User login
Liberalized low–glycemic-index diet effective for seizure reduction
BANGKOK – in a randomized, double-blind, 24-week, noninferiority study.
The low–glycemic-index diet (LGID) was introduced as a kinder, gentler, variant of the classic ketogenic diet for seizure frequency reduction. The ketogenic diet’s efficacy for this purpose is well established, but compliance is a problem and discontinuation rates are high. Yet even though the LGID was designed to be less onerous than the ketogenic diet, many children and parents also find the 7-days-a-week LGID to be excessively burdensome. This was the impetus for pitting the daily LGID against an intermittent version – 5 days on, 2 days off – in a randomized trial, Prateek K. Panda, MD, explained at the International Epilepsy Congress.
The hypothesis of this noninferiority trial was that adherence to the liberalized LGID would be similar to or better than that with the daily LGID regimen, with resultant similar reductions in seizure frequency. And further, that patients on the intermittent LGID would feel better because it would help improve depleted glycogen stores important for daily activity and that the liberalized diet would also be rated more favorably by caregivers, Dr. Panda said at the congress sponsored by the International League Against Epilepsy.
The 24-week, single-center trial included 122 children ages 1-15 years with drug-resistant epilepsy. At baseline they averaged 99 seizures per week by parental diary despite being on a median of four antiepileptic drugs. A total of 88% of participants had some form of structural epilepsy; the rest had a probable or confirmed genetic cause for their seizure disorder, according to Dr. Panda of the All-India Institute of Medical Sciences in New Delhi.
The standard daily LGID was comprised of 10% carbohydrate, 30% protein, and 60% fat, with only low–glycemic-index foods permitted. The cohort randomized to the liberalized diet ate that way on weekdays; however, on Saturdays and Sundays their diet was 20% carbohydrate, 30% protein, and 50% fat, with both medium- and low–glycemic-index foods allowed.
The primary outcome was the mean reduction in seizures per week by caregiver records at 24 weeks. The reduction from baseline was 54% in the strict LGID group and not significantly different at 49% in the intermittent LGID patients. Overall, 54% of patients in the strict LGID arm experienced a greater than 50% reduction in weekly seizure frequency, as did 50% on the liberalized diet, a nonsignificant difference.
There were five study dropouts in the strict LGID group and three in the liberalized LGID cohort. The two groups showed similar improvements over baseline in measures of social function, behavior, and cognition. Parents of children in the liberalized LGID group rated that diet as significantly less difficult to administer than those randomized to the strict LGID therapy.
Mean hemoglobin A1c improved in the strict LGID patients from 5.7% at baseline to 5.1% at both 12 and 24 weeks. The intermittent LGID group went from 5.6% to 5.0% and then to 5.2%. There was no correlation between HbA1c and reduction in seizure frequency. In contrast, serum beta-hydroxybutyrate levels showed a moderate correlation with seizure frequency, a novel finding which if confirmed might render beta-hydroxybutyrate useful as a biomarker, according to Dr. Panda.
Adverse events – mostly dyslipidemia and GI complaints such as vomiting or constipation – occurred in 25% of the strict LGID group and 13% with the intermittent LGID. All adverse events were mild.
Dr. Panda reported having no financial conflicts regarding the study, sponsored by the All-India Institute of Medical Sciences.
SOURCE: Panda PK et al. IEC 2019, Abstract P056.
BANGKOK – in a randomized, double-blind, 24-week, noninferiority study.
The low–glycemic-index diet (LGID) was introduced as a kinder, gentler, variant of the classic ketogenic diet for seizure frequency reduction. The ketogenic diet’s efficacy for this purpose is well established, but compliance is a problem and discontinuation rates are high. Yet even though the LGID was designed to be less onerous than the ketogenic diet, many children and parents also find the 7-days-a-week LGID to be excessively burdensome. This was the impetus for pitting the daily LGID against an intermittent version – 5 days on, 2 days off – in a randomized trial, Prateek K. Panda, MD, explained at the International Epilepsy Congress.
The hypothesis of this noninferiority trial was that adherence to the liberalized LGID would be similar to or better than that with the daily LGID regimen, with resultant similar reductions in seizure frequency. And further, that patients on the intermittent LGID would feel better because it would help improve depleted glycogen stores important for daily activity and that the liberalized diet would also be rated more favorably by caregivers, Dr. Panda said at the congress sponsored by the International League Against Epilepsy.
The 24-week, single-center trial included 122 children ages 1-15 years with drug-resistant epilepsy. At baseline they averaged 99 seizures per week by parental diary despite being on a median of four antiepileptic drugs. A total of 88% of participants had some form of structural epilepsy; the rest had a probable or confirmed genetic cause for their seizure disorder, according to Dr. Panda of the All-India Institute of Medical Sciences in New Delhi.
The standard daily LGID was comprised of 10% carbohydrate, 30% protein, and 60% fat, with only low–glycemic-index foods permitted. The cohort randomized to the liberalized diet ate that way on weekdays; however, on Saturdays and Sundays their diet was 20% carbohydrate, 30% protein, and 50% fat, with both medium- and low–glycemic-index foods allowed.
The primary outcome was the mean reduction in seizures per week by caregiver records at 24 weeks. The reduction from baseline was 54% in the strict LGID group and not significantly different at 49% in the intermittent LGID patients. Overall, 54% of patients in the strict LGID arm experienced a greater than 50% reduction in weekly seizure frequency, as did 50% on the liberalized diet, a nonsignificant difference.
There were five study dropouts in the strict LGID group and three in the liberalized LGID cohort. The two groups showed similar improvements over baseline in measures of social function, behavior, and cognition. Parents of children in the liberalized LGID group rated that diet as significantly less difficult to administer than those randomized to the strict LGID therapy.
Mean hemoglobin A1c improved in the strict LGID patients from 5.7% at baseline to 5.1% at both 12 and 24 weeks. The intermittent LGID group went from 5.6% to 5.0% and then to 5.2%. There was no correlation between HbA1c and reduction in seizure frequency. In contrast, serum beta-hydroxybutyrate levels showed a moderate correlation with seizure frequency, a novel finding which if confirmed might render beta-hydroxybutyrate useful as a biomarker, according to Dr. Panda.
Adverse events – mostly dyslipidemia and GI complaints such as vomiting or constipation – occurred in 25% of the strict LGID group and 13% with the intermittent LGID. All adverse events were mild.
Dr. Panda reported having no financial conflicts regarding the study, sponsored by the All-India Institute of Medical Sciences.
SOURCE: Panda PK et al. IEC 2019, Abstract P056.
BANGKOK – in a randomized, double-blind, 24-week, noninferiority study.
The low–glycemic-index diet (LGID) was introduced as a kinder, gentler, variant of the classic ketogenic diet for seizure frequency reduction. The ketogenic diet’s efficacy for this purpose is well established, but compliance is a problem and discontinuation rates are high. Yet even though the LGID was designed to be less onerous than the ketogenic diet, many children and parents also find the 7-days-a-week LGID to be excessively burdensome. This was the impetus for pitting the daily LGID against an intermittent version – 5 days on, 2 days off – in a randomized trial, Prateek K. Panda, MD, explained at the International Epilepsy Congress.
The hypothesis of this noninferiority trial was that adherence to the liberalized LGID would be similar to or better than that with the daily LGID regimen, with resultant similar reductions in seizure frequency. And further, that patients on the intermittent LGID would feel better because it would help improve depleted glycogen stores important for daily activity and that the liberalized diet would also be rated more favorably by caregivers, Dr. Panda said at the congress sponsored by the International League Against Epilepsy.
The 24-week, single-center trial included 122 children ages 1-15 years with drug-resistant epilepsy. At baseline they averaged 99 seizures per week by parental diary despite being on a median of four antiepileptic drugs. A total of 88% of participants had some form of structural epilepsy; the rest had a probable or confirmed genetic cause for their seizure disorder, according to Dr. Panda of the All-India Institute of Medical Sciences in New Delhi.
The standard daily LGID was comprised of 10% carbohydrate, 30% protein, and 60% fat, with only low–glycemic-index foods permitted. The cohort randomized to the liberalized diet ate that way on weekdays; however, on Saturdays and Sundays their diet was 20% carbohydrate, 30% protein, and 50% fat, with both medium- and low–glycemic-index foods allowed.
The primary outcome was the mean reduction in seizures per week by caregiver records at 24 weeks. The reduction from baseline was 54% in the strict LGID group and not significantly different at 49% in the intermittent LGID patients. Overall, 54% of patients in the strict LGID arm experienced a greater than 50% reduction in weekly seizure frequency, as did 50% on the liberalized diet, a nonsignificant difference.
There were five study dropouts in the strict LGID group and three in the liberalized LGID cohort. The two groups showed similar improvements over baseline in measures of social function, behavior, and cognition. Parents of children in the liberalized LGID group rated that diet as significantly less difficult to administer than those randomized to the strict LGID therapy.
Mean hemoglobin A1c improved in the strict LGID patients from 5.7% at baseline to 5.1% at both 12 and 24 weeks. The intermittent LGID group went from 5.6% to 5.0% and then to 5.2%. There was no correlation between HbA1c and reduction in seizure frequency. In contrast, serum beta-hydroxybutyrate levels showed a moderate correlation with seizure frequency, a novel finding which if confirmed might render beta-hydroxybutyrate useful as a biomarker, according to Dr. Panda.
Adverse events – mostly dyslipidemia and GI complaints such as vomiting or constipation – occurred in 25% of the strict LGID group and 13% with the intermittent LGID. All adverse events were mild.
Dr. Panda reported having no financial conflicts regarding the study, sponsored by the All-India Institute of Medical Sciences.
SOURCE: Panda PK et al. IEC 2019, Abstract P056.
REPORTING FROM IEC 2019
POEM outperforms pneumatic dilation in randomized achalasia trial
Peroral endoscopic myotomy (POEM) had a success rate exceeding 90%, versus just about 50% for standard balloon dilation in what investigators say is, to their knowledge, the first-ever randomized trial to evaluate POEM as a first-line modality for this esophageal motility disorder.
Reflux esophagitis was the major downside of POEM, according to investigators, who reported the complication in 41% of patients at a 2-year follow-up, as compared to just 7% of patients undergoing the standard balloon dilation.
Nevertheless, there were no serious adverse events among 63 POEM-treated patients, while one patient out of 63 undergoing pneumatic dilation had a perforation that required endoscopic closure and hospitalization, according to senior study author Albert J. Bredenoord, MD, PhD, of Amsterdam University Medical Center.
“These findings support consideration of POEM as an initial treatment option for patients with achalasia,” Dr. Bredenoord and coinvestigators said in a report on the study appearing in JAMA.
While endoscopic pneumatic dilation is the usual treatment for achalasia, POEM has become more commonly used following case series showing high rates of efficacy, according to the authors.
The POEM procedure also offers advantages over laparoscopic Heller myotomy, which is invasive and associated with severe complications, including a transmural perforation rate of 4%-10%, they said in their report.
Their randomized trial included 133 adults with newly diagnosed achalasia enrolled at one of six hospitals in Germany, Hong Kong, Italy, Netherlands, and the United States.
Patients were randomly assigned to undergo 1-2 pneumatic dilations performed by an endoscopist who had performed at least 20 such procedures in the past, or to a POEM procedure likewise performed by an expert who had already done more than 20 such procedures.
At baseline, patients’ Eckardt symptom scores ranged from 6 to 9 on a scale with 0 indicating the lowest severity, to 12 indicating the highest. The median Eckardt scores were 8 in the POEM group and 7 in the pneumatic dilation group.
Treatment success, defined as an Eckardt score under 3 and no severe complications or retreatment at 2 years, was achieved by 58 of 63 patients (92%) in the POEM group, compared with 34 of 63 patients (54%) in the pneumatic dilation group (P less than .001), investigators reported.
Reflux esophagitis was observed in 22 of 54 POEM-treated patients (41%) who underwent endoscopy at a 2-year evaluation, compared with only 2 of 29 patients (7%) who had received the balloon dilation procedure (P = .002). In line with that finding, both reflux symptoms and daily proton pump inhibitor use were more common in the POEM group, investigators said.
However, there were no differences between POEM and pneumatic dilation groups in quality of life and other secondary endpoints, including median barium column height and median integrated relaxation pressure, they reported.
Two serious adverse events related to treatment were seen, according to investigators, including one perforation requiring an endoscopic closure plus antibiotics and hospitalization for 13 days, and one hospital admission for a night because of severe chest pain with no signs of perforation.
“Although POEM is more invasive and requires more technical endoscopic skills, the risk of severe complications was not higher than with pneumatic dilation, especially when performed by experienced endoscopists,” Dr. Bredenoord and coauthors said in their report.
However, these results do not imply that the traditional dilation procedure should be abandoned, they said, as POEM is more invasive, more involved, and more likely to result in reflux esophagitis.
“It seems reasonable to offer both options to treatment-naive patients with achalasia and counsel them to select treatment based on the patient’s characteristics, personal preference, comorbidity, and disease subtype,” they said.
Funding for the study came from Fonds NutsOhra and the European Society of Gastrointestinal Endoscopy. Dr. Bredenoord reported disclosures related to Norgine, Laborie, Medtronic, Diversatek, Nutricia, Regeneron, Celgene, Bayer, and Dr. Falk Pharma.
SOURCE: Ponds FA et al. JAMA. 2019;322(2):134-44. doi: 10.1001/jama.2019.8859.
Peroral endoscopic myotomy (POEM) had a success rate exceeding 90%, versus just about 50% for standard balloon dilation in what investigators say is, to their knowledge, the first-ever randomized trial to evaluate POEM as a first-line modality for this esophageal motility disorder.
Reflux esophagitis was the major downside of POEM, according to investigators, who reported the complication in 41% of patients at a 2-year follow-up, as compared to just 7% of patients undergoing the standard balloon dilation.
Nevertheless, there were no serious adverse events among 63 POEM-treated patients, while one patient out of 63 undergoing pneumatic dilation had a perforation that required endoscopic closure and hospitalization, according to senior study author Albert J. Bredenoord, MD, PhD, of Amsterdam University Medical Center.
“These findings support consideration of POEM as an initial treatment option for patients with achalasia,” Dr. Bredenoord and coinvestigators said in a report on the study appearing in JAMA.
While endoscopic pneumatic dilation is the usual treatment for achalasia, POEM has become more commonly used following case series showing high rates of efficacy, according to the authors.
The POEM procedure also offers advantages over laparoscopic Heller myotomy, which is invasive and associated with severe complications, including a transmural perforation rate of 4%-10%, they said in their report.
Their randomized trial included 133 adults with newly diagnosed achalasia enrolled at one of six hospitals in Germany, Hong Kong, Italy, Netherlands, and the United States.
Patients were randomly assigned to undergo 1-2 pneumatic dilations performed by an endoscopist who had performed at least 20 such procedures in the past, or to a POEM procedure likewise performed by an expert who had already done more than 20 such procedures.
At baseline, patients’ Eckardt symptom scores ranged from 6 to 9 on a scale with 0 indicating the lowest severity, to 12 indicating the highest. The median Eckardt scores were 8 in the POEM group and 7 in the pneumatic dilation group.
Treatment success, defined as an Eckardt score under 3 and no severe complications or retreatment at 2 years, was achieved by 58 of 63 patients (92%) in the POEM group, compared with 34 of 63 patients (54%) in the pneumatic dilation group (P less than .001), investigators reported.
Reflux esophagitis was observed in 22 of 54 POEM-treated patients (41%) who underwent endoscopy at a 2-year evaluation, compared with only 2 of 29 patients (7%) who had received the balloon dilation procedure (P = .002). In line with that finding, both reflux symptoms and daily proton pump inhibitor use were more common in the POEM group, investigators said.
However, there were no differences between POEM and pneumatic dilation groups in quality of life and other secondary endpoints, including median barium column height and median integrated relaxation pressure, they reported.
Two serious adverse events related to treatment were seen, according to investigators, including one perforation requiring an endoscopic closure plus antibiotics and hospitalization for 13 days, and one hospital admission for a night because of severe chest pain with no signs of perforation.
“Although POEM is more invasive and requires more technical endoscopic skills, the risk of severe complications was not higher than with pneumatic dilation, especially when performed by experienced endoscopists,” Dr. Bredenoord and coauthors said in their report.
However, these results do not imply that the traditional dilation procedure should be abandoned, they said, as POEM is more invasive, more involved, and more likely to result in reflux esophagitis.
“It seems reasonable to offer both options to treatment-naive patients with achalasia and counsel them to select treatment based on the patient’s characteristics, personal preference, comorbidity, and disease subtype,” they said.
Funding for the study came from Fonds NutsOhra and the European Society of Gastrointestinal Endoscopy. Dr. Bredenoord reported disclosures related to Norgine, Laborie, Medtronic, Diversatek, Nutricia, Regeneron, Celgene, Bayer, and Dr. Falk Pharma.
SOURCE: Ponds FA et al. JAMA. 2019;322(2):134-44. doi: 10.1001/jama.2019.8859.
Peroral endoscopic myotomy (POEM) had a success rate exceeding 90%, versus just about 50% for standard balloon dilation in what investigators say is, to their knowledge, the first-ever randomized trial to evaluate POEM as a first-line modality for this esophageal motility disorder.
Reflux esophagitis was the major downside of POEM, according to investigators, who reported the complication in 41% of patients at a 2-year follow-up, as compared to just 7% of patients undergoing the standard balloon dilation.
Nevertheless, there were no serious adverse events among 63 POEM-treated patients, while one patient out of 63 undergoing pneumatic dilation had a perforation that required endoscopic closure and hospitalization, according to senior study author Albert J. Bredenoord, MD, PhD, of Amsterdam University Medical Center.
“These findings support consideration of POEM as an initial treatment option for patients with achalasia,” Dr. Bredenoord and coinvestigators said in a report on the study appearing in JAMA.
While endoscopic pneumatic dilation is the usual treatment for achalasia, POEM has become more commonly used following case series showing high rates of efficacy, according to the authors.
The POEM procedure also offers advantages over laparoscopic Heller myotomy, which is invasive and associated with severe complications, including a transmural perforation rate of 4%-10%, they said in their report.
Their randomized trial included 133 adults with newly diagnosed achalasia enrolled at one of six hospitals in Germany, Hong Kong, Italy, Netherlands, and the United States.
Patients were randomly assigned to undergo 1-2 pneumatic dilations performed by an endoscopist who had performed at least 20 such procedures in the past, or to a POEM procedure likewise performed by an expert who had already done more than 20 such procedures.
At baseline, patients’ Eckardt symptom scores ranged from 6 to 9 on a scale with 0 indicating the lowest severity, to 12 indicating the highest. The median Eckardt scores were 8 in the POEM group and 7 in the pneumatic dilation group.
Treatment success, defined as an Eckardt score under 3 and no severe complications or retreatment at 2 years, was achieved by 58 of 63 patients (92%) in the POEM group, compared with 34 of 63 patients (54%) in the pneumatic dilation group (P less than .001), investigators reported.
Reflux esophagitis was observed in 22 of 54 POEM-treated patients (41%) who underwent endoscopy at a 2-year evaluation, compared with only 2 of 29 patients (7%) who had received the balloon dilation procedure (P = .002). In line with that finding, both reflux symptoms and daily proton pump inhibitor use were more common in the POEM group, investigators said.
However, there were no differences between POEM and pneumatic dilation groups in quality of life and other secondary endpoints, including median barium column height and median integrated relaxation pressure, they reported.
Two serious adverse events related to treatment were seen, according to investigators, including one perforation requiring an endoscopic closure plus antibiotics and hospitalization for 13 days, and one hospital admission for a night because of severe chest pain with no signs of perforation.
“Although POEM is more invasive and requires more technical endoscopic skills, the risk of severe complications was not higher than with pneumatic dilation, especially when performed by experienced endoscopists,” Dr. Bredenoord and coauthors said in their report.
However, these results do not imply that the traditional dilation procedure should be abandoned, they said, as POEM is more invasive, more involved, and more likely to result in reflux esophagitis.
“It seems reasonable to offer both options to treatment-naive patients with achalasia and counsel them to select treatment based on the patient’s characteristics, personal preference, comorbidity, and disease subtype,” they said.
Funding for the study came from Fonds NutsOhra and the European Society of Gastrointestinal Endoscopy. Dr. Bredenoord reported disclosures related to Norgine, Laborie, Medtronic, Diversatek, Nutricia, Regeneron, Celgene, Bayer, and Dr. Falk Pharma.
SOURCE: Ponds FA et al. JAMA. 2019;322(2):134-44. doi: 10.1001/jama.2019.8859.
FROM JAMA
Sickle cell unit running 24/7 reduces readmissions, emergency visits
FORT LAUDERDALE, FLA. — A dedicated, 24-hour, 7-day-a-week sickle cell inpatient observation unit staffed by a multidisciplinary care team significantly reduced inpatient admissions and emergency department visits per patient, according to an analysis of a Philadelphia program.
“These findings confirm the need for an individualized approach to treatment,” Sanaa Rizk, MD, director of the hereditary anemia program at Thomas Jefferson University Hospital, Philadelphia, said at the annual meeting of the Foundation for Sickle Cell Disease Research. “The potential strength of a multidisciplinary approach and personalized interventions toward high-utilizing subpopulations may offer the greatest impact.”
The study evaluated what Dr. Rizk called “the second clinical transformation” in care of sickle cell disease patients, which Thomas Jefferson University implemented in 2015. The comprehensive sickle cell center first opened in 2003, and the first transformation in November 2013 consisted of opening a four-bed sickle cell day unit to treat uncomplicated sickle cell vaso-occlusive crises with personalized pain treatment protocols (including IV fluids and opioids). It was staffed by a nurse practitioner, medical assistant, and two registered nurses from 8 a.m. to 5 p.m.
The second transformation transferred care to the inpatient observation unit on the hospital floor with access to 12 patient beds. A sickle cell nurse practitioner sees patients for same-day appointments, conducts sick visits, performs outreach, and handles follow-up with patients. The rest of the multidisciplinary team includes hospitalists, hematologists, internists, and a social worker who performs weekly inpatient rounds and meets monthly with ED leaders and pharmacists.
With the first transformation, ED visits per patient fell from 3.67 to 2.14 a year (P less than .001), and inpatient admissions per patient fell from 1.33 to 0.63 (P less than .0001), Dr. Rizk reported.
The second transformation reduced those per-patient rates even further, to 0.47 ED visits (P less than .01) and 0.29 inpatient admissions (P less than .001), she said.
“The expansion of the service reduced admissions and ED use significantly,” Dr. Rizk said.
She added that a subanalysis of the high-utilizer subgroup showed a decrease in average total medical charges by approximately $100,000/patient per year.
Dr. Rizk reported having no relevant financial disclosures.
SOURCE: Rizk S et al. FSCDR 2019, Abstract JSCDH-D-19-00049.
FORT LAUDERDALE, FLA. — A dedicated, 24-hour, 7-day-a-week sickle cell inpatient observation unit staffed by a multidisciplinary care team significantly reduced inpatient admissions and emergency department visits per patient, according to an analysis of a Philadelphia program.
“These findings confirm the need for an individualized approach to treatment,” Sanaa Rizk, MD, director of the hereditary anemia program at Thomas Jefferson University Hospital, Philadelphia, said at the annual meeting of the Foundation for Sickle Cell Disease Research. “The potential strength of a multidisciplinary approach and personalized interventions toward high-utilizing subpopulations may offer the greatest impact.”
The study evaluated what Dr. Rizk called “the second clinical transformation” in care of sickle cell disease patients, which Thomas Jefferson University implemented in 2015. The comprehensive sickle cell center first opened in 2003, and the first transformation in November 2013 consisted of opening a four-bed sickle cell day unit to treat uncomplicated sickle cell vaso-occlusive crises with personalized pain treatment protocols (including IV fluids and opioids). It was staffed by a nurse practitioner, medical assistant, and two registered nurses from 8 a.m. to 5 p.m.
The second transformation transferred care to the inpatient observation unit on the hospital floor with access to 12 patient beds. A sickle cell nurse practitioner sees patients for same-day appointments, conducts sick visits, performs outreach, and handles follow-up with patients. The rest of the multidisciplinary team includes hospitalists, hematologists, internists, and a social worker who performs weekly inpatient rounds and meets monthly with ED leaders and pharmacists.
With the first transformation, ED visits per patient fell from 3.67 to 2.14 a year (P less than .001), and inpatient admissions per patient fell from 1.33 to 0.63 (P less than .0001), Dr. Rizk reported.
The second transformation reduced those per-patient rates even further, to 0.47 ED visits (P less than .01) and 0.29 inpatient admissions (P less than .001), she said.
“The expansion of the service reduced admissions and ED use significantly,” Dr. Rizk said.
She added that a subanalysis of the high-utilizer subgroup showed a decrease in average total medical charges by approximately $100,000/patient per year.
Dr. Rizk reported having no relevant financial disclosures.
SOURCE: Rizk S et al. FSCDR 2019, Abstract JSCDH-D-19-00049.
FORT LAUDERDALE, FLA. — A dedicated, 24-hour, 7-day-a-week sickle cell inpatient observation unit staffed by a multidisciplinary care team significantly reduced inpatient admissions and emergency department visits per patient, according to an analysis of a Philadelphia program.
“These findings confirm the need for an individualized approach to treatment,” Sanaa Rizk, MD, director of the hereditary anemia program at Thomas Jefferson University Hospital, Philadelphia, said at the annual meeting of the Foundation for Sickle Cell Disease Research. “The potential strength of a multidisciplinary approach and personalized interventions toward high-utilizing subpopulations may offer the greatest impact.”
The study evaluated what Dr. Rizk called “the second clinical transformation” in care of sickle cell disease patients, which Thomas Jefferson University implemented in 2015. The comprehensive sickle cell center first opened in 2003, and the first transformation in November 2013 consisted of opening a four-bed sickle cell day unit to treat uncomplicated sickle cell vaso-occlusive crises with personalized pain treatment protocols (including IV fluids and opioids). It was staffed by a nurse practitioner, medical assistant, and two registered nurses from 8 a.m. to 5 p.m.
The second transformation transferred care to the inpatient observation unit on the hospital floor with access to 12 patient beds. A sickle cell nurse practitioner sees patients for same-day appointments, conducts sick visits, performs outreach, and handles follow-up with patients. The rest of the multidisciplinary team includes hospitalists, hematologists, internists, and a social worker who performs weekly inpatient rounds and meets monthly with ED leaders and pharmacists.
With the first transformation, ED visits per patient fell from 3.67 to 2.14 a year (P less than .001), and inpatient admissions per patient fell from 1.33 to 0.63 (P less than .0001), Dr. Rizk reported.
The second transformation reduced those per-patient rates even further, to 0.47 ED visits (P less than .01) and 0.29 inpatient admissions (P less than .001), she said.
“The expansion of the service reduced admissions and ED use significantly,” Dr. Rizk said.
She added that a subanalysis of the high-utilizer subgroup showed a decrease in average total medical charges by approximately $100,000/patient per year.
Dr. Rizk reported having no relevant financial disclosures.
SOURCE: Rizk S et al. FSCDR 2019, Abstract JSCDH-D-19-00049.
REPORTING FROM FSCDR 2019
DECLARE-TIMI58 shows improved kidney function with dapagliflozin
SAN FRANCISCO – and it showed reductions in the relative risk of renal-specific and cardiorenal outcomes of 47% and 24%, respectively, over 4 years, according to an analysis of the DECLARE-TIMI58 trial presented at the annual scientific sessions of the American Diabetes Association.
The findings add to a message physicians are hearing more and more frequently – that SGLT2 inhibitors, which also include empagliflozin (Jardiance) and canagliflozin (Invokana), could be useful for the early prevention of chronic kidney disease in patients who have type 2 diabetes. The main difference between the DECLARE-TIMI58 and previous study data was that most of its population did not have chronic kidney disease.
The drug should be given “at a very early stage of disease – it can reverse the disease and its complications [and] change the outcomes of patients,” said investigator Itamar Raz, MD, of Hebrew University, Jerusalem, in presenting the findings, which were published simultaneously in the Lancet Diabetes & Endocrinology (2019 Jun 10. doi: 10.1016/S2213-8587[19]30180-9).
What’s new in this renal analysis of DECLARE-TIMI58 is that patients were relatively healthy – 60% of them were without established cardiovascular disease and had much better renal function at baseline, compared with patients in other studies. About half of the patients started out with an estimated glomerular filtration rate (eGFR) more than 90 mL/min per 1.73 m2, which means that they had normal kidney function, and most of the rest of the patients had normal to near-normal renal function or mild renal failure. Findings from previous trials with SGLT2 inhibitors that showed renal protection generally included patients with established cardiovascular disease who started out with greater kidney impairment.
Previous findings have also demonstrated cardioprotective effects with SGLT2 inhibitors in patients with type 2 diabetes. For instance, in earlier results from the DECLARE-TIMI58 trial, dapagliflozin reduced the frequency of cardiovascular death or hospitalization for heart failure, compared with placebo (4.9% vs. 5.8%, respectively; New Engl J Med. 2019;380:347-57), although it did not significantly reduce the frequency of stroke, heart attack, or all-cause death, unlike the results from glucagonlike peptide–1 receptor agonists in much sicker patients (Circulation. 2019;139[17]:2022-31).
Taken as a whole, Dr. Raz said that findings for SGLT2 inhibitors, which, like the glucagonlike peptide–1 receptor agonists, are indicated as second-line therapy in type 2 diabetes after metformin, suggest that they should be used sooner in type 2 disease, perhaps in patients with a hemoglobin A1c level as low at 6.5%.
The absolute benefits of dapagliflozin, compared with placebo, notwithstanding, there are safety concerns with SGLT2s, including genitourinary infections, acute kidney injury, Fournier gangrene, diabetic ketoacidosis, bone fractures, and leg amputations (the latter two only with canagliflozin), many of which have been subject to warnings from the Food and Drug Administration.
Safety outcomes for dapagliflozin, compared with placebo, were not tabulated in the new renal report, but the authors noted a previously reported decrease of 30% in risk for acute kidney injury and major hypoglycemia with dapagliflozin over placebo. There was 1 case of Fournier gangrene with the drug versus 5 with placebo; 27 cases of diabetic ketoacidosis versus 12, respectively; 123 amputations versus 113; and 76 genital infections versus 9.
Matthew Riddle, MD, of Oregon Health and Science University, Portland, said SGLT2 studies “have shown important short-term benefit, but we have no information on long-term safety. These drugs are not physiologic; they do something powerful, but it’s nothing you see in nature. We don’t really know what they do yet; the physiology is still being worked out.”
DECLARE-TIMI58 randomized 8,582 patients with type 2 diabetes to receive dapagliflozin 10 mg orally daily and 8,578 patients to receive placebo. The patients remained on routine diabetes and cardiovascular care. Inclusion criteria included either established atherosclerotic cardiovascular disease (41% of patients) or cardiovascular risk factors (almost 60%), and creatinine clearance of at least 60 mL/min. Median follow-up was 4.2 years.
Overall, 4.2% of patients receiving dapagliflozin and 5.3% of those receiving placebo, met a prespecified secondary cardiorenal composite outcome of end-stage renal disease; death from renal or cardiovascular causes; or a decline of at least 40% in eGFR to less than 60 mL/min per 1.73 m2 according to two tests at least 4 weeks apart (hazard ratio, 0.76; P less than .0001).
Similarly, 1.5% of dapagliflozin patients and 2.6% of those on placebo met a prespecified renal-specific composite of those factors minus death from cardiovascular causes (HR, 0.53; P less than .0001).
Among placebo-treated patients, 2.5% had a sustained decline in eGFR of at least 40% to less than 60 mL/min per 1.73 m2, compared with 1.4% in the dapagliflozin group (HR, 0.54; P less than .0001). There were 11 cases of end-stage renal disease or renal death with dapagliflozin (0.1%), compared with 27 (0.3%) with placebo (HR, 0.41; P = .012).
Among patients who entered the trial with significant renal impairment – an eGFR of less than 60 mL/min per 1.73 m2 – the difference in further renal decline with dapagliflozin was not statistically significant against placebo because of the small number of patients, but Dr. Raz said the drug should still be used earlier in type 2 disease.
Dapagliflozin patients fared worse on renal measurements at 6 months, but caught up by year 2, and surpassed placebo at years 3 and 4, the authors wrote in the latest report.
Just more than 60% of participants were men; patients were in their 60s, on average, and overweight. About 80% of the patients were white.
AstraZeneca, which makes dapagliflozin, was involved with study design, data collection, data analysis, interpretation, and writing of the report. Four authors were employees of the company, and all but two of the 17 others, including Dr. Raz, disclosed personal payments from the company and/or research funding. Dr. Riddle disclosed receiving research funding from AstraZeneca.
SAN FRANCISCO – and it showed reductions in the relative risk of renal-specific and cardiorenal outcomes of 47% and 24%, respectively, over 4 years, according to an analysis of the DECLARE-TIMI58 trial presented at the annual scientific sessions of the American Diabetes Association.
The findings add to a message physicians are hearing more and more frequently – that SGLT2 inhibitors, which also include empagliflozin (Jardiance) and canagliflozin (Invokana), could be useful for the early prevention of chronic kidney disease in patients who have type 2 diabetes. The main difference between the DECLARE-TIMI58 and previous study data was that most of its population did not have chronic kidney disease.
The drug should be given “at a very early stage of disease – it can reverse the disease and its complications [and] change the outcomes of patients,” said investigator Itamar Raz, MD, of Hebrew University, Jerusalem, in presenting the findings, which were published simultaneously in the Lancet Diabetes & Endocrinology (2019 Jun 10. doi: 10.1016/S2213-8587[19]30180-9).
What’s new in this renal analysis of DECLARE-TIMI58 is that patients were relatively healthy – 60% of them were without established cardiovascular disease and had much better renal function at baseline, compared with patients in other studies. About half of the patients started out with an estimated glomerular filtration rate (eGFR) more than 90 mL/min per 1.73 m2, which means that they had normal kidney function, and most of the rest of the patients had normal to near-normal renal function or mild renal failure. Findings from previous trials with SGLT2 inhibitors that showed renal protection generally included patients with established cardiovascular disease who started out with greater kidney impairment.
Previous findings have also demonstrated cardioprotective effects with SGLT2 inhibitors in patients with type 2 diabetes. For instance, in earlier results from the DECLARE-TIMI58 trial, dapagliflozin reduced the frequency of cardiovascular death or hospitalization for heart failure, compared with placebo (4.9% vs. 5.8%, respectively; New Engl J Med. 2019;380:347-57), although it did not significantly reduce the frequency of stroke, heart attack, or all-cause death, unlike the results from glucagonlike peptide–1 receptor agonists in much sicker patients (Circulation. 2019;139[17]:2022-31).
Taken as a whole, Dr. Raz said that findings for SGLT2 inhibitors, which, like the glucagonlike peptide–1 receptor agonists, are indicated as second-line therapy in type 2 diabetes after metformin, suggest that they should be used sooner in type 2 disease, perhaps in patients with a hemoglobin A1c level as low at 6.5%.
The absolute benefits of dapagliflozin, compared with placebo, notwithstanding, there are safety concerns with SGLT2s, including genitourinary infections, acute kidney injury, Fournier gangrene, diabetic ketoacidosis, bone fractures, and leg amputations (the latter two only with canagliflozin), many of which have been subject to warnings from the Food and Drug Administration.
Safety outcomes for dapagliflozin, compared with placebo, were not tabulated in the new renal report, but the authors noted a previously reported decrease of 30% in risk for acute kidney injury and major hypoglycemia with dapagliflozin over placebo. There was 1 case of Fournier gangrene with the drug versus 5 with placebo; 27 cases of diabetic ketoacidosis versus 12, respectively; 123 amputations versus 113; and 76 genital infections versus 9.
Matthew Riddle, MD, of Oregon Health and Science University, Portland, said SGLT2 studies “have shown important short-term benefit, but we have no information on long-term safety. These drugs are not physiologic; they do something powerful, but it’s nothing you see in nature. We don’t really know what they do yet; the physiology is still being worked out.”
DECLARE-TIMI58 randomized 8,582 patients with type 2 diabetes to receive dapagliflozin 10 mg orally daily and 8,578 patients to receive placebo. The patients remained on routine diabetes and cardiovascular care. Inclusion criteria included either established atherosclerotic cardiovascular disease (41% of patients) or cardiovascular risk factors (almost 60%), and creatinine clearance of at least 60 mL/min. Median follow-up was 4.2 years.
Overall, 4.2% of patients receiving dapagliflozin and 5.3% of those receiving placebo, met a prespecified secondary cardiorenal composite outcome of end-stage renal disease; death from renal or cardiovascular causes; or a decline of at least 40% in eGFR to less than 60 mL/min per 1.73 m2 according to two tests at least 4 weeks apart (hazard ratio, 0.76; P less than .0001).
Similarly, 1.5% of dapagliflozin patients and 2.6% of those on placebo met a prespecified renal-specific composite of those factors minus death from cardiovascular causes (HR, 0.53; P less than .0001).
Among placebo-treated patients, 2.5% had a sustained decline in eGFR of at least 40% to less than 60 mL/min per 1.73 m2, compared with 1.4% in the dapagliflozin group (HR, 0.54; P less than .0001). There were 11 cases of end-stage renal disease or renal death with dapagliflozin (0.1%), compared with 27 (0.3%) with placebo (HR, 0.41; P = .012).
Among patients who entered the trial with significant renal impairment – an eGFR of less than 60 mL/min per 1.73 m2 – the difference in further renal decline with dapagliflozin was not statistically significant against placebo because of the small number of patients, but Dr. Raz said the drug should still be used earlier in type 2 disease.
Dapagliflozin patients fared worse on renal measurements at 6 months, but caught up by year 2, and surpassed placebo at years 3 and 4, the authors wrote in the latest report.
Just more than 60% of participants were men; patients were in their 60s, on average, and overweight. About 80% of the patients were white.
AstraZeneca, which makes dapagliflozin, was involved with study design, data collection, data analysis, interpretation, and writing of the report. Four authors were employees of the company, and all but two of the 17 others, including Dr. Raz, disclosed personal payments from the company and/or research funding. Dr. Riddle disclosed receiving research funding from AstraZeneca.
SAN FRANCISCO – and it showed reductions in the relative risk of renal-specific and cardiorenal outcomes of 47% and 24%, respectively, over 4 years, according to an analysis of the DECLARE-TIMI58 trial presented at the annual scientific sessions of the American Diabetes Association.
The findings add to a message physicians are hearing more and more frequently – that SGLT2 inhibitors, which also include empagliflozin (Jardiance) and canagliflozin (Invokana), could be useful for the early prevention of chronic kidney disease in patients who have type 2 diabetes. The main difference between the DECLARE-TIMI58 and previous study data was that most of its population did not have chronic kidney disease.
The drug should be given “at a very early stage of disease – it can reverse the disease and its complications [and] change the outcomes of patients,” said investigator Itamar Raz, MD, of Hebrew University, Jerusalem, in presenting the findings, which were published simultaneously in the Lancet Diabetes & Endocrinology (2019 Jun 10. doi: 10.1016/S2213-8587[19]30180-9).
What’s new in this renal analysis of DECLARE-TIMI58 is that patients were relatively healthy – 60% of them were without established cardiovascular disease and had much better renal function at baseline, compared with patients in other studies. About half of the patients started out with an estimated glomerular filtration rate (eGFR) more than 90 mL/min per 1.73 m2, which means that they had normal kidney function, and most of the rest of the patients had normal to near-normal renal function or mild renal failure. Findings from previous trials with SGLT2 inhibitors that showed renal protection generally included patients with established cardiovascular disease who started out with greater kidney impairment.
Previous findings have also demonstrated cardioprotective effects with SGLT2 inhibitors in patients with type 2 diabetes. For instance, in earlier results from the DECLARE-TIMI58 trial, dapagliflozin reduced the frequency of cardiovascular death or hospitalization for heart failure, compared with placebo (4.9% vs. 5.8%, respectively; New Engl J Med. 2019;380:347-57), although it did not significantly reduce the frequency of stroke, heart attack, or all-cause death, unlike the results from glucagonlike peptide–1 receptor agonists in much sicker patients (Circulation. 2019;139[17]:2022-31).
Taken as a whole, Dr. Raz said that findings for SGLT2 inhibitors, which, like the glucagonlike peptide–1 receptor agonists, are indicated as second-line therapy in type 2 diabetes after metformin, suggest that they should be used sooner in type 2 disease, perhaps in patients with a hemoglobin A1c level as low at 6.5%.
The absolute benefits of dapagliflozin, compared with placebo, notwithstanding, there are safety concerns with SGLT2s, including genitourinary infections, acute kidney injury, Fournier gangrene, diabetic ketoacidosis, bone fractures, and leg amputations (the latter two only with canagliflozin), many of which have been subject to warnings from the Food and Drug Administration.
Safety outcomes for dapagliflozin, compared with placebo, were not tabulated in the new renal report, but the authors noted a previously reported decrease of 30% in risk for acute kidney injury and major hypoglycemia with dapagliflozin over placebo. There was 1 case of Fournier gangrene with the drug versus 5 with placebo; 27 cases of diabetic ketoacidosis versus 12, respectively; 123 amputations versus 113; and 76 genital infections versus 9.
Matthew Riddle, MD, of Oregon Health and Science University, Portland, said SGLT2 studies “have shown important short-term benefit, but we have no information on long-term safety. These drugs are not physiologic; they do something powerful, but it’s nothing you see in nature. We don’t really know what they do yet; the physiology is still being worked out.”
DECLARE-TIMI58 randomized 8,582 patients with type 2 diabetes to receive dapagliflozin 10 mg orally daily and 8,578 patients to receive placebo. The patients remained on routine diabetes and cardiovascular care. Inclusion criteria included either established atherosclerotic cardiovascular disease (41% of patients) or cardiovascular risk factors (almost 60%), and creatinine clearance of at least 60 mL/min. Median follow-up was 4.2 years.
Overall, 4.2% of patients receiving dapagliflozin and 5.3% of those receiving placebo, met a prespecified secondary cardiorenal composite outcome of end-stage renal disease; death from renal or cardiovascular causes; or a decline of at least 40% in eGFR to less than 60 mL/min per 1.73 m2 according to two tests at least 4 weeks apart (hazard ratio, 0.76; P less than .0001).
Similarly, 1.5% of dapagliflozin patients and 2.6% of those on placebo met a prespecified renal-specific composite of those factors minus death from cardiovascular causes (HR, 0.53; P less than .0001).
Among placebo-treated patients, 2.5% had a sustained decline in eGFR of at least 40% to less than 60 mL/min per 1.73 m2, compared with 1.4% in the dapagliflozin group (HR, 0.54; P less than .0001). There were 11 cases of end-stage renal disease or renal death with dapagliflozin (0.1%), compared with 27 (0.3%) with placebo (HR, 0.41; P = .012).
Among patients who entered the trial with significant renal impairment – an eGFR of less than 60 mL/min per 1.73 m2 – the difference in further renal decline with dapagliflozin was not statistically significant against placebo because of the small number of patients, but Dr. Raz said the drug should still be used earlier in type 2 disease.
Dapagliflozin patients fared worse on renal measurements at 6 months, but caught up by year 2, and surpassed placebo at years 3 and 4, the authors wrote in the latest report.
Just more than 60% of participants were men; patients were in their 60s, on average, and overweight. About 80% of the patients were white.
AstraZeneca, which makes dapagliflozin, was involved with study design, data collection, data analysis, interpretation, and writing of the report. Four authors were employees of the company, and all but two of the 17 others, including Dr. Raz, disclosed personal payments from the company and/or research funding. Dr. Riddle disclosed receiving research funding from AstraZeneca.
REPORTING FROM ADA 2019
Key clinical point: The sodium-glucose transporter 2 inhibitor dapagliflozin slowed progression of kidney disease in patients with type 2 diabetes.
Major finding: Overall, 4.2% of dapagliflozin and 5.3% of placebo patients met a prespecified secondary cardiorenal composite outcome of end-stage renal disease, death from renal or cardiovascular causes, or a decline of at least 40% in estimated glomerular filtration rate to less than 60 mL/min per 1.73m2 (HR, 0.76; P less than .0001).
Study details: Placebo-controlled trial in more than 17,000 patients with type 2 diabetes
Disclosures: AstraZeneca, the maker of dapagliflozin, funded and conducted the study. Four authors were employees of the company, and all but two of the remaining 17, including Dr. Raz, disclosed personal payments from the company and/or research funding. Dr. Riddle reported receiving research funding from AstraZeneca.
Source: Mosenzon O et al. Lancet Diabetes Endocrinol. 2019 Jun 10. doi: 10.1016/S2213-8587(19)30180-9.
Clinics get more time on Title X changes
Family planning clinics will have more time to comply with a Trump administration rule that prohibits physicians from counseling patients about abortion and bars them from referring women for the procedures.
The Department of Health & Human Services does not intend to bring enforcement actions against taxpayer-funded clinics if they are making good-faith efforts to comply with the new rules, according to a memo issued July 20. Such good faith efforts include a written assurance by Aug. 19 that clinics are not providing abortions nor including abortion in their family planning methods, according to the emailed guidance sent by Diane Foley, MD, HHS Deputy Assistant Secretary at the Office of Population Affairs (OPA). Clinics must also detail an action plan describing the steps they will take to comply with the Title X changes and start those actions immediately, according to the memo, obtained by this news organization.
“In the past, the U.S. Department of Health & Human Services, Office of Population Affairs, has exercised enforcement discretion in appropriate circumstances,” according to the memo. “Given the circumstances surrounding the implementation of the final rule, OPA does not intend to bring enforcement actions against Title X recipients that are making, and continue to make, good-faith efforts to comply with the final rule. OPA is committed to working with grantees to assist them in coming into compliance with the requirements of the final rule.”
The decision comes a week after HHS warned family planning clinics that receive federal money to immediately stop providing referrals and counseling on abortion or face revocation of funding. An email from HHS on July 15 stated that the agency was requiring immediate compliance of the Title X changes consistent with recent court rulings. The warning came just before the start of a national Title X grantee meeting held in Washington.
The changes to the Title X program make health clinics ineligible for funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD testing, cancer screenings, and contraception – to low-income individuals. Under the rule, the government will withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services. The rule also imposes physical separation requirements for health centers that offer abortions.
More than 20 states and several abortion rights organizations sued over the rules in four separate states. District judges in Oregon, Washington, and California temporarily blocked the rules from taking effect. In a June 20 decision, the 9th U.S. Circuit Court of Appeals ruled the federal government may go forward with its plan to restrict Title X funding from clinics that provide abortion counseling or that refer patients for abortion services. The decision overturned the lower court injunctions.
In the July 20 memo, Dr. Foley wrote that, in addition to the Aug. 19 requirements, clinics must send written confirmation by Sep. 18 outlining the steps taken to comply with the Title X changes and provide any relevant documentation needed for HHS to verify the compliance. By March 4, 2020, a written statement must be submitted affirming the clinic is in compliance with the requirement for physical separation between Title X services and abortion services.
The National Family Planning & Reproductive Health Association, a plaintiff in one of the challenges, called the administration’s July 20 memo “wholly insufficient” and said the clinics need more guidance about how to move forward with the rule changes.
“It’s just absurd to think that a few bullet points amount to guidance,” association officials said in a July 21 statement. “We urge [the agency] to take the time to properly expand on and better describe how it will interpret aspects of the rule – using examples that reflect the wide range of provider settings and administrative structures present in Title X. Once again, [the agency] falls far short of linking the rule to day-to-day practice, leaving the entire family planning network in the dark on how they need to operate to stay in the program.”
At presstime, HHS had not responded to a message seeking comment on the requirements. In her note, Dr. Foley wrote that more guidance on the changes were forthcoming and that grantees unable to meet the required time line may request a deadline extension from the agency.
HHS has previously said that the Title X changes ensure that grants and contracts awarded under the program fully comply with the statutory program integrity requirements, “thereby fulfilling the purpose of Title X, so that more women and men can receive services that help them consider and achieve both their short-term and long-term family planning needs.”
Family planning clinics will have more time to comply with a Trump administration rule that prohibits physicians from counseling patients about abortion and bars them from referring women for the procedures.
The Department of Health & Human Services does not intend to bring enforcement actions against taxpayer-funded clinics if they are making good-faith efforts to comply with the new rules, according to a memo issued July 20. Such good faith efforts include a written assurance by Aug. 19 that clinics are not providing abortions nor including abortion in their family planning methods, according to the emailed guidance sent by Diane Foley, MD, HHS Deputy Assistant Secretary at the Office of Population Affairs (OPA). Clinics must also detail an action plan describing the steps they will take to comply with the Title X changes and start those actions immediately, according to the memo, obtained by this news organization.
“In the past, the U.S. Department of Health & Human Services, Office of Population Affairs, has exercised enforcement discretion in appropriate circumstances,” according to the memo. “Given the circumstances surrounding the implementation of the final rule, OPA does not intend to bring enforcement actions against Title X recipients that are making, and continue to make, good-faith efforts to comply with the final rule. OPA is committed to working with grantees to assist them in coming into compliance with the requirements of the final rule.”
The decision comes a week after HHS warned family planning clinics that receive federal money to immediately stop providing referrals and counseling on abortion or face revocation of funding. An email from HHS on July 15 stated that the agency was requiring immediate compliance of the Title X changes consistent with recent court rulings. The warning came just before the start of a national Title X grantee meeting held in Washington.
The changes to the Title X program make health clinics ineligible for funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD testing, cancer screenings, and contraception – to low-income individuals. Under the rule, the government will withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services. The rule also imposes physical separation requirements for health centers that offer abortions.
More than 20 states and several abortion rights organizations sued over the rules in four separate states. District judges in Oregon, Washington, and California temporarily blocked the rules from taking effect. In a June 20 decision, the 9th U.S. Circuit Court of Appeals ruled the federal government may go forward with its plan to restrict Title X funding from clinics that provide abortion counseling or that refer patients for abortion services. The decision overturned the lower court injunctions.
In the July 20 memo, Dr. Foley wrote that, in addition to the Aug. 19 requirements, clinics must send written confirmation by Sep. 18 outlining the steps taken to comply with the Title X changes and provide any relevant documentation needed for HHS to verify the compliance. By March 4, 2020, a written statement must be submitted affirming the clinic is in compliance with the requirement for physical separation between Title X services and abortion services.
The National Family Planning & Reproductive Health Association, a plaintiff in one of the challenges, called the administration’s July 20 memo “wholly insufficient” and said the clinics need more guidance about how to move forward with the rule changes.
“It’s just absurd to think that a few bullet points amount to guidance,” association officials said in a July 21 statement. “We urge [the agency] to take the time to properly expand on and better describe how it will interpret aspects of the rule – using examples that reflect the wide range of provider settings and administrative structures present in Title X. Once again, [the agency] falls far short of linking the rule to day-to-day practice, leaving the entire family planning network in the dark on how they need to operate to stay in the program.”
At presstime, HHS had not responded to a message seeking comment on the requirements. In her note, Dr. Foley wrote that more guidance on the changes were forthcoming and that grantees unable to meet the required time line may request a deadline extension from the agency.
HHS has previously said that the Title X changes ensure that grants and contracts awarded under the program fully comply with the statutory program integrity requirements, “thereby fulfilling the purpose of Title X, so that more women and men can receive services that help them consider and achieve both their short-term and long-term family planning needs.”
Family planning clinics will have more time to comply with a Trump administration rule that prohibits physicians from counseling patients about abortion and bars them from referring women for the procedures.
The Department of Health & Human Services does not intend to bring enforcement actions against taxpayer-funded clinics if they are making good-faith efforts to comply with the new rules, according to a memo issued July 20. Such good faith efforts include a written assurance by Aug. 19 that clinics are not providing abortions nor including abortion in their family planning methods, according to the emailed guidance sent by Diane Foley, MD, HHS Deputy Assistant Secretary at the Office of Population Affairs (OPA). Clinics must also detail an action plan describing the steps they will take to comply with the Title X changes and start those actions immediately, according to the memo, obtained by this news organization.
“In the past, the U.S. Department of Health & Human Services, Office of Population Affairs, has exercised enforcement discretion in appropriate circumstances,” according to the memo. “Given the circumstances surrounding the implementation of the final rule, OPA does not intend to bring enforcement actions against Title X recipients that are making, and continue to make, good-faith efforts to comply with the final rule. OPA is committed to working with grantees to assist them in coming into compliance with the requirements of the final rule.”
The decision comes a week after HHS warned family planning clinics that receive federal money to immediately stop providing referrals and counseling on abortion or face revocation of funding. An email from HHS on July 15 stated that the agency was requiring immediate compliance of the Title X changes consistent with recent court rulings. The warning came just before the start of a national Title X grantee meeting held in Washington.
The changes to the Title X program make health clinics ineligible for funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD testing, cancer screenings, and contraception – to low-income individuals. Under the rule, the government will withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services. The rule also imposes physical separation requirements for health centers that offer abortions.
More than 20 states and several abortion rights organizations sued over the rules in four separate states. District judges in Oregon, Washington, and California temporarily blocked the rules from taking effect. In a June 20 decision, the 9th U.S. Circuit Court of Appeals ruled the federal government may go forward with its plan to restrict Title X funding from clinics that provide abortion counseling or that refer patients for abortion services. The decision overturned the lower court injunctions.
In the July 20 memo, Dr. Foley wrote that, in addition to the Aug. 19 requirements, clinics must send written confirmation by Sep. 18 outlining the steps taken to comply with the Title X changes and provide any relevant documentation needed for HHS to verify the compliance. By March 4, 2020, a written statement must be submitted affirming the clinic is in compliance with the requirement for physical separation between Title X services and abortion services.
The National Family Planning & Reproductive Health Association, a plaintiff in one of the challenges, called the administration’s July 20 memo “wholly insufficient” and said the clinics need more guidance about how to move forward with the rule changes.
“It’s just absurd to think that a few bullet points amount to guidance,” association officials said in a July 21 statement. “We urge [the agency] to take the time to properly expand on and better describe how it will interpret aspects of the rule – using examples that reflect the wide range of provider settings and administrative structures present in Title X. Once again, [the agency] falls far short of linking the rule to day-to-day practice, leaving the entire family planning network in the dark on how they need to operate to stay in the program.”
At presstime, HHS had not responded to a message seeking comment on the requirements. In her note, Dr. Foley wrote that more guidance on the changes were forthcoming and that grantees unable to meet the required time line may request a deadline extension from the agency.
HHS has previously said that the Title X changes ensure that grants and contracts awarded under the program fully comply with the statutory program integrity requirements, “thereby fulfilling the purpose of Title X, so that more women and men can receive services that help them consider and achieve both their short-term and long-term family planning needs.”
New WHO recommendations promote dolutegravir benefits in the face of lowered risk signal for neural tube defects
The risk of neural tube defects linked to dolutegravir exposure during pregnancy is lower than previously signaled, according to new reports that have prompted the World Health Organization (WHO) to confirm that this antiviral medication should be the preferred option across all populations.
The use of dolutegravir (DTG) during pregnancy has been a pressing global health question since May 2018, when an unplanned interim analysis of the Tsepamo surveillance study of birth outcomes in Botswana showed four neural tube defects associated with dolutegravir exposure among 426 infants born to HIV-positive women (0.94%).
With follow-up for additional births, however, just one more neural tube defect was identified out of 1,683 deliveries among women who had taken DTG around the time of conception (0.30%), according to a report just presented here at the at the International AIDS Society Conference on HIV Science.
By comparison, prevalence rates of neural tube defects were 0.10% for mothers taking other antiretroviral therapies at conception, 0.04% for those specifically taking efavirenz at conception, and 0.08% in HIV-uninfected mothers, according to the report, which was simultaneously published in the New England Journal of Medicine.
“While there may be a risk for neural tube defects, this risk is small, and really importantly, needs to be weighed against the large potential benefits of dolutegravir,” investigator Rebecca M. Zash, MD, of Beth Israel Deaconess Medical Center, Boston, said here in Mexico City during an IAS 2019 video press conference.
The WHO had previously sounded a note of caution, saying that DTG could be “considered” in women of childbearing age if other first‐line antiretroviral agents such as efavirenz could not be used.
However, following release of new evidence, including the study by Dr. Zash and colleagues, the WHO has come out with a clear recommendation for HIV drug as “the preferred first-line and second-line treatment for all populations, including pregnant women and those of childbearing potential.”
The updated scientific reports and guidelines have important implications for global health. “Many countries have been working to make dolutegravir-based treatment their preferred first-line regimen, as it’s got several advantages over efavirenz, which people have been using for many years now, including its tolerability and resistance profiles, and its impact on morbidity and mortality,” IAS president Anton Pozniak, MD, said in the press conference.
Some countries paused their plans to roll out dolutegravir-based regimens after the preliminary safety signal from the Tsepamo study was reported, Dr. Pozniak added.
In another study presented at IAS looking at dolutegravir use at conception, investigators described an additional surveillance study in Botswana, conducted independently from the Tsepamo study. One neural tube defect was found among 152 deliveries in mothers who had been taking DTG at conception (0.66%), and two neural tube defects among 2,326 deliveries to HIV-negative mothers (0.09%).
Although the number of deliveries are small in this study, the results suggest a risk of neural tube defects with DTG exposure at conception of less than 1%, said Mmakgomo Mimi Raesima, MD, MPH, public health specialist, Ministry of Health and Wellness, Botswana.
Because neural-tube defects might be related to low folate levels, Dr. Raesima said “conversations are continuing” with regard to folate food fortification in Botswana, a country that does not mandate folate-fortified grains.
“We want to capitalize on the momentum from these results,” Dr. Raesima said in the press conference.
The Tsepamo study was funded by the National Institutes of Health. Dr. Zash reported grants during the conduct of the study from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Zash R et al. N Engl J Med. 2019 Jul 22. doi: 10.1056/NEJMoa1905230.
The risk of neural tube defects linked to dolutegravir exposure during pregnancy is lower than previously signaled, according to new reports that have prompted the World Health Organization (WHO) to confirm that this antiviral medication should be the preferred option across all populations.
The use of dolutegravir (DTG) during pregnancy has been a pressing global health question since May 2018, when an unplanned interim analysis of the Tsepamo surveillance study of birth outcomes in Botswana showed four neural tube defects associated with dolutegravir exposure among 426 infants born to HIV-positive women (0.94%).
With follow-up for additional births, however, just one more neural tube defect was identified out of 1,683 deliveries among women who had taken DTG around the time of conception (0.30%), according to a report just presented here at the at the International AIDS Society Conference on HIV Science.
By comparison, prevalence rates of neural tube defects were 0.10% for mothers taking other antiretroviral therapies at conception, 0.04% for those specifically taking efavirenz at conception, and 0.08% in HIV-uninfected mothers, according to the report, which was simultaneously published in the New England Journal of Medicine.
“While there may be a risk for neural tube defects, this risk is small, and really importantly, needs to be weighed against the large potential benefits of dolutegravir,” investigator Rebecca M. Zash, MD, of Beth Israel Deaconess Medical Center, Boston, said here in Mexico City during an IAS 2019 video press conference.
The WHO had previously sounded a note of caution, saying that DTG could be “considered” in women of childbearing age if other first‐line antiretroviral agents such as efavirenz could not be used.
However, following release of new evidence, including the study by Dr. Zash and colleagues, the WHO has come out with a clear recommendation for HIV drug as “the preferred first-line and second-line treatment for all populations, including pregnant women and those of childbearing potential.”
The updated scientific reports and guidelines have important implications for global health. “Many countries have been working to make dolutegravir-based treatment their preferred first-line regimen, as it’s got several advantages over efavirenz, which people have been using for many years now, including its tolerability and resistance profiles, and its impact on morbidity and mortality,” IAS president Anton Pozniak, MD, said in the press conference.
Some countries paused their plans to roll out dolutegravir-based regimens after the preliminary safety signal from the Tsepamo study was reported, Dr. Pozniak added.
In another study presented at IAS looking at dolutegravir use at conception, investigators described an additional surveillance study in Botswana, conducted independently from the Tsepamo study. One neural tube defect was found among 152 deliveries in mothers who had been taking DTG at conception (0.66%), and two neural tube defects among 2,326 deliveries to HIV-negative mothers (0.09%).
Although the number of deliveries are small in this study, the results suggest a risk of neural tube defects with DTG exposure at conception of less than 1%, said Mmakgomo Mimi Raesima, MD, MPH, public health specialist, Ministry of Health and Wellness, Botswana.
Because neural-tube defects might be related to low folate levels, Dr. Raesima said “conversations are continuing” with regard to folate food fortification in Botswana, a country that does not mandate folate-fortified grains.
“We want to capitalize on the momentum from these results,” Dr. Raesima said in the press conference.
The Tsepamo study was funded by the National Institutes of Health. Dr. Zash reported grants during the conduct of the study from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Zash R et al. N Engl J Med. 2019 Jul 22. doi: 10.1056/NEJMoa1905230.
The risk of neural tube defects linked to dolutegravir exposure during pregnancy is lower than previously signaled, according to new reports that have prompted the World Health Organization (WHO) to confirm that this antiviral medication should be the preferred option across all populations.
The use of dolutegravir (DTG) during pregnancy has been a pressing global health question since May 2018, when an unplanned interim analysis of the Tsepamo surveillance study of birth outcomes in Botswana showed four neural tube defects associated with dolutegravir exposure among 426 infants born to HIV-positive women (0.94%).
With follow-up for additional births, however, just one more neural tube defect was identified out of 1,683 deliveries among women who had taken DTG around the time of conception (0.30%), according to a report just presented here at the at the International AIDS Society Conference on HIV Science.
By comparison, prevalence rates of neural tube defects were 0.10% for mothers taking other antiretroviral therapies at conception, 0.04% for those specifically taking efavirenz at conception, and 0.08% in HIV-uninfected mothers, according to the report, which was simultaneously published in the New England Journal of Medicine.
“While there may be a risk for neural tube defects, this risk is small, and really importantly, needs to be weighed against the large potential benefits of dolutegravir,” investigator Rebecca M. Zash, MD, of Beth Israel Deaconess Medical Center, Boston, said here in Mexico City during an IAS 2019 video press conference.
The WHO had previously sounded a note of caution, saying that DTG could be “considered” in women of childbearing age if other first‐line antiretroviral agents such as efavirenz could not be used.
However, following release of new evidence, including the study by Dr. Zash and colleagues, the WHO has come out with a clear recommendation for HIV drug as “the preferred first-line and second-line treatment for all populations, including pregnant women and those of childbearing potential.”
The updated scientific reports and guidelines have important implications for global health. “Many countries have been working to make dolutegravir-based treatment their preferred first-line regimen, as it’s got several advantages over efavirenz, which people have been using for many years now, including its tolerability and resistance profiles, and its impact on morbidity and mortality,” IAS president Anton Pozniak, MD, said in the press conference.
Some countries paused their plans to roll out dolutegravir-based regimens after the preliminary safety signal from the Tsepamo study was reported, Dr. Pozniak added.
In another study presented at IAS looking at dolutegravir use at conception, investigators described an additional surveillance study in Botswana, conducted independently from the Tsepamo study. One neural tube defect was found among 152 deliveries in mothers who had been taking DTG at conception (0.66%), and two neural tube defects among 2,326 deliveries to HIV-negative mothers (0.09%).
Although the number of deliveries are small in this study, the results suggest a risk of neural tube defects with DTG exposure at conception of less than 1%, said Mmakgomo Mimi Raesima, MD, MPH, public health specialist, Ministry of Health and Wellness, Botswana.
Because neural-tube defects might be related to low folate levels, Dr. Raesima said “conversations are continuing” with regard to folate food fortification in Botswana, a country that does not mandate folate-fortified grains.
“We want to capitalize on the momentum from these results,” Dr. Raesima said in the press conference.
The Tsepamo study was funded by the National Institutes of Health. Dr. Zash reported grants during the conduct of the study from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
SOURCE: Zash R et al. N Engl J Med. 2019 Jul 22. doi: 10.1056/NEJMoa1905230.
FROM IAS 2019
Painless Purple Streaks on the Arms and Chest
The Diagnosis: Factitial Purpura
Factitial dermatologic disorders are characterized by skin findings triggered by deliberate manipulation of the skin with objects to create lesions and feign signs of a dermatologic condition to seek emotional and psychological benefit.1 The etiology of the lesions is unclear, and the patient's history of the injury is hollow.2 Most often, there is sudden onset of the lesions without any warning or symptoms. When giving the history, the patient may appear unemotional, does not report pain, and denies self-infliction.1
In factitial purpura, the purple patches are clearly demarcated from uninvolved skin and have an unusual angular or geometric shape. The pattern typically takes the shape of the object used to create the purpura and lacks the features of recognizable dermatoses.2 In our patient and those with similar linear purpuric streaks, we use the term penny purpura to indicate that the lesions resulted from rubbing with a penny or other blunt object, similar to coining. The lesions occur in areas that are easily accessible and visible such as the arms, chest, or chin. It is suggested that the child unconsciously wants the lesions to be seen. Histologic findings in factitial purpura include disruption of collagen fiber bundles and extravasated red blood cells in the dermis.3 Unfortunately, evolving lesions may give nonspecific histologic findings; when the clinical lesions are typical, skin biopsy usually is unnecessary and may be misleading. Laboratory test results such as complete blood cell count, prothrombin time, and partial thromboplastin time usually are within reference range, as in our patient.
When evaluating these patients, confrontation is not recommended. More than two-thirds of affected patients have a history of trauma such as sexual/physical abuse or neglect, and the lesions typically arise during times of stress.1,3 Thus, treatment includes nonaccusatory measures and referral for psychologic evaluation. The purpura will rapidly heal when covered with an occlusive dressing.2
The differential diagnosis for penny purpura includes lesions that evolve from cupping and coining. Cupping is a type of complementary and alternative medicine that acts by correcting imbalances in the internal biofield and restoring the flow of qi, which determines the state of one's health and life span.4 Cupping is performed by placing a glass cup over a painful body part. A partial vacuum is created by flaming, mechanical withdrawal, or thermal cooling of the entrapped air under the cup. When the flame exhausts the supply of oxygen, the skin is sucked into the mouth of the glass, and the skin is bruised painlessly.4
The differential also includes child maltreatment syndrome and other disorders that would potentiate bruising. Intravascular etiologies include idiopathic thrombocytopenic purpura, leukemia, coagulation disorders, and other causes of thrombocytopenia or platelet dysfunction.3 Extravascular etiologies include hereditary collagen vascular disease (eg, Ehlers-Danlos syndrome), malnutrition, and other disorders associated with a decrease in collagen and other tissues that support cutaneous vessels. Vascular etiologies include infectious (eg, Rocky Mountain spotted fever, meningococcemia) and noninfectious vasculitis (eg, Henoch-Schönlein purpura), leaky capillary syndrome, drug reactions, and other disorders associated with a loss of vascular integrity.3
It is important to be able to differentiate self-inflicted lesions in a person who repeatedly acts as if he/she has a physical disorder from those that are created during the practices of cupping or any other cultural healing practice. Vascular disorders, malnutrition, and child abuse also should be excluded.3
For our patient with factitial purpura, we gently encouraged the family to work with the child's pediatrician and a pediatric psychologist to deal with stress related to the recurrent rash and asked them to think of the rash as a result of an external cause; however, we were careful not to blame anyone for the rash.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372; quiz 373.
- Al Hawsawi K, Pope E. Pediatric psychocutaneous disorders: a review of primary psychiatric disorders with dermatologic manifestations. Am J Clin Dermatol. 2011;12:247-257.
- Ring HC, Miller IM, Benfeldt E, et al. Artefactual skin lesions in children and adolescents: review of the literature and two cases of factitious purpura. Int J Dermatol. 2015;54:E27-E32.
- Mehta P, Dhapte V. Cupping therapy: a prudent remedy for a plethora of medical ailments. J Tradit Complement Med. 2015;5:127-134.
The Diagnosis: Factitial Purpura
Factitial dermatologic disorders are characterized by skin findings triggered by deliberate manipulation of the skin with objects to create lesions and feign signs of a dermatologic condition to seek emotional and psychological benefit.1 The etiology of the lesions is unclear, and the patient's history of the injury is hollow.2 Most often, there is sudden onset of the lesions without any warning or symptoms. When giving the history, the patient may appear unemotional, does not report pain, and denies self-infliction.1
In factitial purpura, the purple patches are clearly demarcated from uninvolved skin and have an unusual angular or geometric shape. The pattern typically takes the shape of the object used to create the purpura and lacks the features of recognizable dermatoses.2 In our patient and those with similar linear purpuric streaks, we use the term penny purpura to indicate that the lesions resulted from rubbing with a penny or other blunt object, similar to coining. The lesions occur in areas that are easily accessible and visible such as the arms, chest, or chin. It is suggested that the child unconsciously wants the lesions to be seen. Histologic findings in factitial purpura include disruption of collagen fiber bundles and extravasated red blood cells in the dermis.3 Unfortunately, evolving lesions may give nonspecific histologic findings; when the clinical lesions are typical, skin biopsy usually is unnecessary and may be misleading. Laboratory test results such as complete blood cell count, prothrombin time, and partial thromboplastin time usually are within reference range, as in our patient.
When evaluating these patients, confrontation is not recommended. More than two-thirds of affected patients have a history of trauma such as sexual/physical abuse or neglect, and the lesions typically arise during times of stress.1,3 Thus, treatment includes nonaccusatory measures and referral for psychologic evaluation. The purpura will rapidly heal when covered with an occlusive dressing.2
The differential diagnosis for penny purpura includes lesions that evolve from cupping and coining. Cupping is a type of complementary and alternative medicine that acts by correcting imbalances in the internal biofield and restoring the flow of qi, which determines the state of one's health and life span.4 Cupping is performed by placing a glass cup over a painful body part. A partial vacuum is created by flaming, mechanical withdrawal, or thermal cooling of the entrapped air under the cup. When the flame exhausts the supply of oxygen, the skin is sucked into the mouth of the glass, and the skin is bruised painlessly.4
The differential also includes child maltreatment syndrome and other disorders that would potentiate bruising. Intravascular etiologies include idiopathic thrombocytopenic purpura, leukemia, coagulation disorders, and other causes of thrombocytopenia or platelet dysfunction.3 Extravascular etiologies include hereditary collagen vascular disease (eg, Ehlers-Danlos syndrome), malnutrition, and other disorders associated with a decrease in collagen and other tissues that support cutaneous vessels. Vascular etiologies include infectious (eg, Rocky Mountain spotted fever, meningococcemia) and noninfectious vasculitis (eg, Henoch-Schönlein purpura), leaky capillary syndrome, drug reactions, and other disorders associated with a loss of vascular integrity.3
It is important to be able to differentiate self-inflicted lesions in a person who repeatedly acts as if he/she has a physical disorder from those that are created during the practices of cupping or any other cultural healing practice. Vascular disorders, malnutrition, and child abuse also should be excluded.3
For our patient with factitial purpura, we gently encouraged the family to work with the child's pediatrician and a pediatric psychologist to deal with stress related to the recurrent rash and asked them to think of the rash as a result of an external cause; however, we were careful not to blame anyone for the rash.
The Diagnosis: Factitial Purpura
Factitial dermatologic disorders are characterized by skin findings triggered by deliberate manipulation of the skin with objects to create lesions and feign signs of a dermatologic condition to seek emotional and psychological benefit.1 The etiology of the lesions is unclear, and the patient's history of the injury is hollow.2 Most often, there is sudden onset of the lesions without any warning or symptoms. When giving the history, the patient may appear unemotional, does not report pain, and denies self-infliction.1
In factitial purpura, the purple patches are clearly demarcated from uninvolved skin and have an unusual angular or geometric shape. The pattern typically takes the shape of the object used to create the purpura and lacks the features of recognizable dermatoses.2 In our patient and those with similar linear purpuric streaks, we use the term penny purpura to indicate that the lesions resulted from rubbing with a penny or other blunt object, similar to coining. The lesions occur in areas that are easily accessible and visible such as the arms, chest, or chin. It is suggested that the child unconsciously wants the lesions to be seen. Histologic findings in factitial purpura include disruption of collagen fiber bundles and extravasated red blood cells in the dermis.3 Unfortunately, evolving lesions may give nonspecific histologic findings; when the clinical lesions are typical, skin biopsy usually is unnecessary and may be misleading. Laboratory test results such as complete blood cell count, prothrombin time, and partial thromboplastin time usually are within reference range, as in our patient.
When evaluating these patients, confrontation is not recommended. More than two-thirds of affected patients have a history of trauma such as sexual/physical abuse or neglect, and the lesions typically arise during times of stress.1,3 Thus, treatment includes nonaccusatory measures and referral for psychologic evaluation. The purpura will rapidly heal when covered with an occlusive dressing.2
The differential diagnosis for penny purpura includes lesions that evolve from cupping and coining. Cupping is a type of complementary and alternative medicine that acts by correcting imbalances in the internal biofield and restoring the flow of qi, which determines the state of one's health and life span.4 Cupping is performed by placing a glass cup over a painful body part. A partial vacuum is created by flaming, mechanical withdrawal, or thermal cooling of the entrapped air under the cup. When the flame exhausts the supply of oxygen, the skin is sucked into the mouth of the glass, and the skin is bruised painlessly.4
The differential also includes child maltreatment syndrome and other disorders that would potentiate bruising. Intravascular etiologies include idiopathic thrombocytopenic purpura, leukemia, coagulation disorders, and other causes of thrombocytopenia or platelet dysfunction.3 Extravascular etiologies include hereditary collagen vascular disease (eg, Ehlers-Danlos syndrome), malnutrition, and other disorders associated with a decrease in collagen and other tissues that support cutaneous vessels. Vascular etiologies include infectious (eg, Rocky Mountain spotted fever, meningococcemia) and noninfectious vasculitis (eg, Henoch-Schönlein purpura), leaky capillary syndrome, drug reactions, and other disorders associated with a loss of vascular integrity.3
It is important to be able to differentiate self-inflicted lesions in a person who repeatedly acts as if he/she has a physical disorder from those that are created during the practices of cupping or any other cultural healing practice. Vascular disorders, malnutrition, and child abuse also should be excluded.3
For our patient with factitial purpura, we gently encouraged the family to work with the child's pediatrician and a pediatric psychologist to deal with stress related to the recurrent rash and asked them to think of the rash as a result of an external cause; however, we were careful not to blame anyone for the rash.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372; quiz 373.
- Al Hawsawi K, Pope E. Pediatric psychocutaneous disorders: a review of primary psychiatric disorders with dermatologic manifestations. Am J Clin Dermatol. 2011;12:247-257.
- Ring HC, Miller IM, Benfeldt E, et al. Artefactual skin lesions in children and adolescents: review of the literature and two cases of factitious purpura. Int J Dermatol. 2015;54:E27-E32.
- Mehta P, Dhapte V. Cupping therapy: a prudent remedy for a plethora of medical ailments. J Tradit Complement Med. 2015;5:127-134.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372; quiz 373.
- Al Hawsawi K, Pope E. Pediatric psychocutaneous disorders: a review of primary psychiatric disorders with dermatologic manifestations. Am J Clin Dermatol. 2011;12:247-257.
- Ring HC, Miller IM, Benfeldt E, et al. Artefactual skin lesions in children and adolescents: review of the literature and two cases of factitious purpura. Int J Dermatol. 2015;54:E27-E32.
- Mehta P, Dhapte V. Cupping therapy: a prudent remedy for a plethora of medical ailments. J Tradit Complement Med. 2015;5:127-134.
A 10-year-old boy presented with painless purple streaks on the arms and chest of 2 months' duration. The rash recurred several times per month and cleared without treatment in 3 to 5 days. There was no history of trauma or medication exposure, and he was growing and developing normally.
FDA approves first generics of pregabalin
The generics were approved to manage neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia, and fibromyalgia, as well as neuropathic pain associated with spinal cord injury, and as an adjunctive therapy for the treatment of partial-onset seizures in patients aged 17 years and older. Approvals were granted to Alembic Pharmaceuticals, Alkem Laboratories, Amneal Pharmaceuticals, Dr. Reddy’s Laboratories, InvaGen Pharmaceuticals, MSN Laboratories, Rising Pharmaceuticals, Sciegen Pharmaceuticals, and Teva Pharmaceuticals.
The most common adverse events associated with pregabalin include dizziness, somnolence, dry mouth, swelling, blurred vision, weight gain, and abnormal thinking. Pregabalin must be dispensed with a patient Medication Guide containing a guide to the drug’s uses and risks. Angioedema, hypersensitivity reactions, increased seizure frequency, increased suicidal behavior, and peripheral edema are all possible.
“Today’s approval of the first generics for pregabalin, a widely used medication, is another example of the FDA’s long-standing commitment to advance patient access to lower-cost, high-quality generic medicines,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a press release.
The generics were approved to manage neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia, and fibromyalgia, as well as neuropathic pain associated with spinal cord injury, and as an adjunctive therapy for the treatment of partial-onset seizures in patients aged 17 years and older. Approvals were granted to Alembic Pharmaceuticals, Alkem Laboratories, Amneal Pharmaceuticals, Dr. Reddy’s Laboratories, InvaGen Pharmaceuticals, MSN Laboratories, Rising Pharmaceuticals, Sciegen Pharmaceuticals, and Teva Pharmaceuticals.
The most common adverse events associated with pregabalin include dizziness, somnolence, dry mouth, swelling, blurred vision, weight gain, and abnormal thinking. Pregabalin must be dispensed with a patient Medication Guide containing a guide to the drug’s uses and risks. Angioedema, hypersensitivity reactions, increased seizure frequency, increased suicidal behavior, and peripheral edema are all possible.
“Today’s approval of the first generics for pregabalin, a widely used medication, is another example of the FDA’s long-standing commitment to advance patient access to lower-cost, high-quality generic medicines,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a press release.
The generics were approved to manage neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia, and fibromyalgia, as well as neuropathic pain associated with spinal cord injury, and as an adjunctive therapy for the treatment of partial-onset seizures in patients aged 17 years and older. Approvals were granted to Alembic Pharmaceuticals, Alkem Laboratories, Amneal Pharmaceuticals, Dr. Reddy’s Laboratories, InvaGen Pharmaceuticals, MSN Laboratories, Rising Pharmaceuticals, Sciegen Pharmaceuticals, and Teva Pharmaceuticals.
The most common adverse events associated with pregabalin include dizziness, somnolence, dry mouth, swelling, blurred vision, weight gain, and abnormal thinking. Pregabalin must be dispensed with a patient Medication Guide containing a guide to the drug’s uses and risks. Angioedema, hypersensitivity reactions, increased seizure frequency, increased suicidal behavior, and peripheral edema are all possible.
“Today’s approval of the first generics for pregabalin, a widely used medication, is another example of the FDA’s long-standing commitment to advance patient access to lower-cost, high-quality generic medicines,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a press release.
New measles outbreaks reported in Los Angeles and El Paso
according to the Centers for Disease Control and Prevention.
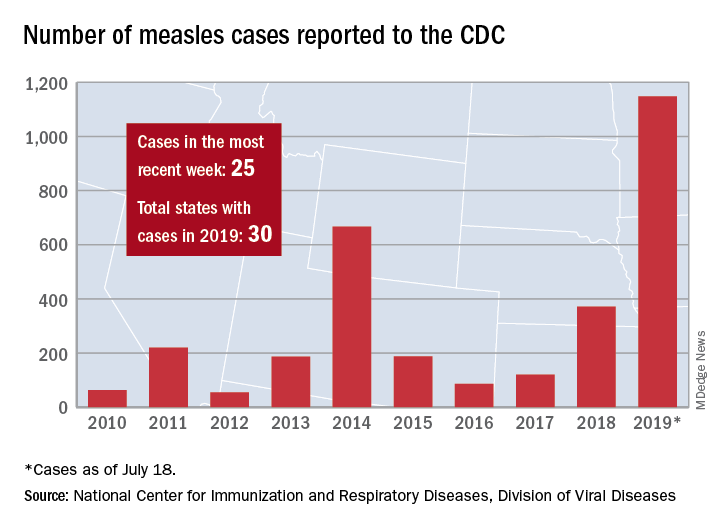
The total number of confirmed cases of measles in the United States is now up to 1,148 for the year, which is 25 more than the previous week, the CDC said on July 22. The highest 1-week total for the year was the 90 cases reported during the week of April 11.
The number of outbreaks is back up to five as California returned to the list after a 1-week absence and El Paso, Tex., made its first appearance of the year. The current outbreak in California – the state’s fifth – is occurring in Los Angeles, which is now up to 16 total cases in 2019. El Paso just reported its fourth case on July 17, and the city’s health department noted that “it had been more than 25 years since El Paso saw its last case of measles before these four recent cases.” Outbreaks also are ongoing in Rockland County, N.Y.; New York City; and three counties in Washington State.
States that joined the ranks of the measles-infected during this most recent reporting week were Alaska and Ohio, which brings the total number to 30 for the year, the CDC said.
The Alaska Department of Health and Social Services said that it “has confirmed a single case of measles in an unvaccinated teenager from the Kenai Peninsula who recently traveled out of state to Arizona via Seattle.” The Ohio case is a “young adult from Stark County [who] recently traveled to a state with confirmed measles cases,” according to the state’s health department.
according to the Centers for Disease Control and Prevention.

The total number of confirmed cases of measles in the United States is now up to 1,148 for the year, which is 25 more than the previous week, the CDC said on July 22. The highest 1-week total for the year was the 90 cases reported during the week of April 11.
The number of outbreaks is back up to five as California returned to the list after a 1-week absence and El Paso, Tex., made its first appearance of the year. The current outbreak in California – the state’s fifth – is occurring in Los Angeles, which is now up to 16 total cases in 2019. El Paso just reported its fourth case on July 17, and the city’s health department noted that “it had been more than 25 years since El Paso saw its last case of measles before these four recent cases.” Outbreaks also are ongoing in Rockland County, N.Y.; New York City; and three counties in Washington State.
States that joined the ranks of the measles-infected during this most recent reporting week were Alaska and Ohio, which brings the total number to 30 for the year, the CDC said.
The Alaska Department of Health and Social Services said that it “has confirmed a single case of measles in an unvaccinated teenager from the Kenai Peninsula who recently traveled out of state to Arizona via Seattle.” The Ohio case is a “young adult from Stark County [who] recently traveled to a state with confirmed measles cases,” according to the state’s health department.
according to the Centers for Disease Control and Prevention.

The total number of confirmed cases of measles in the United States is now up to 1,148 for the year, which is 25 more than the previous week, the CDC said on July 22. The highest 1-week total for the year was the 90 cases reported during the week of April 11.
The number of outbreaks is back up to five as California returned to the list after a 1-week absence and El Paso, Tex., made its first appearance of the year. The current outbreak in California – the state’s fifth – is occurring in Los Angeles, which is now up to 16 total cases in 2019. El Paso just reported its fourth case on July 17, and the city’s health department noted that “it had been more than 25 years since El Paso saw its last case of measles before these four recent cases.” Outbreaks also are ongoing in Rockland County, N.Y.; New York City; and three counties in Washington State.
States that joined the ranks of the measles-infected during this most recent reporting week were Alaska and Ohio, which brings the total number to 30 for the year, the CDC said.
The Alaska Department of Health and Social Services said that it “has confirmed a single case of measles in an unvaccinated teenager from the Kenai Peninsula who recently traveled out of state to Arizona via Seattle.” The Ohio case is a “young adult from Stark County [who] recently traveled to a state with confirmed measles cases,” according to the state’s health department.
Atherosclerotic disease risk persists decades after smoking cessation
Adults who quit smoking reduced their risk for peripheral artery disease in the short term, but remained at increased risk for up to 30 years, compared with never-smokers, based on data from more than 13,000 adults in a community-based study.

Most reports on the impact of smoking cessation on cardiovascular disease have focused on coronary heart disease (CHD), and stroke, while data on the effects of smoking cessation on peripheral artery disease (PAD) are limited, wrote Ning Ding, MBBS, SCM, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, Md., and colleagues.
To compare the impact of smoking on PAD, CHD, and stroke, the researchers used data from the Atherosclerosis Risk in Communities (ARIC) study, which included 15,792 adults aged 45-64 years in four communities. The findings were published in the Journal of the American College of Cardiology.
The study population of 13,355 individuals had no baseline history of PAD, CHD, or stroke. Over a median 26 years of follow-up, the researchers identified 492 cases of PAD, 1,798 cases of CHD, and 1,106 cases of stroke.
The risk of all three conditions began to decline within 5 years of smoking cessation, which could be encouraging to smokers who wish to quit, the researchers noted. In addition, the longer the duration of smoking cessation, the lower the risk for all three conditions (See central illustration).
However, a significantly elevated risk remained for PAD for up to 30 years after smoking cessation and for CHD for up to 20 years after smoking cessation, compared with never-smokers.
The researchers also found a roughly fourfold increased risk for PAD for smokers who smoked for 40 or more pack-years, compared with never-smokers, which was greater than the 2.1 hazard ratio for CHD and 1.8 HR for stroke. In addition, current smokers of at least one pack per day had a significantly greater risk of PAD, compared with never-smokers (HR, 5.36) that was higher than the risk for CHD or stroke (HR, 2.38 and HR, 1.88, respectively).
The study findings were limited by several factors including the reliance on self-reports, potential misclassification of data, and the potential exclusion of mild PAD cases that did not require hospitalization, the researchers noted. However, the results support the value of encouraging smokers to quit and support the need to include PAD risk in public health information, they said. “Although public statements about smoking and [cardiovascular disease] have been focusing on CHD and stroke, our results indicate the need to take account of PAD as well for comprehensively acknowledging the effect of smoking on overall cardiovascular health,” they added.
The ARIC study was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health. Lead author Dr. Ding had no financial conflicts to disclose; coauthors disclosed relationships with Bristol-Myers Squibb and Fukuda Denshi.
SOURCE: Ding N et al. J Am Coll Cardiol. 2019 Jul 22;74:498-507. doi: 10.1016/j.jacc.2019.06.003.
Although the pathophysiology of smoking and cardiovascular disease has yet to be teased out, the current study findings support the public health message that any and all smokers can improve their health by quitting any time: “It is never too early or too late to benefit from quitting,” wrote Nancy A. Rigotti, MD, and Mary M. McDermott, MD, in an accompanying editorial. The editorialists questioned whether the findings were generalizable to patients with mild PAD or those who are not hospitalized. However, they found the data consistent with previous studies suggesting that atherosclerosis is not homogeneous. “Differences in shear stress and hemodynamic forces among the femoral, coronary, and carotid arterial beds may also explain variability in associations of smoking and smoking cessation with the incidence of PAD versus myocardial infarction or stroke,” they said.
The findings also support the need to emphasize PAD in public health messages and provide an opportunity to educate patients about the risks of limb loss and impaired mobility associated with PAD, they said.
Many clinicians put a low priority on smoking cessation, the editorialists wrote, but “long-term tobacco abstinence is achievable using a chronic disease management approach resembling the strategies used to manage other risk factors,” they said. They cited the American College of Cardiology’s recently released “Expert Consensus Decision Pathway on Tobacco Cessation Treatment.” The pathway outlines advice for clinicians, including how to provide a brief intervention and resources along with advice to quit smoking.
Dr. Rigotti is affiliated with Harvard Medical School, Boston. Dr. McDermott is affiliated with Northwestern University, Chicago. Dr. Rigotti disclosed royalties from UpToDate, serving as a consultant for Achieve Life Sciences, and travel expenses from Pfizer for unpaid consulting. Dr. McDermott disclosed research funding from Regeneron, the National Heart, Lung, and Blood Institute, the National Institute on Aging, and the American Heart Association, plus research support from Chromadex, ReserveAge, Hershey, and ViroMed.
Although the pathophysiology of smoking and cardiovascular disease has yet to be teased out, the current study findings support the public health message that any and all smokers can improve their health by quitting any time: “It is never too early or too late to benefit from quitting,” wrote Nancy A. Rigotti, MD, and Mary M. McDermott, MD, in an accompanying editorial. The editorialists questioned whether the findings were generalizable to patients with mild PAD or those who are not hospitalized. However, they found the data consistent with previous studies suggesting that atherosclerosis is not homogeneous. “Differences in shear stress and hemodynamic forces among the femoral, coronary, and carotid arterial beds may also explain variability in associations of smoking and smoking cessation with the incidence of PAD versus myocardial infarction or stroke,” they said.
The findings also support the need to emphasize PAD in public health messages and provide an opportunity to educate patients about the risks of limb loss and impaired mobility associated with PAD, they said.
Many clinicians put a low priority on smoking cessation, the editorialists wrote, but “long-term tobacco abstinence is achievable using a chronic disease management approach resembling the strategies used to manage other risk factors,” they said. They cited the American College of Cardiology’s recently released “Expert Consensus Decision Pathway on Tobacco Cessation Treatment.” The pathway outlines advice for clinicians, including how to provide a brief intervention and resources along with advice to quit smoking.
Dr. Rigotti is affiliated with Harvard Medical School, Boston. Dr. McDermott is affiliated with Northwestern University, Chicago. Dr. Rigotti disclosed royalties from UpToDate, serving as a consultant for Achieve Life Sciences, and travel expenses from Pfizer for unpaid consulting. Dr. McDermott disclosed research funding from Regeneron, the National Heart, Lung, and Blood Institute, the National Institute on Aging, and the American Heart Association, plus research support from Chromadex, ReserveAge, Hershey, and ViroMed.
Although the pathophysiology of smoking and cardiovascular disease has yet to be teased out, the current study findings support the public health message that any and all smokers can improve their health by quitting any time: “It is never too early or too late to benefit from quitting,” wrote Nancy A. Rigotti, MD, and Mary M. McDermott, MD, in an accompanying editorial. The editorialists questioned whether the findings were generalizable to patients with mild PAD or those who are not hospitalized. However, they found the data consistent with previous studies suggesting that atherosclerosis is not homogeneous. “Differences in shear stress and hemodynamic forces among the femoral, coronary, and carotid arterial beds may also explain variability in associations of smoking and smoking cessation with the incidence of PAD versus myocardial infarction or stroke,” they said.
The findings also support the need to emphasize PAD in public health messages and provide an opportunity to educate patients about the risks of limb loss and impaired mobility associated with PAD, they said.
Many clinicians put a low priority on smoking cessation, the editorialists wrote, but “long-term tobacco abstinence is achievable using a chronic disease management approach resembling the strategies used to manage other risk factors,” they said. They cited the American College of Cardiology’s recently released “Expert Consensus Decision Pathway on Tobacco Cessation Treatment.” The pathway outlines advice for clinicians, including how to provide a brief intervention and resources along with advice to quit smoking.
Dr. Rigotti is affiliated with Harvard Medical School, Boston. Dr. McDermott is affiliated with Northwestern University, Chicago. Dr. Rigotti disclosed royalties from UpToDate, serving as a consultant for Achieve Life Sciences, and travel expenses from Pfizer for unpaid consulting. Dr. McDermott disclosed research funding from Regeneron, the National Heart, Lung, and Blood Institute, the National Institute on Aging, and the American Heart Association, plus research support from Chromadex, ReserveAge, Hershey, and ViroMed.
Adults who quit smoking reduced their risk for peripheral artery disease in the short term, but remained at increased risk for up to 30 years, compared with never-smokers, based on data from more than 13,000 adults in a community-based study.

Most reports on the impact of smoking cessation on cardiovascular disease have focused on coronary heart disease (CHD), and stroke, while data on the effects of smoking cessation on peripheral artery disease (PAD) are limited, wrote Ning Ding, MBBS, SCM, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, Md., and colleagues.
To compare the impact of smoking on PAD, CHD, and stroke, the researchers used data from the Atherosclerosis Risk in Communities (ARIC) study, which included 15,792 adults aged 45-64 years in four communities. The findings were published in the Journal of the American College of Cardiology.
The study population of 13,355 individuals had no baseline history of PAD, CHD, or stroke. Over a median 26 years of follow-up, the researchers identified 492 cases of PAD, 1,798 cases of CHD, and 1,106 cases of stroke.
The risk of all three conditions began to decline within 5 years of smoking cessation, which could be encouraging to smokers who wish to quit, the researchers noted. In addition, the longer the duration of smoking cessation, the lower the risk for all three conditions (See central illustration).
However, a significantly elevated risk remained for PAD for up to 30 years after smoking cessation and for CHD for up to 20 years after smoking cessation, compared with never-smokers.
The researchers also found a roughly fourfold increased risk for PAD for smokers who smoked for 40 or more pack-years, compared with never-smokers, which was greater than the 2.1 hazard ratio for CHD and 1.8 HR for stroke. In addition, current smokers of at least one pack per day had a significantly greater risk of PAD, compared with never-smokers (HR, 5.36) that was higher than the risk for CHD or stroke (HR, 2.38 and HR, 1.88, respectively).
The study findings were limited by several factors including the reliance on self-reports, potential misclassification of data, and the potential exclusion of mild PAD cases that did not require hospitalization, the researchers noted. However, the results support the value of encouraging smokers to quit and support the need to include PAD risk in public health information, they said. “Although public statements about smoking and [cardiovascular disease] have been focusing on CHD and stroke, our results indicate the need to take account of PAD as well for comprehensively acknowledging the effect of smoking on overall cardiovascular health,” they added.
The ARIC study was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health. Lead author Dr. Ding had no financial conflicts to disclose; coauthors disclosed relationships with Bristol-Myers Squibb and Fukuda Denshi.
SOURCE: Ding N et al. J Am Coll Cardiol. 2019 Jul 22;74:498-507. doi: 10.1016/j.jacc.2019.06.003.
Adults who quit smoking reduced their risk for peripheral artery disease in the short term, but remained at increased risk for up to 30 years, compared with never-smokers, based on data from more than 13,000 adults in a community-based study.

Most reports on the impact of smoking cessation on cardiovascular disease have focused on coronary heart disease (CHD), and stroke, while data on the effects of smoking cessation on peripheral artery disease (PAD) are limited, wrote Ning Ding, MBBS, SCM, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, Md., and colleagues.
To compare the impact of smoking on PAD, CHD, and stroke, the researchers used data from the Atherosclerosis Risk in Communities (ARIC) study, which included 15,792 adults aged 45-64 years in four communities. The findings were published in the Journal of the American College of Cardiology.
The study population of 13,355 individuals had no baseline history of PAD, CHD, or stroke. Over a median 26 years of follow-up, the researchers identified 492 cases of PAD, 1,798 cases of CHD, and 1,106 cases of stroke.
The risk of all three conditions began to decline within 5 years of smoking cessation, which could be encouraging to smokers who wish to quit, the researchers noted. In addition, the longer the duration of smoking cessation, the lower the risk for all three conditions (See central illustration).
However, a significantly elevated risk remained for PAD for up to 30 years after smoking cessation and for CHD for up to 20 years after smoking cessation, compared with never-smokers.
The researchers also found a roughly fourfold increased risk for PAD for smokers who smoked for 40 or more pack-years, compared with never-smokers, which was greater than the 2.1 hazard ratio for CHD and 1.8 HR for stroke. In addition, current smokers of at least one pack per day had a significantly greater risk of PAD, compared with never-smokers (HR, 5.36) that was higher than the risk for CHD or stroke (HR, 2.38 and HR, 1.88, respectively).
The study findings were limited by several factors including the reliance on self-reports, potential misclassification of data, and the potential exclusion of mild PAD cases that did not require hospitalization, the researchers noted. However, the results support the value of encouraging smokers to quit and support the need to include PAD risk in public health information, they said. “Although public statements about smoking and [cardiovascular disease] have been focusing on CHD and stroke, our results indicate the need to take account of PAD as well for comprehensively acknowledging the effect of smoking on overall cardiovascular health,” they added.
The ARIC study was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health. Lead author Dr. Ding had no financial conflicts to disclose; coauthors disclosed relationships with Bristol-Myers Squibb and Fukuda Denshi.
SOURCE: Ding N et al. J Am Coll Cardiol. 2019 Jul 22;74:498-507. doi: 10.1016/j.jacc.2019.06.003.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY