User login
Consider a Donation to the SVS PAC
The SVS Political Action Committee has set an aggressive fundraising goal for this current 2020 election cycle, and your contribution is critical to helping the PAC to meet this goal. The SVS PAC is one of the most effective tools for communicating with policymakers on Capitol Hill to make the greatest impact on legislative issues that affect your patients and your practices. Contributions allow the PAC Committee to establish strong relationships with members of Congress and cultivate congressional champions for our legislative and regulatory priorities. Make your donation or enhance your previously made donation to the SVS PAC here. Want to learn more? Click here.
The SVS Political Action Committee has set an aggressive fundraising goal for this current 2020 election cycle, and your contribution is critical to helping the PAC to meet this goal. The SVS PAC is one of the most effective tools for communicating with policymakers on Capitol Hill to make the greatest impact on legislative issues that affect your patients and your practices. Contributions allow the PAC Committee to establish strong relationships with members of Congress and cultivate congressional champions for our legislative and regulatory priorities. Make your donation or enhance your previously made donation to the SVS PAC here. Want to learn more? Click here.
The SVS Political Action Committee has set an aggressive fundraising goal for this current 2020 election cycle, and your contribution is critical to helping the PAC to meet this goal. The SVS PAC is one of the most effective tools for communicating with policymakers on Capitol Hill to make the greatest impact on legislative issues that affect your patients and your practices. Contributions allow the PAC Committee to establish strong relationships with members of Congress and cultivate congressional champions for our legislative and regulatory priorities. Make your donation or enhance your previously made donation to the SVS PAC here. Want to learn more? Click here.
Skin safety gap divides white, older from nonwhite, younger
of passersby and skin-cancer screening attendees in Washington, D.C.
“These findings highlight the importance of tailoring free skin cancer screening events for nonwhite and younger populations,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, Washington, said in a statement provided by the institution. “While free screening events are important, we also have to think about comprehensive, community-based solutions that reach broader demographic populations than skin cancer screenings alone.”
For the new study, which appears in the July 2019 issue of Journal of Drugs in Dermatology, researchers led by Emily C. Murphy, BS, of George Washington University, sought to better understand skin safety precautions in the population beyond those who attend skin cancer screenings (J Drugs Dermatol. 2019;18[7]:649-53).
The study authors surveyed 285 passersby at six locations in Washington, D.C. (65% were female, 47% were under age 31, 48% were white, and 29% were black) and 144 attendees at a free skin cancer screening at George Washington University (70% were female, 16% were under 31, 44% were over 60, 73% were white, and 14% were black).
The attendees at the screening event were much more likely to engage in sun safety habits that are linked to lower risk of squamous cell carcinoma, basal cell carcinoma, and melanoma: 34% always used sunscreen vs. 19% of the public group (P = .001), and 52% always sought shade vs. 32% of the public group (P = .002). Seventeen percent of the public group never used sunscreen compared with 8% of the screening group.
Whites and older subjects were more likely to embrace sun-safety practices. When the groups were combined, 84% of whites and 52% of blacks always or sometimes used sunscreen (P less than .0001). Those over 60 were much more likely to always seek shade than were those under 31 (53% vs. 24%, P less than .0001).
“The screening group was older and included more individuals with fair skin, highlighting the need to target younger and nonwhite populations for sun safety education,” the researchers wrote. “Encouraging sun safety in younger populations will decrease the risk of skin cancer for patients now and later in their lives. That said, educating populations who seek skin cancer screenings is still important given 22% of our screening cohort reported rarely or never wearing sunscreen, underscoring this program’s value.”
The researchers noted that the study’s limitations include its small size, the possibility that the public group had higher education rates because they were near a university, and the lack of insight into whether the public group represented the general population.
No study funding was reported. The study authors reported no relevant disclosures.
of passersby and skin-cancer screening attendees in Washington, D.C.
“These findings highlight the importance of tailoring free skin cancer screening events for nonwhite and younger populations,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, Washington, said in a statement provided by the institution. “While free screening events are important, we also have to think about comprehensive, community-based solutions that reach broader demographic populations than skin cancer screenings alone.”
For the new study, which appears in the July 2019 issue of Journal of Drugs in Dermatology, researchers led by Emily C. Murphy, BS, of George Washington University, sought to better understand skin safety precautions in the population beyond those who attend skin cancer screenings (J Drugs Dermatol. 2019;18[7]:649-53).
The study authors surveyed 285 passersby at six locations in Washington, D.C. (65% were female, 47% were under age 31, 48% were white, and 29% were black) and 144 attendees at a free skin cancer screening at George Washington University (70% were female, 16% were under 31, 44% were over 60, 73% were white, and 14% were black).
The attendees at the screening event were much more likely to engage in sun safety habits that are linked to lower risk of squamous cell carcinoma, basal cell carcinoma, and melanoma: 34% always used sunscreen vs. 19% of the public group (P = .001), and 52% always sought shade vs. 32% of the public group (P = .002). Seventeen percent of the public group never used sunscreen compared with 8% of the screening group.
Whites and older subjects were more likely to embrace sun-safety practices. When the groups were combined, 84% of whites and 52% of blacks always or sometimes used sunscreen (P less than .0001). Those over 60 were much more likely to always seek shade than were those under 31 (53% vs. 24%, P less than .0001).
“The screening group was older and included more individuals with fair skin, highlighting the need to target younger and nonwhite populations for sun safety education,” the researchers wrote. “Encouraging sun safety in younger populations will decrease the risk of skin cancer for patients now and later in their lives. That said, educating populations who seek skin cancer screenings is still important given 22% of our screening cohort reported rarely or never wearing sunscreen, underscoring this program’s value.”
The researchers noted that the study’s limitations include its small size, the possibility that the public group had higher education rates because they were near a university, and the lack of insight into whether the public group represented the general population.
No study funding was reported. The study authors reported no relevant disclosures.
of passersby and skin-cancer screening attendees in Washington, D.C.
“These findings highlight the importance of tailoring free skin cancer screening events for nonwhite and younger populations,” senior author Adam Friedman, MD, professor and interim chair of dermatology at George Washington University, Washington, said in a statement provided by the institution. “While free screening events are important, we also have to think about comprehensive, community-based solutions that reach broader demographic populations than skin cancer screenings alone.”
For the new study, which appears in the July 2019 issue of Journal of Drugs in Dermatology, researchers led by Emily C. Murphy, BS, of George Washington University, sought to better understand skin safety precautions in the population beyond those who attend skin cancer screenings (J Drugs Dermatol. 2019;18[7]:649-53).
The study authors surveyed 285 passersby at six locations in Washington, D.C. (65% were female, 47% were under age 31, 48% were white, and 29% were black) and 144 attendees at a free skin cancer screening at George Washington University (70% were female, 16% were under 31, 44% were over 60, 73% were white, and 14% were black).
The attendees at the screening event were much more likely to engage in sun safety habits that are linked to lower risk of squamous cell carcinoma, basal cell carcinoma, and melanoma: 34% always used sunscreen vs. 19% of the public group (P = .001), and 52% always sought shade vs. 32% of the public group (P = .002). Seventeen percent of the public group never used sunscreen compared with 8% of the screening group.
Whites and older subjects were more likely to embrace sun-safety practices. When the groups were combined, 84% of whites and 52% of blacks always or sometimes used sunscreen (P less than .0001). Those over 60 were much more likely to always seek shade than were those under 31 (53% vs. 24%, P less than .0001).
“The screening group was older and included more individuals with fair skin, highlighting the need to target younger and nonwhite populations for sun safety education,” the researchers wrote. “Encouraging sun safety in younger populations will decrease the risk of skin cancer for patients now and later in their lives. That said, educating populations who seek skin cancer screenings is still important given 22% of our screening cohort reported rarely or never wearing sunscreen, underscoring this program’s value.”
The researchers noted that the study’s limitations include its small size, the possibility that the public group had higher education rates because they were near a university, and the lack of insight into whether the public group represented the general population.
No study funding was reported. The study authors reported no relevant disclosures.
FROM THE JOURNAL OF DRUGS IN DERMATOLOGY
Shorter vs. longer DAPT following coronary stent placement
Clinical question: Is 6 months of dual antiplatelet therapy (DAPT) therapy noninferior to 12 months, following ST-elevation myocardial infarction (STEMI) with placement of second-generation drug-eluting stents?
Background: DAPT has been the standard of care to prevent abrupt thrombotic closure of vessels following percutaneous coronary intervention (PCI) and placement of stents. The recommended duration of DAPT was lengthened from at least 30 days in bare metal stents to at least 12 months in earlier-generation drug-eluting stents after observation of high rates of in-stent thrombosis of drug-eluting stents.
Trials have shown that there is no difference in outcomes comparing 6 month vs. 12 months in DAPT for PCI in the cases of non-ST-elevation MI and unstable angina. However, there are no randomized controlled studies comparing 6 vs. 12 months of DAPT with newer drug-eluting stents following STEMI. Newer drug-eluting stents are made of biocompatible polymers with thinner struts and are thought to be fully absorbed by 3 months. International guidelines still recommend 12 months of DAPT following drug-eluting stent placement following STEMI.
Study design: Prospective, unblinded, randomized, multicenter noninferiority trial.
Setting: The study was performed at 17 sites in the Netherlands, Norway, Poland, and Switzerland.
Synopsis: This study enrolled 1100 patients with STEMI started on DAPT during December 2011-June 2015. Overall, 870 patients were randomized to continue DAPT or to change to single antiplatelet therapy (SAPT) at 6 months. Exclusions included embolic events, cardiogenic shock, revascularization, bleeding, or being on anticoagulation. Patients were followed for 24 months.
The primary endpoint was a composite of all-cause mortality, any MI, any revascularization, stroke, or thrombolysis. Incidence of the composite endpoint was 4.8% of SAPT cases, and 6.6% of DAPT cases. Noninferiority was met (P = .004) because the upper 95% confidence interval of 1.27 was smaller than the prespecified noninferiority margin of 1.66. The secondary endpoint of safety and bleeding at 18 months was 3.2% for SAPT, and 4.3% for DAPT with HR of 0.75.
Medtronic’s new stent was used in 92% of the cases of this industry-sponsored study. Despite usage of a composite endpoint, there was no difference in the individual elements of the composite in subgroup analyses. There was a low event rate in both arms likely because of the exclusions that led to a lower-risk population. The individual operators were able to choose the P2Y12 inhibitor.
Bottom line: This industry-sponsored randomized, control trial showed noninferiority of 6 months of DAPT to 12 months of therapy following STEMI to prevent in-stent thrombosis with newer second- generation drug-eluting stents. However, the study’s results may be limited to lower-risk patients, without need for revascularization, oral anticoagulation, or with stroke or cardiogenic shock.
Citation: Kedhi E et al. Six months versus 12 months of dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction (DAPT-STEMI): Randomised, multicenter, noninferiority trial. BMJ. 2018;363:k3793.
Dr. Lennon is an instructor of medicine at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
Clinical question: Is 6 months of dual antiplatelet therapy (DAPT) therapy noninferior to 12 months, following ST-elevation myocardial infarction (STEMI) with placement of second-generation drug-eluting stents?
Background: DAPT has been the standard of care to prevent abrupt thrombotic closure of vessels following percutaneous coronary intervention (PCI) and placement of stents. The recommended duration of DAPT was lengthened from at least 30 days in bare metal stents to at least 12 months in earlier-generation drug-eluting stents after observation of high rates of in-stent thrombosis of drug-eluting stents.
Trials have shown that there is no difference in outcomes comparing 6 month vs. 12 months in DAPT for PCI in the cases of non-ST-elevation MI and unstable angina. However, there are no randomized controlled studies comparing 6 vs. 12 months of DAPT with newer drug-eluting stents following STEMI. Newer drug-eluting stents are made of biocompatible polymers with thinner struts and are thought to be fully absorbed by 3 months. International guidelines still recommend 12 months of DAPT following drug-eluting stent placement following STEMI.
Study design: Prospective, unblinded, randomized, multicenter noninferiority trial.
Setting: The study was performed at 17 sites in the Netherlands, Norway, Poland, and Switzerland.
Synopsis: This study enrolled 1100 patients with STEMI started on DAPT during December 2011-June 2015. Overall, 870 patients were randomized to continue DAPT or to change to single antiplatelet therapy (SAPT) at 6 months. Exclusions included embolic events, cardiogenic shock, revascularization, bleeding, or being on anticoagulation. Patients were followed for 24 months.
