User login
Patients with nonaffective psychosis offer treatment target preferences
‘Worrying less,’ ‘feeling happier,’ and sleeping better were among top targets identified
Patients with nonaffective psychosis want the “causal mechanisms” for their psychotic experiences treated, according to a study conducted by researchers at the University of Oxford (England).
“The findings indicate a need to adopt into services for people with severe mental health difficulties interventions shown by research to treat anxious avoidance, worry, low self-esteem, insomnia, and other such transdiagnostic mechanisms and evaluate the outcomes,” wrote Daniel Freeman, PhD, DClinPsy, of the department of psychiatry at Oxford, and colleagues. The study was published in Schizophrenia Research.
In previous research, Dr. Freeman and colleagues identified factors that appear to maintain symptoms of nonaffective psychosis, such as paranoia (Behav Cogn Psychother. 2016 Sep;44[5]:539-52).Those factors include anxious avoidance, worry, low self-esteem, insomnia, and reasoning bias. In additional research, the researchers demonstrated that addressing those targets can lead to led to reductions in paranoia.
In the current study, 1,809 patients were recruited from 39 National Health Services mental health trusts across England. The researchers analyzed responses to eight clinical assessment tools and a patient-reported questionnaire about treatment preferences.
Dr. Freeman and colleagues found consistency in the relationships observed among the factors and nonaffective psychosis with the theoretical model they had developed, such as positive correlations between paranoia and anxious avoidance (correlation coefficient [r] = 0.37), worry (r = 0.40), and hallucinations (r = 0.54). They found negative correlations between paranoia and levels of positive self-beliefs (r = –0.17), rational reasoning (r = –0.14), and psychological wellbeing (r = –0.29). Those correlations were all statistically significant with P values less than .001.
Most of the patients (90.3%) wanted at least one of the eight problem areas assessed in the study treated, and on average, they wanted more than four (mean, 4.5) treated. The most frequently identified top treatment priorities identified by patients were worrying (50.8%), feeling happier (42.9%), and sleeping better (41.2%).
the researchers wrote.
Among the study limitations: The assessments selected were based on the theoretical model the researchers had developed and therefore may not have captured all difficulties experienced by the patients. Furthermore, although the sample was large, it’s unclear whether it was truly representative because they were drawn from certain mental health services that, for example, patients with milder symptoms might not seek out.
Dr. Freeman is a cofounder and chief clinical officer of Oxford VR. He has received funding from the National Institute of Health Research, Medical Research Council, and Wellcome Trust. No other authors reported conflicts of interest.
SOURCE: Freeman D et al. Schizophr Res. 2019. doi: 10.1016/j.schres.2019.07.016.
‘Worrying less,’ ‘feeling happier,’ and sleeping better were among top targets identified
‘Worrying less,’ ‘feeling happier,’ and sleeping better were among top targets identified
Patients with nonaffective psychosis want the “causal mechanisms” for their psychotic experiences treated, according to a study conducted by researchers at the University of Oxford (England).
“The findings indicate a need to adopt into services for people with severe mental health difficulties interventions shown by research to treat anxious avoidance, worry, low self-esteem, insomnia, and other such transdiagnostic mechanisms and evaluate the outcomes,” wrote Daniel Freeman, PhD, DClinPsy, of the department of psychiatry at Oxford, and colleagues. The study was published in Schizophrenia Research.
In previous research, Dr. Freeman and colleagues identified factors that appear to maintain symptoms of nonaffective psychosis, such as paranoia (Behav Cogn Psychother. 2016 Sep;44[5]:539-52).Those factors include anxious avoidance, worry, low self-esteem, insomnia, and reasoning bias. In additional research, the researchers demonstrated that addressing those targets can lead to led to reductions in paranoia.
In the current study, 1,809 patients were recruited from 39 National Health Services mental health trusts across England. The researchers analyzed responses to eight clinical assessment tools and a patient-reported questionnaire about treatment preferences.
Dr. Freeman and colleagues found consistency in the relationships observed among the factors and nonaffective psychosis with the theoretical model they had developed, such as positive correlations between paranoia and anxious avoidance (correlation coefficient [r] = 0.37), worry (r = 0.40), and hallucinations (r = 0.54). They found negative correlations between paranoia and levels of positive self-beliefs (r = –0.17), rational reasoning (r = –0.14), and psychological wellbeing (r = –0.29). Those correlations were all statistically significant with P values less than .001.
Most of the patients (90.3%) wanted at least one of the eight problem areas assessed in the study treated, and on average, they wanted more than four (mean, 4.5) treated. The most frequently identified top treatment priorities identified by patients were worrying (50.8%), feeling happier (42.9%), and sleeping better (41.2%).
the researchers wrote.
Among the study limitations: The assessments selected were based on the theoretical model the researchers had developed and therefore may not have captured all difficulties experienced by the patients. Furthermore, although the sample was large, it’s unclear whether it was truly representative because they were drawn from certain mental health services that, for example, patients with milder symptoms might not seek out.
Dr. Freeman is a cofounder and chief clinical officer of Oxford VR. He has received funding from the National Institute of Health Research, Medical Research Council, and Wellcome Trust. No other authors reported conflicts of interest.
SOURCE: Freeman D et al. Schizophr Res. 2019. doi: 10.1016/j.schres.2019.07.016.
Patients with nonaffective psychosis want the “causal mechanisms” for their psychotic experiences treated, according to a study conducted by researchers at the University of Oxford (England).
“The findings indicate a need to adopt into services for people with severe mental health difficulties interventions shown by research to treat anxious avoidance, worry, low self-esteem, insomnia, and other such transdiagnostic mechanisms and evaluate the outcomes,” wrote Daniel Freeman, PhD, DClinPsy, of the department of psychiatry at Oxford, and colleagues. The study was published in Schizophrenia Research.
In previous research, Dr. Freeman and colleagues identified factors that appear to maintain symptoms of nonaffective psychosis, such as paranoia (Behav Cogn Psychother. 2016 Sep;44[5]:539-52).Those factors include anxious avoidance, worry, low self-esteem, insomnia, and reasoning bias. In additional research, the researchers demonstrated that addressing those targets can lead to led to reductions in paranoia.
In the current study, 1,809 patients were recruited from 39 National Health Services mental health trusts across England. The researchers analyzed responses to eight clinical assessment tools and a patient-reported questionnaire about treatment preferences.
Dr. Freeman and colleagues found consistency in the relationships observed among the factors and nonaffective psychosis with the theoretical model they had developed, such as positive correlations between paranoia and anxious avoidance (correlation coefficient [r] = 0.37), worry (r = 0.40), and hallucinations (r = 0.54). They found negative correlations between paranoia and levels of positive self-beliefs (r = –0.17), rational reasoning (r = –0.14), and psychological wellbeing (r = –0.29). Those correlations were all statistically significant with P values less than .001.
Most of the patients (90.3%) wanted at least one of the eight problem areas assessed in the study treated, and on average, they wanted more than four (mean, 4.5) treated. The most frequently identified top treatment priorities identified by patients were worrying (50.8%), feeling happier (42.9%), and sleeping better (41.2%).
the researchers wrote.
Among the study limitations: The assessments selected were based on the theoretical model the researchers had developed and therefore may not have captured all difficulties experienced by the patients. Furthermore, although the sample was large, it’s unclear whether it was truly representative because they were drawn from certain mental health services that, for example, patients with milder symptoms might not seek out.
Dr. Freeman is a cofounder and chief clinical officer of Oxford VR. He has received funding from the National Institute of Health Research, Medical Research Council, and Wellcome Trust. No other authors reported conflicts of interest.
SOURCE: Freeman D et al. Schizophr Res. 2019. doi: 10.1016/j.schres.2019.07.016.
FROM SCHIZOPHRENIA RESEARCH
Start From Scratch
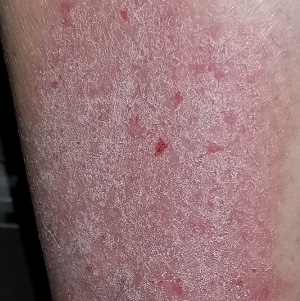
A 50-year-old man presents with complaints of a rash on his right leg that manifested 20 years ago. Although the rash is worrisome, he says the associated pruritus is worse. During the workday, he is able to ignore the itching—but the minute he gets home, he begins to scratch.
He knows the scratching is counterproductive in the long run, but the urge to do it is quite compelling. Sometimes he uses a wet washcloth; other times, he will actually use a hair brush to satiate the itching. The relief is intensely satisfying albeit short lived.
The rash has persisted despite multiple treatment attempts. Tried products include OTC moisturizers and antifungal creams, as well as prescription antifungal creams. None has had an effect.
The patient denies any other skin problems. He does recall having eczema as a child. Although that has long since resolved, he remains quite allergy prone and is particularly sensitive to airborne allergens—a trait that runs strongly in his family.
EXAMINATION
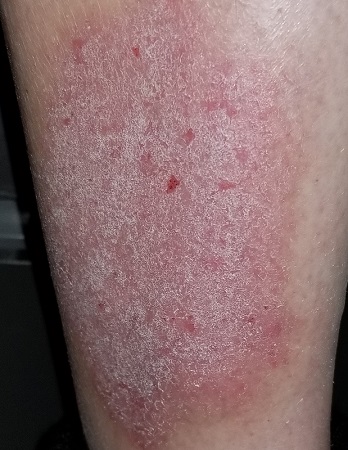
A pink, oval rash covers most of the patient’s right lateral calf. It has a thickened, faintly scaly surface that is uniform and sharply circumscribed. There is no increased warmth or tenderness on palpation. No lymph nodes can be felt in the right groin. A check of the patient’s knees, elbows, scalp, nails, and trunk show no sign of rash or other changes.
What’s the diagnosis?
DISCUSSION
Lichen simplex chronicus (LSC), previously known as neurodermatitis, is quite common but frequently misdiagnosed. Patients often report that their condition started with a bug bite or poison ivy—a provocation that gets the patient in the habit of scratching, which continues long after the initial outbreak has subsided. Thus, LSC is often associated with significant chronicity, as typified by this case.
What patients seldom understand is their own role in the perpetuation of their condition. The urge to scratch is so unbearable that few can resist it. Over time, the scratching creates more nerves that have a lower threshold for itching, and thus the itch-scratch-itch cycle is born. Many LSC patients are atopic, which predisposes them to itching in general and to xerosis especially.
The literature asserts that LSC affects the genders equally, but this ignores the fact that it can present significantly differently in men and women. This patient’s area of involvement is quite typical for men, most of whom never moisturize and for whom the lateral calf is readily accessible. In the author’s experience, the most common location for LSC in women is the nuchal scalp, where heat and sweat appear to play a role, along with ready accessibility to fingernails or the sharp end of a pencil. Other common areas of involvement include the dorsal forearms and the scrotum or vulvae.
Biopsy is seldom necessary, but if performed, it will show a marked thickening of the epidermis, orthokeratosis (normal keratinocytes about to shed), and compacted, elongated rete ridges. These and other changes effectively rule out other items in the differential (eg, psoriasis, simple eczema, fungal infection).
Stopping the itch-scratch-itch cycle with mid-strength topical steroid creams or foams is the first step in treating LSC. But then the patient must be convinced of his contribution to the treatment: leaving the affected sites alone. Truth be told, after 20 years of scratching, the best this patient can look forward to is some relief—not only from the itching, but also from concern about all the terrible things he now knows he doesn’t have.
TAKE-HOME LEARNING POINTS
- Lichen simplex chronicus is quite common in both genders and is typified by longstanding severe itching, usually confined to one area.
- Atopy, xerosis, and stress all appear to contribute to the problem.
- Stopping the itch-scratch-itch cycle with topical steroids is a key component of treatment.
- Patient education—on the nature of the problem and the patient’s role in controlling it—is just as important as any prescribed medication.
A 50-year-old man presents with complaints of a rash on his right leg that manifested 20 years ago. Although the rash is worrisome, he says the associated pruritus is worse. During the workday, he is able to ignore the itching—but the minute he gets home, he begins to scratch.
He knows the scratching is counterproductive in the long run, but the urge to do it is quite compelling. Sometimes he uses a wet washcloth; other times, he will actually use a hair brush to satiate the itching. The relief is intensely satisfying albeit short lived.
The rash has persisted despite multiple treatment attempts. Tried products include OTC moisturizers and antifungal creams, as well as prescription antifungal creams. None has had an effect.
The patient denies any other skin problems. He does recall having eczema as a child. Although that has long since resolved, he remains quite allergy prone and is particularly sensitive to airborne allergens—a trait that runs strongly in his family.

EXAMINATION
A pink, oval rash covers most of the patient’s right lateral calf. It has a thickened, faintly scaly surface that is uniform and sharply circumscribed. There is no increased warmth or tenderness on palpation. No lymph nodes can be felt in the right groin. A check of the patient’s knees, elbows, scalp, nails, and trunk show no sign of rash or other changes.
What’s the diagnosis?
DISCUSSION
Lichen simplex chronicus (LSC), previously known as neurodermatitis, is quite common but frequently misdiagnosed. Patients often report that their condition started with a bug bite or poison ivy—a provocation that gets the patient in the habit of scratching, which continues long after the initial outbreak has subsided. Thus, LSC is often associated with significant chronicity, as typified by this case.
What patients seldom understand is their own role in the perpetuation of their condition. The urge to scratch is so unbearable that few can resist it. Over time, the scratching creates more nerves that have a lower threshold for itching, and thus the itch-scratch-itch cycle is born. Many LSC patients are atopic, which predisposes them to itching in general and to xerosis especially.
The literature asserts that LSC affects the genders equally, but this ignores the fact that it can present significantly differently in men and women. This patient’s area of involvement is quite typical for men, most of whom never moisturize and for whom the lateral calf is readily accessible. In the author’s experience, the most common location for LSC in women is the nuchal scalp, where heat and sweat appear to play a role, along with ready accessibility to fingernails or the sharp end of a pencil. Other common areas of involvement include the dorsal forearms and the scrotum or vulvae.
Biopsy is seldom necessary, but if performed, it will show a marked thickening of the epidermis, orthokeratosis (normal keratinocytes about to shed), and compacted, elongated rete ridges. These and other changes effectively rule out other items in the differential (eg, psoriasis, simple eczema, fungal infection).
Stopping the itch-scratch-itch cycle with mid-strength topical steroid creams or foams is the first step in treating LSC. But then the patient must be convinced of his contribution to the treatment: leaving the affected sites alone. Truth be told, after 20 years of scratching, the best this patient can look forward to is some relief—not only from the itching, but also from concern about all the terrible things he now knows he doesn’t have.
TAKE-HOME LEARNING POINTS
- Lichen simplex chronicus is quite common in both genders and is typified by longstanding severe itching, usually confined to one area.
- Atopy, xerosis, and stress all appear to contribute to the problem.
- Stopping the itch-scratch-itch cycle with topical steroids is a key component of treatment.
- Patient education—on the nature of the problem and the patient’s role in controlling it—is just as important as any prescribed medication.
A 50-year-old man presents with complaints of a rash on his right leg that manifested 20 years ago. Although the rash is worrisome, he says the associated pruritus is worse. During the workday, he is able to ignore the itching—but the minute he gets home, he begins to scratch.
He knows the scratching is counterproductive in the long run, but the urge to do it is quite compelling. Sometimes he uses a wet washcloth; other times, he will actually use a hair brush to satiate the itching. The relief is intensely satisfying albeit short lived.
The rash has persisted despite multiple treatment attempts. Tried products include OTC moisturizers and antifungal creams, as well as prescription antifungal creams. None has had an effect.
The patient denies any other skin problems. He does recall having eczema as a child. Although that has long since resolved, he remains quite allergy prone and is particularly sensitive to airborne allergens—a trait that runs strongly in his family.

