User login
BMI screening trigger for type 2 diabetes is unreliable for at-risk black, Hispanic adults
SAN FRANCISCO – according to findings presented at the annual scientific sessions of the American Diabetes Association.
The conclusions come from a review of 5,656 participants aged 45-84 years in the Multi-Ethnic Study of Atherosclerosis (MESA), an ongoing prospective cohort study. The participants had no signs of cardiovascular disease at enrollment.
The goal of the study reported at the meeting was to see if current ADA screening guidelines are appropriate across racial groups in an increasingly diverse United States. The guidelines recommend body mass index (BMI) as a trigger for screening for type 2 diabetes. The current advice is to screen any adult who has a BMI at or above 25 kg/m2 (23 kg/m2 for Asian Americans) and at least one risk factor for type 2 diabetes, such as hypertension, dyslipidemia, or physical inactivity.
Being black, Asian American, Hispanic, Pacific Islander, or American Indian is itself a risk factor, so “someone from a minority group just needs to meet the BMI criteria,” said lead investigator Luis Rodriguez, a PhD candidate in epidemiology at the University of California, San Francisco.
He and his colleagues focused on 2,383 white, 653 Chinese American, 1,459 black, and 1,161 Hispanic participants in MESA who did not have type 2 diabetes at baseline. Participants were in their early 60s at baseline, on average, and just more than half of them were women. They had five medical exams between 2000 and 2012. The investigators calculated the BMI at which each group hit a 10% risk of developing diabetes within the next 10 years.
In general, the lines crossed at a BMI of about 23 kg/m2 for Chinese Americans; 25 kg/m2 for black and Hispanic participants; and 27 kg/m2 for white participants, which is consistent with ADA advice. The guideline cut points are “appropriate for population-based screening where you may not know if the person in front of you has any diabetes risk factors,” Mr. Rodriguez said.
With known risk factors, however, BMI didn’t hold up. Black participants with one or more risk factor hit the 10% mark at a BMI of 24.7 kg/m2, Hispanics at 23.8 kg/m2, and Chinese Americans at 21.7 kg/m2, all of which are below the recommended cut-points.
“Almost 80%-90% of participants” in minority groups “who had one or more risk factors were above the 10% threshold, so if they have at least one factor, they should pretty much be screened for diabetes, regardless of BMI,” he said.
The 25-kg/m2 threshold worked for white participants; the investigators found that with one or more risk factors, white participants crossed the 10% line at a BMI of 26.2 kg/m2. In addition, white participants with no diabetes risk factors crossed the 10% mark at around a BMI of 30 kg/m2, which the investigators suggested as an appropriate screening trigger.
Participants were in their early 60s at baseline, on average, and just more than half of them were women. In all, 696 cases of type 2 diabetes were diagnosed over a median follow-up of about 9 years, which translated to an incidence rate of 11 cases per 1,000 person-years in white participants, 16 cases for Chinese Americans, 21 for black participants, and 22 for Hispanic participants. Well over half of the participants were overweight or obese at baseline, including about 80% of black and Hispanic participants.
The sample size reported in the abstract was smaller than that in the final presentation because the investigators did not adjust for diet in the final presentation, Mr. Rodriguez explained. Because some study participants did not report diet, by excluding diet from the model adjustment, the number of participants increased, as reflected in this text. Adjusting for diet did not alter the findings.
The National Heart, Lung, and Blood Institute sponsors MESA. Mr. Rodriguez was supported by a grant from the National Institutes of Health/National Institute of Diabetes and Kidney Disease. The other investigators reported having no disclosures.
SOURCE: Rodriguez L et al. ADA 2019, Abstract 115-OR.
SAN FRANCISCO – according to findings presented at the annual scientific sessions of the American Diabetes Association.
The conclusions come from a review of 5,656 participants aged 45-84 years in the Multi-Ethnic Study of Atherosclerosis (MESA), an ongoing prospective cohort study. The participants had no signs of cardiovascular disease at enrollment.
The goal of the study reported at the meeting was to see if current ADA screening guidelines are appropriate across racial groups in an increasingly diverse United States. The guidelines recommend body mass index (BMI) as a trigger for screening for type 2 diabetes. The current advice is to screen any adult who has a BMI at or above 25 kg/m2 (23 kg/m2 for Asian Americans) and at least one risk factor for type 2 diabetes, such as hypertension, dyslipidemia, or physical inactivity.
Being black, Asian American, Hispanic, Pacific Islander, or American Indian is itself a risk factor, so “someone from a minority group just needs to meet the BMI criteria,” said lead investigator Luis Rodriguez, a PhD candidate in epidemiology at the University of California, San Francisco.
He and his colleagues focused on 2,383 white, 653 Chinese American, 1,459 black, and 1,161 Hispanic participants in MESA who did not have type 2 diabetes at baseline. Participants were in their early 60s at baseline, on average, and just more than half of them were women. They had five medical exams between 2000 and 2012. The investigators calculated the BMI at which each group hit a 10% risk of developing diabetes within the next 10 years.
In general, the lines crossed at a BMI of about 23 kg/m2 for Chinese Americans; 25 kg/m2 for black and Hispanic participants; and 27 kg/m2 for white participants, which is consistent with ADA advice. The guideline cut points are “appropriate for population-based screening where you may not know if the person in front of you has any diabetes risk factors,” Mr. Rodriguez said.
With known risk factors, however, BMI didn’t hold up. Black participants with one or more risk factor hit the 10% mark at a BMI of 24.7 kg/m2, Hispanics at 23.8 kg/m2, and Chinese Americans at 21.7 kg/m2, all of which are below the recommended cut-points.
“Almost 80%-90% of participants” in minority groups “who had one or more risk factors were above the 10% threshold, so if they have at least one factor, they should pretty much be screened for diabetes, regardless of BMI,” he said.
The 25-kg/m2 threshold worked for white participants; the investigators found that with one or more risk factors, white participants crossed the 10% line at a BMI of 26.2 kg/m2. In addition, white participants with no diabetes risk factors crossed the 10% mark at around a BMI of 30 kg/m2, which the investigators suggested as an appropriate screening trigger.
Participants were in their early 60s at baseline, on average, and just more than half of them were women. In all, 696 cases of type 2 diabetes were diagnosed over a median follow-up of about 9 years, which translated to an incidence rate of 11 cases per 1,000 person-years in white participants, 16 cases for Chinese Americans, 21 for black participants, and 22 for Hispanic participants. Well over half of the participants were overweight or obese at baseline, including about 80% of black and Hispanic participants.
The sample size reported in the abstract was smaller than that in the final presentation because the investigators did not adjust for diet in the final presentation, Mr. Rodriguez explained. Because some study participants did not report diet, by excluding diet from the model adjustment, the number of participants increased, as reflected in this text. Adjusting for diet did not alter the findings.
The National Heart, Lung, and Blood Institute sponsors MESA. Mr. Rodriguez was supported by a grant from the National Institutes of Health/National Institute of Diabetes and Kidney Disease. The other investigators reported having no disclosures.
SOURCE: Rodriguez L et al. ADA 2019, Abstract 115-OR.
SAN FRANCISCO – according to findings presented at the annual scientific sessions of the American Diabetes Association.
The conclusions come from a review of 5,656 participants aged 45-84 years in the Multi-Ethnic Study of Atherosclerosis (MESA), an ongoing prospective cohort study. The participants had no signs of cardiovascular disease at enrollment.
The goal of the study reported at the meeting was to see if current ADA screening guidelines are appropriate across racial groups in an increasingly diverse United States. The guidelines recommend body mass index (BMI) as a trigger for screening for type 2 diabetes. The current advice is to screen any adult who has a BMI at or above 25 kg/m2 (23 kg/m2 for Asian Americans) and at least one risk factor for type 2 diabetes, such as hypertension, dyslipidemia, or physical inactivity.
Being black, Asian American, Hispanic, Pacific Islander, or American Indian is itself a risk factor, so “someone from a minority group just needs to meet the BMI criteria,” said lead investigator Luis Rodriguez, a PhD candidate in epidemiology at the University of California, San Francisco.
He and his colleagues focused on 2,383 white, 653 Chinese American, 1,459 black, and 1,161 Hispanic participants in MESA who did not have type 2 diabetes at baseline. Participants were in their early 60s at baseline, on average, and just more than half of them were women. They had five medical exams between 2000 and 2012. The investigators calculated the BMI at which each group hit a 10% risk of developing diabetes within the next 10 years.
In general, the lines crossed at a BMI of about 23 kg/m2 for Chinese Americans; 25 kg/m2 for black and Hispanic participants; and 27 kg/m2 for white participants, which is consistent with ADA advice. The guideline cut points are “appropriate for population-based screening where you may not know if the person in front of you has any diabetes risk factors,” Mr. Rodriguez said.
With known risk factors, however, BMI didn’t hold up. Black participants with one or more risk factor hit the 10% mark at a BMI of 24.7 kg/m2, Hispanics at 23.8 kg/m2, and Chinese Americans at 21.7 kg/m2, all of which are below the recommended cut-points.
“Almost 80%-90% of participants” in minority groups “who had one or more risk factors were above the 10% threshold, so if they have at least one factor, they should pretty much be screened for diabetes, regardless of BMI,” he said.
The 25-kg/m2 threshold worked for white participants; the investigators found that with one or more risk factors, white participants crossed the 10% line at a BMI of 26.2 kg/m2. In addition, white participants with no diabetes risk factors crossed the 10% mark at around a BMI of 30 kg/m2, which the investigators suggested as an appropriate screening trigger.
Participants were in their early 60s at baseline, on average, and just more than half of them were women. In all, 696 cases of type 2 diabetes were diagnosed over a median follow-up of about 9 years, which translated to an incidence rate of 11 cases per 1,000 person-years in white participants, 16 cases for Chinese Americans, 21 for black participants, and 22 for Hispanic participants. Well over half of the participants were overweight or obese at baseline, including about 80% of black and Hispanic participants.
The sample size reported in the abstract was smaller than that in the final presentation because the investigators did not adjust for diet in the final presentation, Mr. Rodriguez explained. Because some study participants did not report diet, by excluding diet from the model adjustment, the number of participants increased, as reflected in this text. Adjusting for diet did not alter the findings.
The National Heart, Lung, and Blood Institute sponsors MESA. Mr. Rodriguez was supported by a grant from the National Institutes of Health/National Institute of Diabetes and Kidney Disease. The other investigators reported having no disclosures.
SOURCE: Rodriguez L et al. ADA 2019, Abstract 115-OR.
REPORTING FROM ADA 2019
Key clinical point:
Major finding: Black participants with one or more risk factors had a 10% or greater risk of type 2 diabetes at a BMI of 24.7 kg/m2; Hispanic participants had the same risk at 23.8 kg/m2; and Chinese Americans at 21.7 kg/mg2, all of which are below the BMI cut points recommended by the ADA.
Study details: Review of 5,656 participants in the Multi-Ethnic Study of Atherosclerosis .
Disclosures: The National Heart, Lung, and Blood Institute sponsors MESA. Mr. Rodriguez was supported by grant from the National Institutes of Health/National Institute of Diabetes and Kidney Disease. The other investigators reported having no disclosures.
Source: Rodriguez L et al. ADA 2019, Abstract 115-OR.
Frontline pembro + chemo shows superiority against NSCLC
A combination of pembrolizumab and platinum chemotherapy is the most effective frontline treatment for patients with non–small cell lung cancer (NSCLC), according to a retrospective analysis of more than 16,000 patients.
The study showed that responses to therapy are best predicted by an aggregate of tumor mutational burden (TMB), programmed cell death ligand 1 (PD-L1) expression, and proportion of CD8+ T-cell tumor-infiltrating lymphocytes (TILs), reported Yunfang Yu, MD, of Sun Yat-sen University, Guangzhou, China, and colleagues.
“[I]mmunotherapy has produced inconsistent results in previous randomized clinical trials,” the investigators wrote, citing inconsistent survival outcomes in CheckMate-026 and publications by Takayama and Wu. “Moreover, independent immune-related biomarkers that are currently used, such as PD-L1 and TMB, have achieved clinical relevance for a selection of patients to some extent, but to our knowledge, they are still far from clear and established.” The report is in JAMA Network Open.
To gain clarity, the investigators performed a large-scale meta-analysis (n = 14,395) and individual patient-level study (n = 1,833) involving patients with NSCLC. Data were drawn from a variety of sources, including PubMed, EMBASE, Cochrane, conference proceedings, and others. Primary outcomes were median overall survival and progression-free survival. Secondary outcomes were objective response rate and durable clinical benefit. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) was used as a reporting guideline.
Analysis showed the superiority of immunotherapy to conventional therapy with significantly extended median overall survival and progression-free survival, both with an immunotherapy-favoring hazard ratio (HR) of 0.76 (P less than .001). Immunotherapy survival advantages were also reported individually for checkpoint inhibitors (HR, 0.75), tumor vaccines (HR, 0.83), and cellular immunotherapy (HR, 0.40). Of these three, checkpoint inhibitors and tumor vaccines showed superiority for progression-free survival. For first-line therapy, a combination of a pembrolizumab and chemotherapy was associated with better progression-free and overall survival than were other immunotherapies.
For patients treated with checkpoint inhibitors, higher levels of PD-L1 expression, TMB, or neo-antigen burden (NAB) were each prognostically valuable; however, the most powerful predictive tool was a combination of PD-L1 expression, TMB, and proportion of CD8+ T-cell TILs, with a 3-year overall survival area under the curve of 0.659. In addition, RYR1 and MGAM mutations were independently associated with durable clinical benefits.
“Future development of an optimized, integrated predictive model for immunotherapy should consider the integration of multiple approaches involving biomarkers associated with the T cell–inflamed tumor microenvironment, such as PD-L1 expression, ICs, and those associated with tumor neoepitope burden,” the investigators wrote.
The study was funded by the National Natural Science Foundation of China, Natural Science Foundation of Guangdong Province, and Guangzhou Science and Technology Program. The investigators disclosed no conflicts of interest.
SOURCE: Yu et al. JAMA Open. 2019 Jul 10. doi: 10.1001/jamanetworkopen.2019.6879.
A combination of pembrolizumab and platinum chemotherapy is the most effective frontline treatment for patients with non–small cell lung cancer (NSCLC), according to a retrospective analysis of more than 16,000 patients.
The study showed that responses to therapy are best predicted by an aggregate of tumor mutational burden (TMB), programmed cell death ligand 1 (PD-L1) expression, and proportion of CD8+ T-cell tumor-infiltrating lymphocytes (TILs), reported Yunfang Yu, MD, of Sun Yat-sen University, Guangzhou, China, and colleagues.
“[I]mmunotherapy has produced inconsistent results in previous randomized clinical trials,” the investigators wrote, citing inconsistent survival outcomes in CheckMate-026 and publications by Takayama and Wu. “Moreover, independent immune-related biomarkers that are currently used, such as PD-L1 and TMB, have achieved clinical relevance for a selection of patients to some extent, but to our knowledge, they are still far from clear and established.” The report is in JAMA Network Open.
To gain clarity, the investigators performed a large-scale meta-analysis (n = 14,395) and individual patient-level study (n = 1,833) involving patients with NSCLC. Data were drawn from a variety of sources, including PubMed, EMBASE, Cochrane, conference proceedings, and others. Primary outcomes were median overall survival and progression-free survival. Secondary outcomes were objective response rate and durable clinical benefit. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) was used as a reporting guideline.
Analysis showed the superiority of immunotherapy to conventional therapy with significantly extended median overall survival and progression-free survival, both with an immunotherapy-favoring hazard ratio (HR) of 0.76 (P less than .001). Immunotherapy survival advantages were also reported individually for checkpoint inhibitors (HR, 0.75), tumor vaccines (HR, 0.83), and cellular immunotherapy (HR, 0.40). Of these three, checkpoint inhibitors and tumor vaccines showed superiority for progression-free survival. For first-line therapy, a combination of a pembrolizumab and chemotherapy was associated with better progression-free and overall survival than were other immunotherapies.
For patients treated with checkpoint inhibitors, higher levels of PD-L1 expression, TMB, or neo-antigen burden (NAB) were each prognostically valuable; however, the most powerful predictive tool was a combination of PD-L1 expression, TMB, and proportion of CD8+ T-cell TILs, with a 3-year overall survival area under the curve of 0.659. In addition, RYR1 and MGAM mutations were independently associated with durable clinical benefits.
“Future development of an optimized, integrated predictive model for immunotherapy should consider the integration of multiple approaches involving biomarkers associated with the T cell–inflamed tumor microenvironment, such as PD-L1 expression, ICs, and those associated with tumor neoepitope burden,” the investigators wrote.
The study was funded by the National Natural Science Foundation of China, Natural Science Foundation of Guangdong Province, and Guangzhou Science and Technology Program. The investigators disclosed no conflicts of interest.
SOURCE: Yu et al. JAMA Open. 2019 Jul 10. doi: 10.1001/jamanetworkopen.2019.6879.
A combination of pembrolizumab and platinum chemotherapy is the most effective frontline treatment for patients with non–small cell lung cancer (NSCLC), according to a retrospective analysis of more than 16,000 patients.
The study showed that responses to therapy are best predicted by an aggregate of tumor mutational burden (TMB), programmed cell death ligand 1 (PD-L1) expression, and proportion of CD8+ T-cell tumor-infiltrating lymphocytes (TILs), reported Yunfang Yu, MD, of Sun Yat-sen University, Guangzhou, China, and colleagues.
“[I]mmunotherapy has produced inconsistent results in previous randomized clinical trials,” the investigators wrote, citing inconsistent survival outcomes in CheckMate-026 and publications by Takayama and Wu. “Moreover, independent immune-related biomarkers that are currently used, such as PD-L1 and TMB, have achieved clinical relevance for a selection of patients to some extent, but to our knowledge, they are still far from clear and established.” The report is in JAMA Network Open.
To gain clarity, the investigators performed a large-scale meta-analysis (n = 14,395) and individual patient-level study (n = 1,833) involving patients with NSCLC. Data were drawn from a variety of sources, including PubMed, EMBASE, Cochrane, conference proceedings, and others. Primary outcomes were median overall survival and progression-free survival. Secondary outcomes were objective response rate and durable clinical benefit. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) was used as a reporting guideline.
Analysis showed the superiority of immunotherapy to conventional therapy with significantly extended median overall survival and progression-free survival, both with an immunotherapy-favoring hazard ratio (HR) of 0.76 (P less than .001). Immunotherapy survival advantages were also reported individually for checkpoint inhibitors (HR, 0.75), tumor vaccines (HR, 0.83), and cellular immunotherapy (HR, 0.40). Of these three, checkpoint inhibitors and tumor vaccines showed superiority for progression-free survival. For first-line therapy, a combination of a pembrolizumab and chemotherapy was associated with better progression-free and overall survival than were other immunotherapies.
For patients treated with checkpoint inhibitors, higher levels of PD-L1 expression, TMB, or neo-antigen burden (NAB) were each prognostically valuable; however, the most powerful predictive tool was a combination of PD-L1 expression, TMB, and proportion of CD8+ T-cell TILs, with a 3-year overall survival area under the curve of 0.659. In addition, RYR1 and MGAM mutations were independently associated with durable clinical benefits.
“Future development of an optimized, integrated predictive model for immunotherapy should consider the integration of multiple approaches involving biomarkers associated with the T cell–inflamed tumor microenvironment, such as PD-L1 expression, ICs, and those associated with tumor neoepitope burden,” the investigators wrote.
The study was funded by the National Natural Science Foundation of China, Natural Science Foundation of Guangdong Province, and Guangzhou Science and Technology Program. The investigators disclosed no conflicts of interest.
SOURCE: Yu et al. JAMA Open. 2019 Jul 10. doi: 10.1001/jamanetworkopen.2019.6879.
FROM JAMA NETWORK OPEN
Thelarche and menarche are associated with increased prevalence of migraine
PHILADELPHIA – , according to research presented at the annual meeting of the American Headache Society. The results suggest that earlier exposure to estrogen increases the risk for migraine in adolescent girls, said Vincent Martin, MD, director of the Headache and Facial Pain Center at the University of Cincinnati Gardner Neuroscience Institute.
Previous studies observed an association between earlier onset of menarche and greater prevalence of migraine in adolescent girls, but no investigators had examined the relationship between earlier stages of pubertal development, such as thelarche and pubarche, and migraine.
Dr. Martin and colleagues included participants in the Breast Cancer and Environment Research Program puberty cohort in their study. Physicians examined the girls every 6 to 12 months from the time that they were aged 6-8 years to the time of late adolescence. During the last examination, participants responded to a validated questionnaire to determine whether they met International Classification of Headache Disorders–3 criteria for a diagnosis of migraine. Dr. Martin and colleagues performed logistic regression to examine whether age at thelarche, pubarche, or menarche predicted migraine.
Of 761 girls included in this study, 85 (11.2%) received a diagnosis of migraine. The mean age at which the questionnaire was administered was 15.6 years. After adjusting the data for potential confounders, the researchers found that an earlier age of onset of thelarche and menarche was associated with a higher prevalence of migraine. A 1-year decrease in the age of onset of thelarche or menarche was associated with a 32.8% or 33.8% increase in the odds of migraine headache, respectively. Pubarche was not associated with migraine.
Dr. Martin had no relevant disclosures.
PHILADELPHIA – , according to research presented at the annual meeting of the American Headache Society. The results suggest that earlier exposure to estrogen increases the risk for migraine in adolescent girls, said Vincent Martin, MD, director of the Headache and Facial Pain Center at the University of Cincinnati Gardner Neuroscience Institute.
Previous studies observed an association between earlier onset of menarche and greater prevalence of migraine in adolescent girls, but no investigators had examined the relationship between earlier stages of pubertal development, such as thelarche and pubarche, and migraine.
Dr. Martin and colleagues included participants in the Breast Cancer and Environment Research Program puberty cohort in their study. Physicians examined the girls every 6 to 12 months from the time that they were aged 6-8 years to the time of late adolescence. During the last examination, participants responded to a validated questionnaire to determine whether they met International Classification of Headache Disorders–3 criteria for a diagnosis of migraine. Dr. Martin and colleagues performed logistic regression to examine whether age at thelarche, pubarche, or menarche predicted migraine.
Of 761 girls included in this study, 85 (11.2%) received a diagnosis of migraine. The mean age at which the questionnaire was administered was 15.6 years. After adjusting the data for potential confounders, the researchers found that an earlier age of onset of thelarche and menarche was associated with a higher prevalence of migraine. A 1-year decrease in the age of onset of thelarche or menarche was associated with a 32.8% or 33.8% increase in the odds of migraine headache, respectively. Pubarche was not associated with migraine.
Dr. Martin had no relevant disclosures.
PHILADELPHIA – , according to research presented at the annual meeting of the American Headache Society. The results suggest that earlier exposure to estrogen increases the risk for migraine in adolescent girls, said Vincent Martin, MD, director of the Headache and Facial Pain Center at the University of Cincinnati Gardner Neuroscience Institute.
Previous studies observed an association between earlier onset of menarche and greater prevalence of migraine in adolescent girls, but no investigators had examined the relationship between earlier stages of pubertal development, such as thelarche and pubarche, and migraine.
Dr. Martin and colleagues included participants in the Breast Cancer and Environment Research Program puberty cohort in their study. Physicians examined the girls every 6 to 12 months from the time that they were aged 6-8 years to the time of late adolescence. During the last examination, participants responded to a validated questionnaire to determine whether they met International Classification of Headache Disorders–3 criteria for a diagnosis of migraine. Dr. Martin and colleagues performed logistic regression to examine whether age at thelarche, pubarche, or menarche predicted migraine.
Of 761 girls included in this study, 85 (11.2%) received a diagnosis of migraine. The mean age at which the questionnaire was administered was 15.6 years. After adjusting the data for potential confounders, the researchers found that an earlier age of onset of thelarche and menarche was associated with a higher prevalence of migraine. A 1-year decrease in the age of onset of thelarche or menarche was associated with a 32.8% or 33.8% increase in the odds of migraine headache, respectively. Pubarche was not associated with migraine.
Dr. Martin had no relevant disclosures.
EXPERT ANALYSIS FROM AHS 2019
Abnormal uterine bleeding: When can you forgo biopsy?
NASHVILLE, TENN. – Ultrasound is a reasonable first step for the evaluation of postmenopausal women with abnormal uterine bleeding and endometrial thickness up to 5 mm, according to James Shwayder, MD, JD.
About a third of such patients presenting with abnormal uterine bleeding (AUB) will have an endometrial polyp or submucous myoma, Dr. Shwayder explained during a presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
However, recurrent bleeding warrants further work-up, he said.
The 5-mm value is based on a number of studies, which, in sum, show that the negative predictive value for cancer for endometrial thickness less than 4 mm is greater than 99%, said Dr. Shwayder, chief of the division of gynecology and director of the fellowship in advanced endoscopy obstetrics, gynecology, and women’s health at the University of Louisville (Ky.).
“In fact ... the risk of cancer is 1 in 917,” he added. “That’s pretty good ... that’s a great screening tool.”
The question is whether one can feel comfortable foregoing biopsy in such cases, he said, describing a 61-year-old patient with a 3.9-mm endometrium and AUB for 3 days, 3 weeks prior to her visit.
There are two possibilities: The patient will either have no further bleeding, or she will continue to bleed, he said, noting that a 2003 Swedish study provides some guidance in the case of continued bleeding (Am J Obstet Gynecol. 2003;188:401-8).
The investigators initially looked at histology associated with endometrial thickness in 394 postmenopausal women referred for AUB between 1987 and 1990. Both transvaginal ultrasound and dilatation and curettage were performed, and the findings were correlated.
“But they had the rare opportunity to take patients who had benign evaluations and bring them back 10 years later,” Dr. Shwayder said. “What they found was that, regardless of the endometrial thickness, if it was benign initially and they did not bleed over that 10-year period, no one had cancer.”
However, among the patients followed for 10 years who had recurrent bleeding, 10% had cancer and 12% had hyperplasia. Thus, deciding against a biopsy in this case is supported by good data; if the patient doesn’t bleed, she has an “incredibly low risk of cancer,” he said.
“If they bleed again, you’ve gotta work ‘em up,” he stressed. “Don’t continue to say, ‘Well, let me repeat the ultrasound and see if it’s thinner or thicker.’ No. They need to be evaluated.”
Keep in mind that if a biopsy is performed as the first step, the chances of the results coming back as tissue insufficient for diagnosis (TIFD) are increased, and a repeat biopsy will be necessary because of the inconclusive findings, Dr. Shwayder said. It helps to warn a patient in advance that their thin endometrium makes it highly likely that a repeat biopsy will be necessary, as 90% come back as TIFD or atrophy.
Importantly, though, ACOG says endometrial sampling should be performed first line in patients over age 45 years with AUB.
“I’ll be honest – I use ultrasound in these patients because of the fact that a third will have some sort of structural defect, and the focal abnormalities are things we’re not going to be able to pick up with a straight biopsy, but we have to be cognizant that the college recommends biopsy in this population,” Dr. Shwayder said.
Asymptomatic endometrial thickening
Postmenopausal women are increasingly being referred for asymptomatic endometrial thickening that is found incidentally during an unrelated evaluation, Dr. Shwayder said, noting that evidence to date on how to approach such cases is conflicting.
Although ACOG is working on a recommendation, none is currently available, he said.
However, a 2001 study comparing 123 asymptomatic patients with 90 symptomatic patients, all with an endometrial thickness of greater than 10 mm, found no prognostic advantage to screening versus waiting until bleeding occurred, he noted (Euro J Cancer. 2001;37:64-71).
Overall, 13% of the patients had cancer, 50% had polyps, and 17% had hyperplasia.
“But what they emphasized was ... the length [of time] the patients complained of abnormal uterine bleeding. If it was less than 8 weeks ... there was no statistical difference in outcome, but if it was over 8 weeks there was a statistically significant difference in the grade of disease – a prognostic advantage for those patients who were screened versus symptomatic.”
The overall 5-year disease-free survival was 86% for asymptomatic versus 77% for symptomatic patients; for those with bleeding for less than 8 weeks, it was 98% versus 83%, respectively. The differences were not statistically different. However, for those with bleeding for 8-16 weeks it was 90% versus 74%, and for those with bleeding for more than 16 weeks it was 69% versus 62%, respectively, and those differences were statistically significant.
The problem is that many patients put off coming in for a long time, which means they are in a category with a worse prognosis when they do come in, Dr. Shwayder said. That’s not to say everyone should be screened, but there is no prognostic advantage to screening asymptomatic patients versus symptomatic patients who had bleeding for less than 8 weeks.
“It’s a little clarification, but I think an important one,” he noted.
Another study of 1,607 patients with endometrial thickening, including 233 who were asymptomatic and 1,374 who were symptomatic, found a lower rate of deep invasion with stage 1 disease, but no difference in the rate of more advanced disease, and no association with a more favorable outcome between the groups. (Am J Obstet Gynecol. 2018;219[2]:183e1-6).
Additionally, a study of 42 asymptomatic patients, 95 symptomatic patients with bleeding for less than 3 months, and 83 symptomatic patients with bleeding for more than 3 months showed a nonsignificant trend toward poorer 5-year survival in patients with a longer history of bleeding prior to surgery (Arch Gynecol Obstet 2013;288:1361-4).
“So now the question becomes how thick is too thick [and whether there is] some threshold where we ought to be evaluating patients and some threshold where we’re not,” he said.
The risk of malignancy among symptomatic postmenopausal women with an endometrial thickness greater than 5 mm is 7.3%, and the risk is similar at 6.7% in asymptomatic patients with an endometrial thickness of 11 mm or greater, according to a 2004 study by Smith-Bindman et al. (Ultrasound Obstet Gynecol. 2004;24:558-65).
“So the thought process here is that if a patient is asymptomatic, but the endometrium is over 11 mm, maybe we ought to evaluate that patient, because her risk of cancer is equivalent to that of someone who presents with postmenopausal bleeding and has an endometrium greater than 5 mm,” he explained.
In fact, a practice guideline from the Society of Obstetricians and Gynecologists of Canada recommends that women with endometrial thickness over 11 mm and other risk factors for cancer – such as obesity, hypertension, or late menopause – should be referred to a gynecologist for investigation, Dr. Shwayder said, adding that he also considers increased vascularity, heterogeneity in the endometrium, and fluid seen on a scan as cause for further evaluation.
“But endometrial sampling without bleeding should not be routinely performed,” he said. “So don’t routinely [sample] but based on risk factors and ultrasound findings, you may want to consider evaluating these patients further.”
Dr. Shwayder is a consultant for GE Ultrasound.
NASHVILLE, TENN. – Ultrasound is a reasonable first step for the evaluation of postmenopausal women with abnormal uterine bleeding and endometrial thickness up to 5 mm, according to James Shwayder, MD, JD.
About a third of such patients presenting with abnormal uterine bleeding (AUB) will have an endometrial polyp or submucous myoma, Dr. Shwayder explained during a presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
However, recurrent bleeding warrants further work-up, he said.
The 5-mm value is based on a number of studies, which, in sum, show that the negative predictive value for cancer for endometrial thickness less than 4 mm is greater than 99%, said Dr. Shwayder, chief of the division of gynecology and director of the fellowship in advanced endoscopy obstetrics, gynecology, and women’s health at the University of Louisville (Ky.).
“In fact ... the risk of cancer is 1 in 917,” he added. “That’s pretty good ... that’s a great screening tool.”
The question is whether one can feel comfortable foregoing biopsy in such cases, he said, describing a 61-year-old patient with a 3.9-mm endometrium and AUB for 3 days, 3 weeks prior to her visit.
There are two possibilities: The patient will either have no further bleeding, or she will continue to bleed, he said, noting that a 2003 Swedish study provides some guidance in the case of continued bleeding (Am J Obstet Gynecol. 2003;188:401-8).
The investigators initially looked at histology associated with endometrial thickness in 394 postmenopausal women referred for AUB between 1987 and 1990. Both transvaginal ultrasound and dilatation and curettage were performed, and the findings were correlated.
“But they had the rare opportunity to take patients who had benign evaluations and bring them back 10 years later,” Dr. Shwayder said. “What they found was that, regardless of the endometrial thickness, if it was benign initially and they did not bleed over that 10-year period, no one had cancer.”
However, among the patients followed for 10 years who had recurrent bleeding, 10% had cancer and 12% had hyperplasia. Thus, deciding against a biopsy in this case is supported by good data; if the patient doesn’t bleed, she has an “incredibly low risk of cancer,” he said.
“If they bleed again, you’ve gotta work ‘em up,” he stressed. “Don’t continue to say, ‘Well, let me repeat the ultrasound and see if it’s thinner or thicker.’ No. They need to be evaluated.”
Keep in mind that if a biopsy is performed as the first step, the chances of the results coming back as tissue insufficient for diagnosis (TIFD) are increased, and a repeat biopsy will be necessary because of the inconclusive findings, Dr. Shwayder said. It helps to warn a patient in advance that their thin endometrium makes it highly likely that a repeat biopsy will be necessary, as 90% come back as TIFD or atrophy.
Importantly, though, ACOG says endometrial sampling should be performed first line in patients over age 45 years with AUB.
“I’ll be honest – I use ultrasound in these patients because of the fact that a third will have some sort of structural defect, and the focal abnormalities are things we’re not going to be able to pick up with a straight biopsy, but we have to be cognizant that the college recommends biopsy in this population,” Dr. Shwayder said.
Asymptomatic endometrial thickening
Postmenopausal women are increasingly being referred for asymptomatic endometrial thickening that is found incidentally during an unrelated evaluation, Dr. Shwayder said, noting that evidence to date on how to approach such cases is conflicting.
Although ACOG is working on a recommendation, none is currently available, he said.
However, a 2001 study comparing 123 asymptomatic patients with 90 symptomatic patients, all with an endometrial thickness of greater than 10 mm, found no prognostic advantage to screening versus waiting until bleeding occurred, he noted (Euro J Cancer. 2001;37:64-71).
Overall, 13% of the patients had cancer, 50% had polyps, and 17% had hyperplasia.
“But what they emphasized was ... the length [of time] the patients complained of abnormal uterine bleeding. If it was less than 8 weeks ... there was no statistical difference in outcome, but if it was over 8 weeks there was a statistically significant difference in the grade of disease – a prognostic advantage for those patients who were screened versus symptomatic.”
The overall 5-year disease-free survival was 86% for asymptomatic versus 77% for symptomatic patients; for those with bleeding for less than 8 weeks, it was 98% versus 83%, respectively. The differences were not statistically different. However, for those with bleeding for 8-16 weeks it was 90% versus 74%, and for those with bleeding for more than 16 weeks it was 69% versus 62%, respectively, and those differences were statistically significant.
The problem is that many patients put off coming in for a long time, which means they are in a category with a worse prognosis when they do come in, Dr. Shwayder said. That’s not to say everyone should be screened, but there is no prognostic advantage to screening asymptomatic patients versus symptomatic patients who had bleeding for less than 8 weeks.
“It’s a little clarification, but I think an important one,” he noted.
Another study of 1,607 patients with endometrial thickening, including 233 who were asymptomatic and 1,374 who were symptomatic, found a lower rate of deep invasion with stage 1 disease, but no difference in the rate of more advanced disease, and no association with a more favorable outcome between the groups. (Am J Obstet Gynecol. 2018;219[2]:183e1-6).
Additionally, a study of 42 asymptomatic patients, 95 symptomatic patients with bleeding for less than 3 months, and 83 symptomatic patients with bleeding for more than 3 months showed a nonsignificant trend toward poorer 5-year survival in patients with a longer history of bleeding prior to surgery (Arch Gynecol Obstet 2013;288:1361-4).
“So now the question becomes how thick is too thick [and whether there is] some threshold where we ought to be evaluating patients and some threshold where we’re not,” he said.
The risk of malignancy among symptomatic postmenopausal women with an endometrial thickness greater than 5 mm is 7.3%, and the risk is similar at 6.7% in asymptomatic patients with an endometrial thickness of 11 mm or greater, according to a 2004 study by Smith-Bindman et al. (Ultrasound Obstet Gynecol. 2004;24:558-65).
“So the thought process here is that if a patient is asymptomatic, but the endometrium is over 11 mm, maybe we ought to evaluate that patient, because her risk of cancer is equivalent to that of someone who presents with postmenopausal bleeding and has an endometrium greater than 5 mm,” he explained.
In fact, a practice guideline from the Society of Obstetricians and Gynecologists of Canada recommends that women with endometrial thickness over 11 mm and other risk factors for cancer – such as obesity, hypertension, or late menopause – should be referred to a gynecologist for investigation, Dr. Shwayder said, adding that he also considers increased vascularity, heterogeneity in the endometrium, and fluid seen on a scan as cause for further evaluation.
“But endometrial sampling without bleeding should not be routinely performed,” he said. “So don’t routinely [sample] but based on risk factors and ultrasound findings, you may want to consider evaluating these patients further.”
Dr. Shwayder is a consultant for GE Ultrasound.
NASHVILLE, TENN. – Ultrasound is a reasonable first step for the evaluation of postmenopausal women with abnormal uterine bleeding and endometrial thickness up to 5 mm, according to James Shwayder, MD, JD.
About a third of such patients presenting with abnormal uterine bleeding (AUB) will have an endometrial polyp or submucous myoma, Dr. Shwayder explained during a presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
However, recurrent bleeding warrants further work-up, he said.
The 5-mm value is based on a number of studies, which, in sum, show that the negative predictive value for cancer for endometrial thickness less than 4 mm is greater than 99%, said Dr. Shwayder, chief of the division of gynecology and director of the fellowship in advanced endoscopy obstetrics, gynecology, and women’s health at the University of Louisville (Ky.).
“In fact ... the risk of cancer is 1 in 917,” he added. “That’s pretty good ... that’s a great screening tool.”
The question is whether one can feel comfortable foregoing biopsy in such cases, he said, describing a 61-year-old patient with a 3.9-mm endometrium and AUB for 3 days, 3 weeks prior to her visit.
There are two possibilities: The patient will either have no further bleeding, or she will continue to bleed, he said, noting that a 2003 Swedish study provides some guidance in the case of continued bleeding (Am J Obstet Gynecol. 2003;188:401-8).
The investigators initially looked at histology associated with endometrial thickness in 394 postmenopausal women referred for AUB between 1987 and 1990. Both transvaginal ultrasound and dilatation and curettage were performed, and the findings were correlated.
“But they had the rare opportunity to take patients who had benign evaluations and bring them back 10 years later,” Dr. Shwayder said. “What they found was that, regardless of the endometrial thickness, if it was benign initially and they did not bleed over that 10-year period, no one had cancer.”
However, among the patients followed for 10 years who had recurrent bleeding, 10% had cancer and 12% had hyperplasia. Thus, deciding against a biopsy in this case is supported by good data; if the patient doesn’t bleed, she has an “incredibly low risk of cancer,” he said.
“If they bleed again, you’ve gotta work ‘em up,” he stressed. “Don’t continue to say, ‘Well, let me repeat the ultrasound and see if it’s thinner or thicker.’ No. They need to be evaluated.”
Keep in mind that if a biopsy is performed as the first step, the chances of the results coming back as tissue insufficient for diagnosis (TIFD) are increased, and a repeat biopsy will be necessary because of the inconclusive findings, Dr. Shwayder said. It helps to warn a patient in advance that their thin endometrium makes it highly likely that a repeat biopsy will be necessary, as 90% come back as TIFD or atrophy.
Importantly, though, ACOG says endometrial sampling should be performed first line in patients over age 45 years with AUB.
“I’ll be honest – I use ultrasound in these patients because of the fact that a third will have some sort of structural defect, and the focal abnormalities are things we’re not going to be able to pick up with a straight biopsy, but we have to be cognizant that the college recommends biopsy in this population,” Dr. Shwayder said.
Asymptomatic endometrial thickening
Postmenopausal women are increasingly being referred for asymptomatic endometrial thickening that is found incidentally during an unrelated evaluation, Dr. Shwayder said, noting that evidence to date on how to approach such cases is conflicting.
Although ACOG is working on a recommendation, none is currently available, he said.
However, a 2001 study comparing 123 asymptomatic patients with 90 symptomatic patients, all with an endometrial thickness of greater than 10 mm, found no prognostic advantage to screening versus waiting until bleeding occurred, he noted (Euro J Cancer. 2001;37:64-71).
Overall, 13% of the patients had cancer, 50% had polyps, and 17% had hyperplasia.
“But what they emphasized was ... the length [of time] the patients complained of abnormal uterine bleeding. If it was less than 8 weeks ... there was no statistical difference in outcome, but if it was over 8 weeks there was a statistically significant difference in the grade of disease – a prognostic advantage for those patients who were screened versus symptomatic.”
The overall 5-year disease-free survival was 86% for asymptomatic versus 77% for symptomatic patients; for those with bleeding for less than 8 weeks, it was 98% versus 83%, respectively. The differences were not statistically different. However, for those with bleeding for 8-16 weeks it was 90% versus 74%, and for those with bleeding for more than 16 weeks it was 69% versus 62%, respectively, and those differences were statistically significant.
The problem is that many patients put off coming in for a long time, which means they are in a category with a worse prognosis when they do come in, Dr. Shwayder said. That’s not to say everyone should be screened, but there is no prognostic advantage to screening asymptomatic patients versus symptomatic patients who had bleeding for less than 8 weeks.
“It’s a little clarification, but I think an important one,” he noted.
Another study of 1,607 patients with endometrial thickening, including 233 who were asymptomatic and 1,374 who were symptomatic, found a lower rate of deep invasion with stage 1 disease, but no difference in the rate of more advanced disease, and no association with a more favorable outcome between the groups. (Am J Obstet Gynecol. 2018;219[2]:183e1-6).
Additionally, a study of 42 asymptomatic patients, 95 symptomatic patients with bleeding for less than 3 months, and 83 symptomatic patients with bleeding for more than 3 months showed a nonsignificant trend toward poorer 5-year survival in patients with a longer history of bleeding prior to surgery (Arch Gynecol Obstet 2013;288:1361-4).
“So now the question becomes how thick is too thick [and whether there is] some threshold where we ought to be evaluating patients and some threshold where we’re not,” he said.
The risk of malignancy among symptomatic postmenopausal women with an endometrial thickness greater than 5 mm is 7.3%, and the risk is similar at 6.7% in asymptomatic patients with an endometrial thickness of 11 mm or greater, according to a 2004 study by Smith-Bindman et al. (Ultrasound Obstet Gynecol. 2004;24:558-65).
“So the thought process here is that if a patient is asymptomatic, but the endometrium is over 11 mm, maybe we ought to evaluate that patient, because her risk of cancer is equivalent to that of someone who presents with postmenopausal bleeding and has an endometrium greater than 5 mm,” he explained.
In fact, a practice guideline from the Society of Obstetricians and Gynecologists of Canada recommends that women with endometrial thickness over 11 mm and other risk factors for cancer – such as obesity, hypertension, or late menopause – should be referred to a gynecologist for investigation, Dr. Shwayder said, adding that he also considers increased vascularity, heterogeneity in the endometrium, and fluid seen on a scan as cause for further evaluation.
“But endometrial sampling without bleeding should not be routinely performed,” he said. “So don’t routinely [sample] but based on risk factors and ultrasound findings, you may want to consider evaluating these patients further.”
Dr. Shwayder is a consultant for GE Ultrasound.
EXPERT ANALYSIS FROM ACOG 2019
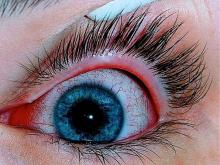
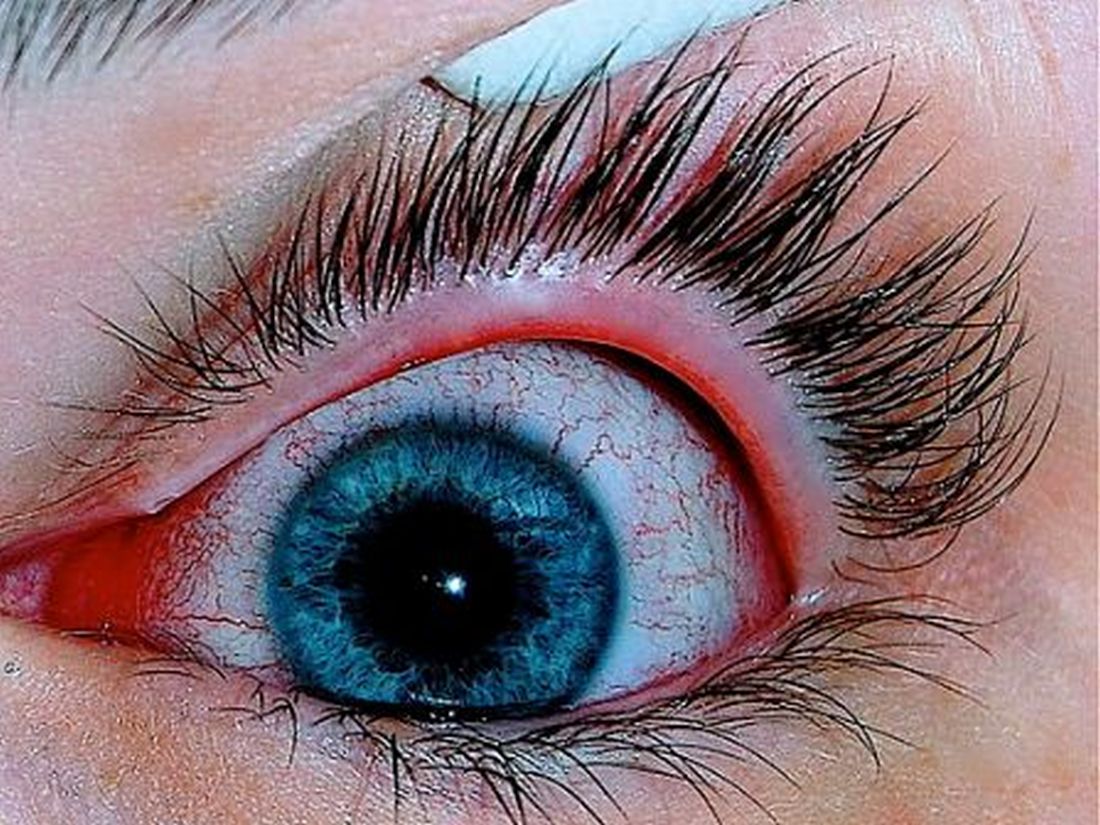
Patients with AD should routinely be asked about conjunctivitis
according to a recent position statement from the International Eczema Council.
Patients with AD who develop conjunctivitis during dupilumab treatment may experience “bilateral inflammation of the anterior conjunctiva and hyperaemia of the limbus, which may cause nodular swelling.”according to the statement, which pertains to conjunctivitis in AD patients, “with and without dupilumab therapy.” (J Eur Acad Dermatol Venereol. 2019 May 6. doi: 10.1111/jdv.15608). Currently, there are no predictive factors of conjunctivitis and no guidance in the literature on how to manage conjunctivitis associated with dupilumab, which in some cases can make it necessary to stop treatment, the authors wrote.
The International Eczema Council (IEC) polled 86 dermatologists in 22 countries who are experts in AD; 46 members responded from 19 countries, including dermatologists from Australia, Canada, Denmark, France, Germany, Japan, Korea, the Netherlands, the United Kingdom, and the United States. The questions centered on the diagnosis and management of conjunctivitis in AD patients, and whether to refer cases to an ophthalmologist. Consensus was achieved if less than 30% of participants disagreed with a statement. IEC members then met in person at a European Academy of Dermatology and Venereology meeting in Paris to discuss the results of the survey. Survey respondents noted they had seen dupilumab-associated conjunctivitis in both pediatric and adult patients.
The IEC members recommended that:
- Patients should be informed about the risks of conjunctivitis before being prescribed dupilumab.
- AD patients should be asked “routinely” about ocular complaints or symptoms.
- AD patients with conjunctivitis should be referred to an ophthalmologist for diagnosis and treatment.
- AD patients with new-onset conjunctivitis during dupilumab treatment always should be referred to an ophthalmologist, especially in more severe cases such as when they do not respond to treatment with antihistamine or artificial tears.
- Dermatologists also should rule out keratoconjunctivitis before treating with dupilumab, as it may cause keratitis and blindness.
- Patients who have had keratoconjunctivitis in the past should not be precluded from treatment with dupilumab, and those who develop conjunctivitis during treatment should be referred to an ophthalmologist – but should stay on treatment while waiting for the consult.
“It was stressed that at this moment there are also no reliable data on the course of atopic keratoconjunctivitis and vernal keratoconjunctivitis during dupilumab treatment,” according to Jacob P. Thyssen, MD, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and coauthors. These patients “should be carefully monitored by an ophthalmologist before and during treatment with dupilumab.”
The recommendations also centered around which treatments should be initiated by dermatologists, and which should be referred to ophthalmologists. Those patients with conjunctivitis should receive eye drops, eye ointment, or oral antihistamines from their dermatologists before an ophthalmology referral, the IEC members said. Dermatologists also should perform, or refer patients for, skin prick testing or specific IgE testing for aeroallergens in patients with AD who have conjunctivitis, and patch testing with an “ophthalmologic series, and native eye drops/ointments to diagnose possible delayed type hypersensitivity reactions to topical ingredients,” they added.
Among the treatments for conjunctivitis best left to ophthalmologists are cyclosporine, tacrolimus, or corticosteroid eye drops.
“Despite the more limited experience with eye drops by dermatologists, rapid access to ophthalmological service may be difficult, sometimes warranting a short course of corticosteroid eye drops without ophthalmological consultations,” Dr. Thyssen and associates said. “However, persistent or recurrent conjunctivitis requiring repeated or prolonged use of corticosteroid, tacrolimus, and ciclosporin-containing eye drops, must be managed by an ophthalmologist, given the risk of glaucoma, cataract, and infections.”
“The AD severity, conjunctivitis severity, possible contraindications, possible effect of dupilumab therapy on concomitant asthma or other comorbidities, as well as other treatment options, should be considered on an individual patient basis,” the authors concluded.
The IEC survey was limited by the small survey response and reliance on expert opinion.
The authors reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speakers bureau positions, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. No funding was obtained for the study.
This article was updated 7/17/19.
according to a recent position statement from the International Eczema Council.
Patients with AD who develop conjunctivitis during dupilumab treatment may experience “bilateral inflammation of the anterior conjunctiva and hyperaemia of the limbus, which may cause nodular swelling.”according to the statement, which pertains to conjunctivitis in AD patients, “with and without dupilumab therapy.” (J Eur Acad Dermatol Venereol. 2019 May 6. doi: 10.1111/jdv.15608). Currently, there are no predictive factors of conjunctivitis and no guidance in the literature on how to manage conjunctivitis associated with dupilumab, which in some cases can make it necessary to stop treatment, the authors wrote.
The International Eczema Council (IEC) polled 86 dermatologists in 22 countries who are experts in AD; 46 members responded from 19 countries, including dermatologists from Australia, Canada, Denmark, France, Germany, Japan, Korea, the Netherlands, the United Kingdom, and the United States. The questions centered on the diagnosis and management of conjunctivitis in AD patients, and whether to refer cases to an ophthalmologist. Consensus was achieved if less than 30% of participants disagreed with a statement. IEC members then met in person at a European Academy of Dermatology and Venereology meeting in Paris to discuss the results of the survey. Survey respondents noted they had seen dupilumab-associated conjunctivitis in both pediatric and adult patients.
The IEC members recommended that:
- Patients should be informed about the risks of conjunctivitis before being prescribed dupilumab.
- AD patients should be asked “routinely” about ocular complaints or symptoms.
- AD patients with conjunctivitis should be referred to an ophthalmologist for diagnosis and treatment.
- AD patients with new-onset conjunctivitis during dupilumab treatment always should be referred to an ophthalmologist, especially in more severe cases such as when they do not respond to treatment with antihistamine or artificial tears.
- Dermatologists also should rule out keratoconjunctivitis before treating with dupilumab, as it may cause keratitis and blindness.
- Patients who have had keratoconjunctivitis in the past should not be precluded from treatment with dupilumab, and those who develop conjunctivitis during treatment should be referred to an ophthalmologist – but should stay on treatment while waiting for the consult.
“It was stressed that at this moment there are also no reliable data on the course of atopic keratoconjunctivitis and vernal keratoconjunctivitis during dupilumab treatment,” according to Jacob P. Thyssen, MD, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and coauthors. These patients “should be carefully monitored by an ophthalmologist before and during treatment with dupilumab.”
The recommendations also centered around which treatments should be initiated by dermatologists, and which should be referred to ophthalmologists. Those patients with conjunctivitis should receive eye drops, eye ointment, or oral antihistamines from their dermatologists before an ophthalmology referral, the IEC members said. Dermatologists also should perform, or refer patients for, skin prick testing or specific IgE testing for aeroallergens in patients with AD who have conjunctivitis, and patch testing with an “ophthalmologic series, and native eye drops/ointments to diagnose possible delayed type hypersensitivity reactions to topical ingredients,” they added.
Among the treatments for conjunctivitis best left to ophthalmologists are cyclosporine, tacrolimus, or corticosteroid eye drops.
“Despite the more limited experience with eye drops by dermatologists, rapid access to ophthalmological service may be difficult, sometimes warranting a short course of corticosteroid eye drops without ophthalmological consultations,” Dr. Thyssen and associates said. “However, persistent or recurrent conjunctivitis requiring repeated or prolonged use of corticosteroid, tacrolimus, and ciclosporin-containing eye drops, must be managed by an ophthalmologist, given the risk of glaucoma, cataract, and infections.”
“The AD severity, conjunctivitis severity, possible contraindications, possible effect of dupilumab therapy on concomitant asthma or other comorbidities, as well as other treatment options, should be considered on an individual patient basis,” the authors concluded.
The IEC survey was limited by the small survey response and reliance on expert opinion.
The authors reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speakers bureau positions, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. No funding was obtained for the study.
This article was updated 7/17/19.
according to a recent position statement from the International Eczema Council.
Patients with AD who develop conjunctivitis during dupilumab treatment may experience “bilateral inflammation of the anterior conjunctiva and hyperaemia of the limbus, which may cause nodular swelling.”according to the statement, which pertains to conjunctivitis in AD patients, “with and without dupilumab therapy.” (J Eur Acad Dermatol Venereol. 2019 May 6. doi: 10.1111/jdv.15608). Currently, there are no predictive factors of conjunctivitis and no guidance in the literature on how to manage conjunctivitis associated with dupilumab, which in some cases can make it necessary to stop treatment, the authors wrote.
The International Eczema Council (IEC) polled 86 dermatologists in 22 countries who are experts in AD; 46 members responded from 19 countries, including dermatologists from Australia, Canada, Denmark, France, Germany, Japan, Korea, the Netherlands, the United Kingdom, and the United States. The questions centered on the diagnosis and management of conjunctivitis in AD patients, and whether to refer cases to an ophthalmologist. Consensus was achieved if less than 30% of participants disagreed with a statement. IEC members then met in person at a European Academy of Dermatology and Venereology meeting in Paris to discuss the results of the survey. Survey respondents noted they had seen dupilumab-associated conjunctivitis in both pediatric and adult patients.
The IEC members recommended that:
- Patients should be informed about the risks of conjunctivitis before being prescribed dupilumab.
- AD patients should be asked “routinely” about ocular complaints or symptoms.
- AD patients with conjunctivitis should be referred to an ophthalmologist for diagnosis and treatment.
- AD patients with new-onset conjunctivitis during dupilumab treatment always should be referred to an ophthalmologist, especially in more severe cases such as when they do not respond to treatment with antihistamine or artificial tears.
- Dermatologists also should rule out keratoconjunctivitis before treating with dupilumab, as it may cause keratitis and blindness.
- Patients who have had keratoconjunctivitis in the past should not be precluded from treatment with dupilumab, and those who develop conjunctivitis during treatment should be referred to an ophthalmologist – but should stay on treatment while waiting for the consult.
“It was stressed that at this moment there are also no reliable data on the course of atopic keratoconjunctivitis and vernal keratoconjunctivitis during dupilumab treatment,” according to Jacob P. Thyssen, MD, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and coauthors. These patients “should be carefully monitored by an ophthalmologist before and during treatment with dupilumab.”
The recommendations also centered around which treatments should be initiated by dermatologists, and which should be referred to ophthalmologists. Those patients with conjunctivitis should receive eye drops, eye ointment, or oral antihistamines from their dermatologists before an ophthalmology referral, the IEC members said. Dermatologists also should perform, or refer patients for, skin prick testing or specific IgE testing for aeroallergens in patients with AD who have conjunctivitis, and patch testing with an “ophthalmologic series, and native eye drops/ointments to diagnose possible delayed type hypersensitivity reactions to topical ingredients,” they added.
Among the treatments for conjunctivitis best left to ophthalmologists are cyclosporine, tacrolimus, or corticosteroid eye drops.
“Despite the more limited experience with eye drops by dermatologists, rapid access to ophthalmological service may be difficult, sometimes warranting a short course of corticosteroid eye drops without ophthalmological consultations,” Dr. Thyssen and associates said. “However, persistent or recurrent conjunctivitis requiring repeated or prolonged use of corticosteroid, tacrolimus, and ciclosporin-containing eye drops, must be managed by an ophthalmologist, given the risk of glaucoma, cataract, and infections.”
“The AD severity, conjunctivitis severity, possible contraindications, possible effect of dupilumab therapy on concomitant asthma or other comorbidities, as well as other treatment options, should be considered on an individual patient basis,” the authors concluded.
The IEC survey was limited by the small survey response and reliance on expert opinion.
The authors reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speakers bureau positions, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. No funding was obtained for the study.
This article was updated 7/17/19.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Smartphone-based system rivals clinical assessments of anxiety in bipolar disorder
In patients with bipolar disorder currently in partial or full remission, self-reporting of anxiety to a smartphone-based system matched clinical evaluations of anxiety, according to Maria Faurholt-Jepsen, MD, and her associates.
A total of 84 patients with bipolar disorder who participated in the randomized, controlled, single-blind, parallel-group MONARCA II trial were included in the study, reported Dr. Faurholt-Jepsen of Rigshospitalet in Copenhagen, and her associates. All patients reported their anxiety to the smartphone-based system every day for a 9-month period; all patients underwent clinical evaluations of anxiety, functioning, patient-reported stress, and quality of life at five fixed time points over the study period. The study was published online by the Journal of Affective Disorders.
Self-reported anxiety was mild, with 19.3% of patients reporting anxiety during the study period, and 2.6% reporting severe anxiety. No association was seen between gender and anxiety days, or between bipolar disorder type and anxiety days. Patients reported depressed mood on 43.2% of the days when anxiety was also reported, and reported mania on 48.0% of the days when anxiety was reported.
Self-reported anxiety scores were positively associated with the anxiety subitems on a key rating scale (P = .0001). In addition, self-reported anxiety was associated with perceived stress, quality of life, and functioning (P = .0001 for all three).
“ Frequent fine-grained monitoring in clinical, high-risk and epidemiological populations provides an opportunity to gain a better understanding of the nature, correlates, and clinical implications of [bipolar disorder],” the investigators wrote.
Three coauthors reported consulting with Eli Lilly, Astra Zeneca, Servier, Bristol-Myers Squibb, Lundbeck, Sunovion, and Medilink. Two coauthors are cofounders and shareholders in Monsenso.
SOURCE: Faurholt-Jepsen M et al. J Affect Disord. 2019. doi: 10.1016/j.jad.2019.07.029.
In patients with bipolar disorder currently in partial or full remission, self-reporting of anxiety to a smartphone-based system matched clinical evaluations of anxiety, according to Maria Faurholt-Jepsen, MD, and her associates.
A total of 84 patients with bipolar disorder who participated in the randomized, controlled, single-blind, parallel-group MONARCA II trial were included in the study, reported Dr. Faurholt-Jepsen of Rigshospitalet in Copenhagen, and her associates. All patients reported their anxiety to the smartphone-based system every day for a 9-month period; all patients underwent clinical evaluations of anxiety, functioning, patient-reported stress, and quality of life at five fixed time points over the study period. The study was published online by the Journal of Affective Disorders.
Self-reported anxiety was mild, with 19.3% of patients reporting anxiety during the study period, and 2.6% reporting severe anxiety. No association was seen between gender and anxiety days, or between bipolar disorder type and anxiety days. Patients reported depressed mood on 43.2% of the days when anxiety was also reported, and reported mania on 48.0% of the days when anxiety was reported.
Self-reported anxiety scores were positively associated with the anxiety subitems on a key rating scale (P = .0001). In addition, self-reported anxiety was associated with perceived stress, quality of life, and functioning (P = .0001 for all three).
“ Frequent fine-grained monitoring in clinical, high-risk and epidemiological populations provides an opportunity to gain a better understanding of the nature, correlates, and clinical implications of [bipolar disorder],” the investigators wrote.
Three coauthors reported consulting with Eli Lilly, Astra Zeneca, Servier, Bristol-Myers Squibb, Lundbeck, Sunovion, and Medilink. Two coauthors are cofounders and shareholders in Monsenso.
SOURCE: Faurholt-Jepsen M et al. J Affect Disord. 2019. doi: 10.1016/j.jad.2019.07.029.
In patients with bipolar disorder currently in partial or full remission, self-reporting of anxiety to a smartphone-based system matched clinical evaluations of anxiety, according to Maria Faurholt-Jepsen, MD, and her associates.
A total of 84 patients with bipolar disorder who participated in the randomized, controlled, single-blind, parallel-group MONARCA II trial were included in the study, reported Dr. Faurholt-Jepsen of Rigshospitalet in Copenhagen, and her associates. All patients reported their anxiety to the smartphone-based system every day for a 9-month period; all patients underwent clinical evaluations of anxiety, functioning, patient-reported stress, and quality of life at five fixed time points over the study period. The study was published online by the Journal of Affective Disorders.
Self-reported anxiety was mild, with 19.3% of patients reporting anxiety during the study period, and 2.6% reporting severe anxiety. No association was seen between gender and anxiety days, or between bipolar disorder type and anxiety days. Patients reported depressed mood on 43.2% of the days when anxiety was also reported, and reported mania on 48.0% of the days when anxiety was reported.
Self-reported anxiety scores were positively associated with the anxiety subitems on a key rating scale (P = .0001). In addition, self-reported anxiety was associated with perceived stress, quality of life, and functioning (P = .0001 for all three).
“ Frequent fine-grained monitoring in clinical, high-risk and epidemiological populations provides an opportunity to gain a better understanding of the nature, correlates, and clinical implications of [bipolar disorder],” the investigators wrote.
Three coauthors reported consulting with Eli Lilly, Astra Zeneca, Servier, Bristol-Myers Squibb, Lundbeck, Sunovion, and Medilink. Two coauthors are cofounders and shareholders in Monsenso.
SOURCE: Faurholt-Jepsen M et al. J Affect Disord. 2019. doi: 10.1016/j.jad.2019.07.029.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Nerve transfer improves function after spinal cord injury
according to research published online July 4 ahead of print in the Lancet. Combining nerve transfer with tendon transfer may maximize the functional benefit of surgery.
The loss of upper extremity function after cervical spinal cord injury can reduce independence and social and vocational engagement. People with tetraplegia rank improvement in hand function as their most important goal. Tendon transfers have been the traditional method of restoring function, but interest in nerve transfers has been increasing with the publication of successful results. Nerve transfers can reanimate several muscles at once and require a smaller incision and shorter immobilization, compared with tendon transfers.
Injury had occurred less than 18 months previously
Natasha van Zyl, MBBS, a plastic and reconstructive surgeon at Austin Health in Melbourne, and colleagues conducted a prospective case series to examine the clinical and functional outcomes of nerve transfer surgery for the reanimation of upper limb function in patients with tetraplegia. The investigators also sought to compare these outcomes with published outcomes for tendon transfer surgery.
Between April 14, 2014, and Nov. 22, 2018, Dr. van Zyl and colleagues recruited consecutive patients of any age with early cervical spinal cord injury of motor level C5 and below. Injury was required to have occurred fewer than 18 months before enrollment. Eligible participants had been referred to a single center for upper extremity reanimation and were considered candidates for nerve transfer.
Every participant underwent single or multiple nerve transfers in one or both upper limbs, and some participants also underwent tendon transfers. The goal of surgery was the restoration of elbow extension, grasp, pinch, and hand opening. An independent assessor evaluated participants at baseline and at 12 months and 24 months after surgery. The primary outcome measures were the action research arm test (ARAT), the grasp release test (GRT), and the spinal cord independence measure (SCIM).
Grasp function improved significantly
Dr. van Zyl and colleagues recruited 16 participants with traumatic spinal cord injury who underwent 59 nerve transfers. Ten participants also underwent tendon transfers. The population’s mean age at time of injury was 27.3 years. Three patients were female. Motor vehicle accidents were the most common cause of injury (31%). Follow-up data at 24 months were unavailable for three patients.
Participants’ median ARAT total score significantly improved from 16.5 at baseline to 34.0 at 24 months. Median GRT total score significantly improved from 35.0 at baseline to 125.2 at 24 months. The population’s mean total SCIM score and mobility in the room and toilet SCIM score improved by more than the minimal detectable change and the minimal clinically important difference. The mean self-care SCIM score improved by more than the minimal detectable change between baseline and 24 months.
The researchers observed six adverse events related to the surgery, but none had sustained functional consequences. No patients had an increase in musculoskeletal or neuropathic pain. Four of the 50 nerve transfers with 24-month follow-up failed.
A novel technique
“This project is the first to comprehensively examine outcomes for early, multiple nerve transfer surgery in the upper limbs of people with tetraplegia following traumatic spinal cord injury and is the largest prospective series of nerve transfers reported in this population to date,” said Dr. van Zyl and colleagues. Study limitations included the small sample size, the high variability of spinal cord injury patterns, and the potential for the multiple procedures that each participant underwent to confound data analysis.
Future research could explore whether nerve transfers are beneficial at more than 24 months after spinal cord injury, wrote the authors. In addition, it is unclear whether function and strength continue to improve beyond 24 months after surgery.
The study was funded by the Institute for Safety, Compensation, and Recovery Research in Australia. The authors had no competing interests.
SOURCE: van Zyl N et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31143-2.
The data from van Zyl et al. suggest that nerve transfers restore more natural movement and finer motor control than tendon transfers do, said Elspeth J.R. Hill, MD, PhD, and Ida K. Fox, MD, plastic and reconstructive surgeons at Washington University in St. Louis, in an accompanying editorial. Patients can engage in light activity immediately after surgery, and cortical plasticity enables function to improve over time. Two disadvantages of nerve transfers, however, are that it takes months before new motion can be observed, and years before full strength can be regained.
The heterogeneity of cervical spinal cord injury requires an individualized approach to surgical assessment and management, they continued. Physicians and patients should make treatment decisions collaboratively. “We envisage a role for nerve transfers in settings where the intensive therapy and immobilization required to optimize complementary tendon transfers are unavailable,” wrote Dr. Hill and Dr. Fox.
Continuing research will be necessary to improve surgical technique and outcomes. “This research should include efforts to compare nerve transfer with tendon transfer, find the optimal timing of such surgeries, and determine which approach produces the greatest functional improvement,” they wrote. “Detailed study of the reasons for nerve transfer failure is also required, as is improving our understanding of the effects of biopsychosocial factors, including access to information and care, psychological readiness, and social support, on patient decision making and outcomes.”
Nerve transfers are a “huge advance” in the restoration of function after spinal cord injury, the authors added. “Surgeons who integrate nerve transfers into their spinal cord injury practice should take a careful and measured approach and rigorously study and disseminate their outcomes to advance this growing field,” they concluded.
The data from van Zyl et al. suggest that nerve transfers restore more natural movement and finer motor control than tendon transfers do, said Elspeth J.R. Hill, MD, PhD, and Ida K. Fox, MD, plastic and reconstructive surgeons at Washington University in St. Louis, in an accompanying editorial. Patients can engage in light activity immediately after surgery, and cortical plasticity enables function to improve over time. Two disadvantages of nerve transfers, however, are that it takes months before new motion can be observed, and years before full strength can be regained.
The heterogeneity of cervical spinal cord injury requires an individualized approach to surgical assessment and management, they continued. Physicians and patients should make treatment decisions collaboratively. “We envisage a role for nerve transfers in settings where the intensive therapy and immobilization required to optimize complementary tendon transfers are unavailable,” wrote Dr. Hill and Dr. Fox.
Continuing research will be necessary to improve surgical technique and outcomes. “This research should include efforts to compare nerve transfer with tendon transfer, find the optimal timing of such surgeries, and determine which approach produces the greatest functional improvement,” they wrote. “Detailed study of the reasons for nerve transfer failure is also required, as is improving our understanding of the effects of biopsychosocial factors, including access to information and care, psychological readiness, and social support, on patient decision making and outcomes.”
Nerve transfers are a “huge advance” in the restoration of function after spinal cord injury, the authors added. “Surgeons who integrate nerve transfers into their spinal cord injury practice should take a careful and measured approach and rigorously study and disseminate their outcomes to advance this growing field,” they concluded.
The data from van Zyl et al. suggest that nerve transfers restore more natural movement and finer motor control than tendon transfers do, said Elspeth J.R. Hill, MD, PhD, and Ida K. Fox, MD, plastic and reconstructive surgeons at Washington University in St. Louis, in an accompanying editorial. Patients can engage in light activity immediately after surgery, and cortical plasticity enables function to improve over time. Two disadvantages of nerve transfers, however, are that it takes months before new motion can be observed, and years before full strength can be regained.
The heterogeneity of cervical spinal cord injury requires an individualized approach to surgical assessment and management, they continued. Physicians and patients should make treatment decisions collaboratively. “We envisage a role for nerve transfers in settings where the intensive therapy and immobilization required to optimize complementary tendon transfers are unavailable,” wrote Dr. Hill and Dr. Fox.
Continuing research will be necessary to improve surgical technique and outcomes. “This research should include efforts to compare nerve transfer with tendon transfer, find the optimal timing of such surgeries, and determine which approach produces the greatest functional improvement,” they wrote. “Detailed study of the reasons for nerve transfer failure is also required, as is improving our understanding of the effects of biopsychosocial factors, including access to information and care, psychological readiness, and social support, on patient decision making and outcomes.”
Nerve transfers are a “huge advance” in the restoration of function after spinal cord injury, the authors added. “Surgeons who integrate nerve transfers into their spinal cord injury practice should take a careful and measured approach and rigorously study and disseminate their outcomes to advance this growing field,” they concluded.
according to research published online July 4 ahead of print in the Lancet. Combining nerve transfer with tendon transfer may maximize the functional benefit of surgery.
The loss of upper extremity function after cervical spinal cord injury can reduce independence and social and vocational engagement. People with tetraplegia rank improvement in hand function as their most important goal. Tendon transfers have been the traditional method of restoring function, but interest in nerve transfers has been increasing with the publication of successful results. Nerve transfers can reanimate several muscles at once and require a smaller incision and shorter immobilization, compared with tendon transfers.
Injury had occurred less than 18 months previously
Natasha van Zyl, MBBS, a plastic and reconstructive surgeon at Austin Health in Melbourne, and colleagues conducted a prospective case series to examine the clinical and functional outcomes of nerve transfer surgery for the reanimation of upper limb function in patients with tetraplegia. The investigators also sought to compare these outcomes with published outcomes for tendon transfer surgery.
Between April 14, 2014, and Nov. 22, 2018, Dr. van Zyl and colleagues recruited consecutive patients of any age with early cervical spinal cord injury of motor level C5 and below. Injury was required to have occurred fewer than 18 months before enrollment. Eligible participants had been referred to a single center for upper extremity reanimation and were considered candidates for nerve transfer.
Every participant underwent single or multiple nerve transfers in one or both upper limbs, and some participants also underwent tendon transfers. The goal of surgery was the restoration of elbow extension, grasp, pinch, and hand opening. An independent assessor evaluated participants at baseline and at 12 months and 24 months after surgery. The primary outcome measures were the action research arm test (ARAT), the grasp release test (GRT), and the spinal cord independence measure (SCIM).
Grasp function improved significantly
Dr. van Zyl and colleagues recruited 16 participants with traumatic spinal cord injury who underwent 59 nerve transfers. Ten participants also underwent tendon transfers. The population’s mean age at time of injury was 27.3 years. Three patients were female. Motor vehicle accidents were the most common cause of injury (31%). Follow-up data at 24 months were unavailable for three patients.
Participants’ median ARAT total score significantly improved from 16.5 at baseline to 34.0 at 24 months. Median GRT total score significantly improved from 35.0 at baseline to 125.2 at 24 months. The population’s mean total SCIM score and mobility in the room and toilet SCIM score improved by more than the minimal detectable change and the minimal clinically important difference. The mean self-care SCIM score improved by more than the minimal detectable change between baseline and 24 months.
The researchers observed six adverse events related to the surgery, but none had sustained functional consequences. No patients had an increase in musculoskeletal or neuropathic pain. Four of the 50 nerve transfers with 24-month follow-up failed.
A novel technique
“This project is the first to comprehensively examine outcomes for early, multiple nerve transfer surgery in the upper limbs of people with tetraplegia following traumatic spinal cord injury and is the largest prospective series of nerve transfers reported in this population to date,” said Dr. van Zyl and colleagues. Study limitations included the small sample size, the high variability of spinal cord injury patterns, and the potential for the multiple procedures that each participant underwent to confound data analysis.
Future research could explore whether nerve transfers are beneficial at more than 24 months after spinal cord injury, wrote the authors. In addition, it is unclear whether function and strength continue to improve beyond 24 months after surgery.
The study was funded by the Institute for Safety, Compensation, and Recovery Research in Australia. The authors had no competing interests.
SOURCE: van Zyl N et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31143-2.
according to research published online July 4 ahead of print in the Lancet. Combining nerve transfer with tendon transfer may maximize the functional benefit of surgery.
The loss of upper extremity function after cervical spinal cord injury can reduce independence and social and vocational engagement. People with tetraplegia rank improvement in hand function as their most important goal. Tendon transfers have been the traditional method of restoring function, but interest in nerve transfers has been increasing with the publication of successful results. Nerve transfers can reanimate several muscles at once and require a smaller incision and shorter immobilization, compared with tendon transfers.
Injury had occurred less than 18 months previously
Natasha van Zyl, MBBS, a plastic and reconstructive surgeon at Austin Health in Melbourne, and colleagues conducted a prospective case series to examine the clinical and functional outcomes of nerve transfer surgery for the reanimation of upper limb function in patients with tetraplegia. The investigators also sought to compare these outcomes with published outcomes for tendon transfer surgery.
Between April 14, 2014, and Nov. 22, 2018, Dr. van Zyl and colleagues recruited consecutive patients of any age with early cervical spinal cord injury of motor level C5 and below. Injury was required to have occurred fewer than 18 months before enrollment. Eligible participants had been referred to a single center for upper extremity reanimation and were considered candidates for nerve transfer.
Every participant underwent single or multiple nerve transfers in one or both upper limbs, and some participants also underwent tendon transfers. The goal of surgery was the restoration of elbow extension, grasp, pinch, and hand opening. An independent assessor evaluated participants at baseline and at 12 months and 24 months after surgery. The primary outcome measures were the action research arm test (ARAT), the grasp release test (GRT), and the spinal cord independence measure (SCIM).
Grasp function improved significantly
Dr. van Zyl and colleagues recruited 16 participants with traumatic spinal cord injury who underwent 59 nerve transfers. Ten participants also underwent tendon transfers. The population’s mean age at time of injury was 27.3 years. Three patients were female. Motor vehicle accidents were the most common cause of injury (31%). Follow-up data at 24 months were unavailable for three patients.
Participants’ median ARAT total score significantly improved from 16.5 at baseline to 34.0 at 24 months. Median GRT total score significantly improved from 35.0 at baseline to 125.2 at 24 months. The population’s mean total SCIM score and mobility in the room and toilet SCIM score improved by more than the minimal detectable change and the minimal clinically important difference. The mean self-care SCIM score improved by more than the minimal detectable change between baseline and 24 months.
The researchers observed six adverse events related to the surgery, but none had sustained functional consequences. No patients had an increase in musculoskeletal or neuropathic pain. Four of the 50 nerve transfers with 24-month follow-up failed.
A novel technique
“This project is the first to comprehensively examine outcomes for early, multiple nerve transfer surgery in the upper limbs of people with tetraplegia following traumatic spinal cord injury and is the largest prospective series of nerve transfers reported in this population to date,” said Dr. van Zyl and colleagues. Study limitations included the small sample size, the high variability of spinal cord injury patterns, and the potential for the multiple procedures that each participant underwent to confound data analysis.
Future research could explore whether nerve transfers are beneficial at more than 24 months after spinal cord injury, wrote the authors. In addition, it is unclear whether function and strength continue to improve beyond 24 months after surgery.
The study was funded by the Institute for Safety, Compensation, and Recovery Research in Australia. The authors had no competing interests.
SOURCE: van Zyl N et al. Lancet. 2019 Jul 4. doi: 10.1016/S0140-6736(19)31143-2.
FROM LANCET
Value-based metrics gain ground in physician employment contracts
Physician employment contracts increasingly include value- and quality-based metrics as bases for production bonuses, according to an analysis of recruitment searches from April 1, 2018, to March 31, 2019.
Metrics such as physician satisfaction rates, proper use of EHRs, following treatment protocols, and others that don’t directly measure volume are becoming more commonplace in employment contracts, though volume measures still are included, according to Phil Miller, vice president of communications at health care recruiting firm Merritt Hawkins and author of the company’s 2019 report on physician and advanced practitioner recruiting incentives, released July 8.
Of 70% of searches that offered a production bonus, 56% featured a bonus based at least in part on quality metrics, up from 43% in 2018. The finding represents the highest percent of contracts offering a quality-based bonus that the company has tracked, according to the report.
Merritt Hawkins’ review is based on a sample of the 3,131 permanent physician and advanced practitioner search assignments that Merritt Hawkins and its sister physician staffing companies at AMN Healthcare have ongoing or were engaged to conduct from April 1, 2018 to March 31, 2019.
Other common value-based metrics include reduction in hospital readmissions, cost containment, and proper coding.
While value-based incentives are on the rise, “facilities that employ physicians want to ensure they stay productive, and ‘productivity’ still is measured in part by what are essentially fee-for-service metrics, including relative value units [RVUs], net collections, and number of patients seen.”
RVUs were used in 70% of production formulas tracked in the 2019 review, up from 50% in the previous year and also a record high.
Mr. Miller noted that employers are seeking the “Goldilocks’ zone,” a balance point between traditional productivity measures and value-based metrics, very much a work in progress right now.
A possible corollary to the increase in production bonuses is a flattening of signing bonuses. During the current review period, 71% of contracts came with a signing bonus, up slightly from 70% in the previous year’s report and down from 76% 2 years ago.
Signing bonuses in the review period for the 2019 report averaged $32,692, down from $33,707 during the 2018 report’s review period.
Overall, family practice physicians remain the highest in demand for job searches, but specialty practice is gaining ground.
For the 2018-2019 review, family medicine was the most requested search by specialty, with 457 searches requested. While the ranking remains No. 1, as it has for the past 13 years, the number of searches has been on a steady decline. Last year, there were 497 searches, which was down from 607 2 years ago and 734 4 years ago.
Mr. Miller said there were a few reasons for the lower number of searches. “One is just the momentum shifts that are kind of inherent to recruiting. People put all of their resources into one area, typically, and in this case it was primary care and they realized, ‘Hey wait a minute, we need some specialists for these doctors to refer to, so now we have to put some of our chips in the specialty basket.’ ”
The Baby Boomers also is having an effect – as they age and are experiencing more health issues, more specialists are needed.
“[Older patients] visit the doctor twice or three times the rate of a younger person and they also generate a much higher percentage of inpatient procedures and tests and diagnoses,” he said.
On the opposite end of the spectrum, “younger people are less likely to have a primary care doctor who coordinates their care,” Mr. Miller said. “What they typically do is go to an urgent care center, a retail clinic, maybe even [use] telemedicine so they are not accessing the system in the same way or necessarily through the same provider.”
Demand for psychiatrists remained strong for the fourth year in a row, but the number of searches has declined for the last several years. For the current review period, there were 199 searches, down from 243 the previous year, 256 2 years ago, and 250 3 years ago.
There is “pretty much a crisis in behavioral health care now because there are so few psychiatrists and the demand has increased,” Mr. Miller noted.
Physician employment contracts increasingly include value- and quality-based metrics as bases for production bonuses, according to an analysis of recruitment searches from April 1, 2018, to March 31, 2019.
Metrics such as physician satisfaction rates, proper use of EHRs, following treatment protocols, and others that don’t directly measure volume are becoming more commonplace in employment contracts, though volume measures still are included, according to Phil Miller, vice president of communications at health care recruiting firm Merritt Hawkins and author of the company’s 2019 report on physician and advanced practitioner recruiting incentives, released July 8.
Of 70% of searches that offered a production bonus, 56% featured a bonus based at least in part on quality metrics, up from 43% in 2018. The finding represents the highest percent of contracts offering a quality-based bonus that the company has tracked, according to the report.
Merritt Hawkins’ review is based on a sample of the 3,131 permanent physician and advanced practitioner search assignments that Merritt Hawkins and its sister physician staffing companies at AMN Healthcare have ongoing or were engaged to conduct from April 1, 2018 to March 31, 2019.
Other common value-based metrics include reduction in hospital readmissions, cost containment, and proper coding.
While value-based incentives are on the rise, “facilities that employ physicians want to ensure they stay productive, and ‘productivity’ still is measured in part by what are essentially fee-for-service metrics, including relative value units [RVUs], net collections, and number of patients seen.”
RVUs were used in 70% of production formulas tracked in the 2019 review, up from 50% in the previous year and also a record high.
Mr. Miller noted that employers are seeking the “Goldilocks’ zone,” a balance point between traditional productivity measures and value-based metrics, very much a work in progress right now.
A possible corollary to the increase in production bonuses is a flattening of signing bonuses. During the current review period, 71% of contracts came with a signing bonus, up slightly from 70% in the previous year’s report and down from 76% 2 years ago.
Signing bonuses in the review period for the 2019 report averaged $32,692, down from $33,707 during the 2018 report’s review period.
Overall, family practice physicians remain the highest in demand for job searches, but specialty practice is gaining ground.
For the 2018-2019 review, family medicine was the most requested search by specialty, with 457 searches requested. While the ranking remains No. 1, as it has for the past 13 years, the number of searches has been on a steady decline. Last year, there were 497 searches, which was down from 607 2 years ago and 734 4 years ago.
Mr. Miller said there were a few reasons for the lower number of searches. “One is just the momentum shifts that are kind of inherent to recruiting. People put all of their resources into one area, typically, and in this case it was primary care and they realized, ‘Hey wait a minute, we need some specialists for these doctors to refer to, so now we have to put some of our chips in the specialty basket.’ ”
The Baby Boomers also is having an effect – as they age and are experiencing more health issues, more specialists are needed.
“[Older patients] visit the doctor twice or three times the rate of a younger person and they also generate a much higher percentage of inpatient procedures and tests and diagnoses,” he said.
On the opposite end of the spectrum, “younger people are less likely to have a primary care doctor who coordinates their care,” Mr. Miller said. “What they typically do is go to an urgent care center, a retail clinic, maybe even [use] telemedicine so they are not accessing the system in the same way or necessarily through the same provider.”
Demand for psychiatrists remained strong for the fourth year in a row, but the number of searches has declined for the last several years. For the current review period, there were 199 searches, down from 243 the previous year, 256 2 years ago, and 250 3 years ago.
There is “pretty much a crisis in behavioral health care now because there are so few psychiatrists and the demand has increased,” Mr. Miller noted.
Physician employment contracts increasingly include value- and quality-based metrics as bases for production bonuses, according to an analysis of recruitment searches from April 1, 2018, to March 31, 2019.
Metrics such as physician satisfaction rates, proper use of EHRs, following treatment protocols, and others that don’t directly measure volume are becoming more commonplace in employment contracts, though volume measures still are included, according to Phil Miller, vice president of communications at health care recruiting firm Merritt Hawkins and author of the company’s 2019 report on physician and advanced practitioner recruiting incentives, released July 8.
Of 70% of searches that offered a production bonus, 56% featured a bonus based at least in part on quality metrics, up from 43% in 2018. The finding represents the highest percent of contracts offering a quality-based bonus that the company has tracked, according to the report.
Merritt Hawkins’ review is based on a sample of the 3,131 permanent physician and advanced practitioner search assignments that Merritt Hawkins and its sister physician staffing companies at AMN Healthcare have ongoing or were engaged to conduct from April 1, 2018 to March 31, 2019.
Other common value-based metrics include reduction in hospital readmissions, cost containment, and proper coding.
While value-based incentives are on the rise, “facilities that employ physicians want to ensure they stay productive, and ‘productivity’ still is measured in part by what are essentially fee-for-service metrics, including relative value units [RVUs], net collections, and number of patients seen.”
RVUs were used in 70% of production formulas tracked in the 2019 review, up from 50% in the previous year and also a record high.
Mr. Miller noted that employers are seeking the “Goldilocks’ zone,” a balance point between traditional productivity measures and value-based metrics, very much a work in progress right now.
A possible corollary to the increase in production bonuses is a flattening of signing bonuses. During the current review period, 71% of contracts came with a signing bonus, up slightly from 70% in the previous year’s report and down from 76% 2 years ago.
Signing bonuses in the review period for the 2019 report averaged $32,692, down from $33,707 during the 2018 report’s review period.
Overall, family practice physicians remain the highest in demand for job searches, but specialty practice is gaining ground.
For the 2018-2019 review, family medicine was the most requested search by specialty, with 457 searches requested. While the ranking remains No. 1, as it has for the past 13 years, the number of searches has been on a steady decline. Last year, there were 497 searches, which was down from 607 2 years ago and 734 4 years ago.
Mr. Miller said there were a few reasons for the lower number of searches. “One is just the momentum shifts that are kind of inherent to recruiting. People put all of their resources into one area, typically, and in this case it was primary care and they realized, ‘Hey wait a minute, we need some specialists for these doctors to refer to, so now we have to put some of our chips in the specialty basket.’ ”
The Baby Boomers also is having an effect – as they age and are experiencing more health issues, more specialists are needed.
“[Older patients] visit the doctor twice or three times the rate of a younger person and they also generate a much higher percentage of inpatient procedures and tests and diagnoses,” he said.
On the opposite end of the spectrum, “younger people are less likely to have a primary care doctor who coordinates their care,” Mr. Miller said. “What they typically do is go to an urgent care center, a retail clinic, maybe even [use] telemedicine so they are not accessing the system in the same way or necessarily through the same provider.”
Demand for psychiatrists remained strong for the fourth year in a row, but the number of searches has declined for the last several years. For the current review period, there were 199 searches, down from 243 the previous year, 256 2 years ago, and 250 3 years ago.
There is “pretty much a crisis in behavioral health care now because there are so few psychiatrists and the demand has increased,” Mr. Miller noted.
Maternal migraine is associated with infant colic
PHILADELPHIA – , according to research presented at the annual meeting of the American Headache Society. Fathers with migraine are not more likely to have children with colic, however. These findings may have implications for the care of mothers with migraine and their children, said Amy Gelfand, MD, associate professor of neurology at the University of California, San Francisco.
Smaller studies have suggested associations between migraine and colic. To examine this relationship in a large, national sample, Dr. Gelfand and her research colleagues conducted a cross-sectional survey of biological parents of 4- to 8-week-olds in the United States. The researchers analyzed data from 1,419 participants – 827 mothers and 592 fathers – who completed online surveys in 2017 and 2018.
Parents provided information about their and their infants’ health. The investigators identified migraineurs using modified International Classification of Headache Disorders 3rd edition criteria and determined infant colic by response to the question, “Has your baby cried for at least 3 hours on at least 3 days in the last week?”
In all, 33.5% of the mothers had migraine or probable migraine, and 20.8% of the fathers had migraine or probable migraine. Maternal migraine was associated with increased odds of infant colic (odds ratio, 1.7). Among mothers with migraine and headache frequency of 15 or more days per month, the likelihood of having an infant with colic was even greater (OR, 2.5).
“The cause of colic is unknown, yet colic is common, and these frequent bouts of intense crying or fussiness can be particularly frustrating for parents, creating family stress and anxiety,” Dr. Gelfand said in a news release. “New moms who are armed with knowledge of the connection between their own history of migraine and infant colic can be better prepared for these often difficult first months of a baby and new mother’s journey.”
PHILADELPHIA – , according to research presented at the annual meeting of the American Headache Society. Fathers with migraine are not more likely to have children with colic, however. These findings may have implications for the care of mothers with migraine and their children, said Amy Gelfand, MD, associate professor of neurology at the University of California, San Francisco.
Smaller studies have suggested associations between migraine and colic. To examine this relationship in a large, national sample, Dr. Gelfand and her research colleagues conducted a cross-sectional survey of biological parents of 4- to 8-week-olds in the United States. The researchers analyzed data from 1,419 participants – 827 mothers and 592 fathers – who completed online surveys in 2017 and 2018.
Parents provided information about their and their infants’ health. The investigators identified migraineurs using modified International Classification of Headache Disorders 3rd edition criteria and determined infant colic by response to the question, “Has your baby cried for at least 3 hours on at least 3 days in the last week?”
In all, 33.5% of the mothers had migraine or probable migraine, and 20.8% of the fathers had migraine or probable migraine. Maternal migraine was associated with increased odds of infant colic (odds ratio, 1.7). Among mothers with migraine and headache frequency of 15 or more days per month, the likelihood of having an infant with colic was even greater (OR, 2.5).
“The cause of colic is unknown, yet colic is common, and these frequent bouts of intense crying or fussiness can be particularly frustrating for parents, creating family stress and anxiety,” Dr. Gelfand said in a news release. “New moms who are armed with knowledge of the connection between their own history of migraine and infant colic can be better prepared for these often difficult first months of a baby and new mother’s journey.”
PHILADELPHIA – , according to research presented at the annual meeting of the American Headache Society. Fathers with migraine are not more likely to have children with colic, however. These findings may have implications for the care of mothers with migraine and their children, said Amy Gelfand, MD, associate professor of neurology at the University of California, San Francisco.
Smaller studies have suggested associations between migraine and colic. To examine this relationship in a large, national sample, Dr. Gelfand and her research colleagues conducted a cross-sectional survey of biological parents of 4- to 8-week-olds in the United States. The researchers analyzed data from 1,419 participants – 827 mothers and 592 fathers – who completed online surveys in 2017 and 2018.
Parents provided information about their and their infants’ health. The investigators identified migraineurs using modified International Classification of Headache Disorders 3rd edition criteria and determined infant colic by response to the question, “Has your baby cried for at least 3 hours on at least 3 days in the last week?”
In all, 33.5% of the mothers had migraine or probable migraine, and 20.8% of the fathers had migraine or probable migraine. Maternal migraine was associated with increased odds of infant colic (odds ratio, 1.7). Among mothers with migraine and headache frequency of 15 or more days per month, the likelihood of having an infant with colic was even greater (OR, 2.5).
“The cause of colic is unknown, yet colic is common, and these frequent bouts of intense crying or fussiness can be particularly frustrating for parents, creating family stress and anxiety,” Dr. Gelfand said in a news release. “New moms who are armed with knowledge of the connection between their own history of migraine and infant colic can be better prepared for these often difficult first months of a baby and new mother’s journey.”
EXPERT ANALYSIS FROM AHS 2019
VIDEO: PsA Fast Facts: Psoriatic arthritis types