The primary endpoint was a composite of all-cause mortality, any MI, any revascularization, stroke, or thrombolysis. Incidence of the composite endpoint was 4.8% of SAPT cases, and 6.6% of DAPT cases. Noninferiority was met (P = .004) because the upper 95% confidence interval of 1.27 was smaller than the prespecified noninferiority margin of 1.66. The secondary endpoint of safety and bleeding at 18 months was 3.2% for SAPT, and 4.3% for DAPT with HR of 0.75.
Medtronic’s new stent was used in 92% of the cases of this industry-sponsored study. Despite usage of a composite endpoint, there was no difference in the individual elements of the composite in subgroup analyses. There was a low event rate in both arms likely because of the exclusions that led to a lower-risk population. The individual operators were able to choose the P2Y12 inhibitor.
Bottom line: This industry-sponsored randomized, control trial showed noninferiority of 6 months of DAPT to 12 months of therapy following STEMI to prevent in-stent thrombosis with newer second- generation drug-eluting stents. However, the study’s results may be limited to lower-risk patients, without need for revascularization, oral anticoagulation, or with stroke or cardiogenic shock.
Citation: Kedhi E et al. Six months versus 12 months of dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction (DAPT-STEMI): Randomised, multicenter, noninferiority trial. BMJ. 2018;363:k3793.
Dr. Lennon is an instructor of medicine at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
Clinical question: Is 6 months of dual antiplatelet therapy (DAPT) therapy noninferior to 12 months, following ST-elevation myocardial infarction (STEMI) with placement of second-generation drug-eluting stents?
Background: DAPT has been the standard of care to prevent abrupt thrombotic closure of vessels following percutaneous coronary intervention (PCI) and placement of stents. The recommended duration of DAPT was lengthened from at least 30 days in bare metal stents to at least 12 months in earlier-generation drug-eluting stents after observation of high rates of in-stent thrombosis of drug-eluting stents.
Trials have shown that there is no difference in outcomes comparing 6 month vs. 12 months in DAPT for PCI in the cases of non-ST-elevation MI and unstable angina. However, there are no randomized controlled studies comparing 6 vs. 12 months of DAPT with newer drug-eluting stents following STEMI. Newer drug-eluting stents are made of biocompatible polymers with thinner struts and are thought to be fully absorbed by 3 months. International guidelines still recommend 12 months of DAPT following drug-eluting stent placement following STEMI.
Study design: Prospective, unblinded, randomized, multicenter noninferiority trial.
Setting: The study was performed at 17 sites in the Netherlands, Norway, Poland, and Switzerland.
Synopsis: This study enrolled 1100 patients with STEMI started on DAPT during December 2011-June 2015. Overall, 870 patients were randomized to continue DAPT or to change to single antiplatelet therapy (SAPT) at 6 months. Exclusions included embolic events, cardiogenic shock, revascularization, bleeding, or being on anticoagulation. Patients were followed for 24 months.
The primary endpoint was a composite of all-cause mortality, any MI, any revascularization, stroke, or thrombolysis. Incidence of the composite endpoint was 4.8% of SAPT cases, and 6.6% of DAPT cases. Noninferiority was met (P = .004) because the upper 95% confidence interval of 1.27 was smaller than the prespecified noninferiority margin of 1.66. The secondary endpoint of safety and bleeding at 18 months was 3.2% for SAPT, and 4.3% for DAPT with HR of 0.75.
Medtronic’s new stent was used in 92% of the cases of this industry-sponsored study. Despite usage of a composite endpoint, there was no difference in the individual elements of the composite in subgroup analyses. There was a low event rate in both arms likely because of the exclusions that led to a lower-risk population. The individual operators were able to choose the P2Y12 inhibitor.
Bottom line: This industry-sponsored randomized, control trial showed noninferiority of 6 months of DAPT to 12 months of therapy following STEMI to prevent in-stent thrombosis with newer second- generation drug-eluting stents. However, the study’s results may be limited to lower-risk patients, without need for revascularization, oral anticoagulation, or with stroke or cardiogenic shock.
Citation: Kedhi E et al. Six months versus 12 months of dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction (DAPT-STEMI): Randomised, multicenter, noninferiority trial. BMJ. 2018;363:k3793.
Dr. Lennon is an instructor of medicine at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
VA Urges All Veterans to Get Tested
The US Department of Veterans Affairs (VA) has a well-established National HIV Program, says Dr. Richard Stone, executive in charge of the VHA. In fact, he notes, the VA is the single largest provider of HIV care in America and has treated 31,000 veterans for HIV.
Thus, the VA plays a critical role in the effort to establish tools and resources to eradicate HIV in the US, Stone says, “one veteran at a time.” To realize this “ambitious but achievable target,” the VA is:
- Offering HIV testing at least once to every veteran and more often to those at risk;
- Rapidly linking those who are diagnosed to effective treatment;
- Deploying an HIV health force to hard-hit areas of the country, expanding timely access to high-quality HIV care and prevention across the VA’s integrated network, with both face-to-face encounters and telehealth; and
- Offering pre-exposure prophylaxis (PrEP) when clinically appropriate.
The primary goal, Stone says, is for veterans with HIV or at risk for HIV to be able to access the best care “safely and free from stigma and discrimination.”
Resources and educational tools are available at www.hiv.va.gov, including recently updated fact sheets and videos for patients about PrEP
The US Department of Veterans Affairs (VA) has a well-established National HIV Program, says Dr. Richard Stone, executive in charge of the VHA. In fact, he notes, the VA is the single largest provider of HIV care in America and has treated 31,000 veterans for HIV.
Thus, the VA plays a critical role in the effort to establish tools and resources to eradicate HIV in the US, Stone says, “one veteran at a time.” To realize this “ambitious but achievable target,” the VA is:
- Offering HIV testing at least once to every veteran and more often to those at risk;
- Rapidly linking those who are diagnosed to effective treatment;
- Deploying an HIV health force to hard-hit areas of the country, expanding timely access to high-quality HIV care and prevention across the VA’s integrated network, with both face-to-face encounters and telehealth; and
- Offering pre-exposure prophylaxis (PrEP) when clinically appropriate.
The primary goal, Stone says, is for veterans with HIV or at risk for HIV to be able to access the best care “safely and free from stigma and discrimination.”
Resources and educational tools are available at www.hiv.va.gov, including recently updated fact sheets and videos for patients about PrEP
The US Department of Veterans Affairs (VA) has a well-established National HIV Program, says Dr. Richard Stone, executive in charge of the VHA. In fact, he notes, the VA is the single largest provider of HIV care in America and has treated 31,000 veterans for HIV.
Thus, the VA plays a critical role in the effort to establish tools and resources to eradicate HIV in the US, Stone says, “one veteran at a time.” To realize this “ambitious but achievable target,” the VA is:
- Offering HIV testing at least once to every veteran and more often to those at risk;
- Rapidly linking those who are diagnosed to effective treatment;
- Deploying an HIV health force to hard-hit areas of the country, expanding timely access to high-quality HIV care and prevention across the VA’s integrated network, with both face-to-face encounters and telehealth; and
- Offering pre-exposure prophylaxis (PrEP) when clinically appropriate.
The primary goal, Stone says, is for veterans with HIV or at risk for HIV to be able to access the best care “safely and free from stigma and discrimination.”
Resources and educational tools are available at www.hiv.va.gov, including recently updated fact sheets and videos for patients about PrEP
Man, 46, With Wrist Laceration
A right hand–dominant 46-year-old man presents to the emergency department (ED) with a 1-cm laceration of his volar right wrist that occurred after he slipped on a wet floor while carrying a ceramic dish. The patient fell with his hand outstretched and landed on the dish as it broke against the floor. The patient has no pain but complains of tingling in his fingers. Past medical history is negative for diabetes, hypertension, or any neurologic disorders. Social history includes smoking one-half pack of cigarettes per day and drinking 6 to 10 12-oz beers each weekend. He works as a machinist.
Physical examination shows no bony tenderness. There is a 1.0-cm transverse laceration at the base of the hand at the midline of the volar wrist crease. Flexion, extension, and strength of the fingers are intact, as are dull and sharp discrimination to the thumb and other fingers. A cotton-tip applicator is used for gross sensory testing. No other neuromuscular assessment of the hand is performed. An x-ray of the hand to rule out a fracture or ceramic foreign body is negative.
The wound is locally anesthetized with 1% xylocaine without epinephrine. The laceration is irrigated with normal saline solution and closed with 4-0 nylon sutures using conventional bedside-suturing technique. A sterile bandage is applied. After-care instructions include wound care and follow-up with the patient’s family physician in 1 week for suture removal.
The patient returns to the ED 4 days later, complaining of increased tingling and weakness of the thumb and index and middle fingers. Repeat neuromuscular examination shows decreased sensation and dull/sharp discrimination, and abnormal static 2-point discrimination of the thumb and index and middle fingers. Based on the location of the laceration, the follow-up provider suspects a median nerve injury. After a telephone consultation with a hand surgeon, the patient is told to come into the office in 2 days.
Subsequent follow-up by the hospital’s risk manager indicates that the hand surgeon found a transected median nerve, requiring surgery to repair it. The patient has resulting deficits in sensation and strength and requires extensive occupational therapy. The risk management team learns that the patient intends to file a malpractice suit.
DISCUSSION
Hand and finger injuries represent about 20% of ED visits and are among the most costly injuries for the employed population.1 Knife and glass lacerations of the fingers are most common.2 Failure to diagnose significant hand and finger injuries is also a major contributor to malpractice claims in the ED.3 It is imperative for the PA or NP working in a high-stress/high-volume environment to perform a thorough neuromuscular and vascular examination when encountering a traumatic hand injury or a laceration. This applies to all frontline practices, including urgent care, ED, and primary care and family practices.
Volar surface lacerations of the wrist and fingers are especially high risk.2 Small lacerations (< 2 cm for fingers and < 3 cm for wrist and forearm) may lead a provider to consider the injury minor; however, these have the greatest potential for missed significant deep injuries.2 Missed median nerve lacerations can result in major complications if not surgically repaired soon after the injury.4
Continue to: With our case patient...
With our case patient, a small glass cut at the volar wrist crease did not cause tendon lacerations or flexor deficits. The patient complained only of mild tingling to the fingers, and a detailed hand-and-finger examination was not performed to isolate further nerve injury.4
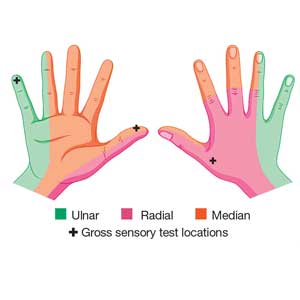
Although most nerve injuries result in a loss in sensory function, motor function must also be evaluated.5 With partial nerve lacerations, subtle loss of motor or sensory function can be missed by the examiner.4 It is imperative to conduct a thorough hand examination (outlined in Tables 1 and 2) to decrease the likelihood of missing a significant nerve or tendon injury.
