User login
Dysregulated sleep is common in children with eosinophilic esophagitis
, Rasintra Siriwat, MD, and colleagues have ascertained.
Children with eosinophilic esophagitis (EoE) also were found to have a high prevalence of atopic diseases, including allergic rhinitis and eczema – findings that could be driving the breathing problems, said Dr. Siriwat, a neurology fellow at the Cleveland Clinic, and coauthors.
The retrospective study comprised 81 children with a diagnosis of EoE who were referred to sleep clinics. In this group, 46 of the children had active EoE (having gastrointestinal symptoms, including feeding difficulties, dysphagia, reflux, nausea/vomiting, or epigastric pain at presentation). The other 35 had an EoE diagnosis but no symptoms on presentation and were categorized as having inactive EoE. Most were male (71.6%) and white (92.5%). The mean age in the cohort was 10 years and the mean body mass index for all subjects was 22 kg/m2. A control group of 192 children without an EoE diagnosis who had overnight polysomnography were included in the analysis.
Allergic-type comorbidities were common among those with active EoE, including allergic rhinitis (55.5%), food allergy (39.5%), and eczema (26%). In addition, a quarter had attention-deficit/hyperactivity disorder, 22% an autism spectrum disorder, 21% a neurological disease, and 29% a psychiatric disorder.
Several sleep complaints were common in the entire EoE cohort, including snoring (76.5 %), restless sleep (66.6%), legs jerking or leg discomfort (43.2%), and daytime sleepiness (58%).
All children underwent an overnight polysomnography. Compared with controls, the children with EoE had significantly higher non-REM2 sleep, significantly lower non-REM3 sleep, lower REM, increased periodic leg movement disorder, and increased arousal index.
“Of note, we found a much higher percentage of [periodic leg movement disorder] in active EoE compared to inactive EoE,” the authors said.
The most common sleep diagnosis for the children with EoE was sleep-disordered breathing. Of 62 children with EoE and sleep disordered breathing, 37% had obstructive sleep apnea (OSA). Two patients had central sleep apnea and five had nocturnal hypoventilation. Children with EoE also reported parasomnia symptoms such as sleep talking (35.8%), sleepwalking (16%), bruxism (23.4%), night terrors (28.4%), and nocturnal enuresis (21.2%).
Of the 59 children with leg movement, 20 had periodic limb movement disorder and 5 were diagnosed with restless leg syndrome. Two were diagnosed with narcolepsy and three with hypersomnia. Four children had a circadian rhythm disorder.
“Notably, the majority of children with EoE had symptoms of sleep-disordered breathing, and more than one-third of total subjects were diagnosed with OSA,” the authors noted. “However, most of them were mild-moderate OSA. It should be noted that the prevalence of OSA in the pediatric population is 1%-5% mostly between the ages of 2-8 years, while the mean age of our subjects was 10 years old. The high prevalence of mild-moderate OSA in the EoE population might be explained by the relationship between EoE and atopic disease.”
Dr. Siriwat had no financial disclosures. The study was supported by Cincinnati Children’s Hospital Research Fund.
SOURCE: Siriwat R et al. Sleep Med. 2019 Sep 11. doi: 10.1016/j.sleep.2019.08.018.
, Rasintra Siriwat, MD, and colleagues have ascertained.
Children with eosinophilic esophagitis (EoE) also were found to have a high prevalence of atopic diseases, including allergic rhinitis and eczema – findings that could be driving the breathing problems, said Dr. Siriwat, a neurology fellow at the Cleveland Clinic, and coauthors.
The retrospective study comprised 81 children with a diagnosis of EoE who were referred to sleep clinics. In this group, 46 of the children had active EoE (having gastrointestinal symptoms, including feeding difficulties, dysphagia, reflux, nausea/vomiting, or epigastric pain at presentation). The other 35 had an EoE diagnosis but no symptoms on presentation and were categorized as having inactive EoE. Most were male (71.6%) and white (92.5%). The mean age in the cohort was 10 years and the mean body mass index for all subjects was 22 kg/m2. A control group of 192 children without an EoE diagnosis who had overnight polysomnography were included in the analysis.
Allergic-type comorbidities were common among those with active EoE, including allergic rhinitis (55.5%), food allergy (39.5%), and eczema (26%). In addition, a quarter had attention-deficit/hyperactivity disorder, 22% an autism spectrum disorder, 21% a neurological disease, and 29% a psychiatric disorder.
Several sleep complaints were common in the entire EoE cohort, including snoring (76.5 %), restless sleep (66.6%), legs jerking or leg discomfort (43.2%), and daytime sleepiness (58%).
All children underwent an overnight polysomnography. Compared with controls, the children with EoE had significantly higher non-REM2 sleep, significantly lower non-REM3 sleep, lower REM, increased periodic leg movement disorder, and increased arousal index.
“Of note, we found a much higher percentage of [periodic leg movement disorder] in active EoE compared to inactive EoE,” the authors said.
The most common sleep diagnosis for the children with EoE was sleep-disordered breathing. Of 62 children with EoE and sleep disordered breathing, 37% had obstructive sleep apnea (OSA). Two patients had central sleep apnea and five had nocturnal hypoventilation. Children with EoE also reported parasomnia symptoms such as sleep talking (35.8%), sleepwalking (16%), bruxism (23.4%), night terrors (28.4%), and nocturnal enuresis (21.2%).
Of the 59 children with leg movement, 20 had periodic limb movement disorder and 5 were diagnosed with restless leg syndrome. Two were diagnosed with narcolepsy and three with hypersomnia. Four children had a circadian rhythm disorder.
“Notably, the majority of children with EoE had symptoms of sleep-disordered breathing, and more than one-third of total subjects were diagnosed with OSA,” the authors noted. “However, most of them were mild-moderate OSA. It should be noted that the prevalence of OSA in the pediatric population is 1%-5% mostly between the ages of 2-8 years, while the mean age of our subjects was 10 years old. The high prevalence of mild-moderate OSA in the EoE population might be explained by the relationship between EoE and atopic disease.”
Dr. Siriwat had no financial disclosures. The study was supported by Cincinnati Children’s Hospital Research Fund.
SOURCE: Siriwat R et al. Sleep Med. 2019 Sep 11. doi: 10.1016/j.sleep.2019.08.018.
, Rasintra Siriwat, MD, and colleagues have ascertained.
Children with eosinophilic esophagitis (EoE) also were found to have a high prevalence of atopic diseases, including allergic rhinitis and eczema – findings that could be driving the breathing problems, said Dr. Siriwat, a neurology fellow at the Cleveland Clinic, and coauthors.
The retrospective study comprised 81 children with a diagnosis of EoE who were referred to sleep clinics. In this group, 46 of the children had active EoE (having gastrointestinal symptoms, including feeding difficulties, dysphagia, reflux, nausea/vomiting, or epigastric pain at presentation). The other 35 had an EoE diagnosis but no symptoms on presentation and were categorized as having inactive EoE. Most were male (71.6%) and white (92.5%). The mean age in the cohort was 10 years and the mean body mass index for all subjects was 22 kg/m2. A control group of 192 children without an EoE diagnosis who had overnight polysomnography were included in the analysis.
Allergic-type comorbidities were common among those with active EoE, including allergic rhinitis (55.5%), food allergy (39.5%), and eczema (26%). In addition, a quarter had attention-deficit/hyperactivity disorder, 22% an autism spectrum disorder, 21% a neurological disease, and 29% a psychiatric disorder.
Several sleep complaints were common in the entire EoE cohort, including snoring (76.5 %), restless sleep (66.6%), legs jerking or leg discomfort (43.2%), and daytime sleepiness (58%).
All children underwent an overnight polysomnography. Compared with controls, the children with EoE had significantly higher non-REM2 sleep, significantly lower non-REM3 sleep, lower REM, increased periodic leg movement disorder, and increased arousal index.
“Of note, we found a much higher percentage of [periodic leg movement disorder] in active EoE compared to inactive EoE,” the authors said.
The most common sleep diagnosis for the children with EoE was sleep-disordered breathing. Of 62 children with EoE and sleep disordered breathing, 37% had obstructive sleep apnea (OSA). Two patients had central sleep apnea and five had nocturnal hypoventilation. Children with EoE also reported parasomnia symptoms such as sleep talking (35.8%), sleepwalking (16%), bruxism (23.4%), night terrors (28.4%), and nocturnal enuresis (21.2%).
Of the 59 children with leg movement, 20 had periodic limb movement disorder and 5 were diagnosed with restless leg syndrome. Two were diagnosed with narcolepsy and three with hypersomnia. Four children had a circadian rhythm disorder.
“Notably, the majority of children with EoE had symptoms of sleep-disordered breathing, and more than one-third of total subjects were diagnosed with OSA,” the authors noted. “However, most of them were mild-moderate OSA. It should be noted that the prevalence of OSA in the pediatric population is 1%-5% mostly between the ages of 2-8 years, while the mean age of our subjects was 10 years old. The high prevalence of mild-moderate OSA in the EoE population might be explained by the relationship between EoE and atopic disease.”
Dr. Siriwat had no financial disclosures. The study was supported by Cincinnati Children’s Hospital Research Fund.
SOURCE: Siriwat R et al. Sleep Med. 2019 Sep 11. doi: 10.1016/j.sleep.2019.08.018.
FROM SLEEP MEDICINE
Set reasonable expectations for managing refractory rheumatoid arthritis
LAS VEGAS – Rheumatoid arthritis that is difficult to treat may occur in about 10% of patients with the disease. The definition of difficult-to-treat rheumatoid arthritis varies among experts, however, and the reasons for refractoriness are often unknown, said Iain McInnes, MD, PhD, professor of experimental medicine and director of the Institute of Infection, Immunity and Inflammation at the University of Glasgow and current president of the European League Against Rheumatism.
There may be an intrinsic refractory disease state, where the disease will manifest despite the use of any existing medications. Patients may have pharmacokinetic refractory disease, which could result from antidrug antibodies. There may be false positives. “It could well be that the patient is sore, but it is not because of the active inflammation,” he said. “It is because of biomechanical disease or pain sensitization. Or it could be that there is osteoarthritis.”
In the end, managing patients with difficult-to-treat rheumatoid arthritis may come down to setting reasonable expectations and addressing practical concerns, such as medication adherence, comorbidities, and adverse drug reactions – “the realpolitik of rheumatological care,” Dr. McInnes said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
Defining difficult to treat
In a recently published study, Dr. McInnes and colleagues surveyed rheumatologists about what characterizes difficult-to-treat rheumatoid arthritis (Ann Rheum Dis. 2018 Dec;77[12]:1705-9).
“There was remarkable variation,” Dr. McInnes said. “Should fatigue be involved? Do the types of drugs that you have failed matter? Should a patient’s need for glucocorticoids be part of the definition? ... We did not really find much of a consensus.”
The presence of cardiovascular disease, extra-articular manifestations, infection, malignancy, diabetes mellitus, and other factors add to the challenge. “These are all very familiar problems that you face in your routine practice, whether you are in rheumatology care primarily or whether you are in family practice,” he said.
Although it is difficult to determine how many patients have refractory rheumatoid arthritis, “the rule of thumb is about 10%,” Dr. McInnes said. Using data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis, researchers estimated that approximately 6% of patients would be refractory to a biologic drug, defined as patients who move on to a third class of biologic therapy (Ann Rheum Dis. 2018 Oct;77[10]:1405-12).
The same study found that, in recent years, patients have cycled through drugs more quickly than they had in the past. Many patients prove difficult to treat within 4 or 5 years, “which is pretty terrifying with a disease whose median age of onset is early 50s, but whose clinics are full of people in their teens, 20s, and 30s,” Dr. McInnes said.
Approaches to treatment
New medications, therapeutic targets, and treatment approaches eventually may help treat or prevent refractory disease. In the meantime, it may be beneficial to “go back to the beginning” when caring for patients with difficult-to-treat rheumatoid arthritis, Dr. McInnes said. “Make absolutely certain that we understand what is going on in that patient. Even just the opportunity to go through the disease course is reassuring for our patients. If nothing else, it tells them that we are listening, paying attention, and we are taking it seriously.”
Physicians may need to recognize the presence of antidrug antibodies. In addition, medication adherence may be a problem. About one-third of patients who are prescribed methotrexate do not take the medication, which may be a difficult topic for them to discuss, he said.
“Detect that inflammation which is there and treat it,” Dr. McInnes said. “And detect that which is not of an inflammatory origin and manage it on its own merits.”
Dr. McInnes is a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Galvani, GlaxoSmithKline, Leo Pharma, Novartis, Pfizer, and UCB. He has received grant or research support from AstraZeneca, Bristol-Myers Squibb, Compugen, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Rheumatoid arthritis that is difficult to treat may occur in about 10% of patients with the disease. The definition of difficult-to-treat rheumatoid arthritis varies among experts, however, and the reasons for refractoriness are often unknown, said Iain McInnes, MD, PhD, professor of experimental medicine and director of the Institute of Infection, Immunity and Inflammation at the University of Glasgow and current president of the European League Against Rheumatism.
There may be an intrinsic refractory disease state, where the disease will manifest despite the use of any existing medications. Patients may have pharmacokinetic refractory disease, which could result from antidrug antibodies. There may be false positives. “It could well be that the patient is sore, but it is not because of the active inflammation,” he said. “It is because of biomechanical disease or pain sensitization. Or it could be that there is osteoarthritis.”
In the end, managing patients with difficult-to-treat rheumatoid arthritis may come down to setting reasonable expectations and addressing practical concerns, such as medication adherence, comorbidities, and adverse drug reactions – “the realpolitik of rheumatological care,” Dr. McInnes said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
Defining difficult to treat
In a recently published study, Dr. McInnes and colleagues surveyed rheumatologists about what characterizes difficult-to-treat rheumatoid arthritis (Ann Rheum Dis. 2018 Dec;77[12]:1705-9).
“There was remarkable variation,” Dr. McInnes said. “Should fatigue be involved? Do the types of drugs that you have failed matter? Should a patient’s need for glucocorticoids be part of the definition? ... We did not really find much of a consensus.”
