User login
<i>Mycobacterium abscessus</i> Infection Following Home Dermabrasion
Case Report
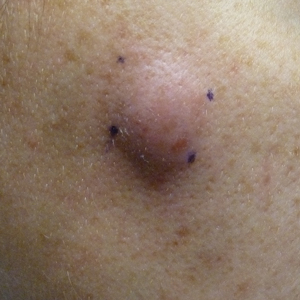
A 32-year-old woman presented to the dermatology clinic with a tender lump overlying the right maxilla of 6 weeks’ duration. The lesion developed acutely 1 to 2 months after the patient began using an at-home microdermabrasion device, which she routinely cleaned with tap water. The physical examination was notable for a 1.5-cm, soft, superficially indurated plaque on the right cheek without associated lymphadenopathy (Figure).
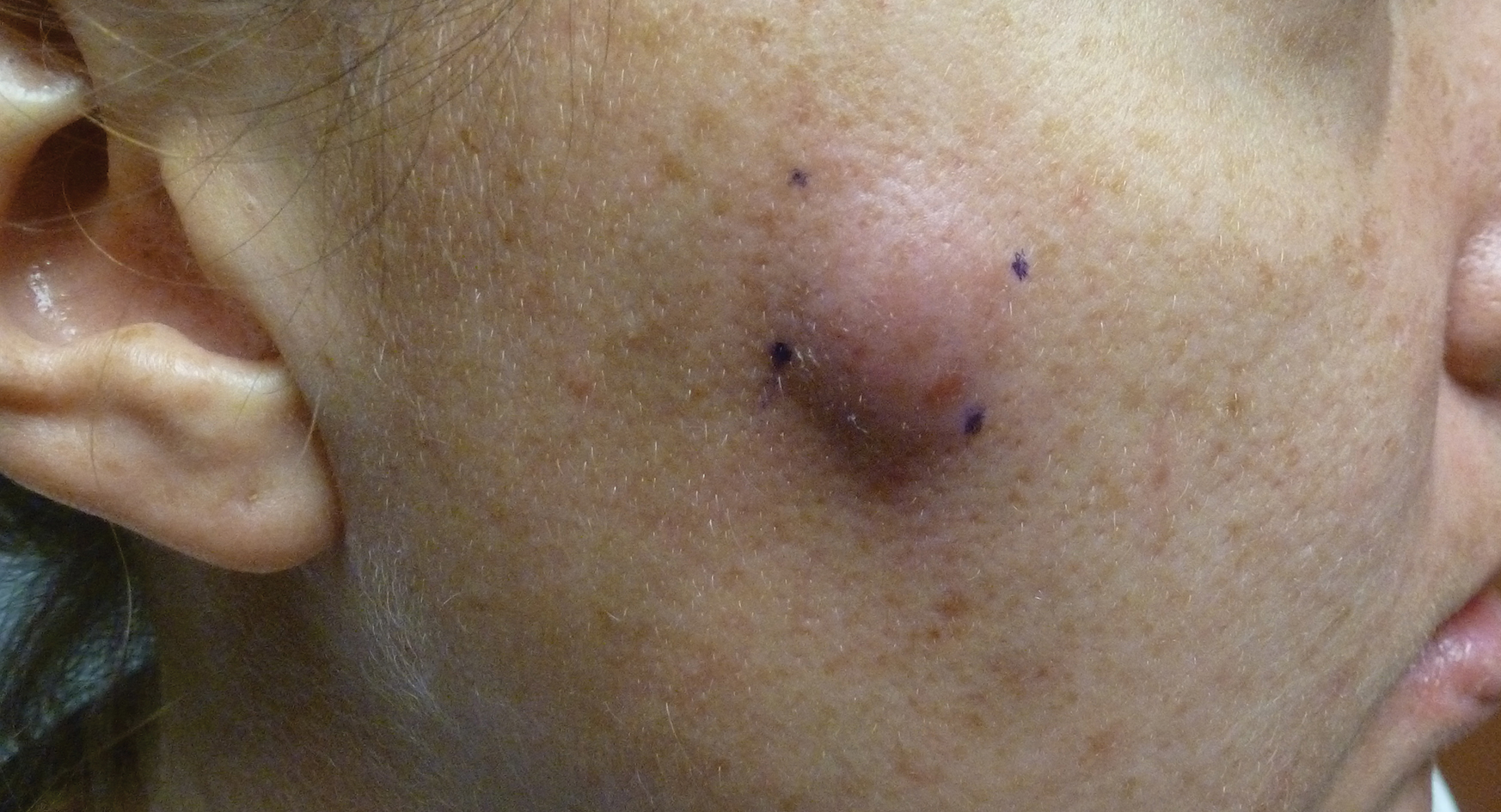
A punch biopsy revealed underlying necrotic fat. Computed tomography of the neck showed 20-mm skin thickening overlying the right zygomatic arch, with minimal adjacent subcutaneous soft tissue stranding and reactive lymph nodes. Further histologic examination of the biopsy specimen revealed inflamed granulation tissue with granulomatous inflammation.
Acid-fast bacterial culture was positive. Subsequent speciation revealed the causal agent to be multidrug-resistant Mycobacterium abscessus. The patient was initially treated with trimethoprim-sulfamethoxazole, which was switched to a combination of doxycycline and levofloxacin a few days later after initial culture returned. The following week, after the specific microorganism was confirmed with specific sensitivity, treatment was changed to intravenous (IV) tigecycline and amikacin. This regimen was continued for 2 more months through a peripherally inserted central catheter, then discontinued after complete resolution of the skin lesion.
Comment
Mycobacterial Infection
Nontuberculous mycobacteria were not identified as human pathogens until the 1950s. They are known to cause skin disease, lymphadenitis, skeletal infection, pulmonary disease, and disseminated infection, with pulmonary disease being the most common clinical form overall.1Mycobacterium abscessus is a member of a more specific group known as rapidly growing nontuberculous mycobacteria, which also includes Mycobacterium fortuitum and Mycobacterium chelonae.2 Commonly found in water, soil, and dust, M abscessus causes skin and soft tissue infection after skin injury by inoculation, minor trauma, or surgery.2-4 An increased rate of infections recently has been attributed to an increase in cosmetic procedures such as tattooing, liposuction, mesotherapy, pedicures, and body piercing. Mycobacterial infections transmitted through acupuncture also have been documented.5,6
Causes of Skin and Soft Tissue Infections
Skin and soft tissue infections due to rapidly growing mycobacteria often are associated with systemic comorbidities that cause immunosuppression and with immunosuppressive medications.7 Our patient did not have a preexisting comorbidity and did not take any long-term medication. When multiple lesions have been reported, patients were more likely to either have a systemic comorbidity or be taking immunosuppressive medication compared to patients with a single lesion. A history of penetrating trauma or an invasive surgical procedure has been reported more often in patients with a single lesion.7
Our patient had a solitary lesion on the face; improper sterile technique while using an at-home microdermabrasion device was thought to be the cause of infection. Although generally considered a minimally abrasive treatment modality, microdermabrasion caused enough trauma to create a nidus of infection in our patient.
Presentation
Cutaneous infection from rapidly growing mycobacteria can manifest as a nonhealing ulceration, subcutaneous abscess, draining sinus, or subcutaneous fluctuant or firm nodules. Erythema may be found in association with ulcers or chronic drainage from a surgical wound.2,7
Histopathologic appearance varies, depending on the evolution of the disease and host immunologic status. Tuberculoid, palisading, and sarcoidlike granulomas; a diffuse infiltrate of histiocytic foamy cells; acute and chronic panniculitis; nonspecific chronic inflammation; cutaneous abscess; suppurative granuloma; and necrotizing folliculitis all can be seen.8 Immunosuppressed patients are less likely to form granulomas.6 Diagnosis often is delayed because acid-fast bacterial culture is not typically performed on skin biopsy specimens or surgical wound infections.7 Fortunately, a high index of suspicion in our patient’s case allowed for prompt diagnosis and expeditious management.
Management
Mycobacterium abscessus tends to be resistant to conventional antituberculous medications; overall, it is considered a highly drug-resistant pathogen that is difficult to treat.9,10 Treatment usually requires 3 to 6 months of therapy, with oral clarithromycin considered the first-line agent for localized infection.5 Because cases of clarithromycin resistance have been reported in patients with M chelonae infection, caution is warranted when deciding between monotherapy or combination therapy.7 Multidrug resistance often necessitates prolonged IV therapy. Amikacin is the mostly commonly used IV agent for M abscessus infection. Adverse effects of treatment are common, often leading to a change in or discontinuation of therapy.11
Our patient was initially given trimethoprim-sulfamethoxazole before being switched to doxycycline and levofloxacin prior to final results of susceptibility testing. Ultimately, due to the multidrug-resistant nature of M abscessus, clarithromycin was not a viable option. Therefore, the patient was administered tigecycline and amikacin through a peripherally inserted central catheter until symptoms fully resolved.
Surgery can be an important adjunctive measure for certain patients, especially those with a single lesion.7 Our patient did well with medical treatment alone.
Conclusion
Given the difficulty of treating skin and soft tissue infections caused by M abscessus and related mycobacteria, it is worth noting that these infections are increasingly caused by procedures generally considered to be minimally invasive. Microdermabrasion—performed at home in an unsterile environment and not by a trained medical professional—was the causal procedure in this case. An important consideration is whether clinicians can be comfortable with the use of these treatments at home or whether they should be advising patients against at-home treatments that have potentially serious complications.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010;37:965-972.
- Fitzgerald DA, Smith AG, Lees A, et al. Cutaneous infection with Mycobacterium abscessus. Br J Dermatol. 1995;132:800-804.
- Moore M, Frerichs JB. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region; report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J Invest Dermatol. 1953;20:133-169.
- Inman PM, Beck A, Brown AE, et al. Outbreak of injection abscesses due to Mycobacterium abscessus. Arch Dermatol. 1969;100:141-147.
- Ryu HJ, Kim WJ, Oh CH, et al. Iatrogenic Mycobacterium abscessus infection associated with acupuncture: clinical manifestations and its treatment. Int J Dermatol. 2005;44:846-850.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
- Bartralot R, Pujol RM, García-Patos V, et al. Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases. comparative study between lesions observed in immunosuppressed patients and normal hosts. J Cutan Pathol. 2000;27:124-129.
- Morris-Jones R, Fletcher C, Morris-Jones S, et al. Mycobacterium abscessus: a cutaneous infection in a patient on renal replacement therapy. Clin Exp Dermatol. 2001;26:415-418.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Novosad SA, Beekmann SE, Polgreen PM, et al. Treatment of Mycobacterium abscessus infection. Emerg Infect Dis. 2016;22:511-514.
Case Report
A 32-year-old woman presented to the dermatology clinic with a tender lump overlying the right maxilla of 6 weeks’ duration. The lesion developed acutely 1 to 2 months after the patient began using an at-home microdermabrasion device, which she routinely cleaned with tap water. The physical examination was notable for a 1.5-cm, soft, superficially indurated plaque on the right cheek without associated lymphadenopathy (Figure).

A punch biopsy revealed underlying necrotic fat. Computed tomography of the neck showed 20-mm skin thickening overlying the right zygomatic arch, with minimal adjacent subcutaneous soft tissue stranding and reactive lymph nodes. Further histologic examination of the biopsy specimen revealed inflamed granulation tissue with granulomatous inflammation.
Acid-fast bacterial culture was positive. Subsequent speciation revealed the causal agent to be multidrug-resistant Mycobacterium abscessus. The patient was initially treated with trimethoprim-sulfamethoxazole, which was switched to a combination of doxycycline and levofloxacin a few days later after initial culture returned. The following week, after the specific microorganism was confirmed with specific sensitivity, treatment was changed to intravenous (IV) tigecycline and amikacin. This regimen was continued for 2 more months through a peripherally inserted central catheter, then discontinued after complete resolution of the skin lesion.
Comment
Mycobacterial Infection
Nontuberculous mycobacteria were not identified as human pathogens until the 1950s. They are known to cause skin disease, lymphadenitis, skeletal infection, pulmonary disease, and disseminated infection, with pulmonary disease being the most common clinical form overall.1Mycobacterium abscessus is a member of a more specific group known as rapidly growing nontuberculous mycobacteria, which also includes Mycobacterium fortuitum and Mycobacterium chelonae.2 Commonly found in water, soil, and dust, M abscessus causes skin and soft tissue infection after skin injury by inoculation, minor trauma, or surgery.2-4 An increased rate of infections recently has been attributed to an increase in cosmetic procedures such as tattooing, liposuction, mesotherapy, pedicures, and body piercing. Mycobacterial infections transmitted through acupuncture also have been documented.5,6
Causes of Skin and Soft Tissue Infections
Skin and soft tissue infections due to rapidly growing mycobacteria often are associated with systemic comorbidities that cause immunosuppression and with immunosuppressive medications.7 Our patient did not have a preexisting comorbidity and did not take any long-term medication. When multiple lesions have been reported, patients were more likely to either have a systemic comorbidity or be taking immunosuppressive medication compared to patients with a single lesion. A history of penetrating trauma or an invasive surgical procedure has been reported more often in patients with a single lesion.7
Our patient had a solitary lesion on the face; improper sterile technique while using an at-home microdermabrasion device was thought to be the cause of infection. Although generally considered a minimally abrasive treatment modality, microdermabrasion caused enough trauma to create a nidus of infection in our patient.
Presentation
Cutaneous infection from rapidly growing mycobacteria can manifest as a nonhealing ulceration, subcutaneous abscess, draining sinus, or subcutaneous fluctuant or firm nodules. Erythema may be found in association with ulcers or chronic drainage from a surgical wound.2,7
Histopathologic appearance varies, depending on the evolution of the disease and host immunologic status. Tuberculoid, palisading, and sarcoidlike granulomas; a diffuse infiltrate of histiocytic foamy cells; acute and chronic panniculitis; nonspecific chronic inflammation; cutaneous abscess; suppurative granuloma; and necrotizing folliculitis all can be seen.8 Immunosuppressed patients are less likely to form granulomas.6 Diagnosis often is delayed because acid-fast bacterial culture is not typically performed on skin biopsy specimens or surgical wound infections.7 Fortunately, a high index of suspicion in our patient’s case allowed for prompt diagnosis and expeditious management.
Management
Mycobacterium abscessus tends to be resistant to conventional antituberculous medications; overall, it is considered a highly drug-resistant pathogen that is difficult to treat.9,10 Treatment usually requires 3 to 6 months of therapy, with oral clarithromycin considered the first-line agent for localized infection.5 Because cases of clarithromycin resistance have been reported in patients with M chelonae infection, caution is warranted when deciding between monotherapy or combination therapy.7 Multidrug resistance often necessitates prolonged IV therapy. Amikacin is the mostly commonly used IV agent for M abscessus infection. Adverse effects of treatment are common, often leading to a change in or discontinuation of therapy.11
Our patient was initially given trimethoprim-sulfamethoxazole before being switched to doxycycline and levofloxacin prior to final results of susceptibility testing. Ultimately, due to the multidrug-resistant nature of M abscessus, clarithromycin was not a viable option. Therefore, the patient was administered tigecycline and amikacin through a peripherally inserted central catheter until symptoms fully resolved.
Surgery can be an important adjunctive measure for certain patients, especially those with a single lesion.7 Our patient did well with medical treatment alone.
Conclusion
Given the difficulty of treating skin and soft tissue infections caused by M abscessus and related mycobacteria, it is worth noting that these infections are increasingly caused by procedures generally considered to be minimally invasive. Microdermabrasion—performed at home in an unsterile environment and not by a trained medical professional—was the causal procedure in this case. An important consideration is whether clinicians can be comfortable with the use of these treatments at home or whether they should be advising patients against at-home treatments that have potentially serious complications.
Case Report
A 32-year-old woman presented to the dermatology clinic with a tender lump overlying the right maxilla of 6 weeks’ duration. The lesion developed acutely 1 to 2 months after the patient began using an at-home microdermabrasion device, which she routinely cleaned with tap water. The physical examination was notable for a 1.5-cm, soft, superficially indurated plaque on the right cheek without associated lymphadenopathy (Figure).

A punch biopsy revealed underlying necrotic fat. Computed tomography of the neck showed 20-mm skin thickening overlying the right zygomatic arch, with minimal adjacent subcutaneous soft tissue stranding and reactive lymph nodes. Further histologic examination of the biopsy specimen revealed inflamed granulation tissue with granulomatous inflammation.
Acid-fast bacterial culture was positive. Subsequent speciation revealed the causal agent to be multidrug-resistant Mycobacterium abscessus. The patient was initially treated with trimethoprim-sulfamethoxazole, which was switched to a combination of doxycycline and levofloxacin a few days later after initial culture returned. The following week, after the specific microorganism was confirmed with specific sensitivity, treatment was changed to intravenous (IV) tigecycline and amikacin. This regimen was continued for 2 more months through a peripherally inserted central catheter, then discontinued after complete resolution of the skin lesion.
Comment
Mycobacterial Infection
Nontuberculous mycobacteria were not identified as human pathogens until the 1950s. They are known to cause skin disease, lymphadenitis, skeletal infection, pulmonary disease, and disseminated infection, with pulmonary disease being the most common clinical form overall.1Mycobacterium abscessus is a member of a more specific group known as rapidly growing nontuberculous mycobacteria, which also includes Mycobacterium fortuitum and Mycobacterium chelonae.2 Commonly found in water, soil, and dust, M abscessus causes skin and soft tissue infection after skin injury by inoculation, minor trauma, or surgery.2-4 An increased rate of infections recently has been attributed to an increase in cosmetic procedures such as tattooing, liposuction, mesotherapy, pedicures, and body piercing. Mycobacterial infections transmitted through acupuncture also have been documented.5,6
Causes of Skin and Soft Tissue Infections
Skin and soft tissue infections due to rapidly growing mycobacteria often are associated with systemic comorbidities that cause immunosuppression and with immunosuppressive medications.7 Our patient did not have a preexisting comorbidity and did not take any long-term medication. When multiple lesions have been reported, patients were more likely to either have a systemic comorbidity or be taking immunosuppressive medication compared to patients with a single lesion. A history of penetrating trauma or an invasive surgical procedure has been reported more often in patients with a single lesion.7
Our patient had a solitary lesion on the face; improper sterile technique while using an at-home microdermabrasion device was thought to be the cause of infection. Although generally considered a minimally abrasive treatment modality, microdermabrasion caused enough trauma to create a nidus of infection in our patient.
Presentation
Cutaneous infection from rapidly growing mycobacteria can manifest as a nonhealing ulceration, subcutaneous abscess, draining sinus, or subcutaneous fluctuant or firm nodules. Erythema may be found in association with ulcers or chronic drainage from a surgical wound.2,7
Histopathologic appearance varies, depending on the evolution of the disease and host immunologic status. Tuberculoid, palisading, and sarcoidlike granulomas; a diffuse infiltrate of histiocytic foamy cells; acute and chronic panniculitis; nonspecific chronic inflammation; cutaneous abscess; suppurative granuloma; and necrotizing folliculitis all can be seen.8 Immunosuppressed patients are less likely to form granulomas.6 Diagnosis often is delayed because acid-fast bacterial culture is not typically performed on skin biopsy specimens or surgical wound infections.7 Fortunately, a high index of suspicion in our patient’s case allowed for prompt diagnosis and expeditious management.
Management
Mycobacterium abscessus tends to be resistant to conventional antituberculous medications; overall, it is considered a highly drug-resistant pathogen that is difficult to treat.9,10 Treatment usually requires 3 to 6 months of therapy, with oral clarithromycin considered the first-line agent for localized infection.5 Because cases of clarithromycin resistance have been reported in patients with M chelonae infection, caution is warranted when deciding between monotherapy or combination therapy.7 Multidrug resistance often necessitates prolonged IV therapy. Amikacin is the mostly commonly used IV agent for M abscessus infection. Adverse effects of treatment are common, often leading to a change in or discontinuation of therapy.11
Our patient was initially given trimethoprim-sulfamethoxazole before being switched to doxycycline and levofloxacin prior to final results of susceptibility testing. Ultimately, due to the multidrug-resistant nature of M abscessus, clarithromycin was not a viable option. Therefore, the patient was administered tigecycline and amikacin through a peripherally inserted central catheter until symptoms fully resolved.
Surgery can be an important adjunctive measure for certain patients, especially those with a single lesion.7 Our patient did well with medical treatment alone.
Conclusion
Given the difficulty of treating skin and soft tissue infections caused by M abscessus and related mycobacteria, it is worth noting that these infections are increasingly caused by procedures generally considered to be minimally invasive. Microdermabrasion—performed at home in an unsterile environment and not by a trained medical professional—was the causal procedure in this case. An important consideration is whether clinicians can be comfortable with the use of these treatments at home or whether they should be advising patients against at-home treatments that have potentially serious complications.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010;37:965-972.
- Fitzgerald DA, Smith AG, Lees A, et al. Cutaneous infection with Mycobacterium abscessus. Br J Dermatol. 1995;132:800-804.
- Moore M, Frerichs JB. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region; report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J Invest Dermatol. 1953;20:133-169.
- Inman PM, Beck A, Brown AE, et al. Outbreak of injection abscesses due to Mycobacterium abscessus. Arch Dermatol. 1969;100:141-147.
- Ryu HJ, Kim WJ, Oh CH, et al. Iatrogenic Mycobacterium abscessus infection associated with acupuncture: clinical manifestations and its treatment. Int J Dermatol. 2005;44:846-850.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
- Bartralot R, Pujol RM, García-Patos V, et al. Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases. comparative study between lesions observed in immunosuppressed patients and normal hosts. J Cutan Pathol. 2000;27:124-129.
- Morris-Jones R, Fletcher C, Morris-Jones S, et al. Mycobacterium abscessus: a cutaneous infection in a patient on renal replacement therapy. Clin Exp Dermatol. 2001;26:415-418.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Novosad SA, Beekmann SE, Polgreen PM, et al. Treatment of Mycobacterium abscessus infection. Emerg Infect Dis. 2016;22:511-514.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010;37:965-972.
- Fitzgerald DA, Smith AG, Lees A, et al. Cutaneous infection with Mycobacterium abscessus. Br J Dermatol. 1995;132:800-804.
- Moore M, Frerichs JB. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region; report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J Invest Dermatol. 1953;20:133-169.
- Inman PM, Beck A, Brown AE, et al. Outbreak of injection abscesses due to Mycobacterium abscessus. Arch Dermatol. 1969;100:141-147.
- Ryu HJ, Kim WJ, Oh CH, et al. Iatrogenic Mycobacterium abscessus infection associated with acupuncture: clinical manifestations and its treatment. Int J Dermatol. 2005;44:846-850.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
- Bartralot R, Pujol RM, García-Patos V, et al. Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases. comparative study between lesions observed in immunosuppressed patients and normal hosts. J Cutan Pathol. 2000;27:124-129.
- Morris-Jones R, Fletcher C, Morris-Jones S, et al. Mycobacterium abscessus: a cutaneous infection in a patient on renal replacement therapy. Clin Exp Dermatol. 2001;26:415-418.
- Jeong SH, Kim SY, Huh HJ, et al. Mycobacteriological characteristics and treatment outcomes in extrapulmonary Mycobacterium abscessus complex infections. Int J Infect Dis. 2017;60:49-56.
- Novosad SA, Beekmann SE, Polgreen PM, et al. Treatment of Mycobacterium abscessus infection. Emerg Infect Dis. 2016;22:511-514.
Practice Points
- Atypical mycobacteria are included in the differential for cutaneous abscesses.
- At-home cosmetic treatments often carry unrecognized risks for adverse events.
- Obtain culture prior to initiation of empiric antibiotics.
Update on Rosacea Classification and Its Controversies
Rosacea is an inflammatory skin condition that affects approximately 5% of the adult population, with the highest prevalence in Europe and North America.1 Despite its prevalence, rosacea remains poorly understood from a pathophysiologic perspective, with no diagnostic laboratory markers.2 Because diagnosis relies on clinical judgment, the nomenclature for describing and characterizing rosacea becomes paramount in ensuring that patients are given an accurate diagnosis and subsequent treatment. We review the shortfalls in the recent history of rosacea classification and discuss their implications.
Subtype to Phenotype Classification
In 2002, the National Rosacea Society (NRS) Expert Committee published a standardized classification schema for rosacea (Table).3 The authors described primary and secondary diagnostic criteria. The presence of 1 or more primary features in a central facial distribution was indicative of rosacea. Primary characteristics included flushing (transient erythema), nontransient erythema, papules and pustules, and telangiectasia. Secondary features, which could occur with or independently of primary features, included burning or stinging of the face, dry appearance, facial edema, ocular manifestations, peripheral (nonfacial) occurrence, phymatous changes, and red facial plaques. Whereas these features often present simultaneously in a characteristic pattern, they were grouped into 4 main subtypes—erythematotelangiectatic (ETR), papulopustular, phymatous, and ocular—and 1 variant, granulomatous rosacea.3
To enhance clinical and research applications of this categorization system as well as offer further standardization, the NRS released a supplementary clinical grading scorecard in 2004 in which each of the primary and secondary characteristics could be assigned a subjective severity score of absent, mild, moderate, or severe. The goal was that the subtype classification and clinical grading system, when used in conjunction with each other, would establish a common language for patients, clinicians, and researchers to describe and further investigate rosacea.4
The 2002 categorization system was certainly an impactful first step in the organization of rosacea. It was not without its critics, however, namely rosacea-oriented dermatologists who were concerned about its lack of specificity.5-7 For instance, the NRS Expert Committee did not address the time frame for flushing, which typically has a long duration in rosacea patients, or for the nontransient erythema; telangiectasia secondary to heliodermatitis; or the often-observed periocular sparing. Additionally, the schema did not account for conditions such as gram-negative folliculitis (pustules characteristically located on the central face) or discuss the need to rule out carcinoid, mastocytosis, or connective-tissue disease, which can lead to nontransient facial erythema. Without strict definitions and exclusions, nonrosacea disorders could be incorrectly labeled as rosacea.
Beyond the lack of specificity, there was additional concern if a subtype system was the ideal way to capture disease presentation and severity. By subtyping, there was unnecessary division of interrelated disease into individual disorders; an individual’s clinical presentation might fall along a spectrum rather than within a discrete box.8
Furthermore, from a research standpoint, subtyping rosacea could hinder or confuse epidemiologic studies. For instance, if patients present with phenotypes from different subtypes, into which subtype would they fall?8-10
The global ROSacea COnsensus (ROSCO) panel, comprising 17 international dermatologists and ophthalmologists, convened in 2016 to address this matter. The panel proposed a new system (published in 2017) based on individual phenotypes.9 In this new system, diagnostic features include persistent centrofacial erythema with periods of increased intensity and phymatous changes. Major features, which are diagnostic when there are at least 2, include flushing (transient erythema), inflammatory papules and pustules, centrofacial telangiectasia, and ocular manifestations. Each feature could then be graded on a severity spectrum independent of concurrent phenotypes (Table).8
The panel concluded that this system would provide a stronger foundation for standardization as new knowledge of rosacea continues to be elucidated.8 In support of their argument, ROSCO also released a treatment algorithm that depended on a phenotype scheme.11 The panel emphasized that by focusing on individual lesions rather than a subtype encompassing many characteristics, treatment could be tailored to the patient. Using this à-la-carte therapy option, physicians could choose those rosacea aspects that are particularly concerning to the patient and manage only those aspects or overlap treatments to improve multiple aspects.11
In 2017, 15 years after the original classification system was proposed, the NRS updated their classification system (published in 2018), taking into consideration some of the criticisms as well as new scientific data on rosacea. Similar to the schema proposed by ROSCO, this system was based on phenotype. Inclusion and exclusion criteria were more robust in this update compared to the original classification in 2002. The criteria provide a timeline for transient flushing—it must occur within seconds or minutes in response to a neurovascular stimulant—and state that it is characteristically prolonged (Table).12
However, the Expert Committee still did not define either the length of time of flushing or nontransient erythema. It also did not specify convex surfaces of the face with periocular sparing as the characteristic pattern or provide additional information on how photoaging fits into the definition. The updated classification stated that centrofacial erythema must not be from cutaneous lupus or seborrheic eczema, and steroid-induced rosacea was still excluded.12 However, there is still the need to exclude other systemic conditions, such as mastocytosis, carcinoid, polycythemia vera, and dermatomyositis. Therefore, the potential for subjective error and inclusion of nonrosacea diseases persists.
A critical change was elimination of the granulomatous rosacea variant. In 2002, this variant was defined by monomorphic, yellow-brown to red papules and nodules that led to scarring. This variant, however, did not share the commonalities of the other subtypes, including persistent facial erythema, limitation to convex surfaces, periocular sparing, and transient flushing.3,13 At the time, Crawford et al6 proposed that the variant be recategorized as granulomatous facial dermatitis. In the updated NRS classification, this variant and phenotypic description was eliminated from the schema.12 It is unclear if it was removed because of these discrepancies or if the NRS panel felt it had a distinct pathogenesis from the proposed rosacea pathophysiology; however, we applaud this change.
Subtype Progression
Both the ROSCO and NRS classification schemes mention progression between the various phenotypes,10,12 suggesting that rosacea phenotypes exist along a continuum, progressing and regressing with disease severity. The main study addressing this point was based on the self-reported retrospective patient memory of disease features in rosacea patients. The authors used a modified criterion of centrofacial erythema alone to define ETR; therefore, a person who began their disease with this finding but then acquired inflammatory lesions or phymas was defined as progressing along a spectrum.14 Given that persistent erythema of convex surfaces of the face is common in all subtypes, we do not find it surprising that the authors found (using their modified criteria) that ETR appeared to progress to papulopustular and phymatous subtypes in a small number of patients. We strongly disagree with their interpretation and conclusion.
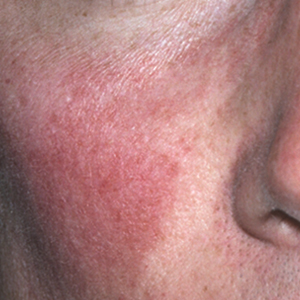
In our experience, ETR patients have fine textured skin without sebaceous quality or a history of extensive acne (Figure 1). Flushing is common and usually lasts 10 minutes to 1 hour. There might be concurrent burning or stinging; however, there is no associated sweating, lightheadedness, palpitations, or diagnostic laboratory findings, which distinguishes ETR from other common causes of flushing. The persistent centrofacial erythema involves convex surfaces, spares periocular skin, and can be best defined as present for longer than 3 months.
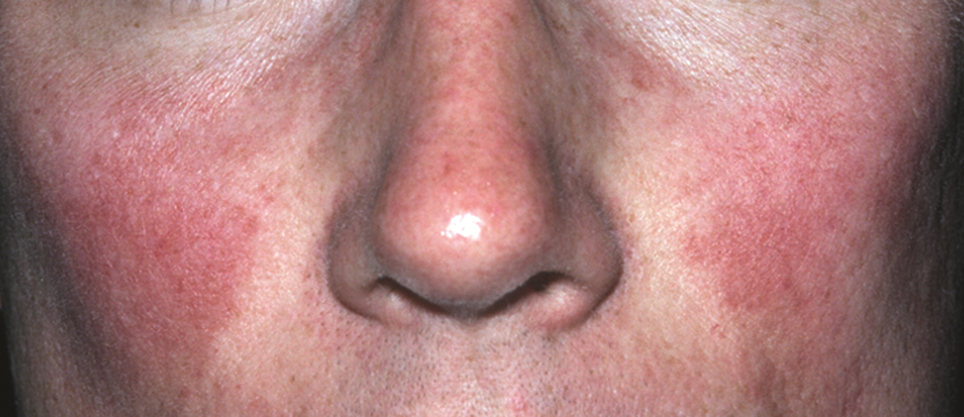
In contrast, phymas occur commonly in patients with thick and sebaceous (glandular) skin (Figure 2).6,15-17 Men are most often affected and usually have a history of moderate to severe acne. It is not uncommon to observe nodules, cysts, and scarring in addition to papules and pustules. These eruptions primarily cluster on the central face and present in areas of nontransient erythema. Flushing, although less prominent than in other phenotypes, also can be seen.
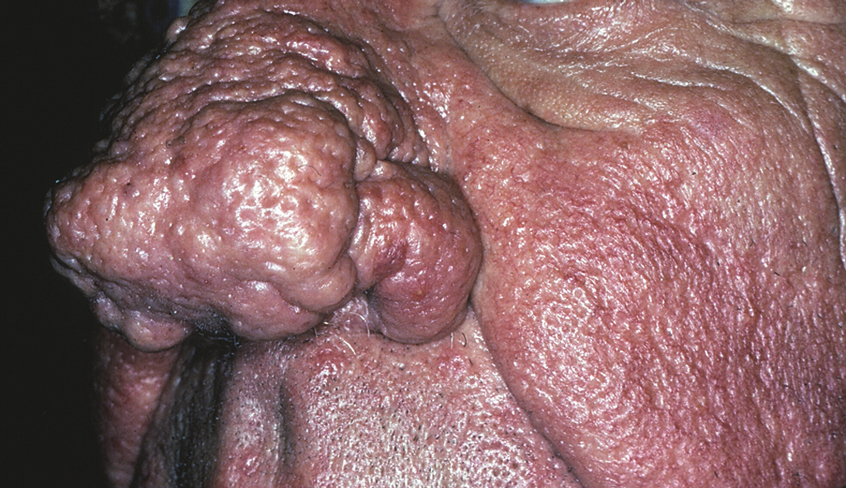
Taken together, we find no convincing evidence from published studies or extensive experience caring for rosacea patients that classic ETR progresses to phymatous rosacea, or the other way around, as displayed in the ROSCO panel report.8 The type of skin seen in Figure 1 will not “progress” to the type seen in Figure 2. Furthermore, treatment will not “reverse” the phymatous skin into thin, ETR-type skin. The implications are important: If a female patient is given a diagnosis of ETR, she will not develop an enlarged phymatous nose. Patients with thick sebaceous skin, as in Figure 2, usually tolerate treatments such as benzoyl peroxide that other rosacea patients do not and frequently respond well to such intervention.
Implications and Future Directions
We present an overview of 2 rosacea classification systems, hoping to stimulate further refinement. Looking forward, there are many directions for further investigation into the pathophysiology of rosacea. From a genetic standpoint, there needs to be continued molecular and epidemiologic data to determine the underlying genetic contributions to disease.
There has been some progress in the realm of understanding the mechanisms of inflammation; we urge further investigation to elucidate how “subclinical neuroinflammation” might lead to glandular hyperplasia.12 We also see value in examining the genetic and hormonal contributions to phymas, as they may be different than those seen in the ETR-type patients. Last, more studies focusing on comorbidities that contribute to or arise from rosacea are welcomed.
The ultimate goal is to develop a classification system that integrates clinical descriptions, pathophysiologic mechanisms, and benchmark indicators of disease. Only then can we have a true gold standard for the diagnosis of rosacea, one that allows for improved personalized treatment and better outcomes.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282-289.
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Saleem MD. Revisiting rosacea criteria: where have we been, where are we going, and how will we get there? Dermatol Clin. 2018;36:161-165.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Tan J, Almeida LMC, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438.
- Wilkin J. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes. Br J Dermatol. 2017;177:597-598.
- Tan J; ROSCO coauthors. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes: reply from the author. Br J Dermatol. 2017;177:598-599.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33:447-455.
- Tan J, Blume‐Peytavi U, Ortonne JP, et al. An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169:555-562.
- James WD, Elston D, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. New York, NY: Elsevier; 2019.
- Reinholz M, Tietze JK, Kilian K, et al. Rosacea - S1 guideline. J Dtsch Dermatol Ges. 2013;11:768-780.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom‐oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
Rosacea is an inflammatory skin condition that affects approximately 5% of the adult population, with the highest prevalence in Europe and North America.1 Despite its prevalence, rosacea remains poorly understood from a pathophysiologic perspective, with no diagnostic laboratory markers.2 Because diagnosis relies on clinical judgment, the nomenclature for describing and characterizing rosacea becomes paramount in ensuring that patients are given an accurate diagnosis and subsequent treatment. We review the shortfalls in the recent history of rosacea classification and discuss their implications.
Subtype to Phenotype Classification
In 2002, the National Rosacea Society (NRS) Expert Committee published a standardized classification schema for rosacea (Table).3 The authors described primary and secondary diagnostic criteria. The presence of 1 or more primary features in a central facial distribution was indicative of rosacea. Primary characteristics included flushing (transient erythema), nontransient erythema, papules and pustules, and telangiectasia. Secondary features, which could occur with or independently of primary features, included burning or stinging of the face, dry appearance, facial edema, ocular manifestations, peripheral (nonfacial) occurrence, phymatous changes, and red facial plaques. Whereas these features often present simultaneously in a characteristic pattern, they were grouped into 4 main subtypes—erythematotelangiectatic (ETR), papulopustular, phymatous, and ocular—and 1 variant, granulomatous rosacea.3
To enhance clinical and research applications of this categorization system as well as offer further standardization, the NRS released a supplementary clinical grading scorecard in 2004 in which each of the primary and secondary characteristics could be assigned a subjective severity score of absent, mild, moderate, or severe. The goal was that the subtype classification and clinical grading system, when used in conjunction with each other, would establish a common language for patients, clinicians, and researchers to describe and further investigate rosacea.4
The 2002 categorization system was certainly an impactful first step in the organization of rosacea. It was not without its critics, however, namely rosacea-oriented dermatologists who were concerned about its lack of specificity.5-7 For instance, the NRS Expert Committee did not address the time frame for flushing, which typically has a long duration in rosacea patients, or for the nontransient erythema; telangiectasia secondary to heliodermatitis; or the often-observed periocular sparing. Additionally, the schema did not account for conditions such as gram-negative folliculitis (pustules characteristically located on the central face) or discuss the need to rule out carcinoid, mastocytosis, or connective-tissue disease, which can lead to nontransient facial erythema. Without strict definitions and exclusions, nonrosacea disorders could be incorrectly labeled as rosacea.
Beyond the lack of specificity, there was additional concern if a subtype system was the ideal way to capture disease presentation and severity. By subtyping, there was unnecessary division of interrelated disease into individual disorders; an individual’s clinical presentation might fall along a spectrum rather than within a discrete box.8
Furthermore, from a research standpoint, subtyping rosacea could hinder or confuse epidemiologic studies. For instance, if patients present with phenotypes from different subtypes, into which subtype would they fall?8-10
The global ROSacea COnsensus (ROSCO) panel, comprising 17 international dermatologists and ophthalmologists, convened in 2016 to address this matter. The panel proposed a new system (published in 2017) based on individual phenotypes.9 In this new system, diagnostic features include persistent centrofacial erythema with periods of increased intensity and phymatous changes. Major features, which are diagnostic when there are at least 2, include flushing (transient erythema), inflammatory papules and pustules, centrofacial telangiectasia, and ocular manifestations. Each feature could then be graded on a severity spectrum independent of concurrent phenotypes (Table).8
The panel concluded that this system would provide a stronger foundation for standardization as new knowledge of rosacea continues to be elucidated.8 In support of their argument, ROSCO also released a treatment algorithm that depended on a phenotype scheme.11 The panel emphasized that by focusing on individual lesions rather than a subtype encompassing many characteristics, treatment could be tailored to the patient. Using this à-la-carte therapy option, physicians could choose those rosacea aspects that are particularly concerning to the patient and manage only those aspects or overlap treatments to improve multiple aspects.11
In 2017, 15 years after the original classification system was proposed, the NRS updated their classification system (published in 2018), taking into consideration some of the criticisms as well as new scientific data on rosacea. Similar to the schema proposed by ROSCO, this system was based on phenotype. Inclusion and exclusion criteria were more robust in this update compared to the original classification in 2002. The criteria provide a timeline for transient flushing—it must occur within seconds or minutes in response to a neurovascular stimulant—and state that it is characteristically prolonged (Table).12
However, the Expert Committee still did not define either the length of time of flushing or nontransient erythema. It also did not specify convex surfaces of the face with periocular sparing as the characteristic pattern or provide additional information on how photoaging fits into the definition. The updated classification stated that centrofacial erythema must not be from cutaneous lupus or seborrheic eczema, and steroid-induced rosacea was still excluded.12 However, there is still the need to exclude other systemic conditions, such as mastocytosis, carcinoid, polycythemia vera, and dermatomyositis. Therefore, the potential for subjective error and inclusion of nonrosacea diseases persists.
A critical change was elimination of the granulomatous rosacea variant. In 2002, this variant was defined by monomorphic, yellow-brown to red papules and nodules that led to scarring. This variant, however, did not share the commonalities of the other subtypes, including persistent facial erythema, limitation to convex surfaces, periocular sparing, and transient flushing.3,13 At the time, Crawford et al6 proposed that the variant be recategorized as granulomatous facial dermatitis. In the updated NRS classification, this variant and phenotypic description was eliminated from the schema.12 It is unclear if it was removed because of these discrepancies or if the NRS panel felt it had a distinct pathogenesis from the proposed rosacea pathophysiology; however, we applaud this change.
Subtype Progression
Both the ROSCO and NRS classification schemes mention progression between the various phenotypes,10,12 suggesting that rosacea phenotypes exist along a continuum, progressing and regressing with disease severity. The main study addressing this point was based on the self-reported retrospective patient memory of disease features in rosacea patients. The authors used a modified criterion of centrofacial erythema alone to define ETR; therefore, a person who began their disease with this finding but then acquired inflammatory lesions or phymas was defined as progressing along a spectrum.14 Given that persistent erythema of convex surfaces of the face is common in all subtypes, we do not find it surprising that the authors found (using their modified criteria) that ETR appeared to progress to papulopustular and phymatous subtypes in a small number of patients. We strongly disagree with their interpretation and conclusion.
In our experience, ETR patients have fine textured skin without sebaceous quality or a history of extensive acne (Figure 1). Flushing is common and usually lasts 10 minutes to 1 hour. There might be concurrent burning or stinging; however, there is no associated sweating, lightheadedness, palpitations, or diagnostic laboratory findings, which distinguishes ETR from other common causes of flushing. The persistent centrofacial erythema involves convex surfaces, spares periocular skin, and can be best defined as present for longer than 3 months.

In contrast, phymas occur commonly in patients with thick and sebaceous (glandular) skin (Figure 2).6,15-17 Men are most often affected and usually have a history of moderate to severe acne. It is not uncommon to observe nodules, cysts, and scarring in addition to papules and pustules. These eruptions primarily cluster on the central face and present in areas of nontransient erythema. Flushing, although less prominent than in other phenotypes, also can be seen.

Taken together, we find no convincing evidence from published studies or extensive experience caring for rosacea patients that classic ETR progresses to phymatous rosacea, or the other way around, as displayed in the ROSCO panel report.8 The type of skin seen in Figure 1 will not “progress” to the type seen in Figure 2. Furthermore, treatment will not “reverse” the phymatous skin into thin, ETR-type skin. The implications are important: If a female patient is given a diagnosis of ETR, she will not develop an enlarged phymatous nose. Patients with thick sebaceous skin, as in Figure 2, usually tolerate treatments such as benzoyl peroxide that other rosacea patients do not and frequently respond well to such intervention.
Implications and Future Directions
We present an overview of 2 rosacea classification systems, hoping to stimulate further refinement. Looking forward, there are many directions for further investigation into the pathophysiology of rosacea. From a genetic standpoint, there needs to be continued molecular and epidemiologic data to determine the underlying genetic contributions to disease.
There has been some progress in the realm of understanding the mechanisms of inflammation; we urge further investigation to elucidate how “subclinical neuroinflammation” might lead to glandular hyperplasia.12 We also see value in examining the genetic and hormonal contributions to phymas, as they may be different than those seen in the ETR-type patients. Last, more studies focusing on comorbidities that contribute to or arise from rosacea are welcomed.
The ultimate goal is to develop a classification system that integrates clinical descriptions, pathophysiologic mechanisms, and benchmark indicators of disease. Only then can we have a true gold standard for the diagnosis of rosacea, one that allows for improved personalized treatment and better outcomes.
Rosacea is an inflammatory skin condition that affects approximately 5% of the adult population, with the highest prevalence in Europe and North America.1 Despite its prevalence, rosacea remains poorly understood from a pathophysiologic perspective, with no diagnostic laboratory markers.2 Because diagnosis relies on clinical judgment, the nomenclature for describing and characterizing rosacea becomes paramount in ensuring that patients are given an accurate diagnosis and subsequent treatment. We review the shortfalls in the recent history of rosacea classification and discuss their implications.
Subtype to Phenotype Classification
In 2002, the National Rosacea Society (NRS) Expert Committee published a standardized classification schema for rosacea (Table).3 The authors described primary and secondary diagnostic criteria. The presence of 1 or more primary features in a central facial distribution was indicative of rosacea. Primary characteristics included flushing (transient erythema), nontransient erythema, papules and pustules, and telangiectasia. Secondary features, which could occur with or independently of primary features, included burning or stinging of the face, dry appearance, facial edema, ocular manifestations, peripheral (nonfacial) occurrence, phymatous changes, and red facial plaques. Whereas these features often present simultaneously in a characteristic pattern, they were grouped into 4 main subtypes—erythematotelangiectatic (ETR), papulopustular, phymatous, and ocular—and 1 variant, granulomatous rosacea.3
To enhance clinical and research applications of this categorization system as well as offer further standardization, the NRS released a supplementary clinical grading scorecard in 2004 in which each of the primary and secondary characteristics could be assigned a subjective severity score of absent, mild, moderate, or severe. The goal was that the subtype classification and clinical grading system, when used in conjunction with each other, would establish a common language for patients, clinicians, and researchers to describe and further investigate rosacea.4
The 2002 categorization system was certainly an impactful first step in the organization of rosacea. It was not without its critics, however, namely rosacea-oriented dermatologists who were concerned about its lack of specificity.5-7 For instance, the NRS Expert Committee did not address the time frame for flushing, which typically has a long duration in rosacea patients, or for the nontransient erythema; telangiectasia secondary to heliodermatitis; or the often-observed periocular sparing. Additionally, the schema did not account for conditions such as gram-negative folliculitis (pustules characteristically located on the central face) or discuss the need to rule out carcinoid, mastocytosis, or connective-tissue disease, which can lead to nontransient facial erythema. Without strict definitions and exclusions, nonrosacea disorders could be incorrectly labeled as rosacea.
Beyond the lack of specificity, there was additional concern if a subtype system was the ideal way to capture disease presentation and severity. By subtyping, there was unnecessary division of interrelated disease into individual disorders; an individual’s clinical presentation might fall along a spectrum rather than within a discrete box.8
Furthermore, from a research standpoint, subtyping rosacea could hinder or confuse epidemiologic studies. For instance, if patients present with phenotypes from different subtypes, into which subtype would they fall?8-10
The global ROSacea COnsensus (ROSCO) panel, comprising 17 international dermatologists and ophthalmologists, convened in 2016 to address this matter. The panel proposed a new system (published in 2017) based on individual phenotypes.9 In this new system, diagnostic features include persistent centrofacial erythema with periods of increased intensity and phymatous changes. Major features, which are diagnostic when there are at least 2, include flushing (transient erythema), inflammatory papules and pustules, centrofacial telangiectasia, and ocular manifestations. Each feature could then be graded on a severity spectrum independent of concurrent phenotypes (Table).8
The panel concluded that this system would provide a stronger foundation for standardization as new knowledge of rosacea continues to be elucidated.8 In support of their argument, ROSCO also released a treatment algorithm that depended on a phenotype scheme.11 The panel emphasized that by focusing on individual lesions rather than a subtype encompassing many characteristics, treatment could be tailored to the patient. Using this à-la-carte therapy option, physicians could choose those rosacea aspects that are particularly concerning to the patient and manage only those aspects or overlap treatments to improve multiple aspects.11
In 2017, 15 years after the original classification system was proposed, the NRS updated their classification system (published in 2018), taking into consideration some of the criticisms as well as new scientific data on rosacea. Similar to the schema proposed by ROSCO, this system was based on phenotype. Inclusion and exclusion criteria were more robust in this update compared to the original classification in 2002. The criteria provide a timeline for transient flushing—it must occur within seconds or minutes in response to a neurovascular stimulant—and state that it is characteristically prolonged (Table).12
However, the Expert Committee still did not define either the length of time of flushing or nontransient erythema. It also did not specify convex surfaces of the face with periocular sparing as the characteristic pattern or provide additional information on how photoaging fits into the definition. The updated classification stated that centrofacial erythema must not be from cutaneous lupus or seborrheic eczema, and steroid-induced rosacea was still excluded.12 However, there is still the need to exclude other systemic conditions, such as mastocytosis, carcinoid, polycythemia vera, and dermatomyositis. Therefore, the potential for subjective error and inclusion of nonrosacea diseases persists.
A critical change was elimination of the granulomatous rosacea variant. In 2002, this variant was defined by monomorphic, yellow-brown to red papules and nodules that led to scarring. This variant, however, did not share the commonalities of the other subtypes, including persistent facial erythema, limitation to convex surfaces, periocular sparing, and transient flushing.3,13 At the time, Crawford et al6 proposed that the variant be recategorized as granulomatous facial dermatitis. In the updated NRS classification, this variant and phenotypic description was eliminated from the schema.12 It is unclear if it was removed because of these discrepancies or if the NRS panel felt it had a distinct pathogenesis from the proposed rosacea pathophysiology; however, we applaud this change.
Subtype Progression
Both the ROSCO and NRS classification schemes mention progression between the various phenotypes,10,12 suggesting that rosacea phenotypes exist along a continuum, progressing and regressing with disease severity. The main study addressing this point was based on the self-reported retrospective patient memory of disease features in rosacea patients. The authors used a modified criterion of centrofacial erythema alone to define ETR; therefore, a person who began their disease with this finding but then acquired inflammatory lesions or phymas was defined as progressing along a spectrum.14 Given that persistent erythema of convex surfaces of the face is common in all subtypes, we do not find it surprising that the authors found (using their modified criteria) that ETR appeared to progress to papulopustular and phymatous subtypes in a small number of patients. We strongly disagree with their interpretation and conclusion.
In our experience, ETR patients have fine textured skin without sebaceous quality or a history of extensive acne (Figure 1). Flushing is common and usually lasts 10 minutes to 1 hour. There might be concurrent burning or stinging; however, there is no associated sweating, lightheadedness, palpitations, or diagnostic laboratory findings, which distinguishes ETR from other common causes of flushing. The persistent centrofacial erythema involves convex surfaces, spares periocular skin, and can be best defined as present for longer than 3 months.

In contrast, phymas occur commonly in patients with thick and sebaceous (glandular) skin (Figure 2).6,15-17 Men are most often affected and usually have a history of moderate to severe acne. It is not uncommon to observe nodules, cysts, and scarring in addition to papules and pustules. These eruptions primarily cluster on the central face and present in areas of nontransient erythema. Flushing, although less prominent than in other phenotypes, also can be seen.

Taken together, we find no convincing evidence from published studies or extensive experience caring for rosacea patients that classic ETR progresses to phymatous rosacea, or the other way around, as displayed in the ROSCO panel report.8 The type of skin seen in Figure 1 will not “progress” to the type seen in Figure 2. Furthermore, treatment will not “reverse” the phymatous skin into thin, ETR-type skin. The implications are important: If a female patient is given a diagnosis of ETR, she will not develop an enlarged phymatous nose. Patients with thick sebaceous skin, as in Figure 2, usually tolerate treatments such as benzoyl peroxide that other rosacea patients do not and frequently respond well to such intervention.
Implications and Future Directions
We present an overview of 2 rosacea classification systems, hoping to stimulate further refinement. Looking forward, there are many directions for further investigation into the pathophysiology of rosacea. From a genetic standpoint, there needs to be continued molecular and epidemiologic data to determine the underlying genetic contributions to disease.
There has been some progress in the realm of understanding the mechanisms of inflammation; we urge further investigation to elucidate how “subclinical neuroinflammation” might lead to glandular hyperplasia.12 We also see value in examining the genetic and hormonal contributions to phymas, as they may be different than those seen in the ETR-type patients. Last, more studies focusing on comorbidities that contribute to or arise from rosacea are welcomed.
The ultimate goal is to develop a classification system that integrates clinical descriptions, pathophysiologic mechanisms, and benchmark indicators of disease. Only then can we have a true gold standard for the diagnosis of rosacea, one that allows for improved personalized treatment and better outcomes.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282-289.
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Saleem MD. Revisiting rosacea criteria: where have we been, where are we going, and how will we get there? Dermatol Clin. 2018;36:161-165.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Tan J, Almeida LMC, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438.
- Wilkin J. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes. Br J Dermatol. 2017;177:597-598.
- Tan J; ROSCO coauthors. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes: reply from the author. Br J Dermatol. 2017;177:598-599.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33:447-455.
- Tan J, Blume‐Peytavi U, Ortonne JP, et al. An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169:555-562.
- James WD, Elston D, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. New York, NY: Elsevier; 2019.
- Reinholz M, Tietze JK, Kilian K, et al. Rosacea - S1 guideline. J Dtsch Dermatol Ges. 2013;11:768-780.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom‐oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282-289.
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Saleem MD. Revisiting rosacea criteria: where have we been, where are we going, and how will we get there? Dermatol Clin. 2018;36:161-165.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Tan J, Almeida LMC, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-438.
- Wilkin J. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes. Br J Dermatol. 2017;177:597-598.
- Tan J; ROSCO coauthors. Updating the diagnosis, classification and assessment of rosacea by effacement of subtypes: reply from the author. Br J Dermatol. 2017;177:598-599.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management and treatment. Dermatol Clin. 2015;33:447-455.
- Tan J, Blume‐Peytavi U, Ortonne JP, et al. An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol. 2013;169:555-562.
- James WD, Elston D, Treat JR, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. New York, NY: Elsevier; 2019.
- Reinholz M, Tietze JK, Kilian K, et al. Rosacea - S1 guideline. J Dtsch Dermatol Ges. 2013;11:768-780.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom‐oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
Practice Points
- Rosacea therapy is based on a phenotype classification system, in which patients can have major and minor features across all previously denoted subtypes. This system allows for greater flexibility in treatment regimens.
- Despite mention of progression between subtypes, there has not been convincing evidence that patients can progress or regress from one end of the rosacea spectrum (erythematotelangiectatic) to the other (phymatous).
The Role of Adolescent Acne Treatment in Formation of Scars Among Patients With Persistent Adult Acne: Evidence From an Observational Study
In the last 20 years, the incidence of acne lesions in adults has markedly increased. 1 Acne affects adults (individuals older than 25 years) and is no longer a condition limited to adolescents and young adults (individuals younger than 25 years). According to Dreno et al, 2 the accepted age threshold for the onset of adult acne is 25 years. 1-3 In 2013, the term adult acne was defined. 2 Among patients with adult acne, there are 2 subtypes: (1) persistent adult acne, which is a continuation or recurrence of adolescent acne, affecting approximately 80% of patients, and (2) late-onset acne, affecting approximately 20% of patients. 4
Clinical symptoms of adult acne and available treatment modalities have been explored in the literature. Daily clinical experience shows that additional difficulties involved in the management of adult acne patients are related mainly to a high therapeutic failure rate in acne patients older than 25 years. 5 Persistent adult acne seems to be noteworthy because it causes long-term symptoms, and patients experience uncontrollable recurrences.
It is believed that adult acne often is resistant to treatment. 2 Adult skin is more sensitive to topical agents, leading to more irritation by medications intended for external use and cosmetics. 6 Scars in these patients are a frequent and undesirable consequence. 3
Effective treatment of acne encompasses oral antibiotics, topical and systemic retinoids, and oral contraceptive pills (OCPs). For years, oral subantimicrobial doses of cyclines have been recommended for acne treatment. Topical and oral retinoids have been successfully used for more than 30 years as important therapeutic options. 7 More recent evidence-based guidelines for acne issued by the American Academy of Dermatology 8 and the European Dermatology Forum 9 also show that retinoids play an important role in acne therapy. Their anti-inflammatory activity acts against comedones and their precursors (microcomedones). Successful antiacne therapy not only achieves a smooth face without comedones but also minimizes scar formation, postinflammatory discoloration, and long-lasting postinflammatory erythema. 10 Oral contraceptives have a mainly antiseborrheic effect. 11
Our study sought to analyze the potential influence of therapy during adolescent acne on patients who later developed adult acne. Particular attention was given to the use of oral antibiotics, isotretinoin, and topical retinoids for adolescent acne and their potential role in diminishing scar formation in adult acne.
Materials and Methods
Patient Demographics and Selection
A population-based study of Polish patients with adult acne was conducted. Patients were included in the study group on a consecutive basis from among those who visited our outpatient dermatology center from May 2015 to January 2016. A total of 111 patients (101 women [90.99%] and 10 men [9.01%]) were examined. The study group comprised patients aged 25 years and older who were treated for adult acne (20 patients [18.02%] were aged 25–29 years, 61 [54.95%] were aged 30–39 years, and 30 [27.02%] were 40 years or older).
The following inclusion criteria were used: observation period of at least 6 months in our dermatologic center for patients diagnosed with adult acne, at least 2 dermatologic visits for adult acne prior to the study, written informed consent for study participation and data processing (the aim of the study was explained to each participant by a dermatologist), and age 25 years or older. Exclusion criteria included those who were younger than 25 years, those who had only 1 dermatologic visit at our dermatology center, and those who were unwilling to participate or did not provide written informed consent. Our study was conducted according to Good Clinical Practice.
Data Collection
To obtain data with the highest degree of reliability, 3 sources of information were used: (1) a detailed medical interview conducted by one experienced dermatologist (E.C.) at our dermatology center at the first visit in all study participants, (2) a clinical examination that yielded results necessary for the assessment of scars using a method outlined by Jacob et al, 12 and (3) information included in available medical records. These data were then statistically analyzed.
Statistical Analysis
The results were presented as frequency plots, and a Fisher exact test was conducted to obtain a statistical comparison of the distributions of analyzed data. Unless otherwise indicated, 5% was adopted as the significance level. The statistical analysis was performed using Stata 14 software (StataCorp LLC, College Station, Texas).
Results
Incidence of Different Forms of Adult Acne
To analyze the onset of acne, patients were categorized into 1 of 2 groups: those with persistent adult acne (81.98%) and those with late-onset adult acne (ie, developed after 25 years of age)(18.02%).
Age at Initiation of Dermatologic Treatment
Of the patients with persistent adult acne, 31.87% first visited a dermatologist the same year that the first acne lesions appeared, 36.26% postponed the first visit by at least 5 years (Figure 1), and 23.08% started treatment at least 10 years after acne first appeared. Among patients with persistent adult acne, 76.92% began dermatologic treatment before 25 years of age, and 23.08% began treatment after 25 years of age. Of the latter, 28.57% did not start therapy until they were older than 35 years.

Severity of Adolescent Acne
In the persistent adult acne group, the severity of adolescent acne was assessed during the medical interview as well as detailed histories in medical records. The activity of acne was evaluated at 2-year intervals with the use of a 10-point scale: 1 to 3 points indicated mild acne (7.69% of patients), 4 to 6 points indicated moderate acne (24.18%), and 7 to 10 points indicated severe acne (68.13%).
Treatment of Persistent Acne in Adolescence
Treatment was comprised of oral therapy with antibiotics, isotretinoin, and/or application of topical retinoids (sometimes supported with OCPs). Monotherapy was the standard of treatment more than 25 years ago when patients with persistent adult acne were treated as adolescents or young adults. As many as 43.96% of patients with persistent adult acne did not receive any of these therapies before 25 years of age; rather, they used antiacne cosmetics or beauty procedures. Furthermore, 50.55% of patients were treated with oral antibiotics (Figure 2). Topical retinoids were used in 19.78% of patients and isotretinoin was used in 16.48%. Incidentally, OCPs were given to 26.5%. In the course of adolescent acne, 31.87% of patients received 2 to 4 courses of treatment with either antibiotics or retinoids (oral or topical), and 5.49% were treated with 5 or more courses of treatment (Figure 3). The analysis of each treatment revealed that only 1 patient received 4 courses of isotretinoin. Five courses of oral antibiotics were given in 1 patient, and 3 courses of topical retinoids were given in the same patient.


Topical Retinoids
In an analysis of the number of treatments with topical retinoids completed by patients with persistent adult acne, it was established that 80.22% of patients never used topical retinoids for acne during adolescence. Additionally, 12.08% of these patients completed 1 course of treatment, and 7.69% completed 2 to 4 treatments. However, after 25 years of age, only 25.27% of the patients with persistent adult acne were not treated with topical retinoids, and 35.16% completed more than 2 courses of treatment.
Duration of Treatment
Because adult acne is a chronic disease, the mean number of years that patients received treatment over the disease course was analyzed. In the case of persistent adult acne, the mean duration of treatment, including therapy received during adolescence, was more than 13 years. At the time of the study, more than 30% of patients had been undergoing treatment of adult acne for more than 20 years. Scars— The proportion of patients with persistent adult acne who experienced scarring was evaluated. In the persistent adult acne group, scars were identified in 53.85% of patients. Scars appeared only during adolescence in 26.37% of patients with persistent adult acne, scars appeared only after 25 years of age in 21.97% of patients, and scars appeared in adolescence as well as adulthood in 30.77% of patients.
In an analysis of patients with persistent adult acne who experienced scarring after 25 years of age, the proportion of patients with untreated adolescent acne and those who were treated with antibiotics only was not significantly different (60% vs 64%; P = .478)(Table). The inclusion of topical retinoids into treatment decreased the proportion of scars (isotretinoin: 20%, P = .009; topical retinoids: 38.89%, P = .114).

Comment
Persistent Adult Acne
Patients with symptoms of persistent adult acne represented 81.98% of the study population, which was similar to a 1999 study by Goulden et al, 1 a 2001 study by Shaw and White, 13 and a 2009 report by Schmidt et al. 14 Of these patients with persistent adult acne, 23.08% initiated therapy after 25 years of age, and 23.08% started treatment at least 10 years after acne lesions first appeared. However, it is noteworthy that 68.13% of all patients with persistent adult acne assessed their disease as severe.
Treatment Modalities for Adult Acne
Over the last 5 years, some researchers have attempted to make recommendations for the treatment of adult acne based on standards adopted for the treatment of adolescent acne. 2,9,15 First-line treatment of patients with adult comedonal acne is topical retinoids. 9 The recommended treatment of mild to moderate adult inflammatory acne involves topical drugs, including retinoids, azelaic acid, or benzoyl peroxide, or oral medications, including antibiotics, OCPs, or antiandrogens. In severe inflammatory acne, the recommended treatment involves oral isotretinoin or combined therapies; the latter seems to be the most effective. 16 Furthermore, this therapy has been adjusted to the patient’s current clinical condition; general individual sensitivity of the skin to irritation and the risk for irritant activity of topical medications; and life situation, such as planned pregnancies and intended use of OCPs due to the risk for teratogenic effects of drugs. 17
To assess available treatment modalities, oral therapy with antibiotics or isotretinoin as well as topical retinoids were selected for our analysis. It is difficult to determine an exclusive impact of OCPs as acne treatment; according to our study, many female patients use hormone therapy for other medical conditions or contraception, and only a small proportion of these patients are prescribed hormone treatment for acne. We found that 43.96% of patients with persistent adult acne underwent no treatment with antibiotics, isotretinoin, or topical retinoids in adolescence. Patients who did not receive any of these treatments came only for single visits to a dermatologist, did not comply to a recommended therapy, or used only cosmetics or beauty procedures. We found that 80.22% of patients with persistent adult acne never used topical retinoids during adolescence and did not receive maintenance therapy, which may be attributed to the fact that there were no strict recommendations regarding retinoid treatment when these patients were adolescents or young adults. Published data indicate that retinoid use for acne treatment is not common. 18 Conversely, among patients older than 25 years with late-onset adult acne, there was only 1 patient (ie, < 1%) who had never received any oral antibiotic or isotretinoin treatment or therapy with topical retinoids. The reason for the lack of medical treatment is unknown. Only 25.27% of patients were not treated with topical retinoids, and 35.16% completed at least 2 courses of treatment.
Acne Scarring
The worst complication of acne is scarring. Scars develop for the duration of the disease, during both adolescent and adult acne. In the group with persistent adult acne, scarring was found in 53.85% of patients. Scar formation has been previously reported as a common complication of acne. 19 The effects of skin lesions that remain after acne are not only limited to impaired cosmetic appearance; they also negatively affect mental health and impair quality of life. 20 The aim of our study was to analyze types of treatment for adolescent acne in patients who later had persistent adult acne. Postacne scars observed later are objective evidence of the severity of disease. We found that using oral antibiotics did not diminish the number of scars among persistent adult acne patients in adulthood. In contrast, isotretinoin or topical retinoid treatment during adolescence decreased the risk for scars occurring during adulthood. In our opinion, these findings emphasize the role of this type of treatment among adolescents or young adults. The decrease of scar formation in adult acne due to retinoid treatment in adolescence indirectly justifies the role of maintenance therapy with topical retinoids. 21,22
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577-580.
- Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
- Preneau S, Dreno B. Female acne--a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
- Goulden V, Clark SM, Cunliffe WJ. Post-adolescent acne: a review of clinical features. Br J Dermatol. 1997;136:66-70.
- Kamangar F, Shinkai K. Acne in the adult female patient: a practical approach. Int J Dermatol. 2012;51:1162-1174.
- Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
- Kligman AM, Fulton JE Jr, Plewig G. Topical vitamin A acid in acne vulgaris. Arch Dermatol. 1969;99:469-476.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945.e33-973.e33.
- Nast A, Dreno B, Bettoli V, et al. European evidence-based guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26(suppl 1):1-29.
- Levin J. The relationship of proper skin cleansing to pathophysiology, clinical benefits, and the concomitant use of prescription topical therapies in patients with acne vulgaris. Dermatol Clin. 2016;34:133-145.
- Savage LJ, Layton AM. Treating acne vulgaris: systemic, local and combination therapy. Expert Rev Clin Pharmacol. 2010;3:563-580.
- Jacob CL, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45:109-117.
- Shaw JC, White LE. Persistent acne in adult women. Arch Dermatol. 2001;137:1252-1253.
- Schmidt JV, Masuda PY, Miot HA. Acne in women: clinical patterns in different age groups. An Bras Dermatol. 2009;84:349-354.
- Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 suppl):1-50.
- Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7:281-290.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Pena S, Hill D, Feldman SR. Use of topical retinoids by dermatologist and non-dermatologist in the management of acne vulgaris. J Am Acad Dermatol. 2016;74:1252-1254.
- Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19;303-308.
- Halvorsen JA, Stern RS, Dalgard F, et al. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population-based study. J Invest Dermatol. 2011;131:363-370.
- Thielitz A, Sidou F, Gollnick H. Control of microcomedone formation throughout a maintenance treatment with adapalene gel, 0.1%. J Eur Acad Dermatol Venereol. 2007;21:747-753.
- Leyden J, Thiboutot DM, Shalita R, et al. Comparison of tazarotene and minocycline maintenance therapies in acne vulgaris: a multicenter, double-blind, randomized, parallel-group study. Arch Dermatol. 2006;142:605-612.
In the last 20 years, the incidence of acne lesions in adults has markedly increased. 1 Acne affects adults (individuals older than 25 years) and is no longer a condition limited to adolescents and young adults (individuals younger than 25 years). According to Dreno et al, 2 the accepted age threshold for the onset of adult acne is 25 years. 1-3 In 2013, the term adult acne was defined. 2 Among patients with adult acne, there are 2 subtypes: (1) persistent adult acne, which is a continuation or recurrence of adolescent acne, affecting approximately 80% of patients, and (2) late-onset acne, affecting approximately 20% of patients. 4
Clinical symptoms of adult acne and available treatment modalities have been explored in the literature. Daily clinical experience shows that additional difficulties involved in the management of adult acne patients are related mainly to a high therapeutic failure rate in acne patients older than 25 years. 5 Persistent adult acne seems to be noteworthy because it causes long-term symptoms, and patients experience uncontrollable recurrences.
It is believed that adult acne often is resistant to treatment. 2 Adult skin is more sensitive to topical agents, leading to more irritation by medications intended for external use and cosmetics. 6 Scars in these patients are a frequent and undesirable consequence. 3
Effective treatment of acne encompasses oral antibiotics, topical and systemic retinoids, and oral contraceptive pills (OCPs). For years, oral subantimicrobial doses of cyclines have been recommended for acne treatment. Topical and oral retinoids have been successfully used for more than 30 years as important therapeutic options. 7 More recent evidence-based guidelines for acne issued by the American Academy of Dermatology 8 and the European Dermatology Forum 9 also show that retinoids play an important role in acne therapy. Their anti-inflammatory activity acts against comedones and their precursors (microcomedones). Successful antiacne therapy not only achieves a smooth face without comedones but also minimizes scar formation, postinflammatory discoloration, and long-lasting postinflammatory erythema. 10 Oral contraceptives have a mainly antiseborrheic effect. 11
Our study sought to analyze the potential influence of therapy during adolescent acne on patients who later developed adult acne. Particular attention was given to the use of oral antibiotics, isotretinoin, and topical retinoids for adolescent acne and their potential role in diminishing scar formation in adult acne.
Materials and Methods
Patient Demographics and Selection
A population-based study of Polish patients with adult acne was conducted. Patients were included in the study group on a consecutive basis from among those who visited our outpatient dermatology center from May 2015 to January 2016. A total of 111 patients (101 women [90.99%] and 10 men [9.01%]) were examined. The study group comprised patients aged 25 years and older who were treated for adult acne (20 patients [18.02%] were aged 25–29 years, 61 [54.95%] were aged 30–39 years, and 30 [27.02%] were 40 years or older).
The following inclusion criteria were used: observation period of at least 6 months in our dermatologic center for patients diagnosed with adult acne, at least 2 dermatologic visits for adult acne prior to the study, written informed consent for study participation and data processing (the aim of the study was explained to each participant by a dermatologist), and age 25 years or older. Exclusion criteria included those who were younger than 25 years, those who had only 1 dermatologic visit at our dermatology center, and those who were unwilling to participate or did not provide written informed consent. Our study was conducted according to Good Clinical Practice.
Data Collection
To obtain data with the highest degree of reliability, 3 sources of information were used: (1) a detailed medical interview conducted by one experienced dermatologist (E.C.) at our dermatology center at the first visit in all study participants, (2) a clinical examination that yielded results necessary for the assessment of scars using a method outlined by Jacob et al, 12 and (3) information included in available medical records. These data were then statistically analyzed.
Statistical Analysis
The results were presented as frequency plots, and a Fisher exact test was conducted to obtain a statistical comparison of the distributions of analyzed data. Unless otherwise indicated, 5% was adopted as the significance level. The statistical analysis was performed using Stata 14 software (StataCorp LLC, College Station, Texas).
Results
Incidence of Different Forms of Adult Acne
To analyze the onset of acne, patients were categorized into 1 of 2 groups: those with persistent adult acne (81.98%) and those with late-onset adult acne (ie, developed after 25 years of age)(18.02%).
Age at Initiation of Dermatologic Treatment
Of the patients with persistent adult acne, 31.87% first visited a dermatologist the same year that the first acne lesions appeared, 36.26% postponed the first visit by at least 5 years (Figure 1), and 23.08% started treatment at least 10 years after acne first appeared. Among patients with persistent adult acne, 76.92% began dermatologic treatment before 25 years of age, and 23.08% began treatment after 25 years of age. Of the latter, 28.57% did not start therapy until they were older than 35 years.

Severity of Adolescent Acne
In the persistent adult acne group, the severity of adolescent acne was assessed during the medical interview as well as detailed histories in medical records. The activity of acne was evaluated at 2-year intervals with the use of a 10-point scale: 1 to 3 points indicated mild acne (7.69% of patients), 4 to 6 points indicated moderate acne (24.18%), and 7 to 10 points indicated severe acne (68.13%).
Treatment of Persistent Acne in Adolescence
Treatment was comprised of oral therapy with antibiotics, isotretinoin, and/or application of topical retinoids (sometimes supported with OCPs). Monotherapy was the standard of treatment more than 25 years ago when patients with persistent adult acne were treated as adolescents or young adults. As many as 43.96% of patients with persistent adult acne did not receive any of these therapies before 25 years of age; rather, they used antiacne cosmetics or beauty procedures. Furthermore, 50.55% of patients were treated with oral antibiotics (Figure 2). Topical retinoids were used in 19.78% of patients and isotretinoin was used in 16.48%. Incidentally, OCPs were given to 26.5%. In the course of adolescent acne, 31.87% of patients received 2 to 4 courses of treatment with either antibiotics or retinoids (oral or topical), and 5.49% were treated with 5 or more courses of treatment (Figure 3). The analysis of each treatment revealed that only 1 patient received 4 courses of isotretinoin. Five courses of oral antibiotics were given in 1 patient, and 3 courses of topical retinoids were given in the same patient.


Topical Retinoids
In an analysis of the number of treatments with topical retinoids completed by patients with persistent adult acne, it was established that 80.22% of patients never used topical retinoids for acne during adolescence. Additionally, 12.08% of these patients completed 1 course of treatment, and 7.69% completed 2 to 4 treatments. However, after 25 years of age, only 25.27% of the patients with persistent adult acne were not treated with topical retinoids, and 35.16% completed more than 2 courses of treatment.
Duration of Treatment
Because adult acne is a chronic disease, the mean number of years that patients received treatment over the disease course was analyzed. In the case of persistent adult acne, the mean duration of treatment, including therapy received during adolescence, was more than 13 years. At the time of the study, more than 30% of patients had been undergoing treatment of adult acne for more than 20 years. Scars— The proportion of patients with persistent adult acne who experienced scarring was evaluated. In the persistent adult acne group, scars were identified in 53.85% of patients. Scars appeared only during adolescence in 26.37% of patients with persistent adult acne, scars appeared only after 25 years of age in 21.97% of patients, and scars appeared in adolescence as well as adulthood in 30.77% of patients.
In an analysis of patients with persistent adult acne who experienced scarring after 25 years of age, the proportion of patients with untreated adolescent acne and those who were treated with antibiotics only was not significantly different (60% vs 64%; P = .478)(Table). The inclusion of topical retinoids into treatment decreased the proportion of scars (isotretinoin: 20%, P = .009; topical retinoids: 38.89%, P = .114).

Comment
Persistent Adult Acne
Patients with symptoms of persistent adult acne represented 81.98% of the study population, which was similar to a 1999 study by Goulden et al, 1 a 2001 study by Shaw and White, 13 and a 2009 report by Schmidt et al. 14 Of these patients with persistent adult acne, 23.08% initiated therapy after 25 years of age, and 23.08% started treatment at least 10 years after acne lesions first appeared. However, it is noteworthy that 68.13% of all patients with persistent adult acne assessed their disease as severe.
Treatment Modalities for Adult Acne
Over the last 5 years, some researchers have attempted to make recommendations for the treatment of adult acne based on standards adopted for the treatment of adolescent acne. 2,9,15 First-line treatment of patients with adult comedonal acne is topical retinoids. 9 The recommended treatment of mild to moderate adult inflammatory acne involves topical drugs, including retinoids, azelaic acid, or benzoyl peroxide, or oral medications, including antibiotics, OCPs, or antiandrogens. In severe inflammatory acne, the recommended treatment involves oral isotretinoin or combined therapies; the latter seems to be the most effective. 16 Furthermore, this therapy has been adjusted to the patient’s current clinical condition; general individual sensitivity of the skin to irritation and the risk for irritant activity of topical medications; and life situation, such as planned pregnancies and intended use of OCPs due to the risk for teratogenic effects of drugs. 17
To assess available treatment modalities, oral therapy with antibiotics or isotretinoin as well as topical retinoids were selected for our analysis. It is difficult to determine an exclusive impact of OCPs as acne treatment; according to our study, many female patients use hormone therapy for other medical conditions or contraception, and only a small proportion of these patients are prescribed hormone treatment for acne. We found that 43.96% of patients with persistent adult acne underwent no treatment with antibiotics, isotretinoin, or topical retinoids in adolescence. Patients who did not receive any of these treatments came only for single visits to a dermatologist, did not comply to a recommended therapy, or used only cosmetics or beauty procedures. We found that 80.22% of patients with persistent adult acne never used topical retinoids during adolescence and did not receive maintenance therapy, which may be attributed to the fact that there were no strict recommendations regarding retinoid treatment when these patients were adolescents or young adults. Published data indicate that retinoid use for acne treatment is not common. 18 Conversely, among patients older than 25 years with late-onset adult acne, there was only 1 patient (ie, < 1%) who had never received any oral antibiotic or isotretinoin treatment or therapy with topical retinoids. The reason for the lack of medical treatment is unknown. Only 25.27% of patients were not treated with topical retinoids, and 35.16% completed at least 2 courses of treatment.
Acne Scarring
The worst complication of acne is scarring. Scars develop for the duration of the disease, during both adolescent and adult acne. In the group with persistent adult acne, scarring was found in 53.85% of patients. Scar formation has been previously reported as a common complication of acne. 19 The effects of skin lesions that remain after acne are not only limited to impaired cosmetic appearance; they also negatively affect mental health and impair quality of life. 20 The aim of our study was to analyze types of treatment for adolescent acne in patients who later had persistent adult acne. Postacne scars observed later are objective evidence of the severity of disease. We found that using oral antibiotics did not diminish the number of scars among persistent adult acne patients in adulthood. In contrast, isotretinoin or topical retinoid treatment during adolescence decreased the risk for scars occurring during adulthood. In our opinion, these findings emphasize the role of this type of treatment among adolescents or young adults. The decrease of scar formation in adult acne due to retinoid treatment in adolescence indirectly justifies the role of maintenance therapy with topical retinoids. 21,22
In the last 20 years, the incidence of acne lesions in adults has markedly increased. 1 Acne affects adults (individuals older than 25 years) and is no longer a condition limited to adolescents and young adults (individuals younger than 25 years). According to Dreno et al, 2 the accepted age threshold for the onset of adult acne is 25 years. 1-3 In 2013, the term adult acne was defined. 2 Among patients with adult acne, there are 2 subtypes: (1) persistent adult acne, which is a continuation or recurrence of adolescent acne, affecting approximately 80% of patients, and (2) late-onset acne, affecting approximately 20% of patients. 4
Clinical symptoms of adult acne and available treatment modalities have been explored in the literature. Daily clinical experience shows that additional difficulties involved in the management of adult acne patients are related mainly to a high therapeutic failure rate in acne patients older than 25 years. 5 Persistent adult acne seems to be noteworthy because it causes long-term symptoms, and patients experience uncontrollable recurrences.
It is believed that adult acne often is resistant to treatment. 2 Adult skin is more sensitive to topical agents, leading to more irritation by medications intended for external use and cosmetics. 6 Scars in these patients are a frequent and undesirable consequence. 3
Effective treatment of acne encompasses oral antibiotics, topical and systemic retinoids, and oral contraceptive pills (OCPs). For years, oral subantimicrobial doses of cyclines have been recommended for acne treatment. Topical and oral retinoids have been successfully used for more than 30 years as important therapeutic options. 7 More recent evidence-based guidelines for acne issued by the American Academy of Dermatology 8 and the European Dermatology Forum 9 also show that retinoids play an important role in acne therapy. Their anti-inflammatory activity acts against comedones and their precursors (microcomedones). Successful antiacne therapy not only achieves a smooth face without comedones but also minimizes scar formation, postinflammatory discoloration, and long-lasting postinflammatory erythema. 10 Oral contraceptives have a mainly antiseborrheic effect. 11
Our study sought to analyze the potential influence of therapy during adolescent acne on patients who later developed adult acne. Particular attention was given to the use of oral antibiotics, isotretinoin, and topical retinoids for adolescent acne and their potential role in diminishing scar formation in adult acne.
Materials and Methods
Patient Demographics and Selection
A population-based study of Polish patients with adult acne was conducted. Patients were included in the study group on a consecutive basis from among those who visited our outpatient dermatology center from May 2015 to January 2016. A total of 111 patients (101 women [90.99%] and 10 men [9.01%]) were examined. The study group comprised patients aged 25 years and older who were treated for adult acne (20 patients [18.02%] were aged 25–29 years, 61 [54.95%] were aged 30–39 years, and 30 [27.02%] were 40 years or older).
The following inclusion criteria were used: observation period of at least 6 months in our dermatologic center for patients diagnosed with adult acne, at least 2 dermatologic visits for adult acne prior to the study, written informed consent for study participation and data processing (the aim of the study was explained to each participant by a dermatologist), and age 25 years or older. Exclusion criteria included those who were younger than 25 years, those who had only 1 dermatologic visit at our dermatology center, and those who were unwilling to participate or did not provide written informed consent. Our study was conducted according to Good Clinical Practice.
Data Collection
To obtain data with the highest degree of reliability, 3 sources of information were used: (1) a detailed medical interview conducted by one experienced dermatologist (E.C.) at our dermatology center at the first visit in all study participants, (2) a clinical examination that yielded results necessary for the assessment of scars using a method outlined by Jacob et al, 12 and (3) information included in available medical records. These data were then statistically analyzed.
Statistical Analysis
The results were presented as frequency plots, and a Fisher exact test was conducted to obtain a statistical comparison of the distributions of analyzed data. Unless otherwise indicated, 5% was adopted as the significance level. The statistical analysis was performed using Stata 14 software (StataCorp LLC, College Station, Texas).
Results
Incidence of Different Forms of Adult Acne
To analyze the onset of acne, patients were categorized into 1 of 2 groups: those with persistent adult acne (81.98%) and those with late-onset adult acne (ie, developed after 25 years of age)(18.02%).
Age at Initiation of Dermatologic Treatment
Of the patients with persistent adult acne, 31.87% first visited a dermatologist the same year that the first acne lesions appeared, 36.26% postponed the first visit by at least 5 years (Figure 1), and 23.08% started treatment at least 10 years after acne first appeared. Among patients with persistent adult acne, 76.92% began dermatologic treatment before 25 years of age, and 23.08% began treatment after 25 years of age. Of the latter, 28.57% did not start therapy until they were older than 35 years.

Severity of Adolescent Acne
In the persistent adult acne group, the severity of adolescent acne was assessed during the medical interview as well as detailed histories in medical records. The activity of acne was evaluated at 2-year intervals with the use of a 10-point scale: 1 to 3 points indicated mild acne (7.69% of patients), 4 to 6 points indicated moderate acne (24.18%), and 7 to 10 points indicated severe acne (68.13%).
Treatment of Persistent Acne in Adolescence
Treatment was comprised of oral therapy with antibiotics, isotretinoin, and/or application of topical retinoids (sometimes supported with OCPs). Monotherapy was the standard of treatment more than 25 years ago when patients with persistent adult acne were treated as adolescents or young adults. As many as 43.96% of patients with persistent adult acne did not receive any of these therapies before 25 years of age; rather, they used antiacne cosmetics or beauty procedures. Furthermore, 50.55% of patients were treated with oral antibiotics (Figure 2). Topical retinoids were used in 19.78% of patients and isotretinoin was used in 16.48%. Incidentally, OCPs were given to 26.5%. In the course of adolescent acne, 31.87% of patients received 2 to 4 courses of treatment with either antibiotics or retinoids (oral or topical), and 5.49% were treated with 5 or more courses of treatment (Figure 3). The analysis of each treatment revealed that only 1 patient received 4 courses of isotretinoin. Five courses of oral antibiotics were given in 1 patient, and 3 courses of topical retinoids were given in the same patient.


Topical Retinoids
In an analysis of the number of treatments with topical retinoids completed by patients with persistent adult acne, it was established that 80.22% of patients never used topical retinoids for acne during adolescence. Additionally, 12.08% of these patients completed 1 course of treatment, and 7.69% completed 2 to 4 treatments. However, after 25 years of age, only 25.27% of the patients with persistent adult acne were not treated with topical retinoids, and 35.16% completed more than 2 courses of treatment.
Duration of Treatment
Because adult acne is a chronic disease, the mean number of years that patients received treatment over the disease course was analyzed. In the case of persistent adult acne, the mean duration of treatment, including therapy received during adolescence, was more than 13 years. At the time of the study, more than 30% of patients had been undergoing treatment of adult acne for more than 20 years. Scars— The proportion of patients with persistent adult acne who experienced scarring was evaluated. In the persistent adult acne group, scars were identified in 53.85% of patients. Scars appeared only during adolescence in 26.37% of patients with persistent adult acne, scars appeared only after 25 years of age in 21.97% of patients, and scars appeared in adolescence as well as adulthood in 30.77% of patients.
In an analysis of patients with persistent adult acne who experienced scarring after 25 years of age, the proportion of patients with untreated adolescent acne and those who were treated with antibiotics only was not significantly different (60% vs 64%; P = .478)(Table). The inclusion of topical retinoids into treatment decreased the proportion of scars (isotretinoin: 20%, P = .009; topical retinoids: 38.89%, P = .114).

Comment
Persistent Adult Acne
Patients with symptoms of persistent adult acne represented 81.98% of the study population, which was similar to a 1999 study by Goulden et al, 1 a 2001 study by Shaw and White, 13 and a 2009 report by Schmidt et al. 14 Of these patients with persistent adult acne, 23.08% initiated therapy after 25 years of age, and 23.08% started treatment at least 10 years after acne lesions first appeared. However, it is noteworthy that 68.13% of all patients with persistent adult acne assessed their disease as severe.
Treatment Modalities for Adult Acne
Over the last 5 years, some researchers have attempted to make recommendations for the treatment of adult acne based on standards adopted for the treatment of adolescent acne. 2,9,15 First-line treatment of patients with adult comedonal acne is topical retinoids. 9 The recommended treatment of mild to moderate adult inflammatory acne involves topical drugs, including retinoids, azelaic acid, or benzoyl peroxide, or oral medications, including antibiotics, OCPs, or antiandrogens. In severe inflammatory acne, the recommended treatment involves oral isotretinoin or combined therapies; the latter seems to be the most effective. 16 Furthermore, this therapy has been adjusted to the patient’s current clinical condition; general individual sensitivity of the skin to irritation and the risk for irritant activity of topical medications; and life situation, such as planned pregnancies and intended use of OCPs due to the risk for teratogenic effects of drugs. 17
To assess available treatment modalities, oral therapy with antibiotics or isotretinoin as well as topical retinoids were selected for our analysis. It is difficult to determine an exclusive impact of OCPs as acne treatment; according to our study, many female patients use hormone therapy for other medical conditions or contraception, and only a small proportion of these patients are prescribed hormone treatment for acne. We found that 43.96% of patients with persistent adult acne underwent no treatment with antibiotics, isotretinoin, or topical retinoids in adolescence. Patients who did not receive any of these treatments came only for single visits to a dermatologist, did not comply to a recommended therapy, or used only cosmetics or beauty procedures. We found that 80.22% of patients with persistent adult acne never used topical retinoids during adolescence and did not receive maintenance therapy, which may be attributed to the fact that there were no strict recommendations regarding retinoid treatment when these patients were adolescents or young adults. Published data indicate that retinoid use for acne treatment is not common. 18 Conversely, among patients older than 25 years with late-onset adult acne, there was only 1 patient (ie, < 1%) who had never received any oral antibiotic or isotretinoin treatment or therapy with topical retinoids. The reason for the lack of medical treatment is unknown. Only 25.27% of patients were not treated with topical retinoids, and 35.16% completed at least 2 courses of treatment.
Acne Scarring
The worst complication of acne is scarring. Scars develop for the duration of the disease, during both adolescent and adult acne. In the group with persistent adult acne, scarring was found in 53.85% of patients. Scar formation has been previously reported as a common complication of acne. 19 The effects of skin lesions that remain after acne are not only limited to impaired cosmetic appearance; they also negatively affect mental health and impair quality of life. 20 The aim of our study was to analyze types of treatment for adolescent acne in patients who later had persistent adult acne. Postacne scars observed later are objective evidence of the severity of disease. We found that using oral antibiotics did not diminish the number of scars among persistent adult acne patients in adulthood. In contrast, isotretinoin or topical retinoid treatment during adolescence decreased the risk for scars occurring during adulthood. In our opinion, these findings emphasize the role of this type of treatment among adolescents or young adults. The decrease of scar formation in adult acne due to retinoid treatment in adolescence indirectly justifies the role of maintenance therapy with topical retinoids. 21,22
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577-580.
- Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
- Preneau S, Dreno B. Female acne--a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
- Goulden V, Clark SM, Cunliffe WJ. Post-adolescent acne: a review of clinical features. Br J Dermatol. 1997;136:66-70.
- Kamangar F, Shinkai K. Acne in the adult female patient: a practical approach. Int J Dermatol. 2012;51:1162-1174.
- Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
- Kligman AM, Fulton JE Jr, Plewig G. Topical vitamin A acid in acne vulgaris. Arch Dermatol. 1969;99:469-476.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945.e33-973.e33.
- Nast A, Dreno B, Bettoli V, et al. European evidence-based guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26(suppl 1):1-29.
- Levin J. The relationship of proper skin cleansing to pathophysiology, clinical benefits, and the concomitant use of prescription topical therapies in patients with acne vulgaris. Dermatol Clin. 2016;34:133-145.
- Savage LJ, Layton AM. Treating acne vulgaris: systemic, local and combination therapy. Expert Rev Clin Pharmacol. 2010;3:563-580.
- Jacob CL, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45:109-117.
- Shaw JC, White LE. Persistent acne in adult women. Arch Dermatol. 2001;137:1252-1253.
- Schmidt JV, Masuda PY, Miot HA. Acne in women: clinical patterns in different age groups. An Bras Dermatol. 2009;84:349-354.
- Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 suppl):1-50.
- Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7:281-290.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Pena S, Hill D, Feldman SR. Use of topical retinoids by dermatologist and non-dermatologist in the management of acne vulgaris. J Am Acad Dermatol. 2016;74:1252-1254.
- Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19;303-308.
- Halvorsen JA, Stern RS, Dalgard F, et al. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population-based study. J Invest Dermatol. 2011;131:363-370.
- Thielitz A, Sidou F, Gollnick H. Control of microcomedone formation throughout a maintenance treatment with adapalene gel, 0.1%. J Eur Acad Dermatol Venereol. 2007;21:747-753.
- Leyden J, Thiboutot DM, Shalita R, et al. Comparison of tazarotene and minocycline maintenance therapies in acne vulgaris: a multicenter, double-blind, randomized, parallel-group study. Arch Dermatol. 2006;142:605-612.
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577-580.
- Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
- Preneau S, Dreno B. Female acne--a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
- Goulden V, Clark SM, Cunliffe WJ. Post-adolescent acne: a review of clinical features. Br J Dermatol. 1997;136:66-70.
- Kamangar F, Shinkai K. Acne in the adult female patient: a practical approach. Int J Dermatol. 2012;51:1162-1174.
- Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
- Kligman AM, Fulton JE Jr, Plewig G. Topical vitamin A acid in acne vulgaris. Arch Dermatol. 1969;99:469-476.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945.e33-973.e33.
- Nast A, Dreno B, Bettoli V, et al. European evidence-based guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26(suppl 1):1-29.
- Levin J. The relationship of proper skin cleansing to pathophysiology, clinical benefits, and the concomitant use of prescription topical therapies in patients with acne vulgaris. Dermatol Clin. 2016;34:133-145.
- Savage LJ, Layton AM. Treating acne vulgaris: systemic, local and combination therapy. Expert Rev Clin Pharmacol. 2010;3:563-580.
- Jacob CL, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45:109-117.
- Shaw JC, White LE. Persistent acne in adult women. Arch Dermatol. 2001;137:1252-1253.
- Schmidt JV, Masuda PY, Miot HA. Acne in women: clinical patterns in different age groups. An Bras Dermatol. 2009;84:349-354.
- Thiboutot D, Gollnick H, Bettoli V, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 suppl):1-50.
- Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7:281-290.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Pena S, Hill D, Feldman SR. Use of topical retinoids by dermatologist and non-dermatologist in the management of acne vulgaris. J Am Acad Dermatol. 2016;74:1252-1254.
- Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19;303-308.
- Halvorsen JA, Stern RS, Dalgard F, et al. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population-based study. J Invest Dermatol. 2011;131:363-370.
- Thielitz A, Sidou F, Gollnick H. Control of microcomedone formation throughout a maintenance treatment with adapalene gel, 0.1%. J Eur Acad Dermatol Venereol. 2007;21:747-753.
- Leyden J, Thiboutot DM, Shalita R, et al. Comparison of tazarotene and minocycline maintenance therapies in acne vulgaris: a multicenter, double-blind, randomized, parallel-group study. Arch Dermatol. 2006;142:605-612.
Practice Points
- Postacne scarring is the most severe complication of acne.
- Isotretinoin or topical retinoid treatment in adolescence decreases the risk for scars during adult acne, justifying the role of maintenance therapy with topical retinoids.
What’s New in the Management of Acne Vulgaris
Inflammation is a backdrop to the commonly cited elements of the pathophysiology of acne: Propionibacterium acnes proliferation, increased sebum production with an increase in circulating androgens, and faulty keratinization.1,2 In fact, research shows that the initiating lesion of acne vulgaris—the microcomedone—is, in essence, an inflammatory lesion.3 This realization has clearly influenced the approach to acne treatment but has not yielded a bevy of new treatments.
A better understanding of acne pathophysiology and the role of inflammation has, however, yielded a better understanding of how existing therapies treat the disease and have led to more comprehensive treatment strategies that are multitargeted. Nonetheless, topical and oral antibiotics remain mainstays of acne therapy, along with topical retinoids and benzoyl peroxide. Current guidelines of care for acne emphasize strategies that reduce dependence on antibiotics and minimize the risk for resistance.4 The therapeutic landscape might at last be shifting, with new chemical entities for acne and several novel formulations in development.
Sarecycline: A Novel Tetracycline
Tetracycline antibiotics have been used to manage acne since the 1950s, but their method of action in the disease has not been fully elucidated.5 In addition to antibiotic effects, tetracyclines have been shown to confer anti-inflammatory properties and other biologic effects.6,7
First-generation tetracycline is broad spectrum. As such, it is associated with increased potential for antibiotic resistance and greater impact on gastrointestinal health. The novel compound sarecycline is a tetracycline with a narrower spectrum of activity compared to other tetracyclines and with reduced activity against enteric gram-negative bacteria8 (Figure 1). Sarecycline recently was approved by the US Food and Drug Administration (FDA) in a once-daily oral formulation for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. Sarecycline is dosed at 1.5 mg/kg daily. The FDA approval marks the first new antibiotic approved for acne in 4 decades.

In 2 phase 3 clinical trials, sarecycline demonstrated efficacy in reducing both inflammatory and noninflammatory lesions.9 At week 12, investigator global assessment (IGA) success (≥2 point reduction in IGA and score 0 [clear] or 1 [almost clear]) rates were 21.9% and 22.6% for active treatment (n=483 and n=519), respectively, in the 2 trials compared to 10.5% and 15.3% (n=485 and n=515), respectively, for controls. Sarecycline demonstrated rapid anti-inflammatory effect. Onset of action against inflammatory lesions was notable by week 3. At week 12, inflammatory lesions were reduced in the active treatment arms by 51.8% and 49.9%, respectively, compared to 35.1% and 35.4%, respectively, for controls.9
The most common reported treatment-emergent adverse events (TEAEs) were nausea, nasopharyngitis, headache, and vomiting.9 Vestibular (dizziness, tinnitus, vertigo) and phototoxic (sunburn, photosensitivity) TEAEs both occurred in 1% or fewer of sarecycline patients. Gastrointestinal TEAE rates for sarecycline were low.9
Sarecycline also was assessed in the 2 trials for efficacy in the treatment of back and chest acne; in the active treatment group, IGA success was achieved by 29.6% and 36.6%, respectively, compared to 19.6% and 21.6%, respectively, of controls.9
Tazarotene Foam in Focus
Topical tazarotene is commercially available in cream, gel, and foam formulations. Tazarotene foam 0.1% was FDA approved in 2012 for the treatment of acne vulgaris in patients 12 years and older. However, the product was recently relaunched to the market and therefore warrants discussion.
Similar to other retinoids, topical tazarotene has been associated with the potential for application-site irritation. This aqueous foam formulation of tazarotene was designed for ease of application and to attempt to impart moisturizing effects to offset potential irritation. It contains noncomedogenic light mineral oil, which is an emollient. The foam spreads easily, including on hair-bearing skin, with demonstrated penetration of the active drug into the epidermis and dermis. Nonetheless, compared to the gel formulation of tazarotene, the foam formulation was associated with reduced systemic exposure.10
The tazarotene foam formulation does not contain alcohol, fragrance, propylene glycol, or parabens. Clinical trial participants, blinded to whether they were on active treatment or vehicle foam, consistently rated the foam formulation favorably for ease of application and spreadability, lack of stickiness or residue, and moisturizing effect. The foam vehicle is suggested to increase compliance and satisfaction in some patients.11The efficacy and tolerability of tazarotene foam 0.1% was investigated in 2 randomized, double-blind, vehicle-controlled, parallel-group studies in the United States and Canada.12 The studies involved participants aged 2 to 45 years who were randomized to receive treatment with either tazarotene foam 0.1% or vehicle foam once daily for 12 weeks (N=1486). Lesion counts, investigator static global assessment, and subject global assessment were evaluated at baseline and at weeks 2, 4, 8, and 12. At week 12, mean reduction from baseline in noninflammatory lesions was 55.9% for active treatment, mean reduction in inflammatory lesions was 56.1%, and mean total lesion reduction was 56% compared to mean reductions of 37.7%, 45.3%, and 40.8%, respectively, for vehicle. In all, 28.2% of participants achieved treatment success with active treatment compared to 14.7% of controls. There was a greater proportion of active-treatment participants with investigator static global assessment scores of 0 or 1 compared to vehicle. The only adverse events reported by more than 5% of participants in the active-treatment groups in both studies were application-site skin irritation and dryness.12
Topical Minocycline
Systemic minocycline is the most commonly prescribed oral antibiotic for acne management.13 Despite its widespread use, it is not without potential safety concerns. Minocycline is distinct among tetracyclines for posing a small risk for systemic lupus erythematosus and autoimmune TEAE.Gastrointestinal side effects and bluish discoloration also are reported.14 Topical application of minocycline for acne would optimize the therapeutic effect while reducing systemic effects. FMX101 4%, an investigational minocycline foam, is being studied for the treatment of moderate to severe acne.
In a pharmacokinetic study, minocycline exposure was 730- to 765-times lower with foam application vs oral minocycline.15 No evidence of minocycline accumulation was identified over the 21 days of application of minocycline foam 4%. Minocycline foam 4% appeared to be safe and well tolerated, without serious TEAEs, treatment-related TEAEs, or TEAEs that led to treatment discontinuation.15
In 2 identical phase 3 studies in which 961 participants were randomized (2:1) to once-daily minocycline foam 4% or foam vehicle for 12 weeks, participants in the active-treatment group demonstrated a significantly greater reduction in both inflammatory and noninflammatory lesions in both studies (both P<.05) and a greater rate of treatment success (≥2 point reduction in IGA and score of 0 [clear] or 1 [almost clear]) in 1 study. Treatment was generally safe and well tolerated, with skin-related adverse events reported in fewer than 1% of participants receiving active treatment.16
In an open-label safety extension study that enrolled 657 patients, treatment with FMX101 continued for as long as 40 weeks.17 In total, 291 participants completed 52 weeks of therapy. Rates and types of reported TEAEs in the open-label extension phase were similar to those seen in the phase 3 trials. Application-site TEAEs occurred in fewer than 2% of participants. Participants reported a high level of treatment satisfaction at week 52.17
In a more recent phase 3 study, 1507 participants were randomized (1:1) to once-daily minocycline foam 4% or foam vehicle for 12 weeks to further evaluate the efficacy and safety of FMX101 4% for moderate to severe acne vulgaris.18 The study met both primary end points: absolute change from baseline in the inflammatory lesion count (−16.93 vs −13.40; P<.0001) and the noninflammatory lesion count (−18.80 vs −15.89; P<.05), as well as percentage of participants with IGA treatment success at week 12 (30.80% vs 19.63%; P<.0001). The percentage reduction in the inflammatory lesion count was statistically significantly greater for minocycline foam 4% compared to vehicle as early as week 3 (P<.0001). The safety profile was found to be consistent with the 2 earlier phase 3 studies.18
Topical Minocycline in Rosacea
A similar foam formulation of minocycline (1.5% concentration) has shown benefit in 2 identical phase 3 studies.19 A total of 1522 participants were enrolled in 2 phase 3, randomized, multicenter, double-blind, vehicle-controlled, 2-arm studies in participants 18 years and older with moderate to severe papulopustular rosacea. Participants were randomized (2:1) to either minocycline foam 1.5% or vehicle once daily to the face for 12 weeks.19
Treatment was associated with a statistically significant reduction in counts of inflammatory lesions of rosacea (Study FX2016-11: −17.57 vs −15.65 [P=.003]; Study FX2016-12: −18.54 vs −14.88 [P<.0001]) and a significantly higher rate of IGA treatment success compared to vehicle (Study FX2016-11: 52.1% vs 43.0% [P=.027]; Study FX2016-12: 49.1% vs 39.0% [P=.008]), highlighting the anti-inflammatory action of the topically applied agent.19
The most common TEAE for both studies was upper respiratory tract infection; there were no serious TEAEs. Overall, 9 participants across both studies discontinued because of a TEAE (foam, 7 participants; vehicle, 2 participants).19
Clascoterone: First-in-Class Topical
Clascoterone cream 1% is a new chemical entity under investigation for the treatment of moderate to severe acne in patients 9 years and older. Clascoterone targets androgen receptors in the skin to block the effects of circulating endogenous androgens; chemically, it shares a 4-ring backbone identical to dihydrotestosterone and spironolactone (Figure 2). Clascoterone competes with dihydrotestosterone for binding to the androgen receptor to limit or block transcription of androgen-responsive genes and modify specific gene expression.20

Androgens are known to promote both sebum production and inflammatory responses within the follicle, contributing to the cycle of acne.21 Antiandrogen therapy would, therefore, inhibit excess sebum production and directly reduce the presence of certain inflammatory mediators in skin. This effect is expected to lead to reduced follicular plugging and a reduction in growth of P acnes and its inflammatory by-products.
Direct and indirect hormonal modulation have been successfully employed to manage acne in women; however, such therapies have not been considered first-line interventions for the disease.22 Although systemic antiandrogens and hormonal modulation are effective for certain women with acne, there may be concerns about systemic exposure23; no hormone-modulating agent has been adopted for use in men with acne.
As an androgen inhibitor, clascoterone is thought to displace androgen hormones from androgen receptors located at the sebaceous gland and hair follicle, thus inhibiting the cycle of physiologic events that leads to acne formation. Clascoterone is applied topically and acts locally on androgen receptors in the skin, with no systemic exposure seen. In phase 2 trials, clascoterone was found to be safe and effective with no systemic exposure and was suggested to have better tolerability than topical tretinoin.
Preliminary individual study analysis of data from 2 phase 3 trials showed that topical clascoterone met its primary end points, achieving statistically significantly greater rates of IGA treatment success (≥2 point reduction in IGA and score of 0 [clear] or 1 [almost clear]) at week 12 (P<.0001).24 Rates of treatment success for actively treated participants were 16.1% and 18.7%, respectively, compared to 7% and 4.7%, respectively, for vehicle. The study population included both males and nonpregnant females 9 years and older who had a baseline IGA score of 3 (moderate) or 4 (severe). At baseline, participants had a mix of inflammatory lesions (≥30, to a maximum of 75) and noninflammatory lesions (≥30, to a maximum of 100).24
Intention-to-treat analysis at week 12 showed a mean total lesion reduction from baseline for active treatment of 37.1% and 37.7%, respectively, compared to 28.5% and 22.2%, respectively, for controls.24 Mean reductions from baseline in noninflammatory lesions for active treatment were 30.7% and 29.3%, respectively, compared to 21.9% and 15.8%, respectively, for controls. Mean reductions from baseline in inflammatory lesions for active treatment were 44.8% and 47%, respectively, compared to 36.6% and 29.8%, respectively, for controls. Similarly low rates of TEAEs were reported in active and placebo groups in both studies. No TEAE suggested systemic antiandrogen exposure.24
Advancements in Cannabinoids
Advancements in pharmaceutical development of cannabinoid compounds have largely coincided with the controversial national movement to legalize medical marijuana and decriminalize recreational marijuana use. Despite the temporal connection, the 2 topics are entirely distinct. Importantly, pharmaceutical development is largely focused on the effects of cannabidiol (CBD), which is 1 of approximately 113 cannabinoids identified from Cannabis sativa. Cannabidiol is not tetrahydrocannabinol, or THC, the compound responsible for marijuana’s psychoactive effects and addictive properties; CBD does not have any psychoactive effects and is not addictive (Figure 3).25

A CBD oral solution agent recently gained FDA approval for seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients 2 years and older; it is estimated that more than 180 trials of CBD are ongoing in the United States for various indications.26 A notable question in the development of CBD-based therapies is: What is the role of natural plant-derived CBD compared to a pure synthetic form of CBD? The latter is akin to a pharmaceutical process in which a single molecule is developed as the active drug.27 Although the potency and composition of plant-derived CBD can vary with crop conditions, plant strains, and the extraction process, a synthetic molecule would allow for consistency in safety, potency, and pharmacokinetic properties, as well as efficacy, as a consequence.26
There are intriguing data to suggest a potential use for topical CBD in the management of skin diseases, including acne vulgaris. Researchers have, for at least a decade, been investigating the role of the endocannabinoid system, which has physiologic regulatory functions in proliferation, differentiation, apoptosis and cytokine, mediator, and hormone production of various cell types in skin, hair follicles, and sebaceous glands.28 Cannabidiol has been shown to suppress proliferation of sebocytes through activation of transient receptor potential vanilloid 4 ion channels and to have anti-inflammatory effects on sebocytes.29 It has been shown to inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism30 and to possess potent antimicrobial activity against gram-positive bacteria such as P acnes.31
Given these effects on sebocytes, modulation of keratinocyte proliferation, and anti-inflammatory and antibacterial effects, CBD could prove beneficial in the management of acne vulgaris. A new synthetic CBD topical formulation, BTX 1503, is under investigation for the treatment of acne vulgaris.
Early clinical data confirm both the anti-inflammatory effects of topical BTX 1503 as well as its effects on noninflammatory lesions, with 4-week reductions in inflammatory lesion counts similar to what are reported in clinical trials for leading FDA-approved topical therapies in the same time frame.
The phase 1b trial was a 4-week, open-label study in participants with moderate to severe acne vulgaris.32 The primary end point was safety, as demonstrated by the incidence of TEAE, laboratory monitoring, and assessment of cutaneous tolerability. Exploratory end points included changes in inflammatory and noninflammatory lesion counts and IGA score. A total of 21 participants aged 18 to 65 years with moderate to severe acne vulgaris were enrolled. BTX 1503 was applied topically twice daily. At baseline, eligible participants had 20 to 50 inflammatory lesions and 20 to 100 noninflammatory acne lesions on the face, an IGA of 3 (moderate) or 4 (severe), and 3 or fewer nodular or cystic lesions (>5 mm in diameter). No serious or severe TEAEs were reported; no participants withdrew due to a TEAE. Slight erythema, slight scaling, slight dryness, and slight burning and stinging were reported; there were no reports of irritant or allergic contact dermatitis. Only 1 TEAE was thought to be possibly related to treatment: mild pain at the application site.32
In addition to presenting a potential new chemical entity for the topical treatment of acne, the novel topical vehicle formulation of BTX 1503 represents an innovative approach to drug delivery. The formulation utilizes proprietary technology to deliver high doses of drug into the skin without controversial penetration enhancers, preservatives, or other potential irritating additives. Instead, volatile excipients are used that evaporate upon application to the skin, leaving a so-called superconcentrated secondary formulation on the skin. The concentration gradient effect then drives the concentrated drug into skin. Although the formulation efficiently delivers active drug into the skin and its appendages, systemic exposure has been reported to be very low. A phase 2 randomized, double-blind, vehicle-controlled trial ongoing in the United States and Australia in 360 patients with moderate to severe acne vulgaris will provide key data to confirm the efficacy and safety of BTX 1503 (ClinicalTrials.gov Identifier NCT03573518).
Conclusion
Drug development continues to focus on the challenge of treating acne effectively and safely. Vehicle innovations are optimizing existing active drugs and creating opportunities to deliver new compounds to the skin. The approval of sarecycline as the first new chemical entity approved for acne in several years may be followed in coming years by other new actives, including clascoterone and CBD.
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Burkhart CN, Gottwald L. Assessment of etiologic agents in acne pathogenesis. Skinmed. 2003;2:222-228.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337-352.
- Griffin MO, Ceballos G, Villarreal FJ. Tetracycline compounds with non-antimicrobial organ protective properties: possible mechanisms of action. Pharmacol Res. 2011;63:102-107.
- Weinberg JM. The anti-inflammatory effects of tetracyclines. Cutis. 2005;75(4 suppl):6-11.
- Leyden JJ, Sniukiene V, Berk DR, et al. Efficacy and safety of sarecycline, a novel, once-daily, narrow spectrum antibiotic for the treatment of moderate to severe facial acne vulgaris: results of a phase 2, dose-ranging study. J Drugs Dermatol. 2018;17:333-338.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Jarratt M, Werner CP, Alió Saenz AB. Tazarotene foam versus tazarotene gel: a randomized relative bioavailability study in acne vulgaris. Clin Drug Investig. 2013;33:283-289.
- Smith JA, Narahari S, Hill D, et al. Tazarotene foam, 0.1%, for the treatment of acne. Expert Opin Drug Saf. 2016;15:99-103.
- Feldman SR, Werner CP, Alió Saenz AB. The efficacy and tolerability of tazarotene foam, 0.1%, in the treatment of acne vulgaris in 2 multicenter, randomized, vehicle-controlled, double-blind studies. J Drugs Dermatol. 2013;12:438-446.
- Lee YH, Liu G, Thiboutot DM, et al. A retrospective analysis of the duration of oral antibiotic therapy for the treatment of acne among adolescents: investigating practice gaps and potential cost-savings. J Am Acad Dermatol. 2014;71:70-76.
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012(8):CD002086.
- Jones TM, Ellman H, deVries T. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177.17.
- Gold LS, Dhawan S, Weiss J, et al. FMX101 4% minocycline foam for the treatment of acne vulgaris: safety and patient satisfaction from the open-label extension of 2 phase 3 studies. Poster presented at: 2018 Winter Clinical Dermatology Conference; January 12-17, 2018; Maui, HI.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Gold LS, Del Rosso JQ, Bhatia ND, et al. Efficacy and safety of FMX103 (1.5% minocycline foam) in the treatment of moderate-to-severe papulopustular rosacea: results from two phase 3 randomized, multicenter, double-blind, vehicle-controlled studies. Poster presented at: 2019 Winter Clinical Dermatology Conference; January 18-23; 2019; Koloa, HI.
- Data on file. CB-03-01 2017. Milan, Italy: Cassiopea SpA; 2017.
- Ju Q, Tao T, Hu T, et al. Sex hormones and acne. Clin Dermatol. 2017;35:130-137.
- Park JH, Bienenfeld A, Orlow SJ, et al. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol. 2018;19:449-455.
- Barros B, Thiboutot D. Hormonal therapies for acne. Clin Dermatol. 2017;35:168-172.
- Hebert A. Clascoterone topical cream, 1%: a novel, topical, local, selective androgen receptor antagonist: results from two phase 3 studies treating children and adult patients with facial acne vulgaris. Presented at: 2019 American Academy of Dermatology Annual Meeting; March 2, 2019; Washington, DC.
- Noreen N, Muhammad F, Akhtar B, et al. Is cannabidiol a promising substance for new drug development? a review of its potential therapeutic applications. Crit Rev Eukaryot Gene Expr. 2018;28:73-86.
- White CM. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential [published online February 7, 2019]. J Clin Pharmacol. 2019;59:923-934.
- Bonn-Miller MO, ElSohly MA, Loflin MJE, et al. Cannabis and cannabinoid drug development: evaluating botanical versus single molecule approaches. Int Rev Psychiatry. 2018;30:277-284.
- Bíró T, Tóth BI, Haskó G, et al. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411-420.
- Oláh A, Tóth BI, Borbíró I, et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest. 2014;124:3713-3724.
- Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci. 2007;45:87-92.
- Appendino G, Gibbons S, Giana A, et al. Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod. 2008;71:1427-1430.
- Spleman L, Sinclair R, Freeman M, et al. The safety of topical cannabidiol (CBD) for the treatment of acne. J Invest Dermatol. 2018;138:S180.
Inflammation is a backdrop to the commonly cited elements of the pathophysiology of acne: Propionibacterium acnes proliferation, increased sebum production with an increase in circulating androgens, and faulty keratinization.1,2 In fact, research shows that the initiating lesion of acne vulgaris—the microcomedone—is, in essence, an inflammatory lesion.3 This realization has clearly influenced the approach to acne treatment but has not yielded a bevy of new treatments.
A better understanding of acne pathophysiology and the role of inflammation has, however, yielded a better understanding of how existing therapies treat the disease and have led to more comprehensive treatment strategies that are multitargeted. Nonetheless, topical and oral antibiotics remain mainstays of acne therapy, along with topical retinoids and benzoyl peroxide. Current guidelines of care for acne emphasize strategies that reduce dependence on antibiotics and minimize the risk for resistance.4 The therapeutic landscape might at last be shifting, with new chemical entities for acne and several novel formulations in development.
Sarecycline: A Novel Tetracycline
Tetracycline antibiotics have been used to manage acne since the 1950s, but their method of action in the disease has not been fully elucidated.5 In addition to antibiotic effects, tetracyclines have been shown to confer anti-inflammatory properties and other biologic effects.6,7
First-generation tetracycline is broad spectrum. As such, it is associated with increased potential for antibiotic resistance and greater impact on gastrointestinal health. The novel compound sarecycline is a tetracycline with a narrower spectrum of activity compared to other tetracyclines and with reduced activity against enteric gram-negative bacteria8 (Figure 1). Sarecycline recently was approved by the US Food and Drug Administration (FDA) in a once-daily oral formulation for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. Sarecycline is dosed at 1.5 mg/kg daily. The FDA approval marks the first new antibiotic approved for acne in 4 decades.

In 2 phase 3 clinical trials, sarecycline demonstrated efficacy in reducing both inflammatory and noninflammatory lesions.9 At week 12, investigator global assessment (IGA) success (≥2 point reduction in IGA and score 0 [clear] or 1 [almost clear]) rates were 21.9% and 22.6% for active treatment (n=483 and n=519), respectively, in the 2 trials compared to 10.5% and 15.3% (n=485 and n=515), respectively, for controls. Sarecycline demonstrated rapid anti-inflammatory effect. Onset of action against inflammatory lesions was notable by week 3. At week 12, inflammatory lesions were reduced in the active treatment arms by 51.8% and 49.9%, respectively, compared to 35.1% and 35.4%, respectively, for controls.9
The most common reported treatment-emergent adverse events (TEAEs) were nausea, nasopharyngitis, headache, and vomiting.9 Vestibular (dizziness, tinnitus, vertigo) and phototoxic (sunburn, photosensitivity) TEAEs both occurred in 1% or fewer of sarecycline patients. Gastrointestinal TEAE rates for sarecycline were low.9
Sarecycline also was assessed in the 2 trials for efficacy in the treatment of back and chest acne; in the active treatment group, IGA success was achieved by 29.6% and 36.6%, respectively, compared to 19.6% and 21.6%, respectively, of controls.9
Tazarotene Foam in Focus
Topical tazarotene is commercially available in cream, gel, and foam formulations. Tazarotene foam 0.1% was FDA approved in 2012 for the treatment of acne vulgaris in patients 12 years and older. However, the product was recently relaunched to the market and therefore warrants discussion.
Similar to other retinoids, topical tazarotene has been associated with the potential for application-site irritation. This aqueous foam formulation of tazarotene was designed for ease of application and to attempt to impart moisturizing effects to offset potential irritation. It contains noncomedogenic light mineral oil, which is an emollient. The foam spreads easily, including on hair-bearing skin, with demonstrated penetration of the active drug into the epidermis and dermis. Nonetheless, compared to the gel formulation of tazarotene, the foam formulation was associated with reduced systemic exposure.10
The tazarotene foam formulation does not contain alcohol, fragrance, propylene glycol, or parabens. Clinical trial participants, blinded to whether they were on active treatment or vehicle foam, consistently rated the foam formulation favorably for ease of application and spreadability, lack of stickiness or residue, and moisturizing effect. The foam vehicle is suggested to increase compliance and satisfaction in some patients.11The efficacy and tolerability of tazarotene foam 0.1% was investigated in 2 randomized, double-blind, vehicle-controlled, parallel-group studies in the United States and Canada.12 The studies involved participants aged 2 to 45 years who were randomized to receive treatment with either tazarotene foam 0.1% or vehicle foam once daily for 12 weeks (N=1486). Lesion counts, investigator static global assessment, and subject global assessment were evaluated at baseline and at weeks 2, 4, 8, and 12. At week 12, mean reduction from baseline in noninflammatory lesions was 55.9% for active treatment, mean reduction in inflammatory lesions was 56.1%, and mean total lesion reduction was 56% compared to mean reductions of 37.7%, 45.3%, and 40.8%, respectively, for vehicle. In all, 28.2% of participants achieved treatment success with active treatment compared to 14.7% of controls. There was a greater proportion of active-treatment participants with investigator static global assessment scores of 0 or 1 compared to vehicle. The only adverse events reported by more than 5% of participants in the active-treatment groups in both studies were application-site skin irritation and dryness.12
Topical Minocycline
Systemic minocycline is the most commonly prescribed oral antibiotic for acne management.13 Despite its widespread use, it is not without potential safety concerns. Minocycline is distinct among tetracyclines for posing a small risk for systemic lupus erythematosus and autoimmune TEAE.Gastrointestinal side effects and bluish discoloration also are reported.14 Topical application of minocycline for acne would optimize the therapeutic effect while reducing systemic effects. FMX101 4%, an investigational minocycline foam, is being studied for the treatment of moderate to severe acne.
In a pharmacokinetic study, minocycline exposure was 730- to 765-times lower with foam application vs oral minocycline.15 No evidence of minocycline accumulation was identified over the 21 days of application of minocycline foam 4%. Minocycline foam 4% appeared to be safe and well tolerated, without serious TEAEs, treatment-related TEAEs, or TEAEs that led to treatment discontinuation.15
In 2 identical phase 3 studies in which 961 participants were randomized (2:1) to once-daily minocycline foam 4% or foam vehicle for 12 weeks, participants in the active-treatment group demonstrated a significantly greater reduction in both inflammatory and noninflammatory lesions in both studies (both P<.05) and a greater rate of treatment success (≥2 point reduction in IGA and score of 0 [clear] or 1 [almost clear]) in 1 study. Treatment was generally safe and well tolerated, with skin-related adverse events reported in fewer than 1% of participants receiving active treatment.16
In an open-label safety extension study that enrolled 657 patients, treatment with FMX101 continued for as long as 40 weeks.17 In total, 291 participants completed 52 weeks of therapy. Rates and types of reported TEAEs in the open-label extension phase were similar to those seen in the phase 3 trials. Application-site TEAEs occurred in fewer than 2% of participants. Participants reported a high level of treatment satisfaction at week 52.17
In a more recent phase 3 study, 1507 participants were randomized (1:1) to once-daily minocycline foam 4% or foam vehicle for 12 weeks to further evaluate the efficacy and safety of FMX101 4% for moderate to severe acne vulgaris.18 The study met both primary end points: absolute change from baseline in the inflammatory lesion count (−16.93 vs −13.40; P<.0001) and the noninflammatory lesion count (−18.80 vs −15.89; P<.05), as well as percentage of participants with IGA treatment success at week 12 (30.80% vs 19.63%; P<.0001). The percentage reduction in the inflammatory lesion count was statistically significantly greater for minocycline foam 4% compared to vehicle as early as week 3 (P<.0001). The safety profile was found to be consistent with the 2 earlier phase 3 studies.18
Topical Minocycline in Rosacea
A similar foam formulation of minocycline (1.5% concentration) has shown benefit in 2 identical phase 3 studies.19 A total of 1522 participants were enrolled in 2 phase 3, randomized, multicenter, double-blind, vehicle-controlled, 2-arm studies in participants 18 years and older with moderate to severe papulopustular rosacea. Participants were randomized (2:1) to either minocycline foam 1.5% or vehicle once daily to the face for 12 weeks.19
Treatment was associated with a statistically significant reduction in counts of inflammatory lesions of rosacea (Study FX2016-11: −17.57 vs −15.65 [P=.003]; Study FX2016-12: −18.54 vs −14.88 [P<.0001]) and a significantly higher rate of IGA treatment success compared to vehicle (Study FX2016-11: 52.1% vs 43.0% [P=.027]; Study FX2016-12: 49.1% vs 39.0% [P=.008]), highlighting the anti-inflammatory action of the topically applied agent.19
The most common TEAE for both studies was upper respiratory tract infection; there were no serious TEAEs. Overall, 9 participants across both studies discontinued because of a TEAE (foam, 7 participants; vehicle, 2 participants).19
Clascoterone: First-in-Class Topical
Clascoterone cream 1% is a new chemical entity under investigation for the treatment of moderate to severe acne in patients 9 years and older. Clascoterone targets androgen receptors in the skin to block the effects of circulating endogenous androgens; chemically, it shares a 4-ring backbone identical to dihydrotestosterone and spironolactone (Figure 2). Clascoterone competes with dihydrotestosterone for binding to the androgen receptor to limit or block transcription of androgen-responsive genes and modify specific gene expression.20

Androgens are known to promote both sebum production and inflammatory responses within the follicle, contributing to the cycle of acne.21 Antiandrogen therapy would, therefore, inhibit excess sebum production and directly reduce the presence of certain inflammatory mediators in skin. This effect is expected to lead to reduced follicular plugging and a reduction in growth of P acnes and its inflammatory by-products.
Direct and indirect hormonal modulation have been successfully employed to manage acne in women; however, such therapies have not been considered first-line interventions for the disease.22 Although systemic antiandrogens and hormonal modulation are effective for certain women with acne, there may be concerns about systemic exposure23; no hormone-modulating agent has been adopted for use in men with acne.
As an androgen inhibitor, clascoterone is thought to displace androgen hormones from androgen receptors located at the sebaceous gland and hair follicle, thus inhibiting the cycle of physiologic events that leads to acne formation. Clascoterone is applied topically and acts locally on androgen receptors in the skin, with no systemic exposure seen. In phase 2 trials, clascoterone was found to be safe and effective with no systemic exposure and was suggested to have better tolerability than topical tretinoin.
Preliminary individual study analysis of data from 2 phase 3 trials showed that topical clascoterone met its primary end points, achieving statistically significantly greater rates of IGA treatment success (≥2 point reduction in IGA and score of 0 [clear] or 1 [almost clear]) at week 12 (P<.0001).24 Rates of treatment success for actively treated participants were 16.1% and 18.7%, respectively, compared to 7% and 4.7%, respectively, for vehicle. The study population included both males and nonpregnant females 9 years and older who had a baseline IGA score of 3 (moderate) or 4 (severe). At baseline, participants had a mix of inflammatory lesions (≥30, to a maximum of 75) and noninflammatory lesions (≥30, to a maximum of 100).24
Intention-to-treat analysis at week 12 showed a mean total lesion reduction from baseline for active treatment of 37.1% and 37.7%, respectively, compared to 28.5% and 22.2%, respectively, for controls.24 Mean reductions from baseline in noninflammatory lesions for active treatment were 30.7% and 29.3%, respectively, compared to 21.9% and 15.8%, respectively, for controls. Mean reductions from baseline in inflammatory lesions for active treatment were 44.8% and 47%, respectively, compared to 36.6% and 29.8%, respectively, for controls. Similarly low rates of TEAEs were reported in active and placebo groups in both studies. No TEAE suggested systemic antiandrogen exposure.24
Advancements in Cannabinoids
Advancements in pharmaceutical development of cannabinoid compounds have largely coincided with the controversial national movement to legalize medical marijuana and decriminalize recreational marijuana use. Despite the temporal connection, the 2 topics are entirely distinct. Importantly, pharmaceutical development is largely focused on the effects of cannabidiol (CBD), which is 1 of approximately 113 cannabinoids identified from Cannabis sativa. Cannabidiol is not tetrahydrocannabinol, or THC, the compound responsible for marijuana’s psychoactive effects and addictive properties; CBD does not have any psychoactive effects and is not addictive (Figure 3).25

A CBD oral solution agent recently gained FDA approval for seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients 2 years and older; it is estimated that more than 180 trials of CBD are ongoing in the United States for various indications.26 A notable question in the development of CBD-based therapies is: What is the role of natural plant-derived CBD compared to a pure synthetic form of CBD? The latter is akin to a pharmaceutical process in which a single molecule is developed as the active drug.27 Although the potency and composition of plant-derived CBD can vary with crop conditions, plant strains, and the extraction process, a synthetic molecule would allow for consistency in safety, potency, and pharmacokinetic properties, as well as efficacy, as a consequence.26
There are intriguing data to suggest a potential use for topical CBD in the management of skin diseases, including acne vulgaris. Researchers have, for at least a decade, been investigating the role of the endocannabinoid system, which has physiologic regulatory functions in proliferation, differentiation, apoptosis and cytokine, mediator, and hormone production of various cell types in skin, hair follicles, and sebaceous glands.28 Cannabidiol has been shown to suppress proliferation of sebocytes through activation of transient receptor potential vanilloid 4 ion channels and to have anti-inflammatory effects on sebocytes.29 It has been shown to inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism30 and to possess potent antimicrobial activity against gram-positive bacteria such as P acnes.31
Given these effects on sebocytes, modulation of keratinocyte proliferation, and anti-inflammatory and antibacterial effects, CBD could prove beneficial in the management of acne vulgaris. A new synthetic CBD topical formulation, BTX 1503, is under investigation for the treatment of acne vulgaris.
Early clinical data confirm both the anti-inflammatory effects of topical BTX 1503 as well as its effects on noninflammatory lesions, with 4-week reductions in inflammatory lesion counts similar to what are reported in clinical trials for leading FDA-approved topical therapies in the same time frame.
The phase 1b trial was a 4-week, open-label study in participants with moderate to severe acne vulgaris.32 The primary end point was safety, as demonstrated by the incidence of TEAE, laboratory monitoring, and assessment of cutaneous tolerability. Exploratory end points included changes in inflammatory and noninflammatory lesion counts and IGA score. A total of 21 participants aged 18 to 65 years with moderate to severe acne vulgaris were enrolled. BTX 1503 was applied topically twice daily. At baseline, eligible participants had 20 to 50 inflammatory lesions and 20 to 100 noninflammatory acne lesions on the face, an IGA of 3 (moderate) or 4 (severe), and 3 or fewer nodular or cystic lesions (>5 mm in diameter). No serious or severe TEAEs were reported; no participants withdrew due to a TEAE. Slight erythema, slight scaling, slight dryness, and slight burning and stinging were reported; there were no reports of irritant or allergic contact dermatitis. Only 1 TEAE was thought to be possibly related to treatment: mild pain at the application site.32
In addition to presenting a potential new chemical entity for the topical treatment of acne, the novel topical vehicle formulation of BTX 1503 represents an innovative approach to drug delivery. The formulation utilizes proprietary technology to deliver high doses of drug into the skin without controversial penetration enhancers, preservatives, or other potential irritating additives. Instead, volatile excipients are used that evaporate upon application to the skin, leaving a so-called superconcentrated secondary formulation on the skin. The concentration gradient effect then drives the concentrated drug into skin. Although the formulation efficiently delivers active drug into the skin and its appendages, systemic exposure has been reported to be very low. A phase 2 randomized, double-blind, vehicle-controlled trial ongoing in the United States and Australia in 360 patients with moderate to severe acne vulgaris will provide key data to confirm the efficacy and safety of BTX 1503 (ClinicalTrials.gov Identifier NCT03573518).
Conclusion
Drug development continues to focus on the challenge of treating acne effectively and safely. Vehicle innovations are optimizing existing active drugs and creating opportunities to deliver new compounds to the skin. The approval of sarecycline as the first new chemical entity approved for acne in several years may be followed in coming years by other new actives, including clascoterone and CBD.
Inflammation is a backdrop to the commonly cited elements of the pathophysiology of acne: Propionibacterium acnes proliferation, increased sebum production with an increase in circulating androgens, and faulty keratinization.1,2 In fact, research shows that the initiating lesion of acne vulgaris—the microcomedone—is, in essence, an inflammatory lesion.3 This realization has clearly influenced the approach to acne treatment but has not yielded a bevy of new treatments.
A better understanding of acne pathophysiology and the role of inflammation has, however, yielded a better understanding of how existing therapies treat the disease and have led to more comprehensive treatment strategies that are multitargeted. Nonetheless, topical and oral antibiotics remain mainstays of acne therapy, along with topical retinoids and benzoyl peroxide. Current guidelines of care for acne emphasize strategies that reduce dependence on antibiotics and minimize the risk for resistance.4 The therapeutic landscape might at last be shifting, with new chemical entities for acne and several novel formulations in development.
Sarecycline: A Novel Tetracycline
Tetracycline antibiotics have been used to manage acne since the 1950s, but their method of action in the disease has not been fully elucidated.5 In addition to antibiotic effects, tetracyclines have been shown to confer anti-inflammatory properties and other biologic effects.6,7
First-generation tetracycline is broad spectrum. As such, it is associated with increased potential for antibiotic resistance and greater impact on gastrointestinal health. The novel compound sarecycline is a tetracycline with a narrower spectrum of activity compared to other tetracyclines and with reduced activity against enteric gram-negative bacteria8 (Figure 1). Sarecycline recently was approved by the US Food and Drug Administration (FDA) in a once-daily oral formulation for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 9 years and older. Sarecycline is dosed at 1.5 mg/kg daily. The FDA approval marks the first new antibiotic approved for acne in 4 decades.

In 2 phase 3 clinical trials, sarecycline demonstrated efficacy in reducing both inflammatory and noninflammatory lesions.9 At week 12, investigator global assessment (IGA) success (≥2 point reduction in IGA and score 0 [clear] or 1 [almost clear]) rates were 21.9% and 22.6% for active treatment (n=483 and n=519), respectively, in the 2 trials compared to 10.5% and 15.3% (n=485 and n=515), respectively, for controls. Sarecycline demonstrated rapid anti-inflammatory effect. Onset of action against inflammatory lesions was notable by week 3. At week 12, inflammatory lesions were reduced in the active treatment arms by 51.8% and 49.9%, respectively, compared to 35.1% and 35.4%, respectively, for controls.9
The most common reported treatment-emergent adverse events (TEAEs) were nausea, nasopharyngitis, headache, and vomiting.9 Vestibular (dizziness, tinnitus, vertigo) and phototoxic (sunburn, photosensitivity) TEAEs both occurred in 1% or fewer of sarecycline patients. Gastrointestinal TEAE rates for sarecycline were low.9
Sarecycline also was assessed in the 2 trials for efficacy in the treatment of back and chest acne; in the active treatment group, IGA success was achieved by 29.6% and 36.6%, respectively, compared to 19.6% and 21.6%, respectively, of controls.9
Tazarotene Foam in Focus
Topical tazarotene is commercially available in cream, gel, and foam formulations. Tazarotene foam 0.1% was FDA approved in 2012 for the treatment of acne vulgaris in patients 12 years and older. However, the product was recently relaunched to the market and therefore warrants discussion.
Similar to other retinoids, topical tazarotene has been associated with the potential for application-site irritation. This aqueous foam formulation of tazarotene was designed for ease of application and to attempt to impart moisturizing effects to offset potential irritation. It contains noncomedogenic light mineral oil, which is an emollient. The foam spreads easily, including on hair-bearing skin, with demonstrated penetration of the active drug into the epidermis and dermis. Nonetheless, compared to the gel formulation of tazarotene, the foam formulation was associated with reduced systemic exposure.10
The tazarotene foam formulation does not contain alcohol, fragrance, propylene glycol, or parabens. Clinical trial participants, blinded to whether they were on active treatment or vehicle foam, consistently rated the foam formulation favorably for ease of application and spreadability, lack of stickiness or residue, and moisturizing effect. The foam vehicle is suggested to increase compliance and satisfaction in some patients.11The efficacy and tolerability of tazarotene foam 0.1% was investigated in 2 randomized, double-blind, vehicle-controlled, parallel-group studies in the United States and Canada.12 The studies involved participants aged 2 to 45 years who were randomized to receive treatment with either tazarotene foam 0.1% or vehicle foam once daily for 12 weeks (N=1486). Lesion counts, investigator static global assessment, and subject global assessment were evaluated at baseline and at weeks 2, 4, 8, and 12. At week 12, mean reduction from baseline in noninflammatory lesions was 55.9% for active treatment, mean reduction in inflammatory lesions was 56.1%, and mean total lesion reduction was 56% compared to mean reductions of 37.7%, 45.3%, and 40.8%, respectively, for vehicle. In all, 28.2% of participants achieved treatment success with active treatment compared to 14.7% of controls. There was a greater proportion of active-treatment participants with investigator static global assessment scores of 0 or 1 compared to vehicle. The only adverse events reported by more than 5% of participants in the active-treatment groups in both studies were application-site skin irritation and dryness.12
Topical Minocycline
Systemic minocycline is the most commonly prescribed oral antibiotic for acne management.13 Despite its widespread use, it is not without potential safety concerns. Minocycline is distinct among tetracyclines for posing a small risk for systemic lupus erythematosus and autoimmune TEAE.Gastrointestinal side effects and bluish discoloration also are reported.14 Topical application of minocycline for acne would optimize the therapeutic effect while reducing systemic effects. FMX101 4%, an investigational minocycline foam, is being studied for the treatment of moderate to severe acne.
In a pharmacokinetic study, minocycline exposure was 730- to 765-times lower with foam application vs oral minocycline.15 No evidence of minocycline accumulation was identified over the 21 days of application of minocycline foam 4%. Minocycline foam 4% appeared to be safe and well tolerated, without serious TEAEs, treatment-related TEAEs, or TEAEs that led to treatment discontinuation.15
In 2 identical phase 3 studies in which 961 participants were randomized (2:1) to once-daily minocycline foam 4% or foam vehicle for 12 weeks, participants in the active-treatment group demonstrated a significantly greater reduction in both inflammatory and noninflammatory lesions in both studies (both P<.05) and a greater rate of treatment success (≥2 point reduction in IGA and score of 0 [clear] or 1 [almost clear]) in 1 study. Treatment was generally safe and well tolerated, with skin-related adverse events reported in fewer than 1% of participants receiving active treatment.16
In an open-label safety extension study that enrolled 657 patients, treatment with FMX101 continued for as long as 40 weeks.17 In total, 291 participants completed 52 weeks of therapy. Rates and types of reported TEAEs in the open-label extension phase were similar to those seen in the phase 3 trials. Application-site TEAEs occurred in fewer than 2% of participants. Participants reported a high level of treatment satisfaction at week 52.17
In a more recent phase 3 study, 1507 participants were randomized (1:1) to once-daily minocycline foam 4% or foam vehicle for 12 weeks to further evaluate the efficacy and safety of FMX101 4% for moderate to severe acne vulgaris.18 The study met both primary end points: absolute change from baseline in the inflammatory lesion count (−16.93 vs −13.40; P<.0001) and the noninflammatory lesion count (−18.80 vs −15.89; P<.05), as well as percentage of participants with IGA treatment success at week 12 (30.80% vs 19.63%; P<.0001). The percentage reduction in the inflammatory lesion count was statistically significantly greater for minocycline foam 4% compared to vehicle as early as week 3 (P<.0001). The safety profile was found to be consistent with the 2 earlier phase 3 studies.18
Topical Minocycline in Rosacea
A similar foam formulation of minocycline (1.5% concentration) has shown benefit in 2 identical phase 3 studies.19 A total of 1522 participants were enrolled in 2 phase 3, randomized, multicenter, double-blind, vehicle-controlled, 2-arm studies in participants 18 years and older with moderate to severe papulopustular rosacea. Participants were randomized (2:1) to either minocycline foam 1.5% or vehicle once daily to the face for 12 weeks.19
Treatment was associated with a statistically significant reduction in counts of inflammatory lesions of rosacea (Study FX2016-11: −17.57 vs −15.65 [P=.003]; Study FX2016-12: −18.54 vs −14.88 [P<.0001]) and a significantly higher rate of IGA treatment success compared to vehicle (Study FX2016-11: 52.1% vs 43.0% [P=.027]; Study FX2016-12: 49.1% vs 39.0% [P=.008]), highlighting the anti-inflammatory action of the topically applied agent.19
The most common TEAE for both studies was upper respiratory tract infection; there were no serious TEAEs. Overall, 9 participants across both studies discontinued because of a TEAE (foam, 7 participants; vehicle, 2 participants).19
Clascoterone: First-in-Class Topical
Clascoterone cream 1% is a new chemical entity under investigation for the treatment of moderate to severe acne in patients 9 years and older. Clascoterone targets androgen receptors in the skin to block the effects of circulating endogenous androgens; chemically, it shares a 4-ring backbone identical to dihydrotestosterone and spironolactone (Figure 2). Clascoterone competes with dihydrotestosterone for binding to the androgen receptor to limit or block transcription of androgen-responsive genes and modify specific gene expression.20

Androgens are known to promote both sebum production and inflammatory responses within the follicle, contributing to the cycle of acne.21 Antiandrogen therapy would, therefore, inhibit excess sebum production and directly reduce the presence of certain inflammatory mediators in skin. This effect is expected to lead to reduced follicular plugging and a reduction in growth of P acnes and its inflammatory by-products.
Direct and indirect hormonal modulation have been successfully employed to manage acne in women; however, such therapies have not been considered first-line interventions for the disease.22 Although systemic antiandrogens and hormonal modulation are effective for certain women with acne, there may be concerns about systemic exposure23; no hormone-modulating agent has been adopted for use in men with acne.
As an androgen inhibitor, clascoterone is thought to displace androgen hormones from androgen receptors located at the sebaceous gland and hair follicle, thus inhibiting the cycle of physiologic events that leads to acne formation. Clascoterone is applied topically and acts locally on androgen receptors in the skin, with no systemic exposure seen. In phase 2 trials, clascoterone was found to be safe and effective with no systemic exposure and was suggested to have better tolerability than topical tretinoin.
Preliminary individual study analysis of data from 2 phase 3 trials showed that topical clascoterone met its primary end points, achieving statistically significantly greater rates of IGA treatment success (≥2 point reduction in IGA and score of 0 [clear] or 1 [almost clear]) at week 12 (P<.0001).24 Rates of treatment success for actively treated participants were 16.1% and 18.7%, respectively, compared to 7% and 4.7%, respectively, for vehicle. The study population included both males and nonpregnant females 9 years and older who had a baseline IGA score of 3 (moderate) or 4 (severe). At baseline, participants had a mix of inflammatory lesions (≥30, to a maximum of 75) and noninflammatory lesions (≥30, to a maximum of 100).24
Intention-to-treat analysis at week 12 showed a mean total lesion reduction from baseline for active treatment of 37.1% and 37.7%, respectively, compared to 28.5% and 22.2%, respectively, for controls.24 Mean reductions from baseline in noninflammatory lesions for active treatment were 30.7% and 29.3%, respectively, compared to 21.9% and 15.8%, respectively, for controls. Mean reductions from baseline in inflammatory lesions for active treatment were 44.8% and 47%, respectively, compared to 36.6% and 29.8%, respectively, for controls. Similarly low rates of TEAEs were reported in active and placebo groups in both studies. No TEAE suggested systemic antiandrogen exposure.24
Advancements in Cannabinoids
Advancements in pharmaceutical development of cannabinoid compounds have largely coincided with the controversial national movement to legalize medical marijuana and decriminalize recreational marijuana use. Despite the temporal connection, the 2 topics are entirely distinct. Importantly, pharmaceutical development is largely focused on the effects of cannabidiol (CBD), which is 1 of approximately 113 cannabinoids identified from Cannabis sativa. Cannabidiol is not tetrahydrocannabinol, or THC, the compound responsible for marijuana’s psychoactive effects and addictive properties; CBD does not have any psychoactive effects and is not addictive (Figure 3).25

A CBD oral solution agent recently gained FDA approval for seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients 2 years and older; it is estimated that more than 180 trials of CBD are ongoing in the United States for various indications.26 A notable question in the development of CBD-based therapies is: What is the role of natural plant-derived CBD compared to a pure synthetic form of CBD? The latter is akin to a pharmaceutical process in which a single molecule is developed as the active drug.27 Although the potency and composition of plant-derived CBD can vary with crop conditions, plant strains, and the extraction process, a synthetic molecule would allow for consistency in safety, potency, and pharmacokinetic properties, as well as efficacy, as a consequence.26
There are intriguing data to suggest a potential use for topical CBD in the management of skin diseases, including acne vulgaris. Researchers have, for at least a decade, been investigating the role of the endocannabinoid system, which has physiologic regulatory functions in proliferation, differentiation, apoptosis and cytokine, mediator, and hormone production of various cell types in skin, hair follicles, and sebaceous glands.28 Cannabidiol has been shown to suppress proliferation of sebocytes through activation of transient receptor potential vanilloid 4 ion channels and to have anti-inflammatory effects on sebocytes.29 It has been shown to inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism30 and to possess potent antimicrobial activity against gram-positive bacteria such as P acnes.31
Given these effects on sebocytes, modulation of keratinocyte proliferation, and anti-inflammatory and antibacterial effects, CBD could prove beneficial in the management of acne vulgaris. A new synthetic CBD topical formulation, BTX 1503, is under investigation for the treatment of acne vulgaris.
Early clinical data confirm both the anti-inflammatory effects of topical BTX 1503 as well as its effects on noninflammatory lesions, with 4-week reductions in inflammatory lesion counts similar to what are reported in clinical trials for leading FDA-approved topical therapies in the same time frame.
The phase 1b trial was a 4-week, open-label study in participants with moderate to severe acne vulgaris.32 The primary end point was safety, as demonstrated by the incidence of TEAE, laboratory monitoring, and assessment of cutaneous tolerability. Exploratory end points included changes in inflammatory and noninflammatory lesion counts and IGA score. A total of 21 participants aged 18 to 65 years with moderate to severe acne vulgaris were enrolled. BTX 1503 was applied topically twice daily. At baseline, eligible participants had 20 to 50 inflammatory lesions and 20 to 100 noninflammatory acne lesions on the face, an IGA of 3 (moderate) or 4 (severe), and 3 or fewer nodular or cystic lesions (>5 mm in diameter). No serious or severe TEAEs were reported; no participants withdrew due to a TEAE. Slight erythema, slight scaling, slight dryness, and slight burning and stinging were reported; there were no reports of irritant or allergic contact dermatitis. Only 1 TEAE was thought to be possibly related to treatment: mild pain at the application site.32
In addition to presenting a potential new chemical entity for the topical treatment of acne, the novel topical vehicle formulation of BTX 1503 represents an innovative approach to drug delivery. The formulation utilizes proprietary technology to deliver high doses of drug into the skin without controversial penetration enhancers, preservatives, or other potential irritating additives. Instead, volatile excipients are used that evaporate upon application to the skin, leaving a so-called superconcentrated secondary formulation on the skin. The concentration gradient effect then drives the concentrated drug into skin. Although the formulation efficiently delivers active drug into the skin and its appendages, systemic exposure has been reported to be very low. A phase 2 randomized, double-blind, vehicle-controlled trial ongoing in the United States and Australia in 360 patients with moderate to severe acne vulgaris will provide key data to confirm the efficacy and safety of BTX 1503 (ClinicalTrials.gov Identifier NCT03573518).
Conclusion
Drug development continues to focus on the challenge of treating acne effectively and safely. Vehicle innovations are optimizing existing active drugs and creating opportunities to deliver new compounds to the skin. The approval of sarecycline as the first new chemical entity approved for acne in several years may be followed in coming years by other new actives, including clascoterone and CBD.
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Burkhart CN, Gottwald L. Assessment of etiologic agents in acne pathogenesis. Skinmed. 2003;2:222-228.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337-352.
- Griffin MO, Ceballos G, Villarreal FJ. Tetracycline compounds with non-antimicrobial organ protective properties: possible mechanisms of action. Pharmacol Res. 2011;63:102-107.
- Weinberg JM. The anti-inflammatory effects of tetracyclines. Cutis. 2005;75(4 suppl):6-11.
- Leyden JJ, Sniukiene V, Berk DR, et al. Efficacy and safety of sarecycline, a novel, once-daily, narrow spectrum antibiotic for the treatment of moderate to severe facial acne vulgaris: results of a phase 2, dose-ranging study. J Drugs Dermatol. 2018;17:333-338.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Jarratt M, Werner CP, Alió Saenz AB. Tazarotene foam versus tazarotene gel: a randomized relative bioavailability study in acne vulgaris. Clin Drug Investig. 2013;33:283-289.
- Smith JA, Narahari S, Hill D, et al. Tazarotene foam, 0.1%, for the treatment of acne. Expert Opin Drug Saf. 2016;15:99-103.
- Feldman SR, Werner CP, Alió Saenz AB. The efficacy and tolerability of tazarotene foam, 0.1%, in the treatment of acne vulgaris in 2 multicenter, randomized, vehicle-controlled, double-blind studies. J Drugs Dermatol. 2013;12:438-446.
- Lee YH, Liu G, Thiboutot DM, et al. A retrospective analysis of the duration of oral antibiotic therapy for the treatment of acne among adolescents: investigating practice gaps and potential cost-savings. J Am Acad Dermatol. 2014;71:70-76.
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012(8):CD002086.
- Jones TM, Ellman H, deVries T. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177.17.
- Gold LS, Dhawan S, Weiss J, et al. FMX101 4% minocycline foam for the treatment of acne vulgaris: safety and patient satisfaction from the open-label extension of 2 phase 3 studies. Poster presented at: 2018 Winter Clinical Dermatology Conference; January 12-17, 2018; Maui, HI.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Gold LS, Del Rosso JQ, Bhatia ND, et al. Efficacy and safety of FMX103 (1.5% minocycline foam) in the treatment of moderate-to-severe papulopustular rosacea: results from two phase 3 randomized, multicenter, double-blind, vehicle-controlled studies. Poster presented at: 2019 Winter Clinical Dermatology Conference; January 18-23; 2019; Koloa, HI.
- Data on file. CB-03-01 2017. Milan, Italy: Cassiopea SpA; 2017.
- Ju Q, Tao T, Hu T, et al. Sex hormones and acne. Clin Dermatol. 2017;35:130-137.
- Park JH, Bienenfeld A, Orlow SJ, et al. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol. 2018;19:449-455.
- Barros B, Thiboutot D. Hormonal therapies for acne. Clin Dermatol. 2017;35:168-172.
- Hebert A. Clascoterone topical cream, 1%: a novel, topical, local, selective androgen receptor antagonist: results from two phase 3 studies treating children and adult patients with facial acne vulgaris. Presented at: 2019 American Academy of Dermatology Annual Meeting; March 2, 2019; Washington, DC.
- Noreen N, Muhammad F, Akhtar B, et al. Is cannabidiol a promising substance for new drug development? a review of its potential therapeutic applications. Crit Rev Eukaryot Gene Expr. 2018;28:73-86.
- White CM. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential [published online February 7, 2019]. J Clin Pharmacol. 2019;59:923-934.
- Bonn-Miller MO, ElSohly MA, Loflin MJE, et al. Cannabis and cannabinoid drug development: evaluating botanical versus single molecule approaches. Int Rev Psychiatry. 2018;30:277-284.
- Bíró T, Tóth BI, Haskó G, et al. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411-420.
- Oláh A, Tóth BI, Borbíró I, et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest. 2014;124:3713-3724.
- Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci. 2007;45:87-92.
- Appendino G, Gibbons S, Giana A, et al. Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod. 2008;71:1427-1430.
- Spleman L, Sinclair R, Freeman M, et al. The safety of topical cannabidiol (CBD) for the treatment of acne. J Invest Dermatol. 2018;138:S180.
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Burkhart CN, Gottwald L. Assessment of etiologic agents in acne pathogenesis. Skinmed. 2003;2:222-228.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337-352.
- Griffin MO, Ceballos G, Villarreal FJ. Tetracycline compounds with non-antimicrobial organ protective properties: possible mechanisms of action. Pharmacol Res. 2011;63:102-107.
- Weinberg JM. The anti-inflammatory effects of tetracyclines. Cutis. 2005;75(4 suppl):6-11.
- Leyden JJ, Sniukiene V, Berk DR, et al. Efficacy and safety of sarecycline, a novel, once-daily, narrow spectrum antibiotic for the treatment of moderate to severe facial acne vulgaris: results of a phase 2, dose-ranging study. J Drugs Dermatol. 2018;17:333-338.
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Jarratt M, Werner CP, Alió Saenz AB. Tazarotene foam versus tazarotene gel: a randomized relative bioavailability study in acne vulgaris. Clin Drug Investig. 2013;33:283-289.
- Smith JA, Narahari S, Hill D, et al. Tazarotene foam, 0.1%, for the treatment of acne. Expert Opin Drug Saf. 2016;15:99-103.
- Feldman SR, Werner CP, Alió Saenz AB. The efficacy and tolerability of tazarotene foam, 0.1%, in the treatment of acne vulgaris in 2 multicenter, randomized, vehicle-controlled, double-blind studies. J Drugs Dermatol. 2013;12:438-446.
- Lee YH, Liu G, Thiboutot DM, et al. A retrospective analysis of the duration of oral antibiotic therapy for the treatment of acne among adolescents: investigating practice gaps and potential cost-savings. J Am Acad Dermatol. 2014;71:70-76.
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012(8):CD002086.
- Jones TM, Ellman H, deVries T. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177.17.
- Gold LS, Dhawan S, Weiss J, et al. FMX101 4% minocycline foam for the treatment of acne vulgaris: safety and patient satisfaction from the open-label extension of 2 phase 3 studies. Poster presented at: 2018 Winter Clinical Dermatology Conference; January 12-17, 2018; Maui, HI.
- Raoof J, Hooper D, Moore A, et al. FMX101 4% topical minocycline foam for the treatment of moderate to severe acne vulgaris: efficacy and safety from a phase 3 randomized, double-blind, vehicle-controlled study. Poster presented at: 2018 Fall Clinical Dermatology Conference; October 18-21, 2018; Las Vegas, NV.
- Gold LS, Del Rosso JQ, Bhatia ND, et al. Efficacy and safety of FMX103 (1.5% minocycline foam) in the treatment of moderate-to-severe papulopustular rosacea: results from two phase 3 randomized, multicenter, double-blind, vehicle-controlled studies. Poster presented at: 2019 Winter Clinical Dermatology Conference; January 18-23; 2019; Koloa, HI.
- Data on file. CB-03-01 2017. Milan, Italy: Cassiopea SpA; 2017.
- Ju Q, Tao T, Hu T, et al. Sex hormones and acne. Clin Dermatol. 2017;35:130-137.
- Park JH, Bienenfeld A, Orlow SJ, et al. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol. 2018;19:449-455.
- Barros B, Thiboutot D. Hormonal therapies for acne. Clin Dermatol. 2017;35:168-172.
- Hebert A. Clascoterone topical cream, 1%: a novel, topical, local, selective androgen receptor antagonist: results from two phase 3 studies treating children and adult patients with facial acne vulgaris. Presented at: 2019 American Academy of Dermatology Annual Meeting; March 2, 2019; Washington, DC.
- Noreen N, Muhammad F, Akhtar B, et al. Is cannabidiol a promising substance for new drug development? a review of its potential therapeutic applications. Crit Rev Eukaryot Gene Expr. 2018;28:73-86.
- White CM. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential [published online February 7, 2019]. J Clin Pharmacol. 2019;59:923-934.
- Bonn-Miller MO, ElSohly MA, Loflin MJE, et al. Cannabis and cannabinoid drug development: evaluating botanical versus single molecule approaches. Int Rev Psychiatry. 2018;30:277-284.
- Bíró T, Tóth BI, Haskó G, et al. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411-420.
- Oláh A, Tóth BI, Borbíró I, et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest. 2014;124:3713-3724.
- Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci. 2007;45:87-92.
- Appendino G, Gibbons S, Giana A, et al. Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod. 2008;71:1427-1430.
- Spleman L, Sinclair R, Freeman M, et al. The safety of topical cannabidiol (CBD) for the treatment of acne. J Invest Dermatol. 2018;138:S180.
Practice Points
- Sarecycline is the first new antibiotic approved for acne in several years.
- Tazarotene foam 0.1% was relaunched to the market. The foam formulation attempts to impart moisturizing effects to offset potential irritation.
- Topical minocycline for acne optimizes the therapeutic effects while reducing systemic effects.
- Clascoterone and cannabidiol currently are under investigation for acne treatment.
Nonsurgical Hair Restoration Treatment
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).
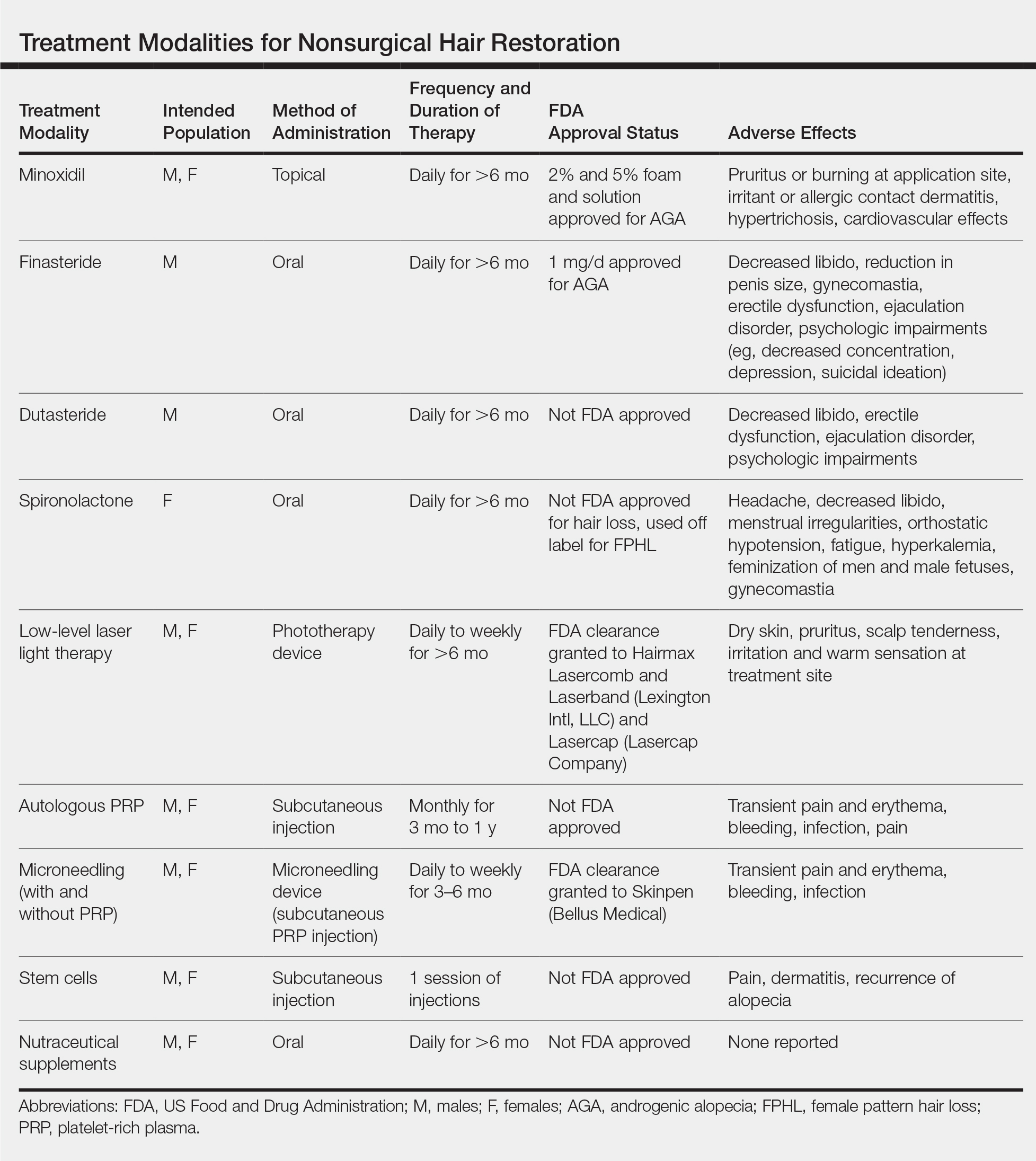
Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).

Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).

Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
Practice Points
- Hair loss is a common phenomenon in both men and women and can seriously impact psychosocial functioning.
- There are numerous US Food and Drug Administration–approved and off-label nonsurgical treatment options for alopecia. Dermatologists should be well versed in these treatment modalities and the associated sideeffect profiles to select the appropriate therapy for each patient.
Consider bleeding risk with oral anticoagulants in patients with GI cancer
MELBOURNE – The treatment of cancer-associated thrombosis may be complicated by increased bleeding risk in patients with gastrointestinal cancer, in whom direct oral anticoagulants may not be the ideal first choice, one expert reported at the International Society on Thrombosis and Haemostasis congress.
Agnes Y.Y. Lee, MD, medical director of the Thrombosis Program at Vancouver General Hospital and the University of British Columbia, spoke about the challenges and necessity of treating cancer-associated thrombosis, pointing out that about 20% of all cases of venous thromboembolism (VTE) are associated with cancer.
“In those with cancer, thrombosis can also interfere with cancer treatment, increases health care costs, and is extraordinarily burdensome to patients and their families,” she said. “Fortunately the most effective way to reduce this burden is to use anticoagulant therapy for prevention and treatment.”
While direct oral anticoagulants have been shown in several studies to be comparable to warfarin in treating most patients with thrombosis, Dr. Lee said there has been a question of how they compare in safety and efficacy to low-molecular-weight heparin in individuals with cancer.
Data from the Hokusai VTE Cancer trial, which compared oral edoxaban with subcutaneous dalteparin in patients with cancer, showed that the two treatments were comparable in time to first occurrence of thrombosis. However, the study did show a fourfold higher risk of bleeding with edoxaban, compared with that of dalteparin, among individuals with gastrointestinal cancers, a difference in bleeding rate that was not seen in patients with nongastrointestinal cancers, Dr. Lee said.
Dr. Lee pointed out that this study also showed a higher bleeding risk in patients with other bleeding risk factors, including those with primary or metastatic brain cancer.
“This study also showed that, when patients developed major bleeding, 60%-80% of them required hospitalization or an ICU stay, so major bleeding is a serious complication and certainly will increase the cost of therapy for these patients,” she said.
In the SELECT-D pilot study, which compared rivaroxaban with dalteparin in patients with cancer, there was a higher risk of bleeding for patients with esophageal or gastroesophageal cancers.
Bleeding risk is generally not well addressed in current guidelines on managing hemostasis in patients with malignancies, partly because it is difficult to quantify bleeding in these patients whose hemoglobin levels would be affected by their disease and their chemotherapy, Dr. Lee said in an interview.
“The bleeding events in cancer patients do get more complicated because there’s all this other noise in the background,” she said.
Commenting on her personal approach to treatment, Dr. Lee said she favors starting patients on low-molecular-weight heparin because it gives her time to understand patients, their disease, and their needs.
“A lot of patients arrive, and they can’t really tell me what their cancer is doing, they can’t really tell me what cancer therapy they’re going through,” she says. “And if they’re on a long list of drugs, then I have to talk to my pharmacist about whether there are drug-drug interactions.”
If patients were well managed on low-molecular-weight heparin without any bleeding, then Dr. Lee said she would consider switching them to direct oral anticoagulants.
Cochair of the session, Ingrid Pabinger, MD, from the Medical University of Vienna commented that vitamin K antagonists should not be forgotten because some patients are unable to afford low-molecular-weight heparin.
However Dr. Lee said these were last on the list for her because of the risk of drug-drug interactions, drug-food interactions, and the issues faced by patients experiencing vomiting or diarrhea with their chemotherapy.
Dr. Lee reported research funding, consultancies, and honoraria from the pharmaceutical sector.
MELBOURNE – The treatment of cancer-associated thrombosis may be complicated by increased bleeding risk in patients with gastrointestinal cancer, in whom direct oral anticoagulants may not be the ideal first choice, one expert reported at the International Society on Thrombosis and Haemostasis congress.
Agnes Y.Y. Lee, MD, medical director of the Thrombosis Program at Vancouver General Hospital and the University of British Columbia, spoke about the challenges and necessity of treating cancer-associated thrombosis, pointing out that about 20% of all cases of venous thromboembolism (VTE) are associated with cancer.
“In those with cancer, thrombosis can also interfere with cancer treatment, increases health care costs, and is extraordinarily burdensome to patients and their families,” she said. “Fortunately the most effective way to reduce this burden is to use anticoagulant therapy for prevention and treatment.”
While direct oral anticoagulants have been shown in several studies to be comparable to warfarin in treating most patients with thrombosis, Dr. Lee said there has been a question of how they compare in safety and efficacy to low-molecular-weight heparin in individuals with cancer.
Data from the Hokusai VTE Cancer trial, which compared oral edoxaban with subcutaneous dalteparin in patients with cancer, showed that the two treatments were comparable in time to first occurrence of thrombosis. However, the study did show a fourfold higher risk of bleeding with edoxaban, compared with that of dalteparin, among individuals with gastrointestinal cancers, a difference in bleeding rate that was not seen in patients with nongastrointestinal cancers, Dr. Lee said.
Dr. Lee pointed out that this study also showed a higher bleeding risk in patients with other bleeding risk factors, including those with primary or metastatic brain cancer.
“This study also showed that, when patients developed major bleeding, 60%-80% of them required hospitalization or an ICU stay, so major bleeding is a serious complication and certainly will increase the cost of therapy for these patients,” she said.
In the SELECT-D pilot study, which compared rivaroxaban with dalteparin in patients with cancer, there was a higher risk of bleeding for patients with esophageal or gastroesophageal cancers.
Bleeding risk is generally not well addressed in current guidelines on managing hemostasis in patients with malignancies, partly because it is difficult to quantify bleeding in these patients whose hemoglobin levels would be affected by their disease and their chemotherapy, Dr. Lee said in an interview.
“The bleeding events in cancer patients do get more complicated because there’s all this other noise in the background,” she said.
Commenting on her personal approach to treatment, Dr. Lee said she favors starting patients on low-molecular-weight heparin because it gives her time to understand patients, their disease, and their needs.
“A lot of patients arrive, and they can’t really tell me what their cancer is doing, they can’t really tell me what cancer therapy they’re going through,” she says. “And if they’re on a long list of drugs, then I have to talk to my pharmacist about whether there are drug-drug interactions.”
If patients were well managed on low-molecular-weight heparin without any bleeding, then Dr. Lee said she would consider switching them to direct oral anticoagulants.
Cochair of the session, Ingrid Pabinger, MD, from the Medical University of Vienna commented that vitamin K antagonists should not be forgotten because some patients are unable to afford low-molecular-weight heparin.
However Dr. Lee said these were last on the list for her because of the risk of drug-drug interactions, drug-food interactions, and the issues faced by patients experiencing vomiting or diarrhea with their chemotherapy.
Dr. Lee reported research funding, consultancies, and honoraria from the pharmaceutical sector.
MELBOURNE – The treatment of cancer-associated thrombosis may be complicated by increased bleeding risk in patients with gastrointestinal cancer, in whom direct oral anticoagulants may not be the ideal first choice, one expert reported at the International Society on Thrombosis and Haemostasis congress.
Agnes Y.Y. Lee, MD, medical director of the Thrombosis Program at Vancouver General Hospital and the University of British Columbia, spoke about the challenges and necessity of treating cancer-associated thrombosis, pointing out that about 20% of all cases of venous thromboembolism (VTE) are associated with cancer.
“In those with cancer, thrombosis can also interfere with cancer treatment, increases health care costs, and is extraordinarily burdensome to patients and their families,” she said. “Fortunately the most effective way to reduce this burden is to use anticoagulant therapy for prevention and treatment.”
While direct oral anticoagulants have been shown in several studies to be comparable to warfarin in treating most patients with thrombosis, Dr. Lee said there has been a question of how they compare in safety and efficacy to low-molecular-weight heparin in individuals with cancer.
Data from the Hokusai VTE Cancer trial, which compared oral edoxaban with subcutaneous dalteparin in patients with cancer, showed that the two treatments were comparable in time to first occurrence of thrombosis. However, the study did show a fourfold higher risk of bleeding with edoxaban, compared with that of dalteparin, among individuals with gastrointestinal cancers, a difference in bleeding rate that was not seen in patients with nongastrointestinal cancers, Dr. Lee said.
Dr. Lee pointed out that this study also showed a higher bleeding risk in patients with other bleeding risk factors, including those with primary or metastatic brain cancer.
“This study also showed that, when patients developed major bleeding, 60%-80% of them required hospitalization or an ICU stay, so major bleeding is a serious complication and certainly will increase the cost of therapy for these patients,” she said.
In the SELECT-D pilot study, which compared rivaroxaban with dalteparin in patients with cancer, there was a higher risk of bleeding for patients with esophageal or gastroesophageal cancers.
Bleeding risk is generally not well addressed in current guidelines on managing hemostasis in patients with malignancies, partly because it is difficult to quantify bleeding in these patients whose hemoglobin levels would be affected by their disease and their chemotherapy, Dr. Lee said in an interview.
“The bleeding events in cancer patients do get more complicated because there’s all this other noise in the background,” she said.
Commenting on her personal approach to treatment, Dr. Lee said she favors starting patients on low-molecular-weight heparin because it gives her time to understand patients, their disease, and their needs.
“A lot of patients arrive, and they can’t really tell me what their cancer is doing, they can’t really tell me what cancer therapy they’re going through,” she says. “And if they’re on a long list of drugs, then I have to talk to my pharmacist about whether there are drug-drug interactions.”
If patients were well managed on low-molecular-weight heparin without any bleeding, then Dr. Lee said she would consider switching them to direct oral anticoagulants.
Cochair of the session, Ingrid Pabinger, MD, from the Medical University of Vienna commented that vitamin K antagonists should not be forgotten because some patients are unable to afford low-molecular-weight heparin.
However Dr. Lee said these were last on the list for her because of the risk of drug-drug interactions, drug-food interactions, and the issues faced by patients experiencing vomiting or diarrhea with their chemotherapy.
Dr. Lee reported research funding, consultancies, and honoraria from the pharmaceutical sector.
EXPERT ANALYSIS FROM 2019 ISTH CONGRESS
Acne in women: What new insights tell us
NEWPORT BEACH, CALIF. – When it comes to acne in adult women, look past the jawline, beyond traditional medications, and toward greater control. That’s the message of a dermatologist who spoke at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“We should be aiming to get our patients to clear or almost clear, and we have the tools necessary to help that happen,” said Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.
she noted. Acne appears to affect 51% of women aged 20-29 years, she said, and prevalence dips to 15% in women older than 50 years.
About 80% of cases continue from adolescence, compared with about 20% that are new-onset during adulthood, she said. According to studies, she added, “most adult women have acne on multiple different areas of their face, not just the jawline. It’s similar to what we see in the adolescent population.”
Dr. Stein Gold offered these tips about treatment in this group of patients:
Inflammation
Researchers now consider that “all acne is inflammatory acne.” Be aggressive with anti-inflammatory treatment, and “continue even after the lesion is resolved” if needed to prevent scarring.
Oral contraceptives (OCs)
OCs can be helpful, but “we have to proceed with caution,” she said. A 2012 Cochrane Library review of 31 trials found that six combination OCs (COCs) “evaluated in placebo-controlled trials are effective in reducing inflammatory and noninflammatory facial acne lesions. Few important and consistent differences were found between COC types in their effectiveness for treating acne,” the review concluded (Cochrane Database Syst Rev. 2012 Jul 11;[7]:CD004425).
Results take time, however, and it “can take 3 months to see an effect, and 6 months for full effect,” Dr. Stein Gold noted.
There are multiple contraindications to the use of OCs, and they’ve been linked – controversially – to an increased risk of blood clots and breast cancer. However, risk of thrombosis also spikes – to significantly higher levels than with OC use – during pregnancy and the postpartum period, she said.
Spironolactone
This antihypertensive drug can be helpful, Dr. Stein Gold noted, although the one study in a 2009 Cochrane review that had acne as an outcome failed to find evidence of efficacy versus placebo (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD000194). Be aware of the boxed warning about links to cancer in rat studies, and consider the risk of potassium elevation in certain populations, she added. Watch the dose: fewer side effects are seen at 50-100 mg daily, although they’re still common, and it can take 3 months or more for improvements to appear, she said.
Truncal acne
Patients may be hesitant to mention they have acne on their chest and back. “They may not tell you about it, and you may not ask about it but [some patients] expect you to know about it and treat it,” Dr. Stein Gold said. She referred to trifarotene, a topical retinoid cream that, although not yet approved, appears to be safe and effective in treating acne on the face and trunk in phase 3 studies.
“Some people will say the trunk will get too irritated if you put a retinoid on it. But it absolutely can be used on the chest and back. The first thing I say to my patients is to expect to have redness and scaling for first 2 weeks. People pay money for that. It’s a chemical peel! It’s okay to have some sloughing; use an oil-free moisturizer.”
Dr. Stein Gold disclosed relationships with Galderma, Foamix, and Sol Gel (investigator, consultant); Valeant (consultant, speaker); and Dermira (investigator, speaker).
SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – When it comes to acne in adult women, look past the jawline, beyond traditional medications, and toward greater control. That’s the message of a dermatologist who spoke at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“We should be aiming to get our patients to clear or almost clear, and we have the tools necessary to help that happen,” said Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.
she noted. Acne appears to affect 51% of women aged 20-29 years, she said, and prevalence dips to 15% in women older than 50 years.
About 80% of cases continue from adolescence, compared with about 20% that are new-onset during adulthood, she said. According to studies, she added, “most adult women have acne on multiple different areas of their face, not just the jawline. It’s similar to what we see in the adolescent population.”
Dr. Stein Gold offered these tips about treatment in this group of patients:
Inflammation
Researchers now consider that “all acne is inflammatory acne.” Be aggressive with anti-inflammatory treatment, and “continue even after the lesion is resolved” if needed to prevent scarring.
Oral contraceptives (OCs)
OCs can be helpful, but “we have to proceed with caution,” she said. A 2012 Cochrane Library review of 31 trials found that six combination OCs (COCs) “evaluated in placebo-controlled trials are effective in reducing inflammatory and noninflammatory facial acne lesions. Few important and consistent differences were found between COC types in their effectiveness for treating acne,” the review concluded (Cochrane Database Syst Rev. 2012 Jul 11;[7]:CD004425).
Results take time, however, and it “can take 3 months to see an effect, and 6 months for full effect,” Dr. Stein Gold noted.
There are multiple contraindications to the use of OCs, and they’ve been linked – controversially – to an increased risk of blood clots and breast cancer. However, risk of thrombosis also spikes – to significantly higher levels than with OC use – during pregnancy and the postpartum period, she said.
Spironolactone
This antihypertensive drug can be helpful, Dr. Stein Gold noted, although the one study in a 2009 Cochrane review that had acne as an outcome failed to find evidence of efficacy versus placebo (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD000194). Be aware of the boxed warning about links to cancer in rat studies, and consider the risk of potassium elevation in certain populations, she added. Watch the dose: fewer side effects are seen at 50-100 mg daily, although they’re still common, and it can take 3 months or more for improvements to appear, she said.
Truncal acne
Patients may be hesitant to mention they have acne on their chest and back. “They may not tell you about it, and you may not ask about it but [some patients] expect you to know about it and treat it,” Dr. Stein Gold said. She referred to trifarotene, a topical retinoid cream that, although not yet approved, appears to be safe and effective in treating acne on the face and trunk in phase 3 studies.
“Some people will say the trunk will get too irritated if you put a retinoid on it. But it absolutely can be used on the chest and back. The first thing I say to my patients is to expect to have redness and scaling for first 2 weeks. People pay money for that. It’s a chemical peel! It’s okay to have some sloughing; use an oil-free moisturizer.”
Dr. Stein Gold disclosed relationships with Galderma, Foamix, and Sol Gel (investigator, consultant); Valeant (consultant, speaker); and Dermira (investigator, speaker).
SDEF and this news organization are owned by the same parent company.
NEWPORT BEACH, CALIF. – When it comes to acne in adult women, look past the jawline, beyond traditional medications, and toward greater control. That’s the message of a dermatologist who spoke at Skin Disease Education Foundation’s Women’s & Pediatric Dermatology Seminar.
“We should be aiming to get our patients to clear or almost clear, and we have the tools necessary to help that happen,” said Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.
she noted. Acne appears to affect 51% of women aged 20-29 years, she said, and prevalence dips to 15% in women older than 50 years.
About 80% of cases continue from adolescence, compared with about 20% that are new-onset during adulthood, she said. According to studies, she added, “most adult women have acne on multiple different areas of their face, not just the jawline. It’s similar to what we see in the adolescent population.”
Dr. Stein Gold offered these tips about treatment in this group of patients:
Inflammation
Researchers now consider that “all acne is inflammatory acne.” Be aggressive with anti-inflammatory treatment, and “continue even after the lesion is resolved” if needed to prevent scarring.
Oral contraceptives (OCs)
OCs can be helpful, but “we have to proceed with caution,” she said. A 2012 Cochrane Library review of 31 trials found that six combination OCs (COCs) “evaluated in placebo-controlled trials are effective in reducing inflammatory and noninflammatory facial acne lesions. Few important and consistent differences were found between COC types in their effectiveness for treating acne,” the review concluded (Cochrane Database Syst Rev. 2012 Jul 11;[7]:CD004425).
Results take time, however, and it “can take 3 months to see an effect, and 6 months for full effect,” Dr. Stein Gold noted.
There are multiple contraindications to the use of OCs, and they’ve been linked – controversially – to an increased risk of blood clots and breast cancer. However, risk of thrombosis also spikes – to significantly higher levels than with OC use – during pregnancy and the postpartum period, she said.
Spironolactone
This antihypertensive drug can be helpful, Dr. Stein Gold noted, although the one study in a 2009 Cochrane review that had acne as an outcome failed to find evidence of efficacy versus placebo (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD000194). Be aware of the boxed warning about links to cancer in rat studies, and consider the risk of potassium elevation in certain populations, she added. Watch the dose: fewer side effects are seen at 50-100 mg daily, although they’re still common, and it can take 3 months or more for improvements to appear, she said.
Truncal acne
Patients may be hesitant to mention they have acne on their chest and back. “They may not tell you about it, and you may not ask about it but [some patients] expect you to know about it and treat it,” Dr. Stein Gold said. She referred to trifarotene, a topical retinoid cream that, although not yet approved, appears to be safe and effective in treating acne on the face and trunk in phase 3 studies.
“Some people will say the trunk will get too irritated if you put a retinoid on it. But it absolutely can be used on the chest and back. The first thing I say to my patients is to expect to have redness and scaling for first 2 weeks. People pay money for that. It’s a chemical peel! It’s okay to have some sloughing; use an oil-free moisturizer.”
Dr. Stein Gold disclosed relationships with Galderma, Foamix, and Sol Gel (investigator, consultant); Valeant (consultant, speaker); and Dermira (investigator, speaker).
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Tildrakizumab shows positive effects in active psoriatic arthritis
MADRID – Tildrakizumab, a high-affinity anti–interleukin-23p19 monoclonal antibody, significantly improved joint and skin manifestations in patients with psoriatic arthritis in an ongoing phase 2b study.
“By week 24, all four doses of tildrakizumab were significantly more efficacious than placebo,” Philip J. Mease, MD, director of the division of rheumatology clinical research at Swedish Medical Center, Seattle, reported at the European Congress of Rheumatology. This included patient-rated pain, he observed.
Furthermore, “there was a clear separation between tildrakizumab and placebo as early as 8 weeks” for the trial’s primary endpoint, a 20% response rate on American College of Rheumatology criteria (ACR20) at 24 weeks.
The study (NCT02980692), which is projected to complete next year, was conducted to demonstrate the safety and efficacy of tildrakizumab in patients with active psoriatic arthritis. Tildrakizumab is already approved for the treatment of moderate to severe plaque psoriasis in multiple countries, Dr. Mease pointed out. Indeed, the drug – which is marketed as Ilumya in the United States and as Ilumetri in the Europe Union – was approved by the Food and Drug Administration in March last year based on the positive results of the phase 3 reSURFACE clinical trials program (Drugs. 2018;78[8]:845-9).
In presenting interim findings from the study, Dr. Mease observed that “it looked like shortening the dosing interval from Q12 to Q4 weeks for the 200-mg dose did not result in a measurable difference in skin or joint responses.”
The trial included 391 of 500 adult patients who were screened and then randomized to one of four tildrakizumab dosing groups or placebo; there were 78 patients treated with tildrakizumab 200 mg once every 4 weeks (Q4W) and 79 who were treated with tildrakizumab 200 mg once every 12 weeks (Q12W). A further 77 patients were treated with a 100 mg tildrakizumab dose Q12W, 78 patients with a 20 mg tildrakizumab dose Q12W, and 79 patients were treated with a placebo Q4W.
The mean age of patients included in the study was around 48 years. A total of 55% were female, and more than 96% were white. Across the groups, patients had a median of 7-8 tender joints and about 14-19 swollen joints, and 53%-70% had at least 3% psoriasis body surface area involvement.
The primary endpoint of ACR20 at 24 weeks was met by 79.5%, 77.2%, 71.4%, and 73.1% of patients in the tildrakizumab 200-mg Q4W, 200-mg Q12W, 100-mg Q12W, and 20-mg Q12W groups, and by 50.6% of the placebo-treated patients. “So even the very low dose had an effect,” Dr. Mease observed, also acknowledging the “very high placebo response.”
An ACR50 response was achieved by a respective 53.6%, 50.6%, 45.5%, 39.7%, 19.7%, and 24.1% of patients. ACR70 response rates were also “proportionately lower” than the ACR20 responses at around 25%-29% for the tildrakizumab groups and 16% for placebo.
“The skin scores were as expected quite high,” Dr. Mease said. The Psoriasis Area and Severity Index (PASI) 75 response rate was 79.6% in the tildrakizumab 200-mg Q12W group, 64.2% in the 200-mg Q4W group, 55.6% in the 100-mg Q12W group, 46.3% in the 20-mg Q12W group, and just 16.7% in the placebo group. The respective percentages of patients achieving a PASI 90 response rate were 50%, 47.2%, 38.9%, 36.6%, and 7.1%.
Patient pain assessment showed a clear reduction with tildrakizumab versus placebo treatment. “We see statistical separation between all of the tildrakizumab arms and placebo,” Dr. Mease said. “A greater than 50% response in pain is considered major clinical improvement, and that was achieved by all of the tildrakizumab arms.”
As for enthesitis, the mean change in Leeds Enthesitis Scores from baseline to week 24 were greater with all tildrakizumab doses than with placebo, although a high placebo response was again apparent.
“In general, the safety profile was very good for this agent,” Dr. Mease said. Any treatment-emergent adverse event (TEAE) occurred in 156 of 317 (49%) tildrakizumab-treated patients and in 34 of 70 (49%) placebo-treated patients. The rates of any severe TEAE were 2.2% for the tildrakizumab arms and 2.5% for placebo. Any TEAE related to treatment occurred in a respective 11.2% and 12.7%, but there were no discontinuations because of adverse events, nor were there any major cardiac adverse events, cases of malignancy, or deaths caused by TEAEs. There was a single serious infection, a case of tonsillitis, which occurred with tildrakizumab treatment.
In response to a question after his presentation, Dr. Measure noted: “In the psoriasis trials with this agent, even a single dose yielded a fairly meaningful PASI 75 responses out for extremely long periods of time, 6–12 months. So, it looks like the Q12 dosing is going to be reasonable and convenient for patients”. He also agreed with a comment that the more frequent dosing seemed to be linked to inferior responses in the skin.
The study was sponsored by Sun Pharmaceutical Industries. Dr. Mease has received research grants, consulting fees, and/or speaker fees from 15 pharmaceutical companies, including Sun Pharmaceutical Industries.
SOURCE: Mease PJ et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):77-9. Abstract LB0002, doi: 10.1136/annrheumdis-2019-eular.8669
MADRID – Tildrakizumab, a high-affinity anti–interleukin-23p19 monoclonal antibody, significantly improved joint and skin manifestations in patients with psoriatic arthritis in an ongoing phase 2b study.
“By week 24, all four doses of tildrakizumab were significantly more efficacious than placebo,” Philip J. Mease, MD, director of the division of rheumatology clinical research at Swedish Medical Center, Seattle, reported at the European Congress of Rheumatology. This included patient-rated pain, he observed.
Furthermore, “there was a clear separation between tildrakizumab and placebo as early as 8 weeks” for the trial’s primary endpoint, a 20% response rate on American College of Rheumatology criteria (ACR20) at 24 weeks.
The study (NCT02980692), which is projected to complete next year, was conducted to demonstrate the safety and efficacy of tildrakizumab in patients with active psoriatic arthritis. Tildrakizumab is already approved for the treatment of moderate to severe plaque psoriasis in multiple countries, Dr. Mease pointed out. Indeed, the drug – which is marketed as Ilumya in the United States and as Ilumetri in the Europe Union – was approved by the Food and Drug Administration in March last year based on the positive results of the phase 3 reSURFACE clinical trials program (Drugs. 2018;78[8]:845-9).
In presenting interim findings from the study, Dr. Mease observed that “it looked like shortening the dosing interval from Q12 to Q4 weeks for the 200-mg dose did not result in a measurable difference in skin or joint responses.”
The trial included 391 of 500 adult patients who were screened and then randomized to one of four tildrakizumab dosing groups or placebo; there were 78 patients treated with tildrakizumab 200 mg once every 4 weeks (Q4W) and 79 who were treated with tildrakizumab 200 mg once every 12 weeks (Q12W). A further 77 patients were treated with a 100 mg tildrakizumab dose Q12W, 78 patients with a 20 mg tildrakizumab dose Q12W, and 79 patients were treated with a placebo Q4W.
The mean age of patients included in the study was around 48 years. A total of 55% were female, and more than 96% were white. Across the groups, patients had a median of 7-8 tender joints and about 14-19 swollen joints, and 53%-70% had at least 3% psoriasis body surface area involvement.
The primary endpoint of ACR20 at 24 weeks was met by 79.5%, 77.2%, 71.4%, and 73.1% of patients in the tildrakizumab 200-mg Q4W, 200-mg Q12W, 100-mg Q12W, and 20-mg Q12W groups, and by 50.6% of the placebo-treated patients. “So even the very low dose had an effect,” Dr. Mease observed, also acknowledging the “very high placebo response.”
An ACR50 response was achieved by a respective 53.6%, 50.6%, 45.5%, 39.7%, 19.7%, and 24.1% of patients. ACR70 response rates were also “proportionately lower” than the ACR20 responses at around 25%-29% for the tildrakizumab groups and 16% for placebo.
“The skin scores were as expected quite high,” Dr. Mease said. The Psoriasis Area and Severity Index (PASI) 75 response rate was 79.6% in the tildrakizumab 200-mg Q12W group, 64.2% in the 200-mg Q4W group, 55.6% in the 100-mg Q12W group, 46.3% in the 20-mg Q12W group, and just 16.7% in the placebo group. The respective percentages of patients achieving a PASI 90 response rate were 50%, 47.2%, 38.9%, 36.6%, and 7.1%.
Patient pain assessment showed a clear reduction with tildrakizumab versus placebo treatment. “We see statistical separation between all of the tildrakizumab arms and placebo,” Dr. Mease said. “A greater than 50% response in pain is considered major clinical improvement, and that was achieved by all of the tildrakizumab arms.”
As for enthesitis, the mean change in Leeds Enthesitis Scores from baseline to week 24 were greater with all tildrakizumab doses than with placebo, although a high placebo response was again apparent.
“In general, the safety profile was very good for this agent,” Dr. Mease said. Any treatment-emergent adverse event (TEAE) occurred in 156 of 317 (49%) tildrakizumab-treated patients and in 34 of 70 (49%) placebo-treated patients. The rates of any severe TEAE were 2.2% for the tildrakizumab arms and 2.5% for placebo. Any TEAE related to treatment occurred in a respective 11.2% and 12.7%, but there were no discontinuations because of adverse events, nor were there any major cardiac adverse events, cases of malignancy, or deaths caused by TEAEs. There was a single serious infection, a case of tonsillitis, which occurred with tildrakizumab treatment.
In response to a question after his presentation, Dr. Measure noted: “In the psoriasis trials with this agent, even a single dose yielded a fairly meaningful PASI 75 responses out for extremely long periods of time, 6–12 months. So, it looks like the Q12 dosing is going to be reasonable and convenient for patients”. He also agreed with a comment that the more frequent dosing seemed to be linked to inferior responses in the skin.
The study was sponsored by Sun Pharmaceutical Industries. Dr. Mease has received research grants, consulting fees, and/or speaker fees from 15 pharmaceutical companies, including Sun Pharmaceutical Industries.
SOURCE: Mease PJ et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):77-9. Abstract LB0002, doi: 10.1136/annrheumdis-2019-eular.8669
MADRID – Tildrakizumab, a high-affinity anti–interleukin-23p19 monoclonal antibody, significantly improved joint and skin manifestations in patients with psoriatic arthritis in an ongoing phase 2b study.
“By week 24, all four doses of tildrakizumab were significantly more efficacious than placebo,” Philip J. Mease, MD, director of the division of rheumatology clinical research at Swedish Medical Center, Seattle, reported at the European Congress of Rheumatology. This included patient-rated pain, he observed.
Furthermore, “there was a clear separation between tildrakizumab and placebo as early as 8 weeks” for the trial’s primary endpoint, a 20% response rate on American College of Rheumatology criteria (ACR20) at 24 weeks.
The study (NCT02980692), which is projected to complete next year, was conducted to demonstrate the safety and efficacy of tildrakizumab in patients with active psoriatic arthritis. Tildrakizumab is already approved for the treatment of moderate to severe plaque psoriasis in multiple countries, Dr. Mease pointed out. Indeed, the drug – which is marketed as Ilumya in the United States and as Ilumetri in the Europe Union – was approved by the Food and Drug Administration in March last year based on the positive results of the phase 3 reSURFACE clinical trials program (Drugs. 2018;78[8]:845-9).
In presenting interim findings from the study, Dr. Mease observed that “it looked like shortening the dosing interval from Q12 to Q4 weeks for the 200-mg dose did not result in a measurable difference in skin or joint responses.”
The trial included 391 of 500 adult patients who were screened and then randomized to one of four tildrakizumab dosing groups or placebo; there were 78 patients treated with tildrakizumab 200 mg once every 4 weeks (Q4W) and 79 who were treated with tildrakizumab 200 mg once every 12 weeks (Q12W). A further 77 patients were treated with a 100 mg tildrakizumab dose Q12W, 78 patients with a 20 mg tildrakizumab dose Q12W, and 79 patients were treated with a placebo Q4W.
The mean age of patients included in the study was around 48 years. A total of 55% were female, and more than 96% were white. Across the groups, patients had a median of 7-8 tender joints and about 14-19 swollen joints, and 53%-70% had at least 3% psoriasis body surface area involvement.
The primary endpoint of ACR20 at 24 weeks was met by 79.5%, 77.2%, 71.4%, and 73.1% of patients in the tildrakizumab 200-mg Q4W, 200-mg Q12W, 100-mg Q12W, and 20-mg Q12W groups, and by 50.6% of the placebo-treated patients. “So even the very low dose had an effect,” Dr. Mease observed, also acknowledging the “very high placebo response.”
An ACR50 response was achieved by a respective 53.6%, 50.6%, 45.5%, 39.7%, 19.7%, and 24.1% of patients. ACR70 response rates were also “proportionately lower” than the ACR20 responses at around 25%-29% for the tildrakizumab groups and 16% for placebo.
“The skin scores were as expected quite high,” Dr. Mease said. The Psoriasis Area and Severity Index (PASI) 75 response rate was 79.6% in the tildrakizumab 200-mg Q12W group, 64.2% in the 200-mg Q4W group, 55.6% in the 100-mg Q12W group, 46.3% in the 20-mg Q12W group, and just 16.7% in the placebo group. The respective percentages of patients achieving a PASI 90 response rate were 50%, 47.2%, 38.9%, 36.6%, and 7.1%.
Patient pain assessment showed a clear reduction with tildrakizumab versus placebo treatment. “We see statistical separation between all of the tildrakizumab arms and placebo,” Dr. Mease said. “A greater than 50% response in pain is considered major clinical improvement, and that was achieved by all of the tildrakizumab arms.”
As for enthesitis, the mean change in Leeds Enthesitis Scores from baseline to week 24 were greater with all tildrakizumab doses than with placebo, although a high placebo response was again apparent.
“In general, the safety profile was very good for this agent,” Dr. Mease said. Any treatment-emergent adverse event (TEAE) occurred in 156 of 317 (49%) tildrakizumab-treated patients and in 34 of 70 (49%) placebo-treated patients. The rates of any severe TEAE were 2.2% for the tildrakizumab arms and 2.5% for placebo. Any TEAE related to treatment occurred in a respective 11.2% and 12.7%, but there were no discontinuations because of adverse events, nor were there any major cardiac adverse events, cases of malignancy, or deaths caused by TEAEs. There was a single serious infection, a case of tonsillitis, which occurred with tildrakizumab treatment.
In response to a question after his presentation, Dr. Measure noted: “In the psoriasis trials with this agent, even a single dose yielded a fairly meaningful PASI 75 responses out for extremely long periods of time, 6–12 months. So, it looks like the Q12 dosing is going to be reasonable and convenient for patients”. He also agreed with a comment that the more frequent dosing seemed to be linked to inferior responses in the skin.
The study was sponsored by Sun Pharmaceutical Industries. Dr. Mease has received research grants, consulting fees, and/or speaker fees from 15 pharmaceutical companies, including Sun Pharmaceutical Industries.
SOURCE: Mease PJ et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):77-9. Abstract LB0002, doi: 10.1136/annrheumdis-2019-eular.8669
REPORTING FROM EULAR 2019 CONGRESS
Key clinical point: Tildrakizumab (Ilumya) significantly improved joint and skin manifestations in patients with psoriatic arthritis.
Major finding: At 24 weeks, more than 70% of patients treated with tildrakizumab versus 50% of those given placebo achieved an American College of Rheumatology (ACR) 20 response.
Study details: Randomized, double-blind, placebo-controlled, multiple-dose, phase 2b study to demonstrate the safety and efficacy of tildrakizumab in patients with active psoriatic arthritis.
Disclosures: The study was sponsored by Sun Pharmaceutical Industries. Dr. Mease has received research grants, consulting fees, speaker fees, or all, from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Galapagos, Genentech, Gilead, Janssen, Leo, Eli Lilly, Merck, Novartis, Pfizer, UCB, and Sun Pharmaceutical Industries.
Source: Mease PJ et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):77-9. Abstract LB0002, doi: 10.1136/annrheumdis-2019-eular.8669
Metformin linked to lower dementia risk in black patients
Black individuals who develop type 2 diabetes are more likely than their white counterparts to develop dementia. Now, findings from a new study point to a possible preventive strategy: Putting older patients on metformin when they are diagnosed could reduce their risk for dementia by as much as 40%, whereas sulfonylureas do not seem to have such an effect.
The researchers did not examine cause and effect, so their findings are not conclusive, and very few women were included in the study. Still, the authors said that their data showing a 29% lower risk of dementia associated with metformin use in black patients aged 65-74 years, and a 40% lower risk in those aged 50-64 years, suggested that “this inexpensive, widely available treatment could be broadly prescribed to substantially reduce the risk of dementia in younger [black] patients with [type 2 diabetes]” (Ann Fam Med. 2019;17:352-62).
Previous findings have suggested that black patients with type 2 diabetes face a 10%-18% higher risk of dementia, compared with white patients (Diabetes Care. 2014; 37[4]:1009-15). Another study linked type 2 diabetes in middle-aged black patients to a 41% decrease in cognition per test results over 14 years. There was no such decrease in white patients (Neuroepidemiology. 2014;43[3-4]: 220-7).
For the new study, researchers led by Jeffrey F. Scherrer, PhD, of Saint Louis University tracked 73,761 patients aged 50 years or older from 2000-2001 (when they were free of dementia and not taking diabetes) to 2015. Among the patients, 86% were white and 14% were black. In the white and black groups, 97% and 95% were men, respectively, and 61% and 55% were obese, respectively.
All participants began metformin (76%) or sulfonylurea (24%) monotherapy after the baseline period. Guidelines recommend metformin as a first-line treatment for type 2 diabetes, whereas sulfonylureas are considered second-line drugs that should be added to metformin.
After adjustment for confounders such as socioeconomic status and other medical conditions, the researchers found a significantly lower risk of dementia in black patients who took metformin, compared with those taking a sulfonylurea (hazard ratio, 0.73; 95% confidence interval, 0.6-0.89). There was no difference between the drugs among white patients (HR, 0.96; 95% CI, 0.9-1.03, both P = .008)
The results were not statistically significant among age groups, but there were trends. In black patients, the dementia-lowering benefit was largest among those aged 50-64 years (HR, 0.6; 95% CI, 0.45-0.81), followed by those aged 65-74 years (HR, 0.71; 95% CI, 0.53-0.94), and there was no benefit among those aged at least 75 (HR, 1.17; 95% CI, 0.73-1.85) all P = .055. There was a slight benefit among white patients in one of the age groups – 65-74 years (HR, 0.9; 95% CI, 0.82-0.99; P = .315).
The authors suggested that the findings could have been the result of an effect of metformin to reduce vascular disease and chronic inflammation in black patients.
They also noted that further research is needed to identify the demographic and clinical subgroups in which metformin is most strongly associated with a reduction in the risk of dementia. In addition, they emphasized that clinical trials are needed to confirm the study findings.
The National Institutes of Health funded the study. The authors report no relevant disclosures.
SOURCE: Scherrer JF et al. Ann Fam Med. 2019;17:352-62.
Black individuals who develop type 2 diabetes are more likely than their white counterparts to develop dementia. Now, findings from a new study point to a possible preventive strategy: Putting older patients on metformin when they are diagnosed could reduce their risk for dementia by as much as 40%, whereas sulfonylureas do not seem to have such an effect.
The researchers did not examine cause and effect, so their findings are not conclusive, and very few women were included in the study. Still, the authors said that their data showing a 29% lower risk of dementia associated with metformin use in black patients aged 65-74 years, and a 40% lower risk in those aged 50-64 years, suggested that “this inexpensive, widely available treatment could be broadly prescribed to substantially reduce the risk of dementia in younger [black] patients with [type 2 diabetes]” (Ann Fam Med. 2019;17:352-62).
Previous findings have suggested that black patients with type 2 diabetes face a 10%-18% higher risk of dementia, compared with white patients (Diabetes Care. 2014; 37[4]:1009-15). Another study linked type 2 diabetes in middle-aged black patients to a 41% decrease in cognition per test results over 14 years. There was no such decrease in white patients (Neuroepidemiology. 2014;43[3-4]: 220-7).
For the new study, researchers led by Jeffrey F. Scherrer, PhD, of Saint Louis University tracked 73,761 patients aged 50 years or older from 2000-2001 (when they were free of dementia and not taking diabetes) to 2015. Among the patients, 86% were white and 14% were black. In the white and black groups, 97% and 95% were men, respectively, and 61% and 55% were obese, respectively.
All participants began metformin (76%) or sulfonylurea (24%) monotherapy after the baseline period. Guidelines recommend metformin as a first-line treatment for type 2 diabetes, whereas sulfonylureas are considered second-line drugs that should be added to metformin.
After adjustment for confounders such as socioeconomic status and other medical conditions, the researchers found a significantly lower risk of dementia in black patients who took metformin, compared with those taking a sulfonylurea (hazard ratio, 0.73; 95% confidence interval, 0.6-0.89). There was no difference between the drugs among white patients (HR, 0.96; 95% CI, 0.9-1.03, both P = .008)
The results were not statistically significant among age groups, but there were trends. In black patients, the dementia-lowering benefit was largest among those aged 50-64 years (HR, 0.6; 95% CI, 0.45-0.81), followed by those aged 65-74 years (HR, 0.71; 95% CI, 0.53-0.94), and there was no benefit among those aged at least 75 (HR, 1.17; 95% CI, 0.73-1.85) all P = .055. There was a slight benefit among white patients in one of the age groups – 65-74 years (HR, 0.9; 95% CI, 0.82-0.99; P = .315).
The authors suggested that the findings could have been the result of an effect of metformin to reduce vascular disease and chronic inflammation in black patients.
They also noted that further research is needed to identify the demographic and clinical subgroups in which metformin is most strongly associated with a reduction in the risk of dementia. In addition, they emphasized that clinical trials are needed to confirm the study findings.
The National Institutes of Health funded the study. The authors report no relevant disclosures.
SOURCE: Scherrer JF et al. Ann Fam Med. 2019;17:352-62.
Black individuals who develop type 2 diabetes are more likely than their white counterparts to develop dementia. Now, findings from a new study point to a possible preventive strategy: Putting older patients on metformin when they are diagnosed could reduce their risk for dementia by as much as 40%, whereas sulfonylureas do not seem to have such an effect.
The researchers did not examine cause and effect, so their findings are not conclusive, and very few women were included in the study. Still, the authors said that their data showing a 29% lower risk of dementia associated with metformin use in black patients aged 65-74 years, and a 40% lower risk in those aged 50-64 years, suggested that “this inexpensive, widely available treatment could be broadly prescribed to substantially reduce the risk of dementia in younger [black] patients with [type 2 diabetes]” (Ann Fam Med. 2019;17:352-62).
Previous findings have suggested that black patients with type 2 diabetes face a 10%-18% higher risk of dementia, compared with white patients (Diabetes Care. 2014; 37[4]:1009-15). Another study linked type 2 diabetes in middle-aged black patients to a 41% decrease in cognition per test results over 14 years. There was no such decrease in white patients (Neuroepidemiology. 2014;43[3-4]: 220-7).
For the new study, researchers led by Jeffrey F. Scherrer, PhD, of Saint Louis University tracked 73,761 patients aged 50 years or older from 2000-2001 (when they were free of dementia and not taking diabetes) to 2015. Among the patients, 86% were white and 14% were black. In the white and black groups, 97% and 95% were men, respectively, and 61% and 55% were obese, respectively.
All participants began metformin (76%) or sulfonylurea (24%) monotherapy after the baseline period. Guidelines recommend metformin as a first-line treatment for type 2 diabetes, whereas sulfonylureas are considered second-line drugs that should be added to metformin.
After adjustment for confounders such as socioeconomic status and other medical conditions, the researchers found a significantly lower risk of dementia in black patients who took metformin, compared with those taking a sulfonylurea (hazard ratio, 0.73; 95% confidence interval, 0.6-0.89). There was no difference between the drugs among white patients (HR, 0.96; 95% CI, 0.9-1.03, both P = .008)
The results were not statistically significant among age groups, but there were trends. In black patients, the dementia-lowering benefit was largest among those aged 50-64 years (HR, 0.6; 95% CI, 0.45-0.81), followed by those aged 65-74 years (HR, 0.71; 95% CI, 0.53-0.94), and there was no benefit among those aged at least 75 (HR, 1.17; 95% CI, 0.73-1.85) all P = .055. There was a slight benefit among white patients in one of the age groups – 65-74 years (HR, 0.9; 95% CI, 0.82-0.99; P = .315).
The authors suggested that the findings could have been the result of an effect of metformin to reduce vascular disease and chronic inflammation in black patients.
They also noted that further research is needed to identify the demographic and clinical subgroups in which metformin is most strongly associated with a reduction in the risk of dementia. In addition, they emphasized that clinical trials are needed to confirm the study findings.
The National Institutes of Health funded the study. The authors report no relevant disclosures.
SOURCE: Scherrer JF et al. Ann Fam Med. 2019;17:352-62.
FROM ANNALS OF FAMILY MEDICINE
Key clinical point:
Major finding: Metformin monotherapy, compared with sulfonylurea monotherapy, was linked to a significantly lower risk for dementia in black patients (HR, 0.73; 95% CI, 0.6-0.89), but not in white patients (HR, 0.96; 95% CI, 0.9-1.03; P = .008).
Study details: Retrospective analysis of 73,761 patients aged 50 years or older in the Veterans Health Administration system who were tracked from 2000-2001 to 2015 and began metformin or sulfonylurea monotherapy after baseline.
Disclosures: The National Institutes of Health funded the study. The authors report no relevant disclosures.
Source: Scherrer JF et al. Ann Fam Med. 2019;17:352-62.
Study: Most patients hospitalized with pneumonia receive excessive antibiotics
Two-thirds of patients hospitalized with pneumonia received an excess duration of antibiotics, according to a recent study of more than 6,000 patients.
.
The findings bolster a growing body of evidence showing that short-course therapy for pneumonia is safe and that longer durations are not only unnecessary, but “potentially harmful,” said Valerie M. Vaughn, MD, assistant professor of medicine at the University of Michigan, Ann Arbor, and coinvestigators.
“Reducing excess treatment durations should be a top priority for antibiotic stewardship nationally,” the investigators wrote in their report, which appears in the Annals of Internal Medicine.
The primary analysis of their retrospective cohort study included 6,481 individuals with pneumonia treated at 43 hospitals participating in a statewide quality initiative designed to improve care for hospitalized medical patients at risk of adverse events. About half of the patients were women, and the median age was 70 years. Nearly 60% had severe pneumonia.
The primary outcome of the study was the rate of excess antibiotic therapy duration beyond the shortest expected treatment duration consistent with guidelines. Patients with community-acquired pneumonia (CAP), representing about three-quarters of the study cohort, were expected to have a treatment duration of at least 5 days, while patients with health care–acquired pneumonia (HCAP) were expected to have at least 7 days of treatment.
Overall, 4,391 patients (67.8%) had antibiotic courses longer than the shortest effective duration, with a median duration of 8 days, and a median excess duration of 2 days, the researchers noted.
The great majority of excess days (93.2%) were due to antibiotic prescribed at discharge, according to Dr. Vaughn and colleagues.
Excess treatment duration was not linked to any improvement in 30-day mortality, readmission rates, or subsequent emergency department visits, they found.
In a telephone call at 30 days, 38% of patients treated to excess said they had gone to the doctor for an antibiotic-associated adverse event, compared with 31% who received appropriate-length courses (P = .003).
Odds of a patient-reported adverse event were increased by 5% for every excess treatment day, the investigators wrote.
Taken together, these findings have implications for patient care, research efforts, and future guidelines, according to Dr. Vaughn and coinvestigators.
“The next iteration of CAP and HCAP guidelines should explicitly recommend (rather than imply) that providers prescribe the shortest effective duration,” they said in a discussion of their study results.
Dr. Vaughn reported no disclosures related to the study. Coauthors reported grants from Blue Cross Blue Shield of Michigan and the Agency for Healthcare Research and Quality, personal fees from Wiley Publishing, and royalties from Wolters Kluwer Publishing and Oxford University Press, among other disclosures.
SOURCE: Vaughn VM et al. Ann Intern Med. 2019;171:153-63. doi: 10.7326/M18-3640.
This study by Vaughn and colleagues adds “valuable insight” to an already considerable body of evidence showing that shorter durations of antibiotic therapy are effective and limit potential harm due to adverse effects, authors of an accompanying editorial said.
“After dozens of randomized, controlled trials and more than a decade since the initial clarion call to move to short-course therapy, it is time to adapt clinical practice for diseases that have been studied and adopt the mantra ‘shorter is better,’ ” Brad Spellberg, MD, and Louis B. Rice, MD, wrote in their editorial.
“It is time for regulatory agencies, payers, and professional societies to align themselves with the overwhelming data and assist in converting practice patterns to short-course therapy,” the authors said.
Brad Spellberg, MD, is with the Los Angeles County–University of Southern California Medical Center, and Louis B. Rice, MD, is with Rhode Island Hospital, Brown University, Providence, R.I. Their editorial appears in Annals of Internal Medicine. The authors reported disclosures outside the submitted work from Alexion, Paratek, TheoremDx, Acurx, Shionogi, Merck, Motif, BioAIM, Mycomed, and ExBaq (Dr. Spellberg); and Zavante Pharmaceuticals and Macrolide (Dr. Rice).
This study by Vaughn and colleagues adds “valuable insight” to an already considerable body of evidence showing that shorter durations of antibiotic therapy are effective and limit potential harm due to adverse effects, authors of an accompanying editorial said.
“After dozens of randomized, controlled trials and more than a decade since the initial clarion call to move to short-course therapy, it is time to adapt clinical practice for diseases that have been studied and adopt the mantra ‘shorter is better,’ ” Brad Spellberg, MD, and Louis B. Rice, MD, wrote in their editorial.
“It is time for regulatory agencies, payers, and professional societies to align themselves with the overwhelming data and assist in converting practice patterns to short-course therapy,” the authors said.
Brad Spellberg, MD, is with the Los Angeles County–University of Southern California Medical Center, and Louis B. Rice, MD, is with Rhode Island Hospital, Brown University, Providence, R.I. Their editorial appears in Annals of Internal Medicine. The authors reported disclosures outside the submitted work from Alexion, Paratek, TheoremDx, Acurx, Shionogi, Merck, Motif, BioAIM, Mycomed, and ExBaq (Dr. Spellberg); and Zavante Pharmaceuticals and Macrolide (Dr. Rice).
This study by Vaughn and colleagues adds “valuable insight” to an already considerable body of evidence showing that shorter durations of antibiotic therapy are effective and limit potential harm due to adverse effects, authors of an accompanying editorial said.
“After dozens of randomized, controlled trials and more than a decade since the initial clarion call to move to short-course therapy, it is time to adapt clinical practice for diseases that have been studied and adopt the mantra ‘shorter is better,’ ” Brad Spellberg, MD, and Louis B. Rice, MD, wrote in their editorial.
“It is time for regulatory agencies, payers, and professional societies to align themselves with the overwhelming data and assist in converting practice patterns to short-course therapy,” the authors said.
Brad Spellberg, MD, is with the Los Angeles County–University of Southern California Medical Center, and Louis B. Rice, MD, is with Rhode Island Hospital, Brown University, Providence, R.I. Their editorial appears in Annals of Internal Medicine. The authors reported disclosures outside the submitted work from Alexion, Paratek, TheoremDx, Acurx, Shionogi, Merck, Motif, BioAIM, Mycomed, and ExBaq (Dr. Spellberg); and Zavante Pharmaceuticals and Macrolide (Dr. Rice).
Two-thirds of patients hospitalized with pneumonia received an excess duration of antibiotics, according to a recent study of more than 6,000 patients.
.
The findings bolster a growing body of evidence showing that short-course therapy for pneumonia is safe and that longer durations are not only unnecessary, but “potentially harmful,” said Valerie M. Vaughn, MD, assistant professor of medicine at the University of Michigan, Ann Arbor, and coinvestigators.
“Reducing excess treatment durations should be a top priority for antibiotic stewardship nationally,” the investigators wrote in their report, which appears in the Annals of Internal Medicine.
The primary analysis of their retrospective cohort study included 6,481 individuals with pneumonia treated at 43 hospitals participating in a statewide quality initiative designed to improve care for hospitalized medical patients at risk of adverse events. About half of the patients were women, and the median age was 70 years. Nearly 60% had severe pneumonia.
The primary outcome of the study was the rate of excess antibiotic therapy duration beyond the shortest expected treatment duration consistent with guidelines. Patients with community-acquired pneumonia (CAP), representing about three-quarters of the study cohort, were expected to have a treatment duration of at least 5 days, while patients with health care–acquired pneumonia (HCAP) were expected to have at least 7 days of treatment.
Overall, 4,391 patients (67.8%) had antibiotic courses longer than the shortest effective duration, with a median duration of 8 days, and a median excess duration of 2 days, the researchers noted.
The great majority of excess days (93.2%) were due to antibiotic prescribed at discharge, according to Dr. Vaughn and colleagues.
Excess treatment duration was not linked to any improvement in 30-day mortality, readmission rates, or subsequent emergency department visits, they found.
In a telephone call at 30 days, 38% of patients treated to excess said they had gone to the doctor for an antibiotic-associated adverse event, compared with 31% who received appropriate-length courses (P = .003).
Odds of a patient-reported adverse event were increased by 5% for every excess treatment day, the investigators wrote.
Taken together, these findings have implications for patient care, research efforts, and future guidelines, according to Dr. Vaughn and coinvestigators.
“The next iteration of CAP and HCAP guidelines should explicitly recommend (rather than imply) that providers prescribe the shortest effective duration,” they said in a discussion of their study results.
Dr. Vaughn reported no disclosures related to the study. Coauthors reported grants from Blue Cross Blue Shield of Michigan and the Agency for Healthcare Research and Quality, personal fees from Wiley Publishing, and royalties from Wolters Kluwer Publishing and Oxford University Press, among other disclosures.
SOURCE: Vaughn VM et al. Ann Intern Med. 2019;171:153-63. doi: 10.7326/M18-3640.
Two-thirds of patients hospitalized with pneumonia received an excess duration of antibiotics, according to a recent study of more than 6,000 patients.
.
The findings bolster a growing body of evidence showing that short-course therapy for pneumonia is safe and that longer durations are not only unnecessary, but “potentially harmful,” said Valerie M. Vaughn, MD, assistant professor of medicine at the University of Michigan, Ann Arbor, and coinvestigators.
“Reducing excess treatment durations should be a top priority for antibiotic stewardship nationally,” the investigators wrote in their report, which appears in the Annals of Internal Medicine.
The primary analysis of their retrospective cohort study included 6,481 individuals with pneumonia treated at 43 hospitals participating in a statewide quality initiative designed to improve care for hospitalized medical patients at risk of adverse events. About half of the patients were women, and the median age was 70 years. Nearly 60% had severe pneumonia.
The primary outcome of the study was the rate of excess antibiotic therapy duration beyond the shortest expected treatment duration consistent with guidelines. Patients with community-acquired pneumonia (CAP), representing about three-quarters of the study cohort, were expected to have a treatment duration of at least 5 days, while patients with health care–acquired pneumonia (HCAP) were expected to have at least 7 days of treatment.
Overall, 4,391 patients (67.8%) had antibiotic courses longer than the shortest effective duration, with a median duration of 8 days, and a median excess duration of 2 days, the researchers noted.
The great majority of excess days (93.2%) were due to antibiotic prescribed at discharge, according to Dr. Vaughn and colleagues.
Excess treatment duration was not linked to any improvement in 30-day mortality, readmission rates, or subsequent emergency department visits, they found.
In a telephone call at 30 days, 38% of patients treated to excess said they had gone to the doctor for an antibiotic-associated adverse event, compared with 31% who received appropriate-length courses (P = .003).
Odds of a patient-reported adverse event were increased by 5% for every excess treatment day, the investigators wrote.
Taken together, these findings have implications for patient care, research efforts, and future guidelines, according to Dr. Vaughn and coinvestigators.
“The next iteration of CAP and HCAP guidelines should explicitly recommend (rather than imply) that providers prescribe the shortest effective duration,” they said in a discussion of their study results.
Dr. Vaughn reported no disclosures related to the study. Coauthors reported grants from Blue Cross Blue Shield of Michigan and the Agency for Healthcare Research and Quality, personal fees from Wiley Publishing, and royalties from Wolters Kluwer Publishing and Oxford University Press, among other disclosures.
SOURCE: Vaughn VM et al. Ann Intern Med. 2019;171:153-63. doi: 10.7326/M18-3640.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Excessive antibiotic therapy was common among patients hospitalized with pneumonia and linked to an increase in patient-reported adverse events.
Major finding: Two-thirds (67.8%) of patients had antibiotic courses longer than the shortest effective duration.
Study details: Retrospective cohort study of 6,481 individuals with pneumonia treated at 43 hospitals participating in a statewide quality initiative.
Disclosures: Study authors reported grants from Blue Cross Blue Shield of Michigan and the Agency for Healthcare Research and Quality, personal fees from Wiley Publishing, and royalties from Wolters Kluwer Publishing and Oxford University Press, among other disclosures.
Source: Vaughn VM et al. Ann Intern Med. 2019;171:153-63. doi: 10.7326/M18-3640.