EXAMINATION
A pink, oval rash covers most of the patient’s right lateral calf. It has a thickened, faintly scaly surface that is uniform and sharply circumscribed. There is no increased warmth or tenderness on palpation. No lymph nodes can be felt in the right groin. A check of the patient’s knees, elbows, scalp, nails, and trunk show no sign of rash or other changes.
What’s the diagnosis?
DISCUSSION
Lichen simplex chronicus (LSC), previously known as neurodermatitis, is quite common but frequently misdiagnosed. Patients often report that their condition started with a bug bite or poison ivy—a provocation that gets the patient in the habit of scratching, which continues long after the initial outbreak has subsided. Thus, LSC is often associated with significant chronicity, as typified by this case.
What patients seldom understand is their own role in the perpetuation of their condition. The urge to scratch is so unbearable that few can resist it. Over time, the scratching creates more nerves that have a lower threshold for itching, and thus the itch-scratch-itch cycle is born. Many LSC patients are atopic, which predisposes them to itching in general and to xerosis especially.
The literature asserts that LSC affects the genders equally, but this ignores the fact that it can present significantly differently in men and women. This patient’s area of involvement is quite typical for men, most of whom never moisturize and for whom the lateral calf is readily accessible. In the author’s experience, the most common location for LSC in women is the nuchal scalp, where heat and sweat appear to play a role, along with ready accessibility to fingernails or the sharp end of a pencil. Other common areas of involvement include the dorsal forearms and the scrotum or vulvae.
Biopsy is seldom necessary, but if performed, it will show a marked thickening of the epidermis, orthokeratosis (normal keratinocytes about to shed), and compacted, elongated rete ridges. These and other changes effectively rule out other items in the differential (eg, psoriasis, simple eczema, fungal infection).
Stopping the itch-scratch-itch cycle with mid-strength topical steroid creams or foams is the first step in treating LSC. But then the patient must be convinced of his contribution to the treatment: leaving the affected sites alone. Truth be told, after 20 years of scratching, the best this patient can look forward to is some relief—not only from the itching, but also from concern about all the terrible things he now knows he doesn’t have.
TAKE-HOME LEARNING POINTS
- Lichen simplex chronicus is quite common in both genders and is typified by longstanding severe itching, usually confined to one area.
- Atopy, xerosis, and stress all appear to contribute to the problem.
- Stopping the itch-scratch-itch cycle with topical steroids is a key component of treatment.
- Patient education—on the nature of the problem and the patient’s role in controlling it—is just as important as any prescribed medication.
Congenital heart disease in children linked to increased autism risk
A new study of children who were born with congenital heart disease (CHD) has found that they have increased odds of developing autism spectrum disorder.
“To our knowledge, this is the only study in which there has been a comparison between [autism spectrum disorder] and multiple CHD subtypes,” wrote Eric R. Sigmon, MD, of Emory University, Atlanta, and coauthors. “Our findings are consistent with previous studies of CHD developmental outcomes, which have shown an increased risk of developmental and academic delay after CHD diagnosis and treatment.” The study was published in Pediatrics.
To further investigate the association between CHD and autism, the researchers performed a case-control study using the Military Health System administrative database. They uncovered 8,760 cases of children with autism spectrum disorder and matched each one with three controls (n = 26,280). From that sample size, they identified 1,063 children with CHD: 401 in the autism spectrum disorder group and 662 in the control group.
Before analysis, children with autism spectrum disorder had an odds ratio of 1.85 of having any form of CHD, compared with controls (95% confidence interval, 1.63-2.10). After adjustment for covariates – including genetic syndromes, maternal age and morbidity, perinatal morbidity, and neonatal complications – the OR was 1.33 (95% CI, 1.16-1.52).
In the sensitivity analysis – which included only 593 children with CHD – the OR was a similar 1.32 (95% CI, 1.10-1.59).
Left heart obstructive lesion was significantly associated with autism spectrum disorder after covariate adjustment (OR, 1.42; 95% CI, 1.04-1.93), but the finding was no longer significant in the sensitivity analysis.
The authors noted the potential limitations of their study, including the general weaknesses of administrative data, which they attempted to counter with the sensitive analysis. In addition, they recognized that children with either autism spectrum disorder or CHD “tend to present for care more frequently,” which could have created an ascertainment bias.
In an accompanying editorial, Johanna Calderon, PhD, David C. Bellinger, PhD, and Jane W. Newburger, MD, MPH, stated that more work needs to be done to further quantify the relationship between CHD and autism spectrum disorder (Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2019-2752). The three authors – all affiliated with Boston Children’s Hospital and Harvard Medical School, also in Boston – reiterated the acknowledgment from Dr. Sigmon and coauthors that the “etiologic pathways that might explain” the link between the two remains unknown. They also noted their surprise that autism spectrum disorder risk appears to be increased in children with modestly severe forms of CHD, stating that this finding required additional investigation.
“Despite the strengths of this study,” they wrote, “it raises more questions than answers.”
The study was funded by the Congressional Directed Medical Research Programs Autism Research Award. The authors reported no conflicts of interest.
SOURCE: Sigmon ER at al. Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2018-4114.
A new study of children who were born with congenital heart disease (CHD) has found that they have increased odds of developing autism spectrum disorder.
“To our knowledge, this is the only study in which there has been a comparison between [autism spectrum disorder] and multiple CHD subtypes,” wrote Eric R. Sigmon, MD, of Emory University, Atlanta, and coauthors. “Our findings are consistent with previous studies of CHD developmental outcomes, which have shown an increased risk of developmental and academic delay after CHD diagnosis and treatment.” The study was published in Pediatrics.
To further investigate the association between CHD and autism, the researchers performed a case-control study using the Military Health System administrative database. They uncovered 8,760 cases of children with autism spectrum disorder and matched each one with three controls (n = 26,280). From that sample size, they identified 1,063 children with CHD: 401 in the autism spectrum disorder group and 662 in the control group.
Before analysis, children with autism spectrum disorder had an odds ratio of 1.85 of having any form of CHD, compared with controls (95% confidence interval, 1.63-2.10). After adjustment for covariates – including genetic syndromes, maternal age and morbidity, perinatal morbidity, and neonatal complications – the OR was 1.33 (95% CI, 1.16-1.52).
In the sensitivity analysis – which included only 593 children with CHD – the OR was a similar 1.32 (95% CI, 1.10-1.59).
Left heart obstructive lesion was significantly associated with autism spectrum disorder after covariate adjustment (OR, 1.42; 95% CI, 1.04-1.93), but the finding was no longer significant in the sensitivity analysis.
The authors noted the potential limitations of their study, including the general weaknesses of administrative data, which they attempted to counter with the sensitive analysis. In addition, they recognized that children with either autism spectrum disorder or CHD “tend to present for care more frequently,” which could have created an ascertainment bias.
In an accompanying editorial, Johanna Calderon, PhD, David C. Bellinger, PhD, and Jane W. Newburger, MD, MPH, stated that more work needs to be done to further quantify the relationship between CHD and autism spectrum disorder (Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2019-2752). The three authors – all affiliated with Boston Children’s Hospital and Harvard Medical School, also in Boston – reiterated the acknowledgment from Dr. Sigmon and coauthors that the “etiologic pathways that might explain” the link between the two remains unknown. They also noted their surprise that autism spectrum disorder risk appears to be increased in children with modestly severe forms of CHD, stating that this finding required additional investigation.
“Despite the strengths of this study,” they wrote, “it raises more questions than answers.”
The study was funded by the Congressional Directed Medical Research Programs Autism Research Award. The authors reported no conflicts of interest.
SOURCE: Sigmon ER at al. Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2018-4114.
A new study of children who were born with congenital heart disease (CHD) has found that they have increased odds of developing autism spectrum disorder.
“To our knowledge, this is the only study in which there has been a comparison between [autism spectrum disorder] and multiple CHD subtypes,” wrote Eric R. Sigmon, MD, of Emory University, Atlanta, and coauthors. “Our findings are consistent with previous studies of CHD developmental outcomes, which have shown an increased risk of developmental and academic delay after CHD diagnosis and treatment.” The study was published in Pediatrics.
To further investigate the association between CHD and autism, the researchers performed a case-control study using the Military Health System administrative database. They uncovered 8,760 cases of children with autism spectrum disorder and matched each one with three controls (n = 26,280). From that sample size, they identified 1,063 children with CHD: 401 in the autism spectrum disorder group and 662 in the control group.
Before analysis, children with autism spectrum disorder had an odds ratio of 1.85 of having any form of CHD, compared with controls (95% confidence interval, 1.63-2.10). After adjustment for covariates – including genetic syndromes, maternal age and morbidity, perinatal morbidity, and neonatal complications – the OR was 1.33 (95% CI, 1.16-1.52).
In the sensitivity analysis – which included only 593 children with CHD – the OR was a similar 1.32 (95% CI, 1.10-1.59).
Left heart obstructive lesion was significantly associated with autism spectrum disorder after covariate adjustment (OR, 1.42; 95% CI, 1.04-1.93), but the finding was no longer significant in the sensitivity analysis.
The authors noted the potential limitations of their study, including the general weaknesses of administrative data, which they attempted to counter with the sensitive analysis. In addition, they recognized that children with either autism spectrum disorder or CHD “tend to present for care more frequently,” which could have created an ascertainment bias.
In an accompanying editorial, Johanna Calderon, PhD, David C. Bellinger, PhD, and Jane W. Newburger, MD, MPH, stated that more work needs to be done to further quantify the relationship between CHD and autism spectrum disorder (Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2019-2752). The three authors – all affiliated with Boston Children’s Hospital and Harvard Medical School, also in Boston – reiterated the acknowledgment from Dr. Sigmon and coauthors that the “etiologic pathways that might explain” the link between the two remains unknown. They also noted their surprise that autism spectrum disorder risk appears to be increased in children with modestly severe forms of CHD, stating that this finding required additional investigation.
“Despite the strengths of this study,” they wrote, “it raises more questions than answers.”
The study was funded by the Congressional Directed Medical Research Programs Autism Research Award. The authors reported no conflicts of interest.
SOURCE: Sigmon ER at al. Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2018-4114.
FROM PEDIATRICS
Key clinical point: Children born with congenital heart disease have higher odds of developing autism, especially with certain forms of CHD, such as atrial and ventricular septal defects.
Major finding: After sensitivity analysis, children with congenital heart disease had increased odds of autism, compared with controls (odds ratio, 1.32; 95% confidence interval, 1.10-1.59).
Study details: A case-control study of children enrolled in the U.S. Military Health System from 2001 to 2013.
Disclosures: The study was funded by the Congressional Directed Medical Research Programs Autism Research Award. The authors reported no conflicts of interest.
Source: Sigmon ER at al. Pediatrics. 2019 Oct 10. doi: 10.1542/peds.2018-4114.
Appeals court to hear prescription drug privacy rights case
An appeals court may soon decide whether federal authorities can access a patient’s medical records through a statewide prescription drug monitoring database without a warrant or if such searches infringe upon privacy rights.
On Oct. 10, the 1st U.S. Circuit Court of Appeals will hear arguments in U.S. Department of Justice v. Jonas, which centers on an investigatory subpoena issued by the U.S. Drug Enforcement Agency in 2018 that sought records about a certain patient from New Hampshire’s Prescription Drug Monitoring Program (PDMP). The subpoena’s recipient, PDMP program manager Michelle Ricco-Jonas, refused to comply, citing state law that prohibits law enforcement from accessing the database without a court order based on probable cause. Releasing PDMP records to authorities without cause violate patients’ privacy protections under the Fourth Amendment, Ms. Jonas and the New Hampshire Attorney General’s Office argued.
The U.S. Department of Justice sued to enforce the subpoena, contending that the DEA’s authority to investigate suspected criminal drug activity preempts New Hampshire’s law under the Supremacy Clause. In November 2018, U.S. Magistrate Judge Andrea K. Johnstone agreed with the DOJ and recommended the district judge grant the government’s motion to enforce the subpoena, a decision affirmed by the U.S. District Court for the District of New Hampshire in January 2019. The court ruled the government met its burden to satisfy the modest requirements for enforcement of the subpoena. Attorneys for New Hampshire appealed to the 1st Circuit.
The case is being closely watched by states, physician groups, pharmacies, and patient advocacy groups. In a joint court brief by the New Hampshire Medical Society and the American Civil Liberties Union, the organizations urged the appeals court to protect patient privacy by finding in favor of New Hampshire.
“The DEA has sought to enforce a subpoena that both injures Jonas and threatens to invade the Fourth Amendment privacy rights of individuals whose private medical information resides in the database, and whose privacy and confidentiality Jonas is statutorily charged with protecting. ... but who have no ability to challenge that impending harm,” the groups wrote in the brief. “The prescription records at issue in this case reveal intimate, private, and potentially stigmatizing details about patients’ health, including details of those patients’ underlying medical conditions. For that reason, as with other medical records, people have a reasonable expectation of privacy in them.”
Oregon’s PDMP faced a similar legal challenge in 2012. In that case, the DEA attempted to access the records of one patient and two prescribing physicians from Oregon’s prescription database. The state sued to prevent release of the records, and the ACLU intervened as a plaintiff on behalf of several unnamed patients and a doctor. A district court ruled in favor of the plaintiffs, finding that the DEA’s actions constituted a Fourth Amendment violation. However, the 9th U.S. Circuit Court in 2017 overturned the decision, ruling that federal law regarding administrative subpoenas trumps Oregon law. In addition, the appeals court ruled that the ACLU lacked standing to intervene and seek relief different from that sought by Oregon. By invalidating ACLU from the suit, the Fourth Amendment argument was never resolved by the 9th Circuit.
New Hampshire is one of 49 states that have Prescription Drug Monitoring Programs in addition to the District of Columbia. The programs collect, monitor, and analyze electronically transmitted prescribing and dispensing data submitted by physicians and pharmacies with the aim of reducing prescription drug abuse and diversion.
An appeals court may soon decide whether federal authorities can access a patient’s medical records through a statewide prescription drug monitoring database without a warrant or if such searches infringe upon privacy rights.
On Oct. 10, the 1st U.S. Circuit Court of Appeals will hear arguments in U.S. Department of Justice v. Jonas, which centers on an investigatory subpoena issued by the U.S. Drug Enforcement Agency in 2018 that sought records about a certain patient from New Hampshire’s Prescription Drug Monitoring Program (PDMP). The subpoena’s recipient, PDMP program manager Michelle Ricco-Jonas, refused to comply, citing state law that prohibits law enforcement from accessing the database without a court order based on probable cause. Releasing PDMP records to authorities without cause violate patients’ privacy protections under the Fourth Amendment, Ms. Jonas and the New Hampshire Attorney General’s Office argued.
The U.S. Department of Justice sued to enforce the subpoena, contending that the DEA’s authority to investigate suspected criminal drug activity preempts New Hampshire’s law under the Supremacy Clause. In November 2018, U.S. Magistrate Judge Andrea K. Johnstone agreed with the DOJ and recommended the district judge grant the government’s motion to enforce the subpoena, a decision affirmed by the U.S. District Court for the District of New Hampshire in January 2019. The court ruled the government met its burden to satisfy the modest requirements for enforcement of the subpoena. Attorneys for New Hampshire appealed to the 1st Circuit.
The case is being closely watched by states, physician groups, pharmacies, and patient advocacy groups. In a joint court brief by the New Hampshire Medical Society and the American Civil Liberties Union, the organizations urged the appeals court to protect patient privacy by finding in favor of New Hampshire.
“The DEA has sought to enforce a subpoena that both injures Jonas and threatens to invade the Fourth Amendment privacy rights of individuals whose private medical information resides in the database, and whose privacy and confidentiality Jonas is statutorily charged with protecting. ... but who have no ability to challenge that impending harm,” the groups wrote in the brief. “The prescription records at issue in this case reveal intimate, private, and potentially stigmatizing details about patients’ health, including details of those patients’ underlying medical conditions. For that reason, as with other medical records, people have a reasonable expectation of privacy in them.”