The presence of cardiovascular disease, extra-articular manifestations, infection, malignancy, diabetes mellitus, and other factors add to the challenge. “These are all very familiar problems that you face in your routine practice, whether you are in rheumatology care primarily or whether you are in family practice,” he said.
Although it is difficult to determine how many patients have refractory rheumatoid arthritis, “the rule of thumb is about 10%,” Dr. McInnes said. Using data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis, researchers estimated that approximately 6% of patients would be refractory to a biologic drug, defined as patients who move on to a third class of biologic therapy (Ann Rheum Dis. 2018 Oct;77[10]:1405-12).
The same study found that, in recent years, patients have cycled through drugs more quickly than they had in the past. Many patients prove difficult to treat within 4 or 5 years, “which is pretty terrifying with a disease whose median age of onset is early 50s, but whose clinics are full of people in their teens, 20s, and 30s,” Dr. McInnes said.
Approaches to treatment
New medications, therapeutic targets, and treatment approaches eventually may help treat or prevent refractory disease. In the meantime, it may be beneficial to “go back to the beginning” when caring for patients with difficult-to-treat rheumatoid arthritis, Dr. McInnes said. “Make absolutely certain that we understand what is going on in that patient. Even just the opportunity to go through the disease course is reassuring for our patients. If nothing else, it tells them that we are listening, paying attention, and we are taking it seriously.”
Physicians may need to recognize the presence of antidrug antibodies. In addition, medication adherence may be a problem. About one-third of patients who are prescribed methotrexate do not take the medication, which may be a difficult topic for them to discuss, he said.
“Detect that inflammation which is there and treat it,” Dr. McInnes said. “And detect that which is not of an inflammatory origin and manage it on its own merits.”
Dr. McInnes is a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Galvani, GlaxoSmithKline, Leo Pharma, Novartis, Pfizer, and UCB. He has received grant or research support from AstraZeneca, Bristol-Myers Squibb, Compugen, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Rheumatoid arthritis that is difficult to treat may occur in about 10% of patients with the disease. The definition of difficult-to-treat rheumatoid arthritis varies among experts, however, and the reasons for refractoriness are often unknown, said Iain McInnes, MD, PhD, professor of experimental medicine and director of the Institute of Infection, Immunity and Inflammation at the University of Glasgow and current president of the European League Against Rheumatism.
There may be an intrinsic refractory disease state, where the disease will manifest despite the use of any existing medications. Patients may have pharmacokinetic refractory disease, which could result from antidrug antibodies. There may be false positives. “It could well be that the patient is sore, but it is not because of the active inflammation,” he said. “It is because of biomechanical disease or pain sensitization. Or it could be that there is osteoarthritis.”
In the end, managing patients with difficult-to-treat rheumatoid arthritis may come down to setting reasonable expectations and addressing practical concerns, such as medication adherence, comorbidities, and adverse drug reactions – “the realpolitik of rheumatological care,” Dr. McInnes said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
Defining difficult to treat
In a recently published study, Dr. McInnes and colleagues surveyed rheumatologists about what characterizes difficult-to-treat rheumatoid arthritis (Ann Rheum Dis. 2018 Dec;77[12]:1705-9).
“There was remarkable variation,” Dr. McInnes said. “Should fatigue be involved? Do the types of drugs that you have failed matter? Should a patient’s need for glucocorticoids be part of the definition? ... We did not really find much of a consensus.”
The presence of cardiovascular disease, extra-articular manifestations, infection, malignancy, diabetes mellitus, and other factors add to the challenge. “These are all very familiar problems that you face in your routine practice, whether you are in rheumatology care primarily or whether you are in family practice,” he said.
Although it is difficult to determine how many patients have refractory rheumatoid arthritis, “the rule of thumb is about 10%,” Dr. McInnes said. Using data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis, researchers estimated that approximately 6% of patients would be refractory to a biologic drug, defined as patients who move on to a third class of biologic therapy (Ann Rheum Dis. 2018 Oct;77[10]:1405-12).
The same study found that, in recent years, patients have cycled through drugs more quickly than they had in the past. Many patients prove difficult to treat within 4 or 5 years, “which is pretty terrifying with a disease whose median age of onset is early 50s, but whose clinics are full of people in their teens, 20s, and 30s,” Dr. McInnes said.
Approaches to treatment
New medications, therapeutic targets, and treatment approaches eventually may help treat or prevent refractory disease. In the meantime, it may be beneficial to “go back to the beginning” when caring for patients with difficult-to-treat rheumatoid arthritis, Dr. McInnes said. “Make absolutely certain that we understand what is going on in that patient. Even just the opportunity to go through the disease course is reassuring for our patients. If nothing else, it tells them that we are listening, paying attention, and we are taking it seriously.”
Physicians may need to recognize the presence of antidrug antibodies. In addition, medication adherence may be a problem. About one-third of patients who are prescribed methotrexate do not take the medication, which may be a difficult topic for them to discuss, he said.
“Detect that inflammation which is there and treat it,” Dr. McInnes said. “And detect that which is not of an inflammatory origin and manage it on its own merits.”
Dr. McInnes is a consultant for AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Galvani, GlaxoSmithKline, Leo Pharma, Novartis, Pfizer, and UCB. He has received grant or research support from AstraZeneca, Bristol-Myers Squibb, Compugen, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB.
Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PRD 2019
Laparoscopic surgery has short-term advantages for elderly patients with colorectal cancer
Sicheng Zhou, and colleagues wrote in BMC Surgery.
“Laparoscopic surgery showed better results than the open surgery in short-term outcomes,” said Dr. Zhou of the Chinese Academy of Medical Sciences, and coauthors. “[Carcinoembryonic antigen] level, III/IV stage, and perineural invasion were all reliable predictors of overall survival and disease-free survival for the treatment of laparoscopic surgery and open surgery for elderly Chinese patients over 80 years old with colorectal cancer.”
The study comprised 313 patients aged 80 years or older who underwent surgery for colorectal cancer. The group was equally divided between those who had laparoscopic and open surgery. They were matched 1:1, for a total of 93 pairs included. The patients’ mean age was 82 years. Medical comorbidities were present in about 63%. The tumor was more likely to present in the in the rectum and right colon (about 34% each). The next most common disease site was the sigmoid colon (22%).
Most tumors were stage III (58%), and II (about 30%). About 70% were moderately differentiated and 20% poorly differentiated. Carcinoembryonic antigen was greater than 5 ng/mL in about three-fourths of the group, and higher in the reminder.
Surgery duration was somewhat shorter in the open group but not significantly so. However, intraoperative complications were higher in the open group, including transfusion (22.6% vs. 16% and blood loss 50.9 vs. 108 mL). There was a lower occurrence of postoperative complications (10.8% vs. 26.9%) in the laparoscopic group.
Intraoperative complications occurred in only one patient, who was in the laparoscopic group, but perioperative complications were significantly more common in the open group (17.2% vs. 6.5%). In the open group these included wound infection (9.7%), followed by ileus (5.4%), anastomosis leakage (4.3%), and delayed gastric emptying (4.3%). In the laparoscopic group, the most common morbidities were anastomosis leakage (2.2%), ileus (2.2%) and pneumonia (2.2%).
The number of retrieved lymph nodes was also significantly higher in the laparoscopic group (20 vs. 17).
Yet, despite the short-term perioperative advantages of the laparoscopic approach in elderly patients, the 3- and 5-year overall and disease-free survival rates were not significantly different in these groups. The investigators concluded “it is noteworthy that the 3-year and 5-year [overall survival] rates, and 3- year and 5-year [disease-free survival] rates of patients in the laparoscopic group were generally higher than the open group. The 5-year [disease-free survival] rate in the laparoscopic group was even higher than that in the open group by more than 10%. This difference might be due to the difference in the number of dissected lymph nodes between the open group and the laparoscopic group. Hence, although there was no significant difference in survival outcomes between the two surgical methods, the laparoscopic surgery in elderly patients with colorectal cancer might achieve better survival outcomes than the open surgery.”
The authors declared that they had no competing interests. This work was supported by the Beijing Hope Run Special Fund of Cancer Foundation of China and Capital Health Research and Development.
SOURCE: Zhou S et al. BMC Surg. 2019;19:137. doi: 10.1186/s12893-019-0596-3.
Sicheng Zhou, and colleagues wrote in BMC Surgery.
“Laparoscopic surgery showed better results than the open surgery in short-term outcomes,” said Dr. Zhou of the Chinese Academy of Medical Sciences, and coauthors. “[Carcinoembryonic antigen] level, III/IV stage, and perineural invasion were all reliable predictors of overall survival and disease-free survival for the treatment of laparoscopic surgery and open surgery for elderly Chinese patients over 80 years old with colorectal cancer.”
The study comprised 313 patients aged 80 years or older who underwent surgery for colorectal cancer. The group was equally divided between those who had laparoscopic and open surgery. They were matched 1:1, for a total of 93 pairs included. The patients’ mean age was 82 years. Medical comorbidities were present in about 63%. The tumor was more likely to present in the in the rectum and right colon (about 34% each). The next most common disease site was the sigmoid colon (22%).
Most tumors were stage III (58%), and II (about 30%). About 70% were moderately differentiated and 20% poorly differentiated. Carcinoembryonic antigen was greater than 5 ng/mL in about three-fourths of the group, and higher in the reminder.
Surgery duration was somewhat shorter in the open group but not significantly so. However, intraoperative complications were higher in the open group, including transfusion (22.6% vs. 16% and blood loss 50.9 vs. 108 mL). There was a lower occurrence of postoperative complications (10.8% vs. 26.9%) in the laparoscopic group.
Intraoperative complications occurred in only one patient, who was in the laparoscopic group, but perioperative complications were significantly more common in the open group (17.2% vs. 6.5%). In the open group these included wound infection (9.7%), followed by ileus (5.4%), anastomosis leakage (4.3%), and delayed gastric emptying (4.3%). In the laparoscopic group, the most common morbidities were anastomosis leakage (2.2%), ileus (2.2%) and pneumonia (2.2%).
The number of retrieved lymph nodes was also significantly higher in the laparoscopic group (20 vs. 17).
Yet, despite the short-term perioperative advantages of the laparoscopic approach in elderly patients, the 3- and 5-year overall and disease-free survival rates were not significantly different in these groups. The investigators concluded “it is noteworthy that the 3-year and 5-year [overall survival] rates, and 3- year and 5-year [disease-free survival] rates of patients in the laparoscopic group were generally higher than the open group. The 5-year [disease-free survival] rate in the laparoscopic group was even higher than that in the open group by more than 10%. This difference might be due to the difference in the number of dissected lymph nodes between the open group and the laparoscopic group. Hence, although there was no significant difference in survival outcomes between the two surgical methods, the laparoscopic surgery in elderly patients with colorectal cancer might achieve better survival outcomes than the open surgery.”
The authors declared that they had no competing interests. This work was supported by the Beijing Hope Run Special Fund of Cancer Foundation of China and Capital Health Research and Development.
SOURCE: Zhou S et al. BMC Surg. 2019;19:137. doi: 10.1186/s12893-019-0596-3.
Sicheng Zhou, and colleagues wrote in BMC Surgery.
“Laparoscopic surgery showed better results than the open surgery in short-term outcomes,” said Dr. Zhou of the Chinese Academy of Medical Sciences, and coauthors. “[Carcinoembryonic antigen] level, III/IV stage, and perineural invasion were all reliable predictors of overall survival and disease-free survival for the treatment of laparoscopic surgery and open surgery for elderly Chinese patients over 80 years old with colorectal cancer.”
The study comprised 313 patients aged 80 years or older who underwent surgery for colorectal cancer. The group was equally divided between those who had laparoscopic and open surgery. They were matched 1:1, for a total of 93 pairs included. The patients’ mean age was 82 years. Medical comorbidities were present in about 63%. The tumor was more likely to present in the in the rectum and right colon (about 34% each). The next most common disease site was the sigmoid colon (22%).
Most tumors were stage III (58%), and II (about 30%). About 70% were moderately differentiated and 20% poorly differentiated. Carcinoembryonic antigen was greater than 5 ng/mL in about three-fourths of the group, and higher in the reminder.
Surgery duration was somewhat shorter in the open group but not significantly so. However, intraoperative complications were higher in the open group, including transfusion (22.6% vs. 16% and blood loss 50.9 vs. 108 mL). There was a lower occurrence of postoperative complications (10.8% vs. 26.9%) in the laparoscopic group.
Intraoperative complications occurred in only one patient, who was in the laparoscopic group, but perioperative complications were significantly more common in the open group (17.2% vs. 6.5%). In the open group these included wound infection (9.7%), followed by ileus (5.4%), anastomosis leakage (4.3%), and delayed gastric emptying (4.3%). In the laparoscopic group, the most common morbidities were anastomosis leakage (2.2%), ileus (2.2%) and pneumonia (2.2%).
The number of retrieved lymph nodes was also significantly higher in the laparoscopic group (20 vs. 17).
Yet, despite the short-term perioperative advantages of the laparoscopic approach in elderly patients, the 3- and 5-year overall and disease-free survival rates were not significantly different in these groups. The investigators concluded “it is noteworthy that the 3-year and 5-year [overall survival] rates, and 3- year and 5-year [disease-free survival] rates of patients in the laparoscopic group were generally higher than the open group. The 5-year [disease-free survival] rate in the laparoscopic group was even higher than that in the open group by more than 10%. This difference might be due to the difference in the number of dissected lymph nodes between the open group and the laparoscopic group. Hence, although there was no significant difference in survival outcomes between the two surgical methods, the laparoscopic surgery in elderly patients with colorectal cancer might achieve better survival outcomes than the open surgery.”
The authors declared that they had no competing interests. This work was supported by the Beijing Hope Run Special Fund of Cancer Foundation of China and Capital Health Research and Development.
SOURCE: Zhou S et al. BMC Surg. 2019;19:137. doi: 10.1186/s12893-019-0596-3.
FROM BMC SURGERY
Consider centralized pain in patients with rheumatic disease
Las Vegas – A fibromyalgia survey may provide important information about the degree to which patients with rheumatic disease experience centralized pain. This information may guide treatment decisions, said Daniel J. Clauw, MD, professor of anesthesiology, rheumatology, and psychiatry and director of the Chronic Pain and Fatigue Research Center at the University of Michigan in Ann Arbor.
