User login
Vaping device marketers take aim at youth through social media
with targeted messages and images, a study of e-cigarette promotion has found.
In 2018, the JUUL company declared a commitment to support efforts to raise the age of legal purchase of tobacco to age 21 years in all U.S. states. In addition, JUUL deleted its official Facebook and Instagram accounts in November 2018, but the promotion of these products has continued through affiliated marketing campaigns from other online vendors.
Vaping among teens has shot up in popularity in recent years. The prevalence of vaping among young people aged 16-19 years has been estimated at 16% in 2018, up from 11% in 2017 (BMJ. 2019 Jun 19. doi: 10.1136/bmj.12219. A study published in JAMA Pediatrics (2019;173[7]:690-92) found that an estimated 81% of users following a popular Twitter account (@JUULvapor) were aged 13-20 years, with 45% in the 13-17 year age range.
Elizabeth C. Hair, PhD, senior vice president of the Truth Initiative Schroeder Institute, and a team of investigators conducted a study of the “proliferation of JUUL-related content across four themes over a 3-month period: overt promotional content, nicotine and addiction-related content, lifestyle content, and content related to youth culture.” The study appeared online in Tobacco Control (2019 Jul 2; doi: 10.1136/tobaccocontrol-2018-054824).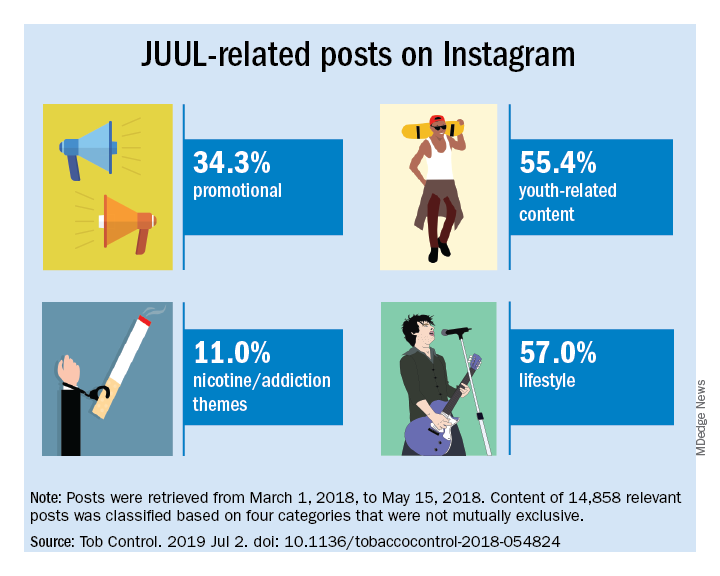
The investigators did a content analysis of social media posts on Instagram related to JUUL and JUUL-like products from March 1 to May 15, 2018. Hash-tag keyword queries of JUUL-related posts on Instagram were collected from the Instagram application programming interface through NUVI, a licensed syndicator of the Instagram firehose. The researchers used 50 hashtags to capture and enumerate individual posts. Examples of the hashtags used are #juul, #juuling, #juulvapor, #juulpod, #switchtojuul, and #juulgang. All posts were included from the official JUUL account and JUUL-related accounts with the highest number of followers at the time of data collection (e.g., @juulcentral, @juulnation, @juul_university, @juul.girls).
The search identified 14,838 posts by 5,201 unique users that featured content relating to product promotion, nicotine and addiction messages, youth culture, and lifestyle themes. Posts were rated promotional incluced branded content, URLs linking to commercial websites, and hashtags indicating affiliations with commercial sites.
Nicotine/addiction posts contained “references to nicotine, including compatible pod-related brand names and nicotine content, as well as any references to addiction or nicotine dependence (e.g., daily use, being an addict, junkie, “nichead,” fiend, maniac), or effects of nicotine use (e.g., “buzz”).
Youth-themed posts included stylistic features such as jargon or slang, acronyms common among youth (e.g., di4j, doit4juul), youth-oriented cartoons, JUUL wrap imagery, youth entertainment, and music. Posts with references to school, the classroom, and other places frequented by youth and youth social networks, family, and peers were included in the youth-themed category.
Lifestyle content referenced "social norms and acceptability-related messages contained any mentions of online or offline communitiesand peer groups (eg, collegelife, juulgirls, juulgang, vapeusa, collegedaily, vapelyfe hashtags) as well as JUUL use during social activities, events, social acceptance of JUULing and any mentions of JUULing as a characteristic of cultural or social identity."
Content analysis of the posts found that 34.3% were promotional, 11% referenced nicotine and addiction themes, and 55.4% featured youth-oriented cultural themes, and 57% featured lifestyle themes. There was overlap among the categories, for example, the 71.9% of the promotional posts had lifestyle messages included and 86.3% of the nicotine/addiction posts contained lifestyle elements. The promotional posts also contained some hashtags referencing cannabis (#420, #710).
An additional feature of the promotional posts is the incentivizing messages. “More than more than a third of JUUL-related posts containing overt promotional content that highlights ways to obtain products at reduced cost, such as giveaways and incentivized friend-tagging. This finding is consistent with previous research which found that Twitter users employed person-tagging (e.g., @username) when purchasing JUUL, suggesting friend-tagging plays an important role in motivating product use,” the researchers wrote.
The study was limited by the short time frame, the analysis of Instagram postings only, and the limitation of only 50 hashtags. These limitations may result in underreporting of the amount of JUUL-related social media messaging that targets youth. In addition, the investigators did not analyze the origin of accounts or the identity of the individuals creating the content.
“The results of this study demonstrate the reach of organic posts that contain JUUL-related content, and posts by third-party vendors of vaping products, who continue to push explicitly youth-targeted advertisements for JUUL and similar e-cigarette products under JUUL-related hashtags,” Dr. Hair wrote. “Our research and studies done by others in the field are one way to build the evidence base to advocate for stricter social media marketing restrictions on tobacco products that are applicable to all players in the field.”
She added that the Food and Drug Administration should use its power to restrict e-cigarette manufacturers from using social media to market to young people. “We also think that social media platforms should do more to adopt and enforce strong and well-enforced policies against the promotion of any tobacco products to young adults,” she concluded.
The study was sponsored by the Truth Initiative. The Truth Initiative was created as a part of the Master Settlement Agreement (MSA) that was negotiated between the tobacco industry and 46 states and the District of Columbia in 1998. The MSA created the American Legacy Foundation (now known as the Truth Initiative), a nonprofit research and educational organization that focuses its efforts on preventing teen smoking and encouraging smokers to quit.
SOURCE: Czaplicki L et al. Tob Control. 2019 Jul 2; doi: 10.1136/tobaccocontrol-2018-054824.
This article was updated 7/17/2019.
with targeted messages and images, a study of e-cigarette promotion has found.
In 2018, the JUUL company declared a commitment to support efforts to raise the age of legal purchase of tobacco to age 21 years in all U.S. states. In addition, JUUL deleted its official Facebook and Instagram accounts in November 2018, but the promotion of these products has continued through affiliated marketing campaigns from other online vendors.
Vaping among teens has shot up in popularity in recent years. The prevalence of vaping among young people aged 16-19 years has been estimated at 16% in 2018, up from 11% in 2017 (BMJ. 2019 Jun 19. doi: 10.1136/bmj.12219. A study published in JAMA Pediatrics (2019;173[7]:690-92) found that an estimated 81% of users following a popular Twitter account (@JUULvapor) were aged 13-20 years, with 45% in the 13-17 year age range.
Elizabeth C. Hair, PhD, senior vice president of the Truth Initiative Schroeder Institute, and a team of investigators conducted a study of the “proliferation of JUUL-related content across four themes over a 3-month period: overt promotional content, nicotine and addiction-related content, lifestyle content, and content related to youth culture.” The study appeared online in Tobacco Control (2019 Jul 2; doi: 10.1136/tobaccocontrol-2018-054824).
The investigators did a content analysis of social media posts on Instagram related to JUUL and JUUL-like products from March 1 to May 15, 2018. Hash-tag keyword queries of JUUL-related posts on Instagram were collected from the Instagram application programming interface through NUVI, a licensed syndicator of the Instagram firehose. The researchers used 50 hashtags to capture and enumerate individual posts. Examples of the hashtags used are #juul, #juuling, #juulvapor, #juulpod, #switchtojuul, and #juulgang. All posts were included from the official JUUL account and JUUL-related accounts with the highest number of followers at the time of data collection (e.g., @juulcentral, @juulnation, @juul_university, @juul.girls).
The search identified 14,838 posts by 5,201 unique users that featured content relating to product promotion, nicotine and addiction messages, youth culture, and lifestyle themes. Posts were rated promotional incluced branded content, URLs linking to commercial websites, and hashtags indicating affiliations with commercial sites.
Nicotine/addiction posts contained “references to nicotine, including compatible pod-related brand names and nicotine content, as well as any references to addiction or nicotine dependence (e.g., daily use, being an addict, junkie, “nichead,” fiend, maniac), or effects of nicotine use (e.g., “buzz”).
Youth-themed posts included stylistic features such as jargon or slang, acronyms common among youth (e.g., di4j, doit4juul), youth-oriented cartoons, JUUL wrap imagery, youth entertainment, and music. Posts with references to school, the classroom, and other places frequented by youth and youth social networks, family, and peers were included in the youth-themed category.
Lifestyle content referenced "social norms and acceptability-related messages contained any mentions of online or offline communitiesand peer groups (eg, collegelife, juulgirls, juulgang, vapeusa, collegedaily, vapelyfe hashtags) as well as JUUL use during social activities, events, social acceptance of JUULing and any mentions of JUULing as a characteristic of cultural or social identity."
Content analysis of the posts found that 34.3% were promotional, 11% referenced nicotine and addiction themes, and 55.4% featured youth-oriented cultural themes, and 57% featured lifestyle themes. There was overlap among the categories, for example, the 71.9% of the promotional posts had lifestyle messages included and 86.3% of the nicotine/addiction posts contained lifestyle elements. The promotional posts also contained some hashtags referencing cannabis (#420, #710).
An additional feature of the promotional posts is the incentivizing messages. “More than more than a third of JUUL-related posts containing overt promotional content that highlights ways to obtain products at reduced cost, such as giveaways and incentivized friend-tagging. This finding is consistent with previous research which found that Twitter users employed person-tagging (e.g., @username) when purchasing JUUL, suggesting friend-tagging plays an important role in motivating product use,” the researchers wrote.
The study was limited by the short time frame, the analysis of Instagram postings only, and the limitation of only 50 hashtags. These limitations may result in underreporting of the amount of JUUL-related social media messaging that targets youth. In addition, the investigators did not analyze the origin of accounts or the identity of the individuals creating the content.
“The results of this study demonstrate the reach of organic posts that contain JUUL-related content, and posts by third-party vendors of vaping products, who continue to push explicitly youth-targeted advertisements for JUUL and similar e-cigarette products under JUUL-related hashtags,” Dr. Hair wrote. “Our research and studies done by others in the field are one way to build the evidence base to advocate for stricter social media marketing restrictions on tobacco products that are applicable to all players in the field.”
She added that the Food and Drug Administration should use its power to restrict e-cigarette manufacturers from using social media to market to young people. “We also think that social media platforms should do more to adopt and enforce strong and well-enforced policies against the promotion of any tobacco products to young adults,” she concluded.
The study was sponsored by the Truth Initiative. The Truth Initiative was created as a part of the Master Settlement Agreement (MSA) that was negotiated between the tobacco industry and 46 states and the District of Columbia in 1998. The MSA created the American Legacy Foundation (now known as the Truth Initiative), a nonprofit research and educational organization that focuses its efforts on preventing teen smoking and encouraging smokers to quit.
SOURCE: Czaplicki L et al. Tob Control. 2019 Jul 2; doi: 10.1136/tobaccocontrol-2018-054824.
This article was updated 7/17/2019.
with targeted messages and images, a study of e-cigarette promotion has found.
In 2018, the JUUL company declared a commitment to support efforts to raise the age of legal purchase of tobacco to age 21 years in all U.S. states. In addition, JUUL deleted its official Facebook and Instagram accounts in November 2018, but the promotion of these products has continued through affiliated marketing campaigns from other online vendors.
Vaping among teens has shot up in popularity in recent years. The prevalence of vaping among young people aged 16-19 years has been estimated at 16% in 2018, up from 11% in 2017 (BMJ. 2019 Jun 19. doi: 10.1136/bmj.12219. A study published in JAMA Pediatrics (2019;173[7]:690-92) found that an estimated 81% of users following a popular Twitter account (@JUULvapor) were aged 13-20 years, with 45% in the 13-17 year age range.
Elizabeth C. Hair, PhD, senior vice president of the Truth Initiative Schroeder Institute, and a team of investigators conducted a study of the “proliferation of JUUL-related content across four themes over a 3-month period: overt promotional content, nicotine and addiction-related content, lifestyle content, and content related to youth culture.” The study appeared online in Tobacco Control (2019 Jul 2; doi: 10.1136/tobaccocontrol-2018-054824).
The investigators did a content analysis of social media posts on Instagram related to JUUL and JUUL-like products from March 1 to May 15, 2018. Hash-tag keyword queries of JUUL-related posts on Instagram were collected from the Instagram application programming interface through NUVI, a licensed syndicator of the Instagram firehose. The researchers used 50 hashtags to capture and enumerate individual posts. Examples of the hashtags used are #juul, #juuling, #juulvapor, #juulpod, #switchtojuul, and #juulgang. All posts were included from the official JUUL account and JUUL-related accounts with the highest number of followers at the time of data collection (e.g., @juulcentral, @juulnation, @juul_university, @juul.girls).
The search identified 14,838 posts by 5,201 unique users that featured content relating to product promotion, nicotine and addiction messages, youth culture, and lifestyle themes. Posts were rated promotional incluced branded content, URLs linking to commercial websites, and hashtags indicating affiliations with commercial sites.
Nicotine/addiction posts contained “references to nicotine, including compatible pod-related brand names and nicotine content, as well as any references to addiction or nicotine dependence (e.g., daily use, being an addict, junkie, “nichead,” fiend, maniac), or effects of nicotine use (e.g., “buzz”).
Youth-themed posts included stylistic features such as jargon or slang, acronyms common among youth (e.g., di4j, doit4juul), youth-oriented cartoons, JUUL wrap imagery, youth entertainment, and music. Posts with references to school, the classroom, and other places frequented by youth and youth social networks, family, and peers were included in the youth-themed category.
Lifestyle content referenced "social norms and acceptability-related messages contained any mentions of online or offline communitiesand peer groups (eg, collegelife, juulgirls, juulgang, vapeusa, collegedaily, vapelyfe hashtags) as well as JUUL use during social activities, events, social acceptance of JUULing and any mentions of JUULing as a characteristic of cultural or social identity."
Content analysis of the posts found that 34.3% were promotional, 11% referenced nicotine and addiction themes, and 55.4% featured youth-oriented cultural themes, and 57% featured lifestyle themes. There was overlap among the categories, for example, the 71.9% of the promotional posts had lifestyle messages included and 86.3% of the nicotine/addiction posts contained lifestyle elements. The promotional posts also contained some hashtags referencing cannabis (#420, #710).
An additional feature of the promotional posts is the incentivizing messages. “More than more than a third of JUUL-related posts containing overt promotional content that highlights ways to obtain products at reduced cost, such as giveaways and incentivized friend-tagging. This finding is consistent with previous research which found that Twitter users employed person-tagging (e.g., @username) when purchasing JUUL, suggesting friend-tagging plays an important role in motivating product use,” the researchers wrote.
The study was limited by the short time frame, the analysis of Instagram postings only, and the limitation of only 50 hashtags. These limitations may result in underreporting of the amount of JUUL-related social media messaging that targets youth. In addition, the investigators did not analyze the origin of accounts or the identity of the individuals creating the content.
“The results of this study demonstrate the reach of organic posts that contain JUUL-related content, and posts by third-party vendors of vaping products, who continue to push explicitly youth-targeted advertisements for JUUL and similar e-cigarette products under JUUL-related hashtags,” Dr. Hair wrote. “Our research and studies done by others in the field are one way to build the evidence base to advocate for stricter social media marketing restrictions on tobacco products that are applicable to all players in the field.”
She added that the Food and Drug Administration should use its power to restrict e-cigarette manufacturers from using social media to market to young people. “We also think that social media platforms should do more to adopt and enforce strong and well-enforced policies against the promotion of any tobacco products to young adults,” she concluded.
The study was sponsored by the Truth Initiative. The Truth Initiative was created as a part of the Master Settlement Agreement (MSA) that was negotiated between the tobacco industry and 46 states and the District of Columbia in 1998. The MSA created the American Legacy Foundation (now known as the Truth Initiative), a nonprofit research and educational organization that focuses its efforts on preventing teen smoking and encouraging smokers to quit.
SOURCE: Czaplicki L et al. Tob Control. 2019 Jul 2; doi: 10.1136/tobaccocontrol-2018-054824.
This article was updated 7/17/2019.
FROM TOBACCO CONTROL
Nearly 20% of migraineurs use opioids for migraine
PHILADELPHIA – People with 4 or more migraine headache days per month are more likely to use opioids, compared with people with fewer migraine headache days per month, researchers said. Opioid use for migraine “remains alarmingly high,” the investigators said at the annual meeting of the American Headache Society.
Although opioid use for the treatment of migraine typically is discouraged, studies indicate that it is common. Evidence suggests that opioids may increase the risk of progression from episodic to chronic migraine.
To evaluate opioid use in people with migraine, Sait Ashina, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center in Boston, and the research colleagues analyzed data from 21,143 people with migraine who participated in the OVERCOME (Observational Survey of the Epidemiology, Treatment and Care of Migraine), a Web-based study of a representative U.S. sample. OVERCOME enrolled participants in the fall of 2018.
The researchers classified self-reported opioid use for migraine as current use in the past 12 months, former use, or never. Participants had a mean age of 42 years, and 74% were female. The researchers used a multivariable logistic regression model adjusted for age and sex in their analyses.
“Strikingly, we were able to find 19% of people with migraine were reporting current use of opioids,” Dr. Ashina said.
Among 12,299 patients with 0-3 migraine headache days per month, 59% were never, 26% former, and 15% current users of opioids for migraine. Among 8,844 patients with 4 or more migraine headache days per month, 44.9% were never, 31.2% former, and 23.9% current users of opioids for migraine.
There was an increased likelihood of opioid use for migraine in people with pain comorbidities such as back pain, neck pain, and fibromyalgia and in people with anxiety and depression.
Approximately 30%-40% of those who used opioids for migraine were using strong opioids, as defined by the World Health Organization, Dr. Ashina noted. Preliminary analyses indicate that patients tended to receive opioids in a primary care setting, he said.
Eli Lilly funded the OVERCOME study. Dr. Ashina has consulted for Novartis, Amgen, Promius, Supernus, Satsuma, and Allergan. He is on the Editorial Advisory Board for Neurology Reviews.
PHILADELPHIA – People with 4 or more migraine headache days per month are more likely to use opioids, compared with people with fewer migraine headache days per month, researchers said. Opioid use for migraine “remains alarmingly high,” the investigators said at the annual meeting of the American Headache Society.
Although opioid use for the treatment of migraine typically is discouraged, studies indicate that it is common. Evidence suggests that opioids may increase the risk of progression from episodic to chronic migraine.
To evaluate opioid use in people with migraine, Sait Ashina, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center in Boston, and the research colleagues analyzed data from 21,143 people with migraine who participated in the OVERCOME (Observational Survey of the Epidemiology, Treatment and Care of Migraine), a Web-based study of a representative U.S. sample. OVERCOME enrolled participants in the fall of 2018.
The researchers classified self-reported opioid use for migraine as current use in the past 12 months, former use, or never. Participants had a mean age of 42 years, and 74% were female. The researchers used a multivariable logistic regression model adjusted for age and sex in their analyses.
“Strikingly, we were able to find 19% of people with migraine were reporting current use of opioids,” Dr. Ashina said.
Among 12,299 patients with 0-3 migraine headache days per month, 59% were never, 26% former, and 15% current users of opioids for migraine. Among 8,844 patients with 4 or more migraine headache days per month, 44.9% were never, 31.2% former, and 23.9% current users of opioids for migraine.
There was an increased likelihood of opioid use for migraine in people with pain comorbidities such as back pain, neck pain, and fibromyalgia and in people with anxiety and depression.
Approximately 30%-40% of those who used opioids for migraine were using strong opioids, as defined by the World Health Organization, Dr. Ashina noted. Preliminary analyses indicate that patients tended to receive opioids in a primary care setting, he said.
Eli Lilly funded the OVERCOME study. Dr. Ashina has consulted for Novartis, Amgen, Promius, Supernus, Satsuma, and Allergan. He is on the Editorial Advisory Board for Neurology Reviews.
PHILADELPHIA – People with 4 or more migraine headache days per month are more likely to use opioids, compared with people with fewer migraine headache days per month, researchers said. Opioid use for migraine “remains alarmingly high,” the investigators said at the annual meeting of the American Headache Society.
Although opioid use for the treatment of migraine typically is discouraged, studies indicate that it is common. Evidence suggests that opioids may increase the risk of progression from episodic to chronic migraine.
To evaluate opioid use in people with migraine, Sait Ashina, MD, of Harvard Medical School and Beth Israel Deaconess Medical Center in Boston, and the research colleagues analyzed data from 21,143 people with migraine who participated in the OVERCOME (Observational Survey of the Epidemiology, Treatment and Care of Migraine), a Web-based study of a representative U.S. sample. OVERCOME enrolled participants in the fall of 2018.
The researchers classified self-reported opioid use for migraine as current use in the past 12 months, former use, or never. Participants had a mean age of 42 years, and 74% were female. The researchers used a multivariable logistic regression model adjusted for age and sex in their analyses.
“Strikingly, we were able to find 19% of people with migraine were reporting current use of opioids,” Dr. Ashina said.
Among 12,299 patients with 0-3 migraine headache days per month, 59% were never, 26% former, and 15% current users of opioids for migraine. Among 8,844 patients with 4 or more migraine headache days per month, 44.9% were never, 31.2% former, and 23.9% current users of opioids for migraine.
There was an increased likelihood of opioid use for migraine in people with pain comorbidities such as back pain, neck pain, and fibromyalgia and in people with anxiety and depression.
Approximately 30%-40% of those who used opioids for migraine were using strong opioids, as defined by the World Health Organization, Dr. Ashina noted. Preliminary analyses indicate that patients tended to receive opioids in a primary care setting, he said.
Eli Lilly funded the OVERCOME study. Dr. Ashina has consulted for Novartis, Amgen, Promius, Supernus, Satsuma, and Allergan. He is on the Editorial Advisory Board for Neurology Reviews.
EXPERT ANALYSIS FROM AHS 2019
A plurality of migraineurs seeks care from primary care physicians
PHILADELPHIA – according to an investigation presented at the annual meeting of the American Headache Society.
The largest group of these patients consults primary care physicians. The acute and preventive migraine treatment that these patients receive vary according to the type of provider that they see. Nevertheless, treatment is generally suboptimal, compared with the standards of current guidelines, said the investigators.
Migraine is underdiagnosed and undertreated, said Dawn C. Buse, PhD, clinical professor of neurology at Albert Einstein College of Medicine, New York, and colleagues. Recent changes in health care policy and the expanded array of treatments for migraine warrant an investigation of the current state of migraine care, they added. They examined survey data to understand where patients with migraine in the United States seek care, which characteristics are associated with seeking care in the previous 12 months, and which treatments are prescribed.
Dr. Buse and colleagues analyzed data from the OVERCOME (Observational Survey of the Epidemiology, Treatment, and Care of Migraine) study. These data were obtained in 2018 using a Web-based survey of a representative U.S. sample of 21,143 patients with migraine. The investigators focused on care seeking and medication use in a subsample of 8,844 patients with 4 or more migraine headache days per month to better understand those with the greatest care needs.
The mean age of this subsample was 42.0 years. Approximately 78% of participants were female, and 74.8% were white. In the preceding 12 months, 61.1% of the patients sought care for migraine; 38.3% sought care from more than two types of provider. Provider types included primary care physicians (45.5%), neurologists (20.2%), emergency medicine clinicians (19.2%), urgent care providers (14.4%), pain specialists (12.8%), headache specialists (12.0%), and retail (nonurgent) clinics (10.4%).
Dr. Buse and colleagues found that sociodemographic factors such as age, sex, education, income, and health insurance type influenced participants’ likelihood of seeking care. Seeking care was positively associated with the number of headache days, pain severity, allodynia, aura, and prodrome. When the researchers examined migraine characteristics, they found that nausea and vomiting (68.8%) was more likely to prompt a patient to seek care, compared with phonophobia and photophobia (64.3%). Participants who sought care from a headache specialist (55.0%) or a neurologist (50.1%) were most likely to be using migraine preventive medication. More than 20% of migraineurs seeking care from primary care, urgent care, or retail clinic professionals were undiagnosed.
Primary care doctors were most likely to prescribe triptans, followed by opioids and preventive medications. Neurologists and headache specialists were most likely to prescribe preventive medications and unlikely to prescribe opioids.
Eli Lilly funds the OVERCOME study. Dr. Buse consults for Lilly on this study, but she and her coauthors who do not work in the industry did not receive any funding for any work related to writing, publishing, or presenting any abstracts, posters, platforms, or manuscripts.
PHILADELPHIA – according to an investigation presented at the annual meeting of the American Headache Society.
The largest group of these patients consults primary care physicians. The acute and preventive migraine treatment that these patients receive vary according to the type of provider that they see. Nevertheless, treatment is generally suboptimal, compared with the standards of current guidelines, said the investigators.
Migraine is underdiagnosed and undertreated, said Dawn C. Buse, PhD, clinical professor of neurology at Albert Einstein College of Medicine, New York, and colleagues. Recent changes in health care policy and the expanded array of treatments for migraine warrant an investigation of the current state of migraine care, they added. They examined survey data to understand where patients with migraine in the United States seek care, which characteristics are associated with seeking care in the previous 12 months, and which treatments are prescribed.
Dr. Buse and colleagues analyzed data from the OVERCOME (Observational Survey of the Epidemiology, Treatment, and Care of Migraine) study. These data were obtained in 2018 using a Web-based survey of a representative U.S. sample of 21,143 patients with migraine. The investigators focused on care seeking and medication use in a subsample of 8,844 patients with 4 or more migraine headache days per month to better understand those with the greatest care needs.
The mean age of this subsample was 42.0 years. Approximately 78% of participants were female, and 74.8% were white. In the preceding 12 months, 61.1% of the patients sought care for migraine; 38.3% sought care from more than two types of provider. Provider types included primary care physicians (45.5%), neurologists (20.2%), emergency medicine clinicians (19.2%), urgent care providers (14.4%), pain specialists (12.8%), headache specialists (12.0%), and retail (nonurgent) clinics (10.4%).
Dr. Buse and colleagues found that sociodemographic factors such as age, sex, education, income, and health insurance type influenced participants’ likelihood of seeking care. Seeking care was positively associated with the number of headache days, pain severity, allodynia, aura, and prodrome. When the researchers examined migraine characteristics, they found that nausea and vomiting (68.8%) was more likely to prompt a patient to seek care, compared with phonophobia and photophobia (64.3%). Participants who sought care from a headache specialist (55.0%) or a neurologist (50.1%) were most likely to be using migraine preventive medication. More than 20% of migraineurs seeking care from primary care, urgent care, or retail clinic professionals were undiagnosed.
Primary care doctors were most likely to prescribe triptans, followed by opioids and preventive medications. Neurologists and headache specialists were most likely to prescribe preventive medications and unlikely to prescribe opioids.
Eli Lilly funds the OVERCOME study. Dr. Buse consults for Lilly on this study, but she and her coauthors who do not work in the industry did not receive any funding for any work related to writing, publishing, or presenting any abstracts, posters, platforms, or manuscripts.
PHILADELPHIA – according to an investigation presented at the annual meeting of the American Headache Society.
The largest group of these patients consults primary care physicians. The acute and preventive migraine treatment that these patients receive vary according to the type of provider that they see. Nevertheless, treatment is generally suboptimal, compared with the standards of current guidelines, said the investigators.
Migraine is underdiagnosed and undertreated, said Dawn C. Buse, PhD, clinical professor of neurology at Albert Einstein College of Medicine, New York, and colleagues. Recent changes in health care policy and the expanded array of treatments for migraine warrant an investigation of the current state of migraine care, they added. They examined survey data to understand where patients with migraine in the United States seek care, which characteristics are associated with seeking care in the previous 12 months, and which treatments are prescribed.
Dr. Buse and colleagues analyzed data from the OVERCOME (Observational Survey of the Epidemiology, Treatment, and Care of Migraine) study. These data were obtained in 2018 using a Web-based survey of a representative U.S. sample of 21,143 patients with migraine. The investigators focused on care seeking and medication use in a subsample of 8,844 patients with 4 or more migraine headache days per month to better understand those with the greatest care needs.
The mean age of this subsample was 42.0 years. Approximately 78% of participants were female, and 74.8% were white. In the preceding 12 months, 61.1% of the patients sought care for migraine; 38.3% sought care from more than two types of provider. Provider types included primary care physicians (45.5%), neurologists (20.2%), emergency medicine clinicians (19.2%), urgent care providers (14.4%), pain specialists (12.8%), headache specialists (12.0%), and retail (nonurgent) clinics (10.4%).
Dr. Buse and colleagues found that sociodemographic factors such as age, sex, education, income, and health insurance type influenced participants’ likelihood of seeking care. Seeking care was positively associated with the number of headache days, pain severity, allodynia, aura, and prodrome. When the researchers examined migraine characteristics, they found that nausea and vomiting (68.8%) was more likely to prompt a patient to seek care, compared with phonophobia and photophobia (64.3%). Participants who sought care from a headache specialist (55.0%) or a neurologist (50.1%) were most likely to be using migraine preventive medication. More than 20% of migraineurs seeking care from primary care, urgent care, or retail clinic professionals were undiagnosed.
Primary care doctors were most likely to prescribe triptans, followed by opioids and preventive medications. Neurologists and headache specialists were most likely to prescribe preventive medications and unlikely to prescribe opioids.
Eli Lilly funds the OVERCOME study. Dr. Buse consults for Lilly on this study, but she and her coauthors who do not work in the industry did not receive any funding for any work related to writing, publishing, or presenting any abstracts, posters, platforms, or manuscripts.
REPORTING FROM AHS 2019
Alzheimer’s disease raises risk for recurrent seizures
LOS ANGELES – Seizures are not uncommon among patients with Alzheimer’s disease – particularly as patients live longer with the disease – and are often associated with worse cognitive and functional performance, according to research findings presented at the Alzheimer’s Association International Conference.
Jonathan Vöglein, MD, of the German Center for Neurodegenerative Diseases and Ludwig-Maximilian University in Munich presented results from a cohort of 9,127 patients with Alzheimer’s disease (AD), of whom 287 had experienced a seizure, and more than 10,000 non-AD control subjects recruited at clinics during 2005-2016.
Dr. Vöglein and colleagues found that seizure risk increased with duration of disease, from 1.5% of patients at 4.8 years with the disease to 5.4% at 11 years, with likelihood of a seizure increasing steadily over time.
Moreover, 70% of AD patients who experienced a seizure had a second one within 7.5 months. People who had seizures fared worse on cognitive and functional tests: a mean 16.6 on the Mini Mental State Examination, compared with 19.6 for patients without seizures. On a severity rating scale, the Clinical Dementia Rating Sum of Boxes, patients with seizures also fared worse, with scores of 9.3, compared with 6.8 for patients without seizures (P less than .0001 for all, with results adjusted for age and disease duration).
“The data of our study show that there’s an association of seizures with worse cognitive and functional performance,” Dr. Vöglein said in an interview.
“It’s important for clinicians to know that Alzheimer’s patients are at an increased risk for seizures,” Dr. Vöglein said. “In my clinical care experience, seizures are rarely the main complaint of patients with Alzheimer’s disease.” Detailed interviews with the patient and a proxy are important, he added, because patients with Alzheimer’s disease may not always remember events that could be a seizure.
Dr. Vöglein noted that, to his knowledge, there are no reliable data showing that treating seizures with antiepileptic drugs slows cognitive decline. “The results of our study suggest that an antiepileptic treatment after a first seizure in patients with Alzheimer’s dementia may be considered,” he said.
Also at the conference, researcher Ruby Castilla-Puentes, MD, DrPH, of Janssen Pharmaceuticals in Hopewell, N.J., along with Miguel Habeych, MD, MPH, of the University of Cincinnati presented findings on dementia and seizure risk from a large U.S. national managed care database of nearly 3 million people aged 60 years and older, of whom 56% were women.
The researchers analyzed this cohort during 2005-2014 and identified 80,000 people (2.8% of the cohort) as having any dementia diagnosis. The overall incidence of new-onset seizures in patients with dementia was 12.3% per year. In general, all subtypes of seizures and epileptic disorders (partial, generalized, or undifferentiated) occurred more frequently in patients with dementia, compared against patients without dementia (P less than .0001).
People with dementia had more than six times greater risk for experiencing recurring epileptic seizures than did people without dementia (95% confidence interval, 4.4-9.5). They were at six times higher risk for partial seizures (95% CI, 5.5-6.6); fivefold higher risk for generalized (95% CI, 4.9-5.5) and undifferentiated epilepsy (95% CI, 4.8-5.2); and 4.75 times higher risk for generalized seizures (95% CI, 4.5-5.0) and partial epilepsy (95% CI, 4.4-5.1).
“Although there are limitations with the use of administrative claims databases to calculate incidence rates, this analysis suggests that patients of 60 years of age or older have higher risks of new-onset seizures associated with a dementia diagnosis,” Dr. Castilla-Puentes commented.
The findings, she said, reinforce the need for clinicians to monitor for seizures to ensure that patients with dementia receive appropriate treatment.
Dr. Vöglein disclosed no financial conflicts of interest. Dr. Castilla-Puentes disclosed being an employee of Janssen, which funded her study.
LOS ANGELES – Seizures are not uncommon among patients with Alzheimer’s disease – particularly as patients live longer with the disease – and are often associated with worse cognitive and functional performance, according to research findings presented at the Alzheimer’s Association International Conference.
Jonathan Vöglein, MD, of the German Center for Neurodegenerative Diseases and Ludwig-Maximilian University in Munich presented results from a cohort of 9,127 patients with Alzheimer’s disease (AD), of whom 287 had experienced a seizure, and more than 10,000 non-AD control subjects recruited at clinics during 2005-2016.
Dr. Vöglein and colleagues found that seizure risk increased with duration of disease, from 1.5% of patients at 4.8 years with the disease to 5.4% at 11 years, with likelihood of a seizure increasing steadily over time.
Moreover, 70% of AD patients who experienced a seizure had a second one within 7.5 months. People who had seizures fared worse on cognitive and functional tests: a mean 16.6 on the Mini Mental State Examination, compared with 19.6 for patients without seizures. On a severity rating scale, the Clinical Dementia Rating Sum of Boxes, patients with seizures also fared worse, with scores of 9.3, compared with 6.8 for patients without seizures (P less than .0001 for all, with results adjusted for age and disease duration).
“The data of our study show that there’s an association of seizures with worse cognitive and functional performance,” Dr. Vöglein said in an interview.
“It’s important for clinicians to know that Alzheimer’s patients are at an increased risk for seizures,” Dr. Vöglein said. “In my clinical care experience, seizures are rarely the main complaint of patients with Alzheimer’s disease.” Detailed interviews with the patient and a proxy are important, he added, because patients with Alzheimer’s disease may not always remember events that could be a seizure.
Dr. Vöglein noted that, to his knowledge, there are no reliable data showing that treating seizures with antiepileptic drugs slows cognitive decline. “The results of our study suggest that an antiepileptic treatment after a first seizure in patients with Alzheimer’s dementia may be considered,” he said.
Also at the conference, researcher Ruby Castilla-Puentes, MD, DrPH, of Janssen Pharmaceuticals in Hopewell, N.J., along with Miguel Habeych, MD, MPH, of the University of Cincinnati presented findings on dementia and seizure risk from a large U.S. national managed care database of nearly 3 million people aged 60 years and older, of whom 56% were women.
The researchers analyzed this cohort during 2005-2014 and identified 80,000 people (2.8% of the cohort) as having any dementia diagnosis. The overall incidence of new-onset seizures in patients with dementia was 12.3% per year. In general, all subtypes of seizures and epileptic disorders (partial, generalized, or undifferentiated) occurred more frequently in patients with dementia, compared against patients without dementia (P less than .0001).
People with dementia had more than six times greater risk for experiencing recurring epileptic seizures than did people without dementia (95% confidence interval, 4.4-9.5). They were at six times higher risk for partial seizures (95% CI, 5.5-6.6); fivefold higher risk for generalized (95% CI, 4.9-5.5) and undifferentiated epilepsy (95% CI, 4.8-5.2); and 4.75 times higher risk for generalized seizures (95% CI, 4.5-5.0) and partial epilepsy (95% CI, 4.4-5.1).
“Although there are limitations with the use of administrative claims databases to calculate incidence rates, this analysis suggests that patients of 60 years of age or older have higher risks of new-onset seizures associated with a dementia diagnosis,” Dr. Castilla-Puentes commented.
The findings, she said, reinforce the need for clinicians to monitor for seizures to ensure that patients with dementia receive appropriate treatment.
Dr. Vöglein disclosed no financial conflicts of interest. Dr. Castilla-Puentes disclosed being an employee of Janssen, which funded her study.
LOS ANGELES – Seizures are not uncommon among patients with Alzheimer’s disease – particularly as patients live longer with the disease – and are often associated with worse cognitive and functional performance, according to research findings presented at the Alzheimer’s Association International Conference.
Jonathan Vöglein, MD, of the German Center for Neurodegenerative Diseases and Ludwig-Maximilian University in Munich presented results from a cohort of 9,127 patients with Alzheimer’s disease (AD), of whom 287 had experienced a seizure, and more than 10,000 non-AD control subjects recruited at clinics during 2005-2016.
Dr. Vöglein and colleagues found that seizure risk increased with duration of disease, from 1.5% of patients at 4.8 years with the disease to 5.4% at 11 years, with likelihood of a seizure increasing steadily over time.
Moreover, 70% of AD patients who experienced a seizure had a second one within 7.5 months. People who had seizures fared worse on cognitive and functional tests: a mean 16.6 on the Mini Mental State Examination, compared with 19.6 for patients without seizures. On a severity rating scale, the Clinical Dementia Rating Sum of Boxes, patients with seizures also fared worse, with scores of 9.3, compared with 6.8 for patients without seizures (P less than .0001 for all, with results adjusted for age and disease duration).
“The data of our study show that there’s an association of seizures with worse cognitive and functional performance,” Dr. Vöglein said in an interview.
“It’s important for clinicians to know that Alzheimer’s patients are at an increased risk for seizures,” Dr. Vöglein said. “In my clinical care experience, seizures are rarely the main complaint of patients with Alzheimer’s disease.” Detailed interviews with the patient and a proxy are important, he added, because patients with Alzheimer’s disease may not always remember events that could be a seizure.
Dr. Vöglein noted that, to his knowledge, there are no reliable data showing that treating seizures with antiepileptic drugs slows cognitive decline. “The results of our study suggest that an antiepileptic treatment after a first seizure in patients with Alzheimer’s dementia may be considered,” he said.
Also at the conference, researcher Ruby Castilla-Puentes, MD, DrPH, of Janssen Pharmaceuticals in Hopewell, N.J., along with Miguel Habeych, MD, MPH, of the University of Cincinnati presented findings on dementia and seizure risk from a large U.S. national managed care database of nearly 3 million people aged 60 years and older, of whom 56% were women.
The researchers analyzed this cohort during 2005-2014 and identified 80,000 people (2.8% of the cohort) as having any dementia diagnosis. The overall incidence of new-onset seizures in patients with dementia was 12.3% per year. In general, all subtypes of seizures and epileptic disorders (partial, generalized, or undifferentiated) occurred more frequently in patients with dementia, compared against patients without dementia (P less than .0001).
People with dementia had more than six times greater risk for experiencing recurring epileptic seizures than did people without dementia (95% confidence interval, 4.4-9.5). They were at six times higher risk for partial seizures (95% CI, 5.5-6.6); fivefold higher risk for generalized (95% CI, 4.9-5.5) and undifferentiated epilepsy (95% CI, 4.8-5.2); and 4.75 times higher risk for generalized seizures (95% CI, 4.5-5.0) and partial epilepsy (95% CI, 4.4-5.1).
“Although there are limitations with the use of administrative claims databases to calculate incidence rates, this analysis suggests that patients of 60 years of age or older have higher risks of new-onset seizures associated with a dementia diagnosis,” Dr. Castilla-Puentes commented.
The findings, she said, reinforce the need for clinicians to monitor for seizures to ensure that patients with dementia receive appropriate treatment.
Dr. Vöglein disclosed no financial conflicts of interest. Dr. Castilla-Puentes disclosed being an employee of Janssen, which funded her study.
REPORTING FROM AAIC 2019
Flurry of new anti–IL-17 monoclonal antibodies show efficacy in axSpA
MADRID – Trial results presented at the European Congress of Rheumatology for three anti–interleukin-17 receptor monoclonal antibodies under investigation for the treatment of axial spondyloarthritis (axSpA), including one for ankylosing spondylitis (AS), appear to support further clinical development and regulatory review to potentially join secukinumab (Cosentyx) and ixekizumab (Taltz) as the only IL-17 inhibitors to be licensed for rheumatic diseases.
Both netakimab and brodalumab (Siliq) achieved positive results in separate phase 3 trials for the treatment of axSpA, while new data from a phase 2b trial of bimekizumab was associated with improvement in the quality of life of patients with AS. Brodalumab is already approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis.
Netakimab
The multinational, double-blind, phase 3 trial with netakimab, called the ASTERA trial, randomized 228 patients with radiographic axSpA to either 120 mg of the experimental agent or placebo, each administered subcutaneously in weekly doses in the first 2 weeks and then every other week thereafter. The primary endpoint was a 40% improvement in Assessment of SpondyloArthritis International Society response criteria (ASAS40) at week 16.
A larger proportion of patients in the netakimab arm met the primary endpoint, compared with those in the placebo arm (40.4% vs. 2.63%, respectively; P less than .0001), reported Inna Gaydukova, MD, of Mechnikov North-Western State Medical University, St. Petersburg, Russia.
“Most of the secondary efficacy endpoints also showed a significant advantage for netakimab relative to placebo by week 4, and these advantages remained significant for the remainder of the study,” she said.
The one serious adverse event in the study occurred in the placebo arm. Although mild to moderate anemia and neutropenia were associated with treatment, the drug was well tolerated overall.
“We did observe a significant reduction in inflammatory activity in the spine with MRI at week 16,” Dr. Gaydukova added. Functional improvements in the experimental arm relative to the placebo arm were also observed, although Dr. Gaydukova acknowledged that longer trials are needed to show that these benefits are durable.
Brodalumab
The results of a multinational, double-blind, phase 3 trial with brodalumab proved similar to those with netakimab. Conducted in Taiwan, Japan, and South Korea, the trial randomized 159 patients to 210 mg of brodalumab or placebo administered subcutaneously. The therapies were administered on the same schedule as in the netakimab trial. The primary outcome was also the same.
At week 16, 43.8% of those on the experimental agent versus 24.1% of those randomized to placebo achieved ASAS40 (P = .018). As in the netakimab study, greater activity with brodalumab than placebo was also seen on several secondary outcomes, such as ASAS20 (67.5% vs. 41.8%).
“In a subgroup analysis, there was an advantage for brodalumab over placebo whether or not patients had prior experience with a TNF [tumor necrosis factor] inhibitor, regardless of baseline hs-CRP [high sensitivity C-reactive protein] level and independent of HLA type,” reported James Cheng-Chung Wei, MD, of Chung Shan Medical University Hospital, Taichung, Taiwan.
There were no significant differences in the types or rates of adverse events, including serious adverse events, in patients assigned to brodalumab relative to placebo. Suicide ideation, which has been associated with some biologics targeting other immunologic mediators, was evaluated but not seen.
“We think brodalumab has the potential to be a new therapeutic option in axSpA,” said Dr. Wei, who reported that studies in AS are also planned.
Bimekizumab
Additional 12-week outcome data from the multinational, double-blind, phase 2b BE AGILE trial of bimekizumab in patients with active AS were presented by Désirée van der Heijde, MD, PhD, of Leiden (the Netherlands) University Medical Center.
Unlike secukinumab and most of the other anti–IL-17 receptor monoclonal antibodies in development, bimekizumab inhibits IL-17F in addition to IL-17A, according to Dr. van der Heijde. She cited experimental evidence suggesting that inhibition of both forms of IL-17 results in greater anti-inflammatory response.
In the initial and previously reported data from this dose-ranging study of 303 AS patients, all four doses of bimekizumab (16 mg, 64 mg, 160 mg, or 320 mg) were superior to placebo for the primary endpoint of ASAS40. However, greater relative benefit was observed for the three highest doses.
In the new analysis, symptoms were evaluated with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). At 12 weeks, 47.5% of patients on the highest dose of bimekizumab versus only 11.9% of patients randomized to placebo achieved a 50% or greater level of improvement on the BASDAI, called BASDAI 50 (P less than .001).
The greater clinical activity of bimekizumab relative to placebo translated into improvement from baseline in Ankylosing Spondylitis Quality of Life scores. Greater reductions in Ankylosing Spondylitis Quality of Life scores relative to placebo, signaling an improved quality of life, were achieved with all doses, but they reached 4.6 points for the highest dose versus only 1.3 for placebo.
When evaluated with Patient Global Assessment of Disease Activity, another tool that reflects perception of disease burden, the score reduction was 3.3 points for the highest dose versus 1.0 points for placebo. Dr. van der Heijde characterized the reductions at the highest doses versus placebo as “significant” although she did not provide P values.
Like the data presented on the other newer anti–IL-17 therapies, bimekizumab was well tolerated with relatively low rates of adverse events, most of which were mild to moderate in severity, according to Dr. van der Heijde.
“The data from the BE AGILE trial supports phase 3 development in AS,” Dr. van der Heijde said. She noted that trials are also being planned in axSpA.
All three presenting authors reported multiple financial relationships with pharmaceutical companies, including, in each case, the pharmaceutical company that sponsored the trial they presented.
SOURCES: Gaydukova I et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):193-4, Abstract OP0232. doi: 10.1136/annrheumdis-2019-eular.6633; Wei JC et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):195, Abstract OP0234. doi: 10.1136/annrheumdis-2019-eular.6888; van der Heijde D et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):193, Abstract OP0231. doi: 10.1136/annrheumdis-2019-eular.6607.
MADRID – Trial results presented at the European Congress of Rheumatology for three anti–interleukin-17 receptor monoclonal antibodies under investigation for the treatment of axial spondyloarthritis (axSpA), including one for ankylosing spondylitis (AS), appear to support further clinical development and regulatory review to potentially join secukinumab (Cosentyx) and ixekizumab (Taltz) as the only IL-17 inhibitors to be licensed for rheumatic diseases.
Both netakimab and brodalumab (Siliq) achieved positive results in separate phase 3 trials for the treatment of axSpA, while new data from a phase 2b trial of bimekizumab was associated with improvement in the quality of life of patients with AS. Brodalumab is already approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis.
Netakimab
The multinational, double-blind, phase 3 trial with netakimab, called the ASTERA trial, randomized 228 patients with radiographic axSpA to either 120 mg of the experimental agent or placebo, each administered subcutaneously in weekly doses in the first 2 weeks and then every other week thereafter. The primary endpoint was a 40% improvement in Assessment of SpondyloArthritis International Society response criteria (ASAS40) at week 16.
A larger proportion of patients in the netakimab arm met the primary endpoint, compared with those in the placebo arm (40.4% vs. 2.63%, respectively; P less than .0001), reported Inna Gaydukova, MD, of Mechnikov North-Western State Medical University, St. Petersburg, Russia.
“Most of the secondary efficacy endpoints also showed a significant advantage for netakimab relative to placebo by week 4, and these advantages remained significant for the remainder of the study,” she said.
The one serious adverse event in the study occurred in the placebo arm. Although mild to moderate anemia and neutropenia were associated with treatment, the drug was well tolerated overall.
“We did observe a significant reduction in inflammatory activity in the spine with MRI at week 16,” Dr. Gaydukova added. Functional improvements in the experimental arm relative to the placebo arm were also observed, although Dr. Gaydukova acknowledged that longer trials are needed to show that these benefits are durable.
Brodalumab
The results of a multinational, double-blind, phase 3 trial with brodalumab proved similar to those with netakimab. Conducted in Taiwan, Japan, and South Korea, the trial randomized 159 patients to 210 mg of brodalumab or placebo administered subcutaneously. The therapies were administered on the same schedule as in the netakimab trial. The primary outcome was also the same.
At week 16, 43.8% of those on the experimental agent versus 24.1% of those randomized to placebo achieved ASAS40 (P = .018). As in the netakimab study, greater activity with brodalumab than placebo was also seen on several secondary outcomes, such as ASAS20 (67.5% vs. 41.8%).
“In a subgroup analysis, there was an advantage for brodalumab over placebo whether or not patients had prior experience with a TNF [tumor necrosis factor] inhibitor, regardless of baseline hs-CRP [high sensitivity C-reactive protein] level and independent of HLA type,” reported James Cheng-Chung Wei, MD, of Chung Shan Medical University Hospital, Taichung, Taiwan.
There were no significant differences in the types or rates of adverse events, including serious adverse events, in patients assigned to brodalumab relative to placebo. Suicide ideation, which has been associated with some biologics targeting other immunologic mediators, was evaluated but not seen.
“We think brodalumab has the potential to be a new therapeutic option in axSpA,” said Dr. Wei, who reported that studies in AS are also planned.
Bimekizumab
Additional 12-week outcome data from the multinational, double-blind, phase 2b BE AGILE trial of bimekizumab in patients with active AS were presented by Désirée van der Heijde, MD, PhD, of Leiden (the Netherlands) University Medical Center.
Unlike secukinumab and most of the other anti–IL-17 receptor monoclonal antibodies in development, bimekizumab inhibits IL-17F in addition to IL-17A, according to Dr. van der Heijde. She cited experimental evidence suggesting that inhibition of both forms of IL-17 results in greater anti-inflammatory response.
In the initial and previously reported data from this dose-ranging study of 303 AS patients, all four doses of bimekizumab (16 mg, 64 mg, 160 mg, or 320 mg) were superior to placebo for the primary endpoint of ASAS40. However, greater relative benefit was observed for the three highest doses.
In the new analysis, symptoms were evaluated with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). At 12 weeks, 47.5% of patients on the highest dose of bimekizumab versus only 11.9% of patients randomized to placebo achieved a 50% or greater level of improvement on the BASDAI, called BASDAI 50 (P less than .001).
The greater clinical activity of bimekizumab relative to placebo translated into improvement from baseline in Ankylosing Spondylitis Quality of Life scores. Greater reductions in Ankylosing Spondylitis Quality of Life scores relative to placebo, signaling an improved quality of life, were achieved with all doses, but they reached 4.6 points for the highest dose versus only 1.3 for placebo.
When evaluated with Patient Global Assessment of Disease Activity, another tool that reflects perception of disease burden, the score reduction was 3.3 points for the highest dose versus 1.0 points for placebo. Dr. van der Heijde characterized the reductions at the highest doses versus placebo as “significant” although she did not provide P values.
Like the data presented on the other newer anti–IL-17 therapies, bimekizumab was well tolerated with relatively low rates of adverse events, most of which were mild to moderate in severity, according to Dr. van der Heijde.
“The data from the BE AGILE trial supports phase 3 development in AS,” Dr. van der Heijde said. She noted that trials are also being planned in axSpA.
All three presenting authors reported multiple financial relationships with pharmaceutical companies, including, in each case, the pharmaceutical company that sponsored the trial they presented.
SOURCES: Gaydukova I et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):193-4, Abstract OP0232. doi: 10.1136/annrheumdis-2019-eular.6633; Wei JC et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):195, Abstract OP0234. doi: 10.1136/annrheumdis-2019-eular.6888; van der Heijde D et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):193, Abstract OP0231. doi: 10.1136/annrheumdis-2019-eular.6607.
MADRID – Trial results presented at the European Congress of Rheumatology for three anti–interleukin-17 receptor monoclonal antibodies under investigation for the treatment of axial spondyloarthritis (axSpA), including one for ankylosing spondylitis (AS), appear to support further clinical development and regulatory review to potentially join secukinumab (Cosentyx) and ixekizumab (Taltz) as the only IL-17 inhibitors to be licensed for rheumatic diseases.
Both netakimab and brodalumab (Siliq) achieved positive results in separate phase 3 trials for the treatment of axSpA, while new data from a phase 2b trial of bimekizumab was associated with improvement in the quality of life of patients with AS. Brodalumab is already approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis.
Netakimab
The multinational, double-blind, phase 3 trial with netakimab, called the ASTERA trial, randomized 228 patients with radiographic axSpA to either 120 mg of the experimental agent or placebo, each administered subcutaneously in weekly doses in the first 2 weeks and then every other week thereafter. The primary endpoint was a 40% improvement in Assessment of SpondyloArthritis International Society response criteria (ASAS40) at week 16.
A larger proportion of patients in the netakimab arm met the primary endpoint, compared with those in the placebo arm (40.4% vs. 2.63%, respectively; P less than .0001), reported Inna Gaydukova, MD, of Mechnikov North-Western State Medical University, St. Petersburg, Russia.
“Most of the secondary efficacy endpoints also showed a significant advantage for netakimab relative to placebo by week 4, and these advantages remained significant for the remainder of the study,” she said.
The one serious adverse event in the study occurred in the placebo arm. Although mild to moderate anemia and neutropenia were associated with treatment, the drug was well tolerated overall.
“We did observe a significant reduction in inflammatory activity in the spine with MRI at week 16,” Dr. Gaydukova added. Functional improvements in the experimental arm relative to the placebo arm were also observed, although Dr. Gaydukova acknowledged that longer trials are needed to show that these benefits are durable.
Brodalumab
The results of a multinational, double-blind, phase 3 trial with brodalumab proved similar to those with netakimab. Conducted in Taiwan, Japan, and South Korea, the trial randomized 159 patients to 210 mg of brodalumab or placebo administered subcutaneously. The therapies were administered on the same schedule as in the netakimab trial. The primary outcome was also the same.
At week 16, 43.8% of those on the experimental agent versus 24.1% of those randomized to placebo achieved ASAS40 (P = .018). As in the netakimab study, greater activity with brodalumab than placebo was also seen on several secondary outcomes, such as ASAS20 (67.5% vs. 41.8%).
“In a subgroup analysis, there was an advantage for brodalumab over placebo whether or not patients had prior experience with a TNF [tumor necrosis factor] inhibitor, regardless of baseline hs-CRP [high sensitivity C-reactive protein] level and independent of HLA type,” reported James Cheng-Chung Wei, MD, of Chung Shan Medical University Hospital, Taichung, Taiwan.
There were no significant differences in the types or rates of adverse events, including serious adverse events, in patients assigned to brodalumab relative to placebo. Suicide ideation, which has been associated with some biologics targeting other immunologic mediators, was evaluated but not seen.
“We think brodalumab has the potential to be a new therapeutic option in axSpA,” said Dr. Wei, who reported that studies in AS are also planned.
Bimekizumab
Additional 12-week outcome data from the multinational, double-blind, phase 2b BE AGILE trial of bimekizumab in patients with active AS were presented by Désirée van der Heijde, MD, PhD, of Leiden (the Netherlands) University Medical Center.
Unlike secukinumab and most of the other anti–IL-17 receptor monoclonal antibodies in development, bimekizumab inhibits IL-17F in addition to IL-17A, according to Dr. van der Heijde. She cited experimental evidence suggesting that inhibition of both forms of IL-17 results in greater anti-inflammatory response.
In the initial and previously reported data from this dose-ranging study of 303 AS patients, all four doses of bimekizumab (16 mg, 64 mg, 160 mg, or 320 mg) were superior to placebo for the primary endpoint of ASAS40. However, greater relative benefit was observed for the three highest doses.
In the new analysis, symptoms were evaluated with the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). At 12 weeks, 47.5% of patients on the highest dose of bimekizumab versus only 11.9% of patients randomized to placebo achieved a 50% or greater level of improvement on the BASDAI, called BASDAI 50 (P less than .001).
The greater clinical activity of bimekizumab relative to placebo translated into improvement from baseline in Ankylosing Spondylitis Quality of Life scores. Greater reductions in Ankylosing Spondylitis Quality of Life scores relative to placebo, signaling an improved quality of life, were achieved with all doses, but they reached 4.6 points for the highest dose versus only 1.3 for placebo.
When evaluated with Patient Global Assessment of Disease Activity, another tool that reflects perception of disease burden, the score reduction was 3.3 points for the highest dose versus 1.0 points for placebo. Dr. van der Heijde characterized the reductions at the highest doses versus placebo as “significant” although she did not provide P values.
Like the data presented on the other newer anti–IL-17 therapies, bimekizumab was well tolerated with relatively low rates of adverse events, most of which were mild to moderate in severity, according to Dr. van der Heijde.
“The data from the BE AGILE trial supports phase 3 development in AS,” Dr. van der Heijde said. She noted that trials are also being planned in axSpA.
All three presenting authors reported multiple financial relationships with pharmaceutical companies, including, in each case, the pharmaceutical company that sponsored the trial they presented.
SOURCES: Gaydukova I et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):193-4, Abstract OP0232. doi: 10.1136/annrheumdis-2019-eular.6633; Wei JC et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):195, Abstract OP0234. doi: 10.1136/annrheumdis-2019-eular.6888; van der Heijde D et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):193, Abstract OP0231. doi: 10.1136/annrheumdis-2019-eular.6607.
REPORTING FROM EULAR 2019 CONGRESS
Title X grantees are told to stop abortion referrals immediately; Wen removed from Planned Parenthood
[UPDATED 5:12 PM]
Effective immediately, recipients of federal family planning dollars under the Title X program can no longer provide referrals for abortion services.
The Health and Human Services department issued the email notification to Title X recipients late in the day on July 15, a day before a planned national Title X grantee meeting in Washington.
The HHS statement cited recent federal appellate court decisions that have declined to stay the ban on abortion referrals for Title X recipients contained in a March 2019 final rule. “Consistent with those rulings, HHS shall now require compliance with the Final Rule,” HHS said in the emailed statement.
Currently, no federal funds can be used to provide abortion services, but Title X recipients have been able to provide referrals for abortions or to perform non–federally funded abortions on the same premises where Title X–funded family planning services are provided. The implementation of the final rule would change this by barring referrals and by imposing physical separation requirements for health centers that offer abortions.
In an email accompanying the notification to Title X grantees, Diane Foley, MD, deputy assistant secretary of the HHS Office of Population Affairs, noted that, “We are aware that many of you have been frustrated with the lack of guidance given to you regarding the 2019 Title X Final Rule that was posted earlier this year.” She added that “The timing of the national grantee meeting will allow us to provide direction to you as well as hopefully answer your questions face to face.”
Providers of family planning and abortion services objected to what they are calling the Title X “gag rule.”
“As we have said all along, the Trump-Pence administration’s Title X gag rule is devastating for the millions of people who rely on this program for cancer screenings, HIV tests, affordable birth control and other critical primary and preventive care,” Leana Wen, MD, president and CEO of the Planned Parenthood Federation of America (PPFA), said in a statement. She went on to say that, “While we are incredibly concerned by this harmful rule, our doors are still open.”
Shortly after that statement was issued, Dr. Wen was removed from her position.
"We thank Dr. Leana Wen for her service to Planned Parenthood in such a pivotal time and extend our best wishes for her continued success," PPFA Board Chair Aimee Cunningham and Planned Parenthood Action Fund Board Chair Jennie Rosenthal said in a joint statement. Alexis McGill Johnson, a PPFA board member and former board chair, was named acting president.
"I am leaving the organization sooner than I'd hoped because of philosophical differences about the direction and future of Planned Parenthood," Dr. Wen said in a post on Twitter. "I came to Planned Parenthood to run a national health care organization and to advocate for the broad range of public health policies that affect our patients' health.... New board leadership has determined that the priorit for Planned Parenthood moving forward is to double down on abortion rights advocacy. Witht he landscape changing dramatically in the last several months and the right to safe, legal abortion care under attack as never before, I understand the shift in the board's prioritization."
The National Family Planning & Reproductive Health Association’s president and CEO Clare Coleman said that, “this rule will shatter the long-standing provider network – leaving hundreds of thousands of vulnerable patients without essential care. Last night’s notice will prompt grantees and other participating entities to withdraw from Title X, and we anticipate withdrawals beginning this week.”
An HHS spokeswoman said additional guidance will be provided to grantees during the meeting.
“While the final rule prohibits referral for abortion as a method of family planning, nondirective counseling on abortion is permitted,” the HHS spokeswoman said. “The final rule protects Title X health care providers so that they are not required to choose between participating in the program and violating their own consciences by providing abortion counseling and referral.”
The American Association of Pro-Life Obstetricians and Gynecologists applauded the implementation of the final rule and the “separation of Title X funds from the provision of abortion.”
Most recently, the federal Ninth Circuit Court of Appeals, in an en banc ruling, denied requests for an administrative stay of the final rule. Previous rulings by the Fourth Circuit, as well as a federal district court in Maine, also have denied requests for injunctions against the final rule.
An additional requirement for physical separation of abortion facilities from other Title X–funded family planning facilities will go into effect on March 4, 2020, according to the HHS communication.
[UPDATED 5:12 PM]
Effective immediately, recipients of federal family planning dollars under the Title X program can no longer provide referrals for abortion services.
The Health and Human Services department issued the email notification to Title X recipients late in the day on July 15, a day before a planned national Title X grantee meeting in Washington.
The HHS statement cited recent federal appellate court decisions that have declined to stay the ban on abortion referrals for Title X recipients contained in a March 2019 final rule. “Consistent with those rulings, HHS shall now require compliance with the Final Rule,” HHS said in the emailed statement.
Currently, no federal funds can be used to provide abortion services, but Title X recipients have been able to provide referrals for abortions or to perform non–federally funded abortions on the same premises where Title X–funded family planning services are provided. The implementation of the final rule would change this by barring referrals and by imposing physical separation requirements for health centers that offer abortions.
In an email accompanying the notification to Title X grantees, Diane Foley, MD, deputy assistant secretary of the HHS Office of Population Affairs, noted that, “We are aware that many of you have been frustrated with the lack of guidance given to you regarding the 2019 Title X Final Rule that was posted earlier this year.” She added that “The timing of the national grantee meeting will allow us to provide direction to you as well as hopefully answer your questions face to face.”
Providers of family planning and abortion services objected to what they are calling the Title X “gag rule.”
“As we have said all along, the Trump-Pence administration’s Title X gag rule is devastating for the millions of people who rely on this program for cancer screenings, HIV tests, affordable birth control and other critical primary and preventive care,” Leana Wen, MD, president and CEO of the Planned Parenthood Federation of America (PPFA), said in a statement. She went on to say that, “While we are incredibly concerned by this harmful rule, our doors are still open.”
Shortly after that statement was issued, Dr. Wen was removed from her position.
"We thank Dr. Leana Wen for her service to Planned Parenthood in such a pivotal time and extend our best wishes for her continued success," PPFA Board Chair Aimee Cunningham and Planned Parenthood Action Fund Board Chair Jennie Rosenthal said in a joint statement. Alexis McGill Johnson, a PPFA board member and former board chair, was named acting president.
"I am leaving the organization sooner than I'd hoped because of philosophical differences about the direction and future of Planned Parenthood," Dr. Wen said in a post on Twitter. "I came to Planned Parenthood to run a national health care organization and to advocate for the broad range of public health policies that affect our patients' health.... New board leadership has determined that the priorit for Planned Parenthood moving forward is to double down on abortion rights advocacy. Witht he landscape changing dramatically in the last several months and the right to safe, legal abortion care under attack as never before, I understand the shift in the board's prioritization."
The National Family Planning & Reproductive Health Association’s president and CEO Clare Coleman said that, “this rule will shatter the long-standing provider network – leaving hundreds of thousands of vulnerable patients without essential care. Last night’s notice will prompt grantees and other participating entities to withdraw from Title X, and we anticipate withdrawals beginning this week.”
An HHS spokeswoman said additional guidance will be provided to grantees during the meeting.
“While the final rule prohibits referral for abortion as a method of family planning, nondirective counseling on abortion is permitted,” the HHS spokeswoman said. “The final rule protects Title X health care providers so that they are not required to choose between participating in the program and violating their own consciences by providing abortion counseling and referral.”
The American Association of Pro-Life Obstetricians and Gynecologists applauded the implementation of the final rule and the “separation of Title X funds from the provision of abortion.”
Most recently, the federal Ninth Circuit Court of Appeals, in an en banc ruling, denied requests for an administrative stay of the final rule. Previous rulings by the Fourth Circuit, as well as a federal district court in Maine, also have denied requests for injunctions against the final rule.
An additional requirement for physical separation of abortion facilities from other Title X–funded family planning facilities will go into effect on March 4, 2020, according to the HHS communication.
[UPDATED 5:12 PM]
Effective immediately, recipients of federal family planning dollars under the Title X program can no longer provide referrals for abortion services.
The Health and Human Services department issued the email notification to Title X recipients late in the day on July 15, a day before a planned national Title X grantee meeting in Washington.
The HHS statement cited recent federal appellate court decisions that have declined to stay the ban on abortion referrals for Title X recipients contained in a March 2019 final rule. “Consistent with those rulings, HHS shall now require compliance with the Final Rule,” HHS said in the emailed statement.
Currently, no federal funds can be used to provide abortion services, but Title X recipients have been able to provide referrals for abortions or to perform non–federally funded abortions on the same premises where Title X–funded family planning services are provided. The implementation of the final rule would change this by barring referrals and by imposing physical separation requirements for health centers that offer abortions.
In an email accompanying the notification to Title X grantees, Diane Foley, MD, deputy assistant secretary of the HHS Office of Population Affairs, noted that, “We are aware that many of you have been frustrated with the lack of guidance given to you regarding the 2019 Title X Final Rule that was posted earlier this year.” She added that “The timing of the national grantee meeting will allow us to provide direction to you as well as hopefully answer your questions face to face.”
Providers of family planning and abortion services objected to what they are calling the Title X “gag rule.”
“As we have said all along, the Trump-Pence administration’s Title X gag rule is devastating for the millions of people who rely on this program for cancer screenings, HIV tests, affordable birth control and other critical primary and preventive care,” Leana Wen, MD, president and CEO of the Planned Parenthood Federation of America (PPFA), said in a statement. She went on to say that, “While we are incredibly concerned by this harmful rule, our doors are still open.”
Shortly after that statement was issued, Dr. Wen was removed from her position.
"We thank Dr. Leana Wen for her service to Planned Parenthood in such a pivotal time and extend our best wishes for her continued success," PPFA Board Chair Aimee Cunningham and Planned Parenthood Action Fund Board Chair Jennie Rosenthal said in a joint statement. Alexis McGill Johnson, a PPFA board member and former board chair, was named acting president.
"I am leaving the organization sooner than I'd hoped because of philosophical differences about the direction and future of Planned Parenthood," Dr. Wen said in a post on Twitter. "I came to Planned Parenthood to run a national health care organization and to advocate for the broad range of public health policies that affect our patients' health.... New board leadership has determined that the priorit for Planned Parenthood moving forward is to double down on abortion rights advocacy. Witht he landscape changing dramatically in the last several months and the right to safe, legal abortion care under attack as never before, I understand the shift in the board's prioritization."
The National Family Planning & Reproductive Health Association’s president and CEO Clare Coleman said that, “this rule will shatter the long-standing provider network – leaving hundreds of thousands of vulnerable patients without essential care. Last night’s notice will prompt grantees and other participating entities to withdraw from Title X, and we anticipate withdrawals beginning this week.”
An HHS spokeswoman said additional guidance will be provided to grantees during the meeting.
“While the final rule prohibits referral for abortion as a method of family planning, nondirective counseling on abortion is permitted,” the HHS spokeswoman said. “The final rule protects Title X health care providers so that they are not required to choose between participating in the program and violating their own consciences by providing abortion counseling and referral.”
The American Association of Pro-Life Obstetricians and Gynecologists applauded the implementation of the final rule and the “separation of Title X funds from the provision of abortion.”
Most recently, the federal Ninth Circuit Court of Appeals, in an en banc ruling, denied requests for an administrative stay of the final rule. Previous rulings by the Fourth Circuit, as well as a federal district court in Maine, also have denied requests for injunctions against the final rule.
An additional requirement for physical separation of abortion facilities from other Title X–funded family planning facilities will go into effect on March 4, 2020, according to the HHS communication.
Racial, ethnic minorities often don’t practice sun protective behaviors
Despite higher rates of skin cancer morbidity and mortality among racial and ethnic minorities, affected adults often are not recognizing their risks or taking preventive measures, said Costner McKenzie, BA, and Roopal V. Kundu, MD of Northwestern University, Chicago.
In a multivariable logistic regression analysis, Mr. Costner and Dr. Kundu sampled data of 33,672 adults included in the 2015 National Health Interview Survey. Data from the 2010 U.S. Census Bureau also were used to develop sample weights representative of the U.S. population. There was a survey of a smaller sample of adults who were determined to have sun-sensitive skin. The findings were published in the Journal of the American Academy of Dermatology.
Sun sensitivity was determined by skin reaction to 1 hour of unprotected sun exposure. Those who self-reported severe sunburn with blisters or moderate sunburn with peeling were determined to be sun sensitive.
The sample surveyed comprised 3,665 women (41%) and 5,287 men (59%). Of these, 82% were white non-Hispanic, 3% black non-Hispanic, 3% Asian non-Hispanic, 11% Hispanic, and 1% other non-Hispanic.
Mr. McKenzie and Dr. Kundu found that (adjusted odds ratio [aOR], 0.43, 0.54, and 0.70, respectively). Non-Hispanic blacks and Hispanics also were less likely to use sunscreen greater than SPF 15 (a0R, 0.39 and 0.64, respectively). Non-Hispanic blacks, non-Hispanic Asians, and Hispanics were less likely to have ever had a total body skin examination (aOR, 0.29, 0.21, and 0.39, respectively).
Yet these same three groups were more likely to wear long sleeves outside (non-Hispanic blacks aOR, 1.96, non-Hispanic Asians aOR, 2.09, and Hispanics aOR, 2.29). In addition, non-Hispanic Asians and Hispanics were more likely to shelter in the shade on warm, sunny days (aOR, 1.63 and 1.85, respectively).
Citing recent literature, the authors noted that although skin cancer is the most commonly diagnosed cancer, it is not typically thought of as a disease that afflicts minority populations, especially among minorities themselves, who do not generally recognize their own risk (Arch Dermatol. 2009;145[2]:207-8). In fact, morbidity and mortality from skin cancer actually are greater in racial and ethnic minorities (J Am Acad Dermatol. 2016;75[5]:983-91; J Am Acad Dermatol. 2006;55[5]:741-60), despite greater incidence of skin cancer among white adults.
“This study highlights the impact of race and ethnicity on sun protective behaviors,” said Mr. McKenzie and Dr. Kundu. Cultural beliefs, stigma, personal preferences, as well as a lack of “knowledge-based interventions” specifically intended for minorities could be responsible for the observed differences between population groups, they speculated.
The primary limitations of the study were its cross-sectional design and the use of self-reported data, the authors noted.
Additional research is needed to fully examine the reasons behind these differences as well as to identify appropriate interventions that promote sun protection, they added.
There was no external funding and the authors had no conflicts of interest to disclose.
SOURCE: McKenzie C and Kundu RV. J Am Acad Dermatol. 2019 Jun 19. doi: 10.1016/j.jaad.2019.06.1306.
Despite higher rates of skin cancer morbidity and mortality among racial and ethnic minorities, affected adults often are not recognizing their risks or taking preventive measures, said Costner McKenzie, BA, and Roopal V. Kundu, MD of Northwestern University, Chicago.
In a multivariable logistic regression analysis, Mr. Costner and Dr. Kundu sampled data of 33,672 adults included in the 2015 National Health Interview Survey. Data from the 2010 U.S. Census Bureau also were used to develop sample weights representative of the U.S. population. There was a survey of a smaller sample of adults who were determined to have sun-sensitive skin. The findings were published in the Journal of the American Academy of Dermatology.
Sun sensitivity was determined by skin reaction to 1 hour of unprotected sun exposure. Those who self-reported severe sunburn with blisters or moderate sunburn with peeling were determined to be sun sensitive.
The sample surveyed comprised 3,665 women (41%) and 5,287 men (59%). Of these, 82% were white non-Hispanic, 3% black non-Hispanic, 3% Asian non-Hispanic, 11% Hispanic, and 1% other non-Hispanic.
Mr. McKenzie and Dr. Kundu found that (adjusted odds ratio [aOR], 0.43, 0.54, and 0.70, respectively). Non-Hispanic blacks and Hispanics also were less likely to use sunscreen greater than SPF 15 (a0R, 0.39 and 0.64, respectively). Non-Hispanic blacks, non-Hispanic Asians, and Hispanics were less likely to have ever had a total body skin examination (aOR, 0.29, 0.21, and 0.39, respectively).
Yet these same three groups were more likely to wear long sleeves outside (non-Hispanic blacks aOR, 1.96, non-Hispanic Asians aOR, 2.09, and Hispanics aOR, 2.29). In addition, non-Hispanic Asians and Hispanics were more likely to shelter in the shade on warm, sunny days (aOR, 1.63 and 1.85, respectively).
Citing recent literature, the authors noted that although skin cancer is the most commonly diagnosed cancer, it is not typically thought of as a disease that afflicts minority populations, especially among minorities themselves, who do not generally recognize their own risk (Arch Dermatol. 2009;145[2]:207-8). In fact, morbidity and mortality from skin cancer actually are greater in racial and ethnic minorities (J Am Acad Dermatol. 2016;75[5]:983-91; J Am Acad Dermatol. 2006;55[5]:741-60), despite greater incidence of skin cancer among white adults.
“This study highlights the impact of race and ethnicity on sun protective behaviors,” said Mr. McKenzie and Dr. Kundu. Cultural beliefs, stigma, personal preferences, as well as a lack of “knowledge-based interventions” specifically intended for minorities could be responsible for the observed differences between population groups, they speculated.
The primary limitations of the study were its cross-sectional design and the use of self-reported data, the authors noted.
Additional research is needed to fully examine the reasons behind these differences as well as to identify appropriate interventions that promote sun protection, they added.
There was no external funding and the authors had no conflicts of interest to disclose.
SOURCE: McKenzie C and Kundu RV. J Am Acad Dermatol. 2019 Jun 19. doi: 10.1016/j.jaad.2019.06.1306.
Despite higher rates of skin cancer morbidity and mortality among racial and ethnic minorities, affected adults often are not recognizing their risks or taking preventive measures, said Costner McKenzie, BA, and Roopal V. Kundu, MD of Northwestern University, Chicago.
In a multivariable logistic regression analysis, Mr. Costner and Dr. Kundu sampled data of 33,672 adults included in the 2015 National Health Interview Survey. Data from the 2010 U.S. Census Bureau also were used to develop sample weights representative of the U.S. population. There was a survey of a smaller sample of adults who were determined to have sun-sensitive skin. The findings were published in the Journal of the American Academy of Dermatology.
Sun sensitivity was determined by skin reaction to 1 hour of unprotected sun exposure. Those who self-reported severe sunburn with blisters or moderate sunburn with peeling were determined to be sun sensitive.
The sample surveyed comprised 3,665 women (41%) and 5,287 men (59%). Of these, 82% were white non-Hispanic, 3% black non-Hispanic, 3% Asian non-Hispanic, 11% Hispanic, and 1% other non-Hispanic.
Mr. McKenzie and Dr. Kundu found that (adjusted odds ratio [aOR], 0.43, 0.54, and 0.70, respectively). Non-Hispanic blacks and Hispanics also were less likely to use sunscreen greater than SPF 15 (a0R, 0.39 and 0.64, respectively). Non-Hispanic blacks, non-Hispanic Asians, and Hispanics were less likely to have ever had a total body skin examination (aOR, 0.29, 0.21, and 0.39, respectively).
Yet these same three groups were more likely to wear long sleeves outside (non-Hispanic blacks aOR, 1.96, non-Hispanic Asians aOR, 2.09, and Hispanics aOR, 2.29). In addition, non-Hispanic Asians and Hispanics were more likely to shelter in the shade on warm, sunny days (aOR, 1.63 and 1.85, respectively).
Citing recent literature, the authors noted that although skin cancer is the most commonly diagnosed cancer, it is not typically thought of as a disease that afflicts minority populations, especially among minorities themselves, who do not generally recognize their own risk (Arch Dermatol. 2009;145[2]:207-8). In fact, morbidity and mortality from skin cancer actually are greater in racial and ethnic minorities (J Am Acad Dermatol. 2016;75[5]:983-91; J Am Acad Dermatol. 2006;55[5]:741-60), despite greater incidence of skin cancer among white adults.
“This study highlights the impact of race and ethnicity on sun protective behaviors,” said Mr. McKenzie and Dr. Kundu. Cultural beliefs, stigma, personal preferences, as well as a lack of “knowledge-based interventions” specifically intended for minorities could be responsible for the observed differences between population groups, they speculated.
The primary limitations of the study were its cross-sectional design and the use of self-reported data, the authors noted.
Additional research is needed to fully examine the reasons behind these differences as well as to identify appropriate interventions that promote sun protection, they added.
There was no external funding and the authors had no conflicts of interest to disclose.
SOURCE: McKenzie C and Kundu RV. J Am Acad Dermatol. 2019 Jun 19. doi: 10.1016/j.jaad.2019.06.1306.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
On leadership, keep your powder dry
My grandfather used to say: “Son, keep your powder dry.” The old aphorism is basically a reminder to keep your power or influence in reserve until you really need it. In my case, he could simply have been trying to quiet an overly talkative kid. But anything my grandfather said that had to do with guns carried great authority with me. He owned a shotgun that had been converted from a flintlock by his grandfather, and we grandchildren were schooled in its use.
I was recently reminded of my grandfather’s advice as I was about to participate in a planned meeting. One of the meeting leaders pulled me aside to tell me that I was not to ask questions, and informed me that if I did ask questions, I would be punished to the fullest extent possible. I also was not to challenge the preordained conclusion.
I was simultaneously aghast and impressed. Never had I heard such a trifecta of terrible leadership uttered in a single breath.
There are basic prerequisites of building consensus and adopting new ideas.
First, you must introduce the wonderful idea and explain it to everyone. It is even better when you are able to back up someone else’s great idea.
Next, you must allow the group to discuss the idea and be open to change. This may involve shelving, or even killing, your shiny new idea.
You must allow time for things to sink in regarding the idea. If you want something to stick, people have to adapt and adopt. If your idea is truly wonderful, you should be able to attract advocates and get a majority to agree to the plan. You should make sure they get to vote on the plan. If you lose the vote, don’t try any maneuvers to undo your loss. Accept it and move on. If your idea is good, it will surface again as someone else’s idea and be a much easier pitch.
Finally, never, ever bully or threaten. Memories are long, and while some may not agree with your idea, they won’t actively try to kill it later or undermine future projects. Pinch yourself every day, and remember that you are a servant of the group. You are a facilitator, not an autocrat.
That said, leaders differ in their styles of leadership. I think the best leaders are not afraid to listen to their boards and membership. Their actions focus thoughts and move things along. If you let your board members own their actions, they will respect you. You will build up tremendous goodwill (and powder). With any luck, you may never need to draw on this goodwill; but if there is a crisis and you need to put a bullet through something quickly, your intentions will not be questioned. At a minimum, you will have plenty of opportunity to explain your actions.
So it saddens me to see leaders burn all their powder and lose their influence. It’s not good for them or their organization, and they will likely have to overcome unexpected resistance in the future.
Leaders, keep your powder dry!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Dr. Coldiron is the chair of SkinPAC for 2019-2021; this is an unpaid volunteer position. Write to him at [email protected].
My grandfather used to say: “Son, keep your powder dry.” The old aphorism is basically a reminder to keep your power or influence in reserve until you really need it. In my case, he could simply have been trying to quiet an overly talkative kid. But anything my grandfather said that had to do with guns carried great authority with me. He owned a shotgun that had been converted from a flintlock by his grandfather, and we grandchildren were schooled in its use.
I was recently reminded of my grandfather’s advice as I was about to participate in a planned meeting. One of the meeting leaders pulled me aside to tell me that I was not to ask questions, and informed me that if I did ask questions, I would be punished to the fullest extent possible. I also was not to challenge the preordained conclusion.
I was simultaneously aghast and impressed. Never had I heard such a trifecta of terrible leadership uttered in a single breath.
There are basic prerequisites of building consensus and adopting new ideas.
First, you must introduce the wonderful idea and explain it to everyone. It is even better when you are able to back up someone else’s great idea.
Next, you must allow the group to discuss the idea and be open to change. This may involve shelving, or even killing, your shiny new idea.
You must allow time for things to sink in regarding the idea. If you want something to stick, people have to adapt and adopt. If your idea is truly wonderful, you should be able to attract advocates and get a majority to agree to the plan. You should make sure they get to vote on the plan. If you lose the vote, don’t try any maneuvers to undo your loss. Accept it and move on. If your idea is good, it will surface again as someone else’s idea and be a much easier pitch.
Finally, never, ever bully or threaten. Memories are long, and while some may not agree with your idea, they won’t actively try to kill it later or undermine future projects. Pinch yourself every day, and remember that you are a servant of the group. You are a facilitator, not an autocrat.
That said, leaders differ in their styles of leadership. I think the best leaders are not afraid to listen to their boards and membership. Their actions focus thoughts and move things along. If you let your board members own their actions, they will respect you. You will build up tremendous goodwill (and powder). With any luck, you may never need to draw on this goodwill; but if there is a crisis and you need to put a bullet through something quickly, your intentions will not be questioned. At a minimum, you will have plenty of opportunity to explain your actions.
So it saddens me to see leaders burn all their powder and lose their influence. It’s not good for them or their organization, and they will likely have to overcome unexpected resistance in the future.
Leaders, keep your powder dry!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Dr. Coldiron is the chair of SkinPAC for 2019-2021; this is an unpaid volunteer position. Write to him at [email protected].
My grandfather used to say: “Son, keep your powder dry.” The old aphorism is basically a reminder to keep your power or influence in reserve until you really need it. In my case, he could simply have been trying to quiet an overly talkative kid. But anything my grandfather said that had to do with guns carried great authority with me. He owned a shotgun that had been converted from a flintlock by his grandfather, and we grandchildren were schooled in its use.
I was recently reminded of my grandfather’s advice as I was about to participate in a planned meeting. One of the meeting leaders pulled me aside to tell me that I was not to ask questions, and informed me that if I did ask questions, I would be punished to the fullest extent possible. I also was not to challenge the preordained conclusion.
I was simultaneously aghast and impressed. Never had I heard such a trifecta of terrible leadership uttered in a single breath.
There are basic prerequisites of building consensus and adopting new ideas.
First, you must introduce the wonderful idea and explain it to everyone. It is even better when you are able to back up someone else’s great idea.
Next, you must allow the group to discuss the idea and be open to change. This may involve shelving, or even killing, your shiny new idea.
You must allow time for things to sink in regarding the idea. If you want something to stick, people have to adapt and adopt. If your idea is truly wonderful, you should be able to attract advocates and get a majority to agree to the plan. You should make sure they get to vote on the plan. If you lose the vote, don’t try any maneuvers to undo your loss. Accept it and move on. If your idea is good, it will surface again as someone else’s idea and be a much easier pitch.
Finally, never, ever bully or threaten. Memories are long, and while some may not agree with your idea, they won’t actively try to kill it later or undermine future projects. Pinch yourself every day, and remember that you are a servant of the group. You are a facilitator, not an autocrat.
That said, leaders differ in their styles of leadership. I think the best leaders are not afraid to listen to their boards and membership. Their actions focus thoughts and move things along. If you let your board members own their actions, they will respect you. You will build up tremendous goodwill (and powder). With any luck, you may never need to draw on this goodwill; but if there is a crisis and you need to put a bullet through something quickly, your intentions will not be questioned. At a minimum, you will have plenty of opportunity to explain your actions.
So it saddens me to see leaders burn all their powder and lose their influence. It’s not good for them or their organization, and they will likely have to overcome unexpected resistance in the future.
Leaders, keep your powder dry!
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Dr. Coldiron is the chair of SkinPAC for 2019-2021; this is an unpaid volunteer position. Write to him at [email protected].
Secukinumab reduced joint pain of psoriatic arthritis in early data from phase 3b trial
MADRID – Secukinumab was superior to placebo for the primary endpoint of 20% improvement in Assessment of SpondyloArthritis international Society criteria (ASAS20), based on the initial 12-week data from the ongoing phase 3b MAXIMISE trial, the first randomized, controlled trial to evaluate a biologic therapy for the treatment of the axial manifestations of psoriatic arthritis (PsA).
“There was rapid and significant clinical improvement as measured with ASAS20 with both of the study doses of secukinumab,” an anti–interleukin-17 monoclonal antibody, reported Xenofon Baraliakos, MD, of Rheumazentrum Ruhrgebliet, Ruhr-University Bochum in Herne, Germany.
At the European Congress of Rheumatology, Dr. Baraliakos said that the 1-year data will be complete before the end of 2019.
In this primary analysis, 498 patients with established PsA were randomized to 150 mg secukinumab, 300 mg secukinumab, or placebo. For enrollment, all patients were required to have substantial axial pain and a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score greater than 4 despite treatment with at least two NSAIDs.
For the first 4 weeks of the trial, patients received their assigned therapies weekly by subcutaneous administration. Treatment thereafter was every 4 weeks.
Almost all patients had active psoriasis and axial symptoms of at least several years duration. The median patient age was 46 years. Approximately one-third were HLA B27 positive.
At baseline, the median back pain score on a visual analog scale of 100 was 73, and the median BASDAI score was greater than 7.0. More than 90% of patients reported back pain worsening with rest.
The proportion of patients achieving ASAS20 at 12 weeks was 63.1% in the group assigned to 300 mg secukinumab, 66.3% in those assigned 150 mg, and 31.1% for those assigned placebo (P less than .0001 for either active therapy versus placebo).
Analyses conducted with multiple imputations and comparing those who were and were not taking methotrexate produced almost the same relative advantage for secukinumab. ASAS20 responses in patients using concomitant methotrexate were 65.1% with 300 mg secukinumab, 67.3% with 150 mg secukinumab, and 33.9% with placebo. Corresponding values in the no-methotrexate group were 60.5%, 64.4%, and 27.1%, respectively.
“There was a good response from either dose of secukinumab no matter what analysis was employed,” reported Dr. Baraliakos, citing an odds ratio of 3.81 for reaching the primary endpoint with secukinumab versus placebo. There were no significant differences in efficacy between the doses of secukinumab.
There was “not much to say about safety,” according to Dr. Baraliakos, as no significant differences in any adverse events were observed between study arms. However, he did caution that longer-term exposure is needed for a more complete analysis of tolerability and safety.
Most patients with PsA are thought to eventually develop axial involvement, which has a major adverse affect on quality of life, according to Dr. Baraliakos. He considers this primary 12-week analysis encouraging, but said the 1-year data will provide more information about whether this therapy should be considered routinely in PsA patients with persistent axial symptoms.
Axial imaging was conducted at study entry even though it was not a criterion for enrollment. Dr. Baraliakos reported that the impact of secukinumab on objective imaging measures of disease activity, if any, is forthcoming.
Imaging data might be needed to establish benefit objectively, judging from a criticism of the study design that arose during discussion after the data were presented. Specifically, it was pointed out that improvement in ASAS20 and BASDAI could occur as a result of improvement in peripheral symptoms, such as enthesitis. The lack of axial-specific outcomes was called a potential weakness of this study.
Dr. Baraliakos countered that BASDAI evaluations did include axial-specific questions, but also confirmed that spine-specific outcomes are included among outcomes to be presented with longer-term analyses.
“These data will come,” said Dr. Baraliakos, referring to imaging as well as other outcomes that will provide more information on the impact of secukinumab in treating the axial involvement of PsA.
Dr. Baraliakos reported multiple financial relationships with pharmaceutical companies, including Novartis, which sponsored this trial.
SOURCE: Ann Rheum Dis. Jun 2019;78(Suppl2):195-6. Abstract OPO235. doi: 10.1136/annrheumdis-2019-eular.2932.
MADRID – Secukinumab was superior to placebo for the primary endpoint of 20% improvement in Assessment of SpondyloArthritis international Society criteria (ASAS20), based on the initial 12-week data from the ongoing phase 3b MAXIMISE trial, the first randomized, controlled trial to evaluate a biologic therapy for the treatment of the axial manifestations of psoriatic arthritis (PsA).
“There was rapid and significant clinical improvement as measured with ASAS20 with both of the study doses of secukinumab,” an anti–interleukin-17 monoclonal antibody, reported Xenofon Baraliakos, MD, of Rheumazentrum Ruhrgebliet, Ruhr-University Bochum in Herne, Germany.
At the European Congress of Rheumatology, Dr. Baraliakos said that the 1-year data will be complete before the end of 2019.
In this primary analysis, 498 patients with established PsA were randomized to 150 mg secukinumab, 300 mg secukinumab, or placebo. For enrollment, all patients were required to have substantial axial pain and a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score greater than 4 despite treatment with at least two NSAIDs.
For the first 4 weeks of the trial, patients received their assigned therapies weekly by subcutaneous administration. Treatment thereafter was every 4 weeks.
Almost all patients had active psoriasis and axial symptoms of at least several years duration. The median patient age was 46 years. Approximately one-third were HLA B27 positive.
At baseline, the median back pain score on a visual analog scale of 100 was 73, and the median BASDAI score was greater than 7.0. More than 90% of patients reported back pain worsening with rest.
The proportion of patients achieving ASAS20 at 12 weeks was 63.1% in the group assigned to 300 mg secukinumab, 66.3% in those assigned 150 mg, and 31.1% for those assigned placebo (P less than .0001 for either active therapy versus placebo).
Analyses conducted with multiple imputations and comparing those who were and were not taking methotrexate produced almost the same relative advantage for secukinumab. ASAS20 responses in patients using concomitant methotrexate were 65.1% with 300 mg secukinumab, 67.3% with 150 mg secukinumab, and 33.9% with placebo. Corresponding values in the no-methotrexate group were 60.5%, 64.4%, and 27.1%, respectively.
“There was a good response from either dose of secukinumab no matter what analysis was employed,” reported Dr. Baraliakos, citing an odds ratio of 3.81 for reaching the primary endpoint with secukinumab versus placebo. There were no significant differences in efficacy between the doses of secukinumab.
There was “not much to say about safety,” according to Dr. Baraliakos, as no significant differences in any adverse events were observed between study arms. However, he did caution that longer-term exposure is needed for a more complete analysis of tolerability and safety.
Most patients with PsA are thought to eventually develop axial involvement, which has a major adverse affect on quality of life, according to Dr. Baraliakos. He considers this primary 12-week analysis encouraging, but said the 1-year data will provide more information about whether this therapy should be considered routinely in PsA patients with persistent axial symptoms.
Axial imaging was conducted at study entry even though it was not a criterion for enrollment. Dr. Baraliakos reported that the impact of secukinumab on objective imaging measures of disease activity, if any, is forthcoming.
Imaging data might be needed to establish benefit objectively, judging from a criticism of the study design that arose during discussion after the data were presented. Specifically, it was pointed out that improvement in ASAS20 and BASDAI could occur as a result of improvement in peripheral symptoms, such as enthesitis. The lack of axial-specific outcomes was called a potential weakness of this study.
Dr. Baraliakos countered that BASDAI evaluations did include axial-specific questions, but also confirmed that spine-specific outcomes are included among outcomes to be presented with longer-term analyses.
“These data will come,” said Dr. Baraliakos, referring to imaging as well as other outcomes that will provide more information on the impact of secukinumab in treating the axial involvement of PsA.
Dr. Baraliakos reported multiple financial relationships with pharmaceutical companies, including Novartis, which sponsored this trial.
SOURCE: Ann Rheum Dis. Jun 2019;78(Suppl2):195-6. Abstract OPO235. doi: 10.1136/annrheumdis-2019-eular.2932.
MADRID – Secukinumab was superior to placebo for the primary endpoint of 20% improvement in Assessment of SpondyloArthritis international Society criteria (ASAS20), based on the initial 12-week data from the ongoing phase 3b MAXIMISE trial, the first randomized, controlled trial to evaluate a biologic therapy for the treatment of the axial manifestations of psoriatic arthritis (PsA).
“There was rapid and significant clinical improvement as measured with ASAS20 with both of the study doses of secukinumab,” an anti–interleukin-17 monoclonal antibody, reported Xenofon Baraliakos, MD, of Rheumazentrum Ruhrgebliet, Ruhr-University Bochum in Herne, Germany.
At the European Congress of Rheumatology, Dr. Baraliakos said that the 1-year data will be complete before the end of 2019.
In this primary analysis, 498 patients with established PsA were randomized to 150 mg secukinumab, 300 mg secukinumab, or placebo. For enrollment, all patients were required to have substantial axial pain and a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score greater than 4 despite treatment with at least two NSAIDs.
For the first 4 weeks of the trial, patients received their assigned therapies weekly by subcutaneous administration. Treatment thereafter was every 4 weeks.
Almost all patients had active psoriasis and axial symptoms of at least several years duration. The median patient age was 46 years. Approximately one-third were HLA B27 positive.
At baseline, the median back pain score on a visual analog scale of 100 was 73, and the median BASDAI score was greater than 7.0. More than 90% of patients reported back pain worsening with rest.
The proportion of patients achieving ASAS20 at 12 weeks was 63.1% in the group assigned to 300 mg secukinumab, 66.3% in those assigned 150 mg, and 31.1% for those assigned placebo (P less than .0001 for either active therapy versus placebo).
Analyses conducted with multiple imputations and comparing those who were and were not taking methotrexate produced almost the same relative advantage for secukinumab. ASAS20 responses in patients using concomitant methotrexate were 65.1% with 300 mg secukinumab, 67.3% with 150 mg secukinumab, and 33.9% with placebo. Corresponding values in the no-methotrexate group were 60.5%, 64.4%, and 27.1%, respectively.
“There was a good response from either dose of secukinumab no matter what analysis was employed,” reported Dr. Baraliakos, citing an odds ratio of 3.81 for reaching the primary endpoint with secukinumab versus placebo. There were no significant differences in efficacy between the doses of secukinumab.
There was “not much to say about safety,” according to Dr. Baraliakos, as no significant differences in any adverse events were observed between study arms. However, he did caution that longer-term exposure is needed for a more complete analysis of tolerability and safety.
Most patients with PsA are thought to eventually develop axial involvement, which has a major adverse affect on quality of life, according to Dr. Baraliakos. He considers this primary 12-week analysis encouraging, but said the 1-year data will provide more information about whether this therapy should be considered routinely in PsA patients with persistent axial symptoms.
Axial imaging was conducted at study entry even though it was not a criterion for enrollment. Dr. Baraliakos reported that the impact of secukinumab on objective imaging measures of disease activity, if any, is forthcoming.
Imaging data might be needed to establish benefit objectively, judging from a criticism of the study design that arose during discussion after the data were presented. Specifically, it was pointed out that improvement in ASAS20 and BASDAI could occur as a result of improvement in peripheral symptoms, such as enthesitis. The lack of axial-specific outcomes was called a potential weakness of this study.
Dr. Baraliakos countered that BASDAI evaluations did include axial-specific questions, but also confirmed that spine-specific outcomes are included among outcomes to be presented with longer-term analyses.
“These data will come,” said Dr. Baraliakos, referring to imaging as well as other outcomes that will provide more information on the impact of secukinumab in treating the axial involvement of PsA.
Dr. Baraliakos reported multiple financial relationships with pharmaceutical companies, including Novartis, which sponsored this trial.
SOURCE: Ann Rheum Dis. Jun 2019;78(Suppl2):195-6. Abstract OPO235. doi: 10.1136/annrheumdis-2019-eular.2932.
REPORTING FROM EULAR 2019 CONGRESS
CARMELINA confirms linagliptin’s renal, CV safety, but it’s still third-line for type 2 diabetes
SAN FRANCISCO – The dipeptidyl peptidase-4 inhibitor linagliptin (Tradjenta) is safe on the kidneys, the cardiovascular system, and in older people with type 2 diabetes, according to findings presented at the annual scientific sessions of the American Diabetes Association.
Investigators in the international Cardiovascular and Renal Microvascular Outcome Study with Linagliptin (CARMELINA) randomized 6,979 patients with type 2 diabetes who also had cardiovascular and/or kidney disease 1:1 to daily oral linagliptin 5 mg or placebo on top of standard of care, and they followed them for a median of 2.2 years. The mean age was 65.9 years, baseline hemoglobin A1c was 8.0%, and disease duration was about 15 years. Almost 63% of the patients were men, and about a quarter had a history of heart failure at baseline (JAMA. 2019;321[1]:69-79).
The study was unusual among other DPP-4 trials in that almost 60% of the patients were older than 65 years and 62.3% had impaired renal function with an estimated glomerular filtration rate (eGFR) of less than 60 ml/min per 1.73 m2.
There was no increased risk with linagliptin, compared with placebo, in the primary composite outcome of cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction (12.4% vs. 12.1%, respectively; hazard ratio, 1.02; P = .74), and there was no difference between the individual components even when broken down by age (younger than 65, 65-75, or older than 75 years) or by renal function (eGFR 60 or more, 45 to less than 60, 30 to less than 45, or less than 30 ml/min per 1.73 m2), according to investigator Mark Cooper, MBBS, PhD, of the department of diabetes at Monash University, Melbourne, who presented the findings.
There was no increase in the number of hospitalizations for heart failure with linagliptin, compared with placebo (6% vs. 6.5%, respectively; HR, 0.90; P = .26) – a concern with some DPP-4 inhibitors – and no increase in hypoglycemia (just over a quarter in both groups), even when broken down by age and renal function.
A decrease in albuminuria with linagliptin held across all renal subgroups. It is not known if that was because of glucose lowering or some other effect, but Dr. Cooper said he believed there was “a modest renal protective effect, [although] not at the level one would expect to translate into hard renal outcomes.”
Robert Eckel, MD, a professor of medicine at the University of Colorado at Denver, Aurora, who moderated the session, said the results were reassuring. “Ultimately, linagliptin seems safe,” even in older people with reduced eGFR. “It does not improve cardiovascular outcomes, but based on many DPP-4 trials, we didn’t expect it to,” he said.
“I don’t think DPP-4s are going to fall into any different place in the [treatment] algorithm” based on these results, he added. The class is currently third-line after metformin or insulin, followed by sodium-glucose cotransporter 2 inhibitors or glucagonlike peptide–1 receptor agonists for cardiovascular protection.
“When we look at [cardiovascular outcomes], ultimately, the SGLT2 inhibitors and the GLP-1 receptor agonists win,” he said. In addition, the blood glucose effects of linagliptin are “pretty modest, so if lowering hemoglobin A1c is the focus, this drug would be lower down on the list.”
Overall, linagliptin “falls into a lesser class, but a safe class for certain circumstances,” said Dr. Eckel, who gave the example of a woman in her late 70s with moderate to severe kidney function, an HbA1c level of 7.9%, and no cardiovascular disease. Her HbA1c might get down to 7.6% or so with linagliptin, he said, “but I’m not sure we have absolute proof of the benefit” of such a modest decline.
Boehringer Ingelheim, the maker of linagliptin, funded the study. The presenter disclosed honoraria, speaking fees, and grants from the company. A number of the investigators were employees of the company.
SAN FRANCISCO – The dipeptidyl peptidase-4 inhibitor linagliptin (Tradjenta) is safe on the kidneys, the cardiovascular system, and in older people with type 2 diabetes, according to findings presented at the annual scientific sessions of the American Diabetes Association.
Investigators in the international Cardiovascular and Renal Microvascular Outcome Study with Linagliptin (CARMELINA) randomized 6,979 patients with type 2 diabetes who also had cardiovascular and/or kidney disease 1:1 to daily oral linagliptin 5 mg or placebo on top of standard of care, and they followed them for a median of 2.2 years. The mean age was 65.9 years, baseline hemoglobin A1c was 8.0%, and disease duration was about 15 years. Almost 63% of the patients were men, and about a quarter had a history of heart failure at baseline (JAMA. 2019;321[1]:69-79).
The study was unusual among other DPP-4 trials in that almost 60% of the patients were older than 65 years and 62.3% had impaired renal function with an estimated glomerular filtration rate (eGFR) of less than 60 ml/min per 1.73 m2.
There was no increased risk with linagliptin, compared with placebo, in the primary composite outcome of cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction (12.4% vs. 12.1%, respectively; hazard ratio, 1.02; P = .74), and there was no difference between the individual components even when broken down by age (younger than 65, 65-75, or older than 75 years) or by renal function (eGFR 60 or more, 45 to less than 60, 30 to less than 45, or less than 30 ml/min per 1.73 m2), according to investigator Mark Cooper, MBBS, PhD, of the department of diabetes at Monash University, Melbourne, who presented the findings.
There was no increase in the number of hospitalizations for heart failure with linagliptin, compared with placebo (6% vs. 6.5%, respectively; HR, 0.90; P = .26) – a concern with some DPP-4 inhibitors – and no increase in hypoglycemia (just over a quarter in both groups), even when broken down by age and renal function.
A decrease in albuminuria with linagliptin held across all renal subgroups. It is not known if that was because of glucose lowering or some other effect, but Dr. Cooper said he believed there was “a modest renal protective effect, [although] not at the level one would expect to translate into hard renal outcomes.”
Robert Eckel, MD, a professor of medicine at the University of Colorado at Denver, Aurora, who moderated the session, said the results were reassuring. “Ultimately, linagliptin seems safe,” even in older people with reduced eGFR. “It does not improve cardiovascular outcomes, but based on many DPP-4 trials, we didn’t expect it to,” he said.
“I don’t think DPP-4s are going to fall into any different place in the [treatment] algorithm” based on these results, he added. The class is currently third-line after metformin or insulin, followed by sodium-glucose cotransporter 2 inhibitors or glucagonlike peptide–1 receptor agonists for cardiovascular protection.
“When we look at [cardiovascular outcomes], ultimately, the SGLT2 inhibitors and the GLP-1 receptor agonists win,” he said. In addition, the blood glucose effects of linagliptin are “pretty modest, so if lowering hemoglobin A1c is the focus, this drug would be lower down on the list.”
Overall, linagliptin “falls into a lesser class, but a safe class for certain circumstances,” said Dr. Eckel, who gave the example of a woman in her late 70s with moderate to severe kidney function, an HbA1c level of 7.9%, and no cardiovascular disease. Her HbA1c might get down to 7.6% or so with linagliptin, he said, “but I’m not sure we have absolute proof of the benefit” of such a modest decline.
Boehringer Ingelheim, the maker of linagliptin, funded the study. The presenter disclosed honoraria, speaking fees, and grants from the company. A number of the investigators were employees of the company.
SAN FRANCISCO – The dipeptidyl peptidase-4 inhibitor linagliptin (Tradjenta) is safe on the kidneys, the cardiovascular system, and in older people with type 2 diabetes, according to findings presented at the annual scientific sessions of the American Diabetes Association.
Investigators in the international Cardiovascular and Renal Microvascular Outcome Study with Linagliptin (CARMELINA) randomized 6,979 patients with type 2 diabetes who also had cardiovascular and/or kidney disease 1:1 to daily oral linagliptin 5 mg or placebo on top of standard of care, and they followed them for a median of 2.2 years. The mean age was 65.9 years, baseline hemoglobin A1c was 8.0%, and disease duration was about 15 years. Almost 63% of the patients were men, and about a quarter had a history of heart failure at baseline (JAMA. 2019;321[1]:69-79).
The study was unusual among other DPP-4 trials in that almost 60% of the patients were older than 65 years and 62.3% had impaired renal function with an estimated glomerular filtration rate (eGFR) of less than 60 ml/min per 1.73 m2.
There was no increased risk with linagliptin, compared with placebo, in the primary composite outcome of cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction (12.4% vs. 12.1%, respectively; hazard ratio, 1.02; P = .74), and there was no difference between the individual components even when broken down by age (younger than 65, 65-75, or older than 75 years) or by renal function (eGFR 60 or more, 45 to less than 60, 30 to less than 45, or less than 30 ml/min per 1.73 m2), according to investigator Mark Cooper, MBBS, PhD, of the department of diabetes at Monash University, Melbourne, who presented the findings.
There was no increase in the number of hospitalizations for heart failure with linagliptin, compared with placebo (6% vs. 6.5%, respectively; HR, 0.90; P = .26) – a concern with some DPP-4 inhibitors – and no increase in hypoglycemia (just over a quarter in both groups), even when broken down by age and renal function.
A decrease in albuminuria with linagliptin held across all renal subgroups. It is not known if that was because of glucose lowering or some other effect, but Dr. Cooper said he believed there was “a modest renal protective effect, [although] not at the level one would expect to translate into hard renal outcomes.”
Robert Eckel, MD, a professor of medicine at the University of Colorado at Denver, Aurora, who moderated the session, said the results were reassuring. “Ultimately, linagliptin seems safe,” even in older people with reduced eGFR. “It does not improve cardiovascular outcomes, but based on many DPP-4 trials, we didn’t expect it to,” he said.
“I don’t think DPP-4s are going to fall into any different place in the [treatment] algorithm” based on these results, he added. The class is currently third-line after metformin or insulin, followed by sodium-glucose cotransporter 2 inhibitors or glucagonlike peptide–1 receptor agonists for cardiovascular protection.
“When we look at [cardiovascular outcomes], ultimately, the SGLT2 inhibitors and the GLP-1 receptor agonists win,” he said. In addition, the blood glucose effects of linagliptin are “pretty modest, so if lowering hemoglobin A1c is the focus, this drug would be lower down on the list.”
Overall, linagliptin “falls into a lesser class, but a safe class for certain circumstances,” said Dr. Eckel, who gave the example of a woman in her late 70s with moderate to severe kidney function, an HbA1c level of 7.9%, and no cardiovascular disease. Her HbA1c might get down to 7.6% or so with linagliptin, he said, “but I’m not sure we have absolute proof of the benefit” of such a modest decline.
Boehringer Ingelheim, the maker of linagliptin, funded the study. The presenter disclosed honoraria, speaking fees, and grants from the company. A number of the investigators were employees of the company.
REPORTING FROM ADA 2019