User login
Clinics get more time on Title X changes
Family planning clinics will have more time to comply with a Trump administration rule that prohibits physicians from counseling patients about abortion and bars them from referring women for the procedures.
The Department of Health & Human Services does not intend to bring enforcement actions against taxpayer-funded clinics if they are making good-faith efforts to comply with the new rules, according to a memo issued July 20. Such good faith efforts include a written assurance by Aug. 19 that clinics are not providing abortions nor including abortion in their family planning methods, according to the emailed guidance sent by Diane Foley, MD, HHS Deputy Assistant Secretary at the Office of Population Affairs (OPA). Clinics must also detail an action plan describing the steps they will take to comply with the Title X changes and start those actions immediately, according to the memo, obtained by this news organization.
“In the past, the U.S. Department of Health & Human Services, Office of Population Affairs, has exercised enforcement discretion in appropriate circumstances,” according to the memo. “Given the circumstances surrounding the implementation of the final rule, OPA does not intend to bring enforcement actions against Title X recipients that are making, and continue to make, good-faith efforts to comply with the final rule. OPA is committed to working with grantees to assist them in coming into compliance with the requirements of the final rule.”
The decision comes a week after HHS warned family planning clinics that receive federal money to immediately stop providing referrals and counseling on abortion or face revocation of funding. An email from HHS on July 15 stated that the agency was requiring immediate compliance of the Title X changes consistent with recent court rulings. The warning came just before the start of a national Title X grantee meeting held in Washington.
The changes to the Title X program make health clinics ineligible for funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD testing, cancer screenings, and contraception – to low-income individuals. Under the rule, the government will withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services. The rule also imposes physical separation requirements for health centers that offer abortions.
More than 20 states and several abortion rights organizations sued over the rules in four separate states. District judges in Oregon, Washington, and California temporarily blocked the rules from taking effect. In a June 20 decision, the 9th U.S. Circuit Court of Appeals ruled the federal government may go forward with its plan to restrict Title X funding from clinics that provide abortion counseling or that refer patients for abortion services. The decision overturned the lower court injunctions.
In the July 20 memo, Dr. Foley wrote that, in addition to the Aug. 19 requirements, clinics must send written confirmation by Sep. 18 outlining the steps taken to comply with the Title X changes and provide any relevant documentation needed for HHS to verify the compliance. By March 4, 2020, a written statement must be submitted affirming the clinic is in compliance with the requirement for physical separation between Title X services and abortion services.
The National Family Planning & Reproductive Health Association, a plaintiff in one of the challenges, called the administration’s July 20 memo “wholly insufficient” and said the clinics need more guidance about how to move forward with the rule changes.
“It’s just absurd to think that a few bullet points amount to guidance,” association officials said in a July 21 statement. “We urge [the agency] to take the time to properly expand on and better describe how it will interpret aspects of the rule – using examples that reflect the wide range of provider settings and administrative structures present in Title X. Once again, [the agency] falls far short of linking the rule to day-to-day practice, leaving the entire family planning network in the dark on how they need to operate to stay in the program.”
At presstime, HHS had not responded to a message seeking comment on the requirements. In her note, Dr. Foley wrote that more guidance on the changes were forthcoming and that grantees unable to meet the required time line may request a deadline extension from the agency.
HHS has previously said that the Title X changes ensure that grants and contracts awarded under the program fully comply with the statutory program integrity requirements, “thereby fulfilling the purpose of Title X, so that more women and men can receive services that help them consider and achieve both their short-term and long-term family planning needs.”
Family planning clinics will have more time to comply with a Trump administration rule that prohibits physicians from counseling patients about abortion and bars them from referring women for the procedures.
The Department of Health & Human Services does not intend to bring enforcement actions against taxpayer-funded clinics if they are making good-faith efforts to comply with the new rules, according to a memo issued July 20. Such good faith efforts include a written assurance by Aug. 19 that clinics are not providing abortions nor including abortion in their family planning methods, according to the emailed guidance sent by Diane Foley, MD, HHS Deputy Assistant Secretary at the Office of Population Affairs (OPA). Clinics must also detail an action plan describing the steps they will take to comply with the Title X changes and start those actions immediately, according to the memo, obtained by this news organization.
“In the past, the U.S. Department of Health & Human Services, Office of Population Affairs, has exercised enforcement discretion in appropriate circumstances,” according to the memo. “Given the circumstances surrounding the implementation of the final rule, OPA does not intend to bring enforcement actions against Title X recipients that are making, and continue to make, good-faith efforts to comply with the final rule. OPA is committed to working with grantees to assist them in coming into compliance with the requirements of the final rule.”
The decision comes a week after HHS warned family planning clinics that receive federal money to immediately stop providing referrals and counseling on abortion or face revocation of funding. An email from HHS on July 15 stated that the agency was requiring immediate compliance of the Title X changes consistent with recent court rulings. The warning came just before the start of a national Title X grantee meeting held in Washington.
The changes to the Title X program make health clinics ineligible for funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD testing, cancer screenings, and contraception – to low-income individuals. Under the rule, the government will withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services. The rule also imposes physical separation requirements for health centers that offer abortions.
More than 20 states and several abortion rights organizations sued over the rules in four separate states. District judges in Oregon, Washington, and California temporarily blocked the rules from taking effect. In a June 20 decision, the 9th U.S. Circuit Court of Appeals ruled the federal government may go forward with its plan to restrict Title X funding from clinics that provide abortion counseling or that refer patients for abortion services. The decision overturned the lower court injunctions.
In the July 20 memo, Dr. Foley wrote that, in addition to the Aug. 19 requirements, clinics must send written confirmation by Sep. 18 outlining the steps taken to comply with the Title X changes and provide any relevant documentation needed for HHS to verify the compliance. By March 4, 2020, a written statement must be submitted affirming the clinic is in compliance with the requirement for physical separation between Title X services and abortion services.
The National Family Planning & Reproductive Health Association, a plaintiff in one of the challenges, called the administration’s July 20 memo “wholly insufficient” and said the clinics need more guidance about how to move forward with the rule changes.
“It’s just absurd to think that a few bullet points amount to guidance,” association officials said in a July 21 statement. “We urge [the agency] to take the time to properly expand on and better describe how it will interpret aspects of the rule – using examples that reflect the wide range of provider settings and administrative structures present in Title X. Once again, [the agency] falls far short of linking the rule to day-to-day practice, leaving the entire family planning network in the dark on how they need to operate to stay in the program.”
At presstime, HHS had not responded to a message seeking comment on the requirements. In her note, Dr. Foley wrote that more guidance on the changes were forthcoming and that grantees unable to meet the required time line may request a deadline extension from the agency.
HHS has previously said that the Title X changes ensure that grants and contracts awarded under the program fully comply with the statutory program integrity requirements, “thereby fulfilling the purpose of Title X, so that more women and men can receive services that help them consider and achieve both their short-term and long-term family planning needs.”
Family planning clinics will have more time to comply with a Trump administration rule that prohibits physicians from counseling patients about abortion and bars them from referring women for the procedures.
The Department of Health & Human Services does not intend to bring enforcement actions against taxpayer-funded clinics if they are making good-faith efforts to comply with the new rules, according to a memo issued July 20. Such good faith efforts include a written assurance by Aug. 19 that clinics are not providing abortions nor including abortion in their family planning methods, according to the emailed guidance sent by Diane Foley, MD, HHS Deputy Assistant Secretary at the Office of Population Affairs (OPA). Clinics must also detail an action plan describing the steps they will take to comply with the Title X changes and start those actions immediately, according to the memo, obtained by this news organization.
“In the past, the U.S. Department of Health & Human Services, Office of Population Affairs, has exercised enforcement discretion in appropriate circumstances,” according to the memo. “Given the circumstances surrounding the implementation of the final rule, OPA does not intend to bring enforcement actions against Title X recipients that are making, and continue to make, good-faith efforts to comply with the final rule. OPA is committed to working with grantees to assist them in coming into compliance with the requirements of the final rule.”
The decision comes a week after HHS warned family planning clinics that receive federal money to immediately stop providing referrals and counseling on abortion or face revocation of funding. An email from HHS on July 15 stated that the agency was requiring immediate compliance of the Title X changes consistent with recent court rulings. The warning came just before the start of a national Title X grantee meeting held in Washington.
The changes to the Title X program make health clinics ineligible for funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD testing, cancer screenings, and contraception – to low-income individuals. Under the rule, the government will withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services. The rule also imposes physical separation requirements for health centers that offer abortions.
More than 20 states and several abortion rights organizations sued over the rules in four separate states. District judges in Oregon, Washington, and California temporarily blocked the rules from taking effect. In a June 20 decision, the 9th U.S. Circuit Court of Appeals ruled the federal government may go forward with its plan to restrict Title X funding from clinics that provide abortion counseling or that refer patients for abortion services. The decision overturned the lower court injunctions.
In the July 20 memo, Dr. Foley wrote that, in addition to the Aug. 19 requirements, clinics must send written confirmation by Sep. 18 outlining the steps taken to comply with the Title X changes and provide any relevant documentation needed for HHS to verify the compliance. By March 4, 2020, a written statement must be submitted affirming the clinic is in compliance with the requirement for physical separation between Title X services and abortion services.
The National Family Planning & Reproductive Health Association, a plaintiff in one of the challenges, called the administration’s July 20 memo “wholly insufficient” and said the clinics need more guidance about how to move forward with the rule changes.
“It’s just absurd to think that a few bullet points amount to guidance,” association officials said in a July 21 statement. “We urge [the agency] to take the time to properly expand on and better describe how it will interpret aspects of the rule – using examples that reflect the wide range of provider settings and administrative structures present in Title X. Once again, [the agency] falls far short of linking the rule to day-to-day practice, leaving the entire family planning network in the dark on how they need to operate to stay in the program.”
At presstime, HHS had not responded to a message seeking comment on the requirements. In her note, Dr. Foley wrote that more guidance on the changes were forthcoming and that grantees unable to meet the required time line may request a deadline extension from the agency.
HHS has previously said that the Title X changes ensure that grants and contracts awarded under the program fully comply with the statutory program integrity requirements, “thereby fulfilling the purpose of Title X, so that more women and men can receive services that help them consider and achieve both their short-term and long-term family planning needs.”
New WHO recommendations promote dolutegravir benefits in the face of lowered risk signal for neural tube defects
The risk of neural tube defects linked to dolutegravir exposure during pregnancy is lower than previously signaled, according to new reports that have prompted the World Health Organization (WHO) to confirm that this antiviral medication should be the preferred option across all populations.
The use of dolutegravir (DTG) during pregnancy has been a pressing global health question since May 2018, when an unplanned interim analysis of the Tsepamo surveillance study of birth outcomes in Botswana showed four neural tube defects associated with dolutegravir exposure among 426 infants born to HIV-positive women (0.94%).
With follow-up for additional births, however, just one more neural tube defect was identified out of 1,683 deliveries among women who had taken DTG around the time of conception (0.30%), according to a report just presented here at the at the International AIDS Society Conference on HIV Science.
By comparison, prevalence rates of neural tube defects were 0.10% for mothers taking other antiretroviral therapies at conception, 0.04% for those specifically taking efavirenz at conception, and 0.08% in HIV-uninfected mothers, according to the report, which was simultaneously published in the New England Journal of Medicine.
“While there may be a risk for neural tube defects, this risk is small, and really importantly, needs to be weighed against the large potential benefits of dolutegravir,” investigator Rebecca M. Zash, MD, of Beth Israel Deaconess Medical Center, Boston, said here in Mexico City during an IAS 2019 video press conference.
The WHO had previously sounded a note of caution, saying that DTG could be “considered” in women of childbearing age if other first‐line antiretroviral agents such as efavirenz could not be used.
However, following release of new evidence, including the study by Dr. Zash and colleagues, the WHO has come out with a clear recommendation for HIV drug as “the preferred first-line and second-line treatment for all populations, including pregnant women and those of childbearing potential.”
The updated scientific reports and guidelines have important implications for global health. “Many countries have been working to make dolutegravir-based treatment their preferred first-line regimen, as it’s got several advantages over efavirenz, which people have been using for many years now, including its tolerability and resistance profiles, and its impact on morbidity and mortality,” IAS president Anton Pozniak, MD, said in the press conference.
Some countries paused their plans to roll out dolutegravir-based regimens after the preliminary safety signal from the Tsepamo study was reported, Dr. Pozniak added.
In another study presented at IAS looking at dolutegravir use at conception, investigators described an additional surveillance study in Botswana, conducted independently from the Tsepamo study. One neural tube defect was found among 152 deliveries in mothers who had been taking DTG at conception (0.66%), and two neural tube defects among 2,326 deliveries to HIV-negative mothers (0.09%).
Although the number of deliveries are small in this study, the results suggest a risk of neural tube defects with DTG exposure at conception of less than 1%, said Mmakgomo Mimi Raesima, MD, MPH, public health specialist, Ministry of Health and Wellness, Botswana.
Because neural-tube defects might be related to low folate levels, Dr. Raesima said “conversations are continuing” with regard to folate food fortification in Botswana, a country that does not mandate folate-fortified grains.
“We want to capitalize on the momentum from these results,” Dr. Raesima said in the press conference.
The Tsepamo study was funded by the National Institutes of Health. Dr. Zash reported grants during the conduct of the study from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Zash R et al. N Engl J Med. 2019 Jul 22. doi: 10.1056/NEJMoa1905230.
The risk of neural tube defects linked to dolutegravir exposure during pregnancy is lower than previously signaled, according to new reports that have prompted the World Health Organization (WHO) to confirm that this antiviral medication should be the preferred option across all populations.
The use of dolutegravir (DTG) during pregnancy has been a pressing global health question since May 2018, when an unplanned interim analysis of the Tsepamo surveillance study of birth outcomes in Botswana showed four neural tube defects associated with dolutegravir exposure among 426 infants born to HIV-positive women (0.94%).
With follow-up for additional births, however, just one more neural tube defect was identified out of 1,683 deliveries among women who had taken DTG around the time of conception (0.30%), according to a report just presented here at the at the International AIDS Society Conference on HIV Science.
By comparison, prevalence rates of neural tube defects were 0.10% for mothers taking other antiretroviral therapies at conception, 0.04% for those specifically taking efavirenz at conception, and 0.08% in HIV-uninfected mothers, according to the report, which was simultaneously published in the New England Journal of Medicine.
