, structure, nor context evince an intent by Congress to empower HHS to issue a rule that compels drug manufacturers to disclose list prices. The rule is therefore invalid,” according to the ruling.
The ruling stems from a case brought against HHS by Merck & Co. Inc., Eli Lilly and Company, and Amgen Inc., along with the National Association of Advertisers Inc., in response to the May 8 final rule that required pharmaceutical manufacturers to include the wholesale acquisition cost (WAC) if above $35, in all television advertising. The final rule also required a disclaimer that if a person had health insurance that covers drugs, “your cost may be different.”
In addition to not having authority, the court took exception to the HHS issuing the rule through the Centers for Medicare & Medicaid Services.
“It has adopted a rule that regulates the conduct of market actors that are not direct participants in the Medicare or Medicaid programs,” the ruling states. “Pharmaceutical manufacturers are not health care providers, private plan carriers, or beneficiaries – each of whom plays a direct role in the public health insurance programs. They do not receive payment for their products from CMS. Their pricing decisions, of course, affect the cost of pharmaceutical benefits offered under the Medicare and Medicaid programs. But those decisions impact the program costs in an indirect way.”
The American Medical Association expressed disappointment that the final rule is not moving forward.
“The AMA supported the Trump Administration’s effort to require pricing information in direct-to-consumer television advertising of prescription drugs,” AMA President Patrice Harris, MD, said in a statement. “Last year, the AMA called for regulations requiring the ads to include the manufacturer’s list price of those drugs, and we have supported similar legislative efforts.”
Dr. Harris noted that having the list price would provide a vital piece of information when patients and their physicians are discussing treatment options, as it would “help patients have a more complete picture when faced with prescription drug ads. While current ads outline the potential benefits and side effects, a crucial factor for patients – the drug’s price – is not included. Patients, especially those who pay a drug’s list price or whose cost-sharing is based on the list price, would benefit by having another tool in their toolbox as they work with their physicians to determine their prescription drug regimens.”
Likewise, AARP also expressed disappointment in the decision, calling it “a step backward in the battle against skyrocketing drug prices and providing more information to consumers. Americans should be trusted to evaluate drug price information and discuss any concerns with their health care providers.”
Judge Mehta noted in his ruling that “the court does not question HHS’ motives in adopting the WAC Disclosure Rule. ... That policy very well could be an effective tool in halting the rising cost of prescription drugs. But no matter how vexing the problem of spiraling drug costs may be, HHS cannot do more than what Congress has authorized. The responsibility rests with Congress to act in the first instance.”
CDC: Look for early symptoms of acute flaccid myelitis, report suspected cases
the CDC said in a telebriefing.
Acute flaccid myelitis (AFM) is defined as acute, flaccid muscle weakness that occurs less than 1 week after a fever or respiratory illness. Viruses, including enterovirus, are believed to play a role in AFM, but the cause still is unknown. The disease appears mostly in children, and the average age of a patient diagnosed with AFM is 5 years.
“Doctors and other clinicians in the United States play a critical role,” Anne Schuchat, MD, principal deputy director of the Centers for Disease Control and Prevention, said in the telebriefing. “We ask for your help with early recognition of patients with AFM symptoms, prompt specimen collection for testing, and immediate reporting of suspected AFM cases to health departments.”
While there is no proven treatment for AFM, early diagnosis is critical to getting patients the best care possible, according to a Vital Signs report released today. This means that clinicians should not wait for the CDC’s case definition before diagnosis, the CDC said.
“When specimens are collected as soon as possible after symptom onset, we have a better chance of understanding the causes of AFM, these recurrent outbreaks, and developing a diagnostic test,” Dr. Schuchat said. “Rapid reporting also helps us to identify and respond to outbreaks early and alert other clinicians and the public.”
AFM appears to follow a seasonal and biennial pattern, with the number of cases increasing mainly in the late summer and early fall. As the season approaches where AFM cases increase, CDC is asking clinicians to look out for patients with suspected AFM so cases can be reported as early as possible.
Since the CDC began tracking AFM, the number of cases has risen every 2 years. In 2018, there were 233 cases in 41 states, the highest number of reported cases since the CDC began tracking AFM following an outbreak in 2014, according to a Vital Signs report. Overall, there have been 570 cases of AFM reported in 48 states and the District of Columbia since 2014.
There is yet to be a confirmatory test for AFM, but clinicians should obtain cerebrospinal fluid, serum, stool and nasopharyngeal swab from patients with suspected AFM as soon as possible, followed by an MRI. AFM has unique MRI features , such as gray matter involvement, that can help distinguish it from other diseases characterized by acute weakness.
In the Vital Signs report, which examined AFM in 2018, 92% of confirmed cases had respiratory symptoms or fever, and 42% of confirmed cases had upper limb involvement. The median time from limb weakness to hospitalization was 1 day, and time from weakness to MRI was 2 days. Cases were reported to the CDC a median of 18 days from onset of limb weakness, but time to reporting ranged between 18 days and 36 days, said Tom Clark, MD, MPH, deputy director of the division of viral diseases at CDC.
“This delay hampers our ability to understand the causes AFM,” he said. “We believe that recognizing AFM early is critical and can lead to better patient management.”
In lieu of a diagnostic test for AFM, clinicians should make management decisions through review of patient symptoms, exam findings, MRI, other test results, and in consulting with neurology experts. The Transverse Myelitis Association also has created a support portal for 24/7 physician consultation in AFM cases.
SOURCE: Lopez A et al. MMWR Morb Mortal Wkly Rep. 2019;68:1-7 .
the CDC said in a telebriefing.

Acute flaccid myelitis (AFM) is defined as acute, flaccid muscle weakness that occurs less than 1 week after a fever or respiratory illness. Viruses, including enterovirus, are believed to play a role in AFM, but the cause still is unknown. The disease appears mostly in children, and the average age of a patient diagnosed with AFM is 5 years.
“Doctors and other clinicians in the United States play a critical role,” Anne Schuchat, MD, principal deputy director of the Centers for Disease Control and Prevention, said in the telebriefing. “We ask for your help with early recognition of patients with AFM symptoms, prompt specimen collection for testing, and immediate reporting of suspected AFM cases to health departments.”
While there is no proven treatment for AFM, early diagnosis is critical to getting patients the best care possible, according to a Vital Signs report released today. This means that clinicians should not wait for the CDC’s case definition before diagnosis, the CDC said.
“When specimens are collected as soon as possible after symptom onset, we have a better chance of understanding the causes of AFM, these recurrent outbreaks, and developing a diagnostic test,” Dr. Schuchat said. “Rapid reporting also helps us to identify and respond to outbreaks early and alert other clinicians and the public.”
AFM appears to follow a seasonal and biennial pattern, with the number of cases increasing mainly in the late summer and early fall. As the season approaches where AFM cases increase, CDC is asking clinicians to look out for patients with suspected AFM so cases can be reported as early as possible.
Since the CDC began tracking AFM, the number of cases has risen every 2 years. In 2018, there were 233 cases in 41 states, the highest number of reported cases since the CDC began tracking AFM following an outbreak in 2014, according to a Vital Signs report. Overall, there have been 570 cases of AFM reported in 48 states and the District of Columbia since 2014.
There is yet to be a confirmatory test for AFM, but clinicians should obtain cerebrospinal fluid, serum, stool and nasopharyngeal swab from patients with suspected AFM as soon as possible, followed by an MRI. AFM has unique MRI features , such as gray matter involvement, that can help distinguish it from other diseases characterized by acute weakness.
In the Vital Signs report, which examined AFM in 2018, 92% of confirmed cases had respiratory symptoms or fever, and 42% of confirmed cases had upper limb involvement. The median time from limb weakness to hospitalization was 1 day, and time from weakness to MRI was 2 days. Cases were reported to the CDC a median of 18 days from onset of limb weakness, but time to reporting ranged between 18 days and 36 days, said Tom Clark, MD, MPH, deputy director of the division of viral diseases at CDC.
“This delay hampers our ability to understand the causes AFM,” he said. “We believe that recognizing AFM early is critical and can lead to better patient management.”
In lieu of a diagnostic test for AFM, clinicians should make management decisions through review of patient symptoms, exam findings, MRI, other test results, and in consulting with neurology experts. The Transverse Myelitis Association also has created a support portal for 24/7 physician consultation in AFM cases.
SOURCE: Lopez A et al. MMWR Morb Mortal Wkly Rep. 2019;68:1-7 .
the CDC said in a telebriefing.

Acute flaccid myelitis (AFM) is defined as acute, flaccid muscle weakness that occurs less than 1 week after a fever or respiratory illness. Viruses, including enterovirus, are believed to play a role in AFM, but the cause still is unknown. The disease appears mostly in children, and the average age of a patient diagnosed with AFM is 5 years.
“Doctors and other clinicians in the United States play a critical role,” Anne Schuchat, MD, principal deputy director of the Centers for Disease Control and Prevention, said in the telebriefing. “We ask for your help with early recognition of patients with AFM symptoms, prompt specimen collection for testing, and immediate reporting of suspected AFM cases to health departments.”
While there is no proven treatment for AFM, early diagnosis is critical to getting patients the best care possible, according to a Vital Signs report released today. This means that clinicians should not wait for the CDC’s case definition before diagnosis, the CDC said.
“When specimens are collected as soon as possible after symptom onset, we have a better chance of understanding the causes of AFM, these recurrent outbreaks, and developing a diagnostic test,” Dr. Schuchat said. “Rapid reporting also helps us to identify and respond to outbreaks early and alert other clinicians and the public.”
AFM appears to follow a seasonal and biennial pattern, with the number of cases increasing mainly in the late summer and early fall. As the season approaches where AFM cases increase, CDC is asking clinicians to look out for patients with suspected AFM so cases can be reported as early as possible.
Since the CDC began tracking AFM, the number of cases has risen every 2 years. In 2018, there were 233 cases in 41 states, the highest number of reported cases since the CDC began tracking AFM following an outbreak in 2014, according to a Vital Signs report. Overall, there have been 570 cases of AFM reported in 48 states and the District of Columbia since 2014.
There is yet to be a confirmatory test for AFM, but clinicians should obtain cerebrospinal fluid, serum, stool and nasopharyngeal swab from patients with suspected AFM as soon as possible, followed by an MRI. AFM has unique MRI features , such as gray matter involvement, that can help distinguish it from other diseases characterized by acute weakness.
In the Vital Signs report, which examined AFM in 2018, 92% of confirmed cases had respiratory symptoms or fever, and 42% of confirmed cases had upper limb involvement. The median time from limb weakness to hospitalization was 1 day, and time from weakness to MRI was 2 days. Cases were reported to the CDC a median of 18 days from onset of limb weakness, but time to reporting ranged between 18 days and 36 days, said Tom Clark, MD, MPH, deputy director of the division of viral diseases at CDC.
“This delay hampers our ability to understand the causes AFM,” he said. “We believe that recognizing AFM early is critical and can lead to better patient management.”
In lieu of a diagnostic test for AFM, clinicians should make management decisions through review of patient symptoms, exam findings, MRI, other test results, and in consulting with neurology experts. The Transverse Myelitis Association also has created a support portal for 24/7 physician consultation in AFM cases.
SOURCE: Lopez A et al. MMWR Morb Mortal Wkly Rep. 2019;68:1-7 .
NEWS FROM THE FDA/CDC
Sicker COPD patients may be more likely to initiate arformoterol
according to study to identify predictors of its use. In addition to being sicker, being treated by a pulmonologist rather than a primary care physician and being white were factors that increased a patient’s likelihood of receiving nebulized arformoterol.
Patients less likely to receive the nebulized version of this long-acting beta2 adrenoreceptor agonist (LABA) were African Americans, patients with psychiatric comorbidities, and patients eligible for both Medicare and Medicaid.
“Studies have shown that 40% to 71% of Medicare beneficiaries receive no maintenance treatment for COPD. Although a recent longitudinal study on Medicare populations reported that use of maintenance medications has been improving, in general, it is recognized that Medicare beneficiaries with COPD remain undertreated,” Todd P. Gilmer, PhD, from the department of family medicine and public health at the University of California, San Diego, and colleagues wrote.
The investigators identified patients with COPD using Medicare administrative data; of these patients, 11,887 were arformoterol users, and 450,178 were control patients who did not use arformoterol. Patients were included in the study if they had at least one claim for COPD medication and were continuously enrolled in Medicare Parts A, B, and D. The cohort consisted of mostly white women aged 70 years or older, and 47% were dual-eligible to receive both Medicare and Medicaid benefits. A subgroup of 1,778 arformoterol users were also identified for analysis who were hospitalized and discharged within 30 days of using arformoterol, as well as a subgroup of 21,910 control patients with hospitalizations.
The researchers found COPD-related hospitalization (odds ratio, 1.31; 95% confidence interval, 1.24-1.39; P less than .001), exacerbation (OR, 1.33; 95% CI, 1.26-1.41; P less than .001), use of a systemic corticosteroid (OR, 1.50; 95% CI, 1.43-1.57; P less than .001) or methylxanthine (OR, 1.37; 95% CI, 1.28-1.47; P less than .001), use of oxygen therapy (OR, 2.01; 95% CI, 1.93-2.09; P less than .001), pulmonologist care (OR, 1.40; 95% CI, 1.34-1.46; P less than .001), and respiratory therapist care (OR, 1.23; 95% CI, 1.11-1.36; P less than .001) strongly predicted arformoterol use, while racial/ethnic minority status, psychiatric comorbidity (OR, 0.65; 95% CI, 0.56-0.76; P less than .001), acquired immune deficiency syndrome (OR, 0.69; 95% CI, 0.52-0.94; P less than .01), and dual-eligibility for Medicare and Medicaid (OR, 0.73; 95% CI, 0.70-0.77; P less than .001) lowered the odds of arformoterol use (P less than .001). In the subgroup of patients with hospitalizations, COPD-related admission (OR, 1.83; 95% CI, 1.55-2.14; P less than .001), exacerbation (OR, 2.62; 95% CI, 1.88-3.63; P less than .001)m and inpatient care from a pulmonologist (OR, 1.78; 95% CI, 1.58-2.01; P less than .001) predicted arformoterol use.
“Given the results of this study, increasing access to nebulized maintenance therapy is warranted for select populations with COPD including racial/ethnic minorities, the dual-eligible, and those with certain comorbidities, such as psychiatric disorders,” Dr. Gilmer and colleagues wrote in their study. “Future studies are needed to explore the optimal time to initiate nebulized maintenance therapy, and the potential differential impact of early versus late initiation on patient outcomes.”
Researchers noted that, although their results may seem initially counterintuitive given that COPD has a higher prevalence in men, 56% of the beneficiaries in their Medicare data were women who were 65 years or older, and the results are consistent with other studies that show similar gender distribution findings for maintenance treatment patterns among COPD patients receiving Medicare.
“Since most Medicare beneficiaries with COPD are older than 70 years of age, the higher percentage of women than men in our two cohorts can be explained by the age distributions that ensued as a result of applying our various inclusion and exclusion criteria,” they said.
This study was funded by Sunovian. Dr. Gilmer and one coauthor are paid employees of University of California San Diego, which receives research funding from Advance Health Solutions. Another coauthor is an advisory board member for Advance Health Solutions and a consultant for GlaxoSmithKline, Boehringer-Ingelheim, Astra Zeneca, Novartis, and Pulmonix. Two other coauthors are paid employees of Advance Health Solutions, and another is a paid employee of Sunovion.
SOURCE: Gilmer TP et al. COPD. 2019 Jun 19. doi: 10.1080/15412555.2019.1618256.
according to study to identify predictors of its use. In addition to being sicker, being treated by a pulmonologist rather than a primary care physician and being white were factors that increased a patient’s likelihood of receiving nebulized arformoterol.
Patients less likely to receive the nebulized version of this long-acting beta2 adrenoreceptor agonist (LABA) were African Americans, patients with psychiatric comorbidities, and patients eligible for both Medicare and Medicaid.
“Studies have shown that 40% to 71% of Medicare beneficiaries receive no maintenance treatment for COPD. Although a recent longitudinal study on Medicare populations reported that use of maintenance medications has been improving, in general, it is recognized that Medicare beneficiaries with COPD remain undertreated,” Todd P. Gilmer, PhD, from the department of family medicine and public health at the University of California, San Diego, and colleagues wrote.
The investigators identified patients with COPD using Medicare administrative data; of these patients, 11,887 were arformoterol users, and 450,178 were control patients who did not use arformoterol. Patients were included in the study if they had at least one claim for COPD medication and were continuously enrolled in Medicare Parts A, B, and D. The cohort consisted of mostly white women aged 70 years or older, and 47% were dual-eligible to receive both Medicare and Medicaid benefits. A subgroup of 1,778 arformoterol users were also identified for analysis who were hospitalized and discharged within 30 days of using arformoterol, as well as a subgroup of 21,910 control patients with hospitalizations.
The researchers found COPD-related hospitalization (odds ratio, 1.31; 95% confidence interval, 1.24-1.39; P less than .001), exacerbation (OR, 1.33; 95% CI, 1.26-1.41; P less than .001), use of a systemic corticosteroid (OR, 1.50; 95% CI, 1.43-1.57; P less than .001) or methylxanthine (OR, 1.37; 95% CI, 1.28-1.47; P less than .001), use of oxygen therapy (OR, 2.01; 95% CI, 1.93-2.09; P less than .001), pulmonologist care (OR, 1.40; 95% CI, 1.34-1.46; P less than .001), and respiratory therapist care (OR, 1.23; 95% CI, 1.11-1.36; P less than .001) strongly predicted arformoterol use, while racial/ethnic minority status, psychiatric comorbidity (OR, 0.65; 95% CI, 0.56-0.76; P less than .001), acquired immune deficiency syndrome (OR, 0.69; 95% CI, 0.52-0.94; P less than .01), and dual-eligibility for Medicare and Medicaid (OR, 0.73; 95% CI, 0.70-0.77; P less than .001) lowered the odds of arformoterol use (P less than .001). In the subgroup of patients with hospitalizations, COPD-related admission (OR, 1.83; 95% CI, 1.55-2.14; P less than .001), exacerbation (OR, 2.62; 95% CI, 1.88-3.63; P less than .001)m and inpatient care from a pulmonologist (OR, 1.78; 95% CI, 1.58-2.01; P less than .001) predicted arformoterol use.
“Given the results of this study, increasing access to nebulized maintenance therapy is warranted for select populations with COPD including racial/ethnic minorities, the dual-eligible, and those with certain comorbidities, such as psychiatric disorders,” Dr. Gilmer and colleagues wrote in their study. “Future studies are needed to explore the optimal time to initiate nebulized maintenance therapy, and the potential differential impact of early versus late initiation on patient outcomes.”
Researchers noted that, although their results may seem initially counterintuitive given that COPD has a higher prevalence in men, 56% of the beneficiaries in their Medicare data were women who were 65 years or older, and the results are consistent with other studies that show similar gender distribution findings for maintenance treatment patterns among COPD patients receiving Medicare.
“Since most Medicare beneficiaries with COPD are older than 70 years of age, the higher percentage of women than men in our two cohorts can be explained by the age distributions that ensued as a result of applying our various inclusion and exclusion criteria,” they said.
This study was funded by Sunovian. Dr. Gilmer and one coauthor are paid employees of University of California San Diego, which receives research funding from Advance Health Solutions. Another coauthor is an advisory board member for Advance Health Solutions and a consultant for GlaxoSmithKline, Boehringer-Ingelheim, Astra Zeneca, Novartis, and Pulmonix. Two other coauthors are paid employees of Advance Health Solutions, and another is a paid employee of Sunovion.
SOURCE: Gilmer TP et al. COPD. 2019 Jun 19. doi: 10.1080/15412555.2019.1618256.
according to study to identify predictors of its use. In addition to being sicker, being treated by a pulmonologist rather than a primary care physician and being white were factors that increased a patient’s likelihood of receiving nebulized arformoterol.
Patients less likely to receive the nebulized version of this long-acting beta2 adrenoreceptor agonist (LABA) were African Americans, patients with psychiatric comorbidities, and patients eligible for both Medicare and Medicaid.
“Studies have shown that 40% to 71% of Medicare beneficiaries receive no maintenance treatment for COPD. Although a recent longitudinal study on Medicare populations reported that use of maintenance medications has been improving, in general, it is recognized that Medicare beneficiaries with COPD remain undertreated,” Todd P. Gilmer, PhD, from the department of family medicine and public health at the University of California, San Diego, and colleagues wrote.
The investigators identified patients with COPD using Medicare administrative data; of these patients, 11,887 were arformoterol users, and 450,178 were control patients who did not use arformoterol. Patients were included in the study if they had at least one claim for COPD medication and were continuously enrolled in Medicare Parts A, B, and D. The cohort consisted of mostly white women aged 70 years or older, and 47% were dual-eligible to receive both Medicare and Medicaid benefits. A subgroup of 1,778 arformoterol users were also identified for analysis who were hospitalized and discharged within 30 days of using arformoterol, as well as a subgroup of 21,910 control patients with hospitalizations.
The researchers found COPD-related hospitalization (odds ratio, 1.31; 95% confidence interval, 1.24-1.39; P less than .001), exacerbation (OR, 1.33; 95% CI, 1.26-1.41; P less than .001), use of a systemic corticosteroid (OR, 1.50; 95% CI, 1.43-1.57; P less than .001) or methylxanthine (OR, 1.37; 95% CI, 1.28-1.47; P less than .001), use of oxygen therapy (OR, 2.01; 95% CI, 1.93-2.09; P less than .001), pulmonologist care (OR, 1.40; 95% CI, 1.34-1.46; P less than .001), and respiratory therapist care (OR, 1.23; 95% CI, 1.11-1.36; P less than .001) strongly predicted arformoterol use, while racial/ethnic minority status, psychiatric comorbidity (OR, 0.65; 95% CI, 0.56-0.76; P less than .001), acquired immune deficiency syndrome (OR, 0.69; 95% CI, 0.52-0.94; P less than .01), and dual-eligibility for Medicare and Medicaid (OR, 0.73; 95% CI, 0.70-0.77; P less than .001) lowered the odds of arformoterol use (P less than .001). In the subgroup of patients with hospitalizations, COPD-related admission (OR, 1.83; 95% CI, 1.55-2.14; P less than .001), exacerbation (OR, 2.62; 95% CI, 1.88-3.63; P less than .001)m and inpatient care from a pulmonologist (OR, 1.78; 95% CI, 1.58-2.01; P less than .001) predicted arformoterol use.
“Given the results of this study, increasing access to nebulized maintenance therapy is warranted for select populations with COPD including racial/ethnic minorities, the dual-eligible, and those with certain comorbidities, such as psychiatric disorders,” Dr. Gilmer and colleagues wrote in their study. “Future studies are needed to explore the optimal time to initiate nebulized maintenance therapy, and the potential differential impact of early versus late initiation on patient outcomes.”
Researchers noted that, although their results may seem initially counterintuitive given that COPD has a higher prevalence in men, 56% of the beneficiaries in their Medicare data were women who were 65 years or older, and the results are consistent with other studies that show similar gender distribution findings for maintenance treatment patterns among COPD patients receiving Medicare.
“Since most Medicare beneficiaries with COPD are older than 70 years of age, the higher percentage of women than men in our two cohorts can be explained by the age distributions that ensued as a result of applying our various inclusion and exclusion criteria,” they said.
This study was funded by Sunovian. Dr. Gilmer and one coauthor are paid employees of University of California San Diego, which receives research funding from Advance Health Solutions. Another coauthor is an advisory board member for Advance Health Solutions and a consultant for GlaxoSmithKline, Boehringer-Ingelheim, Astra Zeneca, Novartis, and Pulmonix. Two other coauthors are paid employees of Advance Health Solutions, and another is a paid employee of Sunovion.
SOURCE: Gilmer TP et al. COPD. 2019 Jun 19. doi: 10.1080/15412555.2019.1618256.
FROM COPD: JOURNAL OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE
CNS-directed therapy appears more effective for synDLBCL
Controlling CNS disease is “paramount” in treating diffuse large B-cell lymphoma with synchronous CNS and systemic disease (synDLBCL), according to researchers.
In a retrospective study, the CNS was the most common site of relapse in patients with synDLBCL, and patients had better outcomes when they received CNS-directed therapy.
The 2-year progression-free survival rate was 50% in patients who received CNS-intensive therapy and 31% in those who received CNS-conservative therapy. The 2-year overall survival rate was 54% and 44%, respectively.
Dr. Joel C. Wight, of Austin Health in Heidelberg, Australia, and colleagues conducted this study and recounted their findings in the British Journal of Haematology.
The researchers retrospectively analyzed 80 patients with synDLBCL treated at 10 centers in Australia and the United Kingdom. Patients had DLBCL not otherwise specified (n = 67); high-grade B-cell lymphoma, including double-hit lymphoma (n = 12); or T-cell histiocyte-rich DLBCL (n = 1).
At baseline, all patients were treatment-naive, they had a median age of 64 years (range, 18-87 years), and 68% were male. Seventy percent of patients had high-risk disease according to the CNS International Prognostic Index (IPI), and 96% had non-CNS extranodal disease. The median number of extranodal sites outside the CNS was 2 (range, 0 to more than 10).
Patients were divided into those who received CNS-intensive therapy (n = 38) and those given CNS-conservative therapy (n = 42). The CNS-conservative group was significantly older (P less than .001), significantly more likely to have high-risk disease according to the National Comprehensive Cancer Network IPI (P = .009) or CNS IPI (P = .01) and significantly more likely to have leptomeningeal disease only (P less than .001).
Treatment
CNS-intensive therapy was defined as any established multiagent IV chemotherapy regimen with two or more CNS-penetrating drugs and cytarabine, with or without intrathecal chemotherapy and/or radiotherapy.
CNS-conservative therapy was defined as one or fewer IV CNS-penetrating chemotherapy agents in induction, with or without intrathecal chemotherapy and/or radiotherapy.
Systemic induction in the CNS-intensive group consisted of R-HyperCVAD (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with IV methotrexate and cytarabine) in 66% of patients and R-CODOX-M/IVAC (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, methotrexate/ifosfamide, etoposide, cytarabine) in 24% of patients.
The most common systemic induction regimens in the CNS-conservative group were R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone) or CHOP-like regimens, given to 83% of patients.
CNS-directed IV therapy was given to 100% of the CNS-intensive group and 60% of the CNS-conservative group. This consisted of IV methotrexate plus cytarabine (97%) or MATRix (methotrexate, cytarabine, and thiotepa; 3%) in the CNS-intensive group and high-dose methotrexate in the conservative group.
Intrathecal chemotherapy was given to 97% of the CNS-intensive group and 60% of the CNS-conservative group. CNS-directed radiation was given to 32% and 19%, respectively.
Thirteen patients in the CNS-intensive group and one in the CNS-conservative group underwent autologous transplant as consolidation.
Outcomes
Dose reductions were more frequent in the CNS-conservative group than in the CNS-intensive group, at 48% and 18% (P = .009), as was early cessation of chemotherapy, at 52% and 18% (P = .002). Rates of treatment-related mortality were similar, at 13% in the CNS-intensive group and 12% in the CNS-conservative group.
At the end of induction, the complete response rate was 69% in the CNS-intensive group and 51% in the CNS-conservative group (P = .16). Primary refractory disease was observed in 19% and 38% of patients, respectively (P = .07).
The CNS was the most common site of relapse or progression (n = 28). CNS progression or relapse occurred in 25% of the CNS-intensive group and 49% of the CNS-conservative group (P = .03).
The 2-year progression-free survival rate was 50% for the CNS-intensive group and 31% for the CNS-conservative group (P = .006). The 2-year overall survival rate was 54% and 44%, respectively (P = .037).
When patients were matched for induction outcomes, consolidative transplant did not improve survival.
“The most significant factor affecting survival was the ability to control the CNS disease, which was improved by the addition of IV cytarabine to [high-dose methotrexate],” the researchers wrote.
“Whilst the younger age and more intensive systemic treatment of the CNS-intensive group may have contributed to the improved survival, it is clear that CNS disease control was substantially improved by the addition of cytarabine with lower rates of CNS relapse/progression observed.”
The researchers noted that “adequate control of the CNS disease is paramount and is best achieved by intensive CNS-directed induction.”
There was no formal funding for this study, and the researchers did not provide financial disclosures.
SOURCE: Wight JC et al. Br J Haematol. 2019 Jun 24. doi: 10.1111/bjh.16064.
Controlling CNS disease is “paramount” in treating diffuse large B-cell lymphoma with synchronous CNS and systemic disease (synDLBCL), according to researchers.
In a retrospective study, the CNS was the most common site of relapse in patients with synDLBCL, and patients had better outcomes when they received CNS-directed therapy.
The 2-year progression-free survival rate was 50% in patients who received CNS-intensive therapy and 31% in those who received CNS-conservative therapy. The 2-year overall survival rate was 54% and 44%, respectively.
Dr. Joel C. Wight, of Austin Health in Heidelberg, Australia, and colleagues conducted this study and recounted their findings in the British Journal of Haematology.
The researchers retrospectively analyzed 80 patients with synDLBCL treated at 10 centers in Australia and the United Kingdom. Patients had DLBCL not otherwise specified (n = 67); high-grade B-cell lymphoma, including double-hit lymphoma (n = 12); or T-cell histiocyte-rich DLBCL (n = 1).
At baseline, all patients were treatment-naive, they had a median age of 64 years (range, 18-87 years), and 68% were male. Seventy percent of patients had high-risk disease according to the CNS International Prognostic Index (IPI), and 96% had non-CNS extranodal disease. The median number of extranodal sites outside the CNS was 2 (range, 0 to more than 10).
Patients were divided into those who received CNS-intensive therapy (n = 38) and those given CNS-conservative therapy (n = 42). The CNS-conservative group was significantly older (P less than .001), significantly more likely to have high-risk disease according to the National Comprehensive Cancer Network IPI (P = .009) or CNS IPI (P = .01) and significantly more likely to have leptomeningeal disease only (P less than .001).
Treatment
CNS-intensive therapy was defined as any established multiagent IV chemotherapy regimen with two or more CNS-penetrating drugs and cytarabine, with or without intrathecal chemotherapy and/or radiotherapy.
CNS-conservative therapy was defined as one or fewer IV CNS-penetrating chemotherapy agents in induction, with or without intrathecal chemotherapy and/or radiotherapy.
Systemic induction in the CNS-intensive group consisted of R-HyperCVAD (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with IV methotrexate and cytarabine) in 66% of patients and R-CODOX-M/IVAC (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, methotrexate/ifosfamide, etoposide, cytarabine) in 24% of patients.
The most common systemic induction regimens in the CNS-conservative group were R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone) or CHOP-like regimens, given to 83% of patients.
CNS-directed IV therapy was given to 100% of the CNS-intensive group and 60% of the CNS-conservative group. This consisted of IV methotrexate plus cytarabine (97%) or MATRix (methotrexate, cytarabine, and thiotepa; 3%) in the CNS-intensive group and high-dose methotrexate in the conservative group.
Intrathecal chemotherapy was given to 97% of the CNS-intensive group and 60% of the CNS-conservative group. CNS-directed radiation was given to 32% and 19%, respectively.
Thirteen patients in the CNS-intensive group and one in the CNS-conservative group underwent autologous transplant as consolidation.
Outcomes
Dose reductions were more frequent in the CNS-conservative group than in the CNS-intensive group, at 48% and 18% (P = .009), as was early cessation of chemotherapy, at 52% and 18% (P = .002). Rates of treatment-related mortality were similar, at 13% in the CNS-intensive group and 12% in the CNS-conservative group.
At the end of induction, the complete response rate was 69% in the CNS-intensive group and 51% in the CNS-conservative group (P = .16). Primary refractory disease was observed in 19% and 38% of patients, respectively (P = .07).
The CNS was the most common site of relapse or progression (n = 28). CNS progression or relapse occurred in 25% of the CNS-intensive group and 49% of the CNS-conservative group (P = .03).
The 2-year progression-free survival rate was 50% for the CNS-intensive group and 31% for the CNS-conservative group (P = .006). The 2-year overall survival rate was 54% and 44%, respectively (P = .037).
When patients were matched for induction outcomes, consolidative transplant did not improve survival.
“The most significant factor affecting survival was the ability to control the CNS disease, which was improved by the addition of IV cytarabine to [high-dose methotrexate],” the researchers wrote.
“Whilst the younger age and more intensive systemic treatment of the CNS-intensive group may have contributed to the improved survival, it is clear that CNS disease control was substantially improved by the addition of cytarabine with lower rates of CNS relapse/progression observed.”
The researchers noted that “adequate control of the CNS disease is paramount and is best achieved by intensive CNS-directed induction.”
There was no formal funding for this study, and the researchers did not provide financial disclosures.
SOURCE: Wight JC et al. Br J Haematol. 2019 Jun 24. doi: 10.1111/bjh.16064.
Controlling CNS disease is “paramount” in treating diffuse large B-cell lymphoma with synchronous CNS and systemic disease (synDLBCL), according to researchers.
In a retrospective study, the CNS was the most common site of relapse in patients with synDLBCL, and patients had better outcomes when they received CNS-directed therapy.
The 2-year progression-free survival rate was 50% in patients who received CNS-intensive therapy and 31% in those who received CNS-conservative therapy. The 2-year overall survival rate was 54% and 44%, respectively.
Dr. Joel C. Wight, of Austin Health in Heidelberg, Australia, and colleagues conducted this study and recounted their findings in the British Journal of Haematology.
The researchers retrospectively analyzed 80 patients with synDLBCL treated at 10 centers in Australia and the United Kingdom. Patients had DLBCL not otherwise specified (n = 67); high-grade B-cell lymphoma, including double-hit lymphoma (n = 12); or T-cell histiocyte-rich DLBCL (n = 1).
At baseline, all patients were treatment-naive, they had a median age of 64 years (range, 18-87 years), and 68% were male. Seventy percent of patients had high-risk disease according to the CNS International Prognostic Index (IPI), and 96% had non-CNS extranodal disease. The median number of extranodal sites outside the CNS was 2 (range, 0 to more than 10).
Patients were divided into those who received CNS-intensive therapy (n = 38) and those given CNS-conservative therapy (n = 42). The CNS-conservative group was significantly older (P less than .001), significantly more likely to have high-risk disease according to the National Comprehensive Cancer Network IPI (P = .009) or CNS IPI (P = .01) and significantly more likely to have leptomeningeal disease only (P less than .001).
Treatment
CNS-intensive therapy was defined as any established multiagent IV chemotherapy regimen with two or more CNS-penetrating drugs and cytarabine, with or without intrathecal chemotherapy and/or radiotherapy.
CNS-conservative therapy was defined as one or fewer IV CNS-penetrating chemotherapy agents in induction, with or without intrathecal chemotherapy and/or radiotherapy.
Systemic induction in the CNS-intensive group consisted of R-HyperCVAD (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with IV methotrexate and cytarabine) in 66% of patients and R-CODOX-M/IVAC (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, methotrexate/ifosfamide, etoposide, cytarabine) in 24% of patients.
The most common systemic induction regimens in the CNS-conservative group were R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone) or CHOP-like regimens, given to 83% of patients.
CNS-directed IV therapy was given to 100% of the CNS-intensive group and 60% of the CNS-conservative group. This consisted of IV methotrexate plus cytarabine (97%) or MATRix (methotrexate, cytarabine, and thiotepa; 3%) in the CNS-intensive group and high-dose methotrexate in the conservative group.
Intrathecal chemotherapy was given to 97% of the CNS-intensive group and 60% of the CNS-conservative group. CNS-directed radiation was given to 32% and 19%, respectively.
Thirteen patients in the CNS-intensive group and one in the CNS-conservative group underwent autologous transplant as consolidation.
Outcomes
Dose reductions were more frequent in the CNS-conservative group than in the CNS-intensive group, at 48% and 18% (P = .009), as was early cessation of chemotherapy, at 52% and 18% (P = .002). Rates of treatment-related mortality were similar, at 13% in the CNS-intensive group and 12% in the CNS-conservative group.
At the end of induction, the complete response rate was 69% in the CNS-intensive group and 51% in the CNS-conservative group (P = .16). Primary refractory disease was observed in 19% and 38% of patients, respectively (P = .07).
The CNS was the most common site of relapse or progression (n = 28). CNS progression or relapse occurred in 25% of the CNS-intensive group and 49% of the CNS-conservative group (P = .03).
The 2-year progression-free survival rate was 50% for the CNS-intensive group and 31% for the CNS-conservative group (P = .006). The 2-year overall survival rate was 54% and 44%, respectively (P = .037).
When patients were matched for induction outcomes, consolidative transplant did not improve survival.
“The most significant factor affecting survival was the ability to control the CNS disease, which was improved by the addition of IV cytarabine to [high-dose methotrexate],” the researchers wrote.
“Whilst the younger age and more intensive systemic treatment of the CNS-intensive group may have contributed to the improved survival, it is clear that CNS disease control was substantially improved by the addition of cytarabine with lower rates of CNS relapse/progression observed.”
The researchers noted that “adequate control of the CNS disease is paramount and is best achieved by intensive CNS-directed induction.”
There was no formal funding for this study, and the researchers did not provide financial disclosures.
SOURCE: Wight JC et al. Br J Haematol. 2019 Jun 24. doi: 10.1111/bjh.16064.
FROM BRITISH JOURNAL OF HAEMATOLOGY