The questionnaire that Dr. Clauw uses is a patient self-report survey for the assessment of fibromyalgia based on criteria in the 2011 modification of the American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. In it, he asks patients to report where they experience pain throughout the body and symptoms such as fatigue, sleep problems, and memory problems. The survey predicts outcomes of surgery for osteoarthritis better than x-rays, MRI scans, or psychological factors do, he said.
Physicians should ask every patient with chronic pain, including patients with OA, rheumatoid arthritis, or lupus, to complete the survey, Dr. Clauw said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “This score will tell you the degree to which their central nervous system is augmenting or amplifying what is going on in their body,” he said. “And the higher their score is, the more you should treat them like you would someone with fibromyalgia, even if their underlying disease might be an autoimmune disease.”
Physicians should not use a cutoff of 13 points on the fibromyalgia measure to define whether a patient has the disease, as has been done in the past, he said. The threshold is arbitrary, he said. “We should not think about fibromyalgia as ‘yes’ or ‘no.’ We should think of the degree of fibromyalgia that people have.”
A poor relationship between pain and imaging
Some patients who have severe knee OA on imaging walk without pain. Other patients have normal x-rays, but severe pain. “There is a terrible relationship between what you see on a knee x-ray or an MRI and whether someone has pain,” Dr. Clauw said. Furthermore, the poor relationship between imaging and pain is common across chronic pain conditions, he said.
This phenomenon may occur because pain manifests in different ways, similar to there being multiple ways to adjust the volume of an electric guitar, he said. How hard the strings are strummed affects the volume. But so does the amplifier setting. “In these centralized pain conditions, the problem is an amplifier problem, not a guitar problem,” he said. “The amplifier, i.e., the central nervous system, is set too high.”
Researchers have found that people who have severe OA of the knee on x-ray but do not experience pain “have a very low amplifier setting,” he said. That is, they are nontender and less sensitive to pain. Most of these patients are men. “On average, men have a much lower amplifier setting than women,” he said. “This is also why ... women have 1.5 to 2 times the rate of any type of chronic pain than men, because on average women have a higher amplifier setting. ... In OA, at any given age, men and women have the exact same percentage of radiographic OA. But if you look at the clinical condition of OA, it is always two-thirds women, one-third men.”
Opioid responsiveness
To examine whether fibromyalgia survey results correlate with outcomes after knee and hip arthroplasty, Dr. Clauw and colleagues conducted a prospective, observational cohort study that included approximately 500 people. Patients completed the questionnaire on the day of surgery.
Patients with higher levels of fibromyalgia were less responsive to opioids. “For each 1-point increase in the fibromyalgia score, people needed about one more hydrocodone tablet in the first 24-48 hours to control their pain,” he said (Anesthesiology. 2013 Dec;119[6]:1434-43). In addition, each 1-point increase in the fibromyalgia score made people about 25% less likely to have a 50% improvement in knee pain level after 6 months (Arthritis Rheumatol. 2015 May;67[5]:1386-94). The correlations were independent of psychological factors. In addition, the associations were linear. “There was nothing magical about a fibromyalgia score of 13,” Dr. Clauw said.
Dr. Clauw is a coauthor of a study to be presented at the 2019 American College of Rheumatology/Association of Rheumatology Professionals annual meeting that found pain centralization in patients with RA is associated with poor response to disease-modifying antirheumatic drugs (DMARDs).
Prior studies in patients with RA have found that the degree of fibromyalgia is a better predictor of pain and disability than erythrocyte sedimentation rate or the number of swollen joints.
Diagnosed cases are the “tip of the iceberg”
Researchers at Dr. Clauw’s institution have identified dozens of patients undergoing knee surgery who met criteria for fibromyalgia but had not received the diagnosis. “This is at the University of Michigan, which is the epicenter for fibromyalgia research. If we are not seeing fibromyalgia superimposed on OA in our patients, no one is seeing it,” he said.
Patients with diagnosed fibromyalgia are “the tip of the iceberg,” he said. “There are far greater numbers of individuals whose primary diagnosis is OA, RA, lupus, ankylosing spondylitis, cancer pain, or sickle cell disease that have the same fundamental problem as fibromyalgia patients. But you do not see it because you label them as having an autoimmune disease or osteoarthritis. And that is at your peril and at their peril. Because treating that individual as if all of their pain and other symptoms are due to a problem out on the periphery will not make that person better.”
Patients with high levels of centralized pain may be less responsive to peripherally directed therapies such as surgery or injections, Dr. Clauw said. Pharmacologic options for patients with centralized pain include gabapentinoids (e.g., pregabalin and gabapentin), serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and milnacipran), and tricyclic compounds (e.g., amitriptyline and cyclobenzaprine), he said. “Opioids are going to be quite unlikely to help these individuals,” he said. “In fact, it is likely that opioids will make this kind of pain worse.”
Dr. Clauw is a consultant for Aptinyx, Daiichi Sankyo, Eli Lilly, Intec Pharma, Pfizer, Samumed, Theravance, Tonix, and Zynerba Pharma. He has received grant or research support from Aptinyx and Pfizer and is an expert witness.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Las Vegas – A fibromyalgia survey may provide important information about the degree to which patients with rheumatic disease experience centralized pain. This information may guide treatment decisions, said Daniel J. Clauw, MD, professor of anesthesiology, rheumatology, and psychiatry and director of the Chronic Pain and Fatigue Research Center at the University of Michigan in Ann Arbor.
The questionnaire that Dr. Clauw uses is a patient self-report survey for the assessment of fibromyalgia based on criteria in the 2011 modification of the American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. In it, he asks patients to report where they experience pain throughout the body and symptoms such as fatigue, sleep problems, and memory problems. The survey predicts outcomes of surgery for osteoarthritis better than x-rays, MRI scans, or psychological factors do, he said.
Physicians should ask every patient with chronic pain, including patients with OA, rheumatoid arthritis, or lupus, to complete the survey, Dr. Clauw said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “This score will tell you the degree to which their central nervous system is augmenting or amplifying what is going on in their body,” he said. “And the higher their score is, the more you should treat them like you would someone with fibromyalgia, even if their underlying disease might be an autoimmune disease.”
Physicians should not use a cutoff of 13 points on the fibromyalgia measure to define whether a patient has the disease, as has been done in the past, he said. The threshold is arbitrary, he said. “We should not think about fibromyalgia as ‘yes’ or ‘no.’ We should think of the degree of fibromyalgia that people have.”
A poor relationship between pain and imaging
Some patients who have severe knee OA on imaging walk without pain. Other patients have normal x-rays, but severe pain. “There is a terrible relationship between what you see on a knee x-ray or an MRI and whether someone has pain,” Dr. Clauw said. Furthermore, the poor relationship between imaging and pain is common across chronic pain conditions, he said.
This phenomenon may occur because pain manifests in different ways, similar to there being multiple ways to adjust the volume of an electric guitar, he said. How hard the strings are strummed affects the volume. But so does the amplifier setting. “In these centralized pain conditions, the problem is an amplifier problem, not a guitar problem,” he said. “The amplifier, i.e., the central nervous system, is set too high.”
Researchers have found that people who have severe OA of the knee on x-ray but do not experience pain “have a very low amplifier setting,” he said. That is, they are nontender and less sensitive to pain. Most of these patients are men. “On average, men have a much lower amplifier setting than women,” he said. “This is also why ... women have 1.5 to 2 times the rate of any type of chronic pain than men, because on average women have a higher amplifier setting. ... In OA, at any given age, men and women have the exact same percentage of radiographic OA. But if you look at the clinical condition of OA, it is always two-thirds women, one-third men.”
Opioid responsiveness
To examine whether fibromyalgia survey results correlate with outcomes after knee and hip arthroplasty, Dr. Clauw and colleagues conducted a prospective, observational cohort study that included approximately 500 people. Patients completed the questionnaire on the day of surgery.
Patients with higher levels of fibromyalgia were less responsive to opioids. “For each 1-point increase in the fibromyalgia score, people needed about one more hydrocodone tablet in the first 24-48 hours to control their pain,” he said (Anesthesiology. 2013 Dec;119[6]:1434-43). In addition, each 1-point increase in the fibromyalgia score made people about 25% less likely to have a 50% improvement in knee pain level after 6 months (Arthritis Rheumatol. 2015 May;67[5]:1386-94). The correlations were independent of psychological factors. In addition, the associations were linear. “There was nothing magical about a fibromyalgia score of 13,” Dr. Clauw said.
Dr. Clauw is a coauthor of a study to be presented at the 2019 American College of Rheumatology/Association of Rheumatology Professionals annual meeting that found pain centralization in patients with RA is associated with poor response to disease-modifying antirheumatic drugs (DMARDs).
Prior studies in patients with RA have found that the degree of fibromyalgia is a better predictor of pain and disability than erythrocyte sedimentation rate or the number of swollen joints.
Diagnosed cases are the “tip of the iceberg”
Researchers at Dr. Clauw’s institution have identified dozens of patients undergoing knee surgery who met criteria for fibromyalgia but had not received the diagnosis. “This is at the University of Michigan, which is the epicenter for fibromyalgia research. If we are not seeing fibromyalgia superimposed on OA in our patients, no one is seeing it,” he said.
Patients with diagnosed fibromyalgia are “the tip of the iceberg,” he said. “There are far greater numbers of individuals whose primary diagnosis is OA, RA, lupus, ankylosing spondylitis, cancer pain, or sickle cell disease that have the same fundamental problem as fibromyalgia patients. But you do not see it because you label them as having an autoimmune disease or osteoarthritis. And that is at your peril and at their peril. Because treating that individual as if all of their pain and other symptoms are due to a problem out on the periphery will not make that person better.”
Patients with high levels of centralized pain may be less responsive to peripherally directed therapies such as surgery or injections, Dr. Clauw said. Pharmacologic options for patients with centralized pain include gabapentinoids (e.g., pregabalin and gabapentin), serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and milnacipran), and tricyclic compounds (e.g., amitriptyline and cyclobenzaprine), he said. “Opioids are going to be quite unlikely to help these individuals,” he said. “In fact, it is likely that opioids will make this kind of pain worse.”
Dr. Clauw is a consultant for Aptinyx, Daiichi Sankyo, Eli Lilly, Intec Pharma, Pfizer, Samumed, Theravance, Tonix, and Zynerba Pharma. He has received grant or research support from Aptinyx and Pfizer and is an expert witness.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Las Vegas – A fibromyalgia survey may provide important information about the degree to which patients with rheumatic disease experience centralized pain. This information may guide treatment decisions, said Daniel J. Clauw, MD, professor of anesthesiology, rheumatology, and psychiatry and director of the Chronic Pain and Fatigue Research Center at the University of Michigan in Ann Arbor.
The questionnaire that Dr. Clauw uses is a patient self-report survey for the assessment of fibromyalgia based on criteria in the 2011 modification of the American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. In it, he asks patients to report where they experience pain throughout the body and symptoms such as fatigue, sleep problems, and memory problems. The survey predicts outcomes of surgery for osteoarthritis better than x-rays, MRI scans, or psychological factors do, he said.
Physicians should ask every patient with chronic pain, including patients with OA, rheumatoid arthritis, or lupus, to complete the survey, Dr. Clauw said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “This score will tell you the degree to which their central nervous system is augmenting or amplifying what is going on in their body,” he said. “And the higher their score is, the more you should treat them like you would someone with fibromyalgia, even if their underlying disease might be an autoimmune disease.”
Physicians should not use a cutoff of 13 points on the fibromyalgia measure to define whether a patient has the disease, as has been done in the past, he said. The threshold is arbitrary, he said. “We should not think about fibromyalgia as ‘yes’ or ‘no.’ We should think of the degree of fibromyalgia that people have.”
A poor relationship between pain and imaging
Some patients who have severe knee OA on imaging walk without pain. Other patients have normal x-rays, but severe pain. “There is a terrible relationship between what you see on a knee x-ray or an MRI and whether someone has pain,” Dr. Clauw said. Furthermore, the poor relationship between imaging and pain is common across chronic pain conditions, he said.
This phenomenon may occur because pain manifests in different ways, similar to there being multiple ways to adjust the volume of an electric guitar, he said. How hard the strings are strummed affects the volume. But so does the amplifier setting. “In these centralized pain conditions, the problem is an amplifier problem, not a guitar problem,” he said. “The amplifier, i.e., the central nervous system, is set too high.”
Researchers have found that people who have severe OA of the knee on x-ray but do not experience pain “have a very low amplifier setting,” he said. That is, they are nontender and less sensitive to pain. Most of these patients are men. “On average, men have a much lower amplifier setting than women,” he said. “This is also why ... women have 1.5 to 2 times the rate of any type of chronic pain than men, because on average women have a higher amplifier setting. ... In OA, at any given age, men and women have the exact same percentage of radiographic OA. But if you look at the clinical condition of OA, it is always two-thirds women, one-third men.”
Opioid responsiveness
To examine whether fibromyalgia survey results correlate with outcomes after knee and hip arthroplasty, Dr. Clauw and colleagues conducted a prospective, observational cohort study that included approximately 500 people. Patients completed the questionnaire on the day of surgery.
Patients with higher levels of fibromyalgia were less responsive to opioids. “For each 1-point increase in the fibromyalgia score, people needed about one more hydrocodone tablet in the first 24-48 hours to control their pain,” he said (Anesthesiology. 2013 Dec;119[6]:1434-43). In addition, each 1-point increase in the fibromyalgia score made people about 25% less likely to have a 50% improvement in knee pain level after 6 months (Arthritis Rheumatol. 2015 May;67[5]:1386-94). The correlations were independent of psychological factors. In addition, the associations were linear. “There was nothing magical about a fibromyalgia score of 13,” Dr. Clauw said.
Dr. Clauw is a coauthor of a study to be presented at the 2019 American College of Rheumatology/Association of Rheumatology Professionals annual meeting that found pain centralization in patients with RA is associated with poor response to disease-modifying antirheumatic drugs (DMARDs).