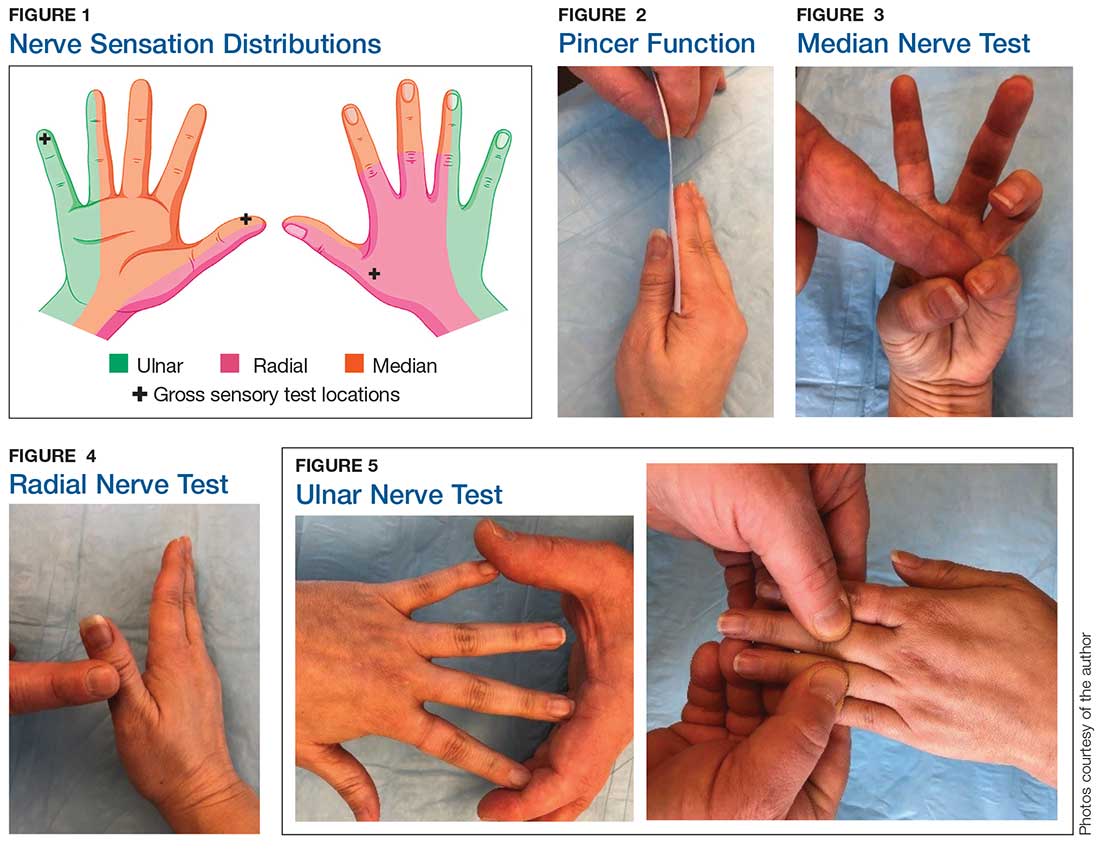
Sensory testing basics
Nerve laceration vs nerve compression disorder. It is important to distinguish sensory testing for a nerve injury or laceration from testing for a nerve compression disorder, such as carpal tunnel syndrome. When examining compression neuropathies, light touch, tuning fork vibration, and monofilament testing are used. When a nerve injury or laceration is suspected, light touch and 2-point discrimination are used.5 Static 2-point discrimination (also known as the Weber static test) will be immediately abnormal if a nerve is lacerated. In a nerve compression disorder, 2-point discrimination is decreased progressively.5
Sensory testing evidence
Comparing light touch, monofilament, and 2-point discrimination. As seen with our case patient, testing dull-sharp discrimination using the cotton-tip applicator for “dull” and the broken end of the wooden applicator stick for “sharp” may not be the most complete way to assess sensation in the hand and fingers. The physical examination should include light touch and 2-point discrimination.5
In one study, tests for sensation compared the gauze test (light touch), the static 2-point discrimination, the moving 2-point discrimination (m2PD; also known as the Weber dynamic test),6 and the monofilament test. The static and m2PD tests were statistically superior to the gauze and monofilament tests (see Table 3).7 Two-point discrimination abnormalities are detected immediately after a nerve is lacerated.5 This suggests performing 2-point discrimination, either moving or static, is superior to dull-sensation testing alone (gauze or cotton-tip applicator). This should be included in the motor and sensory examinations of the hand and fingers seen in Tables 1 and 2.
Continue to: Moving 2-point discrimination test
Moving 2-point discrimination test
The m2PD requires a 2-pointed instrument that can maintain a fixed 5 mm of width, such as a bent paperclip or EKG calipers. Commercially available devices specifically for 2-point discrimination can also be used.
When performing the m2PD test, the provider strokes 1 point in the proximal to distal direction in 5-mm increments on the finger and asks whether the patient feels “1 moving point.” The provider then holds 2 points and moves them in the proximal to distal direction in 5-mm increments and asks whether the patient feels “2 moving points.”
The m2PD test is then conducted comparing the ulnar and radial side of the injured finger with the ipsilateral noninjured finger. This should be done at least 4 times.8 The test is positive if there is a ≥ 2-mm difference between the affected and the unaffected side.7
Wound exploration
Data from a French insurance company indicate that 10% of ED malpractice claims in 2013 were related to inadequately examined hand lacerations. In an analysis of these claims, Mouton et al found that most injuries resulting in claims affected the thumb or the volar aspects of the fingers. Reasons for malpractice claims included residual stiffness, weakness, sensory deficit, retained foreign body, and wound infection. The researchers concluded that inadequate examination of hand wounds “carries a risk of lasting and sometimes severe residual impairment, and generates considerable societal costs.”3
In particular, small penetrating lacerations from broken glass or a knife should be considered high-risk injuries.2 In a study of small (< 2 cm) lacerations of the hand and fingers, 59% of the patients were found to have deep-structure injuries.2 Tuncali et al concluded that small lacerations increase the likelihood of missing deeper structural injuries because of failure to examine the wound.2 Furthermore, with glass lacerations, examiners tend to prioritize ruling out a foreign body and then fail to examine the wound. If a careful examination of the hand and fingers prompts suspicion of a tendon or nerve injury, referral to hand surgery for direct surgical exploration is indicated.
Continue to: CONCLUSION
CONCLUSION
Busy health care providers must be aware that approximately 10% to 15% of the negative outcomes in patient care result from diagnostic errors and are most common in the internal medicine, family medicine, and emergency medicine clinical environments.9 With hand and finger lacerations, small size can give a provider a false sense that the laceration is minor, resulting in a failure to diagnose a deeper injury (eg, tendon or nerve).1
When evaluating a traumatic injury or laceration to the hand or fingers, it is important to conduct a thorough sensory and motor examination. Experts recommend light touch and 2-point discrimination be included in the sensory exam to avoid missing nerve injuries. If a deeper structural injury is suspected, the patient should be referred to hand surgery and the wound surgically explored.2
1. Robinson LS, Sarkies M, Brown T, et al. Direct, indirect and intangible costs of acute hand and wrist injuries: a systematic review. Injury. 2016;47:2614-2626.
2. Tuncali D, Yavuz N, Terzioglu A, Aslan G. The rate of upper-extremity deep-structure injuries through small penetrating lacerations. Ann Plast Surg. 2005;55:146-148.
3. Mouton J, Houdre H, Beccari R, et al. Surgical exploration of hand wounds in the emergency room: preliminary study of 80 personal injury claims. Orthop Traumatol Surg Res. 2016;102:1009-1012.
4. Pederson WC. Median nerve injury and repair. J Hand Surg Am. 2014;39(6): 1216-1222.
5. Kenney RJ, Hammert WC. Physical examination of the hand. J Hand Surg Am. 2014;39(11):2324-2334.
6. Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3(5):474-481.
7. Bijon C, Hidalgo-Diaz JJ, Chiara P, et al. Nerve injuries to the volar aspect of the hand: a comparison of the reliability of the Weber static test versus the gauze test. Injury. 2017;48:2582-2585.
8. Davenport M, Tang P. Injuries to the hand and digits. In: Tintinalli JE, Stapczynski J, Ma OJ, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016:1667.
9. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41:155-162.
10. Madan SS, Pai DR, Kaur A, Dixit R. Injury to the ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7.
A right hand–dominant 46-year-old man presents to the emergency department (ED) with a 1-cm laceration of his volar right wrist that occurred after he slipped on a wet floor while carrying a ceramic dish. The patient fell with his hand outstretched and landed on the dish as it broke against the floor. The patient has no pain but complains of tingling in his fingers. Past medical history is negative for diabetes, hypertension, or any neurologic disorders. Social history includes smoking one-half pack of cigarettes per day and drinking 6 to 10 12-oz beers each weekend. He works as a machinist.
Physical examination shows no bony tenderness. There is a 1.0-cm transverse laceration at the base of the hand at the midline of the volar wrist crease. Flexion, extension, and strength of the fingers are intact, as are dull and sharp discrimination to the thumb and other fingers. A cotton-tip applicator is used for gross sensory testing. No other neuromuscular assessment of the hand is performed. An x-ray of the hand to rule out a fracture or ceramic foreign body is negative.
The wound is locally anesthetized with 1% xylocaine without epinephrine. The laceration is irrigated with normal saline solution and closed with 4-0 nylon sutures using conventional bedside-suturing technique. A sterile bandage is applied. After-care instructions include wound care and follow-up with the patient’s family physician in 1 week for suture removal.
The patient returns to the ED 4 days later, complaining of increased tingling and weakness of the thumb and index and middle fingers. Repeat neuromuscular examination shows decreased sensation and dull/sharp discrimination, and abnormal static 2-point discrimination of the thumb and index and middle fingers. Based on the location of the laceration, the follow-up provider suspects a median nerve injury. After a telephone consultation with a hand surgeon, the patient is told to come into the office in 2 days.
Subsequent follow-up by the hospital’s risk manager indicates that the hand surgeon found a transected median nerve, requiring surgery to repair it. The patient has resulting deficits in sensation and strength and requires extensive occupational therapy. The risk management team learns that the patient intends to file a malpractice suit.
DISCUSSION
Hand and finger injuries represent about 20% of ED visits and are among the most costly injuries for the employed population.1 Knife and glass lacerations of the fingers are most common.2 Failure to diagnose significant hand and finger injuries is also a major contributor to malpractice claims in the ED.3 It is imperative for the PA or NP working in a high-stress/high-volume environment to perform a thorough neuromuscular and vascular examination when encountering a traumatic hand injury or a laceration. This applies to all frontline practices, including urgent care, ED, and primary care and family practices.
Volar surface lacerations of the wrist and fingers are especially high risk.2 Small lacerations (< 2 cm for fingers and < 3 cm for wrist and forearm) may lead a provider to consider the injury minor; however, these have the greatest potential for missed significant deep injuries.2 Missed median nerve lacerations can result in major complications if not surgically repaired soon after the injury.4
Continue to: With our case patient...
With our case patient, a small glass cut at the volar wrist crease did not cause tendon lacerations or flexor deficits. The patient complained only of mild tingling to the fingers, and a detailed hand-and-finger examination was not performed to isolate further nerve injury.4

Although most nerve injuries result in a loss in sensory function, motor function must also be evaluated.5 With partial nerve lacerations, subtle loss of motor or sensory function can be missed by the examiner.4 It is imperative to conduct a thorough hand examination (outlined in Tables 1 and 2) to decrease the likelihood of missing a significant nerve or tendon injury.

Sensory testing basics
Nerve laceration vs nerve compression disorder. It is important to distinguish sensory testing for a nerve injury or laceration from testing for a nerve compression disorder, such as carpal tunnel syndrome. When examining compression neuropathies, light touch, tuning fork vibration, and monofilament testing are used. When a nerve injury or laceration is suspected, light touch and 2-point discrimination are used.5 Static 2-point discrimination (also known as the Weber static test) will be immediately abnormal if a nerve is lacerated. In a nerve compression disorder, 2-point discrimination is decreased progressively.5

Sensory testing evidence
Comparing light touch, monofilament, and 2-point discrimination. As seen with our case patient, testing dull-sharp discrimination using the cotton-tip applicator for “dull” and the broken end of the wooden applicator stick for “sharp” may not be the most complete way to assess sensation in the hand and fingers. The physical examination should include light touch and 2-point discrimination.5
In one study, tests for sensation compared the gauze test (light touch), the static 2-point discrimination, the moving 2-point discrimination (m2PD; also known as the Weber dynamic test),6 and the monofilament test. The static and m2PD tests were statistically superior to the gauze and monofilament tests (see Table 3).7 Two-point discrimination abnormalities are detected immediately after a nerve is lacerated.5 This suggests performing 2-point discrimination, either moving or static, is superior to dull-sensation testing alone (gauze or cotton-tip applicator). This should be included in the motor and sensory examinations of the hand and fingers seen in Tables 1 and 2.

Continue to: Moving 2-point discrimination test
Moving 2-point discrimination test
The m2PD requires a 2-pointed instrument that can maintain a fixed 5 mm of width, such as a bent paperclip or EKG calipers. Commercially available devices specifically for 2-point discrimination can also be used.
When performing the m2PD test, the provider strokes 1 point in the proximal to distal direction in 5-mm increments on the finger and asks whether the patient feels “1 moving point.” The provider then holds 2 points and moves them in the proximal to distal direction in 5-mm increments and asks whether the patient feels “2 moving points.”
