User login
Adolescent lung inflammation may trigger later MS
STOCKHOLM – Scott Montgomery, PhD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
This is speculative, he readily acknowledged, but it is a hypothesis supported by multiple lines of evidence provided by separate Swedish national health care registry studies he has led that showed associations between pneumonia or infectious mononucleosis occurring in early adolescence and increased risk of later MS.
These findings are consistent with the well-established observations that two other causes of lung irritation – cigarette smoking and exposure to organic solvents – are also linked to increased risk of MS (Neurology. 2018 Jul 31;91[5]:e455-62), noted Dr. Montgomery, head of the clinical epidemiology research group at Örebro (Sweden) University.
Moreover, he and his coinvestigators also found in yet another Swedish national registry cohort study that one concussion during adolescence was independently associated with a statistically significant 1.22-fold increased risk of later MS, while two or more were linked to a 2.33-fold increased risk. In contrast, concussions occurring before age 11 years were not associated with any increased risk of MS, which suggests an age-defined period of susceptibility (Ann Neurol. 2017 Oct;82[4]:554-61).
“There seems to be greater brain resilience in childhood as compared to adolescence,” Dr. Montgomery commented.
The new Swedish registry pneumonia study included 6,109 Swedish MS patients and 49,479 controls matched for age, gender, and locale. In an analysis adjusted for education level and history of infectious mononucleosis, history of having pneumonia at age 11-15 years was independently associated with a 2.8-fold increased risk of subsequent MS. Pneumonia occurring at age 16-20 years was associated with a more modest 1.38-fold increased risk, which did not achieve statistical significance, while pneumonia up to age 5 years or at age 6-10 years conferred no increased risk. The investigators restricted their analysis to cases of pneumonia occurring up to age 20 years because that is younger than the typical age of MS onset. The age restriction sidestepped the potential for confounding by reverse causation since it is known that pneumonia occurs with increased frequency in patients with MS.
Because MS patients also have an increased risk of urinary tract infections, Dr. Montgomery and coinvestigators also analyzed the same pediatric data set for UTI rates broken down by 5-year age groups. Rates were similar in individuals who later developed MS and in controls, which suggests that the observed increase in MS risk associated with pneumonia in early adolescence was not an expression of an MS prodromal illness, he explained.
The investigators focused on pneumonia in childhood and adolescence as a potential trigger for MS because pneumonia results in more profound and prolonged inflammation than do other common respiratory illnesses. For example, pneumonia has been shown to be linked to increased risks of cardiovascular disease and chronic kidney disease for up to 5 years after the infection.
Developmentally, age 11-15 years is a period defined by peripubertal reorganization and synaptogenesis, while synaptic pruning and axonal myelination are on the agenda at age 16-20 years, Dr. Montgomery observed.
The study of infectious mononucleosis as a potential risk factor for MS included 4,527 Swedish MS patients and 3.2 million controls, all born during 1970-2000 and followed until 2014. In this analysis, infectious mononucleosis occurring at age 11-15 years was associated with the greatest risk of subsequent MS, with an associated 3.47-fold greater risk of the neurologic disease versus that seen in patients who did not have infectious mononucleosis at age 11-15 years
“It does look like a causal association between Epstein-Barr virus infection and subsequent MS,” according to Dr. Montgomery.
He noted that a plausible mechanism by which lung inflammation could predispose future MS has been put forth by German investigators. Using an animal model, they demonstrated that autoreactive T cells are prepared in bronchus-associated lymphoid tissue and attain a migratory profile allowing them to cross the blood-brain barrier and induce CNS autoimmune disease (Nature. 2012 Aug 30;488[7413]:675-9).
All of this, as Dr. Montgomery emphasized, is speculative at this point in regard to MS pathogenesis. What is not speculative, he continued, is the solid evidence that infection-related mortality after diagnosis of MS has gone down substantially in the current era of newer disease-modifying treatments, as he and his coinvestigators have demonstrated (Neurology. 2017 Aug 8;89[6]:555-62).
“People with MS, compared to the general population, are still at increased risk, but not nearly as much as the infection-related mortality risk present back in the 1960s-80s. So things have improved somewhat,” Dr. Montgomery said.
Which MS patients are at increased risk for mortality caused by infection? His Swedish national registry research demonstrates that the risk is essentially confined to patients with secondary or primary progressive MS or an Expanded Disability Status Scale score of 6 or more.
Another new study he presented at the meeting focused on the types of infections that are more common in a contemporary MS population than in MS-free individuals. This Swedish national cohort study included 6,602 patients diagnosed with MS during 2008-2016 and 61,828 age-, sex-, and location-matched controls. Infections serious enough to have resulted in hospitalization occurred 2.59 times more frequently in the MS population. The risk of meningitis and encephalitis was increased 6.16-fold, opportunistic infections were 2.72-fold more frequent, the risk of urinary tract and kidney infections was increased 2.44-fold, herpes virus infections were increased 2.32-fold, and the combined rate of pneumonia and influenza was roughly double that seen in the matched general population.
Dr. Montgomery reported receiving research funding from F. Hoffmann–La Roche, Novartis, and AstraZeneca and serving on an advisory board for IQVIA.
SOURCE: Montgomery S. ECTRIMS 2019, Abstract 270.
STOCKHOLM – Scott Montgomery, PhD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
This is speculative, he readily acknowledged, but it is a hypothesis supported by multiple lines of evidence provided by separate Swedish national health care registry studies he has led that showed associations between pneumonia or infectious mononucleosis occurring in early adolescence and increased risk of later MS.
These findings are consistent with the well-established observations that two other causes of lung irritation – cigarette smoking and exposure to organic solvents – are also linked to increased risk of MS (Neurology. 2018 Jul 31;91[5]:e455-62), noted Dr. Montgomery, head of the clinical epidemiology research group at Örebro (Sweden) University.
Moreover, he and his coinvestigators also found in yet another Swedish national registry cohort study that one concussion during adolescence was independently associated with a statistically significant 1.22-fold increased risk of later MS, while two or more were linked to a 2.33-fold increased risk. In contrast, concussions occurring before age 11 years were not associated with any increased risk of MS, which suggests an age-defined period of susceptibility (Ann Neurol. 2017 Oct;82[4]:554-61).
“There seems to be greater brain resilience in childhood as compared to adolescence,” Dr. Montgomery commented.
The new Swedish registry pneumonia study included 6,109 Swedish MS patients and 49,479 controls matched for age, gender, and locale. In an analysis adjusted for education level and history of infectious mononucleosis, history of having pneumonia at age 11-15 years was independently associated with a 2.8-fold increased risk of subsequent MS. Pneumonia occurring at age 16-20 years was associated with a more modest 1.38-fold increased risk, which did not achieve statistical significance, while pneumonia up to age 5 years or at age 6-10 years conferred no increased risk. The investigators restricted their analysis to cases of pneumonia occurring up to age 20 years because that is younger than the typical age of MS onset. The age restriction sidestepped the potential for confounding by reverse causation since it is known that pneumonia occurs with increased frequency in patients with MS.
Because MS patients also have an increased risk of urinary tract infections, Dr. Montgomery and coinvestigators also analyzed the same pediatric data set for UTI rates broken down by 5-year age groups. Rates were similar in individuals who later developed MS and in controls, which suggests that the observed increase in MS risk associated with pneumonia in early adolescence was not an expression of an MS prodromal illness, he explained.
The investigators focused on pneumonia in childhood and adolescence as a potential trigger for MS because pneumonia results in more profound and prolonged inflammation than do other common respiratory illnesses. For example, pneumonia has been shown to be linked to increased risks of cardiovascular disease and chronic kidney disease for up to 5 years after the infection.
Developmentally, age 11-15 years is a period defined by peripubertal reorganization and synaptogenesis, while synaptic pruning and axonal myelination are on the agenda at age 16-20 years, Dr. Montgomery observed.
The study of infectious mononucleosis as a potential risk factor for MS included 4,527 Swedish MS patients and 3.2 million controls, all born during 1970-2000 and followed until 2014. In this analysis, infectious mononucleosis occurring at age 11-15 years was associated with the greatest risk of subsequent MS, with an associated 3.47-fold greater risk of the neurologic disease versus that seen in patients who did not have infectious mononucleosis at age 11-15 years
“It does look like a causal association between Epstein-Barr virus infection and subsequent MS,” according to Dr. Montgomery.
He noted that a plausible mechanism by which lung inflammation could predispose future MS has been put forth by German investigators. Using an animal model, they demonstrated that autoreactive T cells are prepared in bronchus-associated lymphoid tissue and attain a migratory profile allowing them to cross the blood-brain barrier and induce CNS autoimmune disease (Nature. 2012 Aug 30;488[7413]:675-9).
All of this, as Dr. Montgomery emphasized, is speculative at this point in regard to MS pathogenesis. What is not speculative, he continued, is the solid evidence that infection-related mortality after diagnosis of MS has gone down substantially in the current era of newer disease-modifying treatments, as he and his coinvestigators have demonstrated (Neurology. 2017 Aug 8;89[6]:555-62).
“People with MS, compared to the general population, are still at increased risk, but not nearly as much as the infection-related mortality risk present back in the 1960s-80s. So things have improved somewhat,” Dr. Montgomery said.
Which MS patients are at increased risk for mortality caused by infection? His Swedish national registry research demonstrates that the risk is essentially confined to patients with secondary or primary progressive MS or an Expanded Disability Status Scale score of 6 or more.
Another new study he presented at the meeting focused on the types of infections that are more common in a contemporary MS population than in MS-free individuals. This Swedish national cohort study included 6,602 patients diagnosed with MS during 2008-2016 and 61,828 age-, sex-, and location-matched controls. Infections serious enough to have resulted in hospitalization occurred 2.59 times more frequently in the MS population. The risk of meningitis and encephalitis was increased 6.16-fold, opportunistic infections were 2.72-fold more frequent, the risk of urinary tract and kidney infections was increased 2.44-fold, herpes virus infections were increased 2.32-fold, and the combined rate of pneumonia and influenza was roughly double that seen in the matched general population.
Dr. Montgomery reported receiving research funding from F. Hoffmann–La Roche, Novartis, and AstraZeneca and serving on an advisory board for IQVIA.
SOURCE: Montgomery S. ECTRIMS 2019, Abstract 270.
STOCKHOLM – Scott Montgomery, PhD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
This is speculative, he readily acknowledged, but it is a hypothesis supported by multiple lines of evidence provided by separate Swedish national health care registry studies he has led that showed associations between pneumonia or infectious mononucleosis occurring in early adolescence and increased risk of later MS.
These findings are consistent with the well-established observations that two other causes of lung irritation – cigarette smoking and exposure to organic solvents – are also linked to increased risk of MS (Neurology. 2018 Jul 31;91[5]:e455-62), noted Dr. Montgomery, head of the clinical epidemiology research group at Örebro (Sweden) University.
Moreover, he and his coinvestigators also found in yet another Swedish national registry cohort study that one concussion during adolescence was independently associated with a statistically significant 1.22-fold increased risk of later MS, while two or more were linked to a 2.33-fold increased risk. In contrast, concussions occurring before age 11 years were not associated with any increased risk of MS, which suggests an age-defined period of susceptibility (Ann Neurol. 2017 Oct;82[4]:554-61).
“There seems to be greater brain resilience in childhood as compared to adolescence,” Dr. Montgomery commented.
The new Swedish registry pneumonia study included 6,109 Swedish MS patients and 49,479 controls matched for age, gender, and locale. In an analysis adjusted for education level and history of infectious mononucleosis, history of having pneumonia at age 11-15 years was independently associated with a 2.8-fold increased risk of subsequent MS. Pneumonia occurring at age 16-20 years was associated with a more modest 1.38-fold increased risk, which did not achieve statistical significance, while pneumonia up to age 5 years or at age 6-10 years conferred no increased risk. The investigators restricted their analysis to cases of pneumonia occurring up to age 20 years because that is younger than the typical age of MS onset. The age restriction sidestepped the potential for confounding by reverse causation since it is known that pneumonia occurs with increased frequency in patients with MS.
Because MS patients also have an increased risk of urinary tract infections, Dr. Montgomery and coinvestigators also analyzed the same pediatric data set for UTI rates broken down by 5-year age groups. Rates were similar in individuals who later developed MS and in controls, which suggests that the observed increase in MS risk associated with pneumonia in early adolescence was not an expression of an MS prodromal illness, he explained.
The investigators focused on pneumonia in childhood and adolescence as a potential trigger for MS because pneumonia results in more profound and prolonged inflammation than do other common respiratory illnesses. For example, pneumonia has been shown to be linked to increased risks of cardiovascular disease and chronic kidney disease for up to 5 years after the infection.
Developmentally, age 11-15 years is a period defined by peripubertal reorganization and synaptogenesis, while synaptic pruning and axonal myelination are on the agenda at age 16-20 years, Dr. Montgomery observed.
The study of infectious mononucleosis as a potential risk factor for MS included 4,527 Swedish MS patients and 3.2 million controls, all born during 1970-2000 and followed until 2014. In this analysis, infectious mononucleosis occurring at age 11-15 years was associated with the greatest risk of subsequent MS, with an associated 3.47-fold greater risk of the neurologic disease versus that seen in patients who did not have infectious mononucleosis at age 11-15 years
“It does look like a causal association between Epstein-Barr virus infection and subsequent MS,” according to Dr. Montgomery.
He noted that a plausible mechanism by which lung inflammation could predispose future MS has been put forth by German investigators. Using an animal model, they demonstrated that autoreactive T cells are prepared in bronchus-associated lymphoid tissue and attain a migratory profile allowing them to cross the blood-brain barrier and induce CNS autoimmune disease (Nature. 2012 Aug 30;488[7413]:675-9).
All of this, as Dr. Montgomery emphasized, is speculative at this point in regard to MS pathogenesis. What is not speculative, he continued, is the solid evidence that infection-related mortality after diagnosis of MS has gone down substantially in the current era of newer disease-modifying treatments, as he and his coinvestigators have demonstrated (Neurology. 2017 Aug 8;89[6]:555-62).
“People with MS, compared to the general population, are still at increased risk, but not nearly as much as the infection-related mortality risk present back in the 1960s-80s. So things have improved somewhat,” Dr. Montgomery said.
Which MS patients are at increased risk for mortality caused by infection? His Swedish national registry research demonstrates that the risk is essentially confined to patients with secondary or primary progressive MS or an Expanded Disability Status Scale score of 6 or more.
Another new study he presented at the meeting focused on the types of infections that are more common in a contemporary MS population than in MS-free individuals. This Swedish national cohort study included 6,602 patients diagnosed with MS during 2008-2016 and 61,828 age-, sex-, and location-matched controls. Infections serious enough to have resulted in hospitalization occurred 2.59 times more frequently in the MS population. The risk of meningitis and encephalitis was increased 6.16-fold, opportunistic infections were 2.72-fold more frequent, the risk of urinary tract and kidney infections was increased 2.44-fold, herpes virus infections were increased 2.32-fold, and the combined rate of pneumonia and influenza was roughly double that seen in the matched general population.
Dr. Montgomery reported receiving research funding from F. Hoffmann–La Roche, Novartis, and AstraZeneca and serving on an advisory board for IQVIA.
SOURCE: Montgomery S. ECTRIMS 2019, Abstract 270.
REPORTING FROM ECTRIMS 2019
Dermatologist who organized PA group faces backlash
Whether a board member for the American Academy of Dermatology and the AAD Association (AAD/A) violated his fiduciary duties by launching an organization that offered board certification to physician assistants (PAs) will soon be decided by AAD members.
In mid-October, AAD President George J. Hruza, MD, said Dr. Dinehart violated his duties when he became sole organizer of the American Board of Dermatology Physician Assistants (ABDPA), an organization that planned to offer board certification to PAs who work with dermatologists, according to a message from Dr. Hruza to members. The use of the words “American Board of Dermatology” in the ABDPA name created confusion to patients and threatened to undermine the value of ABD certification held by AAD/A fellows, Dr. Hruza said.
“Dr. Dinehart’s action to incorporate and organize the for-profit entity ABDPA, LLC., is in direct contradiction to the AAD’s Truth in Advertising and Professional Disclosure policy that states that practitioners should not advertise that they are board certified unless they are certified by an ABMS/AOA [American Board of Medical Specialties or American Osteopathic Association] medical board, such as the American Board of Dermatology,” Dr. Hruza said in an interview with Dermatology News. “This for-profit venture would enable physician assistants to advertise themselves as board certified. The [AAD/A] board in its unanimous decision deemed that the new ABDPA was set up to potentially mislead patients into thinking that physician assistants with this certification would have training and experience equivalent to an ABD-certified dermatologist. This is contrary to the AAD/A’s position on this matter. As such, Dr. Dinehart’s involvement in forming and organizing the ABDPA violated his fiduciary duty to act in the best interests of the AAD/A and to abide by AAD/A policies.”
In a letter to AAD members, Dr. Dinehart called the removal vote a “drastic measure” and said he has done nothing to justify dismissal from the AAD/A board. The ABDPA was intended to improve patient care by establishing certain educational, training, and professional standards for the growing number of physician assistants in dermatology, Dr. Dinehart wrote. That mission was not in conflict with AAD’s values, but rather, the ABDPA would have furthered AAD’s purpose “to promote the highest standards in allied health professionals and services as they relate to dermatology.”
After learning of the board’s concerns, Dr. Dinehart said he discontinued his relationship with the ABDPA and ensured its operations had ended out of respect for the academy. He believes the matter could have been handled much differently.
“I did not violate any bylaws,” Dr. Dinehart said in an interview with Dermatology News. “While I understand that there are certain members of the AAD that personally oppose the growing use of physician assistants in dermatology practices, it is a common practice among many AAD members, and it is not contrary to or detrimental to any of the purposes of the AAD.”
Dr. Dinehart contends he did not run afoul of the AAD/A’s position statement on Truth in Advertising and Professional Credential Disclosure because the statement pertains to AAD/A members and prohibits members from advertising board certifications by nonapproved boards. He emphasized that PAs are not members and said he would never represent or advertise a physician assistant as having a certification equivalent to a board-certified dermatologist or being capable of equivalent credentials.
In addition, he said his involvement with the new organization did not conflict with the AAD’s Practice of Dermatology: Protecting and Preserving Patient Safety and Quality Care position statement, which opposes the independent or unsupervised practice of dermatology procedures by nonphysicians.
“Neither the ADBPA nor the certification it intended to offer would have allowed physician assistants to engage in the independent or unsupervised practice of specific dermatology procedures, and any physician assistant who did so would be subject to the same repercussions that currently exist,” Dr. Dinehart said in the interview.
The ABDPA was formed legally at the end of September and announced its official launch on Oct. 7. The new organization immediately drew criticism from dermatologists and triggered an online petition that denounced the group and called for Dr Dinehart’s removal from the AAD/A board. The petition, started by an anonymous dermatologist, states Dr. Dinehart’s concurrent relationships with the AAD and the ABDPA represent a major conflict of interest. As of Oct. 24, the petition had collected 2,496 signatures.
Monica Madray, MD, a dermatologist based in Georgetown, Tex., who signed the petition, said the ABDPA further muddied the waters in an already confusing world of “providers” and non–dermatology boarded physicians practicing and advertising themselves as dermatologists.
“Patients truly don’t know the difference in training between physicians and midlevels, and by ‘board certifying’ PAs in dermatology, it gets much more confusing,” Dr. Madray said in an interview. “I also think there was a huge financial conflict of interest for Dr. Dinehart to start and run this board as he oversees many midlevels. Frankly, it didn’t pass the sniff test.”
Vivian Bucay, MD, a dermatologist based in San Antonio who signed the petition, said that, if Dr. Dinehart was interested in starting an organization such as the ABDPA, he should have first presented the idea to the AAD/A board and asked for feedback.
“This came [to light] after a press release,” Dr. Bucay said in an interview. “I don’t have an issue with trying to improve and set the bar for what dermatology physician assistants should do. It’s the manner in which it was gone about. In this day and age, we have to disclose any potential conflicts of interest. If we all have to do that as members of the American Society of Dermatologic Surgery or members of the AAD when presenting for continuing medical education, then why would there not be the same standard for a board member to disclose a conflict of interest?”
Dr. Dinehart for his part said the board never gave him an opportunity to respond to its accusations or share his side of the story before initiating the removal vote.
“The board should not have been pressured by a vocal minority of the membership, rushed to judgment, and expedited a special removal vote less than 2 weeks after the board claims it first became aware of the formation of ABDPA,” he said in the interview. “The board should have ... evaluated the alleged conflict and provided me a reasonable opportunity to cure the alleged conflict as provided by the AAD/A’s conflict of interest rules. Had the board allowed me that opportunity, as required, this whole situation could have been resolved.”
Dr. Hruza, however, said the board’s actions came after reviewing evidence with Dr. Dinehart in private, which allowed him to make a statement and provided him a chance to answer questions from the board. He was also afforded several opportunities to resign, which he declined, Dr. Hruza said in the interview.
“I regret that we have been put in this difficult position and that the question must be put to the membership for a painful vote,” Dr. Hruza said. “The academy has a lot of important work to do to advocate for our members and our patients. This issue has been an unfortunate distraction from that important work.”
Daniel M. Siegel, MD, a New York–based dermatologist and former AAD president, said in an interview with Dermatology News that he plans to vote to keep Dr. Dinehart on the board. Dr. Dinehart has been a dedicated advocate for the specialty for more than 30 years and his recent misstep does not rise to the level of board removal, Dr. Siegel said. A social media environment that easily enables people to get fired up about almost any subject and quickly stoke anger in others contributed to overreaction of the situation, he added.
“In retrospect, it was not in the best interests of the AAD, but it was nothing criminal,” Dr. Siegel said in the interview. “He had a business idea, it got a lot negative feedback, and he did the right thing about it. Taking such an aggressive punitive approach for a transgression that was fairly minor is a bad precedent to set.”
Voting on Dr. Dinehart’s position opened on Oct. 21 and will close on Oct. 29. A removal decision requires a two-thirds vote by at least 10% of the voting membership.
Whether a board member for the American Academy of Dermatology and the AAD Association (AAD/A) violated his fiduciary duties by launching an organization that offered board certification to physician assistants (PAs) will soon be decided by AAD members.
In mid-October, AAD President George J. Hruza, MD, said Dr. Dinehart violated his duties when he became sole organizer of the American Board of Dermatology Physician Assistants (ABDPA), an organization that planned to offer board certification to PAs who work with dermatologists, according to a message from Dr. Hruza to members. The use of the words “American Board of Dermatology” in the ABDPA name created confusion to patients and threatened to undermine the value of ABD certification held by AAD/A fellows, Dr. Hruza said.
“Dr. Dinehart’s action to incorporate and organize the for-profit entity ABDPA, LLC., is in direct contradiction to the AAD’s Truth in Advertising and Professional Disclosure policy that states that practitioners should not advertise that they are board certified unless they are certified by an ABMS/AOA [American Board of Medical Specialties or American Osteopathic Association] medical board, such as the American Board of Dermatology,” Dr. Hruza said in an interview with Dermatology News. “This for-profit venture would enable physician assistants to advertise themselves as board certified. The [AAD/A] board in its unanimous decision deemed that the new ABDPA was set up to potentially mislead patients into thinking that physician assistants with this certification would have training and experience equivalent to an ABD-certified dermatologist. This is contrary to the AAD/A’s position on this matter. As such, Dr. Dinehart’s involvement in forming and organizing the ABDPA violated his fiduciary duty to act in the best interests of the AAD/A and to abide by AAD/A policies.”
In a letter to AAD members, Dr. Dinehart called the removal vote a “drastic measure” and said he has done nothing to justify dismissal from the AAD/A board. The ABDPA was intended to improve patient care by establishing certain educational, training, and professional standards for the growing number of physician assistants in dermatology, Dr. Dinehart wrote. That mission was not in conflict with AAD’s values, but rather, the ABDPA would have furthered AAD’s purpose “to promote the highest standards in allied health professionals and services as they relate to dermatology.”
After learning of the board’s concerns, Dr. Dinehart said he discontinued his relationship with the ABDPA and ensured its operations had ended out of respect for the academy. He believes the matter could have been handled much differently.
“I did not violate any bylaws,” Dr. Dinehart said in an interview with Dermatology News. “While I understand that there are certain members of the AAD that personally oppose the growing use of physician assistants in dermatology practices, it is a common practice among many AAD members, and it is not contrary to or detrimental to any of the purposes of the AAD.”
Dr. Dinehart contends he did not run afoul of the AAD/A’s position statement on Truth in Advertising and Professional Credential Disclosure because the statement pertains to AAD/A members and prohibits members from advertising board certifications by nonapproved boards. He emphasized that PAs are not members and said he would never represent or advertise a physician assistant as having a certification equivalent to a board-certified dermatologist or being capable of equivalent credentials.
In addition, he said his involvement with the new organization did not conflict with the AAD’s Practice of Dermatology: Protecting and Preserving Patient Safety and Quality Care position statement, which opposes the independent or unsupervised practice of dermatology procedures by nonphysicians.
“Neither the ADBPA nor the certification it intended to offer would have allowed physician assistants to engage in the independent or unsupervised practice of specific dermatology procedures, and any physician assistant who did so would be subject to the same repercussions that currently exist,” Dr. Dinehart said in the interview.
The ABDPA was formed legally at the end of September and announced its official launch on Oct. 7. The new organization immediately drew criticism from dermatologists and triggered an online petition that denounced the group and called for Dr Dinehart’s removal from the AAD/A board. The petition, started by an anonymous dermatologist, states Dr. Dinehart’s concurrent relationships with the AAD and the ABDPA represent a major conflict of interest. As of Oct. 24, the petition had collected 2,496 signatures.
Monica Madray, MD, a dermatologist based in Georgetown, Tex., who signed the petition, said the ABDPA further muddied the waters in an already confusing world of “providers” and non–dermatology boarded physicians practicing and advertising themselves as dermatologists.
“Patients truly don’t know the difference in training between physicians and midlevels, and by ‘board certifying’ PAs in dermatology, it gets much more confusing,” Dr. Madray said in an interview. “I also think there was a huge financial conflict of interest for Dr. Dinehart to start and run this board as he oversees many midlevels. Frankly, it didn’t pass the sniff test.”
Vivian Bucay, MD, a dermatologist based in San Antonio who signed the petition, said that, if Dr. Dinehart was interested in starting an organization such as the ABDPA, he should have first presented the idea to the AAD/A board and asked for feedback.
“This came [to light] after a press release,” Dr. Bucay said in an interview. “I don’t have an issue with trying to improve and set the bar for what dermatology physician assistants should do. It’s the manner in which it was gone about. In this day and age, we have to disclose any potential conflicts of interest. If we all have to do that as members of the American Society of Dermatologic Surgery or members of the AAD when presenting for continuing medical education, then why would there not be the same standard for a board member to disclose a conflict of interest?”
Dr. Dinehart for his part said the board never gave him an opportunity to respond to its accusations or share his side of the story before initiating the removal vote.
“The board should not have been pressured by a vocal minority of the membership, rushed to judgment, and expedited a special removal vote less than 2 weeks after the board claims it first became aware of the formation of ABDPA,” he said in the interview. “The board should have ... evaluated the alleged conflict and provided me a reasonable opportunity to cure the alleged conflict as provided by the AAD/A’s conflict of interest rules. Had the board allowed me that opportunity, as required, this whole situation could have been resolved.”
Dr. Hruza, however, said the board’s actions came after reviewing evidence with Dr. Dinehart in private, which allowed him to make a statement and provided him a chance to answer questions from the board. He was also afforded several opportunities to resign, which he declined, Dr. Hruza said in the interview.
“I regret that we have been put in this difficult position and that the question must be put to the membership for a painful vote,” Dr. Hruza said. “The academy has a lot of important work to do to advocate for our members and our patients. This issue has been an unfortunate distraction from that important work.”
Daniel M. Siegel, MD, a New York–based dermatologist and former AAD president, said in an interview with Dermatology News that he plans to vote to keep Dr. Dinehart on the board. Dr. Dinehart has been a dedicated advocate for the specialty for more than 30 years and his recent misstep does not rise to the level of board removal, Dr. Siegel said. A social media environment that easily enables people to get fired up about almost any subject and quickly stoke anger in others contributed to overreaction of the situation, he added.
“In retrospect, it was not in the best interests of the AAD, but it was nothing criminal,” Dr. Siegel said in the interview. “He had a business idea, it got a lot negative feedback, and he did the right thing about it. Taking such an aggressive punitive approach for a transgression that was fairly minor is a bad precedent to set.”
Voting on Dr. Dinehart’s position opened on Oct. 21 and will close on Oct. 29. A removal decision requires a two-thirds vote by at least 10% of the voting membership.
Whether a board member for the American Academy of Dermatology and the AAD Association (AAD/A) violated his fiduciary duties by launching an organization that offered board certification to physician assistants (PAs) will soon be decided by AAD members.
In mid-October, AAD President George J. Hruza, MD, said Dr. Dinehart violated his duties when he became sole organizer of the American Board of Dermatology Physician Assistants (ABDPA), an organization that planned to offer board certification to PAs who work with dermatologists, according to a message from Dr. Hruza to members. The use of the words “American Board of Dermatology” in the ABDPA name created confusion to patients and threatened to undermine the value of ABD certification held by AAD/A fellows, Dr. Hruza said.
“Dr. Dinehart’s action to incorporate and organize the for-profit entity ABDPA, LLC., is in direct contradiction to the AAD’s Truth in Advertising and Professional Disclosure policy that states that practitioners should not advertise that they are board certified unless they are certified by an ABMS/AOA [American Board of Medical Specialties or American Osteopathic Association] medical board, such as the American Board of Dermatology,” Dr. Hruza said in an interview with Dermatology News. “This for-profit venture would enable physician assistants to advertise themselves as board certified. The [AAD/A] board in its unanimous decision deemed that the new ABDPA was set up to potentially mislead patients into thinking that physician assistants with this certification would have training and experience equivalent to an ABD-certified dermatologist. This is contrary to the AAD/A’s position on this matter. As such, Dr. Dinehart’s involvement in forming and organizing the ABDPA violated his fiduciary duty to act in the best interests of the AAD/A and to abide by AAD/A policies.”
In a letter to AAD members, Dr. Dinehart called the removal vote a “drastic measure” and said he has done nothing to justify dismissal from the AAD/A board. The ABDPA was intended to improve patient care by establishing certain educational, training, and professional standards for the growing number of physician assistants in dermatology, Dr. Dinehart wrote. That mission was not in conflict with AAD’s values, but rather, the ABDPA would have furthered AAD’s purpose “to promote the highest standards in allied health professionals and services as they relate to dermatology.”
After learning of the board’s concerns, Dr. Dinehart said he discontinued his relationship with the ABDPA and ensured its operations had ended out of respect for the academy. He believes the matter could have been handled much differently.
“I did not violate any bylaws,” Dr. Dinehart said in an interview with Dermatology News. “While I understand that there are certain members of the AAD that personally oppose the growing use of physician assistants in dermatology practices, it is a common practice among many AAD members, and it is not contrary to or detrimental to any of the purposes of the AAD.”
Dr. Dinehart contends he did not run afoul of the AAD/A’s position statement on Truth in Advertising and Professional Credential Disclosure because the statement pertains to AAD/A members and prohibits members from advertising board certifications by nonapproved boards. He emphasized that PAs are not members and said he would never represent or advertise a physician assistant as having a certification equivalent to a board-certified dermatologist or being capable of equivalent credentials.
In addition, he said his involvement with the new organization did not conflict with the AAD’s Practice of Dermatology: Protecting and Preserving Patient Safety and Quality Care position statement, which opposes the independent or unsupervised practice of dermatology procedures by nonphysicians.
“Neither the ADBPA nor the certification it intended to offer would have allowed physician assistants to engage in the independent or unsupervised practice of specific dermatology procedures, and any physician assistant who did so would be subject to the same repercussions that currently exist,” Dr. Dinehart said in the interview.
The ABDPA was formed legally at the end of September and announced its official launch on Oct. 7. The new organization immediately drew criticism from dermatologists and triggered an online petition that denounced the group and called for Dr Dinehart’s removal from the AAD/A board. The petition, started by an anonymous dermatologist, states Dr. Dinehart’s concurrent relationships with the AAD and the ABDPA represent a major conflict of interest. As of Oct. 24, the petition had collected 2,496 signatures.
Monica Madray, MD, a dermatologist based in Georgetown, Tex., who signed the petition, said the ABDPA further muddied the waters in an already confusing world of “providers” and non–dermatology boarded physicians practicing and advertising themselves as dermatologists.
“Patients truly don’t know the difference in training between physicians and midlevels, and by ‘board certifying’ PAs in dermatology, it gets much more confusing,” Dr. Madray said in an interview. “I also think there was a huge financial conflict of interest for Dr. Dinehart to start and run this board as he oversees many midlevels. Frankly, it didn’t pass the sniff test.”
Vivian Bucay, MD, a dermatologist based in San Antonio who signed the petition, said that, if Dr. Dinehart was interested in starting an organization such as the ABDPA, he should have first presented the idea to the AAD/A board and asked for feedback.
“This came [to light] after a press release,” Dr. Bucay said in an interview. “I don’t have an issue with trying to improve and set the bar for what dermatology physician assistants should do. It’s the manner in which it was gone about. In this day and age, we have to disclose any potential conflicts of interest. If we all have to do that as members of the American Society of Dermatologic Surgery or members of the AAD when presenting for continuing medical education, then why would there not be the same standard for a board member to disclose a conflict of interest?”
Dr. Dinehart for his part said the board never gave him an opportunity to respond to its accusations or share his side of the story before initiating the removal vote.
“The board should not have been pressured by a vocal minority of the membership, rushed to judgment, and expedited a special removal vote less than 2 weeks after the board claims it first became aware of the formation of ABDPA,” he said in the interview. “The board should have ... evaluated the alleged conflict and provided me a reasonable opportunity to cure the alleged conflict as provided by the AAD/A’s conflict of interest rules. Had the board allowed me that opportunity, as required, this whole situation could have been resolved.”
Dr. Hruza, however, said the board’s actions came after reviewing evidence with Dr. Dinehart in private, which allowed him to make a statement and provided him a chance to answer questions from the board. He was also afforded several opportunities to resign, which he declined, Dr. Hruza said in the interview.
“I regret that we have been put in this difficult position and that the question must be put to the membership for a painful vote,” Dr. Hruza said. “The academy has a lot of important work to do to advocate for our members and our patients. This issue has been an unfortunate distraction from that important work.”
Daniel M. Siegel, MD, a New York–based dermatologist and former AAD president, said in an interview with Dermatology News that he plans to vote to keep Dr. Dinehart on the board. Dr. Dinehart has been a dedicated advocate for the specialty for more than 30 years and his recent misstep does not rise to the level of board removal, Dr. Siegel said. A social media environment that easily enables people to get fired up about almost any subject and quickly stoke anger in others contributed to overreaction of the situation, he added.
“In retrospect, it was not in the best interests of the AAD, but it was nothing criminal,” Dr. Siegel said in the interview. “He had a business idea, it got a lot negative feedback, and he did the right thing about it. Taking such an aggressive punitive approach for a transgression that was fairly minor is a bad precedent to set.”
Voting on Dr. Dinehart’s position opened on Oct. 21 and will close on Oct. 29. A removal decision requires a two-thirds vote by at least 10% of the voting membership.
Researchers seek to characterize pediatric new daily persistent headache
CHARLOTTE, N.C. – according to research presented at the 48th national meeting of the Child Neurology Society. Most children with NDPH fulfill criteria for its migraine subtype, and one-third of pediatric patients with NDPH have comorbid medication overuse headache (MOH).
NDPH is defined as a daily, unremitting headache that lasts for at least 3 months. “Not many studies on NDPH focus on pediatrics,” said Emily Pierce, from Children’s National Medical Center in Washington. NDPH “is considered to be one of the most intractable headaches in children. Children are able to tell that they’ve had this different type of headache because there’s some kind of onset that is very memorable.”
Ms. Pierce and colleagues conducted an observational study to describe the characteristics of NDPH in pediatric patients who presented to a headache program at a tertiary referral center. The researchers included pediatric patients who visited the headache clinic at Children’s National Medical Center between 2016 and 2018 in their analysis. All patients were enrolled in patient registry that had been approved by an independent review board. Ms. Pierce and colleagues queried the registry for NDPH and reviewed these records to examine participants’ clinical presentations.
The investigators identified 3,260 patient encounters during the study period. Of these encounters, 454 patients (13.9%) were identified as having NDPH. Patients with NDPH were predominantly female (78%) and white (72%). The median age of the sample was 14.8 years.
The frontal head region was the most common location of headache pain, which often had a throbbing quality and was associated with photophobia, phonophobia, nausea, and decreased activity. The median pain intensity was 6 of 10. Approximately 72% of patients had tried abortive medication, and 56% of patients had failed at least one abortive medication. Excedrin, ibuprofen, and acetaminophen were among the common failed abortive medications.
Furthermore, 36% of patients were diagnosed with MOH. The most commonly overused medication was ibuprofen. MOH “is also considered to be intractable for patients with NDPH,” said Ms. Pierce. “Typically, if the patient stops overusing that medication, they’ll find relief from their headaches. However, with our NDPH patients, when they stop overusing that medication, they still are having headaches associated with NDPH.”
The data indicated “a strong difference between our male and female patients,” said Ms. Pierce. Female patients reported significantly more instances of photophobia, phonophobia, nausea, and dizziness than did male patients. Overall, 78% of participants had a diagnosis of an additional comorbidity, such as head trauma (18%), anxiety (14%), depression (8%), or other (37%).
Observational studies of pediatric NDPH offer “a better way for our providers to diagnose these patients, and also to better understand them and help them figure out a treatment that may work,” said Ms. Pierce. In future research, she and her colleagues intend to examine blood work and potential genetic associations in pediatric patients with NDPH.
The study was not supported by funding, and the investigators had no disclosures.
SOURCE: Pierce E et al. CNS 2019, Abstract 100.
CHARLOTTE, N.C. – according to research presented at the 48th national meeting of the Child Neurology Society. Most children with NDPH fulfill criteria for its migraine subtype, and one-third of pediatric patients with NDPH have comorbid medication overuse headache (MOH).
NDPH is defined as a daily, unremitting headache that lasts for at least 3 months. “Not many studies on NDPH focus on pediatrics,” said Emily Pierce, from Children’s National Medical Center in Washington. NDPH “is considered to be one of the most intractable headaches in children. Children are able to tell that they’ve had this different type of headache because there’s some kind of onset that is very memorable.”
Ms. Pierce and colleagues conducted an observational study to describe the characteristics of NDPH in pediatric patients who presented to a headache program at a tertiary referral center. The researchers included pediatric patients who visited the headache clinic at Children’s National Medical Center between 2016 and 2018 in their analysis. All patients were enrolled in patient registry that had been approved by an independent review board. Ms. Pierce and colleagues queried the registry for NDPH and reviewed these records to examine participants’ clinical presentations.
The investigators identified 3,260 patient encounters during the study period. Of these encounters, 454 patients (13.9%) were identified as having NDPH. Patients with NDPH were predominantly female (78%) and white (72%). The median age of the sample was 14.8 years.
The frontal head region was the most common location of headache pain, which often had a throbbing quality and was associated with photophobia, phonophobia, nausea, and decreased activity. The median pain intensity was 6 of 10. Approximately 72% of patients had tried abortive medication, and 56% of patients had failed at least one abortive medication. Excedrin, ibuprofen, and acetaminophen were among the common failed abortive medications.
Furthermore, 36% of patients were diagnosed with MOH. The most commonly overused medication was ibuprofen. MOH “is also considered to be intractable for patients with NDPH,” said Ms. Pierce. “Typically, if the patient stops overusing that medication, they’ll find relief from their headaches. However, with our NDPH patients, when they stop overusing that medication, they still are having headaches associated with NDPH.”
The data indicated “a strong difference between our male and female patients,” said Ms. Pierce. Female patients reported significantly more instances of photophobia, phonophobia, nausea, and dizziness than did male patients. Overall, 78% of participants had a diagnosis of an additional comorbidity, such as head trauma (18%), anxiety (14%), depression (8%), or other (37%).
Observational studies of pediatric NDPH offer “a better way for our providers to diagnose these patients, and also to better understand them and help them figure out a treatment that may work,” said Ms. Pierce. In future research, she and her colleagues intend to examine blood work and potential genetic associations in pediatric patients with NDPH.
The study was not supported by funding, and the investigators had no disclosures.
SOURCE: Pierce E et al. CNS 2019, Abstract 100.
CHARLOTTE, N.C. – according to research presented at the 48th national meeting of the Child Neurology Society. Most children with NDPH fulfill criteria for its migraine subtype, and one-third of pediatric patients with NDPH have comorbid medication overuse headache (MOH).
NDPH is defined as a daily, unremitting headache that lasts for at least 3 months. “Not many studies on NDPH focus on pediatrics,” said Emily Pierce, from Children’s National Medical Center in Washington. NDPH “is considered to be one of the most intractable headaches in children. Children are able to tell that they’ve had this different type of headache because there’s some kind of onset that is very memorable.”
Ms. Pierce and colleagues conducted an observational study to describe the characteristics of NDPH in pediatric patients who presented to a headache program at a tertiary referral center. The researchers included pediatric patients who visited the headache clinic at Children’s National Medical Center between 2016 and 2018 in their analysis. All patients were enrolled in patient registry that had been approved by an independent review board. Ms. Pierce and colleagues queried the registry for NDPH and reviewed these records to examine participants’ clinical presentations.
The investigators identified 3,260 patient encounters during the study period. Of these encounters, 454 patients (13.9%) were identified as having NDPH. Patients with NDPH were predominantly female (78%) and white (72%). The median age of the sample was 14.8 years.
The frontal head region was the most common location of headache pain, which often had a throbbing quality and was associated with photophobia, phonophobia, nausea, and decreased activity. The median pain intensity was 6 of 10. Approximately 72% of patients had tried abortive medication, and 56% of patients had failed at least one abortive medication. Excedrin, ibuprofen, and acetaminophen were among the common failed abortive medications.
Furthermore, 36% of patients were diagnosed with MOH. The most commonly overused medication was ibuprofen. MOH “is also considered to be intractable for patients with NDPH,” said Ms. Pierce. “Typically, if the patient stops overusing that medication, they’ll find relief from their headaches. However, with our NDPH patients, when they stop overusing that medication, they still are having headaches associated with NDPH.”
The data indicated “a strong difference between our male and female patients,” said Ms. Pierce. Female patients reported significantly more instances of photophobia, phonophobia, nausea, and dizziness than did male patients. Overall, 78% of participants had a diagnosis of an additional comorbidity, such as head trauma (18%), anxiety (14%), depression (8%), or other (37%).
Observational studies of pediatric NDPH offer “a better way for our providers to diagnose these patients, and also to better understand them and help them figure out a treatment that may work,” said Ms. Pierce. In future research, she and her colleagues intend to examine blood work and potential genetic associations in pediatric patients with NDPH.
The study was not supported by funding, and the investigators had no disclosures.
SOURCE: Pierce E et al. CNS 2019, Abstract 100.
REPORTING FROM CNS 2019
Many children who present to headache clinics have joint hypermobility
CHARLOTTE, N.C. – according to data presented at the 48th national meeting of the Child Neurology Society. Furthermore, patients with joint hypermobility have a high rate of headache disability, while patients without joint hypermobility have less headache disability, according to Dhwani Sahjwani, MD, a resident at Inova Fairfax Hospital in Falls Church, Va., and colleagues.
While conducting research in the headache clinic at Children’s National Hospital in Washington, D.C., Dr. Sahjwani saw several children with joint hypermobility and a diagnosis of a disorder such as Ehlers-Danlos syndrome. She and her colleagues began analyzing patients to evaluate the potential association between joint hypermobility and headache disability in children. The investigators included pediatric patients examined in the headache clinic at Children’s National Medical Center between October 2018 and January 2019 in their study. All headache clinic patients were enrolled in a patient registry that had been approved by an independent review board.
Dr. Sahjwani and colleagues measured patients’ headache disability with the Headache Impact Test–6 (HIT-6) questionnaire. Scores of 60 or greater on this questionnaire indicate severe headache disability. The researchers assessed joint hypermobility using the Beighton scoring system. In this system, scores greater than 4 indicate joint hypermobility.
Dr. Sahjwani’s group scored 76 patients using the Beighton system and HIT-6 questionnaire. Participants’ median age was 13.7 years. Approximately 26% of patients had Beighton scores that indicated joint hypermobility. About 65% of the patients with joint hypermobility had a diagnosis of migraine without aura. In addition, 80% of patients with joint hypermobility had severe headache disability, according to the HIT-6 disability criteria. The average pain intensity in patients with hypermobile joints was 6.1 out of 10. Among participants without significant joint hypermobility, 90% had mild headache disability.
Patients with joint hypermobility and increased tissue elasticity “tend to have a lower threshold for pain, in general, in all parts of their bodies,” said Dr. Sahjwani. Greater headache severity might be expected in this population, “because they have more pain if they have hypermobile joints or tissue.”
Headache treatments for this population are based solely on the type of headache that each patient has. Patients with joint hypermobility and migraine, for example, are candidates for rescue medication and long-term prophylactic medications. “I don’t think the joint hypermobility is going to change how you manage their headaches,” said Dr. Sahjwani.
The study results suggest that, when children present with severely debilitating headaches, a neurologist should consider examining them for joint hypermobility “to see if they have another diagnosis, such as Ehlers-Danlos syndrome ... that has to be managed in addition to their headaches,” Dr. Sahjwani concluded.
The study was not supported by funding. The authors did not report any disclosures.
SOURCE: Sahjwani D et al. CNS 2019, Abstract 101.
CHARLOTTE, N.C. – according to data presented at the 48th national meeting of the Child Neurology Society. Furthermore, patients with joint hypermobility have a high rate of headache disability, while patients without joint hypermobility have less headache disability, according to Dhwani Sahjwani, MD, a resident at Inova Fairfax Hospital in Falls Church, Va., and colleagues.
While conducting research in the headache clinic at Children’s National Hospital in Washington, D.C., Dr. Sahjwani saw several children with joint hypermobility and a diagnosis of a disorder such as Ehlers-Danlos syndrome. She and her colleagues began analyzing patients to evaluate the potential association between joint hypermobility and headache disability in children. The investigators included pediatric patients examined in the headache clinic at Children’s National Medical Center between October 2018 and January 2019 in their study. All headache clinic patients were enrolled in a patient registry that had been approved by an independent review board.
Dr. Sahjwani and colleagues measured patients’ headache disability with the Headache Impact Test–6 (HIT-6) questionnaire. Scores of 60 or greater on this questionnaire indicate severe headache disability. The researchers assessed joint hypermobility using the Beighton scoring system. In this system, scores greater than 4 indicate joint hypermobility.
Dr. Sahjwani’s group scored 76 patients using the Beighton system and HIT-6 questionnaire. Participants’ median age was 13.7 years. Approximately 26% of patients had Beighton scores that indicated joint hypermobility. About 65% of the patients with joint hypermobility had a diagnosis of migraine without aura. In addition, 80% of patients with joint hypermobility had severe headache disability, according to the HIT-6 disability criteria. The average pain intensity in patients with hypermobile joints was 6.1 out of 10. Among participants without significant joint hypermobility, 90% had mild headache disability.
Patients with joint hypermobility and increased tissue elasticity “tend to have a lower threshold for pain, in general, in all parts of their bodies,” said Dr. Sahjwani. Greater headache severity might be expected in this population, “because they have more pain if they have hypermobile joints or tissue.”
Headache treatments for this population are based solely on the type of headache that each patient has. Patients with joint hypermobility and migraine, for example, are candidates for rescue medication and long-term prophylactic medications. “I don’t think the joint hypermobility is going to change how you manage their headaches,” said Dr. Sahjwani.
The study results suggest that, when children present with severely debilitating headaches, a neurologist should consider examining them for joint hypermobility “to see if they have another diagnosis, such as Ehlers-Danlos syndrome ... that has to be managed in addition to their headaches,” Dr. Sahjwani concluded.
The study was not supported by funding. The authors did not report any disclosures.
SOURCE: Sahjwani D et al. CNS 2019, Abstract 101.
CHARLOTTE, N.C. – according to data presented at the 48th national meeting of the Child Neurology Society. Furthermore, patients with joint hypermobility have a high rate of headache disability, while patients without joint hypermobility have less headache disability, according to Dhwani Sahjwani, MD, a resident at Inova Fairfax Hospital in Falls Church, Va., and colleagues.
While conducting research in the headache clinic at Children’s National Hospital in Washington, D.C., Dr. Sahjwani saw several children with joint hypermobility and a diagnosis of a disorder such as Ehlers-Danlos syndrome. She and her colleagues began analyzing patients to evaluate the potential association between joint hypermobility and headache disability in children. The investigators included pediatric patients examined in the headache clinic at Children’s National Medical Center between October 2018 and January 2019 in their study. All headache clinic patients were enrolled in a patient registry that had been approved by an independent review board.
Dr. Sahjwani and colleagues measured patients’ headache disability with the Headache Impact Test–6 (HIT-6) questionnaire. Scores of 60 or greater on this questionnaire indicate severe headache disability. The researchers assessed joint hypermobility using the Beighton scoring system. In this system, scores greater than 4 indicate joint hypermobility.
Dr. Sahjwani’s group scored 76 patients using the Beighton system and HIT-6 questionnaire. Participants’ median age was 13.7 years. Approximately 26% of patients had Beighton scores that indicated joint hypermobility. About 65% of the patients with joint hypermobility had a diagnosis of migraine without aura. In addition, 80% of patients with joint hypermobility had severe headache disability, according to the HIT-6 disability criteria. The average pain intensity in patients with hypermobile joints was 6.1 out of 10. Among participants without significant joint hypermobility, 90% had mild headache disability.
Patients with joint hypermobility and increased tissue elasticity “tend to have a lower threshold for pain, in general, in all parts of their bodies,” said Dr. Sahjwani. Greater headache severity might be expected in this population, “because they have more pain if they have hypermobile joints or tissue.”
Headache treatments for this population are based solely on the type of headache that each patient has. Patients with joint hypermobility and migraine, for example, are candidates for rescue medication and long-term prophylactic medications. “I don’t think the joint hypermobility is going to change how you manage their headaches,” said Dr. Sahjwani.
The study results suggest that, when children present with severely debilitating headaches, a neurologist should consider examining them for joint hypermobility “to see if they have another diagnosis, such as Ehlers-Danlos syndrome ... that has to be managed in addition to their headaches,” Dr. Sahjwani concluded.
The study was not supported by funding. The authors did not report any disclosures.
SOURCE: Sahjwani D et al. CNS 2019, Abstract 101.
REPORTING FROM CNS 2019
Fluoxetine tied to lower obsessive-compulsive scores among children with ASDs
Impact of the SSRI on those behaviors falls short in multiple secondary analyses
Fluoxetine appeared to lower scores for obsessive-compulsive behaviors among a group of children with autism spectrum disorders (ASDs), but the positive finding fell apart during multiple secondary analyses, Dinah S. Reddihough, MD, and colleagues have reported.
At 16 weeks, children and adolescents randomized to receive the SSRI had about a 2-point improvement on the Children’s Yale-Brown Obsessive Compulsive Scale, modified for pervasive developmental disorder (CYBOCS-PDD), compared with those taking placebo. But the finding lost significance in a multivariate analysis that accounted for a between-group difference in baseline scores – an uncontrollable variable that occurred during randomization, wrote Dr. Reddihough, of the Royal Children’s Hospital in Victoria, Australia, and coauthors.
“Moreover, repeating the analyses with multiple imputation to handle the missing data, arguably a preferable analysis, also failed to show evidence of benefit of fluoxetine compared with placebo irrespective of adjustment for the baseline imbalance,” the team noted. The study was published in JAMA.
Despite the null findings of the additional adjusted analyses, the authors held out hope for fluoxetine.
“Although cautious interpretation of the results from the primary analysis is warranted, all analyses of the primary outcome yielded 95% confidence intervals that extended well above the minimum clinically important difference of 2 points, indicating that fluoxetine may reduce the frequency and severity of obsessive-compulsive behaviors in children and adolescents with ASDs. Given the large amount of missing data, the study may have been underpowered to detect the minimum clinically important difference of 2 points.”
The study comprised 146 children (mean age, 11 years) recruited through three large practices in Australia. Children were randomized to fluoxetine or placebo for 16 weeks. Fluoxetine was weight-dosed and then titrated every week for the first month to a maximum of 20 mg/day.
The primary outcome was the difference between groups in the total score on the CYBOCS-PDD at 16 weeks. Secondary endpoints included changes on the Repetitive Behavior Scale–Revised, the Spence Children’s Anxiety Scale Aberrant Behavior Checklist–Community Version, the Clinical Global Impression Scale–Global Improvement and Efficacy Index, and a Disruptiveness Assessment.
Of the cohort, 85% were male, and 30% had an intellectual disability. The placebo group had higher scores on the Repetitive Behavior Scale–Revised and the Aberrant Behavior Checklist lethargy scale than did the fluoxetine groups.
There was a very high rate of nonadherence to study protocol, with 41% of those in the active group and 30% in the placebo group not completing the treatment regimen. The most often cited reasons for treatment discontinuation included parent decision to drop out (20 fluoxetine, 12 placebo), adverse events (5 fluoxetine, 4 placebo), and clinician decision (2 fluoxetine, 2 placebo).
The primary analysis found that scores on the CYBOCS-PDD were significantly lower in the fluoxetine group at 16 weeks; the fluoxetine group had decreased its score from 12.80 to 9.02, while the placebo group went from 13.13 to 10.89. This mean 2-point difference was statistically significant and, the authors wrote, met the minimum threshold for a clinically significant difference.
But the mean between-group difference decreased to a nonsignificant 1.17 points in the sensitivity analysis that controlled for sex, verbal ability, baseline CYBOCS-PDD, and imbalances found at baseline in some of the measures.
“Moreover, repeating the analyses with multiple imputation to handle the missing data, arguably a preferable analysis, also failed to show evidence of benefit of fluoxetine compared with placebo irrespective of adjustment for the baseline imbalance,” the team said.
There were no significant differences on any of the secondary measures.
Adverse events were similar in the active and placebo groups (45% and 42%, respectively). These included mood disturbances particularly irritability (9 fluoxetine, 12 placebo), gastrointestinal problems such as nausea and diarrhea (10 fluoxetine, 7 placebo), and sleep disorders (13 fluoxetine, 16 placebo). Two patients in the placebo group and none in the active group experienced suicidality.
Dr. Reddihough and coauthors cited the study’s high dropout rate as one of its limitations.
The study was supported by a federal grant from the Australian government. Dr. Reddihough had no financial disclosures.
SOURCE: Reddihough DS et al. JAMA. 2019;322(16):1561-9.
One could certainly take issue with the suggestion that this was presented as a positive trial on two levels: One, the prespecified primary outcome was not met, and two, the clinical significance (as distinct from statistical significance) of a 2-point change on that scale is problematic given the wide range of baseline scores allowed into the study.
The other thing that gets mixed up in this study is: Exactly what are obsessive-compulsive symptoms as distinct from repetitive behaviors? That question is a real challenge in this field when it comes to clinical trials for this target in autism, which tend to lump together heterogeneous repetitive behaviors.
The fact that there was absolutely no signal, or at least not a very strong one, is very challenging, considering how frequently this class of drugs is prescribed in autism.
This is not the first SSRI study for autism that’s come up empty. In this case, though, a negative study is still important because it confirms other negative studies. Another recently published study – the SOFIA fluoxetine study (J Autism Dev Disord. 2019 Jul 2. doi: 10:1007/s10803-019-04120-y) – also came up negative. SOFIA randomized 158 children to 14 weeks of fluoxetine or placebo. There were no significant differences on the primary endpoint, the Children’s Yale-Brown Obsessive-Compulsive Scale, and the placebo response rate was 41%.
However, it was clearly a heroic effort by Dr. Reddihough et al. to get this current study done: It took 7 years to get it over the finish line. This is probably because fluoxetine is so easily available. Why would a parent take a 50% chance of their child not getting a drug that might have some benefit – and that they could get without much trouble? And if it takes 7 years to complete a clinical trial, and we’re sitting around waiting for a definitive one, we are literally looking at potentially decades before we have some real answers that would inform your clinical practice in terms of this commonly prescribed drug.
As far as nailing the coffin shut on fluoxetine, I don’t think that will ever happen because some kids clearly improve. The placebo response in this population is very high. In our citalopram study (JAMA Pediatr. 2013 Nov;167[11]:1045-52), it was close to 33%. The improvement is dramatic and real, no less than any other response. If you see that response as a clinician and parent, it is very difficult to walk away from. Moreover, the population in clinical practice may be different from the population that shows up in a clinical trial specifically focused on restricted, repetitive behaviors.
One reason we may see a response in some is because SSRIs can help with anxiety, which is a common, arguably core symptom of autism. It appears to be part of the reason kids have catastrophic meltdowns when there are any changes in things they have come to expect, like a different route to school or a delay in their favorite TV show coming on. And if anxiety drives that, and an SSRI helps with anxiety, the child might be able to cope with something that would otherwise feel like the end of the world. Maybe that starts a positive feedback loop instead of a negative one, and maybe it propels more changes as the child and family experience success.
So, what are clinicians to do? The answer is still the same – they should use their best judgment about each child’s symptoms and about the risks and benefits that might occur with that individual. The fact that these trials are coming up negative for this indication in autism doesn’t mean that SSRIs might not be helpful for anxiety or depression, just as they are in the general population. I think we are back to basics. Clinicians need to use their best medical judgment according to each child’s unique needs.
These comments were adapted from an interview with Bryan H. King, MD, MBA. Dr. King is a professor of psychiatry at the University of California, San Francisco. He reported receiving personal fees from Genentech. Dr. King also commented on the study in an accompanying editorial (JAMA. 2019;322[16]:1557-8).
Impact of the SSRI on those behaviors falls short in multiple secondary analyses
Impact of the SSRI on those behaviors falls short in multiple secondary analyses
One could certainly take issue with the suggestion that this was presented as a positive trial on two levels: One, the prespecified primary outcome was not met, and two, the clinical significance (as distinct from statistical significance) of a 2-point change on that scale is problematic given the wide range of baseline scores allowed into the study.
The other thing that gets mixed up in this study is: Exactly what are obsessive-compulsive symptoms as distinct from repetitive behaviors? That question is a real challenge in this field when it comes to clinical trials for this target in autism, which tend to lump together heterogeneous repetitive behaviors.
The fact that there was absolutely no signal, or at least not a very strong one, is very challenging, considering how frequently this class of drugs is prescribed in autism.
This is not the first SSRI study for autism that’s come up empty. In this case, though, a negative study is still important because it confirms other negative studies. Another recently published study – the SOFIA fluoxetine study (J Autism Dev Disord. 2019 Jul 2. doi: 10:1007/s10803-019-04120-y) – also came up negative. SOFIA randomized 158 children to 14 weeks of fluoxetine or placebo. There were no significant differences on the primary endpoint, the Children’s Yale-Brown Obsessive-Compulsive Scale, and the placebo response rate was 41%.
However, it was clearly a heroic effort by Dr. Reddihough et al. to get this current study done: It took 7 years to get it over the finish line. This is probably because fluoxetine is so easily available. Why would a parent take a 50% chance of their child not getting a drug that might have some benefit – and that they could get without much trouble? And if it takes 7 years to complete a clinical trial, and we’re sitting around waiting for a definitive one, we are literally looking at potentially decades before we have some real answers that would inform your clinical practice in terms of this commonly prescribed drug.
As far as nailing the coffin shut on fluoxetine, I don’t think that will ever happen because some kids clearly improve. The placebo response in this population is very high. In our citalopram study (JAMA Pediatr. 2013 Nov;167[11]:1045-52), it was close to 33%. The improvement is dramatic and real, no less than any other response. If you see that response as a clinician and parent, it is very difficult to walk away from. Moreover, the population in clinical practice may be different from the population that shows up in a clinical trial specifically focused on restricted, repetitive behaviors.
One reason we may see a response in some is because SSRIs can help with anxiety, which is a common, arguably core symptom of autism. It appears to be part of the reason kids have catastrophic meltdowns when there are any changes in things they have come to expect, like a different route to school or a delay in their favorite TV show coming on. And if anxiety drives that, and an SSRI helps with anxiety, the child might be able to cope with something that would otherwise feel like the end of the world. Maybe that starts a positive feedback loop instead of a negative one, and maybe it propels more changes as the child and family experience success.
So, what are clinicians to do? The answer is still the same – they should use their best judgment about each child’s symptoms and about the risks and benefits that might occur with that individual. The fact that these trials are coming up negative for this indication in autism doesn’t mean that SSRIs might not be helpful for anxiety or depression, just as they are in the general population. I think we are back to basics. Clinicians need to use their best medical judgment according to each child’s unique needs.
These comments were adapted from an interview with Bryan H. King, MD, MBA. Dr. King is a professor of psychiatry at the University of California, San Francisco. He reported receiving personal fees from Genentech. Dr. King also commented on the study in an accompanying editorial (JAMA. 2019;322[16]:1557-8).
One could certainly take issue with the suggestion that this was presented as a positive trial on two levels: One, the prespecified primary outcome was not met, and two, the clinical significance (as distinct from statistical significance) of a 2-point change on that scale is problematic given the wide range of baseline scores allowed into the study.
The other thing that gets mixed up in this study is: Exactly what are obsessive-compulsive symptoms as distinct from repetitive behaviors? That question is a real challenge in this field when it comes to clinical trials for this target in autism, which tend to lump together heterogeneous repetitive behaviors.
The fact that there was absolutely no signal, or at least not a very strong one, is very challenging, considering how frequently this class of drugs is prescribed in autism.
This is not the first SSRI study for autism that’s come up empty. In this case, though, a negative study is still important because it confirms other negative studies. Another recently published study – the SOFIA fluoxetine study (J Autism Dev Disord. 2019 Jul 2. doi: 10:1007/s10803-019-04120-y) – also came up negative. SOFIA randomized 158 children to 14 weeks of fluoxetine or placebo. There were no significant differences on the primary endpoint, the Children’s Yale-Brown Obsessive-Compulsive Scale, and the placebo response rate was 41%.
However, it was clearly a heroic effort by Dr. Reddihough et al. to get this current study done: It took 7 years to get it over the finish line. This is probably because fluoxetine is so easily available. Why would a parent take a 50% chance of their child not getting a drug that might have some benefit – and that they could get without much trouble? And if it takes 7 years to complete a clinical trial, and we’re sitting around waiting for a definitive one, we are literally looking at potentially decades before we have some real answers that would inform your clinical practice in terms of this commonly prescribed drug.
As far as nailing the coffin shut on fluoxetine, I don’t think that will ever happen because some kids clearly improve. The placebo response in this population is very high. In our citalopram study (JAMA Pediatr. 2013 Nov;167[11]:1045-52), it was close to 33%. The improvement is dramatic and real, no less than any other response. If you see that response as a clinician and parent, it is very difficult to walk away from. Moreover, the population in clinical practice may be different from the population that shows up in a clinical trial specifically focused on restricted, repetitive behaviors.
One reason we may see a response in some is because SSRIs can help with anxiety, which is a common, arguably core symptom of autism. It appears to be part of the reason kids have catastrophic meltdowns when there are any changes in things they have come to expect, like a different route to school or a delay in their favorite TV show coming on. And if anxiety drives that, and an SSRI helps with anxiety, the child might be able to cope with something that would otherwise feel like the end of the world. Maybe that starts a positive feedback loop instead of a negative one, and maybe it propels more changes as the child and family experience success.
So, what are clinicians to do? The answer is still the same – they should use their best judgment about each child’s symptoms and about the risks and benefits that might occur with that individual. The fact that these trials are coming up negative for this indication in autism doesn’t mean that SSRIs might not be helpful for anxiety or depression, just as they are in the general population. I think we are back to basics. Clinicians need to use their best medical judgment according to each child’s unique needs.
These comments were adapted from an interview with Bryan H. King, MD, MBA. Dr. King is a professor of psychiatry at the University of California, San Francisco. He reported receiving personal fees from Genentech. Dr. King also commented on the study in an accompanying editorial (JAMA. 2019;322[16]:1557-8).
Fluoxetine appeared to lower scores for obsessive-compulsive behaviors among a group of children with autism spectrum disorders (ASDs), but the positive finding fell apart during multiple secondary analyses, Dinah S. Reddihough, MD, and colleagues have reported.
At 16 weeks, children and adolescents randomized to receive the SSRI had about a 2-point improvement on the Children’s Yale-Brown Obsessive Compulsive Scale, modified for pervasive developmental disorder (CYBOCS-PDD), compared with those taking placebo. But the finding lost significance in a multivariate analysis that accounted for a between-group difference in baseline scores – an uncontrollable variable that occurred during randomization, wrote Dr. Reddihough, of the Royal Children’s Hospital in Victoria, Australia, and coauthors.
“Moreover, repeating the analyses with multiple imputation to handle the missing data, arguably a preferable analysis, also failed to show evidence of benefit of fluoxetine compared with placebo irrespective of adjustment for the baseline imbalance,” the team noted. The study was published in JAMA.
Despite the null findings of the additional adjusted analyses, the authors held out hope for fluoxetine.
“Although cautious interpretation of the results from the primary analysis is warranted, all analyses of the primary outcome yielded 95% confidence intervals that extended well above the minimum clinically important difference of 2 points, indicating that fluoxetine may reduce the frequency and severity of obsessive-compulsive behaviors in children and adolescents with ASDs. Given the large amount of missing data, the study may have been underpowered to detect the minimum clinically important difference of 2 points.”
The study comprised 146 children (mean age, 11 years) recruited through three large practices in Australia. Children were randomized to fluoxetine or placebo for 16 weeks. Fluoxetine was weight-dosed and then titrated every week for the first month to a maximum of 20 mg/day.
The primary outcome was the difference between groups in the total score on the CYBOCS-PDD at 16 weeks. Secondary endpoints included changes on the Repetitive Behavior Scale–Revised, the Spence Children’s Anxiety Scale Aberrant Behavior Checklist–Community Version, the Clinical Global Impression Scale–Global Improvement and Efficacy Index, and a Disruptiveness Assessment.
Of the cohort, 85% were male, and 30% had an intellectual disability. The placebo group had higher scores on the Repetitive Behavior Scale–Revised and the Aberrant Behavior Checklist lethargy scale than did the fluoxetine groups.
There was a very high rate of nonadherence to study protocol, with 41% of those in the active group and 30% in the placebo group not completing the treatment regimen. The most often cited reasons for treatment discontinuation included parent decision to drop out (20 fluoxetine, 12 placebo), adverse events (5 fluoxetine, 4 placebo), and clinician decision (2 fluoxetine, 2 placebo).
The primary analysis found that scores on the CYBOCS-PDD were significantly lower in the fluoxetine group at 16 weeks; the fluoxetine group had decreased its score from 12.80 to 9.02, while the placebo group went from 13.13 to 10.89. This mean 2-point difference was statistically significant and, the authors wrote, met the minimum threshold for a clinically significant difference.
But the mean between-group difference decreased to a nonsignificant 1.17 points in the sensitivity analysis that controlled for sex, verbal ability, baseline CYBOCS-PDD, and imbalances found at baseline in some of the measures.
“Moreover, repeating the analyses with multiple imputation to handle the missing data, arguably a preferable analysis, also failed to show evidence of benefit of fluoxetine compared with placebo irrespective of adjustment for the baseline imbalance,” the team said.
There were no significant differences on any of the secondary measures.
Adverse events were similar in the active and placebo groups (45% and 42%, respectively). These included mood disturbances particularly irritability (9 fluoxetine, 12 placebo), gastrointestinal problems such as nausea and diarrhea (10 fluoxetine, 7 placebo), and sleep disorders (13 fluoxetine, 16 placebo). Two patients in the placebo group and none in the active group experienced suicidality.
Dr. Reddihough and coauthors cited the study’s high dropout rate as one of its limitations.
The study was supported by a federal grant from the Australian government. Dr. Reddihough had no financial disclosures.
SOURCE: Reddihough DS et al. JAMA. 2019;322(16):1561-9.
Fluoxetine appeared to lower scores for obsessive-compulsive behaviors among a group of children with autism spectrum disorders (ASDs), but the positive finding fell apart during multiple secondary analyses, Dinah S. Reddihough, MD, and colleagues have reported.
At 16 weeks, children and adolescents randomized to receive the SSRI had about a 2-point improvement on the Children’s Yale-Brown Obsessive Compulsive Scale, modified for pervasive developmental disorder (CYBOCS-PDD), compared with those taking placebo. But the finding lost significance in a multivariate analysis that accounted for a between-group difference in baseline scores – an uncontrollable variable that occurred during randomization, wrote Dr. Reddihough, of the Royal Children’s Hospital in Victoria, Australia, and coauthors.
“Moreover, repeating the analyses with multiple imputation to handle the missing data, arguably a preferable analysis, also failed to show evidence of benefit of fluoxetine compared with placebo irrespective of adjustment for the baseline imbalance,” the team noted. The study was published in JAMA.
Despite the null findings of the additional adjusted analyses, the authors held out hope for fluoxetine.
“Although cautious interpretation of the results from the primary analysis is warranted, all analyses of the primary outcome yielded 95% confidence intervals that extended well above the minimum clinically important difference of 2 points, indicating that fluoxetine may reduce the frequency and severity of obsessive-compulsive behaviors in children and adolescents with ASDs. Given the large amount of missing data, the study may have been underpowered to detect the minimum clinically important difference of 2 points.”
The study comprised 146 children (mean age, 11 years) recruited through three large practices in Australia. Children were randomized to fluoxetine or placebo for 16 weeks. Fluoxetine was weight-dosed and then titrated every week for the first month to a maximum of 20 mg/day.
The primary outcome was the difference between groups in the total score on the CYBOCS-PDD at 16 weeks. Secondary endpoints included changes on the Repetitive Behavior Scale–Revised, the Spence Children’s Anxiety Scale Aberrant Behavior Checklist–Community Version, the Clinical Global Impression Scale–Global Improvement and Efficacy Index, and a Disruptiveness Assessment.
Of the cohort, 85% were male, and 30% had an intellectual disability. The placebo group had higher scores on the Repetitive Behavior Scale–Revised and the Aberrant Behavior Checklist lethargy scale than did the fluoxetine groups.
There was a very high rate of nonadherence to study protocol, with 41% of those in the active group and 30% in the placebo group not completing the treatment regimen. The most often cited reasons for treatment discontinuation included parent decision to drop out (20 fluoxetine, 12 placebo), adverse events (5 fluoxetine, 4 placebo), and clinician decision (2 fluoxetine, 2 placebo).
The primary analysis found that scores on the CYBOCS-PDD were significantly lower in the fluoxetine group at 16 weeks; the fluoxetine group had decreased its score from 12.80 to 9.02, while the placebo group went from 13.13 to 10.89. This mean 2-point difference was statistically significant and, the authors wrote, met the minimum threshold for a clinically significant difference.
But the mean between-group difference decreased to a nonsignificant 1.17 points in the sensitivity analysis that controlled for sex, verbal ability, baseline CYBOCS-PDD, and imbalances found at baseline in some of the measures.
“Moreover, repeating the analyses with multiple imputation to handle the missing data, arguably a preferable analysis, also failed to show evidence of benefit of fluoxetine compared with placebo irrespective of adjustment for the baseline imbalance,” the team said.
There were no significant differences on any of the secondary measures.
Adverse events were similar in the active and placebo groups (45% and 42%, respectively). These included mood disturbances particularly irritability (9 fluoxetine, 12 placebo), gastrointestinal problems such as nausea and diarrhea (10 fluoxetine, 7 placebo), and sleep disorders (13 fluoxetine, 16 placebo). Two patients in the placebo group and none in the active group experienced suicidality.
Dr. Reddihough and coauthors cited the study’s high dropout rate as one of its limitations.
The study was supported by a federal grant from the Australian government. Dr. Reddihough had no financial disclosures.
SOURCE: Reddihough DS et al. JAMA. 2019;322(16):1561-9.
FROM JAMA
CMS has plan if ACA overturned in court; Verma silent on details
The Trump administration apparently plans to ensure Americans have access to health insurance in the event that the Affordable Care Act is struck down – but officials refuse to share that plan.
“The president has made clear that we will have a plan in action to make sure that Americans have access to affordable coverage” if or when courts negate the ACA, Seema Verma, administrator of the Centers for Medicare & Medicaid Services said Oct. 23 at a House Ways and Means Health Subcommittee hearing. “We do not have that today. There are many Americans today, they are not getting a subsidy. They can’t afford insurance today.”
When asked specifically about the provision to guarantee coverage for those with preexisting conditions, Ms. Verma replied that the president “has made clear that we will do everything we can to ensure that Americans with preexisting conditions maintain the protection that they have today.”
When pressed for details, Ms. Verma dodged the question, first by attempting to tell an anecdote about “a 55-year-old couple making $66,000 a year ...” before getting cut off. When the question was reiterated by Health Subcommittee Chair Diana DeGette (D-Colo.), Ms. Verma replied, “I am not going get into any specifics of a plan.”
Committee Chair Frank Pallone (D-N.J.) said it was “deceptive” that Ms. Verma would not provide any details and openly questioned whether a plan actually existed.
The hearing followed a partisan pattern.
Republican subcommittee members asked questions that allowed Ms Verma to highlight some of the actions taken by the CMS under her watch, such as lowering premiums for exchange plans, increasing the number of available plans and decreasing the number of states that had only one plan option available in the exchange, and other items that are focused on lowering the cost of health care.
“We’re trying to focus on actions that lower the cost of care for Americans,” she said. “If we do that, more people will be able to afford health care.”
Under questioning by panel Democrats, Ms. Verma took a more adversarial tone and tended to deflect rather than answer questions.
When pressed about Medicaid work requirement and the disruption in health care coverage they are causing, Ms. Verma had no answer, instead trying to talking about “community engagement requirements” before being cut off.
Ms. Verma also refused to address the coverage requirements, or lack thereof, of short-term, limited duration plans, which have been expanded under the Trump administration.
When asked whether plans could deny claims based on preexisting conditions, could implement coverage caps, charge more based on age or gender, or ignore other consumer protections in the ACA, she consistently defaulted to a comment that it “depends” on the plan and what they offer, without coming out and simply acknowledging that these plans have it within their power to ignore any and all consumer protections held within the Affordable Care Act.
“None of the actions that we have taken do anything to undermine the protections for people with preexisting conditions,” she said.
“Your testimony is not actually truthful to us today,” Rep. Ann Kuster (D-N.H.) replied.
The Trump administration apparently plans to ensure Americans have access to health insurance in the event that the Affordable Care Act is struck down – but officials refuse to share that plan.
“The president has made clear that we will have a plan in action to make sure that Americans have access to affordable coverage” if or when courts negate the ACA, Seema Verma, administrator of the Centers for Medicare & Medicaid Services said Oct. 23 at a House Ways and Means Health Subcommittee hearing. “We do not have that today. There are many Americans today, they are not getting a subsidy. They can’t afford insurance today.”
When asked specifically about the provision to guarantee coverage for those with preexisting conditions, Ms. Verma replied that the president “has made clear that we will do everything we can to ensure that Americans with preexisting conditions maintain the protection that they have today.”
When pressed for details, Ms. Verma dodged the question, first by attempting to tell an anecdote about “a 55-year-old couple making $66,000 a year ...” before getting cut off. When the question was reiterated by Health Subcommittee Chair Diana DeGette (D-Colo.), Ms. Verma replied, “I am not going get into any specifics of a plan.”
Committee Chair Frank Pallone (D-N.J.) said it was “deceptive” that Ms. Verma would not provide any details and openly questioned whether a plan actually existed.
The hearing followed a partisan pattern.
Republican subcommittee members asked questions that allowed Ms Verma to highlight some of the actions taken by the CMS under her watch, such as lowering premiums for exchange plans, increasing the number of available plans and decreasing the number of states that had only one plan option available in the exchange, and other items that are focused on lowering the cost of health care.
“We’re trying to focus on actions that lower the cost of care for Americans,” she said. “If we do that, more people will be able to afford health care.”
Under questioning by panel Democrats, Ms. Verma took a more adversarial tone and tended to deflect rather than answer questions.
When pressed about Medicaid work requirement and the disruption in health care coverage they are causing, Ms. Verma had no answer, instead trying to talking about “community engagement requirements” before being cut off.
Ms. Verma also refused to address the coverage requirements, or lack thereof, of short-term, limited duration plans, which have been expanded under the Trump administration.
When asked whether plans could deny claims based on preexisting conditions, could implement coverage caps, charge more based on age or gender, or ignore other consumer protections in the ACA, she consistently defaulted to a comment that it “depends” on the plan and what they offer, without coming out and simply acknowledging that these plans have it within their power to ignore any and all consumer protections held within the Affordable Care Act.
“None of the actions that we have taken do anything to undermine the protections for people with preexisting conditions,” she said.
“Your testimony is not actually truthful to us today,” Rep. Ann Kuster (D-N.H.) replied.
The Trump administration apparently plans to ensure Americans have access to health insurance in the event that the Affordable Care Act is struck down – but officials refuse to share that plan.
“The president has made clear that we will have a plan in action to make sure that Americans have access to affordable coverage” if or when courts negate the ACA, Seema Verma, administrator of the Centers for Medicare & Medicaid Services said Oct. 23 at a House Ways and Means Health Subcommittee hearing. “We do not have that today. There are many Americans today, they are not getting a subsidy. They can’t afford insurance today.”
When asked specifically about the provision to guarantee coverage for those with preexisting conditions, Ms. Verma replied that the president “has made clear that we will do everything we can to ensure that Americans with preexisting conditions maintain the protection that they have today.”
When pressed for details, Ms. Verma dodged the question, first by attempting to tell an anecdote about “a 55-year-old couple making $66,000 a year ...” before getting cut off. When the question was reiterated by Health Subcommittee Chair Diana DeGette (D-Colo.), Ms. Verma replied, “I am not going get into any specifics of a plan.”
Committee Chair Frank Pallone (D-N.J.) said it was “deceptive” that Ms. Verma would not provide any details and openly questioned whether a plan actually existed.
The hearing followed a partisan pattern.
Republican subcommittee members asked questions that allowed Ms Verma to highlight some of the actions taken by the CMS under her watch, such as lowering premiums for exchange plans, increasing the number of available plans and decreasing the number of states that had only one plan option available in the exchange, and other items that are focused on lowering the cost of health care.
“We’re trying to focus on actions that lower the cost of care for Americans,” she said. “If we do that, more people will be able to afford health care.”
Under questioning by panel Democrats, Ms. Verma took a more adversarial tone and tended to deflect rather than answer questions.
When pressed about Medicaid work requirement and the disruption in health care coverage they are causing, Ms. Verma had no answer, instead trying to talking about “community engagement requirements” before being cut off.
Ms. Verma also refused to address the coverage requirements, or lack thereof, of short-term, limited duration plans, which have been expanded under the Trump administration.
When asked whether plans could deny claims based on preexisting conditions, could implement coverage caps, charge more based on age or gender, or ignore other consumer protections in the ACA, she consistently defaulted to a comment that it “depends” on the plan and what they offer, without coming out and simply acknowledging that these plans have it within their power to ignore any and all consumer protections held within the Affordable Care Act.
“None of the actions that we have taken do anything to undermine the protections for people with preexisting conditions,” she said.
“Your testimony is not actually truthful to us today,” Rep. Ann Kuster (D-N.H.) replied.
REPORTING FROM a HOUSE ENERGY AND COMMERCE HEALTH SUBCOMMITTEE HEARING
Basal Cell Carcinoma Arising in Nevus Sebaceous During Pregnancy
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.

Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.

Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.

Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
Practice Points
- Benign neoplasms arise more frequently in nevus sebaceous (NS) lesions than do malignant neoplasms.
- The hormonal changes that occur during pregnancy and puberty appear to play a role in the development of neoplasms in NS lesions.
- Monitoring NS lesions more closely during periods of hormonal change may help diagnose malignant transformations in these patients.
Pelosi drug pricing bill passes Ways and Means on party line vote
The House Ways and Means Committee is the latest to pass H.R. 3, a bill aimed at driving the price of prescription drugs down.
During an Oct. 22, 2019, markup of the bill, Republican members criticized committee leadership for abandoning bipartisan efforts to reign in drug prices in favor of a partisan bill that so far gained no support from the minority party. H.R. 3 was passed by the Ways and Means Committee on a 24-17 party line vote.
Both “Democrats and Republicans support lowering drug prices, cracking down on overpriced drugs, giving patients more power to choose affordable medicines, and removing the wrong incentives in federal health programs that reward bad actors for raising prices,” Committee Ranking Member Kevin Brady (R-Tex.) said in his opening statement. In fact, at the request of Committee Chairman Richard Neal (D-Mass.), “both parties in this committee were working together toward that important goal. At least until Speaker Nancy Pelosi (D-Calif.) trashed the bipartisan work and forced through a secretly written, deeply controversial, and highly partisan drug bill to cure political illnesses rather than real ones.”
H.R. 3, recently renamed the Elijah E. Cummings Lower Drug Costs Now Act of 2019, would give the secretary of the Department of Health & Human Services the ability to negotiate drug prices for Medicare Part D (something explicitly banned under current law), implement an excise tax on drugs that see price hikes above the rate of inflation, cap out-of-pocket expenditures annually for Medicare Part D beneficiaries at $2,000, and use an international pricing index to help bring prices for drugs sold in the United States more in line with the lower prices in foreign countries.
But panel Democrats praised the bill as a step forward in helping to lower the cost of prescription drugs.
“H.R. 3 levels the playing field for U.S. consumers who, on average, pay four times more than patients in other countries for the exact same drugs,” Chairman Neal said in a statement following the passage.
He highlighted specifically the provision that caps out-of-pocket expenses in Part D and the HHS’ negotiating power, noting that “more people will be able to afford the drugs they need that they may have previously forgone due to high costs. With more Americans taking the medicines they’re prescribed, families will be healthier, and premiums will go down.”
Republican committee members argued that these same provisions would stifle innovation and ultimately would reduce access to medicine. Most attempts at altering the provisions through amendments were met with strict party line rejection.
The House Ways and Means Committee is the latest to pass H.R. 3, a bill aimed at driving the price of prescription drugs down.
During an Oct. 22, 2019, markup of the bill, Republican members criticized committee leadership for abandoning bipartisan efforts to reign in drug prices in favor of a partisan bill that so far gained no support from the minority party. H.R. 3 was passed by the Ways and Means Committee on a 24-17 party line vote.
Both “Democrats and Republicans support lowering drug prices, cracking down on overpriced drugs, giving patients more power to choose affordable medicines, and removing the wrong incentives in federal health programs that reward bad actors for raising prices,” Committee Ranking Member Kevin Brady (R-Tex.) said in his opening statement. In fact, at the request of Committee Chairman Richard Neal (D-Mass.), “both parties in this committee were working together toward that important goal. At least until Speaker Nancy Pelosi (D-Calif.) trashed the bipartisan work and forced through a secretly written, deeply controversial, and highly partisan drug bill to cure political illnesses rather than real ones.”
H.R. 3, recently renamed the Elijah E. Cummings Lower Drug Costs Now Act of 2019, would give the secretary of the Department of Health & Human Services the ability to negotiate drug prices for Medicare Part D (something explicitly banned under current law), implement an excise tax on drugs that see price hikes above the rate of inflation, cap out-of-pocket expenditures annually for Medicare Part D beneficiaries at $2,000, and use an international pricing index to help bring prices for drugs sold in the United States more in line with the lower prices in foreign countries.
But panel Democrats praised the bill as a step forward in helping to lower the cost of prescription drugs.
“H.R. 3 levels the playing field for U.S. consumers who, on average, pay four times more than patients in other countries for the exact same drugs,” Chairman Neal said in a statement following the passage.
He highlighted specifically the provision that caps out-of-pocket expenses in Part D and the HHS’ negotiating power, noting that “more people will be able to afford the drugs they need that they may have previously forgone due to high costs. With more Americans taking the medicines they’re prescribed, families will be healthier, and premiums will go down.”
Republican committee members argued that these same provisions would stifle innovation and ultimately would reduce access to medicine. Most attempts at altering the provisions through amendments were met with strict party line rejection.
The House Ways and Means Committee is the latest to pass H.R. 3, a bill aimed at driving the price of prescription drugs down.
During an Oct. 22, 2019, markup of the bill, Republican members criticized committee leadership for abandoning bipartisan efforts to reign in drug prices in favor of a partisan bill that so far gained no support from the minority party. H.R. 3 was passed by the Ways and Means Committee on a 24-17 party line vote.
Both “Democrats and Republicans support lowering drug prices, cracking down on overpriced drugs, giving patients more power to choose affordable medicines, and removing the wrong incentives in federal health programs that reward bad actors for raising prices,” Committee Ranking Member Kevin Brady (R-Tex.) said in his opening statement. In fact, at the request of Committee Chairman Richard Neal (D-Mass.), “both parties in this committee were working together toward that important goal. At least until Speaker Nancy Pelosi (D-Calif.) trashed the bipartisan work and forced through a secretly written, deeply controversial, and highly partisan drug bill to cure political illnesses rather than real ones.”
H.R. 3, recently renamed the Elijah E. Cummings Lower Drug Costs Now Act of 2019, would give the secretary of the Department of Health & Human Services the ability to negotiate drug prices for Medicare Part D (something explicitly banned under current law), implement an excise tax on drugs that see price hikes above the rate of inflation, cap out-of-pocket expenditures annually for Medicare Part D beneficiaries at $2,000, and use an international pricing index to help bring prices for drugs sold in the United States more in line with the lower prices in foreign countries.
But panel Democrats praised the bill as a step forward in helping to lower the cost of prescription drugs.
“H.R. 3 levels the playing field for U.S. consumers who, on average, pay four times more than patients in other countries for the exact same drugs,” Chairman Neal said in a statement following the passage.
He highlighted specifically the provision that caps out-of-pocket expenses in Part D and the HHS’ negotiating power, noting that “more people will be able to afford the drugs they need that they may have previously forgone due to high costs. With more Americans taking the medicines they’re prescribed, families will be healthier, and premiums will go down.”
Republican committee members argued that these same provisions would stifle innovation and ultimately would reduce access to medicine. Most attempts at altering the provisions through amendments were met with strict party line rejection.
New drug improves sex drive, at least on paper
The novel drug bremelanotide shows promise in acquired female hypoactive sexual desire disorder, according to the results of two randomized, controlled trials and a 52-week open-label extension study published online in Obstetrics & Gynecology.
Bremelanotide, which received Food and Drug Administration approval for this indication in June 2019, is an analog of the endogenous neuropeptide alpha-melanocyte-stimulating hormone.
Two separate, identically designed phase 3 studies (RECONNECT) were performed by Sheryl Kingsburg, MD, of the Cleveland Medical Center, and associates. Combined, 1,267 premenopausal women in monogamous relationships with acquired hypoactive sexual desire disorder were randomized to bremelanotide or placebo. Women in the treatment arm had significant improvement in female sexual function index–desire domain (FSFI-D) scores from baseline to week 24 (integrated studies: 0.35; P less than .001; effect size, 0.39), compared with placebo. They also experienced significant improvement in the FSFI-desire/arousal/orgasm (FSFI-DAO) domain (integrated studies: –0.33; P less than .001; effect size, 0.27).
The most common adverse events were nausea (integrated: 40% versus 1% in placebo), flushing (20% versus 0.3%), and headache (11% versus 2%). Overall, 77% in the treatment group reported a treatment-emergent adverse event, compared with 58% in the placebo group.
The open-label follow-up study was led by James Simon, MD, of George Washington University and IntimMedicine Specialists, Washington. Of the 684 participants who opted to enter the extension study, 40% completed it. In those who received bremelanotide during the randomized trial, the change in FSFI-D scores from baseline to the end of the open-label study ranged from 1.25 to 1.30, while the change in FSFI-DAO ranged from –1.4 to –1.7. In patients originally on placebo, the changes were 0.70-0.77 and –0.9, respectively.
Both groups surpassed the minimally clinically important difference for the FSFI-D score, which is considered to be 0.6.
“Patients switching from placebo experienced a higher incidence of adverse events than those continuing on bremelanotide during the open-label extension (79% versus 63%, respectively),” Dr. Simon and associates said.
The treatment is subcutaneous and can be self-administered up to about 45 minutes before a sexual event, no more than once during a 24-hour period, and no more than 8 doses per month, according to an FDA press release. The drug is contraindicated for women with cardiovascular disease or uncontrolled hypertension due to observations of transiently, slightly increased blood pressure.
The trials were funded by Palatin Technologies and AMAG Pharmaceuticals. The authors and coauthors have extensive financial relationships with pharmaceutical companies. Dr. Carson reported no financial conflicts.
SOURCE: Obstet Gynecol. 2019 Oct 8. doi: 10.1097/AOG.0000000000003500; Obstet Gynecol. 2019 Oct 8. doi: 10.1097/AOG.0000000000003514.
The results indicate that sex is more satisfying in the treatment arm, but there is no evidence of an increase in the number of sexual events.
But the drug appears safe and offers a second option for women experiencing this concern.
Sandra Ann Carson, MD is in the departments of obstetrics, gynecology, and reproductive sciences, and reproductive endocrinology and infertility, at Yale University, New Haven, Conn. She made these comments in an editorial accompanying the articles by Kingsburg et al. and Simon et al. (Obstet Gynecol. 2019 Nov 134;[5]:897-8). Dr. Carson said she had no financial conflicts.
The results indicate that sex is more satisfying in the treatment arm, but there is no evidence of an increase in the number of sexual events.
But the drug appears safe and offers a second option for women experiencing this concern.
Sandra Ann Carson, MD is in the departments of obstetrics, gynecology, and reproductive sciences, and reproductive endocrinology and infertility, at Yale University, New Haven, Conn. She made these comments in an editorial accompanying the articles by Kingsburg et al. and Simon et al. (Obstet Gynecol. 2019 Nov 134;[5]:897-8). Dr. Carson said she had no financial conflicts.
The results indicate that sex is more satisfying in the treatment arm, but there is no evidence of an increase in the number of sexual events.
But the drug appears safe and offers a second option for women experiencing this concern.
Sandra Ann Carson, MD is in the departments of obstetrics, gynecology, and reproductive sciences, and reproductive endocrinology and infertility, at Yale University, New Haven, Conn. She made these comments in an editorial accompanying the articles by Kingsburg et al. and Simon et al. (Obstet Gynecol. 2019 Nov 134;[5]:897-8). Dr. Carson said she had no financial conflicts.
The novel drug bremelanotide shows promise in acquired female hypoactive sexual desire disorder, according to the results of two randomized, controlled trials and a 52-week open-label extension study published online in Obstetrics & Gynecology.
Bremelanotide, which received Food and Drug Administration approval for this indication in June 2019, is an analog of the endogenous neuropeptide alpha-melanocyte-stimulating hormone.
Two separate, identically designed phase 3 studies (RECONNECT) were performed by Sheryl Kingsburg, MD, of the Cleveland Medical Center, and associates. Combined, 1,267 premenopausal women in monogamous relationships with acquired hypoactive sexual desire disorder were randomized to bremelanotide or placebo. Women in the treatment arm had significant improvement in female sexual function index–desire domain (FSFI-D) scores from baseline to week 24 (integrated studies: 0.35; P less than .001; effect size, 0.39), compared with placebo. They also experienced significant improvement in the FSFI-desire/arousal/orgasm (FSFI-DAO) domain (integrated studies: –0.33; P less than .001; effect size, 0.27).
The most common adverse events were nausea (integrated: 40% versus 1% in placebo), flushing (20% versus 0.3%), and headache (11% versus 2%). Overall, 77% in the treatment group reported a treatment-emergent adverse event, compared with 58% in the placebo group.
The open-label follow-up study was led by James Simon, MD, of George Washington University and IntimMedicine Specialists, Washington. Of the 684 participants who opted to enter the extension study, 40% completed it. In those who received bremelanotide during the randomized trial, the change in FSFI-D scores from baseline to the end of the open-label study ranged from 1.25 to 1.30, while the change in FSFI-DAO ranged from –1.4 to –1.7. In patients originally on placebo, the changes were 0.70-0.77 and –0.9, respectively.
Both groups surpassed the minimally clinically important difference for the FSFI-D score, which is considered to be 0.6.
“Patients switching from placebo experienced a higher incidence of adverse events than those continuing on bremelanotide during the open-label extension (79% versus 63%, respectively),” Dr. Simon and associates said.
The treatment is subcutaneous and can be self-administered up to about 45 minutes before a sexual event, no more than once during a 24-hour period, and no more than 8 doses per month, according to an FDA press release. The drug is contraindicated for women with cardiovascular disease or uncontrolled hypertension due to observations of transiently, slightly increased blood pressure.
The trials were funded by Palatin Technologies and AMAG Pharmaceuticals. The authors and coauthors have extensive financial relationships with pharmaceutical companies. Dr. Carson reported no financial conflicts.
SOURCE: Obstet Gynecol. 2019 Oct 8. doi: 10.1097/AOG.0000000000003500; Obstet Gynecol. 2019 Oct 8. doi: 10.1097/AOG.0000000000003514.
The novel drug bremelanotide shows promise in acquired female hypoactive sexual desire disorder, according to the results of two randomized, controlled trials and a 52-week open-label extension study published online in Obstetrics & Gynecology.
Bremelanotide, which received Food and Drug Administration approval for this indication in June 2019, is an analog of the endogenous neuropeptide alpha-melanocyte-stimulating hormone.
Two separate, identically designed phase 3 studies (RECONNECT) were performed by Sheryl Kingsburg, MD, of the Cleveland Medical Center, and associates. Combined, 1,267 premenopausal women in monogamous relationships with acquired hypoactive sexual desire disorder were randomized to bremelanotide or placebo. Women in the treatment arm had significant improvement in female sexual function index–desire domain (FSFI-D) scores from baseline to week 24 (integrated studies: 0.35; P less than .001; effect size, 0.39), compared with placebo. They also experienced significant improvement in the FSFI-desire/arousal/orgasm (FSFI-DAO) domain (integrated studies: –0.33; P less than .001; effect size, 0.27).
The most common adverse events were nausea (integrated: 40% versus 1% in placebo), flushing (20% versus 0.3%), and headache (11% versus 2%). Overall, 77% in the treatment group reported a treatment-emergent adverse event, compared with 58% in the placebo group.
The open-label follow-up study was led by James Simon, MD, of George Washington University and IntimMedicine Specialists, Washington. Of the 684 participants who opted to enter the extension study, 40% completed it. In those who received bremelanotide during the randomized trial, the change in FSFI-D scores from baseline to the end of the open-label study ranged from 1.25 to 1.30, while the change in FSFI-DAO ranged from –1.4 to –1.7. In patients originally on placebo, the changes were 0.70-0.77 and –0.9, respectively.
Both groups surpassed the minimally clinically important difference for the FSFI-D score, which is considered to be 0.6.
“Patients switching from placebo experienced a higher incidence of adverse events than those continuing on bremelanotide during the open-label extension (79% versus 63%, respectively),” Dr. Simon and associates said.
The treatment is subcutaneous and can be self-administered up to about 45 minutes before a sexual event, no more than once during a 24-hour period, and no more than 8 doses per month, according to an FDA press release. The drug is contraindicated for women with cardiovascular disease or uncontrolled hypertension due to observations of transiently, slightly increased blood pressure.
The trials were funded by Palatin Technologies and AMAG Pharmaceuticals. The authors and coauthors have extensive financial relationships with pharmaceutical companies. Dr. Carson reported no financial conflicts.
SOURCE: Obstet Gynecol. 2019 Oct 8. doi: 10.1097/AOG.0000000000003500; Obstet Gynecol. 2019 Oct 8. doi: 10.1097/AOG.0000000000003514.
FROM OBSTETRICS & GYNECOLOGY
Suicide deaths rising in children aged 10-19 years
according to the National Center for Health Statistics.
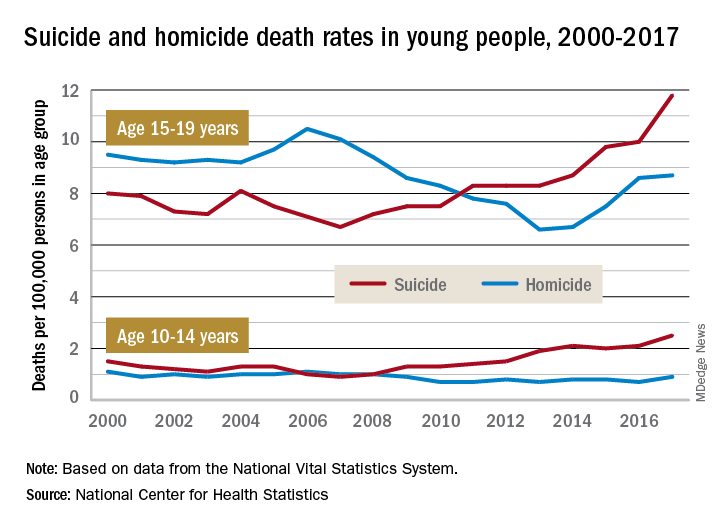
Death rates from suicide for children aged 10-14 years jumped by 178% from 2007 to 2017, while teenagers aged 15-19 years experienced a 76% increase over that period, with both changes reaching significance, the NCHS said in a recent data brief based on data from the National Vital Statistics System.
The actual rate for teens was higher to begin with, however, so in absolute terms the increase is larger for the older group. In 2007, deaths from suicide occurred at a rate of 6.7 per 100,000 persons for persons aged 15-19 years, and by 2017 that rate was up significantly to 11.8 per 100,000. Among children aged 10-14 years, the suicide-related death rate climbed from 0.9 per 100,000 in 2007 to 2.5 in 2014, the NCHS investigators reported.
The news was somewhat better on the other side of the violent death coin. Homicides are down by a significant 18% since 2000 among children aged 10-14 years, as the rate dropped from 1.1 per 100,000 in 2000 to 0.9 in 2017. The homicide rate since 2000 is down slightly for teens aged 15-19 years, but it has risen 32% in recent years, going from 6.6 deaths per 100,000 in 2013 to 8.7 in 2017, they said.
Suicide was the second-leading cause of death in both age groups in 2017, and homicide was third for those aged 15-19 and fifth among 10- to 14-year-olds, the investigators noted.
according to the National Center for Health Statistics.

Death rates from suicide for children aged 10-14 years jumped by 178% from 2007 to 2017, while teenagers aged 15-19 years experienced a 76% increase over that period, with both changes reaching significance, the NCHS said in a recent data brief based on data from the National Vital Statistics System.
The actual rate for teens was higher to begin with, however, so in absolute terms the increase is larger for the older group. In 2007, deaths from suicide occurred at a rate of 6.7 per 100,000 persons for persons aged 15-19 years, and by 2017 that rate was up significantly to 11.8 per 100,000. Among children aged 10-14 years, the suicide-related death rate climbed from 0.9 per 100,000 in 2007 to 2.5 in 2014, the NCHS investigators reported.
The news was somewhat better on the other side of the violent death coin. Homicides are down by a significant 18% since 2000 among children aged 10-14 years, as the rate dropped from 1.1 per 100,000 in 2000 to 0.9 in 2017. The homicide rate since 2000 is down slightly for teens aged 15-19 years, but it has risen 32% in recent years, going from 6.6 deaths per 100,000 in 2013 to 8.7 in 2017, they said.
Suicide was the second-leading cause of death in both age groups in 2017, and homicide was third for those aged 15-19 and fifth among 10- to 14-year-olds, the investigators noted.
according to the National Center for Health Statistics.

Death rates from suicide for children aged 10-14 years jumped by 178% from 2007 to 2017, while teenagers aged 15-19 years experienced a 76% increase over that period, with both changes reaching significance, the NCHS said in a recent data brief based on data from the National Vital Statistics System.
The actual rate for teens was higher to begin with, however, so in absolute terms the increase is larger for the older group. In 2007, deaths from suicide occurred at a rate of 6.7 per 100,000 persons for persons aged 15-19 years, and by 2017 that rate was up significantly to 11.8 per 100,000. Among children aged 10-14 years, the suicide-related death rate climbed from 0.9 per 100,000 in 2007 to 2.5 in 2014, the NCHS investigators reported.
The news was somewhat better on the other side of the violent death coin. Homicides are down by a significant 18% since 2000 among children aged 10-14 years, as the rate dropped from 1.1 per 100,000 in 2000 to 0.9 in 2017. The homicide rate since 2000 is down slightly for teens aged 15-19 years, but it has risen 32% in recent years, going from 6.6 deaths per 100,000 in 2013 to 8.7 in 2017, they said.
Suicide was the second-leading cause of death in both age groups in 2017, and homicide was third for those aged 15-19 and fifth among 10- to 14-year-olds, the investigators noted.