Prior studies in patients with RA have found that the degree of fibromyalgia is a better predictor of pain and disability than erythrocyte sedimentation rate or the number of swollen joints.
Diagnosed cases are the “tip of the iceberg”
Researchers at Dr. Clauw’s institution have identified dozens of patients undergoing knee surgery who met criteria for fibromyalgia but had not received the diagnosis. “This is at the University of Michigan, which is the epicenter for fibromyalgia research. If we are not seeing fibromyalgia superimposed on OA in our patients, no one is seeing it,” he said.
Patients with diagnosed fibromyalgia are “the tip of the iceberg,” he said. “There are far greater numbers of individuals whose primary diagnosis is OA, RA, lupus, ankylosing spondylitis, cancer pain, or sickle cell disease that have the same fundamental problem as fibromyalgia patients. But you do not see it because you label them as having an autoimmune disease or osteoarthritis. And that is at your peril and at their peril. Because treating that individual as if all of their pain and other symptoms are due to a problem out on the periphery will not make that person better.”
Patients with high levels of centralized pain may be less responsive to peripherally directed therapies such as surgery or injections, Dr. Clauw said. Pharmacologic options for patients with centralized pain include gabapentinoids (e.g., pregabalin and gabapentin), serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and milnacipran), and tricyclic compounds (e.g., amitriptyline and cyclobenzaprine), he said. “Opioids are going to be quite unlikely to help these individuals,” he said. “In fact, it is likely that opioids will make this kind of pain worse.”
Dr. Clauw is a consultant for Aptinyx, Daiichi Sankyo, Eli Lilly, Intec Pharma, Pfizer, Samumed, Theravance, Tonix, and Zynerba Pharma. He has received grant or research support from Aptinyx and Pfizer and is an expert witness.
Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PRD 2019
What is the optimal duration of maintenance in myeloma?
SAN FRANCISCO – Should patients with multiple myeloma receive maintenance therapy until progression?
Yvonne A. Efebera, MD, of The Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital in Columbus, and Nina Shah, MD, of the University of California San Francisco Health, faced off on this question at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Dr. Shah said maintenance therapy improves survival in myeloma patients, so it follows that treating them until progression would confer a survival advantage. While Dr. Efebera agreed that maintenance can improve survival, she said the optimal duration of that treatment is unknown.
Treat until progression
Dr. Shah cited studies suggesting that maintenance improves progression-free survival (PFS) and may prolong overall survival (OS) in multiple myeloma.
A meta-analysis of data from the IFM 2005-02, CALGB 100104, and GIMEMA RV-MM-PI-209 trials showed that lenalidomide maintenance prolonged PFS and OS. The median PFS was 52.8 months in patients who received maintenance and 23.5 months in those who received placebo or observation (hazard ratio [HR], 0.48). At a median follow-up of 79.5 months, the median OS was not reached for the maintenance group and was 86.0 months for the no-maintenance group (HR, 0.75; P = .001; J Clin Oncol. 2017 Oct 10;35[29]:3279-89).
In the Myeloma XI trial, maintenance improved PFS, but not OS, in both transplant-eligible and ineligible patients. Overall, the median PFS was 39 months in the lenalidomide maintenance arm and 20 months in the observation arm (P less than .0001). Among transplant-eligible patients, the median PFS was 57 months and 30 months, respectively (P less than .0001). Among transplant-ineligible patients, the median PFS was 26 months and 11 months, respectively (P less than .0001; Lancet Oncol. 2019 Jan;20[1]:57-73).
These data suggest maintenance can improve survival, “but the question is, how long should we have therapy,” Dr. Shah said. “No one has looked at this in a prospective manner, so we really have to look at our retrospective data.”
One study suggested a longer duration of lenalidomide maintenance improves PFS. The HR for progression or death was 0.39 for patients who received maintenance for 12-24 months, compared with those who received maintenance for less than 12 months. The HR was 0.13 for patients who received maintenance for more than 24 months, compared with less than 12 months (Leuk Lymphoma. 2019 Feb;60[2]:511-4).
Dr. Shah also cited a pooled analysis of three phase 3 trials suggesting that continuous therapy is superior to fixed-duration therapy in patients with newly diagnosed myeloma. The median PFS was 32 months with continuous therapy and 16 months with fixed-duration therapy (P less than .001). The 4-year OS was 69% and 60%, respectively (P = .003; J Clin Oncol. 2015 Oct 20;33[30]:3459-66).
These data suggest that “continuous therapy, more therapy, has a survival advantage,” Dr. Shah said.
Don’t treat until progression
Dr. Efebera also discussed data from studies showing that lenalidomide maintenance can prolong survival in multiple myeloma. However, she said, it’s unclear how long maintenance should last.
Different durations of maintenance have proved effective in different trials. In the CALGB 100104 trial, the median duration of maintenance was 31 months (Lancet Haematol. 2017 Sep;4[9]:e431-e442). In the meta-analysis of the CALGB, IFM, and GIMEMA trials, the median duration was 22 months. And in Myeloma XI, the median duration was 18 months.
As there is no randomized trial comparing different durations of maintenance, Dr. Efebera proposed that researchers conduct one. She said this “perfect study” would involve induction with an immunomodulatory agent, a proteasome inhibitor, dexamethasone, and perhaps an anti-CD38 therapy. Transplant-eligible patients would receive four cycles of induction before transplant. Transplant-ineligible patients would receive eight cycles of induction. Then, all patients would be randomized to lenalidomide maintenance for 3 years, 5 years, or 7-10 years.
Until a trial like this reveals the optimal duration of maintenance, we cannot conclude that treating patients until progression is better, Dr. Efebera said.
She added that maintenance has been shown to have detrimental effects, and these should be taken into consideration. For instance, neutropenia, other hematologic adverse events, and second primary malignancies have been shown to be more common among patients who receive lenalidomide maintenance (N Engl J Med. 2012; 366:1782-91).
The cost of maintenance is another factor to consider. Researchers analyzed data from the CALGB 100104 and IFM 2005-02 trials to compare the cost of lenalidomide maintenance with no maintenance. In the CALGB 100104 trial, patients who received lenalidomide maintenance had 5.72 quality-adjusted life years (QALYs), and those who received no maintenance had 4.61 QALYs. The incremental cost-utility ratio (ICUR) was more than 277,000 euros per QALY.
In the IFM2005-02 trial, patients in the lenalidomide group had 5.13 QALYs, and those who didn’t receive maintenance had 4.98 QALYs. The ICUR was more than 1.5 million euros per QALY. The researchers said the high ICURs and budgetary impact add “uncertainty about the maximum prudent duration of the treatment” (Bone Marrow Transplant. 2019 May 31. doi: 10.1038/s41409-019-0574-5).
Dr. Efebera reported relationships with Akcea Therapeutics, Janssen, and Takeda. Dr. Shah reported having no relevant financial relationships.
SAN FRANCISCO – Should patients with multiple myeloma receive maintenance therapy until progression?
Yvonne A. Efebera, MD, of The Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital in Columbus, and Nina Shah, MD, of the University of California San Francisco Health, faced off on this question at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Dr. Shah said maintenance therapy improves survival in myeloma patients, so it follows that treating them until progression would confer a survival advantage. While Dr. Efebera agreed that maintenance can improve survival, she said the optimal duration of that treatment is unknown.
Treat until progression
Dr. Shah cited studies suggesting that maintenance improves progression-free survival (PFS) and may prolong overall survival (OS) in multiple myeloma.
A meta-analysis of data from the IFM 2005-02, CALGB 100104, and GIMEMA RV-MM-PI-209 trials showed that lenalidomide maintenance prolonged PFS and OS. The median PFS was 52.8 months in patients who received maintenance and 23.5 months in those who received placebo or observation (hazard ratio [HR], 0.48). At a median follow-up of 79.5 months, the median OS was not reached for the maintenance group and was 86.0 months for the no-maintenance group (HR, 0.75; P = .001; J Clin Oncol. 2017 Oct 10;35[29]:3279-89).
In the Myeloma XI trial, maintenance improved PFS, but not OS, in both transplant-eligible and ineligible patients. Overall, the median PFS was 39 months in the lenalidomide maintenance arm and 20 months in the observation arm (P less than .0001). Among transplant-eligible patients, the median PFS was 57 months and 30 months, respectively (P less than .0001). Among transplant-ineligible patients, the median PFS was 26 months and 11 months, respectively (P less than .0001; Lancet Oncol. 2019 Jan;20[1]:57-73).
These data suggest maintenance can improve survival, “but the question is, how long should we have therapy,” Dr. Shah said. “No one has looked at this in a prospective manner, so we really have to look at our retrospective data.”
One study suggested a longer duration of lenalidomide maintenance improves PFS. The HR for progression or death was 0.39 for patients who received maintenance for 12-24 months, compared with those who received maintenance for less than 12 months. The HR was 0.13 for patients who received maintenance for more than 24 months, compared with less than 12 months (Leuk Lymphoma. 2019 Feb;60[2]:511-4).
Dr. Shah also cited a pooled analysis of three phase 3 trials suggesting that continuous therapy is superior to fixed-duration therapy in patients with newly diagnosed myeloma. The median PFS was 32 months with continuous therapy and 16 months with fixed-duration therapy (P less than .001). The 4-year OS was 69% and 60%, respectively (P = .003; J Clin Oncol. 2015 Oct 20;33[30]:3459-66).
These data suggest that “continuous therapy, more therapy, has a survival advantage,” Dr. Shah said.
Don’t treat until progression
Dr. Efebera also discussed data from studies showing that lenalidomide maintenance can prolong survival in multiple myeloma. However, she said, it’s unclear how long maintenance should last.
Different durations of maintenance have proved effective in different trials. In the CALGB 100104 trial, the median duration of maintenance was 31 months (Lancet Haematol. 2017 Sep;4[9]:e431-e442). In the meta-analysis of the CALGB, IFM, and GIMEMA trials, the median duration was 22 months. And in Myeloma XI, the median duration was 18 months.
As there is no randomized trial comparing different durations of maintenance, Dr. Efebera proposed that researchers conduct one. She said this “perfect study” would involve induction with an immunomodulatory agent, a proteasome inhibitor, dexamethasone, and perhaps an anti-CD38 therapy. Transplant-eligible patients would receive four cycles of induction before transplant. Transplant-ineligible patients would receive eight cycles of induction. Then, all patients would be randomized to lenalidomide maintenance for 3 years, 5 years, or 7-10 years.
Until a trial like this reveals the optimal duration of maintenance, we cannot conclude that treating patients until progression is better, Dr. Efebera said.
She added that maintenance has been shown to have detrimental effects, and these should be taken into consideration. For instance, neutropenia, other hematologic adverse events, and second primary malignancies have been shown to be more common among patients who receive lenalidomide maintenance (N Engl J Med. 2012; 366:1782-91).
The cost of maintenance is another factor to consider. Researchers analyzed data from the CALGB 100104 and IFM 2005-02 trials to compare the cost of lenalidomide maintenance with no maintenance. In the CALGB 100104 trial, patients who received lenalidomide maintenance had 5.72 quality-adjusted life years (QALYs), and those who received no maintenance had 4.61 QALYs. The incremental cost-utility ratio (ICUR) was more than 277,000 euros per QALY.
In the IFM2005-02 trial, patients in the lenalidomide group had 5.13 QALYs, and those who didn’t receive maintenance had 4.98 QALYs. The ICUR was more than 1.5 million euros per QALY. The researchers said the high ICURs and budgetary impact add “uncertainty about the maximum prudent duration of the treatment” (Bone Marrow Transplant. 2019 May 31. doi: 10.1038/s41409-019-0574-5).
Dr. Efebera reported relationships with Akcea Therapeutics, Janssen, and Takeda. Dr. Shah reported having no relevant financial relationships.
SAN FRANCISCO – Should patients with multiple myeloma receive maintenance therapy until progression?
Yvonne A. Efebera, MD, of The Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital in Columbus, and Nina Shah, MD, of the University of California San Francisco Health, faced off on this question at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Dr. Shah said maintenance therapy improves survival in myeloma patients, so it follows that treating them until progression would confer a survival advantage. While Dr. Efebera agreed that maintenance can improve survival, she said the optimal duration of that treatment is unknown.
Treat until progression
Dr. Shah cited studies suggesting that maintenance improves progression-free survival (PFS) and may prolong overall survival (OS) in multiple myeloma.
A meta-analysis of data from the IFM 2005-02, CALGB 100104, and GIMEMA RV-MM-PI-209 trials showed that lenalidomide maintenance prolonged PFS and OS. The median PFS was 52.8 months in patients who received maintenance and 23.5 months in those who received placebo or observation (hazard ratio [HR], 0.48). At a median follow-up of 79.5 months, the median OS was not reached for the maintenance group and was 86.0 months for the no-maintenance group (HR, 0.75; P = .001; J Clin Oncol. 2017 Oct 10;35[29]:3279-89).
In the Myeloma XI trial, maintenance improved PFS, but not OS, in both transplant-eligible and ineligible patients. Overall, the median PFS was 39 months in the lenalidomide maintenance arm and 20 months in the observation arm (P less than .0001). Among transplant-eligible patients, the median PFS was 57 months and 30 months, respectively (P less than .0001). Among transplant-ineligible patients, the median PFS was 26 months and 11 months, respectively (P less than .0001; Lancet Oncol. 2019 Jan;20[1]:57-73).
These data suggest maintenance can improve survival, “but the question is, how long should we have therapy,” Dr. Shah said. “No one has looked at this in a prospective manner, so we really have to look at our retrospective data.”
One study suggested a longer duration of lenalidomide maintenance improves PFS. The HR for progression or death was 0.39 for patients who received maintenance for 12-24 months, compared with those who received maintenance for less than 12 months. The HR was 0.13 for patients who received maintenance for more than 24 months, compared with less than 12 months (Leuk Lymphoma. 2019 Feb;60[2]:511-4).
Dr. Shah also cited a pooled analysis of three phase 3 trials suggesting that continuous therapy is superior to fixed-duration therapy in patients with newly diagnosed myeloma. The median PFS was 32 months with continuous therapy and 16 months with fixed-duration therapy (P less than .001). The 4-year OS was 69% and 60%, respectively (P = .003; J Clin Oncol. 2015 Oct 20;33[30]:3459-66).
These data suggest that “continuous therapy, more therapy, has a survival advantage,” Dr. Shah said.
Don’t treat until progression
Dr. Efebera also discussed data from studies showing that lenalidomide maintenance can prolong survival in multiple myeloma. However, she said, it’s unclear how long maintenance should last.
Different durations of maintenance have proved effective in different trials. In the CALGB 100104 trial, the median duration of maintenance was 31 months (Lancet Haematol. 2017 Sep;4[9]:e431-e442). In the meta-analysis of the CALGB, IFM, and GIMEMA trials, the median duration was 22 months. And in Myeloma XI, the median duration was 18 months.
As there is no randomized trial comparing different durations of maintenance, Dr. Efebera proposed that researchers conduct one. She said this “perfect study” would involve induction with an immunomodulatory agent, a proteasome inhibitor, dexamethasone, and perhaps an anti-CD38 therapy. Transplant-eligible patients would receive four cycles of induction before transplant. Transplant-ineligible patients would receive eight cycles of induction. Then, all patients would be randomized to lenalidomide maintenance for 3 years, 5 years, or 7-10 years.
Until a trial like this reveals the optimal duration of maintenance, we cannot conclude that treating patients until progression is better, Dr. Efebera said.
She added that maintenance has been shown to have detrimental effects, and these should be taken into consideration. For instance, neutropenia, other hematologic adverse events, and second primary malignancies have been shown to be more common among patients who receive lenalidomide maintenance (N Engl J Med. 2012; 366:1782-91).
The cost of maintenance is another factor to consider. Researchers analyzed data from the CALGB 100104 and IFM 2005-02 trials to compare the cost of lenalidomide maintenance with no maintenance. In the CALGB 100104 trial, patients who received lenalidomide maintenance had 5.72 quality-adjusted life years (QALYs), and those who received no maintenance had 4.61 QALYs. The incremental cost-utility ratio (ICUR) was more than 277,000 euros per QALY.
In the IFM2005-02 trial, patients in the lenalidomide group had 5.13 QALYs, and those who didn’t receive maintenance had 4.98 QALYs. The ICUR was more than 1.5 million euros per QALY. The researchers said the high ICURs and budgetary impact add “uncertainty about the maximum prudent duration of the treatment” (Bone Marrow Transplant. 2019 May 31. doi: 10.1038/s41409-019-0574-5).
Dr. Efebera reported relationships with Akcea Therapeutics, Janssen, and Takeda. Dr. Shah reported having no relevant financial relationships.
EXPERT ANALYSIS FROM NCCN HEMATOLOGIC MALIGNANCIES
Atezolizumab plus chemo gives slight PFS edge in mUC
BARCELONA – Adding the immune checkpoint inhibitor atezolizumab (Tecentriq) to standard platinum-based chemotherapy was associated with a small but statistically significant progression-free survival benefit for patients with previously untreated metastatic urothelial carcinoma, investigators in the IMvigor130 trial found.
Among 1,213 patients with newly diagnosed metastatic urothelial carcinoma assigned to receive either atezolizumab monotherapy or chemotherapy with a platinum compound and gemcitabine plus either atezolizumab or placebo, the median progression-free survival (PFS) at a median follow-up of 11.8 months was 8.2 months with atezolizumab/chemotherapy, compared with 6.3 months with chemotherapy plus placebo, reported Enrique Grande, MD, of MD Anderson Cancer Center Madrid.
“IMvigor130 is the first immune checkpoint inhibitor study to demonstrate an improvement in progression-free survival over the standard of care in first-line metastatic urothelial carcinoma. At this interim analysis, we observed a clinically meaningful improvement in the overall survival for the combination of atezolizumab plus chemotherapy that did not meet the prespecified interim boundary for significance,” he said at the European Society for Medical Oncology Congress.
Median overall survival (OS) at the interim analysis was 16.0 months in the atezolizumab arm, vs. 13.4 months in the placebo arm, translating into a hazard ratio (HR) of 0.83 trending in favor of the combination. But as noted by Dr. Grande, the P value was .027, which did not reach the interim efficacy boundary of .007.
IMvigor130 investigators enrolled patients with locally advanced metastatic urothelial carcinoma with no prior systemic therapy in the metastatic setting who had good performance status (ECOG 2 or less) and were eligible for chemotherapy with either cisplatin or carboplatin plus gemcitabine.
The patients were stratified by programmed death ligand-1 (PD-L1) status, prognostic (Bajorin) risk factor scores, and investigator choice of cisplatin or carboplatin, and then randomized to either atezolizumab plus chemotherapy, atezolizumab monotherapy, or placebo plus chemotherapy.
As noted, the co-primary endpoint of PFS in the chemotherapy arms in the intention-to-treat population was statistically significant at the preplanned interim analysis, but the other primary endpoint of OS had not reached significance.
At the time of the data cutoff in May 2019, the stratified HR for progression with atezolizumab was 0.82 (P = .007).
An analysis by subgroup showed either significant benefit or a trend favoring atezolizumab across all stratification factors, Dr. Grande said.
A hierarchical analysis of atezolizumab monotherapy vs. chemotherapy in the placebo-control arm showed a median OS of 15.7 vs. 13.1 months, respectively, with a hazard ratio of 1.02 (nonsignificant).
The benefit of atezolizumab appeared to be almost entirely among patients whose tumors had higher levels of PD-L1 expression according to immunohistochemistry (IC). The interim OS among patients with PD-L1 IC0/1 was a median of 13.5 months with atezolizumab vs. 12.9 months with chemotherapy alone, with an unstratified HR of 1.07 (nonsignificant). In contrast, among patients with PD-L1 IC2/3 status, the median OS was not reached for patients in the atezolizumab arm, vs. 17.8 months in the chemotherapy alone arm, for a stratified HR of 0.68, although this too did not reach statistical significance.
Responses were similar between the two chemotherapy arms, with an overall response rate (ORR) of 47% with atezolizumab added, vs. 44% with placebo added. The complete response (CR) rates were 13% and 7%, respectively. The ORR in the monotherapy arm was 23%, consisting of 6% complete and 17% partial responses.
Grade 3 or 4 treatment-related adverse events occurred in 81% of patients in each chemotherapy arm, compared with 15% in the atezolizumab monotherapy arm. Nine patients in the atezolizumab/chemotherapy arm died from a treatment-related cause, compared with four in the chemotherapy alone arm, and three in the atezolizumab monotherapy arm.
Adverse events leading to treatment discontinuation occurred in one-fourth of patients in each chemotherapy-containing arm, vs. less than 1% of patients in the monotherapy arm.
“The results from the IMvigor130 trial support the use of atezolizumab in combination with chemotherapy as an important new treatment option for patients with untreated metastatic urothelial carcinoma,” Dr. Grande concluded.
But invited discussant Thomas Powles, MD, of Barts Cancer Institute in London, cautioned that more data are needed to conclude that the addition of atezolizumab should become a standard of care.
“Does this change practice? Well, the for and against: significant delay in PFS, but how meaningful is that? OS trending the right way, but not significant yet. CR rates, yes with 13% CRs, but response rates weren’t very different from one another, and as response rates are similar, it’s hard to argue that the two are synergistic together, for example,” he said.
He added that the adverse event profiles “actually are very acceptable to me, and I’m really looking forward to the quality-of-life data.”
The IMvigor130 study is sponsored by F. Hoffman-La Roche. Dr. Grande disclosed honoraria and research grants from Roche and others. Dr. Powles disclosed research funding, honoraria, and travel costs from Roche and others.
SOURCE: Grande E et al. ESMO 2019. Abstract LBA14_PR.
BARCELONA – Adding the immune checkpoint inhibitor atezolizumab (Tecentriq) to standard platinum-based chemotherapy was associated with a small but statistically significant progression-free survival benefit for patients with previously untreated metastatic urothelial carcinoma, investigators in the IMvigor130 trial found.
Among 1,213 patients with newly diagnosed metastatic urothelial carcinoma assigned to receive either atezolizumab monotherapy or chemotherapy with a platinum compound and gemcitabine plus either atezolizumab or placebo, the median progression-free survival (PFS) at a median follow-up of 11.8 months was 8.2 months with atezolizumab/chemotherapy, compared with 6.3 months with chemotherapy plus placebo, reported Enrique Grande, MD, of MD Anderson Cancer Center Madrid.
“IMvigor130 is the first immune checkpoint inhibitor study to demonstrate an improvement in progression-free survival over the standard of care in first-line metastatic urothelial carcinoma. At this interim analysis, we observed a clinically meaningful improvement in the overall survival for the combination of atezolizumab plus chemotherapy that did not meet the prespecified interim boundary for significance,” he said at the European Society for Medical Oncology Congress.
Median overall survival (OS) at the interim analysis was 16.0 months in the atezolizumab arm, vs. 13.4 months in the placebo arm, translating into a hazard ratio (HR) of 0.83 trending in favor of the combination. But as noted by Dr. Grande, the P value was .027, which did not reach the interim efficacy boundary of .007.
IMvigor130 investigators enrolled patients with locally advanced metastatic urothelial carcinoma with no prior systemic therapy in the metastatic setting who had good performance status (ECOG 2 or less) and were eligible for chemotherapy with either cisplatin or carboplatin plus gemcitabine.
The patients were stratified by programmed death ligand-1 (PD-L1) status, prognostic (Bajorin) risk factor scores, and investigator choice of cisplatin or carboplatin, and then randomized to either atezolizumab plus chemotherapy, atezolizumab monotherapy, or placebo plus chemotherapy.
As noted, the co-primary endpoint of PFS in the chemotherapy arms in the intention-to-treat population was statistically significant at the preplanned interim analysis, but the other primary endpoint of OS had not reached significance.
At the time of the data cutoff in May 2019, the stratified HR for progression with atezolizumab was 0.82 (P = .007).
An analysis by subgroup showed either significant benefit or a trend favoring atezolizumab across all stratification factors, Dr. Grande said.
A hierarchical analysis of atezolizumab monotherapy vs. chemotherapy in the placebo-control arm showed a median OS of 15.7 vs. 13.1 months, respectively, with a hazard ratio of 1.02 (nonsignificant).
The benefit of atezolizumab appeared to be almost entirely among patients whose tumors had higher levels of PD-L1 expression according to immunohistochemistry (IC). The interim OS among patients with PD-L1 IC0/1 was a median of 13.5 months with atezolizumab vs. 12.9 months with chemotherapy alone, with an unstratified HR of 1.07 (nonsignificant). In contrast, among patients with PD-L1 IC2/3 status, the median OS was not reached for patients in the atezolizumab arm, vs. 17.8 months in the chemotherapy alone arm, for a stratified HR of 0.68, although this too did not reach statistical significance.
Responses were similar between the two chemotherapy arms, with an overall response rate (ORR) of 47% with atezolizumab added, vs. 44% with placebo added. The complete response (CR) rates were 13% and 7%, respectively. The ORR in the monotherapy arm was 23%, consisting of 6% complete and 17% partial responses.
Grade 3 or 4 treatment-related adverse events occurred in 81% of patients in each chemotherapy arm, compared with 15% in the atezolizumab monotherapy arm. Nine patients in the atezolizumab/chemotherapy arm died from a treatment-related cause, compared with four in the chemotherapy alone arm, and three in the atezolizumab monotherapy arm.
Adverse events leading to treatment discontinuation occurred in one-fourth of patients in each chemotherapy-containing arm, vs. less than 1% of patients in the monotherapy arm.
“The results from the IMvigor130 trial support the use of atezolizumab in combination with chemotherapy as an important new treatment option for patients with untreated metastatic urothelial carcinoma,” Dr. Grande concluded.
But invited discussant Thomas Powles, MD, of Barts Cancer Institute in London, cautioned that more data are needed to conclude that the addition of atezolizumab should become a standard of care.
“Does this change practice? Well, the for and against: significant delay in PFS, but how meaningful is that? OS trending the right way, but not significant yet. CR rates, yes with 13% CRs, but response rates weren’t very different from one another, and as response rates are similar, it’s hard to argue that the two are synergistic together, for example,” he said.
He added that the adverse event profiles “actually are very acceptable to me, and I’m really looking forward to the quality-of-life data.”
The IMvigor130 study is sponsored by F. Hoffman-La Roche. Dr. Grande disclosed honoraria and research grants from Roche and others. Dr. Powles disclosed research funding, honoraria, and travel costs from Roche and others.
SOURCE: Grande E et al. ESMO 2019. Abstract LBA14_PR.
BARCELONA – Adding the immune checkpoint inhibitor atezolizumab (Tecentriq) to standard platinum-based chemotherapy was associated with a small but statistically significant progression-free survival benefit for patients with previously untreated metastatic urothelial carcinoma, investigators in the IMvigor130 trial found.
Among 1,213 patients with newly diagnosed metastatic urothelial carcinoma assigned to receive either atezolizumab monotherapy or chemotherapy with a platinum compound and gemcitabine plus either atezolizumab or placebo, the median progression-free survival (PFS) at a median follow-up of 11.8 months was 8.2 months with atezolizumab/chemotherapy, compared with 6.3 months with chemotherapy plus placebo, reported Enrique Grande, MD, of MD Anderson Cancer Center Madrid.
“IMvigor130 is the first immune checkpoint inhibitor study to demonstrate an improvement in progression-free survival over the standard of care in first-line metastatic urothelial carcinoma. At this interim analysis, we observed a clinically meaningful improvement in the overall survival for the combination of atezolizumab plus chemotherapy that did not meet the prespecified interim boundary for significance,” he said at the European Society for Medical Oncology Congress.
Median overall survival (OS) at the interim analysis was 16.0 months in the atezolizumab arm, vs. 13.4 months in the placebo arm, translating into a hazard ratio (HR) of 0.83 trending in favor of the combination. But as noted by Dr. Grande, the P value was .027, which did not reach the interim efficacy boundary of .007.