“While there may be a risk for neural tube defects, this risk is small, and really importantly, needs to be weighed against the large potential benefits of dolutegravir,” investigator Rebecca M. Zash, MD, of Beth Israel Deaconess Medical Center, Boston, said here in Mexico City during an IAS 2019 video press conference.
The WHO had previously sounded a note of caution, saying that DTG could be “considered” in women of childbearing age if other first‐line antiretroviral agents such as efavirenz could not be used.
However, following release of new evidence, including the study by Dr. Zash and colleagues, the WHO has come out with a clear recommendation for HIV drug as “the preferred first-line and second-line treatment for all populations, including pregnant women and those of childbearing potential.”
The updated scientific reports and guidelines have important implications for global health. “Many countries have been working to make dolutegravir-based treatment their preferred first-line regimen, as it’s got several advantages over efavirenz, which people have been using for many years now, including its tolerability and resistance profiles, and its impact on morbidity and mortality,” IAS president Anton Pozniak, MD, said in the press conference.
Some countries paused their plans to roll out dolutegravir-based regimens after the preliminary safety signal from the Tsepamo study was reported, Dr. Pozniak added.
In another study presented at IAS looking at dolutegravir use at conception, investigators described an additional surveillance study in Botswana, conducted independently from the Tsepamo study. One neural tube defect was found among 152 deliveries in mothers who had been taking DTG at conception (0.66%), and two neural tube defects among 2,326 deliveries to HIV-negative mothers (0.09%).
Although the number of deliveries are small in this study, the results suggest a risk of neural tube defects with DTG exposure at conception of less than 1%, said Mmakgomo Mimi Raesima, MD, MPH, public health specialist, Ministry of Health and Wellness, Botswana.
Because neural-tube defects might be related to low folate levels, Dr. Raesima said “conversations are continuing” with regard to folate food fortification in Botswana, a country that does not mandate folate-fortified grains.
“We want to capitalize on the momentum from these results,” Dr. Raesima said in the press conference.
The Tsepamo study was funded by the National Institutes of Health. Dr. Zash reported grants during the conduct of the study from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Zash R et al. N Engl J Med. 2019 Jul 22. doi: 10.1056/NEJMoa1905230.
The risk of neural tube defects linked to dolutegravir exposure during pregnancy is lower than previously signaled, according to new reports that have prompted the World Health Organization (WHO) to confirm that this antiviral medication should be the preferred option across all populations.
The use of dolutegravir (DTG) during pregnancy has been a pressing global health question since May 2018, when an unplanned interim analysis of the Tsepamo surveillance study of birth outcomes in Botswana showed four neural tube defects associated with dolutegravir exposure among 426 infants born to HIV-positive women (0.94%).
With follow-up for additional births, however, just one more neural tube defect was identified out of 1,683 deliveries among women who had taken DTG around the time of conception (0.30%), according to a report just presented here at the at the International AIDS Society Conference on HIV Science.
By comparison, prevalence rates of neural tube defects were 0.10% for mothers taking other antiretroviral therapies at conception, 0.04% for those specifically taking efavirenz at conception, and 0.08% in HIV-uninfected mothers, according to the report, which was simultaneously published in the New England Journal of Medicine.
“While there may be a risk for neural tube defects, this risk is small, and really importantly, needs to be weighed against the large potential benefits of dolutegravir,” investigator Rebecca M. Zash, MD, of Beth Israel Deaconess Medical Center, Boston, said here in Mexico City during an IAS 2019 video press conference.
The WHO had previously sounded a note of caution, saying that DTG could be “considered” in women of childbearing age if other first‐line antiretroviral agents such as efavirenz could not be used.
However, following release of new evidence, including the study by Dr. Zash and colleagues, the WHO has come out with a clear recommendation for HIV drug as “the preferred first-line and second-line treatment for all populations, including pregnant women and those of childbearing potential.”
The updated scientific reports and guidelines have important implications for global health. “Many countries have been working to make dolutegravir-based treatment their preferred first-line regimen, as it’s got several advantages over efavirenz, which people have been using for many years now, including its tolerability and resistance profiles, and its impact on morbidity and mortality,” IAS president Anton Pozniak, MD, said in the press conference.
Some countries paused their plans to roll out dolutegravir-based regimens after the preliminary safety signal from the Tsepamo study was reported, Dr. Pozniak added.
In another study presented at IAS looking at dolutegravir use at conception, investigators described an additional surveillance study in Botswana, conducted independently from the Tsepamo study. One neural tube defect was found among 152 deliveries in mothers who had been taking DTG at conception (0.66%), and two neural tube defects among 2,326 deliveries to HIV-negative mothers (0.09%).
Although the number of deliveries are small in this study, the results suggest a risk of neural tube defects with DTG exposure at conception of less than 1%, said Mmakgomo Mimi Raesima, MD, MPH, public health specialist, Ministry of Health and Wellness, Botswana.
Because neural-tube defects might be related to low folate levels, Dr. Raesima said “conversations are continuing” with regard to folate food fortification in Botswana, a country that does not mandate folate-fortified grains.
“We want to capitalize on the momentum from these results,” Dr. Raesima said in the press conference.
The Tsepamo study was funded by the National Institutes of Health. Dr. Zash reported grants during the conduct of the study from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Zash R et al. N Engl J Med. 2019 Jul 22. doi: 10.1056/NEJMoa1905230.
FROM IAS 2019
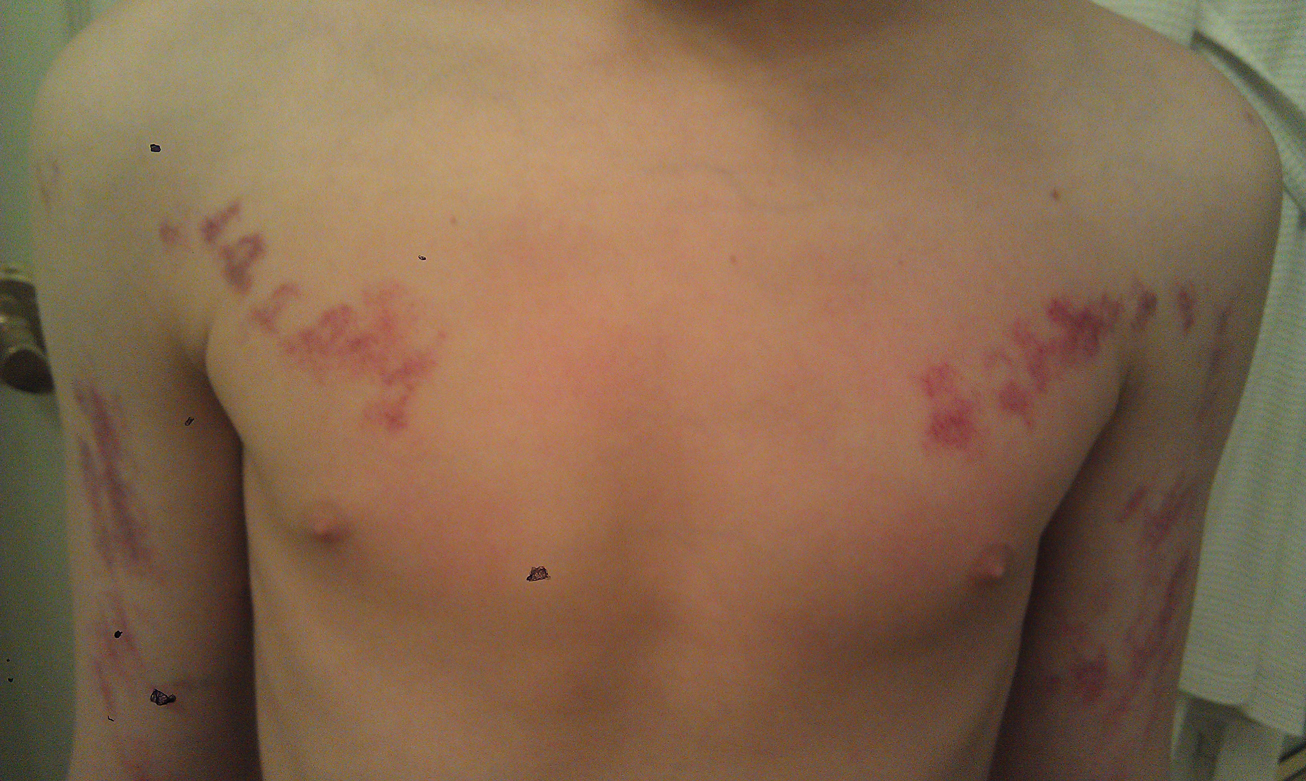
Painless Purple Streaks on the Arms and Chest
The Diagnosis: Factitial Purpura
Factitial dermatologic disorders are characterized by skin findings triggered by deliberate manipulation of the skin with objects to create lesions and feign signs of a dermatologic condition to seek emotional and psychological benefit.1 The etiology of the lesions is unclear, and the patient's history of the injury is hollow.2 Most often, there is sudden onset of the lesions without any warning or symptoms. When giving the history, the patient may appear unemotional, does not report pain, and denies self-infliction.1
In factitial purpura, the purple patches are clearly demarcated from uninvolved skin and have an unusual angular or geometric shape. The pattern typically takes the shape of the object used to create the purpura and lacks the features of recognizable dermatoses.2 In our patient and those with similar linear purpuric streaks, we use the term penny purpura to indicate that the lesions resulted from rubbing with a penny or other blunt object, similar to coining. The lesions occur in areas that are easily accessible and visible such as the arms, chest, or chin. It is suggested that the child unconsciously wants the lesions to be seen. Histologic findings in factitial purpura include disruption of collagen fiber bundles and extravasated red blood cells in the dermis.3 Unfortunately, evolving lesions may give nonspecific histologic findings; when the clinical lesions are typical, skin biopsy usually is unnecessary and may be misleading. Laboratory test results such as complete blood cell count, prothrombin time, and partial thromboplastin time usually are within reference range, as in our patient.
When evaluating these patients, confrontation is not recommended. More than two-thirds of affected patients have a history of trauma such as sexual/physical abuse or neglect, and the lesions typically arise during times of stress.1,3 Thus, treatment includes nonaccusatory measures and referral for psychologic evaluation. The purpura will rapidly heal when covered with an occlusive dressing.2
The differential diagnosis for penny purpura includes lesions that evolve from cupping and coining. Cupping is a type of complementary and alternative medicine that acts by correcting imbalances in the internal biofield and restoring the flow of qi, which determines the state of one's health and life span.4 Cupping is performed by placing a glass cup over a painful body part. A partial vacuum is created by flaming, mechanical withdrawal, or thermal cooling of the entrapped air under the cup. When the flame exhausts the supply of oxygen, the skin is sucked into the mouth of the glass, and the skin is bruised painlessly.4
The differential also includes child maltreatment syndrome and other disorders that would potentiate bruising. Intravascular etiologies include idiopathic thrombocytopenic purpura, leukemia, coagulation disorders, and other causes of thrombocytopenia or platelet dysfunction.3 Extravascular etiologies include hereditary collagen vascular disease (eg, Ehlers-Danlos syndrome), malnutrition, and other disorders associated with a decrease in collagen and other tissues that support cutaneous vessels. Vascular etiologies include infectious (eg, Rocky Mountain spotted fever, meningococcemia) and noninfectious vasculitis (eg, Henoch-Schönlein purpura), leaky capillary syndrome, drug reactions, and other disorders associated with a loss of vascular integrity.3
It is important to be able to differentiate self-inflicted lesions in a person who repeatedly acts as if he/she has a physical disorder from those that are created during the practices of cupping or any other cultural healing practice. Vascular disorders, malnutrition, and child abuse also should be excluded.3
For our patient with factitial purpura, we gently encouraged the family to work with the child's pediatrician and a pediatric psychologist to deal with stress related to the recurrent rash and asked them to think of the rash as a result of an external cause; however, we were careful not to blame anyone for the rash.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372; quiz 373.
- Al Hawsawi K, Pope E. Pediatric psychocutaneous disorders: a review of primary psychiatric disorders with dermatologic manifestations. Am J Clin Dermatol. 2011;12:247-257.
- Ring HC, Miller IM, Benfeldt E, et al. Artefactual skin lesions in children and adolescents: review of the literature and two cases of factitious purpura. Int J Dermatol. 2015;54:E27-E32.
- Mehta P, Dhapte V. Cupping therapy: a prudent remedy for a plethora of medical ailments. J Tradit Complement Med. 2015;5:127-134.
The Diagnosis: Factitial Purpura
Factitial dermatologic disorders are characterized by skin findings triggered by deliberate manipulation of the skin with objects to create lesions and feign signs of a dermatologic condition to seek emotional and psychological benefit.1 The etiology of the lesions is unclear, and the patient's history of the injury is hollow.2 Most often, there is sudden onset of the lesions without any warning or symptoms. When giving the history, the patient may appear unemotional, does not report pain, and denies self-infliction.1
In factitial purpura, the purple patches are clearly demarcated from uninvolved skin and have an unusual angular or geometric shape. The pattern typically takes the shape of the object used to create the purpura and lacks the features of recognizable dermatoses.2 In our patient and those with similar linear purpuric streaks, we use the term penny purpura to indicate that the lesions resulted from rubbing with a penny or other blunt object, similar to coining. The lesions occur in areas that are easily accessible and visible such as the arms, chest, or chin. It is suggested that the child unconsciously wants the lesions to be seen. Histologic findings in factitial purpura include disruption of collagen fiber bundles and extravasated red blood cells in the dermis.3 Unfortunately, evolving lesions may give nonspecific histologic findings; when the clinical lesions are typical, skin biopsy usually is unnecessary and may be misleading. Laboratory test results such as complete blood cell count, prothrombin time, and partial thromboplastin time usually are within reference range, as in our patient.
When evaluating these patients, confrontation is not recommended. More than two-thirds of affected patients have a history of trauma such as sexual/physical abuse or neglect, and the lesions typically arise during times of stress.1,3 Thus, treatment includes nonaccusatory measures and referral for psychologic evaluation. The purpura will rapidly heal when covered with an occlusive dressing.2
The differential diagnosis for penny purpura includes lesions that evolve from cupping and coining. Cupping is a type of complementary and alternative medicine that acts by correcting imbalances in the internal biofield and restoring the flow of qi, which determines the state of one's health and life span.4 Cupping is performed by placing a glass cup over a painful body part. A partial vacuum is created by flaming, mechanical withdrawal, or thermal cooling of the entrapped air under the cup. When the flame exhausts the supply of oxygen, the skin is sucked into the mouth of the glass, and the skin is bruised painlessly.4
The differential also includes child maltreatment syndrome and other disorders that would potentiate bruising. Intravascular etiologies include idiopathic thrombocytopenic purpura, leukemia, coagulation disorders, and other causes of thrombocytopenia or platelet dysfunction.3 Extravascular etiologies include hereditary collagen vascular disease (eg, Ehlers-Danlos syndrome), malnutrition, and other disorders associated with a decrease in collagen and other tissues that support cutaneous vessels. Vascular etiologies include infectious (eg, Rocky Mountain spotted fever, meningococcemia) and noninfectious vasculitis (eg, Henoch-Schönlein purpura), leaky capillary syndrome, drug reactions, and other disorders associated with a loss of vascular integrity.3
It is important to be able to differentiate self-inflicted lesions in a person who repeatedly acts as if he/she has a physical disorder from those that are created during the practices of cupping or any other cultural healing practice. Vascular disorders, malnutrition, and child abuse also should be excluded.3
For our patient with factitial purpura, we gently encouraged the family to work with the child's pediatrician and a pediatric psychologist to deal with stress related to the recurrent rash and asked them to think of the rash as a result of an external cause; however, we were careful not to blame anyone for the rash.
The Diagnosis: Factitial Purpura
Factitial dermatologic disorders are characterized by skin findings triggered by deliberate manipulation of the skin with objects to create lesions and feign signs of a dermatologic condition to seek emotional and psychological benefit.1 The etiology of the lesions is unclear, and the patient's history of the injury is hollow.2 Most often, there is sudden onset of the lesions without any warning or symptoms. When giving the history, the patient may appear unemotional, does not report pain, and denies self-infliction.1
In factitial purpura, the purple patches are clearly demarcated from uninvolved skin and have an unusual angular or geometric shape. The pattern typically takes the shape of the object used to create the purpura and lacks the features of recognizable dermatoses.2 In our patient and those with similar linear purpuric streaks, we use the term penny purpura to indicate that the lesions resulted from rubbing with a penny or other blunt object, similar to coining. The lesions occur in areas that are easily accessible and visible such as the arms, chest, or chin. It is suggested that the child unconsciously wants the lesions to be seen. Histologic findings in factitial purpura include disruption of collagen fiber bundles and extravasated red blood cells in the dermis.3 Unfortunately, evolving lesions may give nonspecific histologic findings; when the clinical lesions are typical, skin biopsy usually is unnecessary and may be misleading. Laboratory test results such as complete blood cell count, prothrombin time, and partial thromboplastin time usually are within reference range, as in our patient.
When evaluating these patients, confrontation is not recommended. More than two-thirds of affected patients have a history of trauma such as sexual/physical abuse or neglect, and the lesions typically arise during times of stress.1,3 Thus, treatment includes nonaccusatory measures and referral for psychologic evaluation. The purpura will rapidly heal when covered with an occlusive dressing.2
The differential diagnosis for penny purpura includes lesions that evolve from cupping and coining. Cupping is a type of complementary and alternative medicine that acts by correcting imbalances in the internal biofield and restoring the flow of qi, which determines the state of one's health and life span.4 Cupping is performed by placing a glass cup over a painful body part. A partial vacuum is created by flaming, mechanical withdrawal, or thermal cooling of the entrapped air under the cup. When the flame exhausts the supply of oxygen, the skin is sucked into the mouth of the glass, and the skin is bruised painlessly.4
The differential also includes child maltreatment syndrome and other disorders that would potentiate bruising. Intravascular etiologies include idiopathic thrombocytopenic purpura, leukemia, coagulation disorders, and other causes of thrombocytopenia or platelet dysfunction.3 Extravascular etiologies include hereditary collagen vascular disease (eg, Ehlers-Danlos syndrome), malnutrition, and other disorders associated with a decrease in collagen and other tissues that support cutaneous vessels. Vascular etiologies include infectious (eg, Rocky Mountain spotted fever, meningococcemia) and noninfectious vasculitis (eg, Henoch-Schönlein purpura), leaky capillary syndrome, drug reactions, and other disorders associated with a loss of vascular integrity.3
It is important to be able to differentiate self-inflicted lesions in a person who repeatedly acts as if he/she has a physical disorder from those that are created during the practices of cupping or any other cultural healing practice. Vascular disorders, malnutrition, and child abuse also should be excluded.3
For our patient with factitial purpura, we gently encouraged the family to work with the child's pediatrician and a pediatric psychologist to deal with stress related to the recurrent rash and asked them to think of the rash as a result of an external cause; however, we were careful not to blame anyone for the rash.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372; quiz 373.
- Al Hawsawi K, Pope E. Pediatric psychocutaneous disorders: a review of primary psychiatric disorders with dermatologic manifestations. Am J Clin Dermatol. 2011;12:247-257.
- Ring HC, Miller IM, Benfeldt E, et al. Artefactual skin lesions in children and adolescents: review of the literature and two cases of factitious purpura. Int J Dermatol. 2015;54:E27-E32.
- Mehta P, Dhapte V. Cupping therapy: a prudent remedy for a plethora of medical ailments. J Tradit Complement Med. 2015;5:127-134.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372; quiz 373.
- Al Hawsawi K, Pope E. Pediatric psychocutaneous disorders: a review of primary psychiatric disorders with dermatologic manifestations. Am J Clin Dermatol. 2011;12:247-257.
- Ring HC, Miller IM, Benfeldt E, et al. Artefactual skin lesions in children and adolescents: review of the literature and two cases of factitious purpura. Int J Dermatol. 2015;54:E27-E32.
- Mehta P, Dhapte V. Cupping therapy: a prudent remedy for a plethora of medical ailments. J Tradit Complement Med. 2015;5:127-134.
A 10-year-old boy presented with painless purple streaks on the arms and chest of 2 months' duration. The rash recurred several times per month and cleared without treatment in 3 to 5 days. There was no history of trauma or medication exposure, and he was growing and developing normally.
FDA approves first generics of pregabalin
The generics were approved to manage neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia, and fibromyalgia, as well as neuropathic pain associated with spinal cord injury, and as an adjunctive therapy for the treatment of partial-onset seizures in patients aged 17 years and older. Approvals were granted to Alembic Pharmaceuticals, Alkem Laboratories, Amneal Pharmaceuticals, Dr. Reddy’s Laboratories, InvaGen Pharmaceuticals, MSN Laboratories, Rising Pharmaceuticals, Sciegen Pharmaceuticals, and Teva Pharmaceuticals.
The most common adverse events associated with pregabalin include dizziness, somnolence, dry mouth, swelling, blurred vision, weight gain, and abnormal thinking. Pregabalin must be dispensed with a patient Medication Guide containing a guide to the drug’s uses and risks. Angioedema, hypersensitivity reactions, increased seizure frequency, increased suicidal behavior, and peripheral edema are all possible.
“Today’s approval of the first generics for pregabalin, a widely used medication, is another example of the FDA’s long-standing commitment to advance patient access to lower-cost, high-quality generic medicines,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a press release.
The generics were approved to manage neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia, and fibromyalgia, as well as neuropathic pain associated with spinal cord injury, and as an adjunctive therapy for the treatment of partial-onset seizures in patients aged 17 years and older. Approvals were granted to Alembic Pharmaceuticals, Alkem Laboratories, Amneal Pharmaceuticals, Dr. Reddy’s Laboratories, InvaGen Pharmaceuticals, MSN Laboratories, Rising Pharmaceuticals, Sciegen Pharmaceuticals, and Teva Pharmaceuticals.
The most common adverse events associated with pregabalin include dizziness, somnolence, dry mouth, swelling, blurred vision, weight gain, and abnormal thinking. Pregabalin must be dispensed with a patient Medication Guide containing a guide to the drug’s uses and risks. Angioedema, hypersensitivity reactions, increased seizure frequency, increased suicidal behavior, and peripheral edema are all possible.
“Today’s approval of the first generics for pregabalin, a widely used medication, is another example of the FDA’s long-standing commitment to advance patient access to lower-cost, high-quality generic medicines,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a press release.
The generics were approved to manage neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia, and fibromyalgia, as well as neuropathic pain associated with spinal cord injury, and as an adjunctive therapy for the treatment of partial-onset seizures in patients aged 17 years and older. Approvals were granted to Alembic Pharmaceuticals, Alkem Laboratories, Amneal Pharmaceuticals, Dr. Reddy’s Laboratories, InvaGen Pharmaceuticals, MSN Laboratories, Rising Pharmaceuticals, Sciegen Pharmaceuticals, and Teva Pharmaceuticals.
The most common adverse events associated with pregabalin include dizziness, somnolence, dry mouth, swelling, blurred vision, weight gain, and abnormal thinking. Pregabalin must be dispensed with a patient Medication Guide containing a guide to the drug’s uses and risks. Angioedema, hypersensitivity reactions, increased seizure frequency, increased suicidal behavior, and peripheral edema are all possible.
“Today’s approval of the first generics for pregabalin, a widely used medication, is another example of the FDA’s long-standing commitment to advance patient access to lower-cost, high-quality generic medicines,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a press release.
New measles outbreaks reported in Los Angeles and El Paso
according to the Centers for Disease Control and Prevention.
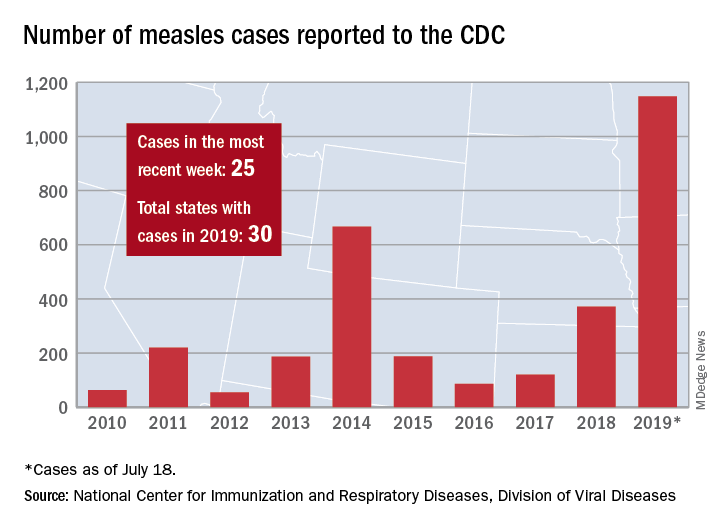
The total number of confirmed cases of measles in the United States is now up to 1,148 for the year, which is 25 more than the previous week, the CDC said on July 22. The highest 1-week total for the year was the 90 cases reported during the week of April 11.
The number of outbreaks is back up to five as California returned to the list after a 1-week absence and El Paso, Tex., made its first appearance of the year. The current outbreak in California – the state’s fifth – is occurring in Los Angeles, which is now up to 16 total cases in 2019. El Paso just reported its fourth case on July 17, and the city’s health department noted that “it had been more than 25 years since El Paso saw its last case of measles before these four recent cases.” Outbreaks also are ongoing in Rockland County, N.Y.; New York City; and three counties in Washington State.
States that joined the ranks of the measles-infected during this most recent reporting week were Alaska and Ohio, which brings the total number to 30 for the year, the CDC said.
The Alaska Department of Health and Social Services said that it “has confirmed a single case of measles in an unvaccinated teenager from the Kenai Peninsula who recently traveled out of state to Arizona via Seattle.” The Ohio case is a “young adult from Stark County [who] recently traveled to a state with confirmed measles cases,” according to the state’s health department.
according to the Centers for Disease Control and Prevention.

The total number of confirmed cases of measles in the United States is now up to 1,148 for the year, which is 25 more than the previous week, the CDC said on July 22. The highest 1-week total for the year was the 90 cases reported during the week of April 11.
The number of outbreaks is back up to five as California returned to the list after a 1-week absence and El Paso, Tex., made its first appearance of the year. The current outbreak in California – the state’s fifth – is occurring in Los Angeles, which is now up to 16 total cases in 2019. El Paso just reported its fourth case on July 17, and the city’s health department noted that “it had been more than 25 years since El Paso saw its last case of measles before these four recent cases.” Outbreaks also are ongoing in Rockland County, N.Y.; New York City; and three counties in Washington State.
States that joined the ranks of the measles-infected during this most recent reporting week were Alaska and Ohio, which brings the total number to 30 for the year, the CDC said.
The Alaska Department of Health and Social Services said that it “has confirmed a single case of measles in an unvaccinated teenager from the Kenai Peninsula who recently traveled out of state to Arizona via Seattle.” The Ohio case is a “young adult from Stark County [who] recently traveled to a state with confirmed measles cases,” according to the state’s health department.
according to the Centers for Disease Control and Prevention.

The total number of confirmed cases of measles in the United States is now up to 1,148 for the year, which is 25 more than the previous week, the CDC said on July 22. The highest 1-week total for the year was the 90 cases reported during the week of April 11.
The number of outbreaks is back up to five as California returned to the list after a 1-week absence and El Paso, Tex., made its first appearance of the year. The current outbreak in California – the state’s fifth – is occurring in Los Angeles, which is now up to 16 total cases in 2019. El Paso just reported its fourth case on July 17, and the city’s health department noted that “it had been more than 25 years since El Paso saw its last case of measles before these four recent cases.” Outbreaks also are ongoing in Rockland County, N.Y.; New York City; and three counties in Washington State.
States that joined the ranks of the measles-infected during this most recent reporting week were Alaska and Ohio, which brings the total number to 30 for the year, the CDC said.
The Alaska Department of Health and Social Services said that it “has confirmed a single case of measles in an unvaccinated teenager from the Kenai Peninsula who recently traveled out of state to Arizona via Seattle.” The Ohio case is a “young adult from Stark County [who] recently traveled to a state with confirmed measles cases,” according to the state’s health department.
Atherosclerotic disease risk persists decades after smoking cessation
Adults who quit smoking reduced their risk for peripheral artery disease in the short term, but remained at increased risk for up to 30 years, compared with never-smokers, based on data from more than 13,000 adults in a community-based study.

Most reports on the impact of smoking cessation on cardiovascular disease have focused on coronary heart disease (CHD), and stroke, while data on the effects of smoking cessation on peripheral artery disease (PAD) are limited, wrote Ning Ding, MBBS, SCM, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, Md., and colleagues.
To compare the impact of smoking on PAD, CHD, and stroke, the researchers used data from the Atherosclerosis Risk in Communities (ARIC) study, which included 15,792 adults aged 45-64 years in four communities. The findings were published in the Journal of the American College of Cardiology.
The study population of 13,355 individuals had no baseline history of PAD, CHD, or stroke. Over a median 26 years of follow-up, the researchers identified 492 cases of PAD, 1,798 cases of CHD, and 1,106 cases of stroke.
The risk of all three conditions began to decline within 5 years of smoking cessation, which could be encouraging to smokers who wish to quit, the researchers noted. In addition, the longer the duration of smoking cessation, the lower the risk for all three conditions (See central illustration).
However, a significantly elevated risk remained for PAD for up to 30 years after smoking cessation and for CHD for up to 20 years after smoking cessation, compared with never-smokers.
The researchers also found a roughly fourfold increased risk for PAD for smokers who smoked for 40 or more pack-years, compared with never-smokers, which was greater than the 2.1 hazard ratio for CHD and 1.8 HR for stroke. In addition, current smokers of at least one pack per day had a significantly greater risk of PAD, compared with never-smokers (HR, 5.36) that was higher than the risk for CHD or stroke (HR, 2.38 and HR, 1.88, respectively).
The study findings were limited by several factors including the reliance on self-reports, potential misclassification of data, and the potential exclusion of mild PAD cases that did not require hospitalization, the researchers noted. However, the results support the value of encouraging smokers to quit and support the need to include PAD risk in public health information, they said. “Although public statements about smoking and [cardiovascular disease] have been focusing on CHD and stroke, our results indicate the need to take account of PAD as well for comprehensively acknowledging the effect of smoking on overall cardiovascular health,” they added.
The ARIC study was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health. Lead author Dr. Ding had no financial conflicts to disclose; coauthors disclosed relationships with Bristol-Myers Squibb and Fukuda Denshi.
SOURCE: Ding N et al. J Am Coll Cardiol. 2019 Jul 22;74:498-507. doi: 10.1016/j.jacc.2019.06.003.
Although the pathophysiology of smoking and cardiovascular disease has yet to be teased out, the current study findings support the public health message that any and all smokers can improve their health by quitting any time: “It is never too early or too late to benefit from quitting,” wrote Nancy A. Rigotti, MD, and Mary M. McDermott, MD, in an accompanying editorial. The editorialists questioned whether the findings were generalizable to patients with mild PAD or those who are not hospitalized. However, they found the data consistent with previous studies suggesting that atherosclerosis is not homogeneous. “Differences in shear stress and hemodynamic forces among the femoral, coronary, and carotid arterial beds may also explain variability in associations of smoking and smoking cessation with the incidence of PAD versus myocardial infarction or stroke,” they said.
The findings also support the need to emphasize PAD in public health messages and provide an opportunity to educate patients about the risks of limb loss and impaired mobility associated with PAD, they said.
Many clinicians put a low priority on smoking cessation, the editorialists wrote, but “long-term tobacco abstinence is achievable using a chronic disease management approach resembling the strategies used to manage other risk factors,” they said. They cited the American College of Cardiology’s recently released “Expert Consensus Decision Pathway on Tobacco Cessation Treatment.” The pathway outlines advice for clinicians, including how to provide a brief intervention and resources along with advice to quit smoking.
Dr. Rigotti is affiliated with Harvard Medical School, Boston. Dr. McDermott is affiliated with Northwestern University, Chicago. Dr. Rigotti disclosed royalties from UpToDate, serving as a consultant for Achieve Life Sciences, and travel expenses from Pfizer for unpaid consulting. Dr. McDermott disclosed research funding from Regeneron, the National Heart, Lung, and Blood Institute, the National Institute on Aging, and the American Heart Association, plus research support from Chromadex, ReserveAge, Hershey, and ViroMed.
Although the pathophysiology of smoking and cardiovascular disease has yet to be teased out, the current study findings support the public health message that any and all smokers can improve their health by quitting any time: “It is never too early or too late to benefit from quitting,” wrote Nancy A. Rigotti, MD, and Mary M. McDermott, MD, in an accompanying editorial. The editorialists questioned whether the findings were generalizable to patients with mild PAD or those who are not hospitalized. However, they found the data consistent with previous studies suggesting that atherosclerosis is not homogeneous. “Differences in shear stress and hemodynamic forces among the femoral, coronary, and carotid arterial beds may also explain variability in associations of smoking and smoking cessation with the incidence of PAD versus myocardial infarction or stroke,” they said.
The findings also support the need to emphasize PAD in public health messages and provide an opportunity to educate patients about the risks of limb loss and impaired mobility associated with PAD, they said.
Many clinicians put a low priority on smoking cessation, the editorialists wrote, but “long-term tobacco abstinence is achievable using a chronic disease management approach resembling the strategies used to manage other risk factors,” they said. They cited the American College of Cardiology’s recently released “Expert Consensus Decision Pathway on Tobacco Cessation Treatment.” The pathway outlines advice for clinicians, including how to provide a brief intervention and resources along with advice to quit smoking.
Dr. Rigotti is affiliated with Harvard Medical School, Boston. Dr. McDermott is affiliated with Northwestern University, Chicago. Dr. Rigotti disclosed royalties from UpToDate, serving as a consultant for Achieve Life Sciences, and travel expenses from Pfizer for unpaid consulting. Dr. McDermott disclosed research funding from Regeneron, the National Heart, Lung, and Blood Institute, the National Institute on Aging, and the American Heart Association, plus research support from Chromadex, ReserveAge, Hershey, and ViroMed.
Although the pathophysiology of smoking and cardiovascular disease has yet to be teased out, the current study findings support the public health message that any and all smokers can improve their health by quitting any time: “It is never too early or too late to benefit from quitting,” wrote Nancy A. Rigotti, MD, and Mary M. McDermott, MD, in an accompanying editorial. The editorialists questioned whether the findings were generalizable to patients with mild PAD or those who are not hospitalized. However, they found the data consistent with previous studies suggesting that atherosclerosis is not homogeneous. “Differences in shear stress and hemodynamic forces among the femoral, coronary, and carotid arterial beds may also explain variability in associations of smoking and smoking cessation with the incidence of PAD versus myocardial infarction or stroke,” they said.
The findings also support the need to emphasize PAD in public health messages and provide an opportunity to educate patients about the risks of limb loss and impaired mobility associated with PAD, they said.
Many clinicians put a low priority on smoking cessation, the editorialists wrote, but “long-term tobacco abstinence is achievable using a chronic disease management approach resembling the strategies used to manage other risk factors,” they said. They cited the American College of Cardiology’s recently released “Expert Consensus Decision Pathway on Tobacco Cessation Treatment.” The pathway outlines advice for clinicians, including how to provide a brief intervention and resources along with advice to quit smoking.
Dr. Rigotti is affiliated with Harvard Medical School, Boston. Dr. McDermott is affiliated with Northwestern University, Chicago. Dr. Rigotti disclosed royalties from UpToDate, serving as a consultant for Achieve Life Sciences, and travel expenses from Pfizer for unpaid consulting. Dr. McDermott disclosed research funding from Regeneron, the National Heart, Lung, and Blood Institute, the National Institute on Aging, and the American Heart Association, plus research support from Chromadex, ReserveAge, Hershey, and ViroMed.
Adults who quit smoking reduced their risk for peripheral artery disease in the short term, but remained at increased risk for up to 30 years, compared with never-smokers, based on data from more than 13,000 adults in a community-based study.

Most reports on the impact of smoking cessation on cardiovascular disease have focused on coronary heart disease (CHD), and stroke, while data on the effects of smoking cessation on peripheral artery disease (PAD) are limited, wrote Ning Ding, MBBS, SCM, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, Md., and colleagues.
To compare the impact of smoking on PAD, CHD, and stroke, the researchers used data from the Atherosclerosis Risk in Communities (ARIC) study, which included 15,792 adults aged 45-64 years in four communities. The findings were published in the Journal of the American College of Cardiology.
The study population of 13,355 individuals had no baseline history of PAD, CHD, or stroke. Over a median 26 years of follow-up, the researchers identified 492 cases of PAD, 1,798 cases of CHD, and 1,106 cases of stroke.
The risk of all three conditions began to decline within 5 years of smoking cessation, which could be encouraging to smokers who wish to quit, the researchers noted. In addition, the longer the duration of smoking cessation, the lower the risk for all three conditions (See central illustration).
However, a significantly elevated risk remained for PAD for up to 30 years after smoking cessation and for CHD for up to 20 years after smoking cessation, compared with never-smokers.
The researchers also found a roughly fourfold increased risk for PAD for smokers who smoked for 40 or more pack-years, compared with never-smokers, which was greater than the 2.1 hazard ratio for CHD and 1.8 HR for stroke. In addition, current smokers of at least one pack per day had a significantly greater risk of PAD, compared with never-smokers (HR, 5.36) that was higher than the risk for CHD or stroke (HR, 2.38 and HR, 1.88, respectively).
The study findings were limited by several factors including the reliance on self-reports, potential misclassification of data, and the potential exclusion of mild PAD cases that did not require hospitalization, the researchers noted. However, the results support the value of encouraging smokers to quit and support the need to include PAD risk in public health information, they said. “Although public statements about smoking and [cardiovascular disease] have been focusing on CHD and stroke, our results indicate the need to take account of PAD as well for comprehensively acknowledging the effect of smoking on overall cardiovascular health,” they added.
The ARIC study was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health. Lead author Dr. Ding had no financial conflicts to disclose; coauthors disclosed relationships with Bristol-Myers Squibb and Fukuda Denshi.
SOURCE: Ding N et al. J Am Coll Cardiol. 2019 Jul 22;74:498-507. doi: 10.1016/j.jacc.2019.06.003.
Adults who quit smoking reduced their risk for peripheral artery disease in the short term, but remained at increased risk for up to 30 years, compared with never-smokers, based on data from more than 13,000 adults in a community-based study.

Most reports on the impact of smoking cessation on cardiovascular disease have focused on coronary heart disease (CHD), and stroke, while data on the effects of smoking cessation on peripheral artery disease (PAD) are limited, wrote Ning Ding, MBBS, SCM, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, Md., and colleagues.
To compare the impact of smoking on PAD, CHD, and stroke, the researchers used data from the Atherosclerosis Risk in Communities (ARIC) study, which included 15,792 adults aged 45-64 years in four communities. The findings were published in the Journal of the American College of Cardiology.
The study population of 13,355 individuals had no baseline history of PAD, CHD, or stroke. Over a median 26 years of follow-up, the researchers identified 492 cases of PAD, 1,798 cases of CHD, and 1,106 cases of stroke.
The risk of all three conditions began to decline within 5 years of smoking cessation, which could be encouraging to smokers who wish to quit, the researchers noted. In addition, the longer the duration of smoking cessation, the lower the risk for all three conditions (See central illustration).
However, a significantly elevated risk remained for PAD for up to 30 years after smoking cessation and for CHD for up to 20 years after smoking cessation, compared with never-smokers.
The researchers also found a roughly fourfold increased risk for PAD for smokers who smoked for 40 or more pack-years, compared with never-smokers, which was greater than the 2.1 hazard ratio for CHD and 1.8 HR for stroke. In addition, current smokers of at least one pack per day had a significantly greater risk of PAD, compared with never-smokers (HR, 5.36) that was higher than the risk for CHD or stroke (HR, 2.38 and HR, 1.88, respectively).
The study findings were limited by several factors including the reliance on self-reports, potential misclassification of data, and the potential exclusion of mild PAD cases that did not require hospitalization, the researchers noted. However, the results support the value of encouraging smokers to quit and support the need to include PAD risk in public health information, they said. “Although public statements about smoking and [cardiovascular disease] have been focusing on CHD and stroke, our results indicate the need to take account of PAD as well for comprehensively acknowledging the effect of smoking on overall cardiovascular health,” they added.
The ARIC study was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health. Lead author Dr. Ding had no financial conflicts to disclose; coauthors disclosed relationships with Bristol-Myers Squibb and Fukuda Denshi.
SOURCE: Ding N et al. J Am Coll Cardiol. 2019 Jul 22;74:498-507. doi: 10.1016/j.jacc.2019.06.003.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Scrotal Ulceration: A Complication of Hyperthermic Intraperitoneal Chemotherapy and Subsequent Treatment With Dimethyl Sulfoxide
To the Editor:
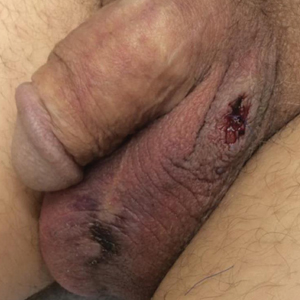
A 54-year-old man with a history of stage IV appendiceal carcinoid adenocarcinoma treated approximately 3 months prior with intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) presented to our clinic with scrotal pain of 5 days’ duration. He had no history of genital herpes, topical contactants, other cutaneous lesions on the body, fever, or chills. On physical examination the patient had an erythematous, purpuric, indurated, tender plaque on the left anterolateral and anterior midline of the scrotum (Figure 1). No other areas of acral purpura or livedoid cutaneous changes were identified. There was no inguinal lymphadenopathy. Biopsy was performed for histologic examination as well as tissue culture. Histology demonstrated epidermal necrosis without evidence of vasculitis. Tissue culture was unremarkable.
Two days after clinic evaluation, the patient presented to the emergency department with progression of the lesions, and he was admitted to the hospital for pain control. Computed tomography of the pelvis showed bilateral hydroceles without evidence of abscess. Ultrasonography showed scrotal thickening without abscess or fluid collection. On day 5 in the hospital, a regimen of topical 60% dimethyl sulfoxide (DMSO) was applied every 8 hours to the affected area. The patient experienced notable pain relief and a decrease in erythema within 7 hours of application (Figure 2). This regimen was continued for 7 days with improvement in surrounding erythema and pain; however, the patient’s pain persisted in the areas of necrosis. Fourteen days following completion of therapy (27 days following presentation), the patient underwent debridement and partial scrotal resection for eschar removal. Histologic examination of the debrided scrotal tissue showed necrosis extending into the dermis and no evidence of vasculitis.
Our case demonstrates a unique presentation of scrotal necrosis secondary to mitomycin C (MitC) extravasation subsequently managed with DMSO. Imaging and biopsy findings effectively ruled out infection or vasculitis and led us to consider extravasation reactions that typically occur at peripheral intravenous (IV) infusion sites. Suspected cases of scrotal necrosis following HIPEC with MitC have been reported in the literature, along with hypothesized pathophysiology.1-3
In consideration of the proposed pathophysiology, individuals with hydroceles may be more likely to experience this complication due to an abnormal but not uncommon communication between the intraperitoneal cavity and the scrotum via a patent processus vaginalis. The location of necrosis on the anterior scrotum remains unexplained. It may be a consequence of the anatomic location of the hydrocele, a collection of fluid within the tunica vaginalis. The tunica vaginalis is composed of an inner visceral and outer parietal layer, enveloping the testis at the anterior border but not the superior or posterior border. Thus, sequestration of MitC in a hydrocele would correlate anatomically to necrosis of the anterior wall of the scrotum.
Akhavan et al1 proposed the testes are unaffected because of the presence of the tough fibrous coat of the tunica albuginea that directly adheres to the testes, in addition to the adjacent visceral layer of the tunica vaginalis. These 2 layers separating the testes and the hydrocele may provide a double barrier of protection for the testes.1
According to a PubMed search of articles indexed for MEDLINE using the terms scrotal or cutaneous, pain or ulceration, and HIPEC or hyperthermic in
Hyperthermic intraperitoneal chemotherapy involves installation of high-concentration chemotherapeutics into the peritoneal cavity at the conclusion of surgical cytoreductive therapy. Cell cycle–nonspecific agents such as MitC commonly are used for this procedure.4 It is classified as a vesicant, which is the designation given to drugs known to produce the most severe extravasation reactions of skin ulceration and necrosis.5,6 Symptoms typically include an early area of localized edema, erythema, and severe pain that progresses to superficial soft tissue and skin necrosis.7 Unfortunately, no well-studied antidote exists for MitC, though empirical guidelines suggest therapeutic management with DMSO and ice packs.6,8
Dimethyl sulfoxide is thought to work as a free radical scavenger as well as a solvent that facilitates diffusion of chemotherapeutics through tissues and thus down a concentration gradient, ideal in the circumstance of an extravasation reaction.8 Topical DMSO has been studied as a nonsurgical treatment in a small number of patients to prevent progression to necrosis following MitC extravasation.5,7 However, these cases only report extravasation reactions from IV infiltration.5,7,9 Dimethyl sulfoxide is rapidly absorbed and acts as a theoretical carrier for MitC as well as other topical substances.5,10,11 Caution is advised when using topical lidocaine or steroids in combination with DMSO, as they will be rapidly absorbed systemically. Patients also should be informed about a mild local burning sensation after DMSO application and a garliclike odor of the breath, which have occurred in 5.5% and 27.5% of patients, respectively (N=144).5 Dimethyl sulfoxide has no known toxic side effects but can cause erythema, pruritus, and very rarely allergic contact dermatitis.5,12 Abdul Aziz et al2 postulated that DMSO might be used as a method to prevent the progression of necrosis in symptomatic patients following HIPEC with MitC. Reports of its use on the scrotum are absent in the current available literature.
Treatment with DMSO was attempted in our patient with limited success secondary to delayed recognition and lack of supporting literature for DMSO treatment of scrotal necrosis. Treatment was delayed by 11 days after the onset of symptoms, which is far beyond the recommendation of starting within 10 minutes.8 Irreversible tissue necrosis had already occurred as evidenced by the presence of eschar. However, it seems apparent that DMSO provided some benefit given the clear improvement in erythema and pain 7 hours after application (Figure 2). It is unknown to what extent the necrosis would have progressed if not treated with DMSO.
Scrotal necrosis following HIPEC with MitC is a rare and incompletely understood but important chemotherapy reaction. The presentation is fairly specific with the presence of intractable and constant scrotal pain along with erythema and induration progressing to eschar. Although DMSO has been found to be effective for certain vesicant extravasation reactions at IV sites, it is not well studied for MitC, and no reports exist regarding its use on the scrotum. The presented characterization and explanation of the pathophysiology of this entity will aid in early recognition and timely institution of topical mitigating agents such as DMSO, which may prevent progression to scrotal necrosis and need for surgical debridement. More effective strategies may be geared toward prevention with thorough washout following HIPEC, preprocedural radiologic imaging or intraoperative visualization of the patent processus vaginalis, internal inguinal canal plugs, and patient education with anticipatory guidance should a reaction occur.2
- Akhavan A, Yin M, Benoit R. Scrotal ulcer after intraperitoneal hyperthermic chemotherapy. Urology. 2007;69:778.E9-E10.
- Abdul Aziz NH, Wang W, Teo MC. Scrotal pain and ulceration post HIPEC: a case report. J Gastrointest Cancer. 2015;46:60-63.
- Silva F, Avancini J, Criado P, et al. Scrotum ulcer developed after intraperitoneal hyperthermic chemotherapy with mitomycin-C [published October 21, 2012]. Bjui International. doi:10.1002/BJUIw-2012-019-web.
- González-Moreno S, González-Bayón LA, Ortega-Pérez G.Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2010;15:68-75.
- Bertelli G, Gozza A, Forno GB, et al. Topical dimethyl sulfoxide for the prevention of soft tissue injury after extravasation of vesicant cytotoxic drugs: a prospective clinical study. J Clin Oncol. 1995;13:2851-2855.
- Bertelli G. Prevention and management of extravasation of cytotoxic drugs. Drug Saf. 1995;12:245-255.
- Alberts DS, Dorr RT. Case report: topical DMSO for mitomycin-C-induced skin ulceration. Oncol Nurs Forum. 1991;18:693-695.
- Pérez Fidalgo JA, García Fabregat L, Cervantes A, et al; ESMO Guidelines Working Group. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012;23(suppl 5):167-173.
- Ludwig CU, Stoll HR, Obrist R, et al. Prevention of cytotoxic drug induced skin ulcers with dimethyl sulfoxide (DMSO) and alpha-tocopherole. Eur J Cancer Clin Oncol. 1987;23:327-329.
- Groel JT. Dimethyl sulfoxide as a vehicle for corticosteroids. a comparison with the occlusive dressing technique. Arch Dermatol. 1968;97:110-114.
- Simon LS, Grierson LM, Naseer Z. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis [published online April 19, 2009]. Pain. 2009;143:238-245.
- Nishimura M, Takano Y, Toshitani S. Systemic contact dermatitis medicamentosa occurring after intravesical dimethyl sulfoxide treatment for interstitial cystitis. Arch Dermatol. 1988;124:182-183.
To the Editor:
A 54-year-old man with a history of stage IV appendiceal carcinoid adenocarcinoma treated approximately 3 months prior with intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) presented to our clinic with scrotal pain of 5 days’ duration. He had no history of genital herpes, topical contactants, other cutaneous lesions on the body, fever, or chills. On physical examination the patient had an erythematous, purpuric, indurated, tender plaque on the left anterolateral and anterior midline of the scrotum (Figure 1). No other areas of acral purpura or livedoid cutaneous changes were identified. There was no inguinal lymphadenopathy. Biopsy was performed for histologic examination as well as tissue culture. Histology demonstrated epidermal necrosis without evidence of vasculitis. Tissue culture was unremarkable.
Two days after clinic evaluation, the patient presented to the emergency department with progression of the lesions, and he was admitted to the hospital for pain control. Computed tomography of the pelvis showed bilateral hydroceles without evidence of abscess. Ultrasonography showed scrotal thickening without abscess or fluid collection. On day 5 in the hospital, a regimen of topical 60% dimethyl sulfoxide (DMSO) was applied every 8 hours to the affected area. The patient experienced notable pain relief and a decrease in erythema within 7 hours of application (Figure 2). This regimen was continued for 7 days with improvement in surrounding erythema and pain; however, the patient’s pain persisted in the areas of necrosis. Fourteen days following completion of therapy (27 days following presentation), the patient underwent debridement and partial scrotal resection for eschar removal. Histologic examination of the debrided scrotal tissue showed necrosis extending into the dermis and no evidence of vasculitis.
Our case demonstrates a unique presentation of scrotal necrosis secondary to mitomycin C (MitC) extravasation subsequently managed with DMSO. Imaging and biopsy findings effectively ruled out infection or vasculitis and led us to consider extravasation reactions that typically occur at peripheral intravenous (IV) infusion sites. Suspected cases of scrotal necrosis following HIPEC with MitC have been reported in the literature, along with hypothesized pathophysiology.1-3
In consideration of the proposed pathophysiology, individuals with hydroceles may be more likely to experience this complication due to an abnormal but not uncommon communication between the intraperitoneal cavity and the scrotum via a patent processus vaginalis. The location of necrosis on the anterior scrotum remains unexplained. It may be a consequence of the anatomic location of the hydrocele, a collection of fluid within the tunica vaginalis. The tunica vaginalis is composed of an inner visceral and outer parietal layer, enveloping the testis at the anterior border but not the superior or posterior border. Thus, sequestration of MitC in a hydrocele would correlate anatomically to necrosis of the anterior wall of the scrotum.
Akhavan et al1 proposed the testes are unaffected because of the presence of the tough fibrous coat of the tunica albuginea that directly adheres to the testes, in addition to the adjacent visceral layer of the tunica vaginalis. These 2 layers separating the testes and the hydrocele may provide a double barrier of protection for the testes.1
According to a PubMed search of articles indexed for MEDLINE using the terms scrotal or cutaneous, pain or ulceration, and HIPEC or hyperthermic in
Hyperthermic intraperitoneal chemotherapy involves installation of high-concentration chemotherapeutics into the peritoneal cavity at the conclusion of surgical cytoreductive therapy. Cell cycle–nonspecific agents such as MitC commonly are used for this procedure.4 It is classified as a vesicant, which is the designation given to drugs known to produce the most severe extravasation reactions of skin ulceration and necrosis.5,6 Symptoms typically include an early area of localized edema, erythema, and severe pain that progresses to superficial soft tissue and skin necrosis.7 Unfortunately, no well-studied antidote exists for MitC, though empirical guidelines suggest therapeutic management with DMSO and ice packs.6,8
Dimethyl sulfoxide is thought to work as a free radical scavenger as well as a solvent that facilitates diffusion of chemotherapeutics through tissues and thus down a concentration gradient, ideal in the circumstance of an extravasation reaction.8 Topical DMSO has been studied as a nonsurgical treatment in a small number of patients to prevent progression to necrosis following MitC extravasation.5,7 However, these cases only report extravasation reactions from IV infiltration.5,7,9 Dimethyl sulfoxide is rapidly absorbed and acts as a theoretical carrier for MitC as well as other topical substances.5,10,11 Caution is advised when using topical lidocaine or steroids in combination with DMSO, as they will be rapidly absorbed systemically. Patients also should be informed about a mild local burning sensation after DMSO application and a garliclike odor of the breath, which have occurred in 5.5% and 27.5% of patients, respectively (N=144).5 Dimethyl sulfoxide has no known toxic side effects but can cause erythema, pruritus, and very rarely allergic contact dermatitis.5,12 Abdul Aziz et al2 postulated that DMSO might be used as a method to prevent the progression of necrosis in symptomatic patients following HIPEC with MitC. Reports of its use on the scrotum are absent in the current available literature.
Treatment with DMSO was attempted in our patient with limited success secondary to delayed recognition and lack of supporting literature for DMSO treatment of scrotal necrosis. Treatment was delayed by 11 days after the onset of symptoms, which is far beyond the recommendation of starting within 10 minutes.8 Irreversible tissue necrosis had already occurred as evidenced by the presence of eschar. However, it seems apparent that DMSO provided some benefit given the clear improvement in erythema and pain 7 hours after application (Figure 2). It is unknown to what extent the necrosis would have progressed if not treated with DMSO.
Scrotal necrosis following HIPEC with MitC is a rare and incompletely understood but important chemotherapy reaction. The presentation is fairly specific with the presence of intractable and constant scrotal pain along with erythema and induration progressing to eschar. Although DMSO has been found to be effective for certain vesicant extravasation reactions at IV sites, it is not well studied for MitC, and no reports exist regarding its use on the scrotum. The presented characterization and explanation of the pathophysiology of this entity will aid in early recognition and timely institution of topical mitigating agents such as DMSO, which may prevent progression to scrotal necrosis and need for surgical debridement. More effective strategies may be geared toward prevention with thorough washout following HIPEC, preprocedural radiologic imaging or intraoperative visualization of the patent processus vaginalis, internal inguinal canal plugs, and patient education with anticipatory guidance should a reaction occur.2
To the Editor:
A 54-year-old man with a history of stage IV appendiceal carcinoid adenocarcinoma treated approximately 3 months prior with intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) presented to our clinic with scrotal pain of 5 days’ duration. He had no history of genital herpes, topical contactants, other cutaneous lesions on the body, fever, or chills. On physical examination the patient had an erythematous, purpuric, indurated, tender plaque on the left anterolateral and anterior midline of the scrotum (Figure 1). No other areas of acral purpura or livedoid cutaneous changes were identified. There was no inguinal lymphadenopathy. Biopsy was performed for histologic examination as well as tissue culture. Histology demonstrated epidermal necrosis without evidence of vasculitis. Tissue culture was unremarkable.
Two days after clinic evaluation, the patient presented to the emergency department with progression of the lesions, and he was admitted to the hospital for pain control. Computed tomography of the pelvis showed bilateral hydroceles without evidence of abscess. Ultrasonography showed scrotal thickening without abscess or fluid collection. On day 5 in the hospital, a regimen of topical 60% dimethyl sulfoxide (DMSO) was applied every 8 hours to the affected area. The patient experienced notable pain relief and a decrease in erythema within 7 hours of application (Figure 2). This regimen was continued for 7 days with improvement in surrounding erythema and pain; however, the patient’s pain persisted in the areas of necrosis. Fourteen days following completion of therapy (27 days following presentation), the patient underwent debridement and partial scrotal resection for eschar removal. Histologic examination of the debrided scrotal tissue showed necrosis extending into the dermis and no evidence of vasculitis.
Our case demonstrates a unique presentation of scrotal necrosis secondary to mitomycin C (MitC) extravasation subsequently managed with DMSO. Imaging and biopsy findings effectively ruled out infection or vasculitis and led us to consider extravasation reactions that typically occur at peripheral intravenous (IV) infusion sites. Suspected cases of scrotal necrosis following HIPEC with MitC have been reported in the literature, along with hypothesized pathophysiology.1-3
In consideration of the proposed pathophysiology, individuals with hydroceles may be more likely to experience this complication due to an abnormal but not uncommon communication between the intraperitoneal cavity and the scrotum via a patent processus vaginalis. The location of necrosis on the anterior scrotum remains unexplained. It may be a consequence of the anatomic location of the hydrocele, a collection of fluid within the tunica vaginalis. The tunica vaginalis is composed of an inner visceral and outer parietal layer, enveloping the testis at the anterior border but not the superior or posterior border. Thus, sequestration of MitC in a hydrocele would correlate anatomically to necrosis of the anterior wall of the scrotum.
Akhavan et al1 proposed the testes are unaffected because of the presence of the tough fibrous coat of the tunica albuginea that directly adheres to the testes, in addition to the adjacent visceral layer of the tunica vaginalis. These 2 layers separating the testes and the hydrocele may provide a double barrier of protection for the testes.1
According to a PubMed search of articles indexed for MEDLINE using the terms scrotal or cutaneous, pain or ulceration, and HIPEC or hyperthermic in
Hyperthermic intraperitoneal chemotherapy involves installation of high-concentration chemotherapeutics into the peritoneal cavity at the conclusion of surgical cytoreductive therapy. Cell cycle–nonspecific agents such as MitC commonly are used for this procedure.4 It is classified as a vesicant, which is the designation given to drugs known to produce the most severe extravasation reactions of skin ulceration and necrosis.5,6 Symptoms typically include an early area of localized edema, erythema, and severe pain that progresses to superficial soft tissue and skin necrosis.7 Unfortunately, no well-studied antidote exists for MitC, though empirical guidelines suggest therapeutic management with DMSO and ice packs.6,8
Dimethyl sulfoxide is thought to work as a free radical scavenger as well as a solvent that facilitates diffusion of chemotherapeutics through tissues and thus down a concentration gradient, ideal in the circumstance of an extravasation reaction.8 Topical DMSO has been studied as a nonsurgical treatment in a small number of patients to prevent progression to necrosis following MitC extravasation.5,7 However, these cases only report extravasation reactions from IV infiltration.5,7,9 Dimethyl sulfoxide is rapidly absorbed and acts as a theoretical carrier for MitC as well as other topical substances.5,10,11 Caution is advised when using topical lidocaine or steroids in combination with DMSO, as they will be rapidly absorbed systemically. Patients also should be informed about a mild local burning sensation after DMSO application and a garliclike odor of the breath, which have occurred in 5.5% and 27.5% of patients, respectively (N=144).5 Dimethyl sulfoxide has no known toxic side effects but can cause erythema, pruritus, and very rarely allergic contact dermatitis.5,12 Abdul Aziz et al2 postulated that DMSO might be used as a method to prevent the progression of necrosis in symptomatic patients following HIPEC with MitC. Reports of its use on the scrotum are absent in the current available literature.
Treatment with DMSO was attempted in our patient with limited success secondary to delayed recognition and lack of supporting literature for DMSO treatment of scrotal necrosis. Treatment was delayed by 11 days after the onset of symptoms, which is far beyond the recommendation of starting within 10 minutes.8 Irreversible tissue necrosis had already occurred as evidenced by the presence of eschar. However, it seems apparent that DMSO provided some benefit given the clear improvement in erythema and pain 7 hours after application (Figure 2). It is unknown to what extent the necrosis would have progressed if not treated with DMSO.
Scrotal necrosis following HIPEC with MitC is a rare and incompletely understood but important chemotherapy reaction. The presentation is fairly specific with the presence of intractable and constant scrotal pain along with erythema and induration progressing to eschar. Although DMSO has been found to be effective for certain vesicant extravasation reactions at IV sites, it is not well studied for MitC, and no reports exist regarding its use on the scrotum. The presented characterization and explanation of the pathophysiology of this entity will aid in early recognition and timely institution of topical mitigating agents such as DMSO, which may prevent progression to scrotal necrosis and need for surgical debridement. More effective strategies may be geared toward prevention with thorough washout following HIPEC, preprocedural radiologic imaging or intraoperative visualization of the patent processus vaginalis, internal inguinal canal plugs, and patient education with anticipatory guidance should a reaction occur.2
- Akhavan A, Yin M, Benoit R. Scrotal ulcer after intraperitoneal hyperthermic chemotherapy. Urology. 2007;69:778.E9-E10.
- Abdul Aziz NH, Wang W, Teo MC. Scrotal pain and ulceration post HIPEC: a case report. J Gastrointest Cancer. 2015;46:60-63.
- Silva F, Avancini J, Criado P, et al. Scrotum ulcer developed after intraperitoneal hyperthermic chemotherapy with mitomycin-C [published October 21, 2012]. Bjui International. doi:10.1002/BJUIw-2012-019-web.
- González-Moreno S, González-Bayón LA, Ortega-Pérez G.Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2010;15:68-75.
- Bertelli G, Gozza A, Forno GB, et al. Topical dimethyl sulfoxide for the prevention of soft tissue injury after extravasation of vesicant cytotoxic drugs: a prospective clinical study. J Clin Oncol. 1995;13:2851-2855.
- Bertelli G. Prevention and management of extravasation of cytotoxic drugs. Drug Saf. 1995;12:245-255.
- Alberts DS, Dorr RT. Case report: topical DMSO for mitomycin-C-induced skin ulceration. Oncol Nurs Forum. 1991;18:693-695.
- Pérez Fidalgo JA, García Fabregat L, Cervantes A, et al; ESMO Guidelines Working Group. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012;23(suppl 5):167-173.
- Ludwig CU, Stoll HR, Obrist R, et al. Prevention of cytotoxic drug induced skin ulcers with dimethyl sulfoxide (DMSO) and alpha-tocopherole. Eur J Cancer Clin Oncol. 1987;23:327-329.
- Groel JT. Dimethyl sulfoxide as a vehicle for corticosteroids. a comparison with the occlusive dressing technique. Arch Dermatol. 1968;97:110-114.
- Simon LS, Grierson LM, Naseer Z. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis [published online April 19, 2009]. Pain. 2009;143:238-245.
- Nishimura M, Takano Y, Toshitani S. Systemic contact dermatitis medicamentosa occurring after intravesical dimethyl sulfoxide treatment for interstitial cystitis. Arch Dermatol. 1988;124:182-183.
- Akhavan A, Yin M, Benoit R. Scrotal ulcer after intraperitoneal hyperthermic chemotherapy. Urology. 2007;69:778.E9-E10.
- Abdul Aziz NH, Wang W, Teo MC. Scrotal pain and ulceration post HIPEC: a case report. J Gastrointest Cancer. 2015;46:60-63.
- Silva F, Avancini J, Criado P, et al. Scrotum ulcer developed after intraperitoneal hyperthermic chemotherapy with mitomycin-C [published October 21, 2012]. Bjui International. doi:10.1002/BJUIw-2012-019-web.
- González-Moreno S, González-Bayón LA, Ortega-Pérez G.Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2010;15:68-75.
- Bertelli G, Gozza A, Forno GB, et al. Topical dimethyl sulfoxide for the prevention of soft tissue injury after extravasation of vesicant cytotoxic drugs: a prospective clinical study. J Clin Oncol. 1995;13:2851-2855.
- Bertelli G. Prevention and management of extravasation of cytotoxic drugs. Drug Saf. 1995;12:245-255.
- Alberts DS, Dorr RT. Case report: topical DMSO for mitomycin-C-induced skin ulceration. Oncol Nurs Forum. 1991;18:693-695.
- Pérez Fidalgo JA, García Fabregat L, Cervantes A, et al; ESMO Guidelines Working Group. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012;23(suppl 5):167-173.
- Ludwig CU, Stoll HR, Obrist R, et al. Prevention of cytotoxic drug induced skin ulcers with dimethyl sulfoxide (DMSO) and alpha-tocopherole. Eur J Cancer Clin Oncol. 1987;23:327-329.
- Groel JT. Dimethyl sulfoxide as a vehicle for corticosteroids. a comparison with the occlusive dressing technique. Arch Dermatol. 1968;97:110-114.
- Simon LS, Grierson LM, Naseer Z. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis [published online April 19, 2009]. Pain. 2009;143:238-245.
- Nishimura M, Takano Y, Toshitani S. Systemic contact dermatitis medicamentosa occurring after intravesical dimethyl sulfoxide treatment for interstitial cystitis. Arch Dermatol. 1988;124:182-183.
Practice Points
- Scrotal ulceration following hyperthermic intraperitoneal chemotherapy has been reported only a few times in the literature and is likely underreported. The presentation in all reported cases was similar, with a delay in symptom onset of weeks to months, involvement of the anterior scrotum, and pain.
- Dimethyl sulfoxide, used in other vesicant reactions, may have a role in mitigating tissue damage. Alternatively, methods to prevent sequestration of vesicants in the potential space of the tunica vaginalis layers can be employed.
Expert shares contact dermatitis trends
AUSTIN – Not long ago, Rajani Katta, MD, received a text message from a friend who expressed concern about a rash that developed in the underarm of her teenage daughter.
The culprit turned out to be the lavender essential oil contained in an “all natural” deodorant that her daughter had recently switched to – a storyline that Dr. Katta encounters with increasing frequency in her role as clinical professor of dermatology at the University of Texas Health Science Center at Houston.
Dr. Katta said at the annual meeting of the Society for Pediatric Dermatology. “When you talk about a natural allergy, it is more likely to occur if your skin barrier is compromised, so I think that’s why we’re seeing it, especially in young girls in the underarm area. If you shave the underarm, you impair that skin barrier and you’re more likely to develop a reaction to something you’re using over it.”
Her list of recommended deodorants includes Almay Roll-On Antiperspirant & Deodorant, Crystal Body Deodorant Stick, Crystal Roll-On Body Deodorant, Vanicream Deodorant for Sensitive Skin (aluminum-free), Vanicream Antiperspirant/Deodorant, and CertainDri Clinical Strength Roll-On. They are fragrance-free and lack propylene glycol, which is a common allergen.
Increasingly, essential oils are being added to lip balms and toothpastes, said Dr. Katta, who is also author of the 2018 book “Glow: The Dermatologist’s Guide to a Whole Foods Younger Skin Diet.” She recalled one patient who presented with chronic chapped lips. “It doesn’t matter how many lip glosses I try; it just keeps getting worse,” the patient told her. The likely culprit turned out to be ingredients contained in flavored lip balm from EOS. Reports of blistering and cracking of the lips from use of the products prompted a class action lawsuit and a notice to consumers from the Food and Drug Administration.
Another patient presented with cracked lips after switching to an “all natural” toothpaste that was labeled “gluten free.”
“It looked great,” Dr. Katta recalled. “Unfortunately it was not flavoring free. She reacted to multiple essential oils, including tea tree oil, contained in the toothpaste. This is being added to a number of toothpastes, and I think we’re going to see more of these types of reactions.”
Other toothpastes contain balsam of Peru, “which is consistently one of the top allergens in patch test clinics,” she said. “One of the components of balsam of Peru is a cinnamon compound, which can be an issue.”
Dr. Katta advises her patients to use Vaseline petroleum jelly as a lip balm and recommends Tom’s of Maine Silly Strawberry Flavor (this flavor only) toothpaste for children.
A few years ago, a teenager presented to Dr. Katta with intense bullae on the dorsum of the foot after wearing shoes without socks. “She was wearing white canvas Keds, which looked very innocuous,” she said. Patch testing revealed that the teen reacted to four different rubber accelerators. “When we contacted the company, they [acknowledged] using rubber cement to make the canvas Keds,” Dr. Katta said. “Rubber cement is an adhesive and it does contain rubber accelerators. Later, I saw two cases of children who had walked around all day at the amusement park wearing their Sperry Topsiders without any socks. We couldn’t get any information from that manufacturer, but I suspect that they also use a rubber-based glue to make those shoes.” She characterized shoes as “a real setup for a foot allergy because you have friction, sweat that’s pulling allergen out of an object, and sweat is carrying it over, especially to the dorsum of the foot.”
Dr. Katta has also noticed an uptick in the number of young patients who develop allergic reactions to dyes used to make workout clothing. “If you ever see rashes that do not involve the axillary vault but do have peraxillary accentuation, think textile allergy,” she said. “We’re seeing a lot of reactions to disperse blue clothing dyes. When you think about textile allergy from the dyes, it tends to be the blue and black clothing. It’s more likely in the setting of synthetic fabrics because they leach out dyes more easily, and it’s more likely in the setting of sweat because sweat helps pull allergen out. I’m seeing it a lot from sports uniforms and tight black leggings and tight sports bras that people are wearing. I’m also seeing some from bathing suits and swim shirts.”
Exposure to products containing the preservative methylisothiazolinone (MI) is also on the rise. It ranks as the second most frequent allergen for which the North American Contact Dermatitis Group is seeing positive results on patch testing, with rates of 13.4%. MI can be found in many skin care products and “probably about half of school glues, fabric glues, and craft glues,” Dr. Katta said. “Stick versus liquid doesn’t make a difference.” Children and teens often use craft glues, laundry detergents, and other products to create “slime” as a way to learn about viscosity, polymers, and chemical reactions. “Sometimes these children have sensitive skin, or they’re using it with prolonged contact, so they may be sensitizing themselves to the MI,” she said.
She concluded her remarks by noting that an increasing number of young patients are developing reactions to wearable medical devices such as insulin pumps and glucose monitors. “With this, the first thing to think about is frictional irritant dermatitis,” she said. “You can put Scanpor medical paper tape on people’s back for 48 hours straight to patch test them. Some people are incredibly reactive to the friction of just that tape. You also have to think about trapped allergen. One of my patients reacted to colophony, fragrance mix, and propylene glycol, all of which were contained in his skin care products. Some people are getting advice from other patients to use Mastisol liquid adhesive to help their glucose monitors stick better. Mastisol has a high rate of cross-reactivity with balsam of Peru, so it’s a fragrance allergen. That’s the first thing you want to ask patients about: what products they’re using.”
One of her patients thought she was reacting to adhesive tape on her skin, but in fact she was reacting to two different acrylates: ethylene glycol dimethacrylate (EGDMA) and hydroxyethyl methacrylate (HEMA). “I know about these allergens because I see reactions from butterfly needles in dialysis patients,” Dr. Katta explained. “What happens is, these acrylates are glues or plastics used somewhere else on the device, and they can migrate through barriers.”
In one published case, a 9-year-old boy developed a reaction to ethyl cyanoacrylate contained in a glucose sensor adhesive (Dermatitis. 2017; 28[4]:289-91). It never touched the boy’s skin directly but was presumed to migrate through that tape. “The bottom line is that acrylates may induce contact dermatitis even through perceived barriers,” she said. “Their use anywhere in medical devices may prove problematic.”
Dr. Katta reported that she is a member of the advisory board for Vichy Laboratories.
AUSTIN – Not long ago, Rajani Katta, MD, received a text message from a friend who expressed concern about a rash that developed in the underarm of her teenage daughter.
The culprit turned out to be the lavender essential oil contained in an “all natural” deodorant that her daughter had recently switched to – a storyline that Dr. Katta encounters with increasing frequency in her role as clinical professor of dermatology at the University of Texas Health Science Center at Houston.
Dr. Katta said at the annual meeting of the Society for Pediatric Dermatology. “When you talk about a natural allergy, it is more likely to occur if your skin barrier is compromised, so I think that’s why we’re seeing it, especially in young girls in the underarm area. If you shave the underarm, you impair that skin barrier and you’re more likely to develop a reaction to something you’re using over it.”
Her list of recommended deodorants includes Almay Roll-On Antiperspirant & Deodorant, Crystal Body Deodorant Stick, Crystal Roll-On Body Deodorant, Vanicream Deodorant for Sensitive Skin (aluminum-free), Vanicream Antiperspirant/Deodorant, and CertainDri Clinical Strength Roll-On. They are fragrance-free and lack propylene glycol, which is a common allergen.
Increasingly, essential oils are being added to lip balms and toothpastes, said Dr. Katta, who is also author of the 2018 book “Glow: The Dermatologist’s Guide to a Whole Foods Younger Skin Diet.” She recalled one patient who presented with chronic chapped lips. “It doesn’t matter how many lip glosses I try; it just keeps getting worse,” the patient told her. The likely culprit turned out to be ingredients contained in flavored lip balm from EOS. Reports of blistering and cracking of the lips from use of the products prompted a class action lawsuit and a notice to consumers from the Food and Drug Administration.
Another patient presented with cracked lips after switching to an “all natural” toothpaste that was labeled “gluten free.”
“It looked great,” Dr. Katta recalled. “Unfortunately it was not flavoring free. She reacted to multiple essential oils, including tea tree oil, contained in the toothpaste. This is being added to a number of toothpastes, and I think we’re going to see more of these types of reactions.”
Other toothpastes contain balsam of Peru, “which is consistently one of the top allergens in patch test clinics,” she said. “One of the components of balsam of Peru is a cinnamon compound, which can be an issue.”
Dr. Katta advises her patients to use Vaseline petroleum jelly as a lip balm and recommends Tom’s of Maine Silly Strawberry Flavor (this flavor only) toothpaste for children.
A few years ago, a teenager presented to Dr. Katta with intense bullae on the dorsum of the foot after wearing shoes without socks. “She was wearing white canvas Keds, which looked very innocuous,” she said. Patch testing revealed that the teen reacted to four different rubber accelerators. “When we contacted the company, they [acknowledged] using rubber cement to make the canvas Keds,” Dr. Katta said. “Rubber cement is an adhesive and it does contain rubber accelerators. Later, I saw two cases of children who had walked around all day at the amusement park wearing their Sperry Topsiders without any socks. We couldn’t get any information from that manufacturer, but I suspect that they also use a rubber-based glue to make those shoes.” She characterized shoes as “a real setup for a foot allergy because you have friction, sweat that’s pulling allergen out of an object, and sweat is carrying it over, especially to the dorsum of the foot.”
Dr. Katta has also noticed an uptick in the number of young patients who develop allergic reactions to dyes used to make workout clothing. “If you ever see rashes that do not involve the axillary vault but do have peraxillary accentuation, think textile allergy,” she said. “We’re seeing a lot of reactions to disperse blue clothing dyes. When you think about textile allergy from the dyes, it tends to be the blue and black clothing. It’s more likely in the setting of synthetic fabrics because they leach out dyes more easily, and it’s more likely in the setting of sweat because sweat helps pull allergen out. I’m seeing it a lot from sports uniforms and tight black leggings and tight sports bras that people are wearing. I’m also seeing some from bathing suits and swim shirts.”
Exposure to products containing the preservative methylisothiazolinone (MI) is also on the rise. It ranks as the second most frequent allergen for which the North American Contact Dermatitis Group is seeing positive results on patch testing, with rates of 13.4%. MI can be found in many skin care products and “probably about half of school glues, fabric glues, and craft glues,” Dr. Katta said. “Stick versus liquid doesn’t make a difference.” Children and teens often use craft glues, laundry detergents, and other products to create “slime” as a way to learn about viscosity, polymers, and chemical reactions. “Sometimes these children have sensitive skin, or they’re using it with prolonged contact, so they may be sensitizing themselves to the MI,” she said.
She concluded her remarks by noting that an increasing number of young patients are developing reactions to wearable medical devices such as insulin pumps and glucose monitors. “With this, the first thing to think about is frictional irritant dermatitis,” she said. “You can put Scanpor medical paper tape on people’s back for 48 hours straight to patch test them. Some people are incredibly reactive to the friction of just that tape. You also have to think about trapped allergen. One of my patients reacted to colophony, fragrance mix, and propylene glycol, all of which were contained in his skin care products. Some people are getting advice from other patients to use Mastisol liquid adhesive to help their glucose monitors stick better. Mastisol has a high rate of cross-reactivity with balsam of Peru, so it’s a fragrance allergen. That’s the first thing you want to ask patients about: what products they’re using.”
One of her patients thought she was reacting to adhesive tape on her skin, but in fact she was reacting to two different acrylates: ethylene glycol dimethacrylate (EGDMA) and hydroxyethyl methacrylate (HEMA). “I know about these allergens because I see reactions from butterfly needles in dialysis patients,” Dr. Katta explained. “What happens is, these acrylates are glues or plastics used somewhere else on the device, and they can migrate through barriers.”
In one published case, a 9-year-old boy developed a reaction to ethyl cyanoacrylate contained in a glucose sensor adhesive (Dermatitis. 2017; 28[4]:289-91). It never touched the boy’s skin directly but was presumed to migrate through that tape. “The bottom line is that acrylates may induce contact dermatitis even through perceived barriers,” she said. “Their use anywhere in medical devices may prove problematic.”
Dr. Katta reported that she is a member of the advisory board for Vichy Laboratories.
AUSTIN – Not long ago, Rajani Katta, MD, received a text message from a friend who expressed concern about a rash that developed in the underarm of her teenage daughter.
The culprit turned out to be the lavender essential oil contained in an “all natural” deodorant that her daughter had recently switched to – a storyline that Dr. Katta encounters with increasing frequency in her role as clinical professor of dermatology at the University of Texas Health Science Center at Houston.
Dr. Katta said at the annual meeting of the Society for Pediatric Dermatology. “When you talk about a natural allergy, it is more likely to occur if your skin barrier is compromised, so I think that’s why we’re seeing it, especially in young girls in the underarm area. If you shave the underarm, you impair that skin barrier and you’re more likely to develop a reaction to something you’re using over it.”
Her list of recommended deodorants includes Almay Roll-On Antiperspirant & Deodorant, Crystal Body Deodorant Stick, Crystal Roll-On Body Deodorant, Vanicream Deodorant for Sensitive Skin (aluminum-free), Vanicream Antiperspirant/Deodorant, and CertainDri Clinical Strength Roll-On. They are fragrance-free and lack propylene glycol, which is a common allergen.
Increasingly, essential oils are being added to lip balms and toothpastes, said Dr. Katta, who is also author of the 2018 book “Glow: The Dermatologist’s Guide to a Whole Foods Younger Skin Diet.” She recalled one patient who presented with chronic chapped lips. “It doesn’t matter how many lip glosses I try; it just keeps getting worse,” the patient told her. The likely culprit turned out to be ingredients contained in flavored lip balm from EOS. Reports of blistering and cracking of the lips from use of the products prompted a class action lawsuit and a notice to consumers from the Food and Drug Administration.
Another patient presented with cracked lips after switching to an “all natural” toothpaste that was labeled “gluten free.”
“It looked great,” Dr. Katta recalled. “Unfortunately it was not flavoring free. She reacted to multiple essential oils, including tea tree oil, contained in the toothpaste. This is being added to a number of toothpastes, and I think we’re going to see more of these types of reactions.”
Other toothpastes contain balsam of Peru, “which is consistently one of the top allergens in patch test clinics,” she said. “One of the components of balsam of Peru is a cinnamon compound, which can be an issue.”
Dr. Katta advises her patients to use Vaseline petroleum jelly as a lip balm and recommends Tom’s of Maine Silly Strawberry Flavor (this flavor only) toothpaste for children.
A few years ago, a teenager presented to Dr. Katta with intense bullae on the dorsum of the foot after wearing shoes without socks. “She was wearing white canvas Keds, which looked very innocuous,” she said. Patch testing revealed that the teen reacted to four different rubber accelerators. “When we contacted the company, they [acknowledged] using rubber cement to make the canvas Keds,” Dr. Katta said. “Rubber cement is an adhesive and it does contain rubber accelerators. Later, I saw two cases of children who had walked around all day at the amusement park wearing their Sperry Topsiders without any socks. We couldn’t get any information from that manufacturer, but I suspect that they also use a rubber-based glue to make those shoes.” She characterized shoes as “a real setup for a foot allergy because you have friction, sweat that’s pulling allergen out of an object, and sweat is carrying it over, especially to the dorsum of the foot.”
Dr. Katta has also noticed an uptick in the number of young patients who develop allergic reactions to dyes used to make workout clothing. “If you ever see rashes that do not involve the axillary vault but do have peraxillary accentuation, think textile allergy,” she said. “We’re seeing a lot of reactions to disperse blue clothing dyes. When you think about textile allergy from the dyes, it tends to be the blue and black clothing. It’s more likely in the setting of synthetic fabrics because they leach out dyes more easily, and it’s more likely in the setting of sweat because sweat helps pull allergen out. I’m seeing it a lot from sports uniforms and tight black leggings and tight sports bras that people are wearing. I’m also seeing some from bathing suits and swim shirts.”
Exposure to products containing the preservative methylisothiazolinone (MI) is also on the rise. It ranks as the second most frequent allergen for which the North American Contact Dermatitis Group is seeing positive results on patch testing, with rates of 13.4%. MI can be found in many skin care products and “probably about half of school glues, fabric glues, and craft glues,” Dr. Katta said. “Stick versus liquid doesn’t make a difference.” Children and teens often use craft glues, laundry detergents, and other products to create “slime” as a way to learn about viscosity, polymers, and chemical reactions. “Sometimes these children have sensitive skin, or they’re using it with prolonged contact, so they may be sensitizing themselves to the MI,” she said.
She concluded her remarks by noting that an increasing number of young patients are developing reactions to wearable medical devices such as insulin pumps and glucose monitors. “With this, the first thing to think about is frictional irritant dermatitis,” she said. “You can put Scanpor medical paper tape on people’s back for 48 hours straight to patch test them. Some people are incredibly reactive to the friction of just that tape. You also have to think about trapped allergen. One of my patients reacted to colophony, fragrance mix, and propylene glycol, all of which were contained in his skin care products. Some people are getting advice from other patients to use Mastisol liquid adhesive to help their glucose monitors stick better. Mastisol has a high rate of cross-reactivity with balsam of Peru, so it’s a fragrance allergen. That’s the first thing you want to ask patients about: what products they’re using.”
One of her patients thought she was reacting to adhesive tape on her skin, but in fact she was reacting to two different acrylates: ethylene glycol dimethacrylate (EGDMA) and hydroxyethyl methacrylate (HEMA). “I know about these allergens because I see reactions from butterfly needles in dialysis patients,” Dr. Katta explained. “What happens is, these acrylates are glues or plastics used somewhere else on the device, and they can migrate through barriers.”
In one published case, a 9-year-old boy developed a reaction to ethyl cyanoacrylate contained in a glucose sensor adhesive (Dermatitis. 2017; 28[4]:289-91). It never touched the boy’s skin directly but was presumed to migrate through that tape. “The bottom line is that acrylates may induce contact dermatitis even through perceived barriers,” she said. “Their use anywhere in medical devices may prove problematic.”
Dr. Katta reported that she is a member of the advisory board for Vichy Laboratories.
EXPERT ANALYSIS FROM SPD 2019
Ibrutinib-venetoclax found highly active in hard-to-treat CLL
The strategy of simultaneously inhibiting proliferation and reactivating apoptosis can eradicate chronic lymphocytic leukemia (CLL) in a large share of patients, suggest results from the phase 2 CLARITY trial.
“Both ibrutinib and venetoclax are active in CLL with improved survival; however, as monotherapies, both currently are given until disease progression,” wrote Peter Hillmen, MBChB, PhD, St. James’s University Hospital, Leeds, England, and his colleagues.
In the single-arm, open-label trial, the investigators treated 53 patients with relapsed or refractory CLL with combination ibrutinib (Imbruvica), a small-molecule inhibitor of Bruton’s tyrosine kinase, and venetoclax (Venclexta), a small molecule inhibitor of the anti-apoptotic protein Bcl-2. The primary endpoint was MRD negativity, defined as presence of fewer than one CLL cell in 10,000 leukocytes, after 12 months of combination therapy.
Results reported in the Journal of Clinical Oncology showed that the combination was highly active, with 53% of patients achieving MRD negativity in the blood and 36% achieving MRD negativity in the marrow.
Most patients, 89%, had a treatment response, and slightly more than half, 51%, achieved a complete remission. With a median 21.1-month follow-up, only a single patient experienced progression and all were still alive.
Adverse effects were generally manageable. Grade 3-4 adverse events of special interest included 34 cases of neutropenia and 1 case of biochemical tumor lysis syndrome that was managed by delaying venetoclax.
“We have demonstrated promising efficacy that indicates potent synergy between ibrutinib and venetoclax for inducing MRD-negative responses with manageable adverse effects,” the investigators wrote. “The observation that a significant proportion of patients experience MRD-negative remission indicates that this combination can be given for a limited period and then stopped after patients achieve a deep remission.”
Whether the combination leads to permanent disease eradication in certain patients is still unclear, the investigators added.
The trial was supported by Bloodwise under the Trials Acceleration Programme, by the National Institute for Health Research Leeds Clinical Research Facility, and by an unrestricted educational grant from Janssen-Cilag and AbbVie. Ibrutinib was provided free of charge by Janssen-Cilag, and venetoclax was provided free of charge by AbbVie. Dr. Hillman reported financial relationships with Janssen, AbbVie, Roche, Pharmacyclics, and Gilead Sciences.
SOURCE: Hillmen P et al. J Clin Oncol. 2019 Jul 11. doi: 10.1200/JCO.19.00894.
The strategy of simultaneously inhibiting proliferation and reactivating apoptosis can eradicate chronic lymphocytic leukemia (CLL) in a large share of patients, suggest results from the phase 2 CLARITY trial.
“Both ibrutinib and venetoclax are active in CLL with improved survival; however, as monotherapies, both currently are given until disease progression,” wrote Peter Hillmen, MBChB, PhD, St. James’s University Hospital, Leeds, England, and his colleagues.
In the single-arm, open-label trial, the investigators treated 53 patients with relapsed or refractory CLL with combination ibrutinib (Imbruvica), a small-molecule inhibitor of Bruton’s tyrosine kinase, and venetoclax (Venclexta), a small molecule inhibitor of the anti-apoptotic protein Bcl-2. The primary endpoint was MRD negativity, defined as presence of fewer than one CLL cell in 10,000 leukocytes, after 12 months of combination therapy.
Results reported in the Journal of Clinical Oncology showed that the combination was highly active, with 53% of patients achieving MRD negativity in the blood and 36% achieving MRD negativity in the marrow.
Most patients, 89%, had a treatment response, and slightly more than half, 51%, achieved a complete remission. With a median 21.1-month follow-up, only a single patient experienced progression and all were still alive.
Adverse effects were generally manageable. Grade 3-4 adverse events of special interest included 34 cases of neutropenia and 1 case of biochemical tumor lysis syndrome that was managed by delaying venetoclax.
“We have demonstrated promising efficacy that indicates potent synergy between ibrutinib and venetoclax for inducing MRD-negative responses with manageable adverse effects,” the investigators wrote. “The observation that a significant proportion of patients experience MRD-negative remission indicates that this combination can be given for a limited period and then stopped after patients achieve a deep remission.”
Whether the combination leads to permanent disease eradication in certain patients is still unclear, the investigators added.
The trial was supported by Bloodwise under the Trials Acceleration Programme, by the National Institute for Health Research Leeds Clinical Research Facility, and by an unrestricted educational grant from Janssen-Cilag and AbbVie. Ibrutinib was provided free of charge by Janssen-Cilag, and venetoclax was provided free of charge by AbbVie. Dr. Hillman reported financial relationships with Janssen, AbbVie, Roche, Pharmacyclics, and Gilead Sciences.
SOURCE: Hillmen P et al. J Clin Oncol. 2019 Jul 11. doi: 10.1200/JCO.19.00894.
The strategy of simultaneously inhibiting proliferation and reactivating apoptosis can eradicate chronic lymphocytic leukemia (CLL) in a large share of patients, suggest results from the phase 2 CLARITY trial.
“Both ibrutinib and venetoclax are active in CLL with improved survival; however, as monotherapies, both currently are given until disease progression,” wrote Peter Hillmen, MBChB, PhD, St. James’s University Hospital, Leeds, England, and his colleagues.
In the single-arm, open-label trial, the investigators treated 53 patients with relapsed or refractory CLL with combination ibrutinib (Imbruvica), a small-molecule inhibitor of Bruton’s tyrosine kinase, and venetoclax (Venclexta), a small molecule inhibitor of the anti-apoptotic protein Bcl-2. The primary endpoint was MRD negativity, defined as presence of fewer than one CLL cell in 10,000 leukocytes, after 12 months of combination therapy.
Results reported in the Journal of Clinical Oncology showed that the combination was highly active, with 53% of patients achieving MRD negativity in the blood and 36% achieving MRD negativity in the marrow.
Most patients, 89%, had a treatment response, and slightly more than half, 51%, achieved a complete remission. With a median 21.1-month follow-up, only a single patient experienced progression and all were still alive.
Adverse effects were generally manageable. Grade 3-4 adverse events of special interest included 34 cases of neutropenia and 1 case of biochemical tumor lysis syndrome that was managed by delaying venetoclax.
“We have demonstrated promising efficacy that indicates potent synergy between ibrutinib and venetoclax for inducing MRD-negative responses with manageable adverse effects,” the investigators wrote. “The observation that a significant proportion of patients experience MRD-negative remission indicates that this combination can be given for a limited period and then stopped after patients achieve a deep remission.”
Whether the combination leads to permanent disease eradication in certain patients is still unclear, the investigators added.
The trial was supported by Bloodwise under the Trials Acceleration Programme, by the National Institute for Health Research Leeds Clinical Research Facility, and by an unrestricted educational grant from Janssen-Cilag and AbbVie. Ibrutinib was provided free of charge by Janssen-Cilag, and venetoclax was provided free of charge by AbbVie. Dr. Hillman reported financial relationships with Janssen, AbbVie, Roche, Pharmacyclics, and Gilead Sciences.
SOURCE: Hillmen P et al. J Clin Oncol. 2019 Jul 11. doi: 10.1200/JCO.19.00894.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
CAR T-cell therapy less effective in transformed follicular lymphoma
All complete responders with FL were still in remission at a median follow-up of 24 months, but the median duration of response was 10.2 months for patients with tFL.
Alexandre V. Hirayama, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues reported these results in Blood.
The trial enrolled 21 adults with relapsed/refractory CD19+ B-cell malignancies, including 8 patients with FL and 13 with tFL. At baseline, the FL/tFL patients had a median age of 56 years (range, 51-62), and 67% were male. Most patients (n = 19) had stage III/IV disease, 17 had extranodal disease, 8 had bulky disease, and 6 had bone marrow involvement. The patients had received a median of 5 prior therapies (range, 2-8), and 13 had received a transplant.
In this study, patients received a lymphodepleting regimen of cyclophosphamide and fludarabine, followed by 2 x 106 CD19 CAR T cells/kg. Five patients (one with FL and four with tFL) also received bridging chemotherapy between leukapheresis and lymphodepletion.
Grade 1-2 cytokine release syndrome occurred in 50% of FL patients and 39% of tFL patients (P = .35). Grade 1-2 neurotoxicity occurred in 50% and 23%, respectively (P = .67). There were no cases of grade 3 or higher cytokine release syndrome or neurotoxicity.
Most FL patients (7 of 8; 88%) achieved a complete response (CR) to treatment, and all of these patients were still in CR at a median follow-up of 24 months (range, 5-37 months). One FL patient received a transplant while in CR.
Six of 13 tFL patients (46%) achieved a CR. At a median follow-up of 38 months (range, 3-39 months), the median duration of response was 10.2 months. The median progression-free survival was 11.2 months in patients who achieved a CR and 1.4 months in all tFL patients.
The researchers noted that peak CAR T-cell counts and the duration of CAR T-cell detection were similar between FL and tFL patients. However, tFL patients had higher serum interleukin-8 concentrations and higher lactate dehydrogenase levels before treatment.
Past research suggested that IL-8 mediates the recruitment of tumor-associated neutrophils, promotes diffuse large B-cell lymphoma progression, and can contribute to local immune suppression. Other studies have linked elevated lactate dehydrogenase to aggressive disease and a more immunosuppressive tumor microenvironment.
“Although these data raise the possibility that differences in the tumor microenvironment may, in part, contribute to differences in outcomes after CAR T-cell immunotherapy in FL and tFL patients, additional studies are required,” the researchers wrote.
This research was supported by the National Institutes of Health, the Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinician Investigator Program, the Fred Hutchinson Cancer Research Center’s Immunotherapy Integrated Research Center, and Juno Therapeutics/Celgene.
The researchers disclosed relationships with Celgene, Juno Therapeutics, Lyell Immunopharma, Adaptive Biotechnologies, Nohla, Kite Pharma, Gilead, Genentech, Novartis, Eureka Therapeutics, Nektar Therapeutics, Caribou Biosciences, Precision Biosciences, Aptevo, Humanigen, and Allogene.
SOURCE: Hirayama AV et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000905
All complete responders with FL were still in remission at a median follow-up of 24 months, but the median duration of response was 10.2 months for patients with tFL.
Alexandre V. Hirayama, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues reported these results in Blood.
The trial enrolled 21 adults with relapsed/refractory CD19+ B-cell malignancies, including 8 patients with FL and 13 with tFL. At baseline, the FL/tFL patients had a median age of 56 years (range, 51-62), and 67% were male. Most patients (n = 19) had stage III/IV disease, 17 had extranodal disease, 8 had bulky disease, and 6 had bone marrow involvement. The patients had received a median of 5 prior therapies (range, 2-8), and 13 had received a transplant.
In this study, patients received a lymphodepleting regimen of cyclophosphamide and fludarabine, followed by 2 x 106 CD19 CAR T cells/kg. Five patients (one with FL and four with tFL) also received bridging chemotherapy between leukapheresis and lymphodepletion.
Grade 1-2 cytokine release syndrome occurred in 50% of FL patients and 39% of tFL patients (P = .35). Grade 1-2 neurotoxicity occurred in 50% and 23%, respectively (P = .67). There were no cases of grade 3 or higher cytokine release syndrome or neurotoxicity.
Most FL patients (7 of 8; 88%) achieved a complete response (CR) to treatment, and all of these patients were still in CR at a median follow-up of 24 months (range, 5-37 months). One FL patient received a transplant while in CR.
Six of 13 tFL patients (46%) achieved a CR. At a median follow-up of 38 months (range, 3-39 months), the median duration of response was 10.2 months. The median progression-free survival was 11.2 months in patients who achieved a CR and 1.4 months in all tFL patients.
The researchers noted that peak CAR T-cell counts and the duration of CAR T-cell detection were similar between FL and tFL patients. However, tFL patients had higher serum interleukin-8 concentrations and higher lactate dehydrogenase levels before treatment.
Past research suggested that IL-8 mediates the recruitment of tumor-associated neutrophils, promotes diffuse large B-cell lymphoma progression, and can contribute to local immune suppression. Other studies have linked elevated lactate dehydrogenase to aggressive disease and a more immunosuppressive tumor microenvironment.
“Although these data raise the possibility that differences in the tumor microenvironment may, in part, contribute to differences in outcomes after CAR T-cell immunotherapy in FL and tFL patients, additional studies are required,” the researchers wrote.
This research was supported by the National Institutes of Health, the Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinician Investigator Program, the Fred Hutchinson Cancer Research Center’s Immunotherapy Integrated Research Center, and Juno Therapeutics/Celgene.
The researchers disclosed relationships with Celgene, Juno Therapeutics, Lyell Immunopharma, Adaptive Biotechnologies, Nohla, Kite Pharma, Gilead, Genentech, Novartis, Eureka Therapeutics, Nektar Therapeutics, Caribou Biosciences, Precision Biosciences, Aptevo, Humanigen, and Allogene.
SOURCE: Hirayama AV et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000905
All complete responders with FL were still in remission at a median follow-up of 24 months, but the median duration of response was 10.2 months for patients with tFL.
Alexandre V. Hirayama, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues reported these results in Blood.
The trial enrolled 21 adults with relapsed/refractory CD19+ B-cell malignancies, including 8 patients with FL and 13 with tFL. At baseline, the FL/tFL patients had a median age of 56 years (range, 51-62), and 67% were male. Most patients (n = 19) had stage III/IV disease, 17 had extranodal disease, 8 had bulky disease, and 6 had bone marrow involvement. The patients had received a median of 5 prior therapies (range, 2-8), and 13 had received a transplant.
In this study, patients received a lymphodepleting regimen of cyclophosphamide and fludarabine, followed by 2 x 106 CD19 CAR T cells/kg. Five patients (one with FL and four with tFL) also received bridging chemotherapy between leukapheresis and lymphodepletion.
Grade 1-2 cytokine release syndrome occurred in 50% of FL patients and 39% of tFL patients (P = .35). Grade 1-2 neurotoxicity occurred in 50% and 23%, respectively (P = .67). There were no cases of grade 3 or higher cytokine release syndrome or neurotoxicity.
Most FL patients (7 of 8; 88%) achieved a complete response (CR) to treatment, and all of these patients were still in CR at a median follow-up of 24 months (range, 5-37 months). One FL patient received a transplant while in CR.
Six of 13 tFL patients (46%) achieved a CR. At a median follow-up of 38 months (range, 3-39 months), the median duration of response was 10.2 months. The median progression-free survival was 11.2 months in patients who achieved a CR and 1.4 months in all tFL patients.
The researchers noted that peak CAR T-cell counts and the duration of CAR T-cell detection were similar between FL and tFL patients. However, tFL patients had higher serum interleukin-8 concentrations and higher lactate dehydrogenase levels before treatment.
Past research suggested that IL-8 mediates the recruitment of tumor-associated neutrophils, promotes diffuse large B-cell lymphoma progression, and can contribute to local immune suppression. Other studies have linked elevated lactate dehydrogenase to aggressive disease and a more immunosuppressive tumor microenvironment.
“Although these data raise the possibility that differences in the tumor microenvironment may, in part, contribute to differences in outcomes after CAR T-cell immunotherapy in FL and tFL patients, additional studies are required,” the researchers wrote.
This research was supported by the National Institutes of Health, the Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinician Investigator Program, the Fred Hutchinson Cancer Research Center’s Immunotherapy Integrated Research Center, and Juno Therapeutics/Celgene.
The researchers disclosed relationships with Celgene, Juno Therapeutics, Lyell Immunopharma, Adaptive Biotechnologies, Nohla, Kite Pharma, Gilead, Genentech, Novartis, Eureka Therapeutics, Nektar Therapeutics, Caribou Biosciences, Precision Biosciences, Aptevo, Humanigen, and Allogene.
SOURCE: Hirayama AV et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000905
FROM BLOOD