Oregon’s PDMP faced a similar legal challenge in 2012. In that case, the DEA attempted to access the records of one patient and two prescribing physicians from Oregon’s prescription database. The state sued to prevent release of the records, and the ACLU intervened as a plaintiff on behalf of several unnamed patients and a doctor. A district court ruled in favor of the plaintiffs, finding that the DEA’s actions constituted a Fourth Amendment violation. However, the 9th U.S. Circuit Court in 2017 overturned the decision, ruling that federal law regarding administrative subpoenas trumps Oregon law. In addition, the appeals court ruled that the ACLU lacked standing to intervene and seek relief different from that sought by Oregon. By invalidating ACLU from the suit, the Fourth Amendment argument was never resolved by the 9th Circuit.
New Hampshire is one of 49 states that have Prescription Drug Monitoring Programs in addition to the District of Columbia. The programs collect, monitor, and analyze electronically transmitted prescribing and dispensing data submitted by physicians and pharmacies with the aim of reducing prescription drug abuse and diversion.
An appeals court may soon decide whether federal authorities can access a patient’s medical records through a statewide prescription drug monitoring database without a warrant or if such searches infringe upon privacy rights.
On Oct. 10, the 1st U.S. Circuit Court of Appeals will hear arguments in U.S. Department of Justice v. Jonas, which centers on an investigatory subpoena issued by the U.S. Drug Enforcement Agency in 2018 that sought records about a certain patient from New Hampshire’s Prescription Drug Monitoring Program (PDMP). The subpoena’s recipient, PDMP program manager Michelle Ricco-Jonas, refused to comply, citing state law that prohibits law enforcement from accessing the database without a court order based on probable cause. Releasing PDMP records to authorities without cause violate patients’ privacy protections under the Fourth Amendment, Ms. Jonas and the New Hampshire Attorney General’s Office argued.
The U.S. Department of Justice sued to enforce the subpoena, contending that the DEA’s authority to investigate suspected criminal drug activity preempts New Hampshire’s law under the Supremacy Clause. In November 2018, U.S. Magistrate Judge Andrea K. Johnstone agreed with the DOJ and recommended the district judge grant the government’s motion to enforce the subpoena, a decision affirmed by the U.S. District Court for the District of New Hampshire in January 2019. The court ruled the government met its burden to satisfy the modest requirements for enforcement of the subpoena. Attorneys for New Hampshire appealed to the 1st Circuit.
The case is being closely watched by states, physician groups, pharmacies, and patient advocacy groups. In a joint court brief by the New Hampshire Medical Society and the American Civil Liberties Union, the organizations urged the appeals court to protect patient privacy by finding in favor of New Hampshire.
“The DEA has sought to enforce a subpoena that both injures Jonas and threatens to invade the Fourth Amendment privacy rights of individuals whose private medical information resides in the database, and whose privacy and confidentiality Jonas is statutorily charged with protecting. ... but who have no ability to challenge that impending harm,” the groups wrote in the brief. “The prescription records at issue in this case reveal intimate, private, and potentially stigmatizing details about patients’ health, including details of those patients’ underlying medical conditions. For that reason, as with other medical records, people have a reasonable expectation of privacy in them.”
Oregon’s PDMP faced a similar legal challenge in 2012. In that case, the DEA attempted to access the records of one patient and two prescribing physicians from Oregon’s prescription database. The state sued to prevent release of the records, and the ACLU intervened as a plaintiff on behalf of several unnamed patients and a doctor. A district court ruled in favor of the plaintiffs, finding that the DEA’s actions constituted a Fourth Amendment violation. However, the 9th U.S. Circuit Court in 2017 overturned the decision, ruling that federal law regarding administrative subpoenas trumps Oregon law. In addition, the appeals court ruled that the ACLU lacked standing to intervene and seek relief different from that sought by Oregon. By invalidating ACLU from the suit, the Fourth Amendment argument was never resolved by the 9th Circuit.
New Hampshire is one of 49 states that have Prescription Drug Monitoring Programs in addition to the District of Columbia. The programs collect, monitor, and analyze electronically transmitted prescribing and dispensing data submitted by physicians and pharmacies with the aim of reducing prescription drug abuse and diversion.
Influenza vaccination modestly reduces risk of hospitalizations in patients with COPD
(COPD), according to data published in the Journal of Infectious Diseases.
“To the best of our knowledge, this is the first large, real-world population study to examine vaccine effectiveness in people with COPD using the test-negative design and influenza-specific study outcomes,” wrote Andrea S. Gershon, MD, of Sunnybrook Health Sciences Center in Toronto and colleagues. “These findings emphasize the need for more effective influenza vaccines for older COPD patients and other preventive strategies.”
A test-negative study design
Data suggest that 70% of COPD exacerbations are caused by infection, and influenza often is identified as the cause. Although all major COPD practice guidelines recommend seasonal influenza vaccination, the evidence indicating that vaccination reduces hospitalizations and death is limited. The inherent or corticosteroid-induced decrease in immune response to vaccination and respiratory infection among patients with COPD may reduce the effectiveness of influenza vaccination, wrote Dr. Gershon and colleagues.
The investigators used a test-negative design to evaluate how effectively influenza vaccination prevents laboratory-confirmed influenza–associated hospitalizations in community-dwelling older patients with COPD. They chose this design because it attenuates biases resulting from misclassification of infection and from differences in health care–seeking behavior between vaccinated and unvaccinated patients.
Dr. Gershon and colleagues examined health care administrative data and respiratory specimens collected from patients who had been tested for influenza during the 2010-2011 to 2015-2016 influenza seasons. Eligible patients were aged 66 years or older, had physician-diagnosed COPD, and had been tested for influenza within 3 days before and during an acute care hospitalization. The researchers determined influenza vaccination status using physician and pharmacist billing claims. They obtained demographic information through linkage with the provincial health insurance database. Multivariable logistic regression allowed Dr. Gershon and colleagues to estimate the adjusted odds ratio of influenza vaccination in people with laboratory-confirmed influenza, compared with those without.
Effectiveness did not vary by demographic factors
The investigators included 21,748 patients in their analysis. Of this population, 3,636 (16.7%) patients tested positive for influenza. Vaccinated patients were less likely than unvaccinated patients to test positive for influenza (15.3% vs. 18.6%). Vaccinated patients also were more likely to be older; live in an urban area; live in a higher income neighborhood; have had more outpatient visits with a physician in the previous year; have received a prescription for a COPD medication in the previous 6 months; have diabetes, asthma, or immunocompromising conditions; have a longer duration of COPD; and have had an outpatient COPD exacerbation in the previous year.
The overall unadjusted estimate of vaccine effectiveness against laboratory-confirmed influenza–associated hospitalizations was 21%. Multivariable adjustment yielded an effectiveness of 22%. When Dr. Gershon and colleagues corrected for misclassification of vaccination status among people with COPD, the effectiveness was estimated to be 43%. Vaccine effectiveness did not vary significantly according to influenza season, nor did it vary significantly by patient-specific factors such as age, sex, influenza subtype, codiagnosis of asthma, duration of COPD, previous outpatient COPD exacerbations, previous COPD hospitalization, previous receipt of inhaled corticosteroids, and previous pneumonia.
One limitation of the study was the possibility that COPD was misclassified because not all participants underwent pulmonary function testing. In addition, the estimates of vaccine effectiveness in the present study are specific to the outcome of influenza hospitalization and may not be generalizable to vaccine effectiveness estimates of outpatient outcomes, said the investigators. Finally, Dr. Gershon and colleagues could not identify the type of vaccine received.
“Given that a large pragmatic randomized controlled trial evaluating influenza vaccination would be unethical, this is likely the most robust estimate of vaccine effectiveness for hospitalizations in the COPD population to guide influenza vaccine recommendations for patients with COPD,” wrote Dr. Gershon and colleagues.
An Ontario Ministry of Health and Long-Term Care Health Systems Research Fund Capacity Grant and a Canadian Institutes of Health Research operating grant funded this research. One investigator received grants from the Canadian Institutes of Health Research during the study, and others received grants from pharmaceutical companies that were unrelated to this study.
SOURCE: Gershon AS et al. J Infect Dis. 2019 Sep 24. doi: 10.1093/infdis/jiz419.
(COPD), according to data published in the Journal of Infectious Diseases.
“To the best of our knowledge, this is the first large, real-world population study to examine vaccine effectiveness in people with COPD using the test-negative design and influenza-specific study outcomes,” wrote Andrea S. Gershon, MD, of Sunnybrook Health Sciences Center in Toronto and colleagues. “These findings emphasize the need for more effective influenza vaccines for older COPD patients and other preventive strategies.”
A test-negative study design
Data suggest that 70% of COPD exacerbations are caused by infection, and influenza often is identified as the cause. Although all major COPD practice guidelines recommend seasonal influenza vaccination, the evidence indicating that vaccination reduces hospitalizations and death is limited. The inherent or corticosteroid-induced decrease in immune response to vaccination and respiratory infection among patients with COPD may reduce the effectiveness of influenza vaccination, wrote Dr. Gershon and colleagues.
The investigators used a test-negative design to evaluate how effectively influenza vaccination prevents laboratory-confirmed influenza–associated hospitalizations in community-dwelling older patients with COPD. They chose this design because it attenuates biases resulting from misclassification of infection and from differences in health care–seeking behavior between vaccinated and unvaccinated patients.
Dr. Gershon and colleagues examined health care administrative data and respiratory specimens collected from patients who had been tested for influenza during the 2010-2011 to 2015-2016 influenza seasons. Eligible patients were aged 66 years or older, had physician-diagnosed COPD, and had been tested for influenza within 3 days before and during an acute care hospitalization. The researchers determined influenza vaccination status using physician and pharmacist billing claims. They obtained demographic information through linkage with the provincial health insurance database. Multivariable logistic regression allowed Dr. Gershon and colleagues to estimate the adjusted odds ratio of influenza vaccination in people with laboratory-confirmed influenza, compared with those without.
Effectiveness did not vary by demographic factors
The investigators included 21,748 patients in their analysis. Of this population, 3,636 (16.7%) patients tested positive for influenza. Vaccinated patients were less likely than unvaccinated patients to test positive for influenza (15.3% vs. 18.6%). Vaccinated patients also were more likely to be older; live in an urban area; live in a higher income neighborhood; have had more outpatient visits with a physician in the previous year; have received a prescription for a COPD medication in the previous 6 months; have diabetes, asthma, or immunocompromising conditions; have a longer duration of COPD; and have had an outpatient COPD exacerbation in the previous year.
The overall unadjusted estimate of vaccine effectiveness against laboratory-confirmed influenza–associated hospitalizations was 21%. Multivariable adjustment yielded an effectiveness of 22%. When Dr. Gershon and colleagues corrected for misclassification of vaccination status among people with COPD, the effectiveness was estimated to be 43%. Vaccine effectiveness did not vary significantly according to influenza season, nor did it vary significantly by patient-specific factors such as age, sex, influenza subtype, codiagnosis of asthma, duration of COPD, previous outpatient COPD exacerbations, previous COPD hospitalization, previous receipt of inhaled corticosteroids, and previous pneumonia.
One limitation of the study was the possibility that COPD was misclassified because not all participants underwent pulmonary function testing. In addition, the estimates of vaccine effectiveness in the present study are specific to the outcome of influenza hospitalization and may not be generalizable to vaccine effectiveness estimates of outpatient outcomes, said the investigators. Finally, Dr. Gershon and colleagues could not identify the type of vaccine received.
“Given that a large pragmatic randomized controlled trial evaluating influenza vaccination would be unethical, this is likely the most robust estimate of vaccine effectiveness for hospitalizations in the COPD population to guide influenza vaccine recommendations for patients with COPD,” wrote Dr. Gershon and colleagues.
An Ontario Ministry of Health and Long-Term Care Health Systems Research Fund Capacity Grant and a Canadian Institutes of Health Research operating grant funded this research. One investigator received grants from the Canadian Institutes of Health Research during the study, and others received grants from pharmaceutical companies that were unrelated to this study.
SOURCE: Gershon AS et al. J Infect Dis. 2019 Sep 24. doi: 10.1093/infdis/jiz419.
(COPD), according to data published in the Journal of Infectious Diseases.
“To the best of our knowledge, this is the first large, real-world population study to examine vaccine effectiveness in people with COPD using the test-negative design and influenza-specific study outcomes,” wrote Andrea S. Gershon, MD, of Sunnybrook Health Sciences Center in Toronto and colleagues. “These findings emphasize the need for more effective influenza vaccines for older COPD patients and other preventive strategies.”
A test-negative study design
Data suggest that 70% of COPD exacerbations are caused by infection, and influenza often is identified as the cause. Although all major COPD practice guidelines recommend seasonal influenza vaccination, the evidence indicating that vaccination reduces hospitalizations and death is limited. The inherent or corticosteroid-induced decrease in immune response to vaccination and respiratory infection among patients with COPD may reduce the effectiveness of influenza vaccination, wrote Dr. Gershon and colleagues.
The investigators used a test-negative design to evaluate how effectively influenza vaccination prevents laboratory-confirmed influenza–associated hospitalizations in community-dwelling older patients with COPD. They chose this design because it attenuates biases resulting from misclassification of infection and from differences in health care–seeking behavior between vaccinated and unvaccinated patients.
Dr. Gershon and colleagues examined health care administrative data and respiratory specimens collected from patients who had been tested for influenza during the 2010-2011 to 2015-2016 influenza seasons. Eligible patients were aged 66 years or older, had physician-diagnosed COPD, and had been tested for influenza within 3 days before and during an acute care hospitalization. The researchers determined influenza vaccination status using physician and pharmacist billing claims. They obtained demographic information through linkage with the provincial health insurance database. Multivariable logistic regression allowed Dr. Gershon and colleagues to estimate the adjusted odds ratio of influenza vaccination in people with laboratory-confirmed influenza, compared with those without.
Effectiveness did not vary by demographic factors
The investigators included 21,748 patients in their analysis. Of this population, 3,636 (16.7%) patients tested positive for influenza. Vaccinated patients were less likely than unvaccinated patients to test positive for influenza (15.3% vs. 18.6%). Vaccinated patients also were more likely to be older; live in an urban area; live in a higher income neighborhood; have had more outpatient visits with a physician in the previous year; have received a prescription for a COPD medication in the previous 6 months; have diabetes, asthma, or immunocompromising conditions; have a longer duration of COPD; and have had an outpatient COPD exacerbation in the previous year.
The overall unadjusted estimate of vaccine effectiveness against laboratory-confirmed influenza–associated hospitalizations was 21%. Multivariable adjustment yielded an effectiveness of 22%. When Dr. Gershon and colleagues corrected for misclassification of vaccination status among people with COPD, the effectiveness was estimated to be 43%. Vaccine effectiveness did not vary significantly according to influenza season, nor did it vary significantly by patient-specific factors such as age, sex, influenza subtype, codiagnosis of asthma, duration of COPD, previous outpatient COPD exacerbations, previous COPD hospitalization, previous receipt of inhaled corticosteroids, and previous pneumonia.
One limitation of the study was the possibility that COPD was misclassified because not all participants underwent pulmonary function testing. In addition, the estimates of vaccine effectiveness in the present study are specific to the outcome of influenza hospitalization and may not be generalizable to vaccine effectiveness estimates of outpatient outcomes, said the investigators. Finally, Dr. Gershon and colleagues could not identify the type of vaccine received.
“Given that a large pragmatic randomized controlled trial evaluating influenza vaccination would be unethical, this is likely the most robust estimate of vaccine effectiveness for hospitalizations in the COPD population to guide influenza vaccine recommendations for patients with COPD,” wrote Dr. Gershon and colleagues.
An Ontario Ministry of Health and Long-Term Care Health Systems Research Fund Capacity Grant and a Canadian Institutes of Health Research operating grant funded this research. One investigator received grants from the Canadian Institutes of Health Research during the study, and others received grants from pharmaceutical companies that were unrelated to this study.