IMvigor130 investigators enrolled patients with locally advanced metastatic urothelial carcinoma with no prior systemic therapy in the metastatic setting who had good performance status (ECOG 2 or less) and were eligible for chemotherapy with either cisplatin or carboplatin plus gemcitabine.
The patients were stratified by programmed death ligand-1 (PD-L1) status, prognostic (Bajorin) risk factor scores, and investigator choice of cisplatin or carboplatin, and then randomized to either atezolizumab plus chemotherapy, atezolizumab monotherapy, or placebo plus chemotherapy.
As noted, the co-primary endpoint of PFS in the chemotherapy arms in the intention-to-treat population was statistically significant at the preplanned interim analysis, but the other primary endpoint of OS had not reached significance.
At the time of the data cutoff in May 2019, the stratified HR for progression with atezolizumab was 0.82 (P = .007).
An analysis by subgroup showed either significant benefit or a trend favoring atezolizumab across all stratification factors, Dr. Grande said.
A hierarchical analysis of atezolizumab monotherapy vs. chemotherapy in the placebo-control arm showed a median OS of 15.7 vs. 13.1 months, respectively, with a hazard ratio of 1.02 (nonsignificant).
The benefit of atezolizumab appeared to be almost entirely among patients whose tumors had higher levels of PD-L1 expression according to immunohistochemistry (IC). The interim OS among patients with PD-L1 IC0/1 was a median of 13.5 months with atezolizumab vs. 12.9 months with chemotherapy alone, with an unstratified HR of 1.07 (nonsignificant). In contrast, among patients with PD-L1 IC2/3 status, the median OS was not reached for patients in the atezolizumab arm, vs. 17.8 months in the chemotherapy alone arm, for a stratified HR of 0.68, although this too did not reach statistical significance.
Responses were similar between the two chemotherapy arms, with an overall response rate (ORR) of 47% with atezolizumab added, vs. 44% with placebo added. The complete response (CR) rates were 13% and 7%, respectively. The ORR in the monotherapy arm was 23%, consisting of 6% complete and 17% partial responses.
Grade 3 or 4 treatment-related adverse events occurred in 81% of patients in each chemotherapy arm, compared with 15% in the atezolizumab monotherapy arm. Nine patients in the atezolizumab/chemotherapy arm died from a treatment-related cause, compared with four in the chemotherapy alone arm, and three in the atezolizumab monotherapy arm.
Adverse events leading to treatment discontinuation occurred in one-fourth of patients in each chemotherapy-containing arm, vs. less than 1% of patients in the monotherapy arm.
“The results from the IMvigor130 trial support the use of atezolizumab in combination with chemotherapy as an important new treatment option for patients with untreated metastatic urothelial carcinoma,” Dr. Grande concluded.
But invited discussant Thomas Powles, MD, of Barts Cancer Institute in London, cautioned that more data are needed to conclude that the addition of atezolizumab should become a standard of care.
“Does this change practice? Well, the for and against: significant delay in PFS, but how meaningful is that? OS trending the right way, but not significant yet. CR rates, yes with 13% CRs, but response rates weren’t very different from one another, and as response rates are similar, it’s hard to argue that the two are synergistic together, for example,” he said.
He added that the adverse event profiles “actually are very acceptable to me, and I’m really looking forward to the quality-of-life data.”
The IMvigor130 study is sponsored by F. Hoffman-La Roche. Dr. Grande disclosed honoraria and research grants from Roche and others. Dr. Powles disclosed research funding, honoraria, and travel costs from Roche and others.
SOURCE: Grande E et al. ESMO 2019. Abstract LBA14_PR.
REPORTING FROM ESMO 2019
A Better Approach to the Diagnosis of PE
Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.

Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).

Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
PRIMA study: Niraparib maintenance improves PFS in advanced OC
BARCELONA – Niraparib significantly improves progression-free survival when given after first-line chemotherapy in patients with advanced ovarian cancer, according to “potentially practice-changing” results from the phase 3 PRIMA/ENGOT-OV26/GOG-3012 study.
Overall progression-free survival (PFS) in 484 patients randomized to receive the poly-ADP ribose polymerase inhibitor (PARPi) niraparib was 13.8 months, compared with 8.2 months in 244 patients who received placebo (hazard ratio, 0.62), Antonio González-Martin, MD, PhD, reported at the European Society for Medical Oncology Congress.
The findings were published simultaneously online in the New England Journal of Medicine (N Engl J Med. 2019 Sep 28. doi: 10.1056/NEJMoa1910962).
In patients at high risk for progression based on homologous recombination deficiency (HRd) – defined by certain tumor factors or the presence of BRCA mutation (BRCAm), PFS was 21.9 vs. 10.4 months in the treatment (n = 245) vs. placebo (n = 125) groups, respectively (HR, 0.43), said Dr. González-Martin of Grupo Español de Investigación en Cáncer de Ovario (GEICO), medical oncology department, Clínica Universidad de Navarra, Madrid.
“At 18 months, which means approximately 2 years after the initiation of chemotherapy, 42% of patients treated with niraparib remained alive and progression free,” he said, adding that 59% of the HRd patients remained alive and progression free at 18 months.
Exploratory analyses showed that the niraparib benefits occurred across all prespecified patient subgroups, including those aged 65 and older vs. those under age 65, those with stage III vs. stage IV disease at diagnosis, those receiving vs. not receiving neoadjuvant chemotherapy, those with complete response (CR) vs. partial response (PR) as their best response to platinum chemotherapy, and those with HRd who had BRCAm vs. BRCA wild type (BRCAwt) tumors, he said.
The hazard ratios for the HRd BRCAm vs. BRCAwt tumors were 0.40 and 0.50, respectively.
“So the benefit of niraparib in the HRd tumor is not driven only by the BRCA-mutated patients,” he said. “Importantly, we also saw benefit in the group of patients with tumors that were [homologous recombination] proficient (HRp), with a reduction in the risk of progression of 32%.”
For the key secondary endpoint of overall survival, a preplanned interim analysis showed that 84% vs. 77% in the niraparib and placebo groups, respectively, were alive at 2 years; in the HRd and HRp groups, those rates were 91% vs. 85% and 81% vs. 59%, respectively.
Participants in the double-blind trial had newly diagnosed, advanced high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube cancer; their mean age was 62 years; and they had experienced a CR (69%) or PR (31%) to first-line platinum-based chemotherapy. Overall, 35% had stage IV disease and 67% received neoadjuvant chemotherapy. They were randomized 2:1 to once-daily niraparib at a starting dose of 300 mg or 200 mg depending on body weight and platelet count, with those weighing 77 kg or greater and with platelet count of 150,000/mcL or less starting at the higher dose, and those weighing less than 77 kg and/or with platelet count less than 150,000/mcL starting at the lower dose.
All subgroups showed a sustained and durable treatment effect, and although most patients experienced treatment-related adverse events (TRAEs), those were “manageable with dose interruption or dose reduction,” Dr. González-Martin said.
Discontinuations due to TRAEs occurred in 12% vs. 2.5% in the treatment vs. placebo groups, and this was consistent with prior niraparib experience, he said, adding that no niraparib-related deaths were reported and no new safety signals were identified.
The findings are notable, because the recurrence rate after standard first-line platinum-based chemotherapy in women with advanced ovarian cancer is estimated at up to 85%, and while certain subgroups of patients have options for maintenance therapy, there remains a high unmet need for others, he explained.
For example olaparib is an option, but only for tumors with BRCA mutation, and bevacizumab can be used, but “may be limited due to safety concerns in some patients and also due to limited data from randomized trials in the neoadjuvant setting,” he said.
As a result, surveillance after chemotherapy is the approach used for many patients, he added.
Niraparib is the first oral PARPi approved for maintenance in patients with recurrent ovarian cancer, regardless of BRCA mutation status; in the NOVA study, it demonstrated efficacy after platinum chemotherapy in all biomarker populations, and in the QUADRA study it showed benefit in patients who received at least three prior therapies.
The current study was designed to test the efficacy and safety of niraparib therapy after response to platinum-based chemotherapy in patients with newly diagnosed advanced ovarian cancer, including those at high risk of relapse.
“Niraparib is the first PARP inhibitor that has demonstrated benefit after front-line platinum-based chemotherapy across all the biomarker subgroups, regardless of BRCA status, consistent with data from the recurrent setting,” Dr. González-Martin said, adding that patients with ovarian cancer at the highest risk of early disease progression obtained significant benefit. “What does this mean for our patients and our practice? Based on these results, niraparib after first-line platinum chemotherapy should be considered a new standard of care.”
Invited discussant Ana Oaknin, MD, PhD, head of the gynecologic cancer program at Vall d’Hebron Institute of Oncology, Vall d’Hebron University Hospital, Barcelona, called the findings “striking” and noted that they, along with those from the PAOLA-1/ENGOT-Ov25 trial demonstrating a PFS benefit with the addition of olaparib to bevacizumab maintenance therapy after first-line platinum-based chemotherapy in advanced ovarian cancer, represent important advances.
“We are witnessing a paradigm shift in the first-line treatment of advanced ovarian cancer patients,” she said.
Are the findings of these trials clinically meaningful enough to justify the addition of PARPi maintenance therapy after first-line chemotherapy therapy as a new standard of care?
“Yes, but while the benefit is clinically meaningful in the overall population, we should consider PFS outcomes according to the biomarker status in the selection of optimal therapy; companion diagnostic tests will be needed,” she said.
The PRIMA/ENGOT-OV26/GOG-3012 study was sponsored by TESARO. Dr. González-Martin reported relationships with numerous pharmaceutical companies.
SOURCE: González-Martin A et al. ESMO 2019: Abstract LBA1.
BARCELONA – Niraparib significantly improves progression-free survival when given after first-line chemotherapy in patients with advanced ovarian cancer, according to “potentially practice-changing” results from the phase 3 PRIMA/ENGOT-OV26/GOG-3012 study.
Overall progression-free survival (PFS) in 484 patients randomized to receive the poly-ADP ribose polymerase inhibitor (PARPi) niraparib was 13.8 months, compared with 8.2 months in 244 patients who received placebo (hazard ratio, 0.62), Antonio González-Martin, MD, PhD, reported at the European Society for Medical Oncology Congress.
The findings were published simultaneously online in the New England Journal of Medicine (N Engl J Med. 2019 Sep 28. doi: 10.1056/NEJMoa1910962).
In patients at high risk for progression based on homologous recombination deficiency (HRd) – defined by certain tumor factors or the presence of BRCA mutation (BRCAm), PFS was 21.9 vs. 10.4 months in the treatment (n = 245) vs. placebo (n = 125) groups, respectively (HR, 0.43), said Dr. González-Martin of Grupo Español de Investigación en Cáncer de Ovario (GEICO), medical oncology department, Clínica Universidad de Navarra, Madrid.
“At 18 months, which means approximately 2 years after the initiation of chemotherapy, 42% of patients treated with niraparib remained alive and progression free,” he said, adding that 59% of the HRd patients remained alive and progression free at 18 months.
Exploratory analyses showed that the niraparib benefits occurred across all prespecified patient subgroups, including those aged 65 and older vs. those under age 65, those with stage III vs. stage IV disease at diagnosis, those receiving vs. not receiving neoadjuvant chemotherapy, those with complete response (CR) vs. partial response (PR) as their best response to platinum chemotherapy, and those with HRd who had BRCAm vs. BRCA wild type (BRCAwt) tumors, he said.
The hazard ratios for the HRd BRCAm vs. BRCAwt tumors were 0.40 and 0.50, respectively.
“So the benefit of niraparib in the HRd tumor is not driven only by the BRCA-mutated patients,” he said. “Importantly, we also saw benefit in the group of patients with tumors that were [homologous recombination] proficient (HRp), with a reduction in the risk of progression of 32%.”
For the key secondary endpoint of overall survival, a preplanned interim analysis showed that 84% vs. 77% in the niraparib and placebo groups, respectively, were alive at 2 years; in the HRd and HRp groups, those rates were 91% vs. 85% and 81% vs. 59%, respectively.
Participants in the double-blind trial had newly diagnosed, advanced high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube cancer; their mean age was 62 years; and they had experienced a CR (69%) or PR (31%) to first-line platinum-based chemotherapy. Overall, 35% had stage IV disease and 67% received neoadjuvant chemotherapy. They were randomized 2:1 to once-daily niraparib at a starting dose of 300 mg or 200 mg depending on body weight and platelet count, with those weighing 77 kg or greater and with platelet count of 150,000/mcL or less starting at the higher dose, and those weighing less than 77 kg and/or with platelet count less than 150,000/mcL starting at the lower dose.
All subgroups showed a sustained and durable treatment effect, and although most patients experienced treatment-related adverse events (TRAEs), those were “manageable with dose interruption or dose reduction,” Dr. González-Martin said.
Discontinuations due to TRAEs occurred in 12% vs. 2.5% in the treatment vs. placebo groups, and this was consistent with prior niraparib experience, he said, adding that no niraparib-related deaths were reported and no new safety signals were identified.
The findings are notable, because the recurrence rate after standard first-line platinum-based chemotherapy in women with advanced ovarian cancer is estimated at up to 85%, and while certain subgroups of patients have options for maintenance therapy, there remains a high unmet need for others, he explained.
For example olaparib is an option, but only for tumors with BRCA mutation, and bevacizumab can be used, but “may be limited due to safety concerns in some patients and also due to limited data from randomized trials in the neoadjuvant setting,” he said.
As a result, surveillance after chemotherapy is the approach used for many patients, he added.
Niraparib is the first oral PARPi approved for maintenance in patients with recurrent ovarian cancer, regardless of BRCA mutation status; in the NOVA study, it demonstrated efficacy after platinum chemotherapy in all biomarker populations, and in the QUADRA study it showed benefit in patients who received at least three prior therapies.
The current study was designed to test the efficacy and safety of niraparib therapy after response to platinum-based chemotherapy in patients with newly diagnosed advanced ovarian cancer, including those at high risk of relapse.
“Niraparib is the first PARP inhibitor that has demonstrated benefit after front-line platinum-based chemotherapy across all the biomarker subgroups, regardless of BRCA status, consistent with data from the recurrent setting,” Dr. González-Martin said, adding that patients with ovarian cancer at the highest risk of early disease progression obtained significant benefit. “What does this mean for our patients and our practice? Based on these results, niraparib after first-line platinum chemotherapy should be considered a new standard of care.”
Invited discussant Ana Oaknin, MD, PhD, head of the gynecologic cancer program at Vall d’Hebron Institute of Oncology, Vall d’Hebron University Hospital, Barcelona, called the findings “striking” and noted that they, along with those from the PAOLA-1/ENGOT-Ov25 trial demonstrating a PFS benefit with the addition of olaparib to bevacizumab maintenance therapy after first-line platinum-based chemotherapy in advanced ovarian cancer, represent important advances.
“We are witnessing a paradigm shift in the first-line treatment of advanced ovarian cancer patients,” she said.
Are the findings of these trials clinically meaningful enough to justify the addition of PARPi maintenance therapy after first-line chemotherapy therapy as a new standard of care?
“Yes, but while the benefit is clinically meaningful in the overall population, we should consider PFS outcomes according to the biomarker status in the selection of optimal therapy; companion diagnostic tests will be needed,” she said.
The PRIMA/ENGOT-OV26/GOG-3012 study was sponsored by TESARO. Dr. González-Martin reported relationships with numerous pharmaceutical companies.
SOURCE: González-Martin A et al. ESMO 2019: Abstract LBA1.
BARCELONA – Niraparib significantly improves progression-free survival when given after first-line chemotherapy in patients with advanced ovarian cancer, according to “potentially practice-changing” results from the phase 3 PRIMA/ENGOT-OV26/GOG-3012 study.
Overall progression-free survival (PFS) in 484 patients randomized to receive the poly-ADP ribose polymerase inhibitor (PARPi) niraparib was 13.8 months, compared with 8.2 months in 244 patients who received placebo (hazard ratio, 0.62), Antonio González-Martin, MD, PhD, reported at the European Society for Medical Oncology Congress.
The findings were published simultaneously online in the New England Journal of Medicine (N Engl J Med. 2019 Sep 28. doi: 10.1056/NEJMoa1910962).
In patients at high risk for progression based on homologous recombination deficiency (HRd) – defined by certain tumor factors or the presence of BRCA mutation (BRCAm), PFS was 21.9 vs. 10.4 months in the treatment (n = 245) vs. placebo (n = 125) groups, respectively (HR, 0.43), said Dr. González-Martin of Grupo Español de Investigación en Cáncer de Ovario (GEICO), medical oncology department, Clínica Universidad de Navarra, Madrid.
“At 18 months, which means approximately 2 years after the initiation of chemotherapy, 42% of patients treated with niraparib remained alive and progression free,” he said, adding that 59% of the HRd patients remained alive and progression free at 18 months.
Exploratory analyses showed that the niraparib benefits occurred across all prespecified patient subgroups, including those aged 65 and older vs. those under age 65, those with stage III vs. stage IV disease at diagnosis, those receiving vs. not receiving neoadjuvant chemotherapy, those with complete response (CR) vs. partial response (PR) as their best response to platinum chemotherapy, and those with HRd who had BRCAm vs. BRCA wild type (BRCAwt) tumors, he said.
The hazard ratios for the HRd BRCAm vs. BRCAwt tumors were 0.40 and 0.50, respectively.
“So the benefit of niraparib in the HRd tumor is not driven only by the BRCA-mutated patients,” he said. “Importantly, we also saw benefit in the group of patients with tumors that were [homologous recombination] proficient (HRp), with a reduction in the risk of progression of 32%.”
For the key secondary endpoint of overall survival, a preplanned interim analysis showed that 84% vs. 77% in the niraparib and placebo groups, respectively, were alive at 2 years; in the HRd and HRp groups, those rates were 91% vs. 85% and 81% vs. 59%, respectively.
Participants in the double-blind trial had newly diagnosed, advanced high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube cancer; their mean age was 62 years; and they had experienced a CR (69%) or PR (31%) to first-line platinum-based chemotherapy. Overall, 35% had stage IV disease and 67% received neoadjuvant chemotherapy. They were randomized 2:1 to once-daily niraparib at a starting dose of 300 mg or 200 mg depending on body weight and platelet count, with those weighing 77 kg or greater and with platelet count of 150,000/mcL or less starting at the higher dose, and those weighing less than 77 kg and/or with platelet count less than 150,000/mcL starting at the lower dose.
All subgroups showed a sustained and durable treatment effect, and although most patients experienced treatment-related adverse events (TRAEs), those were “manageable with dose interruption or dose reduction,” Dr. González-Martin said.
Discontinuations due to TRAEs occurred in 12% vs. 2.5% in the treatment vs. placebo groups, and this was consistent with prior niraparib experience, he said, adding that no niraparib-related deaths were reported and no new safety signals were identified.
The findings are notable, because the recurrence rate after standard first-line platinum-based chemotherapy in women with advanced ovarian cancer is estimated at up to 85%, and while certain subgroups of patients have options for maintenance therapy, there remains a high unmet need for others, he explained.
For example olaparib is an option, but only for tumors with BRCA mutation, and bevacizumab can be used, but “may be limited due to safety concerns in some patients and also due to limited data from randomized trials in the neoadjuvant setting,” he said.
As a result, surveillance after chemotherapy is the approach used for many patients, he added.
Niraparib is the first oral PARPi approved for maintenance in patients with recurrent ovarian cancer, regardless of BRCA mutation status; in the NOVA study, it demonstrated efficacy after platinum chemotherapy in all biomarker populations, and in the QUADRA study it showed benefit in patients who received at least three prior therapies.
The current study was designed to test the efficacy and safety of niraparib therapy after response to platinum-based chemotherapy in patients with newly diagnosed advanced ovarian cancer, including those at high risk of relapse.
“Niraparib is the first PARP inhibitor that has demonstrated benefit after front-line platinum-based chemotherapy across all the biomarker subgroups, regardless of BRCA status, consistent with data from the recurrent setting,” Dr. González-Martin said, adding that patients with ovarian cancer at the highest risk of early disease progression obtained significant benefit. “What does this mean for our patients and our practice? Based on these results, niraparib after first-line platinum chemotherapy should be considered a new standard of care.”
Invited discussant Ana Oaknin, MD, PhD, head of the gynecologic cancer program at Vall d’Hebron Institute of Oncology, Vall d’Hebron University Hospital, Barcelona, called the findings “striking” and noted that they, along with those from the PAOLA-1/ENGOT-Ov25 trial demonstrating a PFS benefit with the addition of olaparib to bevacizumab maintenance therapy after first-line platinum-based chemotherapy in advanced ovarian cancer, represent important advances.
“We are witnessing a paradigm shift in the first-line treatment of advanced ovarian cancer patients,” she said.
Are the findings of these trials clinically meaningful enough to justify the addition of PARPi maintenance therapy after first-line chemotherapy therapy as a new standard of care?
“Yes, but while the benefit is clinically meaningful in the overall population, we should consider PFS outcomes according to the biomarker status in the selection of optimal therapy; companion diagnostic tests will be needed,” she said.
The PRIMA/ENGOT-OV26/GOG-3012 study was sponsored by TESARO. Dr. González-Martin reported relationships with numerous pharmaceutical companies.
SOURCE: González-Martin A et al. ESMO 2019: Abstract LBA1.
REPORTING FROM ESMO 2019
Larval Tick Infestation Causing an Eruption of Pruritic Papules and Pustules
Case Reports
Patient 1
A 65-year-old woman presented to the dermatology clinic in July with a pruritic rash of 2 days’ duration that started on the back and spread diffusely. The patient gardened regularly. Physical examination showed inflammatory papules and pustules on the back (Figure 1), as well as the groin, breasts, and ears. There was a punctate black dot in the center of some papules, and dermoscopy revealed ticks (Figure 2). Removal and microscopic examination confirmed larval-stage lone star ticks (Figure 3). The patient was prescribed topical steroids for pruritus as well as oral doxycycline for prophylaxis against tick-borne illnesses.


Patient 2
A 54-year-old man presented to the same clinic in July with pruritic lesions on the back, legs, ankles, and scrotum of 3 days’ duration that first appeared 24 hours after performing yardwork. Physical examination revealed diffusely distributed papules, pustules, and vesicles on the back (Figure 4). Some papules featured a punctate black dot in the center (similar to patient 1), and dermoscopy again revealed ticks. Removal and microscopic examination confirmed larval-stage ticks. The patient was treated with topical steroids and oral antihistamines for pruritus as well as prophylactic oral doxycycline.
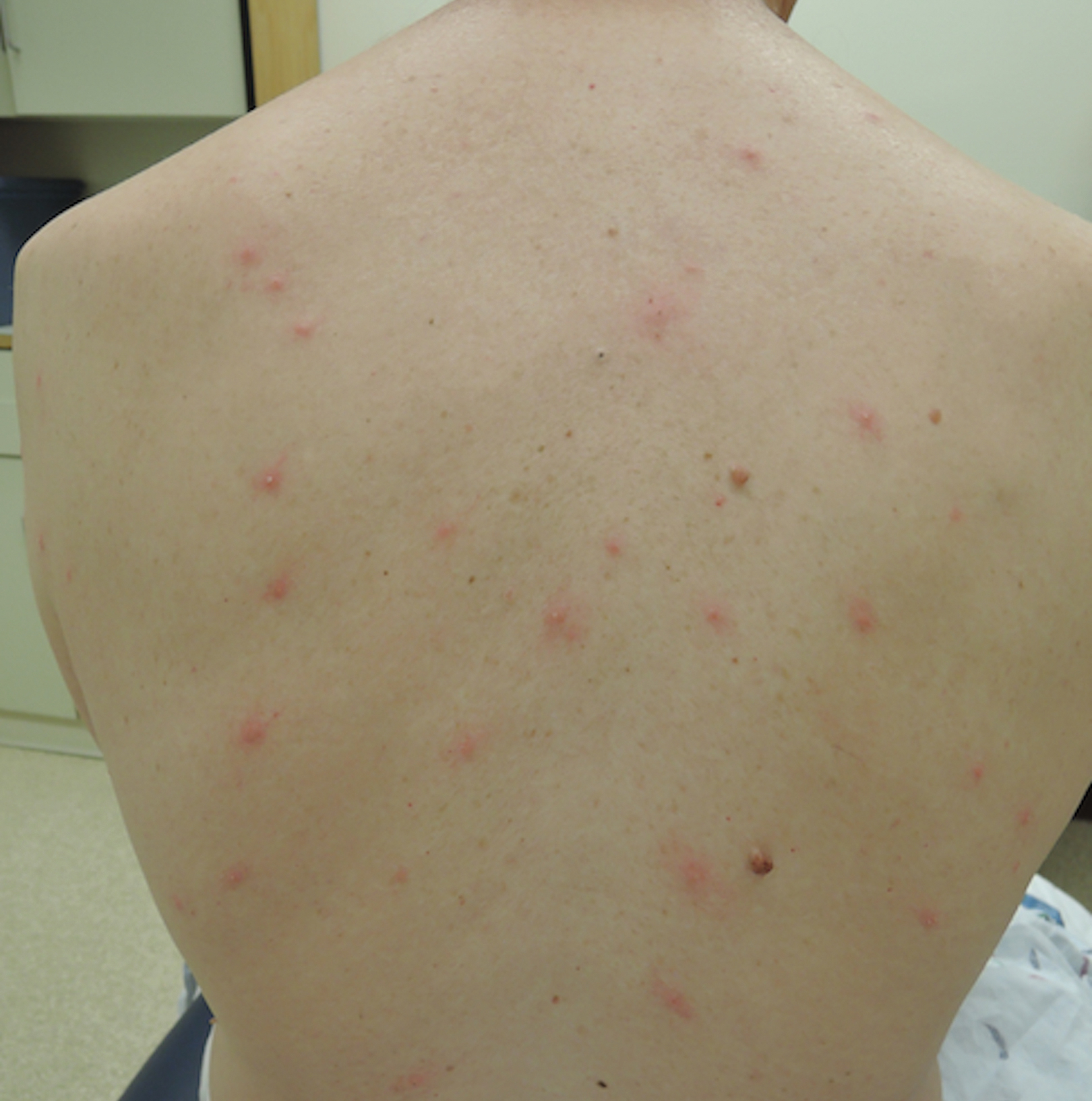
Comment
Ticks are well-known human parasites, representing the second most common vector of human infectious disease.1 Ticks have 3 motile stages: larva (or “seed”), nymph, and adult. They can bite humans during all stages. Larval ticks, distinguished by having 6 legs rather than 8 legs in nymphs and adults, can attack in droves and cause an infestation that presents as diffuse, pruritic, erythematous papules and pustules.2-4 The first report of larval tick infestation in humans may have been in 1728 by William Byrd who described finding ticks on the skin that were too small to see without a microscope.5
Identification
The ticks in both of our cases were lone star ticks (Amblyomma americanum). The larval stage of A americanum is a proven cause of cutaneous reaction.6,7 A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the terms tick, seed tick, or tick bite in combination with rash, eruption, infestation, papule, pustule, or pruritic revealed 6 reported cases of larval tick infestation in the literature (including our case); 5 were caused by A americanum and 1 by Ixodes dammini (now known as Ixodes scapularis); all occurred in July or August.3,7-10 This time frame is consistent with the general tick life cycle across species: Adults feed from April to June, then lay eggs that hatch into larval ticks within 4 to 6 weeks. After hatching, larval ticks climb grass and weeds awaiting a passing host.4
Diagnosis
Larval tick infestation remains a frequently misdiagnosed etiology of diffuse pruritic papules and pustules, especially in urban settings where physicians are less likely to be familiar with this type of manifestation.3,9-11 Larval ticks are submillimeter in size and difficult to appreciate with the naked eye, contributing to misdiagnosis. A punctate black dot may sometimes be seen in papules; however, dermoscopy is critical for accurate diagnosis, as hemorrhagic crust is a frequent misdiagnosis.
