User login
A Call to Action: Hospitalists’ Role in Addressing Substance Use Disorder
In 2017, the death toll from drug overdoses reached a record high, killing more Americans than the entire Vietnam War or the HIV/AIDS epidemic at its peak.1 Up to one-quarter of hospitalized patients have a substance use disorder (SUD) and SUD-related2,3 hospitalizations are surging. People with SUD have longer hospital stays, higher costs, and more readmissions.3,4 While the burden of SUD is staggering, it is far from hopeless. There are multiple evidence-based and highly effective interventions to treat SUD, including medications, behavioral interventions, and harm reduction strategies.
Hospitalization can be a reachable moment to initiate and coordinate addictions care.5 Hospital-based addictions care has the potential to engage sicker, highly vulnerable patients, many who are not engaged in primary care or outpatient addictions care.6 Studied effects of hospital-based addictions care include improved SUD treatment engagement, reduced alcohol and drug use, lower hospital readmissions, and improved provider experience.7-9
Most hospitals, however, do not treat SUD during hospitalization and do not connect people to treatment after discharge. Hospitals may lack staffing or financial resources to implement addiction care, may believe that SUDs are an outpatient concern, may want to avoid caring for people with SUD, or may simply not know where to begin. Whatever the reason, unaddressed SUD can lead to untreated withdrawal, disruptive patient behaviors, failure to complete recommended medical therapy, high rates of against medical advice discharge, poor patient experience, and widespread provider distress.8
Hospitalists—individually and collectively—are uniquely positioned to address this gap. By treating addiction effectively and compassionately, hospitalists can engage patients, improve care, improve patient and provider experience, and lower costs. This paper is a call to action that describes the current state of hospital-based addictions care, outlines key challenges to implementing SUD care in the hospital, debunks common misconceptions, and identifies actionable steps for hospitalists, hospital leaders, and hospitalist organizations.
MODELS TO DELIVER HOSPITAL-BASED ADDICTIONS CARE
Hospital-based addiction medicine consult services are emerging; they include a range of models, with variations in how patients are identified, team composition, service availability, and financing.10 Existing addiction medicine consult services commonly offer SUD assessments, psychological intervention, medical management of SUDs (eg, initiating methadone or buprenorphine), medical pain management, and linkage to SUD care after hospitalization. Some services also explicitly integrate harm reduction principles (eg, naloxone distribution, safe injection education, permitting patients to smoke).11 Additional consult service activities include hospital-wide SUD education, and creation and implementation of hospital guidance documents (eg, methadone policies).10 Some consult services utilize only physicians, while others include interprofessional providers, such as nurses, social workers, and peers with lived experience of addiction. Whereas addiction medicine physicians staff some consult services, hospitalists with less formal addiction credentials staff others.
Broadly, hospital-based addictions care cannot depend solely on consult services. Just as not all hospitals have cardiology consult services, not all hospitals will have addiction consult services. As such, hospitalists can play an even greater role by implementing order sets and guidelines, supporting partnerships with community SUD treatment, and independently initiating evidence-based medications.
CHALLENGES TO ADOPTION AND IMPLEMENTATION OF HOSPITAL-BASED ADDICTIONS CARE
Pervasive individual and structural stigmas12 are perhaps the most critical barriers to incorporating addiction medicine into routine hospital practice, and they are both cause and consequence of our system failures. Most medical schools and residencies lack SUD training, which means that the understanding of addiction as a moral deficiency or lack of willpower may remain unchallenged. Stigma surrounding SUDs contributes to hospitalists’ and hospital leaders’ aversion to treating patients with SUD, and to fears that providing quality SUD care will attract patients suffering from these conditions.
Recent national efforts have focused on the problem of opioid overprescribing. Without an equal emphasis on treatment, this focus can lead to undertreatment of pain and/or opioid use disorder in hospitalized patients, particularly since most hospitalists have little to no training in diagnosing SUD, prescribing life-saving medications for opioid use disorder, or managing acute pain in patients with SUD. The focus on overprescribing also diverts attention away from trends involving stimulants,2 fentanyl contamination of the drug supply,13 and alcohol, all of which have important implications for the care of hospitalized adults.
Hospital policies are often not grounded in evidence (eg, recommending clonidine for first-line treatment of opioid withdrawal and not buprenorphine/methadone), and there are widespread misconceptions about perceived legal barriers to treating opioid use disorder in the hospital, which is both safe and legal.10 People with SUD may be unjustly viewed through a criminal justice lens. Policies focused on controlling visitors and conducting room searches disproportionately burden people with SUD, which may create further harms through reinforcing negative provider cognitive biases about SUDs. Finally, hospitals may lack inpatient social work and pharmacy supports, and they rarely have pathways to connect people to SUD care after discharge.
Funding remains a widespread challenge. While some hospital administrators support addiction medicine services because of the pressing medical need and public health crisis, most services depend on billing or demonstrated savings through reduced hospital days or readmissions.
A CALL TO ACTION: HOW HOSPITALISTS CAN IMPROVE ADDICTION CARE
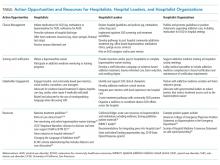
Individual hospitalists, hospitalist leaders, and hospitalist organizations can engage by improving individual practice, driving systems change, and through advocacy and policy change (Table).
Individual Hospitalists
Providing basic addiction medicine care should be a core competency for all hospitalists, just as every hospitalist can initiate a goals-of-care conversation or prescribe insulin. For opioid use disorder, hospitalists should treat withdrawal and offer treatment initiation with opioid agonist therapy (ie, methadone, buprenorphine), which reduces mortality by over half. Commonly, hospitalized patients are subjected to harmful, nonevidence-based treatments, such as mandated rapid methadone tapers,25 which can lead to undertreated withdrawal, increased pain, and opioid cravings. This increases patients’ risk for overdose after discharge and precludes them from receiving life-saving, evidence-based methadone maintenance, or buprenorphine treatment. Though widely misunderstood, prescribing methadone in the hospital is legal, and providers need no special waiver to prescribe buprenorphine during admission. Current laws require that hospitalists have a waiver to prescribe buprenorphine at discharge and prohibit hospitalists (or anyone outside of an opioid treatment program) from prescribing methadone for the treatment of opioid use disorder at discharge. Further, hospitalists should offer medication for alcohol use disorder (eg, naltrexone) and be good stewards of opioids during hospitalization, avoiding intravenous opioids where appropriate and curbing excessive prescribing at discharge. Given high rates of overdose and fentanyl contamination of stimulants, opioids, and benzodiazepines, hospitalists should prescribe naloxone at discharge to every patient with SUD, on chronic opioids, or who uses any nonmedical substances.
Resources exist for individual hospitalists seeking mentorship or additional training (Table). Though not necessary for in-hospital prescribing, hospitalists can obtain a waiver to prescribe buprenorphine at discharge (commonly called the X-waiver). To qualify, physicians must complete eight hours of accredited training (online and/or in-person), after which they must request a waiver from the Drug Enforcement Administration. Advanced-practice practitioners must complete 24 hours of training. Many have argued that policymakers should end this waiver requirement.26 While we support efforts to “X the X” and urgently expand treatment access, additional training can enrich providers’ knowledge and confidence to prescribe buprenorphine, and is a relatively simple way that all hospitalists could act. Finally, by treating addiction and modeling patient-centered addictions care, hospitalists can legitimize and destigmatize the disease of addiction,8 and have the potential to mentor and train students, residents, nurses, and other staff.27
Hospitalist Leaders
As leaders, hospitalists can play a key role in promoting hospital-based addictions care and tailoring solutions to meet local needs. Leaders can promote a cultural shift away from stigma, and promote evidence-based, life-saving care. Hospitalist leaders could require all hospitalists to obtain buprenorphine waivers. Leaders could initiate quality improvement projects related to SUD service delivery, develop policies that support inpatient SUD treatment, develop order sets for medication initiation, engage community substance use treatment partners, build pathways to timely addiction care after discharge, and champion development of addiction medicine consult services.
Hospitalist leaders can reference open-source guidelines, order sets, assessment and treatment tools, patient materials, pharmacy and therapeutics committee materials, and other resources for implementing services for hospitalized patients with SUD (Table).21,22 Hospitalist leaders who understand financial and quality drivers can also champion the business and quality case for hospital-based addictions care, and help pursue local and national funding opportunities.
Hospitalist Organizations
Hospitalist societies could provide training at regional and national conferences to upskill hospitalists to care for people with SUD; support addiction medicine interest groups; and partner with addiction medicine societies, harm reduction organizations, and organizations focused on trauma-informed care. They could endorse practice guidelines and position statements describing the crucial role of hospitalists in addressing the overdose crisis and offering medication for addiction (Table). Hospitalist organizations can engage national and state hospital associations, lobby medical specialties to include addiction medicine competencies in board certification requirements, and advocate with governmental leaders to reduce barriers that restrict treatment access such as the X-waiver.
MOVING FORWARD
Regardless of whether a hospitalist is serving as an individual provider, a hospitalist leader, or as part of a hospitalist organization, hospitalists can take critical steps to advance the care of people with SUD. These steps shift the culture of hospitals from one where patients are afraid to discuss their substance use, to one that creates space for connection, treatment engagement, and healing. By starting medications, utilizing widely accessible resources, and collaborating with community treatment and harm reduction organizations, each one of us can play a part in addressing the epidemic.
Acknowledgments
The authors thank Alisa Patten for help preparing this manuscript. Dr. Englander would like to thank Dr. David Bangsberg and Dr. Christina Nicolaidis for their mentorship.
1. Weiss A, Elixhauser A, Barrett M, Steiner C, Bailey M, O’Malley L. Opioid-related inpatient stays and emergency department visits by state, 2009-2014. Statistical Brief #219. Healthcare Cost and Utilization Project. 2016. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb219-Opioid-Hospital-Stays-ED-Visits-by-State.jsp. Accessed May 21, 2019.
2. Winkelman TA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open. 2018;1(6):e183758. https://doi.org/10.1001/jamanetworkopen.2018.3758.
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
4. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. https://doi.org/10.1097/ADM.0b013e318231de51.
5. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736.
6. Velez C, Nicolaidis C, Korthuis P, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. doi 10.1007/s11606-016-3919-4.
7. Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909-916. https://doi.org/10.1007/s11606-017-4077-z.
8. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993.
9. McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2011;10(8):CD005191 https://doi.org/10.1002/14651858.CD005191.pub3.
10. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496.
11. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001.
12. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment”: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010.
13. Ciccarone D. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int J Drug Policy. 2019. pii: S0955-3959(19)30018-0. [Epub ahead of print]. https://doi.org/10.1016/j.drugpo.2019.01.010.
14. Substance Abuse and Mental Health Services Administration. TIP 63: Medications for Opioid Use Disorder-Executive Summary. February 2018. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Executive-Summary/sma18-5063exsumm. Accessed August 8, 2019.
15. Providers Clinical Support System. Discover the rewards of treating patients with Opioid Use Disorders. https://pcssnow.org/. Accessed August 8, 2019.
16. California Bridge Program. Treatment Starts Here: Resources for the Treatment of Substance Use Disorders from the Acute Care Setting. https://www.bridgetotreatment.org/resources. Accessed August 7, 2019.
17. Clinical Consultation Center. Substance Use Resources. 2019. https://nccc.ucsf.edu/clinical-resources/substance-use-resources/. Accessed August 8, 2019.
18. Thakarar K, Weinstein ZM, Walley AY. Optimising health and safety of people who inject drugs during transition from acute to outpatient care: narrative review with clinical checklist. Postgrad Med J. 2016;92(1088):356-363. https://doi.org/10.1136/postgradmedj-2015-133720.
19. Office of National Drug Control Policy. Changing the Language of Addiction. Washington, D.C. 2017. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Memo%20-%20Changing%20Federal%20Terminology%20Regrading%20Substance%20Use%20and%20Substance%20Use%20Disorders.pdf. Accessed August 8, 2019.
20. The University of New Mexico. Project ECHO: A Revolution in Medical Education and Care Delivery. 2019. https://echo.unm.edu/. Accessed August 8, 2019.
21. Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the Improving Addiction Care Team. J Addict Med. 2019;13(2):85-89. https://doi.org/10.1097/ADM.0000000000000487.
22. Englander H, Gregg J, Gollickson J, et al. Recommendations for intergrating peer mentors in hospital-based addiction care. Subst Abus. In press. https://doi.org/10.1080/08897077.2019.1635968.
23. American College of Medical Toxicology. ACMT Position Statement: Buprenorphine Administration in the Emergency Department. https://www.acep.org/globalassets/sites/acep/media/equal-documents/policy_acmt_bupeadministration.pdf. Accessed May 21, 2019.
24. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980.
25. Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018;13(1):62-64. https://doi.org/10.12788/jhm.2861.
26. Frank JW, Wakeman SE, Gordon AJ. No end to the crisis without an end to the waiver. Subst Abus. 2018;39(3):263-265. https://doi.org/10.1080/08897077.2018.1543382.
27. Gorfinkel L, Klimas J, Reel B, et al. In-hospital training in addiction medicine: a mixed-methods study of health care provider benefits and differences. Subst Abus. 2019. In press. https://doi.org/10.1080/08897077.2018.1561596.
In 2017, the death toll from drug overdoses reached a record high, killing more Americans than the entire Vietnam War or the HIV/AIDS epidemic at its peak.1 Up to one-quarter of hospitalized patients have a substance use disorder (SUD) and SUD-related2,3 hospitalizations are surging. People with SUD have longer hospital stays, higher costs, and more readmissions.3,4 While the burden of SUD is staggering, it is far from hopeless. There are multiple evidence-based and highly effective interventions to treat SUD, including medications, behavioral interventions, and harm reduction strategies.
Hospitalization can be a reachable moment to initiate and coordinate addictions care.5 Hospital-based addictions care has the potential to engage sicker, highly vulnerable patients, many who are not engaged in primary care or outpatient addictions care.6 Studied effects of hospital-based addictions care include improved SUD treatment engagement, reduced alcohol and drug use, lower hospital readmissions, and improved provider experience.7-9
Most hospitals, however, do not treat SUD during hospitalization and do not connect people to treatment after discharge. Hospitals may lack staffing or financial resources to implement addiction care, may believe that SUDs are an outpatient concern, may want to avoid caring for people with SUD, or may simply not know where to begin. Whatever the reason, unaddressed SUD can lead to untreated withdrawal, disruptive patient behaviors, failure to complete recommended medical therapy, high rates of against medical advice discharge, poor patient experience, and widespread provider distress.8
Hospitalists—individually and collectively—are uniquely positioned to address this gap. By treating addiction effectively and compassionately, hospitalists can engage patients, improve care, improve patient and provider experience, and lower costs. This paper is a call to action that describes the current state of hospital-based addictions care, outlines key challenges to implementing SUD care in the hospital, debunks common misconceptions, and identifies actionable steps for hospitalists, hospital leaders, and hospitalist organizations.
MODELS TO DELIVER HOSPITAL-BASED ADDICTIONS CARE
Hospital-based addiction medicine consult services are emerging; they include a range of models, with variations in how patients are identified, team composition, service availability, and financing.10 Existing addiction medicine consult services commonly offer SUD assessments, psychological intervention, medical management of SUDs (eg, initiating methadone or buprenorphine), medical pain management, and linkage to SUD care after hospitalization. Some services also explicitly integrate harm reduction principles (eg, naloxone distribution, safe injection education, permitting patients to smoke).11 Additional consult service activities include hospital-wide SUD education, and creation and implementation of hospital guidance documents (eg, methadone policies).10 Some consult services utilize only physicians, while others include interprofessional providers, such as nurses, social workers, and peers with lived experience of addiction. Whereas addiction medicine physicians staff some consult services, hospitalists with less formal addiction credentials staff others.
Broadly, hospital-based addictions care cannot depend solely on consult services. Just as not all hospitals have cardiology consult services, not all hospitals will have addiction consult services. As such, hospitalists can play an even greater role by implementing order sets and guidelines, supporting partnerships with community SUD treatment, and independently initiating evidence-based medications.
CHALLENGES TO ADOPTION AND IMPLEMENTATION OF HOSPITAL-BASED ADDICTIONS CARE
Pervasive individual and structural stigmas12 are perhaps the most critical barriers to incorporating addiction medicine into routine hospital practice, and they are both cause and consequence of our system failures. Most medical schools and residencies lack SUD training, which means that the understanding of addiction as a moral deficiency or lack of willpower may remain unchallenged. Stigma surrounding SUDs contributes to hospitalists’ and hospital leaders’ aversion to treating patients with SUD, and to fears that providing quality SUD care will attract patients suffering from these conditions.
Recent national efforts have focused on the problem of opioid overprescribing. Without an equal emphasis on treatment, this focus can lead to undertreatment of pain and/or opioid use disorder in hospitalized patients, particularly since most hospitalists have little to no training in diagnosing SUD, prescribing life-saving medications for opioid use disorder, or managing acute pain in patients with SUD. The focus on overprescribing also diverts attention away from trends involving stimulants,2 fentanyl contamination of the drug supply,13 and alcohol, all of which have important implications for the care of hospitalized adults.
Hospital policies are often not grounded in evidence (eg, recommending clonidine for first-line treatment of opioid withdrawal and not buprenorphine/methadone), and there are widespread misconceptions about perceived legal barriers to treating opioid use disorder in the hospital, which is both safe and legal.10 People with SUD may be unjustly viewed through a criminal justice lens. Policies focused on controlling visitors and conducting room searches disproportionately burden people with SUD, which may create further harms through reinforcing negative provider cognitive biases about SUDs. Finally, hospitals may lack inpatient social work and pharmacy supports, and they rarely have pathways to connect people to SUD care after discharge.
Funding remains a widespread challenge. While some hospital administrators support addiction medicine services because of the pressing medical need and public health crisis, most services depend on billing or demonstrated savings through reduced hospital days or readmissions.
A CALL TO ACTION: HOW HOSPITALISTS CAN IMPROVE ADDICTION CARE
Individual hospitalists, hospitalist leaders, and hospitalist organizations can engage by improving individual practice, driving systems change, and through advocacy and policy change (Table).
Individual Hospitalists
Providing basic addiction medicine care should be a core competency for all hospitalists, just as every hospitalist can initiate a goals-of-care conversation or prescribe insulin. For opioid use disorder, hospitalists should treat withdrawal and offer treatment initiation with opioid agonist therapy (ie, methadone, buprenorphine), which reduces mortality by over half. Commonly, hospitalized patients are subjected to harmful, nonevidence-based treatments, such as mandated rapid methadone tapers,25 which can lead to undertreated withdrawal, increased pain, and opioid cravings. This increases patients’ risk for overdose after discharge and precludes them from receiving life-saving, evidence-based methadone maintenance, or buprenorphine treatment. Though widely misunderstood, prescribing methadone in the hospital is legal, and providers need no special waiver to prescribe buprenorphine during admission. Current laws require that hospitalists have a waiver to prescribe buprenorphine at discharge and prohibit hospitalists (or anyone outside of an opioid treatment program) from prescribing methadone for the treatment of opioid use disorder at discharge. Further, hospitalists should offer medication for alcohol use disorder (eg, naltrexone) and be good stewards of opioids during hospitalization, avoiding intravenous opioids where appropriate and curbing excessive prescribing at discharge. Given high rates of overdose and fentanyl contamination of stimulants, opioids, and benzodiazepines, hospitalists should prescribe naloxone at discharge to every patient with SUD, on chronic opioids, or who uses any nonmedical substances.
Resources exist for individual hospitalists seeking mentorship or additional training (Table). Though not necessary for in-hospital prescribing, hospitalists can obtain a waiver to prescribe buprenorphine at discharge (commonly called the X-waiver). To qualify, physicians must complete eight hours of accredited training (online and/or in-person), after which they must request a waiver from the Drug Enforcement Administration. Advanced-practice practitioners must complete 24 hours of training. Many have argued that policymakers should end this waiver requirement.26 While we support efforts to “X the X” and urgently expand treatment access, additional training can enrich providers’ knowledge and confidence to prescribe buprenorphine, and is a relatively simple way that all hospitalists could act. Finally, by treating addiction and modeling patient-centered addictions care, hospitalists can legitimize and destigmatize the disease of addiction,8 and have the potential to mentor and train students, residents, nurses, and other staff.27
Hospitalist Leaders
As leaders, hospitalists can play a key role in promoting hospital-based addictions care and tailoring solutions to meet local needs. Leaders can promote a cultural shift away from stigma, and promote evidence-based, life-saving care. Hospitalist leaders could require all hospitalists to obtain buprenorphine waivers. Leaders could initiate quality improvement projects related to SUD service delivery, develop policies that support inpatient SUD treatment, develop order sets for medication initiation, engage community substance use treatment partners, build pathways to timely addiction care after discharge, and champion development of addiction medicine consult services.
Hospitalist leaders can reference open-source guidelines, order sets, assessment and treatment tools, patient materials, pharmacy and therapeutics committee materials, and other resources for implementing services for hospitalized patients with SUD (Table).21,22 Hospitalist leaders who understand financial and quality drivers can also champion the business and quality case for hospital-based addictions care, and help pursue local and national funding opportunities.
Hospitalist Organizations
Hospitalist societies could provide training at regional and national conferences to upskill hospitalists to care for people with SUD; support addiction medicine interest groups; and partner with addiction medicine societies, harm reduction organizations, and organizations focused on trauma-informed care. They could endorse practice guidelines and position statements describing the crucial role of hospitalists in addressing the overdose crisis and offering medication for addiction (Table). Hospitalist organizations can engage national and state hospital associations, lobby medical specialties to include addiction medicine competencies in board certification requirements, and advocate with governmental leaders to reduce barriers that restrict treatment access such as the X-waiver.
MOVING FORWARD
Regardless of whether a hospitalist is serving as an individual provider, a hospitalist leader, or as part of a hospitalist organization, hospitalists can take critical steps to advance the care of people with SUD. These steps shift the culture of hospitals from one where patients are afraid to discuss their substance use, to one that creates space for connection, treatment engagement, and healing. By starting medications, utilizing widely accessible resources, and collaborating with community treatment and harm reduction organizations, each one of us can play a part in addressing the epidemic.
Acknowledgments
The authors thank Alisa Patten for help preparing this manuscript. Dr. Englander would like to thank Dr. David Bangsberg and Dr. Christina Nicolaidis for their mentorship.
In 2017, the death toll from drug overdoses reached a record high, killing more Americans than the entire Vietnam War or the HIV/AIDS epidemic at its peak.1 Up to one-quarter of hospitalized patients have a substance use disorder (SUD) and SUD-related2,3 hospitalizations are surging. People with SUD have longer hospital stays, higher costs, and more readmissions.3,4 While the burden of SUD is staggering, it is far from hopeless. There are multiple evidence-based and highly effective interventions to treat SUD, including medications, behavioral interventions, and harm reduction strategies.
Hospitalization can be a reachable moment to initiate and coordinate addictions care.5 Hospital-based addictions care has the potential to engage sicker, highly vulnerable patients, many who are not engaged in primary care or outpatient addictions care.6 Studied effects of hospital-based addictions care include improved SUD treatment engagement, reduced alcohol and drug use, lower hospital readmissions, and improved provider experience.7-9
Most hospitals, however, do not treat SUD during hospitalization and do not connect people to treatment after discharge. Hospitals may lack staffing or financial resources to implement addiction care, may believe that SUDs are an outpatient concern, may want to avoid caring for people with SUD, or may simply not know where to begin. Whatever the reason, unaddressed SUD can lead to untreated withdrawal, disruptive patient behaviors, failure to complete recommended medical therapy, high rates of against medical advice discharge, poor patient experience, and widespread provider distress.8
Hospitalists—individually and collectively—are uniquely positioned to address this gap. By treating addiction effectively and compassionately, hospitalists can engage patients, improve care, improve patient and provider experience, and lower costs. This paper is a call to action that describes the current state of hospital-based addictions care, outlines key challenges to implementing SUD care in the hospital, debunks common misconceptions, and identifies actionable steps for hospitalists, hospital leaders, and hospitalist organizations.
MODELS TO DELIVER HOSPITAL-BASED ADDICTIONS CARE
Hospital-based addiction medicine consult services are emerging; they include a range of models, with variations in how patients are identified, team composition, service availability, and financing.10 Existing addiction medicine consult services commonly offer SUD assessments, psychological intervention, medical management of SUDs (eg, initiating methadone or buprenorphine), medical pain management, and linkage to SUD care after hospitalization. Some services also explicitly integrate harm reduction principles (eg, naloxone distribution, safe injection education, permitting patients to smoke).11 Additional consult service activities include hospital-wide SUD education, and creation and implementation of hospital guidance documents (eg, methadone policies).10 Some consult services utilize only physicians, while others include interprofessional providers, such as nurses, social workers, and peers with lived experience of addiction. Whereas addiction medicine physicians staff some consult services, hospitalists with less formal addiction credentials staff others.
Broadly, hospital-based addictions care cannot depend solely on consult services. Just as not all hospitals have cardiology consult services, not all hospitals will have addiction consult services. As such, hospitalists can play an even greater role by implementing order sets and guidelines, supporting partnerships with community SUD treatment, and independently initiating evidence-based medications.
CHALLENGES TO ADOPTION AND IMPLEMENTATION OF HOSPITAL-BASED ADDICTIONS CARE
Pervasive individual and structural stigmas12 are perhaps the most critical barriers to incorporating addiction medicine into routine hospital practice, and they are both cause and consequence of our system failures. Most medical schools and residencies lack SUD training, which means that the understanding of addiction as a moral deficiency or lack of willpower may remain unchallenged. Stigma surrounding SUDs contributes to hospitalists’ and hospital leaders’ aversion to treating patients with SUD, and to fears that providing quality SUD care will attract patients suffering from these conditions.
Recent national efforts have focused on the problem of opioid overprescribing. Without an equal emphasis on treatment, this focus can lead to undertreatment of pain and/or opioid use disorder in hospitalized patients, particularly since most hospitalists have little to no training in diagnosing SUD, prescribing life-saving medications for opioid use disorder, or managing acute pain in patients with SUD. The focus on overprescribing also diverts attention away from trends involving stimulants,2 fentanyl contamination of the drug supply,13 and alcohol, all of which have important implications for the care of hospitalized adults.
Hospital policies are often not grounded in evidence (eg, recommending clonidine for first-line treatment of opioid withdrawal and not buprenorphine/methadone), and there are widespread misconceptions about perceived legal barriers to treating opioid use disorder in the hospital, which is both safe and legal.10 People with SUD may be unjustly viewed through a criminal justice lens. Policies focused on controlling visitors and conducting room searches disproportionately burden people with SUD, which may create further harms through reinforcing negative provider cognitive biases about SUDs. Finally, hospitals may lack inpatient social work and pharmacy supports, and they rarely have pathways to connect people to SUD care after discharge.
Funding remains a widespread challenge. While some hospital administrators support addiction medicine services because of the pressing medical need and public health crisis, most services depend on billing or demonstrated savings through reduced hospital days or readmissions.
A CALL TO ACTION: HOW HOSPITALISTS CAN IMPROVE ADDICTION CARE
Individual hospitalists, hospitalist leaders, and hospitalist organizations can engage by improving individual practice, driving systems change, and through advocacy and policy change (Table).
Individual Hospitalists
Providing basic addiction medicine care should be a core competency for all hospitalists, just as every hospitalist can initiate a goals-of-care conversation or prescribe insulin. For opioid use disorder, hospitalists should treat withdrawal and offer treatment initiation with opioid agonist therapy (ie, methadone, buprenorphine), which reduces mortality by over half. Commonly, hospitalized patients are subjected to harmful, nonevidence-based treatments, such as mandated rapid methadone tapers,25 which can lead to undertreated withdrawal, increased pain, and opioid cravings. This increases patients’ risk for overdose after discharge and precludes them from receiving life-saving, evidence-based methadone maintenance, or buprenorphine treatment. Though widely misunderstood, prescribing methadone in the hospital is legal, and providers need no special waiver to prescribe buprenorphine during admission. Current laws require that hospitalists have a waiver to prescribe buprenorphine at discharge and prohibit hospitalists (or anyone outside of an opioid treatment program) from prescribing methadone for the treatment of opioid use disorder at discharge. Further, hospitalists should offer medication for alcohol use disorder (eg, naltrexone) and be good stewards of opioids during hospitalization, avoiding intravenous opioids where appropriate and curbing excessive prescribing at discharge. Given high rates of overdose and fentanyl contamination of stimulants, opioids, and benzodiazepines, hospitalists should prescribe naloxone at discharge to every patient with SUD, on chronic opioids, or who uses any nonmedical substances.
Resources exist for individual hospitalists seeking mentorship or additional training (Table). Though not necessary for in-hospital prescribing, hospitalists can obtain a waiver to prescribe buprenorphine at discharge (commonly called the X-waiver). To qualify, physicians must complete eight hours of accredited training (online and/or in-person), after which they must request a waiver from the Drug Enforcement Administration. Advanced-practice practitioners must complete 24 hours of training. Many have argued that policymakers should end this waiver requirement.26 While we support efforts to “X the X” and urgently expand treatment access, additional training can enrich providers’ knowledge and confidence to prescribe buprenorphine, and is a relatively simple way that all hospitalists could act. Finally, by treating addiction and modeling patient-centered addictions care, hospitalists can legitimize and destigmatize the disease of addiction,8 and have the potential to mentor and train students, residents, nurses, and other staff.27
Hospitalist Leaders
As leaders, hospitalists can play a key role in promoting hospital-based addictions care and tailoring solutions to meet local needs. Leaders can promote a cultural shift away from stigma, and promote evidence-based, life-saving care. Hospitalist leaders could require all hospitalists to obtain buprenorphine waivers. Leaders could initiate quality improvement projects related to SUD service delivery, develop policies that support inpatient SUD treatment, develop order sets for medication initiation, engage community substance use treatment partners, build pathways to timely addiction care after discharge, and champion development of addiction medicine consult services.
Hospitalist leaders can reference open-source guidelines, order sets, assessment and treatment tools, patient materials, pharmacy and therapeutics committee materials, and other resources for implementing services for hospitalized patients with SUD (Table).21,22 Hospitalist leaders who understand financial and quality drivers can also champion the business and quality case for hospital-based addictions care, and help pursue local and national funding opportunities.
Hospitalist Organizations
Hospitalist societies could provide training at regional and national conferences to upskill hospitalists to care for people with SUD; support addiction medicine interest groups; and partner with addiction medicine societies, harm reduction organizations, and organizations focused on trauma-informed care. They could endorse practice guidelines and position statements describing the crucial role of hospitalists in addressing the overdose crisis and offering medication for addiction (Table). Hospitalist organizations can engage national and state hospital associations, lobby medical specialties to include addiction medicine competencies in board certification requirements, and advocate with governmental leaders to reduce barriers that restrict treatment access such as the X-waiver.
MOVING FORWARD
Regardless of whether a hospitalist is serving as an individual provider, a hospitalist leader, or as part of a hospitalist organization, hospitalists can take critical steps to advance the care of people with SUD. These steps shift the culture of hospitals from one where patients are afraid to discuss their substance use, to one that creates space for connection, treatment engagement, and healing. By starting medications, utilizing widely accessible resources, and collaborating with community treatment and harm reduction organizations, each one of us can play a part in addressing the epidemic.
Acknowledgments
The authors thank Alisa Patten for help preparing this manuscript. Dr. Englander would like to thank Dr. David Bangsberg and Dr. Christina Nicolaidis for their mentorship.
1. Weiss A, Elixhauser A, Barrett M, Steiner C, Bailey M, O’Malley L. Opioid-related inpatient stays and emergency department visits by state, 2009-2014. Statistical Brief #219. Healthcare Cost and Utilization Project. 2016. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb219-Opioid-Hospital-Stays-ED-Visits-by-State.jsp. Accessed May 21, 2019.
2. Winkelman TA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open. 2018;1(6):e183758. https://doi.org/10.1001/jamanetworkopen.2018.3758.
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
4. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. https://doi.org/10.1097/ADM.0b013e318231de51.
5. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736.
6. Velez C, Nicolaidis C, Korthuis P, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. doi 10.1007/s11606-016-3919-4.
7. Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909-916. https://doi.org/10.1007/s11606-017-4077-z.
8. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993.
9. McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2011;10(8):CD005191 https://doi.org/10.1002/14651858.CD005191.pub3.
10. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496.
11. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001.
12. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment”: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010.
13. Ciccarone D. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int J Drug Policy. 2019. pii: S0955-3959(19)30018-0. [Epub ahead of print]. https://doi.org/10.1016/j.drugpo.2019.01.010.
14. Substance Abuse and Mental Health Services Administration. TIP 63: Medications for Opioid Use Disorder-Executive Summary. February 2018. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Executive-Summary/sma18-5063exsumm. Accessed August 8, 2019.
15. Providers Clinical Support System. Discover the rewards of treating patients with Opioid Use Disorders. https://pcssnow.org/. Accessed August 8, 2019.
16. California Bridge Program. Treatment Starts Here: Resources for the Treatment of Substance Use Disorders from the Acute Care Setting. https://www.bridgetotreatment.org/resources. Accessed August 7, 2019.
17. Clinical Consultation Center. Substance Use Resources. 2019. https://nccc.ucsf.edu/clinical-resources/substance-use-resources/. Accessed August 8, 2019.
18. Thakarar K, Weinstein ZM, Walley AY. Optimising health and safety of people who inject drugs during transition from acute to outpatient care: narrative review with clinical checklist. Postgrad Med J. 2016;92(1088):356-363. https://doi.org/10.1136/postgradmedj-2015-133720.
19. Office of National Drug Control Policy. Changing the Language of Addiction. Washington, D.C. 2017. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Memo%20-%20Changing%20Federal%20Terminology%20Regrading%20Substance%20Use%20and%20Substance%20Use%20Disorders.pdf. Accessed August 8, 2019.
20. The University of New Mexico. Project ECHO: A Revolution in Medical Education and Care Delivery. 2019. https://echo.unm.edu/. Accessed August 8, 2019.
21. Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the Improving Addiction Care Team. J Addict Med. 2019;13(2):85-89. https://doi.org/10.1097/ADM.0000000000000487.
22. Englander H, Gregg J, Gollickson J, et al. Recommendations for intergrating peer mentors in hospital-based addiction care. Subst Abus. In press. https://doi.org/10.1080/08897077.2019.1635968.
23. American College of Medical Toxicology. ACMT Position Statement: Buprenorphine Administration in the Emergency Department. https://www.acep.org/globalassets/sites/acep/media/equal-documents/policy_acmt_bupeadministration.pdf. Accessed May 21, 2019.
24. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980.
25. Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018;13(1):62-64. https://doi.org/10.12788/jhm.2861.
26. Frank JW, Wakeman SE, Gordon AJ. No end to the crisis without an end to the waiver. Subst Abus. 2018;39(3):263-265. https://doi.org/10.1080/08897077.2018.1543382.
27. Gorfinkel L, Klimas J, Reel B, et al. In-hospital training in addiction medicine: a mixed-methods study of health care provider benefits and differences. Subst Abus. 2019. In press. https://doi.org/10.1080/08897077.2018.1561596.
1. Weiss A, Elixhauser A, Barrett M, Steiner C, Bailey M, O’Malley L. Opioid-related inpatient stays and emergency department visits by state, 2009-2014. Statistical Brief #219. Healthcare Cost and Utilization Project. 2016. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb219-Opioid-Hospital-Stays-ED-Visits-by-State.jsp. Accessed May 21, 2019.
2. Winkelman TA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open. 2018;1(6):e183758. https://doi.org/10.1001/jamanetworkopen.2018.3758.
3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. https://doi.org/10.1377/hlthaff.2015.1424.
4. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. https://doi.org/10.1097/ADM.0b013e318231de51.
5. Englander H, Weimer M, Solotaroff R, et al. Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12(5):339-342. https://doi.org/10.12788/jhm.2736.
6. Velez C, Nicolaidis C, Korthuis P, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. doi 10.1007/s11606-016-3919-4.
7. Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post-discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32(8):909-916. https://doi.org/10.1007/s11606-017-4077-z.
8. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11):752-758. https://doi.org/10.12788/jhm.2993.
9. McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2011;10(8):CD005191 https://doi.org/10.1002/14651858.CD005191.pub3.
10. Priest KC, McCarty D. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J Addict Med. 2019;13(2):104-112. https://doi.org/10.1097/ADM.0000000000000496.
11. Weinstein ZM, Wakeman SE, Nolan S. Inpatient addiction consult service: expertise for hospitalized patients with complex addiction problems. Med Clin North Am. 2018;102(4):587-601. https://doi.org/10.1016/j.mcna.2018.03.001.
12. McNeil R, Small W, Wood E, Kerr T. Hospitals as a “risk environment”: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. https://doi.org/10.1016/j.socscimed.2014.01.010.
13. Ciccarone D. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int J Drug Policy. 2019. pii: S0955-3959(19)30018-0. [Epub ahead of print]. https://doi.org/10.1016/j.drugpo.2019.01.010.
14. Substance Abuse and Mental Health Services Administration. TIP 63: Medications for Opioid Use Disorder-Executive Summary. February 2018. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Executive-Summary/sma18-5063exsumm. Accessed August 8, 2019.
15. Providers Clinical Support System. Discover the rewards of treating patients with Opioid Use Disorders. https://pcssnow.org/. Accessed August 8, 2019.
16. California Bridge Program. Treatment Starts Here: Resources for the Treatment of Substance Use Disorders from the Acute Care Setting. https://www.bridgetotreatment.org/resources. Accessed August 7, 2019.
17. Clinical Consultation Center. Substance Use Resources. 2019. https://nccc.ucsf.edu/clinical-resources/substance-use-resources/. Accessed August 8, 2019.
18. Thakarar K, Weinstein ZM, Walley AY. Optimising health and safety of people who inject drugs during transition from acute to outpatient care: narrative review with clinical checklist. Postgrad Med J. 2016;92(1088):356-363. https://doi.org/10.1136/postgradmedj-2015-133720.
19. Office of National Drug Control Policy. Changing the Language of Addiction. Washington, D.C. 2017. https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Memo%20-%20Changing%20Federal%20Terminology%20Regrading%20Substance%20Use%20and%20Substance%20Use%20Disorders.pdf. Accessed August 8, 2019.
20. The University of New Mexico. Project ECHO: A Revolution in Medical Education and Care Delivery. 2019. https://echo.unm.edu/. Accessed August 8, 2019.
21. Englander H, Mahoney S, Brandt K, et al. Tools to support hospital-based addiction care: core components, values, and activities of the Improving Addiction Care Team. J Addict Med. 2019;13(2):85-89. https://doi.org/10.1097/ADM.0000000000000487.
22. Englander H, Gregg J, Gollickson J, et al. Recommendations for intergrating peer mentors in hospital-based addiction care. Subst Abus. In press. https://doi.org/10.1080/08897077.2019.1635968.
23. American College of Medical Toxicology. ACMT Position Statement: Buprenorphine Administration in the Emergency Department. https://www.acep.org/globalassets/sites/acep/media/equal-documents/policy_acmt_bupeadministration.pdf. Accessed May 21, 2019.
24. Herzig SJ, Mosher HJ, Calcaterra SL, Jena AB, Nuckols TK. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018;13(4):263-271. https://doi.org/10.12788/jhm.2980.
25. Winetsky D, Weinrieb RM, Perrone J. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018;13(1):62-64. https://doi.org/10.12788/jhm.2861.
26. Frank JW, Wakeman SE, Gordon AJ. No end to the crisis without an end to the waiver. Subst Abus. 2018;39(3):263-265. https://doi.org/10.1080/08897077.2018.1543382.
27. Gorfinkel L, Klimas J, Reel B, et al. In-hospital training in addiction medicine: a mixed-methods study of health care provider benefits and differences. Subst Abus. 2019. In press. https://doi.org/10.1080/08897077.2018.1561596.
© 2020 Society of Hospital Medicine
Turning Your Passion into Action: Becoming a Physician Advocate
I stand in the hospital room of a little girl who was shot in her own home just two weeks ago. She was drawing in her sketchbook when a group of teenagers drove by her apartment and took aim. She was shot twice in the chest. Her life and her health will forever be altered. I am not part of her care team, but I am there because just hours after their arrival to the hospital her mother declared that she was going to do something, that gun violence must be stopped. She wants to speak out and she wants to give her daughter a voice. She does not want this to happen to other little girls. My colleagues know that I can help this woman by elevating her voice, by telling her daughter’s story. I have found a passion in gun violence prevention advocacy and I fight every day for little girls like this.
For almost 10 years, I studied asthma. I presented lectures. I conducted research. I published papers. It was my thing. In fact, it still is my thing. But one day shortly after the shooting at Marjory Stoneman Douglas High School in Parkland, Florida, I was dropping my oldest daughter off at Kindergarten and for the first time, I saw an armed police officer patrolling the drop-off line. It hit me like a ton of bricks. I went home and called my Senators and Representatives. As I was talking to an aide about evidence-based gun safety legislation, I lost it. I started crying. I finished the call and just sat there. I was momentarily frozen, uncertain of what to do next yet compelled to take action. I decided to attend a meeting of a local gun violence prevention group. Maybe this action of going to one meeting would quell the anxiety and fear that was building inside of me. I found my local Moms Demand Action chapter and I went. About halfway through the meeting, the chapter leader began describing their gun safety campaign, Be SMART for kids, and mentioned that they had been trying to make connections with the Children’s Hospital. That is the moment. That is when it clicked. I have a voice that this movement needs. I can help them. And I did.
Gun violence is the second leading cause of death in children.1 Gun violence is a public health epidemic. Every day in America, approximately 100 people are shot and killed.2 The rate of firearm deaths among children and teens in the United States is 36.5 times higher than that of 12 other high-income countries.1 We know that states with stricter gun laws have lower rates of child firearm mortality.3 We also know that safe gun storage practices (storing guns locked, unloaded, and separate from ammunition) reduce the risk of suicide and firearm injuries,4 yet 4.6 million American children live in a home with a loaded, unlocked firearm.5 Promoting safe gun storage practices and advocating for common sense gun safety legislation are two effective ways to address this crisis.
Gun violence prevention is my passion, but it might not be yours. Regardless of your passion, the blueprint for becoming a physician advocate is the same.
WHY DO PHYSICIANS MAKE NATURAL, EFFECTIVE ADVOCATES?
Advocacy, in its most distilled form, is speaking out for something you believe in, often for someone who cannot speak out for themselves. This is at the core of what we, as healthcare providers, do every day. We help people through some of the hardest moments of their lives, when they are sick and vulnerable. Every day, we are faced with problems that need to be solved. Our experience at the bedside helps us understand how policies affect real people. We understand evidence, data, and science. We recognize that anecdotes are powerful but if not backed up with data will be unlikely to lead to meaningful change. Perhaps most importantly, as professional members of the community, we have agency. We can use our voice and our privilege as physicians to elevate the voices of others.
As you go through medical training, you may not even realize that what you are doing on a daily basis is advocacy. But there comes a moment when you realize that the problem is bigger than the individual patient in front of you. There are systems that are broken that, if fixed, could improve the health of patients everywhere and save lives. To create change on a population level, the status quo will need to be challenged and systems may need to be disrupted.
Hospitalists are particularly well positioned to be advocates because we interact with virtually all aspects of the healthcare system either directly or indirectly. We care for patients with a myriad of disease processes and medical needs using varying levels of resources and social support systems. We often see patients in their most dire moments and, unlike outpatient physicians, we have the luxury of time. Hospitalized patients are a captive audience. We have time to educate, assess what patients need, and connect patients with community resources.
HOW TO BECOME A PHYSICIAN ADVOCATE
Find your passion. Often, your passion will find you. When it does, listen to it. Initially, most of your advocacy will be done on your own time. If you are not passionate about your cause, you will struggle and you will be less likely to be an effective advocate. Keep in mind that sometimes the deeper you dig into an issue, the bigger problems you find and, as a result, your passion can grow.
Do your research. Read the literature. Do you really understand the issue? Identify local and national experts, read their work, and follow their careers. You do not need an advanced degree. Your experience as a physician, willingness to learn, and your voice are all you need.
Start small. Do something small every day. Read an article. Make a new contact. Talk to a colleague. Be thoughtful in your approach. Is this a problem that community advocacy can solve? Will legislation be an effective way to achieve my goal? Would state or federal legislation be more appropriate? In most cases, a combination of community advocacy and legislative advocacy is necessary.
Partner with community organizations. Find local organizations that have existing infrastructure and are engaged on the issue and create partnerships. Community organizations are fighting every day and are waiting for a powerful authoritative voice like yours. They want your voice and you need their support.
Find your allies and your challengers. Identify allies in your community, your institution, your field, and in government. Anticipate potential challengers. When you encounter them, work diligently to find common ground and be respectful. If you only talk to people who agree with you, you will not make progress. Tread carefully when necessary. Develop a thick skin. Read people and try to figure out what it is that they want, what is motivating their position. Make your first ask small and as noncontroversial as possible. Stick to the facts. If you keep your patients at the heart of what you are doing, it is hard to go wrong.
Stay focused and disciplined, but do not quiet the anger and frustration that you feel. That is your fuel. Build momentum and build your team. Passion is contagious; when people see that you are making progress, they will want to join you. Together, you can create a dialog that will change minds.
Align advocacy with your other work. Ideally, this work will not be done in isolation from your other professional duties. Advocacy initiatives make excellent quality improvement projects. When you identify holes in the evidence that could potentially inform the policy debate, apply health services research methods and publish. This approach builds the evidence base to affect change and contributes to your professional development. Consider developing an advocacy curriculum for trainees. Identify trainees interested in advocacy and mentor them. Look for opportunities to speak and write on the topic. Use your unique skillset to further your cause.
Work with your employer. Find common ground. Even if they fundamentally disagree with your point of view, you can still speak out as a private citizen. Recognize the difference between speaking as a physician and speaking as an employee of a specific institution. Unless you have explicit permission, you are speaking for yourself, not your institution. Do not be afraid to push leaders at your institution. Help them see why it is important for you to speak up on a particular issue. If your professional organization has a statement on the issue, use it to support your position.
Leverage social media. Social media is a powerful method to amplify your voice. Consider the impact of the #thisisourlane movement. It will connect you with people, across the world, who share similar passions. It will help you identify local allies. It will open opportunities for speaking engagements and publications. It can be a great way to bring positive attention to your institution. It will take time to find your voice. Try to use consistent messaging. Keep it professional. Tag people who you want to see the great work you are doing. It only takes one retweet by someone with hundreds of thousands of followers to get your message in the feed of exponentially more viewers. Tag your institution when you want them to know what you are up to or when you are doing something that you think they should be proud of. Tag the professional organizations that would be interested in your work. Tag community leaders. This can be a great way to elevate their voice with your platform. Include an “opinions my own” statement in your social media profiles. Beware of disinformation. Read articles before retweeting. Ignore the trolls. I repeat, ignore the trolls.
CONCLUSION
I did not start my career with a focus on advocacy and in becoming an advocate, I have not given up my previous focus on asthma research. I did not get an advanced degree or specialized training in advocacy. I let my passion drive me. I am now an active member and leader in our Moms Demand Action chapter. The safe storage campaign in our resident clinic has had significant success. We increased the frequency of discussion of gun safety during well-child visits from 2% to 50% and shared this success at local and national scientific meetings. We have worked with our local media to spread awareness about safe gun storage. We have spent time at the state capital to discuss child access prevention laws with legislators. We have collaborated with community leaders and elected officials for gun violence awareness events. We earned support from leaders at our institution. If you walk through our hospital units, clinics, resident areas, and faculty offices, you will see evidence of our success. Physicians and nurses are still wearing their ribbons from the Wear Orange day on their name badges. “We Can End Gun Violence” signs are hanging on faculty members’ doors. Thanks to local police departments, the clinic has a constant supply of gun locks that are provided to families free of charge. Our residents proudly walk the halls with Be SMART buttons on their badges. These physical reminders of our progress are incredibly motivating as we continue this work. However, it is the quiet moments alone with children and parents who are suffering because of the epidemic of gun violence that really move me. I will not give up this fight until children in our communities are safe.
Acknowledgments
Dr. Andrews wishes to thank Dr. Kelsey Gastineau for her efforts to increase the frequency of gun safety discussions in our Pediatric Primary Care clinic and for her support in all of this work.
1. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Engl J Med. 2018;379(25):2468-2475. https://doi.org/10.1056/NEJMsr1804754.
2. Prevention CfDCa. National centers for injury prevention and control, web-based injury statistics query and reporting system (WISQARS) Fatal Injury Reports. 2013-2017.
3. Goyal MK, Badolato GM, Patel SJ, Iqbal SF, Parikh K, McCarter R. State gun laws and pediatric firearm-related mortality. Pediatrics. 2019;144(2). https://doi.org/10.1542/peds.2018-3283
4. Grossman DC, Mueller BA , Riedy C, Dowd MD, Villaveces A, Prodzinski J, et al. Gun storage practices and risk of youth suicide and unintentional firearm injuries. JAMA. 2005;293(6):707-714. https://doi.org/10.1001/jama.293.6.707.
5. Azrael D, Cohen J, Salhi C, Miller M. Firearm storage in gun-owning households with children: results of a 2015 national survey. J Urban Health. 2018;95(3):295-304. https://doi.org/10.1007/s11524-018-0261-7
I stand in the hospital room of a little girl who was shot in her own home just two weeks ago. She was drawing in her sketchbook when a group of teenagers drove by her apartment and took aim. She was shot twice in the chest. Her life and her health will forever be altered. I am not part of her care team, but I am there because just hours after their arrival to the hospital her mother declared that she was going to do something, that gun violence must be stopped. She wants to speak out and she wants to give her daughter a voice. She does not want this to happen to other little girls. My colleagues know that I can help this woman by elevating her voice, by telling her daughter’s story. I have found a passion in gun violence prevention advocacy and I fight every day for little girls like this.
For almost 10 years, I studied asthma. I presented lectures. I conducted research. I published papers. It was my thing. In fact, it still is my thing. But one day shortly after the shooting at Marjory Stoneman Douglas High School in Parkland, Florida, I was dropping my oldest daughter off at Kindergarten and for the first time, I saw an armed police officer patrolling the drop-off line. It hit me like a ton of bricks. I went home and called my Senators and Representatives. As I was talking to an aide about evidence-based gun safety legislation, I lost it. I started crying. I finished the call and just sat there. I was momentarily frozen, uncertain of what to do next yet compelled to take action. I decided to attend a meeting of a local gun violence prevention group. Maybe this action of going to one meeting would quell the anxiety and fear that was building inside of me. I found my local Moms Demand Action chapter and I went. About halfway through the meeting, the chapter leader began describing their gun safety campaign, Be SMART for kids, and mentioned that they had been trying to make connections with the Children’s Hospital. That is the moment. That is when it clicked. I have a voice that this movement needs. I can help them. And I did.
Gun violence is the second leading cause of death in children.1 Gun violence is a public health epidemic. Every day in America, approximately 100 people are shot and killed.2 The rate of firearm deaths among children and teens in the United States is 36.5 times higher than that of 12 other high-income countries.1 We know that states with stricter gun laws have lower rates of child firearm mortality.3 We also know that safe gun storage practices (storing guns locked, unloaded, and separate from ammunition) reduce the risk of suicide and firearm injuries,4 yet 4.6 million American children live in a home with a loaded, unlocked firearm.5 Promoting safe gun storage practices and advocating for common sense gun safety legislation are two effective ways to address this crisis.
Gun violence prevention is my passion, but it might not be yours. Regardless of your passion, the blueprint for becoming a physician advocate is the same.
WHY DO PHYSICIANS MAKE NATURAL, EFFECTIVE ADVOCATES?
Advocacy, in its most distilled form, is speaking out for something you believe in, often for someone who cannot speak out for themselves. This is at the core of what we, as healthcare providers, do every day. We help people through some of the hardest moments of their lives, when they are sick and vulnerable. Every day, we are faced with problems that need to be solved. Our experience at the bedside helps us understand how policies affect real people. We understand evidence, data, and science. We recognize that anecdotes are powerful but if not backed up with data will be unlikely to lead to meaningful change. Perhaps most importantly, as professional members of the community, we have agency. We can use our voice and our privilege as physicians to elevate the voices of others.
As you go through medical training, you may not even realize that what you are doing on a daily basis is advocacy. But there comes a moment when you realize that the problem is bigger than the individual patient in front of you. There are systems that are broken that, if fixed, could improve the health of patients everywhere and save lives. To create change on a population level, the status quo will need to be challenged and systems may need to be disrupted.
Hospitalists are particularly well positioned to be advocates because we interact with virtually all aspects of the healthcare system either directly or indirectly. We care for patients with a myriad of disease processes and medical needs using varying levels of resources and social support systems. We often see patients in their most dire moments and, unlike outpatient physicians, we have the luxury of time. Hospitalized patients are a captive audience. We have time to educate, assess what patients need, and connect patients with community resources.
HOW TO BECOME A PHYSICIAN ADVOCATE
Find your passion. Often, your passion will find you. When it does, listen to it. Initially, most of your advocacy will be done on your own time. If you are not passionate about your cause, you will struggle and you will be less likely to be an effective advocate. Keep in mind that sometimes the deeper you dig into an issue, the bigger problems you find and, as a result, your passion can grow.
Do your research. Read the literature. Do you really understand the issue? Identify local and national experts, read their work, and follow their careers. You do not need an advanced degree. Your experience as a physician, willingness to learn, and your voice are all you need.
Start small. Do something small every day. Read an article. Make a new contact. Talk to a colleague. Be thoughtful in your approach. Is this a problem that community advocacy can solve? Will legislation be an effective way to achieve my goal? Would state or federal legislation be more appropriate? In most cases, a combination of community advocacy and legislative advocacy is necessary.
Partner with community organizations. Find local organizations that have existing infrastructure and are engaged on the issue and create partnerships. Community organizations are fighting every day and are waiting for a powerful authoritative voice like yours. They want your voice and you need their support.
Find your allies and your challengers. Identify allies in your community, your institution, your field, and in government. Anticipate potential challengers. When you encounter them, work diligently to find common ground and be respectful. If you only talk to people who agree with you, you will not make progress. Tread carefully when necessary. Develop a thick skin. Read people and try to figure out what it is that they want, what is motivating their position. Make your first ask small and as noncontroversial as possible. Stick to the facts. If you keep your patients at the heart of what you are doing, it is hard to go wrong.
Stay focused and disciplined, but do not quiet the anger and frustration that you feel. That is your fuel. Build momentum and build your team. Passion is contagious; when people see that you are making progress, they will want to join you. Together, you can create a dialog that will change minds.
Align advocacy with your other work. Ideally, this work will not be done in isolation from your other professional duties. Advocacy initiatives make excellent quality improvement projects. When you identify holes in the evidence that could potentially inform the policy debate, apply health services research methods and publish. This approach builds the evidence base to affect change and contributes to your professional development. Consider developing an advocacy curriculum for trainees. Identify trainees interested in advocacy and mentor them. Look for opportunities to speak and write on the topic. Use your unique skillset to further your cause.
Work with your employer. Find common ground. Even if they fundamentally disagree with your point of view, you can still speak out as a private citizen. Recognize the difference between speaking as a physician and speaking as an employee of a specific institution. Unless you have explicit permission, you are speaking for yourself, not your institution. Do not be afraid to push leaders at your institution. Help them see why it is important for you to speak up on a particular issue. If your professional organization has a statement on the issue, use it to support your position.
Leverage social media. Social media is a powerful method to amplify your voice. Consider the impact of the #thisisourlane movement. It will connect you with people, across the world, who share similar passions. It will help you identify local allies. It will open opportunities for speaking engagements and publications. It can be a great way to bring positive attention to your institution. It will take time to find your voice. Try to use consistent messaging. Keep it professional. Tag people who you want to see the great work you are doing. It only takes one retweet by someone with hundreds of thousands of followers to get your message in the feed of exponentially more viewers. Tag your institution when you want them to know what you are up to or when you are doing something that you think they should be proud of. Tag the professional organizations that would be interested in your work. Tag community leaders. This can be a great way to elevate their voice with your platform. Include an “opinions my own” statement in your social media profiles. Beware of disinformation. Read articles before retweeting. Ignore the trolls. I repeat, ignore the trolls.
CONCLUSION
I did not start my career with a focus on advocacy and in becoming an advocate, I have not given up my previous focus on asthma research. I did not get an advanced degree or specialized training in advocacy. I let my passion drive me. I am now an active member and leader in our Moms Demand Action chapter. The safe storage campaign in our resident clinic has had significant success. We increased the frequency of discussion of gun safety during well-child visits from 2% to 50% and shared this success at local and national scientific meetings. We have worked with our local media to spread awareness about safe gun storage. We have spent time at the state capital to discuss child access prevention laws with legislators. We have collaborated with community leaders and elected officials for gun violence awareness events. We earned support from leaders at our institution. If you walk through our hospital units, clinics, resident areas, and faculty offices, you will see evidence of our success. Physicians and nurses are still wearing their ribbons from the Wear Orange day on their name badges. “We Can End Gun Violence” signs are hanging on faculty members’ doors. Thanks to local police departments, the clinic has a constant supply of gun locks that are provided to families free of charge. Our residents proudly walk the halls with Be SMART buttons on their badges. These physical reminders of our progress are incredibly motivating as we continue this work. However, it is the quiet moments alone with children and parents who are suffering because of the epidemic of gun violence that really move me. I will not give up this fight until children in our communities are safe.
Acknowledgments
Dr. Andrews wishes to thank Dr. Kelsey Gastineau for her efforts to increase the frequency of gun safety discussions in our Pediatric Primary Care clinic and for her support in all of this work.
I stand in the hospital room of a little girl who was shot in her own home just two weeks ago. She was drawing in her sketchbook when a group of teenagers drove by her apartment and took aim. She was shot twice in the chest. Her life and her health will forever be altered. I am not part of her care team, but I am there because just hours after their arrival to the hospital her mother declared that she was going to do something, that gun violence must be stopped. She wants to speak out and she wants to give her daughter a voice. She does not want this to happen to other little girls. My colleagues know that I can help this woman by elevating her voice, by telling her daughter’s story. I have found a passion in gun violence prevention advocacy and I fight every day for little girls like this.
For almost 10 years, I studied asthma. I presented lectures. I conducted research. I published papers. It was my thing. In fact, it still is my thing. But one day shortly after the shooting at Marjory Stoneman Douglas High School in Parkland, Florida, I was dropping my oldest daughter off at Kindergarten and for the first time, I saw an armed police officer patrolling the drop-off line. It hit me like a ton of bricks. I went home and called my Senators and Representatives. As I was talking to an aide about evidence-based gun safety legislation, I lost it. I started crying. I finished the call and just sat there. I was momentarily frozen, uncertain of what to do next yet compelled to take action. I decided to attend a meeting of a local gun violence prevention group. Maybe this action of going to one meeting would quell the anxiety and fear that was building inside of me. I found my local Moms Demand Action chapter and I went. About halfway through the meeting, the chapter leader began describing their gun safety campaign, Be SMART for kids, and mentioned that they had been trying to make connections with the Children’s Hospital. That is the moment. That is when it clicked. I have a voice that this movement needs. I can help them. And I did.
Gun violence is the second leading cause of death in children.1 Gun violence is a public health epidemic. Every day in America, approximately 100 people are shot and killed.2 The rate of firearm deaths among children and teens in the United States is 36.5 times higher than that of 12 other high-income countries.1 We know that states with stricter gun laws have lower rates of child firearm mortality.3 We also know that safe gun storage practices (storing guns locked, unloaded, and separate from ammunition) reduce the risk of suicide and firearm injuries,4 yet 4.6 million American children live in a home with a loaded, unlocked firearm.5 Promoting safe gun storage practices and advocating for common sense gun safety legislation are two effective ways to address this crisis.
Gun violence prevention is my passion, but it might not be yours. Regardless of your passion, the blueprint for becoming a physician advocate is the same.
WHY DO PHYSICIANS MAKE NATURAL, EFFECTIVE ADVOCATES?
Advocacy, in its most distilled form, is speaking out for something you believe in, often for someone who cannot speak out for themselves. This is at the core of what we, as healthcare providers, do every day. We help people through some of the hardest moments of their lives, when they are sick and vulnerable. Every day, we are faced with problems that need to be solved. Our experience at the bedside helps us understand how policies affect real people. We understand evidence, data, and science. We recognize that anecdotes are powerful but if not backed up with data will be unlikely to lead to meaningful change. Perhaps most importantly, as professional members of the community, we have agency. We can use our voice and our privilege as physicians to elevate the voices of others.
As you go through medical training, you may not even realize that what you are doing on a daily basis is advocacy. But there comes a moment when you realize that the problem is bigger than the individual patient in front of you. There are systems that are broken that, if fixed, could improve the health of patients everywhere and save lives. To create change on a population level, the status quo will need to be challenged and systems may need to be disrupted.
Hospitalists are particularly well positioned to be advocates because we interact with virtually all aspects of the healthcare system either directly or indirectly. We care for patients with a myriad of disease processes and medical needs using varying levels of resources and social support systems. We often see patients in their most dire moments and, unlike outpatient physicians, we have the luxury of time. Hospitalized patients are a captive audience. We have time to educate, assess what patients need, and connect patients with community resources.
HOW TO BECOME A PHYSICIAN ADVOCATE
Find your passion. Often, your passion will find you. When it does, listen to it. Initially, most of your advocacy will be done on your own time. If you are not passionate about your cause, you will struggle and you will be less likely to be an effective advocate. Keep in mind that sometimes the deeper you dig into an issue, the bigger problems you find and, as a result, your passion can grow.
Do your research. Read the literature. Do you really understand the issue? Identify local and national experts, read their work, and follow their careers. You do not need an advanced degree. Your experience as a physician, willingness to learn, and your voice are all you need.
Start small. Do something small every day. Read an article. Make a new contact. Talk to a colleague. Be thoughtful in your approach. Is this a problem that community advocacy can solve? Will legislation be an effective way to achieve my goal? Would state or federal legislation be more appropriate? In most cases, a combination of community advocacy and legislative advocacy is necessary.
Partner with community organizations. Find local organizations that have existing infrastructure and are engaged on the issue and create partnerships. Community organizations are fighting every day and are waiting for a powerful authoritative voice like yours. They want your voice and you need their support.
Find your allies and your challengers. Identify allies in your community, your institution, your field, and in government. Anticipate potential challengers. When you encounter them, work diligently to find common ground and be respectful. If you only talk to people who agree with you, you will not make progress. Tread carefully when necessary. Develop a thick skin. Read people and try to figure out what it is that they want, what is motivating their position. Make your first ask small and as noncontroversial as possible. Stick to the facts. If you keep your patients at the heart of what you are doing, it is hard to go wrong.
Stay focused and disciplined, but do not quiet the anger and frustration that you feel. That is your fuel. Build momentum and build your team. Passion is contagious; when people see that you are making progress, they will want to join you. Together, you can create a dialog that will change minds.
Align advocacy with your other work. Ideally, this work will not be done in isolation from your other professional duties. Advocacy initiatives make excellent quality improvement projects. When you identify holes in the evidence that could potentially inform the policy debate, apply health services research methods and publish. This approach builds the evidence base to affect change and contributes to your professional development. Consider developing an advocacy curriculum for trainees. Identify trainees interested in advocacy and mentor them. Look for opportunities to speak and write on the topic. Use your unique skillset to further your cause.
Work with your employer. Find common ground. Even if they fundamentally disagree with your point of view, you can still speak out as a private citizen. Recognize the difference between speaking as a physician and speaking as an employee of a specific institution. Unless you have explicit permission, you are speaking for yourself, not your institution. Do not be afraid to push leaders at your institution. Help them see why it is important for you to speak up on a particular issue. If your professional organization has a statement on the issue, use it to support your position.
Leverage social media. Social media is a powerful method to amplify your voice. Consider the impact of the #thisisourlane movement. It will connect you with people, across the world, who share similar passions. It will help you identify local allies. It will open opportunities for speaking engagements and publications. It can be a great way to bring positive attention to your institution. It will take time to find your voice. Try to use consistent messaging. Keep it professional. Tag people who you want to see the great work you are doing. It only takes one retweet by someone with hundreds of thousands of followers to get your message in the feed of exponentially more viewers. Tag your institution when you want them to know what you are up to or when you are doing something that you think they should be proud of. Tag the professional organizations that would be interested in your work. Tag community leaders. This can be a great way to elevate their voice with your platform. Include an “opinions my own” statement in your social media profiles. Beware of disinformation. Read articles before retweeting. Ignore the trolls. I repeat, ignore the trolls.
CONCLUSION
I did not start my career with a focus on advocacy and in becoming an advocate, I have not given up my previous focus on asthma research. I did not get an advanced degree or specialized training in advocacy. I let my passion drive me. I am now an active member and leader in our Moms Demand Action chapter. The safe storage campaign in our resident clinic has had significant success. We increased the frequency of discussion of gun safety during well-child visits from 2% to 50% and shared this success at local and national scientific meetings. We have worked with our local media to spread awareness about safe gun storage. We have spent time at the state capital to discuss child access prevention laws with legislators. We have collaborated with community leaders and elected officials for gun violence awareness events. We earned support from leaders at our institution. If you walk through our hospital units, clinics, resident areas, and faculty offices, you will see evidence of our success. Physicians and nurses are still wearing their ribbons from the Wear Orange day on their name badges. “We Can End Gun Violence” signs are hanging on faculty members’ doors. Thanks to local police departments, the clinic has a constant supply of gun locks that are provided to families free of charge. Our residents proudly walk the halls with Be SMART buttons on their badges. These physical reminders of our progress are incredibly motivating as we continue this work. However, it is the quiet moments alone with children and parents who are suffering because of the epidemic of gun violence that really move me. I will not give up this fight until children in our communities are safe.
Acknowledgments
Dr. Andrews wishes to thank Dr. Kelsey Gastineau for her efforts to increase the frequency of gun safety discussions in our Pediatric Primary Care clinic and for her support in all of this work.
1. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Engl J Med. 2018;379(25):2468-2475. https://doi.org/10.1056/NEJMsr1804754.
2. Prevention CfDCa. National centers for injury prevention and control, web-based injury statistics query and reporting system (WISQARS) Fatal Injury Reports. 2013-2017.
3. Goyal MK, Badolato GM, Patel SJ, Iqbal SF, Parikh K, McCarter R. State gun laws and pediatric firearm-related mortality. Pediatrics. 2019;144(2). https://doi.org/10.1542/peds.2018-3283
4. Grossman DC, Mueller BA , Riedy C, Dowd MD, Villaveces A, Prodzinski J, et al. Gun storage practices and risk of youth suicide and unintentional firearm injuries. JAMA. 2005;293(6):707-714. https://doi.org/10.1001/jama.293.6.707.
5. Azrael D, Cohen J, Salhi C, Miller M. Firearm storage in gun-owning households with children: results of a 2015 national survey. J Urban Health. 2018;95(3):295-304. https://doi.org/10.1007/s11524-018-0261-7
1. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Engl J Med. 2018;379(25):2468-2475. https://doi.org/10.1056/NEJMsr1804754.
2. Prevention CfDCa. National centers for injury prevention and control, web-based injury statistics query and reporting system (WISQARS) Fatal Injury Reports. 2013-2017.
3. Goyal MK, Badolato GM, Patel SJ, Iqbal SF, Parikh K, McCarter R. State gun laws and pediatric firearm-related mortality. Pediatrics. 2019;144(2). https://doi.org/10.1542/peds.2018-3283
4. Grossman DC, Mueller BA , Riedy C, Dowd MD, Villaveces A, Prodzinski J, et al. Gun storage practices and risk of youth suicide and unintentional firearm injuries. JAMA. 2005;293(6):707-714. https://doi.org/10.1001/jama.293.6.707.
5. Azrael D, Cohen J, Salhi C, Miller M. Firearm storage in gun-owning households with children: results of a 2015 national survey. J Urban Health. 2018;95(3):295-304. https://doi.org/10.1007/s11524-018-0261-7
© 2019 Society of Hospital Medicine
Rare mixed HCV genotypes found in men who have sex with men
A low percentage of mixed genotypes of hepatitis C virus (HCV) was found in a small study of recently infected HIV+ and HIV– men who have sex with men (MSM) according to a report by Thuy Nguyen, PhD, of the University of North Carolina, Chapel Hill, and colleagues published in the International Journal of Antimicrobial Agents.
The researchers assessed 58 HCV-infected individuals with a median age of 38.5 years, 50 of whom were HIV positive and 18 of whom were HIV negative. Most of the patients were MSM (85.3%), with the rest of unknown sexual orientation. HCV genotyping by Sanger found types GT1a, GT4d, GT3a, and GT2k infection in 47.1%, 41.2%, 8.8%, and 2.9% of the individuals.
After eliminating suspected contaminations, three patients (4.4%) were found with mixed GT infections All three patients were infected with HCV for the first time; two-thirds were coinfected with HIV. The mixed GTs comprised only GT4d and GT1a at different ratios. Mixed infections are potentially problematic when using direct-acting antiviral therapy without broad-spectrum activity, according to the researchers. In this case, however, all HCV patients achieved treatment success.
“From a public health perspective, the MSM population engaging in high-risk behaviors still requires special attention in terms of mixed infections compared with the general HCV-infected population with a regular monitoring of anti-HCV treatment response, particularly when pangenotypic treatment is not used,” the researchers concluded.
The study was funded by the French government; the authors reported having no conflicts.
SOURCE: Nguyen T et al. Int J Antimicrobial Agents. 2019. 54[4]:523-7.
A low percentage of mixed genotypes of hepatitis C virus (HCV) was found in a small study of recently infected HIV+ and HIV– men who have sex with men (MSM) according to a report by Thuy Nguyen, PhD, of the University of North Carolina, Chapel Hill, and colleagues published in the International Journal of Antimicrobial Agents.
The researchers assessed 58 HCV-infected individuals with a median age of 38.5 years, 50 of whom were HIV positive and 18 of whom were HIV negative. Most of the patients were MSM (85.3%), with the rest of unknown sexual orientation. HCV genotyping by Sanger found types GT1a, GT4d, GT3a, and GT2k infection in 47.1%, 41.2%, 8.8%, and 2.9% of the individuals.
After eliminating suspected contaminations, three patients (4.4%) were found with mixed GT infections All three patients were infected with HCV for the first time; two-thirds were coinfected with HIV. The mixed GTs comprised only GT4d and GT1a at different ratios. Mixed infections are potentially problematic when using direct-acting antiviral therapy without broad-spectrum activity, according to the researchers. In this case, however, all HCV patients achieved treatment success.
“From a public health perspective, the MSM population engaging in high-risk behaviors still requires special attention in terms of mixed infections compared with the general HCV-infected population with a regular monitoring of anti-HCV treatment response, particularly when pangenotypic treatment is not used,” the researchers concluded.
The study was funded by the French government; the authors reported having no conflicts.
SOURCE: Nguyen T et al. Int J Antimicrobial Agents. 2019. 54[4]:523-7.
A low percentage of mixed genotypes of hepatitis C virus (HCV) was found in a small study of recently infected HIV+ and HIV– men who have sex with men (MSM) according to a report by Thuy Nguyen, PhD, of the University of North Carolina, Chapel Hill, and colleagues published in the International Journal of Antimicrobial Agents.
The researchers assessed 58 HCV-infected individuals with a median age of 38.5 years, 50 of whom were HIV positive and 18 of whom were HIV negative. Most of the patients were MSM (85.3%), with the rest of unknown sexual orientation. HCV genotyping by Sanger found types GT1a, GT4d, GT3a, and GT2k infection in 47.1%, 41.2%, 8.8%, and 2.9% of the individuals.
After eliminating suspected contaminations, three patients (4.4%) were found with mixed GT infections All three patients were infected with HCV for the first time; two-thirds were coinfected with HIV. The mixed GTs comprised only GT4d and GT1a at different ratios. Mixed infections are potentially problematic when using direct-acting antiviral therapy without broad-spectrum activity, according to the researchers. In this case, however, all HCV patients achieved treatment success.
“From a public health perspective, the MSM population engaging in high-risk behaviors still requires special attention in terms of mixed infections compared with the general HCV-infected population with a regular monitoring of anti-HCV treatment response, particularly when pangenotypic treatment is not used,” the researchers concluded.
The study was funded by the French government; the authors reported having no conflicts.
SOURCE: Nguyen T et al. Int J Antimicrobial Agents. 2019. 54[4]:523-7.
FROM THE INTERNATIONAL JOURNAL OF ANTIMICROBIAL AGENTS
Online resources influencing cosmetic treatment choices
Online resources are affecting most consumers’ selections of cosmetic providers, and social media are now a top-three influence on cosmetic procedure choices and skin care purchases, according to a new survey from the American Society for Dermatologic Surgery.
Almost 70% of respondents said that their use of rate and review websites had an impact on the choice of provider for cosmetic procedures: WebMD was the site most often visited, followed by Facebook, physician websites, and Yelp, the ASDS said based on its annual consumer survey.
For 43% of consumers, the decision to schedule an appointment was influenced by a provider’s social media presence, and 41% of patients said that they follow their current or potential provider on social media, the ASDS said.
“Online resources and social media platforms are clearly influencing consumers’ behavior and perception of skin health,” ASDS President Murad Alam, MD, MBA, chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern University, Chicago, said in a written statement.
Dermatologists, however, remain the leading influence on the decision to have a cosmetic procedure – named as a resource by 34% of respondents, who could select more than one possibility from a list of 15 – but social media moved ahead of primary care physicians into third place (24%), just behind friends (30%), the survey showed. Dermatologists, on the other hand, had polled at 50%-55% for the previous 5 years.
The dermatologists’ lead remained stronger as the top influencer for skin care purchases, selected by 45% of respondents, compared with 32% for friends and 28% for social media. In this category there were 14 factors from which respondents could choose. As for the cost of those skin care products, 48% of consumers spent $1-$50 a month, 31% said that they spent $51-$100 a month, and 12% reported spending $101-$150 a month, the ASDS said.
The society received 3,645 responses to the 2019 Consumer Survey on Cosmetic Dermatologic Procedures, which was conducted online from July 30 to Aug. 27 by Survata.
Online resources are affecting most consumers’ selections of cosmetic providers, and social media are now a top-three influence on cosmetic procedure choices and skin care purchases, according to a new survey from the American Society for Dermatologic Surgery.

Almost 70% of respondents said that their use of rate and review websites had an impact on the choice of provider for cosmetic procedures: WebMD was the site most often visited, followed by Facebook, physician websites, and Yelp, the ASDS said based on its annual consumer survey.
For 43% of consumers, the decision to schedule an appointment was influenced by a provider’s social media presence, and 41% of patients said that they follow their current or potential provider on social media, the ASDS said.
“Online resources and social media platforms are clearly influencing consumers’ behavior and perception of skin health,” ASDS President Murad Alam, MD, MBA, chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern University, Chicago, said in a written statement.
Dermatologists, however, remain the leading influence on the decision to have a cosmetic procedure – named as a resource by 34% of respondents, who could select more than one possibility from a list of 15 – but social media moved ahead of primary care physicians into third place (24%), just behind friends (30%), the survey showed. Dermatologists, on the other hand, had polled at 50%-55% for the previous 5 years.
The dermatologists’ lead remained stronger as the top influencer for skin care purchases, selected by 45% of respondents, compared with 32% for friends and 28% for social media. In this category there were 14 factors from which respondents could choose. As for the cost of those skin care products, 48% of consumers spent $1-$50 a month, 31% said that they spent $51-$100 a month, and 12% reported spending $101-$150 a month, the ASDS said.
The society received 3,645 responses to the 2019 Consumer Survey on Cosmetic Dermatologic Procedures, which was conducted online from July 30 to Aug. 27 by Survata.
Online resources are affecting most consumers’ selections of cosmetic providers, and social media are now a top-three influence on cosmetic procedure choices and skin care purchases, according to a new survey from the American Society for Dermatologic Surgery.

Almost 70% of respondents said that their use of rate and review websites had an impact on the choice of provider for cosmetic procedures: WebMD was the site most often visited, followed by Facebook, physician websites, and Yelp, the ASDS said based on its annual consumer survey.
For 43% of consumers, the decision to schedule an appointment was influenced by a provider’s social media presence, and 41% of patients said that they follow their current or potential provider on social media, the ASDS said.
“Online resources and social media platforms are clearly influencing consumers’ behavior and perception of skin health,” ASDS President Murad Alam, MD, MBA, chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern University, Chicago, said in a written statement.
Dermatologists, however, remain the leading influence on the decision to have a cosmetic procedure – named as a resource by 34% of respondents, who could select more than one possibility from a list of 15 – but social media moved ahead of primary care physicians into third place (24%), just behind friends (30%), the survey showed. Dermatologists, on the other hand, had polled at 50%-55% for the previous 5 years.
The dermatologists’ lead remained stronger as the top influencer for skin care purchases, selected by 45% of respondents, compared with 32% for friends and 28% for social media. In this category there were 14 factors from which respondents could choose. As for the cost of those skin care products, 48% of consumers spent $1-$50 a month, 31% said that they spent $51-$100 a month, and 12% reported spending $101-$150 a month, the ASDS said.
The society received 3,645 responses to the 2019 Consumer Survey on Cosmetic Dermatologic Procedures, which was conducted online from July 30 to Aug. 27 by Survata.
The importance of getting involved for gastroenterology
.
The evening before Advocacy Day, we discussed strategies for having a successful meeting on Capitol Hill with AGA staff (including Kathleen Teixeira, AGA vice president of government affairs, and Jonathan Sollish, AGA senior coordinator, public policy). We discussed having our “asks” supported with evidence, and “getting personal” about how these policy issues directly affect us and our patients. We also had the chance to hear from Rep. Jim McGovern (D-Mass.) and Sen. Roy Blunt (R-Mo.) , both of whom invited our questions. Both congressmen are friends of AGA, with McGovern serving as chair of the House Rules Committee, and Blunt serving as chair of the Senate Labor-HHS Subcommittee on Appropriations.
Advocacy Day began with a group breakfast during which we reviewed some of the policy issues of central importance to gastroenterology:
• Removing Barriers to the Colorectal Cancer Screening Act (HR1570/S668), which enjoys strong bipartisan support, would correct the “cost-sharing” problem of screening colonoscopies turning therapeutic (with polypectomy) for our Medicare patients, by waiving the coinsurance for screening colonoscopies – regardless of whether we remove polyps during these colonoscopies.
• Safe Step Act, HR2279, legislation introduced in the House, facilitates a common-sense and timely (72 hours or 24 hours if life-threatening) appeals process when our patients are subjected to step therapy (“fail first”) by insurers.
• Improving Seniors’ Timely Access to Care Act of 2019, HR3107, legislation in the House, eases onerous prior authorization burdens by promoting an electronic prior authorization process, ensuring requests are approved by qualified medical professionals who have specialty-specific experience, and mandating that plans report their rates of delays and denials.
• NIH research funding facilitates innovative research and supports young investigators in our field.
Full of enthusiasm, our six-strong North Carolina contingent (pictured, L-R, Ziad Gellad, MD, MPH, AGAF; David Leiman, MD, MSPH; Animesh Jain, MD; Anne Finefrock Peery, MD; Lisa Gangarosa, MD, AGAF, chair of the AGA Government Affairs Committee; and Amit Patel, MD) met with the offices of Rep. David Price (D-N.C.), and both North Carolina Senators, Richard Burr (R) and Thom Tillis (R) on Capitol Hill to convey our “asks.”
At Price’s office in the stately Rayburn House Office Building, we thanked his team for cosponsorship of H.R. 1570 and H.R. 2279. We also discussed the importance of increasing research funding by the AGA’s goal of $2.5 billion for NIH for fiscal year 2020, noting that a majority of our delegation has received NIH funding for our training and/or research activities. We also encouraged Price’s office to cosponsor H.R. 3107, sharing our personal experiences about the administrative toll of the prior authorization process for obtaining appropriate and recommended medications for our patients – in my case, swallowed topical corticosteroids for patients with eosinophilic esophagitis.
We moved on to Sen. Tillis’s office, where we thanked his office for cosponsorship of S. 668 but encouraged his office to cosponsor upcoming companion Senate legislation for H.R. 2279 and H.R. 3107. Our colleague capably conveyed how an inflammatory bowel disease (IBD) patient he saw recently may require a colectomy due to delays in appropriate treatment stemming from these regulatory processes. We also showed Tillis’s office how NIH funding generates significant economic activity in North Carolina, supporting jobs in our state.
After a quick stop at the U.S. Senate gift shop in the basement to buy souvenirs for our kids, our last meeting was with Sen. Burr’s office. There, we also thanked his office for cosponsorship of S. 668 but encouraged him to sign the “Dear Colleague” letter that Sen. Sherrod Brown, D-OH, has circulated asking CMS to address the colonoscopy cost-sharing “loophole.” We discussed the importance of cosponsoring upcoming companion Senate legislation for H.R. 2279 and H.R. 3107, sharing stories from our clinical practices about how these regulatory burdens have delayed treatment for our patients.
You can get involved, too.
AGA Advocacy Day was a tremendous experience, but it is not the only way AGA members can get involved and take action. The AGA Advocacy website, gastro.org/advocacy, provides more information on multiple avenues for advocacy. These include an online advocacy tool for sending templated letters on these issues to your elected officials.
Perhaps now more than ever, it is crucial that we get involved to support gastroenterology and advocate for our patients.
Dr. Patel is assistant professor, division of gastroenterology, Duke University, Cary, N.C.; member, AGA Clinical Guidelines Committee.
.
The evening before Advocacy Day, we discussed strategies for having a successful meeting on Capitol Hill with AGA staff (including Kathleen Teixeira, AGA vice president of government affairs, and Jonathan Sollish, AGA senior coordinator, public policy). We discussed having our “asks” supported with evidence, and “getting personal” about how these policy issues directly affect us and our patients. We also had the chance to hear from Rep. Jim McGovern (D-Mass.) and Sen. Roy Blunt (R-Mo.) , both of whom invited our questions. Both congressmen are friends of AGA, with McGovern serving as chair of the House Rules Committee, and Blunt serving as chair of the Senate Labor-HHS Subcommittee on Appropriations.
Advocacy Day began with a group breakfast during which we reviewed some of the policy issues of central importance to gastroenterology:
• Removing Barriers to the Colorectal Cancer Screening Act (HR1570/S668), which enjoys strong bipartisan support, would correct the “cost-sharing” problem of screening colonoscopies turning therapeutic (with polypectomy) for our Medicare patients, by waiving the coinsurance for screening colonoscopies – regardless of whether we remove polyps during these colonoscopies.
• Safe Step Act, HR2279, legislation introduced in the House, facilitates a common-sense and timely (72 hours or 24 hours if life-threatening) appeals process when our patients are subjected to step therapy (“fail first”) by insurers.
• Improving Seniors’ Timely Access to Care Act of 2019, HR3107, legislation in the House, eases onerous prior authorization burdens by promoting an electronic prior authorization process, ensuring requests are approved by qualified medical professionals who have specialty-specific experience, and mandating that plans report their rates of delays and denials.
• NIH research funding facilitates innovative research and supports young investigators in our field.
Full of enthusiasm, our six-strong North Carolina contingent (pictured, L-R, Ziad Gellad, MD, MPH, AGAF; David Leiman, MD, MSPH; Animesh Jain, MD; Anne Finefrock Peery, MD; Lisa Gangarosa, MD, AGAF, chair of the AGA Government Affairs Committee; and Amit Patel, MD) met with the offices of Rep. David Price (D-N.C.), and both North Carolina Senators, Richard Burr (R) and Thom Tillis (R) on Capitol Hill to convey our “asks.”
At Price’s office in the stately Rayburn House Office Building, we thanked his team for cosponsorship of H.R. 1570 and H.R. 2279. We also discussed the importance of increasing research funding by the AGA’s goal of $2.5 billion for NIH for fiscal year 2020, noting that a majority of our delegation has received NIH funding for our training and/or research activities. We also encouraged Price’s office to cosponsor H.R. 3107, sharing our personal experiences about the administrative toll of the prior authorization process for obtaining appropriate and recommended medications for our patients – in my case, swallowed topical corticosteroids for patients with eosinophilic esophagitis.
We moved on to Sen. Tillis’s office, where we thanked his office for cosponsorship of S. 668 but encouraged his office to cosponsor upcoming companion Senate legislation for H.R. 2279 and H.R. 3107. Our colleague capably conveyed how an inflammatory bowel disease (IBD) patient he saw recently may require a colectomy due to delays in appropriate treatment stemming from these regulatory processes. We also showed Tillis’s office how NIH funding generates significant economic activity in North Carolina, supporting jobs in our state.
After a quick stop at the U.S. Senate gift shop in the basement to buy souvenirs for our kids, our last meeting was with Sen. Burr’s office. There, we also thanked his office for cosponsorship of S. 668 but encouraged him to sign the “Dear Colleague” letter that Sen. Sherrod Brown, D-OH, has circulated asking CMS to address the colonoscopy cost-sharing “loophole.” We discussed the importance of cosponsoring upcoming companion Senate legislation for H.R. 2279 and H.R. 3107, sharing stories from our clinical practices about how these regulatory burdens have delayed treatment for our patients.
You can get involved, too.
AGA Advocacy Day was a tremendous experience, but it is not the only way AGA members can get involved and take action. The AGA Advocacy website, gastro.org/advocacy, provides more information on multiple avenues for advocacy. These include an online advocacy tool for sending templated letters on these issues to your elected officials.
Perhaps now more than ever, it is crucial that we get involved to support gastroenterology and advocate for our patients.
Dr. Patel is assistant professor, division of gastroenterology, Duke University, Cary, N.C.; member, AGA Clinical Guidelines Committee.
.
The evening before Advocacy Day, we discussed strategies for having a successful meeting on Capitol Hill with AGA staff (including Kathleen Teixeira, AGA vice president of government affairs, and Jonathan Sollish, AGA senior coordinator, public policy). We discussed having our “asks” supported with evidence, and “getting personal” about how these policy issues directly affect us and our patients. We also had the chance to hear from Rep. Jim McGovern (D-Mass.) and Sen. Roy Blunt (R-Mo.) , both of whom invited our questions. Both congressmen are friends of AGA, with McGovern serving as chair of the House Rules Committee, and Blunt serving as chair of the Senate Labor-HHS Subcommittee on Appropriations.
Advocacy Day began with a group breakfast during which we reviewed some of the policy issues of central importance to gastroenterology:
• Removing Barriers to the Colorectal Cancer Screening Act (HR1570/S668), which enjoys strong bipartisan support, would correct the “cost-sharing” problem of screening colonoscopies turning therapeutic (with polypectomy) for our Medicare patients, by waiving the coinsurance for screening colonoscopies – regardless of whether we remove polyps during these colonoscopies.
• Safe Step Act, HR2279, legislation introduced in the House, facilitates a common-sense and timely (72 hours or 24 hours if life-threatening) appeals process when our patients are subjected to step therapy (“fail first”) by insurers.
• Improving Seniors’ Timely Access to Care Act of 2019, HR3107, legislation in the House, eases onerous prior authorization burdens by promoting an electronic prior authorization process, ensuring requests are approved by qualified medical professionals who have specialty-specific experience, and mandating that plans report their rates of delays and denials.
• NIH research funding facilitates innovative research and supports young investigators in our field.
Full of enthusiasm, our six-strong North Carolina contingent (pictured, L-R, Ziad Gellad, MD, MPH, AGAF; David Leiman, MD, MSPH; Animesh Jain, MD; Anne Finefrock Peery, MD; Lisa Gangarosa, MD, AGAF, chair of the AGA Government Affairs Committee; and Amit Patel, MD) met with the offices of Rep. David Price (D-N.C.), and both North Carolina Senators, Richard Burr (R) and Thom Tillis (R) on Capitol Hill to convey our “asks.”
At Price’s office in the stately Rayburn House Office Building, we thanked his team for cosponsorship of H.R. 1570 and H.R. 2279. We also discussed the importance of increasing research funding by the AGA’s goal of $2.5 billion for NIH for fiscal year 2020, noting that a majority of our delegation has received NIH funding for our training and/or research activities. We also encouraged Price’s office to cosponsor H.R. 3107, sharing our personal experiences about the administrative toll of the prior authorization process for obtaining appropriate and recommended medications for our patients – in my case, swallowed topical corticosteroids for patients with eosinophilic esophagitis.
We moved on to Sen. Tillis’s office, where we thanked his office for cosponsorship of S. 668 but encouraged his office to cosponsor upcoming companion Senate legislation for H.R. 2279 and H.R. 3107. Our colleague capably conveyed how an inflammatory bowel disease (IBD) patient he saw recently may require a colectomy due to delays in appropriate treatment stemming from these regulatory processes. We also showed Tillis’s office how NIH funding generates significant economic activity in North Carolina, supporting jobs in our state.
After a quick stop at the U.S. Senate gift shop in the basement to buy souvenirs for our kids, our last meeting was with Sen. Burr’s office. There, we also thanked his office for cosponsorship of S. 668 but encouraged him to sign the “Dear Colleague” letter that Sen. Sherrod Brown, D-OH, has circulated asking CMS to address the colonoscopy cost-sharing “loophole.” We discussed the importance of cosponsoring upcoming companion Senate legislation for H.R. 2279 and H.R. 3107, sharing stories from our clinical practices about how these regulatory burdens have delayed treatment for our patients.
You can get involved, too.
AGA Advocacy Day was a tremendous experience, but it is not the only way AGA members can get involved and take action. The AGA Advocacy website, gastro.org/advocacy, provides more information on multiple avenues for advocacy. These include an online advocacy tool for sending templated letters on these issues to your elected officials.
Perhaps now more than ever, it is crucial that we get involved to support gastroenterology and advocate for our patients.
Dr. Patel is assistant professor, division of gastroenterology, Duke University, Cary, N.C.; member, AGA Clinical Guidelines Committee.
A letter from Dr. Robert S. Sandler, MPH, AGAF, Chair of the AGA Research Foundation
Dear Colleagues,
Join me in supporting talented investigators through a personal gift to the AGA Research Foundation.
As a member of the GI community, you understand the physical, emotional and financial costs of digestive diseases. And you understand the value of research to advance patient care. The gap in federal funding for research continues to grow. Many well-qualified young investigators cannot get government funding. Gifts to the AGA Research Foundation this year directly supported 52 talented investigators. Despite this success, over 200 other innovative and promising research ideas went unfunded.
That’s why I’m asking for your help.
Securing the future of the field is no small task. Every dollar is a step forward in helping to spark the scientific breakthroughs of today so clinicians will have the tools to improve care tomorrow.
Everyone benefits from GI research developed by dedicated investigators.
I invite you to help the AGA Research Foundation continue our efforts to fund and retain talented GI scientists whose research will impact the future care of patients. Donate today at www.gastro.org/donate.
Thank you for your generosity. Best wishes for a happy, healthy holiday season and successful New Year.
Dear Colleagues,
Join me in supporting talented investigators through a personal gift to the AGA Research Foundation.
As a member of the GI community, you understand the physical, emotional and financial costs of digestive diseases. And you understand the value of research to advance patient care. The gap in federal funding for research continues to grow. Many well-qualified young investigators cannot get government funding. Gifts to the AGA Research Foundation this year directly supported 52 talented investigators. Despite this success, over 200 other innovative and promising research ideas went unfunded.
That’s why I’m asking for your help.
Securing the future of the field is no small task. Every dollar is a step forward in helping to spark the scientific breakthroughs of today so clinicians will have the tools to improve care tomorrow.
Everyone benefits from GI research developed by dedicated investigators.
I invite you to help the AGA Research Foundation continue our efforts to fund and retain talented GI scientists whose research will impact the future care of patients. Donate today at www.gastro.org/donate.
Thank you for your generosity. Best wishes for a happy, healthy holiday season and successful New Year.
Dear Colleagues,
Join me in supporting talented investigators through a personal gift to the AGA Research Foundation.
As a member of the GI community, you understand the physical, emotional and financial costs of digestive diseases. And you understand the value of research to advance patient care. The gap in federal funding for research continues to grow. Many well-qualified young investigators cannot get government funding. Gifts to the AGA Research Foundation this year directly supported 52 talented investigators. Despite this success, over 200 other innovative and promising research ideas went unfunded.
That’s why I’m asking for your help.
Securing the future of the field is no small task. Every dollar is a step forward in helping to spark the scientific breakthroughs of today so clinicians will have the tools to improve care tomorrow.
Everyone benefits from GI research developed by dedicated investigators.
I invite you to help the AGA Research Foundation continue our efforts to fund and retain talented GI scientists whose research will impact the future care of patients. Donate today at www.gastro.org/donate.
Thank you for your generosity. Best wishes for a happy, healthy holiday season and successful New Year.
Top AGA Community patient cases
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Severe ulcerative colitis # IBD – A 41-year-old female patient with ulcerative colitis had a flare that didn’t improve with adalimumab and prednisone, and was admitted to the hospital with bloody stools and abdominal pain. The GI community discussed considerations for next steps and other tests to consider.
2. Unexplained diarrhea – Following the eQ&A with an AGA guideline coauthor on chronic diarrhea, this popular case follows a celiac disease patient on a gluten-free diet who continues to have significant diarrhea and fatigue.
3. Difficult ERCP – How would you handle an ERCP where the papilla is small and in a tricky location? View photos from your colleague’s scope and share your advice with the GI community.
Access these clinical cases and more discussions at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Severe ulcerative colitis # IBD – A 41-year-old female patient with ulcerative colitis had a flare that didn’t improve with adalimumab and prednisone, and was admitted to the hospital with bloody stools and abdominal pain. The GI community discussed considerations for next steps and other tests to consider.
2. Unexplained diarrhea – Following the eQ&A with an AGA guideline coauthor on chronic diarrhea, this popular case follows a celiac disease patient on a gluten-free diet who continues to have significant diarrhea and fatigue.
3. Difficult ERCP – How would you handle an ERCP where the papilla is small and in a tricky location? View photos from your colleague’s scope and share your advice with the GI community.
Access these clinical cases and more discussions at https://community.gastro.org/discussions.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. In case you missed it, here are the most popular clinical discussions shared in the forum recently:
1. Severe ulcerative colitis # IBD – A 41-year-old female patient with ulcerative colitis had a flare that didn’t improve with adalimumab and prednisone, and was admitted to the hospital with bloody stools and abdominal pain. The GI community discussed considerations for next steps and other tests to consider.
2. Unexplained diarrhea – Following the eQ&A with an AGA guideline coauthor on chronic diarrhea, this popular case follows a celiac disease patient on a gluten-free diet who continues to have significant diarrhea and fatigue.
3. Difficult ERCP – How would you handle an ERCP where the papilla is small and in a tricky location? View photos from your colleague’s scope and share your advice with the GI community.
Access these clinical cases and more discussions at https://community.gastro.org/discussions.
Vitamin C–based regimens in sepsis plausible, need more data, expert says
NEW ORLEANS – While further data are awaited on the role of vitamin C, thiamine, and steroids in sepsis, there is at least biologic plausibility for using the combination, and clinical equipoise that supports continued enrollment of patients in the ongoing randomized, controlled VICTAS trial, according to that study’s principal investigator.
“There is tremendous biologic plausibility for giving vitamin C in sepsis,” said Jon Sevransky, MD, professor of medicine at Emory University in Atlanta. But until more data are available on vitamin C–based regimens, those who choose to use vitamin C with thiamine and steroids in this setting need to ensure that glucose is being measured appropriately, he warned.
“If you decide that vitamin C is right for your patient, prior to having enough data – so if you’re doing a Hail Mary, or a ‘this patient is sick, and it’s probably not going to hurt them’ – please make sure that you measure your glucose with something that uses whole blood, which is either a blood gas or sending it down to the core lab, because otherwise, you might get an inaccurate result,” Dr. Sevransky said at the annual meeting of the American College of Chest Physicians.
Results from the randomized, placebo-controlled Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial may be available within the next few months, according to Dr. Sevransky, who noted that the trial was funded for 500 patients, which provides an 80% probability of showing an absolute risk reduction of 10% in mortality.
The primary endpoint of the phase 3 trial is vasopressor and ventilator-free days at 30 days after randomization, while 30-day mortality has been described as “the key secondary outcome” by Dr. Sevransky and colleagues in a recent report on the trial design.
Clinicians have been “captivated” by the potential benefit of vitamin C, thiamine, and hydrocortisone in patients with severe sepsis and septic shock, as published in CHEST in June 2017, Dr. Sevransky said. In that study, reported by Paul E. Marik, MD, and colleagues, hospital mortality was 8.5% for the treatment group, versus 40.4% in the control group, a significant difference.
That retrospective, single-center study had a number of limitations, however, including its before-and-after design and the use of steroids in the comparator arm. In addition, little information was available on antibiotics or fluids given at the time of the intervention, according to Dr. Sevransky.
In results of the CITRIS-ALI randomized clinical trial, just published in JAMA, intravenous administration of high-dose vitamin C in patients with sepsis and acute respiratory distress syndrome (ARDS) failed to significantly reduce organ failure scores or biomarkers of inflammation and vascular injury.
In an exploratory analysis of CITRIS-ALI, mortality at day 28 was 29.8% for the treatment group and 46.3% for placebo, with a statistically significant difference between Kaplan-Meier survival curves for the two arms, according to the investigators.
That exploratory result from CITRIS-ALI, however, is indicative of “something that needs further study,” Dr. Sevransky cautioned. “In summary, I hope I told you that biologic plausibility is present for vitamin C, thiamine, and steroids. I think that, and this is my own personal opinion, that evidence to date allows for randomization of patients, that there’s current equipoise.”
Dr. Sevransky disclosed current grant support from the Biomedical Advanced Research and Development Authority (BARDA) and the Marcus Foundation, as well as a stipend from Critical Care Medicine related to work as an associate editor. He is also a medical advisor to Project Hope and ARDS Foundation and a member of the Surviving Sepsis guideline committees.
SOURCE: Sevransky J et al. Chest 2019.
NEW ORLEANS – While further data are awaited on the role of vitamin C, thiamine, and steroids in sepsis, there is at least biologic plausibility for using the combination, and clinical equipoise that supports continued enrollment of patients in the ongoing randomized, controlled VICTAS trial, according to that study’s principal investigator.
“There is tremendous biologic plausibility for giving vitamin C in sepsis,” said Jon Sevransky, MD, professor of medicine at Emory University in Atlanta. But until more data are available on vitamin C–based regimens, those who choose to use vitamin C with thiamine and steroids in this setting need to ensure that glucose is being measured appropriately, he warned.
“If you decide that vitamin C is right for your patient, prior to having enough data – so if you’re doing a Hail Mary, or a ‘this patient is sick, and it’s probably not going to hurt them’ – please make sure that you measure your glucose with something that uses whole blood, which is either a blood gas or sending it down to the core lab, because otherwise, you might get an inaccurate result,” Dr. Sevransky said at the annual meeting of the American College of Chest Physicians.
Results from the randomized, placebo-controlled Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial may be available within the next few months, according to Dr. Sevransky, who noted that the trial was funded for 500 patients, which provides an 80% probability of showing an absolute risk reduction of 10% in mortality.
The primary endpoint of the phase 3 trial is vasopressor and ventilator-free days at 30 days after randomization, while 30-day mortality has been described as “the key secondary outcome” by Dr. Sevransky and colleagues in a recent report on the trial design.
Clinicians have been “captivated” by the potential benefit of vitamin C, thiamine, and hydrocortisone in patients with severe sepsis and septic shock, as published in CHEST in June 2017, Dr. Sevransky said. In that study, reported by Paul E. Marik, MD, and colleagues, hospital mortality was 8.5% for the treatment group, versus 40.4% in the control group, a significant difference.
That retrospective, single-center study had a number of limitations, however, including its before-and-after design and the use of steroids in the comparator arm. In addition, little information was available on antibiotics or fluids given at the time of the intervention, according to Dr. Sevransky.
In results of the CITRIS-ALI randomized clinical trial, just published in JAMA, intravenous administration of high-dose vitamin C in patients with sepsis and acute respiratory distress syndrome (ARDS) failed to significantly reduce organ failure scores or biomarkers of inflammation and vascular injury.
In an exploratory analysis of CITRIS-ALI, mortality at day 28 was 29.8% for the treatment group and 46.3% for placebo, with a statistically significant difference between Kaplan-Meier survival curves for the two arms, according to the investigators.
That exploratory result from CITRIS-ALI, however, is indicative of “something that needs further study,” Dr. Sevransky cautioned. “In summary, I hope I told you that biologic plausibility is present for vitamin C, thiamine, and steroids. I think that, and this is my own personal opinion, that evidence to date allows for randomization of patients, that there’s current equipoise.”
Dr. Sevransky disclosed current grant support from the Biomedical Advanced Research and Development Authority (BARDA) and the Marcus Foundation, as well as a stipend from Critical Care Medicine related to work as an associate editor. He is also a medical advisor to Project Hope and ARDS Foundation and a member of the Surviving Sepsis guideline committees.
SOURCE: Sevransky J et al. Chest 2019.
NEW ORLEANS – While further data are awaited on the role of vitamin C, thiamine, and steroids in sepsis, there is at least biologic plausibility for using the combination, and clinical equipoise that supports continued enrollment of patients in the ongoing randomized, controlled VICTAS trial, according to that study’s principal investigator.
“There is tremendous biologic plausibility for giving vitamin C in sepsis,” said Jon Sevransky, MD, professor of medicine at Emory University in Atlanta. But until more data are available on vitamin C–based regimens, those who choose to use vitamin C with thiamine and steroids in this setting need to ensure that glucose is being measured appropriately, he warned.
“If you decide that vitamin C is right for your patient, prior to having enough data – so if you’re doing a Hail Mary, or a ‘this patient is sick, and it’s probably not going to hurt them’ – please make sure that you measure your glucose with something that uses whole blood, which is either a blood gas or sending it down to the core lab, because otherwise, you might get an inaccurate result,” Dr. Sevransky said at the annual meeting of the American College of Chest Physicians.
Results from the randomized, placebo-controlled Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial may be available within the next few months, according to Dr. Sevransky, who noted that the trial was funded for 500 patients, which provides an 80% probability of showing an absolute risk reduction of 10% in mortality.
The primary endpoint of the phase 3 trial is vasopressor and ventilator-free days at 30 days after randomization, while 30-day mortality has been described as “the key secondary outcome” by Dr. Sevransky and colleagues in a recent report on the trial design.
Clinicians have been “captivated” by the potential benefit of vitamin C, thiamine, and hydrocortisone in patients with severe sepsis and septic shock, as published in CHEST in June 2017, Dr. Sevransky said. In that study, reported by Paul E. Marik, MD, and colleagues, hospital mortality was 8.5% for the treatment group, versus 40.4% in the control group, a significant difference.
That retrospective, single-center study had a number of limitations, however, including its before-and-after design and the use of steroids in the comparator arm. In addition, little information was available on antibiotics or fluids given at the time of the intervention, according to Dr. Sevransky.
In results of the CITRIS-ALI randomized clinical trial, just published in JAMA, intravenous administration of high-dose vitamin C in patients with sepsis and acute respiratory distress syndrome (ARDS) failed to significantly reduce organ failure scores or biomarkers of inflammation and vascular injury.
In an exploratory analysis of CITRIS-ALI, mortality at day 28 was 29.8% for the treatment group and 46.3% for placebo, with a statistically significant difference between Kaplan-Meier survival curves for the two arms, according to the investigators.
That exploratory result from CITRIS-ALI, however, is indicative of “something that needs further study,” Dr. Sevransky cautioned. “In summary, I hope I told you that biologic plausibility is present for vitamin C, thiamine, and steroids. I think that, and this is my own personal opinion, that evidence to date allows for randomization of patients, that there’s current equipoise.”
Dr. Sevransky disclosed current grant support from the Biomedical Advanced Research and Development Authority (BARDA) and the Marcus Foundation, as well as a stipend from Critical Care Medicine related to work as an associate editor. He is also a medical advisor to Project Hope and ARDS Foundation and a member of the Surviving Sepsis guideline committees.
SOURCE: Sevransky J et al. Chest 2019.
EXPERT ANALYSIS FROM CHEST 2019
FDA transition to disposable component duodenoscopes — talking points for your patients
The U.S. Food and Drug Administration (FDA) recently released a safety communication recommending duodenoscope manufacturers and health care facilities move away from using duodenoscopes with fixed endcaps to those with disposable components that include disposable endcaps – or to fully disposable duodenoscopes when they become available. This announcement may have already produced some questions among your patients when it comes to their procedures that use a duodenoscope, such as endoscopic retrograde cholangiopancreatography (ERCP).
Talking points:
• Duodenoscopes are an important tool used during an ERCP to help localize and treat abnormal issues in your bile duct system and pancreas, and possibly help you avoid surgery.
• The complex design of duodenoscopes can sometimes result in bacteria remaining in a small portion of the duodenoscope (the “elevator channel”) even after careful cleaning according to approved instructions. However, the chance of getting an identified “superbug infection” with a duodenoscope is very low, currently estimated at 1 per 20,000 ERCPs performed in more than 650,000 ERCP procedures each year in the U.S. FDA continues to work with duodenoscope manufacturers to provide strict guidelines for cleaning and disinfection of these tools.
• The switch to new duodenoscopes with disposable components will be slow and orderly to make sure that there are enough duodenoscopes to perform ERCPs so that patients who need this often life-saving procedure will still have access.
• Do not cancel or delay any planned procedure without first discussing the benefits and risks with me or another health care provider, as delaying the procedure and alternatives like surgery or radiologic intervention may be riskier than a timely ERCP.
• The esophagogastroduodenoscopy (EGD) procedure does not use the same tool that is used for ERCP. EGD uses a different endoscope than ERCP and has not been shown to have the same risk of infection because there is no “elevator channel.”
The U.S. Food and Drug Administration (FDA) recently released a safety communication recommending duodenoscope manufacturers and health care facilities move away from using duodenoscopes with fixed endcaps to those with disposable components that include disposable endcaps – or to fully disposable duodenoscopes when they become available. This announcement may have already produced some questions among your patients when it comes to their procedures that use a duodenoscope, such as endoscopic retrograde cholangiopancreatography (ERCP).
Talking points:
• Duodenoscopes are an important tool used during an ERCP to help localize and treat abnormal issues in your bile duct system and pancreas, and possibly help you avoid surgery.
• The complex design of duodenoscopes can sometimes result in bacteria remaining in a small portion of the duodenoscope (the “elevator channel”) even after careful cleaning according to approved instructions. However, the chance of getting an identified “superbug infection” with a duodenoscope is very low, currently estimated at 1 per 20,000 ERCPs performed in more than 650,000 ERCP procedures each year in the U.S. FDA continues to work with duodenoscope manufacturers to provide strict guidelines for cleaning and disinfection of these tools.
• The switch to new duodenoscopes with disposable components will be slow and orderly to make sure that there are enough duodenoscopes to perform ERCPs so that patients who need this often life-saving procedure will still have access.
• Do not cancel or delay any planned procedure without first discussing the benefits and risks with me or another health care provider, as delaying the procedure and alternatives like surgery or radiologic intervention may be riskier than a timely ERCP.
• The esophagogastroduodenoscopy (EGD) procedure does not use the same tool that is used for ERCP. EGD uses a different endoscope than ERCP and has not been shown to have the same risk of infection because there is no “elevator channel.”
The U.S. Food and Drug Administration (FDA) recently released a safety communication recommending duodenoscope manufacturers and health care facilities move away from using duodenoscopes with fixed endcaps to those with disposable components that include disposable endcaps – or to fully disposable duodenoscopes when they become available. This announcement may have already produced some questions among your patients when it comes to their procedures that use a duodenoscope, such as endoscopic retrograde cholangiopancreatography (ERCP).
Talking points:
• Duodenoscopes are an important tool used during an ERCP to help localize and treat abnormal issues in your bile duct system and pancreas, and possibly help you avoid surgery.
• The complex design of duodenoscopes can sometimes result in bacteria remaining in a small portion of the duodenoscope (the “elevator channel”) even after careful cleaning according to approved instructions. However, the chance of getting an identified “superbug infection” with a duodenoscope is very low, currently estimated at 1 per 20,000 ERCPs performed in more than 650,000 ERCP procedures each year in the U.S. FDA continues to work with duodenoscope manufacturers to provide strict guidelines for cleaning and disinfection of these tools.
• The switch to new duodenoscopes with disposable components will be slow and orderly to make sure that there are enough duodenoscopes to perform ERCPs so that patients who need this often life-saving procedure will still have access.
• Do not cancel or delay any planned procedure without first discussing the benefits and risks with me or another health care provider, as delaying the procedure and alternatives like surgery or radiologic intervention may be riskier than a timely ERCP.
• The esophagogastroduodenoscopy (EGD) procedure does not use the same tool that is used for ERCP. EGD uses a different endoscope than ERCP and has not been shown to have the same risk of infection because there is no “elevator channel.”
Talking to your patients about ranitidine
The FDA recently released several safety alerts on ranitidine formulations, including the brand-name drug Zantac, which were found to contain a nitrosamine impurity called N-nitrosodimethylamine (NDMA) at low levels. NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests and animal studies. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables. This contaminant is similar to what was recently found in losartan, an angiotensin II receptor blocker used to treat hypertension, that was recalled by the FDA.
The FDA is continuing to test ranitidine products from multiple manufacturers and is assessing the potential effect on patients who have been taking ranitidine.
With the voluntary recall of 14 lots of prescription ranitidine capsules distributed by Sandoz Inc., as well as the voluntary recall of over-the-counter (OTC) ranitidine tablets (75 mg and 150 mg), labeled by Walgreens, Walmart, and Rite-Aid and manufactured by Apotex Corp, your patients might be asking a lot of questions about whether to continue to using their medicines and what alternatives are available.
Talking to your patients
The FDA safety alerts have been covered by various media outlets since early September. This may cause your patients to question whether they should stay on or start using ranitidine products. When discussing the recall with your patients, let them know that:
• Ranitidine is an H2 blocker (antihistamine) – available OTC and in prescription strength – used to prevent and relieve heartburn associated with acid indigestion and sour stomach. It reduces stomach acid and works longer but not as quickly as antacids.
• Not all ranitidine medicines marketed in the United States are being recalled and the FDA is not recommending individuals stop taking all ranitidine medicines at this time.
• It might be prudent to hold off taking Zantac until a final FDA conclusion is released.
• Multiple drugs are approved for the same or similar uses as ranitidine. Other treatment options are available, both prescription and OTC, for patients who are concerned about ranitidine.
• Life-style modifications may reduce or eliminate the need for heartburn drugs for long-term use. These may include weight loss, avoiding tobacco, or a change in eating patterns. Share AGA’s patient education content on gastroesophageal reflux disease (GERD) for more tips for your patients.
The FDA recently released several safety alerts on ranitidine formulations, including the brand-name drug Zantac, which were found to contain a nitrosamine impurity called N-nitrosodimethylamine (NDMA) at low levels. NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests and animal studies. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables. This contaminant is similar to what was recently found in losartan, an angiotensin II receptor blocker used to treat hypertension, that was recalled by the FDA.
The FDA is continuing to test ranitidine products from multiple manufacturers and is assessing the potential effect on patients who have been taking ranitidine.
With the voluntary recall of 14 lots of prescription ranitidine capsules distributed by Sandoz Inc., as well as the voluntary recall of over-the-counter (OTC) ranitidine tablets (75 mg and 150 mg), labeled by Walgreens, Walmart, and Rite-Aid and manufactured by Apotex Corp, your patients might be asking a lot of questions about whether to continue to using their medicines and what alternatives are available.
Talking to your patients
The FDA safety alerts have been covered by various media outlets since early September. This may cause your patients to question whether they should stay on or start using ranitidine products. When discussing the recall with your patients, let them know that:
• Ranitidine is an H2 blocker (antihistamine) – available OTC and in prescription strength – used to prevent and relieve heartburn associated with acid indigestion and sour stomach. It reduces stomach acid and works longer but not as quickly as antacids.
• Not all ranitidine medicines marketed in the United States are being recalled and the FDA is not recommending individuals stop taking all ranitidine medicines at this time.
• It might be prudent to hold off taking Zantac until a final FDA conclusion is released.
• Multiple drugs are approved for the same or similar uses as ranitidine. Other treatment options are available, both prescription and OTC, for patients who are concerned about ranitidine.
• Life-style modifications may reduce or eliminate the need for heartburn drugs for long-term use. These may include weight loss, avoiding tobacco, or a change in eating patterns. Share AGA’s patient education content on gastroesophageal reflux disease (GERD) for more tips for your patients.
The FDA recently released several safety alerts on ranitidine formulations, including the brand-name drug Zantac, which were found to contain a nitrosamine impurity called N-nitrosodimethylamine (NDMA) at low levels. NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests and animal studies. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables. This contaminant is similar to what was recently found in losartan, an angiotensin II receptor blocker used to treat hypertension, that was recalled by the FDA.
The FDA is continuing to test ranitidine products from multiple manufacturers and is assessing the potential effect on patients who have been taking ranitidine.
With the voluntary recall of 14 lots of prescription ranitidine capsules distributed by Sandoz Inc., as well as the voluntary recall of over-the-counter (OTC) ranitidine tablets (75 mg and 150 mg), labeled by Walgreens, Walmart, and Rite-Aid and manufactured by Apotex Corp, your patients might be asking a lot of questions about whether to continue to using their medicines and what alternatives are available.
Talking to your patients
The FDA safety alerts have been covered by various media outlets since early September. This may cause your patients to question whether they should stay on or start using ranitidine products. When discussing the recall with your patients, let them know that:
• Ranitidine is an H2 blocker (antihistamine) – available OTC and in prescription strength – used to prevent and relieve heartburn associated with acid indigestion and sour stomach. It reduces stomach acid and works longer but not as quickly as antacids.
• Not all ranitidine medicines marketed in the United States are being recalled and the FDA is not recommending individuals stop taking all ranitidine medicines at this time.
• It might be prudent to hold off taking Zantac until a final FDA conclusion is released.
• Multiple drugs are approved for the same or similar uses as ranitidine. Other treatment options are available, both prescription and OTC, for patients who are concerned about ranitidine.
• Life-style modifications may reduce or eliminate the need for heartburn drugs for long-term use. These may include weight loss, avoiding tobacco, or a change in eating patterns. Share AGA’s patient education content on gastroesophageal reflux disease (GERD) for more tips for your patients.