SOURCE: Gershon AS et al. J Infect Dis. 2019 Sep 24. doi: 10.1093/infdis/jiz419.
FROM JOURNAL OF INFECTIOUS DISEASES
Printed meat in space and diesel-pattern baldness
If at first you don’t succeed …
Dozens of new drugs are approved every year, but for every promising new medication, there are far more that fail to get past the clinical trial stage. The causes can vary: The drug didn’t do enough, it caused too many adverse events, and so on.
DNA topoisomerase inhibitors have been extensively researched as an anticancer agent, but many examples failed their clinical trials. These molecules are flat and made up of neatly stacked columns of electrically conductive rings, and operate by inserting themselves into DNA to stop replication.
That molecular structure is what interested a team of researchers from the University of Illinois at Urbana-Champaign. In research published in Nature Communications, the researchers noted that DNA topoisomerase inhibitors are structured much like organic semiconductors, but with a bonus. Those columns that make up the molecule are linked by hydrogen bonds, which allow bridges to form across the entire molecule, transforming the entire thing into a semiconductor.
That property, along with their easy printability and their high-specificity interactions with biological material, make the inhibitors an excellent candidate for use in biosensors or biotransistors.
A word to the manufacturer of those topoisomerase inhibitors, though, now that we’ve gotten you all excited again: We doubt you’ll be able to charge as much for the molecule. Semiconductors are hardly as glamorous as treating cancer.
Houston, we have a cultivated meat product
The Delmonico brothers didn’t do it. Ronald McDonald didn’t do it. Neil Armstrong never even tried. Same goes for Julia Child.
None of these culinary giants or astronauts ever grew beef in space. That honor belongs to Aleph Farms of Rehovot, Israel. The company, a self-proclaimed “global leader in the cultivated meat industry,” collaborated with 3D Bioprinting Solutions of Moscow and others to produce slaughter-free beef in the Russian segment of the International Space Station in September.
The actual process of growing a steak mimics natural “muscle-tissue regeneration occurring inside the cow’s body” and somehow uses a 3-D bioprinter to assemble “a small-scale muscle tissue.” We don’t really understand it, but the scientists and engineers here at LOTME Co. assure us it makes sense.
But why, you may ask, did they do it on the space station? Think of it as a stand-in for New York City. You know … if you can make it there, you can make it anywhere. We’ll let Didier Toubia, cofounder and CEO of Aleph Farms, explain: “In space, we don’t have 10,000 or 15,000 liter (3962.58 gallon) of water available to produce 1 kilogram (2.205 pounds) of beef.” It’s all about sustainable food production.
The next phase of the project, we think, is going to be even more interesting. For a proper fine dining experience in space, they’re going to grow a snooty French waiter to serve cultivated steak to the astronauts.
Pollution Hair Club for Men
By now, most people are well aware that air pollution is linked with cancer. And chronic obstructive pulmonary disease. And asthma. And cardiovascular disease. And Germanic automotive emissions system cheating. Yawn.
But there’s a newly revealed association sure to make even the most jaded health news consumer’s hair stand on end: Exposure to pollution’s particulate matter is linked to ... hair loss.
Researchers from the Future Science Research Center in South Korea exposed cells from the base of human hair follicles to assorted concentrations of diesel and dust particulates. After 24 hours, the pollutants had suppressed production of beta-catenin, the primary protein crucial to the maintenance of George Clooney’s luxurious mane. And three other proteins supporting hair growth and hair retention also went AWOL in the presence of pollutants. Plus more particulate matter meant fewer hair-restoring proteins.
Diesel particulates and baldness? It all makes so much more sense now. Given the pollutant-rich Big Apple air in which lollipop-loving TV detective Theo Kojak’s smooth pate was steeped, is it any wonder he was famously so follicularly challenged? The Clean Air Act – who loves ya, baby?
If at first you don’t succeed …
Dozens of new drugs are approved every year, but for every promising new medication, there are far more that fail to get past the clinical trial stage. The causes can vary: The drug didn’t do enough, it caused too many adverse events, and so on.
DNA topoisomerase inhibitors have been extensively researched as an anticancer agent, but many examples failed their clinical trials. These molecules are flat and made up of neatly stacked columns of electrically conductive rings, and operate by inserting themselves into DNA to stop replication.
That molecular structure is what interested a team of researchers from the University of Illinois at Urbana-Champaign. In research published in Nature Communications, the researchers noted that DNA topoisomerase inhibitors are structured much like organic semiconductors, but with a bonus. Those columns that make up the molecule are linked by hydrogen bonds, which allow bridges to form across the entire molecule, transforming the entire thing into a semiconductor.
That property, along with their easy printability and their high-specificity interactions with biological material, make the inhibitors an excellent candidate for use in biosensors or biotransistors.
A word to the manufacturer of those topoisomerase inhibitors, though, now that we’ve gotten you all excited again: We doubt you’ll be able to charge as much for the molecule. Semiconductors are hardly as glamorous as treating cancer.
Houston, we have a cultivated meat product
The Delmonico brothers didn’t do it. Ronald McDonald didn’t do it. Neil Armstrong never even tried. Same goes for Julia Child.
None of these culinary giants or astronauts ever grew beef in space. That honor belongs to Aleph Farms of Rehovot, Israel. The company, a self-proclaimed “global leader in the cultivated meat industry,” collaborated with 3D Bioprinting Solutions of Moscow and others to produce slaughter-free beef in the Russian segment of the International Space Station in September.
The actual process of growing a steak mimics natural “muscle-tissue regeneration occurring inside the cow’s body” and somehow uses a 3-D bioprinter to assemble “a small-scale muscle tissue.” We don’t really understand it, but the scientists and engineers here at LOTME Co. assure us it makes sense.
But why, you may ask, did they do it on the space station? Think of it as a stand-in for New York City. You know … if you can make it there, you can make it anywhere. We’ll let Didier Toubia, cofounder and CEO of Aleph Farms, explain: “In space, we don’t have 10,000 or 15,000 liter (3962.58 gallon) of water available to produce 1 kilogram (2.205 pounds) of beef.” It’s all about sustainable food production.
The next phase of the project, we think, is going to be even more interesting. For a proper fine dining experience in space, they’re going to grow a snooty French waiter to serve cultivated steak to the astronauts.
Pollution Hair Club for Men
By now, most people are well aware that air pollution is linked with cancer. And chronic obstructive pulmonary disease. And asthma. And cardiovascular disease. And Germanic automotive emissions system cheating. Yawn.
But there’s a newly revealed association sure to make even the most jaded health news consumer’s hair stand on end: Exposure to pollution’s particulate matter is linked to ... hair loss.
Researchers from the Future Science Research Center in South Korea exposed cells from the base of human hair follicles to assorted concentrations of diesel and dust particulates. After 24 hours, the pollutants had suppressed production of beta-catenin, the primary protein crucial to the maintenance of George Clooney’s luxurious mane. And three other proteins supporting hair growth and hair retention also went AWOL in the presence of pollutants. Plus more particulate matter meant fewer hair-restoring proteins.
Diesel particulates and baldness? It all makes so much more sense now. Given the pollutant-rich Big Apple air in which lollipop-loving TV detective Theo Kojak’s smooth pate was steeped, is it any wonder he was famously so follicularly challenged? The Clean Air Act – who loves ya, baby?
If at first you don’t succeed …
Dozens of new drugs are approved every year, but for every promising new medication, there are far more that fail to get past the clinical trial stage. The causes can vary: The drug didn’t do enough, it caused too many adverse events, and so on.
DNA topoisomerase inhibitors have been extensively researched as an anticancer agent, but many examples failed their clinical trials. These molecules are flat and made up of neatly stacked columns of electrically conductive rings, and operate by inserting themselves into DNA to stop replication.
That molecular structure is what interested a team of researchers from the University of Illinois at Urbana-Champaign. In research published in Nature Communications, the researchers noted that DNA topoisomerase inhibitors are structured much like organic semiconductors, but with a bonus. Those columns that make up the molecule are linked by hydrogen bonds, which allow bridges to form across the entire molecule, transforming the entire thing into a semiconductor.
That property, along with their easy printability and their high-specificity interactions with biological material, make the inhibitors an excellent candidate for use in biosensors or biotransistors.
A word to the manufacturer of those topoisomerase inhibitors, though, now that we’ve gotten you all excited again: We doubt you’ll be able to charge as much for the molecule. Semiconductors are hardly as glamorous as treating cancer.
Houston, we have a cultivated meat product
The Delmonico brothers didn’t do it. Ronald McDonald didn’t do it. Neil Armstrong never even tried. Same goes for Julia Child.
None of these culinary giants or astronauts ever grew beef in space. That honor belongs to Aleph Farms of Rehovot, Israel. The company, a self-proclaimed “global leader in the cultivated meat industry,” collaborated with 3D Bioprinting Solutions of Moscow and others to produce slaughter-free beef in the Russian segment of the International Space Station in September.
The actual process of growing a steak mimics natural “muscle-tissue regeneration occurring inside the cow’s body” and somehow uses a 3-D bioprinter to assemble “a small-scale muscle tissue.” We don’t really understand it, but the scientists and engineers here at LOTME Co. assure us it makes sense.
But why, you may ask, did they do it on the space station? Think of it as a stand-in for New York City. You know … if you can make it there, you can make it anywhere. We’ll let Didier Toubia, cofounder and CEO of Aleph Farms, explain: “In space, we don’t have 10,000 or 15,000 liter (3962.58 gallon) of water available to produce 1 kilogram (2.205 pounds) of beef.” It’s all about sustainable food production.
The next phase of the project, we think, is going to be even more interesting. For a proper fine dining experience in space, they’re going to grow a snooty French waiter to serve cultivated steak to the astronauts.
Pollution Hair Club for Men
By now, most people are well aware that air pollution is linked with cancer. And chronic obstructive pulmonary disease. And asthma. And cardiovascular disease. And Germanic automotive emissions system cheating. Yawn.
But there’s a newly revealed association sure to make even the most jaded health news consumer’s hair stand on end: Exposure to pollution’s particulate matter is linked to ... hair loss.
Researchers from the Future Science Research Center in South Korea exposed cells from the base of human hair follicles to assorted concentrations of diesel and dust particulates. After 24 hours, the pollutants had suppressed production of beta-catenin, the primary protein crucial to the maintenance of George Clooney’s luxurious mane. And three other proteins supporting hair growth and hair retention also went AWOL in the presence of pollutants. Plus more particulate matter meant fewer hair-restoring proteins.
Diesel particulates and baldness? It all makes so much more sense now. Given the pollutant-rich Big Apple air in which lollipop-loving TV detective Theo Kojak’s smooth pate was steeped, is it any wonder he was famously so follicularly challenged? The Clean Air Act – who loves ya, baby?
Faster enteral feeding does not up adverse outcomes risk in preterm infants
including moderate or severe neurodevelopmental disability and necrotizing enterocolitis, according to recent research published in the New England Journal of Medicine.
Although some data have shown rapidly increasing the speed of enteral-feeding volumes for preterm infants can raise the risk of necrotizing enterocolitis, these data are from observational case-control and uncontrolled studies, said Jon Dorling, MD, of the division of neonatal–perinatal medicine at Dalhousie University in Halifax, N.S., and colleagues in their study.
Dr. Doring and colleagues randomized 2,804 infants who were either very preterm or with a very low birth weight to receive daily milk increments at different volumes until the infants reached full feeding volume. Infants in the faster-increment group received daily milk at 30 mL per kg of body weight, while the slower-increment group received 18 mL per kg of body weight each day. The researchers analyzed infant survival without moderate or severe neurodevelopmental disability, with secondary outcomes of sepsis, necrotizing enterocolitis, and cerebral palsy at 24 months.
Overall, the researchers had information on the primary outcome for 87.4% of infants in the faster-increment group and 88.7% of infants in the slower-increment group. They found that 65.5% of infants in the faster-increment group and 68.1% of infants in the slower-increment group achieved an outcome of survival without moderate or severe neurodevelopmental disability at 24 months (adjusted risk ratio, 0.96; 95% confidence interval, 0.92-1.01; P equals .16). Secondary outcomes showed similar rates of adverse outcomes in the two groups, with 29.8% of infants in the faster-increment group and 31.1% of infants in the slower-increment group developing late-onset sepsis (aRR, 0.96; 95% CI, 0.86-1.07). Infants in the faster-increment group also had a similar rate of necrotizing enterocolitis (5.0%), compared with infants in the slower-increment group (5.6%) (aRR, 0.88; 95% CI, 0.68-1.16). Motor impairment was higher among infants in the faster-increment group (7.5%), compared with the slow-increment group (5.0%).
In the faster-increment group, the median number of days to reach full milk-feeding volumes was 7 vs. 10 in the slower-increment group.
“Although these feeding outcomes seem to favor faster increments, the risk of moderate or severe motor impairment was unexpectedly higher in the faster-increment group than in the slower-increment group,” the researchers said. “This observation is unexplained, and there were not more cases of late-onset sepsis or necrotizing enterocolitis in the faster-increment group.”
It is possible that it is a chance finding, since it was one of multiple secondary outcomes assessed, but biologically plausible explanations include increased cardiorespiratory events from pressure on the diaphragm or inability to absorb enteral nutrition,” they added.
The researchers said one potential limitation of the study was that it was unblinded.
This study was funded by the Health Technology Assessment Programme of the National Institute for Health Research. The authors reported various relationships with Baxter Bioscience, Chiesi Farmaceutici, Danone Early Life Nutrition, Fresenius Kabi USA LLC, National Institute for Health Research, Nestle Nutrition Institute, Nutrina, Medical Research Council, and Prolacta Biosciences in the form of consultancies, grants, travel reimbursement, board memberships, and editorial board appointments.
SOURCE: Doring J et al. N Eng J Med. 2019. doi: 10.1056/NEJMoa1816654.
including moderate or severe neurodevelopmental disability and necrotizing enterocolitis, according to recent research published in the New England Journal of Medicine.
Although some data have shown rapidly increasing the speed of enteral-feeding volumes for preterm infants can raise the risk of necrotizing enterocolitis, these data are from observational case-control and uncontrolled studies, said Jon Dorling, MD, of the division of neonatal–perinatal medicine at Dalhousie University in Halifax, N.S., and colleagues in their study.
Dr. Doring and colleagues randomized 2,804 infants who were either very preterm or with a very low birth weight to receive daily milk increments at different volumes until the infants reached full feeding volume. Infants in the faster-increment group received daily milk at 30 mL per kg of body weight, while the slower-increment group received 18 mL per kg of body weight each day. The researchers analyzed infant survival without moderate or severe neurodevelopmental disability, with secondary outcomes of sepsis, necrotizing enterocolitis, and cerebral palsy at 24 months.
Overall, the researchers had information on the primary outcome for 87.4% of infants in the faster-increment group and 88.7% of infants in the slower-increment group. They found that 65.5% of infants in the faster-increment group and 68.1% of infants in the slower-increment group achieved an outcome of survival without moderate or severe neurodevelopmental disability at 24 months (adjusted risk ratio, 0.96; 95% confidence interval, 0.92-1.01; P equals .16). Secondary outcomes showed similar rates of adverse outcomes in the two groups, with 29.8% of infants in the faster-increment group and 31.1% of infants in the slower-increment group developing late-onset sepsis (aRR, 0.96; 95% CI, 0.86-1.07). Infants in the faster-increment group also had a similar rate of necrotizing enterocolitis (5.0%), compared with infants in the slower-increment group (5.6%) (aRR, 0.88; 95% CI, 0.68-1.16). Motor impairment was higher among infants in the faster-increment group (7.5%), compared with the slow-increment group (5.0%).
In the faster-increment group, the median number of days to reach full milk-feeding volumes was 7 vs. 10 in the slower-increment group.
“Although these feeding outcomes seem to favor faster increments, the risk of moderate or severe motor impairment was unexpectedly higher in the faster-increment group than in the slower-increment group,” the researchers said. “This observation is unexplained, and there were not more cases of late-onset sepsis or necrotizing enterocolitis in the faster-increment group.”
It is possible that it is a chance finding, since it was one of multiple secondary outcomes assessed, but biologically plausible explanations include increased cardiorespiratory events from pressure on the diaphragm or inability to absorb enteral nutrition,” they added.
The researchers said one potential limitation of the study was that it was unblinded.
This study was funded by the Health Technology Assessment Programme of the National Institute for Health Research. The authors reported various relationships with Baxter Bioscience, Chiesi Farmaceutici, Danone Early Life Nutrition, Fresenius Kabi USA LLC, National Institute for Health Research, Nestle Nutrition Institute, Nutrina, Medical Research Council, and Prolacta Biosciences in the form of consultancies, grants, travel reimbursement, board memberships, and editorial board appointments.
SOURCE: Doring J et al. N Eng J Med. 2019. doi: 10.1056/NEJMoa1816654.
including moderate or severe neurodevelopmental disability and necrotizing enterocolitis, according to recent research published in the New England Journal of Medicine.
Although some data have shown rapidly increasing the speed of enteral-feeding volumes for preterm infants can raise the risk of necrotizing enterocolitis, these data are from observational case-control and uncontrolled studies, said Jon Dorling, MD, of the division of neonatal–perinatal medicine at Dalhousie University in Halifax, N.S., and colleagues in their study.
Dr. Doring and colleagues randomized 2,804 infants who were either very preterm or with a very low birth weight to receive daily milk increments at different volumes until the infants reached full feeding volume. Infants in the faster-increment group received daily milk at 30 mL per kg of body weight, while the slower-increment group received 18 mL per kg of body weight each day. The researchers analyzed infant survival without moderate or severe neurodevelopmental disability, with secondary outcomes of sepsis, necrotizing enterocolitis, and cerebral palsy at 24 months.
Overall, the researchers had information on the primary outcome for 87.4% of infants in the faster-increment group and 88.7% of infants in the slower-increment group. They found that 65.5% of infants in the faster-increment group and 68.1% of infants in the slower-increment group achieved an outcome of survival without moderate or severe neurodevelopmental disability at 24 months (adjusted risk ratio, 0.96; 95% confidence interval, 0.92-1.01; P equals .16). Secondary outcomes showed similar rates of adverse outcomes in the two groups, with 29.8% of infants in the faster-increment group and 31.1% of infants in the slower-increment group developing late-onset sepsis (aRR, 0.96; 95% CI, 0.86-1.07). Infants in the faster-increment group also had a similar rate of necrotizing enterocolitis (5.0%), compared with infants in the slower-increment group (5.6%) (aRR, 0.88; 95% CI, 0.68-1.16). Motor impairment was higher among infants in the faster-increment group (7.5%), compared with the slow-increment group (5.0%).
In the faster-increment group, the median number of days to reach full milk-feeding volumes was 7 vs. 10 in the slower-increment group.
“Although these feeding outcomes seem to favor faster increments, the risk of moderate or severe motor impairment was unexpectedly higher in the faster-increment group than in the slower-increment group,” the researchers said. “This observation is unexplained, and there were not more cases of late-onset sepsis or necrotizing enterocolitis in the faster-increment group.”
It is possible that it is a chance finding, since it was one of multiple secondary outcomes assessed, but biologically plausible explanations include increased cardiorespiratory events from pressure on the diaphragm or inability to absorb enteral nutrition,” they added.
The researchers said one potential limitation of the study was that it was unblinded.
This study was funded by the Health Technology Assessment Programme of the National Institute for Health Research. The authors reported various relationships with Baxter Bioscience, Chiesi Farmaceutici, Danone Early Life Nutrition, Fresenius Kabi USA LLC, National Institute for Health Research, Nestle Nutrition Institute, Nutrina, Medical Research Council, and Prolacta Biosciences in the form of consultancies, grants, travel reimbursement, board memberships, and editorial board appointments.
SOURCE: Doring J et al. N Eng J Med. 2019. doi: 10.1056/NEJMoa1816654.
FROM NEW ENGLAND JOURNAL OF MEDICINE
2019 dermMentors™ Resident of Distinction Award™
The dermMentors™ Resident of Distinction Award™ recognizes top residents in dermatology. DermMentors.org and the dermMentors™ Resident of Distinction Award™ are sponsored by Beiersdorf Inc and administered by DermEd, Inc. The 2019 dermMentors™ Residents of Distinction™ presented new scientific research during the general sessions of the 15th Annual Coastal Dermatology Symposium on October 5, 2019.
Overall Grand Prize
Chronic Inflammatory Skin Diseases Are Associated With Herpes Zoster in US Inpatients
Raj Chovatiya, MD, PhD; Jonathan I. Silverberg MD, PhD, MPH, Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois
Disclosures: None.
Background
Herpes zoster (HZ) is a vaccine-preventable, viral eruption that affects 1 of every 3 people in the United States in their lifetime. Despite evidence-based guidelines and public health advocacy, many high-risk individuals remain unvaccinated and at risk for significant morbidity from HZ. Patients with chronic inflammatory skin diseases (CISDs), including allergic and autoimmune conditions, have potential risk factors for HZ, including long-term use of systemic corticosteroids and immunosuppressants, and immune dysregulation in the skin and periphery. We sought to determine whether CISDs are associated with HZ in hospitalized patients in the US.
Methods
Data were analyzed from the 2002-2012 Nationwide Inpatient Sample, a representative 20% cross-sectional cohort of US hospitalizations (N=68,490,364 children and adults).
Results
The estimated incidence of primary hospitalization for HZ in the US population was 5.0 per 100,000 persons from 2002-2012. In multivariable weighted logistic regression models including age, sex, race/ethnicity, insurance, mean household income, and long-term systemic corticosteroid use, primary hospitalization for HZ was associated with multiple CISDs: atopic dermatitis (adjusted odds ratio [95% confidence interval]: 1.38 [1.14-1.68]), psoriasis (4.78 [2.83-8.08]), pemphigus (1.77 [1.01-3.12]), bullous pemphigoid (1.77 [1.01-3.12]), mycosis fungoides (3.79 [2.55-5.65]), dermatomyositis (7.31 [5.27-10.12]), systemic sclerosis (1.92 [1.47-2.53]), cutaneous lupus erythematosus (1.94 [1.10-3.44]), vitiligo (2.00 [1.04-3.85]), and sarcoidosis (1.52 [1.22-1.90]). Lichen planus (3.01 [1.36-6.67]), Sézary syndrome (12.14 [5.20-28.31]), morphea (2.74 [1.36-5.51]), and pyoderma gangrenosum (2.44 [1.16-5.13]) showed increased odds in bivariable models but not in multivariable models. Whereas, hidradenitis suppurativa, chronic idiopathic/spontaneous urticaria, and alopecia areata were not associated with HZ. Similar results were seen in sensitivity analyses among adults age <60 and <50 years. Significant predictors of primary hospitalization for HZ among patients with CISDs included older age, female sex, non-white race/ethnicity, decreasing number of chronic comorbid conditions, and long-term systemic corticosteroid use. Inpatient length of stay and/or inflation adjusted cost of care were significantly higher in inpatients with CISD with vs. without a primary diagnosis of HZ.
Conclusion
CISDs are associated with increased hospitalization for HZ, prolonged length of inpatient stay and increased cost of hospital care. The associations of CISDs and HZ were significant even below the recommended ages (<60 and <50 years) for vaccination with live and recombinant HZ vaccine, respectively. Additional studies are needed to confirm these findings and determine the mechanisms of VZV reactivation. Patients with CISDs constitute a significant, previously under-recognized group that is high-risk for hospitalization for HZ. Dermatologists are the primary physicians who manage the vulnerable population of CISDs. They should be on the forefront of screening, intervention, and most importantly, vaccination for their patients. Future studies should explore implementation of outpatient HZ vaccination by dermatologists and revised disease-specific guidelines to cover the spectrum of CISDs.
Up next: Buyer Beware or You Might Get Burned...→
Buyer Beware or You Might Get Burned: Unregulated Photosensitizing Agents Available Without Prescription From Major Online Retailers
Kimberly Huerth, MD, MEd, Department of Dermatology, Howard University Hospital, Washington, DC; Olivia Ware, BA, Howard University College of Medicine, Washington, DC; Ginette A. Okoye, MD, Department of Dermatology, Howard University Hospital, Washington, DC; Sharon Bridgeman-Shah, MD, Department of Dermatology, Howard University Hospital, Washington, DC
Disclosures: None.
Background
According to the World Health Organization, the vast majority of the world’s population relies on plant-derived medicine as their primary form of healthcare. Closer to home, these alternative therapies are growing in popularity among American healthcare consumers, in concert with increasing access to such products by means of globalization and the growth of Internet retail. There are numerous reasons why an individual might eschew Western medicine in favor of a more naturopathic approach, including perceived health benefits, cost, prior failure of physician guided treatment, a lack of awareness about the risks these products carry, cultural norms, and desperation born of the psychosocial burden of one’s disease.
A 73-year-old Eritrean man presented to our outpatient dermatology clinic with a severe, acute, phototoxic reaction following the ingestion of Athamanta decoction for the self-directed treatment of vitiligo. The genus Athamanta is in the Apiaceae family and consists of 6 species of flowering plants native to Europe and North Africa. Furanocoumarins are the phototoxic compounds present in these plants, among which psoralen is perhaps the most notorious. The desiccated Athamanta leaves, sought in the absence of medical consultation, had been obtained from a mail order vendor in Egypt which had advertised them as a “miracle effect for curing vitiligo.”
Objective
To determine the extent to which unregulated photosensitizing agents for the treatment of vitiligo are available to consumers from online retailers, including major ones such as Amazon and eBay.
Methods
An online product search of eBay and Amazon, as well as an Internet pharmacy by the name of Cheap Generic, was conducted using search terms “vitiligo treatment,” “psoralen for vitiligo,” “furanocoumarin treatment,” “Athamanta vitiligo,” “Apiaceae vitiligo,” and “leukoderma.” Products generated by the search terms were examined for their route of delivery (topical vs systemic), key photosensitizing ingredient, parent plant, cost to consumer, and country of manufacture. Products that did not identify a known photosensitizing compound, or a plant known to contain a photosensitizing compound, on the list of ingredients were excluded from the analysis.
Results
We identified a total of 11 products—6 listed on Amazon, 3 listed on eBay, and 2 listed by Cheap Generic pharmacy—that had either a photosensitizing compound, or a plant known to contain a photosensitizing compound, on its list of ingredients. 27.3% of products were available in formulations intended for ingestion, such as tablets or powders. 90.9% of products listed psoralen, or a plant known to contain psoralen, as a major ingredient. Of the products that identified the main photosensitizing plant ingredient (72.7%), Psoralea corylifolia was the most common (75%). Products ranged in cost from $3 to $28. The terms “herbal” (54.5%) and “natural” (36.3%) were used most frequently on the packaging and/or website for promotional purposes. While 45.4% of products did not identify a country of origin, of those that did, 66.7% were manufactured in India, while the remainder were prepared in China.
Conclusion
Topically and systemically administered psoralen-containing products for the treatment of vitiligo are widely available without a prescription from several online vendors. These products are affordable and utilize suggestive packaging to tout the benefits of the products, without mention of their risks.
Up next: Collagen VII: A Link Between the Skin and Bone Marrow...→
Collagen VII: A Link Between the Skin and Bone Marrow Via Extracellular Vesicles
Jeffrey D. McBride, MD, PhD, Department of Dermatology and Cutaneous Surgery, University of Miami, Miami, Florida
Disclosures: Dr. McBride has been a consultant with Aegle Therapeutics.
Extracellular vesicles—exosomes, microvesicles, and apoptotic bodies—are ubiquitous in human tissues, circulation, and body fluids. Of these vesicles, exosomes are of growing interest among investigators across multiple fields, including dermatology. The characteristics of exosomes, their associated cargo (nucleic acids, proteins, and lipids), and downstream functions are vastly different, depending on the cell of origin. Specifically, we have discovered that these extracellular vesicles from the bone marrow–derived mesenchymal stem cells stimulate production of basement membrane components by cells that genetically lacked the ability to do so before (ie, collagen VII from fibroblasts derived from patients with recessive dystrophic epidermolysis bullosa). In other words, our work shows that the bone marrow can replenish collagen VII by directly delivering protein but also by transferring messenger RNA, coding for collagen VII, to the epidermolysis bullosa fibroblasts, allowing them to make collagen VII for the first time. Extracellular vesicles, including exosomes, have immediate potential for serving as therapeutics in dermatology over the next decade.
Up next: Genetic Variations in Non-coding Regulatory Regions in Linear Morphea...→
Genetic Variations in Non-coding Regulatory Regions in Linear Morphea
Dawn Zhang Eichenfield, MD, PhD, University of California, San Diego, Medical Center, La Jolla, California
Disclosures: This research was funded by the Women’s Dermatologic Society and the Pediatric Dermatology Research Alliance.
Linear morphea is a chronic inflammatory and pro-fibrotic disease that can be debilitating and disfiguring. It generally presents at an earlier age of onset than other subtypes of morphea. Like other autoimmune diseases, linear morphea presents predominantly in females, has a familial contribution, and has been associated with particular environmental triggers. Beyond this, however, the full pathogenesis of this disease remains unknown. A more thorough understanding of the causes of linear morphea—genetic and environmental—is the first step toward improved diagnosis and treatment.
Lesions of linear morphea predominantly follow Blaschko’s lines, a pattern of lines on the skin thought to represent pathways of epidermal cell migration and proliferation during fetal development. This presentation suggests that genetic mosaicism may be at play, although studies utilizing whole exome sequencing have not found causative somatic genetic mutations in protein-coding regions.1,2 Recent studies, however, have increasingly shown that genetic diseases, including autoimmune disorders, can be caused by mutations in non-protein coding regions. For example, Castellanos-Rubio et al3 recently identified a long non-coding RNA that plays an essential role in the maintenance of intestinal mucosal immune homeostasis and its genetic polymorphisms contribute to inflammation in celiac disease. New sequencing technologies further highlight the complexities of gene regulation. Recent studies of the ENCODE project estimate hundreds of thousands of non-promoter genomic elements that are regulated in a cell-specific and signal-dependent manner through different transcription factors, non-coding RNAs, as well as other transcriptional regulatory elements.4 Taken together, this data suggests that genetic mutations in non-coding regulatory regions have the potential to initiate and perpetuate autoimmune disease.
Our hypothesis is that key somatic genetic mutations in non-coding regulatory regions result in altered transcription factor binding and downstream changes in gene expression, which produce a predisposition to development of linear morphea. To test this hypothesis, we are utilizing whole genome sequencing and Assay for Transposase-Accessible Chromatin using sequencing (ATAC-Seq) on normal and lesional skin in a cohort of patients with linear morphea to identify disease-specific regulatory variants. If initial experiments yield candidate genes or regulatory regions, we will validate these loci through functional assays to identify variants that stimulate a pro-fibrotic response.
Altogether, these studies will help identify new insights into key genetic contributions to the pathogenesis and transcriptional regulation of linear morphea. Understanding the molecular and genetic basis of linear morphea is significant as it is the first step toward improved pharmacological treatments and preventive strategies for linear morphea.
References
- Higgins R, Smith A, Walchli R, et al. Absence of somatic mutations in linear localized scleroderma. J Investig Dermatol. 2017;137:S271.
- Higgins R, Theiler M, Smith A, et al. Genetic architecture of linear localized scleroderma. J Investig Dermatol. 2018;138:S138.
- Castellanos-Rubio A, Fernandez-Jimenez N, Kratchmarov R, et al. A long noncoding RNA associated with susceptibility to celiac disease. Science. 2016;352:91-95.
- Hoffman MM, Ernst J, Wilder SP, et al. Integrative annotation of chromatin elements from ENCODE data. Nucleic Acids Res. 2013;41:827-841.
Up next: Increasing Evidence for Omalizumab in the Treatment of Bullous Pemphigoid→
Increasing Evidence for Omalizumab in the Treatment of Bullous Pemphigoid
Sarah Lonowski, MD, MBA, Division of Dermatology, University of California Los Angeles, Los Angeles, California
Disclosures: None.
Introduction
Bullous pemphigoid (BP) is a common, debilitating autoimmune blistering disease which is often managed with prednisone and other systemic immunosuppressive medications. Here, we present the largest case series to date evaluating the use of omalizumab for the treatment of BP, providing novel insight and further support for use of this well-tolerated, minimally immunosuppressive agent.
Objective
To review and analyze the experience of patients with BP treated with omalizumab at a single academic institution.
Methods
Retrospective chart analysis of eleven patients with BP treated at a single academic institution over a 32-month period (April 2016–December 2018). Data was obtained through comprehensive review of pathology reports, laboratory data, and physician documentation. Standard descriptive statistics were performed on the data.
Results
Eleven patients were included in the analysis, 5 females (45.5%) and 6 males (54.5%) with a mean age of 78 years (range, 57-95 years). Patients had failed a mean of 2.3 systemic agents prior to initiation of omalizumab. Seven of 11 patients (63.6%) had experienced adverse effects related to prednisone prior to initiation of omalizumab. Six patients (54.5%) had complete clearance of skin lesions after a median duration of 4.4 months on omalizumab, 3 patients (27.3%) had partial response, and 2 patients (18.2%) did not respond to treatment. The median duration of treatment in all patients at the time of analysis was 12.6 months. All 10 patients on prednisone at the time of omalizumab initiation were able to reduce the dose of prednisone, and 5 of 10 patients (50%) were able to discontinue systemic steroids completely. Baseline serum immunoglobulin E (IgE) and eosinophil levels did not predict treatment response. There were no serious treatment-related adverse effects during the treatment period.
Conclusions/Relevance
Omalizumab is a well-tolerated, steroid-sparing agent which has a significant disease-modifying effect in the majority of patients. Baseline serum IgE and eosinophil levels do not appear to predict treatment response, therefore patient selection should not hinge on these lab values. Omalizumab should be considered as a treatment option for any patient requiring systemic treatment for BP, but especially for those who have experienced or are at risk for experiencing adverse effects from systemic steroids.
The dermMentors™ Resident of Distinction Award™ recognizes top residents in dermatology. DermMentors.org and the dermMentors™ Resident of Distinction Award™ are sponsored by Beiersdorf Inc and administered by DermEd, Inc. The 2019 dermMentors™ Residents of Distinction™ presented new scientific research during the general sessions of the 15th Annual Coastal Dermatology Symposium on October 5, 2019.
Overall Grand Prize
Chronic Inflammatory Skin Diseases Are Associated With Herpes Zoster in US Inpatients
Raj Chovatiya, MD, PhD; Jonathan I. Silverberg MD, PhD, MPH, Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois
Disclosures: None.
Background
Herpes zoster (HZ) is a vaccine-preventable, viral eruption that affects 1 of every 3 people in the United States in their lifetime. Despite evidence-based guidelines and public health advocacy, many high-risk individuals remain unvaccinated and at risk for significant morbidity from HZ. Patients with chronic inflammatory skin diseases (CISDs), including allergic and autoimmune conditions, have potential risk factors for HZ, including long-term use of systemic corticosteroids and immunosuppressants, and immune dysregulation in the skin and periphery. We sought to determine whether CISDs are associated with HZ in hospitalized patients in the US.
Methods
Data were analyzed from the 2002-2012 Nationwide Inpatient Sample, a representative 20% cross-sectional cohort of US hospitalizations (N=68,490,364 children and adults).
Results
The estimated incidence of primary hospitalization for HZ in the US population was 5.0 per 100,000 persons from 2002-2012. In multivariable weighted logistic regression models including age, sex, race/ethnicity, insurance, mean household income, and long-term systemic corticosteroid use, primary hospitalization for HZ was associated with multiple CISDs: atopic dermatitis (adjusted odds ratio [95% confidence interval]: 1.38 [1.14-1.68]), psoriasis (4.78 [2.83-8.08]), pemphigus (1.77 [1.01-3.12]), bullous pemphigoid (1.77 [1.01-3.12]), mycosis fungoides (3.79 [2.55-5.65]), dermatomyositis (7.31 [5.27-10.12]), systemic sclerosis (1.92 [1.47-2.53]), cutaneous lupus erythematosus (1.94 [1.10-3.44]), vitiligo (2.00 [1.04-3.85]), and sarcoidosis (1.52 [1.22-1.90]). Lichen planus (3.01 [1.36-6.67]), Sézary syndrome (12.14 [5.20-28.31]), morphea (2.74 [1.36-5.51]), and pyoderma gangrenosum (2.44 [1.16-5.13]) showed increased odds in bivariable models but not in multivariable models. Whereas, hidradenitis suppurativa, chronic idiopathic/spontaneous urticaria, and alopecia areata were not associated with HZ. Similar results were seen in sensitivity analyses among adults age <60 and <50 years. Significant predictors of primary hospitalization for HZ among patients with CISDs included older age, female sex, non-white race/ethnicity, decreasing number of chronic comorbid conditions, and long-term systemic corticosteroid use. Inpatient length of stay and/or inflation adjusted cost of care were significantly higher in inpatients with CISD with vs. without a primary diagnosis of HZ.
Conclusion
CISDs are associated with increased hospitalization for HZ, prolonged length of inpatient stay and increased cost of hospital care. The associations of CISDs and HZ were significant even below the recommended ages (<60 and <50 years) for vaccination with live and recombinant HZ vaccine, respectively. Additional studies are needed to confirm these findings and determine the mechanisms of VZV reactivation. Patients with CISDs constitute a significant, previously under-recognized group that is high-risk for hospitalization for HZ. Dermatologists are the primary physicians who manage the vulnerable population of CISDs. They should be on the forefront of screening, intervention, and most importantly, vaccination for their patients. Future studies should explore implementation of outpatient HZ vaccination by dermatologists and revised disease-specific guidelines to cover the spectrum of CISDs.
Up next: Buyer Beware or You Might Get Burned...→
Buyer Beware or You Might Get Burned: Unregulated Photosensitizing Agents Available Without Prescription From Major Online Retailers
Kimberly Huerth, MD, MEd, Department of Dermatology, Howard University Hospital, Washington, DC; Olivia Ware, BA, Howard University College of Medicine, Washington, DC; Ginette A. Okoye, MD, Department of Dermatology, Howard University Hospital, Washington, DC; Sharon Bridgeman-Shah, MD, Department of Dermatology, Howard University Hospital, Washington, DC
Disclosures: None.
Background
According to the World Health Organization, the vast majority of the world’s population relies on plant-derived medicine as their primary form of healthcare. Closer to home, these alternative therapies are growing in popularity among American healthcare consumers, in concert with increasing access to such products by means of globalization and the growth of Internet retail. There are numerous reasons why an individual might eschew Western medicine in favor of a more naturopathic approach, including perceived health benefits, cost, prior failure of physician guided treatment, a lack of awareness about the risks these products carry, cultural norms, and desperation born of the psychosocial burden of one’s disease.
A 73-year-old Eritrean man presented to our outpatient dermatology clinic with a severe, acute, phototoxic reaction following the ingestion of Athamanta decoction for the self-directed treatment of vitiligo. The genus Athamanta is in the Apiaceae family and consists of 6 species of flowering plants native to Europe and North Africa. Furanocoumarins are the phototoxic compounds present in these plants, among which psoralen is perhaps the most notorious. The desiccated Athamanta leaves, sought in the absence of medical consultation, had been obtained from a mail order vendor in Egypt which had advertised them as a “miracle effect for curing vitiligo.”
Objective
To determine the extent to which unregulated photosensitizing agents for the treatment of vitiligo are available to consumers from online retailers, including major ones such as Amazon and eBay.
Methods
An online product search of eBay and Amazon, as well as an Internet pharmacy by the name of Cheap Generic, was conducted using search terms “vitiligo treatment,” “psoralen for vitiligo,” “furanocoumarin treatment,” “Athamanta vitiligo,” “Apiaceae vitiligo,” and “leukoderma.” Products generated by the search terms were examined for their route of delivery (topical vs systemic), key photosensitizing ingredient, parent plant, cost to consumer, and country of manufacture. Products that did not identify a known photosensitizing compound, or a plant known to contain a photosensitizing compound, on the list of ingredients were excluded from the analysis.
Results
We identified a total of 11 products—6 listed on Amazon, 3 listed on eBay, and 2 listed by Cheap Generic pharmacy—that had either a photosensitizing compound, or a plant known to contain a photosensitizing compound, on its list of ingredients. 27.3% of products were available in formulations intended for ingestion, such as tablets or powders. 90.9% of products listed psoralen, or a plant known to contain psoralen, as a major ingredient. Of the products that identified the main photosensitizing plant ingredient (72.7%), Psoralea corylifolia was the most common (75%). Products ranged in cost from $3 to $28. The terms “herbal” (54.5%) and “natural” (36.3%) were used most frequently on the packaging and/or website for promotional purposes. While 45.4% of products did not identify a country of origin, of those that did, 66.7% were manufactured in India, while the remainder were prepared in China.
Conclusion
Topically and systemically administered psoralen-containing products for the treatment of vitiligo are widely available without a prescription from several online vendors. These products are affordable and utilize suggestive packaging to tout the benefits of the products, without mention of their risks.
Up next: Collagen VII: A Link Between the Skin and Bone Marrow...→
Collagen VII: A Link Between the Skin and Bone Marrow Via Extracellular Vesicles
Jeffrey D. McBride, MD, PhD, Department of Dermatology and Cutaneous Surgery, University of Miami, Miami, Florida
Disclosures: Dr. McBride has been a consultant with Aegle Therapeutics.
Extracellular vesicles—exosomes, microvesicles, and apoptotic bodies—are ubiquitous in human tissues, circulation, and body fluids. Of these vesicles, exosomes are of growing interest among investigators across multiple fields, including dermatology. The characteristics of exosomes, their associated cargo (nucleic acids, proteins, and lipids), and downstream functions are vastly different, depending on the cell of origin. Specifically, we have discovered that these extracellular vesicles from the bone marrow–derived mesenchymal stem cells stimulate production of basement membrane components by cells that genetically lacked the ability to do so before (ie, collagen VII from fibroblasts derived from patients with recessive dystrophic epidermolysis bullosa). In other words, our work shows that the bone marrow can replenish collagen VII by directly delivering protein but also by transferring messenger RNA, coding for collagen VII, to the epidermolysis bullosa fibroblasts, allowing them to make collagen VII for the first time. Extracellular vesicles, including exosomes, have immediate potential for serving as therapeutics in dermatology over the next decade.
Up next: Genetic Variations in Non-coding Regulatory Regions in Linear Morphea...→
Genetic Variations in Non-coding Regulatory Regions in Linear Morphea
Dawn Zhang Eichenfield, MD, PhD, University of California, San Diego, Medical Center, La Jolla, California
Disclosures: This research was funded by the Women’s Dermatologic Society and the Pediatric Dermatology Research Alliance.
Linear morphea is a chronic inflammatory and pro-fibrotic disease that can be debilitating and disfiguring. It generally presents at an earlier age of onset than other subtypes of morphea. Like other autoimmune diseases, linear morphea presents predominantly in females, has a familial contribution, and has been associated with particular environmental triggers. Beyond this, however, the full pathogenesis of this disease remains unknown. A more thorough understanding of the causes of linear morphea—genetic and environmental—is the first step toward improved diagnosis and treatment.
Lesions of linear morphea predominantly follow Blaschko’s lines, a pattern of lines on the skin thought to represent pathways of epidermal cell migration and proliferation during fetal development. This presentation suggests that genetic mosaicism may be at play, although studies utilizing whole exome sequencing have not found causative somatic genetic mutations in protein-coding regions.1,2 Recent studies, however, have increasingly shown that genetic diseases, including autoimmune disorders, can be caused by mutations in non-protein coding regions. For example, Castellanos-Rubio et al3 recently identified a long non-coding RNA that plays an essential role in the maintenance of intestinal mucosal immune homeostasis and its genetic polymorphisms contribute to inflammation in celiac disease. New sequencing technologies further highlight the complexities of gene regulation. Recent studies of the ENCODE project estimate hundreds of thousands of non-promoter genomic elements that are regulated in a cell-specific and signal-dependent manner through different transcription factors, non-coding RNAs, as well as other transcriptional regulatory elements.4 Taken together, this data suggests that genetic mutations in non-coding regulatory regions have the potential to initiate and perpetuate autoimmune disease.
Our hypothesis is that key somatic genetic mutations in non-coding regulatory regions result in altered transcription factor binding and downstream changes in gene expression, which produce a predisposition to development of linear morphea. To test this hypothesis, we are utilizing whole genome sequencing and Assay for Transposase-Accessible Chromatin using sequencing (ATAC-Seq) on normal and lesional skin in a cohort of patients with linear morphea to identify disease-specific regulatory variants. If initial experiments yield candidate genes or regulatory regions, we will validate these loci through functional assays to identify variants that stimulate a pro-fibrotic response.
Altogether, these studies will help identify new insights into key genetic contributions to the pathogenesis and transcriptional regulation of linear morphea. Understanding the molecular and genetic basis of linear morphea is significant as it is the first step toward improved pharmacological treatments and preventive strategies for linear morphea.
References
- Higgins R, Smith A, Walchli R, et al. Absence of somatic mutations in linear localized scleroderma. J Investig Dermatol. 2017;137:S271.
- Higgins R, Theiler M, Smith A, et al. Genetic architecture of linear localized scleroderma. J Investig Dermatol. 2018;138:S138.
- Castellanos-Rubio A, Fernandez-Jimenez N, Kratchmarov R, et al. A long noncoding RNA associated with susceptibility to celiac disease. Science. 2016;352:91-95.
- Hoffman MM, Ernst J, Wilder SP, et al. Integrative annotation of chromatin elements from ENCODE data. Nucleic Acids Res. 2013;41:827-841.
Up next: Increasing Evidence for Omalizumab in the Treatment of Bullous Pemphigoid→
Increasing Evidence for Omalizumab in the Treatment of Bullous Pemphigoid
Sarah Lonowski, MD, MBA, Division of Dermatology, University of California Los Angeles, Los Angeles, California
Disclosures: None.
Introduction
Bullous pemphigoid (BP) is a common, debilitating autoimmune blistering disease which is often managed with prednisone and other systemic immunosuppressive medications. Here, we present the largest case series to date evaluating the use of omalizumab for the treatment of BP, providing novel insight and further support for use of this well-tolerated, minimally immunosuppressive agent.
Objective
To review and analyze the experience of patients with BP treated with omalizumab at a single academic institution.
Methods
Retrospective chart analysis of eleven patients with BP treated at a single academic institution over a 32-month period (April 2016–December 2018). Data was obtained through comprehensive review of pathology reports, laboratory data, and physician documentation. Standard descriptive statistics were performed on the data.
Results
Eleven patients were included in the analysis, 5 females (45.5%) and 6 males (54.5%) with a mean age of 78 years (range, 57-95 years). Patients had failed a mean of 2.3 systemic agents prior to initiation of omalizumab. Seven of 11 patients (63.6%) had experienced adverse effects related to prednisone prior to initiation of omalizumab. Six patients (54.5%) had complete clearance of skin lesions after a median duration of 4.4 months on omalizumab, 3 patients (27.3%) had partial response, and 2 patients (18.2%) did not respond to treatment. The median duration of treatment in all patients at the time of analysis was 12.6 months. All 10 patients on prednisone at the time of omalizumab initiation were able to reduce the dose of prednisone, and 5 of 10 patients (50%) were able to discontinue systemic steroids completely. Baseline serum immunoglobulin E (IgE) and eosinophil levels did not predict treatment response. There were no serious treatment-related adverse effects during the treatment period.
Conclusions/Relevance
Omalizumab is a well-tolerated, steroid-sparing agent which has a significant disease-modifying effect in the majority of patients. Baseline serum IgE and eosinophil levels do not appear to predict treatment response, therefore patient selection should not hinge on these lab values. Omalizumab should be considered as a treatment option for any patient requiring systemic treatment for BP, but especially for those who have experienced or are at risk for experiencing adverse effects from systemic steroids.
The dermMentors™ Resident of Distinction Award™ recognizes top residents in dermatology. DermMentors.org and the dermMentors™ Resident of Distinction Award™ are sponsored by Beiersdorf Inc and administered by DermEd, Inc. The 2019 dermMentors™ Residents of Distinction™ presented new scientific research during the general sessions of the 15th Annual Coastal Dermatology Symposium on October 5, 2019.
Overall Grand Prize
Chronic Inflammatory Skin Diseases Are Associated With Herpes Zoster in US Inpatients
Raj Chovatiya, MD, PhD; Jonathan I. Silverberg MD, PhD, MPH, Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois
Disclosures: None.
Background
Herpes zoster (HZ) is a vaccine-preventable, viral eruption that affects 1 of every 3 people in the United States in their lifetime. Despite evidence-based guidelines and public health advocacy, many high-risk individuals remain unvaccinated and at risk for significant morbidity from HZ. Patients with chronic inflammatory skin diseases (CISDs), including allergic and autoimmune conditions, have potential risk factors for HZ, including long-term use of systemic corticosteroids and immunosuppressants, and immune dysregulation in the skin and periphery. We sought to determine whether CISDs are associated with HZ in hospitalized patients in the US.
Methods
Data were analyzed from the 2002-2012 Nationwide Inpatient Sample, a representative 20% cross-sectional cohort of US hospitalizations (N=68,490,364 children and adults).
Results
The estimated incidence of primary hospitalization for HZ in the US population was 5.0 per 100,000 persons from 2002-2012. In multivariable weighted logistic regression models including age, sex, race/ethnicity, insurance, mean household income, and long-term systemic corticosteroid use, primary hospitalization for HZ was associated with multiple CISDs: atopic dermatitis (adjusted odds ratio [95% confidence interval]: 1.38 [1.14-1.68]), psoriasis (4.78 [2.83-8.08]), pemphigus (1.77 [1.01-3.12]), bullous pemphigoid (1.77 [1.01-3.12]), mycosis fungoides (3.79 [2.55-5.65]), dermatomyositis (7.31 [5.27-10.12]), systemic sclerosis (1.92 [1.47-2.53]), cutaneous lupus erythematosus (1.94 [1.10-3.44]), vitiligo (2.00 [1.04-3.85]), and sarcoidosis (1.52 [1.22-1.90]). Lichen planus (3.01 [1.36-6.67]), Sézary syndrome (12.14 [5.20-28.31]), morphea (2.74 [1.36-5.51]), and pyoderma gangrenosum (2.44 [1.16-5.13]) showed increased odds in bivariable models but not in multivariable models. Whereas, hidradenitis suppurativa, chronic idiopathic/spontaneous urticaria, and alopecia areata were not associated with HZ. Similar results were seen in sensitivity analyses among adults age <60 and <50 years. Significant predictors of primary hospitalization for HZ among patients with CISDs included older age, female sex, non-white race/ethnicity, decreasing number of chronic comorbid conditions, and long-term systemic corticosteroid use. Inpatient length of stay and/or inflation adjusted cost of care were significantly higher in inpatients with CISD with vs. without a primary diagnosis of HZ.
Conclusion
CISDs are associated with increased hospitalization for HZ, prolonged length of inpatient stay and increased cost of hospital care. The associations of CISDs and HZ were significant even below the recommended ages (<60 and <50 years) for vaccination with live and recombinant HZ vaccine, respectively. Additional studies are needed to confirm these findings and determine the mechanisms of VZV reactivation. Patients with CISDs constitute a significant, previously under-recognized group that is high-risk for hospitalization for HZ. Dermatologists are the primary physicians who manage the vulnerable population of CISDs. They should be on the forefront of screening, intervention, and most importantly, vaccination for their patients. Future studies should explore implementation of outpatient HZ vaccination by dermatologists and revised disease-specific guidelines to cover the spectrum of CISDs.
Up next: Buyer Beware or You Might Get Burned...→
Buyer Beware or You Might Get Burned: Unregulated Photosensitizing Agents Available Without Prescription From Major Online Retailers
Kimberly Huerth, MD, MEd, Department of Dermatology, Howard University Hospital, Washington, DC; Olivia Ware, BA, Howard University College of Medicine, Washington, DC; Ginette A. Okoye, MD, Department of Dermatology, Howard University Hospital, Washington, DC; Sharon Bridgeman-Shah, MD, Department of Dermatology, Howard University Hospital, Washington, DC
Disclosures: None.
Background
According to the World Health Organization, the vast majority of the world’s population relies on plant-derived medicine as their primary form of healthcare. Closer to home, these alternative therapies are growing in popularity among American healthcare consumers, in concert with increasing access to such products by means of globalization and the growth of Internet retail. There are numerous reasons why an individual might eschew Western medicine in favor of a more naturopathic approach, including perceived health benefits, cost, prior failure of physician guided treatment, a lack of awareness about the risks these products carry, cultural norms, and desperation born of the psychosocial burden of one’s disease.
A 73-year-old Eritrean man presented to our outpatient dermatology clinic with a severe, acute, phototoxic reaction following the ingestion of Athamanta decoction for the self-directed treatment of vitiligo. The genus Athamanta is in the Apiaceae family and consists of 6 species of flowering plants native to Europe and North Africa. Furanocoumarins are the phototoxic compounds present in these plants, among which psoralen is perhaps the most notorious. The desiccated Athamanta leaves, sought in the absence of medical consultation, had been obtained from a mail order vendor in Egypt which had advertised them as a “miracle effect for curing vitiligo.”
Objective
To determine the extent to which unregulated photosensitizing agents for the treatment of vitiligo are available to consumers from online retailers, including major ones such as Amazon and eBay.
Methods
An online product search of eBay and Amazon, as well as an Internet pharmacy by the name of Cheap Generic, was conducted using search terms “vitiligo treatment,” “psoralen for vitiligo,” “furanocoumarin treatment,” “Athamanta vitiligo,” “Apiaceae vitiligo,” and “leukoderma.” Products generated by the search terms were examined for their route of delivery (topical vs systemic), key photosensitizing ingredient, parent plant, cost to consumer, and country of manufacture. Products that did not identify a known photosensitizing compound, or a plant known to contain a photosensitizing compound, on the list of ingredients were excluded from the analysis.
Results
We identified a total of 11 products—6 listed on Amazon, 3 listed on eBay, and 2 listed by Cheap Generic pharmacy—that had either a photosensitizing compound, or a plant known to contain a photosensitizing compound, on its list of ingredients. 27.3% of products were available in formulations intended for ingestion, such as tablets or powders. 90.9% of products listed psoralen, or a plant known to contain psoralen, as a major ingredient. Of the products that identified the main photosensitizing plant ingredient (72.7%), Psoralea corylifolia was the most common (75%). Products ranged in cost from $3 to $28. The terms “herbal” (54.5%) and “natural” (36.3%) were used most frequently on the packaging and/or website for promotional purposes. While 45.4% of products did not identify a country of origin, of those that did, 66.7% were manufactured in India, while the remainder were prepared in China.
Conclusion
Topically and systemically administered psoralen-containing products for the treatment of vitiligo are widely available without a prescription from several online vendors. These products are affordable and utilize suggestive packaging to tout the benefits of the products, without mention of their risks.
Up next: Collagen VII: A Link Between the Skin and Bone Marrow...→
Collagen VII: A Link Between the Skin and Bone Marrow Via Extracellular Vesicles
Jeffrey D. McBride, MD, PhD, Department of Dermatology and Cutaneous Surgery, University of Miami, Miami, Florida
Disclosures: Dr. McBride has been a consultant with Aegle Therapeutics.
Extracellular vesicles—exosomes, microvesicles, and apoptotic bodies—are ubiquitous in human tissues, circulation, and body fluids. Of these vesicles, exosomes are of growing interest among investigators across multiple fields, including dermatology. The characteristics of exosomes, their associated cargo (nucleic acids, proteins, and lipids), and downstream functions are vastly different, depending on the cell of origin. Specifically, we have discovered that these extracellular vesicles from the bone marrow–derived mesenchymal stem cells stimulate production of basement membrane components by cells that genetically lacked the ability to do so before (ie, collagen VII from fibroblasts derived from patients with recessive dystrophic epidermolysis bullosa). In other words, our work shows that the bone marrow can replenish collagen VII by directly delivering protein but also by transferring messenger RNA, coding for collagen VII, to the epidermolysis bullosa fibroblasts, allowing them to make collagen VII for the first time. Extracellular vesicles, including exosomes, have immediate potential for serving as therapeutics in dermatology over the next decade.
Up next: Genetic Variations in Non-coding Regulatory Regions in Linear Morphea...→
Genetic Variations in Non-coding Regulatory Regions in Linear Morphea
Dawn Zhang Eichenfield, MD, PhD, University of California, San Diego, Medical Center, La Jolla, California
Disclosures: This research was funded by the Women’s Dermatologic Society and the Pediatric Dermatology Research Alliance.
Linear morphea is a chronic inflammatory and pro-fibrotic disease that can be debilitating and disfiguring. It generally presents at an earlier age of onset than other subtypes of morphea. Like other autoimmune diseases, linear morphea presents predominantly in females, has a familial contribution, and has been associated with particular environmental triggers. Beyond this, however, the full pathogenesis of this disease remains unknown. A more thorough understanding of the causes of linear morphea—genetic and environmental—is the first step toward improved diagnosis and treatment.
Lesions of linear morphea predominantly follow Blaschko’s lines, a pattern of lines on the skin thought to represent pathways of epidermal cell migration and proliferation during fetal development. This presentation suggests that genetic mosaicism may be at play, although studies utilizing whole exome sequencing have not found causative somatic genetic mutations in protein-coding regions.1,2 Recent studies, however, have increasingly shown that genetic diseases, including autoimmune disorders, can be caused by mutations in non-protein coding regions. For example, Castellanos-Rubio et al3 recently identified a long non-coding RNA that plays an essential role in the maintenance of intestinal mucosal immune homeostasis and its genetic polymorphisms contribute to inflammation in celiac disease. New sequencing technologies further highlight the complexities of gene regulation. Recent studies of the ENCODE project estimate hundreds of thousands of non-promoter genomic elements that are regulated in a cell-specific and signal-dependent manner through different transcription factors, non-coding RNAs, as well as other transcriptional regulatory elements.4 Taken together, this data suggests that genetic mutations in non-coding regulatory regions have the potential to initiate and perpetuate autoimmune disease.
Our hypothesis is that key somatic genetic mutations in non-coding regulatory regions result in altered transcription factor binding and downstream changes in gene expression, which produce a predisposition to development of linear morphea. To test this hypothesis, we are utilizing whole genome sequencing and Assay for Transposase-Accessible Chromatin using sequencing (ATAC-Seq) on normal and lesional skin in a cohort of patients with linear morphea to identify disease-specific regulatory variants. If initial experiments yield candidate genes or regulatory regions, we will validate these loci through functional assays to identify variants that stimulate a pro-fibrotic response.
Altogether, these studies will help identify new insights into key genetic contributions to the pathogenesis and transcriptional regulation of linear morphea. Understanding the molecular and genetic basis of linear morphea is significant as it is the first step toward improved pharmacological treatments and preventive strategies for linear morphea.
References
- Higgins R, Smith A, Walchli R, et al. Absence of somatic mutations in linear localized scleroderma. J Investig Dermatol. 2017;137:S271.
- Higgins R, Theiler M, Smith A, et al. Genetic architecture of linear localized scleroderma. J Investig Dermatol. 2018;138:S138.
- Castellanos-Rubio A, Fernandez-Jimenez N, Kratchmarov R, et al. A long noncoding RNA associated with susceptibility to celiac disease. Science. 2016;352:91-95.
- Hoffman MM, Ernst J, Wilder SP, et al. Integrative annotation of chromatin elements from ENCODE data. Nucleic Acids Res. 2013;41:827-841.
Up next: Increasing Evidence for Omalizumab in the Treatment of Bullous Pemphigoid→
Increasing Evidence for Omalizumab in the Treatment of Bullous Pemphigoid
Sarah Lonowski, MD, MBA, Division of Dermatology, University of California Los Angeles, Los Angeles, California
Disclosures: None.
Introduction
Bullous pemphigoid (BP) is a common, debilitating autoimmune blistering disease which is often managed with prednisone and other systemic immunosuppressive medications. Here, we present the largest case series to date evaluating the use of omalizumab for the treatment of BP, providing novel insight and further support for use of this well-tolerated, minimally immunosuppressive agent.
Objective
To review and analyze the experience of patients with BP treated with omalizumab at a single academic institution.
Methods
Retrospective chart analysis of eleven patients with BP treated at a single academic institution over a 32-month period (April 2016–December 2018). Data was obtained through comprehensive review of pathology reports, laboratory data, and physician documentation. Standard descriptive statistics were performed on the data.
Results
Eleven patients were included in the analysis, 5 females (45.5%) and 6 males (54.5%) with a mean age of 78 years (range, 57-95 years). Patients had failed a mean of 2.3 systemic agents prior to initiation of omalizumab. Seven of 11 patients (63.6%) had experienced adverse effects related to prednisone prior to initiation of omalizumab. Six patients (54.5%) had complete clearance of skin lesions after a median duration of 4.4 months on omalizumab, 3 patients (27.3%) had partial response, and 2 patients (18.2%) did not respond to treatment. The median duration of treatment in all patients at the time of analysis was 12.6 months. All 10 patients on prednisone at the time of omalizumab initiation were able to reduce the dose of prednisone, and 5 of 10 patients (50%) were able to discontinue systemic steroids completely. Baseline serum immunoglobulin E (IgE) and eosinophil levels did not predict treatment response. There were no serious treatment-related adverse effects during the treatment period.
Conclusions/Relevance
Omalizumab is a well-tolerated, steroid-sparing agent which has a significant disease-modifying effect in the majority of patients. Baseline serum IgE and eosinophil levels do not appear to predict treatment response, therefore patient selection should not hinge on these lab values. Omalizumab should be considered as a treatment option for any patient requiring systemic treatment for BP, but especially for those who have experienced or are at risk for experiencing adverse effects from systemic steroids.
California bans “Pay for Delay,” promotes black maternal health, PrEP access
AB 824, the Pay for Delay bill, bans pharmaceutical companies from keeping cheaper generic drugs off the market. The bill prohibits agreements between brand name and generic drug manufacturers to delay the release of generic drugs, defining them as presumptively anticompetitive. A Federal Trade Commission study found that “these anticompetitive deals cost consumers and taxpayers $3.5 billion in higher drug costs every year,” according to a statement from the governor’s office.
The second bill, SB 464, is intended to improve black maternal health care. The bill is designed to reduce preventable maternal mortality among black women by requiring all perinatal health care providers to undergo implicit bias training to curb the effects of bias on maternal health and by improving data collection at the California Department of Public Health to better understand pregnancy-related deaths. “We know that black women have been dying at alarming rates during and after giving birth. The disproportionate effect of the maternal mortality rate on this community is a public health crisis and a major health equity issue. We must do everything in our power to take implicit bias out of the medical system – it is literally a matter of life and death,” said Gov. Newsom.
The third bill, SB 159, aims to facilitate the use of pre-exposure prophylaxis and postexposure prophylaxis against HIV infection. The bill allows pharmacists in the state to dispense PrEP and PEP without a physician’s prescription and prohibits insurance companies from requiring prior authorization for patients to obtain PrEP coverage. “All Californians deserve access to PrEP and PEP, two treatments that have transformed our fight against HIV and AIDS,” Gov. Newsom said in a statement.
AB 824, the Pay for Delay bill, bans pharmaceutical companies from keeping cheaper generic drugs off the market. The bill prohibits agreements between brand name and generic drug manufacturers to delay the release of generic drugs, defining them as presumptively anticompetitive. A Federal Trade Commission study found that “these anticompetitive deals cost consumers and taxpayers $3.5 billion in higher drug costs every year,” according to a statement from the governor’s office.
The second bill, SB 464, is intended to improve black maternal health care. The bill is designed to reduce preventable maternal mortality among black women by requiring all perinatal health care providers to undergo implicit bias training to curb the effects of bias on maternal health and by improving data collection at the California Department of Public Health to better understand pregnancy-related deaths. “We know that black women have been dying at alarming rates during and after giving birth. The disproportionate effect of the maternal mortality rate on this community is a public health crisis and a major health equity issue. We must do everything in our power to take implicit bias out of the medical system – it is literally a matter of life and death,” said Gov. Newsom.
The third bill, SB 159, aims to facilitate the use of pre-exposure prophylaxis and postexposure prophylaxis against HIV infection. The bill allows pharmacists in the state to dispense PrEP and PEP without a physician’s prescription and prohibits insurance companies from requiring prior authorization for patients to obtain PrEP coverage. “All Californians deserve access to PrEP and PEP, two treatments that have transformed our fight against HIV and AIDS,” Gov. Newsom said in a statement.
AB 824, the Pay for Delay bill, bans pharmaceutical companies from keeping cheaper generic drugs off the market. The bill prohibits agreements between brand name and generic drug manufacturers to delay the release of generic drugs, defining them as presumptively anticompetitive. A Federal Trade Commission study found that “these anticompetitive deals cost consumers and taxpayers $3.5 billion in higher drug costs every year,” according to a statement from the governor’s office.
The second bill, SB 464, is intended to improve black maternal health care. The bill is designed to reduce preventable maternal mortality among black women by requiring all perinatal health care providers to undergo implicit bias training to curb the effects of bias on maternal health and by improving data collection at the California Department of Public Health to better understand pregnancy-related deaths. “We know that black women have been dying at alarming rates during and after giving birth. The disproportionate effect of the maternal mortality rate on this community is a public health crisis and a major health equity issue. We must do everything in our power to take implicit bias out of the medical system – it is literally a matter of life and death,” said Gov. Newsom.
The third bill, SB 159, aims to facilitate the use of pre-exposure prophylaxis and postexposure prophylaxis against HIV infection. The bill allows pharmacists in the state to dispense PrEP and PEP without a physician’s prescription and prohibits insurance companies from requiring prior authorization for patients to obtain PrEP coverage. “All Californians deserve access to PrEP and PEP, two treatments that have transformed our fight against HIV and AIDS,” Gov. Newsom said in a statement.
AGA Clinical Practice Update: Surveillance for hepatobiliary cancers in primary sclerosing cholangitis
All adult patients with primary sclerosing cholangitis should be screened at least annually for cholangiocarcinoma and gallbladder cancer, particularly in the first year after their diagnosis, according to a clinical practice update published in Clinical Gastroenterology and Hepatology.
Individuals with primary sclerosing cholangitis have a 400-fold higher risk of cholangiocarcinoma, compared with the general population, and around one-third of cancers are detected within 1 year of the cholangitis diagnosis, Christopher L. Bowlus, MD, of the University of California, Davis, and coauthors wrote.
The clinical practice update from the American Gastroenterological Association was in response to the observation that, while there is significant evidence for an increasing incidence of cirrhosis, hepatic decompensation, hepatocellular carcinoma, and liver transplant listing among patients with primary sclerosing cholangitis, there is a lack of good evidence to guide cholangiocarcinoma surveillance in these patients.
“The low prevalence and long duration of PSC [primary sclerosing cholangitis] present substantial barriers to better understanding risk stratification, developing biomarkers, and measuring the impact surveillance has on clinical outcomes,” they wrote.
The first recommendation was that surveillance for cholangiocarcinoma and gallbladder cancer should be considered in all adult patients with primary sclerosing cholangitis, regardless of their disease stage. The authors especially emphasized the importance of surveillance in the first year after a diagnosis of primary sclerosing cholangitis, in patients who also have ulcerative colitis, and in those diagnosed at an older age.
They cited one study that found regular surveillance of patients with primary sclerosing cholangitis was associated with significantly higher 5-year survival rates, compared with those no regular screening (68% vs. 20%; P less than .0061).
In terms of surveillance modalities, the update suggested 6- to 12-monthly imaging of the biliary tree with ultrasound computed tomography, computed tomography, or magnetic resonance imaging – with or without serum carbohydrate antigen 19-9. However the authors wrote that MRI was often preferred to CT because of its superior sensitivity.
They advised against endoscopic retrograde cholangiopancreatography with brush cytology for routine surveillance because of procedural risks. On the other hand, they suggested this procedure, with or without fluorescence in situ hybridization analysis and/or cholangioscopy, could be used for investigation.
“In addition to ERCP [endoscopic retrograde cholangiopancreatography] with brushings, endoscopic ultrasound, intraductal ultrasonography, and cholangioscopy may be used to direct biopsy sampling,” they wrote. Symptoms such as increasing cholestatic biochemistry values, jaundice, fever, right upper quadrant pain, or pruritus should trigger evaluation for cholangiocarcinoma.
However the authors advised “great caution” with the use of fine-needle aspiration of perihilar biliary strictures in liver transplant candidates because of the risk of tumor seeding if the lesion turned out to be cholangiocarcinoma.
On the question of cholangiocarcinoma surveillance in pediatric patients and those with small-duct primary sclerosing cholangitis, the authors wrote that cholangiocarcinoma was so rare in these patients that routine cholangiocarcinoma surveillance was not required.
The clinical update also looked at the prevalence and risk factors for gallbladder cancer, which affects around 2% of individuals with primary sclerosing cholangitis. Two studies found gallbladder polyps in 10%-17% of patients, but the authors noted that “the optimal modality for diagnosis of gallbladder polyps in PSC remains unknown”.
“Because of the high risk of malignancy in gallbladder mass lesions and a 5-year survival rate of 5% to 10% for gallbladder cancer, patients should undergo annual US [ultrasound] screening,” they wrote.
They said the question of whether to perform a cholecystectomy in patients with gallbladder polyps should be guided by the size and growth of the polyps because there is an increased risk of gallbladder cancer in polyps larger than 8 mm and by the clinical status of the patient.
Finally, the update examined the issue of hepatocellular carcinoma in patients with primary sclerosing cholangitis, which – while rare – may increase with the presence of cirrhosis.
The authors advised that patients with primary sclerosing cholangitis and cirrhosis should undergo surveillance for hepatocellular carcinoma every 6 months with ultrasound, CT, or MRI.
“We anticipate that with the development of large patient cohorts, advances in uncovering genetic and other risk factors for cholangiocarcinoma, and development of effective treatments for PSC, further refinement of this practice update will be required.”
Two authors declared consultancies, grants and research contracts with the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Bowlus C et al. Clin Gastroenterol Hepatol. 2019 Jul 12. doi 10.1016/j.cgh.2019.07.011.
All adult patients with primary sclerosing cholangitis should be screened at least annually for cholangiocarcinoma and gallbladder cancer, particularly in the first year after their diagnosis, according to a clinical practice update published in Clinical Gastroenterology and Hepatology.
Individuals with primary sclerosing cholangitis have a 400-fold higher risk of cholangiocarcinoma, compared with the general population, and around one-third of cancers are detected within 1 year of the cholangitis diagnosis, Christopher L. Bowlus, MD, of the University of California, Davis, and coauthors wrote.
The clinical practice update from the American Gastroenterological Association was in response to the observation that, while there is significant evidence for an increasing incidence of cirrhosis, hepatic decompensation, hepatocellular carcinoma, and liver transplant listing among patients with primary sclerosing cholangitis, there is a lack of good evidence to guide cholangiocarcinoma surveillance in these patients.
“The low prevalence and long duration of PSC [primary sclerosing cholangitis] present substantial barriers to better understanding risk stratification, developing biomarkers, and measuring the impact surveillance has on clinical outcomes,” they wrote.
The first recommendation was that surveillance for cholangiocarcinoma and gallbladder cancer should be considered in all adult patients with primary sclerosing cholangitis, regardless of their disease stage. The authors especially emphasized the importance of surveillance in the first year after a diagnosis of primary sclerosing cholangitis, in patients who also have ulcerative colitis, and in those diagnosed at an older age.
They cited one study that found regular surveillance of patients with primary sclerosing cholangitis was associated with significantly higher 5-year survival rates, compared with those no regular screening (68% vs. 20%; P less than .0061).
In terms of surveillance modalities, the update suggested 6- to 12-monthly imaging of the biliary tree with ultrasound computed tomography, computed tomography, or magnetic resonance imaging – with or without serum carbohydrate antigen 19-9. However the authors wrote that MRI was often preferred to CT because of its superior sensitivity.
They advised against endoscopic retrograde cholangiopancreatography with brush cytology for routine surveillance because of procedural risks. On the other hand, they suggested this procedure, with or without fluorescence in situ hybridization analysis and/or cholangioscopy, could be used for investigation.
“In addition to ERCP [endoscopic retrograde cholangiopancreatography] with brushings, endoscopic ultrasound, intraductal ultrasonography, and cholangioscopy may be used to direct biopsy sampling,” they wrote. Symptoms such as increasing cholestatic biochemistry values, jaundice, fever, right upper quadrant pain, or pruritus should trigger evaluation for cholangiocarcinoma.
However the authors advised “great caution” with the use of fine-needle aspiration of perihilar biliary strictures in liver transplant candidates because of the risk of tumor seeding if the lesion turned out to be cholangiocarcinoma.
On the question of cholangiocarcinoma surveillance in pediatric patients and those with small-duct primary sclerosing cholangitis, the authors wrote that cholangiocarcinoma was so rare in these patients that routine cholangiocarcinoma surveillance was not required.
The clinical update also looked at the prevalence and risk factors for gallbladder cancer, which affects around 2% of individuals with primary sclerosing cholangitis. Two studies found gallbladder polyps in 10%-17% of patients, but the authors noted that “the optimal modality for diagnosis of gallbladder polyps in PSC remains unknown”.
“Because of the high risk of malignancy in gallbladder mass lesions and a 5-year survival rate of 5% to 10% for gallbladder cancer, patients should undergo annual US [ultrasound] screening,” they wrote.
They said the question of whether to perform a cholecystectomy in patients with gallbladder polyps should be guided by the size and growth of the polyps because there is an increased risk of gallbladder cancer in polyps larger than 8 mm and by the clinical status of the patient.
Finally, the update examined the issue of hepatocellular carcinoma in patients with primary sclerosing cholangitis, which – while rare – may increase with the presence of cirrhosis.
The authors advised that patients with primary sclerosing cholangitis and cirrhosis should undergo surveillance for hepatocellular carcinoma every 6 months with ultrasound, CT, or MRI.
“We anticipate that with the development of large patient cohorts, advances in uncovering genetic and other risk factors for cholangiocarcinoma, and development of effective treatments for PSC, further refinement of this practice update will be required.”
Two authors declared consultancies, grants and research contracts with the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Bowlus C et al. Clin Gastroenterol Hepatol. 2019 Jul 12. doi 10.1016/j.cgh.2019.07.011.
All adult patients with primary sclerosing cholangitis should be screened at least annually for cholangiocarcinoma and gallbladder cancer, particularly in the first year after their diagnosis, according to a clinical practice update published in Clinical Gastroenterology and Hepatology.
Individuals with primary sclerosing cholangitis have a 400-fold higher risk of cholangiocarcinoma, compared with the general population, and around one-third of cancers are detected within 1 year of the cholangitis diagnosis, Christopher L. Bowlus, MD, of the University of California, Davis, and coauthors wrote.
The clinical practice update from the American Gastroenterological Association was in response to the observation that, while there is significant evidence for an increasing incidence of cirrhosis, hepatic decompensation, hepatocellular carcinoma, and liver transplant listing among patients with primary sclerosing cholangitis, there is a lack of good evidence to guide cholangiocarcinoma surveillance in these patients.
“The low prevalence and long duration of PSC [primary sclerosing cholangitis] present substantial barriers to better understanding risk stratification, developing biomarkers, and measuring the impact surveillance has on clinical outcomes,” they wrote.
The first recommendation was that surveillance for cholangiocarcinoma and gallbladder cancer should be considered in all adult patients with primary sclerosing cholangitis, regardless of their disease stage. The authors especially emphasized the importance of surveillance in the first year after a diagnosis of primary sclerosing cholangitis, in patients who also have ulcerative colitis, and in those diagnosed at an older age.
They cited one study that found regular surveillance of patients with primary sclerosing cholangitis was associated with significantly higher 5-year survival rates, compared with those no regular screening (68% vs. 20%; P less than .0061).
In terms of surveillance modalities, the update suggested 6- to 12-monthly imaging of the biliary tree with ultrasound computed tomography, computed tomography, or magnetic resonance imaging – with or without serum carbohydrate antigen 19-9. However the authors wrote that MRI was often preferred to CT because of its superior sensitivity.
They advised against endoscopic retrograde cholangiopancreatography with brush cytology for routine surveillance because of procedural risks. On the other hand, they suggested this procedure, with or without fluorescence in situ hybridization analysis and/or cholangioscopy, could be used for investigation.
“In addition to ERCP [endoscopic retrograde cholangiopancreatography] with brushings, endoscopic ultrasound, intraductal ultrasonography, and cholangioscopy may be used to direct biopsy sampling,” they wrote. Symptoms such as increasing cholestatic biochemistry values, jaundice, fever, right upper quadrant pain, or pruritus should trigger evaluation for cholangiocarcinoma.
However the authors advised “great caution” with the use of fine-needle aspiration of perihilar biliary strictures in liver transplant candidates because of the risk of tumor seeding if the lesion turned out to be cholangiocarcinoma.
On the question of cholangiocarcinoma surveillance in pediatric patients and those with small-duct primary sclerosing cholangitis, the authors wrote that cholangiocarcinoma was so rare in these patients that routine cholangiocarcinoma surveillance was not required.
The clinical update also looked at the prevalence and risk factors for gallbladder cancer, which affects around 2% of individuals with primary sclerosing cholangitis. Two studies found gallbladder polyps in 10%-17% of patients, but the authors noted that “the optimal modality for diagnosis of gallbladder polyps in PSC remains unknown”.
“Because of the high risk of malignancy in gallbladder mass lesions and a 5-year survival rate of 5% to 10% for gallbladder cancer, patients should undergo annual US [ultrasound] screening,” they wrote.
They said the question of whether to perform a cholecystectomy in patients with gallbladder polyps should be guided by the size and growth of the polyps because there is an increased risk of gallbladder cancer in polyps larger than 8 mm and by the clinical status of the patient.
Finally, the update examined the issue of hepatocellular carcinoma in patients with primary sclerosing cholangitis, which – while rare – may increase with the presence of cirrhosis.
The authors advised that patients with primary sclerosing cholangitis and cirrhosis should undergo surveillance for hepatocellular carcinoma every 6 months with ultrasound, CT, or MRI.
“We anticipate that with the development of large patient cohorts, advances in uncovering genetic and other risk factors for cholangiocarcinoma, and development of effective treatments for PSC, further refinement of this practice update will be required.”
Two authors declared consultancies, grants and research contracts with the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Bowlus C et al. Clin Gastroenterol Hepatol. 2019 Jul 12. doi 10.1016/j.cgh.2019.07.011.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY