User login
Diagnosis Is an Open Book
ANSWER
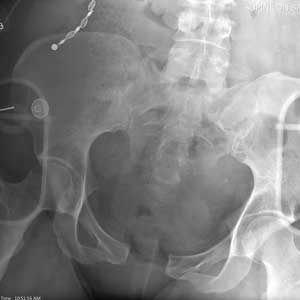
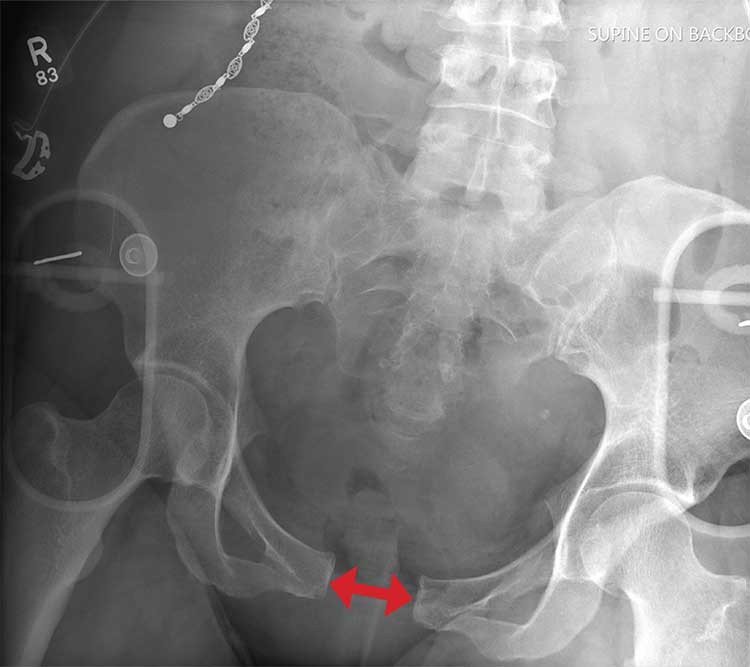
There is evidence of significant widening of the pubic symphysis. This injury results in a disruption of the normal pelvic ring. Such fractures are typically referred to as open-book pelvic fractures. Usually the result of high-energy trauma, they can also be associated with bladder and/or vascular injuries.
Orthopedics was consulted for management of this injury. Prompt stabilization with an external binder may help reduce complications. The patient will ultimately require some form of external or internal fixation.

ANSWER
There is evidence of significant widening of the pubic symphysis. This injury results in a disruption of the normal pelvic ring. Such fractures are typically referred to as open-book pelvic fractures. Usually the result of high-energy trauma, they can also be associated with bladder and/or vascular injuries.
Orthopedics was consulted for management of this injury. Prompt stabilization with an external binder may help reduce complications. The patient will ultimately require some form of external or internal fixation.

ANSWER
There is evidence of significant widening of the pubic symphysis. This injury results in a disruption of the normal pelvic ring. Such fractures are typically referred to as open-book pelvic fractures. Usually the result of high-energy trauma, they can also be associated with bladder and/or vascular injuries.
Orthopedics was consulted for management of this injury. Prompt stabilization with an external binder may help reduce complications. The patient will ultimately require some form of external or internal fixation.
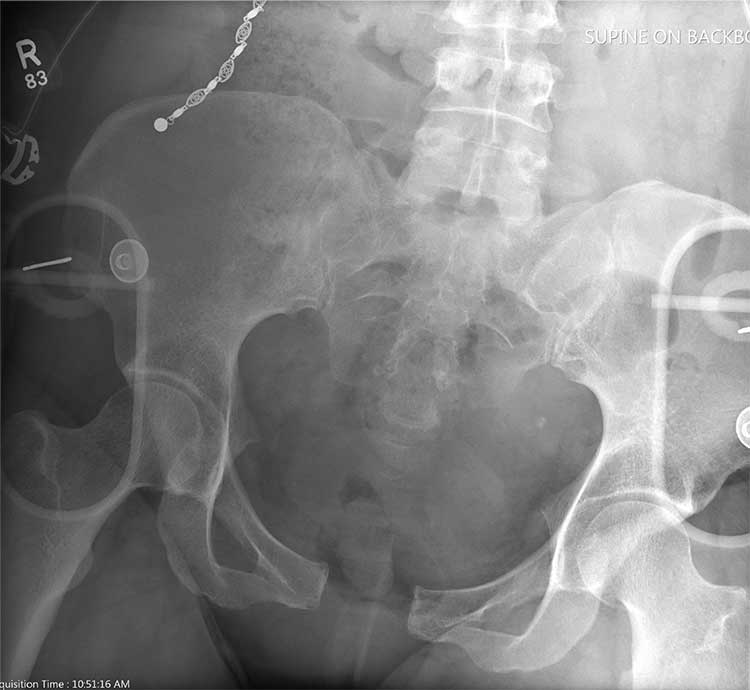
A 50-year-old woman is airlifted to your facility from the scene of an accident. She was riding a motorcycle that was struck by a car at a presumed high rate of speed. There was reportedly a brief loss of consciousness, although when the first responders arrived, the patient was complaining of pain in both her hands and wrists, as well as severe hip pain.
The patient was hemodynamically stable during transport. Blood pressure on arrival is 130/78 mm Hg, with a heart rate of 90 beats/min. Her O2 saturation is 97% with supplemental oxygen administered by nasal cannula.
As you begin your primary survey, the patient responds appropriately to you: She tells you her name and where she is from. Her medical history is significant for migraines, and her surgical history is significant for a prior cholecystectomy and tubal ligation. Primary exam overall appears normal, except for obvious deformities in both wrists.
As you begin your secondary survey, the radiology technicians obtain portable chest and pelvis radiographs (latter shown). What is your impression?
Biogen plans to submit application to FDA for Alzheimer’s drug aducanumab
Biogen aims to file with the Food and Drug Administration for regulatory approval of aducanumab, an antibody under investigation for Alzheimer’s disease, in 2020 following largely positive results of a secondary analysis of two failed phase 3 trials, ENGAGE and EMERGE, the company announced Oct. 22.
Biogen’s plans reverse its March 21, 2019, decision with codeveloper Eisai to discontinue work on the drug after a futility analysis of the trials determined aducanumab was unlikely to yield significant benefit. Biogen announced the plan to file a biologic drug application following a new analysis of additional data that became available after the data lock on the futility analysis. But while primary and secondary endpoints were nearly all positive for EMERGE in the secondary analysis of the larger dataset, the same could not be said for the twin trial, ENGAGE, which had negative results for most of its endpoints. However, Biogen said that “results from a subset of patients in the phase 3 ENGAGE study who received sufficient exposure to high-dose aducanumab support the findings from EMERGE.”
Both the Alzheimer’s Association and researchers interpreted the announcement with a measured tone, saying it offered a hopeful sign for a field continually stymied in its quest for an effective treatment. More than 100 clinical trials have failed over the last 20 years.
“This really is very encouraging news,” Rebecca M. Edelmayer, PhD, director of scientific engagement at the Alzheimer’s Association, said in an interview. The secondary combined analyses showed “the largest reductions in clinical and functional decline we have seen. It’s an important moment for the patients with AD and their families, and for researchers all around the world. This deserves to be discussed and considered by the research community, but we really need to dig deep into the data. We expect to see more of them at the Clinical Trials on Alzheimer’s Disease conference,” which is set for early December.
Paul Aisen, MD, a consultant for Biogen and director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles, was similarly measured.
“There is an enormous amount of data here and they will be very challenging to interpret, especially since both trials were stopped in a futility analysis,” he said in an interview. “We’re now interpreting data that continue to be collected after the initial data lock. But I do believe there is evidence that supports the amyloid hypothesis and the development of aducanumab.”
A deep data dive is in order before the field completely embraces aducanumab’s advancement, agreed Michael Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence.
“We would have to see exactly how they came up with this new data set and analysis. I have felt for many years now that these companies would try to parse and shuffle the data around until they got a statistically significant result, just to get approval. They would make billions per year but not really make a difference in people’s lives. That being said, if aducanumab truly slows the decline in activities of daily living, keeping people independent longer, that would be a worthwhile clinical result.”
Still to be considered is whether aducanumab could confer enough benefits to be worth monthly, potentially lifelong, infusions of a pricey medication that still won’t stop disease progression.
“Whether the clinical impact will be worth the anticipated cost remains to be seen,” Richard J. Caselli, MD, said in an interview. “This is likely to be a very expensive treatment for a subgroup of individuals with the hope of slowing decline, but – unless there is a huge upside surprise in data yet to be released – it is not going to halt progression and will certainly increase the cost of care dramatically.”
Dr. Caselli, clinical core director of the Alzheimer’s Disease Center at the Mayo Clinic in Phoenix, continued: “I imagine if approved, there will be a number of insurance obstacles to overcome regarding who qualifies, for how long, etc. Scientifically, this certainly supports the long-held view that beta amyloid is important in the pathophysiology of Alzheimer’s disease. But, again, unless the impact is unexpectedly huge, I don’t think this quiets those who feel there is more to the story than only a gain of beta amyloid toxicity, though this does support the idea that it plays a role, which should not surprise anyone.”
Dr. Edelmayer said the Alzheimer’s Association has raised the issue of access and cost with Biogen.
“We have had many discussions about their capacity to roll this out,” if aducanumab is approved, she said. “Those who were in the studies will be the first recipients. But Biogen is making plans to move this into the general population if approved, to all patients who meet the diagnostic criteria.”
“There is precedent out there when it comes to doing infusion medicines, and those centers are part of the planning process. In terms of pricing, this is a problem we would be happy to see, because it would mean that we have a treatment. But we’ll cross that bridge when we come to it.”
Secondary analysis results
The new analysis comprised 2,066 patients who had the opportunity to complete the full 18-month trials by March 20, 2019. The full intent-to-treat population of the trials comprised 3,285 patients with mild cognitive impairment caused by Alzheimer’s disease or mild Alzheimer’s disease dementia.
In the secondary analysis of EMERGE’s intent-to-treat population, patients who took the highest dose of aducanumab (10 mg/kg every month) showed 23% lower functional and cognitive decline on the trial’s primary endpoint of Clinical Dementia Rating–Sum of Boxes (CDR-SB) at 18 months when compared with placebo. The rate of decline was slowed by a nonsignificant 14% among users of the lower dose (6 mg/kg monthly).Secondary endpoints for the high-dose group in EMERGE showed 27% slower cognitive decline on the 13-item cognitive subscale of the AD Assessment Scale (ADAS-Cog13) and 40% lower decline in function among patients with mild cognitive impairment based on the MCI version of the AD Cooperative Study–Activities of Daily Living Inventory (ADCS-ADL-MCI).
