User login
Inadequate glycemic control in type 1 diabetes leads to increased fracture risk
A single percentage increase in the level of hemoglobin A1c (HbA1c) in patients with newly diagnosed type 1 diabetes is significantly associated with an increase in fracture risk, according to findings in a study published in Diabetic Medicine.
To determine the effect of glycemic control on fracture risk, Rasiah Thayakaran, PhD, of the University of Birmingham (England) and colleagues analyzed data from 5,368 patients with newly diagnosed type 1 diabetes in the United Kingdom. HbA1c measurements were collected until either fracture or the end of the study, and were then converted from percentages to mmol/mol. Patient age ranged between 1 and 60 years, and the mean age was 22 years.
During 37,830 person‐years of follow‐up, 525 fractures were observed, with an incidence rate of 14 per 1,000 person‐years. The rate among men was 15 per 1,000 person‐years, compared with 12 per 1,000 person‐years among women. There was a significant association between hemoglobin level and risk of fractures (adjusted hazard ratio, 1.007 mmol/mol; 95% confidence interval, 1.002-1.011 mmol/mol), representing an increase of 7% in risk for fracture for each percentage increase in hemoglobin level.
“When assessing an individual with newly diagnosed type 1 diabetes and high HbA1c, increased clinical awareness about the fracture risk may be incorporated in decision‐making regarding the clinical management and even in prompting early antiosteoporotic intervention,” Dr. Thayakaran and coauthors wrote.
The researchers acknowledged the study’s limitations, including a possibility of residual confounding because of their use of observational data. In addition, they could not confirm whether the increase in fracture risk should be attributed to bone fragility or to increased risk of falls. Finally, though they noted using a comprehensive list of codes to identify fractures, they could not verify “completeness of recording ... and therefore reported overall fracture incidence should be interpreted with caution.”
The study was not funded. The authors reported no conflicts of interest.
SOURCE: Thayakaran R et al. Diab Med. 2019 Mar 8. doi: 10.1111/dme.13945.
A single percentage increase in the level of hemoglobin A1c (HbA1c) in patients with newly diagnosed type 1 diabetes is significantly associated with an increase in fracture risk, according to findings in a study published in Diabetic Medicine.
To determine the effect of glycemic control on fracture risk, Rasiah Thayakaran, PhD, of the University of Birmingham (England) and colleagues analyzed data from 5,368 patients with newly diagnosed type 1 diabetes in the United Kingdom. HbA1c measurements were collected until either fracture or the end of the study, and were then converted from percentages to mmol/mol. Patient age ranged between 1 and 60 years, and the mean age was 22 years.
During 37,830 person‐years of follow‐up, 525 fractures were observed, with an incidence rate of 14 per 1,000 person‐years. The rate among men was 15 per 1,000 person‐years, compared with 12 per 1,000 person‐years among women. There was a significant association between hemoglobin level and risk of fractures (adjusted hazard ratio, 1.007 mmol/mol; 95% confidence interval, 1.002-1.011 mmol/mol), representing an increase of 7% in risk for fracture for each percentage increase in hemoglobin level.
“When assessing an individual with newly diagnosed type 1 diabetes and high HbA1c, increased clinical awareness about the fracture risk may be incorporated in decision‐making regarding the clinical management and even in prompting early antiosteoporotic intervention,” Dr. Thayakaran and coauthors wrote.
The researchers acknowledged the study’s limitations, including a possibility of residual confounding because of their use of observational data. In addition, they could not confirm whether the increase in fracture risk should be attributed to bone fragility or to increased risk of falls. Finally, though they noted using a comprehensive list of codes to identify fractures, they could not verify “completeness of recording ... and therefore reported overall fracture incidence should be interpreted with caution.”
The study was not funded. The authors reported no conflicts of interest.
SOURCE: Thayakaran R et al. Diab Med. 2019 Mar 8. doi: 10.1111/dme.13945.
A single percentage increase in the level of hemoglobin A1c (HbA1c) in patients with newly diagnosed type 1 diabetes is significantly associated with an increase in fracture risk, according to findings in a study published in Diabetic Medicine.
To determine the effect of glycemic control on fracture risk, Rasiah Thayakaran, PhD, of the University of Birmingham (England) and colleagues analyzed data from 5,368 patients with newly diagnosed type 1 diabetes in the United Kingdom. HbA1c measurements were collected until either fracture or the end of the study, and were then converted from percentages to mmol/mol. Patient age ranged between 1 and 60 years, and the mean age was 22 years.
During 37,830 person‐years of follow‐up, 525 fractures were observed, with an incidence rate of 14 per 1,000 person‐years. The rate among men was 15 per 1,000 person‐years, compared with 12 per 1,000 person‐years among women. There was a significant association between hemoglobin level and risk of fractures (adjusted hazard ratio, 1.007 mmol/mol; 95% confidence interval, 1.002-1.011 mmol/mol), representing an increase of 7% in risk for fracture for each percentage increase in hemoglobin level.
“When assessing an individual with newly diagnosed type 1 diabetes and high HbA1c, increased clinical awareness about the fracture risk may be incorporated in decision‐making regarding the clinical management and even in prompting early antiosteoporotic intervention,” Dr. Thayakaran and coauthors wrote.
The researchers acknowledged the study’s limitations, including a possibility of residual confounding because of their use of observational data. In addition, they could not confirm whether the increase in fracture risk should be attributed to bone fragility or to increased risk of falls. Finally, though they noted using a comprehensive list of codes to identify fractures, they could not verify “completeness of recording ... and therefore reported overall fracture incidence should be interpreted with caution.”
The study was not funded. The authors reported no conflicts of interest.
SOURCE: Thayakaran R et al. Diab Med. 2019 Mar 8. doi: 10.1111/dme.13945.
FROM DIABETIC MEDICINE
Migrant children need safety net
ACEs tied to traumas threaten the emotional, physical health of a generation
An 11-year-old was caring for his toddler brother. Both were fending for themselves in a cell with dozens of other children. The little one was quiet with matted hair, a hacking cough, muddy pants, and eyes that fluttered with fatigue.
As the two brothers were reportedly interviewed, one fell asleep on two office chairs drawn together, probably the most comfortable bed he had used in weeks. They had been separated from an 18-year-old uncle and sent to the Clint Border Patrol Station in Texas. When they were interviewed in the news report, they had been there 3 weeks and counting.
Per news reports this summer, preteen migrant children have been asked to care for toddlers not related to them with no assistance from adults, and no beds, no food, and no change of clothing. Children were sleeping on concrete floors and eating the same unpalatable and unhealthy foods for close to a month: instant oatmeal, instant soup, and previously frozen burritos. Babies were roaming around in dirty diapers, fending for themselves, foraging for food. Two- and 3-year-old toddlers were sick with no adult comforting them.
When some people visited the border patrol station, they said they saw children trapped in cages like animals. Some were keening in pain while pining for their parents from whom they had been separated.
These children were forcibly separated from parents. In addition, they face living conditions that include hunger, dehydration, and lack of hygiene, to name a few. This sounds like some fantastical nightmare from a war-torn third-world country – but no these circumstances are real, and they are here in the USA.
We witness helplessly the helplessness created by a man-made disaster striking the world’s most vulnerable creature: the human child. This specter afflicting thousands of migrant children either seeking asylum or an immigrant status has far-reaching implications. This is even more ironic, given that, as a nation, we have embraced the concept of adverse childhood experiences (ACEs) and their impact on lifelong health challenges. Most of us reel with horror as these tales make their way to national headlines. But are we as a nation complicit in watching like bystanders while a generation of children is placed at risk from experiencing the long-term effects of ACEs on their physical and emotional health?
Surely if the psychological implications of ACEs do not warrant a change in course, the mere economics of the costs arising from the suffering caused by totally preventable medical problems in adulthood should be considered in policy decisions. However, that is beyond the scope of this commentary.
The human child is so utterly dependent on parents. He does not have the fairly quick physical independence from parents that we see in the animal kingdom. As soon as a child is born, a curious process of attachment begins within the mom and baby dyad, and eventually, this bond engulfs the father as well. The baby depends on the parent to understand his needs: be it when to eat, when he wants to be touched, when he needs to be left alone, when he needs to be cleaned or fed. Optimum crying serves so many purposes, and most parents are exquisitely attuned to the baby’s cry. From this relationship emerges a stable worldview, and, among many things, a stable neuroendocrine system.
Unique cultural backgrounds of individuals create the scaffolding for human variability, which in turn, confers a richness to the human race. However, development proceeds in a fairly uniform and universal fashion for children, regardless of where they come from. The progression of brain and body development moves lockstep with each other responding to a complex interplay between genetics, environment, and neurohormonal factors. It is remarkable just how resilient the human baby is in the face of the challenges that it often faces: accidental injury, illness, and even benign neglect.
However, there comes a breaking point similar to that described in the stories above, where the stress is toxic and intolerable. It is continuous, and it is relentless in its capacity to bathe the developing brain and body of the child with noxious endogenous substances that cause cell death and subsequent atrophy that is potentially irreversible.
We see such children in our clinics downstream: at ages 8, 13, or 16, after they have lost their ability to modulate emotions and are highly aggressive, or are withdrawn and depressed – or in the juvenile justice system after having repeatedly but impulsively violated the law. In other words, repeated trauma changes the wiring of the brain and neuromodulatory capacity. There is literature suggesting that traumatized children carry within them modified genes that affect their capacity to be nurturing parents. In other words, trauma has the potential to lead to multigenerational transmission of the experiences of suffering and often a psychological incapacity to parent – putting subsequent generations at risk.
So what should we do? Be bystanders, or become involved professionals?
The need to create a supportive safety net for these children is essential. Ideally, they should be reunited with their parents. The reunification of children with their parents is an absolute must if it can be done. Their parents are alive somewhere – and the best mitigators of the emotional damage already done. A strong case needs to be made for reunification, otherwise parental separation, deprivation on multiple levels, such as what these children are experiencing, will create a generation of compromised children.
A second-best option is that an emotional and physical safety net should be created that mimics a family for each child. Children need predictability and stability of caregivers with whom they can form an affective bond. This is essential for them to negotiate the cycle of inconsolable weeping, searching for their parent/s, reconciling the loss, and either reaching a level of adaptation or being engulfed in the despair that these toddlers, children, and teens continually face. In addition, these individuals/teams first and foremost should plan on giving equal consideration to the physical and emotional needs of the children.
The damage is done in the form of subjecting children to all that is detrimental to development. Now, steady, regular presence of shift workers who understand the importance of the continuity of relationships and who cannot only advocate for but also provide for the nutritional, sleep, and hygiene needs of the child concurrently is necessary. The children need soft and nurturing touch, predictability of routines, adequate sleep, adequate wholesome nutrition, and familiarity of faces who should make a commitment of spending no less than 6 to 9 months at a stretch in these camps.
Although the task appears herculean, drastic problems need drastic remedies, as the entire life of every child is at stake. These workers should be trained in mental health and physical health first aid, so they can recognize the gradations of despair, detachment, and acting out in children and know how to triage the children to appropriate trained mental health and medical clinicians. It is to be expected that both medical and mental health problems will be concentrated in this population, and planning for staffing such camps should anticipate that. This safety net should be created in all facilities accepting these children.
Dr. Sood is professor of psychiatry and pediatrics, and senior professor of child mental health policy at Virginia Commonwealth University in Richmond.
ACEs tied to traumas threaten the emotional, physical health of a generation
ACEs tied to traumas threaten the emotional, physical health of a generation
An 11-year-old was caring for his toddler brother. Both were fending for themselves in a cell with dozens of other children. The little one was quiet with matted hair, a hacking cough, muddy pants, and eyes that fluttered with fatigue.
As the two brothers were reportedly interviewed, one fell asleep on two office chairs drawn together, probably the most comfortable bed he had used in weeks. They had been separated from an 18-year-old uncle and sent to the Clint Border Patrol Station in Texas. When they were interviewed in the news report, they had been there 3 weeks and counting.
Per news reports this summer, preteen migrant children have been asked to care for toddlers not related to them with no assistance from adults, and no beds, no food, and no change of clothing. Children were sleeping on concrete floors and eating the same unpalatable and unhealthy foods for close to a month: instant oatmeal, instant soup, and previously frozen burritos. Babies were roaming around in dirty diapers, fending for themselves, foraging for food. Two- and 3-year-old toddlers were sick with no adult comforting them.
When some people visited the border patrol station, they said they saw children trapped in cages like animals. Some were keening in pain while pining for their parents from whom they had been separated.
These children were forcibly separated from parents. In addition, they face living conditions that include hunger, dehydration, and lack of hygiene, to name a few. This sounds like some fantastical nightmare from a war-torn third-world country – but no these circumstances are real, and they are here in the USA.
We witness helplessly the helplessness created by a man-made disaster striking the world’s most vulnerable creature: the human child. This specter afflicting thousands of migrant children either seeking asylum or an immigrant status has far-reaching implications. This is even more ironic, given that, as a nation, we have embraced the concept of adverse childhood experiences (ACEs) and their impact on lifelong health challenges. Most of us reel with horror as these tales make their way to national headlines. But are we as a nation complicit in watching like bystanders while a generation of children is placed at risk from experiencing the long-term effects of ACEs on their physical and emotional health?
Surely if the psychological implications of ACEs do not warrant a change in course, the mere economics of the costs arising from the suffering caused by totally preventable medical problems in adulthood should be considered in policy decisions. However, that is beyond the scope of this commentary.
The human child is so utterly dependent on parents. He does not have the fairly quick physical independence from parents that we see in the animal kingdom. As soon as a child is born, a curious process of attachment begins within the mom and baby dyad, and eventually, this bond engulfs the father as well. The baby depends on the parent to understand his needs: be it when to eat, when he wants to be touched, when he needs to be left alone, when he needs to be cleaned or fed. Optimum crying serves so many purposes, and most parents are exquisitely attuned to the baby’s cry. From this relationship emerges a stable worldview, and, among many things, a stable neuroendocrine system.
Unique cultural backgrounds of individuals create the scaffolding for human variability, which in turn, confers a richness to the human race. However, development proceeds in a fairly uniform and universal fashion for children, regardless of where they come from. The progression of brain and body development moves lockstep with each other responding to a complex interplay between genetics, environment, and neurohormonal factors. It is remarkable just how resilient the human baby is in the face of the challenges that it often faces: accidental injury, illness, and even benign neglect.
However, there comes a breaking point similar to that described in the stories above, where the stress is toxic and intolerable. It is continuous, and it is relentless in its capacity to bathe the developing brain and body of the child with noxious endogenous substances that cause cell death and subsequent atrophy that is potentially irreversible.
We see such children in our clinics downstream: at ages 8, 13, or 16, after they have lost their ability to modulate emotions and are highly aggressive, or are withdrawn and depressed – or in the juvenile justice system after having repeatedly but impulsively violated the law. In other words, repeated trauma changes the wiring of the brain and neuromodulatory capacity. There is literature suggesting that traumatized children carry within them modified genes that affect their capacity to be nurturing parents. In other words, trauma has the potential to lead to multigenerational transmission of the experiences of suffering and often a psychological incapacity to parent – putting subsequent generations at risk.
So what should we do? Be bystanders, or become involved professionals?
The need to create a supportive safety net for these children is essential. Ideally, they should be reunited with their parents. The reunification of children with their parents is an absolute must if it can be done. Their parents are alive somewhere – and the best mitigators of the emotional damage already done. A strong case needs to be made for reunification, otherwise parental separation, deprivation on multiple levels, such as what these children are experiencing, will create a generation of compromised children.
A second-best option is that an emotional and physical safety net should be created that mimics a family for each child. Children need predictability and stability of caregivers with whom they can form an affective bond. This is essential for them to negotiate the cycle of inconsolable weeping, searching for their parent/s, reconciling the loss, and either reaching a level of adaptation or being engulfed in the despair that these toddlers, children, and teens continually face. In addition, these individuals/teams first and foremost should plan on giving equal consideration to the physical and emotional needs of the children.
The damage is done in the form of subjecting children to all that is detrimental to development. Now, steady, regular presence of shift workers who understand the importance of the continuity of relationships and who cannot only advocate for but also provide for the nutritional, sleep, and hygiene needs of the child concurrently is necessary. The children need soft and nurturing touch, predictability of routines, adequate sleep, adequate wholesome nutrition, and familiarity of faces who should make a commitment of spending no less than 6 to 9 months at a stretch in these camps.
Although the task appears herculean, drastic problems need drastic remedies, as the entire life of every child is at stake. These workers should be trained in mental health and physical health first aid, so they can recognize the gradations of despair, detachment, and acting out in children and know how to triage the children to appropriate trained mental health and medical clinicians. It is to be expected that both medical and mental health problems will be concentrated in this population, and planning for staffing such camps should anticipate that. This safety net should be created in all facilities accepting these children.
Dr. Sood is professor of psychiatry and pediatrics, and senior professor of child mental health policy at Virginia Commonwealth University in Richmond.
An 11-year-old was caring for his toddler brother. Both were fending for themselves in a cell with dozens of other children. The little one was quiet with matted hair, a hacking cough, muddy pants, and eyes that fluttered with fatigue.
As the two brothers were reportedly interviewed, one fell asleep on two office chairs drawn together, probably the most comfortable bed he had used in weeks. They had been separated from an 18-year-old uncle and sent to the Clint Border Patrol Station in Texas. When they were interviewed in the news report, they had been there 3 weeks and counting.
Per news reports this summer, preteen migrant children have been asked to care for toddlers not related to them with no assistance from adults, and no beds, no food, and no change of clothing. Children were sleeping on concrete floors and eating the same unpalatable and unhealthy foods for close to a month: instant oatmeal, instant soup, and previously frozen burritos. Babies were roaming around in dirty diapers, fending for themselves, foraging for food. Two- and 3-year-old toddlers were sick with no adult comforting them.
When some people visited the border patrol station, they said they saw children trapped in cages like animals. Some were keening in pain while pining for their parents from whom they had been separated.
These children were forcibly separated from parents. In addition, they face living conditions that include hunger, dehydration, and lack of hygiene, to name a few. This sounds like some fantastical nightmare from a war-torn third-world country – but no these circumstances are real, and they are here in the USA.
We witness helplessly the helplessness created by a man-made disaster striking the world’s most vulnerable creature: the human child. This specter afflicting thousands of migrant children either seeking asylum or an immigrant status has far-reaching implications. This is even more ironic, given that, as a nation, we have embraced the concept of adverse childhood experiences (ACEs) and their impact on lifelong health challenges. Most of us reel with horror as these tales make their way to national headlines. But are we as a nation complicit in watching like bystanders while a generation of children is placed at risk from experiencing the long-term effects of ACEs on their physical and emotional health?
Surely if the psychological implications of ACEs do not warrant a change in course, the mere economics of the costs arising from the suffering caused by totally preventable medical problems in adulthood should be considered in policy decisions. However, that is beyond the scope of this commentary.
The human child is so utterly dependent on parents. He does not have the fairly quick physical independence from parents that we see in the animal kingdom. As soon as a child is born, a curious process of attachment begins within the mom and baby dyad, and eventually, this bond engulfs the father as well. The baby depends on the parent to understand his needs: be it when to eat, when he wants to be touched, when he needs to be left alone, when he needs to be cleaned or fed. Optimum crying serves so many purposes, and most parents are exquisitely attuned to the baby’s cry. From this relationship emerges a stable worldview, and, among many things, a stable neuroendocrine system.
Unique cultural backgrounds of individuals create the scaffolding for human variability, which in turn, confers a richness to the human race. However, development proceeds in a fairly uniform and universal fashion for children, regardless of where they come from. The progression of brain and body development moves lockstep with each other responding to a complex interplay between genetics, environment, and neurohormonal factors. It is remarkable just how resilient the human baby is in the face of the challenges that it often faces: accidental injury, illness, and even benign neglect.
However, there comes a breaking point similar to that described in the stories above, where the stress is toxic and intolerable. It is continuous, and it is relentless in its capacity to bathe the developing brain and body of the child with noxious endogenous substances that cause cell death and subsequent atrophy that is potentially irreversible.
We see such children in our clinics downstream: at ages 8, 13, or 16, after they have lost their ability to modulate emotions and are highly aggressive, or are withdrawn and depressed – or in the juvenile justice system after having repeatedly but impulsively violated the law. In other words, repeated trauma changes the wiring of the brain and neuromodulatory capacity. There is literature suggesting that traumatized children carry within them modified genes that affect their capacity to be nurturing parents. In other words, trauma has the potential to lead to multigenerational transmission of the experiences of suffering and often a psychological incapacity to parent – putting subsequent generations at risk.
So what should we do? Be bystanders, or become involved professionals?
The need to create a supportive safety net for these children is essential. Ideally, they should be reunited with their parents. The reunification of children with their parents is an absolute must if it can be done. Their parents are alive somewhere – and the best mitigators of the emotional damage already done. A strong case needs to be made for reunification, otherwise parental separation, deprivation on multiple levels, such as what these children are experiencing, will create a generation of compromised children.
A second-best option is that an emotional and physical safety net should be created that mimics a family for each child. Children need predictability and stability of caregivers with whom they can form an affective bond. This is essential for them to negotiate the cycle of inconsolable weeping, searching for their parent/s, reconciling the loss, and either reaching a level of adaptation or being engulfed in the despair that these toddlers, children, and teens continually face. In addition, these individuals/teams first and foremost should plan on giving equal consideration to the physical and emotional needs of the children.
The damage is done in the form of subjecting children to all that is detrimental to development. Now, steady, regular presence of shift workers who understand the importance of the continuity of relationships and who cannot only advocate for but also provide for the nutritional, sleep, and hygiene needs of the child concurrently is necessary. The children need soft and nurturing touch, predictability of routines, adequate sleep, adequate wholesome nutrition, and familiarity of faces who should make a commitment of spending no less than 6 to 9 months at a stretch in these camps.
Although the task appears herculean, drastic problems need drastic remedies, as the entire life of every child is at stake. These workers should be trained in mental health and physical health first aid, so they can recognize the gradations of despair, detachment, and acting out in children and know how to triage the children to appropriate trained mental health and medical clinicians. It is to be expected that both medical and mental health problems will be concentrated in this population, and planning for staffing such camps should anticipate that. This safety net should be created in all facilities accepting these children.
Dr. Sood is professor of psychiatry and pediatrics, and senior professor of child mental health policy at Virginia Commonwealth University in Richmond.
Sexual Dysfunction in MS
A 37-year-old woman presents to her primary care clinic with a chief complaint of depression. She was diagnosed with relapsing multiple sclerosis (MS) at age 29 and is currently taking an injectable preventive therapy. Over the past 6 months, she has had increased marital strain secondary to losing her job because “I couldn’t mentally keep up with the work anymore.” This has caused financial difficulties for her family. In addition, she tires easily and has been napping in the afternoon. She and her husband are experiencing intimacy difficulties, and she confirms problems with vaginal dryness and a general loss of her sexual drive.
Sexual dysfunction in MS is common, affecting 40% to 80% of women and 50% to 90% of men with MS. It is an “invisible” symptom, similar to fatigue, cognitive dysfunction, and pain.1-3
There are three ways that MS patients can be affected by sexual dysfunction, and they are categorized as primary, secondary, and tertiary. Primary sexual dysfunction results from demyelination/axonal destruction of the central nervous system, which potentially leads to altered genital sensation or paresthesia. Secondary sexual dysfunction stems from nonsexual MS symptoms, such as fatigue, spasticity, tremor, impairments in concentration/attention, and iatrogenic causes (eg, adverse effects of medication). Tertiary sexual dysfunction involves the psychosocial/cultural aspects of the disease that can impact a patient’s sexual drive.
SYMPTOMS
Like many other symptoms associated with MS, the symptoms of sexual dysfunction are highly variable. In women, the most common complaints are fatigue, decrease in genital sensation (27%-47%), decrease in libido (31%-74%) and vaginal lubrication (36%-48%), and difficulty with orgasm.4 In men with MS, in addition to erectile problems, surveys have identified decreased genital sensation, fatigue (75%), difficulty with ejaculation (18%-50%), decreased interest or arousal (39%), and anorgasmia (37%) as fairly common complaints.2
TREATMENT
Managing sexual dysfunction in a patient with MS is dependent on the underlying problem. Some examples include
- For many patients, their disease causes significant anxiety and worry about current and potentially future disability—which can make intimacy more difficult. Sometimes, referral to a mental health professional may be required to help the patient
with individual and/or couples counseling to further elucidate underlying intimacy issues. - For patients experiencing MS-associated fatigue, suggest planning for sexual activity in the morning, since fatigue is known to worsen throughout the day.
- For those who qualify for antidepressant medications, remember that some (eg, selective serotonin reuptake inhibitors) can further decrease libido and therefore should be avoided if possible.
- For women who have difficulty with lubrication, a nonpetroleum-based lubricant may reduce vaginal dryness, while use of a vibrator may assist with genital stimulation.
- For men who cannot maintain erection, phosphodiesterase inhibitor drugs (eg, sildenafil) can be helpful; other options include alprostadil urethral suppositories and intracavernous injections.
The patient is screened for depression using the Patient Health Questionnaire, which yields a score of 17 (moderately severe). You discuss the need for active treatment with her, and she agrees to start an antidepressant medication. Bupropion is chosen, given its effectiveness and lack of adverse effects (including sexual dysfunction). The patient also is encouraged to use nonpetroleum-based lubricants. Finally, a referral is made for couples counseling, and a 6-week follow-up appointment is scheduled.
CONCLUSION
Sexual dysfunction in MS is quite common in both women and men, and the related symptoms are often multifactorial. Strategies to address sexual dysfunction in MS require a tailored approach. Fortunately, any treatments for sexual dysfunction initiated by the patient’s primary care provider will not have an adverse effect on the patient’s outcome with MS. For more complicated cases of MS-associated sexual dysfunction, urology referral is recommended.
1. Foley FW, Sander A. Sexuality, multiple sclerosis and women. Mult Scler Manage. 1997;4:1-9.
2. Calabro RS, De Luca R, Conti-Nibali V, et al. Sexual dysfunction in male patients with multiple sclerosis: a need for counseling! Int J Neurosci. 2014;124(8):547-557.
3. Gava G, Visconti M, Salvi F, et al. Prevalence and psychopathological determinants of sexual dysfunction and related distress in women with and without multiple sclerosis. J Sex Med. 2019;16(6):833-842.
4. Cordeau D, Courtois, F. Sexual disorders in women with MS: assessment and management. Ann Phys Rehabil Med. 2014; 57(5):337-47.
A 37-year-old woman presents to her primary care clinic with a chief complaint of depression. She was diagnosed with relapsing multiple sclerosis (MS) at age 29 and is currently taking an injectable preventive therapy. Over the past 6 months, she has had increased marital strain secondary to losing her job because “I couldn’t mentally keep up with the work anymore.” This has caused financial difficulties for her family. In addition, she tires easily and has been napping in the afternoon. She and her husband are experiencing intimacy difficulties, and she confirms problems with vaginal dryness and a general loss of her sexual drive.
Sexual dysfunction in MS is common, affecting 40% to 80% of women and 50% to 90% of men with MS. It is an “invisible” symptom, similar to fatigue, cognitive dysfunction, and pain.1-3
There are three ways that MS patients can be affected by sexual dysfunction, and they are categorized as primary, secondary, and tertiary. Primary sexual dysfunction results from demyelination/axonal destruction of the central nervous system, which potentially leads to altered genital sensation or paresthesia. Secondary sexual dysfunction stems from nonsexual MS symptoms, such as fatigue, spasticity, tremor, impairments in concentration/attention, and iatrogenic causes (eg, adverse effects of medication). Tertiary sexual dysfunction involves the psychosocial/cultural aspects of the disease that can impact a patient’s sexual drive.
SYMPTOMS
Like many other symptoms associated with MS, the symptoms of sexual dysfunction are highly variable. In women, the most common complaints are fatigue, decrease in genital sensation (27%-47%), decrease in libido (31%-74%) and vaginal lubrication (36%-48%), and difficulty with orgasm.4 In men with MS, in addition to erectile problems, surveys have identified decreased genital sensation, fatigue (75%), difficulty with ejaculation (18%-50%), decreased interest or arousal (39%), and anorgasmia (37%) as fairly common complaints.2
TREATMENT
Managing sexual dysfunction in a patient with MS is dependent on the underlying problem. Some examples include
- For many patients, their disease causes significant anxiety and worry about current and potentially future disability—which can make intimacy more difficult. Sometimes, referral to a mental health professional may be required to help the patient
with individual and/or couples counseling to further elucidate underlying intimacy issues. - For patients experiencing MS-associated fatigue, suggest planning for sexual activity in the morning, since fatigue is known to worsen throughout the day.
- For those who qualify for antidepressant medications, remember that some (eg, selective serotonin reuptake inhibitors) can further decrease libido and therefore should be avoided if possible.
- For women who have difficulty with lubrication, a nonpetroleum-based lubricant may reduce vaginal dryness, while use of a vibrator may assist with genital stimulation.
- For men who cannot maintain erection, phosphodiesterase inhibitor drugs (eg, sildenafil) can be helpful; other options include alprostadil urethral suppositories and intracavernous injections.
The patient is screened for depression using the Patient Health Questionnaire, which yields a score of 17 (moderately severe). You discuss the need for active treatment with her, and she agrees to start an antidepressant medication. Bupropion is chosen, given its effectiveness and lack of adverse effects (including sexual dysfunction). The patient also is encouraged to use nonpetroleum-based lubricants. Finally, a referral is made for couples counseling, and a 6-week follow-up appointment is scheduled.
CONCLUSION
Sexual dysfunction in MS is quite common in both women and men, and the related symptoms are often multifactorial. Strategies to address sexual dysfunction in MS require a tailored approach. Fortunately, any treatments for sexual dysfunction initiated by the patient’s primary care provider will not have an adverse effect on the patient’s outcome with MS. For more complicated cases of MS-associated sexual dysfunction, urology referral is recommended.
A 37-year-old woman presents to her primary care clinic with a chief complaint of depression. She was diagnosed with relapsing multiple sclerosis (MS) at age 29 and is currently taking an injectable preventive therapy. Over the past 6 months, she has had increased marital strain secondary to losing her job because “I couldn’t mentally keep up with the work anymore.” This has caused financial difficulties for her family. In addition, she tires easily and has been napping in the afternoon. She and her husband are experiencing intimacy difficulties, and she confirms problems with vaginal dryness and a general loss of her sexual drive.
Sexual dysfunction in MS is common, affecting 40% to 80% of women and 50% to 90% of men with MS. It is an “invisible” symptom, similar to fatigue, cognitive dysfunction, and pain.1-3
There are three ways that MS patients can be affected by sexual dysfunction, and they are categorized as primary, secondary, and tertiary. Primary sexual dysfunction results from demyelination/axonal destruction of the central nervous system, which potentially leads to altered genital sensation or paresthesia. Secondary sexual dysfunction stems from nonsexual MS symptoms, such as fatigue, spasticity, tremor, impairments in concentration/attention, and iatrogenic causes (eg, adverse effects of medication). Tertiary sexual dysfunction involves the psychosocial/cultural aspects of the disease that can impact a patient’s sexual drive.
SYMPTOMS
Like many other symptoms associated with MS, the symptoms of sexual dysfunction are highly variable. In women, the most common complaints are fatigue, decrease in genital sensation (27%-47%), decrease in libido (31%-74%) and vaginal lubrication (36%-48%), and difficulty with orgasm.4 In men with MS, in addition to erectile problems, surveys have identified decreased genital sensation, fatigue (75%), difficulty with ejaculation (18%-50%), decreased interest or arousal (39%), and anorgasmia (37%) as fairly common complaints.2
TREATMENT
Managing sexual dysfunction in a patient with MS is dependent on the underlying problem. Some examples include
- For many patients, their disease causes significant anxiety and worry about current and potentially future disability—which can make intimacy more difficult. Sometimes, referral to a mental health professional may be required to help the patient
with individual and/or couples counseling to further elucidate underlying intimacy issues. - For patients experiencing MS-associated fatigue, suggest planning for sexual activity in the morning, since fatigue is known to worsen throughout the day.
- For those who qualify for antidepressant medications, remember that some (eg, selective serotonin reuptake inhibitors) can further decrease libido and therefore should be avoided if possible.
- For women who have difficulty with lubrication, a nonpetroleum-based lubricant may reduce vaginal dryness, while use of a vibrator may assist with genital stimulation.
- For men who cannot maintain erection, phosphodiesterase inhibitor drugs (eg, sildenafil) can be helpful; other options include alprostadil urethral suppositories and intracavernous injections.
The patient is screened for depression using the Patient Health Questionnaire, which yields a score of 17 (moderately severe). You discuss the need for active treatment with her, and she agrees to start an antidepressant medication. Bupropion is chosen, given its effectiveness and lack of adverse effects (including sexual dysfunction). The patient also is encouraged to use nonpetroleum-based lubricants. Finally, a referral is made for couples counseling, and a 6-week follow-up appointment is scheduled.
CONCLUSION
Sexual dysfunction in MS is quite common in both women and men, and the related symptoms are often multifactorial. Strategies to address sexual dysfunction in MS require a tailored approach. Fortunately, any treatments for sexual dysfunction initiated by the patient’s primary care provider will not have an adverse effect on the patient’s outcome with MS. For more complicated cases of MS-associated sexual dysfunction, urology referral is recommended.
1. Foley FW, Sander A. Sexuality, multiple sclerosis and women. Mult Scler Manage. 1997;4:1-9.
2. Calabro RS, De Luca R, Conti-Nibali V, et al. Sexual dysfunction in male patients with multiple sclerosis: a need for counseling! Int J Neurosci. 2014;124(8):547-557.
3. Gava G, Visconti M, Salvi F, et al. Prevalence and psychopathological determinants of sexual dysfunction and related distress in women with and without multiple sclerosis. J Sex Med. 2019;16(6):833-842.
4. Cordeau D, Courtois, F. Sexual disorders in women with MS: assessment and management. Ann Phys Rehabil Med. 2014; 57(5):337-47.
1. Foley FW, Sander A. Sexuality, multiple sclerosis and women. Mult Scler Manage. 1997;4:1-9.
2. Calabro RS, De Luca R, Conti-Nibali V, et al. Sexual dysfunction in male patients with multiple sclerosis: a need for counseling! Int J Neurosci. 2014;124(8):547-557.
3. Gava G, Visconti M, Salvi F, et al. Prevalence and psychopathological determinants of sexual dysfunction and related distress in women with and without multiple sclerosis. J Sex Med. 2019;16(6):833-842.
4. Cordeau D, Courtois, F. Sexual disorders in women with MS: assessment and management. Ann Phys Rehabil Med. 2014; 57(5):337-47.
The ABCs of COCs: A Guide for Dermatology Residents on Combined Oral Contraceptives
The American Academy of Dermatology confers combined oral contraceptives (COCs) a strength A recommendation for the treatment of acne based on level I evidence, and 4 COCs are approved for the treatment of acne by the US Food and Drug Administration (FDA).1 Furthermore, when dermatologists prescribe isotretinoin and thalidomide to women of reproductive potential, the iPLEDGE and THALOMID Risk Evaluation and Mitigation Strategy (REMS) programs require 2 concurrent methods of contraception, one of which may be a COC. In addition, COCs have several potential off-label indications in dermatology including idiopathic hirsutism, female pattern hair loss, hidradenitis suppurativa, and autoimmune progesterone dermatitis.
Despite this evidence and opportunity, research suggests that dermatologists underprescribe COCs. The National Ambulatory Medical Care Survey found that between 1993 and 2008, dermatologists in the United States prescribed COCs to only 2.03% of women presenting for acne treatment, which was less often than obstetricians/gynecologists (36.03%) and internists (10.76%).2 More recently, in a survey of 130 US dermatologists conducted from 2014 to 2015, only 55.4% reported prescribing COCs. This survey also found that only 45.8% of dermatologists who prescribed COCs felt very comfortable counseling on how to begin taking them, only 48.6% felt very comfortable counseling patients on side effects, and only 22.2% felt very comfortable managing side effects.3
In light of these data, this article reviews the basics of COCs for dermatology residents, from assessing patient eligibility and selecting a COC to counseling on use and managing risks and side effects. Because there are different approaches to prescribing COCs, readers are encouraged to integrate the information in this article with what they have learned from other sources.
Assess Patient Eligibility
In general, patients should be at least 14 years of age and have waited 2 years after menarche to start COCs. They can be taken until menopause.1,4 Contraindications can be screened for by taking a medical history and measuring a baseline blood pressure (Tables 1 and 2).5 In addition, pregnancy should be excluded with a urine or serum pregnancy test or criteria provided in Box 2 of the 2016 US Selected Practice Recommendations for Contraceptive Use from the Centers for Disease Control and Prevention (CDC).4 Although important for women’s overall health, a pelvic examination is not required to start COCs according to the CDC and the American Academy of Dermatology.1,4


Select the COC
Combined oral contraceptives combine estrogen, usually in the form of ethinyl estradiol, with a progestin. Data suggest that all COCs effectively treat acne, but 4 are specifically FDA approved for acne: ethinyl estradiol–norethindrone acetate–ferrous fumarate, ethinyl estradiol–norgestimate, ethinyl estradiol–drospirenone, and ethinyl estradiol–drospirenone–levomefolate.1 Ethinyl estradiol–desogestrel and ethinyl estradiol–drospirenone are 2 go-to COCs for some of the attending physicians at my residency program. All COCs are FDA approved for contraception. When selecting a COC, one approach is to start with the patient’s drug formulary, then consider the following characteristics.
Monophasic vs Multiphasic
All the hormonally active pills in a monophasic formulation contain the same dose of estrogen and progestin; however, these doses change per pill in a multiphasic formulation, which requires that patients take the pills in a specific order. Given this greater complexity and the fact that multiphasic formulations often are more expensive and lack evidence of superiority, a 2011 Cochrane review recommended monophasic formulations as first line.6 In addition, monophasic formulations are preferred for autoimmune progesterone dermatitis because of the stable progestin dose.
Hormone-Free Interval
Some COCs include placebo pills during which hormone withdrawal symptoms such as bleeding, pelvic pain, mood changes, and headache may occur. If a patient is concerned about these symptoms, choose a COC with no or fewer placebo pills, or have the patient skip the hormone-free interval altogether and start the next pack early7; in this case, the prescription should be written with instructions to allow the patient to get earlier refills from the pharmacy.
Estrogen Dose
To minimize estrogen-related side effects, the lowest possible dose of ethinyl estradiol that is effective and tolerable should be prescribed7,8; 20 μg of ethinyl estradiol generally is the lowest dose available, but it may be associated with more frequent breakthrough bleeding.9 The International Planned Parenthood Federation recommends starting with COCs that contain 30 to 35 μg of estrogen.10 Synthesizing this information, one option is to start with 20 μg of ethinyl estradiol and increase the dose if breakthrough bleeding persists after 3 cycles.
Progestin Type
First-generation progestins (eg, norethindrone), second-generation progestins (eg, norgestrel, levonorgestrel), and third-generation progestins (eg, norgestimate, desogestrel) are derived from testosterone and therefore are variably androgenic; second-generation progestins are the most androgenic, and third-generation progestins are the least. On the other hand, drospirenone, the fourth-generation progestin available in the United States, is derived from 17α-spironolactone and thus is mildly antiandrogenic (3 mg of drospirenone is considered equivalent to 25 mg of spironolactone).
Although COCs with less androgenic progestins should theoretically treat acne better, a 2012 Cochrane review of COCs and acne concluded that “differences in the comparative effectiveness of COCs containing varying progestin types and dosages were less clear, and data were limited for any particular comparison.”11 As a result, regardless of the progestin, all COCs are believed to have a net antiandrogenic effect due to their estrogen component.1
Counsel on Use
Combined oral contraceptives can be started on any day of the menstrual cycle, including the day the prescription is given. If a patient begins a COC within 5 days of the first day of her most recent period, backup contraception is not needed.4 If she begins the COC more than 5 days after the first day of her most recent period, she needs to use backup contraception or abstain from sexual intercourse for the next 7 days.4 In general, at least 3 months of therapy are required to evaluate the effectiveness of COCs for acne.1
Manage Risks and Side Effects
Breakthrough Bleeding
The most common side effect of breakthrough bleeding can be minimized by taking COCs at approximately the same time every day and avoiding missed pills. If breakthrough bleeding does not stop after 3 cycles, consider increasing the estrogen dose to 30 to 35 μg and/or referring to an obstetrician/gynecologist to rule out other etiologies of bleeding.7,8
Nausea, Headache, Bloating, and Breast Tenderness
These symptoms typically resolve after the first 3 months. To minimize nausea, patients should take COCs in the early evening and eat breakfast the next morning.7,8 For headaches that occur during the hormone-free interval, consider skipping the placebo pills and starting the next pack early. Switching the progestin to drospirenone, which has a mild diuretic effect, can help with bloating as well as breast tenderness.7 For persistent symptoms, consider a lower estrogen dose.7,8
Changes in Libido
In a systemic review including 8422 COC users, 64% reported no change in libido, 22% reported an increase, and 15% reported a decrease.12
Weight Gain
Although patients may be concerned that COCs cause weight gain, a 2014 Cochrane review concluded that “available evidence is insufficient to determine the effect of combination contraceptives on weight, but no large effect is evident.”13 If weight gain does occur, anecdotal evidence suggests it tends to be not more than 5 pounds. If weight gain is an issue, consider a less androgenic progestin.8
Venous Thromboembolism
Use the 3-6-9-12 model to contextualize venous thromboembolism (VTE) risk: a woman’s annual VTE risk is 3 per 10,000 women at baseline, 6 per 10,000 women with nondrospirenone COCs, 9 per 10,000 women with drospirenone-containing COCs, and 12 per 10,000 women when pregnant.14 Patients should be counseled on the signs and symptoms of VTE such as unilateral or bilateral leg or arm swelling, pain, warmth, redness, and/or shortness of breath. The British Society for Haematology recommends maintaining mobility as a reasonable precaution when traveling for more than 3 hours.15
Cardiovascular Disease
A 2015 Cochrane review found that the risk for myocardial infarction or ischemic stroke is increased 1.6‐fold in COC users.16 Despite this increased relative risk, the increased absolute annual risk of myocardial infarction in nonsmoking women remains low: increased from 0.83 to 3.53 per 10,000,000 women younger than 35 years and from 9.45 to 40.4 per 10,000,000 women 35 years and older.17
Breast Cancer and Cervical Cancer
Data are mixed on the effect of COCs on the risk for breast cancer and cervical cancer.1 According to the CDC, COC use for 5 or more years might increase the risk of cervical carcinoma in situ and invasive cervical carcinoma in women with persistent human papillomavirus infection.5 Regardless of COC use, women should undergo age-appropriate screening for breast cancer and cervical cancer.
Melasma
Melasma is an estrogen-mediated side effect of COCs.8 A study from 1967 found that 29% of COC users (N=212) developed melasma; however, they were taking COCs with much higher ethinyl estradiol doses (50–100 μg) than typically used today.18 Nevertheless, as part of an overall skin care regimen, photoprotection should be encouraged with a broad-spectrum, water-resistant sunscreen that has a sun protection factor of at least 30. In addition, sunscreens with iron oxides have been shown to better prevent melasma relapse by protecting against the shorter wavelengths of visible light.19
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e933.
- Landis ET, Levender MM, Davis SA, et al. Isotretinoin and oral contraceptive use in female acne patients varies by physician specialty: analysis of data from the National Ambulatory Medical Care Survey. J Dermatolog Treat. 2012;23:272-277.
- Fitzpatrick L, Mauer E, Chen CL. Oral contraceptives for acne treatment: US dermatologists’ knowledge, comfort, and prescribing practices. Cutis. 2017;99:195-201.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2011:CD003553.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Aust Prescr. 2015;38:6-11.
- McKinney K. Understanding the options: a guide to oral contraceptives. https://www.cecentral.com/assets/2097/022%20Oral%20Contraceptives%2010-26-09.pdf. Published November 5, 2009. Accessed June 20, 2019.
- Gallo MF, Nanda K, Grimes DA, et al. 20 microg versus >20 microg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- Terki F, Malhotra U. Medical and Service Delivery Guidelines for Sexual and Reproductive Health Services. London, United Kingdom: International Planned Parenthood Federation; 2004.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- Pastor Z, Holla K, Chmel R. The influence of combined oral contraceptives on female sexual desire: a systematic review. Eur J Contracept Reprod Health Care. 2013;18:27-43.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Birth control pills for acne: tips from Julie Harper at the Summer AAD. Cutis. https://www.mdedge.com/dermatology/article/144550/acne/birth-control-pills-acne-tips-julie-harper-summer-aad. Published August 14, 2017. Accessed June 24, 2019.
- Watson HG, Baglin TP. Guidelines on travel-related venous thrombosis. Br J Haematol. 2011;152:31-34.
- Roach RE, Helmerhorst FM, Lijfering WM, et al. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015:CD011054.
- Acute myocardial infarction and combined oral contraceptives: results of an international multicentre case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1997;349:1202-1209.
- Resnik S. Melasma induced by oral contraceptive drugs. JAMA. 1967;199:601-605.
- Boukari F, Jourdan E, Fontas E, et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72:189-190.e181.
The American Academy of Dermatology confers combined oral contraceptives (COCs) a strength A recommendation for the treatment of acne based on level I evidence, and 4 COCs are approved for the treatment of acne by the US Food and Drug Administration (FDA).1 Furthermore, when dermatologists prescribe isotretinoin and thalidomide to women of reproductive potential, the iPLEDGE and THALOMID Risk Evaluation and Mitigation Strategy (REMS) programs require 2 concurrent methods of contraception, one of which may be a COC. In addition, COCs have several potential off-label indications in dermatology including idiopathic hirsutism, female pattern hair loss, hidradenitis suppurativa, and autoimmune progesterone dermatitis.
Despite this evidence and opportunity, research suggests that dermatologists underprescribe COCs. The National Ambulatory Medical Care Survey found that between 1993 and 2008, dermatologists in the United States prescribed COCs to only 2.03% of women presenting for acne treatment, which was less often than obstetricians/gynecologists (36.03%) and internists (10.76%).2 More recently, in a survey of 130 US dermatologists conducted from 2014 to 2015, only 55.4% reported prescribing COCs. This survey also found that only 45.8% of dermatologists who prescribed COCs felt very comfortable counseling on how to begin taking them, only 48.6% felt very comfortable counseling patients on side effects, and only 22.2% felt very comfortable managing side effects.3
In light of these data, this article reviews the basics of COCs for dermatology residents, from assessing patient eligibility and selecting a COC to counseling on use and managing risks and side effects. Because there are different approaches to prescribing COCs, readers are encouraged to integrate the information in this article with what they have learned from other sources.
Assess Patient Eligibility
In general, patients should be at least 14 years of age and have waited 2 years after menarche to start COCs. They can be taken until menopause.1,4 Contraindications can be screened for by taking a medical history and measuring a baseline blood pressure (Tables 1 and 2).5 In addition, pregnancy should be excluded with a urine or serum pregnancy test or criteria provided in Box 2 of the 2016 US Selected Practice Recommendations for Contraceptive Use from the Centers for Disease Control and Prevention (CDC).4 Although important for women’s overall health, a pelvic examination is not required to start COCs according to the CDC and the American Academy of Dermatology.1,4


Select the COC
Combined oral contraceptives combine estrogen, usually in the form of ethinyl estradiol, with a progestin. Data suggest that all COCs effectively treat acne, but 4 are specifically FDA approved for acne: ethinyl estradiol–norethindrone acetate–ferrous fumarate, ethinyl estradiol–norgestimate, ethinyl estradiol–drospirenone, and ethinyl estradiol–drospirenone–levomefolate.1 Ethinyl estradiol–desogestrel and ethinyl estradiol–drospirenone are 2 go-to COCs for some of the attending physicians at my residency program. All COCs are FDA approved for contraception. When selecting a COC, one approach is to start with the patient’s drug formulary, then consider the following characteristics.
Monophasic vs Multiphasic
All the hormonally active pills in a monophasic formulation contain the same dose of estrogen and progestin; however, these doses change per pill in a multiphasic formulation, which requires that patients take the pills in a specific order. Given this greater complexity and the fact that multiphasic formulations often are more expensive and lack evidence of superiority, a 2011 Cochrane review recommended monophasic formulations as first line.6 In addition, monophasic formulations are preferred for autoimmune progesterone dermatitis because of the stable progestin dose.
Hormone-Free Interval
Some COCs include placebo pills during which hormone withdrawal symptoms such as bleeding, pelvic pain, mood changes, and headache may occur. If a patient is concerned about these symptoms, choose a COC with no or fewer placebo pills, or have the patient skip the hormone-free interval altogether and start the next pack early7; in this case, the prescription should be written with instructions to allow the patient to get earlier refills from the pharmacy.
Estrogen Dose
To minimize estrogen-related side effects, the lowest possible dose of ethinyl estradiol that is effective and tolerable should be prescribed7,8; 20 μg of ethinyl estradiol generally is the lowest dose available, but it may be associated with more frequent breakthrough bleeding.9 The International Planned Parenthood Federation recommends starting with COCs that contain 30 to 35 μg of estrogen.10 Synthesizing this information, one option is to start with 20 μg of ethinyl estradiol and increase the dose if breakthrough bleeding persists after 3 cycles.
Progestin Type
First-generation progestins (eg, norethindrone), second-generation progestins (eg, norgestrel, levonorgestrel), and third-generation progestins (eg, norgestimate, desogestrel) are derived from testosterone and therefore are variably androgenic; second-generation progestins are the most androgenic, and third-generation progestins are the least. On the other hand, drospirenone, the fourth-generation progestin available in the United States, is derived from 17α-spironolactone and thus is mildly antiandrogenic (3 mg of drospirenone is considered equivalent to 25 mg of spironolactone).
Although COCs with less androgenic progestins should theoretically treat acne better, a 2012 Cochrane review of COCs and acne concluded that “differences in the comparative effectiveness of COCs containing varying progestin types and dosages were less clear, and data were limited for any particular comparison.”11 As a result, regardless of the progestin, all COCs are believed to have a net antiandrogenic effect due to their estrogen component.1
Counsel on Use
Combined oral contraceptives can be started on any day of the menstrual cycle, including the day the prescription is given. If a patient begins a COC within 5 days of the first day of her most recent period, backup contraception is not needed.4 If she begins the COC more than 5 days after the first day of her most recent period, she needs to use backup contraception or abstain from sexual intercourse for the next 7 days.4 In general, at least 3 months of therapy are required to evaluate the effectiveness of COCs for acne.1
Manage Risks and Side Effects
Breakthrough Bleeding
The most common side effect of breakthrough bleeding can be minimized by taking COCs at approximately the same time every day and avoiding missed pills. If breakthrough bleeding does not stop after 3 cycles, consider increasing the estrogen dose to 30 to 35 μg and/or referring to an obstetrician/gynecologist to rule out other etiologies of bleeding.7,8
Nausea, Headache, Bloating, and Breast Tenderness
These symptoms typically resolve after the first 3 months. To minimize nausea, patients should take COCs in the early evening and eat breakfast the next morning.7,8 For headaches that occur during the hormone-free interval, consider skipping the placebo pills and starting the next pack early. Switching the progestin to drospirenone, which has a mild diuretic effect, can help with bloating as well as breast tenderness.7 For persistent symptoms, consider a lower estrogen dose.7,8
Changes in Libido
In a systemic review including 8422 COC users, 64% reported no change in libido, 22% reported an increase, and 15% reported a decrease.12
Weight Gain
Although patients may be concerned that COCs cause weight gain, a 2014 Cochrane review concluded that “available evidence is insufficient to determine the effect of combination contraceptives on weight, but no large effect is evident.”13 If weight gain does occur, anecdotal evidence suggests it tends to be not more than 5 pounds. If weight gain is an issue, consider a less androgenic progestin.8
Venous Thromboembolism
Use the 3-6-9-12 model to contextualize venous thromboembolism (VTE) risk: a woman’s annual VTE risk is 3 per 10,000 women at baseline, 6 per 10,000 women with nondrospirenone COCs, 9 per 10,000 women with drospirenone-containing COCs, and 12 per 10,000 women when pregnant.14 Patients should be counseled on the signs and symptoms of VTE such as unilateral or bilateral leg or arm swelling, pain, warmth, redness, and/or shortness of breath. The British Society for Haematology recommends maintaining mobility as a reasonable precaution when traveling for more than 3 hours.15
Cardiovascular Disease
A 2015 Cochrane review found that the risk for myocardial infarction or ischemic stroke is increased 1.6‐fold in COC users.16 Despite this increased relative risk, the increased absolute annual risk of myocardial infarction in nonsmoking women remains low: increased from 0.83 to 3.53 per 10,000,000 women younger than 35 years and from 9.45 to 40.4 per 10,000,000 women 35 years and older.17
Breast Cancer and Cervical Cancer
Data are mixed on the effect of COCs on the risk for breast cancer and cervical cancer.1 According to the CDC, COC use for 5 or more years might increase the risk of cervical carcinoma in situ and invasive cervical carcinoma in women with persistent human papillomavirus infection.5 Regardless of COC use, women should undergo age-appropriate screening for breast cancer and cervical cancer.
Melasma
Melasma is an estrogen-mediated side effect of COCs.8 A study from 1967 found that 29% of COC users (N=212) developed melasma; however, they were taking COCs with much higher ethinyl estradiol doses (50–100 μg) than typically used today.18 Nevertheless, as part of an overall skin care regimen, photoprotection should be encouraged with a broad-spectrum, water-resistant sunscreen that has a sun protection factor of at least 30. In addition, sunscreens with iron oxides have been shown to better prevent melasma relapse by protecting against the shorter wavelengths of visible light.19
The American Academy of Dermatology confers combined oral contraceptives (COCs) a strength A recommendation for the treatment of acne based on level I evidence, and 4 COCs are approved for the treatment of acne by the US Food and Drug Administration (FDA).1 Furthermore, when dermatologists prescribe isotretinoin and thalidomide to women of reproductive potential, the iPLEDGE and THALOMID Risk Evaluation and Mitigation Strategy (REMS) programs require 2 concurrent methods of contraception, one of which may be a COC. In addition, COCs have several potential off-label indications in dermatology including idiopathic hirsutism, female pattern hair loss, hidradenitis suppurativa, and autoimmune progesterone dermatitis.
Despite this evidence and opportunity, research suggests that dermatologists underprescribe COCs. The National Ambulatory Medical Care Survey found that between 1993 and 2008, dermatologists in the United States prescribed COCs to only 2.03% of women presenting for acne treatment, which was less often than obstetricians/gynecologists (36.03%) and internists (10.76%).2 More recently, in a survey of 130 US dermatologists conducted from 2014 to 2015, only 55.4% reported prescribing COCs. This survey also found that only 45.8% of dermatologists who prescribed COCs felt very comfortable counseling on how to begin taking them, only 48.6% felt very comfortable counseling patients on side effects, and only 22.2% felt very comfortable managing side effects.3
In light of these data, this article reviews the basics of COCs for dermatology residents, from assessing patient eligibility and selecting a COC to counseling on use and managing risks and side effects. Because there are different approaches to prescribing COCs, readers are encouraged to integrate the information in this article with what they have learned from other sources.
Assess Patient Eligibility
In general, patients should be at least 14 years of age and have waited 2 years after menarche to start COCs. They can be taken until menopause.1,4 Contraindications can be screened for by taking a medical history and measuring a baseline blood pressure (Tables 1 and 2).5 In addition, pregnancy should be excluded with a urine or serum pregnancy test or criteria provided in Box 2 of the 2016 US Selected Practice Recommendations for Contraceptive Use from the Centers for Disease Control and Prevention (CDC).4 Although important for women’s overall health, a pelvic examination is not required to start COCs according to the CDC and the American Academy of Dermatology.1,4


Select the COC
Combined oral contraceptives combine estrogen, usually in the form of ethinyl estradiol, with a progestin. Data suggest that all COCs effectively treat acne, but 4 are specifically FDA approved for acne: ethinyl estradiol–norethindrone acetate–ferrous fumarate, ethinyl estradiol–norgestimate, ethinyl estradiol–drospirenone, and ethinyl estradiol–drospirenone–levomefolate.1 Ethinyl estradiol–desogestrel and ethinyl estradiol–drospirenone are 2 go-to COCs for some of the attending physicians at my residency program. All COCs are FDA approved for contraception. When selecting a COC, one approach is to start with the patient’s drug formulary, then consider the following characteristics.
Monophasic vs Multiphasic
All the hormonally active pills in a monophasic formulation contain the same dose of estrogen and progestin; however, these doses change per pill in a multiphasic formulation, which requires that patients take the pills in a specific order. Given this greater complexity and the fact that multiphasic formulations often are more expensive and lack evidence of superiority, a 2011 Cochrane review recommended monophasic formulations as first line.6 In addition, monophasic formulations are preferred for autoimmune progesterone dermatitis because of the stable progestin dose.
Hormone-Free Interval
Some COCs include placebo pills during which hormone withdrawal symptoms such as bleeding, pelvic pain, mood changes, and headache may occur. If a patient is concerned about these symptoms, choose a COC with no or fewer placebo pills, or have the patient skip the hormone-free interval altogether and start the next pack early7; in this case, the prescription should be written with instructions to allow the patient to get earlier refills from the pharmacy.
Estrogen Dose
To minimize estrogen-related side effects, the lowest possible dose of ethinyl estradiol that is effective and tolerable should be prescribed7,8; 20 μg of ethinyl estradiol generally is the lowest dose available, but it may be associated with more frequent breakthrough bleeding.9 The International Planned Parenthood Federation recommends starting with COCs that contain 30 to 35 μg of estrogen.10 Synthesizing this information, one option is to start with 20 μg of ethinyl estradiol and increase the dose if breakthrough bleeding persists after 3 cycles.
Progestin Type
First-generation progestins (eg, norethindrone), second-generation progestins (eg, norgestrel, levonorgestrel), and third-generation progestins (eg, norgestimate, desogestrel) are derived from testosterone and therefore are variably androgenic; second-generation progestins are the most androgenic, and third-generation progestins are the least. On the other hand, drospirenone, the fourth-generation progestin available in the United States, is derived from 17α-spironolactone and thus is mildly antiandrogenic (3 mg of drospirenone is considered equivalent to 25 mg of spironolactone).
Although COCs with less androgenic progestins should theoretically treat acne better, a 2012 Cochrane review of COCs and acne concluded that “differences in the comparative effectiveness of COCs containing varying progestin types and dosages were less clear, and data were limited for any particular comparison.”11 As a result, regardless of the progestin, all COCs are believed to have a net antiandrogenic effect due to their estrogen component.1
Counsel on Use
Combined oral contraceptives can be started on any day of the menstrual cycle, including the day the prescription is given. If a patient begins a COC within 5 days of the first day of her most recent period, backup contraception is not needed.4 If she begins the COC more than 5 days after the first day of her most recent period, she needs to use backup contraception or abstain from sexual intercourse for the next 7 days.4 In general, at least 3 months of therapy are required to evaluate the effectiveness of COCs for acne.1
Manage Risks and Side Effects
Breakthrough Bleeding
The most common side effect of breakthrough bleeding can be minimized by taking COCs at approximately the same time every day and avoiding missed pills. If breakthrough bleeding does not stop after 3 cycles, consider increasing the estrogen dose to 30 to 35 μg and/or referring to an obstetrician/gynecologist to rule out other etiologies of bleeding.7,8
Nausea, Headache, Bloating, and Breast Tenderness
These symptoms typically resolve after the first 3 months. To minimize nausea, patients should take COCs in the early evening and eat breakfast the next morning.7,8 For headaches that occur during the hormone-free interval, consider skipping the placebo pills and starting the next pack early. Switching the progestin to drospirenone, which has a mild diuretic effect, can help with bloating as well as breast tenderness.7 For persistent symptoms, consider a lower estrogen dose.7,8
Changes in Libido
In a systemic review including 8422 COC users, 64% reported no change in libido, 22% reported an increase, and 15% reported a decrease.12
Weight Gain
Although patients may be concerned that COCs cause weight gain, a 2014 Cochrane review concluded that “available evidence is insufficient to determine the effect of combination contraceptives on weight, but no large effect is evident.”13 If weight gain does occur, anecdotal evidence suggests it tends to be not more than 5 pounds. If weight gain is an issue, consider a less androgenic progestin.8
Venous Thromboembolism
Use the 3-6-9-12 model to contextualize venous thromboembolism (VTE) risk: a woman’s annual VTE risk is 3 per 10,000 women at baseline, 6 per 10,000 women with nondrospirenone COCs, 9 per 10,000 women with drospirenone-containing COCs, and 12 per 10,000 women when pregnant.14 Patients should be counseled on the signs and symptoms of VTE such as unilateral or bilateral leg or arm swelling, pain, warmth, redness, and/or shortness of breath. The British Society for Haematology recommends maintaining mobility as a reasonable precaution when traveling for more than 3 hours.15
Cardiovascular Disease
A 2015 Cochrane review found that the risk for myocardial infarction or ischemic stroke is increased 1.6‐fold in COC users.16 Despite this increased relative risk, the increased absolute annual risk of myocardial infarction in nonsmoking women remains low: increased from 0.83 to 3.53 per 10,000,000 women younger than 35 years and from 9.45 to 40.4 per 10,000,000 women 35 years and older.17
Breast Cancer and Cervical Cancer
Data are mixed on the effect of COCs on the risk for breast cancer and cervical cancer.1 According to the CDC, COC use for 5 or more years might increase the risk of cervical carcinoma in situ and invasive cervical carcinoma in women with persistent human papillomavirus infection.5 Regardless of COC use, women should undergo age-appropriate screening for breast cancer and cervical cancer.
Melasma
Melasma is an estrogen-mediated side effect of COCs.8 A study from 1967 found that 29% of COC users (N=212) developed melasma; however, they were taking COCs with much higher ethinyl estradiol doses (50–100 μg) than typically used today.18 Nevertheless, as part of an overall skin care regimen, photoprotection should be encouraged with a broad-spectrum, water-resistant sunscreen that has a sun protection factor of at least 30. In addition, sunscreens with iron oxides have been shown to better prevent melasma relapse by protecting against the shorter wavelengths of visible light.19
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e933.
- Landis ET, Levender MM, Davis SA, et al. Isotretinoin and oral contraceptive use in female acne patients varies by physician specialty: analysis of data from the National Ambulatory Medical Care Survey. J Dermatolog Treat. 2012;23:272-277.
- Fitzpatrick L, Mauer E, Chen CL. Oral contraceptives for acne treatment: US dermatologists’ knowledge, comfort, and prescribing practices. Cutis. 2017;99:195-201.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2011:CD003553.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Aust Prescr. 2015;38:6-11.
- McKinney K. Understanding the options: a guide to oral contraceptives. https://www.cecentral.com/assets/2097/022%20Oral%20Contraceptives%2010-26-09.pdf. Published November 5, 2009. Accessed June 20, 2019.
- Gallo MF, Nanda K, Grimes DA, et al. 20 microg versus >20 microg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- Terki F, Malhotra U. Medical and Service Delivery Guidelines for Sexual and Reproductive Health Services. London, United Kingdom: International Planned Parenthood Federation; 2004.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- Pastor Z, Holla K, Chmel R. The influence of combined oral contraceptives on female sexual desire: a systematic review. Eur J Contracept Reprod Health Care. 2013;18:27-43.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Birth control pills for acne: tips from Julie Harper at the Summer AAD. Cutis. https://www.mdedge.com/dermatology/article/144550/acne/birth-control-pills-acne-tips-julie-harper-summer-aad. Published August 14, 2017. Accessed June 24, 2019.
- Watson HG, Baglin TP. Guidelines on travel-related venous thrombosis. Br J Haematol. 2011;152:31-34.
- Roach RE, Helmerhorst FM, Lijfering WM, et al. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015:CD011054.
- Acute myocardial infarction and combined oral contraceptives: results of an international multicentre case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1997;349:1202-1209.
- Resnik S. Melasma induced by oral contraceptive drugs. JAMA. 1967;199:601-605.
- Boukari F, Jourdan E, Fontas E, et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72:189-190.e181.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e933.
- Landis ET, Levender MM, Davis SA, et al. Isotretinoin and oral contraceptive use in female acne patients varies by physician specialty: analysis of data from the National Ambulatory Medical Care Survey. J Dermatolog Treat. 2012;23:272-277.
- Fitzpatrick L, Mauer E, Chen CL. Oral contraceptives for acne treatment: US dermatologists’ knowledge, comfort, and prescribing practices. Cutis. 2017;99:195-201.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2011:CD003553.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Aust Prescr. 2015;38:6-11.
- McKinney K. Understanding the options: a guide to oral contraceptives. https://www.cecentral.com/assets/2097/022%20Oral%20Contraceptives%2010-26-09.pdf. Published November 5, 2009. Accessed June 20, 2019.
- Gallo MF, Nanda K, Grimes DA, et al. 20 microg versus >20 microg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- Terki F, Malhotra U. Medical and Service Delivery Guidelines for Sexual and Reproductive Health Services. London, United Kingdom: International Planned Parenthood Federation; 2004.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- Pastor Z, Holla K, Chmel R. The influence of combined oral contraceptives on female sexual desire: a systematic review. Eur J Contracept Reprod Health Care. 2013;18:27-43.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Birth control pills for acne: tips from Julie Harper at the Summer AAD. Cutis. https://www.mdedge.com/dermatology/article/144550/acne/birth-control-pills-acne-tips-julie-harper-summer-aad. Published August 14, 2017. Accessed June 24, 2019.
- Watson HG, Baglin TP. Guidelines on travel-related venous thrombosis. Br J Haematol. 2011;152:31-34.
- Roach RE, Helmerhorst FM, Lijfering WM, et al. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015:CD011054.
- Acute myocardial infarction and combined oral contraceptives: results of an international multicentre case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1997;349:1202-1209.
- Resnik S. Melasma induced by oral contraceptive drugs. JAMA. 1967;199:601-605.
- Boukari F, Jourdan E, Fontas E, et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72:189-190.e181.
Resident Pearls
- Screen for contraindications to combined oral contraceptives (COCs) by taking a medical history, measuring a baseline blood pressure, and excluding pregnancy. A baseline pelvic examination is unnecessary.
- Characteristics to consider when selecting a COC include the formulation, hormone-free interval, estrogen dose, and progestin type.
- Combined oral contraceptives can be initiated on any day of the menstrual cycle, with the need for backup contraception based on the number of days since the first day of the patient’s most recent period.
- Management of risks and side effects includes simple lifestyle changes, skipping the hormone-free interval, switching the COC, and referring to an obstetrician/gynecologist.
Pharmacist stigma a barrier to rural buprenorphine access
SAN ANTONIO – Most attention paid to barriers for medication-assisted treatment of opioid use disorder has focused on prescribers and patients, but pharmacists are “a neglected link in the chain,” according to Hannah Cooper, ScD, an assistant professor of behavioral sciences and health education at Emory University, Atlanta.
“Pharmacy-based dispensing of buprenorphine is one of the medication’s major advances over methadone,” Dr. Cooper told attendees at the annual meeting of the College on Problems of Drug Dependence. Yet, early interviews she and her colleagues conducted with rural Kentucky pharmacist colleagues in the CARE2HOPE study “revealed that pharmacy-level barriers might also curtail access to buprenorphine.”
Little research has examined those barriers, but Dr. Cooper noted. Further, anecdotal evidence has suggested that wholesaler concerns about Drug Enforcement Administration restrictions on dispensing buprenorphine has caused shortages at pharmacies.
Dr. Cooper and colleagues, therefore, designed a qualitative study aimed at learning about pharmacists’ attitudes and dispensing practices related to buprenorphine. They also looked at whether DEA limits actually exist on dispensing the drug. They interviewed 14 pharmacists operating 15 pharmacies across all 12 counties in two rural Kentucky health districts. Eleven of the pharmacists worked in independent pharmacies; the others worked at chains. Six pharmacies dispensed more than 100 buprenorphine prescriptions a month, five dispensed only several dozen a month, and four refused to dispense it at all.
Perceptions of federal restrictions
“Variations in buprenorphine dispensing did not solely reflect underlying variations in local need or prescribing practices,” Dr. Cooper said. At 12 of the 15 pharmacies, limits on buprenorphine resulted from a perceived DEA “cap” on dispensing the drug or “because of distrust in buprenorphine itself, its prescribers and its patients.”
The perceived cap from the DEA was shrouded in uncertainty: 10 of the pharmacists said the DEA capped the percentage of controlled substances pharmacists could dispense that were opioids, yet the pharmacists did not know what that percentage was.
Five of those interviewed said the cap often significantly cut short how many buprenorphine prescriptions they would dispense. Since they did not know how much the cap was, they internally set arbitrary limits, such as dispensing two prescriptions per day, to avoid risk of the DEA investigating their pharmacy.
Yet, those limits could not meet patient demand, so several pharmacists rationed buprenorphine only to local residents or long-term customers, causing additional problems. That practice strained relationships with prescribers, who then had to call multiple pharmacies to find one that would dispense the drug to new patients. It also put pharmacy staff at risk when a rejection angered a customer and “undermined local recovery efforts,” Dr. Cooper said.
Five other pharmacists, however, did not ration their buprenorphine and did not worry about exceeding the DEA cap.
No numerical cap appears to exist, but DEA regulations and the SUPPORT for Patients and Communities Act do require internal opioid surveillance systems at wholesalers that flag suspicious orders of controlled substances, including buprenorphine. And they enforce it: An $80 million settlement in 2013 resulted from the DEA’s charge that Walgreens distribution centers did not report suspicious drug orders.
Stigma among some pharmacists
Six of the pharmacists had low trust in buprenorphine and in those who prescribed it and used it, Dr. Cooper reported. Three would not dispense the drug at all, and two would not take new buprenorphine patients.
One such pharmacist told researchers: “It is supposed to be the drug to help them [recover.] They want Suboxone worse than they do the hydrocodone. … It’s not what it’s designed to be.”
Those pharmacists also reported believing that malpractice was common among prescribers, who, for example, did not provide required counseling to patients or did not quickly wean them off buprenorphine. The pharmacists perceived the physicians prescribing buprenorphine as doing so only to make more money, just as they had done by prescribing opioids in the first place.
Those pharmacists also believed the patients themselves sold buprenorphine to make money and that opioid use disorder was a choice. They told researchers that dispensing buprenorphine would bring more drug users to their stores and subsequently hurt business.
Yet, those beliefs were not universal among the pharmacists. Eight believed buprenorphine was an appropriate opioid use disorder treatment and had positive attitudes toward patients. Unlike those who viewed the disorder as a choice, those pharmacists saw it as a disease and viewed the patients admirably for their commitment to recovery.
Though a small, qualitative study, those findings suggest a need to more closely examine how pharmacies affect access to medication to treat opioid use disorder, Dr. Cooper said.
“In an epicenter of the U.S. opioid epidemic, policies and stigma curtail access to buprenorphine,” she told attendees. “DEA regulations, the SUPPORT Act, and related lawsuits have led wholesalers to develop proprietary caps that force some pharmacists to ration the number of buprenorphine prescriptions they filled.” Some pharmacists will not dispense the drug at all, while others “limited dispensing to known or local patients and prescribers, a practice that pharmacists recognized hurt patients who had to travel far to reach prescribers.”
The research was funded by the National Institutes of Health through CARE2HOPE, Rural Health Project, and the Emory Center for AIDS Research. The authors reported no disclosures.
SAN ANTONIO – Most attention paid to barriers for medication-assisted treatment of opioid use disorder has focused on prescribers and patients, but pharmacists are “a neglected link in the chain,” according to Hannah Cooper, ScD, an assistant professor of behavioral sciences and health education at Emory University, Atlanta.
“Pharmacy-based dispensing of buprenorphine is one of the medication’s major advances over methadone,” Dr. Cooper told attendees at the annual meeting of the College on Problems of Drug Dependence. Yet, early interviews she and her colleagues conducted with rural Kentucky pharmacist colleagues in the CARE2HOPE study “revealed that pharmacy-level barriers might also curtail access to buprenorphine.”
Little research has examined those barriers, but Dr. Cooper noted. Further, anecdotal evidence has suggested that wholesaler concerns about Drug Enforcement Administration restrictions on dispensing buprenorphine has caused shortages at pharmacies.
Dr. Cooper and colleagues, therefore, designed a qualitative study aimed at learning about pharmacists’ attitudes and dispensing practices related to buprenorphine. They also looked at whether DEA limits actually exist on dispensing the drug. They interviewed 14 pharmacists operating 15 pharmacies across all 12 counties in two rural Kentucky health districts. Eleven of the pharmacists worked in independent pharmacies; the others worked at chains. Six pharmacies dispensed more than 100 buprenorphine prescriptions a month, five dispensed only several dozen a month, and four refused to dispense it at all.
Perceptions of federal restrictions
“Variations in buprenorphine dispensing did not solely reflect underlying variations in local need or prescribing practices,” Dr. Cooper said. At 12 of the 15 pharmacies, limits on buprenorphine resulted from a perceived DEA “cap” on dispensing the drug or “because of distrust in buprenorphine itself, its prescribers and its patients.”
The perceived cap from the DEA was shrouded in uncertainty: 10 of the pharmacists said the DEA capped the percentage of controlled substances pharmacists could dispense that were opioids, yet the pharmacists did not know what that percentage was.
Five of those interviewed said the cap often significantly cut short how many buprenorphine prescriptions they would dispense. Since they did not know how much the cap was, they internally set arbitrary limits, such as dispensing two prescriptions per day, to avoid risk of the DEA investigating their pharmacy.
Yet, those limits could not meet patient demand, so several pharmacists rationed buprenorphine only to local residents or long-term customers, causing additional problems. That practice strained relationships with prescribers, who then had to call multiple pharmacies to find one that would dispense the drug to new patients. It also put pharmacy staff at risk when a rejection angered a customer and “undermined local recovery efforts,” Dr. Cooper said.
Five other pharmacists, however, did not ration their buprenorphine and did not worry about exceeding the DEA cap.
No numerical cap appears to exist, but DEA regulations and the SUPPORT for Patients and Communities Act do require internal opioid surveillance systems at wholesalers that flag suspicious orders of controlled substances, including buprenorphine. And they enforce it: An $80 million settlement in 2013 resulted from the DEA’s charge that Walgreens distribution centers did not report suspicious drug orders.
Stigma among some pharmacists
Six of the pharmacists had low trust in buprenorphine and in those who prescribed it and used it, Dr. Cooper reported. Three would not dispense the drug at all, and two would not take new buprenorphine patients.
One such pharmacist told researchers: “It is supposed to be the drug to help them [recover.] They want Suboxone worse than they do the hydrocodone. … It’s not what it’s designed to be.”
Those pharmacists also reported believing that malpractice was common among prescribers, who, for example, did not provide required counseling to patients or did not quickly wean them off buprenorphine. The pharmacists perceived the physicians prescribing buprenorphine as doing so only to make more money, just as they had done by prescribing opioids in the first place.
Those pharmacists also believed the patients themselves sold buprenorphine to make money and that opioid use disorder was a choice. They told researchers that dispensing buprenorphine would bring more drug users to their stores and subsequently hurt business.
Yet, those beliefs were not universal among the pharmacists. Eight believed buprenorphine was an appropriate opioid use disorder treatment and had positive attitudes toward patients. Unlike those who viewed the disorder as a choice, those pharmacists saw it as a disease and viewed the patients admirably for their commitment to recovery.
Though a small, qualitative study, those findings suggest a need to more closely examine how pharmacies affect access to medication to treat opioid use disorder, Dr. Cooper said.
“In an epicenter of the U.S. opioid epidemic, policies and stigma curtail access to buprenorphine,” she told attendees. “DEA regulations, the SUPPORT Act, and related lawsuits have led wholesalers to develop proprietary caps that force some pharmacists to ration the number of buprenorphine prescriptions they filled.” Some pharmacists will not dispense the drug at all, while others “limited dispensing to known or local patients and prescribers, a practice that pharmacists recognized hurt patients who had to travel far to reach prescribers.”
The research was funded by the National Institutes of Health through CARE2HOPE, Rural Health Project, and the Emory Center for AIDS Research. The authors reported no disclosures.
SAN ANTONIO – Most attention paid to barriers for medication-assisted treatment of opioid use disorder has focused on prescribers and patients, but pharmacists are “a neglected link in the chain,” according to Hannah Cooper, ScD, an assistant professor of behavioral sciences and health education at Emory University, Atlanta.
“Pharmacy-based dispensing of buprenorphine is one of the medication’s major advances over methadone,” Dr. Cooper told attendees at the annual meeting of the College on Problems of Drug Dependence. Yet, early interviews she and her colleagues conducted with rural Kentucky pharmacist colleagues in the CARE2HOPE study “revealed that pharmacy-level barriers might also curtail access to buprenorphine.”
Little research has examined those barriers, but Dr. Cooper noted. Further, anecdotal evidence has suggested that wholesaler concerns about Drug Enforcement Administration restrictions on dispensing buprenorphine has caused shortages at pharmacies.
Dr. Cooper and colleagues, therefore, designed a qualitative study aimed at learning about pharmacists’ attitudes and dispensing practices related to buprenorphine. They also looked at whether DEA limits actually exist on dispensing the drug. They interviewed 14 pharmacists operating 15 pharmacies across all 12 counties in two rural Kentucky health districts. Eleven of the pharmacists worked in independent pharmacies; the others worked at chains. Six pharmacies dispensed more than 100 buprenorphine prescriptions a month, five dispensed only several dozen a month, and four refused to dispense it at all.
Perceptions of federal restrictions
“Variations in buprenorphine dispensing did not solely reflect underlying variations in local need or prescribing practices,” Dr. Cooper said. At 12 of the 15 pharmacies, limits on buprenorphine resulted from a perceived DEA “cap” on dispensing the drug or “because of distrust in buprenorphine itself, its prescribers and its patients.”
The perceived cap from the DEA was shrouded in uncertainty: 10 of the pharmacists said the DEA capped the percentage of controlled substances pharmacists could dispense that were opioids, yet the pharmacists did not know what that percentage was.
Five of those interviewed said the cap often significantly cut short how many buprenorphine prescriptions they would dispense. Since they did not know how much the cap was, they internally set arbitrary limits, such as dispensing two prescriptions per day, to avoid risk of the DEA investigating their pharmacy.
Yet, those limits could not meet patient demand, so several pharmacists rationed buprenorphine only to local residents or long-term customers, causing additional problems. That practice strained relationships with prescribers, who then had to call multiple pharmacies to find one that would dispense the drug to new patients. It also put pharmacy staff at risk when a rejection angered a customer and “undermined local recovery efforts,” Dr. Cooper said.
Five other pharmacists, however, did not ration their buprenorphine and did not worry about exceeding the DEA cap.
No numerical cap appears to exist, but DEA regulations and the SUPPORT for Patients and Communities Act do require internal opioid surveillance systems at wholesalers that flag suspicious orders of controlled substances, including buprenorphine. And they enforce it: An $80 million settlement in 2013 resulted from the DEA’s charge that Walgreens distribution centers did not report suspicious drug orders.
Stigma among some pharmacists
Six of the pharmacists had low trust in buprenorphine and in those who prescribed it and used it, Dr. Cooper reported. Three would not dispense the drug at all, and two would not take new buprenorphine patients.
One such pharmacist told researchers: “It is supposed to be the drug to help them [recover.] They want Suboxone worse than they do the hydrocodone. … It’s not what it’s designed to be.”
Those pharmacists also reported believing that malpractice was common among prescribers, who, for example, did not provide required counseling to patients or did not quickly wean them off buprenorphine. The pharmacists perceived the physicians prescribing buprenorphine as doing so only to make more money, just as they had done by prescribing opioids in the first place.
Those pharmacists also believed the patients themselves sold buprenorphine to make money and that opioid use disorder was a choice. They told researchers that dispensing buprenorphine would bring more drug users to their stores and subsequently hurt business.
Yet, those beliefs were not universal among the pharmacists. Eight believed buprenorphine was an appropriate opioid use disorder treatment and had positive attitudes toward patients. Unlike those who viewed the disorder as a choice, those pharmacists saw it as a disease and viewed the patients admirably for their commitment to recovery.
Though a small, qualitative study, those findings suggest a need to more closely examine how pharmacies affect access to medication to treat opioid use disorder, Dr. Cooper said.
“In an epicenter of the U.S. opioid epidemic, policies and stigma curtail access to buprenorphine,” she told attendees. “DEA regulations, the SUPPORT Act, and related lawsuits have led wholesalers to develop proprietary caps that force some pharmacists to ration the number of buprenorphine prescriptions they filled.” Some pharmacists will not dispense the drug at all, while others “limited dispensing to known or local patients and prescribers, a practice that pharmacists recognized hurt patients who had to travel far to reach prescribers.”
The research was funded by the National Institutes of Health through CARE2HOPE, Rural Health Project, and the Emory Center for AIDS Research. The authors reported no disclosures.
REPORTING FROM CPDD 2019
Durvalumab fails to advance in pancreatic cancer
, according to results of a phase 2 randomized controlled trial.
“Immune checkpoint blockade in PDAC as a single-agent therapy was not currently indicated beyond the subgroup of patients with microsatellite instability or mismatch repair deficiency ... however, a precedent existed for evaluating a combination of 2 immune checkpoint antagonists in this setting,” noted the investigators, led by Eileen M. O’Reilly, MD, gastrointestinal medical oncology, David M. Rubenstein Center for Pancreatic Cancer, Memorial Sloan Kettering Cancer Center and Cornell University, New York. In particular, a combination of agents that inhibit programmed cell death-1 ligand 1 (PD-L1) and the human T-cell receptor protein cytotoxic T-lymphocyte-associated protein 4 (CTLA4), nonredundant mechanisms, has shown promise.
Dr. O’Reilly and coinvestigators treated 65 patients in the trial’s initial cohort who had received only a single first-line fluorouracil- or gemcitabine-based chemotherapy regimen for recurrent or metastatic PDAC. Patients were randomized to combination therapy with durvalumab (Imfinzi), an anti-PD-L1 antibody, and tremelimumab, an investigational anti-CTLA4 antibody, followed by durvalumab alone, or to durvalumab monotherapy.
The objective response rate was just 3.1% with combination therapy and 0% with monotherapy—values that fell far short of the predefined 10% rate needed to initiate a planned expansion cohort. Both groups had a median progression-free survival of 1.5 months, Dr. O’Reilly and associates wrote. Their report is in JAMA Oncology.
The rate of grade 3 or higher treatment-related adverse events was 22% in the combination therapy group and 6% in the monotherapy group. In both groups, the most common events were fatigue and diarrhea. Some 6% and 3% of patients, respectively, stopped treatment because of a treatment-related adverse event.
The small trial population precluded detailed analyses of associations between treatment response and PD-L1 expression or microsatellite instability status.
“The observed efficacy of durvalumab plus tremelimumab therapy and durvalumab monotherapy was reflective of a population of patients with [metastatic PDAC] who had poor prognoses and rapidly progressing disease; however, treatment was well tolerated,” Dr. O’Reilly and coinvestigators wrote.
“Future studies are needed to evaluate how to best combine immune checkpoint blockade with other agents, including cytotoxic and targeted therapies, with the intention of overcoming the unique immunosuppressive, hypoxic, and fibrotic tumor microenvironment of PDAC. Such studies should evaluate biomarker expression to identify patients most likely to benefit from immune checkpoint blockade,” they recommended.
Dr. O’Reilly disclosed holding a consulting or advisory role or receiving grants from numerous pharmaceutical companies, including AstraZeneca, which funded the study.
SOURCE: O’Reilly EM et al. JAMA Oncol. 2019 July 18. doi:10.1001/jamaoncol.2019.1588.
“This study clearly and soberly demonstrates that despite the observed clinical benefits of dual ICI [immune check-point inhibition] therapy appreciated in other tumor types, PDAC remains refractory to standalone dual ICI therapy,” Dan A. Laheru, MD, and colleagues wrote in an invited commentary. “The priming of antitumor T-cell responses in the draining lymph nodes by anti-CTLA-4 therapy, tremelimumab, appears to be insufficient in priming T cells in PDAC for the addition of PD-L1 therapy.”
Current evidence suggests two main challenges will have to be overcome to pave the way for effective ICI therapy in PDAC and similarly nonimmunogenic cancers, they proposed. One will be inducing high-quality effector T cells into the tumor microenvironment (TME); the other will be reprogramming this “extremely immunosuppressive” milieu.
“Although there remains a rationale for testing dual checkpoint blockade therapy in patients with PDAC, this strategy will likely need to include agents that will first trigger the trafficking of T cells into the otherwise T-cell-poor tumor so that T cells are available for activation by ICIs. Furthermore, other agents need to be further tested in combination that would effectively reprogram the otherwise immunosuppressive PDAC TME to optimize T-cell function by turning off inhibitory signals,” Dr. Laheru and colleagues noted. “This study also strongly suggests that we should no longer test stand-alone ICI monotherapy or dual ICI in patients with PDAC without a T-cell inducing agent, whether that is a personalized vaccine-based therapy, small-molecule/antibody immunomodulator, or another immunotherapy agent altogether.
“The road to developing improved immunotherapy for patients with PDAC remains challenging,” they concluded. “Through the results of such work presented by the authors along with a greater understanding of the immune microenvironment, it is our hope that subsequent trials will allow future patients with PDAC to realize the benefits of immunotherapy that have helped so many in other cancer types.”
Arsen Osipov, MD, Neeha Zaidi, MD, and Dan A. Laheru, MD, are with the Skip Viragh Center for Pancreatic Cancer Research and Clinical Care, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
“This study clearly and soberly demonstrates that despite the observed clinical benefits of dual ICI [immune check-point inhibition] therapy appreciated in other tumor types, PDAC remains refractory to standalone dual ICI therapy,” Dan A. Laheru, MD, and colleagues wrote in an invited commentary. “The priming of antitumor T-cell responses in the draining lymph nodes by anti-CTLA-4 therapy, tremelimumab, appears to be insufficient in priming T cells in PDAC for the addition of PD-L1 therapy.”
Current evidence suggests two main challenges will have to be overcome to pave the way for effective ICI therapy in PDAC and similarly nonimmunogenic cancers, they proposed. One will be inducing high-quality effector T cells into the tumor microenvironment (TME); the other will be reprogramming this “extremely immunosuppressive” milieu.
“Although there remains a rationale for testing dual checkpoint blockade therapy in patients with PDAC, this strategy will likely need to include agents that will first trigger the trafficking of T cells into the otherwise T-cell-poor tumor so that T cells are available for activation by ICIs. Furthermore, other agents need to be further tested in combination that would effectively reprogram the otherwise immunosuppressive PDAC TME to optimize T-cell function by turning off inhibitory signals,” Dr. Laheru and colleagues noted. “This study also strongly suggests that we should no longer test stand-alone ICI monotherapy or dual ICI in patients with PDAC without a T-cell inducing agent, whether that is a personalized vaccine-based therapy, small-molecule/antibody immunomodulator, or another immunotherapy agent altogether.
“The road to developing improved immunotherapy for patients with PDAC remains challenging,” they concluded. “Through the results of such work presented by the authors along with a greater understanding of the immune microenvironment, it is our hope that subsequent trials will allow future patients with PDAC to realize the benefits of immunotherapy that have helped so many in other cancer types.”
Arsen Osipov, MD, Neeha Zaidi, MD, and Dan A. Laheru, MD, are with the Skip Viragh Center for Pancreatic Cancer Research and Clinical Care, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
“This study clearly and soberly demonstrates that despite the observed clinical benefits of dual ICI [immune check-point inhibition] therapy appreciated in other tumor types, PDAC remains refractory to standalone dual ICI therapy,” Dan A. Laheru, MD, and colleagues wrote in an invited commentary. “The priming of antitumor T-cell responses in the draining lymph nodes by anti-CTLA-4 therapy, tremelimumab, appears to be insufficient in priming T cells in PDAC for the addition of PD-L1 therapy.”
Current evidence suggests two main challenges will have to be overcome to pave the way for effective ICI therapy in PDAC and similarly nonimmunogenic cancers, they proposed. One will be inducing high-quality effector T cells into the tumor microenvironment (TME); the other will be reprogramming this “extremely immunosuppressive” milieu.
“Although there remains a rationale for testing dual checkpoint blockade therapy in patients with PDAC, this strategy will likely need to include agents that will first trigger the trafficking of T cells into the otherwise T-cell-poor tumor so that T cells are available for activation by ICIs. Furthermore, other agents need to be further tested in combination that would effectively reprogram the otherwise immunosuppressive PDAC TME to optimize T-cell function by turning off inhibitory signals,” Dr. Laheru and colleagues noted. “This study also strongly suggests that we should no longer test stand-alone ICI monotherapy or dual ICI in patients with PDAC without a T-cell inducing agent, whether that is a personalized vaccine-based therapy, small-molecule/antibody immunomodulator, or another immunotherapy agent altogether.
“The road to developing improved immunotherapy for patients with PDAC remains challenging,” they concluded. “Through the results of such work presented by the authors along with a greater understanding of the immune microenvironment, it is our hope that subsequent trials will allow future patients with PDAC to realize the benefits of immunotherapy that have helped so many in other cancer types.”
Arsen Osipov, MD, Neeha Zaidi, MD, and Dan A. Laheru, MD, are with the Skip Viragh Center for Pancreatic Cancer Research and Clinical Care, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore.
, according to results of a phase 2 randomized controlled trial.
“Immune checkpoint blockade in PDAC as a single-agent therapy was not currently indicated beyond the subgroup of patients with microsatellite instability or mismatch repair deficiency ... however, a precedent existed for evaluating a combination of 2 immune checkpoint antagonists in this setting,” noted the investigators, led by Eileen M. O’Reilly, MD, gastrointestinal medical oncology, David M. Rubenstein Center for Pancreatic Cancer, Memorial Sloan Kettering Cancer Center and Cornell University, New York. In particular, a combination of agents that inhibit programmed cell death-1 ligand 1 (PD-L1) and the human T-cell receptor protein cytotoxic T-lymphocyte-associated protein 4 (CTLA4), nonredundant mechanisms, has shown promise.
Dr. O’Reilly and coinvestigators treated 65 patients in the trial’s initial cohort who had received only a single first-line fluorouracil- or gemcitabine-based chemotherapy regimen for recurrent or metastatic PDAC. Patients were randomized to combination therapy with durvalumab (Imfinzi), an anti-PD-L1 antibody, and tremelimumab, an investigational anti-CTLA4 antibody, followed by durvalumab alone, or to durvalumab monotherapy.
The objective response rate was just 3.1% with combination therapy and 0% with monotherapy—values that fell far short of the predefined 10% rate needed to initiate a planned expansion cohort. Both groups had a median progression-free survival of 1.5 months, Dr. O’Reilly and associates wrote. Their report is in JAMA Oncology.
The rate of grade 3 or higher treatment-related adverse events was 22% in the combination therapy group and 6% in the monotherapy group. In both groups, the most common events were fatigue and diarrhea. Some 6% and 3% of patients, respectively, stopped treatment because of a treatment-related adverse event.
The small trial population precluded detailed analyses of associations between treatment response and PD-L1 expression or microsatellite instability status.
“The observed efficacy of durvalumab plus tremelimumab therapy and durvalumab monotherapy was reflective of a population of patients with [metastatic PDAC] who had poor prognoses and rapidly progressing disease; however, treatment was well tolerated,” Dr. O’Reilly and coinvestigators wrote.
“Future studies are needed to evaluate how to best combine immune checkpoint blockade with other agents, including cytotoxic and targeted therapies, with the intention of overcoming the unique immunosuppressive, hypoxic, and fibrotic tumor microenvironment of PDAC. Such studies should evaluate biomarker expression to identify patients most likely to benefit from immune checkpoint blockade,” they recommended.
Dr. O’Reilly disclosed holding a consulting or advisory role or receiving grants from numerous pharmaceutical companies, including AstraZeneca, which funded the study.
SOURCE: O’Reilly EM et al. JAMA Oncol. 2019 July 18. doi:10.1001/jamaoncol.2019.1588.
, according to results of a phase 2 randomized controlled trial.
“Immune checkpoint blockade in PDAC as a single-agent therapy was not currently indicated beyond the subgroup of patients with microsatellite instability or mismatch repair deficiency ... however, a precedent existed for evaluating a combination of 2 immune checkpoint antagonists in this setting,” noted the investigators, led by Eileen M. O’Reilly, MD, gastrointestinal medical oncology, David M. Rubenstein Center for Pancreatic Cancer, Memorial Sloan Kettering Cancer Center and Cornell University, New York. In particular, a combination of agents that inhibit programmed cell death-1 ligand 1 (PD-L1) and the human T-cell receptor protein cytotoxic T-lymphocyte-associated protein 4 (CTLA4), nonredundant mechanisms, has shown promise.
Dr. O’Reilly and coinvestigators treated 65 patients in the trial’s initial cohort who had received only a single first-line fluorouracil- or gemcitabine-based chemotherapy regimen for recurrent or metastatic PDAC. Patients were randomized to combination therapy with durvalumab (Imfinzi), an anti-PD-L1 antibody, and tremelimumab, an investigational anti-CTLA4 antibody, followed by durvalumab alone, or to durvalumab monotherapy.
The objective response rate was just 3.1% with combination therapy and 0% with monotherapy—values that fell far short of the predefined 10% rate needed to initiate a planned expansion cohort. Both groups had a median progression-free survival of 1.5 months, Dr. O’Reilly and associates wrote. Their report is in JAMA Oncology.
The rate of grade 3 or higher treatment-related adverse events was 22% in the combination therapy group and 6% in the monotherapy group. In both groups, the most common events were fatigue and diarrhea. Some 6% and 3% of patients, respectively, stopped treatment because of a treatment-related adverse event.
The small trial population precluded detailed analyses of associations between treatment response and PD-L1 expression or microsatellite instability status.
“The observed efficacy of durvalumab plus tremelimumab therapy and durvalumab monotherapy was reflective of a population of patients with [metastatic PDAC] who had poor prognoses and rapidly progressing disease; however, treatment was well tolerated,” Dr. O’Reilly and coinvestigators wrote.
“Future studies are needed to evaluate how to best combine immune checkpoint blockade with other agents, including cytotoxic and targeted therapies, with the intention of overcoming the unique immunosuppressive, hypoxic, and fibrotic tumor microenvironment of PDAC. Such studies should evaluate biomarker expression to identify patients most likely to benefit from immune checkpoint blockade,” they recommended.
Dr. O’Reilly disclosed holding a consulting or advisory role or receiving grants from numerous pharmaceutical companies, including AstraZeneca, which funded the study.
SOURCE: O’Reilly EM et al. JAMA Oncol. 2019 July 18. doi:10.1001/jamaoncol.2019.1588.
FROM JAMA ONCOLOGY
Follow-up after mental illness admission: State scorecard
Follow-up rates after hospitalization for mental illness varied considerably among the states, ranging from 38% to 89% in 2016, according to the Centers for Medicare & Medicaid Services.
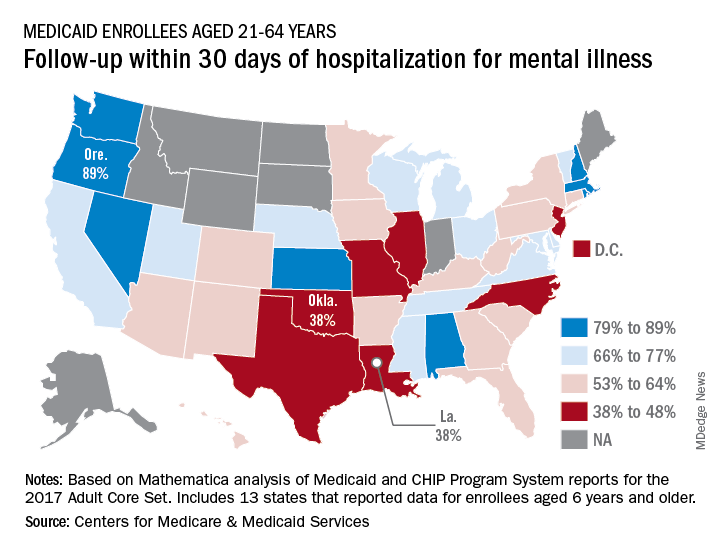
All that variation added up to a national median of 64% of Medicaid enrollees who saw a mental health clinician within 30 days of discharge, CMS reported in its Medicaid and Children’s Health Insurance Program Scorecard.
The other states in the top five were Nevada (85%), Rhode Island (82%), and New Hampshire (80%), scoreboard data show.
In Louisiana and Oklahoma, the states with the lowest rate, only 38% of Medicaid enrollees had a follow-up visit within 30 days of their discharge. The next three rungs on the follow-up ladder were occupied by New Jersey (43%), Illinois (44%), and North Carolina (44%), the CMS said after a recent refresh of data in the scorecard.
“Everyone – whether you are a beneficiary, taxpayer, or lawmaker – deserves to understand the performance of our nation’s largest health coverage programs and often the largest state expenses,” CMS Administrator Seema Verma said in a written statement. “More and more states are voluntarily reporting their health outcomes in the scorecard, and the new data is leading us into an era of increased transparency and accountability, so that together we can improve the quality of care we give to the vulnerable Americans that depend on this vital program.”
Data for this measure “reflect state reporting for Medicaid enrollees ages 21 to 64” years, the CMS noted, but eight states did not report at all. A total of 13 states reported on enrollees aged 6 years and older, 2 states reported results for those aged 21 and older, and 1 state reported data for ages 6-64.
“The included populations … can vary by state. For example, some states report data on certain populations such as those covered under managed care but not those covered under fee for service. This variation in data can affect measure performance and comparisons between states,” the CMS said.
Follow-up rates after hospitalization for mental illness varied considerably among the states, ranging from 38% to 89% in 2016, according to the Centers for Medicare & Medicaid Services.

All that variation added up to a national median of 64% of Medicaid enrollees who saw a mental health clinician within 30 days of discharge, CMS reported in its Medicaid and Children’s Health Insurance Program Scorecard.
The other states in the top five were Nevada (85%), Rhode Island (82%), and New Hampshire (80%), scoreboard data show.
In Louisiana and Oklahoma, the states with the lowest rate, only 38% of Medicaid enrollees had a follow-up visit within 30 days of their discharge. The next three rungs on the follow-up ladder were occupied by New Jersey (43%), Illinois (44%), and North Carolina (44%), the CMS said after a recent refresh of data in the scorecard.
“Everyone – whether you are a beneficiary, taxpayer, or lawmaker – deserves to understand the performance of our nation’s largest health coverage programs and often the largest state expenses,” CMS Administrator Seema Verma said in a written statement. “More and more states are voluntarily reporting their health outcomes in the scorecard, and the new data is leading us into an era of increased transparency and accountability, so that together we can improve the quality of care we give to the vulnerable Americans that depend on this vital program.”
Data for this measure “reflect state reporting for Medicaid enrollees ages 21 to 64” years, the CMS noted, but eight states did not report at all. A total of 13 states reported on enrollees aged 6 years and older, 2 states reported results for those aged 21 and older, and 1 state reported data for ages 6-64.
“The included populations … can vary by state. For example, some states report data on certain populations such as those covered under managed care but not those covered under fee for service. This variation in data can affect measure performance and comparisons between states,” the CMS said.
Follow-up rates after hospitalization for mental illness varied considerably among the states, ranging from 38% to 89% in 2016, according to the Centers for Medicare & Medicaid Services.

All that variation added up to a national median of 64% of Medicaid enrollees who saw a mental health clinician within 30 days of discharge, CMS reported in its Medicaid and Children’s Health Insurance Program Scorecard.
The other states in the top five were Nevada (85%), Rhode Island (82%), and New Hampshire (80%), scoreboard data show.
In Louisiana and Oklahoma, the states with the lowest rate, only 38% of Medicaid enrollees had a follow-up visit within 30 days of their discharge. The next three rungs on the follow-up ladder were occupied by New Jersey (43%), Illinois (44%), and North Carolina (44%), the CMS said after a recent refresh of data in the scorecard.
“Everyone – whether you are a beneficiary, taxpayer, or lawmaker – deserves to understand the performance of our nation’s largest health coverage programs and often the largest state expenses,” CMS Administrator Seema Verma said in a written statement. “More and more states are voluntarily reporting their health outcomes in the scorecard, and the new data is leading us into an era of increased transparency and accountability, so that together we can improve the quality of care we give to the vulnerable Americans that depend on this vital program.”
Data for this measure “reflect state reporting for Medicaid enrollees ages 21 to 64” years, the CMS noted, but eight states did not report at all. A total of 13 states reported on enrollees aged 6 years and older, 2 states reported results for those aged 21 and older, and 1 state reported data for ages 6-64.
“The included populations … can vary by state. For example, some states report data on certain populations such as those covered under managed care but not those covered under fee for service. This variation in data can affect measure performance and comparisons between states,” the CMS said.
Top Qualifications Hospitalist Leaders Seek in Candidates: Results from a National Survey
Hospital Medicine (HM) is medicine’s fastest growing specialty.1 Rapid expansion of the field has been met with rising interest by young physicians, many of whom are first-time job seekers and may desire information on best practices for applying and interviewing in HM.2-4 However, no prior work has examined HM-specific candidate qualifications and qualities that may be most valued in the hiring process.
As members of the Society of Hospital Medicine (SHM) Physicians in Training Committee, a group charged with “prepar[ing] trainees and early career hospitalists in their transition into hospital medicine,” we aimed to fill this knowledge gap around the HM-specific hiring process.
METHODS
Survey Instrument
The authors developed the survey based on expertise as HM interviewers (JAD, AH, CD, EE, BK, DS, and SM) and local and national interview workshop leaders (JAD, CD, BK, SM). The questionnaire focused on objective applicant qualifications, qualities and attributes displayed during interviews (Appendix 1). Content, length, and reliability of physician understanding were assessed via feedback from local HM group leaders.
Respondents were asked to provide nonidentifying demographics and their role in their HM group’s hiring process. If they reported no role, the survey was terminated. Subsequent standardized HM group demographic questions were adapted from the Society of Hospital Medicine (SHM) State of Hospital Medicine Report.5
Survey questions were multiple choice, ranking and free-response aimed at understanding how respondents assess HM candidate attributes, skills, and behavior. For ranking questions, answer choice order was randomized to reduce answer order-based bias. One free-response question asked the respondent to provide a unique interview question they use that “reveals the most about a hospitalist candidate.” Responses were then individually inserted into the list of choices for a subsequent ranking question regarding the most important qualities a candidate must demonstrate.
Respondents were asked four open-ended questions designed to understand the approach to candidate assessment: (1) use of unique interview questions (as above); (2) identification of “red flags” during interviews; (3) distinctions between assessment of long-term (LT) career hospitalist candidates versus short-term (ST) candidates (eg, those seeking positions prior to fellowship); and (4) key qualifications of ST candidates.
Survey Administration
Survey recipients were identified via SHM administrative rosters. Surveys were distributed electronically via SHM to all current nontrainee physician members who reported a United States mailing address. The survey was determined to not constitute human subjects research by the Beth Israel Deaconess Medical Center Committee on Clinical Investigations.
Data Analysis
Multiple-choice responses were analyzed descriptively. For ranking-type questions, answers were weighted based on ranking order.
Responses to all open-ended survey questions were analyzed using thematic analysis. We used an iterative process to develop and refine codes identifying key concepts that emerged from the data. Three authors independently coded survey responses. As a group, research team members established the coding framework and resolved discrepancies via discussion to achieve consensus.
RESULTS
Survey links were sent to 8,398 e-mail addresses, of which 7,306 were undeliverable or unopened, leaving 1,092 total eligible respondents. Of these, 347 (31.8%) responded.
A total of 236 respondents reported having a formal role in HM hiring. Of these roles, 79.0% were one-on-one interviewers, 49.6% group interviewers, 45.5% telephone/videoconference interviewers, 41.5% participated on a selection committee, and 32.1% identified as the ultimate decision-maker. Regarding graduate medical education teaching status, 42.0% of respondents identified their primary workplace as a community/affiliated teaching hospital, 33.05% as a university-based teaching hospital, and 23.0% as a nonteaching hospital. Additional characteristics are reported in Appendix 2.
Quantitative Analysis
Respondents ranked the top five qualifications of HM candidates and the top five qualities a candidate should demonstrate on the interview day to be considered for hiring (Table 1).
When asked to rate agreement with the statement “I evaluate and consider all hospital medicine candidates similarly, regardless of whether they articulate an interest in hospital medicine as a long-term career or as a short-term position before fellowship,” 99 (57.23%) respondents disagreed.
Qualitative Analysis
Thematic analysis of responses to open-ended survey questions identified several “red flag” themes (Table 2). Negative interactions with current providers or staff were commonly noted. Additional red flags were a lack of knowledge or interest in the specific HM group, an inability to articulate career goals, or abnormalities in employment history or application materials. Respondents identified an overly strong focus on lifestyle or salary as factors that might limit a candidate’s chance of advancing in the hiring process.
Responses to free-text questions additionally highlighted preferred questioning techniques and approaches to HM candidate assessment (Appendix 3). Many interview questions addressed candidate interest in a particular HM program and candidate responses to challenging scenarios they had encountered. Other questions explored career development. Respondents wanted LT candidates to have specific HM career goals, while they expected ST candidates to demonstrate commitment to and appreciation of HM as a discipline.
Some respondents described their approach to candidate assessment in terms of investment and risk. LT candidates were often viewed as investments in stability and performance; they were evaluated on current abilities and future potential as related to group-specific goals. Some respondents viewed hiring ST candidates as more risky given concerns that they might be less engaged or integrated with the group. Others viewed the hiring of LT candidates as comparably more risky, relating the longer time commitment to the potential for higher impact on the group and patient care. Accordingly, these respondents viewed ST candidate hiring as less risky, estimating their shorter time commitment as having less of a positive or negative impact, with the benefit of addressing urgent staffing issues or unfilled less desirable positions. One respondent summarized: “If they plan to be a career candidate, I care more about them as people and future coworkers. Short term folks are great if we are in a pinch and can deal with personality issues for a short period of time.”
Respondents also described how valued candidate qualities could help mitigate the risk inherent in hiring, especially for ST hires. Strong interpersonal and teamwork skills were highlighted, as well as a demonstrated record of clinical excellence, evidenced by strong training backgrounds and superlative references. A key factor aiding in ST hiring decisions was prior knowledge of the candidate, such as residents or moonlighters previously working in the respondent’s institution. This allowed for familiarity with the candidate’s clinical acumen as well as perceived ease of onboarding and knowledge of the system.
DISCUSSION
We present the results of a national survey of hospitalists identifying candidate attributes, skills, and behaviors viewed most favorably by those involved in the HM hiring process. To our knowledge, this is the first research to be published on the topic of evaluating HM candidates.
Survey respondents identified demonstrable HM candidate clinical skills and experience as highly important, consistent with prior research identifying clinical skills as being among those that hospitalists most value.6 Based on these responses, job seekers should be prepared to discuss objective measures of clinical experience when appropriate, such as number of cases seen or procedures performed. HM groups may accordingly consider the use of hiring rubrics or scoring systems to standardize these measures and reduce bias.
Respondents also highly valued more subjective assessments of HM applicants’ candidacy. The most highly ranked action item was a candidate’s ability to meaningfully respond to a respondent’s customized interview question. There was also a preference for candidates who were knowledgeable about and interested in the specifics of a particular HM group. The high value placed on these elements may suggest the need for formalized coaching or interview preparation for HM candidates. Similarly, interviewer emphasis on customized questions may also highlight an opportunity for HM groups to internally standardize how to best approach subjective components of the interview.
Our heterogeneous findings on the distinctions between ST and LT candidate hiring practices support the need for additional research on the ST HM job market. Until then, our findings reinforce the importance of applicant transparency about ST versus LT career goals. Although many programs may prefer LT candidates over ST candidates, our results suggest ST candidates may benefit from targeting groups with ST needs and using the application process as an opportunity to highlight certain mitigating strengths.
Our study has limitations. While our population included diverse national representation, the response rate and demographics of our respondents may limit generalizability beyond our study population. Respondents represented multiple perspectives within the HM hiring process and were not limited to those making the final hiring decisions. For questions with prespecified multiple-choice answers, answer choices may have influenced participant responses. Our conclusions are based on the reported preferences of those involved in the HM hiring process and not actual hiring behavior. Future research should attempt to identify factors (eg, region, graduate medical education status, practice setting type) that may be responsible for some of the heterogeneous themes we observed in our analysis.
Our research represents introductory work into the previously unpublished topic of HM-specific hiring practices. These findings may provide relevant insight for trainees considering careers in HM, hospitalists reentering the job market, and those involved in career advising, professional development and the HM hiring process.
Acknowledgments
The authors would like to acknowledge current and former members of SHM’s Physicians in Training Committee whose feedback and leadership helped to inspire this project, as well as those students, residents, and hospitalists who have participated in our Hospital Medicine Annual Meeting interview workshop.
Disclosures
The authors have no conflicts of interest to disclose.
1. Wachter RM, Goldman L. Zero to 50,000-The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
2. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
3. Ratelle JT, Dupras DM, Alguire P, Masters P, Weissman A, West CP. Hospitalist career decisions among internal medicine residents. J Gen Intern Med. 2014;29(7):1026-1030. doi: 10.1007/s11606-014-2811-3.
4. Sweigart JR, Tad-Y D, Kneeland P, Williams MV, Glasheen JJ. Hospital medicine resident training tracks: developing the hospital medicine pipeline. J Hosp Med. 2017;12(3):173-176. doi: 10.12788/jhm.2703.
5. 2016 State of Hospital Medicine Report. 2016. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/. Accessed 7/1/2017.
6. Plauth WH, 3rd, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Emerg Med. 2001;111(3):247-254. doi: https://doi.org/10.1016/S0002-9343(01)00837-3.
Hospital Medicine (HM) is medicine’s fastest growing specialty.1 Rapid expansion of the field has been met with rising interest by young physicians, many of whom are first-time job seekers and may desire information on best practices for applying and interviewing in HM.2-4 However, no prior work has examined HM-specific candidate qualifications and qualities that may be most valued in the hiring process.
As members of the Society of Hospital Medicine (SHM) Physicians in Training Committee, a group charged with “prepar[ing] trainees and early career hospitalists in their transition into hospital medicine,” we aimed to fill this knowledge gap around the HM-specific hiring process.
METHODS
Survey Instrument
The authors developed the survey based on expertise as HM interviewers (JAD, AH, CD, EE, BK, DS, and SM) and local and national interview workshop leaders (JAD, CD, BK, SM). The questionnaire focused on objective applicant qualifications, qualities and attributes displayed during interviews (Appendix 1). Content, length, and reliability of physician understanding were assessed via feedback from local HM group leaders.
Respondents were asked to provide nonidentifying demographics and their role in their HM group’s hiring process. If they reported no role, the survey was terminated. Subsequent standardized HM group demographic questions were adapted from the Society of Hospital Medicine (SHM) State of Hospital Medicine Report.5
Survey questions were multiple choice, ranking and free-response aimed at understanding how respondents assess HM candidate attributes, skills, and behavior. For ranking questions, answer choice order was randomized to reduce answer order-based bias. One free-response question asked the respondent to provide a unique interview question they use that “reveals the most about a hospitalist candidate.” Responses were then individually inserted into the list of choices for a subsequent ranking question regarding the most important qualities a candidate must demonstrate.
Respondents were asked four open-ended questions designed to understand the approach to candidate assessment: (1) use of unique interview questions (as above); (2) identification of “red flags” during interviews; (3) distinctions between assessment of long-term (LT) career hospitalist candidates versus short-term (ST) candidates (eg, those seeking positions prior to fellowship); and (4) key qualifications of ST candidates.
Survey Administration
Survey recipients were identified via SHM administrative rosters. Surveys were distributed electronically via SHM to all current nontrainee physician members who reported a United States mailing address. The survey was determined to not constitute human subjects research by the Beth Israel Deaconess Medical Center Committee on Clinical Investigations.
Data Analysis
Multiple-choice responses were analyzed descriptively. For ranking-type questions, answers were weighted based on ranking order.
Responses to all open-ended survey questions were analyzed using thematic analysis. We used an iterative process to develop and refine codes identifying key concepts that emerged from the data. Three authors independently coded survey responses. As a group, research team members established the coding framework and resolved discrepancies via discussion to achieve consensus.
RESULTS
Survey links were sent to 8,398 e-mail addresses, of which 7,306 were undeliverable or unopened, leaving 1,092 total eligible respondents. Of these, 347 (31.8%) responded.
A total of 236 respondents reported having a formal role in HM hiring. Of these roles, 79.0% were one-on-one interviewers, 49.6% group interviewers, 45.5% telephone/videoconference interviewers, 41.5% participated on a selection committee, and 32.1% identified as the ultimate decision-maker. Regarding graduate medical education teaching status, 42.0% of respondents identified their primary workplace as a community/affiliated teaching hospital, 33.05% as a university-based teaching hospital, and 23.0% as a nonteaching hospital. Additional characteristics are reported in Appendix 2.
Quantitative Analysis
Respondents ranked the top five qualifications of HM candidates and the top five qualities a candidate should demonstrate on the interview day to be considered for hiring (Table 1).
When asked to rate agreement with the statement “I evaluate and consider all hospital medicine candidates similarly, regardless of whether they articulate an interest in hospital medicine as a long-term career or as a short-term position before fellowship,” 99 (57.23%) respondents disagreed.
Qualitative Analysis
Thematic analysis of responses to open-ended survey questions identified several “red flag” themes (Table 2). Negative interactions with current providers or staff were commonly noted. Additional red flags were a lack of knowledge or interest in the specific HM group, an inability to articulate career goals, or abnormalities in employment history or application materials. Respondents identified an overly strong focus on lifestyle or salary as factors that might limit a candidate’s chance of advancing in the hiring process.
Responses to free-text questions additionally highlighted preferred questioning techniques and approaches to HM candidate assessment (Appendix 3). Many interview questions addressed candidate interest in a particular HM program and candidate responses to challenging scenarios they had encountered. Other questions explored career development. Respondents wanted LT candidates to have specific HM career goals, while they expected ST candidates to demonstrate commitment to and appreciation of HM as a discipline.
Some respondents described their approach to candidate assessment in terms of investment and risk. LT candidates were often viewed as investments in stability and performance; they were evaluated on current abilities and future potential as related to group-specific goals. Some respondents viewed hiring ST candidates as more risky given concerns that they might be less engaged or integrated with the group. Others viewed the hiring of LT candidates as comparably more risky, relating the longer time commitment to the potential for higher impact on the group and patient care. Accordingly, these respondents viewed ST candidate hiring as less risky, estimating their shorter time commitment as having less of a positive or negative impact, with the benefit of addressing urgent staffing issues or unfilled less desirable positions. One respondent summarized: “If they plan to be a career candidate, I care more about them as people and future coworkers. Short term folks are great if we are in a pinch and can deal with personality issues for a short period of time.”
Respondents also described how valued candidate qualities could help mitigate the risk inherent in hiring, especially for ST hires. Strong interpersonal and teamwork skills were highlighted, as well as a demonstrated record of clinical excellence, evidenced by strong training backgrounds and superlative references. A key factor aiding in ST hiring decisions was prior knowledge of the candidate, such as residents or moonlighters previously working in the respondent’s institution. This allowed for familiarity with the candidate’s clinical acumen as well as perceived ease of onboarding and knowledge of the system.
DISCUSSION
We present the results of a national survey of hospitalists identifying candidate attributes, skills, and behaviors viewed most favorably by those involved in the HM hiring process. To our knowledge, this is the first research to be published on the topic of evaluating HM candidates.
Survey respondents identified demonstrable HM candidate clinical skills and experience as highly important, consistent with prior research identifying clinical skills as being among those that hospitalists most value.6 Based on these responses, job seekers should be prepared to discuss objective measures of clinical experience when appropriate, such as number of cases seen or procedures performed. HM groups may accordingly consider the use of hiring rubrics or scoring systems to standardize these measures and reduce bias.
Respondents also highly valued more subjective assessments of HM applicants’ candidacy. The most highly ranked action item was a candidate’s ability to meaningfully respond to a respondent’s customized interview question. There was also a preference for candidates who were knowledgeable about and interested in the specifics of a particular HM group. The high value placed on these elements may suggest the need for formalized coaching or interview preparation for HM candidates. Similarly, interviewer emphasis on customized questions may also highlight an opportunity for HM groups to internally standardize how to best approach subjective components of the interview.
Our heterogeneous findings on the distinctions between ST and LT candidate hiring practices support the need for additional research on the ST HM job market. Until then, our findings reinforce the importance of applicant transparency about ST versus LT career goals. Although many programs may prefer LT candidates over ST candidates, our results suggest ST candidates may benefit from targeting groups with ST needs and using the application process as an opportunity to highlight certain mitigating strengths.
Our study has limitations. While our population included diverse national representation, the response rate and demographics of our respondents may limit generalizability beyond our study population. Respondents represented multiple perspectives within the HM hiring process and were not limited to those making the final hiring decisions. For questions with prespecified multiple-choice answers, answer choices may have influenced participant responses. Our conclusions are based on the reported preferences of those involved in the HM hiring process and not actual hiring behavior. Future research should attempt to identify factors (eg, region, graduate medical education status, practice setting type) that may be responsible for some of the heterogeneous themes we observed in our analysis.
Our research represents introductory work into the previously unpublished topic of HM-specific hiring practices. These findings may provide relevant insight for trainees considering careers in HM, hospitalists reentering the job market, and those involved in career advising, professional development and the HM hiring process.
Acknowledgments
The authors would like to acknowledge current and former members of SHM’s Physicians in Training Committee whose feedback and leadership helped to inspire this project, as well as those students, residents, and hospitalists who have participated in our Hospital Medicine Annual Meeting interview workshop.
Disclosures
The authors have no conflicts of interest to disclose.
Hospital Medicine (HM) is medicine’s fastest growing specialty.1 Rapid expansion of the field has been met with rising interest by young physicians, many of whom are first-time job seekers and may desire information on best practices for applying and interviewing in HM.2-4 However, no prior work has examined HM-specific candidate qualifications and qualities that may be most valued in the hiring process.
As members of the Society of Hospital Medicine (SHM) Physicians in Training Committee, a group charged with “prepar[ing] trainees and early career hospitalists in their transition into hospital medicine,” we aimed to fill this knowledge gap around the HM-specific hiring process.
METHODS
Survey Instrument
The authors developed the survey based on expertise as HM interviewers (JAD, AH, CD, EE, BK, DS, and SM) and local and national interview workshop leaders (JAD, CD, BK, SM). The questionnaire focused on objective applicant qualifications, qualities and attributes displayed during interviews (Appendix 1). Content, length, and reliability of physician understanding were assessed via feedback from local HM group leaders.
Respondents were asked to provide nonidentifying demographics and their role in their HM group’s hiring process. If they reported no role, the survey was terminated. Subsequent standardized HM group demographic questions were adapted from the Society of Hospital Medicine (SHM) State of Hospital Medicine Report.5
Survey questions were multiple choice, ranking and free-response aimed at understanding how respondents assess HM candidate attributes, skills, and behavior. For ranking questions, answer choice order was randomized to reduce answer order-based bias. One free-response question asked the respondent to provide a unique interview question they use that “reveals the most about a hospitalist candidate.” Responses were then individually inserted into the list of choices for a subsequent ranking question regarding the most important qualities a candidate must demonstrate.
Respondents were asked four open-ended questions designed to understand the approach to candidate assessment: (1) use of unique interview questions (as above); (2) identification of “red flags” during interviews; (3) distinctions between assessment of long-term (LT) career hospitalist candidates versus short-term (ST) candidates (eg, those seeking positions prior to fellowship); and (4) key qualifications of ST candidates.
Survey Administration
Survey recipients were identified via SHM administrative rosters. Surveys were distributed electronically via SHM to all current nontrainee physician members who reported a United States mailing address. The survey was determined to not constitute human subjects research by the Beth Israel Deaconess Medical Center Committee on Clinical Investigations.
Data Analysis
Multiple-choice responses were analyzed descriptively. For ranking-type questions, answers were weighted based on ranking order.
Responses to all open-ended survey questions were analyzed using thematic analysis. We used an iterative process to develop and refine codes identifying key concepts that emerged from the data. Three authors independently coded survey responses. As a group, research team members established the coding framework and resolved discrepancies via discussion to achieve consensus.
RESULTS
Survey links were sent to 8,398 e-mail addresses, of which 7,306 were undeliverable or unopened, leaving 1,092 total eligible respondents. Of these, 347 (31.8%) responded.
A total of 236 respondents reported having a formal role in HM hiring. Of these roles, 79.0% were one-on-one interviewers, 49.6% group interviewers, 45.5% telephone/videoconference interviewers, 41.5% participated on a selection committee, and 32.1% identified as the ultimate decision-maker. Regarding graduate medical education teaching status, 42.0% of respondents identified their primary workplace as a community/affiliated teaching hospital, 33.05% as a university-based teaching hospital, and 23.0% as a nonteaching hospital. Additional characteristics are reported in Appendix 2.
Quantitative Analysis
Respondents ranked the top five qualifications of HM candidates and the top five qualities a candidate should demonstrate on the interview day to be considered for hiring (Table 1).
When asked to rate agreement with the statement “I evaluate and consider all hospital medicine candidates similarly, regardless of whether they articulate an interest in hospital medicine as a long-term career or as a short-term position before fellowship,” 99 (57.23%) respondents disagreed.
Qualitative Analysis
Thematic analysis of responses to open-ended survey questions identified several “red flag” themes (Table 2). Negative interactions with current providers or staff were commonly noted. Additional red flags were a lack of knowledge or interest in the specific HM group, an inability to articulate career goals, or abnormalities in employment history or application materials. Respondents identified an overly strong focus on lifestyle or salary as factors that might limit a candidate’s chance of advancing in the hiring process.
Responses to free-text questions additionally highlighted preferred questioning techniques and approaches to HM candidate assessment (Appendix 3). Many interview questions addressed candidate interest in a particular HM program and candidate responses to challenging scenarios they had encountered. Other questions explored career development. Respondents wanted LT candidates to have specific HM career goals, while they expected ST candidates to demonstrate commitment to and appreciation of HM as a discipline.
Some respondents described their approach to candidate assessment in terms of investment and risk. LT candidates were often viewed as investments in stability and performance; they were evaluated on current abilities and future potential as related to group-specific goals. Some respondents viewed hiring ST candidates as more risky given concerns that they might be less engaged or integrated with the group. Others viewed the hiring of LT candidates as comparably more risky, relating the longer time commitment to the potential for higher impact on the group and patient care. Accordingly, these respondents viewed ST candidate hiring as less risky, estimating their shorter time commitment as having less of a positive or negative impact, with the benefit of addressing urgent staffing issues or unfilled less desirable positions. One respondent summarized: “If they plan to be a career candidate, I care more about them as people and future coworkers. Short term folks are great if we are in a pinch and can deal with personality issues for a short period of time.”
Respondents also described how valued candidate qualities could help mitigate the risk inherent in hiring, especially for ST hires. Strong interpersonal and teamwork skills were highlighted, as well as a demonstrated record of clinical excellence, evidenced by strong training backgrounds and superlative references. A key factor aiding in ST hiring decisions was prior knowledge of the candidate, such as residents or moonlighters previously working in the respondent’s institution. This allowed for familiarity with the candidate’s clinical acumen as well as perceived ease of onboarding and knowledge of the system.
DISCUSSION
We present the results of a national survey of hospitalists identifying candidate attributes, skills, and behaviors viewed most favorably by those involved in the HM hiring process. To our knowledge, this is the first research to be published on the topic of evaluating HM candidates.
Survey respondents identified demonstrable HM candidate clinical skills and experience as highly important, consistent with prior research identifying clinical skills as being among those that hospitalists most value.6 Based on these responses, job seekers should be prepared to discuss objective measures of clinical experience when appropriate, such as number of cases seen or procedures performed. HM groups may accordingly consider the use of hiring rubrics or scoring systems to standardize these measures and reduce bias.
Respondents also highly valued more subjective assessments of HM applicants’ candidacy. The most highly ranked action item was a candidate’s ability to meaningfully respond to a respondent’s customized interview question. There was also a preference for candidates who were knowledgeable about and interested in the specifics of a particular HM group. The high value placed on these elements may suggest the need for formalized coaching or interview preparation for HM candidates. Similarly, interviewer emphasis on customized questions may also highlight an opportunity for HM groups to internally standardize how to best approach subjective components of the interview.
Our heterogeneous findings on the distinctions between ST and LT candidate hiring practices support the need for additional research on the ST HM job market. Until then, our findings reinforce the importance of applicant transparency about ST versus LT career goals. Although many programs may prefer LT candidates over ST candidates, our results suggest ST candidates may benefit from targeting groups with ST needs and using the application process as an opportunity to highlight certain mitigating strengths.
Our study has limitations. While our population included diverse national representation, the response rate and demographics of our respondents may limit generalizability beyond our study population. Respondents represented multiple perspectives within the HM hiring process and were not limited to those making the final hiring decisions. For questions with prespecified multiple-choice answers, answer choices may have influenced participant responses. Our conclusions are based on the reported preferences of those involved in the HM hiring process and not actual hiring behavior. Future research should attempt to identify factors (eg, region, graduate medical education status, practice setting type) that may be responsible for some of the heterogeneous themes we observed in our analysis.
Our research represents introductory work into the previously unpublished topic of HM-specific hiring practices. These findings may provide relevant insight for trainees considering careers in HM, hospitalists reentering the job market, and those involved in career advising, professional development and the HM hiring process.
Acknowledgments
The authors would like to acknowledge current and former members of SHM’s Physicians in Training Committee whose feedback and leadership helped to inspire this project, as well as those students, residents, and hospitalists who have participated in our Hospital Medicine Annual Meeting interview workshop.
Disclosures
The authors have no conflicts of interest to disclose.
1. Wachter RM, Goldman L. Zero to 50,000-The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
2. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
3. Ratelle JT, Dupras DM, Alguire P, Masters P, Weissman A, West CP. Hospitalist career decisions among internal medicine residents. J Gen Intern Med. 2014;29(7):1026-1030. doi: 10.1007/s11606-014-2811-3.
4. Sweigart JR, Tad-Y D, Kneeland P, Williams MV, Glasheen JJ. Hospital medicine resident training tracks: developing the hospital medicine pipeline. J Hosp Med. 2017;12(3):173-176. doi: 10.12788/jhm.2703.
5. 2016 State of Hospital Medicine Report. 2016. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/. Accessed 7/1/2017.
6. Plauth WH, 3rd, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Emerg Med. 2001;111(3):247-254. doi: https://doi.org/10.1016/S0002-9343(01)00837-3.
1. Wachter RM, Goldman L. Zero to 50,000-The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958.
2. Leyenaar JK, Frintner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018;18(2):200-207. https://doi.org/10.1016/j.acap.2017.05.001.
3. Ratelle JT, Dupras DM, Alguire P, Masters P, Weissman A, West CP. Hospitalist career decisions among internal medicine residents. J Gen Intern Med. 2014;29(7):1026-1030. doi: 10.1007/s11606-014-2811-3.
4. Sweigart JR, Tad-Y D, Kneeland P, Williams MV, Glasheen JJ. Hospital medicine resident training tracks: developing the hospital medicine pipeline. J Hosp Med. 2017;12(3):173-176. doi: 10.12788/jhm.2703.
5. 2016 State of Hospital Medicine Report. 2016. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/. Accessed 7/1/2017.
6. Plauth WH, 3rd, Pantilat SZ, Wachter RM, Fenton CL. Hospitalists’ perceptions of their residency training needs: results of a national survey. Am J Emerg Med. 2001;111(3):247-254. doi: https://doi.org/10.1016/S0002-9343(01)00837-3.
© 2019 Society of Hospital Medicine
Discrepant Advanced Directives and Code Status Orders: A Preventable Medical Error
The United States health system has been criticized for its overuse of aggressive and medically ineffective life-sustaining therapies (LST).1 Some professional societies have elevated dialog about end-of-life (EOL) care to a quality measure,2 expecting that more open discussion will achieve more “goal-concordant care”3 and appropriate use of LST. However, even when Advanced Directives (AD) or Physician Orders for Life-Sustaining Therapy (POLST) have been created, their directions are not always followed in the hospital. This perspective discusses how preventable errors allow for use of LST even when patients designated it as unwanted. Two cases, chosen from several similar ones, are highlighted, demonstrating both human and system errors.
During the time of these events, the hospital policy required admission orders to contain a “code status” designation in the electronic medical record (EMR). All active and historical code status orders were listed chronologically and all AD and POLST documents were scanned into a special section of the EMR. Hospital policy, consistent with professional society guidelines,4,5 stated that patients with AD/POLST limiting EOL support should have individualized discussion about resuscitation options in the event of a periprocedural critical event. Automatic suspension or reinstatement of limited code orders was not permitted.
CASE 1
A 62-year-old woman with refractory heart failure was admitted with recurrence. The admitting code order was “initiate CPR/intubation” even though a POLST order written 10 months earlier indicating “do not intubate” was visible in the EMR. A more recent POLST indicating “No CPR/No intubation” accompanied the patient in the ambulance and was placed at bedside, but not scanned. There was no documented discussion of code status that might have explained the POLST/code order disparity. Notably, during two prior admissions within the year, “full code” orders had also been placed. On the fifth hospital day, the patient was found in respiratory distress and unresponsive. A “code” was called. ICU staff, after confirming full code status, intubated the patient emergently and commenced other invasive ICU interventions. Family members brought the preexisting POLST to medical attention within hours of the code but could not agree on immediate extubation. Over the next week, multiple prognosis discussions were held with the patient (when responsive) and family. Ultimately, the patient failed to improve and indicated a desire to be extubated, dying a few hours later.
CASE 2
A 94-year-old woman was admitted from assisted living with a traumatic subcapital femur fracture. Admission code orders were “initiate CPR/intubation” despite the presence in the EMR of a POLST ordering “no CPR/no intubation.” The patient underwent hemiarthroplasty. There was no documented discussion of AD/POLST by the surgeon, anesthesiologist, or other operating room personnel even though the patient was alert and competent. On postoperative day one, she was found to be bradycardic and hypotensive. A code was called. After confirming full code status in the EMR, cardiac compressions were begun, followed by intubation. Immediately afterward, family members indicated that the patient had a POLST limiting EOL care. When the healthcare proxy was reached hours later, she directed the patient be extubated. The patient died 16 minutes later.
DISCUSSION
Data on the frequency of unwanted CPR/intubation due to medical error are scarce. In the US, several lawsuits arising from unwanted CPR and intubation have achieved notoriety, but registries of legal cases6 probably underestimate the frequency of this harm. In a study of incorrect code status orders at Canadian hospitals, 35% of 308 patients with limited care preferences had full code orders in the chart.7 It is unclear how many of these expressed preferences also had legal documents available. There was considerable variability among hospitals, suggesting that local practices and culture were important factors.
Spot audits of 121 of our own patient charts (median age 77 years) on oncology, geriatrics, and cardiac units at our institution found 36 (30%) with AD/POLST that clearly limited life-sustaining treatments. Of these, 14 (39%) had discrepant full code orders. A review of these discrepant orders showed no medical documentation to indicate that the discrepancy was purposeful.
A root cause analysis (RCA) of cases of unwanted resuscitation, including interviews with involved nurses, medical staff, and operating room, hospitalist, and medical informatics leadership, revealed several types of error, both human and system. These pitfalls are probably common to several hospitals, and the solutions developed may be helpful as well (Table).
ROOT CAUSE 1: HASTE
Haste leads to poor communication with the patient and family. Emergency departments and admitting services can be hectic. Clinicians facing time and acuity pressure may give short shrift to the essential activity of validating patient choices, regardless of whether an AD or POLST is available. Poor communication was the major factor allowing for discrepancy in the Canadian study.7 Avoiding prognostic frankness is a well-known coping strategy for both clinicians and patients8,9 but in all these cases, that obstacle had been overcome earlier in the clinical course of disease, leaving inattention or haste as the most likely culprit.
ROOT CAUSE 2: INADEQUATE COMMUNICATION
“It is not our hospital culture to surveille for code status discrepancies, discuss appropriateness on rounds or at sign out.”
In all reviewed cases of unwanted resuscitation, numerous admitting or attending physicians failed to discuss LST meaningfully despite clinical scenarios that were associated with poor prognosis and should have provoked discussion about medical ineffectiveness. The admitting hospitalist in case 2 stated later that she had listed code choices for the patient who chose full code despite having a POLST stating otherwise. However, that discussion was not in depth, not reviewed for match to her POLST, and not documented.
Moreover, all the cases of AD/POLST and code status discrepancy were on nursing units with daily multidisciplinary rounds and where there had been twice-daily nurse-to-nurse and medical staff–to–medical staff sign out. Queries about code status appropriateness and checks for discrepant AD/POLST and code orders were not standard work. Thus, the medical error was perpetuated.
Analysis of cases of unwanted intubation in postoperative cases indicated that contrary to guidelines,4,5 careful code status review was not part of the preoperative checklist or presurgical discussion.
ROOT CAUSE 3: DECEIVED BY THE EMR
The EMR is a well-recognized source of potential medical error.10,11 Clinicians may rely on the EMR for code status history or as a repository of relevant documents. These are important as a starting place for code status discussions, especially since patients and proxies often cannot accurately recall the existence of an AD/POLST or understand the options being presented.9,12 In case 1, clinicians partially relied upon the erroneous historical code status already in the chart from two prior admissions. This is a dangerous practice since code status choices have several options and depend upon the clinical situation. In the case of paper AD/POLST documents, the EMR is set up poorly to help the medical team find relevant documents. Furthermore, the EMR clinical decision support capabilities do not interact with paper documents, so no assistance in pointing out discrepancies is available. In addition, the scanning process itself can be problematic since scanning of paper documents was not performed until after the patient was discharged, thus hiding the most up-to-date documents from the personnel even if they had sought them. Moreover, our scanning process had been labeling documents with the date of scanning and not the date of completion, making it difficult to find the “active” order.
ROOT CAUSE 4: WE DID NOT KNOW
Interviews with different clinicians revealed widespread knowledge deficits, including appreciation of the POLST as durable across different medical institutions, effective differences between POLST and AD, location of POLST/AD within the EMR, recommendations of professional society guidelines on suspending DNR for procedures, hospital policy on same, the need to check for updates in bedside paper documents, and whether family members can overrule patients’ stated wishes. Education tends to be the most common form of recommendation after RCA and may be the least efficacious in risk mitigation,13 but in this case, education reinforced by new EMR capabilities was an essential part of the solutions bundle (Table).
AD/POLST and similar tools are complex, and the choices are not binary. They are subject to change depending upon the medical context and the patient status and may be poorly understood by patients and clinicians.14 Accordingly, writing a goal-concordant code status order demands time and attention and as much nuanced medical judgment as any other medical problem faced by hospital-based clinicians. Though time-consuming, discussion with the patient or the surrogate should be considered as “standard work.” To facilitate this, a mandatory affirmative statement about review of LST choices was added to admission templates, procedural areas, and clinician sign outs (Table).
Unwanted, and therefore unwarranted, resuscitation violates autonomy and creates distress, anger, and distrust among patients and families. The distress extends also to frontline clinicians who are committed to “do no harm” in every other aspect of their professional lives.
Respecting and translating patients’ AD/POLST or similar tools into goal-concordant code status order is an essential professional commitment. Respect for patient safety and autonomy demands that we do it well, teach it well, and hold each other accountable.
Disclosures
The authors have nothing disclose.
1. Institute of Medicine. Dying in America: improving quality and honoring individual preferences near end of life Washington, DC: National Academies Pr; 2015.
2. ASCO Institute for Quality: QCDR measures. http://www.instituteforquality.org/sites/instituteforquality.org/files/QOPI 2015 QCDR Measures - Narrative_0.pdf. Accessed March 3, 2019.
3. Turnbull AE, Hartog CS. Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med. 2017;43(12):1847-1849. https://doi.org/10.1007/s00134-017-4873-2
4. American Society of Anesthesiology Ethics Committee. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment-last amended October 2013. Accessed March 12, 2019
5. American College of Surgeons Committee on Ethics. Statement on advanced directives by patients: “do not resuscitate” in the operating room. Bull Am Coll Surg. 2014;99(1):42-43
6. Pope TM. Legal briefing: new penalties for disregarding advance directives and do-not-resuscitate orders. J Clin Ethics. 2017;28(1):74-81.
7. Heyland DH, Ilan R, Jiang X, You JJ, Dodek P. The prevalence of medical error related to end-of-life communication in Canadian hospitals: results of a mutlicentre observational study. BMJ Qual Saf. 2016;25:671-679. https://doi.org/10.1136/bmjqs-2015-004567.
8. Robinson JD, Jagsi R. Physician-patient communication—an actionable target for reducing overly aggressive care near the end of life. JAMA Oncol. 2016;2(11):1407-1408. doi:10.1001/jamaoncol.2016.1948
9. Ugalde A, O’Callaghan C, Byard C, et al. Does implementation matter if comprehension is lacking? A qualitative investigation into perceptions of advanced care planning in people with cancer. Support Care Cancer. 2018;26:3765-3771. https://doi.org/10.1007/s00520-018-4241-y.
10. Silversetein S. The Syndrome of inappropriate overconfidence in computing. An invasion of medicine by the information technology industry? J Am Phys Surg. 2009;14:49-50
11. Ratwani RM, Reider, J and Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321(8):743-744. doi:10.1001/jama.2019.0161
12. Turnbull AE, Chessare CM, Coffin RK, Needham DM. More than one in three proxies do not know their loved one’s current code status: an observational study in a Maryland ICU. PLoS ONE. 2019;14(1):e0211531. https//doi.org/10.1371/journal.pone.0211531
13. Wu AW, Lipshutz AKM, Pronovost PJ. Effectiveness and efficiency of root cause analysis in medicine. JAMA. 2008;299(6):685-687. doi:10.1001/jama.299.6.685
14. Mirarchi F, Doshi AA, Zerkle SW, Cooney TE. TRIAD VI: how well do emergency physicians understand Physician Orders for Life-Sustaining Treatment (POLST) forms? J Patient Saf. 2015;11(1):1-8. https://doi.org/10.1097/PTS.0000000000000165.
The United States health system has been criticized for its overuse of aggressive and medically ineffective life-sustaining therapies (LST).1 Some professional societies have elevated dialog about end-of-life (EOL) care to a quality measure,2 expecting that more open discussion will achieve more “goal-concordant care”3 and appropriate use of LST. However, even when Advanced Directives (AD) or Physician Orders for Life-Sustaining Therapy (POLST) have been created, their directions are not always followed in the hospital. This perspective discusses how preventable errors allow for use of LST even when patients designated it as unwanted. Two cases, chosen from several similar ones, are highlighted, demonstrating both human and system errors.
During the time of these events, the hospital policy required admission orders to contain a “code status” designation in the electronic medical record (EMR). All active and historical code status orders were listed chronologically and all AD and POLST documents were scanned into a special section of the EMR. Hospital policy, consistent with professional society guidelines,4,5 stated that patients with AD/POLST limiting EOL support should have individualized discussion about resuscitation options in the event of a periprocedural critical event. Automatic suspension or reinstatement of limited code orders was not permitted.
CASE 1
A 62-year-old woman with refractory heart failure was admitted with recurrence. The admitting code order was “initiate CPR/intubation” even though a POLST order written 10 months earlier indicating “do not intubate” was visible in the EMR. A more recent POLST indicating “No CPR/No intubation” accompanied the patient in the ambulance and was placed at bedside, but not scanned. There was no documented discussion of code status that might have explained the POLST/code order disparity. Notably, during two prior admissions within the year, “full code” orders had also been placed. On the fifth hospital day, the patient was found in respiratory distress and unresponsive. A “code” was called. ICU staff, after confirming full code status, intubated the patient emergently and commenced other invasive ICU interventions. Family members brought the preexisting POLST to medical attention within hours of the code but could not agree on immediate extubation. Over the next week, multiple prognosis discussions were held with the patient (when responsive) and family. Ultimately, the patient failed to improve and indicated a desire to be extubated, dying a few hours later.
CASE 2
A 94-year-old woman was admitted from assisted living with a traumatic subcapital femur fracture. Admission code orders were “initiate CPR/intubation” despite the presence in the EMR of a POLST ordering “no CPR/no intubation.” The patient underwent hemiarthroplasty. There was no documented discussion of AD/POLST by the surgeon, anesthesiologist, or other operating room personnel even though the patient was alert and competent. On postoperative day one, she was found to be bradycardic and hypotensive. A code was called. After confirming full code status in the EMR, cardiac compressions were begun, followed by intubation. Immediately afterward, family members indicated that the patient had a POLST limiting EOL care. When the healthcare proxy was reached hours later, she directed the patient be extubated. The patient died 16 minutes later.
DISCUSSION
Data on the frequency of unwanted CPR/intubation due to medical error are scarce. In the US, several lawsuits arising from unwanted CPR and intubation have achieved notoriety, but registries of legal cases6 probably underestimate the frequency of this harm. In a study of incorrect code status orders at Canadian hospitals, 35% of 308 patients with limited care preferences had full code orders in the chart.7 It is unclear how many of these expressed preferences also had legal documents available. There was considerable variability among hospitals, suggesting that local practices and culture were important factors.
Spot audits of 121 of our own patient charts (median age 77 years) on oncology, geriatrics, and cardiac units at our institution found 36 (30%) with AD/POLST that clearly limited life-sustaining treatments. Of these, 14 (39%) had discrepant full code orders. A review of these discrepant orders showed no medical documentation to indicate that the discrepancy was purposeful.
A root cause analysis (RCA) of cases of unwanted resuscitation, including interviews with involved nurses, medical staff, and operating room, hospitalist, and medical informatics leadership, revealed several types of error, both human and system. These pitfalls are probably common to several hospitals, and the solutions developed may be helpful as well (Table).
ROOT CAUSE 1: HASTE
Haste leads to poor communication with the patient and family. Emergency departments and admitting services can be hectic. Clinicians facing time and acuity pressure may give short shrift to the essential activity of validating patient choices, regardless of whether an AD or POLST is available. Poor communication was the major factor allowing for discrepancy in the Canadian study.7 Avoiding prognostic frankness is a well-known coping strategy for both clinicians and patients8,9 but in all these cases, that obstacle had been overcome earlier in the clinical course of disease, leaving inattention or haste as the most likely culprit.
ROOT CAUSE 2: INADEQUATE COMMUNICATION
“It is not our hospital culture to surveille for code status discrepancies, discuss appropriateness on rounds or at sign out.”
In all reviewed cases of unwanted resuscitation, numerous admitting or attending physicians failed to discuss LST meaningfully despite clinical scenarios that were associated with poor prognosis and should have provoked discussion about medical ineffectiveness. The admitting hospitalist in case 2 stated later that she had listed code choices for the patient who chose full code despite having a POLST stating otherwise. However, that discussion was not in depth, not reviewed for match to her POLST, and not documented.
Moreover, all the cases of AD/POLST and code status discrepancy were on nursing units with daily multidisciplinary rounds and where there had been twice-daily nurse-to-nurse and medical staff–to–medical staff sign out. Queries about code status appropriateness and checks for discrepant AD/POLST and code orders were not standard work. Thus, the medical error was perpetuated.
Analysis of cases of unwanted intubation in postoperative cases indicated that contrary to guidelines,4,5 careful code status review was not part of the preoperative checklist or presurgical discussion.
ROOT CAUSE 3: DECEIVED BY THE EMR
The EMR is a well-recognized source of potential medical error.10,11 Clinicians may rely on the EMR for code status history or as a repository of relevant documents. These are important as a starting place for code status discussions, especially since patients and proxies often cannot accurately recall the existence of an AD/POLST or understand the options being presented.9,12 In case 1, clinicians partially relied upon the erroneous historical code status already in the chart from two prior admissions. This is a dangerous practice since code status choices have several options and depend upon the clinical situation. In the case of paper AD/POLST documents, the EMR is set up poorly to help the medical team find relevant documents. Furthermore, the EMR clinical decision support capabilities do not interact with paper documents, so no assistance in pointing out discrepancies is available. In addition, the scanning process itself can be problematic since scanning of paper documents was not performed until after the patient was discharged, thus hiding the most up-to-date documents from the personnel even if they had sought them. Moreover, our scanning process had been labeling documents with the date of scanning and not the date of completion, making it difficult to find the “active” order.
ROOT CAUSE 4: WE DID NOT KNOW
Interviews with different clinicians revealed widespread knowledge deficits, including appreciation of the POLST as durable across different medical institutions, effective differences between POLST and AD, location of POLST/AD within the EMR, recommendations of professional society guidelines on suspending DNR for procedures, hospital policy on same, the need to check for updates in bedside paper documents, and whether family members can overrule patients’ stated wishes. Education tends to be the most common form of recommendation after RCA and may be the least efficacious in risk mitigation,13 but in this case, education reinforced by new EMR capabilities was an essential part of the solutions bundle (Table).
AD/POLST and similar tools are complex, and the choices are not binary. They are subject to change depending upon the medical context and the patient status and may be poorly understood by patients and clinicians.14 Accordingly, writing a goal-concordant code status order demands time and attention and as much nuanced medical judgment as any other medical problem faced by hospital-based clinicians. Though time-consuming, discussion with the patient or the surrogate should be considered as “standard work.” To facilitate this, a mandatory affirmative statement about review of LST choices was added to admission templates, procedural areas, and clinician sign outs (Table).
Unwanted, and therefore unwarranted, resuscitation violates autonomy and creates distress, anger, and distrust among patients and families. The distress extends also to frontline clinicians who are committed to “do no harm” in every other aspect of their professional lives.
Respecting and translating patients’ AD/POLST or similar tools into goal-concordant code status order is an essential professional commitment. Respect for patient safety and autonomy demands that we do it well, teach it well, and hold each other accountable.
Disclosures
The authors have nothing disclose.
The United States health system has been criticized for its overuse of aggressive and medically ineffective life-sustaining therapies (LST).1 Some professional societies have elevated dialog about end-of-life (EOL) care to a quality measure,2 expecting that more open discussion will achieve more “goal-concordant care”3 and appropriate use of LST. However, even when Advanced Directives (AD) or Physician Orders for Life-Sustaining Therapy (POLST) have been created, their directions are not always followed in the hospital. This perspective discusses how preventable errors allow for use of LST even when patients designated it as unwanted. Two cases, chosen from several similar ones, are highlighted, demonstrating both human and system errors.
During the time of these events, the hospital policy required admission orders to contain a “code status” designation in the electronic medical record (EMR). All active and historical code status orders were listed chronologically and all AD and POLST documents were scanned into a special section of the EMR. Hospital policy, consistent with professional society guidelines,4,5 stated that patients with AD/POLST limiting EOL support should have individualized discussion about resuscitation options in the event of a periprocedural critical event. Automatic suspension or reinstatement of limited code orders was not permitted.
CASE 1
A 62-year-old woman with refractory heart failure was admitted with recurrence. The admitting code order was “initiate CPR/intubation” even though a POLST order written 10 months earlier indicating “do not intubate” was visible in the EMR. A more recent POLST indicating “No CPR/No intubation” accompanied the patient in the ambulance and was placed at bedside, but not scanned. There was no documented discussion of code status that might have explained the POLST/code order disparity. Notably, during two prior admissions within the year, “full code” orders had also been placed. On the fifth hospital day, the patient was found in respiratory distress and unresponsive. A “code” was called. ICU staff, after confirming full code status, intubated the patient emergently and commenced other invasive ICU interventions. Family members brought the preexisting POLST to medical attention within hours of the code but could not agree on immediate extubation. Over the next week, multiple prognosis discussions were held with the patient (when responsive) and family. Ultimately, the patient failed to improve and indicated a desire to be extubated, dying a few hours later.
CASE 2
A 94-year-old woman was admitted from assisted living with a traumatic subcapital femur fracture. Admission code orders were “initiate CPR/intubation” despite the presence in the EMR of a POLST ordering “no CPR/no intubation.” The patient underwent hemiarthroplasty. There was no documented discussion of AD/POLST by the surgeon, anesthesiologist, or other operating room personnel even though the patient was alert and competent. On postoperative day one, she was found to be bradycardic and hypotensive. A code was called. After confirming full code status in the EMR, cardiac compressions were begun, followed by intubation. Immediately afterward, family members indicated that the patient had a POLST limiting EOL care. When the healthcare proxy was reached hours later, she directed the patient be extubated. The patient died 16 minutes later.
DISCUSSION
Data on the frequency of unwanted CPR/intubation due to medical error are scarce. In the US, several lawsuits arising from unwanted CPR and intubation have achieved notoriety, but registries of legal cases6 probably underestimate the frequency of this harm. In a study of incorrect code status orders at Canadian hospitals, 35% of 308 patients with limited care preferences had full code orders in the chart.7 It is unclear how many of these expressed preferences also had legal documents available. There was considerable variability among hospitals, suggesting that local practices and culture were important factors.
Spot audits of 121 of our own patient charts (median age 77 years) on oncology, geriatrics, and cardiac units at our institution found 36 (30%) with AD/POLST that clearly limited life-sustaining treatments. Of these, 14 (39%) had discrepant full code orders. A review of these discrepant orders showed no medical documentation to indicate that the discrepancy was purposeful.
A root cause analysis (RCA) of cases of unwanted resuscitation, including interviews with involved nurses, medical staff, and operating room, hospitalist, and medical informatics leadership, revealed several types of error, both human and system. These pitfalls are probably common to several hospitals, and the solutions developed may be helpful as well (Table).
ROOT CAUSE 1: HASTE
Haste leads to poor communication with the patient and family. Emergency departments and admitting services can be hectic. Clinicians facing time and acuity pressure may give short shrift to the essential activity of validating patient choices, regardless of whether an AD or POLST is available. Poor communication was the major factor allowing for discrepancy in the Canadian study.7 Avoiding prognostic frankness is a well-known coping strategy for both clinicians and patients8,9 but in all these cases, that obstacle had been overcome earlier in the clinical course of disease, leaving inattention or haste as the most likely culprit.
ROOT CAUSE 2: INADEQUATE COMMUNICATION
“It is not our hospital culture to surveille for code status discrepancies, discuss appropriateness on rounds or at sign out.”
In all reviewed cases of unwanted resuscitation, numerous admitting or attending physicians failed to discuss LST meaningfully despite clinical scenarios that were associated with poor prognosis and should have provoked discussion about medical ineffectiveness. The admitting hospitalist in case 2 stated later that she had listed code choices for the patient who chose full code despite having a POLST stating otherwise. However, that discussion was not in depth, not reviewed for match to her POLST, and not documented.
Moreover, all the cases of AD/POLST and code status discrepancy were on nursing units with daily multidisciplinary rounds and where there had been twice-daily nurse-to-nurse and medical staff–to–medical staff sign out. Queries about code status appropriateness and checks for discrepant AD/POLST and code orders were not standard work. Thus, the medical error was perpetuated.
Analysis of cases of unwanted intubation in postoperative cases indicated that contrary to guidelines,4,5 careful code status review was not part of the preoperative checklist or presurgical discussion.
ROOT CAUSE 3: DECEIVED BY THE EMR
The EMR is a well-recognized source of potential medical error.10,11 Clinicians may rely on the EMR for code status history or as a repository of relevant documents. These are important as a starting place for code status discussions, especially since patients and proxies often cannot accurately recall the existence of an AD/POLST or understand the options being presented.9,12 In case 1, clinicians partially relied upon the erroneous historical code status already in the chart from two prior admissions. This is a dangerous practice since code status choices have several options and depend upon the clinical situation. In the case of paper AD/POLST documents, the EMR is set up poorly to help the medical team find relevant documents. Furthermore, the EMR clinical decision support capabilities do not interact with paper documents, so no assistance in pointing out discrepancies is available. In addition, the scanning process itself can be problematic since scanning of paper documents was not performed until after the patient was discharged, thus hiding the most up-to-date documents from the personnel even if they had sought them. Moreover, our scanning process had been labeling documents with the date of scanning and not the date of completion, making it difficult to find the “active” order.
ROOT CAUSE 4: WE DID NOT KNOW
Interviews with different clinicians revealed widespread knowledge deficits, including appreciation of the POLST as durable across different medical institutions, effective differences between POLST and AD, location of POLST/AD within the EMR, recommendations of professional society guidelines on suspending DNR for procedures, hospital policy on same, the need to check for updates in bedside paper documents, and whether family members can overrule patients’ stated wishes. Education tends to be the most common form of recommendation after RCA and may be the least efficacious in risk mitigation,13 but in this case, education reinforced by new EMR capabilities was an essential part of the solutions bundle (Table).
AD/POLST and similar tools are complex, and the choices are not binary. They are subject to change depending upon the medical context and the patient status and may be poorly understood by patients and clinicians.14 Accordingly, writing a goal-concordant code status order demands time and attention and as much nuanced medical judgment as any other medical problem faced by hospital-based clinicians. Though time-consuming, discussion with the patient or the surrogate should be considered as “standard work.” To facilitate this, a mandatory affirmative statement about review of LST choices was added to admission templates, procedural areas, and clinician sign outs (Table).
Unwanted, and therefore unwarranted, resuscitation violates autonomy and creates distress, anger, and distrust among patients and families. The distress extends also to frontline clinicians who are committed to “do no harm” in every other aspect of their professional lives.
Respecting and translating patients’ AD/POLST or similar tools into goal-concordant code status order is an essential professional commitment. Respect for patient safety and autonomy demands that we do it well, teach it well, and hold each other accountable.
Disclosures
The authors have nothing disclose.
1. Institute of Medicine. Dying in America: improving quality and honoring individual preferences near end of life Washington, DC: National Academies Pr; 2015.
2. ASCO Institute for Quality: QCDR measures. http://www.instituteforquality.org/sites/instituteforquality.org/files/QOPI 2015 QCDR Measures - Narrative_0.pdf. Accessed March 3, 2019.
3. Turnbull AE, Hartog CS. Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med. 2017;43(12):1847-1849. https://doi.org/10.1007/s00134-017-4873-2
4. American Society of Anesthesiology Ethics Committee. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment-last amended October 2013. Accessed March 12, 2019
5. American College of Surgeons Committee on Ethics. Statement on advanced directives by patients: “do not resuscitate” in the operating room. Bull Am Coll Surg. 2014;99(1):42-43
6. Pope TM. Legal briefing: new penalties for disregarding advance directives and do-not-resuscitate orders. J Clin Ethics. 2017;28(1):74-81.
7. Heyland DH, Ilan R, Jiang X, You JJ, Dodek P. The prevalence of medical error related to end-of-life communication in Canadian hospitals: results of a mutlicentre observational study. BMJ Qual Saf. 2016;25:671-679. https://doi.org/10.1136/bmjqs-2015-004567.
8. Robinson JD, Jagsi R. Physician-patient communication—an actionable target for reducing overly aggressive care near the end of life. JAMA Oncol. 2016;2(11):1407-1408. doi:10.1001/jamaoncol.2016.1948
9. Ugalde A, O’Callaghan C, Byard C, et al. Does implementation matter if comprehension is lacking? A qualitative investigation into perceptions of advanced care planning in people with cancer. Support Care Cancer. 2018;26:3765-3771. https://doi.org/10.1007/s00520-018-4241-y.
10. Silversetein S. The Syndrome of inappropriate overconfidence in computing. An invasion of medicine by the information technology industry? J Am Phys Surg. 2009;14:49-50
11. Ratwani RM, Reider, J and Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321(8):743-744. doi:10.1001/jama.2019.0161
12. Turnbull AE, Chessare CM, Coffin RK, Needham DM. More than one in three proxies do not know their loved one’s current code status: an observational study in a Maryland ICU. PLoS ONE. 2019;14(1):e0211531. https//doi.org/10.1371/journal.pone.0211531
13. Wu AW, Lipshutz AKM, Pronovost PJ. Effectiveness and efficiency of root cause analysis in medicine. JAMA. 2008;299(6):685-687. doi:10.1001/jama.299.6.685
14. Mirarchi F, Doshi AA, Zerkle SW, Cooney TE. TRIAD VI: how well do emergency physicians understand Physician Orders for Life-Sustaining Treatment (POLST) forms? J Patient Saf. 2015;11(1):1-8. https://doi.org/10.1097/PTS.0000000000000165.
1. Institute of Medicine. Dying in America: improving quality and honoring individual preferences near end of life Washington, DC: National Academies Pr; 2015.
2. ASCO Institute for Quality: QCDR measures. http://www.instituteforquality.org/sites/instituteforquality.org/files/QOPI 2015 QCDR Measures - Narrative_0.pdf. Accessed March 3, 2019.
3. Turnbull AE, Hartog CS. Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med. 2017;43(12):1847-1849. https://doi.org/10.1007/s00134-017-4873-2
4. American Society of Anesthesiology Ethics Committee. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment-last amended October 2013. Accessed March 12, 2019
5. American College of Surgeons Committee on Ethics. Statement on advanced directives by patients: “do not resuscitate” in the operating room. Bull Am Coll Surg. 2014;99(1):42-43
6. Pope TM. Legal briefing: new penalties for disregarding advance directives and do-not-resuscitate orders. J Clin Ethics. 2017;28(1):74-81.
7. Heyland DH, Ilan R, Jiang X, You JJ, Dodek P. The prevalence of medical error related to end-of-life communication in Canadian hospitals: results of a mutlicentre observational study. BMJ Qual Saf. 2016;25:671-679. https://doi.org/10.1136/bmjqs-2015-004567.
8. Robinson JD, Jagsi R. Physician-patient communication—an actionable target for reducing overly aggressive care near the end of life. JAMA Oncol. 2016;2(11):1407-1408. doi:10.1001/jamaoncol.2016.1948
9. Ugalde A, O’Callaghan C, Byard C, et al. Does implementation matter if comprehension is lacking? A qualitative investigation into perceptions of advanced care planning in people with cancer. Support Care Cancer. 2018;26:3765-3771. https://doi.org/10.1007/s00520-018-4241-y.
10. Silversetein S. The Syndrome of inappropriate overconfidence in computing. An invasion of medicine by the information technology industry? J Am Phys Surg. 2009;14:49-50
11. Ratwani RM, Reider, J and Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321(8):743-744. doi:10.1001/jama.2019.0161
12. Turnbull AE, Chessare CM, Coffin RK, Needham DM. More than one in three proxies do not know their loved one’s current code status: an observational study in a Maryland ICU. PLoS ONE. 2019;14(1):e0211531. https//doi.org/10.1371/journal.pone.0211531
13. Wu AW, Lipshutz AKM, Pronovost PJ. Effectiveness and efficiency of root cause analysis in medicine. JAMA. 2008;299(6):685-687. doi:10.1001/jama.299.6.685
14. Mirarchi F, Doshi AA, Zerkle SW, Cooney TE. TRIAD VI: how well do emergency physicians understand Physician Orders for Life-Sustaining Treatment (POLST) forms? J Patient Saf. 2015;11(1):1-8. https://doi.org/10.1097/PTS.0000000000000165.
© 2019 Society of Hospital Medicine
Pain in the United States: Time for a Culture Shift in Expectations, Messaging, and Management
Opioid prescribing has dramatically increased in the United States (US) over the past two decades, fueling the current crisis of opioid-related adverse events and deaths.1 Understanding the potential contributors to this increased prescribing is paramount to developing effective strategies for preventing propagation. In
First, they found that US patients reported greater levels of pain severity than patients hospitalized in other countries, especially among those not taking opioids before admission. However, even after adjusting for these differences in pain severity, opioids were still prescribed more frequently in the US than in other countries. These findings suggest differences in both patients’ experience of pain and physicians’ propensity to prescribe opioids in the US compared with other countries. Furthermore, beliefs and expectations about pain control differed between hospitalized patients in the US versus other countries. For example, patients in other countries were more likely to endorse the statement “Good patients avoid talking about pain” than patients in the US. This may, in part, contribute to the difference in reported pain severity between the US and other countries.
Finally, and perhaps most interestingly, although US patients who were opioid-naive before hospitalization did report greater satisfaction with pain control than patients in other countries, this difference was not attributable to greater opioid receipt. In fact, opioid receipt was not associated with increased satisfaction with pain control, regardless of country. Studies in other settings, such as the emergency department3 and postoperative settings,4 have similarly failed to demonstrate an association between opioid receipt and patient satisfaction. This is not entirely surprising given that studies comparing pain relief between opioid and nonopioid analgesics routinely demonstrate similar efficacy of the two approaches across several conditions.5, 6
This study clearly demonstrates differences in opioid prescribing patterns and patients’ expectations of pain control in sampled hospitals in the US compared to those in other countries; however, there are noteworthy limitations. First, not all regions were sampled within the United States; hospitals in the northeast regions, previously demonstrated to have lower opioid prescribing rates,7 were notably absent. Second, the small number of non-US hospitals and the small sample size in those hospitals limit the ability to draw firm conclusions. The results are nonetheless consistent with anecdotal experience. For example, a recent opinion article in the New York Times describes the experience of a US patient undergoing surgery in Germany;8 the differences the author observes in terms of expectations around pain control, associated messaging, and ultimately, prescribing practices between the two countries are striking.
In response to studies demonstrating underassessment and undertreatment of pain in hospitalized patients in the late 20th century,9 well-intentioned initiatives have promoted more frequent pain assessment and more aggressive pain control. In the context of the current opioid crisis, Burden et al. provide compelling data supporting the idea that the pendulum has swung too far in the US. This international study suggests that curbing the US opioid crisis will require a true culture shift, not just in providers’ analgesic prescribing patterns but also in messaging around pain and patient expectations.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging and R01HS026215 from the Agency for Healthcare Research and Quality.
1. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. Nov 18 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
2. Burden M, Keniston A, Wallace MA, et al. Opioid utilization and perception of pain control in hospitalized patients: a cross-sectional study of 11 sites in 8 countries. J Hosp Med. 2019;14(12):737-745. https://doi.org/10.12788/jhm.3256
3. Schwartz TM, Tai M, Babu KM, Merchant RC. Lack of association between Press Ganey emergency department patient satisfaction scores and emergency department administration of analgesic medications. Ann Emerg Med. 2014;64(5):469-481. https://doi.org/10.1016/j.annemergmed.2014.02.010.
4. Maheshwari K, Cummings KC, 3rd, Farag E, Makarova N, Turan A, Kurz A. A temporal analysis of opioid use, patient satisfaction, and pain scores in colorectal surgery patients. J Clin Anesth. 2016;34:661-667. https://doi.org/10.1016/j.jclinane.2016.07.005.
5. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. https://doi.org/10.1001/jama.2017.16190.
6. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. https://doi.org/10.1002/14651858.CD004137.pub3.
7. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
8. Dumas F. After Surgery in Germany, I Wanted Vicodin, Not Herbal Tea. The New York Times 2018; https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed June 24, 2019.
9. Max MB. Improving outcomes of analgesic treatment: is education enough? Ann Intern Med. 1990;113(11):885-889. https://doi.org/10.7326/0003-4819-113-11-885.
Opioid prescribing has dramatically increased in the United States (US) over the past two decades, fueling the current crisis of opioid-related adverse events and deaths.1 Understanding the potential contributors to this increased prescribing is paramount to developing effective strategies for preventing propagation. In
First, they found that US patients reported greater levels of pain severity than patients hospitalized in other countries, especially among those not taking opioids before admission. However, even after adjusting for these differences in pain severity, opioids were still prescribed more frequently in the US than in other countries. These findings suggest differences in both patients’ experience of pain and physicians’ propensity to prescribe opioids in the US compared with other countries. Furthermore, beliefs and expectations about pain control differed between hospitalized patients in the US versus other countries. For example, patients in other countries were more likely to endorse the statement “Good patients avoid talking about pain” than patients in the US. This may, in part, contribute to the difference in reported pain severity between the US and other countries.
Finally, and perhaps most interestingly, although US patients who were opioid-naive before hospitalization did report greater satisfaction with pain control than patients in other countries, this difference was not attributable to greater opioid receipt. In fact, opioid receipt was not associated with increased satisfaction with pain control, regardless of country. Studies in other settings, such as the emergency department3 and postoperative settings,4 have similarly failed to demonstrate an association between opioid receipt and patient satisfaction. This is not entirely surprising given that studies comparing pain relief between opioid and nonopioid analgesics routinely demonstrate similar efficacy of the two approaches across several conditions.5, 6
This study clearly demonstrates differences in opioid prescribing patterns and patients’ expectations of pain control in sampled hospitals in the US compared to those in other countries; however, there are noteworthy limitations. First, not all regions were sampled within the United States; hospitals in the northeast regions, previously demonstrated to have lower opioid prescribing rates,7 were notably absent. Second, the small number of non-US hospitals and the small sample size in those hospitals limit the ability to draw firm conclusions. The results are nonetheless consistent with anecdotal experience. For example, a recent opinion article in the New York Times describes the experience of a US patient undergoing surgery in Germany;8 the differences the author observes in terms of expectations around pain control, associated messaging, and ultimately, prescribing practices between the two countries are striking.
In response to studies demonstrating underassessment and undertreatment of pain in hospitalized patients in the late 20th century,9 well-intentioned initiatives have promoted more frequent pain assessment and more aggressive pain control. In the context of the current opioid crisis, Burden et al. provide compelling data supporting the idea that the pendulum has swung too far in the US. This international study suggests that curbing the US opioid crisis will require a true culture shift, not just in providers’ analgesic prescribing patterns but also in messaging around pain and patient expectations.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging and R01HS026215 from the Agency for Healthcare Research and Quality.
Opioid prescribing has dramatically increased in the United States (US) over the past two decades, fueling the current crisis of opioid-related adverse events and deaths.1 Understanding the potential contributors to this increased prescribing is paramount to developing effective strategies for preventing propagation. In
First, they found that US patients reported greater levels of pain severity than patients hospitalized in other countries, especially among those not taking opioids before admission. However, even after adjusting for these differences in pain severity, opioids were still prescribed more frequently in the US than in other countries. These findings suggest differences in both patients’ experience of pain and physicians’ propensity to prescribe opioids in the US compared with other countries. Furthermore, beliefs and expectations about pain control differed between hospitalized patients in the US versus other countries. For example, patients in other countries were more likely to endorse the statement “Good patients avoid talking about pain” than patients in the US. This may, in part, contribute to the difference in reported pain severity between the US and other countries.
Finally, and perhaps most interestingly, although US patients who were opioid-naive before hospitalization did report greater satisfaction with pain control than patients in other countries, this difference was not attributable to greater opioid receipt. In fact, opioid receipt was not associated with increased satisfaction with pain control, regardless of country. Studies in other settings, such as the emergency department3 and postoperative settings,4 have similarly failed to demonstrate an association between opioid receipt and patient satisfaction. This is not entirely surprising given that studies comparing pain relief between opioid and nonopioid analgesics routinely demonstrate similar efficacy of the two approaches across several conditions.5, 6
This study clearly demonstrates differences in opioid prescribing patterns and patients’ expectations of pain control in sampled hospitals in the US compared to those in other countries; however, there are noteworthy limitations. First, not all regions were sampled within the United States; hospitals in the northeast regions, previously demonstrated to have lower opioid prescribing rates,7 were notably absent. Second, the small number of non-US hospitals and the small sample size in those hospitals limit the ability to draw firm conclusions. The results are nonetheless consistent with anecdotal experience. For example, a recent opinion article in the New York Times describes the experience of a US patient undergoing surgery in Germany;8 the differences the author observes in terms of expectations around pain control, associated messaging, and ultimately, prescribing practices between the two countries are striking.
In response to studies demonstrating underassessment and undertreatment of pain in hospitalized patients in the late 20th century,9 well-intentioned initiatives have promoted more frequent pain assessment and more aggressive pain control. In the context of the current opioid crisis, Burden et al. provide compelling data supporting the idea that the pendulum has swung too far in the US. This international study suggests that curbing the US opioid crisis will require a true culture shift, not just in providers’ analgesic prescribing patterns but also in messaging around pain and patient expectations.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging and R01HS026215 from the Agency for Healthcare Research and Quality.
1. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. Nov 18 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
2. Burden M, Keniston A, Wallace MA, et al. Opioid utilization and perception of pain control in hospitalized patients: a cross-sectional study of 11 sites in 8 countries. J Hosp Med. 2019;14(12):737-745. https://doi.org/10.12788/jhm.3256
3. Schwartz TM, Tai M, Babu KM, Merchant RC. Lack of association between Press Ganey emergency department patient satisfaction scores and emergency department administration of analgesic medications. Ann Emerg Med. 2014;64(5):469-481. https://doi.org/10.1016/j.annemergmed.2014.02.010.
4. Maheshwari K, Cummings KC, 3rd, Farag E, Makarova N, Turan A, Kurz A. A temporal analysis of opioid use, patient satisfaction, and pain scores in colorectal surgery patients. J Clin Anesth. 2016;34:661-667. https://doi.org/10.1016/j.jclinane.2016.07.005.
5. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. https://doi.org/10.1001/jama.2017.16190.
6. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. https://doi.org/10.1002/14651858.CD004137.pub3.
7. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
8. Dumas F. After Surgery in Germany, I Wanted Vicodin, Not Herbal Tea. The New York Times 2018; https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed June 24, 2019.
9. Max MB. Improving outcomes of analgesic treatment: is education enough? Ann Intern Med. 1990;113(11):885-889. https://doi.org/10.7326/0003-4819-113-11-885.
1. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. Nov 18 2010;363(21):1981-1985. https://doi.org/10.1056/NEJMp1011512.
2. Burden M, Keniston A, Wallace MA, et al. Opioid utilization and perception of pain control in hospitalized patients: a cross-sectional study of 11 sites in 8 countries. J Hosp Med. 2019;14(12):737-745. https://doi.org/10.12788/jhm.3256
3. Schwartz TM, Tai M, Babu KM, Merchant RC. Lack of association between Press Ganey emergency department patient satisfaction scores and emergency department administration of analgesic medications. Ann Emerg Med. 2014;64(5):469-481. https://doi.org/10.1016/j.annemergmed.2014.02.010.
4. Maheshwari K, Cummings KC, 3rd, Farag E, Makarova N, Turan A, Kurz A. A temporal analysis of opioid use, patient satisfaction, and pain scores in colorectal surgery patients. J Clin Anesth. 2016;34:661-667. https://doi.org/10.1016/j.jclinane.2016.07.005.
5. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. 2017;318(17):1661-1667. https://doi.org/10.1001/jama.2017.16190.
6. Holdgate A, Pollock T. Nonsteroidal anti-inflammatory drugs (NSAIDs) versus opioids for acute renal colic. Cochrane Database Syst Rev. 2005:CD004137. https://doi.org/10.1002/14651858.CD004137.pub3.
7. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. https://doi.org/10.1002/jhm.2102.
8. Dumas F. After Surgery in Germany, I Wanted Vicodin, Not Herbal Tea. The New York Times 2018; https://www.nytimes.com/2018/01/27/opinion/sunday/surgery-germany-vicodin.html. Accessed June 24, 2019.
9. Max MB. Improving outcomes of analgesic treatment: is education enough? Ann Intern Med. 1990;113(11):885-889. https://doi.org/10.7326/0003-4819-113-11-885.
© 2019 Society of Hospital Medicine