User login
Liver abnormalities, disease common in patients with psoriatic arthritis
Liver abnormalities in patients with psoriatic arthritis are common and are associated with higher body mass index, more severe disease, and certain therapies, new research suggests.
Patients with psoriatic arthritis (PsA) often have comorbidities such as cardiovascular disease, metabolic syndrome, inflammatory bowel disease, osteoporosis, malignancy, and ophthalmic disease, and liver disease is no exception, wrote Rattapol Pakchotanon, MD, of the department of internal medicine at Phramongkutlao Hospital and College of Medicine, Bangkok, and associates. Their report is in the Journal of Rheumatology.
In psoriasis patients, the prevalence of liver abnormalities has been 24%-36% in previous research, but research regarding liver disease in PsA has been limited.
Of 1,061 patients from the University of Toronto Psoriatic Arthritis Clinic who were included in the study, 343 (32%) had liver abnormalities, including 256 who developed a liver abnormality or disease after their first evaluation at the clinic. Liver abnormality was defined as having aspartate transaminase, alanine transaminase, or alkaline phosphatase levels 1.5 times the upper limit of normal or greater, and liver diseases included drug-induced liver injury, fatty liver, viral hepatitis, autoimmune liver disease, alcoholic liver disease, liver fibrosis, and cirrhosis.
Among the patients with PsA who developed liver abnormalities or disease after their first visit, liver abnormalities occurred after an average of 8.3 years of follow-up and at a mean age of 50.5 years. The average BMI in this group was 29.7 kg/m2, and 11% of patients consumed alcohol daily. A total of 105 patients had recurrent liver abnormalities, and the rest had only one visit with an abnormality; those with transient abnormalities were significantly less likely to have evidence of liver disease (P less than .001).
The most common cause of liver disease was drug-induced hepatitis (14%) and fatty liver (13%). Alcohol-induced hepatitis occurred in 10 patients, and cirrhosis was reported in 2 patients.
In a multivariable analysis, factors found to be independently associated with liver abnormalities in PsA included BMI (odds ratio, 1.07; 95% confidence interval, 1.02-1.12; P = .007), daily alcohol intake (OR, 4.46; 95% CI, 1.30-15.28; P = .02), damaged joint count (OR, 1.04; 95% CI, 1.01-1.08; P = .01), elevated C-reactive protein (OR, 2.00; 95% CI, 1.04-3.85; P = .04), use of methotrexate or leflunomide (OR, 4.39; 95% CI, 1.67-11.54; P = .003), and use of tumor necrosis factor inhibitors (OR, 10.56; 95% CI, 3.63-30.69; P less than .0001).
“We recommend monitoring liver function tests in these high risk PsA patients,” the researchers concluded. “This is important in the management of patients with PsA as many of the therapeutic options may aggravate or even lead to liver abnormalities in this patient population.”
The study was funded in part by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. The investigators reported that they had no conflicts of interest. Dr. Pakchotanon conducted the research while he was at the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital.
SOURCE: Gladman DD et al. J Rheumatol. 2019 Oct 15. doi: 10.3899/jrheum.181312
Liver abnormalities in patients with psoriatic arthritis are common and are associated with higher body mass index, more severe disease, and certain therapies, new research suggests.
Patients with psoriatic arthritis (PsA) often have comorbidities such as cardiovascular disease, metabolic syndrome, inflammatory bowel disease, osteoporosis, malignancy, and ophthalmic disease, and liver disease is no exception, wrote Rattapol Pakchotanon, MD, of the department of internal medicine at Phramongkutlao Hospital and College of Medicine, Bangkok, and associates. Their report is in the Journal of Rheumatology.
In psoriasis patients, the prevalence of liver abnormalities has been 24%-36% in previous research, but research regarding liver disease in PsA has been limited.
Of 1,061 patients from the University of Toronto Psoriatic Arthritis Clinic who were included in the study, 343 (32%) had liver abnormalities, including 256 who developed a liver abnormality or disease after their first evaluation at the clinic. Liver abnormality was defined as having aspartate transaminase, alanine transaminase, or alkaline phosphatase levels 1.5 times the upper limit of normal or greater, and liver diseases included drug-induced liver injury, fatty liver, viral hepatitis, autoimmune liver disease, alcoholic liver disease, liver fibrosis, and cirrhosis.
Among the patients with PsA who developed liver abnormalities or disease after their first visit, liver abnormalities occurred after an average of 8.3 years of follow-up and at a mean age of 50.5 years. The average BMI in this group was 29.7 kg/m2, and 11% of patients consumed alcohol daily. A total of 105 patients had recurrent liver abnormalities, and the rest had only one visit with an abnormality; those with transient abnormalities were significantly less likely to have evidence of liver disease (P less than .001).
The most common cause of liver disease was drug-induced hepatitis (14%) and fatty liver (13%). Alcohol-induced hepatitis occurred in 10 patients, and cirrhosis was reported in 2 patients.
In a multivariable analysis, factors found to be independently associated with liver abnormalities in PsA included BMI (odds ratio, 1.07; 95% confidence interval, 1.02-1.12; P = .007), daily alcohol intake (OR, 4.46; 95% CI, 1.30-15.28; P = .02), damaged joint count (OR, 1.04; 95% CI, 1.01-1.08; P = .01), elevated C-reactive protein (OR, 2.00; 95% CI, 1.04-3.85; P = .04), use of methotrexate or leflunomide (OR, 4.39; 95% CI, 1.67-11.54; P = .003), and use of tumor necrosis factor inhibitors (OR, 10.56; 95% CI, 3.63-30.69; P less than .0001).
“We recommend monitoring liver function tests in these high risk PsA patients,” the researchers concluded. “This is important in the management of patients with PsA as many of the therapeutic options may aggravate or even lead to liver abnormalities in this patient population.”
The study was funded in part by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. The investigators reported that they had no conflicts of interest. Dr. Pakchotanon conducted the research while he was at the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital.
SOURCE: Gladman DD et al. J Rheumatol. 2019 Oct 15. doi: 10.3899/jrheum.181312
Liver abnormalities in patients with psoriatic arthritis are common and are associated with higher body mass index, more severe disease, and certain therapies, new research suggests.
Patients with psoriatic arthritis (PsA) often have comorbidities such as cardiovascular disease, metabolic syndrome, inflammatory bowel disease, osteoporosis, malignancy, and ophthalmic disease, and liver disease is no exception, wrote Rattapol Pakchotanon, MD, of the department of internal medicine at Phramongkutlao Hospital and College of Medicine, Bangkok, and associates. Their report is in the Journal of Rheumatology.
In psoriasis patients, the prevalence of liver abnormalities has been 24%-36% in previous research, but research regarding liver disease in PsA has been limited.
Of 1,061 patients from the University of Toronto Psoriatic Arthritis Clinic who were included in the study, 343 (32%) had liver abnormalities, including 256 who developed a liver abnormality or disease after their first evaluation at the clinic. Liver abnormality was defined as having aspartate transaminase, alanine transaminase, or alkaline phosphatase levels 1.5 times the upper limit of normal or greater, and liver diseases included drug-induced liver injury, fatty liver, viral hepatitis, autoimmune liver disease, alcoholic liver disease, liver fibrosis, and cirrhosis.
Among the patients with PsA who developed liver abnormalities or disease after their first visit, liver abnormalities occurred after an average of 8.3 years of follow-up and at a mean age of 50.5 years. The average BMI in this group was 29.7 kg/m2, and 11% of patients consumed alcohol daily. A total of 105 patients had recurrent liver abnormalities, and the rest had only one visit with an abnormality; those with transient abnormalities were significantly less likely to have evidence of liver disease (P less than .001).
The most common cause of liver disease was drug-induced hepatitis (14%) and fatty liver (13%). Alcohol-induced hepatitis occurred in 10 patients, and cirrhosis was reported in 2 patients.
In a multivariable analysis, factors found to be independently associated with liver abnormalities in PsA included BMI (odds ratio, 1.07; 95% confidence interval, 1.02-1.12; P = .007), daily alcohol intake (OR, 4.46; 95% CI, 1.30-15.28; P = .02), damaged joint count (OR, 1.04; 95% CI, 1.01-1.08; P = .01), elevated C-reactive protein (OR, 2.00; 95% CI, 1.04-3.85; P = .04), use of methotrexate or leflunomide (OR, 4.39; 95% CI, 1.67-11.54; P = .003), and use of tumor necrosis factor inhibitors (OR, 10.56; 95% CI, 3.63-30.69; P less than .0001).
“We recommend monitoring liver function tests in these high risk PsA patients,” the researchers concluded. “This is important in the management of patients with PsA as many of the therapeutic options may aggravate or even lead to liver abnormalities in this patient population.”
The study was funded in part by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. The investigators reported that they had no conflicts of interest. Dr. Pakchotanon conducted the research while he was at the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital.
SOURCE: Gladman DD et al. J Rheumatol. 2019 Oct 15. doi: 10.3899/jrheum.181312
FROM THE JOURNAL OF RHEUMATOLOGY
ACIP plans flu review for older adults
according to data presented at a meeting of the Centers for Disease Control and Prevention’s ACIP.
Lynette Brammer of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD) presented a surveillance update of the flu season in the United States so far. Overall, the influenza A(H3N2) viruses are predominant, although dominance varies in different regions of the country, and it is too soon to predict what strain will dominate later in the season.
“While two of the four vaccine components were updated for the Southern Hemisphere, the components selected for the 2019-2020 Northern Hemisphere vaccine, at this time, look appropriate for the season,” she said.
In other flu news, Lisa Groskopf, MD, of the NCIRD discussed the influenza work group’s plans for a meta-analysis to assess the relative benefit of different vaccines for older adults, in light of the growing variety of products available.
Currently, no preferential recommendations have been made for a specific vaccine for a particular age group. “There’s a dearth of data comparing these vaccines to one another,” said Dr. Groskopf. She added that, because vaccine effectiveness varies by season, the generalizability of effectiveness data is another challenge.
The work group’s systematic review and meta-analysis is designed to compare the high-dose inactivated influenza vaccine (HD-IIV), the adjuvanted inactivated influenza vaccine (aIIV), and the recombinant influenza vaccine (RIV). The study will include adults aged 65 years and older who receive trivalent or quadrivalent HD-IIV, aIIV, or RIV, compared with those who receive another influenza vaccine, a noninfluenza control vaccine, placebo, or no vaccine. The outcomes will include data on safety and effectiveness of the vaccines, Dr. Groskopf said.
In addition to safety and effectiveness, manufacturers such as Sanofi Pasteur continue to collect data on the success of available vaccines and develop new ones. Lee-Jah Chang, MD, of Sanofi Pasteur presented results of a noninferiority study of the company’s investigational high-dose quadrivalent influenza vaccine (QIV-HD; including two prevailing B viruses) versus the high-dose trivalent influenza vaccine (TID-HD). The study was conducted at 35 sites in the United States and included 2,670 adults aged 65 years and older.
Overall, the reactogenicity profile for patients given QIV-HD was similar to that of TID-HD, and approximately 5% of patients in the QIV group reported an immediate adverse event, Dr. Chang said. However, no related deaths or related adverse events of special interest occurred in any of the study groups.
Sanofi plans to pursue licensure of the QIV-HD vaccine, with a Center for Biologics Evaluation and Research action date of Nov. 4, 2019, said Dr. Chang. If the vaccine is licensed, it should be available for purchase by health care providers in the first quarter of 2020.
The ACIP members had no financial conflicts to disclose.
according to data presented at a meeting of the Centers for Disease Control and Prevention’s ACIP.
Lynette Brammer of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD) presented a surveillance update of the flu season in the United States so far. Overall, the influenza A(H3N2) viruses are predominant, although dominance varies in different regions of the country, and it is too soon to predict what strain will dominate later in the season.
“While two of the four vaccine components were updated for the Southern Hemisphere, the components selected for the 2019-2020 Northern Hemisphere vaccine, at this time, look appropriate for the season,” she said.
In other flu news, Lisa Groskopf, MD, of the NCIRD discussed the influenza work group’s plans for a meta-analysis to assess the relative benefit of different vaccines for older adults, in light of the growing variety of products available.
Currently, no preferential recommendations have been made for a specific vaccine for a particular age group. “There’s a dearth of data comparing these vaccines to one another,” said Dr. Groskopf. She added that, because vaccine effectiveness varies by season, the generalizability of effectiveness data is another challenge.
The work group’s systematic review and meta-analysis is designed to compare the high-dose inactivated influenza vaccine (HD-IIV), the adjuvanted inactivated influenza vaccine (aIIV), and the recombinant influenza vaccine (RIV). The study will include adults aged 65 years and older who receive trivalent or quadrivalent HD-IIV, aIIV, or RIV, compared with those who receive another influenza vaccine, a noninfluenza control vaccine, placebo, or no vaccine. The outcomes will include data on safety and effectiveness of the vaccines, Dr. Groskopf said.
In addition to safety and effectiveness, manufacturers such as Sanofi Pasteur continue to collect data on the success of available vaccines and develop new ones. Lee-Jah Chang, MD, of Sanofi Pasteur presented results of a noninferiority study of the company’s investigational high-dose quadrivalent influenza vaccine (QIV-HD; including two prevailing B viruses) versus the high-dose trivalent influenza vaccine (TID-HD). The study was conducted at 35 sites in the United States and included 2,670 adults aged 65 years and older.
Overall, the reactogenicity profile for patients given QIV-HD was similar to that of TID-HD, and approximately 5% of patients in the QIV group reported an immediate adverse event, Dr. Chang said. However, no related deaths or related adverse events of special interest occurred in any of the study groups.
Sanofi plans to pursue licensure of the QIV-HD vaccine, with a Center for Biologics Evaluation and Research action date of Nov. 4, 2019, said Dr. Chang. If the vaccine is licensed, it should be available for purchase by health care providers in the first quarter of 2020.
The ACIP members had no financial conflicts to disclose.
according to data presented at a meeting of the Centers for Disease Control and Prevention’s ACIP.
Lynette Brammer of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD) presented a surveillance update of the flu season in the United States so far. Overall, the influenza A(H3N2) viruses are predominant, although dominance varies in different regions of the country, and it is too soon to predict what strain will dominate later in the season.
“While two of the four vaccine components were updated for the Southern Hemisphere, the components selected for the 2019-2020 Northern Hemisphere vaccine, at this time, look appropriate for the season,” she said.
In other flu news, Lisa Groskopf, MD, of the NCIRD discussed the influenza work group’s plans for a meta-analysis to assess the relative benefit of different vaccines for older adults, in light of the growing variety of products available.
Currently, no preferential recommendations have been made for a specific vaccine for a particular age group. “There’s a dearth of data comparing these vaccines to one another,” said Dr. Groskopf. She added that, because vaccine effectiveness varies by season, the generalizability of effectiveness data is another challenge.
The work group’s systematic review and meta-analysis is designed to compare the high-dose inactivated influenza vaccine (HD-IIV), the adjuvanted inactivated influenza vaccine (aIIV), and the recombinant influenza vaccine (RIV). The study will include adults aged 65 years and older who receive trivalent or quadrivalent HD-IIV, aIIV, or RIV, compared with those who receive another influenza vaccine, a noninfluenza control vaccine, placebo, or no vaccine. The outcomes will include data on safety and effectiveness of the vaccines, Dr. Groskopf said.
In addition to safety and effectiveness, manufacturers such as Sanofi Pasteur continue to collect data on the success of available vaccines and develop new ones. Lee-Jah Chang, MD, of Sanofi Pasteur presented results of a noninferiority study of the company’s investigational high-dose quadrivalent influenza vaccine (QIV-HD; including two prevailing B viruses) versus the high-dose trivalent influenza vaccine (TID-HD). The study was conducted at 35 sites in the United States and included 2,670 adults aged 65 years and older.
Overall, the reactogenicity profile for patients given QIV-HD was similar to that of TID-HD, and approximately 5% of patients in the QIV group reported an immediate adverse event, Dr. Chang said. However, no related deaths or related adverse events of special interest occurred in any of the study groups.
Sanofi plans to pursue licensure of the QIV-HD vaccine, with a Center for Biologics Evaluation and Research action date of Nov. 4, 2019, said Dr. Chang. If the vaccine is licensed, it should be available for purchase by health care providers in the first quarter of 2020.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
Multiple Keratoacanthomas Arising Within Red Tattoo Pigment
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.

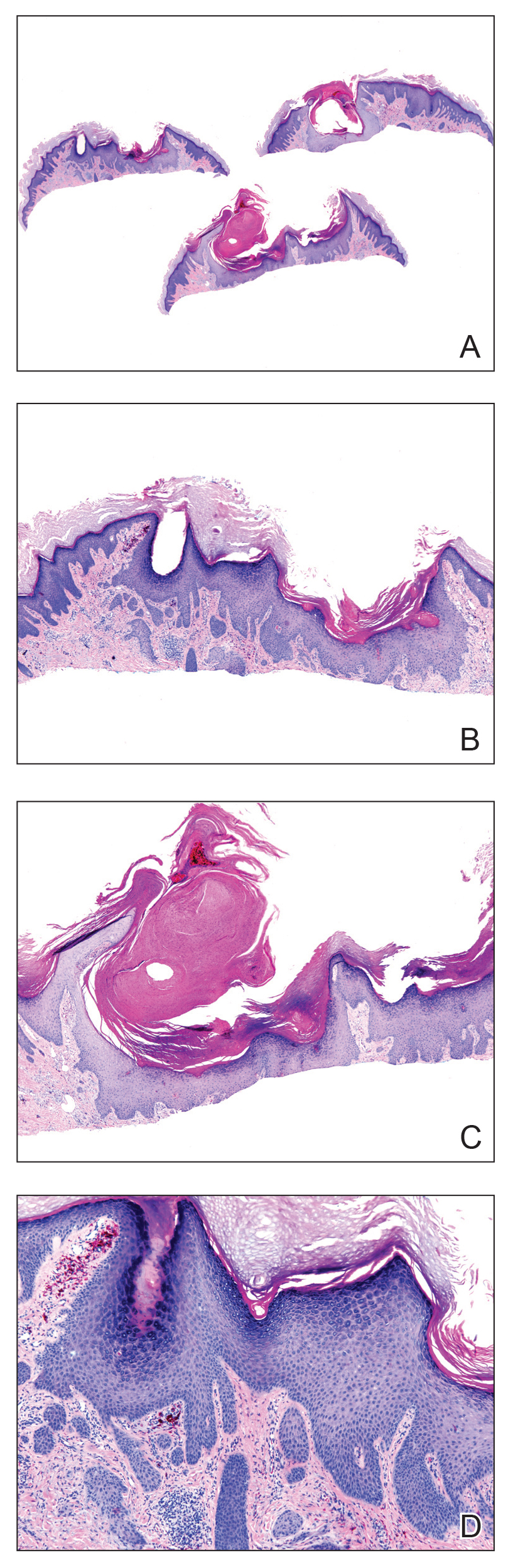
Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.


Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.


Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
Practice Points
- Tattoo reactions range from infectious and inflammatory dermatoses to the development of malignant neoplasms.
- Red pigment is the most common cause of adverse tattoo reactions.
- The management of tattoo-associated keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) has not been widely published, but they can be approached similarly to nontattoo-associated KA-SCCs.
Electrified pathogens and urbanized mosquitoes
Microbes won’t believe this shocking truth!
Pathogens can be tough little critters. Always getting into places they don’t belong, and when they get there, they can be difficult to get rid of, what with their ability to quickly evolve resistance to our best medications. If only there was some shocking new way to tackle those nasty and annoying infections.
Thanks to some engineers and researchers from the University of Pittsburgh, if you’ve got an infection centered on a metal implant, that shocking new treatment won’t be just a figure of speech. They ran a weak electrical current through metal dental implants infected with recurring Candida albicans infection, which damaged the cell membranes of the offending fungal pathogens but left the healthy tissue around the infection alone. That damage increased the pathogen's permeability, making it more susceptible to antimicrobial treatment.
The treatment, also known as electrochemical therapy, is great if you’ve got a recurrent infection. The dormant pathogens responsible for the recurrence, not normally susceptible to treatment, are affected by antifungals or antibiotics after the shock wakes them up. Even bacteria that have evolved drug resistance become vulnerable again after a session with Dr. Electricity.
Unfortunately, while the therapy could certainly be expanded beyond dental implants, shocking yourself when you’ve got a regular old infection probably won’t work. We know – Watt a disappointment.
Blast from the brewing past
What separates a rich Belgian ale from its paler, mass-produced American competitors? Is it the sudsy je ne sais quoi produced by squabbling Walloons and Flemings debating the fine points of the brewing arts over open tanks? Perhaps it’s the signature warm-fermented ways of Trappist monks? Is it possible les Belges have hired the unemployed Artesians who once made Olympia Beer a household name across the American West?
Wrong, wrong, and faux. In fact, Belgium’s finest brews are driven by hybrids. Yeast hybrids. Specifically, rare and unusual forms of hybrid yeasts.
Or, as Belgian researcher and world beer hero Dr. Jan Steensels of VIB-KU Leuven Center for Microbiology explains, “Think of lions and tigers making a super-baby.”
Fearlessly, Dr. Steensels and his intrepid colleagues went hunting for these exotic creatures. They found that the yeasts behind many of Belgium’s finest brews combine the DNA of the traditional, domesticated ale yeast, Saccharomyces cerevisiae, with genetic material from wild yeasts such as Saccharomyces kudriavzevii.
The result? The mighty fermentation strengths of normal beer yeasts are paired with the stress resistance and alluring aromas of feral yeasts that survived mankind’s Medieval brewing endeavors and somehow stumbled into the modern brewery for a drink.
Dr. Steensels’ team is now using its knowledge to craft more yeasty lion-tiger super-babies. We at the Bureau of LOTME look forward to hoisting a pint of this “liger” elixir. We’re certain the brew will bear the name of ligers’ greatest cinematic fan, Napoleon Dynamite, and feature the subtle undertones of tater tots.
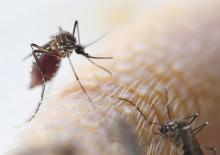
How can mosquitoes be even more fun?
If you’re anything like the gang at LOTME, you’ve spent quite a bit of time wondering which cliché is the best fit for a less-affluent Baltimore neighborhood.
The answer? When it rains, it pours.
We’ll explain. By definition, a less-affluent neighborhood is, well, less affluent, and that lack of affluence has many health consequences for the people who live in those neighborhoods. Today we’re focusing on everyone’s favorite winged disease vector, the mosquito.
It was already known that low-income urban neighborhoods have more mosquitoes than other neighborhoods, and now the Journal of Medical Entomology has published a survey of 13 residential blocks in Baltimore that shows low-income neighborhoods have larger mosquitoes as well.
Trapping took place in five socioeconomically diverse Baltimore neighborhoods during June and July of 2015-2017. (In case you were wondering, the researchers used BG-Sentinel traps baited with CO2 and a 2.0-mL Octenol Lure, which would have been our choice, too). It confirmed that lower affluence correlated with larger mosquito wing size. Wing size, the investigators said in a written statement, “is an accurate proxy for body size in mosquitoes, and body size influences traits that are important to disease transmission.”
So, it seems that larger mosquitoes are more efficient at transmitting diseases, which means more dengue fever, more Zika, more chikungunya, more eastern equine encephalitis, and more West Nile virus. To extend the original cliché a bit, when it rains in Baltimore, the poor neighborhoods get the wettest.
* Correction, 10/24/19: An earlier version of this story misstated the fungal target of the electrical therapy experiment.

Microbes won’t believe this shocking truth!
Pathogens can be tough little critters. Always getting into places they don’t belong, and when they get there, they can be difficult to get rid of, what with their ability to quickly evolve resistance to our best medications. If only there was some shocking new way to tackle those nasty and annoying infections.
Thanks to some engineers and researchers from the University of Pittsburgh, if you’ve got an infection centered on a metal implant, that shocking new treatment won’t be just a figure of speech. They ran a weak electrical current through metal dental implants infected with recurring Candida albicans infection, which damaged the cell membranes of the offending fungal pathogens but left the healthy tissue around the infection alone. That damage increased the pathogen's permeability, making it more susceptible to antimicrobial treatment.
The treatment, also known as electrochemical therapy, is great if you’ve got a recurrent infection. The dormant pathogens responsible for the recurrence, not normally susceptible to treatment, are affected by antifungals or antibiotics after the shock wakes them up. Even bacteria that have evolved drug resistance become vulnerable again after a session with Dr. Electricity.
Unfortunately, while the therapy could certainly be expanded beyond dental implants, shocking yourself when you’ve got a regular old infection probably won’t work. We know – Watt a disappointment.
Blast from the brewing past
What separates a rich Belgian ale from its paler, mass-produced American competitors? Is it the sudsy je ne sais quoi produced by squabbling Walloons and Flemings debating the fine points of the brewing arts over open tanks? Perhaps it’s the signature warm-fermented ways of Trappist monks? Is it possible les Belges have hired the unemployed Artesians who once made Olympia Beer a household name across the American West?
Wrong, wrong, and faux. In fact, Belgium’s finest brews are driven by hybrids. Yeast hybrids. Specifically, rare and unusual forms of hybrid yeasts.
Or, as Belgian researcher and world beer hero Dr. Jan Steensels of VIB-KU Leuven Center for Microbiology explains, “Think of lions and tigers making a super-baby.”
Fearlessly, Dr. Steensels and his intrepid colleagues went hunting for these exotic creatures. They found that the yeasts behind many of Belgium’s finest brews combine the DNA of the traditional, domesticated ale yeast, Saccharomyces cerevisiae, with genetic material from wild yeasts such as Saccharomyces kudriavzevii.
The result? The mighty fermentation strengths of normal beer yeasts are paired with the stress resistance and alluring aromas of feral yeasts that survived mankind’s Medieval brewing endeavors and somehow stumbled into the modern brewery for a drink.
Dr. Steensels’ team is now using its knowledge to craft more yeasty lion-tiger super-babies. We at the Bureau of LOTME look forward to hoisting a pint of this “liger” elixir. We’re certain the brew will bear the name of ligers’ greatest cinematic fan, Napoleon Dynamite, and feature the subtle undertones of tater tots.
How can mosquitoes be even more fun?
If you’re anything like the gang at LOTME, you’ve spent quite a bit of time wondering which cliché is the best fit for a less-affluent Baltimore neighborhood.
The answer? When it rains, it pours.
We’ll explain. By definition, a less-affluent neighborhood is, well, less affluent, and that lack of affluence has many health consequences for the people who live in those neighborhoods. Today we’re focusing on everyone’s favorite winged disease vector, the mosquito.
It was already known that low-income urban neighborhoods have more mosquitoes than other neighborhoods, and now the Journal of Medical Entomology has published a survey of 13 residential blocks in Baltimore that shows low-income neighborhoods have larger mosquitoes as well.
Trapping took place in five socioeconomically diverse Baltimore neighborhoods during June and July of 2015-2017. (In case you were wondering, the researchers used BG-Sentinel traps baited with CO2 and a 2.0-mL Octenol Lure, which would have been our choice, too). It confirmed that lower affluence correlated with larger mosquito wing size. Wing size, the investigators said in a written statement, “is an accurate proxy for body size in mosquitoes, and body size influences traits that are important to disease transmission.”
So, it seems that larger mosquitoes are more efficient at transmitting diseases, which means more dengue fever, more Zika, more chikungunya, more eastern equine encephalitis, and more West Nile virus. To extend the original cliché a bit, when it rains in Baltimore, the poor neighborhoods get the wettest.
* Correction, 10/24/19: An earlier version of this story misstated the fungal target of the electrical therapy experiment.

Microbes won’t believe this shocking truth!
Pathogens can be tough little critters. Always getting into places they don’t belong, and when they get there, they can be difficult to get rid of, what with their ability to quickly evolve resistance to our best medications. If only there was some shocking new way to tackle those nasty and annoying infections.
Thanks to some engineers and researchers from the University of Pittsburgh, if you’ve got an infection centered on a metal implant, that shocking new treatment won’t be just a figure of speech. They ran a weak electrical current through metal dental implants infected with recurring Candida albicans infection, which damaged the cell membranes of the offending fungal pathogens but left the healthy tissue around the infection alone. That damage increased the pathogen's permeability, making it more susceptible to antimicrobial treatment.
The treatment, also known as electrochemical therapy, is great if you’ve got a recurrent infection. The dormant pathogens responsible for the recurrence, not normally susceptible to treatment, are affected by antifungals or antibiotics after the shock wakes them up. Even bacteria that have evolved drug resistance become vulnerable again after a session with Dr. Electricity.
Unfortunately, while the therapy could certainly be expanded beyond dental implants, shocking yourself when you’ve got a regular old infection probably won’t work. We know – Watt a disappointment.
Blast from the brewing past
What separates a rich Belgian ale from its paler, mass-produced American competitors? Is it the sudsy je ne sais quoi produced by squabbling Walloons and Flemings debating the fine points of the brewing arts over open tanks? Perhaps it’s the signature warm-fermented ways of Trappist monks? Is it possible les Belges have hired the unemployed Artesians who once made Olympia Beer a household name across the American West?
Wrong, wrong, and faux. In fact, Belgium’s finest brews are driven by hybrids. Yeast hybrids. Specifically, rare and unusual forms of hybrid yeasts.
Or, as Belgian researcher and world beer hero Dr. Jan Steensels of VIB-KU Leuven Center for Microbiology explains, “Think of lions and tigers making a super-baby.”
Fearlessly, Dr. Steensels and his intrepid colleagues went hunting for these exotic creatures. They found that the yeasts behind many of Belgium’s finest brews combine the DNA of the traditional, domesticated ale yeast, Saccharomyces cerevisiae, with genetic material from wild yeasts such as Saccharomyces kudriavzevii.
The result? The mighty fermentation strengths of normal beer yeasts are paired with the stress resistance and alluring aromas of feral yeasts that survived mankind’s Medieval brewing endeavors and somehow stumbled into the modern brewery for a drink.
Dr. Steensels’ team is now using its knowledge to craft more yeasty lion-tiger super-babies. We at the Bureau of LOTME look forward to hoisting a pint of this “liger” elixir. We’re certain the brew will bear the name of ligers’ greatest cinematic fan, Napoleon Dynamite, and feature the subtle undertones of tater tots.
How can mosquitoes be even more fun?
If you’re anything like the gang at LOTME, you’ve spent quite a bit of time wondering which cliché is the best fit for a less-affluent Baltimore neighborhood.
The answer? When it rains, it pours.
We’ll explain. By definition, a less-affluent neighborhood is, well, less affluent, and that lack of affluence has many health consequences for the people who live in those neighborhoods. Today we’re focusing on everyone’s favorite winged disease vector, the mosquito.
It was already known that low-income urban neighborhoods have more mosquitoes than other neighborhoods, and now the Journal of Medical Entomology has published a survey of 13 residential blocks in Baltimore that shows low-income neighborhoods have larger mosquitoes as well.
Trapping took place in five socioeconomically diverse Baltimore neighborhoods during June and July of 2015-2017. (In case you were wondering, the researchers used BG-Sentinel traps baited with CO2 and a 2.0-mL Octenol Lure, which would have been our choice, too). It confirmed that lower affluence correlated with larger mosquito wing size. Wing size, the investigators said in a written statement, “is an accurate proxy for body size in mosquitoes, and body size influences traits that are important to disease transmission.”
So, it seems that larger mosquitoes are more efficient at transmitting diseases, which means more dengue fever, more Zika, more chikungunya, more eastern equine encephalitis, and more West Nile virus. To extend the original cliché a bit, when it rains in Baltimore, the poor neighborhoods get the wettest.
* Correction, 10/24/19: An earlier version of this story misstated the fungal target of the electrical therapy experiment.

ACIP recommends two options for pertussis vaccination
Either the Tdap or Td vaccine is an acceptable option for pertussis vaccination in most situations, recommended the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 14-0 vote at the October meeting, based on the immunization schedule for persons aged 7 years and older.
Safety data showed no differences in safety concerns between Tdap and Td, including data from pregnant women, said Fiona Havers, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), Atlanta.
Several of the ACIP members noted that the revised language to include both Tdap and Td reflects the increased use of Tdap and allows for maximum flexibility in clinical settings.
The revised language advises that booster doses of “either Td or Tdap” every 10 years throughout life are recommended for continued protection against tetanus and diphtheria. In addition, either Td or Tdap should be used if a tetanus toxoid–containing vaccine is indicated for prophylaxis in nonpregnant individuals.
For catch-up recommendations, which also apply to pregnant women, the committee approved the following wording for a series of three doses for individuals aged 7-18 years and 19 years and older who have never been vaccinated, that “the preferred schedule is a dose of Tdap (preferably the first dose), followed by either Tdap or Td at least 4 weeks afterward and another dose of either Td or Tdap 6-12 months later.” Individuals in these same age groups who are not fully vaccinated should receive one dose of Tdap, and a dose of either Td or Tdap if additional doses are needed.
The committee also voted unanimously 14-0 to accept the updated wording for pertussis vaccination in the Vaccines for Children program.
The ACIP members had no financial conflicts to disclose.
Either the Tdap or Td vaccine is an acceptable option for pertussis vaccination in most situations, recommended the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 14-0 vote at the October meeting, based on the immunization schedule for persons aged 7 years and older.
Safety data showed no differences in safety concerns between Tdap and Td, including data from pregnant women, said Fiona Havers, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), Atlanta.
Several of the ACIP members noted that the revised language to include both Tdap and Td reflects the increased use of Tdap and allows for maximum flexibility in clinical settings.
The revised language advises that booster doses of “either Td or Tdap” every 10 years throughout life are recommended for continued protection against tetanus and diphtheria. In addition, either Td or Tdap should be used if a tetanus toxoid–containing vaccine is indicated for prophylaxis in nonpregnant individuals.
For catch-up recommendations, which also apply to pregnant women, the committee approved the following wording for a series of three doses for individuals aged 7-18 years and 19 years and older who have never been vaccinated, that “the preferred schedule is a dose of Tdap (preferably the first dose), followed by either Tdap or Td at least 4 weeks afterward and another dose of either Td or Tdap 6-12 months later.” Individuals in these same age groups who are not fully vaccinated should receive one dose of Tdap, and a dose of either Td or Tdap if additional doses are needed.
The committee also voted unanimously 14-0 to accept the updated wording for pertussis vaccination in the Vaccines for Children program.
The ACIP members had no financial conflicts to disclose.
Either the Tdap or Td vaccine is an acceptable option for pertussis vaccination in most situations, recommended the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 14-0 vote at the October meeting, based on the immunization schedule for persons aged 7 years and older.
Safety data showed no differences in safety concerns between Tdap and Td, including data from pregnant women, said Fiona Havers, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), Atlanta.
Several of the ACIP members noted that the revised language to include both Tdap and Td reflects the increased use of Tdap and allows for maximum flexibility in clinical settings.
The revised language advises that booster doses of “either Td or Tdap” every 10 years throughout life are recommended for continued protection against tetanus and diphtheria. In addition, either Td or Tdap should be used if a tetanus toxoid–containing vaccine is indicated for prophylaxis in nonpregnant individuals.
For catch-up recommendations, which also apply to pregnant women, the committee approved the following wording for a series of three doses for individuals aged 7-18 years and 19 years and older who have never been vaccinated, that “the preferred schedule is a dose of Tdap (preferably the first dose), followed by either Tdap or Td at least 4 weeks afterward and another dose of either Td or Tdap 6-12 months later.” Individuals in these same age groups who are not fully vaccinated should receive one dose of Tdap, and a dose of either Td or Tdap if additional doses are needed.
The committee also voted unanimously 14-0 to accept the updated wording for pertussis vaccination in the Vaccines for Children program.
The ACIP members had no financial conflicts to disclose.
FROM AN ACIP MEETING
Boy, Is My Face Red!
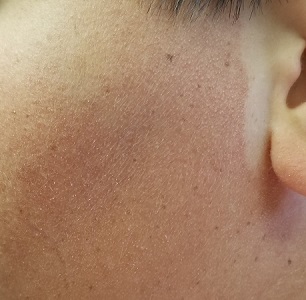
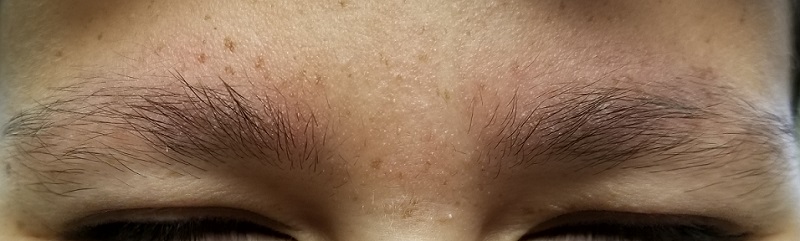
A 17-year-old boy was born with rough skin on his face, arms, legs, thighs, and posterior shoulders. Over the years, his face, especially the posterior lateral aspects, has become progressively redder, while the roughness has increased. The redness is amplified with heat, exertion, anger, or embarrassment. Regarding the latter, mere mention of the condition by his siblings results in worsening of the erythema. Additionally, the skin in his eyebrows is now turning red and scaly.
The patient denies a history of dandruff. His parents, who have accompanied him to the clinic, report a family history of similar skin changes on triceps and thighs, but not on faces. There is no family history of cardiac anomalies or other congenital abnormalities. The boy’s health is otherwise excellent.
EXAMINATION
The patient’s bilateral triceps are covered with fine, rough, follicular papules, which create a faintly erythematous look. Similar lesions are visible on his posterior shoulders and anterior thighs. The skin beneath his eyebrows is faintly erythematous and scaly.
The posterior sides of his face are bright red and covered with the same type of papules. The erythema grows redder as it approaches the immediate preauricular areas, where it ends abruptly, creating a sharp demarcation with the white skin closer to the ears. The visual effect is almost clownish, as if bright red makeup had been applied.
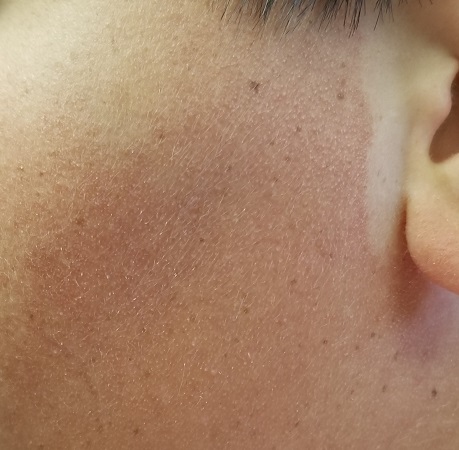
What’s the diagnosis?
DISCUSSION
This young man has all the signs of an extremely rare variant of keratosis pilaris (KP) called ulerythema ophryogenes (UO). In the United States, about 40% of adults have ordinary KP, which usually manifests in childhood (with about 80% of cases occurring in adolescent girls). KP is inherited through autosomal dominance, with highly variable penetrance, though no specific gene has been identified. In this form, KP is considered by most experts to be a minor diagnostic criterion for atopic dermatitis.
However, UO is not merely a variant of KP. Over the decades, it has been connected with more serious conditions, such as cardiofaciocutaneous syndrome, Rubinstein-Taybi syndrome, and Cornelia de Lange disease. Although these conditions are not common, they should be considered when UO is seen.
For this patient, the main concern was his appearance, especially the pronounced erythema around the periphery of his face. This aspect of the problem was addressed with a referral to a provider who can, using an assortment of lasers, try to even out his skin color and hopefully erase the sharp border at the periphery of the affected area.
For the physical discomfort caused by UO, the patient was instructed either to use general moisturizers to reduce dryness or to consider using moisturizers containing salicylic acid, which should help to reduce the prominence of the papules. For the erythema in his brows, he is using 2.5% hydrocortisone ointment two to three times a week.
TAKE-HOME LEARNING POINTS
- Ulerythema ophryogenes is a rarely encountered variant of keratosis pilaris—a condition inherited by autosomal dominance with highly variable penetrance.
- Its main significance, beyond cosmetic concerns, is the possible connection with syndromes that involve heart and structural defects (eg, cardiofaciocutaneous syndrome).
- Treatment options include heavy emollients to soften the scaly papules and laser therapy to reduce the extreme redness seen on the periphery of the face.
A 17-year-old boy was born with rough skin on his face, arms, legs, thighs, and posterior shoulders. Over the years, his face, especially the posterior lateral aspects, has become progressively redder, while the roughness has increased. The redness is amplified with heat, exertion, anger, or embarrassment. Regarding the latter, mere mention of the condition by his siblings results in worsening of the erythema. Additionally, the skin in his eyebrows is now turning red and scaly.
The patient denies a history of dandruff. His parents, who have accompanied him to the clinic, report a family history of similar skin changes on triceps and thighs, but not on faces. There is no family history of cardiac anomalies or other congenital abnormalities. The boy’s health is otherwise excellent.

EXAMINATION
The patient’s bilateral triceps are covered with fine, rough, follicular papules, which create a faintly erythematous look. Similar lesions are visible on his posterior shoulders and anterior thighs. The skin beneath his eyebrows is faintly erythematous and scaly.
The posterior sides of his face are bright red and covered with the same type of papules. The erythema grows redder as it approaches the immediate preauricular areas, where it ends abruptly, creating a sharp demarcation with the white skin closer to the ears. The visual effect is almost clownish, as if bright red makeup had been applied.

What’s the diagnosis?
DISCUSSION
This young man has all the signs of an extremely rare variant of keratosis pilaris (KP) called ulerythema ophryogenes (UO). In the United States, about 40% of adults have ordinary KP, which usually manifests in childhood (with about 80% of cases occurring in adolescent girls). KP is inherited through autosomal dominance, with highly variable penetrance, though no specific gene has been identified. In this form, KP is considered by most experts to be a minor diagnostic criterion for atopic dermatitis.
However, UO is not merely a variant of KP. Over the decades, it has been connected with more serious conditions, such as cardiofaciocutaneous syndrome, Rubinstein-Taybi syndrome, and Cornelia de Lange disease. Although these conditions are not common, they should be considered when UO is seen.
For this patient, the main concern was his appearance, especially the pronounced erythema around the periphery of his face. This aspect of the problem was addressed with a referral to a provider who can, using an assortment of lasers, try to even out his skin color and hopefully erase the sharp border at the periphery of the affected area.
For the physical discomfort caused by UO, the patient was instructed either to use general moisturizers to reduce dryness or to consider using moisturizers containing salicylic acid, which should help to reduce the prominence of the papules. For the erythema in his brows, he is using 2.5% hydrocortisone ointment two to three times a week.
TAKE-HOME LEARNING POINTS
- Ulerythema ophryogenes is a rarely encountered variant of keratosis pilaris—a condition inherited by autosomal dominance with highly variable penetrance.
- Its main significance, beyond cosmetic concerns, is the possible connection with syndromes that involve heart and structural defects (eg, cardiofaciocutaneous syndrome).
- Treatment options include heavy emollients to soften the scaly papules and laser therapy to reduce the extreme redness seen on the periphery of the face.
A 17-year-old boy was born with rough skin on his face, arms, legs, thighs, and posterior shoulders. Over the years, his face, especially the posterior lateral aspects, has become progressively redder, while the roughness has increased. The redness is amplified with heat, exertion, anger, or embarrassment. Regarding the latter, mere mention of the condition by his siblings results in worsening of the erythema. Additionally, the skin in his eyebrows is now turning red and scaly.
The patient denies a history of dandruff. His parents, who have accompanied him to the clinic, report a family history of similar skin changes on triceps and thighs, but not on faces. There is no family history of cardiac anomalies or other congenital abnormalities. The boy’s health is otherwise excellent.

EXAMINATION
The patient’s bilateral triceps are covered with fine, rough, follicular papules, which create a faintly erythematous look. Similar lesions are visible on his posterior shoulders and anterior thighs. The skin beneath his eyebrows is faintly erythematous and scaly.
The posterior sides of his face are bright red and covered with the same type of papules. The erythema grows redder as it approaches the immediate preauricular areas, where it ends abruptly, creating a sharp demarcation with the white skin closer to the ears. The visual effect is almost clownish, as if bright red makeup had been applied.

What’s the diagnosis?
DISCUSSION
This young man has all the signs of an extremely rare variant of keratosis pilaris (KP) called ulerythema ophryogenes (UO). In the United States, about 40% of adults have ordinary KP, which usually manifests in childhood (with about 80% of cases occurring in adolescent girls). KP is inherited through autosomal dominance, with highly variable penetrance, though no specific gene has been identified. In this form, KP is considered by most experts to be a minor diagnostic criterion for atopic dermatitis.
However, UO is not merely a variant of KP. Over the decades, it has been connected with more serious conditions, such as cardiofaciocutaneous syndrome, Rubinstein-Taybi syndrome, and Cornelia de Lange disease. Although these conditions are not common, they should be considered when UO is seen.
For this patient, the main concern was his appearance, especially the pronounced erythema around the periphery of his face. This aspect of the problem was addressed with a referral to a provider who can, using an assortment of lasers, try to even out his skin color and hopefully erase the sharp border at the periphery of the affected area.
For the physical discomfort caused by UO, the patient was instructed either to use general moisturizers to reduce dryness or to consider using moisturizers containing salicylic acid, which should help to reduce the prominence of the papules. For the erythema in his brows, he is using 2.5% hydrocortisone ointment two to three times a week.
TAKE-HOME LEARNING POINTS
- Ulerythema ophryogenes is a rarely encountered variant of keratosis pilaris—a condition inherited by autosomal dominance with highly variable penetrance.
- Its main significance, beyond cosmetic concerns, is the possible connection with syndromes that involve heart and structural defects (eg, cardiofaciocutaneous syndrome).
- Treatment options include heavy emollients to soften the scaly papules and laser therapy to reduce the extreme redness seen on the periphery of the face.
Clinical interventions for global drug use need updating
Public health approach requires greater emphasis on harms, benefits of substance use.
Strategies aimed at reducing drug-related harm should be informed by evidence, and recognize the contribution of social and economic factors to drug use, report the authors of a series of four papers published in The Lancet.
Louisa Degenhardt, PhD, and coauthors wrote in the first paper that, although the availability and use of drugs have been transformed over recent decades – including the emergence of hundreds of new psychoactive substances – professional and public policy has not yet adapted to those new realities (Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9).
, in a way that you don’t see in other areas of public health,” Dr. Degenhardt, of the National Drug and Alcohol Research Centre at the University of New South Wales in Sydney, said in an interview. “There has been an increasing level of awareness of issues but also level of recognition that we need to have hard evidence to work out the best ways to respond.”
The paper by Dr. Degenhardt and coauthors addressed the issue of opioid use and dependence around the world, citing evidence that in 2017, 40.5 million people were dependent on opioids and 109,500 deaths were attributable to opioid overdose. An effective treatment exists in the form of opioid agonists methadone and buprenorphine, both of which are recognized as World Health Organization essential medicines.
While the best evidence for positive outcomes from opioid agonist treatment is in people using illicit opioids such as heroin, there is also evidence for their effectiveness in people with pharmaceutical opioid dependence. A study in Kentucky suggested that scaling up the use and retention of opioid agonist treatment, including in prison, could prevent 57% of overdose deaths among injecting drug users.
“Despite strong evidence for the effectiveness of a range of interventions to improve the health and well-being of people who are dependent on opioids, coverage is low, even in high-income countries,” the authors wrote. They also called for international efforts to eliminate marketing strategies that have contributed to the increase in opioid prescription and harms in North America.
The second paper examined the public health implications of legalizing cannabis for medicinal and recreational use (Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1). Cannabis has been considered an illicit drug for more than 50 years but recently has been decriminalized or legalized in many parts of the world in recognition of the lower levels of harm, compared with other illicit substances.
Cannabis is used to treat a range of medical conditions, including muscle spasticity in multiple sclerosis. It also is used to treat pain, nausea, and vomiting in palliative care, and to reduce seizures in epilepsy. However, the authors noted that the evidence for many medical applications was absent, and that weakly regulated medical cannabis programs in some U.S. states were blurring the boundaries between medicinal and nonmedicinal use.
They also wrote that the public health effects of legalization could not be assessed, because legalization had happened only in the last 5 years.
“A major determinant of the public health effect of cannabis legalization will be the effect that it has on alcohol use,” they wrote. “The substitution of cannabis for alcohol would produce substantial public health gains, but any increase in the combined use of alcohol and cannabis could increase harm.”
The authors also looked at the effect of use of stimulants such as cocaine and amphetamines. While their use is associated with higher mortality, increased incidence of HIV and hepatitis C infection, poor mental health, and increased risk of cardiovascular events, no effective pharmacotherapies are available, and psychosocial interventions such as cognitive-behavioral therapy have only a weak effect.
“Many governments rely on punitive responses, such as involuntary detention in drug centers, despite the absence of evidence for their effectiveness and their potential to increase harm,” the authors wrote. “Substantial research investment is needed to develop more effective, innovative, and impactful prevention and treatment” (Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5).
They focused on interventions to prevent the transmission of blood-borne and sexually transmitted infections – such as the provision of safe injecting equipment, condoms or pre-exposure prophylaxis against HIV – and improve treatment of these, and interventions to prevent and treat overdose, injury, and other harms.
The final paper in the series explored new psychoactive substances, such as synthetic cannabinoids, stimulants, hallucinogens, and dissociative and depressant substances (Peacock A et al. Lancet 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7).
There really needs to be massive change in systems in terms of the way monitoring occurs and the speed with which new drugs are identified, Dr. Degenhardt said in the interview. She also said the risks that are identified need to be communicated more effectively.
“At the moment, the way that drug surveillance works in most countries, things come and then particular drugs may spread in use, cause massive harm, and all of our systems of detecting and responding are not fit to detect those things in a timely way and disseminate information to reduce those risks.”
The papers were supported by European Monitoring Centre on Drugs and Drug Addiction, and the Australian National Drug and Alcohol Research Centre. The authors declared support from a range of institutions and funding bodies, and several also declared unrelated grants, funding, and other support from the pharmaceutical sector.
SOURCES: Degenhardt L et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9; Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1; Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5; and Peacock A et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7.
Public health approach requires greater emphasis on harms, benefits of substance use.
Public health approach requires greater emphasis on harms, benefits of substance use.
Strategies aimed at reducing drug-related harm should be informed by evidence, and recognize the contribution of social and economic factors to drug use, report the authors of a series of four papers published in The Lancet.
Louisa Degenhardt, PhD, and coauthors wrote in the first paper that, although the availability and use of drugs have been transformed over recent decades – including the emergence of hundreds of new psychoactive substances – professional and public policy has not yet adapted to those new realities (Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9).
, in a way that you don’t see in other areas of public health,” Dr. Degenhardt, of the National Drug and Alcohol Research Centre at the University of New South Wales in Sydney, said in an interview. “There has been an increasing level of awareness of issues but also level of recognition that we need to have hard evidence to work out the best ways to respond.”
The paper by Dr. Degenhardt and coauthors addressed the issue of opioid use and dependence around the world, citing evidence that in 2017, 40.5 million people were dependent on opioids and 109,500 deaths were attributable to opioid overdose. An effective treatment exists in the form of opioid agonists methadone and buprenorphine, both of which are recognized as World Health Organization essential medicines.
While the best evidence for positive outcomes from opioid agonist treatment is in people using illicit opioids such as heroin, there is also evidence for their effectiveness in people with pharmaceutical opioid dependence. A study in Kentucky suggested that scaling up the use and retention of opioid agonist treatment, including in prison, could prevent 57% of overdose deaths among injecting drug users.
“Despite strong evidence for the effectiveness of a range of interventions to improve the health and well-being of people who are dependent on opioids, coverage is low, even in high-income countries,” the authors wrote. They also called for international efforts to eliminate marketing strategies that have contributed to the increase in opioid prescription and harms in North America.
The second paper examined the public health implications of legalizing cannabis for medicinal and recreational use (Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1). Cannabis has been considered an illicit drug for more than 50 years but recently has been decriminalized or legalized in many parts of the world in recognition of the lower levels of harm, compared with other illicit substances.
Cannabis is used to treat a range of medical conditions, including muscle spasticity in multiple sclerosis. It also is used to treat pain, nausea, and vomiting in palliative care, and to reduce seizures in epilepsy. However, the authors noted that the evidence for many medical applications was absent, and that weakly regulated medical cannabis programs in some U.S. states were blurring the boundaries between medicinal and nonmedicinal use.
They also wrote that the public health effects of legalization could not be assessed, because legalization had happened only in the last 5 years.
“A major determinant of the public health effect of cannabis legalization will be the effect that it has on alcohol use,” they wrote. “The substitution of cannabis for alcohol would produce substantial public health gains, but any increase in the combined use of alcohol and cannabis could increase harm.”
The authors also looked at the effect of use of stimulants such as cocaine and amphetamines. While their use is associated with higher mortality, increased incidence of HIV and hepatitis C infection, poor mental health, and increased risk of cardiovascular events, no effective pharmacotherapies are available, and psychosocial interventions such as cognitive-behavioral therapy have only a weak effect.
“Many governments rely on punitive responses, such as involuntary detention in drug centers, despite the absence of evidence for their effectiveness and their potential to increase harm,” the authors wrote. “Substantial research investment is needed to develop more effective, innovative, and impactful prevention and treatment” (Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5).
They focused on interventions to prevent the transmission of blood-borne and sexually transmitted infections – such as the provision of safe injecting equipment, condoms or pre-exposure prophylaxis against HIV – and improve treatment of these, and interventions to prevent and treat overdose, injury, and other harms.
The final paper in the series explored new psychoactive substances, such as synthetic cannabinoids, stimulants, hallucinogens, and dissociative and depressant substances (Peacock A et al. Lancet 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7).
There really needs to be massive change in systems in terms of the way monitoring occurs and the speed with which new drugs are identified, Dr. Degenhardt said in the interview. She also said the risks that are identified need to be communicated more effectively.
“At the moment, the way that drug surveillance works in most countries, things come and then particular drugs may spread in use, cause massive harm, and all of our systems of detecting and responding are not fit to detect those things in a timely way and disseminate information to reduce those risks.”
The papers were supported by European Monitoring Centre on Drugs and Drug Addiction, and the Australian National Drug and Alcohol Research Centre. The authors declared support from a range of institutions and funding bodies, and several also declared unrelated grants, funding, and other support from the pharmaceutical sector.
SOURCES: Degenhardt L et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9; Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1; Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5; and Peacock A et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7.
Strategies aimed at reducing drug-related harm should be informed by evidence, and recognize the contribution of social and economic factors to drug use, report the authors of a series of four papers published in The Lancet.
Louisa Degenhardt, PhD, and coauthors wrote in the first paper that, although the availability and use of drugs have been transformed over recent decades – including the emergence of hundreds of new psychoactive substances – professional and public policy has not yet adapted to those new realities (Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9).
, in a way that you don’t see in other areas of public health,” Dr. Degenhardt, of the National Drug and Alcohol Research Centre at the University of New South Wales in Sydney, said in an interview. “There has been an increasing level of awareness of issues but also level of recognition that we need to have hard evidence to work out the best ways to respond.”
The paper by Dr. Degenhardt and coauthors addressed the issue of opioid use and dependence around the world, citing evidence that in 2017, 40.5 million people were dependent on opioids and 109,500 deaths were attributable to opioid overdose. An effective treatment exists in the form of opioid agonists methadone and buprenorphine, both of which are recognized as World Health Organization essential medicines.
While the best evidence for positive outcomes from opioid agonist treatment is in people using illicit opioids such as heroin, there is also evidence for their effectiveness in people with pharmaceutical opioid dependence. A study in Kentucky suggested that scaling up the use and retention of opioid agonist treatment, including in prison, could prevent 57% of overdose deaths among injecting drug users.
“Despite strong evidence for the effectiveness of a range of interventions to improve the health and well-being of people who are dependent on opioids, coverage is low, even in high-income countries,” the authors wrote. They also called for international efforts to eliminate marketing strategies that have contributed to the increase in opioid prescription and harms in North America.
The second paper examined the public health implications of legalizing cannabis for medicinal and recreational use (Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1). Cannabis has been considered an illicit drug for more than 50 years but recently has been decriminalized or legalized in many parts of the world in recognition of the lower levels of harm, compared with other illicit substances.
Cannabis is used to treat a range of medical conditions, including muscle spasticity in multiple sclerosis. It also is used to treat pain, nausea, and vomiting in palliative care, and to reduce seizures in epilepsy. However, the authors noted that the evidence for many medical applications was absent, and that weakly regulated medical cannabis programs in some U.S. states were blurring the boundaries between medicinal and nonmedicinal use.
They also wrote that the public health effects of legalization could not be assessed, because legalization had happened only in the last 5 years.
“A major determinant of the public health effect of cannabis legalization will be the effect that it has on alcohol use,” they wrote. “The substitution of cannabis for alcohol would produce substantial public health gains, but any increase in the combined use of alcohol and cannabis could increase harm.”
The authors also looked at the effect of use of stimulants such as cocaine and amphetamines. While their use is associated with higher mortality, increased incidence of HIV and hepatitis C infection, poor mental health, and increased risk of cardiovascular events, no effective pharmacotherapies are available, and psychosocial interventions such as cognitive-behavioral therapy have only a weak effect.
“Many governments rely on punitive responses, such as involuntary detention in drug centers, despite the absence of evidence for their effectiveness and their potential to increase harm,” the authors wrote. “Substantial research investment is needed to develop more effective, innovative, and impactful prevention and treatment” (Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5).
They focused on interventions to prevent the transmission of blood-borne and sexually transmitted infections – such as the provision of safe injecting equipment, condoms or pre-exposure prophylaxis against HIV – and improve treatment of these, and interventions to prevent and treat overdose, injury, and other harms.
The final paper in the series explored new psychoactive substances, such as synthetic cannabinoids, stimulants, hallucinogens, and dissociative and depressant substances (Peacock A et al. Lancet 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7).
There really needs to be massive change in systems in terms of the way monitoring occurs and the speed with which new drugs are identified, Dr. Degenhardt said in the interview. She also said the risks that are identified need to be communicated more effectively.
“At the moment, the way that drug surveillance works in most countries, things come and then particular drugs may spread in use, cause massive harm, and all of our systems of detecting and responding are not fit to detect those things in a timely way and disseminate information to reduce those risks.”
The papers were supported by European Monitoring Centre on Drugs and Drug Addiction, and the Australian National Drug and Alcohol Research Centre. The authors declared support from a range of institutions and funding bodies, and several also declared unrelated grants, funding, and other support from the pharmaceutical sector.
SOURCES: Degenhardt L et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9; Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1; Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5; and Peacock A et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7.
FROM THE LANCET
Key clinical point: People with drug use disorders around the world need evidence-based and nonjudgmental clinical care.
Major finding: Many interventions aimed at reducing the harm of illicit drug use are not informed by evidence.
Study details: Series of four papers reviewing the evidence on cannabinoids, opioids, new psychoactive substances, and stimulants.
Disclosures: The papers were supported by European Monitoring Centre on Drugs and Drug Addiction, and the Australian National Drug and Alcohol Research Centre. The authors declared support from a range of institutions and funding bodies, and several also declared unrelated grants, funding, and other support from the pharmaceutical sector.
Sources: Degenhardt L et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9; Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1; Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5; and Peacock A et al. Lancet 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7.
Antituberculosis drugs in pregnancy and lactation
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at [email protected].
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at [email protected].
Tuberculosis is one of the top ten causes of death worldwide and the leading cause from a single infectious agent. In the 2012-2017 period, there were more than 9,000 cases of TB each year in the United States. The Centers for Disease Control and Prevention states that untreated TB is a greater hazard to a pregnant woman and her fetus than its treatment.
In the material below, the molecular weights, rounded to the nearest whole number, are shown in parentheses after the drug name. Those less than 1,000 or so suggest that the drug will cross the placenta throughout pregnancy. In the second half of pregnancy, especially in the third trimester, nearly all drugs will cross regardless of their molecular weight.
Para-aminosalicylic acid (Paser) (153) is most frequently used in combination with other agents for the treatment of multidrug-resistant tuberculosis; multidrug-resistant TB (MDR TB) is defined as being caused by TB bacteria that is resistant to at least isoniazid and rifampin, the two most potent TB drugs. The drug has been associated with a marked increased risk of birth defects in some, but not all, studies. Because of this potential risk, the drug is best avoided in the first trimester. The drug is excreted into breast milk, but there are no reports of its use during breastfeeding.
Bedaquiline (Sirturo) (556) is used in combination therapy for patents with multidrug-resistant tuberculosis. One report describing the use of this drug during human pregnancy has been located. Treatment was started in the last 3 weeks of pregnancy and no abnormalities were noted in the child at birth and for 2 years after birth (Emerg Infect Dis. 2017. doi: 10.3201/eid2310.161398). The CDC states that the drug should be used only in a minimum four-drug treatment regimen and administered by direct observation (MMWR Recomm Rep. 2013 Oct 25;62[RR-09]:1-12). The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Capreomycin (Capastat) (653-669) is a polypeptide antibiotic isolated from Streptomyces capreolus that is given intramuscularly. The human pregnancy data are limited to three reports. The toxicity of capreomycin is similar to aminoglycosides (e.g., cranial nerve VIII and renal) and it should not be used with these agents. The CDC has classified the drug as contraindicated in pregnancy. The drug probably is excreted into breast milk, but there are no reports of its use during breastfeeding.
Cycloserine (Seromycin) (102) is a broad spectrum antibiotic. The human pregnancy data are limited but have not shown embryo-fetal harm. Although the best course is to avoid the drug during gestation, it should not be withheld because of pregnancy if the maternal condition requires the antibiotic. The American Academy of Pediatrics classified cycloserine as compatible with breastfeeding.
Ethambutol (Myambutol) (205) should be used in conjunction with other antituberculosis drugs. The human pregnancy data do not suggest an embryo-fetal risk. A frequently used regimen is ethambutol + isoniazid + rifampin. The American Academy of Pediatrics classified ethambutol as compatible with breastfeeding.
Ethionamide (Trecator) (166) is indicated when Mycobacterium tuberculosis is resistant to isoniazid or rifampin, or when the patient is intolerant to other drugs. Although the animal reproductive data suggest risk, the limited human data suggest that the risk is probably low. If indicated, the drug should not be withheld because of pregnancy. Although the molecular weight suggests that the drug will be excreted into breast milk, no reports describing the amount in milk have been located.
Isoniazid (137) is compatible in pregnancy, even though the molecular weight suggests that it will cross the placenta, because the maternal benefit is much greater than the potential embryo-fetal risk. Although the human data are limited, the molecular weight also suggests that the drug will be excreted into breast milk, but it can be considered probably compatible during breastfeeding. No reports of isoniazid-induced effects in the nursing infant have been located, but the potential for interference with nucleic acid function and for hepatotoxicity may exist.
Pyrazinamide (123) is metabolized to an active metabolite. The molecular weight, low plasma protein binding (10%), and prolonged elimination half-life (9-10 hours) suggest that the drug will cross the placenta throughout pregnancy. The drug has been used in human pregnancy without causing embryo-fetal harm. Similar results, although limited, were reported when the drug was used during breastfeeding.
Rifabutin (Mycobutin) (847) has no reported human pregnancy data, but the animal data suggest low risk. The drug probably crosses the placenta throughout pregnancy. The maternal benefit appears to outweigh the unknown risk to the embryo-fetus, so therapy should not be withheld because of pregnancy. The drug probably is excreted into breast milk.
Rifampin (Rifadin) (823) appears to be compatible in pregnancy. Several reviews and reports have concluded that the drug was not a teratogen and recommended use of the drug with isoniazid and ethambutol. However, prophylactic vitamin K1 has been recommended to prevent drug-induced hemorrhagic disease of the newborn. There are no data regarding its use during breastfeeding, but it is probably compatible.
Rifapentine (Priftin) (877) was toxic and teratogenic in two animal species at doses close to those used in humans. In a 2018 study, however, the rates of fetal loss in pregnancies of less than 20 weeks (8/54, 15%) and congenital anomalies in live births (1/37, 3%) were within the expected background rates (Ann Am Thorac Soc. 2018 May;15[4]:570-80). There are no data regarding its use during breastfeeding, but it is probably compatible.
The CDC classifies four antituberculosis and one class of drugs as contraindicated in pregnancy. In addition to capreomycin mentioned above, they are amikacin, fluoroquinolones (ciprofloxacin, gemifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin), kanamycin, and streptomycin. These ten agents are discussed in the 11th edition of my book “Drugs in Pregnancy and Lactation,” (Wolters Kluwer Health: Riverwood, Il., 2017). If they have to be used, checking this source will provide information that has to be discussed with the patient.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs had no disclosures, except for his book. Email him at [email protected].
Adding ramucirumab extends PFS in EGFR-mutated lung cancer
For patients with epidermal growth factor receptor–mutated non–small cell lung cancer (NSCLC), adding the vascular endothelial growth factor receptor 2 (VEGFR-2) inhibitor ramucirumab to standard erlotinib therapy may extend progression-free survival, based on results from the phase 3 RELAY trial.
Considering the acceptable safety profile, this dual regimen should be considered for first-line treatment of epidermal growth factor receptor (EGFR)–mutated disease, according to lead author Kazuhiko Nakagawa, MD, PhD, of the Kindai University faculty of medicine in Osaka, Japan, and colleagues.
Dual blockade of the EGFR and VEGF pathways is supported by previous studies which pointed to efficacy among EGFR-mutated subgroups, the investigators explained in Lancet Oncology, noting that ramucirumab appeared to be the best candidate for VEGF pathway inhibition. “Ramucirumab, a human monoclonal IgG1 antibody, selectively targets VEGFR-2, thereby blocking signaling mediated by VEGF-A, VEGF-C, and VEGF-D in NSCLC,” the investigators wrote. “Therefore, ramucirumab has the potential for broader antitumor activity than inhibitors of VEGF-A.”
The trial involved 449 patients with stage IV NSCLC and an EGFR exon 21 substitution or exon 19 deletion. Patients were randomized in a 1:1 ratio to receive either erlotinib (150 mg/day) plus placebo, or erlotinib plus ramucirumab (10 mg/kg every 2 weeks). The primary endpoint was progression-free survival. Secondary endpoints included safety and toxicity, overall survival, and various measures of response.
After a median follow-up of 20.7 months, the addition of ramucirumab was associated with a significantly better median progression-free survival, at 19.4 months, compared with 12.4 months among patients who received erlotinib alone, which translates to a hazard ratio of 0.59 (P less than .0001). In each cohort, 1% of patients achieved a complete response. Partial responses were also highly similar at 75% for ramucirumab versus 74% for placebo.
Turning to safety, ramucirumab was associated with more safety concerns, including a higher rate of grade 3-4 treatment-emergent adverse events (72% vs. 54%), most often hypertension. Serious adverse events were also more frequent with ramucirumab at a rate of 29%, compared with 21% among those who received erlotinib alone.
Despite these differences, the investigators concluded that the dual regimen still offers acceptable tolerability. “Safety was consistent with the established safety profiles of the individual compounds and a metastatic NSCLC population,” they wrote. “The RELAY regimen is therefore a viable new treatment option for the initial treatment of patients with metastatic EGFR-mutated NSCLC.”
The RELAY trial was funded by Eli Lilly. The investigators reported additional relationships with AstraZeneca, Bristol-Myers Squibb, Novartis, and others.
SOURCE: Nakagawa K et al. Lancet Oncol. 2019 Oct 4. doi: 10.1016/S1470-2045(19)30634-5.
The study by Nakagawa et al. suggests that epidermal growth factor receptor tyrosine kinase inhibitors may feasibly be combined with other drugs, but concerns remain about the infrequency of complete responses, which were uncommon in this trial, at 1%, and only slightly higher, at 7%, in a previous, similar trial that involved the addition of bevacizumab to erlotinib (Lancet Oncol. 2019 Apr 8. doi: 10.1016/S1470-2045(19)30035-X). A lack of complete responses may be caused by vascular endothelial growth factor inhibition, which reduces tumor burden, but also may increase risks of tumor invasion, metastases, hypoxia, and other malignant processes.
Hypothetically, multitargeted receptor tyrosine kinases, such as cabozantinib or foretinib, could counteract the above drawbacks, but this remains to be seen. In the meantime, investigators should aim to design trials based on preclinical data instead of empirical reasoning. Despite the complexity of the molecular pathophysiology involved, research is bringing us closer to a clear understanding of the network of relationships between pathways, which could ultimately enable effective use of available combinations.
Rafael Rosell, MD, PhD, and Carlos Pedraz-Valdunciel are with Germans Trias i Pujol Research Institute and Hospital and the Universitat Autónoma de Barcelona. Both authors declared no competing interests. Their remarks are adapted from an accompanying editorial (Lancet Oncol. 2019 Oct 4. doi: 10.1016/S1470-2045(19)30636-9).
The study by Nakagawa et al. suggests that epidermal growth factor receptor tyrosine kinase inhibitors may feasibly be combined with other drugs, but concerns remain about the infrequency of complete responses, which were uncommon in this trial, at 1%, and only slightly higher, at 7%, in a previous, similar trial that involved the addition of bevacizumab to erlotinib (Lancet Oncol. 2019 Apr 8. doi: 10.1016/S1470-2045(19)30035-X). A lack of complete responses may be caused by vascular endothelial growth factor inhibition, which reduces tumor burden, but also may increase risks of tumor invasion, metastases, hypoxia, and other malignant processes.
Hypothetically, multitargeted receptor tyrosine kinases, such as cabozantinib or foretinib, could counteract the above drawbacks, but this remains to be seen. In the meantime, investigators should aim to design trials based on preclinical data instead of empirical reasoning. Despite the complexity of the molecular pathophysiology involved, research is bringing us closer to a clear understanding of the network of relationships between pathways, which could ultimately enable effective use of available combinations.
Rafael Rosell, MD, PhD, and Carlos Pedraz-Valdunciel are with Germans Trias i Pujol Research Institute and Hospital and the Universitat Autónoma de Barcelona. Both authors declared no competing interests. Their remarks are adapted from an accompanying editorial (Lancet Oncol. 2019 Oct 4. doi: 10.1016/S1470-2045(19)30636-9).
The study by Nakagawa et al. suggests that epidermal growth factor receptor tyrosine kinase inhibitors may feasibly be combined with other drugs, but concerns remain about the infrequency of complete responses, which were uncommon in this trial, at 1%, and only slightly higher, at 7%, in a previous, similar trial that involved the addition of bevacizumab to erlotinib (Lancet Oncol. 2019 Apr 8. doi: 10.1016/S1470-2045(19)30035-X). A lack of complete responses may be caused by vascular endothelial growth factor inhibition, which reduces tumor burden, but also may increase risks of tumor invasion, metastases, hypoxia, and other malignant processes.
Hypothetically, multitargeted receptor tyrosine kinases, such as cabozantinib or foretinib, could counteract the above drawbacks, but this remains to be seen. In the meantime, investigators should aim to design trials based on preclinical data instead of empirical reasoning. Despite the complexity of the molecular pathophysiology involved, research is bringing us closer to a clear understanding of the network of relationships between pathways, which could ultimately enable effective use of available combinations.
Rafael Rosell, MD, PhD, and Carlos Pedraz-Valdunciel are with Germans Trias i Pujol Research Institute and Hospital and the Universitat Autónoma de Barcelona. Both authors declared no competing interests. Their remarks are adapted from an accompanying editorial (Lancet Oncol. 2019 Oct 4. doi: 10.1016/S1470-2045(19)30636-9).
For patients with epidermal growth factor receptor–mutated non–small cell lung cancer (NSCLC), adding the vascular endothelial growth factor receptor 2 (VEGFR-2) inhibitor ramucirumab to standard erlotinib therapy may extend progression-free survival, based on results from the phase 3 RELAY trial.
Considering the acceptable safety profile, this dual regimen should be considered for first-line treatment of epidermal growth factor receptor (EGFR)–mutated disease, according to lead author Kazuhiko Nakagawa, MD, PhD, of the Kindai University faculty of medicine in Osaka, Japan, and colleagues.
Dual blockade of the EGFR and VEGF pathways is supported by previous studies which pointed to efficacy among EGFR-mutated subgroups, the investigators explained in Lancet Oncology, noting that ramucirumab appeared to be the best candidate for VEGF pathway inhibition. “Ramucirumab, a human monoclonal IgG1 antibody, selectively targets VEGFR-2, thereby blocking signaling mediated by VEGF-A, VEGF-C, and VEGF-D in NSCLC,” the investigators wrote. “Therefore, ramucirumab has the potential for broader antitumor activity than inhibitors of VEGF-A.”
The trial involved 449 patients with stage IV NSCLC and an EGFR exon 21 substitution or exon 19 deletion. Patients were randomized in a 1:1 ratio to receive either erlotinib (150 mg/day) plus placebo, or erlotinib plus ramucirumab (10 mg/kg every 2 weeks). The primary endpoint was progression-free survival. Secondary endpoints included safety and toxicity, overall survival, and various measures of response.
After a median follow-up of 20.7 months, the addition of ramucirumab was associated with a significantly better median progression-free survival, at 19.4 months, compared with 12.4 months among patients who received erlotinib alone, which translates to a hazard ratio of 0.59 (P less than .0001). In each cohort, 1% of patients achieved a complete response. Partial responses were also highly similar at 75% for ramucirumab versus 74% for placebo.
Turning to safety, ramucirumab was associated with more safety concerns, including a higher rate of grade 3-4 treatment-emergent adverse events (72% vs. 54%), most often hypertension. Serious adverse events were also more frequent with ramucirumab at a rate of 29%, compared with 21% among those who received erlotinib alone.
Despite these differences, the investigators concluded that the dual regimen still offers acceptable tolerability. “Safety was consistent with the established safety profiles of the individual compounds and a metastatic NSCLC population,” they wrote. “The RELAY regimen is therefore a viable new treatment option for the initial treatment of patients with metastatic EGFR-mutated NSCLC.”
The RELAY trial was funded by Eli Lilly. The investigators reported additional relationships with AstraZeneca, Bristol-Myers Squibb, Novartis, and others.
SOURCE: Nakagawa K et al. Lancet Oncol. 2019 Oct 4. doi: 10.1016/S1470-2045(19)30634-5.
For patients with epidermal growth factor receptor–mutated non–small cell lung cancer (NSCLC), adding the vascular endothelial growth factor receptor 2 (VEGFR-2) inhibitor ramucirumab to standard erlotinib therapy may extend progression-free survival, based on results from the phase 3 RELAY trial.
Considering the acceptable safety profile, this dual regimen should be considered for first-line treatment of epidermal growth factor receptor (EGFR)–mutated disease, according to lead author Kazuhiko Nakagawa, MD, PhD, of the Kindai University faculty of medicine in Osaka, Japan, and colleagues.
Dual blockade of the EGFR and VEGF pathways is supported by previous studies which pointed to efficacy among EGFR-mutated subgroups, the investigators explained in Lancet Oncology, noting that ramucirumab appeared to be the best candidate for VEGF pathway inhibition. “Ramucirumab, a human monoclonal IgG1 antibody, selectively targets VEGFR-2, thereby blocking signaling mediated by VEGF-A, VEGF-C, and VEGF-D in NSCLC,” the investigators wrote. “Therefore, ramucirumab has the potential for broader antitumor activity than inhibitors of VEGF-A.”
The trial involved 449 patients with stage IV NSCLC and an EGFR exon 21 substitution or exon 19 deletion. Patients were randomized in a 1:1 ratio to receive either erlotinib (150 mg/day) plus placebo, or erlotinib plus ramucirumab (10 mg/kg every 2 weeks). The primary endpoint was progression-free survival. Secondary endpoints included safety and toxicity, overall survival, and various measures of response.
After a median follow-up of 20.7 months, the addition of ramucirumab was associated with a significantly better median progression-free survival, at 19.4 months, compared with 12.4 months among patients who received erlotinib alone, which translates to a hazard ratio of 0.59 (P less than .0001). In each cohort, 1% of patients achieved a complete response. Partial responses were also highly similar at 75% for ramucirumab versus 74% for placebo.
Turning to safety, ramucirumab was associated with more safety concerns, including a higher rate of grade 3-4 treatment-emergent adverse events (72% vs. 54%), most often hypertension. Serious adverse events were also more frequent with ramucirumab at a rate of 29%, compared with 21% among those who received erlotinib alone.
Despite these differences, the investigators concluded that the dual regimen still offers acceptable tolerability. “Safety was consistent with the established safety profiles of the individual compounds and a metastatic NSCLC population,” they wrote. “The RELAY regimen is therefore a viable new treatment option for the initial treatment of patients with metastatic EGFR-mutated NSCLC.”
The RELAY trial was funded by Eli Lilly. The investigators reported additional relationships with AstraZeneca, Bristol-Myers Squibb, Novartis, and others.
SOURCE: Nakagawa K et al. Lancet Oncol. 2019 Oct 4. doi: 10.1016/S1470-2045(19)30634-5.
FROM LANCET ONCOLOGY
Etokimab trial highlights IL-33 targeting as potential new AD therapy
Patients with moderate to severe atopic dermatitis who received the immunoglobulin G1 anti–interleukin-33 monoclonal antibody etokimab showed significant disease improvement 29 days after receiving a single systemic administration, according to results from a recent proof-of-concept, phase IIa study.
“Most of the focus for IL-33 pathway inhibition has been on the type 2 cytokine axis because of significant supporting genetic and functional data in both human and murine systems,” Yi-Ling Chen, a DPhil student at the University of Oxford (England), and colleagues wrote in Science Translational Medicine. “Our findings might also provide a further dimension to explain how etokimab, by inhibiting IL-33, could provide such a rapid and persistent clinical benefit as described in this report.”
In their study, 12 patients with moderate to severe AD received etokimab in a single intravenous dose of 300 mg. Prior to receiving etokimab, patients received a placebo dose and then underwent saline or house dust mite intraepidermal challenge, with researchers obtaining blister samples 1 day after challenge following placebo administration and again after patients received etokimab.
At 29 days, 83% of patients reached rapid and sustained clinical benefit under Eczema Area and Severity Index (EASI ) 50 and 33% in EASI 75, including peripheral eosinophil reduction. There also was significant reduction in skin neutrophil infiltration after patients received etokimab and then house dust mite challenge, compared with after they received placebo, .
“These findings open new therapeutic possible applications of etokimab to sterile neutrophilic disease as well as other inflammatory diseases with a clear neutrophilic involvement such as neutrophilic asthma,” the researchers said. “This first-in-class experimental medicine study has shown that in vivo in human tissue IL-33 has key upstream effects which broadly influence different and relevant inflammatory cascades and thus widen the potential of this treatment to a larger than anticipated group of diseases.
“Although excellent previous studies have demonstrated in vitro and in murine models that IL-33 is involved in neutrophil migration, the translation here to human tissue immunology shows that IL-33 plays a dominant role and effects are in part likely to be mediated instead by CXCR1,” concluded Ms. Chen and colleagues. “Therefore, specific IL-33 therapeutic intervention is not targeting a redundant system and is worthy of further investigation.”
This study was funded by AnaptysBio, which was responsible for the conception and design of the study. AnaptysBio also helped in part to analyze and interpret the data from the study, and approved it for submission. Dr. Ogg reported having served on an advisory board, as a consultant, or holds equity in Eli Lilly, Novartis, Janssen, Orbit Discovery, and UCB Pharma. Ms. Marquette and Dr. Londei reported being employees of AnaptysBio.
SOURCE: Chen Y-L et al. Sci Transl Med. 2019. doi: 10.1126/scitranslmed.aax2945.
Patients with moderate to severe atopic dermatitis who received the immunoglobulin G1 anti–interleukin-33 monoclonal antibody etokimab showed significant disease improvement 29 days after receiving a single systemic administration, according to results from a recent proof-of-concept, phase IIa study.
“Most of the focus for IL-33 pathway inhibition has been on the type 2 cytokine axis because of significant supporting genetic and functional data in both human and murine systems,” Yi-Ling Chen, a DPhil student at the University of Oxford (England), and colleagues wrote in Science Translational Medicine. “Our findings might also provide a further dimension to explain how etokimab, by inhibiting IL-33, could provide such a rapid and persistent clinical benefit as described in this report.”
In their study, 12 patients with moderate to severe AD received etokimab in a single intravenous dose of 300 mg. Prior to receiving etokimab, patients received a placebo dose and then underwent saline or house dust mite intraepidermal challenge, with researchers obtaining blister samples 1 day after challenge following placebo administration and again after patients received etokimab.
At 29 days, 83% of patients reached rapid and sustained clinical benefit under Eczema Area and Severity Index (EASI ) 50 and 33% in EASI 75, including peripheral eosinophil reduction. There also was significant reduction in skin neutrophil infiltration after patients received etokimab and then house dust mite challenge, compared with after they received placebo, .
“These findings open new therapeutic possible applications of etokimab to sterile neutrophilic disease as well as other inflammatory diseases with a clear neutrophilic involvement such as neutrophilic asthma,” the researchers said. “This first-in-class experimental medicine study has shown that in vivo in human tissue IL-33 has key upstream effects which broadly influence different and relevant inflammatory cascades and thus widen the potential of this treatment to a larger than anticipated group of diseases.
“Although excellent previous studies have demonstrated in vitro and in murine models that IL-33 is involved in neutrophil migration, the translation here to human tissue immunology shows that IL-33 plays a dominant role and effects are in part likely to be mediated instead by CXCR1,” concluded Ms. Chen and colleagues. “Therefore, specific IL-33 therapeutic intervention is not targeting a redundant system and is worthy of further investigation.”
This study was funded by AnaptysBio, which was responsible for the conception and design of the study. AnaptysBio also helped in part to analyze and interpret the data from the study, and approved it for submission. Dr. Ogg reported having served on an advisory board, as a consultant, or holds equity in Eli Lilly, Novartis, Janssen, Orbit Discovery, and UCB Pharma. Ms. Marquette and Dr. Londei reported being employees of AnaptysBio.
SOURCE: Chen Y-L et al. Sci Transl Med. 2019. doi: 10.1126/scitranslmed.aax2945.
Patients with moderate to severe atopic dermatitis who received the immunoglobulin G1 anti–interleukin-33 monoclonal antibody etokimab showed significant disease improvement 29 days after receiving a single systemic administration, according to results from a recent proof-of-concept, phase IIa study.
“Most of the focus for IL-33 pathway inhibition has been on the type 2 cytokine axis because of significant supporting genetic and functional data in both human and murine systems,” Yi-Ling Chen, a DPhil student at the University of Oxford (England), and colleagues wrote in Science Translational Medicine. “Our findings might also provide a further dimension to explain how etokimab, by inhibiting IL-33, could provide such a rapid and persistent clinical benefit as described in this report.”
In their study, 12 patients with moderate to severe AD received etokimab in a single intravenous dose of 300 mg. Prior to receiving etokimab, patients received a placebo dose and then underwent saline or house dust mite intraepidermal challenge, with researchers obtaining blister samples 1 day after challenge following placebo administration and again after patients received etokimab.
At 29 days, 83% of patients reached rapid and sustained clinical benefit under Eczema Area and Severity Index (EASI ) 50 and 33% in EASI 75, including peripheral eosinophil reduction. There also was significant reduction in skin neutrophil infiltration after patients received etokimab and then house dust mite challenge, compared with after they received placebo, .
“These findings open new therapeutic possible applications of etokimab to sterile neutrophilic disease as well as other inflammatory diseases with a clear neutrophilic involvement such as neutrophilic asthma,” the researchers said. “This first-in-class experimental medicine study has shown that in vivo in human tissue IL-33 has key upstream effects which broadly influence different and relevant inflammatory cascades and thus widen the potential of this treatment to a larger than anticipated group of diseases.
“Although excellent previous studies have demonstrated in vitro and in murine models that IL-33 is involved in neutrophil migration, the translation here to human tissue immunology shows that IL-33 plays a dominant role and effects are in part likely to be mediated instead by CXCR1,” concluded Ms. Chen and colleagues. “Therefore, specific IL-33 therapeutic intervention is not targeting a redundant system and is worthy of further investigation.”
This study was funded by AnaptysBio, which was responsible for the conception and design of the study. AnaptysBio also helped in part to analyze and interpret the data from the study, and approved it for submission. Dr. Ogg reported having served on an advisory board, as a consultant, or holds equity in Eli Lilly, Novartis, Janssen, Orbit Discovery, and UCB Pharma. Ms. Marquette and Dr. Londei reported being employees of AnaptysBio.
SOURCE: Chen Y-L et al. Sci Transl Med. 2019. doi: 10.1126/scitranslmed.aax2945.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point:
Major finding: At 29 days, 83% of patients reached rapid and sustained clinical benefit under Eczema Area and Severity Index (EASI) 50 and 33% in EASI 75, including peripheral eosinophil reduction. There also was significant reduction in skin neutrophil infiltration after patients received etokimab and then house dust mite challenge, compared with the response after they received placebo.
Study details: An analysis of 12 patients with moderate to severe atopic dermatitis who received a single 300 mg IV dose of etokimab, an IgG1 anti–IL-33 monoclonal antibody.
Disclosures: This study was funded by AnaptysBio, which was responsible for the conception and design of the study. AnaptysBio also helped in part to analyze and interpret the data from the study, and approved it for submission. Dr. Ogg reported having served on an advisory board, as a consultant, or holds equity in Eli Lilly, Novartis, Janssen, Orbit Discovery, and UCB Pharma. Ms. Marquette and Dr. Londei reported being employees of AnaptysBio.
Source: Chen Y-L et al. Sci Transl Med. 2019. doi: 10.1126/scitranslmed.aax2945.