Management
In addition to symptomatic therapy, both of our patients received doxycycline as antibiotic prophylaxis for tick-borne illnesses given that a high number of ticks had been attached for more than 2 days.12,13 Antibiotic prophylaxis for tick-borne illness is controversial. The exception is Lyme disease transmitted by nymphal or adult I scapularis when specific conditions are met: the bite must have occurred in an endemic area, doxycycline cannot be contraindicated, estimated duration of attachment is at least 36 hours, and prophylaxis must be started within 72 hours of tick removal.13 There are no official recommendations for the A americanum species or for larval-stage ticks of any species. Larval-stage ticks acting as vectors for disease transmission is not well documented in recent literature, and there currently is limited evidence supporting prophylactic antibiotics for larval tick bites. The presence of spotted fever rickettsioses has been reported (with the exception of Rickettsia rickettsii and Ehrlichia chaffeensis) in larval A americanum ticks, suggesting a theoretical possibility that they could act as disease vectors.3,8,11,14-17 At a minimum, both prompt tick removal and close patient follow-up is warranted.
Conclusion
Human infestation with larval ticks is a common occurrence but can present a diagnostic challenge to an unfamiliar physician. We encourage consideration of larval tick infestation as the etiology of multiple or diffuse pruritic papules with a history of outdoor exposure.
- Sonenshine DE. Biology of Ticks. New York, NY: Oxford University; 1991.
- Alexander JOD. The effects of tick bites. In: Alexander JOD. Arthropods and Human Skin. London, England: Springer London; 1984:363-382.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Cropley TG. William Byrd on ticks, 1728. Arch Dermatol. 2009;145:187.
- Goddard J. A ten-year study of tick biting in Mississippi: implications for human disease transmission. J Agromedicine. 2002;8:25-32.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Fibeger EA, Erickson QL, Weintraub BD, et al. Larval tick infestation: a case report and review of tick-borne disease. Cutis. 2008;82:38-46.
- Jones BE. Human ‘seed tick’ infestation: Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Culp JS. Seed ticks. Am Fam Physician. 1987;36:121-123.
- Perea AE, Hinckley AF, Mead PS. Tick bite prophylaxis: results from a 2012 survey of healthcare providers. Zoonoses Public Health. 2015;62:388-392.
- Tick bites/prevention. Centers for Disease Control and Prevention website. https://www.cdc.gov/ticks/tickbornediseases/tick-bites-prevention.html. Revised January 10, 2019. Accessed September 17, 2019.
- Moncayo AC, Cohen SB, Fritzen CM, et al. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010;83:653-657.
- Castellaw AH, Showers J, Goddard J, et al. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J Med Entomol. 2010;47:473-476.
- Stromdahl EY, Vince MA, Billingsley PM, et al. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 2008;8:15-24.
- Long SW, Zhang X, Zhang J, et al. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 2003;40:1000-1004.
Case Reports
Patient 1
A 65-year-old woman presented to the dermatology clinic in July with a pruritic rash of 2 days’ duration that started on the back and spread diffusely. The patient gardened regularly. Physical examination showed inflammatory papules and pustules on the back (Figure 1), as well as the groin, breasts, and ears. There was a punctate black dot in the center of some papules, and dermoscopy revealed ticks (Figure 2). Removal and microscopic examination confirmed larval-stage lone star ticks (Figure 3). The patient was prescribed topical steroids for pruritus as well as oral doxycycline for prophylaxis against tick-borne illnesses.



Patient 2
A 54-year-old man presented to the same clinic in July with pruritic lesions on the back, legs, ankles, and scrotum of 3 days’ duration that first appeared 24 hours after performing yardwork. Physical examination revealed diffusely distributed papules, pustules, and vesicles on the back (Figure 4). Some papules featured a punctate black dot in the center (similar to patient 1), and dermoscopy again revealed ticks. Removal and microscopic examination confirmed larval-stage ticks. The patient was treated with topical steroids and oral antihistamines for pruritus as well as prophylactic oral doxycycline.

Comment
Ticks are well-known human parasites, representing the second most common vector of human infectious disease.1 Ticks have 3 motile stages: larva (or “seed”), nymph, and adult. They can bite humans during all stages. Larval ticks, distinguished by having 6 legs rather than 8 legs in nymphs and adults, can attack in droves and cause an infestation that presents as diffuse, pruritic, erythematous papules and pustules.2-4 The first report of larval tick infestation in humans may have been in 1728 by William Byrd who described finding ticks on the skin that were too small to see without a microscope.5
Identification
The ticks in both of our cases were lone star ticks (Amblyomma americanum). The larval stage of A americanum is a proven cause of cutaneous reaction.6,7 A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the terms tick, seed tick, or tick bite in combination with rash, eruption, infestation, papule, pustule, or pruritic revealed 6 reported cases of larval tick infestation in the literature (including our case); 5 were caused by A americanum and 1 by Ixodes dammini (now known as Ixodes scapularis); all occurred in July or August.3,7-10 This time frame is consistent with the general tick life cycle across species: Adults feed from April to June, then lay eggs that hatch into larval ticks within 4 to 6 weeks. After hatching, larval ticks climb grass and weeds awaiting a passing host.4
Diagnosis
Larval tick infestation remains a frequently misdiagnosed etiology of diffuse pruritic papules and pustules, especially in urban settings where physicians are less likely to be familiar with this type of manifestation.3,9-11 Larval ticks are submillimeter in size and difficult to appreciate with the naked eye, contributing to misdiagnosis. A punctate black dot may sometimes be seen in papules; however, dermoscopy is critical for accurate diagnosis, as hemorrhagic crust is a frequent misdiagnosis.
Management
In addition to symptomatic therapy, both of our patients received doxycycline as antibiotic prophylaxis for tick-borne illnesses given that a high number of ticks had been attached for more than 2 days.12,13 Antibiotic prophylaxis for tick-borne illness is controversial. The exception is Lyme disease transmitted by nymphal or adult I scapularis when specific conditions are met: the bite must have occurred in an endemic area, doxycycline cannot be contraindicated, estimated duration of attachment is at least 36 hours, and prophylaxis must be started within 72 hours of tick removal.13 There are no official recommendations for the A americanum species or for larval-stage ticks of any species. Larval-stage ticks acting as vectors for disease transmission is not well documented in recent literature, and there currently is limited evidence supporting prophylactic antibiotics for larval tick bites. The presence of spotted fever rickettsioses has been reported (with the exception of Rickettsia rickettsii and Ehrlichia chaffeensis) in larval A americanum ticks, suggesting a theoretical possibility that they could act as disease vectors.3,8,11,14-17 At a minimum, both prompt tick removal and close patient follow-up is warranted.
Conclusion
Human infestation with larval ticks is a common occurrence but can present a diagnostic challenge to an unfamiliar physician. We encourage consideration of larval tick infestation as the etiology of multiple or diffuse pruritic papules with a history of outdoor exposure.
Case Reports
Patient 1
A 65-year-old woman presented to the dermatology clinic in July with a pruritic rash of 2 days’ duration that started on the back and spread diffusely. The patient gardened regularly. Physical examination showed inflammatory papules and pustules on the back (Figure 1), as well as the groin, breasts, and ears. There was a punctate black dot in the center of some papules, and dermoscopy revealed ticks (Figure 2). Removal and microscopic examination confirmed larval-stage lone star ticks (Figure 3). The patient was prescribed topical steroids for pruritus as well as oral doxycycline for prophylaxis against tick-borne illnesses.



Patient 2
A 54-year-old man presented to the same clinic in July with pruritic lesions on the back, legs, ankles, and scrotum of 3 days’ duration that first appeared 24 hours after performing yardwork. Physical examination revealed diffusely distributed papules, pustules, and vesicles on the back (Figure 4). Some papules featured a punctate black dot in the center (similar to patient 1), and dermoscopy again revealed ticks. Removal and microscopic examination confirmed larval-stage ticks. The patient was treated with topical steroids and oral antihistamines for pruritus as well as prophylactic oral doxycycline.

Comment
Ticks are well-known human parasites, representing the second most common vector of human infectious disease.1 Ticks have 3 motile stages: larva (or “seed”), nymph, and adult. They can bite humans during all stages. Larval ticks, distinguished by having 6 legs rather than 8 legs in nymphs and adults, can attack in droves and cause an infestation that presents as diffuse, pruritic, erythematous papules and pustules.2-4 The first report of larval tick infestation in humans may have been in 1728 by William Byrd who described finding ticks on the skin that were too small to see without a microscope.5
Identification
The ticks in both of our cases were lone star ticks (Amblyomma americanum). The larval stage of A americanum is a proven cause of cutaneous reaction.6,7 A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the terms tick, seed tick, or tick bite in combination with rash, eruption, infestation, papule, pustule, or pruritic revealed 6 reported cases of larval tick infestation in the literature (including our case); 5 were caused by A americanum and 1 by Ixodes dammini (now known as Ixodes scapularis); all occurred in July or August.3,7-10 This time frame is consistent with the general tick life cycle across species: Adults feed from April to June, then lay eggs that hatch into larval ticks within 4 to 6 weeks. After hatching, larval ticks climb grass and weeds awaiting a passing host.4
Diagnosis
Larval tick infestation remains a frequently misdiagnosed etiology of diffuse pruritic papules and pustules, especially in urban settings where physicians are less likely to be familiar with this type of manifestation.3,9-11 Larval ticks are submillimeter in size and difficult to appreciate with the naked eye, contributing to misdiagnosis. A punctate black dot may sometimes be seen in papules; however, dermoscopy is critical for accurate diagnosis, as hemorrhagic crust is a frequent misdiagnosis.
Management
In addition to symptomatic therapy, both of our patients received doxycycline as antibiotic prophylaxis for tick-borne illnesses given that a high number of ticks had been attached for more than 2 days.12,13 Antibiotic prophylaxis for tick-borne illness is controversial. The exception is Lyme disease transmitted by nymphal or adult I scapularis when specific conditions are met: the bite must have occurred in an endemic area, doxycycline cannot be contraindicated, estimated duration of attachment is at least 36 hours, and prophylaxis must be started within 72 hours of tick removal.13 There are no official recommendations for the A americanum species or for larval-stage ticks of any species. Larval-stage ticks acting as vectors for disease transmission is not well documented in recent literature, and there currently is limited evidence supporting prophylactic antibiotics for larval tick bites. The presence of spotted fever rickettsioses has been reported (with the exception of Rickettsia rickettsii and Ehrlichia chaffeensis) in larval A americanum ticks, suggesting a theoretical possibility that they could act as disease vectors.3,8,11,14-17 At a minimum, both prompt tick removal and close patient follow-up is warranted.
Conclusion
Human infestation with larval ticks is a common occurrence but can present a diagnostic challenge to an unfamiliar physician. We encourage consideration of larval tick infestation as the etiology of multiple or diffuse pruritic papules with a history of outdoor exposure.
- Sonenshine DE. Biology of Ticks. New York, NY: Oxford University; 1991.
- Alexander JOD. The effects of tick bites. In: Alexander JOD. Arthropods and Human Skin. London, England: Springer London; 1984:363-382.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Cropley TG. William Byrd on ticks, 1728. Arch Dermatol. 2009;145:187.
- Goddard J. A ten-year study of tick biting in Mississippi: implications for human disease transmission. J Agromedicine. 2002;8:25-32.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Fibeger EA, Erickson QL, Weintraub BD, et al. Larval tick infestation: a case report and review of tick-borne disease. Cutis. 2008;82:38-46.
- Jones BE. Human ‘seed tick’ infestation: Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Culp JS. Seed ticks. Am Fam Physician. 1987;36:121-123.
- Perea AE, Hinckley AF, Mead PS. Tick bite prophylaxis: results from a 2012 survey of healthcare providers. Zoonoses Public Health. 2015;62:388-392.
- Tick bites/prevention. Centers for Disease Control and Prevention website. https://www.cdc.gov/ticks/tickbornediseases/tick-bites-prevention.html. Revised January 10, 2019. Accessed September 17, 2019.
- Moncayo AC, Cohen SB, Fritzen CM, et al. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010;83:653-657.
- Castellaw AH, Showers J, Goddard J, et al. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J Med Entomol. 2010;47:473-476.
- Stromdahl EY, Vince MA, Billingsley PM, et al. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 2008;8:15-24.
- Long SW, Zhang X, Zhang J, et al. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 2003;40:1000-1004.
- Sonenshine DE. Biology of Ticks. New York, NY: Oxford University; 1991.
- Alexander JOD. The effects of tick bites. In: Alexander JOD. Arthropods and Human Skin. London, England: Springer London; 1984:363-382.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Cropley TG. William Byrd on ticks, 1728. Arch Dermatol. 2009;145:187.
- Goddard J. A ten-year study of tick biting in Mississippi: implications for human disease transmission. J Agromedicine. 2002;8:25-32.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Fibeger EA, Erickson QL, Weintraub BD, et al. Larval tick infestation: a case report and review of tick-borne disease. Cutis. 2008;82:38-46.
- Jones BE. Human ‘seed tick’ infestation: Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Culp JS. Seed ticks. Am Fam Physician. 1987;36:121-123.
- Perea AE, Hinckley AF, Mead PS. Tick bite prophylaxis: results from a 2012 survey of healthcare providers. Zoonoses Public Health. 2015;62:388-392.
- Tick bites/prevention. Centers for Disease Control and Prevention website. https://www.cdc.gov/ticks/tickbornediseases/tick-bites-prevention.html. Revised January 10, 2019. Accessed September 17, 2019.
- Moncayo AC, Cohen SB, Fritzen CM, et al. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010;83:653-657.
- Castellaw AH, Showers J, Goddard J, et al. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J Med Entomol. 2010;47:473-476.
- Stromdahl EY, Vince MA, Billingsley PM, et al. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 2008;8:15-24.
- Long SW, Zhang X, Zhang J, et al. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 2003;40:1000-1004.
Practice Points
- Larval (“seed”) ticks can attack in droves, causing a widespread rash consisting of pruritic erythematous papules and pustules.
- Tiny black dots can be seen in some papules, which are the seed ticks themselves. Careful dermoscopic examination is critical to avoid easy misdiagnosis as hemorrhagic crust.
- We encourage providers to include larval tick infestation in the differential for eruptive pruritic papules and pustules with a history of outdoor exposure, especially during the summer months.