User login
Expert advice for immediate postpartum LARC insertion
Evidence-based education about long-acting reversible contraception (LARC) for women in the postpartum period can result in the increased continuation of and satisfaction with LARC.1 However, nearly 40% of women do not attend a postpartum visit.2 And up to 57% of women report having unprotected intercourse before the 6-week postpartum visit, which increases the risk of unplanned pregnancy.3 The American College of Obstetricians and Gynecologists (ACOG) supports immediate postpartum LARC insertion as best practice,3 and clinicians providing care for women during the peripartum period can counsel women regarding informed contraceptive decisions and provide guidance regarding both short-acting contraception and LARC.1
Immediate postpartum LARC, using intrauterine devices (IUDs) in particular, has been used around the world for a long time, says Lisa Hofler, MD, MPH, MBA, Chief in the Division of Family Planning at the University of New Mexico School of Medicine in Albuquerque. “Much of our initial data came from other countries, but eventually people in the United States said, ‘This is a great option, why aren't we doing this?’" In addition, although women considering immediate postpartum LARC should be counseled about the theoretical risk of reduced duration of breastfeeding, the evidence overwhelmingly has not shown a negative effect on actual breastfeeding outcomes according to ACOG.3 OBG MANAGEMENT recently met up with Dr. Hofler to ask her which patients are ideal for postpartum LARC, how to troubleshoot common pitfalls, and how to implement the practice within one’s own institution.
OBG Management: Who do you consider to be the ideal patient for immediate postpartum LARC?
Lisa Hofler, MD: The great thing about immediate postpartum LARC (including IUDs and implants) is that any woman is an ideal candidate. We are simply talking about the timing of when a woman chooses to get an IUD or an implant after the birth of her child. There is no one perfect woman; it is the person who chooses the method and wants to use that method immediately after birth. When a woman chooses a LARC, she can be assured that after the birth of her child she will be protected against pregnancy. If she chooses an IUD as her LARC method, she will be comfortable at insertion because the cervix is already dilated when it is inserted.
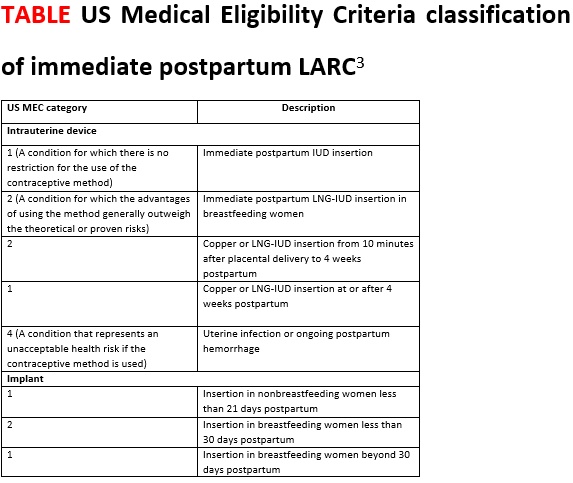
For the implant, the contraindications are the same as in the outpatient setting. The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use covers many medical conditions and whether or not a person might be a candidate for different birth control methods.4 Those same considerations apply for the implant postpartum (TABLE).3
For the IUD, similarly, anyone who would not be a candidate for the IUD in the outpatient setting is not a candidate for immediate postpartum IUD. For instance, if the person has an intrauterine infection, you should not place an IUD. Also, if a patient is hemorrhaging and you are managing the hemorrhage (say she has retained placenta or membranes or she has uterine atony), you are not going to put an IUD in, as you need to attend to her bleeding.
OBG Management: What is your approach to counseling a patient for immediate postpartum LARC?
Dr. Hofler: The ideal time to counsel about postbirth contraception is in the prenatal period, when the patient is making decisions about what method she wants to use after the birth. Once she chooses her preferred method, address timing if appropriate. It is less ideal to talk to a woman about the option of immediate postpartum LARC when she comes to labor and delivery, especially if that is the first time she has heard about it. Certainly, the time to talk about postpartum LARC options is not immediately after the baby is born. Approaching your patient with, "What do you want for birth control? Do you want this IUD? I can put it in right now," can feel coercive. This approach does not put the woman in a position in which she has enough decision-making time or time to ask questions.
OBG Management: What problems do clinicians run into when placing an immediate postpartum IUD, and can you offer solutions?
Dr. Hofler: When placing an immediate postpartum IUD, people might run into a few problems. The first relates to preplacement counseling. Perhaps when making the plan for the postpartum IUD the clinician did not counsel the woman that there are certain conditions that could preclude IUD placement—such as intrauterine infection or postpartum hemorrhage. When dealing with those types of issues, a patient is not eligible for an IUD, and she should be mentally prepared for this type of situation. Let her know during the counseling before the birth that immediately postpartum is a great time and opportunity for effective contraception placement. Tell her that hopefully IUD placement will be possible but that occasionally it is not, and make a back-up plan in case the IUD cannot be placed immediately postpartum.
The second unique area for counseling with immediate postpartum IUDs is a slightly increased risk of expulsion of an IUD placed immediately postpartum compared with in the office. The risk of expulsion varies by type of delivery. For instance, cesarean delivery births have a lower expulsion rate than vaginal births. The expulsion rate seems to vary by type of IUD as well. Copper IUDs seem to have a slightly lower expulsion rate than hormonal IUDs. (See “Levonorgestrel vs copper IUD expulsion rates after immediate postpartum insertion.”) This consideration should be talked about ahead of time, too. Provider training in IUD placement does impact the likelihood of expulsion, and if you place the IUD at the fundus, it is less likely to expel. (See “Inserting the immediate postpartum IUD after vaginal and cesarean birth step by step.”)
A third issue that clinicians run into is actually the systems of care—making sure that the IUD or implant is available when you need it, making sure that documentation happens the way it should, and ensuring that the follow-up billing and revenue cycle happens so that the woman gets the device that she wants and the providers get paid for having provided it. These issues require a multidisciplinary team to work through in order to ensure that postpartum LARC placement is a sustainable process in the long run.
Often, when people think of immediate postpartum LARC they think of postplacental IUDs. However, an implant also is an option, and that too is immediate postpartum LARC. Placing an implant is often a lot easier to do after the birth than placing an IUD. As clinicians work toward bringing an immediate postpartum LARC program to their hospital system, starting with implants is a smart thing to do because clinicians do not have to learn or teach new clinical skills. Because of that, immediate postpartum implants are a good troubleshooting mechanism for opening up the conversation about immediate postpartum LARC at your institution.
OBG MANAGEMENT: What advice do you have for administrators or physicians looking to implement an immediate postpartum LARC program into a hospital setting?
Dr. Hofler: Probably the best single resource is the American College of Obstetricians and Gynecologists’ Postpartum Contraception Access Initiative (PCAI). They have a dedicated website and offer a lot of support and resources that include site-specific training at the hospital or the institution; clinician training on implants and IUDs; and administrator training on some of the systems of care, the billing process, the stocking process, and pharmacy education. They also provide information on all the things that should be included beyond the clinical aspects. I strongly recommend looking at what they offer.
Also, because many hospitals say, "We love this idea. We would support immediate postpartum LARC, we just want to make sure we get paid," the ACOG LARC Program website includes state-specific guidance for how Medicaid pays for LARC devices. There is state-specific guidance about how the device payment can be separated from the global payment for delivery—specific things for each institution to do to get reimbursed.
A 2017 prospective cohort study was the first to directly compare expulsion rates of the levonorgestrel (LNG) intrauterine device (IUD) and the copper IUD placed postplacentally (within 10 minutes of placental delivery). The study investigators found that, among 96 women at 12 weeks, 38% of the LNG-IUD users and 20% of the copper IUD users experienced IUD expulsion (odds ratio, 2.55; 95% confidence interval [CI], 0.99-6.55; P = .05). Women were aged 18 to 40 and had a singleton vaginal delivery at ≥ 35 weeks’ gestation.1 The two study groups were similar except that more copper IUD users were Hispanic (66% vs 38%) and fewer were primiparous (16% vs 31%). The study authors found the only independent predictor of device expulsion to be IUD type.
In a 2019 prospective cohort study, Hinz and colleagues compared the 6-month expulsion rate of IUDs inserted in the immediate postpartum period (within 10 to 15 minutes of placental delivery) after vaginal or cesarean delivery.2 Women were aged 18 to 45 years and selected a LNG 52-mg IUD (75 women) or copper IUD (58 women) for postpartum contraception. They completed a survey from weeks 0 to 5 and on weeks 12 and 24 postpartum regarding IUD expulsion, IUD removal, vaginal bleeding, and breastfeeding. A total of 58 women had a vaginal delivery, and 56 had a cesarean delivery.
At 6 months, the expulsion rates were similar in the two groups: 26.7% of the LNG IUDs expelled, compared with 20.5% of the copper IUDs (P = .38). The study groups were similar, point out the study investigators, except that the copper IUD users had a higher median parity (3 vs. 2; P = .03). In addition, the copper IUDs were inserted by more senior than junior residents (46.2% vs 22.7%, P = .02).
A 2018 systematic review pooled absolute rates of IUD expulsion and estimated adjusted relative risk (RR) for IUD type. A total of 48 studies (rated level I to II-3 of poor to good quality) were included in the analysis, and results indicated that the LNG-IUD was associated with a higher risk of expulsion at less than 4 weeks postpartum than the copper IUD (adjusted RR, 1.91; 95% CI, 1.50-2.43).3
References
1. Goldthwaite LM, Sheeder J, Hyer J, et al. Postplacental intrauterine device expulsion by 12 weeks: a prospective cohort study. Am J Obstet Gynecol. 2017;217:674.e1-674.e8.
2. Hinz EK, Murthy A, Wang B, Ryan N, Ades V. A prospective cohort study comparing expulsion after postplacental insertion: the levonorgestrel versus the copper intrauterine device. Contraception. May 17, 2019. doi: 10.1016/j.contraception.2019.04.011.
3. Jatlaoui TC, Whiteman MK, Jeng G, et al. Intrauterine device expulsion after postpartum placement. Obstet Gynecol. 2018:895-905.
Technique for placing an IUD immediately after vaginal birth
1. Bring supplies for intrauterine device (IUD) insertion: the IUD, posterior blade of a speculum or retractor for posterior vagina, ring forceps, curved Kelly placenta forceps, and scissors.
2. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta. Any perineal lacerations should be repaired after IUD placement.
3. Break down the bed to facilitate placement. If the perineum or vagina is soiled with stool or meconium then consider povodine-iodine prep.
4. Place the posterior blade of the speculum into the vagina and grasp the anterior cervix with the ring forceps.
5. Set up the IUD for insertion: Change into new sterile gloves. Remove the IUD from the inserter. For levonorgestrel IUDs, cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm; copper IUDs do not need strings trimmed. Hold one arm of the IUD with the long Kelly placenta forceps so that the stem of the IUD is approximately parallel to the shaft of the forceps.
6. Insert the IUD: Guide the IUD into the lower uterine segment with the left hand on the cervix ring forceps and the right hand on the IUD forceps. After passing the IUD through the cervix, move the left hand to the abdomen and press the fundus posterior and caudad to straighten the endometrial canal and to feel the IUD at the fundus. With the right hand, guide the IUD to the fundus; this often entails dropping the hand significantly and guiding the IUD much more anteriorly than first expected.
7. Release the IUD with forceps wide open, sweeping the forceps to one side to avoid pulling the IUD out with the forceps. 8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
Troubleshooting tips:
- If you are unable to visualize the anterior cervix, try to place the ring forceps by palpation.
- If you are unable to grasp the cervix with ring forceps by palpation, you may try to place the IUD manually. Hold the IUD between the first and second fingers of the right hand and place the IUD at the fundus. Release the IUD with the fingers wide open and remove the hand without removing the IUD.
Technique for placing an IUD immediately after cesarean birth
1. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta.
2. For levonorgestrel IUDs: Remove the IUD from the inserter. Cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm. Place the IUD at the fundus with a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
3. For copper IUDs: String trimming is not necessary. Place the IUD at the fundus with the IUD inserter or a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
4. Repair the hysterotomy as usual.
1. Dole DM, Martin J. What nurses need to know about immediate postpartum initiation of long-acting reversible contraception. Nurs Womens Health. 2017;21:186-195.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice Bulletin no. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
4. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-104.
Evidence-based education about long-acting reversible contraception (LARC) for women in the postpartum period can result in the increased continuation of and satisfaction with LARC.1 However, nearly 40% of women do not attend a postpartum visit.2 And up to 57% of women report having unprotected intercourse before the 6-week postpartum visit, which increases the risk of unplanned pregnancy.3 The American College of Obstetricians and Gynecologists (ACOG) supports immediate postpartum LARC insertion as best practice,3 and clinicians providing care for women during the peripartum period can counsel women regarding informed contraceptive decisions and provide guidance regarding both short-acting contraception and LARC.1
Immediate postpartum LARC, using intrauterine devices (IUDs) in particular, has been used around the world for a long time, says Lisa Hofler, MD, MPH, MBA, Chief in the Division of Family Planning at the University of New Mexico School of Medicine in Albuquerque. “Much of our initial data came from other countries, but eventually people in the United States said, ‘This is a great option, why aren't we doing this?’" In addition, although women considering immediate postpartum LARC should be counseled about the theoretical risk of reduced duration of breastfeeding, the evidence overwhelmingly has not shown a negative effect on actual breastfeeding outcomes according to ACOG.3 OBG MANAGEMENT recently met up with Dr. Hofler to ask her which patients are ideal for postpartum LARC, how to troubleshoot common pitfalls, and how to implement the practice within one’s own institution.
OBG Management: Who do you consider to be the ideal patient for immediate postpartum LARC?
Lisa Hofler, MD: The great thing about immediate postpartum LARC (including IUDs and implants) is that any woman is an ideal candidate. We are simply talking about the timing of when a woman chooses to get an IUD or an implant after the birth of her child. There is no one perfect woman; it is the person who chooses the method and wants to use that method immediately after birth. When a woman chooses a LARC, she can be assured that after the birth of her child she will be protected against pregnancy. If she chooses an IUD as her LARC method, she will be comfortable at insertion because the cervix is already dilated when it is inserted.
For the implant, the contraindications are the same as in the outpatient setting. The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use covers many medical conditions and whether or not a person might be a candidate for different birth control methods.4 Those same considerations apply for the implant postpartum (TABLE).3
For the IUD, similarly, anyone who would not be a candidate for the IUD in the outpatient setting is not a candidate for immediate postpartum IUD. For instance, if the person has an intrauterine infection, you should not place an IUD. Also, if a patient is hemorrhaging and you are managing the hemorrhage (say she has retained placenta or membranes or she has uterine atony), you are not going to put an IUD in, as you need to attend to her bleeding.

OBG Management: What is your approach to counseling a patient for immediate postpartum LARC?
Dr. Hofler: The ideal time to counsel about postbirth contraception is in the prenatal period, when the patient is making decisions about what method she wants to use after the birth. Once she chooses her preferred method, address timing if appropriate. It is less ideal to talk to a woman about the option of immediate postpartum LARC when she comes to labor and delivery, especially if that is the first time she has heard about it. Certainly, the time to talk about postpartum LARC options is not immediately after the baby is born. Approaching your patient with, "What do you want for birth control? Do you want this IUD? I can put it in right now," can feel coercive. This approach does not put the woman in a position in which she has enough decision-making time or time to ask questions.
OBG Management: What problems do clinicians run into when placing an immediate postpartum IUD, and can you offer solutions?
Dr. Hofler: When placing an immediate postpartum IUD, people might run into a few problems. The first relates to preplacement counseling. Perhaps when making the plan for the postpartum IUD the clinician did not counsel the woman that there are certain conditions that could preclude IUD placement—such as intrauterine infection or postpartum hemorrhage. When dealing with those types of issues, a patient is not eligible for an IUD, and she should be mentally prepared for this type of situation. Let her know during the counseling before the birth that immediately postpartum is a great time and opportunity for effective contraception placement. Tell her that hopefully IUD placement will be possible but that occasionally it is not, and make a back-up plan in case the IUD cannot be placed immediately postpartum.
The second unique area for counseling with immediate postpartum IUDs is a slightly increased risk of expulsion of an IUD placed immediately postpartum compared with in the office. The risk of expulsion varies by type of delivery. For instance, cesarean delivery births have a lower expulsion rate than vaginal births. The expulsion rate seems to vary by type of IUD as well. Copper IUDs seem to have a slightly lower expulsion rate than hormonal IUDs. (See “Levonorgestrel vs copper IUD expulsion rates after immediate postpartum insertion.”) This consideration should be talked about ahead of time, too. Provider training in IUD placement does impact the likelihood of expulsion, and if you place the IUD at the fundus, it is less likely to expel. (See “Inserting the immediate postpartum IUD after vaginal and cesarean birth step by step.”)
A third issue that clinicians run into is actually the systems of care—making sure that the IUD or implant is available when you need it, making sure that documentation happens the way it should, and ensuring that the follow-up billing and revenue cycle happens so that the woman gets the device that she wants and the providers get paid for having provided it. These issues require a multidisciplinary team to work through in order to ensure that postpartum LARC placement is a sustainable process in the long run.
Often, when people think of immediate postpartum LARC they think of postplacental IUDs. However, an implant also is an option, and that too is immediate postpartum LARC. Placing an implant is often a lot easier to do after the birth than placing an IUD. As clinicians work toward bringing an immediate postpartum LARC program to their hospital system, starting with implants is a smart thing to do because clinicians do not have to learn or teach new clinical skills. Because of that, immediate postpartum implants are a good troubleshooting mechanism for opening up the conversation about immediate postpartum LARC at your institution.
OBG MANAGEMENT: What advice do you have for administrators or physicians looking to implement an immediate postpartum LARC program into a hospital setting?
Dr. Hofler: Probably the best single resource is the American College of Obstetricians and Gynecologists’ Postpartum Contraception Access Initiative (PCAI). They have a dedicated website and offer a lot of support and resources that include site-specific training at the hospital or the institution; clinician training on implants and IUDs; and administrator training on some of the systems of care, the billing process, the stocking process, and pharmacy education. They also provide information on all the things that should be included beyond the clinical aspects. I strongly recommend looking at what they offer.
Also, because many hospitals say, "We love this idea. We would support immediate postpartum LARC, we just want to make sure we get paid," the ACOG LARC Program website includes state-specific guidance for how Medicaid pays for LARC devices. There is state-specific guidance about how the device payment can be separated from the global payment for delivery—specific things for each institution to do to get reimbursed.
A 2017 prospective cohort study was the first to directly compare expulsion rates of the levonorgestrel (LNG) intrauterine device (IUD) and the copper IUD placed postplacentally (within 10 minutes of placental delivery). The study investigators found that, among 96 women at 12 weeks, 38% of the LNG-IUD users and 20% of the copper IUD users experienced IUD expulsion (odds ratio, 2.55; 95% confidence interval [CI], 0.99-6.55; P = .05). Women were aged 18 to 40 and had a singleton vaginal delivery at ≥ 35 weeks’ gestation.1 The two study groups were similar except that more copper IUD users were Hispanic (66% vs 38%) and fewer were primiparous (16% vs 31%). The study authors found the only independent predictor of device expulsion to be IUD type.
In a 2019 prospective cohort study, Hinz and colleagues compared the 6-month expulsion rate of IUDs inserted in the immediate postpartum period (within 10 to 15 minutes of placental delivery) after vaginal or cesarean delivery.2 Women were aged 18 to 45 years and selected a LNG 52-mg IUD (75 women) or copper IUD (58 women) for postpartum contraception. They completed a survey from weeks 0 to 5 and on weeks 12 and 24 postpartum regarding IUD expulsion, IUD removal, vaginal bleeding, and breastfeeding. A total of 58 women had a vaginal delivery, and 56 had a cesarean delivery.
At 6 months, the expulsion rates were similar in the two groups: 26.7% of the LNG IUDs expelled, compared with 20.5% of the copper IUDs (P = .38). The study groups were similar, point out the study investigators, except that the copper IUD users had a higher median parity (3 vs. 2; P = .03). In addition, the copper IUDs were inserted by more senior than junior residents (46.2% vs 22.7%, P = .02).
A 2018 systematic review pooled absolute rates of IUD expulsion and estimated adjusted relative risk (RR) for IUD type. A total of 48 studies (rated level I to II-3 of poor to good quality) were included in the analysis, and results indicated that the LNG-IUD was associated with a higher risk of expulsion at less than 4 weeks postpartum than the copper IUD (adjusted RR, 1.91; 95% CI, 1.50-2.43).3
References
1. Goldthwaite LM, Sheeder J, Hyer J, et al. Postplacental intrauterine device expulsion by 12 weeks: a prospective cohort study. Am J Obstet Gynecol. 2017;217:674.e1-674.e8.
2. Hinz EK, Murthy A, Wang B, Ryan N, Ades V. A prospective cohort study comparing expulsion after postplacental insertion: the levonorgestrel versus the copper intrauterine device. Contraception. May 17, 2019. doi: 10.1016/j.contraception.2019.04.011.
3. Jatlaoui TC, Whiteman MK, Jeng G, et al. Intrauterine device expulsion after postpartum placement. Obstet Gynecol. 2018:895-905.
Technique for placing an IUD immediately after vaginal birth
1. Bring supplies for intrauterine device (IUD) insertion: the IUD, posterior blade of a speculum or retractor for posterior vagina, ring forceps, curved Kelly placenta forceps, and scissors.
2. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta. Any perineal lacerations should be repaired after IUD placement.
3. Break down the bed to facilitate placement. If the perineum or vagina is soiled with stool or meconium then consider povodine-iodine prep.
4. Place the posterior blade of the speculum into the vagina and grasp the anterior cervix with the ring forceps.
5. Set up the IUD for insertion: Change into new sterile gloves. Remove the IUD from the inserter. For levonorgestrel IUDs, cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm; copper IUDs do not need strings trimmed. Hold one arm of the IUD with the long Kelly placenta forceps so that the stem of the IUD is approximately parallel to the shaft of the forceps.
6. Insert the IUD: Guide the IUD into the lower uterine segment with the left hand on the cervix ring forceps and the right hand on the IUD forceps. After passing the IUD through the cervix, move the left hand to the abdomen and press the fundus posterior and caudad to straighten the endometrial canal and to feel the IUD at the fundus. With the right hand, guide the IUD to the fundus; this often entails dropping the hand significantly and guiding the IUD much more anteriorly than first expected.
7. Release the IUD with forceps wide open, sweeping the forceps to one side to avoid pulling the IUD out with the forceps. 8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
Troubleshooting tips:
- If you are unable to visualize the anterior cervix, try to place the ring forceps by palpation.
- If you are unable to grasp the cervix with ring forceps by palpation, you may try to place the IUD manually. Hold the IUD between the first and second fingers of the right hand and place the IUD at the fundus. Release the IUD with the fingers wide open and remove the hand without removing the IUD.
Technique for placing an IUD immediately after cesarean birth
1. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta.
2. For levonorgestrel IUDs: Remove the IUD from the inserter. Cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm. Place the IUD at the fundus with a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
3. For copper IUDs: String trimming is not necessary. Place the IUD at the fundus with the IUD inserter or a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
4. Repair the hysterotomy as usual.
Evidence-based education about long-acting reversible contraception (LARC) for women in the postpartum period can result in the increased continuation of and satisfaction with LARC.1 However, nearly 40% of women do not attend a postpartum visit.2 And up to 57% of women report having unprotected intercourse before the 6-week postpartum visit, which increases the risk of unplanned pregnancy.3 The American College of Obstetricians and Gynecologists (ACOG) supports immediate postpartum LARC insertion as best practice,3 and clinicians providing care for women during the peripartum period can counsel women regarding informed contraceptive decisions and provide guidance regarding both short-acting contraception and LARC.1
Immediate postpartum LARC, using intrauterine devices (IUDs) in particular, has been used around the world for a long time, says Lisa Hofler, MD, MPH, MBA, Chief in the Division of Family Planning at the University of New Mexico School of Medicine in Albuquerque. “Much of our initial data came from other countries, but eventually people in the United States said, ‘This is a great option, why aren't we doing this?’" In addition, although women considering immediate postpartum LARC should be counseled about the theoretical risk of reduced duration of breastfeeding, the evidence overwhelmingly has not shown a negative effect on actual breastfeeding outcomes according to ACOG.3 OBG MANAGEMENT recently met up with Dr. Hofler to ask her which patients are ideal for postpartum LARC, how to troubleshoot common pitfalls, and how to implement the practice within one’s own institution.
OBG Management: Who do you consider to be the ideal patient for immediate postpartum LARC?
Lisa Hofler, MD: The great thing about immediate postpartum LARC (including IUDs and implants) is that any woman is an ideal candidate. We are simply talking about the timing of when a woman chooses to get an IUD or an implant after the birth of her child. There is no one perfect woman; it is the person who chooses the method and wants to use that method immediately after birth. When a woman chooses a LARC, she can be assured that after the birth of her child she will be protected against pregnancy. If she chooses an IUD as her LARC method, she will be comfortable at insertion because the cervix is already dilated when it is inserted.
For the implant, the contraindications are the same as in the outpatient setting. The Centers for Disease Control and Prevention’s Medical Eligibility Criteria for Contraceptive Use covers many medical conditions and whether or not a person might be a candidate for different birth control methods.4 Those same considerations apply for the implant postpartum (TABLE).3
For the IUD, similarly, anyone who would not be a candidate for the IUD in the outpatient setting is not a candidate for immediate postpartum IUD. For instance, if the person has an intrauterine infection, you should not place an IUD. Also, if a patient is hemorrhaging and you are managing the hemorrhage (say she has retained placenta or membranes or she has uterine atony), you are not going to put an IUD in, as you need to attend to her bleeding.

OBG Management: What is your approach to counseling a patient for immediate postpartum LARC?
Dr. Hofler: The ideal time to counsel about postbirth contraception is in the prenatal period, when the patient is making decisions about what method she wants to use after the birth. Once she chooses her preferred method, address timing if appropriate. It is less ideal to talk to a woman about the option of immediate postpartum LARC when she comes to labor and delivery, especially if that is the first time she has heard about it. Certainly, the time to talk about postpartum LARC options is not immediately after the baby is born. Approaching your patient with, "What do you want for birth control? Do you want this IUD? I can put it in right now," can feel coercive. This approach does not put the woman in a position in which she has enough decision-making time or time to ask questions.
OBG Management: What problems do clinicians run into when placing an immediate postpartum IUD, and can you offer solutions?
Dr. Hofler: When placing an immediate postpartum IUD, people might run into a few problems. The first relates to preplacement counseling. Perhaps when making the plan for the postpartum IUD the clinician did not counsel the woman that there are certain conditions that could preclude IUD placement—such as intrauterine infection or postpartum hemorrhage. When dealing with those types of issues, a patient is not eligible for an IUD, and she should be mentally prepared for this type of situation. Let her know during the counseling before the birth that immediately postpartum is a great time and opportunity for effective contraception placement. Tell her that hopefully IUD placement will be possible but that occasionally it is not, and make a back-up plan in case the IUD cannot be placed immediately postpartum.
The second unique area for counseling with immediate postpartum IUDs is a slightly increased risk of expulsion of an IUD placed immediately postpartum compared with in the office. The risk of expulsion varies by type of delivery. For instance, cesarean delivery births have a lower expulsion rate than vaginal births. The expulsion rate seems to vary by type of IUD as well. Copper IUDs seem to have a slightly lower expulsion rate than hormonal IUDs. (See “Levonorgestrel vs copper IUD expulsion rates after immediate postpartum insertion.”) This consideration should be talked about ahead of time, too. Provider training in IUD placement does impact the likelihood of expulsion, and if you place the IUD at the fundus, it is less likely to expel. (See “Inserting the immediate postpartum IUD after vaginal and cesarean birth step by step.”)
A third issue that clinicians run into is actually the systems of care—making sure that the IUD or implant is available when you need it, making sure that documentation happens the way it should, and ensuring that the follow-up billing and revenue cycle happens so that the woman gets the device that she wants and the providers get paid for having provided it. These issues require a multidisciplinary team to work through in order to ensure that postpartum LARC placement is a sustainable process in the long run.
Often, when people think of immediate postpartum LARC they think of postplacental IUDs. However, an implant also is an option, and that too is immediate postpartum LARC. Placing an implant is often a lot easier to do after the birth than placing an IUD. As clinicians work toward bringing an immediate postpartum LARC program to their hospital system, starting with implants is a smart thing to do because clinicians do not have to learn or teach new clinical skills. Because of that, immediate postpartum implants are a good troubleshooting mechanism for opening up the conversation about immediate postpartum LARC at your institution.
OBG MANAGEMENT: What advice do you have for administrators or physicians looking to implement an immediate postpartum LARC program into a hospital setting?
Dr. Hofler: Probably the best single resource is the American College of Obstetricians and Gynecologists’ Postpartum Contraception Access Initiative (PCAI). They have a dedicated website and offer a lot of support and resources that include site-specific training at the hospital or the institution; clinician training on implants and IUDs; and administrator training on some of the systems of care, the billing process, the stocking process, and pharmacy education. They also provide information on all the things that should be included beyond the clinical aspects. I strongly recommend looking at what they offer.
Also, because many hospitals say, "We love this idea. We would support immediate postpartum LARC, we just want to make sure we get paid," the ACOG LARC Program website includes state-specific guidance for how Medicaid pays for LARC devices. There is state-specific guidance about how the device payment can be separated from the global payment for delivery—specific things for each institution to do to get reimbursed.
A 2017 prospective cohort study was the first to directly compare expulsion rates of the levonorgestrel (LNG) intrauterine device (IUD) and the copper IUD placed postplacentally (within 10 minutes of placental delivery). The study investigators found that, among 96 women at 12 weeks, 38% of the LNG-IUD users and 20% of the copper IUD users experienced IUD expulsion (odds ratio, 2.55; 95% confidence interval [CI], 0.99-6.55; P = .05). Women were aged 18 to 40 and had a singleton vaginal delivery at ≥ 35 weeks’ gestation.1 The two study groups were similar except that more copper IUD users were Hispanic (66% vs 38%) and fewer were primiparous (16% vs 31%). The study authors found the only independent predictor of device expulsion to be IUD type.
In a 2019 prospective cohort study, Hinz and colleagues compared the 6-month expulsion rate of IUDs inserted in the immediate postpartum period (within 10 to 15 minutes of placental delivery) after vaginal or cesarean delivery.2 Women were aged 18 to 45 years and selected a LNG 52-mg IUD (75 women) or copper IUD (58 women) for postpartum contraception. They completed a survey from weeks 0 to 5 and on weeks 12 and 24 postpartum regarding IUD expulsion, IUD removal, vaginal bleeding, and breastfeeding. A total of 58 women had a vaginal delivery, and 56 had a cesarean delivery.
At 6 months, the expulsion rates were similar in the two groups: 26.7% of the LNG IUDs expelled, compared with 20.5% of the copper IUDs (P = .38). The study groups were similar, point out the study investigators, except that the copper IUD users had a higher median parity (3 vs. 2; P = .03). In addition, the copper IUDs were inserted by more senior than junior residents (46.2% vs 22.7%, P = .02).
A 2018 systematic review pooled absolute rates of IUD expulsion and estimated adjusted relative risk (RR) for IUD type. A total of 48 studies (rated level I to II-3 of poor to good quality) were included in the analysis, and results indicated that the LNG-IUD was associated with a higher risk of expulsion at less than 4 weeks postpartum than the copper IUD (adjusted RR, 1.91; 95% CI, 1.50-2.43).3
References
1. Goldthwaite LM, Sheeder J, Hyer J, et al. Postplacental intrauterine device expulsion by 12 weeks: a prospective cohort study. Am J Obstet Gynecol. 2017;217:674.e1-674.e8.
2. Hinz EK, Murthy A, Wang B, Ryan N, Ades V. A prospective cohort study comparing expulsion after postplacental insertion: the levonorgestrel versus the copper intrauterine device. Contraception. May 17, 2019. doi: 10.1016/j.contraception.2019.04.011.
3. Jatlaoui TC, Whiteman MK, Jeng G, et al. Intrauterine device expulsion after postpartum placement. Obstet Gynecol. 2018:895-905.
Technique for placing an IUD immediately after vaginal birth
1. Bring supplies for intrauterine device (IUD) insertion: the IUD, posterior blade of a speculum or retractor for posterior vagina, ring forceps, curved Kelly placenta forceps, and scissors.
2. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta. Any perineal lacerations should be repaired after IUD placement.
3. Break down the bed to facilitate placement. If the perineum or vagina is soiled with stool or meconium then consider povodine-iodine prep.
4. Place the posterior blade of the speculum into the vagina and grasp the anterior cervix with the ring forceps.
5. Set up the IUD for insertion: Change into new sterile gloves. Remove the IUD from the inserter. For levonorgestrel IUDs, cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm; copper IUDs do not need strings trimmed. Hold one arm of the IUD with the long Kelly placenta forceps so that the stem of the IUD is approximately parallel to the shaft of the forceps.
6. Insert the IUD: Guide the IUD into the lower uterine segment with the left hand on the cervix ring forceps and the right hand on the IUD forceps. After passing the IUD through the cervix, move the left hand to the abdomen and press the fundus posterior and caudad to straighten the endometrial canal and to feel the IUD at the fundus. With the right hand, guide the IUD to the fundus; this often entails dropping the hand significantly and guiding the IUD much more anteriorly than first expected.
7. Release the IUD with forceps wide open, sweeping the forceps to one side to avoid pulling the IUD out with the forceps. 8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
8. Consider use of ultrasound guidance and ultrasound verification of fundal location, especially when first performing postplacental IUD placements.
Troubleshooting tips:
- If you are unable to visualize the anterior cervix, try to place the ring forceps by palpation.
- If you are unable to grasp the cervix with ring forceps by palpation, you may try to place the IUD manually. Hold the IUD between the first and second fingers of the right hand and place the IUD at the fundus. Release the IUD with the fingers wide open and remove the hand without removing the IUD.
Technique for placing an IUD immediately after cesarean birth
1. Determine that the patient still wants the IUD and is still medically eligible for the IUD. Place the IUD as soon as possible following placenta delivery; in most studies IUD placement occurred within 10 minutes of the placenta.
2. For levonorgestrel IUDs: Remove the IUD from the inserter. Cut the strings so that the length of the IUD and strings together is approximately 10 to 12 cm. Place the IUD at the fundus with a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
3. For copper IUDs: String trimming is not necessary. Place the IUD at the fundus with the IUD inserter or a ring forceps and tuck the strings toward the cervix. It is not necessary to open the cervix or to place the strings through the cervix.
4. Repair the hysterotomy as usual.
1. Dole DM, Martin J. What nurses need to know about immediate postpartum initiation of long-acting reversible contraception. Nurs Womens Health. 2017;21:186-195.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice Bulletin no. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
4. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-104.
1. Dole DM, Martin J. What nurses need to know about immediate postpartum initiation of long-acting reversible contraception. Nurs Womens Health. 2017;21:186-195.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice Bulletin no. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
4. Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-104.
First-time fathers at risk of postnatal depressive symptoms
First-time fathers may be at risk of experiencing depressive symptoms as they transition to parenthood – especially if risk factors such as poor sleep are present, results of a prospective study of more than 600 new fathers show.
“Strategies to promote better sleep, mobilize social support, and strengthen the couple relationship may be important to address in innovative interventions tailored to new fathers at risk for depression during the perinatal period,” wrote Deborah Da Costa, PhD, of McGill University, Montreal, and colleagues. The study was published in the Journal of Affective Disorders.
To determine the prevalence of depressive symptoms in first-time fathers and identify notable risk factors, the researchers surveyed 622 Canadian men during their partner’s third trimester. The same group was surveyed again at 2 and 6 months postpartum. Depression was assessed via the Edinburgh Postnatal Depression Scale (EPDS), and additional variables such as sleep quality, social support, and stress were gathered as well.
Of the initial 622 men surveyed, 487 (78.3%) and 375 (60.3%) completed the questionnaires at 2 and 6 months postpartum, respectively. The prevalence of paternal depressive symptoms was 13.76% (95% confidence interval, 10.70-16.82) at 2 months and 13.6% (95% CI, 10.13-17.07) at 6 months. Of the men who reported depressive symptoms at 2 months postpartum, 40.3% also experienced symptoms during the third trimester. Of the men who reported depressive symptoms at 6 months postpartum, 24% experienced symptoms during the third trimester and after 2 months.
At 2 months, the risk of depressive symptoms increased for men with worse sleep quality (odds ratio, 1.25; 95% CI, 1.10-1.42), poorer couple relationship adjustment (OR, 0.97; 95% CI, 0.94-0.99), and higher parenting stress (OR, 1.07; 95% CI, 1.02-1.11). At 6 months, there was a significant association between paternal depressive symptoms and unemployment (OR, 3.75; 95% CI, 1.00-13.72), poorer sleep quality (OR, 1.37; 95% CI, 1.16-1.65), lower social support (OR, 0.92; 95% CI, 0.84-1.00), poorer couple relationship adjustment (OR, 0.95; 95% CI, 0.92-0.98), and higher financial stress (OR, 1.21; 95% CI, 1.04-1.42).
The authors acknowledged their study’s limitations, including a middling response rate that could affect the accuracy of prevalence estimates and a well-educated, largely middle-class sample that could limit generalizability. In addition, they assessed depressive symptoms by self-report and not diagnostic clinical interviews. However, they also noted that “the EPDS is the most widely used tool to assess depressive symptoms in parents during the perinatal period and was validated in expectant and new fathers.”
The study was funded by the Canadian Institutes of Health Research. No conflicts of interest were reported.
SOURCE: Da Costa D et al. J Affect Disord. 2019 Apr 15;249:371-7.
First-time fathers may be at risk of experiencing depressive symptoms as they transition to parenthood – especially if risk factors such as poor sleep are present, results of a prospective study of more than 600 new fathers show.
“Strategies to promote better sleep, mobilize social support, and strengthen the couple relationship may be important to address in innovative interventions tailored to new fathers at risk for depression during the perinatal period,” wrote Deborah Da Costa, PhD, of McGill University, Montreal, and colleagues. The study was published in the Journal of Affective Disorders.
To determine the prevalence of depressive symptoms in first-time fathers and identify notable risk factors, the researchers surveyed 622 Canadian men during their partner’s third trimester. The same group was surveyed again at 2 and 6 months postpartum. Depression was assessed via the Edinburgh Postnatal Depression Scale (EPDS), and additional variables such as sleep quality, social support, and stress were gathered as well.
Of the initial 622 men surveyed, 487 (78.3%) and 375 (60.3%) completed the questionnaires at 2 and 6 months postpartum, respectively. The prevalence of paternal depressive symptoms was 13.76% (95% confidence interval, 10.70-16.82) at 2 months and 13.6% (95% CI, 10.13-17.07) at 6 months. Of the men who reported depressive symptoms at 2 months postpartum, 40.3% also experienced symptoms during the third trimester. Of the men who reported depressive symptoms at 6 months postpartum, 24% experienced symptoms during the third trimester and after 2 months.
At 2 months, the risk of depressive symptoms increased for men with worse sleep quality (odds ratio, 1.25; 95% CI, 1.10-1.42), poorer couple relationship adjustment (OR, 0.97; 95% CI, 0.94-0.99), and higher parenting stress (OR, 1.07; 95% CI, 1.02-1.11). At 6 months, there was a significant association between paternal depressive symptoms and unemployment (OR, 3.75; 95% CI, 1.00-13.72), poorer sleep quality (OR, 1.37; 95% CI, 1.16-1.65), lower social support (OR, 0.92; 95% CI, 0.84-1.00), poorer couple relationship adjustment (OR, 0.95; 95% CI, 0.92-0.98), and higher financial stress (OR, 1.21; 95% CI, 1.04-1.42).
The authors acknowledged their study’s limitations, including a middling response rate that could affect the accuracy of prevalence estimates and a well-educated, largely middle-class sample that could limit generalizability. In addition, they assessed depressive symptoms by self-report and not diagnostic clinical interviews. However, they also noted that “the EPDS is the most widely used tool to assess depressive symptoms in parents during the perinatal period and was validated in expectant and new fathers.”
The study was funded by the Canadian Institutes of Health Research. No conflicts of interest were reported.
SOURCE: Da Costa D et al. J Affect Disord. 2019 Apr 15;249:371-7.
First-time fathers may be at risk of experiencing depressive symptoms as they transition to parenthood – especially if risk factors such as poor sleep are present, results of a prospective study of more than 600 new fathers show.
“Strategies to promote better sleep, mobilize social support, and strengthen the couple relationship may be important to address in innovative interventions tailored to new fathers at risk for depression during the perinatal period,” wrote Deborah Da Costa, PhD, of McGill University, Montreal, and colleagues. The study was published in the Journal of Affective Disorders.
To determine the prevalence of depressive symptoms in first-time fathers and identify notable risk factors, the researchers surveyed 622 Canadian men during their partner’s third trimester. The same group was surveyed again at 2 and 6 months postpartum. Depression was assessed via the Edinburgh Postnatal Depression Scale (EPDS), and additional variables such as sleep quality, social support, and stress were gathered as well.
Of the initial 622 men surveyed, 487 (78.3%) and 375 (60.3%) completed the questionnaires at 2 and 6 months postpartum, respectively. The prevalence of paternal depressive symptoms was 13.76% (95% confidence interval, 10.70-16.82) at 2 months and 13.6% (95% CI, 10.13-17.07) at 6 months. Of the men who reported depressive symptoms at 2 months postpartum, 40.3% also experienced symptoms during the third trimester. Of the men who reported depressive symptoms at 6 months postpartum, 24% experienced symptoms during the third trimester and after 2 months.
At 2 months, the risk of depressive symptoms increased for men with worse sleep quality (odds ratio, 1.25; 95% CI, 1.10-1.42), poorer couple relationship adjustment (OR, 0.97; 95% CI, 0.94-0.99), and higher parenting stress (OR, 1.07; 95% CI, 1.02-1.11). At 6 months, there was a significant association between paternal depressive symptoms and unemployment (OR, 3.75; 95% CI, 1.00-13.72), poorer sleep quality (OR, 1.37; 95% CI, 1.16-1.65), lower social support (OR, 0.92; 95% CI, 0.84-1.00), poorer couple relationship adjustment (OR, 0.95; 95% CI, 0.92-0.98), and higher financial stress (OR, 1.21; 95% CI, 1.04-1.42).
The authors acknowledged their study’s limitations, including a middling response rate that could affect the accuracy of prevalence estimates and a well-educated, largely middle-class sample that could limit generalizability. In addition, they assessed depressive symptoms by self-report and not diagnostic clinical interviews. However, they also noted that “the EPDS is the most widely used tool to assess depressive symptoms in parents during the perinatal period and was validated in expectant and new fathers.”
The study was funded by the Canadian Institutes of Health Research. No conflicts of interest were reported.
SOURCE: Da Costa D et al. J Affect Disord. 2019 Apr 15;249:371-7.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
FDA approves Gadavist for evaluation of supra-aortic, renal artery disease
The Food and Drug Administration has approved gadobutrol (Gadavist) injections, for use in conjunction with magnetic resonance angiography (MRA), to evaluate known or suspected supra-aortic or renal artery disease in adult and pediatric patients.
Approval was based on a pair of open-label, phase 3 studies in which the efficacy of gadobutrol was assessed, based on visualization and performance for distinguishing between normal and abnormal anatomy. MRA with gadobutrol improved visualization by 88%-98%, compared with unenhanced MRA, in which visualization was improved by 24%-82%. Sensitivity and specificity were noninferior to unenhanced MRA.
Gadobutrol was previously indicated for use in diagnostic MRI in both adults and children to detect areas with disrupted blood-brain barrier and/or abnormal vascularity of the central nervous system, and for MRI of the breast to assess the presence and extent of malignant breast disease. The safety profile in the two current trials matched data previously gathered, with the most common adverse events including headache, nausea, and dizziness.
“Until now, no contrast agents were FDA approved for use with MRA of the supra-aortic arteries. With FDA’s action, radiologists now have an approved MRA contrast agent to help visualize supra-aortic arteries in patients with known or suspected supra-aortic arterial disease, including conditions such as prior stroke or transient ischemic attack,” Elias Melhem, MD, chair of the department of diagnostic radiology and nuclear medicine at the University of Maryland, Baltimore, said in the press release.
Find the full release on the Bayer website.
The Food and Drug Administration has approved gadobutrol (Gadavist) injections, for use in conjunction with magnetic resonance angiography (MRA), to evaluate known or suspected supra-aortic or renal artery disease in adult and pediatric patients.
Approval was based on a pair of open-label, phase 3 studies in which the efficacy of gadobutrol was assessed, based on visualization and performance for distinguishing between normal and abnormal anatomy. MRA with gadobutrol improved visualization by 88%-98%, compared with unenhanced MRA, in which visualization was improved by 24%-82%. Sensitivity and specificity were noninferior to unenhanced MRA.
Gadobutrol was previously indicated for use in diagnostic MRI in both adults and children to detect areas with disrupted blood-brain barrier and/or abnormal vascularity of the central nervous system, and for MRI of the breast to assess the presence and extent of malignant breast disease. The safety profile in the two current trials matched data previously gathered, with the most common adverse events including headache, nausea, and dizziness.
“Until now, no contrast agents were FDA approved for use with MRA of the supra-aortic arteries. With FDA’s action, radiologists now have an approved MRA contrast agent to help visualize supra-aortic arteries in patients with known or suspected supra-aortic arterial disease, including conditions such as prior stroke or transient ischemic attack,” Elias Melhem, MD, chair of the department of diagnostic radiology and nuclear medicine at the University of Maryland, Baltimore, said in the press release.
Find the full release on the Bayer website.
The Food and Drug Administration has approved gadobutrol (Gadavist) injections, for use in conjunction with magnetic resonance angiography (MRA), to evaluate known or suspected supra-aortic or renal artery disease in adult and pediatric patients.
Approval was based on a pair of open-label, phase 3 studies in which the efficacy of gadobutrol was assessed, based on visualization and performance for distinguishing between normal and abnormal anatomy. MRA with gadobutrol improved visualization by 88%-98%, compared with unenhanced MRA, in which visualization was improved by 24%-82%. Sensitivity and specificity were noninferior to unenhanced MRA.
Gadobutrol was previously indicated for use in diagnostic MRI in both adults and children to detect areas with disrupted blood-brain barrier and/or abnormal vascularity of the central nervous system, and for MRI of the breast to assess the presence and extent of malignant breast disease. The safety profile in the two current trials matched data previously gathered, with the most common adverse events including headache, nausea, and dizziness.
“Until now, no contrast agents were FDA approved for use with MRA of the supra-aortic arteries. With FDA’s action, radiologists now have an approved MRA contrast agent to help visualize supra-aortic arteries in patients with known or suspected supra-aortic arterial disease, including conditions such as prior stroke or transient ischemic attack,” Elias Melhem, MD, chair of the department of diagnostic radiology and nuclear medicine at the University of Maryland, Baltimore, said in the press release.
Find the full release on the Bayer website.
Periodontal Inflammation in Patients with Migraine
Periodontal inflammation is associated with increased circulating levels of calcitonin gene-related peptide (CGRP) in patients with chronic migraine, a new study found. The cohort included 102 chronic migraineurs and 77 age- and sex-matched individuals free of headache/migraine. Full-mouth periodontal parameters were recorded and the periodontal inflamed surface area (PISA) was calculated to quantify the periodontal inflammatory status for each participant. Researchers found:
- In the chronic migraine group, patients with periodontitis had greater levels of serum CGRP and IL-6, while nonsignificant differences were observed with IL-10 concentrations vs those without periodontitis.
- PISA was independently associated with CGRP in patients with chronic migraine.
Leira Y, et al. Periodontal inflammation is related to increased serum calcitonin gene-related peptide (CGRP) levels in patients with chronic migraine. [Published online ahead of print May 9, 2019]. J Periodontol. doi: 10.1002/JPER.19-0051.
Periodontal inflammation is associated with increased circulating levels of calcitonin gene-related peptide (CGRP) in patients with chronic migraine, a new study found. The cohort included 102 chronic migraineurs and 77 age- and sex-matched individuals free of headache/migraine. Full-mouth periodontal parameters were recorded and the periodontal inflamed surface area (PISA) was calculated to quantify the periodontal inflammatory status for each participant. Researchers found:
- In the chronic migraine group, patients with periodontitis had greater levels of serum CGRP and IL-6, while nonsignificant differences were observed with IL-10 concentrations vs those without periodontitis.
- PISA was independently associated with CGRP in patients with chronic migraine.
Leira Y, et al. Periodontal inflammation is related to increased serum calcitonin gene-related peptide (CGRP) levels in patients with chronic migraine. [Published online ahead of print May 9, 2019]. J Periodontol. doi: 10.1002/JPER.19-0051.
Periodontal inflammation is associated with increased circulating levels of calcitonin gene-related peptide (CGRP) in patients with chronic migraine, a new study found. The cohort included 102 chronic migraineurs and 77 age- and sex-matched individuals free of headache/migraine. Full-mouth periodontal parameters were recorded and the periodontal inflamed surface area (PISA) was calculated to quantify the periodontal inflammatory status for each participant. Researchers found:
- In the chronic migraine group, patients with periodontitis had greater levels of serum CGRP and IL-6, while nonsignificant differences were observed with IL-10 concentrations vs those without periodontitis.
- PISA was independently associated with CGRP in patients with chronic migraine.
Leira Y, et al. Periodontal inflammation is related to increased serum calcitonin gene-related peptide (CGRP) levels in patients with chronic migraine. [Published online ahead of print May 9, 2019]. J Periodontol. doi: 10.1002/JPER.19-0051.
Dry Eye Symptoms in Individuals with Migraine
Individuals with migraine demonstrated a different dry eye (DE) symptom, yet a similar DE sign profile when compared with those without migraine, a new study found. The prospective cross-sectional study of individuals with DE symptoms evaluated symptoms and signs of DE, including symptoms suggestive of nerve dysfunction. Among the details:
- Of 250 individuals, 31 met International Classification of Headache Disorders criteria for migraine based on a validated screen.
- Those with migraine were significantly younger and more likely to be female vs controls.
- Individuals with migraine had more severe DE symptoms and ocular pain vs controls.
- DE symptoms in those with migraine may be driven by nerve dysfunction as opposed to ocular surface abnormalities.
Farhangi M, et al. Individuals with migraine have a different dry eye symptom profile than individuals without migraine. [Published online ahead of print April 30, 2019]. Br J Opthalmol. doi: 10.1136/bjophthalmol-2018-313471.
Individuals with migraine demonstrated a different dry eye (DE) symptom, yet a similar DE sign profile when compared with those without migraine, a new study found. The prospective cross-sectional study of individuals with DE symptoms evaluated symptoms and signs of DE, including symptoms suggestive of nerve dysfunction. Among the details:
- Of 250 individuals, 31 met International Classification of Headache Disorders criteria for migraine based on a validated screen.
- Those with migraine were significantly younger and more likely to be female vs controls.
- Individuals with migraine had more severe DE symptoms and ocular pain vs controls.
- DE symptoms in those with migraine may be driven by nerve dysfunction as opposed to ocular surface abnormalities.
Farhangi M, et al. Individuals with migraine have a different dry eye symptom profile than individuals without migraine. [Published online ahead of print April 30, 2019]. Br J Opthalmol. doi: 10.1136/bjophthalmol-2018-313471.
Individuals with migraine demonstrated a different dry eye (DE) symptom, yet a similar DE sign profile when compared with those without migraine, a new study found. The prospective cross-sectional study of individuals with DE symptoms evaluated symptoms and signs of DE, including symptoms suggestive of nerve dysfunction. Among the details:
- Of 250 individuals, 31 met International Classification of Headache Disorders criteria for migraine based on a validated screen.
- Those with migraine were significantly younger and more likely to be female vs controls.
- Individuals with migraine had more severe DE symptoms and ocular pain vs controls.
- DE symptoms in those with migraine may be driven by nerve dysfunction as opposed to ocular surface abnormalities.
Farhangi M, et al. Individuals with migraine have a different dry eye symptom profile than individuals without migraine. [Published online ahead of print April 30, 2019]. Br J Opthalmol. doi: 10.1136/bjophthalmol-2018-313471.
Atypical Interactions of Cortical Networks in Chronic Migraine
Atypical Interactions of Cortical Networks in Chronic Migraine
The severity of headache is associated with opposite connectivity patterns in frontal executive and dorsal attentional networks in patients with chronic migraine, a new study found. Twenty patients with chronic migraine (CM) without preventive therapy, or acute medication overuse underwent 3T MRI scans and were compared to a group of 20 healthy controls (HC). Researchers used MRI to collect resting-state data in 3 selected networks, identified using group independent component analysis (ICA): the default mode network (DMN), the executive control network (ECN), and the dorsal attention system (DAS). They found:
- Compared to HC, patients with CM had significantly reduced functional connectivity between the DMN and the ECN.
- The DAS showed significantly stronger functional connectivity (FC) with the DMN and weaker FC with the ECN.
- The higher the severity of the headache, the increased strength of DAD connectivity, and the lower the strength of the ECN connectivity.
Coppola G, et al. Aberrant interactions of cortical networks in chronic migraine: A resting-state fMRI study. [Published online ahead of print May 28, 2019]. Neurology. doi: 10.1212/WNL.0000000000007577.
Atypical Interactions of Cortical Networks in Chronic Migraine
The severity of headache is associated with opposite connectivity patterns in frontal executive and dorsal attentional networks in patients with chronic migraine, a new study found. Twenty patients with chronic migraine (CM) without preventive therapy, or acute medication overuse underwent 3T MRI scans and were compared to a group of 20 healthy controls (HC). Researchers used MRI to collect resting-state data in 3 selected networks, identified using group independent component analysis (ICA): the default mode network (DMN), the executive control network (ECN), and the dorsal attention system (DAS). They found:
- Compared to HC, patients with CM had significantly reduced functional connectivity between the DMN and the ECN.
- The DAS showed significantly stronger functional connectivity (FC) with the DMN and weaker FC with the ECN.
- The higher the severity of the headache, the increased strength of DAD connectivity, and the lower the strength of the ECN connectivity.
Coppola G, et al. Aberrant interactions of cortical networks in chronic migraine: A resting-state fMRI study. [Published online ahead of print May 28, 2019]. Neurology. doi: 10.1212/WNL.0000000000007577.
Atypical Interactions of Cortical Networks in Chronic Migraine
The severity of headache is associated with opposite connectivity patterns in frontal executive and dorsal attentional networks in patients with chronic migraine, a new study found. Twenty patients with chronic migraine (CM) without preventive therapy, or acute medication overuse underwent 3T MRI scans and were compared to a group of 20 healthy controls (HC). Researchers used MRI to collect resting-state data in 3 selected networks, identified using group independent component analysis (ICA): the default mode network (DMN), the executive control network (ECN), and the dorsal attention system (DAS). They found:
- Compared to HC, patients with CM had significantly reduced functional connectivity between the DMN and the ECN.
- The DAS showed significantly stronger functional connectivity (FC) with the DMN and weaker FC with the ECN.
- The higher the severity of the headache, the increased strength of DAD connectivity, and the lower the strength of the ECN connectivity.
Coppola G, et al. Aberrant interactions of cortical networks in chronic migraine: A resting-state fMRI study. [Published online ahead of print May 28, 2019]. Neurology. doi: 10.1212/WNL.0000000000007577.
The expert trap
When you fly as a physician, there’s always a chance you’ll get a free drink. It’s not free, of course. For at least a few minutes, you worked. “Is there a physician onboard? – Ah, just how badly do you want that vodka tonic?
I ring my call button, as I’m sure you do. (It’s worth it to see the flight attendant’s face when I reply: “I’m a dermatologist.”) Last time it was for a 68-year-old man who was vomiting. There was no rash.
I responded along with a pediatrician and an ER nurse – gratitude is an ER nurse at 38,000 feet. The patient had chemotherapy-induced nausea. We three managed to get him well enough to finish the flight. Our ER nurse team member ran the show; she was excellent. She asked all the right questions and helped us all make good decisions. Unlike in clinic, I wasn’t an expert here despite my MD.
Several weeks ago, I saw a patient in the office with severe psoriasis. She stood before me erythrodermic. As I was adjusting her orders, I stepped out of the office to call one of my partners for her opinion. She examined the patient and declared: “I don’t think it’s psoriasis. Despite that biopsy, I think this is chronic eczema.” Brilliant.
In contrast to the former story, I was an expert in my office. And yet, success depended in both instances on my recognizing a cognitive bias: I don’t know everything, and worse, I sometimes don’t realize what I don’t know.
. It’s a common mistake and manifests as overconfidence in our own abilities. For example, what decade did Hawaii join the union? Who is on the 20-dollar bill? Which is the farthest planet? You might be 90% confident of your answers, but most of us are more confident than we ought to be. Chances are you’ll be wrong on one. Recognizing this is hard. And yet, it’s what separates the good from the great clinicians.
Short of having your medical assistant whisper in your ear each day “Memento stultus” (remember you’re stupid), avoiding this bias is difficult. Signs that you might be trapped in an expert mindset are: 1. You believe your patients’ failure to improve is due to lack of adherence to your plan. 2. You cannot recall the last time you tried a new treatment. 3. You never ask others for second opinions. 4. Your colleagues stop asking for your opinion. 5. A flight attendant asks if you would mind returning to your seat rather than help with a medical situation.
If you want to be a better doctor, try working on your sense of self-importance. Remember your limitations and those of medicine. Be methodical in questioning your assumptions. Could you be wrong? Could the data you have be misleading? What are you missing? Ask a colleague to review some of your charts or spend time with you during procedures. Join (or start!) a journal club. Share your difficult cases with others and take note of how their advice differs from your approach.
By recognizing when you might be wrong and humbly stepping aside or taking the time to learn, you might just earn that free drink.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
When you fly as a physician, there’s always a chance you’ll get a free drink. It’s not free, of course. For at least a few minutes, you worked. “Is there a physician onboard? – Ah, just how badly do you want that vodka tonic?
I ring my call button, as I’m sure you do. (It’s worth it to see the flight attendant’s face when I reply: “I’m a dermatologist.”) Last time it was for a 68-year-old man who was vomiting. There was no rash.
I responded along with a pediatrician and an ER nurse – gratitude is an ER nurse at 38,000 feet. The patient had chemotherapy-induced nausea. We three managed to get him well enough to finish the flight. Our ER nurse team member ran the show; she was excellent. She asked all the right questions and helped us all make good decisions. Unlike in clinic, I wasn’t an expert here despite my MD.
Several weeks ago, I saw a patient in the office with severe psoriasis. She stood before me erythrodermic. As I was adjusting her orders, I stepped out of the office to call one of my partners for her opinion. She examined the patient and declared: “I don’t think it’s psoriasis. Despite that biopsy, I think this is chronic eczema.” Brilliant.
In contrast to the former story, I was an expert in my office. And yet, success depended in both instances on my recognizing a cognitive bias: I don’t know everything, and worse, I sometimes don’t realize what I don’t know.
. It’s a common mistake and manifests as overconfidence in our own abilities. For example, what decade did Hawaii join the union? Who is on the 20-dollar bill? Which is the farthest planet? You might be 90% confident of your answers, but most of us are more confident than we ought to be. Chances are you’ll be wrong on one. Recognizing this is hard. And yet, it’s what separates the good from the great clinicians.
Short of having your medical assistant whisper in your ear each day “Memento stultus” (remember you’re stupid), avoiding this bias is difficult. Signs that you might be trapped in an expert mindset are: 1. You believe your patients’ failure to improve is due to lack of adherence to your plan. 2. You cannot recall the last time you tried a new treatment. 3. You never ask others for second opinions. 4. Your colleagues stop asking for your opinion. 5. A flight attendant asks if you would mind returning to your seat rather than help with a medical situation.
If you want to be a better doctor, try working on your sense of self-importance. Remember your limitations and those of medicine. Be methodical in questioning your assumptions. Could you be wrong? Could the data you have be misleading? What are you missing? Ask a colleague to review some of your charts or spend time with you during procedures. Join (or start!) a journal club. Share your difficult cases with others and take note of how their advice differs from your approach.
By recognizing when you might be wrong and humbly stepping aside or taking the time to learn, you might just earn that free drink.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
When you fly as a physician, there’s always a chance you’ll get a free drink. It’s not free, of course. For at least a few minutes, you worked. “Is there a physician onboard? – Ah, just how badly do you want that vodka tonic?
I ring my call button, as I’m sure you do. (It’s worth it to see the flight attendant’s face when I reply: “I’m a dermatologist.”) Last time it was for a 68-year-old man who was vomiting. There was no rash.
I responded along with a pediatrician and an ER nurse – gratitude is an ER nurse at 38,000 feet. The patient had chemotherapy-induced nausea. We three managed to get him well enough to finish the flight. Our ER nurse team member ran the show; she was excellent. She asked all the right questions and helped us all make good decisions. Unlike in clinic, I wasn’t an expert here despite my MD.
Several weeks ago, I saw a patient in the office with severe psoriasis. She stood before me erythrodermic. As I was adjusting her orders, I stepped out of the office to call one of my partners for her opinion. She examined the patient and declared: “I don’t think it’s psoriasis. Despite that biopsy, I think this is chronic eczema.” Brilliant.
In contrast to the former story, I was an expert in my office. And yet, success depended in both instances on my recognizing a cognitive bias: I don’t know everything, and worse, I sometimes don’t realize what I don’t know.
. It’s a common mistake and manifests as overconfidence in our own abilities. For example, what decade did Hawaii join the union? Who is on the 20-dollar bill? Which is the farthest planet? You might be 90% confident of your answers, but most of us are more confident than we ought to be. Chances are you’ll be wrong on one. Recognizing this is hard. And yet, it’s what separates the good from the great clinicians.
Short of having your medical assistant whisper in your ear each day “Memento stultus” (remember you’re stupid), avoiding this bias is difficult. Signs that you might be trapped in an expert mindset are: 1. You believe your patients’ failure to improve is due to lack of adherence to your plan. 2. You cannot recall the last time you tried a new treatment. 3. You never ask others for second opinions. 4. Your colleagues stop asking for your opinion. 5. A flight attendant asks if you would mind returning to your seat rather than help with a medical situation.
If you want to be a better doctor, try working on your sense of self-importance. Remember your limitations and those of medicine. Be methodical in questioning your assumptions. Could you be wrong? Could the data you have be misleading? What are you missing? Ask a colleague to review some of your charts or spend time with you during procedures. Join (or start!) a journal club. Share your difficult cases with others and take note of how their advice differs from your approach.
By recognizing when you might be wrong and humbly stepping aside or taking the time to learn, you might just earn that free drink.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Changes in sleep-wake timing accompany cerebral glucose hypometabolism and cognitive function
LOS ANGELES – Dysregulated sleep-wake cycles may be linked to cerebral glucose hypometabolism and subtle cognitive changes, both of which are early signs of Alzheimer’s disease–like neurodegeneration, according to a 2-year study of older Korean adults.
The association was particularly strong in subjects who experienced delayed acrophase, the peak of the normal sleep-wake cycle, So-Yeon Jeon, MD, said at the Alzheimer’s Association International Conference. It’s not yet clear whether the changes are a risk factor for dementia or a prodromal sign of neurodegeneration, but even without full elucidation, the findings could have value as a signal of impending neurodegeneration, said Dr. Jeon of Seoul (South Korea) National University.
“Our findings suggest that delayed acrophase may be used as a predictor for the progression of Alzheimer’s-type neurodegeneration and cognitive decline in the near future in old individuals with diverse cognitive status,” she said. “But the relationship between circadian phases and neurodegeneration is complex and not yet well understood.”
The 24-month study comprised 215 elderly adults enrolled in the Korean Brain Aging Study for the Early Diagnosis and Prediction of Alzheimer’s Disease (KBASE). They were a mean of 70 years old at baseline; 143 were cognitively normal, 40 had mild cognitive impairment, and 32 had Alzheimer’s dementia. Both at baseline and 2 years, everyone underwent a comprehensive neuropsychological assessment, amyloid PET brain imaging with Pittsburgh compound B, and an [18F]-fluorodeoxyglucose PET scan to determine brain glucose metabolic rate.
Before each assessment, the investigators measured sleep and circadian rhythms with 8 days of actigraphy. This assessed sleep variables (total sleep time, sleep latency, sleep efficiency, and wakefulness after sleep); rest-activity rhythm variables (midline estimated statistic of rhythm, amplitude, and acrophase), and some nonparametric values including interdaily stability, intradaily variability, and relative amplitude of sleep cycles. Subjects also completed sleep diaries during these periods.
The study’s main outcomes were 2-year changes in the Mini Mental State Exam (MMSE) score and in Alzheimer’s imaging biomarkers, including glucose metabolism and amyloid deposition. All analyses controlled for age, sex, Clinical Dementia Rating score, apolipoprotein E allele status, and baseline cognition.
At baseline, lower total sleep time was significantly associated with hypometabolism in areas associated with Alzheimer’s pathology as well as lower mean MMSE scores. Circadian variables showed no significant associations with these characteristics. However, the relative amplitude of circadian rhythm was significantly associated with hypometabolism and with lower MMSE score. There were no associations with brain amyloid load.
At 2 years, acrophase was associated with declines in cerebral glucose metabolism and further changes in the MMSE, even after the researchers controlled for the potential confounders. Delayed acrophase, although not associated with either metabolic rate or cognition at baseline, did significantly influence both at 2 years, suggesting a rapidly eroding clinical picture.
“Neurodegeneration over 2 years means the disease is progressing rapidly and subjects are likely to have tauopathies or other proteinopathy,” Dr. Jeon said. “These pathologies may either be resulting in delayed acrophase followed by neurodegeneration, or they may be prodromal symptoms of impending neurodegeneration. Whether they are early symptoms or early risk factors is not currently known, however. Two years is too short of a follow-up to determine these questions.”
Dr. Jeon had no financial declarations.
SOURCE: Jeon SY et al. AAIC 2019, abstract 33543.
LOS ANGELES – Dysregulated sleep-wake cycles may be linked to cerebral glucose hypometabolism and subtle cognitive changes, both of which are early signs of Alzheimer’s disease–like neurodegeneration, according to a 2-year study of older Korean adults.
The association was particularly strong in subjects who experienced delayed acrophase, the peak of the normal sleep-wake cycle, So-Yeon Jeon, MD, said at the Alzheimer’s Association International Conference. It’s not yet clear whether the changes are a risk factor for dementia or a prodromal sign of neurodegeneration, but even without full elucidation, the findings could have value as a signal of impending neurodegeneration, said Dr. Jeon of Seoul (South Korea) National University.
“Our findings suggest that delayed acrophase may be used as a predictor for the progression of Alzheimer’s-type neurodegeneration and cognitive decline in the near future in old individuals with diverse cognitive status,” she said. “But the relationship between circadian phases and neurodegeneration is complex and not yet well understood.”
The 24-month study comprised 215 elderly adults enrolled in the Korean Brain Aging Study for the Early Diagnosis and Prediction of Alzheimer’s Disease (KBASE). They were a mean of 70 years old at baseline; 143 were cognitively normal, 40 had mild cognitive impairment, and 32 had Alzheimer’s dementia. Both at baseline and 2 years, everyone underwent a comprehensive neuropsychological assessment, amyloid PET brain imaging with Pittsburgh compound B, and an [18F]-fluorodeoxyglucose PET scan to determine brain glucose metabolic rate.
Before each assessment, the investigators measured sleep and circadian rhythms with 8 days of actigraphy. This assessed sleep variables (total sleep time, sleep latency, sleep efficiency, and wakefulness after sleep); rest-activity rhythm variables (midline estimated statistic of rhythm, amplitude, and acrophase), and some nonparametric values including interdaily stability, intradaily variability, and relative amplitude of sleep cycles. Subjects also completed sleep diaries during these periods.
The study’s main outcomes were 2-year changes in the Mini Mental State Exam (MMSE) score and in Alzheimer’s imaging biomarkers, including glucose metabolism and amyloid deposition. All analyses controlled for age, sex, Clinical Dementia Rating score, apolipoprotein E allele status, and baseline cognition.
At baseline, lower total sleep time was significantly associated with hypometabolism in areas associated with Alzheimer’s pathology as well as lower mean MMSE scores. Circadian variables showed no significant associations with these characteristics. However, the relative amplitude of circadian rhythm was significantly associated with hypometabolism and with lower MMSE score. There were no associations with brain amyloid load.
At 2 years, acrophase was associated with declines in cerebral glucose metabolism and further changes in the MMSE, even after the researchers controlled for the potential confounders. Delayed acrophase, although not associated with either metabolic rate or cognition at baseline, did significantly influence both at 2 years, suggesting a rapidly eroding clinical picture.
“Neurodegeneration over 2 years means the disease is progressing rapidly and subjects are likely to have tauopathies or other proteinopathy,” Dr. Jeon said. “These pathologies may either be resulting in delayed acrophase followed by neurodegeneration, or they may be prodromal symptoms of impending neurodegeneration. Whether they are early symptoms or early risk factors is not currently known, however. Two years is too short of a follow-up to determine these questions.”
Dr. Jeon had no financial declarations.
SOURCE: Jeon SY et al. AAIC 2019, abstract 33543.
LOS ANGELES – Dysregulated sleep-wake cycles may be linked to cerebral glucose hypometabolism and subtle cognitive changes, both of which are early signs of Alzheimer’s disease–like neurodegeneration, according to a 2-year study of older Korean adults.
The association was particularly strong in subjects who experienced delayed acrophase, the peak of the normal sleep-wake cycle, So-Yeon Jeon, MD, said at the Alzheimer’s Association International Conference. It’s not yet clear whether the changes are a risk factor for dementia or a prodromal sign of neurodegeneration, but even without full elucidation, the findings could have value as a signal of impending neurodegeneration, said Dr. Jeon of Seoul (South Korea) National University.
“Our findings suggest that delayed acrophase may be used as a predictor for the progression of Alzheimer’s-type neurodegeneration and cognitive decline in the near future in old individuals with diverse cognitive status,” she said. “But the relationship between circadian phases and neurodegeneration is complex and not yet well understood.”
The 24-month study comprised 215 elderly adults enrolled in the Korean Brain Aging Study for the Early Diagnosis and Prediction of Alzheimer’s Disease (KBASE). They were a mean of 70 years old at baseline; 143 were cognitively normal, 40 had mild cognitive impairment, and 32 had Alzheimer’s dementia. Both at baseline and 2 years, everyone underwent a comprehensive neuropsychological assessment, amyloid PET brain imaging with Pittsburgh compound B, and an [18F]-fluorodeoxyglucose PET scan to determine brain glucose metabolic rate.
Before each assessment, the investigators measured sleep and circadian rhythms with 8 days of actigraphy. This assessed sleep variables (total sleep time, sleep latency, sleep efficiency, and wakefulness after sleep); rest-activity rhythm variables (midline estimated statistic of rhythm, amplitude, and acrophase), and some nonparametric values including interdaily stability, intradaily variability, and relative amplitude of sleep cycles. Subjects also completed sleep diaries during these periods.
The study’s main outcomes were 2-year changes in the Mini Mental State Exam (MMSE) score and in Alzheimer’s imaging biomarkers, including glucose metabolism and amyloid deposition. All analyses controlled for age, sex, Clinical Dementia Rating score, apolipoprotein E allele status, and baseline cognition.
At baseline, lower total sleep time was significantly associated with hypometabolism in areas associated with Alzheimer’s pathology as well as lower mean MMSE scores. Circadian variables showed no significant associations with these characteristics. However, the relative amplitude of circadian rhythm was significantly associated with hypometabolism and with lower MMSE score. There were no associations with brain amyloid load.
At 2 years, acrophase was associated with declines in cerebral glucose metabolism and further changes in the MMSE, even after the researchers controlled for the potential confounders. Delayed acrophase, although not associated with either metabolic rate or cognition at baseline, did significantly influence both at 2 years, suggesting a rapidly eroding clinical picture.
“Neurodegeneration over 2 years means the disease is progressing rapidly and subjects are likely to have tauopathies or other proteinopathy,” Dr. Jeon said. “These pathologies may either be resulting in delayed acrophase followed by neurodegeneration, or they may be prodromal symptoms of impending neurodegeneration. Whether they are early symptoms or early risk factors is not currently known, however. Two years is too short of a follow-up to determine these questions.”
Dr. Jeon had no financial declarations.
SOURCE: Jeon SY et al. AAIC 2019, abstract 33543.
REPORTING FROM AAIC 2019
Parabens – friend or foe?
Parabens were named nonallergen of the year! It is time that we help consumers understand that the substitutes for parabens are often worse than parabens, and parabens are not as sensitizing as we thought. Preservatives are essential parts of most cosmetics and cosmeceuticals. (I say “most” because many organic products do not have them and consequently have shorter shelf lives.) Without them, products are vulnerable to rapid decomposition and infiltration by bacteria, fungi, and molds. The preservatives that are used in the place of parabens often are sensitizers. What do we tell our patients about the safety of parabens with all of these conflicting reports? This column will focus on current thoughts regarding the safety of parabens used as preservatives. I would love to hear your thoughts.
Background
Parabens are alkyl esters of p-hydroxybenzoic acid and have been used as a class of preservatives since the late 1920s and early 1930s. Parabens are found naturally in raspberries, blackberries, carrots, and cucumbers and are common ingredients in food and pharmaceuticals. They are still widely used in skin, hair, and body care products, despite the public outcry against them.1-4
There are many kinds of parabens such as butylparaben, isobutylparaben, ethylparaben, methylparaben, propylparaben, isopropylparaben, and benzylparaben, each with its own characteristics.5 Parabens are considered ideal preservative ingredients because they exhibit a broad spectrum of antimicrobial activity, stability over a large pH and temperature range, have no odor, do not change color, and are water soluble enough to yield an effective concentration in a hydrophilic formulation.3 As the alkyl chain length of parabens increases, they become less water soluble and more oil soluble. Parabens penetrate the skin barrier in inverse relation to its ester chain length.6 Often, several parabens will be combined to take advantage of each paraben’s solubility characteristics.
Many patients avoid parabens because of “health risks.” Now other preservatives are being substituted for parabens, even though these ingredients may be less studied or even less safe than parabens. It is important not to lump all parabens together as they each have different characteristics. Methylparaben and propylparaben are the most commonly used parabens in skin care products.7 Combinations of parabens are notably more effective than the use of single parabens.3,8 High concentrations of any type of paraben can cause an irritant reaction on the skin, but those with longer ester chain lengths are more likely to cause irritation.
Methylparaben
The methyl ester of p-hydroxybenzoic acid is found in many skin care products. It is readily absorbed through the skin and gastrointestinal tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body. Studies have shown it is nontoxic, nonirritating, and nonsensitizing. It is not teratogenic, embryotoxic, or carcinogenic. Methylparaben, because of its shorter side chain groups and greater lipophilicity, has been shown to be more readily absorbed by the skin than other paraben chemicals.8,9 It is also on the low order of ingredients provoking acute and chronic toxicity.3
Propylparaben
Propylparaben is the ester form of p-hydroxybenzoic acid that has been esterified with n-propanol. It is the most commonly used antimicrobial preservative in foods, cosmetics, and drugs. It is readily absorbed through the skin and GI tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body.
Estrogenic activity of parabens
In a 2004 study, Darbre et al. reported on the discovery of parabens-like substances in breast tissue and published these findings in the Journal of Applied Toxicology.10 The media and public panicked, saying that parabens have estrogenic activity and can cause breast cancer. However, many studies have shown that certain parabens do not have estrogenic activity. Although some parabens have been shown to impart estrogenic effects in vitro, these are very weak. The four most commonly used parabens in cosmetic products are 10,000-fold or less potent than 17beta-estradiol.11 The potential to result in an adverse effect mediated via an estrogen mode of action has not been established in humans.6 Paraben exposure differs geographically. No correlation has been found between the amount of parabens in a geographic location and the incidence of breast cancer. Current scientific knowledge is insufficient to demonstrate a clear cancer risk caused by the topical application of cosmetics that contain parabens on normal intact skin.11
Parabens and contact dermatitis
Paraben compounds are capable of minimal penetrance through intact skin.12 When they are able to penetrate the skin – a capacity that varies among the class – parabens are rapidly metabolized to p-hydroxybenzoic acid and promptly excreted in the urine.3,11 Parabens for many years were thought to cause contact dermatitis, and there are many reports of this. However, the incidence is much lower than previously thought. In fact, parabens were named “Nonallergen of the Year in 2018” because of the low incidence of reactions in patch tests.13 Higher concentrations of parabens applied topically to skin – especially “nonintact” skin – have been shown to cause mild irritant reactions. It is likely that many of these reported cases of “contact dermatitis” were actually irritant dermatitis. Longstanding concerns about the allergenicity of parabens in relation to the skin have been rendered insignificant, as the wealth of evidence reveals little to no support for the cutaneous toxicity of these substances.11 Yim et al. add that parabens remain far less sensitizing than agents newly introduced for use in personal care products.4
Daily average exposure to parabens
It is estimated that parabens are found in 10% of personal care products. In most cases, these products contain 1% or less of parabens. If the average patient uses 50 g of personal care products a day, then the average daily exposure to parabens topically is 0.05 g. Parabens also are found in food and drugs, so the total paraben exposure per day is assumed to be about 1 mg/day. (See the 2002 Food and Chemical Toxicology article for details of how this was calculated.)7 When food, personal care products, and drug exposure rates are added, the average person is exposed to 1.29 mg/kg per day or 77.5 mg/day for a 60-kg individual. You can see that personal care products account for a fraction of exposure, as most paraben exposure comes from food.
Government opinion on the safety of parabens for the skin
Parabens long have been assessed as safe for use in cosmetic products in many countries. The European Commission stipulated a maximum concentration of 0.4% for each paraben and 0.8% for total mixture of paraben esters.4,6 While the Federal Food, Drug, and Cosmetic Act of 1938 prohibits the Food and Drug Administration from ruling on cosmetic ingredients, the industry-sponsored Cosmetic Ingredient Review expert panel has endorsed the European guidelines.4,6 Further, the North American Contact Dermatitis Group has pointed out that parabens continue to demonstrate the lowest prevalence of positivity (0.6%) of any major preservative available on the North American market, which includes over 10,000 cosmetic and personal care products, and remain one of the safest classes of preservatives for the skin.14 Further, the FDA has listed or classified parabens as generally regarded as safe.8
Safety of parabens
Parabens do not accumulate in tissues or organs for any appreciable length of time.6,8 In addition, carcinogenicity, cytotoxicity, or mutagenicity has not been proven in relation to parabens.8 Indeed, classical assays have shown no activity from parabens in terms of mutagenicity or carcinogenicity.11,15 Some estrogenic effects or activity that mimics estrogen have been associated with parabens in vitro, but this activity has been noted as very weak and there are no established reports of human cases in which parabens have elicited an estrogen-mediated adverse event.6,11
Concerns about a possible link between parabens and breast cancer have been largely diminished or relegated to the status of unknown and difficult to ascertain.13 Further, present knowledge provides no established link between the topical application of parabens-containing skin care formulations on healthy skin and cancer risk.10 Only intact skin should come in touch with products containing parabens to prevent irritant reactions.
Conclusion
Despite the fearful hype and reaction to one report 15 years ago, parabens continue to be safely used in numerous topical formulations. Their widespread use and lack of association with adverse events are a testament to their safety. From a dermatologic perspective, this nonallergen of the year deserves a better reputation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. “Goodman and Gilman’s The Pharmacological Basis of Therapeutics,” 6th ed. (New York: Macmillan, 1980, p. 969).
2. Toxicity: The Butyl, Ethyl, Methyl, and Propyl Esters have been found to promote allergic sensitization in humans, in “Dangerous Properties of Industrial Materials,” 4th ed. (New York: Van Nostrand Reinhold, 1975, p. 929).
3. Food Chem Toxicol. 2001 Jun;39(6):513-32.
4. Dermatitis. 2014 Sep-Oct;25(5):215-31.
5. Crit Rev Toxicol. 2005 Jun;35(5):435-58.
6. Int J Toxicol. 2008;27 Suppl 4:1-82.
7. Food Chem Toxicol. 2002 Oct;40(10):1335-73.
8. Dermatitis. 2019 Jan/Feb;30(1):3-31.
9. Exp Dermatol. 2007 Oct;16(10):830-6.
10. J Appl Toxicol. 2004 Jan-Feb;24(1):5-13.
11. Dermatitis. 2019 Jan/Feb;30(1):32-45.
12. Food Chem Toxicol. 2005 Feb;43(2):279-91.
13. Dermatitis. 2018 Dec 18. doi: 10.1097/DER.0000000000000429.
14. Dermatitis. 2018 Nov/Dec;29(6):297-309.
15. Food Chem Toxicol. 2005 Jul;43(7):985-1015.
Parabens were named nonallergen of the year! It is time that we help consumers understand that the substitutes for parabens are often worse than parabens, and parabens are not as sensitizing as we thought. Preservatives are essential parts of most cosmetics and cosmeceuticals. (I say “most” because many organic products do not have them and consequently have shorter shelf lives.) Without them, products are vulnerable to rapid decomposition and infiltration by bacteria, fungi, and molds. The preservatives that are used in the place of parabens often are sensitizers. What do we tell our patients about the safety of parabens with all of these conflicting reports? This column will focus on current thoughts regarding the safety of parabens used as preservatives. I would love to hear your thoughts.
Background
Parabens are alkyl esters of p-hydroxybenzoic acid and have been used as a class of preservatives since the late 1920s and early 1930s. Parabens are found naturally in raspberries, blackberries, carrots, and cucumbers and are common ingredients in food and pharmaceuticals. They are still widely used in skin, hair, and body care products, despite the public outcry against them.1-4
There are many kinds of parabens such as butylparaben, isobutylparaben, ethylparaben, methylparaben, propylparaben, isopropylparaben, and benzylparaben, each with its own characteristics.5 Parabens are considered ideal preservative ingredients because they exhibit a broad spectrum of antimicrobial activity, stability over a large pH and temperature range, have no odor, do not change color, and are water soluble enough to yield an effective concentration in a hydrophilic formulation.3 As the alkyl chain length of parabens increases, they become less water soluble and more oil soluble. Parabens penetrate the skin barrier in inverse relation to its ester chain length.6 Often, several parabens will be combined to take advantage of each paraben’s solubility characteristics.
Many patients avoid parabens because of “health risks.” Now other preservatives are being substituted for parabens, even though these ingredients may be less studied or even less safe than parabens. It is important not to lump all parabens together as they each have different characteristics. Methylparaben and propylparaben are the most commonly used parabens in skin care products.7 Combinations of parabens are notably more effective than the use of single parabens.3,8 High concentrations of any type of paraben can cause an irritant reaction on the skin, but those with longer ester chain lengths are more likely to cause irritation.
Methylparaben
The methyl ester of p-hydroxybenzoic acid is found in many skin care products. It is readily absorbed through the skin and gastrointestinal tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body. Studies have shown it is nontoxic, nonirritating, and nonsensitizing. It is not teratogenic, embryotoxic, or carcinogenic. Methylparaben, because of its shorter side chain groups and greater lipophilicity, has been shown to be more readily absorbed by the skin than other paraben chemicals.8,9 It is also on the low order of ingredients provoking acute and chronic toxicity.3
Propylparaben
Propylparaben is the ester form of p-hydroxybenzoic acid that has been esterified with n-propanol. It is the most commonly used antimicrobial preservative in foods, cosmetics, and drugs. It is readily absorbed through the skin and GI tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body.
Estrogenic activity of parabens
In a 2004 study, Darbre et al. reported on the discovery of parabens-like substances in breast tissue and published these findings in the Journal of Applied Toxicology.10 The media and public panicked, saying that parabens have estrogenic activity and can cause breast cancer. However, many studies have shown that certain parabens do not have estrogenic activity. Although some parabens have been shown to impart estrogenic effects in vitro, these are very weak. The four most commonly used parabens in cosmetic products are 10,000-fold or less potent than 17beta-estradiol.11 The potential to result in an adverse effect mediated via an estrogen mode of action has not been established in humans.6 Paraben exposure differs geographically. No correlation has been found between the amount of parabens in a geographic location and the incidence of breast cancer. Current scientific knowledge is insufficient to demonstrate a clear cancer risk caused by the topical application of cosmetics that contain parabens on normal intact skin.11
Parabens and contact dermatitis
Paraben compounds are capable of minimal penetrance through intact skin.12 When they are able to penetrate the skin – a capacity that varies among the class – parabens are rapidly metabolized to p-hydroxybenzoic acid and promptly excreted in the urine.3,11 Parabens for many years were thought to cause contact dermatitis, and there are many reports of this. However, the incidence is much lower than previously thought. In fact, parabens were named “Nonallergen of the Year in 2018” because of the low incidence of reactions in patch tests.13 Higher concentrations of parabens applied topically to skin – especially “nonintact” skin – have been shown to cause mild irritant reactions. It is likely that many of these reported cases of “contact dermatitis” were actually irritant dermatitis. Longstanding concerns about the allergenicity of parabens in relation to the skin have been rendered insignificant, as the wealth of evidence reveals little to no support for the cutaneous toxicity of these substances.11 Yim et al. add that parabens remain far less sensitizing than agents newly introduced for use in personal care products.4
Daily average exposure to parabens
It is estimated that parabens are found in 10% of personal care products. In most cases, these products contain 1% or less of parabens. If the average patient uses 50 g of personal care products a day, then the average daily exposure to parabens topically is 0.05 g. Parabens also are found in food and drugs, so the total paraben exposure per day is assumed to be about 1 mg/day. (See the 2002 Food and Chemical Toxicology article for details of how this was calculated.)7 When food, personal care products, and drug exposure rates are added, the average person is exposed to 1.29 mg/kg per day or 77.5 mg/day for a 60-kg individual. You can see that personal care products account for a fraction of exposure, as most paraben exposure comes from food.
Government opinion on the safety of parabens for the skin
Parabens long have been assessed as safe for use in cosmetic products in many countries. The European Commission stipulated a maximum concentration of 0.4% for each paraben and 0.8% for total mixture of paraben esters.4,6 While the Federal Food, Drug, and Cosmetic Act of 1938 prohibits the Food and Drug Administration from ruling on cosmetic ingredients, the industry-sponsored Cosmetic Ingredient Review expert panel has endorsed the European guidelines.4,6 Further, the North American Contact Dermatitis Group has pointed out that parabens continue to demonstrate the lowest prevalence of positivity (0.6%) of any major preservative available on the North American market, which includes over 10,000 cosmetic and personal care products, and remain one of the safest classes of preservatives for the skin.14 Further, the FDA has listed or classified parabens as generally regarded as safe.8
Safety of parabens
Parabens do not accumulate in tissues or organs for any appreciable length of time.6,8 In addition, carcinogenicity, cytotoxicity, or mutagenicity has not been proven in relation to parabens.8 Indeed, classical assays have shown no activity from parabens in terms of mutagenicity or carcinogenicity.11,15 Some estrogenic effects or activity that mimics estrogen have been associated with parabens in vitro, but this activity has been noted as very weak and there are no established reports of human cases in which parabens have elicited an estrogen-mediated adverse event.6,11
Concerns about a possible link between parabens and breast cancer have been largely diminished or relegated to the status of unknown and difficult to ascertain.13 Further, present knowledge provides no established link between the topical application of parabens-containing skin care formulations on healthy skin and cancer risk.10 Only intact skin should come in touch with products containing parabens to prevent irritant reactions.
Conclusion
Despite the fearful hype and reaction to one report 15 years ago, parabens continue to be safely used in numerous topical formulations. Their widespread use and lack of association with adverse events are a testament to their safety. From a dermatologic perspective, this nonallergen of the year deserves a better reputation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. “Goodman and Gilman’s The Pharmacological Basis of Therapeutics,” 6th ed. (New York: Macmillan, 1980, p. 969).
2. Toxicity: The Butyl, Ethyl, Methyl, and Propyl Esters have been found to promote allergic sensitization in humans, in “Dangerous Properties of Industrial Materials,” 4th ed. (New York: Van Nostrand Reinhold, 1975, p. 929).
3. Food Chem Toxicol. 2001 Jun;39(6):513-32.
4. Dermatitis. 2014 Sep-Oct;25(5):215-31.
5. Crit Rev Toxicol. 2005 Jun;35(5):435-58.
6. Int J Toxicol. 2008;27 Suppl 4:1-82.
7. Food Chem Toxicol. 2002 Oct;40(10):1335-73.
8. Dermatitis. 2019 Jan/Feb;30(1):3-31.
9. Exp Dermatol. 2007 Oct;16(10):830-6.
10. J Appl Toxicol. 2004 Jan-Feb;24(1):5-13.
11. Dermatitis. 2019 Jan/Feb;30(1):32-45.
12. Food Chem Toxicol. 2005 Feb;43(2):279-91.
13. Dermatitis. 2018 Dec 18. doi: 10.1097/DER.0000000000000429.
14. Dermatitis. 2018 Nov/Dec;29(6):297-309.
15. Food Chem Toxicol. 2005 Jul;43(7):985-1015.
Parabens were named nonallergen of the year! It is time that we help consumers understand that the substitutes for parabens are often worse than parabens, and parabens are not as sensitizing as we thought. Preservatives are essential parts of most cosmetics and cosmeceuticals. (I say “most” because many organic products do not have them and consequently have shorter shelf lives.) Without them, products are vulnerable to rapid decomposition and infiltration by bacteria, fungi, and molds. The preservatives that are used in the place of parabens often are sensitizers. What do we tell our patients about the safety of parabens with all of these conflicting reports? This column will focus on current thoughts regarding the safety of parabens used as preservatives. I would love to hear your thoughts.
Background
Parabens are alkyl esters of p-hydroxybenzoic acid and have been used as a class of preservatives since the late 1920s and early 1930s. Parabens are found naturally in raspberries, blackberries, carrots, and cucumbers and are common ingredients in food and pharmaceuticals. They are still widely used in skin, hair, and body care products, despite the public outcry against them.1-4
There are many kinds of parabens such as butylparaben, isobutylparaben, ethylparaben, methylparaben, propylparaben, isopropylparaben, and benzylparaben, each with its own characteristics.5 Parabens are considered ideal preservative ingredients because they exhibit a broad spectrum of antimicrobial activity, stability over a large pH and temperature range, have no odor, do not change color, and are water soluble enough to yield an effective concentration in a hydrophilic formulation.3 As the alkyl chain length of parabens increases, they become less water soluble and more oil soluble. Parabens penetrate the skin barrier in inverse relation to its ester chain length.6 Often, several parabens will be combined to take advantage of each paraben’s solubility characteristics.
Many patients avoid parabens because of “health risks.” Now other preservatives are being substituted for parabens, even though these ingredients may be less studied or even less safe than parabens. It is important not to lump all parabens together as they each have different characteristics. Methylparaben and propylparaben are the most commonly used parabens in skin care products.7 Combinations of parabens are notably more effective than the use of single parabens.3,8 High concentrations of any type of paraben can cause an irritant reaction on the skin, but those with longer ester chain lengths are more likely to cause irritation.
Methylparaben
The methyl ester of p-hydroxybenzoic acid is found in many skin care products. It is readily absorbed through the skin and gastrointestinal tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body. Studies have shown it is nontoxic, nonirritating, and nonsensitizing. It is not teratogenic, embryotoxic, or carcinogenic. Methylparaben, because of its shorter side chain groups and greater lipophilicity, has been shown to be more readily absorbed by the skin than other paraben chemicals.8,9 It is also on the low order of ingredients provoking acute and chronic toxicity.3
Propylparaben
Propylparaben is the ester form of p-hydroxybenzoic acid that has been esterified with n-propanol. It is the most commonly used antimicrobial preservative in foods, cosmetics, and drugs. It is readily absorbed through the skin and GI tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body.
Estrogenic activity of parabens
In a 2004 study, Darbre et al. reported on the discovery of parabens-like substances in breast tissue and published these findings in the Journal of Applied Toxicology.10 The media and public panicked, saying that parabens have estrogenic activity and can cause breast cancer. However, many studies have shown that certain parabens do not have estrogenic activity. Although some parabens have been shown to impart estrogenic effects in vitro, these are very weak. The four most commonly used parabens in cosmetic products are 10,000-fold or less potent than 17beta-estradiol.11 The potential to result in an adverse effect mediated via an estrogen mode of action has not been established in humans.6 Paraben exposure differs geographically. No correlation has been found between the amount of parabens in a geographic location and the incidence of breast cancer. Current scientific knowledge is insufficient to demonstrate a clear cancer risk caused by the topical application of cosmetics that contain parabens on normal intact skin.11
Parabens and contact dermatitis
Paraben compounds are capable of minimal penetrance through intact skin.12 When they are able to penetrate the skin – a capacity that varies among the class – parabens are rapidly metabolized to p-hydroxybenzoic acid and promptly excreted in the urine.3,11 Parabens for many years were thought to cause contact dermatitis, and there are many reports of this. However, the incidence is much lower than previously thought. In fact, parabens were named “Nonallergen of the Year in 2018” because of the low incidence of reactions in patch tests.13 Higher concentrations of parabens applied topically to skin – especially “nonintact” skin – have been shown to cause mild irritant reactions. It is likely that many of these reported cases of “contact dermatitis” were actually irritant dermatitis. Longstanding concerns about the allergenicity of parabens in relation to the skin have been rendered insignificant, as the wealth of evidence reveals little to no support for the cutaneous toxicity of these substances.11 Yim et al. add that parabens remain far less sensitizing than agents newly introduced for use in personal care products.4
Daily average exposure to parabens
It is estimated that parabens are found in 10% of personal care products. In most cases, these products contain 1% or less of parabens. If the average patient uses 50 g of personal care products a day, then the average daily exposure to parabens topically is 0.05 g. Parabens also are found in food and drugs, so the total paraben exposure per day is assumed to be about 1 mg/day. (See the 2002 Food and Chemical Toxicology article for details of how this was calculated.)7 When food, personal care products, and drug exposure rates are added, the average person is exposed to 1.29 mg/kg per day or 77.5 mg/day for a 60-kg individual. You can see that personal care products account for a fraction of exposure, as most paraben exposure comes from food.
Government opinion on the safety of parabens for the skin
Parabens long have been assessed as safe for use in cosmetic products in many countries. The European Commission stipulated a maximum concentration of 0.4% for each paraben and 0.8% for total mixture of paraben esters.4,6 While the Federal Food, Drug, and Cosmetic Act of 1938 prohibits the Food and Drug Administration from ruling on cosmetic ingredients, the industry-sponsored Cosmetic Ingredient Review expert panel has endorsed the European guidelines.4,6 Further, the North American Contact Dermatitis Group has pointed out that parabens continue to demonstrate the lowest prevalence of positivity (0.6%) of any major preservative available on the North American market, which includes over 10,000 cosmetic and personal care products, and remain one of the safest classes of preservatives for the skin.14 Further, the FDA has listed or classified parabens as generally regarded as safe.8
Safety of parabens
Parabens do not accumulate in tissues or organs for any appreciable length of time.6,8 In addition, carcinogenicity, cytotoxicity, or mutagenicity has not been proven in relation to parabens.8 Indeed, classical assays have shown no activity from parabens in terms of mutagenicity or carcinogenicity.11,15 Some estrogenic effects or activity that mimics estrogen have been associated with parabens in vitro, but this activity has been noted as very weak and there are no established reports of human cases in which parabens have elicited an estrogen-mediated adverse event.6,11
Concerns about a possible link between parabens and breast cancer have been largely diminished or relegated to the status of unknown and difficult to ascertain.13 Further, present knowledge provides no established link between the topical application of parabens-containing skin care formulations on healthy skin and cancer risk.10 Only intact skin should come in touch with products containing parabens to prevent irritant reactions.
Conclusion
Despite the fearful hype and reaction to one report 15 years ago, parabens continue to be safely used in numerous topical formulations. Their widespread use and lack of association with adverse events are a testament to their safety. From a dermatologic perspective, this nonallergen of the year deserves a better reputation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. “Goodman and Gilman’s The Pharmacological Basis of Therapeutics,” 6th ed. (New York: Macmillan, 1980, p. 969).
2. Toxicity: The Butyl, Ethyl, Methyl, and Propyl Esters have been found to promote allergic sensitization in humans, in “Dangerous Properties of Industrial Materials,” 4th ed. (New York: Van Nostrand Reinhold, 1975, p. 929).
3. Food Chem Toxicol. 2001 Jun;39(6):513-32.
4. Dermatitis. 2014 Sep-Oct;25(5):215-31.
5. Crit Rev Toxicol. 2005 Jun;35(5):435-58.
6. Int J Toxicol. 2008;27 Suppl 4:1-82.
7. Food Chem Toxicol. 2002 Oct;40(10):1335-73.
8. Dermatitis. 2019 Jan/Feb;30(1):3-31.
9. Exp Dermatol. 2007 Oct;16(10):830-6.
10. J Appl Toxicol. 2004 Jan-Feb;24(1):5-13.
11. Dermatitis. 2019 Jan/Feb;30(1):32-45.
12. Food Chem Toxicol. 2005 Feb;43(2):279-91.
13. Dermatitis. 2018 Dec 18. doi: 10.1097/DER.0000000000000429.
14. Dermatitis. 2018 Nov/Dec;29(6):297-309.
15. Food Chem Toxicol. 2005 Jul;43(7):985-1015.
Huntington’s symptom domains correlate with structural differences
Differences in the prominence of motor, cognitive, and psychiatric symptoms of Huntington’s disease among individuals can be attributed to differences in gray and white matter structural alterations, according to a neuroimaging study of 43 Huntington’s disease gene carriers conducted by Clara Garcia-Gorro, PhD, of the Bellvitge Institute for Biomedical Research and Bellvitge Hospital, Barcelona, and colleagues.
Their work detected a common neurobiological basis for the carriers’ cognitive and motor symptoms in patterns of reductions in gray matter, cortical thickness, and white matter integrity in cognitive and motor networks. They also found that depressive symptoms were associated with imaging findings primarily characterized by reduced cortical thickness in limbic and paralimbic regions.
“These results are relevant in the context of clinical trials, since they could be used to define specific biomarkers for each symptom profile, even before clinical signs appear. Having more homogeneous groups would potentially increase the likelihood of detecting successful interventions and help to find individualized treatments that target specific cognitive, motor, and psychiatric disturbances,” the authors concluded.
SOURCE: Garcia-Gorro C et al. Neuroimage Clin. 2019 Jun 15. doi: 10.1016/j.nicl.2019.101900.
Differences in the prominence of motor, cognitive, and psychiatric symptoms of Huntington’s disease among individuals can be attributed to differences in gray and white matter structural alterations, according to a neuroimaging study of 43 Huntington’s disease gene carriers conducted by Clara Garcia-Gorro, PhD, of the Bellvitge Institute for Biomedical Research and Bellvitge Hospital, Barcelona, and colleagues.
Their work detected a common neurobiological basis for the carriers’ cognitive and motor symptoms in patterns of reductions in gray matter, cortical thickness, and white matter integrity in cognitive and motor networks. They also found that depressive symptoms were associated with imaging findings primarily characterized by reduced cortical thickness in limbic and paralimbic regions.
“These results are relevant in the context of clinical trials, since they could be used to define specific biomarkers for each symptom profile, even before clinical signs appear. Having more homogeneous groups would potentially increase the likelihood of detecting successful interventions and help to find individualized treatments that target specific cognitive, motor, and psychiatric disturbances,” the authors concluded.
SOURCE: Garcia-Gorro C et al. Neuroimage Clin. 2019 Jun 15. doi: 10.1016/j.nicl.2019.101900.
Differences in the prominence of motor, cognitive, and psychiatric symptoms of Huntington’s disease among individuals can be attributed to differences in gray and white matter structural alterations, according to a neuroimaging study of 43 Huntington’s disease gene carriers conducted by Clara Garcia-Gorro, PhD, of the Bellvitge Institute for Biomedical Research and Bellvitge Hospital, Barcelona, and colleagues.
Their work detected a common neurobiological basis for the carriers’ cognitive and motor symptoms in patterns of reductions in gray matter, cortical thickness, and white matter integrity in cognitive and motor networks. They also found that depressive symptoms were associated with imaging findings primarily characterized by reduced cortical thickness in limbic and paralimbic regions.
“These results are relevant in the context of clinical trials, since they could be used to define specific biomarkers for each symptom profile, even before clinical signs appear. Having more homogeneous groups would potentially increase the likelihood of detecting successful interventions and help to find individualized treatments that target specific cognitive, motor, and psychiatric disturbances,” the authors concluded.
SOURCE: Garcia-Gorro C et al. Neuroimage Clin. 2019 Jun 15. doi: 10.1016/j.nicl.2019.101900.
FROM NEUROIMAGE: CLINICAL