However, data from the ENGAGE trial, which had the same primary endpoint, were not positive. CDR-SB scores worsened 2% more among high-dose aducanumab users but low-dose users slowed decline by a nonsignificant 12% when compared with placebo.“Exposure to high-dose aducanumab was important for efficacy,” the company noted in a slide set presented at an Oct. 22 investors webcast. “Differences in exposure to high-dose aducanumab largely explain the different results between the futility analysis and the new analysis of this larger dataset, as well as the different results between the two studies.”
In EMERGE, changes in secondary endpoints among patients who had the opportunity to complete the full 18-month trials included:
- Mini Mental State Exam (MMSE): Significant 23% decrease in rate of decline in the high-dose group and nonsignificant 3% increase in decline in the low-dose group.
- ADAS-Cog13: Significant 25% decrease for high-dose users and nonsignificant 10% decrease for low dose.
- ADCS-ADL-MCI: Significant decreases of 46% for high-dose and 20% for low-dose users.
The ENGAGE secondary endpoints of high- vs. low-dose patients who completed the full trials were:
- MMSE: Significant 13% increase, nonsignificant 3% decrease.
- ADAS-Cog13: Nonsignificant 2% decrease, nonsignificant 1% decrease.
- ADCS-ADL-MCI: Significant 12% declines in both dosage groups.
All of the positive cognitive and functional results tracked along with results of amyloid PET imaging and CSF biomarkers. In EMERGE, amyloid plaque binding declined about 27% in the high-dose group and about 16% in the low-dose group, reflecting plaque clearance. Phosphorylated tau in CSF decreased by about 17 pg/mL and 10 pg/mL, respectively, and total tau decreased by 160 pg/mL and 120 pg/mL. Tau decreases indicate a slowing of neuronal damage.
In ENGAGE, amyloid plaque binding decreased by about 24% in the high-dose group and by about 16% in the low-dose group. Phosphorylated tau dropped by about 10 pg/mL and 11 pg/mL, respectively. But total tau dropped more in the low-dose group than in the high-dose group (about –100 pg/mL vs. –20 pg/mL).
Biogen said the amyloid PET imaging biomarker results and CDR-SB scores in both studies were consistent with each other in a subset of patients with “sufficient exposure to 10 mg/kg,” which was defined as “10 or more uninterrupted 10-mg/kg dosing intervals at steady-state.”
The most common adverse events were amyloid related imaging abnormalities–edema (ARIA-E), which occurred in 35%, and headache in 20%. The majority of patients who experienced ARIA-E (74%) were asymptomatic; episodes generally resolved within 4-16 weeks, typically without long-term sequelae.
Dr. Aisen is a consultant for Biogen and on the aducanumab steering committee. None of the other sources in this article have any financial relationship with Biogen or Eisai.
This article was updated 10/23/19.
Biogen aims to file with the Food and Drug Administration for regulatory approval of aducanumab, an antibody under investigation for Alzheimer’s disease, in 2020 following largely positive results of a secondary analysis of two failed phase 3 trials, ENGAGE and EMERGE, the company announced Oct. 22.
Biogen’s plans reverse its March 21, 2019, decision with codeveloper Eisai to discontinue work on the drug after a futility analysis of the trials determined aducanumab was unlikely to yield significant benefit. Biogen announced the plan to file a biologic drug application following a new analysis of additional data that became available after the data lock on the futility analysis. But while primary and secondary endpoints were nearly all positive for EMERGE in the secondary analysis of the larger dataset, the same could not be said for the twin trial, ENGAGE, which had negative results for most of its endpoints. However, Biogen said that “results from a subset of patients in the phase 3 ENGAGE study who received sufficient exposure to high-dose aducanumab support the findings from EMERGE.”
Both the Alzheimer’s Association and researchers interpreted the announcement with a measured tone, saying it offered a hopeful sign for a field continually stymied in its quest for an effective treatment. More than 100 clinical trials have failed over the last 20 years.
“This really is very encouraging news,” Rebecca M. Edelmayer, PhD, director of scientific engagement at the Alzheimer’s Association, said in an interview. The secondary combined analyses showed “the largest reductions in clinical and functional decline we have seen. It’s an important moment for the patients with AD and their families, and for researchers all around the world. This deserves to be discussed and considered by the research community, but we really need to dig deep into the data. We expect to see more of them at the Clinical Trials on Alzheimer’s Disease conference,” which is set for early December.
Paul Aisen, MD, a consultant for Biogen and director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles, was similarly measured.
“There is an enormous amount of data here and they will be very challenging to interpret, especially since both trials were stopped in a futility analysis,” he said in an interview. “We’re now interpreting data that continue to be collected after the initial data lock. But I do believe there is evidence that supports the amyloid hypothesis and the development of aducanumab.”
A deep data dive is in order before the field completely embraces aducanumab’s advancement, agreed Michael Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence.
“We would have to see exactly how they came up with this new data set and analysis. I have felt for many years now that these companies would try to parse and shuffle the data around until they got a statistically significant result, just to get approval. They would make billions per year but not really make a difference in people’s lives. That being said, if aducanumab truly slows the decline in activities of daily living, keeping people independent longer, that would be a worthwhile clinical result.”
Still to be considered is whether aducanumab could confer enough benefits to be worth monthly, potentially lifelong, infusions of a pricey medication that still won’t stop disease progression.
“Whether the clinical impact will be worth the anticipated cost remains to be seen,” Richard J. Caselli, MD, said in an interview. “This is likely to be a very expensive treatment for a subgroup of individuals with the hope of slowing decline, but – unless there is a huge upside surprise in data yet to be released – it is not going to halt progression and will certainly increase the cost of care dramatically.”
Dr. Caselli, clinical core director of the Alzheimer’s Disease Center at the Mayo Clinic in Phoenix, continued: “I imagine if approved, there will be a number of insurance obstacles to overcome regarding who qualifies, for how long, etc. Scientifically, this certainly supports the long-held view that beta amyloid is important in the pathophysiology of Alzheimer’s disease. But, again, unless the impact is unexpectedly huge, I don’t think this quiets those who feel there is more to the story than only a gain of beta amyloid toxicity, though this does support the idea that it plays a role, which should not surprise anyone.”
Dr. Edelmayer said the Alzheimer’s Association has raised the issue of access and cost with Biogen.
“We have had many discussions about their capacity to roll this out,” if aducanumab is approved, she said. “Those who were in the studies will be the first recipients. But Biogen is making plans to move this into the general population if approved, to all patients who meet the diagnostic criteria.”
“There is precedent out there when it comes to doing infusion medicines, and those centers are part of the planning process. In terms of pricing, this is a problem we would be happy to see, because it would mean that we have a treatment. But we’ll cross that bridge when we come to it.”
Secondary analysis results
The new analysis comprised 2,066 patients who had the opportunity to complete the full 18-month trials by March 20, 2019. The full intent-to-treat population of the trials comprised 3,285 patients with mild cognitive impairment caused by Alzheimer’s disease or mild Alzheimer’s disease dementia.
In the secondary analysis of EMERGE’s intent-to-treat population, patients who took the highest dose of aducanumab (10 mg/kg every month) showed 23% lower functional and cognitive decline on the trial’s primary endpoint of Clinical Dementia Rating–Sum of Boxes (CDR-SB) at 18 months when compared with placebo. The rate of decline was slowed by a nonsignificant 14% among users of the lower dose (6 mg/kg monthly).Secondary endpoints for the high-dose group in EMERGE showed 27% slower cognitive decline on the 13-item cognitive subscale of the AD Assessment Scale (ADAS-Cog13) and 40% lower decline in function among patients with mild cognitive impairment based on the MCI version of the AD Cooperative Study–Activities of Daily Living Inventory (ADCS-ADL-MCI).
However, data from the ENGAGE trial, which had the same primary endpoint, were not positive. CDR-SB scores worsened 2% more among high-dose aducanumab users but low-dose users slowed decline by a nonsignificant 12% when compared with placebo.“Exposure to high-dose aducanumab was important for efficacy,” the company noted in a slide set presented at an Oct. 22 investors webcast. “Differences in exposure to high-dose aducanumab largely explain the different results between the futility analysis and the new analysis of this larger dataset, as well as the different results between the two studies.”
In EMERGE, changes in secondary endpoints among patients who had the opportunity to complete the full 18-month trials included:
- Mini Mental State Exam (MMSE): Significant 23% decrease in rate of decline in the high-dose group and nonsignificant 3% increase in decline in the low-dose group.
- ADAS-Cog13: Significant 25% decrease for high-dose users and nonsignificant 10% decrease for low dose.
- ADCS-ADL-MCI: Significant decreases of 46% for high-dose and 20% for low-dose users.
The ENGAGE secondary endpoints of high- vs. low-dose patients who completed the full trials were:
- MMSE: Significant 13% increase, nonsignificant 3% decrease.
- ADAS-Cog13: Nonsignificant 2% decrease, nonsignificant 1% decrease.
- ADCS-ADL-MCI: Significant 12% declines in both dosage groups.
All of the positive cognitive and functional results tracked along with results of amyloid PET imaging and CSF biomarkers. In EMERGE, amyloid plaque binding declined about 27% in the high-dose group and about 16% in the low-dose group, reflecting plaque clearance. Phosphorylated tau in CSF decreased by about 17 pg/mL and 10 pg/mL, respectively, and total tau decreased by 160 pg/mL and 120 pg/mL. Tau decreases indicate a slowing of neuronal damage.
In ENGAGE, amyloid plaque binding decreased by about 24% in the high-dose group and by about 16% in the low-dose group. Phosphorylated tau dropped by about 10 pg/mL and 11 pg/mL, respectively. But total tau dropped more in the low-dose group than in the high-dose group (about –100 pg/mL vs. –20 pg/mL).
Biogen said the amyloid PET imaging biomarker results and CDR-SB scores in both studies were consistent with each other in a subset of patients with “sufficient exposure to 10 mg/kg,” which was defined as “10 or more uninterrupted 10-mg/kg dosing intervals at steady-state.”
The most common adverse events were amyloid related imaging abnormalities–edema (ARIA-E), which occurred in 35%, and headache in 20%. The majority of patients who experienced ARIA-E (74%) were asymptomatic; episodes generally resolved within 4-16 weeks, typically without long-term sequelae.
Dr. Aisen is a consultant for Biogen and on the aducanumab steering committee. None of the other sources in this article have any financial relationship with Biogen or Eisai.
This article was updated 10/23/19.
Biogen aims to file with the Food and Drug Administration for regulatory approval of aducanumab, an antibody under investigation for Alzheimer’s disease, in 2020 following largely positive results of a secondary analysis of two failed phase 3 trials, ENGAGE and EMERGE, the company announced Oct. 22.
Biogen’s plans reverse its March 21, 2019, decision with codeveloper Eisai to discontinue work on the drug after a futility analysis of the trials determined aducanumab was unlikely to yield significant benefit. Biogen announced the plan to file a biologic drug application following a new analysis of additional data that became available after the data lock on the futility analysis. But while primary and secondary endpoints were nearly all positive for EMERGE in the secondary analysis of the larger dataset, the same could not be said for the twin trial, ENGAGE, which had negative results for most of its endpoints. However, Biogen said that “results from a subset of patients in the phase 3 ENGAGE study who received sufficient exposure to high-dose aducanumab support the findings from EMERGE.”
Both the Alzheimer’s Association and researchers interpreted the announcement with a measured tone, saying it offered a hopeful sign for a field continually stymied in its quest for an effective treatment. More than 100 clinical trials have failed over the last 20 years.
“This really is very encouraging news,” Rebecca M. Edelmayer, PhD, director of scientific engagement at the Alzheimer’s Association, said in an interview. The secondary combined analyses showed “the largest reductions in clinical and functional decline we have seen. It’s an important moment for the patients with AD and their families, and for researchers all around the world. This deserves to be discussed and considered by the research community, but we really need to dig deep into the data. We expect to see more of them at the Clinical Trials on Alzheimer’s Disease conference,” which is set for early December.
Paul Aisen, MD, a consultant for Biogen and director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles, was similarly measured.
“There is an enormous amount of data here and they will be very challenging to interpret, especially since both trials were stopped in a futility analysis,” he said in an interview. “We’re now interpreting data that continue to be collected after the initial data lock. But I do believe there is evidence that supports the amyloid hypothesis and the development of aducanumab.”
A deep data dive is in order before the field completely embraces aducanumab’s advancement, agreed Michael Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence.
“We would have to see exactly how they came up with this new data set and analysis. I have felt for many years now that these companies would try to parse and shuffle the data around until they got a statistically significant result, just to get approval. They would make billions per year but not really make a difference in people’s lives. That being said, if aducanumab truly slows the decline in activities of daily living, keeping people independent longer, that would be a worthwhile clinical result.”
Still to be considered is whether aducanumab could confer enough benefits to be worth monthly, potentially lifelong, infusions of a pricey medication that still won’t stop disease progression.
“Whether the clinical impact will be worth the anticipated cost remains to be seen,” Richard J. Caselli, MD, said in an interview. “This is likely to be a very expensive treatment for a subgroup of individuals with the hope of slowing decline, but – unless there is a huge upside surprise in data yet to be released – it is not going to halt progression and will certainly increase the cost of care dramatically.”
Dr. Caselli, clinical core director of the Alzheimer’s Disease Center at the Mayo Clinic in Phoenix, continued: “I imagine if approved, there will be a number of insurance obstacles to overcome regarding who qualifies, for how long, etc. Scientifically, this certainly supports the long-held view that beta amyloid is important in the pathophysiology of Alzheimer’s disease. But, again, unless the impact is unexpectedly huge, I don’t think this quiets those who feel there is more to the story than only a gain of beta amyloid toxicity, though this does support the idea that it plays a role, which should not surprise anyone.”
Dr. Edelmayer said the Alzheimer’s Association has raised the issue of access and cost with Biogen.
“We have had many discussions about their capacity to roll this out,” if aducanumab is approved, she said. “Those who were in the studies will be the first recipients. But Biogen is making plans to move this into the general population if approved, to all patients who meet the diagnostic criteria.”
“There is precedent out there when it comes to doing infusion medicines, and those centers are part of the planning process. In terms of pricing, this is a problem we would be happy to see, because it would mean that we have a treatment. But we’ll cross that bridge when we come to it.”
Secondary analysis results
The new analysis comprised 2,066 patients who had the opportunity to complete the full 18-month trials by March 20, 2019. The full intent-to-treat population of the trials comprised 3,285 patients with mild cognitive impairment caused by Alzheimer’s disease or mild Alzheimer’s disease dementia.
In the secondary analysis of EMERGE’s intent-to-treat population, patients who took the highest dose of aducanumab (10 mg/kg every month) showed 23% lower functional and cognitive decline on the trial’s primary endpoint of Clinical Dementia Rating–Sum of Boxes (CDR-SB) at 18 months when compared with placebo. The rate of decline was slowed by a nonsignificant 14% among users of the lower dose (6 mg/kg monthly).Secondary endpoints for the high-dose group in EMERGE showed 27% slower cognitive decline on the 13-item cognitive subscale of the AD Assessment Scale (ADAS-Cog13) and 40% lower decline in function among patients with mild cognitive impairment based on the MCI version of the AD Cooperative Study–Activities of Daily Living Inventory (ADCS-ADL-MCI).
However, data from the ENGAGE trial, which had the same primary endpoint, were not positive. CDR-SB scores worsened 2% more among high-dose aducanumab users but low-dose users slowed decline by a nonsignificant 12% when compared with placebo.“Exposure to high-dose aducanumab was important for efficacy,” the company noted in a slide set presented at an Oct. 22 investors webcast. “Differences in exposure to high-dose aducanumab largely explain the different results between the futility analysis and the new analysis of this larger dataset, as well as the different results between the two studies.”
In EMERGE, changes in secondary endpoints among patients who had the opportunity to complete the full 18-month trials included:
- Mini Mental State Exam (MMSE): Significant 23% decrease in rate of decline in the high-dose group and nonsignificant 3% increase in decline in the low-dose group.
- ADAS-Cog13: Significant 25% decrease for high-dose users and nonsignificant 10% decrease for low dose.
- ADCS-ADL-MCI: Significant decreases of 46% for high-dose and 20% for low-dose users.
The ENGAGE secondary endpoints of high- vs. low-dose patients who completed the full trials were:
- MMSE: Significant 13% increase, nonsignificant 3% decrease.
- ADAS-Cog13: Nonsignificant 2% decrease, nonsignificant 1% decrease.
- ADCS-ADL-MCI: Significant 12% declines in both dosage groups.
All of the positive cognitive and functional results tracked along with results of amyloid PET imaging and CSF biomarkers. In EMERGE, amyloid plaque binding declined about 27% in the high-dose group and about 16% in the low-dose group, reflecting plaque clearance. Phosphorylated tau in CSF decreased by about 17 pg/mL and 10 pg/mL, respectively, and total tau decreased by 160 pg/mL and 120 pg/mL. Tau decreases indicate a slowing of neuronal damage.
In ENGAGE, amyloid plaque binding decreased by about 24% in the high-dose group and by about 16% in the low-dose group. Phosphorylated tau dropped by about 10 pg/mL and 11 pg/mL, respectively. But total tau dropped more in the low-dose group than in the high-dose group (about –100 pg/mL vs. –20 pg/mL).
Biogen said the amyloid PET imaging biomarker results and CDR-SB scores in both studies were consistent with each other in a subset of patients with “sufficient exposure to 10 mg/kg,” which was defined as “10 or more uninterrupted 10-mg/kg dosing intervals at steady-state.”
The most common adverse events were amyloid related imaging abnormalities–edema (ARIA-E), which occurred in 35%, and headache in 20%. The majority of patients who experienced ARIA-E (74%) were asymptomatic; episodes generally resolved within 4-16 weeks, typically without long-term sequelae.
Dr. Aisen is a consultant for Biogen and on the aducanumab steering committee. None of the other sources in this article have any financial relationship with Biogen or Eisai.
This article was updated 10/23/19.
CDC: Don’t vape, especially THC
Federal health officials once again are warning individuals to refrain from using all e-cigarette and vaping products, especially those containing tetrahydrocannabinol (THC).
The restated warning, issued by the Centers for Disease Control and Prevention, is based on a study of 83 patients with e-cigarette, or vaping, product use–associated lung injury (EVALI) in Utah, where researchers found several common characteristics, most strikingly the use of THC-containing products.
Fifty-three patients were interviewed by researchers. Of them, 49 (92%) reported use of THC-containing e-cigarette or vaping products during the 3 months preceding illness; 35 (66%) reported using nicotine-containing products; and 32 (60%) reported using both THC- and nicotine-containing products.
In addition, 17 (32%) patients reported exclusive use of THC-containing products, whereas only 3 (6%) reported exclusive use of nicotine-containing products. Non-medical THC use is illegal in Utah.
The median age of patients was 26 years, 3 years older than the national median; more than one-third were aged 30 years or older, according to the researchers.
Utah is seeing a higher-than-average rate of EVALI cases, with 26/million cases, compared with 4/million nationally.
Vitamin E acetate has been considered to have a suspect role in EVALI and was identified in the majority of THC cartridge samples tested in this study; however, those samples represented only six patients, according to the researchers. They added that testing of different THC cartridge samples by the Food and Drug Administration and other laboratories has shown vitamin E acetate concentrations of 31%-88% and lower-than-expected THC concentrations (14%-76% versus the typically advertised 75%-95%).
“The potential role of vitamin E acetate in lung injury remains unknown; however, the identification of vitamin E acetate among products collected from patients in Utah and elsewhere indicates that the outbreak might be associated with cutting agents or adulterants. Ascertaining the potential contribution of diluents to the current outbreak will require data from multiple states and analysis at the national level,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Lewis N et al. MMWR Morb Mortal Wkly Rep. Early Release. Oct. 22, 2019. 68:1-5.
Federal health officials once again are warning individuals to refrain from using all e-cigarette and vaping products, especially those containing tetrahydrocannabinol (THC).
The restated warning, issued by the Centers for Disease Control and Prevention, is based on a study of 83 patients with e-cigarette, or vaping, product use–associated lung injury (EVALI) in Utah, where researchers found several common characteristics, most strikingly the use of THC-containing products.
Fifty-three patients were interviewed by researchers. Of them, 49 (92%) reported use of THC-containing e-cigarette or vaping products during the 3 months preceding illness; 35 (66%) reported using nicotine-containing products; and 32 (60%) reported using both THC- and nicotine-containing products.
In addition, 17 (32%) patients reported exclusive use of THC-containing products, whereas only 3 (6%) reported exclusive use of nicotine-containing products. Non-medical THC use is illegal in Utah.
The median age of patients was 26 years, 3 years older than the national median; more than one-third were aged 30 years or older, according to the researchers.
Utah is seeing a higher-than-average rate of EVALI cases, with 26/million cases, compared with 4/million nationally.
Vitamin E acetate has been considered to have a suspect role in EVALI and was identified in the majority of THC cartridge samples tested in this study; however, those samples represented only six patients, according to the researchers. They added that testing of different THC cartridge samples by the Food and Drug Administration and other laboratories has shown vitamin E acetate concentrations of 31%-88% and lower-than-expected THC concentrations (14%-76% versus the typically advertised 75%-95%).
“The potential role of vitamin E acetate in lung injury remains unknown; however, the identification of vitamin E acetate among products collected from patients in Utah and elsewhere indicates that the outbreak might be associated with cutting agents or adulterants. Ascertaining the potential contribution of diluents to the current outbreak will require data from multiple states and analysis at the national level,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Lewis N et al. MMWR Morb Mortal Wkly Rep. Early Release. Oct. 22, 2019. 68:1-5.
Federal health officials once again are warning individuals to refrain from using all e-cigarette and vaping products, especially those containing tetrahydrocannabinol (THC).
The restated warning, issued by the Centers for Disease Control and Prevention, is based on a study of 83 patients with e-cigarette, or vaping, product use–associated lung injury (EVALI) in Utah, where researchers found several common characteristics, most strikingly the use of THC-containing products.
Fifty-three patients were interviewed by researchers. Of them, 49 (92%) reported use of THC-containing e-cigarette or vaping products during the 3 months preceding illness; 35 (66%) reported using nicotine-containing products; and 32 (60%) reported using both THC- and nicotine-containing products.
In addition, 17 (32%) patients reported exclusive use of THC-containing products, whereas only 3 (6%) reported exclusive use of nicotine-containing products. Non-medical THC use is illegal in Utah.
The median age of patients was 26 years, 3 years older than the national median; more than one-third were aged 30 years or older, according to the researchers.
Utah is seeing a higher-than-average rate of EVALI cases, with 26/million cases, compared with 4/million nationally.
Vitamin E acetate has been considered to have a suspect role in EVALI and was identified in the majority of THC cartridge samples tested in this study; however, those samples represented only six patients, according to the researchers. They added that testing of different THC cartridge samples by the Food and Drug Administration and other laboratories has shown vitamin E acetate concentrations of 31%-88% and lower-than-expected THC concentrations (14%-76% versus the typically advertised 75%-95%).
“The potential role of vitamin E acetate in lung injury remains unknown; however, the identification of vitamin E acetate among products collected from patients in Utah and elsewhere indicates that the outbreak might be associated with cutting agents or adulterants. Ascertaining the potential contribution of diluents to the current outbreak will require data from multiple states and analysis at the national level,” the researchers concluded.
The authors reported that they had no conflicts.
SOURCE: Lewis N et al. MMWR Morb Mortal Wkly Rep. Early Release. Oct. 22, 2019. 68:1-5.
FROM MMWR
Recent COPD exacerbation did not affect aclidinium’s efficacy in high-risk patients
NEW ORLEANS – A history of recent exacerbations did not significantly affect the safety or efficacy of aclidinium bromide (Tudorza) in patients with moderate to severe chronic obstructive pulmonary disease and high cardiovascular risk, analysis of a postmarketing surveillance trial suggests.
Regardless of exacerbation history, the long-acting muscarinic antagonist reduced the rate of moderate or severe COPD exacerbations versus placebo in this subgroup analysis of the phase IV ASCENT-COPD trial, presented here at the annual meeting of the American College of Chest Physicians.
At the same time, there were no significant increases in the risk of mortality or major cardiac adverse events (MACE) for those patients who had an exacerbation in the past year versus those who did not, according to investigator Robert A. Wise, MD.
Those findings may be reassuring, given that COPD patients commonly have comorbidities and cardiovascular risk factors, according to Dr. Wise, professor of medicine at the Johns Hopkins University, Baltimore.
“There’s a concern and some evidence that patients who have a propensity to COPD exacerbations may also have an increased risk for cardiovascular events,” Dr. Wise said in a podium presentation.
Accordingly, he and coinvestigators sought to tease out the impact of COPD exacerbations on safety as well as efficacy in the randomized, placebo-controlled ASCENT-COPD trial, which included 3,630 patients with moderate to severe COPD plus a cardiovascular disease history or multiple atherothrombotic risk factors.
Of the patients who were analyzed in the study, 1,433 patients had at least one treated COPD exacerbation in the year before screening for the study, while 2,156 had no exacerbations in the prior year, Dr. Wise said.
Top-line results of that study, published several months ago, showed that aclidinium did not increase MACE risk over 3 years, and reduced the rate of moderate to severe COPD exacerbations over the first year (JAMA. 2019 7 May 7;321[17]:1693-701).
In this latest analysis, presented at the meeting, risk of MACE with aclidinium treatment was not increased versus placebo, irrespective of whether they had exacerbations in the prior year (interaction P = .233); likewise, the risk of all-cause mortality was similar between groups (P = .154).
In terms of reduction in moderate or severe COPD exacerbations in the first year, aclidinium was superior to placebo both for the patients who had at least one or exacerbation in the prior year (rate ratio, 0.80) and those who had no exacerbations in the prior year (RR, 0.69).
“This translates into a number-needed-to-treat to prevent one exacerbation of about 11 patients for those without an exacerbation, compared to about 6 patients for those with a prior exacerbation,” Dr. Wise said in his presentation.
The ASCENT-COPD study was funded initially by Forest Laboratories and later by AstraZeneca and Circassia. Dr. Wise provided disclosures related to AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Sunovion, Mylan/Theravance, Contrafect, Pearl, Merck, Verona, Novartis, AbbVie, Syneos, Regeneron, and Kiniksa.
SOURCE: Wise R et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.231.
NEW ORLEANS – A history of recent exacerbations did not significantly affect the safety or efficacy of aclidinium bromide (Tudorza) in patients with moderate to severe chronic obstructive pulmonary disease and high cardiovascular risk, analysis of a postmarketing surveillance trial suggests.
Regardless of exacerbation history, the long-acting muscarinic antagonist reduced the rate of moderate or severe COPD exacerbations versus placebo in this subgroup analysis of the phase IV ASCENT-COPD trial, presented here at the annual meeting of the American College of Chest Physicians.
At the same time, there were no significant increases in the risk of mortality or major cardiac adverse events (MACE) for those patients who had an exacerbation in the past year versus those who did not, according to investigator Robert A. Wise, MD.
Those findings may be reassuring, given that COPD patients commonly have comorbidities and cardiovascular risk factors, according to Dr. Wise, professor of medicine at the Johns Hopkins University, Baltimore.
“There’s a concern and some evidence that patients who have a propensity to COPD exacerbations may also have an increased risk for cardiovascular events,” Dr. Wise said in a podium presentation.
Accordingly, he and coinvestigators sought to tease out the impact of COPD exacerbations on safety as well as efficacy in the randomized, placebo-controlled ASCENT-COPD trial, which included 3,630 patients with moderate to severe COPD plus a cardiovascular disease history or multiple atherothrombotic risk factors.
Of the patients who were analyzed in the study, 1,433 patients had at least one treated COPD exacerbation in the year before screening for the study, while 2,156 had no exacerbations in the prior year, Dr. Wise said.
Top-line results of that study, published several months ago, showed that aclidinium did not increase MACE risk over 3 years, and reduced the rate of moderate to severe COPD exacerbations over the first year (JAMA. 2019 7 May 7;321[17]:1693-701).
In this latest analysis, presented at the meeting, risk of MACE with aclidinium treatment was not increased versus placebo, irrespective of whether they had exacerbations in the prior year (interaction P = .233); likewise, the risk of all-cause mortality was similar between groups (P = .154).
In terms of reduction in moderate or severe COPD exacerbations in the first year, aclidinium was superior to placebo both for the patients who had at least one or exacerbation in the prior year (rate ratio, 0.80) and those who had no exacerbations in the prior year (RR, 0.69).
“This translates into a number-needed-to-treat to prevent one exacerbation of about 11 patients for those without an exacerbation, compared to about 6 patients for those with a prior exacerbation,” Dr. Wise said in his presentation.
The ASCENT-COPD study was funded initially by Forest Laboratories and later by AstraZeneca and Circassia. Dr. Wise provided disclosures related to AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Sunovion, Mylan/Theravance, Contrafect, Pearl, Merck, Verona, Novartis, AbbVie, Syneos, Regeneron, and Kiniksa.
SOURCE: Wise R et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.231.
NEW ORLEANS – A history of recent exacerbations did not significantly affect the safety or efficacy of aclidinium bromide (Tudorza) in patients with moderate to severe chronic obstructive pulmonary disease and high cardiovascular risk, analysis of a postmarketing surveillance trial suggests.
Regardless of exacerbation history, the long-acting muscarinic antagonist reduced the rate of moderate or severe COPD exacerbations versus placebo in this subgroup analysis of the phase IV ASCENT-COPD trial, presented here at the annual meeting of the American College of Chest Physicians.
At the same time, there were no significant increases in the risk of mortality or major cardiac adverse events (MACE) for those patients who had an exacerbation in the past year versus those who did not, according to investigator Robert A. Wise, MD.
Those findings may be reassuring, given that COPD patients commonly have comorbidities and cardiovascular risk factors, according to Dr. Wise, professor of medicine at the Johns Hopkins University, Baltimore.
“There’s a concern and some evidence that patients who have a propensity to COPD exacerbations may also have an increased risk for cardiovascular events,” Dr. Wise said in a podium presentation.
Accordingly, he and coinvestigators sought to tease out the impact of COPD exacerbations on safety as well as efficacy in the randomized, placebo-controlled ASCENT-COPD trial, which included 3,630 patients with moderate to severe COPD plus a cardiovascular disease history or multiple atherothrombotic risk factors.
Of the patients who were analyzed in the study, 1,433 patients had at least one treated COPD exacerbation in the year before screening for the study, while 2,156 had no exacerbations in the prior year, Dr. Wise said.
Top-line results of that study, published several months ago, showed that aclidinium did not increase MACE risk over 3 years, and reduced the rate of moderate to severe COPD exacerbations over the first year (JAMA. 2019 7 May 7;321[17]:1693-701).
In this latest analysis, presented at the meeting, risk of MACE with aclidinium treatment was not increased versus placebo, irrespective of whether they had exacerbations in the prior year (interaction P = .233); likewise, the risk of all-cause mortality was similar between groups (P = .154).
In terms of reduction in moderate or severe COPD exacerbations in the first year, aclidinium was superior to placebo both for the patients who had at least one or exacerbation in the prior year (rate ratio, 0.80) and those who had no exacerbations in the prior year (RR, 0.69).
“This translates into a number-needed-to-treat to prevent one exacerbation of about 11 patients for those without an exacerbation, compared to about 6 patients for those with a prior exacerbation,” Dr. Wise said in his presentation.
The ASCENT-COPD study was funded initially by Forest Laboratories and later by AstraZeneca and Circassia. Dr. Wise provided disclosures related to AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Sunovion, Mylan/Theravance, Contrafect, Pearl, Merck, Verona, Novartis, AbbVie, Syneos, Regeneron, and Kiniksa.
SOURCE: Wise R et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.231.
REPORTING FROM CHEST 2019
Remote ischemic conditioning in STEMI, RIP
PARIS – Remote ischemic condition, long viewed as the best hope for the next breakthrough in improved clinical outcomes in acute MI, is now dead as a broad therapeutic strategy as a result of the resoundingly negative results of the CONDI-2/ERIC-PPCI trial, Hans Erik Bøtker, MD, PhD, declared at the annual congress of the European Society of Cardiology.
“I would not be quite honest if I did not admit that we were somewhat disappointed in these results, given the promising results we have seen in the majority of the proof-of-concept studies,” added Dr. Bøtker, professor of cardiovascular medicine and interventional cardiology at Aarhus (Denmark) University.
Remote ischemic conditioning entails imposing brief cycles of ischemia and reperfusion on an arm or leg prior to or during reperfusion of an occluded artery in the setting of acute MI in order to provide cardioprotection against reperfusion injury. In the small proof-of-concept studies, it reduced infarct size and suggested the possibility of better clinical outcomes. But the four-country, prospective, single-blind, randomized CONDI-2/ERIC-PPCI trial, involving 5,401 patients undergoing primary percutaneous coronary intervention (PCI) for ST-segment elevation MI (STEMI), showed nary a glimmer of the hoped-for clinical benefits from four cycles of 5 minutes of ischemia and 5 minutes of deflation of an automated arm-cuff device.
The primary outcome, a composite of the 12-month rate of cardiac death or heart failure hospitalization, occurred in 9.4% of the remote ischemic conditioning group, which wasn’t significantly different from the 8.6% rate in controls. Nor were there any significant between-group differences in infarct size at 48 hours based upon high-sensitivity troponin T measurement or any of the other prespecified secondary study endpoints.
Discussant Bernard J. Gersh, MB, ChB, PhD, a long-time fervent advocate of remote ischemic conditioning as an important potential advance in MI care, wasn’t an investigator in CONDI-2/ERIC-PPCI, which he characterized as “very well executed, large, and pivotal.”
“The results of this trial are emphatic, and there would appear to be no role for routine remote ischemic conditioning in the management of most – and I would emphasize ‘most,’ perhaps not all, but most – patients with STEMI undergoing primary PCI. The results may not be what we’d hoped to hear, and I share Dr. Bøtker’s disappointment, but the data appear to be conclusive in regard to the majority of the primary PCI population. It is what it is. The only remaining questions are why the result were almost completely universally neutral in CONDI-2/ERIC-PPCI after so many positive experimental models and proof-of-concept studies, and whether are there other patient subsets who might benefit,” according to Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
Pointing to the 30-day cardiac death rate of about 2% in the trial, he commented that the management of STEMI has gotten so good that it becomes very difficult to demonstrate a difference with remote ischemic conditioning, even if a small benefit is actually present.
“I think that’s the era that we now live in,” Dr. Gersh said.
However, he is not ready to throw the final shovelful of dirt on the grave of remote ischemic conditioning.
“There is a group where perhaps there may be a therapeutic benefit: STEMI patients presenting with cardiogenic shock and no cardiac arrest,” he said.
He was a coauthor of a study analyzing the impact of an American Heart Association quality improvement project involving nearly 24,000 STEMI patients. Those with cardiogenic shock but no cardiac arrest had a 23.4% in-hospital mortality (JACC Cardiovasc Interv. 2018 Sep 24;11[18]:1824-33).
“We know that cardiogenic shock is an evolving process, and with this 23% mortality, perhaps myocardial salvage may still make a difference,” Dr. Gersh said.
The full results of CONDI-2/ERIC-PPCI have been published in the Lancet (2019 Oct 19;394[10207]:1415-24). In an accompanying editorial entitled “The Broken Promise of Remote Ischemic Conditioning,” Andrew Peter Vanezis, MD, of Royal Alexandra Hospital in Edmonton, Alta., said that “the role of remote ischemic conditioning in improving the lives of patients with STEMI has been thrown sharply into question. ... It might be time to abandon this form of cardioprotection in favor of more effective therapies” (Lancet. 2019 Oct 19;394:1389-90).
The CONDI-2/ERIC-PPCI study was funded by the British Heart Foundation, University College London, the Danish Innovation Foundation, the Novo Nordisk Foundation, and TrygFonden. Dr. Bøtker reported a financial relationship with CellAegis.
PARIS – Remote ischemic condition, long viewed as the best hope for the next breakthrough in improved clinical outcomes in acute MI, is now dead as a broad therapeutic strategy as a result of the resoundingly negative results of the CONDI-2/ERIC-PPCI trial, Hans Erik Bøtker, MD, PhD, declared at the annual congress of the European Society of Cardiology.
“I would not be quite honest if I did not admit that we were somewhat disappointed in these results, given the promising results we have seen in the majority of the proof-of-concept studies,” added Dr. Bøtker, professor of cardiovascular medicine and interventional cardiology at Aarhus (Denmark) University.
Remote ischemic conditioning entails imposing brief cycles of ischemia and reperfusion on an arm or leg prior to or during reperfusion of an occluded artery in the setting of acute MI in order to provide cardioprotection against reperfusion injury. In the small proof-of-concept studies, it reduced infarct size and suggested the possibility of better clinical outcomes. But the four-country, prospective, single-blind, randomized CONDI-2/ERIC-PPCI trial, involving 5,401 patients undergoing primary percutaneous coronary intervention (PCI) for ST-segment elevation MI (STEMI), showed nary a glimmer of the hoped-for clinical benefits from four cycles of 5 minutes of ischemia and 5 minutes of deflation of an automated arm-cuff device.
The primary outcome, a composite of the 12-month rate of cardiac death or heart failure hospitalization, occurred in 9.4% of the remote ischemic conditioning group, which wasn’t significantly different from the 8.6% rate in controls. Nor were there any significant between-group differences in infarct size at 48 hours based upon high-sensitivity troponin T measurement or any of the other prespecified secondary study endpoints.
Discussant Bernard J. Gersh, MB, ChB, PhD, a long-time fervent advocate of remote ischemic conditioning as an important potential advance in MI care, wasn’t an investigator in CONDI-2/ERIC-PPCI, which he characterized as “very well executed, large, and pivotal.”
“The results of this trial are emphatic, and there would appear to be no role for routine remote ischemic conditioning in the management of most – and I would emphasize ‘most,’ perhaps not all, but most – patients with STEMI undergoing primary PCI. The results may not be what we’d hoped to hear, and I share Dr. Bøtker’s disappointment, but the data appear to be conclusive in regard to the majority of the primary PCI population. It is what it is. The only remaining questions are why the result were almost completely universally neutral in CONDI-2/ERIC-PPCI after so many positive experimental models and proof-of-concept studies, and whether are there other patient subsets who might benefit,” according to Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
Pointing to the 30-day cardiac death rate of about 2% in the trial, he commented that the management of STEMI has gotten so good that it becomes very difficult to demonstrate a difference with remote ischemic conditioning, even if a small benefit is actually present.
“I think that’s the era that we now live in,” Dr. Gersh said.
However, he is not ready to throw the final shovelful of dirt on the grave of remote ischemic conditioning.
“There is a group where perhaps there may be a therapeutic benefit: STEMI patients presenting with cardiogenic shock and no cardiac arrest,” he said.
He was a coauthor of a study analyzing the impact of an American Heart Association quality improvement project involving nearly 24,000 STEMI patients. Those with cardiogenic shock but no cardiac arrest had a 23.4% in-hospital mortality (JACC Cardiovasc Interv. 2018 Sep 24;11[18]:1824-33).
“We know that cardiogenic shock is an evolving process, and with this 23% mortality, perhaps myocardial salvage may still make a difference,” Dr. Gersh said.
The full results of CONDI-2/ERIC-PPCI have been published in the Lancet (2019 Oct 19;394[10207]:1415-24). In an accompanying editorial entitled “The Broken Promise of Remote Ischemic Conditioning,” Andrew Peter Vanezis, MD, of Royal Alexandra Hospital in Edmonton, Alta., said that “the role of remote ischemic conditioning in improving the lives of patients with STEMI has been thrown sharply into question. ... It might be time to abandon this form of cardioprotection in favor of more effective therapies” (Lancet. 2019 Oct 19;394:1389-90).
The CONDI-2/ERIC-PPCI study was funded by the British Heart Foundation, University College London, the Danish Innovation Foundation, the Novo Nordisk Foundation, and TrygFonden. Dr. Bøtker reported a financial relationship with CellAegis.
PARIS – Remote ischemic condition, long viewed as the best hope for the next breakthrough in improved clinical outcomes in acute MI, is now dead as a broad therapeutic strategy as a result of the resoundingly negative results of the CONDI-2/ERIC-PPCI trial, Hans Erik Bøtker, MD, PhD, declared at the annual congress of the European Society of Cardiology.
“I would not be quite honest if I did not admit that we were somewhat disappointed in these results, given the promising results we have seen in the majority of the proof-of-concept studies,” added Dr. Bøtker, professor of cardiovascular medicine and interventional cardiology at Aarhus (Denmark) University.
Remote ischemic conditioning entails imposing brief cycles of ischemia and reperfusion on an arm or leg prior to or during reperfusion of an occluded artery in the setting of acute MI in order to provide cardioprotection against reperfusion injury. In the small proof-of-concept studies, it reduced infarct size and suggested the possibility of better clinical outcomes. But the four-country, prospective, single-blind, randomized CONDI-2/ERIC-PPCI trial, involving 5,401 patients undergoing primary percutaneous coronary intervention (PCI) for ST-segment elevation MI (STEMI), showed nary a glimmer of the hoped-for clinical benefits from four cycles of 5 minutes of ischemia and 5 minutes of deflation of an automated arm-cuff device.
The primary outcome, a composite of the 12-month rate of cardiac death or heart failure hospitalization, occurred in 9.4% of the remote ischemic conditioning group, which wasn’t significantly different from the 8.6% rate in controls. Nor were there any significant between-group differences in infarct size at 48 hours based upon high-sensitivity troponin T measurement or any of the other prespecified secondary study endpoints.
Discussant Bernard J. Gersh, MB, ChB, PhD, a long-time fervent advocate of remote ischemic conditioning as an important potential advance in MI care, wasn’t an investigator in CONDI-2/ERIC-PPCI, which he characterized as “very well executed, large, and pivotal.”
“The results of this trial are emphatic, and there would appear to be no role for routine remote ischemic conditioning in the management of most – and I would emphasize ‘most,’ perhaps not all, but most – patients with STEMI undergoing primary PCI. The results may not be what we’d hoped to hear, and I share Dr. Bøtker’s disappointment, but the data appear to be conclusive in regard to the majority of the primary PCI population. It is what it is. The only remaining questions are why the result were almost completely universally neutral in CONDI-2/ERIC-PPCI after so many positive experimental models and proof-of-concept studies, and whether are there other patient subsets who might benefit,” according to Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
Pointing to the 30-day cardiac death rate of about 2% in the trial, he commented that the management of STEMI has gotten so good that it becomes very difficult to demonstrate a difference with remote ischemic conditioning, even if a small benefit is actually present.
“I think that’s the era that we now live in,” Dr. Gersh said.
However, he is not ready to throw the final shovelful of dirt on the grave of remote ischemic conditioning.
“There is a group where perhaps there may be a therapeutic benefit: STEMI patients presenting with cardiogenic shock and no cardiac arrest,” he said.
He was a coauthor of a study analyzing the impact of an American Heart Association quality improvement project involving nearly 24,000 STEMI patients. Those with cardiogenic shock but no cardiac arrest had a 23.4% in-hospital mortality (JACC Cardiovasc Interv. 2018 Sep 24;11[18]:1824-33).
“We know that cardiogenic shock is an evolving process, and with this 23% mortality, perhaps myocardial salvage may still make a difference,” Dr. Gersh said.
The full results of CONDI-2/ERIC-PPCI have been published in the Lancet (2019 Oct 19;394[10207]:1415-24). In an accompanying editorial entitled “The Broken Promise of Remote Ischemic Conditioning,” Andrew Peter Vanezis, MD, of Royal Alexandra Hospital in Edmonton, Alta., said that “the role of remote ischemic conditioning in improving the lives of patients with STEMI has been thrown sharply into question. ... It might be time to abandon this form of cardioprotection in favor of more effective therapies” (Lancet. 2019 Oct 19;394:1389-90).
The CONDI-2/ERIC-PPCI study was funded by the British Heart Foundation, University College London, the Danish Innovation Foundation, the Novo Nordisk Foundation, and TrygFonden. Dr. Bøtker reported a financial relationship with CellAegis.
REPORTING FROM THE ESC CONGRESS 2019
Macitentan, tadalafil combo found effective for newly diagnosed PAH
NEW ORLEANS – Treatment with macitentan and tadalafil can elicit improvements in patients with newly diagnosed pulmonary arterial hypertension (PAH), trial results suggest.
In the phase 4 OPTIMA trial, the combination significantly improved cardiopulmonary hemodynamics, functional class, 6-minute walk distance, and N-terminal pro B-type natriuretic peptide (NT-proBNP).
Olivier Sitbon, MD, PhD, of Université Paris–Sud in France, presented these results at the annual meeting of the American College of Chest Physicians.
The OPTIMA trial (NCT02968901) enrolled 46 adults who were newly diagnosed with PAH and had medium functional ability (WHO functional class II-III). The patients’ mean age was 57.4 ± 14.9 years, and 65% of them were female.
The mean time from PAH diagnosis was 29.6 ± 55.2 days. Patients had idiopathic PAH (63%), PAH associated with connective tissue disease (19.6%), heritable PAH (6.5%), drug- or toxin-induced PAH (4.4%), HIV-associated PAH (2.2%), and “other” PAH (4.4%).
Patients initially received macitentan at 10 mg once daily and tadalafil at 20 mg once daily. After 8 ± 3 days, the tadalafil dose was increased to 40 mg once daily. The median duration of treatment was 19.9 months.
The researchers assessed efficacy at week 16, but patients were monitored for safety until the study was closed by the sponsor. There were 44 patients who remained on study through week 16, and 39 patients completed the study.
Results
The study’s primary endpoint was the change in pulmonary vascular resistance (PVR). The ratio of week 16 to baseline PVR was 0.53, which translates to a significant 47% reduction in PVR. In fact, 87% of patients had a 30% or greater decrease in PVR from baseline to week 16.
Patients had improvements in other endpoints as well. The mean cardiac index increased from 2.2 to 3.1 L/min/m2 (P less than .0001) from baseline to week 16. The mean pulmonary arterial pressure decreased from 50.0 to 42.2 mm Hg (P = .0002), and the mean right atrial pressure decreased from 8.1 to 7.8 mm Hg (P = .7321).
The mean mixed venous oxygen saturation increased from 63.0% to 68.2% (P = .0003). The mean total pulmonary resistance decreased from 1109.4 to 677.4 dynes/sec/cm-5 (P less than .0001).
NT-proBNP decreased 68% from baseline to week 16. The geometric mean ratio was 0.32 (P less than .0001). The 6-minute walk distance increased from 352.2 to 388.1 m (P = .0008).
None of the patients experienced a worsening of WHO functional class from baseline to week 16, and 63% of patients experienced an improvement.
Nearly 94% of patients (n = 43) had at least one adverse event, 28% (n = 13) had serious adverse events, and 6.5% (n = 3) stopped treatment because of adverse events. The most frequent events were peripheral edema (n = 13), headache (n = 11), diarrhea (n = 9), and dyspnea (n = 7).
Three patients died during follow-up, one due to multiorgan failure and two due to underlying disease.
Actelion Pharmaceuticals funded the trial. Dr. Sitbon disclosed relationships with Actelion, Bayer, GSK, Merck, Arena Pharmaceuticals, Gossamer Bio, and Ferrer.
SOURCE: Sitbon O et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.825.
NEW ORLEANS – Treatment with macitentan and tadalafil can elicit improvements in patients with newly diagnosed pulmonary arterial hypertension (PAH), trial results suggest.
In the phase 4 OPTIMA trial, the combination significantly improved cardiopulmonary hemodynamics, functional class, 6-minute walk distance, and N-terminal pro B-type natriuretic peptide (NT-proBNP).
Olivier Sitbon, MD, PhD, of Université Paris–Sud in France, presented these results at the annual meeting of the American College of Chest Physicians.
The OPTIMA trial (NCT02968901) enrolled 46 adults who were newly diagnosed with PAH and had medium functional ability (WHO functional class II-III). The patients’ mean age was 57.4 ± 14.9 years, and 65% of them were female.
The mean time from PAH diagnosis was 29.6 ± 55.2 days. Patients had idiopathic PAH (63%), PAH associated with connective tissue disease (19.6%), heritable PAH (6.5%), drug- or toxin-induced PAH (4.4%), HIV-associated PAH (2.2%), and “other” PAH (4.4%).
Patients initially received macitentan at 10 mg once daily and tadalafil at 20 mg once daily. After 8 ± 3 days, the tadalafil dose was increased to 40 mg once daily. The median duration of treatment was 19.9 months.
The researchers assessed efficacy at week 16, but patients were monitored for safety until the study was closed by the sponsor. There were 44 patients who remained on study through week 16, and 39 patients completed the study.
Results
The study’s primary endpoint was the change in pulmonary vascular resistance (PVR). The ratio of week 16 to baseline PVR was 0.53, which translates to a significant 47% reduction in PVR. In fact, 87% of patients had a 30% or greater decrease in PVR from baseline to week 16.
Patients had improvements in other endpoints as well. The mean cardiac index increased from 2.2 to 3.1 L/min/m2 (P less than .0001) from baseline to week 16. The mean pulmonary arterial pressure decreased from 50.0 to 42.2 mm Hg (P = .0002), and the mean right atrial pressure decreased from 8.1 to 7.8 mm Hg (P = .7321).
The mean mixed venous oxygen saturation increased from 63.0% to 68.2% (P = .0003). The mean total pulmonary resistance decreased from 1109.4 to 677.4 dynes/sec/cm-5 (P less than .0001).
NT-proBNP decreased 68% from baseline to week 16. The geometric mean ratio was 0.32 (P less than .0001). The 6-minute walk distance increased from 352.2 to 388.1 m (P = .0008).
None of the patients experienced a worsening of WHO functional class from baseline to week 16, and 63% of patients experienced an improvement.
Nearly 94% of patients (n = 43) had at least one adverse event, 28% (n = 13) had serious adverse events, and 6.5% (n = 3) stopped treatment because of adverse events. The most frequent events were peripheral edema (n = 13), headache (n = 11), diarrhea (n = 9), and dyspnea (n = 7).
Three patients died during follow-up, one due to multiorgan failure and two due to underlying disease.
Actelion Pharmaceuticals funded the trial. Dr. Sitbon disclosed relationships with Actelion, Bayer, GSK, Merck, Arena Pharmaceuticals, Gossamer Bio, and Ferrer.
SOURCE: Sitbon O et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.825.
NEW ORLEANS – Treatment with macitentan and tadalafil can elicit improvements in patients with newly diagnosed pulmonary arterial hypertension (PAH), trial results suggest.
In the phase 4 OPTIMA trial, the combination significantly improved cardiopulmonary hemodynamics, functional class, 6-minute walk distance, and N-terminal pro B-type natriuretic peptide (NT-proBNP).
Olivier Sitbon, MD, PhD, of Université Paris–Sud in France, presented these results at the annual meeting of the American College of Chest Physicians.
The OPTIMA trial (NCT02968901) enrolled 46 adults who were newly diagnosed with PAH and had medium functional ability (WHO functional class II-III). The patients’ mean age was 57.4 ± 14.9 years, and 65% of them were female.
The mean time from PAH diagnosis was 29.6 ± 55.2 days. Patients had idiopathic PAH (63%), PAH associated with connective tissue disease (19.6%), heritable PAH (6.5%), drug- or toxin-induced PAH (4.4%), HIV-associated PAH (2.2%), and “other” PAH (4.4%).
Patients initially received macitentan at 10 mg once daily and tadalafil at 20 mg once daily. After 8 ± 3 days, the tadalafil dose was increased to 40 mg once daily. The median duration of treatment was 19.9 months.
The researchers assessed efficacy at week 16, but patients were monitored for safety until the study was closed by the sponsor. There were 44 patients who remained on study through week 16, and 39 patients completed the study.
Results
The study’s primary endpoint was the change in pulmonary vascular resistance (PVR). The ratio of week 16 to baseline PVR was 0.53, which translates to a significant 47% reduction in PVR. In fact, 87% of patients had a 30% or greater decrease in PVR from baseline to week 16.
Patients had improvements in other endpoints as well. The mean cardiac index increased from 2.2 to 3.1 L/min/m2 (P less than .0001) from baseline to week 16. The mean pulmonary arterial pressure decreased from 50.0 to 42.2 mm Hg (P = .0002), and the mean right atrial pressure decreased from 8.1 to 7.8 mm Hg (P = .7321).
The mean mixed venous oxygen saturation increased from 63.0% to 68.2% (P = .0003). The mean total pulmonary resistance decreased from 1109.4 to 677.4 dynes/sec/cm-5 (P less than .0001).
NT-proBNP decreased 68% from baseline to week 16. The geometric mean ratio was 0.32 (P less than .0001). The 6-minute walk distance increased from 352.2 to 388.1 m (P = .0008).
None of the patients experienced a worsening of WHO functional class from baseline to week 16, and 63% of patients experienced an improvement.
Nearly 94% of patients (n = 43) had at least one adverse event, 28% (n = 13) had serious adverse events, and 6.5% (n = 3) stopped treatment because of adverse events. The most frequent events were peripheral edema (n = 13), headache (n = 11), diarrhea (n = 9), and dyspnea (n = 7).
Three patients died during follow-up, one due to multiorgan failure and two due to underlying disease.
Actelion Pharmaceuticals funded the trial. Dr. Sitbon disclosed relationships with Actelion, Bayer, GSK, Merck, Arena Pharmaceuticals, Gossamer Bio, and Ferrer.
SOURCE: Sitbon O et al. CHEST 2019. Abstract, doi: 10.1016/j.chest.2019.08.825.
REPORTING FROM CHEST 2019
In-hospital flu shot reduced readmissions in pneumonia patients
NEW ORLEANS – In-hospital flu shots were rare, yet linked to a lower readmission rate for patients hospitalized with community-acquired pneumonia in a recent retrospective study, suggesting a “missed opportunity” to improve outcomes for these patients, an investigator said.
Less than 2% of patients admitted for community-acquired pneumonia (CAP) received in-hospital influenza vaccination, yet receiving it was linked to a 20% reduction in readmissions, according to investigator Kam Sing Ho, MD, a resident at Mount Sinai St. Luke’s, New York.
Those patients who were readmitted had a significantly higher death rate vs. index admissions, Dr. Ho said in a poster discussion session at the annual meeting of the American College of Chest Physicians.
“I know (vaccines) are pretty much pushed out to the outpatient setting, but given what we showed here in this abstract, I think there’s a role for influenza vaccines to be a discussion in the hospital,” Dr. Ho said in his presentation.
The retrospective analysis was based on 825,906 adult hospital admissions with a primary diagnosis of CAP in data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Of that large cohort, just 14,047 (1.91%) received in-hospital influenza vaccination, according to Dr. Ho.
In-hospital influenza vaccination independently predicted a lower risk of readmission (hazard ratio, 0.821; 95% confidence interval, 0.69-0.98; P less than .02) in a propensity score matching analysis that included 9,777 CAP patients who received the vaccination and 9,777 with similar demographic and clinical characteristics.
Private insurance and high-income status also predicted lower risk of readmission in the analysis, while by contrast, factors associated with higher risk of readmission included advanced age, Medicare insurance, and respiratory failure, among other factors, Dr. Ho reported.
The overall 30-day rate of readmission in the study was 11.9%, and of those readmissions, the great majority (about 80%) were due to pneumonia, he said.
The rate of death in the hospital was 2.96% for CAP patients who were readmitted, versus 1.11% for the index admissions (P less than .001), Dr. Ho reported. Moreover, readmissions were associated with nearly half a million hospital days and $1 billion in costs and $3.67 billion in charges.
Based on these findings, Dr. Ho and colleagues hope to incorporate routine influenza vaccination for all adults hospitalized with CAP.
“We’re always under pressure to do so much for patients that we can’t comprehensively do everything. But the 20% reduction in the risk of coming back, I think that’s significant,” Dr. Ho said in an interview.
The authors reported having no disclosures related to this research.
This article was updated 10/23/2019.
SOURCE: Ho KS, et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.450.
NEW ORLEANS – In-hospital flu shots were rare, yet linked to a lower readmission rate for patients hospitalized with community-acquired pneumonia in a recent retrospective study, suggesting a “missed opportunity” to improve outcomes for these patients, an investigator said.
Less than 2% of patients admitted for community-acquired pneumonia (CAP) received in-hospital influenza vaccination, yet receiving it was linked to a 20% reduction in readmissions, according to investigator Kam Sing Ho, MD, a resident at Mount Sinai St. Luke’s, New York.
Those patients who were readmitted had a significantly higher death rate vs. index admissions, Dr. Ho said in a poster discussion session at the annual meeting of the American College of Chest Physicians.
“I know (vaccines) are pretty much pushed out to the outpatient setting, but given what we showed here in this abstract, I think there’s a role for influenza vaccines to be a discussion in the hospital,” Dr. Ho said in his presentation.
The retrospective analysis was based on 825,906 adult hospital admissions with a primary diagnosis of CAP in data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Of that large cohort, just 14,047 (1.91%) received in-hospital influenza vaccination, according to Dr. Ho.
In-hospital influenza vaccination independently predicted a lower risk of readmission (hazard ratio, 0.821; 95% confidence interval, 0.69-0.98; P less than .02) in a propensity score matching analysis that included 9,777 CAP patients who received the vaccination and 9,777 with similar demographic and clinical characteristics.
Private insurance and high-income status also predicted lower risk of readmission in the analysis, while by contrast, factors associated with higher risk of readmission included advanced age, Medicare insurance, and respiratory failure, among other factors, Dr. Ho reported.
The overall 30-day rate of readmission in the study was 11.9%, and of those readmissions, the great majority (about 80%) were due to pneumonia, he said.
The rate of death in the hospital was 2.96% for CAP patients who were readmitted, versus 1.11% for the index admissions (P less than .001), Dr. Ho reported. Moreover, readmissions were associated with nearly half a million hospital days and $1 billion in costs and $3.67 billion in charges.
Based on these findings, Dr. Ho and colleagues hope to incorporate routine influenza vaccination for all adults hospitalized with CAP.
“We’re always under pressure to do so much for patients that we can’t comprehensively do everything. But the 20% reduction in the risk of coming back, I think that’s significant,” Dr. Ho said in an interview.
The authors reported having no disclosures related to this research.
This article was updated 10/23/2019.
SOURCE: Ho KS, et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.450.
NEW ORLEANS – In-hospital flu shots were rare, yet linked to a lower readmission rate for patients hospitalized with community-acquired pneumonia in a recent retrospective study, suggesting a “missed opportunity” to improve outcomes for these patients, an investigator said.
Less than 2% of patients admitted for community-acquired pneumonia (CAP) received in-hospital influenza vaccination, yet receiving it was linked to a 20% reduction in readmissions, according to investigator Kam Sing Ho, MD, a resident at Mount Sinai St. Luke’s, New York.
Those patients who were readmitted had a significantly higher death rate vs. index admissions, Dr. Ho said in a poster discussion session at the annual meeting of the American College of Chest Physicians.
“I know (vaccines) are pretty much pushed out to the outpatient setting, but given what we showed here in this abstract, I think there’s a role for influenza vaccines to be a discussion in the hospital,” Dr. Ho said in his presentation.
The retrospective analysis was based on 825,906 adult hospital admissions with a primary diagnosis of CAP in data from the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Of that large cohort, just 14,047 (1.91%) received in-hospital influenza vaccination, according to Dr. Ho.
In-hospital influenza vaccination independently predicted a lower risk of readmission (hazard ratio, 0.821; 95% confidence interval, 0.69-0.98; P less than .02) in a propensity score matching analysis that included 9,777 CAP patients who received the vaccination and 9,777 with similar demographic and clinical characteristics.
Private insurance and high-income status also predicted lower risk of readmission in the analysis, while by contrast, factors associated with higher risk of readmission included advanced age, Medicare insurance, and respiratory failure, among other factors, Dr. Ho reported.
The overall 30-day rate of readmission in the study was 11.9%, and of those readmissions, the great majority (about 80%) were due to pneumonia, he said.
The rate of death in the hospital was 2.96% for CAP patients who were readmitted, versus 1.11% for the index admissions (P less than .001), Dr. Ho reported. Moreover, readmissions were associated with nearly half a million hospital days and $1 billion in costs and $3.67 billion in charges.
Based on these findings, Dr. Ho and colleagues hope to incorporate routine influenza vaccination for all adults hospitalized with CAP.
“We’re always under pressure to do so much for patients that we can’t comprehensively do everything. But the 20% reduction in the risk of coming back, I think that’s significant,” Dr. Ho said in an interview.
The authors reported having no disclosures related to this research.
This article was updated 10/23/2019.
SOURCE: Ho KS, et al. CHEST 2019. doi: 10.1016/j.chest.2019.08.450.
REPORTING FROM CHEST 2019
Exercise intolerance linked to neurocognitive dysfunction in ALL
Exercise intolerance was associated with worse neurocognitive function in adult survivors of pediatric acute lymphoblastic leukemia (ALL), according to results from a cross-sectional study.
The findings suggest additional research is needed to better understand the effects of increased exercise capacity on neurocognitive performance in these patients.
“We used a clinically assessed cohort of childhood cancer survivors participating in the St. Jude Lifetime cohort study to determine whether exercise intolerance, expressed as decreased oxygen uptake, is associated with neurocognitive impairments in long-term survivors of childhood ALL and evaluated whether exercise intolerance mediates the association between chronic cardiac or pulmonary conditions and neurocognitive impairment,” wrote Nicholas S. Phillips, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues. The results were published in Cancer.
The cross-sectional cohort study included 341 young adult survivors of pediatric ALL and 288 evaluable control participants. Survivors were recruited from 1980 to 2003.
Eligible participants were 18 years or older, and remained alive for a minimum of 5 years post ALL diagnosis. Control subjects were non–first-degree relatives of ALL survivors.
The researchers evaluated exercise capacity using cardiopulmonary exercise testing, expressed as relative peak volume of oxygen (rpkVO2) max scores. Other tests included self-rated questionnaires, as well as a standardized neuropsychological evaluation.
After analysis, the researchers found that ALL survivors had lower mean rpkVO2 scores, compared with control participants (23.45 vs. 33.03 mL/kg per min; P less than .001).
Survivors also had worse performance on several measures of neurocognitive function, including working memory, verbal intelligence, visual-motor speed, and other math and reading domains, compared with controls (all P less than .001).
The researchers also performed a multivariable analysis and found that a 1-unit metabolic equivalent increase in exercise tolerance was associated with significantly increased performance in some neurocognitive measures, including attention, verbal ability, verbal fluency, motor speed, and academics.
“Our research suggests that a minor improvement in exercise tolerance, such as going from sitting on the couch and watching TV, to walking around the block for 30 minutes a day, can have a significant impact on survivors’ intellectual health,” Dr. Phillips said in a statement.
The researchers noted that recent evidence has shown that structured exercise training may benefit younger survivors. “These studies demonstrate that a low-cost, home-based exercise training program can effectively increase cardiopulmonary fitness during and after completion of therapy,” they wrote.
The team acknowledged a key limitation of the study was the cross-sectional design. As a result, the direction of these associations remains unknown, and warrants future study.
The study was supported by the National Institutes of Health and the American Lebanese Syrian Associated Charities. The researchers did not report conflicts of interest.
SOURCE: Phillips NS et al. Cancer. 2019 Oct 21. doi: 10.1002/cncr.32510.
Exercise intolerance was associated with worse neurocognitive function in adult survivors of pediatric acute lymphoblastic leukemia (ALL), according to results from a cross-sectional study.
The findings suggest additional research is needed to better understand the effects of increased exercise capacity on neurocognitive performance in these patients.
“We used a clinically assessed cohort of childhood cancer survivors participating in the St. Jude Lifetime cohort study to determine whether exercise intolerance, expressed as decreased oxygen uptake, is associated with neurocognitive impairments in long-term survivors of childhood ALL and evaluated whether exercise intolerance mediates the association between chronic cardiac or pulmonary conditions and neurocognitive impairment,” wrote Nicholas S. Phillips, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues. The results were published in Cancer.
The cross-sectional cohort study included 341 young adult survivors of pediatric ALL and 288 evaluable control participants. Survivors were recruited from 1980 to 2003.
Eligible participants were 18 years or older, and remained alive for a minimum of 5 years post ALL diagnosis. Control subjects were non–first-degree relatives of ALL survivors.
The researchers evaluated exercise capacity using cardiopulmonary exercise testing, expressed as relative peak volume of oxygen (rpkVO2) max scores. Other tests included self-rated questionnaires, as well as a standardized neuropsychological evaluation.
After analysis, the researchers found that ALL survivors had lower mean rpkVO2 scores, compared with control participants (23.45 vs. 33.03 mL/kg per min; P less than .001).
Survivors also had worse performance on several measures of neurocognitive function, including working memory, verbal intelligence, visual-motor speed, and other math and reading domains, compared with controls (all P less than .001).
The researchers also performed a multivariable analysis and found that a 1-unit metabolic equivalent increase in exercise tolerance was associated with significantly increased performance in some neurocognitive measures, including attention, verbal ability, verbal fluency, motor speed, and academics.
“Our research suggests that a minor improvement in exercise tolerance, such as going from sitting on the couch and watching TV, to walking around the block for 30 minutes a day, can have a significant impact on survivors’ intellectual health,” Dr. Phillips said in a statement.
The researchers noted that recent evidence has shown that structured exercise training may benefit younger survivors. “These studies demonstrate that a low-cost, home-based exercise training program can effectively increase cardiopulmonary fitness during and after completion of therapy,” they wrote.
The team acknowledged a key limitation of the study was the cross-sectional design. As a result, the direction of these associations remains unknown, and warrants future study.
The study was supported by the National Institutes of Health and the American Lebanese Syrian Associated Charities. The researchers did not report conflicts of interest.
SOURCE: Phillips NS et al. Cancer. 2019 Oct 21. doi: 10.1002/cncr.32510.
Exercise intolerance was associated with worse neurocognitive function in adult survivors of pediatric acute lymphoblastic leukemia (ALL), according to results from a cross-sectional study.
The findings suggest additional research is needed to better understand the effects of increased exercise capacity on neurocognitive performance in these patients.
“We used a clinically assessed cohort of childhood cancer survivors participating in the St. Jude Lifetime cohort study to determine whether exercise intolerance, expressed as decreased oxygen uptake, is associated with neurocognitive impairments in long-term survivors of childhood ALL and evaluated whether exercise intolerance mediates the association between chronic cardiac or pulmonary conditions and neurocognitive impairment,” wrote Nicholas S. Phillips, MD, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues. The results were published in Cancer.
The cross-sectional cohort study included 341 young adult survivors of pediatric ALL and 288 evaluable control participants. Survivors were recruited from 1980 to 2003.
Eligible participants were 18 years or older, and remained alive for a minimum of 5 years post ALL diagnosis. Control subjects were non–first-degree relatives of ALL survivors.
The researchers evaluated exercise capacity using cardiopulmonary exercise testing, expressed as relative peak volume of oxygen (rpkVO2) max scores. Other tests included self-rated questionnaires, as well as a standardized neuropsychological evaluation.
After analysis, the researchers found that ALL survivors had lower mean rpkVO2 scores, compared with control participants (23.45 vs. 33.03 mL/kg per min; P less than .001).
Survivors also had worse performance on several measures of neurocognitive function, including working memory, verbal intelligence, visual-motor speed, and other math and reading domains, compared with controls (all P less than .001).
The researchers also performed a multivariable analysis and found that a 1-unit metabolic equivalent increase in exercise tolerance was associated with significantly increased performance in some neurocognitive measures, including attention, verbal ability, verbal fluency, motor speed, and academics.
“Our research suggests that a minor improvement in exercise tolerance, such as going from sitting on the couch and watching TV, to walking around the block for 30 minutes a day, can have a significant impact on survivors’ intellectual health,” Dr. Phillips said in a statement.
The researchers noted that recent evidence has shown that structured exercise training may benefit younger survivors. “These studies demonstrate that a low-cost, home-based exercise training program can effectively increase cardiopulmonary fitness during and after completion of therapy,” they wrote.
The team acknowledged a key limitation of the study was the cross-sectional design. As a result, the direction of these associations remains unknown, and warrants future study.
The study was supported by the National Institutes of Health and the American Lebanese Syrian Associated Charities. The researchers did not report conflicts of interest.
SOURCE: Phillips NS et al. Cancer. 2019 Oct 21. doi: 10.1002/cncr.32510.
FROM CANCER
FDA approves minocycline foam for moderate, severe acne
The Food and Drug Administration has vulgaris in adults and pediatric patients aged at least 9 years.
FDA approval was based on three phase 3, 12-week, multicenter, randomized, double-blind, vehicle-controlled studies of patients with moderate to severe acne vulgaris who were treated once daily with minocycline 4% plus vehicle or vehicle alone, according to a press release from the manufacturer, Foamix Pharmaceuticals. In all three studies, patients receiving minocycline had a reduction in the number of inflammatory lesions, compared with vehicle alone; in two studies, patients in the minocycline groups had significantly improved Investigator’s Global Assessment scores.
The most commonly reported adverse event reported during the trials was headache. No serious adverse events were reported. The company says it is expected to be available in January 2020.
Find the prescribing information on the FDA website.
The Food and Drug Administration has vulgaris in adults and pediatric patients aged at least 9 years.
FDA approval was based on three phase 3, 12-week, multicenter, randomized, double-blind, vehicle-controlled studies of patients with moderate to severe acne vulgaris who were treated once daily with minocycline 4% plus vehicle or vehicle alone, according to a press release from the manufacturer, Foamix Pharmaceuticals. In all three studies, patients receiving minocycline had a reduction in the number of inflammatory lesions, compared with vehicle alone; in two studies, patients in the minocycline groups had significantly improved Investigator’s Global Assessment scores.
The most commonly reported adverse event reported during the trials was headache. No serious adverse events were reported. The company says it is expected to be available in January 2020.
Find the prescribing information on the FDA website.
The Food and Drug Administration has vulgaris in adults and pediatric patients aged at least 9 years.
FDA approval was based on three phase 3, 12-week, multicenter, randomized, double-blind, vehicle-controlled studies of patients with moderate to severe acne vulgaris who were treated once daily with minocycline 4% plus vehicle or vehicle alone, according to a press release from the manufacturer, Foamix Pharmaceuticals. In all three studies, patients receiving minocycline had a reduction in the number of inflammatory lesions, compared with vehicle alone; in two studies, patients in the minocycline groups had significantly improved Investigator’s Global Assessment scores.
The most commonly reported adverse event reported during the trials was headache. No serious adverse events were reported. The company says it is expected to be available in January 2020.
Find the prescribing information on the FDA website.
FDA approves Trikafta for treatment of cystic fibrosis
in patients aged 12 years or older, the first triple-combination therapy approved for that indication.
Approval for Trikafta was based on results from two clinical trials in patients with cystic fibrosis with an F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. In the first trial, a 24-week, randomized, double-blind, placebo-controlled study of 403 patients, the mean percent predicted forced expiratory volume in 1 second increased by 14% from baseline, compared with placebo. In the second trial, a 4-week, randomized, double-blind, active-controlled study of 107 patients, mean percent predicted forced expiratory volume in 1 second was increased 10% from baseline, compared with tezacaftor/ivacaftor, according to the FDA press release.
In the first trial, patients who received Trikafta also saw improvement in sweat chloride, reduction in the number of pulmonary exacerbations, and reduction of body mass index, compared with placebo.
The most common adverse events associated with Trikafta during the trials were headaches, upper respiratory tract infections, abdominal pains, diarrhea, rashes, and rhinorrhea, among others. The label includes a warning related to elevated liver function tests, use at the same time with products that induce or inhibit a liver enzyme called cytochrome P450 3A4, and cataract risk.
“At the FDA, we’re consistently looking for ways to help speed the development of new therapies for complex diseases, while maintaining our high standards of review. Today’s landmark approval is a testament to these efforts, making a novel treatment available to most cystic fibrosis patients, including adolescents, who previously had no options and giving others in the cystic fibrosis community access to an additional effective therapy,” said acting FDA Commissioner Ned Sharpless, MD.
Find the full press release on the FDA website.
in patients aged 12 years or older, the first triple-combination therapy approved for that indication.
Approval for Trikafta was based on results from two clinical trials in patients with cystic fibrosis with an F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. In the first trial, a 24-week, randomized, double-blind, placebo-controlled study of 403 patients, the mean percent predicted forced expiratory volume in 1 second increased by 14% from baseline, compared with placebo. In the second trial, a 4-week, randomized, double-blind, active-controlled study of 107 patients, mean percent predicted forced expiratory volume in 1 second was increased 10% from baseline, compared with tezacaftor/ivacaftor, according to the FDA press release.
In the first trial, patients who received Trikafta also saw improvement in sweat chloride, reduction in the number of pulmonary exacerbations, and reduction of body mass index, compared with placebo.
The most common adverse events associated with Trikafta during the trials were headaches, upper respiratory tract infections, abdominal pains, diarrhea, rashes, and rhinorrhea, among others. The label includes a warning related to elevated liver function tests, use at the same time with products that induce or inhibit a liver enzyme called cytochrome P450 3A4, and cataract risk.
“At the FDA, we’re consistently looking for ways to help speed the development of new therapies for complex diseases, while maintaining our high standards of review. Today’s landmark approval is a testament to these efforts, making a novel treatment available to most cystic fibrosis patients, including adolescents, who previously had no options and giving others in the cystic fibrosis community access to an additional effective therapy,” said acting FDA Commissioner Ned Sharpless, MD.
Find the full press release on the FDA website.
in patients aged 12 years or older, the first triple-combination therapy approved for that indication.
Approval for Trikafta was based on results from two clinical trials in patients with cystic fibrosis with an F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. In the first trial, a 24-week, randomized, double-blind, placebo-controlled study of 403 patients, the mean percent predicted forced expiratory volume in 1 second increased by 14% from baseline, compared with placebo. In the second trial, a 4-week, randomized, double-blind, active-controlled study of 107 patients, mean percent predicted forced expiratory volume in 1 second was increased 10% from baseline, compared with tezacaftor/ivacaftor, according to the FDA press release.
In the first trial, patients who received Trikafta also saw improvement in sweat chloride, reduction in the number of pulmonary exacerbations, and reduction of body mass index, compared with placebo.
The most common adverse events associated with Trikafta during the trials were headaches, upper respiratory tract infections, abdominal pains, diarrhea, rashes, and rhinorrhea, among others. The label includes a warning related to elevated liver function tests, use at the same time with products that induce or inhibit a liver enzyme called cytochrome P450 3A4, and cataract risk.
“At the FDA, we’re consistently looking for ways to help speed the development of new therapies for complex diseases, while maintaining our high standards of review. Today’s landmark approval is a testament to these efforts, making a novel treatment available to most cystic fibrosis patients, including adolescents, who previously had no options and giving others in the cystic fibrosis community access to an additional effective therapy,” said acting FDA Commissioner Ned Sharpless, MD.
Find the full press release on the FDA website.