User login
Another study supports safety of 2-cm margins for thick melanomas
based on data from a randomized, multicenter trial of 936 patients.
“Over time, and in light of the findings of several randomized studies, less extensive surgery for primary melanoma with tumor thickness greater than 2 mm has become more established,” and most recent guidelines recommend a 2-cm margin for these tumors, wrote Deborah Utjés, MD, of the Karolinska Institute in Stockholm and colleagues.
To reinforce the safety and effectiveness of the 2-cm margin, the researchers conducted an open-label, randomized trial of clinically staged melanoma patients aged 75 years and younger with localized cutaneous melanomas thicker than 2 mm, from January 1992 to May 2004. Patients were treated in Denmark, Estonia, Norway, and Sweden. The findings were published in the Lancet.
Patients were randomized to treatment with a 2-cm (471) or 4-cm excision margin (465). The melanomas were located on the trunk, upper extremities, or lower extremities.
The primary outcome of overall survival was similar between the groups. Over a median 20-year follow-up period, the death rate was approximately 50% in each group (49% in the 2-cm group and 51% in the 4-cm group). Disease-specific survival rates were similar as well. Of the 621 reported deaths, 397 were attributed to melanoma: 192 (48%) in the 2-cm group and 205 (52%) in the 4-cm group.
The study findings were limited by several factors, including a lower-than-expected number of patients, lack of nodal staging during the study period, and a focus only on the surgical margin without recording data on pathological excision margins.
However, the extended follow-up supports the safe use of the 2-cm margin for the treatment of melanomas thicker than 2 mm, the investigators wrote. In addition, results from an ongoing trial comparing 1-cm and 2-cm margins for melanomas at least 1 mm thick may yield more evidence to support still narrower surgical margins for some cutaneous melanomas.
The study notes that guidelines from organizations that include the American National Comprehensive Cancer Network and the American Academy of Dermatology recommend the 2-cm margin for tumors that are thicker than 2 mm.
The study was supported by the Swedish Cancer Society, Stockholm Cancer Society, Swedish Society for Medical Research, and the Stockholm County Council, and by funds from Radiumhemmet Research and Wallström. The authors reported no disclosures.
SOURCE: Utjés D et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31132-8.
based on data from a randomized, multicenter trial of 936 patients.
“Over time, and in light of the findings of several randomized studies, less extensive surgery for primary melanoma with tumor thickness greater than 2 mm has become more established,” and most recent guidelines recommend a 2-cm margin for these tumors, wrote Deborah Utjés, MD, of the Karolinska Institute in Stockholm and colleagues.
To reinforce the safety and effectiveness of the 2-cm margin, the researchers conducted an open-label, randomized trial of clinically staged melanoma patients aged 75 years and younger with localized cutaneous melanomas thicker than 2 mm, from January 1992 to May 2004. Patients were treated in Denmark, Estonia, Norway, and Sweden. The findings were published in the Lancet.
Patients were randomized to treatment with a 2-cm (471) or 4-cm excision margin (465). The melanomas were located on the trunk, upper extremities, or lower extremities.
The primary outcome of overall survival was similar between the groups. Over a median 20-year follow-up period, the death rate was approximately 50% in each group (49% in the 2-cm group and 51% in the 4-cm group). Disease-specific survival rates were similar as well. Of the 621 reported deaths, 397 were attributed to melanoma: 192 (48%) in the 2-cm group and 205 (52%) in the 4-cm group.
The study findings were limited by several factors, including a lower-than-expected number of patients, lack of nodal staging during the study period, and a focus only on the surgical margin without recording data on pathological excision margins.
However, the extended follow-up supports the safe use of the 2-cm margin for the treatment of melanomas thicker than 2 mm, the investigators wrote. In addition, results from an ongoing trial comparing 1-cm and 2-cm margins for melanomas at least 1 mm thick may yield more evidence to support still narrower surgical margins for some cutaneous melanomas.
The study notes that guidelines from organizations that include the American National Comprehensive Cancer Network and the American Academy of Dermatology recommend the 2-cm margin for tumors that are thicker than 2 mm.
The study was supported by the Swedish Cancer Society, Stockholm Cancer Society, Swedish Society for Medical Research, and the Stockholm County Council, and by funds from Radiumhemmet Research and Wallström. The authors reported no disclosures.
SOURCE: Utjés D et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31132-8.
based on data from a randomized, multicenter trial of 936 patients.
“Over time, and in light of the findings of several randomized studies, less extensive surgery for primary melanoma with tumor thickness greater than 2 mm has become more established,” and most recent guidelines recommend a 2-cm margin for these tumors, wrote Deborah Utjés, MD, of the Karolinska Institute in Stockholm and colleagues.
To reinforce the safety and effectiveness of the 2-cm margin, the researchers conducted an open-label, randomized trial of clinically staged melanoma patients aged 75 years and younger with localized cutaneous melanomas thicker than 2 mm, from January 1992 to May 2004. Patients were treated in Denmark, Estonia, Norway, and Sweden. The findings were published in the Lancet.
Patients were randomized to treatment with a 2-cm (471) or 4-cm excision margin (465). The melanomas were located on the trunk, upper extremities, or lower extremities.
The primary outcome of overall survival was similar between the groups. Over a median 20-year follow-up period, the death rate was approximately 50% in each group (49% in the 2-cm group and 51% in the 4-cm group). Disease-specific survival rates were similar as well. Of the 621 reported deaths, 397 were attributed to melanoma: 192 (48%) in the 2-cm group and 205 (52%) in the 4-cm group.
The study findings were limited by several factors, including a lower-than-expected number of patients, lack of nodal staging during the study period, and a focus only on the surgical margin without recording data on pathological excision margins.
However, the extended follow-up supports the safe use of the 2-cm margin for the treatment of melanomas thicker than 2 mm, the investigators wrote. In addition, results from an ongoing trial comparing 1-cm and 2-cm margins for melanomas at least 1 mm thick may yield more evidence to support still narrower surgical margins for some cutaneous melanomas.
The study notes that guidelines from organizations that include the American National Comprehensive Cancer Network and the American Academy of Dermatology recommend the 2-cm margin for tumors that are thicker than 2 mm.
The study was supported by the Swedish Cancer Society, Stockholm Cancer Society, Swedish Society for Medical Research, and the Stockholm County Council, and by funds from Radiumhemmet Research and Wallström. The authors reported no disclosures.
SOURCE: Utjés D et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31132-8.
FROM THE LANCET
Dancing parrots, flying spiders, and ER fish tales
Cockatoo cha-cha-cha
Humans love to dance. Whether it’s just a little bobbing back and forth or a full-on tango, spontaneous dancing is a universal expression of feeling and joy. However, feeling the rhythm is nearly unique to the human species. Not even our closest relatives in the animal kingdom, primates, have been observed busting a move to music.
So who are our ballroom brethren? Parrots, strangely enough. Parrots are nature’s great imitators, so how can we be sure they aren’t just mimicking (or mocking) their human owners’ movements?
Several studies have been conducted on a particularly funky cockatoo named Snowball. He’s a viral Internet sensation, and researchers have been watching him for the past decade as he stomps his feet and bangs his head to all kinds of music – he jams to everything from classic rock to modern pop. Snowball’s owner also assured scientists that her bird does not get his dance moves from her; Snowball is a unique choreographer.
A follow-up study about Snowball was recently published, in which the authors suggested the reason for spontaneous parrot dancing could be the strong auditory-motor connections that exist in parrot brains. They’re similar to humans, but not primates. They also detailed Snowball’s 14 distinct dance moves. Someone get that parrot on America’s Got Talent, ASAP.
It’s a bird, it’s a plane – oh no, it’s a spider
Arachnophobes, just skip this one. If you’re not unsettled by spiders yet, you’re about to be!
In case you didn’t know, spiders can fly. Or rather, they float (spiders with wings would just be straight-up horrifying).
Scientists (and fans of Charlotte’s Web) have known for centuries that spiders can turn into their own personal airships, but it wasn’t always clear how they do it. It was commonly believed that spider-flying, aka “ballooning,” was caused by the spider using its silk as a kind of kite, with the wind catching on the silk and bringing the spider along with it. That explanation, however, doesn’t account for the fact that spiders only balloon during light winds, which are rarely strong enough to carry a spider.
Two intrepid spider fans from the University of Bristol (England) think they have solved the mystery of the flying arachnids.
It turns out that spiders are able to sense the earth’s electric field and use that to soar around. They let loose some silk, which picks up a negative charge. This charge repels the negative charge of whatever they are standing on and can create enough force to launch them into the air. Spiders, those smart little buggers, can increase these charges by climbing onto twigs or grass. Plants rooted in the earth have a strong negative charge but stick up into the positively charged air.
All this fancy science-babble to say: Spiders can fly, and we know how! Stay indoors, folks.
Hospital lures fishermen to ED
There are some things you expect to see when you walk into a hospital: an admitting desk, waiting areas full of people, security guards, bad art work, maybe a friendly greeter at the door (sorry, that’s the Walmart).
The main lobby at Adirondack Medical Center in Saranac Lake, N.Y., however, has something you might not expect: a display of fishing lures.
Each of the more than 100 lures on display originally showed up at the hospital’s emergency department attached to an arm, leg, nose, or lip – pretty much every body part possible, Gary Nye, a physician assistant in the ED, told newyorkupstate.com. He’s even removed lures from several patients’ penises and scrotums. “Usually alcohol has something to do with it,” he noted.
One individual came in with hooks from a lure stuck on both thumbs. He got one set of hooks stuck on one thumb, and as he tried to get it out, he ended up getting the lure’s other set of hooks caught on his other thumb. A nurse who has worked in the ED for 20 years said that alcohol may been involved.
Dr. Michael Pond, the hospital’s former medical director and the one who started the display in 1990, explained why the lures are now kept in a locked case. A number of years ago, when the display was less secure, “someone cut out and stole four to five antique lures out of the case. I mean, these were absolutely gorgeous and expensive lures – Pikie Minnows and [other] things. [They] were made out of wood and painted.”
As with so many fish stories, you should have seen the one that got away.
Inspiration really can come from anywhere
On the surface, a study detailing a new, potentially practice-changing method of converting type A blood to type O doesn’t seem disgusting, silly, or odd enough to make it into Livin’ on the MDedge. But when you dig a little deeper, you’ll see that the method involves a bacteria in the human microbiome.
Now you might be getting a bit suspicious. This is going to be another story about poop, you might be thinking.
And you’d be right!
But the real star of the show isn’t human poop. No, in this case, we owe this groundbreaking discovery to the humble beaver.
In an earlier study published in the ISME Journal, the same group of researchers from the University of British Columbia in Vancouver (of course they’re Canadian) analyzed the microbiomes of beavers to see just how they broke down the complex carbohydrates and glycoproteins found in wood. That was the catalyst that got the researchers thinking about the human microbiome and the problem of stripping red blood cells of their antigens.
Basically, just as the beaver breaks down wood with bacteria in their microbiome, the modified bacteria the researchers conjured up can be used to break down the A and B antigens of red blood cells, leaving a simple type O cell that can be transfused into any patient.
Rumors that all future blood transfusions are required by law to be accompanied by a playing of O Canada remain unsubstantiated.

Cockatoo cha-cha-cha
Humans love to dance. Whether it’s just a little bobbing back and forth or a full-on tango, spontaneous dancing is a universal expression of feeling and joy. However, feeling the rhythm is nearly unique to the human species. Not even our closest relatives in the animal kingdom, primates, have been observed busting a move to music.
So who are our ballroom brethren? Parrots, strangely enough. Parrots are nature’s great imitators, so how can we be sure they aren’t just mimicking (or mocking) their human owners’ movements?
Several studies have been conducted on a particularly funky cockatoo named Snowball. He’s a viral Internet sensation, and researchers have been watching him for the past decade as he stomps his feet and bangs his head to all kinds of music – he jams to everything from classic rock to modern pop. Snowball’s owner also assured scientists that her bird does not get his dance moves from her; Snowball is a unique choreographer.
A follow-up study about Snowball was recently published, in which the authors suggested the reason for spontaneous parrot dancing could be the strong auditory-motor connections that exist in parrot brains. They’re similar to humans, but not primates. They also detailed Snowball’s 14 distinct dance moves. Someone get that parrot on America’s Got Talent, ASAP.
It’s a bird, it’s a plane – oh no, it’s a spider
Arachnophobes, just skip this one. If you’re not unsettled by spiders yet, you’re about to be!
In case you didn’t know, spiders can fly. Or rather, they float (spiders with wings would just be straight-up horrifying).
Scientists (and fans of Charlotte’s Web) have known for centuries that spiders can turn into their own personal airships, but it wasn’t always clear how they do it. It was commonly believed that spider-flying, aka “ballooning,” was caused by the spider using its silk as a kind of kite, with the wind catching on the silk and bringing the spider along with it. That explanation, however, doesn’t account for the fact that spiders only balloon during light winds, which are rarely strong enough to carry a spider.
Two intrepid spider fans from the University of Bristol (England) think they have solved the mystery of the flying arachnids.
It turns out that spiders are able to sense the earth’s electric field and use that to soar around. They let loose some silk, which picks up a negative charge. This charge repels the negative charge of whatever they are standing on and can create enough force to launch them into the air. Spiders, those smart little buggers, can increase these charges by climbing onto twigs or grass. Plants rooted in the earth have a strong negative charge but stick up into the positively charged air.
All this fancy science-babble to say: Spiders can fly, and we know how! Stay indoors, folks.
Hospital lures fishermen to ED
There are some things you expect to see when you walk into a hospital: an admitting desk, waiting areas full of people, security guards, bad art work, maybe a friendly greeter at the door (sorry, that’s the Walmart).
The main lobby at Adirondack Medical Center in Saranac Lake, N.Y., however, has something you might not expect: a display of fishing lures.
Each of the more than 100 lures on display originally showed up at the hospital’s emergency department attached to an arm, leg, nose, or lip – pretty much every body part possible, Gary Nye, a physician assistant in the ED, told newyorkupstate.com. He’s even removed lures from several patients’ penises and scrotums. “Usually alcohol has something to do with it,” he noted.
One individual came in with hooks from a lure stuck on both thumbs. He got one set of hooks stuck on one thumb, and as he tried to get it out, he ended up getting the lure’s other set of hooks caught on his other thumb. A nurse who has worked in the ED for 20 years said that alcohol may been involved.
Dr. Michael Pond, the hospital’s former medical director and the one who started the display in 1990, explained why the lures are now kept in a locked case. A number of years ago, when the display was less secure, “someone cut out and stole four to five antique lures out of the case. I mean, these were absolutely gorgeous and expensive lures – Pikie Minnows and [other] things. [They] were made out of wood and painted.”
As with so many fish stories, you should have seen the one that got away.
Inspiration really can come from anywhere
On the surface, a study detailing a new, potentially practice-changing method of converting type A blood to type O doesn’t seem disgusting, silly, or odd enough to make it into Livin’ on the MDedge. But when you dig a little deeper, you’ll see that the method involves a bacteria in the human microbiome.
Now you might be getting a bit suspicious. This is going to be another story about poop, you might be thinking.
And you’d be right!
But the real star of the show isn’t human poop. No, in this case, we owe this groundbreaking discovery to the humble beaver.
In an earlier study published in the ISME Journal, the same group of researchers from the University of British Columbia in Vancouver (of course they’re Canadian) analyzed the microbiomes of beavers to see just how they broke down the complex carbohydrates and glycoproteins found in wood. That was the catalyst that got the researchers thinking about the human microbiome and the problem of stripping red blood cells of their antigens.
Basically, just as the beaver breaks down wood with bacteria in their microbiome, the modified bacteria the researchers conjured up can be used to break down the A and B antigens of red blood cells, leaving a simple type O cell that can be transfused into any patient.
Rumors that all future blood transfusions are required by law to be accompanied by a playing of O Canada remain unsubstantiated.

Cockatoo cha-cha-cha
Humans love to dance. Whether it’s just a little bobbing back and forth or a full-on tango, spontaneous dancing is a universal expression of feeling and joy. However, feeling the rhythm is nearly unique to the human species. Not even our closest relatives in the animal kingdom, primates, have been observed busting a move to music.
So who are our ballroom brethren? Parrots, strangely enough. Parrots are nature’s great imitators, so how can we be sure they aren’t just mimicking (or mocking) their human owners’ movements?
Several studies have been conducted on a particularly funky cockatoo named Snowball. He’s a viral Internet sensation, and researchers have been watching him for the past decade as he stomps his feet and bangs his head to all kinds of music – he jams to everything from classic rock to modern pop. Snowball’s owner also assured scientists that her bird does not get his dance moves from her; Snowball is a unique choreographer.
A follow-up study about Snowball was recently published, in which the authors suggested the reason for spontaneous parrot dancing could be the strong auditory-motor connections that exist in parrot brains. They’re similar to humans, but not primates. They also detailed Snowball’s 14 distinct dance moves. Someone get that parrot on America’s Got Talent, ASAP.
It’s a bird, it’s a plane – oh no, it’s a spider
Arachnophobes, just skip this one. If you’re not unsettled by spiders yet, you’re about to be!
In case you didn’t know, spiders can fly. Or rather, they float (spiders with wings would just be straight-up horrifying).
Scientists (and fans of Charlotte’s Web) have known for centuries that spiders can turn into their own personal airships, but it wasn’t always clear how they do it. It was commonly believed that spider-flying, aka “ballooning,” was caused by the spider using its silk as a kind of kite, with the wind catching on the silk and bringing the spider along with it. That explanation, however, doesn’t account for the fact that spiders only balloon during light winds, which are rarely strong enough to carry a spider.
Two intrepid spider fans from the University of Bristol (England) think they have solved the mystery of the flying arachnids.
It turns out that spiders are able to sense the earth’s electric field and use that to soar around. They let loose some silk, which picks up a negative charge. This charge repels the negative charge of whatever they are standing on and can create enough force to launch them into the air. Spiders, those smart little buggers, can increase these charges by climbing onto twigs or grass. Plants rooted in the earth have a strong negative charge but stick up into the positively charged air.
All this fancy science-babble to say: Spiders can fly, and we know how! Stay indoors, folks.
Hospital lures fishermen to ED
There are some things you expect to see when you walk into a hospital: an admitting desk, waiting areas full of people, security guards, bad art work, maybe a friendly greeter at the door (sorry, that’s the Walmart).
The main lobby at Adirondack Medical Center in Saranac Lake, N.Y., however, has something you might not expect: a display of fishing lures.
Each of the more than 100 lures on display originally showed up at the hospital’s emergency department attached to an arm, leg, nose, or lip – pretty much every body part possible, Gary Nye, a physician assistant in the ED, told newyorkupstate.com. He’s even removed lures from several patients’ penises and scrotums. “Usually alcohol has something to do with it,” he noted.
One individual came in with hooks from a lure stuck on both thumbs. He got one set of hooks stuck on one thumb, and as he tried to get it out, he ended up getting the lure’s other set of hooks caught on his other thumb. A nurse who has worked in the ED for 20 years said that alcohol may been involved.
Dr. Michael Pond, the hospital’s former medical director and the one who started the display in 1990, explained why the lures are now kept in a locked case. A number of years ago, when the display was less secure, “someone cut out and stole four to five antique lures out of the case. I mean, these were absolutely gorgeous and expensive lures – Pikie Minnows and [other] things. [They] were made out of wood and painted.”
As with so many fish stories, you should have seen the one that got away.
Inspiration really can come from anywhere
On the surface, a study detailing a new, potentially practice-changing method of converting type A blood to type O doesn’t seem disgusting, silly, or odd enough to make it into Livin’ on the MDedge. But when you dig a little deeper, you’ll see that the method involves a bacteria in the human microbiome.
Now you might be getting a bit suspicious. This is going to be another story about poop, you might be thinking.
And you’d be right!
But the real star of the show isn’t human poop. No, in this case, we owe this groundbreaking discovery to the humble beaver.
In an earlier study published in the ISME Journal, the same group of researchers from the University of British Columbia in Vancouver (of course they’re Canadian) analyzed the microbiomes of beavers to see just how they broke down the complex carbohydrates and glycoproteins found in wood. That was the catalyst that got the researchers thinking about the human microbiome and the problem of stripping red blood cells of their antigens.
Basically, just as the beaver breaks down wood with bacteria in their microbiome, the modified bacteria the researchers conjured up can be used to break down the A and B antigens of red blood cells, leaving a simple type O cell that can be transfused into any patient.
Rumors that all future blood transfusions are required by law to be accompanied by a playing of O Canada remain unsubstantiated.

Product News July 2019
AbbVie to Acquire Allergan
AbbVie Inc and Allergan announce that the companies have entered into a definitive transaction agreement under which AbbVie will acquire Allergan in a cash and stock transaction. The combined company will consist of several franchises with leadership positions across immunology, hematologic oncology, medical aesthetics, neuroscience, women’s health, eye care, and virology. Furthermore, Allergan’s product portfolio will be enhanced by AbbVie’s commercial strength, expertise, and international infrastructure. For more information, visit www.abbvie.com and www.allergan.com.
Avène Launches Mineral Sunscreen Fluids
Pierre Fabre Dermo-Cosmetique introduces Avène Mineral Sunscreen Fluids SPF 50+ in both tinted and nontinted varieties. With active ingredients titanium dioxide (11.4%) and zinc oxide (14.6%), both products provide broad-spectrum UVA and UVB protection and are free from octinoxate and oxybenzone (reef friendly). These lightweight lotions are ideal for use on the face; they absorb quickly and can be layered invisibly under makeup. Additionally, the tinted fluid offers protection against blue light. For more information, visit www.aveneusa.com.
CoolTone Device Clearance Expanded
Allergan announces US Food and Drug Administration clearance of the nonsurgical CoolTone device for the improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone also is indicated for the strengthening, toning, and firming of buttocks and thighs. Using magnetic muscle stimulation, CoolTone technology penetrates into the muscle layers and induces involuntary muscle contractions. The body responds to these contractions by strengthening its muscle fibers, resulting in a more defined and toned appearance. For more information, visit www.cooltonebycoolsculpting.com.
Duobrii Lotion for Plaque Psoriasis Now Available
Ortho Dermatologics announces the US launch of Duobrii (halobetasol propionate and tazarotene) Lotion 0.01%/0.045%. Duobrii was approved by the US Food and Drug Administration in April 2019 for the treatment of plaque psoriasis in adult patients, offering psoriasis patients a treatment with strong efficacy and an extended duration of use in a once-daily lotion that can be dosed to clearance. When used separately to treat plaque psoriasis, the duration of use of halobetasol propionate is limited to 2 to 4 weeks, and the use of tazarotene can be limited due to tolerability concerns. By combining halobetasol propionate and tazarotene in a patented once-daily moisturizing lotion, the Duobrii formulation ensures uniform distribution, allowing for simultaneous contact with the skin surface. Unlike other topical products that either contain steroids or are steroids on their own, Duobrii is not restricted to 8 weeks or less of use. The approved labeling for Duobrii does not include a duration limitation; it can be dosed to clearance as long as local skin reactions do not occur, and treatment should be discontinued once clearance is achieved. For more information, visit www.duobrii.com.
Zika Virus Diagnostic Test Receives Marketing Authorization
The US Food and Drug Administration authorizes marketing of the ZIKV Detect 2.0 IgM Capture ELISA (enzymelinked immunosorbent assay)(InBios International, Inc) for the qualitative detection of Zika virus IgM antibodies in human blood. The ZIKV Detect 2.0 IgM Capture ELISA is designed to identify proteins (antibodies) produced by the body’s immune system when it tests for Zika virus infection in the blood; IgM antibodies indicate an early immune response. The test is for use only in patients with clinical signs and symptoms consistent with Zika virus infection and/or those who meet the Centers for Disease Control and Prevention’s Zika virus epidemiologic criteria, such as history of residence in or travel to a geographic region with active Zika transmission at the time of travel. For more information, visit www.inbios.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
AbbVie to Acquire Allergan
AbbVie Inc and Allergan announce that the companies have entered into a definitive transaction agreement under which AbbVie will acquire Allergan in a cash and stock transaction. The combined company will consist of several franchises with leadership positions across immunology, hematologic oncology, medical aesthetics, neuroscience, women’s health, eye care, and virology. Furthermore, Allergan’s product portfolio will be enhanced by AbbVie’s commercial strength, expertise, and international infrastructure. For more information, visit www.abbvie.com and www.allergan.com.
Avène Launches Mineral Sunscreen Fluids
Pierre Fabre Dermo-Cosmetique introduces Avène Mineral Sunscreen Fluids SPF 50+ in both tinted and nontinted varieties. With active ingredients titanium dioxide (11.4%) and zinc oxide (14.6%), both products provide broad-spectrum UVA and UVB protection and are free from octinoxate and oxybenzone (reef friendly). These lightweight lotions are ideal for use on the face; they absorb quickly and can be layered invisibly under makeup. Additionally, the tinted fluid offers protection against blue light. For more information, visit www.aveneusa.com.
CoolTone Device Clearance Expanded
Allergan announces US Food and Drug Administration clearance of the nonsurgical CoolTone device for the improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone also is indicated for the strengthening, toning, and firming of buttocks and thighs. Using magnetic muscle stimulation, CoolTone technology penetrates into the muscle layers and induces involuntary muscle contractions. The body responds to these contractions by strengthening its muscle fibers, resulting in a more defined and toned appearance. For more information, visit www.cooltonebycoolsculpting.com.
Duobrii Lotion for Plaque Psoriasis Now Available
Ortho Dermatologics announces the US launch of Duobrii (halobetasol propionate and tazarotene) Lotion 0.01%/0.045%. Duobrii was approved by the US Food and Drug Administration in April 2019 for the treatment of plaque psoriasis in adult patients, offering psoriasis patients a treatment with strong efficacy and an extended duration of use in a once-daily lotion that can be dosed to clearance. When used separately to treat plaque psoriasis, the duration of use of halobetasol propionate is limited to 2 to 4 weeks, and the use of tazarotene can be limited due to tolerability concerns. By combining halobetasol propionate and tazarotene in a patented once-daily moisturizing lotion, the Duobrii formulation ensures uniform distribution, allowing for simultaneous contact with the skin surface. Unlike other topical products that either contain steroids or are steroids on their own, Duobrii is not restricted to 8 weeks or less of use. The approved labeling for Duobrii does not include a duration limitation; it can be dosed to clearance as long as local skin reactions do not occur, and treatment should be discontinued once clearance is achieved. For more information, visit www.duobrii.com.
Zika Virus Diagnostic Test Receives Marketing Authorization
The US Food and Drug Administration authorizes marketing of the ZIKV Detect 2.0 IgM Capture ELISA (enzymelinked immunosorbent assay)(InBios International, Inc) for the qualitative detection of Zika virus IgM antibodies in human blood. The ZIKV Detect 2.0 IgM Capture ELISA is designed to identify proteins (antibodies) produced by the body’s immune system when it tests for Zika virus infection in the blood; IgM antibodies indicate an early immune response. The test is for use only in patients with clinical signs and symptoms consistent with Zika virus infection and/or those who meet the Centers for Disease Control and Prevention’s Zika virus epidemiologic criteria, such as history of residence in or travel to a geographic region with active Zika transmission at the time of travel. For more information, visit www.inbios.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
AbbVie to Acquire Allergan
AbbVie Inc and Allergan announce that the companies have entered into a definitive transaction agreement under which AbbVie will acquire Allergan in a cash and stock transaction. The combined company will consist of several franchises with leadership positions across immunology, hematologic oncology, medical aesthetics, neuroscience, women’s health, eye care, and virology. Furthermore, Allergan’s product portfolio will be enhanced by AbbVie’s commercial strength, expertise, and international infrastructure. For more information, visit www.abbvie.com and www.allergan.com.
Avène Launches Mineral Sunscreen Fluids
Pierre Fabre Dermo-Cosmetique introduces Avène Mineral Sunscreen Fluids SPF 50+ in both tinted and nontinted varieties. With active ingredients titanium dioxide (11.4%) and zinc oxide (14.6%), both products provide broad-spectrum UVA and UVB protection and are free from octinoxate and oxybenzone (reef friendly). These lightweight lotions are ideal for use on the face; they absorb quickly and can be layered invisibly under makeup. Additionally, the tinted fluid offers protection against blue light. For more information, visit www.aveneusa.com.
CoolTone Device Clearance Expanded
Allergan announces US Food and Drug Administration clearance of the nonsurgical CoolTone device for the improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone also is indicated for the strengthening, toning, and firming of buttocks and thighs. Using magnetic muscle stimulation, CoolTone technology penetrates into the muscle layers and induces involuntary muscle contractions. The body responds to these contractions by strengthening its muscle fibers, resulting in a more defined and toned appearance. For more information, visit www.cooltonebycoolsculpting.com.
Duobrii Lotion for Plaque Psoriasis Now Available
Ortho Dermatologics announces the US launch of Duobrii (halobetasol propionate and tazarotene) Lotion 0.01%/0.045%. Duobrii was approved by the US Food and Drug Administration in April 2019 for the treatment of plaque psoriasis in adult patients, offering psoriasis patients a treatment with strong efficacy and an extended duration of use in a once-daily lotion that can be dosed to clearance. When used separately to treat plaque psoriasis, the duration of use of halobetasol propionate is limited to 2 to 4 weeks, and the use of tazarotene can be limited due to tolerability concerns. By combining halobetasol propionate and tazarotene in a patented once-daily moisturizing lotion, the Duobrii formulation ensures uniform distribution, allowing for simultaneous contact with the skin surface. Unlike other topical products that either contain steroids or are steroids on their own, Duobrii is not restricted to 8 weeks or less of use. The approved labeling for Duobrii does not include a duration limitation; it can be dosed to clearance as long as local skin reactions do not occur, and treatment should be discontinued once clearance is achieved. For more information, visit www.duobrii.com.
Zika Virus Diagnostic Test Receives Marketing Authorization
The US Food and Drug Administration authorizes marketing of the ZIKV Detect 2.0 IgM Capture ELISA (enzymelinked immunosorbent assay)(InBios International, Inc) for the qualitative detection of Zika virus IgM antibodies in human blood. The ZIKV Detect 2.0 IgM Capture ELISA is designed to identify proteins (antibodies) produced by the body’s immune system when it tests for Zika virus infection in the blood; IgM antibodies indicate an early immune response. The test is for use only in patients with clinical signs and symptoms consistent with Zika virus infection and/or those who meet the Centers for Disease Control and Prevention’s Zika virus epidemiologic criteria, such as history of residence in or travel to a geographic region with active Zika transmission at the time of travel. For more information, visit www.inbios.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
CHEST Clinical Perspectives explores the emerging field of precision medicine
For clinicians seeking to provide a pathway to treatment or diagnosis that is individualized to the patient, a recent study found that the issues go beyond awareness or a patient’s degree of comfort – there remains the question of something as simple as: what should we call it?
Clinicians remain uncertain whether to name the new field precision or personalized medicine according the new CHEST Clinical PerspectivesTM white paper, “Precision Medicine: Adoption of Emerging Methods of Evaluation and Therapy.” A survey of leading community clinicians from among CHEST membership found that only 35 % called tailoring medical treatment to the individual characteristics of each patient “precision medicine,” with 24% preferring “personalized” medicine. Thirty-six percent of respondents used the terms interchangeably.
Beyond the communication issues, the study found that most clinicians surveyed did not know enough about precision medicine to adopt it into their practice. Those surveyed reported that they wanted to see more published studies on the effectiveness of the newly available tools before discussing these options with their patients.
The majority of the respondents were general pulmonologists with intensivists and interventional pulmonologists also responding. The study was led by Nichole T. Tanner, MD, MSCR, FCCP, of the Medical University of South Carolina. Dr Tanner will be hosting a webinar to review the conclusions of this paper at 10:00 AM CT on Tuesday, July 30.
More information about CHEST Clinical PerspectivesTM, part of the CHEST Analytics program, can be found at insights.chestnet.org. To suggest a topic to be covered in a future issue, contact Linda Tomczynski, [email protected] or +1 (224) 521-9593. Register today at https://hubs.ly/H0jqCGb0.
For clinicians seeking to provide a pathway to treatment or diagnosis that is individualized to the patient, a recent study found that the issues go beyond awareness or a patient’s degree of comfort – there remains the question of something as simple as: what should we call it?
Clinicians remain uncertain whether to name the new field precision or personalized medicine according the new CHEST Clinical PerspectivesTM white paper, “Precision Medicine: Adoption of Emerging Methods of Evaluation and Therapy.” A survey of leading community clinicians from among CHEST membership found that only 35 % called tailoring medical treatment to the individual characteristics of each patient “precision medicine,” with 24% preferring “personalized” medicine. Thirty-six percent of respondents used the terms interchangeably.
Beyond the communication issues, the study found that most clinicians surveyed did not know enough about precision medicine to adopt it into their practice. Those surveyed reported that they wanted to see more published studies on the effectiveness of the newly available tools before discussing these options with their patients.
The majority of the respondents were general pulmonologists with intensivists and interventional pulmonologists also responding. The study was led by Nichole T. Tanner, MD, MSCR, FCCP, of the Medical University of South Carolina. Dr Tanner will be hosting a webinar to review the conclusions of this paper at 10:00 AM CT on Tuesday, July 30.
More information about CHEST Clinical PerspectivesTM, part of the CHEST Analytics program, can be found at insights.chestnet.org. To suggest a topic to be covered in a future issue, contact Linda Tomczynski, [email protected] or +1 (224) 521-9593. Register today at https://hubs.ly/H0jqCGb0.
For clinicians seeking to provide a pathway to treatment or diagnosis that is individualized to the patient, a recent study found that the issues go beyond awareness or a patient’s degree of comfort – there remains the question of something as simple as: what should we call it?
Clinicians remain uncertain whether to name the new field precision or personalized medicine according the new CHEST Clinical PerspectivesTM white paper, “Precision Medicine: Adoption of Emerging Methods of Evaluation and Therapy.” A survey of leading community clinicians from among CHEST membership found that only 35 % called tailoring medical treatment to the individual characteristics of each patient “precision medicine,” with 24% preferring “personalized” medicine. Thirty-six percent of respondents used the terms interchangeably.
Beyond the communication issues, the study found that most clinicians surveyed did not know enough about precision medicine to adopt it into their practice. Those surveyed reported that they wanted to see more published studies on the effectiveness of the newly available tools before discussing these options with their patients.
The majority of the respondents were general pulmonologists with intensivists and interventional pulmonologists also responding. The study was led by Nichole T. Tanner, MD, MSCR, FCCP, of the Medical University of South Carolina. Dr Tanner will be hosting a webinar to review the conclusions of this paper at 10:00 AM CT on Tuesday, July 30.
More information about CHEST Clinical PerspectivesTM, part of the CHEST Analytics program, can be found at insights.chestnet.org. To suggest a topic to be covered in a future issue, contact Linda Tomczynski, [email protected] or +1 (224) 521-9593. Register today at https://hubs.ly/H0jqCGb0.
Environmental Scan: Drivers of change in health care
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: the CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
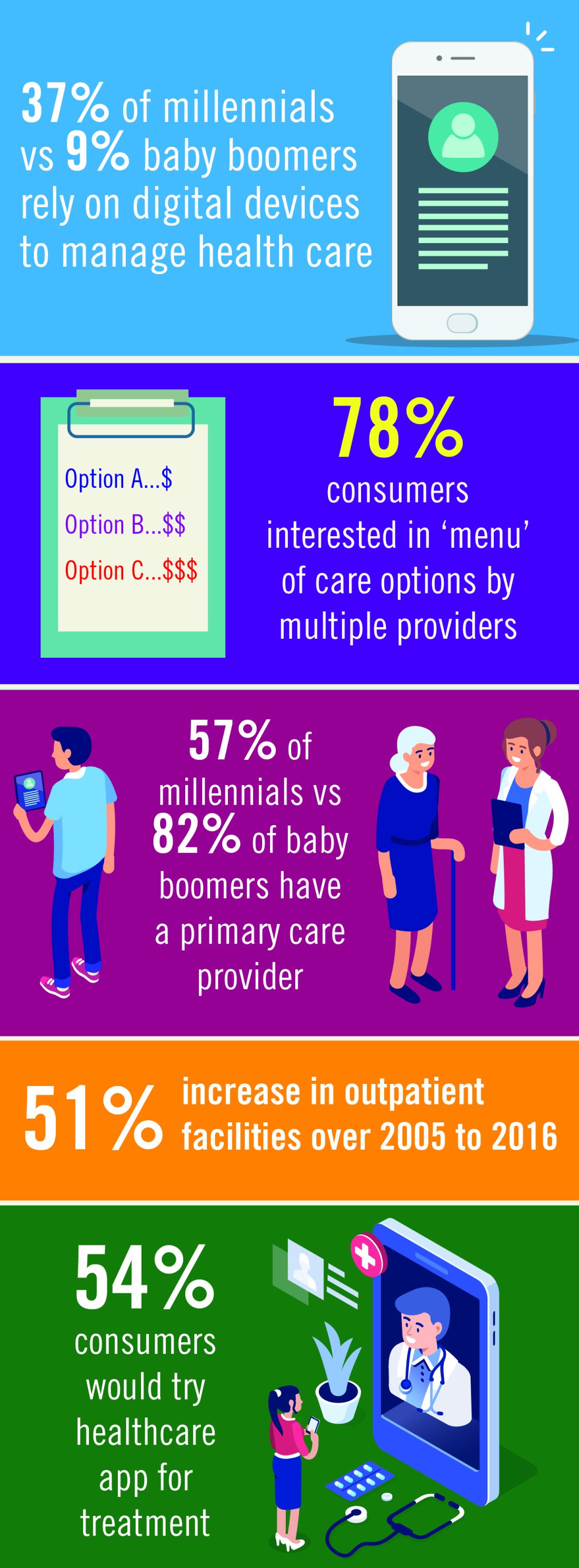
Chest physicians are witnessing a revolution within the environment in which they practice. Information technology, changing consumer behavior, and the social imperative to contain costs are coming together to transform health care.
Innovation in the prevention, diagnosis, and treatment of health-related issues is being fueled by the emergence of accessible and affordable technology-based solutions and changes in patient approaches to health care. Consumers and employers are increasingly motivated to look for cost-effective options for health in care delivery and for economical access to innovations.1 Organizations will need to respond with a strategy that aligns with the changing environment and position physicians to lead these trends in the direction of improved patient care.2
Enabling technologies like electronic health records, blockchain, and artificial intelligence will increase connectivity among all the stakeholders in the health-care system. The exponential increase in connectivity means growing engagement of health systems, health plans, patients, and families in all aspects of health care. For health-care providers, these technologies will mean an acceleration of the requirement to generate data in clinical settings and utilize data for clinical decision making. Easily available data on outcomes and, most importantly, cost of treatment will be expected at point of service.3
Access to information will continue to empower consumers to take an active role in their own health care. More patients will be comfortable with delivery of some health care via digital devices, apps, and virtual access to treatment. The market will respond with technology that helps consumers navigate health-care systems, explore options, and communicate directly with providers. The use of apps and virtual encounters is expected to transform the role of primary care providers: patients will increasingly utilize nonphysician resources in outpatient settings, bypassing primary care physicians and reaching out to specialty care as needed.4
David A. Schulman, MD, FCCP, Professor of Medicine, Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Emory University School of Medicine, Atlanta, and Editor in Chief of CHEST Physician, has seen the transformation of patient behavior and attitudes in his own practice.
“In general, they have done far more research about their health problems before seeking my counsel than patients did previously. Many use the internet not just to read about their symptoms and diseases, but also to connect with others having similar issues, sharing experiences, treatments, outcomes, and emotions; in some ways, this is the new ‘crowdsourcing’ of medicine.”
Patients who do their own “research” can present a challenge for physicians. Dr. Schulman noted, “I am often surprised about the misconceptions about disease that derive from information gleaned from a web-based source. One need not look any farther than the groundswell of misinformation being spread about vaccinations to see the potential downside of the pervasive availability of medical ‘facts’ online. Since we are unlikely to convince our patients to avoid the online milieu entirely, our role as health-care providers is to help our patients process and appropriately weigh the information that they receive, potentially partnering with our national societies to help curate such information.”
Dr. Schulman’s approach to the potential of patient misinformation is to initiate almost all discussions with patients with the question “Have your read or seen anything about this condition?” He said, “It is rare for patients to answer negatively. And listening to them speak about their understanding of their disease provides me with invaluable information about how the remainder of our visit should be spent. Do we need to correct misunderstandings? Are there gaps in the explanation that I can fill? Can we move directly into a conversation about treatment options? Can I provide you with some additional resources that might help to further your knowledge about the condition?”
Generational factors will play a big role in health-care demand and delivery. Health-care companies are already building lower cost delivery models to capture the millennial market.4 Cost-saving digital tools and virtual contacts are currently most commonly used by younger patients.5 Physicians need to understand and be a part of this trend, Dr. Schulman argued. “We should embrace telemedicine and mobile applications to collect data from the patients in their day-to-day lives. While insurance coverage of telemedicine is far from universal at the moment, and the reliability of mobile applications is highly variable, we know that a growing number of our patients are already relying on their digital devices to manage their health. In much the same way that we will need to help patient evaluate online information, we should work with our national societies to support the creation of tools that will allow us to collect data in the home environment in a more robust and reliable fashion.”
The proportion of the US population over the age of 65 is increasing yearly.6 Six out of 10 Americans live with a chronic illness, such as heart disease or diabetes. These and other chronic diseases are the leading drivers of the $3.3 billion annual health-care costs.7 Cost containment for these older patients and those with chronic illness will involve a focus on quality and outcomes data, a drive to deliver treatment in lower cost outpatient settings, and an acceleration of the adoption of value-based models currently underway.8
Taken together, these trends will mean a growing digital interface between physician and patient, a more active consumer-patient, and the availability of a vast array of new tools to access and manage health-care data.
- Delivery of procedures and services will trend from physicians to other members of the health-care team and to lower cost, outpatient settings.9
- Health-care systems will ramp up investment in products and services that improve outcomes and cost effectiveness.10
- Increased regulatory requirements and new payment models mean an ever-growing utilization of information technology by providers to fulfill data imperatives.11
- Physicians will have an increased need for tools that prioritize costs and outcomes data at the point of care.12
- Integration of data from new technologies will touch every aspect of health-care delivery with the objective of improving outcomes and, in turn, reducing costs.13
- Changing consumer attitudes toward delivery of care will be based on a growing familiarity of patients with a digital or virtual interface with providers, facility with health-care apps, and preference for a menu of options for health-care delivery.14
Dr. Schulman concluded, “We can no more expect our patients to ignore the full panoply of medical information on the internet and digital tools on their mobile devices than we can tell the tide not to come in. The die is cast; this is the world within which we must ply our trade. By identifying best practices and sharing our successes, we can come through this revolution better for the experience.”
References
1. https://www.modernhealthcare.com/article/20181220/NEWS/181229992/number-of-outpatient-facilities-surges-as-industry-values-more
2. https://www.accenture.com/us-en/insights/health/digital-health-tech-vision-2018
3. https://www.accenture.com/us-en/insights/health/digital-health-primary-care
4. PcW Health Research Institute: Top health industry issues of 2019
5. https://www.accenture.com/us-en/insights/health/digital-health-primary-care
6. https://www.census.gov/newsroom/press-releases/2017/cb17-100.html
7. https://www.cdc.gov/chronicdisease/index.htm
8. https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/health-care-current-december4-2018.html
9. PcW Health Research Institute Top health industry issues of 2019: The New Health Economy comes of age
10. https://www.accenture.com/us-en/insights/health/digital-health-tech-vision-2018
11. https://www2.deloitte.com/insights/us/en/industry/life-sciences/medtech-research-and-development-innovation.html
12. https://www2.deloitte.com/insights/us/en/industry/health-care/volume-to-value-based-care.html
13. https://www2.deloitte.com/insights/us/en/industry/health-care/volume-to-value-based-care.html
14. https://www.accenture.com/us-en/insights/health/digital-health-primary-care
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: the CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
Chest physicians are witnessing a revolution within the environment in which they practice. Information technology, changing consumer behavior, and the social imperative to contain costs are coming together to transform health care.
Innovation in the prevention, diagnosis, and treatment of health-related issues is being fueled by the emergence of accessible and affordable technology-based solutions and changes in patient approaches to health care. Consumers and employers are increasingly motivated to look for cost-effective options for health in care delivery and for economical access to innovations.1 Organizations will need to respond with a strategy that aligns with the changing environment and position physicians to lead these trends in the direction of improved patient care.2
Enabling technologies like electronic health records, blockchain, and artificial intelligence will increase connectivity among all the stakeholders in the health-care system. The exponential increase in connectivity means growing engagement of health systems, health plans, patients, and families in all aspects of health care. For health-care providers, these technologies will mean an acceleration of the requirement to generate data in clinical settings and utilize data for clinical decision making. Easily available data on outcomes and, most importantly, cost of treatment will be expected at point of service.3
Access to information will continue to empower consumers to take an active role in their own health care. More patients will be comfortable with delivery of some health care via digital devices, apps, and virtual access to treatment. The market will respond with technology that helps consumers navigate health-care systems, explore options, and communicate directly with providers. The use of apps and virtual encounters is expected to transform the role of primary care providers: patients will increasingly utilize nonphysician resources in outpatient settings, bypassing primary care physicians and reaching out to specialty care as needed.4
David A. Schulman, MD, FCCP, Professor of Medicine, Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Emory University School of Medicine, Atlanta, and Editor in Chief of CHEST Physician, has seen the transformation of patient behavior and attitudes in his own practice.
“In general, they have done far more research about their health problems before seeking my counsel than patients did previously. Many use the internet not just to read about their symptoms and diseases, but also to connect with others having similar issues, sharing experiences, treatments, outcomes, and emotions; in some ways, this is the new ‘crowdsourcing’ of medicine.”
Patients who do their own “research” can present a challenge for physicians. Dr. Schulman noted, “I am often surprised about the misconceptions about disease that derive from information gleaned from a web-based source. One need not look any farther than the groundswell of misinformation being spread about vaccinations to see the potential downside of the pervasive availability of medical ‘facts’ online. Since we are unlikely to convince our patients to avoid the online milieu entirely, our role as health-care providers is to help our patients process and appropriately weigh the information that they receive, potentially partnering with our national societies to help curate such information.”
Dr. Schulman’s approach to the potential of patient misinformation is to initiate almost all discussions with patients with the question “Have your read or seen anything about this condition?” He said, “It is rare for patients to answer negatively. And listening to them speak about their understanding of their disease provides me with invaluable information about how the remainder of our visit should be spent. Do we need to correct misunderstandings? Are there gaps in the explanation that I can fill? Can we move directly into a conversation about treatment options? Can I provide you with some additional resources that might help to further your knowledge about the condition?”
Generational factors will play a big role in health-care demand and delivery. Health-care companies are already building lower cost delivery models to capture the millennial market.4 Cost-saving digital tools and virtual contacts are currently most commonly used by younger patients.5 Physicians need to understand and be a part of this trend, Dr. Schulman argued. “We should embrace telemedicine and mobile applications to collect data from the patients in their day-to-day lives. While insurance coverage of telemedicine is far from universal at the moment, and the reliability of mobile applications is highly variable, we know that a growing number of our patients are already relying on their digital devices to manage their health. In much the same way that we will need to help patient evaluate online information, we should work with our national societies to support the creation of tools that will allow us to collect data in the home environment in a more robust and reliable fashion.”
The proportion of the US population over the age of 65 is increasing yearly.6 Six out of 10 Americans live with a chronic illness, such as heart disease or diabetes. These and other chronic diseases are the leading drivers of the $3.3 billion annual health-care costs.7 Cost containment for these older patients and those with chronic illness will involve a focus on quality and outcomes data, a drive to deliver treatment in lower cost outpatient settings, and an acceleration of the adoption of value-based models currently underway.8
Taken together, these trends will mean a growing digital interface between physician and patient, a more active consumer-patient, and the availability of a vast array of new tools to access and manage health-care data.
- Delivery of procedures and services will trend from physicians to other members of the health-care team and to lower cost, outpatient settings.9
- Health-care systems will ramp up investment in products and services that improve outcomes and cost effectiveness.10
- Increased regulatory requirements and new payment models mean an ever-growing utilization of information technology by providers to fulfill data imperatives.11
- Physicians will have an increased need for tools that prioritize costs and outcomes data at the point of care.12
- Integration of data from new technologies will touch every aspect of health-care delivery with the objective of improving outcomes and, in turn, reducing costs.13
- Changing consumer attitudes toward delivery of care will be based on a growing familiarity of patients with a digital or virtual interface with providers, facility with health-care apps, and preference for a menu of options for health-care delivery.14
Dr. Schulman concluded, “We can no more expect our patients to ignore the full panoply of medical information on the internet and digital tools on their mobile devices than we can tell the tide not to come in. The die is cast; this is the world within which we must ply our trade. By identifying best practices and sharing our successes, we can come through this revolution better for the experience.”

References
1. https://www.modernhealthcare.com/article/20181220/NEWS/181229992/number-of-outpatient-facilities-surges-as-industry-values-more
2. https://www.accenture.com/us-en/insights/health/digital-health-tech-vision-2018
3. https://www.accenture.com/us-en/insights/health/digital-health-primary-care
4. PcW Health Research Institute: Top health industry issues of 2019
5. https://www.accenture.com/us-en/insights/health/digital-health-primary-care
6. https://www.census.gov/newsroom/press-releases/2017/cb17-100.html
7. https://www.cdc.gov/chronicdisease/index.htm
8. https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/health-care-current-december4-2018.html
9. PcW Health Research Institute Top health industry issues of 2019: The New Health Economy comes of age
10. https://www.accenture.com/us-en/insights/health/digital-health-tech-vision-2018
11. https://www2.deloitte.com/insights/us/en/industry/life-sciences/medtech-research-and-development-innovation.html
12. https://www2.deloitte.com/insights/us/en/industry/health-care/volume-to-value-based-care.html
13. https://www2.deloitte.com/insights/us/en/industry/health-care/volume-to-value-based-care.html
14. https://www.accenture.com/us-en/insights/health/digital-health-primary-care
CHEST Inspiration is a collection of programmatic initiatives developed by the American College of Chest Physicians leadership and aimed at stimulating and encouraging innovation within the association. One of the components of CHEST Inspiration is the Environmental Scan, a series of articles focusing on the internal and external environmental factors that bear on success currently and in the future. See “Envisioning the Future: the CHEST Environmental Scan,” CHEST Physician, June 2019, p. 44, for an introduction to the series.
Chest physicians are witnessing a revolution within the environment in which they practice. Information technology, changing consumer behavior, and the social imperative to contain costs are coming together to transform health care.
Innovation in the prevention, diagnosis, and treatment of health-related issues is being fueled by the emergence of accessible and affordable technology-based solutions and changes in patient approaches to health care. Consumers and employers are increasingly motivated to look for cost-effective options for health in care delivery and for economical access to innovations.1 Organizations will need to respond with a strategy that aligns with the changing environment and position physicians to lead these trends in the direction of improved patient care.2
Enabling technologies like electronic health records, blockchain, and artificial intelligence will increase connectivity among all the stakeholders in the health-care system. The exponential increase in connectivity means growing engagement of health systems, health plans, patients, and families in all aspects of health care. For health-care providers, these technologies will mean an acceleration of the requirement to generate data in clinical settings and utilize data for clinical decision making. Easily available data on outcomes and, most importantly, cost of treatment will be expected at point of service.3
Access to information will continue to empower consumers to take an active role in their own health care. More patients will be comfortable with delivery of some health care via digital devices, apps, and virtual access to treatment. The market will respond with technology that helps consumers navigate health-care systems, explore options, and communicate directly with providers. The use of apps and virtual encounters is expected to transform the role of primary care providers: patients will increasingly utilize nonphysician resources in outpatient settings, bypassing primary care physicians and reaching out to specialty care as needed.4
David A. Schulman, MD, FCCP, Professor of Medicine, Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Emory University School of Medicine, Atlanta, and Editor in Chief of CHEST Physician, has seen the transformation of patient behavior and attitudes in his own practice.
“In general, they have done far more research about their health problems before seeking my counsel than patients did previously. Many use the internet not just to read about their symptoms and diseases, but also to connect with others having similar issues, sharing experiences, treatments, outcomes, and emotions; in some ways, this is the new ‘crowdsourcing’ of medicine.”
Patients who do their own “research” can present a challenge for physicians. Dr. Schulman noted, “I am often surprised about the misconceptions about disease that derive from information gleaned from a web-based source. One need not look any farther than the groundswell of misinformation being spread about vaccinations to see the potential downside of the pervasive availability of medical ‘facts’ online. Since we are unlikely to convince our patients to avoid the online milieu entirely, our role as health-care providers is to help our patients process and appropriately weigh the information that they receive, potentially partnering with our national societies to help curate such information.”
Dr. Schulman’s approach to the potential of patient misinformation is to initiate almost all discussions with patients with the question “Have your read or seen anything about this condition?” He said, “It is rare for patients to answer negatively. And listening to them speak about their understanding of their disease provides me with invaluable information about how the remainder of our visit should be spent. Do we need to correct misunderstandings? Are there gaps in the explanation that I can fill? Can we move directly into a conversation about treatment options? Can I provide you with some additional resources that might help to further your knowledge about the condition?”
Generational factors will play a big role in health-care demand and delivery. Health-care companies are already building lower cost delivery models to capture the millennial market.4 Cost-saving digital tools and virtual contacts are currently most commonly used by younger patients.5 Physicians need to understand and be a part of this trend, Dr. Schulman argued. “We should embrace telemedicine and mobile applications to collect data from the patients in their day-to-day lives. While insurance coverage of telemedicine is far from universal at the moment, and the reliability of mobile applications is highly variable, we know that a growing number of our patients are already relying on their digital devices to manage their health. In much the same way that we will need to help patient evaluate online information, we should work with our national societies to support the creation of tools that will allow us to collect data in the home environment in a more robust and reliable fashion.”
The proportion of the US population over the age of 65 is increasing yearly.6 Six out of 10 Americans live with a chronic illness, such as heart disease or diabetes. These and other chronic diseases are the leading drivers of the $3.3 billion annual health-care costs.7 Cost containment for these older patients and those with chronic illness will involve a focus on quality and outcomes data, a drive to deliver treatment in lower cost outpatient settings, and an acceleration of the adoption of value-based models currently underway.8
Taken together, these trends will mean a growing digital interface between physician and patient, a more active consumer-patient, and the availability of a vast array of new tools to access and manage health-care data.
- Delivery of procedures and services will trend from physicians to other members of the health-care team and to lower cost, outpatient settings.9
- Health-care systems will ramp up investment in products and services that improve outcomes and cost effectiveness.10
- Increased regulatory requirements and new payment models mean an ever-growing utilization of information technology by providers to fulfill data imperatives.11
- Physicians will have an increased need for tools that prioritize costs and outcomes data at the point of care.12
- Integration of data from new technologies will touch every aspect of health-care delivery with the objective of improving outcomes and, in turn, reducing costs.13
- Changing consumer attitudes toward delivery of care will be based on a growing familiarity of patients with a digital or virtual interface with providers, facility with health-care apps, and preference for a menu of options for health-care delivery.14
Dr. Schulman concluded, “We can no more expect our patients to ignore the full panoply of medical information on the internet and digital tools on their mobile devices than we can tell the tide not to come in. The die is cast; this is the world within which we must ply our trade. By identifying best practices and sharing our successes, we can come through this revolution better for the experience.”

References
1. https://www.modernhealthcare.com/article/20181220/NEWS/181229992/number-of-outpatient-facilities-surges-as-industry-values-more
2. https://www.accenture.com/us-en/insights/health/digital-health-tech-vision-2018
3. https://www.accenture.com/us-en/insights/health/digital-health-primary-care
4. PcW Health Research Institute: Top health industry issues of 2019
5. https://www.accenture.com/us-en/insights/health/digital-health-primary-care
6. https://www.census.gov/newsroom/press-releases/2017/cb17-100.html
7. https://www.cdc.gov/chronicdisease/index.htm
8. https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/health-care-current-december4-2018.html
9. PcW Health Research Institute Top health industry issues of 2019: The New Health Economy comes of age
10. https://www.accenture.com/us-en/insights/health/digital-health-tech-vision-2018
11. https://www2.deloitte.com/insights/us/en/industry/life-sciences/medtech-research-and-development-innovation.html
12. https://www2.deloitte.com/insights/us/en/industry/health-care/volume-to-value-based-care.html
13. https://www2.deloitte.com/insights/us/en/industry/health-care/volume-to-value-based-care.html
14. https://www.accenture.com/us-en/insights/health/digital-health-primary-care
Appeals court may strike down ACA
During the 2-hour hearing, a three-judge panel for the 5th U.S. Circuit Court of Appeals peppered attorneys with questions about whether Congress intended the ACA to function without the individual mandate, and the panel seemed doubtful the law can stand if the regulation is parsed, according to an audio transcript of the arguments. As written, the individual mandate required that all Americans have insurance or pay a tax penalty. However, budget legislation in 2017 zeroed out the penalties associated with the mandate, rendering it unenforceable.
Appeals Judge Kurt Engelhardt, a President Trump appointee, asked defense attorney Samuel Siegel why Congress failed to add a clause in the original law that would have allowed ACA components to be severed if such sectioning was acceptable.
“Congress could have included a severability clause when it adopted the ACA in 2010. Couldn’t it have done so?” Judge Engelhardt asked during oral arguments. “It seems like it did the opposite, where it said, ‘This is a complete overhaul,’ and it set forth a bunch of factual findings. Couldn’t Congress have said, ‘Oh by the way, we think all of these provisions are such excellent ideas and helpful to the public that if any go by the wayside, then we would want the remainder to continue to apply’?”
Congress’s silence on the severing of the ACA does not create a presumption against parsing of the law, argued Mr. Siegel, who is representing the Democratic states suing to retain the ACA in Texas v. United States. He emphasized that in 2017, when Congress terminated the individual mandate penalty, it chose not to repeal preexisting protections or other important reforms instituted by the ACA.
“With that action, your Honor, Congress expressed its views that the individual marketplace and indeed the entire Affordable Care Act can operate without an enforceable individual mandate,” Mr. Siegel said. “We think that’s all this court needs to know to resolve the severability question.”
However, Appellate Judge Jennifer Elrod, a President George W. Bush appointee to the court, questioned whether legislators zeroed out the mandate penalty because they knew the law could not survive without the core provision. She surmised that Congress might have assumed, “Aha, this is the silver bullet that’s going to undo Obamacare.”
Kyle Hawkins, an attorney representing the Republican-led plaintiff states, meanwhile, argued the text of the ACA clearly declares the individual mandate essential to the law and to the goals that Congress intended to achieve.
“The Obama administration thought of that as an inseverable clause,” Mr. Hawkins argued. “The district court directly synthesized those considerations ... and it reached the correct conclusion: The individual mandate is unconstitutional and it is inseverable from the remainder of the law.”
Texas v. United States stems from a legal challenge by a group of 18 Republican state attorneys general and two individuals in 2018 who argue the ACA should be declared unconstitutional. The plaintiffs say that, because budget legislation in 2017 effectively eliminated the penalty associated with the mandate, the requirement itself is invalid. Without the mandate, the entire law must fall, the plaintiffs contend. The Department of Justice declined to fully defend the law, so 16 Democratic state attorneys general intervened. In December 2018, a district court declared the entire ACA to be invalid, a decision immediately appealed to the 5th U.S. Circuit Court of Appeals by the Democratic attorneys general.
The Trump administration initially agreed that the mandate was unconstitutional and should be parsed. Attorneys for the administration said, if the mandate is found unconstitutional, the court should also consider finding two other provisions – the guaranteed issue and community rating requirements – of the ACA invalid. At the time, the Trump administration said the remainder of the ACA can stand without the three linked provisions. The administration later shifted its stance and asserted that much of the ACA should fall because provisions of the law cannot be severed. However, the DOJ expressed support in keeping some provision intact, such as certain criminal statutes that prevent health care fraud.
Most recently, the DOJ has indicated that, if the ACA is struck down or severed, the decision should only apply in the 18 plaintiff states and not to the entire nation. The fickle position of the Trump administration was questioned during the Court of Appeals hearing with judges asking DOJ attorney August Flentje to clarify why a final ruling should not apply nationwide.
“A lot of this stuff would need to get sorted out,” Mr. Flentje responded. “And it’s complicated. How it applies in the states and which parts can’t be applied at all because they would injure the states ... that raises a lot of complicated issues which I think [will be determined after] a final resolution.”
By their line of questioning, the appellate panel appeared to lean toward the plaintiffs’ position more so than toward the defendants’, said Katie Keith, an attorney and health law analyst who writes about Texas v. United States for the Health Affairs Blog.
“At least two of the three judges – the only two that were asking questions – seem very inclined to at, a minimum, strike down the individual mandate itself,” Ms. Keith said in an interview. “The conventional wisdom had been that this court would overturn the lower court’s decision, and I think folks are walking away, myself included, from oral arguments feeling less certain that that’s going to happen.”
Robert Henneke, general counsel for the American Future at the Texas Public Policy Foundation, said that plaintiffs “had a good day in court” and that the defendants’ arguments seemed to “hit a thud with the judges.” Mr. Henneke represents two individual plaintiffs from Texas in the lawsuit.
“Obamacare is still unconstitutional, and the three-judge panel seemed to agree with the trial court that the entirety of the law should be struck down,” Mr. Henneke said in a press conference after oral arguments. “The court really seemed skeptical with the arguments of the other side. We had the chance to tell the story of my clients and how they continue to be hurt by the Affordable Care Act.
Whichever way the Court of Appeals rules, the losing party is expected to appeal to the U.S. Supreme Court, Ms. Keith said. If justices accept the case, a decision could arrive in the summer of 2020, which would coincide with the presidential election. Another options is for the appellate court to send the case back to the lower court for further review, particularly to clear up the DOJ’s murky position, Ms. Keith said.
“They might send it back to [the lower court] and say there’s some questions here about what’s severable,” she said. “The DOJ sort of struggled to explain what they’re talking about. So they could remand the case back to Judge [Reed Charles] O’Connor to say, ‘Figure this out. Work with the parties.’ That’s an option.”
A decision by the Court of Appeals is expected in the next two months.
[email protected]
During the 2-hour hearing, a three-judge panel for the 5th U.S. Circuit Court of Appeals peppered attorneys with questions about whether Congress intended the ACA to function without the individual mandate, and the panel seemed doubtful the law can stand if the regulation is parsed, according to an audio transcript of the arguments. As written, the individual mandate required that all Americans have insurance or pay a tax penalty. However, budget legislation in 2017 zeroed out the penalties associated with the mandate, rendering it unenforceable.
Appeals Judge Kurt Engelhardt, a President Trump appointee, asked defense attorney Samuel Siegel why Congress failed to add a clause in the original law that would have allowed ACA components to be severed if such sectioning was acceptable.
“Congress could have included a severability clause when it adopted the ACA in 2010. Couldn’t it have done so?” Judge Engelhardt asked during oral arguments. “It seems like it did the opposite, where it said, ‘This is a complete overhaul,’ and it set forth a bunch of factual findings. Couldn’t Congress have said, ‘Oh by the way, we think all of these provisions are such excellent ideas and helpful to the public that if any go by the wayside, then we would want the remainder to continue to apply’?”
Congress’s silence on the severing of the ACA does not create a presumption against parsing of the law, argued Mr. Siegel, who is representing the Democratic states suing to retain the ACA in Texas v. United States. He emphasized that in 2017, when Congress terminated the individual mandate penalty, it chose not to repeal preexisting protections or other important reforms instituted by the ACA.
“With that action, your Honor, Congress expressed its views that the individual marketplace and indeed the entire Affordable Care Act can operate without an enforceable individual mandate,” Mr. Siegel said. “We think that’s all this court needs to know to resolve the severability question.”
However, Appellate Judge Jennifer Elrod, a President George W. Bush appointee to the court, questioned whether legislators zeroed out the mandate penalty because they knew the law could not survive without the core provision. She surmised that Congress might have assumed, “Aha, this is the silver bullet that’s going to undo Obamacare.”
Kyle Hawkins, an attorney representing the Republican-led plaintiff states, meanwhile, argued the text of the ACA clearly declares the individual mandate essential to the law and to the goals that Congress intended to achieve.
“The Obama administration thought of that as an inseverable clause,” Mr. Hawkins argued. “The district court directly synthesized those considerations ... and it reached the correct conclusion: The individual mandate is unconstitutional and it is inseverable from the remainder of the law.”
Texas v. United States stems from a legal challenge by a group of 18 Republican state attorneys general and two individuals in 2018 who argue the ACA should be declared unconstitutional. The plaintiffs say that, because budget legislation in 2017 effectively eliminated the penalty associated with the mandate, the requirement itself is invalid. Without the mandate, the entire law must fall, the plaintiffs contend. The Department of Justice declined to fully defend the law, so 16 Democratic state attorneys general intervened. In December 2018, a district court declared the entire ACA to be invalid, a decision immediately appealed to the 5th U.S. Circuit Court of Appeals by the Democratic attorneys general.
The Trump administration initially agreed that the mandate was unconstitutional and should be parsed. Attorneys for the administration said, if the mandate is found unconstitutional, the court should also consider finding two other provisions – the guaranteed issue and community rating requirements – of the ACA invalid. At the time, the Trump administration said the remainder of the ACA can stand without the three linked provisions. The administration later shifted its stance and asserted that much of the ACA should fall because provisions of the law cannot be severed. However, the DOJ expressed support in keeping some provision intact, such as certain criminal statutes that prevent health care fraud.
Most recently, the DOJ has indicated that, if the ACA is struck down or severed, the decision should only apply in the 18 plaintiff states and not to the entire nation. The fickle position of the Trump administration was questioned during the Court of Appeals hearing with judges asking DOJ attorney August Flentje to clarify why a final ruling should not apply nationwide.
“A lot of this stuff would need to get sorted out,” Mr. Flentje responded. “And it’s complicated. How it applies in the states and which parts can’t be applied at all because they would injure the states ... that raises a lot of complicated issues which I think [will be determined after] a final resolution.”
By their line of questioning, the appellate panel appeared to lean toward the plaintiffs’ position more so than toward the defendants’, said Katie Keith, an attorney and health law analyst who writes about Texas v. United States for the Health Affairs Blog.
“At least two of the three judges – the only two that were asking questions – seem very inclined to at, a minimum, strike down the individual mandate itself,” Ms. Keith said in an interview. “The conventional wisdom had been that this court would overturn the lower court’s decision, and I think folks are walking away, myself included, from oral arguments feeling less certain that that’s going to happen.”
Robert Henneke, general counsel for the American Future at the Texas Public Policy Foundation, said that plaintiffs “had a good day in court” and that the defendants’ arguments seemed to “hit a thud with the judges.” Mr. Henneke represents two individual plaintiffs from Texas in the lawsuit.
“Obamacare is still unconstitutional, and the three-judge panel seemed to agree with the trial court that the entirety of the law should be struck down,” Mr. Henneke said in a press conference after oral arguments. “The court really seemed skeptical with the arguments of the other side. We had the chance to tell the story of my clients and how they continue to be hurt by the Affordable Care Act.
Whichever way the Court of Appeals rules, the losing party is expected to appeal to the U.S. Supreme Court, Ms. Keith said. If justices accept the case, a decision could arrive in the summer of 2020, which would coincide with the presidential election. Another options is for the appellate court to send the case back to the lower court for further review, particularly to clear up the DOJ’s murky position, Ms. Keith said.
“They might send it back to [the lower court] and say there’s some questions here about what’s severable,” she said. “The DOJ sort of struggled to explain what they’re talking about. So they could remand the case back to Judge [Reed Charles] O’Connor to say, ‘Figure this out. Work with the parties.’ That’s an option.”
A decision by the Court of Appeals is expected in the next two months.
[email protected]
During the 2-hour hearing, a three-judge panel for the 5th U.S. Circuit Court of Appeals peppered attorneys with questions about whether Congress intended the ACA to function without the individual mandate, and the panel seemed doubtful the law can stand if the regulation is parsed, according to an audio transcript of the arguments. As written, the individual mandate required that all Americans have insurance or pay a tax penalty. However, budget legislation in 2017 zeroed out the penalties associated with the mandate, rendering it unenforceable.
Appeals Judge Kurt Engelhardt, a President Trump appointee, asked defense attorney Samuel Siegel why Congress failed to add a clause in the original law that would have allowed ACA components to be severed if such sectioning was acceptable.
“Congress could have included a severability clause when it adopted the ACA in 2010. Couldn’t it have done so?” Judge Engelhardt asked during oral arguments. “It seems like it did the opposite, where it said, ‘This is a complete overhaul,’ and it set forth a bunch of factual findings. Couldn’t Congress have said, ‘Oh by the way, we think all of these provisions are such excellent ideas and helpful to the public that if any go by the wayside, then we would want the remainder to continue to apply’?”
Congress’s silence on the severing of the ACA does not create a presumption against parsing of the law, argued Mr. Siegel, who is representing the Democratic states suing to retain the ACA in Texas v. United States. He emphasized that in 2017, when Congress terminated the individual mandate penalty, it chose not to repeal preexisting protections or other important reforms instituted by the ACA.
“With that action, your Honor, Congress expressed its views that the individual marketplace and indeed the entire Affordable Care Act can operate without an enforceable individual mandate,” Mr. Siegel said. “We think that’s all this court needs to know to resolve the severability question.”
However, Appellate Judge Jennifer Elrod, a President George W. Bush appointee to the court, questioned whether legislators zeroed out the mandate penalty because they knew the law could not survive without the core provision. She surmised that Congress might have assumed, “Aha, this is the silver bullet that’s going to undo Obamacare.”
Kyle Hawkins, an attorney representing the Republican-led plaintiff states, meanwhile, argued the text of the ACA clearly declares the individual mandate essential to the law and to the goals that Congress intended to achieve.
“The Obama administration thought of that as an inseverable clause,” Mr. Hawkins argued. “The district court directly synthesized those considerations ... and it reached the correct conclusion: The individual mandate is unconstitutional and it is inseverable from the remainder of the law.”
Texas v. United States stems from a legal challenge by a group of 18 Republican state attorneys general and two individuals in 2018 who argue the ACA should be declared unconstitutional. The plaintiffs say that, because budget legislation in 2017 effectively eliminated the penalty associated with the mandate, the requirement itself is invalid. Without the mandate, the entire law must fall, the plaintiffs contend. The Department of Justice declined to fully defend the law, so 16 Democratic state attorneys general intervened. In December 2018, a district court declared the entire ACA to be invalid, a decision immediately appealed to the 5th U.S. Circuit Court of Appeals by the Democratic attorneys general.
The Trump administration initially agreed that the mandate was unconstitutional and should be parsed. Attorneys for the administration said, if the mandate is found unconstitutional, the court should also consider finding two other provisions – the guaranteed issue and community rating requirements – of the ACA invalid. At the time, the Trump administration said the remainder of the ACA can stand without the three linked provisions. The administration later shifted its stance and asserted that much of the ACA should fall because provisions of the law cannot be severed. However, the DOJ expressed support in keeping some provision intact, such as certain criminal statutes that prevent health care fraud.
Most recently, the DOJ has indicated that, if the ACA is struck down or severed, the decision should only apply in the 18 plaintiff states and not to the entire nation. The fickle position of the Trump administration was questioned during the Court of Appeals hearing with judges asking DOJ attorney August Flentje to clarify why a final ruling should not apply nationwide.
“A lot of this stuff would need to get sorted out,” Mr. Flentje responded. “And it’s complicated. How it applies in the states and which parts can’t be applied at all because they would injure the states ... that raises a lot of complicated issues which I think [will be determined after] a final resolution.”
By their line of questioning, the appellate panel appeared to lean toward the plaintiffs’ position more so than toward the defendants’, said Katie Keith, an attorney and health law analyst who writes about Texas v. United States for the Health Affairs Blog.
“At least two of the three judges – the only two that were asking questions – seem very inclined to at, a minimum, strike down the individual mandate itself,” Ms. Keith said in an interview. “The conventional wisdom had been that this court would overturn the lower court’s decision, and I think folks are walking away, myself included, from oral arguments feeling less certain that that’s going to happen.”
Robert Henneke, general counsel for the American Future at the Texas Public Policy Foundation, said that plaintiffs “had a good day in court” and that the defendants’ arguments seemed to “hit a thud with the judges.” Mr. Henneke represents two individual plaintiffs from Texas in the lawsuit.
“Obamacare is still unconstitutional, and the three-judge panel seemed to agree with the trial court that the entirety of the law should be struck down,” Mr. Henneke said in a press conference after oral arguments. “The court really seemed skeptical with the arguments of the other side. We had the chance to tell the story of my clients and how they continue to be hurt by the Affordable Care Act.
Whichever way the Court of Appeals rules, the losing party is expected to appeal to the U.S. Supreme Court, Ms. Keith said. If justices accept the case, a decision could arrive in the summer of 2020, which would coincide with the presidential election. Another options is for the appellate court to send the case back to the lower court for further review, particularly to clear up the DOJ’s murky position, Ms. Keith said.
“They might send it back to [the lower court] and say there’s some questions here about what’s severable,” she said. “The DOJ sort of struggled to explain what they’re talking about. So they could remand the case back to Judge [Reed Charles] O’Connor to say, ‘Figure this out. Work with the parties.’ That’s an option.”
A decision by the Court of Appeals is expected in the next two months.
[email protected]
Appeals court may strike down ACA
During the 2-hour hearing, a three-judge panel for the 5th U.S. Circuit Court of Appeals peppered attorneys with questions about whether Congress intended the ACA to function without the individual mandate, and the panel seemed doubtful the law can stand if the regulation is parsed, according to an audio transcript of the arguments. As written, the individual mandate required that all Americans have insurance or pay a tax penalty. However, budget legislation in 2017 zeroed out the penalties associated with the mandate, rendering it unenforceable.
Appeals Judge Kurt Engelhardt, a President Trump appointee, asked defense attorney Samuel Siegel why Congress failed to add a clause in the original law that would have allowed ACA components to be severed if such sectioning was acceptable.
“Congress could have included a severability clause when it adopted the ACA in 2010. Couldn’t it have done so?” Judge Engelhardt asked during oral arguments. “It seems like it did the opposite, where it said, ‘This is a complete overhaul,’ and it set forth a bunch of factual findings. Couldn’t Congress have said, ‘Oh by the way, we think all of these provisions are such excellent ideas and helpful to the public that if any go by the wayside, then we would want the remainder to continue to apply’?”
Congress’s silence on the severing of the ACA does not create a presumption against parsing of the law, argued Mr. Siegel, who is representing the Democratic states suing to retain the ACA in Texas v. United States. He emphasized that in 2017, when Congress terminated the individual mandate penalty, it chose not to repeal preexisting protections or other important reforms instituted by the ACA.
“With that action, your Honor, Congress expressed its views that the individual marketplace and indeed the entire Affordable Care Act can operate without an enforceable individual mandate,” Mr. Siegel said. “We think that’s all this court needs to know to resolve the severability question.”
However, Appellate Judge Jennifer Elrod, a President George W. Bush appointee to the court, questioned whether legislators zeroed out the mandate penalty because they knew the law could not survive without the core provision. She surmised that Congress might have assumed, “Aha, this is the silver bullet that’s going to undo Obamacare.”
Kyle Hawkins, an attorney representing the Republican-led plaintiff states, meanwhile, argued the text of the ACA clearly declares the individual mandate essential to the law and to the goals that Congress intended to achieve.
“The Obama administration thought of that as an inseverable clause,” Mr. Hawkins argued. “The district court directly synthesized those considerations ... and it reached the correct conclusion: The individual mandate is unconstitutional and it is inseverable from the remainder of the law.”
Texas v. United States stems from a legal challenge by a group of 18 Republican state attorneys general and two individuals in 2018 who argue the ACA should be declared unconstitutional. The plaintiffs say that, because budget legislation in 2017 effectively eliminated the penalty associated with the mandate, the requirement itself is invalid. Without the mandate, the entire law must fall, the plaintiffs contend. The Department of Justice declined to fully defend the law, so 16 Democratic state attorneys general intervened. In December 2018, a district court declared the entire ACA to be invalid, a decision immediately appealed to the 5th U.S. Circuit Court of Appeals by the Democratic attorneys general.
The Trump administration initially agreed that the mandate was unconstitutional and should be parsed. Attorneys for the administration said, if the mandate is found unconstitutional, the court should also consider finding two other provisions – the guaranteed issue and community rating requirements – of the ACA invalid. At the time, the Trump administration said the remainder of the ACA can stand without the three linked provisions. The administration later shifted its stance and asserted that much of the ACA should fall because provisions of the law cannot be severed. However, the DOJ expressed support in keeping some provision intact, such as certain criminal statutes that prevent health care fraud.
Most recently, the DOJ has indicated that, if the ACA is struck down or severed, the decision should only apply in the 18 plaintiff states and not to the entire nation. The fickle position of the Trump administration was questioned during the Court of Appeals hearing with judges asking DOJ attorney August Flentje to clarify why a final ruling should not apply nationwide.
“A lot of this stuff would need to get sorted out,” Mr. Flentje responded. “And it’s complicated. How it applies in the states and which parts can’t be applied at all because they would injure the states ... that raises a lot of complicated issues which I think [will be determined after] a final resolution.”
By their line of questioning, the appellate panel appeared to lean toward the plaintiffs’ position more so than toward the defendants’, said Katie Keith, an attorney and health law analyst who writes about Texas v. United States for the Health Affairs Blog.
“At least two of the three judges – the only two that were asking questions – seem very inclined to at, a minimum, strike down the individual mandate itself,” Ms. Keith said in an interview. “The conventional wisdom had been that this court would overturn the lower court’s decision, and I think folks are walking away, myself included, from oral arguments feeling less certain that that’s going to happen.”
Robert Henneke, general counsel for the American Future at the Texas Public Policy Foundation, said that plaintiffs “had a good day in court” and that the defendants’ arguments seemed to “hit a thud with the judges.” Mr. Henneke represents two individual plaintiffs from Texas in the lawsuit.
“Obamacare is still unconstitutional, and the three-judge panel seemed to agree with the trial court that the entirety of the law should be struck down,” Mr. Henneke said in a press conference after oral arguments. “The court really seemed skeptical with the arguments of the other side. We had the chance to tell the story of my clients and how they continue to be hurt by the Affordable Care Act.
Whichever way the Court of Appeals rules, the losing party is expected to appeal to the U.S. Supreme Court, Ms. Keith said. If justices accept the case, a decision could arrive in the summer of 2020, which would coincide with the presidential election. Another options is for the appellate court to send the case back to the lower court for further review, particularly to clear up the DOJ’s murky position, Ms. Keith said.
“They might send it back to [the lower court] and say there’s some questions here about what’s severable,” she said. “The DOJ sort of struggled to explain what they’re talking about. So they could remand the case back to Judge [Reed Charles] O’Connor to say, ‘Figure this out. Work with the parties.’ That’s an option.”
A decision by the Court of Appeals is expected in the next two months.
AGA calls on Congress to enact legislation that contains essential patient protections and other improvements to ensure affordability, accessibility, and quality health care for all Americans. Learn more at https://www.gastro.org/advocacy-and-policy/issues-and-news/top-issues/patient-protections-and-access-to-care.
[email protected]
During the 2-hour hearing, a three-judge panel for the 5th U.S. Circuit Court of Appeals peppered attorneys with questions about whether Congress intended the ACA to function without the individual mandate, and the panel seemed doubtful the law can stand if the regulation is parsed, according to an audio transcript of the arguments. As written, the individual mandate required that all Americans have insurance or pay a tax penalty. However, budget legislation in 2017 zeroed out the penalties associated with the mandate, rendering it unenforceable.
Appeals Judge Kurt Engelhardt, a President Trump appointee, asked defense attorney Samuel Siegel why Congress failed to add a clause in the original law that would have allowed ACA components to be severed if such sectioning was acceptable.
“Congress could have included a severability clause when it adopted the ACA in 2010. Couldn’t it have done so?” Judge Engelhardt asked during oral arguments. “It seems like it did the opposite, where it said, ‘This is a complete overhaul,’ and it set forth a bunch of factual findings. Couldn’t Congress have said, ‘Oh by the way, we think all of these provisions are such excellent ideas and helpful to the public that if any go by the wayside, then we would want the remainder to continue to apply’?”
Congress’s silence on the severing of the ACA does not create a presumption against parsing of the law, argued Mr. Siegel, who is representing the Democratic states suing to retain the ACA in Texas v. United States. He emphasized that in 2017, when Congress terminated the individual mandate penalty, it chose not to repeal preexisting protections or other important reforms instituted by the ACA.
“With that action, your Honor, Congress expressed its views that the individual marketplace and indeed the entire Affordable Care Act can operate without an enforceable individual mandate,” Mr. Siegel said. “We think that’s all this court needs to know to resolve the severability question.”
However, Appellate Judge Jennifer Elrod, a President George W. Bush appointee to the court, questioned whether legislators zeroed out the mandate penalty because they knew the law could not survive without the core provision. She surmised that Congress might have assumed, “Aha, this is the silver bullet that’s going to undo Obamacare.”
Kyle Hawkins, an attorney representing the Republican-led plaintiff states, meanwhile, argued the text of the ACA clearly declares the individual mandate essential to the law and to the goals that Congress intended to achieve.
“The Obama administration thought of that as an inseverable clause,” Mr. Hawkins argued. “The district court directly synthesized those considerations ... and it reached the correct conclusion: The individual mandate is unconstitutional and it is inseverable from the remainder of the law.”
Texas v. United States stems from a legal challenge by a group of 18 Republican state attorneys general and two individuals in 2018 who argue the ACA should be declared unconstitutional. The plaintiffs say that, because budget legislation in 2017 effectively eliminated the penalty associated with the mandate, the requirement itself is invalid. Without the mandate, the entire law must fall, the plaintiffs contend. The Department of Justice declined to fully defend the law, so 16 Democratic state attorneys general intervened. In December 2018, a district court declared the entire ACA to be invalid, a decision immediately appealed to the 5th U.S. Circuit Court of Appeals by the Democratic attorneys general.
The Trump administration initially agreed that the mandate was unconstitutional and should be parsed. Attorneys for the administration said, if the mandate is found unconstitutional, the court should also consider finding two other provisions – the guaranteed issue and community rating requirements – of the ACA invalid. At the time, the Trump administration said the remainder of the ACA can stand without the three linked provisions. The administration later shifted its stance and asserted that much of the ACA should fall because provisions of the law cannot be severed. However, the DOJ expressed support in keeping some provision intact, such as certain criminal statutes that prevent health care fraud.
Most recently, the DOJ has indicated that, if the ACA is struck down or severed, the decision should only apply in the 18 plaintiff states and not to the entire nation. The fickle position of the Trump administration was questioned during the Court of Appeals hearing with judges asking DOJ attorney August Flentje to clarify why a final ruling should not apply nationwide.
“A lot of this stuff would need to get sorted out,” Mr. Flentje responded. “And it’s complicated. How it applies in the states and which parts can’t be applied at all because they would injure the states ... that raises a lot of complicated issues which I think [will be determined after] a final resolution.”
By their line of questioning, the appellate panel appeared to lean toward the plaintiffs’ position more so than toward the defendants’, said Katie Keith, an attorney and health law analyst who writes about Texas v. United States for the Health Affairs Blog.
“At least two of the three judges – the only two that were asking questions – seem very inclined to at, a minimum, strike down the individual mandate itself,” Ms. Keith said in an interview. “The conventional wisdom had been that this court would overturn the lower court’s decision, and I think folks are walking away, myself included, from oral arguments feeling less certain that that’s going to happen.”
Robert Henneke, general counsel for the American Future at the Texas Public Policy Foundation, said that plaintiffs “had a good day in court” and that the defendants’ arguments seemed to “hit a thud with the judges.” Mr. Henneke represents two individual plaintiffs from Texas in the lawsuit.
“Obamacare is still unconstitutional, and the three-judge panel seemed to agree with the trial court that the entirety of the law should be struck down,” Mr. Henneke said in a press conference after oral arguments. “The court really seemed skeptical with the arguments of the other side. We had the chance to tell the story of my clients and how they continue to be hurt by the Affordable Care Act.
Whichever way the Court of Appeals rules, the losing party is expected to appeal to the U.S. Supreme Court, Ms. Keith said. If justices accept the case, a decision could arrive in the summer of 2020, which would coincide with the presidential election. Another options is for the appellate court to send the case back to the lower court for further review, particularly to clear up the DOJ’s murky position, Ms. Keith said.
“They might send it back to [the lower court] and say there’s some questions here about what’s severable,” she said. “The DOJ sort of struggled to explain what they’re talking about. So they could remand the case back to Judge [Reed Charles] O’Connor to say, ‘Figure this out. Work with the parties.’ That’s an option.”
A decision by the Court of Appeals is expected in the next two months.
AGA calls on Congress to enact legislation that contains essential patient protections and other improvements to ensure affordability, accessibility, and quality health care for all Americans. Learn more at https://www.gastro.org/advocacy-and-policy/issues-and-news/top-issues/patient-protections-and-access-to-care.
[email protected]
During the 2-hour hearing, a three-judge panel for the 5th U.S. Circuit Court of Appeals peppered attorneys with questions about whether Congress intended the ACA to function without the individual mandate, and the panel seemed doubtful the law can stand if the regulation is parsed, according to an audio transcript of the arguments. As written, the individual mandate required that all Americans have insurance or pay a tax penalty. However, budget legislation in 2017 zeroed out the penalties associated with the mandate, rendering it unenforceable.
Appeals Judge Kurt Engelhardt, a President Trump appointee, asked defense attorney Samuel Siegel why Congress failed to add a clause in the original law that would have allowed ACA components to be severed if such sectioning was acceptable.
“Congress could have included a severability clause when it adopted the ACA in 2010. Couldn’t it have done so?” Judge Engelhardt asked during oral arguments. “It seems like it did the opposite, where it said, ‘This is a complete overhaul,’ and it set forth a bunch of factual findings. Couldn’t Congress have said, ‘Oh by the way, we think all of these provisions are such excellent ideas and helpful to the public that if any go by the wayside, then we would want the remainder to continue to apply’?”
Congress’s silence on the severing of the ACA does not create a presumption against parsing of the law, argued Mr. Siegel, who is representing the Democratic states suing to retain the ACA in Texas v. United States. He emphasized that in 2017, when Congress terminated the individual mandate penalty, it chose not to repeal preexisting protections or other important reforms instituted by the ACA.
“With that action, your Honor, Congress expressed its views that the individual marketplace and indeed the entire Affordable Care Act can operate without an enforceable individual mandate,” Mr. Siegel said. “We think that’s all this court needs to know to resolve the severability question.”
However, Appellate Judge Jennifer Elrod, a President George W. Bush appointee to the court, questioned whether legislators zeroed out the mandate penalty because they knew the law could not survive without the core provision. She surmised that Congress might have assumed, “Aha, this is the silver bullet that’s going to undo Obamacare.”
Kyle Hawkins, an attorney representing the Republican-led plaintiff states, meanwhile, argued the text of the ACA clearly declares the individual mandate essential to the law and to the goals that Congress intended to achieve.
“The Obama administration thought of that as an inseverable clause,” Mr. Hawkins argued. “The district court directly synthesized those considerations ... and it reached the correct conclusion: The individual mandate is unconstitutional and it is inseverable from the remainder of the law.”
Texas v. United States stems from a legal challenge by a group of 18 Republican state attorneys general and two individuals in 2018 who argue the ACA should be declared unconstitutional. The plaintiffs say that, because budget legislation in 2017 effectively eliminated the penalty associated with the mandate, the requirement itself is invalid. Without the mandate, the entire law must fall, the plaintiffs contend. The Department of Justice declined to fully defend the law, so 16 Democratic state attorneys general intervened. In December 2018, a district court declared the entire ACA to be invalid, a decision immediately appealed to the 5th U.S. Circuit Court of Appeals by the Democratic attorneys general.
The Trump administration initially agreed that the mandate was unconstitutional and should be parsed. Attorneys for the administration said, if the mandate is found unconstitutional, the court should also consider finding two other provisions – the guaranteed issue and community rating requirements – of the ACA invalid. At the time, the Trump administration said the remainder of the ACA can stand without the three linked provisions. The administration later shifted its stance and asserted that much of the ACA should fall because provisions of the law cannot be severed. However, the DOJ expressed support in keeping some provision intact, such as certain criminal statutes that prevent health care fraud.
Most recently, the DOJ has indicated that, if the ACA is struck down or severed, the decision should only apply in the 18 plaintiff states and not to the entire nation. The fickle position of the Trump administration was questioned during the Court of Appeals hearing with judges asking DOJ attorney August Flentje to clarify why a final ruling should not apply nationwide.
“A lot of this stuff would need to get sorted out,” Mr. Flentje responded. “And it’s complicated. How it applies in the states and which parts can’t be applied at all because they would injure the states ... that raises a lot of complicated issues which I think [will be determined after] a final resolution.”
By their line of questioning, the appellate panel appeared to lean toward the plaintiffs’ position more so than toward the defendants’, said Katie Keith, an attorney and health law analyst who writes about Texas v. United States for the Health Affairs Blog.
“At least two of the three judges – the only two that were asking questions – seem very inclined to at, a minimum, strike down the individual mandate itself,” Ms. Keith said in an interview. “The conventional wisdom had been that this court would overturn the lower court’s decision, and I think folks are walking away, myself included, from oral arguments feeling less certain that that’s going to happen.”
Robert Henneke, general counsel for the American Future at the Texas Public Policy Foundation, said that plaintiffs “had a good day in court” and that the defendants’ arguments seemed to “hit a thud with the judges.” Mr. Henneke represents two individual plaintiffs from Texas in the lawsuit.
“Obamacare is still unconstitutional, and the three-judge panel seemed to agree with the trial court that the entirety of the law should be struck down,” Mr. Henneke said in a press conference after oral arguments. “The court really seemed skeptical with the arguments of the other side. We had the chance to tell the story of my clients and how they continue to be hurt by the Affordable Care Act.
Whichever way the Court of Appeals rules, the losing party is expected to appeal to the U.S. Supreme Court, Ms. Keith said. If justices accept the case, a decision could arrive in the summer of 2020, which would coincide with the presidential election. Another options is for the appellate court to send the case back to the lower court for further review, particularly to clear up the DOJ’s murky position, Ms. Keith said.
“They might send it back to [the lower court] and say there’s some questions here about what’s severable,” she said. “The DOJ sort of struggled to explain what they’re talking about. So they could remand the case back to Judge [Reed Charles] O’Connor to say, ‘Figure this out. Work with the parties.’ That’s an option.”
A decision by the Court of Appeals is expected in the next two months.
AGA calls on Congress to enact legislation that contains essential patient protections and other improvements to ensure affordability, accessibility, and quality health care for all Americans. Learn more at https://www.gastro.org/advocacy-and-policy/issues-and-news/top-issues/patient-protections-and-access-to-care.
[email protected]
Osteoporotic fracture risk appears higher in adults with hemophilia
Adults with newly diagnosed hemophilia may be at a higher risk of developing osteoporotic fractures, according to results from a retrospective study.
Sheng-Hui Tuan, MD, of Cishan Hospital in Kaohsiung, Taiwan, and colleagues conducted a population-based nationwide cohort study that included 75 patients with hemophilia and 300 control subjects without hemophilia matched for age and sex. Data was obtained from a national insurance database in Taiwan from January 2000 to December 2013. The findings were published in Haemophilia.
The primary outcome measured was newly diagnosed osteoporotic fractures, defined as wrist, vertebral, and hip fractures among individuals from both groups. Patients with osteoporotic fractures before hemophilia diagnosis were excluded.
In the analysis, the team calculated hazard ratios and incidence rates of new-onset osteoporotic fractures in both cohorts.
After analysis, the researchers found that the risk of developing new-onset osteoporotic fractures was greater in the hemophilia group versus the comparison group (HR, 5.41; 95% confidence interval, 2.42-12.1; P less than .001).
After adjusting for covariates, such as socioeconomic status, age, sex, and other comorbidities, patients with hemophilia had a 337% higher risk of developing osteoporotic fractures post diagnosis versus matched controls (95% CI, 1.88-10.17; P = .001).
“The risk of osteoporotic fractures following haemophilia increased with time and was significantly higher at 5 years after the diagnosis,” the researchers wrote.
The underlying mechanisms driving these associations remain unknown, according to the authors. Possible risk factors include reduced physical activity, HIV and hepatitis C virus infections, and arthropathy.
The researchers acknowledged that a key limitation of the study was the absence of some relevant clinical information within the database. As a result, information bias could have lowered the accuracy of the analysis.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Tuan S-H et al. Haemophilia. 2019 Jul 7. doi: 10.1111/hae.13814.
Adults with newly diagnosed hemophilia may be at a higher risk of developing osteoporotic fractures, according to results from a retrospective study.
Sheng-Hui Tuan, MD, of Cishan Hospital in Kaohsiung, Taiwan, and colleagues conducted a population-based nationwide cohort study that included 75 patients with hemophilia and 300 control subjects without hemophilia matched for age and sex. Data was obtained from a national insurance database in Taiwan from January 2000 to December 2013. The findings were published in Haemophilia.
The primary outcome measured was newly diagnosed osteoporotic fractures, defined as wrist, vertebral, and hip fractures among individuals from both groups. Patients with osteoporotic fractures before hemophilia diagnosis were excluded.
In the analysis, the team calculated hazard ratios and incidence rates of new-onset osteoporotic fractures in both cohorts.
After analysis, the researchers found that the risk of developing new-onset osteoporotic fractures was greater in the hemophilia group versus the comparison group (HR, 5.41; 95% confidence interval, 2.42-12.1; P less than .001).
After adjusting for covariates, such as socioeconomic status, age, sex, and other comorbidities, patients with hemophilia had a 337% higher risk of developing osteoporotic fractures post diagnosis versus matched controls (95% CI, 1.88-10.17; P = .001).
“The risk of osteoporotic fractures following haemophilia increased with time and was significantly higher at 5 years after the diagnosis,” the researchers wrote.
The underlying mechanisms driving these associations remain unknown, according to the authors. Possible risk factors include reduced physical activity, HIV and hepatitis C virus infections, and arthropathy.
The researchers acknowledged that a key limitation of the study was the absence of some relevant clinical information within the database. As a result, information bias could have lowered the accuracy of the analysis.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Tuan S-H et al. Haemophilia. 2019 Jul 7. doi: 10.1111/hae.13814.
Adults with newly diagnosed hemophilia may be at a higher risk of developing osteoporotic fractures, according to results from a retrospective study.
Sheng-Hui Tuan, MD, of Cishan Hospital in Kaohsiung, Taiwan, and colleagues conducted a population-based nationwide cohort study that included 75 patients with hemophilia and 300 control subjects without hemophilia matched for age and sex. Data was obtained from a national insurance database in Taiwan from January 2000 to December 2013. The findings were published in Haemophilia.
The primary outcome measured was newly diagnosed osteoporotic fractures, defined as wrist, vertebral, and hip fractures among individuals from both groups. Patients with osteoporotic fractures before hemophilia diagnosis were excluded.
In the analysis, the team calculated hazard ratios and incidence rates of new-onset osteoporotic fractures in both cohorts.
After analysis, the researchers found that the risk of developing new-onset osteoporotic fractures was greater in the hemophilia group versus the comparison group (HR, 5.41; 95% confidence interval, 2.42-12.1; P less than .001).
After adjusting for covariates, such as socioeconomic status, age, sex, and other comorbidities, patients with hemophilia had a 337% higher risk of developing osteoporotic fractures post diagnosis versus matched controls (95% CI, 1.88-10.17; P = .001).
“The risk of osteoporotic fractures following haemophilia increased with time and was significantly higher at 5 years after the diagnosis,” the researchers wrote.
The underlying mechanisms driving these associations remain unknown, according to the authors. Possible risk factors include reduced physical activity, HIV and hepatitis C virus infections, and arthropathy.
The researchers acknowledged that a key limitation of the study was the absence of some relevant clinical information within the database. As a result, information bias could have lowered the accuracy of the analysis.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: Tuan S-H et al. Haemophilia. 2019 Jul 7. doi: 10.1111/hae.13814.
FROM HAEMOPHILIA
Novel point-of-care assay appears accurate in monitoring hemophilia A
A novel point-of-care assay demonstrated sensitivity to monitor coagulation factor replacement therapy in patients with severe hemophilia A, with and without inhibitors, according to recent study findings.
The ClotChip assay is a novel factor-replacement therapy monitoring tool that uses a dielectric microsensor to assess whole-blood coagulation in patients with severe hemophilia A.
Debnath Maji of Case Western Reserve University, Cleveland, and colleagues evaluated the novel assay in attempt to address the unmet need of reliable monitoring of coagulation factor–replacement therapy. While global haemostasis assays are available to measure the missing function, these tools require expert interpretation, are technically challenging to operate, and may be unavailable at the bedside, they wrote in Haemophilia.
They evaluated the analytical capabilities of the assay in 6 patients with inhibitors and 12 patients without inhibitors. A total of 50 healthy controls were included in the study.
The team collected whole blood samples pre- and postcoagulation factor–replacement therapy. Measurements were also conducted on the thrombin generation assay (TGA), thromboelastography (TEG), and rotational thromboelastometry (ROTEM) global assays for comparison.
After analysis, the researchers found that ClotChip T-peak parameters showed a significant reduction for samples obtained post–factor replacement therapy versus pretherapy among patients with and without inhibitors (P = .028 and P = .0001, respectively).
Additionally, ClotChip T-peak values exhibited strong correlations with factor VIII clotting activity, clotting time parameters of ROTEM, and peak thrombin and endogenous thrombin potential of TGA.
“Taken together, these data suggest that the ClotChip T-peak parameter is sensitive to detection and correction of coagulopathy in children with haemophilia with and without inhibitors, as indicated by T-peak reaching reference‐range values after coagulation factor replacement therapy,” the researchers wrote.
One key limitation of the study was the small sample size, which may limit the generalizability of the findings.
The novel assay may be an appropriate tool for “real-world” decision making around the use of coagulation factor replacement therapy in patients with severe hemophilia A, they added.
The study was funded by the National Institutes of Health, the American Heart Association, and a Veterans Affairs Research Center of Excellence at Case Western Reserve University. The authors reported financial affiliations with Bayer, Bioverativ, Shire, and XaTek.
SOURCE: Maji D et al. Haemophilia. 2019 Jul 7. doi: 10.1111/hae.13799.
A novel point-of-care assay demonstrated sensitivity to monitor coagulation factor replacement therapy in patients with severe hemophilia A, with and without inhibitors, according to recent study findings.
The ClotChip assay is a novel factor-replacement therapy monitoring tool that uses a dielectric microsensor to assess whole-blood coagulation in patients with severe hemophilia A.
Debnath Maji of Case Western Reserve University, Cleveland, and colleagues evaluated the novel assay in attempt to address the unmet need of reliable monitoring of coagulation factor–replacement therapy. While global haemostasis assays are available to measure the missing function, these tools require expert interpretation, are technically challenging to operate, and may be unavailable at the bedside, they wrote in Haemophilia.
They evaluated the analytical capabilities of the assay in 6 patients with inhibitors and 12 patients without inhibitors. A total of 50 healthy controls were included in the study.
The team collected whole blood samples pre- and postcoagulation factor–replacement therapy. Measurements were also conducted on the thrombin generation assay (TGA), thromboelastography (TEG), and rotational thromboelastometry (ROTEM) global assays for comparison.
After analysis, the researchers found that ClotChip T-peak parameters showed a significant reduction for samples obtained post–factor replacement therapy versus pretherapy among patients with and without inhibitors (P = .028 and P = .0001, respectively).
Additionally, ClotChip T-peak values exhibited strong correlations with factor VIII clotting activity, clotting time parameters of ROTEM, and peak thrombin and endogenous thrombin potential of TGA.
“Taken together, these data suggest that the ClotChip T-peak parameter is sensitive to detection and correction of coagulopathy in children with haemophilia with and without inhibitors, as indicated by T-peak reaching reference‐range values after coagulation factor replacement therapy,” the researchers wrote.
One key limitation of the study was the small sample size, which may limit the generalizability of the findings.
The novel assay may be an appropriate tool for “real-world” decision making around the use of coagulation factor replacement therapy in patients with severe hemophilia A, they added.
The study was funded by the National Institutes of Health, the American Heart Association, and a Veterans Affairs Research Center of Excellence at Case Western Reserve University. The authors reported financial affiliations with Bayer, Bioverativ, Shire, and XaTek.
SOURCE: Maji D et al. Haemophilia. 2019 Jul 7. doi: 10.1111/hae.13799.
A novel point-of-care assay demonstrated sensitivity to monitor coagulation factor replacement therapy in patients with severe hemophilia A, with and without inhibitors, according to recent study findings.
The ClotChip assay is a novel factor-replacement therapy monitoring tool that uses a dielectric microsensor to assess whole-blood coagulation in patients with severe hemophilia A.
Debnath Maji of Case Western Reserve University, Cleveland, and colleagues evaluated the novel assay in attempt to address the unmet need of reliable monitoring of coagulation factor–replacement therapy. While global haemostasis assays are available to measure the missing function, these tools require expert interpretation, are technically challenging to operate, and may be unavailable at the bedside, they wrote in Haemophilia.
They evaluated the analytical capabilities of the assay in 6 patients with inhibitors and 12 patients without inhibitors. A total of 50 healthy controls were included in the study.
The team collected whole blood samples pre- and postcoagulation factor–replacement therapy. Measurements were also conducted on the thrombin generation assay (TGA), thromboelastography (TEG), and rotational thromboelastometry (ROTEM) global assays for comparison.
After analysis, the researchers found that ClotChip T-peak parameters showed a significant reduction for samples obtained post–factor replacement therapy versus pretherapy among patients with and without inhibitors (P = .028 and P = .0001, respectively).
Additionally, ClotChip T-peak values exhibited strong correlations with factor VIII clotting activity, clotting time parameters of ROTEM, and peak thrombin and endogenous thrombin potential of TGA.
“Taken together, these data suggest that the ClotChip T-peak parameter is sensitive to detection and correction of coagulopathy in children with haemophilia with and without inhibitors, as indicated by T-peak reaching reference‐range values after coagulation factor replacement therapy,” the researchers wrote.
One key limitation of the study was the small sample size, which may limit the generalizability of the findings.
The novel assay may be an appropriate tool for “real-world” decision making around the use of coagulation factor replacement therapy in patients with severe hemophilia A, they added.
The study was funded by the National Institutes of Health, the American Heart Association, and a Veterans Affairs Research Center of Excellence at Case Western Reserve University. The authors reported financial affiliations with Bayer, Bioverativ, Shire, and XaTek.
SOURCE: Maji D et al. Haemophilia. 2019 Jul 7. doi: 10.1111/hae.13799.
FROM HAEMOPHILIA
Risk of atrial fibrillation 900% higher with cancer
MELBOURNE – The overall prevalence of atrial fibrillation in people who have or have had cancer is 10 times that of individuals without cancer, according to a study presented at the International Society on Thrombosis and Haemostasis congress.
Cihan Ay, MD, of the division of hematology and hemostaseology at the Medical University of Vienna reported on a nationwide cohort study using health insurance data from more than 8.3 million people in Austria, including roughly 159,000 with a diagnosis of cancer and 113,000 with a diagnosis of atrial fibrillation.
The analysis found that, in individuals whose records showed a diagnosis of cancer, there was a 950% higher relative risk of also having a diagnosis of atrial fibrillation, compared with those with no cancer diagnosis.
The overall prevalence of atrial fibrillation among individuals with a cancer diagnosis was 9.8%, compared with 1.2% in those without cancer.
There was significant variation in relative risk according to age. Although the prevalence of atrial fibrillation increased with age, the highest relative risks were seen in the youngest age groups.
In those aged 12 years or under with a cancer diagnosis, the relative risk of atrial fibrillation was 150 times greater than in those without cancer, and in those aged 13-18 years, it was 200 times higher. At the other end of the age spectrum, individuals aged 70-79 years with a recorded cancer diagnosis, the relative risk of atrial fibrillation was still 130% higher than the noncancer population, and in those aged 80-90 years it was a significant 54% higher.
However, the analysis did not find any effect of gender on the risk of atrial fibrillation associated with cancer, regardless of the age group.
Researchers also examined the influence of different cancer types. They found the highest relative risk of atrial fibrillation was in persons with hematologic malignancies – at nine times the risk in the noncancer population – and the lowest was in the endocrine cancer patients, who had three times the risk.
Dr. Ay told the conference that the association between cancer and atrial fibrillation had been suggested in the literature, but it was still an unexplored field. “The exact magnitude of this association between cancer and atrial fibrillation is still unclear.”
There was also the question of what mechanisms might underlie the association. Dr. Ay pointed out that the health insurance database did not allow researchers to explore the temporal relationship between the two diagnoses, and therefore could not tell which came first.
One audience member queried whether the fact that cancer patients were likely to be visiting a clinician more frequently might mean that the atrial fibrillation would be more likely to be diagnosed.
To that, Dr. Ay suggested the significantly higher relative risk in children was supportive of the notion that cancer itself, or treatment effects, were influencing atrial fibrillation risk.
“There is evidence suggesting that cancer treatments are triggering atrial fibrillation,” he said in an interview. “Also, patients with cancer have situations of in which they are sick – they have neutropenia or sepsis and so on – which can also trigger atrial fibrillation.”
Given the limitations of the retrospective cohort study, Dr. Ay said he was hoping to do a prospective study that would enable baseline measurements of cancer patients to determine how much of the atrial fibrillation was preexisting.
“We have also more and more cancer survivors, and over the years they’re living longer and the likelihood of getting atrial fibrillation increases,” he added.
Commenting on the data, Gerald Soff, MD, chief of hematology at the Memorial Sloan Kettering Cancer Center in New York, said it was very important to quantify the association between cancer and atrial fibrillation.
“What’s striking to me is how many people with cancer come in with preexisting atrial fibrillation,” he said. “It could be that they have cancer and they’re already messed up, but we have, on a given day, several people coming in with newly diagnosed cancers, already on warfarin or apixaban or rivaroxaban because they have atrial fibrillation.”
Dr. Ay reported advisory board positions and speaking engagements for the pharmaceutical sector.
MELBOURNE – The overall prevalence of atrial fibrillation in people who have or have had cancer is 10 times that of individuals without cancer, according to a study presented at the International Society on Thrombosis and Haemostasis congress.
Cihan Ay, MD, of the division of hematology and hemostaseology at the Medical University of Vienna reported on a nationwide cohort study using health insurance data from more than 8.3 million people in Austria, including roughly 159,000 with a diagnosis of cancer and 113,000 with a diagnosis of atrial fibrillation.
The analysis found that, in individuals whose records showed a diagnosis of cancer, there was a 950% higher relative risk of also having a diagnosis of atrial fibrillation, compared with those with no cancer diagnosis.
The overall prevalence of atrial fibrillation among individuals with a cancer diagnosis was 9.8%, compared with 1.2% in those without cancer.
There was significant variation in relative risk according to age. Although the prevalence of atrial fibrillation increased with age, the highest relative risks were seen in the youngest age groups.
In those aged 12 years or under with a cancer diagnosis, the relative risk of atrial fibrillation was 150 times greater than in those without cancer, and in those aged 13-18 years, it was 200 times higher. At the other end of the age spectrum, individuals aged 70-79 years with a recorded cancer diagnosis, the relative risk of atrial fibrillation was still 130% higher than the noncancer population, and in those aged 80-90 years it was a significant 54% higher.
However, the analysis did not find any effect of gender on the risk of atrial fibrillation associated with cancer, regardless of the age group.
Researchers also examined the influence of different cancer types. They found the highest relative risk of atrial fibrillation was in persons with hematologic malignancies – at nine times the risk in the noncancer population – and the lowest was in the endocrine cancer patients, who had three times the risk.
Dr. Ay told the conference that the association between cancer and atrial fibrillation had been suggested in the literature, but it was still an unexplored field. “The exact magnitude of this association between cancer and atrial fibrillation is still unclear.”
There was also the question of what mechanisms might underlie the association. Dr. Ay pointed out that the health insurance database did not allow researchers to explore the temporal relationship between the two diagnoses, and therefore could not tell which came first.
One audience member queried whether the fact that cancer patients were likely to be visiting a clinician more frequently might mean that the atrial fibrillation would be more likely to be diagnosed.
To that, Dr. Ay suggested the significantly higher relative risk in children was supportive of the notion that cancer itself, or treatment effects, were influencing atrial fibrillation risk.
“There is evidence suggesting that cancer treatments are triggering atrial fibrillation,” he said in an interview. “Also, patients with cancer have situations of in which they are sick – they have neutropenia or sepsis and so on – which can also trigger atrial fibrillation.”
Given the limitations of the retrospective cohort study, Dr. Ay said he was hoping to do a prospective study that would enable baseline measurements of cancer patients to determine how much of the atrial fibrillation was preexisting.
“We have also more and more cancer survivors, and over the years they’re living longer and the likelihood of getting atrial fibrillation increases,” he added.
Commenting on the data, Gerald Soff, MD, chief of hematology at the Memorial Sloan Kettering Cancer Center in New York, said it was very important to quantify the association between cancer and atrial fibrillation.
“What’s striking to me is how many people with cancer come in with preexisting atrial fibrillation,” he said. “It could be that they have cancer and they’re already messed up, but we have, on a given day, several people coming in with newly diagnosed cancers, already on warfarin or apixaban or rivaroxaban because they have atrial fibrillation.”
Dr. Ay reported advisory board positions and speaking engagements for the pharmaceutical sector.
MELBOURNE – The overall prevalence of atrial fibrillation in people who have or have had cancer is 10 times that of individuals without cancer, according to a study presented at the International Society on Thrombosis and Haemostasis congress.
Cihan Ay, MD, of the division of hematology and hemostaseology at the Medical University of Vienna reported on a nationwide cohort study using health insurance data from more than 8.3 million people in Austria, including roughly 159,000 with a diagnosis of cancer and 113,000 with a diagnosis of atrial fibrillation.
The analysis found that, in individuals whose records showed a diagnosis of cancer, there was a 950% higher relative risk of also having a diagnosis of atrial fibrillation, compared with those with no cancer diagnosis.
The overall prevalence of atrial fibrillation among individuals with a cancer diagnosis was 9.8%, compared with 1.2% in those without cancer.
There was significant variation in relative risk according to age. Although the prevalence of atrial fibrillation increased with age, the highest relative risks were seen in the youngest age groups.
In those aged 12 years or under with a cancer diagnosis, the relative risk of atrial fibrillation was 150 times greater than in those without cancer, and in those aged 13-18 years, it was 200 times higher. At the other end of the age spectrum, individuals aged 70-79 years with a recorded cancer diagnosis, the relative risk of atrial fibrillation was still 130% higher than the noncancer population, and in those aged 80-90 years it was a significant 54% higher.
However, the analysis did not find any effect of gender on the risk of atrial fibrillation associated with cancer, regardless of the age group.
Researchers also examined the influence of different cancer types. They found the highest relative risk of atrial fibrillation was in persons with hematologic malignancies – at nine times the risk in the noncancer population – and the lowest was in the endocrine cancer patients, who had three times the risk.
Dr. Ay told the conference that the association between cancer and atrial fibrillation had been suggested in the literature, but it was still an unexplored field. “The exact magnitude of this association between cancer and atrial fibrillation is still unclear.”
There was also the question of what mechanisms might underlie the association. Dr. Ay pointed out that the health insurance database did not allow researchers to explore the temporal relationship between the two diagnoses, and therefore could not tell which came first.
One audience member queried whether the fact that cancer patients were likely to be visiting a clinician more frequently might mean that the atrial fibrillation would be more likely to be diagnosed.
To that, Dr. Ay suggested the significantly higher relative risk in children was supportive of the notion that cancer itself, or treatment effects, were influencing atrial fibrillation risk.
“There is evidence suggesting that cancer treatments are triggering atrial fibrillation,” he said in an interview. “Also, patients with cancer have situations of in which they are sick – they have neutropenia or sepsis and so on – which can also trigger atrial fibrillation.”
Given the limitations of the retrospective cohort study, Dr. Ay said he was hoping to do a prospective study that would enable baseline measurements of cancer patients to determine how much of the atrial fibrillation was preexisting.
“We have also more and more cancer survivors, and over the years they’re living longer and the likelihood of getting atrial fibrillation increases,” he added.
Commenting on the data, Gerald Soff, MD, chief of hematology at the Memorial Sloan Kettering Cancer Center in New York, said it was very important to quantify the association between cancer and atrial fibrillation.
“What’s striking to me is how many people with cancer come in with preexisting atrial fibrillation,” he said. “It could be that they have cancer and they’re already messed up, but we have, on a given day, several people coming in with newly diagnosed cancers, already on warfarin or apixaban or rivaroxaban because they have atrial fibrillation.”
Dr. Ay reported advisory board positions and speaking engagements for the pharmaceutical sector.
REPORTING FROM 2019 ISTH CONGRESS