User login
They enrolled in medical school to practice rural medicine. What happened?
SALINA, KAN. – The University of Kansas School of Medicine–Salina opened in 2011 – a one-building campus in the heart of wheat country dedicated to producing the rural doctors that the country needs.
Now, 8 years later, the school’s first graduates are settling into their chosen practices – and locales. And those choices are cause for both hope and despair.
Of the eight graduates, just three chose to go where the shortages are most evident. Two went to small cities with populations of fewer than 50,000. And three chose the big cities of Topeka (estimated 2018 population: 125,904) and Wichita (389,255) instead.
But the mission is critical: About two-thirds of the primary care health professional shortage areas designated by the federal Health Resources and Services Administration in June were in rural or partially rural areas. And it’s only getting worse.
As more baby boomer doctors in rural areas reach retirement age, not nearly enough physicians are willing to take their place. By 2030, the New England Journal of Medicine predicts, nearly a quarter fewer rural physicians will be practicing medicine than today. Over half of rural doctors were at least 50 years old in 2017.
So Salina’s creation of a few rural physicians a year is a start, and, surprisingly, one of the country’s most promising.
Only 40 out of the nation’s more than 180 medical schools offer a rural track. The Association of American Medical Colleges ranked KU School of Medicine, which includes Salina, Wichita and Kansas City campuses, in the 96th percentile last year for producing doctors working in rural settings 10-15 years after graduation.
“The addition of one physician is huge,” said William Cathcart-Rake, MD, the founding dean of the Salina campus. “One physician choosing to come may be the difference of communities surviving or dissolving.”
The draw of rural life
By placing the new campus in Salina (population: 46,716), surrounded by small towns for at least 50 miles in every direction, the university hoped to attract and foster students who had – and would deepen – a bond to rural communities.
And, for some, it worked out pretty much as planned.
One of the school’s first graduates, Sara Ritterling Patry, MD, lives in Hutchinson (population: 40,623). Less than an hour from Wichita, it isn’t the most rural community, but it’s small enough that she still runs into her patients at Dillons, the local grocery store.
“Just being in a smaller community like this feels like to me that I can actually get to know my patients and spend a little extra time with them,” she said.
After all, part of the allure of a rural practice is providing care womb to tomb. The doctor learns how to deliver the town’s babies, while serving as the county coroner and the public health expert all at once, said Robert Moser, MD, the head of the University of Kansas School of Medicine–Salina and former head of the state health department.
He would know – he worked for 22 years in Tribune, Kan. (population: 742).
For another of the original Salina eight, Tyson Wisinger, MD, that calling brought him back to his hometown of Phillipsburg (population: 2,486) after his residency. His kids will go to his old high school, where his graduating class was all of 13 people, and he’ll take care of their baseball teammates. Plus, they’ll grow up living minutes away from generations of extended family.
“I can’t have imagined a situation that could have been more rewarding,” Dr. Wisinger said.
The rural challenge
But the road to rural family medicine also includes a thing called “windshield time” – the amount of time needed to travel between clinics or head to the closest Walmart.
Then there’s figuring out just how far their patients will need to drive to get to the nearest hospital – which for Daniel Linville, MD, and Jill Corpstein Linville, MD, is a solid 4 hours for more advanced care from their new practice in Lakin, Kan. (population: 2,195).
Their outpost in southwestern Kansas can feel a little bit like a fishbowl. “We do life with some of our patients,” Dr. Corpstein Linville said.
Already, the Linvilles have delivered babies and handled a variety of ailments there.
The pair met and married during their 4 years in Salina – they jokingly call it a “full-service med school.” They completed a family medicine residency in Muncie, Ind. Then they were recruited by a rural practice that helped them avoid what Dr. Moser calls the most dreaded words in rural medicine: “solo practice.”
New doctors don’t want to practice alone, especially as they develop their sea legs, because of the strains of constantly being on call and having singular responsibility for a town. Telemedicine, where doctors can easily consult with other physicians around the country via Web video or phone, is helping, as are physician assistants.
Diverging from the path
Claire Hinrichsen Groskurth, MD, another member of the first graduating class, always intended to return to a small town similar to where she grew up.
“The first thing that threw me off was I fell in love with surgery and ob.gyn.,” she said. “Then the second thing that threw me off was marrying another doctor,” whose life goals headed in a different direction.
She’d been a member of the Scholars in Rural Health program at Kansas University that seeks out rural college students who are interested in medicine. She also had committed to the Kansas Medical Student Loan program, which promises to forgive physicians’ tuition and gives a monthly stipend if they agree to work in counties that need physicians, or in other critical capacities.
But when she realized she might specialize, she decided to take out federal loans for her final years. She had to pay back the first year of the special loan with 15% interest.
Plus, her now-husband, who went to Kansas University’s Wichita campus, needed to be in a large enough city to accommodate further training to become a surgeon. So Dr. Hinrichsen Groskurth delivers babies as she thought she would – but in Wichita.
The spousal coin can flip both ways: Dr. Ritterling Patry needed to find a place that worked for her husband’s farming of corn, sorghum, soybeans and wheat. So the smaller city of Hutchinson it was.
Flaws in the pipeline
Most medical school students come from urban areas and are destined to stay there, said Alan Morgan, the head of the National Rural Health Association. Producing doctors for the vast swaths of rural America needs to be more of a priority at every step in the education pipeline, experts said.
Many academic centers sell students on the party line that they’ll be overworked, underappreciated and underpaid, according to Mark Deutchman, MD, director of the University of Colorado School of Medicine’s rural program. “They take people who are interested in primary care or rural and beat it out of them throughout their training,” he said.
And that kind of rhetoric often influences the opinion of their medical school peers, which those in rural health might resent.
“Small does not mean stupid,” Dr. Moser said.
Medical students everywhere should be exposed to rural options, according to Randall Longenecker, MD, who runs Ohio University Heritage College of Osteopathic Medicine’s rural programs in Athens.
“If a medical student never ever goes to a rural place, they never find out,” he said. “That’s why students need to meet rural doctors who love what they do.”
The federal government recently allocated $20 million in grants to help create 27 rural residency programs – institutions where newly minted doctors go for practical training before they can be fully licensed. That’s a big jump from the 92 programs now active.
For Dr. Corpstein Linville, the pipeline also needs to start at more schools like Salina that are promoting rural medicine from day one.
“So when you hear rural medicine, you know that it’s a thing and don’t kind of cringe,” she said. “You don’t think it’s someone taking care of a cow.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
SALINA, KAN. – The University of Kansas School of Medicine–Salina opened in 2011 – a one-building campus in the heart of wheat country dedicated to producing the rural doctors that the country needs.
Now, 8 years later, the school’s first graduates are settling into their chosen practices – and locales. And those choices are cause for both hope and despair.
Of the eight graduates, just three chose to go where the shortages are most evident. Two went to small cities with populations of fewer than 50,000. And three chose the big cities of Topeka (estimated 2018 population: 125,904) and Wichita (389,255) instead.
But the mission is critical: About two-thirds of the primary care health professional shortage areas designated by the federal Health Resources and Services Administration in June were in rural or partially rural areas. And it’s only getting worse.
As more baby boomer doctors in rural areas reach retirement age, not nearly enough physicians are willing to take their place. By 2030, the New England Journal of Medicine predicts, nearly a quarter fewer rural physicians will be practicing medicine than today. Over half of rural doctors were at least 50 years old in 2017.
So Salina’s creation of a few rural physicians a year is a start, and, surprisingly, one of the country’s most promising.
Only 40 out of the nation’s more than 180 medical schools offer a rural track. The Association of American Medical Colleges ranked KU School of Medicine, which includes Salina, Wichita and Kansas City campuses, in the 96th percentile last year for producing doctors working in rural settings 10-15 years after graduation.
“The addition of one physician is huge,” said William Cathcart-Rake, MD, the founding dean of the Salina campus. “One physician choosing to come may be the difference of communities surviving or dissolving.”
The draw of rural life
By placing the new campus in Salina (population: 46,716), surrounded by small towns for at least 50 miles in every direction, the university hoped to attract and foster students who had – and would deepen – a bond to rural communities.
And, for some, it worked out pretty much as planned.
One of the school’s first graduates, Sara Ritterling Patry, MD, lives in Hutchinson (population: 40,623). Less than an hour from Wichita, it isn’t the most rural community, but it’s small enough that she still runs into her patients at Dillons, the local grocery store.
“Just being in a smaller community like this feels like to me that I can actually get to know my patients and spend a little extra time with them,” she said.
After all, part of the allure of a rural practice is providing care womb to tomb. The doctor learns how to deliver the town’s babies, while serving as the county coroner and the public health expert all at once, said Robert Moser, MD, the head of the University of Kansas School of Medicine–Salina and former head of the state health department.
He would know – he worked for 22 years in Tribune, Kan. (population: 742).
For another of the original Salina eight, Tyson Wisinger, MD, that calling brought him back to his hometown of Phillipsburg (population: 2,486) after his residency. His kids will go to his old high school, where his graduating class was all of 13 people, and he’ll take care of their baseball teammates. Plus, they’ll grow up living minutes away from generations of extended family.
“I can’t have imagined a situation that could have been more rewarding,” Dr. Wisinger said.
The rural challenge
But the road to rural family medicine also includes a thing called “windshield time” – the amount of time needed to travel between clinics or head to the closest Walmart.
Then there’s figuring out just how far their patients will need to drive to get to the nearest hospital – which for Daniel Linville, MD, and Jill Corpstein Linville, MD, is a solid 4 hours for more advanced care from their new practice in Lakin, Kan. (population: 2,195).
Their outpost in southwestern Kansas can feel a little bit like a fishbowl. “We do life with some of our patients,” Dr. Corpstein Linville said.
Already, the Linvilles have delivered babies and handled a variety of ailments there.
The pair met and married during their 4 years in Salina – they jokingly call it a “full-service med school.” They completed a family medicine residency in Muncie, Ind. Then they were recruited by a rural practice that helped them avoid what Dr. Moser calls the most dreaded words in rural medicine: “solo practice.”
New doctors don’t want to practice alone, especially as they develop their sea legs, because of the strains of constantly being on call and having singular responsibility for a town. Telemedicine, where doctors can easily consult with other physicians around the country via Web video or phone, is helping, as are physician assistants.
Diverging from the path
Claire Hinrichsen Groskurth, MD, another member of the first graduating class, always intended to return to a small town similar to where she grew up.
“The first thing that threw me off was I fell in love with surgery and ob.gyn.,” she said. “Then the second thing that threw me off was marrying another doctor,” whose life goals headed in a different direction.
She’d been a member of the Scholars in Rural Health program at Kansas University that seeks out rural college students who are interested in medicine. She also had committed to the Kansas Medical Student Loan program, which promises to forgive physicians’ tuition and gives a monthly stipend if they agree to work in counties that need physicians, or in other critical capacities.
But when she realized she might specialize, she decided to take out federal loans for her final years. She had to pay back the first year of the special loan with 15% interest.
Plus, her now-husband, who went to Kansas University’s Wichita campus, needed to be in a large enough city to accommodate further training to become a surgeon. So Dr. Hinrichsen Groskurth delivers babies as she thought she would – but in Wichita.
The spousal coin can flip both ways: Dr. Ritterling Patry needed to find a place that worked for her husband’s farming of corn, sorghum, soybeans and wheat. So the smaller city of Hutchinson it was.
Flaws in the pipeline
Most medical school students come from urban areas and are destined to stay there, said Alan Morgan, the head of the National Rural Health Association. Producing doctors for the vast swaths of rural America needs to be more of a priority at every step in the education pipeline, experts said.
Many academic centers sell students on the party line that they’ll be overworked, underappreciated and underpaid, according to Mark Deutchman, MD, director of the University of Colorado School of Medicine’s rural program. “They take people who are interested in primary care or rural and beat it out of them throughout their training,” he said.
And that kind of rhetoric often influences the opinion of their medical school peers, which those in rural health might resent.
“Small does not mean stupid,” Dr. Moser said.
Medical students everywhere should be exposed to rural options, according to Randall Longenecker, MD, who runs Ohio University Heritage College of Osteopathic Medicine’s rural programs in Athens.
“If a medical student never ever goes to a rural place, they never find out,” he said. “That’s why students need to meet rural doctors who love what they do.”
The federal government recently allocated $20 million in grants to help create 27 rural residency programs – institutions where newly minted doctors go for practical training before they can be fully licensed. That’s a big jump from the 92 programs now active.
For Dr. Corpstein Linville, the pipeline also needs to start at more schools like Salina that are promoting rural medicine from day one.
“So when you hear rural medicine, you know that it’s a thing and don’t kind of cringe,” she said. “You don’t think it’s someone taking care of a cow.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
SALINA, KAN. – The University of Kansas School of Medicine–Salina opened in 2011 – a one-building campus in the heart of wheat country dedicated to producing the rural doctors that the country needs.
Now, 8 years later, the school’s first graduates are settling into their chosen practices – and locales. And those choices are cause for both hope and despair.
Of the eight graduates, just three chose to go where the shortages are most evident. Two went to small cities with populations of fewer than 50,000. And three chose the big cities of Topeka (estimated 2018 population: 125,904) and Wichita (389,255) instead.
But the mission is critical: About two-thirds of the primary care health professional shortage areas designated by the federal Health Resources and Services Administration in June were in rural or partially rural areas. And it’s only getting worse.
As more baby boomer doctors in rural areas reach retirement age, not nearly enough physicians are willing to take their place. By 2030, the New England Journal of Medicine predicts, nearly a quarter fewer rural physicians will be practicing medicine than today. Over half of rural doctors were at least 50 years old in 2017.
So Salina’s creation of a few rural physicians a year is a start, and, surprisingly, one of the country’s most promising.
Only 40 out of the nation’s more than 180 medical schools offer a rural track. The Association of American Medical Colleges ranked KU School of Medicine, which includes Salina, Wichita and Kansas City campuses, in the 96th percentile last year for producing doctors working in rural settings 10-15 years after graduation.
“The addition of one physician is huge,” said William Cathcart-Rake, MD, the founding dean of the Salina campus. “One physician choosing to come may be the difference of communities surviving or dissolving.”
The draw of rural life
By placing the new campus in Salina (population: 46,716), surrounded by small towns for at least 50 miles in every direction, the university hoped to attract and foster students who had – and would deepen – a bond to rural communities.
And, for some, it worked out pretty much as planned.
One of the school’s first graduates, Sara Ritterling Patry, MD, lives in Hutchinson (population: 40,623). Less than an hour from Wichita, it isn’t the most rural community, but it’s small enough that she still runs into her patients at Dillons, the local grocery store.
“Just being in a smaller community like this feels like to me that I can actually get to know my patients and spend a little extra time with them,” she said.
After all, part of the allure of a rural practice is providing care womb to tomb. The doctor learns how to deliver the town’s babies, while serving as the county coroner and the public health expert all at once, said Robert Moser, MD, the head of the University of Kansas School of Medicine–Salina and former head of the state health department.
He would know – he worked for 22 years in Tribune, Kan. (population: 742).
For another of the original Salina eight, Tyson Wisinger, MD, that calling brought him back to his hometown of Phillipsburg (population: 2,486) after his residency. His kids will go to his old high school, where his graduating class was all of 13 people, and he’ll take care of their baseball teammates. Plus, they’ll grow up living minutes away from generations of extended family.
“I can’t have imagined a situation that could have been more rewarding,” Dr. Wisinger said.
The rural challenge
But the road to rural family medicine also includes a thing called “windshield time” – the amount of time needed to travel between clinics or head to the closest Walmart.
Then there’s figuring out just how far their patients will need to drive to get to the nearest hospital – which for Daniel Linville, MD, and Jill Corpstein Linville, MD, is a solid 4 hours for more advanced care from their new practice in Lakin, Kan. (population: 2,195).
Their outpost in southwestern Kansas can feel a little bit like a fishbowl. “We do life with some of our patients,” Dr. Corpstein Linville said.
Already, the Linvilles have delivered babies and handled a variety of ailments there.
The pair met and married during their 4 years in Salina – they jokingly call it a “full-service med school.” They completed a family medicine residency in Muncie, Ind. Then they were recruited by a rural practice that helped them avoid what Dr. Moser calls the most dreaded words in rural medicine: “solo practice.”
New doctors don’t want to practice alone, especially as they develop their sea legs, because of the strains of constantly being on call and having singular responsibility for a town. Telemedicine, where doctors can easily consult with other physicians around the country via Web video or phone, is helping, as are physician assistants.
Diverging from the path
Claire Hinrichsen Groskurth, MD, another member of the first graduating class, always intended to return to a small town similar to where she grew up.
“The first thing that threw me off was I fell in love with surgery and ob.gyn.,” she said. “Then the second thing that threw me off was marrying another doctor,” whose life goals headed in a different direction.
She’d been a member of the Scholars in Rural Health program at Kansas University that seeks out rural college students who are interested in medicine. She also had committed to the Kansas Medical Student Loan program, which promises to forgive physicians’ tuition and gives a monthly stipend if they agree to work in counties that need physicians, or in other critical capacities.
But when she realized she might specialize, she decided to take out federal loans for her final years. She had to pay back the first year of the special loan with 15% interest.
Plus, her now-husband, who went to Kansas University’s Wichita campus, needed to be in a large enough city to accommodate further training to become a surgeon. So Dr. Hinrichsen Groskurth delivers babies as she thought she would – but in Wichita.
The spousal coin can flip both ways: Dr. Ritterling Patry needed to find a place that worked for her husband’s farming of corn, sorghum, soybeans and wheat. So the smaller city of Hutchinson it was.
Flaws in the pipeline
Most medical school students come from urban areas and are destined to stay there, said Alan Morgan, the head of the National Rural Health Association. Producing doctors for the vast swaths of rural America needs to be more of a priority at every step in the education pipeline, experts said.
Many academic centers sell students on the party line that they’ll be overworked, underappreciated and underpaid, according to Mark Deutchman, MD, director of the University of Colorado School of Medicine’s rural program. “They take people who are interested in primary care or rural and beat it out of them throughout their training,” he said.
And that kind of rhetoric often influences the opinion of their medical school peers, which those in rural health might resent.
“Small does not mean stupid,” Dr. Moser said.
Medical students everywhere should be exposed to rural options, according to Randall Longenecker, MD, who runs Ohio University Heritage College of Osteopathic Medicine’s rural programs in Athens.
“If a medical student never ever goes to a rural place, they never find out,” he said. “That’s why students need to meet rural doctors who love what they do.”
The federal government recently allocated $20 million in grants to help create 27 rural residency programs – institutions where newly minted doctors go for practical training before they can be fully licensed. That’s a big jump from the 92 programs now active.
For Dr. Corpstein Linville, the pipeline also needs to start at more schools like Salina that are promoting rural medicine from day one.
“So when you hear rural medicine, you know that it’s a thing and don’t kind of cringe,” she said. “You don’t think it’s someone taking care of a cow.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Trump: No health insurance, no U.S. entry
Health insurance or the ability to pay for care soon will be a requirement for immigrants seeking U.S. visas, under a proclamation from President Trump.
Effective Nov. 3, 2019, visa applicants must demonstrate to immigration authorities that they can obtain coverage by an approved health insurer within 30 days of entering the United States or show evidence they possess the financial resources to pay for foreseeable medical costs. Approved coverage includes, but is not limited to, an employer-sponsored plan, an unsubsidized health plan offered in the individual market, a family member’s plan, or a visitor health insurance plan that provides coverage for at least 364 days, according to the proclamation.
President Trump said that the restriction protects Americans from bearing the burden of uncompensated health care costs generated by immigrants.
“While our health care system grapples with the challenges caused by uncompensated care, the United States government is making the problem worse by admitting thousands of aliens who have not demonstrated any ability to pay for their health care costs,” President Trump said in the proclamation. “Notably, data show that lawful immigrants are about three times more likely than United States citizens to lack health insurance. Immigrants who enter this country should not further saddle our health care system, and subsequently American taxpayers, with higher costs.”
The rule does not apply to refugees or asylum seekers, immigrants holding valid visas prior to Nov. 3, noncitizen children of U.S. citizens, unaccompanied minors, or permanent residents returning to the country after less than a year overseas.
The rule is the latest in a series of immigration restrictions by President Trump. Most recently in August 2019, the Trump administration amended the federal public charge rule, a longstanding policy that allows authorities to refuse to admit immigrants into the United States – or to adjust their legal status – if they are deemed likely to become a public charge. The revised regulation allows officials to consider previously excluded programs in their determination, including nonemergency Medicaid for nonpregnant adults, the Supplemental Nutrition Assistance Program, and several housing programs.
The proclamation is consistent with the President’s recent public charge rule, and it is sound immigration policy, said Dale Wilcox, executive director and general counsel for the Immigration Reform Law Institute.
“If U.S. citizens are responsible for the health care costs of the world, then the world will show up at our doorstep for free health care,” Mr. Wilcox said in an interview. “That is unfair to American citizens and would financially devastate health care providers. The impact of this proclamation would be a more manageable immigration policy that welcomes immigrants who will contribute to our country as well as benefit from it.”
J. Wesley Boyd, MD, of the Center for Bioethics at Harvard University, Boston, said he was saddened to learn about the proclamation, adding that the Trump administration is relying on inaccurate information and falsified facts to justify the new restriction. Dr. Boyd, cofounder of the Human Rights and Asylum Clinic at Cambridge Health Alliance in Cambridge, Mass., coauthored a 2018 study finding that immigrants pay more into the U.S. health care system than they use.
That analysis, which examined 188 peer-reviewed studies related to immigrants and U.S. health care expenditures, found that per capita expenditures from private and public insurance sources were about 40% lower for immigrants, compared with native-born Americans. Expenditures for undocumented immigrants were even lower. Immigrants also made a greater contribution to Medicare’s trust fund than they withdrew, the study found (Int J Health Serv. 2018 Aug. 8 doi: 10.1177/0020731418791963).
Immigrants use less health care resources because they are generally younger and healthier than native-born Americans, and they are less likely to access health care services – regardless of health status, Dr. Boyd said in an interview. In addition, many immigrants who contribute to Medicare through payroll and/or taxes are no longer in the U.S. when they reach Medicare age.
“The Trump administration, in this case, is making yet another set of excuses for why they are trying to keep immigrants out of the country,” Dr. Boyd said. “The potential impact of this restriction is devastating. Obviously, the Trump administration is doing anything that it possibly can to try to limit immigrants from coming into our country and seeking a better life for themselves.”
The proclamation also could have a chilling effect on immigrants currently in the United States who are in need of medical treatment, Dr. Boyd noted.
“The real effect is you’re going to have immigrants who are already here, not [accessing] health care when they need it, for fear they somehow become known to the government,” he said.
Health insurance or the ability to pay for care soon will be a requirement for immigrants seeking U.S. visas, under a proclamation from President Trump.
Effective Nov. 3, 2019, visa applicants must demonstrate to immigration authorities that they can obtain coverage by an approved health insurer within 30 days of entering the United States or show evidence they possess the financial resources to pay for foreseeable medical costs. Approved coverage includes, but is not limited to, an employer-sponsored plan, an unsubsidized health plan offered in the individual market, a family member’s plan, or a visitor health insurance plan that provides coverage for at least 364 days, according to the proclamation.
President Trump said that the restriction protects Americans from bearing the burden of uncompensated health care costs generated by immigrants.
“While our health care system grapples with the challenges caused by uncompensated care, the United States government is making the problem worse by admitting thousands of aliens who have not demonstrated any ability to pay for their health care costs,” President Trump said in the proclamation. “Notably, data show that lawful immigrants are about three times more likely than United States citizens to lack health insurance. Immigrants who enter this country should not further saddle our health care system, and subsequently American taxpayers, with higher costs.”
The rule does not apply to refugees or asylum seekers, immigrants holding valid visas prior to Nov. 3, noncitizen children of U.S. citizens, unaccompanied minors, or permanent residents returning to the country after less than a year overseas.
The rule is the latest in a series of immigration restrictions by President Trump. Most recently in August 2019, the Trump administration amended the federal public charge rule, a longstanding policy that allows authorities to refuse to admit immigrants into the United States – or to adjust their legal status – if they are deemed likely to become a public charge. The revised regulation allows officials to consider previously excluded programs in their determination, including nonemergency Medicaid for nonpregnant adults, the Supplemental Nutrition Assistance Program, and several housing programs.
The proclamation is consistent with the President’s recent public charge rule, and it is sound immigration policy, said Dale Wilcox, executive director and general counsel for the Immigration Reform Law Institute.
“If U.S. citizens are responsible for the health care costs of the world, then the world will show up at our doorstep for free health care,” Mr. Wilcox said in an interview. “That is unfair to American citizens and would financially devastate health care providers. The impact of this proclamation would be a more manageable immigration policy that welcomes immigrants who will contribute to our country as well as benefit from it.”
J. Wesley Boyd, MD, of the Center for Bioethics at Harvard University, Boston, said he was saddened to learn about the proclamation, adding that the Trump administration is relying on inaccurate information and falsified facts to justify the new restriction. Dr. Boyd, cofounder of the Human Rights and Asylum Clinic at Cambridge Health Alliance in Cambridge, Mass., coauthored a 2018 study finding that immigrants pay more into the U.S. health care system than they use.
That analysis, which examined 188 peer-reviewed studies related to immigrants and U.S. health care expenditures, found that per capita expenditures from private and public insurance sources were about 40% lower for immigrants, compared with native-born Americans. Expenditures for undocumented immigrants were even lower. Immigrants also made a greater contribution to Medicare’s trust fund than they withdrew, the study found (Int J Health Serv. 2018 Aug. 8 doi: 10.1177/0020731418791963).
Immigrants use less health care resources because they are generally younger and healthier than native-born Americans, and they are less likely to access health care services – regardless of health status, Dr. Boyd said in an interview. In addition, many immigrants who contribute to Medicare through payroll and/or taxes are no longer in the U.S. when they reach Medicare age.
“The Trump administration, in this case, is making yet another set of excuses for why they are trying to keep immigrants out of the country,” Dr. Boyd said. “The potential impact of this restriction is devastating. Obviously, the Trump administration is doing anything that it possibly can to try to limit immigrants from coming into our country and seeking a better life for themselves.”
The proclamation also could have a chilling effect on immigrants currently in the United States who are in need of medical treatment, Dr. Boyd noted.
“The real effect is you’re going to have immigrants who are already here, not [accessing] health care when they need it, for fear they somehow become known to the government,” he said.
Health insurance or the ability to pay for care soon will be a requirement for immigrants seeking U.S. visas, under a proclamation from President Trump.
Effective Nov. 3, 2019, visa applicants must demonstrate to immigration authorities that they can obtain coverage by an approved health insurer within 30 days of entering the United States or show evidence they possess the financial resources to pay for foreseeable medical costs. Approved coverage includes, but is not limited to, an employer-sponsored plan, an unsubsidized health plan offered in the individual market, a family member’s plan, or a visitor health insurance plan that provides coverage for at least 364 days, according to the proclamation.
President Trump said that the restriction protects Americans from bearing the burden of uncompensated health care costs generated by immigrants.
“While our health care system grapples with the challenges caused by uncompensated care, the United States government is making the problem worse by admitting thousands of aliens who have not demonstrated any ability to pay for their health care costs,” President Trump said in the proclamation. “Notably, data show that lawful immigrants are about three times more likely than United States citizens to lack health insurance. Immigrants who enter this country should not further saddle our health care system, and subsequently American taxpayers, with higher costs.”
The rule does not apply to refugees or asylum seekers, immigrants holding valid visas prior to Nov. 3, noncitizen children of U.S. citizens, unaccompanied minors, or permanent residents returning to the country after less than a year overseas.
The rule is the latest in a series of immigration restrictions by President Trump. Most recently in August 2019, the Trump administration amended the federal public charge rule, a longstanding policy that allows authorities to refuse to admit immigrants into the United States – or to adjust their legal status – if they are deemed likely to become a public charge. The revised regulation allows officials to consider previously excluded programs in their determination, including nonemergency Medicaid for nonpregnant adults, the Supplemental Nutrition Assistance Program, and several housing programs.
The proclamation is consistent with the President’s recent public charge rule, and it is sound immigration policy, said Dale Wilcox, executive director and general counsel for the Immigration Reform Law Institute.
“If U.S. citizens are responsible for the health care costs of the world, then the world will show up at our doorstep for free health care,” Mr. Wilcox said in an interview. “That is unfair to American citizens and would financially devastate health care providers. The impact of this proclamation would be a more manageable immigration policy that welcomes immigrants who will contribute to our country as well as benefit from it.”
J. Wesley Boyd, MD, of the Center for Bioethics at Harvard University, Boston, said he was saddened to learn about the proclamation, adding that the Trump administration is relying on inaccurate information and falsified facts to justify the new restriction. Dr. Boyd, cofounder of the Human Rights and Asylum Clinic at Cambridge Health Alliance in Cambridge, Mass., coauthored a 2018 study finding that immigrants pay more into the U.S. health care system than they use.
That analysis, which examined 188 peer-reviewed studies related to immigrants and U.S. health care expenditures, found that per capita expenditures from private and public insurance sources were about 40% lower for immigrants, compared with native-born Americans. Expenditures for undocumented immigrants were even lower. Immigrants also made a greater contribution to Medicare’s trust fund than they withdrew, the study found (Int J Health Serv. 2018 Aug. 8 doi: 10.1177/0020731418791963).
Immigrants use less health care resources because they are generally younger and healthier than native-born Americans, and they are less likely to access health care services – regardless of health status, Dr. Boyd said in an interview. In addition, many immigrants who contribute to Medicare through payroll and/or taxes are no longer in the U.S. when they reach Medicare age.
“The Trump administration, in this case, is making yet another set of excuses for why they are trying to keep immigrants out of the country,” Dr. Boyd said. “The potential impact of this restriction is devastating. Obviously, the Trump administration is doing anything that it possibly can to try to limit immigrants from coming into our country and seeking a better life for themselves.”
The proclamation also could have a chilling effect on immigrants currently in the United States who are in need of medical treatment, Dr. Boyd noted.
“The real effect is you’re going to have immigrants who are already here, not [accessing] health care when they need it, for fear they somehow become known to the government,” he said.
New consensus recommendations on bleeding in acquired hemophilia
New consensus statements, released by a group of 36 experts, provide specific recommendations related to monitoring bleeding and assessing efficacy of treatment in patients with acquired hemophilia.
A global survey was developed by a nine-member steering committee with expertise in the hemostatic management of patients with acquired hemophilia. The Delphi methodology was used to obtain consensus on a list of statements on the location-specific treatment of bleeding in acquired hemophilia.
“The initial survey was circulated via email for refinement and was formally corroborated at a face-to-face meeting,” wrote Andreas Tiede, MD, PhD, of Hannover (Germany) Medical School and fellow experts. The report is published in Haemophilia.
The key areas outlined include the initial management of bleeding, and management of location-specific bleeding, including urological, gastrointestinal, muscle, and pharyngeal bleeds, as well as intracranial and postpartum hemorrhage.
If an expert hematologist is not available, and the bleeding event is life‐threatening, the emergency physician should initiate treatment in accordance with local or national recommendations, according to the initial management guidelines.
With respect to urological bleeds, the best interval for evaluating successful achievement of hemostasis is every 6-12 hours. The experts also reported that, if first-line hemostatic therapy is not effective, more intensive treatment should be considered every 6-12 hours.
In the management of intracranial hemorrhage, the frequency of clinical evaluation is subject to the particular scenario, and it can vary from every 2 hours (for clinical assessment) to every 24 hours (for imaging studies), they wrote.
If initial hemostatic treatment is not effective, more intensive therapy should be considered every 6 hours, they recommended.
“The statement addressing optimal frequency for assessing hemostasis in intracranial bleeds was the subject of much deliberation among the steering committee regarding timing of assessment,” the experts acknowledged.
The geographic diversity and global representation of expert participants were major strengths of these recommendations. However, these statements did not consider socioeconomic parameters or geopolitical differences that could affect patient care. As a result, they may not be applicable to all patient populations.
The manuscript was funded by Novo Nordisk AG. The authors reported having financial affiliations with Novo Nordisk and several other companies.
SOURCE: Tiede A et al. Haemophilia. 2019 Sep 13. doi: 10.1111/hae.13844.
New consensus statements, released by a group of 36 experts, provide specific recommendations related to monitoring bleeding and assessing efficacy of treatment in patients with acquired hemophilia.
A global survey was developed by a nine-member steering committee with expertise in the hemostatic management of patients with acquired hemophilia. The Delphi methodology was used to obtain consensus on a list of statements on the location-specific treatment of bleeding in acquired hemophilia.
“The initial survey was circulated via email for refinement and was formally corroborated at a face-to-face meeting,” wrote Andreas Tiede, MD, PhD, of Hannover (Germany) Medical School and fellow experts. The report is published in Haemophilia.
The key areas outlined include the initial management of bleeding, and management of location-specific bleeding, including urological, gastrointestinal, muscle, and pharyngeal bleeds, as well as intracranial and postpartum hemorrhage.
If an expert hematologist is not available, and the bleeding event is life‐threatening, the emergency physician should initiate treatment in accordance with local or national recommendations, according to the initial management guidelines.
With respect to urological bleeds, the best interval for evaluating successful achievement of hemostasis is every 6-12 hours. The experts also reported that, if first-line hemostatic therapy is not effective, more intensive treatment should be considered every 6-12 hours.
In the management of intracranial hemorrhage, the frequency of clinical evaluation is subject to the particular scenario, and it can vary from every 2 hours (for clinical assessment) to every 24 hours (for imaging studies), they wrote.
If initial hemostatic treatment is not effective, more intensive therapy should be considered every 6 hours, they recommended.
“The statement addressing optimal frequency for assessing hemostasis in intracranial bleeds was the subject of much deliberation among the steering committee regarding timing of assessment,” the experts acknowledged.
The geographic diversity and global representation of expert participants were major strengths of these recommendations. However, these statements did not consider socioeconomic parameters or geopolitical differences that could affect patient care. As a result, they may not be applicable to all patient populations.
The manuscript was funded by Novo Nordisk AG. The authors reported having financial affiliations with Novo Nordisk and several other companies.
SOURCE: Tiede A et al. Haemophilia. 2019 Sep 13. doi: 10.1111/hae.13844.
New consensus statements, released by a group of 36 experts, provide specific recommendations related to monitoring bleeding and assessing efficacy of treatment in patients with acquired hemophilia.
A global survey was developed by a nine-member steering committee with expertise in the hemostatic management of patients with acquired hemophilia. The Delphi methodology was used to obtain consensus on a list of statements on the location-specific treatment of bleeding in acquired hemophilia.
“The initial survey was circulated via email for refinement and was formally corroborated at a face-to-face meeting,” wrote Andreas Tiede, MD, PhD, of Hannover (Germany) Medical School and fellow experts. The report is published in Haemophilia.
The key areas outlined include the initial management of bleeding, and management of location-specific bleeding, including urological, gastrointestinal, muscle, and pharyngeal bleeds, as well as intracranial and postpartum hemorrhage.
If an expert hematologist is not available, and the bleeding event is life‐threatening, the emergency physician should initiate treatment in accordance with local or national recommendations, according to the initial management guidelines.
With respect to urological bleeds, the best interval for evaluating successful achievement of hemostasis is every 6-12 hours. The experts also reported that, if first-line hemostatic therapy is not effective, more intensive treatment should be considered every 6-12 hours.
In the management of intracranial hemorrhage, the frequency of clinical evaluation is subject to the particular scenario, and it can vary from every 2 hours (for clinical assessment) to every 24 hours (for imaging studies), they wrote.
If initial hemostatic treatment is not effective, more intensive therapy should be considered every 6 hours, they recommended.
“The statement addressing optimal frequency for assessing hemostasis in intracranial bleeds was the subject of much deliberation among the steering committee regarding timing of assessment,” the experts acknowledged.
The geographic diversity and global representation of expert participants were major strengths of these recommendations. However, these statements did not consider socioeconomic parameters or geopolitical differences that could affect patient care. As a result, they may not be applicable to all patient populations.
The manuscript was funded by Novo Nordisk AG. The authors reported having financial affiliations with Novo Nordisk and several other companies.
SOURCE: Tiede A et al. Haemophilia. 2019 Sep 13. doi: 10.1111/hae.13844.
FROM HAEMOPHILIA
Influenza: U.S. activity was low this summer
Influenza activity in the United States was typically low over the summer months, with influenza A(H3N2) viruses predominating, according to the Centers for Disease Control and Prevention.
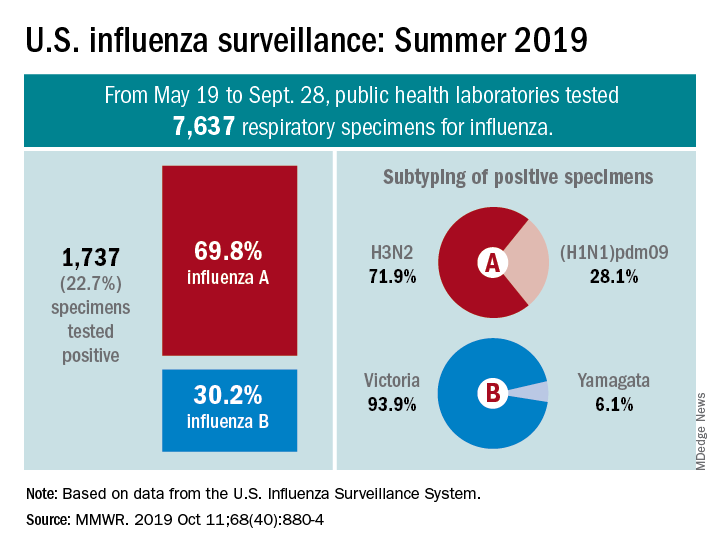
From May 19 to Sept. 28, 2019, weekly flu activity – measured by the percentage of outpatient visits to health care professionals for influenza-like illness (ILI) – was below the national baseline of 2.2%, ranging from 0.7% to 1.4%. Since mid-August, however, when the rate was last 0.7%, it has been climbing slowly but steadily and was up to 1.3% for the week ending Sept. 28, CDC data show.
The various public health laboratories of the U.S. Influenza Surveillance System tested over 7,600 respiratory samples from May 19 to Sept. 28, and 22.7% were positive for influenza viruses, Scott Epperson, DVM, and associates at the CDC’s influenza division said Oct. 10 in the MMWR.
Of the 1,737 samples found to be positive, 69.8% were influenza A and 30.2% were influenza B. The subtype split among specimens positive for Influenza A was 71.9% A(H3N2) and 28.1% A(H1N1)pdm09, while the samples positive for influenza B went 93.9% B/Victoria and 6.1% B/Yamagata, they reported.
Over the same time period in the Southern Hemisphere, “seasonal influenza viruses circulated widely, with influenza A(H3) predominating in many regions; however, influenza A(H1N1)pdm09 and influenza B viruses were predominant in some countries,” the CDC investigators noted.
They also reported the World Health Organization recommendations for the Southern Hemisphere’s 2020 flu vaccines. Components of the egg-based trivalent vaccine are an A/Brisbane/02/2018(H1N1)pdm09-like virus, an A/South Australia/34/2019(H3N2)-like virus, and a B/Washington/02/2019-like virus(B/Victoria lineage). The recommended quadrivalent vaccine adds a B/Phuket/3073/2013-like virus(B/Yamagata lineage), they wrote.
“It is too early in the season to know which viruses will circulate in the United States later this fall and winter or how severe the season might be; however, regardless of what is circulating, the best protection against influenza is an influenza vaccination,” Dr. Epperson and associates wrote.
SOURCE: Epperson S et al. MMWR. 2019 Oct 11;68(40):880-4.
Influenza activity in the United States was typically low over the summer months, with influenza A(H3N2) viruses predominating, according to the Centers for Disease Control and Prevention.

From May 19 to Sept. 28, 2019, weekly flu activity – measured by the percentage of outpatient visits to health care professionals for influenza-like illness (ILI) – was below the national baseline of 2.2%, ranging from 0.7% to 1.4%. Since mid-August, however, when the rate was last 0.7%, it has been climbing slowly but steadily and was up to 1.3% for the week ending Sept. 28, CDC data show.
The various public health laboratories of the U.S. Influenza Surveillance System tested over 7,600 respiratory samples from May 19 to Sept. 28, and 22.7% were positive for influenza viruses, Scott Epperson, DVM, and associates at the CDC’s influenza division said Oct. 10 in the MMWR.
Of the 1,737 samples found to be positive, 69.8% were influenza A and 30.2% were influenza B. The subtype split among specimens positive for Influenza A was 71.9% A(H3N2) and 28.1% A(H1N1)pdm09, while the samples positive for influenza B went 93.9% B/Victoria and 6.1% B/Yamagata, they reported.
Over the same time period in the Southern Hemisphere, “seasonal influenza viruses circulated widely, with influenza A(H3) predominating in many regions; however, influenza A(H1N1)pdm09 and influenza B viruses were predominant in some countries,” the CDC investigators noted.
They also reported the World Health Organization recommendations for the Southern Hemisphere’s 2020 flu vaccines. Components of the egg-based trivalent vaccine are an A/Brisbane/02/2018(H1N1)pdm09-like virus, an A/South Australia/34/2019(H3N2)-like virus, and a B/Washington/02/2019-like virus(B/Victoria lineage). The recommended quadrivalent vaccine adds a B/Phuket/3073/2013-like virus(B/Yamagata lineage), they wrote.
“It is too early in the season to know which viruses will circulate in the United States later this fall and winter or how severe the season might be; however, regardless of what is circulating, the best protection against influenza is an influenza vaccination,” Dr. Epperson and associates wrote.
SOURCE: Epperson S et al. MMWR. 2019 Oct 11;68(40):880-4.
Influenza activity in the United States was typically low over the summer months, with influenza A(H3N2) viruses predominating, according to the Centers for Disease Control and Prevention.

From May 19 to Sept. 28, 2019, weekly flu activity – measured by the percentage of outpatient visits to health care professionals for influenza-like illness (ILI) – was below the national baseline of 2.2%, ranging from 0.7% to 1.4%. Since mid-August, however, when the rate was last 0.7%, it has been climbing slowly but steadily and was up to 1.3% for the week ending Sept. 28, CDC data show.
The various public health laboratories of the U.S. Influenza Surveillance System tested over 7,600 respiratory samples from May 19 to Sept. 28, and 22.7% were positive for influenza viruses, Scott Epperson, DVM, and associates at the CDC’s influenza division said Oct. 10 in the MMWR.
Of the 1,737 samples found to be positive, 69.8% were influenza A and 30.2% were influenza B. The subtype split among specimens positive for Influenza A was 71.9% A(H3N2) and 28.1% A(H1N1)pdm09, while the samples positive for influenza B went 93.9% B/Victoria and 6.1% B/Yamagata, they reported.
Over the same time period in the Southern Hemisphere, “seasonal influenza viruses circulated widely, with influenza A(H3) predominating in many regions; however, influenza A(H1N1)pdm09 and influenza B viruses were predominant in some countries,” the CDC investigators noted.
They also reported the World Health Organization recommendations for the Southern Hemisphere’s 2020 flu vaccines. Components of the egg-based trivalent vaccine are an A/Brisbane/02/2018(H1N1)pdm09-like virus, an A/South Australia/34/2019(H3N2)-like virus, and a B/Washington/02/2019-like virus(B/Victoria lineage). The recommended quadrivalent vaccine adds a B/Phuket/3073/2013-like virus(B/Yamagata lineage), they wrote.
“It is too early in the season to know which viruses will circulate in the United States later this fall and winter or how severe the season might be; however, regardless of what is circulating, the best protection against influenza is an influenza vaccination,” Dr. Epperson and associates wrote.
SOURCE: Epperson S et al. MMWR. 2019 Oct 11;68(40):880-4.
FROM MMWR
Tape strips useful to identify biomarkers in skin of young children with atopic dermatitis
Adhesive according to a study published online on October 9 in JAMA Dermatology.
“Minimally invasive approaches that accurately capture key immune and barrier biomarkers in the skin of patients with early-onset pediatric AD are needed,” wrote Emma Guttman-Yassky, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors. “Because tissue biopsies are considered the criterion standard for evaluating dysregulation in AD lesional and nonlesional skin, it is crucial to understand whether tape-strip profiling can accurately yield key AD-related biomarkers.”
In their cross-sectional study, researchers used large D-Squame tape strips to collect skin samples from 51 children under the age of 5 years (mean, 1.7-1.8 years), including 21 with moderate to severe AD and 30 controls who did not have AD. Samples were collected from lesional skin inside the crook of the elbow and nonlesional skin, on the same arm, then subjected to gene- and protein-expression analysis to identify skin biomarkers of disease.
The participants tolerated the tape stripping well, and there were no clinical effects of the procedure. The authors were able to detect mRNA in 70 of 71 samples.
They then analyzed a panel of 15 cellular markers that assessed markers of monocytes and macrophages, T cells, activated TH2 cells, dendritic cells and dendritic-cell subsets, and Langerhans cells. They found that most showed significant differences between lesional AD skin and normal skin.
They also found that levels of OX40 ligand receptor, a marker associated with atopic dendritic cells, the inducible T-cell costimulatory activation marker, CD209, CD123, and langerin protein, were also significantly higher in nonlesional AD skin.
When comparing lesional and nonlesional skin samples in the AD patients, the authors saw significant differences only in levels of colony-stimulating factor 1 and 2.
The authors noted that some of the mediators detected from the tape-strip samples had not been detected or evaluated in previous studies of the use of tape strips in AD. These included measures of cellular infiltrates, atopic dendritic cells, and key inflammatory markers.
“The novel epidermal cytokines IL [interleukin]–33 and IL-17C, which are currently targeted in clinical trials of patients with AD, were also highlighted as novel tape-strip biomarkers and demonstrated significant correlations with AD severity,” they wrote.
“Because tape stripping is painless, nonscarring, and allows repeated sampling, it may be associated with benefits for longitudinal pediatric studies and clinical trials, in which serial measures are needed to identify predictors of response, course, and comorbidities,” the authors concluded.
The study was supported by the Northwestern University Skin Disease Research Center and the Northwestern University Clinical and Translational Sciences Institute, and partly by a grant to two authors from Regeneron and Sanofi. Dr. Guttman-Yassky reported receiving grants from Regeneron during the study, and had other disclosures related to multiple pharmaceutical companies. Another author also received grants from Regeneron during the study, and another author had disclosures related to various manufacturers; no disclosures were reported for the remaining authors.
SOURCE: Guttman-Yassky E et al. JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2983.
Skin biomarkers of atopic dermatitis (AD) are not well studied in children despite the fact that the disease largely affects this age group. Part of the challenge is the difficulty obtaining samples from children because phlebotomy and skin biopsies can cause trauma and anxiety both in children and their guardians. Better, noninvasive sampling techniques are needed.
This and another recent study show that tape stripping achieves skin samples that can provide clinically relevant AD DNA-expression levels and biomarkers that have been shown in multiple other studies – including some AD biomarkers not previously reported. Importantly, these biomarkers distinguish between children with AD and those without, and even between lesional and nonlesional skin.
While it remains to be seen if these biomarkers can predict disease outcomes or response to medication, this study shows that tape stripping in children with AD is a viable and useful method for future studies.
Leslie Castelo-Soccio, MD, PhD, is with the department of dermatology at the Children’s Hospital of Philadelphia. These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2792). No conflicts of interest were reported.
Skin biomarkers of atopic dermatitis (AD) are not well studied in children despite the fact that the disease largely affects this age group. Part of the challenge is the difficulty obtaining samples from children because phlebotomy and skin biopsies can cause trauma and anxiety both in children and their guardians. Better, noninvasive sampling techniques are needed.
This and another recent study show that tape stripping achieves skin samples that can provide clinically relevant AD DNA-expression levels and biomarkers that have been shown in multiple other studies – including some AD biomarkers not previously reported. Importantly, these biomarkers distinguish between children with AD and those without, and even between lesional and nonlesional skin.
While it remains to be seen if these biomarkers can predict disease outcomes or response to medication, this study shows that tape stripping in children with AD is a viable and useful method for future studies.
Leslie Castelo-Soccio, MD, PhD, is with the department of dermatology at the Children’s Hospital of Philadelphia. These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2792). No conflicts of interest were reported.
Skin biomarkers of atopic dermatitis (AD) are not well studied in children despite the fact that the disease largely affects this age group. Part of the challenge is the difficulty obtaining samples from children because phlebotomy and skin biopsies can cause trauma and anxiety both in children and their guardians. Better, noninvasive sampling techniques are needed.
This and another recent study show that tape stripping achieves skin samples that can provide clinically relevant AD DNA-expression levels and biomarkers that have been shown in multiple other studies – including some AD biomarkers not previously reported. Importantly, these biomarkers distinguish between children with AD and those without, and even between lesional and nonlesional skin.
While it remains to be seen if these biomarkers can predict disease outcomes or response to medication, this study shows that tape stripping in children with AD is a viable and useful method for future studies.
Leslie Castelo-Soccio, MD, PhD, is with the department of dermatology at the Children’s Hospital of Philadelphia. These comments are adapted from an accompanying editorial (JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2792). No conflicts of interest were reported.
Adhesive according to a study published online on October 9 in JAMA Dermatology.
“Minimally invasive approaches that accurately capture key immune and barrier biomarkers in the skin of patients with early-onset pediatric AD are needed,” wrote Emma Guttman-Yassky, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors. “Because tissue biopsies are considered the criterion standard for evaluating dysregulation in AD lesional and nonlesional skin, it is crucial to understand whether tape-strip profiling can accurately yield key AD-related biomarkers.”
In their cross-sectional study, researchers used large D-Squame tape strips to collect skin samples from 51 children under the age of 5 years (mean, 1.7-1.8 years), including 21 with moderate to severe AD and 30 controls who did not have AD. Samples were collected from lesional skin inside the crook of the elbow and nonlesional skin, on the same arm, then subjected to gene- and protein-expression analysis to identify skin biomarkers of disease.
The participants tolerated the tape stripping well, and there were no clinical effects of the procedure. The authors were able to detect mRNA in 70 of 71 samples.
They then analyzed a panel of 15 cellular markers that assessed markers of monocytes and macrophages, T cells, activated TH2 cells, dendritic cells and dendritic-cell subsets, and Langerhans cells. They found that most showed significant differences between lesional AD skin and normal skin.
They also found that levels of OX40 ligand receptor, a marker associated with atopic dendritic cells, the inducible T-cell costimulatory activation marker, CD209, CD123, and langerin protein, were also significantly higher in nonlesional AD skin.
When comparing lesional and nonlesional skin samples in the AD patients, the authors saw significant differences only in levels of colony-stimulating factor 1 and 2.
The authors noted that some of the mediators detected from the tape-strip samples had not been detected or evaluated in previous studies of the use of tape strips in AD. These included measures of cellular infiltrates, atopic dendritic cells, and key inflammatory markers.
“The novel epidermal cytokines IL [interleukin]–33 and IL-17C, which are currently targeted in clinical trials of patients with AD, were also highlighted as novel tape-strip biomarkers and demonstrated significant correlations with AD severity,” they wrote.
“Because tape stripping is painless, nonscarring, and allows repeated sampling, it may be associated with benefits for longitudinal pediatric studies and clinical trials, in which serial measures are needed to identify predictors of response, course, and comorbidities,” the authors concluded.
The study was supported by the Northwestern University Skin Disease Research Center and the Northwestern University Clinical and Translational Sciences Institute, and partly by a grant to two authors from Regeneron and Sanofi. Dr. Guttman-Yassky reported receiving grants from Regeneron during the study, and had other disclosures related to multiple pharmaceutical companies. Another author also received grants from Regeneron during the study, and another author had disclosures related to various manufacturers; no disclosures were reported for the remaining authors.
SOURCE: Guttman-Yassky E et al. JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2983.
Adhesive according to a study published online on October 9 in JAMA Dermatology.
“Minimally invasive approaches that accurately capture key immune and barrier biomarkers in the skin of patients with early-onset pediatric AD are needed,” wrote Emma Guttman-Yassky, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors. “Because tissue biopsies are considered the criterion standard for evaluating dysregulation in AD lesional and nonlesional skin, it is crucial to understand whether tape-strip profiling can accurately yield key AD-related biomarkers.”
In their cross-sectional study, researchers used large D-Squame tape strips to collect skin samples from 51 children under the age of 5 years (mean, 1.7-1.8 years), including 21 with moderate to severe AD and 30 controls who did not have AD. Samples were collected from lesional skin inside the crook of the elbow and nonlesional skin, on the same arm, then subjected to gene- and protein-expression analysis to identify skin biomarkers of disease.
The participants tolerated the tape stripping well, and there were no clinical effects of the procedure. The authors were able to detect mRNA in 70 of 71 samples.
They then analyzed a panel of 15 cellular markers that assessed markers of monocytes and macrophages, T cells, activated TH2 cells, dendritic cells and dendritic-cell subsets, and Langerhans cells. They found that most showed significant differences between lesional AD skin and normal skin.
They also found that levels of OX40 ligand receptor, a marker associated with atopic dendritic cells, the inducible T-cell costimulatory activation marker, CD209, CD123, and langerin protein, were also significantly higher in nonlesional AD skin.
When comparing lesional and nonlesional skin samples in the AD patients, the authors saw significant differences only in levels of colony-stimulating factor 1 and 2.
The authors noted that some of the mediators detected from the tape-strip samples had not been detected or evaluated in previous studies of the use of tape strips in AD. These included measures of cellular infiltrates, atopic dendritic cells, and key inflammatory markers.
“The novel epidermal cytokines IL [interleukin]–33 and IL-17C, which are currently targeted in clinical trials of patients with AD, were also highlighted as novel tape-strip biomarkers and demonstrated significant correlations with AD severity,” they wrote.
“Because tape stripping is painless, nonscarring, and allows repeated sampling, it may be associated with benefits for longitudinal pediatric studies and clinical trials, in which serial measures are needed to identify predictors of response, course, and comorbidities,” the authors concluded.
The study was supported by the Northwestern University Skin Disease Research Center and the Northwestern University Clinical and Translational Sciences Institute, and partly by a grant to two authors from Regeneron and Sanofi. Dr. Guttman-Yassky reported receiving grants from Regeneron during the study, and had other disclosures related to multiple pharmaceutical companies. Another author also received grants from Regeneron during the study, and another author had disclosures related to various manufacturers; no disclosures were reported for the remaining authors.
SOURCE: Guttman-Yassky E et al. JAMA Dermatol. 2019 Oct 9. doi: 10.1001/jamadermatol.2019.2983.
FROM JAMA DERMATOLOGY
Whitening of skin remains charged topic at Skin of Color meeting
NEW YORK – , judging from an informal survey of those attending the Skin of Color Update 2019, where this topic was introduced.
When the Skin of Color conference chair, Eliot Battle, MD, founder of Cultura Dermatology and Laser Center, Washington, asked who in the audience considered total body whitening to be “wrong,” the show of hands was substantial. He then offered some perspective.
“How many think breast augmentation is wrong?” he asked. “How many think changing your hair color is wrong? Before we cast judgment, let’s think a little about how our patients feel.”
Although he acknowledged the difficulty of separating a racial context from the cultural perception of lighter skin as desirable, Dr. Battle contended that choices regarding appearance are complex. He cautioned against moral judgments blind to this complexity.
“As physicians we need to keep ourselves in check, to keep ourselves from making judgments [regarding lightening agents],” he said.
The two other panelists participating in the same session made compatible observations. Although the other two panelists limited most of their presentations to skin lightening for clinical indications, such as melasma and other disorders of hyperpigmentation, they acknowledged and addressed the sense of discomfort the topic raises.
“To many patients, depigmentation is a passport to society,” said Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute, Los Angeles. Although she considers this a global issue, not an issue unique to the black population, she counseled dermatologists to “respect the vicissitudes and issues of pigmentation” that she said include the patient’s concerns about beauty, class, and privilege.
Sensitive to the desire of some patients for lighter skin, Cheryl Burgess, MD, founder of the Center for Dermatology and Dermatologic Surgery, Washington, opened her talk by displaying the Time Magazine cover of O.J. Simpson at the time he was accused of murder. The photo appeared to have been intentionally darkened in an effort that was thought by many to make him appear more sinister.
This might be an appropriate example of what skin pigment represents to some segments of American society, but Dr. Battle said that the quest for lighter skin is a global phenomenon. He claims that Asia, India, and Africa are now among the fastest growing and largest markets for skin lightening strategies. The options in those areas of the world, like the United States, are proliferating quickly.
Many of the rapidly expanding options have not yet proved to be effective or safe. The antioxidant glutathione, which is being used for a long list of proven and unproven indications, is among these, according to Dr. Battle. In many clinics where this drug is administered intravenously to avoid degradation in the gastrointestinal tract, he suggested there is reason to believe the staff has little training in safety monitoring.
There are no long-term studies evaluating the safety and efficacy of glutathione for skin lightening, according to Dr. Battle, but there are many case reports of serious toxicities, including death. He listed thyroid dysfunction, renal impairment, and liver dysfunction among adverse events potentially related to glutathione.
“When I gave this talk a year ago, there were no clinics in Washington [offering glutathione]. Now there are seven,” he said.
Even for those dermatologists uncomfortable offering skin lightening for cosmetic purposes, ignoring the demand is ill advised, he said. Evaluating and advising patients on the safety of these agents is one reason to become involved, said Dr. Battle, who noted that specialists in dermatology are uniquely trained to monitor drugs for this application.
“You can tell a patient to stop, but they won’t stop,” said Dr. Battle. He maintained that organized medicine, including the American Academy of Dermatology, should take a role in evaluating the safety and efficacy of lightening agents even when used only for cosmetic indications.
Currently, there are no Food and Drug Administration–approved therapies for whitening of the skin.
“This is such an important question, and I think we need to figure it out,” Dr. Battle said. “Not a day goes by in our practice when we are not asked about skin lightening.”
Dr. Battle reported no relevant disclosures; Dr. Grimes and Dr. Burgess reported multiple financial relationships with industry that are not necessarily relevant to this topic.
NEW YORK – , judging from an informal survey of those attending the Skin of Color Update 2019, where this topic was introduced.
When the Skin of Color conference chair, Eliot Battle, MD, founder of Cultura Dermatology and Laser Center, Washington, asked who in the audience considered total body whitening to be “wrong,” the show of hands was substantial. He then offered some perspective.
“How many think breast augmentation is wrong?” he asked. “How many think changing your hair color is wrong? Before we cast judgment, let’s think a little about how our patients feel.”
Although he acknowledged the difficulty of separating a racial context from the cultural perception of lighter skin as desirable, Dr. Battle contended that choices regarding appearance are complex. He cautioned against moral judgments blind to this complexity.
“As physicians we need to keep ourselves in check, to keep ourselves from making judgments [regarding lightening agents],” he said.
The two other panelists participating in the same session made compatible observations. Although the other two panelists limited most of their presentations to skin lightening for clinical indications, such as melasma and other disorders of hyperpigmentation, they acknowledged and addressed the sense of discomfort the topic raises.
“To many patients, depigmentation is a passport to society,” said Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute, Los Angeles. Although she considers this a global issue, not an issue unique to the black population, she counseled dermatologists to “respect the vicissitudes and issues of pigmentation” that she said include the patient’s concerns about beauty, class, and privilege.
Sensitive to the desire of some patients for lighter skin, Cheryl Burgess, MD, founder of the Center for Dermatology and Dermatologic Surgery, Washington, opened her talk by displaying the Time Magazine cover of O.J. Simpson at the time he was accused of murder. The photo appeared to have been intentionally darkened in an effort that was thought by many to make him appear more sinister.
This might be an appropriate example of what skin pigment represents to some segments of American society, but Dr. Battle said that the quest for lighter skin is a global phenomenon. He claims that Asia, India, and Africa are now among the fastest growing and largest markets for skin lightening strategies. The options in those areas of the world, like the United States, are proliferating quickly.
Many of the rapidly expanding options have not yet proved to be effective or safe. The antioxidant glutathione, which is being used for a long list of proven and unproven indications, is among these, according to Dr. Battle. In many clinics where this drug is administered intravenously to avoid degradation in the gastrointestinal tract, he suggested there is reason to believe the staff has little training in safety monitoring.
There are no long-term studies evaluating the safety and efficacy of glutathione for skin lightening, according to Dr. Battle, but there are many case reports of serious toxicities, including death. He listed thyroid dysfunction, renal impairment, and liver dysfunction among adverse events potentially related to glutathione.
“When I gave this talk a year ago, there were no clinics in Washington [offering glutathione]. Now there are seven,” he said.
Even for those dermatologists uncomfortable offering skin lightening for cosmetic purposes, ignoring the demand is ill advised, he said. Evaluating and advising patients on the safety of these agents is one reason to become involved, said Dr. Battle, who noted that specialists in dermatology are uniquely trained to monitor drugs for this application.
“You can tell a patient to stop, but they won’t stop,” said Dr. Battle. He maintained that organized medicine, including the American Academy of Dermatology, should take a role in evaluating the safety and efficacy of lightening agents even when used only for cosmetic indications.
Currently, there are no Food and Drug Administration–approved therapies for whitening of the skin.
“This is such an important question, and I think we need to figure it out,” Dr. Battle said. “Not a day goes by in our practice when we are not asked about skin lightening.”
Dr. Battle reported no relevant disclosures; Dr. Grimes and Dr. Burgess reported multiple financial relationships with industry that are not necessarily relevant to this topic.
NEW YORK – , judging from an informal survey of those attending the Skin of Color Update 2019, where this topic was introduced.
When the Skin of Color conference chair, Eliot Battle, MD, founder of Cultura Dermatology and Laser Center, Washington, asked who in the audience considered total body whitening to be “wrong,” the show of hands was substantial. He then offered some perspective.
“How many think breast augmentation is wrong?” he asked. “How many think changing your hair color is wrong? Before we cast judgment, let’s think a little about how our patients feel.”
Although he acknowledged the difficulty of separating a racial context from the cultural perception of lighter skin as desirable, Dr. Battle contended that choices regarding appearance are complex. He cautioned against moral judgments blind to this complexity.
“As physicians we need to keep ourselves in check, to keep ourselves from making judgments [regarding lightening agents],” he said.
The two other panelists participating in the same session made compatible observations. Although the other two panelists limited most of their presentations to skin lightening for clinical indications, such as melasma and other disorders of hyperpigmentation, they acknowledged and addressed the sense of discomfort the topic raises.
“To many patients, depigmentation is a passport to society,” said Pearl Grimes, MD, director of the Vitiligo and Pigmentation Institute, Los Angeles. Although she considers this a global issue, not an issue unique to the black population, she counseled dermatologists to “respect the vicissitudes and issues of pigmentation” that she said include the patient’s concerns about beauty, class, and privilege.
Sensitive to the desire of some patients for lighter skin, Cheryl Burgess, MD, founder of the Center for Dermatology and Dermatologic Surgery, Washington, opened her talk by displaying the Time Magazine cover of O.J. Simpson at the time he was accused of murder. The photo appeared to have been intentionally darkened in an effort that was thought by many to make him appear more sinister.
This might be an appropriate example of what skin pigment represents to some segments of American society, but Dr. Battle said that the quest for lighter skin is a global phenomenon. He claims that Asia, India, and Africa are now among the fastest growing and largest markets for skin lightening strategies. The options in those areas of the world, like the United States, are proliferating quickly.
Many of the rapidly expanding options have not yet proved to be effective or safe. The antioxidant glutathione, which is being used for a long list of proven and unproven indications, is among these, according to Dr. Battle. In many clinics where this drug is administered intravenously to avoid degradation in the gastrointestinal tract, he suggested there is reason to believe the staff has little training in safety monitoring.
There are no long-term studies evaluating the safety and efficacy of glutathione for skin lightening, according to Dr. Battle, but there are many case reports of serious toxicities, including death. He listed thyroid dysfunction, renal impairment, and liver dysfunction among adverse events potentially related to glutathione.
“When I gave this talk a year ago, there were no clinics in Washington [offering glutathione]. Now there are seven,” he said.
Even for those dermatologists uncomfortable offering skin lightening for cosmetic purposes, ignoring the demand is ill advised, he said. Evaluating and advising patients on the safety of these agents is one reason to become involved, said Dr. Battle, who noted that specialists in dermatology are uniquely trained to monitor drugs for this application.
“You can tell a patient to stop, but they won’t stop,” said Dr. Battle. He maintained that organized medicine, including the American Academy of Dermatology, should take a role in evaluating the safety and efficacy of lightening agents even when used only for cosmetic indications.
Currently, there are no Food and Drug Administration–approved therapies for whitening of the skin.
“This is such an important question, and I think we need to figure it out,” Dr. Battle said. “Not a day goes by in our practice when we are not asked about skin lightening.”
Dr. Battle reported no relevant disclosures; Dr. Grimes and Dr. Burgess reported multiple financial relationships with industry that are not necessarily relevant to this topic.
REPORTING FROM SOC 2019
Much work to be done in optimizing treatment for transgender children
ORLANDO – Janet Y. Lee, MD, MPH, said at the annual meeting of the American Society for Bone and Mineral Research.
According to a report from The Williams Institute in 2017, there were approximately 150,000 youth in the United States who identified as transgender, and among studies that measured transgender identity among youth, the percentage who identified as transgender ranged between 1.3% and 3.2% (Herman J et al. “Age of Individuals who Identify as Transgender in the United States.” Los Angeles: The Williams Institute, January 2017).
“If you’ve not seen one of these patients yet, you probably will in your career,” said Dr. Lee of the University of California, San Francisco.
At UCSF, Dr. Lee said the focus of care for transgender youth in early childhood to late childhood is on school resources and social transition, with puberty blockers such as gonadotropin-releasing hormone agents (GnRHa) beginning in late childhood and early puberty at Tanner stage 2. When patients reach early puberty and move to late puberty and adulthood, they usually begin gender-affirming sex hormones such as testosterone for masculinizing hormone therapy or estrogen and spironolactone or bicalutamide for feminizing hormone therapy in transgender women, and consideration of fertility preservation is undertaken. In adulthood, patients can begin gender-affirming surgery.
Dr. Lee said the specific timing of gender-affirming sex hormones is controversial and “seems to be a moving target in our field.” The average age to begin gender-affirming sex hormones at UCSF is 14 years old, but sometimes younger, said Dr. Lee. There also is a question of when to start gender-affirming sex hormones in gender diverse youth. “[They] may not want full adult doses of testosterone or full adult doses of estradiol,” said Dr. Lee. “It’s been very challenging to figure out [appropriate] treatment for these youth.”
In Europe, some studies have shown transwomen have lower bone mineral density (BMD) scores at baseline even after 2.5 years to 5 years of treatment with estradiol, compared with male reference standards. A study by Vlot et al. found transwomen had lower bone turnover markers and bone mineral apparent density in cohorts younger than 15 years old, compared with cohorts 15 years or older (Bone. 2017 Feb. doi: 10.1016/j.bone.2016.11.008).
As in the case of when to start gender-affirming sex hormones, how to approach treatment for gender diverse youth who have a low baseline BMD but are eligible for puberty blockers is debated. “We could go into a whole other talk about this because we have many [patients] who present with low baseline BMD,” said Dr. Lee. “We have to figure out a way to apply treatment without impairing their bone.”
Other questions that have yet to be answered are what dual x-ray absorptiometry standards to be used for transgender individuals and how body composition and height growth are affected by gender-affirming medical therapy, which is currently “really modeled after hypergonadic children,” said Dr. Lee.
Results from a National Institutes of Health–funded longitudinal observational study of transgender youth at UCSF, Children’s Hospital Los Angeles, Boston Children’s Hospital, and Ann & Robert H. Lurie Children’s Hospital of Chicago is currently examining the effect of using gender-affirming medical treatment for patients in early and late puberty and assessing factors such as mental health, psychological well-being, and bone health measures such as dual x-ray absorptiometry and quantitative computed tomography, as well as investigating dietary intake, physical activity and exercise, and vitamin D status. Preliminary findings from the study have shown low BMD z-scores in designated males at birth when compared with designated females at birth, with suboptimal dietary calcium intake in both designated males and designated females.
Dr. Lee reported no relevant conflicts of interest.
ORLANDO – Janet Y. Lee, MD, MPH, said at the annual meeting of the American Society for Bone and Mineral Research.
According to a report from The Williams Institute in 2017, there were approximately 150,000 youth in the United States who identified as transgender, and among studies that measured transgender identity among youth, the percentage who identified as transgender ranged between 1.3% and 3.2% (Herman J et al. “Age of Individuals who Identify as Transgender in the United States.” Los Angeles: The Williams Institute, January 2017).
“If you’ve not seen one of these patients yet, you probably will in your career,” said Dr. Lee of the University of California, San Francisco.
At UCSF, Dr. Lee said the focus of care for transgender youth in early childhood to late childhood is on school resources and social transition, with puberty blockers such as gonadotropin-releasing hormone agents (GnRHa) beginning in late childhood and early puberty at Tanner stage 2. When patients reach early puberty and move to late puberty and adulthood, they usually begin gender-affirming sex hormones such as testosterone for masculinizing hormone therapy or estrogen and spironolactone or bicalutamide for feminizing hormone therapy in transgender women, and consideration of fertility preservation is undertaken. In adulthood, patients can begin gender-affirming surgery.
Dr. Lee said the specific timing of gender-affirming sex hormones is controversial and “seems to be a moving target in our field.” The average age to begin gender-affirming sex hormones at UCSF is 14 years old, but sometimes younger, said Dr. Lee. There also is a question of when to start gender-affirming sex hormones in gender diverse youth. “[They] may not want full adult doses of testosterone or full adult doses of estradiol,” said Dr. Lee. “It’s been very challenging to figure out [appropriate] treatment for these youth.”
In Europe, some studies have shown transwomen have lower bone mineral density (BMD) scores at baseline even after 2.5 years to 5 years of treatment with estradiol, compared with male reference standards. A study by Vlot et al. found transwomen had lower bone turnover markers and bone mineral apparent density in cohorts younger than 15 years old, compared with cohorts 15 years or older (Bone. 2017 Feb. doi: 10.1016/j.bone.2016.11.008).
As in the case of when to start gender-affirming sex hormones, how to approach treatment for gender diverse youth who have a low baseline BMD but are eligible for puberty blockers is debated. “We could go into a whole other talk about this because we have many [patients] who present with low baseline BMD,” said Dr. Lee. “We have to figure out a way to apply treatment without impairing their bone.”
Other questions that have yet to be answered are what dual x-ray absorptiometry standards to be used for transgender individuals and how body composition and height growth are affected by gender-affirming medical therapy, which is currently “really modeled after hypergonadic children,” said Dr. Lee.
Results from a National Institutes of Health–funded longitudinal observational study of transgender youth at UCSF, Children’s Hospital Los Angeles, Boston Children’s Hospital, and Ann & Robert H. Lurie Children’s Hospital of Chicago is currently examining the effect of using gender-affirming medical treatment for patients in early and late puberty and assessing factors such as mental health, psychological well-being, and bone health measures such as dual x-ray absorptiometry and quantitative computed tomography, as well as investigating dietary intake, physical activity and exercise, and vitamin D status. Preliminary findings from the study have shown low BMD z-scores in designated males at birth when compared with designated females at birth, with suboptimal dietary calcium intake in both designated males and designated females.
Dr. Lee reported no relevant conflicts of interest.
ORLANDO – Janet Y. Lee, MD, MPH, said at the annual meeting of the American Society for Bone and Mineral Research.
According to a report from The Williams Institute in 2017, there were approximately 150,000 youth in the United States who identified as transgender, and among studies that measured transgender identity among youth, the percentage who identified as transgender ranged between 1.3% and 3.2% (Herman J et al. “Age of Individuals who Identify as Transgender in the United States.” Los Angeles: The Williams Institute, January 2017).
“If you’ve not seen one of these patients yet, you probably will in your career,” said Dr. Lee of the University of California, San Francisco.
At UCSF, Dr. Lee said the focus of care for transgender youth in early childhood to late childhood is on school resources and social transition, with puberty blockers such as gonadotropin-releasing hormone agents (GnRHa) beginning in late childhood and early puberty at Tanner stage 2. When patients reach early puberty and move to late puberty and adulthood, they usually begin gender-affirming sex hormones such as testosterone for masculinizing hormone therapy or estrogen and spironolactone or bicalutamide for feminizing hormone therapy in transgender women, and consideration of fertility preservation is undertaken. In adulthood, patients can begin gender-affirming surgery.
Dr. Lee said the specific timing of gender-affirming sex hormones is controversial and “seems to be a moving target in our field.” The average age to begin gender-affirming sex hormones at UCSF is 14 years old, but sometimes younger, said Dr. Lee. There also is a question of when to start gender-affirming sex hormones in gender diverse youth. “[They] may not want full adult doses of testosterone or full adult doses of estradiol,” said Dr. Lee. “It’s been very challenging to figure out [appropriate] treatment for these youth.”
In Europe, some studies have shown transwomen have lower bone mineral density (BMD) scores at baseline even after 2.5 years to 5 years of treatment with estradiol, compared with male reference standards. A study by Vlot et al. found transwomen had lower bone turnover markers and bone mineral apparent density in cohorts younger than 15 years old, compared with cohorts 15 years or older (Bone. 2017 Feb. doi: 10.1016/j.bone.2016.11.008).
As in the case of when to start gender-affirming sex hormones, how to approach treatment for gender diverse youth who have a low baseline BMD but are eligible for puberty blockers is debated. “We could go into a whole other talk about this because we have many [patients] who present with low baseline BMD,” said Dr. Lee. “We have to figure out a way to apply treatment without impairing their bone.”
Other questions that have yet to be answered are what dual x-ray absorptiometry standards to be used for transgender individuals and how body composition and height growth are affected by gender-affirming medical therapy, which is currently “really modeled after hypergonadic children,” said Dr. Lee.
Results from a National Institutes of Health–funded longitudinal observational study of transgender youth at UCSF, Children’s Hospital Los Angeles, Boston Children’s Hospital, and Ann & Robert H. Lurie Children’s Hospital of Chicago is currently examining the effect of using gender-affirming medical treatment for patients in early and late puberty and assessing factors such as mental health, psychological well-being, and bone health measures such as dual x-ray absorptiometry and quantitative computed tomography, as well as investigating dietary intake, physical activity and exercise, and vitamin D status. Preliminary findings from the study have shown low BMD z-scores in designated males at birth when compared with designated females at birth, with suboptimal dietary calcium intake in both designated males and designated females.
Dr. Lee reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM ASBMR 2019
Hemophilia prevalence is nearly three times higher than previously reported
The number of people with hemophilia worldwide is higher than previously estimated, and patients still face a shortened life expectancy, according to an international meta-analysis of registry data.
Approximately 1.125 million people have hemophilia worldwide, compared with the previous estimate of 400,000, reported lead author Alfonso Iorio, MD, PhD, of McMaster University, Hamilton, Ont., and colleagues.
The previous estimate, from the early 2000s, was based on prevalence in the United States and the global population at the time, the investigators explained. Their report is in Annals of Internal Medicine.
They noted a lack of clarity in prior estimates concerning type and severity of hemophilia, and aimed to correct this knowledge gap with the present meta-analysis.
Prevalence was estimated using data from registries in Australia, Canada, Italy, France, the United Kingdom, and New Zealand, which are all high-income countries. Prevalence at birth was estimated using the Canadian, French, and British registries, as these are the most established databases, according to the investigators. The World Federation of Hemophilia Annual Global survey was used to estimate the total global number of patients with hemophilia, while national statistics databases were used to determine the number of males and live male births.
Of the 1.125 million cases of hemophilia worldwide, the investigators estimated that 418,000 are likely severe. Proportionally, 17.1 out of 100,000 males have hemophilia A, with 6.0 out of 100,000 males exhibiting severe hemophilia A. Hemophilia B is less common, occurring in 3.8 out of 100,000 males, with a 1.1 out of 100,000 classified as severe.
Turning to prevalence at birth, the investigators estimated that there are 24.6 cases of hemophilia A per 100,000 male births and 5.0 cases of hemophilia B per 100,000 male births.
The associated life expectancy disadvantage in high-income countries is highest for severe hemophilia A (37%), followed by all severities of hemophilia A (30%), severe hemophilia B (27%), and all severities of hemophilia B (24%).
“Having 1,125,000 persons with hemophilia worldwide, of whom about 418,000 have severe and mostly undiagnosed disease, constitutes a formidable challenge and burden for researchers and health care systems, especially because only 196,706 patients have been identified and reported globally,” the investigators wrote. “More efficient diagnostic approaches are needed in less wealthy countries to take advantage of current and future treatment modalities, including gene therapy. Increased demand for care should drive new policy planning and spur renewed effort toward the development and manufacture of new drugs.”
The updated prevalence figures will serve as a valuable roadmap for the future, according to J. Michael Soucie, PhD, of the Centers for Disease Control and Prevention, Atlanta.
“Although the magnitude of the global gaps in care for persons with hemophilia is daunting, country specific data generated by application of the prevalence estimates reported by Iorio and colleagues are an important step toward prioritizing efforts to address these gaps,” Dr. Soucie wrote in an accompanying editorial. “Having more accurate prevalence data might also allow identification of ways in which regional efforts to improve care access could generate considerable benefits for patients and cost savings for countries. Armed with these data for action, we can hope to make substantial progress toward the goal of improving the lives of persons with hemophilia wherever they live.”
The study received no financial support. The investigators reported relationships with Pfizer, Roche, Novo Nordisk, and others. Dr. Soucie reported having no conflicts of interest.
SOURCE: Iorio A et al. Ann Intern Med. 2019 Sept 10. doi: 10.7326/M19-1208.
The number of people with hemophilia worldwide is higher than previously estimated, and patients still face a shortened life expectancy, according to an international meta-analysis of registry data.
Approximately 1.125 million people have hemophilia worldwide, compared with the previous estimate of 400,000, reported lead author Alfonso Iorio, MD, PhD, of McMaster University, Hamilton, Ont., and colleagues.
The previous estimate, from the early 2000s, was based on prevalence in the United States and the global population at the time, the investigators explained. Their report is in Annals of Internal Medicine.
They noted a lack of clarity in prior estimates concerning type and severity of hemophilia, and aimed to correct this knowledge gap with the present meta-analysis.
Prevalence was estimated using data from registries in Australia, Canada, Italy, France, the United Kingdom, and New Zealand, which are all high-income countries. Prevalence at birth was estimated using the Canadian, French, and British registries, as these are the most established databases, according to the investigators. The World Federation of Hemophilia Annual Global survey was used to estimate the total global number of patients with hemophilia, while national statistics databases were used to determine the number of males and live male births.
Of the 1.125 million cases of hemophilia worldwide, the investigators estimated that 418,000 are likely severe. Proportionally, 17.1 out of 100,000 males have hemophilia A, with 6.0 out of 100,000 males exhibiting severe hemophilia A. Hemophilia B is less common, occurring in 3.8 out of 100,000 males, with a 1.1 out of 100,000 classified as severe.
Turning to prevalence at birth, the investigators estimated that there are 24.6 cases of hemophilia A per 100,000 male births and 5.0 cases of hemophilia B per 100,000 male births.
The associated life expectancy disadvantage in high-income countries is highest for severe hemophilia A (37%), followed by all severities of hemophilia A (30%), severe hemophilia B (27%), and all severities of hemophilia B (24%).
“Having 1,125,000 persons with hemophilia worldwide, of whom about 418,000 have severe and mostly undiagnosed disease, constitutes a formidable challenge and burden for researchers and health care systems, especially because only 196,706 patients have been identified and reported globally,” the investigators wrote. “More efficient diagnostic approaches are needed in less wealthy countries to take advantage of current and future treatment modalities, including gene therapy. Increased demand for care should drive new policy planning and spur renewed effort toward the development and manufacture of new drugs.”
The updated prevalence figures will serve as a valuable roadmap for the future, according to J. Michael Soucie, PhD, of the Centers for Disease Control and Prevention, Atlanta.
“Although the magnitude of the global gaps in care for persons with hemophilia is daunting, country specific data generated by application of the prevalence estimates reported by Iorio and colleagues are an important step toward prioritizing efforts to address these gaps,” Dr. Soucie wrote in an accompanying editorial. “Having more accurate prevalence data might also allow identification of ways in which regional efforts to improve care access could generate considerable benefits for patients and cost savings for countries. Armed with these data for action, we can hope to make substantial progress toward the goal of improving the lives of persons with hemophilia wherever they live.”
The study received no financial support. The investigators reported relationships with Pfizer, Roche, Novo Nordisk, and others. Dr. Soucie reported having no conflicts of interest.
SOURCE: Iorio A et al. Ann Intern Med. 2019 Sept 10. doi: 10.7326/M19-1208.
The number of people with hemophilia worldwide is higher than previously estimated, and patients still face a shortened life expectancy, according to an international meta-analysis of registry data.
Approximately 1.125 million people have hemophilia worldwide, compared with the previous estimate of 400,000, reported lead author Alfonso Iorio, MD, PhD, of McMaster University, Hamilton, Ont., and colleagues.
The previous estimate, from the early 2000s, was based on prevalence in the United States and the global population at the time, the investigators explained. Their report is in Annals of Internal Medicine.
They noted a lack of clarity in prior estimates concerning type and severity of hemophilia, and aimed to correct this knowledge gap with the present meta-analysis.
Prevalence was estimated using data from registries in Australia, Canada, Italy, France, the United Kingdom, and New Zealand, which are all high-income countries. Prevalence at birth was estimated using the Canadian, French, and British registries, as these are the most established databases, according to the investigators. The World Federation of Hemophilia Annual Global survey was used to estimate the total global number of patients with hemophilia, while national statistics databases were used to determine the number of males and live male births.
Of the 1.125 million cases of hemophilia worldwide, the investigators estimated that 418,000 are likely severe. Proportionally, 17.1 out of 100,000 males have hemophilia A, with 6.0 out of 100,000 males exhibiting severe hemophilia A. Hemophilia B is less common, occurring in 3.8 out of 100,000 males, with a 1.1 out of 100,000 classified as severe.
Turning to prevalence at birth, the investigators estimated that there are 24.6 cases of hemophilia A per 100,000 male births and 5.0 cases of hemophilia B per 100,000 male births.
The associated life expectancy disadvantage in high-income countries is highest for severe hemophilia A (37%), followed by all severities of hemophilia A (30%), severe hemophilia B (27%), and all severities of hemophilia B (24%).
“Having 1,125,000 persons with hemophilia worldwide, of whom about 418,000 have severe and mostly undiagnosed disease, constitutes a formidable challenge and burden for researchers and health care systems, especially because only 196,706 patients have been identified and reported globally,” the investigators wrote. “More efficient diagnostic approaches are needed in less wealthy countries to take advantage of current and future treatment modalities, including gene therapy. Increased demand for care should drive new policy planning and spur renewed effort toward the development and manufacture of new drugs.”
The updated prevalence figures will serve as a valuable roadmap for the future, according to J. Michael Soucie, PhD, of the Centers for Disease Control and Prevention, Atlanta.
“Although the magnitude of the global gaps in care for persons with hemophilia is daunting, country specific data generated by application of the prevalence estimates reported by Iorio and colleagues are an important step toward prioritizing efforts to address these gaps,” Dr. Soucie wrote in an accompanying editorial. “Having more accurate prevalence data might also allow identification of ways in which regional efforts to improve care access could generate considerable benefits for patients and cost savings for countries. Armed with these data for action, we can hope to make substantial progress toward the goal of improving the lives of persons with hemophilia wherever they live.”
The study received no financial support. The investigators reported relationships with Pfizer, Roche, Novo Nordisk, and others. Dr. Soucie reported having no conflicts of interest.
SOURCE: Iorio A et al. Ann Intern Med. 2019 Sept 10. doi: 10.7326/M19-1208.
FROM ANNALS OF INTERNAL MEDICINE
Pilot program benefits gynecologic cancer patients with malignant bowel obstruction
A pilot program that aims to optimize the care of patients with malignant bowel obstruction is associated with longer survival and shorter cumulative hospital length of stay in the first 60 days after a malignant bowel obstruction diagnosis, according to research published in Journal of Oncology Practice.
Yeh Chen Lee, MBBS, of Princess Margaret Cancer Centre in Toronto, and colleagues retrospectively compared the outcomes of 106 women with advanced gynecologic cancer who were admitted to the hospital with malignant bowel obstruction before the program was implemented, with those of 63 women who were admitted after the program was implemented.
Patients’ median age at diagnosis of malignant bowel obstruction was 62 years (range, 31-91 years). Primary cancer diagnoses included ovarian cancer (73%), uterine cancer (18%), and cervical cancer (9%), and most patients had stage III-IV disease. Most patients had small-bowel obstruction (78%).
In the 2 years before the program, women had an average cumulative length of stay in the hospital of 22 days within the first 60 days of malignant bowel obstruction diagnosis. In the 2 years after, the average length of stay was 13 days. Furthermore, median overall survival, adjusted for initial cancer stage and lines of chemotherapy, increased by about 5 months, from 99 days before the program to 243 days after the program was implemented.
Patients who were treated during the malignant bowel obstruction program were more likely than were patients in the baseline group to receive chemotherapy (83% vs. 56%) and to receive two or more interventions for malignant bowel obstruction, such as surgery, chemotherapy, or total parenteral nutrition (42% vs. 33%). Complications included bowel perforation (13% in the program group vs. 5% in the baseline group) and fistulizing disease (6% in the program group vs. 12% in the baseline group).
In addition, the program was associated with lower costs.
The pilot program was designed “to provide a systematic framework to coordinate care and consensus decision-making among different specialties relevant to [malignant bowel obstruction] management,” Dr. Lee and colleagues said. It includes outpatient care led by oncology nurses through telephone consultations. “Standardized clinical processes, assessment tools, and documentation in the electronic medical record are incorporated to facilitate seamless transition between in- and outpatient care,” the authors said. “Patient educational materials have been developed to empower patients to recognize and effectively communicate their symptoms.”
It is unclear whether other institutions could implement this program, Dr. Lee and colleagues noted. It also is not possible to determine whether differences in survival relate to earlier recognition of malignant bowel obstruction, more effective management, or other factors.
Dr. Lee was supported by funding from Princess Margaret Cancer Foundation and an Australian Government Research Training Program Scholarship. Coauthors disclosed financial relationships with pharmaceutical companies and pending patents related to percutaneous procedures and a surgical device.
SOURCE: Lee YC et al. J Oncol Pract. 2019 Sep 24. doi: 10.1200/JOP.18.00793.
A pilot program that aims to optimize the care of patients with malignant bowel obstruction is associated with longer survival and shorter cumulative hospital length of stay in the first 60 days after a malignant bowel obstruction diagnosis, according to research published in Journal of Oncology Practice.
Yeh Chen Lee, MBBS, of Princess Margaret Cancer Centre in Toronto, and colleagues retrospectively compared the outcomes of 106 women with advanced gynecologic cancer who were admitted to the hospital with malignant bowel obstruction before the program was implemented, with those of 63 women who were admitted after the program was implemented.
Patients’ median age at diagnosis of malignant bowel obstruction was 62 years (range, 31-91 years). Primary cancer diagnoses included ovarian cancer (73%), uterine cancer (18%), and cervical cancer (9%), and most patients had stage III-IV disease. Most patients had small-bowel obstruction (78%).
In the 2 years before the program, women had an average cumulative length of stay in the hospital of 22 days within the first 60 days of malignant bowel obstruction diagnosis. In the 2 years after, the average length of stay was 13 days. Furthermore, median overall survival, adjusted for initial cancer stage and lines of chemotherapy, increased by about 5 months, from 99 days before the program to 243 days after the program was implemented.
Patients who were treated during the malignant bowel obstruction program were more likely than were patients in the baseline group to receive chemotherapy (83% vs. 56%) and to receive two or more interventions for malignant bowel obstruction, such as surgery, chemotherapy, or total parenteral nutrition (42% vs. 33%). Complications included bowel perforation (13% in the program group vs. 5% in the baseline group) and fistulizing disease (6% in the program group vs. 12% in the baseline group).
In addition, the program was associated with lower costs.
The pilot program was designed “to provide a systematic framework to coordinate care and consensus decision-making among different specialties relevant to [malignant bowel obstruction] management,” Dr. Lee and colleagues said. It includes outpatient care led by oncology nurses through telephone consultations. “Standardized clinical processes, assessment tools, and documentation in the electronic medical record are incorporated to facilitate seamless transition between in- and outpatient care,” the authors said. “Patient educational materials have been developed to empower patients to recognize and effectively communicate their symptoms.”
It is unclear whether other institutions could implement this program, Dr. Lee and colleagues noted. It also is not possible to determine whether differences in survival relate to earlier recognition of malignant bowel obstruction, more effective management, or other factors.
Dr. Lee was supported by funding from Princess Margaret Cancer Foundation and an Australian Government Research Training Program Scholarship. Coauthors disclosed financial relationships with pharmaceutical companies and pending patents related to percutaneous procedures and a surgical device.
SOURCE: Lee YC et al. J Oncol Pract. 2019 Sep 24. doi: 10.1200/JOP.18.00793.
A pilot program that aims to optimize the care of patients with malignant bowel obstruction is associated with longer survival and shorter cumulative hospital length of stay in the first 60 days after a malignant bowel obstruction diagnosis, according to research published in Journal of Oncology Practice.
Yeh Chen Lee, MBBS, of Princess Margaret Cancer Centre in Toronto, and colleagues retrospectively compared the outcomes of 106 women with advanced gynecologic cancer who were admitted to the hospital with malignant bowel obstruction before the program was implemented, with those of 63 women who were admitted after the program was implemented.
Patients’ median age at diagnosis of malignant bowel obstruction was 62 years (range, 31-91 years). Primary cancer diagnoses included ovarian cancer (73%), uterine cancer (18%), and cervical cancer (9%), and most patients had stage III-IV disease. Most patients had small-bowel obstruction (78%).
In the 2 years before the program, women had an average cumulative length of stay in the hospital of 22 days within the first 60 days of malignant bowel obstruction diagnosis. In the 2 years after, the average length of stay was 13 days. Furthermore, median overall survival, adjusted for initial cancer stage and lines of chemotherapy, increased by about 5 months, from 99 days before the program to 243 days after the program was implemented.
Patients who were treated during the malignant bowel obstruction program were more likely than were patients in the baseline group to receive chemotherapy (83% vs. 56%) and to receive two or more interventions for malignant bowel obstruction, such as surgery, chemotherapy, or total parenteral nutrition (42% vs. 33%). Complications included bowel perforation (13% in the program group vs. 5% in the baseline group) and fistulizing disease (6% in the program group vs. 12% in the baseline group).
In addition, the program was associated with lower costs.
The pilot program was designed “to provide a systematic framework to coordinate care and consensus decision-making among different specialties relevant to [malignant bowel obstruction] management,” Dr. Lee and colleagues said. It includes outpatient care led by oncology nurses through telephone consultations. “Standardized clinical processes, assessment tools, and documentation in the electronic medical record are incorporated to facilitate seamless transition between in- and outpatient care,” the authors said. “Patient educational materials have been developed to empower patients to recognize and effectively communicate their symptoms.”
It is unclear whether other institutions could implement this program, Dr. Lee and colleagues noted. It also is not possible to determine whether differences in survival relate to earlier recognition of malignant bowel obstruction, more effective management, or other factors.
Dr. Lee was supported by funding from Princess Margaret Cancer Foundation and an Australian Government Research Training Program Scholarship. Coauthors disclosed financial relationships with pharmaceutical companies and pending patents related to percutaneous procedures and a surgical device.
SOURCE: Lee YC et al. J Oncol Pract. 2019 Sep 24. doi: 10.1200/JOP.18.00793.
FROM JOURNAL OF ONCOLOGY PRACTICE
Newly described lung disorder strikes children with systemic juvenile idiopathic arthritis
An uncommon but potentially deadly inflammatory lung disease is emerging among children with systemic juvenile idiopathic arthritis, and its history appears to coincide with the rise of powerful biologics as first-line therapy for children with the disease.
Most confirmed cases of systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) are in the United States. But it’s popping up in other places that have adopted early biologic treatment for sJIA – including Canada, South America, Europe, and the Middle East.
The respiratory symptoms are relatively subtle, so by the time of lung disease detection, the amount of affected lung can be extensive, said Elizabeth Mellins, MD, a Stanford (Calif.) University researcher who, along with first author Vivian Saper, MD, recently published the largest case series comprising reports from 37 institutions (Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040). By the end of follow-up, 22 of the 61 children in her cohort had died, including all 12 patients who demonstrated excessively high neutrophil levels in bronchoalveolar lavage samples.
Another recent report, authored by Grant Schulert, MD, PhD, and colleagues of the Cincinnati Children’s Hospital Medical Center, described 18 patients, 9 of whom were also included in the Stanford cohort (Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073).
Both investigators have now identified new patients.
“We are aware of 60 additional cases beyond what were included in our series,” Dr. Mellins said in an interview, bringing her entire cohort to 121. Dr. Schulert also continues to expand his group, detailing nine new cases at a recent private meeting.
“We are up to 27 now,” he said. “The features of these new patients are all very similar: The children are very young, all have had macrophage activation syndrome in the past and very-difficult-to-control JIA. Reactions to tocilizumab [Actemra] were also not uncommon in this group.”
Dr. Mellins also saw this association with allergic-type tocilizumab reactions, severe delayed hypersensitivity reactions to anakinra (Kineret) or canakinumab (Ilaris). Although serious lung disease in sJIA patients is not unheard of, this phenotype was virtually unknown until about a decade ago. Both investigators said that it’s been rising steadily since 2010 – just about the time that powerful cytokine-inhibiting biologics were changing these patients’ world for the better. After decades of relying almost solely on steroids and methotrexate, with rather poor results and significant long-term side effects, children were not only improving, but thriving. Gone was the life-changing glucocorticoid-related growth inhibition. Biologics could halt fevers, rash, and joint destruction in their tracks.
“For the first time in history, these kids could look forward to a more or less normal life,” Dr. Schulert said.
But the emergence of this particular type of lung disease could throw a pall over that success story, he said. If sJIA-LD is temporally associated with increasing reliance on long-term interleukin-1/IL-6 inhibition in children with early-onset disease, could these drugs actually be the causative agent? The picture remains unclear.
Some of the 18 in his initial series have improved, while 36% of those in the Stanford series died. Most who do recover stay on their IL-1 or IL-6 blocking therapy with good disease control without further lung problems. Both investigators found compelling genetic hints, but nothing conclusive. Children with trisomy 21 appear especially vulnerable. Most patients are very young – around 2 years old – but others are school aged. Some had a history of macrophage activation syndrome. Some had hard-to-control disease and some were clinically well controlled when the lung disease presented.
There are simply no answers yet.
With so many potential links, all unproven, clinicians may question the wisdom of embarking on long-term biologic therapy for their children with sJIA. Peter Nigrovic, MD, of Boston Children’s Hospital, addressed this in an accompanying editorial (Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071).
“My take on this is that it’s a very worrisome trend,” he said in an interview. “We’ve been going full bore toward early biologic therapy in sJIA and at the same time we are seeing more of this lung disease. Is it guilt by association? Or is there something more? The challenge for us is not to jump too soon to that conclusion.”
Although the association is there, he said, association does not equal causation. And there’s no doubt that biologics have vastly improved the lives of sJIA patients. “The drugs might be causal, and I worry about that and think we need to study it. But we absolutely need stronger evidence before we change practice.”
“This is a new manifestation of the disease, and it’s coming at the same time we are changing the treatment paradigm,” Dr. Nigrovic continued. “It could be because of interleukin-1 or interleukin-6 blockade. There is biological plausibility for such a link. It could also be related to the fact that we are using less steroids and methotrexate, which might have been preventing this. The appearance of sJIA lung disease could also be that a distinct secular trend unrelated to treatment, just as we saw amyloid come and go in this population in Europe. These other therapies were actually preventing this. We just don’t know.”
Clinical characteristics
Children presented with similar symptoms. Respiratory symptoms are usually subtle and mild. These can include tachypnea, hypoxia (43% in the Stanford series), and pulmonary hypertension (30% in the Stanford series).
Digital clubbing, often with erythema, was a common finding. Some children showed pruritic, nonevanescent rashes. Eosinophilia occurred in 37% of the Stanford series and severe abdominal pain in 16%, although Dr. Mellins noted that belly pain may be underestimated, as it was only volunteered, not queried, information.
“There are some red flags that should raise suspicion even without obvious respiratory symptoms,” Dr. Mellins said. These include lymphopenia, unexplained abdominal pain, eosinophilia, an unusual rash, and finger clubbing with or without erythema.
Findings on imaging were consistent in both series. Several key clinic features emerged: pleural thickening, septal thickening, bronchial wall or peribronchovascular thickening, “tree-in-bud” opacities, “ground-glass” opacities, peripheral consolidation, and lymphadenopathy.
“The imaging findings correspond to two things,” Dr. Schulert said. “The first is inflammation in the interstitium, which is evidence of chronic and ongoing inflammation. The other thing is that the alveoli are filled with a lipoproteinaceous material which is actually surfactant that’s not being normally recycled by the lung macrophages. You can see these features in other conditions where there’s a problem with lung macrophages, like pulmonary alveolar proteinosis, genetic and autoimmune disorders, infections, or inhalants.”
Pathology showed alveolar filling – a location in the lung that hides usual symptoms until the lung disease is advanced. Prior drug reactions were common. Tocilizumab anaphylaxis occurred in close to 40% of the Stanford series – a surprising finding given the 0.6% reaction incidence in the drug’s sJIA trials. Dr. Schulert saw a similar story.
“In our cohort we also observed a striking number of adverse events to cytokine-targeted biologics exposure,” Dr. Schulert said. “Most of these reactions were to tocilizumab, and were described variously from pain and feeling unwell, to difficulty breathing, to anaphylaxis.”
In a risk analysis, Dr. Schulert determined that adverse events to cytokine-targeting biologics increased the likelihood of lung disease more than 13 times (odds ratio, 13.6).
“We also identified a statistically significant association with history of macrophage activation syndrome when compared to controls (OR, 14.5),” Dr. Schulert and associates wrote.
Genetics
Both the Cincinnati and Stanford teams conducted genetic analyses on some of their patients.
Among eight lung biopsy samples, Dr. Schulert found 37 differentially expressed genes: 36 with increased expression and 1 with decreased expression. Many of the up-regulated genes are involved in interferon-gamma response. Two (CXCL10 and CXCL9) are interferon-induced chemokines associated with macrophage activation syndrome. The down-regulated gene, PADI4, modulates immune response in lupus, and has been associated with the risk of interstitial lung disease in RA.
Dr. Mellins and her team analyzed whole-exome sequencing data from 20 patients and found some rare protein-altering gene variants in genes related to pulmonary alveolar proteinosis, all of which were heterozygous and shared with a healthy parent. But none of them could be directly tied to the disorder.
Another genetic puzzle demands attention, she said. About 10% of the children had trisomy 21 – a stark contrast to the typical 0.2% prevalence among a control group of sJIA patients without any known lung disease in the Childhood Arthritis and Rheumatology Research Alliance Registry cohort, similar to the background population rate. There were suggestions of more aggressive lung disease in all six of these children. Four presented with hypoxia, and two showed advanced interstitial fibrosis. Children with trisomy 21 also seemed more susceptible to infections; 83% had a viral or fungal lung infection at diagnosis, compared with 29% of those without trisomy 21.
Prior exposure to cytokine inhibitors
Parenchymal lung disease and pulmonary hypertension complicating sJIA was first highlighted in a series of 25 cases reported by Kimura et al. in 2013. These authors raised the question of the possible relationship of this and the increasing use of anti–IL-1 and anti–IL-6 biologics in sJIA treatment.
Following this lead, Dr. Mellins started looking into this new clinical entity in 2015. By then, she was identifying some past cases by autopsy records and current cases by clinical presentation. She saw a dramatic shift over time. From 2002 to 2011, she identified four cases, half of which had been exposed to IL-1/IL-6 inhibitors. From 2012 to 2014, eight new cases came to light, and seven had been exposed to those drugs. The crescendo continued from 2015 to 2017. During those years, Dr. Mellins and associates identified 10 new patients, 7 of whom had taken interleukin-inhibiting biologics. The mean time from initial drug exposure to diagnosis was a little more than 1 year.
An adjusted analysis comparing sJIA-LD patients and sJIA patients without lung disease didn’t find any significant difference in drug exposure. However, children with lung disease were more likely to have taken anakinra before the symptoms developed. Additionally, the symptoms of clubbing, abdominal pain, eosinophilia, hyperenhancing lymph nodes, and pulmonary alveolar proteinosis were much more common in children who’d taken the drugs.
The authors pointed out that this association does not prove causality and is confounded by the concomitant reduction in glucocorticoids with IL-1/IL-6 inhibitor use. And the vast majority of children with sJIA take cytokine inhibitors with no problems.
“Possibly, drug exposure may promote lung disease in a subset of children with sJIA, among the substantially larger group of patients who derive striking benefit from these drugs,” Dr. Mellins said, “Importantly, our results argue strongly for more investigation into a possible connection.”
Survival
After a mean follow-up of 1.7 years, the Stanford group saw high mortality. The 5-year survival rate translated to a mortality incidence of 159 deaths per 1,000 person-years, compared with 3.9 per 1,000 person-years in a historical cohort of sJIA patients who required biologic therapy.
Diffuse lung disease was the cause of 12 deaths; 5 of these patients also had macrophage activation syndrome at the time of death. Factors significantly associated with shortened survival included male sex, hypoxia at presentation, and neutrophilic bronchoalveolar lavage with more than 10 times the normal count. In an adjusted analysis, all of these variables fell out. However, none of the children with excessively high neutrophilic bronchoalveolar lavage survived.
Does it affect adults?
Could adults be experiencing the same disorder? There is some evidence to support it: The Food and Drug Administration adverse event website shows alveolar disease or pulmonary hypertension in 39 adults who have been exposed to IL-1 or IL-6 inhibition. Of these, 23 had RA, 11 adult-onset Still’s disease, and 5 unclassified rheumatic disorders.
The research groups were supported by grants from the sJIA Foundation, the Lucile Packard Foundation for Children’s Health, Stanford graduate fellowships, the Life Sciences Research Foundation, the Bill & Melinda Gates Foundation, Cincinnati Children’s Research Foundation, the Childhood Arthritis and Rheumatology Research Alliance, the Arthritis Foundation, and the National Institutes of Health. Many authors on both papers reported financial ties to Genentech, which markets tocilizumab, and other pharmaceutical companies*. Dr. Nigrovic reported receiving consulting fees and research support from Novartis and other companies.
SOURCES: Saper V et al. Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040; Schulert GS et al. Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073; Nigrovic PA. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071.
*Correction, 10/12/19: An earlier version of this article misstated the manufacturer of Actemra (tocilizumab).
This article was updated 10/14/19.
An uncommon but potentially deadly inflammatory lung disease is emerging among children with systemic juvenile idiopathic arthritis, and its history appears to coincide with the rise of powerful biologics as first-line therapy for children with the disease.
Most confirmed cases of systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) are in the United States. But it’s popping up in other places that have adopted early biologic treatment for sJIA – including Canada, South America, Europe, and the Middle East.
The respiratory symptoms are relatively subtle, so by the time of lung disease detection, the amount of affected lung can be extensive, said Elizabeth Mellins, MD, a Stanford (Calif.) University researcher who, along with first author Vivian Saper, MD, recently published the largest case series comprising reports from 37 institutions (Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040). By the end of follow-up, 22 of the 61 children in her cohort had died, including all 12 patients who demonstrated excessively high neutrophil levels in bronchoalveolar lavage samples.
Another recent report, authored by Grant Schulert, MD, PhD, and colleagues of the Cincinnati Children’s Hospital Medical Center, described 18 patients, 9 of whom were also included in the Stanford cohort (Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073).
Both investigators have now identified new patients.
“We are aware of 60 additional cases beyond what were included in our series,” Dr. Mellins said in an interview, bringing her entire cohort to 121. Dr. Schulert also continues to expand his group, detailing nine new cases at a recent private meeting.
“We are up to 27 now,” he said. “The features of these new patients are all very similar: The children are very young, all have had macrophage activation syndrome in the past and very-difficult-to-control JIA. Reactions to tocilizumab [Actemra] were also not uncommon in this group.”
Dr. Mellins also saw this association with allergic-type tocilizumab reactions, severe delayed hypersensitivity reactions to anakinra (Kineret) or canakinumab (Ilaris). Although serious lung disease in sJIA patients is not unheard of, this phenotype was virtually unknown until about a decade ago. Both investigators said that it’s been rising steadily since 2010 – just about the time that powerful cytokine-inhibiting biologics were changing these patients’ world for the better. After decades of relying almost solely on steroids and methotrexate, with rather poor results and significant long-term side effects, children were not only improving, but thriving. Gone was the life-changing glucocorticoid-related growth inhibition. Biologics could halt fevers, rash, and joint destruction in their tracks.
“For the first time in history, these kids could look forward to a more or less normal life,” Dr. Schulert said.
But the emergence of this particular type of lung disease could throw a pall over that success story, he said. If sJIA-LD is temporally associated with increasing reliance on long-term interleukin-1/IL-6 inhibition in children with early-onset disease, could these drugs actually be the causative agent? The picture remains unclear.
Some of the 18 in his initial series have improved, while 36% of those in the Stanford series died. Most who do recover stay on their IL-1 or IL-6 blocking therapy with good disease control without further lung problems. Both investigators found compelling genetic hints, but nothing conclusive. Children with trisomy 21 appear especially vulnerable. Most patients are very young – around 2 years old – but others are school aged. Some had a history of macrophage activation syndrome. Some had hard-to-control disease and some were clinically well controlled when the lung disease presented.
There are simply no answers yet.
With so many potential links, all unproven, clinicians may question the wisdom of embarking on long-term biologic therapy for their children with sJIA. Peter Nigrovic, MD, of Boston Children’s Hospital, addressed this in an accompanying editorial (Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071).
“My take on this is that it’s a very worrisome trend,” he said in an interview. “We’ve been going full bore toward early biologic therapy in sJIA and at the same time we are seeing more of this lung disease. Is it guilt by association? Or is there something more? The challenge for us is not to jump too soon to that conclusion.”
Although the association is there, he said, association does not equal causation. And there’s no doubt that biologics have vastly improved the lives of sJIA patients. “The drugs might be causal, and I worry about that and think we need to study it. But we absolutely need stronger evidence before we change practice.”
“This is a new manifestation of the disease, and it’s coming at the same time we are changing the treatment paradigm,” Dr. Nigrovic continued. “It could be because of interleukin-1 or interleukin-6 blockade. There is biological plausibility for such a link. It could also be related to the fact that we are using less steroids and methotrexate, which might have been preventing this. The appearance of sJIA lung disease could also be that a distinct secular trend unrelated to treatment, just as we saw amyloid come and go in this population in Europe. These other therapies were actually preventing this. We just don’t know.”
Clinical characteristics
Children presented with similar symptoms. Respiratory symptoms are usually subtle and mild. These can include tachypnea, hypoxia (43% in the Stanford series), and pulmonary hypertension (30% in the Stanford series).
Digital clubbing, often with erythema, was a common finding. Some children showed pruritic, nonevanescent rashes. Eosinophilia occurred in 37% of the Stanford series and severe abdominal pain in 16%, although Dr. Mellins noted that belly pain may be underestimated, as it was only volunteered, not queried, information.
“There are some red flags that should raise suspicion even without obvious respiratory symptoms,” Dr. Mellins said. These include lymphopenia, unexplained abdominal pain, eosinophilia, an unusual rash, and finger clubbing with or without erythema.
Findings on imaging were consistent in both series. Several key clinic features emerged: pleural thickening, septal thickening, bronchial wall or peribronchovascular thickening, “tree-in-bud” opacities, “ground-glass” opacities, peripheral consolidation, and lymphadenopathy.
“The imaging findings correspond to two things,” Dr. Schulert said. “The first is inflammation in the interstitium, which is evidence of chronic and ongoing inflammation. The other thing is that the alveoli are filled with a lipoproteinaceous material which is actually surfactant that’s not being normally recycled by the lung macrophages. You can see these features in other conditions where there’s a problem with lung macrophages, like pulmonary alveolar proteinosis, genetic and autoimmune disorders, infections, or inhalants.”
Pathology showed alveolar filling – a location in the lung that hides usual symptoms until the lung disease is advanced. Prior drug reactions were common. Tocilizumab anaphylaxis occurred in close to 40% of the Stanford series – a surprising finding given the 0.6% reaction incidence in the drug’s sJIA trials. Dr. Schulert saw a similar story.
“In our cohort we also observed a striking number of adverse events to cytokine-targeted biologics exposure,” Dr. Schulert said. “Most of these reactions were to tocilizumab, and were described variously from pain and feeling unwell, to difficulty breathing, to anaphylaxis.”
In a risk analysis, Dr. Schulert determined that adverse events to cytokine-targeting biologics increased the likelihood of lung disease more than 13 times (odds ratio, 13.6).
“We also identified a statistically significant association with history of macrophage activation syndrome when compared to controls (OR, 14.5),” Dr. Schulert and associates wrote.
Genetics
Both the Cincinnati and Stanford teams conducted genetic analyses on some of their patients.
Among eight lung biopsy samples, Dr. Schulert found 37 differentially expressed genes: 36 with increased expression and 1 with decreased expression. Many of the up-regulated genes are involved in interferon-gamma response. Two (CXCL10 and CXCL9) are interferon-induced chemokines associated with macrophage activation syndrome. The down-regulated gene, PADI4, modulates immune response in lupus, and has been associated with the risk of interstitial lung disease in RA.
Dr. Mellins and her team analyzed whole-exome sequencing data from 20 patients and found some rare protein-altering gene variants in genes related to pulmonary alveolar proteinosis, all of which were heterozygous and shared with a healthy parent. But none of them could be directly tied to the disorder.
Another genetic puzzle demands attention, she said. About 10% of the children had trisomy 21 – a stark contrast to the typical 0.2% prevalence among a control group of sJIA patients without any known lung disease in the Childhood Arthritis and Rheumatology Research Alliance Registry cohort, similar to the background population rate. There were suggestions of more aggressive lung disease in all six of these children. Four presented with hypoxia, and two showed advanced interstitial fibrosis. Children with trisomy 21 also seemed more susceptible to infections; 83% had a viral or fungal lung infection at diagnosis, compared with 29% of those without trisomy 21.
Prior exposure to cytokine inhibitors
Parenchymal lung disease and pulmonary hypertension complicating sJIA was first highlighted in a series of 25 cases reported by Kimura et al. in 2013. These authors raised the question of the possible relationship of this and the increasing use of anti–IL-1 and anti–IL-6 biologics in sJIA treatment.
Following this lead, Dr. Mellins started looking into this new clinical entity in 2015. By then, she was identifying some past cases by autopsy records and current cases by clinical presentation. She saw a dramatic shift over time. From 2002 to 2011, she identified four cases, half of which had been exposed to IL-1/IL-6 inhibitors. From 2012 to 2014, eight new cases came to light, and seven had been exposed to those drugs. The crescendo continued from 2015 to 2017. During those years, Dr. Mellins and associates identified 10 new patients, 7 of whom had taken interleukin-inhibiting biologics. The mean time from initial drug exposure to diagnosis was a little more than 1 year.
An adjusted analysis comparing sJIA-LD patients and sJIA patients without lung disease didn’t find any significant difference in drug exposure. However, children with lung disease were more likely to have taken anakinra before the symptoms developed. Additionally, the symptoms of clubbing, abdominal pain, eosinophilia, hyperenhancing lymph nodes, and pulmonary alveolar proteinosis were much more common in children who’d taken the drugs.
The authors pointed out that this association does not prove causality and is confounded by the concomitant reduction in glucocorticoids with IL-1/IL-6 inhibitor use. And the vast majority of children with sJIA take cytokine inhibitors with no problems.
“Possibly, drug exposure may promote lung disease in a subset of children with sJIA, among the substantially larger group of patients who derive striking benefit from these drugs,” Dr. Mellins said, “Importantly, our results argue strongly for more investigation into a possible connection.”
Survival
After a mean follow-up of 1.7 years, the Stanford group saw high mortality. The 5-year survival rate translated to a mortality incidence of 159 deaths per 1,000 person-years, compared with 3.9 per 1,000 person-years in a historical cohort of sJIA patients who required biologic therapy.
Diffuse lung disease was the cause of 12 deaths; 5 of these patients also had macrophage activation syndrome at the time of death. Factors significantly associated with shortened survival included male sex, hypoxia at presentation, and neutrophilic bronchoalveolar lavage with more than 10 times the normal count. In an adjusted analysis, all of these variables fell out. However, none of the children with excessively high neutrophilic bronchoalveolar lavage survived.
Does it affect adults?
Could adults be experiencing the same disorder? There is some evidence to support it: The Food and Drug Administration adverse event website shows alveolar disease or pulmonary hypertension in 39 adults who have been exposed to IL-1 or IL-6 inhibition. Of these, 23 had RA, 11 adult-onset Still’s disease, and 5 unclassified rheumatic disorders.
The research groups were supported by grants from the sJIA Foundation, the Lucile Packard Foundation for Children’s Health, Stanford graduate fellowships, the Life Sciences Research Foundation, the Bill & Melinda Gates Foundation, Cincinnati Children’s Research Foundation, the Childhood Arthritis and Rheumatology Research Alliance, the Arthritis Foundation, and the National Institutes of Health. Many authors on both papers reported financial ties to Genentech, which markets tocilizumab, and other pharmaceutical companies*. Dr. Nigrovic reported receiving consulting fees and research support from Novartis and other companies.
SOURCES: Saper V et al. Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040; Schulert GS et al. Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073; Nigrovic PA. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071.
*Correction, 10/12/19: An earlier version of this article misstated the manufacturer of Actemra (tocilizumab).
This article was updated 10/14/19.
An uncommon but potentially deadly inflammatory lung disease is emerging among children with systemic juvenile idiopathic arthritis, and its history appears to coincide with the rise of powerful biologics as first-line therapy for children with the disease.
Most confirmed cases of systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) are in the United States. But it’s popping up in other places that have adopted early biologic treatment for sJIA – including Canada, South America, Europe, and the Middle East.
The respiratory symptoms are relatively subtle, so by the time of lung disease detection, the amount of affected lung can be extensive, said Elizabeth Mellins, MD, a Stanford (Calif.) University researcher who, along with first author Vivian Saper, MD, recently published the largest case series comprising reports from 37 institutions (Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040). By the end of follow-up, 22 of the 61 children in her cohort had died, including all 12 patients who demonstrated excessively high neutrophil levels in bronchoalveolar lavage samples.
Another recent report, authored by Grant Schulert, MD, PhD, and colleagues of the Cincinnati Children’s Hospital Medical Center, described 18 patients, 9 of whom were also included in the Stanford cohort (Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073).
Both investigators have now identified new patients.
“We are aware of 60 additional cases beyond what were included in our series,” Dr. Mellins said in an interview, bringing her entire cohort to 121. Dr. Schulert also continues to expand his group, detailing nine new cases at a recent private meeting.
“We are up to 27 now,” he said. “The features of these new patients are all very similar: The children are very young, all have had macrophage activation syndrome in the past and very-difficult-to-control JIA. Reactions to tocilizumab [Actemra] were also not uncommon in this group.”
Dr. Mellins also saw this association with allergic-type tocilizumab reactions, severe delayed hypersensitivity reactions to anakinra (Kineret) or canakinumab (Ilaris). Although serious lung disease in sJIA patients is not unheard of, this phenotype was virtually unknown until about a decade ago. Both investigators said that it’s been rising steadily since 2010 – just about the time that powerful cytokine-inhibiting biologics were changing these patients’ world for the better. After decades of relying almost solely on steroids and methotrexate, with rather poor results and significant long-term side effects, children were not only improving, but thriving. Gone was the life-changing glucocorticoid-related growth inhibition. Biologics could halt fevers, rash, and joint destruction in their tracks.
“For the first time in history, these kids could look forward to a more or less normal life,” Dr. Schulert said.
But the emergence of this particular type of lung disease could throw a pall over that success story, he said. If sJIA-LD is temporally associated with increasing reliance on long-term interleukin-1/IL-6 inhibition in children with early-onset disease, could these drugs actually be the causative agent? The picture remains unclear.
Some of the 18 in his initial series have improved, while 36% of those in the Stanford series died. Most who do recover stay on their IL-1 or IL-6 blocking therapy with good disease control without further lung problems. Both investigators found compelling genetic hints, but nothing conclusive. Children with trisomy 21 appear especially vulnerable. Most patients are very young – around 2 years old – but others are school aged. Some had a history of macrophage activation syndrome. Some had hard-to-control disease and some were clinically well controlled when the lung disease presented.
There are simply no answers yet.
With so many potential links, all unproven, clinicians may question the wisdom of embarking on long-term biologic therapy for their children with sJIA. Peter Nigrovic, MD, of Boston Children’s Hospital, addressed this in an accompanying editorial (Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071).
“My take on this is that it’s a very worrisome trend,” he said in an interview. “We’ve been going full bore toward early biologic therapy in sJIA and at the same time we are seeing more of this lung disease. Is it guilt by association? Or is there something more? The challenge for us is not to jump too soon to that conclusion.”
Although the association is there, he said, association does not equal causation. And there’s no doubt that biologics have vastly improved the lives of sJIA patients. “The drugs might be causal, and I worry about that and think we need to study it. But we absolutely need stronger evidence before we change practice.”
“This is a new manifestation of the disease, and it’s coming at the same time we are changing the treatment paradigm,” Dr. Nigrovic continued. “It could be because of interleukin-1 or interleukin-6 blockade. There is biological plausibility for such a link. It could also be related to the fact that we are using less steroids and methotrexate, which might have been preventing this. The appearance of sJIA lung disease could also be that a distinct secular trend unrelated to treatment, just as we saw amyloid come and go in this population in Europe. These other therapies were actually preventing this. We just don’t know.”
Clinical characteristics
Children presented with similar symptoms. Respiratory symptoms are usually subtle and mild. These can include tachypnea, hypoxia (43% in the Stanford series), and pulmonary hypertension (30% in the Stanford series).
Digital clubbing, often with erythema, was a common finding. Some children showed pruritic, nonevanescent rashes. Eosinophilia occurred in 37% of the Stanford series and severe abdominal pain in 16%, although Dr. Mellins noted that belly pain may be underestimated, as it was only volunteered, not queried, information.
“There are some red flags that should raise suspicion even without obvious respiratory symptoms,” Dr. Mellins said. These include lymphopenia, unexplained abdominal pain, eosinophilia, an unusual rash, and finger clubbing with or without erythema.
Findings on imaging were consistent in both series. Several key clinic features emerged: pleural thickening, septal thickening, bronchial wall or peribronchovascular thickening, “tree-in-bud” opacities, “ground-glass” opacities, peripheral consolidation, and lymphadenopathy.
“The imaging findings correspond to two things,” Dr. Schulert said. “The first is inflammation in the interstitium, which is evidence of chronic and ongoing inflammation. The other thing is that the alveoli are filled with a lipoproteinaceous material which is actually surfactant that’s not being normally recycled by the lung macrophages. You can see these features in other conditions where there’s a problem with lung macrophages, like pulmonary alveolar proteinosis, genetic and autoimmune disorders, infections, or inhalants.”
Pathology showed alveolar filling – a location in the lung that hides usual symptoms until the lung disease is advanced. Prior drug reactions were common. Tocilizumab anaphylaxis occurred in close to 40% of the Stanford series – a surprising finding given the 0.6% reaction incidence in the drug’s sJIA trials. Dr. Schulert saw a similar story.
“In our cohort we also observed a striking number of adverse events to cytokine-targeted biologics exposure,” Dr. Schulert said. “Most of these reactions were to tocilizumab, and were described variously from pain and feeling unwell, to difficulty breathing, to anaphylaxis.”
In a risk analysis, Dr. Schulert determined that adverse events to cytokine-targeting biologics increased the likelihood of lung disease more than 13 times (odds ratio, 13.6).
“We also identified a statistically significant association with history of macrophage activation syndrome when compared to controls (OR, 14.5),” Dr. Schulert and associates wrote.
Genetics
Both the Cincinnati and Stanford teams conducted genetic analyses on some of their patients.
Among eight lung biopsy samples, Dr. Schulert found 37 differentially expressed genes: 36 with increased expression and 1 with decreased expression. Many of the up-regulated genes are involved in interferon-gamma response. Two (CXCL10 and CXCL9) are interferon-induced chemokines associated with macrophage activation syndrome. The down-regulated gene, PADI4, modulates immune response in lupus, and has been associated with the risk of interstitial lung disease in RA.
Dr. Mellins and her team analyzed whole-exome sequencing data from 20 patients and found some rare protein-altering gene variants in genes related to pulmonary alveolar proteinosis, all of which were heterozygous and shared with a healthy parent. But none of them could be directly tied to the disorder.
Another genetic puzzle demands attention, she said. About 10% of the children had trisomy 21 – a stark contrast to the typical 0.2% prevalence among a control group of sJIA patients without any known lung disease in the Childhood Arthritis and Rheumatology Research Alliance Registry cohort, similar to the background population rate. There were suggestions of more aggressive lung disease in all six of these children. Four presented with hypoxia, and two showed advanced interstitial fibrosis. Children with trisomy 21 also seemed more susceptible to infections; 83% had a viral or fungal lung infection at diagnosis, compared with 29% of those without trisomy 21.
Prior exposure to cytokine inhibitors
Parenchymal lung disease and pulmonary hypertension complicating sJIA was first highlighted in a series of 25 cases reported by Kimura et al. in 2013. These authors raised the question of the possible relationship of this and the increasing use of anti–IL-1 and anti–IL-6 biologics in sJIA treatment.
Following this lead, Dr. Mellins started looking into this new clinical entity in 2015. By then, she was identifying some past cases by autopsy records and current cases by clinical presentation. She saw a dramatic shift over time. From 2002 to 2011, she identified four cases, half of which had been exposed to IL-1/IL-6 inhibitors. From 2012 to 2014, eight new cases came to light, and seven had been exposed to those drugs. The crescendo continued from 2015 to 2017. During those years, Dr. Mellins and associates identified 10 new patients, 7 of whom had taken interleukin-inhibiting biologics. The mean time from initial drug exposure to diagnosis was a little more than 1 year.
An adjusted analysis comparing sJIA-LD patients and sJIA patients without lung disease didn’t find any significant difference in drug exposure. However, children with lung disease were more likely to have taken anakinra before the symptoms developed. Additionally, the symptoms of clubbing, abdominal pain, eosinophilia, hyperenhancing lymph nodes, and pulmonary alveolar proteinosis were much more common in children who’d taken the drugs.
The authors pointed out that this association does not prove causality and is confounded by the concomitant reduction in glucocorticoids with IL-1/IL-6 inhibitor use. And the vast majority of children with sJIA take cytokine inhibitors with no problems.
“Possibly, drug exposure may promote lung disease in a subset of children with sJIA, among the substantially larger group of patients who derive striking benefit from these drugs,” Dr. Mellins said, “Importantly, our results argue strongly for more investigation into a possible connection.”
Survival
After a mean follow-up of 1.7 years, the Stanford group saw high mortality. The 5-year survival rate translated to a mortality incidence of 159 deaths per 1,000 person-years, compared with 3.9 per 1,000 person-years in a historical cohort of sJIA patients who required biologic therapy.
Diffuse lung disease was the cause of 12 deaths; 5 of these patients also had macrophage activation syndrome at the time of death. Factors significantly associated with shortened survival included male sex, hypoxia at presentation, and neutrophilic bronchoalveolar lavage with more than 10 times the normal count. In an adjusted analysis, all of these variables fell out. However, none of the children with excessively high neutrophilic bronchoalveolar lavage survived.
Does it affect adults?
Could adults be experiencing the same disorder? There is some evidence to support it: The Food and Drug Administration adverse event website shows alveolar disease or pulmonary hypertension in 39 adults who have been exposed to IL-1 or IL-6 inhibition. Of these, 23 had RA, 11 adult-onset Still’s disease, and 5 unclassified rheumatic disorders.
The research groups were supported by grants from the sJIA Foundation, the Lucile Packard Foundation for Children’s Health, Stanford graduate fellowships, the Life Sciences Research Foundation, the Bill & Melinda Gates Foundation, Cincinnati Children’s Research Foundation, the Childhood Arthritis and Rheumatology Research Alliance, the Arthritis Foundation, and the National Institutes of Health. Many authors on both papers reported financial ties to Genentech, which markets tocilizumab, and other pharmaceutical companies*. Dr. Nigrovic reported receiving consulting fees and research support from Novartis and other companies.
SOURCES: Saper V et al. Ann Rheum Dis. 2019 Sep 27. doi: 10.1136/annrheumdis-2019-216040; Schulert GS et al. Arthritis Rheumatol. 2019 Aug 5. doi: 10.1002/art.41073; Nigrovic PA. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41071.
*Correction, 10/12/19: An earlier version of this article misstated the manufacturer of Actemra (tocilizumab).
This article was updated 10/14/19.