The m2PD test is then conducted comparing the ulnar and radial side of the injured finger with the ipsilateral noninjured finger. This should be done at least 4 times.8 The test is positive if there is a ≥ 2-mm difference between the affected and the unaffected side.7
Wound exploration
Data from a French insurance company indicate that 10% of ED malpractice claims in 2013 were related to inadequately examined hand lacerations. In an analysis of these claims, Mouton et al found that most injuries resulting in claims affected the thumb or the volar aspects of the fingers. Reasons for malpractice claims included residual stiffness, weakness, sensory deficit, retained foreign body, and wound infection. The researchers concluded that inadequate examination of hand wounds “carries a risk of lasting and sometimes severe residual impairment, and generates considerable societal costs.”3
In particular, small penetrating lacerations from broken glass or a knife should be considered high-risk injuries.2 In a study of small (< 2 cm) lacerations of the hand and fingers, 59% of the patients were found to have deep-structure injuries.2 Tuncali et al concluded that small lacerations increase the likelihood of missing deeper structural injuries because of failure to examine the wound.2 Furthermore, with glass lacerations, examiners tend to prioritize ruling out a foreign body and then fail to examine the wound. If a careful examination of the hand and fingers prompts suspicion of a tendon or nerve injury, referral to hand surgery for direct surgical exploration is indicated.
Continue to: CONCLUSION
CONCLUSION
Busy health care providers must be aware that approximately 10% to 15% of the negative outcomes in patient care result from diagnostic errors and are most common in the internal medicine, family medicine, and emergency medicine clinical environments.9 With hand and finger lacerations, small size can give a provider a false sense that the laceration is minor, resulting in a failure to diagnose a deeper injury (eg, tendon or nerve).1
When evaluating a traumatic injury or laceration to the hand or fingers, it is important to conduct a thorough sensory and motor examination. Experts recommend light touch and 2-point discrimination be included in the sensory exam to avoid missing nerve injuries. If a deeper structural injury is suspected, the patient should be referred to hand surgery and the wound surgically explored.2
A right hand–dominant 46-year-old man presents to the emergency department (ED) with a 1-cm laceration of his volar right wrist that occurred after he slipped on a wet floor while carrying a ceramic dish. The patient fell with his hand outstretched and landed on the dish as it broke against the floor. The patient has no pain but complains of tingling in his fingers. Past medical history is negative for diabetes, hypertension, or any neurologic disorders. Social history includes smoking one-half pack of cigarettes per day and drinking 6 to 10 12-oz beers each weekend. He works as a machinist.
Physical examination shows no bony tenderness. There is a 1.0-cm transverse laceration at the base of the hand at the midline of the volar wrist crease. Flexion, extension, and strength of the fingers are intact, as are dull and sharp discrimination to the thumb and other fingers. A cotton-tip applicator is used for gross sensory testing. No other neuromuscular assessment of the hand is performed. An x-ray of the hand to rule out a fracture or ceramic foreign body is negative.
The wound is locally anesthetized with 1% xylocaine without epinephrine. The laceration is irrigated with normal saline solution and closed with 4-0 nylon sutures using conventional bedside-suturing technique. A sterile bandage is applied. After-care instructions include wound care and follow-up with the patient’s family physician in 1 week for suture removal.
The patient returns to the ED 4 days later, complaining of increased tingling and weakness of the thumb and index and middle fingers. Repeat neuromuscular examination shows decreased sensation and dull/sharp discrimination, and abnormal static 2-point discrimination of the thumb and index and middle fingers. Based on the location of the laceration, the follow-up provider suspects a median nerve injury. After a telephone consultation with a hand surgeon, the patient is told to come into the office in 2 days.
Subsequent follow-up by the hospital’s risk manager indicates that the hand surgeon found a transected median nerve, requiring surgery to repair it. The patient has resulting deficits in sensation and strength and requires extensive occupational therapy. The risk management team learns that the patient intends to file a malpractice suit.
DISCUSSION
Hand and finger injuries represent about 20% of ED visits and are among the most costly injuries for the employed population.1 Knife and glass lacerations of the fingers are most common.2 Failure to diagnose significant hand and finger injuries is also a major contributor to malpractice claims in the ED.3 It is imperative for the PA or NP working in a high-stress/high-volume environment to perform a thorough neuromuscular and vascular examination when encountering a traumatic hand injury or a laceration. This applies to all frontline practices, including urgent care, ED, and primary care and family practices.
Volar surface lacerations of the wrist and fingers are especially high risk.2 Small lacerations (< 2 cm for fingers and < 3 cm for wrist and forearm) may lead a provider to consider the injury minor; however, these have the greatest potential for missed significant deep injuries.2 Missed median nerve lacerations can result in major complications if not surgically repaired soon after the injury.4
Continue to: With our case patient...
With our case patient, a small glass cut at the volar wrist crease did not cause tendon lacerations or flexor deficits. The patient complained only of mild tingling to the fingers, and a detailed hand-and-finger examination was not performed to isolate further nerve injury.4

Although most nerve injuries result in a loss in sensory function, motor function must also be evaluated.5 With partial nerve lacerations, subtle loss of motor or sensory function can be missed by the examiner.4 It is imperative to conduct a thorough hand examination (outlined in Tables 1 and 2) to decrease the likelihood of missing a significant nerve or tendon injury.

Sensory testing basics
Nerve laceration vs nerve compression disorder. It is important to distinguish sensory testing for a nerve injury or laceration from testing for a nerve compression disorder, such as carpal tunnel syndrome. When examining compression neuropathies, light touch, tuning fork vibration, and monofilament testing are used. When a nerve injury or laceration is suspected, light touch and 2-point discrimination are used.5 Static 2-point discrimination (also known as the Weber static test) will be immediately abnormal if a nerve is lacerated. In a nerve compression disorder, 2-point discrimination is decreased progressively.5

Sensory testing evidence
Comparing light touch, monofilament, and 2-point discrimination. As seen with our case patient, testing dull-sharp discrimination using the cotton-tip applicator for “dull” and the broken end of the wooden applicator stick for “sharp” may not be the most complete way to assess sensation in the hand and fingers. The physical examination should include light touch and 2-point discrimination.5
In one study, tests for sensation compared the gauze test (light touch), the static 2-point discrimination, the moving 2-point discrimination (m2PD; also known as the Weber dynamic test),6 and the monofilament test. The static and m2PD tests were statistically superior to the gauze and monofilament tests (see Table 3).7 Two-point discrimination abnormalities are detected immediately after a nerve is lacerated.5 This suggests performing 2-point discrimination, either moving or static, is superior to dull-sensation testing alone (gauze or cotton-tip applicator). This should be included in the motor and sensory examinations of the hand and fingers seen in Tables 1 and 2.

Continue to: Moving 2-point discrimination test
Moving 2-point discrimination test
The m2PD requires a 2-pointed instrument that can maintain a fixed 5 mm of width, such as a bent paperclip or EKG calipers. Commercially available devices specifically for 2-point discrimination can also be used.
When performing the m2PD test, the provider strokes 1 point in the proximal to distal direction in 5-mm increments on the finger and asks whether the patient feels “1 moving point.” The provider then holds 2 points and moves them in the proximal to distal direction in 5-mm increments and asks whether the patient feels “2 moving points.”
The m2PD test is then conducted comparing the ulnar and radial side of the injured finger with the ipsilateral noninjured finger. This should be done at least 4 times.8 The test is positive if there is a ≥ 2-mm difference between the affected and the unaffected side.7
Wound exploration
Data from a French insurance company indicate that 10% of ED malpractice claims in 2013 were related to inadequately examined hand lacerations. In an analysis of these claims, Mouton et al found that most injuries resulting in claims affected the thumb or the volar aspects of the fingers. Reasons for malpractice claims included residual stiffness, weakness, sensory deficit, retained foreign body, and wound infection. The researchers concluded that inadequate examination of hand wounds “carries a risk of lasting and sometimes severe residual impairment, and generates considerable societal costs.”3
In particular, small penetrating lacerations from broken glass or a knife should be considered high-risk injuries.2 In a study of small (< 2 cm) lacerations of the hand and fingers, 59% of the patients were found to have deep-structure injuries.2 Tuncali et al concluded that small lacerations increase the likelihood of missing deeper structural injuries because of failure to examine the wound.2 Furthermore, with glass lacerations, examiners tend to prioritize ruling out a foreign body and then fail to examine the wound. If a careful examination of the hand and fingers prompts suspicion of a tendon or nerve injury, referral to hand surgery for direct surgical exploration is indicated.
Continue to: CONCLUSION
CONCLUSION
Busy health care providers must be aware that approximately 10% to 15% of the negative outcomes in patient care result from diagnostic errors and are most common in the internal medicine, family medicine, and emergency medicine clinical environments.9 With hand and finger lacerations, small size can give a provider a false sense that the laceration is minor, resulting in a failure to diagnose a deeper injury (eg, tendon or nerve).1
When evaluating a traumatic injury or laceration to the hand or fingers, it is important to conduct a thorough sensory and motor examination. Experts recommend light touch and 2-point discrimination be included in the sensory exam to avoid missing nerve injuries. If a deeper structural injury is suspected, the patient should be referred to hand surgery and the wound surgically explored.2
1. Robinson LS, Sarkies M, Brown T, et al. Direct, indirect and intangible costs of acute hand and wrist injuries: a systematic review. Injury. 2016;47:2614-2626.
2. Tuncali D, Yavuz N, Terzioglu A, Aslan G. The rate of upper-extremity deep-structure injuries through small penetrating lacerations. Ann Plast Surg. 2005;55:146-148.
3. Mouton J, Houdre H, Beccari R, et al. Surgical exploration of hand wounds in the emergency room: preliminary study of 80 personal injury claims. Orthop Traumatol Surg Res. 2016;102:1009-1012.
4. Pederson WC. Median nerve injury and repair. J Hand Surg Am. 2014;39(6): 1216-1222.
5. Kenney RJ, Hammert WC. Physical examination of the hand. J Hand Surg Am. 2014;39(11):2324-2334.
6. Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3(5):474-481.
7. Bijon C, Hidalgo-Diaz JJ, Chiara P, et al. Nerve injuries to the volar aspect of the hand: a comparison of the reliability of the Weber static test versus the gauze test. Injury. 2017;48:2582-2585.
8. Davenport M, Tang P. Injuries to the hand and digits. In: Tintinalli JE, Stapczynski J, Ma OJ, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016:1667.
9. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41:155-162.
10. Madan SS, Pai DR, Kaur A, Dixit R. Injury to the ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7.
1. Robinson LS, Sarkies M, Brown T, et al. Direct, indirect and intangible costs of acute hand and wrist injuries: a systematic review. Injury. 2016;47:2614-2626.
2. Tuncali D, Yavuz N, Terzioglu A, Aslan G. The rate of upper-extremity deep-structure injuries through small penetrating lacerations. Ann Plast Surg. 2005;55:146-148.
3. Mouton J, Houdre H, Beccari R, et al. Surgical exploration of hand wounds in the emergency room: preliminary study of 80 personal injury claims. Orthop Traumatol Surg Res. 2016;102:1009-1012.
4. Pederson WC. Median nerve injury and repair. J Hand Surg Am. 2014;39(6): 1216-1222.
5. Kenney RJ, Hammert WC. Physical examination of the hand. J Hand Surg Am. 2014;39(11):2324-2334.
6. Dellon AL. The moving two-point discrimination test: clinical evaluation of the quickly adapting fiber/receptor system. J Hand Surg. 1978;3(5):474-481.
7. Bijon C, Hidalgo-Diaz JJ, Chiara P, et al. Nerve injuries to the volar aspect of the hand: a comparison of the reliability of the Weber static test versus the gauze test. Injury. 2017;48:2582-2585.
8. Davenport M, Tang P. Injuries to the hand and digits. In: Tintinalli JE, Stapczynski J, Ma OJ, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw-Hill; 2016:1667.
9. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41:155-162.
10. Madan SS, Pai DR, Kaur A, Dixit R. Injury to the ulnar collateral ligament of thumb. Orthop Surg. 2014;6:1-7.
Emicizumab follow-up shows further bleeding declines
MELBOURNE – according to data presented at the International Society on Thrombosis and Haemostasis congress.
Michael Callaghan, MD, of the Children’s Hospital of Michigan, Detroit, reported on a pooled analysis of data from 399 patients with hemophilia A who were treated with emicizumab (Hemlibra) for a median duration of 83.1 weeks, representing 650 patient-years of exposure. The studies included pediatric and adult patients, both with and without factor VIII inhibitors.
Patients enrolled in the studies had a median of eight bleeds in the 24 weeks before enrollment, but in the first 24 weeks of treatment with emicizumab, the mean annualized bleed rate dropped to 1.9. During weeks 25-48, this dropped further to 0.8, remained at that level in weeks 49-72, then declined further to 0.3 during weeks 73-96.
During the first 24 weeks of treatment, 70.8% of patients experienced zero bleeds, and 22.5% experienced 1-3 bleeds. By week 96, the number of patients experiencing zero bleeds had increased to 88.6% and nearly 100% of patients had had fewer than three bleeds during that 24-week period.
The study also reported on target joint bleeds and showed the mean annualized bleed rate in target joints decreased from 1.4 in the first 24 weeks of treatment to 0.3 in weeks 73-96, by which time 90.4% of patients reported no target joint bleeds at all. Overall, 99.2% of target joints resolved, which was defined as two or fewer spontaneous bleeding events into a target joint in a year.
“The bleed rate seemed to converge on a low number, suggesting that maybe patients that came with preexisting synovitis or inflamed joints improved over time to resemble the patients who had better joint health at the beginning of the study,” Dr. Callaghan said.
The long-term follow-up did not reveal any major safety concerns. The most common drug-related adverse event was injection site reactions, which just over one-quarter of patients reported. The main serious adverse events were bleeding related.
“With any biologic agent, we were concerned about antidrug antibodies,” Dr. Callaghan told the conference. “At this follow-up point, less than 1% of patients treated with emicizumab in this group have had neutralizing antidrug antibodies.” Most of these antibodies were detected with routine screening, but there was one patient with antidrug antibodies who developed breakthrough bleeding during the study.
In an interview, Dr. Callaghan said emicizumab was “game-changing” therapy, and that the data showed it was efficacious even long term. However, he said there were still some questions to be answered about which patients were most likely to benefit.
“How early do we start this? Do we put previously untreated patients on this, and if we do, how do we expose them to factor VIII?” he said. Other challenging questions are whether to do immune tolerance induction for patients with factor VIII inhibitors and how the drug would work for other patient groups, such as those with comorbidities or who were very active.
The study was sponsored by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Callaghan declared consultancies, grants, clinical trial involvement, speakers bureau engagements, and shares with the pharmaceutical sector.
SOURCE: Callaghan M et al. 2019 ISTH Congress, Abstract OC 60.2.
MELBOURNE – according to data presented at the International Society on Thrombosis and Haemostasis congress.
Michael Callaghan, MD, of the Children’s Hospital of Michigan, Detroit, reported on a pooled analysis of data from 399 patients with hemophilia A who were treated with emicizumab (Hemlibra) for a median duration of 83.1 weeks, representing 650 patient-years of exposure. The studies included pediatric and adult patients, both with and without factor VIII inhibitors.
Patients enrolled in the studies had a median of eight bleeds in the 24 weeks before enrollment, but in the first 24 weeks of treatment with emicizumab, the mean annualized bleed rate dropped to 1.9. During weeks 25-48, this dropped further to 0.8, remained at that level in weeks 49-72, then declined further to 0.3 during weeks 73-96.
During the first 24 weeks of treatment, 70.8% of patients experienced zero bleeds, and 22.5% experienced 1-3 bleeds. By week 96, the number of patients experiencing zero bleeds had increased to 88.6% and nearly 100% of patients had had fewer than three bleeds during that 24-week period.
The study also reported on target joint bleeds and showed the mean annualized bleed rate in target joints decreased from 1.4 in the first 24 weeks of treatment to 0.3 in weeks 73-96, by which time 90.4% of patients reported no target joint bleeds at all. Overall, 99.2% of target joints resolved, which was defined as two or fewer spontaneous bleeding events into a target joint in a year.
“The bleed rate seemed to converge on a low number, suggesting that maybe patients that came with preexisting synovitis or inflamed joints improved over time to resemble the patients who had better joint health at the beginning of the study,” Dr. Callaghan said.
The long-term follow-up did not reveal any major safety concerns. The most common drug-related adverse event was injection site reactions, which just over one-quarter of patients reported. The main serious adverse events were bleeding related.
“With any biologic agent, we were concerned about antidrug antibodies,” Dr. Callaghan told the conference. “At this follow-up point, less than 1% of patients treated with emicizumab in this group have had neutralizing antidrug antibodies.” Most of these antibodies were detected with routine screening, but there was one patient with antidrug antibodies who developed breakthrough bleeding during the study.
In an interview, Dr. Callaghan said emicizumab was “game-changing” therapy, and that the data showed it was efficacious even long term. However, he said there were still some questions to be answered about which patients were most likely to benefit.
“How early do we start this? Do we put previously untreated patients on this, and if we do, how do we expose them to factor VIII?” he said. Other challenging questions are whether to do immune tolerance induction for patients with factor VIII inhibitors and how the drug would work for other patient groups, such as those with comorbidities or who were very active.
The study was sponsored by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Callaghan declared consultancies, grants, clinical trial involvement, speakers bureau engagements, and shares with the pharmaceutical sector.
SOURCE: Callaghan M et al. 2019 ISTH Congress, Abstract OC 60.2.
MELBOURNE – according to data presented at the International Society on Thrombosis and Haemostasis congress.
Michael Callaghan, MD, of the Children’s Hospital of Michigan, Detroit, reported on a pooled analysis of data from 399 patients with hemophilia A who were treated with emicizumab (Hemlibra) for a median duration of 83.1 weeks, representing 650 patient-years of exposure. The studies included pediatric and adult patients, both with and without factor VIII inhibitors.
Patients enrolled in the studies had a median of eight bleeds in the 24 weeks before enrollment, but in the first 24 weeks of treatment with emicizumab, the mean annualized bleed rate dropped to 1.9. During weeks 25-48, this dropped further to 0.8, remained at that level in weeks 49-72, then declined further to 0.3 during weeks 73-96.
During the first 24 weeks of treatment, 70.8% of patients experienced zero bleeds, and 22.5% experienced 1-3 bleeds. By week 96, the number of patients experiencing zero bleeds had increased to 88.6% and nearly 100% of patients had had fewer than three bleeds during that 24-week period.
The study also reported on target joint bleeds and showed the mean annualized bleed rate in target joints decreased from 1.4 in the first 24 weeks of treatment to 0.3 in weeks 73-96, by which time 90.4% of patients reported no target joint bleeds at all. Overall, 99.2% of target joints resolved, which was defined as two or fewer spontaneous bleeding events into a target joint in a year.
“The bleed rate seemed to converge on a low number, suggesting that maybe patients that came with preexisting synovitis or inflamed joints improved over time to resemble the patients who had better joint health at the beginning of the study,” Dr. Callaghan said.
The long-term follow-up did not reveal any major safety concerns. The most common drug-related adverse event was injection site reactions, which just over one-quarter of patients reported. The main serious adverse events were bleeding related.
“With any biologic agent, we were concerned about antidrug antibodies,” Dr. Callaghan told the conference. “At this follow-up point, less than 1% of patients treated with emicizumab in this group have had neutralizing antidrug antibodies.” Most of these antibodies were detected with routine screening, but there was one patient with antidrug antibodies who developed breakthrough bleeding during the study.
In an interview, Dr. Callaghan said emicizumab was “game-changing” therapy, and that the data showed it was efficacious even long term. However, he said there were still some questions to be answered about which patients were most likely to benefit.
“How early do we start this? Do we put previously untreated patients on this, and if we do, how do we expose them to factor VIII?” he said. Other challenging questions are whether to do immune tolerance induction for patients with factor VIII inhibitors and how the drug would work for other patient groups, such as those with comorbidities or who were very active.
The study was sponsored by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Callaghan declared consultancies, grants, clinical trial involvement, speakers bureau engagements, and shares with the pharmaceutical sector.
SOURCE: Callaghan M et al. 2019 ISTH Congress, Abstract OC 60.2.
REPORTING FROM 2019 ISTH CONGRESS
Online ped-derm searches: What are folks looking for?
AUSTIN – After searching online for information about a suspected pediatric dermatologic condition, one in five parents and/or pediatric patients make dermatology appointments sooner than they normally would, results from a novel survey showed.
In an interview at the annual meeting of the Society for Pediatric Dermatology, study author Jamie P. Schlarbaum noted that about one-third of Americans use the Internet to research their condition or symptoms prior to visiting a physician, mostly through Google. “While nearly 50% of parents look up health care information online for their children, rashes were the most common search in pediatrics in 2011,” said Mr. Schlarbaum, who is a fourth-year medical student at the University of Minnesota, Minneapolis. “However, no studies have examined the characteristics and implications of these searches; our study is the first in pediatric dermatology and also adds a new dimension to concern in an online era: How these searches influence health care behaviors.”
During February 2018–February 2019, Kristen Hook, MD, a pediatric dermatologist in Minneapolis and the study’s principal investigator, and Mr. Schlarbaum administered a survey to 220 parents/guardians and pediatric patients who had appointments in pediatric dermatology at a University of Minnesota clinic. The survey consisted of questions about demographics, search tools, search terms, and health care decisions based on this information.
Of the 220 respondents, more than half (59%) did not use an online search engine/tool prior to their appointment. Compared with parents who did not use an online search tool, those who did were slightly younger (34 vs. 36 years, respectively), more likely to be college educated (68% vs. 48%), and less likely to have the patient in question be their first child (37% vs. 52%).
Google ranked as the most common search engine used by the survey respondents (92%), followed distantly by WebMD (18%). About 15% of respondents became more concerned about the pediatric skin condition after searching online, and 20% made appointments sooner because of the information they gleaned from their searches. “Online dermatology clearly has an influence on care today,” Mr. Schlarbaum said. “As we become an even more technologically advanced and dependent society, we anticipate that both of these numbers will grow.”
The researchers also found that (33%), moles (15%), and infections (11%). “The big takeaway [from this study] is to ask your parents and teenagers if they’ve looked up information online,” Mr. Schlarbaum said. “Whether it’s photos of the ‘worst cases’ or concerning differentials that might pop up, it’s worth it to take a few seconds to ask what they’re worried about and why.”
He acknowledged certain limitations of the study, including its small sample size and single-center design. The researchers reported having no financial disclosures.
AUSTIN – After searching online for information about a suspected pediatric dermatologic condition, one in five parents and/or pediatric patients make dermatology appointments sooner than they normally would, results from a novel survey showed.
In an interview at the annual meeting of the Society for Pediatric Dermatology, study author Jamie P. Schlarbaum noted that about one-third of Americans use the Internet to research their condition or symptoms prior to visiting a physician, mostly through Google. “While nearly 50% of parents look up health care information online for their children, rashes were the most common search in pediatrics in 2011,” said Mr. Schlarbaum, who is a fourth-year medical student at the University of Minnesota, Minneapolis. “However, no studies have examined the characteristics and implications of these searches; our study is the first in pediatric dermatology and also adds a new dimension to concern in an online era: How these searches influence health care behaviors.”
During February 2018–February 2019, Kristen Hook, MD, a pediatric dermatologist in Minneapolis and the study’s principal investigator, and Mr. Schlarbaum administered a survey to 220 parents/guardians and pediatric patients who had appointments in pediatric dermatology at a University of Minnesota clinic. The survey consisted of questions about demographics, search tools, search terms, and health care decisions based on this information.
Of the 220 respondents, more than half (59%) did not use an online search engine/tool prior to their appointment. Compared with parents who did not use an online search tool, those who did were slightly younger (34 vs. 36 years, respectively), more likely to be college educated (68% vs. 48%), and less likely to have the patient in question be their first child (37% vs. 52%).
Google ranked as the most common search engine used by the survey respondents (92%), followed distantly by WebMD (18%). About 15% of respondents became more concerned about the pediatric skin condition after searching online, and 20% made appointments sooner because of the information they gleaned from their searches. “Online dermatology clearly has an influence on care today,” Mr. Schlarbaum said. “As we become an even more technologically advanced and dependent society, we anticipate that both of these numbers will grow.”
The researchers also found that (33%), moles (15%), and infections (11%). “The big takeaway [from this study] is to ask your parents and teenagers if they’ve looked up information online,” Mr. Schlarbaum said. “Whether it’s photos of the ‘worst cases’ or concerning differentials that might pop up, it’s worth it to take a few seconds to ask what they’re worried about and why.”
