User login
Acne and Rosacea - July 2019 Supplement
The 2019 Acne & Rosacea supplement features a selection of articles on these two topics published in Dermatology News during the previous year, with commentaries by dermatologists Hilary E. Baldwin, MD, and Julie C. Harper, M.D., both past presidents of the American Acne & Rosacea Society. They were also both members of the American Academy of Dermatology work group that developed the AAD’s updated guidelines on the management of acne vulgaris, published in 2016.
Highlights include:
- The global incidence of rosacea
- Rosacea likely undertreated in skin of color
- The impact of isotretinoin on the dermal microbiome
- Laser treatments of acne and rosacea
The 2019 Acne & Rosacea supplement features a selection of articles on these two topics published in Dermatology News during the previous year, with commentaries by dermatologists Hilary E. Baldwin, MD, and Julie C. Harper, M.D., both past presidents of the American Acne & Rosacea Society. They were also both members of the American Academy of Dermatology work group that developed the AAD’s updated guidelines on the management of acne vulgaris, published in 2016.
Highlights include:
- The global incidence of rosacea
- Rosacea likely undertreated in skin of color
- The impact of isotretinoin on the dermal microbiome
- Laser treatments of acne and rosacea
The 2019 Acne & Rosacea supplement features a selection of articles on these two topics published in Dermatology News during the previous year, with commentaries by dermatologists Hilary E. Baldwin, MD, and Julie C. Harper, M.D., both past presidents of the American Acne & Rosacea Society. They were also both members of the American Academy of Dermatology work group that developed the AAD’s updated guidelines on the management of acne vulgaris, published in 2016.
Highlights include:
- The global incidence of rosacea
- Rosacea likely undertreated in skin of color
- The impact of isotretinoin on the dermal microbiome
- Laser treatments of acne and rosacea
COPD eosinophil counts predict steroid responders
Triple therapy with an inhaled corticosteroid is particularly helpful for patients with chronic obstructive pulmonary disease (COPD) who have high baseline eosinophil counts, a trial involving more than 10,000 patients found.
Former smokers received greater benefit from inhaled corticosteroids (ICS) than did current smokers, reported lead author Steven Pascoe, MBBS, of GlaxoSmithKline and colleagues. The investigators noted that these findings can help personalize therapy for patients with COPD, which can be challenging to treat because of its heterogeneity. The study was published in Lancet Respiratory Medicine.
The phase 3 IMPACT trial compared single-inhaler fluticasone furoate–umeclidinium–vilanterol with umeclidinium-vilanterol and fluticasone furoate–vilanterol in patients with moderate to very severe COPD at high risk of exacerbation. Of the 10,333 patients involved, approximately one-quarter (26%) had one or more severe exacerbations in the previous year and half (47%) had two or more moderate exacerbations in the same time period. All patients were symptomatic and were aged 40 years or older. A variety of baseline and demographic patient characteristics were recorded, including blood eosinophil count, smoking status, and others. Responses to therapy were measured with trough forced expiratory volume in 1 second (FEV1), symptom scoring, and a quality of life questionnaire.
After 52 weeks, results showed that higher baseline eosinophil counts were associated with progressively greater benefits in favor of triple therapy. For patients with baseline blood eosinophil counts of at least 310 cells per mcL, triple therapy was associated with about half as many moderate and severe exacerbations as treatment with umeclidinium-vilanterol (rate ratio = 0.56; 95% confidence interval, 0.47-0.66). For patients with less than 90 cells per mcL at baseline, the rate ratio for the same two regimens was 0.88, but with a confidence interval crossing 1 (0.74-1.04). For fluticasone furoate–vilanterol vs. umeclidinium-vilanterol, high baseline eosinophil count again demonstrated its predictive power for ICS efficacy, again with an associated rate ratio of 0.56 (0.47-0.66), compared with 1.09 (0.91-1.29) for patients below the lower threshold. Symptom scoring, quality of life, and FEV1 followed a similar trend, although the investigators noted that this was “less marked” for FEV1. Although the trend held regardless of smoking status, benefits were more pronounced among former smokers than current smokers.
“In former smokers, ICS benefits were observed at all blood eosinophil counts when comparing triple therapy with umeclidinium-vilanterol, whereas in current smokers no ICS benefit was observed at lower eosinophil counts, less than approximately 200 eosinophils per [mcL],” the investigators wrote.
“Overall, these results show the potential use of blood eosinophil counts in conjunction with smoking status to predict the magnitude of ICS response within a dual or triple-combination therapy,” the investigators concluded. “Future approaches to the pharmacological management of COPD should move beyond the simple dichotomization of each clinical or biomarker variable, toward more complex algorithms that integrate the interactions between important variables including exacerbation history, smoking status, and blood eosinophil counts.”
The study was funded by GlaxoSmithKline. The investigators disclosed additional relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, CSA Medical, and others.
SOURCE: Pascoe S et al. Lancet Resp Med. 2019 Jul 4. doi: 10.1016/S2213-2600(19)30190-0.
Triple therapy with an inhaled corticosteroid is particularly helpful for patients with chronic obstructive pulmonary disease (COPD) who have high baseline eosinophil counts, a trial involving more than 10,000 patients found.
Former smokers received greater benefit from inhaled corticosteroids (ICS) than did current smokers, reported lead author Steven Pascoe, MBBS, of GlaxoSmithKline and colleagues. The investigators noted that these findings can help personalize therapy for patients with COPD, which can be challenging to treat because of its heterogeneity. The study was published in Lancet Respiratory Medicine.
The phase 3 IMPACT trial compared single-inhaler fluticasone furoate–umeclidinium–vilanterol with umeclidinium-vilanterol and fluticasone furoate–vilanterol in patients with moderate to very severe COPD at high risk of exacerbation. Of the 10,333 patients involved, approximately one-quarter (26%) had one or more severe exacerbations in the previous year and half (47%) had two or more moderate exacerbations in the same time period. All patients were symptomatic and were aged 40 years or older. A variety of baseline and demographic patient characteristics were recorded, including blood eosinophil count, smoking status, and others. Responses to therapy were measured with trough forced expiratory volume in 1 second (FEV1), symptom scoring, and a quality of life questionnaire.
After 52 weeks, results showed that higher baseline eosinophil counts were associated with progressively greater benefits in favor of triple therapy. For patients with baseline blood eosinophil counts of at least 310 cells per mcL, triple therapy was associated with about half as many moderate and severe exacerbations as treatment with umeclidinium-vilanterol (rate ratio = 0.56; 95% confidence interval, 0.47-0.66). For patients with less than 90 cells per mcL at baseline, the rate ratio for the same two regimens was 0.88, but with a confidence interval crossing 1 (0.74-1.04). For fluticasone furoate–vilanterol vs. umeclidinium-vilanterol, high baseline eosinophil count again demonstrated its predictive power for ICS efficacy, again with an associated rate ratio of 0.56 (0.47-0.66), compared with 1.09 (0.91-1.29) for patients below the lower threshold. Symptom scoring, quality of life, and FEV1 followed a similar trend, although the investigators noted that this was “less marked” for FEV1. Although the trend held regardless of smoking status, benefits were more pronounced among former smokers than current smokers.
“In former smokers, ICS benefits were observed at all blood eosinophil counts when comparing triple therapy with umeclidinium-vilanterol, whereas in current smokers no ICS benefit was observed at lower eosinophil counts, less than approximately 200 eosinophils per [mcL],” the investigators wrote.
“Overall, these results show the potential use of blood eosinophil counts in conjunction with smoking status to predict the magnitude of ICS response within a dual or triple-combination therapy,” the investigators concluded. “Future approaches to the pharmacological management of COPD should move beyond the simple dichotomization of each clinical or biomarker variable, toward more complex algorithms that integrate the interactions between important variables including exacerbation history, smoking status, and blood eosinophil counts.”
The study was funded by GlaxoSmithKline. The investigators disclosed additional relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, CSA Medical, and others.
SOURCE: Pascoe S et al. Lancet Resp Med. 2019 Jul 4. doi: 10.1016/S2213-2600(19)30190-0.
Triple therapy with an inhaled corticosteroid is particularly helpful for patients with chronic obstructive pulmonary disease (COPD) who have high baseline eosinophil counts, a trial involving more than 10,000 patients found.
Former smokers received greater benefit from inhaled corticosteroids (ICS) than did current smokers, reported lead author Steven Pascoe, MBBS, of GlaxoSmithKline and colleagues. The investigators noted that these findings can help personalize therapy for patients with COPD, which can be challenging to treat because of its heterogeneity. The study was published in Lancet Respiratory Medicine.
The phase 3 IMPACT trial compared single-inhaler fluticasone furoate–umeclidinium–vilanterol with umeclidinium-vilanterol and fluticasone furoate–vilanterol in patients with moderate to very severe COPD at high risk of exacerbation. Of the 10,333 patients involved, approximately one-quarter (26%) had one or more severe exacerbations in the previous year and half (47%) had two or more moderate exacerbations in the same time period. All patients were symptomatic and were aged 40 years or older. A variety of baseline and demographic patient characteristics were recorded, including blood eosinophil count, smoking status, and others. Responses to therapy were measured with trough forced expiratory volume in 1 second (FEV1), symptom scoring, and a quality of life questionnaire.
After 52 weeks, results showed that higher baseline eosinophil counts were associated with progressively greater benefits in favor of triple therapy. For patients with baseline blood eosinophil counts of at least 310 cells per mcL, triple therapy was associated with about half as many moderate and severe exacerbations as treatment with umeclidinium-vilanterol (rate ratio = 0.56; 95% confidence interval, 0.47-0.66). For patients with less than 90 cells per mcL at baseline, the rate ratio for the same two regimens was 0.88, but with a confidence interval crossing 1 (0.74-1.04). For fluticasone furoate–vilanterol vs. umeclidinium-vilanterol, high baseline eosinophil count again demonstrated its predictive power for ICS efficacy, again with an associated rate ratio of 0.56 (0.47-0.66), compared with 1.09 (0.91-1.29) for patients below the lower threshold. Symptom scoring, quality of life, and FEV1 followed a similar trend, although the investigators noted that this was “less marked” for FEV1. Although the trend held regardless of smoking status, benefits were more pronounced among former smokers than current smokers.
“In former smokers, ICS benefits were observed at all blood eosinophil counts when comparing triple therapy with umeclidinium-vilanterol, whereas in current smokers no ICS benefit was observed at lower eosinophil counts, less than approximately 200 eosinophils per [mcL],” the investigators wrote.
“Overall, these results show the potential use of blood eosinophil counts in conjunction with smoking status to predict the magnitude of ICS response within a dual or triple-combination therapy,” the investigators concluded. “Future approaches to the pharmacological management of COPD should move beyond the simple dichotomization of each clinical or biomarker variable, toward more complex algorithms that integrate the interactions between important variables including exacerbation history, smoking status, and blood eosinophil counts.”
The study was funded by GlaxoSmithKline. The investigators disclosed additional relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, CSA Medical, and others.
SOURCE: Pascoe S et al. Lancet Resp Med. 2019 Jul 4. doi: 10.1016/S2213-2600(19)30190-0.
FROM LANCET RESPIRATORY MEDICINE
Cathepsin Z identified as a potential biomarker for osteoporosis
The presence of cathepsin Z messenger RNA in peripheral blood mononuclear cells of people with osteopenia, osteoporosis, and women with osteoporosis and older than 50 years could be used as a biomarker to help diagnose osteoporosis, according to a recent study published in Scientific Reports.
Dong L. Barraclough, PhD, of the Institute of Ageing and Chronic Disease at the University of Liverpool, England, and colleagues studied the expression of cathepsin Z messenger RNA (mRNA) in peripheral blood mononuclear cells (PBMCs) of 88 participants (71 women, 17 men). The participants were grouped according to their bone mineral density and T score, where a T score of −1.0 or higher was considered nonosteoporotic, a score between −1.0 and −2.5 was classified as osteopenia, and −2.5 or less was classified as osteoporosis.
Overall, there were 48 participants with osteopenia (38 women, 10 men; 55% of total participants; average age, 65 years), 23 participants with osteoporosis (19 women, 4 men; 26%; 69 years), and 17 participants in the nonosteoporotic control group (14 women, 3 men; 19%; 56 years), with 88% of the total number of participants aged 50 years and older (82% women, 18% men).
The researchers found significantly higher differential expression of cathepsin Z mRNA in PBMCs when comparing the nonosteoporotic control group and participants with osteopenia (95% confidence interval, −0.32 to −0.053; P = .0067), the control group with participants with osteoporosis (95% CI, −0.543 to −0.24; P less than .0001), and participants with osteopenia and those with osteoporosis (95% CI, −0.325 to −0.084; P = .0011).
That association also was seen in women with osteoporosis who were older than 50 years (P = .0016) and did not change when participants were excluded for receiving treatment for osteoporosis, the authors wrote.
There also was an inverse association between cathepsin Z mRNA levels and bone mineral density (P = .0149) as well as inversely associated with lumbar spine L2-L4 and femoral neck T-scores (P = .0002 and P = .0139, respectively) and fragility fracture (P = .0018) in participants with osteopenia, osteoporosis, and women with osteoporosis older than 50 years.
Patients with chronic inflammatory disease sometimes have “osteoporosis-like conditions,” the authors noted. “However, there was no significant difference in cathepsin Z mRNA levels between osteopenia and osteoporosis patients who were also suffering from chronic inflammatory disorders and those [who] were not,” either when all osteopenia and osteoporosis participants were included (P = .774), or when only women participants with osteopenia or osteoporosis and older than 50 years were included (P = .666).
“The observation that [participants] with osteopenia also showed a significant increase in cathepsin Z mRNA, compared [with] nonosteoporotic controls, strongly suggests that, if replicated in a larger study, the cathepsin Z mRNA in patients’ PBMC preparations could form the basis of a test for osteoporosis, which could aid in the detection of osteoporosis before a critical and expensive fragility fracture occurs,” the authors wrote.
The authors reported no relevant conflicts of interest.
SOURCE: Dera AA et al. Sci Rep. 2019 Jul 5. doi: 10.1038/s41598-019-46068-0.
The presence of cathepsin Z messenger RNA in peripheral blood mononuclear cells of people with osteopenia, osteoporosis, and women with osteoporosis and older than 50 years could be used as a biomarker to help diagnose osteoporosis, according to a recent study published in Scientific Reports.
Dong L. Barraclough, PhD, of the Institute of Ageing and Chronic Disease at the University of Liverpool, England, and colleagues studied the expression of cathepsin Z messenger RNA (mRNA) in peripheral blood mononuclear cells (PBMCs) of 88 participants (71 women, 17 men). The participants were grouped according to their bone mineral density and T score, where a T score of −1.0 or higher was considered nonosteoporotic, a score between −1.0 and −2.5 was classified as osteopenia, and −2.5 or less was classified as osteoporosis.
Overall, there were 48 participants with osteopenia (38 women, 10 men; 55% of total participants; average age, 65 years), 23 participants with osteoporosis (19 women, 4 men; 26%; 69 years), and 17 participants in the nonosteoporotic control group (14 women, 3 men; 19%; 56 years), with 88% of the total number of participants aged 50 years and older (82% women, 18% men).
The researchers found significantly higher differential expression of cathepsin Z mRNA in PBMCs when comparing the nonosteoporotic control group and participants with osteopenia (95% confidence interval, −0.32 to −0.053; P = .0067), the control group with participants with osteoporosis (95% CI, −0.543 to −0.24; P less than .0001), and participants with osteopenia and those with osteoporosis (95% CI, −0.325 to −0.084; P = .0011).
That association also was seen in women with osteoporosis who were older than 50 years (P = .0016) and did not change when participants were excluded for receiving treatment for osteoporosis, the authors wrote.
There also was an inverse association between cathepsin Z mRNA levels and bone mineral density (P = .0149) as well as inversely associated with lumbar spine L2-L4 and femoral neck T-scores (P = .0002 and P = .0139, respectively) and fragility fracture (P = .0018) in participants with osteopenia, osteoporosis, and women with osteoporosis older than 50 years.
Patients with chronic inflammatory disease sometimes have “osteoporosis-like conditions,” the authors noted. “However, there was no significant difference in cathepsin Z mRNA levels between osteopenia and osteoporosis patients who were also suffering from chronic inflammatory disorders and those [who] were not,” either when all osteopenia and osteoporosis participants were included (P = .774), or when only women participants with osteopenia or osteoporosis and older than 50 years were included (P = .666).
“The observation that [participants] with osteopenia also showed a significant increase in cathepsin Z mRNA, compared [with] nonosteoporotic controls, strongly suggests that, if replicated in a larger study, the cathepsin Z mRNA in patients’ PBMC preparations could form the basis of a test for osteoporosis, which could aid in the detection of osteoporosis before a critical and expensive fragility fracture occurs,” the authors wrote.
The authors reported no relevant conflicts of interest.
SOURCE: Dera AA et al. Sci Rep. 2019 Jul 5. doi: 10.1038/s41598-019-46068-0.
The presence of cathepsin Z messenger RNA in peripheral blood mononuclear cells of people with osteopenia, osteoporosis, and women with osteoporosis and older than 50 years could be used as a biomarker to help diagnose osteoporosis, according to a recent study published in Scientific Reports.
Dong L. Barraclough, PhD, of the Institute of Ageing and Chronic Disease at the University of Liverpool, England, and colleagues studied the expression of cathepsin Z messenger RNA (mRNA) in peripheral blood mononuclear cells (PBMCs) of 88 participants (71 women, 17 men). The participants were grouped according to their bone mineral density and T score, where a T score of −1.0 or higher was considered nonosteoporotic, a score between −1.0 and −2.5 was classified as osteopenia, and −2.5 or less was classified as osteoporosis.
Overall, there were 48 participants with osteopenia (38 women, 10 men; 55% of total participants; average age, 65 years), 23 participants with osteoporosis (19 women, 4 men; 26%; 69 years), and 17 participants in the nonosteoporotic control group (14 women, 3 men; 19%; 56 years), with 88% of the total number of participants aged 50 years and older (82% women, 18% men).
The researchers found significantly higher differential expression of cathepsin Z mRNA in PBMCs when comparing the nonosteoporotic control group and participants with osteopenia (95% confidence interval, −0.32 to −0.053; P = .0067), the control group with participants with osteoporosis (95% CI, −0.543 to −0.24; P less than .0001), and participants with osteopenia and those with osteoporosis (95% CI, −0.325 to −0.084; P = .0011).
That association also was seen in women with osteoporosis who were older than 50 years (P = .0016) and did not change when participants were excluded for receiving treatment for osteoporosis, the authors wrote.
There also was an inverse association between cathepsin Z mRNA levels and bone mineral density (P = .0149) as well as inversely associated with lumbar spine L2-L4 and femoral neck T-scores (P = .0002 and P = .0139, respectively) and fragility fracture (P = .0018) in participants with osteopenia, osteoporosis, and women with osteoporosis older than 50 years.
Patients with chronic inflammatory disease sometimes have “osteoporosis-like conditions,” the authors noted. “However, there was no significant difference in cathepsin Z mRNA levels between osteopenia and osteoporosis patients who were also suffering from chronic inflammatory disorders and those [who] were not,” either when all osteopenia and osteoporosis participants were included (P = .774), or when only women participants with osteopenia or osteoporosis and older than 50 years were included (P = .666).
“The observation that [participants] with osteopenia also showed a significant increase in cathepsin Z mRNA, compared [with] nonosteoporotic controls, strongly suggests that, if replicated in a larger study, the cathepsin Z mRNA in patients’ PBMC preparations could form the basis of a test for osteoporosis, which could aid in the detection of osteoporosis before a critical and expensive fragility fracture occurs,” the authors wrote.
The authors reported no relevant conflicts of interest.
SOURCE: Dera AA et al. Sci Rep. 2019 Jul 5. doi: 10.1038/s41598-019-46068-0.
FROM SCIENTIFIC REPORTS
Stillbirth linked to nearly fivefold increase in maternal morbidity risk
, according to research in Obstetrics & Gynecology.
Citing major increases in risk for a host of serious complications, the authors of the large population-based study urge those caring for women experiencing stillbirth to be vigilant for trouble.
Severe maternal morbidity among mothers experiencing stillbirth occurred in 578 cases per 10,000 deliveries, compared with 99 cases per 10,000 live deliveries, wrote Elizabeth Wall-Wieler, PhD, and coauthors. After statistical adjustment, the relative risk (RR) for severe maternal morbidity in a stillbirth compared with a live delivery was 4.77 (95% confidence interval, 4.53-5.02).
“Our findings indicate that nearly 1 in 17 women who deliver a stillbirth in California experience severe maternal morbidity. Furthermore, the risk of severe maternal morbidity was more than fourfold higher for women undergoing stillbirth delivery than live birth delivery,” the investigators wrote.
Major maternal organ dysfunction or failure – including acute renal failure, adult respiratory distress syndrome, disseminated intravascular coagulation, sepsis, or shock – all were more common in stillbirth deliveries, noted Dr. Wall-Wieler and colleagues. Hysterectomy, likely performed to control major loss of blood, also was more likely in stillbirth deliveries.
“Minimal attention has been given to maternal outcomes and acute complications experienced by women who have a stillbirth,” wrote Dr. Wall-Wieler, a postdoctoral research fellow in developmental and neonatal medicine, and colleagues at Stanford (Calif.) University. This is so because many analyses of maternal morbidity exclude stillbirth deliveries, or lump them with term deliveries, she and coauthors explained.
Using data from the Office of Statewide Health Planning and Development in California, Dr. Wall-Wieler and colleagues examined a total of 6,459,842 deliveries occurring in the state during 1999-2011; of these, 25,997 (0.4%) were stillbirths. For the cross-sectional study, the investigators included only deliveries for which fetal or neonatal vital records could be linked with the maternal hospital record.
Stillbirth was defined in the study as a fetal death delivered at or after 20 weeks’ gestation, so deliveries at less than 20 weeks’ gestation were excluded, as were any deliveries recorded as being at or after 45 weeks’ gestation, because the latter set were considered likely to be data entry errors.
Deliveries were considered to have severe maternal morbidity if any of the 18 indicators identified by the Centers for Disease Control and Prevention were coded in the medical record. The most common severe morbidities seen in stillbirth were blood transfusion, disseminated intravascular coagulation, and acute renal failure (adjusted RRs 5.38, 8.78, and 13.22, respectively). Although absolute occurrences were less frequent, relative risk for sepsis and shock were more than 14 times higher for stillbirths than for live birth deliveries.
“Taken together, these findings suggest the morbidity associated with obstetric hemorrhage and preeclampsia among women hospitalized for stillbirth delivery is a serious concern,” wrote Dr. Wall-Wieler and coauthors. They called for prospective studies to clarify cause and effect between stillbirth and these morbidities and to look into whether women carrying a nonviable fetus or with known fetal demise are managed differently than those with a viable fetus.
Overall, stillbirth deliveries were more likely for women who were older, for non-Hispanic black women, for those who did not have a college education, and those who did not have private insurance. Preexisting diabetes and hypertension, as well as a vaginal delivery, also upped the risk for stillbirth.
For reasons that are not completely clear, the risk for severe maternal morbidity with stillbirth climbed after 30 weeks’ gestation. Dr. Wall-Wieler and collaborators conducted an exploratory analysis that dichotomized deliveries for both stillbirth and live births into those occurring at fewer than 30 weeks’ gestation, or at or after 30 weeks’. They found no increased risk for severe maternal morbidity earlier than 30 weeks, but an RR of 5.4 for stillbirth at or after 30 weeks.
A reported cause of fetal demise was available for 71% of deliveries, with umbilical cord anomalies, obstetric complications, and placental conditions collectively accounting for almost half (46%) of the identified causes of demise. Severe maternal morbidity was most common in deaths related to hypertensive disorders, at 24/100, and least common in deaths from major fetal structural or genetic problems, at 1/100.
The size of the study strengthens the findings, said the investigators, but the large amount of missing data in recording fetal deaths does introduce some limitations. These include the inability to distinguish between intrapartum and antepartum fetal death, as well as the fact that cause of fetal death was not recorded for over one in four stillbirths.
“Given the recent calls to reduce the national rate of severe maternal morbidity, new public health initiatives and practice guidelines are needed to highlight and address the morbidity risk associated with stillbirth identified in this study,” wrote Dr. Wall-Wieler and colleagues.
The study was funded by the National Institutes of Health and by Stanford University. Ronald S. Gibbs, MD, reported receiving money from Novavax/ACI. Alexander J. Butwick, MD, reported receiving money from Cerus Corp. and Instrumentation Laboratory. The other coauthors reported no relevant financial conflicts of interest.
SOURCE: Wall-Wieler E et al. Obstet Gynecol. 2019 Aug. 134:2;310-7.
, according to research in Obstetrics & Gynecology.
Citing major increases in risk for a host of serious complications, the authors of the large population-based study urge those caring for women experiencing stillbirth to be vigilant for trouble.
Severe maternal morbidity among mothers experiencing stillbirth occurred in 578 cases per 10,000 deliveries, compared with 99 cases per 10,000 live deliveries, wrote Elizabeth Wall-Wieler, PhD, and coauthors. After statistical adjustment, the relative risk (RR) for severe maternal morbidity in a stillbirth compared with a live delivery was 4.77 (95% confidence interval, 4.53-5.02).
“Our findings indicate that nearly 1 in 17 women who deliver a stillbirth in California experience severe maternal morbidity. Furthermore, the risk of severe maternal morbidity was more than fourfold higher for women undergoing stillbirth delivery than live birth delivery,” the investigators wrote.
Major maternal organ dysfunction or failure – including acute renal failure, adult respiratory distress syndrome, disseminated intravascular coagulation, sepsis, or shock – all were more common in stillbirth deliveries, noted Dr. Wall-Wieler and colleagues. Hysterectomy, likely performed to control major loss of blood, also was more likely in stillbirth deliveries.
“Minimal attention has been given to maternal outcomes and acute complications experienced by women who have a stillbirth,” wrote Dr. Wall-Wieler, a postdoctoral research fellow in developmental and neonatal medicine, and colleagues at Stanford (Calif.) University. This is so because many analyses of maternal morbidity exclude stillbirth deliveries, or lump them with term deliveries, she and coauthors explained.
Using data from the Office of Statewide Health Planning and Development in California, Dr. Wall-Wieler and colleagues examined a total of 6,459,842 deliveries occurring in the state during 1999-2011; of these, 25,997 (0.4%) were stillbirths. For the cross-sectional study, the investigators included only deliveries for which fetal or neonatal vital records could be linked with the maternal hospital record.
Stillbirth was defined in the study as a fetal death delivered at or after 20 weeks’ gestation, so deliveries at less than 20 weeks’ gestation were excluded, as were any deliveries recorded as being at or after 45 weeks’ gestation, because the latter set were considered likely to be data entry errors.
Deliveries were considered to have severe maternal morbidity if any of the 18 indicators identified by the Centers for Disease Control and Prevention were coded in the medical record. The most common severe morbidities seen in stillbirth were blood transfusion, disseminated intravascular coagulation, and acute renal failure (adjusted RRs 5.38, 8.78, and 13.22, respectively). Although absolute occurrences were less frequent, relative risk for sepsis and shock were more than 14 times higher for stillbirths than for live birth deliveries.
“Taken together, these findings suggest the morbidity associated with obstetric hemorrhage and preeclampsia among women hospitalized for stillbirth delivery is a serious concern,” wrote Dr. Wall-Wieler and coauthors. They called for prospective studies to clarify cause and effect between stillbirth and these morbidities and to look into whether women carrying a nonviable fetus or with known fetal demise are managed differently than those with a viable fetus.
Overall, stillbirth deliveries were more likely for women who were older, for non-Hispanic black women, for those who did not have a college education, and those who did not have private insurance. Preexisting diabetes and hypertension, as well as a vaginal delivery, also upped the risk for stillbirth.
For reasons that are not completely clear, the risk for severe maternal morbidity with stillbirth climbed after 30 weeks’ gestation. Dr. Wall-Wieler and collaborators conducted an exploratory analysis that dichotomized deliveries for both stillbirth and live births into those occurring at fewer than 30 weeks’ gestation, or at or after 30 weeks’. They found no increased risk for severe maternal morbidity earlier than 30 weeks, but an RR of 5.4 for stillbirth at or after 30 weeks.
A reported cause of fetal demise was available for 71% of deliveries, with umbilical cord anomalies, obstetric complications, and placental conditions collectively accounting for almost half (46%) of the identified causes of demise. Severe maternal morbidity was most common in deaths related to hypertensive disorders, at 24/100, and least common in deaths from major fetal structural or genetic problems, at 1/100.
The size of the study strengthens the findings, said the investigators, but the large amount of missing data in recording fetal deaths does introduce some limitations. These include the inability to distinguish between intrapartum and antepartum fetal death, as well as the fact that cause of fetal death was not recorded for over one in four stillbirths.
“Given the recent calls to reduce the national rate of severe maternal morbidity, new public health initiatives and practice guidelines are needed to highlight and address the morbidity risk associated with stillbirth identified in this study,” wrote Dr. Wall-Wieler and colleagues.
The study was funded by the National Institutes of Health and by Stanford University. Ronald S. Gibbs, MD, reported receiving money from Novavax/ACI. Alexander J. Butwick, MD, reported receiving money from Cerus Corp. and Instrumentation Laboratory. The other coauthors reported no relevant financial conflicts of interest.
SOURCE: Wall-Wieler E et al. Obstet Gynecol. 2019 Aug. 134:2;310-7.
, according to research in Obstetrics & Gynecology.
Citing major increases in risk for a host of serious complications, the authors of the large population-based study urge those caring for women experiencing stillbirth to be vigilant for trouble.
Severe maternal morbidity among mothers experiencing stillbirth occurred in 578 cases per 10,000 deliveries, compared with 99 cases per 10,000 live deliveries, wrote Elizabeth Wall-Wieler, PhD, and coauthors. After statistical adjustment, the relative risk (RR) for severe maternal morbidity in a stillbirth compared with a live delivery was 4.77 (95% confidence interval, 4.53-5.02).
“Our findings indicate that nearly 1 in 17 women who deliver a stillbirth in California experience severe maternal morbidity. Furthermore, the risk of severe maternal morbidity was more than fourfold higher for women undergoing stillbirth delivery than live birth delivery,” the investigators wrote.
Major maternal organ dysfunction or failure – including acute renal failure, adult respiratory distress syndrome, disseminated intravascular coagulation, sepsis, or shock – all were more common in stillbirth deliveries, noted Dr. Wall-Wieler and colleagues. Hysterectomy, likely performed to control major loss of blood, also was more likely in stillbirth deliveries.
“Minimal attention has been given to maternal outcomes and acute complications experienced by women who have a stillbirth,” wrote Dr. Wall-Wieler, a postdoctoral research fellow in developmental and neonatal medicine, and colleagues at Stanford (Calif.) University. This is so because many analyses of maternal morbidity exclude stillbirth deliveries, or lump them with term deliveries, she and coauthors explained.
Using data from the Office of Statewide Health Planning and Development in California, Dr. Wall-Wieler and colleagues examined a total of 6,459,842 deliveries occurring in the state during 1999-2011; of these, 25,997 (0.4%) were stillbirths. For the cross-sectional study, the investigators included only deliveries for which fetal or neonatal vital records could be linked with the maternal hospital record.
Stillbirth was defined in the study as a fetal death delivered at or after 20 weeks’ gestation, so deliveries at less than 20 weeks’ gestation were excluded, as were any deliveries recorded as being at or after 45 weeks’ gestation, because the latter set were considered likely to be data entry errors.
Deliveries were considered to have severe maternal morbidity if any of the 18 indicators identified by the Centers for Disease Control and Prevention were coded in the medical record. The most common severe morbidities seen in stillbirth were blood transfusion, disseminated intravascular coagulation, and acute renal failure (adjusted RRs 5.38, 8.78, and 13.22, respectively). Although absolute occurrences were less frequent, relative risk for sepsis and shock were more than 14 times higher for stillbirths than for live birth deliveries.
“Taken together, these findings suggest the morbidity associated with obstetric hemorrhage and preeclampsia among women hospitalized for stillbirth delivery is a serious concern,” wrote Dr. Wall-Wieler and coauthors. They called for prospective studies to clarify cause and effect between stillbirth and these morbidities and to look into whether women carrying a nonviable fetus or with known fetal demise are managed differently than those with a viable fetus.
Overall, stillbirth deliveries were more likely for women who were older, for non-Hispanic black women, for those who did not have a college education, and those who did not have private insurance. Preexisting diabetes and hypertension, as well as a vaginal delivery, also upped the risk for stillbirth.
For reasons that are not completely clear, the risk for severe maternal morbidity with stillbirth climbed after 30 weeks’ gestation. Dr. Wall-Wieler and collaborators conducted an exploratory analysis that dichotomized deliveries for both stillbirth and live births into those occurring at fewer than 30 weeks’ gestation, or at or after 30 weeks’. They found no increased risk for severe maternal morbidity earlier than 30 weeks, but an RR of 5.4 for stillbirth at or after 30 weeks.
A reported cause of fetal demise was available for 71% of deliveries, with umbilical cord anomalies, obstetric complications, and placental conditions collectively accounting for almost half (46%) of the identified causes of demise. Severe maternal morbidity was most common in deaths related to hypertensive disorders, at 24/100, and least common in deaths from major fetal structural or genetic problems, at 1/100.
The size of the study strengthens the findings, said the investigators, but the large amount of missing data in recording fetal deaths does introduce some limitations. These include the inability to distinguish between intrapartum and antepartum fetal death, as well as the fact that cause of fetal death was not recorded for over one in four stillbirths.
“Given the recent calls to reduce the national rate of severe maternal morbidity, new public health initiatives and practice guidelines are needed to highlight and address the morbidity risk associated with stillbirth identified in this study,” wrote Dr. Wall-Wieler and colleagues.
The study was funded by the National Institutes of Health and by Stanford University. Ronald S. Gibbs, MD, reported receiving money from Novavax/ACI. Alexander J. Butwick, MD, reported receiving money from Cerus Corp. and Instrumentation Laboratory. The other coauthors reported no relevant financial conflicts of interest.
SOURCE: Wall-Wieler E et al. Obstet Gynecol. 2019 Aug. 134:2;310-7.
FROM OBSTETRICS & GYNECOLOGY
Gaps in patient-provider survivorship communication persist
There has been little to no recent improvement in the large share of cancer patients who are not receiving detailed information about survivorship care, suggests a nationally representative cross-sectional survey.
In 2006, the Institute of Medicine issued a seminal report recommending survivorship care planning to address the special needs of this patient population, noted the investigators, led by Ashish Rai, PhD, American Cancer Society, Framingham, Mass. Other organizations have since issued guidelines and policies in this area.
For the study, Dr. Rai and colleagues analyzed data from 2,266 survivors who completed the 2011 or 2016 Medical Expenditure Panel Survey – Experiences with Cancer questionnaire. Survivors were asked whether any clinician had ever discussed various aspects of survivorship care; responses were dichotomized as having had detailed discussion versus not (brief or no discussion, or not remembering).
Between 2011 and 2016, there was minimal change in the percentage of survivors who reported not receiving detailed information on follow-up care (from 35.1% to 35.4%), late or long-term adverse effects (from 54.2% to 55.5%), lifestyle recommendations (from 58.9% to 57.8%), and emotional or social needs (from 69.2% to 68.2%), the investigators wrote. Their report is in Journal of Oncology Practice.
When analyses were restricted to only those survivors who had received cancer-directed treatment within 3 years of the survey, findings were essentially the same.
About one-quarter of survivors reported having detailed discussions about all four topics in both 2011 (24.4%) and 2016 (21.9%).
In 2016, nearly half of survivors, 47.6%, reported not having detailed discussions with their providers about a summary of their cancer treatments. (This question was not asked in 2011.)
“Despite national efforts and organizations promoting survivorship care planning and highlighting the need for improved quality of survivorship care delivery, clear gaps in quality of communication between survivors of cancer and providers persist,” Dr. Rai and colleagues said.
“Continued efforts are needed to promote communication about survivorship issues, including implementation and evaluation of targeted interventions in key survivorship care areas,” they recommended. “These interventions may consist of furnishing guidance on optimal ways to identify and address survivors’ communication needs, streamlining the flow of information across provider types, ensuring better integration of primary care providers with the survivorship care paradigm, and augmenting the use of health information technology for collection and dissemination of information across the cancer control continuum.”
Dr. Rai did not disclose any relevant conflicts of interest. The study did not receive specific funding.
SOURCE: Rai A et al. J Oncol Pract. 2019 July 2. doi: 10.1200/JOP.19.00157.
There has been little to no recent improvement in the large share of cancer patients who are not receiving detailed information about survivorship care, suggests a nationally representative cross-sectional survey.
In 2006, the Institute of Medicine issued a seminal report recommending survivorship care planning to address the special needs of this patient population, noted the investigators, led by Ashish Rai, PhD, American Cancer Society, Framingham, Mass. Other organizations have since issued guidelines and policies in this area.
For the study, Dr. Rai and colleagues analyzed data from 2,266 survivors who completed the 2011 or 2016 Medical Expenditure Panel Survey – Experiences with Cancer questionnaire. Survivors were asked whether any clinician had ever discussed various aspects of survivorship care; responses were dichotomized as having had detailed discussion versus not (brief or no discussion, or not remembering).
Between 2011 and 2016, there was minimal change in the percentage of survivors who reported not receiving detailed information on follow-up care (from 35.1% to 35.4%), late or long-term adverse effects (from 54.2% to 55.5%), lifestyle recommendations (from 58.9% to 57.8%), and emotional or social needs (from 69.2% to 68.2%), the investigators wrote. Their report is in Journal of Oncology Practice.
When analyses were restricted to only those survivors who had received cancer-directed treatment within 3 years of the survey, findings were essentially the same.
About one-quarter of survivors reported having detailed discussions about all four topics in both 2011 (24.4%) and 2016 (21.9%).
In 2016, nearly half of survivors, 47.6%, reported not having detailed discussions with their providers about a summary of their cancer treatments. (This question was not asked in 2011.)
“Despite national efforts and organizations promoting survivorship care planning and highlighting the need for improved quality of survivorship care delivery, clear gaps in quality of communication between survivors of cancer and providers persist,” Dr. Rai and colleagues said.
“Continued efforts are needed to promote communication about survivorship issues, including implementation and evaluation of targeted interventions in key survivorship care areas,” they recommended. “These interventions may consist of furnishing guidance on optimal ways to identify and address survivors’ communication needs, streamlining the flow of information across provider types, ensuring better integration of primary care providers with the survivorship care paradigm, and augmenting the use of health information technology for collection and dissemination of information across the cancer control continuum.”
Dr. Rai did not disclose any relevant conflicts of interest. The study did not receive specific funding.
SOURCE: Rai A et al. J Oncol Pract. 2019 July 2. doi: 10.1200/JOP.19.00157.
There has been little to no recent improvement in the large share of cancer patients who are not receiving detailed information about survivorship care, suggests a nationally representative cross-sectional survey.
In 2006, the Institute of Medicine issued a seminal report recommending survivorship care planning to address the special needs of this patient population, noted the investigators, led by Ashish Rai, PhD, American Cancer Society, Framingham, Mass. Other organizations have since issued guidelines and policies in this area.
For the study, Dr. Rai and colleagues analyzed data from 2,266 survivors who completed the 2011 or 2016 Medical Expenditure Panel Survey – Experiences with Cancer questionnaire. Survivors were asked whether any clinician had ever discussed various aspects of survivorship care; responses were dichotomized as having had detailed discussion versus not (brief or no discussion, or not remembering).
Between 2011 and 2016, there was minimal change in the percentage of survivors who reported not receiving detailed information on follow-up care (from 35.1% to 35.4%), late or long-term adverse effects (from 54.2% to 55.5%), lifestyle recommendations (from 58.9% to 57.8%), and emotional or social needs (from 69.2% to 68.2%), the investigators wrote. Their report is in Journal of Oncology Practice.
When analyses were restricted to only those survivors who had received cancer-directed treatment within 3 years of the survey, findings were essentially the same.
About one-quarter of survivors reported having detailed discussions about all four topics in both 2011 (24.4%) and 2016 (21.9%).
In 2016, nearly half of survivors, 47.6%, reported not having detailed discussions with their providers about a summary of their cancer treatments. (This question was not asked in 2011.)
“Despite national efforts and organizations promoting survivorship care planning and highlighting the need for improved quality of survivorship care delivery, clear gaps in quality of communication between survivors of cancer and providers persist,” Dr. Rai and colleagues said.
“Continued efforts are needed to promote communication about survivorship issues, including implementation and evaluation of targeted interventions in key survivorship care areas,” they recommended. “These interventions may consist of furnishing guidance on optimal ways to identify and address survivors’ communication needs, streamlining the flow of information across provider types, ensuring better integration of primary care providers with the survivorship care paradigm, and augmenting the use of health information technology for collection and dissemination of information across the cancer control continuum.”
Dr. Rai did not disclose any relevant conflicts of interest. The study did not receive specific funding.
SOURCE: Rai A et al. J Oncol Pract. 2019 July 2. doi: 10.1200/JOP.19.00157.
FROM THE JOURNAL OF ONCOLOGY PRACTICE
Measles cases have slowed but not stopped
The United States continues to slowly add new cases of measles to 2019’s postelimination-record total, but California was officially removed from the outbreak list this week, according to the Centers for Disease Control and Prevention.
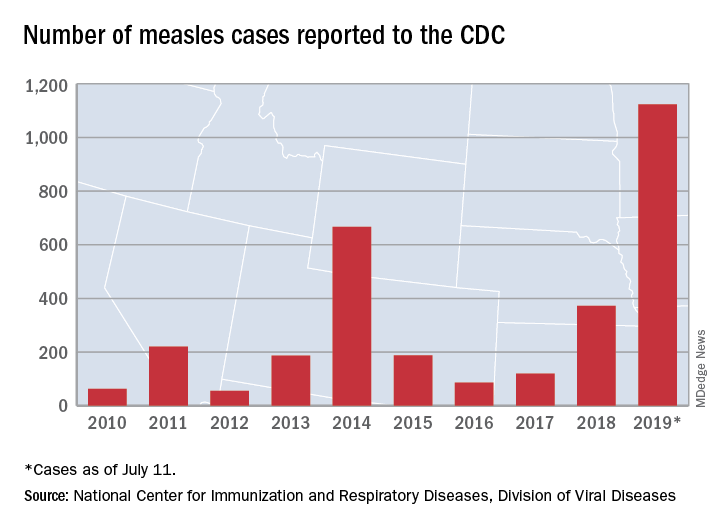
That is the highest number of cases reported since measles was declared eliminated in 2000 and the most in a single year since 1992.
The end of outbreak-related activity in California leaves three locations still dealing with ongoing cases: Rockland County, N.Y.; New York City; and King, Pierce, and Snohomish Counties in Washington, the CDC said.
Those three jurisdictions currently report the following:
- reported four new cases from July 3 to July 11 and is up to 175 cases for the year.
- had one new case from July 1 to July 8 and is now at 564 for the year.
- reported two cases from July 1 to July 10 and is now at 10 for the year (the other two counties have a total of three cases). Clark County in Washington reported 71 cases in an earlier, unrelated outbreak.
The United States continues to slowly add new cases of measles to 2019’s postelimination-record total, but California was officially removed from the outbreak list this week, according to the Centers for Disease Control and Prevention.

That is the highest number of cases reported since measles was declared eliminated in 2000 and the most in a single year since 1992.
The end of outbreak-related activity in California leaves three locations still dealing with ongoing cases: Rockland County, N.Y.; New York City; and King, Pierce, and Snohomish Counties in Washington, the CDC said.
Those three jurisdictions currently report the following:
- reported four new cases from July 3 to July 11 and is up to 175 cases for the year.
- had one new case from July 1 to July 8 and is now at 564 for the year.
- reported two cases from July 1 to July 10 and is now at 10 for the year (the other two counties have a total of three cases). Clark County in Washington reported 71 cases in an earlier, unrelated outbreak.
The United States continues to slowly add new cases of measles to 2019’s postelimination-record total, but California was officially removed from the outbreak list this week, according to the Centers for Disease Control and Prevention.

That is the highest number of cases reported since measles was declared eliminated in 2000 and the most in a single year since 1992.
The end of outbreak-related activity in California leaves three locations still dealing with ongoing cases: Rockland County, N.Y.; New York City; and King, Pierce, and Snohomish Counties in Washington, the CDC said.
Those three jurisdictions currently report the following:
- reported four new cases from July 3 to July 11 and is up to 175 cases for the year.
- had one new case from July 1 to July 8 and is now at 564 for the year.
- reported two cases from July 1 to July 10 and is now at 10 for the year (the other two counties have a total of three cases). Clark County in Washington reported 71 cases in an earlier, unrelated outbreak.
Overreliance on DAS scores undermines rheumatoid arthritis management
MADRID – Two major changes that improved RA management in recent years – the introduction of potent biologic and targeted synthetic drugs to control inflammatory disease, and the treat-to-target strategy – have also produced an unanticipated snag in the care patients receive. Their persistent comorbidities and their more atypical rheumatoid manifestations often go overlooked and untreated.
The situation has been dubbed “DAS blindness,” when clinicians caring for patients with RA are so focused on a patient’s disease activity score (DAS), measured by counting their swollen and tender joints (usually 28 joints to tally the DAS28 score), that they lose sight of other important features of a RA patient’s disease such as pain and fatigue, Ruth Williams, MBChB, said in an invited talk at the European Congress of Rheumatology.
“There is so much focus on the DAS28 that people are blinded by it. Clinicians concentrate too much on the primary physical condition” of RA, “and they miss important functional, psychological, and social impacts of the disease,” said Dr. Williams, a general-practice physician who is also a long-time RA patient who works as a patient representative and RA researcher at King’s College London.
In Dr. William’s extended personal experience as an RA patient (she was first diagnosed in 1966 as a child), management of the disease changed dramatically with the relatively recent, widespread adoption of the DAS28 score in routine clinical practice in Europe and the United States, migrating from its initial use in research studies. Once her clinicians began to use the DAS28 “I felt that perhaps I wasn’t being seen anymore. It was just the biology of my disease being noted rather than me as an individual,” Dr. Williams said in an interview. Clinicians “need to discuss with patients what remission means to them, and their objectives” from treatment, because a patient’s treatment goals may go beyond just reducing the number of swollen or tender joints they total in the DAS28 assessment.
Rheumatologists also have begun to recognize this common disconnect between both the assessment and the antirheumatoid treatment that RA patients routinely receive, and the symptoms that cause problems for RA patients that are not directly tied to their inflammatory disease. Patients can present with remission-level responses in their tender and swollen joint counts and in their serum level of C-reactive protein or erythrocyte sedimentation rate but still score high on the patient global assessment (PGA) scale, a residual consequence of RA that places them out of remission range based on the 2011 “Boolean” criteria for RA remission in trials endorsed by the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) (Arthritis Rheum. 2011 Mar;63[3]:373-86).
In a review of 411 RA patients who met three of the four ACR/EULAR criteria that collectively define remission, 61% missed on the PGA measure (Ann Rheum Dis. 2012 Oct;71[10]:1702-5), noted Joan M. Bathon, MD, professor of medicine and director of rheumatology at Columbia University, New York, in a talk during the Congress. Another review of 273 RA patients who missed on one of the four criteria showed 80% missing because of their PGA score (Arthritis Res Ther. 2013;15:R221). The specific clinical features that triggered high PGAs in these patients were things like fibromyalgia, back pain, anxiety, depression, and rheumatoid activity in joints not included in the DAS28 score, Dr. Bathon noted. The PGA can have poor correlation with the other three measures, but that is a strength because it reflects different dimensions of RA that are important to patients. When the PGA is discordant with the other three measures of remission, it may not make sense to try to improve it by simply using more immunosuppressive treatment.
The solution to the dilemma of what remission target to aim for when treating to target is to apply common sense to existing guidelines and recommendations and tailor management to each patient, she concluded. “The worst thing we can do is to take criteria meant for clinical rials and for patients with average scores and apply them to every individual patient,” she said. Remission guidelines are good for large populations, “but we shouldn’t apply them to every single patient without thinking.”
A similar plea for thoughtful use of the treat-to-target model and immunomodulatory treatment came in a separate talk from Laure Gossec, MD, a professor of rheumatology at Pitie-Salpétriere Hospital and Sorbonne University in Paris.
The challenge of DAS28 is that it was a remission criteria developed by the ACR and EULAR to use in clinical trials that was coopted for use in routine practice. Despite that, Dr. Gossec believes that DAS28 largely succeeded in this transition. “The DAS28 performs well, it has good prognostic capacity and is widely used.” In her practice, Dr. Gossec relies on the DAS28 score as her primary tool to track disease status in RA patients. “It’s not perfect, but I’m familiar with it, and I work with it,” she said.
It’s undeniable, she acknowledged, that a high PGA often stands between a patient and remission. PGA “is hard to use to guide anti-inflammatory treatment. Many patients have high PGA scores even though they have no inflammation.” Discrepancies like this create a case for dual-treatment targets, both a low swollen and tender joint count and low PGA, as separate and equal treatment goals, Dr. Gossec said, an approach she and her associates proposed in a recent article (Arthritis Care Res. 2018 Mar;709[3]:369-78).
Dr. Williams had no disclosures. Dr. Bathon has been a consultant to AbbVie and has received research funding from Bristol-Myers Squibb and Pfizer. Dr. Gossec has been a consultant to and has received research funding from several companies.
MADRID – Two major changes that improved RA management in recent years – the introduction of potent biologic and targeted synthetic drugs to control inflammatory disease, and the treat-to-target strategy – have also produced an unanticipated snag in the care patients receive. Their persistent comorbidities and their more atypical rheumatoid manifestations often go overlooked and untreated.
The situation has been dubbed “DAS blindness,” when clinicians caring for patients with RA are so focused on a patient’s disease activity score (DAS), measured by counting their swollen and tender joints (usually 28 joints to tally the DAS28 score), that they lose sight of other important features of a RA patient’s disease such as pain and fatigue, Ruth Williams, MBChB, said in an invited talk at the European Congress of Rheumatology.
“There is so much focus on the DAS28 that people are blinded by it. Clinicians concentrate too much on the primary physical condition” of RA, “and they miss important functional, psychological, and social impacts of the disease,” said Dr. Williams, a general-practice physician who is also a long-time RA patient who works as a patient representative and RA researcher at King’s College London.
In Dr. William’s extended personal experience as an RA patient (she was first diagnosed in 1966 as a child), management of the disease changed dramatically with the relatively recent, widespread adoption of the DAS28 score in routine clinical practice in Europe and the United States, migrating from its initial use in research studies. Once her clinicians began to use the DAS28 “I felt that perhaps I wasn’t being seen anymore. It was just the biology of my disease being noted rather than me as an individual,” Dr. Williams said in an interview. Clinicians “need to discuss with patients what remission means to them, and their objectives” from treatment, because a patient’s treatment goals may go beyond just reducing the number of swollen or tender joints they total in the DAS28 assessment.
Rheumatologists also have begun to recognize this common disconnect between both the assessment and the antirheumatoid treatment that RA patients routinely receive, and the symptoms that cause problems for RA patients that are not directly tied to their inflammatory disease. Patients can present with remission-level responses in their tender and swollen joint counts and in their serum level of C-reactive protein or erythrocyte sedimentation rate but still score high on the patient global assessment (PGA) scale, a residual consequence of RA that places them out of remission range based on the 2011 “Boolean” criteria for RA remission in trials endorsed by the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) (Arthritis Rheum. 2011 Mar;63[3]:373-86).
In a review of 411 RA patients who met three of the four ACR/EULAR criteria that collectively define remission, 61% missed on the PGA measure (Ann Rheum Dis. 2012 Oct;71[10]:1702-5), noted Joan M. Bathon, MD, professor of medicine and director of rheumatology at Columbia University, New York, in a talk during the Congress. Another review of 273 RA patients who missed on one of the four criteria showed 80% missing because of their PGA score (Arthritis Res Ther. 2013;15:R221). The specific clinical features that triggered high PGAs in these patients were things like fibromyalgia, back pain, anxiety, depression, and rheumatoid activity in joints not included in the DAS28 score, Dr. Bathon noted. The PGA can have poor correlation with the other three measures, but that is a strength because it reflects different dimensions of RA that are important to patients. When the PGA is discordant with the other three measures of remission, it may not make sense to try to improve it by simply using more immunosuppressive treatment.
The solution to the dilemma of what remission target to aim for when treating to target is to apply common sense to existing guidelines and recommendations and tailor management to each patient, she concluded. “The worst thing we can do is to take criteria meant for clinical rials and for patients with average scores and apply them to every individual patient,” she said. Remission guidelines are good for large populations, “but we shouldn’t apply them to every single patient without thinking.”
A similar plea for thoughtful use of the treat-to-target model and immunomodulatory treatment came in a separate talk from Laure Gossec, MD, a professor of rheumatology at Pitie-Salpétriere Hospital and Sorbonne University in Paris.
The challenge of DAS28 is that it was a remission criteria developed by the ACR and EULAR to use in clinical trials that was coopted for use in routine practice. Despite that, Dr. Gossec believes that DAS28 largely succeeded in this transition. “The DAS28 performs well, it has good prognostic capacity and is widely used.” In her practice, Dr. Gossec relies on the DAS28 score as her primary tool to track disease status in RA patients. “It’s not perfect, but I’m familiar with it, and I work with it,” she said.
It’s undeniable, she acknowledged, that a high PGA often stands between a patient and remission. PGA “is hard to use to guide anti-inflammatory treatment. Many patients have high PGA scores even though they have no inflammation.” Discrepancies like this create a case for dual-treatment targets, both a low swollen and tender joint count and low PGA, as separate and equal treatment goals, Dr. Gossec said, an approach she and her associates proposed in a recent article (Arthritis Care Res. 2018 Mar;709[3]:369-78).
Dr. Williams had no disclosures. Dr. Bathon has been a consultant to AbbVie and has received research funding from Bristol-Myers Squibb and Pfizer. Dr. Gossec has been a consultant to and has received research funding from several companies.
MADRID – Two major changes that improved RA management in recent years – the introduction of potent biologic and targeted synthetic drugs to control inflammatory disease, and the treat-to-target strategy – have also produced an unanticipated snag in the care patients receive. Their persistent comorbidities and their more atypical rheumatoid manifestations often go overlooked and untreated.
The situation has been dubbed “DAS blindness,” when clinicians caring for patients with RA are so focused on a patient’s disease activity score (DAS), measured by counting their swollen and tender joints (usually 28 joints to tally the DAS28 score), that they lose sight of other important features of a RA patient’s disease such as pain and fatigue, Ruth Williams, MBChB, said in an invited talk at the European Congress of Rheumatology.
“There is so much focus on the DAS28 that people are blinded by it. Clinicians concentrate too much on the primary physical condition” of RA, “and they miss important functional, psychological, and social impacts of the disease,” said Dr. Williams, a general-practice physician who is also a long-time RA patient who works as a patient representative and RA researcher at King’s College London.
In Dr. William’s extended personal experience as an RA patient (she was first diagnosed in 1966 as a child), management of the disease changed dramatically with the relatively recent, widespread adoption of the DAS28 score in routine clinical practice in Europe and the United States, migrating from its initial use in research studies. Once her clinicians began to use the DAS28 “I felt that perhaps I wasn’t being seen anymore. It was just the biology of my disease being noted rather than me as an individual,” Dr. Williams said in an interview. Clinicians “need to discuss with patients what remission means to them, and their objectives” from treatment, because a patient’s treatment goals may go beyond just reducing the number of swollen or tender joints they total in the DAS28 assessment.
Rheumatologists also have begun to recognize this common disconnect between both the assessment and the antirheumatoid treatment that RA patients routinely receive, and the symptoms that cause problems for RA patients that are not directly tied to their inflammatory disease. Patients can present with remission-level responses in their tender and swollen joint counts and in their serum level of C-reactive protein or erythrocyte sedimentation rate but still score high on the patient global assessment (PGA) scale, a residual consequence of RA that places them out of remission range based on the 2011 “Boolean” criteria for RA remission in trials endorsed by the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) (Arthritis Rheum. 2011 Mar;63[3]:373-86).
In a review of 411 RA patients who met three of the four ACR/EULAR criteria that collectively define remission, 61% missed on the PGA measure (Ann Rheum Dis. 2012 Oct;71[10]:1702-5), noted Joan M. Bathon, MD, professor of medicine and director of rheumatology at Columbia University, New York, in a talk during the Congress. Another review of 273 RA patients who missed on one of the four criteria showed 80% missing because of their PGA score (Arthritis Res Ther. 2013;15:R221). The specific clinical features that triggered high PGAs in these patients were things like fibromyalgia, back pain, anxiety, depression, and rheumatoid activity in joints not included in the DAS28 score, Dr. Bathon noted. The PGA can have poor correlation with the other three measures, but that is a strength because it reflects different dimensions of RA that are important to patients. When the PGA is discordant with the other three measures of remission, it may not make sense to try to improve it by simply using more immunosuppressive treatment.
The solution to the dilemma of what remission target to aim for when treating to target is to apply common sense to existing guidelines and recommendations and tailor management to each patient, she concluded. “The worst thing we can do is to take criteria meant for clinical rials and for patients with average scores and apply them to every individual patient,” she said. Remission guidelines are good for large populations, “but we shouldn’t apply them to every single patient without thinking.”
A similar plea for thoughtful use of the treat-to-target model and immunomodulatory treatment came in a separate talk from Laure Gossec, MD, a professor of rheumatology at Pitie-Salpétriere Hospital and Sorbonne University in Paris.
The challenge of DAS28 is that it was a remission criteria developed by the ACR and EULAR to use in clinical trials that was coopted for use in routine practice. Despite that, Dr. Gossec believes that DAS28 largely succeeded in this transition. “The DAS28 performs well, it has good prognostic capacity and is widely used.” In her practice, Dr. Gossec relies on the DAS28 score as her primary tool to track disease status in RA patients. “It’s not perfect, but I’m familiar with it, and I work with it,” she said.
It’s undeniable, she acknowledged, that a high PGA often stands between a patient and remission. PGA “is hard to use to guide anti-inflammatory treatment. Many patients have high PGA scores even though they have no inflammation.” Discrepancies like this create a case for dual-treatment targets, both a low swollen and tender joint count and low PGA, as separate and equal treatment goals, Dr. Gossec said, an approach she and her associates proposed in a recent article (Arthritis Care Res. 2018 Mar;709[3]:369-78).
Dr. Williams had no disclosures. Dr. Bathon has been a consultant to AbbVie and has received research funding from Bristol-Myers Squibb and Pfizer. Dr. Gossec has been a consultant to and has received research funding from several companies.
REPORTING FROM EULAR 2019 CONGRESS
There’s Mischief Afoot

ANSWER
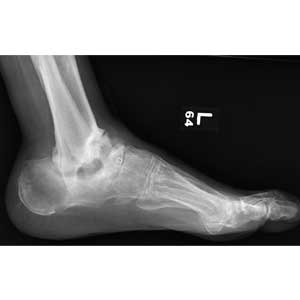
The radiograph demonstrates no evidence of an acute fracture or soft-tissue gas to suggest an abscess. Of note, though, within the tibiotalar joint, the patient has bony destruction and settling of the articular surfaces of both the distal tibia and fibula into the talus and calcaneus.
This finding is typically associated with neuropathic arthropathy (also known as a Charcot joint). This pathologic process is typically seen in a weight-bearing joint that develops progressive degeneration from chronic loss of sensation.

ANSWER
The radiograph demonstrates no evidence of an acute fracture or soft-tissue gas to suggest an abscess. Of note, though, within the tibiotalar joint, the patient has bony destruction and settling of the articular surfaces of both the distal tibia and fibula into the talus and calcaneus.
This finding is typically associated with neuropathic arthropathy (also known as a Charcot joint). This pathologic process is typically seen in a weight-bearing joint that develops progressive degeneration from chronic loss of sensation.

ANSWER
The radiograph demonstrates no evidence of an acute fracture or soft-tissue gas to suggest an abscess. Of note, though, within the tibiotalar joint, the patient has bony destruction and settling of the articular surfaces of both the distal tibia and fibula into the talus and calcaneus.
This finding is typically associated with neuropathic arthropathy (also known as a Charcot joint). This pathologic process is typically seen in a weight-bearing joint that develops progressive degeneration from chronic loss of sensation.

A 70-year-old man presents for evaluation of left foot pain, redness, and swelling. He reports injuring the foot a week ago; he went to the emergency department for evaluation of the cut he had sustained, which required stapling.
The patient has a chronic foot ulcer for which a home health aide provides wound care and dressing changes. His medical history is significant for hypertension, stroke with chronic left-sided weakness, congestive heart failure, and chronic renal insufficiency. He admits to daily tobacco use, and his medical record reflects a history of drug use.
On physical exam, you note an elderly, chronically ill male in no obvious distress. His vital signs are stable, and he is afebrile. Inspection of his left foot shows generalized swelling and redness. Good distal pulses are appreciated. On the dorsal aspect, there is a healing wound with a single staple present. On the heel is a 2-cm stage 2 ulcer with some scant purulent drainage.
Bloodwork and a radiograph of the left foot are ordered; lateral view is shown. What is your impression?
Microbiome – Impact on health and disease
The gut microbiota influences our biology through our mucosal immune system as well as by leading to the production of bioactive small molecules. I’ll describe how gut microbiota influences colon cancer, liver disease, the production of bioactive compounds, as well as the current status and future prospects of microbiota therapeutics.
The gut microbiota may be a factor in colon cancer. Studies have shown that bacterial biofilms are associated with right-sided colon cancers in humans. More recently, a study has shown that mucosal biofilm formation is carcinogenic in an animal model, suggesting that such biofilms may play a role in the disease pathogenesis. From the standpoint of the liver, the microbiome may be a biomarker for diseases such as cirrhosis and fibrosis in patients with nonalcoholic steatohepatitis. Therapeutically, a recent study suggests that the function of gut microbiota can be altered by introducing an engineered Escherichia coli bacterial strain to treat hyperammonemia by modifying its metabolism to overproduce arginine, thereby sequestering ammonia produced by gut bacteria into the amino acids (Sci Transl Med. 2019 Jan 16;11[475]. doi: 10.1126/scitranslmed.aau7975). Drug metabolism also can be influenced by the gut microbiota and vice versa. For example, drugs such as metformin have effects on the composition of the gut microbiota in humans. In turn, the gut microbiota and its metabolites can have an influence on hepatic drug metabolism, thereby altering xenobiotic pharmacokinetics and pharmacodynamics.
The production of bioactive small molecules by bacterial metabolism is a topic of intense interest in the microbiome field. Such small molecules have been shown to act as antibiotics, neurotransmitters, immune modulators, and ligands for host receptors. Some of these small metabolites are generated through the dietary aromatic amino acids in which the bacterial enzymatic pathways are being elucidated. Such small molecules have a myriad of functions. For example, indole propionic acid, a bacterial metabolite of tryptophan, can activate the pregnane X receptor to fortify intestinal epithelial barrier function, a pathway that may have relevance to inflammatory bowel disease.
Probiotics that are found in dietary supplements represent our currently available strategy for the prevention and/or treatment of disease through the delivery of specific live microbes. However, there are limitations to their effectiveness since none have been approved for the prevention or treatment of any disease process. Via an intensive human subject study, (Cell. 2018 Sep 6;174[6]:1388-405) investigators have shown that the mucosally associated microbiota was a better biomarker for probiotic engraftment than stool was, where the response was very personalized. It’s possible that the personalized nature of probiotic engraftment may indicate that “one size may not fit all.” There will be a technical review and guideline document published by the American Gastroenterological Association early in 2020.
Currently, the only effective therapeutic modality for the treatment of a human disease by deeply altering the composition of the gut microbiota is the use of fecal microbiota transplantation (FMT) for the treatment of recurrent Clostridium difficile infection (CDI). However, there is now early evidence that FMT might have efficacy in the treatment of a disease other than recurrent CDI, namely ulcerative colitis. Although the short-term risks for FMT are low and quantifiable and long-term risks are largely hypothetical, there is a need for caution and regulation in the practice of FMT. Indeed, long-term engraftment of bacterial strains from the donor into the recipient has been demonstrated. Ultimately, as the science in the microbiota field moves forward together with product development, more sophisticated microbiota-based therapeutics will be generated. During this interim period, the AGA and partner national societies have developed an FMT National Registry to gather information on FMT practice, assess effectiveness as well as short- and long-term safety, and promote scientific investigation.
In conclusion, the field of gut microbiome research is very dynamic and exciting with tremendous opportunities at the intersection between fabulous science and technology, clinical practice, and federal regulation involving the practice of FMT, concurrent in a significant interest in intellectual property and business.
Dr. Wu is the Ferdinand G. Weisbrod Professor in Gastroenterology at the University of Pennsylvania, Philadelphia. He has received research funding from Seres Therapeutics, Intercept Pharmaceuticals, and Takeda; is on the scientific advisory board for Danone and Biocodex; and does consulting for Hitachi High-Technologies. Dr. Wu made these comments during the AGA Institute Presidential Plenary at the annual Digestive Disease Week®.
The gut microbiota influences our biology through our mucosal immune system as well as by leading to the production of bioactive small molecules. I’ll describe how gut microbiota influences colon cancer, liver disease, the production of bioactive compounds, as well as the current status and future prospects of microbiota therapeutics.
The gut microbiota may be a factor in colon cancer. Studies have shown that bacterial biofilms are associated with right-sided colon cancers in humans. More recently, a study has shown that mucosal biofilm formation is carcinogenic in an animal model, suggesting that such biofilms may play a role in the disease pathogenesis. From the standpoint of the liver, the microbiome may be a biomarker for diseases such as cirrhosis and fibrosis in patients with nonalcoholic steatohepatitis. Therapeutically, a recent study suggests that the function of gut microbiota can be altered by introducing an engineered Escherichia coli bacterial strain to treat hyperammonemia by modifying its metabolism to overproduce arginine, thereby sequestering ammonia produced by gut bacteria into the amino acids (Sci Transl Med. 2019 Jan 16;11[475]. doi: 10.1126/scitranslmed.aau7975). Drug metabolism also can be influenced by the gut microbiota and vice versa. For example, drugs such as metformin have effects on the composition of the gut microbiota in humans. In turn, the gut microbiota and its metabolites can have an influence on hepatic drug metabolism, thereby altering xenobiotic pharmacokinetics and pharmacodynamics.
The production of bioactive small molecules by bacterial metabolism is a topic of intense interest in the microbiome field. Such small molecules have been shown to act as antibiotics, neurotransmitters, immune modulators, and ligands for host receptors. Some of these small metabolites are generated through the dietary aromatic amino acids in which the bacterial enzymatic pathways are being elucidated. Such small molecules have a myriad of functions. For example, indole propionic acid, a bacterial metabolite of tryptophan, can activate the pregnane X receptor to fortify intestinal epithelial barrier function, a pathway that may have relevance to inflammatory bowel disease.
Probiotics that are found in dietary supplements represent our currently available strategy for the prevention and/or treatment of disease through the delivery of specific live microbes. However, there are limitations to their effectiveness since none have been approved for the prevention or treatment of any disease process. Via an intensive human subject study, (Cell. 2018 Sep 6;174[6]:1388-405) investigators have shown that the mucosally associated microbiota was a better biomarker for probiotic engraftment than stool was, where the response was very personalized. It’s possible that the personalized nature of probiotic engraftment may indicate that “one size may not fit all.” There will be a technical review and guideline document published by the American Gastroenterological Association early in 2020.
Currently, the only effective therapeutic modality for the treatment of a human disease by deeply altering the composition of the gut microbiota is the use of fecal microbiota transplantation (FMT) for the treatment of recurrent Clostridium difficile infection (CDI). However, there is now early evidence that FMT might have efficacy in the treatment of a disease other than recurrent CDI, namely ulcerative colitis. Although the short-term risks for FMT are low and quantifiable and long-term risks are largely hypothetical, there is a need for caution and regulation in the practice of FMT. Indeed, long-term engraftment of bacterial strains from the donor into the recipient has been demonstrated. Ultimately, as the science in the microbiota field moves forward together with product development, more sophisticated microbiota-based therapeutics will be generated. During this interim period, the AGA and partner national societies have developed an FMT National Registry to gather information on FMT practice, assess effectiveness as well as short- and long-term safety, and promote scientific investigation.
In conclusion, the field of gut microbiome research is very dynamic and exciting with tremendous opportunities at the intersection between fabulous science and technology, clinical practice, and federal regulation involving the practice of FMT, concurrent in a significant interest in intellectual property and business.
Dr. Wu is the Ferdinand G. Weisbrod Professor in Gastroenterology at the University of Pennsylvania, Philadelphia. He has received research funding from Seres Therapeutics, Intercept Pharmaceuticals, and Takeda; is on the scientific advisory board for Danone and Biocodex; and does consulting for Hitachi High-Technologies. Dr. Wu made these comments during the AGA Institute Presidential Plenary at the annual Digestive Disease Week®.
The gut microbiota influences our biology through our mucosal immune system as well as by leading to the production of bioactive small molecules. I’ll describe how gut microbiota influences colon cancer, liver disease, the production of bioactive compounds, as well as the current status and future prospects of microbiota therapeutics.
The gut microbiota may be a factor in colon cancer. Studies have shown that bacterial biofilms are associated with right-sided colon cancers in humans. More recently, a study has shown that mucosal biofilm formation is carcinogenic in an animal model, suggesting that such biofilms may play a role in the disease pathogenesis. From the standpoint of the liver, the microbiome may be a biomarker for diseases such as cirrhosis and fibrosis in patients with nonalcoholic steatohepatitis. Therapeutically, a recent study suggests that the function of gut microbiota can be altered by introducing an engineered Escherichia coli bacterial strain to treat hyperammonemia by modifying its metabolism to overproduce arginine, thereby sequestering ammonia produced by gut bacteria into the amino acids (Sci Transl Med. 2019 Jan 16;11[475]. doi: 10.1126/scitranslmed.aau7975). Drug metabolism also can be influenced by the gut microbiota and vice versa. For example, drugs such as metformin have effects on the composition of the gut microbiota in humans. In turn, the gut microbiota and its metabolites can have an influence on hepatic drug metabolism, thereby altering xenobiotic pharmacokinetics and pharmacodynamics.
The production of bioactive small molecules by bacterial metabolism is a topic of intense interest in the microbiome field. Such small molecules have been shown to act as antibiotics, neurotransmitters, immune modulators, and ligands for host receptors. Some of these small metabolites are generated through the dietary aromatic amino acids in which the bacterial enzymatic pathways are being elucidated. Such small molecules have a myriad of functions. For example, indole propionic acid, a bacterial metabolite of tryptophan, can activate the pregnane X receptor to fortify intestinal epithelial barrier function, a pathway that may have relevance to inflammatory bowel disease.
Probiotics that are found in dietary supplements represent our currently available strategy for the prevention and/or treatment of disease through the delivery of specific live microbes. However, there are limitations to their effectiveness since none have been approved for the prevention or treatment of any disease process. Via an intensive human subject study, (Cell. 2018 Sep 6;174[6]:1388-405) investigators have shown that the mucosally associated microbiota was a better biomarker for probiotic engraftment than stool was, where the response was very personalized. It’s possible that the personalized nature of probiotic engraftment may indicate that “one size may not fit all.” There will be a technical review and guideline document published by the American Gastroenterological Association early in 2020.
Currently, the only effective therapeutic modality for the treatment of a human disease by deeply altering the composition of the gut microbiota is the use of fecal microbiota transplantation (FMT) for the treatment of recurrent Clostridium difficile infection (CDI). However, there is now early evidence that FMT might have efficacy in the treatment of a disease other than recurrent CDI, namely ulcerative colitis. Although the short-term risks for FMT are low and quantifiable and long-term risks are largely hypothetical, there is a need for caution and regulation in the practice of FMT. Indeed, long-term engraftment of bacterial strains from the donor into the recipient has been demonstrated. Ultimately, as the science in the microbiota field moves forward together with product development, more sophisticated microbiota-based therapeutics will be generated. During this interim period, the AGA and partner national societies have developed an FMT National Registry to gather information on FMT practice, assess effectiveness as well as short- and long-term safety, and promote scientific investigation.
In conclusion, the field of gut microbiome research is very dynamic and exciting with tremendous opportunities at the intersection between fabulous science and technology, clinical practice, and federal regulation involving the practice of FMT, concurrent in a significant interest in intellectual property and business.
Dr. Wu is the Ferdinand G. Weisbrod Professor in Gastroenterology at the University of Pennsylvania, Philadelphia. He has received research funding from Seres Therapeutics, Intercept Pharmaceuticals, and Takeda; is on the scientific advisory board for Danone and Biocodex; and does consulting for Hitachi High-Technologies. Dr. Wu made these comments during the AGA Institute Presidential Plenary at the annual Digestive Disease Week®.
Hiring the right employees
Many of the personnel questions I receive concern the dreaded “marginal employee” – a person who has never done anything truly heinous to merit firing, but neither anything special to merit continued employment. I advise getting rid of such people and then changing the hiring criteria that bring you marginal employees in the first place.
Most bad hires come about because employers do not have a clear vision of the kind of employee they want. Many office manuals do not contain detailed job descriptions. If you don’t know exactly what you are looking for, your entire selection process will be inadequate from initial screening of applicants through assessments of their skills and personalities. Many physicians compound the problem with poor interview techniques and inadequate verification.
. Take a hard look at your job descriptions, and update them if necessary. A good job description lists the major responsibilities of the position, with the relative importance of each duty and the critical knowledge, skills, and education levels necessary for each function. In other words, it describes, accurately and in detail, exactly what you expect from the employee you will hire to perform that job.
Once you have a clear job description in mind (and in print), take all the time you need to find the best possible match for it. This is not a place to cut corners. Screen your candidates carefully and avoid lowering your expectations. This is the point at which it might be tempting to settle for a marginal candidate, just to get the process over with.
It also is tempting to hire the candidate that you have the “best feeling” about, even though he or she is a poor match for the job, and then try to mold the job to that person. Every doctor knows that hunches are no substitute for hard data.
Be alert for red flags in résumés: significant time gaps between jobs; positions at companies that are no longer in business, or are otherwise impossible to verify; job titles that don’t make sense, given the applicant’s qualifications.
Background checks are a dicey subject, but publicly available information can be found, cheaply or free, on multiple websites created for that purpose. Be sure to tell applicants that you will be verifying facts in their résumés; it’s usually wise to get their written consent to do so.
Many employers skip the essential step of verification; many applicants know that. (I once actually overheard a new hire say, “I won’t be here long if they check my references.” And by golly, she was right!) If a reference is reluctant to tell you anything substantive, ask, “Would you hire this person again?” You can interpret a lot from the answer – or lack of one.
Interviews often get short shrift as well. Many doctors tend to do all the talking. The purpose of an interview is to allow you to size up the prospective employee, not to deliver a lecture on the sterling attributes of your office. Important interview topics include educational background, skills, experience, and unrelated job history.
By law, you cannot ask an applicant’s age, date of birth, sex, creed, color, religion, or national origin. Other forbidden subjects include disabilities, marital status, military record, number of children (or who cares for them), addiction history, citizenship, criminal record, psychiatric history, absenteeism, or workers’ compensation.
There are acceptable alternatives to some of those questions, however: You can ask if applicants have ever gone by another name (for your background check), for example. You can ask if they are legally authorized to work in this country, and whether they will be physically able to perform the duties specified in the job description. While past addictions are off limits, you do have a right to know about current addictions to illegal substances.
Once you have hired people whose skills and personalities best fit your needs, train them well, and then give them the opportunity to succeed. “The best executive,” wrote Theodore Roosevelt, “is the one who has sense enough to pick good [people] to do what he [or she] wants done, and self-restraint enough to keep from meddling with them while they do it.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Many of the personnel questions I receive concern the dreaded “marginal employee” – a person who has never done anything truly heinous to merit firing, but neither anything special to merit continued employment. I advise getting rid of such people and then changing the hiring criteria that bring you marginal employees in the first place.
Most bad hires come about because employers do not have a clear vision of the kind of employee they want. Many office manuals do not contain detailed job descriptions. If you don’t know exactly what you are looking for, your entire selection process will be inadequate from initial screening of applicants through assessments of their skills and personalities. Many physicians compound the problem with poor interview techniques and inadequate verification.
. Take a hard look at your job descriptions, and update them if necessary. A good job description lists the major responsibilities of the position, with the relative importance of each duty and the critical knowledge, skills, and education levels necessary for each function. In other words, it describes, accurately and in detail, exactly what you expect from the employee you will hire to perform that job.
Once you have a clear job description in mind (and in print), take all the time you need to find the best possible match for it. This is not a place to cut corners. Screen your candidates carefully and avoid lowering your expectations. This is the point at which it might be tempting to settle for a marginal candidate, just to get the process over with.
It also is tempting to hire the candidate that you have the “best feeling” about, even though he or she is a poor match for the job, and then try to mold the job to that person. Every doctor knows that hunches are no substitute for hard data.
Be alert for red flags in résumés: significant time gaps between jobs; positions at companies that are no longer in business, or are otherwise impossible to verify; job titles that don’t make sense, given the applicant’s qualifications.
Background checks are a dicey subject, but publicly available information can be found, cheaply or free, on multiple websites created for that purpose. Be sure to tell applicants that you will be verifying facts in their résumés; it’s usually wise to get their written consent to do so.
Many employers skip the essential step of verification; many applicants know that. (I once actually overheard a new hire say, “I won’t be here long if they check my references.” And by golly, she was right!) If a reference is reluctant to tell you anything substantive, ask, “Would you hire this person again?” You can interpret a lot from the answer – or lack of one.
Interviews often get short shrift as well. Many doctors tend to do all the talking. The purpose of an interview is to allow you to size up the prospective employee, not to deliver a lecture on the sterling attributes of your office. Important interview topics include educational background, skills, experience, and unrelated job history.
By law, you cannot ask an applicant’s age, date of birth, sex, creed, color, religion, or national origin. Other forbidden subjects include disabilities, marital status, military record, number of children (or who cares for them), addiction history, citizenship, criminal record, psychiatric history, absenteeism, or workers’ compensation.
There are acceptable alternatives to some of those questions, however: You can ask if applicants have ever gone by another name (for your background check), for example. You can ask if they are legally authorized to work in this country, and whether they will be physically able to perform the duties specified in the job description. While past addictions are off limits, you do have a right to know about current addictions to illegal substances.
Once you have hired people whose skills and personalities best fit your needs, train them well, and then give them the opportunity to succeed. “The best executive,” wrote Theodore Roosevelt, “is the one who has sense enough to pick good [people] to do what he [or she] wants done, and self-restraint enough to keep from meddling with them while they do it.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Many of the personnel questions I receive concern the dreaded “marginal employee” – a person who has never done anything truly heinous to merit firing, but neither anything special to merit continued employment. I advise getting rid of such people and then changing the hiring criteria that bring you marginal employees in the first place.
Most bad hires come about because employers do not have a clear vision of the kind of employee they want. Many office manuals do not contain detailed job descriptions. If you don’t know exactly what you are looking for, your entire selection process will be inadequate from initial screening of applicants through assessments of their skills and personalities. Many physicians compound the problem with poor interview techniques and inadequate verification.
. Take a hard look at your job descriptions, and update them if necessary. A good job description lists the major responsibilities of the position, with the relative importance of each duty and the critical knowledge, skills, and education levels necessary for each function. In other words, it describes, accurately and in detail, exactly what you expect from the employee you will hire to perform that job.
Once you have a clear job description in mind (and in print), take all the time you need to find the best possible match for it. This is not a place to cut corners. Screen your candidates carefully and avoid lowering your expectations. This is the point at which it might be tempting to settle for a marginal candidate, just to get the process over with.
It also is tempting to hire the candidate that you have the “best feeling” about, even though he or she is a poor match for the job, and then try to mold the job to that person. Every doctor knows that hunches are no substitute for hard data.
Be alert for red flags in résumés: significant time gaps between jobs; positions at companies that are no longer in business, or are otherwise impossible to verify; job titles that don’t make sense, given the applicant’s qualifications.
Background checks are a dicey subject, but publicly available information can be found, cheaply or free, on multiple websites created for that purpose. Be sure to tell applicants that you will be verifying facts in their résumés; it’s usually wise to get their written consent to do so.
Many employers skip the essential step of verification; many applicants know that. (I once actually overheard a new hire say, “I won’t be here long if they check my references.” And by golly, she was right!) If a reference is reluctant to tell you anything substantive, ask, “Would you hire this person again?” You can interpret a lot from the answer – or lack of one.
Interviews often get short shrift as well. Many doctors tend to do all the talking. The purpose of an interview is to allow you to size up the prospective employee, not to deliver a lecture on the sterling attributes of your office. Important interview topics include educational background, skills, experience, and unrelated job history.
By law, you cannot ask an applicant’s age, date of birth, sex, creed, color, religion, or national origin. Other forbidden subjects include disabilities, marital status, military record, number of children (or who cares for them), addiction history, citizenship, criminal record, psychiatric history, absenteeism, or workers’ compensation.
There are acceptable alternatives to some of those questions, however: You can ask if applicants have ever gone by another name (for your background check), for example. You can ask if they are legally authorized to work in this country, and whether they will be physically able to perform the duties specified in the job description. While past addictions are off limits, you do have a right to know about current addictions to illegal substances.
Once you have hired people whose skills and personalities best fit your needs, train them well, and then give them the opportunity to succeed. “The best executive,” wrote Theodore Roosevelt, “is the one who has sense enough to pick good [people] to do what he [or she] wants done, and self-restraint enough to keep from meddling with them while they do it.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].