He acknowledged certain limitations of the study, including its small sample size and single-center design. The researchers reported having no financial disclosures.
AUSTIN – After searching online for information about a suspected pediatric dermatologic condition, one in five parents and/or pediatric patients make dermatology appointments sooner than they normally would, results from a novel survey showed.
In an interview at the annual meeting of the Society for Pediatric Dermatology, study author Jamie P. Schlarbaum noted that about one-third of Americans use the Internet to research their condition or symptoms prior to visiting a physician, mostly through Google. “While nearly 50% of parents look up health care information online for their children, rashes were the most common search in pediatrics in 2011,” said Mr. Schlarbaum, who is a fourth-year medical student at the University of Minnesota, Minneapolis. “However, no studies have examined the characteristics and implications of these searches; our study is the first in pediatric dermatology and also adds a new dimension to concern in an online era: How these searches influence health care behaviors.”
During February 2018–February 2019, Kristen Hook, MD, a pediatric dermatologist in Minneapolis and the study’s principal investigator, and Mr. Schlarbaum administered a survey to 220 parents/guardians and pediatric patients who had appointments in pediatric dermatology at a University of Minnesota clinic. The survey consisted of questions about demographics, search tools, search terms, and health care decisions based on this information.
Of the 220 respondents, more than half (59%) did not use an online search engine/tool prior to their appointment. Compared with parents who did not use an online search tool, those who did were slightly younger (34 vs. 36 years, respectively), more likely to be college educated (68% vs. 48%), and less likely to have the patient in question be their first child (37% vs. 52%).
Google ranked as the most common search engine used by the survey respondents (92%), followed distantly by WebMD (18%). About 15% of respondents became more concerned about the pediatric skin condition after searching online, and 20% made appointments sooner because of the information they gleaned from their searches. “Online dermatology clearly has an influence on care today,” Mr. Schlarbaum said. “As we become an even more technologically advanced and dependent society, we anticipate that both of these numbers will grow.”
The researchers also found that (33%), moles (15%), and infections (11%). “The big takeaway [from this study] is to ask your parents and teenagers if they’ve looked up information online,” Mr. Schlarbaum said. “Whether it’s photos of the ‘worst cases’ or concerning differentials that might pop up, it’s worth it to take a few seconds to ask what they’re worried about and why.”
He acknowledged certain limitations of the study, including its small sample size and single-center design. The researchers reported having no financial disclosures.
REPORTING FROM SPD 2019
How are your otoscopy skills?
If the name Michael E. Pichichero, MD, is unfamiliar, you haven’t been reading some of the best articles on this website . Dr. Pichichero, an infectious disease specialist at the Research Institute at the Rochester General Hospital in New York, reports in his most recent ID Consult column on new research presented at the June 2019 meeting of the International Society for Otitis Media, including topics such as transtympanic antibiotic delivery, biofilms, probiotics, and biomarkers.
Dr. Pichichero described work he and his colleagues have been doing on the impact of overdiagnosis of acute otitis media (AOM). They found when “validated otoscopists” evaluated children, half as many reached the diagnostic threshold of being labeled “otitis prone” as when community-based pediatricians performed the exams.
Looking around at the colleagues with whom you share patients, do you find that some of them diagnose AOM much more frequently than does the coverage group average? How often do you see a child a day or two after he has been diagnosed with AOM by a colleague and find that the child’s tympanic membranes are transparent and mobile? Do you or your practice group keep track of each provider’s diagnostic tendencies? If these data exists, is there a mechanism for addressing apparent outliers? I suspect that the answer to those last two questions is a firm “No.”
I don’t have the stomach this morning to open those two cans of worms. But certainly Dr. Pichichero’s findings suggest that these are issues that need to be addressed. How the process should proceed in a nonthreatening way is a story for another day. But I’m not sure that involving your community ear, nose, and throat (ENT) specialist as a resource is the best answer. The scenarios in which pediatricians and ENTs perform otoscopies couldn’t be more different. In the pediatrician’s office, the child is generally sick, feverish, and possibly in pain. In the ENT’s office, the acute process has probably passed and the assessment may lean more heavily on history. The child is more likely to accept the exam without resistance, and the findings are not those of AOM but of a chronic process. The fact that Dr. Pichichero has been able to find and train “validated otoscopists” suggests that improving the quality of otoscopy among the physicians in communities like yours and mine is achievable.
How are your otoscopy skills? Do feel comfortable that you can do a good exam and accurately diagnose AOM? When did you acquire that comfort level? Probably not in medical school. More likely as a house officer when you were guided by a more senior house officer who may nor not been a master otoscopist. How would you rate your training? Or were you self-taught? Do you insufflate? Are you a skilled cerumen extractor? Or do you give up after one attempt? Be honest. How is your equipment? Are the bulbs and batteries fresh? Do you find yourself frustrated by an otoscope that is tethered to the wall charger by a cord that ensnarls you, the parent, and the patient? Have you complained to the practice administrators that your otoscopes are inadequate?
These are not minor issues. It is clear that overdiagnosis of AOM happens. It may occur even more often than Dr. Pichichero suggests, but I doubt it is less. Overdiagnosis can result in overtreatment with antibiotics, and the cascade of consequences both for the patient, the community, and the environment. Overdiagnosis can be the first step on the path to unnecessary surgery. It is incumbent on all of us to make sure that our otoscopy skills and those of our colleagues are sharp, that our equipment is well maintained and that we remain abreast of the latest developments in the diagnosis and treatment of AOM.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
If the name Michael E. Pichichero, MD, is unfamiliar, you haven’t been reading some of the best articles on this website . Dr. Pichichero, an infectious disease specialist at the Research Institute at the Rochester General Hospital in New York, reports in his most recent ID Consult column on new research presented at the June 2019 meeting of the International Society for Otitis Media, including topics such as transtympanic antibiotic delivery, biofilms, probiotics, and biomarkers.
Dr. Pichichero described work he and his colleagues have been doing on the impact of overdiagnosis of acute otitis media (AOM). They found when “validated otoscopists” evaluated children, half as many reached the diagnostic threshold of being labeled “otitis prone” as when community-based pediatricians performed the exams.
Looking around at the colleagues with whom you share patients, do you find that some of them diagnose AOM much more frequently than does the coverage group average? How often do you see a child a day or two after he has been diagnosed with AOM by a colleague and find that the child’s tympanic membranes are transparent and mobile? Do you or your practice group keep track of each provider’s diagnostic tendencies? If these data exists, is there a mechanism for addressing apparent outliers? I suspect that the answer to those last two questions is a firm “No.”
I don’t have the stomach this morning to open those two cans of worms. But certainly Dr. Pichichero’s findings suggest that these are issues that need to be addressed. How the process should proceed in a nonthreatening way is a story for another day. But I’m not sure that involving your community ear, nose, and throat (ENT) specialist as a resource is the best answer. The scenarios in which pediatricians and ENTs perform otoscopies couldn’t be more different. In the pediatrician’s office, the child is generally sick, feverish, and possibly in pain. In the ENT’s office, the acute process has probably passed and the assessment may lean more heavily on history. The child is more likely to accept the exam without resistance, and the findings are not those of AOM but of a chronic process. The fact that Dr. Pichichero has been able to find and train “validated otoscopists” suggests that improving the quality of otoscopy among the physicians in communities like yours and mine is achievable.
How are your otoscopy skills? Do feel comfortable that you can do a good exam and accurately diagnose AOM? When did you acquire that comfort level? Probably not in medical school. More likely as a house officer when you were guided by a more senior house officer who may nor not been a master otoscopist. How would you rate your training? Or were you self-taught? Do you insufflate? Are you a skilled cerumen extractor? Or do you give up after one attempt? Be honest. How is your equipment? Are the bulbs and batteries fresh? Do you find yourself frustrated by an otoscope that is tethered to the wall charger by a cord that ensnarls you, the parent, and the patient? Have you complained to the practice administrators that your otoscopes are inadequate?
These are not minor issues. It is clear that overdiagnosis of AOM happens. It may occur even more often than Dr. Pichichero suggests, but I doubt it is less. Overdiagnosis can result in overtreatment with antibiotics, and the cascade of consequences both for the patient, the community, and the environment. Overdiagnosis can be the first step on the path to unnecessary surgery. It is incumbent on all of us to make sure that our otoscopy skills and those of our colleagues are sharp, that our equipment is well maintained and that we remain abreast of the latest developments in the diagnosis and treatment of AOM.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
If the name Michael E. Pichichero, MD, is unfamiliar, you haven’t been reading some of the best articles on this website . Dr. Pichichero, an infectious disease specialist at the Research Institute at the Rochester General Hospital in New York, reports in his most recent ID Consult column on new research presented at the June 2019 meeting of the International Society for Otitis Media, including topics such as transtympanic antibiotic delivery, biofilms, probiotics, and biomarkers.
Dr. Pichichero described work he and his colleagues have been doing on the impact of overdiagnosis of acute otitis media (AOM). They found when “validated otoscopists” evaluated children, half as many reached the diagnostic threshold of being labeled “otitis prone” as when community-based pediatricians performed the exams.
Looking around at the colleagues with whom you share patients, do you find that some of them diagnose AOM much more frequently than does the coverage group average? How often do you see a child a day or two after he has been diagnosed with AOM by a colleague and find that the child’s tympanic membranes are transparent and mobile? Do you or your practice group keep track of each provider’s diagnostic tendencies? If these data exists, is there a mechanism for addressing apparent outliers? I suspect that the answer to those last two questions is a firm “No.”
I don’t have the stomach this morning to open those two cans of worms. But certainly Dr. Pichichero’s findings suggest that these are issues that need to be addressed. How the process should proceed in a nonthreatening way is a story for another day. But I’m not sure that involving your community ear, nose, and throat (ENT) specialist as a resource is the best answer. The scenarios in which pediatricians and ENTs perform otoscopies couldn’t be more different. In the pediatrician’s office, the child is generally sick, feverish, and possibly in pain. In the ENT’s office, the acute process has probably passed and the assessment may lean more heavily on history. The child is more likely to accept the exam without resistance, and the findings are not those of AOM but of a chronic process. The fact that Dr. Pichichero has been able to find and train “validated otoscopists” suggests that improving the quality of otoscopy among the physicians in communities like yours and mine is achievable.
How are your otoscopy skills? Do feel comfortable that you can do a good exam and accurately diagnose AOM? When did you acquire that comfort level? Probably not in medical school. More likely as a house officer when you were guided by a more senior house officer who may nor not been a master otoscopist. How would you rate your training? Or were you self-taught? Do you insufflate? Are you a skilled cerumen extractor? Or do you give up after one attempt? Be honest. How is your equipment? Are the bulbs and batteries fresh? Do you find yourself frustrated by an otoscope that is tethered to the wall charger by a cord that ensnarls you, the parent, and the patient? Have you complained to the practice administrators that your otoscopes are inadequate?
These are not minor issues. It is clear that overdiagnosis of AOM happens. It may occur even more often than Dr. Pichichero suggests, but I doubt it is less. Overdiagnosis can result in overtreatment with antibiotics, and the cascade of consequences both for the patient, the community, and the environment. Overdiagnosis can be the first step on the path to unnecessary surgery. It is incumbent on all of us to make sure that our otoscopy skills and those of our colleagues are sharp, that our equipment is well maintained and that we remain abreast of the latest developments in the diagnosis and treatment of AOM.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
First adult APS recommendations released by European society
MADRID – Low-dose aspirin is recommended for the primary prevention of antiphospholipid syndrome (APS) in patients at high risk for developing the condition, according to new recommendations developed by the European League Against Rheumatism (EULAR).
Indeed, the NSAID should be given at a dose of between 75 mg and 100 mg per day, in patients with a “high risk” antiphospholipid (aPL) antibody profile, including asymptomatic aPL antibody carriers, patients with systemic lupus erythematosus (SLE) without APS, and in women who are not pregnant but who have a history of obstetric APS.
The recommendations aim to help guide practice and ultimately to improve the quality of care for patients and their outcomes following treatment, Maria G. Tektonidou, MD, PhD, said at the European Congress of Rheumatology.
The guidance is necessary as “clinical practice in APS remains highly variable,” said Dr. Tektonidou of the National and Kapodistrian University of Athens. This is perhaps because APS is a “rare disease and also because it’s a newly recognized disease – it’s only 35 years old – and knowledge about the clinical spectrum, classification, and management is continuously advancing.”
Dr. Tektonidou, who was the convener of the EULAR Task Force that wrote the recommendations, noted that they were now published in Annals of the Rheumatic Diseases and considered three main groups of patients: those with thrombotic APS, those with obstetric APS, and those with catastrophic APS (CAPS). There are three overarching principles, 12 recommendations, and 29 graded statements, she said.
The three overarching principles concerned risk stratification, general measures for managing patients who test positive for aPL antibodies, and patient education and counseling on various topics, such as treatment adherence, therapeutic drug monitoring, contraceptive use, and lifestyle interventions.
Dr. Tektonidou highlighted how risk stratification was important and that a high-risk aPL profile was defined as the presence of lupus anticoagulant (LA) on at least two occasions, measured 12 weeks apart according to International Society on Thrombosis and Haemostasis guidelines, or the presence of two or even three aPL antibodies, or persistently high aPL antibody titers. By contrast, a low-risk aPL profile was defined as the isolated presence of anticardiolipin (aCL) or anti–beta-2 glycoprotein I antibodies at low-medium titers, particularly if transiently positive.
“Risk stratification should include the determination of the high-risk aPL profile; a prior history of thrombotic or obstetric [APS]; the coexistence of other systemic autoimmune diseases, and the presence of traditional cardiovascular risk factors,” Dr. Tektonidou said.
Four of the recommendations focus on the secondary prevention of APS, giving guidance on anticoagulant treatment with definite APS, first provoked or unprovoked venous thrombosis, and how to manage recurrent venous thrombosis. There also is a recommendation for the management of patients with definite APS and a first arterial thrombosis, outlining the type and intensity of anticoagulant therapy that should be given. Another four of the recommendations focus on the management of obstetric APS, with a focus on how to manage the various types of complications seen in pregnant women. Then the final recommendation concerns CAPS, it’s prevention and first-line treatment, and how to manage refractory patients.
With regards to CAPS, Ricard Cervera, MD, PhD, of the Hospital Clinic of Barcelona, this is “terrible” but “thankfully rare” form of APS that was first described in the early 1990s.
Although fewer than 1% of the APS adult population have CAPS (Arthritis Rheum. 2002;46[4]:1019-27), it’s a condition in which several thrombotic events occur simultaneously, affecting multiple systems or organs and which can be life threatening if not treated quickly.
New treatment guidelines for catastrophic APS
During a separate clinical science session at the conference, Dr. Cervera discussed the development of treatment guidelines for CAPS, noting that this had been one of the focus points of the McMaster RARE-Bestpractices project group in 2016. The group selected CAPS for a pilot exercise in guideline development for a rare disease and published their recommendations in 2018 (J Thromb Haemost. 2018;16:1656-64). Ten recommendations were developed, most of which were conditional, Dr. Cervera said, due to the lack of, or very low certainty, of the evidence.
The new EULAR 2019 adult APS recommendations now include CAPS and recommendation number 12 is split into two parts. The first, part A, states that prompt treatment of infections is needed in all patients positive for aPL antibodies and that anticoagulation should have minimal interruption or be used at level to help prevent the development of CAPS.
The second, part B, states that the first-line treatment of CAPS should be a triple combination therapy of glucocorticoids, heparin, and plasma exchange, or intravenous immunoglobulins, rather than single-agent treatment. Plus, it says that any triggering factor should be treated accordingly.
“Finally,” Dr. Cervera said, “in patients with refractory CAPS, B-cell depletion with rituximab or complement inhibitors, for example eculizumab, may be considered.”
The adult APS recommendations project was funded by EULAR. Dr. Tektonidou and Dr. Cervera reported having no relevant conflicts of interest.
SOURCES: Tektonidou M. Ann Rheum Dis. Jun 2019;78(Suppl 2):59-60. Abstract SP0191, doi: 0.1136/annrheumdis-2019-eular.8601; and Cervera R et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):62. Abstract SP0201. doi: 10.1136/annrheumdis-2019-eular.8444.
MADRID – Low-dose aspirin is recommended for the primary prevention of antiphospholipid syndrome (APS) in patients at high risk for developing the condition, according to new recommendations developed by the European League Against Rheumatism (EULAR).
Indeed, the NSAID should be given at a dose of between 75 mg and 100 mg per day, in patients with a “high risk” antiphospholipid (aPL) antibody profile, including asymptomatic aPL antibody carriers, patients with systemic lupus erythematosus (SLE) without APS, and in women who are not pregnant but who have a history of obstetric APS.
The recommendations aim to help guide practice and ultimately to improve the quality of care for patients and their outcomes following treatment, Maria G. Tektonidou, MD, PhD, said at the European Congress of Rheumatology.
The guidance is necessary as “clinical practice in APS remains highly variable,” said Dr. Tektonidou of the National and Kapodistrian University of Athens. This is perhaps because APS is a “rare disease and also because it’s a newly recognized disease – it’s only 35 years old – and knowledge about the clinical spectrum, classification, and management is continuously advancing.”
Dr. Tektonidou, who was the convener of the EULAR Task Force that wrote the recommendations, noted that they were now published in Annals of the Rheumatic Diseases and considered three main groups of patients: those with thrombotic APS, those with obstetric APS, and those with catastrophic APS (CAPS). There are three overarching principles, 12 recommendations, and 29 graded statements, she said.
The three overarching principles concerned risk stratification, general measures for managing patients who test positive for aPL antibodies, and patient education and counseling on various topics, such as treatment adherence, therapeutic drug monitoring, contraceptive use, and lifestyle interventions.
Dr. Tektonidou highlighted how risk stratification was important and that a high-risk aPL profile was defined as the presence of lupus anticoagulant (LA) on at least two occasions, measured 12 weeks apart according to International Society on Thrombosis and Haemostasis guidelines, or the presence of two or even three aPL antibodies, or persistently high aPL antibody titers. By contrast, a low-risk aPL profile was defined as the isolated presence of anticardiolipin (aCL) or anti–beta-2 glycoprotein I antibodies at low-medium titers, particularly if transiently positive.
“Risk stratification should include the determination of the high-risk aPL profile; a prior history of thrombotic or obstetric [APS]; the coexistence of other systemic autoimmune diseases, and the presence of traditional cardiovascular risk factors,” Dr. Tektonidou said.
Four of the recommendations focus on the secondary prevention of APS, giving guidance on anticoagulant treatment with definite APS, first provoked or unprovoked venous thrombosis, and how to manage recurrent venous thrombosis. There also is a recommendation for the management of patients with definite APS and a first arterial thrombosis, outlining the type and intensity of anticoagulant therapy that should be given. Another four of the recommendations focus on the management of obstetric APS, with a focus on how to manage the various types of complications seen in pregnant women. Then the final recommendation concerns CAPS, it’s prevention and first-line treatment, and how to manage refractory patients.
With regards to CAPS, Ricard Cervera, MD, PhD, of the Hospital Clinic of Barcelona, this is “terrible” but “thankfully rare” form of APS that was first described in the early 1990s.
Although fewer than 1% of the APS adult population have CAPS (Arthritis Rheum. 2002;46[4]:1019-27), it’s a condition in which several thrombotic events occur simultaneously, affecting multiple systems or organs and which can be life threatening if not treated quickly.
New treatment guidelines for catastrophic APS
During a separate clinical science session at the conference, Dr. Cervera discussed the development of treatment guidelines for CAPS, noting that this had been one of the focus points of the McMaster RARE-Bestpractices project group in 2016. The group selected CAPS for a pilot exercise in guideline development for a rare disease and published their recommendations in 2018 (J Thromb Haemost. 2018;16:1656-64). Ten recommendations were developed, most of which were conditional, Dr. Cervera said, due to the lack of, or very low certainty, of the evidence.
The new EULAR 2019 adult APS recommendations now include CAPS and recommendation number 12 is split into two parts. The first, part A, states that prompt treatment of infections is needed in all patients positive for aPL antibodies and that anticoagulation should have minimal interruption or be used at level to help prevent the development of CAPS.
The second, part B, states that the first-line treatment of CAPS should be a triple combination therapy of glucocorticoids, heparin, and plasma exchange, or intravenous immunoglobulins, rather than single-agent treatment. Plus, it says that any triggering factor should be treated accordingly.
“Finally,” Dr. Cervera said, “in patients with refractory CAPS, B-cell depletion with rituximab or complement inhibitors, for example eculizumab, may be considered.”
The adult APS recommendations project was funded by EULAR. Dr. Tektonidou and Dr. Cervera reported having no relevant conflicts of interest.
SOURCES: Tektonidou M. Ann Rheum Dis. Jun 2019;78(Suppl 2):59-60. Abstract SP0191, doi: 0.1136/annrheumdis-2019-eular.8601; and Cervera R et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):62. Abstract SP0201. doi: 10.1136/annrheumdis-2019-eular.8444.
MADRID – Low-dose aspirin is recommended for the primary prevention of antiphospholipid syndrome (APS) in patients at high risk for developing the condition, according to new recommendations developed by the European League Against Rheumatism (EULAR).
Indeed, the NSAID should be given at a dose of between 75 mg and 100 mg per day, in patients with a “high risk” antiphospholipid (aPL) antibody profile, including asymptomatic aPL antibody carriers, patients with systemic lupus erythematosus (SLE) without APS, and in women who are not pregnant but who have a history of obstetric APS.
The recommendations aim to help guide practice and ultimately to improve the quality of care for patients and their outcomes following treatment, Maria G. Tektonidou, MD, PhD, said at the European Congress of Rheumatology.
The guidance is necessary as “clinical practice in APS remains highly variable,” said Dr. Tektonidou of the National and Kapodistrian University of Athens. This is perhaps because APS is a “rare disease and also because it’s a newly recognized disease – it’s only 35 years old – and knowledge about the clinical spectrum, classification, and management is continuously advancing.”
Dr. Tektonidou, who was the convener of the EULAR Task Force that wrote the recommendations, noted that they were now published in Annals of the Rheumatic Diseases and considered three main groups of patients: those with thrombotic APS, those with obstetric APS, and those with catastrophic APS (CAPS). There are three overarching principles, 12 recommendations, and 29 graded statements, she said.
The three overarching principles concerned risk stratification, general measures for managing patients who test positive for aPL antibodies, and patient education and counseling on various topics, such as treatment adherence, therapeutic drug monitoring, contraceptive use, and lifestyle interventions.
Dr. Tektonidou highlighted how risk stratification was important and that a high-risk aPL profile was defined as the presence of lupus anticoagulant (LA) on at least two occasions, measured 12 weeks apart according to International Society on Thrombosis and Haemostasis guidelines, or the presence of two or even three aPL antibodies, or persistently high aPL antibody titers. By contrast, a low-risk aPL profile was defined as the isolated presence of anticardiolipin (aCL) or anti–beta-2 glycoprotein I antibodies at low-medium titers, particularly if transiently positive.
“Risk stratification should include the determination of the high-risk aPL profile; a prior history of thrombotic or obstetric [APS]; the coexistence of other systemic autoimmune diseases, and the presence of traditional cardiovascular risk factors,” Dr. Tektonidou said.
Four of the recommendations focus on the secondary prevention of APS, giving guidance on anticoagulant treatment with definite APS, first provoked or unprovoked venous thrombosis, and how to manage recurrent venous thrombosis. There also is a recommendation for the management of patients with definite APS and a first arterial thrombosis, outlining the type and intensity of anticoagulant therapy that should be given. Another four of the recommendations focus on the management of obstetric APS, with a focus on how to manage the various types of complications seen in pregnant women. Then the final recommendation concerns CAPS, it’s prevention and first-line treatment, and how to manage refractory patients.
With regards to CAPS, Ricard Cervera, MD, PhD, of the Hospital Clinic of Barcelona, this is “terrible” but “thankfully rare” form of APS that was first described in the early 1990s.
Although fewer than 1% of the APS adult population have CAPS (Arthritis Rheum. 2002;46[4]:1019-27), it’s a condition in which several thrombotic events occur simultaneously, affecting multiple systems or organs and which can be life threatening if not treated quickly.
New treatment guidelines for catastrophic APS
During a separate clinical science session at the conference, Dr. Cervera discussed the development of treatment guidelines for CAPS, noting that this had been one of the focus points of the McMaster RARE-Bestpractices project group in 2016. The group selected CAPS for a pilot exercise in guideline development for a rare disease and published their recommendations in 2018 (J Thromb Haemost. 2018;16:1656-64). Ten recommendations were developed, most of which were conditional, Dr. Cervera said, due to the lack of, or very low certainty, of the evidence.
The new EULAR 2019 adult APS recommendations now include CAPS and recommendation number 12 is split into two parts. The first, part A, states that prompt treatment of infections is needed in all patients positive for aPL antibodies and that anticoagulation should have minimal interruption or be used at level to help prevent the development of CAPS.
The second, part B, states that the first-line treatment of CAPS should be a triple combination therapy of glucocorticoids, heparin, and plasma exchange, or intravenous immunoglobulins, rather than single-agent treatment. Plus, it says that any triggering factor should be treated accordingly.
“Finally,” Dr. Cervera said, “in patients with refractory CAPS, B-cell depletion with rituximab or complement inhibitors, for example eculizumab, may be considered.”
The adult APS recommendations project was funded by EULAR. Dr. Tektonidou and Dr. Cervera reported having no relevant conflicts of interest.
SOURCES: Tektonidou M. Ann Rheum Dis. Jun 2019;78(Suppl 2):59-60. Abstract SP0191, doi: 0.1136/annrheumdis-2019-eular.8601; and Cervera R et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):62. Abstract SP0201. doi: 10.1136/annrheumdis-2019-eular.8444.
REPORTING FROM THE EULAR 2019 Congress
Medication overuse prevalent among U.S. migraine patients
PHILADELPHIA – according to findings from an analysis of 16,789 people with migraine.
About 18% of the people identified with migraine in the study cohort reported a drug consumption pattern that met the prespecified definition of “medication overuse,” Todd J. Schwedt, MD, and his associates reported in a poster at the annual meeting of the American Headache Society. Supplying each migraine patient with a “comprehensive treatment plan” along with “improved acute treatment options ... may help reduce the prevalence and associated burden of medication overuse,” said Dr. Schwedt, a professor of neurology at the Mayo Clinic in Phoenix. The analysis also showed that medication overuse (MO) significantly linked with several markers of worse clinical status.
If patients have “an effective preventive treatment that reduces headaches and migraine attacks then they will, in general, use less acute medications. Many people with migraine never even get diagnosed, and patients who qualify for preventive treatment never get it,” Dr. Schwedt noted in an interview. He described a comprehensive treatment plan as a management strategy that includes lifestyle modifications, a migraine-prevention agent, and the availability of an effective acute treatment for a patient to use when a migraine strikes along with clear instructions on how to appropriately self-administer the medication. Only a small fraction of U.S. migraine patients currently receive this complete package of care, he said.
The analysis he ran used data collected in the CaMEO (Chronic Migraine Epidemiology and Outcomes) study, which used an Internet-based survey to collect data from a representative 58,000-person sample of U.S. residents, which included 16,789 who met the applied migraine definition, with 91% having fewer than 15 headaches/month and the remaining 9% with a monthly headache average of 15 or more (Cephalagia. 2015 Jun;35[7]:563-78).
The researchers defined overuse of a single medication as use 15 times or more a month of an NSAID, aspirin, or acetaminophen, or use at least 10 times a month of a triptan, ergotamine, or opioid. They also had a prespecified definition of multidrug overuse that applied similar monthly thresholds. The patients averaged about 41 years old, three-quarters were women, and 85% were white. Patients identified with MO had a substantially higher rate of headaches per month: an average of nearly 12, compared with an average of about 4 per month among those without overuse. Almost two-thirds of the patients with MO reported having been formally diagnosed as having migraine headaches, compared with 41% of those without overuse.
Among the 13,749 patients (82%) on some headache medication, 67% were on a nonopioid analgesic, including 61% on an NSAID. MO among all people on nonopioid analgesics was 16%, and 12% among those who used NSAIDS. The most overused drug in this subgroup were combination analgesics, overused by 18% of those taking these drugs.
The drug class with the biggest MO rate was opioids, used by 12% of those on any medication and overused by 22% of those taking an opioid. Triptans were taken by 11%, with an MO rate of 11% among these users. Ergotamine was used by less than 1% of all patients, and those taking this drug tallied a 19% MO rate.
“Opioids were the class most often overused, more evidence that opioids should rarely if ever be used to treat migraine,” Dr. Schwedt said.
The analysis also showed that patients who had MO has multiple signs of worse clinical status. Patients with MO had a significantly higher rate of diagnosed depression, 54%, compared with 28% in those without MO; anxiety, 49% compared with 26%; migraine-associated disability, 73% compared with 32%; migraine-associated functional impairment (Migraine Interictal Burden Scale), 65% compared with 32%; and emergency department or urgent care use, 13% compared with 3%. All these between-group differences were statistically significant.
CaMEO was funded by Allergan. Dr. Schwedt has been a consultant to Allergan, and also to Alder, Amgen, Cipla, Dr. Reddy’s, Ipsen, Lilly, Novartis, and Teva. He has stock ownership in Aural Analytics, Nocira, and Second Opinion, and he has received research funding from Amgen.
SOURCE: Schwedt TJ et al. Headache. 2019 June;59[S1]:83-4, Abstract P92.
PHILADELPHIA – according to findings from an analysis of 16,789 people with migraine.
About 18% of the people identified with migraine in the study cohort reported a drug consumption pattern that met the prespecified definition of “medication overuse,” Todd J. Schwedt, MD, and his associates reported in a poster at the annual meeting of the American Headache Society. Supplying each migraine patient with a “comprehensive treatment plan” along with “improved acute treatment options ... may help reduce the prevalence and associated burden of medication overuse,” said Dr. Schwedt, a professor of neurology at the Mayo Clinic in Phoenix. The analysis also showed that medication overuse (MO) significantly linked with several markers of worse clinical status.
If patients have “an effective preventive treatment that reduces headaches and migraine attacks then they will, in general, use less acute medications. Many people with migraine never even get diagnosed, and patients who qualify for preventive treatment never get it,” Dr. Schwedt noted in an interview. He described a comprehensive treatment plan as a management strategy that includes lifestyle modifications, a migraine-prevention agent, and the availability of an effective acute treatment for a patient to use when a migraine strikes along with clear instructions on how to appropriately self-administer the medication. Only a small fraction of U.S. migraine patients currently receive this complete package of care, he said.
The analysis he ran used data collected in the CaMEO (Chronic Migraine Epidemiology and Outcomes) study, which used an Internet-based survey to collect data from a representative 58,000-person sample of U.S. residents, which included 16,789 who met the applied migraine definition, with 91% having fewer than 15 headaches/month and the remaining 9% with a monthly headache average of 15 or more (Cephalagia. 2015 Jun;35[7]:563-78).
The researchers defined overuse of a single medication as use 15 times or more a month of an NSAID, aspirin, or acetaminophen, or use at least 10 times a month of a triptan, ergotamine, or opioid. They also had a prespecified definition of multidrug overuse that applied similar monthly thresholds. The patients averaged about 41 years old, three-quarters were women, and 85% were white. Patients identified with MO had a substantially higher rate of headaches per month: an average of nearly 12, compared with an average of about 4 per month among those without overuse. Almost two-thirds of the patients with MO reported having been formally diagnosed as having migraine headaches, compared with 41% of those without overuse.
Among the 13,749 patients (82%) on some headache medication, 67% were on a nonopioid analgesic, including 61% on an NSAID. MO among all people on nonopioid analgesics was 16%, and 12% among those who used NSAIDS. The most overused drug in this subgroup were combination analgesics, overused by 18% of those taking these drugs.
The drug class with the biggest MO rate was opioids, used by 12% of those on any medication and overused by 22% of those taking an opioid. Triptans were taken by 11%, with an MO rate of 11% among these users. Ergotamine was used by less than 1% of all patients, and those taking this drug tallied a 19% MO rate.
“Opioids were the class most often overused, more evidence that opioids should rarely if ever be used to treat migraine,” Dr. Schwedt said.
The analysis also showed that patients who had MO has multiple signs of worse clinical status. Patients with MO had a significantly higher rate of diagnosed depression, 54%, compared with 28% in those without MO; anxiety, 49% compared with 26%; migraine-associated disability, 73% compared with 32%; migraine-associated functional impairment (Migraine Interictal Burden Scale), 65% compared with 32%; and emergency department or urgent care use, 13% compared with 3%. All these between-group differences were statistically significant.
CaMEO was funded by Allergan. Dr. Schwedt has been a consultant to Allergan, and also to Alder, Amgen, Cipla, Dr. Reddy’s, Ipsen, Lilly, Novartis, and Teva. He has stock ownership in Aural Analytics, Nocira, and Second Opinion, and he has received research funding from Amgen.
SOURCE: Schwedt TJ et al. Headache. 2019 June;59[S1]:83-4, Abstract P92.
PHILADELPHIA – according to findings from an analysis of 16,789 people with migraine.
About 18% of the people identified with migraine in the study cohort reported a drug consumption pattern that met the prespecified definition of “medication overuse,” Todd J. Schwedt, MD, and his associates reported in a poster at the annual meeting of the American Headache Society. Supplying each migraine patient with a “comprehensive treatment plan” along with “improved acute treatment options ... may help reduce the prevalence and associated burden of medication overuse,” said Dr. Schwedt, a professor of neurology at the Mayo Clinic in Phoenix. The analysis also showed that medication overuse (MO) significantly linked with several markers of worse clinical status.
If patients have “an effective preventive treatment that reduces headaches and migraine attacks then they will, in general, use less acute medications. Many people with migraine never even get diagnosed, and patients who qualify for preventive treatment never get it,” Dr. Schwedt noted in an interview. He described a comprehensive treatment plan as a management strategy that includes lifestyle modifications, a migraine-prevention agent, and the availability of an effective acute treatment for a patient to use when a migraine strikes along with clear instructions on how to appropriately self-administer the medication. Only a small fraction of U.S. migraine patients currently receive this complete package of care, he said.
The analysis he ran used data collected in the CaMEO (Chronic Migraine Epidemiology and Outcomes) study, which used an Internet-based survey to collect data from a representative 58,000-person sample of U.S. residents, which included 16,789 who met the applied migraine definition, with 91% having fewer than 15 headaches/month and the remaining 9% with a monthly headache average of 15 or more (Cephalagia. 2015 Jun;35[7]:563-78).
The researchers defined overuse of a single medication as use 15 times or more a month of an NSAID, aspirin, or acetaminophen, or use at least 10 times a month of a triptan, ergotamine, or opioid. They also had a prespecified definition of multidrug overuse that applied similar monthly thresholds. The patients averaged about 41 years old, three-quarters were women, and 85% were white. Patients identified with MO had a substantially higher rate of headaches per month: an average of nearly 12, compared with an average of about 4 per month among those without overuse. Almost two-thirds of the patients with MO reported having been formally diagnosed as having migraine headaches, compared with 41% of those without overuse.
Among the 13,749 patients (82%) on some headache medication, 67% were on a nonopioid analgesic, including 61% on an NSAID. MO among all people on nonopioid analgesics was 16%, and 12% among those who used NSAIDS. The most overused drug in this subgroup were combination analgesics, overused by 18% of those taking these drugs.
The drug class with the biggest MO rate was opioids, used by 12% of those on any medication and overused by 22% of those taking an opioid. Triptans were taken by 11%, with an MO rate of 11% among these users. Ergotamine was used by less than 1% of all patients, and those taking this drug tallied a 19% MO rate.
“Opioids were the class most often overused, more evidence that opioids should rarely if ever be used to treat migraine,” Dr. Schwedt said.
The analysis also showed that patients who had MO has multiple signs of worse clinical status. Patients with MO had a significantly higher rate of diagnosed depression, 54%, compared with 28% in those without MO; anxiety, 49% compared with 26%; migraine-associated disability, 73% compared with 32%; migraine-associated functional impairment (Migraine Interictal Burden Scale), 65% compared with 32%; and emergency department or urgent care use, 13% compared with 3%. All these between-group differences were statistically significant.
CaMEO was funded by Allergan. Dr. Schwedt has been a consultant to Allergan, and also to Alder, Amgen, Cipla, Dr. Reddy’s, Ipsen, Lilly, Novartis, and Teva. He has stock ownership in Aural Analytics, Nocira, and Second Opinion, and he has received research funding from Amgen.
SOURCE: Schwedt TJ et al. Headache. 2019 June;59[S1]:83-4, Abstract P92.
REPORTING FROM AHS 2019